Abstract
Molecular adaptation to hypoxia depends on the binding of hypoxia-inducible factor 1 (HIF-1) to cognate response elements in oxygen-regulated genes. In addition, adjacent sequences are required for hypoxia-inducible transcription. To investigate the mechanism of interaction between these cis-acting sequences, the multiprotein complex binding to the lactate dehydrogenase A (LDH-A) promoter was characterized. The involvement of HIF-1, CREB-1/ATF-1, and p300/CREB binding protein (CBP) was demonstrated by techniques documenting in vitro binding, in combination with transient transfections that test the in vivo functional importance of each protein. In both the LDH-A promoter and the erythropoietin 3′ enhancer, formation of multiprotein complexes was analyzed by using biotinylated probes encompassing functionally critical cis-acting sequences. Strong binding of p300/CBP required interactions with multiple DNA binding proteins. Thus, the necessity of transcription factor binding sites adjacent to a HIF-1 site for hypoxically inducible transcription may be due to the requirement of p300 to interact with multiple transcription factors for high-affinity binding and activation of transcription. Since it has been found to interact with a wide range of transcription factors, p300 is likely to play a similar role in other genes, mediating interactions between DNA binding proteins, thereby activating stimulus-specific and tissue-specific gene transcription.
Tissue oxygen concentration is an important regulatory stimulus for many physiological and pathological processes (6). Adaptation to hypoxia depends in part on appropriate alterations in the expression of a number of physiologically relevant genes. Induction of the erythropoietin (Epo) gene by hypoxia is central to the regulation of the oxygen-carrying capacity of the blood (24); hypoxic induction of genes encoding angiogenic growth factors leads to new blood vessel formation in development, wound repair, and tumor growth (20, 21, 32); and hypoxic induction of genes encoding specific glycolytic isoenzymes and glucose transporters contributes to long-term adaptation of energy metabolism to decreased oxygen tension (12, 13, 16, 42). In particular, increased expression of the lactate dehydrogenase A (LDH-A) gene in hypoxic cells plays a critical role in the switch from aerobic to anaerobic energy metabolism, resulting in increased production of lactate and regeneration of NAD+ (17).
Most if not all mammalian cell types share a common mechanism of oxygen sensing and signal transduction (33), enabling hypoxia-induced activation of the transcription factor hypoxia-inducible factor 1 (HIF-1) (45). HIF-1 is a heterodimer comprised of HIF-1α and ARNT, two basic helix-loop-helix proteins in the PAS family (46). While ARNT mRNA and protein and HIF-1α mRNA are unaffected by oxygen tension, the level of HIF-1α protein increases markedly in hypoxic cells (23, 25, 37). In normoxia, HIF-1α is rapidly degraded by the proteasome; in hypoxia, this degradation is blocked, and HIF-1α accumulates (22). ARNT cannot form an active homodimer, so functional HIF-1 heterodimer is present only under hypoxic conditions. The coactivator protein p300 has been shown to interact with HIF-1α, thereby enabling enhanced transcription of Epo and vascular epithelial growth factor in response to hypoxia (1).
Despite similarities among hypoxia-responsive genes, there are important differences. While Epo expression can be induced as much as 100-fold, other responses to hypoxia are less robust. In each oxygen-regulated gene, hypoxic inducibility appears to be modulated by other environmental stimuli as well as tissue-specific cues. Some of these effects are far from HIF-1 sites, such as distant cis-acting elements (41) and sequences that regulate RNA stability (10, 29).
Although HIF-1 sites appear to be necessary for the function of many if not most oxygen-regulated genes, a single HIF-1 site in isolation is not very active in inducing gene expression in response to hypoxia (36). In promoters and enhancers which have been analyzed in detail, sequences close to HIF-1 binding sites have been identified as being necessary for hypoxic inducibility. For example, in the LDH-A promoter, mutations at three separate sites abolished hypoxic induction (17). Moreover, none of the three individual functionally critical sites, even when concatemerized, were capable of high-level hypoxic induction. Thus, each site is necessary but not sufficient for hypoxic inducibility. One site, located approximately 20 bp upstream of the TATA box, is a consensus cyclic AMP (cAMP) response element (CRE). The use of a cAMP agonist and a cAMP antagonist, which modulate the activity of proteins binding at the CRE, also modulated the level of hypoxic response. Characterization of the Epo enhancer revealed a similar tripartite array of sites except that instead of the CRE found in the LDH-A promoter, the Epo enhancer has a nuclear hormone receptor element (HRE) (5, 18, 36, 43).
In general, gene expression is not determined by the simple additive influences of individual transcription factor binding sites. Adjacent sites interact with each other to produce effects which range from repressive to highly synergistic. Multiprotein complexes involving transcription factors, coactivator proteins, and other proteins are thought to integrate signals and create the specificity and control required for precise regulation of gene transcription. HIF-1 may thus interact with adjacent transcription factors and coactivating proteins to form multiprotein complexes which are different in each oxygen-regulated gene, depending on adjacent cis-acting sequences. The specific complex formed in a given gene may contribute to the unique regulation of that gene with respect to its level of induction by hypoxia and interactions with other stimuli.
To investigate the manner in which hypoxically inducible transcription is coordinated through multiple interacting transcription factor binding sites, we analyzed the multiprotein complex which forms in the LDH-A promoter. Both in vitro DNA binding and functional evidence demonstrated that the complex includes HIF-1, CREB-1/ATF-1, and p300/CREB binding protein (CBP). Using a biotinylated DNA probe to capture the multiprotein complex, we demonstrated that all three functionally important sites must be intact for high-affinity binding of p300. Applying the same technique to the Epo 3′ enhancer, we demonstrated that both the HIF-1 site and the adjacent, functionally critical nuclear HRE must be intact for high-affinity binding of p300 in this complex as well. Thus, in both the LDH-A promoter and the Epo enhancer, multiple sites are required for recruitment of p300 to the hypoxically inducible complex.
MATERIALS AND METHODS
Cell culture.
