Abstract
Evaluating persistent trace organic chemicals (TOrCs) and transformation products (TPs) in membrane bioreactors (MBRs) is essential, given that MBRs are now widely implemented for wastewater treatment and water reuse. This research applied comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS)-based nontargeted analysis to compare the effectiveness of parallel aerobic and anaerobic MBRs (AeMBRs and AnMBRs, respectively), treating the same municipal wastewater. The average total chromatographic feature peak area abundances were significantly reduced by 84% and 72% from influent to membrane permeate in both the AeMBR and AnMBR (p < 0.05), respectively. However, the reduction of the average number of chromatographic features was significant for only AeMBR treatment (p = 0.006). A similar number of TPs were generated during both AeMBR and AnMBR treatments (165 vs 171 compounds, respectively). The overall results suggest that the AeMBR was more effective for reducing the diversity of TOrCs than the AnMBR, but both aerobic and anaerobic processes had a similar reduction of TOrC abundance. Suspect screening analysis using GC×GC/TOF-MS, which resulted in the tentative identification of 351 TOrCs, proved to be a powerful approach for uncovering compounds previously unreported in wastewater, including many fragrances and personal care products.
Keywords: wastewater treatment, membrane bioreactors, suspect screening, chemicals of emerging concern, transformation products
Short abstract
This study compares the persistence, removal, and transformation of organic chemicals in parallel aerobic and anaerobic membrane bioreactor systems treating municipal wastewater using a nontargeted analysis based on GC×GC/TOF-MS.
Introduction
Trace organic chemicals (TOrCs) refer to a wide range of compounds, including pharmaceuticals, personal care products, agrochemicals, cosmetics, food additives, and flame retardants found at low concentrations in environmental systems.1 A major source of TOrC input to aquatic environments is wastewater treatment facilities (WWTFs), which were not traditionally designed to remove TOrCs.2 Some TOrCs can be highly persistent,3 appearing in different water matrices, including treated wastewater,2 surface water,2 groundwater,4 seawater,2 and drinking water.5,6 Persistent TOrCs can bioaccumulate and biomagnify, having sublethal toxic effects on aquatic food chains.7−9
Nevertheless, TOrC removal from the aqueous phase has been reported in activated sludge treatment processes due primarily to biodegradation or sorption to biosolids.10−13 Membrane bioreactors (MBRs) combine an activated sludge biological treatment with a membrane filtration component and have been applied for both potable and nonpotable water reuses with more frequent applications in decentralized systems.14,15 While many studies have demonstrated instances where aerobic MBR (AeMBR) and anaerobic MBR (AnMBR) treatments achieve greater than 90% removal of some TOrCs, including pharmaceuticals, plasticizers, and industrial chemicals,10−13,16 few compare the two treatment trains side by side to differentiate aerobic and anaerobic processes. To the best of our knowledge, only one laboratory-scale study compared TOrC removal in an AeMBR and an AnMBR operating in parallel, but this study utilized synthetic wastewater spiked with 15 commonly detected wastewater TOrCs.13
Additionally, there are over 350,000 chemicals registered for commercial use in the global market, many of which are unidentified, making them difficult to detect using conventional targeted analytical (TA) methods.1 Most studies assessing TOrCs in real wastewater treatment systems, including MBR systems and sludge digestion, have employed TA methods based on gas chromatography (GC) or liquid chromatography (LC), mostly coupled to mass spectrometry (MS).17 However, TA approaches miss chemicals that were untargeted and unhypothesized in advance, such as transformation products during wastewater treatment. Nontargeted analysis (NTA) has been successfully applied to detect and identify a large number of compounds in wastewater samples using either a comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS)-based approach18,19 or a liquid chromatography (LC)-based mass spectrometry (MS) approach.2,20−23 GC×GC/TOF-MS provides high resolution and sensitivity compared to GC-MS24 and has been identified as highly complementary to LC-based NTA approaches.19 LC-based approaches, compared to GC-based approaches, are more frequently used for detecting more polar TOrCs in water and wastewater.17
For this reason, in a review by Meyers et al.25 where authors conducted a meta-analysis of 6157 abstracts to review trends in the diversity of compounds reported, the authors found that GC-amenable compounds such as fragrances were under-represented in the environmental pharmaceuticals and personal care product (PPCP) literature compared to pharmaceuticals. The GC×GC/TOF-MS approach paired with suspect screening allows for tentative identification of both expected and unexpected contaminants, including many under-represented compounds, thereby elucidating the complex mixtures present in wastewater and aerobic and anaerobic MBR systems.
