Abstract
Diet, serving as a vital source of nutrients, exerts a profound influence on human health and disease progression. Recently, dietary interventions have emerged as promising adjunctive treatment strategies not only for cancer but also for neurodegenerative diseases, autoimmune diseases, cardiovascular diseases, and metabolic disorders. These interventions have demonstrated substantial potential in modulating metabolism, disease trajectory, and therapeutic responses. Metabolic reprogramming is a hallmark of malignant progression, and a deeper understanding of this phenomenon in tumors and its effects on immune regulation is a significant challenge that impedes cancer eradication. Dietary intake, as a key environmental factor, can influence tumor metabolism. Emerging evidence indicates that dietary interventions might affect the nutrient availability in tumors, thereby increasing the efficacy of cancer treatments. However, the intricate interplay between dietary interventions and the pathogenesis of cancer and other diseases is complex. Despite encouraging results, the mechanisms underlying diet-based therapeutic strategies remain largely unexplored, often resulting in underutilization in disease management. In this review, we aim to illuminate the potential effects of various dietary interventions, including calorie restriction, fasting-mimicking diet, ketogenic diet, protein restriction diet, high-salt diet, high-fat diet, and high-fiber diet, on cancer and the aforementioned diseases. We explore the multifaceted impacts of these dietary interventions, encompassing their immunomodulatory effects, other biological impacts, and underlying molecular mechanisms. This review offers valuable insights into the potential application of these dietary interventions as adjunctive therapies in disease management.
Subject terms: Cancer metabolism, Immunotherapy, Cancer imaging
Introduction
Nutrients play a crucial role in regulating various physiological processes.1 The main source of nutrients is usually considered to be diet. The quantity, quality, and composition of the food consumed, as well as the timing of meals, directly impact human health by influencing the availability of nutrients.2 Although there have been advancements in understanding the link between diet and disease in recent years, there is still much to learn about how specific dietary components affect disease risk and prevention.3
Epidemiological studies have linked various dietary patterns to cancer and other diseases.4 For instance, diets high in saturated fats and sugars have been associated with an increased risk of cardiovascular diseases (CVD) and type 2 diabetes.5 Conversely, diets rich in fiber, fruits, and vegetables are associated with a lower risk of these conditions.6 Similarly, conditions such as osteoporosis and certain neurological disorders have also shown links to dietary patterns, highlighting the broad influence of diet on overall health.7,8 In the context of cancer, increased consumption of alcohol and red or processed meat is associated with a heightened risk of cancer, whereas adherence to a Mediterranean dietary pattern—characterized by high intake of fruits, vegetables, whole grains, legumes, fish, and olive oil, along with moderate consumption of dairy products such as yogurt—may confer protective effects against carcinogenesis.9,10 Similarly, a strong adherence to the plant-based Paleolithic diet and a Paleolithic-like lifestyle has been found to significantly reduce the risk of colorectal cancer (CRC), especially in individuals with a body mass index (BMI) less than 30.11 Although many cancer patients are interested in using dietary intervention to improve cancer therapy outcomes or even using it as a key component of the therapeutic process,12 there is currently no solid evidence showing that any nutrition-related regimen can be a primary treatment for cancer.13 However, preclinical studies suggest that calorie and energy restrictions can hinder tumor growth and progression and increase the efficacy of chemotherapy and radiotherapy.14,15 A rising number of clinical trials are exploring the impact of dietary interventions or nutritional supplements in conjunction with standard antitumor therapies, with some showing clinical benefits.16,17
Diet is a crucial source of nutrients for tumors and has emerged as a key component in determining whole-body metabolism.18 The nutrients in the tumor microenvironment (TME) largely regulate tumor cell and immune cell metabolism.19 Recent evidence suggests that metabolic reprogramming, a crucial hallmark of cancer, involves several metabolic adaptations by tumor cells to sustain proliferation and metastasis in the TME.19–21 The TME constitutes a multifaceted and dynamic ecosystem comprising an assortment of cell types, including tumor cells, immune cells, and stromal cells, in addition to components of the extracellular matrix. The interplay among these constituents, along with the challenging environmental conditions, exerts a significant influence on the growth trajectory and progression of tumors.22 For example, oxygen levels within the TME can vary due to increased metabolic demand from rapidly proliferating tumor cells, resulting in low oxygen tension, known as hypoxia, in tissues. In addition, nutrient availability, including the availability of glucose, fatty acids, and amino acids, can vary within the TME, impacting metabolic processes and energy production. The accumulation of metabolic waste products and alterations in pH can further contribute to a hostile TME, which can impair immune function and promote tumor progression.23 These factors, along with dynamic interactions within the TME, play crucial roles in influencing tumor proliferation and the effectiveness of antitumor immune responses.24
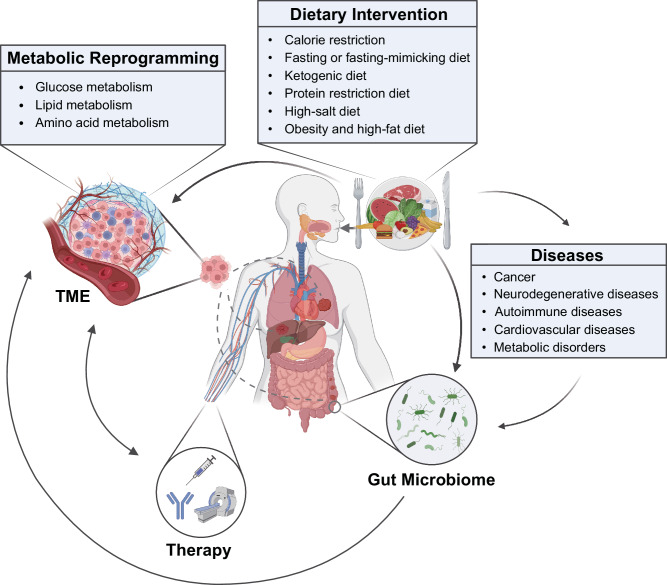
As our understanding of the complex relationships between diet, metabolic reprogramming, and various diseases continues to evolve, it becomes increasingly evident that dietary components and patterns significantly influence disease risk, prevention, and progression. This review delves into the unique metabolic characteristics and nutrient availability of tumors. Furthermore, we investigate recent evidence and emerging trends concerning the effects of dietary interventions on both cancer and other diseases, underscoring the potential therapeutic benefits these dietary strategies may offer to a wide range of patients (Fig. 1).
Fig. 1.
Overview of the relationship between dietary interventions and diseases. The cellular microenvironment, including the tumor microenvironment (TME), plays a crucial role in disease biology, and diet serves as a vital source of nutrients that can influence these microenvironments. Metabolic reprogramming, a prominent feature associated with disease progression, can affect cell metabolism and immune function. Dietary interventions, such as caloric restriction (CR), fasting-mimicking diet (FMD), and ketogenic diet (KD), can modulate the progression and treatment sensitivity of various diseases, including cancer. Additionally, dietary interventions can alter the composition and functional capacity of the gut microbiome, thereby indirectly influencing the progression and treatment of diseases. These direct and indirect effects of dietary interventions can influence metabolic reprogramming, modulate immune responses, and potentially enhance the clinical efficacy of treatments for various diseases. This figure was created with BioRender.com
Metabolic characteristics and nutrient availability in the tumor
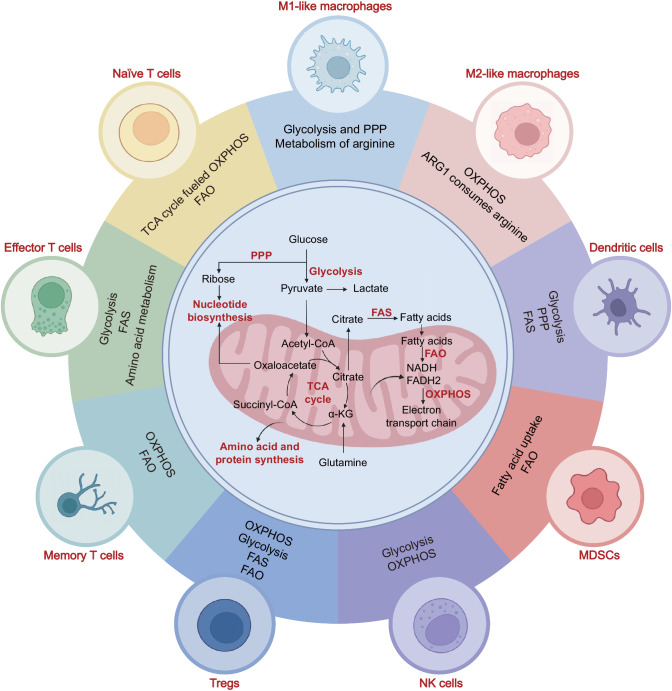
Cellular metabolism encompasses a complex array of biochemical reactions that utilize specific nutrients, including carbohydrates, fatty acids, and amino acids. These nutrients are the primary sources for maintaining energy homeostasis and synthesizing macromolecules.25 Our focus here is on cancer metabolism, which differs from that in corresponding healthy tissues in terms of nutrient levels and metabolic demands.26 Within the TME, cancer cells can establish an immunosuppressive metabolic microenvironment by depriving immune cells of vital metabolites such as glucose and oxygen while also elevating the levels of mediators such as lactate and adenosine that limit the function of immune cells.27 Therefore, different subsets of immune cells undergo metabolic reprogramming in tumors, and specific nutrients are required for these metabolic programs.28,29 Generally, the metabolic programs that play vital roles in immune cells include glycolysis, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS), the pentose phosphate pathway (PPP), fatty acid oxidation (FAO), fatty acid synthesis (FAS) and the amino acid metabolic pathway30 (Fig. 2).
Fig. 2.
Major metabolic pathways associated with different immune cell subtypes within the tumor microenvironment (TME). Summary of the main metabolic pathways of immune cells, highlighting the distinctive metabolic characteristics and requirements of different subsets of immune cells. This figure was created with BioRender.com
Glucose metabolism
Glucose serves as a vital energy source, facilitating the functioning of immune cells. Once transported across the plasma membrane, glucose is metabolically processed via three distinct pathways: glycolysis, the PPP, and the TCA cycle. Glycolysis, which occurs in the cytosol, transforms glucose into pyruvate and lactate, simultaneously generating adenosine triphosphate (ATP). Under aerobic conditions, pyruvate is channeled into the TCA cycle, where OXPHOS occurs, yielding additional ATP. Moreover, glucose-6-phosphate, a derivative of glycolysis, fuels the PPP, culminating in the production of ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH). Recent research has indicated a marked disparity in energy consumption between immune cells in resting and activated states.18 Although glycolysis does not generate as many ATP molecules as OXPHOS, glycolysis produces ATP more rapidly, which is important to metabolically active immune cells.
Cancer cells are characterized by their rapid proliferation, primarily fueled by the consumption of glucose as an energy source. Intriguingly, these cells continue to rely on glycolysis for energy production even in the presence of ample oxygen, a phenomenon referred to as the “Warburg effect”.31 This unique phenomenon leads to glucose depletion and lactic acid (LA) accumulation in the microenvironment, ultimately inhibiting antitumor responses.32 High glycolytic rates in triple-negative breast cancer cells promote the infiltration of myeloid-derived suppressor cells (MDSCs) and suppress T-cell function, while suppressing glycolysis inhibits tumor colony-stimulating factor (CSF) expression and MDSC development.33 Cancer cells produce LA through glycolysis, which reduces the antitumor activity of CD8+ T cells and natural killer (NK) cells. However, the activation of LA metabolism pathways in regulatory T cells (Tregs) is increased, and these cells adapt to high-LA conditions.34,35 Furthermore, cancer cells can take advantage of immune cells by utilizing their metabolic byproducts. LA can shift tumor-associated macrophages (TAMs) from a proinflammatory (M1-like) to an anti-inflammatory (M2-like) phenotype in the TME. Notably, lactate-activated TAMs enhance cancer cell adhesion, migration, invasion in vitro, and promote metastasis in vivo.36
T cells play crucial roles in the TME. Upon activation, these cells undergo metabolic reprogramming, which subsequently yields diverse functional outcomes. Naïve T cells, which are metabolically quiescent, exhibit basic nutrient intake rates and low glycolysis rates. They primarily generate ATP through TCA cycle-fueled OXPHOS.37 The activation of specific membrane receptors triggers the differentiation of naïve T cells into effector T cells, also known as Teff cells. This process is accompanied by a pronounced increase in both energy demand and biosynthetic activity within Teff cells. In Teff cells, the metabolic state is changed to increasingly rely on glycolysis, as these cells upregulate GLUT1, increase glucose intake.38–41 Simultaneously, this metabolic alteration benefits Teff cells by reducing their reliance on oxygen for energy production, which enables them to maintain cytokine production and cytolytic activity even when they migrate into microenvironments within solid tumors that have low oxygen levels.42 In contrast to naïve and Teff cells, memory T cells undergo a metabolic rewiring process that leads them to enter a quiescent state characterized by elevated OXPHOS rates compared to the glycolysis rate.43 Tregs, known for their suppressive function, exhibit decreased glycolysis rates and primarily rely on OXPHOS to support their function, while glycolysis is crucial for their migration.44 It has been reported that the Treg-specific transcription factor FOXP3 reprograms Treg metabolism by suppressing Myc expression and glycolysis while promoting OXPHOS and NAD(H) oxidation. This adaptation enables Tregs to be more adaptable to low-glucose and/or lactate-rich microenvironments.45
There are several other types of cells within the TME that exhibit distinct metabolic functions. In the case of NK cells, glycolysis and OXPHOS play important roles in maintaining their cytotoxicity, as indicated by the inhibition of these processes leading to diminished expression of IFNγ and Fas ligands.46 Researchers have shown that transcription factor-controlled glucose metabolism, specifically by sterol regulatory element-binding proteins (SREBPs), which conventionally control lipid synthesis, is essential for metabolic reprogramming in activated NK cells.47 Dendritic cells (DCs), on the other hand, rely on glycolysis and the PPP for energy production to sustain their function, including cytokine production, antigen processing and presentation, and the stimulation of T cells.48 Furthermore, different subsets of macrophages present distinct metabolic functions. M1-like macrophages predominantly utilize anabolic metabolism, specifically glycolysis and the PPP, to generate energy and synthesize cellular components, whereas M2-like macrophages are more reliant on OXPHOS, particularly through the enhancement of FAO.49
Lipid metabolism
Lipids, such as fatty acids, triglycerides, cholesterol, phospholipids, and sphingolipids, play crucial roles as precursors to many important biological molecules.50 Lipids, including substances such as cholesterol and fatty acids that are widely distributed in organelles, are key components of internal cellular membranes. Moreover, lipids are essential biological molecules that provide energy during nutrient deficiency, participate in the synthesis of complex fat-containing substances, and aid in cellular signal transmission as second messengers.51 Lipids within the microenvironment profoundly influence the proliferation of cancer cells and regulate the functional activity of immune cells.
Cancer cells undergo metabolic reprogramming of lipids in the tumor niche. The activation of adipocytes triggers the lipolysis of stored triglycerides and secretion of fatty acids. Cancer cells can then take up these fatty acids to fulfill their lipid requirements for rapid growth.52 Research has also demonstrated that ovarian cancer cells stimulate membrane cholesterol efflux from TAMs, fostering an environment that promotes tumor growth by enhancing interleukin (IL)-4-mediated reprogramming and suppressing IFNγ-induced gene expression. The deletion of ABC transporters, responsible for cholesterol efflux, reversed the tumor-promoting functions of TAMs, leading to reduced tumor progression.53
Furthermore, elevated cholesterol levels in the microenvironment stimulate the expression of immune checkpoints, including PD-1, 2B4, TIM-3, and LAG-3, in T cells, driving T-cell exhaustion via the activation of the endoplasmic reticulum stress response.54 In contrast to the negative effects of reprogramming T-cell lipid metabolism on antitumor immunity, the inhibition of ACAT1, a pivotal enzyme responsible for cholesterol esterification in CD8+ T cells, results in elevated cholesterol levels in the plasma membrane. This increase subsequently amplifies TCR signaling and promotes antitumor activity. These findings highlight the complex mechanisms through which cholesterol regulates T-cell function.55
For efficient tumor antigen processing and presentation to T cells, activated DCs need high rates of cell surface or secretory protein biosynthesis, which is partly regulated by FAS-induced increases in cytokine production.56 Teff cells depend mainly on FAS to support inflammatory cytokine secretion and proliferation, while naïve T cells and memory T cells maintain their basic functions by increasing the FAO rate.57–59 Although Teff cells rely mainly on glycolysis for energy, CD8+ T cells that undergo enhanced FAO exhibit stable antitumor functions even under conditions of low glucose and oxygen levels. By promoting fatty acid catabolism, CD8+ T cells exhibit increased functionality, and the efficacy of immunotherapy in patients with melanoma can thus increase.60
While these studies indicate a positive influence of lipids on the functionality and metabolism of CD8+ T cells in the TME, it is important to note that alterations to T-cell lipid metabolism might attenuate their antitumoral effects. In obesity-related breast cancer murine models, the activation of STAT3 triggered an increase in FAO in CD8+ T cells, which suppressed glycolysis and weakened their tumor-suppressing ability.61 Moreover, enhanced lipid uptake and peroxidation can result in high oxidative stress, which leads to CD8+ T cell dysfunction. CD36, a fatty acid scavenger receptor, facilitates the incorporation of arachidonic acid into CD8+ T cells. This process subsequently triggers lipid peroxidation and ferroptosis, events that cumulatively attenuate the antitumor immune response and reduce the efficacy of immunotherapy.62–64
Lipid metabolism also plays an active role in regulating Treg function. Fatty acid synthase (FASN)-mediated FAS contributes to the proliferation and maturation of Tregs, and FAO provides the energy crucial for Treg infiltration into the TME.65 Research has shown that OX40 plays a role in modifying the lipid composition of Tregs, leading to the proliferation of OX40+ Tregs in the TME. This effect is achieved through increased FAS expression and glycolysis rate in Tregs.66 CD36, via the peroxisome proliferator-activated receptor-β (PPAR) signaling pathway, maintains the mitochondrial fitness of Tregs, promoting Treg viability and inhibitory functions.67 SREBPs have been found to show increased activity in Tregs that infiltrate tumors. Inhibiting FAS and metabolic signaling by targeting SREBPs has been shown to effectively activate the antitumor immune response without causing autoimmune toxicity. When the SREBP-SCAP axis was inhibited, in addition to tumor growth attenuation, immunotherapy effectiveness was boosted. These findings suggest that SREBPs may be promising targets for cancer therapy.68
High expression of FASN in TAMs promotes the accumulation of fatty acids, leading to enhanced tumor immune tolerance via the FAO pathway.69 Notably, lipid metabolism differs between M1-like and M2-like macrophages. M1-like macrophages prevalently engage the FAS pathway, while M2-like macrophages predominantly utilize the mitochondrial FAO pathway for their bioenergetic demands.70,71 Receptor-interacting protein kinase 3 (RIPK3), which is crucial for necroptosis, is found to be diminished in hepatocellular carcinoma (HCC)-associated macrophages, leading to inhibited caspase1-mediated cleavage of PPAR, a process vital for enhancing fatty acid metabolism, including FAO. This metabolic shift results in increased accumulation and polarization of M2-like macrophages in the TME, contributing to accelerated HCC growth.72
MDSCs also exert a substantial influence in suppressing antitumor immunity in the microenvironment, and they can be categorized into monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (PMN-MDSCs).73 Tumor-infiltrating MDSCs increase fatty acid uptake and induce FAO.74 The accumulation of lipids in MDSCs increases oxidative metabolism, resulting in MDSC acquisition of an immunosuppressive and anti-inflammatory phenotype.75
Amino acid metabolism
Amino acids are the primary substrates for protein biosynthesis, and recent evidence emphasizes the critical role of amino acid availability and metabolism in the regulation of antitumor immunity.
Glutamine is the most abundant amino acid and a crucial energy substrate, as well as an important nitrogen and carbon donor for various biosynthetic precursors.76 Teff cells require higher levels of glutamine than naïve T cells due to their rapid proliferation and demand for sufficient raw materials for macromolecule synthesis and cytokine secretion.77 Cancer cells have been shown to exhibit the highest glutamine uptake capacity and consume most of the glutamine in the microenvironment.76 In turn, elevated glutamine consumption by cancer cells diminishes the glutamine supply necessary for T cells, consequently impeding the antitumor immune response.78 In the microenvironment, cancer cells consume glutamine to synthesize γ-aminobutyric acid (GABA) via glutamate decarboxylase 1 (GAD1). By activating the GABAB receptor, GABA inhibits GSK-3β activity, which enhances β-catenin signaling, promoting cancer cell proliferation while suppressing intratumoral infiltration of CD8+ T cells.79 Furthermore, elimination of glutaminase, a vital enzyme for glutamine metabolism, within tumor cells stimulates T-cell activation and augments the efficacy of antitumor immune responses. The compound V-9302, an inhibitor of the glutamine transporter, selectively impedes glutamine uptake in cancer cells while simultaneously enhancing both glutamine assimilation and glutathione synthesis in Teff cells, ultimately enhancing their function.80
Tryptophan is another essential amino acid. Following its entry into eukaryotic cells via the transport proteins SLC1A5 or SLC7A5, tryptophan is primarily subjected to three primary metabolic pathways: incorporation into protein synthesis, metabolism via the kynurenine (Kyn) pathway, or conversion through the serotonin pathway.81 Notably, a substantial fraction of tryptophan is directed through the Kyn pathway, culminating in the production of a suite of metabolites with significant physiological implications.82 Tryptophan plays a crucial role in determining the strength and effectiveness of the T cell response by affecting its availability in the microenvironment.83 However, within the tumor niche, cancer cells, MDSCs, TAMs, suppressive DCs, and cancer-associated fibroblasts, among other cell types, exhibit upregulated expression of indoleamine 2,3-dioxygenase (IDO), which metabolizes tryptophan into suppressive kynurenine to promote Tregs and suppress CD8+ T cell function.84–86 Most cancer cells overexpress IDO, and the level of kynurenine in the microenvironment is associated with poor prognosis in multiple solid and hematological malignancies.87 Kynurenine has been found to bind to the aryl hydrocarbon receptor (AHR) in naïve CD4+ T cells, which promotes Treg differentiation.87
An additional metabolite generated through the Kyn pathway is the essential redox cofactor nicotinamide adenine dinucleotide (NAD+), a molecule of fundamental importance for the maintenance of cellular homeostasis.88 In particular, cancer cells heavily depend on NAD+ to promote metabolic reprogramming and meet higher demands for ATP. Elevated NAD+ levels have been demonstrated to promote the proliferation of cancer cells.89 Although the majority of studies suggest that an increase in NAD+ drives cellular proliferation, prior investigations have proposed that a decrease in NAD+ levels can lead to genomic instability, subsequently instigating liver tumorigenesis.90 Moreover, tryptophan metabolism mediated by IDO affects not only the Kyn pathway but also other pathways, such as the purine, nicotinamide, and pyrimidine metabolism pathways, ultimately leading to decreased T-cell function.91 In addition to IDO, another enzyme, tryptophan 2,3-dioxygenase (TDO), is involved in tryptophan catabolism. High TDO expression has been shown to impair T-cell antitumor immunity and to be correlated with poor clinical prognosis. Suppressing TDO expression can increase the antitumor efficacy of immune checkpoint inhibitors (ICIs).92
In addition to the aforementioned amino acids, other amino acids play crucial roles in regulating tumor metabolism. T-cell proliferation relies heavily on arginine consumption. L-arginine supplementation has been shown to facilitate the metabolic shift from glycolysis to OXPHOS, enhancing T-cell survival and boosting antitumor responses of CD8+ tumor infiltrating lymphocytes (TILs).93 Notably, the functional differences resulting from TAM polarization partially depend on arginine metabolism. In macrophages with the M1-like phenotype, arginine is converted into nitric oxide (NO) and citrulline via inducible nitric oxide synthase (iNOS), and this anabolic pathway is closely associated with macrophage cytotoxicity and antitumor effects. Conversely, in macrophages with the M2-like phenotype, arginine is hydrolyzed to yield ornithine and urea through arginase 1 (Arg1).94 This metabolic shift affects arginine availability, which in turn impacts the activation and proliferation of T cells and NK cells, leading to immune suppression within the microenvironment. Notably, Arg1 expression in MDSCs contributes to arginine depletion in the microenvironment, further inhibiting T-cell antitumor function and reducing their survival.95,96 In addition, depletion of cystine and cysteine is also linked to the immunosuppressive effect of MDSCs. T cells are unable to synthesize the essential amino acid cysteine from substances such as cystine or methionine, necessitating its import from external sources for their functionality.97 MDSCs import cystine but do not release cysteine, thus the levels of cysteine in the microenvironment are regulated, inhibiting T-cell activation.98 Asparagine is another amino acid that significantly boosts CD8+ T-cell activation and antitumor responses. Restricting dietary asparagine or inhibiting its uptake impaired T-cell activation and differentiation into memory-like cells.99 Cancer cells consume higher levels of methionine due to increased expression of its transporter (SLC43A2), which inhibits methionine metabolism and function in CD8+ T cells by altering histone methylation patterns.100
Organ-specific metabolic profiles
Understanding the metabolic differences between various organs is critical for developing targeted therapeutic strategies in cancer treatment. Each organ has unique metabolic demands and pathways that can be dysregulated in cancer, leading to distinct metabolic profiles for different types of tumors.101 This organ-specific metabolic reprogramming plays a key role in cancer progression and survival, and its understanding could be leveraged for therapeutic benefits.
