Highlights
-
•
Obesity related Chronic Disease (ORCKD) is highly prevalent.
-
•
Primary ORCKD includes Obesity related glomerulopathy (ORG) and focal segmental glomerulosclerosis.
-
•
ORCKD is associated with a multiplicity of comorbidities and complications especially in the cardio-metabolic domain.
-
•
Strategies for CKD modulation in non-obese patients are also effective in ORCKD.
-
•
Evidence suggests that weight loss and management strategies provide added benefit to renal function and cardio-metabolic risk reduction in ORCKD.
Keywords: Obesity related Chronic kidney disease (ORCKD), Obesity related Glomerulopathy (ORG), Fatty kidney, Renal Steatosis, Renal Fatty infiltration, Renal Lipotoxicity, Focal segmental glomerulosclerosis, Chronic kidney disease, Metabolic associated fatty kidney disease (MAFKD)
Abstract
Obesity and chronic kidney disease are two ongoing progressive clinical pandemics of major public health and clinical care significance. Because of their growing prevalence, chronic indolent course and consequent complications both these conditions place significant burden on the health care delivery system especially in developed countries like the United States. Beyond the chance coexistence of both of these conditions in the same patient based on high prevalence it is now apparent that obesity is associated with and likely has a direct causal role in the onset, progression and severity of chronic kidney disease. The causes and underlying pathophysiology of this are myriad, complicated and multi-faceted. In this review, continuing the theme of this special edition of the journal on “ The Cross roads between Endocrinology and Nephrology” we review the epidemiology of obesity related chronic kidney disease (ORCKD), and its various underlying causes and pathophysiology. In addition, we delve into the consequent comorbidities and complications associated with ORCKD with particular emphasis on the cardio metabolic consequences and then review the current body of evidence for available strategies for chronic kidney disease modulation in ORCKD as well as the potential unique role of weight reduction and management strategies in its improvement and risk reduction.
Introduction
Obesity represents a major ongoing world-wide epidemic with increasing global prevalence [1], [2]. The GBD 2015 obesity collaborators review of over 65 million subjects worldwide spanning ∼ 195 countries and including both adults and children from 1980 to 2015 demonstrate that the prevalence of obesity doubled in more than 70 of these countries (including the United States). Furthermore, while the prevalence of childhood obesity is obviously less than in adults, the rate of increase of childhood obesity prevalence outpaces adult obesity with potentially grave public health and clinical consequences for the near future [1], [2], [3], [4].
The disease morbidity and mortality associated with obesity has been exhaustively documented [1], [2], [3], [4]. This is known to be consequent upon the direct effects of obesity itself as well as its associated comorbidities and complications which are protean. Conservative estimates suggest that obesity accounts for at least 4 million deaths annually worldwide and > two thirds of these deaths are due to cardiovascular mortality which is one of the many complications obesity brings in its wake [1], [2], [3], [4], [5].
It is generally less appreciated that beyond the scope and impact of obesity on public health and clinical morbidity and mortality there is a second (often silent) tandem global epidemic of chronic kidney disease (CKD) [6], [7], [8], [9], [10], [11], [12]. Current conservative estimates suggest that CKD afflicts > 1in 7 adults in the United States with an estimated prevalence of at least ∼ 37 million. Among “enriched” populations this CKD risk is even greater. Among the dominant known risk factors for CKD are diabetes mellitus (with ∼ 30 % CKD disease risk), essential hypertension(∼20 % disease risk), atherosclerotic vascular disease (AVD), family history of CKD and geriatric populations (>65 years old) [6], [7], [8], [9], [10], [11], [12]. Available cross sectional and cohort study data also indicate a distinct racial discrepancy in CKD prevalence with African American and Black subjects have a greater prevalence (∼20 %) compared to Hispanic (14 %) non-Hispanic Asian (∼14 %) and Non-Hispanic Caucasians (∼12 %) subjects [6], [7], [8], [9], [10], [11], [12]. As with obesity but to an even greater extent, CKD portends major associated morbidity and mortality risk with the greatest mortality risk being due to the associated accelerated AVD and acute coronary syndromes (ACS) [6], [7], [11]. Conservative estimates ascribe ∼ 1.5million deaths to CKD between 1990 and 2017, but more tellingly, the CKD mortality rate has increased by > 40 % over this period despite advances in standards of care and resources. The mortality rate from CKD is ∼ 1.28–1.3 times higher in males than females overall. In addition, conservative estimates suggest that ∼ 7.3 million years aggregate of living with disability (YLDs), ∼ 28.5 million aggregate years of life lost (YLLs) and ∼ 35.8 million disability adjusted life years (DALYs) are attributable to CKD.
While just based on the high prevalence of the twin epidemics of obesity and CKD it would be anticipated that there would be a sizeable degree of overlap between the populations and patients burdened by obesity and CKD it is now very apparent that the cohabitation of obesity and CKD in large populations of patients is not simply from statistical happenstance. It is now well established that obesity is an independent risk factor for CKD onset and progression with growing body of evidence suggesting even more provocatively that it is an independent etio-pathogenic cause for CKD like essential hypertension, diabetes and chronic nonsteroidal anti-inflammatory drug (NSAID) use [6], [11], [12]. The recognition of the distinct entity of obesity related chronic kidney disease (ORCKD) has spurred growing interest in this unique subgroup of the CKD disease burden population both to better understand its underlying pathophysiology, management strategies and to contrast its clinical course and prognosis to the more commonly known forms of CKD. Conceptually CKD identified in obese patients can be sub-stratified into primary (where obesity appears to be the only identifiable clinical etiology) and secondary ORCKD. Included in the subset of secondary ORCKD are Hypertensive CKD, Diabetic Nephropathy with CKD and Renal stone associated CKD all of which are seen with significantly greater prevalence in obese patients than in the general population [8], [9], [11], [13]. For the sake of clarity, secondary ORCKD can also be referred to as Obesity associated CKD and is not the focus of this review.
Primary ORCKD which is the main focus of this review is a complex multifaceted condition which on closer review may be better described as a syndrome with possibly several different etiopathogenetic pathways, presentations and clinical manifestations [14], [15], [16], [17], [18].
Among the identified pathophysiologic mechanisms that drive the development of primary ORCKD are altered renal and glomerular hemodynamics with hyperfiltration, chronic renal inflammation, oxidative stress and activation of the renin-angiotensin-aldosterone-Kallikrein system (RAAKS) [17], [19].
Upon microscopic evaluation, patients with primary ORCKD often display glomerulomegaly and focal segmental glomerulosclerosis often referred to as Obesity related glomerulopathy (ORG) [17], [19]. These patients are generally asymptomatic or with non-specific atypical symptoms early in the evolution of this disease. The main clinical feature to screen for prior to onset of functional renal decline is proteinuria which can range from microalbuminuria through sub-nephrotic to nephrotic range proteinuria [17], [19].
Other contributory factors to the onset and progression of primary ORCKD include lipotoxicity, adipocytokine dysregulation, glomerular hypertension and ectopic fat accumulation in the kidneys resulting in pathologic evidence of renal steatosis (fatty kidney) [14], [15], [17], [19], [20], [21], [22].
It is now apparent that well before the onset/development of diabetes in obese patients there is a continuum of progressive decline in beta cell function along with insulin resistance. This “prediabetic” state is often associated with the dysmetabolic syndrome and is now known to be an independent risk factor for ORCKD [15], [17], [19]. Even among obese patients with little to no dysmetabolic derangements (so called metabolically healthy obese patients) there is evidence a distinct, independent CKD risk and ORCKD cohort still persists [20], [23].
To provide an in-depth review of primary ORCKD we discuss in depth the epidemiology of obesity, CKD and their coexistence. Furthermore, we review the pathophysiology of ORCKD and its consequent cardiometabolic comorbidities and complications as well as review the body of evidence for CKD management in ORCKD. In addition, we also explore the unique role that weight loss and management can play in the management of ORCKD and its comorbidities and complications.
Epidemiology of obesity and CKD
In clinical practice, the widely accepted definition for overweight is a BMI of 25 – 29.9 kg/m2, obesity being defined a BMI > 30 kg/m2 and severe obesity classified as BMI at or above 40.0 kg/m2.
Although BMI is widely used as a measure of body fat, it fails to account for factors like muscle mass, bone mass or fat distribution. At a given BMI level, body fat may vary by sex, age, and race. Additionally, the validity of some of the existing BMI cutoffs have been questioned, as they were not developed using diverse populations. The relationship between BMI and mortality is likely to be similar for all races and ethnicities, but the threshold BMI where excess risk begins may differ.
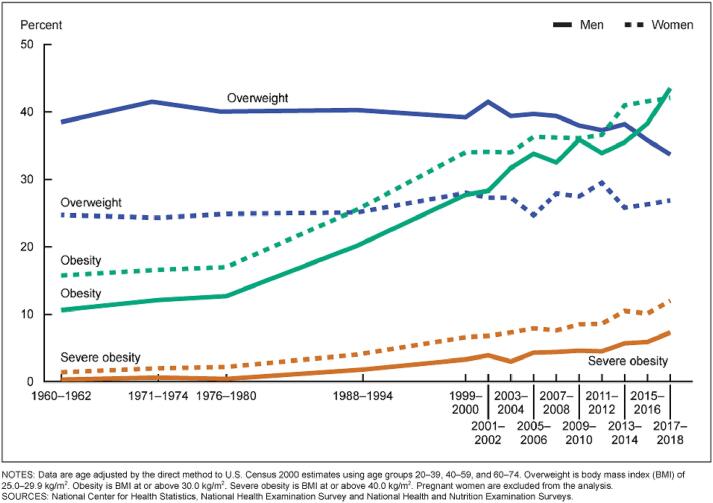
Results from the 2017–2018 National Health and Nutrition Examination Survey (NHANES), using measured heights and weights, indicate that an estimated 42.5 % of U.S. adults aged 20 and over have obesity, including 9.0 % with severe obesity, and another 31.1 % are overweight. This is detailed below in Fig. 1 [24].
Fig. 1.
NHANES Time Course Trends in Obesity and Overweight Prevalence by Gender Strata.
Non-Hispanic blacks have the highest age-adjusted rates of obesity (48.1 %) followed by Hispanics (42.5 %), non-Hispanic whites (34.5 %), and non-Hispanic Asians (11.7 %) [25].
Obesity is higher among middle-aged adults (age 40–59 years; 40.2 %) and older adults (age 60 and over; 37.0 %) than among younger adults (age 20–39; 32.3 %) [25].
The estimates presented in the 5th annual World Obesity Atlas displayed above (Fig. 2 above) suggest that, on current trends, overweight and obesity will cost the global economy over US$4 trillion of potential income in 2035, nearly 3 % of current global gross domestic product (GDP) [26].
Fig. 2.
World Obesity Prevalence Distribution.
The estimates for global levels of overweight and obesity (BMI ≥ 25 kg/m2), suggest that over 4 billion people may be affected by 2035, compared with over 2.6 billion in 2020. This reflects an increase from 38 % of the world’s population in 2020 to over 50 % by 2035.
The prevalence of obesity (BMI ≥ 30 kg/m2) alone is anticipated to rise from 14 % to 24 % of the population over the same period, affecting nearly 2 billion adults, children and adolescents by 2035 [26].
In some but certainly not all higher income countries, the rate at which obesity prevalence levels are rising appears to be slowing down. In lower income countries there are many reasons to expect rising obesity prevalence, including (a) trends in dietary preferences towards more highly processed foods, (b) trends towards greater levels of sedentary behavior, (c) weaker policies to control the food supply and food marketing and (d) less well-resourced healthcare services to assist in weight management and in health education in the population – all of which can continue to stimulate an increase in obesity prevalence.
CKD is associated with age-related renal functional decline and is accelerated in settings of hypertension, diabetes, obesity and primary renal disorders [27].
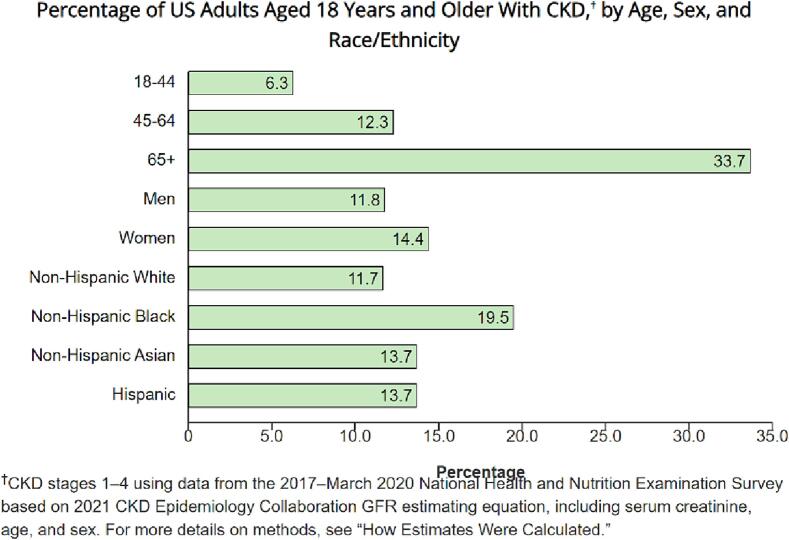
According to current estimates from NHANES data from 2017 to 2020, more than 1 in 7 US adults–about 35.5 million people, or 14 %–are estimated to have CKD [12], [28]. Fig. 3 details the breakdown from the NHANES cohort regarding CKD prevalence percentages based on age, sex and race/ethnicity strata [12].
Fig. 3.
NHANES CKD Prevalence percentages by age and Race + Ethnicity Strata.
A systematic review and meta-analysis estimating global CKD prevalence measured by all 5 stages of CKD between 11 and 13 %, with the majority being stage 3 [29]. In 2017, in the Global Burdan of disease study, 697·5 million cases of all-stage CKD were recorded, for a global prevalence of 9·1% [30], [31].
A meta-analysis of 25 cohorts, 3 cross-sectional and 19 case-control studies that met inclusion criteria confirmed that obesity increases the risk of CKD in the general population [32], suggesting a pathogenic association between both conditions. This systemic disease can affect the kidneys by at least two mechanisms: Indirectly through diabetes mellitus (DM) and hypertension and directly through adipokine secretion. Excessive adipose tissue is associated with insulin resistance, oxidative stress (OS) and visceral adiposity promotes hyper filtration and hyperperfusion, decreased podocyte density, increased foot processes which may lead to glomerular hypertrophy, and the appearance of microalbuminuria, activates the renin-angiotensin-aldosterone system and is associated with high levels of pro-inflammatory cytokines [33], [34].
The Framingham Offspring study cohort consisted of 1223 males and 1362 females who were initially free of preexisting kidney disease. After a mean follow-up of 18.5 years, 244 participants (9.4 %) had developed kidney disease (defined as Modification of Diet in Renal Disease [MDRD] estimated glomerular filtration rate [eGFR] of < 64 and 59 mL/min/1.73 m2 for males and females, respectively). The development of CKD was associated with increased age, diabetes, hypertension, smoking, obesity, and lower baseline glomerular filtration rate [35].
Ejerblad et al, [36] showed that patients without diabetes or hypertension still had a threefold increased risk for CKD if they were overweight at age 20 years. Hsu et al, [37] showed that higher baseline BMI remained an independent predictor for ESRD after adjustments for BP and diabetes mellitus.
Using the Global Burden of Disease study data and methods the health effects of overweight and obesity in 195 countries over 25 years showed that CKD was the second leading cause of BMI-related disability-adjusted life-years in 2015; 18.0 % of disability-adjusted life-years occurred at a BMI of 30 or more and 7.2 % at a BMI of less than 30 [2].
Simply from the high population prevalence of both obesity and CKD the confluence of both in individual patients and subpopulations is expected, anticipated and inevitable. Beyond that though, the commonalities of etiologic causes and contributors to the ongoing twin global pandemics of both obesity and CKD result in a prevalence of ORCKD that far exceeds just statistical coexistence of both conditions [6], [10], [11], [12], [17], [28], [38], [39], [40].
It is now apparent that the association of obesity and CKD can be subdivided into primary (or so called idiopathic ORCKD) where the exact etio-pathogenic are still somewhat unclear and subject to speculation and likely multiple possibly co-interacting mechanisms (some of which are detailed below) and secondary ORCKD where well established known secondary diseases known to be associated with CKD coexist in patients with obesity. As a group secondary ORCKD is more prevalent and thus has greater public health import but this does not diminish the importance of the need for a better understanding of the both the pathophysiologic basis and long term management strategies for primary ORCKD [13], [19], [34], [36], [37], [41]. Prominent among the entities embodied in secondary ORCKD (which wont be further discussed in this review) are Hypertensive Obesity related CKD, Diabetic related Obesity related CKD, Renal stone disease related Obesity CKD, NSAID and other medication related Obesity CKD and acquired cystic Obesity related kidney disease [6], [13], [39], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50].
