Abstract
Background
Whether physical activity could mitigate the adverse impacts of sugar-sweetened beverages (SSBs) or artificially sweetened beverages (ASBs) on incident cardiovascular disease (CVD) remains uncertain.
Objectives
This study aimed to examine the independent and joint associations between SSB or ASB consumption and physical activity and risk of CVD, defined as fatal and nonfatal coronary artery disease and stroke, in adults from 2 United States-based prospective cohort studies.
Methods
Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% CIs between SSB or ASB intake and physical activity with incident CVD among 65,730 females in the Nurses’ Health Study (1980–2016) and 39,418 males in the Health Professional’s Follow-up Study (1986–2016), who were free from chronic diseases at baseline. SSBs and ASBs were assessed every 4-y and physical activity biannually.
Results
A total of 13,269 CVD events were ascertained during 3,001,213 person-years of follow-up. Compared with those who never/rarely consumed SSBs or ASBs, the HR for CVD for participants consuming ≥2 servings/d was 1.21 (95% CI: 1.12, 1.32; P-trend < 0.001) for SSBs and 1.03 (95% CI: 0.97, 1.09; P-trend = 0.06) for those consuming ≥2 servings/d of ASBs. The HR for CVD per 1 serving increment of SSB per day was 1.18 (95% CI: 1.10, 1.26) and 1.12 (95% CI: 1.04, 1.20) for participants meeting and not meeting physical activity guidelines (≥7.5 compared with <7.5 MET h/wk), respectively. Compared with participants who met physical activity guidelines and never/rarely consumed SSBs, the HR for CVD was 1.47 (95% CI: 1.37, 1.57) for participants not meeting physical activity guidelines and consuming ≥2 servings/wk of SSBs. No significant associations were observed for ASB when stratified by physical activity.
Conclusions
Higher SSB intake was associated with CVD risk regardless of physical activity levels. These results support current recommendations to limit the intake of SSBs even for physically active individuals.
Keywords: coronary heart disease, stroke, soda, diet soda, public health, exercise
Introduction
Sugar-sweetened beverages (SSBs) have a detrimental impact on health [[1], [2], [3], [4], [5]] and are associated with a higher risk of cardiovascular disease (CVD) [1,[6], [7], [8], [9], [10], [11]], the leading cause of mortality worldwide [12]. The underlying biological mechanism by which SSB intake is associated with an increased risk of CVD includes not only their capacity to induce weight gain but also the high amounts of quickly absorbable carbohydrates (i.e., sugar or high-fructose corn syrup), contributing to an increase in blood glucose and insulin levels and thereby glycemic load [13]. This process exacerbates inflammatory biomarkers and overall inflammation, which are linked to atherosclerosis, ultimately leading to risk of CVD [13].
Although there has been a nationwide decline in SSB consumption since 2000 [14], they remain the single largest source of calories and added sugar in the American diet [15]. In contrast to SSBs, the intake of artificially sweetened beverages (ASBs) has increased in the United States [16], and they are used as “healthier” alternatives to SSBs as an effective strategy to help control caloric intake [17]. Evidence on the long-term health impact of ASBs on incident CVD is still inconclusive owing to biases, such as reverse causation and residual confounding [5,[18], [19], [20], [21]].
Physical activity is cardioprotective [22,23], yet in the relationship between SSB or ASB intake and risk of CVD, it has been mostly considered as a confounder [3,5,7,8,10,[24], [25], [26]]. A possible argument is that physical activity could mitigate the potential health risk associated with higher intakes of SSB. Thus, the inference is that with identical SSB intake, those who meet physical activity recommendations would have reduced the health risk associated with higher intakes of SSBs. Although there have been observational epidemiology studies [[27], [28], [29], [30]] that have examined the independent association between physical activity and risk of CVD and the combined associations of physical activity and other behavioral factors including diet and CVD mortality [[31], [32], [33], [34]], none have addressed the combined associations of physical activity and SSB or ASB intake and incident CVD. In addition, only 1 study [8] has addressed the possible effect modification by physical activity, reporting no significant multiplicative interaction. This study contributes to the SSB and ASB intake literature by determining the combined associations between intakes of SSB or ASB and physical activity, going beyond how physical activity has been previously considered, as a confounder or as part of a multiplicative interaction.
Therefore, this study aimed to examine the independent and joint associations between SSB or ASB consumption and physical activity in relation to the risk of total CVD, as a composite of coronary artery disease (CAD) and stroke, in males and females from 2 large United States prospective cohort studies. We hypothesize that SSB intake will be independently associated with a higher risk of total CVD, CAD, and stroke, whereas ASB will independently have a null or slightly higher risk of total CVD, CAD, and stroke. Moreover, physical activity will be independently associated with a lower risk of total CVD, CAD, and stroke. Furthermore, meeting physical activity guidelines will not lower the adverse risk of total CVD, CAD, and stroke associated with SSB or ASB intake in joint analysis.
Methods
Study population and design
The Nurses’ Health Study (NHS), a prospective study initiated in 1976, enrolled 121,701 registered nurses aged 30–55 y. The Health Professionals Follow-up Study (HPFS), a prospective study established in 1986, enrolled 51,529 United States male health professionals aged 40–75 y. Baseline and follow-up questionnaires were sent to participants every 2 y to update medical, lifestyle, and health information. Follow-up rates exceeded 90% for each 2-year cycle. Diet was assessed using a self-administered food frequency questionnaire every 4 y, first collected in 1980 in NHS and 1986 for HPFS, used as this study’s baseline cycles. A detailed description of the 2 cohorts has been previously reported [35,36].
We excluded individuals with a history of diabetes mellitus, CVD (defined as a history of CAD, including fatal and nonfatal myocardial infarction, stroke, and coronary artery bypass grafting) or cancer at or before baseline; died at or before baseline and missing records; missing information regarding both SSB and physical activity; or implausible caloric intake (<500 or >3500 kcal/d in females, and <800 kcal or >4200 kcal/d in males). The final analysis included 65,730 females and 39,418 males (Supplemental Figure 1). The protocol was approved by the institutional review board of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health. All participants gave informed consent.
Dietary assessment and SSB and ASB intake
Intakes of SSBs and ASBs were assessed using a validated and reproducible food frequency questionnaire (FFQ) designed to assess the usual diet over the previous year, completed every 4 y in both cohorts. The reproducibility and validity of these instruments have been described previously [37,38]. Participants were asked how often, on average, they consumed a standard portion of foods and beverages (1 standard glass, bottle, or can), with possible responses ranging from “never or less than once/mo” to “≥6 times/d.” SSBs were defined as carbonated SSBs (caffeinated colas, caffeine-free colas, or other) and noncarbonated SSBs (fruit punches, lemonades, or other fruit drinks). Pure fruit juices were not considered SSBs because sugar is naturally present. ASBs were defined as caffeinated, caffeine-free, and noncarbonated low-calorie or diet beverages.
Physical activity assessment
Participants’ physical activity was assessed biannually, starting in 1986 for both cohorts, by reporting the average time per week spent in various moderate-intensity or vigorous-intensity leisure-time activities in the preceding year, including walking, jogging, running, swimming, bicycling, calisthenics and other aerobic exercises, squash/racquetball, tennis, lower-intensity exercise, weightlifting, and outdoor work, using a validated self-reported questionnaire [39]. Up to 15 repeated measures of physical activity were available and used, and the same questionnaires were used over the follow-up, except that weight training information was added beginning from 1990 for HPFS and 2000 for NHS. Based on the intensity and duration of each activity, weekly expenditure in metabolic equivalent tasks (MET h/wk) was calculated and summed over all activities for total physical activity for each assessment. Moderate activity was defined as activities <6 MET including walking, lower-intensity exercise, weightlifting, and calisthenics. Vigorous activity was defined as activities ≥6 MET including jogging, running, swimming, bicycling, and other aerobic exercises, squash/racquetball, tennis, outdoor work, and stair climbing. Moderate and vigorous physical activities were calculated by summing the corresponding physical activities in MET-hours per week.
Ascertainment of CVD
The primary outcome was incident CVD, defined as a composite of fatal and nonfatal CAD (including myocardial infarction) and fatal and nonfatal stroke. Secondary outcomes were CAD, defined as fatal CAD or nonfatal myocardial infarction, and stroke. In both the NHS and HPFS cohorts, when a participant (or family members) reported an incident event, permission was requested to examine their medical records by the study investigator-physicians who were blinded to the participant’s risk factor status. For each end point, the month and year of diagnosis were recorded as the diagnosis date. Nonfatal events were confirmed through a review of medical records. Myocardial infarction was defined according to the WHO criteria [40]. Strokes were confirmed if data in the medical records fulfilled the National Survey of Stroke criteria [41]. Strokes were classified as ischemic stroke (thrombotic or embolic occlusion of a cerebral artery), hemorrhagic stroke (subarachnoid and intraparenchymal hemorrhage), or stroke of probable and/or unknown subtype (subtype data not available). Death ascertainment was performed by searching the National Death Index [42], by family members’ responses to follow-up questionnaires, or by reports from participants’ professional organizations. We requested access to medical records, autopsy reports, and death certificates to confirm all suspected deaths due to myocardial infarction. Fatal myocardial infarction was confirmed by medical records or autopsy reports. We included all confirmed and probable cases in our report because results were similar after probable cases were excluded (data not shown). Follow-up for deaths was >98% complete.
