Abstract
We have previously shown that binding of human immunodeficiency virus type 1 (HIV-1) virions to CD4 receptors stimulates association of Lck with Raf-1 and results in the activation of Raf-1 kinase in a Ras-independent manner. In the present study, we demonstrate that HIV-1 envelope glycoproteins of both T-cell-tropic and macrophagetropic strains rapidly activate the ERK/mitogen-activated protein (MAP) kinase pathway and the binding of nuclear transcription factors (AP-1, NF-κB, and C/EBP) and stimulate expression of cytokine and chemokine genes. The activation of this signaling pathway requires functional CD4 receptors and is independent of binding to CXCR4. Binding of the natural ligand stromal cell-derived factor 1 (SDF-1) to CXCR4, which inhibits entry of T-cell-tropic HIV-1, activates also the ERK/MAP kinase pathway. However, SDF-1 did not affect the CD4-mediated expression of cytokine and chemokine genes. These results provide firm molecular evidence that binding of HIV-1 envelope glycoproteins to CD4 receptor initiates a signaling pathway(s) independent of the binding to the chemokine receptor that leads to the aberrant expression of inflammatory genes and may contribute significantly to HIV-1 replication as well as to deregulation of the immune system.
Chronic activation of the immune system is commonly observed in AIDS, and aberrant expression of inflammatory cytokines observed during progression of human immunodeficiency virus type 1 (HIV-1) disease has been implicated in the pathogenicity of AIDS (24, 25). Elevated levels of cytokines were detected in serum (7, 27, 35) as well as in T lymphocytes infiltrating lymph nodes of HIV-infected individuals (22). However, the molecular mechanism by which HIV-1 modulates the expression of cytokine genes is not completely understood. The HIV-1-mediated changes in cellular signaling may occur as a consequence of HIV-1 binding to its receptors as well as of viral replication. The Nef protein, encoded by an early viral gene, was shown to interact with several cellular proteins such as tyrosine kinases Hck (36) and Lck (17, 30), as well as cellular serine/threonine kinases (38, 40), and to induce synthesis of interleukin-6 (IL-6) in peripheral blood mononuclear cells (PBMC) (14). Overexpression of another HIV-1 regulatory protein, Tat, induced both tumor necrosis factor alpha (TNF-α) (10) and gamma interferon (IFN-γ) (48). Furthermore, the observation that upregulation of chemokine gene production in PBMC requires productive infection implies involvement of HIV-1-encoded proteins (52). However, binding of HIV-1 virions to their receptors alone can also induce cellular signaling since both the primary CD4 receptor and the chemokine coreceptors (19, 59) can trigger the signaling pathway upon ligand binding. The signaling potential of CD4 is mediated by its association with a cytoplasmic Src-like tyrosine kinase p56Lck (50). Although CD4 generally functions by association with the T-cell receptor, it has been also identified as a receptor for IL-16 (12, 18), suggesting that it can transmit signals independently of the T-cell receptor.
Binding of HIV-1 to CD4 is necessary but not sufficient for productive infection, and chemokine receptors CCR5 and CXCR4 were first identified as HIV-1 coreceptors. These receptors belong to the superfamily of seven-transmembrane G-protein-coupled receptors. Binding of HIV-1 to either CXCR4 or CCR5 receptors generally determines the tropism of HIV-1 strains either for T cells or macrophages, respectively. The CC chemokines RANTES, MIP-1α, and MIP-1β were found to suppress the macrophagetropic HIV-1 infection (16), and this effect is related to both the ligand occupancy and downregulation of receptors (1, 2).
As a part of the studies of the role of cytokines in HIV-1 pathogenesis, we investigated the very early events in HIV-1 replication and showed that cross-linking of the CD4 receptors, induced by binding of HIV-1 virions to T cells, enhanced association of Lck with Raf-1 and consequently activated the Raf-1 kinase (47). Surprisingly, the HIV-1-mediated signaling did not result in the activation of Ras GTP-binding activity or its association with Raf-1. Since the signaling pathway generated by HIV-1 binding is not identical to the classical Ras/Raf-1 pathway, in the present study we examined (i) whether this pathway is functional and results in the stimulation of transcriptional nuclear factors and activation of cytokine genes and (ii) whether the binding of HIV-1 virions to the chemokine coreceptors contributes to CD4-mediated signaling. We demonstrate that binding of HIV-1 to CD4 receptors activates the MEK/ERK kinase pathway, stimulates the expression of nuclear factors (AP-1, NF-κB, and C/EBP), and results in the expression of inflammatory genes. We also show that this signaling pathway is independent of HIV-1 binding to the chemokine receptors and that it can be induced in CD4-positive cells by both T-cell-tropic and macrophagetropic HIV-1 variants.
MATERIALS AND METHODS
Reagents.
Human stromal cell-derived factor 1α (SDF-1α) was prepared as described previously (32). Mouse monoclonal anti-human CD4 (Q4120), control mouse immunoglobulin G1, and goat anti-mouse antibodies were from Sigma. Recombinant gp120 envelope glycoprotein from the T-cell-tropic HIV-1 IIIB (gp120 IIIB) and mouse anti-gp120 monoclonal antibodies were purchased from Intracel (Cambridge, Mass.). Phosphoprotein-specific antibodies detecting MEK1/2 when activated by phosphorylation at Ser217/221 and ERK1/2 (p44/p42 mitogen-activated protein [MAP] kinase) when activated by phosphorylation at Thr202 and Tyr204 as well as antibodies detecting total levels of MEK1/2 and ERK1/2 were purchased from New England Biolabs (Beverly, Mass.).
Cell cultures.
Jurkat T cells, clone E6-1 (ATCC TIB-152), and A2.01/CD4.401 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Sigma), 2 mM l-glutamine, and gentamicin (50 μg/ml). Before using in experiments, the cells were starved for 24 h in RPMI medium supplemented with 0.5% FCS.
Virus stock preparation and virus titer.
