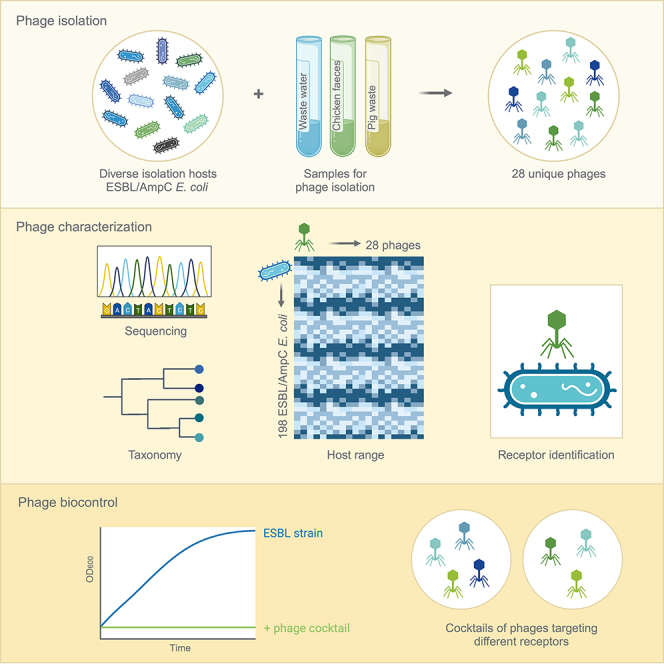
Summary
Novel solutions are needed to reduce the risk of transmission of extended spectrum β-lactamase (ESBL) and AmpC β-lactamase producing Escherichia coli (ESBL/AmpC E. coli) from livestock to humans. Given that phages are promising biocontrol agents, a collection of 28 phages that infect ESBL/AmpC E. coli were established. Whole genome sequencing showed that all these phages were unique and could be assigned to 15 different genera. Host range analysis showed that 82% of 198 strains, representing the genetic diversity of ESBL/AmpC E. coli, were sensitive to at least one phage. Identifying receptors used for phage binding experimentally as well as in silico predictions, allowed us to combine phages into two different cocktails with broad host range targeting diverse receptors. These phage cocktails efficiently inhibit the growth of ESBL/AmpC E. coli in vitro, thus suggesting the potential of phages as promising biocontrol agents.
Subject areas: Biological sciences, Microbiology, Bacteriology, Virology
Graphical abstract
Highlights
-
•
28 unique phages infecting ESBL/AmpC E. coli were isolated and characterized
-
•
Broad host range phages targeting different receptors were used to compose phage cocktails
-
•
Phage cocktails efficiently inhibit the growth of ESBL/AmpC E. coli in vitro
Biological sciences; Microbiology; Bacteriology; Virology
Introduction
The rise of antibiotic resistance and emergence of multi-drug resistant bacteria is a global problem. Of special concern are extended spectrum β-lactamase (ESBL) and AmpC β-lactamase (AmpC) producing E. coli (hereafter referred to as ESBL/AmpC E. coli) that are resistant to a broad spectrum of antibiotics, including penicillin and third generation cephalosporins.1 ESBL/AmpC E. coli show large genomic diversity and are represented in all phylogroups and sequence types (STs) of E. coli.2,3,4 In accordance, the O-antigen of the lipopolysaccharides displayed by ESBL are highly diverse, representing a large proportion of the 185 different O-antigens found in E. coli.5 The AmpC β-lactamases are chromosomally encoded genes showing upregulated expression in resistant strains,6 whereas ESBL genes are mainly associated with a wide range of conjugative plasmids.7,8,9,10 Due to the transmissible nature of these plasmids, ESBL genes may spread both in vitro and in vivo.11 In livestock ESBL/AmpC E. coli is mainly commensal but may transfer antibiotic resistance genes into pathogenic E. coli as well as related pathogens to the human reservoir through contaminated foods.12,13 Thus, applying a One Health approach reducing the prevalence of ESBL/AmpC E. coli in animal reservoirs may minimize emergence of antibiotic resistant pathogenic E. coli.14 Different decolonization approaches have been proposed to reduce ESBL/AmpC E. coli prevalence in poultry flocks and pig pens.15 Among these approaches are diverse cleaning and disinfection agents, attempting competitive exclusion using probiotic cultures, and specific feed additives showed strong effect in prevention in some studies (reviewed in Becker et al.,16) However, complete decolonization of animals has proven challenging, and, in most cases, the applied approaches were ineffective against ESBL/AmpC E. coli.15,16 There is therefore a need for alternative methods to reduce the numbers of ESBL/AmpC E. coli in livestock.
Bacteriophages (phages) are viruses that infect and kill bacteria and have been used for biocontrol purposes as well as phage therapy targeting pathogenic bacteria (reviewed in Wittebole et al.,17). Phages are host-specific, often infecting only specific species or even strains, leaving the rest of the microbiota unharmed. Additionally, they are self-replicating and self-limiting as they replicate only in the presence of a suitable host.18 Many diverse phages infecting E. coli have been described and diverse collections are well characterized, providing an insight into their diversity, genomics, and interactions with their E. coli host.19,20,21,22,23,24,25,26 These studies show that coliphages are found in diverse environments, including feces, wastewater, soil, and water and have host ranges infecting specific E. coli strains. The host specificity is highly influenced by receptor binding proteins (RBPs) forming tail fibers or tail spike proteins located at the distal tail allowing binding to specific host receptors.27 For coliphages receptors may be proteins residing in the outer membrane, most of which forms a β-barrel structure and serve as permeability channels for nutrients, toxins, and antibiotics.28,29 For example, phage T2 recognize the outer membrane protein Tsx of E. coli.30 In addition, surface carbohydrates like capsular polysaccharides, enterobacterial common antigen and lipopolysaccharides carrying the highly diverse O-antigen may serve as receptors for E. coli phages.25 However, the capsule as well as the long chains of O-antigen may also mask the outer membrane including the protein receptors and prevent infection, thus playing a dual role in phage susceptibility of E. coli.31 Thus, through evolution phages have developed diverse RBPs for binding different receptors and ensuring recognition of their host bacteria.
Still, for phages infecting ESBL, receptors have not been identified yet as only a few studies using existing coliphage collections with limited characterization have been used to determine phage susceptibility of ESBL/AmpC E. coli. Two studies showed that phages isolated using environmental E. coli strains can infect and kill ESBL/AmpC E. coli with varying but low efficiency.24,32 In addition, we previously determined the ability of 16 coliphages isolated on the E. coli Reference collection (ECOR) to infect and form plaques on a large diverse ESBL/AmpC E. coli collection.4 However, these phages were only able to infect 23% of the 198 strains in the collection with varying efficacy, suggesting a need for other phages to cover the diversity of ESBL/AmpC E. coli.4 Other studies isolated phages using ESBL/AmpC E. coli as hosts, but only determined lysis on bacterial lawns adding phages in high concentration and true phage infection were not demonstrated.33,34,35,36 Thus, studies characterizing phage infection of ESBL/AmpC E. coli as well as their potential for biocontrol are limited. To increase the diversity of phages infecting ESBL/AmpC E. coli and provide well-characterized phages for biocontrol, we isolated and characterize phages infecting diverse ESBL/AmpC E. coli. Subsequently, the collection was used to compose two different phage cocktails to explore the possibility of using them for biocontrol of ESBL/AmpC E. coli.
Results
Isolation of phages infecting ESBL/AmpC E. coli using a diverse set of strains and samples
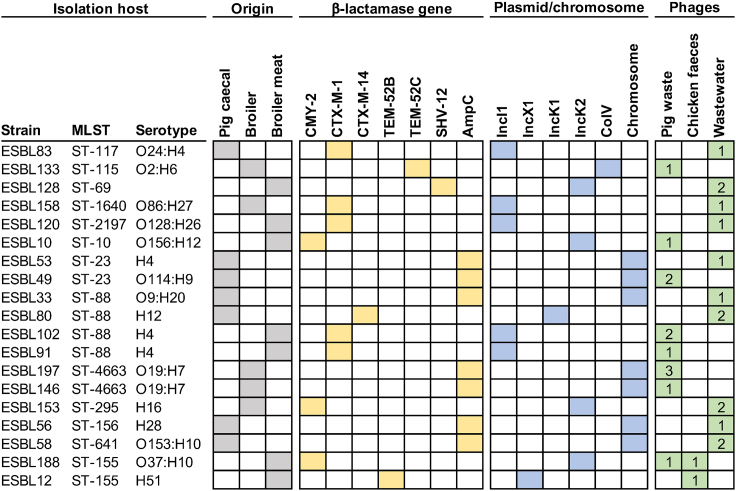
For isolating phages infecting ESBL/AmpC E. coli, we took advantage of our previously established large collection of 198 ESBL/AmpC E. coli covering the genetic diversity of this group of bacteria.4 To maximize the chances of isolating diverse phages, 19 diverse strains were selected as isolation hosts based on different genetic background defined by multi-locus sequence typing (MLST) and carriers of diverse β-lactamase genes and plasmids (Figure 1). The samples for phage isolation were collected from environments expected to contain ESBL/AmpC E. coli including five samples of pig waste from a biogas production plant and two samples of broiler feces. Additionally, two samples from aeration tanks in a wastewater treatment plant were collected to increase the likelihood of capturing diverse phages, as wastewater has proven to be a rich source of phages.23 A total of 28 phages were isolated either by direct plating or by enrichment in the presence of one of the 19 different isolation host (Figure 1). Most phages were isolated from wastewater (n = 14) and pig waste (n = 12), whereas only two phages were isolated from broiler feces. There were no apparent correlations between the origin of isolation hosts and samples, as host strains originating from pig mostly isolated phages from wastewater and two phages from pig waste, whereas broiler strains isolated phages from all sources. Finally, most strains isolated only 1–2 phages, thus indicating potentially diverse phages.
Figure 1.
Diverse ESBL/AmpC E. coli are used for phage isolation from animal and wastewater samples
The MLST as well as O- and H- antigen was extracted from Enterobase and a phylogenetic tree was made and visualized using iTOL v6.37 Columns indicate ST using MLST, serotypes, origin of strain (gray), type of β-lactamase gene (yellow) carried on specific plasmid types or the chromosome (blue) obtained from Vitt et al.,4 Number of phages isolated from either pig waste, chicken feces, or wastewater on each isolation host is indicted (green).
