Abstract
The pursuit to discover the fundamental biology and mechanisms of aging within the context of the physical and social environment is critical to designing interventions to prevent and treat its complex phenotypes. Aging research is critically linked to understanding health disparities because these inequities shape minority aging, which may proceed on a different trajectory than the overall population. Health disparities are characteristically seen in commonly occurring age-associated diseases such as cardiovascular and cerebrovascular disease as well as diabetes mellitus and cancer. The early appearance and increased severity of age-associated disease among African American and low socioeconomic status (SES) individuals suggests that the factors contributing to the emergence of health disparities may also induce a phenotype of ‘premature aging’ or ‘accelerated aging’ or ‘weathering’. In marginalized and low SES populations with high rates of early onset age-associated disease the interaction of biologic, psychosocial, socioeconomic and environmental factors may result in a phenotype of accelerated aging biologically similar to premature aging syndromes with increased susceptibility to oxidative stress, premature accumulation of oxidative DNA damage, defects in DNA repair and higher levels of biomarkers of oxidative stress and inflammation. Health disparities, therefore, may be the end product of this complex interaction in populations at high risk. This review will examine the factors that drive both health disparities and the accelerated aging phenotype that ultimately contributes to premature mortality.
Keywords: Minority aging, Health disparities, African Americans, Hispanics, Inflammation, Age, Genetics, Epigenetics
1. Introduction
The purpose of this review is to discuss and review aging research in the context of minority health and health disparities. This issue is of increasing importance as we approach intersecting demographic shifts in the United States. There has been considerable attention paid to the aging of the U.S. population as 1 in 5 Americans will be 65 and older by 2030 (Vespa et al., 2020). The other demographic challenge super-imposed on the aging of the U.S. population is the majority-minority shift which demographers predict will occur by 2060 when non-Hispanic White individuals will no longer be the majority population (Colby and Ortman, 2014). The U.S. population overall will become more racially and ethnically diverse as the non-Hispanic White population contracts due to low birth rates and rising death dates among this group. Those who identify as themselves as ‘Two or More Races’ will be the fastest growing racial/ethnic group over the coming decades followed by the Asian population (Colby and Ortman, 2014). In 2060, 56.4% of the U.S. population will be members of a minority group (Hispanic Americans 28.6%, African Americans 13.0%, Asian Americans 9.1%, and those who identify themselves as Two or More Races 4.9%) (Colby and Ortman, 2014).
As the overall U.S. population ages and becomes more diverse, the segment of the population over age 65 years will also become more racially and ethnically diverse. By 2040, it is estimated that 34% of older adults will be members of a racial or ethnic minority group (2020b). While African American individuals comprised 9% of the older population in 2017, in 2060 this group is projected to be 13% of U.S. older adults (2019b). American Indian and Alaska Native individuals made up 0.5% of the over 65 years of age U.S. population in 2017; in 2060 the percentage will increase slightly to 0.7% (2019a). The Asian American population percentages are expected to double between 2017 and 2060 going from 4% of the older population to 8% (2019c). The largest percentage increase is predicted for older Hispanic Americans. In 2017 Hispanic American individuals comprised 8% of the U.S. older population; however, in 2060 they are predicted to comprise 21% of older U.S. adults (2019d).
These shifting demographics among the population overall and particularly among older Americans requires that the field indemnify efforts to understand aging in minority populations and the environmental and social factors that influence differential aging trajectories. Therefore, it is critical for researchers to consider their human aging research pursuits in the context of the social construct of race as well as ethnicity along with the social determinants of health (SDOH) which influence the course of aging, life expectancy and age-related chronic disease (Fig. 1). The SDOH are important root causes of health disparities and contribute to the accelerated aging phenotype. The SDOH which result from the political determinants of health (Dawes, 2020) may have their effects through biologic transduction pathways that include but are not limited to genomic factors including the epigenome and genetics, DNA damage and repair pathways, immune and inflammatory response pathways, as well as alterations in gene expression (Fig. 2).
Fig. 1.
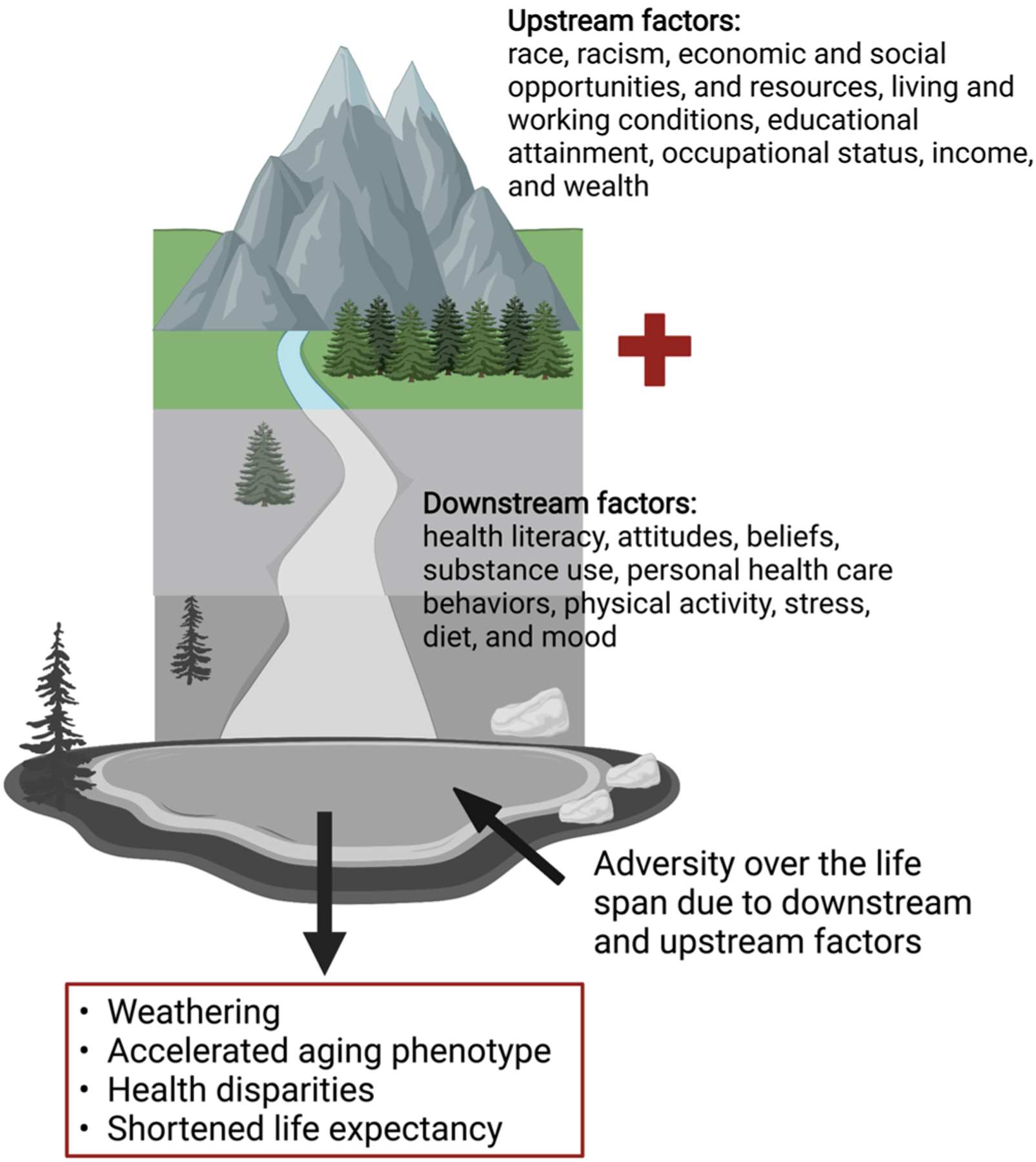
Upstream and downstream determinants of health influence outcomes over the lifespan.
Fig. 2.
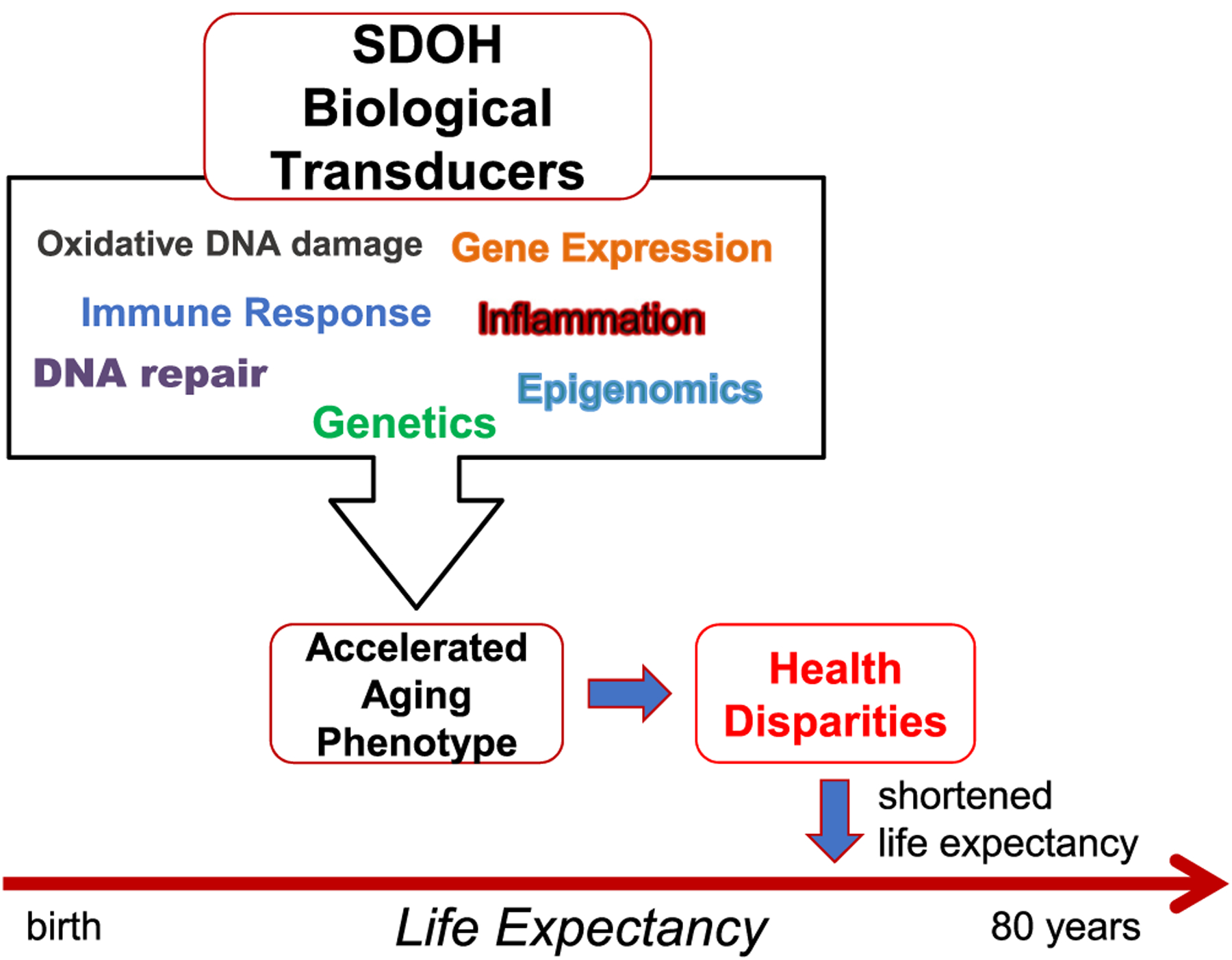
Social determinants of health biologic transducers that lead to the accelerated aging phenotype and health disparities in aging and age-related disease.
Incorporation of minority study participants, minority biological samples and minority investigators has been increasing but uneven historically. Minority scholars, particularly African American scholars, have played a critical role in highlighting the importance of studying minority aging. Beginning in the early 20th century, clinical research in Alzheimer’s disease was conducted by African American psychiatrist Solomon Carter Fuller (Brown et al., 2014). He and others recognized the need to examine and to understand the characteristics of aging that are shared but also to investigate characteristics that do not overlap and may be influenced by race as a surrogate for social and environmental circumstances. It was many years before literature reviews revealed the dearth of aging research in the African American population. Jacquelyne Johnson Jackson highlighted the need for further research on African American adults, challenging gerontologists to conduct aging research on African American adults with focus on psychosocial patterns of aging that might elucidate how race and social class factors may influence aging and the need to develop relevant methods and techniques for this research (Jackson, 1967, 1974). African American researchers have made fundamental contributions to building theoretical frameworks that are integral in understanding the multifaceted interplay of SDOH such as health care access and cultural competence in aging minority populations (Campinha-Bacote, 2002, 2009).
However, it was African American sociologist, Rose C. Gordon, who was among the first who examined both White and African American populations to examine the relationships between race, sex, and socioeconomic factors over the lifespan. She pursued and developed the concept of ‘double jeopardy’ leading to disparities in aging among older White and African American adults and advocated for intragroup causation studies (Gibson, 1988, 1994). Her work laid the foundation for several African American scholars whose work truly propelled the broadening of aging research to more thoroughly include minority populations and the special factors that are relevant in examining differences in the trajectory of aging. These scholars include Reginald L. Jones, who felt that life course theory should be applied to African American populations over the lifespan, had the foresight to bring together fellow African American academics in gerontology and other fields to co-author Black Adult Development and Aging (Jones, 1989). The coauthors, including Linda Chatters, James S. Jackson and Robert Joseph Taylor, went on to make important observations in minority aging over the life course. For example, James S. Jackson (2011) developed the Law of Small Effects in Race Related Outcomes. This predicted that no single factor produced disparities among racial and ethnic sub-populations. The disparities are due to a group of small differences accumulating over the life course that produce disparate health outcomes in adulthood and in older minority populations. To a large extent, Jackson’s theory has been proven correct since we know that a multifactorial and multilevel network operates to generate health disparities at all stages of life and is only one of his groundbreaking contributions to the field of aging. Jackson’s work facilitated the work of other minority aging scholars including Keith E. Whitfield and Jennifer J. Manly who began investigating cognitive aging among older African American adults (Manly and Espino, 2004; Whitfield et al., 2004).
This review highlights the important work which has addressed these issues and underscores the need for a multi-level approach because it is the accumulation of ‘small effects’, be they sociologic or biologic, that make the difference in promoting disparity.
2. Health disparities in the United States
Health disparities in the United States related to race, ethnicity, and socioeconomic status are widespread. The governmental definition of health disparity is “a particular type of health difference that is closely linked with social, economic, and/or environmental disadvantage. Health disparities adversely affect groups of people who have systematically experienced greater obstacles to health based on their racial or ethnic group; religion; socioeconomic status; gender; age; mental health; cognitive, sensory, or physical disability; sexual orientation or gender identity; geographic location; or other characteristics historically linked to discrimination or exclusion” (2010).
Health disparities are evident in numerous epidemiologic health statistics. Life expectancy at birth reveals persistent and, in some cases, expanding gaps related to race. This was highlighted in 2018 where a glaring life expectancy gap was reported between Hispanic women, non-Hispanic White women, African American women, non-Hispanic White men, and African American men who had the lowest life expectancy, 71.3 years compared to 79.1 years for Hispanic men who also outpaced non-Hispanic White men. In fact, the life expectancy for Hispanic men slightly exceeds that of African American women whose life expectancy is 78.0 (Arias and Xu, 2020). The differential health impact of COVID-19 has reduced life expectancy significantly for African American and Hispanic individuals. The recent National Center for Health Statistics report reveals a disproportionate decline in US life expectancy for the first half of 2020. Overall, African American individuals lost 2.7 years of life expectancy (72 years) and Hispanic individuals lost 1.9 years (79.9 years) (Arias et al., 2021).
For the African American population, this is the lowest life expectancy estimate reported since 2001. Survivorship is also significantly influenced by race. Before and after age 65, Hispanic individuals have a distinct survival advantage known as the “Hispanic paradox” (see “The Hispanic (Immigrant) Paradox” section for more details). More than 87% of Hispanic individuals survive to age 65 compared to 83% of non-Hispanic White individuals and 76% of non-Hispanic African American individuals. More than 52% of Hispanic individuals survive to age 85 compared to 42% of non-Hispanic White individuals, and only 33% of non-Hispanic African American individuals (Arias and Xu, 2020). It is important to note that U.S. mortality data reveals an African American-White mortality crossover among adults near age 85 with African American men and women surviving longer than their age matched White counterparts (Arias, 2006; Johnson, 2000). These findings may be due to poor quality data, population heterogeneity or cohort effects (Masters, 2012).
Age-adjusted death rates for 2019 also reflect significant disparities particularly for non-Hispanic African American men whose age-adjusted death rate is 1092/100,000 compared to 869/100,000 for non-Hispanic White men and 633/100,00 for Hispanic men (Kochanek et al., 2020). Unsurprisingly, the pandemic influenced the 2020 provisional mortality rates. The age-adjusted death rate increased by 15% with 11% of those increased deaths attributed to COVID-19. The racial disparities in overall mortality and COVID-19 related mortality are prominent with non-Hispanic African American individuals having the highest age-adjusted death followed by non-Hispanic American Indian individuals or Alaska Native American individuals (Ahmad et al., 2021). However, overall statistics reveal that American Indians/Alaska Natives and Hispanic American individuals have higher incidence, hospitalization, and mortality rates. As expected, adults ≥ 85 years had the highest mortality with the highest mortality mirroring the racial/ethnic disparities seen for the overall population.
There are also notable disparities in age-associated chronic disease incidence and prevalence. For the most commonly occurring age-associated chronic disease there are significant health disparities. American Indians/Alaska Native individuals have the highest prevalence of diabetes mellitus followed by Hispanic and African American individuals. The incidence of newly diagnosed diabetes among adults reveals the highest incidence rates for African American and Hispanic individuals (2020a). African American individuals are more likely to have hypertension while non-Hispanic White individuals were more likely to have cardiovascular disease. However African American individuals have the highest age-adjusted death rate for cardiovascular disease. Non-Hispanic African American individuals are more than twice as likely to die from cardiovascular disease when compared to Asian individuals and Pacific Islander individuals. In addition, African American and Hispanic individuals were more likely to be obese (BMI >30.0) than non-Hispanic White individuals (2021b). Racial and ethnic minority groups are also more likely to report the presence of multiple age-related disease co-morbidities including diabetes, cardiovascular disease, cerebrovascular disease, and hypertension (Ahmed and Conway, 2020).
Cancer racial/ethnic health disparities, though declining, are still stark. African American individuals bear a significantly disproportionate cancer burden with the highest death rates and shortest survival than other population groups (2021a). For example, although African American women have a lower incidence of breast cancer, their breast cancer mortality is higher than White women. African American men have both higher incidence and mortality from prostate cancer when compared to all U.S. population groups.
3. Social and political determinants of health and aging
Non-medical social, political and economic factors have both direct and indirect effects on health. The Institute on Medicine has identified 12 key SDOH risk factors from 5 domains (sociodemographic, psychological, behavioral, individual level social relationships and living conditions, and neighborhoods and communities) in the context of health outcomes including morbidity and mortality. The specific factors include: neighborhoods and communities compositional characteristics, social connections and social isolation, exposure to violence, dietary patterns, physical activity, tobacco use and exposure, alcohol abuse, health literacy stress, negative mood and affect (depression and anxiety), country of origin, education, employment and financial resources/strain (2015).
These non-medical factors influence health and can be divided into upstream and downstream factors. Downstream factors or determinants are those that are spatially or temporally close to the health outcome observed. These downstream factors include but are not limited to health literacy, attitudes, beliefs, substance use, personal health care behaviors, physical activity, stress, diet, and mood (Braveman et al., 2011) (Fig. 1). Although these downstream factors may be the most apparent and immediate, they are not the fundamental causes of health disparities over the lifespan. Downstream determinants are molded by upstream determinants of health which though indirect are fundamental causes of disparities because these are the initiating factors of multiple intricate causal pathways to result in differential health outcomes. The upstream determinants of health include social and political factors including race, racism, economic and social opportunities, and resources, living and working conditions, educational attainment, occupational status, income, and wealth (Fig. 1).
The upstream and downstream determinants are linked through a complex and interconnected network that interdigitates with other factors such as lifespan as well (Fig. 1). Upstream factors may be particularly important in aging because they are operational at every stage of life. Adversity experienced through living and working conditions, educational attainment, limited resources, racism, or any of the other upstream factors will likely have cumulative effects over the lifespan influencing health span, quality of life in old age, and longevity itself. These factors may be rapid acting exposures with immediate effects; however, the ultimate effects may not manifest until late in the lifespan and accelerate the premature development of age-associated chronic disease and disability (Braveman and Gottlieb, 2014). Geronimus proposed the ‘weathering hypothesis’ in 1992 as she examined disparities in maternal child health surmising that these disparities may be the result of the “physical consequences of cumulative socioeconomic disadvantage” (Geronimus, 1992). Lifelong stress induced by the need to cope with or overcome the adversity and marginalization triggered by race and various forms of racism is a particularly important factor throughout the lifespan for racial and ethnic minorities that also ultimately influences their health status and outcomes.
