Abstract
Objective
Animal studies suggest that prebiotic, plant-derived nutrients could improve homoeostatic and hedonic brain functions through improvements in microbiome–gut–brain communication. However, little is known if these results are applicable to humans. Therefore, we tested the effects of high-dosed prebiotic fibre on reward-related food decision-making in a randomised controlled within-subject cross-over study and assayed potential microbial and metabolic markers.
Design
59 overweight young adults (19 females, 18–42 years, body mass index 25–30 kg/m2) underwent functional task MRI before and after 14 days of supplementary intake of 30 g/day of inulin (prebiotics) and equicaloric placebo, respectively. Short chain fatty acids (SCFA), gastrointestinal hormones, glucose/lipid and inflammatory markers were assayed in fasting blood. Gut microbiota and SCFA were measured in stool.
Results
Compared with placebo, participants showed decreased brain activation towards high-caloric wanted food stimuli in the ventral tegmental area and right orbitofrontal cortex after prebiotics (preregistered, family wise error-corrected p <0.05). While fasting blood levels remained largely unchanged, 16S-rRNA sequencing showed significant shifts in the microbiome towards increased occurrence of, among others, SCFA-producing Bifidobacteriaceae, and changes in >60 predicted functional signalling pathways after prebiotic intake. Changes in brain activation correlated with changes in Actinobacteria microbial abundance and associated activity previously linked with SCFA production, such as ABC transporter metabolism.
Conclusions
In this proof-of-concept study, a prebiotic intervention attenuated reward-related brain activation during food decision-making, paralleled by shifts in gut microbiota.
Trial registration number
Keywords: BRAIN/GUT INTERACTION, BRAIN IMAGING, DIETARY FIBRE, OBESITY, SHORT CHAIN FATTY ACIDS
WHAT IS ALREADY KNOWN ON THIS TOPIC
Targeting high-caloric food craving and unhealthy eating behaviour is crucial for prevention and treatment of the worldwide obesity pandemic. The gut microbiome has been implicated in feeding behaviour through modifying gut-brain crosstalk, for example, short chain fatty acid production.
WHAT THIS STUDY ADDS
We here present causal evidence for effects of supplementary prebiotics on reward-related food decision making in a group of 59 well-characterised overweight adults. Leveraging advanced neuroimaging, next-generation sequencing and multiomics, our results suggest functional microbial changes that underly these effects.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings strengthen the hypothesis that dietary prebiotics cause a reduction of reward-related brain activation in response to high-caloric food stimuli. A better understanding of underlying microbiome–gut–brain mechanisms could help to develop novel strategies towards fostering healthier eating behaviour in humans.
Introduction
Plant-based diets, recognised as a major effector of planetary health,1 are more beneficial for cardiovascular and brain health compared with conventional Western diets.2 3 Plant-based food and related prebiotic nutrients are less dense in calories and have been claimed to modulate brain function4 including feeding5 and psychological functioning6 via the microbiota–gut–brain axis, however, direct experimental evidence is still limited.
Microbiota-derived metabolites of plant-based dietary fibre such as short-chain fatty acids (SCFA), can cross the blood–brain barrier7 to modulate hypothalamic signalling.8 First experimental studies showed that oral intake of the SCFA butyrate or of the butyrate-producing bacteria Akkermansia spp lowered body weight (in humans9) and restored obesity-induced functional brain changes (in mice10). Moreover, 1 week of colonic SCFA delivery modulated hypothalamic-pituitary-adrenal axis-dependent stress-induced cortisol response in a study including 66 healthy men,11 and intake of autologous faeces-derived microbiota from a dietary weight-loss period enhanced weight loss maintenance in humans.12
Earlier trials in humans showed that supplementary intake of prebiotic fibre such as inulin-type fructans reduced subjective hunger and improved gut hormonal-driven appetite regulation through changes in postprandial glucagon-like peptide (GLP)-1, neuropeptide y (PYY)13 (n=10) and ghrelin14 15 (both n<50). In another randomised clinical trial (RCT) in >100 patients with obesity, inulin compared with placebo induced greater weight loss16 and exploratory results indicated mood improvements in a microbiota-based subgroup with elevated relative Coprococcus abundance at baseline.17 Own results from two cross-sectional analyses indicated that habitual overall dietary fibre intake links to specific microbiota genera including Parabacteriodes, which in turn explained variance in eating behaviour in adults with overweight and treatment success after bariatric surgery.18
However, neuroimaging evidence of how prebiotic diets and diet-related microbial changes affect the brain with regard to eating behaviour remains to be shown. At the brain level, food decision-making is thought to rely on a complex interplay of homoeostatic and hedonic signalling, orchestrated by a variety of subcortical and cortical networks involving the brainstem and hypothalamus, striatum and prefrontal cortex areas.19 The neurobiological underpinnings of (unhealthy) eating behaviour and their neuroimaging correlates, however, have not been fully understood. Functional MRI (fMRI) studies indicated that presentation of highly palatable food cues leads to a stronger brain response in reward areas than equicaloric, non-palatable food cues.20 In parallel, disinhibition and unhealthy food craving, sometimes controversially described as food addiction,21 have been linked with subtle structural differences in the reward network22 23 and with differential brain activation in the ventromedial prefrontal cortex (vmPFC) in response to high-caloric food stimuli.24 Whether these effects can be mitigated by prebiotic dietary targeting the gut–brain axis25 is yet unknown.
We here aimed to test the hypothesis that a high-dosed prebiotic fibre intervention can alter the gut microbiome and thereby neural activation patterns of food reward in a population at risk for weight gain and insulin resistance. To this end, we conducted an RCT in overall healthy adults in a randomised within-subject cross-over design and assessed food wanting using fMRI before and after 14 days of daily 30 g supplementary intake of inulin (prebiotic fibre) and equicaloric maltodextrin (placebo), respectively. Suggested microbial and metabolic mediators of potential effects were measured using faeces and serum proxies collected at all four timepoints. The study and analyses were preregistered at ClinicalTrials.gov/NCT03829189 and osf.io/ynkxw.
Methods
Study design
In this within-subject cross-over design, participants underwent screening and, if eligible, received both verum and placebo in a randomised order (two arms) for 14 days each, separated by a wash-out period of at least 2 weeks (figure 1). Verum (prebiotic fibre) consisted of 30 g inulin (63 kcal, 26.7 g fibre, Orafti Beneo Synergy1, BENEO, Mannheim, Germany) per day compared with calorie-matched placebo consisting of 16 g maltodextrin (63 kcal, 0 g fibre), each provided as two sachets per day.
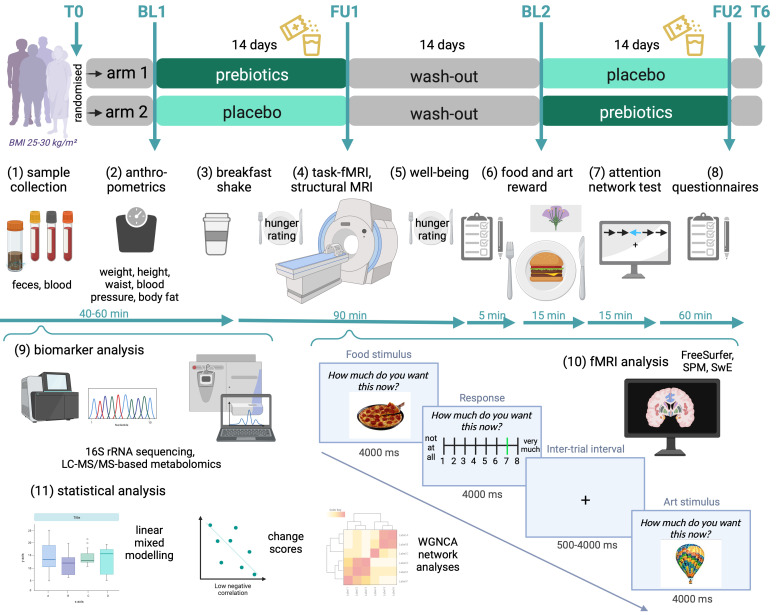
Figure 1.
