Abstract
The endocrine system is a fundamental type of long-range cell-cell communication that is important for maintaining metabolism, physiology, and other aspects of organismal homeostasis. Endocrine signaling is mediated by diverse blood-borne ligands, also called hormones, including metabolites, lipids, steroids, peptides, and proteins. The size and structure of these hormones are fine-tuned to make them bioactive, responsive, and adaptable to meet the demands of changing environments. Why has nature selected such diverse ligand types to mediate communication in the endocrine system? What is the chemical, signaling, or physiologic logic of these ligands? What fundamental principles from our knowledge of endocrine communication can be applied as we continue as a field to uncover additional new circulating molecules that are claimed to mediate long-range cell and tissue crosstalk? This review provides a framework based on the biochemical logic behind this crosstalk with respect to their chemistry, temporal regulation in physiology, specificity, signaling actions, and evolutionary development.
Keywords: endocrine, ligand size, metabolites, signaling, evolution
Background
The endocrine system
The endocrine system is a large network of organs in the body that produces, stores, and secretes blood-borne factors called hormones. These hormones serve as the central long-range communication system of the body and maintain various physiological states. The discovery of endocrine organs and endocrine hormones dates back to the observation that the removal of specific organs would induce dramatic phenotypes, including disease or even death. Remarkably, these phenotypes could be entirely reversed by re-administration of crude biochemical preparations of the organs that were excised. These observations gave rise to the hypothesis that specific “factors” secreted by these excised organs were important for normal homeostasis and health. However, within the endocrine system, the roles of molecules traditionally labeled as “signaling metabolites” or “hormones” are not strictly defined, as they share many similarities. Distinctive features, such as their contribution to metabolic reactions—where metabolites often serve as precursors or intermediates and hormones exert regulatory control—provide a basis for differential consideration. Additionally, the specificity and affinity of receptor interactions offer further delineation; metabolites generally exhibit lower affinity and broader receptor interactions, contrasting with the high-affinity, targeted receptor binding characteristic of hormones. However, contextual factors may confer attributes of both systems to each class. The eventual biochemical purification and identification of such factors, which include insulin from the pancreas 1,2, thyroid hormone from the thyroid gland 3,4, glucocorticoids from the adrenal gland 5,6, and sex hormones from the reproductive organs 7, provided direct evidence of the presence of endocrine hormones, and by extension, endocrine communication.
In addition to the traditional biochemical, activity-guided fractionation methods, newer genetic techniques have also brought forth a new era of endocrine hormone discovery and research into endocrine communication. For instance, hormones such as fibroblast growth factor 21 (FGF21) 8,9, leptin 10, and growth differentiation factor 15 (GDF15) 11-14 were identified, not from organ resections, but through genetic screening and characterization. Interestingly, unlike classical endocrine organs like the thyroid or pancreas, the cell types and organs or tissues producing these hormones cannot be easily resected or even clearly delineated. This highlights the increasing complexity of the origins of endocrine hormones, with many tissues contributing to organ communication beyond classical glands alone.
Why does the body rely on endocrine signaling?
French physiologist Claude Bernard was the first to suggest that the exchange of chemical messengers is essential for maintaining the stability of the internal environment, or “milieu interieur” 15. Signaling involves a complex series of interactions beginning with the release of a secreted signaling molecule or ligand. The ligand travels by blood to interact with a specific receptor on a target cell. This interaction triggers a cascade of intracellular events, often mediated by second messengers, leading ultimately to a specific cellular response. The response might be a change in gene expression, protein function, or cell behavior 16,17. The endocrine system serves to integrate multiple physiological processes, ensuring that organs and tissues function in a coordinated manner, especially in response to external or internal challenges.
Disruptions of endocrine communication can result in various disorders. The translation of endocrine hormones to new therapeutics has dramatically improved human health. Most classically, deficiency of insulin from the pancreas results in diabetes, leading to persistently elevated blood glucose levels due to impaired glucose uptake in peripheral tissues, particularly in muscle and adipose tissues 18,19. The absence of insulin is central to diabetes, a condition that was once invariably lethal. The discovery that diabetic symptoms could be managed, or even reversed, by injecting insulin paved the way for endocrine research 2. Abnormalities in adrenal, parathyroid, and reproductive function can lead to disorders such as adrenal disorders, parathyroid disorders, reproductive disorders, and tumors. Another example is leptin deficiency, where deficiency leads to abnormal energy metabolism, hyperphagia, and obesity 10. Likewise, defects in growth hormone (GH) or insulin-like growth factor (IGF) can lead to dwarfism. Therapeutically, synthetic GH addresses growth deficiencies in children and adults, while leptin therapy can normalize body weight in cases of congenital leptin deficiency.
Recent advances and the need for a new framework
Over the past few decades, the development of more sensitive mass spectrometry methods, including proteomics, peptidomics, lipidomics, and metabolomics 20,21 in combination with genetic approaches has accelerated the discovery of signaling molecules. The success of such techniques in screening novel molecules is exemplified by the fact that novel adipokines such as isthmin-1 (ISM-1) and ependymin related protein 1 (EPDR1) have been discovered with the aid of proteomics-based mapping of secretomes 22,23. In parallel, N glucosyl taurine and supra basin derived peptides have been discovered by metabolomics and peptidomics respectively 24,25. This development has enabled an expanded understanding of intercellular communication. However, since each new molecule brings a unique aspect of biology, our current classification and frameworks primarily based on organ expression might not fully encompass the physiological relevance, regulation, and function of these signaling molecules. While our present knowledge has provided a solid foundation, it is helpful to have a comprehensive framework that also incorporates an understanding of their differences in production, their modes of action, their targets, and their regulatory processes. Moving forward, the line among traditional hormones, cytokines, and other signaling molecules may blur, broadening our understanding of how cells communicate over long distance. Here, we discuss a structured way to study these molecules, helping to understand their roles in health and disease.
Functions, specificity, and selectivity of cellular targets
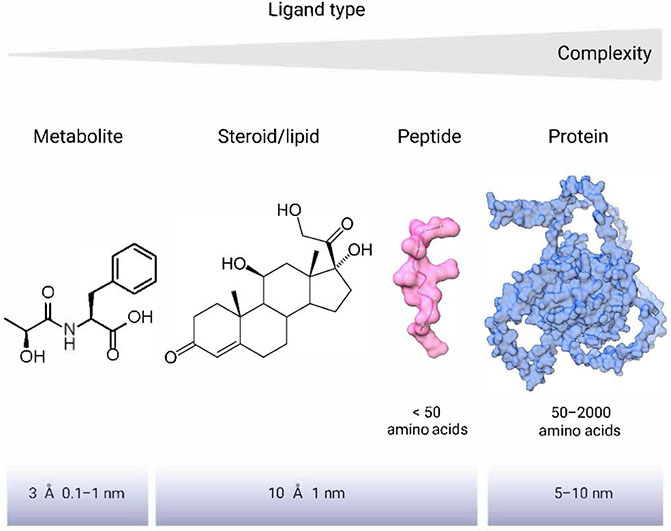
Small molecules (metabolites, lipids), peptides, and proteins are all essential for various biological processes and cellular signaling, but they exhibit differences in their structure, size, function, and mechanisms of action (Fig. 1). Secreted proteins have diverse functions, including enzymatic, signaling, and regulation of gene expression. They are large, often complex molecules composed of several chains of amino acids and have a molecular weight that can range from a few thousand to several million daltons. Given that proteins have unique three-dimensional structures that determine their function and interactions with other molecules, they generally act through specific protein-protein interactions, which depend on the structural complementarity between the interacting partners 26. These interactions can be transient or stable, depending on the nature of the proteins involved and the cellular context. Peptides (< 50 amino acids in size), small molecules, and lipids, on the other hand, are low-molecular weight compounds. Small molecules typically have a molecular weight of less than 900 Da 27. They consist of a limited number of atoms and are composed of relatively simple structures, such as sugars, lipids, and amino acids. Secreted small molecules play a wide range of roles in biological processes, including energy metabolism, cellular signaling, and serving as building blocks. They can act as neurotransmitters, hormones, or secondary messengers in cell signaling pathways (Fig. 1).
