Abstract
The most prevalent type of cancer among males is prostate cancer. Survival is considered quite good, but it can be further improved when risk factors are optimized. One of these factors is micronutrients, including Se and Zn. To our knowledge, the interaction between Se and Zn and prostate cancer remains undescribed. This study aimed to investigate the optimal levels of selenium (Se) and zinc (Zn) and their impact on the survival of individuals diagnosed with prostate cancer. A total of 338 prostate cancer patients were enrolled in this study, which was conducted in Poland between 2009 and 2015. Mass spectrometry, which uses inductively coupled plasma mass, was used to assess serum element levels before treatment. The study participants were categorized into quartiles (QI-QIV) based on the distributions of Se and Zn levels observed among surviving participants. Cox regression was used to assess the association between serum Se and Zn levels and the survival of prostate cancer patients. Our results reveal the effect of combined Se and Zn levels on survival in prostate cancer patients (SeQI-ZnQI vs. SeQIV-ZnQIV; HR = 20.9). These results need further research to establish Se/Zn norms for different populations.
Keywords: selenium, zinc, prostate cancer, survival
1. Introduction
Prostate cancer ranks as the most prevalent cancer in men and stands as the second primary cause of mortality. In the year 2020, a worldwide assessment reported 1,414,259 newly diagnosed cases and 375,304 deaths linked to this particular malignancy [1]. Survival is relatively high, as demonstrated by the EUROCARE-5 study, which revealed an overall 5-year survival rate of 83% [2]; for US patients, the 5-year survival is even better at ~97% [3], but it could still be improved.
A notable problem in the management of prostate cancer involves the identification of the determinants affecting survival. Generally, factors affecting survival vary between regions and cultures and can interact and complement each other. The most frequent of these include cardiovascular diseases, diabetes, obesity, respiratory insufficiency/illnesses [4], disease progression, lifestyle choices (alcohol/tobacco use, physical activity levels), and environmental factors. Among the plethora of environmental factors influencing survival are some elements that have been studied, such as iron (Fe) [5,6,7], cadmium (Cd) [8], mercury (Hg) [8], selenium (Se) [9,10,11,12,13,14,15,16], and zinc (Zn) [17].
Se is a crucial element necessary for the optimal physiological functioning of diverse organisms. The exact mechanisms by which serum Se influences survival remain uncertain, and the association between an unfavorable prognosis and diminished Se levels is a subject of contention. To date, Se has been shown to be associated with survival in several malignancies, which include laryngeal, breast, lung, and colorectal cancers and malignant melanoma [9,10,11,12,13,14,15,16,17,18,19].
Zn is an essential trace element widely distributed throughout the environment that plays a pivotal role in human metabolism. Moreover, Zn is a required cofactor for the activation of over 300 enzymes. Additionally, it forms an integral part of structural and regulatory proteins, including transcription factors, establishing “zinc fingers” that facilitate DNA binding. Zn levels have been linked to survival in prostate, breast, lung, and laryngeal cancers [17,20,21].
It is well-established that the effect of a single element can vary depending on the levels of other micronutrients. Molecular and cellular investigations suggest that Se and Zn exhibit reciprocal effects influenced by variations in Zn level [22]. The low-dose Se and Zn supplementation model can be used to reduce the risk of prostate cancer and overall mortality [21,23,24]. However, it should be remembered that supplementation should be performed carefully after studying the level of micronutrients in the patient’s blood/serum. The optimal level should be targeted, because both deficiency and excess can adversely affect the patient’s prognosis [25,26,27,28].
To the best of our knowledge, the interaction between Se and Zn and cancer survival remains undescribed. Hence, our study aimed to examine the optimal levels of Se and Zn and their contribution to survival in individuals diagnosed with prostate cancer.
2. Methods and Materials
2.1. Study Cohort
A total of 338 unselected patients with prostate cancer were enrolled in this prospective study. The diagnosis of prostate cancer was always based on the result of histopathological examination. Cases originated from the Department of Urology and the Urological Clinical Hospital of the Pomeranian Medical University in Szczecin. Blood samples were taken from cases shortly after diagnosis and prior to treatment (between 2009 and 2015). All blood samples were collected fasting. All patients with complete information regarding their age at diagnosis (≤60/>60), Gleason (<7/7/>7), PSA (<4/4–10/>10), and vital status during follow up (alive/dead) were taken into account in the final calculations. Ethical approval for this study was obtained from the Ethics Committee of the Pomeranian Medical University in Szczecin under the reference number KB-0012/73/10 dated 21 June 2010, and the research adhered to the principles outlined in the Helsinki Declaration. Written and informed consent was obtained from all enrolled participants.
2.2. Methodology for Measurements
2.2.1. Sample Storage and Collection
Serum samples were collected using the Vacutainer® System (BD, Plymouth, UK). Blood for the serum was collected in tubes with a clot activator, incubated for a minimum of 30 min at room temperature for clotting, and then centrifuged at 1300× g for 12 min. The obtained serum was aliquoted into new cryovials and stored at −80 °C until analysis. On the analysis day, sera were thawed, vortex-mixed, and centrifuged at 5000× g for 5 min.
2.2.2. Measurement Methodology
Determination of 80Se and 66Zn was conducted using an inductively coupled plasma (ICP) mass spectrometer ELAN DRC-e (PerkinElmer, Concord, Ontario, Canada). Calibration of the instrument was performed daily, and oxygen served as the reaction gas. The spectrometer was calibrated using an external calibration technique with freshly prepared daily standards from Multi-Element Calibration Standard 3 (PerkinElmer Pure Plus, Shelton, CT, USA). A 30-fold dilution of serum in a blank reagent was assumed for the analysis, consisting of high-purity water, TMAH (AlfaAesar, Kandel, Germany), Triton X-100 (PerkinElmer, Shelton, CT, USA), n-butanol (Merck, Darmstadt, Germany), and EDTA (Merck, Darmstadt, Germany). Matrix-matched calibration was performed.
2.2.3. Quality Control
The precision and accuracy of measurements were assessed using certified reference material (CRM), Clincheck Plasmonorm Serum Trace Elements Level 1 (Recipe, Munich, Germany).
2.3. Statistical Analysis
The study cohort (n = 338) was categorized into quartiles (QI-QIV) based on Se and Zn serum levels within the subgroup of living patients (n = 246). Fourth quartiles (QIV) were chosen as the reference group. The characteristics of the study group were analyzed using the chi-square and Kruskal–Wallis tests for qualitative and quantitative data, respectively. Data normality was assessed using the Anderson–Darling test. Univariable and multivariable COX proportional hazard regression models were calculated to estimate the association between serum Se and Zn levels and prostate cancer survival considering certain variables, such as age at diagnosis (≤60/>60), Gleason (<7/7/>7), and PSA (<4/4–10/>10). The survival of alive patients was the difference between the final follow-up date (20 July 2022) and the date of prostate cancer diagnosis. The survival of deceased patients was the difference between the date of death and the date of diagnosis. For calculation purposes, a survival time longer than or equal to 5 years was treated as exactly 5 years of observation time. Kaplan–Meier curves were utilized to present univariable survival based on Se and Zn serum levels. All calculations were performed and all graphics were created using the R statistical environment (R Foundation for Statistical Computing, Vienna, Austria 2023; R version: 4.3.2).