HeLa cells were maintained in Dulbecco’s modified Earle’s medium with 5% fetal calf serum, penicillin (50 IU/ml), and streptomycin sulfate (50 μg/ml) at 37°C. The mouse hepatoma cell line Hepa-1 and the ARNT-deficient cell line Hepa-1 c4 were maintained in minimal essential medium alpha supplemented with 10% fetal calf serum, penicillin, and streptomycin sulfate. Cells were routinely cultured under a humidified atmosphere of 5% CO2–95% air (normoxia); for exposure to hypoxia, cells were incubated under a humidified atmosphere of 1% O2–5% CO2–94% N2 in an Espec BNP-210 incubator.
Nuclear extract and electrophoretic mobility shift assays (EMSAs).
HeLa cells were harvested after a 12-h incubation in normoxia or hypoxia. Nuclear extract was prepared by using a modification of the protocol described by Dignam et al. (11). Sequences of oligonucleotides derived from the mouse LDH-A promoter are portrayed in Fig. 1A. Each 24-bp oligonucleotide and its complementary 24-mer were purified by polyacrylamide gel electrophoresis (PAGE). Probes were labeled with [γ-32P]ATP by means of T4 polynucleotide kinase and annealed with a fourfold molar excess of the unlabeled complementary strand. Equimolar concentrations of complementary strands were annealed for use as competitors.
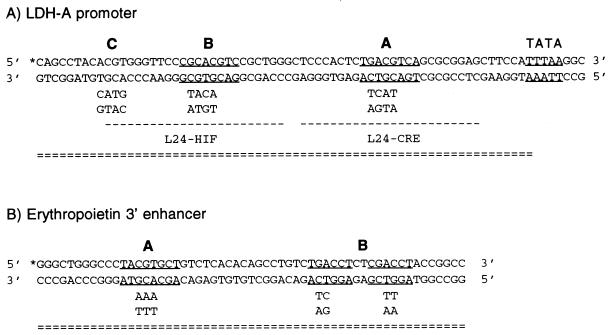
FIG. 1.
Summary of probes. EMSA probes, labeled L24-HIF and L24-CRE, biotinylated probes (= = =), and biotin label (∗) are indicated. (A) The CRE (site A), HIF-1 consensus sequence (sites B and C), and TATA consensus sequence are underlined. The sequence of mutations in sites A, B, and C are shown below their respective sites. (B) The HIF-1 site (site A) and both half sites of the direct repeat steroid hormone receptor consensus sequence (site B) are underlined. Mutations in sites A and B are shown below the sites; site B is mutated by two base pairs in two locations.
HeLa nuclear extract (5 μg) was preincubated in a 20-μl reaction mixture containing 50 mM KCl, 1 mM MgCl2, 0.5 mM EDTA, 5 mM dithiothreitol (DTT), 5% (vol/vol) glycerol, and 250 ng of sonicated poly(dI-dC) for 5 min at room temperature. Following this, 0.1 ng of radiolabeled probe was added, and the mixture was incubated for a further 10 min. For supershift assays, 1 μg of monoclonal antibody or 0.1 μg of polyclonal antibody was added, and the binding reaction mixtures were incubated at 4°C for 30 min to overnight. Binding reaction mixtures were loaded on a 5% polyacrylamide gel and electrophoresed at 4°C in 0.5× TBE (45 mM Tris, 45 mM boric acid, 1.0 mM EDTA [pH 8.3]). CREB and ATF antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-phospho-CREB antibody was obtained from Upstate Biotechnology Inc. (UBI). Procedures for production of HIF-1α antibodies and p300/CBP are described elsewhere (1).
Plasmids and transient transfections.
The LDH-growth hormone (GH) test plasmid (17) contains 233 bp of the mouse LDH-A promoter, extending from positions −186 to +47 relative to the transcription initiation site, inserted upstream of the human GH gene. The sequences of mutations in the LDH promoter are illustrated in Fig. 1A. The α1-globin control plasmid encodes the human α1-globin promoter and gene. Plasmid pRc/RSV-CREB encodes CREB341 (28). The mutated form of CREB, CREBM1, contains a mutation in serine 133, the single protein kinase A (PK-A) phosphoacceptor site (44). pRC/CMV E1A encodes the 12S form of E1A, pRC/CMV E1A CR1 encodes the 12S form of E1A with a mutation in the CR1 site which is important for p300 binding, and pRC/CMV is the empty expression vector (14).
All transfections were performed by electroporation. In experiments using PK-A or CREB expression plasmids, HeLa cells were transfected with 25 μg of LDH-GH test plasmid, 25 μg of α1-globin control plasmid, and 40 μg of expression plasmid. In experiments using E1A expression plasmids, HeLa cells were transfected with 30 μg of LDH-GH test plasmid, 20 μg of α1-globin control plasmid, and 40 μg of expression plasmid. Hepa-1 and Hepa-1 c4 cells were transfected with 30 μg of LDH-GH test plasmid and 20 μg of α1-globin control plasmid. In all experiments, pools of transfected cells were split equally and incubated in parallel under normoxic or hypoxic conditions for 16 h.
RNA preparation and analysis.
RNA was extracted by a modified acid-guanidinium thiocyanate-phenol-chloroform method (RNA STAT-60; Tel-Test “B,” Inc., Friendswood, Tex.) and dissolved in hybridization buffer [80% formamide, 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 400 mM sodium chloride, 1 mM EDTA (pH 8)]. RNase protection assays were performed with 5 to 10 μg of RNA, using a double hybridization with probes that protect 120 bp of GH mRNA and 120 bp of α-globin mRNA. For quantitation of gels, a PhosphorImager was used with ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). Results are stated as the mean ± standard error of the mean of three independent experiments.
Biotinylated probe pull-down and Western blot assays.