Study design. Within-subject cross-over dietary intervention design with two study arms and up to six measurement timepoints (upper panel, T0: screening; BL1/2: baseline 1/2, FU1/2: follow-up 1/2, T6: additional follow-up). Participants were randomly assigned to receive first prebiotics and second placebo (arm 1), or vice versa (arm 2), for 14 days each, separated by a 14-day wash-out period. Following the same timeline, at BL1, FU1, BL2 and FU2, participants provided stool samples and underwent fasting blood draw (1), anthropometric measurements (2), received a standard breakfast shake (3) and MRI assessments (4), followed by brief surveys (5), food remuneration (6) and further tests and questionnaires (7–8). Steps (9–11) indicate data processing and statistical analysis. Screens give fMRI wanting task paradigm scheme and timing. BL, baseline; FU, follow-up; fMRI, functional magnetic resonance imaging (MRI); LC-MS/MS, liquid chromatography–mass spectrometry; SPM, statistical parametric mapping, SwE, sandwich estimator, WGNCA, weighted graph network correlational analysis. Created with BioRender.com.
Data acquisition took place between 2019 and 2022 with some breaks due to lockdown regulations during the SARS-CoV-2 pandemic. All participants were invited to baseline and follow-up visits for each condition, resulting in four study visits with faeces and fasting blood sample collection, fMRI and questionnaires. Briefly, after fasting blood draw and anthropometrics (~45 min), participants received a neutral drink covering 10% of their individual daily energy requirement. Right after, the MRI assessment followed (~2 hours), which was then followed by further computer-based assessments (~1.5 hours) (see online supplemental file_general for further details).
gutjnl-2023-330365supp001.pdf (177.5KB, pdf)
Participants
Volunteers of all gender were recruited via online and local advertisements and the institute’s local database. Inclusion criteria were a body mass index of 25–30 kg/m2, no MRI contraindications, aged 18–45 years, women: intake of oral contraceptives. Exclusion criteria were: neurological or psychiatric disease; intake of medication acting on the central nervous system; diabetes mellitus type 2; severe untreated internal disease including the gastrointestinal tract, lung, heart, vasculature, liver and kidneys; eating disorder or unconventional eating habits; women: pregnancy, breastfeeding as well as daily consumption of >50 g alcohol, >10 cigarettes, or >6 cups of coffee. Out of 106 initially recruited volunteers with screening assessment, 59 participants (19 women, 40 men) took part in the study, with 45 completing all 4 measurement visits (figure 2). For power analysis and sample size rationale, see online supplemental file_general.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Participants underwent a randomised controlled dietary intervention trial in a within-subject cross-over design. BMI, body mass index; fMRI, functional MRI.
Registration and blinding
Participants received a small reimbursement of €9–€10/hour for testing days and additionally €30 for study completion. The study was registered at https://clinicaltrials.gov/ct2/show/NCT03829189 and https://osf.io/f6qz5 (14 January 2019) prior to recruitment and data acquisition. Additionally, details on fMRI (pre)processing were uploaded before the start of data analysis https://osf.io/ynkxw (11 May 2021). Participants and staff members were blinded regarding the study intervention/placebo allocation. Sachets were labelled with either A or B through a random assignment performed by author AVW, who was not involved in data collection, prior to the study. Allocation to the A-B or B-A study arm was determined following a randomised order generated using the R software’s ‘sample()’ function by author RT. Authors EM and RT enrolled participants and assigned them to the intervention arm accordingly.
Patient and public involvement
The authors acknowledge a missed opportunity of not following a tailored approach to involve patients or the public in the design of the study. We invited and collected comments and assessments from all participants throughout the study to inform the design of upcoming research studies.
MRI
MRI was performed on a 3T Siemens Prismafit scanner with a 32-channel head coil. FMRI was done in an event-related design assessing wanting of food and art, respectively. Participants were presented with four sets of images across four sessions (randomised order). Each stimulus was shown for 4000 ms with the question ‘How much do you want this now?’, followed by a 4000 ms response period, followed by 500–4000 ms interstimulus interval with a 500 ms jitter until the next stimulus was presented (figure 1). Wanting ratings were done on a 8-point Likert scale with 1 labelled as ‘not at all’ and 8 as ‘absolutely’. Participants were informed about receiving a reward right after the scanning session outside the scanner, for food and art, respectively, based on their highest ratings in that session. The reward was given as a dish to eat right away and as a carton-based art print to take home with.
Preprocessing was done using fMRIPprep V.1.2.5.26 As preregistered, first-level contrasts of interest were global difference between food and art viewing, food compared with art wanting slope, and wanting modulation (design A), food wanting by caloric or fibre density (design B) and considering liking ratings as modulator (design C). See online supplemental file_fMRI for further details.
gutjnl-2023-330365supp002.pdf (15.5MB, pdf)
Additional behavioural assessments
Dietary habits, lifestyle factors including gastrointestinal quality of life, sleep, physical activity, mental well-being and mood were assessed at each timepoint. Additionally, we assessed potential traits associated with food decision-making at baseline, that is, on personality, eating behaviour, anxiety and well-being, as well as on art knowledge (see online supplemental file_behav for details).
gutjnl-2023-330365supp003.pdf (567.2KB, pdf)
Blood and faeces markers
To assess serum SCFA, gut hormones (ghrelin, GLP-1, PYY), markers of glucose/lipid metabolism (glucose, insulin, glycated haemoglobin A1c, high and low density lipoprotein, triglycerides), inflammatory markers (high sensitive C reactive protein, interleukin-6, TNFalpha) and other markers (trimethylamine-n-oxid and amino acids), blood was obtained in fasting state (12.5±2.2 hours fasted) at the same time per participant for each session. Stool samples were taken within 1–2 days before the testing day to assess faecal SCFA and microbial markers.
Microbial analysis
For 16S-rRNA gene profiling, DNA was extracted and V3–V4 variable regions of the 16S-rRNA genes were amplified by PCR and a library was constructed, followed by paired-end 2×250 bp Illumina sequencing. Raw sequencing data analysis was done on the inhouse Galaxy server using a pipeline implemented with the DADA2 R-package processed data in fastq format.27 For further details, see online supplemental file_microbiome.
gutjnl-2023-330365supp004.pdf (837.4KB, pdf)
gutjnl-2023-330365supp005.pdf (2.1MB, pdf)
gutjnl-2023-330365supp006.pdf (5.5MB, pdf)
Statistical analysis
On a behavioural level, we hypothesised that participant’s wanting ratings scored higher for food compared with art (H_behav_1), and that wanting would change after prebiotic intervention (H_behav_2), dependent on caloric density of the food item (H_behav_3). Linear mixed models were performed in R (version>3.6) using lmer(), for a model-of-interest and a null model for each effect of interest. Model residuals were tested for normal distribution using the R package performance() with the command check_normality(x, effects=‘random’), see online supplemental file_behav for details.
On a neural level, we hypothesised that food evaluation elicits different regional brain activation compared with art evaluation (H_neural_1), and that this differential brain response changes after prebiotic intervention (H_neural_2). Inference tests were performed using a homoeostatic and reward-related region-of-interest brain mask on first-level contrasts (designs A–C) and second-level factors time (baseline, follow-up), group (prebiotics/placebo), and time×group interactions, using the Sandwich Estimator (SwE V.2.2.2, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Swe, implemented in SPM V.12.7486 run in MATLAB V.>9.0) and R (V.>3.6). All main analyses were run in a homoeostatic regulation and reward-related region-of-interest brain mask defined by a combination of two meta-analyses of available previous independent studies at neurosynth.org using the keywords ‘hypothalmus’ and ‘reward’, respectively, integrating functional brain responses of 922 and 98 studies, respectively (created in April 2021; figure 3 in online supplemental file_fMRI). Significant results were reported according to threshold-free cluster enhancement methods with alpha <0.05 and family wise error (FWE) correction for multiple comparisons. For details, see fMRI preregistration and online supplemental file_fMRI.