These fundamental upstream pathways are intricately emmeshed with biological transduction pathways through which these determinants result in differential health outcomes (Seeman and Crimmins, 2001). Chronic exposure to environmental stressors viewed in the broadest context can alter physiologic function as well as biologic systems and processes. The term ‘allostatic load’ refers to the accumulative weight of persistent stress and life experiences (Gehlert et al., 2008; McEwen and Gianaros, 2010; McEwen and Seeman, 1999; Miller et al., 2009; Seeman et al., 2010). The ‘allostatic load’ construct encompasses many bio-physiologic processes and regulatory systems and is assessed through selected biomarkers and clinical criteria. Allostatic load is an important factor in age-related health disparities (Geronimus et al., 2006). It is associated with frailty as well as cognitive function, brain structure, mortality, physical function and personality characteristics [For review (Guidi et al., 2021)]. This ‘load’ resulting from aspects of the SDOH is dynamic across the lifespan. The SDOH exert their effects in a stepwise gradient through which health status is linked to incremental improvement in social position and socioeconomic status up to a point. The socioeconomic gradients are particularly potent factors in aging. For example, functional limitations and disability among those 65–74 years of age are significantly influenced by socioeconomic gradients (Minkler et al., 2006). Not only are the gradients of socioeconomic status important in aging but also the cumulative risk associated with a wide range of SDOH. A cumulative risk score using data from the National Health and Nutrition Examination Survey (NHANES) comprised of SDOH, self-rated health, and functional capacity found that not only did SDOH-related Black-White disparities persist throughout the lifespan but also that older African American individuals have a higher mean cumulative risk score than White individuals in comparable age groups (Rhee et al., 2021). This study also demonstrated that SDOH Index scores were higher among older African American and White adults with functional limitations. In the Midlife Development in the United States (MIDUS) study, African American participants reported a higher level of exposure to stressors including childhood, work, financial stress as well as perceived discrimination compared to White participants (Chen et al., 2021). This increase in cumulative stress was associated with lower levels of executive function and episodic memory in African American participants compared to White participants. As much as 8% of the disparity in executive function and over 13% of the disparity in episodic memory was accounted for by cumulative stress exposure. This finding is in line with multiple other studies that have demonstrated higher levels of cumulative stress among African American individuals including the Health and Retirement study, the Chicago Community Adult Health Study and the Women’s Health Study (Burroughs Pena et al., 2019; Sternthal et al., 2011; Williams and Mohammed, 2009). However, improvement in income and social status do not completely ameliorate disparate health outcomes among African American individuals and other minorities (Institute of Medicine, 2000).
Among the upstream determinants of health are politically driven social and economic policies and actions that directly influence longevity and survival particularly among marginalized population segments (Dawes, 2020). Since the Nation has failed to guarantee equal protection under law, it has simultaneously failed to provide for the general welfare and good health of the entire population. This failure is particularly perilous over the lifespan as the accumulated stress, deprivation, and marginalization exact a toll on health span, possibly promotes the development of the accelerated aging phenotype, and contributes to truncation of lifespan.
3.1. Race as a social determinant of health
There is much debate about the role of race and ethnicity in health. Many believe that it is not possible to define a single concept of race or ethnicity. Most studies use race as a psychosocial construct to contextualize life experience since race is a powerful social category that through history has been used by societies to shape human experience. Race conveys power and privilege as well as oppression and disenfran-chisement. The social determinism of race is present at every stage of life accompanying the processes of maturation as well as aging. From the perspective of social scientists, race may be considered a “…subjective social construct based on ascribed characteristics that have acquired socially significant meaning…” (2004). Although the social consequences of race working through interdigitating biological pathways likely influences health outcomes there is no objective scientific or genetic data to support a genetic or biologic basis of race. Although early generations of biologists and naturalists generated biologic classifications of race based on observable differences including skin color, physiognomy, and physique from the genetic perspective there is no proven genetic basis of race [for review (Muller-Wille, 2014)]. What the genome does reveal to us is genetic variation or clustering based on geographic origin (Keita et al., 2004). We all have a shared genetic heritage revealed by global sequencing data that supports the hypothesis that humans likely originated in Africa. The documented genetic variations are likely evidence of descent with variation (Cooper and Rotimi, 2020). The value of studying genetic variation in the context of biology and clinical medicine is that the genetic variation noted may provide information on human disease risk and resiliency as well as avenues for potential treatment. Race as a social construct may further contextualize the interaction between the environment and the genome. Race is a risk factor for disparate health outcomes that must be considered with SDOH including social position, income, access to necessary resources (health care, transportation, employment), education, place of residence and contextualized with ancestry and genomic variation (Morning, 2011).
3.2. The Hispanic (Immigrant) paradox
Hispanic individuals have greater lifespans and reduced mortality rates compared to White and African American individuals despite having similar socioeconomic conditions as African American individuals (Markides and Coreil, 1986; Markides and Rote, 2019). These observations were first described in 1986 as the “Hispanic paradox” (Markides and Coreil, 1986) and has been extended to include observations that Hispanic individuals have similar health profiles as White individuals (Garcia et al., 2018; Markides and Rote, 2019), though this aspect has been debated (Boen and Hummer, 2019; Crimmins et al., 2007; Espinoza et al., 2013; Tarraf et al., 2020). The Hispanic paradox is even more apparent when examining health status differences in native versus foreign-born Hispanic individuals, where Hispanic immigrants have relatively better health outcomes and lower mortality rates compared to U.S. native-born Hispanic individuals (Boen and Hummer, 2019; Markides and Rote, 2019) and White individuals (Lariscy et al., 2015). For example, foreign-born Hispanic men and women 65 years of age and older had significantly reduced mortality rates compared to White men and women, while native-born Hispanic individuals in the same age range had grossly similar mortality rates as White individuals (Lariscy et al., 2015). This same study also revealed that foreign-born Hispanic individuals have reduced cause-specific mortality rates such as heart disease, cancer, and respiratory diseases compared to White individuals, while native-born Hispanic individuals only had reduced mortality rates for lung cancer and respiratory diseases relative to White individuals (Lariscy et al., 2015).
Potential contributing factors to the Hispanic paradox include migration selection, diet and other health behaviors, sociocultural factors, differential stress exposure, genetics, and ethnicity misclassification on death certificates (Boen and Hummer, 2019; Markides and Rote, 2019). Age at migration has also been found to be correlated with the Hispanic paradox (Gubernskaya, 2015), where immigrants migrating to the U.S. after 24 years old confer an immigrant mortality advantage (Markides and Rote, 2019). However, one study found an immigrant health advantage was only present in foreign-born Hispanic individuals that migrated to the U.S. between the ages of 18 and 34 years old (Gubernskaya, 2015). Interestingly, rates of decline in self-reported health at 50 years of age were significantly different based on the age of migration, but steeply decreased self-reported health rates were observed in all Hispanic immigrants after the age of 50 regardless of their age at migration (Gubernskaya, 2015).
Since its initial description, current studies seem to support the Hispanic paradox (Fenelon et al., 2017; Garcia et al., 2018; Lariscy et al., 2015), yet other studies found conflicting results (Boen and Hummer, 2019; Crimmins et al., 2007; Espinoza et al., 2013; Tarraf et al., 2020). An early study revealed both native and foreign-born Hispanic individuals had greater biological risk scores and factors compared to White individuals, and no significant differences were observed between native and foreign-born Hispanic individuals (Crimmins et al., 2007). In this study, biological risk scores were defined as a combination of 3 different clinical measurements: 1) blood pressure (pulse rate and systolic and diastolic blood pressure), 2) metabolic function (body mass index, total and high-density lipoprotein cholesterol, and glycated hemoglobin), and 3) inflammation (fibrinogen, C-reactive protein, and albumin) (Crimmins et al., 2007). The San Antonio Longitudinal Study of Aging (SALSA) detected higher mortality rates in older Mexican American adults compared to White adults after adjusting for sex and age, but was no longer significant when adjusted for socioeconomic status (Espinoza et al., 2013). A recent study found that the Hispanic paradox does not encompass health risk markers in native and foreign-born Hispanic individuals relative to White individuals and concluded there was no apparent “healthy immigrant effect” (Boen and Hummer, 2019). For example, the authors found that both foreign-born and native Hispanic individuals had greater functional limitations than White individuals, but foreign-born Hispanic individuals had greater C-reactive protein (CRP) concentrations compared to White individuals while no significant differences were observed between native Hispanic individuals and White individuals (Boen and Hummer, 2019). Interestingly, this study also observed disparities associated with functional limitations decreased with age in foreign-born Hispanic individuals versus White individuals, while the disparities between native-born Hispanic individuals and White individuals was consistent (Boen and Hummer, 2019). Another recent study found that both native and foreign-born Hispanic individuals were just as likely to be categorized as “healthy” compared to White individuals only when health habits and adult achievements variables were adjusted (Tarraf et al., 2020). Yet this same study also discovered that both native and foreign-born Hispanic individuals also had a similar or increased likelihood of having high morbidity and cognitive decline with aging compared to White individuals, suggesting that nativity may not influence health status (Tarraf et al., 2020).
While most studies on the Hispanic paradox have focused on either combined Hispanic populations or Mexican American subpopulations, recent studies have examined additional Hispanic subpopulations such as Puerto Rican and Cuban individuals (Fenelon et al., 2017; Garcia and Ailshire, 2019; Garcia et al., 2018). One study examined mortality rates associated with foreign-born and U.S. native-born Hispanic individuals broken down by 6 countries/regions of origin (Mexican, Puerto Rican, Cuban, Dominican, Central/South American, and other Hispanic) (Fenelon et al., 2017). When adjusted for socioeconomic covariates, all foreign-born Hispanic subgroups had a mortality advantage at ages 65 years and older compared to White individuals, while the only native-born mortality advantages were observed for native Mexican, Cuban, and other Hispanic men aged 65 years and older relative to White men (Fenelon et al., 2017). Garcia and colleagues reported that foreign-born Mexican, foreign-born Cuban, and island-born Puerto Rican women had greater life expectancies compared to White women, while native-born Puerto Rican and foreign-born Mexican men had greater life expectancies compared to White men (Garcia et al., 2018). Island-born Puerto Rican women had the most years living with morbidity compared to White women and the other Hispanic subgroups, while U.S. native-born Puerto Rican men had the greatest years living with morbidity compared to White men and the other Hispanic subgroups (Garcia et al., 2018). Foreign-born Cuban men and women spent the most years of life without morbidity compared to White individuals and the remaining Hispanic subgroups (Garcia et al., 2018). A recent study investigating biological risk profiles demonstrated that foreign-born Mexican, island-born Puerto Rican, foreign-born “other” Hispanic, and U.S. native-born Mexican individuals demonstrated greater biological risk compared to White individuals (Garcia and Ailshire, 2019). The authors also used the same parameters as the Crimmins et al. (2007) study to define biological risk factors, with the following modifications: 1) the metabolic function component also included cystatin C measurements, and 2) the inflammatory component only included C-reactive protein measurements (Garcia and Ailshire, 2019). After adjusting for socioeconomic status, health behaviors, and access to care, native-born Mexican individuals had significantly greater metabolic risk while island-born Puerto Rican individuals had significantly increased risk of inflammation relative to White individuals (Garcia and Ailshire, 2019).
Several questions surrounding the Hispanic paradox still remain. Current literature has largely focused on lifespan and mortality rather than health span (Boen and Hummer, 2019). While few studies have examined morbidity associated with life expectancies (Garcia et al., 2018), more studies are needed to confirm these results and expand to other Hispanic subpopulations residing in various geographical regions within the U.S. As socioeconomic status appears to explain part of the Hispanic paradox (Espinoza et al., 2013; Fenelon et al., 2017; Garcia and Ailshire, 2019; Lariscy et al., 2015), the behavioral, environmental, and psychosocial exposures contributing to the Hispanic paradox also require further investigation. Additionally, longitudinal studies on how the Hispanic paradox evolves over time are limited (Boen and Hummer, 2019; Tarraf et al., 2020). Furthermore, more studies need to examine the Hispanic paradox in additional Hispanic nationalities, as current studies have only examined, Mexican American, Puerto Rican, and Cuban American populations.
4. Genetics and other biomarkers of aging in minority populations
4.1. Genetics of aging in minority populations
Many studies have examined the genetic contributions to human aging and longevity, which have been reviewed elsewhere (Melzer et al., 2020; Morris et al., 2019). Most recent heritability estimates of longevity range from < 10–16% (Melzer et al., 2020; Morris et al., 2019), and previous genome-wide association studies (GWAS) found that genetic variants have small to moderate effects on longevity, parental lifespan, and health span (Melzer et al., 2020; Morris et al., 2019). However, the majority of these genetic studies have focused on predominantly aging European ancestry populations (Melzer et al., 2020; Morris et al., 2019). Given the expected rise of aging non-European populations within the next few decades, understanding the genetic components of aging in racial and ethnic minority populations will be crucial. In this section, we will summarize current genetic studies of longevity, health span, and lifespan in racial and ethnic minorities.
Lee and colleagues estimated heritability of lifespan and survival in African American, Caribbean Hispanic, and White adults aged 65 years and older from the Washington Heights-Inwood Columbia Aging Project (WHICAP) (Lee et al., 2004). When adjusted for sex and birth cohort, heritability of lifespan for deceased relatives was statistically significant in Caribbean Hispanic adults (h2 = 0.29) and White adults (h2 = 0.26), while heritability estimates in African American adults were low and not significant (h2 = 0.035) (Lee et al., 2004). As heritability estimates cannot identify specific genetic variants or affected biological pathways, genotyping, GWAS and other large-scale genomics platforms have been utilized to identify novel genomic variants associated with aging. An early genotyping study in 2003 examined the relationship between the human homolog of the Drosophila Indy lifespan gene and human longevity (Lee et al., 2003). The Indy V80L variant had a low frequency and was only present in African American individuals (Lee et al., 2003). Another study evaluated the relationship between CRP genotype and the number of years and healthy years lived as well as all-cause mortality in White and African American participants in the Cardiovascular Health Study (CHS) cohort (Hindorff et al., 2008). They found that CRP haplotypes were not associated with years of life, years of healthy life, or all-cause mortality in African American or White participants (Hindorff et al., 2008). However, more copies of the CRP HapD haplotype were correlated with reduced risk of all-cause mortality in African American participants (Hindorff et al., 2008).
The first genetic association study of aging in racial and ethnic minority populations was based on the Women’s Health Initiative (WHI) cohort, which took a candidate gene approach to evaluate variants associated with healthy aging and survival in postmenopausal African American, Hispanic, and White women (Shadyab et al., 2017). Fourteen single nucleotide polymorphisms (SNPs) from previous GWAS studies of longevity in primarily European populations were specifically evaluated to determine if these SNPs were also associated with longevity in postmenopausal women (Shadyab et al., 2017). None of the selected SNPs were significantly associated with longevity in African American or Hispanic women in fully adjusted models, but when models were adjusted only for population stratification and age, 7 SNPs were significantly associated with survival to 85 years of age in Hispanic women (Shadyab et al., 2017). These SNPs were in linkage disequilibrium with the rs2149954 variant, which was previously associated with longevity in a separate study of European ancestry. This SNP had the opposite direction of effect on both longevity and heart disease risk in women of European ancestry (T allele associated with increased longevity and lower cardiovascular mortality risk) and Hispanic women (T allele associated with decreased longevity and higher coronary heart disease risk) (Shadyab et al., 2017).
GWAS studies have provided unique advantages and greater power compared to earlier candidate gene approaches, such as having larger sample sizes and multi-cohort designs. GWAS examining aging and longevity have included African American and Hispanic individuals as part of a trans-ethnic meta-analysis as well as individual cohorts (Deelen et al., 2019; Joshi et al., 2017; Tanaka et al., 2017; Wright et al., 2019). In a trans-ethnic meta-analysis study examining longevity variants in European, East Asian, and African American populations from 20 cohorts, APOE ε4 rs429358 and APOE ε2 rs7412 variants were associated with reduced and increased survival odds, respectively, to the 90th and 99th age percentile in each individual study cohort including African American individuals from CHS (Deelen et al., 2019). APOE serves an important role in cholesterol metabolism and transport in the brain (Deelen et al., 2019). The APOE ε4 rs429358 variant has been previously correlated with higher risk of Alzheimer’s disease and cardiovascular disease, while the APOE ε2 rs7412 variant has the opposite effect (Deelen et al., 2019).
A GWAS examining parental lifespan in European and African ancestry populations from UK Biobank and LifeGen found 1 significant variant, rs10198124, located within the intergenic region of chromosome 2, that was associated with paternal lifespan in African American individuals (hazard ratio = 1.22) (Joshi et al., 2017). The function of the rs10198124 variant remains unknown. No significant SNPs were associated with combined parental lifespans or maternal lifespans in African American individuals (Joshi et al., 2017). Another parental lifespan GWAS study was performed in European and African ancestry participants from the Health and Retirement Study (HRS), where significant variants were only identified from the trans-ethnic meta-analysis and not ancestry-specific analyses (Tanaka et al., 2017). The authors found the rs35715456 variant within the SMAD7 gene had the greatest significant association with having a long-lived parent but could not replicate this finding in the Framingham or InCHIANTI studies (Tanaka et al., 2017). SMAD7 is involved in TGFβ signaling inhibition, and SMAD7 expression was previously found to be increased with chronological age in a large European cohort (Tanaka et al., 2017).
Finally, a GWAS from the AncestryDNA database examined the relationship between genetic ancestry, rather than race and ethnicity, and parental lifespan, but only found significant correlations in European ancestry groups (Wright et al., 2019). Of note, while not significant after correcting for multiple testing, the Native American ancestral group had a negative correlation between genetic ancestral admixture and maternal lifespan (Wright et al., 2019). No significant associations between parental lifespan and genetic variants were identified in Sub-Saharan African or Native American/ East Asian ancestry populations (Wright et al., 2019). The authors attributed these findings to the smaller sample sizes in these respective populations, and thus were underpowered (Wright et al., 2019).
In the above described GWAS studies, the African American and Hispanic populations were largely underpowered and/or had small to modest sample sizes, which could explain the lack of identified significant variants associated with longevity and lifespan. This is in line with other published GWAS results of complex traits and diseases in racial and ethnic populations (Daya et al., 2019; Kang et al., 2010; Ke et al., 2018; Salinas et al., 2016). Additionally, we cannot exclude the possibility that genetic variants associated with longevity, health span, and/or lifespan are shared across multiple populations, but the allele frequencies may differ across the populations. For example, Lee and colleagues found the I550V variant in the Indy gene was shared between non-Hispanic White, Hispanic, Asian, and African American populations at similar gene frequency levels (Lee et al., 2003). While not statistically significant, differences in CRP haplotype frequencies were observed between White and African American participants in the CHS cohort (Hindorff et al., 2008). To the best of our knowledge, no other genetic studies of aging have examined allelic or genotypic frequency differences between geographic populations. More genetic studies including diverse, racial and ethnic populations will be required to identify allele and/or genotype frequencies associated with healthy aging that could be enriched in certain populations. The limited number of currently identified genetic variants also suggests environmental and psychosocial factors or gene-gene interactions may play a larger role in aging for diverse populations. Furthermore, these collective results suggest that genetic variants and predictors identified from European ancestral populations cannot be extrapolated to non-European populations (Geoffroy et al., 2020; Mogil et al., 2018; Wojcik et al., 2019).
African ancestry populations have the highest levels of genomic diversity (reviewed in (Campbell and Tishkoff, 2008)), while Hispanic populations have varying degrees of genomic admixture (Banda et al., 2015; Bryc et al., 2010b; Conomos et al., 2016; Price et al., 2007), adding another level of complexity into understanding the genetic contributions to aging in Hispanic populations. Hispanic and Latino populations have varying proportions of European, African, and Native American ancestries, which differ between and within Latin American populations (Bryc et al., 2010b; Conomos et al., 2016; Price et al., 2007). For example, Mexican individuals tend to have higher levels of Native American ancestry, Cuban individuals have higher European ancestry, and Puerto Rican and Dominican populations have higher African ancestry (Banda et al., 2015; Bryc et al., 2010b; Conomos et al., 2016; Price et al., 2007). African American individuals also have varying proportions of genetic admixture, composed primarily of African and European ancestry with low levels of Native American ancestry (Bryc et al., 2010a; Tishkoff et al., 2009), and ancestry also varies by geographical region (Baharian et al., 2016).
Interestingly, studies have also shown associations between genetic ancestry levels and phenotypic markers and diseases. For example, an early study found that African genetic ancestry, in addition to other sociodemographic variables, was correlated with years of life and fasting blood glucose levels in African American participants from the CHS cohort (Reiner et al., 2005). The WHI examined whether ancestry-specific genetic variants were correlated with the aging associated biomarker CRP levels in postmenopausal African American and Hispanic women (Reiner et al., 2012). When CRP levels were adjusted for age, the authors found a negative correlation between European ancestry and CRP in African American and Hispanic women, and a positive correlation between Native American ancestry and CRP in Hispanic women (Reiner et al., 2012). A genome-wide admixture scan in African American women identified multiple significant variants in loci such as 1q23 and 6p21 that were specific to African ancestral populations, where local African ancestry was associated with higher CRP concentrations (Reiner et al., 2012). Despite current evidence demonstrating genetic heterogeneity both within and between Hispanic and African American populations, an overwhelming number of studies group these respective populations into a single, generalized “Hispanic” or “African American” group. Thus, it is possible that key genetic variants and biological processes associated with aging are not captured due to these generalizations and population stratification confounding effects. Future studies should investigate genetic variants associated with aging in Hispanic populations by country of origin rather than as a single combined ethnic group.
