Abstract
Increasing research links the gut microbiome to neurodegenerative disorders. The gut microbiome communicates with the central nervous system via the gut–brain axis and affects behavioral and cognitive phenotypes. Dysbiosis (a dysfunctional microbiome) drives increased intestinal permeability and inflammation that can negatively affect the brain via the gut–brain axis. Healthier metabolic and lipid profiles and cognitive phenotypes are observed in individuals with more distinct microbiomes. In this review, we discuss the role of the gut microbiome and gut–brain axis in neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease and related animal models, in cancer and cancer treatments, and in metabolic syndrome. We also discuss strategies to improve the gut microbiome and ultimately brain function. Because healthier cognitive phenotypes are observed in individuals with more distinct microbiomes, increased efforts are warranted to develop therapeutic strategies for those at increased risk of developing neurological disorders and patients diagnosed with those disorders.
Keywords: gut microbiome, Parkinson’s disease, Alzheimer’s disease, radiation, Western diet
The gut microbiome plays an important role in absorption of nutrients and minerals; synthesis of enzymes, amino acids, and neurotransmitters; production of metabolites promoting epithelial barrier integrity; and immune modulation.1,2 These various functions of the gut microbiome can impact brain functioning and ultimately cognition and behavior through a variety of bidirectional, physiological mechanisms, known as the gut–brain axis, which include vagal nerve innervation, neurotransmitter production, endocrine signaling, and inflammation.3–8 Dysbiosis, wherein the composition of the gut microbiome changes into a state that maladaptively contributes to physiology, can result in a variety of pathologies, including increased intestinal permeability and inflammation that can negatively affect the brain via the gut–brain axis. In humans, gut microbiomes diversify with age and can predict survival.9 Healthy aging is associated with an age-related diversification of the gut microbiome toward a more distinct compositional state, whereas unhealthy aging, including unhealthy brain aging, is not. Corresponding with these compositional differences in the gut microbiome overall, specific gut microbial taxa also link to Parkinson’s disease (PD)10–13 and Alzheimer’s disease (AD).14,15 Healthier metabolic and lipid profiles also are observed in individuals with more distinct microbiomes,9 indicating the importance of including metabolomics and lipidomics analyses as part of assessments of alterations in the gut microbiome and gut–brain axis. The gut microbiome becomes more distinctive at midlife in those with healthy aging. Considering the age at onset of neurodegenerative diseases such as PD and AD, developing safe and effective therapeutic strategies to improve the gut microbiome when the earliest clinical symptoms appear is critical.
Preclinical animal models afford tremendous opportunity to improve our understanding of the gut microbiome’s link to specific neurodegenerative phenotypes, as well as its role in the manifestation of these phenotypes. In particular, these model organisms afford more invasive analyses of the enteric and central nervous systems under environmentally controlled experimental conditions, which is a critical feature given the gut microbiome’s sensitivity to environmental variation. In this review, we discuss the role of the gut microbiome and gut–brain axis in neurodegenerative diseases, such as PD and AD and their related animal models. In addition, we discuss the role of the gut microbiome in brain function in cancer and cancer treatments, including radiation and immunotherapy, all of which are associated with detrimental effects on the brain. We also discuss the role of the gut microbiome and gut–brain axis in metabolic syndrome, also associated with impaired cognitive function. Finally, we discuss strategies to improve the gut microbiome, including using exercise and food supplements, and ultimately to improve gut–brain axis and brain function.
Parkinson’s Disease
PD is a progressive neurological disease without a cure. In PD, cognitive symptoms are often poorly recognized and treated, although they account for disability and reduced life expectancy.16 We need to advance our understanding of the pathways involved in the cognitive impairments in PD so that we can ultimately develop novel therapeutic interventions to mitigate them and alter the course of the disease. The gut has not historically been considered a major contributor to PD, but many PD patients have digestive symptoms years before they exhibit neurological symptoms, and composition of the gut microbiome of PD patients differs from that of healthy controls.17 In addition, a recent meta-analysis revealed significant alterations in the PD-associated microbiome.18 Enrichment of the genera Lactobacillus, Akkermansia, and Bifidobacterium and depletion of bacteria belonging to the Lachnospiraceae family and the Faecalibacterium genus, both important short-chain fatty acid (SCFA) producers, were revealed as the most consistent alterations in the PD gut microbiome. In another recent meta-analysis, mucin-degrading Akkermansia was increased and SCFA-producing bacteria were decreased in PD. An increase in Akkermansia, coupled with a decrease in SCFAs, may enhance intestinal permeability and facilitate exposure of the intestinal neural plexus to environmental toxins and cause aggregation of α-synuclein (aSyn),18 which is a major constituent of Lewy bodies, the pathological hallmark of PD.19 In a recent study involving 490 PD patients and 234 controls, the PD microbiome was characterized by an overabundance of pathogens and immunogenic components, increased production of toxicants and molecules that induce aSyn pathology, dysregulated neuroactive signaling, and a reduction in anti-inflammatory and neuroprotective factors.20 Consistent with these human studies, gut microbiota regulate motor impairments and neuroinflammation in mice overexpressing aSyn.21
Environmental factors contribute to PD.22 Therefore, it is important to investigate them in both preclinical and clinical studies. Although the neurotoxic herbicide paraquat (N, N′-dimethyl-4,4′-bipyridinium dichloride; PQ) is made in England, it cannot be purchased there or in the European Union. However, it is still widely used in the United States and other parts of the world. When weeds become resistant to glyphosate (Monsanto’s Roundup), PQ is used as an alternative herbicide. In 2015, 7 million pounds of PQwere used in the United States on nearly 15 million acres. PQ increases the risk of developing PD in most studies.22 The effects of PQ are not limited to the gut microbiome and gut–brain axis, but include other systemic effects. For example, flies mutant for parkin, important in PD pathogenesis and in gut enterocytes for the maintenance of microbial load homeostasis,23 are more sensitive to PQ.24 This sensitivity is reduced in germ-free flies.23 Additionally, in rats, vagotomy prevents the development of PD symptoms and constrains the appearance of misfolded aSyn to myenteric neurons following co-administration of subthreshold doses of PQ and lectins.25 These data support a role for the gut microbiome and gut–brain axis in mediating the effects of PQ. Relatedly, we showed that the effects of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on cognitive performance may, at least in part, be mediated by the gut microbiome.26 Like PQ, MPTP also causes a dose-dependent dopamine neuronal pathology, reduces locomotor activity, and increases the risk of developing PD symptoms in most studies. Collectively, these studies indicate the importance of considering how other types of environmental exposures may interact with the gut microbiome to influence PD (Fig. 1).
Fig. 1.
