ABSTRACT
Background
The oral microbiome is a complex and dynamic assemblage of microorganisms that colonize different sites of the oral cavity maintaining both oral and systemic health. Therefore, when its composition is altered, oral diseases occur. Among oral inflammatory pathologies, periodontal diseases affect the tissues surrounding the teeth, representing the main cause of tooth loss and one of the most important threats to the oral health. Lifestyle and eating habits influence the composition of the human oral microbiota and the development and progression of oral diseases. In this context, the Mediterranean Diet (MD) model, comprising both healthy dietary choices and lifestyle, is linked to the prevention of several metabolic and chronic-degenerative pathological processes, including oral diseases. Indeed, the MD is a plant-based diet, enriched of anti-inflammatory and antioxidant nutrients, which may induce beneficial effects against dental caries and periodontal diseases.
Aim
This review summarizes the role of the oral microbiome in the development of the oral diseases and the potential of MD in modulating the oral microbiome leading to implications for oral health.
Conclusions
The data collected highlight the need to promote the MD pattern along with the correct hygiene habits to prevent the development of oral diseases.
KEYWORDS: Mediterranean Diet, oral microbiome, oral diseases, periodontal diseases, chronic metabolic diseases, dental plaque, biofilm, nutrients, oral health implications
3 key messages
The oral microbiome is a complex and dynamic assemblage of microorganisms that colonize different sites of the oral cavity maintaining both oral and systemic health.
The Mediterranean Diet (MD) model, comprising both healthy dietary choices and lifestyle, is linked to the prevention of several metabolic and chronic degenerative pathological processes, including oral diseases.
The MD may represent a potential player in the link between oral microbiome and oral diseases.
Introduction
Oral microbiome, representing the second largest and diverse microbiome after the gut, refers to the collective microorganisms seeded in the oral cavity. It accounts for approximately 700 species of bacteria, fungi, viruses, archaea, and protozoa [1], which colonize teeth, tongue, cheeks, gingival sulcus, tonsils, hard and soft palate, as well as the surfaces of the oral cavity [2,3]. The tropism of each species of microorganism to the surface of the mouth, i.e. the ability of the microorganism to interact positively with the host surface, depends on the expression of adhesion proteins, which recognize the receptors on the oral surface and, depending on the substrate, it expresses a different pathogenic potential [4]. The Human Microbiome Project has identified an oral core microbiome, shared among individuals that changes across a life-history stages of the host [5]. In addition, a variable microbiome unique for each subject, which depends on the lifestyle and genotypic determinants, has been found [6]. The oral microbiota interacts with the host to reflect the immunity and metabolic status through two-way communication along the oral cavity and the systemic organs. These phenomena are extremely important in maintaining the balance of the microbiota, such as the ratio of ‘commensals’ Veilonelle, which produce vitamin K used by B. melaninogenicus, or the ‘antagonistic’ relationship of different Streptococci, which produce bacteriocins capable of being active on bacteria of the same or different species [7]. Thus, the oral cavity is one of the most important interaction windows between the human body and the environment [8]. Alterations of the oral microbiome led to a wide range of pathological conditions, including oral and systemic diseases [9,10]. Several exogenous factors, such as diet, can influence the composition of the microbiome [11], suggesting the important role of a healthy dietary pattern in maintaining eubiosis of the oral microbiota.
The Mediterranean Diet (MD) is a plant-based diet, which has been recognized as one of the healthiest dietary patterns worldwide [12]. MD showed beneficial effects in the prevention and development of several diseases, including the non-communicable diseases (NCDs), such as diabetes, metabolic syndrome, cardiovascular and neurodegenerative diseases, and cancer [13]. It has been found that oral diseases are associated with the most common NCDs [14]. Thus, the oral health needs to be promoted in order to decrease the development of NCDs. Interestingly, the MD showed positive effects against oral diseases because of the bioactive molecules characterizing the Mediterranean foods [15,16]. However, it is still not completely understood whether the MD might modify the oral microbiome composition affecting the oral diseases.
Here, we summarize the role of the oral microbiome in the development of the oral diseases and the potential of MD in modulating the oral microbiome leading to implications for oral health.
The MD pattern
The MD is one of the most studied dietary patterns associated with many benefits for human health worldwide [12]. The origins of the traditional MD were found in the civilizations surrounding the Mediterranean Sea, closely reflecting the social behaviors and lifestyles of that geographic area [17]. Although the different food cultures vary according to the Mediterranean region, a basic Mediterranean dietary pattern can be identified, making the MD a diversified heritage [17]. The MD eating pattern is characterized by abundance of plant food consumption represented by fruits, vegetables, breads and other cereals (traditionally minimally refined), legumes, nuts, seeds, and extra virgin olive oil (EVO) [18]. MD pattern also includes the consumption of moderate amounts of eggs and dairy products (principally low-fat cheese and yogurt), low-to-moderate amounts of fish and poultry, low amounts of red meat, and modest consumption of wine, normally with meals [19,20]. The MD food recommendations are graphed using the typical MD pyramid, which indicates the weekly and daily consumption frequencies of each food of the MD pattern [20]. Particularly, the food groups that should be consumed in greatest quantities appear in the largest section of the pyramid; conversely, foods that should be consumed in small quantities are represented in the narrowest part of the pyramid [18]. According to the European and US Dietary Guidelines, a healthy diet is based on approximately 55–60% of carbohydrates mainly represented by high-complex carbohydrate intake, like grains and legumes, 10–15% of proteins with lower consumption of animal than plant origin proteins, and 25–30% of total dietary fat intake. Regarding fats, no more than 7–8% of total calories should be saturated fats and cholesterol less than 300 mg/day with moderate consumption of EVO as main source of monounsaturated fatty acids. In addition, both omega-3 and omega-6 polyunsaturated fatty acids have to be ingested through diet, predominantly from marine organisms or from seeds [21].
After the recognition of the MD as an Intangible Cultural Heritage of Humanity by UNESCO in 2010, new food recommendations have been proposed resulting in a new revised graphic representation of the pyramid [22]. Particularly, the scientific experts of the Mediterranean Diet Foundation’s International Scientific Committee together with the Forum on Mediterranean Food Cultures, giving the changes in the lifestyle, dietary, socio-cultural, and environmental habits of the global population, have adapted the food recommendations considering the various geographical, socio-economic, and cultural contexts of each Mediterranean region [18]. As a consequence, cultural and lifestyle elements were introduced in the MD pyramid as important elements of the MD pattern. These concepts include physical activity, adequate rest, seasonality, biodiversity, traditional and local food products, and socialization (Figure 1).
Figure 1.

Mediterranean Diet pyramid and daily recommended macronutrient intake.
Interestingly, the MD has been proposed as a ‘gold standard’ diet not only due to its major health and nutrition benefits but also its lower environmental impact and richness in biodiversity, sociocultural food value, and its positive local economic returns. Indeed, since MD is mainly a plant-based diet with low consumption of animal products, it is characterized by a smaller water footprint and lower greenhouse gas emissions compared with other current dietary patterns [23–27].
Despite the recommendation of the MD pattern, about 70% of the population in Mediterranean countries exceeds in the consumption of saturated fats, sodium, and added sugars, highlighting the need to promote the adoption of a healthy eating pattern in the general population [28].
The beneficial effects of the tablet against NCDs
NCDs, including diabetes, cardiovascular and neurodegenerative diseases, as well as cancers, are global health problems since they have an important impact on the quality and life expectancy and cause death and disability to millions of people worldwide [22]. NCDs share four major risk factors: smoking, physical inactivity, alcohol consumption, and unhealthy diets [29]. Controlling these risk factors represents the goal to reduce the burden of NCDs [29,30].
Since the pioneer Seven Countries Study by Ancel Keys [31], many scientific evidences highlighted the protective effects of the MD on metabolic as well as chronic and degenerative diseases [32–37]. Indeed, it has been demonstrated that several healthy foods and nutrients included in MD have been inversely associated with incidence of type-2 diabetes [38–40]. Similarly, a high adherence to the MD pattern is associated with a significant reduction in mortality from cardiovascular and neurodegenerative diseases and cancer [41–45]. Particularly, a critical review by Martinez Gonzales et al. reported strong evidences supporting MD as an ideal approach for cardiovascular health [46]. Findings from a recent randomized clinical trial, the CORDIOPREV study, demonstrated that MD prevented major cardiovascular events respect to a low-fat diet, suggesting the adoption of this eating model in long-term secondary prevention of cardiovascular disease [47]. Interestingly, a high adherence to the MD showed beneficial effects in population regardless ethnic groups, geographical, and socio-cultural settings [48]. For example, a lower score for MD adherence was found in inhabitants from the southeastern Spain compared to other Spanish areas, which was strongly associated with increased prevalence of hypertension [49]. Interestingly, a study conducted in a cohort of Anglo-Celts and Greek-Australians living in Melbourne showed that MD adherence was associated with longer survival even in a non-Mediterranean country [50]. Data on the degree of MD adherence across adults from different United States regions demonstrated that the adherence to MD was geospatially different affecting the prevalence of NCDs [51].
The beneficial effects of the MD foods depend on the presence of phytochemical compounds, which can modulate different biological functions in normal and altered cells. Several molecules found in the MD foods exert antioxidant, anti-inflammatory, antibacterial, pro or antiapoptotic, and vasodilatory activities, mediating anti-tumor, cardioprotective, and neuroprotective actions [52]. For example, it has been demonstrated that polyphenols and other phenylpropanoids present in fruits, vegetables, cereals, legumes, olive oil, cocoa, and plants-based drinks (for example tea, wine, and coffee), acting independently or synergistically, prevent the onset of several NCDs [53,54].
