Abstract
Simple Summary
Necrotic enteritis (NE) is mainly caused by coccidia and Clostridium perfringens (CCP), which can induce intestine injury and oxidative stress in broilers. Tannic acids (TA) are natural polyphenolic compounds with anti-bacterial, anti-inflammatory, and anti-oxidation functions. It has been demonstrated that dietary supplementation with hydrolyzable TA has beneficial effects on the growth and antioxidant capacity of broilers. However, the effects of TA on intestinal health and antioxidative function in broilers with NE conditions still need to be clarified. Thus, this study aimed to evaluate the effects of TA on the antioxidant function, immunity, and intestinal barrier in broilers co-infected with CCP. The results showed that the addition of 1000 mg/kg TA to the diet could improve the jejunal barrier, attenuate the inflammatory response of the jejunum, and increase the antioxidant capacity of the liver and jejunum through the activation of the transcription factor Nrf2 downstream of the Nrf2-Keap1 pathway in CCP infected broilers.
Abstract
The purpose of this study was to determine the efficacy of tannic acid on the antioxidative function, immunity, and intestinal barrier of broilers co-infected with coccidia and Clostridium perfringens (CCP). A total of 294 1-day-old arbor acres(AA) broilers were divided into three groups: control group (CON), CCP co-infected group (CCP), and 1000 mg/kg TA + CCP co-infected group (CTA). This trial lasted for 28 days. The results showed that the CCP group decreased the activity of glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), catalase (CAT), and total antioxidant capacity (T-AOC) levels and increased the contents of hydrogen peroxide (H2O2) and malondialdehyde (MDA) in the jejunum (p < 0.05). The mRNA levels of GSH-Px3 and CAT in the liver and jejunum, and the mRNA levels of GSH-Px3, SOD, HO-1, and NAD(P)H quinone oxidoreductase I (NQO1) in the liver were down-regulated by CCP challenge (p < 0.05). In addition, the Keap1 and Nrf2 mRNA levels in the liver and jejunum, jejunal glutathione S-transferase (GST), and heme-oxygenase-1 (HO-1) were upregulated in the CCP group compared with CON (p < 0.05). The mRNA levels of interleukin 8 (IL-8), IL-1β, inducible nitric oxide synthase (iNOS), and interferon γ (IFN-γ) in the jejunum were elevated, and jejunal mRNA levels of IL-10, zonula occludens protein1 (ZO-1), claudin-1, claudin-2, and occludin were decreased in the CCP treatment (p < 0.05). Dietary supplementation with 1000 mg/kg TA increased the activity of GSH-Px, T-SOD, CAT, and T-AOC and decreased the contents of H2O2 and MDA in the jejunum (p < 0.05). Compared with the CCP group, TA decreased the mRNA level of Keap1 and Nrf2 in the liver and jejunum, increased the GSH-Px3, SOD, and CAT mRNA in the liver, and alleviated the rise of IL-8, IL-1β, iNOS, and IFN-γ and decrease in IL-10, occludin gene expression in the jejunum (p < 0.05). In conclusion, the addition of 1000 mg/kg TA to the diet improved the jejunal barrier, mitigated the jejunal inflammation, and increased the antioxidant capacity of the liver and jejunum through the activation of the transcription factor Nrf2 downstream of the Nrf2-Keap1 pathway in broilers with NE condition.
Keywords: antioxidant, broiler, intestinal barrier, necrotic enteritis, tannic acid
1. Introduction
Necrotic enteritis (NE) is a prevalent infectious gastrointestinal illness in broiler production, predominantly caused by Clostridium perfringen. C. perfringens is secondary to coccidia (coccidia is primary), or they may be co-infected, resulting in an inflammatory reaction of the intestinal mucosa and oxidative stress, which can cause a tremendous economic loss in the poultry industry [1,2,3,4]. Oxidative stress and inflammation disrupt mucosal cells and tight junction proteins, impeding the self-repair mechanisms of the intestinal barrier [5,6]. Antibiotics protect broilers from intestinal diseases and increase productivity. However, antibiotics in animal feed have been limited or outlawed in many countries due to bacteria and antibiotic resistance. Therefore, it is urgent to find natural and environmentally friendly feed additives to control the growing prevalence of NE in poultry effectively.
Tannins are natural polyphenolic substances in numerous plants with anti-bacterial, anti-viral, anti-inflammatory, and anti-oxidation functions [7,8]. Research has demonstrated that dietary supplementation with hydrolyzed tannins can reduce oxidative stress and improve antioxidant capacities in broilers [9,10]. Dietary TA can also alleviate tissue inflammation and protect intestinal health by lowering the mRNA levels of tissue inflammation elements and increasing the mRNA levels of intestinal intact proteins in broilers [11,12,13]. It has been shown that TA can alleviate the inflammation of broilers with NE conditions and enhance the antioxidative ability by activating the Nrf2-Keap1 signaling pathway in rats [14,15,16,17]. However, whether TA could improve antioxidant capacity and alleviate oxidative stress through the Nrf2-Keap1 signaling pathway in broilers with NE condition has yet to be reported.
Therefore, this study aimed to investigate the efficacy of TA on antioxidant functions, immune, and gut barrier of broilers co-infected with CCP to provide some data references and theoretical backup for protecting TA against poultry with NE condition.
2. Materials and Methods
2.1. Animal Care and Diet
The study was performed for 28 days in a one-way, fully randomized design. A total of 294 1-day-old AA broilers, with an initial BW of 46.40 ± 0.40 g, were randomized to 3 treatment groups with 7 replicates of 14 broilers in each pen (7 males and 7 females). Treatments were as follows: the control group without CCP challenge (CON), the co-infected group with CCP challenge (CCP), and the 1000 mg/kg TA (≥80% the hydrolysable TA from Chinese gallnut, Wufeng Chicheng Biotechnology Company Limited, Yichang, China) + co-infected group with CCP challenge (CTA). The diets were fed in Phase 1 (Day 1 to Day 14) and Phase 2 (Day 15 to Day 28). The basal diets are formulated concerning and in conjunction with the Arbor Acres Broiler Nutrition Specifications (2019); the compositions and nutrition contents of the basal diets are summarized in Table 1. On days 7 and 10 of the study, broilers in the CCP and CTA groups were orally injected with 1 mL of quadrivalent anti-coccidial vaccine (purchased from Foshan Zhengdian Biotechnology Co., Ltd., Foshan, Guangdong, China). The quadrivalent vaccine consisted of 1 × 105 oocysts of the E. acervuline strain PAHY, plus the 5 × 104 oocysts of E. necatrix strain PNHZ, E. tenella strain PTMZ, and E. maxima strain PMHY. The recommended inoculation dose for each bird was 1100 ± 110 sporulated oocysts, and the dose used in the present study was 30 times the recommended dose. Meanwhile, the CON group received an equivalent amount of saline. From day 16 to day 20, birds in the CCP and CTA groups per bird per day were orally given 1 mL of fresh C. perfringens (1 × 108 CFU/mL, gavage using a 1 mL pipette gun, the C. perfringens, CVCC2030, was obtained from the China Veterinary Microbial Culture Collection and Management Center, Beijing, China), while birds in the CON group were orally administered an equivalent amount of sterile broth. All broilers were kept in cages with free access to feed and water throughout the trial. The ambient temperature was maintained at 33 ± 2 °C for the first week and then progressively lowered to 22 °C until the experiment ended. The broilers were exposed to 23 h of light and 1 h of darkness per day throughout the experimental period.
