Abstract
The structure of a sialoglycan can be translated into to a biological response when it binds to a specific endogenous lectin. Among endogenous sialic acid-binding lectins in humans are those comprising the 15-member Siglec family, most of which are expressed on overlapping sets of immune cells. Endogenous Siglec ligands are sialoglycolipids (gangliosides) and/or sialoglycoproteins, on cell surfaces or in the extracellular milieu, that bind to and initiate signaling by cell surface Siglecs. In the nervous system, where gangliosides are the predominant sialoglycans, Siglec-4 (myelin-associated glycoprotein) on myelinating cells binds to gangliosides GD1a and GT1b on nerve cell axons to ensure stable and productive axon-myelin interactions. In the immune system, Siglec-7 on natural killer cells binds to gangliosides GD3 and GD2 to inhibit immune signaling. Expression of GD3 and GD2 on cancer cells can lead to tumor immune evasion. Siglec-1 (sialoadhesin, CD169) on macrophages binds to gangliosides on tumors and enveloped viruses. This may enhance antigen presentation in some cases, or increase viral distribution in others. Several other Siglecs bind to gangliosides in vitro, the biological significance of which has yet to be fully established. Gangliosides, which are found on all human cells and tissues in cell-specific distributions, are functional Siglec ligands with varied roles driving Siglec-mediated signaling.
Keywords: Sialic acid, Myelin-associated glycoprotein, CD33, Sialoadhesin, Natural killer cells, Macrophages
Introduction
Sialic acid, because of its carboxylate, N-acyl group, and glycerol side chain, is particularly well suited for molecular recognition [1]. The history of sialic acid’s discovery is intertwined with its role as a ligand for sialic acid binding proteins [2]. Dr. Roland Schauer provided an account of that history in a recent review [3]. At a conference in Cambridge in 1949 Gunnar Blix (Uppsala Sweden), who first described the chemical properties of sialic acid isolated from submaxillary mucin [4], spoke with Alfred Gottschalk (Melbourne Australia) about the chemical properties of small molecules that Gottschalk found were released from natural sources when incubated with intact influenza virus [5]. Blix wrote later “It struck me immediately that some of the properties Gottschalk mentioned strongly reminded me about those of sialic acid. When I got home I mailed Gottschalk what we had written about sialic acid and told him that we intended to test the matter closer.” The molecule released by influenza virus was indeed sialic acid. The enzyme that released it was the influenza neuraminidase, and sialic acid was established as the cell surface ligand for influenza hemagglutinin [3, 6, 7]. These discoveries raised interest in sialic acids as molecular recognition molecules and are part of the extensive literature on sialic acids as pathogen receptors for viruses, bacteria and protozoa [8]. This raises the question of why sialic acid is maintained in evolution despite being usurped by deadly pathogens. In this chapter we review some endogenous functions of sialoglycans, with a specific focus on gangliosides, with one family of native human sialic acid binding proteins, Siglecs.
Human Siglecs, Siglec ligands and Siglec functions
In the early 1990’s Sørge Kelm and Paul Crocker discovered that “a subgroup of the immunoglobulin superfamily can mediate diverse biological processes through recognition of specific sialylated glycans on cell surfaces” [9]. At that time, the subgroup included the macrophage surface protein sialoadhesin (Siglec-1), the B-cell surface protein CD22 (Siglec-2), and myelin-associated glycoprotein (MAG, Siglec4), expressed on myelinating cells of the nervous system. The discovery that these three proteins constituted a structurally related family of sialic acid binding proteins was the birth of research on what are now called Siglecs, sialic acid-binding immunoglobulin-like lectins [10], of which there are 15 in humans [11]. Each Siglec is a single-pass transmembrane protein with variable length short intracellular domains and variable numbers of extracellular immunoglobulin (Ig)-like domains. In each Siglec, the outermost Ig-like domain has the highest sequence similarity to other Siglecs, and contains a shallow surface that binds sialic acid via a conserved arginine residue that engages the sialic acid carboxylate in context of the larger glycan on which it is carried (Fig. 1).
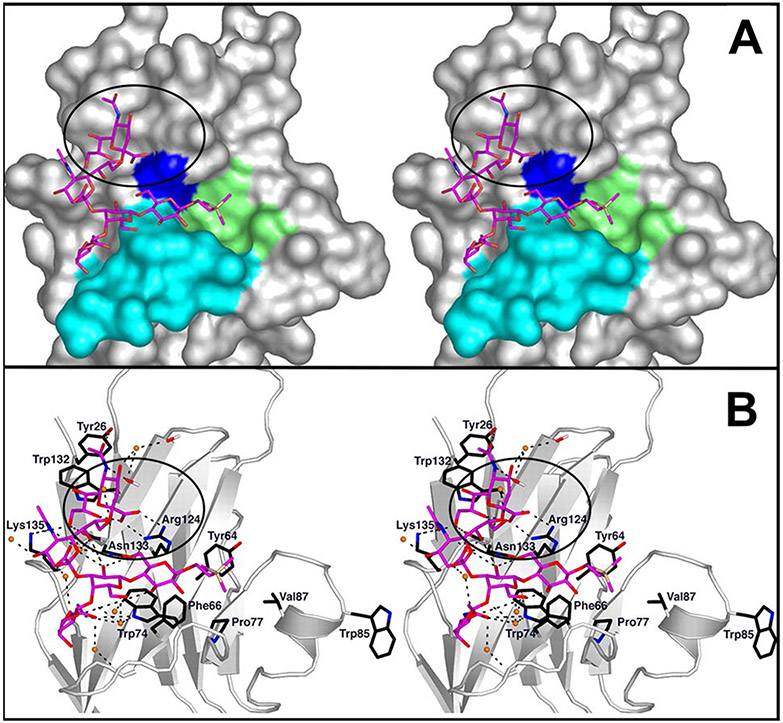
Fig. 1.