While race and ethnicity have often been connected to genetic ancestry (Borrell et al., 2021), it is important to emphasize that race is a social construct, not a biological construct. In contrast, genetic ancestry relies on “the genetic origin of one’s population” and is more precise in predicting the presence of genetic variants in specific populations compared to race and ethnicity categories (Borrell et al., 2021). However, genetic ancestry only partially explains biological variation observed in diverse populations (Borrell et al., 2021). Furthermore, while some genetic variants have been linked to racial and ethnic differences in various diseases and conditions, these variants may not necessarily be directly causal of health disparities (Borrell et al., 2021). These observations could be due to various factors such as unknown non-genetic contributors, and/or the lack of diverse populations included in genetic studies (Borrell et al., 2021). Regardless of whether genetic variants directly influence health or disease outcomes, studying genetic variation in racial and ethnic minority populations will still be crucial to understand the relationship between variant effect sizes, allelic and genotypic frequencies, and health and disease risk across diverse populations (Borrell et al., 2021). As previously stated, genetic studies have overwhelmingly focused on White or European populations, and there is still much to be learned about genetic variation in racial and ethnic minority populations. Racial and ethnic minorities have not been included in many genotyping, transcriptomic, proteomic, and epigenetic studies resulting in a nonrepresentative cohort -omic data set. Until more racial and ethnic minority populations have more genetic and other “-omic” sequencing data collected, caution should be used when interpreting GWAS and other genetic association results as they relate to causality of health and disease outcomes in non-White populations (Borrell et al., 2021).
To the best of our knowledge, no GWAS studies have been performed in aging Native American or American Indian populations. Furthermore, no genetic studies have been performed using whole exome or whole genome sequencing (WGS) technologies in any aging racial and ethnic minority population. WGS captures more genetic variation compared to traditional GWAS and could serve as a powerful tool to identify low frequency and rare variants in diverse ancestral populations. Given that knowledge on rare variants in racial and ethnic minority populations remain significantly understudied, whether rare variants could explain longevity differences in diverse minority populations requires additional investigation. Future studies should utilize WGS to investigate the interplay between genetic variants, genetic ancestry, and aging aspects such as longevity, lifespan, and health span in aging ethnic and racial minority populations. The ultimate goal would be to incorporate these genetic findings into precision medicine applications at the individual level. By gaining a more thorough understanding of genetic variation at the population level, we can begin to realize the full potential of precision medicine for aging individuals from all racial and ethnic populations.
4.2. Other molecular biomarkers in minority populations
Criteria for biomarkers of aging have been defined by the American Federation for Aging Research as follows: 1) can model the rate of aging, 2) represent a core biological process of healthy aging, 3) can be frequently tested in humans without harm, and 4) can be identified and manipulated in humans and animal models (Xia et al., 2017). Our current understanding of potential biomarkers of aging come from the traditional hallmarks of aging, which include epigenetic changes, genomic instability, telomere shortening or attrition, dysregulated nutrient sensing, cellular senescence, loss of proteostasis, mitochondrial dysfunction, stem cell exhaustion, and altered intercellular communication (reviewed in (Lopez-Otin et al., 2013)). To our knowledge, biomarkers associated with the latter 4 hallmarks of aging have not been examined in healthy, normally aging racial and ethnic populations. As epigenetics and genomic instability concepts will be covered in later sections of this review, we highlight current knowledge of telomere shortening, nutrient sensing and other metabolic processes, cellular senescence, and other aging associated biomarkers associated with healthy aging in racial and ethnic minority populations.
A recent study explored the correlation and co-dependency between 50 clinical biomarkers with age, race and sex using the National Health and Nutrition Examination Survey (NHANES) data set (Le Goallec and Patel, 2019). Differences in biomarker correlations were identified by ethnicity, with 58 correlation differences in White vs Hispanic individuals and 113 correlation differences in White vs African American individuals (Le Goallec and Patel, 2019). The authors also found few alterations in correlations of age trajectories between the ethnic groups, with only 3 correlation differences observed between White and Hispanic individuals, and 8 correlation differences between White and African American individuals (Le Goallec and Patel, 2019). When evaluating the predictability of biomarkers with age, 3 biomarkers had greater predictability in White participants compared to Hispanic participants, while 9 biomarkers had a higher prediction value in Hispanic participants (Le Goallec and Patel, 2019). Six biomarkers had improved prediction in White participants compared to African American participants, while 10 biomarkers had improved prediction in African American participants (Le Goallec and Patel, 2019). When examining the predictability trajectories of biomarkers with age by ethnicity, only 3 differences were found between White and Hispanic individuals and 1 difference between White and African American individuals (Le Goallec and Patel, 2019). The authors have provided an interactive website for readers to further evaluate biomarker correlations and predictabilities at http://apps.chiragjpgroup.org/Aging_Biomarkers_Co-Dependencies/ (Le Goallec and Patel, 2019). However, the authors noted that the biomarkers used in this study were not considered to be commonly utilized chronological age biomarkers like DNA methylation clocks, telomere length and inflammatory markers, but rather a collection of blood biomarkers (such as glucose, blood cell counts, and lipid levels) and anthropometric values (such as body mass index, height, and blood pressure) (Le Goallec and Patel, 2019).
4.3. Telomere shortening and attrition
Telomeres, especially telomere length, are considered an important biomarker of aging. Previous studies found heritability estimates of telomere length range between 34% and 82% (Melzer et al., 2020). Differences in telomere length and shortening by race and ethnicity, sex, and age have been documented with conflicting results (Brown et al., 2017; Diez Roux et al., 2009; Fitzpatrick et al., 2011; Needham et al., 2019; Song et al., 2018). In the CHS cohort, average telomere length was significantly longer in African American adults aged 65 years and older (Fitzpatrick et al., 2011). Investigators using data from the HRS study examined telomere length and interactions between age and race, and found African American women had larger decreases in telomere length as age increased compared to White women (Brown et al., 2017). No significant differences in telomere length and aging were observed in Hispanic women relative to White women (Brown et al., 2017). Interestingly, Hispanic men had a significantly steep reduction in telomere length with increasing age, while no significant differences were observed between African American and White men (Brown et al., 2017).
The associations with telomere length and race/ethnicity and age were investigated in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort (Diez Roux et al., 2009). When adjusted for the sociodemographic covariates age, sex, physical activity, body mass index, diet, smoking, income, and education, Hispanics and African American participants had marginally but significantly shorter telomeres than White participants (Diez Roux et al., 2009). Additionally, there were robust associations with age and telomere attrition for both sexes in African American and Hispanic participants compared to White participants, however the racial and ethnic differences were significantly higher in women (Diez Roux et al., 2009). A follow-up study from MESA assessed telomere length changes over a 10-year period and whether sex, age, and race and ethnicity could be used as predictors of telomere shortening (Needham et al., 2019). While White participants had longer telomeres at the initial time point visit, the average telomere length at the 10-year follow-up visit was not significantly different between the three racial and ethnic groups (Needham et al., 2019). The estimated change in 10-year telomere shortening for African American and Hispanic participants were 0.05 and 0.04 units, respectively, lower than White participants (Needham et al., 2019). No significant differences in telomere shortening were observed between African American and Hispanic participants (Needham et al., 2019).
Connecting telomere length with other potential biomarkers such as hormones, the WHI cohort evaluated the relationship between telomere length and circulating sex hormone concentrations (Song et al., 2018). No significant associations were found between telomere length and estradiol levels (Song et al., 2018). However, there was a significant opposite association between telomere length and free and total testosterone in Asian/Pacific Islander women, where higher levels of testosterone were associated with shorter telomeres (Song et al., 2018).
4.4. Nutrient sensing and other metabolic biomarkers
The most well-known nutrient sensing pathways and regulators associated with aging include the insulin and insulin-like growth factor 1 (IGF-1) signaling (IIS) pathway, mTOR, AMPK, and sirtuins (Lopez-Otin et al., 2013; Xia et al., 2017). Previous studies have shown that mutations in mTOR, IGF-1 and insulin receptors, and growth hormones were associated with longevity (Lopez-Otin et al., 2013). The IIS pathway, responsible for sensing glucose levels, decreases with normal aging, yet constitutive reduction of IIS activity prolongs longevity (Lopez-Otin et al., 2013; Xia et al., 2017). mTOR, responsible for detecting high amino acid concentrations, activity increases with aging, and inhibiting the mTOR complex increases lifespan (Lopez-Otin et al., 2013; Xia et al., 2017). AMPK and sirtuins function to detect nutrient scarceness and low-energy states based on high AMP and NAD+ concentrations, respectively (Lopez-Otin et al., 2013; Xia et al., 2017). Increased activity of AMPK has been linked to extending lifespan, while sirtuins have decreased expression during aging and overexpression of the sirtuin family member SIRT6 led to longer lifespan (Lopez-Otin et al., 2013; Xia et al., 2017).
Currently published studies associated with nutrient sensing processes in older racial and ethnic populations have been on circulating hormones and metabolites. It should be noted that the majority of the studies described in this section examined biomarkers in older and relatively healthy adults but did not examine biomarkers directly in the context of aging. For example, the Endogenous Hormones Nutritional Biomarkers and Prostate Cancer Collaborative Group investigated the relationship between circulating sex hormones and various sociodemographic, anthropometric, and behavioral factors in healthy men aged 25–85 years old (Watts et al., 2017). They found African American men had greater estrone, estradiol, and free estradiol concentrations compared to White men, while Hispanic men had approximately 12% higher A-diol-G concentrations as White men (Watts et al., 2017). Another study from the same cohort found that African American men had reduced levels of IGFBP-1, −2, and −3 as well as IGF-II, while Hispanic men had reduced levels of IGFBP-2 and − 3 as well as IGF-I and -II compared to White men (Watts et al., 2019). This is in line with previous studies showing decreased levels of growth hormone and IGF-1 during normal aging (Lopez-Otin et al., 2013). It would be interesting to differentiate whether these hormones and proteins are constitutively reduced over time, as previous studies have demonstrated constitutive reductions of the IIS pathway increase longevity (Lopez-Otin et al., 2013). Future studies should examine longitudinal changes in IGF-1 and other IIS pathway regulators’ concentrations in racial and ethnic minority groups to determine whether changes in concentrations confer beneficial or detrimental effects to longevity and health span.
Lipids and lipid metabolism have recently received attention as potential biomarkers of aging. Lipids play key roles in biological processes such as inflammation and cellular stress that change with age, and lipid profiles have been shown to alter with age (Almeida et al., 2021). The WHICAP cohort compared lipids and lipoproteins in White, African American, and Hispanic participants 65 years old and above, and found that race and ethnicity was a separate predictor of lipid and lipoprotein concentrations (Rodriguez et al., 2002). African American participants had higher levels of HDL cholesterol, and lower levels of total/HDL cholesterol ratio and triglycerides compared to White participants, while Hispanic participants had lower levels of total, HDL, and LDL cholesterol compared to White participants (Rodriguez et al., 2002). Another study from WHICAP performed a 3-year follow-up of lipid levels for the same cohort, and found Hispanic participants had significantly reduced HDL-C concentrations and African Americans participants had significantly greater triglyceride concentrations compared to White participants (Schupf et al., 2005). The WHICAP cohort also found African American and White participants in the lowest quartiles of non-HDL, LDL, and total cholesterol had a higher risk of death, while no significant differences were observed in Hispanic participants (Akerblom et al., 2008). A study from the Massachusetts Hispanic Elders Study examined how diet and ethnicity affect lipid concentrations in elderly Caribbean Hispanic and White participants (Bermudez et al., 2002). The authors found that White participants had higher HDL cholesterol, while Hispanic participants had higher LDL cholesterol (Bermudez et al., 2002), opposite to findings from the WHICAP studies (Rodriguez et al., 2002; Schupf et al., 2005). However, Hispanic women had significantly lower concentrations of apolipoproteins A-I and B, as well as HDL, LDL, and total cholesterol compared to White women, while Hispanic men had higher levels of apolipoprotein A-I than White men (Bermudez et al., 2002).
The observed LDL reductions in Hispanic participants are in opposition to previous studies showing LDL increases with age (Morgan et al., 2016). However, increased LDL concentrations were correlated with reduced mortality risk in an elderly Chinese cohort (Morgan et al., 2016). HDL levels have been shown to decrease with age (Morgan et al., 2016), and decreased HDL concentrations were observed in Hispanic populations from WHICAP (Rodriguez et al., 2002; Schupf et al., 2005) and the Massachusetts Hispanic Elders Study (Bermudez et al., 2002). However, HDL levels were increased in African American participants from the WHICAP cohort (Rodriguez et al., 2002). A previous study has shown African American participants tend to absorb more cholesterol compared to White participants, and higher HDL levels have been proposed to extend longevity (Morgan et al., 2016). It would be tempting to speculate that reduced LDL in Hispanic participants and higher HDL in African American participants could serve as protective aging factors in these respective populations. More studies examining longitudinal changes in cholesterol and lipoproteins concentrations in aging racial and ethnic minority populations are needed.
The Baltimore Longitudinal Study of Aging (BLSA) examined plasma concentrations of sphingomyelin species in participants 55+ years old (Mielke et al., 2015). African American participants from the BLSA cohort displayed greater concentrations of circulating sphingomyelin and nearly all dihydrosphingomyelins compared to White participants (Mielke et al., 2015). This is in line with previous studies showing sphingomyelins increase with aging (Almeida et al., 2021). Interestingly, GWAS studies have linked genetic variants to various circulating lipids and metabolites. One study from the CHARGE consortium identified genetic variants associated with circulating trans fatty acids in European ancestry populations, plus African American, Hispanic, and Chinese American populations (Mozaffarian et al., 2015). One SNP (rs174548) in FADS1/2 was correlated with cis/trans-18:2 in Hispanic populations, while the rs174579 variant in FADS2 was correlated with the same trans fatty acid in African American populations (Mozaffarian et al., 2015). However, after adjusting for cis-20:4n-6 phospholipid concentrations, the association between rs174548 and cis/trans-18:2 was not significant (Mozaffarian et al., 2015). No other studies have examined cis/trans-18:2 in the context of aging, and thus require additional studies to understand how this fatty acid contributes to healthy aging. Another GWAS study identified genetic variants associated with homocysteine levels in African American and Yoruba cohorts from the Indianapolis-Ibadan Dementia Project (Kim et al., 2016). Homocysteine plays a key role in methionine metabolism (Kim et al., 2016), has been shown to increase with age (Ostrakhovitch and Tabibzadeh, 2019), and associated with age-related diseases and conditions such as cognitive decline and Alzheimer’s disease (Kim et al., 2016; Ostrakhovitch and Tabibzadeh, 2019). They found 4 significant SNPs in and surrounding CBS and 1 significant SNP in the intronic region of CD2AP (Kim et al., 2016). The CBS gene codes for an enzyme that converts homocysteine to cystathionine, which represents the first step of the trans-sulfuration pathway (Kim et al., 2016). CD2AP has multiple biological roles including cell adhesion and cytoskeletal reorganization, which are processes also regulated by homocysteine (Kim et al., 2016). However, the mechanistic relationship between CD2AP and homocysteine remains unclear (Kim et al., 2016). Variants in CBS and CD2AP were associated with decreased homocysteine levels, suggesting that these variants could confer protection against increasing homocysteine levels (Kim et al., 2016).
Overall, more studies are needed to evaluate hormone, lipid, and other circulating metabolite profiles in aging racial and ethnic minority populations. Longitudinal studies examining how these metabolic profiles change over the course of healthy aging will be crucial in determining whether metabolic biomarkers can predict longevity and/or mortality in racial and ethnic minorities. This will also require a better understanding of how environmental, socioeconomic, and psychosocial factors influence metabolic profile changes in these aging populations.
4.5. Cellular senescence biomarkers
Inflammation and cellular senescence are closely linked, where senescent cells can release proinflammatory cytokines, leading to an increased inflammatory response and premature aging (Lopez-Otin et al., 2013). No studies have directly examined cellular senescent biomarkers in racial and ethnic minority populations, but few studies have examined inflammatory biomarkers. One study has examined the relationship between immigration history of multi-generation Mexican-origin immigrants over the ages of 60 years old from the SALSA study and inflammatory biomarkers (Martin et al., 2018). This study found 3rd generation immigrants had the highest levels of CRP, leptin, IL-6, sTNF-R1, and sTNF-R2, in which CRP levels were significantly higher compared to 1st generation immigrants that have lived in the U.S. for less than 15 years (Martin et al., 2018). Additionally, in comparison to 1st generation immigrants, leptin was significantly higher for all generational immigrants (Martin et al., 2018). Second generation immigrants had roughly 20% higher levels of IL-6, and 3rd generation immigrants had approximately 31% higher IL-6 concentrations (Martin et al., 2018). Similar trends were found for CRP, sTNF-R1, sTNF-R2 levels in 2nd and 3rd generation immigrants (Martin et al., 2018).
Interestingly, 2 complementary studies from the Health, Aging, and Body Composition (Health ABC) study have examined the relationship between genetic admixture and various inflammatory biomarkers in elderly African American participants (Reich et al., 2007; Wassel Fyr et al., 2007). One study found increased IL-6 soluble receptor concentrations were significantly associated with higher European ancestry, while increased CRP concentrations were significantly associated with higher African ancestry (Reich et al., 2007). Additionally, the authors identified a novel association between the SNP rs8192284 and higher IL-6 concentrations in African American and White participants (Reich et al., 2007). A companion study investigated the association between adipocytokines and genetic ancestry, and found adiposity, other mediators of metabolic syndrome, and higher European ancestry were significantly correlated with increased IL-2 soluble receptor, IL-6 soluble receptor, TNF-α soluble receptor II, and adiponectin as well as decreased CRP levels (Wassel Fyr et al., 2007).
Collectively, these studies suggest inflammatory biomarkers increase in older Hispanic and African American populations, consistent with the previous literature demonstrating rising inflammatory responses with aging (Lopez-Otin et al., 2013). More studies are needed to investigate longitudinal changes of inflammatory and cellular senescent biomarkers in Native American, Hispanic, African American, and other racial and ethnic minority populations. It would also be interesting to further delineate between multi-generational Hispanic immigrant populations by studying individual Hispanic sub-populations based on country of origin. The previously identified relationship between genetic admixture and some inflammatory biomarker levels (Reich et al., 2007; Reiner et al., 2012; Wassel Fyr et al., 2007) reinforces the need to further explore the role of genetic admixture in aging racial and ethnic minorities sub-populations from distinct geographical locations. It is also likely that non-biological factors could also contribute to changes in inflammatory and cellular senescent biomarkers and require future investigation.
4.6. Extracellular vesicles as biomarkers
Emerging evidence indicates that extracellular vesicles may be promising, readily accessible biomarkers since these small nano-sized vesicles are released by cells into the circulation. EVs are membrane-bound and contain bioactive cargo such as nucleic acids, proteins, and lipids. EVs can serve as messengers by interacting with cell-surface receptors and through transferring cargo to recipient cells. Through these interactions, EVs play a role in a myriad of biological processes (Kalluri and LeBleu, 2020; Yanez-Mo et al., 2015). Currently, EVs are being pursued as diagnostic and prognostic factors for various age-related diseases including cancer, and neurodegenerative, metabolic and cardiovascular diseases (Kalluri and LeBleu, 2020; Noren Hooten and Evans, 2020; Yanez-Mo et al., 2015). For EVs to be utilized as a biomarker, they must be characterized across the lifespan and in different racial groups. However, there are limited studies that have examined EVs in minority populations.
We have characterized EVs in the context of age in a racially diverse cohort of participants from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study (Eitan et al., 2017). Plasma EV concentration decreases with advancing age in both White and African American participants. There were no racial differences in EV concentration or size. In this sub-cohort, we also reported the presence of circulating cell-free mitochondrial DNA (mtDNA) in EVs and that EV mtDNA levels decline with human age (Lazo et al., 2021). There were no significant differences in EV mtDNA levels between races (Lazo et al., 2021). We designed a specific sub-cohort to address whether there were differences in EV characteristics and protein cargo with race (Noren Hooten et al., 2019). No racial differences were observed in EV concentration or size. Examination of EV protein cargo revealed higher levels of phospho-p53, total p53, cleaved caspase 3, ERK1/2 and phospho-AKT in White individuals compared to African American individuals (Noren Hooten et al., 2019). In this study, the relationship between EV protein cargo, race and clinical markers of mortality were also analyzed. The association of EV characteristics with many mortality markers differs by race. These data highlight the importance of examining EVs and their associated cargo in racially diverse populations.
5. Epigenomics, transcriptomics and social genomics and aging in minority populations
5.1. Epigenetic age in minority populations
Minority populations are exposed to environmental and lifestyle-related factors that can impact health in part through affecting transcriptional programs. Many mechanisms regulate gene expression in cells. One of the most characterized, especially in minority populations, is DNA methylation (DNAm). This epigenetic modification alters gene expression without changing the DNA sequence. DNA methylation is heritable and also influenced by age, environmental insults and lifestyle factors (Fig. 3) (Alegría-Torres et al., 2011; Noroozi et al., 2021; Peters et al., 2021). Therefore, this points to DNA methylation as an important epigenomic process that may be modified as a consequence of the SDOH. Here we will discuss the status of epigenetic studies in minority populations.
Fig. 3.
