Abstract
We have constructed transgenic (Tg) mice expressing the entire human immunodeficiency virus type 1 (HIV-1) coding sequences in cells targeted by HIV-1 infection in humans. These Tg mice developed a severe AIDS-like disease leading to early death (<1 month). They developed muscle wasting, severe atrophy and fibrosis of lymphoid organs, tubulointerstitial nephritis, and lymphoid interstitial pneumonitis. In addition the expression of RANTES was increased in various tissues of these Tg mice relative to that in the normal controls. Disease appearance was correlated with the levels of transgene expression. The numerous pathologies observed in these mice are remarkably similar to those observed in human AIDS and, more specifically, in pediatric AIDS.
Several investigators have constructed transgenic (Tg) mice to model human immunodeficiency virus type 1 (HIV-1)-induced diseases (32). Expression of the whole HIV-1 genome in Tg mice led to the development of an AIDS-like syndrome, but this phenotype was observed in only a single line (35). More recently, it was found that the expression of the whole HIV-1 genome harboring a modified long terminal repeat (LTR) induced cataracts, weeping eye, and wasting in Tg mice (14). Expression of the 3′ half of the HIV-1 genome in Tg mice induced a severe nephropathy (13, 33). Expression of either Nef (15) or Tat (57) in Tg mice was found to induce epidermal hyperplasia, while expression of Nef, Gag, or protease in lens fiber cells was responsible for the development of cataracts (2, 12, 27, 56). Tg mice expressing HIV-1 Nef under the regulation of the T-cell-specific CD3 δ promoter-enhancer element (52), the T-cell receptor beta (TCRβ) chain enhancer-promoter (36), or the CD2 regulatory elements (4) showed various degrees of depletion of CD4+ thymocytes and of peripheral T cells. However, additional important characteristics of AIDS were not observed in these mice, while other features, such as a large increase of the B-cell population, that are not seen in human AIDS were observed (36).
In an attempt to develop a more relevant model of HIV-1 infection, we set out to express HIV-1 gene products in the same cells of Tg mice as those usually found infected in HIV-1-positive individuals, i.e., CD4+ T cells and cells of the dendritic/macrophage lineage. We used the human CD4 gene promoter sequences flanked by the enhancer of the mouse CD4 gene to express the whole HIV-1 coding sequences in Tg mice. We have previously shown that these enhancer-promoter sequences can direct expression of a surrogate gene specifically in CD4+ CD8+ and CD4+ CD8− thymic T cells, in peripheral CD4+ CD8− T cells, and in macrophages (23). Thus, expression of HIV-1 with this promoter should mimic more closely the expression of HIV-1 detected in HIV-1-infected individuals. In this report, we present evidence that indeed these Tg mice develop a severe AIDS-like disease which is dependent on the levels of HIV-1 expression.
MATERIALS AND METHODS
Generation of Tg mice.
The CD4A (12.5-kbp) and CD4C (14.4-kbp) promoters have been described previously (23). Each was fused to an 8.8-kbp BssHII-SacI fragment of the HIV-1 pNL4-3 clone (1) and to simian virus 40 polyadenylation sequences as described before (21) and cloned in the pBR322 vector to generate CD4A/HIVWT and CD4C/HIVWT transgenes, respectively. The transgene DNAs were excised with AatII, purified by agarose gel electrophoresis, and microinjected into fertilized (C57BL/6 × C3H)F2 oocytes, as described before (21). Chimeric mice were generated by inoculation of ES cell clones containing the CD4C/HIVWT transgene into C57BL/6 blastocysts as described elsewhere (42). Mice were bred as heterozygotes with C3H or CD1 mice obtained from Charles River Canada (St. Constant, Quebec, Canada). The presence of the transgene was confirmed by Southern blot hybridization of tail DNA with 32P-labeled total HIV-1 sequences as a probe, as described previously (21). The Tg mice and their non-Tg littermates were housed in the same cages.
RNA purification and Northern blot analysis.
RNA was isolated by the method of Chomczynski and Sacchi (6) from different tissues, and 10 μg from each sample was electrophoresed on formaldehyde agarose gels and processed for hybridization with a 32P-labeled 8.8-kbp BssHII-SacI HIV-1 probe, as previously described (21).
Flow cytometry.
Cell suspensions were prepared from lymphoid organs and stained with antibodies, as previously described (23). Fluorescein isothiocyanate (FITC)-coupled anti-mouse CD4, phycoerythrin-coupled anti-mouse CD8, and FITC-coupled anti-mouse TCRαβ antibodies were purchased from Cederlane Laboratories. Monoclonal antibodies RA3-6B2 (murine anti-B220), kindly provided by R. Coffman (DNAX Research Institute of Cellular and Molecular Biology, Palo Alto, Calif.), and Mac-1 (Boehringer Mannheim Inc., Montreal, Quebec, Canada) were used in an indirect assay. The second antibody was FITC-conjugated anti-rat immunoglobulin G (IgG) (mouse absorbed; Kirkegaard and Perry, Inc.). Cytometric analyses were performed with a FACscan (Becton Dickinson) as described previously (23).
Peritoneal macrophage preparation.
Mice were injected intraperitoneally with 1 ml of mineral oil 2 days prior to sacrifice. Peritoneal cells were harvested and plated on petri dishes. The attached macrophages were washed and processed for in situ hybridization (ISH) or collected for RNA extraction.
Detection of Igs.
Total Ig levels were measured by two-antibody assays with affinity-purified goat anti-Ig antibodies as described previously (24).
Microscopic analysis.
Mice were anesthetized with avertin, sera were collected, and the animals were exsanguinated with phosphate-buffered saline. Lymphoid organs were collected and immersion fixed in paraformaldehyde or periodate-lysine-paraformaldehyde fixative (38). The remainder of the animal was then perfusion fixed with paraformaldehyde or periodate-polylysine-paraformaldehyde fixative. Organs to be assessed were embedded in parraffin, sectioned at 5 μm, and stained with hematoxylin and eosin, as described previously (21). Tg and control non-Tg tissues were assessed blindly by two investigators (S.J. and D.G.K.).
ISH.
ISH was performed on paraffin-embedded tissues, using 35S-UTP-labeled antisense and control sense RNA probes as described previously (21). A mixture of two probes was used: the 1.4-kbp HindIII-SacI fragment (nucleotides 8131 to 9566) of the pNL4-3 clone (GenBank accession number M19921) and the 627-bp HindIII fragment (nucleotides 407 to 1034) of the HIV-1 BH102 clone (GenBank accession number M15654). Tissues from non-Tg control animals hybridized with antisense probes, as well as Tg animal tissues hybridized with sense probes, failed to exhibit any specific hybridization signal. RANTES expression was detected by using a 373-bp murine cDNA probe cloned by reverse transcription-PCR from thymus RNA, with the sense primer 5′-CTCTGCCGCGGGTACCATGAAG and the antisense primer 5′-GTGGCATCCCCAAGCTGGCTAG.
RESULTS
Construction of Tg mice and mosaicism of founder mice.
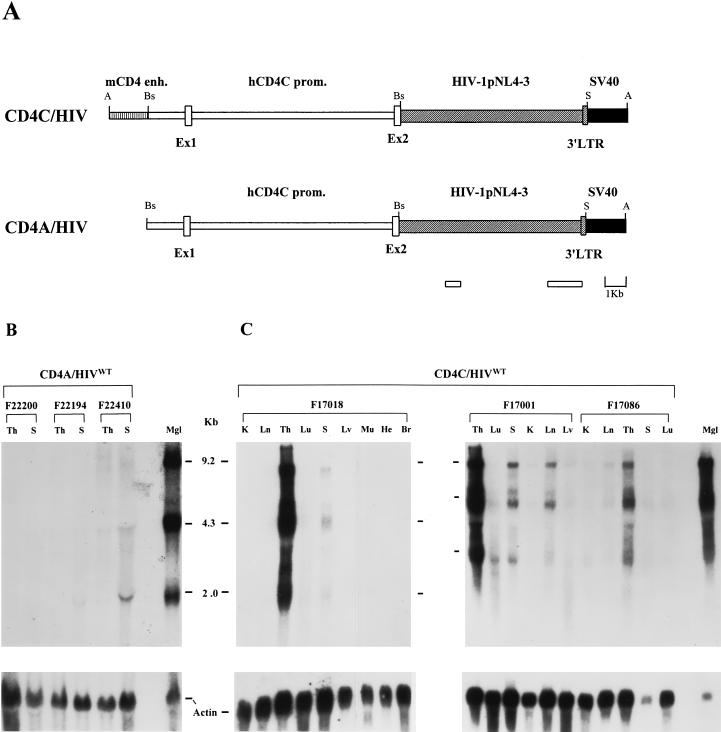
Five CD4C/HIVWT founders (F15564, F17001, F17018, F17027, and F17086) and four CD4A/HIVWT founders (F21093, F22194, F22200, and F22410) were produced. Southern blot analysis indicated that the structures of both transgenes (Fig. 1A) appeared to be grossly intact (data not shown).