Consider primary brain tumors as an example. These tumors, often found nestled within the intricate neural networks of the brain, exhibit a remarkable metabolic flexibility.102 They are known to express elevated levels or alternative isoforms of glycolytic enzymes, a trait that points towards a potential therapeutic opportunity.103 Specifically, the therapeutic strategy of glucose deprivation could selectively starve brain tumor cells while sparing healthy neurons, which are capable of surviving on alternative fuels such as ketone bodies.104 Similarly, HCC cells undergo a significant metabolic shift from glucose production (a state known as gluconeogenesis) to glucose usage.105 HCC cells also exhibit a marked increase in amino acid metabolism, particularly in the metabolism of glutamine.106 Additionally, studies have shown that HCC cells often exhibit abnormal lipid accumulation, increased FAS, and enhanced cholesterol metabolism. These changes contribute to the aggressive and metastatic behaviors of HCC.107
Moreover, hormone-sensitive tissues such as the breast, endometrium, and prostate also exhibit significant metabolic fluctuations in response to hormone levels.101 Hyperactivation of the PI3K pathway, a lipid kinase that promotes proliferation and nutrient uptake in response to growth signals, has been implicated in breast and endometrial cancers, providing a possible mechanism for hormonal therapy evasion.107 This pathway could be a potential target for therapeutic interventions, particularly in hormone therapy-resistant cancers.
In summary, understanding organ-specific metabolic profiles and their dysregulation in cancer can open up new avenues for targeted cancer therapy. By exploiting these unique metabolic dependencies of tumors, more effective and personalized treatment strategies can be developed.
Targeted dietary interventions and mechanistic insights into their impact on cancer
Understanding the metabolic pathways of glucose, lipids, and amino acids lays a crucial foundation for exploring the effects of various dietary restrictions. Macronutrients, including carbohydrates, fats, and proteins, are the primary sources of energy for our bodies, and they each follow distinct metabolic pathways. By manipulating the relative intake of these macronutrients, we can influence the metabolic pathways they utilize and thereby exert control over our systemic metabolism. This concept forms the basis for various dietary restrictions and special diets, such as caloric restriction (CR), fasting or fasting-mimicking diet (FMD), ketogenic diet (KD), high-fat diet (HFD), or amino acid-defined diet. Moreover, high-salt diet (HSD), although not directly involving macronutrients, is noteworthy due to its potential impact on tumor biology. Therefore, an in-depth discussion on the role of HSD in cancer research and treatment is included in our exploration.
The connections between various dietary patterns and cancer risk are likely rooted in several biological mechanisms, such as inflammation and immune function; specific factors, such as the gut microbiota and their metabolites; unfavorable events, such as certain epigenetic changes and metabolic or hormonal disruptions; and stress, such as oxidative stress.108 Alterations in dietary composition impact not only the availability of nutrients within tumor cells but also the surrounding microenvironment, thereby offering potential opportunities to impede tumor growth109 (Table 1).
Table 1.
Preclinical studies supporting dietary interventions in cancer
| Dietary intervention | Cancer type | Results | References |
|---|---|---|---|
| Calorie restriction | Breast cancer |
Ly6C+-expressing memory T cells (CD4+Ly6C+)↑, Ly6C+CD8+ cells↑, CD103+CD4+ cells↑, CD103+CD8+ cells↑ FOXP3+CD8+ Tregs↓, MDSCs↓, PMN-MDSC↓, TAMs↓ |
118 |
|
(1) ER (energy restriction) vs. AL: the expression of Aicda, Pdcd1, Ifng, Foxp3, and Ido1↓ (2) PA (physical activity) + ER vs. SED (sedentary)+ AL: (i) For tumors at equal size: CD8+ T cells↑, CD4+ T cells↑, CD8/total MDSC ratio↑, MSDCs↓, M-MSDCs↓ (ii) For tumors at day 35 post-tumor implantation: CD8+ T cells↑, CD8/total MDSC ratio↑, MSDCs↓, M-MSDCs↓, PMN-MSDCs↓ |
124 | ||
| Colorectal cancer | CD8+ T cells↑, IFNγ+CD8+ T cells↑ | 290 | |
| Fasting-mimicking diet | Breast cancer |
CTLA-4+ Tregs↑, PD-1+ Tregs↑ Myeloid cells↓, M2-like macrophages↓, PMN-MDSCs↓, M-MDSCs↓, PD-L1+ PMN-MDSCs↓, PD-L1+ M-MDSCs↓ |
236 |
| Alternate day fasting | Colorectal cancer | TAMs↓, M2-like macrophages↓ | 137 |
| Ketogenic diet | Colorectal cancer |
Macrophages↑, M2 to M1 TAM polarization↑ Lactate↓ |
151 |
| Hopx activation↑, colonic crypt cell proliferation↓, tumor growth↓ | 147 | ||
| Non-small cell lung cancer | Per↑, AMPK activation↑, SIRT1↑, tumor cell apoptosis↑, tumor cell growth↓ | 148 | |
| Neuroendocrine cancer | PI3K-Akt-mTOR signaling↓, tumor growth↓ | 149 | |
| Glioma |
CD4+ T cells↑, CD4+ T cells/Tregs ratio↑, IFNγ+CD8+ T cells↑, TNF+CD8+ T cells↑, IL-2+CD8+ T cells↑, cytotoxic capability of CD8+ T cells↑, IFNγ+ NK cells↑, TNF+ NK cells↑ PD-1+CD8+ T cells↓, CTLA-4+CD8+ T cells↓, IL-10+ Tregs↓ |
152 | |
|
Colorectal cancer Adrenocortical cancer |
Tumor cell ferroptosis↑, cachexia onset↑, overall survival↓ | 156 | |
| Dietary restriction of protein/80% methionine-restricted diet | Prostate cancer |
M1-like macrophages↑, M1-like macrophages linked proteins (CXCL11/I-TAC, IL-1α, IL-1β, IL-12p40, M-CSF, and IL-17A) ↑, CD8+ T cells↑, granzyme B+CD8+ T cells↑ M2-like macrophages↓, PMN-MDSCs↓, M-MDSCs↓, M2-like macrophages linked proteins (C-reactive protein, FGF acidic, IL-33, leptin, and MMP9) ↓ |
163 |
| Dietary methionine restriction | Colorectal cancer |
CD8+ T cells↑, GZMB+CD8+ T cells↑, IFNγ+CD8+ T cells↑ L-cystathionine (LCYH)↓, SAM↓, 5’-methylthioadenosine (MTA)↓, S-adenosylhomocysteine (SAH)↓, glutathione (GSH)↓, L-methionine (Met)↓, homoserine↓ |
162 |
| Dietary serine and glycine restriction |
Intestinal cancer Lymphoma |
Anti-oxidant response↑ | 161 |
| Low-protein diet | Breast cancer | mTORC1 signaling↓, TFEB↑, TFE3↑, mTORC1↑, tumor-associated macrophages↑ | 164 |
|
Breast cancer Melanoma |
IGF-1↓ | 157 | |
| Low-protein isocaloric diet | Melanoma | NK cells↑, CD3+ cells↑, CD8+ cells↑ | 165 |
| High-protein diet | Bladder cancer | Urinary urea↑, intracellular deposition of ammonia↑, tumor growth↓ | 166 |
| Overactivation of CRP, MCPT2, MCPT9, EPXH2, SERPING1, SRGN, CDKN1C, CDK6, CCNB1, PCNA, BAX, MAGEB16, SERPINE1, HSPA2, and FOS | 167 | ||
| High-salt diet |
Melanoma Lung cancer |
The expression of Tnfα, Ifnγ, and Nos2↑ Suppressive function of MDSCs↓ |
184 |
|
Breast cancer Melanoma |
IL-12p40↑, ICAM-1↑, IFNγ↑, TNFα↑, macrophages↑, M-MDSCs differentiation into antitumor macrophages↑, functions of PMN-MDSCs switch from immunosuppressive to proinflammatory and antitumor↑, CD4+ T cells↑, CD8+ T cells↑, IFNγ+CD4+ T cells↑, IFNγ+CD8+ T cells↑, Th17 cells↑, TNFα+ Th17 cells↑ IL-6↓, IL-10↓, GM-CSF↓, MDSCs↓, M-MDSCs↓, Tregs↓ |
185 | |
| Melanoma |
NK cells↑, CD107a+ NK cells↑, IFNγ+ NK cells↑, Bifidobacterium↑ PD-1+ NK cells↓, CTLA-4↓, PD-1↓ |
183 | |
| Breast cancer | Hyperosmotic stress↑, lung metastasis↑ | 187 | |
| Th17 cells↑, the expression of Il17f, Il21, Il22 and Rorγt↑ | 190 | ||
| γENaC mediated chronic inflammatory response↑, RNS/ROS↑, IL-6↑, TNFα↑, tumor growth↑ | 191 | ||
| High-fat diet | Nonalcoholic steatohepatitis and hepatocarcinogenesis |
Hepatic unconventional prefoldin RPB5 interactor (URI) ↑, Th17↑, IL-17A↑ Neutrophil infiltration into white adipose tissue, causing insulin resistance and release of fatty acids |
199 |
| Helicobacter-induced chronic gastric inflammation and gastric carcinogenesis | Immature myeloid cells↑, CD4+ T Cells↑, IL-17A↑, granulocyte macrophage colony-stimulating factor↑, phosphorylated STAT3↑ | 200 | |
| Colorectal cancer |
Myeloid cells↑, MDSCs↑, TAMs↑, IL-2+CD8+ T cells↑, triglyceride↑, diglyceride↑ CD8+ T cells↓, leukocyte/tumor cell ratio↓, CD8+ T cells/tumor cell ratio↓, Ki67+CD8+ T cells↓, CD8+/Treg ratio↓, ICOS+CD8+ T cells↓, PD-1+CD8+ T cells↓, GZMB+CD8+ T cells↓, fatty acid↓ |
207 | |
|
PD-1highCD4+ T cells↑, PD-1highCD8+ T cells↑ CD4+ T cells↓, CD8+ T cells↓, central memory CD4+ T cell↓, effector memory CD4+ T cell↓, CD107a+CD4+ T cells↓, CD107a+CD8+ T cells↓, TNFα+CD4+ T cells↓, IFNγ+CD4+ T cells↓, TNFα+CD8+ T cells↓, IFNγ+CD8+ T cells↓ |
209 | ||
| IL-6↑, M2-like TAMs↑, CCL20↑, B cells↑, Tregs↑, αβT cells↑, γδT cells↑ | 203 | ||
|
Total macrophages↑, M2-like macrophages↑ M1-like macrophages↓ |
296 | ||
| EVs↑, YAP signaling↑, CYR61↑, M2-like macrophages↑, liver metastasis↑ | 212 | ||
| Oral squamous cell carcinoma |
CD45+ cells↑, myeloid cells↑, MDSCs (mainly PMN-MDSCs) ↑, CCR1+ PMN-MDSCs↑, Arg1+ MDSCs↑, the expression of Arg1 and S100a9↑ T cells↓ |
205 | |
| Prostate cancer | MDSCs↑, M2/M1 macrophage ratio↑, the expression of Il6, Il1b, Il13, and Il17a↑, pSTAT3+ cells/tumor cells ratio↑ | 202 | |
| SREBP prometastatic lipogenic program↑, lipid↑ | 213 | ||
| Breast cancer |
MDSCs↑, effector CD8+ T cells↑, PD-1+CD8+ T cells↑, Ki-67+CD8+ T cells↑, IFNγ+CD8+ T cells↑, apoptotic CD8+ T cells↑, Fas+CD8+ T cells↑, PMN-MDSCs↑, the expression of MDSC-related cyto/chemokines (Il1b, Cxcl1, Cxcl3, S100a8, and Csf3) ↑, CXCL1↑, per cell expression of FasL in PMN-MDSCs↑ naïve CD8+ T cells↓, IFNγ↓, Bcl-2+CD8+ T cells↓ |
206 | |
| Overexpression of nitric oxide synthase, NO↑, recruitment of macrophages↑ | 201 | ||
| Palmitate↑, acetyl-CoA↑, lysine acetyltransferase 2a↑, nuclear factor-kappaB subunit p65 acetylation↑, lung and liver metastasis↑ | 211 | ||
| CD36 palmitoylation↑, MUFAs intake↑, palmitate-induced lipotoxicity↓ | 215 | ||
| Colorectal cancer |
Valine↑, leucine↑ CD45+ cells↓, CD8+ T cells↓, IFNγ+CD8+ T cells↓, IFNγ+TNF+CD8+ T cells↓, GZMB+CD8+ T cells↓, Ki67+CD8+ T cells↓, PD-1+CD8+ T cells↓, CD98+CD8+ T cells↓, pS6+CD8+ T cells↓, glutamine↓, arginine↓, ornithine↓, kynurenic acid↓ |
208 | |
| Melanoma | CD45+ cells↓, CD8+ T cells↓, CD4+ T cells↓, NK cells↓, CD49d+CD8+ T cells↓, CXCR3+CD8+ T cells↓, the expression pf Cxcl9 and Cxcl10↓, IFNγ+TNF+CD8+ T cells↓, lipidtox+CD8+ T cells↓ | ||
| Pancreatic cancer | TAMs↑, IL-1β↑, IL-4↑, IL-5↑, IL-2↑ | 204 | |
| CD45+ cells↑, myeloid cells↑, MDSCs↑, tumor-associated neutrophils↑, IL-1β↑ | 210 | ||
| Melanoma |
PD-1+CD8+ T cells↑, Tim3+CD8+ T cells↑, Lag3+CD8+ T cells↑, expression of Cpt1a↑ Ki67+CD8+ T cells↓ |
246 | |
| High-cholesterol diet | Colorectal cancer | IL-1β↑, IL-6↑, TNFα↑, macrophages↑, NLRP3 inflammasome activation↑ | 217 |
|
Macrophages↑ IFNγ+CD8+ T cells↓ |
218 | ||
| Hepatocellular carcinoma | NK cells↑, NK cells↑, effector function of NK cells↑, CD8+ T cells↑ | 221 | |
| Fish oil high-fat diet (vs. cocoa butter high-fat diet) | Breast cancer |
ROS production in TAMs↑ TAMs↓ |
222 |
| Fish oil high-fat diet (vs. corn oil high-fat diet) | Prostate cancer |
M1-like macrophages↑ M2-like macrophages↓, the expression of CD206, Arg1, TNFα, CCL2, CCL22, MMP-9, VEGF↓ |
223 |
| Safflower oil high-fat diet (vs. olive oil high-fat diet) | Breast cancer | CD8+ T cells↓, CD4+ T cells↓, TNFα+CD8+ T cells↓, TNFα+CD4+ T cells↓ | 224 |
| High-fiber diet | Lymphoma | DCs↑, cDC1↑, Ifnb1+ monocytes↑, Xcl1 on NK cells↑ | 301 |
| Colorectal cancer |
DCs↑, monocytes↑ Macrophages↓ |
Calorie restriction
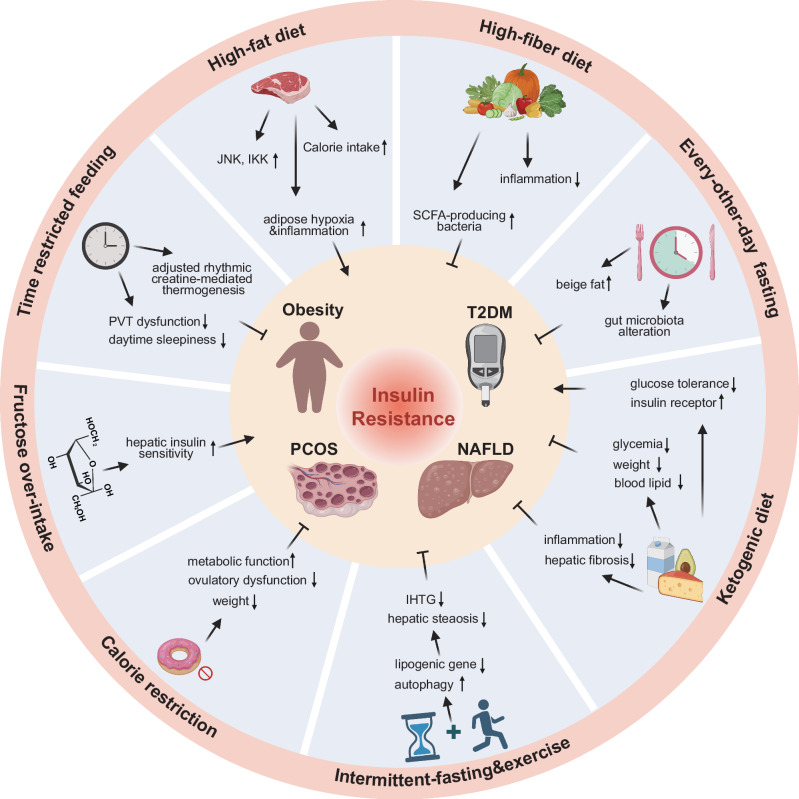
Effective CR is a dietary intervention that reduces energy intake by approximately 15–30% while maintaining a balanced proportion of macronutrients and preventing malnutrition.110 CR has been shown to prolong life and reduce age-related diseases, including cancer, in experimental models.111
Although the antitumor effect of CR has been confirmed, the underlying mechanism remains unclear. Nonetheless, it is believed that the tumor-inhibiting effect is partially mediated by several biological changes, such as increased apoptosis rates in cancer cells, decreased circulating blood glucose levels, inhibited insulin-like growth factor 1 (IGF-1) signaling, reduced insulin levels, and mediators that regulate metabolic pathway activation and inhibit angiogenesis.112 In particular, controlling IGF-1 signal transduction is a critical component underlying the antitumor effects of CR. The IGF-1 signaling pathway is frequently activated in cancer cells, and it shifts metabolic resources toward growth and proliferation. Therefore, the reduction in IGF-1 levels in response to CR leads to attenuated tumor growth and progression.113 The impact of CR on cancer is also interconnected with mutations and oncogenic pathways. A study showed that CR results in a reduction of insulin levels, thereby diminishing tumor PI3K signaling.114 CR has also been found to suppress xenograft tumor growth by upregulating the aldolase A (ALDOA)/DNA-PK/p53 pathway, with ALDOA acting as a potential oncogene that can also activate the tumor suppressor p53.115 Moreover, CR has been shown to modify the cancer stem cell (CSC) phenotype, reducing their carcinogenic and metastatic potential. Notably, in MMTV-ErbB2 transgenic mice, the CSC subpopulation was most affected by CR, as shown by a reduction of luminal cells (CD24high/CD49flow), putative mammary reconstituting unit subpopulations (CD24high/CD49fhigh) and luminal progenitor cells (CD61high/CD49fhigh). These effects were largely attributed to the concurrent inhibition of estrogen receptor and ErbB2 signaling.116
CR has been shown to shape the TME in several ways, including through the specific reduction in the number of TAMs, increase in the formation of CD8+ cytotoxic T cells and memory T cells, and negative modulation of immunosuppressive Treg cell activity and immunosuppressive cytokine levels.117 Additionally, CR promotes favorable changes in the immune signature, providing enhanced protection against tumor growth and metastasis, possibly in part by remodeling the TME. In mice, no impact of a CR diet was observed on the number of CD4+ or CD8+ cells in the TME; however, the cytotoxic killing potential of these cells was elevated. Notably, higher expression of CD103+, a marker of crucial tissue-resident memory T cells that possess enhanced cytotoxic capacity and can contribute to tissue protection against tumor cell invasion, was found. Additionally, a downward trend in the frequency of Tregs was observed, and a significant reduction in the total number of MDSCs was detected.118 Hence, it was concluded that CR not only inhibits cancer cell proliferation but also helps maintain antitumor immunity.
Furthermore, research has shown that fasting, CR, and caloric restriction mimetics (CRMs) can promote T-cell-mediated tumor cytotoxicity, alter NK cell function, and potentially trigger immunogenic cell death, thereby stimulating cancer immunosurveillance pathways.119 CRMs are pharmacological agents or natural compounds that imitate the biochemical effects of CR by reducing the lysine acetylation rates of cellular proteins.120 Examples of CRMs include hydroxycitrate (an inhibitor of ATP citrate lyase), spermidine (an inhibitor of EP300 acetyl transferase activity), and resveratrol (an activator of sirtuin-1 deacetylase activity).121 Treatment with CRMs has been found to decrease the concentration of free IGF-1, promote autophagy in cancer cells, and improve the antitumor immune response, resulting in a reduction in tumor growth when combined with immunogenic chemotherapeutics.119 CRM hydroxycitrate has been found to stimulate autophagy in U2OS osteosarcoma cells in vitro, thereby increasing antitumor immunosurveillance and reducing tumor mass in mice with autophagy-competent mutant KRAS-induced lung cancers.122 Moreover, in vitro treatment with resveratrol inhibits mitochondrial respiration in breast cancer cell lines through a SIRT1-dependent mechanism, diminishes the expression of markers associated with breast CSCs, and promotes their differentiation.123 Collectively, these findings suggest that CRMs may enhance antitumor immunosurveillance in preclinical models.
Moderate physical activity, energy restriction, and their combination can also affect tumor growth. In fact, the combined effects of moderate physical activity and 10% energy restriction (PA + ER) have been shown to significantly delay primary tumor growth, reduce spontaneous metastases, and prolong survival. These effects on tumor progression and survival are accompanied by beneficial changes in immune cell infiltrates within the microenvironment. Specifically, the PA + ER combination leads to an increase in the percentage of CD8+ T cells and a decrease in the percentage of total MDSCs and MDSC subsets within tumors.124
Nevertheless, it is crucial to emphasize that there are established nutritional recommendations for cancer care, and the weight loss or reduction in protein intake often associated with CR may conflict with these guidelines.125 These dietary practices could exacerbate the risk of malnutrition, sarcopenia, fatigue, delayed wound healing, and impaired immunity, particularly in cancer patients who are already at an increased age-associated risk for these conditions.126 Therefore, while exploring dietary interventions for cancer treatment, the potential adverse effects on overall patient health and nutritional status must be carefully considered.
Fasting or fasting-mimicking diet
In addition to CR, alternative approaches such as intermittent fasting (IF), including short-term fasting (STF), intake of an FMD, and time-restricted feeding (TRF), which limits food consumption to a specific time window each day, are being condisered.127,128 The term “fasting” has a broad definition, encompassing a range of eating patterns, including complete and voluntary deprivation of food with no restriction on drinking water.129 An FMD is based on a regimen of low-calorie and low-protein foods that mimics the effects of fasting but induces fewer side effects. This approach retains the benefits of traditional fasting methods while minimizing their potential drawbacks.130
Fasting or intake of an FMD can cause various metabolic changes, including alterations in the systemic levels of hormones and growth factors such as insulin, glucagon, growth hormone, IGF-1, glucocorticoids or adrenaline.131 In response to these changes, normal cells activate protective mechanisms against stress and toxic insults, thereby reducing their metabolic requirements and cell division rate. On the other hand, because fasting or FMDs reduce tumor growth-promoting nutrients and factors, cancer cells struggle to manage metabolite deprivation and thus develop greater sensitivity to cancer therapies.132 In obesity-driven postmenopausal cancer mouse models, TRF was shown to delay the onset of tumors and reduce lung metastasis. Moreover, TRF was found to increase systemic insulin sensitivity and decrease hyperinsulinemia. Importantly, TRF could also restore the circadian rhythm of gene expression within tumors while attenuating both tumor growth and insulin signal transduction.133 Fasting can cause an “anti-Warburg effect” by reducing aerobic glycolysis and glutaminolysis while increasing OXPHOS uncoupled from ATP synthesis.134 In cancer cells, OXPHOS increases reactive oxygen species (ROS) production and leads to oxidative stress, activation of p53 signaling and DNA damage, particularly when combined with chemotherapy or other cancer therapies.135 Therefore, the unique metabolic vulnerabilities of cancer cells, which differ from those of normal cells, can be strategically targeted to develop novel and effective therapeutic interventions. According to a recent study, the combination of chemical treatment with an FMD reduces the expression of heme oxygenase-1 (HO-1), which is a stress-responsive enzyme that protects cancer cells against oxidative damage and apoptosis in vivo. Interestingly, this combination treatment resulted in upregulated HO-1 expression in normal cells. The downregulation of HO-1 production in cancer cells, in part, facilitated FMD-induced chemosensitization of cancer cells by boosting CD8+ TIL-dependent cytotoxicity, which was possibly facilitated by decreased Tregs.136 A separate study conducted with mouse models of colon cancer indicated that alternate day fasting for 2 weeks triggered autophagy in cancer cells, which in turn downregulated CD73 expression. As a result, the production of immunosuppressive adenosine in cancer cells was reduced, ultimately preventing macrophages from acquiring an M2 immunosuppressive phenotype.137
Clinical experiments have suggested that intake of an FMD can induce metabolic changes and increase antitumor immunity in cancer patients. In fact, the final outcomes of an FMD-treated clinical trial (NCT03340935) demonstrated that a severely calorie-restricted, five-day FMD regimen was well tolerated and resulted in substantial systemic metabolic changes in patients with different tumor types who were concurrently receiving antitumor therapies.138,139 In another clinical trial called DigesT (NCT03454282), a five-day FMD regimen was found to broadly reshape intratumor immunity in breast cancer patients. Specifically, the FMD was shown to promote the infiltration of activated and cytotoxic immune cell populations, including total and activated intratumoral CD8+ T cells, M1-like macrophages, aDCs, and NK cells. These changes were paralleled by an increase in immune signatures associated with improved clinical outcomes in cancer patients.138
Ketogenic diet
A KD comprises a high-fat component, very low carbohydrate levels, and low to moderate protein levels, as explained in a recent study.140 A traditional KD is typically formulated at a 4:1 ratio of fat:carbohydrate plus protein.141 In this classical formulation, 80–85% of calories are derived from fat, 10–15% from protein, and less than 5% from carbohydrates.142 A KD is known to be effective at treating epilepsy, lowering glucose levels, and producing ketone bodies in vivo.143 There is increasing evidence to support the use of KD as a potential tumor treatment or prevention method, either as a standalone approach or in combination with other medicines.144
The Warburg effect indicates that lower intratumoral glucose levels can impede tumor growth, which can be achieved through pharmacological intervention and dietary changes such as a KD. Cancer cells, unable to utilize ketone bodies produced by KD for energy due to their aberrant mitochondrial function and diminished enzyme activity, can essentially be “starved” of glucose. Hence, KD emerges as a potentially promising strategy for cancer prevention.145 One of the primary ways in which a KD potentially promotes potential anticancer effects is by increasing the levels of β-hydroxybutyrate (β-HB), which is the most abundant ketone body.146 For instance, β-HB has been proven to inhibit CRC by activating the transcriptional regulator Hopx through the surface receptor Hcar2, thereby reducing the proliferation of colonic crypt cells and suppressing tumor growth.147 Another antitumoral effect of KD is upregulating the expression of the circadian clock gene Per (Period) by activating AMPK and upregulating SIRT1 (Sirtuin1), resulting in enhanced apoptosis and growth delay in tumor cells.148 KD also decreases insulin-regulated PI3K-Akt-mTOR signaling, which is overactivated in pancreatic neuroendocrine tumors (PanNETs), resulting in decreased blood glucose levels and a suppressive effect on the development and progression of PanNETs.149
Emerging evidence suggests that a KD may be a valuable clinical tool to enhance T-cell-mediated antitumor immune responses. In vitro and in vivo studies have shown that KD intake markedly increased the specific responses of human T cells, resulting in enhanced CD4+, CD8+, and Treg capacity, as well as augmented T memory cell formation. Under conditions of KD intake, CD8+ T cells undergo metabolic reprogramming to rely on OXPHOS in response to increased ketone bodies, leading to enhanced cellular energy and respiratory reserve, potentially improving their functionality.150 In addition, KD intake prevented the progression of colon tumors by inducing tumor cell oxidative stress, inhibiting MMP-9 expression, and promoting M2 to M1 TAM polarization.151 In a mouse model of malignant glioma, KD feeding led to significantly enhanced innate and adaptive tumor-specific immune responses. Mice fed a KD showed increased cytokine production (IFNγ, TNF, and IL-2) and greater tumor-reactive CD8+ T-cell cytotoxicity. Moreover, the mice maintained on a KD presented with a higher number of immune cells and a higher ratio of CD4+ T cells to Tregs, while the functionality of the Tregs was weakened. Feeding mice with the KD resulted in a noteworthy decrease in the expression of immune inhibitory receptors (PD-1 and CTLA-4) on CD8+ TILs, as well as a reduction in the expression of inhibitory ligands (CD86 and PD-L1) on cancer cells.152 These findings suggest that a KD has the potential to attenuate tumor-induced T-cell suppression by decreasing the population of cells susceptible to the inhibitory PD-1 pathway.