Table 1.
Compositions and nutrient content of the basal diet (%).
| Items | 1 to 14 Days | 15 to 28 Days |
|---|---|---|
| Ingredients | ||
| Corn | 10.00 | 10.00 |
| Wheat | 55.42 | 58.90 |
| Soybean meal | 23.50 | 19.37 |
| Fish meal | 5.00 | 5.00 |
| Soybean oil | 2.50 | 3.80 |
| CaHPO4 | 1.05 | 0.75 |
| Limestone | 1.00 | 0.80 |
| NaCl | 0.35 | 0.35 |
| L-lysine•HCl | 0.35 | 0.25 |
| DL-methionine | 0.20 | 0.15 |
| D-threonine | 0.15 | 0.15 |
| Choline chloride | 0.25 | 0.25 |
| Premix a | 0.23 | 0.23 |
| Total | 100.00 | 100.00 |
| Nutrient levels b | ||
| ME/(MJ/kg) | 12.55 | 12.95 |
| Crude protein | 21.53 | 20.14 |
| Ca | 0.96 | 0.86 |
| Non-phytate phosphorus | 0.45 | 0.40 |
| Digestible lysine | 1.25 | 1.05 |
| Digestible methionine | 0.53 | 0.46 |
| Digestible threonine | 0.81 | 0.76 |
| Digestible tryptophan | 0.23 | 0.22 |
a The premix provided the following per kg of diets: Cu 10 mg, Zn 100 mg, Fe 80 mg, Mn 100 mg, Se 0.3 mg, I 0.7 mg, VA 12,000 IU, VD3 3000 IU, VK3 3.2 mg, VB1 3 mg, VB2 8.0 mg, VB12 0.025 mg, VE 44 IU, biotin 0.0325 mg, folic acid 2.00 mg, pantothenic acid 15 mg, and nicotinic acid 15 mg. b Non-phytate phosphorus, ME and digestible amino acids were calculated values, while Ca and CP were measured values.
2.2. Sample Collection
On days 14, 21, and 28, two broilers of average weight were chosen in each replicate, which was euthanized by cervical dislocation and then promptly slaughtered for sampling. Liver and jejunum(mid-jejunum) were collected and frozen in liquid nitrogen, then transferred to a freezer at −80 °C for storage.
2.3. Antioxidant Indexes
Liver and jejunal samples were placed in a mortar, to which liquid nitrogen was added and then crushed with a pestle. A total of 0.1 g of liver and jejunum samples were placed in 1.5 mL centrifuge tubes, respectively, and 0.9 mL of saline was injected into the sample centrifuge tubes using a pipette, followed by centrifugation in a centrifuge (Fresco 21, Thermo Scientific, Wilmington, DE, USA) for 15 min at 3500× g at 4 °C, and the supernatant was collected. Off-the-shelf test kits were purchased from Nanjing Jianjian Bioengineering Institute, Nanjing, China. The activity of GSH-Px, T-SOD, and CAT, the concentration of H2O2 and MDA, and T-AOC in the liver and jejunum, were tested following the manufacturer’s directions.
2.4. RNA Isolation and Quantitative Real-Time PCR
RNA isolation and quantitative real-time PCR of liver and jejunal samples were performed, as reported by Guo and Li [18,19]. The SYBR Premix Ex Taq kit (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China) used cDNA from each sample and primers. The qPCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA, USA). The relative expression of each gene was analyzed using the 2−ΔΔCt method (Relative quantification) [20]. β-actin was used as the endogenous reference gene for all the genes tested. Table 2 lists the sequences of the relevant assay genes and internal reference primers used in this study.
Table 2.
List of gene primer sequences a.
| Gene | Primer Sequence (5′ to 3′) | Product Length | NCBI Number |
|---|---|---|---|
| β-actin | F-ACTCTGGTGATGGTGTTAC | 497 | NM 205518.2 |
| R-GGCTGTGATCTCCTTCTG | |||
| Nrf-2 | F-ATCACCTCTTCTGCACCGAA | 229 | NM 205117.2 |
| R-GCTTTCTCCCGCTCTTTCTG | |||
| Keap1 | F-CAACTTCGCCGAGCAGA | 179 | KU 321503179 |
| R-CGTGGAACACCTCCGACT | |||
| GST | F-AGAGTCGAAGCCTGATGCAC | 220 | NM_001001777.2 |
| R-CACTCCGCTTATCAGCAAACA | |||
| HO-1 | F-ACGAGTTCAAGCTGGTCACG | 244 | NM_205344.2 |
| R-GGATGCTTCTTGCCAACGAC | |||
| NQO1 | F-TCGCCGAGCAGAAGAAGATTGAAG | 191 | NM_001277621.1 |
| R-GGTGGTGAGTGACAGCATGGC | |||
| GSH-px1 | F-GACCAACCCGCAGTACATCA | 204 | NM_001277853.3 |
| R-GAGGTGCGGGCTTTCCTTTA | |||
| GSH-px3 | F-AAGTGCCAGGTGAACGGGAAGG | 204 | NM 001163232.3 |
| R-AGGGCTGTAGCGGCGGAAAG | |||
| SOD | F-GGTGCTCACTTTAATCCTG | 109 | NM 205064.2 |
| R-CTACTTCTGCCACTCCTCC | |||
| CAT | F-GGTTCGGTGGGGTTGTCTTT | 213 | NM_001031215.2 |
| R-CACCAGTGGTCAAGGCATCT | |||
| iNOS | F-CCTGGAGGTCCTGGAAGAGT | 82 | NM_204961.2 |
| R-CCTGGGTTTCAGAAGTGGC | |||
| TNF-α | F-GAGCGTTGACTTGGCTGTC | 64 | NM_204267.2 |
| R-AAGCAACAACCAGCTATGCAC | |||
| IFN-γ | F-AGCTGACGGTGGACCTATTATT | 259 | Y07922.1 |
| R-GGCTTTGCGCTGGATTC | |||
| IL-1β | F-ACTGGGCATCAAGGGCTA | 131 | NM_204524.2 |
| R-GGTAGAAGATGAAGCGGGTC | |||
| IL-8 | F-GCAAGGTAGGACGCTGGTAA | 107 | NM_205498.2 |
| R-GCGTCAGCTTCACATCTTGA | |||
| TGF-β4 | F-CGGGACGGATGAGAAGAAC | 258 | M31160.1 |
| R-CGGCCCACGTAGTAAATGAT | |||
| ZO-1 | F-CTTCAGGTGTTTCTCTTCCTCCTC | 131 | XM_413773.4 |
| R-CTGTGGTTTCATGGCTGGATC | |||
| Claudin-1 | F-CATACTCCTGGGTCTGGTTGGT | 100 | AY750897.1 |
| R-GACAGCCATCCGCATCTTCT | |||
| Claudin-2 | F-CAACTGGAAGATCAGCTCCT | 119 | NM_001277622.1 |
| R-TGTAGATGTCGCACTGAGTG | |||
| Occludin | F-ACGGCAGCACCTACCTCAA | 123 | D21837.1 |
| R-GGGCGAAGAAGCAGATGAG | |||
| Mucin-2 | F-TTCATGATGCCTGCTCTTGTG | 93 | XM_421035.2 |
| R-CCTGAGCCTTGGTACATTCTTGT |
F Upstream primer, R Downstream primers. a The primers were synthesized by Shanghai Shenggong Biotechnology Co., Ltd. (Shanghai, China)
2.5. Statistical Analysis
Data from this trial were analyzed by one-way ANOVA conducted using least significant difference (LSD) multiple comparisons using SPSS Statistics 25 (SPSS Inc., Chicago, IL, USA). Data presented as mean ± standard deviation (SD). Statistically significant at p < 0.05. The graphs were created using GraphPad Prism 10.0 software (GraphPad Software, LLC, San Diego, CA, USA).
