Abstract
This study explores the interplay between executive functions and body weight, examining both the influence of biological factors, specifically sex, and methodological issues, such as the choice between Body Mass Index (BMI) and waist circumference (WC) as the primary anthropometric measure. A total of 386 participants (222 females, mean age = 45.98 years, SD = 17.70) were enrolled, from whom sociodemographic (sex, age, years of formal education) and anthropometric (BMI and WC) data were collected. Executive functions were evaluated using the Frontal Assessment Battery–15 (FAB15). The results showed the increased effectiveness of WC over BMI in examining the relationships between executive functions, sex differences, and body weight. In particular, this study revealed that there was a significant moderating effect of sex at comparable levels of executive functioning. Specifically, women with higher executive performance had lower WCs than their male counterparts, suggesting that executive function has a greater impact on WC in women than in men. Our findings highlight the importance of conducting more in-depth investigations of the complex relationship between cognitive deficits and weight gain, considering confounding variables of behavioral, psychobiological, and neurophysiological origin.
Keywords: obesity, executive functions, sex differences, Body Mass Index, waist circumference, moderation analysis
1. Introduction
Obesity affects about 650 million adults globally, transcending geographical boundaries. Given its contribution to the pathogenesis of several chronic diseases, within which it increases mortality rates [1,2,3,4,5], obesity represents a major public health challenge [6,7,8].
Over the past few decades, obesity has been associated with reduced cognitive performance. Consistently, it is now recognized as a risk factor involved in the onset of the majority of neurocognitive disorders [9,10,11,12,13,14,15,16,17,18,19].
Among the most extensively investigated cognitive domains, executive functions—higher-order cognitive functions associated with neural activity in the prefrontal cortex—appear to play a central role in the regulation of body weight. In general, these functions enable individuals to deal effectively with environmental challenges, particularly in novel or conflictual situations [20,21,22,23,24].
In the context of research on weight-related issues, previous behavioral studies have found that executive functions may predict body weight variability, dietary inhibition, appetite regulation, energy expenditure, adherence to a healthy diet, and outcomes of interventions aimed at weight reduction [25,26,27,28,29,30]. Congruently, neurophysiological evidence has identified the prefrontal cortex as a critical hub in regulating dietary self-control, operating in tandem with the mesocorticolimbic pathway to modulate reward mechanisms and the hedonic aspects of food choices [31,32,33]. Still, previous research in cognitive neuroscience has highlighted a significant association between impaired working memory and diminished gray matter volume in the left dorsal striatum, as well as alterations in the white matter microstructure of the left inferior longitudinal fasciculus along the occipitotemporal/ventral visual stream in individuals with higher body weight [34,35]. Given the involvement of the dorsal striatum in learning action–reward associations [36,37] and the role of the ventral visual stream in reward-cueing-related processes during spatial working memory tasks [38], this pattern of results may imply a significant relationship between reward processing mechanisms and obesity-related phenomena.
Other studies have focused on decision making. For example, structural neuroimaging studies in obese individuals have reported reduced gray matter density in key frontal regions responsible for decision making, such as the frontal operculum, middle frontal gyrus, and orbitofrontal cortex [39,40,41]. In addition, a previous fMRI study on obese women participating in a monetary decision-making task showed that greater task complexity was associated with increased activation in the frontal cortex. Furthermore, reduced brain activity in the same regions predicted an increased rate of weight gain over the following 3 years [42]. Taken together, this brief overview only partially explains the relationship between executive functions, brain activity, and obesity.
Noteworthy are the “paradoxical” findings, such as those underpinning the so-called “obesity paradox”. This paradox implies a potential positive association between obesity and psychophysical well-being, including cognitive functioning, particularly in the older adult population [43,44,45]. Once again, these counterintuitive findings not only highlight the intrinsic complexity of the relationship between cognitive functions, obesity, and health status but also suggest the need for further research aimed at exploring this topic [46,47,48,49,50,51,52,53].
A number of factors can definitely influence the relationship between executive functions and obesity. These include the different methods used for assessing body weight and the role of some confounding variables [54,55]. The Body Mass Index (BMI), which is commonly used to assess weight status, has faced criticism as a more imperfect indicator for individual evaluation, particularly when measuring regional adiposity [56,57,58,59]. This may lead to distortions in evaluating obesity-related effects [58]. In contrast, waist circumference (WC) appears to be a more sensitive measure of visceral obesity, with greater reliability in predicting obesity-related health risk factors, such as cardiovascular diseases, diabetes, and mortality [60,61,62,63].
In addition to methodological issues, the interaction between executive functions and obesity may be further complicated by biological sex differences [64,65,66]. On the one hand, such differences may influence the prevalence of obesity; on the other hand, sex may influence raw performance in neuropsychological tests assessing executive functions [67,68,69,70,71,72]. Hence, it should be recommended to treat sex as a potential covariate to be kept under control in psychometric models attempting to explore the association between executive functions and obesity.
The impact of sex on obesity prevalence is a stark global reality. Obesity is more prevalent among women in several geographic regions, including Sub-Saharan Africa, East Asia, the United States, and Europe [64,65,66]. This likely results from the interplay of sociocultural, environmental, and genetic factors [73]. Regarding neuropsychological outcomes, different studies have detected a significant concomitant effect of sex on tests exploring executive functioning. For instance, men and women exhibit distinct abilities in specific executive subdomains such as attention, planning, inhibition, and verbal fluency, and these differences manifest across different life stages [67,68,69,70,71,72]. In particular, increased impulsivity and shorter reaction times have been found in men as compared with women [68,72,74]. Conversely, better planning [75,76,77], attention [75,77], decision making [78,79], and working memory skills have been observed in female as compared with male individuals [80,81,82,83]. Nevertheless, it is important to stress that sex differences in executive functioning do not necessarily imply systematic disparities between sexes; rather, they may reflect differences in the cognitive strategies employed when required to perform a cognitive task [84,85].
Sex differences in neuropsychological test scores may extend to a lower level of abstraction and relate to variability in anatomofunctional brain characteristics. Indeed, men and women seem to employ different brain networks when performing a number of cognitive tasks, and may exhibit different activity across neurotransmitter systems, including dopamine and serotonin [86,87,88,89,90,91].
The present study was designed to further explore the intricate relationship between executive functions and body weight, taking into account the critical issues highlighted above. In particular, two primary objectives were outlined: (1) to assess the predictive value of executive functions in explaining the variance of different anthropometric measures (BMI vs. WC) and (2) to explore the potential moderating role of sex in the relationship between executive functions and body weight. In this regard, we expect that (1) executive functions will show a significant association with anthropometry and (2) sex will significantly moderate the relationship between executive functions and body weight. More specifically, we hypothesize that higher executive performance will be associated with lower body weight. Furthermore, we predict that sex will demonstrate a moderating effect on the association between executive functions and body weight.
2. Materials and Methods
2.1. Participants
A total of 386 participants (222 females) were involved in this study. Italian volunteers were recruited through the convenience sampling method from various districts in the Campania, Calabria, and Puglia regions of Southern Italy. The inclusion criteria for participation in this study were as follows: being at least 18 years old and having a minimum of 5 years of formal education (equivalent to completing primary school). Moreover, according to the normative datasets detailed in the Materials and Procedure section, participants who scored insufficiently on the cognitive batteries administered were excluded from the study. Additional exclusion criteria were past or current history of intellectual and/or linguistic deficits, or neurocognitive, psychiatric, or psychopathological disorders. None of the participants had a history of alcohol or substance abuse/addiction, and they were not receiving treatment with drugs known to affect cognitive performance. To prevent a “hyper normality” bias [92], individuals with chronic medical conditions that were managed pharmacologically, such as hypertension, cardiovascular diseases, or type II diabetes, were not excluded.