Taxonomic assignment and overall genetic comparison of ESBL/AmpC E. coli phages
To assign the phages into current taxonomic phage genera, all 28 isolated phages were subjected to whole genome sequencing and compared to existing phage genomes at the NCBI database using whole genome BLAST similarity search. Our analyses showed that the phages could be assigned to four different families: Ackermannviridae, Autographiviridae, Drexlerviridae, and Straboviridae as well as several different subfamilies and 15 genera. Among the families, Straboviridae phages were the most abundant, represented by 16 phages belonging to the genera Krischvirus, Mosigvirus, and Tequatrovirus. The majority of the 28 phages showed high nucleotide similarity (96–99% identity, 92–98% coverage) to other phages infecting E. coli (Table 1). Exceptions were Rosemountvirus AV127 showing similarities to phages infecting Salmonella and phage AV124 to Klebsiella phage Seu621 belonging to the genus Mydovirus. The lowest sequence similarity to other known phages was observed for phage AV104, sharing only 88% nucleotide identity over 75% coverage to its closest relative phage fFiEco02, a Kagunavirus infecting E. coli. Thus, while a few phages were genetically distinct from previously sequenced phages, most of the phages in our collection are genetically related to known phages.
Table 1.
Genome characteristics of phages infecting ESBL/AmpC E. coli
| Phage | Isolation host | Isolation source | PredictedFamily | PredictedSubfamily | PredictedGenus | Genome size (bp) | ORFsb | tRNA | G + C (%) | GenBank acc. no. | Query cover | % identity | Name of the closest relative | GenBank acc. no. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV101a | ESBL58 | Wastewater | Ackermannviridae | Aglimvirinae | Agtevirus | 156759 | 199 | 4 | 49.0 | OQ973471 | 0.85 | 0.976 | Escherichia phage vB_EcoM-ZQ1 | MW650886.1 |
| AV102a | ESBL53 | Wastewater | Autographviridae | Slopekvirinae | Drulisvirus | 43345 | 59 | NDc | 51.4 | OR352933 | 0.95 | 0.965 | Escherichia phage Minorna | NC_048172.1 |
| AV103a | ESBL58 | Wastewater | Autographviridae | Studiervirinae | Teseptimavirus | 39735 | 53 | ND | 48.6 | OR352934 | 0.89 | 0.960 | Escherichia phage JeanTinguely | MZ501081.1 |
| AV104 | ESBL128 | Wastewater | none | Guernseyvirinae | Kagunavirus | 43102 | 76 | ND | 50.7 | OR352935 | 0.75 | 0.888 | Escherichia phage vB_EcoS_fFiEco02 | MT711523.1 |
| AV105a | ESBL128 | Wastewater | Drexlerviridae | Tempevirinae | Warwickvirus | 49722 | 87 | ND | 44.5 | OR352936 | 0.93 | 0.989 | Escherichia phage ityhuna | MN850582.1 |
| AV106 | ESBL80 | Wastewater | Drexlerviridae | Tunavirinae | Tunavirus | 50922 | 83 | ND | 45.6 | OR352937 | 0.94 | 0.927 | Shigella phage SH6 | NC_047785.1 |
| AV108 | ESBL10 | Pig waste | Straboviridae | Tevenvirinae | Krischvirus | 166043 | 264 | ND | 40.4 | OR352938 | 0.93 | 0.978 | Enterobacteria phage GEC-3S | HE978309.1 |
| AV109 | ESBL91 | Pig waste | Straboviridae | Tevenvirinae | Mosigvirus | 168521 | 260 | 2 | 37.4 | OR352939 | 0.92 | 0.984 | Escherichia phage vB_EcoM_G2469 | MK327934.1 |
| AV110 | ESBL102 | Pig waste | Straboviridae | Tevenvirinae | Mosigvirus | 170007 | 269 | ND | 37.6 | OR352940 | 0.97 | 0.986 | Escherichia phage vB_EcoM_WFbE185 | MK373778.1 |
| AV111 | ESBL158 | Wastewater | Straboviridae | Tevenvirinae | Mosigvirus | 172602 | 268 | 3 | 37.6 | OR352941 | 0.93 | 0.975 | Escherichia phage SF | NC_055749.1 |
| AV112 | ESBL153 | Wastewater | Straboviridae | Tevenvirinae | Mosigvirus | 167943 | 260 | 10 | 37.7 | OR352942 | 0.96 | 0.980 | Escherichia phage ST0 | NC_041990.1 |
| AV113 | ESBL56 | Wastewater | Straboviridae | Tevenvirinae | Mosigvirus | 167942 | 260 | 10 | 37.7 | OR352943 | 0.96 | 0.980 | Escherichia phage ST0 | NC_041990.1 |
| AV114 | ESBL197 | Pig waste | Straboviridae | Tevenvirinae | Mosigvirus | 168522 | 259 | 2 | 37.5 | OR352944 | 0.92 | 0.984 | Escherichia phage vB_EcoM_G2469 | MK327934.1 |
| AV115 | ESBL197 | Pig waste | Straboviridae | Tevenvirinae | Mosigvirus | 167064 | 260 | 2 | 37.5 | OR352945 | 0.92 | 0.986 | Escherichia phage vB_EcoM_MM02 | MK373784.1 |
| AV116 | ESBL188 | Pig waste | Straboviridae | Tevenvirinae | Mosigvirus | 167816 | 263 | 2 | 37.4 | OR352946 | 0.93 | 0.984 | Escherichia phage vB_EcoM_G2469 | MK327934.1 |
| AV117 | ESBL153 | Wastewater | Straboviridae | Tevenvirinae | Mosigvirus | 168908 | 266 | 2 | 37.7 | OR352947 | 0.98 | 0.979 | Escherichia phage vB_EcoM_JS09 | KF582788.2 |
| AV118 | ESBL102 | Pig waste | Straboviridae | Tevenvirinae | Mosigvirus | 169872 | 266 | 2 | 37.4 | OR352948 | 0.93 | 0.982 | Escherichia phage vB_EcoM_MM02 | MK373784.1 |
| AV119 | ESBL146 | Pig waste | Straboviridae | Tevenvirinae | Tequatrovirus | 169342 | 277 | 8 | 35.3 | OR352949 | 0.94 | 0.967 | Escherichia phage vB_EcoM_F1 | NC_054912.1 |
| AV120 | ESBL197 | Pig waste | Straboviridae | Tevenvirinae | Tequatrovirus | 169342 | 275 | 8 | 35.3 | OR352950 | 0.94 | 0.967 | Escherichia phage vB_EcoM_F1 | NC_054912.1 |
| AV121 | ESBL133 | Pig waste | Straboviridae | Tevenvirinae | Tequatrovirus | 169535 | 274 | 11 | 35.3 | OR352951 | 0.95 | 0.999 | Escherichia phage vB_EcoM_G4500 | MK327945.1 |
| AV122a | ESBL83 | Wastewater | Straboviridae | Tevenvirinae | Tequatrovirus | 167386 | 269 | 10 | 35.4 | OR352952 | 0.97 | 0.979 | Escherichia phage vB_EcoM_R5505 | MK373786.1 |
| AV123 | ESBL12 | Broiler feces | none | Stephanstirmvirinae | Justusliebigvirus | 146801 | 252 | 13 | 37.4 | OR352953 | 0.97 | 0.988 | Escherichia phage EmilieFrey | MZ501063.1 |
| AV124a | ESBL33 | Wastewater | none | Vequintavirinae | Mydovirus | 144944 | 240 | 14 | 44.7 | OR352954 | 0.80 | 0.966 | Klebsiella phage vB_KpnM_Seu621 | MT939253.1 |
| AV125 | ESBL49 | Pig waste | none | Stephanstirmvirinae | Phapecoctavirus | 152808 | 278 | 11 | 39.0 | OR352955 | 0.93 | 0.994 | Escherichia phage ESCO13 | NC_047770.1 |
| AV126a | ESBL80 | Wastewater | none | Stephanstirmvirinae | Phapecoctavirus | 149254 | 272 | 11 | 39.1 | OR352956 | 0.94 | 0.984 | Escherichia phage ukendt | NC_052661.1 |
| AV127 | ESBL188 | Broiler feces | none | none | Rosemountvirus | 53045 | 75 | ND | 46.0 | OR352957 | 0.98 | 0.974 | Salmonella phage ciri | MT074442.1 |
| AV128 | ESBL120 | Wastewater | none | none | Wifcevirus | 68483 | 103 | ND | 46.2 | OR352958 | 0.94 | 0.960 | Escherichia phage vB_EcoM_WFH | NC_048194.1 |
| AV129 | ESBL49 | Pig waste | none | none | Dhillonvirus | 45321 | 63 | ND | 54.5 | OR352959 | 0.93 | 0.926 | Escherichia phage vb_EcoS_bov22_1 | MT884014.1 |
Phage isolated by direct plating on the isolation host; the remaining phages were isolated after enrichment in the presence of the isolation host.
ORF: open reading frames.
ND: no tRNAs were identified.