A meta-analysis of 2650 patients showed that the MD provided a more robust reduction in cardiovascular disease risk factors and inflammatory markers because of the consumption of the marine omega-3 fatty acids, which intake is widely recommended in the MD pattern [55]. The mechanisms by which fish prevent cardiovascular diseases are, at least in part, understood and include the inhibition of inflammation, oxidation, and coagulation processes, which lead to the improvement of the lipid profiles and the reduction of the blood pressure [56–58]. Particularly, the biomolecules responsible for these activities are mainly represented by omega-3 fatty acids, which modulate the activation of intracellular signaling pathways involved in different pathophysiological conditions [59].
Alzheimer’s, Parkinson’s, and other forms of cognitive decline extending from mild-cognitive impairment to vascular dementia represent some of the most concerning neurodegenerative diseases involving over million people [60,61]. Several Mediterranean dietary components have been studied to evaluate whether their potential antioxidant, anti-inflammatory, and vasodilatory effects may modify cognitive decline, as revised by Dominguez et al. [62]. Among the micronutrients of the MD foods, vitamins B6, B12, C, D, E, beta-carotene, folic acid, omega-3 fatty acids, zinc, and magnesium have shown neuroprotective effects and may be considered as optimal compounds for the maintenance of brain health [63].
A meta-analysis including over 1.7 million participants have reported that high adherence to MD was associated with a significantly lower risk of all-cause of mortality for different types of cancer, including breast, gastrointestinal (such as liver and pancreas), head and neck, prostate, and pulmonary cancer [64]. Particularly, data from PREDIMED trial have demonstrated that the highest nuts consumption, typical component of MD model, was associated with a 40% decrease in cancer mortality compared to the lowest consumption, as well as a long-term dietary intervention with an MD supplemented with EVO may reduce the risk of breast cancer [65,66].
MD and oral diseases
Among NCDs, chronic oral diseases are largely preventable pathological conditions, which represent a serious health and economic burdens worldwide [67]. In 2017, approximately 50% of the world’s population suffered from oral diseases [68]. Specifically, dental caries, periodontal diseases, teeth loss, and cancers of the lips and oral cavity are the most prevalent and concerning issues [68]. Adhering to a healthy eating pattern might prevent the onset of this type of disorders [69]. In particular, the MD rich in anti-inflammatory and antioxidant foods has beneficial effects in patients with periodontal inflammation. Indeed, Bartha et al. have demonstrated a significant decrease in the bleeding on probing, gingival index, and periodontal inflamed surface in periodontal inflammatory patients following the MD for 6 months [70]. In contrast, an increased gingival inflammatory response was observed in people following a Western-type diet, characterized by high consumption of refined grains, sugar, and omega-6 fatty acids [71,72]. Interestingly, reduced serum omega-6 levels were detected, determining a lower omega-6/omega-3 ratio, which may be responsible for the improvement in their gingival inflammatory parameters [73]. Supporting the importance of consuming omega-3 fatty acids, results from a cross-sectional study demonstrated that consumption of olive oil showed protective effects against periodontitis in young Moroccan, although no significant association was found between adherence to the MD and periodontitis [74]. As above reported, the MD is not only a diet, but it is a healthy lifestyle based on a balanced eating habit and regular physical activity [75]. Marruganti et al. demonstrated an inverse correlation among the MD adherence, physical activity, and periodontitis onset. In particular, subjects with lower MD adherence and moderate physical activity showed worse biometric and inflammatory parameters along with increased mobility and teeth loss due to periodontitis than higher MD adherers [76]. The negative correlation between Stage-III/IV periodontitis and the MD adherence in patients was mainly due to their reduced consumption of wholegrain products. Of note, lower adherers to the MD declaring lower wholegrain product intakes showed eightfold increase of Stage-III/IV periodontitis, along with decreasing insulin sensitivity and increasing low-grade systemic inflammation [77]. It is therefore possible to suppose, from this point of view, a correlation between periodontal pathology, the anti-inflammatory power of various foods, antioxidant molecules contained in them, which can have an action not only through topical application but also systemically through the blood circulation. Indeed, in a recent systematic review, the positive effects of the natural phytochemicals, due to their documented antioxidant properties, have been correlated with improvements in periodontitis [78]. To date, it has been well addressed the correlation of single components or nutrients of the MD and the risk of oral pharyngeal cancer (OCP) [79]. However, only few studies have analyzed the impact of the MD pattern on the OCP cancer risk [80]. Specifically, Filomeno et al. reported that MD pattern reduced upper aerodigestive tract (UADT) cancer risk, whereas the impact of the individual dietary components of the MD was not sufficient to prevent UADT cancers, including oral cavity and oropharynx, larynx, and oesophagus cancers [80]. The protective role of MD in preventing OCP cancer is linked to the high intakes of foods enriched of biological compounds, which exert healthy effects. In fact, fruits and vegetables have reduced the risk of OCP cancer [81–83] because of their high content of carotenoids, vitamin C and E, as well as flavonoids [84]. In addition, EVO, enriched of antioxidant compounds including polyphenols, has a positive influence in reducing the risk of UADT cancers, including the OCP cancer [85]. Consistent with these data, dietary polyphenols have been found to prevent the periodontal tissue destruction maintaining the balance between oxidative stress and antioxidant activity in the oral cavity [86].
From the point of view of the development of carious pathology, with a multifactorial etiology, it has been demonstrated that MD is able to significantly reduce the appearance of carious lesions even at an early age [87]. Considering the relevant public health problem, to correlate the risk factors of tooth decay with a diet capable of limiting the acid action and fermentation of simple sugars by the oral microbiota, modulating it and making the systemic supply of protective factors constant represent a topic of great interest [88].
Despite the health benefits of the MD in the prevention of oral diseases, it is worth to note that regular oral hygiene practices, such as regular brushing and dental check-ups, are essential for maintaining oral health [89]. In this context, it has been observed that poor adherence to MD is associated with insufficient oral hygiene, characterized by higher bleeding on probing and plaque indexes, regardless to age, sex, physical activity, and diabetes mellitus as variables in multiple-regression analysis [89]. In addition, a positive association between the eating habits mirroring the MD food choices and healthy oral habits was found among kidney transplant recipients, which usually suffer from severe forms of periodontitis [90]. Specifically, patients with a higher MD adherence score had a higher number of teeth as well as an increased dental plaque removal [90]. These findings suggest that maintaining a proper dietary regimen, such as the MD, may contribute to improve oral health outcomes. Collectively, the health benefits of the MD on metabolic and chronic diseases, including oral diseases, are summarized in Figure 2.
Figure 2.
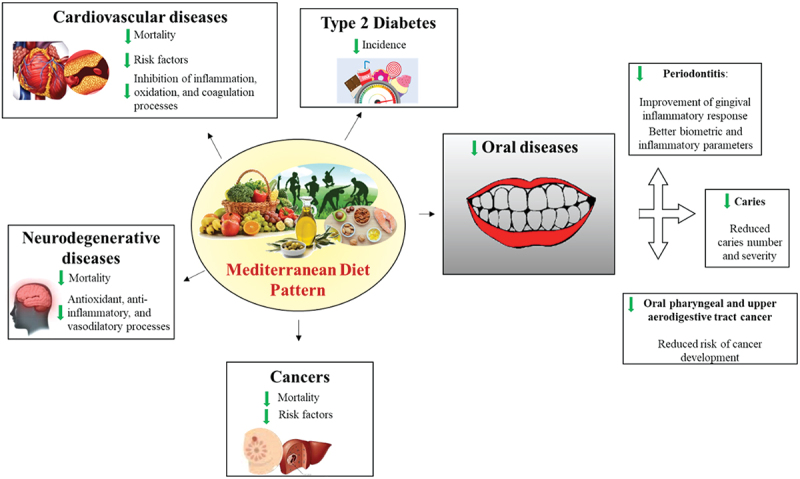
Impact of the MD pattern on the metabolic and chronic diseases, including oral diseases.
Etiology of oral diseases
Periodontal diseases are oral inflammatory diseases affecting the tissues supporting and surrounding the teeth and include gingivitis and periodontitis [91]. This same pathological condition, but in a clinically more serious and accelerated form, also occurs at the dental implant level, known as mucositis and peri-implantitis, similar to gingivitis and periodontitis, which significantly compromise the outcome of the rehabilitation therapy [92–94]. Although gingivitis is associated with bleeding, swollen gums, and pain in its progression, periodontitis is related to the loss of periodontal attachment and supporting bone, often asymptomatic [95]. However, if untreated, periodontitis can lead to tooth loss, with a consequent impairment in mastication function, esthetics, self-confidence, and quality of life [96,97]. The same important limitations can be caused by the progression and development of the carious pathology, which demonstrates a different multifactorial etiology compared to periodontitis and compresses the hard tissues of the tooth, often keeping the supporting bone and periodontal ligament intact [96].