FIG. 1.
(A) Structure and expression of CD4A/HIVWT and CD4C/HIVWT transgenes. The enhancer fragment of the mouse CD4 gene (mCD4 enh.) (vertically hatched bar), the human CD4 promoter (hCD4C prom.) (white bars and boxes), the HIV-1 pNL4-3 DNA fragment (diagonally hatched bars and boxes), simian virus 40 (SV40) polyadenylation sequences (black boxes), and the HIV-1 fragments cloned in GEM-4 (Promega) and used for riboprobes (lower white bars) are illustrated. Restriction sites: A, AatII; Bs, BssHII, S, SacI. (B and C) Transgene RNA expression in CD4A/HIVWT and CD4C/HIVWT mice. Northern blot analysis was carried out on total RNAs (10 μg) extracted from different organs of CD4A/HIVWT mice (from lines F22200, F22194, and F22410) (B) or CD4C/HIVWT mice (from lines F17018, F17001, and F17086) (C). K, kidney; Ln, lymph node; Th, thymus; Lu, lung; S, spleen; Lv, liver: Mu, muscle; He, heart; Br, brain; Mgl, mammary gland of a mouse mammary tumor virus/HIV Tg mouse (28) used as a positive control. Hybridization was performed with a 32P-labeled 8.9-kbp SacI fragment from the HIV-1 genomic sequences. The filters were then washed and rehybridized with an actin probe.
CD4C/HIVWT founders were bred with C3H or CD1 mice for the first generation (N1). All founder animals produced normal-size N1 litters, but the frequency of Tg animals in these litters was significantly lower than expected, except for one founder (F17018), suggesting that these founders were mosaic (Table 1). The CD4C/HIVWT founder F17027 produced only non-Tg pups and non-Tg 12- to 19-day-old fetuses (data not shown), confirming that this founder was indeed mosaic. The CD4C/HIVWT F17018 male founder died suddenly 3.5 months after birth, and no further analysis was carried out due to autolysis. The CD4C/HIVWT F17086 female founder became ill 5 months after birth from an intestinal obstruction caused by a large lymphoma which did not express the transgene (data not shown). The other three CD4C/HIVWT founders appeared to be healthy during a 12-month period of observation in terms of development, growth, body weight, and fertility. Tg lines could not be established from any of these CD4C/HIVWT Tg founders. Members of the first generation (N1) of CD4C/HIVWT Tg pups either died early or were sacrificed before attaining sexual maturity due to the development of severe disease. Only two N2 and no N3 Tg mice could be produced. The diseases arising in N1 Tg mice of the C3H or CD1 background were indistinguishable.
TABLE 1.
Characteristics of the CD4C/HIVWT founders
| Founder | Sexa | Clinical disease | No. of Tg mice/total no. of mice born (%) | Mosa- icism | N1 Tg pups surviving >15 days |
|---|---|---|---|---|---|
| 15564 | M | No | 3/69 (4.3) | Yes | Nob |
| 17001 | M | No | 7/212 (3.3) | Yes | Yesc |
| 17018 | M | Yes (sudden death) | 13/32 (40) | No | Yesc |
| 17027 | M | No | 0/98 (0) | Yes | NAd |
| 17086 | F | Yes (intestinal lymphoma) | 3/20 (15) | Yes | Yesc |
M, male; F, female.
Tg animals were found dead at a very early age (<15 days), and the assessment of their pathology (if any) could not be made.
These mice survived and later developed severe disease, as described in the text.
NA, not applicable.
CD4A/HIVWT founders were bred with C3H mice for several generations, and Tg lines could be established. The CD4A/HIVWT founders and their progeny remained healthy during the 24-month period of observation.
Clinical phenotype and assessment of CD4A/HIVWT Tg mice.
No clinical phenotype was observed in any of the 76 CD4A/HIVWT mice observed for a period of up to 24 months. Macroscopic and histological examinations were also negative for 20 animals sacrificed at 12 to 14 months, except for 1 animal which exhibited enlarged lymphoid tissues and a lung tumor (data not shown). Fluorescence-activated cell sorter (FACS) analysis (CD4/CD8 ratio) was also normal. The expression of the transgene in the thymuses and the spleens of these mice was barely detectable by Northern blot analysis (Fig. 1B). The levels of transgene RNA were estimated to be ∼20- to 60-fold lower than those in CD4C/HIVWT Tg mice. This low level of transgene expression may explain the absence of an obvious phenotype or signs of disease.
Clinical phenotype of CD4C/HIVWT Tg mice.
The time course of disease development was similar in all four CD4C/HIVWT lines. Over the first 2 weeks of life, no clinical abnormalities could be detected in the Tg mice compared to non-Tg controls. In the third week, the Tg mice were readily distinguishable from their non-Tg littermates by their lower body weight (Tg, 7.4 ± 0.8 g [n = 8]; non-Tg, 12.4 ± 2.1 g [n = 4]), slow movements, and hypoactivity. At later stages, the diseased animals had developed weakness, severe tremors, ruffled hair, and feeding problems. Some mice also developed diarrhea (Table 2). The hematocrit measured in a few Tg mice (n = 3) overlapped that seen in control non-Tg mice (n = 3). All affected N1 (n = 25) and two N2 animals from four different founders showed the same phenotype. None of the control non-Tg littermates (n = 127) kept in the same cages as the Tg mice exhibited a similar phenotype or detectable abnormalities, indicating that this phenotype was transgene specific.
TABLE 2.
Incidence of disease in CD4C/HIVWT Tg mice
| Pathology observeda | No. with trait/total no. studiedb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15564
|
17001
|
17018
|
17086
|
Totale
|
||||||
| + (15 ± 1.7)c | −d | + (30.3 ± 5) | − | + (25.2 ± 7) | − | + (21) | − | + | − | |
| Macroscopic | ||||||||||
| Small body size, ruffled fur, and hypoactivity | 3/3f | 0/23 | 7/7 | 0/52 | 13/13 | 0/32 | 2/2 | 0/20 | 25/25 | 0/127 |
| Diarrhea | 0/1 | 0/23 | 3/7 | 0/52 | 2/4 | 0/32 | 1/2 | 0/20 | 6/14 | 0/127 |
| Edema | 0/1 | 2/7 | 0/7 | 2/4 | 0/4 | 1/2 | 0/2 | 5/14 | 0/13 | |
| Low body wt | 1/1 | 7/7 | 0/7 | 4/4 | 0/4 | 2/2 | 0/2 | 14/14 | 0/13 | |
| Small or absent thymus | 1/1 | 7/7 | 0/7 | 4/4 | 0/4 | 2/2 | 0/2 | 14/14 | 0/13 | |
| Small or absent lymph nodes | 1/1 | 7/7 | 0/7 | 3/4 | 0/4 | 1/2 | 0/2 | 12/14 | 0/13 | |
| Small pale spleen | 1/1 | 7/7 | 0/7 | 3/4 | 0/4 | 1/2 | 0/2 | 12/14 | 0/13 | |
| Small and/or mottled kidney | 1/1 | 6/7 | 0/7 | 2/4 | 0/4 | 2/2 | 0/2 | 11/14 | 0/13 | |
| Microscopic | ||||||||||
| Thymus (hypocellularityg) | 1/2 | 0/6 | 4/4 | 0/4 | 1/2 | 0/2 | 6/8 | 0/12 | ||
| Spleen (hypocellularityg) | 4/5 | 1/6 | 2–3/3 | 0/4 | 1/2 | 0/2 | 7/10 | 1/12 | ||
| Lymph node (hypocellularityg) | 1/1 | 0/2 | 4/4 | NAh | 0/1 | 0/1 | 5/6 | 0/3 | ||
| Kidney (tubular interstitial nephritis) | 6/7 | 0/7 | 2–3/3 | 0/3 | 1–2/2 | 0/1 | 9/12 | 0/11 | ||
| Lung (interstitial pneumonitis) | 1/6 | 0/5 | 3/4 | 0/4 | 2/2 | 0/1 | 6/12 | 0/10 | ||
See the text for a detailed description of the disease.