3. Results
The characteristics of the study group are shown in Table 1.
Table 1.
Characteristics of the study population (n = 338 prostate cancer patients).
| Se | Zn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 338 | QI 41.58–72.03 (65.06), n = 107 | QII 72.05–78.07 (75.14), n = 83 | QIII 78.08–87.63 (82.82), n = 73 | QIV (Reference) 87.96–138.27 (94.78), n = 75 | p | QI 515.70–784.83 (701.67), n = 112 | QII 785.22–863.23 (820.73), n = 80 | QIII 863.76–944.15 (906.19), n = 76 | QIV (Reference) 944.26–1339.61 (1036.22), n = 70 | p |
| Status | <0.001 | <0.001 | |||||||||
| Alive | 246 (73%) | 62 (58%) | 61 (73%) | 61 (84%) | 62 (83%) | 62 (55%) | 61 (76%) | 61 (80%) | 62 (89%) | ||
| Dead | 92 (27%) | 45 (42%) | 22 (27%) | 12 (16%) | 13 (17%) | 50 (45%) | 19 (24%) | 15 (20%) | 8 (11%) | ||
| Age | 0.025 | 0.059 | |||||||||
| ≤60 (reference) 41.00–60.00 (56.57) | 77 (23%) | 17 (16%) | 22 (27%) | 13 (18%) | 25 (33%) | 16 (14%) | 20 (25%) | 20 (26%) | 21 (30%) | ||
| >60 61.00–86.00 (68.45) | 261 (77%) | 90 (84%) | 61 (73%) | 60 (82%) | 50 (67%) | 96 (86%) | 60 (75%) | 56 (74%) | 49 (70%) | ||
| Gleason | 0.3 | 0.5 | |||||||||
| <7 | 114 (34%) | 43 (40%) | 22 (27%) | 28 (38%) | 21 (28%) | 43 (38%) | 25 (31%) | 27 (36%) | 19 (27%) | ||
| 7 | 166 (49%) | 46 (43%) | 49 (59%) | 33 (45%) | 38 (51%) | 47 (42%) | 41 (51%) | 37 (49%) | 41 (59%) | ||
| >7 | 58 (17%) | 18 (17%) | 12 (14%) | 12 (16%) | 16 (21%) | 22 (20%) | 14 (18%) | 12 (16%) | 10 (14%) | ||
| PSA | 0.2 | 0.2 | |||||||||
| <4 | 19 (5.6%) | 5 (4.7%) | 5 (6.0%) | 5 (6.8%) | 4 (5.3%) | 6 (5.4%) | 3 (3.8%) | 7 (9.2%) | 3 (4.3%) | ||
| 4–10 | 185 (55%) | 48 (45%) | 47 (57%) | 47 (64%) | 43 (57%) | 53 (47%) | 44 (55%) | 47 (62%) | 41 (59%) | ||
| >10 | 134 (40%) | 54 (50%) | 31 (37%) | 21 (29%) | 28 (37%) | 53 (47%) | 33 (41%) | 22 (29%) | 26 (37%) | ||
| Prostatectomy | 0.001 | <0.001 | |||||||||
| No | 68 (20%) | 31 (29%) | 16 (19%) | 11 (15%) | 10 (13%) | 34 (30%) | 17 (21%) | 12 (16%) | 5 (7.1%) | ||
| Yes | 250 (74%) | 63 (59%) | 64 (77%) | 60 (82%) | 63 (84%) | 65 (58%) | 60 (75%) | 62 (82%) | 63 (90%) | ||
| Missing | 20 (5.9%) | 13 (12%) | 3 (3.6%) | 2 (2.7%) | 2 (2.7%) | 13 (12%) | 3 (3.8%) | 2 (2.6%) | 2 (2.9%) | ||
| Radiotherapy | 0.092 | 0.023 | |||||||||
| No | 149 (44%) | 39 (36%) | 40 (48%) | 32 (44%) | 38 (51%) | 45 (40%) | 31 (39%) | 40 (53%) | 33 (47%) | ||
| Yes | 146 (43%) | 46 (43%) | 34 (41%) | 35 (48%) | 31 (41%) | 43 (38%) | 41 (51%) | 30 (39%) | 32 (46%) | ||
| Missing | 43 (13%) | 22 (21%) | 9 (11%) | 6 (8.2%) | 6 (8.0%) | 24 (21%) | 8 (10%) | 6 (7.9%) | 5 (7.1%) | ||
| Chemotherapy | 0.011 | 0.007 | |||||||||
| No | 257 (76%) | 68 (64%) | 66 (80%) | 57 (78%) | 66 (88%) | 75 (67%) | 61 (76%) | 62 (82%) | 59 (84%) | ||
| Yes | 20 (5.9%) | 11 (10%) | 4 (4.8%) | 4 (5.5%) | 1 (1.3%) | 4 (3.6%) | 7 (8.8%) | 6 (7.9%) | 3 (4.3%) | ||
| Missing | 61 (18%) | 28 (26%) | 13 (16%) | 12 (16%) | 8 (11%) | 33 (29%) | 12 (15%) | 8 (11%) | 8 (11%) | ||
| Hormonotherapy | 0.035 | 0.10 | |||||||||
| No | 197 (58%) | 49 (46%) | 50 (60%) | 48 (66%) | 50 (67%) | 55 (49%) | 46 (58%) | 51 (67%) | 45 (64%) | ||
| Yes | 107 (32%) | 41 (38%) | 24 (29%) | 21 (29%) | 21 (28%) | 39 (35%) | 27 (34%) | 20 (26%) | 21 (30%) | ||
| Missing | 34 (10%) | 17 (16%) | 9 (11%) | 4 (5.5%) | 4 (5.3%) | 18 (16%) | 7 (8.8%) | 5 (6.6%) | 4 (5.7%) | ||
Q—quartile; PSA—prostate specific antigen.
The median Se level for the entire group (n = 338) was 76.85 µg/L (IQR = 15.55), with a mean Se level of 77.97 µg/L (±2.16). The median Zn level for the entire group was 830.40 µg/L (IQR = 169.26), and the mean level of Zn was 845.12 µg/L (±135.90).