Viral stock of the NL4-3 clone of HIV-1 was prepared as previously described (47). Viral stock of macrophagetropic clone AD8 (57), obtained from F. Maldarelli (National Institutes of Health, Bethesda), was produced by electroporation of the molecular clone into Jurkat cells and expansion in phytohemagglutinin (PHA)-stimulated PBMC. HIV-1 Ba-L (28) stock was generated by infecting PHA-stimulated PBMC with cell-free virus (obtained from S. Gartner, Johns Hopkins University, Baltimore, Md.). Viral stocks of the replication-competent NL4-3-derived envelope mutants with significantly reduced binding to CD4, NL-D368E and NL-D368P (obtained from D. Kabat, Oregon Health Sciences University, Portland), were produced by transfection of 293T cells with plasmid DNA and collecting culture supernatants 48 h later. All culture supernatants containing HIV-1 were clarified by low-speed centrifugation and filtration through a 0.45-μm-pore-size filter and kept frozen at −80°C, or virus was concentrated and purified by ultracentrifugation through a cushion of sucrose buffer as described previously (47). Pelleted virus was used immediately in experiments after resuspension in RPMI–0.5% FCS. The virus titer was monitored by the reverse transcriptase (RT) activity assay (55) and the infectivity titer (47). In addition, the levels of gp120 envelope in various viral preparations were determined by Western blot analysis. In the induction protocol, the cells (1 × 107 to 2 × 107 cells/ml in RPMI–0.5% FCS) were preincubated on ice for 60 min with the purified virus (5 RT units/cell) and then transferred immediately to 37°C and incubated for the indicated times, followed by washing twice in ice-cold phosphate-buffered saline and lysis in protein or RNA lysis buffer.
Western blot analysis.
Cell aliquots (107 cells) were solubilized in ice-cold lysis buffer containing 1% Triton X-100, 50 mM HEPES (pH 7.4), 100 mM NaCl, 10% glycerol, 5 mM MgCl2, and 1 mM EGTA, supplemented with the protease inhibitors leupeptin (10 μg/ml) and aprotinin (10 μg/ml) and the phosphatase inhibitors sodium orthovanadate (1 mM), sodium fluoride (50 mM), and β-glycerophosphate (20 mM). After 30 min on ice, the lysates were clarified by ultracentrifugation. Protein concentrations of the lysates were determined with the Pierce (Rockford, Ill.) bicinchoninic acid protein assay reagent. Proteins (50 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide gel), transferred to supported nitrocellulose membranes, probed with specific antibodies (usually diluted 1:1,000), and then incubated with secondary horseradish peroxidase-conjugated antibody (1:100,000). Bound antibodies were detected with the SuperSignal ULTRA chemiluminescent substrate (Pierce).
RT-PCR analysis of cytokine and chemokine mRNA expression.
Total RNA was isolated by using RNeasy Total RNA purification system (Qiagen), and 1 μg of DNase-treated RNA was used for cDNA synthesis using Superscript II RNase H RT and oligo(dT)12–18 primers (Gibco-BRL); 1/10 of this reaction product was used as a template for PCR amplification with Taq polymerase (Supermix; Gibco-BRL). The mRNA for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or Raf-1 kinase served as internal control. Primers for CXCR4, GAPDH (39), CCR5 (20), MIP-1α, MIP-1β, and TNF-α (60) as well as amplification conditions were described elsewhere. Primers for IL-2 were from Stratagene. The following primers were used: for IFN-γ (413 bp), 5′-CTGTTACTGCCAGGACCCAT-3′ (sense) and 5′-GCATCTGACTCCTTTTTCGC-3′ (antisense); for IL-6 (347 bp), 5′-GAACTCCTTCTCCACAAGCG-3′ (sense) and 5′-TGATGATTTTCACCAGGCAA-3′ (antisense); for Fas antigen (357 bp), 5′-ATAAGCCCTGTCCTCCAGGT-3′ (sense) and 5′-GACAAAGCCACCCCAAGTTA-3′ (antisense); and for Fas ligand (439 bp), 5′-CACCTACAGAAGGAGCTGGC-3′ (sense) and 5′-TAAGATTGAACACTGCCCCC-3′ (antisense). These cDNAs were amplified for 30 cycles (94°C for 45 s, 60°C for 45 s, and 72°C for 1.5 min), and PCR products were resolved by electrophoresis in 2% agarose gels and visualized by ethidium bromide staining.
Electrophoretic mobility shift assay.
Nuclear extracts for electrophoretic mobility shift assay were prepared as described previously (45). Double-stranded oligonucleotides representing consensus and mutated binding motifs for transcription factors NF-κB, AP-1, and C/EBP (Santa Cruz Biotechnology) were end labeled by using T4 polynucleotide kinase and [γ-32P]ATP. Specificity of the binding of nuclear proteins to consensus oligonucleotides was determined by the analysis of the complex formation in the presence of 10- or 50-fold molar excess of the unlabeled corresponding oligonucleotide or by using oligonucleotides with mutated binding sites. Protein composition of the formed complexes was identified in a supershift assay using specific antibodies (Santa Cruz Biotechnology).
RESULTS
Binding of HIV-1 to CD4+ T cells induces rapid accumulation of nuclear transcription factors.
