Abstract
The class II transactivator (CIITA) is a key regulatory factor that controls expression of the major histocompatibility complex (MHC) class II genes that are essential components for antigen presentation and thus regulation of the immune response. We show here that the adenovirus E1A protein interferes with the action of CIITA and inhibits both B-cell-specific and gamma interferon (IFN-γ)-induced expression of MHC class II promoters. Transfection studies provide evidence for the functional role of the CREB-binding protein (CBP) in IFN-γ and CIITA-mediated MHC class II promoter activation. We demonstrate that the N-terminally located transcription activation domain of CIITA physically interacts with both the N-terminal and the E1A-binding (C/H3) regions of CBP. These results suggest the involvement of a multisubunit complex, which contains the gene-specific coactivator CIITA and the versatile coactivator CBP, in MHC class II gene regulation, which may be responsible for both high-level expression and modulation by different signaling pathways.
Surface expression of major histocompatibility complex (MHC) molecules enables cells to acquire antigen-presenting capability that is essential for the function of the immune system. Regulation of transcription of MHC class II genes has served as a model system to study both cell-specific and inducible gene expression. These genes are constitutively expressed in B cells and their expression can be induced in a variety of cell types upon gamma interferon (IFN-γ) treatment (13). The DNA regulatory region responsible for the complex pattern of MHC class II expression has been identified. This region is remarkably complex, consisting of an array of functional elements (H/Z/W, X, and Y) that are conserved both in sequence and spacing among the different human and mouse genes (13). Although many of the transcription factors that bind to these elements have been identified (24, 41), their presence is not sufficient for the regulated expression of these genes. Specificity of expression is achieved by recruitment to the promoter of the class II transactivator (CIITA), which acts as a gene-specific coactivator and whose expression pattern parallels exactly that of class II gene expression. Thus, CIITA expression is constitutive in B lymphocytes and other antigen-presenting cells and is IFN-γ inducible in various cell types (24, 40, 41). MHC class II promoter activation by CIITA requires primarily the X1-X2 region and secondarily the Y and H boxes (35, 45). CIITA does not bind DNA on its own but is recruited to the promoter via protein-protein interactions that are documented to involve at least the RFX5 factor (37) and possibly other proteins bound to the class II conserved elements (X, Y, and H boxes). Functional dissection of CIITA revealed the presence of an amino-terminal acidic region that can function as an autonomous activation domain and a carboxy-terminal region that is required for the recruitment of CIITA to the MHC class II promoters (35, 45).
Although a lot of information exists regarding the positive regulation of MHC class II genes either in B cells or in other cell types which are inducible by IFN-γ, the mechanism of action of negatively acting agents is poorly understood. Several substances that inhibit MHC class II genes are known (13). Glucocorticoids and prostaglandins down regulate MHC class II genes in B cells (7, 13, 16, 38). In macrophages, glucocorticoids, prostaglandins, and IFN-α/β antagonize the action of IFN-γ, which together with interleukin 4 is the main positive regulator of MHC class II genes (11, 13). The adenoviral oncoprotein E1A has a strong inhibitory effect on IFN-inducible gene activity in many systems, including the MHC class II genes (14, 18). E1A oncoprotein is a pleiotropic molecule able to modulate the expression of various cellular genes (3). Some of the effects of E1A have been attributed to its interactions with the versatile coactivators CREB-binding protein (CBP) and p300 (17, 39). The amino-terminal region and conserved region 1 (CR1) of E1A have been shown to be involved in interactions with the C/H3 regions of CBP and p300 coactivators, resulting in inhibition of transcription from various cellular enhancers and promoters requiring CBP-p300 (1, 10, 23). CBP-p300 coactivators interact with a large number of activators and potentiate their activity by recruitment of the basic transcriptional machinery and via histone acetylation (2, 17, 33, 39, 43). Since CBP-p300-dependent transcriptional activators mediate the effects of diverse signal transduction pathways, competition for limiting amounts of CBP-p300 by different activators can account for the specificity of cellular responses to extracellular signals (15, 19). In an attempt to analyze the mechanism of action of positively or negatively acting agents, we investigated the involvement of CBP coactivator in the regulation of MHC class II expression.
In this paper we show that CBP is required for both IFN-γ-inducible and constitutive B cell expression of MHC class II promoters and that E1A inhibits both expression pathways. We demonstrate that CBP is recruited to the MHC class II promoter by interaction with the N-terminal activation domain of CIITA. Thus, activation of MHC class II gene expression requires the interplay between the gene-specific coactivator CIITA and the versatile coactivator CBP.
MATERIALS AND METHODS
Cell culture and transfections.
HeLa and COS1 cell lines were maintained and transfected as previously described (42). Lymphoid (Raji) cells were maintained as before (42) and were transfected by electroporation at 210 V and 960 μF with a Bio-Rad apparatus. Chloramphenicol acetyltransferase (CAT) assays were performed as described earlier (42). Cells were harvested 48 h after transfection. When indicated, cells were treated with 50 U of IFN-γ (R & D) per ml for the last 20 to 24 h before harvesting.
Plasmids.