Figure 1.
Structures and complexities of selected ligand types. The figure illustrates a spectrum of molecular complexities and sizes, displaying from right to left. On the far right, the structure of the secreted protein ISM-1 is shown, representing the larger and more complex end of the spectrum. Moving leftward, the structure of an 11-mer peptide is depicted, which is smaller and less complex than proteins. Further left, the structure of cortisol represents a middle-of-the-range molecule in terms of size and complexity. On the far left, the structure of Lac-Phe is presented, highlighting a relatively simple metabolite. The diversity in size, complexity, and formation of each structure underscores the sophisticated nature of intercellular and inter-organ communication.
Selectivity and specificity
There are several reasons why nature utilizes different chemical modalities for cell and tissue crosstalk. The selection of ligands, determined by their size and other chemical properties, dictates the tissue-specific expression and functionality of proteins and small molecules. This diversity allows for a high degree of specificity and selectivity in interactions between signaling molecules and their target receptors 28. With this large collection of molecular sizes and structures available, cells can fine-tune their interactions, ensuring that each signaling molecule can selectively activate or inhibit its target without disrupting unrelated pathways. The minimal specificity characteristic of the receptors for these ligands allows for specific interactions, which is a cornerstone of pharmacological interventions 29. While small molecules easily diffuse through extracellular spaces to reach their target cells, larger peptides and proteins often require specific transport mechanisms such as receptor-mediated internalization, vesicle-mediated transport, or carrier proteins. Small molecules and lipids can often diffuse through cell membranes and extracellular spaces, allowing for rapid and direct interactions with target molecules or receptors. Smaller signaling molecules frequently bind to protein targets via non-covalent interactions, such as hydrogen bonding, ionic interactions, or hydrophobic interactions. For instance, FGF21, FGF19, and leptin are proteins that act as hormones and signal through specific receptors. FGF21 and FGF19 act via the FGF receptors (FGFRs) 1c, 2c, and 3c in conjunction with the obligate co-receptor β-Klotho 9,30. FGFRs, 1, 2, and 3 are predominantly expressed in white adipose, brown adipose, and brain tissues. Interestingly, the activity of FGF19 for FGFR1c/β-Klotho is regulated by a single amino acid in the C-terminus of FGF19 31. β-Klotho, a shared co-receptor of FGF19 and FGF21 mediates its pharmacological functions in tissue-specific manner. For example, β-Klotho in liver and adipocytes is dispensable for the effects of FGF19 and FGF21 on weight loss 32. However, it is indispensable in neurons, where FGF19, FGF21, and bFKB1 (bispecific FGFR1/β-Klotho-activating antibody) require the β-Klotho receptor complex to exert their weight loss functions.
Similarly, leptin is a highly selective signaling molecule, binding to the leptin receptor (LEPR) with high specificity 33. The structural features of leptin, including its four-helix bundle configuration, allow it to interact only with LEPR. Glucagon-like peptide-1 (GLP-1) and neuropeptide Y (NPY) are peptide hormones, and they also signal through specific receptors, commonly G-protein coupled receptors (GPCR). GLP-1’s selectivity is due to its unique peptide sequence fitting into the binding pocket of GLP-1 receptor (GLP-1R). NPY signals via multiple GPCRs (Y1, Y2, Y4, Y5), showing selectivity based on sequence variations between different NPY family peptides 34. On the other hand, prostaglandins (PGs) are lipid-based signaling molecules that interact with a series of GPCRs (PGD2 receptor (DP), PGE2 receptors (EP1–4), PGF2α receptor (FP), PGI2 receptor (IP), thromboxane A2 receptor (TP), chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2)). The diversity of receptors allows for high selectivity based on the specific structure of each PG. Fatty acid esters of hydroxy fatty acids (FAHFAs), and palmitic acid esters of hydroxy stearic acids (PAHSAs) are specific types of lipids that have been identified as signaling molecules involved in various metabolic processes, including insulin sensitivity, insulin secretion, and thermogenesis 35,36. FAHFA/PAHSA lipids signal via multiple receptors, including GPCRs and nuclear receptors. The specificity of these interactions depends on the chain length and degree of saturation of fatty acids. Lastly, lactate and serotonin, while both being metabolites, differ significantly in their signaling mechanisms. As a signaling molecule, lactate exhibits low specificity and interacts with a broad spectrum of protein receptors, G protein-coupled receptor 81 (GPR81, also known as hydroxycarboxylic acid receptor 1 (HCA1)) in certain tissues. Its signaling is less specific than the proteins and peptides described above, likely due to its simpler molecular structure. Serotonin (5-hydroxytryptamine) is a biogenic amine that signals via an array of GPCRs and ligand-gated ion channels, making it a highly specific and versatile signaling molecule 37,38.
To understand the development of specificity, it is helpful to elucidate its origin from an evolutionary perspective. The synthesis of signaling molecules requires a certain metabolic state and a considerable amount of energy. Thus, cells have evolved mechanisms to select signal molecules that are metabolically economical and space-saving. It is interesting to observe a conserved uniformity among organisms in employing smaller signaling molecules for quick and frequent responses like neuronal transmission and muscle contraction 39. Why does an organism recruit different sizes of signal molecules? The answer might lie in versatility contributed by the availability of different signal molecules where size plays an important role. For example, neurotransmitters like dopamine and γ-aminobutyric acid (GABA) can diffuse in less than 1 millisecond across 20 nm synaptic cleft 40. Smaller molecules confer a great advantage to an organism in mediating a rapid signaling event because smaller molecules are mobile and can be stored in higher amounts as a readily accessible signal pool. Moreover, the synthesis of signaling molecules demands a particular metabolic stage and requires a large amount of energy 41.
In summary, the nature of the ligand (protein, peptide, lipid, metabolite) greatly influences its receptor specificity and signaling selectivity (Table 1). These characteristics, in turn, reflect the complexity of the molecular structures and the specific needs of the tissues in which they function.
Table 1.