Binding of a ganglioside GT1b analog to Siglec7. A Stereo image of the outermost Ig-like domain of Siglec-7 bound to a synthetic GT1b analog. Five of the seven sugars of GT1b are resolved at the binding site in the crystal structure (Neu5Acα2-8Neu5Acα2–3[GalNAcβ1-4Galβ1-4Glcβ-R). The terminal sialic acid (Neu5Ac) binds to the conserved Arg residue, R124 (blue). The circled area shows the terminal sialic acid binding via its carboxylate to R124. A convex shelf (marine blue), forms the base of the binding site over which the rest of the glycan lies. The synthetic trimethylsilyl aglycone (yellow) lies in a hydrophobic cup (green). B Stereo image of the network of potential hydrogen bonds (black-dashed lines) at the binding site. Stably associated water molecules are shown as orange spheres. Adapted from reference [12]
The 15 members of the human Siglec family are notable for their variety of sialic acid binding specificities [11]. Different members of the family take advantage of different ways in which sialic acids are presented on larger glycans. For example, Siglec-1 binds selectively to α2–3 linked sialic acids, Siglec-2 is specific for α2–6 linked sialic acids, and Siglec-7 binds preferentially to α2–8 linked sialic acids (along with certain branched α2,6-sialyl structures) [13]. Binding specificity may also extend further down the glycan chain [9] and include additional glycan constituents, such as sulfation [14]. Human Siglec-XII fails to bind sialic acid (thus its designation by roman numeral), and is included in the family based on its sequence similarity and evolutionary correspondence to sialic acid binding orthologs in primates [15].
Siglecs function in molecular recognition and cellular regulation [16]. Of the 15 human Siglecs, 13 are expressed in overlapping sets of immune cells. Nine of these have one or more immunoreceptor tyrosine-based inhibitory motifs on their intracellular domains that down-regulate immune responses. Three others have basic residues in their transmembrane domains that associate with immune-activating adaptor proteins. A prevailing hypothesis is that Siglecs on the surface of immune cells encounter and bind to specific sialoglycan ligands in their local environment to initiate signaling pathways that down- or up-regulate of the immune response depending on the Siglec(s) engaged. From this perspective, most of the Siglec family evolved to keep immune responses properly tuned. The one Siglec that is not expressed on immune cells is MAG (Siglec-4), which is exclusively expressed on myelinating cells in the nervous system, as will be detailed further below.
Siglec ligands are sialoglycans that engage specific Siglecs. Endogenous human Siglec ligands range from the smallest class, gangliosides (sialylated glycosphingolipids, ~ 1.5–2.5 kDa) to the largest class, mucins (which may exceed 4 MDa) [17]. In each case, a lipid or protein is decorated in the Golgi apparatus with specific Siglec-targeting sialoglycans. The sialoglycan synthetic machinery is regulated in some diseases resulting in altered expression of Siglec ligands [18-20], and is altered in certain congenital disorders of glycosylation resulting in Siglec-related genetic diseases [21].
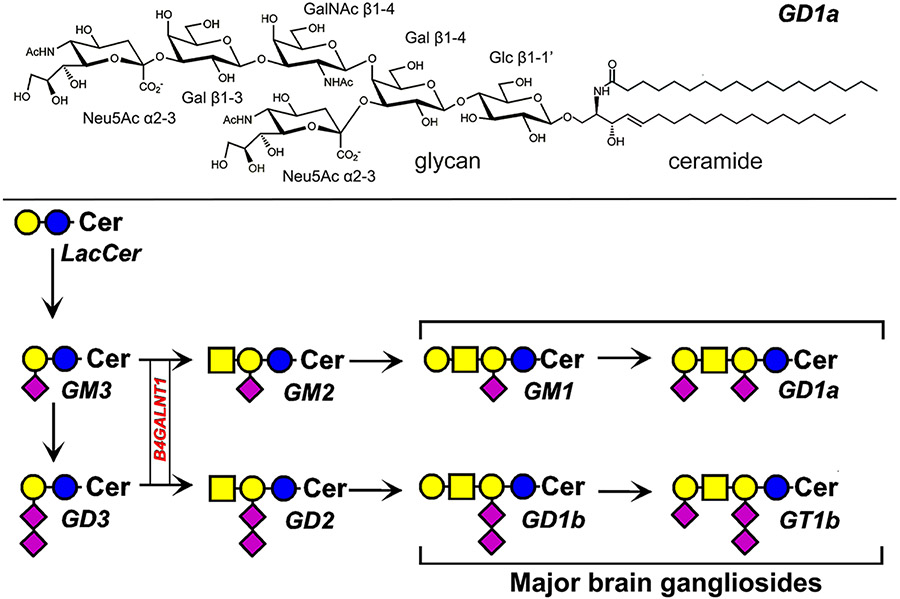
Among endogenous Siglec ligands are gangliosides, defined as sialylated glycosphingolipids. All human cells and tissues express gangliosides, with quantities and structures that vary among cell types. Each ganglioside has a hydrophobic lipid moiety, ceramide, firmly embedded in the membrane (primarily the outer leaflet of the plasma membrane) and a glycan that typically extends outward from the cell surface. While there are hundreds of unique ganglioside glycans [22], and even more variation in ganglioside structures based on differences in their ceramide lipids [23], 8 glycan structures make up the majority of gangliosides in human tissues (Fig. 2). These range from the trisaccharide ganglioside GM3 common to many non-neuronal tissues to the four major brain gangliosides (GM1, GD1a, GD1b, GT1b) that comprise > 97% of the gangliosides and the majority of sialoglycans in the human brain [24]. Ganglioside biosynthesis is stepwise (Fig. 2), and mutations in two genes exclusive for glycolipid biosynthesis result in rare congenital disorders. In addition, some gangliosides are overexpressed in cancer, such as GD3 and GD2 in melanoma and neuroblastoma respectively [25, 26]. Changes in ganglioside structures and expression levels impact Siglec-ganglioside interactions and result in human pathology.