Assessment of covariates
Information on lifestyle and CVD risk factors was assessed and updated every other year and included the following: age; weight; smoking status; use of aspirin, multivitamins, postmenopausal hormone-replacement therapy, and oral contraceptives; menopausal status; and hypertension or hypercholesterolemia that had been recently diagnosed by a physician; and a family history of chronic diseases. Height was ascertained for females in 1976 and for males in 1986. Height and body weight were used to calculate BMI [weight in kilograms divided by height in meters squared (kg/m2)]. Alcohol intake was assessed, and updated, from the FFQs every 4 y. Ancestry information was collected in1986 for males and in 1992 for females, and the self-reported categories in the questionnaire included Southern European/Mediterranean, Caucasian/Scandinavian, other Caucasian, African American, Hispanic, Asian, American Indian, and other. Because the data on race and ethnicity in our cohorts were collected >30 y ago, they were not consistent with the current standard classifications. The Alternate Healthy Eating Index (AHEI, range 0–90; higher score indicating adherence to 2015–2020 Dietary Guidelines for Americans) was calculated from FFQ data, without the exposure (SSBs) and alcohol (which was considered as a separate confounder) components, to indicate participant’s overall diet quality [43].
Statistical analysis
Person-years of follow-up were calculated from the return of the baseline questionnaire to the date of diagnosis of CVD, death, or end of follow-up (June 30, 2016, for the NHS, and January 31, 2016, for the HPFS) whichever came first. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs of developing CVD according to the independent and joint associations of SSB or ASB intakes and physical activity.
For the independent association analysis between SSB or ASB intakes and risk of CVD, we calculated the cumulative averages of SSBs and ASBs, and subtypes, at each updated FFQ to approximate long-term habitual intakes. This minimizes within-person variation by using all data from baseline to follow-up date, providing a statistically more powerful test of disease-exposure association. Beverage intake was categorized by frequency: <1/mo (reference), 1–4/mo, 2–6/wk, 1 to <2/d, and ≥2/d, and linear trends were evaluated using the Wald test on a continuous variable representing median intakes of each category. The same categorization was applied to SSB and ASB subtypes. We also evaluated the association for 1 serving increment of SSB and ASB intakes per day in the multivariable models.
For the independent association analysis between physical activity and risk of CVD, we calculated the cumulative averages of repeated measures of physical activity to capture the long-term physical activity expenditure and to reduce within-person measurement error. We then determined quintiles of expenditure for each cohort.
The first multivariable model was adjusted for age (continuous, years); race [White compared with Other (Black, American Indian or Alaska Native, Asian, Native Hawaiian, and other)]; ancestry [Caucasian compared with other (Southern European/Mediterranean, Hispanic, African American, Asian, American Indian, and other)]; alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥15.0 g/d); smoking status (never, former, and current: 1.0–14.0, 15.0–24.0, or ≥25.0 cigarettes/d); physical activity (for independent SSB and ASB models, <3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, and ≥27.0 MET h/wk); a family history of myocardial infarction (yes/no); baseline hypertension or antihypertensive medication (yes/no); baseline hypercholesterolemia or cholesterol-lowering medication (yes/no); multivitamin and aspirin use (yes/no); menopausal status and use of hormone-replacement therapy [in females, premenopausal, postmenopausal (no, past, and current hormone use)]; total energy intake (continuous, kilocalories per day); and BMI (<23.0, ≥23.0 to <25.0, ≥25.0 to <30, ≥30 to <35, and ≥35 kg/m2). In the second multivariable model, we additionally adjusted for the AHEI (in quintiles, without SSBs and alcohol; not applicable in the independent physical activity models). The time-varying variables used in the models were updated throughout the follow-up, and the questionnaire year determined the time point and included all mentioned variables except for a family history of myocardial infarction, baseline hypertension or antihypertensive medication, and baseline hypercholesterolemia or cholesterol-lowering medication. The physical activity models included the same set of covariables.
We conducted a series of stratified analyses for continuous servings per day of SSBs or ASBs with incidence CVD by prespecified subgroups based on previous publications and median values. We calculated P values for heterogeneity with Wald tests for the multiplication interaction of SSB or ASB exposures (continuous variable) with the effect modifier variables.
For the joint analyses, SSB or ASB intakes were combined into 3 categories: 1) never or <1 serving/mo, 2) 1–4 servings/mo, and 3) ≥2 servings/wk (based on the distribution of intake in our cohorts), with physical activity dichotomized according to the current aerobic physical activity guidelines for adults (<7.5 MET h/wk is equivalent to 150 min/wk of moderate-intensity physical activity) [44]: <7.5 MET h/wk compared with. ≥7.5 MET h/wk. We set participants who reported never/rarely consuming SSBs or ASBs and meeting physical activity guidelines as the reference category. We tested the multiplicative interaction with the cross-product interaction term between continuous servings per day of SSB or ASB intakes × total physical activity added to the main effects models. To assess the additive interaction, we derived the probability value of the relative excess risk due to interaction as an index of additive interaction, along with SSB and ASB intakes and physical activity as continuous variables [[45], [46], [47]].
Because participants may alter dietary patterns after the diagnosis of major illness, we stopped updating dietary variables when participants reported a diagnosis of coronary artery bypass, angina, or cancer, although follow-up continued until CVD endpoint occurrence, death, or the end of the study period [48]. If FFQ exposure data were missing at a given updated time point, we carried forward the exposure level from the previous cumulative cycles. This was applied to all missing data from other variables.
Sensitivity analyses
We also ran a series of sensitivity analyses to assess the robustness of results in independent and joint associations. First, the diet was continuously updated until the end of follow-up. Second, we excluded BMI from the models. Third, models were adjusted for median household income and education. Fourth, instead of adjusting for AHEI, we adjusted for a set of dietary variables. Finally, we further examined 2 subgroups: 1) heavy SSB or ASB consumers (top 25% SSB or ASB consumers compared with not) and the dichotomized physical activity variable, and 2) heavy SSB or ASB consumers and high physical activity level (top 25% physical activity compared with not).
All analyses were performed separately for each cohort and then pooled using inverse variance-weighted fixed-effect meta-analysis and variance-weighted meta-analysis. Statistical tests were 2-sided, and P values of <0.05 were considered to indicate statistical significance. Data were analyzed with the SAS package, version 9.4 (SAS Institute).
Results
In these 2 United States-based cohorts, 13,269 CVD cases (8438 CAD; 4997 strokes) were documented, 6156 in males and 7113 in females, during a maximum follow-up of 30 (median 13.4) and 36 (median 17.9) years, respectively. Study participants were middle-aged, White, health professionals, with 62.5% of the total sample being females. Males and females in the reference group, who jointly met physical activity guidelines and consumed <1 SSB serving/mo at baseline (21% females and 23% males), had on average lower total energy intake and higher diet quality (Table 1). By contrast, males and females who did not meet physical activity guidelines and consumed ≥2 servings/wk of SSBs at baseline had a higher total energy intake and lower AHEI diet quality score. When participants were compared according to their jointly baseline ASB consumption and physical activity level, concomitantly, differences were consistent (Supplemental Table 1). Differences in characteristics of participants followed similar trends when they were compared based on only on their level of SSB or ASB consumption or on their level of physical activity (Supplemental Table 2).
TABLE 1.