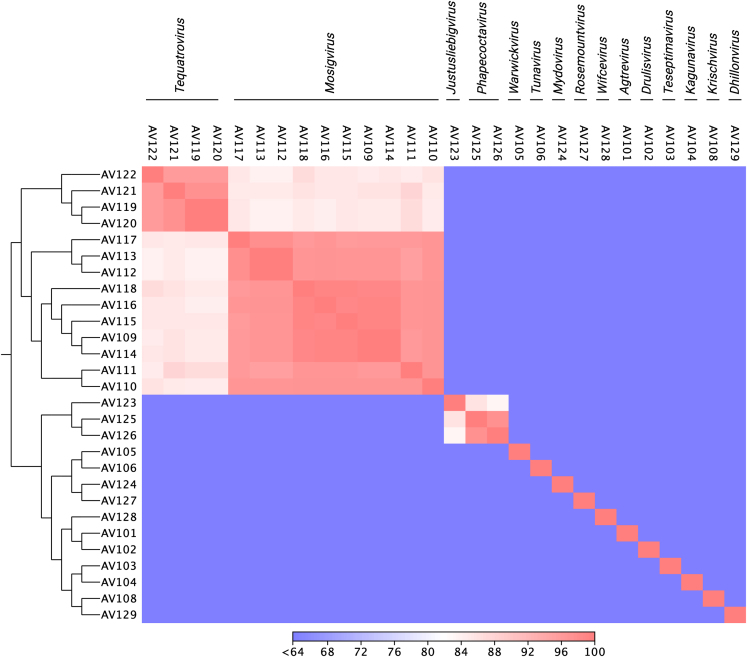
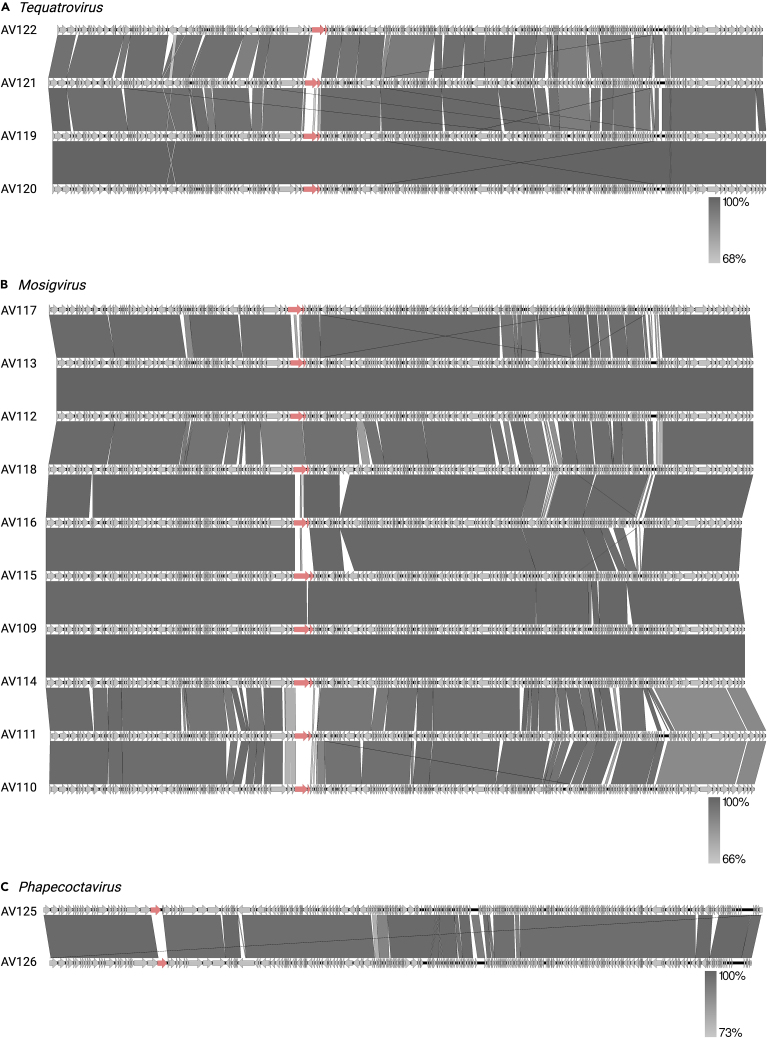
To understand the genetic diversity within the phage collection, all genomes were compared at nucleotide level constructing an identity matrix based on the whole genome sequencing (WGS) data (Figure 2). This analysis showed that the phages are distinct from each other and confirmed that they cluster according to their predicted taxonomic classification. Further genomic analysis revealed varying number of open reading frames (ORFs), tRNAs as well as guanine-cytosine (GC) content associated with the assigned genus (Table 1). Functional annotation of the larger group of phages belonging to the subfamily of Tevenvirinae demonstrated high overall similarity and synteny in genome organization (Figures 3A and 3B). These phages contain the typical features of Tevenvirinae including genome sizes of 162–250 kb, a genomic organization of clusters of early, middle, and late genes, a varying number of homing endonucleases, the presence of several tRNAs and hyper modification of cytosine residues to protect the phage against different restriction-modification systems of the host.38,39,40 Comparative genomics of Tevenvirinae phages within Mosigvirus and Tequatrovirus showed minor variations in all parts of the genome. In addition, the region encoding the long tail fiber gp37 and the gp38 adhesins known recognize host receptors in Tequatrovirus phages T4 and T2 and T6, respectively, varies between phages (Figures 3A and 3B). Finally, functional annotation of the more rarely isolated Phapecoctavirus phages AV125 and AV126 identified large numbers of uncharacterized ORFs and hypothetical genes. For example, 253 of 278 genes are annotated as hypothetical genes in AV125. Moreover, we found only few differences between these two phages including a specific putative RBP present in region encoding several tail fibers and tail spikes (Figure 3C). Based on the genome annotations, none of the phages in the collection encode integrases or potential repressors to maintain lysogeny, suggesting that all phages are virulent. Finally, screening all phage genomes for virulence factors and antibiotic resistance genes using the program VirulenceFinder41 suggested that none of the phages encode virulence genes and thus may be for biocontrol applications targeting ESBL/AmpC E. coli.
Figure 2.
Genomic similarity among isolated phages infecting ESBL/AmpC E. coli
Alle phage genomes were aligned, and the average nucleotide identity (ANI) was calculated for each pair of genomes based on all aligned regions of the whole genome alignment. The color bar indicates ANI as the percentage of exactly matching nucleotides.
Figure 3.
Comparative genomics of phages infecting ESBL/AmpC E. coli
The ESBL/AmpC E. coliphages belong to (A) Mosigvirus, (B) Tequatrovirus, and (C) Phapecoctavirus. Putative receptor binding proteins as gp37 and gp38 for Mosigvirus and Tequatrovirus as well as diverging genes in the tail fiber locus of Phapecoctavirus are indicated by red arrows.
Phage host range of a large collection representing the diversity of ESBL/AmpC E. coli
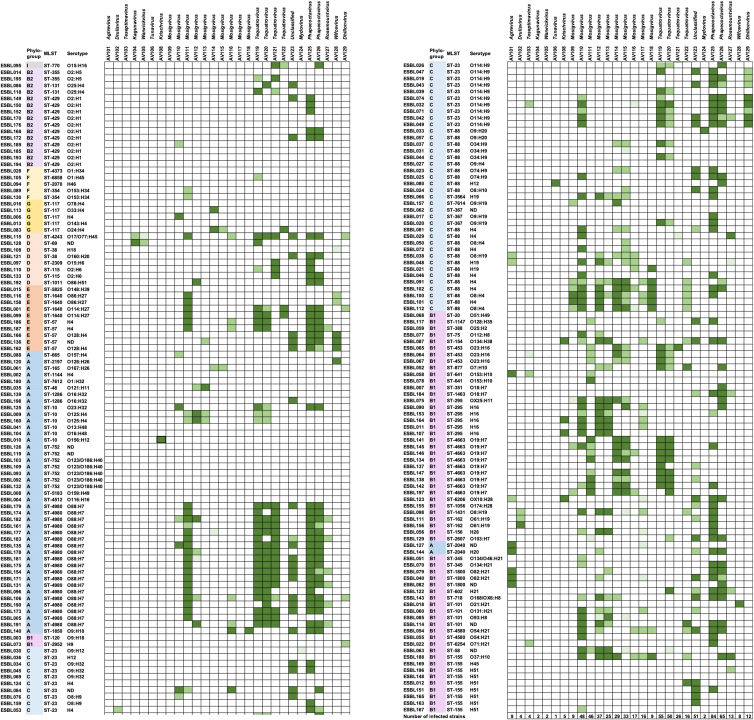
To determine the ability of the isolated phages to infect diverse ESBL/AmpC E. coli, we performed an extended host range analysis determining plaque formation using our large collection of ESBL/AmpC E. coli. This collection contains 198 commensal ESBL/AmpC E. coli from two animal reservoirs including pigs and broilers and the meats hereof, representing all known phylogroups as well as 65 STs and 49 different O-antigens.4 Despite the genomic diversity of the ESBL/AmpC E. coli collection, a total of 162 strains (82% of all 198 strains) showed susceptibility to at least one phage in our collection (Figure 4). The sensitive ESBL/AmpC E. coli belongs to phylogroups A, B1, and C that mainly are represented by commensal E. coli and phylogroups B2, D, E, F, G, and clade I that may carry virulence genes and are potentially pathogenic42 (Figure 4). For example, Mosigvirus were the only phages infecting strains of phylogroup G, while strains in phylogroup B2 were only infected by Tequatrovirus, Justusliebigvirus, and Phapecoctavirus phages (Table S2). In addition, the sensitive ESBL/AmpC E. coli belongs to diverse STs and carries different O-antigens showing no obvious correlation to phage sensitivity (Figure 4). Overall, the host range of the entire phage collection span across most STs and phylogroups infecting many diverse serotypes, thus demonstrating phage infection of genetically diverse ESBL/AmpC E. coli.
Figure 4.
Phage host range analysis using plaque assay on 198 diverse ESBL/AmpC E. coli
Strains are grouped according to phylogeny with phylogroups marked with different colors.4 Columns indicate ST using MLST and serotypes (O- and H-antigen) extracted from Enterobase. ND: Could not be determined. Information about isolation hosts can be found in Table 1. More than 108 pfu per mL (dark green). Between105 to 9.99 × 107 pfu per mL (medium green). Less than 9.99 × 104 pfu per mL (light green). No plaques observed (white).
The host range analysis also demonstrated that each phage showing unique host range profile. While some phages were infecting only one or a few ESBL/AmpC E. coli, others formed plaques on nearly 40% of the entire strain collection (Figure 4). Several phages like for example Agtrevirus AV101, Mosigvirus AV109, and Wificvirus AV127 showed narrow host ranges with specificity only toward their isolation host and a few other strains (Figure 4). In contrast, other phages showed broad host ranges infecting ESBL/AmpC E. coli across most phylogroups and many different STs (Figure 4; Table S2). For example, Mosigvirus AV110 and AV111, Tequatrovirus AV119 and AV120 as well as Justusliebigvirus AV123 infects between 46 and 55 strains of ESBL/AmpC E. coli (Figure 4). Finally, Phapecoctavirus AV125 and AV126 displayed the broadest host range of all phages in the collection, infecting 84 and 65 ESBL/AmpC E. coli, respectively (Figure 4). Remarkably, these phages were not only infecting closely related strains but also a wide range of genetically diverse isolates belonging to 28 to 35 different STs and six phylogroups (Table S2). Overall, the broad complementary and diverse host ranges suggest that single phages may be combined into a cocktail covering the majority of ESBL/AmpC E. coli diversity.
