Abstract
Purpose
To investigate the various strategies used for the treatment of premature ejaculation (PE); these encompassed behavioral, drug and surgical interventions.
Materials and Methods
We retrieved data from electronic literature searches of PubMed and Cochrane library using the MeSH (Medical Subject Headings terms) and text keywords from the earliest available date of indexing through September 2022. The subject headings and text keywords included those related to the population (male patients with PE), interventions & comparisons (mono and combination treatment), and outcomes (ejaculation latency time, ELT).
Results
The initial search identified a total of 454 articles from electronic databases. Finally, a total of 10,474 patients from 59 direct comparison trials were included 143 effect sizes with 43 treatments. Of these, 9 of mono treatments and 4 of combination treatments were statistically significant. Pharmaceutical agents commonly used for patients with PE are prescribed off-label, except for dapoxetine. The surface under the cumulative ranking curve values of ranking probabilities for each treatment performance, which indicated that tramadol 100 mg ranked first in terms of ELT.
Conclusions
Medications recommended by the American Urological Association and the Sexual Medicine Society of North America were all incorporated within the present review, together with additional management approaches that have been evaluated in randomized controlled trials. The findings indicated that in addition to SSRIs, tramadol, clomipramine, topical agents and PDE5 inhibitors could be used in the therapy of PE.
Keywords: Drug therapy; Ejaculation; Premature ejaculation; Sexual dysfunction, physiological
INTRODUCTION
One of the most frequently arising reasons for male sexual dysfunction, premature ejaculation (PE) occurs in about 20% to 30% of men in sexually active age group [1]. Previously, this pathology and the disease processes underlying it have been poorly described, but in 2014, an initial evidence-based and standardized description of PE was presented [2]. According to the recommendations published by the International Society for Sexual Medicine (ISSM), PE includes a triad of features: (i) ejaculation which, in the majority of instances, takes place within the first minute after vaginal entry from the initial sexual encounter, or a notably short ejaculation latency time (ELT), i.e. of under 3 minutes; (ii) lack of ability to delay ejaculation on the majority of vaginal penetrations; and (iii) a negative impact on the affected individual’s psychology, causing them to become upset and vexed, and to shy away from sexual intercourse [3].
At present, the Food and Drug Administration (FDA) in the United States (US) has failed to sanction any treatments for PE [4,5,6]. The ELT, as quantified by a stopwatch, has been used as a measure in numerous clinical trials and observational research into PE, but has not been incorporated into the general treatment of PE [7].
There have been a number of recent advances in drugs and surgical strategies for the management of PE [8]. Pharmaceutical approaches predominantly comprise the prescription of selective serotonin reuptake inhibitors (SSRIs), e.g. sertraline, fluoxetine, dapoxetine and paroxetine, phosphodiesterase type 5 (PDE5) inhibitors, e.g. tadalafil or sildenafil, as well as alternative medications, which include tramadol [9].
The current systematic review and network meta-analysis (NMA) has been conducted in order to investigate the different management choices for PE, which include behavioral therapy, systemic and topical drug therapy, and surgical interventions.
MATERIALS AND METHODS
Our study used publicly available data and did not include human participant research. As per 45 CFR §46.102(f), this study was not submitted for institutional review board approval and did not require informed consent procedures.
This systematic review and NMA was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) extension for NMA of healthcare interventions guidelines [10]. The protocol pertaining to this study was registered on PROSPERO (CRD42022374835) before the study.
1. Data sources and literature searches
We retrieved data from electronic literature searches of PubMed and Cochrane library using the MeSH (Medical Subject Headings terms) and text keywords from the earliest available date of indexing through September 2022. The subject headings and text keywords included those related to the population (male patients with PE), interventions & comparisons (20 mono treatment: citalopram, cligosiban, clomiphene, clomipramine, dapoxetine, and duloxetine, ED agent [sildenafil and tadarafil], epelsiban, escitalopram, fluoxetine, fluvoxamine, modafinil, nefazodone, ointment, physical therapy, placebo, pregabalin, paroxetine, sertraline, and tramadol; and 9 combination treatment: dapoxetine-physical therapy, dapoxetine-ED agent, dapoxetine-mirodenafil, fluoxetine-ED agent, paroxetine-ED agent, paroxetine-pindolol, ointment-ED agent, other medicaiton, and other-therapy), and outcomes: intravaginal ejaculation latency time (IELT). The search terms were grouped according to Boolean operators (AND, OR, NOT). The same search strategy was adopted for EMBASE using Emtree (EMBASE subject headings) (Supplement Table 1). Additional studies were screened by two independent investigators (SR Shim and JH Kim) through manual search of clinical trial databases and previous study reference lists (Supplement Table 2).