The mice studied include the Tg founder itself as well as Tg (+) and non-Tg (−) offspring derived from each founder (F15564, F17001, F17018, and F17086).
Numbers in parentheses are the mean age in days (± standard deviation) at which N1 animals died of disease or were sacrificed due to severe illness. A total of 3, 7, 13, and 2 mice from founders F15564, F17001, F17018, and F17086, respectively, were assessed.
For all Tg lines studied, non-Tg mice (−) were littermates of Tg mice kept in the same cages and sacrificed on the same day as the Tg mice. Equal number of Tg and non-Tg mice were autopsied.
Total numbers of animals assessed in all lines.
Hypoactivity was not assessed for these three mice.
Disorganized architecture accompanied hypocellularity in most organs assessed.
NA, not assessed.
Pathological assessment of CD4C/HIVWT Tg mice.
At autopsy, macroscopic observation revealed severe wasting, edema, and abnormalities of several organs (lymphoid organs, kidney, and lungs) in most Tg mice compared to control non-Tg littermates (Table 2). However, signs of opportunistic infections (such as abcess, granuloma, or parisitic proliferation) were not observed. Generally, when pathological changes were severe in one organ, other organs of the same mouse were also affected.
(i) Wasting.
Severe wasting (loss of both fat and lean body mass) was observed in all the Tg mice. The muscle mass was atrophic, no fat was visible, and the bones (ribs) were thin and friable compared to the case for normal animals of the same size.
(ii) Lymphoid organs.
All lymphoid organs (thymus, spleen, and lymph nodes) were atrophic; Tg thymuses contained 0.7 × 106 ± 0.7 × 106 cells (n = 5), compared to 137 × 106 ± 72 × 106 cells (n = 7) in control non-Tg littermates, Tg spleens had 4.2 × 106 ± 2.9 × 106 cells (n = 7), compared to 72 × 106 ± 52 × 106 cells (n = 7) in control littermates, and Tg mesenteric lymph nodes contained 1.4 × 106 ± 0.7 × 106 cells (n = 5), compared to 28 × 106 ± 19.5 × 106 cells (n = 5) in control littermates (± equals standard deviation). This atrophy of lymphoid organs was also observed in two animals kept in a specific-pathogen-free facility.
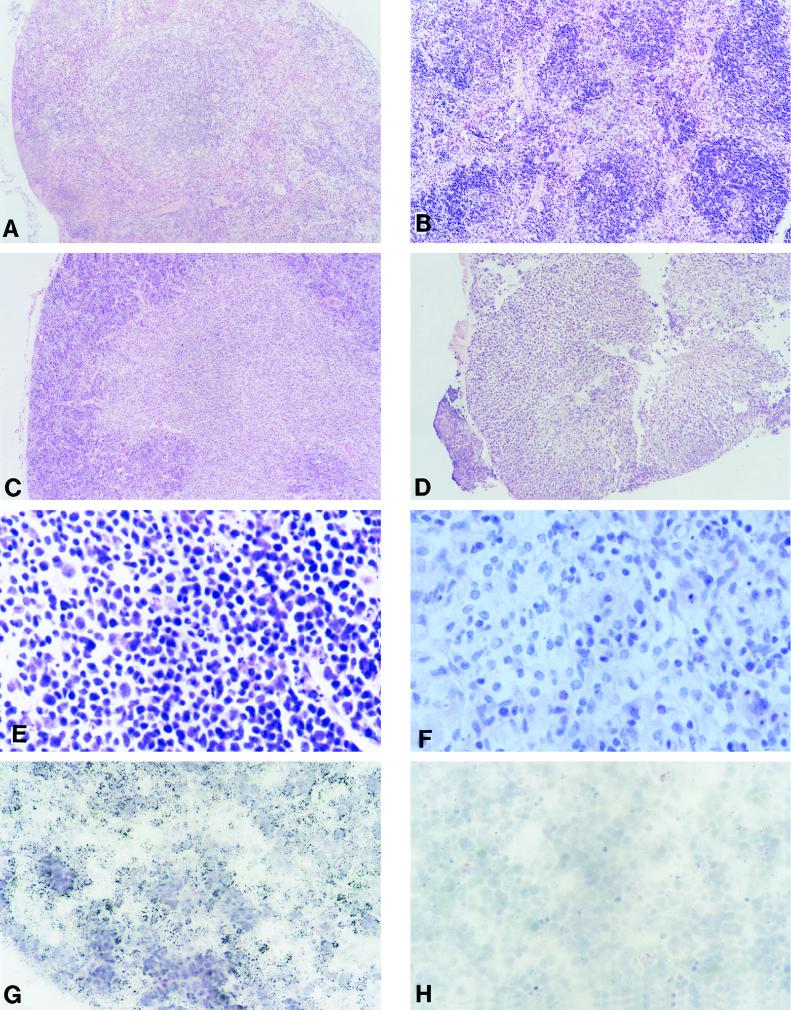
Histological examination revealed abnormalities in the spleens of these Tg mice (Table 2): partial to extensive loss of splenic architecture, frequent hypocellularity, and occasionally fibrosis (Fig. 2B). Changes in the thymus, consisting of loss of architecture and hypocellularity, were noted (Table 2; Fig. 2D). Finally, the mesenteric lymph nodes were hypocellular, and their architecture was disorganized (Fig. 2F). Lymphoid organs from non-Tg littermates exhibited normal histology (Fig. 2A, C, and E), with the exception of 1 of 12 spleens assessed, which showed follicular regression. Thus, similar if not identical histopathologies were observed in the majority of lymphoid organs examined (18 of 24 combined organs) of Tg mice from three independent CD4C/HIVWT founder lines (Table 2). Such extensive destruction of the lymphoid organs is likely to severely affect the function of the immune system.
FIG. 2.
Pathology and transgene expression in lymphoid tissues from CD4C/HIVWT Tg mice. (A to F) Light micrographs of various lymphoid tissues. (A and B) Non-Tg and Tg (F17001) spleens, respectively. (C and D) Non-Tg and Tg (F17018) thymuses, respectively. Note the small size of the Tg thymus. (E and F) Non-Tg and Tg (F17001) lymph nodes, respectively. Note the tissue disorganization and hypocellularity of the Tg organ. (G and H) Thymus from a Tg animal (F17001). ISH was performed with antisense (G) or sense (H) probes. Note that in panel G the vast majority of the cells are ISH positive and are of lymphoid morphology. The lymphoid morphology is best shown in panel H on an adjacent section of the same tissue in the absence of a cell-specific hybridization signal when exposed to the sense probe. Magnifications, ×80 (A to D), ×390 (E and F), and ×320 (G and H). The counterstain was hematoxylin and eosin.
(iii) Kidneys.