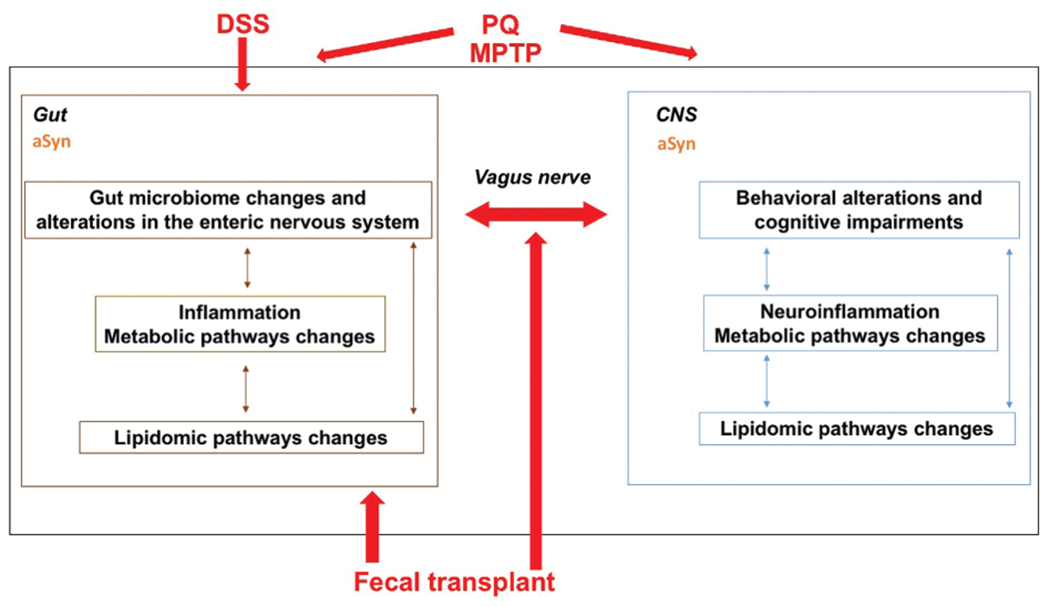
While the gut has historically not been considered a major contributor to PD, many PD patients have digestive symptoms years before they exhibit neurological symptoms, and composition of the gut microbiome of PD patients differs from that of healthy controls. It has been hypothesized that environmental toxins such as PQ or PD-related genetic mutations causing aggregation of aSyn, a major constituent of Lewy bodies and the pathological hallmark of PD, might be the initial trigger causing PD. Consistent with these human studies, gut microbiota regulate motor impairments and neuroinflammation in mice overexpressing aSyn. Moreover, flies mutant for parkin, important in PD pathogenesis and in gut enterocytes for the maintenance of microbial load homeostasis, are more sensitive to PQ and this sensitivity is reduced in germ-free flies. There is evidence that the vagus nerve is critical for mediating these gut–brain axis effects and for spreading aSyn pathology to the brain; vagotomy prevents the development of PD symptoms and constrains the appearance of misfolded aSyn to myenteric neurons following co-administration of subthreshold doses of PQ and lectins. Additionally, when transgenic mice displaying aSyn overexpression due to the A53T PD-associated mutation are chronically exposed to DSS, there is an invasion of macrophages into the lining of the gut wall, accumulation of aSyn in enteric neurons within the submucosal plexus, greater behavioral impairments, brain pathology, and inflammation in the gut and the brain. Consistent with these data, the effects of the neurotoxin MPTP on cognitive performance may, at least in part, be mediated by the gut microbiome. In the gut and the brain, gut microbiome changes might be mediated by inflammation involving alterations in metabolic pathways and lipidomic pathway changes. Fecal transplants of PD patients can induce PD phenotype in mice and fecal transplants of healthy people might be able to modulate PD phenotypes in PD patients. As the gut–brain axis involves bidirectional communication, PD pathology in the brain might also enhance PD pathology in the gut.
aSyn may interact with the gut microbiome in the development of PD, since aSyn participates in the immune response of the gut, and may protect against invading microbes. Following exposure to certain types of bacteria, aSyn levels in the gut and brain are increased. Also, in children with gut infections, aSyn levels in the gut are increased and correlate with the degree of inflammation.27 Expression of aSyn in the neuronal processes of the vascular layer of the gut epithelium correlates with the influx of macrophages and dendritic immune cells. Elevated accumulation of aSyn, due to chronic infection or deficient clearance, may lead to aSyn pathology in the gut, which then spreads via the vagus nerve to connected brain regions, thereby driving PD pathogenesis.12 Consistent with this notion, when young transgenic mice, displaying aSyn overexpression due to the A53T PD-associated mutation, are chronically exposed to dextran sulfate sodium (DSS), the result includes the following: invasion of macrophages into the lining of the gut wall; accumulation of aSyn in enteric neurons within the submucosal plexus; and greater behavioral impairments, brain pathology, and inflammation in the gut and the brain, compared with control littermates at 3 months28 or 21 months29,30 following initiation of the DSS exposure. Via the gut–brain axis, the gut microbiome can also affect stress-related behaviors, anxiety, and depression.3–5 For example, DSS administration increases measures of anxiety in female and male C57BL/6J mice.31,32
Alzheimer’s Disease
AD is a major neurodegenerative disorder and one of the most burdensome diseases facing society. It is the most common form of dementia33 and the seventh leading cause of death in the United States.34 AD robs people of their cognitive capacity; it destroys memory as well as thinking and motor skills.35,36 Consequently, AD places great strain on health care resources in the form of long-term patient care.33 As it is a disease of aging, the volume of AD patients—and consequently the strain on our health care system—is expected to substantially increase in coming years, alongside a rapidly aging population.35 These challenges have motivated extensive research into AD etiology. Although much has been learned, we still struggle to understand the mechanisms underlying AD, how the disease progresses, and how to treat it.37
Recent work raises the possibility that the gut microbiome influences the severity of AD and the notion that genotype impacts the microbiome’s contribution to cognitive aging38–40 (Fig. 2). Several studies have demonstrated statistical associations between the composition of the gut microbiome and AD in humans41–43 as well as mouse models.14,15 For example, using 6-month old knock-in mice expressing human amyloid precursor protein (APP) with two (AppNL-F, or three AppNL-G-F, dominant AD mutations, we showed that the microbiome’s composition, as well as its association with mouse cognition, differs based on APP genotype and DNA methylation of the Apoe gene in the hippocampus.43 In humans, there are three isoforms of apolipoprotein E: E2, E3, and E4.44,45 Compared with E3, E4 increases the risk to develop age-related cognitive decline, AD, and cognitive impairments following various environmental challenges, whereas E2 is protective. ApoE is involved in cholesterol and lipid homeostasis and synaptic functions.44 The role of apoE in immunomodulation, especially in the brain, has been recognized.46 Even among healthy middle-aged populations, compared with E3, carrying E4 is associated with accelerated cognitive decline.47 Compared with E3, E2 is associated with a relative protective effect regarding AD risk, but is also associated with an increased propensity toward developing more severe symptoms in survivors with posttraumatic stress disorder.48 How apoE isoforms impact disease outcomes remains unclear. In addition to its expression in the brain and liver, apoE is expressed in the gut and the abundance of specific microbiota in the gut varies in humans, based on APOE genotypes suggesting that the gut microbiome and gut-brain axis might mediate apoE isoform-dependent effects on disease outcomes.49
Fig. 2.