The designs of wild-type and mutant biotinylated probes from the LDH-A promoter and Epo enhancer are illustrated in Fig. 1. Oligonucleotides, biotinylated at the 5′ end, were purified by PAGE, and equimolar amounts of complementary strands were annealed. Dynabeads M-280 Streptavidin (Dynal, Inc., Lake Success, N.Y.) were prepared by washing three times in phosphate-buffered saline (pH 7.4) containing 0.1% bovine serum albumin and two times with Tris-EDTA containing 1 M NaCl. Between each wash, beads were pulled down with a Dynal magnetic particle concentrator. Double-stranded, biotinylated DNA probe was added to the washed beads, and the beads were incubated for 20 min at room temperature with mixing to keep the beads in solution. The beads were then washed three times in Tris-EDTA with 1 M NaCl and two times in 1× binding buffer (10 mM Tris [pH 7.5], 50 mM KCl, 1 mM MgCl2, 1 mM EDTA, 5.5 mM DTT, 5% glycerol, .03% Nonidet P-40). Nuclear extract (50 μg) was preincubated for 5 min at room temperature in a 100-μl reaction volume containing 1× binding buffer, 2.5 μg of poly(dI-dC), and 1 mM sodium orthovanadate (Sigma, St. Louis, Mo.). The binding reaction was then mixed with 250 μg of Dynabeads M-280 Streptavidin bound to 10 pmol of probe and incubated for a further 20 min at room temperature. Proteins bound to the probe were pulled down with a magnetic particle concentrator, and unbound proteins were washed away. For high-stringency wash in p300 experiments, the beads were washed three times in 1× binding buffer with 0.5 μg of poly(dI-dC) per ml. For all other experiments, the beads were washed once in 0.5× buffer (5 mM Tris [pH 7.5], 25 mM KCl, 0.95 mM EDTA, 5 mM DTT, 0.03% Nonidet P-40) containing 0.5 μg of poly(dI-dC) per ml. After the binding reaction and after each wash, proteins bound to the probe were pulled down with a magnetic particle concentrator.
Beads were resuspended in 1× sample buffer, boiled for 5 min, and resolved by sodium dodecyl sulfate-PAGE. Gels were transblotted overnight to Immobilon-P transfer membranes (Millipore, Bedford, Mass.) by using a Mini Trans-Blot electrophoresis transfer cell (Bio-Rad, Hercules, Calif.). Membranes were probed with a monoclonal ATF-1/CREB-1 cross-reactive antibody (25C10G; Santa Cruz Biotechnology), an ARNT polyclonal antibody, or a mixture of p300/CBP cross-reactive monoclonal antibodies (AC240, AC26, and RW 128, as described previously [4]). Antigen-antibody complexes were visualized by enhanced chemiluminescence (NEN, Boston, Mass.).
RESULTS
Identification of proteins binding to functionally critical sites in the LDH-A promoter.
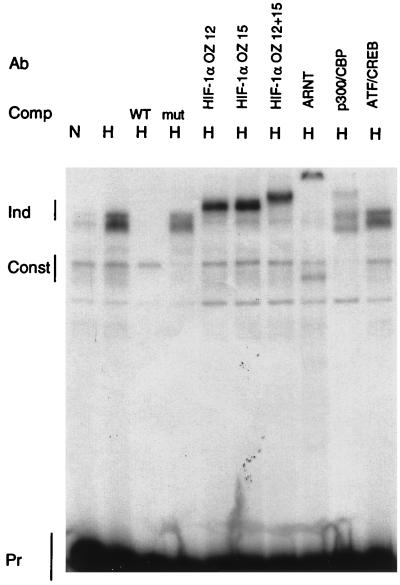
To determine which proteins bind to the functionally critical sites, EMSAs were performed with the addition of antibodies to detect specific DNA binding or coactivator proteins. As shown in Fig. 2, the wild-type probe L24-HIF, encompassing one full HIF-1 consensus sequence, is shifted by both a hypoxia-dependent binding activity and a weaker, constitutive binding activity (lanes 1 and 2). An excess of wild-type L24-HIF competed for binding of the inducible complex, while a mutant competitor in which the HIF-1 site was mutated, mut B, did not compete. Two monoclonal antibodies to HIF-1α supershifted the entire hypoxically inducible binding activity, as did a polyclonal antibody to ARNT, thus demonstrating that this inducible binding activity consists of the HIF-1α–ARNT heterodimer. An antibody to the coactivator protein, p300, produced a supershift, consistent with the finding that p300 interacts with HIF-1α. p300 and CBP are homologous proteins, both by sequence and by functional analysis (31), and the antibodies used are cross-reactive. Monoclonal antibody 25C10G, which cross-reacts with both CREB-1 and ATF-1, was previously shown to supershift the constitutive binding activity observed in mobility shift assays of the HIF-1 site from the Epo enhancer (27). In EMSAs of the HIF-1 site from the LDH-A promoter, however, this same antibody did not supershift the constitutive binding activity (Fig. 2). Specificity of the supershifts was tested by using nonspecific monoclonal antibodies and the preimmune serum for the ARNT polyclonal antiserum. Neither had any effect on the electrophoretic mobility shifts (data not shown).
FIG. 2.
Binding of proteins to the HIF-1 site. EMSAs were performed with the L24-HIF probe with the addition of nuclear extract from HeLa cells incubated in normoxia (N) or hypoxia (H). Inducible (Ind) and constitutive (Const) complexes, as well as the free probe (Pr), are labeled. The addition of competitor (Comp) or antibody (Ab) is noted. OZ12 and OZ15 are monoclonal antibodies to HIF-1α; the combination of both antibodies caused an additive supershift. Supershifts were also observed with an ARNT polyclonal and a p300/CBP monoclonal antibody (AC240) (4) but not with an ATF-1/CREB-1 monoclonal antibody (25C10G; Santa Cruz). WT, wild type; mut, mutant.
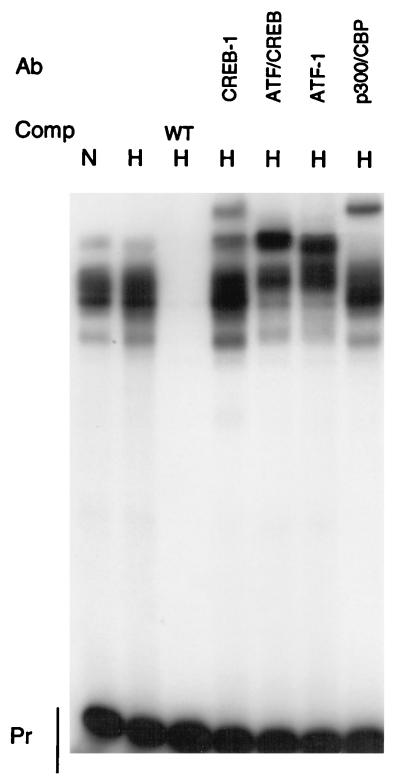
Similar experiments were performed with the probe L24-CRE, which encompasses the consensus CRE in the LDH-A promoter (Fig. 3). Although several proteins bound to the wild-type probe, no differences in binding activities were noted between normoxic and hypoxic nuclear extracts. Wild-type L24-CRE competed for binding of all of the proteins. Antibodies specific to CREB-1 and ATF-1 produced supershifts, as did an antibody that cross-reacts with both CREB-1 and ATF-1. CREB-1 and ATF-1 are both capable of forming homodimers and heterodimers. The CRE is important for the regulation of LDH-A by cAMP. Both CREB-1 and ATF-1 are cAMP responsive (30), but a large number of other proteins which bind to sites similar to a CRE have been identified. It is likely that a variety of these proteins can potentially bind to the LDH-A CRE under different conditions.