3. Results
3.1. Antioxidant Indexes
In our previous study [21], we found that the hepatic CAT and T-SOD activity was decreased, and the MDA and H2O2 contents in the liver were decreased in the CCP group than those in the CON and CTA groups (p < 0.05).
Jejunal antioxidant capacity results are shown in Table 3. Jejunal GSH-Px and CAT activities were lower in the CCP group than in the CON and CTA groups on day 14 (p < 0.05). The GSH-Px level of jejunum was decreased in the CCP group compared with the CON and CTA, and T-SOD activity in CCP was lower than the CTA on day 21 (p < 0.05). Jejunal T-AOC, T-SOD, and CAT levels were lower in the CCP group than in the CON and CTA groups on day 28 (p < 0.05). On days 14 and 21, the content of jejunal H2O2 was increased in the CCP compared with the CON and CTA groups, and MDA content was more significant than the CON, and MDA content on day 21 was markedly decreased in the CTA group (p < 0.05).
Table 3.
Effects of TA on jejunal antioxidant capacity of broilers co-infected with CCP.
| Item | CON | CCP | CTA | p-Value |
|---|---|---|---|---|
| Day 14 | ||||
| GSH-Px(U/mg prot) | 21.97 ± 4.40 b | 16.90 ± 4.45 c | 26.96 ± 2.03 a | <0.001 |
| T-SOD (U/mg prot) | 192.04 ± 19.56 | 181.88 ± 13.81 | 180.72 ± 28.72 | 0.322 |
| CAT (U/mg prot) | 1.31 ± 0.12 a | 0.82 ± 0.23 c | 1.13 ± 0.10 b | <0.001 |
| H2O2 (mmol/g prot) | 1.88 ± 0.22 b | 3.03 ± 0.29 a | 2.01 ± 0.40 b | <0.001 |
| MDA (nmol/mg prot) | 7.15 ± 1.42 b | 10.52 ± 1.11 a | 9.37 ± 3.06 a | <0.001 |
| T-AOC (mmol/g prot) | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.052 |
| Day 21 | ||||
| GSH-Px (U/mg prot) | 28.82 ± 3.75 b | 24.12 ± 2.17 c | 31.18 ± 2.76 a | <0.001 |
| T-SOD (U/mg prot) | 178.16 ± 12.25 ab | 167.95 ± 11.94 b | 184.79 ± 18.14 a | 0.013 |
| CAT (U/mg prot) | 2.27 ± 0.39 | 2.30 ± 0.32 | 2.37 ± 0.35 | 0.747 |
| H2O2 (mmol/g prot) | 6.16 ± 0.50 b | 7.62 ± 1.94 a | 5.38 ± 1.35 b | 0.001 |
| MDA (nmol/mg prot) | 6.09 ± 0.91 b | 7.03 ± 1.39 a | 5.83 ± 1.14 b | 0.024 |
| T-AOC (mmol/g prot) | 0.15 ± 0.01 | 0.15 ± 0.02 | 0.16 ± 0.02 | 0.084 |
| Day 28 | ||||
| GSH-Px (U/mg prot) | 68.42 ± 9.34 | 63.18 ± 9.80 | 64.33 ± 13.58 | 0.428 |
| T-SOD (U/mg prot) | 364.21 ± 21.01 a | 313.83 ± 32.65 c | 339.47 ± 28.04 b | <0.001 |
| CAT (U/mg prot) | 6.32 ± 0.99 a | 5.07 ± 0.38 b | 5.85 ± 0.81 a | <0.001 |
| H2O2 (mmol/g prot) | 2.58 ± 0.60 | 3.08 ± 0.76 | 2.64 ± 0.50 | 0.086 |
| MDA (nmol/mg prot) | 5.85 ± 1.63 | 7.58 ± 1.56 | 7.12 ± 1.56 | 0.076 |
| T-AOC (mmol/g prot) | 0.17 ± 0.05 a | 0.12 ± 0.01 b | 0.17 ± 0.06 a | 0.028 |
Numbers are expressed as mean and SD, n = 14. a,b,c Values not sharing a superscript in the same row are markedly different.
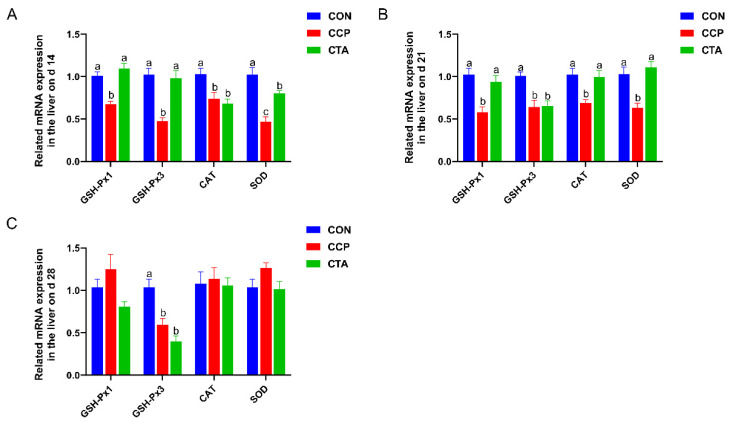
3.2. The mRNA Levels of Antioxidant Enzymes in the Liver
As shown in Figure 1, hepatic GSH-Px1, GSH-Px3, CAT, and SOD mRNA levels on days 14 and 21 were reduced by CCP challenge (Figure 1A,B, p < 0.05); GSH-Px3 mRNA levels in the liver on day 28 were markedly greater in the CON group than in the CTA and CCP groups (Figure 1C, p < 0.05). The CTA could alleviate the downregulation of hepatic GSH-Px1, CAT, and SOD mRNA levels of broilers challenged by CCP (p < 0.05).
Figure 1.
Antioxidant enzymes mRNA expression in the liver ((A) on day 14, (B) on day 21, (C) on day 28). Numbers are expressed as mean and SD, n = 14. a,b,c Values not sharing a superscript in the same row are markedly different.