Baseline1 characteristics of participants according to sugar-sweetened beverage intake and physical activity joint association categories in 2 large United States cohorts
| Meets the United States Physical Activity Guidelines2 |
Does Not Meet the United States Physical Activity Guidelines2 |
|||||
|---|---|---|---|---|---|---|
| Never or <1 SSB serving/mo | 1–4 SSB servings/mo | ≥2 SSB servings/wk | Never or <1 SSB serving/mo | 1–4 SSB servings/mo | ≥2 SSB servings/wk | |
| Nurses’ Health Study | ||||||
| Participants (n) | 13,895 | 7636 | 12,014 | 11,331 | 7367 | 13,487 |
| Age (y) | 47 ± 7 | 47 ± 7 | 45 ± 7 | 48 ± 7 | 47 ± 7 | 45 ± 7 |
| BMI (kg/m2) | 23.7 ± 3.7 | 23.6 ± 3.8 | 24.0 ± 4.2 | 24.6 ± 4.5 | 24.5 ± 4.4 | 24.9 ± 4.9 |
| Physical activity (MET h/wk) | 26.1 ± 24.5 | 24.3 ± 24.2 | 23.9 ± 22.3 | 2.8 ± 2.0 | 2.8 ± 2.0 | 2.8 ± 1.9 |
| Family history of myocardial infarction (%) | 39.6 | 38.5 | 37.5 | 39.5 | 37.7 | 37.1 |
| Family history of diabetes (%) | 28.2 | 27.9 | 27.8 | 30.3 | 29.5 | 30.5 |
| Ethnicity, white (%) | 98.7 | 97.9 | 97.4 | 98.7 | 98.0 | 97.0 |
| Current smoker (%) | 24.3 | 22.1 | 25.7 | 31.9 | 28.3 | 29.8 |
| Hypertension (%) | 14.1 | 12.5 | 14.1 | 16.3 | 14.6 | 16.6 |
| Hypercholesterolemia (%) | 5.0 | 4.6 | 4.5 | 5.7 | 5.0 | 4.7 |
| Multivitamin supplement use (%) | 37.8 | 36.0 | 34.5 | 33.1 | 31.9 | 31.0 |
| Aspirin use (%) | 44.2 | 46.5 | 49.1 | 46.2 | 45.9 | 49.6 |
| Current menopausal hormone use (%) | 7.3 | 6.8 | 6.3 | 7.1 | 6.8 | 6.3 |
| Total energy intake (kcal/d) | 1444 ± 461 | 1534 ± 470 | 1718 ± 500 | 1445 ± 470 | 1506 ± 473 | 1705 ± 510 |
| Artificially sweetened beverages (servings/d) | 0.6 ± 1.0 | 0.3 ± 0.7 | 0.3 ± 0.6 | 0.6 ± 1.1 | 0.3 ± 0.7 | 0.3 ± 0.6 |
| Sugar-sweetened beverages (servings/d) | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.9 ± 0.9 | 0.0 ± 0.0 | 0.1 ± 0.0 | 1.0 ± 1.0 |
| Total beverages (servings/d) | 5.4 ± 2.5 | 5.2 ± 2.4 | 5.7 ± 2.6 | 5.5 ± 2.6 | 5.3 ± 2.5 | 5.8 ± 2.6 |
| Alcohol (g/d) | 8.0 ± 11.0 | 6.3 ± 9.6 | 5.5 ± 9.2 | 8.0 ± 12.3 | 5.5 ± 9.9 | 5.0 ± 9.7 |
| Red and processed meat (servings/d) | 1.2 ± 0.8 | 1.4 ± 0.8 | 1.5 ± 0.8 | 1.3 ± 0.8 | 1.4 ± 0.8 | 1.6 ± 0.8 |
| Nuts (servings/d) | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 |
| Whole grains (servings/d) | 2.3 ± 2.0 | 2.2 ± 1.9 | 2.0 ± 1.8 | 2.1 ± 2.0 | 2.1 ± 1.9 | 1.9 ± 1.8 |
| Fruits and vegetables (servings/d) | 4.5 ± 2.2 | 4.2 ± 2.0 | 4.1 ± 2.0 | 4.0 ± 2.1 | 3.7 ± 1.9 | 3.6 ± 1.9 |
| AHEI3 | 44.6 ± 9.0 | 42.3 ± 8.5 | 40.3 ± 8.3 | 41.2 ± 8.7 | 39.4 ± 8.2 | 37.7 ± 7.8 |
| Health Professionals Follow-up Study | ||||||
| Participants (n) | 9062 | 5903 | 10,015 | 4520 | 3308 | 6610 |
| Age (y) | 54 ± 9 | 53 ± 9 | 51 ± 9 | 56 ± 9 | 54 ± 9 | 51 ± 9 |
| BMI (kg/m2) | 25.1 ± 3.1 | 25.2 ± 3.1 | 25.2 ± 3.1 | 26.2 ± 3.7 | 25.9 ± 3.5 | 25.9 ± 3.5 |
| Physical activity (MET h/wk) | 32.6 ± 27.3 | 30.4 ± 25.5 | 30.7 ± 26.0 | 3.0 ± 2.2 | 3.1 ± 2.1 | 3.0 ± 2.1 |
| Family history of myocardial infarction (%) | 33.6 | 30.4 | 30.7 | 33.1 | 31.2 | 31.6 |
| Family history of diabetes (%) | 20.0 | 18.4 | 16.9 | 20.3 | 19.2 | 18.3 |
| Ethnicity, white (%) | 95.7 | 96.2 | 94.1 | 96.2 | 95.5 | 94.0 |
| Current smoker (%) | 6.7 | 7.4 | 9.1 | 12.1 | 11.6 | 13.6 |
| Hypertension (%) | 21.2 | 19.2 | 19.6 | 24.5 | 22.9 | 22.5 |
| Hypercholesterolemia (%) | 11.0 | 11.0 | 9.6 | 11.5 | 10.2 | 9.4 |
| Multivitamin supplement use (%) | 46.5 | 43.8 | 41.5 | 41.1 | 37.4 | 36.7 |
| Aspirin use (%) | 25.6 | 25.2 | 27.5 | 27.0 | 26.6 | 27.3 |
| Total energy intake (kcal/d) | 1821 ± 558 | 1944 ± 575 | 2219 ± 632 | 1738 ± 545 | 1872 ± 556 | 2163 ± 639 |
| Artificially sweetened beverages (servings/d) | 0.7 ± 1.1 | 0.5 ± 0.8 | 0.3 ± 0.7 | 0.7 ± 1.2 | 0.5 ± 1.0 | 0.3 ± 0.7 |
| Sugar-sweetened beverages (servings/d) | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.8 ± 0.8 |
| Total beverages (servings/d) | 7.2 ± 3.0 | 7.2 ± 2.8 | 7.7 ± 2.9 | 7.2 ± 3.0 | 7.1 ± 2.9 | 7.4 ± 3.0 |
| Alcohol (g/d) | 12.8 ± 16.2 | 11.6 ± 14.7 | 10.8 ± 14.3 | 12.4 ± 16.9 | 11.2 ± 16.0 | 10.0 ± 15.4 |
| Red and processed meat (servings/d) | 0.9 ± 0.8 | 1.0 ± 0.8 | 1.3 ± 0.8 | 1.1 ± 0.8 | 1.2 ± 0.8 | 1.4 ± 0.9 |
| Nuts (servings/d) | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 |
| Whole grains (servings/d) | 1.9 ± 1.6 | 1.8 ± 1.5 | 1.6 ± 1.4 | 1.5 ± 1.4 | 1.5 ± 1.3 | 1.4 ± 1.2 |
| Fruits and vegetables (servings/d) | 5.3 ± 2.9 | 5.2 ± 2.9 | 5.1 ± 2.7 | 4.4 ± 2.6 | 4.3 ± 2.4 | 4.3 ± 2.4 |
| AHEI3 | 48.2 ± 10.2 | 45.9 ± 9.8 | 43.1 ± 9.4 | 43.9 ± 10.1 | 41.8 ± 9.6 | 39.6 ± 9.1 |
Values are mean ± SD or % and are standardized to the age distribution of the study population. Total sample size, n = 105,148 (Health Professionals Follow-up Study, n = 39,418; Nurses’ Health Study, n = 65,730).
Abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index; MET, metabolic equivalent task.
Baseline for Nurses’ Health Study is 1980 and for Health Professionals Follow-up Study is 1986.
United States physical activity guidelines were met if participant reported ≥150 min/wk of moderate–vigorous physical activity.
AHEI range 0–90; sugar-sweetened beverages and alcohol were removed from the index calculation.
Table 2 summarizes the pooled associations for intakes of SSBs, ASBs, and physical activity with CVD, CAD, and stroke. In multivariable model 2, compared with participants who never/rarely consumed SSBs, those consuming ≥2 SSB servings/d had a 21% higher risk of CVD (pooled HR, 1.21; 95% CI: 1.12, 1.32; P-trend < 0.001). Regarding per serving increment of SSB intake per day, the pooled HR for CVD was 1.15 (95% CI: 1.09, 1.20); for CAD, 1.17 (95% CI: 1.10, 1.24); and for stroke, 1.11 (95% CI: 1.02, 1.20). No evidence of significant differences in ASB intake and risk of CVD or CAD was observed, except for stroke where per serving increment of ASB intake per day was 1.05 (95% CI: 1.01, 1.10; P-trend = 0.03). Comparing the highest with the lowest quintile of physical activity, the pooled hazard ratios were 0.66 (95% CI: 0.63, 0.70) for CVD, 0.67 (95% CI: 0.63, 0.72) for CAD, and 0.64 (95% CI: 0.59, 0.71) for stroke. The relationships between SSB, ASB, and physical activity and stroke subtypes were assessed, and only evidence of an inverse association between physical activity practice and ischemic and hemorrhagic stroke was observed (Supplemental Table 3). Supplemental Table 4 tabulates the results for the association between SSB or ASB subtypes and CVD. In the SSB subtype pooled analysis, per serving increment of cola and noncola carbonated drink intake per day was associated with a 15% (pooled HR, 1.15; 95% CI: 1.08, 1.23; P-trend < 0.0001) and a 12% (pooled HR, 1.12; 95% CI: 1.04, 1.21; P-trend = 0.004) higher risk of CVD, respectively. The associations between SSB, ASB, and physical activity with CVD risks were consistent across subgroups (Table 3). When stratifying for physical activity, the CVD risk according to 1 serving increment of SSB per day was 12% (pooled HR, 1.12; 95% CI: 1.04, 1.20) for those participants who did not meet physical activity guidelines and 18% (pooled HR, 1.18; 95% CI: 1.10, 1.26) for those who met the guidelines. CAD and stroke outcomes showed a similar pattern as CVD. No significant associations were observed for ASB when stratifying for physical activity. Cohort-specific analyses provided similar and consistent results across subgroups (Supplemental Table 5).