The prevalence of periodontal diseases is estimated to range from 20 to 50% worldwide, emerging as the 11th most prevalent condition in the world as reported by the Global Burden of Disease Study of 2016 [29]. Heterogeneity among studies analyzed by Borg-Bartolo et al. among the different regions of the world was 99.7% (prevalence of edentulism) and 99.9% (prevalence of dental caries) [98]. The global estimated random-effects pooled prevalence of edentulism was 22%, which allows defining this pathology as a public health problem [98]. Based on this information, to understand what role the MD can have, it is necessary to understand which mechanisms underlie the etiopathogenesis of periodontal diseases and caries, also considering the different oral pathogens involved. Periodontal diseases have been shown to be related to low-grade systemic inflammation, potentially driven by several inflammatory mediators [91,99,100]. The possibility of studying the levels of peripheral tissue inflammation, before the signs of periodontal pathology are manifest (marginal bone loss, increased probing depth), represents to date the most important possibility for avoiding irreversible damage to the supporting tissues. In this context, early diagnosis performed through atraumatic analysis of mediators of peripheral tissue inflammation represents the main option to achieve an early diagnosis, and from this perspective, further studies are necessary to achieve an even more defined correlation relationship between the different markers of inflammation and clinical indicators of pathology [101]. Recent studies showed that patients affected by periodontal diseases are characterized by higher circulating levels of C-reactive protein, fibrinogen, neutrophils, and indirect systemic inflammatory markers, such as tumor necrosis factor and interleukin (IL) 1, IL-6, and IL-8 [102,103]. It is interesting to note that, in oral tissues, nutrition exerts a combined action, both at local and systemic level [104]. Excluding the process of formation of dental tissues, which can undergo significant alterations for various reasons and lead to conditions that are cariogenic, the caries process derives from the absorption of fermentable carbohydrates, including sucrose, glucose, fructose, lactose, maltose, and starch. These fermentable carbohydrates may have both local and systemic effects on dental caries, and these effects differ considerably depending on the conditions of the subjects, in particular depending on the age and the dental surfaces exposed (root/crown) [104]. Considering the complex action that macro and micronutrients carry out at a systemic level, the maintenance of periodontal health depends on the optimal nutrition with respect to both. Clearly, different foods, by local and systemic action, exert more complex interactions for the development of the carious process [105]. As gingival tissues are sentinel of overall systemic health, the absence of both dental caries and gum bleeding in the absence of oral hygiene could be considered a potentially sensitive indicator for an overall healthy diet [104].
Oral microbiome in the development of oral diseases
Research has focused more on studying periodontitis and systemic diseases, exploring the role of oral inflammation and microbiota in conditions like atherosclerosis, diabetes mellitus, pneumonia, chronic obstructive pulmonary disease, rheumatoid arthritis, and Alzheimer’s disease [106–108]. While both dental caries and periodontitis are biofilm-mediated diseases, their pathogenesis differs. Dental caries involves multiple factors leading to localized demineralization of teeth, and the potential systemic consequences of untreated dental caries and the associated oral microbial-inflammatory process require further investigation through human and animal studies. The spread of the oral microbiome into the systemic circulation from dental caries is plausible, and parallel mechanisms could have observed in periodontal diseases. In dental caries, the root canal space or marginal periodontium are the most likely pathways for direct systemic extension of oral microbiota [9]. The oral microbial ecosystem is constantly exposed to exogenous substances, which concur to maintain the fine-tuned equilibrium of the oral health, or presents opportunities for oral microbial dysbiosis leading to oral and systemic diseases [109].
Starting from this point of view, dental plaque is defined as a biofilm composed of complex polymicrobial communities. This biofilm, responsible for various alterations at the dental level, receives most of its nourishment from the diet, which provides nutritional resources for the oral microbiota and serves also as a selective pressure by enriching for organisms best adapted to utilize specific host-derived dietary resources [110]. Major historical dietary shifts throughout evolution are accompanied by significant changes in the oral microbiota [111], which led to a selection of acid-producing and acid-tolerant organisms and periodontal pathogens [112]. Often, these bacteria represent pathobionts, capable of playing different and synergistic roles in the development of oral or systemic diseases depending on the general state of health of the host. It is also interesting to note how the conditions that modify the environment and the nourishment granted to the microbiome are different and vary throughout life, and in any case significantly during ageing [109]. It is possible to define carious or periodontal oral pathologies as a microbiome dysbiosis of the site under consideration, which can be caused by site-specific factors, systemic factors, or a combination of both [113]. Dental caries is particularly related to a high dietary intake of carbohydrates, leading to an increased production of acid by microbes (which hinder the buffering capabilities of saliva), a decrease in salivary pH, a greater production of biofilm exopolysaccharide matrix (that entraps and concentrates acids on enamel surfaces), and induction of positive-feedback loops that encourage outgrowth of aciduric and acidogenic species, including Streptococcus mutans and Lactobacillus species [113,114]. Although the evaluation of the microbiome involved in the etiopathogenesis of carious pathology is complex, that involved in periodontal pathology is even more complex, also considering the mutual interactions between the different bacteria it contains [115]. Taking into consideration for example the colonization by Corynebacterium, long filaments are developed on which distinct microenvironments are created, and streptococci at the periphery of oral biofilms produces lactate, which serves as a preferred substrate for catabolism by Veillonella, Corynebacterium, and Eubacterium species [113].
Dental caries is mainly caused by oral biofilm acid, and composite dental restorations are the most common treatment [116]. The main cause of failure is secondary caries adjacent to the restoration, and long-term survival of dental materials is improved by the presence of antibacterial agents, which selectively inhibit bacterial growth or survival [116,117]. Chemical, natural, and biomaterials have been studied for their antimicrobial activities and antibacterial bonding agents have been improved, in order to obtain an important antibacterial potential at the weakest interface of the restorative complex [118]. Through these new findings, it is possible to study characteristics, epidemiology, risk factors, clinical manifestations, diagnosis, and innovative treatments for several types of oral infectious diseases [119].
To date, we can define that the sub-gingival biofilm is also absolutely dependent on the biofilm found at the supra-gingival level, from which it is modified depending on the type of bacteria present and their metabolism depending on the external stimuli to which they are subjected [120]. Recently, it has been found that the Bifidobacterium animalis subsp. lactis (B. lactis) HN019 decreased the periodontal biofilm virulence, the gingival inflammation as well as the number of pathogenic bacteria characterizing the periodontal diseases [121–123]. In a randomized controlled trial, (B. lactis) HN019 reduced the bleeding in patients presenting with peri-implant mucositis, decreasing the levels of pro-inflammatory cytokines in the peri-implant crevicular fluid [124]. The bacteria that coexist at the periodontal level, including periodontopathogens, have been cataloged into different ‘complexes’, depending on their danger and effectiveness in altering the state of periodontal health [125]. To make this evaluation very complex, it is necessary to consider how, for example, Streptococcus gordonii has been shown to increase the virulence of Aggregatibacter Actinomycetem comitans by producing lactate via streptococcal carbon metabolism [126]. The potential interrelationships among microbes make it difficult to evaluate the behavior of a single pathogen, and definitively focus attention on the biofilm and the microbiome, a condition that significantly alters the response of some pathogenic species, which can even enhance the outgrowth of other pathogens through symbiotic relationships. This interplay demonstrates the role of microbial interactions in promoting diseases via multifaceted physical and metabolic interactions that underlie the polymicrobial synergy and dysbiosis model [127].
Due to the shortcomings of several antimicrobial medications frequently prescribed in dentistry, the lack of resources in developing countries, the prevalence of oral inflammatory conditions in diverse oral pathologies, and the ever-increasing growth of bacterial antibiotic resistance, there is a need for reliable, efficient, and affordable alternative solutions for the prevention and treatment of periodontal diseases [128]. Several accessible chemical agents can alter the oral microbiota, although these substances also have unfavorable symptoms, mainly caused by the imbalance of the oral/intestinal microbiota, such as vomiting, diarrhea, and tooth discoloration [129]. Precisely in light of this information, the natural phytochemicals generated from plants that have historically been used as medicines are categorized as prospective alternatives due to the ongoing quest for substitute products, ensuring greater compatibility of treatments [130]. A summary of the main virulence mechanisms of microorganisms most frequently involved in the development of oral diseases is reported in Table 1.
Table 1.
Summary of the main virulence factors of the principle periodontal pathogens.
| Microorganisms | Description | Associated Oral Condition | Ref. | |
|---|---|---|---|---|
| Streptococcus mutans | Primary bacterium linked to dental caries. Flourishes in the acidic environment, producing acids damaging tooth enamel | Dental caries | [131] | |
| Lactobacillus | Contributes to dental caries by producing acids that erode dental enamel | Dental caries | [132] | |
| Bifidobacterium | It can contribute to acid production and tooth enamel damage in dental caries | Dental caries | [133] | |
| Porphyromonas gingivalis | Bacterium associated with gingivitis. Produces enzymes damaging gum tissues, leading to inflammation | Gingivitis | [134] | |
| Treponema denticola | Associated with the progression of gingivitis, producing enzymes breaking down gum tissues and contributing to plaque formation/ Associated with severe periodontitis, produces enzymes contributing to gum tissue destruction and inflammation |
Gingivitis/ periodontitis |
[135,136] | |
| Tannerella forsythia | Causes gum inflammation, generating toxins and enzymes that harm tissues, provoking an inflammatory response/ Implicated in the progression of periodontal disease by producing enzymes and toxins damaging gum tissues |
Gingivitis/ periodontitis |
[135,136] | |
|
Aggregatibacter actinomycetemcomitans |
Linked to gingivitis and periodontitis, produces toxins and proteins damaging gum tissues/ Implicated in aggressive periodontitis, producing toxins causing tissue damage and bone resorption |
Gingivitis/ periodontitis |
[136,137] | |
| Fusobacterium nucleatum | Commonly found in bacterial plaque, exacerbating gum inflammation and contributing to the formation of harmful biofilms/ Commonly found in periodontal pockets, co-aggregating with other pathogenic bacteria, contributing to the progression of the disease |
Gingivitis/ periodontitis |
[136,138] | |
| Prevotella intermedia | Associated with gum inflammation, producing toxins that damage gum tissues and trigger an inflammatory response/ Associated with periodontal disease progression, produces enzymes and toxins contributing to tissue damage |
Gingivitis/ periodontitis |
[136,138] | |
| Porphyromonas gingivalis | Bacterium associated with periodontitis. Produces enzymes and toxins leading to breakdown of gum tissues and bone. | Periodontitis | [136] | |
| Veillonella parvula | Component of a specific bacterial complex observed in the microbial communities of subgingival plaque | Periodontitis | [139] | |
| Corynebacterium | Component of a synergistic and dysbiotic microbial community triggering periodontal disease | Periodontitis | [113] | |
| Eubacterium | Component of a synergistic and dysbiotic microbial community triggering periodontal disease | Periodontitis | [113] | |
Potential role of MD in modulating oral microbiome in oral diseases
Many investigations focused on the discovery of the specific composition and microbial diversity, while only a few studies have investigated the impact that host genetics and environmental factors, including lifestyle, can have on the biological function of the oral microbiota. Based on the data reported in literature, diet could influence the composition of the oral microbiota and the development and progression of oral diseases [140].