2.2. Materials and Procedure
Participants were accommodated in a soundproof room where anamnestic information was collected, and anthropometric and neuropsychological measurements were performed. Specifically, the initial phase involved gathering sociodemographic data such as sex, age, and years of formal education. This was followed by anthropometric assessments, including measurements of weight, height (measured using a weight scale with a stadiometer), and waist circumference (WC). The Body Mass Index (BMI) value was calculated using Quetelet’s formula (expressed as kg/m2). WC was measured by encircling a tape measure horizontally around the abdomen, positioned approximately 2 cm above the navel. Global cognitive functioning was assessed by administering the Mini-Mental State Examination [93,94]. Executive functions were evaluated using the Frontal Assessment Battery–15 (FAB15) [69]. This is a short screening battery designed to assess general frontal/executive functioning. The FAB15 explores various executive domains, such as abstraction, generativity, set-shifting, planning, sensitivity to interference, and inhibitory control. The battery showed a robust psychometric architecture, supported by adequate internal consistency (Cronbach’s alpha = 0.72), a parsimonious unifactorial structure, and excellent interrater (ICC = 0.99) and test–retest (ICC = 0.98) reliability. Regression-based normative data are available from a cohort of 1187 healthy individuals [69]. The scoring range is 0–15, with higher scores indicating a better general executive functioning. Following correction of raw scores for sex, age, and education, participants scoring below 23.8 on the MMSE and 9.36 on the FAB15 were excluded.
2.3. Statistical Analyses
Descriptive statistics were expressed as frequency for categorical variables and mean (M) and standard deviation (SD) for continuous variables. According to biological sex, between-group equivalence in age, education, and FAB15 scores was checked by univariate Analysis of Variance (ANOVA). Theoretically speaking, considering the potential differences in body composition between men and women, including the distribution of adipose tissue (specifically, visceral adiposity around the abdomen), sex disparities might be more pronounced in WC than BMI measurements. To mitigate this bias, we used a standardized regression-driven psychometric algorithm to remove the effects of possible linear associations between sex and anthropometric measures. Specifically, two simple linear regression models were constructed by independently entering BMI and WC as outcome variables and sex as a predictor. Based on the results of regression analyses, the following correction equation was solved:
where refers to the unstandardized regression coefficient, which represents the average change in the dependent variable associated with moving from one sex category to the other, and to the sex dummy variable (coded as 1 = female, 0 = male). Subsequently, the SPSS Macro PROCESS was employed for running moderation analysis with 5000 bootstrap samples. Two moderation models were tested. BMI- and WC-adjusted values entered each model as outcome variables, the FAB15 score as a predictor, and sex as a moderator, respectively. The nominal α level = 0.05 was adjusted according to Bonferroni’s correction for multiple comparisons, if appropriate. Overall, statistical analyses were performed by means of IBM SPSS Statistics v. 27.
3. Results
3.1. Preliminary Data Analysis: Normality Assumptions and Missing Data
Univariate outliers, i.e., z-scores higher than three in absolute terms, were deleted. Thereafter, univariate normality was assessed according to skewness and kurtosis indexes. In particular, values ranging from −2 to +2 indicate the absence of appreciable deviations from normality. For diagnostics of multivariate outliers, Mahalanobis’ distance was used. No multivariate outliers were detected. Multivariate normality was assumed by Mardia’s kurtosis coefficient.
3.2. Descriptive Statistics
Data from 386 participants (222 females; M age = 41.80 years, SD = 18.32, range = 18–86 years; M education = 15.11 years, SD = 3.96, range = 5–24) were analyzed. Mean BMI and WC values were 25.32 (SD = 4.53, range = 18.03–39.51) and 86.11 (SD = 14.83, range = 60–130), respectively. The mean MMSE score was 29.11 (SD = 1.29, range = 23–30) while the mean FAB15 score was 13.56 (SD = 1.62, range = 7–15). No sex differences were detected for age [F(1, 385) = 1.152, p = 0.284], education [F(1, 385) = 0.129, p = 0.720], MMSE [F(1, 385) = 0.110, p = 0.740], or FAB15 score [F(1, 385) = 0.909, p = 0.341]. As anticipated, no between-group differences were detected for BMI [F(1, 385) = 1.267, p = 0.21], while they emerged for WC [F(1, 385) = 20.910, p < 0.001]. Sex-group differences are reported in Table 1 for descriptive purposes. Based on the regression analyses’ results (BMI: = −0.525, SE = 0.47, t = −1.126, p = 0.26; WC: = −6.906, SE = 1.51, t = −4.573, p < 0.001), and taking into account an of 0.58, correction equations were derived, par condicio, to adjust both BMI and WC values. In both instances, this led to a significant selective reduction in sex differences (ps > 0.99).
Table 1.
Mean (SD) by sex groups.
| Whole Sample (N = 386) |
Females (n = 222) |
Males (n = 164) |
p-Value | |
|---|---|---|---|---|
| Age (years) | 41.80 (18.32) | 42.66 (18.18) | 40.63 (18.50) | ns |
| Education (years) | 15.11 (3.96) | 15.17 (4.03) | 15.02 (3.89) | ns |
| MMSE | 29.11 (1.29) | 29.09 (1.40) | 29.13 (1.12) | ns |
| FAB15 | 13.56 (1.62) | 13.64 (1.72) | 13.46 (1.47) | ns |
| BMI | 25.32 (4.53) | 25.10 (4.81) | 25.62 (4.13) | ns |
| WC | 86.11 (14.83) | 83.26 (15.07) | 90.17 (13.53) | <0.001 |
MMSE: Mini-Mental State Examination; FAB15: Frontal Assessment Battery–15; BMI: Body Mass Index; WC: waist circumference.
3.3. Moderation Analysis
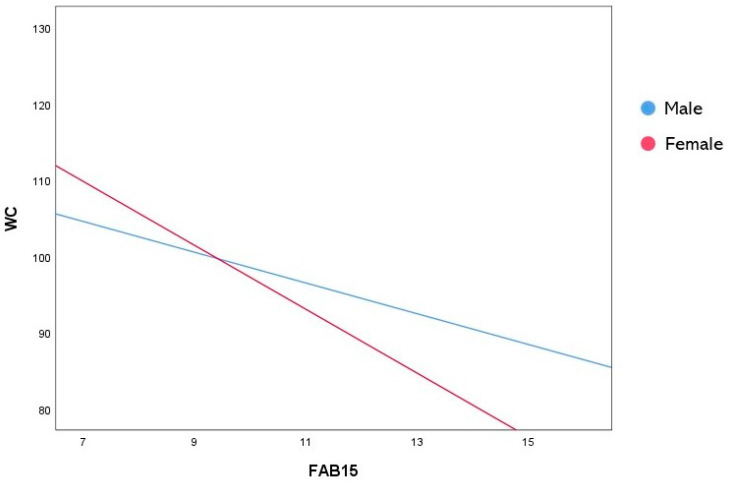
To test the moderating role of sex in the relationship between the FAB15 and anthropometric measures, two moderation analyses with 5000 bootstrap samples were performed, where BMI- and WC-adjusted values were entered as dependent variables, respectively. The first model was statistically significant (R2 = 0.172, F(3, 382) = 22.802, p < 0.001) and showed a main effect of the FAB15 score only (b = −0.787, 95% CI [−1.246, −0.329], SE = 0.233, t = −3.376, p < 0.001) (Table 2). Similarly, the second model explained a significant amount of WC variance (R2 = 0.188, F(3, 382) = 25.324, p <0.001). In addition, both a main effect of the FAB15 score (b = −2.014, 95% CI [−3.426, −0.603], SE = 0.717, t = −2.808, p = 0.005) and a significant interaction between sex and the FAB15 (b = −2.172, 95% CI [−3.917, −0.427], SE = 0.887, t = −2.448, p = 0.015) were found (Table 3). This result suggests that sex significantly moderated the relationship between the FAB15 and WC. More specifically, as revealed by the analysis of the conditional effects at each moderator’s level (Table 4), female subjects with higher FAB15 scores had lower WCs than males (Figure 1).
Table 2.
Results of moderation analysis of BMI-adjusted-by-sex values.
| b | SE | t | p | LLCI | ULCI | |
|---|---|---|---|---|---|---|
| Constant | 36.213 | 3.159 | 11.462 | <0.001 | 29.998 | 42.428 |
| FAB15 | −0.787 | 0.233 | −3.376 | <0.001 | −1.246 | −0.329 |
| Sex | 5.939 | 3.937 | 1.508 | 0.132 | −1.806 | 13.685 |
| FAB15×Sex | −0.476 | 0.289 | −1.647 | 0.100 | −1.045 | 0.092 |
FAB15: Frontal Assessment Battery–15; LLCI: Lower Level of Confidence Interval; ULCI: Upper Level of Confidence Interval.
Table 3.