Pathophysiology and pathobiology of Primary Obesity related chronic kidney disease
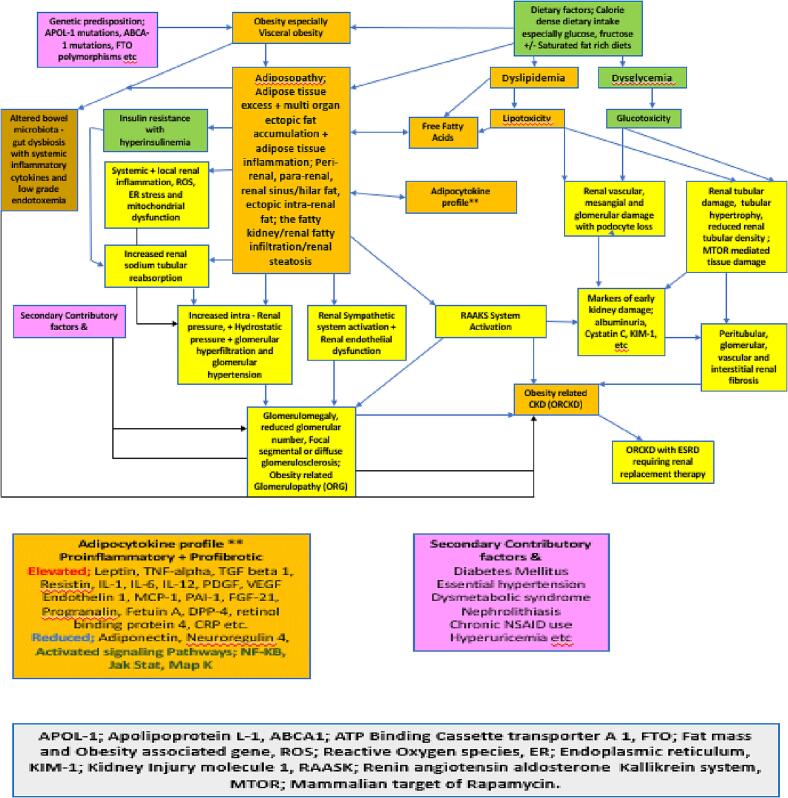
The exact etiopathogenesis of primary ORCKD is unknown but accumulating evidence from cellular basic, translational, animal model and various forms of human clinical studies and observations are progressively increasing the accumulated body of evidence in this regard and paint a complex multifaceted tapestry with different degrees of contributory factors in individual patients and subpopulation groups. To varying degrees it is now apparent that ORCKD involves elements of glomerular, tubular and renal parenchymal injury [18], [20], [51].
Among the main established pathophysiologic mechanisms involved in the development of ORCKD are the following that are discussed below.
Renal fat excess and ectopic accumulation
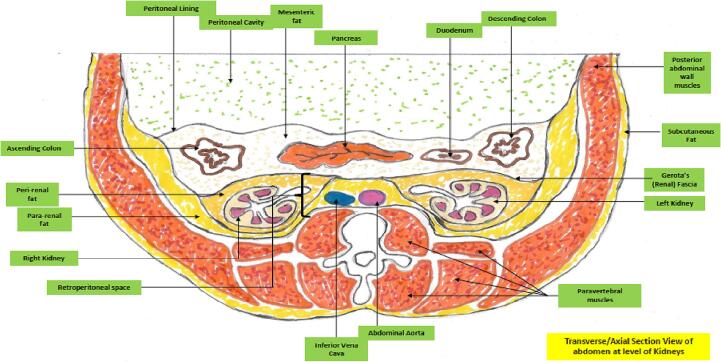
As Fig. 4, Fig. 5 illustrate, the kidney is associated with multiple distinct fat depots in and around them. There is now evidence that excessive amounts of fat in any of these locations with or without ectopic parenchymal renal fat accumulation akin to that seen in states of metabolic associated fatty liver disease can result in the renal dysfunction associated with ORCKD [14], [20], [22]. Ectopic fatty disease accumulation in the kidney is now well demonstrated to be pathogenic and is variously referred to as fatty kidney disease, renal steatosis and renal fatty infiltration [14], [15], [20], [21], [22]. There is some evidence from both human studies and animal models that renal sinus/hilum ectopic fat by sheer mechanical effects and compression can impair renal tissue perfusion as this is the entry point to the rest of the renal parenchyma for the renal artery, vein and lymphatics as well as the ureter and renal pelvis. The elevated hydrostatic pressure can also result in a degree of renal outflow obstruction [52], [53], [54], [55], [56]. The increased renal hilum/sinus fat accumulation and resultant intra-renal hypertension has also been demonstrated to be associated with local renal hypoxia indicated by increased production of hypoxia inducible factor 1 alpha and consequent ischemic renal tissue damage [57]. Both peri and pararenal fat depots have been shown to positively correlate with degree of blood pressure elevation, degree of insulin resistance, Hemoglobin A1C, albuminuria and presence of the dysmetabolic syndrome [58], [59]. Fig. 6 provides illustrative examples of renal parenchymal and tubular fatty infiltration.
Fig. 4.
Longitudinal Section of Kidney showing fat depots.
Fig. 5.
Transverse abdominal section at level of Kidneys showing fat depots.
Fig. 6.
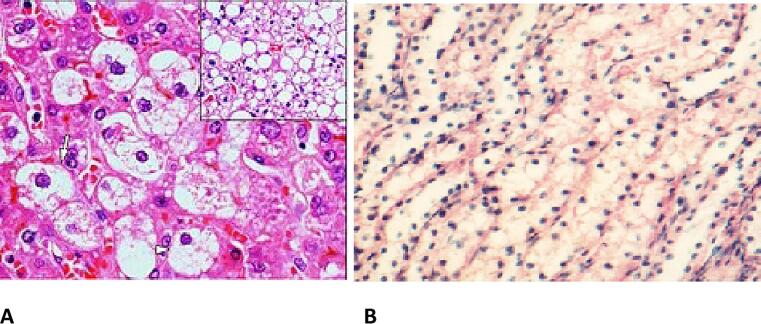
Renal Steatosis/Fatty renal infiltration; A; Renal parenchymal steatosis, B; Renal tubular steatosis.
Among the established imaging modalities by which renal adiposity can be visualized and semi quantified are targeted abdominal sonography, Computed tomography (including renal tissue density measurements using Hounsfield unit scores), magnetic resonance imaging (MRI), nuclear magnetic resonance spectroscopy (NMRS) and renal elastography [14], [15], [20], [21], [22]. These methods provide in-vivo means of renal adipose tissue visualization, localization and semi-quantification that have substantively reduced the need for percutaneous renal biopsies which while being the gold standard for renal adiposity documentation is seldom performed because of its invasive nature. Just as has been recognized with fatty liver disease which can have alcohol and non-alcohol related etiologies that is now described as metabolic associated fatty liver disease (MAFLD) a subset of patients with ORCKD with demonstrable ectopic and excess intrarenal fat accumulation can be described as having metabolic associated fatty kidney disease (MAFKD).
Altered renal hemodynamics
Weisinger and colleagues were among the earliest to document the association between marked obesity and intra-renal venous hypertension which is associated with reversible proteinuria and which is one of the first pathophysiological steps in the onset of ORCKD [21], [60]. Intra-renal venous hypertension is known to result in glomerular hyperfiltration as has been demonstrated in both diabetic and hypertensive nephrosclerosis. This sustained over time results in a sustained glomerulopathy that ultimately leads to renal functional decline and CKD with end stage glomerular hypofiltration and chronic renal failure requiring renal replacement therapy [33], [61], [62], [63].
Obesity Related Glomerulopathy (ORG)
Subsequent studies have demonstrated the association of obesity with the development of a distinctive glomerulopathy characterized by glomerulomegaly and focal segmental glomerulosclerosis now commonly referred to as ORG [18], [21], [61], [64], [65].
The degree of proteinuria associated with ORG is variable and can range from mild to nephrotic range depending on various other factors and variables [21], [40], [48], [60]. The evolution of ORG also includes glomerular basement membrane expansion, podocyte hypertrophy and detachment with ultimate consequent renal functional decline accompanied with progressively worsening proteinuria [33], [34], [36], [61], [62], [63], [64].
Renin Angiotensin Aldosterone Kallikrein System (RAAKS) Activation
Consequent upon the renal hemodynamic pertubations detailed above, ORCKD has been demonstrated to be associated with both systemic and local intra-renal paracrine activation the RAASK system. The glomerular hyperfiltration in particular results in elevated proximal tubular reabsorption of sodium and water with consequent reduced sodium delivery to the macular densa, reduced afferent arteriolar vascular resistance and inhibited tubuloglomerular feedback. The fact that several components of the RAASK system (including renin and aldosterone) are known adipokines provides an additional accelerant source of these hemodynamic changes in ORCKD [45], [64], [66], [67].
Tissue inflammation and oxidative stress
The Adipocyte excess and ectopic expression associated with fatty kidneys are associated with a local and systemic pro inflammatory state also associated with oxidative stress. This is mediated at least in part with the various inflammatory adipokines produced by these adipocytes [21], [68], [69]. Among the putative pro inflammatory mediators produced from the adipocytes are leptin, resistin, free fatty acids, TNF alpha, interleukin-6 and several other pro inflammatory interleukins as well as reduced production of the anti-inflammatory, insulin sensitizing adipokine, adiponectin [21], [68], [69], [70]. The diseased adipocyte tissue depots in ORCKD (sometimes referred to as adiposopathy is also associated with cellular endoplasmic reticular stress (ER stress) and over expression of TGF-beta which is partially mediated by leptin [14], [34], [64], [71], [72], [73], [74]. Chronic inflammation with ongoing oxidative and ER stress eventually leads to progressive renal fibrosis which appears to be at least TGF-beta mediated and can involve the entirety of the renal parenchyma including the glomeruli, renal tubules and renal interstitium resulting in permanent renal dysfunction and potentially end stage renal disease (ESRD).
Renal sympathetic nervous system activation
ORCKD is also known to be associated with activation and overactivity of the local renal sympathetic nervous system. While the full details of the cause for this is unclear, leptin does appear to be contributory [21], [22], [75].
Role of lipotoxicity and myriad CKD risk mediators
Beyond the induction of local and systemic inflammation as previously detailed, ectopic renal fat can also induce direct nephrotoxicity due to specific nephrotoxicity of ectopic fat and their expressed adipokines [14], [21], [22], [73], [74], [75], [76], [77], [78], [79]. It has also been shown that the presence of certain apolipoprotein L1 (APOL1) genetic variants in patients appears to be associated with development of ORCKD as has already been demonstrated in the development of both HIV associated nephropathy and idiopathic focal segmental glomerulosclerosis (FSGS) [80], [81]. These APOL1 gene polymorphisms may impair reverse cholesterol transfer and downregulate cholesterol efflux transporters resulting in toxic tissue cholesterol accumulation in renal tissue especially within the podocytes [20], [80], [81].
As with most other forms of CKD, ORCKD is typically indolent in its natural history of progression over time. About ∼ 10–33 % of ORCKD patients in the absence of d interventions typically progress to ESRD requiring renal transplant therapy [61], [64], [82], [83].
Hyperinsulinemia and insulin resistance
Insulin resistance and the compensatory hyperinsulinemia associated with it are common in obese patients. This is known to be associated with preglomerular vasodilatation and intraglomerular hypertension. This is also consequently associated with albuminuria and possible renal functional decline. Insulin has also been demonstrated to play a role in normal podocyte function, morphology and function. Insulin resistance is consequently associated with podocyte apoptosis and hypertrophy [84], [85], [86], [87].There is some suggestion that Fetuin A may also be an intermediary mediating the transition from insulin resistance to ectopic fat accumulation in the liver (resulting in metabolically associated fatty liver disease) as well as potentially fatty kidney with possible progression to ORCKD [88], [89], [90], [91], [92], [93].
Early markers of renal injury and functional deficits
Well before the onset of measurable proteinuria and renal functional decline shown by elevated serum creatinine, Cystatin C and/or creatinine clearance decline, hypoxemic renal tissue damage which early on is predominantly in the proximal convoluted tubules is characterized by increased production of kidney injury molecule −1 (KIM 1) and fibroblast growth factor 21 (FGF-21). Chronic renal expression of both these markers over time ultimately leads to renal fibrosis [94]. Urine neutrophil gelatinase associated lipocalin, urinary Cystatin C, urinary N-acetyl Beta D aminoglucosidase and serum interleukin 18 levels have also been reported to be elevated in subjects with early ORCKD [17], [19], [95], [96], [97], [98]. Other potentially useful markers for use as biomarkers of early kidney disease in ORCKD are podocin, nephrin, podocin/nephrin ratio, podocalyxin, urinary alanyl aminopeptidase, urinary glutamyl aminopeptidase, urinary klotho, urinary osteopontin and netrin-1 [99], [100], [101], [102], [103], [104]. There is also increasing evidence suggesting a potential role and place for proteomics and metabolomic indices as early markers of renal injury in ORCKD [17]. Controversies and contention still exist regarding the accuracy of clinical prediction equations of creatinine clearance based on serum creatinine and/or cystatin C levels in patients with ORCKD compared to the general population [17], [105], [106], [107], [108].
Dysbiosis of gut microbiota and ORCKD
Derangements of normal gut microbiota profiles have recently emerged as an important factor in the onset and development of several chronic metabolic disease. The extent to which gut microbiota changes observed in obesity are etiologic as opposed to correlative, associative or consequent complications of obesity is the subject of ongoing research [109], [110], [111], [112]. Beyond the known changes in microbiota associated with obesity however, it is also now apparent that patients with CKD and ESRD have demonstrable quantitative and qualitative changes in gut microbiota compared to controls. It is also apparent that toxic products of this dysbiosis can aggravate CKD and CKD related complications [113], [114]. In particular, with ESRD serum urea levels increase and is secreted into the intestines where the urease expressed by several gut microbes increase local ammonia production resulting in elevation of the local bowel Ph. This environmental change can inhibit the normal growth and proliferation of normal bowel commensals resulting in both qualitative and quantitative changes to the bowel microbiota including significantly increase aerobic and anaerobic bacteria in the duodenum and jejunum which are not significantly colonized by bacteria In normal healthy subjects [115], [116]. Altered microbiota, low grade endotoxemia and “leaky gut syndrome” associated with uremia can initiate a systemic inflammatory state which can both initiate and/or aggravate the cascade leading to ORCKD development in obese subjects [117], [118], [119].
Histopathologic features of ORCKD
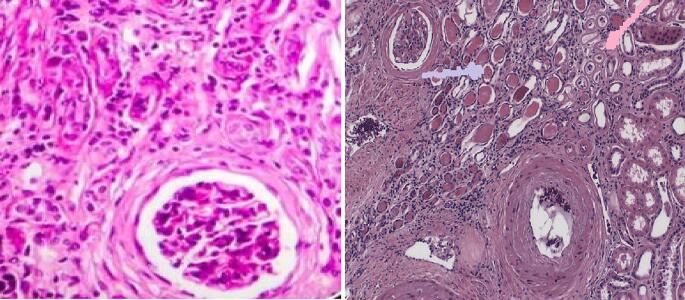
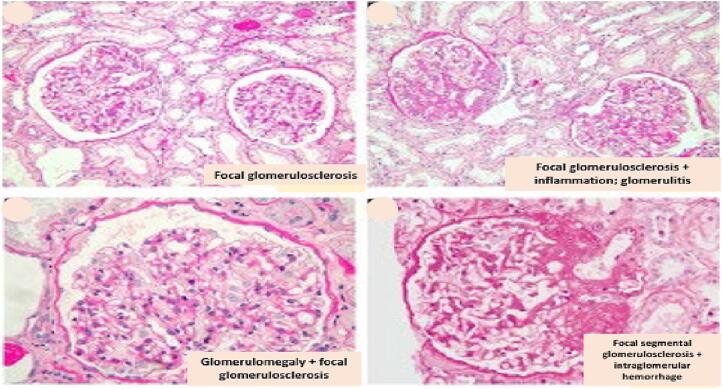
Among the myriad potential findings associated with ORCKD on histologic examination of renal biopsy tissue include glomerulomegaly, global or FSGS, reduced glomerular density, podocyte injury and loss (on Electron microscopy), glomerulosclerosis/fibrosis, mesangial and/or glomerular basement membrane expansion (on Electron microscopy), tubule-interstitial fibrosis, tubular atrophy, proximal tubular epithelial hypertrophy, and tubular cell apoptosis [17], [120], [121], [122], [123]. Fig. 7 illustrates some of these findings while Fig. 8 illustrates the salient findings of obesity related glomerulopathy (ORG). Fig. 9 summarizes the salient aspects of the known pathophysiology and pathobiology of ORCKD.
Fig. 7.
Renal Glomerular and interstitial fibrosis.
Fig. 8.