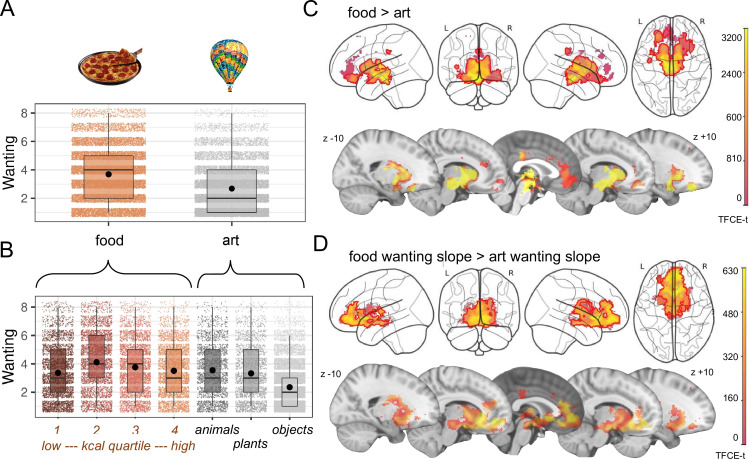
Figure 3.
Behavioural (A, B) and neural response (C, D) to food and art stimuli in overweight adults during decision-making. Participants responded to food with higher wanting scores compared with art (nobs=32 111, nsubj=59) (A), showing highest mean values for moderately high caloric stimuli, and lowest mean values for art objects (nobs=32 111, nsubj=59) (B). Food compared with art valuation elicited stronger brain activation particularly in subcortical areas of the reward network (nsubj=57) (C), while additional parametric modulation with wanting scores indicated a stronger brain activation in ventromedial prefrontal cortex and orbitofrontal cortex when comparing food versus art (nsubj=57) (D). Statistics were done with linear mixed effect modelling, up to 4 time points per participant×120 stimuli on wanting scores (main analysis) (A), (exploratory analysis) (B) and on voxel-wise blood-oxygen-level-dependent signal using the sandwich estimator toolbox with threshold-free cluster enhancement (TFCE) family wise-error correction (FWE) of multiple comparisons (C,D),(main analyses) (C,D). Colour bars depict parametric TFCE statistic (TFCE-t >50 for visualisation purposes) with wild-boot strapped pFWE<0.05 marked in red outline.
Further exploratory analyses were done with the aim to generate hypotheses on potential mechanisms between changes in the microbiome/metabolism and changes in brain activation. First, intervention effects versus placebo were explored in anthropometrics and blood and faeces markers according to mixed effects inference with (restricted) maximum likelihood fitting and χ2 test for comparison. Microbiome composition and predicted functional pathways based on Kyoto Encyclopaedia of Genes and Genomes (KEGG 28) were analysed using Stress test on non-metric multidimensional scaling (NMDS) prior to individual genera/pathway testing with linear mixed effects modelling. Second, bivariate correlation analyses were done on the difference (delta) post versus pre after prebiotic treatment, in those outcomes that showed a significant group×time interaction effect only. Significance threshold for exploratory analyses was set at p<0.05, follow-up microbiome analyses were corrected for multiple comparisons using false discovery rate.
Data and code availability
Data are available at https://doi.org/10.17605/OSF.IO/FC4 and code is available at https://gitlab.gwdg.de/gut_brain_study/food-wanting/task-fmri-behavior-analysis and https://gitlab.gwdg.de/gut_brain_study/food-wanting/fmri-analysis.29 Statistical MRI maps are available at https://identifiers.org/neurovault.collection:14111.
Results
A total of 59 well-characterised overweight/obese adults were included in main analyses (19 women, 40 men, mean age 28 years±6.2 SD, body mass index (BMI) 27.3 kg/m2±1.4 SD, socioeconomic status 14.2±3.2; table 1, online supplemental file_general-table1).
Table 1.
Baseline characteristics
| n=59 | ||
| Gender (n) | Women | 19 |
| Men | 40 | |
| Age (years) | Mean (SD) | 28.3 (6.55) |
| Median (min, max) | 28 (19.0, 45.0) | |
| BMI (kg/m2) | Mean (SD) | 27.3 (1.51) |
| Median (min, max) | 27.0 (25 30) | |
| SES index (score) | Mean (SD) | 14.5 (2.98) |
| Median (min, max) | 14.4 (5.10, 19.2) | |
| Habitual dietary fibre (g/day) | Mean (SD) | 16.3 (6.31) |
| Median (min, max) | 15.4 (1.54, 30.5) | |
| Blood HbA1c (%) | Mean (SD) | 5.31 (0.20) |
| Median (min, max) | 5.30 (4.6, 5.8) | |
| Missing | 2 (3.4%) |
BMI, body mass index; HbA1c, glycated haemoglobin A1c; Max, maximum; Min, minimum; SD, standard deviation; SES, socioeconomic status.
Neurobehavioural correlates of reward-related decision-making
Overall, wanting and liking ratings in the fMRI preference task were higher for food than for art stimuli (H_behav_1; nobs=32 111, nsubj=59, b=1.03, t=7.78, 8, p<0.001, figure 3A,B, online supplemental file_behav-table 2). Food evaluation activated large parts of the reward network, including ventral tegmental area (VTA), hypothalamus, nucleus accumbens (NAc), basal ganglia and ventromedial thalamus, as well as anterior insula, amygdala, cingulate, vmPFC and distinct parts of the orbitofrontal cortex (OFC) (H_neural_1, n=57, design A, pFWE<0.05; figure 3C). Similarly, higher wanting ratings for food compared with art elicited higher brain activation ubiquitously across these brain areas, yet particularly in the vmPFC and OFC (design A, pFWE<0.05; figure 3D).
Effect of prebiotics on food decision-making
At the behavioural level, individuals’ overall wanting scores were not different after the 2-week prebiotic intervention regarding food versus art and when accounting for calories or fibre, contrary to our hypothesis (H_behav_2+3; nobs=32 111/16,071, nsubj=59, ball < ∣0.07∣, tall < ∣1.0∣; pall>0.32, online supplemental file_behav tables 7a, 10 and 11). Exploratory analysis showed however that prebiotics compared with placebo led to significantly lower overall wanting scores (online supplemental file_behav table 7b). That is, when looking at stimulus subcategory, participants reported decreases in wanting for very low and very high caloric content as well as for plants after prebiotics (approximatively −0.3 points on the Likert scale, figure 4A; nobs=32 111, nsubj=59, group×time×subcategory: all p<0.01; online supplemental file_fMRI-Results).
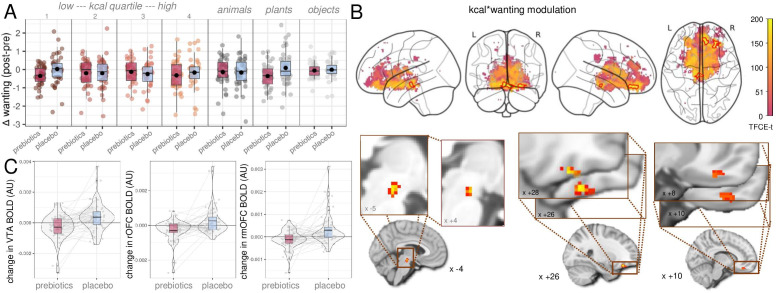
Figure 4.
Effects of prebiotic intervention on food decision-making. After the intervention, participants decreased wanting scores for food from caloric quartiles 1 and 4 as well as animals (exploratory analysis, nsubj=59, (A). At the neural level, brain activation decreased in the ventral tegmental area (VTA) and in two clusters in the orbitofrontal cortex (OFC) towards high-caloric, wanted food stimuli (main analysis, nsubj=57, (B, C). Statistics according to linear mixed effects modelling, up to 4 time points per participant×120 stimuli on wanting scores and on voxel-wise blood-oxygen-level-dependent signal using the sandwich estimator toolbox with threshold-free cluster enhancement (TFCE) family wise-error correction (FWE) of multiple comparisons. Colour bars depict parametric TFCE statistic with wild-boot strapped pFWE<0.05 marked in red outline (upper right panel) and as enlargement (lower right panel).