2. Inclusion and exclusion criteria for study selection
The inclusion criteria for relevant studies were as follows: (1) patients with a diagnosis of PE; (2) the outcome was IELT, the assessment time after entering the vagina in the first sexual contact; (3) duplicated publications were excluded, as were publications that did not contain original data, such as review articles, case reports, conference abstracts, editorials, letters, and guidelines; (4) human-based randomized control trials (RCTs) rather than cross-over design. Exclusion criteria were as follow: (1) non-human RCTs; and (2) no use of ELT; and (3) no comparison groups. Two independent investigators independently screened the titles and abstracts of all the articles using the predefined inclusion criteria. All of the researchers independently reviewed the full-text articles to see if they met the inclusion criteria. Furthermore, two independent investigators independently extracted data using a data extraction form. The final inclusion of each article was determined by all investigators through evaluation and discussion. To ensure the integrity of the meta-analysis and the absence of overlapping data, the references and data for each included study were cross-checked.
3. Data extraction and measurement outcomes
Details of the basic study information (first author, year of publication, country, and follow-up period), patient characteristics (number of patients and age range), and technical aspects (inclusion & exclusion criteria, and treatments) were extracted from the included articles, using a predefined data extraction form. In one study, when there were several treatment durations, the effect size was calculated in the last part, and if there were several dose ranges, the highest dose was set, and the dose was converted based on daily dosage. Only studies providing complete information were included in the final meta-analysis.
4. Data analysis
We performed a Bayesian NMA, using the R version 4.2.1 “gemtc” package. The analysis pooled the mean difference of IELTs and 95% credible intervals (CrI). A two-sided p value of <0.05 was considered statistically significant. We assessed publication bias (or small-study effects) using a funnel plot. We also used the Egger test (i.e., linear regression test of funnel plot asymmetry) when assessing the publication bias.
To compare the forty-three treatments including specific, the prior distribution and likelihood were fed into a Markov chain Monte Carlo (MCMC) simulation, and the distribution with the best convergence of the posterior distribution was chosen. The MCMC simulation was used to determine the probability of a stable distribution and the area under the posterior distribution function. Finally, the posterior distribution was used to perform statistical reasoning for the treatment effect. We performed node-splitting assessments to determine the association between the direct and indirect evidence for the consistency test. The surface under the cumulative ranking curve (SUCRA) was used to calculate the probability of each index test being the most effective treatment method based on a Bayesian approach using probability values to facilitate interpretation of treatment performance; the larger the SUCRA value was, the higher the rank of the intervention [11,12].
5. Quality assessment
The Cochrane Collaboration risk of-bias 2.0 tool was used to assess the risk of bias and methodological quality [13,14]. We evaluated five parameters: 1) randomization process; 2) deviations from the intended interventions; 3) missing outcome data; 4) measurement of the outcome; and 5) selection of the reported result. Each domain was assigned a risk of bias rating of high, low, or unclear. The overall risk of bias was considered to be low if all domains were rated as "Low," some concerns if even one domain was rated as "Some concerns," and high risk if even one domain was rated as "High," or more than two domains were received the "Some concerns."