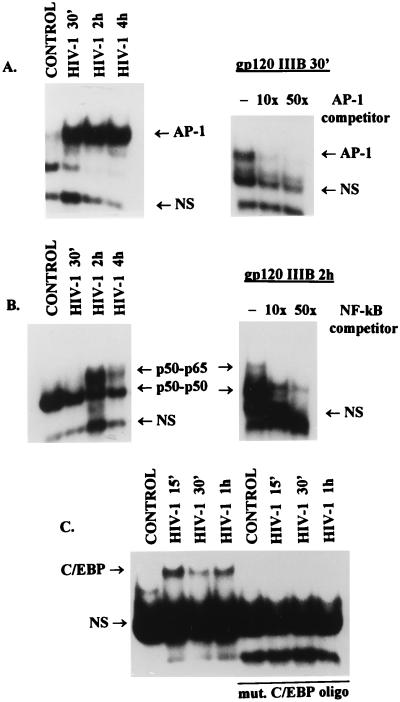
We have previously shown that binding of HIV-1 virions to CD4 receptors stimulates association of tyrosine kinase p56Lck with serine/threonine kinase Raf-1 and activates Raf-1 in a Ras-independent manner (47). To determine whether this Raf-1-mediated signaling is functional, we examined, by a gel retardation assay, the accumulation of transcription factors in the nucleus of Jurkat T cells stimulated with T-cell-tropic NL4-3 HIV-1. The results show a rapid nuclear accumulation of AP-1 in Jurkat T cells as early as 30 min after HIV-1 binding (Fig. 1A). Similarly, binding of gp120 IIIB to Jurkat cells also resulted in the activation of AP-1. In addition, the binding of AP-1 was specific and was inhibited in the presence of 10- or 50-fold molar excess of an unlabeled oligonucleotide representing AP-1 binding site (Fig. 1A). The nuclear extracts from uninduced Jurkat T cells show a presence of a single NF-κB complex representing p50 homodimers (as determined in supershift assay [data not shown]). The presence of the p50/p65 NF-κB heterodimers was detected only after the stimulation with NL4-3 virions or gp120 IIIB and reached a maximum by 2 h (Fig. 1B). The binding was specific and could be abolished in the presence of 10- or 50-fold molar excess of an unlabeled NF-κB consensus oligonucleotide. Stimulation of NF-κB binding was also abolished when the HIV-1 virions were pretreated with soluble CD4 (data not shown), treatment that also abolished Raf-1 activation (47). These results are in accordance with results reported by others (4, 9, 13, 15). In addition to the induction of AP-1 and NF-κB, we have found that binding of HIV-1 virions to Jurkat cells resulted also in a rapid nuclear accumulation of C/EBP (NF-IL6) (Fig. 1C), a transcription factor that is required for the induction of promoters of several early-response genes and is implicated in the regulation of HIV-1 long terminal repeat activity (51). Binding of C/EBP was specific since it was not observed with oligonucleotide representing mutated C/EBP binding. Together, these results indicate that interaction of HIV-1 with CD4 receptor results in the stimulation of Raf-1 activity and nuclear accumulation of transcription factors that participate in the regulation of the inflammatory genes as well as the HIV-1 promoter.
FIG. 1.
Binding of T-cell-tropic NL4-3 or IIIB glycoprotein gp120 to CD4+ Jurkat T cells induces nuclear accumulation of transcription factors. Nuclear extracts were prepared from unstimulated (control) cells or cells exposed to NL4-3 (HIV-1) or gp120 IIIB (1 μg/ml) for the indicated times (45). Radiolabeled double-stranded oligonucleotides representing consensus binding sites for AP-1 (A), NF-κB (B), or C/EBP (C) were incubated with nuclear extracts (6 μg), and formed DNA-protein complexes were resolved on a 4% polyacrylamide gel as described in Materials and Methods. Specificity of induction of AP-1 and NF-κB was determined by competition with 10- and 50-fold molar excesses of unlabeled oligonucleotides representing appropriate consensus binding sites. Protein composition of the NF-κB complexes was determined in a supershift assay using specific antibodies against p50 or p65 (data not shown). Specificity of the formation of C/EBP was determined by using end-labeled oligonucleotides with mutated binding sites (mut. C/EBP oligo). NS represents nonspecific binding.
Anti-CD4 receptor antibodies induce the expression of inflammatory cytokine and chemokine genes.
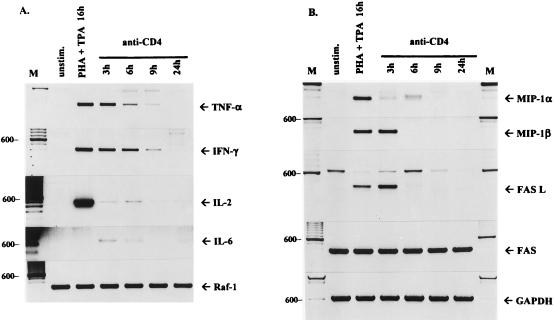
Productive viral replication has been implicated in the induction of proinflammatory cytokines in infected monocytes (52). However, it was recently shown that cross-linking of CD4 receptors on unfractionated PBMC was sufficient to induce IFN-γ and TNF-α gene expression (43). To determine whether the HIV-1-stimulated pathway leads to the expression of cytokine genes, we first analyzed the relative levels of cytokine mRNA in stimulated and unstimulated CD4-positive Jurkat T cells. Since Raf-1 activity can be effectively induced in Jurkat cells by cross-linking of CD4 receptors (47), the cells were stimulated with monoclonal anti-CD4 antibody Q4120 and the expression of inflammatory genes was analyzed by semiquantitative RT-PCR (Fig. 2). To control for possible differences in RNA levels, we analyzed the relative levels of Raf-1 and GAPDH RNAs as internal controls. Stimulation with the anti-CD4 antibody induced high levels of TNF-α and IFN-γ transcripts that could be detected for up to 9 h after stimulation (Fig. 2A). In unstimulated cells exposed to isotype-matched control mouse antibody, the expression of these cytokine genes was very low or undetectable. Cells stimulated with PHA and phorbol ester (tetradecanoyl phorbol acetate [TPA]) served as positive controls and showed high levels of TNF-α, IFN-γ, and IL-2 mRNAs. Interestingly, cross-linking of CD4 receptors also induced expression of chemokine MIP-1β and low levels of MIP-1α genes that were not expressed in uninduced cells but were induced by PHA and TPA stimulation (Fig. 2B). Stimulation of the CD4-dependent pathway also increased the relative levels of RANTES mRNA, although RANTES mRNA could also be detected in unstimulated Jurkat cells (data not shown). Promoters of RANTES, TNF-α, and IL-6 genes contain cis-acting elements that may serve as binding sites for NF-κB, AP-1, and C/EBP that are induced in HIV-1-stimulated cells. It is therefore conceivable that CD4-mediated binding of HIV-1 virions to the cells alone stimulates transcription of these inflammatory genes. Furthermore, we have detected the constitutive expression of Fas antigen in Jurkat T cells; however, the expression of Fas ligand was transient and was significantly upregulated by anti-CD4 antibody (Fig. 2B).
FIG. 2.
RT-PCR analysis of cytokine, chemokine, and FasL/Fas mRNA expression stimulated by anti-CD4 antibody. Total RNA was extracted from Jurkat T cells unstimulated (unstim.), treated for 16 h with PHA (5 μg/ml) and TPA (50 ng/ml), or stimulated with anti-CD4 antibody Q4120 (10 μg/ml) for the indicated times. RT-PCR-assisted amplification of cytokine (A) and chemokine and FasL/Fas (B) mRNAs was performed as described in Materials and Methods. PCR products were resolved by electrophoresis in 2% agarose gels and stained with ethidium bromide. Raf-1 and GAPDH represent internal controls. M, 100-bp molecular size markers (600-bp band marked on the left).
