Abstract
The treatment of digestive system tumors presents challenges, particularly in immunotherapy, owing to the advanced immune tolerance of the digestive system. Nanomaterials have emerged as a promising approach for addressing these challenges. They provide targeted drug delivery, enhanced permeability, high bioavailability, and low toxicity. Additionally, nanomaterials target immunosuppressive cells and reshape the tumor immune microenvironment (TIME). Among the various cells in the TIME, tumor-associated macrophages (TAMs) are the most abundant and play a crucial role in tumor progression. Therefore, investigating the modulation of TAMs by nanomaterials for the treatment of digestive system tumors is of great significance. Here, we present a comprehensive review of the utilization of nanomaterials to modulate TAMs for the treatment of gastric cancer, colorectal cancer, hepatocellular carcinoma, and pancreatic cancer. We also investigated the underlying mechanisms by which nanomaterials modulate TAMs to treat tumors in the digestive system. Furthermore, this review summarizes the role of macrophage-derived nanomaterials in the treatment of digestive system tumors. Overall, this research offers valuable insights into the development of nanomaterials tailored for the treatment of digestive system tumors.
Keywords: Digestive system tumors, Tumor-associated macrophages, Nanomaterials, Tumor immune microenvironment, Therapy
Graphical abstract
Highlights
-
•
Nanomaterials modulate TAMs, inhibiting M2 polarization.
-
•
Nanomaterials prevent TAM infiltration.
-
•
Nanomaterials depleting TAMs using oxygen.
-
•
Macrophage-derived nanomaterials target TME.
-
•
The activation of NF-κB pathway reprogram TAMs.
Abbreviations
- HCC
hepatocellular carcinoma
- RT
radiotherapy
- TIME
tumor immune microenvironment
- TME
tumor microenvironment
- TAMs
tumor-associated macrophages
- TNF-α
tumor necrosis factor-α
- IL
interleukin
- CCR2
CC chemokine receptor 2
- GC
gastric cancer
- CRC
colorectal cancer
- DAMPs
damage-associated molecular patterns
- ROS
reactive oxygen species
- NPs
nanoparticles
- OXA
oxaliplatin
- ABCG2
ATP-binding cassette subfamily G member 2
- siRNA
short interfering RNA
- TLR
toll-like receptors
- HA
hyaluronic acid
- ICD
immunogenic cell death
- IMD
imiquimod
- ox-mtDNA
oxidized mitochondrial DNA
- PDT
photodynamic therapy
- NIR
near-infrared
- IRFs
interferon regulatory factors
- dsRNA
double-stranded RNA
- CSF-1
colony-stimulating factor-1
- CSF–1R
CSF-1 receptor
- PTT
photothermal therapy
- CAC
colitis-associated colorectal cancer
- PHD
propylhydroxylase domain enzyme
- LDHA
lactate dehydrogenase A
- SUCNR1
succinate receptor 1
- IFN-γ
interferon gamma
- Hsps
heat-shock proteins
- DOX
doxorubicin
- SIRP-α
signal regulatory protein α
- BPQD
black phosphorous quantum dot
- DC
dendritic cell
- PLGA
poly-lactic-co-glycolic acid
- SDF1
stromal derived factor 1
- CCL2
cc-motif ligand 2
- SOR
sorafenib
- AMP
adenosine monophosphate
- UCNP
upconversion NPs
- S. aureus
Staphylococcus aureus
- MPLA
monophosphoryl lipid A
- LPS
lipopolysaccharide
- CpG-ODNs
cytosine-phosphate-guanine oligodeoxynucleotides
- PPAR-γ
peroxisome proliferator–activated receptor γ
- DMXAA
5,6-dimethylxanthenone-4-acetic acid
- IONPs
iron oxide NPs
- M2pep
M2 macrophage-binding peptide
- GEM
gemcitabine
- nab-paclitaxel
nanoparticle albumin-bound paclitaxel
- miRNAs
microRNAs
- MGLL
monoaylglycerol lipase
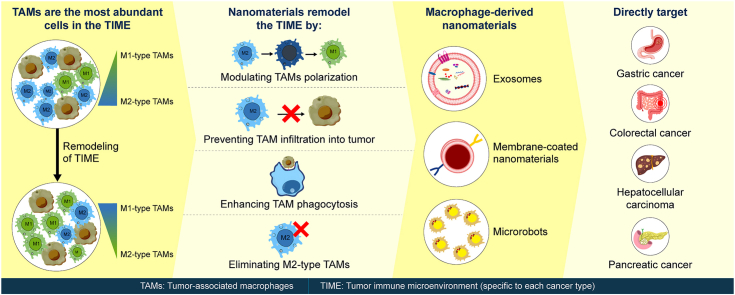
1. Introduction
Digestive system tumors, including esophageal cancer, gastric cancer, colorectal cancer, pancreatic cancer, and hepatocellular carcinoma (HCC), show high incidence, strong invasiveness, and poor prognosis [1]. Owing to the lack of noticeable early symptoms and difficulties in their detection, most patients are diagnosed at an advanced or late stage [[2], [3], [4], [5], [6]]. Digestive system tumors lack the driver of specific gene mutations; therefore, targeted therapies are less effective and reliance is more on chemotherapy and anti-angiogenic treatments. The digestive system has a higher immune tolerance than other systems of the human body [7], rendering immunotherapy particularly crucial for its treatment [[8], [9], [10], [11]]. Therefore, chemotherapy combined with immunotherapy has become the first-line treatment option for tumors of the digestive system. However, most free drugs show low solubility, rapid metabolism, poor cellular uptake, non-specific tissue distribution, and strong off-target toxic side effects [12,13].
With the development of nanomaterials, this situation is expected to change. Nanomaterials are typically prepared from natural or synthetic polymer materials using traditional chemical methods [14]. A variety of nanoparticles (NPs) of different material classes, including organic nanomaterials (e.g., lipids/liposomes, polymeric micelles, and polymeric NPs), inorganic nanomaterials (e.g., carbon-based NPs, silicon-based NPs and metal-based NPs (gold, manganese, zinc and iron), and those with biological/natural carriers, have been used [15]. Drugs are loaded inside or on the surface of nanomaterials through encapsulation, intercalation, adsorption, polymerization, condensation, or coupling reactions [16]. Modified nanomaterials can target immunosuppressive cells and reshape the tumor immune microenvironment (TIME) [17]. Nanomaterial-loaded drugs exhibit targeted drug delivery, high permeability, high bioavailability, low toxicity, and reticuloendothelial system escape [18,19]. The release of these drugs at a specific time and space is controlled through sound, light, heat, and magnetic systems, significantly increasing the drug efficacy [20]. Nanomaterials can carry multiple therapeutic drugs simultaneously to achieve combined treatment of cancer [21]. Therefore, nanomaterials have unique advantages in treating digestive system tumors.
Immunotherapy mainly includes cytokine therapy, immune checkpoint blockade therapy, and adoptive T cell therapy [22]. Most of them generally follow the same pathway to generate immune-activated cytotoxic T lymphocytes [15]. However, the clinical outcomes of patients with cancer are affected by immune infiltration in the tumor microenvironment (TEM) [23]. Among the immune cells recruited to the tumor site, tumor-associated macrophages (TAMs) are the most abundant throughout the tumor progression [24]. TAMs are a subset of cells in the TIME that originate from peripheral blood mononuclear cells and tissue-resident macrophages [24]. TAMs can be classified into classically and alternatively activated M1-and M2-type, with opposing roles in the tumors [25]. M1-type TAMs express CD80, CD86, inducible nitric oxide synthase, and secrete interleukin-6 (IL-6), IL-12, and tumor necrosis factor-α (TNF-α) to kill tumor cells [26,27]. In contrast, M2-type TAMs inhibit the expression of pro-inflammatory factors through the high expression of CD206, CD163, CC chemokine receptor 2 (CCR2), Arginase-1, IL-10, Ym-1, Fizz-1, and other molecules, thereby promoting tumor cell proliferation and metastasis [28]. In the TIME, M2-type TAMs are predominant [29]. Moreover, massive infiltration of M2-like TAMs is associated with poor prognosis in patients with digestive system tumors [[30], [31], [32], [33]]. Targeting and reactivating M2-type TAMs is necessary to reverse the immunosuppressive state and stimulate immune defense for effective tumor clearance [34]. Dynamic changes between M1-type and M2-type TAMs are important in the development and metastasis of digestive system tumors [35,36]. Therefore, effectively regulating TAMs is of great significance for treating digestive system tumors.
Nanomaterials primarily regulate TAMs by preventing TAM infiltration, eliminating TAMs, modulating TAM polarization, and enhancing TAM phagocytosis [37]. This reshapes the TIME in the digestive system and inhibits tumor development [15]. Esophageal cancer is the sixth most malignant digestive system tumor with the highest mortality rate in the world [38]. However, research on nanomaterials regulating TAMs for treating esophageal cancer is lacking. Hence, this review summarizes the use of nanomaterials to modulate TAMs in the treatment of gastric cancer (GC), colorectal cancer (CRC), HCC, and pancreatic cancer (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Table 1). Although nanomaterial design focuses primarily on targeting TAMs, the mechanisms underlying the targeting process are often overlooked. Hence, this study also investigated the relative mechanism by which nanomaterials modulate TAMs in the treatment of digestive system tumors. Macrophage-based microrobots, macrophage-derived exosomes, and macrophage-coated nanomaterials have demonstrated promising efficacy in drug delivery [39]. Consequently, this study also presents a comprehensive overview of the role played by these macrophage-derived nanomaterials in the context of digestive system tumors (Fig. 14, Fig. 15, Table 2). Ultimately, the findings of this study contribute to the establishment of a theoretical foundation and provide valuable insights into the development of more effective nanomaterials for treating digestive system tumors.
Fig. 1.
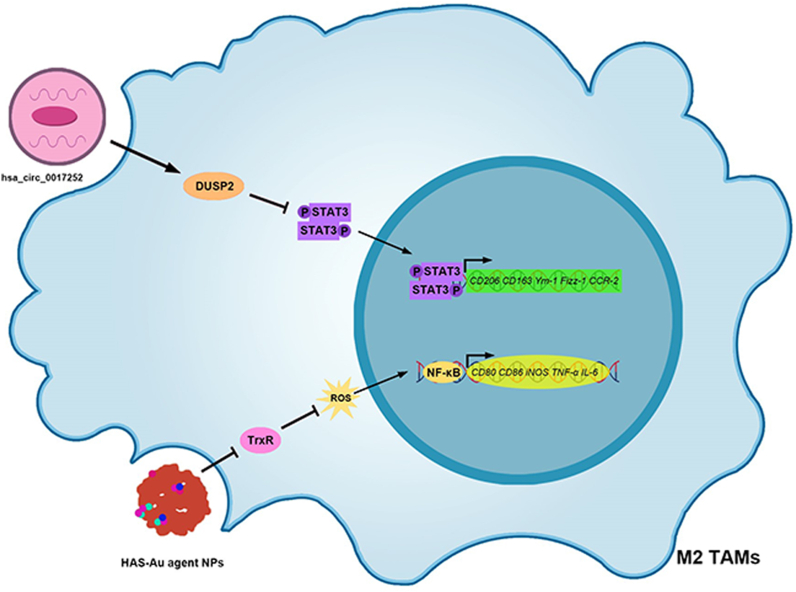
Nanomaterials modulating TAMs for the treatment of GC. (A) Nanomaterials modulating TAMs polarization for GC treatment. hsa_circ_0017,252 promotes DUSP2 and inhibits the activation of the STAT3 signaling pathway, thereby inhibiting M2-type TAM polarization in GC tissues. In addition, HAS-Au agent NPs inhibit TrxR, increase ROS content, activate the NF-κB signaling pathway, and promote M2-type TAM polarization to M1-type in GC tissues. TAMs, Tumor-associated macrophages; GC, Gastric cancer; DUSP2, Dual specificity phosphatase 2; STAT3, Signal transducer of activators of transcription 3; HAS-Au agent NPs, HAS-Au (III) thiosemicarbazone agent NP; TrxR, Thioredoxin reductase; ROS, Reactive oxygen species; NF-kB, Nuclear factor-kappaB.
Fig. 2.
Nanomaterials modulating TAMs polarization for the treatment of CRC.
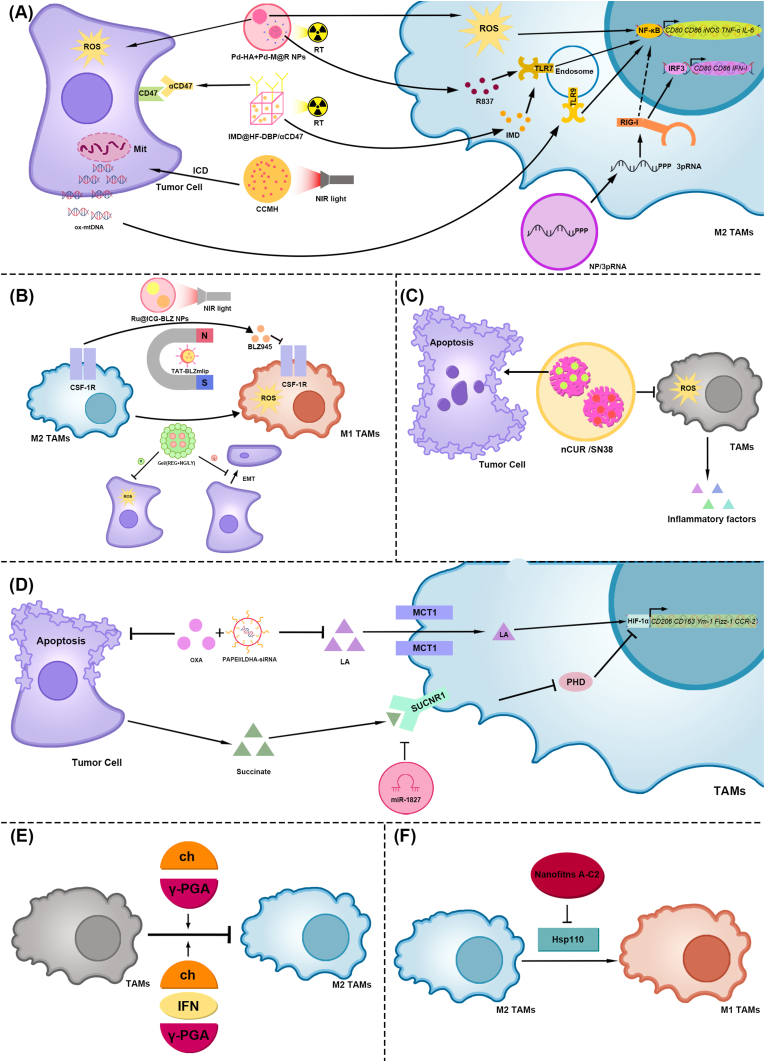
(A) The NF-kB signaling pathway., (1) Pd-HA NPs promote ROS production in M2-type TAMs and CRC cells during RT. Pd-M@R NPs release the TLR7/8 agonist R837, promote TLR7 in M2-type TAMs, and activate the NF-κB signaling pathway, thereby promoting M2-type TAMs polarization to M1-type in CRC tissue., (2) IMD@HF-DBP/αCD47 releases αCD47 and IMD during RT. αCD47 binds to CD47 on CRC cells and enhances TAM phagocytosis. IMD promotes TLR7 in M2-type TAMs, activates the NF-κB signaling pathway, and promotes M2-type TAM polarization to the M1 type in CRC tissues., (3) CCMH promoted ICD in CRC cells under NIR light irradiation, producing ox-mtDNA. Ox-mtDNA promotes TLR9 in M2-type TAMs, activates the NF-κB signaling pathway, and promotes M2-type TAM polarization to M1-type in CRC tissue., (4) NP/3pRNA releases 3pRNA into M2-type TAMs and activates RIG-I. RIG-I promotes IRF3 activation, thereby increasing M1-type TAMs. In addition, the NF-κB signaling pathway may be involved.
(B) The CSF-1/CSF–1R signaling pathway, (1) TAT-BLZmlip releases BLZ945 under the action of an alternating magnetic field, inhibits CSF–1R, and generates ROS, thereby promoting M2-type TAM polarization to M1-type in CRC tissues, (2) Ru@ICG-BLZ NPs produced similar biological effects under NIR light irradiation, (3) Gel/(REG + NG/LY) release regorafenib and product ROS, which inhibits CRC and promotes M2-type TAM polarization to M1-type proliferation. Gel/(REG + NG/LY) also release LY3200882 and inhibits the EMT of CRC
(C) ROS. nCUR/SN38 inhibits ROS production, which in turn reduces the release of inflammatory factors in TAMs. Additionally, nCUR/SN38 induces apoptosis in CRC cells
(D) The HIF-1α signaling pathway, (1) PAPEI/LDHA-siRNA inhibits LA production in CRC tissues, thereby reducing the uptake of LA by M2-type TAMs, inhibiting the activation of the HIF-1α signaling pathway, and preventing TAMs from polarizing toward M2. In addition, PAPEI/LDHA-siRNA combined with OXA promoted the apoptosis of CRC cells, (2) Succinate released by tumor cells activates SUCNR1, inhibits PHD, leads to the activation of the HIF-1α signaling pathway, and promotes TAMs polarization to M2. However, hUCMSC-Exos carrying miR-1827 effectively inhibited SUCNR1, thereby inhibiting TAM polarization to M2, (E) Immune adjuvants: chitosan and γ-PGA. Both Ch/γ-PGA NPs and IFN-γ-incorporated Ch/γ-PGA NPs can inhibit TAM polarization to M2 phenotype in CRC tissue, (F) Hsp110. Nanofitins A-C2 inhibit Hsp110, thereby promoting M2-type TAM polarization to M1-type in CRC tissues. TAMs, Tumor-associated macrophages; CRC, Colorectal cancer; NF-kB, Nuclear factor-kappaB; Pd-HA + Pd-M@R NPs, Bidirectional anisotropic Pd nanoclusters; RT, radiotherapy; ROS, Reactive oxygen species; TLR, Toll-like receptor; IMD@HF-DBP/αCD47, Hf-DBP nMOF(Nanoscale metal-organic frameworks) for the co-delivery of imiquimod (IMD), and anti-CD47 antibody (αCD47); CCMH, CaO2@CuS–MnO2@HA; Ox-mtDNA, Oxidized mitochondrial DNA; NP/3pRNA, nanoparticles delivery 5′ triphosphate, short, double-stranded RNA; IRF3, Interferon Regulatory Factor 3; RIG-I, Retinoic acid-inducible gene I; CSF-1/CSF–1R, Colony stimulating factor-1/CSF-1 receptor; TAT-BLZmlip, Transcriptional activator protein-BLZ945magnetic liposomal; Ru@ICG-BLZ NPs, Ru encapsulated to ICG (Indocyanine green) and BLZ945; Near-infrared, NIR; Gel/(REG + NG/LY), ROS-responsive nanogels loaded with regorafenib and LY3200882; EMT, Epithelial-mesenchymal transition; nCUR/SN38, Chitosan loaded Curcumin and SN38; HIF-1α, Hypoxia-inducible factor-1α; OXA, Oxaliplatin, PAPEI/LDHA-siRNA, APEG-PAsp(PEI)/Lactic acid-short interfering RNA; LA, Lactic acid; SUCNR1, Succinate receptor 1; PHD, Prolyl hydroxylase domain enzyme; hUCMSC-Exos, Human umbilical cord mesenchymal stem cell-derived exosomes; Ch/γ-PGA NPs Ch, Chitosan; γ-PGA, Poly-γ-glutamic acid; IFN-γ, Interferon-γ; Hsp110, Heat-shock proteins 110.
Fig. 3.
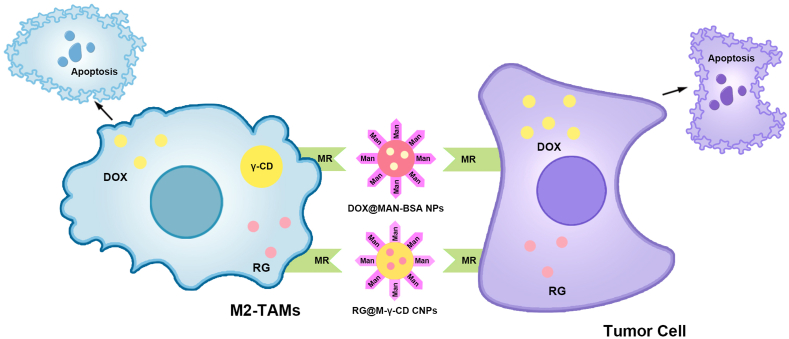
Nanomaterials depleting TAMs for the treatment of CRC via MR.
(1) DOX@MAN-BSA NPs simultaneously targeted M2-type TAMs and CRC cells via the interaction between Man and MR, thereby delivering DOX. This causes CRC cell apoptosis and the deletion of M2-type TAMs. (2) RG@M-γ-CD CNPs also produced similar biological effects. TAMs, Tumor-associated macrophages; CRC, Colorectal cancer; MR, Mannose receptor; DOX@MAN-BSA NPs, Doxorubicin encapsulated to mannose-modified bovine serum albumin nanoparticles; Man, Mannose; DOX, Doxorubicin; RG@M-γ-CD CNPs, Regorafenib encapsulated to mannose-modified γ-cyclodextrin non-covalent channel-type nanoparticles; RG, Regorafenib; γ-CD, γ-cyclodextrin.
Fig. 4.
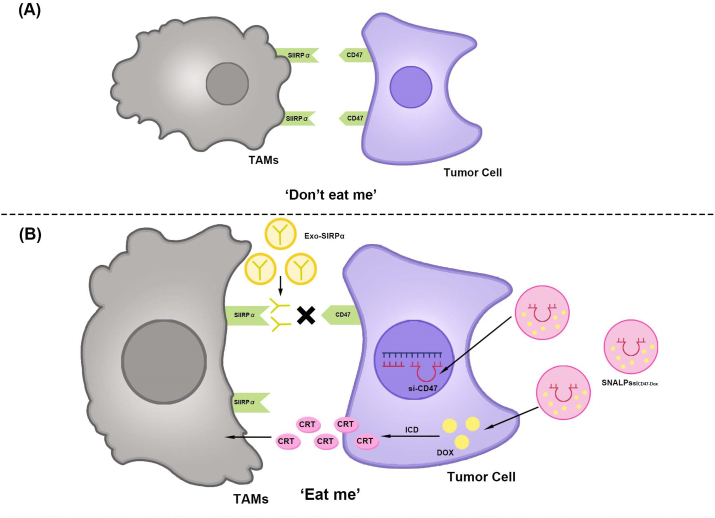
Nanomaterials enhancing TAMs phagocytosis for the treatment of CRC via the CD47-SIRPα signaling pathway.
(A) Do not eat me. The SIRPα receptor in macrophages binds to CD47 overexpressed by tumor cells and sends a “do not eat me” signal, leading to phagocytic resistance and immune evasion of cancer cells.
(B) Nanomaterials enhancing TAMs phagocytosis for the treatment of CRC via the CD47-SIRPα signaling pathway. (1) Exo-SIRPα binds to CD47 on tumor cells, blocks the CD47-SIRPα signaling pathway, and enhances the tumor phagocytosis ability of macrophages. (2) SNALPssiCD47-Dox knocks out CD47 in tumor cells and induces ICD. ICD promotes CRT production and synergistically enhances TAM phagocytosis. TAMs, Tumor-associated macrophages; CRC, Colorectal cancer; SIRPα, Signal regulatory protein α; Exo-SIRPα, Exosomes-SIRPα; SNALPssiCD47-Dox, Stable nucleic acid-lipid particles loaded with short interfering RNA CD47and Doxorubicin; DOX, Doxorubicin; ICD, Immunogenic cell death; CRT, Calreticulin.
Fig. 5.
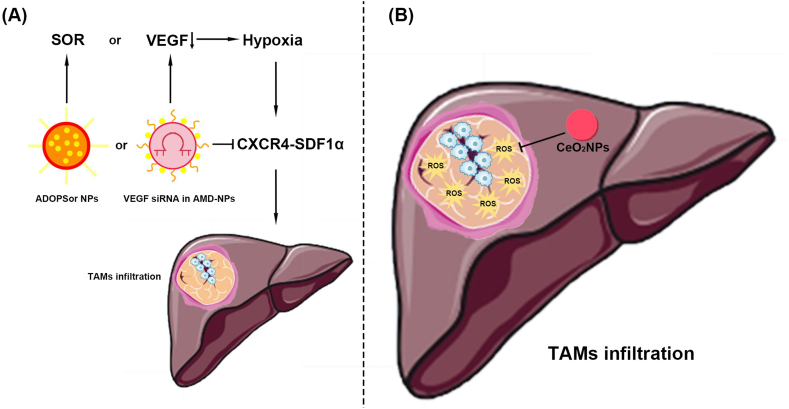
Nanomaterials preventing TAM infiltration for the treatment of HCC.
(A) The CXCR4-SDF1α signaling pathway. (1) ADOPSor NPs inhibits CXCR4, thereby blocking SDF1α and reducing TAMs infiltration caused by SOR. (2) VEGF siRNA in AMD-NPs inhibits CXCR4, thereby blocking SDF1α and reducing TAM infiltration caused by VEGF reduction.
(B) ROS. CeO2NPs reduce TAM infiltration by inhibiting ROS in HCC tissues. CeO2NPs reduces TAMs infiltration by inhibiting ROS in HCC tissues. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; CXCR4, C-X-C chemokine receptor type 4; SDF1α, Stromal-derived-factor1α; ADOPSor NPs, AMD3100 coated DOPA-PLGA nanoparticles containing sorafenib; SOR, Sorafenib; VEGF siRNA in AMD-NPs, Vascular endothelial-derived growth factor short interfering RNA in AMD3100-nanoparticles; ROS, Reactive oxygen species; CeO2NPs, Cerium oxide nanoparticles.
Fig. 6.
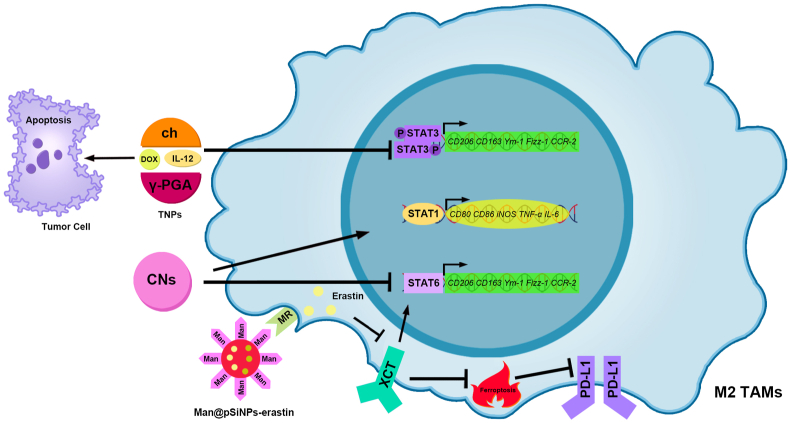
Nanomaterials modulating TAM polarization for the treatment of HCC via the STAT signaling pathway.
(1) IL-12 and DOX in TNPs inhibit STAT3, thereby inhibiting M2 TAM polarization. DOX in TNPs promoted apoptosis in HCC cells. (2) CNs promote STAT1 and inhibit STAT6, thereby promoting TAM polarization to the M1-type. (3) Man@pSiNPs-erastin targets M2 type TAMs through the MR and released erastin. Erastin inhibits xCT, thereby weakening STAT6 and reducing TAM polarization to the M2-type. In addition, the inhibition of xCT can promote TAM ferroptosis, thereby inhibiting PD-L1 expression. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; STAT, Signal transducer of activators of transcription; TNPs, Therapeutic nanoparticles; IL-12, Interleukin-12; DOX, Doxorubicin; CNs, chitosan-based nanoparticles; Man@pSiNPs-erastin, Mannose encapsulated to functionalized porous silicon nanoparticles-erastin; MR, Mannose receptor; xCT, Cysteine/glutamate transporter; PD-L1, Programmed cell death-ligand 1.
Fig. 7.
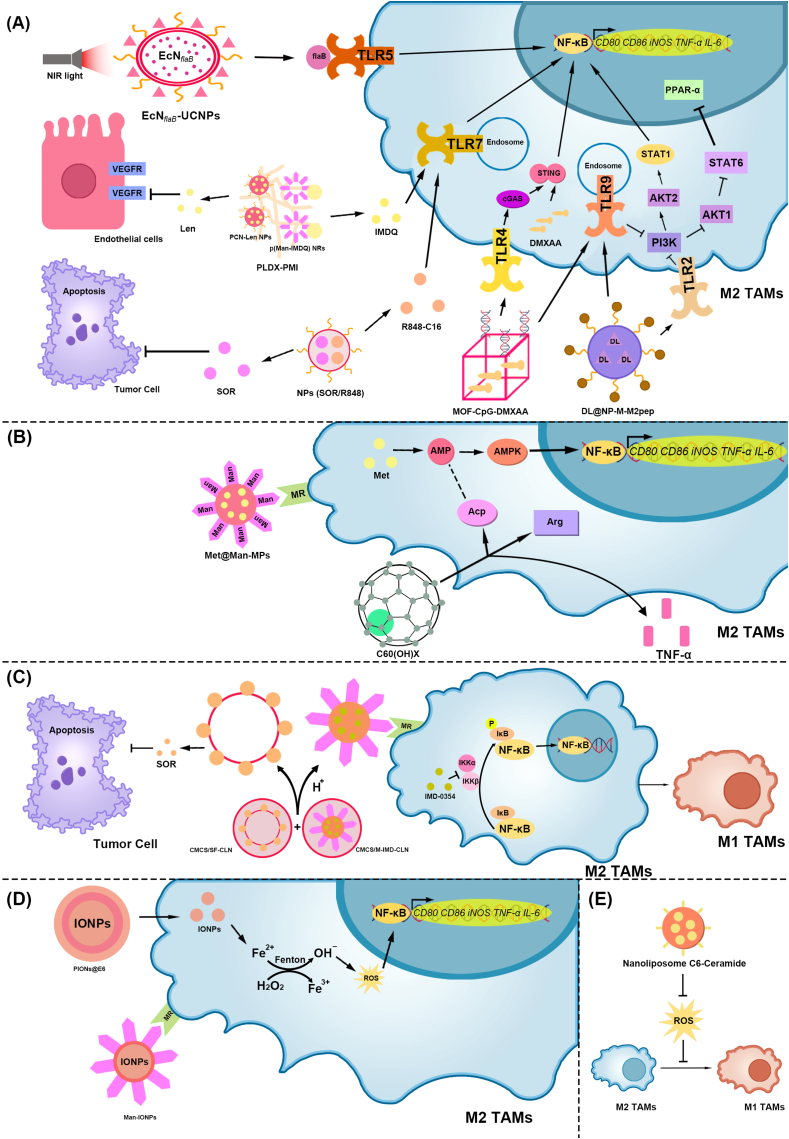
Nanomaterials modulating TAMs polarization for the treatment of HCC via the NF-κB signaling pathway.
(A) TLR. (1) Under NIR irradiation, EcNflaB-UCNPs released flaB. FlaB binds to TLR5, activates the NF-κB signaling pathway, and promotes M2-type TAM polarization to the M1-type. (2) PLDX-PMI was composed of PCN-Len NPs and p (Man-IMDQ) NRs. p (Man-IMDQ) NRs release IMDQ, promote TLR7, and then activate the NF-κB signaling pathway, promoting M2-type TAM polarization to M1-type. PCN-Len NPs released Len, thereby inhibiting VEGFR in vascular endothelial cells and reducing tumor microvessel density., (3) NPs (SOR/R848) released SOR and R848-C16. SOR causes apoptosis in HCC cells, and R848-C16 promotes TLR7, thereby promoting M2-type TAM polarization to the M1 type., (4) MOF-CpG-DMXAA promotes the cGAS-STING–NF–κB pathway through TLR4 and promotes M2-type TAM polarization to M1-type, (5) DL@NP-M-M2 pep promotes M2-type TAM polarization to M1-type via TLR2 and TLR9, regulating PPAR-γ and NF-κB
(B) AMP, (1) Met@Man-MPs target M2-type TAMs via Man, releasing Met and promoting AMP production. AMP activates AMPK and initiates NF-κB-mediated transcription, promoting M2-type TAM polarization to the M1-type, (2) C60(OH)x increases Arg and Acp activities in TAMs and promotes TNF-α secretion. Acp activity may reflect changes in AMP levels
(C) IKKβ. The CMCS/M-IMD-CLN released CLN through H+ ions in the TME. CMCS and M-IMD target HCC cells and M2-type TAMs, respectively, releasing SOR and IMD-0354. SOR induces the apoptosis of HCC cells. IMD-0354 inhibits IKKβ, thereby inhibiting IκB phosphorylation and NF-κB-mediated transcription and increasing the content of M1-type TAMs
(D) Positive role of ROS, (1) PIONs@E6 releases IONPs, generates ROS through the Fenton reaction, and activates the NF-κB signaling pathway to promote TAM polarization to M1-type. (2) Man-IONPs target M2-type TAMs through Man, release IONPs, increase the content of ROS in cells, and promote TAM polarization to the M1 type.