ORG showing glomerulomegaly and focal segmental glomerulosclerosis.
Fig. 9.
Summary Schema of the pathophysiology of ORCKD.
The comorbidities and complications of obesity and CKD with emphasis on cardiometabolic aspects
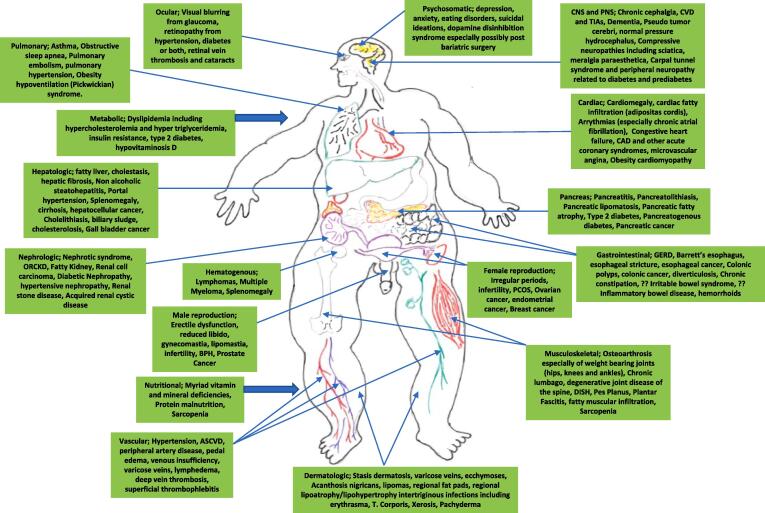
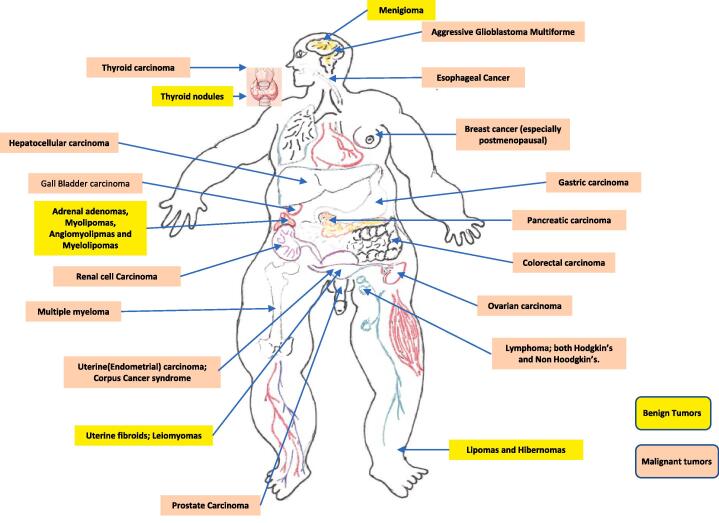
Both CKD and obesity are associated with myriad endocrine and metabolic comorbidities are complications of which the cardiovascular consequences are arguably the most impactful as far as chronic morbidity and mortality potential [124], [125], [126], [127], [128]. As numerous and complex as the associated endocrine and cardiometabolic comorbidities associated with CKD and obesity are separately, it is now well established that the coexistence of both in ORCKD exacerbates both in prevalence and intensity the consequent litany of endocrine, hormonal and cardiometabolic complications and comorbidities [6], [17], [33], [34], [38], [39], [124], [125], [126], [127], [128], [129]. Table 1 below summarizes the major established comorbidities associated with CKD and by extension ORCKD [6], [17], [33], [34], [38], [39], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138]. Even more protean are the various comorbidities and complications known to be associated with obesity. There are > 200 distinct complications and comorbidities associated with obesity and while this review wont be discussing them in depth as related to ORCKD the cardiometabolic and endocrine are of particular interest and importance [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155]. Fig. 10 provides a panoramic view of the most common comorbidities and complications of obesity which affect virtually every organ system of the body [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155]. Of particular note and interest as well is the growing body of evidence demonstrating the association of obesity with increased cancer risk, prevalence and consequent morbidity and mortality [1], [2], [140], [144], [146], [147], [150], [154], [155], [156]. In relation to ORCKD in particular the associated increased risk for renal cell carcinoma among other increased cancer risks and it is also notable to appreciate that from a modifiable risk perspective, obesity is now the second most prevalent risk factor for oncogenesis in humans exceeded only by tobacco abuse and exposure [1], [2], [140], [144], [146], [147], [150], [154], [155], [156]. Figs. 11 shows the cancers and benign tumors with the most robust available data to date of being obesity related and enabled.
Table 1.
Endocrine and Cardiometabolic comorbidities and complications of CKD and ORCKD.
| Organ/System | Manifestations | Suggested Pathophysiologic Mechanisms | Other Comments |
|---|---|---|---|
| Hypothalamic Pituitary Axis | Hyperprolactinemia, Growth hormone deficiency and/or Growth hormone resistance, Short Stature + growth retardation on children, secondary hypogonadism. | ?? Mediated by uremia and other Accumulated renal excretory metabolites | |
| Calcium/Phosphate, Mineral Balance + Skeletal System | Hypovitaminosis D, Secondary hyperparathyroidism, renal Osteodystrophy, Osteomalacia, Osteoporosis, Hyperuricemia, Gouty arthropathy | Reduced renal clearance | |
| Skeletal Muscle System | Sarcopenia Deconditioning Asthenia, Malaise, Chronic fatigue etc | Altered and impaired myokine Balance including irisin, Electrolyte Derangements including hypokalemia, Hypophosphatemia, hypocalcemia etc | |
| Adipocyte tissue Depots | Obesity, Visceral fat Accumulation, Cachexia, Protein energy Malnutrition | ?? Mediated by uremia and other Accumulated renal excretory metabolites | |
| Thyroid | Subclinical hypothyroidism, Low T3 syndrome | Altered Deiodinase function and distribution, Cytokine mediated non thyroidal illness effect on TSH secretion and action. | |
| FGF - 21 and FGF- 23 | FGF - 21 involved in modulation of dysglycemia, insulin resistance and dyslipidemia, FGF- 23 (Phosphatonin) involved in phosphaturia + hypophosphatemia | Impaired renal clearance and elevated serum levels of both FGF- 21 and FGF- 23 as well other pro- inflammatory cytokines | |
| Hematopoietic system + Bone Marrow | Normocytic anemia | Ineffective erythropoiesis and reduced erythropoietin production. | |
| Cardiovascular System | Atherosclerotic Cardiovascular disease (ASCVD) ; Chronic disease, acute vascular events, hypertension, RAAKS activation | Vascular calcification, reduced endothelial reactivity, effects of dysglycemia, dyslipidemia and insulin resistance on vascular function. | |
| Reproductive System | Menstrual irregularities, premature menopause, Male and female hypogonadism (primary, secondary or mixed), erectile dysfunction | ?? Mediated by uremia and other Accumulated renal excretory metabolites | |
| Multi - functional / Multi - System | Chronic Fatigue, Malaise, Asthenia, deconditioning, Depression etc | ?? Mediated by uremia and other Accumulated renal excretory metabolites. ?? inflammatory cytokine mediated. | |
| Dermatologic | Acanthosis Nigricans, Xerosis, Chronic pruritus | ?? Mediated by uremia and other Accumulated renal excretory metabolites. Insulin resistance. | |
| Endocrine Pancreas | Insulin resistance, hyperinsulinemia, Dysglycemia, Nephrogenic and Transplant associated Diabetes | ?? Mediated by uremia and other Accumulated renal excretory metabolites. In Transplant patients; effects of transplant related medications. | |
| Adrenal | Secondary adrenal insufficiency, ?? Primary Adrenal insufficiency, Hyporeninemic hypoaldosteronism | Potential roles for Renal, Adrenal and Systemic RAAKS activation, Adrenomedullin and renal prostaglandins. | Problems with measurement and assays of adrenal medullary and Adreno - cortical hormones and Metabolites |
Fig. 10.
Complications and Comorbidities of Obesity.
Fig. 11.
Obesity associated cancers and benign tumors.
Cardiometabolic burden of ORCKD
Disease burden and complications of obesity
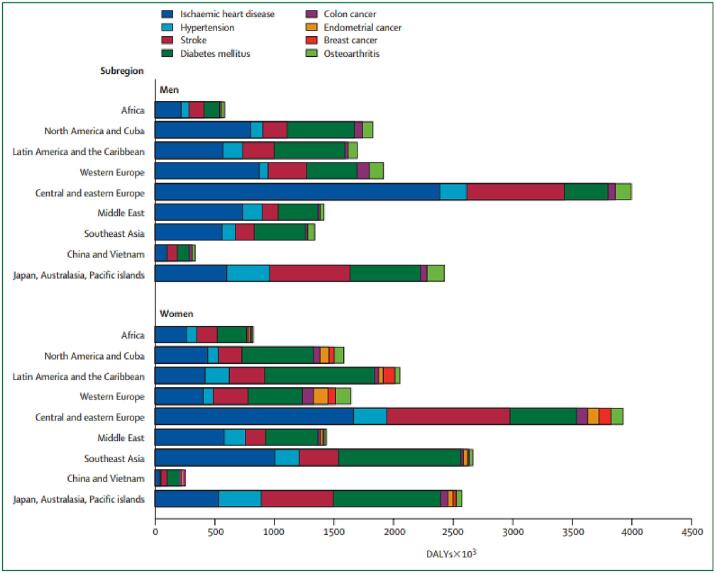
Obesity is a medical disorder that leads to many comorbidities. This association is profoundly important for the affected individuals, but the associated morbidity is also economically damaging for society. Detailed estimates of the years of ill health and lives lost between the ages of 30 years and 75 years because of excess weight are shown for the subregions of the world in Fig. 12.
Fig. 12.
Disability-adjusted life-years (DALYs) lost as a result of obesity in men and women world-wide. Derived from James and colleagues [174].
Obesity causes cardiovascular and renal diseases through several mechanisms including hypertension, hyperglycemia, dyslipidemia, inflammation, and atherosclerosis. These disorders often coexist, especially when there is excess visceral fat, and have often been referred to as the “metabolic syndrome.” However, there is substantial evidence that excess visceral fat is the main driving force for almost all of the disorders associated with the metabolic syndrome, including CKD [157], [158].
-
1)
Hypertension: Obesity is an important cause of hypertension as evident by multiple studies. Although the mechanisms responsible for obesity-induced hypertension are not fully understood, there is considerable evidence that abnormal kidney function plays a key role. Obesity causes excess renal sodium reabsorption, impaired renal-pressure natriuresis, and expansion of extracellular fluid volume, which lead to increased arterial blood pressure [159], [160]. Chronically elevated blood pressure coupled with renal vasodilation and glomerular hyperfiltration, SNS and RAAS activation, inflammation and metabolic derangements eventually causes renal injury which, in turn, further impairs renal-pressure natriuresis and exacerbates hypertension and kidney injury. Structural changes in the kidneys occur within a few weeks after rapid weight gain. These glomerular changes, if progressive, could eventually impinge on the glomerular lumen and reduce filtration surface area, initiating positive feedback that further increases blood pressure [161].
-
2)
Coronary artery disease and stroke: Cardiovascular disease (CVD) is a major cause of morbidity and mortality among patients with obesity as well as patients with CKD [162]. In the general population, obesity is associated with cardiovascular morbidity, and abdominal fat content predicts cardiovascular risk and CV death. The presence of overt metabolic syndrome further intensifies the CV risks. Metabolic syndrome is present in 30.5 % of stage 4 or 5 CKD patients and is an independent predictor of cardiovascular death, acute coronary syndrome, revascularization, non-fatal stroke and amputation [163], [164]. Obesity itself probably imposes a small additional cardiovascular risk to patients with mild to moderate CKD, but the effect of obesity on the cardiovascular risk of patients with advanced CKD is probably the result of the concomitant metabolic syndrome and coexisting cardiovascular risk factors [165].
-
3)
Type 2 Diabetes Mellitus: Excess body weight and obesity are significant risk factors for type 2 diabetes mellitus (T2DM). The lifetime diabetes risk in men older than 18 years increases from 7 % to 70 % when BMI increases from less than 18.5 kg/m to more than 35 kg/m. Similarly, the lifetime diabetes risk in females increases from 12 % to 74 % with the same BMI values [166]. Diabetes mellitus is among the leading causes of chronic kidney disease and end-stage kidney disease in the western world. Control of diabetes mellitus is more challenging in patient with CKD and the risk for severe hypoglycemic episodes increases when GFR falls below 45 mL/min. Hypoglycemic episodes may develop due to reduced gluconeogenesis and counter-regulation in the kidneys [167], [168].
-
4)
Non-alcoholic fatty liver disease (NAFLD): NAFLD is characterized by excessive fat accumulation in hepatocytes and may progress to non-alcoholic steatohepatitis (NASH), ultimately leading to advanced fibrosis and cirrhosis [169]. Experimental and epidemiological data reveal some pathophysiological links between them and support the assertion that NAFLD may be a pathogenic factor of CKD, wherein CKD accelerates the progression of NAFLD [170], [171]. Generation of lipotoxic metabolites of fatty acids typically occurred in parallel with lipid accumulation, which plays a critical role in the pathogenesis of NAFLD and CKD [171].
-
5)
Cancers and reproduction: Obesity is one of the most important known preventable causes of cancer [172]. About 10 % of all cancer deaths among non-smokers are related to obesity including renal cancer. Obesity has been associated with an increased risk of kidney malignancy. Several studies have concluded the increment of risk associated with obesity and it has been estimated that 20 % of renal cancer patients were obese [173].
In addition, both Obesity and CKD can cause disruption of the hypothalamic-pituitary-ovarian axis resulting in an abnormal reproductive hormone profile, where the degree of disruption increases with CKD progression [173].
The impact of weight loss interventions in modulating obesity and CKD cardiometabolic comorbidities and complications
Dietary changes with calorie restriction have long been the cornerstone for weight loss interventions, but use of dietary changes alone typically only results in modest weight loss and is frequently associated with weight regain [175], [176], [177], [178], [179]. There are few studies of the effect of calorie restriction on improvements in kidney function and proteinuria with mixed results. One study by Praga, et al. achieved an 86 % reduction in proteinuria via a 12 % reduction in BMI using a low-calorie diet, which was similar to the reduction in proteinuria seen in the control group who was treated with captopril [180]. A meta-analysis by Navaneethan, et al. showed that caloric restricted diets were associated with a BMI reduction of 3.67 kg/m2 as well as a reduction in proteinuria [181]. However, no difference has been seen in glomerular filtration rate, creatinine clearance, or rate in progression of CKD across multiple studies [51], [180], [181], [182], [183].
Dietary interventions that achieve short-term weight loss consistently show improvement in multiple cardiometabolic comorbidities and complications. In the Diabetes Prevention Program, achieving a weight loss of about 7 % resulted in about 55 % lower risk of developing type 2 diabetes mellitus after 3 years [184]. The data on weight loss through dietary interventions appears mixed, with some studies showing weight loss reduces both systolic and diastolic blood pressures independently and other studies showing that the blood pressure reduction is only associated with dietary sodium restriction [185], [186], [187], [188], [189], [190]. There does not appear to be any studies showing weight loss intervention through caloric restriction and reduction in risk of atherosclerotic cardiovascular diseases [191], [192], [193]. However, a post-hoc analysis of the Look Ahead trial showed a reduction in risk of coronary artery disease events of about 20 % in the population of participants who achieved at least a 10 % weight loss during the trial [194]. However, this was in a trial of participants with type 2 diabetes mellitus and only a small percentage of patients with CKD [194]. In addition, there are a few studies that suggest that cardiometabolic parameters worsen as weight is regained (which is common with dietary interventions alone), including insulin sensitivity and blood pressure [177], [180], [182], [195], [196].
The current recommendation is to start medication to assist with weight loss in people with a BMI greater than 30 kg/m2, or a BMI greater than 27 kg/m2 with associated comorbidities related to obesity [197], [198]. There are multiple different classes of medications that are FDA-approved to be used for long-term medical management of obesity, including orlistat, naltrexone-bupropion, phentermine-topiramate, and incretin mimetics. The trials for orlistat, naltrexone-bupropion, and phentermine-topiramate concluded that these medications did not significantly increase cardiovascular events, but none of these studies showed reduction in event rates [199], [200], [201]. These medications are associated with modest weight loss benefit of about 3–8 % [202], [203], [204], [205], [206], [207], [208]. Orlistat and phentermine-topiramate were consistently associated with reduction in systolic blood pressure without change in diastolic blood pressure as well as reduce the risk of developing type 2 diabetes mellitus [200], [202], [203], [204], [205], [208], [209], [210]. Only orlistat was consistently shown to reduce total and LDL cholesterol levels and improve metabolic-associated fatty liver disease, with limited data for naltrexone-bupropion [200], [202], [203], [204], [205], [208], [209], [210]. In addition, orlistat was able to achieve these cardiometabolic benefits independent of weight loss [205], [210]. However, there is not enough data published to determine the effect of these medications on progression of CKD and proteinuria in participants without type 2 diabetes mellitus [51], [183].