Another advantage of NTA is that the increased number of chemicals identified can be utilized to inform models predicting TOrC removal and transformation in MBRs. Previous studies, using either ultrapure water or synthetic wastewater, spiked with TOrCs at concentrations higher than those naturally found in wastewater, have suggested that the log of the octanol–water partition coefficient (log P), functional groups, and the number of nitrogen or sulfur atoms may influence a compound’s biodegradation in real wastewater.10−13 NTA makes it possible to test the hypotheses put forth in previous studies by accessing a larger set of tentatively identified TOrCs at their innate concentrations and to evaluate relationships between compound structure and removal by wastewater treatment processes.19 The combination of NTA and QSPR modeling has not (to the best of our knowledge) been applied for evaluating the basis for compound removal in AeMBR and AnMBR systems.
Therefore, this study seeks to apply a GC-based approach with NTA to uncover and interrogate a wider breadth of persistent and removed TOrCs and transformation products at their innate concentrations in AeMBR and AnMBR systems. Our objectives were to (1) conduct AeMBR and AnMBR systems in parallel, treating the same real wastewater, (2) evaluate and compare TOrC removal and transformation between the systems using NTA, (3) identify removed and persistent TOrCs and transformation products, and (4) assess relationships between the tentatively identified chemicals’ physicochemical properties and removal by the AeMBR and AnMBR systems.
Methods
Description of MBR Systems
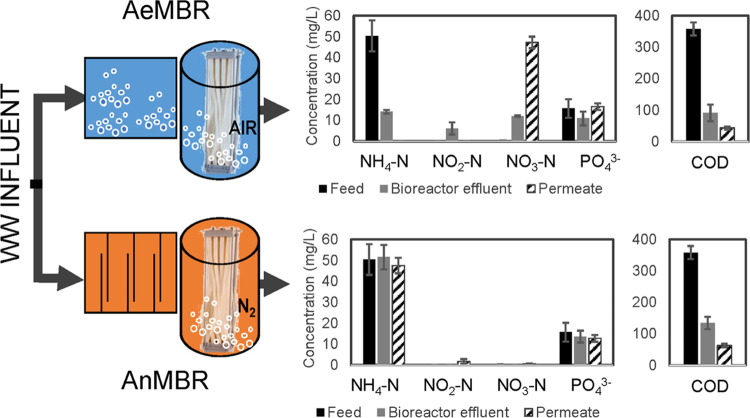
Two laboratory-scale AeMBR and AnMBR systems, sharing one feed tank (Figure 1), were operated in parallel. For biological treatment, the AnMBR utilized an anaerobic baffled reactor (ABR) seeded with waste-activated sludge obtained from the San Elijo Water Reclamation Facility (WRF), and the AeMBR utilized an aeration tank, seeded with aerobic biosolids from a local brewery. The aerobic bioreactor operated for 8 months, and the anaerobic bioreactor operated >2 years, prior to being connected to the membrane tanks. Over a 6 month period, both reactors were transitioned from treating full synthetic wastewater to eventually 100% domestic wastewater. Prior to sampling, the AeMBR and AnMBR systems operated for 6 weeks using 100% domestic wastewater until steady-state conditions were achieved (consistent chemical oxygen demand (COD) removal in both systems).
Figure 1.
Schematic of the laboratory-scale AeMBR and AnMBR treatment systems and their average nutrient and COD concentrations for N = 3 treatment cycles. For AeMBR, an aeration tank preceded the submerged membrane bioreactor (MBR) tank, while for AnMBR, an anaerobic baffled reactor preceded the MBR tank. For each experiment, a 48 h composite sample of wastewater influent was collected. The duration of the composite sampling of the aerobic and anaerobic membrane permeates corresponded to their hydraulic retention times (24 h for the AeMBR and 48 h for the AnMBR).