(E) Negative role of ROS. Nanoliposome C6-Ceramide can inhibit the ROS of macrophages, effectively reduce the number of M2-type TAMs, and increase the number of M1-type TAMs in tumor tissues. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; NF-kB, Nuclear factor-kappaB; TLR, Toll-like receptor; Near-infrared, NIR; EcNflaB-UCNPs, Lanthanide upconversion nanoparticles-conjugated engineered Escherichia coli Nissle 1917; FlaB, Flagellin B; PLDX-PMI, p (Man-IMDQ) NRs encapsulated in PCN-Len/DX hydrogel; p (Man-IMDQ) NRs, p (Mannose-imidazoquinoline) nanoregulators; IMDQ, Imidazoquinoline; PCN-Len NPs, lenvatinib-loaded nanomedicines; Len, Lenvatinib; VEGFR, Vascular endothelial growth factor receptor; NPs (SOR/R848), Nanoparticles (Sorafenib/Resiquimod); SOR, Sorafenib; R848-C16, Modified resiquimod; MOF-CpG-DMXAA, Metal-organic framework-801-cytosine-phosphate-guanine oligodeoxynucleotides −5, 6-dimethylxanthenone-4-acetic acid; cGAS, cyclic GMP-AMP synthase; STING, Stimulator of interferon genes; DL@NP-M-M2 pep, Lactide-glycolide copolymer nanoparticles to load d-lactate, and modified the DL-loaded NP with HCC membrane and M2 macrophage-binding peptide; PPAR-γ, peroxisome proliferator–activated receptor γ; STAT, Signal transducer and activator of transcription; PI3K, Phosphoinositide 3-kinase; AKT, AGC serine/threonine kinases; AMP, Adenosine monophosphate; AMPK, AMP-activated protein kinase; Met@Man-MPs, Metformin encapsulated to Mannose-cellular microparticles; Arg, Arginine; Acp, Acid phosphatase; TNF-α, Tumor Necrosis Factor-α; CLN CMCS/M-IMD-CLN, Cationic lipid-based nanoparticles o-carboxymethyl-chitosan/Mannose- Imiquimod- Cationic lipid-based nanoparticles; CLN, Cationic lipid-based nanoparticles; CMCS, O-carboxymethyl-chitosan; M-IMD, Mannose- Imiquimod- Cationic lipid-based nanoparticles; IKKα, IkappaB kinase α; IKKβ, IkappaB kinase β; IκB, NF-κB inhibitor; ROS, reactive oxygen species; PIONs@E6, Exosomes synergized with pegylated IONs loaded with chlorin E6; IONPs, Iron oxide nanoparticles; Man-IONPs, Mannose- Iron oxide nanoparticles; Man, Mannose.
Fig. 8.
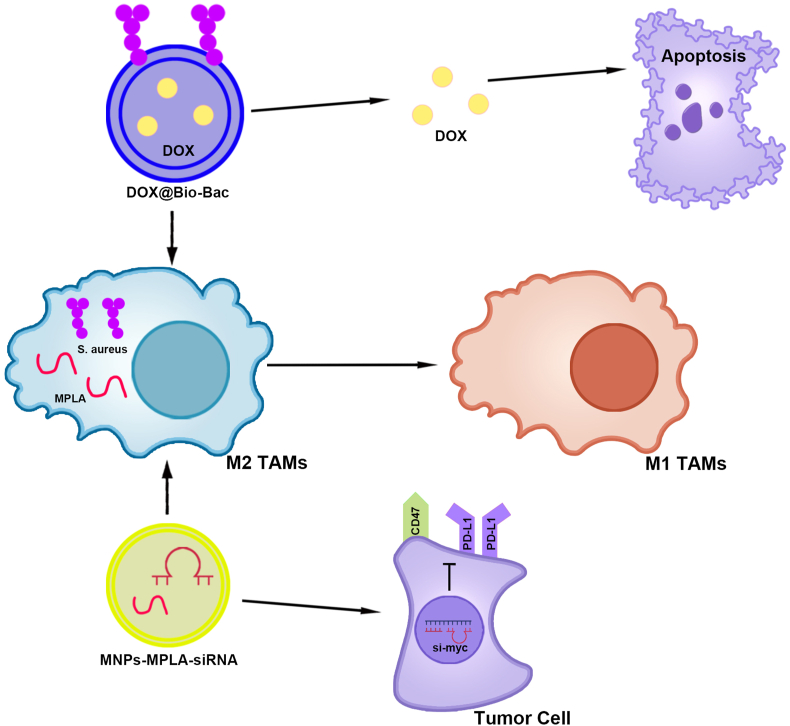
Nanomaterials modified with bacterial derivatives modulating TAM polarization for the treatment of HCC.
(1) DOX@Bio-Bac promoted M2-type TAM polarization to the M1-type through S. aureus and induced apoptosis of liver cancer cells through DOX. (2) MPLA in MNPs-MPLA-siRNA promoted M2-type TAM polarization to M1-type. MNP-MPLA-siRNA inhibits CD47 and PD-L1 expression in HCC cells by silencing the proto-oncogene c-Myc. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; DOX@Bio-Bac, Doxorubicin encapsulated to liposome-based bionic bacteria; S. aureus, Staphylococcus aureus; DOX, Doxorubicin; MNPs-MPLA-siRNA, Cell membrane-derived nanoparticles-monophosphoryl lipid A-short interfering RNA; PD-L1, Programmed cell death-ligand 1.
Fig. 9.
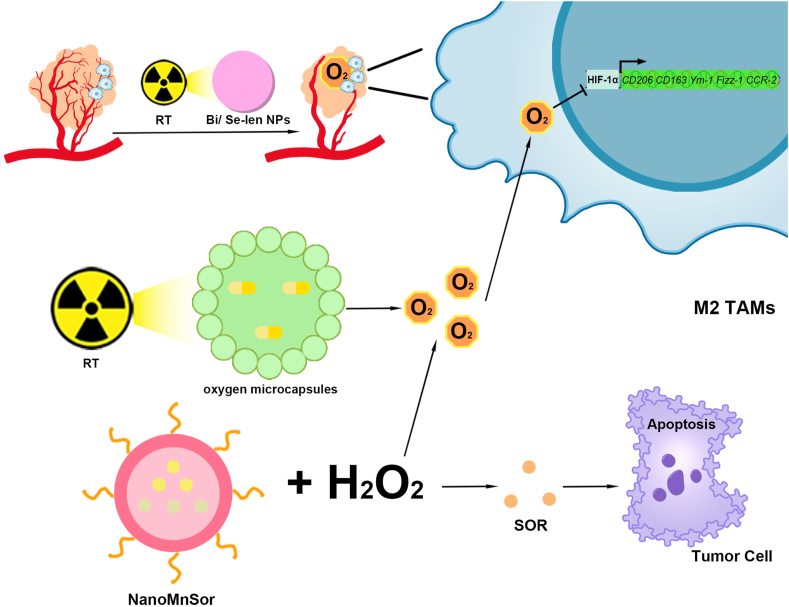
Nanomaterials modulating TAMs polarization for the treatment of HCC via the HIF-1α signaling pathway.
(1) Bi/Se-Len NPs promoted M2-type TAM polarization to the M1-type by normalizing tumor blood vessels through RT, alleviating hypoxia, and inhibiting HIF-1α expression. (2) RT prompted oxygen microcapsules release of oxygen, inhibited the expression of HIF-1α expression, and promoted M2-type TAM polarization to M1-type. (3) NanoMnSor promotes M2-type TAM polarization to M1-type by catalyzing H2O2 to generate oxygen in the TME. In addition, NanoMnSor releases SOR, which causes apoptosis in HCC cells. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; HIF-1α, Hypoxia-inducible factor-1α; Bi/Se-Len, Bi/Se -Lenvatinib nanoparticles; RT, radiotherapy; NanoMnSor, nanomaterials loaded with MnO2 and Sorafenib; TME, Tumor microenvironment; Sor, Sorafenib.
Fig. 10.
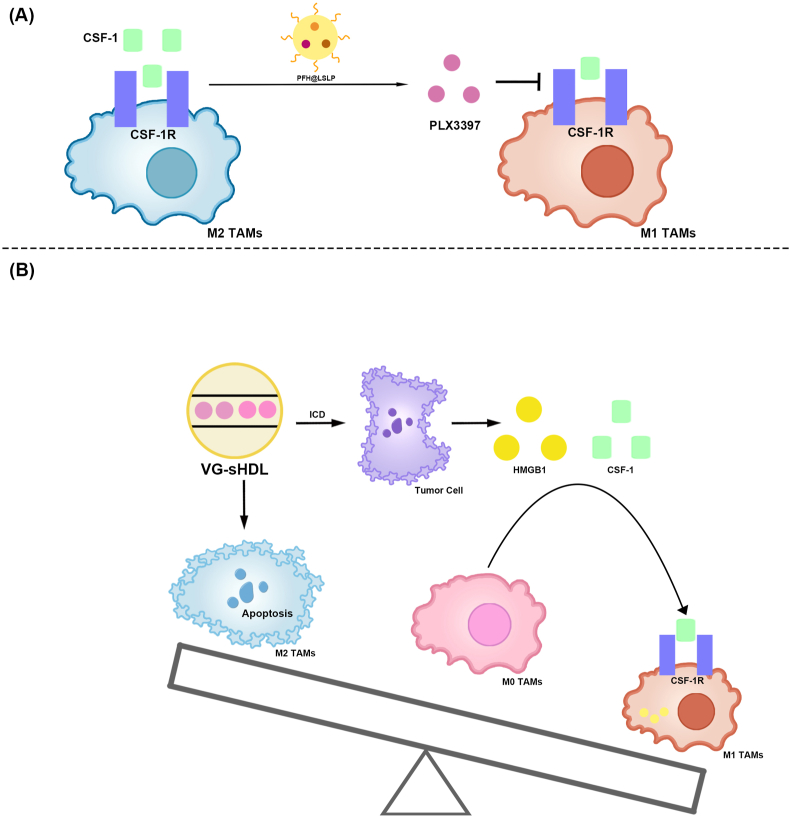
Nanomaterials modulating TAM polarization for the treatment of HCC via the CSF-1/CSF–1R signaling pathway.
(A) PFH@LSLP inhibits the CSF-1/CSF–1R signaling pathway by releasing PLX3397 blockade, which in turn promotes M2-type TAM polarization to M1.
(B) VG-sHDL targets HCC cells and M2-type TAMs, resulting in cell death. ICD released CSF-1 and HMGB1 from HCC cells and promoted monocyte differentiation into M1-type. VG-sHDL reduced the content of M2-type TAMs and increased the content of M1-type TAMs. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; CSF-1/CSF–1R, Colony stimulating factor-1/CSF-1 receptor; PFH@LSLP, An oxygensaturated perfluorohexane-cored liposome, with LFC131 peptides modifying on the surface to deliver sorafenib and PLX3397; VG-sHDL, Vadimezan and Gemcitabine-Synthetic high-density lipoproteins; ICD, Immunogenic cell death; CSF-1, Colony stimulating factor-1; High mobility group box-1 protein, HMGB1.
Fig. 11.
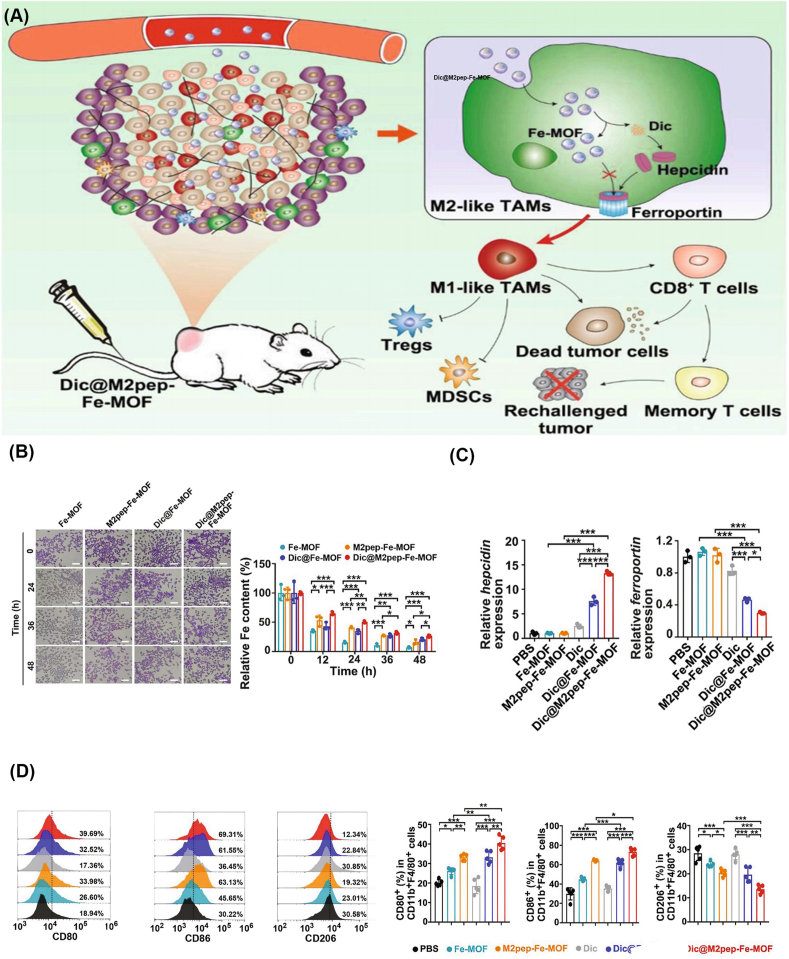
Nanomaterials modulating TAMs polarization for the treatment of HCC via iron metabolisms.
(A) DIC@M2pep-Fe-MOF effectively targets M2-type TAMs and promotes Hepcidin, thereby inhibiting ferroportin expression, reducing the iron efflux of M2-type TAMs, enhancing intracellular aggregation, and promoting M2-type macrophage polarization. M1-type TAMs promote CD8+ T cells and inhibit Tregs and MDSCs.
(B) Relative intracellular Fe content in M2-type macrophage after treatment with Dic@M2pep-FeMOF.
(C) Relative intracellular Fe content in M2-type macrophage after treatment with Dic@M2pep-FeMOF.
(D) Efficient repolarization of M2-type TAMs by Dic@M2pep-Fe-MOF in H22 tumor-bearing mice [204].Copyright 2021 Elsevier. TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; Fe-MOF, Fe-Metal-organic framework; M2pep, M2 macrophage-binding peptide; Dic, Diclofenac; DIC@M2pep-Fe-MOF, Diclofenac encapsulated to M2pep-Fe-MOF; Tregs, Regulatory T cells; BMDCs, Bone marrow-derived myeloid cells.
Fig. 12.
Nanomaterials depleting TAMs for the treatment of HCC using oxygen.
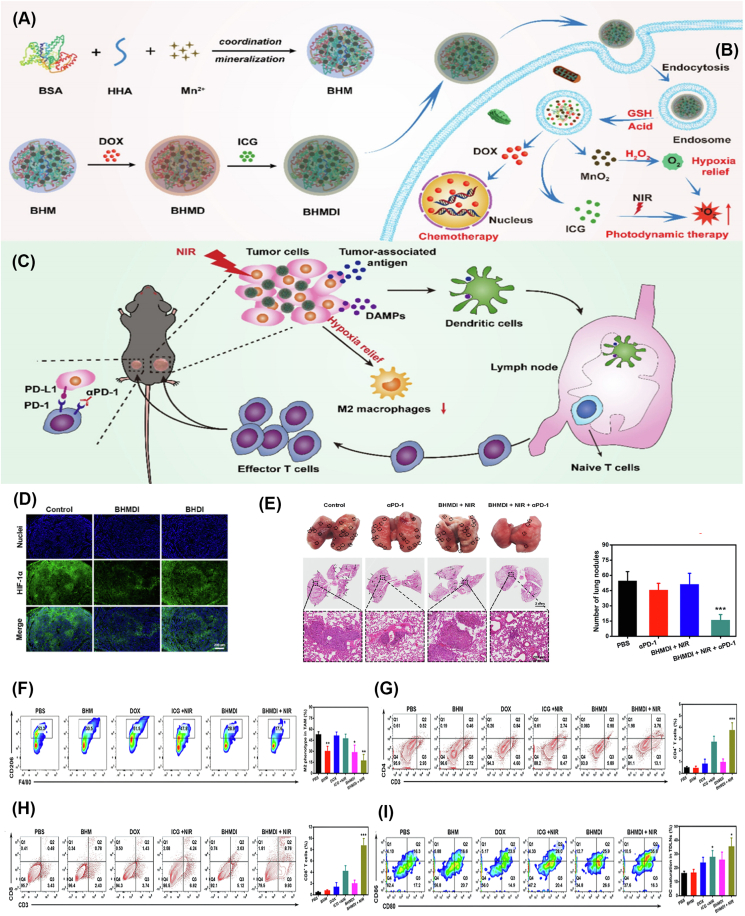
(A) BSA, HHA, and Mn2+ synthesize BHM. BHM is loaded with DOX and ICG and prepared into BHMDI.
(B) BHMDI decomposes into MnO2, ICG, and DOX in GSH and the acidic environment after being engulfed by TAMs. MnO2 catalyzes H2O2 decomposition into O2 and effectively alleviates tumor deficiency. Oxygen reduces the number of M2-type TAMs and significantly improves the efficacy of ICG-mediated PDT. In addition, DOX promotes cell death.
(C) Relief of hypoxia reduces M2-type TAMs. PDT induces ICD in HCC cells, releases tumor-associated antigens and DAMPs and promotes DC and effector T cell maturation. The combined application of BHMDI and anti-PD-1 exerts a good effect and further inhibits tumor growth.
(D) Representative fluorescence images reflecting HIF-1a levels in tumors receiving different treatments. Scale bar: 200 μm
(E) Photographs and H&E-stained images of lungs of mice receiving different treatments, and numbers of lung nodules in each group.
(F) Flow cytometry plots of CD11c+ F4/80+ CD206+ cells in tumors in different groups
(G) Flow cytometry plots of CD3+ CD4+ T cells in tumors in different groups
(H) Flow cytometry plots of CD3+ CD8+ T cells in tumors in different groups
(I) In vivo maturation and maturation rates of DCs in lymph nodes from mice in different groups [206]. Copyright 2022 Elsevier.
TAMs, Tumor-associated macrophages; HCC, Hepatocellular carcinoma; BSA, Bovine serum albumin; HHA, hydrazided hyaluronan; BHM, Manganese oxide-crosslinked bovine albumin/hyaluronic acid nanoparticles; DOX, Doxorubicin; ICG, Indocyanine green; BHMDI, DOX/ICG-coloaded BHM nanoplatform; GSH, Glutathione; PDT, Photodynamic therapy; DAMPs, Damage-associated molecular patterns; DC, Dendritic cell; PD-1, Programmed Cell Death Ligand-1; Near-infrared, NIR; H&E, Hematoxylin-eosin staining; DCs, Dendritic cells.
Fig. 13.
Nanomaterials modulating TAMs polarization for the treatment of pancreatic cancer.
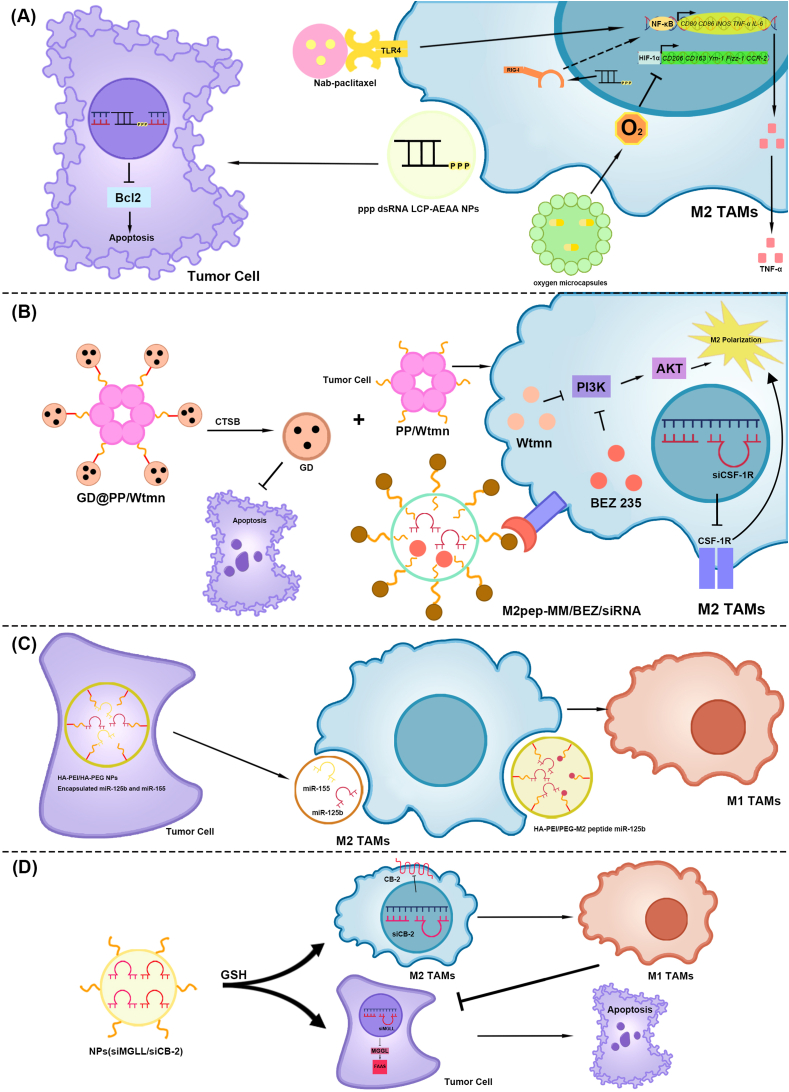
(A) The NF-κB and HIF-1αsignaling pathway. (1) TAMs phagocytose nab-paclitaxel. Nab-paclitaxel promotes TLR4, activates the NF-κB signaling pathway, and induces M2-type TAM polarization to M1-type. (2) ppp dsRNA LCP-AEAA NPs knock out Bcl2 in pancreatic cancer cells and induce apoptosis. In addition, ppp dsRNA activates RIG-I, induces M2-type TAM polarization to M1-type, and releases TNF-α. The NF-κB signaling pathway may be involved. (3) Oxygen microcapsules release the oxygen, which inhibit the HIF-1αsignaling pathway and the polarization of M2 type TAM.
(B) The PI3K/AKT signaling pathway. (1) GD@PP/Wtmn decomposed into GD and PP/Wtmn via CTSB in the pancreatic tumor tissue. GD induces apoptosis in pancreatic cancer cells. Wtmn in PP/Wtmn mice inhibited PI3K and subsequently AKT in M2-type TAMs, impairing TAM polarization to M2-type TAMs. (2) M2pep-MM/BEZ/siRNA targets M2-type TAMs to release BEZ 235 and CSF-1RsiRNA. BEZ 235 inhibited the PI3K/AKT signaling pathway in M2-type TAMs and weakened M2-type TAM polarization. CSF–1R knockdown using CSF–1R siRNA attenuates M2-type TAM polarization.
(C) MicroRNAs: miR-155 and miR-125b. (1) HA-PEI/HA-PEG nanoparticles-encapsulated miR-155 and miR-125b were phagocytosed by pancreatic cancer cells to produce exosomes containing miR-155 and miR-125b. After being engulfed by M2-type TAMs, they promote M2-type TAM polarization to M1-type. (2) The HA-PEl/PEG-M2 peptide miR-125b targets M2 type TAMs through M2pep, and miR-125b promotes M2-type TAM repolarization to M1-type.
(D) CB-2. siMGLL/siCB-2 high GSH concentration in the cytoplasm promoted the release of siMGLL and siCB-2. siMGLL inhibited MGLL expression in pancreatic cancer cells, thereby inhibiting FFAs. siCB-2 inhibits CB-2 expression in M2-type TAMs, thereby promoting M2-type TAM polarization to M1-type and inhibits pancreatic cancer cells. TAMs, Tumor-associated macrophages; NF-kB, Nuclear factor-kappaB; HIF-1α, Hypoxia-inducible factor-1α; Nab-paclitaxel, Nanoparticle albumin-bound paclitaxel; TLR4, Toll-like receptor 4; ppp dsRNA LCP-AEAA NPs, 5′ triphosphate double-stranded RNA Lipid calcium phosphate-targeted with aminoethyl anisamide nanoparticles; Bcl2, B-cell lymphoma 2; ppp dsRNA, 5′ triphosphate double-stranded RNA; RIG-I, Retinoic acid-inducible gene I; TNF-α, Tumor necrosis factor-α; PI3K, Phosphatidylinositol-3hydroxykinase; AKT, AGC serine/threonine kinases; GD@PP/Wtmn, GEM conjugated dendritic poly-lysine DGL encapsulated to polycaprolactone-polyethylene glycol micelles loaded with wortmannin; CTSB, Cathepsin B; GD, GEM conjugated dendritic poly-lysine DGL; PP/Wtmn, polycaprolactone-polyethylene glycol micelles loaded with wortmannin; Wtmn, Wortmannin; M2pep-MM/BEZ/siRNA, M2 macrophage-binding peptide- Mixed micelle/BEZ 235/short interfering RNA; HA-PEI/HA-PEG NP-Encapsulated miR-155 and miR-125b, Hyaluronic acid-poly (ethylene imine)/hyaluronic acid-poly (ethylene glycol) Nanoparticles-Encapsulated miR-155 and miR-125b; M2pep, M2 macrophage-binding peptide; CSF-1RsiRNA, Colony stimulating factor-1 receptor siRNA; CSF–1R, Colony stimulating factor-1 receptor; HAPEI/PEG-M2peptide miR-125b NPs, Hyaluronic acid-poly (ethylene imine)/hyaluronic acid-poly (ethylene glycol)- M2peptide miR-125b Nanoparticles; CB-2, Endocannabinoid receptor-2; NPs(siMGLL/siCB-2), NPs for co-delivery of MGLL siRNA and CB-2 siRNA; GSH, Glutathione; FFAs, Free fatty acids.
Table 1.
Strategies of TAM regulated by nanomaterials in the treatment of digestive system tumors.
