Abstract
Background
Admission avoidance hospital at home provides active treatment by healthcare professionals in the patient's home for a condition that would otherwise require acute hospital inpatient care, and always for a limited time period. This is the fourth update of this review.
Objectives
To determine the effectiveness and cost of managing patients with admission avoidance hospital at home compared with inpatient hospital care.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and CINAHL on 24 February 2022, and checked the reference lists of eligible articles. We sought ongoing and unpublished studies by searching ClinicalTrials.gov and WHO ICTRP, and by contacting providers and researchers involved in the field.
Selection criteria
Randomised controlled trials recruiting participants aged 18 years and over. Studies comparing admission avoidance hospital at home with acute hospital inpatient care.
Data collection and analysis
We followed the standard methodological procedures expected by Cochrane and the Effective Practice and Organisation of Care (EPOC) Group. We performed meta‐analysis for trials that compared similar interventions, reported comparable outcomes with sufficient data, and used individual patient data when available. We used the GRADE approach to assess the certainty of the body of evidence for the most important outcomes.
Main results
We included 20 randomised controlled trials with a total of 3100 participants; four trials recruited participants with chronic obstructive pulmonary disease; two trials recruited participants recovering from a stroke; seven trials recruited participants with an acute medical condition who were mainly older; and the remaining trials recruited participants with a mix of conditions. We assessed the majority of the included studies as at low risk of selection, detection, and attrition bias, and unclear for selective reporting and performance bias.
For an older population, admission avoidance hospital at home probably makes little or no difference on mortality at six months' follow‐up (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.68 to 1.13; P = 0.30; I2 = 0%; 5 trials, 1502 participants; moderate‐certainty evidence); little or no difference on the likelihood of being readmitted to hospital after discharge from hospital at home or inpatient care within 3 to 12 months' follow‐up (RR 1.14, 95% CI 0.97 to 1.34; P = 0.11; I2 = 41%; 8 trials, 1757 participants; moderate‐certainty evidence); and probably reduces the likelihood of living in residential care at six months' follow‐up (RR 0.53, 95% CI 0.41 to 0.69; P < 0.001; I2 = 67%; 4 trials, 1271 participants; moderate‐certainty evidence).
Hospital at home probably results in little to no difference in patient's self‐reported health status (2006 patients; moderate‐certainty evidence). Satisfaction with health care received may be improved with admission avoidance hospital at home (1812 participants; low‐certainty evidence); few studies reported the effect on caregivers. Hospital at home reduced the initial average hospital length of stay (2036 participants; low‐certainty evidence), which ranged from 4.1 to 18.5 days in the hospital group and 1.2 to 5.1 days in the hospital at home group. Hospital at home length of stay ranged from an average of 3 to 20.7 days (hospital at home group only). Admission avoidance hospital at home probably reduces costs to the health service compared with hospital admission (2148 participants; moderate‐certainty evidence), though by a range of different amounts and using different methods to cost resource use, and there is some evidence that it decreases overall societal costs to six months' follow‐up.
Authors' conclusions
Admission avoidance hospital at home, with the option of transfer to hospital, may provide an effective alternative to inpatient care for a select group of older people who have been referred for hospital admission. The intervention probably makes little or no difference to patient health outcomes; may improve satisfaction; probably reduces the likelihood of relocating to residential care; and probably decreases costs.
Keywords: Humans, Health Facilities, Home Care Services, Hospitalization, Hospitals, Inpatients, Patient Discharge
Plain language summary
'Hospital at home' services to avoid admission to hospital
What is the aim of this review?
The aim of this Cochrane Review was to find out if providing health care in an admission avoidance hospital at home setting improves patient health outcomes and reduces health service costs.
Key messages
Admission avoidance hospital at home probably makes little or no difference to risk of death; probably increases the chances of living at home at six months' follow‐up; and may be slightly less expensive.
What was studied in this review?
There continues to be more demand for acute hospital beds than there are beds available. One way to reduce reliance on hospital beds is to provide people with acute health care at home, sometimes called 'admission avoidance hospital at home'. In contrast, 'early discharge hospital at home' refers to patients being discharged early from hospital to be treated at home; this topic has been reviewed separately.
What did we want to find out?
We wanted to find out if hospital at home makes a difference to patient health outcomes and to living independently at home. We also wanted to find out if it was less expensive than hospital care, and if it affects length of stay in treatment and patient satisfaction.
What did we do?
We searched for studies that compared hospital at home treatment for an acute health event with inpatient hospital care. We compared and summarised the results of the studies, and rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find?
We found 20 studies, of which four were identified for this update, with a total of 3100 patients with a range of acute conditions. Four studies recruited participants with chronic obstructive (lung) disease; two studies recruited participants recovering from a stroke; seven studies recruited participants with a (sudden or short‐term) medical condition who were mainly older; and the remaining studies recruited participants with a mix of conditions.
When compared to in‐hospital care, admission avoidance hospital at home services for a select group of patients probably make little or no difference to risk of death or to the likelihood of being taken to hospital in the next 3 to 12 months, and probably increase the chances of living at home at six months' follow‐up. Patients who receive care at home may have increased satisfaction compared to those in hospital; however, the effects of this type of care on the caregivers who support them are unclear. Hospital at home probably results in little to no difference in patients' health status. Hospital at home decreases the amount of time patients spend in hospital, while length of stay in hospital at home tended to be longer than a typical hospital stay. Admission avoidance hospital at home probably decreases treatment costs, though by a range of different amounts.
What are the limitations of the evidence?
Due to the small size of most of the studies, we are moderately confident that admission avoidance hospital at home does not make a difference to the number of people who died when compared to in‐hospital care. Our confidence in the evidence for readmission and living in residential care was reduced to moderate because the lengths of follow‐up differed among studies. We are moderately confident in the evidence for patient‐reported health status, as participants were aware of which treatment they were getting, which could have influenced the results. We have little confidence in the evidence on patient satisfaction because not many studies reported this outcome, and on length of stay because length of stay varied across studies. We are moderately confident in the evidence for cost because only three trials looked at this fully.
How up‐to‐date is the review?
We searched for studies published up to February 2022.
Summary of findings
Summary of findings 1. Admission avoidance hospital at home compared with inpatient admission for older people requiring admission to hospital.
| Admission avoidance hospital at home compared with inpatient admission for older people requiring admission to hospital | |||||
|
Patient or population: older people requiring hospital admission Settings: home Intervention: admission avoidance hospital at home Comparison: inpatient care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Inpatient care | Admission avoidance hospital at home | ||||
|
Mortality (6 months' follow‐up) (using data from trialists and published data) |
Study population |
RR 0.88 (0.68 to 1.13) |
1502 (5 studies)A | ⊕⊕⊕⊝a Moderate | |
| 208 per 1000 | 183 per 1000 (141 to 235) | ||||
|
Admission to hospital (3 to 12 months' follow‐up) (using individual patient data and published data) |
Study population |
RR 1.14 (0.97 to 1.34 |
1757 (8 studies)B | ⊕⊕⊕⊝b Moderate | |
| 407 per 1000 | 464 per 1000 (395 to 546) | ||||
|
Living in residential care at follow‐up (6 months' follow‐up) |
Study population |
RR 0.53 (0.41 to 0.69) |
1271 (4 studies)C | ⊕⊕⊕⊝b Moderate |
|
| 124 per 1000 | 66 per 1000 (51 to 85) | ||||
| Patient self‐reported health status | Patient‐reported health status was largely the same for participants treated in hospital at home and hospital, with some reporting higher quality of life or better health status in hospital at home.D | ‐ | 2006 (9 studies) |
⊕⊕⊕⊝c Moderate |
|
| Patient satisfaction | Patients allocated to hospital at home reported higher levels of satisfaction on average; a small proportion preferred hospital, or satisfaction was equal between groups.E | ‐ | 1812 (8 studies) |
⊕⊕⊝⊝d Low |
|
| Length of stay in hospital and hospital at home | Hospital at home reduced average length of stay in hospital, which ranged from an average of 4.1 to 18.5 days in the hospital group to 1.2 to 5.1 days in the hospital at home group.F Hospital at home length of stay ranged from an average of 3 to 20.7 days (hospital at home group only).G Length of stay for the acute episode ranged from a mean increase of 0.7 to 9.1 daysF for the hospital at home group compared to the hospital group. |
‐ | 2036 (11 studies) |
⊕⊕⊝⊝e Low |
|
| Cost and resource use | Hospital at home was generally less costly than hospital care, with a range of estimates for the mean reduction per episode with different levels of certainty, from USD −215 (P = 0.38) to GBP −1981 (95% CI −2551 to −1411).H Estimates for the difference in total health and social care costs for a variety of follow‐up durations also varied, ranging from GBP −1015.7 (95% CI −2735.5 to 644.8) to GBP −2265 (95% CI −4279 to −252).I |
‐ | 2148 (12 studies) |
⊕⊕⊕⊝f Moderate |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded the certainty of the evidence by one level to moderate due to imprecision of the estimate. bWe downgraded the certainty of the evidence by one level to moderate due to indirect comparisons between studies. cWe downgraded the certainty of the evidence by one level to moderate due to risk of performance bias since patients cannot be blinded to the intervention. dWe downgraded the certainty of the evidence by two levels to low as only 35% of the studies reported this outcome, and there is a risk of detection bias due to subjective reporting of this outcome. eWe downgraded the certainty of the evidence by two levels to low due to imprecision and indirect comparisons between studies. fWe downgraded the certainty of the evidence by one level to moderate since only three trials reported a full cost analysis.
ACaplan 1999; Ricauda 2008; Shepperd 2021; Tibaldi 2009; Wilson 1999. BCaplan 1999; Davies 2000; Harris 2005; Mendoza 2009; Ricauda 2008; Shepperd 2021; Tibaldi 2009; Wilson 1999. CRicauda 2008; Shepperd 2021; Tibaldi 2004; Tibaldi 2009. DCorwin 2005; Echevarria 2018; Mendoza 2009; Ricauda 2008; Richards 2005; Shepperd 2021; Talcott 2011; Tibaldi 2009; Wilson 1999. ECaplan 1999; Corwin 2005; Levine 2018; Levine 2020; Ricauda 2008; Richards 2005; Shepperd 2021; Wilson 1999. FDavies 2000; Echevarria 2018; Shepperd 2021; Wilson 1999. GHarris 2005; Levine 2018; Levine 2020; Mendoza 2009; Ricauda 2008; Tibaldi 2009; Wilson 1999. HCaplan 1999; Nicholson 2001; Ricauda 2004; Ricauda 2008; Richards 2005; Shepperd 2021; Wilson 1999. IEchevarria 2018; Mendoza 2009; Shepperd 2021.
Background
In the last 20 years, efforts to manage the steady increase in hospital admissions have included expanding out‐of‐hospital services. Examples include hospital at home services, which are designed to avoid a hospital admission or to provide early supported discharge from hospital (Leong 2021; Oliver 2021). Possible benefits of these services include releasing hospital beds; reducing the risk of adverse events associated with time in hospital (Rafter 2015); loss of independence associated with prolonged hospitalisations (Loyd 2020); receiving rehabilitation within the home environment (Kimmel 2020); and improved patient satisfaction and communication (Leff 2006).
Recent developments in remote monitoring technology, as well as pressures on health systems caused by the COVID‐19 pandemic, have motivated more countries to prioritise hospital at home services. Examples include Queensland Australia Hospital in the Home (Mackay 2023; Queensland Government 2022), Spain (Nogues 2021), and the UK, where NHS England has committed to funding the set‐up of virtual wards (otherwise known as 'hospital at home'), and Integrated Care Systems have been asked to deliver capacity equivalent to 40 to 50 virtual ward 'beds' per 100,000 population (NHS England 2021). In Scotland, health boards are required to provide hospital at home services, and some of these services have been running for many years (British Geriatrics Society 2022). In Spain, hospital at home units became popular in the 1990s, and gradually progressed to most of the country (de Sousa Vale 2020). In Australia, 'hospital in the home' activity is also growing, accounting for 3.7% of admissions from 2011 to 2017, and there are calls for more systematic monitoring and oversight (Montalto 2020).
The type of patient treated in hospital at home services varies, as does the use of technology, similar to the variation in hospitals. Some services are designed to care for specific conditions, such as chronic obstructive pulmonary disease, or to provide specific skills such as parenteral nutrition (Kumpf 2019). These services usually have close ties with acute hospitals and may be encouraged by the different structure of incentives in insurance‐based systems of health care by providing the type of service that is reimbursed.
Description of the condition
The demographic shift of a rising number of older people has increased the demand for hospital‐level care. For example, in the UK more than 40% of people admitted to hospital are over 65 years of age (NHS Digital 2019; WHO 2021 to 2030), and between 2006 and 2018 there was a 59% increase in the number of people aged over 85 who required emergency hospital admission (Steventon 2018). Healthcare decision‐makers in a number of countries are attempting to reconfigure services to deal with a year‐on‐year increase in hospital admissions, often with an inadequate evidence base (Nolte 2008; Steventon 2018). These changes have raised concerns that the pressure of delivering health care to greater numbers may be at odds with the provision of person‐centred, high‐quality care (RCP 2017). In addition to ageing, other factors such as remote monitoring are driving the adoption of hospital at home services.
Description of the intervention
The majority of admission avoidance hospital at home services provide co‐ordinated, multidisciplinary health care in the home for people who would otherwise be admitted to hospital (Arsenault‐Lapierre 2021; Leff 2009). Similar to hospitals, services are adapted to suit the patient population. People are admitted to admission avoidance hospital at home after assessment in the community by their primary care physician or in the emergency department or a medical admissions unit. Hospital at home may also provide hospital‐level care following early discharge from hospital (we have conducted a parallel systematic review of early discharge hospital at home, recently updated with no new studies identified (Gonçalves‐Bradley 2017), and a review of home‐based end‐of‐life care (Shepperd 2016b).
In single‐payer systems, hospital at home is commonly integrated with existing services, for example using telehealth that is routinely available, or existing emergency services or out‐of‐hours stand‐by services to provide publicly funded 24‐hour cover for patients if they deteriorate. This is the case in the UK, Spain, and Canada.
How the intervention might work
As well as reducing the demand for acute hospital beds, receiving hospital at home may lower the risk of functional decline from the limited mobility that can occur during an admission to hospital. This may be particularly beneficial for older people living with frailty, by providing co‐ordinated health care in the less restrictive home environment and thereby providing patients with the opportunity for continued involvement in activities of daily living (Covinsky 2003).
Why it is important to do this review
With the current policy emphasis on care closer to home (WHO 2021 to 2030), and concern about the steadily increasing demand for hospital bed‐based care (Monitor 2015; Virtual Wards 2022), we are updating this review to incorporate new randomised evidence. Along with more widespread use of admission avoidance hospital at home services, concerns have been raised about standards of care, lack of data, oversight systems, and the role of financial incentives in insurance‐based health systems that motivate providers to establish these services (Batt 2023). An up‐to‐date systematic review of the global evidence is needed to establish whether hospital at home is effective and cost‐effective when compared with bed‐based hospital care, or if there is a risk that it reduces the quality of care (Batt 2023). This is the fourth update of this review (Shepperd 2016a).
Objectives
To determine the effectiveness and cost of managing patients with admission avoidance hospital at home compared with inpatient hospital care.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
This review included evaluations of admission avoidance hospital at home schemes involving people aged 18 years and over. We did not include people with long‐term care needs unless they required admission to hospital for an acute episode of care. We excluded evaluations of obstetric, paediatric, and mental health hospital at home schemes from the review as our preliminary literature searches suggested that separate reviews would be justified for each of these groups. For the purposes of this review, we defined older patients as those aged 65 years and older.
Types of interventions
Studies comparing admission avoidance hospital at home with acute hospital inpatient care. The admission avoidance hospital at home studies may have admitted patients directly from the community, thereby avoiding physical contact with the hospital, or may have admitted from the emergency room or an acute assessment unit. We used the following definition to determine whether studies should be included in the review: hospital at home is a service that can avoid the need for hospital admission by providing active treatment by healthcare professionals (including doctors) in the patient's home for a condition that would otherwise require acute hospital inpatient care, and always for a limited time period. In particular, hospital at home has to offer a specific service to patients in their home requiring healthcare professionals to take an active part in the patient's care. If hospital at home were not available, then the patient would be admitted to an acute hospital ward. We have therefore excluded the following services from this review:
services providing long‐term care;
services provided in outpatient settings or postdischarge from hospital; and
self‐care by the patient in their home such as self‐administration of an intravenous infusion.
Types of outcome measures
Primary outcomes
Mortality.
Admission to hospital.
Secondary outcomes
Living in residential care at follow‐up.
Patient self‐reported health status: quality of life, functional status, psychological health.
Satisfaction: patient, caregiver, health professionals.
Length of stay in hospital and hospital at home.
Cost and resource use.
Clinical outcomes.
Search methods for identification of studies
Electronic searches
For this update we searched the following databases on 24 February 2022 for references published since 2 March 2016, the last version of this review:
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 2);
MEDLINE (Ovid) (MEDALL);
Embase (Ovid);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCOhost).
We sought ongoing and unpublished studies by searching ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and by contacting providers and researchers involved in the field.
Search strategies are comprised of natural language and controlled vocabulary terms. Search terms for this update were revised based on terminology used in studies included in previous versions of the review. We applied no limits on language. We ran searches from 2015 onwards, the date of publication of the previous version of the review. In databases where it was possible and appropriate, study design filters for randomised trials were used; in MEDLINE we used a modified version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2021). Limits were used in Embase to remove MEDLINE records in order to avoid duplication in downloaded results. Remaining results were deduplicated in EndNote against each other and against results from searches conducted for previous versions of the review. All search strategies used in this version of the review are provided in Appendix 1. Search strategies and search methods used in previous versions of the review are published within those prior publications.
Searching other resources
We searched the following trial registries on 14 November 2022 (Appendix 1):
WHO ICTRP (trialsearch.who.int);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov).
We checked the reference lists of articles identified electronically for evaluations of hospital at home and obtained potentially relevant articles. We checked relevant systematic reviews for other relevant studies.
Data collection and analysis
For a previous version of this review we contacted the investigators of 10 of the included trials that recruited similar populations, inviting them to contribute individual patient data (IPD) to the hospital at home admission avoidance collaborative review (Shepperd 2005), and had access to IPD for one new study for this update (Shepperd 2021).
Selection of studies
Three review authors (KE, SS, DGB) read all the abstracts in the records retrieved by the electronic searches to identify potentially eligible publications. We retrieved the full‐text papers for these publications, and two review authors (KE, SS, SI or DGB) independently assessed their eligibility. We selected studies for the review according to the prespecified inclusion criteria and resolved any disagreements by discussion. As an author of one of the studies (Shepperd 2021), SS was not involved in assessing their own study for inclusion, risk of bias, or data extraction.
Data extraction and management
Four review authors (SS, SI, DGB, KE) independently completed data extraction using a good‐practice extraction form developed by Cochrane that was modified and amended for the purposes of this review (EPOC 2015a).
Assessment of risk of bias in included studies
Four review authors (SS, SI, DGB, KE) independently assessed risk of bias in the included studies using the suggested risk of bias criteria for Cochrane Effective Practice and Organisation of Care (EPOC) reviews (EPOC 2015b):
random sequence generation;
allocation concealment;
baseline outcome measurement;
baseline characteristics;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting of outcomes.
Measures of treatment effect
We used IPD data and published data to conduct meta‐analyses on the outcomes of mortality, admission to hospital after discharge from hospital at home or inpatient care, and place of residence (living in residential care). We used a two‐stage approach: first we obtained or calculated the study treatment effects and their standard errors, and then subsequently combined them.
For mortality at three months, treatment effects adjusted for age and sex were estimated in a previous version of the review, based on IPD received from three trialists (Davies 2000; Harris 2005; Wilson 1999). These risk ratios were then combined using fixed‐effect inverse variance meta‐analysis (Deeks 2001). The pooled effect is expressed as the risk ratio for hospital at home compared with usual hospital care, with 95% confidence interval.
For mortality at six months, we extracted numbers of dead and alive in each group from published data from three studies, Caplan 1999; Ricauda 2008; Tibaldi 2009, and from IPD from two studies (Shepperd 2021; Wilson 1999), and combined this information as unadjusted risk ratios using a fixed‐effect model with inverse variance weights. Though studies published some adjusted risk ratios, they varied in the covariates they adjusted for, and therefore it was most straightforward to use only unadjusted estimates.
We analysed the effect of admission avoidance hospital at home on admission to hospital after discharge from hospital at home or inpatient care using IPD received from five trialists (Davies 2000; Harris 2005; Mendoza 2009; Shepperd 2021; Wilson 1999), and published data from three studies (Caplan 1999; Ricauda 2008; Tibaldi 2009), again combined using a fixed‐effect model and inverse variance weights. This outcome describes subsequent admission to inpatient hospital care after discharge (from either hospital at home or hospital) for a range of follow‐up times (3 to 12 months). We also extracted and presented data for transfer to hospital during the hospital at home episode, but as this only applies to the intervention group, we did not meta‐analyse these data.
There were insufficient IPD for living in residential care, therefore for this outcome we used available published data from three studies, Ricauda 2008; Tibaldi 2004; Tibaldi 2009, combined with the unadjusted risk ratio obtained from (Shepperd 2021), using a fixed‐effect model with inverse variance weights. The analyses in this review were carried out in Stata 16.1.
Our statistical analyses sought to include all randomised participants, using the intention‐to‐treat principle. We relied on published data when the IPD did not include the relevant outcomes.
Unit of analysis issues
The unit of allocation was the participant in all trials.
Dealing with missing data
In one data set contributing to the IPD meta‐analysis (Davies 2000), some dates were missing for known events, and so we gave the missing event a time at the midpoint between randomisation and last follow‐up, or as the midpoint between follow‐up times if these were known. For one trial where follow‐up was 90 days, we set the time to event as 45 days for three cases in the admission avoidance hospital at home arm and for one case in the control group where we knew death had occurred but we did not have a date (Davies 2000).
Assessment of heterogeneity
We quantified heterogeneity by Cochran's Q and the I2 statistic (Cochran 1954), the latter quantifying the percentage of the total variation across studies that is due to heterogeneity rather than chance (Higgins 2003); smaller percentages suggest less observed heterogeneity.