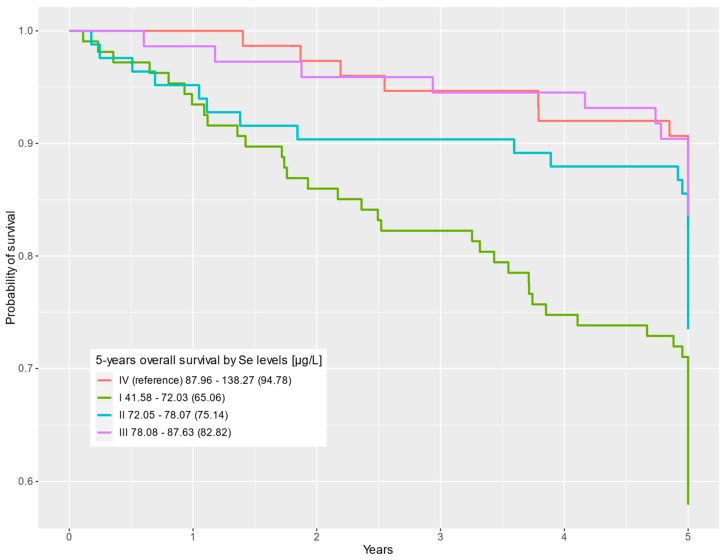
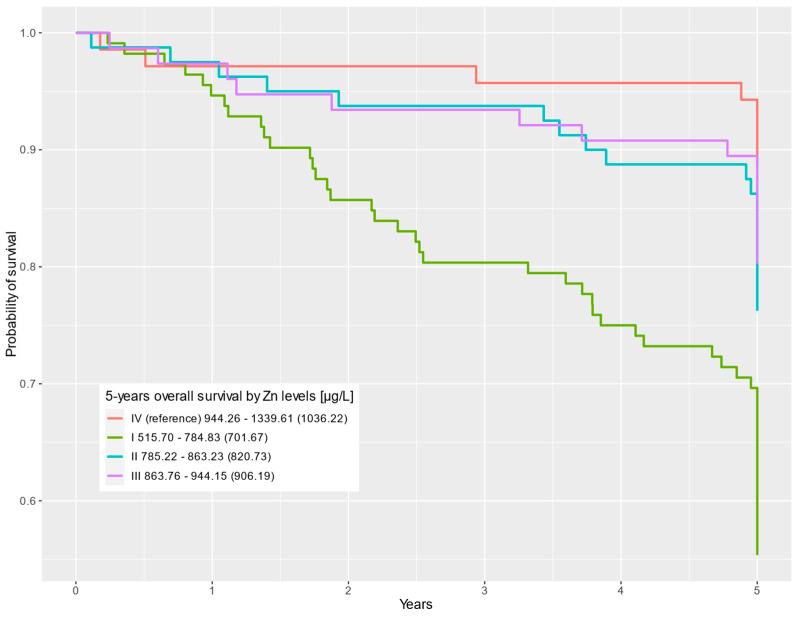
In the multivariable Cox regression, statistically significant differences were observed for the first-quartile Se level compared to the fourth Se quartile (HR = 2.43; 95% CI = 1.29–4.57; p = 0.006). Statistically significant differences in survival were also observed for the first Zn quartile compared to the highest Zn levels in the fourth quartile (HR = 4.11; 95% CI = 1.93–8.74; p < 0.001). The results for the uni- and multivariable Cox regression analyses are shown in Table 2. Survival curves depending on Se and Zn levels are presented in Figure 1 and Figure 2, respectively.
Table 2.
Survival of prostate cancer patients according to serum Se and Zn levels.
| Frequency | Univariable Cox Regression | Multivariable Cox Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 338 | Alive, n = 246 | Dead, n = 92 | HR | 95% CI | p | HR | 95% CI | p |
| Se | |||||||||
| Se level | |||||||||
| QI 41.58–72.03 (65.06) | 107 (32%) | 62 (25%) | 45 (49%) | 2.94 | 1.59, 5.45 | <0.001 | 2.43 | 1.29, 4.57 | 0.006 |
| QII 72.05–78.07 (75.14) | 83 (25%) | 61 (25%) | 22 (24%) | 1.63 | 0.82–3.24 | 0.2 | 1.84 | 0.92–3.67 | 0.085 |
| QIII 78.08–87.63 (82.82) | 73 (22%) | 61 (25%) | 12 (13%) | 0.95 | 0.43–2.08 | 0.9 | 1.01 | 0.45–2.23 | >0.9 |
| QIV (reference) 87.96–138.27 (94.78) | 75 (22%) | 62 (25%) | 13 (14%) | — | — | — | — | ||
| Age | |||||||||
| ≤60 (reference) 41.00–60.00 (56.57) | 77 (23%) | 66 (27%) | 11 (12%) | — | — | — | — | ||
| >60 61.00–86.00 (68.45) | 261 (77%) | 180 (73%) | 81 (88%) | 2.37 | 1.26–4.45 | 0.007 | 2.04 | 1.07–3.87 | 0.030 |
| Gleason | |||||||||
| <7 | 114 (34%) | 80 (33%) | 34 (37%) | — | — | — | — | ||
| 7 | 166 (49%) | 135 (55%) | 31 (34%) | 0.60 | 0.37–0.98 | 0.040 | 0.62 | 0.38–1.01 | 0.056 |
| >7 | 58 (17%) | 31 (13%) | 27 (29%) | 1.84 | 1.11–3.04 | 0.018 | 1.54 | 0.92–2.59 | 0.10 |
| PSA | |||||||||
| <4 | 19 (5.6%) | 13 (5.3%) | 6 (6.5%) | — | — | — | — | ||
| 4–10 | 185 (55%) | 156 (63%) | 29 (32%) | 0.45 | 0.19–1.09 | 0.076 | 0.50 | 0.21–1.21 | 0.12 |
| >10 | 134 (40%) | 77 (31%) | 57 (62%) | 1.46 | 0.63–3.38 | 0.4 | 1.32 | 0.56–3.08 | 0.5 |
| Zn | |||||||||
| Zn level | |||||||||
| QI 515.70–784.83 (701.67) | 112 (33%) | 62 (25%) | 50 (54%) | 4.91 | 2.33–10.4 | <0.001 | 4.11 | 1.93–8.74 | <0.001 |
| QII 785.22–863.23 (820.73) | 80 (24%) | 61 (25%) | 19 (21%) | 2.22 | 0.97–5.08 | 0.058 | 2.08 | 0.91–4.75 | 0.084 |
| QIII 863.76–944.15 (906.19) | 76 (22%) | 61 (25%) | 15 (16%) | 1.81 | 0.77–4.27 | 0.2 | 1.87 | 0.79–4.42 | 0.2 |
| QIV (reference) 944.26–1339.61 (1036.22) | 70 (21%) | 62 (25%) | 8 (8.7%) | — | — | — | — | ||
| Age | |||||||||
| ≤60 (reference) 41.00–60.00 (56.57) | 77 (23%) | 66 (27%) | 11 (12%) | — | — | — | — | ||
| >60 61.00–86.00 (68.45) | 261 (77%) | 180 (73%) | 81 (88%) | 2.37 | 1.26–4.45 | 0.007 | 1.87 | 0.99–3.54 | 0.053 |
| Gleason | |||||||||
| <7 | 114 (34%) | 80 (33%) | 34 (37%) | — | — | — | — | ||
| 7 | 166 (49%) | 135 (55%) | 31 (34%) | 0.60 | 0.37–0.98 | 0.040 | 0.66 | 0.41–1.08 | 0.10 |
| >7 | 58 (17%) | 31 (13%) | 27 (29%) | 1.84 | 1.11–3.04 | 0.018 | 1.52 | 0.91–2.53 | 0.11 |
| PSA | |||||||||
| <4 | 19 (5.6%) | 13 (5.3%) | 6 (6.5%) | — | — | — | — | ||
| 4–10 | 185 (55%) | 156 (63%) | 29 (32%) | 0.45 | 0.19–1.09 | 0.076 | 0.53 | 0.22–1.30 | 0.2 |
| >10 | 134 (40%) | 77 (31%) | 57 (62%) | 1.46 | 0.63–3.38 | 0.4 | 1.54 | 0.66–3.58 | 0.3 |
HR—hazard ratio; CI—confidence interval; Q—quartile; PSA—prostate specific antigen.
Figure 1.
Five-year overall survival by Se level (µg/L) categorized into quartiles (QI–QIV).
Figure 2.