| Strategy | Signal pathway | Target | Nanomaterial | Nanomaterial type | Drug or adjuvants | Component | Caner type | Ref. |
|---|---|---|---|---|---|---|---|---|
| TAMs polarization |
STAT | DUSP2 | Hsa_circ_0017,252 | Natural carrier | circ_0017,252 | Exosome from GC cell circ_0017,252 | GC | [42] |
| STAT | STAT3 | Therapeutic NPs | Polymeric NPs | IL-12 and Dox | PGA Dox Chitosan IL-12 |
HCC | [181] | |
| STAT | STAT1 and STAT6 | Chitosan-based NPs | Polymeric NPs | Chitosan | Deacetylated chitosan Pentasodium Tripolyphosphate |
HCC | [182] | |
| STAT and ferroptosis | xCT | Man@pSiNPs-erastin | Si-based NPs | Erastin | Mannose Porous silicon NPs Erastin |
HCC | [183] | |
| NF-κB | TrxR | HAS-Au agent NPs | HAS NPs | Au(III) thiosemicarbazone agent | HSA N-heterocyclic thiosemicarbazone agent Au(III) thiosemicarbazone agent |
GC | [43] | |
| NF-κB | ROS | PIONs@E6 | Metal-based NPs | IONPs | GAL-DSPE-PEG Chlorin e6 Oleic acid coated IONPs |
HCC | [197] | |
| NF-κB | ROS | Man-IONPs | Metal-based NPs | IONPs | Mannose IONPs |
HCC | [195] | |
| NF-κB | ROS | Nanoliposome C6-Ceramide | Lipids/liposome | C6-ceramid | DSPC DOPE DSPEPEG C6-ceramid PEG |
HCC | [198] | |
| PPAR-γ and NF-κB | TLR2 and TLR9 | DL@NP-M-M2 pep | Polymeric NPs | d-lactate | d-lactate PLAG Plasma membrane from hepatic carcinoma DSPE-PEG-M2pep |
HCC | [205] | |
| NF-κB | TLR4 | Nab-paclitaxel | HAS NPs | Nab-paclitaxel | Paclitaxel HAS |
Pancreatic cancer | [312] | |
| NF-κB | TLR4 and TLR9 | MOF-CpG-DMXAA | MOF | CpG-ODN and DMXAA | MOF-801 CpG-ODN DMXAA |
HCC | [190] | |
| NF-κB | TLR5 | EcNflaB-UCNPs | Natural carrier | flaB | UCNPs ECN contained flaB |
HCC | [185] | |
| NF-κB | TLR7 | Pd-M@R NPs | Pd-based NPs | R837 | Pd (acac)2 Mannose R837 |
CRC | [64] | |
| NF-κB and CD47− SIRPα | TLR7 and CD47 | IMD@Hf-DBP/αCD47 | MOF | Imiquimod and αCD47 | Imiquimod Hf-5,15-DBP modified nMOF αCD47 |
CRC | [65] | |
| NF-κB | TLR7 | p (Man-IMDQ) NRs | Polymeric NPs | IMDQ | DEAEMA Polymannose IMDQ |
HCC | [188] | |
| NF-κB | TLR7 | NPs (SOR/R848) | Polymeric NPs | SOR and R848 | PEG PLGA SOR R848 |
HCC | [189] | |
| NF-κB | ox-mtDNA-TLR9 | CCMH | Metal-based NPs | CaO2 and CuS | CaO2 BSA CuS MnO2 HA |
CRC | [66] | |
| NF-κB and IRF3 | RIG-I | NP/3pRNA | Polymeric micelle | 5′ppp-RNA | DMAEMA BMA PAA 5′ppp-RNA |
CRC | [67] | |
| NF-κB | RIG-I | ppp dsRNA LCP-AEAA NPs | Lipids/liposome | ppp dsRNA | ppp dsRNA DOPA CaP Cationic outer leaflet lipids DOTAP Lipid cholesterol DSPE-PEG AEAA |
Pancreatic cancer | [313] | |
| NF-κB | AMP | Met@Man-MPs | Natural carrier | Met | Met Mannose Cellular microparticles from macrophage |
HCC | [192] | |
| NF-κB | AMP | C60(OH)x | C-based NPs | / | C60(OH)x | HCC | [194] | |
| NF-κB | IKKβ | CMCS/M-IMD-CLN | Lipids/liposome | IMD-0354 | CMCS Mannose-DOPE Soylecithin DOTAP IMD-0354 |
HCC | [196] | |
| CSF-1/CSF–1R | CSF–1R | PFH@LSLP | Lipids/liposome | SOR, PLX3397, oxygen, and LFC131 | DSPE-PEG DSPE-PEG-LFC131 Cholesterol SPC SOR PLX3397 PFH Oxygen |
HCC | [203] | |
| CSF-1/CSF–1R | CSF–1R | VG-sHDL | Lipids/liposome | Vadimezan and GEM | DMPC Cholesterol oleate Vadimezan GEM ApoA-1 mimetic peptide |
HCC | [202] | |
| CSF-1/CSF–1R | CSF–1R and ROS | TAT-BLZmlip | Lipids/liposome | BLZ945 | TAT BLZ945 Superparamagnetic NPs Liposome |
CRC | [68] | |
| CSF-1/CSF–1R | CSF–1R and ROS | Ru@ICG-BLZ NPs | Ru-based NPs | BLZ945 | RuCl3 TGMs ICG BLZ945 |
CRC | [69] | |
| / | ROS | Gel/(REG + NG/LY) | Polymeric NPs | Regorafenib and LY3200882 | mPEG-b-PAla hydrogel Regorafenib Semi-deprotected poly (l-lysine) nanogel Thioketal LY3200882 |
CRC | [75] | |
| / | ROS | nCUR/SN38 | Polymeric NPs | Curcumin and SN38 | Deacetylated Chitosan Curcumin SN38 |
CRC | [122] | |
| HIF-1α | Lactic acid | PAPEI/LDHA-siRNA | Polymeric NPs | LDHA-siRNA | Cationic PAPEI LDHA-siRNA |
CRC | [70] | |
| HIF-1α | SUCNR1 | hUCMSC-Exos | Natural carrier | miR-1827 | hUC-MSCs-Exos miR-1827 |
CRC | [71] | |
| HIF-1α | HIF-1α | Bi/Se-Len NPs | Metal-based NPs | Lenvatinib | Bi quantum dots Na2SeO3 Lenvatinib |
HCC | [199] | |
| HIF-1α | HIF-1α | oxygen microcapsules | Polymeric NPs | Oxygen | Dopamine Chitosan polylysine Oxygen |
HCC | [200] | |
| HIF-1α | HIF-1α | NanoMnSor | Metal-based NPs | SOR and MnO2 | DSPE-PEG DSPE-PEG-SP94 DOPC DOPA Cholesterol PLGA TPGS SOR MnO2 |
HCC | [201] | |
| HIF-1α | HIF-1α | Oxygen microcapsules | Polymeric NPs | Oxygen | Dopamine Chitosan polylysine Oxygen |
Pancreatic cancer | [319] | |
| PI3K-AKT | PI3K | GD@PP/Wtmn | Polymeric micelle | GEM and Wtmn | PCL-PEG-PEP-DGL-GEM PCL-PEG-OCH3 Wtmn |
Pancreatic cancer | [315] | |
| PI3K-AKT and CSF-1/CSF–1R | PI3K and CSF–1R | M2pep-MM/BEZ/siRNA | Polymeric micelle | BEZ 235 and siCSF-1R | M2pep PEI-SA DSPE-PEG BEZ 235 siCSF-1R |
Pancreatic cancer | [314] | |
| Hepcidin/ferroportin | Hepcidin | Dic@M2pep-Fe-MOF | MOF | Diclofenac | Fe-MOF M2pep Diclofenac |
HCC | [204] | |
| / | Hsp110 | Nanofitins A-C2 | Nanofitin | A-C2 | A-C2 | CRC | [74] | |
| / | miR-125b and miR-155 | HA-PEI/HA-PEG NPs- encapsulated miR-155 and miR-125b | Polymeric micelle | miR-125b and miR-155 | HA-PEI/HA-PEG miR-155 miR-125b |
Pancreatic cancer | [316] | |
| / | miR-125b | HAPEI/PEG-M2peptide miR-125b NPs | Polymeric micelle | miR-125b | HA-PEI/HA-PEG M2pep miR-125b |
Pancreatic cancer | [317] | |
| / | CB-2 | NPs(siMGLL/siCB-2) | Polymeric NPs | siMGLL and siCB-2 | PDSA DSPE-PEG siMGLL siCB-2 |
Pancreatic cancer | [318] | |
| / | / | Ch/γ-PGA NPs | Polymeric NPs | Chitosan and γ-PGA | Chitosan γ-PGA PEMs |
CRC | [73] | |
| / | / | Ch/γ-PGA NPs incorporated with IFN-γ | Polymeric NPs | Chitosan, γ-PGA and IFN-γ | Chitosan γ-PGA PEMs IFN-γ |
CRC | [72] | |
| / | / | DOX@Bio-Bac | Lipids/liposome | Dox and S. aureus | Dox S. aureus Soybean lecithin Cholesterol |
HCC | [186] | |
| / | / | MNPs-MPLA-siRNA | Natural carrier | MPLA and siC-MYC | Plasma membrane from hepatic carcinoma MPLA siC-MYC |
HCC | [187] | |
| Depleting TAMs |
/ | Mannose Receptor | DOX@MAN-BSA NPs | Polymeric NPs | DOX | Dox Mannose BSA |
CRC | [76] |
| / | Mannose Receptor | RG@M-γ-CD CNPs | Polymeric NPs | Regorafenib | Regorafenib Mannose γ-CD |
CRC | [77] | |
| / | Oxygen | BHMDI | Metal-based NPs | MnO2, DOX and ICG | BSA HHA MnO2 DOX ICG |
HCC | [206] | |
| Enhancing TAMs phagocytosis |
CD47-SIRPα | SIRPα | Exo-SIRPα | Natural carrier | Anti-SIRPα | Exosome from HEK293T cells anti-SIRPα |
CRC | [78] |
| CD47-SIRPα and CRT-CD91 |
CD47 and CRT |
SNALPssiCD47-Dox |
Polymeric NPs |
siCD47 and DOX |
Cholesterol DSPC DOTAP C16-PEG-Ceramide siCD47 DOX |
CRC |
[79] |
|
| Preventing TAMs infiltration | CXCR4-SDF1α | CXCR4 | ADOPSor NPs | Polymeric NPs | SOR and AMD3100 | AMD3100 DOPA-PLGA SOR |
HCC | [178] |
| CXCR4-SDF1α | CXCR4 | VEGF siRNA in AMD-NPs | Lipids/liposome | siVEGF and AMD3100 | siVEGF AMD3100 Protamine Nucleic acids DOPC DOPA Cholesterol DSPE-PEG |
HCC | [179] | |
| / | ROS | CeO2NPs | Metal-based NPs | CeO2NPs | CeO2 | HCC | [180] | |
Abbreviations: TAMs, Tumor-associated macrophages; STAT, Signal transducer of activators of transcription; DUSP2, Dual specificity phosphatase 2; GC, Gastric cancer; NPs, nanoparticles; IL-12, Interleukin-12; DOX, Doxorubicin; PGA, Poly(glutamic acid); HCC, Hepatocellular carcinoma; xCT, Cysteine/glutamate transporter; NF-kB, Nuclear factor-kappaB; Man@pSiNPs-erastin, Mannose encapsulated to functionalized porous silicon nanoparticles-erastin; HAS-Au agent NPs, HAS-Au (III) thiosemicarbazone agent NP; TrxR, Thioredoxin reductase; HAS NPs, Human Serum Albumin NPs; HAS, Human Serum Albumin; ROS, Reactive oxygen species; PIONs@E6, Exosomes synergized with pegylated IONs loaded with chlorin E6; IONPs, Iron oxide nanoparticles; GAL-DSPE-PEG, Galactose conjugated to 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-succinyl(polyethylene glycol)-2000; Man-IONPs, Mannose-Iron oxide nanoparticles; DSPC, 1,2-distearoyl-snglycero-3-phosphocholine; DOPE, 1,2-dioleoyl-sn-glycero3-phosphoethanolamine; DSPEPEG, 1,2-distearoyl-sn-glycero3-phosphoethanolamine-polyethylene glycol; PEG, Polyethylene glycol; PPAR-γ, peroxisome proliferator–activated receptor γ; TLR, Toll-like receptor; DL@NP-M-M2 pep, Lactide-glycolide copolymer nanoparticles to load d-lactate, and modified the DL-loaded NP with HCC membrane and M2 macrophage-binding peptide; DSPE-PEG- M2 pep, 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (poly ethylene glycol)-M2 macrophage-binding peptide; Nab-paclitaxel, Nanoparticle albumin-bound paclitaxel; MOF-CpG-DMXAA, Metal-organic framework-801-cytosine-phosphate-guanine oligodeoxynucleotides−5, 6-dimethylxanthenone-4-acetic acid; MOF, Metal-organic framework; CpG-ODN, cytosin-phosphate-guanine oligodeoxynucleotides; DMXAA, 5, 6-dimethylxanthenone-4-acetic acid; EcNflaB-UCNPs, Lanthanide upconversion nanoparticles-conjugated engineered Escherichia coli Nissle 1917; flaB, Flagellin B; EcN, Escherichia coli Nissle 1917; UCNPs, rare-earth upconversion NPs; Pd-M@R NPs, Pd-Man NPs loaded with R837; CRC, Colorectal cancer; SIRPα, Signal regulatory protein α; IMD@HF-DBP/αCD47, Hf-DBP nanoscale MOF for the co-delivery of imiquimod, and αCD47; Hf-5,15-DBP modified nMOF, Hf-5,15-di(pbenzoato)porphyrin modified nMOF; p(Man-IMDQ) NRs, p(Mannose-imidazoquinoline) nanoregulators; IMDQ, Imidazoquinoline; DMAEMA, dimethylaminoethyl methacrylate; NPs (SOR/R848), Nanoparticles(Sorafenib/Resiquimod); SOR, Sorafenib; R848-C16, Modified resiquimod; PLGA, polylactic-co-glycolic-acid; CCMH, CaO2@CuS–MnO2@HA; ox-mtDNA, oxidized mitochondrial DNA; HA, Hyaluronic Acid; IRF3, Interferon Regulatory Factor 3; RIG-I, Retinoic acid-inducible gene I; NP/3pRNA, NPs delivery 5′ triphosphate, short, double-stranded RNA; DMAEMA, dimethylaminoethyl methacrylate; BMA, butyl methacrylate; PAA, propylacrylic acid; ppp dsRNA LCP-AEAA NPs, 5′ triphosphate double-stranded RNA Lipid calcium phosphate-targeted with aminoethyl anisamide nanoparticles; ppp dsRNA, 5′ ppp double-stranded RNA; DOPA, 1,2-dioleoyl-sn-glycero-3-phosphate; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DSPE-PEG, 1,2-distearoryl-sn-glycero-3phosphoethanolamine-N-[methoxy(polyethyleneglycol-2000)] ammonium salt; AEAA, Aminoethyl anisamide; AMP, Adenosine monophosphate; Met@Man-MPs, Metformin encapsulated to Mannose-cellular microparticles; Met, Metformin; IKKβ, IkappaB kinase β; CLN CMCS/M-IMD-CLN, Cationic lipid-based nanoparticles o-carboxymethyl-chitosan/Mannose-Imiquimod-Cationic lipid-based nanoparticles; CLN, Cationic lipid-based nanoparticles; CMCS, O-carboxymethyl-chitosan; CSF-1/CSF–1R, Colony stimulating factor-1/CSF-1 receptor; PFH@LSLP, Oxygen saturated perfluorohexane-cored liposome, with LFC131 peptides modifying on the surface to deliver sorafenib and PLX3397; PFH, perfluorohexane; VG-sHDL, Vadimezan and Gemcitabine-Synthetic high-density lipoproteins; GEM, Gemcitabine; DMPC, dimyristoylphosphatidylcholine; TAT-BLZmlip, Transcriptional activator protein-BLZ945magnetic liposomal; Ru@ICG-BLZ NPs, Ru encapsulated to Indocyanine green and BLZ945; Gel/(REG + NG/LY), ROS-responsive nanogels loaded with regorafenib and LY3200882; mPEG-b-Pala, methoxy poly(ethylene glycol)-block-poly(l-alanine); nCUR/SN38, Chitosan loaded Cur and SN38; SN38, 7-ethyl-10-hydroxycamptothecin; HIF-1α, Hypoxia-inducible factor-1α; PAPEI/LDHA-siRNA, APEG-PAsp(PEI)/Lactic acid-short interfering RNA; PAPEI,APEG-PAsp(PEI); SUCNR1, Succinate receptor 1; hUCMSC-Exos, Human umbilical cord mesenchymal stem cell-derived exosomes; Bi/Se-Len, Bi/Se- Lenvatinib nanoparticles; NanoMnSor, Nanomaterials loaded with MnO2 and Sorafenib; DSPE-PEG-SP94, DSPE-PEG-SFSIIHTPILPL peptide; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; TPGS, D-α-tocopherol polyethylene glycol 1000 succinate; PI3K, Phosphatidylinositol-3hydroxykinase; AKT, AGC serine/threonine kinases; GD@PP/Wtmn, GEM conjugated dendritic poly-lysine DGL encapsulated to polycaprolactone-polyethylene glycol micelles loaded with wortmannin; PCL-PEG-PEP-DGL-GEM, polycaprolacton-poly(ethylene glycol)-peptide-dendrigraft poly-l-lysine-GEM; PCL-PEG-OCH3, polycaprolacton-poly(ethylene glycol)-OCH3; Wtmn, wortmannin; M2pep-MM/BEZ/siRNA, M2 macrophage-binding peptide-Mixed micelle/BEZ 235/short interfering RNA; PEI-SA, polyethyleneimin-stearic acid; DIC@M2pep-Fe-MOF, Diclofenac encapsulated to M2pep-Fe-MOF; Hsp110, Heat-shock proteins 110; HA-PEI/HA-PEG NPs-Encapsulated miR-155 and miR-125b, Hyaluronic acid-poly (ethylene imine)/hyaluronic acid-poly (ethylene glycol) Nanoparticles-Encapsulated miR-155 and miR-125b; HAPEI/PEG-M2peptide miR-125b NPs, Hyaluronic acid-poly (ethylene imine)/hyaluronic acid-poly (ethylene glycol)-M2peptide miR-125b Nanoparticles; CB-2, Endocannabinoid receptor-2; NPs(siMGLL/siCB-2), NPs for co-delivery of MGLL siRNA and CB-2 siRNA; PDSA, poly (disulfide amide); γ-PGA, Poly-γ-glutamic acid; IFN-γ, Interferon-γ; PEMs, Polyelectrolyte multi-layered films; DOX@Bio-Bac, Doxorubicin encapsulated to liposome-based bionic bacteria; S. aureus, Staphylococcus aureus; DOX, Doxorubicin; MNPs-MPLA-siRNA, Cell membrane-derived nanoparticles-monophosphoryl lipid A-short interfering RNA; MPLA, Monophosphoryl lipid A; DOX@MAN-BSA NPs, Doxorubicin encapsulated to mannose-modified bovine serum albumin NPs; BSA, bovine serum albumin; RG@M-γ-CD CNPs, Regorafenib encapsulated to mannose-modified γ-cyclodextrin non-covalent channel-type NPs; γ-CD, γ-cyclodextrin; BHMDI, DOX/ICG-coloaded BHM nanoplatform; ICG, Indocyanine green; HHA, hydrazided hyaluronan; Exo-SIRPα, Exosomes-SIRPα; SNALPssiCD47-Dox, Stable nucleic acid-lipid particles loaded with short interfering RNA CD47 and Doxorubicin; CRT, Calreticulin; CXCR4, C-X-C chemokine receptor type 4; SDF1α, Stromal-derived-factor1α; ADOPSor NPs, AMD3100 coated DOPA-PLGA nanoparticles containing sorafenib; VEGF siRNA in AMD-NPs, Vascular endothelial-derived growth factor short interfering RNA in AMD3100-NPs.
Fig. 14.
Macrophage-derived nanomaterials for the treatment of digestive system tumor.
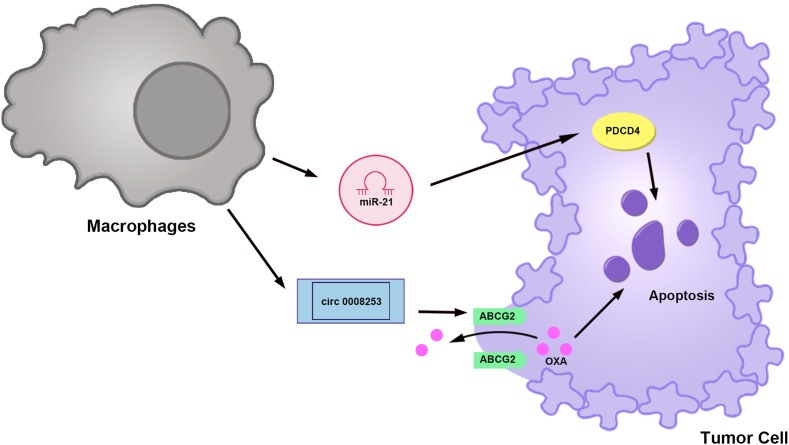
(1) Exosomes secreted by macrophages deliver miR-21, which increases PDCD4 expression and leads to the apoptosis of gastric cancer cells. (2) circ 0008253 exosomes secreted by macrophages increase ABCG2 expression. ABCG2 transports OXA out of gastric cancer cells, leading to OXA resistance. PDCD4, Programmed cell death 4; ABCG2, ATP binding cassette subfamily G member 2; OXA, Oxaliplatin.
Fig. 15.
Macrophage membrane-coated nanomaterials and macrophage-based microrobot for the treatment of digestive system tumor.
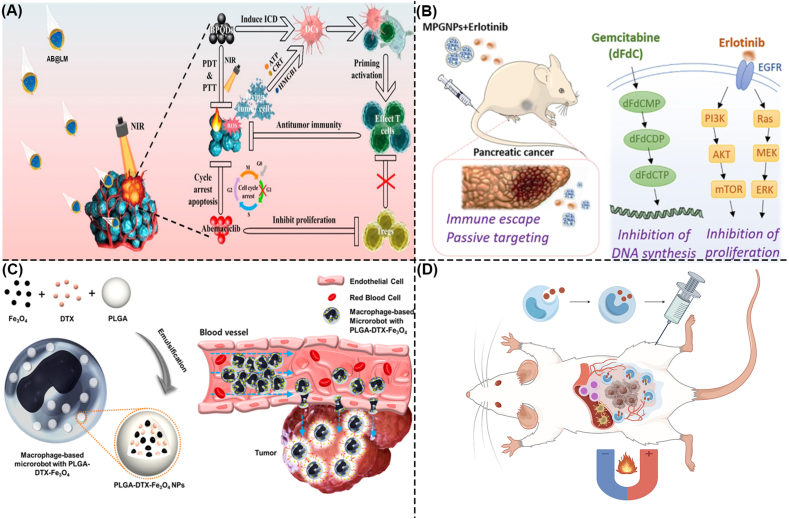
(A) Macrophage membrane-coated nanomaterials for the treatment of CRC. AB@LM targets CRC tissue and releases BPQD and Abemaciclib. Under NIR light irradiation, BPQD generates ROS to induce ICD and activate DC and effector T cells. Abemaciclib induces direct apoptosis of colon cancer cells and inhibition of Tregs [353]. Copyright 2022, American Chemical Society.
(B) Macrophage membrane-coated nanomaterials for the treatment of pancreatic cancer. MPGNPs and erlotinib target tumor tissues. MPGNPs released Gem and inhibited DNA synthesis in pancreatic cancer cells by promoting the dFdCMP-dFdCDP-dFdCTP signaling pathway. Erlotinib binds to EGFR and activates the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways, thereby inhibiting the proliferation of pancreatic cancer cells [354]. Copyright 2021, American Chemical Society.
(C) Macrophage-based microrobot for the treatment of CRC. Macrophages phagocytized PLGA-DTX-Fe3O4 to form a mixed-driven microrobot. Under the action of the EMA system, PLGA-DTX-Fe3O4 targets tumor spheres and delivers DTX, thereby leading to CRC cell apoptosis [356].Copyright 2016, Jiwon Han et al.
(D) Macrophage-based microrobot for the treatment of pancreatic cancer. Macrophages engulf core/shell iron/iron oxide nanoparticles to form macrophage-based microrobots. Macrophage-based microrobots were injected into mice in a disseminated peritoneal pancreatic cancer model. Under the action of AMF, macrophage-based microrobots target the tumor tissue, generate heat, induce pancreatic cancer cell apoptosis, and promote immune cell infiltration. By FigDraw. CRC, Colorectal cancer; AB@LM, Artificial Assembled Macrophages; BPQD, Black phosphorus quantum dot; NIR, Near-infrared; ROS, Reactive oxygen species; ICD, Immunogenic cell death; DC, Dendritic cell; Tregs, Regulatory T cells; MPGNPs, Gemcitabine-loaded PLGA NPs with macrophage membrane coating; dFdCMP, Gemcitabine monophosphate; dFdCDP, Gemcitabine diphosphate; dFdCTP, gemcitabine triphosphate; EGFR, Epidermal Growth Factor Receptor; PI3K, Phosphoinositide 3-kinase; AKT, AGC serine/threonine kinases; mTOR, Mammalian/mechanistic target of rapamycin; Ras, Rat sarcoma; Raf, Rapidly accelerated fibrosarcoma; MEK, Mitogen-activated protein kinase; ERK, Extracellular signal-regulated kinase; PLGA, Poly-lactic-co-glycolic-acid; DTX, Docetaxel; PLGA-DTX-Fe3O4 NPs, Poly-lactic-co-glycolic-acid-Docetaxel-Fe3O4 nanoparticles.
Table 2.
Macrophage-derived nanomaterials for the treatment of digestive system tumors.
| Category | Nanomaterials | Drug or adjuvants | Caner type | Results | Ref. |
|---|---|---|---|---|---|
| Exosomes derived from macrophages | Exosomes miR-21 | miR-21 | GC | Promote PDCD4, leading to apoptosis of gastric cancer cells | [344] |
| Exosomes circ 0008253 | circ 0008253 | GC | Promote ABCG2, leading to resistance of gastric adenocarcinoma cells to Oxaliplatin | [345] | |
| Macrophage membrane-coated nanomaterials | AB@LM | BPQD and Abemaciclib | CRC | Induce ICD of CRC cells, activate DC and effector T cells, and inhibit Tregs. | [353] |
| MPGNPs | GEM | Pancreatic cancer | Inhibit the DNA synthesis of pancreatic cancer cell | [354] | |
| Macrophage-based microrobot | PLGA-DTX-Fe3O4 | DTX | CRC | Induce apoptosis of CRC cells | [356] |
| Paramagnetic iron/iron oxide NPs | Fe and Fe3O4 | Pancreatic cancer | Induce apoptosis of pancreatic cancer cells | [357] |
Abbreviations: GC, Gastric cancer; PDCD4, Programmed cell death 4; ABCG2, ATP binding cassette subfamily G member 2; CRC, Colorectal cancer; AB@LM, Artificial Assembled Macrophages; BPQD, Black phosphorus quantum dot; ICD, Immunogenic cell death; DC, Dendritic cell; Tregs, Regulatory T cells; MPGNPs, Gemcitabine-loaded PLGA NPs with macrophage membrane coating; DTX, Docetaxel; PLGA-DTX-Fe3O4 NPs, Poly-lactic-co-glycolic-acid-Docetaxel-Fe3O4 nanoparticles.
2. Modulation of TAMs by nanomaterials for the treatment of GC
GC is associated with high morbidity and mortality worldwide [40]. High infiltration of M2-type TAMs is an important factor associated with poor prognosis in patients with GC [30]. M2-type TAMs contribute to the growth and spread of GCs by promoting blood and lymphatic vessel formation [41]. Nanomaterials effectively regulate TAM polarization, thus preventing the occurrence and development of GC [42,43] (Fig. 1, Table 1). Therefore, we summarized the methods by which nanomaterials regulate TAMs to treat GC, providing new ideas and directions for its treatment.
2.1. Nanomaterials modulate TAM polarization for GC treatment
2.1.1. STAT signaling pathway
The signal transducer and activator of the transcription (STAT) signaling pathway is a crucial communication network for cellular functions [44]. In this pathway, STAT is phosphorylated, leading to its dimerization and translocation to the nucleus through the nuclear membrane [45]. STAT regulates the expression of related genes in the nucleus [45]. Among the STAT family members, STAT3 is significantly involved in macrophage polarization. The STAT3 pathway contributes to GC metastasis by promoting TAM polarization to the M2 type [30,46].
Interaction between dual-specificity phosphatase 2 (DUSP2) and STAT3 results in STAT3 dephosphorylation, thereby reducing its activity [[47], [48], [49]]. Jin et al. discovered that hsa_circ_0017,252 exosomes upregulate DUSP2 in M2-type TAMs, which, in turn, inhibits p-STAT3 expression [42]. Consequently, polarization to the M2 phenotype is reduced, inhibiting GC cell invasion and migration [42]. Exosomes, which are natural nanomaterials [50], are discussed in detail in Section 6.1.
2.1.2. Nuclear factor-kappa B signaling pathway
The nuclear factor-kappaB (NF-κB) pathway is a well-known pro-inflammatory signaling pathway that primarily stimulates the expression of pro-inflammatory genes, such as cytokines, chemokines, and adhesion molecules [51]. In its inactive state, NF-κB forms a complex with the NF-κB inhibitor (IκB) and remains in the cytosol [52]. Pro-inflammatory signals, such as cytokines, pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs), trigger IκB degradation [52]. This releases NF-κB into the nucleus, thereby increasing pro-inflammatory gene expression [52]. Reactive oxygen species (ROS) are highly reactive molecules that contain oxidizing agents generated in cells through various mechanisms [53]. ROS can induce macrophage polarization to the M1 phenotype by facilitating NF-κB entry into the nucleus and promoting the expression of M1-type macrophage-related proteins [54].
In GC tissues, the ROS content in TAMs is reduced, leading to TAM polarization towards the M2 phenotype and promoting GC invasion and metastasis [55]. However, the cationic organogold (III) complex [Au(III)] promotes thioredoxin reductase (TrxR) inactivation and reduces ROS scavenging, thereby increasing intracellular ROS levels [56,57]. Zhang et al. developed NPs consisting of a human serum albumin-Au(III) thiosemicarbazone agent (HAS-Au NPs) [43]. HAS-Au NPs augmented ROS generation by M2-type macrophages, thereby upregulating NF-κB expression and polarizing macrophages into the M1-type [43]. The use of HAS-Au NPs increased the M1-type TAM population within GC tissues, which subsequently activated and recruited CD4+ T, CD8+ T, and natural killer cells [43].
3. Modulation of TAMs by nanomaterials for the treatment of CRC
The prevalence and fatality rates of CRC have substantially increased recently [58,59]. The TME actively participates in CRC initiation and progression [60]. TAMs are prevalent in the TME of patients with CRC [31]. TAMs in the TME are predominantly the M2-type, which are important in cancer cell proliferation and metastasis [61]. A low M1/M2 ratio indicates poor prognosis and facilitates tumor cell metastasis [31,62,63]. Nanomaterials modulate TAM polarization [[64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]], eliminate M2-type TAMs within tumor tissues [76,77], and enhance TAMs phagocytic capacity [78,79], thereby effectively impeding CRC progression (Fig. 2, Fig. 3, Fig. 4, Table 1). Consequently, we present a comprehensive overview of the use of nanomaterials through various methods to regulate TAMs in the treatment of CRC, offering novel insights and avenues for therapeutic interventions.
3.1. Nanomaterials modulate TAM polarization for CRC treatment
3.1.1. NF-kB signaling pathway
In Section 2.1.2, the NF-κB signaling pathway influence on macrophage polarization and its involvement in GC was comprehensively explained. In CRC, regulating the NF-κB signaling pathway in TAMs also holds significant importance. Nanomaterials can activate the NF-κB signaling pathway by regulating TLR7, TLR9, and RIG-I (Retinoic acid-inducible gene I), thereby facilitating the polarization of M2-type TAMs into M1-type TAMs in the TIME of CRC [[64], [65], [66], [67]] (Fig. 2A).
3.1.1.1. TLR
Toll-like receptors (TLRs), classified as pattern recognition receptors (PRRs), play a crucial role in recognizing DAMPs and PAMPs and subsequently initiate innate immune responses [80]. All TLR signaling pathways ultimately activate NF-κB and promote the expression of a series of genes encoding inflammatory cytokines [81]. TLR7, a member of the TLR family, is expressed in endosomal membranes [82]. TLR7 can recognize single-stranded RNA and short interfering RNA (siRNA), leading to a cascade of signal transduction events [83] and is involved in M2-type macrophage polarization towards the M1 phenotype [84]. Reduced TLR7 expression is associated with progression of colorectal polyps in CRC [85]. Conversely, elevated levels of TLR7 in the CRC tissues of patients are associated with a more favorable prognosis [86].
TLR7/8 agonists have been identified as anti-tumor medications that induce the release of pro-inflammatory cytokines by activating NF-κB via TLR7/8, consequently influencing the immune status of the TME and exhibiting anti-tumor properties [[87], [88], [89]]. Zhang et al. conducted a study which revealed that TLR7/8 agonists facilitate TAM polarization from the M2-type to the M1-type by activating the NF-κB pathway, effectively impeding melanoma progression [90]. Similarly, Liu et al. conducted a study, which demonstrated that TLR7/8 agonists promote the differentiation of myeloid-derived suppressor cells into M1-type TAMs, thereby reversing oxaliplatin (OXA) resistance in CRC [91]. However, the low solubility and potent systemic toxicity of TLR7/8 agonists necessitate the exploration of strategies to enhance their efficacy and safety [92]. One such approach involves the use of bioconjugates and NP formulations to modify pharmacokinetics, biodistribution, and cell targeting [88]. In this light, Chen et al. developed bidirectional anisotropic palladium (Pd) nanocluster Pd-HA + Pd-M@R NPs, which possessed simultaneous targeting capabilities for CRC cells and M2-type TAMs through hyaluronic acid (HA) and Man modifications [64]. Pd nanozymes exhibit significant peroxidase and catalase activities, leading to ROS generation through RT-radiodynamic therapy, thereby directly inducing CRC cell death [64]. Pd-M@R NPs effectively delivered the TLR7/8 agonist R837 and induced ROS production, resulting in M2-type TAM polarization to the M1 phenotype and reversal of the immunosuppressive TIME [64].
In addition, Ni et al. developed IMD@HF-DBP/αCD47, which is a hafnium-DBP nanoscale metal-organic-framework (HF-DBP nMOFs) equipped with the TLR7 agonist IMD and the anti-CD47 antibody (αCD47) [65]. HF-DBP nMOFs are effective sensitizers for RT-radiodynamic therapy [93]. Consequently, when exposed to X-rays, IMD@HF-DBP/αCD47 induced immunogenic cell death (ICD), polarized M2-type TAMs to M1 phenotype and enhanced TAMs' phagocytic activity by blocking CD47 on tumor cells [65]. Notably, TLR7 is involved in the liver metastasis of CRC. The expression of miR-21 in plasma-derived exosomes is positively associated with liver metastasis in patients with CRC [94]. These miR-21-enriched exosomes specifically target the liver tissue and bind to TLR7 receptors on liver macrophages, leading to IL-6 secretion by macrophages and the induction of inflammatory premetastatic niches [94]. Consequently, the development of TLR7 agonists requires the targeting of specific tissues, a characteristic that can be achieved using nanomaterials. Additionally, owing to the limited literature on TLR8 involvement in CRC, our study specifically focused on investigating TLR7.
TLR9, a member of the TLR family, can identify DNA fragments released by host cells, thereby inducing inflammatory reactions [95]. Its predominant expression was observed in immune cells, particularly macrophages [96]. ICD induces oxidative damage to mitochondrial DNA in tumor cells, resulting in the generation of DAMPs known as oxidized mitochondrial DNA (ox-mtDNA) [97]. TLR9 can recognize this ox-mtDNA and subsequently activate myeloid differentiation primary response 88, leading to NF-κB activation [97]. This activation process promotes TAM polarization towards the M1 phenotype, triggering the release of inflammatory factors, stimulating immune responses, and restraining pancreatic cancer growth [97].
Photodynamic therapy (PDT) is an invasive approach to treating cancer that involves the utilization of red or near-infrared (NIR) light to activate photosensitizers that accumulate in tumors [98,99]. Upon activation, photosensitizers react with oxygen to ROS, thereby inducing ICD [98,99]. However, PDT efficacy is hindered by the hypoxic TME [100]. To address this issue, copper-based nanomaterials have been investigated because of their favorable NIR light absorption properties and ability to synergize with hydrogen peroxide (H2O2) within the TME, resulting in oxygen production and ultimately enhancing PDT efficacy [101]. Furthermore, CaO2 nanomaterials generate O2 and H2O2 while releasing Ca2+ ions to induce calcium overload and facilitate apoptosis in tumor cells [102]. Consequently, Huang et al. developed CaO2@CuS–MnO2@HA (CCMH) nanocomposites to induce ICD through PDT, leading to mitochondrial impairment and ox-mtDNA liberation [66]. The presence of ox-mtDNA facilitates the conversion of M2-type TAMs into M1-type TAMs in CRC tissues [66]. Notably, a correlation was observed between increased TLR9 expression and tumor differentiation, invasion, and liver metastasis in CRC [103]. Furthermore, CRC cells exhibit elevated TLR9 levels, potentially facilitating tumor growth and invasion [104]. TLR9 expression in CRC cells promotes CRC, whereas TLR9 expression in TAMs impedes CRC development. This study employed the characteristics of nanomaterials to augment the efficacy of ICD, resulting in the apoptosis of CRC cells and increased ox-mtDNA levels. The presence of ox-mtDNA triggers TLR9 activation, facilitating the transformation of TAMs into the M1-type, thereby further impeding CRC progression.
3.1.1.2. RIG-I
Nanomaterials stimulate interferon regulatory factors (IRFs) via RIG-I and induce M2-type TAM polarization. The involvement of the NF-κB signaling pathway in this process has also been suggested (Fig. 2A). RIG-I, a cytoplasmic innate immune receptor, recognizes double-stranded RNA (dsRNA) [105]. Following this recognition, RIG-I recruits specific intracellular adapter proteins, thereby initiating signaling pathways that ultimately activate NF-κB and IRFs [106]. Consequently, transcription of type I interferons and other inflammatory factors are promoted [106]. Artificially synthesized triphosphorylated stem-loop RNAs function as RIG-I agonists, thereby promoting macrophage polarization towards the M1 phenotype [107]. Despite the effective regulation of macrophage polarization by RNA RIG-I agonists, their delivery is hindered by lysosomal degradation, leading to reduced efficacy [108,109].
To address this issue, a pH-responsive nanomicelle was engineered to enhance the RNA delivery capability [[110], [111], [112]]. Under physiological pH conditions, the nanomicelles maintained an almost charge-neutral state [113]. In lysosomes, a decrease in pH causes significant alteration in micelles, resulting in a positive charge and increased hydrophobicity [113]. Disruption of the lysosomal membrane allows micelles to diffuse into the cytosol [113]. Max et al. used micelles to load the 3pRNA RIG-I ligand (NP/3pRNA), which effectively enhanced 3pRNA delivery to macrophages and activated RIG-I [67]. RIG-I activation subsequently promoted IRF3 activation, leading to increased expression of TAMs M1-type markers CD80 and CD86 and elevated secretion of IFN-I, IL-6, and TNFα in CRC tissue [67]. While this study did not encompass NF-κB, the secretion of IL-6 and TNF-α, driven by NF-κB, increased [67]. Consequently, the involvement of the NF-κB signaling pathway may be implicated in TAM polarization mediated by RIG-1 in CRC.