The bioreactors were connected to ultrafiltration membranes (0.04 μm nominal pore size, poly(vinylidene fluoride) membrane, Zeeblok-2.5 modules, Suez Water Technologies) submerged inside a 1.2 m tall fabricated cylindrical tank (Figure 1). The aerobic and anaerobic membrane tanks used air and nitrogen gas, respectively, for membrane scouring, both supplied at a rate of 0.01 m3/min/flow rate (as per the manufacturer’s recommendations). The systems operated with permeation–relaxation cycles of 9:1 and 8:2 min for the aerobic and anaerobic membrane tanks, respectively. The membrane permeate flow rate was 30 mL/min for the AeMBR and 13.9 mL/min for the AnMBR, which equates to an observed flux of 9.80 and 4.9 L/m2h, respectively, well below the maximum flux requirements set by the manufacturer. The hydraulic residence time (HRT) and solid retention time (SRT) for the AeMBR were approximately 24 h and 3.97 days, respectively. For the AnMBR, the HRT was 48 h, and no wasting was needed due to low sludge production; therefore, the SRT is estimated based on a conservative value of 1 L wasted per 30 days and is approximately 960 days. Aerobic transmembrane pressure operated at 2.07 kPa, and anaerobic membrane pressure operated around 0.69 kPa within the recommended operating range; this could be due to a thicker cake layer formation on the aerobic membrane compared to that of the anaerobic membrane (Figure S1). Membrane cleanings were performed weekly to reduce their susceptibility to clogging following standard maintenance cleaning guidelines described in the ZeeBlok Installation and Operating Manual (2017).
Operation and Sample Collection of MBR Systems
The parallel treatment systems were tested for 3 treatment cycles (referred to as treatment cycle “I”, “II”, or “III”), which achieved average total suspended solid (TSS) removals of 97 ± 2.7 and 89 ± 0.7% and average COD reductions of 88 ± 0.0 and 83 ± 0.02% for the AeMBR and AnMBR, respectively (Table S1). The following protocol was followed for each treatment cycle. The MBR systems were run continuously from Monday to Friday (approximately 100 h), with large volume batches of wastewater fed to the system on Monday morning and Tuesday afternoon. Operation of the parallel MBR systems was paused on Saturdays and Sundays and restarted on Mondays with the new wastewater batch. The feed consisted of untreated, screened influent (120 L per batch). The first batch was collected from San Elijo WRF on Mondays between 6:00 and 8:00 in the morning, and the second batch was collected on Tuesdays between 11:00 and 13:00 in the afternoon to capture diurnal variability in wastewater flows. Wastewater from this facility corresponds to a small sewershed (serves a population of ∼40,000 with a 19,900 m3/d average treatment capacity), containing mostly domestic and institutional (hospital, schools) wastewater sources, and excludes major industrial inputs.
Sampling of the parallel MBR treatment train was conducted as follows: grab samples of the influent and MBR permeates were collected hourly using an autosampler; the influent was collected for 48 h, while the MBR permeates were sampled for a duration corresponding to their hydraulic retention times (24 h for AeMBR and 48 h for AnMBR). The grab samples were combined to create composite samples for each of the influents, aerobic membrane permeate (AeMP), and anaerobic membrane permeate (AnMP) for NTA analyses. Throughout the sampling period, composite samples were kept in an ice bath (8.5 ± 2.5 °C). The composite sample for AnMP, during treatment cycle I, was composited for only 24 h, rather than the 48 h duration used in the other two treatment cycles, treatment cycles II and III. The first composite sample was included for selecting the 1,094 chromatographic features according to the criteria described below. After the experimental work concluded, it was determined that the 24 h composite AnMP sample was substantially lower in the number of chromatographic features and total peak areas compared to the 48 h composite AnMP samples, for which sample averages and relative standard deviations are reported in Table S2. Therefore, the first AnMP sample was excluded from comparisons of MBR treatment efficiencies and from evaluations of relationships between compound removal and physicochemical properties of TOrCs.
Bulk Water Quality
Conductivity, total dissolved solids, and pH were recorded (Fisherbrand Accumet AP85 portable pH and conductivity meter) routinely on grab samples to monitor the performance. Dissolved oxygen was measured using a ProODO YSI meter. COD, ammonium (NH4-N), nitrite (NO2-N), nitrate (NO3-N), and total phosphorus (PO4) were analyzed using HACH TNTplus Vial Test kits and a HACH DR 3900 spectrophotometer. Samples collected for TSS analysis were either analyzed right away or stored refrigerated at 6 °C and then analyzed later following Method 2540.26 COD and nutrient removal efficiencies for each MBR system are illustrated in Figure 1. Detailed information on the performance of the MBR systems (in terms of COD and TSS removal), mixed liquor suspended solids concentrations, sludge blanket TSS concentrations (Table S1), and the other water quality parameters (Table S3) are presented in Supporting Information I.