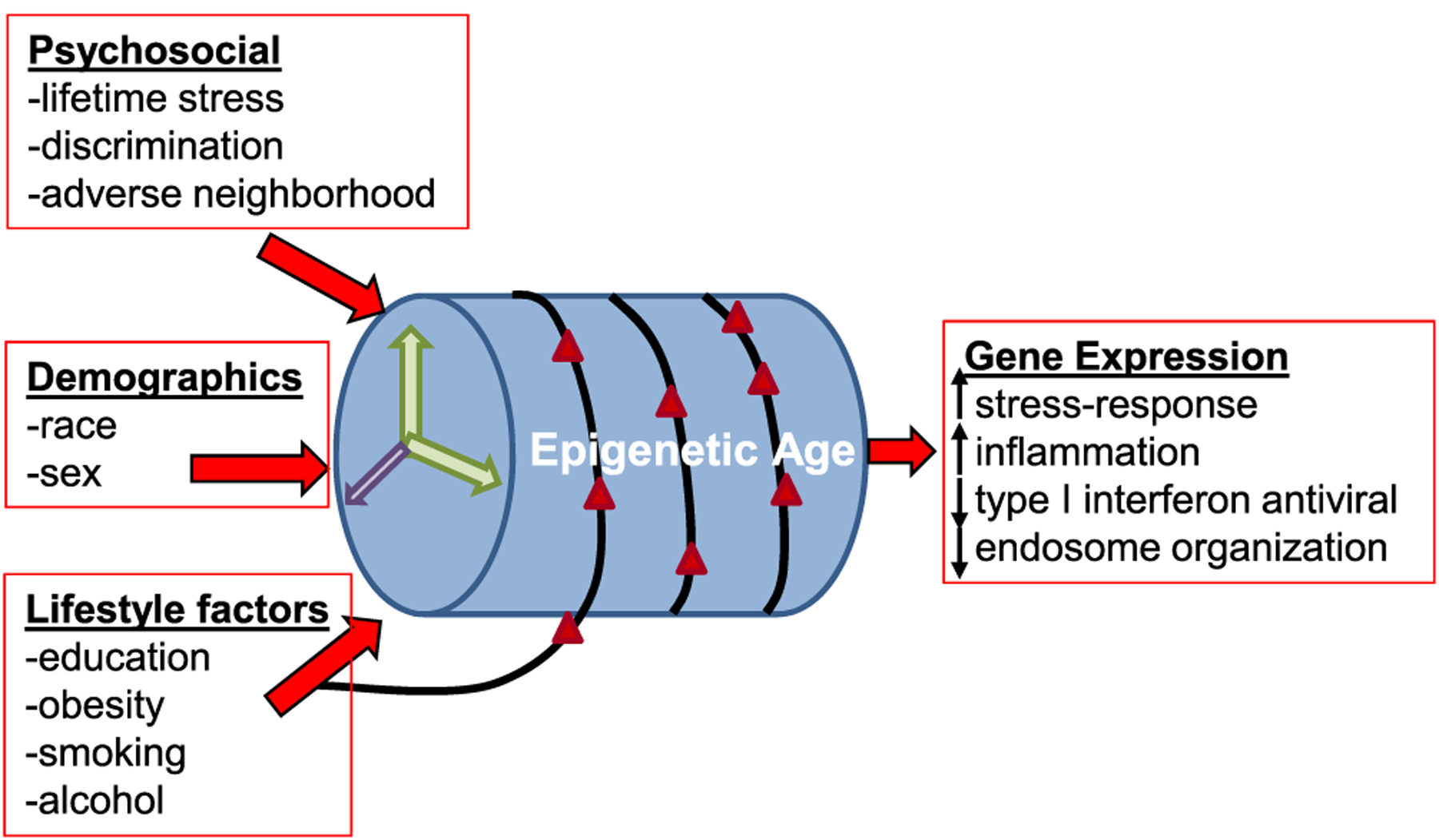
Factors leading to epigenetic age acceleration in minority populations. Psychosocial, lifestyle factors as well as demographics contribute to changes in DNA methylation that have been categorized as accelerated epigenetic aging. These epigenetic alterations may result in the observed gene expression changes in minority populations in response to the social determinants of health.
DNAm at select CpG sites across the genome has been used in mathematical models to determine the DNAm age, also referred to as epigenetic age or epigenetic clock, which has been used to predict biological age but also highly correlates with chronological age. Using these epigenetic clocks, epigenetic age acceleration can be estimated, which generally is the difference between DNAm age and chronological age (Noroozi et al., 2021). Epigenetic age acceleration is typically calculated as the residuals from regressing epigenetic age on chronological age after controlling for various covariates (Noroozi et al., 2021). Additional epigenetic age estimators have also been developed that measure intrinsic epigenetic age acceleration (IEAA) and extrinsic epigenetic age acceleration (EEAA). IEAA captures aspects of the aging process independent of changes in white blood cell composition. EEAA estimates aging of the immune system since it considers age-dependent changes in white blood cell composition (Noroozi et al., 2021).
Studies have shown that African American and White men have faster universal age acceleration versus African American women (Tajuddin et al., 2019). Ten times more age-associated differentially methylated CpG positions (aDMPs) were reported in African American individuals (4930) versus White individuals (469), suggesting more widespread DNAm changes in African American individuals. Two studies have reported that African American individuals have slower extrinsic age acceleration than White individuals (Horvath et al., 2016; Tajuddin et al., 2019). Racial differences in differentially methylated sites have also been reported in the context of metabolic syndrome (Chitrala et al., 2020).
One outstanding question in the field is to whether the various epigenetic age measures capture aspects of biological age. There are several studies that support this idea (Fig. 3). Epigenetic age acceleration was associated with faster rates of cognitive decline in African American and White men (Beydoun et al., 2020; Bressler et al., 2020). In a recent meta-analysis containing cohorts that included White, Hispanic and African American individuals, epigenetic age acceleration measures were predictive of all-cause mortality (Chen et al., 2016). In agreement with this study, recent evidence suggests that markers of target organ damage were associated with intrinsic epigenetic age acceleration, but the majority of these relationships were attenuated after adjustment for blood pressure and anti-hypertensives (Smith et al., 2019). Examination of education and lifestyle factors in African American participants of the Genetic Epidemiology Network of Arteriopathy found that gender, education, BMI, smoking, and alcohol consumption were all independently associated with GrimAge acceleration (Zhao et al., 2019), which is a composite biomarker that includes surrogate DNAm biomarkers of 7 different plasma proteins and a DNAm-based estimator of smoking pack-years (Lu et al., 2019). In this African American cohort with a high prevalence of hypertension, several cardiovascular risk factors were also associated with various measures of epigenetic age acceleration as well (Ammous et al., 2021). In African American mothers enrolled in the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) study, obesity was associated with epigenetic age acceleration (Li et al., 2019).
Most recently a new epigenetic clock was developed that incorporates additional clinical measures of phenotypic age and DNAm termed “PhenoAge” (Levine et al., 2018). PhenoAge predicts all-cause mortality across all racial groups examined including Black, White and Hispanic individuals. It also outperformed previous epigenetic clocks (Ex: DNAmAge Hannum and DNAmAge Horvath) at predicting age-related clinical outcomes. Racial/ethnic differences were also observed in PhenoAge as non-Hispanic Black individuals had the highest DNAm PhenoAge while non-Hispanic White individuals had the lowest. These data point to PhenoAge as a promising indicator of biological age in minority populations. As studies that incorporate these “second generation epigenetic clocks” (ex: GrimAge and PhenoAge) in minority populations is limited, we are only beginning to understand which of these epigenetic measures may be better at predicting biological aging and age-related health outcomes in the setting of race/ethnicity.
Psychosocial stress has also been shown to impact epigenetic age in African American individuals (Fig. 3). Epigenetic age acceleration was associated with cumulative lifetime stress in a cohort of urban African Americans individuals (Zannas et al., 2015). Given this data, the authors examined the CpG sites in the Horvath epigenetic clock and found that one third of these sites were located in glucocorticoid response elements (Zannas et al., 2015). Furthermore, glucocorticoid activation altered DNAm and transcription of stress-response genes. These stress-response genes were enriched for association with aging-related diseases (Zannas et al., 2015). Adverse neighborhood environment has also been linked to faster epigenetic aging in African American individuals (Lei et al., 2017; Martin et al., 2021). These data point to changes in DNAm as a biological consequence of the social determinants of health (Fig. 3).
Few studies have examined epigenetic age measures in other minority populations. In a large study of race/ethnicity, it was reported that Hispanic individuals have a lower intrinsic age (“younger”) but higher (“older”) extrinsic epigenetic age rates than White individuals. Age acceleration was also higher in Hispanic men compared to Hispanic women (Horvath et al., 2016). Lower extrinsic age acceleration was also associated with higher education level in Hispanic and African American individuals (Horvath et al., 2016). In Mexican American individuals, 10 CpG sites were differentially methylated in controls versus individuals with mild cognitive impairment (Pathak et al., 2019). It is important to include multi-ethnic cohorts into epigenetic age studies as results are not always replicated in different ethnic cohorts, but this may also be due to underpowering of these cohorts (Ma et al., 2020) or different mechanisms that drive aging and age-related disease in minority populations.
The inclusion of minority populations in more and more epigenetic studies will surely enhance our understanding about whether epigenomic processes are modified as a consequence of the SDOH. The advent of new epigenetic clocks that incorporate DNAm and clinical measures will also be beneficial.
5.2. Transcriptomics and social genomics in minority populations
Alterations in DNAm of epigenetic age CpG sites or other CpG sites may result in differences in gene expression in minority populations. Indeed, one area of research in the field of social genomics has focused on how social adversity leads to changes in gene expression (Fig. 3). Cole and colleagues have reported changes in immune cell gene expression profiles from individuals facing a variety of adverse social conditions, which has been termed the Conserved Transcriptional Response to Adversity (CTRA) (Cole, 2019). This CTRA pattern describes an upregulation of specific inflammatory-associated genes and downregulation of genes in the Type I interferon antiviral response pathway (Fig. 3) (Cole, 2019). Consistent with this data, our laboratory reported differences in long noncoding RNA (lncRNA) and gene expression in individuals living above or below poverty (Noren Hooten and Evans, 2019). Top pathways that were enriched in individuals living above poverty were those involved in response to stress, immune stimuli, and viral infection (Noren Hooten and Evans, 2019). These pathways were also reported in another independent study examining SES in African Americans (Gaye et al., 2017). This transcriptomic analysis also reported higher abundance of genes in inflammatory pathways (IL-8; Nf-kB; Colony-stimulating factor 2 (CSF2)) in low SES African Americans as part of a molecular profile of SES (Gaye et al., 2017).
The effects of various social stresses on gene expression are context-dependent and therefore it is important to examine other gene networks. For example, there was no difference in the CTRA profile between low-income Black mothers living in neighborhoods with high violence versus low violence (Lee et al., 2021). Greater neighborhood stress and racial discrimination were associated with higher glucocorticoid receptor response genes (Lee et al., 2021; Thames et al., 2019), which is consistent with the epigenetic modifications of these response genes in African American individuals with cumulative lifetime stress (Zannas et al., 2015). Comparisons of African American men and women living above and below poverty identified other pathways that may also underlie the effects of poverty. In African American women, pathways related to long chain fatty-acyl-CoA biosynthetic processes, B-cell differentiation, genetic imprinting, and interleukin-12-mediated signaling were significantly different in African American women living above or below poverty (Arnold et al., 2020). Pathways related to neutrophil degranulation, toll-like receptor signaling, antigen processing and presentation and MyD88-dependent toll-like receptor signaling were significantly different between African American men living above and below poverty (Arnold et al., 2020). Endosome organization was the one overlapping pathway comparing both African American men and women living above or below poverty (Arnold et al., 2020). These studies highlight the commonalities among pathways that are regulated by psychosocial stress, but also highlight that these effects are contextual and may lead to different biological consequences over the lifespan.
The importance of acquiring more transcriptomic data sets of minority populations is highlighted by a recent study showing that datasets trained using individuals of European descent largely are not generalizable to African American individuals (Keys et al., 2020). In response to this, a recent study incorporated ~ 50,000 Hispanic/Latino, African American, Asian, Native Hawaiian, and Native American individuals as part of the Population Architecture using Genomics and Epidemiology (PAGE) study. The authors utilized this large GWAS dataset to perform transcriptome-wide association studies (TWAS) to predict gene expression levels and gene-trait associations (Geoffroy et al., 2020). Twenty-eight different traits in the following categories were examined: inflammatory, lipid, lifestyle, glycemic, electrocardiogram, blood pressure, anthropometric and kidney. There were gene-trait associations identified for 14 out of the 28 traits using three different models incorporating different populations: the African American and Hispanic/Latino (AFHI) model, the European (EUR) model, and the African American, Hispanic/Latino, and European (ALL) model. All together there were 206 genes and 240 unique gene-trait pairs identified. However, their AFHI model, incorporating data from African American and Hispanic/Latino individuals, discovered more gene-trait associations that replicated in larger cohorts, indicating the importance of including matched populations of similar ancestry in transcriptome prediction model studies (Geoffroy et al., 2020). Furthermore, since this study was prediction-based it highlights the need for additional transcriptomic studies in diverse, minority populations as they age.
6. DNA damage/repair and aging in minority populations
Both the free radical and the DNA damage theory of aging posit that aging is caused by accumulation of DNA damage and reduced DNA repair (Gensler and Bernstein, 1981; Harman, 1956). Increased DNA damage is correlated with age-related diseases such as cancer, inversely, premature aging disorders are associated with impaired DNA repair systems and accumulation of DNA damage (Chen et al., 2020; Niedernhofer et al., 2018). Overall, there is a correlation between age and general DNA damage, although specific studies may not reflect a significant correlation depending on the methods employed to measure DNA damage or the particular variety of DNA damage investigated (Jacob et al., 2013; Soares et al., 2014).
DNA damage can be caused by exogenous or endogenous DNA damaging agents. Exogenous DNA damage occurs after exposure to environmental sources such as pollutants or ionizing radiation. Endogenous damaging agents primarily include reactive alkylating agents or oxygen species (ROS) that are naturally produced within cells, although DNA damage may also occur during replication or spontaneously (Chatterjee and Walker, 2017). Both endogenous and exogenous damaging agents may cause strand breaks or oxidative damage to DNA. The most studied ROS include hydrogen peroxide, superoxide radicals, and the hydroxyl radical. Produced in the mitochondria, ROS can interact with nearby proteins or genes to cause damage and further increase production of ROS (Niedernhofer et al., 2018). ROS and oxidative damage increase as organisms, including humans, age (Jacob et al., 2013). Spontaneous DNA mutations are typically stochastic, but evidence shows that mutation accumulation increases with age (Ramsey et al., 1995). DNA strand breaks are highly toxic and thus difficult to measure precisely at low levels; however, multiple studies confirm that single- and double-strand breaks increase in cells taken from the elderly compared to younger populations (Chevanne et al., 2003; Rube et al., 2011; Sedelnikova et al., 2008). Conversely, long-lived humans display similar amounts of strand breaks as young adults (Chevanne et al., 2003).
DNA repair systems normally compensate for the damage that takes place daily. However, individual repair capacity may differ from the population average, overwhelming amounts of damage will exhaust repair capacity, and DNA repair efficacy appears to decline with age (Chen et al., 2020; Nagel et al., 2014; Niedernhofer et al., 2018). Studies have shown a decline with age in the expression of DNA repair proteins involved in multiple repair pathways, although results conflict depending on the cell type investigated (Zhang et al., 2020; Li et al., 2016). Nucleotide excision repair has been shown to decline with age in several studies (Goukassian et al., 2000; Moriwaki et al., 1996; Yamada et al., 2006). Base excision repair has also been shown to decline in a variety of human cell types from aged individuals (Atamna et al., 2000; Xu et al., 2015; Zhang et al., 2020). Studies investigating strand break repair have produced conflicting results (Jacob et al., 2013). While some labs have found that single- or double-strand break repair declines with age in leukocytes or human-derived cell lines (Li et al., 2016; Sedelnikova et al., 2008; Trzeciak et al., 2008), conflicting results have also been published showing no decline (Garm et al., 2013; Zhang et al., 2020). An age-related decline in repair capacity may be tissue-specific or require more precise study design to reveal repair capacity declines in certain populations (Chen et al., 2020; Jacob et al., 2013).
Research has suggested that both levels of baseline DNA damage and DNA repair capacity are not equal among all human populations (Chatterjee and Walker, 2017; Jacob et al., 2013). Measurement of DNA damage is frequently performed on cohorts stratified by age and often sex. The interplay between race and DNA damage in the process of aging is rarely studied (Møller, 2006; Neri et al., 2015). Differences in DNA damage and repair may explain some age-related health disparities observed in minority populations. This section will summarize studies on DNA damage and repair related to aging in minority populations.
Studies on DNA damage have revealed race-dependent differences in baseline DNA damage and DNA repair capacity. Lahiri et al. demonstrated an increased resistance to oxidative stress in African individuals relative to White individuals in a pilot study (Lahiri et al., 1999). In a recruitment study for breast cancer, oxidative damage was shown to be highly variable in African American patients (SD = 224.1) compared to White patients (SD = 57.5, p < 0.001) (Simon et al., 2000). Another study investigating the effect of antioxidants on oxidative damage noted a difference in baseline oxidative DNA damage between African American and White individuals (Huang et al., 2000). Lowered baseline urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG, ng/mg creatinine) was associated with race (15.6 ± 0.8 in African American individuals versus 20.3 ± 1.2 in White individuals, mean ± SE; p = 0.001), antioxidant supplementation, and exercise (Huang et al., 2000). A study on women launderers and dry cleaners reported a difference in oxidative damage between African American and White groups (Toraason et al., 2003). White women displayed a significantly higher level of leukocyte 8-OHdG (17.8 ± 7.4, mean ± SD) that was significantly greater than the observed damage in control African American women (11.8 ± 5.9, mean ± SD) (Toraason et al., 2003). In a study investigating the correlation between antioxidant intake and DNA damage measured by the alkaline comet assay, African American individuals were reported to have lower levels of oxidative DNA damage compared to White individuals (1.404 versus 1.559, mean tail moment; p = 0.005) (Watters et al., 2007). In later follow-up studies, regression models examining demographic and behavioral correlates with oxidative DNA damage levels explained more of the damage variance in White individuals than in African American individuals (27% vs 19%) (Watters et al., 2007). Finally, the same samples were examined with alternative oxidative stress and DNA damage assays urinary F2-isoprostanes; the comet assay with formamidopyrimidine DNA glycosylase (FPG); and 8-oxo-7, 8-dihydro-2′-deoxyguanosine (8-oxo-dG)) (Watters et al., 2007). Results for the first two assays matched previous results: African American individuals had lower oxidative DNA damage and stress levels as measured by the comet assay (p = 0.0003) and F2-isoprostanes (p = 0.27), however damage measured by 8-oxo-dG was higher (p = 0.05) than White individuals (Watters et al., 2007).
In a longitudinal study from the HANDLS cohort, our laboratory reported findings that matched previous observed differences in DNA damage and repair levels between African American and White participants (Trzeciak et al., 2008). The fast component of single-strand break repair was shown to increase with age only in White women (Trzeciak et al., 2008). When comparing DNA repair levels between African American and White women, the fast component of single-strand break repair was lowered with age in African American women compared to White women (Trzeciak et al., 2008). In a follow-up study, overall single-strand break repair was shown to be higher in women than in men (p = 0.013); the difference persisted in African American women versus African American men (p = 0.029) but not in White women versus White men (p = 0.164) (Trzeciak et al., 2012). Repair of toxic double-strand breaks has also been examined in a racially diverse cohort (Sharma et al., 2015). No statistical difference in baseline double-strand breaks was found for a specific racial group, however, a significant interaction between age and race was observed for Hispanic participants (Sharma et al., 2015). Double-strand break repair rates were overall positively associated with age (p = 0.025) and specifically for White participants (p = 0.007) (Sharma et al., 2015).
Current limitations in correlating measured DNA damage and repair capacity to demographic or behavioral factors illustrate the need for more racially diverse studies to improve our knowledge of DNA damage response mechanisms, aging, and health disparities in all populations (Nagel et al., 2017).
7. Discrimination and accelerated aging phenotype
In the United States, the deeply rooted system of racism has permeated our culture leading to long standing effects. Prolonged negative social experiences including psychological stresses can cause detrimental health outcomes. Discrimination is a psychological stress that is defined as the prejudice and unfair treatment given to people due to their membership in a certain group (Williams and Mohammed, 2009). These include, but are not limited to, minority racial status, and can be characterized on multiple levels ranging from an individual perspective to society in general. Discrimination as a form of social exclusion can be felt short term (everyday) or long term (lifetime) leading to adverse physiological responses.
Discrimination has been associated with several age-related health outcomes (Fig. 4). Many studies have examined the link between discrimination and risk factors and outcomes for cardiovascular disease (CVD) (Lewis et al., 2014). For example, among women, everyday discrimination predicts higher levels of blood pressure, which is mediated by adiposity (Moody et al., 2018). In the MESA and JHS studies, lifetime discrimination was associated with higher incidence of hypertension among Black Americans (Forde et al., 2021, 2020; Sims et al., 2012). Inflammation increases with age and is associated with CVD. Specifically, the inflammatory marker interleukin-6 (IL-6) and the acute phase protein CRP are risk factors for CVD. Higher levels of IL-6, but not CRP, were associated with women experiencing greater everyday discrimination and lifetime discrimination (Kershaw et al., 2016). Discrimination and race-related stressors were shown to be associated with increases in inflammatory markers even in African American children, with early exposure to discrimination also predicting later levels of inflammation in adulthood (Simons et al., 2018). This increase in chronic inflammation has been predicted to lead to accelerated aging (Levine and Crimmins, 2014; Simons et al., 2018). Another study found that older African American adults experiencing everyday discrimination had higher levels of CRP and this was modified by obesity (Lewis et al., 2010). The effect of discrimination may be modulated by socioeconomic status (SES). Several studies investigated potential effects of SES on inflammation among diverse populations. African American individuals of higher SES have been shown to report higher discrimination than African American individuals of lower SES (Borrell et al., 2013; Vines et al., 2006). Low SES was associated with overexpression of pro-inflammatory pathways in a cohort of African American individuals (Gaye et al., 2017). SES discrimination was reported to be associated with higher levels of CRP in higher educated African American individuals (Van Dyke et al., 2017). Lower educated African American and White individuals of either education level did not display the same association (Van Dyke et al., 2017). These studies, and others, indicate that the link between discrimination and CVD risk factors is complex and may be modified by other factors (Lewis et al., 2014). Importantly, recent data has shown that lifetime discrimination increased CVD risk (Everson-Rose et al., 2015).