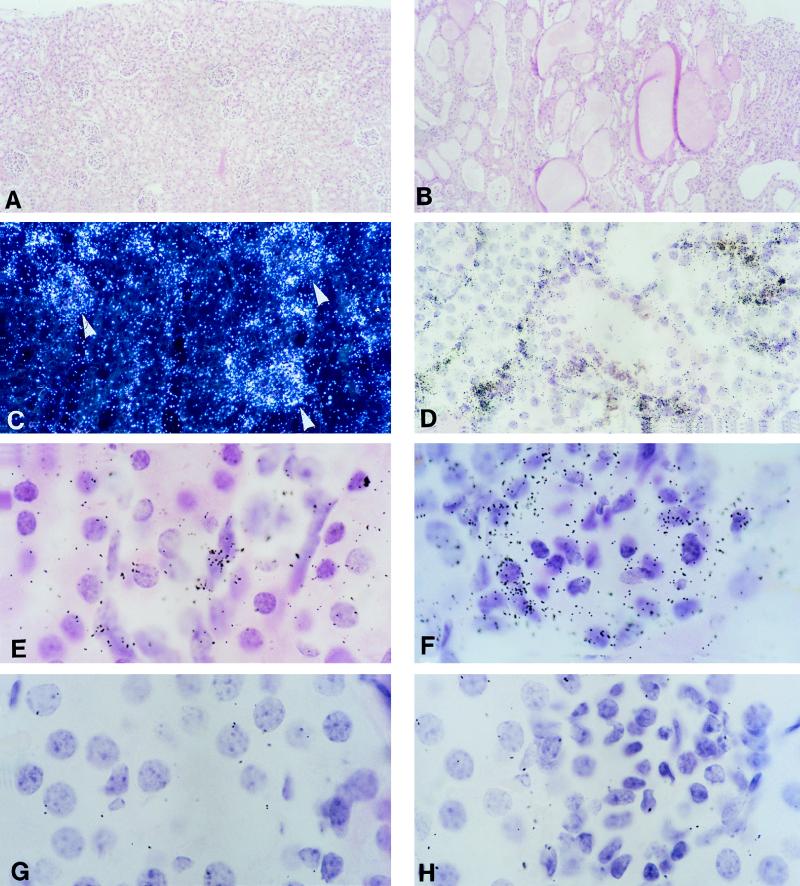
In most Tg mice, kidneys were markedly atrophic and paler than those in the control mice. In more severely affected mice, the kidneys had an irregular cortical surface (Table 2). Five of these mice also exhibited edema, consistent with renal failure. The histological changes in 10 of 14 mice examined were severe, consisting of marked tubular atrophy and interstitial fibrosis associated with interstitial inflammation (Fig. 3B and D). The lumen of the atrophic tubules was markedly dilated, and their epithelium was thinned. There were no significant glomerular changes.
FIG. 3.
Pathology and transgene expression in kidneys of CD4C/HIVWT Tg mice. (A and B) Light micrographs of kidney, in longitudinal section, from normal (A) and Tg (F17018) (B) mice. Note the large number of dilated tubules lined by atrophic epithelial cells. (C) Low-power, dark-field image showing ISH for transgene expression in Tg animal F17001 with extensive pathology. Note expression of the transgene in areas corresponding to glomeruli (arrowheads) as well as ductal regions. (D) Low-power, bright-field image of ISH-positive interstitial infiltrating cells (F17001) in kidney. (E and F) high-power images of ISH-positive interstitial infiltrating cells (E) and cells in glomeruli (F). (G and H) Regions equivalent to those in panels E and F, respectively, but from a non-Tg animal hybridized with an antisense probe. Magnifications, ×80 (A and B), ×200 (C and D), and ×830 (E to H). The counterstain was hematoxylin and eosin.
(iv) Lungs.
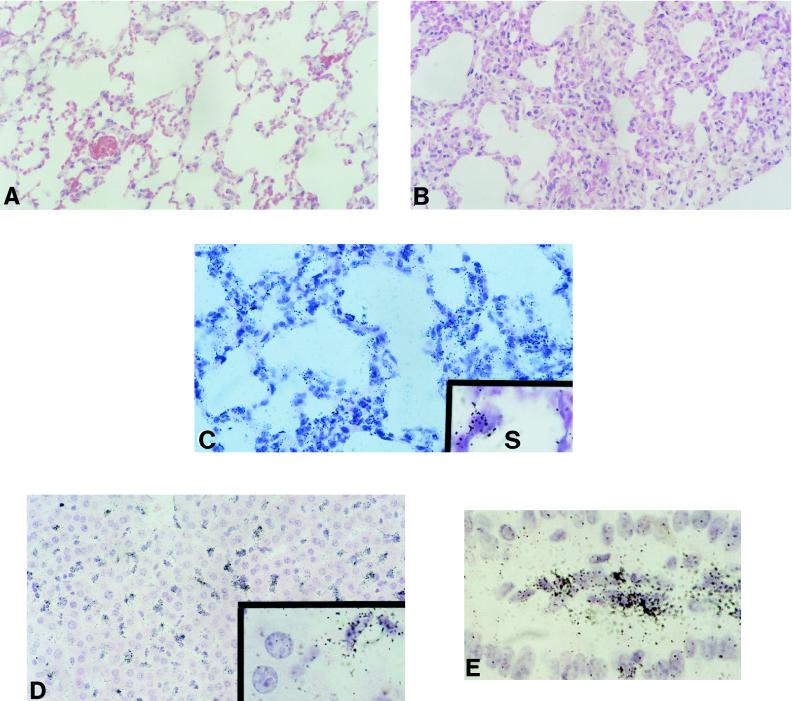
The lungs of some Tg mice were firm compared to those of non-Tg mice. Histological examination showed a marked thickening of alveolar walls by infiltrating mononuclear cells in some Tg animals (Fig. 4B). Such severe interstitial infiltration was not observed in non-Tg littermates (Fig. 4A). No evidence of airspace disease was noticed in Tg mice. Two Tg mice with extensive lung disease were assessed for Pneumocystis carinii infection by Grocott’s stain of lung sections and were found to be negative.
FIG. 4.
Pathology and transgene expression in livers, lungs, and intestines of CD4C/HIVWT Tg mice. (A and B) Non-Tg and Tg (F17018) lungs, respectively. Note the extensive interstitial pneumonitis in the Tg lung. (C to E) Tg expression is seen in interstitial infiltrating cells and alveolar macrophages in the lung (F17001) (C) (S, alveolar space), in Kupffer cells (liver macrophages) (F17001) (D), and in cells in the lamina propria of the small intestine (F17018) (E). Magnifications, ×190 (A to D) and ×400 (insets to panels C and D panel E). The counterstain was hematoxylin and eosin.
FACS analysis of lymphoid organs from CD4C/HIVWT Tg mice.
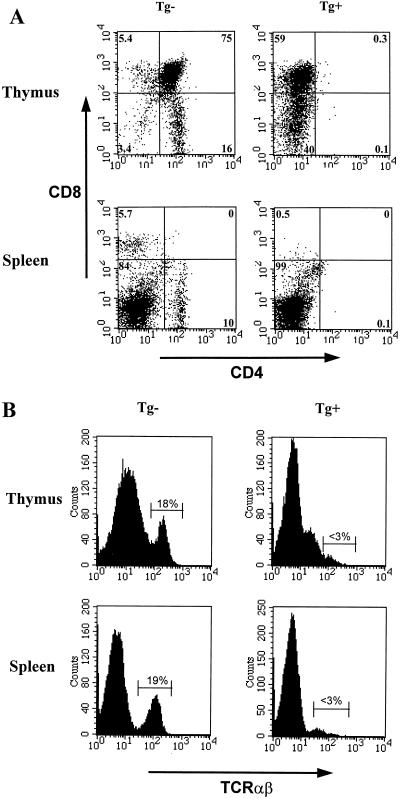
FACS analysis performed on thymocytes of six Tg mice from founder F17001 with antibodies against CD4, CD8, and TCR showed dramatic changes: the CD4+ CD8+ and CD4+ CD8− cells were almost absent in all Tg mice, while CD4− CD8+ and CD4− CD8− T cells constituted the largest populations (Fig. 5). Interestingly, only a minority of thymocytes (<0.1%) were found to express TCR, an indication that the large population of remaining CD4− CD8+ thymocytes did not have the phenotype of mature T cells. They most likely represent double-positive CD4+ CD8+ immature T cells in which the CD4 molecule may well have been downregulated by HIV-1 gene products, possibly Nef (19). A similar analysis carried out on spleen and lymph node cells of Tg mice showed a very low percentage of mature CD4+ (0 to 6%) and CD8+ (1 to 3.8%) T cells and a reduction in the number of TCR-expressing cells (<3%, versus a control value of ∼18%). Other N1 offspring (n = 2) from other lines (F17018 and F17086) showed a similar but variable depletion of CD4+ T cells in peripheral lymphoid organs.
FIG. 5.
Cytofluorometric analysis of thymocytes and splenocytes of a young CD4C/HIVWT Tg mouse. Thymocytes and splenocytes from a Tg mouse (F17001) and a non-Tg control mouse were analyzed by two-color flow cytometry for the expression of CD4 and CD8 (A) and TCRαβ (B). The percentages of cells found in each quadrant are indicated; 104 cells were analyzed.