Although KD has shown various potential benefits to tumor patients with its promising effects of inhibiting tumor cell growth and activating immune response, there is still limitation in its clinical application owing to its inevitable side effects.153 It should be considered that KD also presents some risks, as they are typically high in saturated fats and may lack a substantial amount of nutrients, specifically carbohydrates and dietary fiber, as well as micronutrients such as calcium, magnesium, potassium and vitamins A, B and B6.154,155 According to a recent research, KD delayed tumor growth but meanwhile accelerated cachexia onset, therefore shortening survival in a mouse model of IL-6-producing cancer. Excitingly, the same research group found that applying dexamethasone during KD treatment might delay cachexia onset without affecting the inhibition of tumor growth, providing fundamental insight into reversing the limitations of the clinical application of KD.156
Protein restriction diet
The prevailing notion suggests that high protein intake, particularly among individuals under the age of 65, potentially escalates the risk of overall and cancer-related mortality.157 To establish a protein restriction diet, either dietary protein intake or the number of amino acids can be reduced.140 Recent research has demonstrated that dietary protein restriction is linked with a reduced incidence of tumor occurrence and a decreased risk of mortality.158
Dietary restriction of protein and certain amino acids, including serine, methionine, and branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine, has been shown to impede tumor growth.159 One mechanism through which protein restriction may inhibit tumor growth is via the IGF-1 signaling pathway. In melanoma and breast cancer mouse models, it has been observed that mice fed a low-protein diet (4% kcal protein) exhibit reduced IGF-1 levels and slower tumor progression compared to those fed a high-protein diet (18% kcal protein). A low-protein diet has been associated with reduced IGF-1 levels in patients aged 50–65 years, subsequently decreasing their risk of death from cancer. Conversely, a low-protein diet has been linked with an increased mortality rate in older patients (aged 65 and above), suggesting that a life-stage-specific approach to protein intake could optimize healthspan and longevity.157 Other potential mechanisms for cancer prevention that are mediated by protein restriction could involve mTOR signaling, amino acid metabolic programming, FGF21, and autophagy.158 In addition to these general effects, specific dietary restrictions on certain amino acids, such as serine and glycine, have been associated with prolonged survival in mouse models of various tumor types. The mechanisms underlying this observed survival benefit could include the correction of abnormal cellular nucleotide, protein, and lipid synthesis; improved mitochondrial function; and changes in epigenetic modifications.160,161
The antitumoral effect of a low-protein diet also hinges on promoting immunosurveillance against cancer, while the dietary restriction of amino acids may adversely affect the metabolic reprogramming of the TME in various ways. In multiple mouse models, reducing dietary methionine inhibited tumor growth and boosted antitumor immunity by increasing the quantity and cytotoxicity of tumor-infiltrating CD8+ T cells.162 Moreover, restricted intake of dietary protein or methionine/cystine has been shown to modify the infiltration and tumoricidal capacity of TAMs, leading to a significant increase in tumor-infiltrating CD8+ T cells and a decrease in the number of infiltrating MDSCs. Mechanistically, a protein-restricted diet inhibited mTOR pathway activation and increased macrophage acquisition of an antitumor phenotype by increasing the number of macrophages undergoing polarization to the M1 type.163 Macrophages might sense diet-derived cytosolic amino acids via the GTPase Rag, which subsequently regulates the expression of TFEB, TFE3 and mTORC1 when activated.164 Furthermore, an isocaloric diet that moderately reduced protein intake (by 25%) was shown to trigger an unfolded protein response (UPR) that depended on IRE1α in cancer cells. The increase in UPR activation, in turn, led to an increase in the recruitment of CD8+ T cells and enhanced antitumor immunosurveillance. Notably, intake of a low-carbohydrate diet did not exert the same effect.165 Although a low-protein isocaloric diet has been proven to reduce the concentration of amino acids in tumor tissues, it remains uncertain whether this reduction is limited to certain amino acids. Thus, further research is needed to explore the correlation between a low-protein isocaloric diet and the decrease in the levels of specific amino acids in tumors.
Interestingly, several studies have shown that high-protein diets may also benefit the restriction of tumor growth or clinical outcoming of cancer patients, which seem contradictory to the findings of the protein restriction diet discussed above. However, the underlying mechanisms are totally different. A high-protein diet increased the production of urinary urea in a tumor protein 53 (TP53)-mutated orthotopic bladder tumor mouse model, leading to the cascade modulation of ammonia in tumor cells, which induces tumor apoptosis.166 These findings challenge the former hypothesis that high urinary urea concentrations caused by a high-protein diet might serve as a potential carcinogenic factor in the bladder, suggesting the urgent need for further investigation.167 Applying a high-protein diet may improve the overall survival of older outpatients with advanced gastrointestinal cancer, which may improve the nutritional state of these patients with poor digestive system function.168
Moreover, there have been efforts to develop a series of drugs that mimic amino acid restriction. One focus of researchers in the cancer therapy field has been on glutamine metabolism, as cancer cells rely heavily on glutamine. Glutaminase inhibitors, for instance, have been shown to decrease tumor burden.169,170 The use of 6-diazo-5-L-oxo-norleucine (DON) promoted antitumor immunity by greatly favoring OXPHOS over glycolysis in CD8+ T cells while disrupting the metabolism of cancer cells.171 Notably, DON showed the ability to significantly inhibit the generation and recruitment of MDSCs and to reprogram M2-like TAMs into proinflammatory TAMs, which increased tumor antigen cross-presentation to T cells and enhanced the efficacy of immune checkpoint blockade (ICB).172 In addition, CB-839, which is considered the most effective glutaminase inhibitor, can be utilized alone or in combination with PD-1 inhibitors to treat solid or hematological malignancies.173–175 As previously mentioned, IDO and TDO are tryptophan catabolism enzymes, and inhibitors of these enzymes have been developed and evaluated in various clinical trials.176 For example, epacadostat is a novel compound that serves as an IDO1 inhibitor, suppressing systemic tryptophan catabolism.177 Both in vitro and in vivo studies have demonstrated that epacadostat can reduce tumor growth and promote the proliferation of T cells and NK cells.178 Furthermore, cyst(e)inase, a glutathione inhibitor that degrades cysteine and cystine, reduces tumor progression by elevating ROS levels and inducing tumor cell-selective ferroptosis.179,180
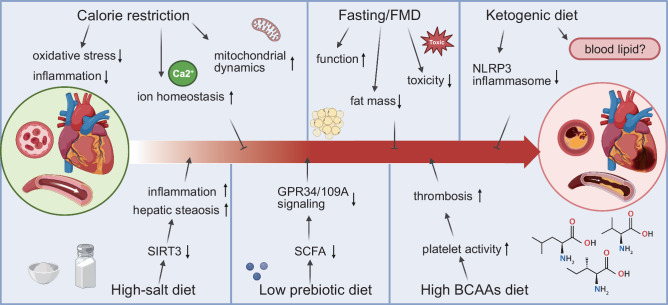
High-salt diet
HSD has long been considered as a risk factor and trigger of malignancies. However, recent studies have provided new insights into the effect of sodium intake. As research continues, it is becoming increasingly clear that salt can accumulate in the interstitium and modulate immune cell differentiation, activation, and function through the effects of extracellular hypersalinity.181 In addition, consumption of a HSD can lead to elevated tissue sodium concentrations and affect immune responses within microenvironments, ultimately impacting the development of immune-regulated diseases such as infections and cancer.182
HSD, comprising 4% sodium chloride (NaCl), is recognized as a robust immunomodulator that is capable of eliciting a substantial inflammatory response.183 Indeed, research has shown that high salt conditions can inhibit tumor growth by enhancing antitumor immunity, particularly through the modulation of MDSC functions.184 According to a recent study, an HSD reduced the production of cytokines essential for the expansion of MDSCs and thus attenuated the accumulation of MDSCs within the tumor niche. As a result, the two primary types of MDSCs acquired different phenotypes: M-MDSCs differentiated into antitumor macrophages, and PMN-MDSCs adopted a proinflammatory phenotype, which led to the reactivation of T-cell antitumor functions.185 Furthermore, a high salt level has been found to induce the transformation of anti-inflammatory Tregs into proinflammatory Th1 cells, which led to the secretion of the inflammatory cytokine IFNγ.186 In another study, salt functioned as an adjuvant that enhanced the effectiveness of anti-PD-1 immunotherapy in tumor regression. Specifically, an HSD induces NK cell-mediated tumor immunity by suppressing PD-1 expression while increasing IFNγ levels and the serum hippurate concentration. Notably, hippurate is a microbial benzoate metabolism product that has been identified as a metabolic marker of effective PD-1 immunotherapy in responsive patients.183 Although the major antitumoural effect of HSD is modulating immune cell function, mechanisms other than immunomodulation have also been discovered. For instance, HSD suppressed tumor growth and lung metastasis in a murine model of breast cancer, possibly by inducing hyperosmotic stress or through mimicking CR.187
Nevertheless, despite the potential benefits of salt intake on cancer treatment effectiveness, high salt intake can also lead to the development of a proinflammatory state, which can negatively impact cancer outcomes.188 High salt intake is a risk factor for various types of cancer in humans, including lung, testicular, bladder, renal cell, pancreatic, esophageal, and gastric cancer.182 HSD has been shown to induce chronic inflammation, which may in turn incite continuous cell proliferation, DNA damage, or cancer transformation. However, whether there is a connection remains uncertain.188 IL-17, specifically IL-17A, plays an important role in the mechanism of action of HSD. Evidence suggests that high salt intake can induce the differentiation of Th17 cells, a prominent source of IL-17A.189 The overproduction of IL-17A can lead to inflammation and other immune responses that contribute to various pathologies. Furthermore, in the case of breast cancer, an HSD has been found to promote tumor progression and lung metastasis, increase the proportion of Th17 cells, and activate the MAPK/ERK signaling pathway in breast cancer cells through the secretion of IL-17F. The increase in the secreted IL-17F level results in the unregulated expression of protumor genes and the induced inflammatory responses, ultimately accelerating the proliferation, migration and invasion of breast tumors.190 In addition, the combination of high NaCl concentrations with subeffective IL-17 has been proven to reduce reactive nitrogen and oxygen species (RNS/ROS) levels and enhance the growth of breast cancer cells.191,192 Recent research has also demonstrated that intake of an HSD can disrupt the development and function of NK cells in mice.193 Therefore, it can be concluded that dietary salt may exert dual effects on tumorigenesis, and the contradictory results obtained may be due to variations in the effects of high salt concentrations on tumors in different tissues and during different phases of tumor development.
Obesity and high-fat diet
Obesity, a serious health issue characterized by excessive body fat, is a known risk factor for multiple types of cancer. It can be induced or exacerbated by HFD, characterized by the consumption of foods rich in saturated fats and cholesterol.194 Obesity can induce systemic metabolic disruptions within the body, leading to dyslipidemia, hypercholesterolemia, insulin resistance, alterations in hormone levels, and changes in the baseline inflammation status.195 Conversely, a low-fat diet, typically associated with reduced total fat intake, can potentially lower the risk of certain types of cancer.196,197 Given that both HFD and obesity are major factors influencing cancer risk, the forthcoming discussion will primarily focus on these aspects. By diving deeper into the mechanisms by which HFD and obesity affect cancer development and progression, we aim to provide a more comprehensive understanding of this intricate relationship.
Dietary obesity is associated with multiple factors related to cancer occurrence and exacerbation of immune suppression in tumor niches.198 In the context of obesity, increased hepatic expression of the unconventional prefoldin RPB5 interactor (URI) has been shown to couple nutrient surplus with inflammation, leading to nonalcoholic steatohepatitis (NASH) and consequent HCC. This process involves URI-induced DNA damage in hepatocytes triggering Th17 lymphocyte-mediated inflammation, and subsequent IL-17A-induced adipose tissue neutrophil infiltration, which promotes insulin resistance and hepatic fat accumulation, thereby inducing NASH and HCC.199 Notably, obesity also accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing the trafficking of immature myeloid cells and the Th17 response. This exacerbates proinflammatory immune responses, characterized by cross-talk between inflamed gastric and adipose tissues, thereby contributing to a protumorigenic gastric microenvironment.200
Diet-induced obesity has been shown to elevate nitric oxide (NO) production, which enhances tumor growth. This is primarily due to the recruitment of macrophages and the overexpression of inducible NO synthase as a result of HFD.201 Additionally, in response to HFD intake, IL-6-mediated inflammation has been shown to accelerate prostate cancer tumor growth and increase the fraction of MDSCs and the M2/M1 macrophage ratio.202 The effects of diet-induced obesity extend to the microenvironment of colitis-associated CRC. Here, diet-induced obesity has been shown to increase IL-6 expression and promote the polarization of macrophages into M2-like macrophages, enhancing the production of CC-chemokine-ligand (CCL) 20. CCL20 recruits CC-chemokine receptor 6 (CCR6)-expressing B cells and γδ T cells, ultimately leading to colitis-associated CRC progression.203 In animal models of HFD-induced obesity, the infiltration rate of TAMs and the expression of cytokines in M2-like macrophages were increased, enhancing tumor growth and metastasis. However, ablation of VEGFR-1 signaling can reverse the abnormal TME associated with obesity and reprogram TAMs to promote their acquisition of the M1 phenotype.204
The intake of an HFD has been shown to significantly increase the incidence of oral squamous cell carcinoma (OSCC) by expanding MDSCs within the local immune microenvironment.205 Obesity induced by diet can also trigger the accumulation of PMN-MDSCs, leading to Fas/FasL-mediated apoptosis of tumor-infiltrating CD8+ T cells and causing resistance to immunotherapy in breast cancer treatment.206 Obesity has been shown to suppress the infiltration and function of CD8+ T cells, which was linked to decreased chemokine production, reduced fatty acid availability, and alterations in amino acid metabolism.207,208 Moreover, based on findings from mouse models, obesity reduced the number and function of CD4+ T cells in the TME of CRC, leading to a compromised antitumor response of both CD4+ and CD8+ T cells and ultimately accelerating disease progression.209 Furthermore, considerable evidence shows that obesity-associated adipocytes in pancreatic ductal adenocarcinoma can secrete IL-1β to attract tumor-associated neutrophils (TANs), which subsequently activate pancreatic stellate cells and contribute to tumor growth.210
HFD or diet-induced obesity may induce tumor metastasis. HFD has been proven to increase palmitate secretion from alveolar type 2 cells and nuclear factor-kappaB subunit p65 acetylation in the lung to prepare a premetastatic niche.211 HFD-induced fatty liver may promote liver metastasis by facilitating the secretion of hepatocyte-derived extracellular vesicles (EVs), which transfer Yes-associated protein (YAP) signaling-regulating microRNAs, hence elevating nuclear YAP expression, CYR61 expression, and M2-like macrophage infiltration.212 Another mechanism of HFD-induced liver metastasis is the upregulation of NOD-like receptor C4 (NLRC4), which further induces M2-like macrophage activation and IL-1β processing. An alteration from an indolent to a metastatic state may be stimulated by HFD-induced lipid accumulation in prostate tumors, the mechanism of which may be related to the sterol regulatory element-binding protein (SREBP)-related prometastatic lipogenic program.213 In addition, it is widely acknowledged that the fatty acid receptor CD36 plays an important role in HFD-related metastasis promotion by enhancing the metastatic potential of CD36+ metastasis-initiating cells.214 However, a recent study revealed that CD36 may prevent palmitate-induced lipotoxicity rather than facilitating HFD-driven metastasis, suggesting that further investigations of the dual effects of CD36 are needed.215
An elevated cholesterol level is an obesity comorbidity, and studies suggest that the effects of obesity on cancer may be partly mediated by increased cholesterol levels.216 In fact, a high-cholesterol diet (HCD) alone has been shown to promote macrophage infiltration and significantly enhance the growth of CRC tumors.217 One mechanism by which HCD promotes CRC progression is through the inhibition of the CD8+ T-cell response. Specifically, macrophages with infiltration driven by HCD can secrete CCL5, which obstructs the activation of CD8+ T cells, thereby facilitating the evasion of immune system surveillance by CRC cells.218 27-Hydroxycholesterol (27-HC) is a crucial mediator of the effects of dietary cholesterol on cancer metastasis. This oxysterol is synthesized through the action of the CYP27A1 enzyme and is present at high levels in the circulatory system.219 Oxysterol has been shown to modulate the TME by recruiting immunosuppressive neutrophils to the metastatic niche, facilitating cancer progression.220 However, some studies have reported conflicting findings regarding the effects of high serum cholesterol levels on cancer progression. For instance, one study showed that high serum levels of cholesterol attributed to HCD intake increased the accumulation of NK cells and promoted their effector functions to reduce the growth of liver tumors in mice.221 However, further studies are needed to understand these conflicting findings.
In expanding on the relationship between HFD and tumor promotion, it is worth noting that the tumor-promoting effect of HFD is not universal and depends largely on the subtype of fatty acids involved. Mouse models of breast cancer developed comparable obesity levels from an HFD of either cocoa butter or fish oil. However, the consumption of the cocoa butter HFD, which is high in saturated fatty acids, led to faster mammary tumor growth and increased protumor macrophages and IL-10 expression while reducing B-cell and CD8+ T-cell infiltration. On the other hand, the fish oil HFD, which is rich in omega-3 fatty acids, disrupted the typical obesity-tumor growth link and reduced the number of protumor macrophages.222 This effect of dietary omega-3 fatty acids is mediated by host GPR120 and has also been shown to inhibit prostate cancer.223 Moreover, oleic acid (OA) and linoleic acid (LA) are the most common unsaturated fatty acids in dietary oils. While both an HFD rich in OA and an HFD rich in LA can similarly induce obesity in mice, a diet high in LA specifically encourages the growth of mammary tumors. Furthermore, an LA-rich HFD can impair antitumor T-cell responses via the induction of mitochondrial dysfunction.224 Based on these findings, it appears that modulating dietary oil composition may constitute a promising strategy for enhancing immune function in both the prevention and treatment of obesity-associated cancers. By carefully selecting and balancing the types of fatty acids in HFDs, it may be possible to reduce the tumor-promoting effects of obesity while simultaneously increasing immune responses against tumors. Further research in this area may help to identify more precise dietary interventions that can ultimately improve outcomes for individuals at risk of developing obesity-associated cancers.
Potential role of dietary factors in cancer treatment
Immunotherapy
Recent studies have highlighted the pivotal influence of the TME on the efficacy of immunotherapy in cancer treatment.225 Immunotherapy, recognized as a substantial advance in cancer treatment, has revolutionized the field of oncology by augmenting the body’s innate defenses to effectively target and eliminate malignant cells.226 Various forms of cancer immunotherapy have been developed, including oncolytic virus therapies, cancer vaccines, cytokine therapies, adoptive cell transfer, and ICIs, all of which have shown promise in clinical practice.227 Among these therapies, ICIs are perhaps the most important, as they are antibody-based drugs that can eliminate the influence of tumor-specific CD8+T cells.228 In particular, ICIs targeting PD-1 or its ligand PD-L1 have demonstrated notable clinical efficacy in the treatment of various advanced cancers.229
Extensive research has been conducted to identify the effects of various dietary substances and patterns on tumor growth, metastasis and TME reprogramming, which has led to the consideration of nutritional intervention as a possible strategy for increasing the efficacy of tumor treatment230,231 (Tables 2, 3). The decline in T-cell functionality with aging, a widely documented phenomenon, is linked to a reduced efficacy of anti-OX40 immunotherapy in murine models.232 CR not only preserves T-cell function but also improves the response of aged CD4+ T-cell populations to anti-OX40 therapy.233 When used in combination with immunogenic cell death (ICD)-inducing chemotherapy and immunotherapy, CRMs potentially enhance the efficacy of cancer treatments through synergistic effects.234 Preclinical studies have shown that STF, which serves as an adjunct to various cancer treatments, may bolster antitumor immunity by attenuating immunosuppressive conditions and amplifying CD8+ T-cell cytotoxicity.235 For example, an experimental study of non-small cell lung cancer demonstrated that STF sensitized cancer cells to anti-PD-1 therapy. The antitumor efficacy of combination therapy was achieved by inhibiting IGF-1-IGF-1R signaling in cancer cells, boosting the intratumoral CD8 cell: Treg ratio in the TME.132 Furthermore, intake of an FMD has been shown to enhance the effectiveness of immunotherapy against triple-negative breast cancer with low immunogenicity by affecting the TME. Specifically, intake of an FMD has been shown to reactivate Teff cells that underwent early exhaustion, shift cancer metabolism from glycolytic to OXPHOS, and reduce the collagen deposition rate.236 These effects led to the increased efficacy of anti-PD-L1 and anti-OX40 immunotherapy. These results suggest that combining immunotherapy with dietary restriction may lead to profound synergistic effects.
Table 2.
Preclinical studies showing effects of dietary intervention on cancer therapy
| Dietary intervention | Cancer type | Comparison of treatment | Results | References |
|---|---|---|---|---|
| Calorie restriction | Breast cancer | Radiotherapy |
Teff/Tregs ratio↑, PD-1+CD8+ T cells↑ Tregs↓ |
261 |
| CRM hydroxycitrate |
Fibrosarcomas Colorectal cancer Lung cancer |
Chemotherapy |
Autophagy in tumor cells↑ Tregs↓ |
122 |
| Fasting or fasting-mimicking diet | Lung cancer | Immunotherapy |
CD8/Treg ratio↑, NK cells↑ Tregs↓, CD19+ B cells↓, PD-1+CD8+ T cells↓, PD-1+CD4+ T cells↓ |
132 |
| Pancreatic cancer | Chemotherapy |
Levels of equilibrative nucleoside transporter (hENT1) in tumor cells↑ Levels of ribonucleotide reductase M1 (RRM1) in tumor cells↓ |
251 | |
| Breast cancer | Chemotherapy | ROS in tumor cells↑ | 252 | |
| Immunotherapy |
T cells↑, CD8+ T cells↑, GZMB+CD8+ T cell↑, Ki67+CD8+ T cells↑, γδ T cells↑, GZMB+ γδ T cells↑, Ki67+ γδ T cells↑, Ki67+FOXP3-CD4+ T cells↑, OX40+FOXP3-CD4+ T cells↑, PD-1+FOXP3-CD4+ T cells↑, Ki67+ Tregs↑, OX40+ Tregs↑, PD-L1+ PMN-MDSCs↑, macrophages↑, PD-L1+ macrophages↑, ToxintCD8+ T cells↑, ToxintPD-1intCD39lowCD8+ T cells↑ PMN-MDSCs↓, M2-like macrophages↓ |
236 | ||
| Endocrine therapy | Circulating IGF1, insulin, and leptin levels↓, AKT-mTOR signaling↓ | 265 | ||
|
Breast cancer Melanoma |
Chemotherapy |
CD8+ T cells↑, CD3+ T cells↑, granzyme-B↑ HO-1↓, Tregs↓ |
136 | |
|
Lung cancer Breast cancer Colorectal cancer |
TKIs |
E2F-dependent transcription inhibition↑ MAPK signaling pathway↓ |
266 | |
| Hepatocellular carcinoma | TKIs | Glucose↓, AKT/mTOR signaling↓ | 267 | |
| Fasting-mimicking diet+ vitamin C | Colorectal cancer | Chemotherapy | Reactive iron and oxygen species in tumor cells↑ | 253 |
| Fasting-mimicking diet+ ferroptosis inducer | Colorectal cancer | Chemotherapy | Autophagy in tumor cells↑ | 254 |
| Ketogenic diet | Colorectal cancer | Immunotherapy | IFNγ+CD8+ T cells↑, TNFα+CD8+ T cells↑ | 238 |
| Neuroblastoma | Chemotherapy | Tumor burden↓ | 258 | |
| Chemotherapy |
Serine, glutamine and glycine↑ Tumor blood-vessel density and intratumoral hemorrhage↓, serum levels of essential amino acids↓ |
259 | ||
| Pancreatic cancer | Chemotherapy | Tumor NADH levels↑ | 260 | |
| Radiotherapy | Oxidative stress in tumor cells↑ | 263 | ||
| PI3K inhibitors | Hyperglycemia↓, insulin secretion↓, mTORC1 signaling↓ | 268 | ||
| Lung cancer | Radiation or radio-chemotherapy | Oxidative stress in tumor cells↑ | 262 | |
| Dietary restriction of protein/80% methionine-restricted diet | Prostate cancer | Immunotherapy |
CD8+ T cells↑, CD8+ T cells/M1-like ratio↑, CD8+ T cells/M2-like ratio↑, CD8+ T cells/M-MDSC ratio↑, CD8+ T cells/PMN-MDSC ratio↑ M2-like TAMs↓ |
163 |
| Dietary methionine restriction | Colorectal cancer | Immunotherapy | CD8+ T cells↑ | 162 |
|
Colorectal cancer Soft-tissue sarcoma |
Radiotherapy Antimetabolite chemotherapy. |
Alterations in one-carbon metabolism | 264 | |
| Dietary serine deprivation |
Lung cancer Colorectal cancer |
Biguanide treatment | Serine↓, glycolysis↓ | 269 |
| High-salt diet |
Breast cancer Melanoma |
Immunotherapy | CD4+ T cells↑, CD8+ T cells↑ | 185 |
| Melanoma | Immunotherapy | PD-1+ NK cells↓ | 183 | |
| High-fat diet | Breast cancer | Immunotherapy | PMN-MDSCs↑, the expression of Cxcl1↑ | 206 |
| Melanoma | Immunotherapy | CD3+ T cells↑, CD8+ T cells↑, CD8+/CD4+ ratio↑ | 246 | |
| Ovarian cancer | Chemotherapy | Fibrosis↑, M1-like macrophages↓, M2-like macrophages↓, M2/M1 macrophage ratio↑ | 442 | |
| Inulin gel | Colorectal cancer | Immunotherapy |
CD8+ cells↑, AH1 tetramer+CD8+ T cells↑, DCs↑, CD4+ T cells↑, Tcf1+PD-1+CD8+ T cells↑, neutrophils↑, M1/M2 macrophages ratio↑ PD-1+CD8+ T cells↓, M2-like macrophages↓, Tregs↓ |
303 |
| Pectin | Colorectal cancer | Immunotherapy | CD4+ T cells↑, CD8+ T cells↑, IFNγ+CD8+ T cells↑ | 304 |
Table 3.