3.1.2. Colony-stimulating factor-1 (CSF-1)/CSF-1 receptor (CSF–1R) signaling pathway
The CSF-1/CSF–1R signaling pathway plays a crucial role in regulating TAM polarization in CRC. This signaling pathway influences TAMs in the digestive system and contributes to the initiation and progression of digestive system tumors [114]. Notably, CSF–1R inhibitors can induce the conversion of M2-type TAMs to M1-type TAMs, leading to anti-tumor effects [84,115,116]. Moreover, in primary CRC, CSF–1R overexpression is strongly associated with unfavorable patient survival outcomes [117]. Hence, nanomaterials containing CSF–1R inhibitors have the potential to induce the conversion of M2-type TAMs to the M1 phenotype and impede CRC progression [68,69] (Fig. 2B). Additionally, these nanomaterials possess optical, thermal, and magnetic properties that enable them to generate ROS and facilitate TAM polarization.
Fang et al. developed a magnetic liposome, denoted as TAT-BLZmlips, which was modified with a cell-penetrating TAT peptide and loaded with the CSF–1R inhibitor BLZ945 [68]. TAT-BLZmlips target the tumor site under the action of an external fixed magnetic field [68]. TAT-BLZmlips were then subjected to heat by applying an alternating magnetic field, which promoted BLZ945 release and ROS production [68]. BLZ945 and ROS promote M2-type TAM polarization, leading to ICD in CRC cells [68]. In addition, Liu et al. synthesized ruthenium (Ru) NPs loaded with indocyanine green (ICG) and BLZ945, which are denoted as Ru@ICG-BLZ NPs [69]. Through the application of NIR light, Ru@ICG-BLZ NPs induced apoptosis in CRC cells via photothermal therapy (PTT) and PDT, resulting in high temperatures, ROS generation, and BLZ945 release [69]. The combined effects of BLZ945 and ROS facilitate the polarization of M2-type TAMs to the M1 phenotype [69]. Similar to PDT, PTT employs NIR light to irradiate a photothermal agent, thereby inducing heat generation for tumor eradication [118]. ICG possesses the dual attributes of PA and PS, enabling tumor elimination through both PTT and PDT [119]. However, ICG is unstable [120]. Consequently, Ru-based nanomaterials were used as carriers for ICG, resulting in a substantial enhancement in stability and efficacy [66]. It is worth noting that nanomaterials achieve sequential release of drugs by regulating ROS. Li et al. encapsulated LY3200882, a selective transforming growth factor-β inhibitor, in ROS-responsive nanogels (Gel/NG) and loaded with regorafenib (Gel/(REG + NG/LY)) [75](Fig. 2B). Gel/(REG + NG/LY) preferentially releases regorafenib, inhibits CRC proliferation, and promotes ROS production, followed by subsequent release of LY3200882 from ROS-responsive NG/LY [75]. LY3200882 inhibits the epithelial-mesenchymal transition of CRC, thereby inhibiting metastasis [75]. In addition, Gel/(REG + NG/LY) also promotes the transformation of M2-type TAMs into M1-type [75].
ROS also plays a detrimental role in CRC. ROS accumulation has long been recognized as a pathogenic factor in colitis-associated colorectal cancer (CAC) [121]. Nanomaterials loaded with curcumin (Cur) and C7-ethyl-10-hydroxycamptothecin (SN38) inhibit ROS production in TAMs and suppress CAC growth [122] (Fig. 2C). Cur, derived from the traditional Chinese medicine turmeric, exhibits scavenging properties against ROS, inhibits NF-κB activation, reduces pro-inflammatory gene expression, and mitigates inflammatory responses [123,124]. Moreover, Cur effectively inhibits CAC development in mice [125]. However, the efficacy of Cur is limited by its inadequate solubility and bioavailability [126]. SN38, known for its potent inhibition of DNA topoisomerase I [127], can induce apoptosis in CRC cells [[128], [129], [130]]. Similarly, SN38 encounters challenges related to its poor solubility and stability [131]. In contrast, chitosan possesses attributes such as water solubility, adhesion, and adsorption enhancement, rendering it suitable for encapsulating water-insoluble drugs within Ch-based NPs and facilitating efficient intestinal absorption [[132], [133], [134]]. Wang et al. carried Cur and SN38 in Ch scaffolds and assembled them into the oral nanomedicine nCUR/SN38 [122]. nCUR/SN38 administration effectively suppressed the generation of ROS and inflammatory mediators in TAMs and impeded CAC growth [122].
3.1.3. Hypoxia-inducible factor-1α signaling pathway
Hypoxia-inducible factor-1α (HIF-1α) is a crucial transcription factor involved in tumor progression and targeted therapy [135]. HIF-1α is a subunit sensitive to oxygen levels, and its expression is induced under hypoxic conditions [135]. Under normoxia, prolyl hydroxylase domain (PHD) enzymes alter the HIF-1α hydroxylation process, resulting in HIF-1α degradation [[136], [137], [138]]. Conversely, under hypoxia, PHD activity diminishes, leading to increased HIF-1α levels and its subsequent translocation into the nucleus, where it upregulates oncogenes expression [[139], [140], [141]]. Within solid tumors, HIF-1α plays a crucial role in regulating metabolic alterations that contribute to tumor angiogenesis and invasion [142]. Furthermore, metabolites present in the TME can activate the HIF-1α signaling pathway and facilitate TAM polarization towards the M2 phenotype [143,144]. In the treatment of CRC, nanomaterials exert their inhibitory effects on the HIF-1α signaling pathway by modulating the levels of metabolites such as lactic acid or succinate, consequently impeding TAMs polarization towards the M2 type [70,71] (Fig. 2D).
3.1.3.1. Lactic acid
Lactic acid, a metabolic byproduct of tumor cells during aerobic or anaerobic glycolysis, is internalized by TAMs via monocarboxylate transporter 1 [145,146]. This uptake of lactic acid by TAMs triggers the activation of the HIF-1α pathway, leading to TAM polarization towards the M2-type, promotion of neovascularization, and ultimately tumor growth [145,146]. Notably, lactic acid accumulation is inversely associated with anti-tumor immunity in CRC [147]. Furthermore, lactic acid produced by rectal cancer cells exacerbates TAM polarization towards the M2 phenotype, thereby exacerbating carcinogenic behavior in cancer cells [148]. Therefore, inhibiting lactic acid in the TME of CRC hinders TAM polarization towards the M2 phenotype. Hu et al. synthesized cationic polymers APEG-PAsp(PEI) (PAPEI) to deliver siRNA targeting the lactate dehydrogenase A (LDHA) gene (LDHA-siRNA) [70]. PAPEI-mediated LDHA-siRNA delivery (PAPEI/LDHA-siRNA) effectively reduced lactate accumulation in the TME and inhibited TAM polarization towards the M2 phenotype [70]. Furthermore, combining PAPEI/LDHA-siRNA and OXA treatment enhanced the apoptosis of colon cancer cells and improved therapeutic outcomes in CRC [70].
3.1.3.2. Succinate
In addition to lactic acid, succinate induces macrophage polarization towards the M2 phenotype. Tumor cells release succinate, which activates succinate receptor 1 (SUCNR1), thereby inhibiting PHD in TAMs and subsequently activating HIF-1α signaling [143]. This activation promotes TAM polarization towards the M2 phenotype [143]. Additionally, nude mice bearing CRC tumors and overexpressing SUCNR1 exhibited increased expression of M2 phenotypic markers [71]. Consequently, targeting SUCNR1 in TAMs to prevent their polarization towards the M2 phenotype holds promise as an effective therapeutic approach for CRC. Exosomes derived from human umbilical cord mesenchymal stem cells (hUCMSC-Exos) carrying miR-1827 demonstrated a significant inhibitory effect on SUCNR1 expression [71]. This intervention effectively impaired M2-type TAM polarization and subsequently hindered liver metastasis in nude mice with CRC [71]. In summary, nanomaterials are capable of impeding CRC progression by inhibiting metabolites or associated receptors, suppressing the HIF-1α signaling pathway, and preventing TAM polarization towards the M2 phenotype.
3.1.4. Immune adjuvants: Ch and γ-polyglutamic acid (γ-PGA)
Immune adjuvants are crucial in promoting TAM polarization towards the M1 phenotype, thereby inhibiting CRC development [72,73]. Consequently, the formulation of nanomaterials containing two adjuvants with opposing charges can effectively modulate macrophage polarization and impede CRC progression [72,73] (Fig. 2E). Specifically, Ch, a linear aminopolysaccharide with a positive charge [149], serves as an adjuvant to stimulate immune activation [150] and regulates macrophage polarization [151]. Furthermore, γ-PGA, a biodegradable and non-toxic polyamino acid with a negative charge [152], functions as an immune adjuvant that regulates the production of inflammatory factors by macrophages [153]. Owing to their opposite charges, Ch and γ-PGA undergo electrostatic interactions and form Ch/γ-PGA nanocomposites (Ch/γ-PGA NPs) [154]. These Ch/γ-PGA NPs impede TAM polarization into M2 phenotype in CRC tissue [72]. Additionally, the cytokine interferon-γ (IFN-γ) facilitates macrophage polarization into M1 phenotype via the STAT1 signaling pathway [[155], [156], [157]]. However, unlike Ch/γ-PGA NPs, there was no further increase in the expression of M1-type TAMs phenotypic markers when stimulated with IFN-γ [73]. This is because of the lower STAT1 activity observed in macrophages stimulated with IFN-γ in Ch/γ-PGA NPs compared to those stimulated with IFN-γ alone [73].
3.1.5. Other target: Hsp110
Nanomaterials facilitate M2-type TAM polarization and hinder CRC development by regulating the proteins within TAMs [74] (Fig. 2F). Heat-shock proteins (Hsps) are a large class of proteins involved in protein folding and maturation [158]. Heat shock and other stress-inducing factors trigger Hsp expression [159]. Notably, Hsp110 upregulation has been observed in patients with CRC and is associated with metastasis [160]. The secretion of Hsp110 by CRC cells facilitates the conversion of TAMs into the M2-type and contributes to CRC progression [161]. Thus, Marcion et al. developed nanofitins A-C2, which effectively inhibited Hsp110 [74]. This inhibition promotes the transition of M2-type TAMs to M1-type TAMs in CRC mice [74]. Nevertheless, further investigation is required to fully understand the precise mechanism by which Hsp110 regulates macrophage polarization.
3.2. Nanomaterials depleting TAMs for CRC treatment
3.2.1. Mannose receptors
In addition to regulating TAM polarization, the utilization of nanomaterials to eliminate M2-type TAMs in CRC tissues serves as an effective approach to regulating TAM polarization [76,77] (Fig. 3). M2-type TAMs and CRC cells exhibit high expression of the mannose receptor, whereas M1 TAMs do not possess this receptor [[162], [163], [164]]. Consequently, modifying nanomaterials with humans enables the specific targeting of M2-type TAMs and CRC cells. Additionally, humans have an enhanced uptake capacity for both TAMs and CRC cells [165]. Zeng et al. synthesized DOX@MAN-BSA NPs, which are human-modified bovine serum albumin NPs that carry the chemotherapeutic drug doxorubicin (DOX) [76]. DOX@MAN-BSA NPs can concurrently eradicate M2-type TAMs and CRC cells expressing the mannose receptor [76]. Additionally, regorafenib can reduce the number of tumor-infiltrating macrophages in CRC [166]. Hence, Bai et al. combined human-modified γ-cyclodextrin (M-γ-CD) and RG with channel-type NPs (CNPs) to create RG@M-γ-CD CNPs [77]. Consequently, RG@M-γ-CD CNPs induce apoptosis in CRC cells [77]. Moreover, RG@M-γ-CD CNPs effectively suppressed the activity of M2-type TAMs, leading to a decline in their abundance [77]. This decline may be attributed to the demise of quiescent M2-type TAMs, resulting in a reduction in their quantity. However, the precise mechanisms underlying this mode of cell death require further investigation.
3.3. Nanomaterials enhance TAM phagocytosis for CRC treatment
3.3.1. Signaling pathway: CD47-signal regulatory protein α
Although M2-type TAMs have a pro-tumor role, properly activated TAMs can effectively engulf tumor cells [167]. Consequently, interest in enhancing the phagocytic capacity of TAMs as a potential therapeutic strategy for cancer is growing. However, cancer cells often overexpress CD47, a molecule that binds to the signal regulatory protein α (SIRPα) receptor found on myeloid immune cells such as macrophages and dendritic cells (DCs) [168]. This interaction triggers a “do not eat me” signal, resulting in phagocytosis resistance and immune evasion by cancer cells [168] (Fig. 4A). Conversely, blocking the CD47-SIRPα interaction promotes the clearance of cancer cells [169]. Nanomaterials carrying SIRPα variants bind to CD47 on tumor cells, unblocking the “do not eat me” signal in the treatment of other solid tumors [170,171]. CRC development involves an increase in macrophages that specifically express SIRPα and upregulation of CD47 expression in tumor cells [172]. Consequently, nanomaterials inhibit the CD47-SIRPα signaling pathway, enhancing TAM phagocytosis and ultimately exerting a tumor suppressor effect on CRC [78,79] (Fig. 4B).
Cho et al. developed an exosome, referred to as Exo-SIRPα, which could bind to SIRPα in TAMs [78]. This binding action effectively blocked the CD47-SIRPα signaling pathway and enhanced macrophage phagocytosis [78]. In CRC mice, Exo-SIRPα administration enhanced macrophage phagocytosis and inhibited tumor growth [78]. Abdel-Bar et al. used nucleic acid-lipid NPs (SNALPs) to load DOX and siCD47, resulting in the formation of SNALPssiCD47-Dox [79]. SNALPssiCD47-Dox administration effectively suppressed CD47 expression in CRC cells and triggered ICD to release calreticulin [79]. Combined blockade of the CD47-SIRPα signaling pathway and calreticulin upregulation significantly enhanced macrophage phagocytosis, thereby efficiently eradicating CRC in murine models [79]. Calreticulin probably enhances macrophage phagocytosis by binding to CD91 on phagocytes, subsequently initiating the “eat me” signal [173].
4. Modulation of TAMs by nanomaterials for HCC treatment
Liver cancer is a common malignancy of the digestive system [174]. HCC is the most common type of liver cancer, accounting for 75–85% of cases [38]. The TME is of paramount importance in HCC advancement and progression [175]. TAMs, highly abundant immune cells infiltrating the TME at all stages of HCC progression, have emerged as prime targets for immunotherapy [176]. Nanomaterials offer several advantages, including liver tissue targeting, enhanced drug bioavailability, and minimal adverse effects [177]. Consequently, TAM modulation using nanomaterials provides new possibilities for HCC treatment. Nanomaterials impede TAM infiltration [[178], [179], [180]], modulate their polarization [[181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205]], and eliminate M2-type TAMs within tumor tissues [206], thereby effectively impeding HCC onset and progression (Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Table 1). Below, we present a comprehensive overview of the strategies used in modifying nanomaterials to regulate TAMs for HCC treatment with the aim of offering novel insights and avenues for HCC therapy.
4.1. Nanomaterials prevent TAM infiltration for HCC treatment
TAM infiltration is associated with a negative prognosis in patients diagnosed with HCC [207]. Hepatocytes recruit peripheral monocytes through the secretion of chemokines, such as stromal-derived-factor1 (SDF1) and C–C motif ligand 2 (CCL2) [208,209]. This recruitment process leads to TAM infiltration, which, in turn, contributes to HCC development and metastasis [208,209]. Consequently, inhibiting SDF1 and CCL2 binding to their respective receptors on macrophages, CXCR4, and CCR2 reduces macrophage infiltration and effectively hinders HCC cell growth and metastasis [208,210,211]. Furthermore, in other solid tumors, ROS depletion leads to decreased CCL2 secretion by tumor cells, consequently inhibiting TAM infiltration [212]. A potential strategy to impede TAM infiltration involves reducing the levels of ROS in HCC tissues, thereby diminishing the secretion of chemokines, such as CCL2. Consequently, nanomaterials inhibit the CXCR4-SDF1α pathway and reduce ROS levels in HCC tissues, leading to reduced TAM infiltration and ultimately HCC treatment [[178], [179], [180]] (Fig. 5).
4.1.1. CXCR4-SDF1α signaling pathway
Sorafenib (SOR), a multi-kinase inhibitor, induces tumor cell apoptosis, suppresses angiogenesis, and hinders tumor cell proliferation [213]. SOR is the primary treatment option for HCC [175]. However, while SOR effectively inhibits tumor angiogenesis, it also induces tumor tissue hypoxia and triggers SDF1α release from HCC and stromal cells [214]. Consequently, SDF1α binds to CXCR4 in macrophages, leading to TAM infiltration [214]. CXCR4 antagonist plerixafor (AMD3100) inhibits the reduction of TAMs infiltration caused by hypoxia induced by SOR or VEGF knockout, thereby reducing the side effects of targeted drugs [178](Fig. 5A).
AMD3100, the only CXCR4 antagonist currently approved for marketing [215], exhibits limitations in its long-term administration owing to unfavorable pharmacokinetics, severe toxicity, and side effects, thereby restricting its application in solid tumors [208,216,217]. However, nanotechnology has facilitated the utilization of nanoformulations for AMD3100 in the treatment of diverse cancers with the objective of enhancing drug stability and bioavailability, ultimately improving therapeutic outcomes [218]. Gao et al. used polylactic-co-glycolic-acid (PLGA) nanomaterials modified with AMD3100 as SOR carriers to obtain ADOPSor NPs [178]. ADOPSor NPs effectively inhibited TAM infiltration in HCC tissue [178]. In addition, Liu et al. developed an AMD3100-modified multifunctional lipid-based NP carrying siVEGF, namely, VEGF siRNA, in AMD-NPs [179]. In vivo experiments demonstrated therapeutic effects similar to those of ADOPSor NPs [179].
4.1.2. ROS
ROS has dual functions in HCC progression [219]. Elevated ROS levels in HCC cells facilitate ferroptosis and effectively impede HCC onset and progression [[220], [221], [222]]. However, elevated ROS levels within HCC cells induce oxidative stress, which triggers the accumulation of misfolded or unfolded proteins within the mitochondrial matrix, resulting in the activation of the mitochondrial unfolded protein response (UPRmt) [223]. The UPRmt subsequently upregulates the expression of fibroblast growth factor 21 and growth differentiation factor 15, both of which are positively associated with HCC occurrence, progression, and metastasis [223]. Furthermore, ROS within tumor tissues stimulates chemokine secretion, thereby promoting TAM infiltration [212,224,225]. Hence, Guillermo et al. used cerium oxide NPs (CeO2NPs), an anti-inflammatory agent, to mitigate ROS levels in HCC tissues [180] (Fig. 5B). This intervention effectively impedes TAM infiltration [180]. Notably, the catalytic activity of CeO2NPs was solely activated under extreme ROS conditions. Consequently, maintaining a balanced ROS level is crucial for preventing its tumor-promoting properties.
4.2. Nanomaterials modulate TAM polarization for HCC treatment
4.2.1. STAT signaling pathway
In Section 2.2.1, we introduced the STAT signaling pathway and the impact of nanomaterials on CRC therapy through STAT3 regulation. Nanomaterials also regulate TAM polarization by modulating STAT3, STAT1, and STAT6, thereby inhibiting HCC progression [[181], [182], [183]] (Fig. 6). STAT3 is highly expressed in patients with HCC [226], and its activated signaling pathway is involved in HCC development and progression [227]. STAT3 also plays a role in TAM polarization within the HCC TIME. IL-12 induces TAM polarization towards the M1 phenotype by downregulating STAT3, thereby inhibiting HCC growth [228]. Li et al. exploited the ionic interactions between Ch and PGA to synthesize therapeutic NPs to deliver DOX and IL-12 [181]. The accumulation of therapeutic NPs at the tumor site results in the sustained release of IL-12 and DOX [181]. IL-12 promotes M2-type TAM polarization, and DOX promotes HCC cell apoptosis, thereby exerting anti-tumor effects [181].
STAT1 and STAT6 are also involved in regulating macrophage polarization. Specifically, the STAT1 pathway facilitates macrophage polarization to the M1 phenotype [[229], [230], [231], [232], [233]], whereas the STAT6 pathway promotes macrophage polarization to the M2 phenotype [[234], [235], [236]]. Upregulation of STAT6 expression in TAMs leads to their polarization to the M2 phenotype and plays a role in regulating tumor angiogenesis [237]. Jiang et al. discovered that chitosan-based NPs can promote TAMs to M1 phenotype by enhancing STAT1 activity and inhibiting STAT6 activity [182]. The administration of chitosan-based NPs decreased the population of M2-type TAMs and increased the number of M1-type TAMs within HCC tissues [182]. Tang et al. developed man-modified porous silicon NPs loaded with the ferroptosis inducer erastin (Man@pSiNP-erastin) to specifically target M2-type TAMs [183]. The application of Man@pSiNP-erastin successfully inhibited cysteine/glutamate transporter (xCT), which inhibited STAT6 expression in M2-type TAMs, thereby preventing their polarization towards the M2 phenotype [183]. Additionally, the inhibition of xCT facilitated ferroptosis induction in M2-type TAMs, resulting in reduced PD-L1 expression [183]. Combined treatment with Man@pSiNPs-erastin and anti-PD-L1 potently inhibited HCC progression [183]. However, another study discovered that augmenting STAT1 activity in TAMs resulted in their polarization towards the M2 phenotype, thereby facilitating HCC metastasis and immune evasion [238]. This observation could be potentially attributed to variations in the cell lines and methodology used to construct the mouse HCC model. Consequently, selecting an appropriate HCC model was of paramount significance when conducting this investigation.
4.2.2. NF-κB signaling pathway
In Sections 3, 2.1.2.1.1, nanomaterials effectively treated GC and CRC by regulating the NF-kB signaling pathway in M2-type TAMs. In HCC treatment, nanomaterials have been specifically engineered to target TAMs, thereby regulating the Nf-κb signaling pathway by modulating TLR, adenosine monophosphate (AMP), IKKβ (IkappaB kinaseβ), and ROS in TAMs [[184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198],205] (Fig. 7). This regulation facilitates M2-type TAM polarization and effectively inhibits HCC progression and metastasis.
4.2.2.1. TLR
TLR5, a pattern recognition receptor, exhibits a specific affinity for flagellin [239]. Within the hepatic context, TLR5 elicits inflammatory reactions via NF-κB signaling cascades [240,241]. Notably, variations in TLR5 expression have been linked to HCC arising from cirrhosis induced by steatohepatitis [242,243]. In TLR5 knockout mice, gut microbiota dysbiosis occurs, culminating in cholestasis and, ultimately, HCC [244,245]. In solid tumors, activation of TLR5-Nf-κb signaling in TAMs promotes TAM polarization towards the M1 phenotype [246]. These findings could be considered valuable references for HCC treatment (Fig. 7A).
Zhu et al. utilized bacterial therapy by combining Escherichia coli Nissle 1917 (ECN), which senses blue light and released flagellin B (flaB) with rare-earth upconversion NPs (UCNPs) [185]. This led to the generation of UCNP-conjugated engineered ECNs (EcNflaB-UCNPs) [185]. Upon NIR light irradiation, EcNflaB-UCNPs convert red light to blue light, thereby activating ECN to secrete flaB [185]. Subsequently, flaB binds to TLR5 receptors present in TAMs, activating NF-κB signaling and subsequently inducing TAM polarization towards the M1 phenotype [185]. EcNflaB-UCNPs administration promotes TAM polarization towards the M1 phenotype and effectively inhibits HCC growth in mice [185]. Bacterial therapy offers novel strategies for targeting tumors and stimulating both the innate and adaptive immune systems, resulting in altered TME immunodynamics [247]. In the following sections, we discuss the inhibitory effects of nanomaterials modified with bacterial derivatives on HCC growth via TAM polarization [186,187] (Fig. 8).
Meng et al. combined chemotherapeutic drugs with biomimetic bacteria to induce TAM polarization into the M1 phenotype and suppress invasion and metastasis in HCC [186]. They developed a liposome-based bionic bacterium by incorporating the cell wall of Staphylococcus aureus (S. aureus) into liposomes and encapsulating DOX to create DOX@Bio-Bac [186]. In a mouse model of HCC lymphatic metastasis, DOX@Bio-Bac facilitated TAM polarization towards the M1 phenotype and triggered apoptosis in HCC cells [186]. Edson et al. used cell membrane-derived NPs loaded with si-Myc and monophosphoryl lipid A (MPLA) derived from lipopolysaccharide (LPS) to produce MNPs-MPLA-siRNA, which specifically targets HCC cells and monocytes [187]. MNP-MPLA-siRNA effectively suppresses the activity of the c-Myc oncogene and downregulates CD47 and PD-L1 expression in HCC cells [187]. Additionally, treatment with MNP-MPLA-siRNA decreased M2-type TAMs and increased M1-type TAMs [187]. Bacteria and their derivatives have strong penetrating power, and the hypoxic tumor-targeting core has a great advantage in activating the immune response. HCC treatment and drug delivery have great potential [248].
In Section 3.1.1, we elucidated the significance of incorporating TLR7/8 agonists loaded with nanomaterials to inhibit CRC. TLR7/8 agonists have also demonstrated the potential to impede HCC onset and progression. A phase 1/2 human multicenter cancer vaccine trial (NCT03203005) has commenced to evaluate the efficacy of an HCC vaccine that combines TLR7/8 agonists [249]. Nanomaterials containing TLR7/8 agonists exhibit therapeutic effects in HCC treatment [250]. Furthermore, the activation of TLR7/NF-κB signaling pathway induces macrophage polarization towards the M1 phenotype, which effectively impedes HCC proliferation in mice [251]. Consequently, manipulation of macrophage polarization using nanomaterials containing TLR7/8 agonists can inhibit HCC development [188,189] (Fig. 7A).
Liu et al. developed a supramolecular hydrogel delivery system, PLDX-PMI, for treating HCC [188]. This delivery system was composed of lenvatinib-loaded nanomaterials (PCN-Len NPs), oxidized dextran, and polyTLR7/8a agonist IMDQ nanomodulators (p (Man-IMDQ) NRs), which underwent co-assembly to form PLDX-PMI [188]. PCN-Len NPs specifically target vascular endothelial cell tyrosine kinases and inhibit the vascular endothelial growth factor receptor signaling pathway [188]. Additionally, p (Man-IMDQ) NRs polarized M2-type TAMs to M1-type TAMs, thereby suppressing tumor angiogenesis [188]. PLDX-PM significantly reduces tumor microvessel density, promotes tumor vascular network maturation, and increases M1-type TAMs [188]. Furthermore, Huang et al. employed pH-responsive nanomaterials to concurrently deliver the SOR and TLR7/8 agonist R848-C16, resulting in the formation of NPs denoted as NPs(SOR/R848) [189]. Upon intravenous administration, these NPs significantly accumulate within tumor tissues and undergo degradation of surface polyethylene glycol chains within the low-pH TME, thereby facilitating their uptake by TAMs and HCC cells [189]. The NPs(SOR/R848) subsequently induces TAM polarization towards the M1 phenotype, leading to HCC cell apoptosis and growth suppression [189].
In Section 3.1.1, we introduced the concept of nanomaterials regulating TLR9, thereby promoting the polarization of M2-type TAM to M1 type. In the treatment of HCC, nanomaterials activate the NF-κB signaling pathway by regulating TLR9, thereby regulating TAM polarization [190,205] (Fig. 7A). TLR9 activates humoral and cellular immunity by binding to synthetic oligonucleotide adjuvants cytosin-phosphate-guanine oligodeoxynucleotides (CpG-ODNs) [191], thereby showing the potential to prevent and treat cancer [252]. Furthermore, CpG-ODNs inhibit TAM polarization towards the M2 phenotype within the TME, consequently impeding HCC progression [253]. However, CpG-ODNs have a short half-life in serum and are susceptible to degradation [254]. Complexes formed between nanomaterial and CpG-ODNs enhance cellular uptake and demonstrate greater adjuvant effects than free CpG-ODNs [255]. 5, 6-dimethylxanthenone-4-acetic acid (DMXAA), an anti-vascular agent, specifically targets the endothelial cells of existing tumor blood vessels, leading to distortion or damage and subsequently reducing tumor blood flow [256]. Additionally, DMXAA acts as a STING activator, stimulating STING-dependent NF-κB pathway signaling and secretion of inflammatory factors [257,258]. However, owing to the primary targeting of the central vasculature in solid tumors, the impact of DMXAA on the surrounding tissues is limited [259]. This limitation can be overcome by combining DMXAA with immunotherapy [259].
Chen et al. introduced CpG-ODNs and DMXAA into MOF-801, which resulted in its self-assembly [190]. MOF-CpG-DMXAA facilitated M2-type TAM polarization, DC maturation, and tumor blood vessel destruction, thereby synergistically improving the TME of HCC [190]. Despite the observed increase in TLR4, -8, and -9 in macrophages stimulated by MOF-CpG-DMXAA, only inhibited TLR4 hindered macrophage polarization [190]. Hence, the activation of the NF-κB pathway and the promotion of macrophage polarization by MOF-CpG-DMXAA primarily occur through the TLR4 receptor. Notably, TLR4 primarily engages in innate immunity and facilitates inflammatory responses by recognizing LPS and bacterial endotoxins [260]. Therefore, the mechanism through which MOF-CpG-DMXAA stimulates TLR4 requires further investigation. In addition, Han et al. used lactide-glycolide copolymer NPs to load d-lactate, and modified the DL-loaded NP with HCC membrane and M2 pep to form the nanomaterial DL@ NP-M-M2 pep [205] DL@NP-M-M2 pep combines with TLR2 and TLR9 in macrophages, activates the PI3K/AKT signaling pathway, and then regulates STAT1 and STAT6, ultimately regulating peroxisome proliferator–activated receptor γ (PPAR-γ) and NF-κB, and promotes the polarization of M2 type TAM to M1 type [205]. The combined application of DL@NP-M-M2 pep and α-CD47 improves the tumor killing ability of TAM and reverses the immune microenvironment of HCC [205].
4.2.2.2. AMP
Nanomaterials loaded with metformin (Met) are effective in treating HCC through the regulation of the AMP-AMPK–NF–kB pathway and promotion of M2-type TAM polarization into M1-type TAMs [[192], [193], [194]] (Fig. 7B). Met, an oral antidiabetic drug widely used in clinical practice [261], is associated with beneficial effects in cancer prevention and treatment according to epidemiological studies, which have sparked interest in its potential as an anticancer agent [262]. The underlying mechanism of action of Met involves AMPK activation [263]. Met effectively induces M2-type TAM polarization to the M1 phenotype through activation of the AMPK–NF–κB signaling pathway, thereby inhibiting breast cancer growth [264]. Furthermore, the use of nanomaterials for the delivery of Met enhances its bioavailability, reduces the frequency of administration, mitigates gastrointestinal side effects and toxicity, and facilitates the efficacious application of Met in cancer treatment [265]. In the treatment of HCC, Met may potentially decrease disease risk [266], inhibit HCC cell proliferation [267,268], enhance SOR sensitivity [269], decrease PD-L1 expression [270], facilitate T cells infiltration [271], regulate macrophage phenotype [271], and impede the infiltration of bone marrow-derived myeloid cells [272].
Wei et al. developed man-modified macrophage-derived microparticles loaded with Met (Met@Man-MPs) to specifically target M2-type TAMs and induce their polarization into M1-type TAMs [192]. The collagen-degrading ability of man-MPs facilitates CD8+T cell recruitment by M1-type TAMs, leading to their infiltration into tumors and promotion of the delivery of anti-PD-1 antibodies within the TME of HCC [192]. Moreover, the combined administration of Met@Man-MPs and liposomal dox inhibited HCC and induced long-term immune memory in HCC mice [193]. Notably, Met is not effective against late-stage cancer metastasis and may even exacerbate cancer-related mortality [273]. The underlying factors contributing to these distinct effects of Met remain uncertain, that is, whether they are attributed to the cancer stage severity or potential drug interactions between Met and other medications [273]. Additionally, Zhu et al. observed that the nanomaterial C60(OH)x stimulates TNF-α release from macrophages and impedes HCC growth in mice [194]. C60(OH)x increased the activity of Arg and acid phosphatase (Acp) in macrophages [194]. Notably, the Acp activity may reflect changes in AMP levels [274]. Additionally, TNF-α is a marker of M1-type macrophage, and its production depends on NF-kB [27,67]. Therefore, nanomaterials regulate TAM polarization through the AMP-AMPK- NF-kB pathway and inhibit HCC growth.