According to fMRI (H_neural_2), we did not observe changes in regional brain response after prebiotics in food compared with art viewing, food compared with art wanting slope, or wanting modulation (design A). However, brain activation towards wanted, high-caloric food (design B) decreased after prebiotics compared with placebo in three clusters, in the VTA (pFWE-corr=0.042), in the right OFC (rOFC, pFWE-corr<0.05) and in the right medial OFC (rmOFC, pFWE-corr<0.05) (n=57, figure 4B,C, table 2). In addition, art liking compared with food liking increased in a small cluster in the right NAc after prebiotics compared with placebo (design C, table 2). See online supplemental file_fMRI-Results for secondary and sensitivity results.
Table 2.
Localisation of significant changes in brain activation to visual food and art stimuli during functional MRI, after prebiotic compared with placebo intervention (main analysis)
| Prebiotic compared with placebo | TFCE P (FWE-corr) | TFCE cluster size | Peak Z | Peak P(unc) | X (mm) |
Y (mm) |
Z (mm) |
Region |
| Parametric modulation, food wanting×kcal; decreases in activation | 0.038 | 51 | 3.595 | 0.002 | 26 | 32 | 16 | Right OFC |
| 0.042 | 43 | 3.388 | 0.001 | 4 | 20 | 14 | VTA | |
| 0.043 | 41 | 3.075 | 0.003 | 10 | 36 | 20 | Right (medial) OFC | |
| Art liking>food liking slope; increases in activation | 0.039 | 3 | 3.827 | 0.001 | 8 | 16 | 6 | Right NAc |
NAc, nucleus accumbens; OFC, orbitofrontal cortex; TFCE, threshold-free cluster enhancement; unc, uncorrected; VTA, ventral tegmental area.
In addition, after prebiotics, participants reported less subjective hunger during the fMRI task, compared with placebo (exploratory analyses, ß=−0.39, p<0.001; figure 5A, online supplemental file _behav tables 18 and 19).
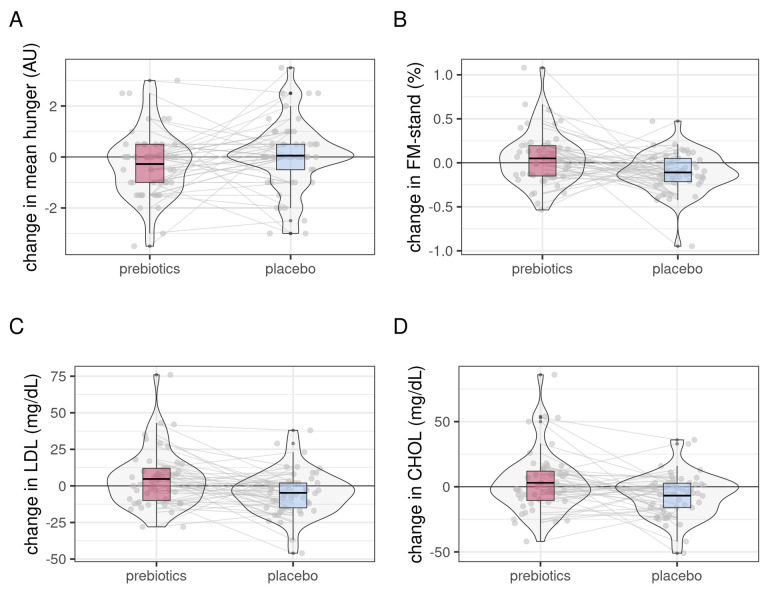
Figure 5.
Changes in secondary eating behaviour-related outcomes after prebiotic (red) compared with placebo condition (light blue). Hunger ratings during fMRI significantly decreased after prebiotics (A), while gender-standardised body fat mass (FM-stand, B), serum lipid markers low-density lipoprotein (LDL, C) and cholesterol (CHOL, D) significantly decreased after placebo (linear mixed effects modelling, all p<0.05, exploratory analyses). FMRI, functional magnetic resonance imaging.
While both intervention and placebo supplements contained, the same amounts of calories and participants reported equally high compliance in taking the daily supplements, we observed in exploratory analysis decreases in body fat after placebo (timepoint×intervention, b=0.16, p=0.005; figure 5B). In addition, lipid markers were significantly lower after placebo intake compared with prebiotics, as well as alanin-aminotransferase (ball>0.09, tall>2.4, pall<0.013; examples figure 5C,D). BMI, waist-to-hip ratio, and blood pressure did not change significantly, which was also true for fasting ghrelin, GLP-1 and PYY, glucose, insulin, amino acids, as well as inflammatory markers (see online supplemental file_general tables 2–5).
In exploratory bivariate correlation analysis on change scores after the prebiotic intervention, mean bold activation in the three outlined VTA and OFC clusters decreased in correlation with decreases in fasting PYY (Spearman’s rall>0.32, pall<0.05).
Changes in gut microbiota and parameters
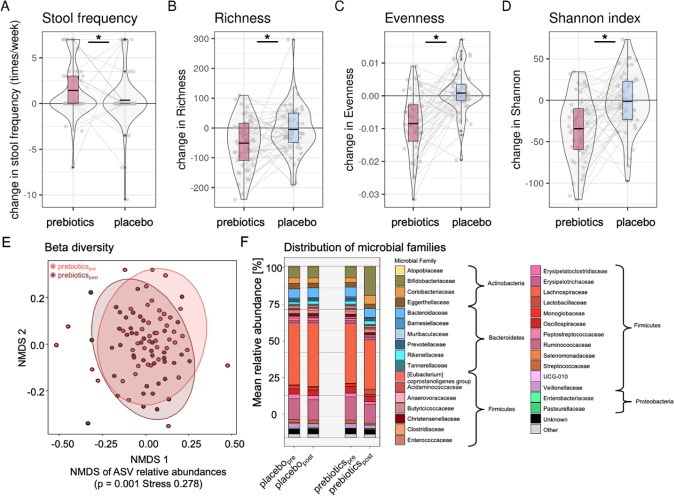
The prebiotic intervention led to increases in stool frequency (b=1.2, t=2.1, p=0.04, figure 6A). Through 16S-rRNA analysis, we detected significantly decreased richness, evenness and alpha diversity after prebiotics compared with placebo (nobs=200, nsubj=57, all p<0.001 figure 6B–D online supplemental file_microbiome_table 1). Beta diversity on Amplicon Sequencing Variant was significantly different after prebiotic intervention (NMDS, prebiotics: padj=0.001; figure 6E), and there were abundance changes in families of Actinobacteria and Firmicutes (all padj<0.02, figure 6F). Zooming at the genera level, prebiotics induced significant shifts in various abundances, including profound increases in Bifidobacteria (table 3, online supplemental file_microbiome table 2).
Figure 6.
Microbiota-related shifts after 2-week prebiotic intervention (exploratory analyses). Increases in stool frequency (A) and decreases in (B) microbiota richness, (C) evenness, (D) Shannon index, (E) beta diversity changes compared by dissimilarity gradients according to group and timepoint after prebiotics (pink) compared with placebo (blue), and (F) shifts in microbial family distribution. Asterisks in (A–D) indicating significant ANOVA results for null-full model comparisons (p<0.05). ANOVA, analysis of variance; ASV, amplicon sequencing variant; NMDS, non-metric multidimensional scaling.
Table 3.