FIG. 3.
Binding of proteins to the CRE. EMSAs were performed with the L24-CRE probe with the addition of HeLa nuclear extract from normoxic (N) or hypoxic (H) cells. Free probe (Pr), competitors (Comp), and antibodies (Ab) are noted. Supershifts were observed with antibodies to CREB-1 (C-21; Santa Cruz), ATF-1/CREB-1 (25C10G; Santa Cruz), ATF-1 (C41-5.1; Santa Cruz), and p300/CBP. WT, wild type.
The coactivator protein CBP binds to CREB-1 and ATF-1 to activate transcription (28), and the highly homologous protein p300 also binds to CREB-1 (31). An antibody which cross-reacts with both CBP and p300 produced a supershift with the L24-CRE probe.
Functional importance of HIF-1, CREB-1/ATF-1, and p300 in the LDH-A promoter.
Results of EMSAs presented above indicate that HIF-1α, ARNT, CREB-1, ATF-1, and p300/CBP bind to sites in the LDH-A promoter in vitro. To assess the functional contributions of these molecules to the regulation of the LDH-A promoter by hypoxia, the abundance or activity of these proteins was modified in tissue culture cells which were transiently transfected with a plasmid containing the LDH promoter inserted upstream of a GH reporter gene.
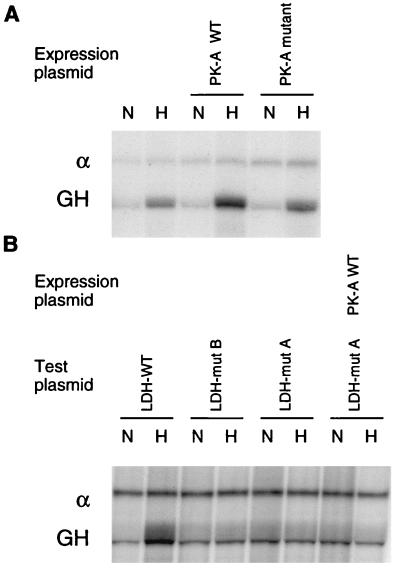
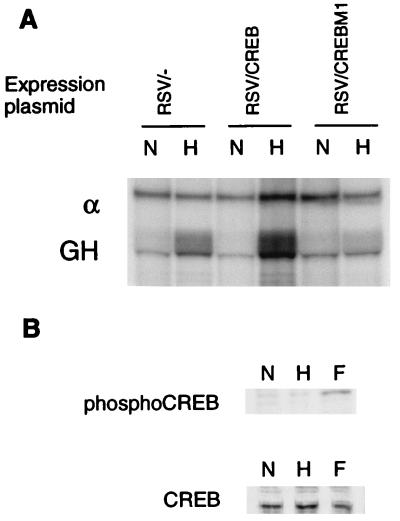
The functional importance of CREB-1 or ATF-1 binding to the CRE was tested by cotransfection of an expression plasmid encoding PK-A. PK-A phosphorylates CREB-1 at serine 133, enabling the activation of transcription by CREB-1 via CBP (28). As shown in Fig. 4A, expression of PK-A increased hypoxic induction of the GH reporter from 5.4 ± 0.3-fold to 7.3 ± 0.9-fold (mean ± standard error of three independent experiments). Expression of a mutated form of PK-A which is functionally inactive did not affect hypoxic induction (5.6 ± 0.5-fold). As shown in Fig. 4B, mutations of either the CRE or HIF-1 sites abrogated hypoxic induction (1.1 ± 0.2 and 0.9 ± 0.1-fold, respectively), and mutation of the CRE abrogated the enhancement by PK-A (1.0 ± 0.2-fold).
FIG. 4.
PK-A expression increases hypoxic inducibility of the LDH-A promoter. Cells were cotransfected with a test plasmid, encoding the LDH-A promoter and GH reporter gene, and a control plasmid, encoding the α-globin promoter (α) and gene. Transfected cells were incubated in parallel in normoxia (N) and hypoxia (H), and expression of GH and α-globin was analyzed by RNase protection assay. (A) Expression of wild-type (WT) PK-A increased hypoxic induction of the GH reporter, while expression of a functionally inactive PK-A mutant did not affect hypoxic induction. (B) Mutations in either site B (HIF-1) or site A (CRE) abrogated hypoxic induction, and mutation of site A blocked the effect of PK-A as well.
Cotransfection of an expression vector encoding CREB-1 caused an increase in hypoxic induction of the reporter gene to 5.1 ± 1.6-fold, compared with 2.9 ± 0.5-fold in cells cotransfected with an empty expression vector (Fig. 5A). CREBM1 is a dominant negative form of CREB-1 in which serine 133 is mutated, thus preventing phosphorylation and transcriptional activation (44). When a plasmid expressing CREBM1 was cotransfected into HeLa cells, hypoxic induction of the LDH-A promoter was decreased to 1.4 ± 0.3-fold (Fig. 5A), indicating that blocking the function of CREB-1 and ATF-1 is effectively equivalent to mutating the CRE.
FIG. 5.
Effect of CREB overexpression and CREBM1 on the LDH-A promoter. (A) Hypoxic induction of the transfected GH reporter, relative to α-globin control (α), was measured by RNase protection assay. Hypoxic induction was increased by cotransfection of a CREB expression plasmid and decreased by expression of a dominant negative protein, CREBM1. (B) To test whether hypoxia influences the phosphorylation of CREB, Western blot analyses were performed with nuclear extracts from HeLa cells incubated in normoxia (N), hypoxia (H), or 20 μM forskolin (F). Blots were probed with an anti-phospho-CREB antibody (UBI) or an anti-CREB antibody (25C10G; Santa Cruz).