4.2.2.3. IKKβ
The regulation of TAM polarization and the inhibition of HCC development can be achieved through the utilization of nanomaterials that carry IKKβ inhibitors [196] (Fig. 7C). NF-κB activation depends on IκB phosphorylation and degradation, a process that is controlled by two kinases, namely IKKα and IKKβ [81]. The IKKβ inhibitor IMD-0354 effectively hinders the progression and metastasis of solid tumors [[275], [276], [277]]. To facilitate the delivery of IMD-0354, Wang et al. developed a man-modified pH-responsive charge-reversal polymer O-carboxymethyl-chitosan (CMCS)-coated cationic lipid-based NPs (CLN), referred to as CMCS/M-IMD-CLN, as well as a CMCS-coated CLN for SOR delivery, known as CMCS/SF-CLN [196]. In an acidic TME with a low pH, the conversion of negatively charged CMCS into positively charged CMCS occurs through the protonation of amino groups and inhibition of carboxyl hydrolysis [278]. Positively charged CMCS, SF-CLN, and IMD-CLN exhibit repulsion towards each other [196]. CMCS releases SF-CLN and M-IMD-CLN, which selectively target HCC cells and M2-type TAMs, respectively [196]. SF-CLN and M-IMD-CLNs exhibit inhibitory effects on tumor growth and promote TAM polarization towards the M1-type, effectively suppressing HCC development in mice [196]. Notably, IMD-0354 inhibits NF-κB from entering the nucleus to initiate transcription by inhibiting IKKβ. Consequently, further investigation is required to understand the mechanism by which inhibition of the NF-κB signaling pathway contributes to M2-type TAM polarization towards the M1 phenotype.
4.2.2.4. ROS
In Sections 2.1.2, 3.2.1, the role of nanomaterials in regulating ROS and the impact on NF-κB signaling in the treatment of GC and CRC were introduced. Additionally, Section 4.1.2 introduced the dual role of ROS in HCC. Regulating ROS and NF-κB signaling in TAMs, which promotes M2-type TAM polarization to M1-type, can prevent HCC development [279]. Consequently, the development of nanomaterials that can effectively regulate ROS, target TAMs, and modulate their phenotype holds promise for HCC treatment [195,197,198] (Fig. 7D and E).
Iron oxide NPs (IONPs) are magnetic iron oxide cores with surface-modified coatings that can be synthesized using various chemical methods [[280], [281], [282]]. These IONPs exhibit biocompatibility, versatile surface chemistry, and magnetic properties that make them suitable for contrast-enhanced magnetic resonance imaging, thus indicating their potential application in immunotherapy [283]. IONPs undergo phagocytosis by macrophages and are subsequently degraded by lysosomes [284]. This releases Fe2+ into the cytoplasm, which then participates in the Fenton reaction (Fe2++ H2O2→ Fe3++ OH−), resulting in ROS generation [284]. ROS activation triggers the activation of NF-kB and subsequently promotes macrophage polarization towards the M1-type [284]. In addition, IONPs possess hepatoprotective properties against metastatic seeds and can enhance macrophage-regulated cancer immunotherapy [285]. Therefore, the promotion of ROS production using IONPs can effectively induce M2-type TAM polarization towards the M1 type, presenting a promising therapeutic approach for HCC treatment.
Chen et al. synthesized exosomes combined with PEGylated iron oxide NPs loaded with chlorine E6 (PIONs@E6) [197]. The application of PIONs@E6 results in ROS generation, which facilitates TAM conversion to the M1 phenotype, thereby promoting HCC growth [197]. Cui et al. developed D-Man-chelated iron oxide NPs (Man-IONPs) to specifically target M2-type TAM polarization towards the M1 phenotype and impede residual HCC progression following microwave ablation [195]. However, ROS is also detrimental to macrophage regulation. In a study conducted by Li et al. the utilization of nanoliposome-loaded C6-Ceramide effectively suppressed ROS levels in macrophages [198]. This intervention demonstrated ROS's ability to significantly decrease the population of M2-type TAMs within tumor tissues while concurrently increasing the number of M1-type TAMs [198]. Considering the dual role of ROS in CRC and HCC treatment, it is imperative to quantitatively assess the localized distribution of ROS and the redox status to develop more tailored and rational treatment strategies [286].
4.2.3. HIF-1α signaling pathway
Hypoxia is a prevalent feature in most solid tumors [287]. The presence of abnormal tumor vasculature can diminish oxygen and nutrient availability and hinder drug transportation [288]. Hypoxia facilitates tumor invasion, heightened metastasis, angiogenesis, resistance to chemotherapy, and increased tolerance to radiation [289]. At the cellular level, gene transcription in the hypoxic milieu occurs primarily via the interaction between HIF and hypoxia-responsive elements [290]. Inactivating the HIF-1 pathway effectively addresses the disadvantages of tumor hypoxia [291].
In Section 3.1.3, we introduced the HIF-1α signaling pathway and the use of nanomaterials to inhibit this pathway by regulating metabolites. This regulation ultimately promotes M2-type TAM polarization to M1 type, thereby inhibiting CRC progression. In HCC, the HIF-1α signaling pathway plays a crucial role in promoting tumor proliferation, metastasis, and angiogenesis [292]. Under normal aerobic conditions, HIF-1α is degraded [[136], [137], [138]]. However, under hypoxic conditions, the level of HIF-1α increases, thereby upregulating cancer-promoting genes [[139], [140], [141]]. Therefore, alleviating tumor tissue hypoxia can inhibit the HIF-1α signaling pathway and inhibit HCC development. Nanomaterials enhance tumor oxygenation through two main approaches: the first involves the normalizing tumor blood vessels to regulate oxygen levels, while the second focuses on reoxygenating tumor tissue using oxygen-loaded nanocarriers, natural/artificial oxygen nanocarriers, and oxygen generators [293]. In HCC treatment, nanomaterials alleviate tissue hypoxia through the two mechanisms above, inhibit the HIF-1α signaling pathway, and facilitate TAM polarization into M2-type [[199], [200], [201]] (Fig. 9).
Liu et al. employed a normalization strategy to regulate oxygen levels by targeting tumor blood vessels. They developed Bi/Se-Len NPs, which are Bi/Se NPs loaded with Len [199]. In vivo experiments involved Bi/Se-Len NP administration followed by stereotactic body RT, resulting in the reshaping and normalization of tumor blood vessels, reduction of tumor hypoxia, suppression of HIF-1α expression, promotion of M2-type TAM polarization into M1-type, and consequent inhibition of HCC growth [199]. Dai et al. utilized a second approach to preparing nanocarriers loaded with oxygen to reoxygenate tumor tissues. This study aimed to develop oxygen microcapsules stabilized by dopamine NPs to rapidly increase the oxygen concentration in a low-oxygen environment and sustain it over an extended period [200]. The combination of oxygen microcapsules and RT suppressed HIF-1α expression and induced M2-type TAM polarization into M1-type [200]. Notably, the hypoxic TME significantly impedes the efficacy of RT, and the RT process further depletes oxygen within the tumor tissue [294]. Hence, through hypoxia mitigation, nanomaterials serve as inhibitors of the HIF-1α signaling pathway and demonstrate a significant enhancement in RT efficacy. Furthermore, Chang et al. employed an alternative approach to fabricate an oxygen generator to reoxygenate tumor tissue. They successfully developed NanoMnSor, a nanomaterial comprising a MnO2 core and a lipid- and SOR-loaded PLGA shell, which effectively co-delivered oxygen-generating MnO2 and SOR into the HCC tissues of mice [201]. The generation of oxygen was catalyzed by NanoMnSor to H2O2 in the TME, which subsequently released SOR [201]. Treatment with NanoMnSor promotes M2-type TAM polarization into M1-type TAMs, induces apoptosis in HCC cells, and alleviates hypoxia in tumor tissues [201]. It is important to highlight that the benefits of alleviating HCC hypoxia extend beyond inhibiting HIF-1α and enhancing RT efficacy. Reoxygenation also reducts of M2-type TAMs and improves of PDT efficiency have also been discussed [206], and further details are provided in Section 4.3.1.
4.2.4. CSF-1/CSF–1R signaling pathway
In Section 3.1.2, we elucidated that inhibition of the CSF-1/CSF–1R axis can facilitate TAM polarization into the M1 phenotype, thereby offering a potential therapeutic approach for CRC. Similarly, in HCC, inhibition of the CSF-1/CSF–1R axis effectively promotes M2-type TAM polarization towards the M1 phenotype, resulting in diminished resistance to anti-PD-1 [295]. Consequently, the use of nanomaterials can stimulate TAM polarization towards the M1 phenotype and impede HCC progression by modulating the CSF-1/CSF–1R axis [202,203] (Fig. 10).
Wang et al. developed PFH@LSLP, a liposome formulation incorporating oxygen-saturated perfluorohexane as the core, which was surface-modified with the CXCR4 antagonist LFC131 and simultaneously delivered SOR and the CSF-1/CSF–1R inhibitor PLX3397 [203]. PFH@LSLP effectively alleviated tumor hypoxia and disrupted the CXCR4-SDF1α axis in HCC cells, thereby overcoming SOR resistance and inducing apoptosis [203]. Furthermore, PFH@LSLP inhibited the CSF-1/CSF–1R signaling pathway, leading to the inhibition of TAM polarization into the M2 phenotype [203]. However, other studies have indicated that CSF-1 may positively impact macrophage polarization towards the M1 subtype. VG-sHDL, which refers to the functional high-density lipoproteins of vadimezan and gemcitabine (GEM), were prepared [202]. These VG-sHDLs were specifically designed to target HCC cells and M2-type TAMs, resulting in cell death [202]. As a consequence of cell death, HCC cells release CSF-1 and High mobility group box-1 protein, which facilitate TAM differentiation into the M1 subtype [202].
4.2.5. Iron metabolisms
Macrophages are responsible for the clearance of senescent red blood cells, which are vital for iron recycling and the maintenance of homeostasis [296]. Alterations in iron homeostasis also influence macrophage polarization [281]. The M1 macrophages retain iron by exhibiting low ferroportin and high ferritin expression [297,298]. Conversely, M2 macrophages release iron by displaying high or low ferroportin expression [297]. Iron promotes TAM polarization into the M1 subtype, thereby impeding HCC growth in mice [299]. Nevertheless, the administration of iron through direct injection harms endothelial cells and leads to cirrhosis [299]. To address this issue, Fe-based nanomaterials have emerged as promising solutions [300].
Wei et al. developed a MOF called Dic@M2pep-Fe-MOF by modifying the M2 macrophage-binding peptide (M2pep) with an iron-based metal and loading it with diclofenac [204] (Fig. 11). Diclofenac can enhance the synthesis of hepcidin [301], which inhibits the excessive release of cellular iron by interacting with and inducing ferroportin internalization and degradation [302]. Consequently, Dic@M2pep-Fe-MOF effectively targets M2-type TAMs and suppresses ferroportin expression, leading to reduced iron outflow [204]. This, in turn, promotes M2-type TAM polarization into M1-type TAMs [204]. Dic@M2pep-Fe-MOF can reverse the suppressive TIME, effectively induce apoptosis in HCC cells, and impede tumor recurrence [204]. Notably, owing to rapid proliferation and DNA synthesis, tumor cells require more iron than normal cells [303]. Moreover, M2-type TAMs release iron, thereby providing an additional source of iron for tumor cells [300]. Consequently, modulating iron recycling to promote M2-type TAM polarization towards M1-type TAMs can reshape the TIME and suppress tumor cell proliferation.
4.3. Nanomaterials deplete TAMs for HCC treatment
4.3.1. Oxygen
In Section 4.2.3, we elucidated the use of nanomaterials for effectively treating HCC by alleviating HCC hypoxia and modulating M2-type TAM polarization towards the M1 phenotype. Furthermore, oxygen decreases the population of M2-type TAMs [206] (Fig. 12). Hou et al. developed BHMDI, a nanoplatform composed of manganese dioxide-crosslinked bovine albumin/HA NPs loaded with both DOX and ICG [206]. BHMDI effectively alleviated hypoxia in HCC tissues, facilitated PDT, and released DOX [206]. This process induces ICD in HCC cells, thereby enhancing DC and effector T-cell maturation [206]. Additionally, alleviation of hypoxia diminishes the presence of M2-type TAMs [206]. The concurrent administration of BHMDI and anti-PD-1 demonstrated remarkable efficacy in eradicating primary HCC, preventing recurrence, and effectively inhibiting distant tumor growth [206].
5. Modulation of TAMs by nanomaterials for the treatment of pancreatic cancer
Pancreatic cancer, a malignancy of the digestive system, is characterized by poor prognosis [38]. Within pancreatic cancer tissues, a notable infiltration of M2-type TAMs exists, which contributes to the establishment of an immunosuppressive TIME and subsequently promote tumor progression [304]. Moreover, the presence of M2-type TAMs is strongly correlated with metastasis and an unfavorable prognosis in patients with pancreatic cancer [305,306]. Inhibiting TAM polarization towards the M2 phenotype can effectively impede the growth, invasion, and metastasis of pancreatic cancer [[307], [308], [309]]. However, factors such as extensive desmoplastic stroma and hypoxic microenvironment in pancreatic cancer pose significant obstacles to efficient drug delivery [310].
Nanomaterials have the potential to enhance therapeutic efficacy by reducing the tumor matrix, inhibiting cancer-associated fibroblasts (CAF), and improving the hypoxic environment [311]. Consequently, manipulation of TAMs using nanomaterials presents novel prospects for the treatment of pancreatic cancer. Nanomaterials are effective in impeding the progression of pancreatic cancer by facilitating the polarization of M2-type TAMs to the M1 phenotype [[312], [313], [314], [315], [316], [317], [318], [319]] (Fig. 13, Table 1). Therefore, we aimed to provide insight into the treatment of pancreatic cancer by summarizing the approaches employed to regulate TAMs using nanomaterials.
5.1. Nanomaterials modulating TAM polarization for the treatment of pancreatic cancer
5.1.1. NF-κB signaling pathway
In Section 4.2.2, we introduced the fact that nanomaterials regulate TLR4 or RIG-I, subsequently promoting the NF-κB signaling pathway. The activation of NF-κB signaling pathway impedes HCC progression by controlling TAM polarization. Similarly, nanomaterials hinder pancreatic cancer growth by promoting TLR4 and RIG-I, which subsequently modulate the TAM phenotype [312,313] (Fig. 13A). The NF-κB signaling pathway may also be involved in these processes.
5.1.1.1. TLR
Multiple studies have indicated that TLR4 recognizes LPS and triggers the activation of the NF-κB signaling pathway, promoting macrophage polarization into the M1 phenotype [[320], [321], [322]]. The combination of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) and GEM is the primary treatment approach for patients with advanced pancreatic cancer [323]. Macrophages phagocytose nab-paclitaxel, which mimics LPS and stimulates TLR4, consequently inducing M2 macrophage polarization to the M1 phenotype [312]. Furthermore, the combination of nab-paclitaxel and GEM enhanced the presence of M1-type TAMs within orthotopic pancreatic ductal adenocarcinoma tissues in mice [312].
5.1.1.2. RIG-I
In Section 3.1.1, we presented the finding that NP/3pRNA exerts control over the NF-κB and IRFs signaling pathways through the regulation of RIG-I, subsequently influencing TAM polarization and inhibiting the development of CRC. In the context of pancreatic cancer treatment, Manisit et al. developed PPP dsRNA LCP - AEAA NPs, which carry 5′ triphosphate Bcl2 specificity at the terminus of short interference dsRNA lipid phosphate NPs [313]. The administration of ppp dsRNA LCP-AEAA NPs suppresses Bcl2 expression and induces apoptosis in pancreatic cancer cells [313]. Furthermore, ppp dsRNA LCP-AEAA NPs activated RIG-I, diminished M2-type TAMs, and enhanced the production of M1-type TAMs [313].
Although NF-κB was not included in the research on nanomaterials regulating TLR4 or IRFs to treat pancreatic cancer, both of them did detect an elevation in TNF-α secretion. Given that TNF-α is reliant on NF-κB for its expression [324], TNF-α upregulation is closely associated with NF-κB activation. Consequently, regulation of TAM polarization by nanomaterials occurs through TLR4 and RIG-I modulation, leading to the activation of the NF-κB signaling pathway.
5.1.2. HIF-1α signaling pathway
In Sections 4, 3.1.3.2.3, we introduced in detail the process of HIF-1α signaling pathway promoting the polarization of macrophages into M2 type. In addition, we introduced the process of oxygen microcapsules relieving TME hypoxia and inhibiting HIF-1α signaling pathway by carrying oxygen [200]. In the treatment of pancreatic cancer, oxygen microcapsules inhibit the expression of HIF-1α, reduce and promote the polarization of M2 type TAM to M1 type [319] (Fig. 13A). The combined use of oxygen microcapsules and anti-PD-1 antibodies increases the proportion of helper T subtype 1 cells and cytotoxic T lymphocytes, and improves the anti-tumor immune response [319].
5.1.3. PI3K-AKT signaling pathway
The phosphatidylinositol-3hydroxykinase (PI3K)-AGC serine/threonine kinases (AKT) signaling pathway plays a crucial role in various inflammatory and metabolic pathways in macrophages, resulting in phenotypic alterations [325]. Recent studies have demonstrated that inhibiting the PI3K-AKT pathway can effectively impede TAM polarization towards the M2 phenotype, consequently hindering pancreatic cancer progression [307]. Consequently, the use of nanomaterials as carriers of PI3K inhibitors presents a promising approach to inhibit the PI3K-AKT signaling pathway, promote M2 TAM polarization towards the M1 phenotype, and ultimately suppress pancreatic cancer development [314,315] (Fig. 13B).
Li et al. utilized GEM-conjugated dendritic polylysine DGL (GD) NPs and PI3K inhibitor Wtmn-coated polycaprolactone-polyethylene glycol micelles, connected through a cathepsin B substrate peptide to synthesize GD@PP/Wtmn micelles [315]. High expression of cathepsin B in pancreatic tumor tissues facilitates the release of GD and PP/Wtmn from micelles upon reaching the TME [315]. GD effectively penetrates the tumor-stromal barrier and induces apoptosis in pancreatic cancer cells, whereas PP/Wtmn remains localized in the perivascular area enriched with TAMs [315]. By inhibiting PI3K, PP/Wtmn suppressed AKT phosphorylation, promoted M2-type TAM polarization, and synergistically inhibited pancreatic cancer growth in conjunction with GD [315].
A different study was conducted to develop a nanomaterial known as M2pep-MM/BEZ/siRNA, which consists of a mixed micelle formed by M2pep-modified polyethylenimine-stearic acid (PEI-SA) and 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-succinyl (polyethylene glycol) (DSPE-PEG) and equipped with the PI3K inhibitors BEZ235 and CSF-1RsiRNA [314]. M2pep-MM/BEZ/siRNA nanomaterials specifically target M2-type TAMs and promote their polarization towards the M1 phenotype by blocking the PI3K-AKT and CSF-1/CSF–1R signaling pathways [314]. Notably, free PI3K inhibitors have low solubility, instability, and rapid clearance from the bloodstream, resulting in poor in vivo efficacy [326]. Nanomaterial can enhance the bioavailability of PI3K inhibitors, improve their pharmacokinetic properties, and yield significant pharmacodynamic effects [327].
5.1.4. MicroRNAs: miR-125b and miR-155
MicroRNAs (miRNAs) are a class of small non-coding RNAs that regulate gene expression at the post-transcriptional level [328]. Within the TME, miRNAs influence tumor initiation and progression by modulating TAM polarization [329]. Analysis of miRNA expression profiles revealed that mir-155 and miR-125b actively promote TAM polarization towards the M1 phenotype [330]. However, several physiological barriers pose challenges to the efficacy of miRNA interference technologies [331]. The use of hyaluronic acid-poly (ethylene imine)/hyaluronic acid-poly (ethylene glycol) (HA-PEI/HA-PEG) polymers for miRNA encapsulation can overcome these obstacles, leading to enhanced delivery and transfection efficiency [332,333]. Consequently, the targeted delivery of miR-125b and miR-155 via nanomaterials effectively facilitated M2-type TAM polarization, thereby offering novel therapeutic avenues for treating pancreatic cancer [316,317] (Fig. 13C).
Su et al. used HA-PEI/HA-PEG polymers carrying miR-125b and miR-155 to introduce pancreatic cancer cells to promote the expression of exosomes containing miR-125b and miR-155 [316]. After macrophages phagocytose exosomes containing miR-125b and miR-155, they promote the transformation of M2-type human macrophages into M1-type [316]. Additionally, Neha et al. conjugated M2 targeting peptide and miR-125b with an HA-PEI/HA-PEG polymer to form self-assembled NPs (HAPEI/PEG-M2peptide miR-125b NPs) [317]. The HAPEI/PEG-M2peptide miR-125b NPs specifically target M2-type TAMs within pancreatic cancer tissues and induce their polarization towards the M1 phenotype [317]. In addition to miR-125b and miR-155, miRNA expression profiles have shown that miR-127 also plays a significant role in promoting TAM polarization into the M1 phenotype [330]. Additionally, the utilization of nanomaterials loaded with miR-127 induces M2-type TAM polarization towards the M1 phenotype in breast cancer tissues [334]. Consequently, the regulation of TAM polarization through miR-127 warrants consideration for the treatment of pancreatic cancer.
5.1.5. Other target: Endocannabinoid receptor-2 (CB-2)
CB-2, a member of the G-protein-coupled receptor (GPCR) family, primarily participates in immune responses [335]. It inhibits pro-inflammatory cytokines and pro-apoptotic factors, and the downregulation of its activity marks the beginning of the inflammatory response [336]. Numerous studies have substantiated that CB-2 inhibition induces macrophage polarization towards the M1 phenotype [[337], [338], [339]]. In patients with pancreatic cancer, monoacylglycerol lipase (MGLL) and CB-2 are highly expressed in pancreatic cancer cells and TAMs, respectively [318]. MGLL plays a crucial role in regulating the cancer-promoting fatty acid network and promoting various processes, such as tumor migration, invasion, survival, and growth [340]. Consequently, MGLL and CB-2 inhibition in pancreatic cancer cells and TAMs, respectively, through the use of nanomaterials effectively hindered pancreatic cancer development and metastasis [318] (Fig. 13D).
Cao et al. devised a nanoplatform called NPs(siMGLL/siCB-2) that utilizes a reduction reaction-based polydisulfide amide to deliver MGLL and CB-2 siRNAs [318]. After NPs(siMGLL/siCB-2) internalization by pancreatic cancer cells and TAMs, abundant glutathione in the cytoplasm reacts with polydisulfide amide, leading to siRNA liberation [318]. MGLL siRNA effectively hinders the presence of free fatty acids in pancreatic cancer cells, consequently impeding the provision of nutrients to the tumor cells [318]. In contrast, CB-2 siRNA effectively suppresses the expression of CB-2 in TAMs, thereby inducing the polarization of M2-type TAMs [318].
6. Macrophage-derived nanomaterials for treating digestive cancer
Macrophages can prolong drug circulation and release, improve drug stability, and reduce immunogenicity [341]. They have demonstrated good biocompatibility and degradability and provide abundant surface receptors for targeted delivery of several drugs [341]. Therefore, combining macrophages and nanomaterials to form macrophage-derived nanomaterials can improve the targeting and utilization of anti-tumor drugs. Macrophage-derived nanomaterials mainly include macrophage-derived exosomes, macrophage membrane-coated nanomaterials, and macrophage-based microrobot [39]. Next, we introduce the role of these three types of macrophage-derived nanomaterials in the treatment of digestive system tumors (Fig. 14, Fig. 15, Table 2).
6.1. Macrophage-derived exosomes
Exosomes are natural nanomaterials, pivotal for safeguarding and transporting endogenous macromolecules over considerable distances [50]. Moreover, exosomes exhibit remarkable permeability and retention capabilities, making them highly suitable for targeted drug delivery [342]. Given their ability to be secreted naturally by various cells, exosomes offer a cost-effective approach to produce multifunctional nanomaterials [343]. Macrophage-derived exosomes regulate GC development [344,345] (Fig. 14). Wang et al. discovered that exosomes released by human macrophages facilitate the delivery of microRNA-21 (miR-21) inhibitors, further inhibiting human GC cell migration and upregulating programmed cell death 4 (PDCD4) to promote apoptosis in GC cells [344]. Conversely, Yu et al. observed that exosomes derived from M2-type TAMs circ 0008253 inhibited the apoptosis of gastric adenocarcinoma cells following treatment with oxaliplatin [345]. This effect was attributed to increased expression of ATP-binding cassette subfamily G member 2 (ABCG2) [345]. ABCG2 facilitates OXA efflux from tumor cells, decreasing intracellular OXA levels [346]. Tumor volume in mice pretreated with circ 0008253 significantly increased after treatment with OXA [345]. Consequently, the specific content of exosomes is crucial for determining their effect on tumors, rendering their universal application challenging.
6.2. Macrophage membrane-coated nanomaterials
Cell membrane-coating nanotechnology is an emerging nanomaterial modification strategy comprising a natural cell membrane layer surface and a synthetic NP core [347]. Recently, NPs derived from various sources, including cancer cells, bacteria, red blood cells, leukocytes, and macrophage membranes, have been used to deliver small-molecule drugs, antibodies, and vaccines, thereby evading immune system clearance [348]. Compared with alternative cell membrane types, macrophage membranes possess several advantages, including prolonged circulation within the bloodstream, enhanced antigen recognition for improved targeting, gradual drug release, and reduced in vivo toxicity [[349], [350], [351], [352]]. Consequently, nanomaterials modified with macrophage membranes may be used for treating CRC and pancreatic cancer [353,354] (Fig. 15 A and B).
Fang et al. employed an extrusion technique to merge macrophage membranes with abemaciclip-loaded liposomes (A-Lip) and black phosphorus quantum dot (BPQD) liposomes, resulting in the fabrication of artificially assembled macrophages (AB@LM) [353]. AB@LM specifically targets CRC tissue through the macrophage membrane and subsequently releases BPQD and abemaciclib upon NIR light irradiation [353]. The use of BPQD-based PDT and PTT facilitates ROS generation, thereby inducing ICD and promoting DC maturation, antigen presentation, and T-cell proliferation [353]. Abemaciclib, a potent inhibitor of cyclin-dependent kinases 4 and -6, exerts its therapeutic effects by inducing G1 phase arrest and apoptosis in tumor cells [353]. It suppresses the proliferation of regulatory T cells (Tregs), thereby augmenting their anti-tumor efficacy [353].
In the treatment of pancreatic cancer, the utilization of GEM-loaded PLGA NPs with a macrophage membrane coating (MPGNPs) enables the evasion of phagocytosis, thereby facilitating the passive targeting of pancreatic tumors [354]. The combination of MPGNPs and erlotinib effectively impedes DNA synthesis and proliferation of pancreatic cancer cells, consequently inhibiting pancreatic cancer growth in mouse models [354]. Notably, culture, purification, and sterilization processes of macrophages can lead to phenotypic changes or epigenetic alterations, which may decrease the reproducibility of macrophage membranes across different batches [355]. Furthermore, the activity and function of macrophages are primarily affected by factor, including race, age, sex, and health status, which pose challenges in formulating allogeneic transfusions [355]. Despite the promising efficacy of macrophage membrane-coated nanomaterials in treating digestive system tumors, challenges must be addressed in future research.
6.3. Macrophage-based microrobot
Macrophages that engulf nanomaterials can function as macrophage robots, targeting CRC and pancreatic cancer cells, transporting anti-tumor drugs, and inducing apoptosis [356,357] (Fig. 15C and D). Macrophage phagocytosis involves the incorporation of docetaxel, PLGA NPs, and Fe3O4 magnetic NPs, forming a hybrid-driven macrophage microrobot [356]. Under the effect of an electromagnetic actuation system, these macrophage microrobots target tumor spheroids, deliver docetaxel, and promote apoptosis in CRC cells [356].
The mouse monocyte/macrophage-derived cell line, RAW264.7, selectively infiltrates pancreatic tumors without affecting other organs [357]. Consequently, RAW264.7 cells were employed to engulf paramagnetic iron/iron oxide NPs to create a macrophage-based microrobot exerting a magnetocaloric effect [357]. In a mouse model of peritoneal pancreatic cancer, a macrophage-based microrobot generated heat when exposed to an alternating magnetic field, leading to tumor cell apoptosis [357]. Among the three categories of nanomaterials derived from macrophages, macrophage-based microrobots exhibit greater ease of preparation and enhanced targeting efficacy [39]. Nevertheless, the loaded nanomaterials may induce toxicity in macrophages, resulting in a comparatively lower drug-loading capacity for the macrophage-based microrobot compared to macrophage membrane-coated nanomaterials and macrophage-derived exosomes [39].
7. Conclusion and perspective
Digestive system tumors are a prevalent form of malignant tumors in clinical settings. Immune tolerance in the digestive system poses challenges to achieving satisfactory outcomes with RT, chemotherapy, and immunotherapy. TAMs significantly contribute to the initiation, progression, and metastasis of digestive system tumors and are abundant within the TIME. Nanomaterials can counteract the immunosuppressive TIME by modulating the population of tumor-promoting M2-type TAMs (through deletion, polarization, and prevention of infiltration), promoting the presence of tumor-suppressing M1-type TAMs (through polarization) and enhancing the phagocytic activity of TAMs. This multifaceted approach effectively inhibited the development and metastasis of digestive system tumors. Although nanomaterials can be taken up by macrophages, nanomaterials targeting M2-type TAMs can improve specificity. Haptoglobin [358,359], mannose [188,360], M2pep [361], dextra [362], galactose [363], folate [364], and other substances targeting M2-type TAM can be modified on the surface of nanomaterials. Highly targeted nanomaterials can be designed by taking advantage of the high affinity of high-density lipoprotein for macrophages [202]. The above-mentioned strategy of targeting M2 deserves further promotion to improve drug delivery efficiency. Notably, the primary emphasis in the design of nanomaterials has been on the observed phenomena, with little consideration to the underlying mechanisms that give rise to these phenomena. Consequently, this article introduced TAM regulation by nanomaterials and discusses the mechanisms underlying this regulation.
Furthermore, the application of macrophage membrane-coated nanomaterials, macrophage-derived exosomes, and macrophage-based microrobots derived from macrophages has demonstrated remarkable efficacy in the treatment of digestive system tumors. Notably, macrophage therapies, including macrophage-based microrobots and chimeric antigen receptor-macrophages (CAT-M), have shown great therapeutic potential in treating tumors [365]. However, in the treatment of solid tumors, CAT-M has the defect of reacquiring the M2 phenotype in TEM, difficulty penetrating through the stroma, and inability to recognize heterogeneous surface antigens [366,367]. Macrophage-based microrobots can be driven by biology, chemistry, acoustics, and magnetism to precisely target cancer sites [368]. Nanomaterials in macrophage-based microrobots promote their maintenance of M1 phenotype and exert anticancer effects [369]. Therefore, the combination of CAT-M and nanomaterials to maximize the advantages of immunotherapy is a direction worth exploring in the future treatment of digestive system tumors.
Each digestive system tumor possesses a distinct TME because TAMs are not present in isolation. Therefore, this review discussed the role of these targets in their respective TMEs to the greatest extent possible. This study provides a theoretical foundation for enhancing the efficacy of nanomaterials and minimizing their potential adverse effects. Notably, using detection techniques such as single-cell sequencing has changed how TAMs are categorized, moving away from the traditional M0, M1, and M2 classification. TAMs with distinctive phenotypes may exert significant effect on tumor initiation and progression. A comprehensive understanding of the signaling pathways and biological characteristics associated with TAMs will prove advantageous for developing nanomaterials with enhanced properties. Using the characteristics of nanomaterials carrying multiple drugs, multiple targets of TAMs can be controlled simultaneously to promote the polarization of TAMs to the M1 type and enhance their phagocytic ability [65]. Moreover, the combined application of nanomaterials that regulate TAM and immune checkpoint inhibitors targeting PD-1 achieves multi-target synergistic treatment and has shown good results in animal experiments [65,206]. Studies of multi-target synergistic therapy have focused on combinations of approved treatments aimed at further increasing positive outcomes and survival [370]. However, nanomaterials that regulate TAM and are approved for marketing are absent. Imaging TAMs using nanomaterials can provide reference information for tumor diagnosis, guide tumor biopsy, analyze changes in TAM content within tumors or between metastases, define tumor edges, and quantify them by specifically analyzing apoptosis-initiated macrophage recruitment to evaluate the therapeutic effect of tumors [371,372]. Magnetic resonance imaging-compatible nanomaterials can specifically target macrophages and evaluate their accumulation at specific targets in real time [373]. Positron emission tomography contrast agents facilitate the determination of systemic biomarkers [374]. Non-invasive TAM directional imaging is a promising strategy for selecting appropriate nanomaterials trials, monitoring RT processes, and immunotherapy responses [375]. In summary, developing nanomaterials that promote the anti-tumor function of TAMs along with imaging capabilities and promoting their clinical translation are future research directions.
Funding
This work is supported by National Natural Science Foundation of China, 62371474 (MN), Natural Science Foundation of Liaoning Province, 2023JH2/101600006 (MN).
Ethics approval and consent to participate
The manuscript is a review article. The authors declare no experimentation on human or animals were designed.
CRediT authorship contribution statement
Hao Li: Writing – original draft. Shuai Wang: Writing – original draft. Zhengqiang Yang: Writing – review & editing. Xianwei Meng: Writing – review & editing. Meng Niu: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
All authors contributed to the article and approved the submitted version.
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Hao Li, Email: 269182498@qq.com.
Shuai Wang, Email: wangshuai2011@foxmail.com.
Zhengqiang Yang, Email: yangzhengqiang@jsph.org.cn.