Five-year overall survival by Zn level (µg/L) categorized into quartiles (QI–QIV).
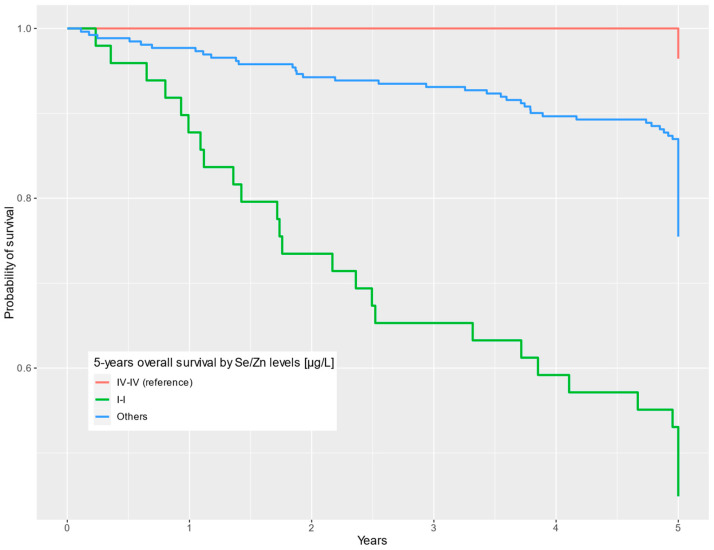
Among the total number of 338 prostate cancer patients, there were 77 (22.8%) patients with corresponding extreme quartiles of Se and Zn, whereas 49 (14.5%) patients had corresponding first quartiles of Se and Zn (SeQI-ZnQI) and 28 (8.28%) patients had corresponding fourth quartiles of Se and Zn (SeQIV-ZnQIV).
In the multivariable model, our findings show that individuals who were in both the lowest Se quartile and the lowest Zn quartile (SeQI-ZnQI) had almost a 21-fold lower chance of 5-year survival compared to patients in the highest Se and Zn quartiles (SeQIV-ZnQIV) (HR = 20.9; 95% CI = 2.80–156; p = 0.003).
Results for uni- and multivariable Cox analyses are presented in Table 3. Survival curves depending on Se and Zn levels combined are presented in Figure 3.
Table 3.
Prostate cancer patients’ survival—combined effect of Se and Zn blood levels by quartiles.
| Univariable Cox Regression Models | Multivariable Cox Regression Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile No. | Se Level (µg/L) | Zn Level (µg/L) | Alive | Dead | HR | 95% CI | p | HR | 95% CI | p |
| SeQI-ZnQI vs. SeQIV-ZnQIV | ||||||||||
| SeQI-ZnQI | 41.58–71.79 | 515.70–784.83 | 22 | 27 | 24.5 | 3.32–180 | 0.002 | 20.9 | 2.80–156 | 0.003 |
| Others | 52.77–138.27 | 541.75–1339.61 | 197 | 64 | 7.71 | 1.07–55.6 | 0.043 | 6.52 | 0.90–47.2 | 0.063 |
| SeQIV-ZnQIV | 88.78–104.22 | 946.99–1225.28 | 27 | 1 | — | — | — | — | ||
HR—hazard ratio; CI—confidence interval; SeQ—selenium quartile; ZnQ—zinc quartile.
Figure 3.
Five-year overall survival by Se/Zn levels (µg/L) categorized into common quartiles.
4. Discussion
Some key elements have been associated with cancer risk and progression. For this reason, research is being conducted to determine if they can be used as survival markers. Our previous reports examining survival in breast, lung, and laryngeal cancer and malignant melanoma patients showed an increased risk of death with low Se levels [9,10,11,12,13]. Several previously published studies have found a moderate correlation between diminished serum Se levels and survival outcomes in individuals diagnosed with breast and colorectal cancers [15,16]. We also found that high Zn levels correlated with prolonged survival in laryngeal [17], lung, breast, and prostate cancer patients [20]. Likewise, low Fe levels can increase the risk of death, as we have shown among a cohort of patients with malignant melanoma and lung cancer [5,12]. In addition, low Fe levels slightly increase the likelihood of mortality in individuals diagnosed with oral cancer [6]. We reported that blood Cd levels below 1.97 μg/L and Hg levels below 0.44 μg/L showed a connection with increased survival rates among individuals diagnosed with stage IA lung cancer [8]. In Table 4, we have listed selected studies (inclusion criteria: study cohort n ≥ 100, HR > 1.3) examining the effect of micronutrient levels on cancer survival.
Table 4.
Levels of selected micronutrients and their impact on cancer survival.
| Study | Group (n) | Element | Survival | Cancer | Sample |
|---|---|---|---|---|---|
| Kornitzer et al. 2003 [14] | 139 | Se (≤72 vs. ≥85 μg/L *) | HR = 2.2; 95% CI = 1.3–3.7; p = 0.011 | All | Blood serum |
| Lubiński et al. 2018 [9] | 296 | Se (<50 vs. >66.8 μg/L *) | HR = 3.07; 95% CI = 1.59–5.94; p = 0.0009 | Laryngeal | Blood serum |
| Lubiński et al. 2018 [10] | 546 | Se (<81.0 vs. >81.0 μg/L *) | HR = 2.49; 95% CI = 1.53–4.04; p = 0.0002 | Breast | Blood serum |
| Pietrzak et al. 2019 [11] | 302 | Se (<57.91 vs. >69 μg/L *) | HR = 2.73; 95% CI = 1.21–6.11; p = 0.01) | Lung | Blood serum |
| Sandsveden et al. 2020 [15] | 1066 | Se (≤81 vs. ≥100.01 μg/L *) | HR = 1.67; 95% CI = 0.37–0.98 | Breast | Blood serum |
| Baker et al. 2021 [16] | 995 | Se (≤67.5 vs. ≥100 μg/L *) | HR = 1.37; 95% CI = 0.98–1.92; p = 0.06 | Colorectal | Blood serum |
| Rogoża-Janiszewska et al. 2021 [12] | 375 | Se (<76.23 vs. >96.15 μg/L *) | HR = 5.83; 95% CI = 1.32–25.8; p = 0.02 | Melanoma | Blood serum |
| Szwiec et al. 2021 [13] | 538 | Se (52.1–76.7 vs. 94.7–171.5 μg/L *) | HR = 2.31; 95% CI = 1.24–4.31; p = 0.008 | Breast | Blood serum |
| Lubiński et al. 2021 [17] | 300 | Zn (<579 vs. >688 μg/L *) | HR = 2.32;95% CI = 1.47–3.69; p < 0.01 | Laryngeal | Blood serum |
| Sukiennicki et al. 2021 [5] | 200 | Fe (<959.92 vs. >1628.62μg/L *) | HR = 1.67; 95% CI = 0.96–2.86; p = 0.07 | Lung | Blood serum |
| Lin et al. 2021 [6] | 747 | Fe (≤15.3 vs. >15.3 μmol/L *) | HR = 1.39; 95% CI = 1.11–1.92 | Oral | Blood serum |
| Rowińska et al. 2022 [7] | 375 | Fe (<893.05 vs. ≥1348.63 μg/L *) | HR = 4.66; 95% CI = 1.28–16.9; p = 0.019 | Melanoma | Blood serum |
| Pietrzak et al. 2021 [8] | 336 | Cd (<0.57 * vs. >1.97 μg/L) | HR = 7.36; 95% CI: 2.14–25.25; p < 0.01 | Lung | Blood |
| Hg (<0.44 vs. >1.30 μg/L *) | HR = 1.55; 95% CI = 1.03–2.34; p = 0.04 |
HR—hazard ratio; CI—confidence interval; *—reference group.