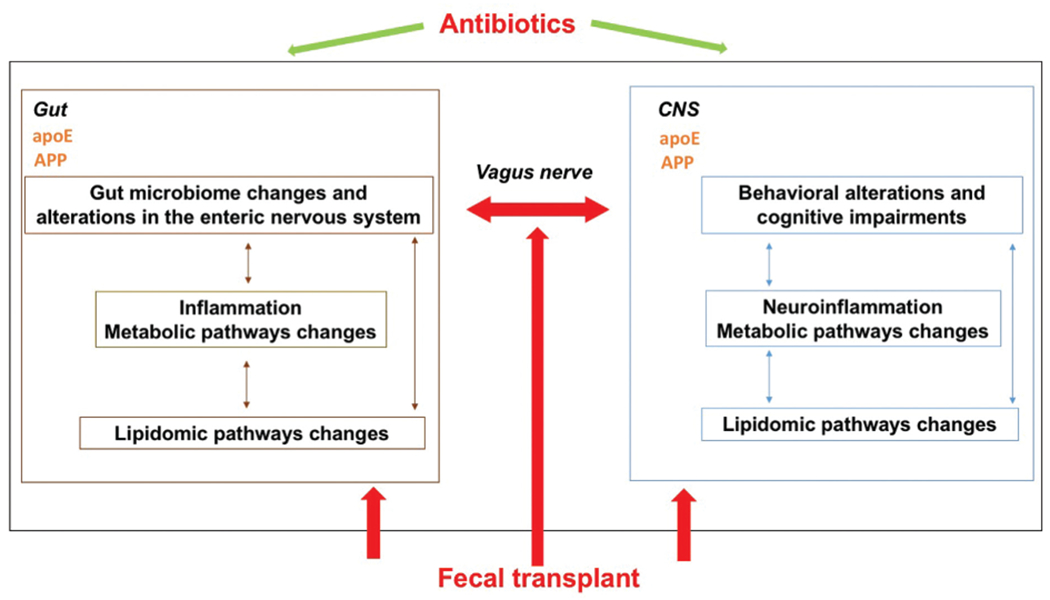
The gut–brain axis might play an important role in AD as well. APP and apoE are both expressed in the gut. The gut microbiome influences the severity of AD and the composition of the gut microbiome is associated with AD in humans and mouse models. In addition, transplanting microbiomes from AppNL-G-F mice, and from AppNL-G-F mice expressing E4, into germ-free wild-type mice yields donor genotype–dependent differences in recipient mouse behavioral and cognitive performance and amyloid pathology in brain. An antibiotic cocktail (ABX)–perturbed gut microbiome is associated with reduced amyloid-β (Aβ) plaque pathology and astrogliosis in the male, but not female, amyloid precursor protein (APP)SWE/presenilin 1 (PS1)ΔE9 and APPSWE/PS1L166P (APPPS1-21) transgenic models of Aβ amyloidosis. The data suggest that the link between the gut microbiome and behavior is genotype- and sex-dependent. Consequently, host genotype and sex may need to be carefully considered when developing novel therapeutic strategies targeting the gut microbiome in AD and other neurodegenerative disorders.
Fecal transplants in germ-free mice can be used to determine the causal impact of changes to the gut microbiome on brain function. Our studies have demonstrated that transplanting microbiomes from AppNL-G-F mice, and from AppNL-G-F mice expressing E4 as well, into germ-free wild-type mice yielded donor genotype–dependent differences in recipient mouse behavioral and cognitive performance.41 These fecal transplants also induced amyloid pathology in brain, a hallmark of AD pathology, and this, in turn, correlated with specific behavioral measures. Insoluble Aβ40 levels, a marker of AD pathology, were detected in the cortex of recipient mice receiving fecal matter from AppNL-G-F and AppNL-G-F mice expressing E4. Recipient mice receiving fecal matter from AppNL-G-F mice had cortical insoluble Aβ40 levels that positively correlated with activity levels on the first and second day of open field testing. For recipient mice, regression models demonstrated a statistical interaction between donor genotype and several behavioral scores linked to gut microbiome alpha-diversity. Two behavioral performance measures, distance moved on the first day of open field testing (a measure of exploratory behavior in a novel environment) and performance in the wire hang test (a measure of motor function), significantly associated with microbiome composition in recipient mice.41 However, this association was dependent on the donor genotype. These data suggest that the link between the gut microbiome and behavior is genotype-dependent. Consequently, host genotype may need to be carefully considered when developing novel therapeutic strategies targeting the gut microbiome in AD and other neurodegenerative disorders.
Besides genotype, the relationships we revealed between the gut microbiome and behavioral measures in our studies were sex-dependent. Consistent with these data, an antibiotic cocktail (ABX)–perturbed gut microbiome is associated with reduced amyloid-β (Aβ) plaque pathology and astrogliosis in the male amyloid precursor protein (APP)SWE/presenilin 1 (PS1)ΔE9 and APPSWE/PS1L166P (APPPS1-21) transgenic models of Aβ amyloidosis.50 These alterations are only seen in brains of male, but not female, mice, and transplants of fecal microbiota from age-matched APPPS1-21 male mice into ABX-treated APPPS1-21 males restore the gut microbiome and partially restore Aβ pathology. Consistent with an important role of the gut microbiome in AD pathology, there is a reduction of cerebral Aβ plaques and neurofibrillary tangles pathology in germ-free 3 × Tg mice, compared with specific pathogen–free mice, while fecal transplantation of stool from AD patients exacerbated AD pathologies in the 3 × Tg mice, associated with cognitive impairments, compared with those receiving stool from healthy donors.51 In the absence of gut microbiota, inflammatory pathway signaling and insulin/IGF-1 signaling in 3 × Tg mice brain are altered and polyunsaturated fatty acid metabolites and their oxidative enzymes are elevated, corresponding with microglia activation and inflammation. These data also highlight the complex beneficial and detrimental roles of neuroinflammation in AD. Consistent with the role of the gut microbiome in 3 × Tg mice, antibiotic treatment also reduced amyloid pathology and improved cognitive function in the 5 × FAD mouse model of amyloidosis.52 The prebiotic, R13, reduced amyloid pathology, gut leakage, and oxidative stress in the gut, further supporting a role for the gut microbiome and pathology in the gut in AD.52
Bile acids and ceramides might be involved in apoE isoform-dependent impacts on the brain involving the gut microbiome and gut–liver–brain axis. In young adults, apoE isoform associates with seven ceramides connected to atherogenically potent macrophages and/or lipoprotein particles53 and apoE is a major determinant of hepatic bile homeostasis.54 Lipids, especially ceramides, and bile acids have been implicated in AD-related neurodegeneration55,56 and are impacted by the gut microbiota.57 However, it is not known if the gut microbiome’s impact on these compounds influences AD outcomes.