Binding of gp120 and anti-CD4 antibodies activates the MAP kinase pathway.
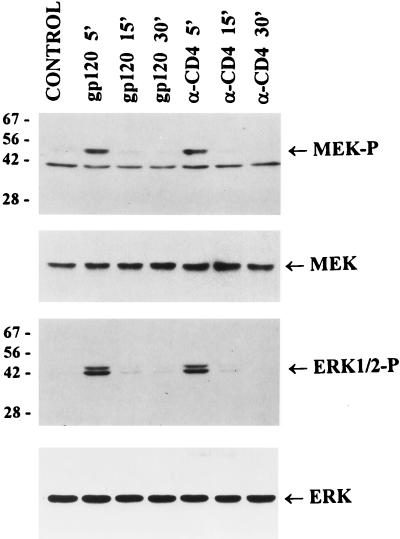
To determine whether the binding of gp120 or anti-CD4 antibodies to CD4 receptors activates MAP kinase pathway, we analyzed the levels of phosphorylated MEK1/2 (a direct cellular substrate for activated Raf-1) and ERK1/2 kinases in Jurkat cells stimulated for different times with recombinant gp120 IIIB or anti-CD4 (Q4120) antibodies. Using antibodies which can specifically recognize the activated and phosphorylated forms of MEK1/2 and ERK1/2 kinases, we found that stimulation with gp120 or anti-CD4 antibodies that induced cytokine and chemokine gene expression (Fig. 2) also resulted in a fast and transient phosphorylation of MEK1/2 and ERK1/2 (Fig. 3). These results support the concept that HIV-1 binding to CD4 receptor activates the expression of cytokine and chemokine genes through the Raf-1-activated MAP kinase pathway.
FIG. 3.
Activation of MEK and ERK/MAP kinases in response to T-cell-tropic gp120 or anti-CD4 antibodies. Jurkat cells were preincubated on ice for 60 min with recombinant gp120 IIIB (1 μg/ml) or anti-CD4 (α-CD4) antibody (5 μg/ml) or left untreated (control) and then incubated at 37°C for the indicated times before lysis. Proteins (50 μg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with antibodies specific for phosphorylated forms of MEK1/2 (MEK-P) and ERK1/2 (ERK1/2-P) as well as with antibodies specific for total MEK and ERK. The positions of molecular weight markers (in kilodaltons) and kinases are indicated.
Both T-cell-tropic and macrophagetropic HIV-1 can activate the MAP kinase pathway and expression of cytokine and chemokine genes in T cells.
Although results obtained with anti-CD4 antibodies show that CD4 receptor alone may transduce signals required for the expression of cytokine/chemokine genes independently of chemokine receptor signaling, we could not exclude the possibility that the interaction of HIV-1 with chemokine coreceptors and subsequent entry of the virus also affect cellular signaling and modulate the expression of inflammatory genes. We therefore analyzed the contribution of chemokine receptor occupancy in HIV-1-mediated stimulation of inflammatory gene expression. By RT-PCR analysis, we did not detect in Jurkat cells expression of the CCR5 gene encoding the major coreceptor for macrophagetropic viruses (20), while these cells expressed CXCR4, a major coreceptor for T-cell-tropic HIV-1 (5), and were fully permissive for the infection with T-cell-tropic HIV-1 NL4-3 (data not shown). To examine whether HIV-1 binding to a chemokine coreceptor contributes to the CD4-mediated expression of cytokine genes, we used macrophagetropic viruses Ba-L and AD8. Since CCR5 is not expressed in Jurkat cells and these viruses do not enter and infect these cells, the induction of inflammatory genes could be attributed solely to the interaction of the HIV-1 with the CD4 receptor.
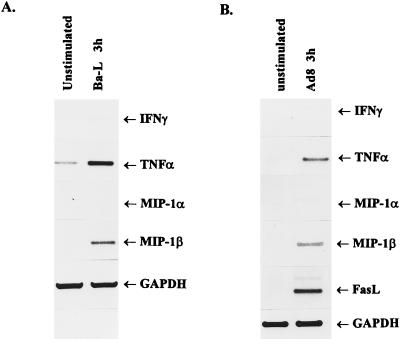
The results show that binding of Ba-L or AD8 to Jurkat T cells significantly upregulated the expression of TNF-α and MIP-1β genes (Fig. 4). In addition, AD8 also stimulated the expression of Fas ligand gene (Fig. 4B). However, in contrast to stimulation with anti-CD4 antibody, binding of macrophagetropic viruses stimulated the expression of MIP-1α and IFN-γ genes much less effectively. Although their significance is unclear, these differences may reflect different binding affinities of HIV-1 and anti-CD4 antibody. Carroll et al. (11) have recently shown that Ba-L binds to CD4+ T cells in the absence of the expression of CCR5 and viral entry, substantiating our conclusion that virus binding to CD4 is sufficient for the induction of cytokine and chemokine genes in T cells. These experiments have not excluded, however, the possibility that macrophagetropic viruses can bind to the receptor(s) of the CCR family present in Jurkat cells (37).
FIG. 4.
Binding of macrophagetropic viruses to CCR5-negative Jurkat T cells upregulates expression of cytokine, chemokine, and FasL genes. The cells were incubated for 3 h at 37°C with sucrose gradient-purified Ba-L (A) or AD8 (B) virions. Total RNA was isolated, and mRNA expression was analyzed by RT-PCR using primers described in Materials and Methods. GAPDH served as an internal control. PCR products were resolved on 2% agarose gels and stained with ethidium bromide.