We tested whether CREB phosphorylation is altered by hypoxia by probing Western blots with an antibody specific for the form of CREB which has been phosphorylated at serine 133 (UBI). The same degree of phosphorylation was observed in extracts from HeLa cells incubated in normoxia and hypoxia (Fig. 5B).
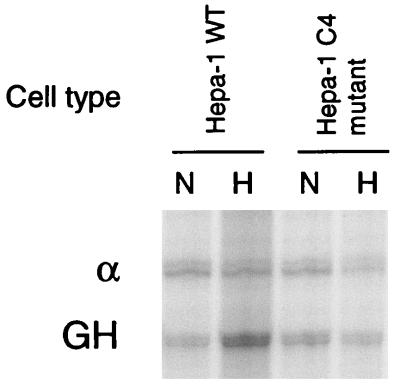
To provide functional evidence for the role of HIF-1 in the LDH-A promoter, activity of the LDH-A promoter was analyzed in Hepa-1 c4 cells, a mouse hepatoma cell line which lacks functional ARNT. Nuclear extracts from Hepa-1 c4 cells lack HIF-1 DNA binding activity, and the endogenous LDH-A gene does not respond to hypoxia in Hepa-1 c4 cells (48). As illustrated in Fig. 6, hypoxic induction of the reporter gene was intact in wild-type Hepa-1 cells (3.1 ± 1.0-fold), but hypoxic inducibility was not present in the ARNT-deficient Hepa-1 c4 cell line (0.9 ± 0.2-fold).
FIG. 6.
The LDH-A promoter is not regulated by oxygen in ARNT-deficient cells. Hypoxic induction of the transfected GH reporter, relative to α-globin control, was measured by RNase protection assay. Hypoxic inducibility of the LDH-A promoter was intact in wild-type Hepa-1 cells, but in the ARNT-deficient cell line Hepa-1 c4, the LDH-A promoter was not responsive to hypoxia. For abbreviations, see the legend to Fig. 4.
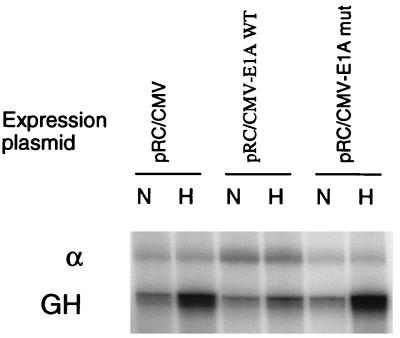
The functional role of p300/CBP in the LDH promoter was tested by using the adenovirus E1A protein. Overexpression of E1A by adenovirus infection or transfection of an E1A expression vector has been shown to block many of the roles of p300, including its function as a coactivator (1). As shown in Fig. 7, in cells cotransfected with an empty expression vector, the reporter gene was induced 6.9 ± 0.9-fold by hypoxia; but in cells cotransfected with an E1A expression vector, this response was nearly eliminated (1.7 ± 0.4-fold). In contrast, cotransfection of an expression vector encoding a mutated form of E1A, which does not interact with p300/CBP, did not diminish hypoxic induction of the LDH-A promoter (6.8 ± 1.2-fold).
FIG. 7.
Requirement of p300 for oxygen regulation. Cells were cotransfected with a vector expressing wild-type (WT) E1A or a vector expressing a mutant (mut) form of E1A which does not bind to p300. Hypoxic induction of the reporter, relative to α-globin control (α), was measured by RNase protection assay. Expression of E1A nearly abolished induction by hypoxia, whereas the mutated E1A did not affect hypoxic inducibility of the LDH-A promoter.
Formation of a hypoxically inducible complex in the LDH-A promoter.
Our EMSA and transient transfection experiments provide evidence for the binding and functional importance of HIF-1, CREB-1/ATF-1, and p300/CBP in the regulation of the LDH-A promoter by hypoxia. To investigate how these proteins interact, we used a technique which permits the analysis of a multiprotein complex. A double-stranded DNA probe which encompasses all three functionally important sites in the LDH-A promoter was synthesized, and one strand was 5′ biotinylated (Fig. 1A). The probe was bound to streptavidin-coated magnetic beads and then incubated with nuclear extract, enabling the multiprotein complex to form. Beads, probe, and associated proteins were pulled down with a magnet, and unbound proteins were washed away. The remaining, purified proteins which were bound to the probe were loaded on a denaturing gel and analyzed by Western blotting.
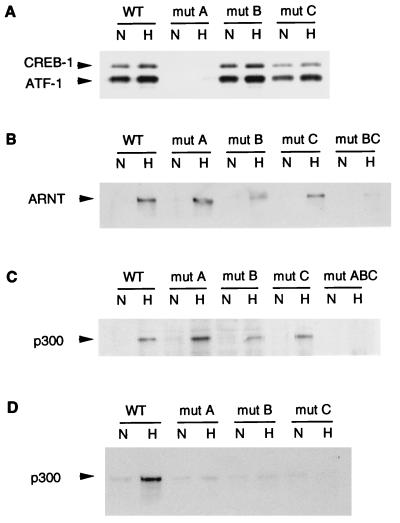
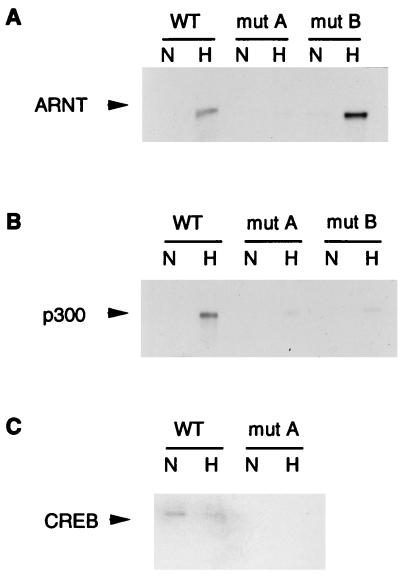
Both CREB-1 and ATF-1 bound to the wild-type LDH-A probe (Fig. 8A). However, with a probe in which the CRE was mutated (mut A), CREB-1 and ATF-1 binding was completely eliminated. Mutation of either of the HIF-1 sites did not significantly affect CREB-1 or ATF-1 binding. As mentioned above, it has been proposed that CREB-1 and ATF-1 bind constitutively to the HIF-1 site in the Epo enhancer (27). As shown in Fig. 8A, the mut A probe has an intact HIF-1 site and a mutated CRE, but binding of CREB-1 and ATF-1 was completely eliminated. Thus, both EMSA supershift experiments and experiments using a biotinylated probe to analyze complex formation indicate that neither CREB-1 nor ATF-1 interacts with either of the HIF-1 sites in the LDH-A promoter.