Ligand size of different classes of secreted signaling molecules and their established roles in metabolism. A, angstrom; nm, nano meter; kDa, kilo dalton.
| Class | Molecule | Ligand size |
Source | Known role in metabolism | References |
|---|---|---|---|---|---|
| Metabolites | Acetylcholine | 0.5 Å | Neurons | Stimulates muscle contraction, involved in learning- and memory | 76,77 |
| Serotonin | 0.5 Å | Brain, gut | Regulates mood, appetite, and sleep, also affects memory and learning | 37,38 | |
| Norepinephrine | 0.5 Å | Brain, sympathetic nervous system | Affects attention and response actions, involved in the fight-or-flight response | 78,79 | |
| Lactate | 0.5 Å | Muscles, red blood cells, brain, other tissues | Waste product of anaerobic metabolism, also used as an energy source by other tissues | 51,80 | |
| Succinate | 0.5 Å | Mitochondria | Involved in the citric acid cycle, signal hypoxia and inflammation | 81 | |
| β-hydroxybutyrate | 0.5 Å | Liver | Used as an energy source during periods of fasting or intense exercise | 82 | |
| Lac-Phe | 1 Å | Macrophages | Secreted after exercise, known to suppress appetite | 83 | |
| T4 | 1 nm | Thyroid gland | Regulates metabolism and growth, controls rate of energy use | 3,84 | |
| Kynurenine | 1 Å | Liver, other tissues | Plays a role in the regulation of immune responses and neuroactive signaling | 85 | |
| Acylcarnitines | 1.5 Å | Various tissues | Involved in fatty acid metabolism, transport fatty acids into mitochondria for β-oxidation | 86,87 | |
| Steroids/lipids | Resolvins/maresins | 0.5 nm | White blood cells, certain tissues | Involved in the resolution of inflammation, promote tissue regeneration | 88,89 |
| Prostaglandins | 1.5 nm | Various cells | Regulate inflammation, involved in the regulation of bone metabolism | 90,91 | |
| Sphingolipids | 1.5 nm | Various cells | Regulate cellular processes including differentiation, proliferation, cell signaling, and apoptosis | 92,93 | |
| Cholesterol | 1 nm | Liver, dietary intake | Function as structural molecule in cell membranes and precursor for steroid hormones, regulates SREBP transport | 94,95 | |
| FAHFA | 1.5 nm | Various tissues | Modulate insulin sensitivity, inflammation, and thermogenesis | 35 | |
| PAHSA | 1.5 nm | Various tissues | Potent regulators of glucose homeostasis and insulin secretion | 36 | |
| Testosterone | 1.5 nm | Testes, adrenal glands | Promotes muscle and bone growth, stimulates production of red blood cells | 96 | |
| Estrogen | 1.5 nm | Ovaries, adrenal glands | Plays a role in energy balance and glucose homeostasis | 7,97 | |
| Glucocorticoids (Cortisol) | 2 nm | Adrenal gland | Regulates metabolism and immune response, helps body respond to stress | 6,98 | |
| Peptides | GLP-1 | 30 AA | Intestine | Enhances insulin secretion and reduces glucagon secretion | 99,100 |
| Ghrelin | 28 AA | Stomach | Stimulates appetite and food intake, also plays a role in energy balance | 101 | |
| NPY | 36 AA | Brain, nervous system | Potent stimulator of food intake, influences energy homeostasis | 102,103 | |
| CCK | 58 AA | Intestine | Stimulates digestion of fat and protein through the release of digestive enzymes from the pancreas | 48,49 | |
| Oxytocin | 9 AA | Hypothalamus | Stimulates contraction of uterus and milk ejection in breastfeeding, promotes bonding and social behavior | 104,105 | |
| Secretin | 27 AA | S cells of the small intestine | Regulates water homeostasis and secretion of gastric juice | 106,107 | |
| Amylin | 37 AA | Beta cells of the pancreas | Slows gastric emptying, promotes satiety | 108,109 | |
| PYY | 36 AA | L cells in the ileum and colon | Reduces appetite, slows gastric emptying | 110 | |
| Proteins | Glucagon | 3.5 kDa | Pancreas | Stimulates conversion of stored glucose (glycogen) in the liver into glucose | 111,112 |
| Insulin | 5.8 kDa | Pancreas | Regulates glucose metabolism, promotes the storage of glucose | 1,113 | |
| Leptin | 16 kDa | Adipose tissue | Regulates appetite and energy expenditure | 10 | |
| FGF21 | 22.3 kDa | Liver | Regulates glucose and lipid metabolism | 9,114 | |
| FGF19 | 21.8 kDa | Ileum | Regulates bile acid synthesis and energy homeostasis | 115,116 | |
| GDF15 | 35 kDa | Multiple | Suppresses appetite, enhances glucose and lipid metabolism | 11-14 | |
| NRG4 | 12 kDa | Liver, brown adipose tissue | Regulates hepatic lipogenesis and systemic energy metabolism | 117 | |
| Adiponectin | 28 kDa | Adipose tissue | Regulates glucose levels and fatty acid breakdown | 118 | |
| ISM-1 | 52 kDa | Adipose tissue, various tissues | Regulates glucose uptake and lipid synthesis | 23 | |
| PM20D1 | 55 kDa | Various tissues | Involved in the biosynthesis of N-acyl amino acids and energy expenditure | 119 | |
| Dkk3 | 38 | Skeletal muscle | Involved in muscle differentiation and regeneration | 120 | |
| Apo-B48 | 240 kDa | Intestine | Involved in transport of dietary lipids from intestines to peripheral tissues (primarily adipose and skeletal muscle) | 121 | |
| Apo-B100 | 550 kDa | Liver | Involved in liver-mediated removal and metabolism of LDL | 45 |
Modulation of signaling strength and duration: spatial, temporal, and chemical modalities
Key elements such as the spatial distribution, temporal aspects, and chemical nature of signals determine the routing of information and its physiological impact. The spatial distribution, timing, and chemical nature of signals can change both the intensity and durability, which is fundamental for managing complex cellular activities. Cells employ different modes of signaling to adapt to these temporal variations: paracrine for short distances and endocrine for longer distances. Both the size and structure of the signaling molecules contribute to the versatility and specificity of cellular responses, with smaller molecules being advantageous in mediating rapid signaling events due to their high mobility and greater storage capacity. For instance, the molecular size of endocrine peptides, such as triiodothyronine (T3), thyrotropin-releasing hormone (TRH), and met-enkephalin correlates inversely with their signaling speed, enabling rapid diffusion and receptor engagement. Small peptides likely can quickly traverse biological fluids and initiate fast physiological responses due to reduced steric hindrance and high receptor affinity. In terms of time scale, small molecules typically work faster as they are often under enzymatic regulation, providing rapid control of production and degradation. Larger molecules are often subject to transcriptional regulation and usually respond at a slower pace. Small molecules such as neurotransmitters (e.g., dopamine, serotonin, and acetylcholine) diffuse rapidly, leading to rapid and frequent responses such as neuronal transmission and muscle contraction. On the other hand, larger molecules, including proteins (e.g., insulin, glucagon, and growth factors) and larger peptides, exhibit slower diffusion rates, allowing for sustained signaling. These larger molecules often modulate processes requiring prolonged regulation such as metabolism, growth, and development.