Cancer and Cancer Treatments
The number of cancer survivors in the United States continues to increase, due to a growing aging population and improvements in cancer detection and treatment. As of January 2019, there were an estimated 17 million cancer survivors living in the United States. Within 10 years, the number of cancer survivors in the United States is projected to increase by 31%,58 meaning more people will be living longer with the adverse effects of cancer and cancer treatment. Many of these cancer survivors will be elderly and may experience reduced quality of life from a preventable or treatable toxicity related to cancer or cancer treatment. Therefore, it is important to understand how individual genetic susceptibility factors influence both symptom burden and efficacy of mitigation strategies in cancer survivors in order to best tailor rehabilitation programs. Cancer survivors often experience impaired functioning even years after cancer treatment.59 Frequently reported symptoms include behavioral and cognitive changes, such as difficulty concentrating, memory impairment, fatigue, and increased anxiety.60
Genetic factors might modulate these symptoms in cancer survivors. Little is known regarding the role of apoE isoform in influencing cancer survivorship. Several studies have identified an association between E4 and increased vulnerability to cognitive dysfunction after cancer treatment in survivors with breast cancer, lymphoma, and testicular cancer receiving chemotherapy.61 APOE genotype may modulate long-term cancer-related toxicity through a number of pathways. E3 has been described as functioning in an antitumor capacity through suppression of angiogenesis and cell invasion,62 whereas E2 has been associated with decreased risk of gastric cancer.62 A recent study found that E4 was associated with prolonged survival in survivors with melanoma, while E2 was associated with shorter survival.63 APOE genotype also modulates aggressive behavior in prostate cancer cells by modulating cholesterol metabolism.64
Prior studies have identified the impact of lifestyle-related factors on mediating the relationship between the apoE isoform and long-term cancer-related toxicity. That said, despite recognition of exercise as a salient protective factor against functional decline in E4 carriers in the setting of other medical comorbidities, this relationship has not yet been explored in the context of cancer. Exercise may not only curb adverse effects during active cancer treatment, but also lower the risk of cancer recurrence and improve quality of life in cancer survivors.65 Exercise has cardiometabolic benefits and may also attenuate the increased risk of cardiovascular disease following cancer treatment, which is now a competing cause of morbidity and mortality for female cancer survivors.66 In a study of fall prevention exercise in posttreatment female cancer survivors aged 50 to 75 years, we recently reported that APOE genotype modulates cancer treatment-related adverse effects and symptoms, along with response to exercise intervention.67 It is unclear whether similar effects occur in men. The beneficial effects of moderate exercise in cancer survivors, as well as in PD and AD, might be mediated by the gut microbiome (Fig. 3). Moderate exercise results in positive changes in the composition of the gut microbiome, which might be related to reduced inflammation in the gut (for recent reviews, see Clauss et al68 Monda et al69 and Mailing et al70).
Fig. 3.
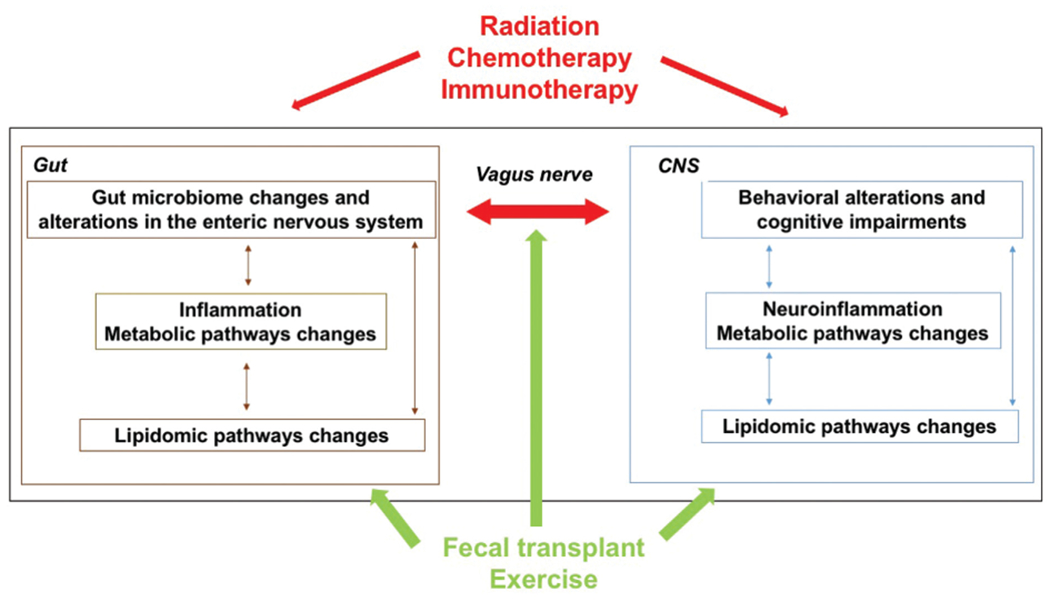
Effects of moderate exercise in cancer survivors might be mediated by the gut microbiome. Moderate exercise results in positive changes in the composition of the gut microbiome, which might be related to reduced inflammation in the gut. The gut microbiome may be critical in prostate cancer. In prostate cancer patients on androgen-deprivation therapy (ADT), or in castrated mice, the gut microbiome can generate testosterone and fuel prostate cancer growth, thus contributing to treatment resistance. There may be long-term effects on the brain as men who receive ADT for prostate cancer have an increased risk of dementia and/or AD, compared with men who do not receive ADT. The gut microbiome is also critical in modulating the treatment response of cancer patients to some therapeutic interventions. For example, the gut microbiome is key in modulating the response of cancer patients to immune activation using checkpoint inhibitors. While some cancer patients are cured following treatment with checkpoint inhibitors, others show resistance to treatment or become resistant to this treatment. Some melanoma and renal cell carcinoma patients become susceptible to treatment following a fecal transplant from patients who do respond to checkpoint inhibitors, supporting that the gut microbiome drives the sensitivity of these treatments. The gut microbiome is also affected by cancer treatments, such as chemotherapy and irradiation. Chemotherapy induces acute dysbiosis, and in different types of cancer, the gut microbiome is associated with chemotherapy toxicity and efficacy. Based on the relationship between the gut microbiome and cancer treatments such as chemotherapy and immunotherapy, there is increased interest in developing and testing therapeutic strategies to manipulate the gut microbiome to reduce tumor burden and improve the quality of life in cancer patients.