Fig. 2.
Ganglioside structure and biosynthesis. The structure of disialo ganglioside GD1a (top). Biosynthesis of major human gangliosides (bottom) using symbol nomenclature for glycans [27]. The biosynthetic gene B4GALNT1 discussed in the text is boxed. Cer = ceramide
Siglec-4 (myelin-associated glycoprotein, MAG)
Among the first proteins identified as a member of the Siglec family was the previously well-characterized nervous system protein MAG (myelin-associated glycoprotein) [9]. MAG is the only human Siglec that is not expressed on immune cells, but instead is found on myelinating cells in the central and peripheral nervous systems (oligodendrocytes and Schwann cells respectively) [28]. MAG has 5 extracellular Ig-like domains and an intracellular domain of variable length that contains a site for Fyn tyrosine kinase phosphorylation. It was implicated as a sialic acid binding protein based on its sequence similarly to sialoadhesin and CD22 [9]. This was confirmed by demonstrating that an expressed soluble tagged form MAG bound to human erythrocytes in a sialidase-sensitive manner. Erythrocyte re-sialylation using sialyltransferases with different specificities revealed that MAG failed to bind to α2–6 linked sialic acids and had a strong preference for the “3-O” sialoglycan structure: Neu5Acα2-3Galβ1-3GalNAc. Gangliosides are quantitatively the major sialoglycans in the brain [24], and some carry the “3-O” structure (Fig. 2). Subsequent studies confirmed that MAG binds to gangliosides in a physiologically and pathologically relevant interaction revealed by biochemistry [29], mouse genetics [30], and human congenital disorders of glycosylation [21].
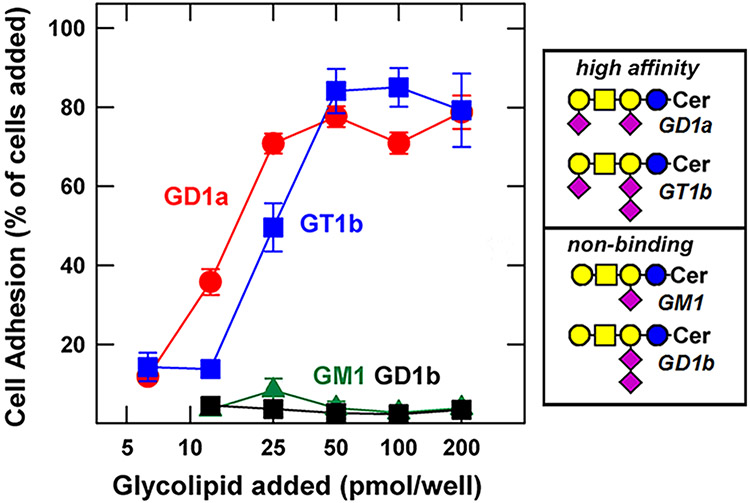
Gangliosides are the major sialoglycans in the human brain, carrying over 75% of the total brain sialic acid [24]. Half of that sialic acid is found on two gangliosides, GD1a and GT1b, that carry the “3-O” terminus (Neu5Acα2-3Galβ1-3GalNAc). When gangliosides were stably adsorbed to microwells, they supported binding of cells engineered to express MAG on their surface (Fig. 3). As predicted, GD1a and GT1b supported robust MAG-mediated binding, whereas the gangliosides GM1 and GD1b, which lack the “3-O” terminus, did not. To test the functional roles of MAG-ganglioside binding in vivo, genetically altered mice were used. Mice with a disrupted B4galnt1 gene (Fig. 2) lack the “3-O” terminus specifically on gangliosides. These mice lack all of the major brain gangliosides and instead express comparable concentrations of the truncated gangliosides behind the biosynthetic block, GM3 and GD3 [31, 32]. B4galnt1-null mice demonstrated the pathologies of Mag-null mice when compared side-by-side, including central and peripheral nervous system axon degeneration that resulted in loss of sensory and motor signal integrity leading to progressive motor behavioral deficits and hindlimb paralysis [30]. Subsequently, similar pathology was discovered in rare human congenital disorders targeting the B4GALNT1 gene and the MAG gene [21]. Mutations in the B4GALNT1 gene result in complex hereditary spastic paraplegia (SPG26) in several dozen individuals associated with 12 family pedigrees with 12 different B4GALNT1 gene mutations. The disorder is characterized by weakness and spasticity of the lower limbs with onset typically in childhood and progressing to spastic gait, dysreflexia, muscle atrophy, and speech deficits. Nerve histology revealed axonal neuropathy. In another study, a very rare gene mutation was identified in siblings suffering from a progressive gait disorder and cognitive impairment who were diagnosed with progressive axonal sensorimotor polyneuropathy [33]. The mutation was a single amino acid substitution, R118H, in MAG. That arginine is the very same residue that engages the terminal sialic acid carboxylate of gangliosides. Together, these data support the hypothesis that the “3-O” terminus of gangliosides GD1a and GT1b engage MAG (Siglec-4) to support axon-myelin interactions essential to long term axonal survival. Congenital loss of either the sialoglycan terminus of gangliosides or the amino acid on MAG that binds them results in axonal loss, progressive motor behavioral deterioration and intellectual disability.
Fig. 3.