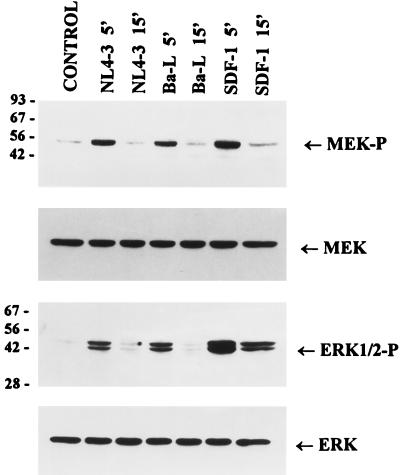
To determine whether the activation of cytokine gene expression by T-cell-tropic and macrophagetropic viruses in Jurkat cells correlates with the induction of the MAP kinase pathway, we analyzed the levels of phosphorylated and activated MEK1/2 and ERK1/2 MAP kinases upon binding of these viruses to Jurkat cells. The results in Fig. 5 show that binding of both NL4-3 and Ba-L strains of HIV-1 to Jurkat cells results in fast and transient phosphorylation and activation of both MEK and ERK1/2. Similarly, activation of ERK2 by HIV-1 LAI was shown recently (4). Surprisingly, however, MEK and ERK1/2 kinases could also be activated upon binding of the natural ligand SDF-1 to the CXCR4 receptor. Induction of the MAP kinase pathway upon binding of SDF-1 to CXCR4 has not been reported previously.
FIG. 5.
Activation of MEK and ERK/MAP kinases in response to the binding of T-cell-tropic or macrophagetropic HIV-1 or SDF-1. Jurkat cells were preincubated on ice for 60 min with purified virions or SDF-1 (5 μg/ml) or left untreated (control) and then incubated at 37°C for the indicated times before lysis. Proteins (50 μg) were resolved by SDS-PAGE and analyzed as described in the legend to Fig. 3. The positions of phosphorylated forms of MEK1/2 (MEK-P) and ERK1/2 (ERK1/2-P) as well as total MEK and ERK are indicated. Sizes are indicated in kilodaltons.
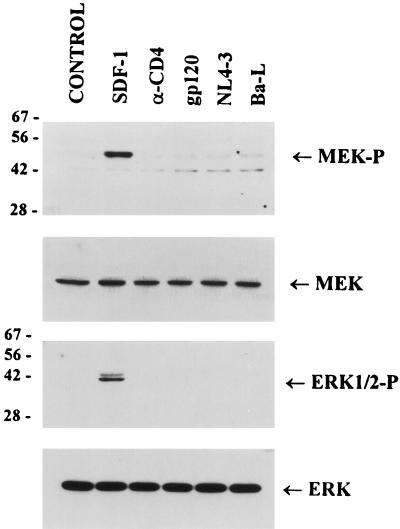
Activation of the MAP kinase pathway by gp120 or HIV-1 is dependent on the presence of cytoplasmic domain of CD4 that associates with Lck.
Since the binding of SDF-1 to CXCR4 receptor activated the MAP kinase pathway in Jurkat cells, we needed to establish whether the activation of the MAP kinase pathway observed upon binding of T-cell-tropic gp120 IIIB or NL4-3 is mediated by CD4 or CXCR4 receptors. We have used A2.01/CD4.401 cells expressing a truncated form of CD4 lacking the whole cytoplasmic domain of the receptor (4) and therefore unable to associate with Lck and induce signaling (58). Analysis of the relative levels of activated MEK1/2 and ERK1/2 MAP kinase (Fig. 6) shows that in these cells, neither anti-CD4 nor gp120 IIIB, NL4-3, or Ba-L antibodies were able to activate the MAP kinase pathway. Similarly, no induction of cytokine gene expression upon binding of NL4-3, Ba-L, or anti-CD4 antibodies could be detected by RT-PCR analysis in these cells (data not shown). Binding of SDF-1 ligand, however, activated the phosphorylation of MEK1/2 and ERK1/2 in these cells as effectively as in Jurkat cells. These results suggest that binding of T-cell-tropic NL4-3 or gp120 IIIB to the CXCR4 receptor does not activate the MEK/ERK signal transduction pathway as does the binding of the natural ligand SDF-1. These data correlate with the recent results of others, who have shown that T-cell-tropic gp160 derived from NL4-3 as well as gp120 IIIB did not induce a calcium flux in CD4+ T cells (59).
FIG. 6.
Activation of the MEK/ERK kinase pathway by T-cell-tropic or macrophagetropic HIV-1 or anti-CD4 antibody depends on the presence of cytoplasmic domain of CD4 receptor. A2.01/CD4.401 cells expressing truncated CD4 receptors were left untreated (control) or preincubated on ice for 60 min with SDF-1 (5 μg/ml), anti-CD4 antibody (5 μg/ml), gp120 IIIB (1 μg/ml), or purified NL4-3 or Ba-L (5 RT cpm/cell) and then incubated at 37°C for 5 min before lysis. Proteins (50 μg) were resolved by SDS-PAGE and analyzed as described in the legend to Fig. 3. The positions of phosphorylated forms of MEK1/2 (MEK-P) and ERK1/2 (ERK1/2-P) as well as total MEK and ERK are indicated. Sizes are indicated in kilodaltons.
SDF-1 neither induces nor inhibits HIV-1-stimulated expression of cytokine and chemokine genes in CD4+ T cells.
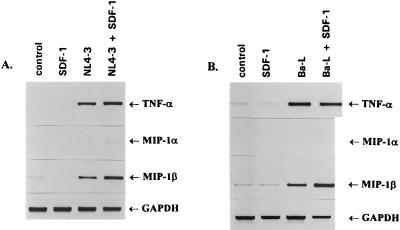
To further investigate the role of a chemokine coreceptor in the HIV-1-mediated induction of cytokine and chemokine gene expression in T cells, we examined the effect of binding the CXCR4 natural ligand, SDF-1, on T-cell-tropic NL4-3 virus-mediated cytokine gene induction (Fig. 7A). Binding of NL4-3 to Jurkat cells stimulated expression of TNF-α and MIP-1β genes, while the expression of MIP-1α and IFN-γ (not shown) was undetectable. The binding of SDF-1 to CXCR4 receptor, which prevents entry of T-cell line-tropic viruses (6, 41), did not stimulate transcription of these genes despite the fact that SDF-1 was able to activate the MAP kinase pathway (Fig. 5). Furthermore, binding of SDF-1 to CXCR4 receptor did not significantly affect the expression of cytokine and chemokine genes induced by NL4-3 (Fig. 7A). The lack of effect of SDF-1 on CD4-mediated signaling was not restricted to Jurkat T cells, as binding of NL4-3 to purified CD4+ peripheral blood T lymphocytes stimulated the expression of TNF-α and MIP-1β independently of the presence of SDF-1 (data not shown). As expected, binding of SDF-1 to CXCR4 receptor also did not affect stimulation of cytokine genes by a macrophagetropic virus (Fig. 7B). Since SDF-1 was able to block NL4-3 infection, these findings clearly demonstrate that induction of the early cytokine and chemokine genes in T cells is determined primarily by the interaction of the HIV-1 envelope with CD4 receptor and that viral tropism and/or viral entry does not play a critical role in CD4-mediated signaling.