Receptor identification
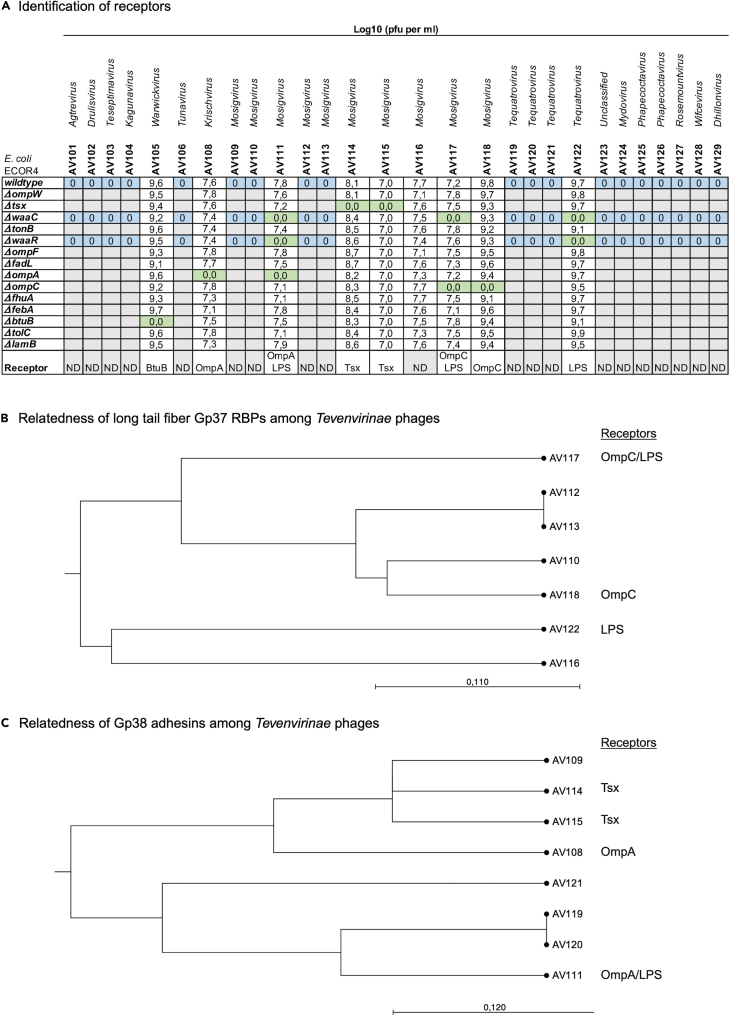
To determine the bacterial receptors used for phage binding, 10-fold serial dilutions of phage stocks were spotted on lawns of wild type E. coli ECOR4 and as well as defined deletion mutants of known phage receptors (Figure 5A). This identified the phage receptors among outer membrane proteins BtuB, OmpA, OmpC, and Tsx as well as lipopolysaccharide (LPS) for nine phages that were able to infect wild-type E. coli ECOR4 (Figure 5A). The LPS mutants waaC lack the conserved inner and outer LPS core and waaR only lack the distal glucose and heptose of the outer LPS core. However, phages not infecting wild type ECOR4 could still not infect these mutants, demonstrating that the core LPS did not mask infection of phages of the wild type (Figure 5A). In contrast, phages like AV117 infecting waaR but not waaC may bind to remaining conserved residues of the inner or outer LPS core of ECOR4. In contrast, phages like AV111 and AV122 not infecting either of the LPS mutants requires either the distal glucose and heptose of the outer LPS core or the O-antigen. However, ECOR4 is reported to lack the O-antigen,43 suggesting that the receptors for AV111 and AV122 may be residues of the conserved inner or outer core. Thus, the 19 phages that cannot infect ECOR4 may be dependent on the highly diverse O-antigen of E. coli, as this is quite common for E. coli phages.25
Figure 5.
Identification of bacterial receptors
(A) Log10(PFU per mL) obtained from plaque assay on lawns of E. coli wild-type ECOR4 as well as ECOR4 deletion mutants as indicated. Blue: No infection of wild type, waaC and waaR mutants; Green: No infection of receptor mutant.
(B) Phylogenetic relatedness of long tail fibers gp37 of Mosigvirus phages AV110, AV112, AV113, AV116, AV117, and AV118 as well as Tequatrovirus phage AV122.
(C) Phylogenetic relatedness of gp38 adhesins of Krischvirus phage AV108, Mosigvirus phages AV109, AV111, AV114, and AV11 as well as Tequatrovirus phages AV119, AV120, and AV121.
To propose receptors for the remaining Tevenvirinae phages, extracted sequences of predicted RBPs were compared by BLASTp analysis. The well-studied Tevenvirinae phage T4 uses long tail fibers (Gp37) to interact with the host receptor.44 In addition, in phage T4 Gp38 serves as chaperone for Gp37 folding, whereas Gp38 homologues of related Tevenvirinae phages T2 and T6 encode an adhesin responsible for binding to the bacterial receptors.30 Amino acid alignment demonstrated that seven phages encode long tail fibers similar to phage T4 (Figure S1) as well as a chaperone showing 83–100% similarity to Gp38 of T4 (Figure S2). These phages include mosigvirus AV110, AV112, AV113, AV116, AV117, and AV118 as well as tequatrovirus AV122, hence predicted to use their Gp37 long tail fibers to bind their receptors. Alignment analysis identified the major differences were observed within the Gp37 head domain (corresponding to 918–973 of gp37 of T4) of the tail tip, previously demonstrated to be responsible for receptor binding of phage T445 (Figure S1). Phylogenetic analysis indicated that Gp37 of phages AV110, AV112, and AV113 may bind to OmpC, as confirmed experimentally for phages AV117 and AV118 (Figures 5A and 5B). However, the involvement of LPS cannot be ruled out by this analysis. In contrast, phage AV116 may be dependent on LPS for infection like AV122, but an unidentified outer membrane protein cannot be ruled out as a secondary receptor (Figure 5B).
The remaining Tevenvirinae eight phages showed similarity to the Gp38 adhesin of T2 and T6 responsible for host recognition (Figure S3). The Gp38 adhesin of Tevenvirinae phages T2 and T6 consist of an N-terminal attaching to the long tail fibers and a C-terminal comprised five conserved glycine-rich motifs (GRMs) and four hypervariable segments (HVSs).30 Alignment analysis demonstrated that mosigvirus AV111 as well as tequatrovirus AV119, AV120, and AV121 are most similar to the Gp38 adhesin of T6, whereas Krischvirus AV108 and mosigvirus AV109, AV114, and AV115 showed similarity to the Gp38 adhesin of T2 (Figure S3). In general, the main differences among all Gp38 homologues were found within the HVSs responsible for host recognition (Figure S3). Phylogenetic analysis showed that the Gp38 adhesin of phages AV119, AV120, and AV121 are most closely related to Gp38 of phage AV111 dependent on OmpA and LPS for infection, suggesting that these phages depend on both receptors for infection of ECOR4 (Figures 5A and 5C). Phylogenetic analysis confirmed that the Gp38 adhesin of phage AV109 is closely related to phages AV114 and AV115, shown to recognize Tsx, thus suggesting that phage AV109 may use this receptor as well (Figures 5A and 5C). In contrast, phage AV108 uses OmpA as receptor and while its Gp38 adhesin showed similarities to the adhesins of AV109, AV114, and AV115, differences within the HVS3 and HVS4 may be responsible for binding to two different receptors (Figure S3). Overall, our phage collection may target at least six different receptors, some predicted by bioinformatic analysis while others were demonstrated experimentally.
Phage-mediated growth inhibition of ESBL/AmpC E. coli in vitro
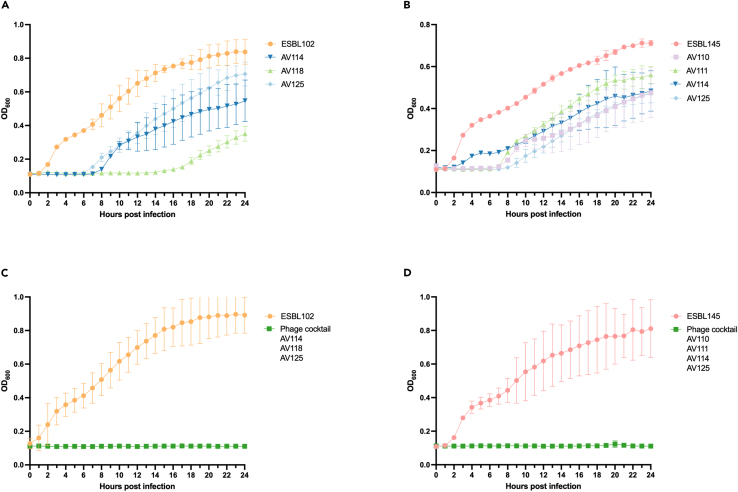
To demonstrate the therapeutic potential of our phages, we tested the ability of selected individual phages to inhibit growth in vitro of two different strains; ESBL102 that carries a CTX-M-1 β-lactamase on an IncI1 plasmid and ESBL145 expressing a chromosomal upregulated AmpC. Moreover, ESBL102 and ESBL145 belong to phylogroups C and B1, as well as ST-88 and ST4663, respectively, thus representing diverse ESBL/AmpC E. coli. At MOI of 10, single phages AV110, AV111, AV114, AV118, and AV125 inhibited growth for up to 8 h whereafter growth was initiated probably due to development of resistance (Figures 6A and 6B). At lower MOIs similar patterns were observed, but with a tendency of an earlier onset of growth and thus resistance development (data not shown). Thus, to prevent resistance development, two phage cocktails consisting of phages targeting different receptors were designed. In both cases phapecoctavirus AV125 were included in the cocktail, as this phage infects the most strains (n = 84) (Figure 3). A remarkable feature of the Stephanstirmvirinae phages like phapecoctavirus AV125, is the presence of four different sets of tail fibers and two tail spike proteins that form a structure resembling an open “nanosized Swiss army knife” with tail fibers pointing in three directions.46 Such tail structure is suggested to provide broad host specificity proposed to target polysaccharides of the capsule, enterobacterial common antigen and lipopolysaccharides as receptors.25,46 We composed one cocktail of mosigvirus AV114 (infecting 29 strains using Tsx as receptor), mosigvirus AV118 (infecting 9 strains using OmpC as receptor) and phapecoctavirus AV125. The second cocktail was composed of mosigvirus AV110 (infecting 48 strains with the predicted receptors LPS and OmpC), mosigvirus AV111 (infecting 46 strains using OmpA as receptor), mosigvirus AV114 (infecting 29 strains using Tsx as receptor), and phapecoctavirus AV125. Interestingly, both cocktails were able to inhibit growth of their target strain for entire 24 h of the experiment (Figures 6C and 6D). Overall, the results suggested that phage cocktails targeting different receptors can be used to inhibit the growth of ESBL/AmpC E. coli in vitro and could potentially be used for biocontrol.
Figure 6.
Phage-mediated growth inhibition of ESBL/AmpC E. coli in vitro
(A) Growth of ESBL102 in the absence (orange) of phages and presence of phage mosigvirus AV114 (dark blue), mosigvirus AV118 (light green), and phapecoctavirus AV125 (light blue).