There are currently three FDA-approved incretin mimetics for treatment of obesity: liraglutide, semaglutide, and tirzepatide. These medications are helpful in achieving and maintaining significant weight loss while being taken, especially semaglutide and tirzepatide, which helped at least 70 % of participants achieve at least 10 % weight loss from baseline during the trial periods [211], [212], [213], [214]. Each of these medications is consistently associated with reduction in systolic blood pressure, total cholesterol, LDL cholesterol, and impaired glucose tolerance [209], [211], [212], [213]. Recent studies with semaglutide showed about a 20 % reduction in composite MACE endpoint over placebo, which appears to have mainly be driven by reductions in nonfatal myocardial infarction [214]. The findings of the study also showed about a 20 % reduction in the composite nephropathy endpoint, which included death from renal causes, initiation of renal replacement therapy, or onset of persistent macroalbuminuria, but only about 20 % of the participants had an eGFR below 60 mL/min/1.73 m2 [214], [215]. Unfortunately, most of the studies of incretin mimetic therapy as regards improved renal outcomes in a CKD population have so far been exclusively in participants with type 2 diabetes [216], [217], [218]. In addition, discontinuation of these medications results in mostly complete reversal of both the weight loss and the prior noted improvements in cardiometabolic parameters [219].
The current recommendation to qualify for bariatric surgery is a BMI greater than 40 kg/m2, or a BMI greater than 35 kg/m2 with at least one associated comorbidity related to obesity [197], [220]. A meta-analysis by Navaneethan, et al. showed that bariatric surgery was associated with a BMI reduction of 16.53 kg/m2 and showed a significant improvement in glomerular filtration rate with the majority of patients included achieving normalization of their glomerular filtration rate [181]. However, there was heterogeneity between the studies due to different types of bariatric surgery approaches being studied, but the findings for kidney function were consistent across all studies included [181]. Multiple more recent studies continue to show the benefit of bariatric surgery at improving proteinuria, improving glomerular filtration rate, and slowing the progression of CKD by about 40 % [183], [221], [222], [223], [224], [225].
Bariatric surgery consistently shows significant improvements in multiple cardiometabolic complications and comorbidities, including hypertension, hyperlipidemia, ASCVD, and impaired glucose tolerance [181], [183], [221], [226]. Multiple studies have shown significant reduction in systolic blood pressures, with the meta-analysis by Navaneethan, et al. showing an average reduction of about 22 mmHg [181], [226]. Multiple studies have shown significant reduction in cardiovascular events in patients undergoing bariatric surgery, compared to those who were receiving medical therapy for weight loss [227], [228], [229], [230], [231]. In addition, the Swedish Obese Subjects study showed a reduction in all-cause mortality by about 29 % [232]. These benefits are potentially due to rapid changes in weight loss which promotes significant improvement in inflammation, insulin sensitivity, gut microbiome, and activation of renin-angiotensin-aldosterone system [233], [234], [235], [236], [237].
Weight loss thus appears to consistently improve kidney function and reduce proteinuria in direct proportion to the amount of weight loss achieved [181], [183], [214], [215], [221], [222], [223], [224], [225]. However, the improvement in other cardiometabolic diseases associated with CKD and obesity appear to be only with the use of certain weight loss medications and bariatric surgery, which may be due to higher likelihood of achieving and maintaining significant weight loss [230]. However, there are no prospective randomized studies that specifically look at the effects of weight loss on cardiometabolic comorbidities in an obese population with chronic kidney disease (that is specifically for an ORCKD population). Most of the studies included obese patients with chronic kidney disease, but these patients made up a very small portion of the overall population, so it makes it difficult to be able to make definitive inferences about the interaction and to enable firm evidence based clinical practice recommendations in this regard [183].
Strategies for CKD modulation in patients with ORCKD
Beyond the propensity of data described above that suggest the utility of interventions geared at significant weight loss in positively modulating kidney function and its cardiometabolic consequences in patients with ORCKD, other medical and surgical interventions already established for management of CKD are equally applicable to patients with ORCKD [19], [32], [39], [182], [183], [238], [239], [240], [241], [242], [243], [244], [245], [246], [247], [248], [249].
Among the well established strategies in this regard are use of RAAKS inhibitor medications including ACE inhibitors, Angiotensin receptor blockers (ARBs), direct renin inhibitors and potentially Kallikrein inhibitors [32], [67], [250], [251], [252].
It is evident that early recognition and detection of ORCKD using various biomarkers by enabling early multi-pronged interventions offers the best prognosis for prevention of CKD progression and the potential for CKD reversal [14], [22], [34], [35], [72], [76], [79], [95], [96], [101], [117], [120], [122], [253], [254], [255], [256], [257].
There is some available data mostly from post hoc data analyses suggesting the potential utility of PPAR alpha agonists (particularly fenofibrate) in positively modulating ORCKD. The suggested putative mechanism for this effect is the ability of PPAR alpha agonists to inhibit lipolysis and beta oxidation with the potential of thus positively impacting renal lipotoxicity [258], [259], [260], [261].
There is also some evidence that suggests mineralocorticoid receptor blockers like spironolactone, eplerenone and in particular the non steroidal mineralocorticoid receptor antagonist (MRA) finerenone can also have positive modulating capacity in ORCKD management especially among patients with secondary ORCKD in the setting of coexisting diabetes mellitus [262], [263], [264].
In addition, a growing body of evidence suggests that vasopressin receptor antagonists (aka aquaretics) especially tolvaptan which is already FDA approved for modulating CKD due to autosomal dominant polycystic kidney disease (ADPKD) may also have utility in management of ORCKD [265], [266], [267], [268], [269].
The rapidly expanding body of evidence for the utility of SGLT-2 inhibitor’s positive impact in modulating CKD beyond their antidiabetic utility and the fact that these effects have been consistently demonstrated in both diabetic and non-diabetic patient populations now make them another important tool in the armamentarium of ORCKD management [39], [69], [270], [271], [272]. Beyond their glycosuric capacity, SGLT-2 inhibitors have been shown to inhibit the expression of Hypoxia inducible factor alpha (HIF-1 alpha) in both murine and human kidney tissue samples. This is of significance as HIF-1 alpha is established as the metabolic switch responsible for transition from lipid beta oxidation to glycolysis thus reducing intra-renal lipid accumulation [273], [274], [275], [276]. Furthermore, SGLT-2 inhibitors are known to reduce systemic and local tissue insulin to glucagon ratios resulting in a consequent renal tissue metabolic flux towards lipolysis as opposed to lipogenesis and of glycolysis as opposed to gluconeogenesis with the consequent reduction in renal tissue lipid and glucose accumulation as well as potentially of associated renal lipo and glucotoxicity [277], [278], [279], [280].
Among the growing list of potential therapeutic options and targets suggested for positive modulation and management of ORCKD are CD-36 inhibitors. Though human studies are still pending, the observation that CD −36 is involved in renal cholesterol and lipoprotein uptake into the renal parenchyma suggests that its modulation may have therapeutic utility [281], [282]. This is further bolstered by the finding of CD-36 inhibitors in mice having positive effects on the development of renal inflammation and fibrosis [281], [282].
The potential utility of statins for the specific management of ORCKD is still somewhat controversial as the body of evidence available has yielded somewhat conflicting and non-definitive results to firmly establish independent protective effects [283], [284], [285]. This however doesn’t in anyway preclude their use in patients with ORCKD and dyslipidemia and/or ASCVD for which they would be otherwise indicated [283], [284], [285].
As has been the case with SGLT-2 inhibitors, GLP-1 agonists have been shown with a broad range of studies to have positive effects in CKD as a whole as well as ORCKD in particular that extend beyond their effects as antidiabetic medications. While some of these effects are clearly related to the significant weight loss they induce in patients, there is also accumulating evidence suggesting that this is also related to other pleotropic effects including redistribution of renal adipose tissue, reduced renal triglyceride, free fatty acid and cholesterol accumulation with consequent reduction in renal fat infiltration, and improved mitochondrial function via modulation of the Sirt1/AMPK/PGC1 alpha cascade pathway [286], [287], [288], [289], [290], [291], [292], [293], [294]. Their capacity to improve insulin sensitivity both systemically and intrinsic to the kidney also likely plays a role in this observed reno-protective effect.
Based on the known pathophysiology and pathobiology of ORCKD the potential utility of insulin sensitizers to counteract the associated insulin resistance in ORCKD as well as anti-inflammatory agents to inhibit the systemic and local intra-renal inflammatory state in ORCKD may have utility but it is difficult to distinguish these effects independent of the various weight reduction strategies known to positively impact these pathogenic mechanisms.
The potential role of gut microbiota modulation as a strategy for positively impacting ORCKD is still preliminary but a recent meta-analysis suggests that it may have a unique niche especially in non diabetic ORCKD patients as well as ORCKD patients with ESRD on dialysis [295], [296].
Another area of clinical investigation with potential clinical utility for ORCKD management that has recently emerged is melatonin supplementation and melatonin receptor modulation. While the majority of the available data on this at the moment is animal derived it does raise appealing prospects because of the relative ease and safety of its modulation and the suggested multifaceted effects it seems to have on improving ORCKD including inhibition of the NF-kappa B pathway (with consequent reduction of expression of inflammatory cytokines like IL-1β, IL-6 and TNF-α), downregulation of the RAAKS cascade and reduction of reactive oxygen species (ROS) expression resulting in reduction of systemic and local renal oxidative stress as well as reduced expression of the fibrogenic fibronectin (Transforming growth factor beta −1; TGF-β1) [297], [298], [299], [300], [301], [302], [303]. Melatonin has pleotropic effects including circadian modulating effects, anti-inflammatory, anti-apoptotic and myriad lipid and adipocyte modulating effects including enhancing brown adipocyte tissue growth and beigeing of white adipose tissue. Via its effects of adipocyte distribution and typing it can influence energy expenditure and has been shown in murine models as well as obese diabetic and Zucker diabetic rats to increase expression of glutathione peroxidase, super oxide dismutase (SOD) and catalase (all of which have anti-oxidant effects) [297], [298], [299], [300], [301], [302], [303], [304], [305], [306], [307].
Among the other numerous potential target pathways and systems involved in renal adiposogenesis, lipid metabolism, lipid accumulation, disposition and signal transduction that offer other potential therapeutic options for ORCKD management are farnesoid X receptor (FXR) activators, anti-oxidants, endothelin receptor blockade, vitamin D receptor modulators such as doxecalciferol, M−Tor inhibitors and Sterol regulatory element binding protein 1 (SREBP-1) modulators [88], [250], [308], [309], [310], [311], [312], [313], [314], [315]. These various targets which are the subject of various ongoing in vitro and in vivo studies and trials offer considerable promise to improving the prognosis and clinical course of ORCKD and its complications in the near future. Table 2 provides a global summary of the established and putative management strategies for management of ORCKD in clinical practice.
Table 2.
Established and future Management strategies for ORCKD.
| Antilpidemics; Triglyceride lowering medications | Particularly in ORCKD patients with known persistent hypertriglyceridemia and/or confirmed renal steatosis on imaging/renal biopsy; Fibrates, niacin, fish oil analogs and derivatives, icosapent ethyl etc |
|
|---|---|---|
| Uric acid modulators; Xanthine oxidase inhibitors and uricosurics | Particularly in ORCKD patients with known nephrolithiasis, nephrocalcinois, known hyperuricemia and/or hyperuricosuria; Allopurinol, febuxostat, probenecid etc. |
|
|
Putative and Experimental adjuncts |
||
| Anti-inflammatory agents | Limited human clinical data; No FDA approved options;?? Colchine utility based on small case series |
|
|
Endothelin Receptor blockers |
Clinical trials; No clinically available nor FDA approved options |
|
| CD-36 inhibitors | Pre-Clinical and animal model trials | |
| Vasopressin Receptor antagonists (V1a, V1b and V2 receptor blockers) | May have potential utility in ORCKD patients with associated polycystic kidney disease and particularly in ADPKD patients with superimposed ORCKD; tolvaptan, satavaptan, lixivaptan and conivaptan |
|
| Fecal microbiota modulation | Limited clinical data and no clinical practice guidelines; use of pre-pro and post biotic supplements, potential of fecal transplantation in select patients. Clinical trial stage. |
|
| Melatonin supplementation and Melatonin receptor modulation | Limited human clinical data and no clinical practice guideline. Not FDA approved?? utility of low to mid dose melatonin replacement therapy. Early clinical trial stage. |
|
| FXR receptor activation | Preclinical trial stage; experimental | |
| Anti-oxidants | Limited clinical data. No clinical practice guidelines and no FDA approved options. |
|
| SREBP-1 modulators | Preclinical trial stage; experimental | |
| M−Tor inhibitors | Preclinical trial stage; experimental | |
| Aldosterone Synthase inhibitors | Limited available human clinical data. No FDA approved options; early stages of clinical development. May have particular utility in ORCKD patients with coexisting primary aldosteronism. Osilodrostat, Baxdrostat, Lorundrostat etc. |
|
| Vitamin D receptor agonists and modulators | Limited available clinical trial data. In early stages of clinical development. May unique niche of utility in ORCKD patients with coexisting secondary or tertiary hyperparathyroidism. Calcitriol, paricalcitol, doxercalciferol, alfacalcidol etc. |
|
| Patients with ORCKD and ESRD | Renal Replacement therapy | Hemo and/or peritoneal dialysis with the unique difficulties and challenges of the obese ORCKD patient. Renal transplant. |
Concluding remarks
The ongoing epidemics of obesity and CKD has resulted in the increased prevalence of ORCKD which is a multifactorial disease entity associated with multiple comorbidities and complications. The cardiometabolic complications of ORCKD in particular, have considerable morbidity and mortality consequence with implications for both individual patient and public health care.
The growing range and effectiveness of medical and other management options for obesity add an important therapeutic option to the management strategies for effective ORCKD management. Prevention of obesity, early recognition of ORCKD before it can progress to ESRD and aggressive multi-pronged clinical interventions can ameliorate the clinical course and prognosis of this increasingly prevalent cause of CKD. Ongoing studies offer the promise for greater range of therapeutic options with greater treatment efficacy both for obesity and for renal functional preservation in patients with ORCKD.
CRediT authorship contribution statement
Mariam M. Ali: Writing – review & editing, Writing – original draft. Sanober Parveen: Writing – review & editing, Writing – original draft. Vanessa Williams: Writing – review & editing, Writing – original draft. Robert Dons: Writing – original draft, Visualization, Conceptualization. Gabriel I. Uwaifo: Writing – review & editing, Writing – original draft, Visualization, Supervision, Investigation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: There are no relevant relationships to declare. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to acknowledge the entire clinical team at the Southern Illinois University Department of Medicine Endocrinology Division including but not restricted to our patients, nursing and medical assistant staff, the medical students and residents who rotate through our system, our clinical fellows, clinical consultants and research collaborators, internal and external. We also want to acknowledge our section lead Administrator Jodi Humphries and our section Chief, Dr Michael Jakoby for their support of this work. It really does take a “village” to enable this sort of work become reality.
Source of funding
None.
The preparation of this manuscript was not funded nor supported by any specific grant nor funding agency in the public, commercial nor private or not-for-profit funding sectors.
Contributor Information
Mariam M. Ali, Email: mmurtazaali67@siumed.edu.
Sanober Parveen, Email: sparveen28@siumed.edu.
Vanessa Williams, Email: vwilliams83@siumed.edu.
Robert Dons, Email: rdons25@siumed.edu.
Gabriel I. Uwaifo, Email: guwaifo93@siumed.edu.
References
- 1.Afshin A., Reitsma M.B., Murray C.J.L. Health effects of overweight and obesity in 195 countries. N Engl J Med. 2017;377(15):1496–1497. doi: 10.1056/NEJMc1710026. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators G.B.D.O., Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., et al. Health effects of overweight and obesity in 195 countries over 25 Years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwyer-Lindgren L., Freedman G., Engell R.E., Fleming T.D., Lim S.S., Murray C.J., et al. Prevalence of physical activity and obesity in US counties, 2001–2011: a road map for action. Popul Health Metr. 2013;11:7. doi: 10.1186/1478-7954-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth G.A., Huffman M.D., Moran A.E., Feigin V., Mensah G.A., Naghavi M., et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132(17):1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 6.Falodia J.S., Mk. CKD epidemiology and risk factors. clinical queries. Nephrology. 2012;1(4):249–252. [Google Scholar]
- 7.Kovesdy C.P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011l. 2022;12(1):7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundstrom J., Bodegard J., Bollmann A., Vervloet M.G., Mark P.B., Karasik A., et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2.4 million patients from 11 countries: the CaReMe CKD study. Lancet Reg Health Eur. 2022;20 doi: 10.1016/j.lanepe.2022.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampmann J.D., Heaf J.G., Mogensen C.B., Mickley H., Wolff D.L., Brandt F. Prevalence and incidence of chronic kidney disease stage 3–5 - results from KidDiCo. BMC Nephrol. 2023;24(1):17. doi: 10.1186/s12882-023-03056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIDDK NIoH. Kidney disease Statistics for the United States 2023.