FIG. 7.
SDF-1 does not affect the HIV-1-stimulated expression of cytokine and chemokine genes in Jurkat T cells. The cells were left untreated (control) or were stimulated with sucrose gradient-purified NL4-3 (A) or Ba-L virions (B), SDF-1 (5 μg/ml), or a combination of HIV-1 and SDF-1. After 3 h at 37°C, total RNA was isolated and expression of cytokine and chemokine genes was analyzed by RT-PCR as described in the legend to Fig. 2. GAPDH served as an internal control.
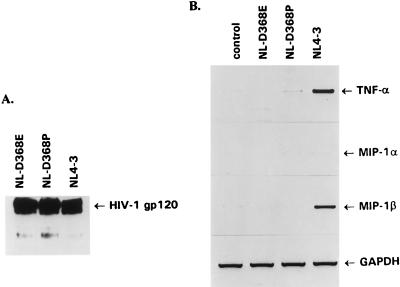
To determine whether the induction of cytokine gene expression is dependent on the affinity of HIV-1 binding to the CD4 receptor, we used two T-cell-tropic NL4-3-derived viruses that were shown to have a significantly reduced CD4 binding affinity (44) and compared their abilities to induce TNF-α and MIP-1β gene expression with that of NL4-3. To ensure that all of these viral preparations used in experiments contained the same amount of gp120, we analyzed the levels of gp120 in pelleted virions by Western blot analysis (Fig. 8A). Despite the fact that pelleted virions contained similar amounts of gp120, HIV-1 NL4-3 but neither clone NL-D368E nor clone NL-D368P was able to induce the expression of the TNF-α or MIP-1β gene (Fig. 8B). Thus, the ability of HIV-1 to induce cytokine gene expression is also determined by the affinity of binding of HIV-1 virions to CD4 receptors.
FIG. 8.
Induction of cytokine and chemokine gene expression is dependent on the affinity of HIV-1 binding to CD4 receptor. (A) The levels of gp120 envelope in pelleted virion preparations (108 RT cpm) of NL4-3 and NL4-3-derived clones NL-D368E and NL-D368P were determined by Western blot analysis using mouse anti-gp120 monoclonal antibodies. (B) Binding of NL4-3 but not NL-D368E and NL-D368P (45) induced expression of cytokine and chemokine genes in Jurkat cells. Purified virions (108 RT cpm) were incubated for 3 h at 37°C, total RNA was isolated, and expression of cytokine and chemokine genes was analyzed by RT-PCR as described in the legend to Fig. 2. GAPDH served as an internal control.
DISCUSSION
In this study, we have shown that binding of HIV-1 envelope glycoprotein gp120 to CD4 receptors on T cells results in the activation of the ERK/MAP kinase pathway and induction of transcription of cytokine and chemokine genes. Several lines of evidence indicate that the binding of HIV-1 envelope to CD4 receptors is essential in the induction of expression of the early inflammatory genes and that CXCR4 does not participate in this signaling. First, in Jurkat T cells that neither expressed CCR5 nor supported replication of macrophagetropic Ba-L and AD8, these two HIV-1 variants were still able to activate the ERK/MAP kinase pathway and upregulate expression of TNF-α and MIP-1β. Second, neither activation of the ERK/MAP kinase pathway nor induction of cytokine genes was detected in cells expressing a truncated CD4 receptor that is unable to associate with Lck and induce cellular signaling. However, since the extracellular part of CD4 receptor is not altered, these cells are still able to bind HIV-1 and support HIV-1 replication (4). Third, binding of SDF-1, a natural ligand for the CXCR4 receptor which inhibits replication of T-cell-tropic HIV-1 NL4-3 in Jurkat cells (data not shown) and CD4+ T cells (1), did not affect HIV-1-mediated activation of cytokine gene expression. The observation that downregulation of CCR5 coreceptors that prevented entry of macrophagetropic virus into CD4+ cells (11) did not affect virus binding is in accordance with our results.
These data strongly indicate the critical role of the CD4-mediated signal transduction pathway in the aberrant expression of cytokine and chemokine genes and extend previous observations that the binding of HIV-1 envelope glycoprotein gp120 or cross-linking of CD4 receptors with anti-CD4 antibodies induces synthesis of several cytokines, including TNF-α and IFN-γ (43, 56) as well as IL-10 (53) and IL-6 (42). We have, however, observed quantitative differences between the levels of cytokines induced by binding of anti-CD4 antibodies and different envelope mutants of T-cell-tropic NL4-3. While both the anti-CD4 antibody and wild-type NL4-3 stimulate the expression of TNF-α and MIP-1β, IFN-γ and MIP-1α were induced more effectively by anti-CD4 antibody than by the virus. Two NL4-3-derived clones, NL-D368E and NL-D368P, that have altered CD4 binding region of gp120 and consequently significantly reduced affinity for CD4 receptors (44) were not able to stimulate transcription of cytokine genes in Jurkat cells. Since the infectivity of these poorly CD4 binding virions was linearly dependent on the cell surface density of CD4 (33, 34), our data suggest that the ability of primary HIV-1 isolates to induce cytokine gene expression in vivo may be determined both by the affinity of viral glycoproteins for CD4 and by the number of CD4 receptors.