MAG binding to major brain gangliosides. Fibroblasts were transfected to express full-length MAG on their surface, then were placed in microwells adsorbed with the indicated concentrations of the indicated gangliosides. MAG-mediated cell adhesion is expressed as a percent of the MAG-transfected cells added to each well. Ganglioside structures are shown using symbol nomenclature for glycans [27]. Image adapted from reference [24]
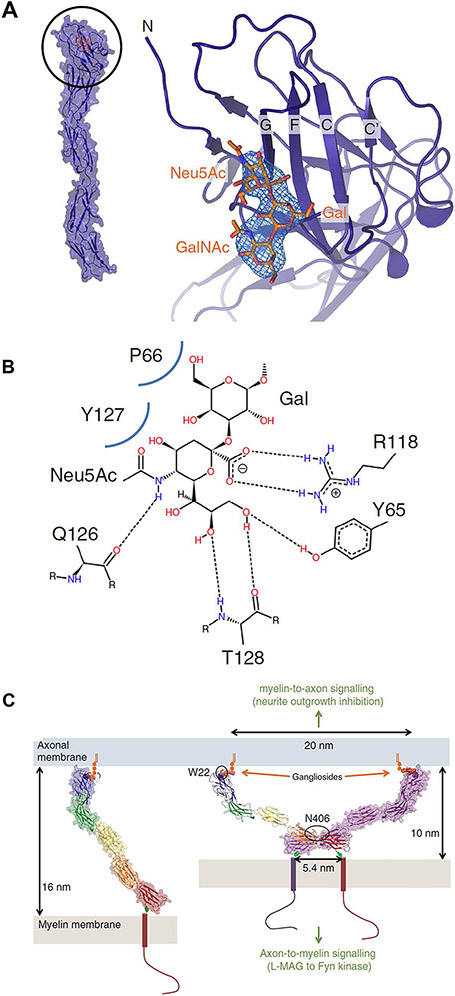
The molecular basis for the functional interaction of MAG with gangliosides GD1a and GT1b has been solved (Fig. 4) [34]. The outermost N-terminal Ig-like domain has a shallow surface that binds the terminal Neu5Ac of the “3-O” sequence via a salt bridge to a conserved arginine (R118). When the elongated 5 Ig-like domain structure binds to ganglioside and dimerizes, it decreases the distance between the axon and myelin membranes and facilitates axon-myelin signaling.
Fig. 4.
MAG-ganglioside binding and a model for myelin-axon engagement. A crystal structure of MAG and its terminal Ig-like domain binding to the “3-O” trisaccharide (Neu5Acα2,3Galβ1-3GalNAc, orange). B Protein-ligand interactions with hydrogen bonds indicated by dashes and Van der Waals’ contacts by curved blue lines, C Model for MAG-mediated myelin-axon engagement and signaling. Dimerization of MAG restricts the distance between the innermost myelin sheath and axon membrane to 10 nm. Reproduced from reference [34]
Siglec-7
Siglec-7 is expressed by human natural killer (NK) cells, and subsets of myeloid and dendritic cells [35]. It is an immune checkpoint (inhibitory) receptor on cells that functions in tumor surveillance. If sialoglycans expressed on the surface of cancer cells engage Siglec-7 on NK cells, the cancer cells may avoid immune surveillance and replicate. This concept is supported by the consistent finding of enhanced sialylation in many cancers, evidence that has led to the search for Siglec-7 ligands on human cancer cells [36, 37].
Using synthetic glycan arrays, Siglec-7 binds to disialoglycans, including the α2–8 linked disialic acid moieties on gangliosides GD3, GD2, GD1b and GT1b (Fig. 2) [38]. Gangliosides GD3 and GD2 are robustly overexpressed on certain cancers [26] and are target epitopes for melanoma and neuroblastoma immunotherapies [39, 40]. Evidence that cancer cell disialogangliosides engage Siglec-7 to inhibit immune responses was obtained using human neuroblastoma cells [41]. In a multidrug protocol, anti-GD2 antibody sensitized human cancer cells to macrophage-mediated phagocytosis by blocking Siglec-7 binding.
Structural studies revealed the sites on Siglec7 responsible for enhanced binding [12, 13, 42]. In one study [12], the structure of the outermost Siglec-7 Ig-like domain bound to a synthetic analog of GT1b was reported (Fig. 1). The bound disialo moiety and core ganglioside disaccharide (Galβ1-4Glc) were well defined in the crystal such that the terminal sialic acid engaged the conserved arginine residue of Siglecs (R124) with the core disaccharide in a sharply bent configuration relative to the disialo moiety. Binding of the ganglioside mimetic resulted in a large conformational shift that opened up a large hydrophobic patch. It is inviting to speculate that glycan binding, notably of the Neu5Acα2-8Neu5Acα2-3Galβ1-4Glc of gangliosides GD3, GD2, GD1b and GT1b, then enhances engagement with the hydrophobic ceramide. This speculation is supported by a recent study that found that cell surface GD3 containing normal ceramide (see Fig. 2) supports Siglec-7 binding, whereas GD3 containing a ceramide with an extra hydroxyl group on sphingosine C4 (phytoceramide) or a 2-hydroxl group on the fatty acid amide failed to support functional Siglec-7 engagement [43]. These data imply that adding a hydrophilic hydroxyl group near the top of the ceramide may alter the way Siglec-7 interacts with disialo-bearing gangliosides.