Sample Preparation
A solid-phase extraction (SPE) method was used to prepare samples, including three laboratory blanks composed of liquid chromatography mass spectrometry (LC-MS) grade water for GC×GC/TOF-MS analyses. Prior to extraction, samples were filtered through precombusted 1.6 μm Whatman GF-A filters. The filtrate was preserved with ascorbic acid (0.05 g/L) and sodium azide (1 g/L) and frozen in amber bottles until it was ready for analysis. The frozen samples were defrosted prior to SPE extraction and extracted following the procedures described in Chang et al.27 Briefly, the filtrate was run through an Oasis hydrophilic–lipophilic balance (200 mg, HLB) glass cartridge (5 mL, Waters Corporation, Milford, MA). In this study, recovery efficiencies were not evaluated; however, many studies report that OASIS HLB cartridges are generally robust, achieve high recovery, and perform consistently across different water matrices including wastewater influent and effluent.28−31 The extract was dried with sodium sulfate, and then additional drying was conducted with an SPE of a 6 mL UCT EnviroClean muffled sodium sulfate glass cartridge (United Chemical Technologies, Inc., Bristol, PA). The final extracts were subsequently concentrated and evaporated to 400 μL with a Zymark TurboVap LV Evaporator (Gen Scientific, Hopkinton, MA) with nitrogen gas in a 40 °C water bath. The final extracts were spiked with the internal standard mixture of acenaphthene-d10, chrysene-d12, 1,4-dichlorobenzene-d4, naphthalene-d8, perylene-d12, and phenanthrene-d10 and stored at −20 °C until they were ready for GC×GC/TOF-MS analysis.
Nontargeted Chemical Analysis Using GC×GC/TOF-MS
Samples were analyzed using Pegasus 4D GC×GC/TOF-MS (LECO, St. Joesph, MI); detailed instrumental conditions are listed in Table S4. Data processing was conducted using the ChromaTOF software (version 4.72.0), yielding a list of features with distinct chromatographic peaks and associated fragmentation mass spectra. The total number of chromatographic features detected per treatment cycle is listed in Table S5. Peak area abundances for all chromatographic features were normalized by dividing each of the chromatographic feature’s peak area by the volume of sample extracted (influent, 500 mL; AeMP and AnMP, 1000 mL each). Then, the average total number of chromatographic features and the average total peak area abundances per sample across the experiments (influent, n = 3; AeMP, n = 3; AnMP, n = 2) were each evaluated using an analysis of variance (ANOVA) followed by a Bonferroni adjusted post hoc test (Tables S6–S9). The ANOVA and post hoc tests were conducted by using IBM SPSS software (version 27).
Selection of Chromatographic Features
To compare the chemical constituents across subsets of the samples, LECO’s software add-in “Statistical Compare” was used to align the features across samples in a peak table based on mass spectral and retention time similarity. Specific conditions used for Statistical Compare are listed in Table S10. Analytes that met the following conditions were considered in the following analyses in order to focus on the compounds that behaved in a reproducible manner: (1) the analyte was detected in at least one of the following sample types: influent, AeMP, or AnMP, (2) the analyte was detected in the sample type for at least two out of three treatment cycles, and (3) the analyte was not detected in any of the laboratory blanks. As a quality assurance measure, compounds meeting the inclusion criteria were manually reviewed to rule out false positives. The peak abundances of the resulting 1094 selected chromatographic features were normalized using a method similar to that described in Shaul et al.,32 by dividing the peak area of each analyte by the peak area of the internal standard (phenanthrene-d10) in that sample and then dividing by the sample volume (mL) extracted. The normalized peak areas were used to calculate the percent change of compounds. The hierarchical clustering heatmap of those 1094 compounds was generated from R (version 4.1.1), with the R package latticeExtra (version 0.6–30), for which instances of nondetection were assigned as zero, and normalized peak areas were transformed using eq 1. These transformed normalized peak areas were only used for visualizing data via the heatmap.
| 1 |
Comparing MBR Treatment of Individual TOrCs
The percent change was calculated from the untransformed normalized peak areas using eq 2
| 2 |
where Ainf is the normalized peak area in an influent sample and Aeff is the normalized peak area in a membrane permeate sample.
Compounds were classified as removed for the following conditions: (1) if it was detected in the influent and not detected in the effluent (−100% change) and (2) if it had a percent change between −99.9 and −90% from influent to membrane permeate across all treatment cycles. Persistent compounds refer to the compounds that had any percent change greater than −90% from influent to membrane permeates across all treatment cycles. Therefore, persistent compounds include compounds that were partially removed (>−90 to 0% change) as well as compounds that increased in the effluents (>0% change), and it excludes any compounds that were not originally detected in the influents (newly formed transformation products).