FIG. 8.
Hypoxically inducible complex in the LDH-A promoter. Nuclear extract from normoxic (N) or hypoxic (H) HeLa cells was incubated with a double-stranded, biotinylated probe bound to streptavidin-coated magnetic beads. Multiprotein complexes were purified by using a magnet, washed, and analyzed by Western blotting. Binding was compared between wild-type (WT) LDH-A probe and probes in which functionally critical sites were mutated. In the probe denoted mut BC, both sites B and C were mutated; in mut ABC, sites A, B, and C were mutated. Western blots were probed with antibodies to CREB-1/ATF-1 (25C10G; Santa Cruz) (A), ARNT (B), or p300 (C and D). In panel C, protein complexes bound to the probe and beads were washed with low stringency; in panel D, protein complexes were washed with high stringency.
ARNT binding was present only in hypoxic nuclear extracts and was unaffected by mutation of the CRE (Fig. 8B). ARNT binding was significantly decreased but was still present when site B, the HIF-1 consensus site, was mutated. Moreover, ARNT binding was also preserved when site A was mutated. In contrast, when a probe in which both sites B and C were mutated (mut BC) was tested, ARNT binding was nearly abolished. Site C is slightly different from HIF-1 sites in other genes. EMSAs have been used to show that site C in the LDH-A promoter binds HIF-1 weakly as well as an unknown, constitutive binding activity (40). Results of assays using the biotinylated probe confirm that HIF-1 binds both sites B and C but binds more strongly to site B. Similar results were obtained in experiments analyzing binding of HIF-1α (data not shown).
The use of a large biotinylated probe permits an analysis of multiple contacts between p300 and DNA-bound proteins. As shown in Fig. 8C, p300 was recruited to the multiprotein complex only under hypoxic conditions. Under conditions in which the beads were washed with low stringency, individual mutations of any of the three sites did not significantly affect p300 binding to the complex, but a triple mutation (mut ABC), in which all three functionally critical sites were mutated, did eliminate p300 binding. Under high-stringency conditions, p300 binding was again hypoxically inducible, but in contrast with results of assays using low-stringency conditions, p300 binding was substantially decreased by any of the single mutations (Fig. 8D). Thus, if single sites are mutated, p300 can still bind weakly to the intact sites. High-affinity binding of p300, however, requires multiple contacts with proteins binding at each of the functionally critical sites.
Similar results were obtained in assays using an independent set of biotinylated probes generated by PCR rather than by direct oligonucleotide synthesis.
Formation of a hypoxically inducible complex in the Epo enhancer.
In the Epo enhancer, the layout of functionally critical sites is remarkably similar to the organization of interacting sites in the LDH-A promoter (5, 36, 43). In the Epo enhancer, as in the LDH-A promoter, there are three sites which are necessary for oxygen regulation. One site binds HIF-1, and one site is a direct repeat consensus for a nuclear HRE and binds the orphan nuclear receptor HNF-4 (18).
To investigate interacting proteins binding to the Epo enhancer, a biotinylated probe encompassing this region was synthesized (Fig. 1B). Similar to results obtained with LDH-A, ARNT binding was oxygen dependent and was unaffected by mutation of the HRE, whereas mutation of the HIF-1 site eliminated ARNT binding (Fig. 9A). p300 binding, under stringent wash conditions, was oxygen dependent and was substantially decreased by mutation of either the HIF-1 or the HRE site (Fig. 9B). Thus, in the Epo enhancer, as in the LDH-A promoter, strong binding of p300 is dependent on multiple adjacent sites.
FIG. 9.
Hypoxically inducible complex in the Epo 3′ enhancer. Nuclear extract from normoxic (N) or hypoxic (H) HeLa cells was incubated with a biotinylated probe derived from the Epo 3′ enhancer. Multiprotein complexes were pulled down and analyzed on Western blots probed with antibodies to ARNT (A), p300 (B), or CREB-1/ATF-1 (25C10G; Santa Cruz) (C). Binding to the wild-type (WT) probe was compared with binding to probes with mutations in the HIF-1 site (mut A) or HRE (mut B). p300 binding was tested under high-stringency wash conditions.
Since CREB had previously been shown to bind constitutively to the Epo HIF-1 site (27), we examined the binding of CREB to the Epo enhancer. Consistent with the findings of Kvietikova et al. (27), CREB bound constitutively to the wild-type Epo probe but did not bind to a probe in which the HIF-1 site was mutated (Fig. 9C).
DISCUSSION
In this study, we used a combination of in vitro binding assays and in vivo functional assays to demonstrate that HIF-1, CREB-1/ATF-1, and p300 participate in a cooperative, hypoxically inducible complex that interacts with the LDH-A promoter.
HIF-1 binding was demonstrated by EMSA. Antibodies to both HIF-1α and ARNT supershifted the hypoxically inducible binding activity. Use of a longer, biotinylated probe to investigate HIF-1 binding in the context of a larger complex revealed that HIF-1 binds to two of the functional sites in the LDH-A promoter. Although mutation of either site did not completely block HIF-1 binding, hypoxic induction of the reporter gene was abrogated. Mutation of both sites fully eliminates HIF-1 binding. Since the LDH-A promoter was not regulated by oxygen in ARNT-deficient cells, HIF-1 was shown to be functionally necessary for hypoxic induction of the LDH-A promoter.