TABLE 2.
Pooled Risk1 of cardiovascular events according to sugar-sweetened or artificially sweetened beverage intake categories and physical activity2 quintiles in 2 large United States cohorts
| Sugar-sweetened beverages | HR (95% CI) |
P-trend | HR (95% CI) per serving increment of SSB3 intake per day | |||||
|---|---|---|---|---|---|---|---|---|
| Never or <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | 1 to <2 servings/d | ≥2 servings/d | ||||
| Total cardiovascular disease | ||||||||
| HPFS (cases/person-years) | 1354/187,404 | 1906/266,714 | 2195/338,803 | 276/41,556 | 425/74,663 | |||
| NHS (cases/person-years) | 991/392,997 | 2669/727,712 | 2789/735,164 | 238/85,332 | 426/150,868 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 1.00 (0.95, 1.05) | 1.10 (1.05, 1.16) | 1.26 (1.15, 1.39) | 1.34 (1.24, 1.45) | <0.0001 | 1.26 (1.20, 1.32) | |
| Multivariable model | 1.00 | 1.04 (0.99, 1.10) | 1.13 (1.07, 1.19) | 1.23 (1.12, 1.36) | 1.25 (1.16, 1.36) | <0.0001 | 1.17 (1.12, 1.23) | |
| Multivariable model + AHEI | 1.00 | 1.04 (0.98, 1.09) | 1.11 (1.05, 1.17) | 1.20 (1.08, 1.32) | 1.21 (1.12, 1.32) | <0.0001 | 1.15 (1.09, 1.20) | |
| coronary artery disease | ||||||||
| HPFS (cases/person-years) | 1001/187,692 | 1435/267,136 | 1649/339,278 | 220/41,622 | 336/74,765 | |||
| NHS (cases/person-years) | 548/393,295 | 1389/728,575 | 1477/736,011 | 138/85,404 | 245/151,000 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 1.01 (0.94, 1.07) | 1.11 (1.04, 1.18) | 1.34 (1.19, 1.51) | 1.41 (1.28, 1.55) | <0.0001 | 1.30 (1.23, 1.38) | |
| Multivariable model | 1.00 | 1.06 (1.00, 1.13) | 1.14 (1.07, 1.22) | 1.30 (1.16, 1.46) | 1.31 (1.19, 1.45) | <0.0001 | 1.21 (1.14, 1.28) | |
| Multivariable model + AHEI | 1.00 | 1.05 (0.99, 1.12) | 1.12 (1.05, 1.19) | 1.25 (1.11, 1.41) | 1.25 (1.13, 1.39) | <0.0001 | 1.17 (1.10, 1.24) | |
| Stroke | ||||||||
| HPFS (cases/person-years) | 353/187,871 | 471/267,471 | 546/339,696 | 56/41,678 | 89/74,850 | |||
| NHS (cases/person-years) | 476/393,327 | 1326/728,488 | 1381/735,934 | 107/85,423 | 192/151,020 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.97 (0.89, 1.06) | 1.09 (1.00, 1.19) | 1.14 (0.96, 1.34) | 1.21 (1.05, 1.39) | <0.0001 | 1.18 (1.09, 1.28) | |
| Multivariable model | 1.00 | 0.99 (0.91, 1.08) | 1.10 (1.01, 1.20) | 1.11 (0.93, 1.31) | 1.13 (0.98, 1.31) | 0.01 | 1.11 (1.02, 1.21) | |
| Multivariable model + AHEI | 1.00 | 0.99 (0.91, 1.08) | 1.09 (1.00, 1.19) | 1.10 (0.92, 1.30) | 1.12 (0.97, 1.29) | 0.02 | 1.11 (1.02, 1.20) | |
| Artificially sweetened beverages | HR (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Never or <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | 1 to <2 servings/d | ≥2 servings/d | P-trend | HR (95% CI) per serving increment of ASB3 intake per day | ||
| Total cardiovascular disease | ||||||||
| HPFS (cases/person-years) | 1906/266,941 | 1378/188,107 | 1774/266,083 | 296/50,244 | 802/137,764 | |||
| NHS (cases/person-years) | 2692/774,993 | 1199/344,166 | 1585/487,216 | 632/184,325 | 1005/301,373 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.91 (0.87, 0.96) | 0.93 (0.89, 0.97) | 1.04 (0.97, 1.12) | 1.09 (1.04, 1.16) | <0.0001 | 1.07 (1.04, 1.10) | |
| Multivariable model | 1.00 | 0.94 (0.90, 0.99) | 0.94 (0.90, 0.98) | 1.02 (0.95, 1.10) | 1.02 (0.96, 1.08) | 0.11 | 1.02 (0.99, 1.05) | |
| Multivariable model + AHEI | 1.00 | 0.95 (0.90, 1.00) | 0.95 (0.91, 0.99) | 1.03 (0.96, 1.10) | 1.03 (0.97, 1.09) | 0.06 | 1.03 (1.00, 1.06) | |
| coronary artery disease | ||||||||
| HPFS (cases/person-years) | 1448/267,352 | 1059/188,387 | 1308/266,499 | 219/50,313 | 607/137,939 | |||
| NHS (cases/person-years) | 1452/775,826 | 629/344,545 | 849/487,721 | 329/184,504 | 538/301,689 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.91 (0.86, 0.97) | 0.92 (0.87, 0.97) | 1.01 (0.92, 1.11) | 1.10 (1.03, 1.18) | 0.0003 | 1.07 (1.03, 1.11) | |
| Multivariable model | 1.00 | 0.95 (0.89, 1.01) | 0.92 (0.87, 0.98) | 0.97 (0.88, 1.07) | 1.00 (0.93, 1.08) | 0.61 | 1.01 (0.97, 1.05) | |
| Multivariable model + AHEI | 1.00 | 0.95 (0.90, 1.02) | 0.94 (0.88, 0.99) | 0.98 (0.89, 1.08) | 1.01 (0.94, 1.09) | 0.42 | 1.02 (0.98, 1.05) | |
| Stroke | ||||||||
| HPFS (cases/person-years) | 458/267,729 | 319/188,627 | 466/266,738 | 77/50,370 | 195/138,102 | |||
| NHS (cases/person-years) | 1292/775,823 | 596/344,501 | 781/487,702 | 321/184,495 | 492/301,670 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.92 (0.85, 1.00) | 0.96 (0.89, 1.03) | 1.11 (1.00, 1.24) | 1.09 (1.00, 1.20) | 0.002 | 1.07 (1.03, 1.12) | |
| Multivariable model | 1.00 | 0.95 (0.87, 1.03) | 0.98 (0.91, 1.06) | 1.11 (1.00, 1.24) | 1.06 (0.97, 1.17) | 0.03 | 1.05 (1.00, 1.10) | |
| Multivariable model + AHEI |
1.00 |
0.95 (0.88, 1.03) |
0.98 (0.91, 1.06) |
1.12 (1.00, 1.25) |
1.07 (0.97, 1.17) |
0.03 |
1.05 (1.01, 1.10) |
|
| Physical activity |
HR (95% CI) | |||||||
| Quintile 1 (0 to ≤3.0) |
Quintile 2 (>3.0 to ≤8.4) |
Quintile 3 (>8.4 to ≤17.8) |
Quintile 4 (>17.8 to ≤35.1) |
Quintile 5 (>35.1) |
P-trend |
|||
| Total cardiovascular disease | ||||||||
| HPFS (cases/person-years) | 1924/178,321 | 1239/181,529 | 1105/182,492 | 990/183,382 | 898/183,415 | |||
| NHS (cases/person-years) | 2205/413,395 | 1520/418,327 | 1262/417,294 | 1163/421,176 | 963/421,881 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.72 (0.69, 0.76) | 0.66 (0.62, 0.69) | 0.61 (0.58, 0.64) | 0.54 (0.51, 0.57) | <0.0001 | ||
| Multivariable model | 1.00 | 0.78 (0.74, 0.82) | 0.74 (0.70, 0.78) | 0.71 (0.67, 0.75) | 0.65 (0.61, 0.69) | <0.0001 | ||
| Multivariable model + AHEI | 1.00 | 0.78 (0.74, 0.82) | 0.74 (0.71, 0.78) | 0.72 (0.68, 0.76) | 0.66 (0.63, 0.70) | <0.0001 | ||
| coronary artery disease | ||||||||
| HPFS (cases/person-years) | 1524/178,645 | 916/181,811 | 847/182,731 | 697/183,661 | 657/183,644 | |||
| NHS (cases/person-years) | 1221/414,038 | 806/418,789 | 657/417,706 | 589/421,559 | 524/422,193 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.68 (0.64, 0.73) | 0.63 (0.59, 0.67) | 0.55 (0.52, 0.59) | 0.52 (0.49, 0.56) | <0.0001 | ||
| Multivariable model | 1.00 | 0.75 (0.71, 0.80) | 0.73 (0.68, 0.78) | 0.67 (0.62, 0.71) | 0.65 (0.61, 0.70) | <0.0001 | ||
| Multivariable model + AHEI | 1.00 | 0.76 (0.71, 0.80) | 0.74 (0.70, 0.79) | 0.68 (0.64, 0.73) | 0.67 (0.63, 0.72) | <0.0001 | ||
| Stroke | ||||||||
| HPFS (cases/person-years) | 400/178,905 | 323/182,010 | 258/182,987 | 293/183,828 | 241/183,837 | |||
| NHS (cases/person-years) | 1033/413,964 | 746/418,790 | 634/417,681 | 608/421,538 | 461/422,220 | |||
| Pooled risk | ||||||||
| Age-adjusted model | 1.00 | 0.79 (0.73, 0.86) | 0.70 (0.64, 0.76) | 0.72 (0.66, 0.78) | 0.58 (0.53, 0.63) | <0.0001 | ||
| Multivariable model | 1.00 | 0.83 (0.76, 0.90) | 0.75 (0.69, 0.82) | 0.79 (0.73, 0.86) | 0.64 (0.59, 0.71) | <0.0001 | ||
| Multivariable model + AHEI | 1.00 | 0.83 (0.77, 0.90) | 0.75 (0.69, 0.82) | 0.80 (0.73, 0.87) | 0.64 (0.59, 0.71) | <0.0001 | ||
Total sample size, n = 105,148 (HPFS, n = 39,418; NHS, n = 65,730). Sugar-sweetened beverages and alcohol intake were removed from the AHEI calculation.