Clinical trials of dietary intervention combined with cancer treatment
| Dietary intervention | NCT | Therapeutic intervention | Disease | Status |
|---|---|---|---|---|
| Calorie restriction | NCT01819233 | Surgery and radiotherapy | Stage 0-I breast cancer | Completed |
| NCT02792270 | Pre-operative radiotherapy | Sarcoma | Unknown | |
| NCT01802346 | Chemotherapy | Breast or prostate cancer | Recruiting | |
| Cyclic, 5-day calorie restriction | NCT05703997 | Atezolizumab | Small cell lung cancer | Not yet recruiting |
| Calorie restriction and exercise | NCT03131024 | Anthracycline-containing chemotherapy | Breast cancer | Completed |
| Intermittent calorie restriction and plant-based diet | NCT05359848 | Chemotherapy | Cancer | Recruiting |
| Low-carbohydrate diet | NCT02149459 | Radiotherapy with or without metformin | Recurrent brain cancer | Unknown |
| Very low carbohydrate diet | NCT04035096 | High dose intravenous vitamin C | Stage IV colon cancer with KRAS and BRAF mutation | Unknown |
| Intermittent fasting | NCT01175837 | Chemotherapy | Cancer | Completed |
| NCT02607826 | Chemotherapy | Solid tumors | Unknown | |
| NCT06015087 | Chemotherapy | Breast cancer | Recruiting | |
| NCT01304251 | Chemotherapy | Breast cancer | Completed | |
| NCT05722288 | Radiotherapy and/or chemotherapy and/or hormone therapy | Prostate, cervical or rectal cancer | Recruiting | |
| NCT04247464 | Chemotherapy | Colorectal cancer | Enrolling by invitation | |
| Nightly fasting | NCT05023967 | Chemotherapy and metformin | Early breast cancer | Recruiting |
| Prolonged nightly fasting | NCT05083416 | Immunotherapy | Advanced head & neck cancer | Active, not recruiting |
| Alternate day fasting | NCT05990426 | Chemotherapy | Endometrial, ovarian, fallopian tube or primary peritoneal cancer | Not yet recruiting |
| 5:2 intermittent fasting | NCT05861362 | Radiotherapy | Breast Cancer | Completed |
| Time restricted eating with or without Mediterranian diet | NCT05259410 | Chemotherapy | Breast Cancer | Recruiting |
| Intermittent fasting or vegan diet | NCT03162289 | Chemotherapy | Gynecological cancer | Active, not recruiting |
| Intermittent fasting or Mediterranian diet | NCT02710721 | Chemotherapy with or without hormone therapy | Advanced metastatic prostate cancer | Completed |
| Fasting-mimicking diet | NCT03595540 | Active cancer treatment | Cancer | Completed |
| NCT03709147 | Metformin hydrochloride, cisplatin, carboplatin, pemetrexed, pembrolizumab | Advanced LKB1-inactive lung adenocarcinoma | Unknown | |
| NCT05763992 | Chemoimmunotherapy | Triple-negative breast cancer | Recruiting | |
| NCT05503108 | Neoadjuvant chemotherapy (ddAC, T) | HR+, HER2- breast cancer | Recruiting | |
| NCT02126449 | Neoadjuvant chemotherapy (AC>T) | HER2- breast cancer | Completed | |
| NCT04248998 | Neoadjuvant chemotherapy (AC>T) with or without metformin | Triple-negative breast cancer | Active, not recruiting | |
| NCT03340935 | Standard cancer treatment | Malignancies with the exception of small cell neuroendocrine tumors | Completed | |
| NCT05921149 | Carboplatin and paclitaxel | Advanced or recurrent ovarian, fallopian tube and primary peritoneal cancer | Not yet recruiting | |
| Ketogenic diet | NCT05119010 | Nivolumab and ipilimumab | Metastatic renal cell carcinoma | Recruiting |
| NCT05938322 | Neoadjuvant radiotherapy | Locally advanced nonmetastatic rectal adenocarcinoma | Not yet recruiting | |
| NCT04316520 | Nivolumab + ipilimumab, pembrolizumab + axitinib, sunitinib or pazopanib | Metastatic renal cell carcinoma | Recruiting | |
| NCT03962647 | Letrozole | Early-stage ER+, HER2- breast cancer | Active, not recruiting | |
| NCT03535701 | Paclitaxel | Stage IV breast cancer | Completed | |
| NCT05234502 | Neoadjuvant chemotherapy (AC>T) | Breast cancer | Not yet recruiting | |
| NCT05708352 | Chemotherapy and/or radiotherapy | Glioblastoma | Recruiting | |
| NCT03451799 | Radiotherapy and temozolomide | Glioblastoma | Active, not recruiting | |
| NCT02302235 | Radiotherapy and temozolomide | Glioblastoma multiforme | Completed | |
| NCT02939378 | Chemotherapy | Recurrent glioblastoma multiforme | Unknown | |
| NCT04631445 | Nab-paclitaxel, gemcitabine, and cisplatin | Metastatic pancreatic ductal adenocarcinoma | Recruiting | |
| NCT02516501 | Radio(chemo)therapy | Breast, head & neck or colorectal carcinoma | Completed | |
| NCT02983942 | Chemotherapy (HD-MTX) | Primary central nervous system lymphoma | Unknown | |
| Energy restricted ketogenic diet | NCT01535911 | Chemotherapy and radiotherapy | Glioblastoma multiforme | Active, not recruiting |
| Intermittent fasting and time-restricted modified ketogenic diet between fasts | NCT04730869 | Chemoradiotherapy and adjuvant chemotherapy | Glioblastoma multiforme | Recruiting |
| Modified ketogenic diet or medium chain triglyceride ketogenic diet | NCT03075514 | Chemotherapy, radiotherapy or chemoradiotherapy | Glioblastoma | Completed |
| Modified Atkins diet | NCT02768389 | Bevacizumab | Recurrent glioblastoma or other grade IV malignant glioma | Completed |
| NCT03278249 | Temodar and radiotherapy | Primary malignant glioma | Active, not recruiting | |
| Low protein diet | NCT03329742 | Sipuleucel-T | Metastatic castrate-resistant prostate cancer | Completed |
| NCT05356182 | Immunotherapy | Solid tumors | Recruiting | |
| High protein diet | NCT05677958 | Chemo(radio)therapy or immunotherapy | Colorectal or non-small cell lung cancer | Completed |
| NCT03559881 | Chemotherapy and/or immunotherapy | Non-small cell lung cancer | Completed | |
| Supplementation of poly-unsaturated n-3 fatty acids and high-protein diet | NCT04965129 | Immunotherapy, chemotherapy and tyrosine kinase Inhibitors | Non-small cell lung cancer | Recruiting |
| Mediterranean diet | NCT04045392 | Adjuvant hormone therapy | Breast cancer | Unknown |
| NCT04534738 | Chemotherapy | Cancer | Completed | |
| Mediterranean diet with or without exercise | NCT05839210 | Chemotherapy | Lymphoma | Recruiting |
| Plant-based Mediterranean diet (olive oil supplementary) | NCT01083771 | Androgen deprivation therapy | Prostate cancer | Completed |
| Mediterranean-DASH intervention for neurodegenerative delay | NCT05984888 | Chemotherapy, targeted therapies or endocrine therapy | HR+ breast cancer | Recruiting |
| High-fiber diet | NCT05805319 | Immune checkpoint inhibition | Non-small cell lung cancer | Recruiting |
| NCT04534075 | Radiotherapy | Gynecological, colorectal, anal, prostate, or urinary bladder cancer | Recruiting | |
| NCT01549782 | Radiotherapy | Gynecological cancer | Completed | |
| NCT00888147 | Radiotherapy | Head & neck cancer | Completed | |
| Low-fiber diet or high-fiber diet | NCT01170299 | Radiotherapy | Gynecological, urological (bladder), colorectal or anal cancer | Completed |
| Isocaloric high-fiber diet | NCT04645680 | Pembrolizumab or nivolumab | Unresectable melanoma | Recruiting |
| High-fiber, plant-based diet and exercise | NCT04866810 | ipilimumab + nivolumab, relatlimab + nivolumab, pembrolizumab or nivolumab | Melanoma | Recruiting |
| Calorie-restricted plant-based diet and exercise | NCT04298086 | Anastrozole, letrozole or exemestane | HR+ breast cancer | Active, not recruiting |
| Low fat diet | NCT00002564 | Adjuvant therapy with or without either chemotherapy or endocrine therapy | Early-stage breast cancer | Completed |
| Carbohydrate-restricted, high-fat diet | NCT04253808 | Radiotherapy | Head & neck cancer | Completed |
| Oral vitamins A and E | NCT00228319 | Chemotherapy | Ovarian cancer | Completed |
| Oral vitamin A and folic acid | NCT05720559 | Oxaliplatin, cetuximab and metronidazole | Prehepatic CTC+ colorectal cancer | Not yet recruiting |
| NCT05774964 | Oxaliplatin, cetuximab and metronidazole | Colorectal cancer with liver metastases | Not yet recruiting | |
| Vitamins B6 and B12 supplementation | NCT00659269 | Chemotherapy | Cancer | Completed |
| Vitamin B12 and folic acid supplementation | NCT02679443 | Chemotherapy | Non-small cell lung cancer | Completed |
| NCT00609518 | Pemetrexed and dexamethasone | Non-small cell lung cancer | Completed | |
| NCT00216099 | Pemetrexed | Hormone refractory prostate cancer | Completed | |
| Oral vitamin D | NCT03467789 | Radiotherapy | Basal cell carcinoma | Recruiting |
| NCT04864431 | Chemotherapy | Epithelial ovarian cancer | Recruiting | |
| NCT02603757 | Chemotherapy | Colorectal cancer | Completed | |
| NCT04091178 | Chemotherapy | Breast cancer | Completed | |
| NCT04677816 | Chemotherapy | Triple-negative breast cancer | Recruiting | |
| NCT03331562 | Pembrolizumab | Metastatic pancreatic ductal adenocarcinoma | Completed | |
| Oral vitamin D and Omega-3 | NCT05331807 | Chemotherapy | Breast cancer | Recruiting |
| Oral vitamin E | NCT03613389 | Chemotherapy | Pediatric cancer | Unknown |
| NCT00363129 | Chemotherapy | Cancer | Completed | |
| Oral vitamin E and Hydrogen-rich water | NCT04713332 | Radiotherapy | Rectal cancer | Unknown |
| Antioxidant-deficient diet | NCT00486304 | Chemotherapy and radiotherapy | Oropharyngeal cancer | Completed |
| Anti-inflammatory diet | NCT03994055 | Chemo-radiotherapy and brachytherapy | Cervical cancer | Active, not recruiting |
| Low copper diet | NCT00003751 | Radiotherapy and penicillamine | Glioblastoma multiforme | Completed |
| Paleolithic diet and exercise | NCT04574323 | Radiotherapy | Breast cancer | Completed |
| Low-residue diet | NCT00258401 | Radiotherapy | Uterine, cervical or prostate cancer | Completed |
KD also enhances the antitumor effects of PD-1 blockade alone or in combination with anti-CTLA-4 antibodies. Mechanistically, the principal ketone body 3-hydroxybutyrate (3HB) in a KD prevented the ICB-mediated upregulation of PD-L1 on myeloid cells while simultaneously promoting the expansion of CXCR3+ T cells.237 Similarly, KD enhanced the effectiveness of anti-CTLA-4 immunotherapy by reducing PD-L1 protein levels and augmenting the expression of interferons and antigen presentation-related genes. When combined with immunotherapy, the intake of a KD can reshape the TME by increasing the population of CD8+ TILs, macrophages and CD86+ DCs. Mechanistically, the activation of AMPK via KD intake is the key molecular event that promotes immunotherapy efficacy. This activated AMPK phosphorylates PD-L1 on Ser283, which interrupts its association with CMTM4 and results in PD-L1 degradation. Furthermore, AMPK phosphorylates EZH2, which impedes polycomb repressive complex 2 (PRC2), leading to an increase in interferons and antigen-presenting gene expression.238
Combining a protein-restricted diet with a vaccine or anti-PD-1 therapy has been shown to significantly inhibit tumor growth and prolong survival.239 Notably, treatment with a methionine-/cystine-restricted diet significantly increased the number of tumor-infiltrating CD8+ T cells and cytotoxic granzyme B+CD8+ T cells, which was further enhanced when combined with immunotherapy.163 Another study confirmed the inhibitory effect of dietary methionine restriction on tumor growth and its ability to synergize with PD-1 blockers to increase tumor control. Mechanistically, this dietary approach reduced the number of metabolites, such as S-adenosylmethionine (SAM), which controls N6-methyladenosine (m6A) methylation reactions, in cancer cells. A reduction in the SAM level altered the m6A modification rate and decreased the expression of PD-L1 and V-domain Ig suppressor of T-cell activation (VISTA) in cancer cells.162 Moreover, the enzyme cyst(e)inase breaks down cystine and cysteine, thereby bolstering T-cell-mediated antitumor immunity and inducing ferroptosis in tumor cells when combined with PD-L1 blockade.240 IDO1 is a critical enzyme in the tryptophan–kynurenine pathway and has been identified as a promising immunomodulatory target.241 A phase 1/2 (ECHO-202/KEYNOTE-037) trial evaluating the effectiveness of the IDO1 inhibitor epacadostat combined with pembrolizumab on advanced solid tumors showed a high objective response rate (ORR) of 40.3% overall and 61.9% in malignant melanoma patients, demonstrating promising antitumor efficacy.242 Unfortunately, phase 3 trials failed to confirm these benefits. The ECHO-301/KEYNOTE-252 trial showed that combining epacadostat with pembrolizumab failed to prolong progression-free survival (PFS) or overall survival (OS) compared to pembrolizumab alone in patients with advanced melanoma.243
Despite being linked to T-cell dysfunction and poor cancer prognosis, obesity has paradoxically been shown to enhance the response to anti-PD-1/PD-L1 immunotherapy.244 Recent research suggests that immunotherapy yielded superior outcomes in obese patients, evidenced by an improved response rate and extended PFS and OS, in comparison to lean patients.245 However, obesity also promoted tumor growth and T-cell exhaustion, leading to increased PD-1 expression and dysfunction, partly due to high leptin levels. Despite this outcome, PD-1-mediated T-cell dysfunction in individuals with obesity was found to significantly enhance tumor responsiveness to PD-1/PD-L1 inhibitors, as confirmed by preclinical and clinical data.246 Therefore, obesity seems to be a double-edged sword for cancer immunotherapy, and the underlying mechanisms remain unclear and require further investigation.
Chemotherapy
Chemotherapy, a cornerstone of traditional cancer treatment, employs drugs to destroy rapidly dividing cells, a defining characteristic of cancer.247 Despite its widespread use and undeniable efficacy in many cases, chemotherapy often has substantial side effects due to its impact on healthy cells.248 Additionally, individual responses to chemotherapy can vary greatly and are influenced by a multitude of factors, including genetics, tumor characteristics, and, intriguingly, diet.15 A growing body of research now highlights the role of dietary interventions in modulating the effectiveness of chemotherapy, emphasizing the need to further understand these interactions for improved therapeutic outcomes.
Due to their expression of oncogenes, cancer cells are more susceptible to the effects of fasting and CR than are normal cells, an effect termed ‘differential stress resistance’.14,249,250 Based on this characteristic, CRM hydroxycitrate has been shown to increase sensitivity to chemotherapy by eliciting an adaptive cellular immune response, resulting in a decrease in the number of tumor-infiltrating Tregs into the tumor niche in various tumor models.122
Emerging research also suggests a profound influence of fasting or FMD on the efficacy of chemotherapy. In vitro studies indicate that fasting cycles not only retard tumor growth but also sensitize a wide array of cancer cell types to chemotherapy.14 This heightened sensitivity has been observed in various contexts, including the enhancement of gemcitabine efficacy in mice with prostate cancer xenografts and the increased efficacy of chemotherapy in triple-negative breast cancer via the upregulation of ROS.251,252 FMD combined with vitamin C can potentially increase the effectiveness of chemotherapy for treating KRAS-mutant cancer cells by reversing the vitamin C-induced upregulation of HO-1 and ferritin.253 Furthermore, when combined with a ferroptosis inducer, FMD can effectively eliminate slow-cycling, chemotherapy-resistant cells, suggesting a potential strategy for enhancing the sensitivity of certain difficult-to-treat cancers to chemotherapy through dietary interventions.254 Interestingly, fasting can also counteract certain adverse effects of chemotherapy. For instance, it has been demonstrated to enhance self-renewal in hematopoietic stem cells and mitigate the immunosuppression induced by cyclophosphamide chemotherapy in mice.255 In tumor-bearing mice, both prolonged fasting and FMDs can induce specific stress resistance responses, enhancing chemotoxicity in cancer cells while protecting normal cells.256 This dual action is partly mediated by the reduction in IGF-1 and glucose levels, thus shielding normal cells and organs from chemical toxicity.250 The potential of FMD in clinical settings has been supported by the ‘DIRECT’ study involving HER2-negative stage II/III breast cancer patients. This study revealed that treatment with FMD, administered three days prior to and during neoadjuvant chemotherapy, enhanced therapeutic efficacy without increasing toxicity or reducing chemotherapy-induced DNA damage in T cells.257 Collectively, these findings highlight the potential of fasting and FMD as adjuncts to chemotherapy, warranting further exploration and clinical testing.
In addition to slowing tumor growth, KD also sensitizes tumor cells to classic chemotherapy. For example, the combination of KD with metronomic cyclophosphamide significantly enhances antitumor effects, resulting in the regression of neuroblastoma tumors.258,259 Similarly, in pancreatic cancer, cotreatment with KD and cytotoxic chemotherapy substantially elevates tumor NADH levels, synergistically suppressing tumor growth and tripling survival benefits compared to chemotherapy alone.260
Radiotherapy
Dietary interventions have emerged as promising strategies for enhancing the efficacy of radiotherapy in cancer treatment. For instance, CR combined with radiotherapy, has been shown to modulate the TME in a triple-negative breast cancer model by decreasing the number of intratumoral Tregs, increasing the CD8+ cell: Treg ratio, and upregulating PD-1 expression on CD8+ T cells. Furthermore, compared with patients who received radiotherapy alone, breast cancer patients who underwent CR concurrently with radiotherapy exhibited a significant reduction in the serum levels of immunosuppressive cytokines, suggesting potential benefits of CR in mitigating radiation-induced immunosuppression.261
When combined with radiation or radiochemotherapy, KD slows tumor growth in lung cancer xenografts, potentially through a mechanism involving increased oxidative stress.262 Additionally, KD was shown to enhance radiation sensitivity in a pancreatic cancer xenograft model, suggesting potential improvements in therapeutic outcomes. However, phase 1 clinical trials in patients with locally advanced non-small cell lung cancer and pancreatic cancer showed suboptimal compliance with the diet, indicating challenges in practical application.263
Moreover, other dietary restrictions, such as methionine deprivation, have shown promising results in enhancing the efficacy of radiation and antimetabolite chemotherapy. In patient-derived xenograft and autochthonous tumor mouse models, methionine restriction sensitized tumor cells to these treatments, possibly via alterations in one-carbon metabolism.264
Other therapies
In hormone receptor-positive breast cancer mouse models, periodic fasting or an FMD can enhance the therapeutic effects of endocrine agents such as tamoxifen and fulvestrant. This enhancement is believed to occur through a reduction in circulating IGF1, insulin, and leptin levels and suppression of AKT-mTOR signaling. Concurrent administration of these dietary strategies with a therapeutic regimen of fulvestrant and palbociclib has been associated with prolonged tumor regression and reversal of treatment resistance. Analogous metabolic alterations found in patients on an FMD during estrogen therapy suggest the potential of diet as an adjuvant in treating hormone receptor-positive breast cancer.265
In addition to their effects on hormone-driven cancers, fasting or FMD has also been shown to enhance the efficacy of tyrosine kinase inhibitors (TKIs) across different cancer cell lines. Mechanistically, these effects are attributed to the increased ability of TKIs to block cancer cell growth and inhibit the MAPK signaling pathway under starvation conditions.266 Another study reported that in HCC cells, xenografts, and patient-derived organoids, fasting improved the therapeutic response to sorafenib through the regulation of glucose transporters and proapoptotic protein expression by p53.267
KD has also shown promise in supporting the effectiveness of phosphatidylinositol 3 kinase (PI3K) inhibitors and overcoming drug resistance in various mouse cancer models, including pancreatic, bladder, endometrial, and breast cancer models, as well as acute myeloid leukemia.145 KD appears to enhance this effectiveness by decreasing hyperglycemia and reducing insulin secretion, actions correlated with a decrease in mTORC1 signaling within the tumor.268
Finally, the combination of serine deprivation and biguanide treatment, such as phenformin and metformin, can lead to metabolic stress in cancer cells. This stress arises from the forced upregulation of glycolysis due to the biguanide-induced reduction in OXPHOS. Under conditions of serine deficiency, this stress may exceed the metabolic flexibility of cancer cells, leading to their potential death and, consequently, enhanced anticancer effects.269
In summary, these findings underscore the potential of dietary interventions to modulate the therapeutic landscape of cancer treatment, enhancing the effectiveness of drugs and potentially overcoming resistance mechanisms. However, it should be viewed with cautious optimism. The biological plausibility of diet modifying treatment efficacy and resistance is compelling; however, the translation of this concept into clinical practice requires rigorous validation. It is critical to remain grounded in evidence-based medicine, recognizing that dietary strategies are adjuncts, not replacements, for established therapeutic regimens. Further exploration and clinical validation are necessary to fully understand these interactions and to integrate dietary strategies into standard cancer care effectively and safely.
Diet changes the gut microbiome in conjunction with antitumor effects and cancer treatment
The gut microbiome encompasses the genetic makeup of all species within the gut, such as bacteria, viruses, yeasts, protozoans, fungi, and archaea, and can be affected by a range of internal and external factors.270 The gut microbiota plays a significant role in influencing the health and disease status of the host. The constituents of the gut microbiome and their interactions with the host immune system can impact the development of tumors and carcinogenesis.271 Various dietary patterns have been found to significantly influence the composition and functionality of the gut microbiome.272,273 It is through these changes in the gut microbiome that dietary patterns can indirectly influence the outcomes of cancer patients.274
In recent early studies, several interventional strategies, ranging from dietary interventions to fecal microbiome transplant (FMT) and prebiotic, probiotic and antibiotic treatments, have shown promise in altering the composition or functional capacity of the gut microbiome.275 Two prospective cohort studies have suggested that diet-related inflammation can alter the gut microbiome, leading to the development of CRC by suppressing adaptive antitumor immune responses.276,277 Other prospective cohort studies have revealed the associations between prudent diets (rich in whole grains and dietary fiber) and Western diets (rich in red and processed meat, refined grains, and desserts) with CRC risk and indicated that the effect of these diets may differ based on the presence of Fusobacterium nucleatum in tumor tissue.278,279 Specifically, these studies showed that, compared with a Western diet, adhering to a long-term prudent diet is associated with a reduced risk of F. nucleatum-positive CRC; however, it does not appear to mitigate the risk of F. nucleatum-negative CRC.278 A recent study investigated the impact of the gut microbiota and dietary patterns on the response to ICIs in patients with melanoma. The present study revealed that patients with microbiomes dominated by the Ruminococcaceae family had greater response rates than did those with microbiomes dominated by the Bacteroidaceae family. Furthermore, another finding revealed that a poor response was associated with decreased intake of fiber and omega-3 fatty acids.280 These results suggest that dietary interventions may be promising for improving cancer treatment outcomes.