Binding of CREB-1 and ATF-1 to the functionally critical CRE in the LDH-A promoter was demonstrated both by EMSA and by pulling these proteins down with a biotinylated probe. Overexpressing CREB-1 and increasing the phosphorylation of CREB-1 by PK-A overexpression both increased the magnitude of hypoxic induction. Expression of a CREB dominant negative protein eliminated this response. The ability to influence hypoxic inducibility by manipulating activity of proteins binding to the CRE provides further evidence for an interaction in vivo between proteins binding at the CRE and proteins binding at the HIF-1 site.
Finally, the binding and function of p300 were demonstrated. In EMSAs, antibodies to p300 supershifted complexes binding to the L24-HIF probe and complexes binding to the L24-CRE probe. p300 was pulled down as part of a hypoxically inducible multiprotein complex bound to a biotinylated DNA probe. Expression of the E1A protein, which binds to p300 and prevents its action as a transcriptional coactivator, eliminated hypoxic inducibility of the LDH-A promoter.
Use of biotinylated probes encompassing all of the functionally critical sites permitted an analysis of the formation of a hypoxically inducible complex including both DNA binding proteins and coactivator proteins. Techniques currently used to detect binding between specific proteins may be confounded by false-positive results arising from interactions between irrelevant and nonphysiological orientations. By pulling down the complex with a biotinylated probe, binding of coactivator proteins was investigated in a more native context in which transcription factors are fixed in orientation by binding to DNA. By varying conditions for washing away unbound proteins, the relative affinity of coactivator binding was tested. In these experiments, mutation of one site did not affect the binding of proteins to an adjacent site, indicating that interactions between adjacent transcription factors was not essential for binding of these proteins to DNA. Since p300 is known to interact with both CREB-1 and HIF-1, it is not surprising that p300 was capable of binding with low affinity when single cis-acting sequences were mutated. In contrast, high-affinity binding was blocked by mutation of any of the three functionally critical sites, implying that p300 contacts DNA-bound proteins binding at each of these sites. Thus, cooperation between these three loci for maximal p300 binding is consistent with the functional experiments which demonstrate that these three sites are required for hypoxic induction (17).
The arrangement of functional cis-acting sequences in the LDH-A promoter is analogous to the layout of sites in other genes. The Epo 3′ enhancer is very similar except that an HRE is present instead of a CRE (18). Consistent with findings for the LDH-A promoter, high-affinity binding of p300 to the hypoxically inducible complex in the Epo enhancer was blocked by mutation of either the HIF-1 site or the HRE. The dependence of p300 binding on occupancy of the HRE is consistent with finding that nuclear hormone receptors interact with p300 (7, 26). The tyrosine hydroxylase gene, which encodes the rate-limiting enzyme in dopamine synthesis, is regulated by hypoxia and has adjacent HIF-1 and AP-1 binding sites, both of which play a role in oxygen-dependent expression of tyrosine hydroxylase (34). AP-1 proteins have been shown to interact with p300 to activate transcription (3), and so it is likely that HIF-1, p300, and AP-1 form a complex at the tyrosine hydroxylase promoter. p300 has also been shown to interact with many other transcription factors, including STAT1 and -2 (4, 50), the p65 subunit of NF-κB (19, 35), MyoD and MEF2C (15, 38, 39, 49), and p53 (2). Given the broad range of transcription factors with which p300 interacts, it is likely that HIF-1 sites in other genes are adjacent to sites for transcription factors which interact with p300. Thus, a common theme in oxygen-regulated gene expression may be the formation of a hypoxically inducible complex including HIF-1 and another transcription factor which binds to a site adjacent to HIF-1, both of which interact with p300.
The LDH-A promoter has two adjacent sites which bind HIF-1, and mutation of either of these sites abrogates both hypoxically inducible function of the promoter (17) and high-affinity binding of p300. In addition to LDH-A, two adjacent HIF-1 sites, arranged as either direct or inverted repeats, have been noted in several oxygen-regulated genes, including those encoding enolase 1 and phosphoglycerate kinase 1 (40). When the activity of a 25-bp sequence containing a single HIF-1 site was compared to a dimer of this sequence, hypoxic induction of a reporter gene increased from less than 5-fold induction to greater than 50-fold induction (36). The correlation between high-affinity p300 binding and hypoxically inducible activity of tandem HIF-1 sites implies that synergy between tandem HIF-1 sites may be mediated by the coactivator, p300.
p300 is a large molecule with multiple protein binding domains. HIF-1α binds to the first cysteine/histidine-rich region of p300, encompassing amino acids 346 to 410 (1). Distinct from the HIF-1α binding domain, the CREB binding domain lies between amino acids 462 and 661 (9). The nuclear receptor ligand binding domain interacts with the amino-terminal 170 amino acids of CBP as well as possibly the first cysteine/histidine-rich region (7). Other regions of p300 interact with TATA binding protein (49) and TFIIB (28). Given the distinct binding sites on the p300 molecule for HIF-1 and CREB, it is possible that a single p300 molecule binds to multiple transcription factors in the LDH-A promoter and contacts the basal transcription machinery.
In addition to its function as a transcriptional coactivator, p300 itself and two proteins with which p300 interacts, ACTR and P/CAF, have histone acetyltransferase activity (8). Core histone hyperacetylation is linked to activation of gene expression (47). HIF-1 activation and induction of gene expression by hypoxia is maximal by 4 h and does not diminish for over 24 h. Thus, formation of a hypoxically inducible complex involving HIF-1 and p300 may further regulate gene expression by influencing chromatin structure.
By using a long, biotinylated probe, we were able to capture and analyze an intact, hypoxically inducible complex of proteins including both DNA binding proteins and the coactivator, p300. Mutation of functionally critical sites blocked high-affinity binding of p300 to both the LDH-A promoter and the Epo enhancer. The correlation between high-affinity binding of p300 and hypoxically inducible functional activity as well as the abrogation of functional activity by E1A expression indicate that p300 binding is central to activation of transcription by hypoxia. The requirement of multiple interactions for recruitment of p300 and the importance of p300 for transcriptional activation may contribute significantly to highly specific and precise regulation of gene expression in response to hypoxia and other stimuli.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (DK41234) to H.F.B.