Since the discovery that the chemokine receptors that belong to a family of seven-transmembrane-domain G-protein-coupled receptors serve as HIV-1 coreceptors, numerous studies have addressed the question of whether the signaling pathway mediated by these receptors is required for viral infectivity. All of these studies indicate that G-protein-mediated signaling and intracellular calcium mobilization are not required for HIV-1 membrane fusion and entry of HIV-1 into the cells (3, 23, 29). The ability to uncouple chemokine receptor-mediated signaling from its ability to function as a coreceptor for HIV-1 entry suggests that internalization of HIV-1 proceeds through a novel mechanism that is unrelated to the signaling function of these receptors. However, recent data from our laboratories as well as data of others indicate that HIV-1 gp120 can function as a ligand for the chemokine receptors. Thus, we have demonstrated that gp120 IIIB induces migration of human neurons in a dose-dependent manner and that this chemotactic response can be blocked by CXCR4-specific antibodies. Furthermore, both gp120 IIIB and SDF-1 can directly induce apoptosis in human neurons (reference 32 and unpublished data). Similarly, Weissman et al. (59) have recently shown that macrophagetropic HIV-1 and simian immunodeficiency virus envelopes mediate signaling through CCR5 receptors and induce chemotaxis in T cells. In contrast, no signaling or chemotaxis was observed upon binding of gp120 IIIB from T-cell-tropic HIV-1 to CXCR4 receptors. We have shown in this study that binding of SDF-1 ligand to CXCR4 receptor stimulates MEK/ERK kinase pathway; however, binding of neither T-cell-tropic NL4-3 virions nor gp120 IIIB envelope glycoprotein could induce this pathway in the absence of signaling from CD4 receptor, indicating that binding of gp120 and SDF-1 to CXCR4 does not necessarily lead to the activation of the same signaling pathway (Fig. 6). While this study was in progress, Davis et al. (19) showed that binding of SDF-1 and RANTES to CXCR4 and CCR5, respectively, induces tyrosine phosphorylation of Pyk2 kinase in HL60 cells as well as in T-cell clone DU6. Tyrosine phosphorylation of Pyk2 was also detected in HL60 cells after addition of monomeric gp120 (BH10). Activation of Pyk2 by gp120 (BH10) was inhibited by pretreatment with monoclonal antibodies to CXCR4 or by pertussin toxin, and the authors concluded that for activation of Pyk2, a functional G protein is required. The Pyk2 kinase was previously shown to link G-coupled receptors with Grb and Sos and activate MAP kinase in PC12 cells (21). It will be interesting to see whether the SDF-1-mediated activation of Pyk2 can be linked to the MAP kinase signaling pathway described in this study. Although we have observed the activation of the MAP kinase pathway only in the presence of functional CD4 receptor, we cannot eliminate the possibility that gp120 derived from another strain of HIV-1, which may bind to another domain of the CXCR4 receptor (8, 39, 54), will activate the MAP kinase pathway independently of CD4 signaling. Further studies are under way to explore this possibility.
We have shown that activation of Raf-1 and the MAP kinase pathway by binding of HIV-1 envelope glycoproteins to CD4 receptors leads to the transcriptional activation of expression of the early inflammatory genes. We have also shown that T cells overexpressing activated Raf-1 support replication of HIV-1 more effectively than parental cells (47) and that some of the early inflammatory gene products such as TNF-α can support HIV-1 replication in the absence of HIV-1-encoded transactivator Tat (46). These data altogether suggest that the activation of cellular signaling pathways that lead to the production of cytokine and chemokine genes is used by the virus to support its first cycle of replication that occurs before the Tat transactivator is synthesized. Interestingly, the activation of the MAP kinase pathway by binding of SDF-1 to CXCR4 receptor does not lead to the stimulation of cytokine gene expression (Fig. 7). These results suggest that binding of HIV-1 envelope glycoproteins to CD4 may generate a second signal (possibly by the involvement of Lck) which when coupled to the MAP kinase signaling pathway stimulates the transcription of the early inflammatory genes. The observations that over 99% of plasma virions are not infectious (26) and that a large amount of HIV-1 virions are associated with follicular dendritic cells in lymphoid tissues (31), where they come in contact with migrating CD4 T cells, suggest that CD4+ T cells may become aberrantly activated. It was also shown recently (49) that HIV-1 antigens, including gp160, stimulate production of IFN-γ, RANTES, and MIP-1 in CD4+ T cells from individuals chronically infected with HIV-1, which may contribute to the augmentation of humoral immune response and control of viremia. Thus, the interaction of both T-cell-tropic and macrophagetropic HIV-1 envelope glycoproteins with CD4 receptors can, even in the absence of HIV-1 entry and replication, result in the activation of signal transduction pathways and the aberrant production of cytokines and chemokines and contribute significantly to the replicative ability of HIV-1 variants as well as to a modulation of the immune responses.
ACKNOWLEDGMENTS
We thank D. Kabat for the NL-D368E and NL-D368P HIV-1 clones, F. Maldarelli for AD8 HIV-1, S. Gartner for Ba-L HIV-1, and K.-T. Jeang for A2.01/CD4.401 cells.
This research was supported by grants from the National Institutes of Health (AI26123 and AI40838 to P.M.P. and AI42557 to W.P.).
REFERENCES
- 1.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos F, Virelizier J L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 3.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 4.Benkirane M, Schmid-Antomarchi H, Littman D R, Hirn M, Rossi B, Devaux C. The cytoplasmic tail of CD4 is required for inhibition of human immunodeficiency virus type 1 replication by antibodies that bind to the immunoglobulin CDR3-like region in domain 1 of CD4. J Virol. 1995;69:6904–6910. doi: 10.1128/jvi.69.11.6904-6910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Breen E C, Rezai A R, Nakajima K, Beall G N, Mitsuyasu R T, Hirano T, Kishimoto T, Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–484. [PubMed] [Google Scholar]
- 8.Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR-4 in human immunodeficiency virus coreceptor activity. J Virol. 1997;71:4744–4751. doi: 10.1128/jvi.71.6.4744-4751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briant L, Coudronniere N, Robert-Hebmann V, Benkirane M, Devaux C. Binding of HIV-1 virions or gp120-anti-gp120 immune complexes to HIV-1-infected quiescent peripheral blood mononuclear cells reveals latent infection. J Immunol. 1996;156:3994–4004. [PubMed] [Google Scholar]
- 10.Buonaguro L, Barillari G, Chang H K, Bohan C A, Kao V, Morgan R, Gallo R C, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein W, Weislow O S, Brown C R, Berger E A, June C H, St. Louis D C. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 12.Center D M, Kornfeld H, Cruikshank W W. Interleukin 16 and its function as a CD4 ligand. Immunol Today. 1996;17:476–481. doi: 10.1016/0167-5699(96)10052-i. [DOI] [PubMed] [Google Scholar]