Abbreviations: AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent task; NHS, Nurses’ Health Study.
HRs and 95% CIs derived from the age-adjusted and multivariable model adjusted for age; race; ethnicity; physical activity (excluded in the physical activity as main exposure models); smoking status; alcohol intake; multivitamin use; in women, postmenopausal status and menopausal hormone use; family history of myocardial infarction; baseline hypertension; baseline hypercholesterolemia; calorie intake; and body mass index.
Physical activity was measured in MET h/wk.
One serving increment of sugar-sweetened or artificially sweetened beverage intake per day, where appropriate.
TABLE 3.
Pooled subgroup analyses for risk1 of cardiovascular events according to 1 serving increment in sugar-sweetened or artificially sweetened beverage intake per day2 in 2 large United States cohorts
| Cardiovascular disease |
Coronary artery disease |
Stroke |
||||
|---|---|---|---|---|---|---|
| Pooled Adjusted HR (95% CI) | p-value for interaction | Pooled Adjusted HR (95% CI) | p-value for interaction | Pooled Adjusted HR (95% CI) | p-value for interaction | |
| Sugar-sweetened beverages | ||||||
| Age (y) | ||||||
| <65 | 1.15 (1.06, 1.25) | 0.05 | 1.21 (1.10, 1.34) | 0.04 | 1.01 (0.87, 1.18) | 0.94 |
| ≥65 | 1.12 (1.05, 1.20) | 1.12 (1.03, 1.21) | 1.14 (1.03, 1.26) | |||
| Body mass index (kg/m2) | ||||||
| <25 | 1.20 (1.12, 1.28) | 0.76 | 1.24 (1.14, 1.35) | 0.45 | 1.12 (0.99, 1.26) | 0.69 |
| ≥25 | 1.09 (1.02, 1.17) | 1.09 (1.00, 1.19) | 1.10 (0.97, 1.24) | |||
| Physical activity, meet United States guidelines | ||||||
| No (<150 min/wk MVPA) | 1.12 (1.04, 1.20) | 0.14 | 1.12 (1.03, 1.23) | 0.07 | 1.09 (0.97, 1.24) | 0.63 |
| Yes (≥150 min/wk MVPA) | 1.18 (1.10, 1.26) | 1.22 (1.12, 1.32) | 1.12 (1.00, 1.26) | |||
| Television watching (h/wk) | ||||||
| <8 | 1.18 (1.08, 1.29) | 0.45 | 1.22 (1.10, 1.36) | 0.59 | 1.12 (0.96, 1.30) | 0.75 |
| 8–15.5 | 1.12 (1.00, 1.26) | 1.13 (0.98, 1.30) | 1.13 (0.93, 1.37) | |||
| ≥15.5 | 1.15 (1.07, 1.24) | 1.18 (1.07, 1.29) | 1.12 (0.98, 1.26) | |||
| Smoking status | ||||||
| Never | 1.13 (1.05, 1.21) | 0.01 | 1.16 (1.06, 1.28) | 0.08 | 1.07 (0.94, 1.21) | 0.10 |
| Former/current | 1.20 (1.13, 1.29) | 1.21 (1.12, 1.31) | 1.19 (1.06, 1.33) | |||
| Family history of myocardial infarction | ||||||
| No | 1.17 (1.09, 1.25) | 0.89 | 1.19 (1.10, 1.29) | 0.98 | 1.13 (1.02, 1.26) | 0.69 |
| Yes | 1.11 (1.03, 1.20) | 1.15 (1.05, 1.26) | 1.06 (0.93, 1.22) | |||
| Calories per day (kcal) | ||||||
| <Median | 1.19 (1.10, 1.29) | 0.17 | 1.17 (1.06, 1.29) | 0.67 | 1.24 (1.09, 1.41) | 0.06 |
| ≥Median | 1.09 (1.02, 1.16) | 1.12 (1.03, 1.21) | 1.03 (0.92, 1.15) | |||
| AHEI | ||||||
| <Median | 1.13 (1.06, 1.20) | 0.26 | 1.12 (1.04, 1.21) | 0.02 | 1.15 (1.03, 1.28) | 0.34 |
| ≥Median | 1.20 (1.11, 1.30) | 1.30 (1.18, 1.43) | 1.05 (0.92, 1.21) | |||
| Artificially sweetened beverages | ||||||
| Age (y) | ||||||
| <65 | 0.99 (0.93, 1.04) | 0.52 | 0.95 (0.89, 1.01) | 0.16 | 1.08 (0.99, 1.19) | 0.07 |
| ≥65 | 1.04 (1.01, 1.08) | 1.05 (1.00, 1.10) | 1.04 (0.99, 1.10) | |||
| Body mass index (kg/m2) | ||||||
| <25 | 1.05 (1.01, 1.10) | 0.72 | 1.02 (0.96, 1.08) | 0.46 | 1.10 (1.03, 1.18) | 0.25 |
| ≥25 | 1.03 (0.99, 1.07) | 1.04 (0.99, 1.09) | 1.02 (0.96, 1.09) | |||
| Physical activity, meet United States guidelines | ||||||
| No (<150 min/wk MVPA) | 1.04 (1.00, 1.09) | 0.70 | 1.04 (0.98, 1.10) | 0.60 | 1.05 (0.98, 1.13) | 0.88 |
| Yes (≥150 min/wk MVPA) | 1.02 (0.98, 1.06) | 1.00 (0.95, 1.05) | 1.06 (0.99, 1.13) | |||
| Television watching (h/wk) | ||||||
| <8 | 1.09 (1.04, 1.15) | 0.01 | 1.10 (1.03, 1.18) | 0.004 | 1.08 (1.00, 1.18) | 0.62 |
| 8–15.5 | 1.03 (0.96, 1.10) | 1.03 (0.95, 1.12) | 1.03 (0.93, 1.14) | |||
| ≥15.5 | 0.98 (0.93, 1.03) | 0.96 (0.90, 1.02) | 1.01 (0.94, 1.09) | |||
| Smoking status | ||||||
| Never | 1.06 (1.02, 1.11) | 0.0004 | 1.05 (0.99, 1.11) | 0.002 | 1.08 (1.01, 1.16) | 0.10 |
| Former/current | 1.02 (0.98, 1.07) | 1.03 (0.97, 1.09) | 1.03 (0.96, 1.11) | |||
| Family history of myocardial infarction | ||||||
| No | 1.03 (0.99, 1.07) | 0.96 | 1.01 (0.96, 1.06) | 1.00 | 1.06 (1.00, 1.13) | 0.98 |
| Yes | 1.03 (0.98, 1.07) | 1.02 (0.96, 1.08) | 1.04 (0.97, 1.12) | |||
| Calories per day (kcal) | ||||||
| <Median | 1.05 (1.01, 1.09) | 0.21 | 1.05 (0.99, 1.10) | 0.10 | 1.06 (1.00, 1.13) | 0.75 |
| ≥Median | 1.01 (0.96, 1.05) | 0.98 (0.93, 1.04) | 1.04 (0.97, 1.11) | |||
| AHEI | ||||||
| <Median | 1.03 (0.98, 1.07) | 0.97 | 1.01 (0.96, 1.07) | 0.99 | 1.05 (0.98, 1.12) | 0.84 |
| ≥Median | 1.03 (0.99, 1.07) | 1.02 (0.96, 1.07) | 1.06 (0.99, 1.13) | |||
Total sample size, n = 105,148 (Health Professionals Follow-up Study, n = 39,418; Nurses’ Health Study, n = 65,730). Sugar-sweetened beverages and alcohol intake were removed from the AHEI calculation.