Results of moderation analysis of WC-adjusted-by-sex values.
| b | SE | t | p | LLCI | ULCI | |
|---|---|---|---|---|---|---|
| Constant | 118.653 | 9.702 | 12.229 | <0.001 | 99.567 | 137.739 |
| FAB15 | −2.014 | 0.717 | −2.808 | 0.005 | −3.426 | −0.603 |
| Sex | 20.444 | 12.065 | 1.694 | 0.091 | −3.290 | 44.179 |
| FAB15×Sex | −2.172 | 0.887 | −2.448 | 0.015 | −3.917 | −0.427 |
FAB15: Frontal Assessment Battery–15; LLCI: Lower Level of Confidence Interval; ULCI: Upper Level of Confidence Interval.
Table 4.
Results of analysis of conditional effects of FAB15 scores at sex levels.
| b | SE | t | p | LLCI | ULCI | |
|---|---|---|---|---|---|---|
| Male | −2.014 | 0.717 | −2.808 | 0.005 | −3.426 | −0.603 |
| Female | −4.187 | 0.522 | −8.023 | <0.001 | −5.213 | −3.160 |
LLCI: Lower Level of Confidence Interval; ULCI: Upper Level of Confidence Interval.
Figure 1.
Interaction plot representing the significant moderating effect of sex in the relationship between FAB15 and WC. FAB15: Frontal Assessment Battery–15; WC: waist circumference.
4. Discussion
In the current study, we examined the relationship between executive functioning, sex differences, and two different anthropometric indexes, namely BMI and WC. Our results showed the following: (1) executive functions, as measured by the FAB15, are significantly associated with body weight; (2) WC is a more reliable measure than BMI for examining the relationship between FAB15 score, sex, and body weight; and (3) biological sex exerts a moderating effect in the relationship between FAB15 score and WC. Specifically, we found that the association between waist circumference and the FAB15 was more significant in women, suggesting that executive function has a greater impact on WC in women than in men. Taken together, these findings provide additional insights into the interplay between executive functions and body weight. Considering the growing scientific interest regarding this topic [95,96,97,98,99], our results may offer meaningful contributions to this area of discussion by incorporating the role of sex-related physiological variability. However, only a few studies have explored this issue in the scientific literature [100]. Therefore, the interpretation of our findings draws predominantly from the realm of speculation and thus requires further investigations. In fact, the hypotheses that will follow are built upon spurious pathways.
Executive functions rely on the activation of various brain regions, particularly the prefrontal cortex, encompassing both the medial [101,102,103] and orbitofrontal regions [104,105,106,107]. Overall, the prefrontal cortex plays a critical role in executive processes like attention, action planning, and working memory. Still, the prefrontal cortex relies on a delicate balance between excitatory glutamatergic neurons [108,109] and inhibitory GABAergic neurons to function properly [101,109,110]. Similarly, neurotransmitters such as dopamine, endogenous opioids [102,111,112], and acetylcholine [113] have been associated with proficient executive functioning. To understand how sex differences might interact with cognition, it is essential to discuss the physiological variations occurring in neurotransmitter systems, particularly within the prefrontal cortex and striatum.
Previous research in animal models has highlighted innate differences in serotonin and dopamine levels in the prefrontal cortex between sexes [114], as well as different behavioral responses following excitation of nicotinic receptors [115]. Additionally, sex differences have been observed in the expression of dopaminergic receptors into the striatum [116]. In humans, fluoxetine, a selective serotonin reuptake inhibitor, has been shown to enhance attention in women but not in men [117]. From the perspective of cognitive functioning, and moving from the molecular level to the “raw” gray matter, it is important to acknowledge how sex differences in structural brain development may determine sex differences in executive functions. Indeed, during the early stages of brain development, there is a proliferation of neurons and synapses, followed by a process of synaptic pruning, in which less active neural connections are eliminated [118].
Sex differences in brain development, in terms of both timing and complexity, are evident, with variations observed in both cortical maturation [119] and neuronal structural complexity [120]. These variations may influence different cognitive and behavioral patterns in each sex. Still, microglia and astrocytes, which play a key role in synaptic pruning, interact with some components of the immune system, known as the complement system, including specific proteins such as C1q and C3 [121,122]. Sex differences have been shown in these components, which appear, moreover, sensitive to environmental factors including stress [123,124] and alcohol exposure [125].
In the field of cognitive neuroscience, the impact of sex hormones on both brain connectivity and cognitive abilities is widely recognized, exhibiting significant variations depending on age and sex. Specifically, in women, sex hormones may affect brain connectivity, especially during the natural aging process and menopause. This seems to have a detrimental effect on cognitive performance [126,127]. In men, elevated testosterone levels were associated with reduced performance in specific executive tasks involving domains like error monitoring and set-shifting [128]. This pattern of results highlights how sex differences may exert a selective impact on cognitive functions [129].
Focusing on the link binding sex differences and executive functions to obesity, the role played by the Central Autonomic Network (CAN) is likely pivotal. This network encompasses brain regions such as the insula and ventromedial prefrontal cortex, interacting with the cingulate cortex and sensorimotor areas. Such interactions exert a significant impact on both autonomic nervous system activation and central brain processing. Thus, the CAN’s neural activity influences cognition, with a particular focus on executive functions [100,130].
Intrinsic activity within the CAN shows variations between male and female individuals due to their respective metabolic and hormonal differences [129]. These variations affect reward sensitivity, potentially influencing eating behavior. Moreover, they may be further exacerbated by limited awareness of internal bodily signals (interoceptive awareness) and difficulties in impulse control [53,131]. The dynamics of these mechanisms, in analogy with Damasio’s Somatic Marker Hypothesis [132], suggest that eating behavior and weight control abilities may vary between sexes according to different levels of CAN activation. This would imply a difference in the ability to effectively evaluate the long-term consequences of dietary choices, with the potential for dysfunctional eating behaviors. However, it is important to note that this hypothesis requires further in-depth investigation. Despite our study’s results showing that female sex might be considered a “protective” factor in the context of weight-related cognitive issues, executive functions impacted body weight in both sexes. In this vein, research in the field of physiology has unveiled a potential mechanistic pathway involving metabolic, immune, and neurological systems [133,134,135].
It is well known that obesity is strongly linked to metabolic dysregulation [136]. The relationship between cognitive dysfunctions and body weight may involve the alteration of brain glucose metabolism and mitochondrial function in obese individuals [137,138]. In addition, these individuals demonstrate a higher uptake of brain fatty acids compared to their lean counterparts [139]. In this context, the impact of sex as a biological modifier is relevant. Indeed, sex differences have been found in glycolysis and mitochondrial metabolism [140,141].
Still, adipose tissue, through the production of cytokines such as interleukin (IL1ß, IL6), interferon γ (IFNγ), tumor necrosis factor α (TNFα), and monocyte chemoattractant protein 1 (MCP1), contributes to the establishment of a chronic low-grade inflammation [142,143,144,145,146]. This persistent state of inflammation exerts its influence on the blood–brain barrier, resulting in endothelial dysfunction, neuroinflammation, and heightened oxidative stress, all of which can culminate in cognitive deficits [147,148]. Given that the blood–brain barrier interfaces with the central nervous system, it constitutes a key element in bridging the gap between peripheral inflammation and weight-related cognitive blunting, potentially extending to executive functions.
It should not be overlooked that differences in the association between executive functions and body weight between men and women may arise from the interaction of environmental, cultural, and social factors. For instance, in Western cultures, pervasive societal ideals of thinness for women and muscularity for men may influence individuals’ perceptions of body image [149,150,151,152,153], potentially impacting executive functioning differently between sexes. Conversely, in many African societies, where larger body sizes are often favored in women [154,155,156], different outcomes in executive functions may be observed in the female sex due to greater body satisfaction in response to greater body fat.
Notwithstanding the aforementioned hypotheses, it is crucial to underscore that sex differences in the relationship between executive functions and body weight may be attributed to sex-related variability in strategies for task resolution and preferences for different outcomes rather than clear disparities in cognitive abilities between sexes [85]. In this regard, note that our assessment of executive functions exclusively relies on the FAB15. While this constitutes a neuropsychological battery, we cannot disregard the possibility that alternative measures, particularly domain-specific ones, may provide different insights, given the multifaceted nature of executive functions.