Assessment of reporting biases
If we identified an adequate number of studies (more than 10) and included these in a meta‐analysis, we explored publicaion bias using a funnel plot to visually assess funnel plot asymmetry (Higgins 2019).
Data synthesis
We used IPD when this information was available for studies that recruited similar populations (Davies 2000; Harris 2005; Mendoza 2009; Shepperd 2021; Wilson 1999). The pooled effect is expressed as the risk ratio for hospital at home compared with usual hospital care. Throughout the analyses, we took statistical significance at the two‐sided 5% level (P < 0.05), presenting data as the estimated effect with 95% confidence intervals. For this update, we conducted the analysis using Review Manager 5 (Review Manager 2020).
When combining outcome data was not possible because of differences in the measurement or reporting of outcomes, or in the case of outcomes that only applied to the intervention group, we presented data from individual studies in tables. Although planned, we did not attempt a direct comparison of costs because the trials collected data on different resources and used different methods to calculate costs.
Subgroup analysis and investigation of heterogeneity
We grouped studies by study population to reduce the amount of variation in the analysis.
Sensitivity analysis
We did not conduct a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We assessed our confidence in the evidence by creating a summary of findings table using the approach recommended by the GRADE Working Group, in Guyatt 2008, and specific guidance developed by EPOC (EPOC 2017), employing GRADEpro GDT software (GRADEpro GDT 2022). We included the main outcomes of mortality and admission to hospital, as well as living in residential care at follow‐up, patient satisfaction, length of stay, patient self‐reported health status, and cost and resource use. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and risk of bias) to assess the certainty of the evidence as it relates to the main outcomes (Guyatt 2008). We used the methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Results
Description of studies
We identified 20 trials that randomised individual participants (N = 3100), of which four were located in this update (Echevarria 2018; Levine 2018 (a pilot study); Levine 2020; Shepperd 2021) (Characteristics of included studies).
Results of the search
The updated search retrieved 2982 records from the electronic databases. We found 10 additional records from other sources, for a total of 2992 records after duplicates were removed, of which 2973 were ineligible. We obtained the full texts for the remaining 19 records, four of which fulfilled the inclusion criteria (four trials, five records), bringing the total number of trials included in the review to 20 (Figure 1). We excluded 12 studies with reasons provided (Excluded studies). We also identified two ongoing trials (NCT03156686; Pouw 2018; see Characteristics of ongoing studies).
1.
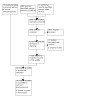
PRISMA flow diagram.
Included studies
See Characteristics of included studies.
For a previous version of this review, we contacted the investigators of 10 of the included trials that recruited similar populations, inviting them to contribute IPD to the hospital at home admission avoidance collaborative review (Shepperd 2005). We used summaries of this information from the previous review for the three‐month mortality comparison using three studies (Davies 2000; Harris 2005; Wilson 1999), and had access to IPD for one new study (Shepperd 2021). We have summarised each study in Table 2, including details of the intervention and population in each study.
1. Details of each hospital at home study.
| N | Length of follow‐up | Population | Conditions | Intervention | Control | Location | Mean age (SD) | 24‐hour care provision | |
| Andrei 2011 | 45 | 12 months | Patients with chronic heart failure that had deteriorated at a minimum of 1 week prior to recruitment | Chronic heart failure | Admission avoidance hospital at home; the first 48 hours of treatment was in the ED | Unknown | Romania | Unknown | Not reported |
| Caplan 1999 | T: 51 C: 49 |
6 months | Patients attended casualty | Range of acute conditions | Hospital community outreach team | Hospital care | Australia | T: 73 (median) C: 79 (median) |
Not reported |
| Corwin 2005 | T: 98 C: 96 |
6 days | Patients attended emergency department | Cellulitis | Hospital at home admission avoidance from the ED by GP and community care nursing staff | Hospital care | New Zealand | T: 54.6 (20.6) C: 48.4 (19.0) |
Not reported |
| Davies 2000 | T: 100 C: 50 |
3 months | Patients attended A&E with chronic obstructive airways disease | COPD | Admission avoidance hospital at home by outreach specialist nurses and GP/community nurses | Hospital care | UK | Unknown | District nurses |
| Echevarria 2018 | T: 62 C: 58 |
90 days | Patients over 35 years of age admitted to hospital with COPD | COPD | Once‐ or twice‐daily vists from respiratory specialist nurse under remote supervision from consultant | Hospital care | UK | T: 71.0 (9.6) C: 68.7 (10.5) |
24/7 contact with HAH team available |
| Harris 2005 | T: 39 C: 37 |
90 days | Patients attended emergency department or acute assessment ward | Range of acute conditions | Hospital outreach programme; nurse‐led team provided care and rehab in patients' homes | Hospital care | New Zealand | 80.0 | 24‐hour on‐call geriatrician |
| Kalra 2000 | T: 153 C: 152 |
12 months | Patients within 72 hours of stroke onset | Moderately severe stroke | Hospital outreach admission avoidance multidisciplinary care | Hospital care, stroke unit care | UK | T: 77.7 C: 77.3 (medians) |
Not reported |
| Levine 2018 | T: 9 C: 11 |
30 days | Patients over 18 years of age attending emergency department | Infection, heart failure, COPD, asthma exacerbation | Hospital at home; at least 1 daily visit from general internist, 2 daily visits from nurse | Hospital care | USA | T: 65 (28) C: 60 (29) median (IQR) |
Attending physician available 24/7 |
| Levine 2020 | T: 43 C: 48 |
30 days | Patients over 18 years of age attending emergency department | Infection, heart failure, COPD, asthma exacerbation | Hospital at home; at least 1 daily visit from general internist, 2 daily visits from nurse | Hospital care | USA | T: 80 (19) C: 72 (23) median (IQR) |
Attending physician available 24/7 |
| Mendoza 2009 | T: 37 C: 34 |
1 year | Patients in A&E with acute decompensation of chronic heart failure | Heart failure | Admission avoidance hospital at home; hospital outreach model | Hospital care | Spain | 79 | Emergency services |
| Nicholson 2001 | T: 13 C: 12 |
Duration of treatment | Patients over 45 years of age with COPD referred by GP or emergency staff | COPD | Hospital at home | Hospital care | Australia | Unknown | 24‐hour telephone support by hospital staff |
| Ricauda 2004 | T: 60 C: 60 |
6 months | Patients admitted to hospital within 24 hours of onset of stroke symptoms | Stroke | Hospital outreach admission avoidance | Hospital care | Italy | T: 82.5 (8.6) C: 79.5 (6.7) |
Physician and nurse available 24 hours |
| Ricauda 2008 | T: 52 C: 52 |
6 months | Patients admitted to hospital for acute exacerbation of COPD | COPD | Physician‐led admission avoidance hospital outreach service | Hospital care | Italy | T: 80.1 (3.2) C: 79.2 (3.1) |
HAH staff available 24 hours |
| Richards 2005 | T: 24 C: 25 |
6 weeks | Patients presented to emergency room with pneumonia | Community‐acquired pneumonia | Hospital at home: admission avoidance from emergency room | Hospital care | New Zealand | T: 50.1 C: 49.8 |
24‐hour emergency contact number |
| Shepperd 2021 | T: 687 C: 345 |
12 months | Patients over 65 years of age referred to HAH | Range of acute conditions | Admission avoidance hospital at home; geriatrician‐led multidisciplinary team | Hospital care | UK | T: 83.3 (7.0) C: 83.3 (6.9) |
NHS telephone out‐of‐hours service, plus site‐specific arrangements for overnight care |
| Talcott 2011 | T: 47 C: 66 |
Duration of acute episode | Patients who had chemotherapy | Febrile neutropenia | Admission avoidance hospital at home; commercial home care provider | Hospital care | USA | 47 (median) 20 to 81 (range) |
Not reported |
| Tibaldi 2004 | T: 56 C: 53 |
Until discharge | Patients with advanced dementia | Range of acute conditions | Hospital at home; geriatric home hospitalisation service | Hospital care | Italy | T: 82.9 (7.9) C: 84.1 (7.5) |
Not reported |
| Tibaldi 2009 | T: 48 C: 53 |
6 months | Patients presented to emergency department | Chronic heart failure | Admission avoidance hospital at home; hospital outreach | Hospital care | Italy | 81 | HAH staff available 24 hours |
| Vianello 2013 | T: 26 C: 27 |
3 months | Patients with neuromuscular disease | Acute respiratory tract infection | Hospital at home; portable ventilator, respiratory therapist daily visits | Hospital care | Italy | T: 44.6 (20.4) C: 46.7 (20.2) |
Pulmonologist available by telephone |
| Wilson 1999 | T: 102 C: 97 |
3 months | Majority elderly, referred by GP to Bed Bureau | Range of acute conditions | Admission avoidance hospital at home | Hospital care | UK | 84 (median) | 24‐hour care available |
A&E: accident & emergency department C: control COPD: chronic obstructive pulmonary disease ED: emergency department GP: general practitioner HAH: hospital at home IQR: interquartile range SD: standard deviation T: treatment
Study populations
Four trials recruited participants with chronic obstructive pulmonary disease (COPD), with an average age range of 69.9 to 81 years (Davies 2000; Echevarria 2018; Nicholson 2001; Ricauda 2008). Two trials recruited participants recovering from a moderately severe stroke who were clinically stable, with an average age range of 77.5 to 81 years (Kalra 2000; Ricauda 2004). Seven trials recruited participants with an acute medical condition who were mainly elderly, with an average age range of 76 to 84 years (Andrei 2011; Caplan 1999; Harris 2005; Mendoza 2009; Shepperd 2021; Tibaldi 2009; Wilson 1999). Harris 2005 included two treatment groups and a control (hospital) group. One treatment group was an "admission prevention" group, and the other was an "early discharge" group. Harris provided IPD data for the admission prevention and control groups only. Two trials, one of which was the pilot for the main effectiveness trial, recruited adults attending the emergency department with a primary diagnosis of any infection, heart failure exacerbation, COPD exacerbation, or asthma exacerbation, with an average age of each 62.5 and 65 years respectively (Levine 2018; Levine 2020). There was one trial each for participants with cellulitis, average age 51.5 years (Corwin 2005), community‐acquired pneumonia, average age 50 (Richards 2005), fever and neutropenia, average age 47 (Talcott 2011), frail elderly participants with dementia, average age 83.5 (Tibaldi 2004), and neuromuscular disease, average age 45.7 years (Vianello 2013). The 20 trials were conducted in seven countries: Australia (two trials), Italy (five trials), New Zealand (three trials), Romania (one trial), Spain (one trial), the UK (five trials), and the USA (three trials).
Interventions
Details of the intervention components are described in Table 2 and Table 3. Participants were admitted to hospital at home from the emergency room (Andrei 2011; Caplan 1999; Corwin 2005; Davies 2000; Levine 2018; Levine 2020; Mendoza 2009; Nicholson 2001; Ricauda 2004; Ricauda 2008; Richards 2005; Tibaldi 2004; Tibaldi 2009; Vianello 2013), after a hospital assessment (within 24 hours) (Echevarria 2018), the community following referral by their primary care physician (Harris 2005; Kalra 2000; Wilson 1999), an outpatient department (Talcott 2011), and a hospital acute assessment unit or primary care (Shepperd 2021). For participants allocated to hospital at home, health care was provided by a hospital outreach team (Caplan 1999; Echevarria 2018; Harris 2005; Mendoza 2009; Ricauda 2004; Ricauda 2008; Talcott 2011; Tibaldi 2004; Tibaldi 2009), a mix of outreach and community staff (Davies 2000; Kalra 2000; Levine 2018; Levine 2020; Nicholson 2001; Shepperd 2021; Vianello 2013), or by the general practitioner (GP) and community nursing staff (Corwin 2005; Richards 2005; Wilson 1999). For one trial it was not clear who provided care (Andrei 2011). In two trials, the intervention was provided by Pegasus Health, an independent association of GPs (Corwin 2005; Richards 2005). One trial was a three‐group comparison of stroke unit care, inpatient stroke team, and hospital at home (Kalra 2000); we selected the inpatient stroke team as the comparison group, as this was most similar to the comparator in the other trials.
2. Referral, hospital at home provision, and types of care in the included studies.
| Andrei 2011 | Caplan 1999 | Corwin 2005 | Davies 2000 | Echevarria 2018 | Harris 2005 | Kalra 2000 | Levine 2018 | Levine 2020 | Mendoza 2009 | Nicholson 2001 | Ricauda 2004 | Ricauda 2008 | Richards 2005 | Shepperd 2021 | Talcott 2011 | Tibaldi 2004 | Tibaldi 2009 | Vianello 2013 | Wilson 1999 | |
| Mode of referral | ||||||||||||||||||||
| Emergency room | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Community (by primary care physician) | X | X | X | |||||||||||||||||
| Outpatient department | X | |||||||||||||||||||
| From admission < 24 hours | X | |||||||||||||||||||
| Acute assessment unit/home | X | |||||||||||||||||||
| Hospital at home provision | ||||||||||||||||||||
| Hospital outreach team | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Mix of outreach/community staff | X | X | X | X | X | |||||||||||||||
| GP/community nursing staff | X | X | X | |||||||||||||||||
| Unclear | X | |||||||||||||||||||
| Types of care | ||||||||||||||||||||
| Physiotherapy | X | X | X | X | X | X | X | X | X | |||||||||||
| Social worker | X | X | X | X | X | X | X | X | X | |||||||||||
| Occupational therapy | X | X | X | X | X | X | X | X | ||||||||||||
| Counsellor | X | |||||||||||||||||||
| Speech therapist | X | X | ||||||||||||||||||
| Cultural link worker | X | |||||||||||||||||||
| Portable ventilator | X | |||||||||||||||||||
GP: general practitioner
Physiotherapy care was described in 10 of the interventions (Harris 2005; Kalra 2000; Levine 2018; Levine 2020; Nicholson 2001; Ricauda 2004; Ricauda 2008; Shepperd 2021; Tibaldi 2004; Wilson 1999), and occupational therapist care in seven (Harris 2005; Kalra 2000; Levine 2018; Levine 2020; Nicholson 2001; Shepperd 2021; Wilson 1999). A social worker was part of the hospital at home team in 10 of the interventions (Davies 2000; Harris 2005; Kalra 2000; Levine 2018; Levine 2020; Ricauda 2004; Shepperd 2021; Talcott 2011; Tibaldi 2004; Wilson 1999), and a counsellor in one (Talcott 2011). Access to a speech therapist was described in three of the interventions (Kalra 2000; Ricauda 2004; Wilson 1999). In two trials, participants could access a home health aide and medical meals, if required (Levine 2018; Levine 2020). One trial described access to a cultural link worker (Wilson 1999). The intervention in one trial included the use of a portable ventilator; a respiratory therapist made daily visits for the first three days of home care, and district nurses and caregivers were trained in the application of the device and on assisting with coughing (Vianello 2013). District nurses visited daily until recovery from the respiratory tract infection; participants also had telephone access to pulmonary specialists (Vianello 2013).
Excluded studies
The main reason for exclusion was that the trial tested the feasibility of introducing technologies for ameliorating a condition at home (four trials), Duiverman 2019; Hazenberg 2014; Mascardi 2016; NCT02363413, and not hospital at home (see Characteristics of excluded studies).
Risk of bias in included studies
See Characteristics of included studies. Summaries of the risk of bias assessments for the included studies are presented in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 15 studies concealment of allocation was adequate (Figure 2; Figure 3) (Caplan 1999; Corwin 2005; Davies 2000; Echevarria 2018; Harris 2005; Kalra 2000; Levine 2018; Levine 2020; Mendoza 2009; Ricauda 2008; Richards 2005; Shepperd 2021; Talcott 2011; Tibaldi 2009; Wilson 1999), and in 12 studies sequence generation was adequately described (Caplan 1999; Echevarria 2018; Harris 2005; Kalra 2000; Levine 2018; Levine 2020; Mendoza 2009; Ricauda 2004; Ricauda 2008; Richards 2005; Shepperd 2021; Talcott 2011).
Blinding
We assessed seven studies as having a low risk of performance bias (Davies 2000; Echevarria 2018; Harris 2005; Kalra 2000; Ricauda 2008; Shepperd 2021; Wilson 1999). Three studies were at unclear risk of bias for the measurement of objective outcomes, and nine studies were at low risk of bias for the measurement of subjective outcomes (Figure 3).
Incomplete outcome data
Most studies had a low risk of attrition bias, with seven studies having an unclear risk (Andrei 2011; Mendoza 2009; Nicholson 2001; Ricauda 2004; Richards 2005; Tibaldi 2004; Tibaldi 2009).
Selective reporting
Nine studies were at low risk of bias for selective reporting (Davies 2000; Echevarria 2018; Harris 2005; Kalra 2000; Levine 2020; Ricauda 2008; Shepperd 2021; Tibaldi 2009; Wilson 1999).
Other potential sources of bias
Other sources of bias were not assessed.
Effects of interventions
See: Table 1
See Table 1 for the main comparison admission avoidance hospital at home versus inpatient admission for older people requiring admission to hospital.
Mortality
We combined IPD for a subset of three studies (N = 420), adjusted for age and sex, for mortality at three months' follow‐up (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.55 to 1.45; P = 0.64; N = 420 participants; moderate‐certainty evidence) (Davies 2000; Harris 2005; Wilson 1999) (Analysis 1.1). We combined published data from three studies, Caplan 1999; Ricauda 2008; Tibaldi 2009, with data received from trialists of two studies, Shepperd 2021; Wilson 1999, for mortality at six months (RR 0.88, 95% CI 0.68 to 1.13; P = 0.30; N = 1502 participants; moderate‐certainty evidence) (Analysis 1.2). Results indicated that admission avoidance hospital at home probably makes little to no difference to mortality when compared with in‐hospital care.
1.1. Analysis.

Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 1: Mortality at 3 months using IPD
1.2. Analysis.

Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 2: Mortality at 6 months' follow‐up (using published data, and IPD from Wilson and Shepperd)
Admission to hospital
We analysed the effect of admission avoidance hospital at home on hospital admission after discharge from hospital at home or inpatient care at 3 to 12 months' follow‐up using data received from five trialists, Davies 2000; Harris 2005; Mendoza 2009; Shepperd 2021; Wilson 1999, and published data from three studies, Caplan 1999; Ricauda 2008; Tibaldi 2009. Results indicated that admission avoidance hospital at home probably makes little to no difference to hospital admission (RR 1.14, 95% CI 0.97 to 1.34; P = 0.11; I2 = 41%; N = 1757 participants; moderate‐certainty evidence) (Analysis 1.3). Four trials reported transfer to hospital while receiving hospital at home (Analysis 1.4) (Corwin 2005; Ricauda 2008; Richards 2005; Talcott 2011).
1.3. Analysis.

Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 3: Readmission to hospital after discharge from hospital at home or inpatient care (3 to 12 months' follow‐up)
1.4. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 4: Transfer to hospital while receiving hospital at home
| Transfer to hospital while receiving hospital at home | ||
| Study | Outcomes | Results |
| Corwin 2005 | Transfer to hospital | T= 11/98 |
| Ricauda 2008 | Transfer to acute hospital | T= 3/52 |
| Richards 2005 | Transfer to hospital | T= 2/24 |
| Talcott 2011 | Readmission to hospital while receiving hospital at home | T= 4/47 |
Living in residential care at follow‐up
Admission avoidance probably reduces the likelihood of living in residential care, measured at discharge to six months' follow‐up (RR 0.53, 95% CI 0.41 to 0.69; P < 0.001; I2 = 67%; 4 trials; N = 1271 participants; moderate‐certainty evidence) (Analysis 1.5) (Ricauda 2008; Shepperd 2021; Tibaldi 2004; Tibaldi 2009).
1.5. Analysis.

Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 5: Living in residential care at follow‐up
Patient self‐reported health status
Quality of life
Nine trials assessed health status or quality of life using different measures, as described below (Analysis 1.6).
1.6. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 6: Quality of life/health status
| Quality of life/health status | |||
| Study | Outcomes | Results | Notes |
| Admission avoidance quality of life | |||
| Corwin 2005 | SF 36 Physical functioning Role physical Pain | SF 36 Physical functioning Day 3 T= 37 (29.1), C= 41 (28.3) Mean difference ‐1.9, 95% CI ‐10.7 to 6.9 Day 6 T=50.7 (33.7), C=50.9 (31.6) Mean difference ‐5.2, 95% CI ‐13.7 to 3.2 Role physical Day 3 T= 5.4 (18.8), C=5.5 (19.7) Mean difference ‐1.8 95% CI ‐13.1 to 9.4 Day 6 T=21.1 (36.9), C=18.4 (36.5) Mean difference 2.2, 95% CI ‐10.7 to 15.1 Pain Day 3 T=57 (28.8), C=55.9 (25.4) Mean difference ‐2.5 95% CI ‐10.1 to 5.1 Day 6 T=69.8 (26.4), C=64.8 (25.6) Mean difference ‐3.8 95% CI ‐10.6 to 3.0 | Differences calculated on absolute differences between day 0 & day 3, or day 0 & day 6.