(B) Growth of ESBL145 in the absence (light red) of phages or in the presence of mosigvirus AV110 (light purple), mosigvirus AV111 (light green), mosigvirus AV114 (dark blue), and phapecoctavirus AV125 (light blue).
(C) Growth of ESBL102 in the presence (dark green) or absence (orange) of phage cocktail consisting of mosigvirus AV114 (Tsx receptor), mosigvirus AV118 (OmpC receptor) and phapecoctavirus AV125. (D) Growth of ESBL145 in the presence (dark green) or absence (light red) of phage cocktail consisting of mosigvirus AV110 (predicted OmpC), mosigvirus AV111 (OmpA receptor), mosigvirus AV114 (Tsx receptor), and phapecoctavirus AV125.
All experiments were done in triplicates and the mean and error bars are visualized in the graphs.
Discussion
The rise of antibiotic resistance is a global problem within human and veterinary medicine. Resistance to broad spectrum β-lactam antibiotics is found among highly diverse ESBL/AmpC E. coli with the main reservoir in livestock. Thus, there is a risk of transmission of resistant bacteria as well as antibiotic resistance genes to farmers and consumers through direct contact to animals and food. Thus, reducing ESBL/AmpC E. coli in livestock and foods may prevent spreading of antibiotic resistance to the human reservoir and thus positively impact human health. Since phages are a promising approach to reduce ESBL/AmpC E. coli, we established and characterized a collection of 28 diverse phages targeting a broad range of ESBL/AmpC E. coli representing the genetic diversity found worldwide.4 Subsequently, we demonstrated application of phages as a promising approach for biocontrol of ESBL/AmpC E. coli.
Although most of our phages fall into established genera of coliphages, exhibiting high overall nucleotide similarity over 90%, they are distinct from their closest relatives and genus/subfamily representatives, thus expanding on coliphage diversity. In addition, the closest relatives of several phages showed similarities to other phages isolated from wastewater,21,23,25 highlighting the abundance of these phages in wastewater treatment plants across different geographical locations. Interestingly, phages AV124 and AV104 showed low overall nucleotide similarity of 77% (96.5% identity, 80% coverage) and 66% (88.8% identity, 75% coverage), respectively, to known phages. AV124 were classified within the Vequintavirinae subfamily (likely Mydovirus genus), whereas AV104 belongs to Kagunavirus of Drexlerviridae. Interestingly, the few known kagunavirus were identified through metagenomics data originating from microbiome studies.47 Phage AV124, on the other hand, only had close relatives of phages infecting Klebsiella, thus likely explaining its narrow host range within the ESBL/AmpC E. coli. Similarly, another narrow host range phage AV127, belonging to Rosemountvirus genus, and showed similarity to Salmonella phages only. The host ranges of the coliphages in our collection may therefore extend to other species of Enterobactericeae such as Enterobacteria, Klebsiella, Salmonella, and Shigella yet most of the closely related phages infect E. coli.
Phages for biocontrol or phage therapy must be specific against the target bacterium and preferably have a broad host range to cover the diversity of the target bacterial population. Importantly, phages of our collection combined infect all phylogroups and most of the 65 ST groups as well as diverse O-antigens of our ESBL/AmpC E. coli collection, thus showing a broad coverage of the diversity of ESBL/AmpC E. coli. Furthermore, isolating phages specifically targeting ESBL/AmpC E. coli significantly increased the coverage from 23% in our previous study to 82% in the present work.4 Still, the overall susceptibility of ESBL/AmpC E. coli could be further improved by isolating phages using ESBL/AmpC E. coli know to be resistant to phage infection. Among E. coli up to 185 diverse O-antigens of LPS have been identified.5 Thus, some phages of our collection may have a limited host range, potentially due to targeting specific O-antigens, but this remains to be verified. However, the long chains of O-antigen may also prevent infection by masking outer membrane protein receptors as demonstrated previously,31 thus influencing the host ranges. Interestingly, this study demonstrated that several phages within the Tevenvirinae subfamily as well as phages belonging to Stephanstrimvirinae had broad host ranges covering all E. coli phylogroups and various STs not limited to specific O-antigen of the host. To some extent, such a wide host range may be attributed to protection against anti-phage defense mechanisms by genomic modifications, such as hyper modification of cytosine characteristic for Tevenvirinae phages also identified in our phages and rhamnose modification in Stephanstrimvirinae phages.21,23,25,48 While the cytosine hyper modification has proved to be efficient against RM-systems for phages of Tevenvirinae subfamily, some Stephastirmvirinae phages were shown to be sensitive to many RM-systems.21,23,25,48 Thus, suggesting that other mechanisms potentially encoded by some of the many unknown genes may allow successful infection of a wide host range of strains.
Phages are equipped with tail fibers or tail spikes that may allow recognition of diverse surface structures displayed by ESBL/AmpC E. coli.49,50 In addition to binding specificity, tail spikes hold enzymatic activity toward their polysaccharide receptor.51,52 In contrast, tail fibers are more diverse, with some encoding depolymerase activity for digesting surface polysaccharides and allowing the phage to reach the bacterial surface and eventually bind a second receptor.25,46 Stephanstirmvirinae phages encode three conserved tail fibers carrying glycosidase and colanidase activity, some phages encode an additional tail spike with N-acetylneuraminidase activity but is not found in phages AV125 and AV126 (data not shown). So far, the specific receptors were not determined for our Stephanstrimvirinae, but other studies have suggested that these phages may initially bind to polysaccharides of enterobacterial common antigen and then to the outer core of lipopolysaccharide for DNA injection, but the role of the specific tail fibers in host binding have not been elucidated yet.46 However, the tail fibers carrying glycosidase and colanidase activity may be involved in degrading surface polysaccharides otherwise masking access to the receptor, thus broaden the host range of the Stephanstrimvirinae phages. The Tevenvirinae phages of our collection encode tail fibers with similarity to homologues of either gp37 of T4 or the gp38 adhesin homologues of T2 or T6. The diversity of the tail fibers was mainly due to variations within the hypervariable segments (HVSs) of the gp38 homologues of T2 or T6 and in the head domain of the tail tip of the homologues of gp37 of T4, known to influence phage binding to the host. In accordance, phages AV110, AV112, and AV113 encoding highly similar long tail fibers showed similar host range profiles, whereas the remaining phages using gp37 for host binding showed differences in tail fibers as well as host ranges. Interestingly, phages AV111, AV119, and AV120 showed the broadest host range within our Tevenvirinae phages and were encoding highly similar gp38 adhesins possibly targeting OmpA independently of LPS, which may explain their relatively brad host ranges. The host ranges among these phages were quite similar, but minor differences were observed that may be due to amino acid substitutions in HVS1 and the C-terminal of the three phages. Yet, it should be noted that some Tevenvirinae phages may recognize different receptors when infecting diverse strains and that a few amino acid differences of the receptor binding domains may change the receptor recognized by Tevenvirinae.53 Overall, the broad host range of some phages, like the Justusliebigvirus, Phapecoctavirus, and Tevenvirinae phages suggest that they may be promising candidates for biocontrol of ESBL/AmpC E. coli.
Notably, a few studies have tested the efficacy of commercially available phage cocktails against ESBL/AmpC E. coli, including the Intesti bacteriophage cocktail consisting of at least 23 phages infecting different bacterial species causing intestinal, urinary tract, and oral cavity infections caused by E. coli among other bacteria.54 Yet, the cocktail has not been tested systematically against ESBL/AmpC E. coli. Here, we designed two different phage cocktails and tested their ability to inhibit growth of ESBL/AmpC E. coli in vitro using knowledge of host ranges to select phages infecting the target strain. Similarly, for specific applications, phages for cocktails may be selected based on knowledge of the target ESBL/AmpC E. coli strain. To compose the most efficient phage cocktails, we used the obtained data to select phages that binds to diverse receptors when infecting ESBL/AmpC E. coli, thus reducing the chances of phage resistance development. Indeed, in vitro experiments demonstrated that the phage cocktails could inhibit the growth of ESBL/AmpC E. coli strains ESBL102 and ESBL145 over 24 h without phage resistant development. In contrast, treatment by single phages leads to resistance development within up to 8 h, thus demonstrating the power of phage cocktails in preventing resistance development.55,56,57,58,59 In conclusion, the present work demonstrates that phages in our collection are promising to target diverse ESBL/AmpC E. coli and have thus laid the foundation for further development for phage cocktails used for biocontrol of ESBL/AmpC E. coli.