So far, much of the research has focused on the impact of the MD in the gut microbiome, demonstrating that a high adherence to the MD leads to the microbiota eubiosis reestablishment, increased microbiota diversity as well as an improved gut-barrier function and permeability, decreasing the risk of chronic diseases such as cardiovascular diseases [141]. Recently, it has been reported that the MD positively affects resting metabolic rate and salivary microbiota enhancing the percentage of Subflava and Prevotella species with respect to Vegan Diet in humans [142]. Notably, the main difference between the two diet regimens is represented by lower protein intake in the Vegan Diet and by higher content of fibers in fruits and vegetables in MD, which explain the abundance of Subflava and Prevotella [142]. Particularly, some nutrients, such as fiber, positively influence the oral eubiosis suggesting the beneficial effects of the MD [143]. The oral cavity serves as a reservoir of Staphylococcus aureus for infection of the lower respiratory tract and cross-infection, suggesting that Staphylococcus aureus continues to be a frequent isolate in the oral cavity and perioral region [144]. Thus, the role of Staphylococcus aureus in the pathogenesis of certain oral diseases should also be considered as part of a complete differential diagnosis [145].
Moreover, it has been found that the consumption of foods and beverage characterized by a high content of polyphenols has antimicrobial activities on oral pathogenic bacteria such as the Staphylococcus aureus and Porphyromonas gingivalis [146,147]. However, some studies have primarily examined the effects of specific micro or macro-nutrients from the MD on the microbiome, rather than considering the overall dietary pattern. The association between micro and macronutrients and the main microorganisms is summarized in Table 2.
Table 2.
Associations between nutrients and oral microbiome.
| Dietary sources | Nutrients | Associated microrganisms | Ref. |
|---|---|---|---|
| Micronutrients | |||
| Meats, fish, and whole grains | Vitamin B1 |
Neisseriaceae Gemellaceae Fusobacterium nucleatum |
[148–151] |
| Meats, green vegetables, dairy products | Vitamin B2 | [149,150] | |
| Eggs, fish, meat, mushrooms, nuts | Vitamin B3 | [149,150] | |
| Beef, chicken, organ meats, broccoli, avocados | Vitamin B5 | [150,152,153] | |
| Meat, vegetables, nuts, banana | Vitamin B6 | [150,154] | |
| Leafy vegetables, peanuts, raw egg, liver, | Vitamin B7 | [150,155] | |
| Leafy vegetables, cereals, legumes, nuts and seeds | Vitamin B9 | [150,151] | |
| Fish, meat, poultry, eggs, and dairy products | Vitamin B12 | [150,156] | |
| Green vegetables (kale, spinach, cabbage, lettuces), egg yolk | Vitamin K | Not identified | [157] |
| Fish eggs, liver, mushrooms, milk | Vitamin D | Fusobacterium nucleatum, Porphyromonas gingivalis, Pseudomonas aeruginosa, Candida spp. | [150,158–162] |
| Citrus fruits, fruits (oranges, kiwi, lemon, grapefruit), kiwis and strawberries; Cruciferous vegetables (broccoli, brussels sprout, cabbage, cauliflower); tomatoes, bell peppers and white potatoes |
Vitamin C |
Neisseriaceae Lepto-trichiaceae Lachnospiraceae Fusobacterium nucleatum |
[74,148,163–167] |
| Dark leafy greens vegetables, egg yolk, vegetable oils, nuts, sunflower seeds, pumpkin | Vitamin E |
Neisseriaceae Fusobacterium nucleatum Gemellaceae |
[74,148,163–168] |
| Vitamin A | Not identified | [74,164] | |
| Milk, dairy products, fish, eggs, leafy vegetables, nuts, seeds |
Calcium | [169,170] | |
| Green leafy vegetables, legumes, nuts, seeds, whole grains | Magnesium | Not identified | [169] |
| Grapefruits, nuts cocoa, tea, dried fruits, oatmeal | Fluoride | [171,172] | |
| Meat, dry beans, spinach, shellfish | Iron | [173] | |
| Fish meat, seafood, spinach, grains | Zinc | [174,175] | |
| Macronutrients | |||
| Fish, meat, milk, dairy products, eggs and pulses | Proteins | Not identified | [176–179] |
| Cereal and derivates (pasta, rice, bread) potatoes, legumes, vegetables, fruits | Carbohydrates |
Lacto-bacillaceae Fusobacterium nucleatum |
[104,148,180–184] |
| Oily fish (salmon, sardines, mackerel, herring, cod liver oil); Vegetables (olive oil, oilseeds, nuts grape seed oil, soya oil, sunflower oil); Butter, cheese, yogurt |
Fats |
Fusobacteria nucleatum Neisseriaceae |
[74,148,161,164,185] |
Despite these findings that support the potential positive effects of the MD in modulating the oral microbiome, our understanding still remains limited. The wide variability in genetic and environmental factors including dietary habits and lifestyle represents a limitation for study design and finding interpretation making difficult to enhance our knowledge in this field. Specifically, further research will be needed to shed light on the mechanisms by which nutrition protects oral microbiome eubiosis maintaining oral health.
Conclusions
A healthy lifestyle, based on the Mediterranean model, impacts on the general health status of human beings, including oral health. Overall, MD is rich of anti-inflammatory and antioxidant compounds, which may induce beneficial effects in patients suffering of oral diseases, mainly represented by dental caries and periodontal diseases and also positively affects oral microbiome. Conversely, an unhealthy diet affects the composition of the oral microbiota, leading to dysbiosis. To date, an interesting and unexplored area regards the potential link among MD, oral diseases, and oral microbiome, which deserves to be further investigated.
The dentist plays a fundamental role in promoting and disseminating the correct dietary habits based on healthy food choices among the population that, together with lifestyle, may significantly improve their general and oral health status.
Funding Statement
This work was supported by the EU Regional Operational Programme Calabria, Italy (POR Calabria FESR-FSE 2014–2020) DI. ME. NU. (prot. #52243/2017) at the Department of Pharmacy, and the Health and Nutritional Sciences, University of Calabria, Italy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Deo PN, Deshmukh R.. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23(1):122–16. doi: 10.4103/jomfp.JOMFP_304_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010, 192(19):5002–5017. doi 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhao H, Chu M, Huang Z, et al. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7(1):11773. doi: 10.1038/s41598-017-11779-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li X, Liu Y, Yang X, et al. The oral microbiota: community composition, influencing factors, Pathogenesis, and interventions. Front Microbiol. 2022;13:895537. doi: 10.3389/fmicb.2022.895537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zarco MF, Vess TJ, Ginsburg GS: The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012, 18(2):109–120. doi: 10.1111/j.1601-0825.2011.01851.x [DOI] [PubMed] [Google Scholar]
- [7].Baty JJ, Stoner SN, Scoffield JA, et al. Oral commensal streptococci: gatekeepers of the oral cavity. J Bacteriol. 2022;204(11):e0025722. doi: 10.1128/jb.00257-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peng X, Cheng L, You Y, et al. Oral microbiota in human systematic diseases. Int J Oral Sci. 2022;14(1):14. doi: 10.1038/s41368-022-00163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sampaio-Maia B, Caldas IM, Pereira ML, et al. The oral microbiome in health and its implication in oral and systemic diseases. Adv Appl Microbiol. 2016, 97:171–210. [DOI] [PubMed] [Google Scholar]
- [10].Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol. 2019;431(16):2957–2969. doi: 10.1016/j.jmb.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McLean JS. Advancements toward a systems level understanding of the human oral microbiome. Front Cell Infect Microbiol. 2014;4:98. doi: 10.3389/fcimb.2014.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Martini D. Health benefits of Mediterranean diet. Nutrients. 2019;11(8):11. doi: 10.3390/nu11081802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mentella MC, Scaldaferri F, Ricci C, et al. Cancer and mediterranean diet: a review. Nutrients. 2019, 11(9):11. doi: 10.3390/nu11092059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wolf TG, Cagetti MG, Fisher JM, et al. Non-communicable diseases and oral health: an overview. Front Oral Health. 2021;2:725460. doi: 10.3389/froh.2021.725460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maruca A, Catalano R, Bagetta D, et al. The mediterranean diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur J Med Chem. 2019;181:111579. doi: 10.1016/j.ejmech.2019.111579 [DOI] [PubMed] [Google Scholar]
- [16].Javier Marhuenda H, Pilar Zafrilla R M. Bioactive compounds contained in mediterranean diet and their effects on neurodegenerative diseases. In: Naofumi S, editor. Current topics on superfoods. Rijeka: IntechOpen; 2018. Chapter 2. doi: 10.5772/intechopen.740 [DOI] [Google Scholar]
- [17].Urquiaga I, Echeverria G, Dussaillant C, et al. Origen, componentes y posibles mecanismos de acción de la dieta mediterránea. Rev Med Chil. 2017, 145(1):85–95. doi: 10.4067/S0034-98872017000100012 [DOI] [PubMed] [Google Scholar]