An example of these processes can be seen in lipolysis, where the body breaks down fats to generate lipids that are subsequently used as precursors in various biological processes. One example where lipids serve as precursors involves the synthesis and secretion of eicosanoids, a family of hormone-like lipids, such as PGs. PGs are derived from arachidonic acid, a polyunsaturated fatty acid released from the phospholipid layer of cell membranes during lipolysis. This fatty acid is then converted into PGE2 through a series of enzymatic reactions involving cyclooxygenase enzymes (COX-1 and COX-2) and PGE synthase 42. Once produced, PGE2 is secreted from the cell and can bind to its specific receptors. PGs play key roles in the metabolic crosstalk among adipose tissue, the immune system, and the liver. In the context of obesity, PGs released from adipose tissue contribute to inflammation by recruiting immune cells to the adipose tissue, promoting a chronic low-grade inflammatory state often seen in metabolic syndrome. Additionally, PGE2 communicates with the liver to regulate lipid metabolism 43. This example illustrates how lipolysis can rapidly initiate the production of lipid-derived secreted signaling molecules that can control a wide range of physiological processes.
Biosynthesis, production, and degradation
Protein and peptide biosynthesis
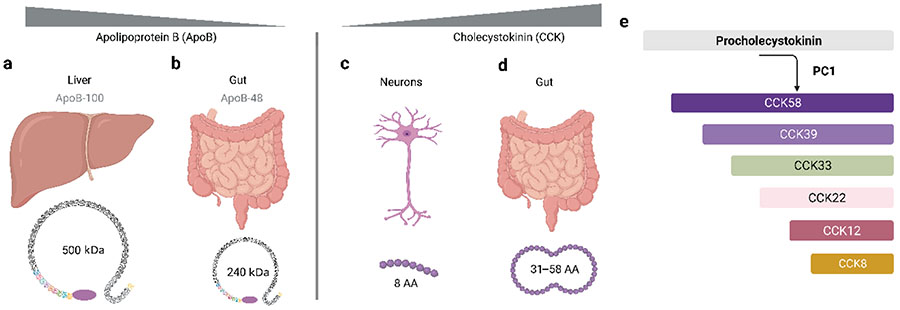
Mechanistic understanding of the synthesis of secreted molecules is important to appreciate their regulation and physiological function. Secreted proteins are synthesized based on the genetic information encoded in mRNA. They have an N-terminal signal sequence for secretion following synthesis through translation on ribosomes. Processing of precursor proteins can give rise to distinct peptides with different functions via two mechanisms: mRNA splicing by mRNA editing enzymes, or posttranslational processing by enzymes in the endoplasmic reticulum. Peptide hormones are most often synthesized as prohormones (precursor proteins) which are then cleaved to generate the active hormone 44. For posttranslational processing, the precursor protein has an N-terminal signal sequence, and the secreted chain is flanked with specific proteolytic enzyme sites. Cell-type specific enzyme expression enables the posttranslational generation of fragments of the precursor proteins to generate specific proteins or peptides. Apolipoprotein (Apo)-B100 and Apo-B48 are two distinctive apolipoproteins encoded from the same gene, APOB 45. Apo-B48 is a protein with a molecular weight of 240 kDa (Fig. 2a), and ApoB100 is a very large protein with a molecular weight of > 550 kDa (Fig. 2b). The exclusive presence of the mRNA editing enzyme Apobec-1 in intestinal cells of most vertebrates truncates the APOB gene for efficient chylomicron formation and lipid absorption 46. While both facilitating fatty acid transport by forming lipoprotein complexes, Apo-B100 is exclusively present in the liver and Apo-B48 in the intestine 47. Cholecystokinin (CCK), a peptide hormone composed of only 8 amino acids, acts centrally as a neurotransmitter and regulates food intake (Fig. 2c). In contrast, in the gastrointestinal tract, the size of CCK is larger around 31–58 amino acids, and it acts as a peptide hormone involved in digestion (Fig. 2d) 48,49 In addition, several other CCK peptides have been reported (Fig. 2e).
Figure 2.
Tissue-specific contrast of size and structure. Evolutionary processes build proteins and synthesize peptides with specific characteristic features fine-tuned to make them bioactive, versatile, and dynamically regulated. (a and b) Splicing of apolipoprotein generates two specific proteins, ApoB-100 of 500 kDa in the liver (a) and ApoB-48 of 240 kDa in the intestine (b). (c and d) CCK, a proteolytically processed peptide hormone composed of 8 amino acids, acts as a neurotransmitter in the brain (c). In contrast, in the gastrointestinal tract, the size of CCK varies from 31–58 amino acids and it functions as a digestive peptide hormone (d). (e) The enzyme prohormone convertase 1 (PC1) serially cleaves the prohormone, procholecystokinin (proCCK), into peptides of different size.
Metabolite and lipid biosynthesis
Metabolites and lipids are synthesized through metabolic pathways involving a series of enzymatic reactions. They can be degraded by enzymes or spontaneously decompose. Lactate, once considered to be a waste product and fatigue agent, has proved to be the metabolite phoenix as major energy fuel and primary precursor for gluconeogenesis 50. While not functioning as an endocrine factor, lactate does bridge the gap between glycolysis and oxidative metabolism and also fuels the oxidative machinery of mitochondria in glycolytically active cells. The lactate shuttle hypothesis considers this interconnection to occur under aerobic conditions within and among cells, tissues, and organs 51. Examples of this lactate shuttle phenomenon can be seen in the exchange between skeletal muscle and other tissues such as the brain, liver, heart, and kidney. This shuttling is regulated through concentration gradients and is mediated by the monocarboxylate transporter (MCT) family, ensuring that lactate fulfills its role in metabolic coordination without invoking the specialized signaling typically associated with hormones. Beyond its role as an energy substrate, lactate contributes to the biosynthesis of L-lactate-derived amino acids, which could be considered endocrine molecules. Li et al. demonstrated that exercise-induced secretion of N-lactoyl-phenylalanine (Lac-Phe) helps in reducing adiposity and body weight by suppressing appetite52. Lac-Phe synthesis, a reaction catalyzed by the cytosolic enzyme carnosine dipeptidase 2 (CN2 or CNDP2), involves the condensation of lactate and phenylalanine. Intriguingly, the non-specificity of CNDP2 allows it to catalyze the condensation of different amino acids (such as leucine, isoleucine, and valine), leading to the synthesis of diverse N-lactoyl-amino acids. The utilization of the highly dynamic substrate lactate to generate metabolites not only broadens the responsive elements to physical activity but also contributes to long-lasting endocrine effects. This example of a substrate-driven production of bioactive metabolites represents a large, understudied area where many similar metabolites as expected to exist. Another example is the synthesis of T3 (and thyroxine (T4)). This process is unique because it involves the transformation of a protein, thyroglobulin, into a metabolite hormone 53. T3 synthesis starts with the iodination of tyrosine residues within the thyroglobulin protein, a step that requires the specialized enzyme thyroperoxidase. The iodinated tyrosine residues within the thyroglobulin molecule are then coupled to form the hormones T3 and T4. The thyroid gland has a remarkable ability to concentrate iodine, which is essential for the iodination of tyrosine residues on thyroglobulin, a precursor protein in the thyroid hormone synthesis process. Few metabolic pathways are directly dependent on a specific dietary mineral. Second, the biologically active form of the hormone, T3, is predominantly formed outside the thyroid gland through a process known as peripheral deiodination. This is an unusual feature in hormone metabolism because the full activation of the hormone occurs not at the site of its synthesis, but in the peripheral tissues where it exerts its effect. The flexibility of this process allows the body to fine-tune the amount of active T3 hormone available based on the metabolic demands of the body, further emphasizing the unique nature of thyroid hormone synthesis and regulation. Therefore, large quantities of T3 and T4 can be stored within the thyroid gland as part of the thyroglobulin protein, and then released when needed. This unique aspect of thyroid hormone synthesis and storage allows for the rapid release of hormones when the body's metabolic demands increase 4.