Accumulating data suggest that alterations in the gut microbiome primarily contribute to the progression, prognosis, and treatment of cancer, primarily through interactions with the immune system. Metabolites produced by the microbiota play important roles in modulating antitumor immunity.281,282 Microbiota-derived metabolites have been demonstrated to influence the efficacy of tumor immunotherapy. Short-chain fatty acids (SCFAs) are produced primarily by the fermentation of nondigestible carbohydrates, such as dietary fiber, by the microbiota. The main SCFAs include acetate, propionate, and butyrate.283,284 The gut microbiota, which is mediated by SCFAs, can potentiate the antitumor activity of CD8+ T cells, thereby influencing the efficacy of tumor immunotherapy both in vitro and in vivo.285 Metabolic and epigenetic reprogramming enables pentanoate and butyrate to enhance the effectiveness of cancer immunotherapy by boosting the antitumor activity of antigen-specific cytotoxic T lymphocytes and ROR1-targeting chimeric antigen receptor (CAR)-T cells.286 Inosine is another important metabolite produced by the microbiome and is closely associated with immunotherapy. Intestinal Bifidobacterium pseudolongum promoted Th1 cell transcriptional differentiation and antitumor activity to increase the efficacy of immunotherapy, mainly through the action of inosine.287 Inosine is instrumental in enhancing antitumor therapy by serving as a carbon source for CD8+ T cells in glucose-restricted microenvironments, facilitating their growth and optimal functioning.288 Moreover, engineered bacteria can modify the concentration of metabolites in the microenvironment, thereby altering the composition of the TME. For instance, the genetically engineered probiotic strain Escherichia coli Nissle 1917 colonizes tumor sites and continuously converts ammonia metabolites into L-arginine. When injected into the tumor, this strain has been shown to increase the concentration of L-arginine within the microenvironment, leading to increased infiltration of tumor-infiltrating T cells, sustained effector T-cell functions, increased tumor-specific T-cell memory formation, and enhanced efficacy of PD-L1-blocking antibodies.289
Recent research has highlighted the role of the gut microbiota in the antitumor effects of dietary intervention (Fig. 3). Specifically, enrichment of Bifidobacterium bifidum after CR increases acetate levels, which in turn elevates IFNγ+CD8+ T cells in the TME. In contrast, the antitumor effect of IF was not mediated by the gut microbiome, as it was not abrogated after the microbiota was depleted.290 Similarly, recent studies have revealed that KD significantly influences the gut microbiota, inducing a shift from a population dominated by tolerogenic bacteria (Lactobacilli spp., Clostridium asparagiforme) toward a population dominated by an increase in immunogenic bacteria (such as Akkermansia muciniphila).237 It has been reported that a shift in the gut microbiota is partially attributable to the host’s production of ketone bodies due to the intake of a KD. Among these ketone bodies, β-HB selectively suppresses the proliferation of Bifidobacterium. This suppression subsequently leads to a reduction in intestinal Th17 immune cells.291 Dietary methionine/cystine restriction has been shown to alter the gut microbiota and potentially contribute to immune system alterations. Specifically, this type of diet restriction promoted a significant decrease in the relative abundance of multiple Ruminococcaceae and Prevotellaceae families while increasing the presence of members of the Lactobacillaceae family.163 Consumption of an HSD promotes an increase in the abundance of Bifidobacterium, which, due to enhanced gut permeability, infiltrates tumors, subsequently augmenting the functionality of NK cells and ultimately contributing to tumor regression. These results suggest that HSD intake modulates the gut microbiome, which may stimulate NK cell-dependent tumor immunity, thereby providing potential implications for the development of novel therapeutic interventions.183 The intake of HSD has also been shown to inhibit enterotoxigenic Bacteroides fragilis (ETBF)-promoted colon carcinogenesis by decreasing the expression of IL-17A and iNOS, thereby inhibiting inflammation.292 However, intake of an HSD can exacerbate Helicobacter pylori infection, contributing to gastric carcinogenesis.293 In a mouse model of Barrett’s esophagus, feeding an HFD was observed to promote dysplasia and carcinogenesis by modulating the esophageal microenvironment and gut microbiome, thereby inducing inflammation and promoting stem cell proliferation.294 The bile salt hydrolase (BSH) enzyme expressed by Bacteroides was also found to play a crucial role in CRC progression in overweight patients and in model mice with HFD-induced CRC. High BSH activity activates the β-catenin/CCL28 axis, resulting in an increase in immunosuppressive Tregs and accelerated CRC progression.295 Moreover, HFD feeding can reduce the level of SCFA-producing bacteria and the rate of SCFA production, leading to decreased levels of SCFAs that can activate the MCP-1/CCR2 axis. This effect promotes M2 TAM recruitment and polarization, ultimately contributing to CRC progression.296
Fig. 3.
Mechanisms by which diet modulates antitumor effects and cancer treatment via modulation of the gut microbiome. a Calorie restriction (CR) elevates IFNγ+CD8+ T cells in the tumor microenvironment (TME) by enriching Bifidobacterium bifidum and increasing acetate levels. b Ketogenic diet (KD) induces a shift from tolerogenic (Lactobacilli spp., Clostridium asparagiforme) toward immunogenic bacteria (such as Akkermansia muciniphila) driven by host production of ketone bodies, of which β-HB selectively inhibits the growth of bifidobacteria, resulting in KD-associated decreases in intestinal Th17 cell levels. c High-salt diet (HSD) increases the abundance of Bifidobacterium and leads to intratumoral localization of Bifidobacterium, further enhancing NK cell functions and tumor regression. HSD decreases the expression of IL-17A and iNOS and inhibits inflammation, which reduces enterotoxigenic Bacteroides fragilis (ETBF)-promoted colon carcinogenesis. HSD exacerbates Helicobacter pylori infection and promotes gastric carcinogenesis. d High-fat diet (HFD), through augmentation of queuosine-producing gut bacteria, can incite chemotherapy resistance in pancreatic cancer patients. HFD reduces SCFA-producing bacteria and SCFA production, leading to decreased levels of short-chain fatty acids (SCFAs) that activate the MCP-1/CCR2 axis, which promotes M2 TAM recruitment and polarization, ultimately contributing to colorectal cancer (CRC) progression. High bile salt hydrolase (BSH) enzyme activity in an HFD mouse model activates the β-catenin/CCL28 axis, further inducing immunosuppressive Tregs and accelerating CRC progression. e Dietary intake rich in tryptophan stimulates certain Bacteroides to produce the metabolite indole-3-acetic acid (3-IAA). Increased levels of 3-IAA enhance the efficacy of chemotherapy treatment. Dietary intake rich in tryptophan, through the action of the probiotic Lactobacillus reuteri (Lr), leads to the production of the metabolite indole-3-aldehyde (I3A). This metabolite promotes the production of IFNγ from CD8+ T cells, thereby enhancing antitumor immunity and the efficacy of immune checkpoint inhibitors (ICIs). f High-fiber diet enriches Akkermansia muciniphila which produces the microbiota-derived STING agonist c-di-AMP, inducing type I interferon (IFN-I) production by intratumoural monocytes, resulting in various TME modulation pathways, including reprogramming of mononuclear phagocytes into immunostimulatory monocytes and DCs, promoting macrophage polarization toward an antitumor phenotype and stimulating crosstalk between NK cells and DCs, further enhancing the therapeutic effect of immunotherapy. Dietary fiber inulin can enhance the effectiveness of anti-PD-1 therapy by increasing the abundance of beneficial commensal microbes (e.g., Akkermansia, Lactobacillus and Roseburia) and SCFAs, further increasing the number of stem-like T-cell factor-1 (Tcf1)+PD-1+CD8+ T cells numbers. Dietary fiber pectin can improve the effectiveness of anti-PD-1 therapy by increasing the abundance of butyrate-producing bacteria, further promoting T-cell infiltration and activation in the TME. This figure was created with BioRender.com
Studies suggest that the gut microbiota plays a crucial role in modulating the therapeutic response to immunotherapy.297,298 In fact, specific gut microbial signatures have been shown to differentiate responders from nonresponders across various epithelial tumor types in cohorts treated with ICB.299 Considering the profound impact of the gut microbiota on the immune system, research investigating the modulation of the gut microbiota via dietary interventions to optimize cancer treatment efficacy has been predominantly centered around immunotherapy. A high-fiber dietary intervention has been associated with significantly prolonged PFS in melanoma patients receiving ICB treatment.300 Microbiota-derived STING agonists, specifically c-di-AMP, induce the production of type I interferon (IFN-I) in intratumoral monocytes. This activation results in the transformation of mononuclear phagocytes within the TME into immunostimulatory monocytes and DCs. Additionally, it promotes the polarization of macrophages to antitumor macrophages and stimulates crosstalk between NK cells and DCs. A high-fiber diet can trigger this mechanism by enriching the population of Akkermansia muciniphila, which produces c-di-AMP and enhances the therapeutic effect of ICB in melanoma patients.301 The presence of Akkermansia, a mucin-degrading bacterium, is strongly associated with favorable outcomes in cancer patients.302 Moreover, inulin, a polysaccharide dietary fiber, can enhance the effectiveness of anti-PD-1 therapy by increasing the abundance of beneficial commensal microbiota genera (e.g., Akkermansia, Lactobacillus and Roseburia) and SCFAs, further increasing the number of stem-like T-cell factor-1 (Tcf1)+PD-1+CD8+ T cells.303 Similarly, oral administration of pectin, another dietary polysaccharide fiber, can largely improve the efficacy of anti-PD-1 mAbs by increasing the number of butyrate-producing bacteria, which is sufficient to promote T-cell infiltration and activation in the TME.304
Although research into the antitumor or protumor effects of the intratumor microbiome is still in its early stages, recent studies have started to focus on how the intratumor microbiome can influence the effectiveness of immunotherapy. The colonization of Bifidobacterium in the microenvironment, combined with anti-CD47 monoclonal antibody treatment, stimulates the STING signaling pathway and enhances the cross-priming of DCs to upregulate CD8+ T cells.305 The probiotic Lactobacillus reuteri (Lr) within melanoma promotes the local generation of IFNγ by CD8+ T cells through the release of its tryptophan breakdown metabolite, indole-3-aldehyde (I3A), thus enhancing ICI efficacy. Dietary intake rich in tryptophan boosts the antitumor immunity induced by Lr and ICI, which is dependent on the CD8+ T-cell AhR signaling pathway.306
Apart from immunotherapy, recent research has also started to investigate how diet, by influencing the gut microbiota, could affect other forms of cancer treatment. By enriching the gut microbiome with queuosine-producing bacteria, HFD can induce chemotherapy resistance in pancreatic cancer through the upregulation of the oxidative stress protector PRDX1. This resistance can be counteracted by SAM, which is typically produced by bacteria in lean diets, highlighting the influence of diet on chemotherapy effectiveness via gut microbiome adjustments.307 Expanding on the theme of diet’s influence on chemotherapy effectiveness in pancreatic cancer, another study revealed that the microbiota-derived tryptophan metabolite indole-3-acetic acid (3-IAA) is enriched in patients responsive to chemotherapy. Through dietary manipulation of tryptophan, an increase in 3-IAA production enhances chemotherapy efficacy by disrupting cancer cell metabolic fitness via increased reactive oxygen species and reduced autophagy.308 These findings further emphasize the crucial role of gut microbiota modulation via dietary interventions in cancer treatment outcomes.
Despite the significant progress in this field, the complex relationships among dietary factors, the gut microbiota, and cancer treatment still need to be understood. Each individual’s microbiome is unique, influenced by genetics, diet, environment, and lifestyle, which adds layers of complexity to the task of identifying universally beneficial interventions. Additionally, the development of high-throughput technologies and bioinformatics tools for microbiome analysis will be vital in deciphering these complex interactions. These advancements could enable the identification of biomarkers for microbiome-related treatment responses and the customization of diet-based interventions to enhance the efficacy of cancer therapies. The identification of specific dietary factors and gut microbiota constituents that can enhance the effectiveness of cancer therapies may lead to the development of personalized treatments to improve therapeutic outcomes for cancer patients.
Implications of dietary intervention for other diseases
Dietary interventions may induce, prevent or delay the progression of various diseases in addition to cancer, which also influence human health and longevity. Healthy dietary patterns that are rich in fiber and beneficial nutrients may reduce the risk of disease, while unhealthy dietary patterns may increase the risk of disease and worsen clinical outcomes.309 Here, we summarize preclinical and human studies revealing the implications and mechanisms of various dietary patterns on other diseases in addition to cancer, including neurodegenerative diseases, autoimmune diseases, CVD, and metabolic disorders.
Neurodegenerative diseases
Several neurodegenerative diseases (NDs), such as epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS), which feature chronic progressive damage to the nervous system, have been proven to be tightly connected with nutrient availability and dietary patterns.310 The underlying mechanisms of various dietary interventions mainly include altering neurotransmitters, remodeling, interfering with brain energy metabolism and mitochondrial function, and altering inflammation and oxidative stress. The underlying mechanisms also include altering the composition and balance of the gut microbiome, which further influence the process of neurodegeneration via the gut-brain axis (Fig. 4).
Fig. 4.
Impact of different diets on neurodegenerative diseases. The ketogenic diet (KD) can enhance inhibitory neurotransmission and anti-inflammatory effects in epilepsy, influence the gut microbiota, and elevate beneficial metabolites. KD is particularly beneficial for treating pediatric drug-resistant epilepsy with elevated specific Bifidobacteria and TNF. In Alzheimer’s disease (AD) and Parkinson’s disease (PD), KD could counteract decreased β-HB levels, inhibit the NLRP3 inflammasome, reduce pathology, and alleviate symptoms by inhibiting microglial activation. Fasting mimicking diet (FMD) enhances the gut microbiota composition and metabolites, inhibiting neuroinflammation. This results in the attenuated loss of dopaminergic neurons in the substantia nigra in patients with PD. Caloric restriction (CR) may prevent AD by lowering serum tyrosine levels, reversing the exhaustion of tyrosyl-tRNA synthetase (TyrRS), and upregulating the sirtuin pathway, which attenuates the amyloidogenic processing of amyloid-β protein precursor (APP). Dietary restriction can increase brain-derived neurotrophic factor (BDNF) and chaperone heat-shock protein-70 (HSP70) levels in the striatum and cortex, which are relevant to Huntington’s disease (HD). High-fat diet (HFD) can accelerate recognition-memory impairment in an AD mouse model by increasing blood N-acetylneuraminic acid (NANA) levels, leading to systemic immune exhaustion. Conversely, the Mediterranean diet (MD) may protect against memory decline and mediotemporal atrophy by lowering amyloid-β protein and phosphorylated tau levels, reducing AD risk. This figure was created with BioRender.com
KD has been clinically applied for nearly a century as alternative therapy for childhood intractable epilepsy, but there is sufficient evidence that a modified Atkins diet (MAD) is more tolerable and has a greater probability of causing seizure reduction than a classical KD according to a systematic review.311–313 Increased levels of the inhibitory neurotransmitter GABA can be observed in preclinical KD models and patient cerebrospinal fluid (CSF), dampening neuronal excitability.314–316 An increase in peroxisome proliferator activated receptor gamma 2 (PPARγ2) and upregulation of hippocampal catalase in KD-fed rats are observed, which may increase anti-inflammatory and antioxidant activity.317 In addition, a KD may upregulate potassium channels that are sensitive to ATP opening, reducing the electrical excitability of the brain and increasing the seizure threshold.318 The gut microbiota, which includes Akkermansia, Parabacteroides, and Bifidobacteria, also contributes to the neuroprotective effects of KD on epilepsy.319,320
Epidemiologic evidence indicates that obesity is an independent risk factor for AD, while HFD is closely associated with an increased risk of obesity.321 Recognition-memory impairment in an AD mouse model (5xFAD) can be accelerated by high-fat obesogenic diet by increasing blood levels of the metabolite N-acetylneuraminic acid (NANA), which results in systemic immune exhaustion.322 HFD may also enhance neuroinflammation by increasing circulating free fatty acids and cytokines, which may lead to cognitive impairment.323 Conversely, healthy dietary interventions, including the Mediterranean diet (MD), CR, and KD, may prevent AD progression.324–326 Adhering to MD may act as a protective factor against memory decline and mediotemporal atrophy, as indicated by decreased levels of amyloid-β protein and phosphorylated tau, reducing the risk of AD.327 CR may prevent AD by lowering serum tyrosine levels to reverse the exhaustion of tyrosyl-tRNA synthetase (TyrRS) and upregulating the sirtuin pathway, which attenuates the amyloidogenic processing of amyloid-β protein precursor (APP), as confirmed by in vivo and in vitro models.328,329 KD may reverse the decreased β-HB levels in red blood cells and the brain parenchyma of AD patients, hence inhibiting NLRP3 inflammasome activation and reducing AD pathology.330 In addition, diet can influence AD by modulating the gut microbiome and metabolites. For instance, a Mediterranean-ketogenic diet (MMKD) is associated with improved AD biomarkers in CSF, as indicated by increased Akkermansia muciniphila levels, which modulate GABA levels and gut transit time.331,332
Gut microenvironmental changes may trigger the development of PD through the gut-brain axis, as determined by the presence of α-synuclein and Lewy bodies in the enteric nervous system and the convincing association between PD and gut inflammation.333,334 Research has revealed changes in the gut microbiome in PD patients compared to healthy volunteers, highlighting the potential benefits of dietary interventions in treating PD patients.335 High serum sodium is associated with cognitive decline, as observed in the aged population.336 However, a recent study denies the association between HSD and neurodegeneration or α-synuclein accumulation in a PLP-hαSyn model, suggesting that the mechanism of HSD needs further exploration.337 Adhering to MD is associated with a decreased incidence of PD, the mechanisms of which may include reducing neuroinflammation, similar to AD.338,339 KD ameliorates motor and nonmotor symptoms in PD patients by inhibiting microglial activation340. FMD promotes a favorable gut microbiota composition and metabolites and inhibits neuroinflammation, consequently attenuating the loss of dopaminergic neurons in the substantia nigra in a PD model.341
Other neurodegenerative diseases with lower incidence rates are also relevant to dietary interventions. A clinical trial suggested that increased consumption of dairy products may increase the risk of phenoconversion, resulting in earlier onset of HD.342 In addition, high antigliadin antibody titers in patients with HD suggest the potential value of applying gluten-free diet in HD patients.343 A dietary restriction regimen retarded the progression of neuropathological, behavioral, and metabolic abnormalities in an HD model, resulting in an extension of life span by increasing brain-derived neurotrophic factor and chaperone heat-shock protein-70 (HSP70) levels in the striatum and cortex, the mechanisms of which still need further explanation.344 A cross-sectional baseline analysis revealed that a higher intake of antioxidants and carotenes may result in greater ALS function.345 Another meta-analysis revealed that a greater intake of ω-3 PUFAs is associated with a reduced risk of ALS.346 Although weight loss has been identified as a negative prognostic factor, high-calorie fatty acid diet provides a significant survival benefit for patients in the subgroup of fast-progressing ALS patients only.347
Autoimmune diseases
Different types of autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), Hashimoto’s thyroiditis (HT), and multiple sclerosis (MS), can cause distinct clinical features from abnormal activation of the immune system that erroneously attacks healthy host cells and tissues. Impaired gut barrier function, also referred to as a “leaky gut”, which may disrupt the balance between tolerance and immunity to non-self-antigens, is often observed in autoimmune diseases.348 This finding suggested a close relationship between diet, the gut, and autoimmune diseases. Dietary interventions may influence the susceptibility, progression and treatment response of these autoimmune diseases through various mechanisms, from adjusting inflammation levels and immune cell composition to adjusting the gut microbiome composition (Fig. 5).
Fig. 5.
Impact of different diets on autoimmune diseases. Extravirgin olive oil (EVOO) can reduce joint inflammation and degradation in rheumatoid arthritis (RA) due to its phenolic compounds. However, the protective effects of a high-fiber diet can be reversed by Prevotella copri colonization, which promotes proinflammatory responses. Fish oil supplementation can suppress proinflammatory cytokines and cartilage degradation, improving RA outcomes. Vitamin D can inhibit the proliferation, differentiation, and function of B and T cells, potentially reducing inflammatory cytokine expression in systemic lupus erythematosus (SLE) patients. A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can alleviate gut symptoms in quiescent inflammatory bowel disease (IBD) patients, possibly by regulating the immune response through reducing fecal microbial abundance. However, a high-fat diet (HFD) can exacerbate pre-IBD inflammation by impairing epithelial mitochondrial bioenergetics and triggering microbiota disruptions, especially when combined with antibiotics. High salt diet (HSD) can exacerbate autoimmune conditions such as multiple sclerosis (MS) by promoting the induction of pathogenic Th17 cells. Intermittent fasting (IF) can improve MS by reducing the number of IL-17-producing T cells, increasing the number of Tregs in the gut, and enhancing antioxidative microbial metabolic pathways. However, the Western diet can impair myelin-debris clearance in microglia, hindering lesion recovery after demyelination and potentially contributing to MS induction. This figure was created with BioRender.com
A healthy MD may benefit RA by reducing inflammatory activity and increasing physical function.349 Phenolic compounds in extravirgin olive oil (EVOO), an essential component of the MD, can decrease joint edema, cell migration, cartilage degradation and bone erosion by reducing the levels of proinflammatory cytokines and prostaglandin E2 in the joint.350 However, the protective effect of high-fiber diet may be reversed if there exists colonization of Prevotella copri, which leads to the overproduction of organic acids, including fumarate and succinate, during the digestion of complex fibers and the promotion of proinflammatory responses in macrophages, exacerbating arthritis in an RA model.351 In addition, abundant supplementation of fish oil benefits the clinical outcome of RA by suppressing the production of proinflammatory cytokines and cartilage degradative enzymes.352 The erythrocyte level of ω-6 PUFAs acts as a biomarker that inverses the risk of RA, and the remission rate of RA increases when ω-3 PUFAs are added to disease-modifying anti-rheumatic drug (DMARD) treatment.353,354
Dysbiosis of the gut microbiome can be observed in SLE patients, including a decreased richness and diversity of the gut microbiota and a reduced proportion of Firmicutes/Bacteroides (F/B); the latter may promote lymphocyte activation and Th17 differentiation from naïve CD4+ lymphocytes.355,356 Blooming of Ruminococcus (blautia) gnavus occurs at times of high disease activity and during lupus nephritis, indicating that it is the driver of often remitting-relapsing SLE.357 Another analysis showed that Veillonella dispar has a positive association with the activity of SLE.358 According to a systematic review, nutritional support in the SLE population is focused mainly on interventions involving ω-3 and vitamin D.359 The anti-inflammatory effect of ω-3 may contribute to its clinical function, similar to that of RA.360 Vitamin D blocks the proliferation, differentiation and function of B cells and T cells, which may attenuate the expression of inflammatory cytokines in patients with SLE.361 Inadequate levels of serum vitamin D have been observed in SLE patients, suggesting the importance of supplementing their diet with vitamin D362. Dietary patterns other than single nutrients as supplementary treatments for SLE still require further investigation.363
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major clinical phenotypes of IBD. Dietary management and microbiota modulation have been clinically recommended for IBD treatment according to clinical guidelines.364 Obesity is a risk factor for IBD, especially for CD.365 As a potential trigger of obesity, HFD, together with antibiotics, exacerbates inflammation in pre-IBDs by impairing epithelial mitochondrial bioenergetics and triggering microbiota disruptions in mouse models.366 However, IBD increases the risk of malnutrition, which triggers inflammatory responses and subsequently leads to poor clinical outcomes.367 Therefore, dietary interventions and nutritional care should be planned according to the precise nutritional assessment and dietary assessment for IBD patients.368 Exclusive enteral nutrition (EEN), the first-line therapy in pediatric patients with active CD, can effectively decrease clinical activity and reduce the complications of CD simultaneously, but its benefit in adults still lacks competent evidence.369 Similarly, CD exclusion diet (CDED) positively correlates with the clinical remission of pediatric patients with active CD.370 In addition, diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can relieve the gut symptoms of patients with quiescent IBD, possibly reducing the fecal abundance of microbes and thereby regulating the immune response of the host.371
Dietary interventions may also influence the risk and clinical outcome of other autoimmune diseases. A recent study on HT suggested that low intake of animal foods, mainly meat, has a protective effect on thyroid autoimmunity and potentially has a positive influence on redox balance, which further reduces oxidative stress-related disorders.372 Improvement in HT has also been observed in other dietary interventions, including elimination of gluten or lactose, energy restriction, and consumption of Nigella sativa, suggesting the potential benefit of diet as a complementary treatment for HT.373 MS is more common in western countries, suggesting diet as a potential risk factor.374 Western diet triggers impaired myelin-debris clearance in microglia, thereby impairing lesion recovery after demyelination, which may explain its role in MS induction.375 Moreover, an elevated intake of dietary salt can exacerbate autoimmune conditions by promoting the induction of pathogenic Th17 cells, contributing to MS.376 Conversely, IF diet ameliorates the clinical course and pathology of MS by reducing the number of IL-17-producing T cells, increasing the number of Tregs in the gut and increasing the richness of gut bacteria, which enhance antioxidative microbial metabolic pathways.377 Vitamin D supplementation has been shown to lower the incidence and benefit MS patients with sufficient evidence, and a “Coimbra Protocol” referring to daily doses up to 1000 I.U. vitamin D3 per kg body weight is clinically applied to treat patients with MS.378,379
Cardiovascular diseases (CVD)
According to epidemiological studies, obesity and unhealthy diet are risk factors for CVD. Greater dietary fiber intake from cereal, vegetables and fruits is associated with a lower risk of CVD, suggesting that high-fiber diet is a potential protective factor.380 An experimental model fed with diet lack of prebiotic fiber induces hypertension through inducing deficiency of SCFA production and GPR43/109A signaling, suggesting the underlying mechanisms of dietary fiber.381 Besides, high-fiber diet and acetate supplementation can lead to changes in the gut microbiota, particularly an increase in Bacteroides acidifaciens, which is protective against the development of CVD.382 Other healthy dietary patterns, including the Nordic diet, the Dietary Approaches to Stop Hypertension (DASH) diet, the MD, and the vegetarian diet, also have protective effects on CVD risk.383 High sodium intake is the leading dietary risk factor for CVD.384 High salt load may induce persistent hepatic steatosis and inflammation by inhibiting SIRT3 expression, thereby contributing to cardiovascular damage.385 Conversely, a low-sodium diet may dampen the risk of CVD, which is highly recommended by current dietary guidelines.386 Amino acids play different roles in the progression of CVD. Diet with high-unsaturated fatty acid composition and less saturated fat might be cardioprotective.387 In contrast, higher intake of BCAAs is associated with increased platelet activity and arterial thrombosis formation; therefore, BCAA levels are associated with the risk of CVD.388
Therapeutic implications of diet for CVD treatment have also been a focus of recent studies. CR attenuates hypertension, left ventricular remodeling and diastolic dysfunction in DS/obese rats by reducing cardiac oxidative stress and inflammation.389 In addition, a combination of CR and exercise can improve cardiac mitochondrial dynamics, decrease cardiac apoptosis, and maintain cardiac [Ca2+]i homeostasis in obese insulin-resistant rats.390 CR also helps to maintain the iron homeostasis of cardiomyocytes.391 These findings suggest the function of CR in cardiac protection. However, strictly adhering to CR is very difficult for most patients. IF is easier to perform than CR and has similar potential clinical value.392 FMD, a 5-day fasting dietary pattern, increases cardiac vascularity and function and resistance to cardiotoxins in a high-fat, high-calorie diet (HFCD) mouse model, thereby postponing the process of cardiac aging.393 Alternate day fasting (ADF) improves cardiovascular marker levels, including reduced fat mass, an improved fat-to-lean ratio, and increased β-HB-hydroxybutyrate levels, suggesting its clinical relevance for CVD intervention.394 KD has a beneficial effect on the blood lipid profile, the NLRP3 inflammasome, myocardial energy metabolism, and the vascular endothelium, benefiting CVD patients.395 However, research on healthy individuals has reported that lipid profiles deteriorate in response to a KD, suggesting that its role in preventing CVD in the normal population needs further inquiry (Fig. 6).396
Fig. 6.