Full-length CIITA or its derivatives were expressed from pCDNA3 or pRC-RSV expression vectors. pRSV5E1A, expressing the 13S product, and pRSVmCR1, -CR2, and -CR3, expressing molecules with deletions in these domains (amino acids 38 to 65, 125 to 133, and 140 to 185, respectively), were provided by A. van der Eb (32). An N-terminal E1A deletion mutant missing amino acids 2 to 20 was generated by removing sequences upstream of the PvuII site and providing a new initiation codon. All E1A products were expressed from a Rous sarcoma virus (RSV)-based vector and their expression was verified by immunofluorescence analysis of transiently transfected COS1 cells with a rabbit anti-E1A antibody (Santa Cruz) and fluorochrome-coupled secondary antibody. CBP expression plasmids have been described (27). GAL4 fusion products were expressed on pBXG1 (36) or its derivative pRXG1, produced by replacing the simian virus 40 promoter with the RSV long terminal repeat. The class II −353 Eα CAT construct has been described (42). Expression of CIITA or its segments was established by immunofluorescence and Western blotting analysis with antibodies against the GAL4 DNA-binding domain (sc-577; Santa Cruz) or the hemagglutinin epitope (sc-805; Santa Cruz) tag that was introduced at the N-terminal end of the coding region. Intact and truncated CIITA molecules were also produced as fusions with the green fluorescent protein (GFP) by using the EGFPC1 plasmid (Clontech), and their expression was detected by fluorescence of living cells. All constructions were verified by dideoxy sequencing.
In vitro protein-protein interaction experiments.
Fragments of CBP were subcloned into pGEX vectors (Pharmacia) in frame with glutathione S-transferase (GST) and were expressed in Escherichia coli DH5α. Approximately 2 μg of fusion proteins was immobilized on glutathione-Sepharose beads and incubated with in vitro-translated and 35S-labeled (TNT; Promega) CIITA protein in a buffer containing 150 mM KCl, 20 mM HEPES (pH 7.9), 0.1% Nonidet P-40, 5 mM MgCl2, and 0.2% bovine serum albumin and supplemented with protease inhibitors. Reactions were carried out at 4°C for 5 h, and the mixtures were washed three times in the same buffer without bovine serum albumin. Bound proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected by autoradiography.
Immunoprecipitation and Western blotting.
Whole-cell extracts were prepared from transiently transfected COS cells in lysis buffer containing 10 mM Tris HCl (pH 8), 170 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, 1 mM dithiothreitol, and protease inhibitors. Extracts equivalent to about 5 × 106 cells were incubated for 16 h at 4°C with the rabbit anti-CBP antibodies C-20 and A-22 (sc-583 and sc-369; Santa Cruz). After a 3-h incubation with 50 μl of protein A-agarose beads (Pharmacia), the immunoprecipitated samples were washed four times with lysis buffer containing 250 mM NaCl and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting analysis was performed with either rabbit anti-CBP antibodies or a mouse monoclonal antibody directed against GFP (Clontech).
RESULTS
E1A inhibits MHC class II gene transcription and interferes with CIITA action.
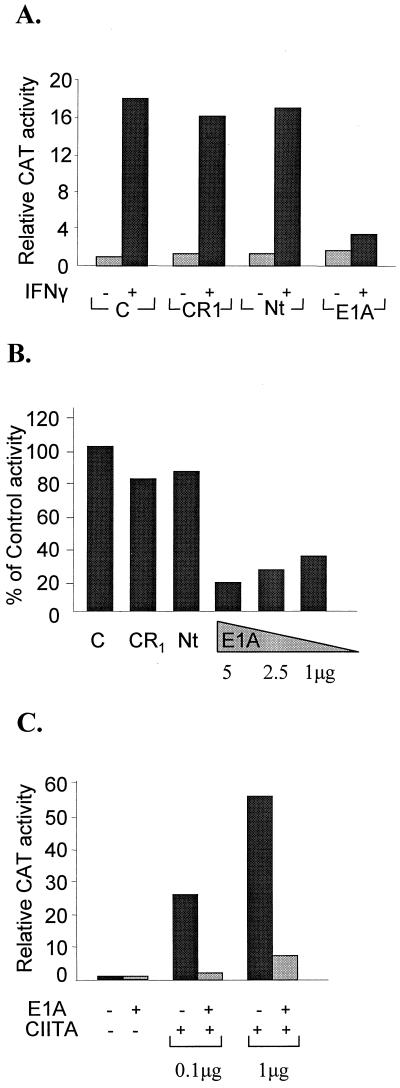
To investigate the role of the E1A oncoprotein in MHC class II gene expression, we carried out transient-transfection experiments with a reporter construct bearing the mouse Eα class II gene promoter (−353 to +14) fused to the CAT gene along with cotransfected E1A expression plasmids. As shown in Fig. 1A, IFN-γ treatment resulted in a 16-fold transcriptional activation of the transfected Eα gene promoter in HeLa cells. However, cotransfection of the E1A expression plasmid significantly decreased the IFN-γ-stimulated transcriptional activation of the Eα promoter but had no effect on the basal level activity. Furthermore, E1A strongly inhibited the high-level expression of the Eα gene in Raji B cells (Fig. 1B). Mutations in the E1A protein that removed CR1 or the 22 amino-terminal amino acids (Nt) did not affect either IFN-γ- or B-cell-specific transcription (Fig. 1A and B). In contrast, deletions that removed CR2 or CR3 had no effect on the ability of E1A to inhibit class II gene expression (data not shown). Similar results were obtained with reporter constructs bearing the mouse Eβ or human DRα promoters (data not shown). Previous studies have established that CIITA is required for both IFN-γ- and B-cell-specific expression of the class II genes (24, 40, 41). In addition, ectopic expression of CIITA in HeLa cells is sufficient to direct high levels of MHC expression even in the absence of IFN-γ treatment (9, 21a). Therefore, we investigated the effect of E1A on the ability of ectopically expressed CIITA to activate the Eα promoter in HeLa cells. Transfected CIITA strongly activated class II expression in HeLa cells (up to 58-fold) under these experimental conditions (Fig. 1C). Remarkably, cotransfection of the intact E1A practically abolished CIITA-dependent activation of the Eα gene promoter (Fig. 1C), whereas its Nt or CR1 mutants could not affect the action of CIITA (data not shown). These results strongly suggest that CIITA may be one of the targets of E1A action.