Metabolites as biomarkers
With the advancements in metabolomics techniques, the identification of small metabolites has exponentially increased and enabled us to discover the novel biomarkers involved in the pathophysiology of metabolic and non-metabolic diseases. Changes and perturbations in the metabolite signature of organisms could help in advance diagnosis of diseases and drug designing. Molecular metabolite profiling will enable a deeper understanding of the metabolic aspects of diseases and develop early therapeutic interventions 54. For instance, the galactose/glycerolipid metabolic pathway is disturbed in diabetic kidney disease (DKD) suggesting glycerol-3-galactoside as a potential biomarker 55. Circulating metabolites, hexanoylcarnitine, kynurenine, and tryptophan have been associated with improvement in the prediction of all-cause mortality in type 2 diabetes 56.
Degradation
The initiation of a signaling event to facilitate a specific cellular response necessitates the termination of that signal. In circulation, proteins and peptides undergo degradation either extracellularly via proteases or intracellularly through proteases or the ubiquitin-proteasome pathway. Molecules exposed to extracellular spaces are vulnerable to enzymatic degradation, which can impact the signal range 57,58. However, stable signal molecules can traverse longer distances. For instance, metabolites like lactate can rapidly diffuse out of the cellular environment. This speed is dependent on their concentration gradient and the availability of transporters. Additionally, the enzymatic conversion of lactate back to pyruvate can occur within seconds to minutes, contingent upon enzymatic activity and substrate availability. Similarly, lipids like PGE2 are rapidly degraded, with a half-life of less than a few minutes in circulation and in tissues 59. Uptake of PGE2 by cells can occur within seconds, and its enzymatic degradation by 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in the cytoplasm can also occur within seconds to minutes. Peptides such as GLP-1 are degraded and inactivated extremely quickly by dipeptidyl peptidase-4 (DPP-4), resulting in a half-life of only about 1–2 min 60. This rapid degradation ensures that insulin secretion is tightly regulated and can quickly respond to changes in blood glucose levels. In contrast, proteins like leptin, due to their larger size and complexity, have a slower degradation rate, with a half-life of 40 min in mice 61. Their degradation, occurring either through proteolysis in the kidneys and liver or via receptor-mediated endocytosis in target cells, can take minutes to hours. This duration depends on factors such as protein stability and the availability of receptors and proteolytic enzymes. These examples underscore the wide range of speeds at which different types of signaling molecules can be degraded, ranging from seconds for small metabolites and lipids to minutes or even hours for larger proteins.
Evolutionary adaptability of endocrine hormones
Diversification of signaling molecules and adaptability in evolution
Small molecules could be considered more ancient in the context of cellular signaling and the evolution of life. Before the emergence of complex life forms, primitive cells relied on simple chemical interactions to facilitate cellular processes and communication. Small molecules would have been more accessible and easier for these primitive cells to synthesize and utilize than larger, more complex molecules such as peptides and proteins. As life evolved and organisms became more complex, so did the signaling molecules used for communication between cells and organs. The emergence of peptides and proteins as signaling molecules would have allowed for greater specificity, selectivity, and versatility in cellular communication and regulation of physiological processes. However, small molecules have remained essential components of cellular signaling and metabolic processes in all organisms, from bacteria to humans. They continue to serve as neurotransmitters, hormones, and secondary messengers, playing a critical role in various biological processes such as energy metabolism, cell growth and differentiation, and immune responses.
Diversification of signaling molecules forms the bedrock of biological diversity. The emergence of a diverse range of ligands exerts significant selection pressure on enzymes to evolve 62. The accumulation of a ligand library, a consequence of evolutionary exposure spanning billions of years, introduces incremental advancements. For instance, gene superfamilies encoding conus peptides rapidly evolve through gene duplications, enzyme mutations, and functional deletions in response to environmental changes 63. The ability to use a variety of signaling molecules allows for the development of complex, adaptable regulatory systems that can evolve to meet new challenges.
A great mystery: why are there so many different chemical types of endocrine hormones?
In mammals, secreted signaling molecules are diverse, ranging from small molecules such as metabolites (e.g., catecholamines), lipids and steroids (e.g., PGs and corticosteroids), peptides (2–50 amino acids in size, e.g., GLP-1 and neuropeptides), to larger protein molecules (> 50 amino acids in size, e.g., leptin, insulin and other tyrosine kinase receptor ligands). Why has nature evolved such chemical diversity in endocrine hormones, and what functional relevance does that have to their signaling? One might speculate that the size, structure, and properties of these molecules have been shaped by evolution to facilitate nuanced control over countless biological processes. The evolution from simpler life forms to multicellular organisms has led to increased diversity in signaling molecules and complexity in signal transduction pathways, driving a dynamic process of intercellular communication. Metabolites, due to their small size and direct dependence on substrate availability, can serve as quick response messengers to changes in cellular metabolic status, such as during exercise 52,64,65 or fasting 66. Lipids and steroids, due to their lipophilic nature, can pass through cell membranes and exert potent modulatory effects on intracellular targets such as nuclear receptors 27. Peptides, falling between small molecules and proteins in size, not only often act as rapid local regulators (e.g., neuropeptides) but also serve as important hormonal regulators (e.g., incretins) 67,68. Finally, proteins, the largest and most diverse group of signaling molecules, can exert a wide array of effects on cells due to their structural and functional diversity 33,69,70. This diversity ensures that the endocrine system can fine-tune its responses according to the distinct needs of different physiological states.