Impact of different diets on cardiovascular diseases. Calorie restriction (CR) can reduce cardiac oxidative stress and inflammation, improve cardiac mitochondrial dynamics and maintain cardiac ion homeostasis, which may be protective against cardiovascular disease (CVD) in obese and/or insulin-resistant models. Fast-mimicking diet (FMD) increases cardiac vascularity and function and resistance to cardiotoxins in a high-fat, high-calorie diet (HFCD) mouse model. Alternate day fasting (ADF) improves cardiovascular markers, for example, reduced fat mass. Ketogenic diet (KD) inhibits the NLRP3 inflammasome and improves the blood lipid profile but may lead to impaired blood lipid profiles in healthy individuals. High-salt diet (HSD) can inhibit SIRT3 expression and induce persistent hepatic steatosis and inflammation, thereby contributing to cardiovascular damage. A diet lacking prebiotic fiber induces hypertension through inducing a deficiency in short-chain fatty acid (SCFA) production and GPR43/109A signaling. High branched-chain amino acid (BCAA) intake is associated with increased platelet activity and arterial thrombosis formation. This figure was created with BioRender.com
Metabolic disorders
Overnutrition is a driving factor for obesity and related metabolic disorders, mainly including type 2 diabetes mellitus (T2DM), metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), and polycystic ovarian syndrome (PCOS).397 In addition, these metabolic disorders have a complicated internal relation, for instance, T2DM and NAFLD are independent factors for each other, and PCOS is closely related to insulin resistance and T2DM.398,399 These epidemiological characteristics suggest a high correlation between dietary patterns and multiple metabolic disorders (Fig. 7). Changes in the gut microbiome may also explain the etiology of metabolic disorders by altering the levels of metabolites, such as SCFAs and succinate.400
Fig. 7.
Impact of different diets on metabolic disorders. High-fat diet (HFD) can directly increase caloric intake, induce inflammatory mediators such as JNK and IκB kinase (IKK) to promote hypothalamic inflammation, and contribute to adipose tissue hypoxia and inflammation, which all lead to the development of obesity and/or insulin resistance. Over-intake of fructose can also increase caloric intake and induce obesity by impairing hepatic insulin sensitivity. However, time-restricted feeding (TRF) with equivalent caloric intake from HFD can adjust various signaling pathways and rhythmic creatine-mediated thermogenesis and reverse excessive daytime sleepiness induced by paraventricular thalamic nucleus (PVT) dysfunction, resulting in a protective effect on HFD-induced obesity. High-fiber diet can reduce inflammation and insulin resistance by influencing the gut microbiota and associated molecules, for instance, SCFA-producing bacteria. Every-other-day fasting (EODF) regimen can also shift the gut microbiota composition and stimulate beige fat development within white adipose tissue to inhibit insulin resistance. Ketogenic diet (KD) is clinically beneficial for the glycemic control of type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD). However, in experimental models, KD can decrease sensitivity to peripheral insulin by upregulating insulin receptors. Intermittent fasting (IF) alone or combined with exercise can reduce intrahepatic triglyceride (IHTG) levels and hepatic steatosis in NAFLD patients by downregulating hepatic inflammatory pathways, modifying lipogenic gene expression and inducing autophagy. Calorie restriction (CR) can be effective at reducing weight loss and reversing ovulatory/metabolic dysfunction in polycystic ovarian syndrome (PCOS) patients. This figure was created with BioRender.com
HFD is the standard method to induce obesity in animal models and results from the overconsumption of fat, which directly increases caloric intake. The elevation of inflammatory mediators such as JNK and IκB kinase (IKK) in hypothalamic inflammation may also explain the obesity induced by HFD.401 Interestingly, a TRF with equivalent caloric intake from HFD has been shown to have a protective effect on HFD-induced obesity and associated complications by adjusting various signaling pathways and causing rhythmic creatine-mediated thermogenesis, which may further improve nutrient utilization and energy expenditure and reverse excessive daytime sleepiness induced by paraventricular thalamic nucleus (PVT) dysfunction.402–404 Adipose tissue hypoxia and inflammation may lead to adipocyte dysfunction and obesity-induced insulin resistance in HFD-fed models, as indicated by increased infiltration of adipose tissue macrophages (ATMs), activation of the NLRP3 inflammasome and increased levels of proinflammatory cytokines.405–407 In addition to fat intake, the overintake of fructose may also impair hepatic insulin sensitivity, and several metabolic pathways are independent of increased weight gain and caloric intake.408 Within this complex interplay of diet, metabolism, and inflammation, IL-17 has been identified as a key player in metabolic dysregulation associated with HFD, where inhibiting IL-17A production or blocking its receptor can attenuate obesity by enhancing adipose tissue browning and energy dissipation.409 Complementarily, IL-17F promote the expression of TGFβ1 in adipocytes, which fosters sympathetic innervation and suggests a novel therapeutic target for obesity that could stimulate thermogenic activity in fat tissue, thereby improving metabolic health and providing a potential treatment strategy for obesity and its related metabolic disorders.410
Cohort studies have demonstrated that healthy diets, including the Portfolio diet, DASH diet, and MD, are associated with a decreased risk of T2DM.411–413 The promotion of SCFA-producing bacteria induced by dietary fibers observed in T2DM patients suggests the potential value of fiber supplementation in clinical practice.414 In addition, increased fiber consumption is associated with decreased insulin resistance, the mechanism of which mainly includes the gut microbiota and associated molecules.415,416 IF is an effective strategy for controlling weight and increasing insulin sensitivity in patients with diabetes and can also improve cardiometabolic outcomes.417,418 The every-other-day fasting (EODF) regimen selectively stimulates beige fat development within white adipose tissue and shifts the gut microbiota composition in experimental models, explaining the mechanism through which IF ameliorates obesity, insulin resistance, and hepatic steatosis.419 KD has therapeutic effects on glycemia, lipid control, and weight reduction in T2DM patients.420 However, KD may contribute to decreased sensitivity to peripheral insulin and impaired glucose tolerance by upregulating insulin receptors, as determined by previous studies, which contradicts clinical findings.421
NAFLD features hepatic steatosis or adiposity with a potential risk of developing into inflammation, fibrosis, and cancer. MD, as the most recommended dietary pattern for NAFLD, can reduce liver steatosis and improve insulin sensitivity even without weight loss in an insulin-resistant population.422 Reduced liver fat may be associated with ameliorated inflammation induced by antioxidants, low glycemic response induced by dietary fiber, and improved hepatic lipid metabolism.423 KD is more clinically meaningful for glycemic control in individuals with T2DM and NAFLD than low-calorie diet or high-carbohydrate, low-fat (HCLF) diet.424,425 Mechanistically, ketone bodies may modulate inflammation and fibrosis in hepatic cells.426 IF alone or combined with exercise is effective at lowering intrahepatic triglyceride (IHTG) levels and reducing hepatic steatosis in patients with NAFLD, possibly by downregulating hepatic inflammatory pathways, modifying lipogenic gene expression and increasing levels of autophagy.427,428
PCOS features a series of metabolic irregularities, mainly androgen excess and ovarian dysfunction. A meta-analysis showed that women with PCOS have a lower overall diet quality with higher cholesterol, lower magnesium and lower zinc intake.429 Dietary modification with lower caloric intake to achieve weight loss is recommended as a first-line therapy for managing PCOS, and higher supplementary nutrient intake, including vitamin D, chromium and ω-3, may also benefit patients suffering from PCOS.430 MD, KD and their combination can all lead to significant improvements in body weight, metabolic function and ovulatory dysfunction in PCOS patients.431–433 In addition, IF may be beneficial for treating anovulatory PCOS by reducing body fat and improving menstruation, hyperandrogenemia, insulin resistance and chronic inflammation.434 CR may also improve weight and metabolic disorders in patients with PCOS, alone or in combination with supplementation.435 However, the exact mechanisms of these dietary interventions remain unclear and need further exploration.
While the potential of dietary interventions to influence systemic diseases of the whole body is supported by various studies, a critical outlook reveals the necessity for more rigorous, long-term clinical trials to validate these findings. It is essential to approach these interventions with caution, considering individual differences and the intricate balance of potential benefits against nutritional deficiencies or other risks.
Conclusions and perspectives
Our review provides compelling evidence that dietary interventions, including calorie restriction, fasting or FMD, KD, protein restriction diet, HSD, HFD, and high-fiber diet, have substantial potential for modulating metabolism, redirecting disease progression, and enhancing therapeutic responses. These findings highlight the pivotal role of diet, an important environmental factor, in influencing tumor metabolism and the course of various diseases, such as cancer, neurodegenerative diseases, autoimmune diseases, CVD, and metabolic disorders.
Despite compelling evidence, the potential impact of dietary interventions on disease treatment, particularly cancer treatment, is not fully understood.436 The latest American Society of Clinical Oncology (ASCO) guidelines suggest that “there is currently insufficient evidence to recommend for or against dietary interventions such as ketogenic or low-carbohydrate diets, low-fat diets, functional foods, or fasting to improve outcomes related to quality of life (QoL), treatment toxicity, or cancer control”.437 The intricate relationship between dietary interventions and treatment outcomes can be influenced by numerous factors, such as overall lifestyle habits, health status, specific disease type and its corresponding treatment, degree of dietary alterations, and patient adherence. A comprehensive assessment of these variables is crucial for understanding the precise impact of diet on treatment efficacy.438,439
With the recognition of metabolic reprogramming inherent in disease progression, particularly in malignancies, it is becoming essential to explore the value of implementing dietary interventions and translating the evidence into practice. Future research should focus on unraveling the specific molecular mechanisms involved, which will enable the development of more effective, personalized dietary interventions that serve as adjunct therapies in comprehensive disease management.
Building upon the initial observation, it is crucial to interpret and apply these findings with caution due to potential variations and discrepancies. The efficacy of dietary interventions may vary significantly, for instance, depending on the mouse model used.440 Each model might have unique metabolic and immune responses that could influence the outcome of dietary interventions. Similarly, the type of cancer cells used to induce tumor formation, whether primary cells derived directly from patient tissues or cultured cell lines, can have profound impacts on the experimental results.441 Orthotopic or heterotopic transplantation technique is another significant factor that can influence how tumors respond to dietary interventions. Furthermore, the duration of treatment and the specifics of dietary interventions can substantially influence the results, as short-term interventions might not yield the same results as long-term interventions, and different dietary components could have varying effects on tumor growth and progression.120 Therefore, future research in this field should carefully consider the design of animal models and the specifics of dietary interventions to ensure that the findings are robust and translatable to human cancer treatment.
Additionally, clinical trials with larger sample sizes and longer follow-up periods are needed to further validate the efficacy of these strategies and to identify potential side effects and contraindications. It is important for these trials to be designed to represent diverse population groups, including elderly and obese individuals, as these groups may respond differently to dietary modifications. The safety of dietary interventions is another key consideration. While dietary changes generally cause fewer side effects than pharmacological treatments, potential risks should not be overlooked. For instance, severe dietary restrictions may lead to malnutrition or other health complications, particularly in vulnerable population groups. Therefore, in addition to efficacy, these trials should systematically evaluate the safety of dietary interventions, identifying any potential side effects and contraindications.
In conclusion, dietary interventions hold great promise as a novel approach to disease management. However, to realize their full potential, it is essential to continue rigorous scientific investigations into their mechanisms of action, safety profiles, and efficacy in different patient populations. With further research, dietary interventions could become integral components of personalized medicine, providing a new avenue for the prevention and treatment of a myriad of diseases.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82103369) and the China Postdoctoral Science Foundation (2022M710757). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. The figures were created with Biorender.com.
Author contributions
Y.-Z.J., Z.-M.S., Y.-L.X., and Y.G. designed and finalized the study. Y.-L.X., Y.G., and Y.-J.Q. wrote and edited the paper and generated the figures. All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
Consent for publication
The content of this manuscript has not been previously published and is not under consideration for publication elsewhere. All authors are aware of and agree to the content of the paper and are listed as coauthors of the paper.
Footnotes
These authors contributed equally: Yu-Ling Xiao, Yue Gong, Ying-Jia Qi
References
- 1.Collins N, Belkaid Y. Control of immunity via nutritional interventions. Immunity. 2022;55:210–223. doi: 10.1016/j.immuni.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Gao ZJ, Yu X, Wang P. Dietary regulation in health and disease. Signal Transduct. Target. Ther. 2022;7:252. doi: 10.1038/s41392-022-01104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman MJ. Nutrition and cancer: prevention and survival. Br. J. Nutr. 2019;122:481–487. doi: 10.1017/S0007114518002222. [DOI] [PubMed] [Google Scholar]
- 4.Jochems SH, et al. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: a systematic review of current epidemiological literature. BMJ Open. 2018;8:e014530. doi: 10.1136/bmjopen-2016-014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab U, et al. Dietary fat intakes and cardiovascular disease risk in adults with type 2 diabetes: a systematic review and meta-analysis. Eur. J. Nutr. 2021;60:3355–3363. doi: 10.1007/s00394-021-02507-1. [DOI] [PubMed] [Google Scholar]
- 6.Partula V, et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020;112:195–207. doi: 10.1093/ajcn/nqaa063. [DOI] [PubMed] [Google Scholar]
- 7.Ellouze I, Sheffler J, Nagpal R, Arjmandi B. Dietary patterns and Alzheimer’s disease: an updated review linking nutrition to neuroscience. Nutrients. 2023;15:3204. doi: 10.3390/nu15143204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986. doi: 10.3390/nu12071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ubago-Guisado E, et al. Evidence update on the relationship between diet and the most common cancers from the European prospective investigation into cancer and nutrition (EPIC) study: a systematic review. Nutrients. 2021;13:3582. doi: 10.3390/nu13103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan Z, et al. Healthy eating patterns and risk of total and cause-specific mortality. JAMA Intern. Med. 2023;183:142–153,. doi: 10.1001/jamainternmed.2022.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y, et al. Adherence to the Paleolithic diet and Paleolithic-like lifestyle reduce the risk of colorectal cancer in the United States: a prospective cohort study. J. Transl Med. 2023;21:482. doi: 10.1186/s12967-023-04352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia T, et al. Association of healthy diet and physical activity with breast cancer: lifestyle interventions and oncology education. Front. Public Health. 2022;10:797794. doi: 10.3389/fpubh.2022.797794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernieri C, et al. Diet and supplements in cancer prevention and treatment: clinical evidences and future perspectives. Crit. Rev. Oncol. Hematol. 2018;123:57–73. doi: 10.1016/j.critrevonc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee C, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl Med. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercier BD, et al. Dietary interventions in cancer treatment and response: a comprehensive review. Cancers. 2022;14:5149. doi: 10.3390/cancers14205149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anic K, et al. Intermittent fasting-short- and long-term quality of life, fatigue, and safety in healthy volunteers: a prospective, clinical trial. Nutrients. 2022;14:4216. doi: 10.3390/nu14194216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim EM, Al-Foheidi MH, Al-Mansour MM. Energy and caloric restriction, and fasting and cancer: a narrative review. Support Care Cancer. 2021;29:2299–2304. doi: 10.1007/s00520-020-05879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia L, et al. The cancer metabolic reprogramming and immune response. Mol. Cancer. 2021;20:28. doi: 10.1186/s12943-021-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PH, et al. Metabolic diversity in human non-small cell lung cancer cells. Mol. Cell. 2019;76:838–851.e835. doi: 10.1016/j.molcel.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan C, et al. Emerging role of metabolic reprogramming in tumor immune evasion and immunotherapy. Sci. China Life Sci. 2021;64:534–547. doi: 10.1007/s11427-019-1735-4. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 22.Petitprez F, et al. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front. Immunol. 2020;11:784. doi: 10.3389/fimmu.2020.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Pansy K, et al. Immune regulatory processes of the tumor microenvironment under malignant conditions. Int. J. Mol. Sci. 2021;22:13311. doi: 10.3390/ijms222413311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad F, Cherukuri MK, Choyke PL. Metabolic reprogramming in prostate cancer. Br. J. Cancer. 2021;125:1185–1196. doi: 10.1038/s41416-021-01435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanderson SM, Gao X, Dai Z, Locasale JW. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat. Rev. Cancer. 2019;19:625–637. doi: 10.1038/s41568-019-0187-8. [DOI] [PubMed] [Google Scholar]
- 27.Jia Q, et al. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp. Hematol. Oncol. 2022;11:24. doi: 10.1186/s40164-022-00277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrejeva G, Rathmell JC. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab. 2017;26:49–70. doi: 10.1016/j.cmet.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas SK. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43:435–449. doi: 10.1016/j.immuni.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Domínguez-Amorocho O, Takiishi T, da Cunha FF, Camara NOS. Immunometabolism: a target for the comprehension of immune response toward transplantation. World J. Transplant. 2019;9:27–34. doi: 10.5500/wjt.v9.i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ping Y, Shen C, Huang B, Zhang Y. Reprogramming T-Cell metabolism for better anti-tumor immunity. Cells. 2022;11:3103. doi: 10.3390/cells11193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab. 2018;28:87–103.e106. doi: 10.1016/j.cmet.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson MJ, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591:645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apostolova P, Pearce EL. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022;43:969–977. doi: 10.1016/j.it.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl Acad. Sci. USA. 2017;114:580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangel Rivera GO, et al. Fundamentals of T Cell metabolism and strategies to enhance cancer immunotherapy. Front. Immunol. 2021;12:645242. doi: 10.3389/fimmu.2021.645242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019;150:104511. doi: 10.1016/j.phrs.2019.104511. [DOI] [PubMed] [Google Scholar]
- 40.Siska PJ, et al. Suppression of glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T Cell impairment in B cell leukemia. J. Immunol. 2016;197:2532–2540. doi: 10.4049/jimmunol.1502464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho PC, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turbitt WJ, Buchta Rosean C, Weber KS, Norian LA. Obesity and CD8 T cell metabolism: implications for anti‐tumor immunity and cancer immunotherapy outcomes. Immunol Rev. 2020;295:203–219. doi: 10.1111/imr.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrado M, Pearce EL. Targeting memory T cell metabolism to improve immunity. J. Clin. Investig. 2022;132:e148546. doi: 10.1172/JCI148546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J. Hematol. Oncol. 2022;15:104. doi: 10.1186/s13045-022-01322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelin A, et al. Foxp3 reprograms T Cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–1293.e1287. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol. Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assmann N, et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 2017;18:1197–1206. doi: 10.1038/ni.3838. [DOI] [PubMed] [Google Scholar]
- 48.Peng X, et al. Metabolism of dendritic cells in tumor microenvironment: for immunotherapy. Front. Immunol. 2021;12:613492. doi: 10.3389/fimmu.2021.613492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer K, et al. Immunometabolism in cancer at a glance. Dis. Model. Mech. 2018;11:dmm034212. doi: 10.1242/dmm.034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA. The role of lipids in cancer progression and metastasis. Cell Metab. 2022;34:1675–1699. doi: 10.1016/j.cmet.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Cockcroft S. Mammalian lipids: structure, synthesis and function. Essays Biochem. 2021;65:813–845. doi: 10.1042/EBC20200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chae HS, Hong ST. Overview of cancer metabolism and signaling transduction. Int. J. Mol. Sci. 2022;24:12. doi: 10.3390/ijms24010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goossens P, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29:1376–1389.e1374. doi: 10.1016/j.cmet.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Ma X, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e145. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang W, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du X, Chapman NM, Chi H. Emerging roles of cellular metabolism in regulating dendritic cell subsets and function. Front. Cell. Dev. Biol. 2018;6:152. doi: 10.3389/fcell.2018.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin Z, et al. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J. Exp. Clin. Cancer Res. 2019;38:403. doi: 10.1186/s13046-019-1409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Sullivan D. The metabolic spectrum of memory T cells. Immunol. Cell Biol. 2019;97:636–646. doi: 10.1111/imcb.12274. [DOI] [PubMed] [Google Scholar]
- 59.Raynor JL, Chapman NM, Chi H. Metabolic control of memory T-cell generation and stemness. Cold Spring Harb. Perspect. Biol. 2021;13:a037770. doi: 10.1101/cshperspect.a037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377–391.e379. doi: 10.1016/j.ccell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C, et al. STAT3 activation-induced fatty acid oxidation in CD8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 2020;31:148–161.e145. doi: 10.1016/j.cmet.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prendeville H, Lynch L. Diet, lipids, and antitumor immunity. Cell Mol. Immunol. 2022;19:432–444. doi: 10.1038/s41423-021-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma X, et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012.e1005. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu S, et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561–1577.e1567. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M, Wei T, Zhang X, Guo D. Targeting lipid metabolism reprogramming of immunocytes in response to the tumor microenvironment stressor: a potential approach for tumor therapy. Front. Immunol. 2022;13:937406. doi: 10.3389/fimmu.2022.937406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pacella I, et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc. Natl Acad. Sci. USA. 2018;115:E6546–e6555. doi: 10.1073/pnas.1720113115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020;21:298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim SA, et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306–311. doi: 10.1038/s41586-021-03235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, et al. Metabolism and polarization regulation of macrophages in the tumor microenvironment. Cancer Lett. 2022;543:215766. doi: 10.1016/j.canlet.2022.215766. [DOI] [PubMed] [Google Scholar]
- 70.Ocaña MC, Martínez-Poveda B, Quesada AR, Medina M. Metabolism within the tumor microenvironment and its implication on cancer progression: an ongoing therapeutic target. Med. Res. Rev. 2019;39:70–113. doi: 10.1002/med.21511. [DOI] [PubMed] [Google Scholar]
- 71.Luo Q, et al. Lipid accumulation in macrophages confers protumorigenic polarization and immunity in gastric cancer. Cancer Sci. 2020;111:4000–4011. doi: 10.1111/cas.14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu L, et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol. Res. 2020;8:710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 73.Yang X, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol. Res. 2020;8:1440–1451. doi: 10.1158/2326-6066.CIR-20-0111. [DOI] [PubMed] [Google Scholar]
- 74.Hossain F, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol. Res. 2015;3:1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan D, et al. Lipid metabolic pathways confer the immunosuppressive function of myeloid-derived suppressor cells in tumor. Front. Immunol. 2019;10:1399. doi: 10.3389/fimmu.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinfeld BI, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–288. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma G, et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun. Signal. 2022;20:114. doi: 10.1186/s12964-022-00909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lian X, et al. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol. Cancer. 2022;21:27. doi: 10.1186/s12943-021-01486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang D, et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol. 2022;24:230–241. doi: 10.1038/s41556-021-00820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edwards DN, et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 2021;131:e140100. doi: 10.1172/JCI140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perez-Castro L, et al. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J. 2023;290:7–27. doi: 10.1111/febs.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gouasmi R, et al. The kynurenine pathway and cancer: why keep it simple when you can make it complicated. Cancers. 2022;14:2793. doi: 10.3390/cancers14112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat. Rev. Cancer. 2020;20:516–531. doi: 10.1038/s41568-020-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021;21:785–797. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu M, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J. Hematol. Oncol. 2018;11:100. doi: 10.1186/s13045-018-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang F, et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct. Target. Ther. 2022;7:311. doi: 10.1038/s41392-022-01127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heintzman DR, Fisher EL, Rathmell JC. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell Mol. Immunol. 2022;19:316–326. doi: 10.1038/s41423-021-00833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savitz J. The kynurenine pathway: a finger in every pie. Mol. Psychiatry. 2020;25:131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiarugi A, Dölle C, Felici R, Ziegler M. The NAD metabolome–a key determinant of cancer cell biology. Nat, Rev, Cancer. 2012;12:741–752. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- 90.Tummala KS, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Amobi-McCloud A, et al. IDO1 expression in ovarian cancer induces PD-1 in T cells via aryl hydrocarbon receptor activation. Front. Immunol. 2021;12:678999. doi: 10.3389/fimmu.2021.678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schramme F, et al. Inhibition Of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol. Res. 2020;8:32–45. doi: 10.1158/2326-6066.CIR-19-0041. [DOI] [PubMed] [Google Scholar]
- 93.Geiger R, et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842.e813. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arlauckas SP, et al. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics. 2018;8:5842–5854. doi: 10.7150/thno.26888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grzywa TM, et al. Myeloid cell-derived arginase in cancer immune response. Front. Immunol. 2020;11:938. doi: 10.3389/fimmu.2020.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sosnowska A, et al. Inhibition of arginase modulates T-cell response in the tumor microenvironment of lung carcinoma. Oncoimmunology. 2021;10:1956143. doi: 10.1080/2162402X.2021.1956143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu H, et al. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol. Med. 2021;27:856–867. doi: 10.1016/j.molmed.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 98.Srivastava MK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu J, et al. Asparagine enhances LCK signalling to potentiate CD8(+) T-cell activation and anti-tumour responses. Nat.Cell Biol. 2021;23:75–86. doi: 10.1038/s41556-020-00615-4. [DOI] [PubMed] [Google Scholar]