Tentative Identification of Compounds
A suspect screening approach, defined in Newton et al.,33 was used to manually review the 1094 chromatographic features for tentative identification. The National Institute of Standards and Technology (version 2017) Electron Impact Mass Spectral Library was assessed for tentative compound identification. A compound to be considered tentatively identifiable must meet the following criteria, also used in Mladenov et al.18 (1) the similarity score >600, (2) the molecular ion (M+) is present, and (3) the top three most prominent ions were present in the mass spectrum. This process resulted in the tentative identification of 351 compounds. These compounds and their normalized peak areas are listed in Table S11, and their mass spectra are presented in Supporting Information II. The overall data processing steps for selecting chromatographic features and tentatively identifiable compounds are pictured in Figure S2.
The rate of success for verifying tentatively identified compounds against synthetic standards ranged from 88 to 100% in our previous studies, using a similar GC×GC/TOF-MS method and search against the NIST mass spectral library.18,34,35 In this study, 15 chemicals were selected from the set of compounds commonly identified across three different WWTF influents in Mladenov et al.,18 one of which provided the influent for the current study. One hundred percent of the compounds were verified with their authentic standard, and their treatment by the MBR systems and the full-scale WWTFs from Mladenov et al.18 were compared. A list of the 15 compounds with their vendor information is listed in Table S12.
Physicochemical Analysis
Physicochemical data were retrieved from the Environmental Protection Agency (EPA) Comptox Chemicals Dashboard36 (access date: 09/20/2020) and the online service ChemMine Tools.37 These tools and descriptors are described in our previous work.18 For the 50 compounds not found in the EPA Comptox Dashboard, their canonical SMILES strings were retrieved from PubChem. Two compounds were not found in PubChem database (benzamide, 3-methyl-N-methyl-N-propyl- and carbonic acid, monoamide, N-butyl-, allyl ester), and these compounds were manually drawn using PubChem Sketcher V2.438 to generate their SMILES strings (C1=CC(=CC(=C1)C(=O)[N](CCC)C)C and C=CCOC(=O)N(CCCC)[H], respectively). The SMILES strings were used for input into the online service ChemMine Tools,37 where molecular descriptors were retrieved from the cheminformatics libraries JOELib39 and ChemmineR40 for all 351 tentatively identified compounds. The relationship between the tentatively identified compounds’ molecular descriptors and their removal was investigated using principal component analysis (PCA). PCA has been used to reduce the dimensionality of data sets and generate mathematically independent variables as a precursor to statistical experimental designs that investigate if a molecule’s behavior in a water treatment system is predictable based on its structural properties.41
Results and Discussion
Chromatographic Features Detected in MBR Systems
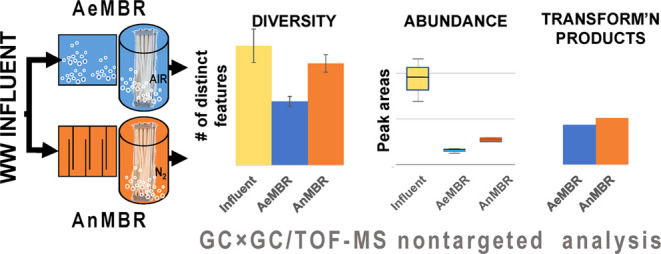
An average number of chromatographic features was calculated in the wastewater influent (10,009 ± 1393, relative standard deviation (RSD) = 13.9%, n = 3 composite samples), AeMP (5357 ± 421, RSD = 7.9%, n = 3 composite samples), and AnMP (8533 ± 730, RSD = 8.6%, n = 2 composite samples). A one-way ANOVA and Bonferroni post hoc test were used to compare the mean number of chromatographic features between the three sample types (Tables S6 and S7). The number of chromatographic features detected in the AeMP was significantly lower (p = 0.006) compared to the wastewater influent, and the difference was not significant between the wastewater influent and the AnMP (p = 0.475). Between the AeMP and AnMP, a significantly lower number of chromatographic features were detected in the AeMP than in the AnMP (p = 0.049). Therefore, AeMBR treatment was observed to be more effective at removing a greater number of different TOrCs compared to AnMBR. One limitation to consider here is that the small sample size lowers the statistical power.