Evolutionary contrast in insulin size: comparing humans and cone snails
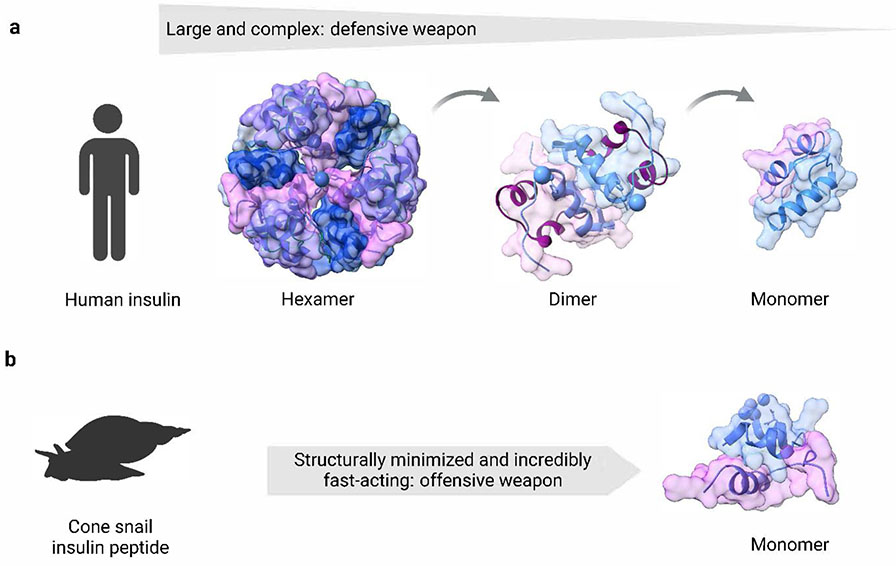
The discovery of insulin, a critical hormone for blood glucose regulation, is a milestone in the advancement of drug discovery and enhancing the longevity of humans 2. Insulin varies remarkably between humans and other species, including, drosophila 71 and cone snails 72. In humans, insulin is a 51 amino acid (5.7 kDa) protein hormone secreted by pancreatic β-cells and stored in secretory vesicles as hexamers (Fig. 3a). The formation of the hexamer starts with the oligomerization of three dimers held together by zinc ions. Storing insulin as hexamers in secretory vesicles is critical as it protects the insulin from degradation and fibrillation 73. To facilitate glucose uptake into cells and lower systemic blood glucose levels, the insulin hexamer molecules must dissociate into dimers, and finally into the functional monomer. This insulin monomer not only acts as a signaling ligand for insulin receptors but also has faster pharmacokinetics, resulting in lower stability in circulation. The hexamer-to-monomer transition is a slow process and this predicament has spurred the engineering of a fast-acting monomer insulin analog for diabetes 74. The C-terminus of B chain in the insulin molecule facilitates the dimerization process and also confers receptor activation 75. Intriguingly, the engineering of a fast-acting insulin analog has been done by nature thousands of years ago. In contrast to human insulin, insulin present in the venom of the cone snails, Conus. geographus is comparatively a smaller peptide of 10–40 amino acids and is typically monomeric in structure. Cone snail insulin also lacks the C-terminus region responsible for dimerization in human insulin. Interestingly, cone snail insulin is extremely potent and fast-acting in lowering blood glucose in vertebrates, including humans 63. Cone snails use insulin in their venom as an offensive weapon to catch the prey by causing hypoglycemia that sedates the prey (Fig. 3b) 72. It is fascinating that to target the different insulin receptors, different fish species fine-tune the ligand specificity by producing different versions of insulin (cone snail insulins (Con-Ins) G1 and Con-Ins G3) (Fig. 3). The rationale for such divergence is the evolutionary pressures stemming from constantly changing prey-predator relationships. Cone snail insulin peptides and other constituents of their venom are the most rapidly diverging and evolving ligands in biology 72. However, the prey of cone snails is constrained to evolve their insulin receptor specificity. So the evolutionary contrast of insulin in humans and cone snails exemplifies the essence of ligand size in nature. In this example, insulin is united by function and divided by structure and action – used as an offensive weapon in cone snails and defensive in humans.
Figure 3.
Insulin size architecture tailored by nature. Human insulin has acquired a propensity to self-assemble into dimers and hexamers, protecting itself from enzymatic degradation. To facilitate the glucose uptake of cells and lower circulating blood glucose levels, human insulin hexamer molecules have to first dissociate into dimers and functional monomers (a). In contrast, in cone snails, nature has designed insulin molecules with minimum structure and yet it is fully functional and incredibly fast-acting. The cone snail insulin is readily secreted as a monomeric unit and, in contrast to human insulin, is used as an offensive weapon (b).
Future perspectives
As we continue to deconstruct the biology of cellular communication, we acknowledge the presence of an extensive array of distinct signaling molecules, each possessing unique characteristics and functions. These entities, defined by their individual structure, size, and physicochemical properties, not only carry out designated roles but also contribute to the multidimensional architecture of intercellular and intracellular signaling pathways. Despite the considerable advancements in our understanding of these processes, it is apparent that we are only beginning to explore this vast landscape of signaling biochemistry. The acceleration in the discovery of signaling molecules, propelled by advancements in high-resolution mass spectrometry methodologies, mandates the development of an inclusive and evolving classification system. This system should move beyond the traditional organ-specific classification and include the synthesis, regulation, specificity, and signaling responses of these molecules, thus providing a more integrative view of their physiological implications. As advancements in detection methodologies continue to evolve, we anticipate the identification of an even more diverse collection of these molecules, further enriching our understanding of the intricate landscape of cellular signaling pathways and mechanisms. High-throughput metabolomics combined with deep mining of data from biobanks, such as the UKBioBank, and where feasible, the use of genetic models will be crucial for the discovery and elucidation of the roles of these signaling molecules within a spectrum of physiological and pathological conditions, and for confirming their therapeutic potential. Therefore, the continuous endeavor to identify novel signaling molecules and subsequent elucidation of their physiological roles will remain a central theme in metabolism in the coming years.
Acknowledgements
Illustrations were created with Biorender.com. Structures were generated with ChimeraX-1.5. This work was funded by NIH R01DK125260 and AHA 23IPA1042031 (K.J.S) and NIH DK124265 and DK136526 (J.Z.L.).
Abbreviations
- Apo-B48
apolipoprotein B48
- Apo-B100
apolipoprotein B100
- CCK
cholecystokinin
- Dkk3
dickkopf-related protein 3
- FAHFA
fatty acid esters of hydroxy fatty acids
- FGF19
fibroblast growth factor 19
- FGF21
fibroblast growth factor 21
- GDF15
growth differentiation factor 15
- GLP-1
glucagon-like peptide 1
- ISM-1
isthmin-1
- Lac-Phe
N-lactoyl-phenylalanine
- LDL
low density lipoprotein
- NPY
neuropeptide Y
- NRG4
neuregulin 4
- PAHSA
palmitic acid esters of hydroxy stearic acids
- PM20D1
peptidase M20 domain containing 1
- PYY
peptide YY
- SREBP
sterol regulatory element-binding protein
- T4
Thyroxine
Footnotes
Conflict of interest
The authors declares that no conflict of interest exists.