Xianwei Meng, Email: mengxw@mail.ipc.ac.cn.
Meng Niu, Email: niumeng@cmu.edu.cn.
References
- 1.Almhanna K. Immunotherapy for gastrointestinal malignancies: the journey does not end here. Transl. Gastroenterol. Hepatol. 2020;5:2. doi: 10.21037/tgh.2019.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian S., Peng P., Li J., Deng H., Zhan N., Zeng Z., Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/beta-catenin signaling pathway. Aging (Albany NY) 2020;12(4):3574–3593. doi: 10.18632/aging.102831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Tan F., Liu X., Yi R., Zhao X. Exploring the antioxidant effects and periodic regulation of cancer cells by polyphenols produced by the fermentation of grape skin by lactobacillus plantarum KFY02. Biomolecules. 2019;9(10) doi: 10.3390/biom9100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H., Zheng L.Y., Wu L.Y., Chen J., Xu N., Mi S.C. The immune escape signature predicts the prognosis and immunotherapy sensitivity for pancreatic ductal adenocarcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.978921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L., Jiang Q., Li D.Z., Zhou X., Yu D.S., Zhong J. TIMP1 mRNA in tumor-educated platelets is diagnostic biomarker for colorectal cancer. Aging (Albany NY) 2019;11(20):8998–9012. doi: 10.18632/aging.102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdal E., Haider S., Rehwinkel J., Harris A.L., McHugh P.J. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 2017;31(4):353–369. doi: 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Sun J., Song Y., Gao P., Wang X., Chen M., Li Y., Wu Z. Roles of fusion genes in digestive system cancers: dawn for cancer precision therapy. Crit. Rev. Oncol. Hematol. 2022;171 doi: 10.1016/j.critrevonc.2022.103622. [DOI] [PubMed] [Google Scholar]
- 8.Morrison A.H., Byrne K.T., Vonderheide R.H. Immunotherapy and prevention of pancreatic cancer. Trends In Cancer. 2018;4(6):418–428. doi: 10.1016/j.trecan.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., Pikarsky E., Zhu A.X., Finn R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022;19(3):151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 10.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A. Immunotherapy in colorectal cancer: rationale, challenges and potential, Nature Reviews. Gastroenterol. Hepatol. 2019;16(6):361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.-L., He Y., Xu R.-H. Gastric cancer treatment: recent progress and future perspectives. J. Hematol. Oncol. 2023;16(1):57. doi: 10.1186/s13045-023-01451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SreeHarsha N., Hiremath J.G., Chilukuri S., Aitha R.K., Al-Dhubiab B.E., Venugopala K.N., Alzahrani A.M., Meravanige G. An approach to enhance dissolution rate of tamoxifen citrate. BioMed Res. Int. 2019 doi: 10.1155/2019/2161348. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Z., Han K., Dai X., Han H. Precisely striking tumors without adjacent normal tissue damage via mitochondria-templated accumulation. ACS Nano. 2018;12(6):6252–6262. doi: 10.1021/acsnano.8b03212. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Ding J., Xiao C., Zhuang X., Chen X. Emerging antitumor applications of extracellularly reengineered polymeric nanocarriers. Biomater. Sci. 2015;3(7) doi: 10.1039/c5bm00044k. [DOI] [PubMed] [Google Scholar]
- 15.Wei X., Wang J., Liang M., Song M. Development of functional nanomedicines for tumor associated macrophages-focused cancer immunotherapy. Theranostics. 2022;12(18):7821–7852. doi: 10.7150/thno.78572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Zhang P., Li Z., Feng X., Lv C., Zhang H., Xiao H., Ding J., Chen X. Evaluation of polymer nanoformulations in hepatoma therapy by established rodent models. Theranostics. 2019;9(5):1426–1452. doi: 10.7150/thno.31683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J., Zhang C. Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy. Wiley Interdiscipl. Rev. Nanomed. Nanobiotechnol. 2020;12(4) doi: 10.1002/wnan.1612. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Z., Li M., Dey R., Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol. Oncol. 2021;14(1):85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang H., Yhee J.Y., Jang G.H., You D.G., Ryu J.H., Choi Y., Na J.H., Park J.H., Lee K.H., Choi K., Kim K., Kwon I.C. Predicting the in vivo accumulation of nanoparticles in tumor based on in vitro macrophage uptake and circulation in zebrafish. J. Contr. Release : Off. J. Controlled Release Soc. 2016;244(Pt B):205–213. doi: 10.1016/j.jconrel.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Mostafa N., Salem A., Mansour S.Z., El-Sonbaty S.M., Moawed F.S.M., Kandil E.I. Rationale for tailoring an alternative oncology trial using a novel gallium-based nanocomplex: mechanistic insights and preclinical challenges. Technol. Cancer Res. Treat. 2022;21 doi: 10.1177/15330338221085376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C., Chan C., Weichselbaum R.R., Fleming G.F., Yamada S.D., Lin W. Nanomedicine for combination therapy of cancer. EBioMedicine. 2015;2(5):366–367. doi: 10.1016/j.ebiom.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy, Nature Reviews. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawicka D., Hryniewicka A., Gohal S., Sadowska A., Pryczynicz A., Guzinska-Ustymowicz K., Sokolowska E., Morzycki J.W., Car H. Establishment of in vitro and in vivo anticolorectal cancer efficacy of lithocholic acid-based imidazolium salts. Int. J. Mol. Sci. 2022;23(13) doi: 10.3390/ijms23137019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y.J., Oh S.G., Singh T.D., Ha J.H., Kim D.W., Lee S.W., Jeong S.Y., Ahn B.C., Lee J., Jeon Y.H. Visualization of the biological behavior of tumor-associated macrophages in living mice with colon cancer using multimodal optical reporter gene imaging. Neoplasia. 2016;18(3):133–141. doi: 10.1016/j.neo.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G., Lei H., Yang Y., Zhong X., Gong F., Gong Y., Zhou Y., Zhang Y., Shi H., Xiao Z., Dong Z., Cheng L. Titanium sulfide nanosheets serve as cascade bioreactors for H(2) S-mediated programmed gas-sonodynamic cancer therapy. Adv. Sci. 2022;9(30) doi: 10.1002/advs.202201069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 28.Ngambenjawong C., Gustafson H.H., Pun S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017;114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 30.Li W., Zhang X., Wu F., Zhou Y., Bao Z., Li H., Zheng P., Zhao S. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10(12):918. doi: 10.1038/s41419-019-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vayrynen J.P., Haruki K., Lau M.C., Vayrynen S.A., Zhong R., Dias Costa A., Borowsky J., Zhao M., Fujiyoshi K., Arima K., Twombly T.S., Kishikawa J., Gu S., Aminmozaffari S., Shi S., Baba Y., Akimoto N., Ugai T., Da Silva A., Guerriero J.L., Song M., Wu K., Chan A.T., Nishihara R., Fuchs C.S., Meyerhardt J.A., Giannakis M., Ogino S., Nowak J.A. The prognostic role of macrophage polarization in the colorectal cancer microenvironment. Cancer Immunol. Res. 2021;9(1):8–19. doi: 10.1158/2326-6066.CIR-20-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong P., Ma L., Liu L., Zhao G., Zhang S., Dong L., Xue R., Chen S. CD86⁺/CD206⁺, diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int. J. Mol. Sci. 2016;17(3):320. doi: 10.3390/ijms17030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi B., Chu J., Huang T., Wang X., Li Q., Gao Q., Xia Q., Luo S. The scavenger receptor MARCO expressed by tumor-associated macrophages are highly associated with poor pancreatic cancer prognosis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.771488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao L., Zhuo Z., Tang J., Huang X., Liu J., Wang H.Y., Xia H., He J. FABP4 deactivates NF-kappaB-IL1alpha pathway by ubiquitinating ATPB in tumor-associated macrophages and promotes neuroblastoma progression. Clin. Transl. Med. 2021;11(4):e395. doi: 10.1002/ctm2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laviron M., Boissonnas A. Ontogeny of tumor-associated macrophages. Front. Immunol. 2019;10:1799. doi: 10.3389/fimmu.2019.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Y., Chen J.X., Li M., Xiang Z., Wu J., Wang Y.J. Role of tumor-associated macrophages in common digestive system malignant tumors. World J. Gastrointest. Oncol. 2023;15(4):596–616. doi: 10.4251/wjgo.v15.i4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ovais M., Guo M., Chen C. Tailoring nanomaterials for targeting tumor-associated macrophages. Adv. Mater. 2019;31(19) doi: 10.1002/adma.201808303. [DOI] [PubMed] [Google Scholar]
- 38.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y., Rao L., Yao H., Wang Z., Ning P., Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 2020;32(40) doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Li Z., Li C., Huang H., Ren Y., Li Z., Hu Y., Guo W. Manganese-containing polydopamine nanoparticles as theranostic agents for magnetic resonance imaging and photothermal/chemodynamic combined ferroptosis therapy treating gastric cancer. Drug Deliv. 2022;29(1):1201–1211. doi: 10.1080/10717544.2022.2059124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi T., Fushida S., Yamamoto Y., Tsukada T., Kinoshita J., Oyama K., Miyashita T., Tajima H., Ninomiya I., Munesue S., Harashima A., Harada S., Yamamoto H., Ohta T. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19(4):1052–1065. doi: 10.1007/s10120-015-0579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J., Xu X., He S., Wang N., Bai Y., Li B., Zhang S. Exosomal hsa_circ_0017252 attenuates the development of gastric cancer via inhibiting macrophage M2 polarization. Hum. Cell. 2022;35(5):1499–1511. doi: 10.1007/s13577-022-00739-9. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Jiang M., Li S., Zhang Z., Sun H., Yang F., Liang H. Developing a novel anticancer gold(III) agent to integrate chemotherapy and immunotherapy. J. Med. Chem. 2021;64(10):6777–6791. doi: 10.1021/acs.jmedchem.1c00050. [DOI] [PubMed] [Google Scholar]
- 44.Hu X., Li J., Fu M., Zhao X., Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Targeted Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin P., Xu X., Deng C., Liu S., Wang Y., Zhou X., Ma H., Wei D., Sun S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharm. 2020;80 doi: 10.1016/j.intimp.2020.106210. [DOI] [PubMed] [Google Scholar]
- 46.Mu G., Zhu Y., Dong Z., Shi L., Deng Y., Li H. Calmodulin 2 facilitates angiogenesis and metastasis of gastric cancer via STAT3/HIF-1A/VEGF-A mediated macrophage polarization. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.727306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao K.Y., Chang N., Tsai J.L., Lin S.C., Tsai S.J., Wu M.H. Hypoxia-inhibited DUSP2 expression promotes IL-6/STAT3 signaling in endometriosis. Am. J. Reprod. Immunol. 2017;78(4) doi: 10.1111/aji.12690. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Chen J., Liu L. DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway. Open Life Sci. 2023;18(1) doi: 10.1515/biol-2022-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu D., Liu L., Ji X., Gao Y., Chen X., Liu Y., Liu Y., Zhao X., Li Y., Li Y., Jin Y., Zhang Y., McNutt M.A., Yin Y. The phosphatase DUSP2 controls the activity of the transcription activator STAT3 and regulates TH17 differentiation. Nat. Immunol. 2015;16(12):1263–1273. doi: 10.1038/ni.3278. [DOI] [PubMed] [Google Scholar]
- 50.Alhasan A.H., Patel P.C., Choi C.H., Mirkin C.A. Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agents. Small. 2014;10(1):186–192. doi: 10.1002/smll.201302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspect. Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giridharan S., Srinivasan M. Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018;11:407–419. doi: 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreira H., Szyjka A., Paliszkiewicz K., Barg E. Prooxidative activity of celastrol induces apoptosis, DNA damage, and cell cycle arrest in drug-resistant human colon cancer cells. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/6793957. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C., Hu F., Jiao G., Guo Y., Zhou P., Zhang Y., Zhang Z., Yi J., You Y., Li Z., Wang H., Zhang X. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFkappaB P65 signaling pathway after spinal cord injury. J. Nanobiotechnol. 2022;20(1):65. doi: 10.1186/s12951-022-01273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J., Li T., Chen H., Jiang Y., Zhao Y., Huang J., Chen Z., Tang X., Huang Z., Yang Z. ADAMTS10 inhibits aggressiveness via JAK/STAT/c-MYC pathway and reprograms macrophage to create an anti-malignant microenvironment in gastric cancer. Gastric Cancer. 2022;25(6):1002–1016. doi: 10.1007/s10120-022-01319-4. [DOI] [PubMed] [Google Scholar]
- 56.Shaik N., Martinez A., Augustin I., Giovinazzo H., Varela-Ramirez A., Sanau M., Aguilera R.J., Contel M. Synthesis of apoptosis-inducing iminophosphorane organogold(III) complexes and study of their interactions with biomolecular targets. Inorg. Chem. 2009;48(4):1577–1587. doi: 10.1021/ic801925k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan K.T., Wang K.H., Chang W.H., Yang J.C., Yeh C.F., Cheng K.T., Hung S.C., Chen Y.R. Application of data-independent acquisition approach to study the proteome change from early to later phases of tomato pathogenesis responses. Int. J. Mol. Sci. 2019;20(4) doi: 10.3390/ijms20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ottaiano A., De Stefano A., Capozzi M., Nappi A., De Divitiis C., Romano C., Silvestro L., Cassata A., Casaretti R., Tafuto S., Caraglia M., Berretta M., Nasti G., Avallone A. First biologic drug in the treatment of RAS wild-type metastatic colorectal cancer: anti-EGFR or bevacizumab? Results from a meta-analysis. Front. Pharmacol. 2018;9:441. doi: 10.3389/fphar.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segev L., Kalady M.F., Church J.M. Left-sided dominance of early-onset colorectal cancers: a rationale for screening flexible sigmoidoscopy in the young. Dis. Colon Rectum. 2018;61(8):897–902. doi: 10.1097/DCR.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 60.Qi W., Zhang Q. Identification and validation of immune molecular subtypes and immune landscape based on colon cancer cohort. Front. Med. 2022;9 doi: 10.3389/fmed.2022.827695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao P., Wang B., Zhang Z., Zhang W., Liu Y. Response gene to complement 32 expression in macrophages augments paracrine stimulation-mediated colon cancer progression. Cell Death Dis. 2019;10(10):776. doi: 10.1038/s41419-019-2006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrera M., Herrera A., Dominguez G., Silva J., Garcia V., Garcia J.M., Gomez I., Soldevilla B., Munoz C., Provencio M., Campos-Martin Y., Garcia de Herreros A., Casal I., Bonilla F., Pena C. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013;104(4):437–444. doi: 10.1111/cas.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Algars A., Irjala H., Vaittinen S., Huhtinen H., Sundstrom J., Salmi M., Ristamaki R., Jalkanen S. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int. J. Cancer. 2012;131(4):864–873. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- 64.Chen X., Jia Z., Wen Y., Huang Y., Yuan X., Chen Y., Liu Y., Liu J. Bidirectional anisotropic palladium nanozymes reprogram macrophages to enhance collaborative chemodynamic therapy of colorectal cancer. Acta Biomater. 2022;151:537–548. doi: 10.1016/j.actbio.2022.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Ni K., Luo T., Culbert A., Kaufmann M., Jiang X., Lin W. Nanoscale metal-organic framework Co-delivers TLR-7 agonists and anti-CD47 antibodies to modulate macrophages and orchestrate cancer immunotherapy. J. Am. Chem. Soc. 2020;142(29):12579–12584. doi: 10.1021/jacs.0c05039. [DOI] [PubMed] [Google Scholar]
- 66.Huang C., Lin B., Chen C., Wang H., Lin X., Liu J., Ren Q., Tao J., Zhao P., Xu Y. Synergistic reinforcing of immunogenic cell death and transforming tumor-associated macrophages via a multifunctional cascade bioreactor for optimizing cancer immunotherapy. Adv. Mater. 2022;34(51) doi: 10.1002/adma.202207593. [DOI] [PubMed] [Google Scholar]
- 67.Jacobson M.E., Wang-Bishop L., Becker K.W., Wilson J.T. Delivery of 5'-triphosphate RNA with endosomolytic nanoparticles potently activates RIG-I to improve cancer immunotherapy. Biomater. Sci. 2019;7(2):547–559. doi: 10.1039/c8bm01064a. [DOI] [PubMed] [Google Scholar]
- 68.Fang Y., He Y., Wu C., Zhang M., Gu Z., Zhang J., Liu E., Xu Q., Asrorov A.M., Huang Y. Magnetism-mediated targeting hyperthermia-immunotherapy in "cold" tumor with CSF1R inhibitor. Theranostics. 2021;11(14):6860–6872. doi: 10.7150/thno.57511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Wen Y., Chen X., Zhu X., Yu Q., Gong Y., Yuan G., Liu J., Qin X. Inflammation-responsive functional Ru nanoparticles combining a tumor-associated macrophage repolarization strategy with phototherapy for colorectal cancer therapy. J. Mater. Chem. B. 2019;7(40):6210–6223. doi: 10.1039/c9tb01613a. [DOI] [PubMed] [Google Scholar]
- 70.Hu L., Huang S., Chen G., Li B., Li T., Lin M., Huang Y., Xiao Z., Shuai X., Su Z. Nanodrugs incorporating LDHA siRNA inhibit M2-like polarization of TAMs and amplify autophagy to assist oxaliplatin chemotherapy against colorectal cancer. ACS Appl. Mater. Interfaces. 2022;14(28):31625–31633. doi: 10.1021/acsami.2c05841. [DOI] [PubMed] [Google Scholar]
- 71.Chen J., Li Z., Yue C., Ma J., Cao L., Lin J., Zhu D., An R., Lai J., Guo Y., Gu B. Human umbilical cord mesenchymal stem cell-derived exosomes carrying miR-1827 downregulate SUCNR1 to inhibit macrophage M2 polarization and prevent colorectal liver metastasis. Apoptosis. 2023;28(3–4):549–565. doi: 10.1007/s10495-022-01798-x. [DOI] [PubMed] [Google Scholar]
- 72.Castro F., Pinto M.L., Silva A.M., Pereira C.L., Teixeira G.Q., Gomez-Lazaro M., Santos S.G., Barbosa M.A., Goncalves R.M., Oliveira M.J. Pro-inflammatory chitosan/poly(gamma-glutamic acid) nanoparticles modulate human antigen-presenting cells phenotype and revert their pro-invasive capacity. Acta Biomater. 2017;63:96–109. doi: 10.1016/j.actbio.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 73.Castro F., Pinto M.L., Almeida R., Pereira F., Silva A.M., Pereira C.L., Santos S.G., Barbosa M.A., Goncalves R.M., Oliveira M.J. Chitosan/poly(gamma-glutamic acid) nanoparticles incorporating IFN-gamma for immune response modulation in the context of colorectal cancer. Biomater. Sci. 2019;7(8):3386–3403. doi: 10.1039/c9bm00393b. [DOI] [PubMed] [Google Scholar]
- 74.Marcion G., Hermetet F., Neiers F., Uyanik B., Dondaine L., Dias A.M.M., Da Costa L., Moreau M., Bellaye P.S., Collin B., Gobbo J., Briand L., Seigneuric R., Kitten O., Cinier M., Garrido C. Nanofitins targeting heat shock protein 110: an innovative immunotherapeutic modality in cancer. Int. J. Cancer. 2021;148(12):3019–3031. doi: 10.1002/ijc.33485. [DOI] [PubMed] [Google Scholar]
- 75.Li Z., Xu W., Yang J., Wang J., Wang J., Zhu G., Li D., Ding J., Sun T. A tumor microenvironments-adapted polypeptide hydrogel/nanogel composite boosts antitumor molecularly targeted inhibition and immunoactivation. Adv. Mater. 2022;34(21) doi: 10.1002/adma.202200449. [DOI] [PubMed] [Google Scholar]
- 76.Zeng J., Sun Y., Sun S., Jiang M., Zhang D., Li W., Liu Z., Shang H., Guan X., Zhang W. Leveraging nanodrug delivery system for simultaneously targeting tumor cells and M2 tumor-associated macrophages for efficient colon cancer therapy. ACS Appl. Mater. Interfaces. 2022;14(45):50475–50484. doi: 10.1021/acsami.2c11534. [DOI] [PubMed] [Google Scholar]
- 77.Bai H., Wang J., Phan C.U., Chen Q., Hu X., Shao G., Zhou J., Lai L., Tang G. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021;12(1):759. doi: 10.1038/s41467-021-21071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho E., Nam G.H., Hong Y., Kim Y.K., Kim D.H., Yang Y., Kim I.S. Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J. Contr. Release. 2018;279:326–335. doi: 10.1016/j.jconrel.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 79.Abdel-Bar H.M., Walters A.A., Lim Y., Rouatbi N., Qin Y., Gheidari F., Han S., Osman R., Wang J.T., Al-Jamal K.T. An "eat me" combinatory nano-formulation for systemic immunotherapy of solid tumors. Theranostics. 2021;11(18):8738–8754. doi: 10.7150/thno.56936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Kawai T., Akira S. Signaling to NF-kappaB by toll-like receptors. Trends Mol. Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Ding F.P., Tian J.Y., Wu J., Han D.F., Zhao D. Identification of key genes as predictive biomarkers for osteosarcoma metastasis using translational bioinformatics. Cancer Cell Int. 2021;21(1):640. doi: 10.1186/s12935-021-02308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shafeghat M., Kazemian S., Aminorroaya A., Aryan Z., Rezaei N. Toll-like receptor 7 regulates cardiovascular diseases. Int. Immunopharm. 2022;113(Pt A) doi: 10.1016/j.intimp.2022.109390. [DOI] [PubMed] [Google Scholar]
- 84.Cai H., Zhang Y., Wang J., Gu J. Defects in macrophage reprogramming in cancer therapy: the negative impact of PD-L1/PD-1. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.690869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eiro N., Gonzalez L., Gonzalez L.O., Andicoechea A., Fernandez-Diaz M., Altadill A., Vizoso F.J. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J. Clin. Immunol. 2012;32(4):848–854. doi: 10.1007/s10875-012-9666-3. [DOI] [PubMed] [Google Scholar]
- 86.Beilmann-Lehtonen I., Hagstrom J., Kaprio T., Stenman U.H., Strigard K., Palmqvist R., Gunnarsson U., Bockelman C., Haglund C. The relationship between the tissue expression of TLR2, TLR4, TLR5, and TLR7 and systemic inflammatory responses in colorectal cancer patients. Oncology. 2021;99(12):790–801. doi: 10.1159/000518397. [DOI] [PubMed] [Google Scholar]
- 87.Schon M.P., Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27(2):190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 88.Bhagchandani S., Johnson J.A., Irvine D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: from antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021;175 doi: 10.1016/j.addr.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu H. TLR agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Front. Immunol. 2014;5:83. doi: 10.3389/fimmu.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Feng Z., Liu J., Li H., Su Q., Zhang J., Huang P., Wang W., Liu J. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact. Mater. 2022;16:359–371. doi: 10.1016/j.bioactmat.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Z., Xie Y., Xiong Y., Liu S., Qiu C., Zhu Z., Mao H., Yu M., Wang X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020;469:173–185. doi: 10.1016/j.canlet.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 92.Varshney D., Qiu S.Y., Graf T.P., McHugh K.J. Employing drug delivery strategies to overcome challenges using TLR7/8 agonists for cancer immunotherapy. AAPS J. 2021;23(4):90. doi: 10.1208/s12248-021-00620-x. [DOI] [PubMed] [Google Scholar]
- 93.Ni K., Lan G., Song Y., Hao Z., Lin W. Biomimetic nanoscale metal-organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chem. Sci. 2020;11(29):7641–7653. doi: 10.1039/d0sc01949f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shao Y., Chen T., Zheng X., Yang S., Xu K., Chen X., Xu F., Wang L., Shen Y., Wang T., Zhang M., Hu W., Ye C., Yu X., Shao J., Zheng S. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39(11):1368–1379. doi: 10.1093/carcin/bgy115. [DOI] [PubMed] [Google Scholar]
- 95.Nishimoto S., Fukuda D., Sata M. Emerging roles of Toll-like receptor 9 in cardiometabolic disorders. Inflamm. Regen. 2020;40:18. doi: 10.1186/s41232-020-00118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karapetyan L., Luke J.J., Davar D. Toll-like receptor 9 agonists in cancer. OncoTargets Ther. 2020;13:10039–10060. doi: 10.2147/OTT.S247050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang H., Guo Y., Wei C., Hu P., Shi J. Nanocatalytic innate immunity activation by mitochondrial DNA oxidative damage for tumor-specific therapy. Adv. Mater. 2021;33(20) doi: 10.1002/adma.202008065. [DOI] [PubMed] [Google Scholar]
- 98.Jin F., Liu D., Xu X., Ji J., Du Y. Nanomaterials-based photodynamic therapy with combined treatment improves antitumor efficacy through boosting immunogenic cell death. Int. J. Nanomed. 2021;16:4693–4712. doi: 10.2147/IJN.S314506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamblin M.R., Abrahamse H. Factors affecting photodynamic therapy and anti-tumor immune response. Anti Cancer Agents Med. Chem. 2021;21(2):123–136. doi: 10.2174/1871520620666200318101037. [DOI] [PubMed] [Google Scholar]
- 100.Alzeibak R., Mishchenko T.A., Shilyagina N.Y., Balalaeva I.V., Vedunova M.V., Krysko D.V. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J. Immunother. Cancer. 2021;9(1) doi: 10.1136/jitc-2020-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuo X., Liu Z., Aishajiang R., Wang T., Yu D. Recent progress of copper-based nanomaterials in tumor-targeted photothermal therapy/photodynamic therapy. Pharmaceutics. 2023;15(9) doi: 10.3390/pharmaceutics15092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He C., Zhang S., Liu X., Wang J., Huang Y., Zhang A., Zhang X. CaO2 nanomedicines: a review of their emerging roles in cancer therapy. Nanotechnology. 2023 doi: 10.1088/1361-6528/acf381. [DOI] [PubMed] [Google Scholar]
- 103.Gao C., Qiao T., Zhang B., Yuan S., Zhuang X., Luo Y. TLR9 signaling activation at different stages in colorectal cancer and NF-kappaB expression. OncoTargets Ther. 2018;11:5963–5971. doi: 10.2147/OTT.S174274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shahriari S., Rezaeifard S., Moghimi H.R., Khorramizadeh M.R., Faghih Z. Cell membrane and intracellular expression of toll-like receptor 9 (TLR9) in colorectal cancer and breast cancer cell-lines. Cancer Biomarkers. 2017;18(4):375–380. doi: 10.3233/CBM-160260. [DOI] [PubMed] [Google Scholar]
- 105.Kell A.M., Gale M., Jr. RIG-I in RNA virus recognition. Virology. 2015;479–480:110–121. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kawai T., Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 107.Wu W., Zhang W., Alexandar J.S., Booth J.L., Miller C.A., Xu C., Metcalf J.P. RIG-I agonist SLR10 promotes macrophage M1 polarization during influenza virus infection. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1177624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 109.Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 110.Duvall C.L., Convertine A.J., Benoit D.S., Hoffman A.S., Stayton P.S. Intracellular delivery of a proapoptotic peptide via conjugation to a RAFT synthesized endosomolytic polymer. Mol. Pharm. 2010;7(2):468–476. doi: 10.1021/mp9002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson J.T., Keller S., Manganiello M.J., Cheng C., Lee C.C., Opara C., Convertine A., Stayton P.S. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7(5):3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Keller S., Wilson J.T., Patilea G.I., Kern H.B., Convertine A.J., Stayton P.S. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses. J. Contr. Release. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Convertine A.J., Diab C., Prieve M., Paschal A., Hoffman A.S., Johnson P.H., Stayton P.S. pH-responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules. 2010;11(11):2904–2911. doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H.W., Tang S.L. Colony stimulating factor-1 and its receptor in gastrointestinal malignant tumors. J. Cancer. 2021;12(23):7111–7119. doi: 10.7150/jca.60379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cannarile M.A., Weisser M., Jacob W., Jegg A.M., Ries C.H., Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer. 2017;5(1):53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., Olson O.C., Quick M.L., Huse J.T., Teijeiro V., Setty M., Leslie C.S., Oei Y., Pedraza A., Zhang J., Brennan C.W., Sutton J.C., Holland E.C., Daniel D., Joyce J.A. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shi X., Kaller M., Rokavec M., Kirchner T., Horst D., Hermeking H. Characterization of a p53/miR-34a/CSF1R/STAT3 feedback loop in colorectal cancer. Cell Mol. Gastroenterol. Hepatol. 2020;10(2):391–418. doi: 10.1016/j.jcmgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duan S., Hu Y., Zhao Y., Tang K., Zhang Z., Liu Z., Wang Y., Guo H., Miao Y., Du H., Yang D., Li S., Zhang J. Nanomaterials for photothermal cancer therapy. RSC Adv. 2023;13(21):14443–14460. doi: 10.1039/d3ra02620e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dash B.S., Das S., Chen J.P. Photosensitizer-functionalized nanocomposites for light-activated cancer theranostics. Int. J. Mol. Sci. 2021;22(13) doi: 10.3390/ijms22136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang C., Zhao Y., Zhang H., Chen X., Zhao N., Tan D., Zhang H., Shi C. The application of heptamethine cyanine dye DZ-1 and indocyanine green for imaging and targeting in xenograft models of hepatocellular carcinoma. Int. J. Mol. Sci. 2017;18(6) doi: 10.3390/ijms18061332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z., Li S., Cao Y., Tian X., Zeng R., Liao D.F., Cao D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/9875298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han W., Xie B., Li Y., Shi L., Wan J., Chen X., Wang H. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics. 2019;9(24):7458–7473. doi: 10.7150/thno.38081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perez-Torres I., Castrejon-Tellez V., Soto M.E., Rubio-Ruiz M.E., Manzano-Pech L., Guarner-Lans V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017;60(5):1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo Y., Wu R., Gaspar J.M., Sargsyan D., Su Z.Y., Zhang C., Gao L., Cheng D., Li W., Wang C., Yin R., Fang M., Verzi M.P., Hart R.P., Kong A.N. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis. 2018;39(5):669–680. doi: 10.1093/carcin/bgy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng T., Wei Y., Lee R.J., Zhao L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017;12:6027–6044. doi: 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6(10):789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 128.Mattinzoli D., Cacioppo M., Ikehata M., Armelloni S., Alfieri C.M., Castellano G., Barilani M., Arcudi F., Messa P., Prato M. Carbon dots conjugated to SN38 for improved colorectal anticancer therapy. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang C., Xia A.J., Du C.H., Hu M.X., Gong Y.L., Tian R., Jiang X., Xie Y.M. Discovery of highly potent and selective 7-ethyl-10-hydroxycamptothecin-glucose conjugates as potential anti-colorectal cancer agents. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1014854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nagheh Z., Irani S., Mirfakhraie R., Dinarvand R. SN38-PEG-PLGA-verapamil nanoparticles inhibit proliferation and downregulate drug transporter ABCG2 gene expression in colorectal cancer cells. Prog. Biomater. 2017;6(4):137–145. doi: 10.1007/s40204-017-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang J., Jia L., He Z., Wang Y. Recent advances in SN-38 drug delivery system. Int. J. Pharm. 2023;637 doi: 10.1016/j.ijpharm.2023.122886. [DOI] [PubMed] [Google Scholar]