Analysis of B cells with the B220 marker showed the presence of B cells and indicated that they represented on average a higher proportion of the cells of the spleen (54%) (n = 7) and of the lymph nodes (58%) (n = 4) in these Tg mice than in normal mice (40 and 13%, respectively), as expected for a T-cell-depleted lymphoid organ. Similarly, cells of the monocyte/macrophage/dendritic lineage, detected with anti-Mac-1, were found to constitute a higher proportion of the remaining spleen cells in Tg mice (n = 3) than in control mice.
B cells in CD4C/HIVWT Tg mice (n = 7) did not appear to function normally, as the levels of serum Igs (1.9 ± 0.9 mg/ml) were on average only 10% of those observed in non-Tg control animals (n = 6) (24.1 ± 15.5 mg/ml).
Expression of the transgene in CD4C/HIVWT Tg mice.
Northern blot analysis revealed the three main transcripts of HIV-1 (8.8-kb full length, 4.3-kb env specific, and 2.0-kb multiply spliced) at high levels in the thymus and at moderate levels in the spleen and lymph nodes (Fig. 1C). These organs are known to support transcription of surrogate genes from this CD4C promoter (23). A weak but detectable signal was also observed in other organs, such as kidneys, lungs, intestines, and livers (Fig. 1C). This hybridization signal could originate from circulating T lymphoid cells and/or resident macrophages, which are known to support the expression from this CD4C promoter (23). No expression was detected in testis, skin, muscle, heart, and brain (Fig. 1C).
Transgene expression was further evaluated by ISH with 35S-labeled HIV-1-specific antisense and control sense riboprobes. On a per-cell basis, moderate to strong expression of the transgene was observed in each of the four Tg lines in various organs assessed. In lymphoid tissues, expression was seen in diseased organs but was rarely observed in normal-appearing tissues, indicating that expression of HIV-1 gene products was correlated with development of pathological lesions. In the spleen, the numbers of transgene-expressing cells varied from several tens to hundreds per histological section. Where sufficient splenic architecture was conserved, transgene expression was found to be concentrated in the red pulp areas (data not shown). In the lymph nodes, again the disorganization of tissue architecture made it impossible to determine the localization of the expressing cells (data not shown). In the thymuses whose architecture was not too distorted, transgene expression was found to be concentrated in the cortical region, while fewer medullary cells expressed the transgene (Fig. 2G), consistent with an expression in CD4+ CD8+ (cortical) and CD4+ CD8− (medullary) T cells. Interestingly, in some diseased spleens, thymuses, and lymph nodes, no transgene expression could be detected, suggesting either that the lesions were induced indirectly or, more likely, that transgene-expressing cells were already depleted in these mice.
Transgene expression was also observed in nonlymphoid tissues. In five diseased kidneys, cells within the glomeruli (Fig. 3C and F) and interstitial, most likely infiltrating mononuclear leukocytes (Fig. 3C to E) were found to express the transgene. It was not possible to determine whether expressing cells within glomeruli were normal components of the glomerulus or were infiltrating cells, although cells around the periphery of glomeruli, where epithelial cells are located, often had elevated levels of transgene expression. Transgene expression was undetectable in two other kidneys with minimal pathological changes and in one exhibiting normal morphology. These results strongly suggested that the kidney lesions in these mice were related to the number of cells expressing the transgene.
Transgene expression was also observed in mononuclear cells in several other organs, such as the lamina propria of the intestine (Fig. 4E), the livers, and the lungs. Liver Kupffer cells (Fig. 4D) and lung interstitial infiltrating cells and alveolar macrophages (Fig. 4C) were positive for transgene expression. In addition, peritoneal macrophages from a mouse of line F17001 were found to express the transgene, as expected (data not shown). Other cell types not expected to express the transgene were negative by ISH. Thus, the epithelial cells, smooth muscle and connective tissue cells of the gastrointestinal tract, tubular epithelial cells of the kidney, pneumocytes, hepatocytes, myocytes of the heart and skeletal muscle, seminiferous tubules, and spermatocytes, as well as vasculature in these organs, were all negative for transgene expression. Together, these results are consistent with the specificity of the CD4C promoter for CD4+ T lymphocytes and for cells of the macrophage lineage.
Studies of CD4C/HIVWT chimeric mice.
Because the Tg founders which were mosaic survived longer than their nonmosaic Tg offspring, we decided to generate chimeric CD4C/HIVWT Tg mice. Possibly, these animals may remain viable for a longer period of time and thus develop pathologies resulting from a more chronic exposure to HIV-1 gene products. Cells from two ES cell clones harboring the transfected CD4C/HIVWT transgene (J1-1838-2 and J1-1838-5) were used to generate chimeric mice. Twelve and 15 of 35 and 21 born mice, respectively, showed coat color chimerism (Table 3). Southern blot analysis with an HIV-1 probe also demonstrated variable contributions of ES cells to multiple tissues (data not shown). Five of 15 chimeric mice derived from clone J1-1838-5 exhibited disease early in life and were sacrificed at 18 to 32 days after birth. This disease was similar to that described above for CD4C/HIVWT Tg mice. The phenotype included low body weight (6 to 8 g [n = 4], compared to 13 g for age-matched nonchimeric controls), wasting, slow movement, hypoactivity, and early death. This phenotype was not observed in nonchimeric mice, suggesting that it was transgene specific.
TABLE 3.
Characteristics of CD4C/HIVWT chimeric mice
| ES clone inoculated | No. with the following trait/total no.
|
||||
|---|---|---|---|---|---|
| Coat color chimerism | Clinical disease
|
Pathological lesions in chimeric mice | Transgene expression | ||
| Chimeric mice | Nonchimeric mice | ||||
| J1-1838-2 | 12/35 | 1/12 | 0/23 | 0/8 | 1/8 |
| J1-1838-5 | 15/21 | 5/15 | 0/6 | 4/13a | 5/13b |
The four chimeric mice with pathological lesions had no. 20882, 20883, 20886, and 20887. See the text for a description.
The mice which showed the highest number of in situ positive cells (no. 20886 and 20887) also demonstrated the highest degree of histopathology.
Transgene expression was assessed by ISH for several tissues (kidney, spleen, lymph node, and thymus) of chimeric mice. Five chimeric mice derived from clone J1-1838-5 harbored HIV-1-expressing cells in several organs, and the level of expression per cell was relatively high (data not shown), being comparable to that observed for Tg animals. Generally, higher numbers of ISH-positive cells correlated with histopathology. In three animals, transgene expression was observed in the kidneys, in cells of several glomeruli as well as in interstitial mononuclear cells. Two of these mice showed clear evidence of kidney disease. The pathology observed was indistinguishable from that observed in the CD4C/HIVWT Tg animals. Here, greater pathology was observed in the animal exhibiting the highest number of ISH-positive cells. With the exception of one thymus, which exhibited a loss of corticomedullary junction, lymphoid tissues from three chimeric animals with demonstrated lower numbers of transgene-expressing cells had no detectable pathological lesions. Among the eight chimeric mice derived from the other clone (J1-1838-2), none showed any evidence of pathology in their tissues, and only one animal showed minimal evidence of transgene expression in lymphoid tissues. However, reverse transcription-PCR performed on peritoneal macrophages isolated from three chimeric animals indicated that there was expression in these cells (data not shown), and this was confirmed by ISH for one animal. These results suggest that the absence of a phenotype in these chimeric mice may be related to the low levels of transgene expression.
Together, these results extend and confirm our data on the mosaic founder mice. They show that the presence of high numbers of cells expressing moderate to high levels of this transgene correlates with pathology.
Overexpression of RANTES in CD4C/HIVWT Tg mice.