In the present investigation, our team evaluated whether the Se and Zn serum levels’ combined effect could be related to the survival of patients with prostate cancer.
The precise mechanisms through which these elements affect prognosis are not fully understood. Se and Zn levels can certainly serve as biomarkers, although it cannot be excluded that they contribute directly to the progression of disease. Se and Zn are involved in various metabolic mechanisms that could potentially impact prognosis. It is plausible that the progression may be driven by the activity of conjugate proteins, with metal levels potentially playing a role in facilitating this process. Se, through its incorporation into selenoproteins, contributes to maintaining cellular redox balance, which is closely connected to MAPK signaling. Similarly, recent findings have linked Zn to MAPK signaling and the oncogene BRAF, which is relevant to prostate cancer [29,30,31].
The relationship between Zn and Se and many processes involved in cancer progression has been extensively studied (Table 5). It seems that, generally, micronutrients act dependently on one another. Actually, in our studies, the correlation between Se and Zn levels is moderate (correlation coefficient = 0.32; p < 0.001), and for this reason, their effects appear multiplicative. However, it cannot be excluded that in the same processes involving Zn and Se, they act in opposite directions. The relationship between overall survival or cancer progression and serum Se and/or Zn levels is poorly described in the literature. To date, some reports about micronutrients affecting survival have been forthcoming, but so far, no combined effects of Se and Zn have been reported with respect to cancer progression. In this report, we would like to draw attention to the importance of the potential benefits of optimizing these essential micronutrients and implementing this information into daily life/clinical practice. Our study, as far as we are aware, is the initial report of this correlation. The results we present (SeQI-ZnQI vs. SeQIV-ZnQIV; HR = 20.9; 95% CI = 2.80–156; p = 0.003) point towards a tremendous potential for improving patient outcomes.
Table 5.
Mechanisms of micronutrients in survival.
| Mechanism of Action | Se | Zn |
|---|---|---|
| Generating oxygen free radicals/involved in oxidative stress/antioxidant | ||
| Neoplastic growth | ||
| DNA repair | ||
| Apoptosis and cell signaling | ||
| Maintaining DNA integrity in humans |
|
|
| Inflammation suppression | ||
| Immune response enhancement |
|
|
| Protein kinase C inactivation |
|
|
| DNA methylation alteration | ||
| Angiogenesis inhibition |
|
|
| Cell cycle blockage |
|
|
| Telomere length—preserving telomere length leads to a decrease in the occurrence of age-related chronic diseases and cancers |
|
|
| Regulation of thyroid function |
|
|
| Cardiovascular disease |
5. Conclusions
Our results show the impact of combined Se and Zn levels on survival in prostate cancer patients. Even though the effect of Zn has already been well-established, our data strongly indicate that it is more beneficial to optimize both Se and Zn levels. Therefore, we are going to establish a trial to prove this statement. Certainly, such a trial should be based on careful, systematic measurements of Se and Zn serum levels. At the same time, we want to draw the attention of scientists around the world to conduct similar studies to determine the best element levels for people in different regions of the world, knowing that background levels of Se and Zn vary from continent to continent.
6. Patents
Based on the results presented in the following paper, a patent application has been submitted to the Patent Office of the Republic of Poland (application ID P.446712).
Author Contributions
Conceptualization, S.P., M.R.L. and J.L.; methodology, W.M., R.D. and P.B; software, P.B.; validation, W.M. and R.D.; formal analysis, P.B. and J.L.; investigation, S.P., W.M. and R.D.; resources, A.S., J.G., M.S., C.C., A.G., T.H., T.D. and T.K.; data curation, S.P., W.M., R.D., P.B., M.B., J.G., C.C., T.H., T.D., M.R.L., A.J. and J.L.; writing—original draft preparation, S.P., W.M., M.M., A.K., P.B., R.J.S. and J.L.; writing—review and editing, S.P., W.M., M.M., A.K., P.B., R.J.S. and J.L; visualization, S.P., P.B. and J.L; supervision, J.L.; project administration, J.L.; funding acquisition, A.J. and J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Pomeranian Medical University in Szczecin (Poland) under the number KB-0012/73/10 (26 June 2010).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data supporting the reported results are available from the corresponding author upon request from all interested researchers.
Conflicts of Interest
Jan Lubiński is the CEO of Read-Gene SA, which offers measurements on micro- and macro-elemental levels. These authors are part-time employees of Read-Gene: W.M., R.D., P.B., J.G., C.C., and T.H. The other authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the program of the Minister of Science and Higher Education called the “Regional Excellence Initiative” in 2019–2023 (grant number 002/RID/2018/19).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Trama A., Foschi R., Larrañaga N., Sant M., Fuentes-Raspall R., Serraino D., Tavilla A., Van Eycken L., Nicolai N., Hackl M., et al. Survival of Male Genital Cancers (Prostate, Testis and Penis) in Europe 1999–2007: Results from the EUROCARE-5 Study. Eur. J. Cancer. 2015;51:2206–2216. doi: 10.1016/j.ejca.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 4.Sears S.P., Carr G., Bime C. Oncologic Critical Care. Springer International Publishing; Cham, Switzerland: 2020. Acute and Chronic Respiratory Failure in Cancer Patients; pp. 445–475. [DOI] [Google Scholar]