Abbreviations: AHEI, Alternative Healthy Eating Index; MVPA, moderate–vigorous physical activity.
HRs and 95% CI derived from the multivariable model adjusted for age; race; ethnicity; physical activity (excluded in the physical activity as main exposure models); smoking status; alcohol intake; multivitamin use; in women, postmenopausal status and menopausal hormone use; family history of myocardial infarction; baseline hypertension; baseline hypercholesterolemia; calorie intake; body mass index; and AHEI.
One serving of sugar-sweetened or artificially sweetened beverage. HRs for 1 serving increment of sugar-sweetened or artificially sweetened beverage intake per day in each subgroup category.
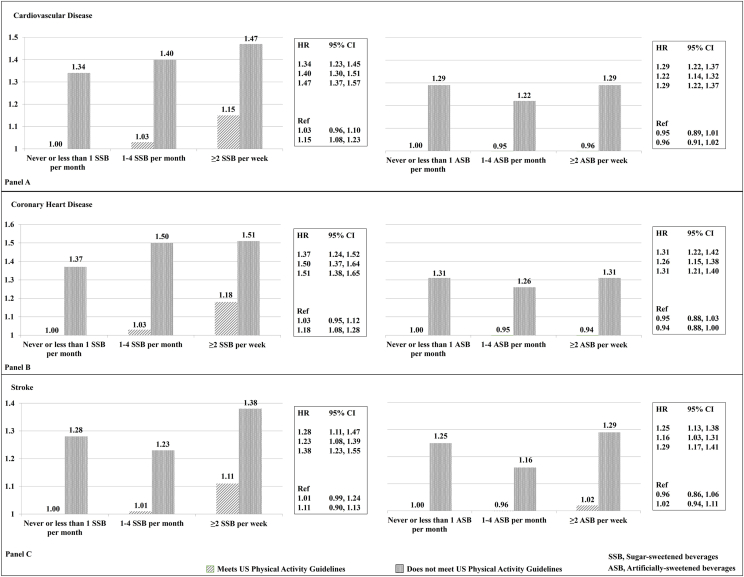
Compared with the reference group (met physical activity guidelines and consumed SSBs <1/mo), participants who did not meet the physical activity guidelines and consumed ≥2 servings/wk of SSBs had a pooled HR of 1.47 (95% CI: 1.37, 1.57) higher risk of CVD (Figure 1A). There was also a higher risk of CVD among those who met physical activity guidelines and consumed ≥2 SSB servings/d (pooled HR, 1.15; 95% CI: 1.08, 1.23), with similar patterns for CAD (Figure 1B) and stroke (Figure 1C). Participants who did not meet physical activity guidelines and consumed ASBs <1/month had a 29% (pooled HR, 1.29; 95% CI: 1.22, 1.37) higher risk of CVD (Figure 1A), which was consistent with those who did not meet the physical activity guidelines and consumed ≥2 servings/wk of ASBs (pooled HR, 1.29; 95% CI: 1.22, 1.37), compared with the reference group. Cohort-specific results analyses for joint associations provided similar findings than the pooled results (Supplemental Table 6). Supplemental Table 7 documents that not meeting physical activity guidelines and consuming >2 servings/d of SSB or ASB is associated with a higher risk of ischemic and hemorrhagic stroke. Supplemental Table 8 summarizes the results for the joint associations between SSB or ASB subtypes and physical activity and risk of CVD. Although there was no evidence of statistically significant multiplicative interactions between SSB or ASB and physical activity for any outcome, we observed a significant additive interaction between SSB intake and physical activity and risk of CVD (P < 0.001) and CAD (P < 0.001) (Supplemental Table 9). We also observed a significant additive interaction between the intake of ASBs and physical activity and the risk of CVD (P = 0.03) and stroke (P = 0.04).
Figure 1.
Pooled risk1 of cardiovascular events associated with the joint associations of sugar-sweetened or artificially sweetened beverages and physical activity2 in 2 large us cohorts. Total sample size, n = 105,148 (Health Professionals Follow-up Study, n = 39,418; Nurses’ Health Study, n = 65,730). Sugar-sweetened beverages and alcohol intake were removed from the AHEI calculation. 1HRs ratios and 95% CIs derived from the multivariable model adjusted for the following: age, ethnicity/race; smoking status; alcohol intake; multivitamin use; in women, postmenopausal status and menopausal hormone use; family history of myocardial infarction; baseline hypertension; baseline hypercholesterolemia; calorie intake; body mass index; and AHEI. 2Physical activity was dichotomized to meet or do not meet the United States Physical activity guidelines of ≥150 min/wk of moderate–vigorous physical activity recommendations (corresponds to ≥7.5 MET h/wk or ≥450 MET min/wk). AHEI, Alternative Healthy Eating Index; MET, metabolic equivalent task.
The results of the sensitivity analyses were consistent with those from primary analyses. When we selectively stop updating diet, the independent associations between SSB or ASB consumption and risk of CVD and CAD, were slightly attenuated, whereas the independent association between SSB intake and risk of stroke remained consistent (Supplemental Table 10). The results of the independent association between physical activity and risk of CVD remained unchanged (Supplemental Table 10). The SSB or ASB intakes and physical activity joint associations and risk of CVD findings were consistent after we stopped updating diet (Supplemental Table 11). The independent and joint association results persisted after we excluded BMI from the final model (Supplemental Tables 12 and 13), except for the ASBs and physical activity joint association results that were strengthened (Supplemental Table 13). Results remained consistent when we additionally adjusted for socioeconomic status (Supplemental Tables 14–15) and when we adjusted for dietary factors in lieu of AHEI (Supplemental Tables 16 and 17). The pooled HRs for risk of CVD in heavy SSB consumers who met and did not meet the physical activity guidelines were 1.14 (95% CI: 1.08, 1.21) and 1.44 (95% CI: 1.35, 1.53), respectively (Supplemental Table 18). The pooled HRs for heavy SSB or ASB consumers and who were in the top 25% of physical activity (high physical activity level) were slightly attenuated—compared with. using dichotomized physical activity—for all outcomes (Supplemental Table 19).
Discussion
In 2 large prospective cohorts of United States males and females followed up for >30 y, we found positive linear associations between SSB consumption and the risk of CVD and CAD, whereas physical activity was inversely associated with CVD, CAD, and stroke risk, after adjusting for risk factors and other dietary variables. Marginally, significant associations were observed per serving increment per day of ASB intake and the risk of CVD. Most importantly, our stratified analyses showed that there was a positive association between SSB intake and physical activity levels. Moreover, our joint analysis showed that individuals who met physical activity guidelines and consumed >2 servings/d of SSBs had a higher risk of CVD than those who met physical activity guidelines and consumed SSBs <1/mo. To our knowledge, this study is the first large prospective study to examine the joint associations of SSB or ASB consumption and physical activity and the incidence of CVD. Overall, our study suggests that the protective effects of physical activity do not mitigate the detrimental impact of SSB consumption on cardiovascular health.
Before discussing the joint association results, we would like to briefly address the results from the independent associations. Compared with those who never/rarely consumed SSBs, we found a 21% higher incidence of CVD in participants who reported consuming ≥2 servings/d of SSBs. These results are consistent with findings from several meta-analyses showing that SSB intake is associated with CVD risk in adults, after adjustment for physical activity [3,5,26]. The most recent meta-analysis (7 prospective cohorts with 16,937,316 person-years of follow-up and 16,915 incident CVD cases), found that SSB consumption was associated with a 9% higher risk of CVD (95% CI: 1.01, 1.18) when extreme categories of intake (none or <1 per month compared with 1 or more per day) were compared [5]. Regarding ASBs, we found a weaker association, a 3% higher risk of CVD, with marginal significance, and a 5% higher risk of stroke. A meta-analysis that included 6 prospective studies (16,281,005 person-years of follow-up with 18,077 incident CVD events) showed a slightly higher risk, whereby the per serving increment of ASBs per day was associated with a 7% higher risk of incidence of CVD (95% CI: 1.05, 1.10) [5]. In addition, each serving increment of ASBs per day was associated with a 6% higher risk of CAD and a 9% higher risk of stroke. Overall, the relationship between ASBs and CVD is less clear compared with the consistent evidence for SSBs.
Concerning physical activity, we observed a 34% lower risk in the incidence of CVD in those in the highest quintile of physical activity, when compared with the lowest quintile and after accounting for demographic and lifestyle factors. A consistent and strong protective association has been reported with leisure time (relative risk: 0.76; 95% CI: 0.70, 0.82 in males 0.73; 95% CI: 0.68, 0.78 in females) and occupational physical activity (relative risk: 0.89; 95% CI: 0.82, 0.97 in males and 0.83; 95% CI: 0.67, 1.03 in females) [49]. Furthermore, a recent systematic review and meta-analysis (33 studies with 1,683,693 participants and 89,493 events, follow-up = 12.8 y) reported that an increase from being inactive to attaining recommended physical activity levels was associated with a lower risk of CVD incidence by 17% (95% CI: 0.77, 0.89), after adjusting for body weight [50].