FIG. 1.
E1A represses IFN-γ-mediated, constitutive B-lymphoid-cell, and CIITA-inducible class II promoter activity. (A) Samples of 200 ng of plasmids encoding wild-type (E1A) or proteins with deletions of the indicated conserved domains (CR1 and Nt) or vector control (C) were cotransfected with 2 μg of an Eα CAT class II plasmid into HeLa cells. Basal and IFN-γ activities were assayed 24 h after IFN-γ addition. Results are averages of five experiments with variability less than 15%. The vector control CAT activity was set at 1. (B) Raji cells were transfected with 5 μg of Eα CAT reporter and 5 μg of vector control (C), CR1 plasmid, or Nt plasmid or the indicated amounts of E1A wild-type plasmids. The activity of the vector control was set at 100%. (C) Eα CAT activity was evaluated in the presence of 200 ng of E1A and either 0.1 or 1 μg of a CIITA expression plasmid (CIITA) in HeLa cells. In this and all subsequent experiments the vector control value was set at 1 and corresponded to at least 500 cpm above background level.
CBP is required for CIITA-dependent activation of MHC class II promoters.
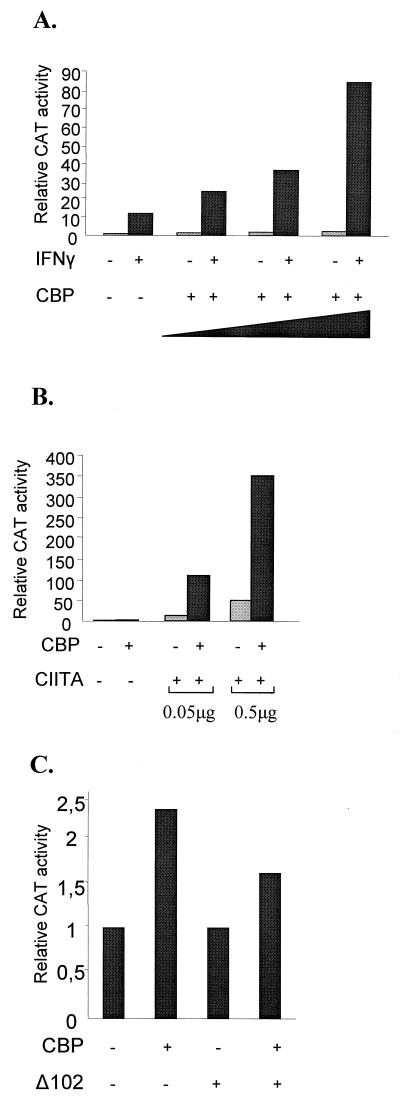
We have shown that both CR1 and the amino-terminal regions of E1A are required for inhibition of class II gene expression. Interestingly, the same regions of E1A are also required for the pleiotropic effect of E1A on various unrelated activators. This effect is due to the interaction of E1A with the CBP/p300 class of coactivators (1, 10, 23). Therefore, we initially investigated whether CBP is involved in class II gene expression. HeLa cells were transiently transfected with the Eα promoter construct along with increasing amounts of a CBP-expressing plasmid, and CAT reporter activity was determined in extracts derived from either untreated or IFN-γ-treated cells. The data in Fig. 2A show that transfection of increasing amounts of the CBP plasmid resulted in a dose-dependent potentiation (up to sevenfold) of the IFN-γ-induced transcription and had no significant effect (up to 2.2-fold) on the basal level of expression. Thus, the CBP coactivator is required for maximal levels of IFN-γ-induced class II gene transcription.
FIG. 2.
CBP augments the IFN-γ and CIITA activation of a class II promoter. (A) CAT activity from uninduced and IFN-γ-induced HeLa cells transfected with an Eα CAT plasmid was assayed in the absence or the presence of 1, 2, or 3 μg of a CBP-expressing plasmid. (B) CAT activity of the Eα CAT promoter in HeLa cells after introduction of CIITA (0.05 or 0.5 μg), CBP (4 μg), or both expression plasmids. (C) Potentiation of the action of CIITA by CBP requires the N-terminal activation domain of CIITA. CAT activity of the Eα CAT promoter in HeLa cells after introduction of the CIITA truncation Δ102 mutant (0.5 μg), CBP (4 μg), or both expression plasmids is shown.