Numbers vary due to missing data (high score=better health) |
| Echevarria 2018 | EQ‐5D‐5L utility 14 day EQ‐5D‐5L utility 90 day |
EQ‐5D‐5L utility (SD), mean 14 day unit change from baseline T = 0.091 (0.249) C = 0.055 (0.316) EQ‐5D‐5L utility (SD), mean 90 day unit change from baseline T = 0.003 (0.287) C = 0.007 (0.338) |
|
| Mendoza 2009 | SF 36 Physical component Mental component |
Physical component T= 3.6 (‐0.5 to 7.7), C= 2.2 (‐1.9 to 6.4), P = 0.47 Mental component T= 4.0 (‐0.9 to 8.9), C= 2.8 (‐2.4 to 8.0), P = 0.38 |
Score at 1 year (adjusted for baseline differences) |
| Ricauda 2008 | Nottingham Health Profile | 6 months, mean (SD) T= 3.6 (7.9), C= 0.8 (4.5), P = 0.04 |
Changes at 6 months |
| Richards 2005 | SF‐12 Mean physical and mental component score |
Physical component At 2 weeks T= 38.1, C= 40.2, P = 0.45 At 6 weeks T= 42.2, C=45.8, P = 0.18 Mental component At 2 weeks T=48.3, C=48.6, P = 0.91 At 6 weeks T = 50.4, C=51.0, P = 0.81 |
higher score=better health |
| Shepperd 2021 | Health status EQ‐5D‐5L utility Barthel Index |
Mean EQ‐5D‐5L utility 6 months (SD) T = 0.451 (0.324) C = 0.457 (0.340) Difference in means (95% CI) ‐0.006 (‐0.053, 0.041) Mean (SD) Barthel Index at 6 months T = 15.8 (4.4) C = 15.6 (4.9) Adjusted mean difference (95% CI) 0.24 (‐0.33, 0.80) P=0.41 |
|
| Talcott 2011 | Quality of life EORTC QLQ C‐30 |
Role Function T= 0.58, C= 0.78, P = 0.05 Emotional Function T= 3.27, C= ‐6.94, P = 0.04 |
Quality of life data were collected at the time of consent to join the study, as soon as possible after the resolution of the episode. Data were collected for the first study episode. Change score |
| Tibaldi 2009 | Nottingham Health Profile | 6 months, mean (SD) T= +1.09 (2.57), C= +0.18 (1.94), P = 0.046 |
|
| Wilson 1999 | Sickness Impact Profile (SIP) Euroqol | SIP, median (IQR) T= 24 (20‐31), C= 26 (20‐31) Difference ‐2 (95% CI ‐4 to 4), P = 0.73 Euroqol, median T= 0.64, C= 0.63 Difference 0.01 (95% CI ‐0.12 to 0.09), P = 0.94 | At 3 months follow‐up |
One trial that recruited people with cellulitis reported 36‐Item Short Form Health Survey (SF‐36) scores at six days' follow‐up. The difference in score from day 0 was compared between the treatment groups; each item is scored between 0 and 100, with a higher score indicating better health; a difference above 0 favours the treatment group (physical component scale mean difference (MD) −5.2, 95% CI −13.7 to 3.2; role physical scale MD 2.2, 95% CI −10.7 to 15.1; pain scale MD −3.8, 95% CI −10.6 to 3.0) (Corwin 2005).
A second trial measuring health status with the SF‐36 reported follow‐up data at one year for the physical component scale (treatment group (T): 3.6 (−0.5 to 7.7), control group (C): 2.2 (−1.9 to 6.4); P = 0.47) and the mental component scale (T: 4.0 (−0.9 to 8.9), C: 2.8 (−2.4 to 8.0); P = 0.38) (Mendoza 2009). One trial measured quality of life with the SF‐12 at six weeks' follow‐up and reported similar scores for each group on the physical component (T: 42.2, C: 45.8; P = 0.18) and mental component scale (T: 50.4, C: 51.0; P = 0.81) (Richards 2005).
Two trials assessed quality of life using the Nottingham Health Profile at six months' follow‐up. Yes/no answers are given for 38 items, which are then weighted to give a score between 0 and 100, with a higher score indicating better health. In these two trials, the change from baseline to six months was compared between treatment groups (T: +1.09 (standard deviation (SD) 2.57) N = 48, C: +0.18 (SD 1.94); P = 0.046) (Tibaldi 2009) and (T: 3.6 (SD 7.9), C: 0.8 (SD 4.5); P = 0.04) (Ricauda 2008).
One trial assessed change from baseline in quality of life when a participant had a health event using the EORTC QLQ‐C30, measuring the change before and after the episode (range 0 to 100, higher is better quality of life) (T: 0.58, C: 0.78, P = 0.05; emotional function hospital at home 3.27, hospital −6.94; P = 0.04) (Talcott 2011). One trial reported median values at three months' follow‐up for the Sickness Impact Profile (range 0 to 100, higher score is better health) (T: 24 (interquartile range (IQR) 20 to 31), C: 26 (IQR 20 to 31); MD −2, 95% CI −4 to 4; P = 0.73) and the EuroQol (utility score anchored at 0 for death and 1 for perfect health) (T: 0.64, n = 73, C: 0.63, n = 96; MD 0.01, 95% CI −0.12 to 0.09; P = 0.94) (Wilson 1999). Two trials reported mean changes for the EQ‐5D: Echevarria 2018 reported small changes from baseline to 14‐ and 90‐day follow‐up, and Shepperd 2021 reported that there was likely little or no difference between groups at six months' follow‐up.
Functional status
Ten trials reported measures of functional ability, for which higher scores indicate greater independence (see Analysis 1.7 for specific details on the scales used), described as follows.
1.7. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 7: Functional status
| Functional status | ||
| Study | Functional ability | Results |
| Admission avoidance patients with a medical condition ‐ functional ability | ||
| Caplan 1999 | Change in Barthel score from admission to discharge (high score=greater independence) Instrumental activities of daily living score from admission to discharge (higher score=greater independence) | Mean (SEM) T= 0.37 (0.27), C= ‐0.04 (0.27), NS Mean (SEM) T= 0.65 (0.23), C= ‐0.88 (0.26), P = 0.037 |
| Davies 2000 | St Georges' respiratory questionnaire (to a random sub‐group of 90 participants). High score indicates poorer health related quality of life. A minimum change in score of 4 units is clinically relevant. Forced expiratory volume in one second (FEV1) |
Baseline scores
T= 71.5 (43.4 to 99.6), C= 71.0 (43.4 to 98.6)
Mean (SD) change at 3 months
T= 0.48 (16.92) C= 3.13 (14.02) Forced expiratory volume in 1 second (FEV1) At 3 months: T= 41.5% (95% CI 8.2% to 74.8%) C= 41.9% (95% CI 6.2% to 77.6%) |
| Levine 2018 | Activities of daily living Instrumental activities of daily living |
ADLs worse at discharge T = 9 (0%) C = 11 (9%) IADLs worse at discharge T = 9 (0%) C = 11 (18%) |
| Levine 2020 | Instrumental activities of daily living Activities of daily living |
IADLs worse: admission to discharge (%) T = 11 (26) N=42 C = 14 (31) N=45 IADLs worse: admission to 30d after discharge (%) T = 14 (37) N=42 C = 13 (34) N=38 ADLs worse: admission to discharge (%) T = 6 (14) N=42 C = 6 (13) N=45 ADLs worse: admission to 30d after discharge (%) T = 4 (11) N=42 C = 6 (16) N=38 |
| Mendoza 2009 | Activities of daily living |
Mean score Barthel Index at 1 year (adjusted for baseline differences) T= 4.0 (‐0.9 to 8.9) C= 4.7 (‐2.2 to 11.5) P = 0.21 |
| Ricauda 2008 | Change in ADL (score 0 to 6) | At 6 months, mean (SD) T= 0.12 (0.64), C= 0.08 (0.73), P = 0.81 |
| Shepperd 2021 | Activities of daily living | Mean score Barthel Index at 6 months (SD) T = 15.8 (4.4) C = 15.6 (4.9) Adjusted mean difference (95% CI) 0.24 (‐0.33, 0.80) P=0.41 |
| Tibaldi 2004 | Behavioural disturbances | Sleeping disorders T= 5/56 (9%), C= 23/53 (43%), MD: ‐34%, 95% CI ‐50% to ‐19%, P < 0.001 Agitation/aggressiveness T= 5 /56 (9%), C= 22/53 (41.5%), MD ‐33% 95% CI ‐48% to ‐17%, P<0.001 Feeding disorders T= 5 /56 (9%), C= 21/53 (40%), MD ‐31% 95% CI ‐46% to ‐16%, P < 0.001 |
| Tibaldi 2009 | Activities of daily living Barthel Index |
ADL at 6 months mean change T= ‐1.95 (9.61) N=48, C= ‐0.30 (10.12) N=53, |
| Wilson 1999 | Barthel Index |
Barthel Index At 3 months (Median (IQR)) T= 16 (13‐19), C= 16 (12‐20) Barthel Index ‐ number (%) not assessed: T= 21 (28%), C= 18 (28%) Sickness Impact Profile: At 3 months (Median (IQR)) T= 24 (20‐31), C= 26 (20‐31) Sickness Impact Profile ‐ no (%) not assessed T= 31 (41%), C= 30 (46%) |
Caplan 1999 reported scores for instrumental activities of daily living between admission and discharge (MD −0.23, P = 0.04) and the Barthel Index (high score = greater independence) (hospital at home (T): 0.37 (0.27), hospital (C): −0.04 (0.27)). A trial that recruited participants with dementia reported that fewer participants in the hospital at home group had problems with sleep (difference 34%, P < 0.001), agitation and aggression (difference 32.5%, P < 0.001), and feeding (difference 31%, P < 0.001) (Tibaldi 2004).
One trial recruiting participants who had had a stroke reported the number of participants with a favourable outcome measured by the Barthel Index (score of 15 to 20) at three months (T: 106/145 (73%), C: 106/151 (70%); RR 0.96, 95% CI 0.83 to 1.11; P = 0.58) (Kalra 2000). Ricauda 2004, which also recruited participants with a stroke, reported activities of daily living (scale 0 to 6) at six months (median (IQR), T: 4 (2 to 5), C: 4 (2 to 6); P = 0.57). Two trials recruiting participants with COPD reported follow‐up data: Ricauda 2008 reported change in activities of daily living at six months (score 0 to 6) (T: 0.12 (SD) 0.64, C: 0.08 (SD 0.73); P = 0.81), and Davies 2000 reported forced expiratory volume in 1 second (FEV1) at three months' follow‐up (T: 41.5%, 95% CI 8.2% to 74.8%; C: 41.9%, 95% CI 6.2% to 77.6%).
Two studies recruiting participants with heart failure reported little or no change in activities of daily living measured by the Barthel Index at baseline and six months' follow‐up (mean T: −1.95 (SD 9.61), C: −0.30 (SD 10.12)) (Tibaldi 2009); and at one year (T: 4.0, 95% CI −0.9 to 8.9; C: 4.7, 95% CI −2.2 to 11.5; P = 0.21), adjusted for baseline differences (Mendoza 2009). Wilson 1999, which recruited older people with a mix of conditions, assessed functional ability at three months using the Barthel Index (median (IQR) T: 16 (13 to 19), C: 16 (12 to 20)). Levine 2020 reported that patients who received their health care at home were less sedentary than those in hospital, and that reductions in functional status at 30 days after discharge were similar in both groups. Shepperd 2021 reported little or no difference between groups in activities of daily living measured by the Barthel Index at six months' follow‐up (Analysis 1.7).
Psychological health
Seven trials measured cognitive function and depression, detailed as follows (Analysis 1.8).
1.8. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 8: Psychological health
| Psychological health | ||
| Study | Outcomes | Results |
| admission avoidance ‐ cognitive function/well being | ||
| Caplan 1999 | Mental status questionnaire score from admission to discharge (maximum score 10); Number with confusion | Mean (SEM) T= 0.43 (0.12), C= 0.27 (0.12), NS Number with confusion T=0/51, C=10/49 |
| Echevarria 2018 | Hospital Anxiety and Depression Scale score (HADS) | Number analysed: T=60; C=58 HADS ‐ Anxiety, 14 day (IQR), median unit change from baseline T = ‐1.0 (‐3 to 1.75) C = 0.5 (‐3 to 2) HADS ‐ Anxiety, 90 day (IQR), median unit change from baseline T = 0 (‐2 to 3) C = 0 (‐3 to 2) HADS ‐ Depression, 14 day (IQR), median unit change from baseline T = ‐1.0 (‐3 to 1) C = 0 (‐2 to 3) HADS ‐ Depression, 90 day (IQR), median unit change from baseline T = ‐0.5 (‐3 to 1.25) C = 0 (‐2 to 3) |
| Ricauda 2004 | Change in geriatric Depression Scale score (range 0‐30) higher scores indicate depression (people recovering from a stroke) | At 6 months, median IQR T=10 (5 to 15), C=17 (13 to 20) p<0.001 |
| Ricauda 2008 | Change in geriatric Depression Scale score (range 0‐30) higher scores indicate depression (people with COPD) | At 6 months, mean (SD) T= ‐3.1 (4.7), C=0.7 (3.2), P < 0.001 |
| Shepperd 2021 | Montreal Cognitive Assessment (MoCA) score (range 0‐30) Confusion Assessment Method (CAM) (Y/N for delirium) |
At 6 months (%) T: Abnormal (score of <26): 273/407 (67.1) Normal (score of >=26): 134/183 (32.9) C: Abnormal: 115 (62.8) Normal: 68 (37.2) Adjusted RR (95% CI): 1.06 (0.93, 1.21) P = 0.36 CAM (presence/absence of delirium) (%) 3 days T = 25/645 (3.9) C = 11/312 (3.5) RR: 1.12 (0.54, 2.29) P=0.76 5 days T = 17/638 (2.7) C = 9/308 (3.0) RR: 0.93 (0.34, 2.47) P=0.87 1 month T = 10/602 (1.7) C = 13/297 (4.4) Relative risk: 0.38 (0.19, 0.76) P=0.006 |
| Tibaldi 2009 | Mini Mental State Exam (MMSE) Geriatric Depression Scale |
At 6 months, mean change (SD) T= +0.07 (1.38), C= +0.08 (1.36), P = 0.97 At 6 months, mean change (SD) T= +1.48 (1.86), C= +0.12 (3.36), P = 0.02 |
| Wilson 1999 | Philadelphia Geriatric Morale Scale | At 3 months, median (IQR) T= 37 (30‐42), C= 37 (31‐43), Difference 0, 95% CI ‐4.1 to 4.1 |
One trial that recruited participants recovering from a stroke reported that hospital at home may lead to lower scores on the Geriatric Depression Scale (GDS) (lower scores = fewer symptoms) (MD 7 points on a 0‐to‐30‐point scale, P < 0.001) (Ricauda 2004), and one trial reported a lower score at six months for participants who had COPD and were allocated to hospital at home (T: −3.1 (SD 4.7), C: 0.7 (SD 3.2); P < 0.001) (Ricauda 2008). One trial that recruited participants with acute chronic heart failure reported fewer depressive symptoms at six months follow‐up (measured by the GDS) for those allocated to admission avoidance hospital at home (mean change T: 1.48 (SD 1.86), C: 0.12 (SD 3.36); P = 0.02) (Tibaldi 2009). Wilson 1999 reported median (IQR) scores for the Philadelphia Geriatric Morale Scale at three months, finding little to no difference between groups (T: 37 (30 to 42), C: 37 (31 to 43); MD 0, 95% CI −4.1 to 4.1). Echevarria 2018 and Shepperd 2021, using the Hospital Anxiety and Depression Scale and the Montreal Cognitive Assessment (MoCA) questionnaire, respectively, reported little or no differences between groups.
Two trials used the Mini‐Mental State Examination (max score 30) to assess cognitive functioning at six months' follow‐up and reported little to no difference between groups (T: −0.4 (SD 4.0), C: −0.5 (SD 1.8); P = 0.88) (Ricauda 2004); and (T: 0.07 (SD 1.38), C: 0.08 (SD 1.36); P = 0.97) (Tibaldi 2009). One trial that recruited participants with a mix of conditions reported cognitive function scores: mean T: 0.43 (standard error of the mean (SEM) 0.12), C: 0.27 (SEM 0.12); and that fewer people receiving hospital at home care experienced short‐term confusion during an episode of care (MD −20.4%, 95% CI −32% to −9%) (Caplan 1999). One trial used the Confusion Assessment Method (CAM) to screen for delirium at baseline, three days, five days, and one month, and reported a difference at one month (T: 10/602 (1.7%), C: 13/297 (4.4%), RR 0.38 (0.19, 0.76); P = 0.006) (Shepperd 2021).
Satisfaction: patient, caregiver, and health professionals
Admission avoidance may increase patient satisfaction with the health care received. Participants allocated to hospital at home care reported higher levels of patient satisfaction across a range of different conditions (8 studies; 1812 participants; low‐certainty evidence). Twenty‐seven per cent (P < 0.001) more participants with cellulitis in the hospital at home group reported increased satisfaction with their location of care compared with those admitted to hospital (Corwin 2005), and 40% (P < 0.001) more participants with community‐acquired pneumonia allocated to hospital at home reported that they were happy with their care (Richards 2005). Two trials (recruiting mainly older participants with a mix of medical conditions) also reported increased levels of satisfaction for those allocated to hospital at home care (median difference of 3 on a 0‐to‐18‐point scale, P < 0.001 (Wilson 1999); and MD of 0.9 on a 4‐point scale, P < 0.001 (Caplan 1999)). However, in the latter trial, there was a low response rate for the control group: 40% compared with 78% in the hospital at home group (Caplan 1999).
Some participants (6/101; 6%) refused hospital at home care and were admitted to hospital, and a greater number of participants allocated to hospital care (23/97; 24%) were not admitted because of refusal by the participant, caregiver, or general practitioner (Wilson 1999). One trial recruiting participants with COPD reported the number of participants assessing satisfaction with care as very good or excellent (hospital at home 49/52 (94%), hospital 46/52 (88%); P = 0.83) (Analysis 1.9) (Ricauda 2008), and one trial reported that overall satisfaction scores favoured hospital at home (Shepperd 2021). Two trials (one pilot study, Levine 2018) found that patients in both groups had the same or similar median global satisfaction score, both indicating high satisfaction, though one point lower in the hospital group in Levine 2020.
1.9. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 9: Patient satisfaction
| Patient satisfaction | |||
| Study | Outcomes | Results | Notes |
| Caplan 1999 | Satisfaction rated on a 4 point scale: 1=excellent, 2=good, 3=fair, 4=poor. | Mean score T= 1.1, C= 2.0, P < 0.0001 | Response rates were 78% for the treatment group, and 40% for the control. |
| Corwin 2005 | Patient satisfaction questionnaire (not described) |
Overall
T= 87/91 (96%), C=87/96 (96%), P = 0.12
Satisfaction with location of care
T= 85/91 (93%), C= 59/88 (66%), P < 0.0001
Location preference
In the hospital
T= 5/91 (5%), C= 27/88 (31%)
In the community
T= 78/91 (86%), C= 31/88 (35%)
No preference
T= 8/91 (9%), C= 30/88 (34%) P < 0.0001 |
Numbers for control group vary between 88 and 91 due to missing data Proportion of participants satisfied or very satisfied |
| Levine 2018 | Global satisfaction score; | Median global satisfaction score (IQR) T = 10 (1) C = 10 (2) P=0.67 |
|
| Levine 2020 | Global satisfaction score; range of scores from 0 to 10, high scores equal high satisfaction | Median global satisfaction score (IQR) T = 10 (1) N=42 C = 9 (1) N=38 |
|
| Ricauda 2008 | Patient satisfaction questionnaire (not described) | T= 49/52 (94%), C= 46/52 (88%), P = 0.83 | Proportion of participants rating satisfaction as very good/excellent at discharge |
| Richards 2005 | Outcome not described | T= 24/24 (100%), C= 14/24 (60%), P = 0.001 | Proportion of patients very happy with care |
| Shepperd 2021 | Patient‐reported experience questionnaire at 1 month, developed by the Picker Institute Europe (Oxford, UK) | Patient satisfaction in favour of CGA HAH | |
| Wilson 1999 | Patient satisfaction, scale 0 to 18 | Median (IQR) T= 15 (13 to 16.5), C= 12 (11 to 14), P < 0.0001 | At 2 weeks, or discharge Reported in ⛔ Wilson 2002 |
One trial reported that caregivers in the hospital at home group had significantly higher levels of satisfaction compared with those in the hospital group (difference −0.8 on a 4‐point scale, P < 0.001) (Caplan 1999), although the response rate was 27% in the hospital group and 55% in the hospital at home group. A second trial assessed caregiver satisfaction through semi‐structured interviews; caregivers reported that although hospital would potentially relieve them from caring, the upheaval of visiting hospital and the accompanying anxiety was a less satisfactory option (Wilson 1999). One trial recruiting participants with COPD reported change in relatives' stress at six months (mean scores (SD) T: 4.6 (5.6), C: 2.6 (6.1); P = 0.16) (Analysis 1.10) (Ricauda 2008).
1.10. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 10: Caregiver satisfaction
| Caregiver satisfaction | |||
| Study | Outcomes | Results | Notes |
| Care giver satisfaction | |||
| Caplan 1999 | Carer satisfaction | Mean score T= 1.1, C= 1.9, P < 0.0001 | Satisfaction rated on a 4 point scale: 1=excellent, 2=good, 3=fair, 4=poor |
| Ricauda 2008 | Change in Relative’s Stress Scale Score |
At 6 months, mean (SD) T= 4.6 (5.6), C= 2.6 (6.1), P = 0.16 |
|
Health professionals' views
One trial evaluated general practitioners' satisfaction with the service (T: 1.17, C: 1.8, score of 1 to 4, high score = excellent, low score = poor); the response rate was poor: 63% in the hospital at home group and 37% in the control group (Analysis 1.11) (Caplan 1999).
1.11. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 11: Health professional satisfaction
| Health professional satisfaction | |||
| Study | Outcomes | Results | Notes |
| Caplan 1999 | GP satisfaction | Mean score (95% CI) T= 1.7 (1.4 to 2.0), C= 1.8 (1.4 to 2.2), Difference: NS | Higher scores indicate higher satisfaction Response rate: T: 63%, C: 37% |
Length of stay in hospital and hospital at home
Eleven trials reported the effect of admission avoidance hospital at home on length of hospital stay or hospital at home stay, or both, with differing results (Analysis 1.12). Four trials reported length of stay in hospital, for both the intervention and control groups (Davies 2000; Echevarria 2018; Shepperd 2021; Wilson 1999). Hospital length of stay ranged from an average of 4.1 days, Echevarria 2018, to 18.5 days, Wilson 1999, in the hospital group, and 1.2 days, Echevarria 2018, to 5.1 days, Wilson 1999, in the hospital at home group. Hospital at home length of stay ranged from an average of 3 to 20.7 days (hospital at home group only) (Harris 2005; Levine 2018; Levine 2020; Mendoza 2009; Ricauda 2008; Tibaldi 2009; Wilson 1999). One trial (Singh 2022 for Shepperd 2021) reported a reduction in hospital length of stay of just over a day at six months follow‐up for those allocated to hospital at home.