Significant shifts in microbiota relative abundances on the genera level after prebiotic intervention, according to 16S-rRNA sequencing and linear mixed effects modelling after FDR-correction for multiple comparisons
| Interaction effect time (follow-up)×intervention (prebiotic) | ANOVA null model comparison | |||
| Increased abundance | b | t | P value | padj |
| Anaerostipes | 0.73 | 3.01 | 0.003 | 0.017 |
| Bifidobacterium | 9.82 | 10.42 | <0.001 | <0.001 |
| Collinsella | 2.66 | 4.96 | <0.001 | <0.001 |
| Holdemanella | 0.37 | 3.13 | 0.002 | 0.011 |
| Lachnospiraceae FCS020 group | 0.21 | 3.31 | 0.001 | 0.006 |
| Lacticaseibacillus | 0.10 | 2.05 | <0.001 | 0.002 |
| Lactiplantibacillus | 0.03 | 2.82 | <0.001 | <0.001 |
| Lactobacillus* | 2.08 | 2.65 | 0.008 | 0.045 |
| Ligilactobacillus | 0.28 | 2.67 | 0.008 | 0.045 |
| Limosilactobacillus | 0.28 | 5.10 | <0.001 | <0.001 |
| Decreased abundance | ||||
| Desulfovibrio | 0.20 | 3.41 | 0.001 | 0.006 |
| Eggerthella | 0.33 | 3.46 | 0.001 | 0.006 |
| Eubacterium brachy group | 0.11 | 3.18 | 0.002 | 0.011 |
| Eubacterium eligens group | 0.21 | 2.76 | 0.006 | 0.033 |
| Roseburia | 1.10 | 3.86 | <0.001 | 0.001 |
| Ruminococcus gauvreauii group | 0.69 | 3.86 | <0.001 | 0.001 |
| Shuttleworthia | 0.08 | 2.78 | 0.006 | 0.033 |
| Subdoligranulum | 1.30 | 2.82 | 0.005 | 0.028 |
Linear mixed effects modelling outcome compared to null model and model of interest as follows (ANOVA model comparison with p<0.05): with the Formula: bacterial_genus_of_interest−time point×intervention+time point+intervention+(1+(intervention+time point)|subject). All models run on nobs =204 in nsubj=58 and listed in alphabetical order of genera of interest.
*Statistics refer to models without random slopes due to non-convergence.
ANOVA, analysis of variance; FDR, false-discovery rate.
Changes in microbiota genera link to changes in neurobehavioural outcomes
We further explored whether the observed changes in microbial genera predicted intervention-induced changes in neurobehaviour. According to these exploratory analyses, a less severe decrease in Subdoligranulum correlated with intervention-induced decreases in VTA brain activation towards wanted, high caloric food stimuli after prebiotic intervention (r=−0.38, p=0.01). Note that bacterial abundance was measured in percentage, thus a relative decrease in Subdoligranulum does not necessarily display absolute decrease after prebiotics. Additionally, increases in Lactiplantibacillus (lactic acid producing bacteria) were significantly related to increases in rmOFC activation (r=0.40, p=0.008), however abundance of this bacterium did not change in all participants.
Complementary weighted network analyses in a subgroup of available participant data from all four timepoints (n=35) did not provide compelling evidence that clusters of microbial taxa related to neurobehavioural outcomes (exploratory analyses, online supplemental file_microbiome).
SCFA and microbial functional capacity prediction
We could not detect changes in SCFA acetate, butyrate and propionate after intervention, neither in fasting serum nor in faecal concentrations (exploratory analyses, nobs≥122, nsubj≥40, pall>0.39, table 4).
Table 4.
Concentrations of faeces and serum SCFA levels
| Total (μmol/g) | Butyrate (μmol/g) | Acetate (μmol/g) | Propionate (μmol/g) | |||||||||||||||||||||
| n | Pre, mean±SD | n | Post, mean±SD | Change (%) | P value | n | Pre, mean±SD | n | Post, mean±SD | Change (%) | P value | n | Pre, mean±SD | n | Post, mean±SD | Change (%) | P value | n | Pre, mean±SD | n | Post, mean±SD | Change (%) | P value | |
| Faeces | ||||||||||||||||||||||||
| Prebiotics | 42 | 26.4±10.9 | 40 | 24.6±8.5 | −6.8 | 0.39 | 42 | 18.3±8.7 | 41 | 16.8±8.0 | −8.2 | 0.70 | 42 | 7.9±2.7 | 41 | 8.1±2.4 | 2.5 | 0.78 | 42 | 7.1±2.4 | 41 | 6.2±3.2 | −12.7 | 0.60 |
| Placebo | 42 | 23.9±8.8 | 42 | 23.2±12.2 | −2.9 | 42 | 16.2±6.9 | 42 | 15.5±9.4 | −4.3 | 42 | 7.4±2.4 | 42 | 7.4±3.2 | 0.0 | 42 | 7.1±3.1 | 42 | 6.6±3.4 | −7.0 | ||||
| Serum | ||||||||||||||||||||||||
| Prebiotics | 27 | 5.8±1.3 | 27 | 5.5±1.5 | −5.2 | 0.65 | 36 | 0.5±0.3 | 34 | 0.5±0.4 | 0.0 | 0.59 | 30 | 3.1±3.2 | 32 | 3.8±3.0 | 22.6 | 0.88 | 28 | 0.5±0.5 | 24 | 0.6±0.6 | 20.0 | 0.84 |
| Placebo | 29 | 5.5±1.4 | 25 | 5.3±1.5 | −3.6 | 34 | 0.5±0.4 | 37 | 0.4±0.3 | −20.0 | 32 | 2.4±2.3 | 28 | 3.1±3.2 | 29.2 | 27 | 0.4±0.4 | 28 | 0.4±0.4 | 0.0 | ||||
P, according to ANOVA null-full model p value, total=sum of butyrate, acetate and proprionate.
ANOVA, analysis of variance; SCFA, short chain fatty acid.
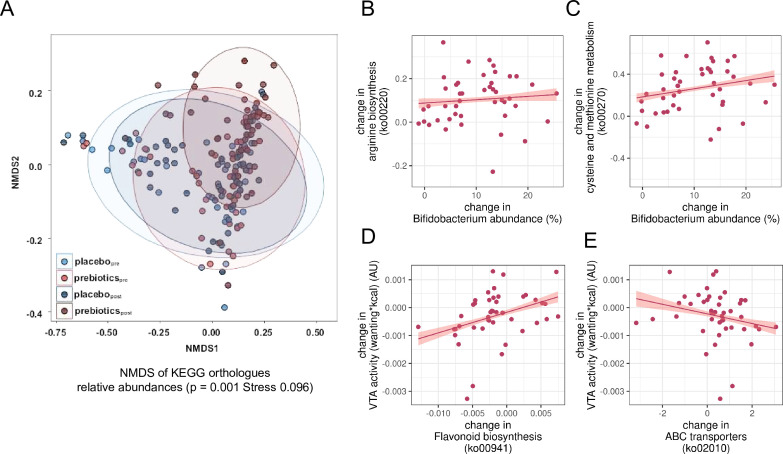
Next, we explored changes induced by microbial shifts on the metagenomic level according to KEGG28 analysis. Changes in KEGG orthologue relative abundance were significantly different after prebiotics (figure 7A, NMDS: prebiotics padj=0.001, placebo padj=0.99, posthoc-pairwise permutational multivariate analysis of variance (PERMANOVA): F=11.46, padj=0.002, online supplemental file_microbiome table 3). The KEGG orthologues were annotated to 158 pathways out of which about 44%, that is, 69 were significantly altered in relative abundance after prebiotic intervention compared with placebo, including pathays related to carbohydrate, protein and fat metabolism, plant degradation or cell repair (padj<0.05, online supplemental file_microbiome table 4).
Figure 7.