- 132.Zhang X., Yang X., Ji J., Liu A., Zhai G. Tumor targeting strategies for chitosan-based nanoparticles. Colloids Surf. B Biointerfaces. 2016;148:460–473. doi: 10.1016/j.colsurfb.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 133.Pathomthongtaweechai N., Muanprasat C. Potential applications of chitosan-based nanomaterials to surpass the gastrointestinal physiological obstacles and enhance the intestinal drug absorption. Pharmaceutics. 2021;13(6) doi: 10.3390/pharmaceutics13060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen M.C., Mi F.L., Liao Z.X., Hsiao C.W., Sonaje K., Chung M.F., Hsu L.W., Sung H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013;65(6):865–879. doi: 10.1016/j.addr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 135.Masoud G.N., Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Negri A.L. Role of prolyl hydroxylase/HIF-1 signaling in vascular calcification. Clin. Kidney J. 2023;16(2):205–209. doi: 10.1093/ckj/sfac224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J.M., Lane W.S., Kaelin W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 138.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 139.Kunze R., Zhou W., Veltkamp R., Wielockx B., Breier G., Marti H.H. Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia. Stroke. 2012;43(10):2748–2756. doi: 10.1161/STROKEAHA.112.669598. [DOI] [PubMed] [Google Scholar]
- 140.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Z., Yao L., Yang J., Wang Z., Du G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol. Med. Rep. 2018;18(4):3547–3554. doi: 10.3892/mmr.2018.9375. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cowman S.J., Koh M.Y. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer. 2022;8(1):28–42. doi: 10.1016/j.trecan.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu J.Y., Huang T.W., Hsieh Y.T., Wang Y.F., Yen C.C., Lee G.L., Yeh C.C., Peng Y.J., Kuo Y.Y., Wen H.T., Lin H.C., Hsiao C.W., Wu K.K., Kung H.J., Hsu Y.J., Kuo C.C. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol. Cell. 2020;77(2):213–227 e5. doi: 10.1016/j.molcel.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 144.Jiang H., Wei H., Wang H., Wang Z., Li J., Ou Y., Xiao X., Wang W., Chang A., Sun W., Zhao L., Yang S. Zeb1-induced metabolic reprogramming of glycolysis is essential for macrophage polarization in breast cancer. Cell Death Dis. 2022;13(3):206. doi: 10.1038/s41419-022-04632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J., Muri J., Fitzgerald G., Gorski T., Gianni-Barrera R., Masschelein E., D'Hulst G., Gilardoni P., Turiel G., Fan Z., Wang T., Planque M., Carmeliet P., Pellerin L., Wolfrum C., Fendt S.M., Banfi A., Stockmann C., Soro-Arnaiz I., Kopf M., De Bock K. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metabol. 2020;31(6):1136–1153 e7. doi: 10.1016/j.cmet.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., Cline G.W., Phillips A.J., Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J., Guo Y., Liu J., Guo F., Du L., Yang Y., Li X., Ma Y. Depicting the landscape of gut microbial-metabolic interaction and microbial-host immune heterogeneity in deficient and proficient DNA mismatch repair colorectal cancers. J. Immunother. Cancer. 2023;11(8) doi: 10.1136/jitc-2023-007420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao X., Zhou S., Qin Z., Li D., Zhu Y., Ma D. Upregulation of HMGB1 in tumor-associated macrophages induced by tumor cell-derived lactate further promotes colorectal cancer progression. J. Transl. Med. 2023;21(1):53. doi: 10.1186/s12967-023-03918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hembach L., Cord-Landwehr S., Moerschbacher B.M. Enzymatic production of all fourteen partially acetylated chitosan tetramers using different chitin deacetylases acting in forward or reverse mode. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-17950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van der Lubben I.M., Verhoef J.C., Borchard G., Junginger H.E. Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 2001;52(2):139–144. doi: 10.1016/s0169-409x(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 151.Vasconcelos D.P., Fonseca A.C., Costa M., Amaral I.F., Barbosa M.A., Aguas A.P., Barbosa J.N. Macrophage polarization following chitosan implantation. Biomaterials. 2013;34(38):9952–9959. doi: 10.1016/j.biomaterials.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 152.Halmschlag B., Putri S.P., Fukusaki E., Blank L.M. Identification of key metabolites in poly-gamma-glutamic acid production by tuning gamma-PGA synthetase expression. Front. Bioeng. Biotechnol. 2020;8:38. doi: 10.3389/fbioe.2020.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ahn H., Kang S.G., Yoon S.I., Kim P.H., Kim D., Lee G.S. Poly-gamma-glutamic acid from Bacillus subtilis upregulates pro-inflammatory cytokines while inhibiting NLRP3, NLRC4 and AIM2 inflammasome activation. Cell. Mol. Immunol. 2018;15(2):111–119. doi: 10.1038/cmi.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pereira C.L., Antunes J.C., Goncalves R.M., Ferreira-da-Silva F., Barbosa M.A. Biosynthesis of highly pure poly-gamma-glutamic acid for biomedical applications. J. Mater. Sci. Mater. Med. 2012;23(7):1583–1591. doi: 10.1007/s10856-012-4639-x. [DOI] [PubMed] [Google Scholar]
- 155.Ji L., Zhao X., Zhang B., Kang L., Song W., Zhao B., Xie W., Chen L., Hu X. Slc6a8-Mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity. 2019;51(2):272–284 e7. doi: 10.1016/j.immuni.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 156.Yu T., Zuo Y., Cai R., Huang X., Wu S., Zhang C., Chin Y.E., Li D., Zhang Z., Xia N., Wang Q., Shen H., Yao X., Zhang Z.Y., Xue S., Shen L., Cheng J. SENP1 regulates IFN-gamma-STAT1 signaling through STAT3-SOCS3 negative feedback loop. J. Mol. Cell Biol. 2017;9(2):144–153. doi: 10.1093/jmcb/mjw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu J., Li P., Li Z., Li Y., Luo J., Su W., Liang D. Topical administration of 0.3% tofacitinib suppresses M1 macrophage polarization and allograft corneal rejection by blocking STAT1 activation in the rat cornea. Transl. Vis. Sci. Technol. 2022;11(3):34. doi: 10.1167/tvst.11.3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu J., Liu T., Rios Z., Mei Q., Lin X., Cao S. Heat shock proteins and cancer. Trends Pharmacol. Sci. 2017;38(3):226–256. doi: 10.1016/j.tips.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 159.Saini J., Sharma P.K. Clinical, prognostic and therapeutic significance of heat shock proteins in cancer. Curr. Drug Targets. 2018;19(13):1478–1490. doi: 10.2174/1389450118666170823121248. [DOI] [PubMed] [Google Scholar]
- 160.Slaby Significant overexpression of Hsp110 gene during colorectal cancer progression. Oncol. Rep. 2009;21(5) doi: 10.3892/or_00000346. [DOI] [PubMed] [Google Scholar]
- 161.Berthenet K., Boudesco C., Collura A., Svrcek M., Richaud S., Hammann A., Causse S., Yousfi N., Wanherdrick K., Duplomb L., Duval A., Garrido C., Jego G. Extracellular HSP110 skews macrophage polarization in colorectal cancer. OncoImmunology. 2016;5(7) doi: 10.1080/2162402X.2016.1170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang C., Yu X., Gao L., Zhao Y., Lai J., Lu D., Bao R., Jia B., Zhong L., Wang F., Liu Z. Noninvasive imaging of cd206-positive M2 macrophages as an early biomarker for post-chemotherapy tumor relapse and lymph node metastasis. Theranostics. 2017;7(17):4276–4288. doi: 10.7150/thno.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiong M., Lei Q., You X., Gao T., Song X., Xia Y., Ye T., Zhang L., Wang N., Yu L. Mannosylated liposomes improve therapeutic effects of paclitaxel in colon cancer models. J. Microencapsul. 2017;34(6):513–521. doi: 10.1080/02652048.2017.1339739. [DOI] [PubMed] [Google Scholar]
- 165.Su F.Y., Srinivasan S., Lee B., Chen J., Convertine A.J., West T.E., Ratner D.M., Skerrett S.J., Stayton P.S. Macrophage-targeted drugamers with enzyme-cleavable linkers deliver high intracellular drug dosing and sustained drug pharmacokinetics against alveolar pulmonary infections. J. Contr. Release. 2018;287:1–11. doi: 10.1016/j.jconrel.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hoff S., Grünewald S., Röse L., Zopf D. Immunomodulation by regorafenib alone and in combination with anti PD1 antibody on murine models of colorectal cancer. Ann. Oncol. 2017;28 doi: 10.1093/annonc/mdx376.060. [DOI] [Google Scholar]
- 167.Mantovani A., Allavena P., Marchesi F., Garlanda C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022;21(11):799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Duijn A., Van der Burg S.H., Scheeren F.A. CD47/SIRPalpha axis: bridging innate and adaptive immunity. J. Immunother. Cancer. 2022;10(7) doi: 10.1136/jitc-2022-004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jia X., Yan B., Tian X., Liu Q., Jin J., Shi J., Hou Y. CD47/SIRPalpha pathway mediates cancer immune escape and immunotherapy. Int. J. Biol. Sci. 2021;17(13):3281–3287. doi: 10.7150/ijbs.60782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rao L., Wu L., Liu Z., Tian R., Yu G., Zhou Z., Yang K., Xiong H.G., Zhang A., Yu G.T., Sun W., Xu H., Guo J., Li A., Chen H., Sun Z.J., Fu Y.X., Chen X. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 2020;11(1):4909. doi: 10.1038/s41467-020-18626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meng Q.-F., Zhao Y., Dong C., Liu L., Pan Y., Lai J., Liu Z., Yu G.-T., Chen X., Rao L. Genetically programmable fusion cellular vesicles for cancer immunotherapy. Angew. Chem. 2021;60(50):26320–26326. doi: 10.1002/anie.202108342. [DOI] [PubMed] [Google Scholar]
- 172.Roelands J., van der Ploeg M., Ijsselsteijn M.E., Dang H., Boonstra J.J., Hardwick J.C.H., Hawinkels L., Morreau H., de Miranda N. Transcriptomic and immunophenotypic profiling reveals molecular and immunological hallmarks of colorectal cancer tumourigenesis. Gut. 2023;72(7):1326–1339. doi: 10.1136/gutjnl-2022-327608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hernandez C., Huebener P., Schwabe R.F. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35(46):5931–5941. doi: 10.1038/onc.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ma X., Cui H., Sun M., Liu Q., Liu X., Li G., Wei Y., Fu Q., Liu S., Cao L. Fasting blood glucose, cholesterol, and risk of primary liver cancer: the kailuan study. Cancer Res. Treat. 2021;53(4):1113–1122. doi: 10.4143/crt.2020.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., Pikarsky E., Zhu A.X., Finn R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022;19(3):151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 176.Cheng K., Cai N., Zhu J., Yang X., Liang H., Zhang W. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Commun. 2022;42(11):1112–1140. doi: 10.1002/cac2.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou F., Teng F., Deng P., Meng N., Song Z., Feng R. Recent progress of nano-drug delivery system for liver cancer treatment. Anti Cancer Agents Med. Chem. 2018;17(14):1884–1897. doi: 10.2174/1871520617666170713151149. [DOI] [PubMed] [Google Scholar]
- 178.Gao D.Y., Lin Ts T., Sung Y.C., Liu Y.C., Chiang W.H., Chang C.C., Liu J.Y., Chen Y. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials. 2015;67:194–203. doi: 10.1016/j.biomaterials.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 179.Liu J.Y., Chiang T., Liu C.H., Chern G.G., Lin T.T., Gao D.Y., Chen Y. Delivery of siRNA using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol. Ther. 2015;23(11):1772–1782. doi: 10.1038/mt.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fernandez-Varo G., Perramon M., Carvajal S., Oro D., Casals E., Boix L., Oller L., Macias-Munoz L., Marfa S., Casals G., Morales-Ruiz M., Casado P., Cutillas P.R., Bruix J., Navasa M., Fuster J., Garcia-Valdecasas J.C., Pavel M.C., Puntes V., Jimenez W. Bespoken nanoceria: an effective treatment in experimental hepatocellular carcinoma. Hepatology. 2020;72(4):1267–1282. doi: 10.1002/hep.31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li T., Liu Z., Fu X., Chen Y., Zhu S., Zhang J. Co-delivery of Interleukin-12 and doxorubicin loaded Nano-delivery system for enhanced immunotherapy with polarization toward M1-type Macrophages. Eur. J. Pharm. Biopharm. 2022;177:175–183. doi: 10.1016/j.ejpb.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 182.Jiang L., Wang Y., Wei X., Yang L., Liu S., Wang Y., Xu Y., Wang Z., Zhang C., Zhang M., Zhang Y., Jin F., Yin X. Improvement in phenotype homeostasis of macrophages by chitosan nanoparticles and subsequent impacts on liver injury and tumor treatment. Carbohydr. Polym. 2022;277 doi: 10.1016/j.carbpol.2021.118891. [DOI] [PubMed] [Google Scholar]
- 183.Tang B., Zhu J., Wang Y., Chen W., Fang S., Mao W., Xu Z., Yang Y., Weng Q., Zhao Z., Chen M., Ji J. Targeted xCT-mediated ferroptosis and protumoral polarization of macrophages is effective against HCC and enhances the efficacy of the anti-PD-1/L1 response. Adv. Sci. 2023;10(2) doi: 10.1002/advs.202203973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Q., Huang W., Liang W., Ye Q. Current strategies for modulating tumor-associated macrophages with biomaterials in hepatocellular carcinoma. Molecules. 2023;28(5) doi: 10.3390/molecules28052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu X., Chen S., Hu X., Zhao L., Wang Y., Huang J., Chen J., Qiu Y., Zhang X., Wang M., Yang X., Zhang Y., Zhu Y. Near-infrared nano-optogenetic activation of cancer immunotherapy via engineered bacteria. Adv. Mater. 2023;35(8) doi: 10.1002/adma.202207198. [DOI] [PubMed] [Google Scholar]
- 186.Meng J., Li L., Xu Y., Wei X., Chen D., Chen C., Liu S., Wang Z., Shi G., Wang S., Zhou X., Jiang L. A simple liposome-based bionic bacterium for tumor treatment by re-education of tumor-associated microphages in combination with chemotherapy. Colloids Surf. B Biointerfaces. 2023;222 doi: 10.1016/j.colsurfb.2022.113069. [DOI] [PubMed] [Google Scholar]
- 187.Comparetti E.J., Lins P.M.P., Quitiba J., Zucolotto V. Cancer cell membrane-derived nanoparticles block the expression of immune checkpoint proteins on cancer cells and coordinate modulatory activity on immunosuppressive macrophages. J. Biomed. Mater. Res. 2022;110(8):1499–1511. doi: 10.1002/jbm.a.37387. [DOI] [PubMed] [Google Scholar]
- 188.Liu X., Huangfu Y., Wang J., Kong P., Tian W., Liu P., Fang C., Li S., Nie Y., Feng Z., Huang P., Shi S., Zhang C., Dong A., Wang W. Supramolecular polymer-nanomedicine hydrogel loaded with tumor associated macrophage-reprogramming polyTLR7/8a nanoregulator for enhanced anti-angiogenesis therapy of orthotopic hepatocellular carcinoma. Adv. Sci. 2023;10(22) doi: 10.1002/advs.202300637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang L., Xu R., Li W., Lv L., Lin C., Yang X., Yao Y., Saw P.E., Xu X. Repolarization of macrophages to improve sorafenib sensitivity for combination cancer therapy. Acta Biomater. 2023;162:98–109. doi: 10.1016/j.actbio.2023.03.014. [DOI] [PubMed] [Google Scholar]
- 190.Chen X., Tang Q., Wang J., Zhou Y., Li F., Xie Y., Wang X., Du L., Li J., Pu J., Hu Q., Gu Z., Liu P. A DNA/DMXAA/Metal-Organic framework activator of innate immunity for boosting anticancer immunity. Adv. Mater. 2023;35(15) doi: 10.1002/adma.202210440. [DOI] [PubMed] [Google Scholar]
- 191.Ahmed K.K., Geary S.M., Salem A.K. Applying biodegradable particles to enhance cancer vaccine efficacy. Immunol. Res. 2014;59(1–3):220–228. doi: 10.1007/s12026-014-8537-9. [DOI] [PubMed] [Google Scholar]
- 192.Wei Z., Zhang X., Yong T., Bie N., Zhan G., Li X., Liang Q., Li J., Yu J., Huang G., Yan Y., Zhang Z., Zhang B., Gan L., Huang B., Yang X. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 2021;12(1):440. doi: 10.1038/s41467-020-20723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X., Wei Z., Ding Z., Lv W., Li J., Li X., Liu H., Yu P., Yang X., Gan L. Boosting doxil-based chemoimmunotherapy via reprogramming tumor-associated macrophages. Chem. Eng. J. 2023;451 doi: 10.1016/j.cej.2022.138971. [DOI] [Google Scholar]
- 194.Zhu J., Ji Z., Wang J., Sun R., Zhang X., Gao Y., Sun H., Liu Y., Wang Z., Li A., Ma J., Wang T., Jia G., Gu Y. Tumor-inhibitory effect and immunomodulatory activity of fullerol C60(OH)x. Small. 2008;4(8):1168–1175. doi: 10.1002/smll.200701219. [DOI] [PubMed] [Google Scholar]
- 195.Cui R., Wang L., Zhang D., Zhang K., Dou J., Dong L., Zhang Y., Wu J., Tan L., Yu J., Liang P. Combination therapy using microwave ablation and d-mannose-chelated iron oxide nanoparticles inhibits hepatocellular carcinoma progression. Acta Pharm. Sin. B. 2022;12(9):3475–3485. doi: 10.1016/j.apsb.2022.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang T., Zhang J., Hou T., Yin X., Zhang N. Selective targeting of tumor cells and tumor associated macrophages separately by twin-like core-shell nanoparticles for enhanced tumor-localized chemoimmunotherapy. Nanoscale. 2019;11(29):13934–13946. doi: 10.1039/c9nr03374b. [DOI] [PubMed] [Google Scholar]
- 197.Chen H., Jiang S., Zhang P., Ren Z., Wen J. Exosomes synergized with PIONs@E6 enhance their immunity against hepatocellular carcinoma via promoting M1 macrophages polarization. Int. Immunopharm. 2021;99 doi: 10.1016/j.intimp.2021.107960. [DOI] [PubMed] [Google Scholar]
- 198.Li G., Liu D., Kimchi E.T., Kaifi J.T., Qi X., Manjunath Y., Liu X., Deering T., Avella D.M., Fox T., Rockey D.C., Schell T.D., Kester M., Staveley-O'Carroll K.F. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology. 2018;154(4):1024–1036 e9. doi: 10.1053/j.gastro.2017.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu J., Chen J., Liu H., Zhang K., Zeng Q., Yang S., Jiang Z., Zhang X., Chen T., Li D., Shan H. Bi/Se-Based nanotherapeutics sensitize CT image-guided stereotactic body radiotherapy through reprogramming the microenvironment of hepatocellular carcinoma. ACS Appl. Mater. Interfaces. 2021;13(36):42473–42485. doi: 10.1021/acsami.1c11763. [DOI] [PubMed] [Google Scholar]
- 200.Dai X., Ruan J., Guo Y., Sun Z., Liu J., Bao X., Zhang H., Li Q., Ye C., Wang X., Zhao C.-X., Zhou F., Sheng J., Chen D., Zhao P. Enhanced radiotherapy efficacy and induced anti-tumor immunity in HCC by improving hypoxia microenvironment using oxygen microcapsules. Chem. Eng. J. 2021;422 doi: 10.1016/j.cej.2021.130109. [DOI] [Google Scholar]
- 201.Chang C.C., Dinh T.K., Lee Y.A., Wang F.N., Sung Y.C., Yu P.L., Chiu S.C., Shih Y.C., Wu C.Y., Huang Y.D., Wang J., Lu T.T., Wan D., Chen Y. Nanoparticle delivery of MnO(2) and antiangiogenic therapy to overcome hypoxia-driven tumor escape and suppress hepatocellular carcinoma. ACS Appl. Mater. Interfaces. 2020;12(40):44407–44419. doi: 10.1021/acsami.0c08473. [DOI] [PubMed] [Google Scholar]
- 202.Wang J., Zheng C., Zhai Y., Cai Y., Lee R.J., Xing J., Wang H., Zhu H.H., Teng L., Li Y., Zhang P. High-density lipoprotein modulates tumor-associated macrophage for chemoimmunotherapy of hepatocellular carcinoma. Nano Today. 2021;37 doi: 10.1016/j.nantod.2020.101064. [DOI] [Google Scholar]
- 203.Wang Y., Wang Z., Jia F., Xu Q., Shu Z., Deng J., Li A., Yu M., Yu Z. CXCR4-guided liposomes regulating hypoxic and immunosuppressive microenvironment for sorafenib-resistant tumor treatment. Bioact. Mater. 2022;17:147–161. doi: 10.1016/j.bioactmat.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wei Z., Zhang X., Zhang Z., Yong T., Zhan G., Lv W., Ding Z., Sun K., Yang X., Gan L. Engineered Iron-Based nanoplatform amplifies repolarization of M2-Like Tumor-Associated Macrophages for enhanced cancer immunotherapy. Chem. Eng. J. 2022;433 doi: 10.1016/j.cej.2021.133847. [DOI] [Google Scholar]
- 205.Han S., Bao X., Zou Y., Wang L., Li Y., Yang L., Liao A., Zhang X., Jiang X., Liang D., Dai Y., Zheng Q.C., Yu Z., Guo J. d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci. Adv. 2023;9(29) doi: 10.1126/sciadv.adg2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hou G., Qian J., Guo M., Xu W., Wang J., Wang Y., Suo A. Hydrazide-manganese coordinated multifunctional nanoplatform for potentiating immunotherapy in hepatocellular carcinoma. J. Colloid Interface Sci. 2022;628(Pt B):968–983. doi: 10.1016/j.jcis.2022.08.091. [DOI] [PubMed] [Google Scholar]
- 207.Tian Z., Hou X., Liu W., Shao C., Gao L., Jiang J., Zhang L., Han Z., Wei L. Targeted blocking of CCR2 and CXCR2 improves the efficacy of transarterial chemoembolization of hepatocarcinoma. Cancer Cell Int. 2022;22(1):362. doi: 10.1186/s12935-022-02771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Song J.S., Chang C.C., Wu C.H., Dinh T.K., Jan J.J., Huang K.W., Chou M.C., Shiue T.Y., Yeh K.C., Ke Y.Y., Yeh T.K., Ta Y.N., Lee C.J., Huang J.K., Sung Y.C., Shia K.S., Chen Y. A highly selective and potent CXCR4 antagonist for hepatocellular carcinoma treatment. Proc. Natl. Acad. Sci. U. S. A. 2021;118(13) doi: 10.1073/pnas.2015433118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bao D., Zhao J., Zhou X., Yang Q., Chen Y., Zhu J., Yuan P., Yang J., Qin T., Wan S., Xing J. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene. 2019;38(25):5007–5020. doi: 10.1038/s41388-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yao W., Ba Q., Li X., Li H., Zhang S., Yuan Y., Wang F., Duan X., Li J., Zhang W., Wang H. A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine. 2017;22:58–67. doi: 10.1016/j.ebiom.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huang W., Chen Z., Zhang L., Tian D., Wang D., Fan D., Wu K., Xia L. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149(4):1053–1067 e14. doi: 10.1053/j.gastro.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 212.Zhang Y., Sang R., Bao J., Jiang Z., Qian D., Zhou Y., Su W., Wei J., Zhao L., Wei Z., Zhao Y., Shi M., Chen G. Schwann cell-derived CXCL2 contributes to cancer pain by modulating macrophage infiltration in a mouse breast cancer model. Brain Behav. Immun. 2023;109:308–320. doi: 10.1016/j.bbi.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 213.Tang W., Chen Z., Zhang W., Cheng Y., Zhang B., Wu F., Wang Q., Wang S., Rong D., Reiter F.P., De Toni E.N., Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct. Targeted Ther. 2020;5(1):87. doi: 10.1038/s41392-020-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen Y., Ramjiawan R.R., Reiberger T., Ng M.R., Hato T., Huang Y., Ochiai H., Kitahara S., Unan E.C., Reddy T.P., Fan C., Huang P., Bardeesy N., Zhu A.X., Jain R.K., Duda D.G. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rioja-Blanco E., Gallardo A., Arroyo-Solera I., Alamo P., Casanova I., Unzueta U., Serna N., Sanchez-Garcia L., Quer M., Villaverde A., Vazquez E., Leon X., Alba-Castellon L., Mangues R. A novel CXCR4-targeted diphtheria toxin nanoparticle inhibits invasion and metastatic dissemination in a head and neck squamous cell carcinoma mouse model. Pharmaceutics. 2022;14(4) doi: 10.3390/pharmaceutics14040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Choi H.Y., Yong C.S., Yoo B.K. Plerixafor for stem cell mobilization in patients with non-Hodgkin's lymphoma and multiple myeloma. Ann. Pharmacother. 2010;44(1):117–126. doi: 10.1345/aph.1M380. [DOI] [PubMed] [Google Scholar]
- 217.Tsou L.K., Huang Y.H., Song J.S., Ke Y.Y., Huang J.K., Shia K.S. Harnessing CXCR4 antagonists in stem cell mobilization, HIV infection, ischemic diseases, and oncology. Med. Res. Rev. 2018;38(4):1188–1234. doi: 10.1002/med.21464. [DOI] [PubMed] [Google Scholar]
- 218.Zhao R., Liu J., Li Z., Zhang W., Wang F., Zhang B. Recent advances in CXCL12/CXCR4 antagonists and nano-based drug delivery systems for cancer therapy. Pharmaceutics. 2022;14(8) doi: 10.3390/pharmaceutics14081541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xing L., Tang Y., Li L., Tao X. ROS in hepatocellular carcinoma: what we know. Arch. Biochem. Biophys. 2023;744 doi: 10.1016/j.abb.2023.109699. [DOI] [PubMed] [Google Scholar]
- 220.Zhang B., Bao W., Zhang S., Chen B., Zhou X., Zhao J., Shi Z., Zhang T., Chen Z., Wang L., Zheng X., Chen G., Wang Y. LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis. 2022;13(8):734. doi: 10.1038/s41419-022-05173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang Y., Luo M., Cui X., O'Connell D., Yang Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022;29(9):1850–1863. doi: 10.1038/s41418-022-00970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim K.M., Cho S.S., Ki S.H. Emerging roles of ferroptosis in liver pathophysiology. Arch Pharm. Res. (Seoul) 2020;43(10):985–996. doi: 10.1007/s12272-020-01273-8. [DOI] [PubMed] [Google Scholar]
- 223.Lee H.Y., Nga H.T., Tian J., Yi H.S. Mitochondrial metabolic signatures in hepatocellular carcinoma. Cells. 2021;10(8) doi: 10.3390/cells10081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mao W., Xiong G., Wu Y., Wang C., St Clair D., Li J.D., Xu R. RORalpha suppresses cancer-associated inflammation by repressing respiratory complex I-dependent ROS generation. Int. J. Mol. Sci. 2021;22(19) doi: 10.3390/ijms221910665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zheng H., Yu S., Zhu C., Guo T., Liu F., Xu Y. HIF1alpha promotes tumor chemoresistance via recruiting GDF15-producing TAMs in colorectal cancer. Exp. Cell Res. 2021;398(2) doi: 10.1016/j.yexcr.2020.112394. [DOI] [PubMed] [Google Scholar]
- 226.Calvisi D.F., Ladu S., Gorden A., Farina M., Conner E.A., Lee J.S., Factor V.M., Thorgeirsson S.S. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130(4):1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 227.Huang B., Lang X., Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1023177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Q., Cheng F., Ma T.T., Xiong H.Y., Li Z.W., Xie C.L., Liu C.Y., Tu Z.G. Interleukin-12 inhibits the hepatocellular carcinoma growth by inducing macrophage polarization to the M1-like phenotype through downregulation of Stat-3. Mol. Cell. Biochem. 2016;415(1–2):157–168. doi: 10.1007/s11010-016-2687-0. [DOI] [PubMed] [Google Scholar]
- 229.Liang Y.B., Tang H., Chen Z.B., Zeng L.J., Wu J.G., Yang W., Li Z.Y., Ma Z.F. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type. Mol. Med. Rep. 2017;16(5):6405–6411. doi: 10.3892/mmr.2017.7384. [DOI] [PubMed] [Google Scholar]
- 230.Hao X., Luan J., Jiao C., Ma C., Feng Z., Zhu L., Zhang Y., Fu J., Lai E., Zhang B., Wang Y., Kopp J.B., Pi J., Zhou H. LNA-anti-miR-150 alleviates renal interstitial fibrosis by reducing pro-inflammatory M1/M2 macrophage polarization. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.913007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lu Y., Chen Y., Li Y., Xu S., Lian D., Liang J., Jiang D., Chen S., Hou S. Monotropein inhibits colitis associated cancer through VDR/JAK1/STAT1 regulation of macrophage polarization. Int. Immunopharm. 2023;124(Pt A) doi: 10.1016/j.intimp.2023.110838. [DOI] [PubMed] [Google Scholar]
- 232.Bi J., Liu J., Chen X., Shi N., Wu H., Tang H., Mao J. MiR-155-5p-SOCS1/JAK1/STAT1 participates in hepatic lymphangiogenesis in liver fibrosis and cirrhosis by regulating M1 macrophage polarization. Hum. Exp. Toxicol. 2023;42 doi: 10.1177/09603271221141695. [DOI] [PubMed] [Google Scholar]
- 233.Ma C., Wang C., Zhang Y., Li Y., Fu K., Gong L., Zhou H., Li Y. Phillygenin inhibited M1 macrophage polarization and reduced hepatic stellate cell activation by inhibiting macrophage exosomal miR-125b-5p. Biomed. Pharmacother. 2023;159 doi: 10.1016/j.biopha.2023.114264. [DOI] [PubMed] [Google Scholar]
- 234.Xu L., Zhang Y., Dai Q., Lin N., Guan T., Song X., Hong S. Scorpion venom polypeptide governs alveolar macrophage M1/M2 polarization to alleviate pulmonary fibrosis. Tissue Cell. 2022;79 doi: 10.1016/j.tice.2022.101939. [DOI] [PubMed] [Google Scholar]
- 235.Wang F., Zhang S., Vuckovic I., Jeon R., Lerman A., Folmes C.D., Dzeja P.P., Herrmann J. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metabol. 2018;28(3):463–475 e4. doi: 10.1016/j.cmet.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang X., Chen S., Lu R., Sun Y., Song T., Nie Z., Yu C., Gao Y. Adipose-derived stem cell-secreted exosomes enhance angiogenesis by promoting macrophage M2 polarization in type 2 diabetic mice with limb ischemia via the JAK/STAT6 pathway. Heliyon. 2022;8(11) doi: 10.1016/j.heliyon.2022.e11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Han C., Yang Y., Sheng Y., Wang J., Li W., Zhou X., Guo L. The mechanism of lncRNA-CRNDE in regulating tumour-associated macrophage M2 polarization and promoting tumour angiogenesis. J. Cell Mol. Med. 2021;25(9):4235–4247. doi: 10.1111/jcmm.16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen F., Yang L., Peng X., Mao M., Liu X., Song J., Hu J. Histone deacetylase 2 regulates STAT1-dependent upregulation of atypical chemokine receptor 3 to induce M2 macrophage migration and immune escape in hepatocellular carcinoma. Mol. Immunol. 2022;151:204–217. doi: 10.1016/j.molimm.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 239.Feng S., Zhang C., Chen S., He R., Chao G., Zhang S. TLR5 signaling in the regulation of intestinal mucosal immunity. J. Inflamm. Res. 2023;16:2491–2501. doi: 10.2147/JIR.S407521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yan J., Shen S., He Y., Li Z. TLR5 silencing reduced hyperammonaemia-induced liver injury by inhibiting oxidative stress and inflammation responses via inactivating NF-kappaB and MAPK signals. Chem. Biol. Interact. 2019;299:102–110. doi: 10.1016/j.cbi.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 241.Shu M., Huang D.D., Hung Z.A., Hu X.R., Zhang S. Inhibition of MAPK and NF-kappaB signaling pathways alleviate carbon tetrachloride (CCl4)-induced liver fibrosis in Toll-like receptor 5 (TLR5) deficiency mice. Biochem. Biophys. Res. Commun. 2016;471(1):233–239. doi: 10.1016/j.bbrc.2016.01.119. [DOI] [PubMed] [Google Scholar]
- 242.Nischalke H.D., Fischer J., Kluners A., Matz-Soja M., Kramer B., Langhans B., Goeser F., Soyka M., Stickel F., Spengler U., Nattermann J., Strassburg C.P., Berg T., Lutz P. A genetic variant in toll-like receptor 5 is linked to chemokine levels and hepatocellular carcinoma in steatohepatitis. Liver Int. 2021;41(9):2139–2148. doi: 10.1111/liv.14980. [DOI] [PubMed] [Google Scholar]
- 243.Cao L., Zhang T., Zhu J., Li A., Zheng K., Zhang N., Su B., Xia W., Wu H., Li N., He Q. Polymorphism of TLR5 rs5744174 is associated with disease progression in Chinese patients with chronic HBV infection. APMIS. 2017;125(8):708–716. doi: 10.1111/apm.12707. [DOI] [PubMed] [Google Scholar]
- 244.Golonka R.M., Yeoh B.S., Saha P., Gohara A., Tummala R., Stepkowski S., Tiwari A.K., Joe B., Gonzalez F.J., Gewirtz A.T., Vijay-Kumar M. Loss of toll-like receptor 5 potentiates spontaneous hepatocarcinogenesis in farnesoid X receptor-deficient mice. Hepatol. Commun. 2023;7(6) doi: 10.1097/HC9.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Singh V., Yeoh B.S., Abokor A.A., Golonka R.M., Tian Y., Patterson A.D., Joe B., Heikenwalder M., Vijay-Kumar M. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microb. 2020;11(4):1077–1091. doi: 10.1080/19490976.2020.1743492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jiang H., Zhou L., Shen N., Ning X., Wu D., Jiang K., Huang X. M1 macrophage-derived exosomes and their key molecule lncRNA HOTTIP suppress head and neck squamous cell carcinoma progression by upregulating the TLR5/NF-kappaB pathway. Cell Death Dis. 2022;13(2):183. doi: 10.1038/s41419-022-04640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Howell L.M., Forbes N.S. Bacteria-based immune therapies for cancer treatment. Semin. Cancer Biol. 2022;86(Pt 2):1163–1178. doi: 10.1016/j.semcancer.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 248.Ye Z., Liang L., Lu H., Shen Y., Zhou W., Li Y. Nanotechnology-employed bacteria-based delivery strategy for enhanced anticancer therapy. Int. J. Nanomed. 2021;16:8069–8086. doi: 10.2147/IJN.S329855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Loffler M.W., Gori S., Izzo F., Mayer-Mokler A., Ascierto P.A., Konigsrainer A., Ma Y.T., Sangro B., Francque S., Vonghia L., Inno A., Avallone A., Ludwig J., Alcoba D.D., Flohr C., Aslan K., Mendrzyk R., Schuster H., Borrelli M., Valmori D., Chaumette T., Heidenreich R., Gouttefangeas C., Forlani G., Tagliamonte M., Fusco C., Penta R., Inarrairaegui M., Gnad-Vogt U., Reinhardt C., Weinschenk T., Accolla R.S., Singh-Jasuja H., Rammensee H.G., Buonaguro L. Phase I/II multicenter trial of a novel therapeutic cancer vaccine, HepaVac-101, for hepatocellular carcinoma. Clin. Cancer Res. 2022;28(12):2555–2566. doi: 10.1158/1078-0432.CCR-21-4424. [DOI] [PubMed] [Google Scholar]