Next, we examined whether the two coactivators CBP and CIITA synergize in the context of the class II promoter. The data in Fig. 2B show that transfection of either small (50 ng) or large (500 ng) amounts of a CIITA-expressing plasmid resulted in 11- and 51-fold activation, respectively, of the Eα promoter in HeLa cells. However, coexpression of CBP with CIITA activated the Eα promoter 111- and 350-fold, respectively, thus resulting in a 7- to 10-fold synergy. The fact that CBP alone minimally affects Eα promoter activity suggests that recruitment of CBP to the promoter requires the presence of CIITA. The latter is recruited to the promoter either following ectopic expression or by the induction of its synthesis by IFN-γ. It has been previously shown that amino-terminal deletions of CIITA fail to activate class II promoters and exhibit a dominant negative function (5, 21a, 46). Interestingly, such a deletion mutant of CIITA lacking the 102 amino-terminal amino acids (Δ102 mutant), which is devoid of transcriptional activation capability, could not synergize with CBP (Fig. 2C). Thus, potentiation of CIITA action on a class II promoter by CBP depends on the presence of the N-terminal activation domain of CIITA.
CBP interacts directly with CIITA.
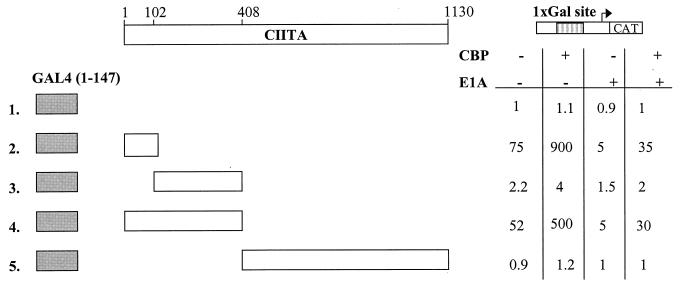
To examine whether the transcriptional synergy between CIITA and CBP on transcriptional activation of the class II promoters is due to their direct interaction, we used a mammalian two-hybrid assay approach. Different portions of CIITA were linked to the yeast GAL4 DNA-binding domain, and their transcriptional activities were determined either in the presence or in the absence of cotransfected CBP with a reporter bearing a single GAL4 site upstream of the CAT gene. Fusion proteins containing the amino-terminal amino acids 1 to 114 or 1 to 408 of CIITA increased reporter gene expression 75- and 52-fold in agreement with earlier reports (35, 45), and these activities were further enhanced by CBP up to 900- and 500-fold above basal levels, respectively (Fig. 3, lines 2 and 4). In addition, E1A strongly decreased the intrinsic activation function of the amino-terminal fragments of CIITA, which was partially restored by coexpression of CBP (Fig. 3, lines 2 and 4). The fragments of CIITA containing the region spanning amino acids 102 to 408 or 408 to 1130 neither activated transcription nor synergized with CBP (Fig. 3, lines 3 and 5). These data show that the 102 amino-terminal amino acids of CIITA are involved in the interaction with CBP.
FIG. 3.
CBP interacts with the N-terminal domain of CIITA. Cells were transfected with 1 μg of plasmids expressing hybrids of the GAL4 DNA binding domain fused to the indicated segments of CIITA and 1.5 μg of a minimal promoter CAT reporter carrying a single GAL4 binding site shown diagrammatically on top. Results are averages of triplicate experiments in HeLa cells in the presence or the absence of 6 μg of CBP and/or 0.5 μg of E1A expression plasmids.
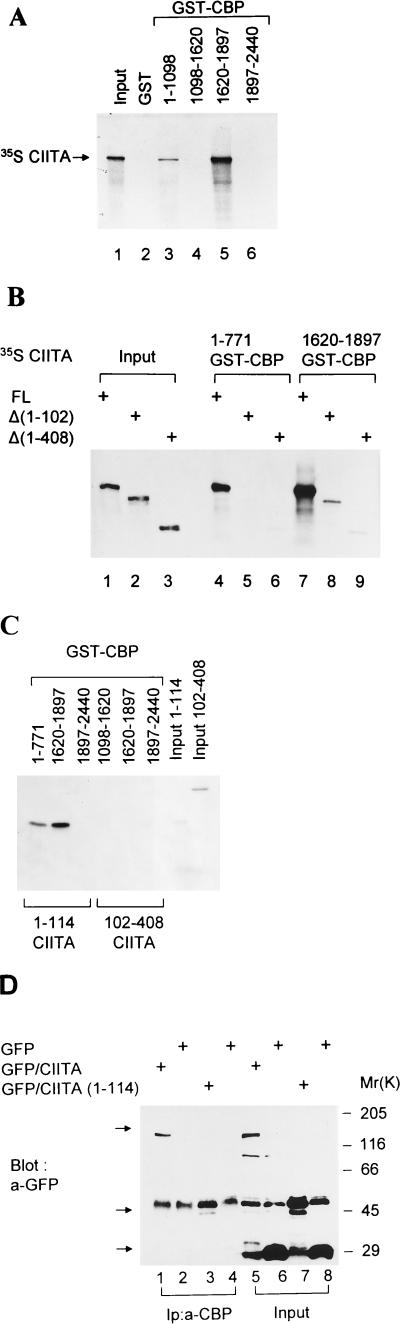
In vitro evidence for a physical interaction between CIITA and CBP was obtained by GST pull-down experiments. In vitro translated and 35S-labeled CIITA was tested for its ability to specifically interact with several fragments of CBP immobilized on glutathione-Sepharose beads. We found that CIITA interacted with two different regions of CBP. The first, which spans amino acids 1620 to 1897 (Fig. 4A, lane 5), contains the E1A-binding region of CBP, whereas the second is located within the first 1,098 amino acids of CBP (Fig. 4A, lane 3). The specificity of these interactions was underscored by the inability of CIITA to