- 5.Sukiennicki G.M., Marciniak W., Muszyńska M., Baszuk P., Gupta S., Białkowska K., Jaworska-Bieniek K., Durda K., Lener M., Pietrzak S., et al. Iron Levels, Genes Involved in Iron Metabolism and Antioxidative Processes and Lung Cancer Incidence. PLoS ONE. 2019;14:e0208610. doi: 10.1371/journal.pone.0208610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Y., Bao X., Li J., Pan C., Qian J., Lin L., Qiu Y., Shi B., Liu F., Chen F., et al. Correlation between Serum Iron Level and Overall Survival of Oral Cancer. Wei Sheng Yan Jiu. 2021;50:756–762. doi: 10.19813/j.cnki.weishengyanjiu.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Rowińska K., Baszuk P., Rogoża-Janiszewska E., Deptuła J., Marciniak W., Derkacz R., Lener M., Cybulski C., Kiedrowicz M., Boer M., et al. Serum Iron Level and 10-Year Survival after Melanoma. Biomedicines. 2022;10:3018. doi: 10.3390/biomedicines10123018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrzak S., Wójcik J., Baszuk P., Marciniak W., Wojtyś M., Dębniak T., Cybulski C., Gronwald J., Alchimowicz J., Masojć B., et al. Influence of the Levels of Arsenic, Cadmium, Mercury and Lead on Overall Survival in Lung Cancer. Biomolecules. 2021;11:1160. doi: 10.3390/biom11081160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubiński J., Marciniak W., Muszynska M., Jaworowska E., Sulikowski M., Jakubowska A., Kaczmarek K., Sukiennicki G., Falco M., Baszuk P., et al. Serum Selenium Levels and the Risk of Progression of Laryngeal Cancer. PLoS ONE. 2018;1:e0184873. doi: 10.1371/journal.pone.0184873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubinski J., Marciniak W., Muszynska M., Huzarski T., Gronwald J., Cybulski C., Jakubowska A., Debniak T., Falco M., Kladny J., et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res. Treat. 2018;16:591–598. doi: 10.1007/s10549-017-4525-9. [DOI] [PubMed] [Google Scholar]
- 11.Pietrzak S., Wójcik J., Scott R.J., Kashyap A., Grodzki T., Baszuk P., Bielewicz M., Marciniak W., Wójcik N., Dębniak T., et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J. Trace Elem. Med. Biol. 2019;56:46–51. doi: 10.1016/j.jtemb.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Rogoża-Janiszewska E., Malińska K., Baszuk P., Marciniak W., Derkacz R., Lener M., Jakubowska A., Cybulski C., Huzarski T., Masojć B., et al. Serum Selenium Level and 10-Year Survival after Melanoma. Biomedicines. 2021;9:991. doi: 10.3390/biomedicines9080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szwiec M., Marciniak W., Derkacz R., Huzarski T., Gronwald J., Cybulski C., Dębniak T., Jakubowska A., Lener M., Falco M., et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients. 2021;13:953. doi: 10.3390/nu13030953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kornitzer M., Valente F., De Bacquer D., Neve J., De Backer G. Serum Selenium and Cancer Mortality: A Nested Case–Control Study within an Age- and Sex-Stratified Sample of the Belgian Adult Population. Eur. J. Clin. Nutr. 2004;58:98–104. doi: 10.1038/sj.ejcn.1601754. [DOI] [PubMed] [Google Scholar]
- 15.Sandsveden M., Nilsson E., Borgquist S., Rosendahl A.H., Manjer J. Prediagnostic Serum Selenium Levels in Relation to Breast Cancer Survival and Tumor Characteristics. Int. J. Cancer. 2020;147:2424–2436. doi: 10.1002/ijc.33031. [DOI] [PubMed] [Google Scholar]
- 16.Baker J.R., Umesh S., Jenab M., Schomburg L., Tjønneland A., Olsen A., Boutron-Ruault M.C., Rothwell J.A., Severi G., Katzke V., et al. Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations. Biomedicines. 2021;9:1521. doi: 10.3390/biomedicines9111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubiński J., Jaworowska E., Derkacz R., Marciniak W., Białkowska K., Baszuk P., Scott R.J., Lubiński J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules. 2021;11:865. doi: 10.3390/biom11060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psathakis D., Wedemeyer N., Oevermann E., Krug F., Siegers C.P., Bruch H.P. Blood Selenium and Glutathione Peroxidase Status in Patients with Colorectal Cancer. Dis. Colon Rectum. 1998;41:328–335. doi: 10.1007/BF02237487. [DOI] [PubMed] [Google Scholar]
- 19.Meyer H.A., Endermann T., Stephan C., Stoedter M., Behrends T., Wolff I., Jung K., Schomburg L. Selenoprotein P Status Correlates to Cancer-Specific Mortality in Renal Cancer Patients. PLoS ONE. 2012;7:e46644. doi: 10.1371/journal.pone.0046644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubiński J., Lener M.R., Marciniak W., Pietrzak S., Derkacz R., Cybulski C., Gronwald J., Dębniak T., Jakubowska A., Huzarski T., et al. Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients. 2023;15:2611. doi: 10.3390/nu15112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein M.M., Kasperzyk J.L., Andrén O., Giovannucci E.L., Wolk A., Håkansson N., Andersson S.O., Johansson J.E., Fall K., Mucci L.A. Dietary Zinc and Prostate Cancer Survival in a Swedish Cohort. Am. J. Clin. Nutr. 2011;93:586–593. doi: 10.3945/ajcn.110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maret W. Metallothionein Redox Biology in the Cytoprotective and Cytotoxic Functions of Zinc. Exp. Gerontol. 2008;43:363–369. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Stopsack K.H., Wu K., Song M., Mucci L.A., Giovannucci E. Post-Diagnostic Zinc Supplement Use and Prostate Cancer Survival Among Men With Nonmetastatic Prostate Cancer. J. Urol. 2023;209:549–556. doi: 10.1097/JU.0000000000003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayehmiri K., Azami M., Mohammadi Y., Soleymani A., Tardeh Z. The Association between Selenium and Prostate Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2018;19:1431–1437. doi: 10.22034/APJCP.2018.19.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer F., Galan P., Douville P., Bairati I., Kegle P., Bertrais S., Estaquio C., Hercberg S. Antioxidant Vitamin and Mineral Supplementation and Prostate Cancer Prevention in the SU.VI.MAX Trial. Int. J. Cancer. 2005;116:182–186. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 26.Leitzmann M.F., Stampfer M.J., Wu K., Colditz G.A., Willett W.C., Giovannucci E.L. Zinc Supplement Use and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 27.Kenfield S.A., Van Blarigan E.L., DuPre N., Stampfer M.J., Giovannucci E.L., Chan J.M. Selenium Supplementation and Prostate Cancer Mortality. JNCI J. Natl. Cancer Inst. 2014;107:dju360. doi: 10.1093/jnci/dju360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Song M., Mucci L.A., Giovannucci E.L. Zinc Supplement Use and Risk of Aggressive Prostate Cancer: A 30-Year Follow-up Study. Eur. J. Epidemiol. 2022;37:1251–1260. doi: 10.1007/s10654-022-00922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashtchizadeh N., Karimi P., Dehgan P., Salimi Movahed M. Effects of Selenium in the MAPK Signaling Cascade. J. Cardiovasc. Thorac. Res. 2015;7:107–112. doi: 10.15171/jcvtr.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marranci A., Prantera A., Masotti S., De Paolo R., Baldanzi C., Podda M.S., Mero S., Vitiello M., Franchin C., Laezza M., et al. PARP1 Negatively Regulates MAPK Signaling by Impairing BRAF-X1 Translation. J. Hematol. Oncol. 2023;16:33. doi: 10.1186/s13045-023-01428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spick M., Muazzam A., Pandha H., Michael A., Gethings L.A., Hughes C.J., Munjoma N., Plumb R.S., Wilson I.D., Whetton A.D., et al. Multi-Omic Diagnostics of Prostate Cancer in the Presence of Benign Prostatic Hyperplasia. Heliyon. 2023;9:e22604. doi: 10.1016/j.heliyon.2023.e22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayman M.P. Selenium in Cancer Prevention: A Review of the Evidence and Mechanism of Action. Proc. Nutr. Soc. 2005;64:527–542. doi: 10.1079/PNS2005467. [DOI] [PubMed] [Google Scholar]