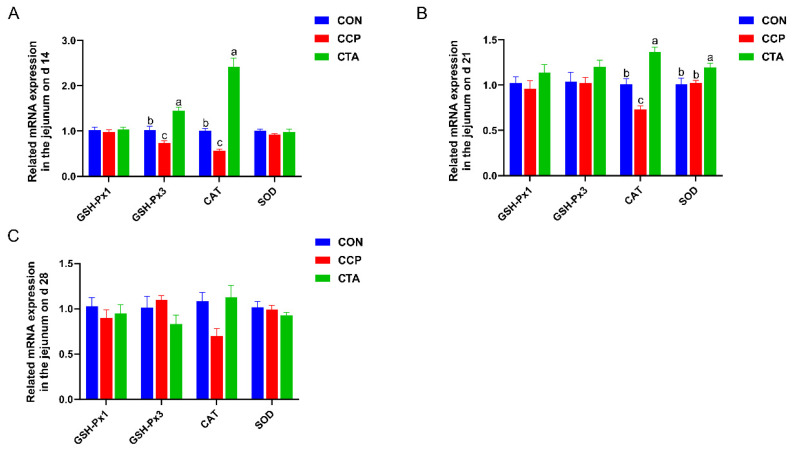
3.3. The mRNA Levels of Antioxidant Enzymes in the Jejunum
The results of jejunal antioxidant enzyme mRNA levels are shown in Figure 2. Compared to the CON and CTA groups, the CCP significantly lowered jejunal GSH-Px3 and CAT mRNA levels on day 14 (Figure 2A, p < 0.05). The SOD mRNA level in the CTA group on day 21 was markedly higher than those in the CON and CCP groups. Additionally, the CCP significantly downregulated the CAT mRNA level in the jejunum on day 21 compared with the CON and CTA (Figure 2B, p < 0.05). There was no significant difference in relevant antioxidant enzymes in the jejunum among treatments on day 28 (Figure 2C, p > 0.05).
Figure 2.
Jejunal antioxidant enzyme mRNA expression ((A) on day 14, (B) on day 21, (C) on day 28). Numbers are expressed as mean and SD, n = 14. a,b,c Values not sharing a superscript in the same row are markedly different.
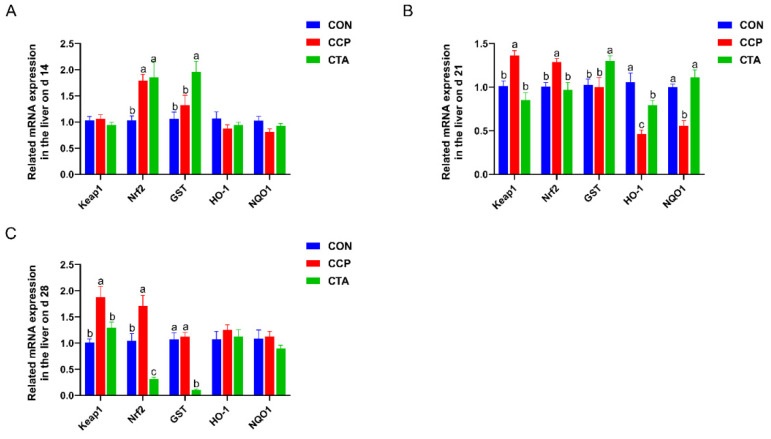
3.4. The mRNA Levels of the Antioxidant Pathway in the Liver
As presented in Figure 3, on day 14, the Nrf2 mRNA level was lower in the CON in the liver than in the CTA and CCP groups, and the GST mRNA level was markedly higher in CTA than in CON and CCP (Figure 3A, p < 0.05). On day 21, the CCP significantly increased the Keap1 and Nrf2 mRNA levels and significantly reduced the mRNA levels of HO-1 and NQO1 compared to the CON; compared to the CCP, the CTA markedly reduced the mRNA levels of Keap1 and Nrf2 and significantly increased the mRNA levels of GST and NQO1(Figure 3B, p < 0.05). On day 28, the CCP significantly enhanced the mRNA levels of Keap1 and Nrf2 compared to the CON; the CTA markedly decreased the mRNA levels of Keap1, Nrf2, and GST compared to the CCP (Figure 3C, p < 0.05).
Figure 3.
Antioxidant pathway mRNA expression in the liver ((A) on day 14, (B) on day 21, (C) on day 28). Numbers are expressed as mean and SD, n = 14. a,b,c Values not sharing a superscript in the same row are markedly different.
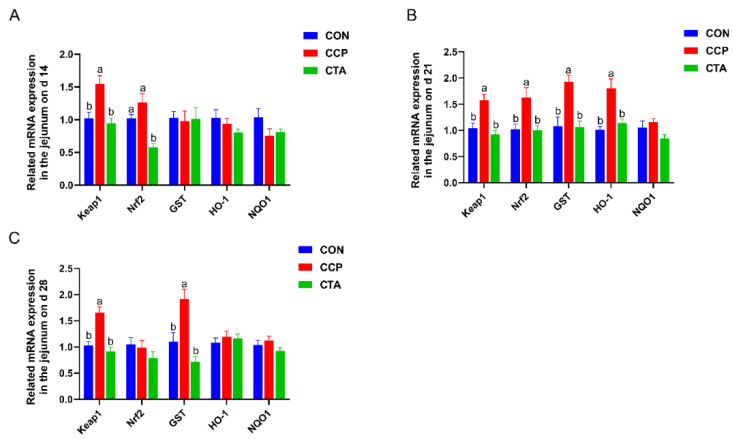
3.5. The mRNA Levels of the Antioxidant Pathway in the Jejunum
The results of jejunal antioxidant pathways mRNA levels are shown in Figure 4. On day 14, the mRNA level of Keap1 was markedly more significant in the CCP in the jejunum than in the CON and CTA; the mRNA level of Nrf2 was markedly lower in the CTA in the jejunum than in COT and CCP (Figure 4A, p < 0.05). The mRNA levels of Keap1, Nrf2, GST, and HO-1 in the jejunum on day 21 were upregulated by the CCP challenge (Figure 4B, p < 0.05); the mRNA levels of Keap1 and GST in the jejunum on day 28 were increased with CCP challenge (Figure 4C, p < 0.05); The CTA could alleviate the upregulation of Keap1, Nrf2, GST and HO-1 in the jejunum on days 21 and 28 in the CCP-challenged broilers (Figure 4B,C, p < 0.05).
Figure 4.
Antioxidant pathway mRNA expression in the jejunum ((A) on day 14, (B) on day 21, (C) on day 28). Numbers are expressed as mean and SD, n = 14. a,b Values not sharing a superscript in the same row are markedly different.