- [18].Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today Science and cultural updates. Public Health Nutr. 2011, 14(12A):2274–2284. doi: 10.1017/S1368980011002515 [DOI] [PubMed] [Google Scholar]
- [19].Kafatos A, Verhagen H, Moschandreas J, et al. Mediterranean diet of Crete: foods and nutrient content. J Am Diet Assoc. 2000, 100(12):1487–1493. doi: 10.1016/S0002-8223(00)00416-8 [DOI] [PubMed] [Google Scholar]
- [20].Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402s–1406s. doi: 10.1093/ajcn/61.6.1402S [DOI] [PubMed] [Google Scholar]
- [21].Russo GL, Siani A, Fogliano V, et al. The Mediterranean diet from past to future: key concepts from the second “ancel keys” international seminar ” International Seminar Nutr Metab Cardiovasc Dis. 2021, 31(3):717–732. doi: 10.1016/j.numecd.2020.12.020 [DOI] [PubMed] [Google Scholar]
- [22].Mediterranean diet, UNESCO . [accessed 2023 Nov]. https://ich.unesco.org/en/RL/mediterranean-diet-00884
- [23].Dernini S, Berry EM, Serra-Majem L, et al. Med diet 4.0: the Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017;20(7):1322–1330. doi: 10.1017/S1368980016003177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heller MC, Keoleian GA, Willett WC. Toward a life cycle-based, diet-level framework for food environmental impact and nutritional quality assessment: a critical review. Environ Sci Technol. 2013;47(22):12632–12647. doi: 10.1021/es4025113 [DOI] [PubMed] [Google Scholar]
- [25].Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515(7528):518–522. doi: 10.1038/nature13959 [DOI] [PubMed] [Google Scholar]
- [26].van Dooren C, Marinussen M, Blonk H, et al. Exploring dietary guidelines based on ecological and nutritional values: a comparison of six dietary patterns. Food Policy. 2014;44:36–46. doi: 10.1016/j.foodpol.2013.11.002 [DOI] [Google Scholar]
- [27].Vanham D, Del Pozo S, Pekcan AG, et al. Water consumption related to different diets in Mediterranean cities. Sci Total Environ. 2016;573:96–105. doi: 10.1016/j.scitotenv.2016.08.111 [DOI] [PubMed] [Google Scholar]
- [28].Vitale M, Racca E, Izzo A, et al. Adherence to the traditional Mediterranean diet in a population of south of Italy: factors involved and proposal of an educational field-based survey tool. Int J Food Sci Nutr. 2019;70(2):195–201. doi: 10.1080/09637486.2018.1481202 [DOI] [PubMed] [Google Scholar]
- [29].Vos T, Abajobir AA, Abate KH. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017, 390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Béland M, Lavoie KL, Briand S, et al. Aerobic exercise alleviates depressive symptoms in patients with a major non-communicable chronic disease: a systematic review and meta-analysis. Br J Sports Med. 2020;54(5):272–278. doi: 10.1136/bjsports-2018-099360 [DOI] [PubMed] [Google Scholar]
- [31].Keys A. Coronary heart disease in seven countries. 1970. Nutrition. 1997;13(3):250–252. discussion 249, 253. doi: 10.1016/S0899-9007(96)00410-8 [DOI] [PubMed] [Google Scholar]
- [32].Augimeri G, Avolio E, Caparello G, et al. Serum from adolescents with high polyphenol intake exhibits improved lipid profile and prevents lipid accumulation in HepG2 human liver cells. Oxid Med Cell Longev. 2023;2023:1555942. doi: 10.1155/2023/1555942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Augimeri G, Galluccio A, Caparello G, et al. Potential antioxidant and anti-inflammatory properties of serum from healthy adolescents with optimal mediterranean diet adherence: findings from DIMENU cross-sectional study. Antioxidants (Basel). 2021;10(8):10. doi: 10.3390/antiox10081172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Augimeri G, Montalto FI, Giordano C, et al. Nutraceuticals in the Mediterranean Diet: potential avenues for breast cancer treatment. Nutrients. 2021, 13(8): 13. doi: 10.3390/nu13082557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ceraudo F, Caparello G, Galluccio A, et al. Impact of mediterranean diet food choices and physical activity on serum metabolic profile in healthy adolescents: findings from the DIMENU Project. Nutrients. 2022, 14(4): 881. doi: 10.3390/nu14040881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x [DOI] [PubMed] [Google Scholar]
- [37].Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- [38].Dominguez LJ, Bes-Rastrollo M, Basterra-Gortari FJ, et al. Association of a dietary score with incident type 2 diabetes: the dietary-based diabetes-risk score (DDS). PLoS One. 2015, 10(11):e0141760. doi: 10.1371/journal.pone.0141760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008, 336(7657):1348–1351. doi: 10.1136/bmj.39561.501007.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann internal med. 2014;160(1):1–10. doi: 10.7326/M13-1725 [DOI] [PubMed] [Google Scholar]
- [41].Lim GB. Mediterranean diet superior to low-fat diet for secondary prevention of CVD. Nat Rev Cardiol. 2022;19(7):432. doi: 10.1038/s41569-022-00727-4 [DOI] [PubMed] [Google Scholar]
- [42].Buckland G, Travier N, Cottet V, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer. 2013;132(12):2918–2927. doi: 10.1002/ijc.27958 [DOI] [PubMed] [Google Scholar]
- [43].Giacosa A, Barale R, Bavaresco L, et al. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev. 2013;22(1):90–95. doi: 10.1097/CEJ.0b013e328354d2d7 [DOI] [PubMed] [Google Scholar]
- [44].Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, et al. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr. 2014;99(1):172–180. doi: 10.3945/ajcn.113.068106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis12. Am J Clin Nutr. 2010;92(5):1189–1196. doi: 10.3945/ajcn.2010.29673 [DOI] [PubMed] [Google Scholar]
- [46].Martinez-Gonzalez MA, Gea A, Ruiz-Canela M. The mediterranean diet and cardiovascular health. Circ Res. 2019;124(5):779–798. doi: 10.1161/CIRCRESAHA.118.313348 [DOI] [PubMed] [Google Scholar]
- [47].Delgado-Lista J, Alcala-Diaz JF, Torres-Pena JD, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399(10338):1876–1885. doi: 10.1016/S0140-6736(22)00122-2 [DOI] [PubMed] [Google Scholar]
- [48].Mattavelli E, Olmastroni E, Bonofiglio D, et al. Adherence to the Mediterranean diet: impact of geographical location of the observations. Nutrients. 2022;14(10):2040. doi: 10.3390/nu14102040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abellán Alemán J, Zafrilla Rentero MP, Montoro-García S, et al. Adherence to the “Mediterranean diet” in Spain and its relationship with cardiovascular risk (DIMERICA study). Nutrients. 2016;8(11):8. doi: 10.3390/nu8110680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kouris-Blazos A, Gnardellis C, Wahlqvist ML, et al. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br J Nutr. 1999;82(1):57–61. doi: 10.1017/S0007114599001129 [DOI] [PubMed] [Google Scholar]
- [51].Chen M, Creger T, Howard V, et al. Geospatial analysis of Mediterranean diet adherence in the United States. Public Health Nutr. 2021;24(10):2920–2928. doi: 10.1017/S1368980020001135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Varoni EM, Lodi G, Sardella A, et al. Plant polyphenols and oral health: old phytochemicals for new fields. Curr Med Chem. 2012;19(11):1706–1720. doi: 10.2174/092986712799945012 [DOI] [PubMed] [Google Scholar]
- [53].Rana A, Samtiya M, Dhewa T, et al. Health benefits of polyphenols: a concise review. J Food Biochem. 2022;46(10):e14264. doi: 10.1111/jfbc.14264 [DOI] [PubMed] [Google Scholar]
- [54].Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81(1):215s–217s. doi: 10.1093/ajcn/81.1.215S [DOI] [PubMed] [Google Scholar]
- [55].Nordmann AJ, Suter-Zimmermann K, Bucher HC, et al. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med. 2011, 124(9):841–851.e842. doi: 10.1016/j.amjmed.2011.04.024 [DOI] [PubMed] [Google Scholar]
- [56].Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189(1):19–30. doi: 10.1016/j.atherosclerosis.2006.02.012 [DOI] [PubMed] [Google Scholar]
- [57].Calder PC: N –3 fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond). 2004, 107(1):1–11. doi: 10.1042/CS20040119 [DOI] [PubMed] [Google Scholar]
- [58].Harris WS, Miller M, Tighe AP, et al. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12–24. doi: 10.1016/j.atherosclerosis.2007.11.008 [DOI] [PubMed] [Google Scholar]
- [59].Kar A, Ghosh P, Patra P, et al. Omega-3 fatty acids mediated cellular signaling and its regulation in human health. Clin Nutri Open Sci. 2023;52:72–86. doi: 10.1016/j.nutos.2023.10.004 [DOI] [Google Scholar]
- [60].Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the Major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4):10. doi: 10.1101/cshperspect.a033118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lamptey RNL, Chaulagain B, Trivedi R, et al. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of Nanotherapeutics. Int J Mol Sci. 2022;23(3):1851. doi: 10.3390/ijms23031851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dominguez LJ, Barbagallo M, Muñoz-Garcia M, et al. Dietary patterns and cognitive decline: key features for prevention. Curr Pharm Des. 2019;25(22):2428–2442. doi: 10.2174/1381612825666190722110458 [DOI] [PubMed] [Google Scholar]