5. Conclusions
In this study, we found that WC may serve as a more effective body weight index than BMI in exploring the relationship between executive functions, sex, and body weight. Furthermore, our moderation model unveiled a significant modulation effect of sex. In particular, female participants, who scored higher in executive function assessments, exhibited a comparatively lower standardized WC than their male counterparts. This suggests that the relationship between executive function and WC is more pronounced in women than in men. These observations might be particularly relevant in the current scientific context, where the link between executive functions and obesity remains an area of active research and inquiry.
Author Contributions
Conceptualization, C.R.I., A.M. (Antonietta Monda), G.D.M. and M.L.M.; methodology, C.R.I. and M.L.M.; software, C.R.I. and W.S.; formal analysis, C.R.I., A.I., G.F. and M.L.M.; investigation, M.C. and V.A.; data curation, S.C. and A.M. (Antonietta Messina); writing—original draft preparation, C.R.I., A.M. (Antonietta Monda), G.D.M. and M.L.M.; writing—review and editing, V.M. and I.V.; supervision, M.S. and M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli” (protocol code 126380/2022-UNA2CLE-0126380 ID2354069 and date of approval 27 July 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Polito R., Scarinci A., Ambrosi A., Tartaglia N., Tafuri D., Monda M., Messina A., Cimmino F., Catapano A., Sessa F., et al. The Beneficial Effects of Physical Activity and Weight Loss on Human Colorectal Carcinoma Cell Lines. J. Hum. Sport Exerc. 2020;15:S252–S260. doi: 10.14198/jhse.2020.15.Proc2.16. [DOI] [Google Scholar]
- 2.The GBD 2015 Obesity Collaborators Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarısoy G., Kaçar Ö.F., Pazvantoğlu O., Öztürk A., Korkmaz I.Z., Kocamanoğlu B., Böke Ö., Sahin A.R. Temperament and Character Traits in Patients with Bipolar Disorder and Associations with Attempted Suicide. Compr. Psychiatry. 2012;53:1096–1102. doi: 10.1016/j.comppsych.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Hruby A., Hu F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelaal M., le Roux C.W., Docherty N.G. Morbidity and Mortality Associated with Obesity. Ann. Transl. Med. 2017;5:161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blüher M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 7.Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organ. Tech. Rep. Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 8.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma W., Zhang H., Wu N., Liu Y., Han P., Wang F., Wang J., Xie F., Niu S., Hu H., et al. Relationship between Obesity-Related Anthropometric Indicators and Cognitive Function in Chinese Suburb-Dwelling Older Adults. PLoS ONE. 2021;16:e0258922. doi: 10.1371/journal.pone.0258922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson D., Rothenberg E., Blennow K., Steen B., Skoog I. An 18-Year Follow-up of Overweight and Risk of Alzheimer Disease. Arch. Intern. Med. 2003;163:1524. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 11.Rosengren A. Body Mass Index, Other Cardiovascular Risk Factors, and Hospitalization for Dementia. Arch. Intern. Med. 2005;165:321. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Panza F., D’Introno A., Colacicco A.M., Capurso C., Pichichero G., Capurso S.A., Capurso A., Solfrizzi V. Lipid Metabolism in Cognitive Decline and Dementia. Brain Res. Rev. 2006;51:275–292. doi: 10.1016/j.brainresrev.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Whitmer R.A., Gustafson D.R., Barrett-Connor E., Haan M.N., Gunderson E.P., Yaffe K. Central Obesity and Increased Risk of Dementia More than Three Decades Later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 14.Beydoun M.A., Beydoun H.A., Wang Y. Obesity and Central Obesity as Risk Factors for Incident Dementia and Its Subtypes: A Systematic Review and Meta-Analysis. Obes. Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitmer R.A., Gunderson E.P., Barrett-Connor E., Quesenberry C.P., Yaffe K. Obesity in Middle Age and Future Risk of Dementia: A 27 Year Longitudinal Population Based Study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller A.A., Spencer S.J. Obesity and Neuroinflammation: A Pathway to Cognitive Impairment. Brain Behav. Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Bischof G.N., Park D.C. Obesity and Aging: Consequences for Cognition, Brain Structure, and Brain Function. Psychosom. Med. 2015;77:697–709. doi: 10.1097/PSY.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dye L., Boyle N.B., Champ C., Lawton C. The Relationship between Obesity and Cognitive Health and Decline. Proc. Nutr. Soc. 2017;76:443–454. doi: 10.1017/S0029665117002014. [DOI] [PubMed] [Google Scholar]
- 19.Leigh S.-J., Morris M.J. Diet, Inflammation and the Gut Microbiome: Mechanisms for Obesity-Associated Cognitive Impairment. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020;1866:165767. doi: 10.1016/j.bbadis.2020.165767. [DOI] [PubMed] [Google Scholar]
- 20.Chieffi S., Castaldi C., Di Maio G., La Marra M., Messina A., Monda V., Villano I. Attentional Bias in the Radial and Vertical Dimensions of Space. Comptes Rendus Biol. 2019;342:97–100. doi: 10.1016/j.crvi.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Ardila A. On the Evolutionary Origins of Executive Functions. Brain Cogn. 2008;68:92–99. doi: 10.1016/j.bandc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Burgess P.W., Simons J.S. Theories of Frontal Lobe Executive Function: Clinical Applications. In: Halligan P.W., Wade D.T., editors. The Effectiveness of Rehabilitation for Cognitive Deficits. Oxford University Press; Oxford, UK: 2005. pp. 211–231. [Google Scholar]
- 23.Gilbert S.J., Burgess P.W. Executive Function. Curr. Biol. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Diamond A. Executive Functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigue C., Ouellette A.-S., Lemieux S., Tchernof A., Biertho L., Bégin C. Executive Functioning and Psychological Symptoms in Food Addiction: A Study among Individuals with Severe Obesity. Eat. Weight. Disord. 2018;23:469–478. doi: 10.1007/s40519-018-0530-1. [DOI] [PubMed] [Google Scholar]
- 26.Sims R.C., Bennett N.-K., Mwendwa D.T., Ali M.K., Levy S.-A.T., Callender C.O., Campbell A.L. Executive Function and Negative Eating Behaviors in Severely Obese African Americans. Ethn. Dis. 2014;24:328–334. [PubMed] [Google Scholar]
- 27.Riggs N., Chou C.-P., Spruijt-Metz D., Pentz M.A. Executive Cognitive Function as a Correlate and Predictor of Child Food Intake and Physical Activity. Child Neuropsychol. 2010;16:279–292. doi: 10.1080/09297041003601488. [DOI] [PubMed] [Google Scholar]
- 28.Powell D.J.H., McMinn D., Allan J.L. Does Real Time Variability in Inhibitory Control Drive Snacking Behavior? An Intensive Longitudinal Study. Health Psychol. 2017;36:356–364. doi: 10.1037/hea0000471. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Shields G.S., Guo C., Liu Y. Executive Function Performance in Obesity and Overweight Individuals: A Meta-Analysis and Review. Neurosci. Biobehav. Rev. 2018;84:225–244. doi: 10.1016/j.neubiorev.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Dassen F.C.M., Houben K., Allom V., Jansen A. Self-Regulation and Obesity: The Role of Executive Function and Delay Discounting in the Prediction of Weight Loss. J. Behav. Med. 2018;41:806–818. doi: 10.1007/s10865-018-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polito R., Francavilla V.C., Ambrosi A., Tartaglia N., Tafuri D., Monda M., Messina A., Sessa F., Di Maio G., Ametta A., et al. The Orexin-A Serum Levels Are Strongly Modulated by Physical Activity Intervention in Diabetes Mellitus Patients. J. Hum. Sport Exerc. 2020;15:244–251. [Google Scholar]