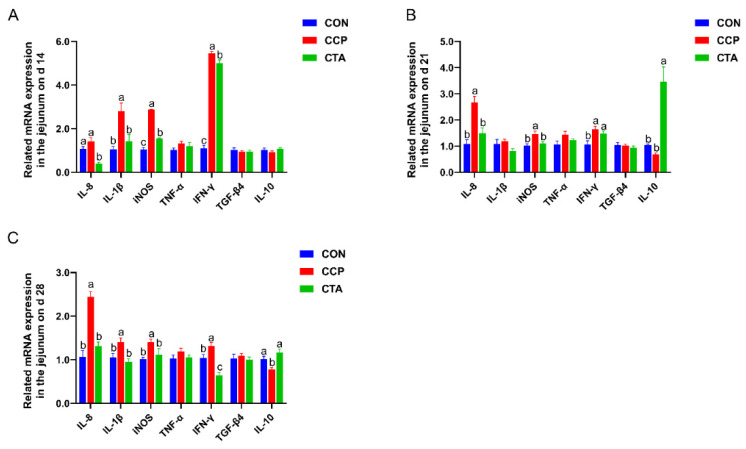
3.6. The mRNA Levels of the Cytokines in the Jejunum
As shown in Figure 5, on day 14, the CCP markedly elevated the mRNA levels of IL-8, IL-1β, iNOS, and IFN-γ in the jejunum compared with the CON and CTA groups (Figure 5A, p < 0.05). The CTA markedly elevated the mRNA levels of IL-10 in the jejunum on day 21 compared with the CON and CCP groups (Figure 5B, p < 0.05). The mRNA levels of IL-8, iNOS, and IFN-γ in the jejunum on days 21 and 28 were elevated by CCP challenge (Figure 5A,B, p < 0.05). Alternatively, the mRNA level of IL-1β was elevated, and the mRNA level of IL-10 was reduced in the jejunum on day 28 with CCP challenge (Figure 5C, p < 0.05). The CTA could alleviate the increase in IL-8, IL-1β, iNOS, and IFN-γ mRNA levels and the decrease in IL-10 mRNA level in the jejunum in the CCP-challenged broilers (p < 0.05).
Figure 5.
The cytokines mRNA expression in the jejunum ((A) on day 14, (B) on day 21, (C) on day 28). Numbers are expressed as mean and SD, n = 14. a,b,c Values not sharing a superscript in the same row are markedly different.
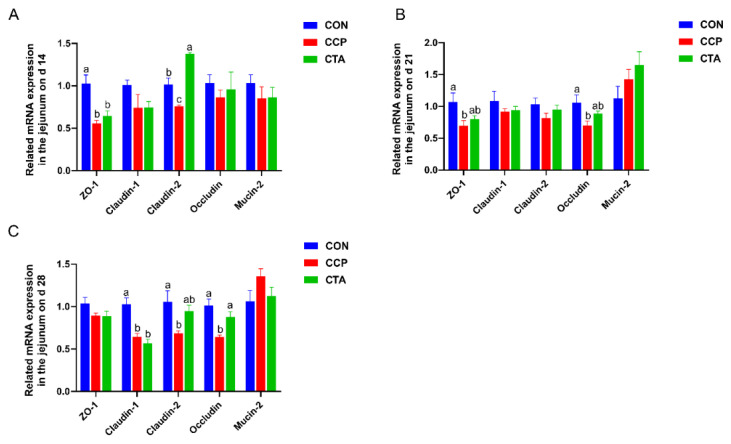
3.7. The mRNA Levels of Barrier Factors in the Jejunum
According to Figure 6. The ZO-1 mRNA level in the jejunum on day 14 was reduced by CCP challenge in comparison with the CON group; the CCP markedly reduced the mRNA levels of Claudin-2 in the jejunum on day 14 compared to the CON and CTA groups (Figure 6A, p < 0.05). Jejunal mRNA levels of ZO-1 and Occludin were markedly reduced by CCP challenge on day 21 compared to the CON group (Figure 6B, p < 0.05). The mRNA levels of Claudin-1, Claudin-2, and Occludin in the jejunum on day 28 were significantly reduced by CCP challenge (Figure 6C, p < 0.05). The CTA could alleviate the decrease in Claudin-2 and Occludin mRNA in the jejunum in the CCP-challenged broilers (p < 0.05).
Figure 6.
Barrier factors mRNA expression in the jejunum ((A) on day 14, (B) on day 21, (C) on day 28). Numbers are expressed as mean and SD, n = 14. a,b,c Values not sharing a superscript in the same row are markedly different.
4. Discussion
The NE is a prevalent infectious gastrointestinal illness in broiler production, predominantly associated with C. perfringens types A and G. C. perfringens is secondary to coccidia (coccidia is primary), or they may be co-infected. This study used the NE model constructed by coccidia and C. perfringens type A. In our previous study, we found that the infection of coccidia and C. perfringens reduced the growth performance and caused intestinal morphological structure damage in broilers [21]. Therefore, the infection model is well-established. We also found significantly decreased levels of high-density lipoprotein (HDL), gamma-glutamyl transferase (GGT), and lactate dehydrogenase (LDH) in serum in broilers under NE conditions compared with the control group. HDL is associated with lipid metabolism, and GGT and LDH are associated with liver function [21]. Therefore, in this study, we investigated the effects of TA on hepatic antioxidant function and jejunal function in broilers with NE conditions.
The antioxidant system consists of several components, including enzymatic species such as SOD, CAT, and GSH-Px, as well as non-enzymatic species such as T-AOC [22,23]. When animals are exposed to pathogens, viruses, and harsh environments, the body produces large amounts of reactive oxygen species (ROS) and MDA, which may cause oxidative stress [24]. It has been shown that broilers with NE condition reduced antioxidant function, induced inflammatory response, and impaired intestinal barrier function [19,25,26]. Consistent with previous findings, we found that Jejunal H2O2 and MDA contents were increased, and T-AOC, SOD, CAT, and GSH-Px levels were decreased by CCP challenge, which indicated that CCP co-infection caused oxidative stress in the broiler. Dietary supplementation with TA has been shown to enhance the activities of intestinal GSH-Px, SOD, and CAT, and to decrease H2O2 and MDA contents, thereby increasing intestinal antioxidant capacity and protecting the intestinal tract from damage caused by oxidative stress [9,10,27]. In the present study, we found that dietary supplementation with 1000 mg/kg TA reduced the increase in peroxides caused by CCP challenge and enhanced the activities of jejunal antioxidant enzymes. In our previous study, dietary supplementation with 1000 mg/kg TA could alleviate the decrease in antioxidant enzyme levels and increase H2O2 and MDA contents in the liver by CCP challenge [21]. These results indicated that dietary supplementation with 1000 mg/kg TA could alleviate the oxidative stress in the liver and jejunum of broilers caused by CCP challenge.
The Nrf2-Keap1 system is a significant regulatory pathway against oxidative stress [28]. The activation of the Nrf2-Keap1 pathway enhances antioxidant defense factors-associated genes, which include the GSH-Px1, GSH-Px3, SOD, CAT, HO-1, NQO1, and GST [29]. GSH-Px1 and GSH-Px3 belong to selenium-containing GSH-Px, which have the function of cleaning up reactive oxygen species, with the difference that GSH-Px1 exists in the cytoplasm and mitochondria, and GSH-Px3 exists in the extracellular [30,31,32,33]. Invasion by pathogens can upregulate the expression of the Nrf2 and keap1 genes, which causes oxidative stress in the organism and promotes the entry of Nrf2 into the nucleus to participate in the synthesis of several antioxidant enzymes in reaction to oxidative stress [19,34,35]. In our study, Keap1 and Nrf2 mRNA levels were elevated in the liver and jejunum, and jejunal GST and HO-1 mRNA levels were increased in the CCP-challenged broilers, suggesting that CCP infection induces oxidative stress in broilers. However, we found that the mRNA levels of GSH-Px1, CAT, and SOD in the liver and jejunal CAT were decreased, and jejunal H2O2 and MDA content in broilers with the CCP challenge were increased. It showed that CCP-infected broilers responded but could not successfully increase antioxidant enzymes to scavenge free radicals, causing oxidative damage to the liver and jejunum. Studies have shown that tannins can enhance the mRNA levels of antioxidant enzymes and reduce oxidative stress to protect the health of the body [36,37,38]. Consistent with these studies, we found that dietary supplementation with 1000 mg/kg TA could alleviate the up-regulation of Keap1 and Nrf2 mRNA levels and enhance the mRNA levels of GSH-Px1, SOD, CAT in the liver and jejunal CAT in the CCP-challenged broilers, which suggested that dietary supplementation with 1000 mg/kg TA could enhance antioxidant enzyme activities through the activation of the transcription factor Nrf2 downstream of the Nrf2-Keap1 pathway to alleviate oxidative damage in the liver and jejunum caused in the CCP-challenged broilers.