Siglec-1 (sialoadhesin)
Sialoadhesin (Siglec-1, CD169) is selectively expressed on human macrophages where it engages self sialoglycans in the extracellular milieu as well as sialoglycans on human pathogens [44, 45]. It does not have immune inhibitory domains, and enhances macrophage phagocytosis of sialoglycan-bearing cargo. Binding to microwell-adsorbed gangliosides revealed that sialoadhesin binds to several gangliosides, with near equivalent binding to GM3, GD1a, GD1b and GT1b [46]. Since pathogenic viruses bud from cells that express some of these gangliosides, binding of viral surface gangliosides to sialoadhesin is implicated in viral uptake by macrophages [45]. This has dual effects depending on the virus and context. Sialoadhesin binding to viral surface gangliosides can result in phagocytosis, degradation, antigen presentation and enhanced viral clearing. Alternatively, gangliosides on opportunistic viruses may enhance viral entry into macrophages and viral dissemination. Understanding both of these pathways has implications for understanding viral pathogenesis.
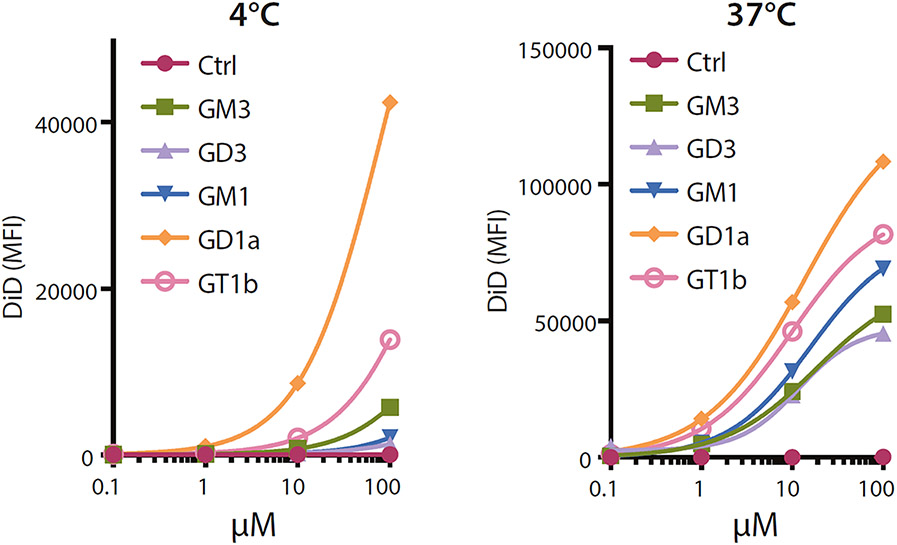
Whether helpful or harmful, the above observations led to the use of gangliosides in biotechnology to target nanoparticles to macrophages [47, 48]. A study of nanoparticles decorated with different gangliosides revealed selective binding of GD1a by sialoadhesin-expressing human cells (Fig. 5), and the ability of several gangliosides to enhance nanoparticle internalization [48]. Notably, adding gangliosides to nanoparticles carrying human tumor antigens enhanced the immune response of sialoadhesin-expressing antigen-presenting cells. These findings emphasize that ganglioside binding to Siglecs is a useful cell-targeting biotechnology with therapeutic potential.
Fig. 5.
Ganglioside-liposomes bind Siglec-1 on THP-1 human monocyte/macrophage cells and are internalized. Fluorescently-labeled ganglioside-liposomes were incubated with THP1 cells overexpressing Siglec-1, and binding at 4 °C or uptake at 37 °C determined by flow cytometry. Binding or uptake of ganglioside-liposomes at different concentrations are shown. Data are from reference [48]
Other ganglioside-binding siglecs
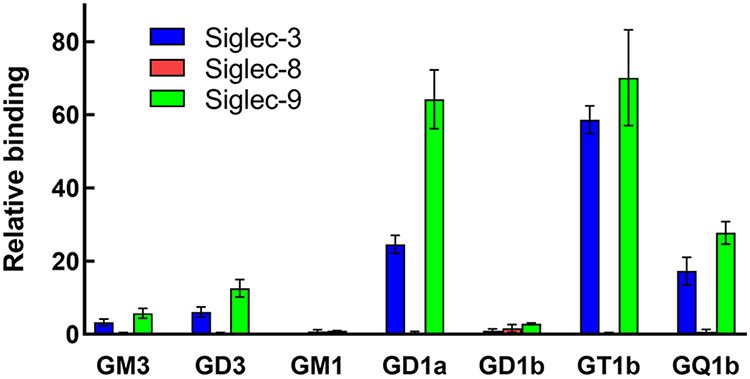
Siglec binding to gangliosides adsorbed on microwells was determined for a subset of CD33-related human Siglecs. A summary of the results based on those data are shown in Table 1. While the functional significance of these particular Siglecs binding to gangliosides has yet to be fully explored, it is notable that each tested Siglec had a different binding pattern. As expected, Siglec-7 bound to gangliosides having the α2–8 linked disialo group. Siglec-5 bound preferentially to GQ1b, which has α2–8 disialo groups on both the internal and external Gal residues, whereas Siglec-10 bound to GT1b but not the closely related structures GD1a or GQ1b. Siglec-9 bound to most of the gangliosides tested. In a separate set of studies using microwell-adsorbed gangliosides, Siglec-3 and Siglec-9 bound similar gangliosides, whereas Siglec-8 failed to bind any (Fig. 6). The implications of these findings for Siglec function are the subject of ongoing studies [49, 50].
Table 1.
Siglec-ganglioside binding based on reference [51]
| Ganglioside | Siglec-5 | Siglec-7 | Siglec-9 | Siglec-10 |
|---|---|---|---|---|
| GM3 | − | − | + | − |
| GM2 | − | − | − | − |
| GD3 | − | ++ | + | − |
| GD1a | − | − | − | − |
| GT1b | +/− | + | + | ++ |
| GQ1b | + | ++ | + | − |
Fig. 6.