be retained by GST alone (Fig. 4A, lane 2) or by other regions of CBP (Fig. 4A, lanes 4 and 6). The amino-terminal region able to interact with CIITA was further restricted to the first 771 amino acids of CBP (data not shown). The data in Fig. 4B show that deletion of the amino-terminal 102 amino acids of CIITA greatly diminished its ability to interact with both domains of CBP (compare lanes 4 with 5 and 7 with 8). Significantly, the 102 amino-terminal amino acids of CIITA are sufficient for interaction with both regions of CBP (Fig. 4C). These results demonstrate that the amino terminus of CIITA, which contains an intrinsic activating function, directly contacts CBP at two different regions. One of these regions coincides with the E1A-binding domain of CBP, whereas the second region includes amino acids 1 to 771 of CBP. The association of E1A with CBP/p300 requires both N-terminal and CR1 sequences (1, 10, 23). The inability of the E1A mutants lacking either of these regions (Nt or CR1) to affect IFN-γ or CIITA action correlates with their inability to bind CBP. Thus, the action of E1A may be due to competition with CIITA for CBP binding or to the formation of a ternary complex that is transcriptionally inactive.
FIG. 4.
CIITA physically associates with CBP. (A) In vitro translated 35S-radiolabeled full-length CIITA was used in a GST pull-down assay with equal amounts of GST alone (lane 2) or GST fused with the indicated parts of CBP (lanes 3 to 6). Lane 1 contains 10% of input CIITA. (B) GST pull-down assays using the CBP regions of amino acids 1 to 771 (lanes 4 to 6) and 1620 to 1897 (lanes 7 to 9) and in vitro-translated full-length (FL) or truncated CIITA molecules. Lanes 1 to 3 contain 10% of input CIITA. (C) In vitro translated and radiolabeled regions of CIITA spanning amino acids 1 to 114 and 102 to 408 were incubated with fusions of GST to amino acids 1 to 771, 1620 to 1897, and 1897 to 2440 of CBP. (D) CBP and CIITA form a complex in vivo. Whole-cell extracts from COS1 cells transfected with the indicated constructs were immunoprecipitated with anti-CBP (a-CBP) antibodies, followed by Western blot analysis with anti-GFP antibodies. Lanes 1 to 4, immunoprecipitation (Ip) with anti-CBP; lanes 5 to 8, extract without immunoprecipitation. Constructs used for transfections were GFP alone (lanes 2, 4, 6, and 8), GFP fused to the intact CIITA, GFP-CIITA (lanes 1 and 5), and its 114 N-terminal amino acids [GFP/CIITA(1–114)] (lanes 3 and 7). Arrows point to the aforementioned proteins.
In order to detect the association of CIITA with CBP in vivo, we used immunoprecipitation and Western blotting experiments (Fig. 4D). The full-length CIITA protein or just the 114 amino-terminal amino acids were produced in COS cells as fusions with GFP. Lysates from transfected COS cells were analyzed by Western blotting with a monoclonal antibody specific for GFP with (Fig. 4D, lanes 1 to 4) or without (lanes 5 to 8) previous immunoprecipitation with anti-CBP antibodies. The efficiency of immunoprecipitation was tested on a Western blot with anti-CBP antibodies (data not shown). Both the intact CIITA (Fig. 4D, lane 1) and a short CIITA molecule containing the first 114 amino acids (lane 3) were coimmunoprecipitated by the anti-CBP antibodies, as opposed to GFP alone (lanes 2 and 4). A fusion of GFP to a CIITA molecule lacking the 102 N-terminal amino acids could not be immunoprecipitated by anti-CBP antisera (data not shown). Thus, in agreement with the in vitro experiment, we demonstrated that CIITA also interacts with CBP in cells and that the first 114 amino-terminal amino acids of CIITA are sufficient for this interaction.