Fig. 4.
Discrimination lays the roots for health disparities in health outcomes.
Discrimination may also impact metabolic diseases. In a longitudinal cohort of US women from the Study of Women’s Health Across the Nation (SWAN), everyday discrimination predicted a greater incidence of metabolic syndrome in all women, but was more prominent in Black, Hispanic, and Japanese women (Beatty Moody et al., 2018). Major experiences of discrimination, but not everyday discrimination, also predicted greater risk of incident of type 2 diabetes mellitus in another study (Whitaker et al., 2017). Although most studies have focused on discrimination in African American individuals, it has been reported that Hispanic individuals experiencing ethnic discrimination have higher odds of diabetes but only in those individuals with higher depressive symptoms (McCurley et al., 2019).
African American individuals bear a disparate burden of poor brain health outcomes, which may be a consequence of discrimination. Black individuals experiencing more everyday discrimination have poorer episodic memory (Barnes et al., 2012a) and faster episodic memory decline (Zahodne et al., 2017). Although we are only beginning to understand how discrimination leads to deficits in cognition, one study indicated that inflammation, as measured by CRP, mediated the effects on baseline memory but not memory decline (Zahodne et al., 2019a). Using neuroimaging, other data suggests that discrimination can affect the structure of the brain. Older African American adults experiencing greater lifetime and racial discrimination had increased White matter lesion volume (WMLV) while younger African American adults with lower racial discrimination was associated with increased WMLV (Beatty Moody et al., 2019b). WMLV is a subclinical prognostic indicator in the brain associated with future stroke, dementia and cognitive decline. In cohort studies from the Rush Alzheimer’s Disease Center, experience of discrimination in older Black adults was associated with differences in functional connectivity of the insula, a region of the brain that regulates emotions and trust (Han et al., 2020). Furthermore, lifetime discrimination in African American individuals with depressive symptoms had increased carotid intimal-medial thickness, which is a subclinical marker of atherosclerosis and stroke incidence (Beatty Moody et al., 2020).
Telomere attrition is a hallmark of aging and the accelerated aging phenotype. Women with high SES that experienced greater lifetime discrimination, racial discrimination and gender discrimination had shorter telomeres (Beatty Moody et al., 2019a; Pantesco et al., 2018). Interestingly, this observation was not observed in men or women with lower SES. In another study, African American men experiencing greater racial discrimination had shorter telomere length (Chae et al., 2014). In a follow up study, it was reported that African American men with lower depressive symptoms had shorter telomere length (Chae et al., 2016). In addition to telomere attrition, oxidative stress is an important driver of the aging process. Red blood cell oxidative stress was shown to be significantly associated with racial discrimination (Szanton et al., 2012). These data indicate that African American individuals experiencing psychosocial stress due to discrimination can cause biological consequences that promote accelerated aging and point to mechanisms that may explain health disparities in aging (Fig. 4).
One important question is whether experiencing discrimination affects longevity and aging. In the Chicago Health and Aging Project, older adults exposed to greater discrimination had increased mortality (Barnes et al., 2008). Consistent with this idea, data from the HRS found that everyday discrimination was associated with increased risk for all-cause mortality (Farmer et al., 2019). In both the HRS and in the Black Women’s Health study, racial discrimination was not associated with all-cause mortality (Albert et al., 2010; Farmer et al., 2019). In agreement with this data, studies from the JHS indicate that everyday discrimination was not associated with CVD outcomes and surprisingly with lower all-cause mortality, which was partially mediated by perceived stress (Dunlay et al., 2017).
Combined these studies point to discrimination as a psychosocial stress that effects biological pathways that impact aging and age-related diseases (Fig. 4). This area of research is complex and may be mediated by a variety of susceptibility factors that have yet to be explored fully. Therefore, additional studies that include minority populations and discrimination data are needed to understand the physiological impacts of discrimination on age-related clinical health outcomes.
8. Inflamm-aging and minority populations
Acute and chronic stress in life cause physiological changes in the body, and even traumatic experiences or stress in childhood have been shown to alter the immune system and inflammation responses in adulthood (Emeny et al., 2021). The normal process of aging is also accompanied by a 2- to 4-fold increase in plasma/serum levels of proinflammatory cytokines in the elderly compared to younger individuals (Ferrucci et al., 2010; Michaud et al., 2013). Elevated circulating inflammatory markers are associated with frailty, risk of morbidity, and mortality in aged individuals (Michaud et al., 2013). Low-level, chronic inflammation in the context of aged individuals, or inflamm-aging, is implicated as a marker or cause of a wide variety of age-related diseases and premature biological aging (Beydoun et al., 2019; Ferrucci et al., 2010; Franceschi et al., 2000, 2018). Minority populations are exposed to stressful life events and experiences such as discrimination, leading to increased inflammation (Cuevas et al., 2020; Djuric et al., 2008; Schmeer and Tarrence, 2018). The strength of the inflammatory response has also been shown to differ by ethnicity (Nédélec et al., 2016; Yeyeodu et al., 2019). The contribution of inflammation to the process of aging must be clarified to understand the physiological impacts of inequality on aging and age-related health disparities. Here we will discuss recent studies examining the role of inflamm-aging in the aging of minority populations.
8.1. Inflammatory markers and aging in minority populations
Immune system function differs among populations and changes with age. Race/ethnicity likely modifies inflammatory responses, as African American individuals with neuroinflammatory disorders have been shown to display more severe clinical manifestations than White individuals with the same diagnosis (Cree et al., 2009; Wharton et al., 2019). Research is ongoing to tease apart meaningful differences in immune system marker baseline levels and function that may translate to diverse rates of aging.
Circulating levels of proinflammatory factors such as interleukins, CRP, and TNF-α generally increase with age. Higher levels of inflammatory markers have been linked with age-related diseases such as cancer and frailty, although evidence is mixed based on the populations studied and study design (Jacob et al., 2013). Conflicting results associated with IL-6 levels may be attributable to differing sources of samples (e.g., blood, serum, plasma, or cerebrospinal fluid), the age of the cohort in the study, or the methods used to measure IL-6. In a middle-aged cohort, frail White individuals displayed higher IL-6 expression in PBMCs than African American individuals (Prince et al., 2019). In a small pilot study with only older White participants, serum IL-6 levels correlated with frailty and a reduction in hemoglobin and hematocrit (Leng et al., 2002). In contrast, studies on populations of older African American and White women observed higher serum IL-6 levels in African American women (Allison et al., 2006; Walston et al., 2005). No significant differences in serum IL-6 were observed in African American versus White individuals in a mixed gender sample aged 70–79 (Yaffe et al., 2003). A study balanced between African American and White participants who were middle-aged and older did not find an association between CRP levels and race (Waldstein et al., 2016). A large meta-analysis in 2016 found that pre-frailty and frailty were associated with IL-6 and CRP in cross-sectional studies, however, the three longitudinal studies in the meta-analysis did not display a correlation between elevated inflammatory markers and future development of frailty (Soysal et al., 2016). Data from the diverse Cardiovascular Health Study (CHS) All Stars Study showed that a subset of aged participants developed higher IL-6 and CRP levels with increasing age (IL-6: 23%, CRP: 21%) (Jenny et al., 2012). In addition, a doubling in either IL-6 or CRP levels was correlated with higher risk for physical or mental impairment (IL-6 odds ratio, 1.45; 95% confidence interval 1.20–1.76; CRP odds ratio 1.29, 95% confidence interval 1.15–1.45) (Jenny et al., 2012). However, data from the CHS All Stars Study was not broken down for potential associations between levels of inflammatory markers and race (Jenny et al., 2012). A study based on the longitudinal HRS cohort found that African American individuals displayed higher CRP levels than both White and Hispanic individuals (Mitchell and Aneshensel, 2017). CRP levels of Hispanic individuals did not differ from White individuals (Mitchell and Aneshensel, 2017). A later study also performed on data from the HRS showed both higher baseline levels of CRP and greater increases in CRP over the study’s period in African American participants compared to White participants (Zahodne et al., 2019b). Hispanic participants also showed a higher baseline CRP than White participants but no difference in CRP over time was found (Zahodne et al., 2019b). Significant differences in other inflammatory markers, including receptor for advanced glycation end products, intracellular adhesion molecule-1, vascular cell adhesion protein-1, and monocyte chemo-attractant protein-1, have also been reported between African American and White women (Noren Hooten et al., 2012).
8.2. Oxidative stress, DNA damage, and inflamm-aging in minority populations
Inflammation that increases the pace of biological aging can arise from many potential pathways. Oxidative stress, and the DNA damage and damage-associated molecular patterns (DAMPs) that subsequently occur, has been linked to inflammation (Goldberg and Dixit, 2015; Kapetanovic et al., 2015). Inflammation is a response to oxidative damage, and often additional oxidative stress will occur from the inflammatory response (Venkataraman et al., 2013). Of interest is a potential difference in oxidative stress levels in varied populations, and how these differences contribute to inflamm-aging and development of age-related disease, especially given the unequal burden of age-related diseases in minority populations. African American individuals have been reported to have higher levels of oxidative stress compared to White individuals, even when differences in inflammation levels and risk factors for cardiovascular disease were controlled for (Morris et al., 2012). Chronic self-reported racial discrimination has been linked to markers of oxidative stress such as fluorescent heme degradation products in a diverse cohort from the HANDLS study (Szanton et al., 2012). Of interest is the observation that the results were only significant for African American individuals when the cohort was stratified by race (Szanton et al., 2012). Higher levels of oxidative stress in certain populations can lead to patterns of DNA damage or reduced DNA repair capacity. Fluorescent heme degradation products were found to be correlated with DNA single-strand breaks in African American men but not women (Trzeciak et al., 2012). Other markers of oxidative stress may or may not be associated with DNA damage: CRP was found to induce DNA base lesions but not single- or double-strand breaks in vitro (Noren Hooten et al., 2012). However, in a diverse cohort of women, the base lesion and oxidative stress marker 8-oxo-dG was significantly associated with serum CRP levels (Trzeciak et al., 2012). Finally, a racially diverse cohort of retired football players was examined for inflammatory markers and their association with atherosclerosis (Virani et al., 2012), as inflammation plays a key role in the development of atherosclerosis (Hansson, 2005; Libby et al., 2011). Atherosclerosis was associated with measures of atherogenic cholesterol and lipoproteins (Virani et al., 2012). CRP levels were not associated with atherosclerosis in the study; however, CRP was higher in individuals with metabolic syndrome (Virani et al., 2012).
The process of aging is complex and multifaceted. Aging is influenced by physical as well as social determinants of health. The contribution of inflammation to the process of aging must be clarified to better understand aging in underserved and disadvantaged populations.
9. Conclusions
Here, we have discussed factors that contribute to the accelerated aging phenotype frequently manifest in minority populations. The interplay of the upstream and downstream SDOH with complex biological factors and pathways are the root causes of disparate health outcomes with aging. Aging and health disparities operate through common converging pathways that ultimately result in similar health outcomes just with different trajectories. The different trajectories may be influenced by many factors including dysfunctional gene-environment interactions, the social and political determinants of health and numerous biologic transduction pathways that may be modulated through adversity. Although there is evidence that inflammation, oxidative stress, immune function and DNA repair may all play a role in the development of the accelerated aging phenotype of health disparities phenotype, the multi-level interacting factors and pathways are incompletely understood. Equally important in future research will be examination and identification of resilience factors that are present and may explain factors that underlie the Black-White cross over and the Hispanic survival paradox. However, we have yet to completely understand this process. Observational, longitudinal, and interdisciplinary studies that include minority populations with clinical, psychosocial and health outcome data are required as important cornerstones that would propel this area of research. As a guide for aging researchers interested in minority aging, we have included a table of studies cited in this review that include minority populations (Table 1). We hope this review and the information it provides will spur additional focus on the health disparities in aging and age-related diseases in minority populations.
Table 1.
Cohort studies with minority populations.
| Race/ethnicity distribution | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Full study name | Study website | Total | White | African American | Hispanic | Asian | NA/PIa | Otherb | Reference |
| Age/Aging-related | |||||||||
| Americans’ Changing Lives study | ACL | 3617 | 2443 | 1174 | (House, 2018) | ||||
| Advanced Cognitive Training for Independent and Vital Elderly | ACTIVE | 73.3% | 26% | 0.7% | (Tennstedt and Unverzagt, 2013) | ||||
| National Longitudinal Study of Adolescent to Adult Healthc | Add Health | 15,140 | 8372 | 3312 | 2393 | 1063 | (Popkin and Udry, 1998) | ||
| Baltimore Longitudinal Study of Aging | BLSA | 70% | 25% | 5% | (Shock, 1984) | ||||
| Chicago Health, Aging, and Social Relations Study | CHASRS | 229 | 35.8% | 35.4% | 28.8% | (Cacioppo and Cacioppo, 2018) | |||
| Healthy Aging in Neighborhoods of Diversity across the Life Span | HANDLS | 3720 | 1520 | 2200 | (Evans et al., 2010) | ||||
| Health and Retirement Study (total) | HRS | 42,226 | 29,148 | 7877 | 5201 | (Sonnega et al., 2014) | |||
| Long Life Family Study | LLFS | 3690 | 99.3% | 0.7% | (Newman et al., 2011) | ||||
| Hispanic Community Health Study/Study of Latinos | HCHS/SOL | 16,415 | 16,415d | (Lavange et al., 2010) | |||||
| Minority Aging Research Study | MARS | 366 | 366 | (Lewis et al., 2010) | |||||
| Minority Health Genomics and Translational Research Bio-Repository Database | MH-GRID | 1637 | 1637 | (Zilbermint et al., 2019) | |||||
| Mid-life in the United States Wave 1 + 2 | MIDUS | 7138 | 5860 | 962 | 316 | (Brim et al., 2020) | |||
| National Health and Nutrition Examination Survey | NHANES III | 18,825 | 83% | 12% | 5% | (Ezzati et al., 1992) | |||
| National Health and Nutrition Examination Survey | NHANES IV | 11,432 | 75.6% | 10.9% | 13.4% | (Liu et al., 2018) | |||
| National Health Interview Surveye | NHIS | 72,831 | 46,222 | 8487 | 12,481 | 4455 | 1186 | ((NCHS), 2018) | |
| Washington Heights and Inwood Community Aging projectf | WHICAP | −6000 | 19.4–29.5% | 30.7–34.1% | 38.5–47% | (Manly et al., 2005; Tang et al., 2001) | |||
| Women’s Health and Aging Studyg | WHAS | 1002 | 71.2% | 28.3% | (Onder et al., 2002) | ||||
| Age-related diseases | |||||||||
| Atherosclerosis Risk in Communities | ARIC | 15,792 | 4328 | (The ARIC investigators, 1989) | |||||
| Genetics of Pancreatic β-cell Failure in Mexican-Americans | BetaGene study | −2000 | 2000 | (Watanabe et al., 2007) | |||||
| Bogalusa Heart study | BHS | 12,138 | 7786 | 4352 | (Berenson, 2001) | ||||
| Boston Puerto Rican Health Study | BPRHS | 1200 | 1200h | (Tucker, 2005) | |||||
| Cardiovascular Health Study (+ All Stars) | CHS (+ All Stars) | 5888 | 16% | (Fried et al., 1991) | |||||
| DIet, Supplements, and Health Study | DISH | 155 | 79 | 76 | (Watters et al., 2007) | ||||
| Coronary Artery Risk Development in Young Adults | CARDIA | 5116 | 2472 | 2644 | (Friedman et al., 1988) | ||||
| Genetic Epidemiology Network of Arteriopathy | GENOA | 1583 | 1854 | 1812 | (Daniels et al., 2004) | ||||
| Health, Aging, and Body Composition | Health ABC | 3075 | 1794 | 1281 | (Santanasto et al., 2017) | ||||
| The Health & Aging Brain Study-Health Disparities | HABS-HD | 2000 | 1000 | 1000i | 1000 | (O’Bryant et al., 2013) | |||
| Indianapolis-Ibadan Dementia Project | IIDP | 8537 | 8537j | (Hendrie et al., 2001, 1995) | |||||
| Jackson Heart Study | JHS | 5301 | 5301 | (Fox et al., 2016) | |||||
| Massachusetts Hispanic Elders Study | MAHES | 1030 | 154 | 876 | (Falcon et al., 1997; Gao et al., 2004) | ||||
| Rush Memory and Aging Project | MAP | 1407 | 1298 | 109 | (Barnes et al., 2012b) | ||||
| MAP - Clinical Core of the Rush Alzheimer’s Disease Core Center | MAP-RADC Clinical Core | 218 | 218 | (Barnes et al., 2012b) | |||||
| Multi-Ethnic Study of Atherosclerosis | MESA | 6500 | 2623 | 1891 | 1496 | 804 | (Bild et al., 2002) | ||
| Morehouse and Emory Team up to Eliminate Health Disparities | META-Health | 3391 | 1936 | 1455 | (Morris et al., 2011, 2012) | ||||
| San Antonio Longitudinal Study of Agingk | SALSA (v2) | 749 | 355 | 394 | (Espinoza et al., 2010, 2013) | ||||
| Women’s Health Initiative baseline | WHI | 27,347 | 22,027 | 2741 | 1543 | 527 | 378 | 131 | (The Women’s Health Initiative Study Group, 1998) |
| Women’s Health Initiativel | WHI | 67,140 | 87% | 7% | 3% | 2% | 0% | 1% | (The Women’s Health Initiative Study Group, 1998) |
| Psychosocial factors | |||||||||
| Detroit Neighborhood Health Study | DNHS | 2081 | 192 | 1757 | 10 | 21 | 101 | (Goldmann et al., 2011) | |
| Family and Community Health Study | FCHS | 889 | 889 | (Simons et al., 2016) | |||||
| Grady Trauma Project | GTP | 12,000 | 12,000 | (Gillespie et al., 2009) | |||||
| Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure | InterGEN | 250 | 250 | (Taylor et al., 2016) | |||||
| Study of Women’s Health Across the Nation | SWAN | 3302 | 1550 | 935 | 286 | 531 | (Sowers et al., 2000) | ||
| Sacramento Area Latino Study on Aging | SALSA (v1) | 1789 | 1789 | (Haan et al., 2003) | |||||
| Veterans Aging Cohort Study | VACS | 5998 | 23.5% | 66.7% | 9.5% | (Justice et al., 2006) | |||
Study cohort information was based on publications, websites and in some cases communication from the study investigators.
Native American, American Indian, Alaskan Native, or Pacific Islander
Other/Missing/”Non-W”/Multi-Racial
DNA archived Wave IV
Hispanic or Latino
Taken from the NHIS 2018 Data Release, Person file variable frequencies document (“HHC.200_01.000: Race/ethnicity recode- HISCODI3” variable); sample sizes vary by years
A range of percentages were based on recent cohort description papers.
First WHAS cohort (only 3 years)
Hispanic adults of Puerto Rican origin
Future recruitment
Baseline enrollment: 2212 AA, 2494 Nigerians; 2nd enrollment stage: 1892 AA, 1939 Nigerians
The SALSA cohort participants were recruited from the San Antonio Heart Study (SAHS) cohort (PMID: 6507426) and numbers were estimated based on publications.
Numbers were taken from the “About WHI” on WHI website, and reflect Active WHI participants as of March 2019
Future directions
Aging research must be a place of equity for groups from all segments of society, and we must also focus our research efforts to be inclusive of all; racial/ethnic minorities, low SES, as well as considerations of geographic location. We should actively pursue facilitated discussions to make aging researchers leaders in the important step on this journey to diversify the biomedical workforce and to understand the differential influence of SDOH and political determinants of health on aging. The ultimate goal is to inform appropriate interventions to ameliorate these factors, improve health span, and lengthen lifespan. Addressing upstream SDOH especially structural racism will hopefully reduce the harmful stress exposures among all minority groups African Americans, Hispanics, Native Americans/Alaska Natives, and Asian/Native Hawaiians and Pacific islanders over the lifespan and eliminate health disparities and insure health equity for all.
Acknowledgements
This work was funded by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. NLP was also funded by a postdoctoral fellowship through the National Institute on Minority Health and Health Disparities (NIMHD) Intramural Research Program. The authors wish to thank past and present members of the laboratory and HANDLS staff and participants for their contributions to past and ongoing research. Some figures were created with BioRender. com.