- 33.Kim B.Y., Jang S.Y., Choi D.H., Jung C.H., Mok J.O., Kim C.H. Anti-Inflammatory and Antioxidant Effects of Selenium on Orbital Fibroblasts of Patients with Graves Ophthalmopathy. Ophthalmic Plast. Reconstr. Surg. 2021;37:476–481. doi: 10.1097/IOP.0000000000001931. [DOI] [PubMed] [Google Scholar]
- 34.Alam S., Kelleher S.L. Cellular Mechanisms of Zinc Dysregulation: A Perspective on Zinc Homeostasis as an Etiological Factor in the Development and Progression of Breast Cancer. Nutrients. 2012;4:875–903. doi: 10.3390/nu4080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skrajnowska D., Bobrowska-Korczak B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients. 2019;11:2273. doi: 10.3390/nu11102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone N., Courbon D., Ducimetiere P., Zureik M. Zinc, Copper, and Magnesium and Risks for All-Cause, Cancer, and Cardiovascular Mortality. Epidemiology. 2006;17:308–314. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 37.Demircan K., Bengtsson Y., Sun Q., Brange A., Vallon-Christersson J., Rijntjes E., Malmberg M., Saal L.H., Rydén L., Borg Å., et al. Serum Selenium, Selenoprotein P and Glutathione Peroxidase 3 as Predictors of Mortality and Recurrence Following Breast Cancer Diagnosis: A Multicentre Cohort Study. Redox Biol. 2021;47:102145. doi: 10.1016/j.redox.2021.102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang C., Worley B.L., Phaëton R., Hempel N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers. 2020;12:2197. doi: 10.3390/cancers12082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selenius M., Rundlöf A.K., Olm E., Fernandes A.P., Björnstedt M. Selenium and the Selenoprotein Thioredoxin Reductase in the Prevention, Treatment and Diagnostics of Cancer. Antioxid. Redox Signal. 2010;12:867–880. doi: 10.1089/ars.2009.2884. [DOI] [PubMed] [Google Scholar]
- 40.Gao K., Zhang Y., Niu J., Nie Z., Liu Q., Lv C. Zinc Promotes Cell Apoptosis via Activating the Wnt-3a/β-Catenin Signaling Pathway in Osteosarcoma. J. Orthop. Surg. Res. 2020;15:57. doi: 10.1186/s13018-020-01585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.To P.K., Do M.H., Cho J.H., Jung C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020;21:2991. doi: 10.3390/ijms21082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes A.P., Gandin V. Selenium Compounds as Therapeutic Agents in Cancer. Biochim. Biophys. Acta BBA—Gen. Subj. 2015;1850:1642–1660. doi: 10.1016/j.bbagen.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Bera S., De Rosa V., Rachidi W., Diamond A.M. Does a Role for Selenium in DNA Damage Repair Explain Apparent Controversies in Its Use in Chemoprevention? Mutagenesis. 2013;28:127–134. doi: 10.1093/mutage/ges064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad A.S., Beck F.W.J., Snell D.C., Kucuk O. Zinc in Cancer Prevention. Nutr. Cancer. 2009;61:879–887. doi: 10.1080/01635580903285122. [DOI] [PubMed] [Google Scholar]
- 45.Falchuk K.H. The Molecular Basis for the Role of Zinc in Developmental Biology. Mol. Cell. Biochem. 1998;188:41–48. doi: 10.1023/A:1006808119862. [DOI] [PubMed] [Google Scholar]
- 46.Prasad A.S. Zinc Deficiency in Humans: A Neglected Problem. J. Am. Coll. Nutr. 1998;17:542–543. doi: 10.1080/07315724.1998.10718800. [DOI] [PubMed] [Google Scholar]
- 47.Ho E. Zinc Deficiency, DNA Damage and Cancer Risk. J. Nutr. Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Yan M., Song Y., Wong C.P., Hardin K., Ho E. Zinc Deficiency Alters DNA Damage Response Genes in Normal Human Prostate Epithelial Cells3. J. Nutr. 2008;138:667–673. doi: 10.1093/jn/138.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith M.L., Lancia J.K., Mercer T.I., Ip C. Selenium Compounds Regulate P53 by Common and Distinctive Mechanisms. Anticancer Res. 2004;24:1401–1408. [PubMed] [Google Scholar]
- 50.Qi L., Wang Y., Su S., Wang M., Jablonska E., Jia Y., Wang R., Hao S., Feng C., Li G., et al. Sodium Selenite Inhibits Cervical Cancer Growth via ROS Mediated AMPK/FOXO3a /GADD45a Axis. Chem. Biol. Interact. 2022;367:110171. doi: 10.1016/j.cbi.2022.110171. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y., Pu Q., Zhang Q., Liu Y., Ma Y., Yuan Y., Liu L., Zhu W. Selenium-binding Protein 1 Inhibits Malignant Progression and Induces Apoptosis via Distinct Mechanisms in Non-small Cell Lung Cancer. Cancer Med. 2023;12:17149–17170. doi: 10.1002/cam4.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo C.H., Wang S.Y., Chung C.H., Shih M.Y., Li W.C., Chen P.C., Lee S.Y., Hsia S. Selenium Modulates AR/IGF-1R/EGFR and TROP2 Signaling Pathways and Improves Anticancer Efficacy in Murine Mammary Carcinoma 4T1. J. Nutr. Biochem. 2023;120:109417. doi: 10.1016/j.jnutbio.2023.109417. [DOI] [PubMed] [Google Scholar]
- 53.Cheng Z., Yu S., He W., Li J., Xu T., Xue J., Shi P., Chen S., Li Y., Hong S., et al. Selenite Induces Cell Cycle Arrest and Apoptosis via Reactive Oxygen Species-Dependent Inhibition of the AKT/MTOR Pathway in Thyroid Cancer. Front. Oncol. 2021;11:668424. doi: 10.3389/fonc.2021.668424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang T., Zhao G., Zhu X., Jiang K., Wu H., Deng G., Qiu C. Sodium Selenite Induces Apoptosis via ROS-mediated NF-κB Signaling and Activation of the Bax–Caspase-9–Caspase-3 Axis in 4T1 Cells. J. Cell. Physiol. 2019;234:2511–2522. doi: 10.1002/jcp.26783. [DOI] [PubMed] [Google Scholar]
- 55.Ye Q., Liu J., Xie K. Zinc Finger Proteins and Regulation of the Hallmarks of Cancer. Histol. Histopathol. 2019;34:1097–1109. doi: 10.14670/HH-18-121. [DOI] [PubMed] [Google Scholar]
- 56.Takatani-Nakase T. Zinc Transporters and the Progression of Breast Cancers. Biol. Pharm. Bull. 2018;41:1517–1522. doi: 10.1248/bpb.b18-00086. [DOI] [PubMed] [Google Scholar]
- 57.Franklin R.B., Costello L.C. Zinc as an Anti-Tumor Agent in Prostate Cancer and in Other Cancers. Arch. Biochem. Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y., Chung C.S., Bruno R.S., Traber M.G., Brown K.H., King J.C., Ho E. Dietary Zinc Restriction and Repletion Affects DNA Integrity in Healthy Men. Am. J. Clin. Nutr. 2009;90:321–328. doi: 10.3945/ajcn.2008.27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joray M.L., Yu T.W., Ho E., Clarke S.L., Stanga Z., Gebreegziabher T., Hambidge K.M., Stoecker B.J. Zinc Supplementation Reduced DNA Breaks in Ethiopian Women. Nutr. Res. 2015;35:49–55. doi: 10.1016/j.nutres.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bevinakoppamath S., Saleh Ahmed A.M., Ramachandra S.C., Vishwanath P., Prashant A. Chemopreventive and Anticancer Property of Selenoproteins in Obese Breast Cancer. Front. Pharmacol. 2021;12:618172. doi: 10.3389/fphar.2021.618172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mou D., Ding D., Yang M., Jiang X., Zhao L., Che L., Fang Z., Xu S., Lin Y., Zhuo Y., et al. Maternal Organic Selenium Supplementation during Gestation Improves the Antioxidant Capacity and Reduces the Inflammation Level in the Intestine of Offspring through the NF-ΚB and ERK/Beclin-1 Pathways. Food Funct. 2021;12:315–327. doi: 10.1039/D0FO02274H. [DOI] [PubMed] [Google Scholar]