RESULTS
1. Study selection
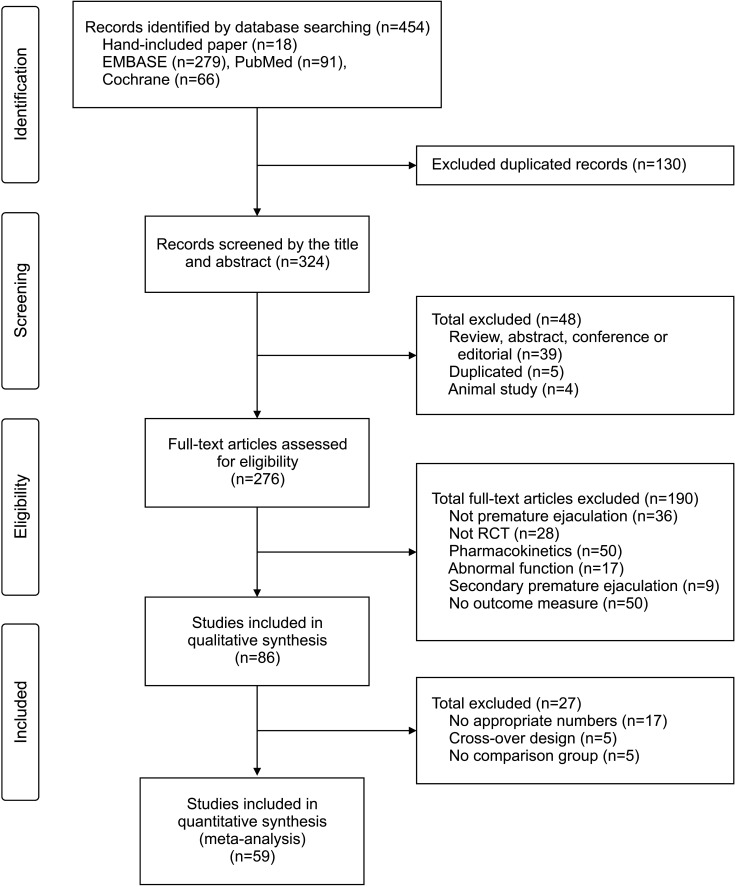
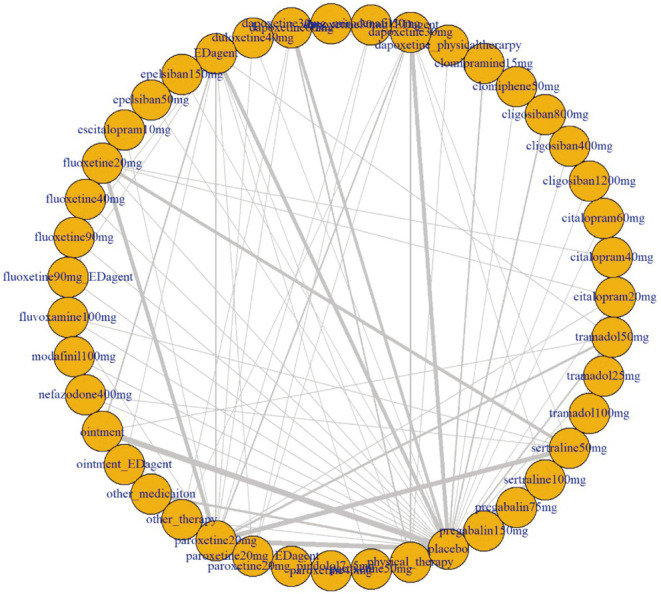
The initial search identified a total of 454 articles from electronic databases (PubMed, 91; Cochrane, 66; EMBASE, 279; manual searching, 18), of which 178 were unrelated to the topic, contained overlapping data, or appeared in more than one database and were excluded. After a more detailed review, an additional 187 papers that were no PE, no RCT, pharmacokinetics only, abnormal function, secondary PE, and no outcome measure were eliminated. After full-text assessments, 86 studies were eligible for systematic review and meta-analysis. Of these, 27 were further excluded for the following reasons: no appropriate numbers (n=17), no comparison group (n=5), and cross-over design (n=5) (Fig. 1). Finally, 59 studies including 10,474 participants met our selection criteria for NMA, among which 2-arm, 3-arm, 4-arm, and 5-arm studies were 33, 18, 6, and 2 (Fig. 2).
Fig. 1. Flow chart. RCT: randomized control trial.
Fig. 2. Network plot.
2. Quality assessment
We evaluated the eight-nine PE studies using the five RoB 2 domains to determine the risk of bias for the included studies. In D1, one study was classified as "High”. In D2, six studies were classified as "High." In D3, one study was classified as "High." In D4 and D5, all studies were classified as "Low" or "Some concerns." Based on these evaluations, the overall risk of bias was ranked. Thirty-nine studies were classified as "Low," twenty-three studies as "Some concerns," and twenty-seven studies as "High" (Supplement Fig. 1).