- 11.Obrador G. Epidemiology of Chronic Kidney Disease. In: Curhan GT, M.; Taylor, EN.UpToDate https://www.uptodate.com/contents/epidemiology-of-chronic-kidney-disease/;2023[cited.
- 12.Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2023. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2023.
- 13.Carbone A., Al Salhi Y., Tasca A., Palleschi G., Fuschi A., De Nunzio C., et al. Obesity and kidney stone disease: a systematic review. Minerva Urol Nefrol. 2018;70(4):393–400. doi: 10.23736/S0393-2249.18.03113-2. [DOI] [PubMed] [Google Scholar]
- 14.de Vries A.P., Ruggenenti P., Ruan X.Z., Praga M., Cruzado J.M., Bajema I.M., et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417–426. doi: 10.1016/S2213-8587(14)70065-8. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N., Artunc F., Heyne N., Machann J., Schleicher E.D., Haring H.U. Obesity and renal disease: not all fat is created equal and not all obesity is harmful to the kidneys. Nephrol Dial Transplant. 2016;31(5):726–730. doi: 10.1093/ndt/gfu081. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X., Han L., Shen S., Wu W. Association between visceral adiposity index and chronic kidney disease: evidence from the China health and retirement longitudinal study. Nutr Metab Cardiovasc Dis. 2022;32(6):1437–1444. doi: 10.1016/j.numecd.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z., Wang Y., Zhao X., Cui H., Han M., Ren X., et al. Obesity and chronic kidney disease. Am J Physiol Endocrinol Metab. 2023;324(1):E24–E41. doi: 10.1152/ajpendo.00179.2022. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Montoro J.I., Morales E., Cornejo-Pareja I., Tinahones F.J., Fernandez-Garcia J.C. Obesity-related glomerulopathy: current approaches and future perspectives. Obes Rev. 2022;23(7):e13450. doi: 10.1111/obr.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehus E. Obesity and chronic kidney disease. Curr Opin Pediatr. 2018;30(2):241–1226. doi: 10.1097/MOP.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 20.Kanbay M., Copur S., Demiray A., Sag A.A., Covic A., Ortiz A., et al. Fatty kidney: a possible future for chronic kidney disease research. Eur J Clin Invest. 2022;52(6):e13748. doi: 10.1111/eci.13748. [DOI] [PubMed] [Google Scholar]
- 21.Hti Lar Seng N.S., Lohana P., Chandra S., Jim B. The fatty kidney and beyond: a silent epidemic. Am J Med. 2023;136(10):965–974. doi: 10.1016/j.amjmed.2023.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Verde L., Luca S., Cernea S., Sulu C., Yumuk V.D., Jenssen T.G., et al. The fat kidney. Curr Obes Rep. 2023;12(2):86–98. doi: 10.1007/s13679-023-00500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanbay M., Copur S., Siriopol D., Yildiz A.B., Berkkan M., Tuttle K.R., et al. The risk for chronic kidney disease in metabolically healthy obese patients: a systematic review and meta-analysis. Eur J Clin Invest. 2023;53(1):e13878. doi: 10.1111/eci.13878. [DOI] [PubMed] [Google Scholar]
- 24.Fryar CD CM, Afful J. Prevalence of overweight, obesity and severe obesity among adults aged 20 and over: United States, 1960-62 through 2017-2018. NCHS Health E-Stats2020.
- 25.Field A.E., Coakley E.H., Must A., Spadano J.L., Laird N., Dietz W.H., et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 26.https://ourworldindata.org/obesityWorld Health Organization - Global Health Observatory (2024) – processed by Our World in Data. “Prevalence of obesity among adults, BMI >= 30 (crude estimate) (%) - Sex: both sexes - Age group: 18+ years” . World Health Organization, “Global Health Observatory”. (Chart 6 of 25) Accessed on 03/11/23.
- 27.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., Jafar T.H., Heerspink H.J., Mann J.F., et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 28.Prevention CfDCCa. Chronic Kidney Diseae in the United States https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html.2023.
- 29.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O'Callaghan C.A., Lasserson D.S., et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas B., Matsushita K., Abate K.H., Al-Aly Z., Arnlov J., Asayama K., et al. Global Cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28(7):2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenvinkel P., Zoccali C., Ikizler T.A. Obesity in CKD–what should nephrologists know? J Am Soc Nephrol. 2013;24(11):1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alicic R.Z., Patakoti R., Tuttle K.R. Direct and indirect effects of obesity on the kidney. Adv Chronic Kidney Dis. 2013;20(2):121–127. doi: 10.1053/j.ackd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Wickman C., Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33(1):14–22. doi: 10.1016/j.semnephrol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Fox C.S., Larson M.G., Leip E.P., Culleton B., Wilson P.W., Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 36.Ejerblad E., Fored C.M., Lindblad P., Fryzek J., McLaughlin J.K., Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 37.Hsu C.Y., McCulloch C.E., Iribarren C., Darbinian J., Go A.S. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 38.Hojs R., Ekart R., Bevc S., Vodosek H.N. Chronic kidney disease and obesity. Nephron. 2023 doi: 10.1159/000531379. [DOI] [PubMed] [Google Scholar]
- 39.Kreiner F.F., Schytz P.A., Heerspink H.J.L., von Scholten B.J., Idorn T. Obesity-related kidney disease: current understanding and future perspectives. Biomedicines. 2023;11(9).10.3390/biomedicines11092498 doi: 10.3390/biomedicines11092498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nawaz S., Chinnadurai R., Al-Chalabi S., Evans P., Kalra P.A., Syed A.A., et al. Obesity and chronic kidney disease: a current review. Obes Sci Pract. 2023;9(2):61–74. doi: 10.1002/osp4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhowmik D., Tiwari S.C. Metabolic syndrome and chronic kidney disease. Indian J Nephrol. 2008;18(1):1–4. doi: 10.4103/0971-4065.41279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lea J., Cheek D., Thornley-Brown D., Appel L., Agodoa L., Contreras G., et al. Metabolic syndrome, proteinuria, and the risk of progressive CKD in hypertensive african Americans. Am J Kidney Dis. 2008;51(5):732–740. doi: 10.1053/j.ajkd.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Domingos F., Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26(3):864–868. doi: 10.1093/ndt/gfq501. [DOI] [PubMed] [Google Scholar]
- 44.Leonardis D., Mallamaci F., Enia G., Postorino M., Tripepi G., Zoccali C., et al. The MAURO study: baseline characteristics and compliance with guidelines targets. J Nephrol. 2012;25(6):1081–1090. doi: 10.5301/jn.5000239. [DOI] [PubMed] [Google Scholar]
- 45.Hall J.E., Mouton A.J., da Silva A.A., Omoto A.C.M., Wang Z., Li X., et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res. 2021;117(8):1859–1876. doi: 10.1093/cvr/cvaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oluyombo R., Banjo Oguntade H., Soje M., Obajolowo O., Karim M. Obesity and CKD in sub-Saharan Africa: a Narrative review. Kidney Med. 2022;4(2) doi: 10.1016/j.xkme.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polito L., Bortolotti M., Battelli M.G., Bolognesi A. Chronic kidney disease: which role for xanthine oxidoreductase activity and products? Pharmacol Res. 2022;184 doi: 10.1016/j.phrs.2022.106407. [DOI] [PubMed] [Google Scholar]
- 48.Stasi A., Cosola C., Caggiano G., Cimmarusti M.T., Palieri R., Acquaviva P.M., et al. Obesity-related chronic kidney disease: principal mechanisms and new approaches in nutritional Management. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.925619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fotheringham A.K., Gallo L.A., Borg D.J., Forbes J.M. Advanced glycation end products (AGEs) and chronic kidney disease: does the modern diet AGE the kidney? Nutrients. 2022;14(13).10.3390/nu14132675 doi: 10.3390/nu14132675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanbay M., Yildiz A.B., Yavuz F., Covic A., Ortiz A., Siriopol D. The role of body mass index on IgA nephropathy prognosis: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54(10):2567–2579. doi: 10.1007/s11255-022-03160-1. [DOI] [PubMed] [Google Scholar]
- 51.Sandino J., Martin-Taboada M., Medina-Gomez G., Vila-Bedmar R., Morales E. Novel insights in the physiopathology and Management of Obesity-Related Kidney Disease. Nutrients. 2022;14(19) doi: 10.3390/nu14193937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faegenburg D., Bosniak M., Evans J.A. Renal sinus lipomatosis: its demonstration by nephrotomography. Radiology. 1964;83:987–998. doi: 10.1148/83.6.987. [DOI] [PubMed] [Google Scholar]
- 53.Dwyer T.M., Carroll J.F., Mizelle H.L., Cockrell K. Renal size and composition in hypertensive, obese rabbits. Int J Obes Relat Metab Disord. 1998;22(9):935–938. doi: 10.1038/sj.ijo.0800677. [DOI] [PubMed] [Google Scholar]
- 54.Chughtai H.L., Morgan T.M., Rocco M., Stacey B., Brinkley T.E., Ding J., et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56(5):901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irazabal M.V., Eirin A. Role of renal sinus adipose tissue in obesity-induced renal injury. EBioMedicine. 2016;13:21–22. doi: 10.1016/j.ebiom.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishna S., Schieda N., Flood T.A., Shanbhogue A.K., Ramanathan S., Siegelman E. Magnetic resonance imaging (MRI) of the renal sinus. Abdom Radiol (NY) 2018;43(11):3082–3100. doi: 10.1007/s00261-018-1593-1. [DOI] [PubMed] [Google Scholar]
- 57.Wang S.S., Gu Q., Liu N., Li J., Liu X. Aerobic exercise attenuates ectopic renal sinus adipose tissue accumulation-related renal hypoxia injury in obese mice. Life Sci. 2021;279 doi: 10.1016/j.lfs.2021.119106. [DOI] [PubMed] [Google Scholar]
- 58.De Pergola G., Campobasso N., Nardecchia A., Triggiani V., Caccavo D., Gesualdo L., et al. Para- and perirenal ultrasonographic fat thickness is associated with 24-hours mean diastolic blood pressure levels in overweight and obese subjects. BMC Cardiovasc Disord. 2015;15:108. doi: 10.1186/s12872-015-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricci M.A., Scavizzi M., Ministrini S., De Vuono S., Pucci G., Lupattelli G. Morbid obesity and hypertension: the role of perirenal fat. J Clin Hypertens (Greenwich) 2018;20(10):1430–11147. doi: 10.1111/jch.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weisinger J.R., Kempson R.L., Eldridge F.L., Swenson R.S. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81(4):440–447. doi: 10.7326/0003-4819-81-4-440. [DOI] [PubMed] [Google Scholar]
- 61.Kambham N., Markowitz G.S., Valeri A.M., Lin J., D'Agati V.D. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 62.Adebayo O.C., Nkoy A.B., van den Heuvel L.P., Labarque V., Levtchenko E., Delanaye P., et al. Glomerular hyperfiltration: part 2-clinical significance in children. Pediatr Nephrol. 2023;38(8):2529–2547. doi: 10.1007/s00467-022-05826-5. [DOI] [PubMed] [Google Scholar]
- 63.Pottel H., Adebayo O.C., Nkoy A.B., Delanaye P. Glomerular hyperfiltration: part 1 - defining the threshold - is the sky the limit? Pediatr Nephrol. 2023;38(8):2523–12257. doi: 10.1007/s00467-022-05827-4. [DOI] [PubMed] [Google Scholar]
- 64.D'Agati V.D., Chagnac A., de Vries A.P., Levi M., Porrini E., Herman-Edelstein M., et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 65.Herman-Edelstein M., Weinstein T., Chagnac A. Obesity-related Glomerulopathy: clinical Management. Semin Nephrol. 2021;41(4):358–370. doi: 10.1016/j.semnephrol.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Toto R.D., Greene T., Hebert L.A., Hiremath L., Lea J.P., Lewis J.B., et al. Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: results from the african American study of kidney disease and hypertension (AASK) cohort. Am J Kidney Dis. 2010;56(5):896–906. doi: 10.1053/j.ajkd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallamaci F., Ruggenenti P., Perna A., Leonardis D., Tripepi R., Tripepi G., et al. ACE inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol. 2011;22(6):1122–1128. doi: 10.1681/ASN.2010090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang J., Yan H., Zhuang S. Inflammation and oxidative stress in obesity-related glomerulopathy. Int J Nephrol. 2012;2012 doi: 10.1155/2012/608397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei L., Li Y., Yu Y., Xu M., Chen H., Li L., et al. Obesity-related Glomerulopathy: from mechanism to therapeutic Target. Diabetes Metab Syndr Obes. 2021;14:4371–4380. doi: 10.2147/DMSO.S334199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma K. The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int. 2009;76(2):145–148. doi: 10.1038/ki.2009.137. [DOI] [PubMed] [Google Scholar]
- 71.Scholze A., Rattensperger D., Zidek W., Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15(6):1617–1622. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 72.Suthanthiran M., Gerber L.M., Schwartz J.E., Sharma V.K., Medeiros M., Marion R., et al. Circulating transforming growth factor-beta1 levels and the risk for kidney disease in african Americans. Kidney Int. 2009;76(1):72–80. doi: 10.1038/ki.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Przybycinski J., Dziedziejko V., Puchalowicz K., Domanski L., Pawlik A. Adiponectin in chronic kidney disease. Int J Mol Sci. 2020;21(24) doi: 10.3390/ijms21249375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammoud S.H., AlZaim I., Al-Dhaheri Y., Eid A.H., El-Yazbi A.F. Perirenal adipose tissue inflammation: novel insights linking metabolic dysfunction to renal diseases. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salgado J.V., Goes M.A., Salgado F.N. FGF21 and chronic kidney disease. Metabolism. 2021;118 doi: 10.1016/j.metabol.2021.154738. [DOI] [PubMed] [Google Scholar]
- 76.Castro B.B.A., Foresto-Neto O., Saraiva-Camara N.O., Sanders-Pinheiro H. Renal lipotoxicity: insights from experimental models. Clin Exp Pharmacol Physiol. 2021;48(12):1579–1588. doi: 10.1111/1440-1681.13556. [DOI] [PubMed] [Google Scholar]
- 77.Grigoras A., Balan R.A., Caruntu I.D., Giusca S.E., Lozneanu L., Avadanei R.E., et al. Perirenal adipose tissue-current knowledge and future opportunities. J Clin Med. 2021;10(6) doi: 10.3390/jcm10061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canbakan M., Bakkaloglu O.K., Atay K., Koroglu E., Tuncer M.M., Canbakan B., et al. The liver-kidney axis: is serum leptin a potential link in non-alcoholic fatty liver disease-associated chronic kidney disease? Arab J Gastroenterol. 2023;24(1):52–57. doi: 10.1016/j.ajg.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Ren L., Cui H., Wang Y., Ju F., Cai Y., Gang X., et al. The role of lipotoxicity in kidney disease: from molecular mechanisms to therapeutic prospects. Biomed Pharmacother. 2023;161 doi: 10.1016/j.biopha.2023.114465. [DOI] [PubMed] [Google Scholar]
- 80.Ryu J.H., Ge M., Merscher S., Rosenberg A.Z., Desante M., Roshanravan H., et al. APOL1 renal risk variants promote cholesterol accumulation in tissues and cultured macrophages from APOL1 transgenic mice. PLoS One. 2019;14(4):e0211559. doi: 10.1371/journal.pone.0211559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valdez Imbert R., Hti Lar Seng N.S., Stokes M.B., Jim B. Obesity-related glomerulopathy in the presence of APOL1 risk alleles. BMJ Case Rep. 2022;15(8) doi: 10.1136/bcr-2022-249624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Praga M., Hernandez E., Morales E., Campos A.P., Valero M.A., Martinez M.A., et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16(9):1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 83.Tsuboi N., Koike K., Hirano K., Utsunomiya Y., Kawamura T., Hosoya T. Clinical features and long-term renal outcomes of japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol. 2013;17(3):379–385. doi: 10.1007/s10157-012-0719-y. [DOI] [PubMed] [Google Scholar]
- 84.Mykkanen L., Zaccaro D.J., Wagenknecht L.E., Robbins D.C., Gabriel M., Haffner S.M. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47(5):793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 85.Kurella M., Lo J.C., Chertow G.M. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16(7):2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 86.Welsh G.I., Hale L.J., Eremina V., Jeansson M., Maezawa Y., Lennon R., et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Artunc F., Schleicher E., Weigert C., Fritsche A., Stefan N., Haring H.U. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 88.Schafer C., Heiss A., Schwarz A., Westenfeld R., Ketteler M., Floege J., et al. The serum protein alpha 2-heremans-schmid glycoprotein/fetuin-a is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112(3):357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stefan N., Hennige A.M., Staiger H., Machann J., Schick F., Krober S.M., et al. Alpha2-heremans-schmid glycoprotein/fetuin-a is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29(4):853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 90.Li M., Xu M., Bi Y., Song A., Liu Y., Li X., et al. Association between higher serum fetuin-a concentrations and abnormal albuminuria in middle-aged and elderly chinese with normal glucose tolerance. Diabetes Care. 2010;33(11):2462–2464. doi: 10.2337/dc10-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haukeland J.W., Dahl T.B., Yndestad A., Gladhaug I.P., Loberg E.M., Haaland T., et al. Fetuin a in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol. 2012;166(3):503–510. doi: 10.1530/EJE-11-0864. [DOI] [PubMed] [Google Scholar]
- 92.Stefan N., Haring H.U. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9(3):144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 93.Siegel-Axel D.I., Ullrich S., Stefan N., Rittig K., Gerst F., Klingler C., et al. Fetuin-a influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia. 2014;57(5):1057–1066. doi: 10.1007/s00125-014-3177-0. [DOI] [PubMed] [Google Scholar]