- 32.Ribeiro O., Carmo I.D., Paiva T., Figueira M.L. Neuropsychological Profile, Cognitive Reserve and Emotional Distress in a Portuguese Sample of Severely Obese Patients. Acta Med. Port. 2020;33:38. doi: 10.20344/amp.12233. [DOI] [PubMed] [Google Scholar]
- 33.Gluck M.E., Viswanath P., Stinson E.J. Obesity, Appetite, and the Prefrontal Cortex. Curr. Obes. Rep. 2017;6:380–388. doi: 10.1007/s13679-017-0289-0. [DOI] [PubMed] [Google Scholar]
- 34.Horstmann A., Busse F.P., Mathar D., Müller K., Lepsien J., Schlögl H., Kabisch S., Kratzsch J., Neumann J., Stumvoll M., et al. Obesity-Related Differences between Women and Men in Brain Structure and Goal-Directed Behavior. Front. Hum. Neurosci. 2011;5:58. doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alarcón G., Ray S., Nagel B.J. Lower Working Memory Performance in Overweight and Obese Adolescents Is Mediated by White Matter Microstructure. J. Int. Neuropsychol. Soc. 2016;22:281–292. doi: 10.1017/S1355617715001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balleine B.W., Delgado M.R., Hikosaka O. The Role of the Dorsal Striatum in Reward and Decision-Making: Figure 1. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander G.E., Crutcher M.D. Functional Architecture of Basal Ganglia Circuits: Neural Substrates of Parallel Processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- 38.Cho Y.T., Moujaes F., Schleifer C.H., Starc M., Ji J.L., Santamauro N., Adkinson B., Kolobaric A., Flynn M., Krystal J.H., et al. Reward and Loss Incentives Improve Spatial Working Memory by Shaping Trial-by-Trial Posterior Frontoparietal Signals. NeuroImage. 2022;254:119139. doi: 10.1016/j.neuroimage.2022.119139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassenstab J.J., Sweet L.H., Del Parigi A., McCaffery J.M., Haley A.P., Demos K.E., Cohen R.A., Wing R.R. Cortical Thickness of the Cognitive Control Network in Obesity and Successful Weight Loss Maintenance: A Preliminary MRI Study. Psychiatry Res. Neuroimaging. 2012;202:77–79. doi: 10.1016/j.pscychresns.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pannacciulli N., Del Parigi A., Chen K., Le D.S.N.T., Reiman E.M., Tataranni P.A. Brain Abnormalities in Human Obesity: A Voxel-Based Morphometric Study. NeuroImage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 41.Shott M.E., Cornier M.-A., Mittal V.A., Pryor T.L., Orr J.M., Brown M.S., Frank G.K.W. Orbitofrontal Cortex Volume and Brain Reward Response in Obesity. Int. J. Obes. 2015;39:214–221. doi: 10.1038/ijo.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishinevsky F.I., Cox J.E., Murdaugh D.L., Stoeckel L.E., Cook E.W., Weller R.E. fMRI Reactivity on a Delay Discounting Task Predicts Weight Gain in Obese Women. Appetite. 2012;58:582–592. doi: 10.1016/j.appet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Artham S.M., Lavie C.J., Milani R.V., Ventura H.O. The Obesity Paradox: Impact of Obesity on the Prevalence and Prognosis of Cardiovascular Diseases. Postgrad. Med. 2008;120:34–41. doi: 10.3810/pgm.2008.07.1788. [DOI] [PubMed] [Google Scholar]
- 44.Childers D.K., Allison D.B. The ‘Obesity Paradox’: A Parsimonious Explanation for Relations among Obesity, Mortality Rate and Aging? Int. J. Obes. 2010;34:1231–1238. doi: 10.1038/ijo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flegal K.M., Kit B.K., Orpana H., Graubard B.I. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-Analysis. JAMA. 2013;309:71. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzpatrick S., Gilbert S., Serpell L. Systematic Review: Are Overweight and Obese Individuals Impaired on Behavioural Tasks of Executive Functioning? Neuropsychol. Rev. 2013;23:138–156. doi: 10.1007/s11065-013-9224-7. [DOI] [PubMed] [Google Scholar]
- 47.Boeka A., Lokken K. Neuropsychological Performance of a Clinical Sample of Extremely Obese Individuals. Arch. Clin. Neuropsychol. 2008;23:467–474. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Gunstad J., Lhotsky A., Wendell C.R., Ferrucci L., Zonderman A.B. Longitudinal Examination of Obesity and Cognitive Function: Results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartanto A., Yong J., Toh W. Bidirectional Associations between Obesity and Cognitive Function in Midlife Adults: A Longitudinal Study. Nutrients. 2019;11:2343. doi: 10.3390/nu11102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skinner J.S., Abel W.M., McCoy K., Wilkins C.H. Exploring the “Obesity Paradox” as a Correlate of Cognitive and Physical Function in Community-Dwelling Black and White Older Adults. Ethn. Dis. 2017;27:387. doi: 10.18865/ed.27.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkow N.D., Wang G.-J., Fowler J.S., Telang F. Overlapping Neuronal Circuits in Addiction and Obesity: Evidence of Systems Pathology. Phil. Trans. R. Soc. B. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monica D., Paulo M., Appolinário J.C., de Freitas S.R., Coutinho G., Santos C., Coutinho W. Assessment of Executive Functions in Obese Individuals with Binge Eating Disorder. Rev. Bras. Psiquiatr. 2010;32:381–388. doi: 10.1590/S1516-44462010000400011. [DOI] [PubMed] [Google Scholar]
- 53.Favieri F., Forte G., Casagrande M. The Executive Functions in Overweight and Obesity: A Systematic Review of Neuropsychological Cross-Sectional and Longitudinal Studies. Front. Psychol. 2019;10:2126. doi: 10.3389/fpsyg.2019.02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Marra M., Ilardi C.R., Villano I., Carosella M., Staiano M., Iavarone A., Chieffi S., Messina G., Polito R., Scarinci A., et al. Functional Relationship between Inhibitory Control, Cognitive Flexibility, Psychomotor Speed and Obesity. Brain Sci. 2022;12:1080. doi: 10.3390/brainsci12081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La Marra M., Villano I., Ilardi C.R., Carosella M., Staiano M., Iavarone A., Chieffi S., Messina G., Polito R., Porro C., et al. Executive Functions in Overweight and Obese Treatment-Seeking Patients: Cross-Sectional Data and Longitudinal Perspectives. Brain Sci. 2022;12:777. doi: 10.3390/brainsci12060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens J., McClain J.E., Truesdale K.P. Selection of Measures in Epidemiologic Studies of the Consequences of Obesity. Int. J. Obes. 2008;32:S60–S66. doi: 10.1038/ijo.2008.88. [DOI] [PubMed] [Google Scholar]
- 57.Gallagher D., Visser M., Sepulveda D., Pierson R.N., Harris T., Heymsfield S.B. How Useful Is Body Mass Index for Comparison of Body Fatness across Age, Sex, and Ethnic Groups? Am. J. Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 58.Rothman K.J. BMI-Related Errors in the Measurement of Obesity. Int. J. Obes. 2008;32:S56–S59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 59.Alves Junior C.A., Mocellin M.C., Gonçalves E.C.A., Silva D.A., Trindade E.B. Anthropometric Indicators as Body Fat Discriminators in Children and Adolescents: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017;8:718–727. doi: 10.3945/an.117.015446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villano I., La Marra M., Messina A., Di Maio G., Moscatelli F., Chieffi S., Monda M., Messina G., Monda V. Effects of Vegetarian and Vegan Nutrition on Body Composition in Competitive Futsal Athletes. Prog. Nutr. 2021;23:e2021126. doi: 10.23751/pn.v23i2.11366. [DOI] [Google Scholar]
- 61.Zhu S., Heymsfield S.B., Toyoshima H., Wang Z., Pietrobelli A., Heshka S. Race-Ethnicity–Specific Waist Circumference Cutoffs for Identifying Cardiovascular Disease Risk Factors. Am. J. Clin. Nutr. 2005;81:409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 62.Janssen I., Katzmarzyk P.T., Ross R. Waist Circumference and Not Body Mass Index Explains Obesity-Related Health Risk. Am. J. Clin. Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 63.Price G.M., Uauy R., Breeze E., Bulpitt C.J., Fletcher A.E. Weight, Shape, and Mortality Risk in Older Persons: Elevated Waist-Hip Ratio, Not High Body Mass Index, Is Associated with a Greater Risk of Death. Am. J. Clin. Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.2.449. [DOI] [PubMed] [Google Scholar]