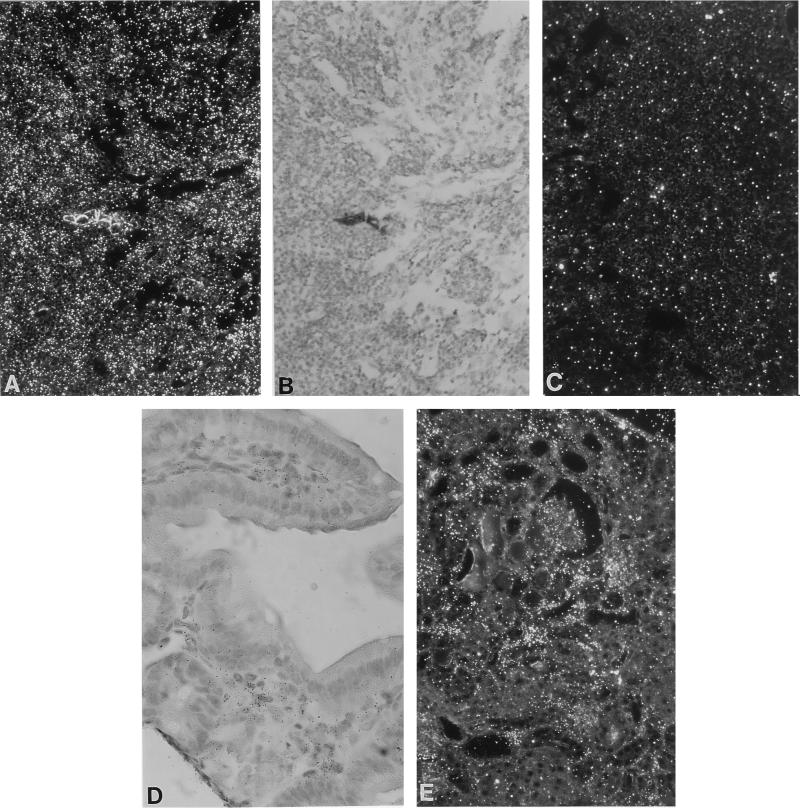
HIV-1 infection has been found to upregulate β-chemokine expression in monocytes in vitro and in vivo (46). To determine whether the expression of β-chemokines was affected in CD4C/HIVWT Tg mice, we measured RANTES expression in several tissues of Tg mice by using ISH. The pattern of RANTES expression mimicked that of transgene expression. In four of seven Tg animals examined, moderate to high numbers of RANTES-expressing cells were detected. Expression was detected in lymphoid tissues, with the highest levels seen in lymph nodes (Fig. 6A to C). Expression was also detected in cells of the lamina propria of the gastrointestinal tract (Fig. 6D) and in interstitial infiltrating and glomerular cells of the kidney (Fig. 6E), as well as in cells infiltrating the lung and in Kupffer cells (data not shown). In control non-Tg littermates, only very low levels of RANTES expression (occasional expressing cells) were detected in lymphoid tissues. These results suggest that RANTES may play some role in the HIV-1-induced changes seen in these mice.
FIG. 6.
Elevated levels of RANTES expression in CD4C/HIVWT Tg mice. Assessment was done by ISH. (A to C) Lymph node (F17018). Dark-field (A) and bright-field (B) images of the same field show tissue hybridized with the antisense probe. (C) Hybridization with the control sense probe. Note the hypocellularity of the node in panel B. (D and E) ISH-positive cells of the lamina propria of the intestine (F17001) (D) and in interstitial cells in the kidney (F17001) (E). Magnifications, ×180 (A to C and E) and ×380 (D). The counterstain was hematoxylin and eosin.
DISCUSSION
Expression of the transgene in target cells for HIV-1 infection.
Our results show that Tg mice expressing the complete HIV-1 coding sequences under the regulation of the CD4C promoter develop a severe AIDS-like disease followed by early death. We have previously reported that this promoter, derived from the human and mouse CD4 genes, allows expression of surrogate genes in CD4+ CD8+ and CD4+ thymocytes, in peripheral CD4+ T cells, and in macrophages (23). Expression of the transgene in CD4C/HIVWT mice was found to be apparently in the same cells, namely, T cells and macrophages. In addition, we have recently documented by further examination of CD4C/CD4 Tg mice that this promoter is active in spleen dendritic cells (41a). These represent the specific subsets of cells which are normally targeted for infection by HIV-1 in humans (10, 16, 40, 47, 48). Expression of HIV-1 in these specific cell populations of Tg mice appears to be important to elicit this AIDS-like disease, since our other mice similarly expressing the whole HIV-1 genome at high levels in different cell populations and through different promoters did not develop any apparent abnormal phenotype of the immune system (28, 55). Although both the T cells and the dendritic/macrophage cell lineages express the HIV-1 genome in the CD4C/HIVWT Tg mice, it is not clear whether expression in each of these lineages is required for induction of each of the phenotypes observed. In fact, expression of HIV-1 in dendritic/macrophage cells may be sufficient to elicit several of the phenotypes seen in these mice. On the other hand, expression of a specific factor by HIV-1-expressing CD4+ T cells may be an absolute requirement for development of some or all phenotypes. Additional work is needed to approach this question.
To date, only mice from one other Tg line expressing the whole HIV-1 genome under the regulation of the HIV-1 LTR have been reported to develop an AIDS-like disease characterized by early death and pathological changes in several organs (35). Some of these changes (early death, lymphadenopathy, thymus hypoplasia, and lung lesions) were similar to those seen in CD4C/HIVWT Tg mice, while others (epidermal hyperplasia and absence of detectable HIV-1 RNA in spleen by ISH) were different or (kidney disease) absent. However, since only one line (no. 13) of Tg mice developed this disease, an insertional mutation by the transgene contributing to the phenotype could not be ruled out.
Levels of HIV-1 expression and development of diseases.
In addition to the specific cell populations expressing the transgene, another parameter which may have influenced the development of disease in CD4C/HIVWT Tg mice is the level of HIV-1 expression. In these Tg mice, it appears that a relatively high level of viral RNA expression was required for the development of the disease. Four of five Tg founder mice which were mosaic for the transgene, and presumably harbored lower numbers of HIV-1-expressing cells, failed to develop disease or developed the disease much later than their nonmosaic N1 offspring. Similarly, only chimeric mice having the highest number of HIV-1-expressing cells developed histopathological lesions. Finally, the CD4A/HIVWT Tg mice, which expressed HIV-1 at much lower levels, did not develop disease even after a long period of observation. Therefore, as in human AIDS (25), the viral RNA load appears to be an important determinant of disease in CD4C/HIVWT Tg mice. Since no reinfection cycle occurs in these mice, this viral RNA load is likely to mimic a steady-state load of virus expression in humans, although more work will be needed to determine whether these two parameters reflect the same pathogenesis.
AIDS-like pathologies in Tg mice.
Several pathological changes observed in these CD4C/HIVWT Tg mice are similar to those found in individuals with AIDS, and especially in pediatric AIDS (5), consistent with the fact that the transgene is expressed early in life.
Early death, which constitutes the most dramatic phenotype in these mice, is also seen in a high percentage of infants (49) and in some adults (17, 43) with AIDS. Except in some mice with a severe lung or kidney disease, the exact cause of death remains obscure. Death is unlikely to result from only bacterial or viral infections, since no evidence of bacteremia or extensive viral infection was observed in these mice. Additionally, early death was also observed in two animals kept in a specific-pathogen-free facility. Death is also unlikely to be exclusively the result of the loss of T cells, since nude mice kept in the same rooms survived much longer than these CD4C/HIVWT Tg mice. The kidney or lung diseases also cannot account for all of these early deaths, since severe and life-threatening kidney or lung lesions were not present in all mice which died early. This early death may be related to the severe wasting observed in the majority of these mice. In HIV-1-infected humans, wasting has been found to be a strong predictor of survival (54).
Wasting indeed represents the second-most-striking phenotype in these mice. It has been observed in a relatively high percentage of AIDS patients (9, 22), and its pathogenesis is not well understood but it is thought to be multifactorial (22). A similar cachexia syndrome has been described for other Tg mice expressing the whole or the 3′ half of the HIV-1 genome (14, 44). However, in one of those studies, it was observed only in mice homozygous for the transgene (44) and may have resulted from an insertional mutation by the transgene. Therefore, the wasting seen in the heterozygous CD4C/HIVWT Tg mice represents a very good model of HIV-1-associated wasting in humans.