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
References
- Ahmad FB, Cisewski JA, Minino A, Anderson RN, 2021. Provisional mortality data - United States, 2020. MMWR Morb. Mortal. Wkly Rep 70, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Conway C, 2020. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J. Soc., Behav., Health Sci 14, 153–168. [Google Scholar]
- Akerblom JL, Costa R, Luchsinger JA, Manly JJ, Tang MX, Lee JH, Mayeux R, Schupf N, 2008. Relation of plasma lipids to all-cause mortality in caucasian, African-American and Hispanic elders. Age Ageing 37, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA, Cozier Y, Ridker PM, Palmer JR, Glynn RJ, Rose L, Halevy N, Rosenberg L, 2010. Perceptions of race/ethnic discrimination in relation to mortality among black women: results from the black women’s health study. Arch. Intern. Med 170, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegría-Torres JA, Baccarelli A, Bollati V, 2011. Epigenetics and lifestyle. Epigenomics 3, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S, 2006. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol 48, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Almeida I, Magalhaes S, Nunes A, 2021. Lipids: biomarkers of healthy aging. Biogerontology. doi: 10.1007/s10522-021-09921-2. Epub 2021 Apr 10. [DOI] [PubMed] [Google Scholar]
- Ammous F, Zhao W, Ratliff SM, Mosley TH, Bielak LF, Zhou X, Peyser PA, Kardia SLR, Smith JA, 2021. Epigenetic age acceleration is associated with cardiometabolic risk factors and clinical cardiovascular disease risk scores in African Americans. Clin. Epigenetics 13, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021b 2021b. Health, United States, 2019. National Center for Health Statistics, Hyattsville, Maryland. [PubMed] [Google Scholar]
- (NCHS), N.C.f.H.S., 2018. 2018 Data Release. Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- 2019a 2019a. 2018 Profile for American Indians and Alaska Natives Age 65 and over. Administration for Community Living Administration on Aging. [Google Scholar]
- 2020a. National Diabetes Statistics Report 2020. Centers of Disease Control and Prevention, Atlanta, Georgia. [Google Scholar]
- 2019d 2019d. 2018 Profile of Hispanic Americans Age 65 and over. Administration for Community Living, Administration on Aging, Washington, D.C. [Google Scholar]
- 2021a. Cancer Facts and Figures for African Americans 2019–2021 In: Society, A.C. (Ed.), Cancer Facts and Figures, Atlanta, Georgia. [Google Scholar]
- 2020b. Profile of Older Americans 2019. Administration for Community Living. 2020b. Profile of Older Americans 2019. Administration for Community Living. [Google Scholar]
- 2019c 2019c. 2018 Profile of Asian Americans Age 65 and over Administration for Community Living, Administration on Aging; Washington, D.C. [Google Scholar]
- 2019b 2019b. 2018 Profile of African Americans Age 65 and over. Administration for Community Living Administration on Aging; Washington, D.C. [Google Scholar]
- 2015 2015. Capturing social and behavioral domains and measures in electronic health records National Academy of Sciences, Medicine, and Engineering, Washington, D.C. [Google Scholar]
- 2010 2010. Recommendations for the framework and format of Healthy People 2020, Washington, D.C. [Google Scholar]
- Arias E, 2006. United States life tables, 2003. Natl. Vital. Stat. Rep 54, 1–40. [PubMed] [Google Scholar]
- Arias E, Xu J, 2020. United States Life Tables, 2018. Natl. Vital. Stat. Rep 69, 1–45. [PubMed] [Google Scholar]
- Arias E, Tejada-Vera B, Ahmad F, 2021. Provisional Life Expectancy Estimates for January through June 2020, Vital Statistics Rapid Release. National Center for Health Statistics, Hyattsville, Maryland. [Google Scholar]
- Arnold NS, Noren Hooten N, Zhang Y, Lehrmann E, Wood W 3rd, Camejo Nunez W, Thorpe RJ Jr., Evans MK, Dluzen DF, 2020. The association between poverty and gene expression within peripheral blood mononuclear cells in a diverse Baltimore City cohort. In: PLoS One, 15, e0239654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H, Cheung I, Ames BN, 2000. A method for detecting abasic sites in living cells: age-dependent changes in base excision repair. Proc. Natl. Acad. Sci. USA 97, 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharian S, Barakatt M, Gignoux CR, Shringarpure S, Errington J, Blot WJ, Bustamante CD, Kenny EE, Williams SM, Aldrich MC, Gravel S, 2016. The great migration and African-American genomic diversity. PLoS Genet. 12, e1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda Y, Kvale MN, Hoffmann TJ, Hesselson SE, Ranatunga D, Tang H, Sabatti C, Croen LA, Dispensa BP, Henderson M, Iribarren C, Jorgenson E, Kushi LH, Ludwig D, Olberg D, Quesenberry CP Jr., Rowell S, Sadler M, Sakoda LC, Sciortino S, Shen L, Smethurst D, Somkin CP, Van Den Eeden SK, Walter L, Whitmer RA, Kwok PY, Schaefer C, Risch N, 2015. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics 200, 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, de Leon CFM, Lewis TT, Bienias JL, Wilson RS, Evans DA, 2008. Perceived discrimination and mortality in a population-based study of older adults. Am. J. Public Health 98, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, Wilson RS, 2012a. Perceived discrimination and cognition in older African Americans. J. Int. Neuropsychol. Soc 18, 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA, 2012b. The minority aging research study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr. Alzheimer Res 9, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Chang Y, Brown C, Bromberger JT, Matthews KA, 2018. Everyday discrimination and metabolic syndrome incidence in a racially/ethnically diverse sample: study of women’s health across the nation. Psychosom. Med 80, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Leibel DK, Darden TM, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, Weng N-P, Evans MK, Zonderman AB, 2019a. Interpersonal-level discrimination indices, sociodemographic factors, and telomere length in African-Americans and Whites. Biol. Psychol 141, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Taylor AD, Leibel DK, Al-Najjar E, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, Kouo T, Erus G, Rosenberger WF, Evans MK, Zonderman AB, Waldstein SR, 2019b. Lifetime discrimination burden, racial discrimination, and subclinical cerebrovascular disease among African Americans. Health Psychol. 38, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Leibel DK, Pantesco EJ, Wendell CR, Waldstein SR, Evans MK, Zonderman AB, 2020. Interactive relations across dimensions of interpersonal-level discrimination and depressive symptoms to carotid intimal-medial thickening in African Americans. Psychosom. Med 82, 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson GS, 2001. Bogalusa heart study: a long-term community study of a rural biracial (black/white) population. Am. J. Med. Sci 322, 293–300. [PubMed] [Google Scholar]
- Bermudez OI, Velez-Carrasco W, Schaefer EJ, Tucker KL, 2002. Dietary and plasma lipid, lipoprotein, and apolipoprotein profiles among elderly Hispanics and non-Hispanics and their association with diabetes. Am. J. Clin. Nutr 76, 1214–1221. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Weiss J, Obhi HK, Beydoun HA, Dore GA, Liang H, Evans MK, Zonderman AB, 2019. Cytokines are associated with longitudinal changes in cognitive performance among urban adults. Brain Behav. Immun 80, 474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Shaked D, Tajuddin SM, Weiss J, Evans MK, Zonderman AB, 2020. Accelerated epigenetic age and cognitive decline among urban-dwelling adults. Neurology 94, e613–e625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP, 2002. Multi-ethnic study of atherosclerosis: objectives and design. Am. J. Epidemiol 156, 871–881. [DOI] [PubMed] [Google Scholar]
- 2004. Measuring Racial Discrimination.Panel on Methods for Assessing Discrimination, in: Blank R,M, Dabady M, Citro C,F (Eds.). National Research Council, Washington, D.C. [Google Scholar]
- Boen CE, Hummer RA, 2019. Longer-but harder-lives?: the hispanic health paradox and the social determinants of racial, ethnic, and immigrant-native health disparities from midlife through late life. J. Health Soc. Behav 60, 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Kiefe CI, Diez-Roux AV, Williams DR, Gordon-Larsen P, 2013. Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA study. Ethn. Health 18, 227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, Bibbins-Domingo K, Rodríguez-Santana JR, Lenoir MA, Gavin JR, Kittles RA, Zaitlen NA, Wilkes DS, Powe NR, Ziv E, Burchard EG, 2021. Race and genetic ancestry in medicine — a time for reckoning with racism. N. Engl. J. Med 384, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P, Gottlieb L, 2014. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 129 (Suppl 2), 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P, Egerter S, Williams DR, 2011. The social determinants of health: coming of age. Annu. Rev. Public Health 32, 381–398. [DOI] [PubMed] [Google Scholar]
- Bressler J, Marioni RE, Walker RM, Xia R, Gottesman RF, Windham BG, Grove ML, Guan W, Pankow JS, Evans KL, McIntosh AM, Deary IJ, Mosley TH, Boerwinkle E, Fornage M, 2020. Epigenetic age acceleration and cognitive function in african american adults in midlife: the atherosclerosis risk in communities study. J. Gerontol. A Biol. Sci. Med. Sci 75, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, Kessler RC, Lachman ME, Markus HR, Marmot MG, Rossi AS, Ryff CD, Shweder RA, 2020. Midlife in the United States (MIDUS 1), 1995–1996. Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- Brown CS, Baker TA, Mingo CA, Harden JT, Whitfield K, Aiken-Morgan AT, Phillips KL, Washington T, 2014. A review of our roots: blacks in gerontology. Gerontologist 54, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Needham B, Ailshire J, 2017. Telomere length among older u.s. adults: differences by race/ethnicity, gender, and age. J. Aging Health 29, 1350–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryc K, Auton A, Nelson MR, Oksenberg JR, Hauser SL, Williams S, Froment A, Bodo JM, Wambebe C, Tishkoff SA, Bustamante CD, 2010a. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc. Natl. Acad. Sci. U.S.A 107, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryc K, Velez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, Hammer M, Bustamante CD, Ostrer H, 2010b. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc. Natl. Acad. Sci. USA 107 (2), 8954–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs Pena MS, Mbassa RS, Slopen NB, Williams DR, Buring JE, Albert MA, 2019. Cumulative psychosocial stress and ideal cardiovascular health in older women. Circulation 139, 2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, 2018. The population-based longitudinal Chicago Health, Aging, and Social Relations Study (CHASRS): study description and predictors of attrition in older adults. Arch. Sci. Psychol 6, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, Tishkoff SA, 2008. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev. Genom. Hum. Genet 9, 403–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campinha-Bacote J, 2002. The process of cultural competence in the delivery of healthcare services: a model of care. J. Transcult. Nurs 13, 181–184 discussion 200–181. [DOI] [PubMed] [Google Scholar]
- Campinha-Bacote J, 2009. A culturally competent model of care for African Americans. Urol. Nurs 29, 49–54. [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, Epel ES, 2014. Discrimination, racial bias, and telomere length in African-American men. Am. J. Prev. Med 46, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, Blackburn EH, Thomas SB, 2016. Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology 63, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Walker GC, 2017. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen 58, 235–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Guan W, Bressler J, Fornage M, Studenski S, Vandiver AR, Moore AZ, Tanaka T, Kiel DP, Liang L, Vokonas P, Schwartz J, Lunetta KL, Murabito JM, Bandinelli S, Hernandez DG, Melzer D, Nalls M, Pilling LC, Price TR, Singleton AB, Gieger C, Holle R, Kretschmer A, Kronenberg F, Kunze S, Linseisen J, Meisinger C, Rathmann W, Waldenberger M, Visscher PM, Shah S, Wray NR, McRae AF, Franco OH, Hofman A, Uitterlinden AG, Absher D, Assimes T, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Carty CL, LaCroix AZ, Reiner AP, Spector TD, Feinberg AP, Levy D, Baccarelli A, van Meurs J, Bell JT, Peters A, Deary IJ, Pankow JS, Ferrucci L, Horvath S, 2016. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8, 1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Weuve J, Misra S, Cuevas A, Kubzansky LD, Williams DR, 2021. Racial disparities in cognitive function among middle-aged and older adults: the roles of cumulative stress exposures across the life course. J. Gerontol. Ser. A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Geng A, Zhang W, Qian Z, Wan X, Jiang Y, Mao Z, 2020. Fight to the bitter end: DNA repair and aging. Ageing Res. Rev 64, 101154. [DOI] [PubMed] [Google Scholar]
- Chevanne M, Caldini R, Tombaccini D, Mocali A, Gori G, Paoletti F, 2003. Comparative levels of DNA breaks and sensitivity to oxidative stress in aged and senescent human fibroblasts: a distinctive pattern for centenarians. Biogerontology 4, 97–104. [DOI] [PubMed] [Google Scholar]
- Chitrala KN, Hernandez DG, Nalls MA, Mode NA, Zonderman AB, Ezike N, Evans MK, 2020. Race-specific alterations in DNA methylation among middle-aged African Americans and Whites with metabolic syndrome. Epigenetics 15, 462–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SL, Ortman JM, 2014. Projections of the Size and Composition of the U.S. Population: 2014–2060, in: Bureau, U.S.C. (Ed.), Washington, D.C. [Google Scholar]
- Cole SW, 2019. The conserved transcriptional response to adversity. Curr. Opin. Behav. Sci 28, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conomos MP, Laurie CA, Stilp AM, Gogarten SM, McHugh CP, Nelson SC, Sofer T, Fernandez-Rhodes L, Justice AE, Graff M, Young KL, Seyerle AA, Avery CL, Taylor KD, Rotter JI, Talavera GA, Daviglus ML, Wassertheil-Smoller S, Schneiderman N, Heiss G, Kaplan RC, Franceschini N, Reiner AP, Shaffer JR, Barr RG, Kerr KF, Browning SR, Browning BL, Weir BS, Aviles-Santa ML, Papanicolaou GJ, Lumley T, Szpiro AA, North KE, Rice K, Thornton TA, Laurie CC, 2016. Genetic diversity and association studies in us hispanic/latino populations: applications in the hispanic community health study/study of latinos. Am. J. Hum. Genet 98, 165–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RS, Rotimi CN, 2020. The practice of anti-racist science requires an internationalist perspective. Am. J. Hum. Genet 107, 793–796. [Google Scholar]
- Cree BA, Reich DE, Khan O, De Jager PL, Nakashima I, Takahashi T, Bar-Or A, Tong C, Hauser SL, Oksenberg JR, 2009. Modification of multiple sclerosis phenotypes by African Ancestry at HLA. Arch. Neurol 66, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T, 2007. Hispanic paradox in biological risk profiles. Am. J. Public Health 97, 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas AG, Ong AD, Carvalho K, Ho T, Chan SW, Allen JD, Chen R, Rodgers J, Biba U, Williams DR, 2020. Discrimination and systemic inflammation: a critical review and synthesis. Brain Behav. Immun 89, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels PR, Kardia SL, Hanis CL, Brown CA, Hutchinson R, Boerwinkle E, Turner ST, 2004. Familial aggregation of hypertension treatment and control in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Am. J. Med 116, 676–681. [DOI] [PubMed] [Google Scholar]
- Dawes DE, 2020. The Political Determinants of Health. Johns Hopkins University Press, Baltimore, Maryland. [Google Scholar]
- Daya M, Rafaels N, Brunetti TM, Chavan S, Levin AM, Shetty A, Gignoux CR, Boorgula MP, Wojcik G, Campbell M, Vergara C, Torgerson DG, Ortega VE, Doumatey A, Johnston HR, Acevedo N, Araujo MI, Avila PC, Belbin G, Bleecker E, Bustamante C, Caraballo L, Cruz A, Dunston GM, Eng C, Faruque MU, Ferguson TS, Figueiredo C, Ford JG, Gan W, Gourraud PA, Hansel NN, Hernandez RD, Herrera-Paz EF, Jimenez S, Kenny EE, Knight-Madden J, Kumar R, Lange LA, Lange EM, Lizee A, Maul P, Maul T, Mayorga A, Meyers D, Nicolae DL, O’Connor TD, Oliveira RR, Olopade CO, Olopade O, Qin ZS, Rotimi C, Vince N, Watson H, Wilks RJ, Wilson JG, Salzberg S, Ober C, Burchard EG, Williams LK, Beaty TH, Taub MA, Ruczinski I, Mathias RA, Barnes KC, Caapa, 2019. Association study in African-admixed populations across the Americas recapitulates asthma risk loci in non-African populations. Nat. Commun 10, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Evans DS, Arking DE, Tesi N, Nygaard M, Liu X, Wojczynski MK, Biggs ML, van der Spek A, Atzmon G, Ware EB, Sarnowski C, Smith AV, Seppala I, Cordell HJ, Dose J, Amin N, Arnold AM, Ayers KL, Barzilai N, Becker EJ, Beekman M, Blanche H, Christensen K, Christiansen L, Collerton JC, Cubaynes S, Cummings SR, Davies K, Debrabant B, Deleuze JF, Duncan R, Faul JD, Franceschi C, Galan P, Gudnason V, Harris TB, Huisman M, Hurme MA, Jagger C, Jansen I, Jylha M, Kahonen M, Karasik D, Kardia SLR, Kingston A, Kirkwood TBL, Launer LJ, Lehtimaki T, Lieb W, Lyytikainen LP, Martin-Ruiz C, Min J, Nebel A, Newman AB, Nie C, Nohr EA, Orwoll ES, Perls TT, Province MA, Psaty BM, Raitakari OT, Reinders MJT, Robine JM, Rotter JI, Sebastiani P, Smith J, Sorensen TIA, Taylor KD, Uitterlinden AG, van der Flier W, van der Lee SJ, van Duijn CM, van Heemst D, Vaupel JW, Weir D, Ye K, Zeng Y, Zheng W, Holstege H, Kiel DP, Lunetta KL, Slagboom PE, Murabito JM, 2019. A meta-analysis of genome-wide association studies identifies multiple longevity genes. Nat. Commun 10, 3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T, 2009. Race/ethnicity and telomere length in the multi-ethnic study of atherosclerosis. Aging Cell 8, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Bird CE, Furumoto-Dawson A, Rauscher GH, Ruffin M.Tt, Stowe RP, Tucker KL, Masi CM, 2008. Biomarkers of psychological stress in health disparities research. Open Biomark. J 1, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlay SM, Lippmann SJ, Greiner MA, O’Brien EC, Chamberlain AM, Mentz RJ, Sims M, 2017. Perceived discrimination and cardiovascular outcomes in older African Americans: insights from the jackson heart study. Mayo Clin. Proc 92, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Green J, Bodogai M, Mode NA, Bæk R, Jørgensen MM, Freeman DW, Witwer KW, Zonderman AB, Biragyn A, Mattson MP, Noren Hooten N, Evans MK, 2017. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci. Rep 1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeny RT, Carpenter DO, Lawrence DA, 2021. Health disparities: intracellular consequences of social determinants of health. Toxicol. Appl. Pharmacol 416, 115444. [DOI] [PubMed] [Google Scholar]
- Espinoza SE, Jung I, Hazuda H, 2010. Lower frailty incidence in older Mexican Americans than in older European Americans: the san antonio longitudinal study of aging. J. Am. Geriatr. Soc 58, 2142–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza SE, Jung I, Hazuda H, 2013. The Hispanic paradox and predictors of mortality in an aging biethnic cohort of Mexican Americans and European Americans: the san antonio longitudinal study of aging. J. Am. Geriatr. Soc 61, 1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB, 2010. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn. Dis 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, Lutsey PL, Roetker NS, Lewis TT, Kershaw KN, Alonso A, Diez Roux AV, 2015. Perceived discrimination and incident cardiovascular events: the multi-ethnic study of atherosclerosis. Am. J. Epidemiol 182, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR, 1992. Sample design: third national health and nutrition examination survey. Vital. Health Stat 2, 1–35. [PubMed] [Google Scholar]
- Falcon L, Tucker K, Bermudez O, 1997. Correlates of Poverty and Participation in Food Assistance Programs among Hispanic Elders in Massachusetts. University of Wisconsin Institute for Research on Poverty, Institute for Research on Poverty Discussion Papers. [Google Scholar]
- Farmer HR, Wray LA, Thomas JR, 2019. Do race and everyday discrimination predict mortality risk? evidence from the health and retirement study. Gerontol. Geriatr. Med 5, 2333721419855665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon A, Chinn JJ, Anderson RN, 2017. A comprehensive analysis of the mortality experience of hispanic subgroups in the United States: variation by age, country of origin, and nativity. SSM Popul. Health 3, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, Sun K, Woodman RC, Andrews NC, Cotter RJ, Ganz T, Nemeth E, Longo DL, 2010. Proinflammatory state, hepcidin, and anemia in older persons. Blood 115, 3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A, 2011. Leukocyte telomere length and mortality in the cardiovascular health study. J. Gerontol. A Biol. Sci. Med. Sci 66, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde AT, Sims M, Muntner P, Lewis T, Onwuka A, Moore K, Diez Roux AV, 2020. Discrimination and hypertension risk among African Americans in the jackson heart study. Hypertension 76, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde AT, Lewis TT, Kershaw KN, Bellamy SL, Roux AVD, 2021. Perceived discrimination and hypertension risk among participants in the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc 10, e019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ER, Samdarshi TE, Musani SK, Pencina MJ, Sung JH, Bertoni AG, Xanthakis V, Balfour PC Jr., Shreenivas SS, Covington C, Liebson PR, Sarpong DF, Butler KR, Mosley TH, Rosamond WD, Folsom AR, Herrington DM, Vasan RS, Taylor HA, 2016. Development and validation of risk prediction models for cardiovascular events in black adults: the jackson heart study cohort. JAMA Cardiol. 1, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N Y Acad. Sci 908, 244–254. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A, 2018. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol 14, 576–590. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, 1991. The cardiovascular health study: design and rationale. Ann. Epidemiol 1, 263–276. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K, Savage PJ, 1988. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol 41, 1105–1116. [DOI] [PubMed] [Google Scholar]