- [63].Muth AK, Park SQ. The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr. 2021;40(6):3999–4010. doi: 10.1016/j.clnu.2021.04.043 [DOI] [PubMed] [Google Scholar]
- [64].Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4(12):1933–1947. doi: 10.1002/cam4.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Guasch-Ferré M, Bulló M, Martínez-González M, et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11(1):164. doi: 10.1186/1741-7015-11-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Toledo E, Salas-Salvadó J, Donat-Vargas C, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED Trial.J Reine und Angew Math. 2015;175(11):1752–1760. doi: 10.1001/jamainternmed.2015.4838 [DOI] [PubMed] [Google Scholar]
- [67].Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–260. doi: 10.1016/S0140-6736(19)31146-8 [DOI] [PubMed] [Google Scholar]
- [68].Watt RG, Daly B, Allison P, et al. Ending the neglect of global oral health: time for radical action. Lancet. 2019;394(10194):261–272. doi: 10.1016/S0140-6736(19)31133-X [DOI] [PubMed] [Google Scholar]
- [69].Molendijk M, Molero P, Ortuno Sanchez-Pedreno F, et al. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. Journal Of Affective Disorders. 2018;226:346–354. doi: 10.1016/j.jad.2017.09.022 [DOI] [PubMed] [Google Scholar]
- [70].Bartha V, Exner L, Schweikert D, et al. Effect of the Mediterranean diet on gingivitis: a randomized controlled trial. J Clinic Periodontol. 2022, 49(2):111–122. doi: 10.1111/jcpe.13576 [DOI] [PubMed] [Google Scholar]
- [71].Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51(5):794–811. doi: 10.1016/j.immuni.2019.09.020 [DOI] [PubMed] [Google Scholar]
- [72].Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005, 81(2):341–354. doi: 10.1093/ajcn.81.2.341 [DOI] [PubMed] [Google Scholar]
- [73].Bartha V, Exner L, Basrai M, et al. Changes in serum omega fatty acids on a Mediterranean diet intervention in patients with gingivitis: an exploratory study. J Periodontal Res. 2022;57(6):1198–1209. doi: 10.1111/jre.13056 [DOI] [PubMed] [Google Scholar]
- [74].Iwasaki M, Ennibi OK, Bouziane A, et al. Association between periodontitis and the Mediterranean diet in young Moroccan individuals. J Periodontal Res. 2021, 56(2):408–414. doi: 10.1111/jre.12833 [DOI] [PubMed] [Google Scholar]
- [75].Salas-Salvado J, Diaz-Lopez A, Ruiz-Canela M, et al. Effect of a lifestyle intervention program with energy-restricted mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus trial. Diabetes Care. 2019, 42(5):777–788. doi: 10.2337/dc18-0836 [DOI] [PubMed] [Google Scholar]
- [76].Pavlidou E, Papadopoulou SK, Alexatou O, et al. Childhood mediterranean diet adherence is associated with lower prevalence of childhood obesity, Specific Sociodemographic, and lifestyle factors: a cross-sectional study in pre-school children. Epidemiologia (Basel). 2023;5(1):11–28. doi: 10.3390/epidemiologia5010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Marruganti C, Traversi J, Gaeta C, et al. Adherence to Mediterranean diet, physical activity level, and severity of periodontitis: results from a university-based cross-sectional study. J Periodontol. 2022;93(8):1218–1232. doi: 10.1002/JPER.21-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Inchingolo AD, Inchingolo AM, Malcangi G, et al. Effects of resveratrol, curcumin and quercetin supplementation on bone metabolism—a systematic review. Nutrients. 2022, 14(17): 3519. doi: 10.3390/nu14173519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schwingshackl L, Schwedhelm C, Galbete C, et al. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9(10):9. doi: 10.3390/nu9101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Filomeno M, Bosetti C, Garavello W, et al. The role of a Mediterranean diet on the risk of oral and pharyngeal cancer. Br J Cancer. 2014;111(5):981–986. doi: 10.1038/bjc.2014.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Boeing H, Dietrich T, Hoffmann K, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control. 2006, 17(7):957–969. doi: 10.1007/s10552-006-0036-4 [DOI] [PubMed] [Google Scholar]
- [82].Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–671. doi: 10.1093/jn/nxz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lucenteforte E, Bosetti C, Gallus S, et al. Macronutrients, fatty acids and cholesterol intake and stomach cancer risk. Ann Oncol. 2009, 20(8):1434–1438. doi: 10.1093/annonc/mdp009 [DOI] [PubMed] [Google Scholar]
- [84].Rossi M, Garavello W, Talamini R, et al. Flavonoids and the risk of oral and pharyngeal cancer: a case-control study from Italy. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1621–1625. doi: 10.1158/1055-9965.EPI-07-0168 [DOI] [PubMed] [Google Scholar]
- [85].Pelucchi C, Bosetti C, Rossi M, et al. Selected aspects of mediterranean diet and cancer risk. Nutr Cancer. 2009;61(6):756–766. doi: 10.1080/01635580903285007 [DOI] [PubMed] [Google Scholar]
- [86].Naureen Z, Medori MC, Dhuli K, et al. Polyphenols and Lactobacillus reuteri in oral health. J Prev Med Hyg. 2022;63(2 Suppl 3):E246–E254. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Marqués-Martínez L, Pérez-Bermejo M, Lairón-Peris AR, et al. Association between the severity of dental caries and the degree of adherence to the Mediterranean diet in the pediatric population. Nutrients. 2022;14(17):3622. doi: 10.3390/nu14173622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kale S, Kakodkar P, Shetiya S, et al. Prevalence of dental caries among children aged 5–15 years from 9 countries in the eastern mediterranean region: a meta-analysis. East Mediterr Health J. 2020, 26(6):726–735. doi: 10.26719/emhj.20.050 [DOI] [PubMed] [Google Scholar]
- [89].Altun E, Walther C, Borof K, et al. Association between dietary pattern and periodontitis—a cross-sectional study. Nutrients. 2021, 13(11): 13. doi: 10.3390/nu13114167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Radić J, Vučković M, Gelemanović A, et al. Interconnectedness between periodontitis stage, oral hygiene habits, adherence to the Mediterranean diet and nutritional status in dalmatian kidney transplant recipients: a cross-sectional study. Sci Rep. 2022;12(1):11614. doi: 10.1038/s41598-022-15589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3(1):17038. doi: 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- [92].Guarnieri R, Reda R, Di Nardo D, et al. Clinical, radiographic, and biochemical evaluation of two-piece versus one-piece single implants with a laser-microgrooved collar surface after 5 years of functional loading. Clin Implant Dent Relat Res. 2022;24(5):676–682. doi: 10.1111/cid.13118 [DOI] [PubMed] [Google Scholar]
- [93].Guarnieri R, Miccoli G, Reda R, et al. Sulcus fluid volume, IL-6, and il-1b concentrations in periodontal and peri-implant tissues comparing machined and laser-microtextured collar/abutment surfaces during 12 weeks of healing: a split-mouth RCT. Clin Oral Implants Res. 2022, 33(1):94–104. doi: 10.1111/clr.13868 [DOI] [PubMed] [Google Scholar]
- [94].Guarnieri R, Reda R, Zanza A, et al. Can peri-implant marginal bone loss progression and a-MMP-8 Be considered indicators of the subsequent onset of peri-implantitis? A 5-year study. Diagn (Basel). 2022;12(11):2599. doi: 10.3390/diagnostics12112599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].World Health Organization in Oral Health. Geneva, Switzerland: World Health Organization; 2018. [accessed 2023 Nov]. https://www.who.int/news-room/fact-sheets/detail/oral-health [Google Scholar]
- [96].Ferreira MC, Dias-Pereira AC, LS BDA, et al. Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res. 2017, 52(4):651–665. doi: 10.1111/jre.12436 [DOI] [PubMed] [Google Scholar]
- [97].Tonetti MS, Jepsen S, Jin L, et al. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44(5):456–462. doi: 10.1111/jcpe.12732 [DOI] [PubMed] [Google Scholar]
- [98].Borg-Bartolo R, Roccuzzo A, Molinero-Mourelle P, et al. Global prevalence of edentulism and dental caries in middle-aged and elderly persons: a systematic review and meta-analysis. J Dent. 2022;127:104335. doi: 10.1016/j.jdent.2022.104335 [DOI] [PubMed] [Google Scholar]
- [99].Bui FQ, CLC ADS, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019, 42(1):27–35. doi: 10.1016/j.bj.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Carrizales-Sepúlveda EF, Ordaz- FaríFaríAs A, Vera-Pineda R, et al. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27(11):1327–1334. doi: 10.1016/j.hlc.2018.05.102 [DOI] [PubMed] [Google Scholar]
- [101].Ansar W, Ghosh S. Inflammation and inflammatory diseases, markers, and mediators: role of CRP in some inflammatory diseases. Biology of C Reactive Protein In Health And Disease. 2016 Mar 24;67–107. doi: 10.1007/978-81-322-2680-2_4 [DOI] [Google Scholar]
- [102].Miller CS, Ding X, Dawson DR EJ 3rd: Salivary biomarkers for discriminating periodontitis in the presence of diabetes. J Clin Periodontol. 2021, 48(2):216–225. doi: 10.1111/jcpe.13393 [DOI] [PubMed] [Google Scholar]