References
- 1.Banting FG, Best CH, Collip JB, Campbell WR & Fletcher AA Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J 12, 141–146 (1922). [PMC free article] [PubMed] [Google Scholar]
- 2.Vecchio I, Tornali C, Bragazzi NL & Martini M The discovery of insulin: An important milestone in the history of medicine. Frontiers in Endocrinology 9, 613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen BM Experiments in the Transplantation of the Hypophysis of Adult Rana pipiens to Tadpoles. Science 52, 274–276 (1920). [DOI] [PubMed] [Google Scholar]
- 4.Citterio CE, Targovnik HM & Arvan P The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 15, 323–338 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Kendall EC ISOLATION OF THE IODINE COMPOUND WHICH OCCURS IN THE THYROID: First Paper. Journal of Biological Chemistry 39, 125–147 (1919). [Google Scholar]
- 6.Kuo T, McQueen A, Chen T-C & Wang J-C Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol 872, 99–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauvais-Jarvis F, Clegg DJ & Hevener AL The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr Rev 34, 309–338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams AC et al. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Molecular Metabolism 2, 31–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura T, Nakatake Y, Konishi M & Itoh N Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 1492, 203–206 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Friedman J The long road to leptin. J Clin Invest 126, 4727–4734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmerson PJ et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nature Medicine 23, 1215–1219 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Hsu JY et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550, 255–259 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Macia L et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS ONE 7, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metabolism 29, 707–718.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard C On the production of sugar in the liver of man and animals. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 2, 326–330 (1851). [Google Scholar]
- 16.Lemmon MA & Schlessinger J Cell Signaling by Receptor Tyrosine Kinases. Cell 141, 1117–1134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning BD & Toker A AKT/PKB Signaling: Navigating the Network. Cell 169, 381–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang L, Chiang S-H & Saltiel AR Insulin Signaling and the Regulation of Glucose Transport. Molecular Medicine 10, 65–71 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel VT & Shulman GI The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. Journal of Clinical Investigation 126, 12–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown KJ et al. Advances in the proteomic investigation of the cell secretome. Expert Rev Proteomics 9, 337–345 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen X et al. Multi-omics microsampling for the profiling of lifestyle-associated changes in health. Nature Biomedical Engineering (2023) doi: 10.1038/s41551-022-00999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshmukh AS et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metabolism 30, 963–975.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Jiang Z et al. Isthmin-1 is an adipokine that promotes glucose uptake and improves glucose tolerance and hepatic steatosis. Cell Metab 33, 1836–1852.e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L et al. Metabolite discovery through global annotation of untargeted metabolomics data. Nat Methods 18, 1377–1385 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taguchi T et al. Suprabasin-derived bioactive peptides identified by plasma peptidomics. Sci Rep 11, 1047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojtowicz WM et al. A Human IgSF Cell-Surface Interactome Reveals a Complex Network of Protein-Protein Interactions. Cell 182, 1027–1043.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang NJ & Hinner MJ Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Methods Mol Biol 1266, 29–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglass J, Civelli O & Herbert E Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annual review of biochemistry 53, 665–715 (1984). [DOI] [PubMed] [Google Scholar]
- 29.Eick GN, Colucci JK, Harms MJ, Ortlund EA & Thornton JW Evolution of minimal specificity and promiscuity in steroid hormone receptors. PLoS Genet 8, e1003072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foltz IN et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the βKlotho/FGFR1c receptor complex. Science Translational Medicine 4, 162ra153–162ra153 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Agrawal A et al. Molecular elements in FGF19 and FGF21 defining KLB/FGFR activity and specificity. Mol Metab 13, 45–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan T et al. FGF19, FGF21 and an FGFR1/β-Klotho-activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab 26, 709–718.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caron A, Lee S, Elmquist JK & Gautron L Leptin and brain-adipose crosstalks. Nature Reviews Neuroscience 19, 153–165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatemoto K, Carlquist M & Mutt V Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660 (1982). [DOI] [PubMed] [Google Scholar]
- 35.Yore MM et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou P et al. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J Clin Invest 129, 4138–4150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapport MM, Green AA & Page IH Crystalline Serotonin. Science 108, 329–330 (1948). [DOI] [PubMed] [Google Scholar]
- 38.Twarog BM & Page IH Serotonin Content of Some Mammalian Tissues and Urine and a Method for Its Determination. American Journal of Physiology-Legacy Content 175, 157–161 (1953). [DOI] [PubMed] [Google Scholar]
- 39.Physical Biology of the Cell. Routledge & CRC Press; https://www.routledge.com/Physical-Biology-of-the-Cell/Garcia-Phillips-Kondev-Theriot-Phillips-Kondev-Garcia/p/book/9780815344506. [Google Scholar]
- 40.Rusakov DA, Savtchenko LP, Zheng K & Henley JM Shaping the synaptic signal: molecular mobility inside and outside the cleft. Trends Neurosci 34, 359–369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez JM et al. Genome-scale reconstructions of the mammalian secretory pathway predict metabolic costs and limitations of protein secretion. Nat Commun 11, 68 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami M et al. Regulation of Prostaglandin E2 Biosynthesis by Inducible Membrane-associated Prostaglandin E2 Synthase That Acts in Concert with Cyclooxygenase-2*. Journal of Biological Chemistry 275, 32783–32792 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Henkel J et al. Stimulation of fat accumulation in hepatocytes by PGE2-dependent repression of hepatic lipolysis, β-oxidation and VLDL-synthesis. Laboratory Investigation 92, 1597–1606 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Brakch N et al. Role of prohormone convertases in pro-neuropeptide Y processing: coexpression and in vitro kinetic investigations. Biochemistry 36, 16309–16320 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Welty FK, Lichtenstein AH, Barrett PHR, Dolnikowski GG & Schaefer EJ Human Apolipoprotein (Apo) B-48 and ApoB-100 Kinetics With Stable Isotopes. Arteriosclerosis, Thrombosis, and Vascular Biology 19, 2966–2974 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Lo C-M et al. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol 294, G344–352 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Lo CC & Coschigano KT ApoB48 as an Efficient Regulator of Intestinal Lipid Transport. Front Physiol 11, 796 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivy AC & Oldberg E A hormone mechanism for gall-bladder contraction and evacuation. American Journal of Physiology-Legacy Content 86, 599–613 (1928). [Google Scholar]
- 49.Rehfeld JF Cholecystokinin—From Local Gut Hormone to Ubiquitous Messenger. Front Endocrinol (Lausanne) 8, 47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks GA Cell-cell and intracellular lactate shuttles. J Physiol 587, 5591–5600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasserman K The anaerobic threshold measurement to evaluate exercise performance. Am Rev Respir Dis 129, S35–40 (1984). [DOI] [PubMed] [Google Scholar]
- 52.Li VL et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606, 785–790 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Jeso B & Arvan P Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr Rev 37, 2–36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu S et al. Small molecule metabolites: discovery of biomarkers and therapeutic targets. Sig Transduct Target Ther 8, 1–37 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S et al. Serum integrative omics reveals the landscape of human diabetic kidney disease. Mol Metab 54, 101367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarale MG et al. Circulating Metabolites Associate With and Improve the Prediction of All-Cause Mortality in Type 2 Diabetes. Diabetes 71, 1363–1370 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Lu P, Takai K, Weaver VM & Werb Z Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3, a005058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholpp S & Brand M Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr Biol 14, 1834–1841 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Eguchi N, Hayashi H, Urade Y, Ito S & Hayaishi O Central action of prostaglandin E2 and its methyl ester in the induction of hyperthermia after their systemic administration in urethane-anesthetized rats. J Pharmacol Exp Ther 247, 671–679 (1988). [PubMed] [Google Scholar]