- Gao X, Bermudez OI, Tucker KL, 2004. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in hispanic and non-hispanic white elders. J. Nutr 134, 913–918. [DOI] [PubMed] [Google Scholar]
- Garcia C, Ailshire JA, 2019. Biological risk profiles among latino subgroups in the health and retirement study. Innov. Aging 3 igz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Garcia C, Chiu CT, Raji M, Markides KS, 2018. A comprehensive analysis of morbidity life expectancies among older hispanic subgroups in the united states: variation by nativity and country of origin. Innov. Aging 2 igy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garm C, Moreno-Villanueva M, Bürkle A, Petersen I, Bohr VA, Christensen K, Stevnsner T, 2013. Age and gender effects on DNA strand break repair in peripheral blood mononuclear cells. Aging Cell 12, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaye A, Gibbons GH, Barry C, Quarells R, Davis SK, 2017. Influence of socioeconomic status on the whole blood transcriptome in African Americans. PLoS One 12, e0187290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert S, Sohmer D, Sacks T, Mininger C, McClintock M, Olopade O, 2008. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff. 27, 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensler HL, Bernstein H, 1981. DNA damage as the primary cause of aging. Q. Rev. Biol 56, 279–303. [DOI] [PubMed] [Google Scholar]
- Geoffroy E, Gregga I, Wheeler HE, 2020. Population-matched transcriptome prediction increases twas discovery and replication rate. Science 23, 101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, 1992. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn. Dis 2, 207–221. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J, 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 96, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RC, 1988. Minority aging research: opportunity and challenge. Gerontologist 28, 559–560. [DOI] [PubMed] [Google Scholar]
- Gibson RC, 1994. The age-by-race gap in health and mortality in the older population: a social science research agenda. Gerontologist 34, 454–462. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ, 2009. Trauma exposure and stress-related disorders in inner city primary care patients. Gen. Hosp. Psychiatry 31, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EL, Dixit VD, 2015. Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev 265, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, Galea S, 2011. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: the detroit neighborhood health study. J. Trauma Stress 24, 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goukassian D, Gad F, Yaar M, Eller MS, Nehal US, Gilchrest BA, 2000. Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J. 14, 1325–1334. [DOI] [PubMed] [Google Scholar]
- Gubernskaya Z, 2015. Age at migration and self-rated health trajectories after age 50: understanding the older immigrant health paradox. J. Gerontol. B Psychol. Sci. Soc. Sci 70, 279–290. [DOI] [PubMed] [Google Scholar]
- Guidi J, Lucente M, Sonino N, Fava GA, 2021. Allostatic load and its impact on health: a systematic review. Psychother. Psychosom 90, 11–27. [DOI] [PubMed] [Google Scholar]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ, 2003. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J. Am. Geriatr. Soc 51, 169–177. [DOI] [PubMed] [Google Scholar]
- Han SD, Lamar M, Fleischman D, Kim N, Bennett DA, Lewis TT, Arfanakis K, Barnes LL, 2020. Self-reported experiences of discrimination in older black adults are associated with insula functional connectivity. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, 2005. Inflammation, atherosclerosis, and coronary artery disease. New Engl. J. Med 352, 1685–1695. [DOI] [PubMed] [Google Scholar]
- Harman D, 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol 11, 298–300. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, Gureje O, Rodenberg CA, Baiyewu O, Musick BS, 1995. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am. J. Psychiatry 152, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Gao S, Evans RM, Ogunseyinde AO, Adeyinka AO, Musick B, Hui SL, 2001. Incidence of dementia and Alzheimer disease in 2 communities: yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA 285, 739–747. [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Rice KM, Lange LA, Diehr P, Halder I, Walston J, Kwok P, Ziv E, Nievergelt C, Cummings SR, Newman AB, Tracy RP, Psaty BM, Reiner AP, 2008. Common variants in the CRP gene in relation to longevity and cause-specific mortality in older adults: the cardiovascular health study. Atherosclerosis 197, 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, Ritz BR, Chen B, Lu AT, Rickabaugh TM, Jamieson BD, Sun D, Li S, Chen W, Quintana-Murci L, Fagny M, Kobor MS, Tsao PS, Reiner AP, Edlefsen KL, Absher D, Assimes TL, 2016. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 17, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, 2018. Americans’ Changing Lives: Waves I, II, III, IV, and V, 1986, 1989, 1994, 2002, and 2011. Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- Huang H-Y, Helzlsouer KJ, Appel LJ, 2000. The effects of vitamin C and vitamin E on oxidative DNA damage: results from a randomized controlled trial. Cancer epidemiol. Biomark. Prev 9, 647–652. [PubMed] [Google Scholar]
- Institute of Medicine, 2000. Promoting Health Intervention Strategies from Social and Behavioral Research. The National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- Jackson JJ, 1967. Social gerontology and the Negro: a review. Gerontologist 7, 168–178. [DOI] [PubMed] [Google Scholar]
- Jackson JJ, 1974. The national center on black aged: a challenge to gerontologists. Gerontologist 14 (194), 196. [DOI] [PubMed] [Google Scholar]
- Jackson JS, 2011. Racial and Ethnic Minority Group Disparities and the Affordance Model. The Gerontological Society of America, Boston, MA. [Google Scholar]
- Jacob KD, Noren Hooten N, Trzeciak AR, Evans MK, 2013. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev 134, 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PHM, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, Sarnak MJ, Newman AB, 2012. Long-term assessment of inflammation and healthy aging in late life: the cardiovascular health study all stars. J. Gerontol. Ser. A 67, 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NE, 2000. The racial crossover in comorbidity, disability, and mortality. Demography 37, 267–283. [PubMed] [Google Scholar]
- Jones L,R, 1989. Black Adult Development and Aging. Cobb and Henry, Berkeley, California. [Google Scholar]
- Joshi PK, Pirastu N, Kentistou KA, Fischer K, Hofer E, Schraut KE, Clark DW, Nutile T, Barnes CLK, Timmers P, Shen X, Gandin I, McDaid AF, Hansen TF, Gordon SD, Giulianini F, Boutin TS, Abdellaoui A, Zhao W, Medina-Gomez C, Bartz TM, Trompet S, Lange LA, Raffield L, van der Spek A, Galesloot TE, Proitsi P, Yanek LR, Bielak LF, Payton A, Murgia F, Concas MP, Biino G, Tajuddin SM, Seppala I, Amin N, Boerwinkle E, Borglum AD, Campbell A, Demerath EW, Demuth I, Faul JD, Ford I, Gialluisi A, Gogele M, Graff M, Hingorani A, Hottenga JJ, Hougaard DM, Hurme MA, Ikram MA, Jylha M, Kuh D, Ligthart L, Lill CM, Lindenberger U, Lumley T, Magi R, Marques-Vidal P, Medland SE, Milani L, Nagy R, Ollier WER, Peyser PA, Pramstaller PP, Ridker PM, Rivadeneira F, Ruggiero D, Saba Y, Schmidt R, Schmidt H, Slagboom PE, Smith BH, Smith JA, Sotoodehnia N, Steinhagen-Thiessen E, van Rooij FJA, Verbeek AL, Vermeulen SH, Vollenweider P, Wang Y, Werge T, Whitfield JB, Zonderman AB, Lehtimaki T, Evans MK, Pirastu M, Fuchsberger C, Bertram L, Pendleton N, Kardia SLR, Ciullo M, Becker DM, Wong A, Psaty BM, van Duijn CM, Wilson JG, Jukema JW, Kiemeney L, Uitterlinden AG, Franceschini N, North KE, Weir DR, Metspalu A, Boomsma DI, Hayward C, Chasman D, Martin NG, Sattar N, Campbell H, Esko T, Kutalik Z, Wilson JF, 2017. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat. Commun 8, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, Bryant K, 2006. Veterans Aging Cohort Study (VACS): overview and description. Med. Care 44, S13–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, LeBleu VS, 2020. The biology, function, and biomedical applications of exosomes. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Chiang CW, Palmer CD, Tayo BO, Lettre G, Butler JL, Hackett R, Adeyemo AA, Guiducci C, Berzins I, Nguyen TT, Feng T, Luke A, Shriner D, Ardlie K, Rotimi C, Wilks R, Forrester T, McKenzie CA, Lyon HN, Cooper RS, Zhu X, Hirschhorn JN, 2010. Genome-wide association of anthropometric traits in African- and African-derived populations. Hum. Mol. Genet 19, 2725–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic R, Bokil NJ, Sweet MJ, 2015. Innate immune perturbations, accumulating DAMPs and inflammasome dysregulation: A ticking time bomb in ageing. Ageing Res. Rev 24, 40–53. [DOI] [PubMed] [Google Scholar]
- Ke W, Rand KA, Conti DV, Setiawan VW, Stram DO, Wilkens L, Le Marchand L, Assimes TL, Haiman CA, 2018. Evaluation of 71 coronary artery disease risk variants in a multiethnic cohort. Front Cardiovasc. Med 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita SO, Kittles RA, Royal CD, Bonney GE, Furbert-Harris P, Dunston GM, Rotimi CN, 2004. Conceptualizing human variation. Nat. Genet 36, S17–S20. [DOI] [PubMed] [Google Scholar]
- Kershaw KN, Lewis TT, Diez Roux AV, Jenny NS, Liu K, Penedo FJ, Carnethon MR, 2016. Self-reported experiences of discrimination and inflammation among men and women: the multi-ethnic study of atherosclerosis. Health Psychol. 35, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys KL, Mak ACY, White MJ, Eckalbar WL, Dahl AW, Mefford J, Mikhaylova AV, Contreras MG, Elhawary JR, Eng C, Hu D, Huntsman S, Oh SS, Salazar S, Lenoir MA, Ye JC, Thornton TA, Zaitlen N, Burchard EG, Gignoux CR, 2020. On the cross-population generalizability of gene expression prediction models. PLoS Genet 16, e1008927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Nho K, Ramanan VK, Lai D, Foroud TM, Lane K, Murrell JR, Gao S, Hall KS, Unverzagt FW, Baiyewu O, Ogunniyi A, Gureje O, Kling MA, Doraiswamy PM, Kaddurah-Daouk R, Hendrie HC, Saykin AJ, 2016. Genetic influences on plasma homocysteine levels in African Americans and Yoruba Nigerians. J. Alzheimers Dis 49, 991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek KD, Xu J, Arias E, 2020. Mortality in the United States, 2019. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Lahiri DK, Xu Y, Klaunig J, Baiyewu O, Ogunniyi A, Hall K, Hendrie H, Sahota A, 1999. Effect of oxidative stress on DNA damage and beta-amyloid precursor proteins in lymphoblastoid cell lines from a Nigerian population. Ann. N Y Acad. Sci 893, 331–336. [DOI] [PubMed] [Google Scholar]
- Lariscy JT, Hummer RA, Hayward MD, 2015. Hispanic older adult mortality in the United States: new estimates and an assessment of factors shaping the Hispanic paradox. Demography 52, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP, 2010. Sample design and cohort selection in the Hispanic community health study/study of latinos. Ann. Epidemiol 20, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo S, Noren Hooten N, Green J, Eitan E, Mode NA, Liu Q-R, Zonderman AB, Ezike N, Mattson MP, Ghosh P, Evans MK, 2021. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 20, e13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goallec A, Patel CJ, 2019. Age-dependent co-dependency structure of biomarkers in the general population of the United States. Aging 11, 1404–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Flaquer A, Costa R, Andrews H, Cross P, Lantigua R, Schupf N, Tang MX, Mayeux R, 2004. Genetic influences on life span and survival among elderly African-Americans, Caribbean Hispanics, and Caucasians. Am. J. Med. Genet A 128A, 159–164. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Rittschof CC, Greenlee AJ, Turi KN, Rodriguez-Zas SL, Robinson GE, Cole SW, Mendenhall R, 2021. Transcriptomic analyses of black women in neighborhoods with high levels of violence. Psychoneuroendocrinology 127, 105174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Ho N, Gelbart T, Beutler E, 2003. Polymorphisms in the human homologue of the drosophila Indy (I’m not dead yet) gene. Mech. Ageing Dev 124, 897–902. [DOI] [PubMed] [Google Scholar]
- Lei M-K, Simons RL, Beach SRH, Philibert RA, 2017. Neighborhood disadvantage and biological aging: using marginal structural models to assess the link between neighborhood census variables and epigenetic aging. J. Gerontol. Ser. B 74, e50–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng S, Chaves P, Koenig K, Walston J, 2002. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J. Am. Geriatr. Soc 50, 1268–1271. [DOI] [PubMed] [Google Scholar]
- Levine ME, Crimmins EM, 2014. Evidence of accelerated aging among African Americans and its implications for mortality. Soc. Sci. Med 118, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S, 2018. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL, 2010. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav. Immun 24, 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Williams DR, Tamene M, Clark CR, 2014. Self-reported experiences of discrimination and cardiovascular disease. Curr. Cardiovasc. Risk Rep 8, 365–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang W, Chen Y, Guo W, Zhang J, Tang H, Xu Z, Zhang H, Tao Y, Wang F, Jiang Y, Sun FL, Mao Z, 2016. Impaired DNA double-strand break repair contributes to the age-associated rise of genomic instability in humans. Cell Death Differ. 23, 1765–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK, 2011. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325. [DOI] [PubMed] [Google Scholar]
- Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M, 2018. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 15, e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S, 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Rebholz CM, Braun KVE, Reynolds LM, Aslibekyan S, Xia R, Biligowda NG, Huan T, Liu C, Mendelson MM, Joehanes R, Hu EA, Vitolins MZ, Wood AC, Lohman K, Ochoa-Rosales C, Meurs Jv, Uitterlinden A, Liu Y, Elhadad MA, Heier M, Waldenberger M, Peters A, Colicino E, Whitsel EA, Baldassari A, Gharib SA, Sotoodehnia N, Brody JA, Sitlani CM, Tanaka T, Hill WD, Corley J, Deary IJ, Zhang Y, Schöttker B, Brenner H, Walker ME, Ye S, Nguyen S, Pankow J, Demerath EW, Zheng Y, Hou L, Liang L, Lichtenstein AH, Hu FB, Fornage M, Voortman T, Levy D, 2020. Whole blood DNA methylation signatures of diet are associated with cardiovascular disease risk factors and all-cause mortality. Circ. Genom. Precis. Med 13, e002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Espino DV, 2004. Cultural influences on dementia recognition and management. Clin. Geriatr. Med 20, 93–119. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R, 2005. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch. Neurol 62, 1739–1746. [DOI] [PubMed] [Google Scholar]
- Markides KS, Coreil J, 1986. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 101, 253–265. [PMC free article] [PubMed] [Google Scholar]
- Markides KS, Rote S, 2019. The healthy immigrant effect and aging in the United States and other western countries. Gerontologist 59, 205–214. [DOI] [PubMed] [Google Scholar]
- Martin CL, Haan MN, Fernandez-Rhodes L, Lee A, Aiello AE, 2018. Association between immigration history and inflammatory marker profiles among older adult Mexican Americans. Biodemography Soc. Biol 64, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CL, Ward-Caviness CK, Dhingra R, Zikry TM, Galea S, Wildman DE, Koenen KC, Uddin M, Aiello AE, 2021. Neighborhood environment, social cohesion, and epigenetic aging. Aging 13, 7883–7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters RK, 2012. Uncrossing the U.S black-white mortality crossover: the role of cohort forces in life course mortality risk. Demography 49, 773–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley JL, Gutierrez AP, Bravin JI, Schneiderman N, Reina SA, Khambaty T, Castañeda SF, Smoller S, Daviglus ML, O’Brien MJ, Carnethon MR, Isasi CR, Perreira KM, Talavera GA, Yang M, Gallo LC, 2019. Association of social adversity with comorbid diabetes and depression symptoms in the hispanic community health study/study of latinos sociocultural ancillary study: a syndemic framework. Ann. Behav. Med 53, 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ, 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N Y Acad. Sci 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T, 1999. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N Y Acad. Sci 896, 30–47. [DOI] [PubMed] [Google Scholar]
- Melzer D, Pilling LC, Ferrucci L, 2020. The genetics of human ageing. Nat. Rev. Genet 21, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F, 2013. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc 14, 877–882. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ, 2015. Factors affecting longitudinal trajectories of plasma sphingomyelins: the baltimore longitudinal study of aging. Aging Cell 14, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW, 2009. Health psychology: developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol 60, 501–524. [DOI] [PubMed] [Google Scholar]
- Minkler M, Fuller-Thomson E, Guralnik JM, 2006. Gradient of disability across the socioeconomic spectrum in the United States. N. Engl. J. Med 355, 695–703. [DOI] [PubMed] [Google Scholar]
- Mitchell UA, Aneshensel CS, 2017. Social inequalities in inflammation: age variations in older persons. J. Aging Health 29, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil LS, Andaleon A, Badalamenti A, Dickinson SP, Guo X, Rotter JI, Johnson WC, Im HK, Liu Y, Wheeler HE, 2018. Genetic architecture of gene expression traits across diverse populations. PLoS Genet 14, e1007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P, 2006. Assessment of reference values for DNA damage detected by the comet assay in human blood cell DNA. Mutat. Res. / Rev. Mutat. Res 612, 84–104. [DOI] [PubMed] [Google Scholar]
- Moody DLB, Chang Y-F, Pantesco EJ, Darden TM, Lewis TT, Brown C, Bromberger JT, Matthews KA, 2018. Everyday discrimination prospectively predicts blood pressure across 10 years in racially/ethnically diverse midlife women: study of women’s health across the nation. Ann. Behav. Med 53, 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AE, Mooney KM, Wilkinson SJ, Pickles NA, Mc Auley MT, 2016. Cholesterol metabolism: a review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res. Rev 27, 108–124. [DOI] [PubMed] [Google Scholar]
- Moriwaki S, Ray S, Tarone RE, Kraemer KH, Grossman L, 1996. The effect of donor age on the processing of UV-damaged DNA by cultured human cells: reduced DNA repair capacity and increased DNA mutability. Mutat. Res 364, 117–123. [DOI] [PubMed] [Google Scholar]
- Morning A, 2011. The Nature of Race University of California Press, Berkley and Los Angeles. [Google Scholar]
- Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, Gibbons G, Din-Dzietham R, Quyyumi A, Vaccarino V, 2011. Association between depression and inflammation–differences by race and sex: the META-Health study. Psychosom. Med 73, 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, Gibbons GH, Vaccarino V, Din-Dzietham R, Quyyumi AA, 2012. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab. Syndr. Relat. Disord 10, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, Willcox BJ, Donlon TA, 2019. Genetic and epigenetic regulation of human aging and longevity. Biochim. Biophys. Acta Mol. Basis Dis 1865, 1718–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Kabagambe EK, Johnson CO, Lemaitre RN, Manichaikul A, Sun Q, Foy M, Wang L, Wiener H, Irvin MR, Rich SS, Wu H, Jensen MK, Chasman DI, Chu AY, Fornage M, Steffen L, King IB, McKnight B, Psaty BM, Djousse L, Chen IY, Wu JH, Siscovick DS, Ridker PM, Tsai MY, Rimm EB, Hu FB, Arnett DK, 2015. Genetic loci associated with circulating phospholipid trans fatty acids: a meta-analysis of genome-wide association studies from the CHARGE consortium. Am. J. Clin. Nutr 101, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Wille S, 2014. Race and history: comments from an epistemological point of view. Sci. Technol. Hum. Values 39, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel ZD, Chaim IA, Samson LD, 2014. Inter-individual variation in DNA repair capacity: a need for multi-pathway functional assays to promote translational DNA repair research. DNA Repair 19, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel ZD, Engelward BP, Brenner DJ, Begley TJ, Sobol RW, Bielas JH, Stambrook PJ, Wei Q, Hu JJ, Terry MB, Dilworth C, McAllister KA, Reinlib L, Worth L, Shaughnessy DT, 2017. Towards precision prevention: technologies for identifying healthy individuals with high risk of disease. Mutat. Res 800–802, 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, Grenier J-C, Freiman A, Sams AJ, Hebert S, Pagé Sabourin A, Luca F, Blekhman R, Hernandez RD, Pique-Regi R, Tung J, Yotova V, Barreiro LB, 2016. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 167, 657–669 e621. [DOI] [PubMed] [Google Scholar]
- Needham BL, Wang X, Carroll JE, Barber S, Sanchez BN, Seeman TE, Diez Roux AV, 2019. Sociodemographic correlates of change in leukocyte telomere length during mid- to late-life: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 102, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri M, Milazzo D, Ugolini D, Milic M, Campolongo A, Pasqualetti P, Bonassi S, 2015. Worldwide interest in the comet assay: a bibliometric study. Mutagenesis 30, 155–163. [DOI] [PubMed] [Google Scholar]