Limitations of the study
This study describe isolation of 28 phages infecting ESBL/AmpC E. coli and provide a comprehensive host range analysis as well as receptor identification. However, further studies are needed to better understand the biology and potential applications of these phages, for example identification of the receptors of broad host range Phapecoctavirus as well as functional assignment of their unknown ORF. Future studies could as well focus on the host range determinants of phages in the collection, including the role of O-antigen as well as their ability to infect other commensals E. coli that may be beneficial for the gut health or alternatively pathogenic strains of E. coli. Additionally, the potential for biocontrol could be further investigated by determining safety aspects as well as the ability of the phage cocktails to decolonize animals using mice models or farm animals as well as phage resistant development in vivo.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli strain ECOR 4 | Ochman et al. 198460 | N/A |
| ECOR4 ΔompW | This study | N/A |
| ECOR4 Δtsx | This study | N/A |
| ECOR4 ΔwaaC | This study | N/A |
| ECOR4 ΔtonB | This study | N/A |
| ECOR4 ΔwaaR | This study | N/A |
| ECOR4 ΔompF | This study | N/A |
| ECOR4 ΔfadL | This study | N/A |
| ECOR4 ΔompA | This study | N/A |
| ECOR4 ΔompC | This study | N/A |
| ECOR4 ΔfhuA | This study | N/A |
| ECOR4 ΔfepA | This study | N/A |
| ECOR4 ΔbtuB | This study | N/A |
| ECOR4 ΔtolC | This study | N/A |
| ECOR4 ΔlamB | This study | N/A |
| ESBL001-ESBL198 | Vitt et al.4 | ESBL strains |
| AV101 | This study | Genbank: OQ973471 |
| AV102 | This study | Genbank: OR352933 |
| AV103 | This study | Genbank: OR352934 |
| AV104 | This study | Genbank: OR352935 |
| AV105 | This study | Genbank: OR352936 |
| AV106 | This study | Genbank: OR352937 |
| AV108 | This study | Genbank: OR352938 |
| AV109 | This study | Genbank: OR352939 |
| AV110 | This study | Genbank: OR352940 |
| AV111 | This study | Genbank: OR352941 |
| AV112 | This study | Genbank: OR352942 |
| AV113 | This study | Genbank: OR352943 |
| AV114 | This study | Genbank: OR352944 |
| AV115 | This study | Genbank: OR352945 |
| AV116 | This study | Genbank: OR352946 |
| AV117 | This study | Genbank: OR352947 |
| AV118 | This study | Genbank: OR352948 |
| AV119 | This study | Genbank: OR352949 |
| AV120 | This study | Genbank: OR352950 |
| AV121 | This study | Genbank OR352951 |
| AV122 | This study | Genbank: OR352952 |
| AV123 | This study | Genbank: OR352953 |
| AV124 | This study | Genbank: OR352954 |
| AV125 | This study | Genbank: OR352955 |
| AV126 | This study | Genbank: OR352956 |
| AV127 | This study | Genbank: OR352957 |
| AV128 | This study | Genbank: OR352958 |
| AV129 | This study | Genbank: OR352959 |
| Chemicals, peptides, and recombinant proteins | ||
| Lysogeny Broth | Oxid | Cat# CM1023 |
| Brain Heart Infusion broth | Oxid | Cat# CM1135 |
| Cefotaxime | Sigma | Cat# C7912 |
| DNase I (1 U/μl) | Thermo Fisher Scientific | Cat# EN0521 |
| RNase A (10 mg/ml) | Thermo Fisher Scientific | Cat# EN0531 |
| Proteinase K 50 μg/ml | Thermo Fisher Scientific | Cat# EO0491 |
| Glycogen | Thermo Fisher Scientific | Cat# R0551 |
| Ammonium acetate | Sigma | Cat#A1542 |
| Kanamycin | Sigma | Cat# BP906 |
| Ampicillin | Sigma | Cat# A0166 |
| L-arabinose | Sigma | Cat# A3256 |
| CaCl2 | Sigma | Cat# C3306 |
| Water, nuclease-free | Thermo Fisher Scientific | Cat# R0582 |
| Critical commercial assays | ||
| DNA Clean & Concentrator-25 | Zymo Research | Cat# D4011 |
| Nextera XT v.3 | Illumina | Cat# 15031942 |
| Quick & Easy E. coli Gene Deletion Kit | Gene Bridges | Cat# K006 |
| Amplicon Taq 2x Master Mix Red | Amplicon | Cat# A190301 |
| GeneJET PCR Purification Kit | Thermo Fischer Scientific | Cat# K0702 |
| Oligonucleotides | ||
| Primers for deletion fragment amplification | See Table S4 | N/A |
| Primers for deletion control | See Table S5 | N/A |
| Software and algorithms | ||
| CLC Genomics Workbench v. 9.5.3 | Qiagen | N/A |
| CLC Workbench v. 21 | Qiagen | N/A |
| RAST v. 2.0 | Aziz et al.61 | https://rast.nmpdr.org |
| ARAGORN software v. 2.4.1 | Laslett et al.62 | http://www.ansikte.se/ARAGORN/ |
| BLAST v. 2.15.0 | NCBI | https://blast.ncbi.nlm.nih.gov/Blast.cgi |
| EasyFig version v. 2.2.2 | Sullivan et al.63 | http://mjsull.github.io/Easyfig/files.html |
| iTOL v.6 | Söding et al.37 | https://itol.embl.de |
| VirulenceFinder v. 2.0.3 | Joensen et al.41 | https://cge.food.dtu.dk/services/VirulenceFinder/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Lone Brøndsted (lobr@sund.ku.dk).
Materials availability
Bacterial isolates and bacteriophages are available by request from the lead contact under the conditions of a material transfer agreement (MTA).
Data and code availability
-
•
Assembled bacteriophage genomes have been deposited at NCBI and are publicly available as of the date of publication. For accession numbers for phage genomes see key resources table. Bacterial genomes are available at NCBI under designated Bioprojects as noted in Table S1.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Bacterial strains
ESBL/AmpC E. coli strains (n=198) (Table S1) originating from poultry, broiler meat or pig caecum were collected as part of Danish surveillance program and previously characterized.4 E. coli strains were cultured in Lysogeny Broth (LB) and LB agar (LA) (Oxoid, Roskilde, Denmark). LA plates were supplemented with cefotaxime (Sigma Aldrich, Copenhagen, Denmark) at final concentration of 1 μg/ml. Overnight cultures were prepared in LB with 2-3 colonies shaking at 200 rpm at 37°C for 16-20 hours. Among 198 ESBL/AmpC E. coli, 19 strains were chosen for the isolation of the phages based on their characteristics as summarized in Figure 1.
Method details
Processing of animal and wastewater samples
Samples were collected from broiler faeces (n=6), pig waste (n=4); wastewater (n=2) was used to isolate phages.26 Faeces were diluted (1:10 w/v) in sterile SM buffer (0.1 M NaCl, 8 mM MgSO4, 50 mM Tris-HCl, pH 7.5), vortexed and centrifuged at 10.000 x g for 10 min at room temperature. Waste and wastewater samples were centrifuged at 6000 x g for 10 min. Following centrifugation, all supernatants were filtered through 0.45 μm filters and stored at 4°C.
Phage isolation, purification, and propagation
For isolation and purification and propagation we followed a previous established method.26 Depending on the size of the Petri dish used, the bacterial lawns were prepared from 100 or 300 μl overnight cultures of isolation strains that were mixed with 4 or 10 ml of molten overlay agar (LBov; LB broth with 0,6% Agar bacteriological no.1 (Oxoid)) and spread on 9 or 12 cm LA (LB with 1,2% agar) plates, respectively. Bacterial lawns were allowed to settle for 15 minutes and then dried in a laminar hood for 45 min to be used immediately thereafter. For phage isolation, a total of 5 drops of 10 μl of sample were spotted on the lawns of isolation host strains and were incubated ON at 37°C aerobically. When no plaques were detected, the samples were subjected to selective enrichment with the isolation strains: 500 μl filtered sample, 500 μl ON isolation host culture and 1 ml LB mixed and incubated ON at 37°C with shaking at 180 rpm. The following day, the enrichment inoculums were centrifuged at 10.000 x g for 10 min and ten-fold serial dilutions in SM buffer were spotted on a lawn of the enrichment strain. Up to three plaques with different and consistent plaque morphologies were picked with a pipette tip and suspended in 200-400 μl of SM buffer, vortexed and ten-fold diluted. A 100 μl aliquot of selected dilution(s) were mixed with 100 μl of the isolation strain in 4 ml LBov and spread on LA plates. Each single plaque was purified for at least three rounds. Single plaques from the final purification steps were used for phage propagation on the isolation strain and phage stocks were prepared by plate lysis method adapted from64 with modifications. Briefly, 100 μl of predetermined phage dilution, corresponding to a confluent lysis, was mixed with 100 μl of ON inoculum of the host strain, prepared as described above. After 10 min, 4 ml molten LBov was added, mixed gently, and poured over a pre-made LA plate. After settling of the overlay agar, the plates were incubated ON at 37°C. The next day, plates were examined, the layer with overlay agar was scraped off with a sterile inoculation loop, collected into a centrifuge tube and mixed with 5 ml SM-buffer. After thorough vortexing, the mixture was centrifuged at 8000 x g for 10 min at 4°C and the supernatant filtered once through 0.22 μm filters and stored at 4°C.
Phage plaque assay
A double layered plaque assay was used to determine phage titers.65 Briefly, ten-fold serial dilutions (up to 10-7 - 10-8) of the phage stocks in SM buffer were prepared and 3 droplets of 10 μl aliquots were spotted on pre-made plates of bacterial lawns. Following an overnight incubation at 37°C, plaques were counted and plaque forming units per ml (PFU ml-1) were calculated for each strain.
Host range analysis
Phage host range was determined in two steps: spot assay and, if lysis spots were observed, plaque assay was performed.65 For the spot assay, bacterial lawns were prepared in 12 cm round plates as described above. Brain Heart Infusion (BHI) (Oxoid) with 1.2 % agar for the basal plates and 0.6% agar for the overlays were used throughout the host range experiments. 10 μl of ten-fold diluted phage stocks (titers above 108 PFU ml-1) were spotted on the air-dried bacterial lawns prepared with BHI 1.2 and 0.6 % agar and incubated 18-24 h at room temperature. To confirm phage infection for the spots with lysis, plaque assay was performed with one spot of 10 μl of each dilution. The plates were incubated 18-24 h at room temperature, plaques were counted and plaque forming units (PFU) ml-1 were calculated.
Determination of receptors in E. coli strain ECOR4
Specific gene knockout strains were obtained with the Quick & Easy E. coli Gene Deletion Kit (Gene Bridges). Linear gene deletion fragments were generated by PCR (Amplicon Taq 2x Master Mix Red) using primers designed to match the FRT-PGK-gb2-neo-FRT cassette supplied with the mutagenesis kit. Each of the primer pairs were equipped with 50 bp 5’ extensions matching the terminal nucleotides of the respective genes of the ECOR4 chromosome. PCR products were purified and concentrated using the GeneJET PCR Purification Kit (Thermo Fischer Scientific) by elution in 10 μl nuclease-free water. First, E. coli ECOR4 were made competent by inoculation of 1 ml of an overnight culture in 100 ml fresh LB and incubation for 2.5 hours at 37°C with shaking at 180 rpm. The cells were put on ice for 10 min and collected by centrifugation at 4°C for 3 min at 4500 x g followed by two washes in 20 ml ice-cold 0.1 M CaCl2 before resuspension in 5 ml ice-cold 0.1 M CaCl2. 100 μl of competent cells were incubated on ice with 1 μl purified pRedET (amp) plasmid for 30 min before heat shock for 1 min at 42°C and addition of 900 μl LB followed by incubation for 1 hour at 30°C with shaking at 180 rpm. Cells were plated on LA containing 100 μg/ml ampicillin and incubated overnight at 30°C. Next, ECOR4 bearing the Red/ET expression plasmid were grown in a shaking flask in LB plus 100 μg/ml ampicillin at 30°C until an OD600 of 0.3 followed by addition of L-arabinose at 0.35% final concentration and continued growth at 37°C for 1 hour. Cells were cooled on ice and washed four times in ice-cold water (2 times 1 volume, 1 time ½ the volume, and 1 time ¼ the volume) and gently resuspended in 1/100 the volume of ice-cold water. 50 μl of cells were added 2 μl of concentrated deletion fragment and electroporated in pre-chilled 0.2 cm electroporation cuvettes using a MicroPulser Electroporator (Bio-Rad) at Ec2 settings. Fresh LB was added, and cells were incubated at 37°C for 2-3 hours before plating on LA containing 50 μg/ml kanamycin and overnight incubation at 37°C. Successful gene knockout was confirmed with gene-specific primers for respective target genes. After the construction of the ECOR4 mutants, the phage infectivity was evaluated using a normal plaque assay (see STAR Methods above).