- 64.Stival C., Lugo A., Odone A., van den Brandt P.A., Fernandez E., Tigova O., Soriano J.B., José López M., Scaglioni S., Gallus S., et al. Prevalence and Correlates of Overweight and Obesity in 12 European Countries in 2017–2018. Obes. Facts. 2022;15:655–665. doi: 10.1159/000525792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Restrepo B.J. Obesity Prevalence Among U.S. Adults During the COVID-19 Pandemic. Am. J. Prev. Med. 2022;63:102–106. doi: 10.1016/j.amepre.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lartey S.T., Magnussen C.G., Si L., Boateng G.O., de Graaff B., Biritwum R.B., Minicuci N., Kowal P., Blizzard L., Palmer A.J. Rapidly Increasing Prevalence of Overweight and Obesity in Older Ghanaian Adults from 2007–2015: Evidence from WHO-SAGE Waves 1 & 2. PLoS ONE. 2019;14:e0215045. doi: 10.1371/journal.pone.0215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lange E.H., Nesvåg R., Ringen P.A., Hartberg C.B., Haukvik U.K., Andreassen O.A., Melle I., Agartz I. One Year Follow-up of Alcohol and Illicit Substance Use in First-Episode Psychosis: Does Gender Matter? Compr. Psychiatry. 2014;55:274–282. doi: 10.1016/j.comppsych.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 68.Bezdjian S., Baker L.A., Lozano D.I., Raine A. Assessing Inattention and Impulsivity in Children during the Go/NoGo Task. Br. J. Dev. Psychol. 2009;27:365–383. doi: 10.1348/026151008X314919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ilardi C.R., Chieffi S., Scuotto C., Gamboz N., Galeone F., Sannino M., Garofalo E., La Marra M., Ronga B., Iavarone A. The Frontal Assessment Battery 20 Years Later: Normative Data for a Shortened Version (FAB15) Neurol. Sci. 2022;43:1709–1719. doi: 10.1007/s10072-021-05544-0. [DOI] [PubMed] [Google Scholar]
- 70.Giambra L.M., Quilter R.E. Sex Differences in Sustained Attention across the Adult Life Span. J. Appl. Psychol. 1989;74:91–95. doi: 10.1037/0021-9010.74.1.91. [DOI] [PubMed] [Google Scholar]
- 71.Riley N., Lubans D.R., Holmes K., Morgan P.J. Findings From the EASY Minds Cluster Randomized Controlled Trial: Evaluation of a Physical Activity Integration Program for Mathematics in Primary Schools. J. Phys. Act. Health. 2016;13:198–206. doi: 10.1123/jpah.2015-0046. [DOI] [PubMed] [Google Scholar]
- 72.Barnett J.H., Jones P.B., Robbins T.W., Müller U. Effects of the Catechol-O-Methyltransferase Val158Met Polymorphism on Executive Function: A Meta-Analysis of the Wisconsin Card Sort Test in Schizophrenia and Healthy Controls. Mol. Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- 73.Muscogiuri G., Verde L., Vetrani C., Barrea L., Savastano S., Colao A. Obesity: A Gender-View. J. Endocrinol. Investig. 2023;47:299–306. doi: 10.1007/s40618-023-02196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conners C.K., Epstein J.N., Angold A., Klaric J. Continuous Performance Test Performance in a Normative Epidemiological Sample. J. Abnorm. Child. Psychol. 2003;31:555–562. doi: 10.1023/A:1025457300409. [DOI] [PubMed] [Google Scholar]
- 75.Naglieri J.A., Rojahn J. Gender Differences in Planning, Attention, Simultaneous, and Successive (PASS) Cognitive Processes and Achievement. J. Educ. Psychol. 2001;93:430–437. doi: 10.1037/0022-0663.93.2.430. [DOI] [Google Scholar]
- 76.Bardos A.N., Naglieri J.A., Prewett P.N. Gender Differences on Planning, Attention, Simultaneous, and Successive Cognitive Processing Tasks. J. Sch. Psychol. 1992;30:293–305. doi: 10.1016/0022-4405(92)90012-T. [DOI] [Google Scholar]
- 77.Warrick P.D., Naglieri J.A. Gender Differences in Planning, Attention, Simultaneous, and Successive (PASS) Cognitive Processes. J. Educ. Psychol. 1993;85:693–701. doi: 10.1037/0022-0663.85.4.693. [DOI] [Google Scholar]
- 78.Overman W.H., Pierce A. Iowa Gambling Task with Non-Clinical Participants: Effects of Using Real + Virtual Cards and Additional Trials. Front. Psychol. 2013;4:64997. doi: 10.3389/fpsyg.2013.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Den Bos R., Homberg J., De Visser L. A Critical Review of Sex Differences in Decision-Making Tasks: Focus on the Iowa Gambling Task. Behav. Brain Res. 2013;238:95–108. doi: 10.1016/j.bbr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Reed J.L., Gallagher N.M., Sullivan M., Callicott J.H., Green A.E. Sex Differences in Verbal Working Memory Performance Emerge at Very High Loads of Common Neuroimaging Tasks. Brain Cogn. 2017;113:56–64. doi: 10.1016/j.bandc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Rahman Q., Abrahams S., Jussab F. Sex Differences in a Human Analogue of the Radial Arm Maze: The “17-Box Maze Test”. Brain Cogn. 2005;58:312–317. doi: 10.1016/j.bandc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Duff S.J., Hampson E. A Sex Difference on a Novel Spatial Working Memory Task in Humans. Brain Cogn. 2001;47:470–493. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- 83.Lejbak L., Vrbancic M., Crossley M. The Female Advantage in Object Location Memory Is Robust to Verbalizability and Mode of Presentation of Test Stimuli. Brain Cogn. 2009;69:148–153. doi: 10.1016/j.bandc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Gaillard A., Fehring D.J., Rossell S.L. A Systematic Review and Meta-analysis of Behavioural Sex Differences in Executive Control. Eur. J. Neurosci. 2021;53:519–542. doi: 10.1111/ejn.14946. [DOI] [PubMed] [Google Scholar]
- 85.Grissom N.M., Reyes T.M. Let’s Call the Whole Thing off: Evaluating Gender and Sex Differences in Executive Function. Neuropsychopharmacology. 2019;44:86–96. doi: 10.1038/s41386-018-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbruggen F., Logan G.D. Response Inhibition in the Stop-Signal Paradigm. Trends Cogn. Sci. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cahill L. Why Sex Matters for Neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 88.Cummings J.L. Frontal-Subcortical Circuits and Human Behavior. Arch. Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 89.Miller E.K., Cohen J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 90.Bonté E., Flemming T., Fagot J. Executive Control of Perceptual Features and Abstract Relations by Baboons (Papio Papio) Behav. Brain Res. 2011;222:176–182. doi: 10.1016/j.bbr.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 91.Mansouri F.A., Tanaka K., Buckley M.J. Conflict-Induced Behavioural Adjustment: A Clue to the Executive Functions of the Prefrontal Cortex. Nat. Rev. Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 92.Ilardi C.R., La Marra M., Amato R., Di Cecca A., Di Maio G., Ciccarelli G., Migliaccio M., Cavaliere C., Federico G. The “Little Circles Test” (LCT): A Dusted-off Tool for Assessing Fine Visuomotor Function. Aging Clin. Exp. Res. 2023;35:2807–2820. doi: 10.1007/s40520-023-02571-z. [DOI] [PubMed] [Google Scholar]
- 93.Measso G., Cavarzeran F., Zappalà G., Lebowitz B.D., Crook T.H., Pirozzolo F.J., Amaducci L.A., Massari D., Grigoletto F. The Mini-mental State Examination: Normative Study of an Italian Random Sample. Dev. Neuropsychol. 1993;9:77–85. doi: 10.1080/87565649109540545. [DOI] [Google Scholar]