1.12. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 12: Length of stay
| Length of stay | |||
| Study | Results | Outcomes | Notes |
| Hospital and hospital at home length of stay | |||
| Davies 2000 | Hospital length of stay | Median (IQR) 5 days (4 to 7) N=100 Mean (SD) 6.72 days (4.3) N=100 |
Data for the control group only |
| Echevarria 2018 | Length of hospital stay at 90 days Length of hospital stay (index admission) Length of stay within HAH |
Mean length of hospital stay (index admission) T = 1.2 (2.1) C = 4.1 (4.6) Mean length of hospital stay at 90 days (SD) T = 6.1 (9.7) C = 10.3 (15.8) Median length of stay within HAH (IQR) T = 4 (2‐5) C = NA |
|
| Harris 2005 | Average length of stay for the index episode until discharge from hospital or hospital at home (days) |
T= 11.33 days (SD 11.14) N=39 C= 7.83 days (7.35) N=37 Mean difference 3.5 95% CI ‐0.80 to 7.80 |
IPD |
| Levine 2018 | Length of stay during acute care episode | Median length of stay during acute care episode (IQR) T = 3 (1) C = 3 (3) P=0.79 |
|
| Levine 2020 | Length of stay during acute care episode | Mean length of stay (95% CI) (days) T = 4.5 (3.9, 5.0) C = 3.8 (3.3, 4.4) |
|
| Mendoza 2009 | Average length of stay for the index episode (days) | T= 10.9 (SD 5.9) N=37 C= 7.9 (SD 3.0), P = 0.01 N=34 |
|
| Ricauda 2008 | Hospital at home and hospital length of stay (days) Total length of stay to include hospital transfers for the hospital at home group |
Total days of care (hospital plus hospital at home), mean (SD) T= 15.5 (SD 9.5) N=52 C= 52 (SD 7.9) Difference 4.50, 95% CI 1.14, 7.86 |
|
| Richards 2005 | Median number of days to discharge | T=4 (range 1‐14) N=24 C= 2 (range 0‐10) N=25 |
|
| Shepperd 2021 | Average length of hospital stay | Mean length of initial stay (SD) (complete cases) T = 1.43 (4.84) N=563 C = 4.92 (7.64) N=274 Mean length of hospital length of stay at six months follow‐up T=9.47 (18.41) N=563 C=10.58 (19.49) N=274 |
|
| Tibaldi 2009 | Time in the emergency department (hours) Length of stay (days) |
Time in ED, mean (SD) T= 14.6 (3.4), C= 16.3 (3.0) Length of treatment, mean (SD) T= 20.7 (6.9) N=48, C= 11.6 (10.7) N=53, P = 0.001 |
|
| Wilson 1999 | Length of stay | Treatment N=102 Control N=97 Length of hospital stay in days, median T= 5.1 (13.53), C= 18.5 (18.51) days, P = 0.026 Total days of care (hospital plus hospital at home), median T= 9, C= 16 days; P = 0.031 Total days of care (hospital plus hospital at home and readmission days), mean (SD) T= 13.33 (17.26), C= 21.42 (25.46) Difference ‐8.09 95% CI ‐14.34 to ‐1.85 |
|
For the total length of stay in the acute episode, admission avoidance hospital at home increased the length of stay or made no difference. The increase ranged from 0.7 days, in Levine 2020, to 9.1 days, in Tibaldi 2009. In one study there was no difference in length of stay between treatment groups (Levine 2018).
Cost and resource use
Three trials reported a full evaluation of healthcare resources and costs (Echevarria 2018; Shepperd 2021; Wilson 1999); one of these trials included informal‐care costs (Shepperd 2021). Four trials reported the difference in mean cost per initial acute health episode (Caplan 1999; Nicholson 2001; Shepperd 2021; Wilson 1999); three trials reported the mean cost per patient (Ricauda 2004; Ricauda 2008; Richards 2005); and two trials reported the percentage reduction in median cost of episode and subsequent 30 days (Levine 2018; Levine 2020) (Analysis 1.13).
1.13. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 13: Cost and resource use
| Cost and resource use | |||
| Study | Outcomes | Results | Notes |
| Health service resources and costs | |||
| Caplan 1999 | Cost | Average cost per episode, mean (SD)
T= $1,764 ($1,253), C= $3,775 ($2,496)
Mean difference per episode $‐2011
Cost per day, mean (SD)
T= $191 ($58), C= $484 ($67.23) Mean difference per day ‐$293 |
Cost data financial year 1995/1996 |
| Corwin 2005 | Days on oral antibiotics | HR 1.09 (0.82 to 1.45), P = 0.56 | |
| Echevarria 2018 | Health and formal social care costs | Mean health and formal social care costs (SD) T = 3857.8 (3199.6) C = 4873.5 (5631.1) Bootstrapped mean difference (95% CI) ‐1015.7 (‐2735.5, 644.8) |
|
| Levine 2018 | Relative cost reduction of acute care episode Relative cost reduction of acute care episode and 30 days after acute care episode |
Relative cost reduction of acute care episode, % 52% (IQR, 28%; p = 0.05) Relative cost reduction of acute care episode and 30 days after, % 67% (IQR, 77%; p<0.01) |
% lower than for control patients Change in median cost |
| Levine 2020 | Relative cost reduction of acute care episode (including physician cost, Appendix Table 7 (Levine 2020) Relative cost reduction of acute care episode and 30 days after acute care episode |
With physician labour Adjusted relative reduction in cost of acute care episode, % (95% CI) 19 (4, 31) P=0.017 Adjusted relative reduction in cost of acute care episode and 30 days after, % (95% CI) 25 (10,38) P<0.001 |
Positive means home group costs less Relative reduction in mean cost (%) |
| Mendoza 2009 | Cost | Mean (SD) T= €2,541 (1,334), C= €4,502 (2,153) Difference €1,961 P < 0.0001 |
Difference attributed to fewer investigations. Costs include health service costs used during follow‐up period of 1 year, excludes informal care. |
| Nicholson 2001 | Costs | Cost per episode, mean (95% C) T= $745 ($595 to $895),C= $2543 ($1766 to $3321) Difference $1798, P < 0.01 Hospital at home costs 29% of the average hospital managed patient episode. Reported cost effectiveness ratio of 3:1 T + C costs GP 10% of costs, Domiciliary allied health 21% of costs, community nursing 28% of costs = 59% of costs and hospital care 41% of costs. If C=$895 then T= $1287 (59% of costs) Total costs=$2182 per patient episode of care |
Costs based on financial year 99/00; Used average DRG costs (Australian $), patient data for ED costs, and modelled costs for OPD clinic visits. HAH care individual costs, included direct and non direct costs. GP costs at $91.00 per hour. |
| Ricauda 2004 | Mean total cost (EUR converted to US$ 1 Euro=$1.3) | T= $6 413.5 per patient, C= $6 504.8 per patient Cost per patient per day (SD) T= $163 (20.5), C= $275.6 (27.7) P < 0.001 | |
| Ricauda 2008 | Hospital at home resources Total costs |
Nursing visits (range) T= 14.1 (3 to 38) Physician visits T= 9.9 (2 to 28) Visits to hospital for diagnosis T= 11 Total mean cost per patient T=$1,175.9, C= $1,390.9, P = 0.38 Total mean cost per day (SD) T= $101.4 (61.3), C= 151.7 (96.4) |
|
| Richards 2005 | Cost based on DRGs for control and actual cost for intervention | Mean cost per patient NZ$ T= $1157.9, C= $1556.28 | |
| Shepperd 2021 | Health and social care costs | Mean cost of initial admission (SD) T = 1742 (3234) C = 3723 (5095) Mean difference: ‐1981 (‐2551, ‐1411) Mean Health and Social care cost (adjusted), baseline to 6 months follow up T = 15,124 C = 17,390 Mean difference: ‐2265 (‐4279, ‐252) Mean Societal costs (adjusted), baseline to 6 month follow up T = 19,067 C = 21,907 Mean difference: ‐2,840 (‐5,495, ‐185) |
|
| Wilson 1999 | Cost | Cost of initial episode (95% CI) T= £2,568.9 (2,089.3 to 2,972.1) C= £2,880.6 (2,316.1 to 3,547.8) Difference ‐311.7, P > 0.43 Bootstrap difference using 1000 subsamples: ‐304.72 (‐1,112.4 to 447.9). Mean cost per day (95% CI) T= £204.6 (91.5 to 118.4) C= £104.9 £ (181.1 to 228.22) Mean difference £99.71 P < 0.001 Cost at 3 months (95% CI) T= £3,671.3 (3,140.5 to 4,231.3) C= £3,876.9 (3,224.51 to 4,559.6) Difference ‐205.7, P > 0.65 Bootstrap difference using 1000 subsamples: ‐210.9 (‐1,025 to 635.5) COSTS EXCLUDING REFUSERS Cost of initial episode, mean (95% CI) T= £2,594.4 (£2,170.36 to £3,143.5) C= £3,659.20 (£3,140.46 to £4,231.28) Mean difference ‐£1,064.79, P < 0.01. Bootstrap mean difference £1070.53, (95% CI‐£1843.2 to ‐£245.73) 95% CI derived using bootstrap method with 1000 subsamples Cost per day, mean (95% CI) T= £206.68 (£183.21 to £230.14) C= £133.7 (£124.6 to £142.8) Mean difference £72.98, P < 0.001 Cost at 3 months, mean (95% CI) T= £3,697.5 (£3136.13 to £4330.66) C= £4,761.3 (£4105.6 to £5476.6) Mean difference ‐£1,063.8, p = 0.025 Bootstrap mean difference: £1,063.45 (95% CI ‐£2043.8 to ‐£162.7) | Cost data financial year 1995/1996 BNF for medicines 1995 |
| Use of other social services | |||
| Davies 2000 | While receiving hospital at home care, or on discharge from hospital | Referred for increased social support T= 24/100 (24%), C= 3/50 (6%) Difference 18%, 95% CI 7.3% to 28.6% |
|
| Echevarria 2018 | Patients with a social care package post discharge | Patients with a social care package post discharge (%) T = 7 (11.7) C = 5 (8.6) |
|
| Informal care inputs | |||
| Kalra 2000 | Informal care inputs | Received informal care: T= 100/140 (71%), C= 98/147 (67%), Difference 4.8%, 95% CI ‐5.9% to 15.3% Total from co residents over 12 months, hours (SD) T= 899.18 (1760), C= 718 (6778), P = 0.75 Total hours per average week from co residents (SD) T=46.38 (48.15), C= 33.71 (44.35), P = 0.02 Total hours from nonresidents over 12 months (SD) T= 79.7 (283), C= 127.44 (348), P = 0.27 Total average hours per week from non residents T= 4.79 (16.51), C= 5.03 (11.54), P = 0.88 Total hours over 12 months (SD) T= 979 (1749), C= 846 (1549), P = 0.49 | |
| Shepperd 2021 | Informal care | Mean total number of hours of unpaid help over the last 6 months (SD) T = 594.89 (1093.63) C = 657.64 (1170.87) Difference in means (95% CI): ‐62.76 (‐224.61, 99.09) |
|
Admission avoidance hospital at home probably decreases healthcare costs (2148 participants, moderate‐certainty evidence) (Caplan 1999; Corwin 2005; Echevarria 2018; Levine 2018; Levine 2020; Mendoza 2009; Nicholson 2001; Ricauda 2004; Ricauda 2008; Richards 2005; Shepperd 2021; Wilson 1999), though by a range of different amounts, and there is some evidence that it decreases overall societal costs to six months' follow‐up (Shepperd 2021).
Older participants with a medical condition
One trial reported a cost minimisation analysis (Wilson 1999), finding an initial increase in the mean cost per day for hospital at home (difference GBP 99.71, P < 0.001) for all those randomised, and little or no difference in cost at three months' follow‐up (GBP −210.9, 95% CI GBP −1025 to GBP 635.47). When participants refusing their allocated place of care (T: 6/101, C: 23/97) were removed from the analysis, there was a reduction in costs for those receiving hospital at home for the initial episode of care (difference GBP −1070.53, 95% CI GBP −1843.2 to GBP −245.73) and at three months' follow‐up (difference GBP −1063.45, 95% CI GBP −2043 to GBP −162.7). The difference in mean cost per day between hospital at home and hospital care was reduced, although hospital at home care remained more costly per day (GBP 206.68 versus GBP 133.7, MD GBP 72.98, P < 0.001).
Another trial, recruiting mainly older participants with a mix of conditions, examined health service costs using average costs (Board 2000 secondary publication to Caplan 1999), and reported reduced health service costs for the intervention group (T: AUD 1764 (SD AUD 1253), C: AUD 3775 (SD AUD 2496) for an episode of care, MD per episode AUD −2011) and cost per day (T: AUD 191 (SD AUD 58), C: AUD 484 (SD AUD 67.23); MD AUD 293). The costs of the nurse co‐ordinator and hospital doctor involved were excluded from this analysis (see Analysis 1.13.1). Mendoza 2009 reported the mean (SD) cost at one‐year follow‐up (T: EUR 2541 (1334), C: EUR 4502 (2153); difference EUR 1961, P < 0.001).
Singh 2022 for Shepperd 2021 compared total health and social care costs, and total societal costs (includes the productivity loss of informal carers costed by the hour). They reported reduced health and social care costs for the intervention group from baseline to six months' follow‐up (T: GBP 15,124, C: GBP 17,390; difference GBP −2265, 95% CI GBP −4279 to GBP −252), adjusting for gender, cognitive decline, utilities, pre‐randomisation costs, and site. Total adjusted societal costs were also reduced for the intervention group at six months' follow‐up (T: GBP 19,067, C: GBP 21,907; difference GBP −2840, 95% CI GBP −5495 to GBP −185).
Participants recovering from a stroke
A trial recruiting participants recovering from a stroke compared stroke unit care, inpatient stroke team care, and hospital at home. Regarding immediate care, hospital at home care was less costly than inpatient stroke team care (MD GBP −2096, 95% CI GBP −3272 to GBP −920). The inclusion of costs of informal care, based on the minimum wage, resulted in an MD of GBP −2216 (95% CI GBP −4771 to GBP 339) (Analysis 1.13) (Patel 2004 for Kalra 2000). In another trial recruiting participants with a stroke, a small reduction in mean cost per patient was reported for those allocated to hospital at home (USD 6413.5 versus USD 6504.8) (Ricauda 2004), which translated to a lower cost per day for hospital at home of USD 112.00 (USD 163.0, SD 20.5 versus USD 275.6, SD 27.7; P < 0.001).
COPD and community‐acquired pneumonia
One trial recruiting participants with COPD reported a lower mean health service cost for participants allocated to hospital at home; hospital costs were based on an average DRG (a diagnostic‐related group categorised by resource use) cost per bed day (cost per episode MD GBP −1798, P < 0.01) (Nicholson 2001). Another trial recruiting participants with community‐acquired pneumonia, again using DRG costs for the control and actual resource use for costing the intervention, reported a reduced cost for those allocated to hospital at home (mean cost per patient T: NZD 1157.9, C: NZD 1556.28) (Richards 2005). Ricauda 2008 reported the total mean cost per patient (T: USD 1175.9, C: USD 1390.9, P = 0.38) and the total mean cost per day (T: USD 101.4 (SD 61.3), C: USD 151.7 (SD 96.4)).
Use of other health services and informal care
Davies 2000 reported an increase in referrals for social support for participants with COPD who were allocated to hospital at home. This occurred during the time they were receiving hospital at home or when the control group had been discharged from hospital (24% versus 6%, difference 18%, 95% CI 7.3% to 28.6%) (Analysis 1.13). One trial recruiting participants recovering from a stroke reported that 71% (100/140) of those allocated to hospital at home received informal care, compared with 67% (98/147) receiving care from the inpatient stroke team (Patel 2004). This translated into 979 hours (SD 1749) versus 846 hours (SD 1549) of care over a 12‐month period (Analysis 1.13). Singh 2022 for Shepperd 2021 found no significant difference in the total hours of informal care between the treatment groups up to six months' follow‐up (mean (SD) T: 594.89 (1093.63), C: 657.64 (1170.87); difference −62.76, 95% CI −224.61 to 99.09).
Clinical outcomes
One trial measured clinical complications, with fewer participants allocated to hospital at home reporting bowel complications (difference −22.5%, 95% CI −34% to −10.8%) or urinary complications (difference −14.4%, 95% CI −25.4% to −3.3%) (Caplan 1999). In a trial recruiting participants with dementia, fewer participants in the hospital at home group were prescribed antipsychotic drugs at discharge (difference −14%, 95% CI −28% to 0.3%) (Tibaldi 2004). One trial that recruited people with cellulitis reported risk of advancement of cellulitis (hazard ratio 0.98, 95% CI 0.73 to 1.32) (Corwin 2005), and one trial recruiting participants with COPD reported that more participants allocated to hospital at home were prescribed an antibiotic (difference 18%, 95% CI 1.4% to 34.6%) (Davies 2000). Talcott 2011 reported the difference in major complications during the episode of care (difference 1%, 95% CI −10% to 13%) (Analysis 1.14).
1.14. Analysis.
Comparison 1: Admission avoidance hospital at home versus inpatient care, Outcome 14: Clinical outcomes
| Clinical outcomes | ||
| Study | Outcomes | Results |
| Clinical outcomes | ||
| Corwin 2005 | No advancement of cellulitis (indelible line drawn around peripheral margin of the cellulitis and dated) |
Mean (SD) days T= 1.5 (0.11), C= 1.49 (0.10), Mean difference 0.01 days, 95% CI ‐0.3 to 0.28 Days of no advancement of cellulites HR 0.98, 95% CI 0.73 to 1.32, P = 0.90 Days on intravenous antibiotics HR 0.84, 95% CI 0.63 to 1.12, P = 0.23 Days to discharge HR 0.93, 95% CI 0.70 to 1.23, P = 0.60 Days on oral antibiotics HR 1.09, 95% CI 0.82 to 1.45, P = 0.56 |
| Davies 2000 | Proportion of patients prescribed an antibiotic at 3 months | T= 56/100 (56%), C= 19/50 (38%), Difference 18%, 95% CI 1.4 to 34.6% |
| Echevarria 2018 | COPD Assessment Tool (CAT) at 14 and 90 days | CAT, 14‐day (IQR) T = ‐4.0 (‐9.5, 0) C = ‐3.0 (‐7, 1) CAT, 90 day (IQR) T = ‐3.0 (‐8, 1) C = ‐1.0 (‐6, 1) |
| Levine 2020 | Any safety event Median pain score Inappropriate medication use Urinary catheter use Restraint use |
Any safety event (%) T = 4 (9) C = 7 (15) Median pain score (IQR) T = 0 (1) C = 0 (3) Inappropriate medication use T = 0 (0) C = 5 (10) Urinary catheter use T = 0 (0) C = 2 (4) Restraint use T = 0 (0) C = 0 (0) |
| Shepperd 2021 | Charlson Comorbidity Index score | Mean at 6 months (SD) T = 6.17 (1.94) C = 6.00 (1.93) Adjusted mean difference (95% CI): 0.0002 (‐0.1452 to 0.1455) |
| Talcott 2011 | Major medical complications during care in hospital at home or hospital | T= 4/47 (9%), C= 5/66 (8%), Difference 1%, 95% CI ‐10 to13% |
| Tibaldi 2004 | Use of antipsychotic drugs | On admission T= 26/56 (46.4%), C= 18/56 (32%), Difference 14.3%, 95% CI ‐3.7% to 31.1% On discharge T= 6/56 (11%), C = 13/53 (25%), Difference 14%, 95% CI ‐28% to 0.3% |
Discussion
Summary of main results
Admission avoidance hospital at home probably makes little or no difference to risk of death at six months' follow‐up and to readmission to hospital after discharge from hospital at home or inpatient care within 3 to 12 months (range of follow‐up times from the included studies), and probably reduces the likelihood of relocating from home to residential care at six months. Admission avoidance hospital at home probably results in little to no difference in patient‐reported health status, and the evidence suggests that it may increase patient satisfaction and reduce hospital length of stay; that total length of stay for hospital at home may be greater than for those allocated to hospital; and that hospital at home can be less costly than in‐hospital care (Table 1).
Patients valued the quality of communication and personal care received in a hospital at home setting (Shepperd 2021; Wilson 1999). However, the increased satisfaction reported by patients must be balanced against the potential burden on caregivers; for example, interviews with caregivers showed that their contributions might be required to facilitate an episode of hospital at home (Makela 2020), and that this can place an additional burden on families. Comparing cost data from the different studies was limited by the different methods used to cost resources, and follow‐up times that ranged from 1 to 12 months. Three trials conducted a full economic evaluation (Jones 1999 for Wilson 1999; Singh 2022 for Shepperd 2021; and Patel 2004 for Kalra 2000), reporting that hospital at home may lead to a small reduction in health service cost, a reduction in societal costs, and can be a cost‐effective alternative to hospital admission for select groups of patients.
There was some variation in the way the admission avoidance hospital at home services operated that reflected different health systems, existing services available, and financing. Services admitted patients directly from the community (Harris 2005; Kalra 2000; Vianello 2013; Wilson 1999), outpatients (Talcott 2011), and from an accident and emergency department or medical assessment unit (Andrei 2011; Caplan 1999; Corwin 2005; Davies 2000; Echevarria 2018; Levine 2018; Levine 2020; Mendoza 2009; Nicholson 2001; Ricauda 2004; Ricauda 2008; Richards 2005; Shepperd 2021; Tibaldi 2004; Tibaldi 2009). Six trials evaluated interventions where the patient could be living alone, and five trials required a caregiver to be either living with the patient or nearby. This variation reflects the nature of complex interventions (i.e. similar to stroke units, case management, telemedicine), and we did not combine data from studies with a high level of clinical heterogeneity. There were some important common features across the different hospital at home services; these included access to a doctor, co‐ordinated care provided by a multidisciplinary team, the provision of 24‐hour cover if required, and a safe home environment being a requirement for the provision of hospital at home. Inevitably, hospital at home does not function as an isolated intervention, with the organisation of services reflecting local health systems, workforce, and available social care services. For some countries, integrating hospital at home into existing services is the most efficient way to deliver these services.