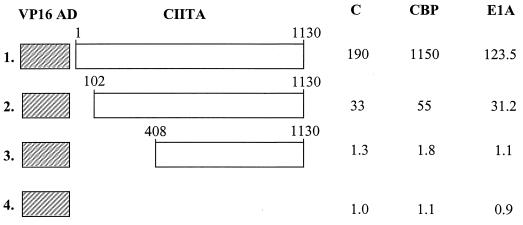
To investigate the biological significance of these interactions in the activation of the MHC class II genes, we examined the effects of CIITA constructs bearing deletions at their CBP-interacting domains. Previous studies have shown that amino-terminal deletions of CIITA abolished its ability to activate MHC class II gene expression, presumably because the transcriptional activation function is located in this region (35, 45). Therefore, we generated chimeric CIITA constructs bearing the strong and CBP-unresponsive VP16 activation domain and tested their ability to recruit CBP in vivo. The data in Fig. 5 show that transfection of the VP16-CIITA fusion activated transcription from a class II promoter that was further potentiated by coexpression of CBP (line 1). Interestingly, a construct bearing a deletion of the 102 amino-terminal amino acids of CIITA did not respond to CBP, although it was able to activate a class II promoter (line 2). Further deletion up to amino acid 408 resulted in transcriptionally inactive molecules, presumably due to their inability to be recruited to the promoter in the presence or the absence of ectopically expressed CBP (Fig. 5, line 3). The same VP16-CIITA fusion products were then tested for inhibition or noninhibition by E1A (Fig. 5). Cotransfected E1A inhibited the VP16 fusions of the intact CIITA and Δ102 mutant by 35 and 5%, respectively (Fig. 5, lines 1 and 2), under conditions in which the activity of the unfused CIITA was inhibited by more than 95% of the control (data not shown). Thus, the addition of the CBP-independent VP16 activation domain to the intact CIITA or the replacement of the 114 amino-terminal amino acids of CIITA by VP16 renders CIITA resistant to the suppressive effect of E1A.
FIG. 5.
The Δ102 mutant of CIITA missing its amino-terminal activation domain is recruited to the promoter but is not affected by coexpression of CBP or E1A. HeLa cells were transfected with 1 μg of class II CAT reporter, 1 μg of empty VP16 vector (line 4), or the indicated CIITA fusions (lines 1 to 3). Cells were cotransfected with either 0.5 μg of E1A, 4 μg of CBP expression plasmid (CBP), or the empty vector (C) alone. Shown are average activities from six experiments with less than 25% variability. The activity of the empty VP16 plasmid was set at 1.
These results strongly indicate that the interaction between CBP and the 102 amino-terminal amino acids of CIITA is essential for high levels of transcription from the MHC class II promoters.
DISCUSSION
We have studied the mechanism by which the CIITA protein functions to stimulate transcription of the class II histocompatibility genes. We demonstrate that CIITA works by recruiting the versatile coactivator CBP. The E1A oncoprotein inhibits MHC class II transcription by association with CBP, thus preventing the interaction between CIITA and CBP. The 102 amino-terminal amino acids of CIITA, which function as an autonomous activation domain, are required for recruitment of CBP.
CIITA is a gene-specific activator protein required for both B-lymphocyte-specific and IFN-γ-inducible expression of MHC class II genes. Previous studies have shown that, although the activity of CIITA depends on the presence of the well-characterized, conserved regulatory elements of MHC class II genes, CIITA itself is not a DNA-binding protein. This led to the hypothesis that CIITA functions as a gene-specific coactivator to increase transcriptional activation of the MHC class II genes (24, 41). CIITA is recruited to the MHC promoter via specific protein-protein interactions with the MHC activators (37). Similar to effects described in other IFN-responsive systems (14, 18), the E1A protein, as we show here, can suppress IFN-γ-induced transcription of the Eα MHC class II promoter. In addition, E1A strongly inhibits the activity of the same promoter in B lymphoid cells. In both cases inhibition depended on the presence of Nt and CR1 sequences of E1A. E1A also efficiently blocked promoter activation by exogenous CIITA, indicating that CIITA is a direct target of E1A. Since we could not detect interaction between CIITA and E1A (data not shown), repression by E1A can be explained by its ability to bind and inhibit the coactivators CBP and p300, in analogy to previously described cases (1, 10, 23).
In this study we demonstrate that the action of CIITA is potentiated by CBP and leads to increased expression of MHC class II promoters both constitutively in B lymphoid cells and following IFN-γ stimulation in non-B cells.
With a mammalian two-hybrid approach as well as GST pull-down and immunoprecipitation experiments, we were able to detect direct interaction between CBP and CIITA that leads to synergistic transcriptional activation. We demonstrated that CIITA can physically interact with the C/H3 domain (amino acids 1620 to 1897) and the 771 N-terminal amino acids of CBP. Dissection of the CIITA molecule showed that its N-terminal region (amino acids 1 to 102) interacts with both regions of CBP. Thus, the versatile coactivator CBP achieves gene type specificity by interacting with the gene-specific coactivator CIITA.
Previous reports described interactions of CIITA with components of the basal transcription machinery TAFII32 (12), TAFII250 (25), and TFIIB (25). The interaction of CIITA with CBP described here provides a direct promoter link with the RNA polymerase II to which CBP is complexed (20, 31). Therefore, CIITA may activate transcription through recruitment of TFIID-TFIIB complex and CBP-RNA polymerase II in a way reminiscent of the CREB activator (31). Comparison of the N-terminal activation domain of CIITA to the activation domain of VP16 showed that they are of equal potency when fused to the GAL4 DNA-binding domain (data not shown). However, CIITA, but not the VP16 activation domain, was further potentiated by CBP. Conversely, replacement of the CIITA activation domain with that of VP16, which is also known to directly contact the basal transcription apparatus, generates a molecule with a sixfold decrease of its MHC-specific transcriptional activation potency and with no significant response to CBP. Thus, the activation domain of CIITA is not interchangeable with the activation domain of VP16 in the context of the class II promoter because the former but not the latter, recruits CBP.
cis-acting promoter analysis and protein-DNA binding studies suggest that transcription of class II genes depends on the cooperative binding of factors that interact with at least the H/S, X, and Y elements (22, 29, 34). The assembly of a high-affinity multiprotein-DNA complex is necessary for the recruitment of CIITA to the promoter (24, 41). The multiprotein class II promoter complex may generate novel contact surfaces to facilitate the binding of not only CIITA but also CBP in a way similar to that of the well-characterized IFN-β gene (27). CBP recruitment, through its intrinsic and/or associated histone acetylase activity (2, 33, 43), could subsequently alter chromatin structure to strengthen the formation of the stereospecific complex (6, 21).