- 62.Liu S., Chen Q., Yan L., Ren Y., Fan J., Zhang X., Zhu S. Phytosomal Tripterine with Selenium Modification Attenuates the Cytotoxicity and Restrains the Inflammatory Evolution via Inhibiting NLRP3 Inflammasome Activation and Pyroptosis. Int. Immunopharmacol. 2022;108:108871. doi: 10.1016/j.intimp.2022.108871. [DOI] [PubMed] [Google Scholar]
- 63.Makita S., Takatori H., Nakajima H. Post-Transcriptional Regulation of Immune Responses and Inflammatory Diseases by RNA-Binding ZFP36 Family Proteins. Front. Immunol. 2021;12:711633. doi: 10.3389/fimmu.2021.711633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiremidjian-Schumacher L., Roy M., Glickman R., Schneider K., Rothstein S., Cooper J., Hochster H., Kim M., Newman R. Selenium and Immunocompetence in Patients with Head and Neck Cancer. Biol. Trace Elem. Res. 2000;73:97–112. doi: 10.1385/BTER:73:2:97. [DOI] [PubMed] [Google Scholar]
- 65.Dharmalingam K., Birdi A., Tomo S., Sreenivasulu K., Charan J., Yadav D., Purohit P., Sharma P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian J. Clin. Biochem. 2021;36:416–426. doi: 10.1007/s12291-021-00961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gopalakrishna R., Gundimeda U. Antioxidant Regulation of Protein Kinase C in Cancer Prevention. J. Nutr. 2002;132:3819S–3823S. doi: 10.1093/jn/132.12.3819S. [DOI] [PubMed] [Google Scholar]
- 67.Nasir A., Bullo M.M.H., Ahmed Z., Imtiaz A., Yaqoob E., Safdar M., Ahmed H., Afreen A., Yaqoob S. Nutrigenomics: Epigenetics and Cancer Prevention: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2020;60:1375–1387. doi: 10.1080/10408398.2019.1571480. [DOI] [PubMed] [Google Scholar]
- 68.Davis C.D., Uthus E.O. Dietary Folate and Selenium Affect Dimethylhydrazine-Induced Aberrant Crypt Formation, Global DNA Methylation and One-Carbon Metabolism in Rats. J. Nutr. 2003;133:2907–2914. doi: 10.1093/jn/133.9.2907. [DOI] [PubMed] [Google Scholar]
- 69.Azimi Z., Isa M.R., Khan J., Wang S.M., Ismail Z. Association of Zinc Level with DNA Methylation and Its Consequences: A Systematic Review. Heliyon. 2022;8:e10815. doi: 10.1016/j.heliyon.2022.e10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandaviya P.R., Joehanes R., Brody J., Castillo-Fernandez J.E., Dekkers K.F., Do A.N., Graff M., Hänninen I.K., Tanaka T., de Jonge E.A.L., et al. Association of Dietary Folate and Vitamin B-12 Intake with Genome-Wide DNA Methylation in Blood: A Large-Scale Epigenome-Wide Association Analysis in 5841 Individuals. Am. J. Clin. Nutr. 2019;110:437–450. doi: 10.1093/ajcn/nqz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGee M., Bainbridge S., Fontaine-Bisson B. A Crucial Role for Maternal Dietary Methyl Donor Intake in Epigenetic Programming and Fetal Growth Outcomes. Nutr. Rev. 2018;76:469–478. doi: 10.1093/nutrit/nuy006. [DOI] [PubMed] [Google Scholar]
- 72.Łoboś P., Regulska-Ilow B. Link between Methyl Nutrients and the DNA Methylation Process in the Course of Selected Diseases in Adults. Rocz. Państwowego Zakładu Hig. 2021;72:123–136. doi: 10.32394/rpzh.2021.0157. [DOI] [PubMed] [Google Scholar]
- 73.Jiang C., Kim K.H., Wang Z., Lu J. Methyl Selenium-Induced Vascular Endothelial Apoptosis Is Executed by Caspases and Principally Mediated by P38 MAPK Pathway. Nutr. Cancer. 2004;49:174–183. doi: 10.1207/s15327914nc4902_9. [DOI] [PubMed] [Google Scholar]
- 74.Davis C.D., Finley J.W. Functional Foods & Nutraceuticals in Cancer Prevention. Iowa State Press; Ames, IA, USA: 2003. Chemical Versus Food Forms of Selenium in Cancer Prevention; pp. 55–86. [DOI] [Google Scholar]
- 75.Rello-Varona S. Mitotic Catastrophe Induced in HeLa Cells by Photodynamic Treatment with Zn(II)-Phthalocyanine. Int. J. Oncol. 1992;32:1189–1196. doi: 10.3892/ijo_32_6_1189. [DOI] [PubMed] [Google Scholar]
- 76.Shu Y., Wu M., Yang S., Wang Y., Li H. Association of Dietary Selenium Intake with Telomere Length in Middle-Aged and Older Adults. Clin. Nutr. 2020;39:3086–3091. doi: 10.1016/j.clnu.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Shi H., Li X., Yu H., Shi W., Lin Y., Zhou Y. Potential Effect of Dietary Zinc Intake on Telomere Length: A Cross-Sectional Study of US Adults. Front. Nutr. 2022;9:993425. doi: 10.3389/fnut.2022.993425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thvilum M., Brandt F., Almind D., Christensen K., Hegedüs L., Brix T.H. Excess Mortality in Patients Diagnosed With Hypothyroidism: A Nationwide Cohort Study of Singletons and Twins. J. Clin. Endocrinol. Metab. 2013;98:1069–1075. doi: 10.1210/jc.2012-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Genchi G., Lauria G., Catalano A., Sinicropi M.S., Carocci A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023;24:2633. doi: 10.3390/ijms24032633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Binitha M., Sarita S., Betsy A. Zinc Deficiency Associated with Hypothyroidism: An Overlooked Cause of Severe Alopecia. Int. J. Trichol. 2013;5:40. doi: 10.4103/0974-7753.114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkins D.J., Kitts D., Giovannucci E.L., Sahye-Pudaruth S., Paquette M., Blanco Mejia S., Patel D., Kavanagh M., Tsirakis T., Kendall C.W., et al. Selenium, Antioxidants, Cardiovascular Disease, and All-Cause Mortality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2020;112:1642–1652. doi: 10.1093/ajcn/nqaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z., Chang C., Zhang Y., Chai Z., Li J., Qiu C. The Association between Serum Selenium Concentration and Prognosis in Patients with Heart Failure in a Chinese Population. Sci. Rep. 2021;11:14533. doi: 10.1038/s41598-021-93873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakatani S., Mori K., Shoji T., Emoto M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients. 2021;13:1680. doi: 10.3390/nu13051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akbari G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol. Trace Elem. Res. 2020;196:1–9. doi: 10.1007/s12011-019-01892-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the reported results are available from the corresponding author upon request from all interested researchers.