Single bacteriophage and bacteriophage cocktail growth inhibition assay
Growth inhibition effect of ESBL/AmpC E. coli strains was performed following previous described method.59 Firstly, ESBL/AmpC E. coli strains ESBL102 and ESBL145 was chosen as hosts because of their genetic diversity. Secondly, Phages AV114, AV118 and AV125 were chosen as phage cocktail against ESBL102 whereas phages AV110, AV111, AV114 and AV125 were combined against ESBL145. Both single phages and the cocktail were evaluated for their growth inhibition ability against the strains. A single colony of each strain were inoculated in 5 mL LB media and incubated overnight at 37°C and 180 rpm. The following morning, the cultures were diluted to 1∗10ˆ8 CFU/mL and 100 μL of the culture was added to 96 wells plates (TPP). Afterwards, the phages (MOI of 10) were added to the samples and the plates were incubated in Gen5 plate reader (Agilent BioTek) for 24 hours at 37°C and OD600 values were determinate every 15 minutes. ESBL102 and ESBL145 without phages were used as negative controls. The growth inhibition assay was done in three triplicates and the standard deviations were calculated in GraphPad Prism9 (version 9.5.0).
DNA extraction and sequencing
High titer (>108 PFU ml-1) phage stocks were subjected to DNA extraction and purification by ethanol precipitation with modifications.66 Briefly, phage stocks were treated with RNase A (Thermo Fischer Scientific, Waltham, MA, USA) and DNAse I (Thermo Fischer Scientific) at final concentrations of 10 and 20 μg ml-1 and incubated at 37°C for 1-2 h. Phage DNA was released from capsids by treatment with proteinase K (50 μg/ml, Thermo Fischer Scientific) in the presence of SDS (0.5%) at 56°C overnight. After cooling the samples to room temperature, DNA was precipitated with 0.1 volume of 3 M sodium acetate (pH 5.5), glycogen (final concentration of 0.05 μg/μl, Thermo Fischer Scientific) and 2.5 volumes of ice-cold ethanol (96%) were added and incubated 2-6 days at -20°C. Precipitated DNA was centrifuged at 10000rpm for 30 min and washed two times with 70% ice-cold ethanol (10000rpm, 20 min). Pellets with DNA were air-dried at 37°C and dissolved in 10 mM Tris-HCl (pH 8) at 4°C overnight. Dissolved DNA was further purified using DNA Clean & Concentrator-25 (Zymo Research) following manufacturer’s instructions with elution in 50-150 μl of 10 mM Tris-HCl (pH 8). DNA concentrations were measured using Qubit (Thermo Fischer Scientific) and DNA libraries were prepared using Nextera XT v.3 (Illumina, San Diego, CA, USA) kit. Next generation sequencing was performed using MiSeq (Illumina) platform with paired-end (2 X 250-bp) mode.
Quantification and statistical analysis
Genome assembly and analyses
The sequences were de novo assembled using CLC Genomics Workbench 9.5.3 (Qiagen Digital Insights, Aarhus, Denmark). Open reading frames and tRNAs were detected and annotated automatically using Rapid Annotation Subsystem Technology RAST version 2.061[NO_PRINTED_FORM]. tRNAs were additionally determined with ARAGORN software.62 All tools were run with default parameters. The phages were assigned their taxonomy by overall genome BLAST similarities to their closest phage genome available at the NCBI.
Bioinformatics analyses
The 19 isolating ESBL/AmpC E. coli genomes was extracted from Enterobase and a phylogenetic tree based on their cgMLST was visualized in iTOL v. 6 using default settings.37 CLC Workbench version 22 (Qiagen Digital Insights, Aarhus, Denmark) was used to alignment of all phage genomes with the default settings (minimum initial seed length: 15, allow mismatches in seeds: yes and minimum alignment block length: 100). Pairwise comparison of the analysis was conducted to create a heatmap displaying the average nucleotide identity (ANI) using default settings (table types: ANI, distance measure: euclidean distance and linkage criteria: complete linkage). Easyfig version 2.2.5 with 0.4 minimum identity for BLAST setting was used to align and visualize the phage genomes in the Tequatrovirus, Mosigvirus and Phapecoctavirus genera (Sullivan, Petty, and Beatson 2011). Furthermore, ClustalO67 available in CLC was used for alignment of receptor binding protein sequences (Gp37 and Gp38) of the phages in the Tevenvirnae subfamily with default settings; Gap cost 10, gap extension cost 1, end gap cost: as any others and alignment mode: very accurate. VirulenceFinder version 241 was used to estimate if any virulence genes were present in the phage genomes. The Gp37 of phage T4 (Accession number MT984581) and Gp38 of phage T2 (Accession number MH751506) and T6 (Accession number AP018814) was used for alignment of the Gp37 and Gp38 of Tevenvirinae phages isolated in the study.
Acknowledgments
This project has received funding from Promilleafgiftsfonden, Denmark to A.R.V. and A.N.S. was supported by the Danish Council for Independent Research (2035-00112B).
Author contributions
Conceptualization, A.R.V., M.C.H.S., V.B., A.N.S., M.S.B., and L.B.; methodology, A.R.V., A.N.S., M.S.B., and L.B.; investigation, A.R.V., A.N.S., M.S.B., and L.B.; resources, L.B.; writing – original draft, A.R.V. and L.B.; writing – review and editing, A.N.S. and L.B.; funding acquisition, L.B.; resources, L.B.; supervision, M.C.H.S., V.B., and L.B.
Declaration of interests
The authors declare no competing interests.
Published: January 17, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.108826.
Supplemental information
References
- 1.Paterson D.L., Bonomo R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clermont O., Bonacorsi S., Bingen E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touchon M., Perrin A., De Sousa J.A.M., Vangchhia B., Burn S., O’Brien C.L., Denamur E., Gordon D., Rocha E.P. Phylogenetic background and habitat drive the genetic diversification of Escherichia coli. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitt A.R., Sørensen M.C.H., Bortolaia V., Brøndsted L. A Representative Collection of Commensal Extended-Spectrum- and AmpC-β-Lactamase-Producing Escherichia coli of Animal Origin for Phage Sensitivity Studies. Phage. 2023;4:35–45. doi: 10.1089/phage.2023.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu B., Furevi A., Perepelov A.V., Guo X., Cao H., Wang Q., Reeves P.R., Knirel Y.A., Wang L., Widmalm G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020;44:655–683. doi: 10.1093/femsre/fuz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby G.A. β-lactamase nomenclature. Antimicrob. Agents Chemother. 2006;50:1123–1129. doi: 10.1128/AAC.50.4.1123-1129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen K.H., Bortolaia V., Nielsen C.A., Nielsen J.B., Schønning K., Agersø Y., Guardabassi L. Host-specific patterns of genetic diversity among IncI1-Iγ and IncK plasmids encoding CMY-2 β -lactamase in Escherichia coli isolates from humans, poultry meat, poultry, and dogs in Denmark. Appl. Environ. Microbiol. 2016;82:4705–4714. doi: 10.1128/AEM.00495-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dame-Korevaar A., Fischer E.A.J., van der Goot J., Velkers F., van den Broek J., Veldman K., Ceccarelli D., Mevius D., Stegeman A. Effect of challenge dose of plasmid-mediated extended-spectrum β-lactamase and AmpC β-lactamase producing Escherichia coli on time-until-colonization and level of excretion in young broilers. Vet. Microbiol. 2019;239 doi: 10.1016/j.vetmic.2019.108446. [DOI] [PubMed] [Google Scholar]
- 9.Carattoli A., Villa L., Fortini D., García-Fernández A. Contemporary IncI1 plasmids involved in the transmission and spread of antimicrobial resistance in Enterobacteriaceae. Plasmid. 2021;118 doi: 10.1016/j.plasmid.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Leverstein-van Hall M.A., Dierikx C.M., Cohen Stuart J., Voets G.M., van den Munckhof M.P., van Essen-Zandbergen A., Platteel T., Fluit A.C., van de Sande-Bruinsma N., Scharinga J., et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 2011;17:873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 11.Benz F., Huisman J.S., Bakkeren E., Herter J.A., Stadler T., Ackermann M., Diard M., Egli A., Hall A.R., Hardt W.D., Bonhoeffer S. Plasmid- and strain-specific factors drive variation in ESBL-plasmid spread in vitro and in vivo. ISME J. 2021;15:862–878. doi: 10.1038/s41396-020-00819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dame-Korevaar A., Fischer E.A.J., van der Goot J., Stegeman A., Mevius D. Transmission routes of ESBL/pAmpC producing bacteria in the broiler production pyramid, a literature review. Prev. Vet. Med. 2019;162:136–150. doi: 10.1016/j.prevetmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Carattoli A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008;14:117–123. doi: 10.1111/j.1469-0691.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 14.European Food Safety Authority, European Centre for Disease Prevention and Control The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022;20 doi: 10.2903/j.efsa.2022.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tacconelli E., Mazzaferri F., de Smet A.M., Bragantini D., Eggimann P., Huttner B.D., Kuijper E.J., Lucet J.C., Mutters N.T., Sanguinetti M., et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 2019;25:807–817. doi: 10.1016/j.cmi.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Becker E., Projahn M., Burow E., Käsbohrer A. Are there effective intervention measures in broiler production against the esbl/ampc producer escherichia coli? Pathogens. 2021;10 doi: 10.3390/pathogens10050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittebole X., De Roock S., Opal S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutter E., Hoyle N., Eisner W., Kuhl S., Alavidze Z., Blasdel B.G. Phage Therapy: Bacteriophages as Natural, Self-Limiting Antibiotics. Textb. Nat. Med. 2020;777:777–787.e3. [Google Scholar]