- 94.Folstein M.F., Folstein S.E., McHugh P.R. Mini-Mental State. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 95.La Marra M., Ilardi C.R., Villano I., Polito R., Sibillo M.R., Franchetti M., Caggiano A., Strangio F., Messina G., Monda V., et al. Higher General Executive Functions Predicts Lower Body Mass Index by Mitigating Avoidance Behaviors. Front. Endocrinol. 2022;13:1048363. doi: 10.3389/fendo.2022.1048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.La Marra M., Messina A., Ilardi C.R., Verde G., Amato R., Esposito N., Troise S., Orlando A., Messina G., Monda V., et al. The Neglected Factor in the Relationship between Executive Functioning and Obesity: The Role of Motor Control. Healthcare. 2022;10:1775. doi: 10.3390/healthcare10091775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vainik U., Dagher A., Dubé L., Fellows L.K. Neurobehavioural Correlates of Body Mass Index and Eating Behaviours in Adults: A Systematic Review. Neurosci. Biobehav. Rev. 2013;37:279–299. doi: 10.1016/j.neubiorev.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Emery R.L., Levine M.D. Questionnaire and Behavioral Task Measures of Impulsivity Are Differentially Associated with Body Mass Index: A Comprehensive Meta-Analysis. Psychol. Bull. 2017;143:868–902. doi: 10.1037/bul0000105. [DOI] [PubMed] [Google Scholar]
- 99.Gettens K.M., Gorin A.A. Executive Function in Weight Loss and Weight Loss Maintenance: A Conceptual Review and Novel Neuropsychological Model of Weight Control. J. Behav. Med. 2017;40:687–701. doi: 10.1007/s10865-017-9831-5. [DOI] [PubMed] [Google Scholar]
- 100.Favieri F., Forte G., Pazzaglia M., Chen E.Y., Casagrande M. High-Level Executive Functions: A Possible Role of Sex and Weight Condition in Planning and Decision-Making Performances. Brain Sci. 2022;12:149. doi: 10.3390/brainsci12020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jupp B., Caprioli D., Saigal N., Reverte I., Shrestha S., Cumming P., Everitt B.J., Robbins T.W., Dalley J.W. Dopaminergic and GABA -ergic Markers of Impulsivity in Rats: Evidence for Anatomical Localisation in Ventral Striatum and Prefrontal Cortex. Eur. J. Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- 102.Selleck R.A., Lake C., Estrada V., Riederer J., Andrzejewski M., Sadeghian K., Baldo B.A. Endogenous Opioid Signaling in the Medial Prefrontal Cortex Is Required for the Expression of Hunger-Induced Impulsive Action. Neuropsychopharmacology. 2015;40:2464–2474. doi: 10.1038/npp.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feja M., Koch M. Ventral Medial Prefrontal Cortex Inactivation Impairs Impulse Control but Does Not Affect Delay-Discounting in Rats. Behav. Brain Res. 2014;264:230–239. doi: 10.1016/j.bbr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Chieffi S., Messina G., La Marra M., Iavarone A., Viggiano A., De Luca V., Monda M. Horizons in Neuroscience Research. Volume 13. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2014. Distractor Interference in Visual Motor Tasks; pp. 151–160. [Google Scholar]
- 105.Ilardi C.R., Di Maio G., Villano I., Messina G., Monda V., Messina A., Porro C., Panaro M.A., Gamboz N., Iavarone A., et al. The Assessment of Executive Functions to Test the Integrity of the Nigrostriatal Network: A Pilot Study. Front. Psychol. 2023;14:1121251. doi: 10.3389/fpsyg.2023.1121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yates J.R., Darna M., Beckmann J.S., Dwoskin L.P., Bardo M.T. Individual Differences in Impulsive Action and Dopamine Transporter Function in Rat Orbitofrontal Cortex. Neuroscience. 2016;313:122–129. doi: 10.1016/j.neuroscience.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tessitore A., Giordano A., Russo A., Tedeschi G. Structural Connectivity in Parkinson’s Disease. Park. Relat. Disord. 2016;22:S56–S59. doi: 10.1016/j.parkreldis.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 108.Benn A., Robinson E.S.J. Investigating Glutamatergic Mechanism in Attention and Impulse Control Using Rats in a Modified 5-Choice Serial Reaction Time Task. PLoS ONE. 2014;9:e115374. doi: 10.1371/journal.pone.0115374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murphy-Royal C., Dupuis J., Groc L., Oliet S.H.R. Astroglial Glutamate Transporters in the Brain: Regulating Neurotransmitter Homeostasis and Synaptic Transmission. J. Neurosci. Res. 2017;95:2140–2151. doi: 10.1002/jnr.24029. [DOI] [PubMed] [Google Scholar]
- 110.Di Maio G., Villano I., Ilardi C.R., Messina A., Monda V., Iodice A.C., Porro C., Panaro M.A., Chieffi S., Messina G., et al. Mechanisms of Transmission and Processing of Pain: A Narrative Review. Int. J. Environ. Res. Public Health. 2023;20:3064. doi: 10.3390/ijerph20043064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.D’Amour-Horvat V., Leyton M. Impulsive Actions and Choices in Laboratory Animals and Humans: Effects of High vs. Low Dopamine States Produced by Systemic Treatments given to Neurologically Intact Subjects. Front. Behav. Neurosci. 2014;8:432. doi: 10.3389/fnbeh.2014.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez-Roige S., Ripley T.L., Stephens D.N. Alleviating Waiting Impulsivity and Perseverative Responding by μ-Opioid Receptor Antagonism in Two Inbred Mouse Strains. Psychopharmacology. 2015;232:1483–1492. doi: 10.1007/s00213-014-3786-9. [DOI] [PubMed] [Google Scholar]
- 113.Kolisnyk B., Al-Onaizi M.A., Hirata P.H.F., Guzman M.S., Nikolova S., Barbash S., Soreq H., Bartha R., Prado M.A.M., Prado V.F. Forebrain Deletion of the Vesicular Acetylcholine Transporter Results in Deficits in Executive Function, Metabolic, and RNA Splicing Abnormalities in the Prefrontal Cortex. J. Neurosci. 2013;33:14908–14920. doi: 10.1523/JNEUROSCI.1933-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Staiti A.M., Morgane P.J., Galler J.R., Grivetti J.Y., Bass D.C., Mokler D.J. A Microdialysis Study of the Medial Prefrontal Cortex of Adolescent and Adult Rats. Neuropharmacology. 2011;61:544–549. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alves N.C., Bailey C.D.C., Nashmi R., Lambe E.K. Developmental Sex Differences in Nicotinic Currents of Prefrontal Layer VI Neurons in Mice and Rats. PLoS ONE. 2010;5:e9261. doi: 10.1371/journal.pone.0009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andersen S.L., Teicher M.H. Sex Differences in Dopamine Receptors and Their Relevance to ADHD. Neurosci. Biobehav. Rev. 2000;24:137–141. doi: 10.1016/S0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 117.LaRoche R.B., Morgan R.E. Adolescent Fluoxetine Exposure Produces Enduring, Sex-Specific Alterations of Visual Discrimination and Attention in Rats. Neurotoxicol. Teratol. 2007;29:96–107. doi: 10.1016/j.ntt.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Kolb B., Mychasiuk R., Muhammad A., Li Y., Frost D.O., Gibb R. Experience and the Developing Prefrontal Cortex. Proc. Natl. Acad. Sci. USA. 2012;109:17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGrath J., Saha S., Welham J., El Saadi O., MacCauley C., Chant D. A Systematic Review of the Incidence of Schizophrenia: The Distribution of Rates and the Influence of Sex, Urbanicity, Migrant Status and Methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu G., Qi F., Shen D. Learning-Based Deformable Registration of MR Brain Images. IEEE Trans. Med. Imaging. 2006;25:1145–1157. doi: 10.1109/TMI.2006.879320. [DOI] [PubMed] [Google Scholar]
- 121.Villano I., La Marra M., Di Maio G., Monda V., Chieffi S., Guatteo E., Messina G., Moscatelli F., Monda M., Messina A. Physiological Role of Orexinergic System for Health. Int. J. Environ. Res. Public Health. 2022;19:8353. doi: 10.3390/ijerph19148353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 123.Bollinger J.L., Bergeon Burns C.M., Wellman C.L. Differential Effects of Stress on Microglial Cell Activation in Male and Female Medial Prefrontal Cortex. Brain Behav. Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bollinger J.L., Collins K.E., Patel R., Wellman C.L. Behavioral Stress Alters Corticolimbic Microglia in a Sex- and Brain Region-Specific Manner. PLoS ONE. 2017;12:e0187631. doi: 10.1371/journal.pone.0187631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barton E., Baker C., Leasure J. Investigation of Sex Differences in the Microglial Response to Binge Ethanol and Exercise. Brain Sci. 2017;7:139. doi: 10.3390/brainsci7100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gurvich C., Le J., Thomas N., Thomas E.H.X., Kulkarni J. Vitamins and Hormones. Volume 115. Elsevier; Amsterdam, The Netherlands: 2021. Sex Hormones and Cognition in Aging; pp. 511–533. [DOI] [PubMed] [Google Scholar]