The gut microbiome might also mediate the apoE isoform-dependent effects on the brain in cancer survivors. For example, the gut microbiome may be critical in prostate cancer. In prostate cancer patients on androgen-deprivation therapy (ADT), or in castrated mice, the gut microbiome can generate testosterone and fuel prostate cancer growth, thus contributing to treatment resistance.71 There also may be long-term effects on the brain. Men who receive ADT for prostate cancer have an increased risk of dementia and/or AD, compared with men who do not receive ADT, and this is more pronounced when ADT is administered for longer than 12 months.72 It is currently unknown if the gut microbiome plays a role in these long-term effects. Interestingly, the increased risk of E4 carriers to develop AD might be related to testosterone.73
The gut microbiome is also critical in modulating the treatment response of cancer patients to some therapeutic interventions. For example, the gut microbiome is key in modulating the response of cancer patients to immune activation using checkpoint inhibitors (for a recent review, see Li et al74). Although some cancer patients are cured following treatment with checkpoint inhibitors, many show resistance to treatment or become resistant to this treatment. That said, some melanoma and renal cell carcinoma patients become susceptible to treatment following a fecal transplant from patients who do respond to checkpoint inhibitors.74 Similar studies are ongoing in other cancers, including gastrointestinal and prostate cancer.74 The gut microbiome appears to drive the sensitivity of these treatments. Germ-free mice receiving fecal transplants from patients responsive to programmed cell death protein 1 (PD-1) checkpoint inhibitor blockade were responsive to antitumor immunity and responsive to anti-PD-1 therapy, but germ-free mice receiving fecal transplants from nonresponsive patients were not responsive to PD-1 blockade.75 Similarly, the gut microbiome and the growth and immune cells infiltration of the tumor in germ-free mice that received fecal transplants from long-term pancreatic adenocarcinoma survivors were distinct from that in germ-free mice that received fecal transplants from short-term survivors.76
The gut microbiome is also affected by cancer treatments, such as chemotherapy and irradiation. Chemotherapy induces acute dysbiosis and it is not clear how long those effects last.77 In different types of cancer (e.g., colorectal cancer, acute myeloid leukemia, non-Hodgkin’s lymphoma, breast cancer, lung cancer, ovarian cancer, and liver cancer), the gut microbiome is associated with chemotherapy toxicity and efficacy.78 Based on the relationship between the gut microbiome and cancer treatments such as chemotherapy and immunotherapy, there is increased interest in developing and testing therapeutic strategies to manipulate the gut microbiome to reduce tumor burden and improve the quality of life in cancer patients.79–81
Radiation therapy also causes dysbiosis in gut microbiota, often producing a decrease in beneficial and increase in harmful bacteria, and also a change in SCFAs.82 These radiation effects on the gut microbiome are not restricted to clinical radiation. Environmental conditions at the International Space Station, including radiation, can alter microbial ecosystems that, in turn, may pose hazards to astronaut health, particularly during long-term missions, such as those planned to Mars. These effects might involve alterations in the diversity and composition of the gut microbiome and the gut–brain axis (reviewed in Ritchie et al83). The mouse gut microbiome is impacted by 13 days of space flight84 and by 16O ion irradiation (600 MeV/n, 0.1 and 0.25 Gy).85 In our ground-based simulated space radiation research, sequential three- and six-beam irradiation impacted the diversity and composition of the gut microbiome, with dose- and sex-dependent impacts and alterations to the relative abundance of several gut genera.86 Our analyses also identified associations between the gut microbiome and measures of behavioral and cognitive performance. These alterations to the gut microbiome may help explain some of the observed behavioral and cognitive impacts of radiation, given the microbiome’s influence on the brain and behavioral and cognitive performance.87,88
Metabolic Syndrome
Metabolic syndrome, or insulin resistance syndrome, which increases the risk of developing cardiovascular disease, stroke, type 2 diabetes, nonalcoholic fatty liver disease, cognitive impairment, and AD, also is associated with alterations in the gut microbiome.89 Elevated levels of hepatic enzymes, such as gamma-glutamyl transpeptidase, which are associated with impaired glucose tolerance90 and risk of diabetes,91 may play a role in patients becoming more severely affected by metabolic syndrome.92 The liver also may play a role in the effects of the gut microbiome on the brain via the gut–liver–brain axis.93 The liver and liver-generated factors are hypothesized to play an important role in AD. Brain phenotypes evident in mice with humanized livers are examples of this.94,95 Further examples that are consistent with a role for the liver in the development of brain phenotypes include the AD neurodegenerative phenotype in mice that generates human Aβ only in the liver,96 altered levels of Aβ-degrading enzymes in the livers of AD patients,97 and the association between altered liver enzymes with an AD diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers.98 Since gut microbiota can either positively associate with healthy metabolism or associate with unhealthy metabolic conditions, there is growing interest in developing microbiota-targeted interventions aimed at optimizing metabolic health.99
Strategies to Improve the Gut Microbiome, the Gut–Brain Axis, and Brain Function
Both exercise, especially moderate anaerobic exercise, and a healthy diet alter the diversity of the gut microbiome, improve barrier permeability, and are associated with increased levels of SCFA-producing bacteria in humans.100–102 Therefore, a variety of human studies involving exercise, diet, and dietary supplements in older study participants have been completed or are ongoing (Fig. 4). For example, a Mediterranean diet, involving increased consumption of nonrefined grains, fruits and vegetables, legumes, nuts, and fish, along with reduced intake of red meat and processed foods, has been associated with improved cognitive performance, reduced AD risk, and reduced gut permeability and inflammation.103,104 Another study assessing a diet characterized by increased consumption of cranberry, which is rich in polyphenols and associated with more healthy gut microbiota, documented enhanced visual episodic memory and decreased low-density lipoprotein cholesterol levels in 50- to 80-year-old people.105 An example of work in progress is a study involving a low-fat vegan diet, aerobic and resistance exercise, stress management, and group support, which are expected to have beneficial effects on the gut microbiome, gut–brain axis, and cognition.106 These human intervention studies are important to confirm the promising environmentally controlled preclinical studies involving exercise and diet supplements aimed at improving gut and brain function.
Fig. 4.
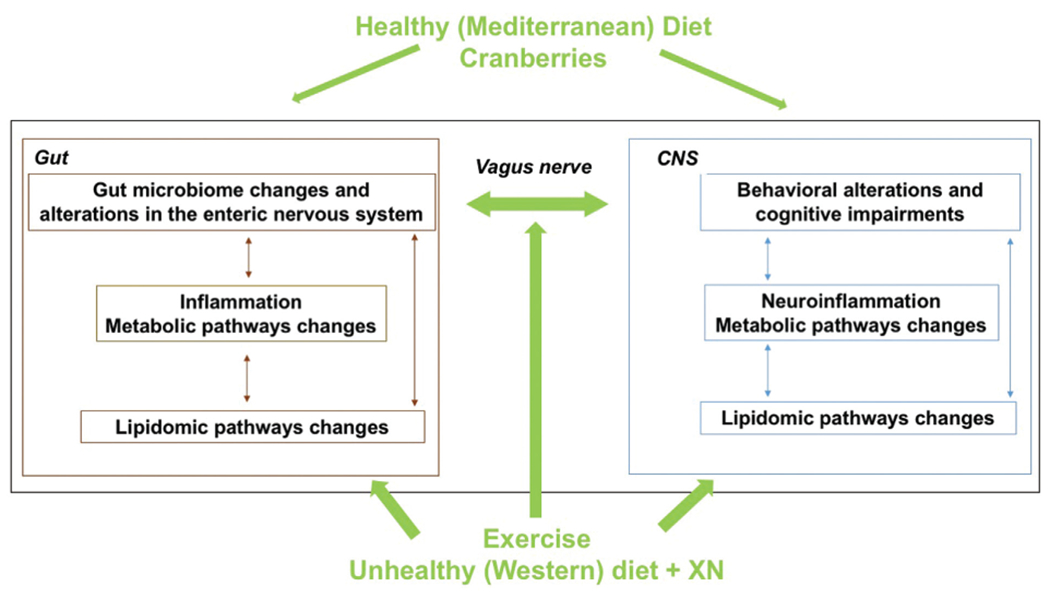
Exercise and a healthy diet alter the diversity of the gut microbiome, improve barrier permeability, and are associated with increased levels of SCFA-producing bacteria in humans. Therefore, a variety of human studies involving exercise, diet, and dietary supplements in older study participants have been completed or are ongoing. For example, a Mediterranean diet, involving increased consumption of nonrefined grains, fruits and vegetables, legumes, nuts, and fish, along with reduced intake of red meat and processed foods, has been associated with improved cognitive performance, reduced AD risk, and reduced gut permeability and inflammation. Another study assessing a diet characterized by increased consumption of cranberry, which is rich in polyphenols and associated with more healthy gut microbiota, documented enhanced visual episodic memory and decreased low-density lipoprotein cholesterol levels in 50- to 80-year-old people. Recognizing that it is hard for most people to alter their diet or adhere to regular exercise training, some dietary supplements have been studied in preclinical animal models consuming an unhealthy Western diet, which in humans typically involves consumption of foods high in saturated fats and simple sugars and low in fiber, and is associated with cognitive impairments and increased AD risk. For example, XN, a prenylated flavonoid found in the hop plant, Humulus lupulu, improves metabolic outcomes, including total body weight, fasting glucose, and plasma triglyceride levels, in a rodent model of obesity and metabolic syndrome. XN decreases plasma markers of reactive oxygen species and peripheral markers of dysfunctional lipid oxidation, increases uncoupled cellular respiration in obese male rats, and alters bile acid composition in mice. Dietary supplementation with XN also improves cognitive performance and affects the gut microbiome and improved cognitive performance in a mouse model of AD.