Intestinal immune barrier homeostasis is maintained by releasing inflammation and anti-inflammation factors by bowel-related lymphoid tissues composed of various cells that prevent pathogen invasion [39]. This study aimed to determine the expression of specific immune genes in the intestine, mainly belonging to pro-inflammatory (IL-8, IL-1β, iNOS, IFN-γ, TNF-α) and anti-inflammatory (TGF-β4, IL-10) mediators. Pro- and anti-inflammatory cytokines are essential to immune response homeostasis and inflammation [40]. It has been found that when inflammation occurs in the gut upon invasion by pathogens, there is a significant elevation in the expression of the pro-inflammatory genes, which may or may not coincide with a reduction in the anti-inflammatory gene expression [18,41,42]. This study found that jejunal pro-inflammatory mediators (IL-8, IL-1β, iNOS, IFN-γ) were significantly upregulated, the anti-inflammatory mediator (IL-10) was significantly downregulated in broilers with the CCP challenge, and these results suggested that CCP-infected broilers elicited an intestinal inflammatory response in the jejunum. In our study, dietary supplementation with 1000 mg/kg TA significantly diminished pro-inflammatory mediators and elevated anti-inflammatory mediators with CCP challenge, consistent with previous studies [43,44,45]. These results suggest that dietary supplementation with 1000 mg/kg TA is beneficial in alleviating intestinal inflammatory responses in broilers co-infected with CCP.
Tight junction proteins are essential for maintaining the epithelial barrier, which maintains the diffusion barrier and closes the cell gap. ZO-1, occludin, and claudins are the most critical proteins of the intestinal barrier [42]. It was found that both inflammatory mediators and cytokines result in abnormal expression of tight junction proteins such as ZO-1, claudin-1, and occludin, elevating intestinal mucosal permeability and impairing intestinal barrier function [46,47,48,49,50]. It was shown that CCP infection of broilers resulted in significant downregulation of tight junction proteins, causing intestinal damage in broilers [51,52]. In the present study, the mRNA levels of ZO-1, claudin-1, claudin-2, and occludin in the jejunum were significantly downregulated in CCP-infected broilers, which suggested that the jejunal barrier is impaired in CCP-infected broilers. Tannins significantly upregulate ZO-1 levels to protect against jejunal barrier damage. Research has shown that dietary TA supplementation can increase the mRNA levels of ZO-1, claudin-1, claudin-2, and occludin to mitigate intestinal barrier damage [53,54]. In line with prior research, we found that dietary supplementation with 1000 mg/kg TA raised the mRNA levels of claudin-2 and occludin, which suggested that dietary supplementation with TA is beneficial in ameliorating jejunal barrier damage in CCP-challenged broilers.
In this study, dietary supplementation with 1000 mg/kg TA could improve liver and jejunal function in broilers with NE conditions. However, we also found that TA increases GST mRNA levels, which may cause some adverse effects on the liver in broilers. The reason may be related to TA’s origin, dose, and degree of polymerization of TA, which are closely related to its bioavailability. Studies have shown that highly polymerized tannins, with high molecular weight, are more poorly absorbed in the small intestine [55], and hydrolyzed tannins are oligomers of gallic acid, ellagic acid, and glucose that are partially hydrolyzed by digestive acids in the small intestine for easier absorption [56,57]. However, adding high doses of TA to diets may negatively affect performance, lymphoid organ weights, and ileal digestibility of amino acids in broilers and cause liver damage in mice [58,59]. Therefore, the increase in the GST mRNA level in the liver in this trial may be related to the dose of TA. We will further investigate the optimal dose of TA added to the ration to improve the health of broilers with NE conditions.
5. Conclusions
The co-infection of CCP reduced antioxidant capacity, induced intestinal inflammatory response, and impaired the intestinal barrier functions of broilers. Dietary supplementation with 1000 mg/kg TA could protect the intestinal barrier, mitigate the inflammatory response, and enhance antioxidant capacity by the activation of the transcription factor Nrf2 downstream of the Nrf2-Keap1 pathway, thereby protecting the intestinal health of co-infected broilers with CCP.
Acknowledgments
We thank our students and technicians for contributing to this research.
Abbreviations
TA: tannic acid; NE: Necrotic enteritis; CCP: coccidia and Clostridium perfringens; MDA: malondialdehyde; H2O2: hydrogen peroxide; T-SOD: total superoxide dismutase; CAT: catalase; T-AOC: total antioxidant capacity; GSH-Px: glutathione peroxidase; Keap1: Kelch-like-ECH-associated protein1; Nrf2: nuclear factor erythroid 2-related factor 2; GSH-Px1: glutathione peroxidase1; GSH-Px3: glutathione peroxidase3; SOD: superoxide dismutase; GST: glutathione S-transferase; HO-1: heme-oxygenase-1; NQO1: NAD(P)H quinone oxidoreductase I; iNOS: inducible nitric oxide synthase; TNF-α: tumor necrosis factor α; IL-1β interleukin 1β; IL-8: interleukin 8; IL-10: interleukin 10; TGF-β4: ransforming growth factorβ4; IFN-γ: Interferon γ; ZO-1: zonula occludens protein1.