Overall completeness and applicability of evidence
The evidence indicates that admission avoidance hospital at home may provide an effective alternative to inpatient care for a select group of patients requiring hospital admission. The majority of the included trials recruited participants who were older, with an average age that ranged from 70 to over 80 years, and who had experienced a medical event that required admission to hospital. All trials but one, Andrei 2011, were conducted in high‐income countries. Eight trials excluded patients who did not have continuous family support (Caplan 1999; Mendoza 2009; Ricauda 2004; Ricauda 2008; Richards 2005; Tibaldi 2004; Tibaldi 2009; Vianello 2013). Four trials reported the number of participants recruited from ethnic minority groups; this ranged from 14% to 20% of all recruited participants (Corwin 2005; Levine 2020; Richards 2005; Talcott 2011). In one trial, hospital at home included remote monitoring for heart rate, respiratory rate, telemetry, movement, falls, and sleep via a small skin patch; cost was the primary outcome (Levine 2020), and the study was stopped early by the funder (who was also the service provider) due to the cost benefit of the intervention.
The 20 trials included in this review were conducted in Australia, Italy, New Zealand, Romania, Spain, the UK, and the USA. There is growing interest from other health systems in testing the delivery of hospital‐level care in the home. For example, in Singapore a large study that seeks to recruit 2000 participants is under way (Chong 2022; NCT04330378), and a large hospital in Pune, India expects to increase its capacity by 40% to 60% using remote patient monitoring (Mernin 2021). A large hospital in Bangkok, Thailand has also set up an @Home service, suitable for patients after surgery or acute illness, patients with chronic conditions who need regular follow‐up, and the dependent elderly (Bumrungrad 2023). Such services were also set up in the Philippines during COVID‐19 (CNN Philippines 2021). Additional large, high‐quality randomised trials will improve the certainty of the evidence and generalisability of these findings, and be a valuable guide to the development of out‐of‐hospital services to increase the capacity of health systems.
We defined admission avoidance hospital at home as: a service that can avoid the need for hospital admission by providing active treatment by healthcare professionals in the patient's home for a condition that would otherwise require acute hospital inpatient care, and always for a limited time period. All of the included studies met these criteria, and the evidence applies to services that also meet these criteria and do not provide long‐term care, outpatient care, or self‐administration of treatment. The studies varied in the amount of time that patients were in the emergency department before being admitted to hospital at home or a hospital bed; this was due to the type of illness and severity (and was not often analysed) and the ongoing demands on the health system in question. We did not apply a time threshold in the emergency room for the study to qualify as admission avoidance. If we had, it would likely have resulted in the exclusion of patients in the most pressured health systems, where evidence is arguably most needed, and have risked imposing an artificial time limit that isn't experienced on the ground, and thus limited the applicability of the evidence.
Hospital at home services inevitably place demands on those who live with the person receiving care, and family and friends who live elsewhere. Safety in the home, cultural considerations, and health problems experienced by the main carer pose further challenges (Simon 2022). An additional concern is inequality in the resources available to unpaid carers across different populations, which might increase the potential burden of receiving hospital at home (Vlachantoni 2012). A Cochrane qualitative evidence synthesis of factors that influence the implementation of hospital at home reports in detail on the barriers that limit implementation, as well as factors that support the implementation of sustainable hospital at home services (Wallis 2024). A limitation of the trials included in this review is that few reported if deaths occurred during the hospital at home admission, and if they were unexpected and related to the hospital at home intervention. The reason for this is likely the small sample size of many of the studies, and early death being a rare event. Mortality occurring during an episode of hospital at home or hospital (control group) admission might not be related to hospital at home or hospital care, and the cause would have to be assessed case by case. The cause of such adverse events was not always clearly reported in the included studies; further investigation would provide clarity on the causes of death and any links with the intervention.
Certainty of the evidence
While we assessed the overall risk of bias as low, most of the included studies were small. Shepperd 2021 was the largest trial, recruiting 1032 participants who contributed to the analysis. The meta‐analysis for the main outcome included a subgroup of five trials recruiting participants with similar conditions (older patients with a mix of medical conditions, excluding stroke) to limit heterogeneity. We downgraded the certainty of the evidence for mortality due to imprecision of the estimate: the result could be consistent with hospital at home being associated with both higher or lower risk of mortality. We downgraded for readmission to hospital and relocating to residential care, because the studies evaluated these outcomes at different lengths of follow‐up, therefore the comparison was indirect. We downgraded for patient satisfaction because only 35% of the studies evaluated this outcome, and patients/researchers were not blinded. We downgraded for patient self‐reported health status due to non‐blinding, and for length of stay because the time frame for measuring this outcome differed among studies, and the estimates covered a wide range. We downgraded for cost because only three trials did a full cost analysis (Kalra 2000; Shepperd 2021; Wilson 1999).
Potential biases in the review process
We limited publication bias by conducting an extensive search that included different databases of published articles and sources of unpublished literature; this was facilitated by a long‐established international network of people working in the field who alert us to new randomised controlled trials. Three review authors screened the search results of potentially eligible studies to reduce the risk of missing any eligible studies. To check that inclusion criteria had been applied consistently, eligibility of studies was discussed with the review team. As an author of one of the included studies (Shepperd 2021), review author SS was not involved in assessing their own study for inclusion, risk of bias assessment, or data extraction.
Agreements and disagreements with other studies or reviews
Other reviews have looked at the effect of hospital at home schemes for end‐of‐life care (Shepperd 2021), and hospital at home for early discharge (Gonçalves‐Bradley 2017). A review of a few studies of end‐of‐life care at home found that such programmes increase the chance of dying at home rather than in hospital, and patient satisfaction may be higher at one‐month follow‐up (Shepperd 2021). A review of early discharge hospital at home services found insufficient evidence for economic benefit, or improved health outcomes (Gonçalves‐Bradley 2017). The search for this review was updated in March 2020 and identified no new studies.
Systematic reviews of hospital at home services for patients with specific conditions, such as COPD and heart failure, have been published (Jeppesen 2012; Qaddoura 2015). For patients with COPD, it was reported that hospital at home reduced the number of readmissions when compared with hospital care, with uncertain evidence for mortality, health‐related quality of life, cost, and clinical outcomes (Jeppesen 2012). A review of a few studies that specifically recruited patients with heart failure found a slight increase in time to readmission, improved health‐related quality of life, and reduced index costs, with limited evidence for mortality for those allocated to hospital at home. The authors judged these studies to be of modest quality (Qaddoura 2015). A systematic review of studies of hospital at home for chronic disease identified nine studies: five trials of COPD, two of heart failure, one of stroke, and one of neuromuscular disease (Arsenault‐Lapierre 2021). We included all studies included in the Arsenault‐Lapierre 2021 review in the current review update except for Hernandez 2003, which did not compare admission avoidance hospital at home with inpatient care. In Hernandez 2003, patients who required immediate hospitalisation were excluded, and the control group was evaluated by a physician who decided on inpatient admission or discharge. The review found no significant difference in mortality for a range of follow‐up times, and a lower risk of readmission in the hospital at home group, using the longest follow‐up time for each study. In addition, similar to the current review, Arsenault‐Lapierre 2021 reported that length of treatment was longer in the hospital at home group, and relocation to residential care was less likely in the hospital at home group than in the in‐hospital group; study authors did not analyse mortality occurring during the hospital or hospital at home admission.
Authors' conclusions
Implications for practice.
Admission avoidance hospital at home, with the option of transfer to hospital, may provide an effective alternative to inpatient care for selected patients who require hospital admission. The 20 trials included in this review were conducted in several different countries. Although the health systems in these countries vary with respect to the way healthcare financing is structured, the policy objectives are the same, with admission avoidance hospital at home being provided to control costs and reduce demand for inpatient hospital beds (Aviv 2021; Naik 2006; Oliver 2021). The level of primary care in a country, and the enthusiasm of local clinicians and healthcare managers, may determine the degree to which admission avoidance hospital at home operates as an outreach model or is run by supplementing existing primary care services. Other aspects that might vary by health system include the level of integration with existing services to avoid duplication of services, for example using existing out‐of‐hours services as in a single‐payer system with free access at the point of care versus setting up a new out‐of‐hours service for patients that do not have access to this through their usual healthcare coverage (such as in a multipayer health system). Admission avoidance hospital at home may not completely substitute for hospital, as hospital admission remains an option if required, for example patients whose condition unexpectedly deteriorated or who could no longer be managed at home had access to transfer to a traditional acute hospital ward. Policymakers should consider what type of hospital care is planned to be substituted with hospital at home, and the impact this will have on cost‐effectiveness.
The way health care for the control group is organised will have an impact on cost‐effectiveness, for example the routine use of comprehensive geriatric assessment to structure hospital care will provide an additional layer of geriatrician‐led multidisciplinary assessment and co‐ordination of care that can improve outcomes (Ellis 2017). The entry criteria required that patients be clinically stable and not require specialist diagnostic investigation or emergency interventions. Patients eligible for the trials included in this review did not include those whose condition was so severe that death was an expected outcome. Furthermore, patients whose condition deteriorated or who could no longer be managed at home had access to hospital admission.
Although admission avoidance hospital at home provides an alternative to inpatient admission for some patients, the volume of such patients recruited to the included trials was comparatively low, and some of these patients required access to hospital services, which might make the closure of a ward or hospital in favour of hospital at home an unrealistic option. In Shepperd 2021, 37/687 (5%) participants allocated to hospital at home received required admission to hospital bed‐based care, and 76/345 (22%) participants allocated to hospital instead received hospital at home care. This indicates that some patients require a greater intensity of care due to their condition deteriorating, that others have a preference for health care in their own home, and at times when no hospital beds were available for those randomised to hospital admission, hospital at home admission was required. Being less likely to be relocated to residential care at follow‐up might be due to the location of the patient influencing the decision to move to residential care, or be related to the patient's maintaining their usual routines at home while avoiding some of the harms associated with the inpatient environment such as sleep deprivation, poor nutrition, confusion, and falls and infection risk (Mudge 2019). A reduction in relocating to residential care may contribute to cost savings from hospital at home.
Many of the studies in this review are over 10 years old and do not reflect the recent improvement of remote patient monitoring technology. These developments are advancing rapidly and may allow hospital at home to care for different groups of patients. However, there have been concerns about excluding patients based on their or their caregiver's low technological literacy (British Geriatrics Society 2022).
Implications for research.
Over the last 20 years the randomised evidence has grown from 1 to 20 trials, using a pragmatic randomised trial design that includes a process evaluation to inform real‐world implementation.
Future research of admission avoidance hospital at home should assess the impact of hospital at home services in more disadvantaged populations, who are more likely to have a higher percentage of informal caregivers (Young 2005). Mortality should be measured, in particular during admission to hospital at home and hospital, along with transfer to hospital during a hospital at home admission, readmission to hospital after discharge from hospital at home or inpatient care, relocation to residential care at follow‐up, and cost‐effectiveness. Studies should clarify if mortality during the acute episode was assessed as related to the hospital at home intervention or hospital control and unexpected.
There is little evidence on the impact of hospital at home on patients, caregivers and their networks, despite the potential for them to have a significant role when health care is being delivered in the patient's home. A qualitative study linked to the randomised trial by Shepperd 2021 (Makela 2020) describes how patients and caregivers facilitate the delivery of hospital at home care, and this includes monitoring the patient. A recent study found that caregiver burden should be a key aspect to assessing appropriate social support during hospital at home (Levine 2021). Additional evidence on how staff experience hospital at home, the training required, and how roles evolve would help with workforce planning (e.g. Karacaoglu 2021; Leary 2022), and if hospital at home services represent a shift in care provision to patients' families and lower skilled workers (Batt 2023). A recent survey identified several domains for future research, including defining the type of hospital at home care, defining optimal study outcomes, patient and caregiver experience, the education of hospital at home clinicians, and the use of technology and telehealth, among others (Leff 2022). Other important outcomes for future research should include the assessment of unintended impacts, person‐centred care, advanced care planning, and the impact on unpaid carers and their networks. As hospital at home services are more widely implemented, evidence on the impact on caregiver outcomes that includes burden, experience, satisfaction, and quality of life becomes increasingly important.
The potential for admission avoidance hospital at home to increase the capacity of health systems was explored during the COVID‐19 pandemic (Aviv 2021; Nundy 2020; Oliver 2021). Further study has been done regarding 'virtual wards' and remote monitoring in this context (Thornton 2020), with a review of 27 studies finding that patient/carer training was a determining factor of success, but there was uncertainty of evidence around patient safety or deterioration (Vindrola‐Padros 2021). This is another area that requires more research, as there is a risk that remote monitoring might not be used effectively.
What's new
| Date | Event | Description |
|---|---|---|
| 24 February 2022 | New citation required but conclusions have not changed | We identified four new trials in this update (Echevarria 2018; Levine 2018; Levine 2020; Shepperd 2021). |
| 24 February 2022 | New search has been performed | New searches performed, four new trials added to the review. The review now includes 20 trials. In this update we removed data reported by Kalra 2000 and Ricauda 2004 from the meta‐analysis, as evidence recommends that people with a suspected stroke be directly admitted to a specialist acute stroke unit after initial assessment (Langhorne 2020; Langhorne 2021). An acute stroke unit is a discrete area in the hospital that is staffed by a specialist stroke multidisciplinary team, with access to equipment for monitoring and rehabilitating patients (NICE 2019). |
History
Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 6 July 2011 | Amended | Reference revised to published review. |
| 8 June 2011 | Amended | Title changed for consistency and changes to published notes. |
| 17 February 2010 | Amended | Change to published notes. |
| 1 August 2008 | New search has been performed | This review is an updated search and partial update from the original review (Shepperd 1998). Shepperd 1998 has been split into three reviews, of which this is one. |
| 10 July 2008 | Amended | Converted to new review format. |
Notes
This review is the fourth update; the original review was first published in Issue 1, 1998 of the Cochrane Library (Shepperd 1998). The original review has been separated into three distinct reviews: Hospital at home admission avoidance, Hospital at home early discharge, and Hospital at home: home‐based end‐of‐life care. The titles have been changed for consistency. Hospital at home early discharge, Gonçalves‐Bradley 2017, and Hospital at home: home‐based end‐of‐life care, Shepperd 2016b, are published in the Cochrane Library.
Acknowledgements
We thank peer reviewers for their comments on previous versions (updates and the first version) of this review, and acknowledge the following individuals for providing individual patient data for the 2008 version: Dr Roger Harris and Joanne Broad, Dr Lalit Kalra, Dr Aimonino Ricauda, and Professor Andy Wilson. Robert M Angus for collaborating on previous versions of the review, and Nia Roberts and Paul Miller for updating and conducting the searches.
This review was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding and a Cochrane programme grant.
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Rebecca Fortescue (née Normansell), Population Health Research Institute, St George’s, University of London;
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Luisa M Fernandez Mauleffinch, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments, and supported the editorial team): Leticia Rodrigues, Cochrane Central Editorial Service;
Copy Editor (copy editing and production): Lisa Winer, Cochrane Central Production Service;
Peer reviewers (provided comments and recommended an editorial decision): Jennifer Hilgart, Cochrane (methods); Steve McDonald, Cochrane Australia (search); Jonathan M Fuchs, FACHE Kidder Street Consulting Group (consumer); Bruce Leff, MD, Johns Hopkins University School of Medicine (clinical); Jorge Arias de la Torre, King's College London (clinical); Yi Feng Lai, MOH Office for Healthcare Transformation, Singapore (clinical); Maria Cristina Martin (clinical); and Víctor J González‐Ramallo, Hospital at Home Service, Hospital General Universitario Gregorio Marañón, Madrid, Spain (clinical). One additional peer reviewer provided clinical peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
Medline, OVID (MEDALL)
Search date: 24 February 2022
| No. | Search terms | Results |
| 1 | home care services, hospital‐based/ | 1882 |
| 2 | home care services/ and (hospital* or unit? or ward? or institution*).ti,ab,kf. | 7847 |
| 3 | home health nursing/ | 329 |
| 4 | (hospital* adj2 home).ti,ab,kf. | 5192 |
| 5 | virtual ward?.ti,ab,kf. | 36 |
| 6 | ((early or earlier or supported or assisted) adj2 discharge?).ti,ab,kf. | 5380 |
| 7 | ((hospice* or terminal or end of life or palliative) adj3 home).ti,ab,kf. | 3217 |
| 8 | or/1‐7 | 21053 |
| 9 | exp randomized controlled trial/ | 503167 |
| 10 | controlled clinical trial.pt. | 93583 |
| 11 | randomi#ed.ti,ab. | 611437 |
| 12 | placebo.ab. | 206278 |
| 13 | randomly.ti,ab. | 330738 |
| 14 | Clinical Trials as topic.sh. | 190496 |
| 15 | trial.ti. | 215710 |
| 16 | or/9‐15 | 1321613 |
| 17 | exp animals/ not humans/ | 4681451 |
| 18 | 16 not 17 | 1218072 |
| 19 | 8 and 18 | 2721 |
| 20 | (2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021* or 2022*).dt,dp,ed,ep,yr. | 7140476 |
| 21 | 19 and 20 | 873 |
Embase, OVID (1974 ‐ )
Search date: 24 February 2022
| No. | Search terms | Results |
| 1 | exp *home care/ | 35966 |
| 2 | (hospital* or unit? or ward? or institution*).ti,ab,kw. | 2899467 |
| 3 | 1 and 2 | 8758 |
| 4 | (hospital* adj2 home).ti,ab,kw. | 7434 |
| 5 | virtual ward?.ti,ab,kw. | 65 |
| 6 | ((early or earlier or supported or assisted) adj2 discharge?).ti,ab,kw. | 8645 |
| 7 | ((hospice* or terminal or end of life or palliative) adj3 home).ti,ab,kw. | 4892 |
| 8 | or/3‐7 | 26968 |
| 9 | random*.ti,ab. | 1515492 |
| 10 | factorial*.ti,ab. | 37539 |
| 11 | (crossover* or cross over*).ti,ab. | 105414 |
| 12 | ((doubl* or singl*) adj blind*).ti,ab. | 230030 |
| 13 | (assign* or allocat* or volunteer* or placebo*).ti,ab. | 1023714 |
| 14 | crossover procedure/ | 62570 |
| 15 | single blind procedure/ | 38333 |
| 16 | randomized controlled trial/ | 596110 |
| 17 | double blind procedure/ | 170759 |
| 18 | or/9‐17 | 2297858 |
| 19 | exp animal/ not human/ | 4747743 |
| 20 | 18 not 19 | 2068138 |
| 21 | 8 and 20 | 3938 |
| 22 | limit 21 to yr="2015 ‐Current" | 1376 |
| 23 | limit 22 to embase | 687 |
CENTRAL, Wiley
Search date: 24 February 2022
| No. | Search terms | Results |
| #1 | [mh "home care services, hospital‐based"] | 237 |
| #2 | [mh ^"home care services"] and (hospital* or unit? or ward? or institution*):ti,ab,kw | 770 |
| #3 | [mh "home health nursing"] | 7 |
| #4 | (hospital* near/2 home):ti,ab,kw | 1634 |
| #5 | (virtual next ward?):ti,ab,kw | 7 |
| #6 | ((early or earlier or supported or assisted) next discharge*):ti,ab,kw | 1035 |
| #7 | ((hospice* or terminal* or "end of life" or palliative) near/3 home*):ti,ab,kw | 304 |
| #8 | {or #1‐#7} | 3564 |
| #9 | {or #1‐#7} with Cochrane Library publication date Between Jan 2015 and Feb 2022 | 1879 |
CINAHL, EBSCO
Search date: 24 February 2022
| No. | Search terms | Results |
| S1 | (MH "Home Health Care+") | 47,909 |
| S2 | (hospital* or unit? or ward? or institution*) | 770,406 |
| S3 | S1 AND S2 | 9,381 |
| S4 | TI (hospital* N2 home) OR AB (hospital* N2 home) | 6,432 |
| S5 | TI (virtual ward?) OR AB (virtual ward?) | 52 |
| S6 | TI ((early or earlier or supported or assisted) N2 discharge?) OR AB ((early or earlier or supported or assisted) N2 discharge?) | 2,507 |
| S7 | TI ((hospice* or terminal or end of life or palliative) N3 home) OR AB ((hospice* or terminal or end of life or palliative) N3 home) | 3,957 |
| S8 | S3 OR S4 OR S5 OR S6 OR S7 | 19,369 |
| S9 | PT randomized controlled trial | 129,461 |
| S10 | PT clinical trial | 108,609 |
| S11 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 317,084 |
| S12 | (MH "Clinical Trials+") | 314,688 |
| S13 | (MH "Random Assignment") | 67,288 |
| S14 | S9 OR S10 OR S11 OR S12 OR S13 | 490,912 |
| S15 | S8 AND S14 | 2,149 |
| S16 | S15 | 772 |
| S17 | S16 Limiters ‐ Published Date: 20150101‐20221231 | 378 |
ClinicalTrials.gov
Search date: 14 November 2022
| Search terms | Results |
| Interventional Studies | (intervention/treatment) early supported discharge OR "hospital at home" OR virtual ward | 20 |
| Interventional Studies | (title) home AND hospital | 88 |
| Total = | 108 |
WHO ICTRP
Search date: 14 November 2022
| Search terms | Results |
| hospital at home | 91 |
| early supported discharge | 25 |
| virtual ward* | 3 |
| TITLE (advanced search): hospital AND home | 75 |
| Total = | 194 |
Data and analyses
Comparison 1. Admission avoidance hospital at home versus inpatient care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality at 3 months using IPD | 3 | 420 | mortality (IV, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| 1.2 Mortality at 6 months' follow‐up (using published data, and IPD from Wilson and Shepperd) | 5 | 1502 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.68, 1.13] |
| 1.3 Readmission to hospital after discharge from hospital at home or inpatient care (3 to 12 months' follow‐up) | 8 | 1757 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.3.1 Readmission for older patients with a medical condition using IPD and published data. N=1856 | 8 | 1757 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.4 Transfer to hospital while receiving hospital at home | 0 | Other data | No numeric data | |
| 1.5 Living in residential care at follow‐up | 4 | 1271 | Risk Ratio (IV, Fixed, 95% CI) | 0.53 [0.41, 0.69] |
| 1.5.1 With a medical condition (6 months' follow‐up) | 4 | 1271 | Risk Ratio (IV, Fixed, 95% CI) | 0.53 [0.41, 0.69] |
| 1.6 Quality of life/health status | 9 | Other data | No numeric data | |
| 1.6.1 Admission avoidance quality of life | 9 | Other data | No numeric data | |
| 1.7 Functional status | 10 | Other data | No numeric data | |
| 1.7.2 Admission avoidance patients with a medical condition ‐ functional ability | 10 | Other data | No numeric data | |
| 1.8 Psychological health | 7 | Other data | No numeric data | |
| 1.8.1 admission avoidance ‐ cognitive function/well being | 7 | Other data | No numeric data | |
| 1.9 Patient satisfaction | 0 | Other data | No numeric data | |
| 1.10 Caregiver satisfaction | 2 | Other data | No numeric data | |
| 1.10.1 Care giver satisfaction | 2 | Other data | No numeric data | |
| 1.11 Health professional satisfaction | 0 | Other data | No numeric data | |
| 1.12 Length of stay | 11 | Other data | No numeric data | |
| 1.12.1 Hospital and hospital at home length of stay | 11 | Other data | No numeric data | |
| 1.13 Cost and resource use | 14 | Other data | No numeric data | |
| 1.13.1 Health service resources and costs | 12 | Other data | No numeric data | |
| 1.13.2 Use of other social services | 2 | Other data | No numeric data | |
| 1.13.3 Informal care inputs | 2 | Other data | No numeric data | |
| 1.14 Clinical outcomes | 7 | Other data | No numeric data | |
| 1.14.1 Clinical outcomes | 7 | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrei 2011.