Predicted functional shifts and their correlations with changes in microbiota genera and in reward-related brain activation after prebiotic intervention (exploratory analyses). (A) Dissimilarity of functional composition of microbiome preprebiotic to versus postprebiotic intervention based on NMDS stress test (p=0.001) and principal component analysis of relative abundance of predicted KEGG orthologues statistics, calculated by PERMANOVA (padj=0.002). (B) Change scores of Bifidobacterium abundance and arginine biosynthesis (ko00220), (C) Bifidobacterium abundance and cysteine and methionine metbolism (ko00270), (D) flavonoid biosynthesis (ko00941) and changes in reward-related brain response, (E) stilbenoid, gingerol biosynthesis (ko00945) and reward-related brain response. (B–E), all r>0.32, all p<0.05 according to Spearman’s correlation, line gives regression fit with 95% CI. KEGG, Kyoto Encyclopaedia of Genes and Genomes; NMDS, non-metric multidimensional scaling; PERMANOVA, permutational multivariate analysis of variance; VTA, ventral tegmental area.
More specifically, exploratory analyses indicated that increases in relative abundance of Bifidobacteria correlated significantly with increases in metabolic pathways related to taurine, seleno compounds, nicotinate and amino acids, and with decreases related to porphyrin metabolism, steroid degradation, (unsaturated) fatty acid biosynthesis, and DNA repair functions (exemplary figure 7B,C; Spearman’s rall>0.32, pall<0.05). In addition, increases in Lactobacillus and decreases in Gordonibacter correlated with increases in pyruvate metabolism pathway, a precursor of SCFA (note that not all participants changed in Lactobacillus and Gordonibacter abundance, though). Further exploratory analyses indicated that decreases in VTA brain activation after prebiotic intervention correlated with intervention-induced significant decreases in pathways involved in flavonoid and stilbenoid biosynthesis, two-component signal transduction, biofilm formation, amino sugar and nucleotide sugar metabolism, citrate cycle (rall>0.37, pall<0.05), and with significant increases in ATP-binding cassette transporters (ABC, r=−0.39, p<0.05, exemplary figure 7D,E). Decreases in rOFC activation after prebiotics correlated with significant decreases in aromatic hydrocarbon degradation (r=0.32, p<0.05). Decreases in rmOFC activation after prebiotics correlated with significant increases in oxidative phosphorylation (r=−0.31, p<0.05). For details, see online_supplemental_file_microbiome figure 2a and 2b.
Discussion
In this proof-of-concept study, we tested the effects of a prebiotic intervention on food decision-making in a randomised within-subject cross-over design including 59 well-characterised, overweight adults. In preregistered analyses, we found that 14 days of high-dose dietary prebiotics, compared with placebo, led to decreases in bold-related brain activation towards high caloric, wanted food in the VTA and right OFC measured using 3T fMRI. In parallel, prebiotics led to significant shifts in relative abundance of the gut microbiota, including increases in SCFA-producers such as Bifidobacteria and Collinsella. Exploratory analyses indicated intervention-induced changes in relative abundance and predicted metabolic pathways correlated with changes in VTA brain activation. While fasting gut hormones, inflammatory markers and SCFA in blood and faeces remained unchanged, we observed that prebiotics-induced decreases in brain activation in reward areas related to decreases in fasting PYY.
Changes in functional brain activation
Only few studies with moderate sample size have addressed whether manipulating the microbiome can alter brain functions. A parallel trial in 34 females indicated that 4 weeks of fermented milk consumption (including Bifidobacteria) induced resting-state functional connectivity changes in the midbrain.30 Another randomised trial reported that 4 weeks of probiotic supplementary powder containing Bifidobacteria and Lactobacillae resulted in changes in microbial genera abundance that correlated with improvements of emotional attention and memory, paralleled by differences in related brain activation.31 Our findings now present prebiotics-induced changes in brain activation with potential implications for food craving and decision-making: While the neuronal processes underlying human eating behaviour are far from fully understood,32 neuroimaging studies indicate neural responses within VTA and OFC to underly dopamine-related reward anticipation and subjective value attribution of food, respectively, linking stronger BOLD-related activation to higher reward values and decision-making.33 Indeed, midbrain and medial OFC activation during fMRI in response to milkshake taste predicted the amount of milkshake intake after the scan.34
Consistently, drivers of reward considering food (such as caloric content) modulate subjective value particularly in the OFC,35 and the right OFC has been specifically implicated in food-related motivation.36 Notably, decreases in brain activation towards high caloric food cues such as ice-cream in the OFC has for example been shown using fMRI when participants were instructed to consider health aspects or long-term consequences of consumption, compared with ‘naive’ viewing.37 The intervention-induced decreases in VTA and rOFC in the current study might thus indicate a diminished anticipation of reward, and a smaller subjective value attribution to high-caloric wanted foods after prebiotic treatment, potentially translating in a subtle reduction of the desire for high-caloric food. At the behavioural level, we could not confirm a general reduction in food wanting ratings, yet exploratory analysis indicated less wanting of very high and very low caloric food, as well as certain art objects, and less hunger after prebiotics. We also observed a marginal increase in body fat after prebiotics which was not statistically significant when comparing pre versus post, but in the interaction model, that is, when taking into account a marginal decrease after placebo (discussed below). This anthropometric data might speak against a significant translation of the observed changes in brain activation to healthier eating behaviour, however two weeks may be too short to generate robust trends in body composition and studies incorporating longer durations are needed.
Microbiota-related mechanisms
The gut microbiome has only recently been shown to be relevant for host nutritional foraging in rats, for example, through changing circulating amino acids and bacterial tryptophan.38 Another faecal transplantation rat study indicated that microbiota from obese donors resulted in changes in food preference and expression of dopaminergic markers in the striatum.39 In humans, a single-group study in 26 females suggested that increased consumption of vegetables rich in inulin-type fructans over two weeks increased Bifidobacteria and decreased the desire to eat sweet, salty, and fatty food.40 In the current study, we similarly observed changes in multiple bacterial genera abundances after prebiotics compared with placebo, mainly increases in Actinobacteria phylum (eg, Bifidobacteria) and Firmicutes phylum (eg, Lactobacillus). This suggests a marked increase in fiber-degrading, SCFA producing bacteria that are present in the gut, which is in line with previous human trials.16 41–44
Functional capacity prediction analyses further yielded a multitude of different pathways that were selectively changed after prebiotic intervention, among them pathways involved in SCFA production capable to modify systemic SCFA signalling. For example, one of the most strongly upregulated pathways related to the ABC transporters (ko02010). It has been shown that Lactobacillus use dietary fibre (e.g., inulin) via ABC transporters to produce acetate,45 which can be further degraded to butyrate.46 Multiple of the upregulated microbiota genera after prebiotics have been classified in previous studies to produce SCFA, eg, Anaerostipes, Bifidobacterium and Holdemanella.47 Moreover, pointing to a dose–effect relationship, a less severe decrease in relative Subdoligranulum abundance (also SCFA producers), as well as the increases in prebiotics-induced upregulation of ABC transporters, correlated with significant decreases in prebiotics-induced VTA brain activation in the current study. This may suggest a potential mechanistic route of higher SCFA production leading to lessened reward anticipation, however, these considerations need to be taken with caution due to the lack of direct evidence.
In contrast to our a priori hypothesis, we did not observe changes in faecal or fasting blood levels of acetate, butyrate or propionate, suggesting that, in principle, changes in brain activity may have been driven by other indirect factors. Similar to our trial, previous small scale studies could not show increases in faecal SCFA after inulin, for example, in healthy young adults (subgroup, n=49).48 Others observed SCFA increases, for example, in type 2 diabetes mellitus (n=25,49), or even decreases in faecal SCFA (n=30).42 These conflicting results might be explained by unknown complexity of local and systemic microbial effects, and/or by pre-existing differences such as microbiota patterns at baseline (note higher relative Firmicutes in our overweight/obese group compared with obesity studies), or differences in stool frequency, weight and fluidity (note significant changes in Bristol stool scale after prebiotics in the current study). The latter opens the possibility that changes in, for example, gut motility (specifically anticipatory contractions on seeing food stimuli in the scanner) may underlie the observed changes in brain responses.