The third striking phenotype observed in these Tg mice is the small size of the lymphoid organs, with the loss of their normal architecture accompanied by a severe depletion of thymocytes and peripheral T cells. Severe premature involution of the thymus with depletion of both lymphoid and epithelial thymic cell populations, and specifically of Hassall’s corpuscules, is a feature of both pediatric and adult AIDS in humans (20, 29, 50). In the thymuses of the Tg mice, CD4+ CD8+ immature and CD4+ CD8− mature T cells appear to be initially preferentially lost. Human CD4+ CD8+ thymic T cells have been reported to be infectable with HIV-1 in vitro (11, 47). The loss of T cells in peripheral organs of these mice is likely to reflect the virtual absence of precursor T cells in the thymus. Recent evidence indicates that a reduction in early life of both CD4+ and CD8+ T cells in HIV-1-infected children (a DiGeorge-like immunophenotype) is associated with a rapid progression to AIDS (34). The loss of the architecture of the spleen and lymph nodes associated with fibrosis and hypocellularity also mimics the case for the lymphoid organs of adults and, especially, children with advanced AIDS (29). Thymic atrophy and the loss of T cells in peripheral lymphoid organs have previously been reported to occur in Tg mice expressing only the HIV-1 nef gene under the regulation of T-cell-specific promoters (4, 36, 52), suggesting that the thymic phenotype in the CD4C/HIVWT Tg mice may be caused primarily by the expression of the nef gene in T cells. The construction of additional Tg mice expressing mutant HIV-1 genomes is under way to test this hypothesis.
The fourth-most-important pathology seen in these Tg mice affects the kidneys. Kidney disease in AIDS patients is relatively frequent (41, 51), particularly in children (53). A broad spectrum of renal lesions have been described, including focal and segmental glomerulosclerosis, mesangial hyperplasia, and tubulointerstitial nephritis, but the pathogenesis of these changes is not well understood (26, 41). In the CD4C/HIVWT Tg mice, the predominant features of the kidney disease (tubulointerstitial nephritis) mimic what has been observed in humans. Expression of the HIV-1 transgene in glomerular and tubular epithelial kidney cells was not expected to occur with the CD4C promoter used here. In fact, most of the cells expressing the transgene in the kidney appear to be infiltrating interstitial mononuclear cells, some with a lymphocyte cell morphology. Cells of unknown identity were also found to express the transgene in glomeruli. This is consistent with the suggestion that glomerular cells may express CD4 in humans (30). Cells of the macrophage lineage which express the transgene are also likely to be present in the kidneys. A role for macrophages in HIV-1-associated kidney diseases has previously been postulated (3). In addition, the expression of HIV-1 gene products may directly affect epithelial kidney cells. A severe kidney disease has previously been found to develop in Tg mice expressing the 3′ half of the HIV-1 genome under the regulation of the HIV-1 LTR (13, 33). The kidneys were larger than those of non-Tg controls, and most of their glomeruli were severely sclerotic. In contrast, in CD4C/HIVWT Tg mice, the kidneys were smaller than normal and tubulointerstitial nephritis was the histological change most frequently observed. The identity of the cell population expressing the 3′-half HIV-1 transgene in these mice was not reported, and it is unclear whether the pathogeneses of these two distinct kidney diseases in these mice and in the CD4C/HIVWT Tg mice are the same or different.
The lung lesions constitute the fifth main phenotype detected in these Tg mice. This phenotype does not seem to be caused by P. carinii, which was not found in these tissues. It resembles the pulmonary lymphoid hyperplasia/lymphoid interstitial pneumonitis observed in children, and rarely in adults, with AIDS (5, 29, 39), whose origin is unknown but is thought to be immune mediated (7).
Finally, the overexpression of RANTES in various tissues of these Tg mice represents a sixth phenotype which may be relevant to the human disease. Although MIP-1α and MIP-1β have been the main β-chemokines found to be elevated in HIV-1-infected monocytes (46), the levels of RANTES and other β-chemokines have been found to be elevated in lymph nodes of macaques infected with the pathogenic SIVmac239 but not in animals infected with its nef-deleted derivative (58) and in brains of SIVmac251-inoculated macaques exhibiting encephalitis (45). Moreover, these β-chemokines appear to represent the major soluble factors responsible for HIV-1-suppressive effects secreted by CD4+ and CD8+ T cells derived from HIV-1-infected individuals (8, 31). Overexpression of RANTES has also been reported in inflammatory diseases (37). In addition, high levels of RANTES would be expected to compete with the macrophage-tropic HIV-1 virions for the CCR-5 receptor and thus exert a strong selection pressure for the emergence of T-cell variants of HIV-1 utilizing another coreceptor, such as CXCR4/fusin (18). Our results suggest that this may be amenable to experimentation in vivo.
In summary, our results show that the expression of HIV-1 gene products in specific subsets of cells which are normally targeted for HIV-1 infection in humans can be particularly pathogenic in mice. These mice developed a systemic disease characterized by several pathological changes strikingly similar to those observed in human AIDS. Such a constellation of pathologies has not been reported for other Tg mice expressing HIV-1 through different promoters, except in a single line derived by Leonard et al. (35). Therefore, the CD4C/HIVWT Tg mice described here represent the best model of Tg mice yet available for human AIDS.
ACKNOWLEDGMENTS
Zaher Hanna and Denis G. Kay contributed equally to this work.
This work was supported by grants from the Medical Research Council of Canada to P.J. and from the National Health Research and Development Program to Z.H. D.G.K. was supported by a career development award from the Fonds de la Recherche en Santé du Québec.
We gratefully acknowledge Nathalie Gauthier, Karina Lamarre, Ginette Masse, Benoît Laganière, Michel Robillard, and Michel Ste-Marie for excellent technical assistance. We also thank Sonia Colombi for some of the FACS analysis on CD4A/HIVWT Tg lines. Finally, we thank Rita Gingras for typing the manuscript.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettelheim F A, Zeng F F, Bia Y, Tumminia S J, Russell P. Lens hydration in transgenic mice containing HIV-1 protease linked to the lens alpha A-crystallin promoter. Arch Biochem Biophys. 1995;324:223–227. doi: 10.1006/abbi.1995.0034. [DOI] [PubMed] [Google Scholar]
- 3.Bodi I, Abraham A, Kimmel P L. Macrophages in human immunodeficiency virus-associated kidney diseases. Am J Kidney Dis. 1994;24:762–767. doi: 10.1016/s0272-6386(12)80669-x. [DOI] [PubMed] [Google Scholar]
- 4.Brady H J, Pennington D J, Miles C G, Dzierzak E A. CD4 cell surface downregulation in HIV-1 Nef transgenic mice is a consequence of intracellular sequestration. EMBO J. 1993;12:4923–4932. doi: 10.1002/j.1460-2075.1993.tb06186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvelli T A, Rubinstein A. Pediatric HIV infection: a review. Immunodefic Rev. 1990;2:83–127. [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Clarke J R, Robinson D S, Coker R J, Miller R F, Mitchell D M. Role of the human immunodeficiency virus within the lung. Thorax. 1995;50:567–576. doi: 10.1136/thx.50.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 9.Coodley G O, Loveless M O, Merrill T M. The HIV wasting syndrome: a review. J Acquired Immune Defic Syndr. 1994;7:681–694. [PubMed] [Google Scholar]
- 10.Crowe S M, Kornbluth R S. Overview of HIV interactions with macrophages and dendritic cells: the other infection in AIDS. J Leukocyte Biol. 1994;56:215–217. doi: 10.1002/jlb.56.3.215. [DOI] [PubMed] [Google Scholar]
- 11.De Rossi A, Calabro M L, Panozzo M, Bernardi D, Caruso B, Tridente G, Chieco-Bianchi L. In vitro studies of HIV-1 infection in thymic lymphocytes: a putative role of the thymus in AIDS pathogenesis. AIDS Res Hum Retroviruses. 1990;6:287–298. doi: 10.1089/aid.1990.6.287. [DOI] [PubMed] [Google Scholar]
- 12.Dickie P. HIV type 1 Nef perturbs eye lens development in transgenic mice. AIDS Res Hum Retroviruses. 1996;12:177–189. doi: 10.1089/aid.1996.12.177. [DOI] [PubMed] [Google Scholar]