- 127.Sultana F., Davis S.R., Murray A.M., Woods R.L., McNeil J.J., Islam R.M. Sex Hormones, SHBG and Cognitive Performance among Older Australian Women: An Observational Study. Climacteric. 2023;26:121–128. doi: 10.1080/13697137.2023.2166824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen T.-V., Lew J., Albaugh M.D., Botteron K.N., Hudziak J.J., Fonov V.S., Collins D.L., Ducharme S., McCracken J.T. Sex-Specific Associations of Testosterone with Prefrontal-Hippocampal Development and Executive Function. Psychoneuroendocrinology. 2017;76:206–217. doi: 10.1016/j.psyneuen.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sie J.-H., Chen Y.-H., Shiau Y.-H., Chu W.-C. Gender- and Age-Specific Differences in Resting-State Functional Connectivity of the Central Autonomic Network in Adulthood. Front. Hum. Neurosci. 2019;13:369. doi: 10.3389/fnhum.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Derntl B., Pintzinger N., Kryspin-Exner I., Schöpf V. The Impact of Sex Hormone Concentrations on Decision-Making in Females and Males. Front. Neurosci. 2014;8:111184. doi: 10.3389/fnins.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rotge J.-Y., Poitou C., Fossati P., Aron-Wisnewsky J., Oppert J.-M. Decision-making in Obesity without Eating Disorders: A Systematic Review and Meta-analysis of Iowa Gambling Task Performances. Obes. Rev. 2017;18:936–942. doi: 10.1111/obr.12549. [DOI] [PubMed] [Google Scholar]
- 132.Damasio A.R. On Some Functions of the Human Prefrontal Cortexa. Ann. N. Y. Acad. Sci. 1995;769:241–252. doi: 10.1111/j.1749-6632.1995.tb38142.x. [DOI] [PubMed] [Google Scholar]
- 133.Villano I., La Marra M., Allocca S., Ilardi C.R., Polito R., Porro C., Chieffi S., Messina G., Monda V., Di Maio G., et al. The Role of Nutraceutical Supplements, Monacolin K and Astaxanthin, and Diet in Blood Cholesterol Homeostasis in Patients with Myopathy. Biomolecules. 2022;12:1118. doi: 10.3390/biom12081118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villano I., Ilardi C.R., Arena S., Scuotto C., Gleijeses M.G., Messina G., Messina A., Monda V., Monda M., Iavarone A., et al. Obese Subjects without Eating Disorders Experience Binge Episodes Also Independently of Emotional Eating and Personality Traits among University Students of Southern Italy. Brain Sci. 2021;11:1145. doi: 10.3390/brainsci11091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.La Marra M., Messina A., Ilardi C.R., Staiano M., Di Maio G., Messina G., Polito R., Valenzano A., Cibelli G., Monda V., et al. Factorial Model of Obese Adolescents: The Role of Body Image Concerns and Selective Depersonalization—A Pilot Study. Int. J. Environ. Res. Public Health. 2022;19:11501. doi: 10.3390/ijerph191811501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Val-Laillet D., Layec S., Guérin S., Meurice P., Malbert C. Changes in Brain Activity After a Diet-Induced Obesity. Obesity. 2011;19:749–756. doi: 10.1038/oby.2010.292. [DOI] [PubMed] [Google Scholar]
- 137.Morella I.M., Brambilla R., Morè L. Emerging Roles of Brain Metabolism in Cognitive Impairment and Neuropsychiatric Disorders. Neurosci. Biobehav. Rev. 2022;142:104892. doi: 10.1016/j.neubiorev.2022.104892. [DOI] [PubMed] [Google Scholar]
- 138.Iozzo P., Guzzardi M.A. Imaging of Brain Glucose Uptake by PET in Obesity and Cognitive Dysfunction: Life-Course Perspective. Endocr. Connect. 2019;8:R169–R183. doi: 10.1530/EC-19-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rebelos E., Hirvonen J., Bucci M., Pekkarinen L., Nyman M., Hannukainen J.C., Iozzo P., Salminen P., Nummenmaa L., Ferrannini E., et al. Brain Free Fatty Acid Uptake Is Elevated in Morbid Obesity, and Is Irreversible 6 Months after Bariatric Surgery: A Positron Emission Tomography Study. Diabetes Obes. Metab. 2020;22:1074–1082. doi: 10.1111/dom.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee J.W., Profant M., Wang C. Metabolic Sex Dimorphism of the Brain at the Gene, Cell, and Tissue Level. J. Immunol. 2022;208:212–220. doi: 10.4049/jimmunol.2100853. [DOI] [PubMed] [Google Scholar]
- 141.Gaignard P., Fréchou M., Liere P., Thérond P., Schumacher M., Slama A., Guennoun R. Sex Differences in Brain Mitochondrial Metabolism: Influence of Endogenous Steroids and Stroke. J. Neuroendocrinol. 2018;30:e12497. doi: 10.1111/jne.12497. [DOI] [PubMed] [Google Scholar]
- 142.Francavilla V.C., Genovesi F., Asmundo A., Di Nunno N.R., Ambrosi A., Tartaglia N., Tafuri D., Monda V., Monda M., Messina A., et al. Fascia and Movement: The Primary Link in the Prevention of Accidents in Soccer. Revision and Models of Intervention. Med. Sport. 2020;73:291–301. doi: 10.23736/S0025-7826.20.03677-7. [DOI] [Google Scholar]
- 143.Murray P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 144.Guillemot-Legris O., Muccioli G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di Maio G., Alessio N., Demirsoy I.H., Peluso G., Perrotta S., Monda M., Di Bernardo G. Evaluation of Browning Agents on the White Adipogenesis of Bone Marrow Mesenchymal Stromal Cells: A Contribution to Fighting Obesity. Cells. 2021;10:403. doi: 10.3390/cells10020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castanon N., Luheshi G., Layé S. Role of Neuroinflammation in the Emotional and Cognitive Alterations Displayed by Animal Models of Obesity. Front. Neurosci. 2015;9:146137. doi: 10.3389/fnins.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tucsek Z., Toth P., Tarantini S., Sosnowska D., Gautam T., Warrington J.P., Giles C.B., Wren J.D., Koller A., Ballabh P., et al. Aging Exacerbates Obesity-Induced Cerebromicrovascular Rarefaction, Neurovascular Uncoupling, and Cognitive Decline in Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mask L., Blanchard C.M. The Protective Role of General Self-Determination against ‘Thin Ideal’ Media Exposure on Women’s Body Image and Eating-Related Concerns. J. Health Psychol. 2011;16:489–499. doi: 10.1177/1359105310385367. [DOI] [PubMed] [Google Scholar]
- 150.Groesz L.M., Levine M.P., Murnen S.K. The Effect of Experimental Presentation of Thin Media Images on Body Satisfaction: A Meta-analytic Review. Int. J. Eat. Disord. 2002;31:1–16. doi: 10.1002/eat.10005. [DOI] [PubMed] [Google Scholar]
- 151.Posavac S.S., Posavac H.D. Predictors of Women’s Concern with Body Weight: The Roles of Perceived Self-Media Ideal Discrepancies and Self-Esteem. Eat. Disord. 2002;10:153–160. doi: 10.1002/erv.462. [DOI] [PubMed] [Google Scholar]
- 152.Sands E.R., Wardle J. Internalization of Ideal Body Shapes in 9–12-year-old Girls. Int. J. Eat. Disord. 2003;33:193–204. doi: 10.1002/eat.10121. [DOI] [PubMed] [Google Scholar]
- 153.Ahern A.L., Bennett K.M., Kelly M., Hetherington M.M. A Qualitative Exploration of Young Women’s Attitudes towards the Thin Ideal. J. Health Psychol. 2011;16:70–79. doi: 10.1177/1359105310367690. [DOI] [PubMed] [Google Scholar]
- 154.Pradeilles R., Holdsworth M., Olaitan O., Irache A., Osei-Kwasi H.A., Ngandu C.B., Cohen E. Body Size Preferences for Women and Adolescent Girls Living in Africa: A Mixed-Methods Systematic Review. Public Health Nutr. 2022;25:738–759. doi: 10.1017/S1368980021000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rguibi M., Belahsen R. Body Size Preferences and Sociocultural Influences on Attitudes towards Obesity among Moroccan Sahraoui Women. Body Image. 2006;3:395–400. doi: 10.1016/j.bodyim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 156.Rguibi M., Belahsen R. Fattening Practices among Moroccan Saharawi Women. East. Mediterr. Health J. 2006;12:619–624. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.