| Study characteristics | ||
| Methods | Parallel randomised trial | |
| Participants | Setting: Romania People with chronic heart failure who had deteriorated at a minimum of 1 week prior to recruitment. Number of participants in each group was not reported, a total of 45 participants recruited. |
|
| Interventions | Admission avoidance hospital at home; the first 48 hours of treatment was in the emergency department | |
| Outcomes | Mortality, biological measures, and cost | |
| Notes | Follow‐up at 1, 3, 6, and 12 months Funding: the study was published as an abstract, details not reported Conflicts of interest: details not reported Ethical approval: details not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Allocation concealment (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Baseline outcome measurements (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Baseline characteristics (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Methods not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study was published as an abstract, details not reported |
| Selective reporting (reporting bias) | Unclear risk | The study was published as an abstract, details not reported |
Caplan 1999.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between October 1995 and February 1997. |
|
| Participants | Setting: Australia Range of acute conditions requiring admission to hospital; participants recruited from casualty Number recruited: hospital at home: 51; hospital: 49 |
|
| Interventions | Hospital community outreach team Type of service: hospital community outreach team. Clinical responsibility by GP, or hospital doctor if GP declined |
|
| Outcomes | Functional status, mental status, clinical complications, patient and caregiver satisfaction, GP views | |
| Notes | Follow‐up: 1 and 6 months Funding: not reported Conflicts of interest: not reported Ethical approval: hospital ethical committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, stratified by participant's residence and if they had a deep vein thrombosis |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional and mental status, and diagnoses; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of treatment and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Unclear risk for functional status, mental status, clinical complications, patient and caregiver satisfaction, GP view |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, readmission |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Corwin 2005.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between July 2002 and June 2003. |
|
| Participants | Setting: New Zealand Patients with cellulitis Number recruited: treatment: 98, control: 96 Ages, mean (SD): T: 54.6 (20.6), C: 48.4 (19) European: T: 77/98 (79%), C: 78/96 (81%) Maori: T: 10/98 (10%), C: 5/96 (5%) Pacific: T: 2/98 (2%), C: 1/96 (1%) Other: T: 9/98 (9%), C: 13/96 (12%) |
|
| Interventions | Hospital at home admission avoidance from the emergency department. Run by Pegasus Health, an independent practitioner's association for 230 GPs in Christchurch, New Zealand. Care provided by GP and community care nursing staff. Patients required IV antibiotics for cellulitis. |
|
| Outcomes | Advancement of cellulitis, readmission, days on IV antibiotics, functional outcomes (SF‐36), patient satisfaction | |
| Notes | Follow up: 3 and 6 days Funding: Pegasus Health Conflicts of interest: none declared Ethical approval: local ethical committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list produced by SAS code |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation service |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional outcomes; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of treatment and control groups are reported and similar for main characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Unclear risk for pain, functional and physical health (SF‐36), and satisfaction |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for advancement of cellulitis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 from each group excluded at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Davies 2000.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between February 1998 and August 1999. |
|
| Participants | Setting: UK Patients with chronic obstructive airways disease Number recruited: hospital at home: 100, hospital: 50 |
|
| Interventions | Hospital at home Type of service: admission avoidance from A&E. Care provided by outreach specialist nurses and GP and community nurses if required. |
|
| Outcomes | Respiratory function, readmission, quality of life | |
| Notes | Few details on measure of quality of life Follow up: 2 weeks and 3 months Funding: North Mersey Community (NHS) Trust and University of Liverpool (UK) Conflicts of interest: none declared Ethical approval: district ethical committee This author contributed IPD for a previous update of this review (Shepperd 2016a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Ratio of 2:1 (hospital at home: hospital) |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for respiratory function; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel not possible Hospital readmission was a primary outcome, with decision to admit made by hospital staff |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | A small group of participants completed the St George's Respiratory Questionnaire |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for admission to hospital, changes in FEV1 score |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: hospital at home: 7/100; hospital: 5/50 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
Echevarria 2018.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between June 2014 and January 2016. |
|
| Participants | Setting: UK Patients aged >= 35 years, admitted to hospital with COPD exacerbation and low mortality risk. Those with life expectancy < 1 year due to illness other than COPD, long‐term ventilation, and coexistent secondary diagnosis necessitating admission were excluded. Number recruited: hospital at home: 62; usual care: 58 (analysed: hospital at home: 60; usual care: 58) Mean age (SD): T: 71 (9.6), C: 68.7 (10.5) Female: T: 32/60 (53%), C: 30/58 (52%) Approximately 1/5 of participants also had ischaemic heart disease and depression. |
|
| Interventions | The intervention consisted of once‐ or twice‐daily visits from a respiratory specialist nurse, under remote supervision from a respiratory consultant. An emergency contact number allowed patients to contact the team 24/7. Patients were monitored daily, and blood sampling was taken as required. Other available services included oral and IV therapies, acute controlled oxygen therapy, physiotherapy, psychology, occupational therapy, and formal social care. Comparison: usual hospital care |
|
| Outcomes | Main outcomes: health and social care costs over 90 days (non‐inferiority analysis) Other outcomes: survival; all‐cause and respiratory readmission rates; bed days over a) acute period of care, and b) postdischarge to 90 days; caregiver and patient preference; COPD exacerbations; unplanned health resource use; HADS score; quality of life; caregiver burden; perceptions of health care of patients and their caregivers and health professionals regarding the use of the clinical score for group allocation |
|
| Notes | Follow‐up: 90 days Trial registry: ISRCTN29082260 Funding: National Institute for Health Research (NIHR, UK) Conflicts of interest: "SCB reports grants from NIHR: Research for Patient Benefit Programme, during the conduct of the study; HTA funding, grants from Philips Respironics and Pfizer Open Air, personal fees from Pfizer and AstraZeneca, outside the submitted work. JG reports grants from NIHR Research for Patient Benefit, during the conduct of the study. CE, GJG, TH, AJS and JS have no competing interests to declare." Ethical approval: local ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Allocation to HAH or UC was based on 1:1 randomisation, performed by minimisation undertaken by an external, independent agency (sealedenvelope. com)” |
| Allocation concealment (selection bias) | Low risk | Quote: “The researchers were blind to the method of allocation for individual patients. For the primary cost analysis, the health economist was blinded to group allocation” |
| Baseline outcome measurements (selection bias) | Low risk | Comment: similar proportion of ECOPD treatment prior to admissions, Hospital Anxiety and Depression score, COPD assessment tool, and utility scores (EQ‐5D‐5L) |
| Baseline characteristics (selection bias) | Low risk | Quote: “Groups were well matched with respect to minimisation indices” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “For the primary cost analysis, the health economist was blinded to group allocation.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: subjective outcome was 'stated preference for HAH care day 14' (subjective to patient, did not require subjective judgement by researcher) Quote: “Patients in both arms maintained a diary of all health and social care visits and attendances, and were phoned every 2 weeks to prompt completion and collect data.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “For the primary cost analysis, the health economist was blinded to group allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: sensitivity analysis carried out for missing data |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes are consistent between trial registry and published results. |
Harris 2005.
| Study characteristics | ||
| Methods | Parallel randomised trial Dates of study not reported. |
|
| Participants | Setting: New Zealand Patients had a broad range of diagnoses: fractures (28%); miscellaneous medical problems (18%); respiratory problems (16%); stroke and neurological diagnoses (14%); falls and injuries (11%); cardiac diagnoses (8%); and rehabilitation and other problems (5%). Number recruited: hospital at home: 39, hospital: 37 |
|
| Interventions | Operated as a hospital outreach programme under the management of Auckland Hospital from the emergency department or acute assessment ward. A nurse‐led multidisciplinary team (physiotherapy, occupational therapy, social work) co‐ordinated care and rehabilitation for the patient within the patient's own home. There was a daily nursing review. Clinical responsibility was held by a dedicated hospital at home registrar, a consultant geriatrician, and in some cases the patient's GP, with 24‐hour on‐call medical cover. The service provided care 7 days a week with 10 hours of nursing care a day available, and a 24‐hour live‐in home caregiver if required. There was a daily nursing review, and a discharge handover to ongoing support services. The study included 2 treatment groups and a control (hospital) group. 1 treatment group was an "admission prevention" group and the other an "early discharge" group. We received IPD for the admission prevention and control groups only from the trialist. |
|
| Outcomes | Activities of daily living, cognitive function, instrumental activities of daily living | |
| Notes | Follow‐up: 90 days Funding: Northern Regional Health Authority (New Zealand) Conflicts of interest: not reported Ethical approval: local ethics committee This author contributed IPD for a previous update of this review (Shepperd 2016a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation service |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation service |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for activities of daily living, instrumental activities of daily living, and cognitive functioning; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel not possible Research staff did not make decision to admit to hospital |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Trained researchers not involved in patient care assessed participants for functional independence (FIM), cognitive function (MMSE), and instrumental activities of daily living (OARS) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, readmission, and measurement and valuation of costs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants withdrawing: hospital at home: 4/143; hospital: 10/124 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
Kalra 2000.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between April 1995 and October 1999. |
|
| Participants | Setting: UK Patients recovering from a moderately severe stroke Number recruited: hospital at home: 153; stroke unit care: 152; hospital care: 152 Median age (IQR): T: 75 (72 to 84), C: 77.7 (67 to 83) Living alone: T: 50/148 (34%), C: 50/149 (34%) |
|
| Interventions | Hospital outreach admission avoidance multidisciplinary with joint care from community services | |
| Outcomes | Mortality, institutionalisation, level of independence, activities of daily living, treatment inputs, readmission, hospital length of stay, cost | |
| Notes | Follow‐up: 3, 6, and 12 months Funding: NHS R&D Executive's Health Technology Assessment Programme; Stroke Association; Bromley Health Authority (UK) Conflicts of interest: not reported Ethical approval: local ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for level of independence and activities of daily living; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel not possible Primary outcome: death |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | A trained researcher, independent of the health care provided and unaware of treatment allocation, assessed functional status (Barthel and Rankin scale) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, institutionalisation, resource use and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: hospital at home: 9/144; hospital: 3/152 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
Levine 2018.
| Study characteristics | ||
| Methods | Parallel pilot randomised trial Study conducted between September and November 2016. |
|
| Participants | Setting: USA Patients aged >= 18 years, attending the emergency department with a primary diagnosis of any infection, heart failure exacerbation, COPD exacerbation, or asthma exacerbation. Those residing in a facility that provided on‐site medical care or who were at high risk for clinical deterioration were excluded. Number recruited: hospital at home: 9; usual care: 11 Median age (IQR): T: 65 (28), C: 60 (29) Female: T: 2/9 (22%), C: 8/11 (73%) Participants had on average 6 comorbidities and took 9 medications. |
|
| Interventions | The intervention was tailored to the patient's needs. There was at least 1 daily visit from an attending general internist and 2 daily visits from a home health registered nurse; additional services included medical meals and the services of a home health aide, social worker, physical therapist, and/or occupational therapist. Home services included oxygen and respiratory therapies, radiology, and point‐of‐care blood diagnostics; patients were remotely monitored for heart rate, respiratory rate, telemetry, movement, falls, and sleep via a small skin patch. Control group: usual hospital care; patients were also monitored using the same skin patch. |
|
| Outcomes | Main outcome: total cost of the hospitalisation Other outcomes: length of stay, readmissions, healthcare use, quality of life, activities of daily living, satisfaction with care |
|
| Notes | Follow‐up: 30 days Funding: Partners HealthCare Population Health Management; Institutional National Research Service Award; Ryoichi Sasakawa Fellowship Fund (USA) Conflicts of interest: none reported Ethical approval: approved by local ethical committee Trial registry: NCT02864420 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “stratified by condition with randomly selected block sizes between 4 and 6” |
| Allocation concealment (selection bias) | Low risk | Quote: “allocation concealment via sealed envelopes” |
| Baseline outcome measurements (selection bias) | Unclear risk | Comment: participants allocated to the control group reported more depression symptoms and lower self‐assessed quality of life. There was little or no difference between groups for hospital admission and ED visits in the past 6 months |
| Baseline characteristics (selection bias) | Unclear risk | Comment: participants allocated to the control group were younger, more likely to be female and speak English, more educated and less likely to be privately insured. Although these were sub‐threshold values, the sample is very small. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “All measures were derived from the EHR, except falls, physical activity, and sleep, which were observed via the skin patch.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: very low attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary and secondary outcomes are the same as per trial registry. There are other outcomes mentioned in the trial registry but not in the publication. |
Levine 2020.
| Study characteristics | ||
| Methods | Parallel pilot randomised trial Study conducted between June 2017 and January 2018; follow‐up ended on 17 February 2018. |
|
| Participants | Setting: USA Patients aged >= 18 years, attending the emergency department with a primary diagnosis of any infection, heart failure exacerbation, COPD exacerbation, or asthma exacerbation. Those residing in a residential or rehabilitation facility or who were at high risk for clinical deterioration were excluded. Number recruited: hospital at home: 43; usual care: 48 (all randomised) Median age (IQR): T: 80 (19), C: 72 (23) Female: T: 15/43 (35%), C: 18/48 (38%) Patients were generally frail and chronically ill and used hospital care frequently. |
|
| Interventions | The intervention was tailored to the patient's needs. There was at least 1 daily visit from an attending general internist and 2 daily visits from a home health registered nurse; additional services included medical meals and the services of a home health aide, social worker, physical therapist, and/or occupational therapist. Home services included oxygen and respiratory therapies, radiology, and point‐of‐care blood diagnostics; patients were remotely monitored for heart rate, respiratory rate, telemetry, movement, falls, and sleep via a small skin patch. Care was available 24/7 from the attending physician. Control group: usual hospital care; sleep was monitored using the same skin patch as the intervention group. |
|
| Outcomes | Main outcome: total cost of the hospitalisation Other outcomes: length of stay, readmissions, healthcare use, quality of life, activities of daily living, satisfaction with care |
|
| Notes | Follow‐up: 30 days Funding: Partners HealthCare Center for Population Health and internal departmental funds (USA) Conflicts of interest: "Dr. Levine reports grants from Biofourmis outside the submitted work. Dr. Blanchfield reports consulting income from Verily, GreyBird Ventures, and Atlas5D outside the submitted work. Dr. Schnipper reports grants from Mallinckrodt Pharmaceuticals and Portola Pharmaceuticals outside the submitted work." Ethical approval: approved by local ethical committee Trial registry: NCT03203759 The study was stopped early after enrolling 91 patients (76% of intended sample) "in light of local operational needs to quickly increase home hospital capacity after positive interim outcomes were presented to hospital leadership”. 63% of patients approached and possibly eligible refused to participate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An outside statistician generated the randomization using SAS (SAS Institute). Randomization was stratified by infection, heart failure, chronic obstructive pulmonary disease or asthma, and other diagnosis; block sizes between 4 and 6 were randomly selected, |
| Allocation concealment (selection bias) | Low risk | Comment: allocation was concealed via sealed opaque envelopes. |
| Baseline outcome measurements (selection bias) | Low risk | Comment: the primary outcome was the direct cost of an acute care episode, data were obtained from the electronic record |
| Baseline characteristics (selection bias) | Unclear risk | Comment: a few differences between groups, with the largest difference being for the numbers who recorded having a home health aide at baseline: HAH 17/43 (40%) vs control 10/48 (21%) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: patients, study staff and physicians were not blinded to allocation status. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: unclear risk of bias for unblinded assessment of physical activity, patient experience, and quality during the acute care episode |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: the primary outcome was the direct cost of an acute care episode; secondary outcomes were health care use, physical activity, patient experience, safety (medication and delirium), and quality of care during the acute care episode |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: “If patients could not be reached 30 days after discharge (8 total patients, 1 in the home group and 7 in the control group), we used EHR data alone to estimate health care use and readmission rates and did not measure patient experience.” (page 79) |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes are consistent between trial registry and published results. |
Mendoza 2009.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between May 2006 and March 2007. |
|
| Participants | Setting: Spain Patients in A&E with acute decompensation of chronic heart failure Inclusion criteria: > 65 years of age, with heart failure for at least 12 months prior to study, NYHA functional class II or III prior to acute episode, all‐day supervision available, telephone, < 10 kilometres from hospital Age > 65 years, mean age 79. 29% female in hospital arm, 51% female in hospital at home arm Number recruited (between May 2006 and March 2007): hospital at home: 37; hospital: 34 |
|
| Interventions | Admission avoidance hospital at home; patients admitted from emergency departments, hospital outreach model. Hospital at home nurse visited within 12 to 24 hours of admission to hospital at home and then daily visits. Care available between 8 a.m. and 9 p.m.; patients called emergency services outside these hours. Hospital specialist visited daily or every other day, depending on patient’s condition. Control group: admitted to hospital |
|
| Outcomes | Mortality, readmission, functional status, general health status, length of stay, costs | |
| Notes | Follow‐up: 1 year after discharge Funding: Caja Vital Kutxa (Spain) Conflicts of interest: none declared Ethical approval: approved by the hospital ethical committee This author contributed IPD for a previous update of this review (Shepperd 2016a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation described as "externally generated sequence." |
| Allocation concealment (selection bias) | Low risk | Random sequence hidden from the physician until patient had consented to participate |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional status and general health status; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | A trained researcher, independent of the health care provided, assessed functional status (Barthel Index), health‐related quality of life (SF‐36) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for death, readmission |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 of 80 did not complete the study (2 in hospital at home group, 7 in hospital group) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Nicholson 2001.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between October 1999 and October 2000. |
|
| Participants | Setting: Australia Patients with COPD Inclusion criteria: age > 45 years, COPD, current or ex‐smoker, FEV1 < 60% predicted, admission requested by GP or OPD clinic staff or ED staff, telephone at home Number recruited: hospital at home: 13; hospital: 12 |
|
| Interventions | Hospital at home (discharge from emergency department) Patients retained in patient status and received clinical supervision from hospital specialist, and hospital had legal and financial responsibility; also received care from GP, community nursing, and domiciliary care. Hospital medical staff provided 24‐hour telephone support. |
|
| Outcomes | Cost to the health service | |
| Notes | Follow‐up: duration of care in hospital at home or inpatient care Funding: the Commonwealth Department of Health and Aged Care, National Demonstration Hospitals Program Phase 3 Conflicts of interest: not reported Ethical approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Baseline outcome measurements (selection bias) | Unclear risk | Baseline outcome measurements not reported |
| Baseline characteristics (selection bias) | Unclear risk | Baseline characteristics of the study and control groups not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | None reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for resource use and cost |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to allocate low or high risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Ricauda 2004.
| Study characteristics | ||
| Methods | Parallel randomised trial Participants recruited from January 1997 to February 1998. |
|
| Participants | Setting: Italy Patients recovering from a stroke Eligibility criteria: patients admitted to hospital within 24 hours of the onset of symptoms and evaluated for at least 24 hours Age (IQR): 76 to 88, median 82 Number recruited: hospital at home: 60; hospital: 60 |
|
| Interventions | Hospital outreach admission avoidance. 24‐hour care available from a multidisciplinary team: physiotherapist, occupational therapist, nursing, hospital geriatrician, social worker, speech therapist, psychologist |
|
| Outcomes | Length of treatment, mortality, activities of daily living, functional impairment, depression, costs | |
| Notes | Follow‐up: 6 months External funding not reported. Conflicts of interest: not reported Ethical approval: obtained from the San Giovanni Battista Hospital ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for activities of daily living, functional impairment, and depression; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcomes assessor blinded to the study allocation, and used validated measures of outcome |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes assessor blinded to the study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Ricauda 2008.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between April 2004 and April 2005. |
|
| Participants | Setting: Italy Patients requiring acute hospitalisation for acute exacerbation of COPD; care supervision at home; telephone connection; living in the catchment area; family or social support Age (SD): T: 80.1 (3.2), C: 79.2 (3.1) Number recruited: hospital at home: 52; inpatient: 52 |
|
| Interventions | Physician‐led admission avoidance hospital outreach service; GHHS of a regional hospital. The home care programme emphasised patient and caregiver education about the disease, advice about smoking cessation, nutrition, management of activities of daily living and energy conservation, understanding and use of drugs, health maintenance, and early recognition of triggers of exacerbation that require medical intervention. In the first days after admission to GHHS, physicians and nurses visited each patient at home daily, then daily visits by the nurse and visits by the doctor every 2 to 3 days or less. Blood tests, electrocardiogram, spirometry, pulse oximetry, oxygen, IV fluids, antimicrobials and other medications, blood transfusions, surgical treatment for pressure ulcers were available. Control group: inpatient hospital care |
|
| Outcomes | Mortality, readmission, health status, satisfaction, residential care, length of stay, resource use and cost, caregiver outcomes | |
| Notes | Follow‐up: 6 months Funding: S. Giovanni Battista Hospital of Torino Conflict of interest: public funds of S. Giovanni Battista Hospital of Torino were used in this study. The editor‐in‐chief has reviewed the conflict of interest checklists provided by the authors and determined that none of the authors have any financial or any other kind of personal conflicts with this manuscript. Ethical approval: ethical approval was obtained from the hospital ethics committee. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | The project manager randomly allocated participants using a numbered set of sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for health status; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Data collected by independent postgraduate physicians who were not involved in the care of patients or the research team |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Standard set of outcomes reported |
Richards 2005.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between July 2002 and October 2003. |
|
| Participants | Setting: New Zealand Patients with community‐acquired pneumonia Age: T: 50.1, C: 49.8 Number recruited: hospital at home: 24; hospital: 25 |
|
| Interventions | Hospital at home: admission avoidance from emergency room. Run by Pegasus Health, an independent practitioner's association for 230 GPs in Christchurch, New Zealand. Care provided by GP and community care nursing staff. | |
| Outcomes | Median number of days to discharge, days of IV antibiotics, functional outcomes, mortality, readmission, patient satisfaction, costs | |
| Notes | Follow‐up: 2 and 6 weeks Funding: not reported Conflicts of interest: none reported Ethical approval: local ethics committee Canterbury Ethics Committee, Christchurch, New Zealand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional outcomes; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Patient‐rated symptoms, satisfaction |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Days on IV antibiotics, admissions extracted from clinical records |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 exclusions after randomisation, no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Shepperd 2021.