- 13.Dickie P, Felser J, Eckhaus M, Bryant J, Silver J, Marinos N, Notkins A L. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185:109–119. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- 14.Dickie P, Gazzinelli R, Chang L J. Models of HIV type 1 proviral gene expression in wild-type HIV and MLV/HIV transgenic mice. AIDS Res Hum Retroviruses. 1996;12:1103–1116. doi: 10.1089/aid.1996.12.1103. [DOI] [PubMed] [Google Scholar]
- 15.Dickie P, Ramsdell F, Notkins A L, Venkatesan S. Spontaneous and inducible epidermal hyperplasia in transgenic mice expressing HIV-1 Nef. Virology. 1993;197:431–438. doi: 10.1006/viro.1993.1607. [DOI] [PubMed] [Google Scholar]
- 16.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 17.Enger C, Graham N, Peng Y, Chmiel J S, Kingsley L A, Detels R, Munoz A. Survival from early, intermediate, and late stages of HIV infection. JAMA. 1996;275:1329–1334. [PubMed] [Google Scholar]
- 18.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 19.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 20.Gaulton G N, Scobie J V, Rosenzweig M. HIV-1 and the thymus. AIDS. 1997;11:403–414. doi: 10.1097/00002030-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Goudreau G, Carpenter S, Beaulieu N, Jolicoeur P. Vacuolar myelopathy in transgenic mice expressing human immunodefiency virus type 1 proteins under the regulation of the myelin basic protein gene promoter. Nat Med. 1996;2:655–661. doi: 10.1038/nm0696-655. [DOI] [PubMed] [Google Scholar]
- 22.Grunfeld C, Feingold K R. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- 23.Hanna Z, Simard C, Jolicoeur P. Specific expression of the human CD4 gene in mature CD4+ CD8− and immature CD4+ CD8+ T cells and in macrophages of transgenic mice. Mol Cell Biol. 1994;14:1084–1094. doi: 10.1128/mcb.14.2.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 25.Ho D D. Viral counts count in HIV infection. Science. 1996;272:1124–1125. doi: 10.1126/science.272.5265.1124. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys M H. Human immunodeficiency virus-associated glomerulosclerosis. Kidney Int. 1995;48:311–320. doi: 10.1038/ki.1995.299. [DOI] [PubMed] [Google Scholar]
- 27.Iwakura Y, Shioda T, Tosu M, Yoshida E, Hayashi M, Nagata T, Shibuta H. The induction of cataracts by HIV-1 in transgenic mice. AIDS. 1992;6:1069–1075. doi: 10.1097/00002030-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Jolicoeur P, Laperrière A, Beaulieu N. Efficient production of human immunodeficiency virus proteins in transgenic mice. J Virol. 1992;66:3904–3908. doi: 10.1128/jvi.66.6.3904-3908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi V. Pathology of acquired immunodeficiency syndrome in children. Pediatr Hematol Oncol. 1994;11:351–355. doi: 10.3109/08880019409140534. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson-Parra A, Dimeny E, Fellstrom B, Klareskog L. HIV receptors (CD4 antigen) in normal human glomerular cells. N Engl J Med. 1989;320:741. doi: 10.1056/NEJM198903163201119. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 31.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous beta-chemokines and the viral inductive effects of other endogenous cytokines. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klotman P E, Rappaport J, Ray P, Kopp J B, Franks R, Bruggeman L A, Notkins A L. Transgenic models of HIV-1. AIDS. 1995;9:313–324. [PubMed] [Google Scholar]
- 33.Kopp J B, Klotman M E, Adler S H, Bruggeman L A, Dickie P, Marinos N J, Eckhaus M, Bryant J L, Notkins A L, Klotman P E. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kourtis A P, Ibegbu C, Nahmias A J, Lee F K, Clark W S, Sawyer M K, Nesheim S. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 35.Leonard J M, Abramczuk J W, Pezen D S, Rutledge R, Belcher J H, Hakim F S, Lamperth G L, Travis W, Fredrickson T, et al. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988;242:1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- 36.Lindemann D, Wilhelm R, Renard P, Althage A, Zinkernagel R, Mous J. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J Exp Med. 1994;179:797–807. doi: 10.1084/jem.179.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H E, Hess M W, Laissue J A, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201–206. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.McLean I W, Nakane P K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 39.McSherry G D. Human immunodeficiency-virus-related pulmonary infections in children. Semin Respir Infect. 1996;11:173–183. [PubMed] [Google Scholar]
- 40.Pantaleo G, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 41.Rappaport J, Kopp J B, Klotman P E. Host virus interactions and the molecular regulation of HIV-1: role in the pathogenesis of HIV-associated nephropathy. Kidney Int. 1994;46:16–27. doi: 10.1038/ki.1994.240. [DOI] [PubMed] [Google Scholar]
- 41a.Rebai, N., Z. Hanna, and P. Jolicoeur. Unpublished data.
- 42.Robertson E J. Teratocarcinomas and embryonic stem cells: a practical approach. Oxford, United Kingdom: IRL Press Ltd.; 1987. [Google Scholar]
- 43.Rothenberg R, Woelfel M, Stoneburner R, Milberg J, Parker R, Truman B. Survival with the acquired immunodeficiency syndrome. Experience with 5833 cases in New York City. N Engl J Med. 1987;317:1297–1302. doi: 10.1056/NEJM198711193172101. [DOI] [PubMed] [Google Scholar]
- 44.Santoro T J, Bryant J L, Pellicoro J, Klotman M E, Kopp J B, Bruggeman L A, Franks R R, Notkins A L, Klotman P E. Growth failure and AIDS-like cachexia syndrome in HIV-1 transgenic mice. Virology. 1994;201:147–151. doi: 10.1006/viro.1994.1276. [DOI] [PubMed] [Google Scholar]
- 45.Sasseville V G, Smith M M, Mackay C R, Pauley D R, Mansfield K G, Ringler D J, Lackner A A. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnittman S M, Denning S M, Greenhouse J J, Justement J S, Baseler M K, Haynes J B F, Fauci A S. Evidence for susceptibility of intrathymic T-cell precursors and their progeny carrying T-cell antigen receptor phenotypes TCR alpha beta + and TCR gamma delta + to human immunodeficiency virus infection: a mechanism for CD4+ (T4) lymphocyte depletion. Proc Natl Acad Sci USA. 1990;87:7727–7731. doi: 10.1073/pnas.87.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnittman S M, Psallidopoulos M C, Lane H C, Thompson L, Baseler M M, Fox F C H, Salzman N P, Fauci A S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- 49.Scott G B, Hutto C, Makuch R W, Mastrucci M T, O’Connor T, Mitchell C D, Trapido E J, Parks W P. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med. 1989;321:1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 50.Seemayer T A, Laroche A C, Russo P, Malebranche R, Arnoux E, Guerin J M, Pierre G, Dupuy J M, Gartner J G, Lapp W S, et al. Precocious thymic involution manifest by epithelial injury in the acquired immune deficiency syndrome. Hum Pathol. 1984;15:469–474. doi: 10.1016/s0046-8177(84)80082-9. [DOI] [PubMed] [Google Scholar]
- 51.Seney F D, Jr, Burns D K, Silva F G. Acquired immunodeficiency syndrome and the kidney. Am J Kidney Dis. 1990;16:1–13. doi: 10.1016/s0272-6386(12)80779-7. [DOI] [PubMed] [Google Scholar]
- 52.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauss J, Abitbol C, Zilleruelo G, Scott G, Paredes A, Malaga S M, Mitchell B C, Parks W, Pardo V. Renal disease in children with the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:625–630. doi: 10.1056/NEJM198909073211001. [DOI] [PubMed] [Google Scholar]
- 54.Suttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Muller M J. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:239–246. doi: 10.1097/00042560-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 55.Thomas F P, Chalk C, Lalonde R, Robitaille Y, Jolicoeur P. Expression of human immunodeficiency virus type 1 in the nervous system of transgenic mice leads to neurological disease. J Virol. 1994;68:7099–7107. doi: 10.1128/jvi.68.11.7099-7107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tumminia S J, Jonak G J, Focht R J, Cheng Y S, Russell P. Cataractogenesis in transgenic mice containing the HIV-1 protease linked to the lens alpha A-crystallin promoter. J Biol Chem. 1996;271:425–431. doi: 10.1074/jbc.271.1.425. [DOI] [PubMed] [Google Scholar]
- 57.Vogel J, Hinrichs S H, Reynolds R K, Luciw P A, Jay G. The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature. 1988;335:606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- 58.Zou W, Lackner A A, Simon M, Durand-Gasselin I, Galanaud P, Desrosiers R C, Emilie D. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]