Author Contributions
B.D.: conceptualization, study design, methodology, and supervision; Z.Z. and P.X.: formal analysis and writing the original draft; C.L. and J.C.: validation and formal analysis; B.R., E.D. and S.G.: investigation and data curation; P.L. and L.L.: statistical analysis and manuscript review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Animal Care and Use Committee at Wuhan Polytechnic University (NO.WPU202204152). All animal experiments were conducted following the guidelines of the Research Ethics Committee of the College of Animal Science and Nutritional Engineering, Wuhan Polytechnic University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
This study was funded by the Natural Science Foundation of Hubei Province (2022CFB396), the Innovative Group Program of the Natural Science Foundation of Hubei Province (2023AFA018), the Scientific and Technological Projects of Hubei Province (2023EHA041), and the PhD Start-up Program of Wuhan Polytechnic University (2022RZ068).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Emami N.K., Dalloul R.A. Centennial Review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021;100:101330. doi: 10.1016/j.psj.2021.101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu C., Lillehoj H.S., Sun Z., Lee Y., Zhao H., Xianyu Z., Yan X., Wang Y., Lin S., Liu L., et al. Characterization of virulent netB+/tpeL+ Clostridium perfringens strains from necrotic enteritis-affected broiler chicken farms. Avian Dis. 2019;63:461–467. doi: 10.1637/11973-092018-Reg.1. [DOI] [PubMed] [Google Scholar]
- 3.Mehdizadeh Gohari I., A Navarro M., Li J., Shrestha A., Uzal F., A McClane B. Pathogenicity, and virulence of Clostridium perfringens. Virulence. 2021;12:723–753. doi: 10.1080/21505594.2021.1886777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu C., Tong Y., Li Q., Wang T., Yang Z. Trans-anethole ameliorates intestinal injury through activation of Nrf2 signaling pathway in subclinical necrotic enteritis-induced broilers. Front. Vet. Sci. 2022;9:877066. doi: 10.3389/fvets.2022.877066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Tommaso N., Gasbarrini A., Ponziani F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public. Health. 2021;18:12836. doi: 10.3390/ijerph182312836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing W., Xiaolan C., Yu C., Feng Q., Haifeng Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022;154:113561. doi: 10.1016/j.biopha.2022.113561. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin A., Booth B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022;36:1503–1523. doi: 10.1177/08853282211058099. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z.F., Xi Y., Wang S.T., Zheng L.Y., Qi Y., Guo S.S., Ding B.Y. Effects of Chinese gallnut tannic acid on growth performance, blood parameters, antioxidative status, intestinal histomorphology, and cecal microbial shedding in broilers challenged with aflatoxin B1. J. Anim. Sci. 2022;100:skac099. doi: 10.1093/jas/skac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi Y., Chen J., Guo S., Wang S., Liu Z., Zheng L., Qi Y., Xu P., Li L., Zhang Z., et al. Effects of tannic acid on growth performance, relative organ weight, antioxidative status, and intestinal histomorphology in broilers exposed to aflatoxin B1. Front. Vet. Sci. 2022;9:1037046. doi: 10.3389/fvets.2022.1037046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H.W., Li K., Zhao J.S., Deng W. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J Anim. Physiol. Anim. Nutr. 2018;102:717–726. doi: 10.1111/jpn.12839. [DOI] [PubMed] [Google Scholar]
- 12.Guo P., Tong Y., Yang R., Zhang M., Lin Q., Lin S., Wang C. Effects of hydrolyzed gallotannin on intestinal physical barrier, immune function, and microbiota structure of yellow-feather broilers. Poult. Sci. 2023;102:103010. doi: 10.1016/j.psj.2023.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui X., Zhang S., Jiang S., Gou Z., Wang Y. Dietary protocatechuic acid ameliorates ileal mucosal barrier injury and inflammatory response and improves intestinal microbiota composition in Yellow chickens challenged with Salmonella typhimurium. Poult. Sci. 2023;102:102496. doi: 10.1016/j.psj.2023.102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H., Fu J., Luo Y., Li P., Song B., Lv Z., Guo Y. Effects of tannic acid on the immunity and intestinal health of broiler chickens with necrotic enteritis infection. J. Anim. Sci. Biotechnol. 2023;14:72. doi: 10.1186/s40104-023-00867-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H., Zhang X., Li P., Luo Y., Fu J., Gong L., Lv Z., Guo Y. Effects of tannic acid supplementation on the intestinal health, immunity, and antioxidant function of broilers challenged with necrotic enteritis. Antioxidants. 2023;12:1476. doi: 10.3390/antiox12071476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M., Liu P., Xue Y., Liang Y., Shi J., Han X., Zhang J., Chu X., Chu L. Tannic acid attenuates hepatic oxidative stress, apoptosis and inflammation by activating the Keap1-Nrf2/ARE signaling pathway in arsenic trioxide-toxicated rats. Oncol. Rep. 2020;44:2306–2316. doi: 10.3892/or.2020.7764. [DOI] [PubMed] [Google Scholar]
- 17.Jin W., Xue Y., Xue Y., Han X., Song Q., Zhang J., Li Z., Cheng J., Guan S., Sun S., et al. Tannic acid ameliorates arsenic trioxide-induced nephrotoxicity, contribution of NF-κB and Nrf2 pathways. Biomed. Pharmacother. 2020;126:110047. doi: 10.1016/j.biopha.2020.110047. [DOI] [PubMed] [Google Scholar]
- 18.Guo S., Xi Y., Xia Y., Wu T., Zhao D., Zhang Z., Ding B. Dietary Lactobacillus fermentum and Bacillus coagulans supplementation modulates intestinal immunity and microbiota of broiler chickens challenged by Clostridium perfringens. Front. Vet. Sci. 2021;8:680742. doi: 10.3389/fvets.2021.680742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P., Liu C., Niu J., Zhang Y., Li C., Zhang Z., Guo S., Ding B. Effects of dietary supplementation with vitamin A on antioxidant and intestinal barrier function of broilers co-Infected with coccidia and Clostridium perfringens. Animals. 2022;12:3431. doi: 10.3390/ani12233431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Xu P., Chen Y., Chen J., He L., Liu C., Guo S., Zhang Z., Ding B. Effects of tannic acid on growth performance, antioxidant function, and intestinal morphology of broilers infected with coccidia and Clostridium perfringens. Chin. J. Anim. Nutr. 2023;35:7036–7048. doi: 10.12418/CJAN2023.641. [DOI] [Google Scholar]
- 22.Demirci-Çekiç S., Özkan G., Avan A.N., Uzunboy S., Çapanoğlu E., Apak R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022;209:114477. doi: 10.1016/j.jpba.2021.114477. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S., Wang C., Sun Y., Wang G., Chen H., Li D., Yu X., Chen G. Xylanase and fermented polysaccharide of hericium caputmedusae reduce pathogenic infection of broilers by improving antioxidant and anti-Inflammatory properties. Oxidative Med. Cell Longev. 2018;2018:4296985. doi: 10.1155/2018/4296985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelic M.D., Mandic A.D., Maricic S.M., Srdjenovic B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021;17:22–28. doi: 10.4103/jcrt.JCRT_862_16. [DOI] [PubMed] [Google Scholar]