The joint associations between SSB or ASB intakes and physical activity and risk of CVD findings are novel. Findings provide evidence against the argument that physical activity could counterbalance the potential health risk that would have been induced by SSB or ASB intake. Participants who did not meet the physical activity recommendations had a higher risk of CVD at all levels of SSB; however, participants who not only consumed ≥2 servings SSBs/wk, compared with never/rarely consumers of SSBs, but also met physical activity guidelines, had a 15% higher risk of CVD. Thus, meeting the physical activity guidelines while consuming SSBs twice per week was still harmful. Of note, stratified analysis by physical activity guideline cut points showed that both inactive and active participants had a higher risk of CVD and CAD with higher intake of SSBs, thus providing strong support for the aforementioned findings. However, we did not observe a similar pattern with ASB intake, although ASB intake has been associated with obesity, chronic disease incidence, and even all-cause and CVD-specific mortality in meta-analyses [51,52] and a recent umbrella review [53]. A possible explanation for this finding can be that physical activity might be counterbalancing the noxious influence of ASBs compared with SSBs and that ASBs have fewer or no calories/sugar (compared with SSBs), so exercise/movement weakens the risk of CVD associated by ASB intake alone. Moreover, we must also consider the possibility of reverse causation in this observational epidemiology study. ASB consumers could choose these beverages because they developed a cardiometabolic condition, became ill, or even noticed weight gain, which would drive them to switch from SSBs to ASBs, affecting the association we might expect and see. The increased risk between ASB and CVD, when combined with inactivity, was primarily attributable to the lack of physical activity.
Our study findings align with the results by Ding et al. [34], who used UK Biobank data and examined independent and interactive associations of physical activity and diet with all-cause, CVD, and physical activity, diet, and adiposity-related cancer mortality, reporting that when comparing across physical activity and diet combinations, the lowest risk combinations consistently included the higher levels of physical activity and the highest diet quality score. Although we did not address diet quality or mortality, we can accentuate that high SSB intake is part of a suboptimal diet and is associated with poor diet quality [54,55]. Therefore, our joint SSB and physical activity analysis suggest the same signal as Ding et al. [34], where we observed that a lower intake of SSBs and adequate physical activity combinations were associated with a lower risk of CVD. Although we did not observe a multiplicative interaction between SSB or ASB and physical activity and risk of CVD, we did detect significant additive interactions between SSB and physical activity and risk of CVD and CAD and between ASB and physical activity and risk of CVD and stroke. Of note, in a previous study in males, De Koning et al. [8] reported no significant interaction between SSB and physical activity. However, our study considers another 10 y of follow-up with additional accrued cases. However, the impact of SSBs on CVD risk is still observed even in participants who met physical activity recommendations and were in the top 25% of physical activity levels. Moreover, Ding et al. [34] did not observe additive or multiplicative interactions between physical activity categories and dietary quality combinations.
Strengths of this study include large sample size, prospective population-based design with long and high rates of follow-up, well-defined outcomes, and the use of repeated and validated measurements of diet and other lifestyle data. Limitations of this study include the difficulty in inferring causality due to the observational design. In addition, residual confounding remains a possibility although the analyses were extensively adjusted for potential confounders. Nonetheless, we acknowledge that socioeconomic factors may impact the reported results because it is possible that different levels of physical activity and SSB or ASB intakes are closely related to ethnic background, income, and deprivation index. In addition, reverse causation in the ASB analyses is a plausible limitation. Moreover, the dietary information was self-reported, and our assessment of SSBs and ASBs and physical activity inevitably had some degree of measurement error. However, the use of repeated measurements lessens random measurement error caused by within-person variation. Furthermore, our study population consisted of primarily non-Hispanic White health professionals, thus the generalizability of our results to other populations may be limited.
A higher consumption of SSBs was independently associated with a higher risk of CVD, whereas physical activity was independently associated with a lower risk of CVD. A higher intake of SSB was associated with risk of CVD regardless of physical activity levels. Even when individuals are physically active, higher consumption of SSBs is associated with a higher risk of CVD. A greater intake of SSBs combined with physical inactivity conferred the highest risk. Our results underscore the adverse health impact of both SSBs consumption and being physically inactive on CVD risk and support public health recommendations and policies to limit the intake of SSBs and maintain adequate physical activity levels.
Authors’ contributions
The authors’ responsibilities were as follows – LSP, FBH, MGF: participated in project conception and development of research methods; LSP: primarily analyzed the data and performed analysis; MGF, FBH, DKT, SNB, J-PD-C: provided oversight; LSP: drafted the first draft of the manuscript, with supervision of MG-F; LSP, FBH, MGF: had primary responsibility for final content; and all authors: significantly contributed to the final draft of the manuscript and provided final approval of the version to be published. LSP, as corresponding author, attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare the following: support from the National Institutes of Health (NIH) for the submitted work; LSP has been supported by grants T32 DK007703-26 from NIH and Harvard Chan Yerby Fellowship at Harvard T.H. Chan School of Public Health as a research fellow for the current work, and discloses awards from NHLBI and from the Nutrition Obesity Research Center at Harvard; DKT has been supported by grant R01DK125803 from NIH and is currently a member of the 2025 United States Dietary Guidelines Scientific Advisory Committee, reviewing beverages and cardiovascular disease; SNB has been supported by grants R01DK131753 and R01DK120560 from NIH and discloses a pending patent “system and methods for conducting nutritional health risk assessments and adapting risk analyses based on actual health outcomes” (provisional application filed on May 20, 2022; United States Application No. 63/344,293); WCW is part of the EAT Foundation Advisory Board (unpaid position) and receives royalties for the textbook Nutritional Epidemiology, Oxford University Press; DSL discloses royalties for books that recommend a carbohydrate-modified diet; CBE has been supported by grant R01DK125273 from NIH and from Thrasher Research Fund, New Balance Foundation, and Arnold Ventures, and honoraria from Columbia Cornell Obesity course and University of Arkansas for Medical Sciences (speaker); J-PD-C has been supported by grants and received payments (research grant review committee), from the Dairy Farmers of Canada. DEH reports grants from NIH (T32CA009001) and the Boston Nutrition Obesity Research Center and received honoraria and consulting fees from Boston University. MGF reports support from Novo Nordisk Foundation (grant NNF18CC0034900). This study was supported by the aforementioned sources of funding, with no additional sponsors. All other authors report no conflicts of interest. DKT is the Academic Editorial and DSL is an Associate Editor for The American Journal of Clinical Nutrition and played no role in the Journal’s evaluation of the manuscript.
Funding
The research reported in this manuscript was supported by National Institutes of Health grants UM1 CA186107, U01 CA167552, R01 HL034594, R01 HL088521, and R01 HL35464. Moreover, this work was supported by grant T32 DK007703-26 from the National Institute of Diabetes and Digestive and Kidney Diseases and the Harvard Chan Yerby Fellowship at Harvard T.H. Chan School of Public Health (to LSP).