Body fat and lipid markers slightly improved after placebo condition and worsened after prebiotics in the current study. While we did not observe changes in lifestyle habits according to questionnaires, beneficial effects of for example increased energy expenditure in the placebo phase cannot be ruled out. Also, inulin, particularly at high doses, might challenge liver cholesterol metabolism, as postulated in mice under certain conditions.50 A recent human study further reported spikes in liver enzymes, cytokines and cholesterol in some participants after 30 g/day inulin,51 underlining the possibility that the dosage of inulin in the current study might have exceeded optimal levels. For serum SCFA, others did find short-term increases in SCFA after inulin,52 and the postprandial increase in SCFA correlated with decreases in serum ghrelin.53 Prebiotics and SCFA also stimulate the expression of PYY and GLP-1 in the gut,54 that may contribute to changes in central reward-related food responses. In humans, PYY injections induced changes in BOLD-related fMRI signalling in the hypothalamus, VTA and OFC.55 56 We found that decreases in fasting PYY correlated with decreases in brain activation in the VTA and OFC clusters after intervention, pointing to a similar mechanisms. However, a (postprandial) increase in serum SCFA or gut hormones due to prebiotics in our sample might have been masked after overnight fasting.
Limitations
Our study should be discussed in light of several limitations. First, 14 days of intervention can be considered too short to induce long-lasting effects on neuronal processes involved in eating behaviour. Also, secondary analyses did not replicate the exact same activation clusters at the whole brain level or when further constraining fMRI analyses to very small peak areas of the reward network. By following recommendations to fully preregister the applied brain mask and statistical thresholding in addition to further preprocessing steps, we, however, aimed to ensure confidence in the robustness of the observed effects. Exploratory analyses need to be interpreted with caution due to their non-confirmative nature. In addition, KEGG analyses need to be considered indirect only and microbiome samples were not time-locked to MRI sessions. Due to the within-subject cross-over design, however, interindividual differences at baseline determining microbiota responses could be kept to a minimum. Also, participants belonged to a Western, Educated, Industrialised, Rich and Democratic society and we did not recruit representative shares of female and diverse gender, limiting generalisability of results difficult.
Conclusions
According to preregistered RCT analysis of advanced 3T-fMRI, this proof-of-concept study suggests that a high-dosed microbiome-changing prebiotic intervention decreases brain responses to high-caloric food cues during decision-making within 2 weeks in overweight adults. Based on 16S-rRNA combined with functional pathway prediction and metabolomics, exploratory findings offer the possibility of a mechanistic link between prebiotic dietary intake, related changes in SCFA production, gut motility or PYY and reduced reward-related brain activation during food-decision making. While the current data does not allow us to conclude that the prebiotic treatment-induced changes in brain responses were beneficial for behavioural control, neural response in reward-related areas during fMRI have previously shown to predict behaviour change,57 underlining implications for the treatment of unhealthy eating behaviours or overnutrition using microbiome-changing interventions. Future studies are needed to explore whether such treatments could open avenues for less invasive approaches to obesity.
Acknowledgments
We thank all the individuals who took part in the study. For participant support, we thank Maria Dreyer, Ramona Menger, Bettina Johst and Susan Prejawa and for technical support at the MRI we thank all MTAs and specifically Nicole Pampus, Sylvie Neubert, Mandy Jochemko, Anke Kummer and Domenica Klank, and Torsten Schlumm. For fMRI analysis support we thank Hannah Sophie Heinrichs. For lab support we thank Laura Hesse, Charlotte Wiegank, Lina Eisenberg, Emmy Töws and Anna-Luise Wehle. For all other data collection, we highly appreciate the support of all our interns and student assistants Leonie Disch, Lukas Recker, Emira Shehabi, Niklas Hlubek, Lynn Mosesku, Larissa de Biasi, Hannah Stock, Lennard Schneidewind, Christian Schneider and Anne-Kathrin Brecht. We thank Lorenz Lemcke and Anna Bujanow for medical assistance. For SCFA analysis, we thank Beatrice Engelmann and for blood analysis we thank Nicole Krebs and Anja Willenberg. For help with formatting and project coordination, we thank Silke Friedrich.
Footnotes
Twitter: @EvelynMedawar, @witte1veronica
Contributors: Study conceptualisation and design: EM, MvB, MS, AV and VW. Code for tasks: RT. Data collection: EM and RT. Behavioural and fMRI analysis: EM, FB and VW. Code review: RT, FB. Serum analysis: MR. Microbiome analysis: S-BH. Metabolomics analysis: URK. Network analysis: S-BH, EM, VW and RC. Manuscript draft and guarantor: EM and VW. Revision: FB, RT, RC, MvB, MS, EM and VW. All authors agreed on the content of the material.
Funding: This work was supported by grants of the German Research Foundation (DFG), contract grant number 209933838 CRC1052-03 A1 to VW and MS, and by the Berlin School of Mind and Brain (stipend for EM) and the German Foundation for Environment (stipend for EM). The inulin supplement was sponsored by the manufacturer BENEO, Mannheim, Germany.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data and code availability. Data are available at 10.17605/OSF.IO/FC2G4 and code is available at https://gitlab.gwdg.de/gut_brain_study/food-wanting/task-fmri-behavior-analysis and https://gitlab.gwdg.de/gut_brain_study/food-wanting/fmri-analysis. Statistical MRI maps are available at https://identifiers.org/neurovault.collection:14111.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The ethics board of the Medical Faculty of the University of Leipzig, Germany, raised no concerns regarding the study protocol (228/18-ek) and all participants provided written informed consent.
References
- 1. Springmann M, Wiebe K, Mason-D’Croz D, et al. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country-level detail. Lancet Planet Health 2018;2:e451–61. 10.1016/S2542-5196(18)30206-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medawar E, Huhn S, Villringer A, et al. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry 2019;9:226. 10.1038/s41398-019-0552-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen X, Maguire B, Brodaty H, et al. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimers Dis 2019;67:583–619. 10.3233/JAD-180468 [DOI] [PubMed] [Google Scholar]
- 4. Berding K, Carbia C, Cryan JF. Going with the grain: fiber, cognition, and the microbiota-gut-brain-axis. Exp Biol Med (Maywood) 2021;246:796–811. 10.1177/1535370221995785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu KB, Hsiao EY. Roles for the gut microbiota in regulating neuronal feeding circuits. J Clin Invest 2021;131:e143772. 10.1172/JCI143772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalile B, Van Oudenhove L, Vervliet B, et al. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 2019;16:461–78. 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 7. Hoyles L, Snelling T, Umlai U-K, et al. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome 2018;6:55. 10.1186/s40168-018-0439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anastasovska J, Arora T, Sanchez Canon GJ, et al. Fermentable carbohydrate alters hypothalamic neuronal activity and protects against the obesogenic environment. Obesity (Silver Spring) 2012;20:1016–23. 10.1038/oby.2012.6 [DOI] [PubMed] [Google Scholar]
- 9. Depommier C, Everard A, Druart C, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25:1096–103. 10.1038/s41591-019-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnoldussen IAC, Wiesmann M, Pelgrim CE, et al. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int J Obes (Lond) 2017;41:935–44. 10.1038/ijo.2017.52 [DOI] [PubMed] [Google Scholar]
- 11. Dalile B, Vervliet B, Bergonzelli G, et al. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacology 2020;45:2257–66. 10.1038/s41386-020-0732-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rinott E, Youngster I, Yaskolka Meir A, et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology 2021;160:158–73. 10.1053/j.gastro.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cani PD, Lecourt E, Dewulf EM, et al. Gut Microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009;90:1236–43. 10.3945/ajcn.2009.28095 [DOI] [PubMed] [Google Scholar]
- 14. Hume MP, Nicolucci AC, Reimer RA. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am J Clin Nutr 2017;105:790–9. 10.3945/ajcn.116.140947 [DOI] [PubMed] [Google Scholar]