3. Outcomes
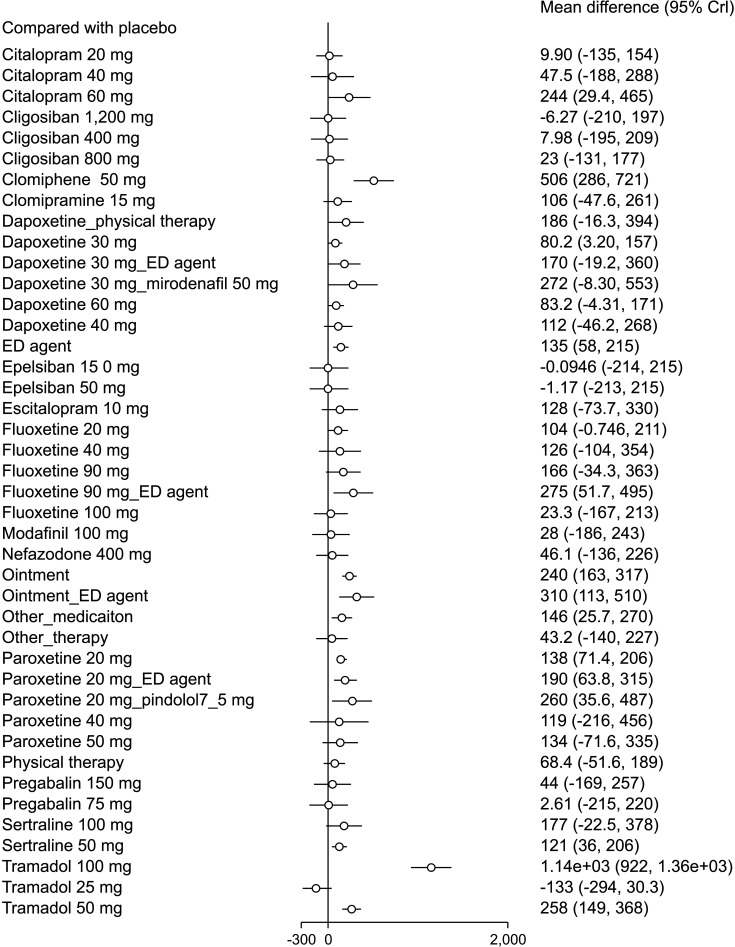
A total of 10,474 patients from 59 direct comparison trials were included 143 effect sizes with 43 treatments. Of these, 9 of mono treatments and 4 of combination treatments were statistically significant. The pooled overall mean differences of IELT (sec) were 1,140 (95% CrI: 922, 1,360) in tramadol 100 mg, 506 (95% CrI: 286, 721) in clomiphene 50 mg, 310 (95% CrI: 113, 510) in ointment-ED agent, 275 (95% CrI: 51.7, 495) in fluoxetine 90 mg-ED agent, 260 (95% CrI: 35.6, 487) in paroxetine 20 mg-pindolol 7.5 mg, 258 (95% CrI: 149, 368) in tramadol 50 mg, 244 (95% CrI: 29.4, 465) in citalopram 60 mg, 240 (95% CrI: 163, 317) in ointment, 190 (95% CrI: 63.8, 315) in paroxetine 20 mg-ED agent, 138 (95% CrI: 71.4, 206) in paroxetine 20 mg, 135 (95% CrI: 58.0, 215) in ED agent, 121 (95% CrI: 36.0, 206) in sertraline 50 mg, 80.2 (95% CrI: 3.20, 157) in dapoxetine 30 mg (Fig. 3).
Fig. 3. Forest plot. Crl: credible intervals.
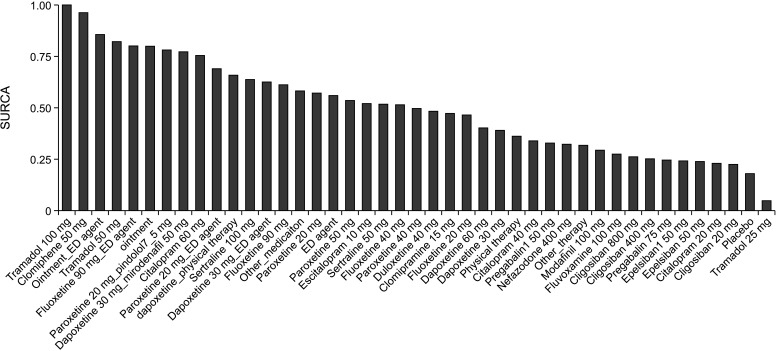
Fig. 4 shows the SUCRA values of ranking probabilities for each treatment performance, which indicated that tramadol 100 mg ranked first in terms of IELT.