- 100.Bian Y, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585:277–282. doi: 10.1038/s41586-020-2682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taylor SR, Falcone JN, Cantley LC, Goncalves MD. Developing dietary interventions as therapy for cancer. Nat. Rev. Cancer. 2022;22:452–466. doi: 10.1038/s41568-022-00485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Badr CE, Silver DJ, Siebzehnrubl FA, Deleyrolle LP. Metabolic heterogeneity and adaptability in brain tumors. Cell Mol. Life Sci. 2020;77:5101–5119. doi: 10.1007/s00018-020-03569-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Venneti S, Thompson CB. Metabolic reprogramming in brain tumors. Annu. Rev. Pathol. 2017;12:515–545. doi: 10.1146/annurev-pathol-012615-044329. [DOI] [PubMed] [Google Scholar]
- 104.Maurer GD, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11:315. doi: 10.1186/1471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elia I, Schmieder R, Christen S, Fendt SM. Organ-specific cancer metabolism and its potential for therapy. Handb. Exp. Pharmacol. 2016;233:321–353. doi: 10.1007/164_2015_10. [DOI] [PubMed] [Google Scholar]
- 106.Dai W, et al. OGDHL silencing promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J. Hepatol. 2020;72:909–923. doi: 10.1016/j.jhep.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 107.Sangineto M, et al. Lipid metabolism in development and progression of hepatocellular carcinoma. Cancers. 2020;12:1419. doi: 10.3390/cancers12061419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat. Rev. Cancer. 2020;20:125–138. doi: 10.1038/s41568-019-0227-4. [DOI] [PubMed] [Google Scholar]
- 109.Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. 2020;579:507–517. doi: 10.1038/s41586-020-2124-0. [DOI] [PubMed] [Google Scholar]
- 110.Lean MEJ, Astrup A, Roberts SB. Making progress on the global crisis of obesity and weight management. Bmj. 2018;361:k2538. doi: 10.1136/bmj.k2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Z, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22:165–187. doi: 10.1007/s10522-021-09910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Flanagan CH, Smith LA, McDonell SB, Hursting SD. When less may be more: calorie restriction and response to cancer therapy. BMC Med. 2017;15:106. doi: 10.1186/s12916-017-0873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salvadori G, Mirisola MG, Longo VD. Intermittent and periodic fasting, hormones, and cancer prevention. Cancers. 2021;13:4587. doi: 10.3390/cancers13184587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma D, et al. Upregulation of the ALDOA/DNA-PK/p53 pathway by dietary restriction suppresses tumor growth. Oncogene. 2018;37:1041–1048. doi: 10.1038/onc.2017.398. [DOI] [PubMed] [Google Scholar]
- 116.Ma Z, et al. Caloric restriction inhibits mammary tumorigenesis in MMTV-ErbB2 transgenic mice through the suppression of ER and ErbB2 pathways and inhibition of epithelial cell stemness in premalignant mammary tissues. Carcinogenesis. 2018;39:1264–1273. doi: 10.1093/carcin/bgy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vidoni C, et al. Calorie restriction for cancer prevention and therapy: mechanisms, expectations, and efficacy. J. Cancer Prev. 2021;26:224. doi: 10.15430/JCP.2021.26.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pomatto-Watson LCD, et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat. Commun. 2021;12:6201. doi: 10.1038/s41467-021-26431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pistollato F, et al. Effects of caloric restriction on immunosurveillance, microbiota and cancer cell phenotype: possible implications for cancer treatment. Semin. Cancer Biol. 2021;73:45–57. doi: 10.1016/j.semcancer.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 120.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 2019;29:592–610. doi: 10.1016/j.cmet.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 121.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat. Immunol. 2017;18:843–850. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 122.Pietrocola F, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deus CM, et al. Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch. Toxicol. 2017;91:1261–1278. doi: 10.1007/s00204-016-1784-x. [DOI] [PubMed] [Google Scholar]
- 124.Turbitt WJ, et al. Physical activity plus energy restriction prevents 4T1.2 mammary tumor progression, MDSC accumulation, and an immunosuppressive tumor microenvironment. Cancer Prev. Res. 2019;12:493–506. doi: 10.1158/1940-6207.CAPR-17-0233. [DOI] [PubMed] [Google Scholar]
- 125.Caccialanza R, Aprile G, Cereda E, Pedrazzoli P. Fasting in oncology: a word of caution. Nat. Rev. Cancer. 2019;19:177. doi: 10.1038/s41568-018-0098-0. [DOI] [PubMed] [Google Scholar]
- 126.Castejón M, et al. Energy restriction and colorectal cancer: a call for additional research. Nutrients. 2020;12:114. doi: 10.3390/nu12010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petersen MC, et al. Complex physiology and clinical implications of time-restricted eating. Physiol. Rev. 2022;102:1991–2034. doi: 10.1152/physrev.00006.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turbitt WJ, Demark-Wahnefried W, Peterson CM, Norian LA. Targeting glucose metabolism to enhance immunotherapy: emerging evidence on intermittent fasting and calorie restriction mimetics. Front. Immunol. 2019;10:1402. doi: 10.3389/fimmu.2019.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Isaac-Lam MF, DeMichael KM. Calorie restriction and breast cancer treatment: a mini-review. J. Mol. Med. 2022;100:1095–1109. doi: 10.1007/s00109-022-02226-y. [DOI] [PubMed] [Google Scholar]
- 130.Zhang J, Deng Y, Khoo BL. Fasting to enhance cancer treatment in models: the next steps. J. Biomed. Sci. 2020;27:58. doi: 10.1186/s12929-020-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat. Rev. Cancer. 2018;18:707–719. doi: 10.1038/s41568-018-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ajona D, et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat. Cancer. 2020;1:75–85. doi: 10.1038/s43018-019-0007-9. [DOI] [PubMed] [Google Scholar]
- 133.Das M, et al. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nat. Commun. 2021;12:565. doi: 10.1038/s41467-020-20743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bianchi G, et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6:11806–11819. doi: 10.18632/oncotarget.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blaževitš O, Di Tano M, Longo VD. Fasting and fasting mimicking diets in cancer prevention and therapy. Trends Cancer. 2023;9:212–222. doi: 10.1016/j.trecan.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Di Biase S, et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell. 2016;30:136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun P, et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8:74649–74660. doi: 10.18632/oncotarget.20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vernieri C, et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. 2022;12:90–107. doi: 10.1158/2159-8290.CD-21-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ligorio F, et al. Exceptional tumour responses to fasting-mimicking diet combined with standard anticancer therapies: a sub-analysis of the NCT03340935 trial. Eur. J. Cancer. 2022;172:300–310. doi: 10.1016/j.ejca.2022.05.046. [DOI] [PubMed] [Google Scholar]
- 140.Zhang X, et al. Impact of diets on response to immune checkpoint inhibitors (ICIs) Therapy against tumors. Life. 2022;12:409. doi: 10.3390/life12030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thau-Zuchman O, et al. A new ketogenic formulation improves functional outcome and reduces tissue loss following traumatic brain injury in adult mice. Theranostics. 2021;11:346. doi: 10.7150/thno.48995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bandera-Merchan B, et al. Ketotherapy as an epigenetic modifier in cancer. Rev. Endocr. Metab. Disord. 2020;21:509–519. doi: 10.1007/s11154-020-09567-4. [DOI] [PubMed] [Google Scholar]
- 143.Simeone TA, Simeone KA, Stafstrom CE, Rho JM. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology. 2018;133:233–241. doi: 10.1016/j.neuropharm.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Talib WH, et al. Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr. Issues Mol. Biol. 2021;43:558–589. doi: 10.3390/cimb43020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu H, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022;7:11. doi: 10.1038/s41392-021-00831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shah UA, Iyengar NM. Plant-based and ketogenic diets as diverging paths to address cancer: a review. JAMA Oncol. 2022;8:1201–1208. doi: 10.1001/jamaoncol.2022.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dmitrieva-Posocco O, et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature. 2022;605:160–165. doi: 10.1038/s41586-022-04649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li B, et al. Glucose restriction induces AMPK-SIRT1-mediated circadian clock gene per expression and delays NSCLC progression. Cancer Lett. 2023;576:216424. doi: 10.1016/j.canlet.2023.216424. [DOI] [PubMed] [Google Scholar]
- 149.Chen Y, et al. Metabolic intervention by low carbohydrate diet suppresses the onset and progression of neuroendocrine tumors. Cell Death Dis. 2023;14:597. doi: 10.1038/s41419-023-06123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hirschberger S, et al. Very-low-carbohydrate diet enhances human T-cell immunity through immunometabolic reprogramming. EMBO Mol. Med. 2021;13:e14323. doi: 10.15252/emmm.202114323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang N, et al. Ketogenic diet elicits antitumor properties through inducing oxidative stress, inhibiting MMP-9 expression, and rebalancing M1/M2 Tumor-associated macrophage phenotype in a mouse model of colon cancer. J. Agric. Food Chem. 2020;68:11182–11196. doi: 10.1021/acs.jafc.0c04041. [DOI] [PubMed] [Google Scholar]
- 152.Lussier DM, et al. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:310. doi: 10.1186/s12885-016-2337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim AJ, Hong DS, George GC. Dietary influences on symptomatic and non-symptomatic toxicities during cancer treatment: a narrative review. Cancer Treat. Rev. 2022;108:102408. doi: 10.1016/j.ctrv.2022.102408. [DOI] [PubMed] [Google Scholar]
- 154.Kenig S, et al. Assessment of micronutrients in a 12-wk ketogenic diet in obese adults. Nutrition. 2019;67:110522. doi: 10.1016/j.nut.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 155.Manolis AS, Manolis TA, Manolis AA, Melita H. Diet and sudden death: how to reduce the risk. Curr. Vasc. Pharmacol. 2022;20:383–408. doi: 10.2174/1570161120666220621090343. [DOI] [PubMed] [Google Scholar]
- 156.Ferrer M, et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 2023;35:1147–1162.e1147. doi: 10.1016/j.cmet.2023.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Levine ME, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yin J, et al. Protein restriction and cancer. Biochim. Biophys. Acta Rev. Cancer. 2018;1869:256–262. doi: 10.1016/j.bbcan.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 159.Jiménez-Alonso JJ, López-Lázaro M. Dietary manipulation of amino acids for cancer therapy. Nutrients. 2023;15:2879. doi: 10.3390/nu15132879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shunxi W, et al. Serine metabolic reprogramming in tumorigenesis, tumor immunity, and clinical treatment. Adv. Nutr. 2023;14:1050–1066. doi: 10.1016/j.advnut.2023.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maddocks ODK, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- 162.Li T, et al. Methionine deficiency facilitates antitumour immunity by altering m(6)A methylation of immune checkpoint transcripts. Gut. 2023;72:501–511. doi: 10.1136/gutjnl-2022-326928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Orillion A, et al. Dietary protein restriction reprograms tumor-associated macrophages and enhances immunotherapy. Clin. Cancer Res. 2018;24:6383–6395. doi: 10.1158/1078-0432.CCR-18-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang X, et al. Reprogramming tumour-associated macrophages to outcompete cancer cells. Nature. 2023;619:616–623. doi: 10.1038/s41586-023-06256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rubio-Patiño C, et al. Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metab. 2018;27:828–842.e827. doi: 10.1016/j.cmet.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 166.Jing W, et al. Metabolic modulation of intracellular ammonia via intravesical instillation of nanoporter-encased hydrogel eradicates bladder carcinoma. Adv. Sci. 2023;10:e2206893. doi: 10.1002/advs.202206893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu M, et al. Elevated urinary urea by high-protein diet could be one of the inducements of bladder disorders. J. Transl Med. 2016;14:53. doi: 10.1186/s12967-016-0809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pimentel GD, Pichard C, Laviano A, Fernandes RC. High protein diet improves the overall survival in older adults with advanced gastrointestinal cancer. Clin. Nutr. 2021;40:1376–1380. doi: 10.1016/j.clnu.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 169.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp. Mol. Med. 2020;52:15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Du H, et al. Detachable MOF-based core/shell nanoreactor for cancer dual-starvation therapy with reversing glucose and glutamine metabolisms. Small. 2023;19:e2303253. doi: 10.1002/smll.202303253. [DOI] [PubMed] [Google Scholar]
- 171.Leone RD, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oh MH, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Investig. 2020;130:3865–3884. doi: 10.1172/JCI131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gross MI, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 174.Tannir NM, et al. CANTATA: a randomized phase 2 study of CB-839 in combination with cabozantinib vs. placebo with cabozantinib in patients with advanced/metastatic renal cell carcinoma. J. Clin. Oncol. 2018;36:TPS4601–TPS4601. doi: 10.1200/JCO.2018.36.15_suppl.TPS4601. [DOI] [Google Scholar]
- 175.Wu Q, et al. Metabolic regulation in the immune response to cancer. Cancer Commun. 2021;41:661–694. doi: 10.1002/cac2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guo Y, et al. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials. 2021;276:121018. doi: 10.1016/j.biomaterials.2021.121018. [DOI] [PubMed] [Google Scholar]
- 177.Tang K, Wu YH, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021;14:68. doi: 10.1186/s13045-021-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jochems C, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget. 2016;7:37762–37772. doi: 10.18632/oncotarget.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cramer SL, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017;23:120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Badgley MA, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wilck N, et al. The role of sodium in modulating immune cell function. Nat. Rev. Nephrol. 2019;15:546–558. doi: 10.1038/s41581-019-0167-y. [DOI] [PubMed] [Google Scholar]
- 182.Li X, et al. The modulatory effect of high salt on immune cells and related diseases. Cell Prolif. 2022;55:e13250. doi: 10.1111/cpr.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rizvi ZA, et al. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci. Adv. 2021;7:eabg5016. doi: 10.1126/sciadv.abg5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Willebrand R, et al. High salt inhibits tumor growth by enhancing anti-tumor immunity. Front. Immunol. 2019;10:1141. doi: 10.3389/fimmu.2019.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He W, et al. High-salt diet inhibits tumour growth in mice via regulating myeloid-derived suppressor cell differentiation. Nat. Commun. 2020;11:1732. doi: 10.1038/s41467-020-15524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hernandez AL, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015;125:4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xu Y, et al. High salt intake attenuates breast cancer metastasis to lung. J. Agric. Food Chem. 2018;66:3386–3392. doi: 10.1021/acs.jafc.7b05923. [DOI] [PubMed] [Google Scholar]
- 188.Allu AS, Tiriveedhi V. Cancer salt nostalgia. Cells. 2021;10:1285. doi: 10.3390/cells10061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen J, et al. High salt diet may promote progression of breast tumor through eliciting immune response. Int. Immunopharmacol. 2020;87:106816. doi: 10.1016/j.intimp.2020.106816. [DOI] [PubMed] [Google Scholar]
- 191.Amara S, Ivy MT, Myles EL, Tiriveedhi V. Sodium channel γENaC mediates IL-17 synergized high salt induced inflammatory stress in breast cancer cells. Cell Immunol. 2016;302:1–10. doi: 10.1016/j.cellimm.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huangfu L, Li R, Huang Y, Wang S. The IL-17 family in diseases: from bench to bedside. Signal Transduct. Target. Ther. 2023;8:402. doi: 10.1038/s41392-023-01620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zeng X, et al. A high-salt diet disturbs the development and function of natural killer cells in mice. J. Immunol. Res. 2020;2020:6687143. doi: 10.1155/2020/6687143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu W, et al. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J. Hematol. Oncol. 2021;14:187. doi: 10.1186/s13045-021-01200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deng T, et al. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 196.Peng L, et al. Association between low-fat diet and liver cancer risk in 98,455 participants: results from a prospective study. Front. Nutr. 2022;9:1013643. doi: 10.3389/fnut.2022.1013643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chlebowski RT, et al. Low-fat dietary pattern and breast cancer mortality in the women’s health initiative randomized controlled trial. J. Clin. Oncol. 2017;35:2919–2926. doi: 10.1200/JCO.2016.72.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barbi J, et al. Visceral obesity promotes lung cancer progression-toward resolution of the obesity paradox in lung cancer. J. Thorac. Oncol. 2021;16:1333–1348. doi: 10.1016/j.jtho.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gomes AL, et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2016;30:161–175. doi: 10.1016/j.ccell.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 200.Ericksen RE, et al. Obesity accelerates helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut. 2014;63:385–394. doi: 10.1136/gutjnl-2013-305092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yadav AK, et al. Activity-based NIR bioluminescence probe enables discovery of diet-induced modulation of the tumor microenvironment via nitric oxide. ACS Cent. Sci. 2022;8:461–472. doi: 10.1021/acscentsci.1c00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hayashi T, et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin. Cancer Res. 2018;24:4309–4318. doi: 10.1158/1078-0432.CCR-18-0106. [DOI] [PubMed] [Google Scholar]
- 203.Wunderlich CM, et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat. Commun. 2018;9:1646. doi: 10.1038/s41467-018-03773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Incio J, et al. PlGF/VEGFR-1 signaling promotes macrophage polarization and accelerated tumor progression in obesity. Clin. Cancer Res. 2016;22:2993–3004. doi: 10.1158/1078-0432.CCR-15-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Peng J, et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021;12:946. doi: 10.1038/s41419-021-04217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gibson JT, et al. Obesity-associated myeloid-derived suppressor cells promote apoptosis of tumor-infiltrating CD8 T cells and immunotherapy resistance in breast cancer. Front. Immunol. 2020;11:590794. doi: 10.3389/fimmu.2020.590794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ringel AE, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183:1848–1866.e1826. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dyck L, et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med. 2022;219:e20210042. doi: 10.1084/jem.20210042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yamada K, et al. Reduced number and immune dysfunction of CD4+ T cells in obesity accelerate colorectal cancer progression. Cells. 2022;12:86. doi: 10.3390/cells12010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Incio J, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Altea-Manzano P, et al. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation resulting in pro-metastatic NF-κB signaling. Nat. Cancer. 2023;4:344–364. doi: 10.1038/s43018-023-00513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang Z, et al. Extracellular vesicles in fatty liver promote a metastatic tumor microenvironment. Cell Metab. 2023;35:1209–1226.e1213. doi: 10.1016/j.cmet.2023.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen M, et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018;50:206–218. doi: 10.1038/s41588-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pascual G, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 215.Terry AR, et al. CD36 maintains lipid homeostasis via selective uptake of monounsaturated fatty acids during matrix detachment and tumor progression. Cell Metab. 2023;35:2060–2076.e2069. doi: 10.1016/j.cmet.2023.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Garcia-Estevez L, Moreno-Bueno G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21:35. doi: 10.1186/s13058-019-1124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Du Q, et al. Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem. Pharmacol. 2016;105:42–54. doi: 10.1016/j.bcp.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 218.Liu C, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020;27:1765–1781. doi: 10.1038/s41418-019-0460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Asghari A, Umetani M. Obesity and cancer: 27-Hydroxycholesterol, the missing link. Int. J. Mol. Sci. 2020;21:4822. doi: 10.3390/ijms21144822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Baek AE, et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017;8:864. doi: 10.1038/s41467-017-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Qin WH, et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158:1713–1727. doi: 10.1053/j.gastro.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 222.Liu L, et al. Consumption of the fish oil high-fat diet uncouples obesity and mammary tumor growth through induction of reactive oxygen species in protumor macrophages. Cancer Res. 2020;80:2564–2574. doi: 10.1158/0008-5472.CAN-19-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liang P, et al. Role of host GPR120 in mediating dietary omega-3 fatty acid inhibition of prostate cancer. J. Natl Cancer Inst. 2019;111:52–59. doi: 10.1093/jnci/djy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jin R, et al. Dietary fats high in linoleic acids impair antitumor T-cell responses by inducing E-FABP-mediated mitochondrial dysfunction. Cancer Res. 2021;81:5296–5310. doi: 10.1158/0008-5472.CAN-21-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Plesca I, et al. Characteristics of tumor-infiltrating lymphocytes prior to and during immune checkpoint inhibitor therapy. Front. Immunol. 2020;11:364. doi: 10.3389/fimmu.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021;14:45. doi: 10.1186/s13045-021-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Luo C, et al. Progress and prospect of immunotherapy for triple-negative breast cancer. Front. Oncol. 2022;12:919072. doi: 10.3389/fonc.2022.919072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shergold AL, Millar R, Nibbs RJ. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol. Res. 2019;145:104258. doi: 10.1016/j.phrs.2019.104258. [DOI] [PubMed] [Google Scholar]
- 229.Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist. 2019;24:S31–S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Spyrou N, Vallianou N, Kadillari J, Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin. Cancer Biol. 2021;73:356–376. doi: 10.1016/j.semcancer.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 231.Zitvogel L, Kroemer G. Boosting the immunotherapy response by nutritional interventions. J. Clin. Investig. 2022;132:e161483. doi: 10.1172/JCI161483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Coleman MF, et al. Cell intrinsic and systemic metabolism in tumor immunity and immunotherapy. Cancers. 2020;12:852. doi: 10.3390/cancers12040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Farazi M, et al. Caloric restriction maintains OX40 agonist-mediated tumor immunity and CD4 T cell priming during aging. Cancer Immunol. Immunother. 2014;63:615–626. doi: 10.1007/s00262-014-1542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lévesque S, et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology. 2019;8:e1657375. doi: 10.1080/2162402X.2019.1657375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.de Gruil N, Pijl H, van der Burg SH, Kroep JR. Short-term fasting synergizes with solid cancer therapy by boosting antitumor immunity. Cancers. 2022;14:1390. doi: 10.3390/cancers14061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cortellino S, et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 2022;40:111256. doi: 10.1016/j.celrep.2022.111256. [DOI] [PubMed] [Google Scholar]
- 237.Ferrere G, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207. doi: 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dai X, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell. 2021;81:2317–2331.e2316. doi: 10.1016/j.molcel.2021.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yue T, et al. Hydrogen sulfide creates a favorable immune microenvironment for colon cancer. Cancer Res. 2023;83:595–612. doi: 10.1158/0008-5472.CAN-22-1837. [DOI] [PubMed] [Google Scholar]
- 240.Wang W, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fujiwara Y, et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022;110:102461. doi: 10.1016/j.ctrv.2022.102461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mitchell TC, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037) J. Clin. Oncol. 2018;36:3223–3230. doi: 10.1200/JCO.2018.78.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Long GV, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–1097. doi: 10.1016/S1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 244.Assumpção JAF, et al. The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J. Biomed. Sci. 2022;29:12. doi: 10.1186/s12929-022-00796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Murphy WJ, Longo DL. The surprisingly positive association between obesity and cancer immunotherapy efficacy. JAMA. 2019;321:1247–1248. doi: 10.1001/jama.2019.0463. [DOI] [PubMed] [Google Scholar]
- 246.Wang Z, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019;25:141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhao B, et al. Research progress of conjugated nanomedicine for cancer treatment. Pharmaceutics. 2022;14:1522. doi: 10.3390/pharmaceutics14071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review) Int. J. Oncol. 2019;54:407–419. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Raffaghello L, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl Acad. Sci. USA. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lee C, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.D’Aronzo M, et al. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget. 2015;6:18545–18557. doi: 10.18632/oncotarget.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pateras IS, et al. Short term starvation potentiates the efficacy of chemotherapy in triple negative breast cancer via metabolic reprogramming. J. Transl Med. 2023;21:169. doi: 10.1186/s12967-023-03935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Di Tano M, et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 2020;11:2332. doi: 10.1038/s41467-020-16243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu X, et al. Fasting-mimicking diet synergizes with ferroptosis against quiescent, chemotherapy-resistant cells. EBioMedicine. 2023;90:104496. doi: 10.1016/j.ebiom.2023.104496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cheng CW, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022;23:56–73. doi: 10.1038/s41580-021-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.de Groot S, et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020;11:3083. doi: 10.1038/s41467-020-16138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Morscher RJ, et al. Combination of metronomic cyclophosphamide and dietary intervention inhibits neuroblastoma growth in a CD1-nu mouse model. Oncotarget. 2016;7:17060–17073. doi: 10.18632/oncotarget.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Aminzadeh-Gohari S, et al. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget. 2017;8:64728–64744. doi: 10.18632/oncotarget.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang L, et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3:119–136,. doi: 10.1016/j.medj.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Manukian G, et al. Caloric restriction impairs regulatory T cells within the tumor microenvironment after radiation and primes effector T cells. Int. J. Radiat. Oncol. Biol. Phys. 2021;110:1341–1349. doi: 10.1016/j.ijrobp.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Allen BG, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res. 2013;19:3905–3913. doi: 10.1158/1078-0432.CCR-12-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zahra A, et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the university of iowa experience of two phase 1 clinical trials. Radiat. Res. 2017;187:743–754. doi: 10.1667/RR14668.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gao X, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397–401. doi: 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Caffa I, et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583:620–624. doi: 10.1038/s41586-020-2502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Caffa I, et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget. 2015;6:11820–11832. doi: 10.18632/oncotarget.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Krstic J, et al. Fasting improves therapeutic response in hepatocellular carcinoma through p53-dependent metabolic synergism. Sci. Adv. 2022;8:eabh2635. doi: 10.1126/sciadv.abh2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hopkins BD, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gravel SP, et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 2014;74:7521–7533. doi: 10.1158/0008-5472.CAN-14-2643-T. [DOI] [PubMed] [Google Scholar]