- 15. Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr 2009;89:1751–9. 10.3945/ajcn.2009.27465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiel S, Gianfrancesco MA, Rodriguez J, et al. Link between gut microbiota and health outcomes in inulin -treated obese patients: lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr 2020;39:3618–28. 10.1016/j.clnu.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 17. Leyrolle Q, Cserjesi R, D G H Mulders M, et al. Prebiotic effect on mood in obese patients is determined by the initial gut microbiota composition: a randomized, controlled trial. Brain Behav Immun 2021;94:289–98. 10.1016/j.bbi.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 18. Medawar E, Haange S-B, Rolle-Kampczyk U, et al. Gut Microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behaviour. Nutrition [Preprint] 2020. 10.1101/2021.02.18.21251818 [DOI] [PMC free article] [PubMed]
- 19. Berthoud H-R, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 2017;152:1728–38. 10.1053/j.gastro.2016.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DiFeliceantonio AG, Coppin G, Rigoux L, et al. Supra-additive effects of combining fat and carbohydrate on food reward. Cell Metab 2018;28:33–44. 10.1016/j.cmet.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 21. Fletcher PC, Kenny PJ. Food addiction: a valid concept. Neuropsychopharmacology 2018;43:2506–13. 10.1038/s41386-018-0203-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beyer F, García-García I, Heinrich M, et al. Neuroanatomical correlates of food addiction symptoms and body mass index in the general population. Hum Brain Mapp 2019;40:2747–58. 10.1002/hbm.24557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng-Li D, Sørensen TA, Li Y, et al. Systematically lower structural brain connectivity in individuals with elevated food addiction symptoms. Appetite 2020;155. 10.1016/j.appet.2020.104850 [DOI] [PubMed] [Google Scholar]
- 24. Schulte EM, Yokum S, Jahn A, et al. Food cue reactivity in food addiction: a functional magnetic resonance imaging study. Physiol Behav 2019;208:112574. 10.1016/j.physbeh.2019.112574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta A, Osadchiy V, Mayer EA. Brain-gut-Microbiome interactions in obesity and food addiction. Nat Rev Gastroenterol Hepatol 2020;17:655–72. 10.1038/s41575-020-0341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 2019;16:111–6. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 2016;13:581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogata H, Goto S, Fujibuchi W, et al. Computation with the KEGG pathway database. Biosystems 1998;47:119–28. 10.1016/s0303-2647(98)00017-3 [DOI] [PubMed] [Google Scholar]
- 29. Medawar E, Thieleking R, Beyer F. Data from Gut-Brain study: Effects of prebiotic intervention on the food wanting in overweight adults. A double-blind cross-over randomized intervention study. osf.io, 2023. [Google Scholar]
- 30. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–401. 10.1053/j.gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018;9:486–96. 10.1080/19490976.2018.1460015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stover PJ, Field MS, Andermann ML, et al. Neurobiology of eating behavior, nutrition, and health. J Intern Med July 9, 2023. 10.1111/joim.13699 [DOI] [PubMed] [Google Scholar]
- 33. Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013;76:412–27. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nolan-Poupart S, Veldhuizen MG, Geha P, et al. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite 2013;60:168–74. 10.1016/j.appet.2012.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang DW, Fellows LK, Dagher A. Behavioral and neural valuation of foods is driven by implicit knowledge of caloric content. Psychol Sci 2014;25:2168–76. 10.1177/0956797614552081 [DOI] [PubMed] [Google Scholar]
- 36. Wang G-J, Volkow ND, Telang F, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 2004;21:1790–7. 10.1016/j.neuroimage.2003.11.026 [DOI] [PubMed] [Google Scholar]
- 37. Hollmann M, Hellrung L, Pleger B, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012;36:648–55. 10.1038/ijo.2011.125 [DOI] [PubMed] [Google Scholar]
- 38. Trevelline BK, Kohl KD. The gut microbiome influences host diet selection behavior. Proc Natl Acad Sci U S A 2022;119:e2117537119. 10.1073/pnas.2117537119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Wouters d’Oplinter A, Rastelli M, Van Hul M, et al. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021;13:1959242. 10.1080/19490976.2021.1959242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hiel S, Bindels LB, Pachikian BD, et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr 2019;109:1683–95. 10.1093/ajcn/nqz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013;62:1112–21. 10.1136/gutjnl-2012-303304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salazar N, Dewulf EM, Neyrinck AM, et al. Inulin-type fructans modulate intestinal bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr 2015;34:501–7. 10.1016/j.clnu.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 43. Hess AL, Benítez-Páez A, Blædel T, et al. The effect of inulin and resistant maltodextrin on weight loss during energy restriction: a randomised, placebo-controlled, double-blinded intervention. Eur J Nutr 2020;59:2507–24. 10.1007/s00394-019-02099-x [DOI] [PubMed] [Google Scholar]
- 44. Vandeputte D, Falony G, Vieira-Silva S, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017;66:1968–74. 10.1136/gutjnl-2016-313271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barrangou R, Altermann E, Hutkins R, et al. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by lactobacillus acidophilus. Proc Natl Acad Sci U S A 2003;100:8957–62. 10.1073/pnas.1332765100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Louis P, Young P, Holtrop G, et al. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-coa:acetate coa-transferase gene. Environ Microbiol 2010;12:304–14. 10.1111/j.1462-2920.2009.02066.x [DOI] [PubMed] [Google Scholar]
- 47. Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:277. 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baxter NT, Schmidt AW, Venkataraman A, et al. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019;10:e02566-18. 10.1128/mBio.02566-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Birkeland E, Gharagozlian S, Birkeland KI, et al. Prebiotic effect of inulin‑type fructans on faecal microbiota and short‑chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur J Nutr 2020;59:3325–38. 10.1007/s00394-020-02314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pauly MJ, Rohde JK, John C, et al. Inulin supplementation disturbs hepatic cholesterol and bile acid metabolism independent from housing temperature. Nutrients 2020;12:3200. 10.3390/nu12103200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lancaster SM, Lee-McMullen B, Abbott CW, et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 2022;30:848–62. 10.1016/j.chom.2022.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rahat-Rozenbloom S, Fernandes J, Cheng J, et al. The acute effects of inulin and resistant starch on postprandial serum short-chain fatty acids and second-meal glycemic response in lean and overweight humans. Eur J Clin Nutr 2017;71:227–33. 10.1038/ejcn.2016.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rahat-Rozenbloom S, Fernandes J, Cheng J, et al. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur J Clin Nutr 2017;71:953–8. 10.1038/ejcn.2016.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 2004;92:521–6. 10.1079/bjn20041225 [DOI] [PubMed] [Google Scholar]
- 55. Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007;450:106–9. 10.1038/nature06212 [DOI] [PubMed] [Google Scholar]
- 56. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3-36 And GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 2011;14:700–6. 10.1016/j.cmet.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giuliani NR, Merchant JS, Cosme D, et al. Neural predictors of eating behavior and dietary change. Ann N Y Acad Sci 2018;1428:208–20. 10.1111/nyas.13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2023-330365supp001.pdf (177.5KB, pdf)
gutjnl-2023-330365supp002.pdf (15.5MB, pdf)
gutjnl-2023-330365supp003.pdf (567.2KB, pdf)
gutjnl-2023-330365supp004.pdf (837.4KB, pdf)
gutjnl-2023-330365supp005.pdf (2.1MB, pdf)
gutjnl-2023-330365supp006.pdf (5.5MB, pdf)
Data Availability Statement
Data are available at https://doi.org/10.17605/OSF.IO/FC4 and code is available at https://gitlab.gwdg.de/gut_brain_study/food-wanting/task-fmri-behavior-analysis and https://gitlab.gwdg.de/gut_brain_study/food-wanting/fmri-analysis.29 Statistical MRI maps are available at https://identifiers.org/neurovault.collection:14111.
Data are available in a public, open access repository. Data and code availability. Data are available at 10.17605/OSF.IO/FC2G4 and code is available at https://gitlab.gwdg.de/gut_brain_study/food-wanting/task-fmri-behavior-analysis and https://gitlab.gwdg.de/gut_brain_study/food-wanting/fmri-analysis. Statistical MRI maps are available at https://identifiers.org/neurovault.collection:14111.