Binding of select Siglecs to major gangliosides. Gangliosides were adsorbed to microwell plates and overlaid with soluble expressed Siglec-Fc chimeras precomplexed to alkaline-phosphatase(AP)-labeled anti-Fc antibody. After incubation and washing, bound Siglec was determined by measuring AP colorimetrically. Data are from references [52] and [53]
Concluding statement
The 15 human Siglecs, most of which are immune regulatory receptors, have a variety of endogenous ligands that include sialoglycolipids and sialoglycoproteins that have evolved to serve specific functions in the tissues or on the cells where they are expressed [17]. Strong evidence indicates that certain Siglecs, such as Siglec-4 (MAG) and Siglec-7, are engaged by endogenous gangliosides to trigger important physiological and pathophysiological signaling events. For other Siglecs, the roles of gangliosides as ligands in the context of particular cells, tissues, and biological outcomes have yet to be established. Ongoing studies will provide insights to understand the roles of gangliosides in Siglec signaling. Whatever their endogenous functions, the ability to synthesize gangliosides and incorporate them into nanoparticles and even directly into cell membranes make them inviting Siglec targets for biomedical discovery and possibly therapeutics.
Acknowledgements
This review is dedicated to the memory of a founder and leader in our field, Roland Schauer. His insights, rigor, openness and support have been highlights of my career. Although we are not related, the similarity of our names (Ronald Schnaar and Roland Schauer, 64% sequence identical) has at times led strangers to mistake me for Roland. For this I was always grateful and aspired to be worthy of their error. As the field of sialobiology rapidly expands, Roland’s early appreciation of its potential and highly significant contributions to its progress remain a foundation on which the field is built.
Funding
The writing of this manuscript was supported by National Institutes of Health Grants CA241953 and AI136443.
Footnotes
Conflicts of interest The authors declare that they have no conflicts of interest.
Ethical approval This review article reports only on human and animal studies that were previously published. No new data are reported.
Competing interests The author reports no competing interests.
References
- 1.Varki A, Schnaar RL, Schauer R: Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (eds.) Essentials of glycobiology, 3rd edn., pp. 179–195. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; (2017). https://www.ncbi.nlm.nih.gov/books/NBK579918/ [Google Scholar]
- 2.Lundblad A: Gunnar Blix and his discovery of sialic acids. Fascinating molecules in glycobiology. Ups J. Med. Sci 120(2), 104–112 (2015). 10.3109/03009734.2015.1027429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schauer R, Kamerling JP: Exploration of the sialic acid world. Sialic Acids, Pt I: Historical Background and Development, and Chemical Synthesis 75, 1–213 (2018). 10.1016/bs.accb.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blix G: Über die Kohlenhydratgruppen des Submaxillarismucins [Concerning the carbohydrate groups of submaxillary mucin]. Hoppe Seylers Z. Physiol. Chem 240, 43–54 (1936). 10.1515/bchm2.1936.240.1-2.43 [DOI] [Google Scholar]
- 5.Gottschalk A, Lind PE: Product of interaction between influenza virus enzyme and ovomucin. Nature 164(4162), 232 (1949). 10.1038/164232a0 [DOI] [PubMed] [Google Scholar]
- 6.von Itzstein M: The war against influenza: discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov 6(12), 967–974 (2007). 10.1038/nrd2400 [DOI] [PubMed] [Google Scholar]
- 7.Baum LG, Paulson JC: Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl 40, 35–38 (1990) [PubMed] [Google Scholar]
- 8.Kelm S, Schauer R: Sialic acids in molecular and cellular interactions. Int. Rev. Cytol 175, 137–240 (1997). 10.1016/s0074-7696(08)62127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelm S, Pelz A, Schauer R, Filbin MT, Song T, de Bellard ME, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, Crocker PR: Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol 4, 965–972 (1994). 10.1016/S0960-9822(00)00220-7 [DOI] [PubMed] [Google Scholar]
- 10.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, Kelm S, Le Douarin N, Powell L, Roder J, Schnaar RL, Sgroi DC, Stamenkovic K, Schauer R, Schachner M, van den Berg TK, van der Merwe PA, Watt SM, Varki A: Siglecs: a family of sialic-acid binding lectins. Glycobiology 8(2), v (1998). 10.1093/oxfordjournals.glycob.a018832 [DOI] [PubMed] [Google Scholar]
- 11.Duan S, Paulson JC: Siglecs as immune cell checkpoints in disease. Annu. Rev. Immunol 38, 365–395 (2020). 10.1146/annurev-immunol-102419-035900 [DOI] [PubMed] [Google Scholar]
- 12.Attrill H, Imamura A, Sharma RS, Kiso M, Crocker PR, van Aalten DM: Siglec-7 undergoes a major conformational change when complexed with the alpha(2,8)-disialylganglioside GT1b. J. Biol. Chem 281(43), 32774–32783 (2006). 10.1074/jbc.M601714200 [DOI] [PubMed] [Google Scholar]