- 270.Lee KA, et al. Role of the gut microbiome for cancer patients receiving immunotherapy: dietary and treatment implications. Eur. J. Cancer. 2020;138:149–155. doi: 10.1016/j.ejca.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 271.Ogunrinola GA, Oyewale JO, Oshamika OO, Olasehinde GI. The human microbiome and its impacts on health. Int. J. Microbiol. 2020;2020:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Greathouse KL, et al. Diet-microbiome interactions in cancer treatment: opportunities and challenges for precision nutrition in cancer. Neoplasia. 2022;29:100800. doi: 10.1016/j.neo.2022.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zeng X, et al. Gut bacterial nutrient preferences quantified in vivo. Cell. 2022;185:3441–3456.e3419. doi: 10.1016/j.cell.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Soldati L, et al. The influence of diet on anti-cancer immune responsiveness. J. Transl Med. 2018;16:75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Duan H, et al. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 2022;62:1427–1452. doi: 10.1080/10408398.2020.1843396. [DOI] [PubMed] [Google Scholar]
- 276.Liu L, et al. Association between inflammatory diet pattern and risk of colorectal carcinoma subtypes classified by immune responses to tumor. Gastroenterology. 2017;153:1517–1530.e1514. doi: 10.1053/j.gastro.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liu L, et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain fusobacterium nucleatum. Clin. Gastroenterol. Hepatol. 2018;16:1622–1631.e1623. doi: 10.1016/j.cgh.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mehta RS, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3:921–927. doi: 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Shimomura Y, et al. Mediation effect of intestinal microbiota on the relationship between fiber intake and colorectal cancer. Int. J. Cancer. 2023;152:1752–1762. doi: 10.1002/ijc.34398. [DOI] [PubMed] [Google Scholar]
- 280.Simpson RC, et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 2022;28:2344–2352. doi: 10.1038/s41591-022-01965-2. [DOI] [PubMed] [Google Scholar]
- 281.Lu Y, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022;15:47. doi: 10.1186/s13045-022-01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Klement RJ, Pazienza V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina. 2019;55:84. doi: 10.3390/medicina55040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Luu M, Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 2019;49:842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 284.Martin-Gallausiaux C, et al. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 285.Dong Y, et al. Gut microbiota-derived short-chain fatty acids regulate gastrointestinal tumor immunity: a novel therapeutic strategy? Front. Immunol. 2023;14:1158200. doi: 10.3389/fimmu.2023.1158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Luu M, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021;12:4077. doi: 10.1038/s41467-021-24331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mager LF, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 288.Wang T, et al. Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat. Metab. 2020;2:635–647. doi: 10.1038/s42255-020-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Canale FP, et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598:662–666. doi: 10.1038/s41586-021-04003-2. [DOI] [PubMed] [Google Scholar]
- 290.Mao YQ, et al. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat. Metab. 2023;5:96–110. doi: 10.1038/s42255-022-00716-4. [DOI] [PubMed] [Google Scholar]
- 291.Ang QY, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181:1263–1275.e1216. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hwang S, et al. Dietary salt administration decreases enterotoxigenic bacteroides fragilis (ETBF)-promoted tumorigenesis via inhibition of colonic inflammation. Int. J. Mol. Sci. 2020;21:8034. doi: 10.3390/ijms21218034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gaddy JA, et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect. Immun. 2013;81:2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Münch NS, et al. High-fat diet accelerates carcinogenesis in a mouse model of Barrett’s esophagus via interleukin 8 and alterations to the gut microbiome. Gastroenterology. 2019;157:492–506.e492. doi: 10.1053/j.gastro.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sun L, et al. Bile salt hydrolase in non-enterotoxigenic bacteroides potentiates colorectal cancer. Nat. Commun. 2023;14:755. doi: 10.1038/s41467-023-36089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Liu T, et al. High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence. J. Cell Mol. Med. 2020;24:2648–2662. doi: 10.1111/jcmm.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Villemin C, et al. The heightened importance of the microbiome in cancer immunotherapy. Trends Immunol. 2023;44:44–59. doi: 10.1016/j.it.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 298.Singh A, Alexander SG, Martin S. Gut microbiome homeostasis and the future of probiotics in cancer immunotherapy. Front. Immunol. 2023;14:1114499. doi: 10.3389/fimmu.2023.1114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Park EM, et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022;28:690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 300.Spencer CN, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lam KC, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338–5356.e5321. doi: 10.1016/j.cell.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Paden H, et al. Dietary impacts on changes in diversity and abundance of the murine microbiome during progression and treatment of cancer. Nutrients. 2023;15:724. doi: 10.3390/nu15030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Han K, et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng. 2021;5:1377–1388. doi: 10.1038/s41551-021-00749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang SL, et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics. 2021;11:4155–4170. doi: 10.7150/thno.54476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Shi Y, et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J. Exp. Med. 2020;217:e20192282. doi: 10.1084/jem.20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bender MJ, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862.e1826. doi: 10.1016/j.cell.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kesh K, et al. Obesity enriches for tumor protective microbial metabolites and treatment refractory cells to confer therapy resistance in PDAC. Gut Microbes. 2022;14:2096328. doi: 10.1080/19490976.2022.2096328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tintelnot J, et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615:168–174. doi: 10.1038/s41586-023-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kurowska A, Ziemichód W, Herbet M, Piątkowska-Chmiel I. The role of diet as a modulator of the inflammatory process in the neurological diseases. Nutrients. 2023;15:1436. doi: 10.3390/nu15061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Chu CQ, et al. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022;135:104556. doi: 10.1016/j.neubiorev.2022.104556. [DOI] [PubMed] [Google Scholar]
- 311.Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, Pluta R. Ketogenic diet and epilepsy. Nutrients. 2019;11:2510. doi: 10.3390/nu11102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 313.Devi N, et al. Efficacy and safety of dietary therapies for childhood drug-resistant epilepsy: a systematic review and network meta-analysis. JAMA Pediatr. 2023;177:258–266. doi: 10.1001/jamapediatrics.2022.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Calderón N, Betancourt L, Hernández L, Rada P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: a microdialysis study. Neurosci. Lett. 2017;642:158–162. doi: 10.1016/j.neulet.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 315.Rudy L, et al. Anticonvulsant mechanisms of the ketogenic diet and caloric restriction. Epilepsy Res. 2020;168:106499. doi: 10.1016/j.eplepsyres.2020.106499. [DOI] [PubMed] [Google Scholar]
- 316.Napolitano A, et al. The ketogenic diet increases in vivo glutathione levels in patients with epilepsy. Metabolites. 2020;10:504. doi: 10.3390/metabo10120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Knowles S, et al. Ketogenic diet regulates the antioxidant catalase via the transcription factor PPARγ2. Epilepsy Res. 2018;147:71–74. doi: 10.1016/j.eplepsyres.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yellen G. Ketone bodies, glycolysis, and KATP channels in the mechanism of the ketogenic diet. Epilepsia. 2008;49:80–82. doi: 10.1111/j.1528-1167.2008.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Olson CA, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173:1728–1741.e1713. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Dahlin M, et al. Higher levels of Bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. EBioMedicine. 2022;80:104061. doi: 10.1016/j.ebiom.2022.104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Nianogo RA, et al. Risk factors associated with alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 2022;79:584–591,. doi: 10.1001/jamaneurol.2022.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Suzzi S, et al. N-acetylneuraminic acid links immune exhaustion and accelerated memory deficit in diet-induced obese Alzheimer’s disease mouse model. Nat. Commun. 2023;14:1293. doi: 10.1038/s41467-023-36759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pan W, et al. Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice. Microbiome. 2023;11:30. doi: 10.1186/s40168-023-01471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Phillips MCL, et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 2021;13:51. doi: 10.1186/s13195-021-00783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Coelho-Júnior HJ, Trichopoulou A, Panza F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: a systematic review and meta-analysis. Ageing Res. Rev. 2021;70:101395. doi: 10.1016/j.arr.2021.101395. [DOI] [PubMed] [Google Scholar]
- 326.Elias A, Padinjakara N, Lautenschlager NT. Effects of intermittent fasting on cognitive health and Alzheimer’s disease. Nutr. Rev. 2023;81:1225–1233. doi: 10.1093/nutrit/nuad021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ballarini T, et al. Mediterranean diet, Alzheimer disease biomarkers and brain atrophy in old age. Neurology. 2021;96:e2920–e2932. doi: 10.1212/WNL.0000000000012067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Jhanji M, et al. Cis- and trans-resveratrol have opposite effects on histone serine-ADP-ribosylation and tyrosine induced neurodegeneration. Nat. Commun. 2022;13:3244. doi: 10.1038/s41467-022-30785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Bonda DJ, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10:275–279. doi: 10.1016/S1474-4422(11)70013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shippy DC, et al. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation. 2020;17:280. doi: 10.1186/s12974-020-01948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Nagpal R, et al. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–542. doi: 10.1016/j.ebiom.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Dilmore AH, et al. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimers Dement. 2023;19:4805–4816. doi: 10.1002/alz.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Travagli RA, Browning KN, Camilleri M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2020;17:673–685. doi: 10.1038/s41575-020-0339-z. [DOI] [PubMed] [Google Scholar]
- 334.Augustin A, et al. Faecal metabolite deficit, gut inflammation and diet in Parkinson’s disease: Integrative analysis indicates inflammatory response syndrome. Clin. Transl Med. 2023;13:e1152. doi: 10.1002/ctm2.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lin CH, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation. 2019;16:129. doi: 10.1186/s12974-019-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Nowak KL, et al. Serum sodium and cognition in older community-dwelling men. Clin. J. Am. Soc. Nephrol. 2018;13:366–374. doi: 10.2215/CJN.07400717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Heras-Garvin A, et al. High-salt diet does not boost neuroinflammation and neurodegeneration in a model of α-synucleinopathy. J. Neuroinflammation. 2020;17:35. doi: 10.1186/s12974-020-1714-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sofi F, et al. Adherence to Mediterranean diet and health status: meta-analysis. Bmj. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sampson TR, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Phillips MCL, et al. Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov. Disord. 2018;33:1306–1314. doi: 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhou ZL, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson’s disease mice via gut microbiota and metabolites. Neurotherapeutics. 2019;16:741–760. doi: 10.1007/s13311-019-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Marder K, et al. Relationship of Mediterranean diet and caloric intake to phenoconversion in huntington disease. JAMA Neurol. 2013;70:1382–1388. doi: 10.1001/jamaneurol.2013.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bushara KO, Nance M, Gomez CM. Antigliadin antibodies in huntington’s disease. Neurology. 2004;62:132–133. doi: 10.1212/WNL.62.1.132. [DOI] [PubMed] [Google Scholar]
- 344.Duan W, et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl Acad. Sci. USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Nieves JW, et al. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 2016;73:1425–1432,. doi: 10.1001/jamaneurol.2016.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Fitzgerald KC, et al. Dietary ω-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:1102–1110,. doi: 10.1001/jamaneurol.2014.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ludolph AC, et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann. Neurol. 2020;87:206–216. doi: 10.1002/ana.25661. [DOI] [PubMed] [Google Scholar]
- 348.Fasano A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012;42:71–78. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 349.Sköldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003;62:208–214. doi: 10.1136/ard.62.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Rosillo M, et al. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014;25:1275–1281. doi: 10.1016/j.jnutbio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 351.Jiang L, et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell Mol. Immunol. 2022;19:1414–1424. doi: 10.1038/s41423-022-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Cleland LG, James MJ, Proudman SM. The role of fish oils in the treatment of rheumatoid arthritis. Drugs. 2003;63:845–853. doi: 10.2165/00003495-200363090-00001. [DOI] [PubMed] [Google Scholar]
- 353.de Pablo P, et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case-control study. Ann. Rheum. Dis. 2018;77:981–987. doi: 10.1136/annrheumdis-2017-212274. [DOI] [PubMed] [Google Scholar]
- 354.Proudman SM, et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann. Rheum. Dis. 2015;74:89–95. doi: 10.1136/annrheumdis-2013-204145. [DOI] [PubMed] [Google Scholar]
- 355.Toumi E, et al. Gut microbiota in systemic lupus erythematosus patients and lupus mouse model: a cross species comparative analysis for biomarker discovery. Front. Immunol. 2022;13:943241. doi: 10.3389/fimmu.2022.943241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.López P, et al. Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci. Rep. 2016;6:24072. doi: 10.1038/srep24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Azzouz DF, et al. Longitudinal gut microbiome analyses and blooms of pathogenic strains during lupus disease flares. Ann. Rheum. Dis. 2023;82:1315–1327. doi: 10.1136/ard-2023-223929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Li Y, et al. Disordered intestinal microbes are associated with the activity of systemic lupus erythematosus. Clin Sci. 2019;133:821–838. doi: 10.1042/CS20180841. [DOI] [PubMed] [Google Scholar]
- 359.de Medeiros MCS, et al. Dietary intervention and health in patients with systemic lupus erythematosus: a systematic review of the evidence. Crit. Rev. Food Sci. Nutr. 2019;59:2666–2673. doi: 10.1080/10408398.2018.1463966. [DOI] [PubMed] [Google Scholar]
- 360.Duarte-García A, et al. Effect of omega-3 fatty acids on systemic lupus erythematosus disease activity: a systematic review and meta-analysis. Autoimmun. Rev. 2020;19:102688. doi: 10.1016/j.autrev.2020.102688. [DOI] [PubMed] [Google Scholar]
- 361.Shoenfeld Y, et al. Vitamin D and systemic lupus erythematosus - the hype and the hope. Autoimmun. Rev. 2018;17:19–23. doi: 10.1016/j.autrev.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 362.Islam MA, et al. Vitamin D status in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Autoimmun. Rev. 2019;18:102392. doi: 10.1016/j.autrev.2019.102392. [DOI] [PubMed] [Google Scholar]
- 363.Hsieh CC, Lin BF. Dietary factors regulate cytokines in murine models of systemic lupus erythematosus. Autoimmun. Rev. 2011;11:22–27. doi: 10.1016/j.autrev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 364.Bischoff SC, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2023;42:352–379. doi: 10.1016/j.clnu.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 365.Chan SSM, et al. Obesity is associated with increased risk of Crohn’s disease, but not ulcerative colitis: a pooled analysis of five prospective cohort studies. Clin. Gastroenterol. Hepatol. 2022;20:1048–1058. doi: 10.1016/j.cgh.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 366.Lee JY, et al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe. 2020;28:273–284.e276. doi: 10.1016/j.chom.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Massironi S, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023;8:579–590. doi: 10.1016/S2468-1253(23)00011-0. [DOI] [PubMed] [Google Scholar]
- 368.Lamb CA, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Hansen T, Duerksen DR. Enteral nutrition in the management of pediatric and adult Crohn’s disease. Nutrients. 2018;10:537. doi: 10.3390/nu10050537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sigall Boneh R, et al. Dietary therapies induce rapid response and remission in pediatric patients with active Crohn’s disease. Clin. Gastroenterol. Hepatol. 2021;19:752–759. doi: 10.1016/j.cgh.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 371.Cox SR, et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158:176–188.e177. doi: 10.1053/j.gastro.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 372.Ruggeri RM, et al. Influence of dietary habits on oxidative stress markers in Hashimoto’s Thyroiditis. Thyroid. 2021;31:96–105. doi: 10.1089/thy.2020.0299. [DOI] [PubMed] [Google Scholar]
- 373.Osowiecka K, Myszkowska-Ryciak J. The influence of nutritional intervention in the treatment of Hashimoto’s Thyroiditis-a systematic review. Nutrients. 2023;15:1041. doi: 10.3390/nu15041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Manzel A, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014;14:404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bosch-Queralt M, et al. Diet-dependent regulation of TGFβ impairs reparative innate immune responses after demyelination. Nat. Metab. 2021;3:211–227. doi: 10.1038/s42255-021-00341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kleinewietfeld M, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Cignarella F, et al. Intermittent fasting confers protection in CNS Autoimmunity by altering the gut microbiota. Cell Metab. 2018;27:1222–1235.e1226. doi: 10.1016/j.cmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Evans E, Piccio L, Cross AH. Use of vitamins and dietary supplements by patients with multiple sclerosis: a review. JAMA Neurol. 2018;75:1013–1021. doi: 10.1001/jamaneurol.2018.0611. [DOI] [PubMed] [Google Scholar]
- 379.Lemke D, et al. Vitamin D resistance as a possible cause of autoimmune diseases: a hypothesis confirmed by a therapeutic high-dose vitamin D protocol. Front. Immunol. 2021;12:655739. doi: 10.3389/fimmu.2021.655739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Threapleton DE, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Bmj. 2013;347:f6879. doi: 10.1136/bmj.f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Kaye DM, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141:1393–1403. doi: 10.1161/CIRCULATIONAHA.119.043081. [DOI] [PubMed] [Google Scholar]
- 382.Marques FZ, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 383.Zampelas A, Magriplis E. Dietary patterns and risk of cardiovascular diseases: a review of the evidence. Proc. Nutr. Soc. 2020;79:68–75. doi: 10.1017/S0029665119000946. [DOI] [PubMed] [Google Scholar]
- 384.GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet393, 1958–1972 (2019). [DOI] [PMC free article] [PubMed]
- 385.Gao P, et al. Salt-induced hepatic inflammatory memory contributes to cardiovascular damage through epigenetic modulation of SIRT3. Circulation. 2022;145:375–391. doi: 10.1161/CIRCULATIONAHA.121.055600. [DOI] [PubMed] [Google Scholar]
- 386.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–989. doi: 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Jayachandran M, Chung SSM, Xu B. A critical review on diet-induced microbiota changes and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2020;60:2914–2925. doi: 10.1080/10408398.2019.1666792. [DOI] [PubMed] [Google Scholar]
- 388.Xu Y, et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing Tropomodulin-3 Propionylation in platelets. Circulation. 2020;142:49–64. doi: 10.1161/CIRCULATIONAHA.119.043581. [DOI] [PubMed] [Google Scholar]
- 389.Takatsu M, et al. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension. 2013;62:957–965. doi: 10.1161/HYPERTENSIONAHA.113.02093. [DOI] [PubMed] [Google Scholar]
- 390.Palee S, et al. Combination of exercise and calorie restriction exerts greater efficacy on cardioprotection than monotherapy in obese-insulin resistant rats through the improvement of cardiac calcium regulation. Metabolism. 2019;94:77–87. doi: 10.1016/j.metabol.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 391.An HS, et al. Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signaling mice. Sci. Rep. 2020;10:7176. doi: 10.1038/s41598-020-64201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Fontana L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 2018;15:566–577. doi: 10.1038/s41569-018-0026-8. [DOI] [PubMed] [Google Scholar]
- 393.Mishra A, et al. Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat. Metab. 2021;3:1342–1356. doi: 10.1038/s42255-021-00469-6. [DOI] [PubMed] [Google Scholar]
- 394.Stekovic S, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–476.e466. doi: 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 395.Dyńka D, Kowalcze K, Charuta A, Paziewska A. The ketogenic diet and cardiovascular diseases. Nutrients. 2023;15:3368. doi: 10.3390/nu15153368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Burén J, Ericsson M, Damasceno NRT, Sjödin A. A Ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. 2021;13:814. doi: 10.3390/nu13030814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Chen XW, Ding G, Xu L, Li P. A glimpse at the metabolic research in China. Cell Metab. 2021;33:2122–2125. doi: 10.1016/j.cmet.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 398.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 399.Wang C, et al. Mendelian randomization analyses for PCOS: evidence, opportunities, and challenges. Trends Genet. 2022;38:468–482. doi: 10.1016/j.tig.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 400.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 401.Jais A, Brüning JC. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017;127:24–32. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Hepler C, et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science. 2022;378:276–284. doi: 10.1126/science.abl8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wang X, et al. Time-restricted feeding is an intervention against excessive dark-phase sleepiness induced by obesogenic diet. Natl Sci. Rev. 2023;10:nwac222. doi: 10.1093/nsr/nwac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Lee YS, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157:1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Cao S, et al. EGFR-mediated activation of adipose tissue macrophages promotes obesity and insulin resistance. Nat. Commun. 2022;13:4684. doi: 10.1038/s41467-022-32348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rheinheimer J, et al. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism. 2017;74:1–9. doi: 10.1016/j.metabol.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 408.Softic S, et al. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020;57:308–322. doi: 10.1080/10408363.2019.1711360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Teijeiro A, et al. Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat. Metab. 2021;3:496–512. doi: 10.1038/s42255-021-00371-1. [DOI] [PubMed] [Google Scholar]
- 410.Hu B, et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 2020;578:610–614. doi: 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Glenn AJ, et al. The portfolio diet and incident type 2 diabetes: findings from the women’s health initiative prospective cohort study. Diabetes Care. 2023;46:28–37. doi: 10.2337/dc22-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Hashemi R, Rahimlou M, Baghdadian S, Manafi M. Investigating the effect of DASH diet on blood pressure of patients with type 2 diabetes and prehypertension: randomized clinical trial. Diabetes Metab. Syndr. 2019;13:1–4. doi: 10.1016/j.dsx.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 413.Salas-Salvadó J, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160:1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 414.Zhao L, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 415.Tucker LA. Fiber intake and insulin resistance in 6374 adults: the role of abdominal obesity. Nutrients. 2018;10:237. doi: 10.3390/nu10020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Deehan EC, et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome. 2022;10:77. doi: 10.1186/s40168-022-01248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Herz D, et al. Efficacy of fasting in type 1 and type 2 diabetes mellitus: a narrative review. Nutrients. 2023;15:3525. doi: 10.3390/nu15163525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Patikorn C, et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw. Open. 2021;4:e2139558. doi: 10.1001/jamanetworkopen.2021.39558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Li G, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26:672–685.e674. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Yuan X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr. Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Kinzig KP, Honors MA, Hargrave SL. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology. 2010;151:3105–3114. doi: 10.1210/en.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ryan MC, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 423.Godos J, Federico A, Dallio M, Scazzina F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017;68:18–27. doi: 10.1080/09637486.2016.1214239. [DOI] [PubMed] [Google Scholar]
- 424.Browning JD, et al. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011;93:1048–1052. doi: 10.3945/ajcn.110.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Hansen CD, et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease : a randomized controlled trial. Ann. Intern. Med. 2023;176:10–21. doi: 10.7326/M22-1787. [DOI] [PubMed] [Google Scholar]
- 426.Watanabe M, et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes. Rev. 2020;21:e13024. doi: 10.1111/obr.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Ezpeleta M, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab. 2023;35:56–70.e53. doi: 10.1016/j.cmet.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Marjot T, Tomlinson JW, Hodson L, Ray DW. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut. 2023;72:1607–1619. doi: 10.1136/gutjnl-2023-329998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Kazemi M, et al. Comparison of dietary and physical activity behaviors in women with and without polycystic ovary syndrome: a systematic review and meta-analysis of 39 471 women. Hum. Reprod. Update. 2022;28:910–955. doi: 10.1093/humupd/dmac023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Faghfoori Z, Fazelian S, Shadnoush M, Goodarzi R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab. Syndr. 2017;11:S429–s432. doi: 10.1016/j.dsx.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 431.Barrea L, et al. Adherence to the Mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS) Nutrients. 2019;11:2278. doi: 10.3390/nu11102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Magagnini MC, et al. Does the ketogenic diet improve the quality of ovarian function in obese women? Nutrients. 2022;14:4147. doi: 10.3390/nu14194147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Paoli A, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl Med. 2020;18:104. doi: 10.1186/s12967-020-02277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Li C, et al. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl Med. 2021;19:148. doi: 10.1186/s12967-021-02817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Tabrizi FPF, Farhangi MA, Vaezi M, Hemmati S. The effects of spinach-derived thylakoid supplementation in combination with calorie restriction on anthropometric parameters and metabolic profiles in obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutr. J. 2020;19:82. doi: 10.1186/s12937-020-00601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Goncalves MD, Maddocks OD. Engineered diets to improve cancer outcomes. Curr. Opin. Biotechnol. 2021;70:29–35. doi: 10.1016/j.copbio.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ligibel JA, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J. Clin. Oncol. 2022;40:2491–2507. doi: 10.1200/JCO.22.00687. [DOI] [PubMed] [Google Scholar]
- 438.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20:e77–e91. doi: 10.1016/S1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]
- 439.Mayne ST, Playdon MC, Rock CL. Diet, nutrition, and cancer: past, present and future. Nat. Rev. Clin. Oncol. 2016;13:504–515. doi: 10.1038/nrclinonc.2016.24. [DOI] [PubMed] [Google Scholar]
- 440.Preguiça I, et al. Diet-induced rodent models of obesity-related metabolic disorders- a guide to a translational perspective. Obes. Rev. 2020;21:e13081. doi: 10.1111/obr.13081. [DOI] [PubMed] [Google Scholar]
- 441.Fenton JI, Hord NG. Stage matters: choosing relevant model systems to address hypotheses in diet and cancer chemoprevention research. Carcinogenesis. 2006;27:893–902. doi: 10.1093/carcin/bgi355. [DOI] [PubMed] [Google Scholar]
- 442.Liu Y, et al. Host obesity alters the ovarian tumor immune microenvironment and impacts response to standard of care chemotherapy. J. Exp. Clin. Cancer Res. 2023;42:165. doi: 10.1186/s13046-023-02740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]