- 25.Daneshmand A., Kermanshahi H., Mohammed J., Sekhavati M.H., Javadmanesh A., Ahmadian M., Alizadeh M., Razmyar J., Kulkarni R.R. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 2022;101:101652. doi: 10.1016/j.psj.2021.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Zhao Q., Ci X., Chen S., Xie Z., Li H., Zhang H., Chen F., Xie Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020;99:6606–6618. doi: 10.1016/j.psj.2020.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M., Huang H., Liu S., Zhuang Y., Yang H., Li Y., Chen S., Wang L., Yin L., Yao Y., et al. Tannic acid modulates intestinal barrier functions associated with intestinal morphology, antioxidative activity, and intestinal tight junction in a diquat-induced mouse model. RSC Adv. 2019;9:31988–31998. doi: 10.1039/C9RA04943F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu H., Dai S., Li J., Wen A., Bai X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020;99:1454–1461. doi: 10.1016/j.psj.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Li X., Shi S., Zhou Y., Zhang K., Wang Y., Zhao J. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult. Sci. 2022;101:102036. doi: 10.1016/j.psj.2022.102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flohe L., Günzler W.A., Schock H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K., Avissar N., Whitin J., Cohen H. Purification and characterization of human plasma glutathione peroxidase: A selenoglycoprotein distinct from the known cellular enzyme. Arch. Biochem. Biophys. 1987;256:677–686. doi: 10.1016/0003-9861(87)90624-2. [DOI] [PubMed] [Google Scholar]
- 32.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Handy D.E., Loscalzo J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022;188:146–161. doi: 10.1016/j.freeradbiomed.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He F., Antonucci L., Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S., Pi J., Zhang Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway. Redox Biol. 2022;54:102389. doi: 10.1016/j.redox.2022.102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei X., Luo C., He Y., Huang H., Ran F., Liao W., Tan P., Fan S., Cheng Y., Zhang D., et al. Hepatoprotective effects of different extracts from triphala against CCl4-Induced acute liver injury in mice. Front. Pharmacol. 2021;12:664607. doi: 10.3389/fphar.2021.664607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kizir D., Karaman M., Ceylan H. Tannic acid may ameliorate doxorubicin-induced changes in oxidative stress parameters in rat spleen. Naunyn Schmiedebergs Arch. Pharmacol. 2023;396:3605–3613. doi: 10.1007/s00210-023-02563-w. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z., Guo L., Ding X., Li F., Xu H., Li S., Wang X., Li K., Yue X. Supplementation of chestnut tannins in diets can improve meat quality and antioxidative capability in Hu lambs. Meat Sci. 2023;206:109342. doi: 10.1016/j.meatsci.2023.109342. [DOI] [PubMed] [Google Scholar]
- 39.Broom L.J., Kogut M.H. Gut immunity: Its development and reasons and opportunities for modulation in monogastric production animals. Anim. Health Res. Rev. 2018;19:46–52. doi: 10.1017/S1466252318000026. [DOI] [PubMed] [Google Scholar]
- 40.Kolehmainen M., Mykkänen O., Kirjavainen P.V., Leppänen T., Moilanen E., Adriaens M., Laaksonen D.E., Hallikainen M., Puupponen-Pimiä R., Pulkkinen L., et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012;56:1501–1510. doi: 10.1002/mnfr.201200195. [DOI] [PubMed] [Google Scholar]
- 41.Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Y., Zhang X., Wang Y., Guo Y., Zhu P., Li G., Zhang J., Ma Q., Zhao L. Dietary ellagic acid ameliorated Clostridium perfringens-induced subclinical necrotic enteritis in broilers via regulating inflammation and cecal microbiota. J. Anim. Sci. Biotechnol. 2022;13:47. doi: 10.1186/s40104-022-00694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing C., Niu J., Liu Y., Jiao N., Huang L., Jiang S., Yan L., Yang W., Li Y. Tannic acid extracted from galla chinensis supplementation in the diet improves intestinal development through suppressing inflammatory responses via blockage of NF-κB in broiler chickens. Animals. 2022;12:2397. doi: 10.3390/ani12182397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S., Wang K., Lin S., Zhang Z., Cheng M., Hu S., Hu H., Xiang J., Chen F., Li G., et al. Comparison of the effects between tannins extracted from different natural plants on growth performance, antioxidant capacity, immunity, and intestinal flora of broiler chickens. Antioxidants. 2023;12:441. doi: 10.3390/antiox12020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X., Chen Y., Chen X., Zhao X., Zou Y., Li L., Zhou X., Li M., Zhang D., Ye G., et al. Exosomes from tannic acid-stimulated macrophages accelerate wound healing through miR-221-3p mediated fibroblasts migration by targeting CDKN1b. Int. J. Biol. Macromol. 2023;244:125088. doi: 10.1016/j.ijbiomac.2023.125088. [DOI] [PubMed] [Google Scholar]
- 46.Liao P., Liao M., Li L., Tan B., Yin Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol Res. 2017;6:866–877. doi: 10.1039/C7TX00202E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019;9:10256. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J., Wang P., Liu Y., Zhao L., Li Z., Xue Y. Krüppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5. J. Cell. Physiol. 2014;229:916–926. doi: 10.1002/jcp.24523. [DOI] [PubMed] [Google Scholar]
- 49.Qiu K., Li C.L., Wang J., Qi G.H., Gao J., Zhang H.J., Wu S.G. Effects of dietary supplementation with Bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front. Nutr. 2021;8:786878. doi: 10.3389/fnut.2021.786878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajasekaran S.A., Barwe S.P., Gopal J., Ryazantsev S., Schneeberger E.E., Rajasekaran A.K. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G124–G133. doi: 10.1152/ajpgi.00297.2006. [DOI] [PubMed] [Google Scholar]
- 51.Xu X., Yang S., Olajide J.S., Qu Z., Gong Z., Wang J., Zhang Y., Wang H., Xiong L., Zhang K., et al. Clostridium butyricum supplement can ameliorate the intestinal barrier roles in broiler chickens experimentally infected with Clostridium perfringens. Front. Physiol. 2021;12:737481. doi: 10.3389/fphys.2021.737481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li P., Zheng L., Qi Y., Liu Z., Du E., Wei J., Zhang Z., Guo S., Ding B. Dietary Lactobacillus fermentum and Lactobacillus paracasei improve the intestinal health of broilers challenged with coccidia and Clostridium perfringens. Front. Vet. Sci. 2022;9:1025677. doi: 10.3389/fvets.2022.1025677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X., Sun R., Liu Q., Gong Y., Ou Y., Qi Q., Xie Y., Wang X., Hu C., Jiang S., et al. Effects of dietary supplementation with dandelion tannins or soybean isoflavones on growth performance, antioxidant function, intestinal morphology, and microbiota composition in Wenchang chickens. Front. Vet. Sci. 2023;9:1073659. doi: 10.3389/fvets.2022.1073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu J., Song Y., Yu B., He J., Zheng P., Mao X., Huang Z., Luo Y., Luo J., Yan H., et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J. Anim. Sci. Biotechnol. 2020;11:87. doi: 10.1186/s40104-020-00496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landete J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011;44:1150–1160. doi: 10.1016/j.foodres.2011.04.027. [DOI] [Google Scholar]
- 56.Fraga-Corral M., Otero P., Cassani L., Echave J., Garcia-Oliveira P., Carpena M., Chamorro F., Lourenço-Lopes C., Prieto M.A., Simal-Gandara J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods. 2021;10:251. doi: 10.3390/foods10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hidayat C., Irawan A., Jayanegara A., Sholikin M.M., Prihambodo T.R., Yanza Y.R., Wina E., Sadarman S., Krisnan R., Isbandi I. Effect of dietary tannins on the performance, lymphoid organ weight, and amino acid ileal digestibility of broiler chickens: A meta-analysis. Vet. World. 2021;14:1405–1411. doi: 10.14202/vetworld.2021.1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin L.S., Zhao H.P., Zhao Y.L., Ma Z.J., Zeng L.N., Zhang Y.M., Zhang P., Yan D., Bai Z.F., Li Y., et al. Protection and bidirectional effect of rhubarb anthraquinone and tannins for rats’ liver. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:698–703. (In Chinese) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.