| Study characteristics | ||
| Methods | Parallel randomised trial First participant was recruited 14 March 2015, last participant was recruited 18 June 2018. |
|
| Participants | Setting: UK Patients aged >= 65 years, who have been referred to the geriatrician‐led admission avoidance hospital at home service and would otherwise require hospital admission for an acute medical event. Patients were excluded if they had acute coronary syndrome, suspected stroke, lived in residential settings, required palliative care, or were assessed as high risk. Age: T: 83.3 (7.0), C: 83.3 (6.9) Number recruited: hospital at home: 700; hospital: 355 |
|
| Interventions | Intervention is geriatrician‐led co‐ordinated, multidisciplinary health care in the home, delivered by a team including nurses, physiotherapists, occupational therapists, and social workers who are either part of the primary healthcare team or dedicated staff and can refer the patient to other services as needed (e.g. mental health or social work services, pharmacy support). Health care was provided 7 days per week, and emergency medical cover was available 24 hours/day. Control group: hospital‐based inpatient, with comprehensive geriatric assessment when available |
|
| Outcomes | Main outcome: living at home Other outcomes: activities of daily living, cognitive impairment, delirium, mortality, new long‐term residential care, quality of life, resource use, transfer to hospital |
|
| Notes | Follow‐up: 6 and 12 months Trial registry: ISRCTN60477865 Funding: NIHR Health Services and Delivery Research Programme (UK) Conflicts of interest: none declared Ethical approval: Research Ethics Committee England, Wales and Northern Ireland, and Scotland Primary reference is Annals of Internal Medicine article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was stratified by site, sex, and cognitive status measured before randomization using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)” |
| Allocation concealment (selection bias) | Low risk | Quote: “ Eligible participants who provided informed consent were randomly allocated to either CGA HAH or hospital admission using Sortition, a validated, secure online randomization system developed by the University of Oxford's Primary Care Clinical Trials Unit.” |
| Baseline outcome measurements (selection bias) | Low risk | Comment: Patients had similar mean MoCA scores, Barthel scores, comorbidity, delirium, and EQ‐5D‐5L. |
| Baseline characteristics (selection bias) | Low risk | Comment: Patients had similar proportions of the presenting problems, diagnoses. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Main outcome (inverse of death or new admission to long‐term residential care) is objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: Questionnaire outcomes collected by study staff aware of allocation. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: Main outcome (inverse of death or new admission to long‐term residential care) is objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Sensitivity analyses showed little or no change when missing data were imputed with different outcomes.” |
| Selective reporting (reporting bias) | Low risk | Comment: Primary outcome was changed to be measured at 6 months not 12 months, agreed on by trial steering committee. |
Talcott 2011.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between September 1994 and January 1999. |
|
| Participants | Setting: USA Participants: recruited from outpatients with postchemotherapy febrile neutropenia and assessed as low risk if there was no indication for hospitalisation other than fever and neutropenia (such as systemic hypotension, altered mental status, respiratory failure, or inadequate oral fluid intake during 24‐hour observation; and had adequately controlled cancer) Age 20 to 81 years, median 47 years Number recruited: hospital at home: 47; inpatient: 66 |
|
| Interventions | Admission avoidance hospital at home, patients recruited from outpatient clinic. Provided by commercial home care provider who agreed to provide protocol care for patients without out‐of‐pocket charges; daily visits by a home care nurse who followed a protocol/standard checklist and contacted the primary care physician if there were abnormal findings. Daily blood tests. 24‐hour care available. Hospital specialist examined the patient 2 to 4 days following discharge and then at least weekly. Home IV available. Control group received care in oncology units in general hospitals. |
|
| Outcomes | Major medical complications, readmission to hospital, quality of life | |
| Notes | Folllow‐up time for each episode was the resolution of fever, neutropenia, and any complications arising during the episode. Quality of life data were collected at the time of consent to join the study and as soon as possible after resolution of the episode. Funding: National Cancer Institute (USA) Conflicts of interest: authors indicated no potential conflicts of interest. Ethical approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks of random numbers stratified by colony‐stimulating factors, institution, and whether random assignment occurred on weekends, holidays, or after hours |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for clinical characteristics and quality of life; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar for all main characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Any medical event requiring urgent diagnostic or therapeutic intervention. Predefined complications included systemic hypotension (systolic blood pressure > 90 mmHg), respiratory failure (partial pressure of oxygen < 60 torr adjusted for hyperventilation) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Predefined medical complications using blinded review |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up, 5 withdrawn/excluded (3 withdrew consent, 2 excluded as neutropenia had resolved at recruitment) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Tibaldi 2004.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between February 1999 and April 2002. |
|
| Participants | Setting: Italy Patients: elderly with advanced dementia Mean age (SD): T: 82.9 (7.9), C: 84.1 (7.5) Number recruited: hospital at home: 56; inpatient: 53 |
|
| Interventions | Hospital at home run by S. Giovanni Battista Hospital, Turin, Italy: GHHS, patients referred from emergency department. 24‐hour‐a‐day care available, home nursing multidisciplinary care, rapid access to equipment. |
|
| Outcomes | Behavioural disturbances, number of patients treated with antipsychotic drugs on admission and on discharge, mortality, length of stay, place of discharge (home or to a nursing home) | |
| Notes | Follow‐up: to discharge from service 4 participants admitted from hospital at home to hospital for new medical problems. Funding: not reported Conflicts of interest: not reported Ethical approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for cognitive status, severity of disease, and activities of daily living; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Assessment method not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Low risk for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Tibaldi 2009.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between April 2004 and April 2005. |
|
| Participants | Setting: Italy People with acute decompensation of chronic heart failure recruited within 12 to 24 hours of admission to the emergency department. Care supervision possible at home, telephone at home, need for IV infusions, living in catchment area, at least 1 previous admission for chronic heart failure Age: 75 years and over, mean age 81 Number recruited: hospital at home: 48; hospital: 53 |
|
| Interventions | Admission avoidance hospital at home, hospital outreach (hospital maintains legal and financial responsibility); 24‐hour care available 7 days a week; 4 specialist geriatricians, home care nurses, physiotherapist, social worker, counsellor, IV infusions available. Control group: admission to San Giovanni Battista Hospital, Turin, Italy |
|
| Outcomes | Mortality, readmission, length of stay, residential care, health status, psychological well‐being | |
| Notes | Funding: not reported Conflicts of interest: none reported Ethical approval: hospital ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for health status and psychological well‐being; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Standard set of outcomes reported |
Vianello 2013.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between January 2009 and December 2011. |
|
| Participants | Setting: Italy Patients with neuromuscular disease and who had an acute respiratory tract infection and required hospital admission; recruited between January 2009 and December 2011. Number recruited: hospital at home: 26; inpatient hospital: 27 |
|
| Interventions | The use of a portable ventilator; a respiratory therapist made daily visits for the first 3 days of home care, and district nurses and caregivers were trained in the application of the device and on assisting with coughing. District nurses visited daily until recovery from the respiratory tract infection, participants also had telephone access to pulmonary specialists. | |
| Outcomes | Recovery from exacerbation, defined as relief of respiratory distress and return of SpO2 level | |
| Notes | Follow‐up: 3 months Funding: Associazione Distrofia Muscolare, Associazione Sclerosi Laterale Amiotrofica, and Unione Italiana Lotta Alla Distrofia Muscolare (Italy) Conflicts of interest: none reported Ethical approval: institutional review board ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for respiratory distress and SpO2 level; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Subjective outcomes not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, need for intubation, and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up data reported in Table 3, page 2066; the authors did not report loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Wilson 1999.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between November 1995 and May 1997. |
|
| Participants | Setting: UK Patients with a mix of conditions (majority elderly) referred by GP to Bed Bureau Number recruited: hospital at home: 102; inpatient: 97 6 patients refused hospital at home care and were admitted to hospital. |
|
| Interventions | Hospital at home (admission avoidance) Type of service: multidisciplinary team (nurses, therapy, generic health workers, cultural link worker) Referred by a GP, who maintains medical responsibility Maximum of 5 patients at a time Control group: inpatient hospital care |
|
| Outcomes | Mortality, readmission, functional status, quality of life, patient satisfaction | |
| Notes | Follow‐up: 3 days, 2 weeks, 3 months Funding: National R&D Programme, Primary‐Secondary Care Interface, NHS Executive, North Thames (UK) Conflicts of interest: none reported Ethical approval: local ethics committee This author contributed IPD for a previous update of this review (Shepperd 2016a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional status and quality of life; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome assessments by independent research staff, decision to admit made by hospital staff, not research team |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Low risk for health status (Sickness Impact Profile 68), cognitive function (Clifton Assessment Procedures for the Elderly), functional status (Barthel Index), and quality of life (EuroQol) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, readmission, resource use and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: hospital at home: 8/87; hospital: 5/80 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
A&E: accident & emergency department C: control COPD: chronic obstructive pulmonary disease ED: emergency department FEV1: forced expiratory volume in 1 second GHHS: geriatric home hospitalisation service GP: general practitioner HADS: Hospital Anxiety and Depression Scale IPD: individual patient data IQR: interquartile range IV: intravenous MMSE: Mini‐Mental State Examination NIHR: National Institute for Health and Care Research NYHA: New York Heart Association OPD: outpatient department SD: standard deviation SF‐12: 12‐Item Short Form Health Survey SF‐36: 36‐Item Short Form Health Survey SpO2: oxygen saturation T: treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cabrol 2000 | Compares inpatient and outpatient (ambulatory) treatment |
| Duiverman 2019 | Tests the feasibility of home initiation of chronic non‐invasive ventilation to ameliorate chronic respiratory failure at home, using telemedicine |
| Hazenberg 2014 | Tests the feasibility of introducing technologies that ameliorate chronic respiratory failure, at home. For such patients initiation of the treatment has normally been in hospital, but may be feasible and safe at home. |
| Hill 1978 | Patients with myocardial infarction are not suitable to be treated at home because they require urgent treatment (www.nhs.uk/conditions/heart-attack/treatment/). |
| King 2000 | Cross‐over randomised controlled trial evaluating chemotherapy provided in a home setting versus an outpatient clinic setting |
| Levine 2021 | Remote physician care versus daily in‐person physician hospital at home, with no hospital comparison |
| Mascardi 2016 | Tests the feasibility of introducing technologies that ameliorate chronic respiratory failure, at home. For such patients initiation of the treatment has normally been in hospital, but may be feasible and safe at home. |
| Mather 1976 | Patients with myocardial infarction are not suitable to be treated at home because they require urgent treatment (www.nhs.uk/conditions/heart-attack/treatment/). |
| NCT02363413 | Tests the feasibility of introducing technologies that ameliorate chronic respiratory failure, at home. For such patients initiation of the treatment has normally been in hospital, but may be feasible and safe at home. |
| NCT03490084 | Not randomised |
| Wade 1985 | Clinical controlled trial Compared 2 districts, 1 with a domiciliary stroke service and 1 without |
| Wolfe 2000 | Intervention does not substitute for inpatient care. |
Characteristics of ongoing studies [ordered by study ID]
NCT03156686.
| Study name | SAFEty and Efficacy of HOME‐based hospitalization versus inpatient care for patients with acute heart failure in chronic heart failure. (SAFE‐HOME) |
| Methods | Parallel randomised trial |
| Participants | Inclusion: adults aged >= 18 years diagnosed with acute heart failure, eligible for hospital home within 4 days of hospitalisation, affiliated with social security and complementary health insurance Exclusion: severe cognitive or behavioural disorders, no caregiver, other chronic conditions |
| Interventions | Intervention: home‐based hospitalisation with intravenous diuretics Comparison: usual hospital care |
| Outcomes | Main outcome: time to rehospitalisation (3 months) Other outcomes: adverse events, quality of life, nutritional status, mortality, cost‐effectiveness, time to rehospitalisation (12 months) Follow‐up: 3 and 12 months |
| Starting date | June 2017 |
| Contact information | Thibaud Damy, Hôpital Henri Mondor, France |
| Notes |
Pouw 2018.
| Study name | Hospital at home care for older patients with cognitive impairment and an acute medical illness |
| Methods | Parallel randomised trial |
| Participants | Inclusion: adults aged >= 65 who present to the emergency department with a defined acute illness; diagnosed with dementia, delirium, or other cause of cognitive impairment; caregiver present, living within the catchment area, and with adequate living arrangements Exclusion: hospitalised during the previous 7 days, nursing home residents or awaiting a placement, need for palliative care, other acute or chronic conditions |
| Interventions | Intervention: hospital‐level care provided at the patient's own home Comparison: usual hospital care |
| Outcomes | Main outcomes: mortality, institutionalisation, ADL‐functioning, prevalence of hospital‐associated geriatric syndromes, length of stay in hospital or in hospital at home care programme, contact with healthcare professionals Other outcomes: time spent at home (home‐time), total number of days alive and out of the hospital or a skilled nursing facility, costs Follow‐up: 6 months |
| Starting date | December 2017 |
| Contact information | Maaike A Pouw Department of Geriatrics, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands |
| Notes | Trial registry: NTR6581 |
ADL: activities of daily living
Differences between protocol and review
We updated the methods to align with current Cochrane guidance (MECIR 2012).
Contributions of authors
KE screened records, extracted data, assessed risk of bias, analysed the results for this update, and contributed to the writing of the review.
SI screened records, extracted data, and commented on each draft of the review.
HD and MJC contributed to the interpretation of data and writing of the review for the 2008 update.
DGB screened records, extracted data, and contributed to the writing of the review.
EW commented on the drafts and final draft of the review.
SS co‐ordinated the review, screened records, extracted data, analysed the results (with HD) for the 2008 update with individual patient data, and led the writing of the review. SS did not assess risk of bias or extract data from the Shepperd 2021 study.
Sources of support
Internal sources
-
Nuffield Department of Population Health, University of Oxford, UK
Sasha Shepperd
External sources
-
National Institute for Health and Care Research (NIHR), UK
Funding from the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the EPOC Group supported the conduct of this review, the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the National Institute of Health Research or the Department of Health and Social Care. A previous version of the review was supported by an NIHR Research Scientist in Evidence Synthesis Award to Sasha Shepperd.
-
The Healthcare Improvement Studies Institute (THIS), UK
Kate Edgar is funded by THIS Institute as part of a doctoral fellowship. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of The Healthcare Improvement Studies Institute.
Declarations of interest
KE: none known.
SI: none known.
HD: none known.
MC: is a Cochrane Editor, but was not involved in the editorial process of this review.
DGB: is a Cochrane Editor, but was not involved in the editorial process of this review.
EW: no declarations of interest.
SS is author of one of the included studies (Shepperd 2021); she did not perform risk of bias assessment, data extraction, or GRADE assessment for that study. SS is a Cochrane Editor, but was not involved in the editorial process of this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrei 2011 {published data only}
- Andrei CL, Sinescu CJ, Ianula RM, Popa AI, Chioncel VP, Mischie AN, et al. Can be the home care of the heart failure patients a better economic alternative? European Journal of Heart Failure Supplements (Abstract) 2011;10(S1):S29. [Google Scholar]
Caplan 1999 {published data only}
- Board N, Brennan N, Caplan G. A randomised controlled trial of the costs of hospital as compared with hospital at home for acute medical patients. Australian and New Zealand Journal of Public Health 2000;24(3):305-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Caplan GA, Coconis J, Woods J. Effect of hospital in the home treatment on physical and cognitive function: a randomized controlled trial. Journal of Gerontology 2005;60(8):1035-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Caplan GA, Ward JA, Brennan NJ, Coconis J, Board N, Brown A. Hospital in the home: a randomised controlled trial. Medical Journal of Australia 1999;170(4):156-60. [PMID: ] [PubMed] [Google Scholar]
Corwin 2005 {published data only}
- Corwin P, Toop L, McGeoch G, Than M, Wynn-Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005;330(7483):129. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2000 {published and unpublished data}
- Davies L, Wilkinson M, Bonner S, Calverley PM, Angus RM. Hospital at home versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. BMJ 2000;321(7271):1265-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Echevarria 2018 {published data only}
- Dismore LL, Echevarria C, Wersch A, Gibson J, Bourke S. What are the positive drivers and potential barriers to implementation of hospital at home selected by low-riskDECAF score in the UK: a qualitative study embedded within a randomised controlled trial. BMJ Open 2019;9:e026609. [DOI: 10.1136/bmjopen-2018-026609] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria C, Gray J, Hartley T, Steer J, Miller J, Simpson AJ, et al. Home treatment of COPD exacerbation selected by DECAF score: a non-inferiority, randomised controlledtrial and economic evaluation. Thorax 2018;73:713-22. [DOI: 10.1136/thoraxjnl-2017-211197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2005 {published and unpublished data}
- Harris R, Ashton T, Broad J, Connolly G, Richmond D. The effectiveness, acceptability and costs of a hospital-at-home service compared with acute hospital care: a randomised controlled trial. Journal of Health Services and Research Policy 2005;10(3):158-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kalra 2000 {published data only}
- Kalra L, Evans A, Perez I, Knapp M, Donaldson N, Swift CG. Alternative strategies for stroke care: a prospective randomised controlled trial. Lancet 2000;356(9233):894-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost effectiveness and cost-utility analyses from a prospective randomized controlled trial. Stroke 2004;35(1):196-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Levine 2018 {published data only}
- Levine DM, Ouchi K, Blanchfield B, Diamond K, Licurse A, Pu CT, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. Journal of General Internal Medicine 2018;33(5):729-36. [DOI: 10.1007/s11606-018-4307-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2020 {published data only}
- Levine DM, Ouchi K, Blanchfield B, Saenz A, Burke K, Paz M, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Annals of Internal Medicine 2020;172(2):77-92. [DOI: 10.7326/M19-0600] [DOI] [PubMed] [Google Scholar]
- Levine DM, Pian J, Mahendrakumar K, Patel A, Saenz A, Schnipper JL. Hospital-level care at home for acutely ill adults: a qualitative evaluation of a randomized controlled trial. Journal of General Internal Medicine 2021;36(7):1965-73. [DOI: 10.1007/s11606-020-06416-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendoza 2009 {published and unpublished data}
- Mendoza H, Martin MJ, Garcia A, Aros F, Aizpuru F, Regalado de Los Cobos J, et al. Hospital at home care model as an effective alternative in the management of decompensated chronic heart failure. European Journal of Heart Failure 2009;11:1208-13. [DOI] [PubMed] [Google Scholar]
Nicholson 2001 {published data only}
- Nicholson C, Bowler S, Jackson C, Schollay D, Tweeddale M, O'Rourke P. Cost comparison of hospital- and home-based treatment models for acute chronic obstructive pulmonary disease. Australian Health Review 2001;24(4):181-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ricauda 2004 {published data only}
- Ricauda NA, Bo M, Molaschi M, Massaia M, Salerno K, Amati D, et al. Home hospitalization service for acute uncomplicated first ischemic stroke in elderly patients: a randomized trial. Journal of the American Geriatrics Society 2004;52(2):278-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ricauda NA, Pla LF, Marinello M, Molaschi M, Fabris F. Feasibility of an acute stroke home care service for elderly patients. Archives of Gerontology and Geriatrics 1998;26(Suppl 6):17-22. [EMBASE: 1998333220] [Google Scholar]
Ricauda 2008 {published data only}
- Ricauda NA, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive hospital at home versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Journal of the American Geriatrics Society 2008;56:493-500. [DOI] [PubMed] [Google Scholar]
Richards 2005 {published data only}
- Richards DA, Toop LJ, Epton MJ, McGeoch RB, Town GI, Wynn-Thomas SM, et al. Home management of mild to moderately severe community-acquired pneumonia: a randomised controlled trial. Medical Journal of Australia 2005;183(5):235-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shepperd 2021 {published and unpublished data}
- Mäkelä P, Godfrey M, Cradduck-Bamford C, Ellis G, Shepperd S. A protocol for the process evaluation of a multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 2018;19:569. [DOI: 10.1186/s13063-018-2929-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older persons?: a randomized trial. Annals of Internal Medicine 2021;174(7):889-98. [DOI: 10.7326/M20-5688] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd S, Cradduck-Bamford A, Butler C, Ellis G, Goddfrey M, Gray A, et al. A multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 201;18:491. [DOI: 10.1186/s13063-017-2214-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Gray A, Shepperd S, Stott DJ, Ellis G, Hemsley A, et al. Is comprehensive geriatric assessment hospital at home a cost-effective alternative to hospital admission for older people? Age and Ageing 2022;51(1):afab220. [DOI: 10.1093/ageing/afab220] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talcott 2011 {published data only}
- Hendricks AM, Loggers ET, Talcott JA. Costs of home versus inpatient treatment for fever and neutropenia: analysis of a multicenter randomized trial. Journal of Clinical Oncology 2011;29:3984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott JA, Yeap JA, Siegal RD, Loggers ET, Lu C, Godley PA. Safety of early discharge for low risk patients with febrile neutropenia: A multicentre randomised controlled trial. Journal of Clinical Oncology 2011;29:3977-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tibaldi 2004 {published data only}
- Tibaldi V, Aimonino N, Ponzetto M, Stasi MF, Amati D, Raspo S, et al. A randomized controlled trial of a home hospital intervention for frail elderly demented patients: behavioral disturbances and caregiver's stress. Archives of Gerontology and Geriatrics - Supplement 2004;38(9):431-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tibaldi 2009 {published data only}
- Tibaldi V, Isaia G, Scarafiotti C, Gariglio F, Zanocchi M, Bo M, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Archives of Internal Medicine 2009;169:1569-75. [DOI] [PubMed] [Google Scholar]
Vianello 2013 {published data only}
- Vianello A, Savoia F, Pipitone E, Nordio B, Gallina G, Paladini L, et al. “Hospital at home” for neuromuscular disease patients with respiratory tract infection: a pilot study. Respiratory Care 2013;12(58):2061-8. [DOI] [PubMed] [Google Scholar]
Wilson 1999 {published and unpublished data}
- Jones J, Wilson A, Parker H, Wynn A, Jagger C, Spiers N, et al. Economic evaluation of hospital at home versus hospital care: cost minimisation analysis of data from randomised controlled trial. BMJ 1999;319(7224):1547-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Parker H, Wynn A, Jagger C, Spiers N, Jones J, et al. Randomised controlled trial of effectiveness of Leicester hospital at home scheme compared with hospital care. BMJ 1999;319(7224):1542-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Wynn A, Parker H. Patient and carer satisfaction with 'hospital at home': quantitative and qualitative results from a randomised controlled trial. British Journal of General Practice 2002;52(474):9-13. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Cabrol 2000 {published data only}
- Cabrol J, Güell M, Brullet E, Campo R, Calvet X, Laporte E. Home versus hospital treatment of patients with uncomplicated upper gastrointestinal bleeding. Prospective randomized study [Tratamiento domicilario versus ingreso hospitalario de los pacientes con hemorragia digestiva alta no complicada: Estudio prospectivo y randomizado]. Revista Española of Enfermedades Digestivas 2000;92(Suppl. 1):31. [Google Scholar]
Duiverman 2019 {published data only}
- Duiverman ML, Vonk JM, Bladder G, Van Melle JP, Nieuwenhuis J, Hazenberg A, et al. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial. Thorax 2019;75:244-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazenberg 2014 {published data only}
- Hazenberg A, Kerstjens HA, Prins SC, Vermeulen KM, Wijkstra PJ. Initiation of home mechanical ventilation at home: a randomised controlled trial of efficacy, feasibility and costs. Respiratory Medicine 2014;108(9):1387-95. [DOI] [PubMed] [Google Scholar]
Hill 1978 {published data only}
- Hill JD, Hampton JR, Mitchell JR. A randomised trial of home-versus-hospital management for patients with suspected myocardial infarction. Lancet 1978;1(8069):837-41. [DOI] [PubMed] [Google Scholar]
King 2000 {published data only}
- King MT, Hall J, Caleo S, Gurney HP, Harnett PR. Home or hospital? An evaluation of the costs, preferences and outcomes of domiciliary chemotherapy. International Journal of Health Services 2000;30:557-79. [DOI] [PubMed] [Google Scholar]
Levine 2021 {published data only}
- Levine DM, Schnipper JL. Remote physician care for home hospital patients: a randomized controlled noninferiority trial. Journal of General Internal Medicine 2021;36:S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascardi 2016 {published data only}
- Mascardi V, Grecchi B, Barlascini C, Banfi P, Nicolini A. Effectiveness of temporary positive expiratory pressure (T-PEP) at home and at hospital in patients with severe chronic obstructive pulmonary disease. Journal of Thoracic Disease 2016;8(10):2895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mather 1976 {published data only}
- Mather HG, Morgan DC, Pearson NG, Read KL, Shaw DB, Steed GR, et al. Myocardial infarction: a comparison between home and hospital care for patients. BMJ 1976;1(6015):925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02363413 {published data only}
- NCT02363413. Initiation of non-invasive ventilation at home versus hospital among patients with overlap syndrome (OVNI-Dom). clinicaltrials.gov/ct2/show/NCT02363413 (first received 16 February 2015).