Recognizing that it is hard for most people to alter their diet or adhere to regular exercise training, some dietary supplements have been studied in preclinical animal models consuming an unhealthy Western diet, which in humans typically involves consumption of foods high in saturated fats and simple sugars and low in fiber, and is associated with cognitive impairments and increased AD risk.107,108 For example, xanthohumol (XN), a prenylated flavonoid found in the hop plant, Humulus lupulus,109 improves metabolic outcomes, including total body weight, fasting glucose, and plasma triglyceride levels, in a rodent model of obesity and metabolic syndrome.110 The beneficial effects of XN in response to a high-fat diet are also evident when XN does not significantly decrease body weight gain.11 XN significantly decreases plasma markers of reactive oxygen species and peripheral markers of dysfunctional lipid oxidation, increases uncoupled cellular respiration in obese male rats,111 and alters bile acid composition in mice.112 Dietary supplementation with XN also improves cognitive performance.113,114 XN affects the gut microbiome and improved cognitive performance in a mouse model of AD.115
Conclusions
Gut microbiome dysbiosis may be a component that drives the development of cognitive and behavioral pathologies, including that evident in neurodegenerative disorders such as PD and AD (Fig. 5). However, the complex phenotype of brain functioning and neurodegeneration also appears to depend upon genotypic and environmental variables. Preclinical models can afford a powerful opportunity to control these sources of variation and determine how their interaction drives behavioral and cognitive outcomes. Given the threat of increased burden of neurodegenerative disorders, including those that emerge from non–age-related sources (e.g., radiation), it is imperative that we resolve the underlying mechanisms of these conditions so that we can ultimately derive therapeutics for them. Based on the literature reviewed here, strong leads for understanding the basis of neurodegeneration may emerge from consideration of the microbiome alongside genetic factors such as APOE, lifestyle factors such as diet and exercise regime, environmental factors such as neurotoxicants and radiation, and host metabolic and lipid profiles. Ultimately, the integration of these various points of perspective about the host–microbiome relationship across preclinical models and human cohort studies can help disentangle the mechanisms through which neurodegenerative conditions arise.
Fig. 5.
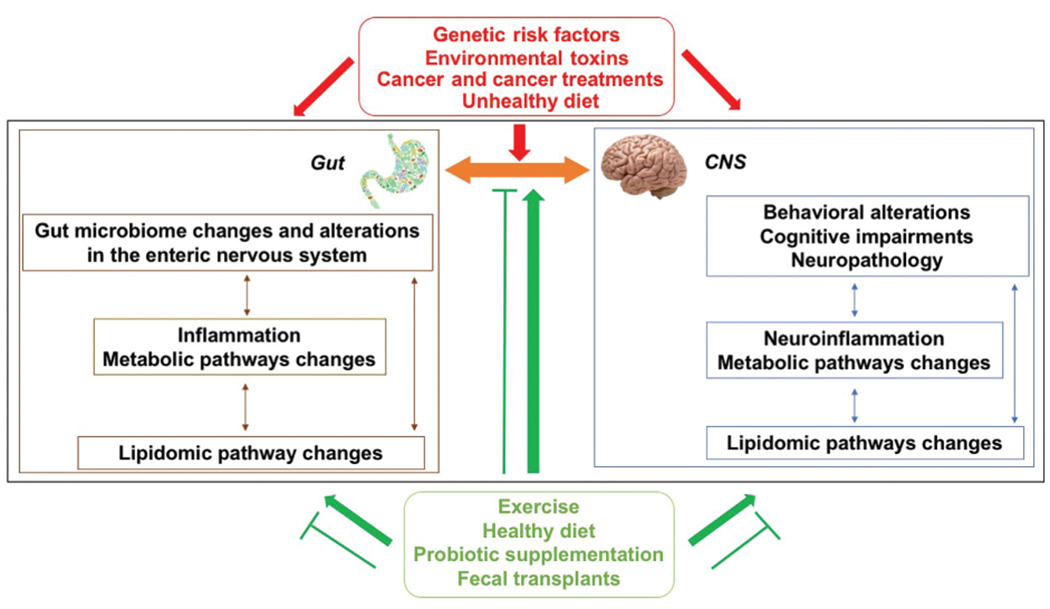
The gut microbiome communicates with the brain via the gut–brain axis and affects behavioral and cognitive phenotypes pertinent to neurological disorders. Dysbiosis (gut microbial imbalance) is associated with increased intestinal permeability and inflammation that can negatively affect the brain via the gut–brain axis. Inflammation in the gut and brain involve metabolic pathway changes and are associated with lipidomic pathway changes. While healthier metabolic and lipid profiles and cognitive phenotypes are observed in individuals with more unique microbiomes as they age, unhealthier metabolic and lipid profiles and cognitive phenotypes are observed in individuals with less unique microbiome as they age. The relationships between the gut microbiome and the brain are modulated by genotype and sex. Genetic risk factors, environmental toxins pertinent to PD, cancer and cancer treatments, and an unhealthy diet are associated with gut dysbiosis, cognitive injury, and neuropathology (red arrows). Exercise, especially anaerobic exercise, a healthy diet, probiotic supplementation, and fecal transplants from healthy donors are associated with a healthy gut and brain function and with the ability to antagonize the detrimental factors indicated in red (green arrows).
Funding
This work was partially supported by NIH RF1 AG059088, R21 AG065914, BrightFocus A2019444S, NASA 80NSSC19K0498 –P00001, and NIH NIEHS R01 ES030226.
Footnotes
Conflict of Interest
None declared.