Expression of CIITA after IFN-γ treatment is dependent on the Jak-Stat pathway. CIITA synthesis cannot be induced in Jak1-deficient cells (8) or in cells derived from Stat1−/− mice (26). Stat1 binds to the IFN-γ-responsive promoter of CIITA in cooperation with USF-1 (30). Interestingly, gene activation by Stat1 and Stat2 requires the coactivators CBP and p300 (4, 15, 44). Since the effect of CBP on the action of transfected CIITA was also observed in the mutant cell line RJ225 (data not shown), lacking endogenous CIITA, potentiation does not involve CBP-mediated production of endogenous CIITA. Therefore, the CBP coactivator is involved in at least two steps that lead to MHC class II gene activation: first, in the expression of CIITA mediated by Stat1 (15, 44), and second, in the action of CIITA to transcriptionally activate class II target genes that we demonstrate here.
CBP and p300 serve as integrators of numerous signal-dependent pathways that control a multitude of genes. The involvement of CBP in MHC class II gene expression may thus explain the action of certain stimuli that negatively control them, such as glucocorticoids, prostaglandins (which raise intracellular cyclic AMP levels), and IFN-α/β (7, 11, 13, 16, 38). The glucocorticoid receptor (19), the CREB activator (28), and Stat2 (4) might compete with Stat1 and CIITA for CBP and thus limit the expression of class II genes.
The functional interaction between two non-DNA-binding regulatory proteins described in this paper provides further insights into the complex mechanism involved in the activation of expression of MHC class II genes as well as its modulation in various signal transduction pathways.
ACKNOWLEDGMENTS
We thank T. Makatounakis for expert technical assistance and L. Kalogeraki for photographic work. We thank A. van der Eb for E1A plasmids. We are grateful to D. Thanos for plasmids, helpful discussions, and advice and to J. Talianidis and C. Mamalaki for critical reading.
This work was funded by the Greek Secretariat General for Research through institutional funds and by grant 236.234.603 of the European Union Program EPET II.
REFERENCES
- 1.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Bayley S T, Mymryk J S. Adenovirus E1A proteins and transformation. Int J Oncol. 1994;5:425–444. doi: 10.3892/ijo.5.3.425. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, Livingston D M. Cooperation of Stat2 and p300/CBP in signaling induced by interferon-α. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 5.Bontron S, Ucla C, Mach B, Steimle V. Efficient repression of endogenous major histocompatibility complex class II expression through dominant negative CIITA mutants isolated by a functional selection strategy. Mol Cell Biol. 1997;17:4249–4258. doi: 10.1128/mcb.17.8.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 7.Celada A, McKercher S, Maki R A. Repression of major histocompatibility complex IA expression by glucocorticoids: the glucocorticoid receptor inhibits the DNA binding of the X box DNA binding protein. J Exp Med. 1993;177:691–698. doi: 10.1084/jem.177.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C H, Fontes J D, Peterlin M, Flavell R A. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C H, Flavell R A. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med. 1995;18:765–767. doi: 10.1084/jem.181.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckner R, Ewen M, Newsome D, Gerdes M, DeCaprio J, Lawrence J, Livingstone D. Molecular cloning and functional analysis of the adenovirus E1A associated 300-Kd (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 11.Fertsch-Ruggio D, Schoenberg D R, Vogel S N. Induction of macrophage Ia antigen expression by rIFN-gamma and down-regulation by IFN-alpha/beta and dexamethasone are regulated transcriptionally. J Immunol. 1988;141:1582–1589. [PubMed] [Google Scholar]
- 12.Fontes J D, Jiang B, Peterlin B M. The class II trans-activator CIITA interacts with the TBP-associated factor TAFII32. Nucleic Acids Res. 1997;25:2522–2528. doi: 10.1093/nar/25.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glimcher L H, Kara C J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 14.Gutch M J, Reich N C. Repression of the interferon signal transduction pathway by the adenovirus E1A oncogene. Proc Natl Acad Sci USA. 1991;88:7913–7917. doi: 10.1073/pnas.88.18.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvai A E, Xu L, Korzus E, Brard G, Kalafus D, Mullen T M, Rose D W, Rosenfeld M G, Glass C K. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc Natl Acad Sci USA. 1997;94:1074–1079. doi: 10.1073/pnas.94.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivashkiv L B, Glimcher L H. Repression of class II major histocompatibility complex genes by cyclic AMP is mediated by conserved promoter elements. J Exp Med. 1991;174:1583–1592. doi: 10.1084/jem.174.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janknecht R, Hunter T. Transcriptional control: versatile molecular glue. Curr Biol. 1996;6:951–954. doi: 10.1016/s0960-9822(02)00636-x. [DOI] [PubMed] [Google Scholar]
- 18.Kalvakolanu D V R, Bandyopadhyay S K, Harter M L, Sen G C. Inhibition of interferon-inducible gene expression by adenovirus E1A proteins: block in transcriptional complex formation. Proc Natl Acad Sci USA. 1991;88:7459–7463. doi: 10.1073/pnas.88.17.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 20.Kee B L, Arias J, Montminy M R. Adaptor-mediated recruitment of RNA polymerase II to a signal-dependent activator. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 21.Kim T K, Maniatis T. The mechanism of transcriptional synergy of an in vitro assembled interferon-β enhanceosome. Mol Cell. 1997;1:119–129. doi: 10.1016/s1097-2765(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 21a.Kretsovali, A. Unpublished data.