- 13.Chirmule N, Goonewardena H, Pahwa S, Pasieka R, Kalyanaraman V S, Pahwa S. HIV-1 envelope glycoproteins induce activation of activated protein-1 in CD4+ T cells. J Biol Chem. 1995;270:19364–19369. doi: 10.1074/jbc.270.33.19364. [DOI] [PubMed] [Google Scholar]
- 14.Chirmule N, Oyaizu N, Saxinger C, Pahwa S. Nef protein of HIV-1 has B-cell stimulatory activity. AIDS. 1994;8:733–734. doi: 10.1097/00002030-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chirmule N, Kalyanaraman V S, Pahwa S. Signals transduced through the CD4 molecule on T lymphocytes activate NF-κB. Biochem Biophys Res Commun. 1994;203:498–505. doi: 10.1006/bbrc.1994.2210. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 17.Collette Y, Dutartre H, Benziane A, Ramos-Morales, Benarous R, Harris M, Olive D. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. J Biol Chem. 1996;15:6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 18.Cruikshank W W, Center D M, Nisar N, Wu M, Natke B, Theodore A C, Kornfeld H. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci USA. 1994;91:5109–5113. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 21.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 22.Emilie D, Peuchmaur M, Maillot M C, Crevon M C, Brousse N, Delfraissy J F, Dormont J, Galanaud P. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J Clin Invest. 1990;86:148–159. doi: 10.1172/JCI114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1β-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 24.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 25.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs D, Hausen A, Reibnegger G, Werner E R, Werner-Felmayer G, Dierich M P, Wachter H. Interferon-γ concentration are increased in sera from individuals infected with human immunodeficiency virus type 1. J Acquired Immune Defic Syndr. 1989;2:158–162. [PubMed] [Google Scholar]
- 28.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 29.Gosling J, Monteclaro F S, Atchison R E, Arai H, Tsou C L, Goldsmith M A, Charo I F. Molecular uncoupling of C-C chemokine receptor 5-induced chemotaxis and signal transduction from HIV-1 coreceptor activity. Proc Natl Acad Sci USA. 1997;94:5061–5066. doi: 10.1073/pnas.94.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenway A, Azad A, Mills J, McPhee D. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J Virol. 1996;70:6701–6708. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath S L, Tew J G, Szakal A K, Burton G F. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 32.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 33.Kabat D, Kozak S L, Wehrly K, Chasebro B. Differences in CD4 dependence for infection of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol. 1994;68:2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahdevirta J, Maury C P, Teppo A M, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85:289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 36.Lee C H, Leung B, Lemmon M A, Zheng J, Cowburn D, Kuriyan J, Saksela K. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J. 1995;14:5006–5015. doi: 10.1002/j.1460-2075.1995.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo T, Garcia J V. The association of Nef with a cellular serine/threonine kinase and its enhancement of infectivity are viral isolate dependent. J Virol. 1996;70:6493–6496. doi: 10.1128/jvi.70.9.6493-6496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunn M F, Marsh J W. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 42.Oyaizu N, Chirmule N, Ohnishi Y, Kalyanaraman V S, Pahwa S. Human immunodeficiency virus type 1 envelope glycoproteins gp120 and gp160 induce interleukin-6 production in CD4+ T-cell clones. J Virol. 1991;65:6277–6282. doi: 10.1128/jvi.65.11.6277-6282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyaizu N, McCloskey T W, Than S, Hu R, Kalyanaraman V S, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 44.Platt E J, Madani N, Kozak S L, Kabat D. Infectious properties of human immunodeficiency virus type 1 mutants with distinct affinities for the CD4 receptor. J Virol. 1997;71:883–890. doi: 10.1128/jvi.71.2.883-890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popik W, Pitha P M. Inhibition by interferon of herpes simplex virus type 1-activated transcription of tat-defective provirus. Proc Natl Acad Sci USA. 1991;88:9573–9577. doi: 10.1073/pnas.88.21.9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popik W, Pitha P M. Role of tumor necrosis factor alpha in activation and replication of the tat-defective human immunodeficiency virus type 1. J Virol. 1993;67:1094–1099. doi: 10.1128/jvi.67.2.1094-1099.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popik W, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rautonen N, Rautonen J, Martin N L, Wara D W. HIV-1 Tat induces cytokine synthesis by uninfected mononuclear cells. AIDS. 1994;8:1504–1506. [PubMed] [Google Scholar]
- 49.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 50.Rudd C E, Trevillyan J M, Dasgupta J D, Wong L L, Schlossman S F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci USA. 1988;85:5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruocco M R, Chen X, Ambrosino C, Dragonetti E, Liu W, Mallardo M, De Falco G, Palmieri C, Franzoso G, Quinto I, Venuta S, Scala G. Regulation of HIV-1 long terminal repeats by interaction of C/EBP(NF-IL6) and NF-kappa B/Rel transcription factors. J Biol Chem. 1996;271:22479–22486. doi: 10.1074/jbc.271.37.22479. [DOI] [PubMed] [Google Scholar]
- 52.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schols D, De Clercq E. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin-10 production. J Virol. 1996;70:4953–4960. doi: 10.1128/jvi.70.8.4953-4960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strizki J M, Turner J D, Collman R G, Hoxie J A, Gonzalez-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with the dual-tropic human immunodeficiency virus type 1 isolate HIV-1 (89.6) but not the T-tropic isolate HIV-1(HxB) J Virol. 1997;71:5678–5683. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su Y, Popik W, Pitha P M. Inhibition of human immunodeficiency virus type 1 replication by a Tat-activated, transduced interferon gene: targeted expression to human immunodeficiency virus type 1-infected cells. J Virol. 1995;69:110–121. doi: 10.1128/jvi.69.1.110-121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamma S M L, Chirmule N, Yagura H, Oyaizu N, Kalyanaraman V, Pahwa S. CD4 cross-linking (CD4XL) induces Ras activation and tumor necrosis factor-α secretion in CD4+ T cells. Blood. 1997;90:1588–1593. [PubMed] [Google Scholar]
- 57.Theodore T S, Englund G, Buckler-White A, Buckler C E, Martin M A, Peden K W C. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res Hum Retroviruses. 1996;12:191–194. doi: 10.1089/aid.1996.12.191. [DOI] [PubMed] [Google Scholar]
- 58.Veillette A, Bookman M A, Horak E M, Samelson L E, Bolen J B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 59.Weissman D, Rabin R L, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber J M, Fauci A S. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 60.Zhou L-J, Tedder T F. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood. 1995;86:3295–3301. [PubMed] [Google Scholar]