- 13.Yamakawa N, Yasuda Y, Yoshimura A, Goshima A, Crocker PR, Vergoten G, Nishiura Y, Takahashi T, Hanashima S, Matsumoto K, Yamaguchi Y, Tanaka H, Kitajima K, Sato C,: Discovery of a new sialic acid binding region that regulates Siglec-7. Sci. Rep 10(1), 8647 (2020). 10.1038/s41598-020-64887-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung J, Enterina JR, Bui DT, Mozaneh F, Lin PH, Nitin, Kuo CW, Rodrigues E, Bhattacherjee A, Raeisimakiani P, Daskhan GC, Laurent S, Khoo CD, Mahal KH, Zandberg LK, Huang WF, Klassen X, Macauley JS: Carbohydrate sulfation as a mechanism for fine-tuning Siglec ligands. ACS Chem. Biol 16(11), 2673–2689 (2021). 10.1021/acschembio.1c00501 [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui SS, Vaill M, Do R, Khan N, Verhagen AL, Zhang W, Lenz HJ, Johnson-Pais TL, Leach RJ, Fraser G, Wang C, Feng GS, Varki N, Varki A: Human-specific polymorphic pseudogenization of SIGLEC12 protects against advanced cancer progression. FASEB Bioadv 3(2), 69–82 (2021). 10.1096/fba.2020-00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crocker PR, Paulson JC, Varki A: Siglecs and their roles in the immune system. Nat. Rev. Immunol 7(4), 255–266 (2007). https://www.nature.com/articles/nri2056 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Gil A, Li TA, Kim J, Schnaar RL: Human sialoglycan ligands for immune inhibitory siglecs. Mol. Aspects Med 101110 (2022). 10.1016/j.mam.2022.101110 [DOI] [PubMed] [Google Scholar]
- 18.Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS, Kern RC, Schleimer RP, Schnaar RL: Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J. Allergy Clin. Immunol 135(3), 799–810 (2015). 10.1016/j.jaci.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HS, Gonzalez-Gil A, Drake V, Li TA, Schnaar RL, Kim J: Induction of the endogenous sialoglycan ligand for eosinophil death receptor Siglec-8 in chronic rhinosinusitis with hyperplastic nasal polyposis. Glycobiology 31(8), 1026–1036 (2021). 10.1093/glycob/cwab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim J, Sari-Ak D, Bagga T: Siglecs as therapeutic targets in cancer. Biology (Basel) 10(11), 1178 (2021). 10.3390/biology10111178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li TA, Schnaar RL: Congenital disorders of Ganglioside Biosynthesis. Prog Mol. Biol. Transl Sci 156, 63–82 (2018). 10.1016/bs.pmbts.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 22.Yu RK, Tsai YT, Ariga T, Yanagisawa M: Structures, biosynthesis, and functions of gangliosides-an overview. J. Oleo Sci 60(10), 537–544 (2011). 10.5650/jos.60.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez PH, Aja S, Aoki K, Seldin MM, Lei X, Ronnett GV, Wong GW, Schnaar RL: Mice lacking sialyltransferase ST3Gal-II develop late-onset obesity and insulin resistance. Glycobiology 27(2), 129–139 (2017). 10.1093/glycob/cww098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnaar RL, Gerardy-Schahn R, Hildebrandt H: Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease and regeneration. Physiol. Rev 94, 461–518 (2014). 10.1152/physrev.00033.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan GC, Chan CM: Anti-GD2 Directed immunotherapy for high-risk and metastatic neuroblastoma. Biomolecules. 12(3) (2022). 10.3390/biom12030358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasprowicz A, Sophie GD, Lagadec C, Delannoy P: Role of GD3 synthase ST8Sia I in cancers. Cancers (Basel) 14(5), 1299 (2022). 10.3390/cancers14051299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, Prestegard JJ, Schnaar RL, Freeze HH, Marth JD, Bertozzi CR, Etzler ME, Frank M, Vliegenthart JF, Lutteke T, Perez S , Bolton E, Rudd P, Paulson J, Kanehisa M, Toukach P, Aoki-Kinoshita KF, Dell A, Narimatsu H, York W, Taniguchi N, Kornfeld S: Symbol nomenclature for graphical representations of glycans. Glycobiology 25(12), 1323–1324 (2015). 10.1093/glycob/cwv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quarles RH: Myelin-associated glycoprotein (MAG): past, present and beyond. J. Neurochem 100(6), 1431–1448 (2007). 10.1111/j.1471-4159.2006.04319.x [DOI] [PubMed] [Google Scholar]
- 29.Yang LJ-S, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, Schnaar RL: Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Natl. Acad. Sci. U. S. A 93, 814–818 (1996). 10.1073/pnas.93.2.814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan B, Fromholt SE, Hess EJ, Crawford TO, Griffin JW, Sheikh KA, Schnaar RL: Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice. Exp. Neurol 195(1), 208–217 (2005). 10.1016/j.expneurol.2005.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Wada R, Kawai H, Sango K, Deng C, Tai T, McDonald MP, Araujo K, Crawley JN, Bierfreund U, Sandhoff K, Suzuki K, Proia RL: A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest 103, 497–505 (1999). 10.1172/JCI5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Shaper NL, Itonori S, Heffer-Lauc M, Sheikh KA, Schnaar RL: Myelin-associated glycoprotein (Siglec-4) expression is progressively and selectively decreased in the brains of mice lacking complex gangliosides. Glycobiology 14(9), 851–857 (2004). 10.1093/glycob/cwh107 [DOI] [PubMed] [Google Scholar]
- 33.Roda RH, FitzGibbon EJ, Boucekkine H, Schindler AB, Blackstone C: Neurologic syndrome associated with homozygous mutation at MAG sialic acid binding site. Ann. Clin. Transl Neurol 3(8), 650–654 (2016). 10.1002/acn3.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ: Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun 7, 13584 (2016). 10.1038/ncomms13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S , Mattei MG, Crocker PR: Identification and characterization of a novel siglec, Siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem 274(48), 34089–34095 (1999). 10.1074/jbc.274.48.34089 [DOI] [PubMed] [Google Scholar]