- 22.Louis-Plence P, Moreno C S, Boss J M. Formation of a regulatory factor X/X2 box-binding protein/nuclear factor-Y multiprotein complex on the conserved regulatory regions of HLA Class II genes. J Immunol. 1997;159:3899–3909. [PubMed] [Google Scholar]
- 23.Lundblad J R, Kwok R P S, Laurance M E, Harter M L, Goodman R H. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 24.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 25.Mahanta S K, Scholl T, Yang F C, Strominger J L. Transactivation by CIITA, the type II bare lymphocyte syndrome-associated factor, requires participation of multiple regions of the TATA box binding protein. Proc Natl Acad Sci USA. 1997;94:6324–6329. doi: 10.1073/pnas.94.12.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meraz M A, White J M, Sheehan K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 27.Merika M, Williams A J, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFNβ enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:1–20. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 28.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 29.Moreno C S, Emery P, West J E, Durand B, Reith W, Mach B. Purified X2BP cooperatively binds the class II MHC X box region in the presence of purified RFX, the X box factor deficient in the bare lymphocyte syndrome. J Immunol. 1995;155:4313–4321. [PubMed] [Google Scholar]
- 30.Muhlethaler-Mottet A, Di Berardino W, Otten L A, Mach B. Activation of the MHC class II transactivator CIITA by interferon-γ requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima T, Uchida C, Anderson S F, Parvin J D, Montminy M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 32.Offringa R, Gebel S, van Dam H, Timmers M, Smits A, Zwart R, Stein B, Bos J L, van der Eb A, Herrlich P. A novel function of the transforming domain of Ela: repression of AP-1 activity. Cell. 1990;62:527–538. doi: 10.1016/0092-8674(90)90017-9. [DOI] [PubMed] [Google Scholar]
- 33.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatami Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 34.Reith W, Kobr M, Emery P, Durand B, Siegrist C A, Mach B. Cooperative binding between factors RFX and X2bp to the X and X2 boxes of MHC class II promoters. J Immunol. 1994;31:20020–20025. [PubMed] [Google Scholar]
- 35.Riley J L, Westerheide S D, Price J A, Brown J A, Boss J M. Activation of class II MHC genes requires both the X box region and the class II transactivator (CIITA) Immunity. 1995;2:533–543. doi: 10.1016/1074-7613(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 36.Sadowski I, Ptashne M. A vector for expressing GAL4(1–147) fusions in mammalian cells. Nucleic Acids Res. 1989;18:7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholl T, Mahanta S K, Strominger J L. Specific complex formation between the type II bare lymphocyte syndrome-associated transactivators CIITA and RFX5. Proc Natl Acad Sci USA. 1997;94:6330–6334. doi: 10.1073/pnas.94.12.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwiebert L M, Schleimer R P, Radka S F, Ono S J. Modulation of MHC class II expression in human cells by dexamethasone. Cell Immunol. 1995;165:12–19. doi: 10.1006/cimm.1995.1181. [DOI] [PubMed] [Google Scholar]
- 39.Shikama N, Lyon J, Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 40.Steimle V, Siegrist C A, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 41.Steimle V, Reith W, Mach B. Major histocompatibility complex class II deficiency: a disease of gene regulation. Adv Immunol. 1996;61:327–340. doi: 10.1016/s0065-2776(08)60870-6. [DOI] [PubMed] [Google Scholar]
- 42.Thanos D, Mavrothalassitis G, Papamatheakis J. Multiple regulatory regions on the 5′ side of the mouse E alpha gene. Proc Natl Acad Sci USA. 1988;85:3075–3079. doi: 10.1073/pnas.85.9.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatami Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J J, Vinkemeier U, Gu W, Chakravarti D, Horvath C M, Darnell J E. Two contact regions between Stat1 and CBP/p300 in interferon γ signaling. Proc Natl Acad Sci USA. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H, Glimcher L H. Human MHC class II gene transcription directed by the carboxyl terminus of CIITA, one of the defective genes in type II MHC combined immune deficiency. Immunity. 1995;2:545–553. doi: 10.1016/1074-7613(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Su H S, Zhang X, Douhan III J, Glimcher L H. CIITA-dependent and -independent class II MHC expression revealed by a dominant negative mutant. J Immunol. 1997;158:4741–4749. [PubMed] [Google Scholar]