- 94.Krievina G., Tretjakovs P., Skuja I., Silina V., Keisa L., Krievina D., et al. Ectopic adipose tissue storage in the left and the right renal sinus is Asymmetric and associated with serum kidney injury Molecule-1 and fibroblast growth Factor-21 levels increase. EBioMedicine. 2016;13:274–283. doi: 10.1016/j.ebiom.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao N., Devarajan P., Inge T.H., Jenkins T.M., Bennett M., Mitsnefes M.M. Subclinical kidney injury before and 1 year after bariatric surgery among adolescents with severe obesity. Obesity (Silver Spring) 2015;23(6):1234–1238. doi: 10.1002/oby.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding W., Mak R.H. Early markers of obesity-related renal injury in childhood. Pediatr Nephrol. 2015;30(1):1–4. doi: 10.1007/s00467-014-2976-3. [DOI] [PubMed] [Google Scholar]
- 97.Mori Y, Ajay AK, Chang JH, Mou S, Zhao H, Kishi S, et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 2021;33(5):1042-61 e7.10.1016/j.cmet.2021.04.004. [DOI] [PMC free article] [PubMed]
- 98.Safaeian B., Nickavar A., Zaeri H., Lahootian L., Behnampour N. Utility of urine N-acetyl-beta-D-glucosaminidase for prediction of renal damage in obese children. Saudi J Kidney Dis Transpl. 2021;32(3):699–702. doi: 10.4103/1319-2442.336764. [DOI] [PubMed] [Google Scholar]
- 99.Pereira S.V., Dos Santos M., Rodrigues P.G., do Nascimento J.F., Timm J.R., Zancan R., et al. Increased urine podocyte-associated messenger RNAs in severe obesity are evidence of podocyte injury. Obesity (Silver Spring) 2015;23(8):1643–1649. doi: 10.1002/oby.21156. [DOI] [PubMed] [Google Scholar]
- 100.Ovunc Hacihamdioglu D., Hacihamdioglu B., Altun D., Muftuoglu T., Karademir F., Suleymanoglu S. Urinary Netrin-1: a new Biomarker for the Early diagnosis of renal damage in obese children. J Clin Res Pediatr Endocrinol. 2016;8(3):282–287. doi: 10.4274/jcrpe.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suwanpen C., Nouanthong P., Jaruvongvanich V., Pongpirul K., Pongpirul W.A., Leelahavanichkul A., et al. Urinary podocalyxin, the novel biomarker for detecting early renal change in obesity. J Nephrol. 2016;29(1):37–44. doi: 10.1007/s40620-015-0199-8. [DOI] [PubMed] [Google Scholar]
- 102.Montoro-Molina S., Lopez-Carmona A., Quesada A., O'Valle F., Martin-Morales N., Osuna A., et al. Klotho and aminopeptidases as Early Biomarkers of renal injury in zucker obese rats. Front Physiol. 2018;9:1599. doi: 10.3389/fphys.2018.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Minakawa A., Fukuda A., Sato Y., Kikuchi M., Kitamura K., Wiggins R.C., et al. Podocyte hypertrophic stress and detachment precedes hyperglycemia or albuminuria in a rat model of obesity and type2 diabetes-associated nephropathy. Sci Rep. 2019;9(1):18485. doi: 10.1038/s41598-019-54692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vianello E., Kalousova M., Dozio E., Tacchini L., Zima T., Corsi Romanelli M.M., et al. The Molecular bridge between fat and Cardiac-renal Disorders. Int J Mol Sci. 2020;21(15) doi: 10.3390/ijms21155568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Porrini E., Ruggenenti P., Luis-Lima S., Carrara F., Jimenez A., de Vries A.P.J., et al. Reply to 'strengths and limitations of estimated and measured GFR'. Nat Rev Nephrol. 2019;15(12):785–1776. doi: 10.1038/s41581-019-0214-8. [DOI] [PubMed] [Google Scholar]
- 106.Porrini E., Ruggenenti P., Luis-Lima S., Carrara F., Jimenez A., de Vries A.P.J., et al. Author correction: estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15(2):121. doi: 10.1038/s41581-018-0105-4. [DOI] [PubMed] [Google Scholar]
- 107.Porrini E., Ruggenenti P., Luis-Lima S., Carrara F., Jimenez A., de Vries A.P.J., et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15(3):177–190. doi: 10.1038/s41581-018-0080-9. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Martinez M., Luis-Lima S., Morales E., Navarro-Diaz M., Negrin-Mena N., Folgueras T., et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int J Obes (Lond) 2020;44(5):1129–1140. doi: 10.1038/s41366-019-0476-z. [DOI] [PubMed] [Google Scholar]
- 109.Gomes A.C., Hoffmann C., Mota J.F. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. 2018;9(4):308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsumoto M., Kibe R., Ooga T., Aiba Y., Kurihara S., Sawaki E., et al. Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep. 2012;2:233. doi: 10.1038/srep00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nallu A., Sharma S., Ramezani A., Muralidharan J., Raj D. Gut microbiome in chronic kidney disease: challenges and opportunities. Transl Res. 2017;179:24–37. doi: 10.1016/j.trsl.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lovre D., Shah S., Sihota A., Fonseca V.A. Managing diabetes and Cardiovascular risk in chronic kidney disease patients. Endocrinol Metab Clin North Am. 2018;47(1):237–257. doi: 10.1016/j.ecl.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu I.W., Hsu K.H., Lee C.C., Sun C.Y., Hsu H.J., Tsai C.J., et al. p-cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Popkov V.A., Zharikova A.A., Demchenko E.A., Andrianova N.V., Zorov D.B., Plotnikov E.Y. Gut Microbiota as a source of uremic toxins. Int J Mol Sci. 2022;23(1) doi: 10.3390/ijms23010483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramezani A., Massy Z.A., Meijers B., Evenepoel P., Vanholder R., Raj D.S. Role of the gut microbiome in uremia: a potential therapeutic Target. Am J Kidney Dis. 2016;67(3):483–498. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rysz J., Franczyk B., Lawinski J., Olszewski R., Cialkowska-Rysz A., Gluba-Brzozka A. The impact of CKD on uremic toxins and gut Microbiota. Toxins (Basel) 2021;13(4) doi: 10.3390/toxins13040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borges N.A., Barros A.F., Nakao L.S., Dolenga C.J., Fouque D., Mafra D. Protein-bound uremic toxins from gut Microbiota and inflammatory Markers in chronic kidney disease. J Ren Nutr. 2016;26(6):396–400. doi: 10.1053/j.jrn.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Antza C., Stabouli S., Kotsis V. Gut microbiota in kidney disease and hypertension. Pharmacol Res. 2018;130:198–203. doi: 10.1016/j.phrs.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 119.Rukavina Mikusic N.L., Kouyoumdzian N.M., Choi M.R. Gut microbiota and chronic kidney disease: evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflugers Arch. 2020;472(3):303–320. doi: 10.1007/s00424-020-02352-x. [DOI] [PubMed] [Google Scholar]
- 120.Serra A., Romero R., Lopez D., Navarro M., Esteve A., Perez N., et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73(8):947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 121.Tsuboi N., Utsunomiya Y., Kanzaki G., Koike K., Ikegami M., Kawamura T., et al. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7(5):735–741. doi: 10.2215/CJN.07270711. [DOI] [PubMed] [Google Scholar]
- 122.Tsuboi N., Utsunomiya Y., Hosoya T. Obesity-related glomerulopathy and the nephron complement. Nephrol Dial Transplant. 2013;28 doi: 10.1093/ndt/gft258. Suppl 4:iv108-13.10.1093/ndt/gft258. [DOI] [PubMed] [Google Scholar]
- 123.Choung H.G., Bomback A.S., Stokes M.B., Santoriello D., Campenot E.S., Batal I., et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int. 2019;95(3):647–654. doi: 10.1016/j.kint.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 124.Gracia-Iguacel C., Gonzalez-Parra E., Perez-Gomez M.V., Mahillo I., Egido J., Ortiz A., et al. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia. 2013;33(4):495–505. doi: 10.3265/Nefrologia.pre2013.Apr.11979. [DOI] [PubMed] [Google Scholar]
- 125.Ros S., Carrero J.J. Endocrine alterations and cardiovascular risk in CKD: is there a link? Nefrologia. 2013;33(2):181–187. doi: 10.3265/Nefrologia.pre2012.Oct.11710. [DOI] [PubMed] [Google Scholar]
- 126.Kuczera P., Adamczak M., Wiecek A. Endocrine abnormalities in patients with chronic kidney disease. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015;36(2):109–118. doi: 10.1515/prilozi-2015-0059. [DOI] [PubMed] [Google Scholar]
- 127.Cho Y.K., Jung C.H. Metabolically healthy obesity: epidemiology, criteria, and implications in chronic kidney disease. J Obes Metab Syndr. 2022;31(3):208–216. doi: 10.7570/jomes22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaka N., Sethi Y., Patel N., Kaiwan O., Al-Inaya Y., Manchanda K., et al. Endocrine manifestations of chronic kidney disease and their evolving management: a systematic review. Dis Mon. 2022;68(12) doi: 10.1016/j.disamonth.2022.101466. [DOI] [PubMed] [Google Scholar]
- 129.Vinhas J., Gardete-Correia L., Boavida J.M., Raposo J.F., Mesquita A., Fona M.C., et al. Prevalence of chronic kidney disease and associated risk factors, and risk of end-stage renal disease: data from the PREVADIAB study. Nephron Clin Pract. 2011;119(1):c35–c40. doi: 10.1159/000324218. [DOI] [PubMed] [Google Scholar]
- 130.Weidmann P., Reinhart R., Maxwell M.H., Rowe P., Coburn J.W., Massry S.G. Syndrome of hyporeninemic hypoaldosteronism and hyperkalemia in renal disease. J Clin Endocrinol Metab. 1973;36(5):965–977. doi: 10.1210/jcem-36-5-965. [DOI] [PubMed] [Google Scholar]
- 131.Oh M.S., Carroll H.J., Clemmons J.E., Vagnucci A.H., Levison S.P., Whang E.S. A mechanism for hyporeninemic hypoaldosteronism in chronic renal disease. Metabolism. 1974;23(12):1157–1166. doi: 10.1016/0026-0495(74)90032-8. [DOI] [PubMed] [Google Scholar]
- 132.Lim V.S., Kathpalia S.C., Henriquez C. Endocrine abnormalities associated with chronic renal failure. Med Clin North Am. 1978;62(6):1341–1361. doi: 10.1016/s0025-7125(16)31740-0. [DOI] [PubMed] [Google Scholar]
- 133.Norby L.H., Weidig J., Ramwell P., Slotkoff L., Flamenbaum W. Possible role for impaired renal prostaglandin production in pathogenesis of hyporeninaemic hypoaldosteronism. Lancet. 1978;2(8100):1118–1122. doi: 10.1016/s0140-6736(78)92275-4. [DOI] [PubMed] [Google Scholar]
- 134.Rosman P.M., Farag A., Peckham R., Benn R., Tito J., Bacci V., et al. Pituitary-adrenocortical function in chronic renal failure: blunted suppression and early escape of plasma cortisol levels after intravenous dexamethasone. J Clin Endocrinol Metab. 1982;54(3):528–533. doi: 10.1210/jcem-54-3-528. [DOI] [PubMed] [Google Scholar]
- 135.Mooradian A.D., Morley J.E. Endocrine dysfunction in chronic renal failure. Arch Intern Med. 1984;144(2):351–353. [PubMed] [Google Scholar]
- 136.Clodi M., Riedl M., Schmaldienst S., Vychytil A., Kotzmann H., Kaider A., et al. Adrenal function in patients with chronic renal failure. Am J Kidney Dis. 1998;32(1):52–55. doi: 10.1053/ajkd.1998.v32.pm9669424. [DOI] [PubMed] [Google Scholar]
- 137.Arregger A.L., Cardoso E.M., Zucchini A., Aguirre E.C., Elbert A., Contreras L.N. Adrenocortical function in hypotensive patients with end stage renal disease. Steroids. 2014;84:57–63. doi: 10.1016/j.steroids.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 138.Sogbe-Diaz M.E., Diaz-Lopez E.E. Adrenomedullin in the kidney: physiology and pathophysiology. Invest Clin. 2016;57(1):66–76. [PubMed] [Google Scholar]
- 139.Bray G.A. Risks of obesity. Prim Care. 2003;30(2):281–299. doi: 10.1016/s0095-4543(03)00008-3. v–vi.10.1016/s0095-4543(03)00008–3. [DOI] [PubMed] [Google Scholar]
- 140.Modesitt S.C., van Nagell Jr. J.R. The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60(10):683–692. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 141.Cawley J., Sweeney M.J., Kurian M., Beane S. New York state Bariatric surgery W. predicting complications after bariatric surgery using obesity-related co-morbidities. Obes Surg. 2007;17(11):1451–1456. doi: 10.1007/s11695-008-9422-1. [DOI] [PubMed] [Google Scholar]
- 142.Cali A.M., Bonadonna R.C., Trombetta M., Weiss R., Caprio S. Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab. 2008;93(5):1767–1773. doi: 10.1210/jc.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cali A.M., Caprio S. Obesity in children and adolescents. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S31–S36. doi: 10.1210/jc.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pischon T., Nothlings U., Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67(2):128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 145.Randeva H.S., Tan B.K., Weickert M.O., Lois K., Nestler J.E., Sattar N., et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33(5):812–841. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmitz K.H., Neuhouser M.L., Agurs-Collins T., Zanetti K.A., Cadmus-Bertram L., Dean L.T., et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105(18):1344–1354. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katsareli E.A., Dedoussis G.V. Biomarkers in the field of obesity and its related comorbidities. Expert Opin Ther Targets. 2014;18(4):385–401. doi: 10.1517/14728222.2014.882321. [DOI] [PubMed] [Google Scholar]
- 148.Segula D. Complications of obesity in adults: a short review of the literature. Malawi Med J. 2014;26(1):20–24. [PMC free article] [PubMed] [Google Scholar]
- 149.Abate N., Chandalia M. Risk of obesity-related Cardiometabolic complications in special populations: a crisis in asians. Gastroenterology. 2017;152(7):1647–1655. doi: 10.1053/j.gastro.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 150.Salaun H., Thariat J., Vignot M., Merrouche Y., Vignot S. Obesity and cancer. Bull Cancer. 2017;104(1):30–41. doi: 10.1016/j.bulcan.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 151.Jakobsen G.S., Smastuen M.C., Sandbu R., Nordstrand N., Hofso D., Lindberg M., et al. Association of Bariatric Surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291–301. doi: 10.1001/jama.2017.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kinlen D., Cody D., O'Shea D. Complications of obesity. QJM. 2018;111(7):437–443. doi: 10.1093/qjmed/hcx152. [DOI] [PubMed] [Google Scholar]
- 153.Delpino F.M., Dos Santos Rodrigues A.P., Petarli G.B., Machado K.P., Flores T.R., Batista S.R., et al. Overweight, obesity and risk of multimorbidity: a systematic review and meta-analysis of longitudinal studies. Obes Rev. 2023;24(6):e13562. doi: 10.1111/obr.13562. [DOI] [PubMed] [Google Scholar]
- 154.Yuen M.M.A. Health complications of obesity: 224 obesity-associated comorbidities from a mechanistic perspective. Gastroenterol Clin North Am. 2023;52(2):363–380. doi: 10.1016/j.gtc.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X., Ha S., Lau H.C., Yu J. Excess body weight: novel insights into its roles in obesity comorbidities. Semin Cancer Biol. 2023;92:16–27. doi: 10.1016/j.semcancer.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 156.Venkatesh N., Martini A., McQuade J.L., Msaouel P., Hahn A.W. Obesity and renal cell carcinoma: biological mechanisms and perspectives. Semin Cancer Biol. 2023;94:21–33. doi: 10.1016/j.semcancer.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hall J.E., Henegar J.R., Dwyer T.M., Liu J., Da Silva A.A., Kuo J.J., et al. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11(1):41–54. doi: 10.1053/j.arrt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 158.Tchernof A., Despres J.P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 159.Hall J.E., Crook E.D., Jones D.W., Wofford M.R., Dubbert P.M. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324(3):127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 160.Hall J.E., da Silva A.A., do Carmo J.M., Dubinion J., Hamza S., Munusamy S., et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Henegar J.R., Bigler S.A., Henegar L.K., Tyagi S.C., Hall J.E. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12(6):1211–11127. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 162.Fox C.S., Matsushita K., Woodward M., Bilo H.J., Chalmers J., Heerspink H.J., et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ervin R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;13:1–7. [PubMed] [Google Scholar]
- 164.Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Than W.H.C., C.k., et al. The role of obesity on chronic kidney disease development, progression and cardiovascular complications. Advances in Biomarker Sciences and Technology. 2020;2:24–34. [Google Scholar]
- 166.Narayan K.M., Boyle J.P., Thompson T.J., Gregg E.W., Williamson D.F. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30(6):1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 167.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nordheim E., Geir J.T. Chronic kidney disease in patients with diabetes mellitus. Endocr Connect. 2021;10(5):R151–R159. doi: 10.1530/EC-21-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou J., Zhou F., Wang W., Zhang X.J., Ji Y.X., Zhang P., et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71(5):1851–1864. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 170.Musso G., Gambino R., Tabibian J.H., Ekstedt M., Kechagias S., Hamaguchi M., et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang M., Geng C.A., Liu X., Guan M. Lipid Disorders in NAFLD and chronic kidney disease. Biomedicines. 2021;9(10).10.3390/biomedicines9101405 doi: 10.3390/biomedicines9101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pati S., Irfan W., Jameel A., Ahmed S., Shahid R.K. Obesity and cancer: a current overview of epidemiology, pathogenesis. Outcomes, and Management Cancers (Basel) 2023;15(2).10.3390/cancers15020485 doi: 10.3390/cancers15020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kotsis V., Martinez F., Trakatelli C., Redon J. Impact of obesity in kidney diseases. Nutrients. 2021;13(12).10.3390/nu13124482 doi: 10.3390/nu13124482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.James WPTJ-L, R.; Ni Mhurchu, C.; et al. Overweight and Obesity (High Body mass index). In: Ezzati ML, A.D.; Rodgers, A.; Murray, C.J.L., editor. Comparative quantification of health risks; global and regional burden of disease attributable to selected major risk factors.Geneva: WHO; 2004. p. 497 - 596.