- Newman AB, Glynn NW, Taylor CA, Sebastiani P, Perls TT, Mayeux R, Christensen K, Zmuda JM, Barral S, Lee JH, Simonsick EM, Walston JD, Yashin AI, Hadley E, 2011. Health and function of participants in the long life family study: a comparison with other cohorts. Aging 3, 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Gurkar AU, Wang Y, Vijg J, Hoeijmakers JHJ, Robbins PD, 2018. Nuclear genomic instability and aging. Annu. Rev. Biochem 87, 295–322. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N, Evans MK, 2019. Age and poverty status alter the coding and noncoding transcriptome. Aging 11 (4), 1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Evans MK, 2020. Extracellular vesicles as signaling mediators in type 2 diabetes mellitus. Am. J. Physiol. Cell Physiol 318, C1189–C1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Ejiogu N, Zonderman AB, Evans MK, 2012. Association of oxidative DNA damage and C-reactive protein in women at risk for cardiovascular disease. Arterioscler. Thromb. Vasc. Biol 32, 2776–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, McFarland MH, Freeman DW, Mode NA, Ezike N, Zonderman AB, Evans MK, 2019. Association of extracellular vesicle protein cargo with race and clinical markers of mortality. Sci. Rep 9, 17582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroozi R, Ghafouri-Fard S, Pisarek A, Rudnicka J, Spólnicka M, Branicki W, Taheri M, Pośpiech E, 2021. DNA methylation-based age clocks: From age prediction to age reversion. Ageing Res. Rev 68, 101314. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, Devous M, Cushings B, Knebl J, Hall J, 2013. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis 33, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G, Penninx BWJH, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, Pahor M, 2002. Change in physical performance over time in older women: the women’s health and aging study. J. Gerontol. Ser. A 57, M289–M293. [DOI] [PubMed] [Google Scholar]
- Ostrakhovitch EA, Tabibzadeh S, 2019. Homocysteine and age-associated disorders. Ageing Res. Rev 49, 144–164. [DOI] [PubMed] [Google Scholar]
- Pantesco EJ, Leibel DK, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, Weng NP, Evans MK, Zonderman AB, Beatty Moody DL, 2018. Multiple forms of discrimination, social status, and telomere length: interactions within race. Psychoneuroendocrinology 98, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak GA, Silzer TK, Sun J, Zhou Z, Daniel AA, Johnson L, O’Bryant S, Phillips NR, Barber RC, 2019. Genome-wide methylation of mild cognitive impairment in Mexican Americans highlights genes involved in synaptic transport, Alzheimer’s disease-precursor phenotypes, and metabolic morbidities. J. Alzheimer’s Dis 72, 733–749. [DOI] [PubMed] [Google Scholar]
- Peters A, Nawrot TS, Baccarelli AA, 2021. Hallmarks of environmental insults. Cell 184, 1455–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Udry JR, 1998. Adolescent obesity increases significantly in second and third generation U.S. immigrants: the national longitudinal study of adolescent health. J. Nutr 128, 701–706. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson N, Yu F, Cox DR, Waliszewska A, McDonald GJ, Tandon A, Schirmer C, Neubauer J, Bedoya G, Duque C, Villegas A, Bortolini MC, Salzano FM, Gallo C, Mazzotti G, Tello-Ruiz M, Riba L, Aguilar-Salinas CA, Canizales-Quinteros S, Menjivar M, Klitz W, Henderson B, Haiman CA, Winkler C, Tusie-Luna T, Ruiz-Linares A, Reich D, 2007. A genomewide admixture map for Latino populations. Am. J. Hum. Genet 80, 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CS, Noren Hooten N, Mode NA, Zhang Y, Ejiogu N, Becker KG, Zonderman AB, Evans MK, 2019. Frailty in middle age is associated with frailty status and race-specific changes to the transcriptome. Aging 11, 5518–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MJ, Moore DH 2nd, Briner JF, Lee DA, Olsen L, Senft JR, Tucker JD, 1995. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat. Res 338, 95–106. [DOI] [PubMed] [Google Scholar]
- Reich D, Patterson N, Ramesh V, De Jager PL, McDonald GJ, Tandon A, Choy E, Hu D, Tamraz B, Pawlikowska L, Wassel-Fyr C, Huntsman S, Waliszewska A, Rossin E, Li R, Garcia M, Reiner A, Ferrell R, Cummings S, Kwok PY, Harris T, Zmuda JM, Ziv E, Health A, Body Composition S, 2007. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am. J. Hum. Genet 80, 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner AP, Ziv E, Lind DL, Nievergelt CM, Schork NJ, Cummings SR, Phong A, Burchard EG, Harris TB, Psaty BM, Kwok PY, 2005. Population structure, admixture, and aging-related phenotypes in African American adults: the cardiovascular health study. Am. J. Hum. Genet 76, 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner AP, Beleza S, Franceschini N, Auer PL, Robinson JG, Kooperberg C, Peters U, Tang H, 2012. Genome-wide association and population genetic analysis of C-reactive protein in African American and Hispanic American women. Am. J. Hum. Genet 91, 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee TG, Lee K, Schensul JJ, 2021. Black-white disparities in social and behavioral determinants of health index and their associations with self-rated health and functional limitations in older adults. J. Gerontol. A Biol. Sci. Med. Sci 76, 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Pablos-Mendez A, Palmas W, Lantigua R, Mayeux R, Berglund L, 2002. Comparison of modifiable determinants of lipids and lipoprotein levels among African-Americans, Hispanics, and Non-Hispanic Caucasians > or =65 years of age living in New York City. Am. J. Cardiol 89, 178–183. [DOI] [PubMed] [Google Scholar]
- Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, Rube C, 2011. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 6, e17487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas YD, Wang L, DeWan AT, 2016. Multiethnic genome-wide association study identifies ethnic-specific associations with body mass index in Hispanics and African Americans. BMC Genet. 17, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto AJ, Goodpaster BH, Kritchevsky SB, Miljkovic I, Satterfield S, Schwartz AV, Cummings SR, Boudreau RM, Harris TB, Newman AB, 2017. Body Composition Remodeling and Mortality: The Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci 72, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeer KK, Tarrence J, 2018. Racial-ethnic disparities in inflammation: evidence of weathering in childhood? J. Health Soc. Behav 59, 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R, 2005. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J. Am. Geriatr. Soc 53, 219–226. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM, 2008. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell 7, 89–100. [DOI] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS, 2010. Socioeconomic differentials in peripheral biology: cumulative allostatic load. Ann. N Y Acad. Sci 1186, 223–239. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, 2001. Social environment effects on health and aging: integrating epidemiologic and demographic approaches and perspectives. Ann. N Y Acad. Sci 954, 88–117. [DOI] [PubMed] [Google Scholar]
- Shadyab AH, Kooperberg C, Reiner AP, Jain S, Manson JE, Hohensee C, Macera CA, Shaffer RA, Gallo LC, LaCroix AZ, 2017. Replication of genome-wide association study findings of longevity in white, African American, and Hispanic Women: the women’s health initiative. J. Gerontol. A Biol. Sci. Med. Sci 72, 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PM, Ponnaiya B, Taveras M, Shuryak I, Turner H, Brenner DJ, 2015. High throughput measurement of γH2AX DSB repair kinetics in a healthy human population. PLoS One 10, e0121083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock NW, 1984. Normal human aging: The Baltimore longitudinal study of aging.
- Simon MS, Heilbrun LK, Stephens D, Lababidi S, Djuric Z, 2000. Recruitment for a pilot case control study of oxidative DNA damage and breast cancer risk. Am. J. Clin. Oncol 23, 283–287. [DOI] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SR, Philibert RA, Cutrona CE, Gibbons FX, Barr A, 2016. Economic hardship and biological weathering: the epigenetics of aging in a U. S. sample of black women. Soc. Sci. Med 150, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Barr AB, Simons LG, Gibbons FX, Philibert RA, 2018. Discrimination, segregation, and chronic inflammation: testing the weathering explanation for the poor health of Black Americans. Dev. Psychol 54, 1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, James SA, Robinson JC, Williams DR, Taylor HA, 2012. Perceived discrimination and hypertension among African Americans in the jackson heart study. Am. J. Public Health 102, S258–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Raisky J, Ratliff SM, Liu J, Kardia SLR, Turner ST, Mosley TH, Zhao W, 2019. Intrinsic and extrinsic epigenetic age acceleration are associated with hypertensive target organ damage in older African Americans. BMC Med. Genom 12, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JP, Cortinhas A, Bento T, Leitão JC, Collins AR, Gaivão I, Mota MP, 2014. Aging and DNA damage in humans: a meta-analysis study. Aging 6, 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Cho M, Brennan KM, Chen BH, Song Y, Manson JE, Hevener AL, You NY, Butch AW, Liu S, 2018. Relationships of sex hormone levels with leukocyte telomere length in Black, Hispanic, and Asian/Pacific Islander postmenopausal women. J. Diabetes 10, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR, 2014. Cohort profile: the Health and Retirement Study (HRS). Int J. Epidemiol 43, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J, 2000. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. Sybil L. Crawford [Google Scholar]
- Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu Y-T, Veronese N, 2016. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res. Rev 31, 1–8. [DOI] [PubMed] [Google Scholar]
- Sternthal MJ, Slopen N, Williams DR, 2011. Racial disparities in health: how much does stress really matter? Du Bois Rev. 8, 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanton SL, Rifkind JM, Mohanty JG, Miller ER, Thorpe RJ, Nagababu E, Epel ES, Zonderman AB, Evans MK, 2012. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int. J. Behav. Med 19, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin SM, Hernandez DG, Chen BH, Noren Hooten N, Mode NA, Nalls MA, Singleton AB, Ejiogu N, Chitrala KN, Zonderman AB, Evans MK, 2019. Novel age-associated DNA methylation changes and epigenetic age acceleration in middle-aged African Americans and whites. Clin. Epigenetics 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Dutta A, Pilling LC, Xue L, Lunetta KL, Murabito JM, Bandinelli S, Wallace R, Melzer D, Ferrucci L, 2017. Genome-wide ASsociation Study of Parental Life Span. J. Gerontol. A Biol. Sci. Med. Sci 72, 1407–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R, 2001. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 56, 49–56. [DOI] [PubMed] [Google Scholar]
- Tarraf W, Jensen GA, Dillaway HE, Vasquez PM, Gonzalez HM, 2020. Trajectories of aging among u.s. older adults: mixed evidence for a hispanic paradox. J. Gerontol. B Psychol. Sci. Soc. Sci 75, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JY, Wright ML, Crusto CA, Sun YV, 2016. The intergenerational impact of genetic and psychological factors on blood pressure (interGEN) study: design and methods for complex DNA analysis. Biol. Res. Nurs 18, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennstedt SL, Unverzagt FW, 2013. The ACTIVE study: study overview and major findings. J. Aging Health 25, 3s–20s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Irwin MR, Breen EC, Cole SW, 2019. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology 106, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARIC investigators, 1989. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. the ARIC investigators. Am. J. Epidemiol 129, 687–702. [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group, 1998. Design of the women’s health initiative clinical trial and observational study. Control. Clin. Trials 19, 61–109. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M, Juma AT, Kotze MJ, Lema G, Moore JH, Mortensen H, Nyambo TB, Omar SA, Powell K, Pretorius GS, Smith MW, Thera MA, Wambebe C, Weber JL, Williams SM, 2009. The genetic structure and history of Africans and African Americans. Science 324, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraason M, Butler MA, Ruder A, Forrester C, Taylor L, Ashley DL, Mathias P, Marlow KL, Cheever KL, Krieg E, Wey H, 2003. Effect of perchloroethylene, smoking, and race on oxidative DNA damage in female dry cleaners. Mutat. Res 539, 9–18. [DOI] [PubMed] [Google Scholar]
- Trzeciak AR, Barnes J, Ejiogu N, Foster K, Brant LJ, Zonderman AB, Evans MK, 2008. Age, sex, and race influence single-strand break repair capacity in a human population. Free Radic. Biol. Med 45, 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciak AR, Mohanty JG, Jacob KD, Barnes J, Ejiogu N, Lohani A, Zonderman AB, Rifkind JM, Evans MK, 2012. Oxidative damage to DNA and single strand break repair capacity: relationship to other measures of oxidative stress in a population cohort. Mutat. Res 736, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, 2005. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J. Med. Investig 52 (Suppl), 252–258. [DOI] [PubMed] [Google Scholar]
- Van Dyke ME, Vaccarino V, Dunbar SB, Pemu P, Gibbons GH, Quyyumi AA, Lewis TT, 2017. Socioeconomic status discrimination and C-reactive protein in African-American and White adults. Psychoneuroendocrinology 82, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman K, Khurana S, Tai TC, 2013. Oxidative stress in aging–matters of the heart and mind. Int. J. Mol. Sci 14, 17897–17925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa J, Medina L, Armstrong D, 2020. Demographic turning points for the United States for 2020–2060. In: U.S.C. (Ed.), Proceedings of the Bureau, Washington, D.C. [Google Scholar]
- Vines AI, Baird DD, McNeilly M, Hertz-Picciotto I, Light KC, Stevens J, 2006. Social correlates of the chronic stress of perceived racism among black women. Ethn. Dis 16, 101–107. [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Pompeii L, Lincoln AE, Dunn RE, Tucker AM, Nambi V, Nasir K, Vogel RA, Boone JL, Roberts AJ, Ballantyne CM, 2012. Association between traditional cholesterol parameters, lipoprotein particle concentration, novel biomarkers and carotid plaques in retired National Football League players. Atherosclerosis 222, 551–556. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Moody DLB, McNeely JM, Allen AJ, Sprung MR, Shah MT, Al’Najjar E, Evans MK, Zonderman AB, 2016. Cross-sectional relations of race and poverty status to cardiovascular risk factors in the Healthy Aging in Neighborhoods of Diversity across the Lifespan (HANDLS) study. BMC Public Health 16, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Arking DE, Fallin D, Li T, Beamer B, Xue Q, Ferrucci L, Fried LP, Chakravarti A, 2005. IL-6 gene variation is not associated with increased serum levels of IL-6, muscle, weakness, or frailty in older women. Exp. Gerontol 40, 344–352. [DOI] [PubMed] [Google Scholar]
- Wassel Fyr CL, Kanaya AM, Cummings SR, Reich D, Hsueh WC, Reiner AP, Harris TB, Moffett S, Li R, Ding J, Miljkovic-Gacic I, Ziv E, Health ABCS, 2007. Genetic admixture, adipocytokines, and adiposity in black Americans: the health, aging, and body composition study. Hum. Genet 121, 615–624. [DOI] [PubMed] [Google Scholar]
- Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA, 2007. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 56, 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JL, Satia JA, Kupper LL, Swenberg JA, Schroeder JC, Switzer BR, 2007. Associations of antioxidant nutrients and oxidative DNA damage in healthy African-American and White adults. Cancer Epidemiol. Biomark. Prev 16, 1428–1436. [DOI] [PubMed] [Google Scholar]
- Watts EL, Appleby PN, Albanes D, Black A, Chan JM, Chen C, Cirillo PM, Cohn BA, Cook MB, Donovan JL, Ferrucci L, Garland CF, Giles GG, Goodman PJ, Habel LA, Haiman CA, Holly JMP, Hoover RN, Kaaks R, Knekt P, Kolonel LN, Kubo T, Le Marchand L, Luostarinen T, MacInnis RJ, Maenpaa HO, Mannisto S, Metter EJ, Milne RL, Nomura AMY, Oliver SE, Parsons JK, Peeters PH, Platz EA, Riboli E, Ricceri F, Rinaldi S, Rissanen H, Sawada N, Schaefer CA, Schenk JM, Stanczyk FZ, Stampfer M, Stattin P, Stenman UH, Tjonneland A, Trichopoulou A, Thompson IM, Tsugane S, Vatten L, Whittemore AS, Ziegler RG, Allen NE, Key TJ, Travis RC, 2017. Circulating sex hormones in relation to anthropometric, sociodemographic and behavioural factors in an international dataset of 12,300 men. PLoS One, e0187741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts EL, Perez-Cornago A, Appleby PN, Albanes D, Ardanaz E, Black A, Buenode-Mesquita HB, Chan JM, Chen C, Chubb SAP, Cook MB, Deschasaux M, Donovan JL, English DR, Flicker L, Freedman ND, Galan P, Giles GG, Giovannucci EL, Gunter MJ, Habel LA, Haggstrom C, Haiman C, Hamdy FC, Hercberg S, Holly JM, Huang J, Huang WY, Johansson M, Kaaks R, Kubo T, Lane JA, Layne TM, Le Marchand L, Martin RM, Metter EJ, Mikami K, Milne RL, Morris HA, Mucci LA, Neal DE, Neuhouser ML, Oliver SE, Overvad K, Ozasa K, Pala V, Pernar CH, Pollak M, Rowlands MA, Schaefer CA, Schenk JM, Stattin P, Tamakoshi A, Thysell E, Touvier M, Trichopoulou A, Tsilidis KK, Van Den Eeden SK, Weinstein SJ, Wilkens L, Yeap BB, Key TJ, Allen NE, Travis RC, 2019. The associations of anthropometric, behavioural and sociodemographic factors with circulating concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a pooled analysis of 16,024 men from 22 studies. Int. J. Cancer 145, 3244–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton W, Kollhoff AL, Gangishetti U, Verble DD, Upadhya S, Zetterberg H, Kumar V, Watts KD, Kippels AJ, Gearing M, Howell JC, Parker MW, Hu WT, 2019. Interleukin 9 alterations linked to alzheimer disease in african americans. Ann. Neurol 86, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker KM, Everson-Rose SA, Pankow JS, Rodriguez CJ, Lewis TT, Kershaw KN, Diez Roux AV, Lutsey PL, 2017. Experiences of discrimination and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Epidemiol 186, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield KE, Allaire JC, Wiggins SA, 2004. Relationships among health factors and everyday problem solving in african americans. Health Psychol. 23, 641–644. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, 2009. Discrimination and racial disparities in health: evidence and needed research. J. Behav. Med 32, 20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik GL, Graff M, Nishimura KK, Tao R, Haessler J, Gignoux CR, Highland HM, Patel YM, Sorokin EP, Avery CL, Belbin GM, Bien SA, Cheng I, Cullina S, Hodonsky CJ, Hu Y, Huckins LM, Jeff J, Justice AE, Kocarnik JM, Lim U, Lin BM, Lu Y, Nelson SC, Park SL, Poisner H, Preuss MH, Richard MA, Schurmann C, Setiawan VW, Sockell A, Vahi K, Verbanck M, Vishnu A, Walker RW, Young KL, Zubair N, Acuna-Alonso V, Ambite JL, Barnes KC, Boerwinkle E, Bottinger EP, Bustamante CD, Caberto C, Canizales-Quinteros S, Conomos MP, Deelman E, Do R, Doheny K, Fernandez-Rhodes L, Fornage M, Hailu B, Heiss G, Henn BM, Hindorff LA, Jackson RD, Laurie CA, Laurie CC, Li Y, Lin DY, Moreno-Estrada A, Nadkarni G, Norman PJ, Pooler LC, Reiner AP, Romm J, Sabatti C, Sandoval K, Sheng X, Stahl EA, Stram DO, Thornton TA, Wassel CL, Wilkens LR, Winkler CA, Yoneyama S, Buyske S, Haiman CA, Kooperberg C, Le Marchand L, Loos RJF, Matise TC, North KE, Peters U, Kenny EE, Carlson CS, 2019. Genetic analyses of diverse populations improves discovery for complex traits. Nature 570, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Rand KA, Kermany A, Noto K, Curtis D, Garrigan D, Slinkov D, Dorfman I, Granka JM, Byrnes J, Myres N, Ball CA, Ruby JG, 2019. A prospective analysis of genetic variants associated with human lifespan. G3 9, 2863–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Chen W, McDermott J, Han JJ, 2017. Molecular and phenotypic biomarkers of aging. F1000Reserch 6, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zhang L, Zhang W, Meng D, Zhang H, Jiang Y, Xu X, Van Meter M, Seluanov A, Gorbunova V, Mao Z, 2015. SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner. Cell Cycle 14, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T, 2003. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61, 76–80. [DOI] [PubMed] [Google Scholar]
- Yamada M, Udono MU, Hori M, Hirose R, Sato S, Mori T, Nikaido O, 2006. Aged human skin removes UVB-induced pyrimidine dimers from the epidermis more slowly than younger adult skin in vivo. Arch. Dermatol. Res 297, 294–302. [DOI] [PubMed] [Google Scholar]
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O, 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeyeodu ST, Kidd LR, Kimbro KS, 2019. Protective innate immune variants in racial/ethnic disparities of breast and prostate cancer. Cancer Immunol. Res 7, 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Sol K, Kraal Z, 2017. Psychosocial pathways to racial/ethnic inequalities in late-life memory trajectories. J. Gerontol. Ser. B 74, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Kraal AZ, Sharifian N, Zaheed AB, Sol K, 2019a. Inflammatory mechanisms underlying the effects of everyday discrimination on age-related memory decline. Brain Behav. Immun 75, 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Kraal AZ, Zaheed A, Farris P, Sol K, 2019b. Longitudinal effects of race, ethnicity, and psychosocial disadvantage on systemic inflammation. SSM Popul. Health 7, 100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A, Lange JF, Brückl T, Ising M, Wray NR, Erhardt A, Binder EB, Mehta D, 2015. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 16, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cai B, Geng A, Tang H, Zhang W, Li S, Jiang Y, Tan R, Wan X, Mao Z, 2020. Base excision repair but not DNA double-strand break repair is impaired in aged human adipose-derived stem cells. Aging Cell 19, e13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Ammous F, Ratliff S, Liu J, Yu M, Mosley TH, Kardia SLR, Smith JA, 2019. Education and lifestyle factors are associated with DNA methylation clocks in older African Americans. Int. J. Environ. Res. Public Health 16, 3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbermint M, Gaye A, Berthon A, Hannah-Shmouni F, Faucz FR, Lodish MB, Davis AR, Gibbons GH, Stratakis CA, 2019. ARMC5 variants and risk of hypertension in blacks: MH-GRID study. J. Am. Heart Assoc 8, e012508. [DOI] [PMC free article] [PubMed] [Google Scholar]