- 250.Zhang L., Huang J., Chen X., Pan C., He Y., Su R., Guo D., Yin S., Wang S., Zhou L., Chen J., Zheng S., Qiao Y. Self-assembly nanovaccine containing TLR7/8 agonist and STAT3 inhibitor enhances tumor immunotherapy by augmenting tumor-specific immune response. J. Immunother. Cancer. 2021;9(8) doi: 10.1136/jitc-2021-003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Han Z., Liu S., Lin H., Trivett A.L., Hannifin S., Yang D., Oppenheim J.J. Inhibition of murine hepatoma tumor growth by cryptotanshinone involves TLR7-dependent activation of macrophages and induction of adaptive antitumor immune defenses. Cancer Immunol. Immunother. 2019;68(7):1073–1085. doi: 10.1007/s00262-019-02338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chen W., Jiang M., Yu W., Xu Z., Liu X., Jia Q., Guan X., Zhang W. CpG-based nanovaccines for cancer immunotherapy. Int. J. Nanomed. 2021;16:5281–5299. doi: 10.2147/IJN.S317626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jiang Y., Han Q., Zhao H., Zhang J. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/beta-catenin/c-Myc signaling and reprogramming glycolysis. J. Exp. Clin. Cancer Res. 2021;40(1):13. doi: 10.1186/s13046-020-01808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang Z., Kuo J.C., Yao S., Zhang C., Khan H., Lee R.J. CpG oligodeoxynucleotides for anticancer monotherapy from preclinical stages to clinical trials. Pharmaceutics. 2021;14(1) doi: 10.3390/pharmaceutics14010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hanagata N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int. J. Nanomed. 2017;12:515–531. doi: 10.2147/IJN.S114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Baguley B.C. Antivascular therapy of cancer: DMXAA. Lancet Oncol. 2003;4(3):141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 257.Cui X., Zhang R., Cen S., Zhou J. STING modulators: predictive significance in drug discovery. Eur. J. Med. Chem. 2019;182 doi: 10.1016/j.ejmech.2019.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sun J., Zhou Y.Q., Xu B.Y., Li J.Y., Zhang L.Q., Li D.Y., Zhang S., Wu J.Y., Gao S.J., Ye D.W., Mei W. STING/NF-kappaB/IL-6-Mediated inflammation in microglia contributes to spared nerve injury (SNI)-Induced pain initiation. J. Neuroimmune Pharmacol. 2022;17(3–4):453–469. doi: 10.1007/s11481-021-10031-6. [DOI] [PubMed] [Google Scholar]
- 259.Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 2021;6(7):1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang Y., Liang X., Bao X., Xiao W., Chen G. Toll-like receptor 4 (TLR4) inhibitors: current research and prospective. Eur. J. Med. Chem. 2022;235 doi: 10.1016/j.ejmech.2022.114291. [DOI] [PubMed] [Google Scholar]
- 261.Cejuela M., Martin-Castillo B., Menendez J.A., Pernas S. Metformin and breast cancer: where are we now? Int. J. Mol. Sci. 2022;23(5) doi: 10.3390/ijms23052705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 262.Morales D.R., Morris A.D. Metformin in cancer treatment and prevention. Annu. Rev. Med. 2015;66:17–29. doi: 10.1146/annurev-med-062613-093128. [DOI] [PubMed] [Google Scholar]
- 263.Podhorecka M., Ibanez B., Dmoszynska A. Metformin - its potential anti-cancer and anti-aging effects. Postepy Hig. Med. Dosw. 2017;71(0):170–175. doi: 10.5604/01.3001.0010.3801. [DOI] [PubMed] [Google Scholar]
- 264.Chiang C.F., Chao T.T., Su Y.F., Hsu C.C., Chien C.Y., Chiu K.C., Shiah S.G., Lee C.H., Liu S.Y., Shieh Y.S. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-kappaB signaling. Oncotarget. 2017;8(13):20706–20718. doi: 10.18632/oncotarget.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cetin M., Sahin S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Deliv. 2016;23(8):2796–2805. doi: 10.3109/10717544.2015.1089957. [DOI] [PubMed] [Google Scholar]
- 266.Plaz Torres M.C., Jaffe A., Perry R., Marabotto E., Strazzabosco M., Giannini E.G. Diabetes medications and risk of HCC. Hepatology. 2022;76(6):1880–1897. doi: 10.1002/hep.32439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cauchy F., Mebarki M., Leporq B., Laouirem S., Albuquerque M., Lambert S., Bourgoin P., Soubrane O., Van Beers B.E., Faivre S., Bedossa P., Paradis V. Strong antineoplastic effects of metformin in preclinical models of liver carcinogenesis. Clin. Sci. (Lond.) 2017;131(1):27–36. doi: 10.1042/CS20160438. [DOI] [PubMed] [Google Scholar]
- 268.Cheng J., Huang T., Li Y., Guo Y., Zhu Y., Wang Q., Tan X., Chen W., Zhang Y., Cheng W., Yamamoto T., Jing X., Huang J. AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lai H.Y., Tsai H.H., Yen C.J., Hung L.Y., Yang C.C., Ho C.H., Liang H.Y., Chen F.W., Li C.F., Wang J.M. Metformin resensitizes sorafenib-resistant HCC cells through AMPK-dependent autophagy activation. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.596655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cha J.H., Yang W.H., Xia W., Wei Y., Chan L.C., Lim S.O., Li C.W., Kim T., Chang S.S., Lee H.H., Hsu J.L., Wang H.L., Kuo C.W., Chang W.C., Hadad S., Purdie C.A., McCoy A.M., Cai S., Tu Y., Litton J.K., Mittendorf E.A., Moulder S.L., Symmans W.F., Thompson A.M., Piwnica-Worms H., Chen C.H., Khoo K.H., Hung M.C. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. Cell. 2018;71(4):606–620 e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.de Oliveira S., Houseright R.A., Graves A.L., Golenberg N., Korte B.G., Miskolci V., Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J. Hepatol. 2019;70(4):710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang Y., Wang H., Xiao H. Metformin actions on the liver: protection mechanisms emerging in hepatocytes and immune cells against NASH-related HCC. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22095016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen P.H., Zhu G.Z., Li L., Chen Y., Dai H.Q., Su W.J., Wu Y., Zhou J. Distinct effects of anti-diabetic medications on liver cancer risk and patient survival. J BUON. 2020;25(5):2147–2153. [PubMed] [Google Scholar]
- 274.Kondomerkos D.J., Kalamidas S.A., Kotoulas O.B. In vitro effects of hormones and autacoids on the hydrogen peroxide production and the morphology of endotoxin-activated rat peritoneal macrophages. Histol. Histopathol. 2003;18(1):55–65. doi: 10.14670/HH-18.55. [DOI] [PubMed] [Google Scholar]
- 275.Kinose Y., Sawada K., Makino H., Ogura T., Mizuno T., Suzuki N., Fujikawa T., Morii E., Nakamura K., Sawada I., Toda A., Hashimoto K., Isobe A., Mabuchi S., Ohta T., Itai A., Morishige K., Kurachi H., Kimura T. IKKbeta regulates VEGF expression and is a potential therapeutic target for ovarian cancer as an antiangiogenic treatment. Mol. Cancer Therapeut. 2015;14(4):909–919. doi: 10.1158/1535-7163.MCT-14-0696. [DOI] [PubMed] [Google Scholar]
- 276.Gomez-Cabrero A., Wrasidlo W., Reisfeld R.A. IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0073607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kim S., Ko D., Lee Y., Jang S., Lee Y., Lee I.Y., Kim S. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-46447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Liu T., Wang M., Wang T., Yao Y., Zhang N. Co-delivery of doxorubicin and siRNA by a simplified platform with oligodeoxynucleotides as a drug carrier. Colloids Surf. B Biointerfaces. 2015;126:531–540. doi: 10.1016/j.colsurfb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 279.Lee J., Lim K.T. SJSZ glycoprotein (38 kDa) modulates macrophage type 1/2-related factors at hepatocarcinogenic stage in N-nitrosodiethylamine-treated Balb/c. Mol. Cell. Biochem. 2013;372(1–2):17–26. doi: 10.1007/s11010-012-1441-5. [DOI] [PubMed] [Google Scholar]
- 280.Laurent S., Forge D., Port M., Roch A., Robic C., Vander Elst L., Muller R.N. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108(6):2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 281.Dadfar S.M., Roemhild K., Drude N.I., von Stillfried S., Knuchel R., Kiessling F., Lammers T. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019;138:302–325. doi: 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Geppert M., Himly M. Iron oxide nanoparticles in bioimaging - an immune perspective. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.688927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chung S., Revia R.A., Zhang M. Iron oxide nanoparticles for immune cell labeling and cancer immunotherapy. Nanoscale Horiz. 2021;6(9):696–717. doi: 10.1039/d1nh00179e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mulens-Arias V., Rojas J.M., Barber D.F. The use of iron oxide nanoparticles to reprogram macrophage responses and the immunological tumor microenvironment. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.693709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A., Pajarinen J.S., Nejadnik H., Goodman S., Moseley M., Coussens L.M., Daldrup-Link H.E. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11(11):986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-Based nanomedicine. Chem. Rev. 2019;119(8):4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 287.Patel A., Sant S. Hypoxic tumor microenvironment: opportunities to develop targeted therapies. Biotechnol. Adv. 2016;34(5):803–812. doi: 10.1016/j.biotechadv.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sharma A., Arambula J.F., Koo S., Kumar R., Singh H., Sessler J.L., Kim J.S. Hypoxia-targeted drug delivery. Chem. Soc. Rev. 2019;48(3):771–813. doi: 10.1039/c8cs00304a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 290.Greco O., Marples B., Joiner M.C., Scott S.D. How to overcome (and exploit) tumor hypoxia for targeted gene therapy. J. Cell. Physiol. 2003;197(3):312–325. doi: 10.1002/jcp.10374. [DOI] [PubMed] [Google Scholar]
- 291.Bischoff P., Altmeyer A., Dumont F. Radiosensitising agents for the radiotherapy of cancer: advances in traditional and hypoxia targeted radiosensitisers. Expert Opin. Ther. Pat. 2009;19(5):643–662. doi: 10.1517/13543770902824172. [DOI] [PubMed] [Google Scholar]
- 292.Chu Q., Gu X., Zheng Q., Zhu H. Regulatory mechanism of HIF-1alpha and its role in liver diseases: a narrative review. Ann. Transl. Med. 2022;10(2):109. doi: 10.21037/atm-21-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jahanban-Esfahlan R., de la Guardia M., Ahmadi D., Yousefi B. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J. Cell. Physiol. 2018;233(3):2019–2031. doi: 10.1002/jcp.25859. [DOI] [PubMed] [Google Scholar]
- 294.Zu Y., Wang Z., Yao H., Yan L. Oxygen-generating biocatalytic nanomaterials for tumor hypoxia relief in cancer radiotherapy. J. Mater. Chem. B. 2023;11(14):3071–3088. doi: 10.1039/d2tb02751h. [DOI] [PubMed] [Google Scholar]
- 295.Wei C.Y., Zhu M.X., Zhang P.F., Huang X.Y., Wan J.K., Yao X.Z., Hu Z.T., Chai X.Q., Peng R., Yang X., Gao C., Gao J., Wang S.W., Zheng Y.M., Tang Z., Gao Q., Zhou J., Fan J.B., Ke A.W., Fan J. PKCalpha/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022;77(1):163–176. doi: 10.1016/j.jhep.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 296.Wang S., Liu R., Yu Q., Dong L., Bi Y., Liu G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019;452:14–22. doi: 10.1016/j.canlet.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 297.Cairo G., Recalcati S., Mantovani A., Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32(6):241–247. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 298.Recalcati S., Gammella E., Cairo G. Ironing out macrophage immunometabolism. Pharmaceuticals. 2019;12(2) doi: 10.3390/ph12020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhou Y., Que K.T., Zhang Z., Yi Z.J., Zhao P.X., You Y., Gong J.P., Liu Z.J. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7(8):4012–4022. doi: 10.1002/cam4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jung M., Weigert A., Mertens C., Rehwald C., Brune B. Iron handling in tumor-associated macrophages-is there a new role for lipocalin-2? Front. Immunol. 2017;8:1171. doi: 10.3389/fimmu.2017.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mleczko-Sanecka K., da Silva A.R., Call D., Neves J., Schmeer N., Damm G., Seehofer D., Muckenthaler M.U. Imatinib and spironolactone suppress hepcidin expression. Haematologica. 2017;102(7):1173–1184. doi: 10.3324/haematol.2016.162917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Singh B., Arora S., Agrawal P., Gupta S.K. Hepcidin: a novel peptide hormone regulating iron metabolism. Clin. Chim. Acta. 2011;412(11–12):823–830. doi: 10.1016/j.cca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 303.Zheng J., Wang J.J., Ma H.M., Shen M.Q., Qian Z.M., Bao Y.X. Norcantharidin down-regulates iron contents in the liver and spleen of lipopolysaccharide-treated mice. Redox Rep. 2022;27(1):119–127. doi: 10.1080/13510002.2022.2088011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Du Y., Dong S., Jiang W., Li M., Li W., Li X., Zhou W. Integration of single-cell RNA sequencing and bulk RNA sequencing reveals that TAM2-driven genes affect immunotherapeutic response and prognosis in pancreatic cancer. Int. J. Mol. Sci. 2023;24(16) doi: 10.3390/ijms241612787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hu H., Hang J.J., Han T., Zhuo M., Jiao F., Wang L.W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016;37(7):8657–8664. doi: 10.1007/s13277-015-4741-z. [DOI] [PubMed] [Google Scholar]
- 306.Yoshikawa K., Mitsunaga S., Kinoshita T., Konishi M., Takahashi S., Gotohda N., Kato Y., Aizawa M., Ochiai A. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 2012;103(11):2012–2020. doi: 10.1111/j.1349-7006.2012.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Xu X., Cui L., Zhang L., Yang L., Zhuo Y., Li C. Saikosaponin d modulates the polarization of tumor-associated macrophages by deactivating the PI3K/AKT/mTOR pathway in murine models of pancreatic cancer. Int. Immunopharm. 2023;122 doi: 10.1016/j.intimp.2023.110579. [DOI] [PubMed] [Google Scholar]
- 308.Hu W.M., Liu S.Q., Zhu K.F., Li W., Yang Z.J., Yang Q., Zhu Z.C., Chang J. The ALOX5 inhibitor Zileuton regulates tumor-associated macrophage M2 polarization by JAK/STAT and inhibits pancreatic cancer invasion and metastasis. Int. Immunopharm. 2023;121 doi: 10.1016/j.intimp.2023.110505. [DOI] [PubMed] [Google Scholar]
- 309.Dai E., Han L., Liu J., Xie Y., Kroemer G., Klionsky D.J., Zeh H.J., Kang R., Wang J., Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yang F., Jin C., Subedi S., Lee C.L., Wang Q., Jiang Y., Li J., Di Y., Fu D. Emerging inorganic nanomaterials for pancreatic cancer diagnosis and treatment. Cancer Treat Rev. 2012;38(6):566–579. doi: 10.1016/j.ctrv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 311.Jia M., Zhang D., Zhang C., Li C. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J. Nanobiotechnol. 2021;19(1):384. doi: 10.1186/s12951-021-01134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cullis J., Siolas D., Avanzi A., Barui S., Maitra A., Bar-Sagi D. Macropinocytosis of nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol. Res. 2017;5(3):182–190. doi: 10.1158/2326-6066.CIR-16-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Das M., Shen L., Liu Q., Goodwin T.J., Huang L. Nanoparticle delivery of RIG-I agonist enables effective and safe adjuvant therapy in pancreatic cancer. Mol. Ther. 2019;27(3):507–517. doi: 10.1016/j.ymthe.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li M., Li M., Yang Y., Liu Y., Xie H., Yu Q., Tian L., Tang X., Ren K., Li J., Zhang Z., He Q. Remodeling tumor immune microenvironment via targeted blockade of PI3K-gamma and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J. Contr. Release. 2020;321:23–35. doi: 10.1016/j.jconrel.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 315.Li T., Chen D., Liu H., Tao Y., He X., Zang S., Li J., Zhang L., Li M., Liu J., He Q. Spatially targeting and regulating tumor-associated macrophages using a raspberry-like micellar system sensitizes pancreatic cancer chemoimmunotherapy. Nanoscale. 2022;14(36):13098–13112. doi: 10.1039/d2nr03053e. [DOI] [PubMed] [Google Scholar]
- 316.Su M.J., Aldawsari H., Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci. Rep. 2016;6 doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Parayath N.N., Hong B.V., Mackenzie G.G., Amiji M.M. Hyaluronic acid nanoparticle-encapsulated microRNA-125b repolarizes tumor-associated macrophages in pancreatic cancer. Nanomedicine. 2021;16(25):2291–2303. doi: 10.2217/nnm-2021-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cao S., Saw P.E., Shen Q., Li R., Liu Y., Xu X. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121264. [DOI] [PubMed] [Google Scholar]
- 319.Wu J., Wang X., Chen L., Wang J., Zhang J., Tang J., Ji Y., Song J., Wang L., Zhao Y., Zhang H., Li T., Sheng J., Chen D., Zhang Q., Liang T. Oxygen microcapsules improve immune checkpoint blockade by ameliorating hypoxia condition in pancreatic ductal adenocarcinoma. Bioact. Mater. 2023;20:259–270. doi: 10.1016/j.bioactmat.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhou W., Hong J., Liu T., Li M., Jin H., Wang X. Polygonatum polysaccharide regulates macrophage polarization and improves LPS-induced acute lung injury through TLR4-MAPK/NF-kappaB pathway. Cancer Res. J. 2022;2022 doi: 10.1155/2022/2686992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lee H., Han J.H., An K., Kang Y.J., Hwangbo H., Heo J.H., Choi B.H., Kim J.J., Kim S.R., Lee S.Y., Hur J. Recombinant human KAI1/CD82 attenuates M1 macrophage polarization on LPS-stimulated RAW264.7 cells via blocking TLR4/JNK/NF-kappaB signal pathway. BMB Rep. 2023;56(6):359–364. doi: 10.5483/BMBRep.2022-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Abdollahi E., Keyhanfar F., Delbandi A.A., Falak R., Hajimiresmaiel S.J., Shafiei M. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-kappaB activation in human endothelial cells and differentiated macrophages. Eur. J. Pharmacol. 2022;918 doi: 10.1016/j.ejphar.2021.174715. [DOI] [PubMed] [Google Scholar]
- 323.Mizrahi J.D., Surana R., Valle J.W., Shroff R.T. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 324.Nakahara T., Tanaka K., Ohno S., Egawa N., Yugawa T., Kiyono T. Activation of NF-kappaB by human papillomavirus 16 E1 limits E1-dependent viral replication through degradation of E1. J. Virol. 2015;89(9):5040–5059. doi: 10.1128/JVI.00389-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198(3):1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 326.Lu X.Y., Ciraolo E., Stefenia R., Chen G.Q., Zhang Y., Hirsch E. Sustained release of PI3K inhibitor from PHA nanoparticles and in vitro growth inhibition of cancer cell lines. Appl. Microbiol. Biotechnol. 2011;89(5):1423–1433. doi: 10.1007/s00253-011-3101-1. [DOI] [PubMed] [Google Scholar]
- 327.Khan M.A., Jain V.K., Rizwanullah M., Ahmad J., Jain K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov. Today. 2019;24(11):2181–2191. doi: 10.1016/j.drudis.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 328.Saliminejad K., Khorram Khorshid H.R., Soleymani Fard S., Ghaffari S.H. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019;234(5):5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 329.Mohapatra S., Pioppini C., Ozpolat B., Calin G.A. Non-coding RNAs regulation of macrophage polarization in cancer. Mol. Cancer. 2021;20(1):24. doi: 10.1186/s12943-021-01313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Essandoh K., Li Y., Huo J., Fan G.C. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122–131. doi: 10.1097/SHK.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pecot C.V., Calin G.A., Coleman R.L., Lopez-Berestein G., Sood A.K. RNA interference in the clinic: challenges and future directions. Nat. Rev. Cancer. 2011;11(1):59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lee S.W.L., Paoletti C., Campisi M., Osaki T., Adriani G., Kamm R.D., Mattu C., Chiono V. MicroRNA delivery through nanoparticles. J. Contr. Release. 2019;313:80–95. doi: 10.1016/j.jconrel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Aldawsari H.M., Dhaliwal H.K., Aljaeid B.M., Alhakamy N.A., Banjar Z.M., Amiji M.M. Optimization of the conditions for plasmid DNA delivery and transfection with self-assembled hyaluronic acid-based nanoparticles. Mol. Pharm. 2019;16(1):128–140. doi: 10.1021/acs.molpharmaceut.8b00904. [DOI] [PubMed] [Google Scholar]
- 334.Deci M.B., Liu M., Gonya J., Lee C.J., Li T., Ferguson S.W., Bonacquisti E.E., Wang J., Nguyen J. Carrier-free CXCR4-targeted nanoplexes designed for polarizing macrophages to suppress tumor growth. Cell. Mol. Bioeng. 2019;12(5):375–388. doi: 10.1007/s12195-019-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Malfitano A.M., Basu S., Maresz K., Bifulco M., Dittel B.N. What we know and do not know about the cannabinoid receptor 2 (CB2) Semin. Immunol. 2014;26(5):369–379. doi: 10.1016/j.smim.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kumawat V.S., Kaur G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019;862 doi: 10.1016/j.ejphar.2019.172628. [DOI] [PubMed] [Google Scholar]
- 337.Rzeczycki P., Rasner C., Lammlin L., Junginger L., Goldman S., Bergman R., Redding S., Knights A.J., Elliott M., Maerz T. Cannabinoid receptor type 2 is upregulated in synovium following joint injury and mediates anti-inflammatory effects in synovial fibroblasts and macrophages. Osteoarthritis Cartilage. 2021;29(12):1720–1731. doi: 10.1016/j.joca.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Luo X.Q., Li A., Yang X., Xiao X., Hu R., Wang T.W., Dou X.Y., Yang D.J., Dong Z. Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin. Med. 2018;13:14. doi: 10.1186/s13020-018-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Braun M., Khan Z.T., Khan M.B., Kumar M., Ward A., Achyut B.R., Arbab A.S., Hess D.C., Hoda M.N., Baban B., Dhandapani K.M., Vaibhav K. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018;68:224–237. doi: 10.1016/j.bbi.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140(1):49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Liang T., Zhang R., Liu X., Ding Q., Wu S., Li C., Lin Y., Ye Y., Zhong Z., Zhou M. Recent advances in macrophage-mediated drug delivery systems. Int. J. Nanomed. 2021;16:2703–2714. doi: 10.2147/IJN.S298159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Liu Y., Bai L., Guo K., Jia Y., Zhang K., Liu Q., Wang P., Wang X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics. 2019;9(18):5261–5281. doi: 10.7150/thno.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lee E.S., Cha B.S., Kim S., Park K.S. Synthesis of exosome-based fluorescent gold nanoclusters for cellular imaging applications. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang J.J., Wang Z.Y., Chen R., Xiong J., Yao Y.L., Wu J.H., Li G.X. Macrophage-secreted exosomes delivering miRNA-21 inhibitor can regulate BGC-823 cell proliferation. Asian Pac. J. Cancer Prev. APJCP. 2015;16(10):4203–4209. doi: 10.7314/apjcp.2015.16.10.4203. [DOI] [PubMed] [Google Scholar]
- 345.Yu D., Chang Z., Liu X., Chen P., Zhang H., Qin Y. Macrophage-derived exosomes regulate gastric cancer cell oxaliplatin resistance by wrapping circ 0008253. Cell Cycle. 2023;22(6):705–717. doi: 10.1080/15384101.2022.2146839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hsu H.H., Chen M.C., Baskaran R., Lin Y.M., Day C.H., Lin Y.J., Tu C.C., Vijaya Padma V., Kuo W.W., Huang C.Y. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell. Physiol. 2018;233(7):5458–5467. doi: 10.1002/jcp.26406. [DOI] [PubMed] [Google Scholar]
- 347.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Cao X., Tan T., Zhu D., Yu H., Liu Y., Zhou H., Jin Y., Xia Q. Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int. J. Nanomed. 2020;15:1915–1928. doi: 10.2147/IJN.S244849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ji B., Cai H., Yang Y., Peng F., Song M., Sun K., Yan F., Liu Y. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020;111:363–372. doi: 10.1016/j.actbio.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 350.Ma X., Yao M., Gao Y., Yue Y., Li Y., Zhang T., Nie G., Zhao X., Liang X. Functional immune cell-derived exosomes engineered for the trilogy of radiotherapy sensitization. Adv. Sci. 2022;9(23) doi: 10.1002/advs.202106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Nai J., Zhang J., Li J., Li H., Yang Y., Yang M., Wang Y., Gong W., Li Z., Li L., Gao C. Macrophage membrane- and cRGD-functionalized thermosensitive liposomes combined with CPP to realize precise siRNA delivery into tumor cells. Mol. Ther. Nucleic Acids. 2022;27:349–362. doi: 10.1016/j.omtn.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Chen C., Song M., Du Y., Yu Y., Li C., Han Y., Yan F., Shi Z., Feng S. Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett. 2021;21(13):5522–5531. doi: 10.1021/acs.nanolett.1c00818. [DOI] [PubMed] [Google Scholar]
- 353.Fang Y., Zhang Z., Liu Y., Gao T., Liang S., Chu Q., Guan L., Mu W., Fu S., Yang H., Zhang N., Liu Y. Artificial assembled macrophage Co-deliver black phosphorus quantum dot and CDK4/6 inhibitor for colorectal cancer triple-therapy. ACS Appl. Mater. Interfaces. 2022;14(18):20628–20640. doi: 10.1021/acsami.2c01305. [DOI] [PubMed] [Google Scholar]
- 354.Cai H., Wang R., Guo X., Song M., Yan F., Ji B., Liu Y. Combining gemcitabine-loaded macrophage-like nanoparticles and erlotinib for pancreatic cancer therapy. Mol. Pharm. 2021;18(7):2495–2506. doi: 10.1021/acs.molpharmaceut.0c01225. [DOI] [PubMed] [Google Scholar]
- 355.Khatoon N., Zhang Z., Zhou C., Chu M. Macrophage membrane coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater. Sci. 2022;10(5):1193–1208. doi: 10.1039/d1bm01664d. [DOI] [PubMed] [Google Scholar]
- 356.Han J., Zhen J., Du Nguyen V., Go G., Choi Y., Ko S.Y., Park J.O., Park S. Hybrid-actuating macrophage-based microrobots for active cancer therapy. Sci. Rep. 2016;6 doi: 10.1038/srep28717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Basel M.T., Balivada S., Wang H., Shrestha T.B., Seo G.M., Pyle M., Abayaweera G., Dani R., Koper O.B., Tamura M., Chikan V., Bossmann S.H., Troyer D.L. Cell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer model. Int. J. Nanomed. 2012;7:297–306. doi: 10.2147/IJN.S28344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Wang Y., Yu J., Luo Z., Shi Q., Liu G., Wu F., Wang Z., Huang Y., Zhou D. Engineering endogenous tumor-associated macrophage-targeted biomimetic nano-RBC to reprogram tumor immunosuppressive microenvironment for enhanced chemo-immunotherapy. Adv. Mater. 2021;33(39) doi: 10.1002/adma.202103497. [DOI] [PubMed] [Google Scholar]
- 359.Sun J.H., Liang X., Cai M., Yan L., Chen Z., Guo L., Jing L., Wang Y., Zhou D. Protein-crowned micelles for targeted and synergistic tumor-associated macrophage reprogramming to enhance cancer treatment. Nano Lett. 2022;22(11):4410–4420. doi: 10.1021/acs.nanolett.2c00901. [DOI] [PubMed] [Google Scholar]
- 360.Matos A.I., Peres C., Carreira B., Moura L.I.F., Acurcio R.C., Vogel T., Wegener E., Ribeiro F., Afonso M.B., Santos F.M.F., Martinez-Barriocanal A., Arango D., Viana A.S., Gois P.M.P., Silva L.C., Rodrigues C.M.P., Graca L., Jordan R., Satchi-Fainaro R., Florindo H.F. Polyoxazoline-based nanovaccine synergizes with tumor-associated macrophage targeting and anti-PD-1 immunotherapy against solid tumors. Adv. Sci. 2023;10(25) doi: 10.1002/advs.202300299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Cieslewicz M., Tang J., Yu J.L., Cao H., Zavaljevski M., Motoyama K., Lieber A., Raines E.W., Pun S.H. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Natl. Acad. Sci. U. S. A. 2013;110(40):15919–15924. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rodell C.B., Arlauckas S.P., Cuccarese M.F., Garris C.S., Li R., Ahmed M.S., Kohler R.H., Pittet M.J., Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018;2(8):578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Liu L., Yi H., He H., Pan H., Cai L., Ma Y. Tumor associated macrophage-targeted microRNA delivery with dual-responsive polypeptide nanovectors for anti-cancer therapy. Biomaterials. 2017;134:166–179. doi: 10.1016/j.biomaterials.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 364.Deng C., Zhang Q., Jia M., Zhao J., Sun X., Gong T., Zhang Z. Tumors and their microenvironment dual-targeting chemotherapy with local immune adjuvant therapy for effective antitumor immunity against breast cancer. Adv. Sci. 2019;6(6) doi: 10.1002/advs.201801868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lee S., Kivimae S., Dolor A., Szoka F.C. Macrophage-based cell therapies: the long and winding road. J. Contr. Release. 2016;240:527–540. doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Na Y.R., Kim S.W., Seok S.H. A new era of macrophage-based cell therapy. Exp. Mol. Med. 2023;55(9):1945–1954. doi: 10.1038/s12276-023-01068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Anderson N.R., Minutolo N.G., Gill S., Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81(5):1201–1208. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 368.Nguyen V.D., Park J.O., Choi E. Macrophage-based microrobots for anticancer therapy: recent progress and future perspectives. Biomimetics. 2023;8(7) doi: 10.3390/biomimetics8070553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Dogan N.O., Ceylan H., Suadiye E., Sheehan D., Aydin A., Yasa I.C., Wild A.M., Richter G., Sitti M. Remotely guided immunobots engaged in anti-tumorigenic phenotypes for targeted cancer immunotherapy. Small. 2022;18(46) doi: 10.1002/smll.202204016. [DOI] [PubMed] [Google Scholar]
- 370.Khair D.O., Bax H.J., Mele S., Crescioli S., Pellizzari G., Khiabany A., Nakamura M., Harris R.J., French E., Hoffmann R.M., Williams I.P., Cheung A., Thair B., Beales C.T., Touizer E., Signell A.W., Tasnova N.L., Spicer J.F., Josephs D.H., Geh J.L., MacKenzie Ross A., Healy C., Papa S., Lacy K.E., Karagiannis S.N. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front. Immunol. 2019;10:453. doi: 10.3389/fimmu.2019.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Weissleder R., Nahrendorf M., Pittet M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014;13(2):125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 372.Zanganeh S., Spitler R., Hutter G., Ho J.Q., Pauliah M., Mahmoudi M. Tumor-associated macrophages, nanomedicine and imaging: the axis of success in the future of cancer immunotherapy. Immunotherapy. 2017;9(10):819–835. doi: 10.2217/imt-2017-0041. [DOI] [PubMed] [Google Scholar]
- 373.Daldrup-Link H., Coussens L.M. MR imaging of tumor-associated macrophages. OncoImmunology. 2012;1(4):507–509. doi: 10.4161/onci.19456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Keliher E.J., Yoo J., Nahrendorf M., Lewis J.S., Marinelli B., Newton A., Pittet M.J., Weissleder R. 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging. Bioconjugate Chem. 2011;22(12):2383–2389. doi: 10.1021/bc200405d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Zhao C., Pang X., Yang Z., Wang S., Deng H., Chen X. Nanomaterials targeting tumor associated macrophages for cancer immunotherapy. J. Contr. Release. 2022;341:272–284. doi: 10.1016/j.jconrel.2021.11.028. [DOI] [PubMed] [Google Scholar]