- 175.Elfhag K., Rössner S. Who succeeds in maintaining weight loss? a conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 176.Jeffery R.W., Drewnowski A., Epstein L.H., Stunkard A.J., Wilson G.T., Wing R.R., et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1s):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 177.Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R., et al. Persistent metabolic adaptation 6 years after “the biggest loser” competition. Obesity (Silver Spring) 2016;24(8):1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klos LA, Greenleaf C, Paly N, Kessler MM, Shoemaker CG, Suchla EA. Losing Weight on Reality TV: A Content Analysis of the Weight Loss Behaviors and Practices Portrayed on The Biggest Loser. J Health Commun. 2015;20(6):639-46.10.1080/10810730.2014.965371. [DOI] [PubMed]
- 179.Hall K.D. Diet versus exercise in “the biggest loser” weight loss competition. Obesity (Silver Spring) 2013;21(5):957–959. doi: 10.1002/oby.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Praga M., Hernández E., Andrés A., León M., Ruilope L.M., Rodicio J.L. Effects of body-weight loss and captopril treatment on proteinuria associated with obesity. Nephron. 1995;70(1):35–41. doi: 10.1159/000188541. [DOI] [PubMed] [Google Scholar]
- 181.Navaneethan S.D., Yehnert H., Moustarah F., Schreiber M.J., Schauer P.R., Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–1574. doi: 10.2215/cjn.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Y., Dabbas W., Gangemi A., Benedetti E., Lash J., Finn P.W., et al. Obesity Management and chronic kidney disease. Semin Nephrol. 2021;41(4):392–402. doi: 10.1016/j.semnephrol.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 183.Chintam K., Chang A.R. Strategies to treat obesity in patients with CKD. Am J Kidney Dis. 2021;77(3):427–439. doi: 10.1053/j.ajkd.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fagerberg B., Andersson O.K., Isaksson B., Björntorp P. Blood pressure control during weight reduction in obese hypertensive men: separate effects of sodium and energy restriction. Br Med J (Clin Res Ed) 1984;288(6410):11–14. doi: 10.1136/bmj.288.6410.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dahl L.K., Silver L., Christie R.W. The role of salt in the fall of blood pressure accompanying reduction in obesity. N Engl J Med. 1958;258(24):1186–1192. doi: 10.1056/nejm195806122582402. [DOI] [PubMed] [Google Scholar]
- 187.Maxwell M.H., Kushiro T., Dornfeld L.P., Tuck M.L., Waks A.U. BP changes in obese hypertensive subjects during rapid weight loss. Comparison of restricted v unchanged salt intake. Arch Intern Med. 1984;144(8):1581–1584. [PubMed] [Google Scholar]
- 188.Tuck M.L., Sowers J., Dornfeld L., Kledzik G., Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304(16):930–933. doi: 10.1056/nejm198104163041602. [DOI] [PubMed] [Google Scholar]
- 189.Reisin E., Abel R., Modan M., Silverberg D.S., Eliahou H.E., Modan B. Effect of weight loss without salt restriction on the reduction of blood pressure in overweight hypertensive patients. N Engl J Med. 1978;298(1):1–6. doi: 10.1056/nejm197801052980101. [DOI] [PubMed] [Google Scholar]
- 190.Morales E., Praga M. The effect of weight loss in obesity and chronic kidney disease. Curr Hypertens Rep. 2012;14(2):170–176. doi: 10.1007/s11906-012-0247-x. [DOI] [PubMed] [Google Scholar]
- 191.Wing R.R., Bolin P., Brancati F.L., Bray G.A., Clark J.M., Coday M., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ma C., Avenell A., Bolland M., Hudson J., Stewart F., Robertson C., et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359 doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sierra-Johnson J., Romero-Corral A., Somers V.K., Lopez-Jimenez F., Thomas R.J., Squires R.W., et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15(3):336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 194.Gregg E.W., Jakicic J.M., Blackburn G., Bloomquist P., Bray G.A., Clark J.M., et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. doi: 10.1016/s2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Knowler W.C., Fowler S.E., Hamman R.F., Christophi C.A., Hoffman H.J., Brenneman A.T., et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/s0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Group TLAR Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22(1):5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garvey W.T., Mechanick J.I., Brett E.M., Garber A.J., Hurley D.L., Jastreboff A.M., et al. American Association of Clinical Endocrinologists and American College of endocrinology comprehensive clinical Practice guidelines for medical Care of Patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/ep161365.Gl. [DOI] [PubMed] [Google Scholar]
- 198.Apovian C.M., Aronne L.J., Bessesen D.H., McDonnell M.E., Murad M.H., Pagotto U., et al. Pharmacological Management of Obesity: an Endocrine Society clinical Practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 199.Nissen S.E., Wolski K.E., Prcela L., Wadden T., Buse J.B., Bakris G., et al. Effect of Naltrexone-bupropion on major adverse Cardiovascular events in overweight and obese patients with Cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 200.Filippatos T.D., Derdemezis C.S., Gazi I.F., Nakou E.S., Mikhailidis D.P., Elisaf M.S. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31(1):53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 201.Patel D.K., Stanford F.C. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130(2):173–182. doi: 10.1080/00325481.2018.1435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blüher M., Aras M., Aronne L.J., Batterham R.L., Giorgino F., Ji L., et al. New insights into the treatment of obesity. Diabetes Obes Metab. 2023;25(8):2058–2072. doi: 10.1111/dom.15077. [DOI] [PubMed] [Google Scholar]
- 203.Montan P.D., Sourlas A., Olivero J., Silverio D., Guzman E., Kosmas C.E. Pharmacologic therapy of obesity: mechanisms of action and cardiometabolic effects. Ann Transl Med. 2019;7(16):393. doi: 10.21037/atm.2019.07.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sweeting A.N., Tabet E., Caterson I.D., Markovic T.P. Management of obesity and cardiometabolic risk - role of phentermine/extended release topiramate. Diabetes Metab Syndr Obes. 2014;7:35–44. doi: 10.2147/dmso.S38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Torgerson J.S., Hauptman J., Boldrin M.N., Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 206.Greenway F.L., Fujioka K., Plodkowski R.A., Mudaliar S., Guttadauria M., Erickson J., et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/s0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 207.Apovian C.M., Aronne L., Rubino D., Still C., Wyatt H., Burns C., et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Garvey W.T., Ryan D.H., Look M., Gadde K.M., Allison D.B., Peterson C.A., et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ahmad N.N., Robinson S., Kennedy-Martin T., Poon J.L., Kan H. Clinical outcomes associated with anti-obesity medications in real-world practice: a systematic literature review. Obes Rev. 2021;22(11):e13326. doi: 10.1111/obr.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rössner S., Sjöström L., Noack R., Meinders A.E., Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. european orlistat obesity study group. Obes Res. 2000;8(1):49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- 211.Rubino D.M., Greenway F.L., Khalid U., O'Neil P.M., Rosenstock J., Sørrig R., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 213.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 214.Lincoff A.M., Brown-Frandsen K., Colhoun H.M., Deanfield J., Emerson S.S., Esbjerg S., et al. Semaglutide and Cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023 doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 215.Lingvay I., Brown-Frandsen K., Colhoun H.M., Deanfield J., Emerson S.S., Esbjerg S., et al. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity (Silver Spring) 2023;31(1):111–122. doi: 10.1002/oby.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mann J.F.E., Ørsted D.D., Brown-Frandsen K., Marso S.P., Poulter N.R., Rasmussen S., et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 217.Shaman A.M., Bain S.C., Bakris G.L., Buse J.B., Idorn T., Mahaffey K.W., et al. Effect of the glucagon-like Peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145(8):575–585. doi: 10.1161/circulationaha.121.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Heerspink H.J.L., Sattar N., Pavo I., Haupt A., Duffin K.L., Yang Z., et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(11):774–785. doi: 10.1016/s2213-8587(22)00243-1. [DOI] [PubMed] [Google Scholar]
- 219.Wilding J.P.H., Batterham R.L., Davies M., Van Gaal L.F., Kandler K., Konakli K., et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553–1564. doi: 10.1111/dom.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.10.1016/j.jacc.2013.11.004. [DOI] [PubMed]
- 221.Palomar R., Fernández-Fresnedo G., Domínguez-Diez A., López-Deogracias M., Olmedo F., Martín de Francisco A.L., et al. Effects of weight loss after biliopancreatic diversion on metabolism and cardiovascular profile. Obes Surg. 2005;15(6):794–798. doi: 10.1381/0960892054222687. [DOI] [PubMed] [Google Scholar]
- 222.Nehus E.J., Khoury J.C., Inge T.H., Xiao N., Jenkins T.M., Moxey-Mims M.M., et al. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. 2017;91(2):451–1448. doi: 10.1016/j.kint.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Imam T.H., Fischer H., Jing B., Burchette R., Henry S., DeRose S.F., et al. Estimated GFR before and after Bariatric surgery in CKD. Am J Kidney Dis. 2017;69(3):380–1338. doi: 10.1053/j.ajkd.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li K., Zou J., Ye Z., Di J., Han X., Zhang H., et al. Effects of Bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One. 2016;11(10):e0163907. doi: 10.1371/journal.pone.0163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Carlsson L.M., Romeo S., Jacobson P., Burza M.A., Maglio C., Sjöholm K., et al. The incidence of albuminuria after bariatric surgery and usual care in swedish obese subjects (SOS): a prospective controlled intervention trial. Int J Obes (Lond) 2015;39(1):169–175. doi: 10.1038/ijo.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hallersund P., Sjöström L., Olbers T., Lönroth H., Jacobson P., Wallenius V., et al. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis - long term results from the swedish obese subjects (SOS) study. PLoS One. 2012;7(11):e49696. doi: 10.1371/journal.pone.0049696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Powell-Wiley T.M., Poirier P., Burke L.E., Després J.P., Gordon-Larsen P., Lavie C.J., et al. Obesity and Cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/cir.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Adams T.D., Gress R.E., Smith S.C., Halverson R.C., Simper S.C., Rosamond W.D., et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 229.Sjöström L., Peltonen M., Jacobson P., Sjöström C.D., Karason K., Wedel H., et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 230.Batsis J.A., Sarr M.G., Collazo-Clavell M.L., Thomas R.J., Romero-Corral A., Somers V.K., et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102(7):930–937. doi: 10.1016/j.amjcard.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fisher D.P., Johnson E., Haneuse S., Arterburn D., Coleman K.J., O'Connor P.J., et al. Association between Bariatric surgery and Macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570–1582. doi: 10.1001/jama.2018.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sjöström L. Review of the key results from the swedish obese subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 233.Biobaku F., Ghanim H., Monte S.V., Caruana J.A., Dandona P. Bariatric surgery: remission of inflammation. Cardiometabolic Benefits, and Common Adverse Effects J Endocr Soc. 2020;4(9):bvaa049.10.1210/jendso/bvaa049. doi: 10.1210/jendso/bvaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Holdstock C., Lind L., Engstrom B.E., Ohrvall M., Sundbom M., Larsson A., et al. CRP reduction following gastric bypass surgery is most pronounced in insulin-sensitive subjects. Int J Obes (Lond) 2005;29(10):1275–1280. doi: 10.1038/sj.ijo.0803000. [DOI] [PubMed] [Google Scholar]
- 235.Fenske W.K., Dubb S., Bueter M., Seyfried F., Patel K., Tam F.W., et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9(4):559–568. doi: 10.1016/j.soard.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 236.Jørgensen N.B., Jacobsen S.H., Dirksen C., Bojsen-Møller K.N., Naver L., Hvolris L., et al. Acute and long-term effects of roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 237.Shen N., Caixàs A., Ahlers M., Patel K., Gao Z., Dutia R., et al. Longitudinal changes of microbiome composition and microbial metabolomics after surgical weight loss in individuals with obesity. Surg Obes Relat Dis. 2019;15(8):1367–1373. doi: 10.1016/j.soard.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Merion R.M., Twork A.M., Rosenberg L., Ham J.M., Burtch G.D., Turcotte J.G., et al. Obesity and renal transplantation. Surg Gynecol Obstet. 1991;172(5):367–376. [PubMed] [Google Scholar]
- 239.Morales E., Valero M.A., Leon M., Hernandez E., Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41(2):319–327. doi: 10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 240.MacLaughlin H.L., Cook S.A., Kariyawasam D., Roseke M., van Niekerk M., Macdougall I.C. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55(1):69–76. doi: 10.1053/j.ajkd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 241.Harcourt B.E., Sourris K.C., Coughlan M.T., Walker K.Z., Dougherty S.L., Andrikopoulos S., et al. Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int. 2011;80(2):190–198. doi: 10.1038/ki.2011.57. [DOI] [PubMed] [Google Scholar]
- 242.Tirosh A., Golan R., Harman-Boehm I., Henkin Y., Schwarzfuchs D., Rudich A., et al. Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care. 2013;36(8):2225–2232. doi: 10.2337/dc12-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Saleh F., Kim S.J., Okrainec A., Jackson T.D. Bariatric surgery in patients with reduced kidney function: an analysis of short-term outcomes. Surg Obes Relat Dis. 2015;11(4):828–835. doi: 10.1016/j.soard.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 244.Choudhury D., Yalamanchili H.B., Hasan A. Dialysis of the obese patient: meeting needs for a growing epidemic. Semin Nephrol. 2021;41(4):371–1339. doi: 10.1016/j.semnephrol.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 245.Friedman A.N., Kaplan L.M., le Roux C.W., Schauer P.R. Management of Obesity in adults with CKD. J Am Soc Nephrol. 2021;32(4):777–790. doi: 10.1681/ASN.2020101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sandino J., Cordero Garcia-Galan L., Aubert Girbal L., Praga M., Pascual J., Morales E. Anything new in the treatment of obesity in obese patients with CKD? Nephron. 2022;146(6):616–623. doi: 10.1159/000524201. [DOI] [PubMed] [Google Scholar]
- 247.Ardiles L.G. Obesity and renal disease: benefits of bariatric surgery. Front Med (Lausanne) 2023;10:1134644. doi: 10.3389/fmed.2023.1134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bosch C., Carriazo S., Soler M.J., Ortiz A., Fernandez-Fernandez B. Tirzepatide and prevention of chronic kidney disease. Clin Kidney J. 2023;16(5):797–808. doi: 10.1093/ckj/sfac274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chang J.H., Mushailov V., Mohan S. Obesity and kidney transplantation. Curr Opin Organ Transplant. 2023;28(2):149–155. doi: 10.1097/MOT.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 250.Lee T.M., Chung T.H., Lin S.Z., Chang N.C. Endothelin receptor blockade ameliorates renal injury by inhibition of RhoA/Rho-kinase signalling in deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2014;32(4):795–805. doi: 10.1097/HJH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 251.Papageorgiou PC, Chan CT, Yeo EL, Backx PH, Floras JS. Coagulation factor XIIa-kinin-mediated contribution to hypertension of chronic kidney disease. J Hypertens. 2014;32(7):1523-33; discussion 33.10.1097/HJH.0000000000000192. [DOI] [PubMed]
- 252.Haerteis S., Schork A., Dorffel T., Bohnert B.N., Nacken R., Worn M., et al. Plasma kallikrein activates the epithelial sodium channel in vitro but is not essential for volume retention in nephrotic mice. Acta Physiol (Oxf) 2018;224(1):e13060. doi: 10.1111/apha.13060. [DOI] [PubMed] [Google Scholar]
- 253.Bello A.K., Peters J., Wight J., El Nahas M. The kidney evaluation and Awareness program in Sheffield (KEAPS): a community-based screening for microalbuminuria in a british population. Nephron Clin Pract. 2010;116(2):c95–c. doi: 10.1159/000314658. [DOI] [PubMed] [Google Scholar]
- 254.Kassam A.F., Mirza A., Kim Y., Hanseman D., Woodle E.S., Quillin R.C., 3rd, et al. Long-term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am J Transplant. 2020;20(2):422–429. doi: 10.1111/ajt.15650. [DOI] [PubMed] [Google Scholar]
- 255.Potrykus M., Czaja-Stolc S., Malgorzewicz S., Proczko-Stepaniak M., Debska-Slizien A. Diet Management of Patients with chronic kidney disease in Bariatric surgery. Nutrients. 2022;15(1).10.3390/nu15010165 doi: 10.3390/nu15010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang M., Wang Z., Chen Y., Dong Y. Kidney damage caused by obesity and its feasible treatment drugs. Int J Mol Sci. 2022;23(2).10.3390/ijms23020747 doi: 10.3390/ijms23020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Verma N., Despa F. The association between renal accumulation of pancreatic amyloid-forming amylin and renal hypoxia. Front Endocrinol (Lausanne) 2023;14:1104662. doi: 10.3389/fendo.2023.1104662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tanaka Y., Kume S., Araki S., Isshiki K., Chin-Kanasaki M., Sakaguchi M., et al. Fenofibrate, a PPARalpha agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 2011;79(8):871–882. doi: 10.1038/ki.2010.530. [DOI] [PubMed] [Google Scholar]
- 259.Mychaleckyj J.C., Craven T., Nayak U., Buse J., Crouse J.R., Elam M., et al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care. 2012;35(5):1008–1014. doi: 10.2337/dc11-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Frazier R., Mehta R., Cai X., Lee J., Napoli S., Craven T., et al. Associations of fenofibrate therapy with incidence and progression of CKD in patients with type 2 diabetes. Kidney Int Rep. 2019;4(1):94–102. doi: 10.1016/j.ekir.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yen C.L., Fan P.C., Lin M.S., Lee C.C., Tu K.H., Chen C.Y., et al. Fenofibrate delays the need for dialysis and reduces Cardiovascular risk among patients with advanced CKD. J Clin Endocrinol Metab. 2021;106(6):1594–1605. doi: 10.1210/clinem/dgab137. [DOI] [PubMed] [Google Scholar]
- 262.Ortiz A., Ferro C.J., Balafa O., Burnier M., Ekart R., Halimi J.M., et al. Mineralocorticoid receptor antagonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. Nephrol Dial Transplant. 2023;38(1):10–25. doi: 10.1093/ndt/gfab167. [DOI] [PubMed] [Google Scholar]
- 263.Faulkner J.L., Bruder-Nascimento T., Belin de Chantemele E.J. The regulation of aldosterone secretion by leptin: implications in obesity-related cardiovascular disease. Curr Opin Nephrol Hypertens. 2018;27(2):63–69. doi: 10.1097/MNH.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.(NIH) NIoH. A trial to learn how well finerenoneworks and how safe it is in adult participants with non diabetic chronic kidney disease. https://clinicaltrials.gov/study/NCT05047263?intr=Finerenone&page=2&rank=15.2024 cited 2024 01-21-2024].