- 19.Smith R., O’Hara M., Hobman J.L., Millard A.D. Draft Genome Sequences of 14 Escherichia coli Phages Isolated from Cattle Slurry. Genome Announc. 2015;3 doi: 10.1128/genomeA.01364-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurczak-Kurek A., Gąsior T., Nejman-Faleńczyk B., Bloch S., Dydecka A., Topka G., Necel A., Jakubowska-Deredas M., Narajczyk M., Richert M., et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016;6 doi: 10.1038/srep34338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korf I.H.E., Meier-Kolthoff J.P., Adriaenssens E.M., Kropinski A.M., Nimtz M., Rohde M., van Raaij M.J., Wittmann J. Still something to discover: Novel insights into Escherichia coli phage diversity and taxonomy. Viruses. 2019;11:454. doi: 10.3390/v11050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sørensen P.E., Van Den Broeck W., Kiil K., Jasinskyte D., Moodley A., Garmyn A., Ingmer H., Butaye P. New insights into the biodiversity of coliphages in the intestine of poultry. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen N.S., Forero-Junco L., Kot W., Hansen L.H. Exploring the Remarkable Diversity of Culturable Escherichia coli Phages in the Danish Wastewater Environment. Viruses. 2020;12:986. doi: 10.3390/v12090986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan Mirzaei M., Nilsson A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maffei E., Shaidullina A., Burkolter M., Heyer Y., Estermann F., Druelle V., Sauer P., Willi L., Michaelis S., Hilbi H., et al. Systematic exploration of Escherichia coli phage-host interactions with the BASEL phage collection. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitt A.R., Ahern S.J., Gambino M., Sørensen M.C.H., Brøndsted L. Genome Sequences of 16 Escherichia coli Bacteriophages Isolated from Wastewater, Pond Water, Cow Manure, and Bird Feces. Microbiol. Resour. Announc. 2022;11 doi: 10.1128/mra.00608-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobrega F.L., Vlot M., de Jonge P.A., Dreesens L.L., Beaumont H.J.E., Lavigne R., Dutilh B.E., Brouns S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018;16:760–773. doi: 10.1038/s41579-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y.F., Yan J.J., Lei H.Y., Teng C.H., Wang M.C., Tseng C.C., Wu J.J. Loss of outer membrane protein C in Escherichia coli contributes to both antibiotic resistance and escaping antibody-dependent bactericidal activity. Infect. Immun. 2012;80:1815–1822. doi: 10.1128/IAI.06395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kortright K.E., Chan B.K., Turner P.E. High-throughput discovery of phage receptors using transposon insertion sequencing of bacteria. Proc. Natl. Acad. Sci. USA. 2020;117:18670–18679. doi: 10.1073/pnas.2001888117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trojet S.N., Caumont-Sarcos A., Perrody E., Comeau A.M., Krisch H.M. The gp38 adhesins of the T4 superfamily: A complex modular determinant of the Phage’s host specificity. Genome Biol. Evol. 2011;3:674–686. doi: 10.1093/gbe/evr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulikov E.E., Golomidova A.K., Prokhorov N.S., Ivanov P.A., Letarov A.V. High-throughput LPS profiling as a tool for revealing of bacteriophage infection strategies. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-39590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skaradzińska A., Śliwka P., Kuźmińska-Bajor M., Skaradziński G., Rząsa A., Friese A., Roschanski N., Murugaiyan J., Roesler U.H. The efficacy of isolated bacteriophages from pig farms against ESBL/AmpC-producing Escherichia coli from pig and Turkey farms. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald-Hughes D., Bolkvadze D., Balarjishvili N., Leshkasheli L., Ryan M., Burke L., Stevens N., Humphreys H., Kutateladze M. Susceptibility of extended-spectrum- β-lactamase-producing Escherichia coli to commercially available and laboratory-isolated bacteriophages. J. Antimicrob. Chemother. 2014;69:1148–1150. doi: 10.1093/jac/dkt453. [DOI] [PubMed] [Google Scholar]
- 34.Gundogdu A., Bolkvadze D., Kilic H. In vitro effectiveness of commercial bacteriophage cocktails on diverse extended-spectrum beta-lactamase producing Escherichia coli strains. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erol H.B., Kaskatepe B., Ozturk S., Safi Oz Z. The comparison of lytic activity of isolated phage and commercial Intesti bacteriophage on ESBL producer E. coli and determination of Ec_P6 phage efficacy with in vivo Galleria mellonella larvae model. Microb. Pathog. 2022;167 doi: 10.1016/j.micpath.2022.105563. [DOI] [PubMed] [Google Scholar]
- 36.Hoang Minh D., Hoang Minh S., Honjoh K.I., Miyamoto T. Isolation and bio-control of Extended Spectrum Beta-Lactamase (ESBL)-producing Escherichia coli contamination in raw chicken meat by using lytic bacteriophages. Lebensm. Wiss. Technol. 2016;71:339–346. [Google Scholar]
- 37.Letunic I., Bork P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolan J.M., Petrov V., Bertrand C., Krisch H.M., Karam J.D. Genetic diversity among five T4-like bacteriophages. Virol. J. 2006;3:30. doi: 10.1186/1743-422X-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas J.A., Orwenyo J., Wang L.X., Black L.W. The odd “RB” phage—identification of arabinosylation as a new epigenetic modification of DNA in T4-like phage RB69. Viruses. 2018;10:313. doi: 10.3390/v10060313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller E.S., Kutter E., Mosig G., Arisaka F., Kunisawa T., Rüger W. Bacteriophage T4 Genome. Microbiol. Mol. Biol. Rev. 2003;67:86–156. doi: 10.1128/MMBR.67.1.86-156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joensen K.G., Scheutz F., Lund O., Hasman H., Kaas R.S., Nielsen E.M., Aarestrup F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014;52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clermont O., Christenson J.K., Denamur E., Gordon D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- 43.Johnson J.R., Delavari P., Kuskowski M., Stell A.L. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 2001;183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 44.Yu F., Mizushima S. Roles of Lipopolysaccharide and Outer Membrane Protein OmpC of Escherichia coli K-12 in the Receptor Function for Bacteriophage T4. J. Bacteriol. 1982;151:718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartual S.G., Otero J.M., Garcia-Doval C., Llamas-Saiz A.L., Kahn R., Fox G.C., van Raaij M.J. Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proc. Natl. Acad. Sci. USA. 2010;107:20287–20292. doi: 10.1073/pnas.1011218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarzer D., Buettner F.F.R., Browning C., Nazarov S., Rabsch W., Bethe A., Oberbeck A., Bowman V.D., Stummeyer K., Mühlenhoff M., et al. A Multivalent Adsorption Apparatus Explains the Broad Host Range of Phage phi92: a Comprehensive Genomic and Structural Analysis. J. Virol. 2012;86:10384–10398. doi: 10.1128/JVI.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tisza M.J., Buck C.B. A catalog of tens of thousands of viruses from human metagenomes reveals hidden associations with chronic diseases. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2023202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kutter E., Bryan D., Ray G., Brewster E., Blasdel B., Guttman B. From host to phage metabolism: Hot tales of phage T4’s takeover of E. coli. Viruses. 2018;10 doi: 10.3390/v10070387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertozzi Silva J., Storms Z., Sauvageau D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016;363 doi: 10.1093/femsle/fnw002. [DOI] [PubMed] [Google Scholar]
- 50.Hantke K. Compilation of Escherichia coli K-12 outer membrane phage receptors – their function and some historical remarks. FEMS Microbiol. Lett. 2020;367 doi: 10.1093/femsle/fnaa013. [DOI] [PubMed] [Google Scholar]
- 51.Knecht L.E., Veljkovic M., Fieseler L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2019;10:2949. doi: 10.3389/fmicb.2019.02949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leiman P.G., Molineux I.J. Evolution of a new enzyme activity from the same motif fold. Mol. Microbiol. 2008;69:287–290. doi: 10.1111/j.1365-2958.2008.06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taslem Mourosi J., Awe A., Guo W., Batra H., Ganesh H., Wu X., Zhu J. Understanding Bacteriophage Tail Fiber Interaction with Host Surface Receptor: The Key “Blueprint” for Reprogramming Phage Host Range. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zschach H., Joensen K.G., Lindhard B., Lund O., Goderdzishvili M., Chkonia I., Jgenti G., Kvatadze N., Alavidze Z., Kutter E.M., et al. What can we learn from a metagenomic analysis of a georgian bacteriophage cocktail? Viruses. 2015;7:6570–6589. doi: 10.3390/v7122958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mutalik V.K., Adler B.A., Rishi H.S., Piya D., Zhong C., Koskella B., Kutter E.M., Calendar R., Novichkov P.S., Price M.N., et al. High-throughput mapping of the phage resistance landscape in E. coli. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zulk J.J., Clark J.R., Ottinger S., Ballard M.B., Mejia M.E., Mercado-Evans V., Heckmann E.R., Sanchez B.C., Trautner B.W., Maresso A.W., et al. Phage Resistance Accompanies Reduced Fitness of Uropathogenic Escherichia coli in the Urinary Environment. mSphere. 2022;7 doi: 10.1128/msphere.00345-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez B.C., Heckmann E.R., Green S.I., Clark J.R., Kaplan H.B., Ramig R.F., Hines-Munson C., Skelton F., Trautner B.W., Maresso A.W. Development of Phage Cocktails to Treat E. coli Catheter-Associated Urinary Tract Infection and Associated Biofilms. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.796132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y., Shen W., Zhong Q., Chen Q., He X., Baker J.L., Xiong K., Jin X., Wang J., Hu F., Le S. Development of a Bacteriophage Cocktail to Constrain the Emergence of Phage-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J., Park H., Ryu S., Jeon B. Inhibition of Antimicrobial-Resistant Escherichia coli Using a Broad Host Range Phage Cocktail Targeting Various Bacterial Phylogenetic Groups. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.699630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochman H., Selander R.K. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 1984;157:690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A., Formsma K., Gerdes S., Glass E.M., Kubal M., et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laslett D., Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004;32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carey-Smith G.V., Billington C., Cornelius A.J., Hudson J.A., Heinemann J.A. Isolation and characterization of bacteriophages infecting Salmonella spp. FEMS Microbiol. Lett. 2006;258:182–186. doi: 10.1111/j.1574-6968.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 65.Gencay Y.E., Gambino M., Prüssing T.F., Brøndsted L. The genera of bacteriophages and their receptors are the major determinants of host range. Environ. Microbiol. 2019;21:2095–2111. doi: 10.1111/1462-2920.14597. [DOI] [PubMed] [Google Scholar]
- 66.Sambrook J., Russell D.W. Purification of Nucleic Acids by Extraction with Phenol:Chloroform. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4455. [DOI] [PubMed] [Google Scholar]
- 67.Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Assembled bacteriophage genomes have been deposited at NCBI and are publicly available as of the date of publication. For accession numbers for phage genomes see key resources table. Bacterial genomes are available at NCBI under designated Bioprojects as noted in Table S1.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.