- [103].Wang IC, Sugai JV, Majzoub J, et al. Pro-inflammatory profiles in cardiovascular disease patients with peri-implantitis. J Periodontol. 2022;93(6):824–836. doi: 10.1002/JPER.21-0419 [DOI] [PubMed] [Google Scholar]
- [104].Hujoel PP, Lingström P: Nutrition, dental caries and periodontal disease: a narrative review. J Clin Periodontol. 2017, 44 Suppl S18:S79–s84. doi: 10.1111/jcpe.12672 [DOI] [PubMed] [Google Scholar]
- [105].Machado V, Botelho J, Viana J, et al. Association between dietary Inflammatory Index and periodontitis: a cross-sectional and mediation analysis. Nutrients. 2021;13(4):13. doi: 10.3390/nu13041194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 2018, 45 S20:S219–s229. doi: 10.1111/jcpe.12951 [DOI] [PubMed] [Google Scholar]
- [107].Jia G, Zhi A, Lai PFH, et al. The oral microbiota – a mechanistic role for systemic diseases. Br Dent J. 2018, 224(6):447–455. doi: 10.1038/sj.bdj.2018.217 [DOI] [PubMed] [Google Scholar]
- [108].Zaura E, Keijser BJF, Huse SM, et al. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sedghi L, DiMassa V, Harrington A, et al. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 2000. 2021;87(1):107–131. doi: 10.1111/prd.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Moye ZD, Zeng L, Burne RA. Fueling the caries process: carbohydrate metabolism and gene regulation by Streptococcus mutans. J Oral Microbiol. 2014;6(1):6. doi: 10.3402/jom.v6.24878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Adler CJ, Malik R, Browne GV, et al. Diet may influence the oral microbiome composition in cats. Microbiome. 2016;4(1):23. doi: 10.1186/s40168-016-0169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Adler CJ, Dobney K, Weyrich LS, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and industrial revolutions. Nature Genet. 2013;45(4):450–455. doi: 10.1038/ng.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. doi: 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Koo H, Xiao J, Klein MI, et al. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192(12):3024–3032. doi: 10.1128/JB.01649-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Mahendra J, Mahendra L, Mugri MH, et al. Role Of periodontal bacteria, viruses, and placental mir155 in chronic periodontitis and preeclampsia—a genetic microbiological study. CIMB. 2021, 43(2):831–844. doi: 10.3390/cimb43020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tebyaniyan H, Hussain A, Vivian M: Current antibacterial agents in dental bonding systems: a comprehensive overview. Future Microbiol. 2023, 18(12):825–844. doi: 10.2217/fmb-2022-0203 [DOI] [PubMed] [Google Scholar]
- [117].Hakim LK, Yazdanian M, Alam M, et al. Biocompatible and biomaterials application in drug delivery system in oral cavity. Evid Based Complement Alternat Med. 2021;2021:9011226. doi: 10.1155/2021/9011226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Motallaei MN, Yazdanian M, Tebyanian H, et al. The current strategies in controlling oral diseases by herbal and chemical materials. Evid Based Complement Alternat Med. 2021;2021:3423001. doi: 10.1155/2021/3423001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tahmasebi E, Keshvad A, Alam M, et al. Current infections of the orofacial region: treatment, diagnosis, and epidemiology. Life. 2023;13(2):13. doi: 10.3390/life13020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cekici A, Kantarci A, Hasturk H, et al. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64(1):57–80. doi: 10.1111/prd.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Haas AN, Furlaneto F, Gaio EJ, et al. New tendencies in non-surgical periodontal therapy. Braz Oral Res. 2021;35(suppl 2):e095. doi: 10.1590/1807-3107bor-2021.vol35.0095 [DOI] [PubMed] [Google Scholar]
- [122].Wang J, Liu Y, Wang W, et al. The rationale and potential for using lactobacillus in the management of periodontitis. J Microbiol. 2022;60(4):355–363. doi: 10.1007/s12275-022-1514-4 [DOI] [PubMed] [Google Scholar]
- [123].Wu F, Fang B, Wuri G, et al. Metagenomic analysis reveals a mitigating role for Lactobacillus paracasei and Bifidobacterium animalis in experimental periodontitis. Nutrients. 2022;14(10):2125. doi: 10.3390/nu14102125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Santana SI, Silva PHF, Salvador SL, et al. Adjuvant use of multispecies probiotic in the treatment of peri-implant mucositis: A randomized controlled trial. J Clinic Periodontol. 2022, 49(8):828–839. doi: 10.1111/jcpe.13663 [DOI] [PubMed] [Google Scholar]
- [125].Jiang Y, Song B, Brandt BW, et al. Comparison of red-complex bacteria between saliva and subgingival plaque of periodontitis patients: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2021;11:727732. doi: 10.3389/fcimb.2021.727732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Van Dyke TE, Bartold PM, Reynolds EC. The nexus between periodontal inflammation and dysbiosis. Front Immunol. 2020;11:511. doi: 10.3389/fimmu.2020.00511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sedghi LM, Bacino M, Kapila YL. Periodontal disease: the good, the bad, and the unknown. Front Cell Infect Microbiol. 2021;11:766944. doi: 10.3389/fcimb.2021.766944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Seifi Kafshgari H, Yazdanian M, Ranjbar R, et al. The effect of citrullus colocynthis extracts on Streptococcus mutans, Candida albicans, normal gingival fibroblast and breast cancer cells. J Biol Res. 2019, 92(1):92. doi: 10.4081/jbr.2019.8201 [DOI] [Google Scholar]
- [129].Yazdanian M, Rostamzadeh P, Alam M, et al. Evaluation of antimicrobial and cytotoxic effects of Echinacea and Arctium extracts and Zataria essential oil. AMB Express. 2022;12(1):75. doi: 10.1186/s13568-022-01417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Mosaddad SA, Hussain A, Tebyaniyan H. Green Alternatives as antimicrobial agents in mitigating periodontal diseases: a narrative review. Microorganisms. 2023;11(5):11. doi: 10.3390/microorganisms11051269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lemos JA, Palmer SR, Zeng L, et al. The biology of streptococcus mutans. Microbiol Spectr. 2019;7(1):7. doi: 10.1128/microbiolspec.GPP3-0051-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Zhang J, Xu X. Research progress in the relationship between lactobacillus and dental caries. Sichuan Da Xue Xue Bao Yi Xue Ban. 2022;53(5):929–934. doi: 10.12182/20220960103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Manome A, Abiko Y, Kawashima J, et al. Acidogenic potential of oral bifidobacterium and its high fluoride tolerance. Front Microbiol. 2019;10:1099. doi: 10.3389/fmicb.2019.01099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Huck O, Mulhall H, Rubin G, et al. Akkermansia muciniphila reduces porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol. 2020, 47(2):202–212. doi: 10.1111/jcpe.13214 [DOI] [PubMed] [Google Scholar]
- [135].Mineoka T, Awano S, Rikimaru T, et al. Site-specific development of periodontal disease is associated with increased levels of porphyromonas gingivalis, treponema denticola, and Tannerella forsythia in subgingival plaque. J Periodontol. 2008;79(4):670–676. doi: 10.1902/jop.2008.070398 [DOI] [PubMed] [Google Scholar]
- [136].Yasui M, Ryu M, Sakurai K, et al. Colonisation of the oral cavity by periodontopathic bacteria in complete denture wearers. Gerodontology. 2012;29(2):e494–502. doi: 10.1111/j.1741-2358.2011.00506.x [DOI] [PubMed] [Google Scholar]
- [137].Pietiäinen M, Kopra KAE, Vuorenkoski J, et al. Aggregatibacter actinomycetemcomitans serotypes associate with periodontal and coronary artery disease status. J Clinic Periodontol. 2018, 45(4):413–421. doi: 10.1111/jcpe.12873 [DOI] [PubMed] [Google Scholar]
- [138].Blasco-Baque V, Garidou L, Pomié C, et al. Periodontitis induced by porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut. 2017;66(5):872–885. doi: 10.1136/gutjnl-2015-309897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x [DOI] [PubMed] [Google Scholar]
- [140].Lugones-Sánchez C, Santos-Mínguez S, Salvado R, et al. Lifestyles, arterial aging, and its relationship with the intestinal and oral microbiota (MIVAS III study): a research protocol for a cross-sectional multicenter study. Front Public Health. 2023;11:1164453. doi: 10.3389/fpubh.2023.1164453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Merra G, Noce A, Marrone G, et al. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020;13(1):13. doi: 10.3390/nu13010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Daniele S, Scarfò G, Ceccarelli L, et al. The mediterranean diet positively affects resting metabolic rate and salivary microbiota in human subjects: a comparison with the vegan regimen. Biology. 2021;10(12):10. doi: 10.3390/biology10121292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hansen TH, Kern T, Bak EG, et al. Impact of a vegan diet on the human salivary microbiota. Sci Rep. 2018;8(1):5847. doi: 10.1038/s41598-018-24207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Lam OL, McGrath C, Bandara HM, et al. Oral health promotion interventions on oral reservoirs of staphylococcus aureus: a systematic review. Oral Dis. 2012, 18(3):244–254. doi: 10.1111/j.1601-0825.2011.01874.x [DOI] [PubMed] [Google Scholar]