- 265.Oh Y.K. Vasopressin and vasopressin receptor antagonists. Electrolyte Blood Press. 2008;6(1):51–55. doi: 10.5049/EBP.2008.6.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Torres V.E., Chapman A.B., Devuyst O., Gansevoort R.T., Perrone R.D., Koch G., et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 267.Raina R., Houry A., Rath P., Mangat G., Pandher D., Islam M., et al. Clinical utility and tolerability of tolvaptan in the treatment of autosomal dominant polycystic kidney disease (ADPKD) Drug Healthc Patient Saf. 2022;14:147–159. doi: 10.2147/DHPS.S338050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mekahli D., Guay-Woodford L.M., Cadnapaphornchai M.A., Greenbaum L.A., Litwin M., Seeman T., et al. Tolvaptan for children and adolescents with autosomal dominant polycystic kidney disease: randomized controlled trial. Clin J Am Soc Nephrol. 2023;18(1):36–46. doi: 10.2215/CJN.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jynarque (tolvaptan) for Autosomal Dominant Polycystic Kidney Disease (ADPKD) www.jynarquehcp.com.2024.
- 270.Garcia-Carro C., Vergara A., Bermejo S., Azancot M.A., Sellares J., Soler M.J. A nephrologist perspective on obesity: from kidney injury to clinical Management. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.655871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lim L.L., Chow E., Chan J.C.N. Cardiorenal diseases in type 2 diabetes mellitus: clinical trials and real-world practice. Nat Rev Endocrinol. 2023;19(3):151–163. doi: 10.1038/s41574-022-00776-2. [DOI] [PubMed] [Google Scholar]
- 272.Mark P.B., Sarafidis P., Ekart R., Ferro C.J., Balafa O., Fernandez-Fernandez B., et al. SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: a comprehensive review by EURECA-m and ERBP working groups of ERA. Nephrol Dial Transplant. 2023;38(11):2444–2455. doi: 10.1093/ndt/gfad112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chang Y.K., Choi H., Jeong J.Y., Na K.R., Lee K.W., Lim B.J., et al. Correction: dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016;11(7):e0160478. doi: 10.1371/journal.pone.0160478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chang Y.K., Choi H., Jeong J.Y., Na K.R., Lee K.W., Lim B.J., et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016;11(7):e0158810. doi: 10.1371/journal.pone.0158810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cai T., Ke Q., Fang Y., Wen P., Chen H., Yuan Q., et al. Sodium-glucose cotransporter 2 inhibition suppresses HIF-1alpha-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis. 2020;11(5):390. doi: 10.1038/s41419-020-2544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kanbay M., Demiray A., Afsar B., Karakus K.E., Ortiz A., Hornum M., et al. Sodium-glucose cotransporter 2 inhibitors for diabetes mellitus control after kidney transplantation: review of the current evidence. Nephrology (Carlton) 2021;26(12):1007–1017. doi: 10.1111/nep.13941. [DOI] [PubMed] [Google Scholar]
- 277.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 278.Cherney D.Z., Kanbay M., Lovshin J.A. Renal physiology of glucose handling and therapeutic implications. Nephrol Dial Transplant. 2020;35(Suppl 1):i3–i12. doi: 10.1093/ndt/gfz230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Opingari E., Verma S., Connelly K.A., Mazer C.D., Teoh H., Quan A., et al. The impact of empagliflozin on kidney injury molecule-1: a subanalysis of the effects of empagliflozin on Cardiac structure, function, and circulating Biomarkers in patients with type 2 diabetes CardioLink-6 trial. Nephrol Dial Transplant. 2020;35(5):895–997. doi: 10.1093/ndt/gfz294. [DOI] [PubMed] [Google Scholar]
- 280.Szekeres Z., Toth K., Szabados E. The effects of SGLT2 inhibitors on lipid metabolism. Metabolites. 2021;11(2).10.3390/metabo11020087 doi: 10.3390/metabo11020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bessi V.L., Labbe S.M., Huynh D.N., Menard L., Jossart C., Febbraio M., et al. EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc Res. 2012;96(1):99–108. doi: 10.1093/cvr/cvs225. [DOI] [PubMed] [Google Scholar]
- 282.Souza A.C., Bocharov A.V., Baranova I.N., Vishnyakova T.G., Huang Y.G., Wilkins K.J., et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int. 2016;89(4):809–822. doi: 10.1016/j.kint.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Baigent C., Landray M.J., Reith C., Emberson J., Wheeler D.C., Tomson C., et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of Heart and renal protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Koch C.G. Statin therapy. Curr Pharm Des. 2012;18(38):6284–6290. doi: 10.2174/138161212803832335. [DOI] [PubMed] [Google Scholar]
- 285.Hu P.J., Wu M.Y., Lin T.C., Chen T.T., Wu Y.C., Su S.L., et al. Effect of statins on renal function in chronic kidney disease patients. Sci Rep. 2018;8(1):16276. doi: 10.1038/s41598-018-34632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Morano S., Romagnoli E., Filardi T., Nieddu L., Mandosi E., Fallarino M., et al. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: an ultrasonography study. Acta Diabetol. 2015;52(4):727–732. doi: 10.1007/s00592-014-0710-z. [DOI] [PubMed] [Google Scholar]
- 287.Seghieri M., Christensen A.S., Andersen A., Solini A., Knop F.K., Vilsboll T. Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor co-agonists in the treatment of NAFLD. Front Endocrinol (Lausanne) 2018;9:649. doi: 10.3389/fendo.2018.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang C., Li L., Liu S., Liao G., Li L., Chen Y., et al. GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS One. 2018;13(3):e0193473. doi: 10.1371/journal.pone.0193473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang Q., Liu L., Gao L., Li Q. Cardiovascular safety of GLP-1 receptor agonists for diabetes patients with high cardiovascular risk: a meta-analysis of cardiovascular outcomes trials. Diabetes Res Clin Pract. 2018;143:34–42. doi: 10.1016/j.diabres.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 290.Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP-1 Receptor Agonists and Kidney Protection. Medicina (Kaunas). 2019;55(6).10.3390/medicina55060233. [DOI] [PMC free article] [PubMed]
- 291.Zhang Y., Liu S., Fan C., Zeng Y., Li J., Xie C., et al. Effect of glucagon-like peptide 1 receptor agonists on body fat redistribution and muscle mass in overweight and obese type 2 diabetic patients. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39(4):450–505. doi: 10.12122/j.issn.1673-4254.2019.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lv X., Dong Y., Hu L., Lu F., Zhou C., Qin S. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for the management of nonalcoholic fatty liver disease (NAFLD): a systematic review. Endocrinol Diabetes Metab. 2020;3(3):e00163. doi: 10.1002/edm2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mosterd C.M., Bjornstad P., van Raalte D.H. Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol. 2020;33(5):965–975. doi: 10.1007/s40620-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ertuglu L.A., Porrini E., Hornum M., Demiray A., Afsar B., Ortiz A., et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors for diabetes after solid organ transplantation. Transpl Int. 2021;34(8):1341–1359. doi: 10.1111/tri.13883. [DOI] [PubMed] [Google Scholar]
- 295.Yang H.L., Feng P., Xu Y., Hou Y.Y., Ojo O., Wang X.H. The role of Dietary fiber supplementation in regulating uremic toxins in patients with chronic kidney disease: a meta-analysis of randomized controlled trials. J Ren Nutr. 2021;31(5):438–447. doi: 10.1053/j.jrn.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 296.Liu J., Zhong J., Yang H., Wang D., Zhang Y., Yang Y., et al. Biotic supplements in patients with chronic kidney disease: meta-analysis of randomized controlled trials. J Ren Nutr. 2022;32(1):10–21. doi: 10.1053/j.jrn.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Winiarska K., Focht D., Sierakowski B., Lewandowski K., Orlowska M., Usarek M. NADPH oxidase inhibitor, apocynin, improves renal glutathione status in zucker diabetic fatty rats: a comparison with melatonin. Chem Biol Interact. 2014;218:12–19. doi: 10.1016/j.cbi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 298.Liu Z., Gan L., Xu Y., Luo D., Ren Q., Wu S., et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J Pineal Res. 2017;63(1).10.1111/jpi.12414 doi: 10.1111/jpi.12414. [DOI] [PubMed] [Google Scholar]
- 299.Xu P., Wang J., Hong F., Wang S., Jin X., Xue T., et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62(4).10.1111/jpi.12399 doi: 10.1111/jpi.12399. [DOI] [PubMed] [Google Scholar]
- 300.Liu Z., Gan L., Zhang T., Ren Q., Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2018;64(1).10.1111/jpi.12455 doi: 10.1111/jpi.12455. [DOI] [PubMed] [Google Scholar]
- 301.Yin J., Li Y., Han H., Chen S., Gao J., Liu G., et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65(4):e12524. doi: 10.1111/jpi.12524. [DOI] [PubMed] [Google Scholar]
- 302.Tofte N., Suvitaival T., Trost K., Mattila I.M., Theilade S., Winther S.A., et al. Metabolomic assessment reveals alteration in polyols and branched chain amino acids associated with present and future renal impairment in a discovery cohort of 637 persons with type 1 diabetes. Front Endocrinol (Lausanne) 2019;10:818. doi: 10.3389/fendo.2019.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Liu D.J.X., Stock E., Broeckx B.J.G., Daminet S., Meyer E., Delanghe J.R., et al. Weight-gain induced changes in renal perfusion assessed by contrast-enhanced ultrasound precede increases in urinary protein excretion suggestive of glomerular and tubular injury and normalize after weight-loss in dogs. PLoS One. 2020;15(4):e0231662. doi: 10.1371/journal.pone.0231662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bhattacharya S., Patel K.K., Dehari D., Agrawal A.K., Singh S. Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem. 2019;462(1–2):133–155. doi: 10.1007/s11010-019-03617-5. [DOI] [PubMed] [Google Scholar]
- 305.de Souza C.A.P., Gallo C.C., de Camargo L.S., de Carvalho P.V.V., Olescuck I.F., Macedo F., et al. Melatonin multiple effects on brown adipose tissue molecular machinery. J Pineal Res. 2019;66(2):e12549. doi: 10.1111/jpi.12549. [DOI] [PubMed] [Google Scholar]
- 306.Karamitri A., Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15(2):105–125. doi: 10.1038/s41574-018-0130-1. [DOI] [PubMed] [Google Scholar]
- 307.Yawoot N., Govitrapong P., Tocharus C., Tocharus J. Ischemic stroke, obesity, and the anti-inflammatory role of melatonin. Biofactors. 2021;47(1):41–58. doi: 10.1002/biof.1690. [DOI] [PubMed] [Google Scholar]
- 308.Jiang T., Wang Z., Proctor G., Moskowitz S., Liebman S.E., Rogers T., et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280(37):32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 309.Wang X.X., Jiang T., Shen Y., Caldas Y., Miyazaki-Anzai S., Santamaria H., et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59(11):2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gai Z., Gui T., Hiller C., Kullak-Ublick G.A. Farnesoid X receptor protects against kidney injury in uninephrectomized obese mice. J Biol Chem. 2016;291(5):2397–2411. doi: 10.1074/jbc.M115.694323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cho H., Um J., Lee J.H., Kim W.H., Kang W.S., Kim S.H., et al. ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, alleviates the symptoms of type 2 diabetes. Sci Rep. 2017;7:44186. doi: 10.1038/srep44186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Jang H.S., Noh M.R., Kim J., Padanilam B.J. Defective mitochondrial fatty acid oxidation and lipotoxicity in kidney diseases. Front Med (Lausanne) 2020;7:65. doi: 10.3389/fmed.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Szeto C.C., Li P.K. The use of vitamin D analogues in chronic kidney diseases: possible mechanisms beyond bone and mineral metabolism. NDT Plus. 2009;2(3):205–212. doi: 10.1093/ndtplus/sfp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jean G., Souberbielle J.C., Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients. 2017;9(4).10.3390/nu9040328 doi: 10.3390/nu9040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zand L., Kumar R. The use of vitamin D metabolites and analogues in the treatment of chronic kidney disease. Endocrinol Metab Clin North Am. 2017;46(4):983–1007. doi: 10.1016/j.ecl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]