NCT03490084 {published data only}
- NCT03490084. Impacts on health outcomes and resources utilization of hospital-at-home for elderly patients with multiple myeloma (PAMM-HAD1). clinicaltrials.gov/ct2/show/NCT03490084 (first received 27 February 2020).
Wade 1985 {published data only}
- Wade DT, Langton-Hewer R, Skilbeck CE, Bainton D, Burns-Cox C. Controlled trial of a home-care service for acute stroke patients. Lancet 1985;1(8424):323-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wolfe 2000 {published data only}
- Wolfe CDA, Tilling K, Rudd AG. The effectiveness of community-based rehabilitation for stroke patients who remain at home: a pilot randomized trial. Clinical Rehabilitation 2000;14(6):563-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03156686 {published data only}
- NCT03156686. SAFEty and Efficacy of HOME-based hospitalization versus inpatient care for patients with acute heart failure in chronic heart failure. (SAFE-HOME). clinicaltrials.gov/ct2/show/NCT03156686 (first received 17 May 2017).
Pouw 2018 {published data only}
- Pouw MA, Calf AH, Munster BC, Ter Maaten JC, Smidt N, Rooij SE. Hospital at Home care for older patients with cognitive impairment: a protocol for a randomised controlled feasibility trial. BMJ Open 2018;8(3):e020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Arsenault‐Lapierre 2021
- Arsenault-Lapierre G, Henein M, Gaid D, Le Berre M, Gore G, Vedel I. Hospital-at-home interventions vs in-hospital stay for patients with chronic disease who present to the emergency department: a systematic review and meta-analysis. JAMA Network Open 2021;4(6):e2111568. [DOI: 10.1001/jamanetworkopen.2021.11568] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aviv 2021
- Aviv R. Intensive home care for COVID-19 to reduce admissions. Home Health Care Management & Practice 2021;33(4):320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batt 2023
- Batt R, Appelbaum E. The new hospital at home movement: opportunity or threat for patient care? Public Policy & Aging Report 2023;33(2):63-9. [DOI: 10.1093/ppar/prad004] [DOI] [Google Scholar]
Board 2000
- Board N, Brennan N, Caplan G. A randomised controlled trial of the costs of hospital as compared with hospital at home for acute medical patients. Australian and New Zealand Journal of Public Health 2000;24(3):305-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
British Geriatrics Society 2022
- British Geriatrics Society. Bringing hospital care home: Virtual Wards and Hospital at Home for older people; August 2022. www.bgs.org.uk/virtualwards (accessed prior to 3 November 2023).
Bumrungrad 2023
- Bumrungrad International Hospital. Bumrungrad @ Home Service Center. www.bumrungrad.com/en/centers/home-service-center (accessed prior to 23 August 2023).
Chong 2022
- Chong C. Some patients can now be hospitalised at home and be cared for via teleconsultations, home visits; October 2022. www.straitstimes.com/singapore/some-patients-can-now-be-hospitalised-at-home-and-be-cared-for-via-teleconsultations-home-visits (accessed prior to 3 November 2023).
CNN Philippines 2021
- CNN Philippines. Healthcare firm offers home services for patients; April 2021. www.cnnphilippines.com/news/2021/4/23/Healthcare-firm-offers-home-services-for-patients.html.
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29. [PsycINFO: 1955-00087-001] [Google Scholar]
Covinsky 2003
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic DM, et al. Loss of independence in activities of daily living in older adults hospitalised with medical illness: increased vulnerability with age. Journal of the American Geriatrics Society 2003;51:51-8. [DOI] [PubMed] [Google Scholar]
de Sousa Vale 2020
- Sousa Vale J, Franco AI, Oliveira CV, Araújo I, Sousa D. Hospital at home: an overview of literature. Home Health Care Management & Practice 2020;32(2):118-23. [Google Scholar]
Deeks 2001
- Deeks JJ, Altman D, Bradburn MJ. Chapter 15. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd edition. Wiley, 2001:285-312. [DOI: 10.1002/9780470693926.ch15] [DOI] [Google Scholar]
Ellis 2017
- Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD006211. [DOI: 10.1002/14651858.CD006211.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote [Computer program]
- EndNote 20. Philadelphia, PA: Clarivate, 2013.
EPOC 2015a
- Effective Practice and Organisation of Care (EPOC). Oslo: Norwegian Knowledge Centre for the Health Services. Data collection form. EPOC Resources for review authors. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed September 2015).
EPOC 2015b
- Effective Practice and Organisation of Care (EPOC). Oslo: Norwegian Knowledge Centre for the Health Services. Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed September 2015).
EPOC 2017
- Effective Practice and Organisation of Care (EPOC). Oslo: Norwegian Knowledge Centre for the Health Services. EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed July 2022).
Gonçalves‐Bradley 2017
- Gonçalves-Bradley DC, Iliffe S, Doll HA, Broad J, Gladman J, Langhorne P, et al. Early discharge hospital at home. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD000356. [DOI: 10.1002/14651858.CD000356.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2022 [Computer program]
- GRADEpro GDT. Version accessed 29 December 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2022. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, the GRADE Working Group. What is ‘quality of evidence’ and why is it important to clinicians? BMJ 2008;336:995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2003
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. European Respiratory Journal 2003;21(1):58-67. [DOI: 10.1183/09031936.03.00015603] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Compston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Jeppesen 2012
- Jeppesen E, Bruberg KG, Vist GE, Wedzicha JA, Wright JJ, Greenstone M, et al. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD003573. [DOI: 10.1002/14651858.CD003573.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1999
- Jones J, Wilson A, Parker H, Wynn A, Jagger C, Spiers N, et al. Economic evaluation of hospital at home versus hospital care: cost minimisation analysis of data from randomised controlled trial. BMJ 1999;319(7224):1547-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karacaoglu 2021
- Karacaoglu K. Staff views of a hospital at home model implemented in a Scottish care setting. AIMS Public Health 2021;8(3):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimmel 2020
- Kimmel LA, Burge A, Watterson D, Wolters C, Holland A, Reed M, et al. Substituting inpatient rehabilitation beds for home‐based multidisciplinary rehabilitation: a qualitative study of patient perceptions. Australasian Journal on Ageing 2021;40(3):275-82. [DOI: 10.1111/ajag.12883] [DOI] [PubMed] [Google Scholar]
Kumpf 2019
- Kumpf VJ. Challenges and obstacles of long-term home parenteral nutrition. Nutrition in Clinical Practice 2019;34(2):196-203. [DOI: 10.1002/ncp.10258] [DOI] [PubMed] [Google Scholar]
Langhorne 2020
- Langhorne P, Ramachandra S. Organised inpatient (stroke unit) care for stroke: network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD000197. [DOI: 10.1002/14651858.CD000197.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langhorne 2021
- Langhorne P. The Stroke Unit Story: where have we been and where are we going? Cerebrovascular Diseases 2021;50(6):636-43. [DOI: 10.1159/000518934] [DOI] [PubMed] [Google Scholar]
Leary 2022
- Leary A. Saved bed days: the ultimate currency. BMJ 2022;378:o1686. [DOI: 10.1136/bmj.o1686] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Leff 2006
- Leff B, Burton L, Mader S, Naughton B, Burl J, Clark R, et al. Satisfaction with hospital at home care. Journal of the American Geriatrics Society 2006;54(9):1355-63. [DOI: 10.1111/j.1532-5415.2006.00855.x] [DOI] [PubMed] [Google Scholar]
Leff 2009
- Leff B. Defining and disseminating the hospital-at-home model. CMAJ: Canadian Medical Association Journal 2009;180(2):156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leff 2022
- Leff B, DeCherrie LV, Montalto M, Levine DM. A research agenda for hospital at home. Journal of the American Geriatrics Society 2022;70(4):1060-9. [DOI: 10.1111/jgs.17715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leong 2021
- Leong MQ, Lim CW, Lai YF. Comparison of Hospital-at-Home models: a systematic review of reviews. BMJ Open 2021;11(1):e043285. [DOI: 10.1136/bmjopen-2020-043285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2021
- Levine DM, Pian J, Mahendrakumar K, Patel A, Saenz A, Schnipper JL. Hospital-level care at home for acutely ill adults: a qualitative evaluation of a randomized controlled trial. Journal of General Internal Medicine 2021;36(7):1965-73. [DOI: 10.1007/s11606-020-06416-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loyd 2020
- Loyd C, Larkland AD, Zhang Y, Fowler M, Harper S, Wright NC, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. Journal of the American Medical Directors Association 2020;21(4):455-461.e5. [DOI: 10.1016/j.jamda.2019.09.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackay 2023
- Mackay I, France M, McAuley D, Wing S, Wheeldon M, Britton S, et al. COVID‐19 (Omicron strain) hospital admissions from a virtual ward – who required further care? Influenza and Other Respiratory Viruses 2023;17(3):e13108. [DOI: 10.1111/irv.13108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makela 2020
- Mäkelä P, Stott D, Godfrey M, Ellis G, Schiff R, Shepperd S. The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age and Ageing 2020;49(5):856-64. [DOI: 10.1093/ageing/afaa085] [DOI] [PMC free article] [PubMed] [Google Scholar]
MECIR 2012
- Standards for the reporting of new Cochrane Intervention Reviews. web.archive.org/web/20230328142021/https://community.cochrane.org/mecir-manual/standards-reporting-new-cochrane-intervention-reviews-r1-r109 (accessed 19 February 2016).
Mernin 2021
- Mernin A. 'Hospital at home’ technology to expand care capacity in India; November 2021. www.htworld.co.uk/news/hospital-at-home-india/#:~:text=The%20'hospital%20at%20home'%20system,deteriorating%20and%20high%2Drisk%20patients (accessed prior to 3 November 2023).
Monitor 2015
- Monitor: Making the health sector work for patients. Moving healthcare closer to home: Literature review of clinical impacts. www.gov.uk/government/uploads/system/uploads/attachment_data/file/459268/Moving_healthcare_closer_to_home_clinical_review.pdf (accesed 17 August 2015).
Montalto 2020
- Montalto M, McElduff P, Hardy K. Home ward bound: features of hospital in the home use by major Australian hospitals, 2011–2017. Medical Journal of Australia 2020;213(1):22-7. [DOI: 10.5694/mja2.50599] [DOI] [PubMed] [Google Scholar]
Mudge 2019
- Mudge AM, McRae P, Hubbard RE, Peel NM, Lim WK, Barnett AG, et al. Hospital‐associated complications of older people: a proposed multicomponent outcome for acute care. Journal of the American Geriatrics Society 2019;67(2):352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naik 2006
- Naik G. Portland hospital gives acutely ill a homecare option; 19 April 2006. www.wsj.com/articles/SB114540442717929396 (accessed prior to 3 November 2023).
NCT04330378
- NCT04330378. A hospital-at-home pilot in Singapore: a prospective quasi-experimental cohort study. clinicaltrials.gov/study/NCT04330378 (first received 31 March 2020).
NHS Digital 2019
- NHS Digital. Hospital admitted patient care and adult critical care activity. digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19#summary (accessed 22 February 2021).
NHS England 2021
- NHS England. Guidance note: Frailty virtual ward (Hospital at Home for those living with frailty); December 2021. www.england.nhs.uk/publication/guidance-note-frailty-virtual-ward-hospital-at-home-for-those-living-with-frailty/ (accessed prior to 3 November 2023).
NICE 2019
- National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management NICE guideline NG128; May 2019. www.nice.org.uk/guidance/ng128/chapter/recommendations#specialist-care-for-people-with-acute-stroke (accessed prior to 3 November 2023).
Nogues 2021
- Nogués X, Sánchez-Martinez F, Castells X, Díez-Pérez A, Sabaté RA, Petit I, et al. Hospital-at-Home expands hospital capacity during COVID-19 pandemic. Journal of the American Medical Directors Association 2021;22(5):939-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolte 2008
- Nolte E, McKee M. Caring for People With Chronic Conditions: A Health System Perspective. Maidenhead: McGraw Hill Open University Press, 2008. [Google Scholar]
Nundy 2020
- Nundy S, Patel KK. Hospital-at-home to support COVID-19 surge – time to bring down the walls? JAMA Health Forum 2020;1(5):e200504. [DOI: 10.1001/jamahealthforum.2020.0504] [DOI] [PubMed] [Google Scholar]
Oliver 2021
- Oliver D. David Oliver: the pandemic has delivered clinical service innovations worth keeping. BMJ 2021;373:n1306. [DOI: 10.1136/bmj.n1306] [DOI] [PubMed] [Google Scholar]
Patel 2004
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost effectiveness and cost utility analysis from a prospective randomised controlled trial. Stroke 2004;35(1):196-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qaddoura 2015
- Qaddoura A, Yazdan-Ashoori P, Kabali C, Thabane L, Haynes RB, Connolly SJ, et al. Efficacy of hospital at home in patients with heart failure: a systematic review and meta-analysis. PLOS One 2015;10(6):e0129282. [DOI: 10.1371/journal.pone.0129282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Queensland Government 2022
- Queensland Government. Hospital in the home. www.health.qld.gov.au/system-governance/policies-standards/guidelines/hospital-in-the-home (accessed prior to 3 November 2023).
Rafter 2015
- Rafter N, Hickey A, Condell S, Conroy R, O'Connor P, Vaughan D, et al. Adverse events in healthcare: learning from mistakes. QJM 2015;108(4):273-7. [DOI] [PubMed] [Google Scholar]
RCP 2017
- Royal College of Physicians. Delivering the future hospital: full report. www.rcplondon.ac.uk/projects/outputs/future-hospital-programme-delivering-future-hospital (accessed 22 February 2021).
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Shepperd 2016b
- Shepperd S, Gonçalves-Bradley DC, Wee B, Straus SE. Hospital at home: home-based end of life care. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD009231. [DOI: 10.1002/14651858.CD009231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2022
- Simon DA, Cohen IG, Balatbat C, Offodile AC 2nd. The hospital-at-home presents novel liabilities for physicians, hospitals, caregivers, and patients. Nature Medicine 2022;28(3):438-41. [DOI: 10.1038/s41591-022-01697-3] [DOI] [PubMed] [Google Scholar]
Singh 2022
- Singh S, Gray A, Shepperd S, Stott DJ, Ellis G, Hemsley A, et al. Is comprehensive geriatric assessment hospital at home a cost-effective alternative to hospital admission for older people? Age and Ageing 2022;51(1):afab220. [DOI: 10.1093/ageing/afab220] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata 16.1 [Computer program]
- Stata Statistical Software: Release 16.. StataCorp. College Station, TX: StataCorp LLC., 2019.
Steventon 2018
- Steventon A, Deeny S, Friebel R, Gardner T, Thorlby R. Emergency hospital admissions in England: Which may be avoidable and how? www.health.org.uk/publications/emergency-hospital-admissions-in-england-which-may-be-avoidable-and-how (accessed 22 February 2021).
Thornton 2020
- Thornton J. The 'virtual wards' supporting patients with covid-19 in the community. BMJ 2020;369:m2119. [DOI: 10.1136/bmj.m2119] [DOI] [PubMed] [Google Scholar]
Vindrola‐Padros 2021
- Vindrola-Padros C, Singh KE, Sidhu MS, Georghiou T, Sherlaw-Johnson C, Tomini SM, et al. Remote home monitoring (virtual wards) for confirmed or suspected COVID-19 patients: a rapid systematic review. EClinicalMedicine 2021;37:100965. [DOI: 10.1016/j.eclinm.2021.100965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Virtual Wards 2022
- NHS England. Bringing hospital care home: Virtual Wards and Hospital at Home for older people. www.england.nhs.uk/virtual-wards/ (accessed 2 December 2022).
Vlachantoni 2012
- Vlachantoni A. Financial inequality and gender in older people. Maturitas 2012;72(2):104-7. [DOI: 10.1016/j.maturitas.2012.02.015] [DOI] [PubMed] [Google Scholar]
Wallis 2024
- Wallis JA, Shepperd S, Makela P, Han JX, Tripp EM, Gearon E, et al. Factors influencing the implementation of early discharge hospital at home and admission avoidance hospital at home: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2024, Issue 3. Art. No: CD014765. [DOI: 10.1002/14651858.CD014765.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2021 to 2030
- World Health Organization. WHO's work on the UN Decade of Healthy Ageing (2021-2030). www.who.int/initiatives/decade-of-healthy-ageing (accessed 3 May 2023).
Young 2005
- Young H, Grundy E, Kalogirou S. Who cares? Geographic variation in unpaid caregiving in England and Wales: evidence from the 2001 census. Population Trends 2005;120:23-34. [PubMed] [Google Scholar]
References to other published versions of this review
Shepperd 1998
- Shepperd S, Iliffe S. Effectiveness of hospital at home compared to in-patient hospital care. Cochrane Database of Systematic Reviews 1998, Issue 1. Art. No: CD000356. [DOI: 10.1002/14651858.CD000356] [DOI] [Google Scholar]
Shepperd 2005
- Shepperd S, Iliffe S. Hospital at home versus in-patient hospital care. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD000356. [DOI: 10.1002/14651858.CD000356.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shepperd 2008
- Shepperd S, Doll H, Angus RM, Clarke MJ, Illife S, Kalra L, et al. Hospital at home admission avoidance. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD007491. [DOI: 10.1002/14651858.CD007491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 2016a
- Shepperd S, Iliffe S, Doll HA, Clarke MJ, Karla L, Wilson AD, et al. Admission avoidance hospital at home. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD007491. [DOI: 10.1002/14651858.CD007491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