We thank the following for providing reagents: David Livingston for HIF-1α antibodies, p300 antibodies, and E1A expression plasmids; Oliver Hankinson for the ARNT antibody and Hepa-1 c4 cells; Marc Montminy for the CREBM1 expression plasmid and PK-A expression plasmids; and Richard Goodman for the CREB expression plasmid. We thank Maureen Schau for technical assistance, and we thank Eric Huang, Jie Gu, Peter Ratcliffe, and Tucker Collins for helpful advice and critical review of the manuscript.
REFERENCES
- 1.Arany Z, Huang L E, Eckner R, Bhattacharya S, Jiang C, Goldberg M A, Bunn H F, Livingston D M. Participation by the p300/CBP family of proteins in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 3.Bannister A J, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995;14:4758–4762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, Livingston D M. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard K L, Acquaviva A M, Galson D L, Bunn H F. Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol Cell Biol. 1992;12:5373–5385. doi: 10.1128/mcb.12.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunn H F, Poyton R O. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/p300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Lin R J, Schlitz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 9.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 10.Czyzyk-Krzeska M F, Dominski Z, Kole R, Millhorn D E. Hypoxia stimulates binding of the cytoplasmic protein to a pyrimidine-rich sequence in the 3′-untranslated region of rat tyrosine hydroxylase mRNA. J Biol Chem. 1994;269:9940–9945. [PubMed] [Google Scholar]
- 11.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebert B L, Firth J D, Ratcliffe P J. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J Biol Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- 13.Ebert B L, Gleadle J M, O’Rourke J F, Bartlett S M, Poulton J, Ratcliffe P J. Isoenzyme-specific regulation of genes involved in energy metabolism by hypoxia: similarities with the regulation of erythropoietin. Biochem J. 1996;313:809–814. doi: 10.1042/bj3130809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 15.Eckner R, Yao T-P, Oldread E, Oldread D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 16.Firth J D, Ebert B L, Pugh C W, Ratcliffe P J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firth J D, Ebert B L, Ratcliffe P J. Hypoxic regulation of lactate dehydrogenase A: interaction between hypoxia inducible factor 1 and cAMP response elements. J Biol Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- 18.Galson D L, Tsuchiya T, Tendler D S, Huang L E, Ren Y, Ogura T, Bunn H F. The orphan receptor hepatic nuclear factor 4 functions as a transcriptional activator for tissue-specific and hypoxia-specific erythropoetin gene expression and is antagonized by EAR3/COUP-TF1. Mol Cell Biol. 1995;15:2135–2144. doi: 10.1128/mcb.15.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleadle J M, Ebert B L, Firth J D, Ratcliffe P J. Regulation of angiogenic growth factor expression by hypoxia, transition metals and chelating agents. Am J Physiol. 1995;268:C1362–C1368. doi: 10.1152/ajpcell.1995.268.6.C1362. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg M A, Schneider T J. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- 22.Huang, L., J. Gu, and H. Bunn. Regulation of hypoxia-inducible factor 1α is mediated by its oxygen-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 23.Huang L E, Arany Z, Livingston D M, Bunn H F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its a subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 24.Jelkmann W. Erythropietin: structure, control of production, and function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 25.Kallio P J, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1a: posttranslational regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94:5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 27.Kvietikova I, Wenger R H, Marti H H, Gassman M. The transcription factors ATF-1 and CREB-1 bind constitutively to the hypoxia-inducible factor-1 (HIF-1) DNA recognition site. Nucleic Acids Res. 1995;23:4542–4550. doi: 10.1093/nar/23.22.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 29.Levy A P, Levy N S, Goldberg M A. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Thompson M A, Wagner S, Greenberg M E, Green M R. Activating transcription factor-1 can mediate Ca2+- and cAMP-inducible transcriptional activation. J Biol Chem. 1993;268:6714–6720. [PubMed] [Google Scholar]
- 31.Lundblad J R, Kwok R P S, Laurance M E, Harter M L, Goodman R H. Adenovirus E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell P H, Dachs G U, Gleadle J M, Nicholls L G, Harris A L, Stratford I J, Hankinson O, Pugh C W, Ratcliffe P J. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell P H, Pugh C W, Ratcliffe P J. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norris M L, Millhorn D E. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 35.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 36.Pugh C W, Ebert B L, Ebrahim O, Ratcliffe P J. Characterisation of functional domains within the mouse erythropoietin 3′ enhancer conveying oxygen-regulated responses in different cell lines. Biochim Biophys Acta. 1994;1217:297–306. doi: 10.1016/0167-4781(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 37.Pugh C W, O’Rourke J F, Nagao M, Gleadle J M, Ratcliffe P J. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the a subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 38.Puri P L, Avantaggiati M L, Balsano C, Sang N, Graessmann A, Giordano A, Levrero M. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 1997;16:369–383. doi: 10.1093/emboj/16.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartobelli V, Huang J, Hamamori Y, Kedes L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol Cell Biol. 1997;17:1010–1026. doi: 10.1128/mcb.17.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza G L, Jiang B-H, Leung S W, Passatino R, Concordet J-P, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 41.Semenza G L, Koury S T, Nejfelt M K, Gearhart J D, Antonarakis S E. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci USA. 1991;88:8725–8729. doi: 10.1073/pnas.88.19.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semenza G L, Roth P H, Fang H-M, Wang G L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 43.Semenza G L, Wang G L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Struthers R S, Vale W W, Arias C, Sawchenko P E, Montminy M R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature. 1991;350:622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- 45.Wang G L, Semenza G L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang G L, Semenza G L. Purification and characterization of hypoxia-inducible factor-1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 47.Wolffe A P, Pruss D. Targeting chromatin disruption: transcriptional regulators that acetylate histones. Cell. 1996;84:817–819. doi: 10.1016/s0092-8674(00)81059-4. [DOI] [PubMed] [Google Scholar]
- 48.Wood S M, Gleadle J M, Pugh C W, Hankinson O, Ratcliffe P J. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. J Biol Chem. 1996;269:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- 49.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–9013. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J J, Vinkemeier U, Gu W, Chakravarti D, Horvath C M, Darnell J E. Two contact regions between Stat1 and CBP/p300 in interferon-γ signalling. Proc Natl Acad Sci USA. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]