Data availability
Data described in the manuscript, code book, and analytic code will be made available on request pending. Please visit the Nurses’ Health Study and Health Professionals Follow-Up Study sites where sharing mechanisms are described: https://www.nurseshealthstudy.org or https://www.hsph.harvard.edu/hpfs/.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2024.01.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Malik V.S., Hu F.B. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11:1840. doi: 10.3390/nu11081840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik V.S., Hu F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022;18(4):205–218. doi: 10.1038/s41574-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narain A., Kwok C.S., Mamas M.A. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int. J. Clin. Pract. 2016;70(10):791–805. doi: 10.1111/ijcp.12841. [DOI] [PubMed] [Google Scholar]
- 4.Malik V.S., Pan A., Willett W.C., Hu F.B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin J., Zhu Y., Malik V., Li X., Peng X., Zhang F.F., et al. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv. Nutr. 2021;12(1):89–101. doi: 10.1093/advances/nmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik V.S., Popkin B.M., Bray G.A., Després J.-P., Willett W.C., Hu F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung T.T., Malik V., Rexrode K.M., Manson J.E., Willett W.C., Hu F.B. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009;89(4):1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Koning L., Malik V.S., Kellogg M.D., Rimm E.B., Willett W.C., Hu F.B. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125(14):1735–1741. doi: 10.1161/CIRCULATIONAHA.111.067017. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco L.S., Lacey J.V., Martinez M.E., Lemus H., Araneta M.R.G., Sears D.D., et al. Sugar-sweetened beverage intake and cardiovascular disease risk in the california teachers study. J. Am. Heart. Assoc. 2020;9(10) doi: 10.1161/JAHA.119.014883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein A.M., de Koning L., Flint A.J., Rexrode K.M., Willett W.C. Soda consumption and the risk of stroke in men and women. Am. J. Clin. Nutr. 2012;95(5):1190–1199. doi: 10.3945/ajcn.111.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson S.C., Akesson A., Wolk A. Sweetened beverage consumption is associated with increased risk of stroke in women and men. J. Nutr. 2014;144(6):856–860. doi: 10.3945/jn.114.190546. [DOI] [PubMed] [Google Scholar]
- 12.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik V.S., Popkin B.M., Bray G.A., Després J.-P., Hu F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J., Soto M.J., Dunn C.G., Bleich S.N. Trends and patterns in sugar-sweetened beverage consumption among children and adults by race and/or ethnicity, 2003–2018. Public Health Nutr. 2021;24(9):2405–2410. doi: 10.1017/S1368980021001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu F.B. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013;14(8):606–619. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhouri T.H., Kit B.K., Ogden C.L. NCHS Data Brief; 2012. Consumption of diet drinks in the United States, 2009–2010; pp. 1–8. [PubMed] [Google Scholar]
- 17.Johnson R.K., Lichtenstein A.H., Anderson C.A.M., Carson J.A., Després J.P., Hu F.B., et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138 doi: 10.1161/CIR.0000000000000569. e126–e140. [DOI] [PubMed] [Google Scholar]
- 18.Mossavar-Rahmani Y., Kamensky V., Manson Joann E., Silver B., Rapp S.R., Haring B., et al. Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women’s Health Initiative. Stroke. 2019;50:555–562. doi: 10.1161/STROKEAHA.118.023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chazelas E., Debras C., Srour B., Fezeu L.K., Julia C., Hercberg S., et al. Sugary drinks, artificially-sweetened beverages, and cardiovascular disease in the NutriNet-Santé cohort. J. Am. Coll. Cardiol. 2020;76(18):2175–2177. doi: 10.1016/j.jacc.2020.08.075. [DOI] [PubMed] [Google Scholar]
- 20.Toews I., Lohner S., Küllenberg De Gaudry D., Sommer H., Meerpohl J.J. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364 doi: 10.1136/bmj.k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng Y., Li S., Khan J., Dai Z., Li C., Hu X., et al. Sugar- and artificially sweetened beverages consumption linked to type 2 diabetes, cardiovascular diseases, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutrients. 2021;13(8):2636. doi: 10.3390/nu13082636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin B.A., Thompson C.P.D., Al-Zaiti S.S., Albert C.M., Hivert M.F., Levine B.D., et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective—an update: a scientific statement from the American Heart Association. Circulation. 2020;141:e705. doi: 10.1161/CIR.0000000000000749. –e736. [DOI] [PubMed] [Google Scholar]
- 23.Tian D., Meng J. Exercise for prevention and relief of cardiovascular disease: prognoses, mechanisms, and approaches. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3756750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q., Zhang Z., Gregg E.W., Flanders W.D., Merritt R., Hu F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014;174(4):516–524. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik V.S., Li Y., Pan A., De Koning L., Schernhammer E., Willet W.C., et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113–2125. doi: 10.1161/CIRCULATIONAHA.118.037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C., Huang J., Tian Y., Yang X., Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis. 2014;234(1):11–16. doi: 10.1016/j.atherosclerosis.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Stampfer M.J., Hu F.B., Manson J.E., Rimm E.B., Willett W.C. Primary prevention of coronary heart disease in women through diet and lifestyle. N. Engl. J. Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 28.Chiuve S.E., McCullough M.L., Sacks F.M., Rimm E.B. Healthy lifestyle factors in the primary prevention of coronary heart disease among men. Circulation. 2006;114(2):160–167. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 29.Chiuve S.E., Rexrode K.M., Spiegelman D., Logroscino G., Manson J.E., Rimm E.B. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118(9):947–954. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chomistek A.K., Chiuve S.E., Eliassen A.H., Mukamal K.J., Willett W.C., Rimm E.B. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J. Am. Coll. Cardiol. 2015;65(1):43–51. doi: 10.1016/j.jacc.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards M.K., Shivappa N., Mann J.R., Hébert J.R., Wirth M.D., Loprinzi P.D. The association between physical activity and dietary inflammatory index on mortality risk in U.S. adults. Phys. Sportsmed. 2018;46(2):249–254. doi: 10.1080/00913847.2018.1443665. [DOI] [PubMed] [Google Scholar]
- 32.Brown J.C., Harhay M.O., Harhay M.N. Physical activity, diet quality, and mortality among community-dwelling prefrail and frail older adults. J. Nutr. Gerontol. Geriatr. 2016;35(4):253–266. doi: 10.1080/21551197.2016.1247022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez-Alvarez I., Zazpe I., Pérez de Rojas J., Bes-Rastrollo M., Ruiz-Canela M., Fernandez-Montero A., et al. Mediterranean diet, physical activity and their combined effect on all-cause mortality: the Seguimiento Universidad de Navarra (SUN) cohort. Prev. Med. 2018;106:45–52. doi: 10.1016/j.ypmed.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Ding D., Van B.J., Nguyen B., Stamatakis E., Elbarbary M., Veronese N., et al. Physical activity, diet quality and all-cause cardiovascular disease and cancer mortality: a prospective study of 346 627 UK Biobank participants. Br. J. Sports Med. 2022:1–10. doi: 10.1136/bjsports-2021-105195. [DOI] [PubMed] [Google Scholar]
- 35.Colditz G.A., Manson J.E., Hankinson S.E. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J. Women’s Heal. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 36.Rimm E.B., Giovannucci E.L., Willett W.C., Colditz G.A., Ascherio A., Rosner B., et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 37.Yuan C., Spiegelman D., Rimm E.B., Rosner B.A., Stampfer M.J., Barnett J.B., et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am. J. Epidemiol. 2018;187(5):1051–1063. doi: 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willett W.C., Sampson L., Stampfer M.J., Rosner B., Bain C., Witschi J., et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 39.Wolf A.M., Hunter D.J., Colditz G.A., Manson J.E., Stampfer M.J., Corsano K.A., et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int. J. Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 40.Rose G. World Health Organization; Albany, NY: 1982. Cardiovascular survey methods. WHO Publications Centre distributor. [PubMed] [Google Scholar]
- 41.Walker A.E., Robbins M., Weinfeld F.D. The National Survey of Stroke. Clinical findings. Stroke. 1981;12(2 Pt 2 Suppl 1):l13–l44. [PubMed] [Google Scholar]
- 42.Stampfer M.J., Willett W.C., Speizer F.E., Dysert D.C., Lipnick R., Rosner B., et al. Test of the National Death Index. Am. J. Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 43.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., Mccullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US H.H.S. 2nd ed. US Department of Health and Human Services; Washington, DC: 2018. Physical Activity Guidelines for Americans. [Google Scholar]
- 45.Vanderweele T.J., Knol M.J. A tutorial on interaction. Epidemiol. Methods. 2014;3(1):33–72. [Google Scholar]
- 46.Vanderweele T.J., Tchetgen Tchetgen E.J. Attributing effects to interactions. Epidemiology. 2014;25(5):711. doi: 10.1097/EDE.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li R., Chambless L. Test for additive interaction in proportional hazards models. Ann. Epidemiol. 2007;17(3):227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Hu F.B., Stampfer M.J., Rimm E., Ascherio A., Rosner B.A., Spiegelman D., et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 49.Li J., Siegrist J. Physical activity and risk of cardiovascular disease—a meta-analysis of prospective cohort studies. Int. J. Environ. Res. Public Health. 2012;9(2):391–407. doi: 10.3390/ijerph9020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahid A., Manek N., Nichols M., Kelly P., Foster C., Webster P., et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J. Am. Heart Assoc. 2016;5(9) doi: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin P., Li Q., Zhao Y., Chen Q., Sun X., Liu Y., et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020;35(7):655–671. doi: 10.1007/s10654-020-00655-y. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y.B., Jiang Y.W., Chen J.X., Xia P.F., Pan A. Association of consumption of sugar-sweetened beverages or artificially sweetened beverages with mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Adv. Nutr. 2021;12(2):374–383. doi: 10.1093/advances/nmaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diaz C., Rezende L.F.M., Sabag A., Lee D.H., Ferrari G., Giovannucci E.L., et al. Artificially sweetened beverages and health outcomes: an umbrella review. Adv. Nutr. 2023;14(4):710–717. doi: 10.1016/j.advnut.2023.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leung C.W., DiMatteo S.G., Gosliner W.A., Ritchie L.D. Sugar-sweetened beverage and water intake in relation to diet quality in U.S. children. Am. J. Prev. Med. 2018;54(3):394–402. doi: 10.1016/j.amepre.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doherty A.M., Lacko A.M., Popkin B.M. Sugar-sweetened beverage (SSB) consumption is associated with lower quality of the non-SSB diet in US adolescents and young adults. Am. J. Clin. Nutr. 2021;113(3):657–664. doi: 10.1093/ajcn/nqaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available on request pending. Please visit the Nurses’ Health Study and Health Professionals Follow-Up Study sites where sharing mechanisms are described: https://www.nurseshealthstudy.org or https://www.hsph.harvard.edu/hpfs/.