References
- 1.Davidson GL, Cooke AC, Johnson CN, Quinn JL. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos Trans R Soc Lond B Biol Sci 2018; 373(1756):20170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gareau MG. Cognitive function and the microbiome. Int Rev Neurobiol 2016;131:227–246 [DOI] [PubMed] [Google Scholar]
- 3.Foster JA, McVey Neufeld K-A. gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36(05):305–312 [DOI] [PubMed] [Google Scholar]
- 4.Allen AP, Dinan TG, Clarke G, Cryan JF. A psychology of the human brain-gut-microbiome axis. Soc Personal Psychol Compass 2017;11(04):e12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch JB, Hsiao EY. Microbiomes as sources of emergent host phenotypes. Science 2019;365(6460):1405–1409 [DOI] [PubMed] [Google Scholar]
- 7.Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbbiome and host behavior. Annu Rev Neurosci 2017;40:21–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004;558(Pt 1):263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilmanski T, Diener C, Rappaport N, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab 2021;3(02):274–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A Jr. The gut and Parkinson’s disease- a birectional pathway. Front Neurol 2019;10:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elfil M, Kamel S, Kandil M, Koo BB, Schaefer SM. Implications of the gut microbiome in Parkinson’s disease. Mov Disord 2020;35(06):921–933 [DOI] [PubMed] [Google Scholar]
- 12.Keshavarzian A, Engen P, Bonvegna S, Cilia R. The gut microbiome in Parkinson’s disease: a culprit or a bystander? Prog Brain Res 2020;252:357–450 [DOI] [PubMed] [Google Scholar]
- 13.Koutzoumis DN, Vergara M, Pino J, et al. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp Neurol 2020;325:113159. [DOI] [PubMed] [Google Scholar]
- 14.Askarova S, Umbayev B, Masoud A-R, et al. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front Cell Infect Microbiol 2020;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022;71(11):2233–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhuri KR, Healy DG, Schapira AHNational Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006;5(03):235–245 [DOI] [PubMed] [Google Scholar]
- 17.Fan H-X, Sheng S, Zhang F. New hope for Parkinson’s disease treatment: targeting gut microbiota. CNS Neurosci Ther 2022;28(11):1675–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirayama M, Ohno K. Parkinson’s disease and gut microbiota. Ann Nutr Metab 2021;77(Suppl 2):28–35 [DOI] [PubMed] [Google Scholar]
- 19.McCormack AL, Thiruchelvam M, Manning-Bog AB, et al. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002;10(02):119–127 [DOI] [PubMed] [Google Scholar]
- 20.Wallen ZD, Demirkan A, Twa G, et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun 2022;13(01):6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neurinflammation in a model of Parkinson’s disease. Cell 2016;167(06):1469–1480.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball N, Teo W-P, Chandra S, Chapman J. Parkinson’s disease and the environment. Front Neurol 2019;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feltzin V, Wan K, Cleniker S, et al. Role and impact of the gut microbiota in a Drosophila model for parkinsonism. bioRxiv 2019. Doi: 10.1101/718825 [DOI] [Google Scholar]
- 24.Pesah Y, Pham T, Burgess H, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 2004;131(09):2183–2194 [DOI] [PubMed] [Google Scholar]
- 25.Anselmi L, Bove C, Coleman FH, et al. Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. NPJ Parkinsons Dis 2018;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres ERS, Akinyeke T, Stagaman K, et al. Effects of sub-chronic MPTP exposure on behavioral and cognitive performance and the microbiome of wild-type and mGlu8 knockout female and male mice. Front Behav Neurosci 2018;12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labrie V, Brundin P. Alpha-synuclein to the rescue: immune cell recruitment by alpha-synuclein during gastrointestinal infection. J Innate Immun 2017;9(05):437–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishimoto Y, Zhu W, Hosoda W, Sen JM, Mattson MP. Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in alpha-synulcein mutant mice. Neuromolecular Med 2019;21(03):239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AlzForum AR. Do immune cells promote the spread of α-synuclein pathology? Available at: https://www.alzforum.org/news/conference-coverage/do-immune-cells-promote-spread-synuclein-pathology. Accessed May 10, 2019
- 30.Grathwohl S, Quansah E, Maroof N, et al. Experimental colitis drives enteric alpha-synuclein accumulation and Parkinson-like brain pathology. bioRxiv 2018. Doi: 10.1101/505164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyuyki KD, Cluny NL, Swain MG, Sharkey KA, Pittman QJ. Altered brain excitability and increased anxiety in mice with experimental colitis: consideration of hyperalgesia and sex difference. Front Behav Neurosci 2018;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei D, Zhao N, Xie L, et al. Electroacupuncture and moxibustion improved anxiety behavior in DSS-induced colitis mice. Gastroenterol Res Pract 2019;2019:2345890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet 2021;397(10284):1577–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AF Fact Sheet. Accessed at: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet 2023
- 35.Nandi A, Counts N, Chen S, et al. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: a value of statistical life approach. J eClin Med 2022;51:101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers 2021;7(01):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell KL. Alzheimer’s research reset. Science 2019;366:140–142 [Google Scholar]
- 38.Chandra S, Sisodia SS, Vassar RJ. The gut microbiome in Alzheimer’s disease: what we know and what remains to be explored. Mol Neurodegener 2023;18(01):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varesi A, Pierella E, Romeo M, et al. The potential role of gut microbiota in Alzheimer’s disease: from diagnosis to treatment. Nutrients 2022;14(03):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bairamian D, Sha S, Rolhion N, et al. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol Neurodegener 2022;17(01):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kundu P, Stagaman K, Kasschau K, et al. Fecal implants from AppNL-G-F and AppNL-G-F/E4 donor mice sufficient to induce behavioral phenotypes in germ-free mice. Front Behav Neurosci 2022;16:791128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundu P, Torres ERS, Stagaman K, et al. Integrated analysis of behavioral, epigenetic, and gut microbiome analyses in AppNL-G-F, AppNL-F, and wild type mice. Sci Rep 2021;11(01):4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marizzoni M, Cattaneo A, Mirabelli P, et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis 2020;78(02):683–697 [DOI] [PubMed] [Google Scholar]
- 44.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 2004;25(05):641–650 [DOI] [PubMed] [Google Scholar]
- 45.Mahley RW, Apolipoprotein E. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988;240(4852):622–630 [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol 2018;18(12):759–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE epsilon4 on object recognition and spatial navigation in the elderly. Neuroscience 2007;147(01):6–17 [DOI] [PubMed] [Google Scholar]
- 48.Torres ERS, Luo J, Boehnlein JK, et al. Apolipoprotein E isoform-specific changes related to stress and trauma exposure. Transl Psychiatry 2022;12(01):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran TTT, Corsini S, Kellingray L, et al. APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J 2019;33(07):8221–8231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dodiya HB, Kuntz T, Shaik SM, et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J Exp Med 2019;216(07):1542–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022;71(11):2233–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C, Ahn EH, Kang SS, Liu X, Alam A, Ye K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci Adv 2020;6(31):eaba0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karjalainen J-P, Mononen N, Hutri-Kähönen N, et al. New evidence from plasma ceramides links apoE polymorphism to greater risk of coronary artery disease in Finnish adults. J Lipid Res 2019;60(09):1622–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Hardenberg S, Gnewuch C, Schmitz G, Borlak J. ApoE is a major determinant of hepatic bile acid homeostasis in mice. J Nutr Biochem 2018;52:82–91 [DOI] [PubMed] [Google Scholar]
- 55.MahmoudianDehkordi S, Arnold M, Nho K, et al. ; Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement 2019;15(01):76–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kao Y-C, Ho P-C, Tu Y-K, Jou IM, Tsai KJ. Lipids and Alzheimer’s disease. Int J Mol Sci 2020;21(04):1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol 2019;10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69(05):363–385 [DOI] [PubMed] [Google Scholar]
- 59.Hardy SJ, Krull KR, Wefel JS, Janelsins M. Cognitive changes in cancer survivors. Am Soc Clin Oncol Educ Book 2018;38:795–806 [DOI] [PubMed] [Google Scholar]
- 60.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res 2009;15(17):5534–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003;12(06):612–619 [DOI] [PubMed] [Google Scholar]