- [145].McCormack MG, Smith AJ, Akram AN, et al. Staphylococcus aureus and the oral cavity: an overlooked source of carriage and infection? Am J Infect Control. 2015;43(1):35–37. doi: 10.1016/j.ajic.2014.09.015 [DOI] [PubMed] [Google Scholar]
- [146].Musarra-Pizzo M, Ginestra G, Smeriglio A, et al. The antimicrobial and antiviral activity of polyphenols from almond (prunus dulcis L.) skin. Nutrients. 2019;11(10):11. doi: 10.3390/nu11102355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Tsou SH, Hu SW, Yang JJ, et al. Potential oral health care agent from coffee against virulence factor of periodontitis. Nutrients. 2019, 11(9):11. doi: 10.3390/nu11092235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kato I, Vasquez A, Moyerbrailean G, et al. Nutritional correlates of human oral microbiome. J Am Coll Nutr. 2017, 36(2):88–98. doi: 10.1080/07315724.2016.1185386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lee JH, Lee SA, Kim HD. Periodontitis and intake of thiamine, riboflavin and niacin among Korean adults. Community Dent Oral Epidemiol. 2020;48(1):21–31. doi: 10.1111/cdoe.12496 [DOI] [PubMed] [Google Scholar]
- [150].Neiva RF, Al-Shammari K, Nociti FH Jr., et al. Effects of vitamin-B complex supplementation on periodontal wound healing. J Periodontol. 2005;76(7):1084–1091. doi: 10.1902/jop.2005.76.7.1084 [DOI] [PubMed] [Google Scholar]
- [151].Pack AR: Folate mouthwash: effects on established gingivitis in periodontal patients. J Clin Periodontol. 1984, 11(9):619–628. doi: 10.1111/j.1600-051X.1984.tb00914.x [DOI] [PubMed] [Google Scholar]
- [152].Herrala M, Turunen S, Hanhineva K, et al. Low-dose doxycycline treatment normalizes levels of some salivary metabolites associated with oral microbiota in patients with primary Sjögren’s syndrome. Metabolites. 2021;11(9):11. doi: 10.3390/metabo11090595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lassalle F, Spagnoletti M, Fumagalli M, et al. Oral microbiomes from hunter-gatherers and traditional farmers reveal shifts in commensal balance and pathogen load linked to diet. Mol Ecol. 2018;27(1):182–195. doi: 10.1111/mec.14435 [DOI] [PubMed] [Google Scholar]
- [154].Ikeda E, Shiba T, Ikeda Y, et al. Japanese subgingival microbiota in health vs disease and their roles in predicted functions associated with periodontitis. Odontology. 2020;108(2):280–291. doi: 10.1007/s10266-019-00452-4 [DOI] [PubMed] [Google Scholar]
- [155].Voland L, Yuan M, Lecoutre S, et al. Tissue pleiotropic effect of biotin and prebiotic supplementation in established obesity. Am J Physiol Endocrinol Metab. 2023;325(4):E390–E405. doi: 10.1152/ajpendo.00295.2022 [DOI] [PubMed] [Google Scholar]
- [156].Green R, Allen LH, Bjørke-Monsen AL, et al. Vitamin B(12) deficiency. Nat Rev Dis Primers. 2017, 3(1):17040. doi: 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- [157].Aral K, Alkan BA, Saraymen R, et al. Therapeutic effects of systemic vitamin k2 and vitamin d3 on gingival inflammation and alveolar bone in rats with experimentally induced periodontitis. J Periodontol. 2015, 86(5):666–673. doi: 10.1902/jop.2015.140467 [DOI] [PubMed] [Google Scholar]
- [158].Antonoglou GN, Knuuttila M, Niemelä O, et al. Low serum level of 1,25(OH)2 D is associated with chronic periodontitis. J Periodontal Res. 2015;50(2):274–280. doi: 10.1111/jre.12207 [DOI] [PubMed] [Google Scholar]
- [159].Bashutski JD, Eber RM, Kinney JS, et al. The impact of vitamin D status on periodontal surgery outcomes. J Dent Res. 2011, 90(8):1007–1012. doi: 10.1177/0022034511407771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Dietrich T, Nunn M, Dawson-Hughes B, et al. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82(3):575–580. doi: 10.1093/ajcn/82.3.575 [DOI] [PubMed] [Google Scholar]
- [161].Stein SH, Tipton DA: Vitamin D and its impact on oral health–an update. J Tenn Dent Assoc. 2011, 91(2):30–33; quiz 34–35. [PubMed] [Google Scholar]
- [162].Zanetta P, Squarzanti DF, Sorrentino R, et al. Oral microbiota and vitamin D impact on oropharyngeal squamous cell carcinogenesis: a narrative literature review. Crit Rev Microbiol. 2021;47(2):224–239. doi: 10.1080/1040841X.2021.1872487 [DOI] [PubMed] [Google Scholar]
- [163].Abou Sulaiman AE, Shehadeh RM: Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J Periodontol. 2010, 81(11):1547–1554. doi: 10.1902/jop.2010.100173 [DOI] [PubMed] [Google Scholar]
- [164].Dodington DW, Fritz PC, Sullivan PJ, et al. Higher intakes of fruits and vegetables, β-carotene, vitamin C, α-tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J Nutr. 2015;145(11):2512–2519. doi: 10.3945/jn.115.211524 [DOI] [PubMed] [Google Scholar]
- [165].García-Closas R, Berenguer A, José Tormo M, et al. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br J Nutr. 2004;91(6):1005–1011. doi: 10.1079/BJN20041130 [DOI] [PubMed] [Google Scholar]
- [166].Staudte H, Sigusch BW, Glockmann E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br Dent J. 2005;199(4):213–217. discussion 210. doi: 10.1038/sj.bdj.4812613 [DOI] [PubMed] [Google Scholar]
- [167].Woelber JP, Tennert C: Chapter 13: diet and periodontal diseases. Monogr Oral Sci. 2020, 28:125–133. [DOI] [PubMed] [Google Scholar]
- [168].Cerná H, Fiala B, Fingerová H, et al. Contribution to indication of total therapy with vitamin E in chronic periodontal disease (pilot study). Acta Univ Palacki Olomuc Fac Med. 1984, 107:167–170. [PubMed] [Google Scholar]
- [169].Adegboye AR, Christensen LB, Holm-Pedersen P, et al. Intake of dairy products in relation to periodontitis in older Danish adults. Nutrients. 2012, 4(9):1219–1229. doi: 10.3390/nu4091219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Garcia MN, Hildebolt CF, Miley DD, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol. 2011;82(1):25–32. doi: 10.1902/jop.2010.100207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Khurshid Z, Zafar MS, Zohaib S, et al. Green tea (camellia sinensis): chemistry and oral health. Open Dent J. 2016;10(1):166–173. doi: 10.2174/1874210601610010166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Maguire A. ADA clinical recommendations on topical fluoride for caries prevention. Evid Based Dent. 2014;15(2):38–39. doi: 10.1038/sj.ebd.6401019 [DOI] [PubMed] [Google Scholar]
- [173].Chakraborty S, Tewari S, Sharma RK, et al. Impact of iron deficiency anemia on chronic periodontitis and superoxide dismutase activity: a cross-sectional study. J Periodontal Implant Sci. 2014;44(2):57–64. doi: 10.5051/jpis.2014.44.2.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Jentsch HF, Knöfler GU, Purschwitz RE, et al. Periodontal dressing as an adjunct after scaling and root planing–a useful preventive tool?. Oral Health Prev Dent. 2016, 14(2):101–109. doi: 10.3290/j.ohpd.a35612 [DOI] [PubMed] [Google Scholar]
- [175].Pushparani DS. Serum zinc and β D glucuronidase enzyme level in type 2 diabetes mellitus with periodontitis. Curr Diabetes Rev. 2016;12(4):449–453. doi: 10.2174/1573399811666150724094525 [DOI] [PubMed] [Google Scholar]
- [176].Najeeb S, Zafar MS, Khurshid Z, et al. The role of nutrition in periodontal health: an update. Nutrients. 2016;8(9):8. doi: 10.3390/nu8090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Richter CK, Skulas-Ray AC, Champagne CM, et al. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk?. Adv Nutr. 2015;6(6):712–728. doi: 10.3945/an.115.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Stahl SS, Sandler HC, Cahn L. The effects of protein deprivation upon the oral tissues of the rat and particularly upon periodontal structures under irritation. Oral Surg Oral Med Oral Pathol. 1955;8(7):760–768. doi: 10.1016/0030-4220(55)90040-2 [DOI] [PubMed] [Google Scholar]
- [179].Staufenbiel I, Weinspach K, Förster G, et al. Periodontal conditions in vegetarians: a clinical study. Eur J Clin Nutr. 2013;67(8):836–840. doi: 10.1038/ejcn.2013.101 [DOI] [PubMed] [Google Scholar]
- [180].Baumgartner S, Imfeld T, Schicht O, et al. The impact of the stone age diet on gingival conditions in the absence of oral hygiene. J Periodontol. 2009;80(5):759–768. doi: 10.1902/jop.2009.080376 [DOI] [PubMed] [Google Scholar]
- [181].Chapple ILC, Bouchard P, Cagetti MG, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clinic Periodontol. 2017, 44(S18):S39–S51. doi: 10.1111/jcpe.12685 [DOI] [PubMed] [Google Scholar]
- [182].Lula EC, Ribeiro CC, Hugo FN, et al. Added sugars and periodontal disease in young adults: an analysis of NHANES III data. Am J Clin Nutr. 2014;100(4):1182–1187. doi: 10.3945/ajcn.114.089656 [DOI] [PubMed] [Google Scholar]
- [183].Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014, 384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Woelber JP, Bremer K, Vach K, et al. An oral health optimized diet can reduce gingival and periodontal inflammation in humans - a randomized controlled pilot study. BMC Oral Health. 2016, 17(1):28. doi: 10.1186/s12903-016-0257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Juan PM F-S: Trans fatty acids (tFA): sources and intake levels, biological effects and content in commercial Spanish food. Nutr Hosp. 2009, 24(5):515–520. [PubMed] [Google Scholar]