Fig. 4. The surface under the cumulative ranking curve (SUCRA) plot.
4. Inconsistency test
The inconsistency tests for NMA assumption were analyzed using the node-splitting method, and the results indicated consistency among the direct and indirect evidence of all outcomes. Therefore, a consistency model was applied in the current study (all p>0.05).
5. Publication bias
The statistical approaches for the detection of publication bias or small-study effect in fifty-nine studies are shown in Supplement Fig. 2. Egger’s regression (two-tailed p=0.120) and a visual inspection of symmetry graphic in the funnel plot suggested that there was no evidence of publication bias or small-study effect in this NMA.
DISCUSSION
The most contemporary relevant clinical recommendations have been published by the American Urological Association and the Sexual Medicine Society of North America [6]. A number of approaches to PE are broached, which include psychological health, behavioral and drug-related strategies, although the latter are not yet sanctioned for utilization in PE by the US FDA; such use is deemed to be ‘off-label’. The initial drugs advised in the recommendations encompass clomipramine, sertraline, fluoxetine and citalopram; further second-line options are tramadol, terazosin, alfuzosin, silodosin, tamsulosin and doxazosin. These medications were all incorporated within the present review, together with additional management approaches, such as behavioral, topical and surgical treatment options that have been evaluated in randomized controlled trials. The findings indicated that in addition to SSRIs, tramadol, clomipramine, topical agents and PDE5 inhibitors could be used in the therapy of PE.
Two behavioral strategies which are most commonly advocated included the ‘stop-and-start’ method and the‘squeeze’ technique, reported by Semans in 1956 [8], and Masters and Johnson in 1970 [9], respectively. The rationale underlying these methods are distraction and a diminution of sexual arousal or stimulation; however, these approaches may lead to lower levels of sexual satisfaction [5]. Nevertheless, the findings of this study have demonstrated that the use of behavioral techniques alone or in combination with therapies, such as SSRIs, lack efficacy in the management of PE.
Clinical studies evaluating PE have conventionally investigated the use of tricyclic antidepressants, e.g. clomipramine and SSRIs. Indications for clomipramine include the management of major episodes of depression, secondary depression, panic disorders in combination with agoraphobia, generalized anxiety and obsessive-compulsive disorders [15]. Small doses of clomipramine appear to be promising as a therapeutically efficacious option for individuals with PE [16]. The mechanism of action of a number of anti-depressive agents, e.g. clomipramine, in this context has been proposed to be a reduced serotonin or 5-hydroxytryptamine (5-HT) reuptake, which delays ejaculation. Additionally, these drugs may cause an increased sensory threshold for genital stimulation [17]. Successful PE management was achieved in one study following ingestion of a 25 mg oral dose of clomipramine 4 hours prior to sexual engagement [18]. The current review demonstrated a beneficial effect of 50 mg clomipramine when judged against a placebo in individuals with PE.
A contemporary view is that PE reflects a neurobiological issue related to a neurotransmission abnormality at serotonin and 5-HT receptors in the central nervous system [19]. This underlies the ISSM advice that SSRIs should be the principal pharmaceutical agents offered for the management of PE [3]. SSRIs may also inhibit the 5-HT transporter and activate the 5-HT 2C receptor, an action which diminishes the operation of the 5-HT 1A receptor, thus reattaining equilibrium between the actions of the 5-HT 1A and 5-HT 2C receptors and causing 5-HT titers within the synapses to rise [20].
It was noted in the current study that when compared against a placebo, a number of SSRIs, i.e. paroxetine, dapoxetine, sertraline and citalopram, demonstrated greater efficacy for the therapy of PE. Typically used as an anti-depressant agent, paroxetine is therapeutically indicated in major depressive, panic, obsessive-compulsive and social anxiety disorders [21]. Primary PE was improved with a 20 mg dose of paroxetine; higher doses led to additional prolongations of IELT [22]. The current study revealed that 20 mg paroxetine alone, or together with either PDE5 inhibitors or the beta-blocker, pindolol, were also superior to placebo for the treatment of PE. Pharmaceutical agents and their doses for which similar results were demonstrated included 30 mg dapoxetine, 60 mg citalopram, 50 mg sertraline and 90 mg fluoxetine, together with a PDE5 inhibitor.