- 36.Hugonnet M, Singh P, Haas Q, von Gunten S: The distinct roles of Sialyltransferases in cancer biology and onco-immunology. Front. Immunol 12, 799861 (2021). 10.3389/fimmu.2021.799861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly J, Carlsten M, O’Dwyer M: Sugar free: novel immunotherapeutic approaches targeting siglecs and sialic acids to enhance natural killer cell cytotoxicity against cancer. Front. Immunol 10, 1047 (2019). 10.3389/fimmu.2019.01047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y: A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha2,8-disialyl and branched alpha2,6-sialyl residues: a comparison with Siglec-9. J. Biol. Chem 277, 6324–6332 (2002). 10.1074/jbc.M110146200 [DOI] [PubMed] [Google Scholar]
- 39.Furukawa K, Hamamura K, Nakashima H, Furukawa K: Molecules in the signaling pathway activated by gangliosides can be targets of therapeutics for malignant melanomas. Proteomics 8(16), 3312–3316 (2008). 10.1002/pmic.200800228 [DOI] [PubMed] [Google Scholar]
- 40.Qiu B, Matthay KK: Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol (2022). 10.1038/s41571-022-00643-z [DOI] [PubMed] [Google Scholar]
- 41.Theruvath J, Menard M, Smith BAH, Linde MH, Coles GL, Dalton GN, Wu W, Kiru L, Delaidelli A, Sotillo, , Silberstein JL, Geraghty AC, Banuelos A, Radosevich MT, Dhingra S, Heitzeneder S, Tousley A, Lattin J, Xu P, Huang J, Nasholm N, He A, Kuo TC, Sangalang ERB, Pons J, Barkal A, Brewer RE, Marjon KD, Vilches-Moure JG, Marshall PL, Fernandes R, Monje M, Cochran JR, Sorensen PH, Daldrup-Link HE, Weissman IL, Sage J, Majeti R, Bertozzi CR, Weiss WA, Mackall CL, Majzner RG, : Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med 28(2), 333–344 (2022). 10.1038/s41591-021-01625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attrill H, Takazawa H, Witt S, Kelm S, Isecke R, Brossmer R, Ando T, Ishida H, Kiso M, Crocker PR, van Aalten DM: The structure of Siglec-7 in complex with sialosides: leads for rational structure-based inhibitor design. Biochem. J 397(2), 271–278 (2006). 10.1042/BJ20060103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto N, Ito S, Tsuchida A, Bhuiyan RH, Okajima T , Yamamoto A, Furukawa K, Ohmi Y, Furukawa K: The ceramide moiety of disialoganglioside (GD3) is essential for GD3 recognition by the sialic acid-binding lectin SIGLEC7 on the cell surface. J. Biol. Chem 294(28), 10833–10845 (2019). 10.1074/jbc.RA118.007083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klaas M, Crocker PR: Sialoadhesin in recognition of self and non-self. Semin Immunopathol 34(3), 353–364 (2012) [DOI] [PubMed] [Google Scholar]
- 45.Perez-Zsolt D, Martinez-Picado J, Izquierdo-Useros N: When dendritic cells go viral: the role of Siglec-1 in host defense and dissemination of enveloped viruses. Viruses. 12(1) (2019). 10.3390/v12010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins BE, Kiso M, Hasegawa A, Tropak MB, Roder JC, Crocker PR, Schnaar RL: Binding specificities of the sialoadhesin family of I-type lectins. Sialic acid linkage and substructure requirements for binding of myelin-associated glycoprotein, Schwann cell myelin protein, and sialoadhesin. J. Biol. Chem 272(27), 16889–16895 (1997). 10.1074/jbc.272.27.16889 [DOI] [PubMed] [Google Scholar]
- 47.Zang H, Siddiqui M, Gummuluru S, Wong WW, Reinhard BM: Ganglioside-functionalized nanoparticles for chimeric antigen receptor T-Cell activation at the immunological synapse. ACS Nano (2022). 10.1021/acsnano.2c06516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Affandi AJ, Grabowska J, Olesek K, Lopez Venegas M, Barbaria A, Rodriguez E, Mulder PPG, Pijffers HJ, Ambrosini M, Kalay H, O’Toole T, Zwart ES, Kazemier G, Nazmi K, Bikker FJ, Stockl J, van den Eertwegh AJM, de Gruijl TD, Storm G, van Kooyk Y, den Haan JMM: Selective tumor antigen vaccine delivery to human CD169(+) antigen-presenting cells using ganglioside-liposomes. Proc. Natl. Acad. Sci. U. S. A 117(44), 27528–27539 (2020). 10.1073/pnas.2006186117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ledeen RW, Kopitz J, Abad-Rodríguez J, Gabius HJ: Glycan chains of gangliosides: functional ligands for tissue lectins (Siglecs/Galectins). Prog. Mol. Biol. Transl. Sci 156, 289–324 (2018). 10.1016/bs.pmbts.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 50.Linnartz-Gerlach B, Mathews M, Neumann H: Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin-like lectins. Neuroscience 275, 113–124 (2014). 10.1016/j.neuroscience.2014.05.061 [DOI] [PubMed] [Google Scholar]
- 51.Rapoport E, Mikhalyov I, Zhang J, Crocker P, Bovin N: Ganglioside binding pattern of CD33-related siglecs. Bioorg. Med. Chem. Lett 13(4), 675–678 (2003). 10.1016/S0960-894X(02)00998-8 [DOI] [PubMed] [Google Scholar]
- 52.Bull C, Nason R, Sun L, Van Coillie J, Madriz Sorensen D, Moons SJ, Yang Z, Arbitman S, Fernandes SM, Furukawa S, McBride R, Nycholat CM, Adema GJ, Paulson JC, Schnaar RL, Boltje TJ, Clausen H, Narimatsu Y: Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc. Natl. Acad. Sci. U. S. A 118(17), e2026102118 (2021). 10.1073/pnas.2026102118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, Paulson JC, Nycholat CM, Schnaar RL: Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 27(7), 657–668 (2017). 10.1093/glycob/cwx026 [DOI] [PMC free article] [PubMed] [Google Scholar]