- 60.Deacon CF Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nature reviews. Endocrinology 16, 642–653 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Burnett LC, Skowronski AA, Rausch R, LeDuc CA & Leibel RL Determination of the half-life of circulating leptin in the mouse. Int J Obes (Lond) 41, 355–359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen K & Arnold FH Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide. Proceedings of the National Academy of Sciences 90, 5618–5622 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahorukomeye P et al. Fish-hunting cone snail venoms are a rich source of minimized ligands of the vertebrate insulin receptor. Elife 8, e41574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schranner D, Kastenmüller G, Schönfelder M, Römisch-Margl W & Wackerhage H Metabolite Concentration Changes in Humans After a Bout of Exercise: a Systematic Review of Exercise Metabolomics Studies. Sports Med Open 6, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Q, Vijayakumar A & Kahn BB Metabolites as regulators of insulin sensitivity and metabolism. Nature reviews. Molecular cell biology 19, 654–672 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teruya T, Chaleckis R, Takada J, Yanagida M & Kondoh H Diverse metabolic reactions activated during 58-hr fasting are revealed by non-targeted metabolomic analysis of human blood. Scientific Reports 9, 854 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crown A, Clifton DK & Steiner RA Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 86, 175–182 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Campbell JE & Drucker DJ Pharmacology, physiology, and mechanisms of incretin hormone action. Cell metabolism 17, 819–837 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Boucher J, Kleinridders A & Ronald Kahn C Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor Perspectives in Biology 6, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao M, Jung Y, Jiang Z & Svensson KJ Regulation of Energy Metabolism by Receptor Tyrosine Kinase Ligands. Frontiers in Physiology 11, 354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H et al. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc Natl Acad Sci U S A 106, 19617–19622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Safavi-Hemami H et al. Venom Insulins of Cone Snails Diversify Rapidly and Track Prey Taxa. Mol Biol Evol 33, 2924–2934 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee S, Mondal S, Deshmukh AA, Gopal B & Bagchi B What Gives an Insulin Hexamer Its Unique Shape and Stability? Role of Ten Confined Water Molecules. J Phys Chem B 122, 1631–1637 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Elleri D, Dunger DB & Hovorka R Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med 9, 120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menting JG et al. Protective hinge in insulin opens to enable its receptor engagement. Proc Natl Acad Sci U S A 111, E3395–E3404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dale HH & Dudley HW The presence of histamine and acetylcholine in the spleen of the ox and the horse. The Journal of Physiology 68, 97–123 (1929). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fatt P & Katz B Spontaneous subthreshold activity at motor nerve endings. J Physiol 117, 109–128 (1952). [PMC free article] [PubMed] [Google Scholar]
- 78.Axelrod J, Hertting G & Potter L Effect of Drugs on the Uptake and Release of 3H-Norepinephrine in the Rat Heart. Nature 194, 297–297 (1962). [DOI] [PubMed] [Google Scholar]
- 79.Euler, U. S. v. A Specific Sympathomimetic Ergone in Adrenergic Nerve Fibres (Sympathin) and its Relations to Adrenaline and Nor-Adrenaline. Acta Physiologica Scandinavica 12, 73–97 (1946). [Google Scholar]
- 80.Needham DM Machina Carnis: The Biochemistry of Muscular Contraction in Its Historical Development. (Cambridge University Press, 1971). [Google Scholar]
- 81.Reddy A et al. pH-Gated Succinate Secretion Regulates Muscle Remodeling in Response to Exercise. Cell 183, 62–75.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cahill GF Starvation in Man. N Engl J Med 282, 668–675 (1970). [DOI] [PubMed] [Google Scholar]
- 83.Li VL et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606, 785–790 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johann K et al. Thyroid-Hormone-Induced Browning of White Adipose Tissue Does Not Contribute to Thermogenesis and Glucose Consumption. Cell Rep 27, 3385–3400.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Agudelo LZ et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Newgard CB Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metabolism 15, 606–614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Randle PJ Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14, 263–283 (1998). [DOI] [PubMed] [Google Scholar]
- 88.Dalli J & Serhan CN Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serhan CN et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 206, 15–23 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferreira SH, Moncada S & Vane JR Prostaglandins and the mechanism of analgesia produced by aspirin-like drugs. British Journal of Pharmacology 120, 401–412 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawaguchi H, Pilbeam CC, Harrison JR & Raisz LG The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res 36–46 (1995). [PubMed] [Google Scholar]
- 92.Fan W et al. SIRT1 regulates sphingolipid metabolism and neural differentiation of mouse embryonic stem cells through c-Myc-SMPDL3B. eLife 10, e67452 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hannun YA & Obeid LM Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9, 139–150 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Devaux PF Lipid transmembrane asymmetry and flip-flop in biological membranes and in lipid bilayers: Current Opinion in Structural Biology 1993, 3:489–494. Current Opinion in Structural Biology 3, 489–494 (1993). [Google Scholar]
- 95.Sun L-P, Seemann J, Goldstein JL & Brown MS Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proceedings of the National Academy of Sciences 104, 6519–6526 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Griggs RC et al. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol (1985) 66, 498–503 (1989). [DOI] [PubMed] [Google Scholar]
- 97.Geary N, Asarian L, Korach KS, Pfaff DW & Ogawa S Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 142, 4751–4757 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Morgan SA et al. 11beta-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle. Diabetes 58, 2506–2515 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dupre J, Ross SA, Watson D & Brown JC Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37, 826–828 (1973). [DOI] [PubMed] [Google Scholar]
- 100.Pi-Sunyer X et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med 373, 11–22 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Kojima M et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999). [DOI] [PubMed] [Google Scholar]
- 102.Clark JT, Kalra PS, Crowley WR & Kalra SP Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (1984). [DOI] [PubMed] [Google Scholar]
- 103.Tatemoto K, Carlquist M & Mutt V Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660 (1982). [DOI] [PubMed] [Google Scholar]
- 104.Brindley BA & Sokol RJ Induction and Augmentation of Labor: Basis and Methods for Current Practice. Obstetrical & Gynecological Survey 43, 730 (1988). [DOI] [PubMed] [Google Scholar]
- 105.Matsunaga M et al. Breastfeeding dynamically changes endogenous oxytocin levels and emotion recognition in mothers. Biology Letters 16, 20200139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feldberg W & Gaddum JH The chemical transmitter at synapses in a sympathetic ganglion. The Journal of Physiology 81, 305–319 (1934). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tam JKV, Lee LTO, Jin J & Chow BKC MOLECULAR EVOLUTION OF GPCRS: Secretin/secretin receptors. Journal of Molecular Endocrinology 52, T1–T14 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Clark A et al. ISLET AMYLOID FORMED FROM DIABETES-ASSOCIATED PEPTIDE MAY BE PATHOGENIC IN TYPE-2 DIABETES. The Lancet 330, 231–234 (1987). [DOI] [PubMed] [Google Scholar]
- 109.Westermark P, Wernstedt C, Wilander E & Sletten K A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochemical and Biophysical Research Communications 140, 827–831 (1986). [DOI] [PubMed] [Google Scholar]
- 110.Batterham RL et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4, 223–233 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Gerich JE et al. Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N Engl J Med 291, 544–547 (1974). [DOI] [PubMed] [Google Scholar]
- 112.Kimball CP & Murlin JR AQUEOUS EXTRACTS OF PANCREAS: III. SOME PRECIPITATION REACTIONS OF INSULIN. Journal of Biological Chemistry 58, 337–346 (1923). [Google Scholar]
- 113.Goeddel DV et al. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proceedings of the National Academy of Sciences 76, 106–110 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Markan KR et al. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes 63, 4057–4063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishimura T, Utsunomiya Y, Hoshikawa M, Ohuchi H & Itoh N Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim Biophys Acta 1444, 148–151 (1999). [DOI] [PubMed] [Google Scholar]
- 116.Potthoff MJ et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab 13, 729–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang G-X et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuating hepatic lipogenesis. Nat Med 20, 1436–1443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scherer PE, Williams S, Fogliano M, Baldini G & Lodish HF A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270, 26746–26749 (1995). [DOI] [PubMed] [Google Scholar]
- 119.Long JZ et al. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell 166, 424–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu J et al. Myofiber Baf60c controls muscle regeneration by modulating Dkk3-mediated paracrine signaling. J Exp Med 220, e20221123 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tso P & Balint JA Formation and transport of chylomicrons by enterocytes to the lymphatics. American Journal of Physiology-Gastrointestinal and Liver Physiology 250, G715–G726 (1986). [DOI] [PubMed] [Google Scholar]