Despite the fact that SSRIs are frequently advised as the initial form of therapy in PE, they are associated with a number of drawbacks. Their side effects encompass diminished libido and erectile dysfunction [23]. They also interact with numerous other medications. When more than one serotonergic drug is used simultaneously, a constellation of symptoms referred to as serotonin syndrome may occur, e.g. in the context when SSRIs are prescribed in combination with lithium, tryptophan or monoamine oxidase inhibitors [24]. The use of SSRIs is also linked with a risk of hemorrhage, as hemostasis relies on the liberation of serotonin from platelets.
Sildenafil, a PDE5 inhibitor, has been evaluated as a therapeutic agent in PE in a number of clinical trials. PDE5 inhibitors are considered to be effective in PE owing to their actions both centrally and peripherally. The latter may slow the ejaculation time by impacting the contractile reactions of the vas deferens, seminal vesicles, prostate and urethra, generating an analgesic effect, and lengthening erection duration. The former actions may reflect an attenuation of sympathetic drive [25]. The current review demonstrated that PDE5 inhibitors, both as lone agents and together with SSRIs or topical agents, were superior to a placebo for the treatment of PE.
The time to ejaculation has been prolonged by the use of topical agents, e.g. lidocaine-prilocaine cream (5%) or spray, local Severance Secret cream and dyclonine-alprostadil cream, which reduce the degree of stimulation in relation to the penis. The present study indicated that these topical formulations were more effective in PE than a placebo either alone or when used as an adjunct to either SSRIs or PDE5 inhibitors.
An analgesic which works within the central nervous system, tramadol has two main effects, impacting the mu opioid receptor, as well as reducing the reuptake of norepinephrine and serotonin. The exact way in which it works in relation to PE is unclear, although both these mechanisms of action may be involved, the first diminishing sensitivity and the second causing a delay in ejaculation. Its safety data and adverse event frequency are acceptable [26]. When contrasted against a placebo, one study demonstrated that 50 mg tramadol taken 2 hours prior to sexual intercourse over an 8-week period increased ELT and sexual satisfaction [27]. The current study demonstrated that 50 mg or 100 mg tramadol taken as required was also superior to a placebo in patients with PE. The latter mode of delivery has been used in the majority of studies which have evaluated tramadol owing to the potential for substance abuse and the development of drug dependence [5]. The likelihood of opioid addiction in this context requires determination following additional longitudinal research.
There have been few studies on surgical strategies for PE management. We included studies on surgical intervention in this analysis, but due to the small number of studies, they were not represented in SUCRA values of ranking probabilities for each treatment performance. The RCTs described in this review were all high-quality studies. However, a number of limitations of the current study were identified. Not all the studies used a double-blind design; additionally, there was some dropout of study subjects owing to failure to withstand the intervention. The findings will therefore be influenced by these biases.
CONCLUSIONS
The current review aimed to describe the various strategies used for treating PE; these encompassed behavioral, drug and surgical interventions. Of note is that commonly utilized pharmaceutical agents in these patients are prescribed off-label. The exception is dapoxetine, which has recently been approved in over 50 countries worldwide and offers an efficacious as required preparation that is well tolerated. Promising alternative de novo therapeutic strategies identified in this review for PE encompass tramadol as required, clomiphene and topical treatments, PDE5 inhibitors and SSRIs.
Acknowledgements
None.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by the Soonchunhyang University Research Fund.
- Conceptualization: JHK.
- Data curation: SRS.
- Formal analysis: HYL.
- Methodology: SRS.
- Project administration: JHP.
- Resources: HYL.
- Visualization: JHP.
- Writing – original draft: HYL.
- Writing – review & editing: JHK.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.230030.
Risk-of-bias assessment for individual studies.
Funnel plot for publication bias.