- 62.Pencheva N, Tran H, Buss C, et al. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell 2012;151(05):1068–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ostendorf BN, Bilanovic J, Adaku N, et al. Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat Med 2020;26(07):1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ifere GO, Desmond R, Demark-Wahnefried W, Nagy TR. Apolipoprotein E gene polymorphism influences aggressive behavior in prostate cancer cells by deregulating cholesterol homeostasis. Int J Oncol 2013;43(04):1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 2019;51(11):2391–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bardia A, Arieas ET, Zhang Z, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat 2012;131(03):907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGinnis GJ, Holden S, Yu B, et al. Association of fall rate and functional status by APOE genotype in cancer survivors after exercise intervention. Oncotarget 2022;13:1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clauss M, Gérard P, Mosca A, Leclerc M. Interplay between exercise and gut microbiome in the context of human health and performance. Front Nutr 2021;8:637010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017;2017:3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev 2019;47(02):75–85 [DOI] [PubMed] [Google Scholar]
- 71.Pernigoni N, Zagato E, Calcinotto A, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 2021;374(6564):216–224 [DOI] [PubMed] [Google Scholar]
- 72.Sari Motlagh R, Quhal F, Mori K, et al. The risk of new onset dementia and/or Alzheimer disease among patients with prostate cancer treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol 2021;205(01):60–67 [DOI] [PubMed] [Google Scholar]
- 73.Raber J. Differential gene actions of polymorphic alleles at the APOE locus; potential role of androgens and androgen receptor-mediated signaling. Science of Aging (SAGE) Knowledge Environment (KE) 2004; posted March 17, 2004. Available at: 10.1126/sageke.2004.11.re2 [DOI] [Google Scholar]
- 74.Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine 2022;82:104163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359(6371):91–97 [DOI] [PubMed] [Google Scholar]
- 76.Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019;178(04):795–806.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deleemans JM, Chleilat F, Reimer RA, et al. The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019;19(01):1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh B, Boyle F, Pavlakis N, et al. Emerging evidence of the gut microbiome in chemotherapy: a clinical review. Front Oncol 2021;11:706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang J, Liu W, Kang W, et al. Effects of microbiota on anticancer drugs: current knowledge and potential applications. EBioMedicine 2022;83:104197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chrysostomou D, Roberts LA, Marchesi JR, Kinross JM. Gut microbiota modulation of efficacy and toxicity of cancer chemotherapy and immunotherapy. Gastroenterology 2023;164(02):198–213 [DOI] [PubMed] [Google Scholar]
- 81.Lee KA, Luong MK, Shaw H, Nathan P, Bataille V, Spector TD. The gut microbiome: what the oncologist ought to know. Br J Cancer 2021;125(09):1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, Zhang Y, Wei K, et al. Review: effect of gut microbiota and its metabolite SCFAs on radiation-induced intestinal injury. Front Cell Infect Microbiol 2021;11:577236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cervantes JL, Hong BY. Dysbiosis and immune dysregulation in outer space. Int Rev Immunol 2016;35(01):67–82 [DOI] [PubMed] [Google Scholar]
- 84.Ritchie LE, Taddeo SS, Weeks BR, et al. Space environmental factor impacts upon murine colon microbiota and mucosal homeostasis. PLoS One 2015;10(06):e0125792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casero D, Gill K, Sridharan V, et al. Space-type radiation induces multimodal responses in the mouse gut microbiome and metabolome. Microbiome 2017;5(01):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raber J, Yamazaki J, Torres ERS, et al. Combined effects of three high energy charged particle beams important for space flight on brain, behavioral and cognitive endpoints in B6D2F1 female and male mice. Front Physiol 2019;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 2011;23(03):187–192 [DOI] [PubMed] [Google Scholar]
- 88.Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol 2020;19(02):179–194 [DOI] [PubMed] [Google Scholar]
- 89.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019;129(10):4050–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franzini M, Fornaciari I, Rong J, et al. Correlates and reference limits of plasma gamma-glutamyltransferase fractions from the Framingham Heart Study. Clin Chim Acta 2013;417:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiraishi M, Tanaka M, Okada H, et al. Potential impact of the joint association of total bilirubin and gamma-glutamyltransferase with metabolic syndrome. Diabetol Metab Syndr 2019;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheng S, Yan S, Chen J, et al. Gut microbiome is associated with metabolic syndrome accompanied by elevated gamma-glutamyl transpeptidase in men. Front Cell Infect Microbiol 2022;12:946757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding J-H, Jin Z, Yang XX, et al. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol 2020;26(40):6141–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giannisis A, Patra K, Edlund AK, et al. Brain integrity is altered by hepatic APOE ε4 in humanized-liver mice. Mol Psychiatry 2022;27(08):3533–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kessler K, Giannisis A, Bial G, Foquet L, Nielsen HM, Raber J. Behavioral and cognitive performance of humanized APOEε3/ε3 liver mice in relation to plasma apolipoprotein E levels. Sci Rep 2023;13(01):1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lam V, Takechi R, Hackett MJ, et al. Synthesis of human amyloid restricted to liver results in an Alzheimer disease-like neurodegenerative phenotype. PLoS Biol 2021;19(09):e3001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maarouf CL, Walker JE, Sue LI, Dugger BN, Beach TG, Serrano GE. Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS One 2018;13(09):e0203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nho K, Kueider-Paisley A, Ahmad S, et al. ; Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium. Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open 2019;2(07):e197978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19(01):55–71 [DOI] [PubMed] [Google Scholar]
- 100.Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018;50(04):747–757 [DOI] [PubMed] [Google Scholar]
- 101.Taniguchi H, Tanisawa K, Sun X, et al. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol Rep 2018;6(23):e13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morita E, Yokoyama H, Imai D, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients 2019;11(04):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease–a review. Adv Nutr 2019;10(06):1040–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65(11):1812–1821 [DOI] [PubMed] [Google Scholar]
- 105.Flanagan E, Cameron D, Sobhan R, et al. Chronic consumption of cranberries (Vaccinium macrocarpon) for 12 weeks improves episodic memory and regional brain perfusion in healthy older adults: a randomised, placebo-controlled, parallel-groups feasibility study. Front Nutr 2022;9:849902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koblinsky ND, Power KA, Middleton L, Ferland G, Anderson ND. The role of the gut microbiome in diet and exercise effects on cognition: a review of the intervention literature. J Gerontol A Biol Sci Med Sci 2023;78(02):195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite 2013;63:119–128 [DOI] [PubMed] [Google Scholar]
- 108.Parrott MD, Carmichael PH, Laurin D, et al. The association between dietary pattern adherence, cognitive stimulating lifestyle, and cognitive function among older adults from the Quebec Longitudinal Study on Nutrition and Successful Aging. J Gerontol B Psychol Sci Soc Sci 2021;76(03):444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health!. Phytochemistry 2004;65(10):1317–1330 [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Bobe G, Revel JS, et al. Improvements in metabolic syndrome by xanthohumol derivatives are linked to altered gut microbiota and bile acid metabolism. Mol Nutr Food Res 2020;64(01):e1900789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kirkwood JS, Legette LL, Miranda CL, Jiang Y, Stevens JF. A metabolomics-driven elucidation of the anti-obesity mechanisms of xanthohumol. J Biol Chem 2013;288(26):19000–19013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis 2021;7(01):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miranda CL, Johnson LA, de Montgolfier O, et al. Non-estrogenic xanthohumol derivatives mitigate insulin resistance and cognitive impairment in high-fat diet-induced obese mice. Sci Rep 2018;8(01):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kundu P, Holden S, Paraiso IL, et al. ApoE isoform-dependent effects of xanthohumol on high fat diet-induced cognitive impairments and hippocampal metabolic pathways. Front Pharmacol 2022;13:954980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu W, He K, Wu D, et al. Natural dietary compound xanthohumol regulates the gut microbiota and its metabolic profile in a mouse model of Alzheimer’s disease. Molecules 2022;27(04):1281. [DOI] [PMC free article] [PubMed] [Google Scholar]