Search queries
Characteristics of all studies included in meta-analysis
References
- 1.Raveendran AV, Agarwal A. Premature ejaculation - current concepts in the management: a narrative review. Int J Reprod Biomed. 2021;19:5–22. doi: 10.18502/ijrm.v19i1.8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serefoglu EC, McMahon CG, Waldinger MD, Althof SE, Shindel A, Adaikan G, et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. Sex Med. 2014;2:41–59. doi: 10.1002/sm2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE) J Sex Med. 2014;11:1392–1422. doi: 10.1111/jsm.12504. [DOI] [PubMed] [Google Scholar]
- 4.Wang WF, Chang L, Minhas S, Ralph DJ. Selective serotonin reuptake inhibitors in the treatment of premature ejaculation. Chin Med J (Engl) 2007;120:1000–1006. [PubMed] [Google Scholar]
- 5.Saleh R, Majzoub A, Abu El-Hamd M. An update on the treatment of premature ejaculation: a systematic review. Arab J Urol. 2021;19:281–302. doi: 10.1080/2090598X.2021.1943273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindel AW, Althof SE, Carrier S, Chou R, McMahon CG, Mulhall JP, et al. Disorders of ejaculation: an AUA/SMSNA guideline. J Urol. 2022;207:504–512. doi: 10.1097/JU.0000000000002392. [DOI] [PubMed] [Google Scholar]
- 7.Althof SE, Abdo CH, Dean J, Hackett G, McCabe M, McMahon CG, et al. International Society for Sexual Medicine. International Society for Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med. 2010;7:2947–2969. doi: 10.1111/j.1743-6109.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 8.Semans JH. Premature ejaculation: a new approach. South Med J. 1956;49:353–358. doi: 10.1097/00007611-195604000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Human sexual inadequacy. Med J Aust. 1971;1:3–4. doi: 10.5694/j.1326-5377.1971.tb87410.x. [DOI] [PubMed] [Google Scholar]
- 10.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 11.Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. doi: 10.4178/epih.e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane Methods Bias. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials [Internet] London: Cochrane; [cited 2022 Jun 6]. Available from: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials . [Google Scholar]
- 15.Koen N, Stein DJ. Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin Neurosci. 2011;13:423–437. doi: 10.31887/DCNS.2011.13.4/nkoen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segraves RT, Saran A, Segraves K, Maguire E. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Ther. 1993;19:198–200. doi: 10.1080/00926239308404904. [DOI] [PubMed] [Google Scholar]
- 17.Colpi GM, Fanciullacci F, Aydos K, Grugnetti C. Effectiveness mechanism of chlomipramine by neurophysiological tests in subjects with true premature ejaculation. Andrologia. 1991;23:45–47. doi: 10.1111/j.1439-0272.1991.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 18.Waldinger MD, Zwinderman AH, Olivier B. On-demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol. 2004;46:510–515. doi: 10.1016/j.eururo.2004.05.005. discussion 516. [DOI] [PubMed] [Google Scholar]
- 19.Coskuner ER, Ozkan B. Premature ejaculation and endocrine disorders: a literature review. World J Mens Health. 2022;40:38–51. doi: 10.5534/wjmh.200184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldinger MD, Olivier B. Utility of selective serotonin reuptake inhibitors in premature ejaculation. Curr Opin Investig Drugs. 2004;5:743–747. [PubMed] [Google Scholar]
- 21.Green B. Focus on paroxetine. Curr Med Res Opin. 2003;19:13–21. doi: 10.1185/030079902125001353. [DOI] [PubMed] [Google Scholar]
- 22.Waldinger MD, Hengeveld MW, Zwinderman AH. Ejaculation-retarding properties of paroxetine in patients with primary premature ejaculation: a double-blind, randomized, dose-response study. Br J Urol. 1997;79:592–595. doi: 10.1046/j.1464-410x.1997.00102.x. [DOI] [PubMed] [Google Scholar]
- 23.Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry. 2001;62 Suppl 3:10–21. [PubMed] [Google Scholar]
- 24.Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13:533–540. [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Hamid IA. Phosphodiesterase 5 inhibitors in rapid ejaculation: potential use and possible mechanisms of action. Drugs. 2004;64:13–26. doi: 10.2165/00003495-200464010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Salem EA, Wilson SK, Bissada NK, Delk JR, Hellstrom WJ, Cleves MA. Tramadol HCL has promise in on-demand use to treat premature ejaculation. J Sex Med. 2008;5:188–193. doi: 10.1111/j.1743-6109.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 27.Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18:164–169. doi: 10.1038/sj.ijir.3901384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk-of-bias assessment for individual studies.
Funnel plot for publication bias.
Search queries
Characteristics of all studies included in meta-analysis