In addition, we compared the mean total peak area abundance of the chromatographic features among the three sample types using a one-way ANOVA with a Bonferroni post hoc test (Tables S8 and S9). The average total peak abundance per sample for each membrane permeate was significantly lower compared with that of the influent. The average total peak abundances decreased by 84% from the influent to the AeMP (p-value = 0.004) and 72% from the influent to the AnMP (p-value = 0.014). The average total peak abundances of the AeMP and AnMP, which had RSDs of 13.8% and 7.9%, respectively, were not significantly different (p = 1.0). This suggests that both MBR treatments similarly reduced the total amount of chemicals detected from the influent to membrane permeates.
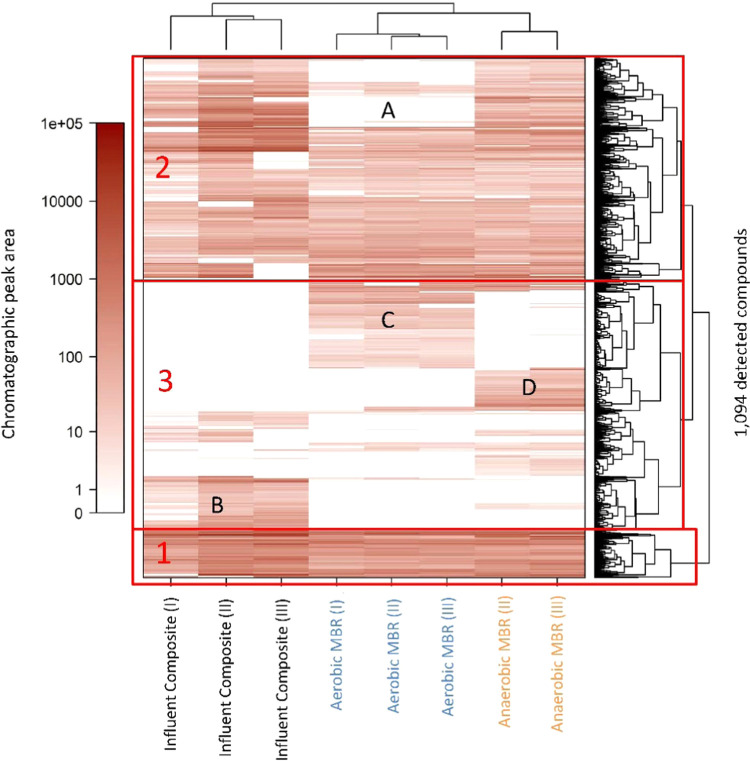
Comparison of Individual Compounds across Repeated Treatment Cycles
The normalized peak areas of the 1,094 compounds, selected based on the criteria described in the methods, were compared among the three sample types across the repeated treatment cycles via the hierarchical clustering heatmap (Figure 2). The dendrogram on the y-axis highlights three major clusters of compounds. The compounds in cluster 1 were highly persistent through both MBR treatments. Cluster 2 contains some persistent compounds as well as a group of compounds (A) that were completely removed through AeMBR treatment but removed to a lesser extent by AnMBR treatment. The compounds in cluster 2 are relatively low in abundance compared to those in cluster 1. Cluster 3 shows some persistent compounds and three other groups of compounds: (B) compounds that were completely removed by both MBR treatments, (C) compounds that newly formed through AeMBR treatment, and (D) compounds that newly formed through AnMBR treatment.
Figure 2.
Hierarchical clustering heatmap of the log-transformed normalized GC×GC/TOF-MS peak area abundance of N = 1,094 unique compounds detected in the influent composite, aerobic membrane permeate (AeMP), and anaerobic membrane permeate (AnMP) samples. Clusters 1–3 and groups A–D are discussed in the text. Each of the three treatment cycles is an independent experiment and is referred to by I, II, and III.
Comparison of AeMBR and AnMBR TOrC Treatment at Various Removal Efficiencies
Whereas the data analysis presented above focused on compounds detected in at least two of three treatment cycles, we selected the compounds detected in all treatment cycles for further data analysis to increase certainty. Our analysis revealed that 493 compounds were detected across all three influents. In addition, there were a high number of compounds (n = 312) that newly formed in either AeMP or AnMP across all treatment cycles (N = 3 for AeMP, N = 2 for AnMP). The number of compounds treated at various efficiency categories is shown in Figure 3, and the corresponding data points are listed in Table S13.
Figure 3.
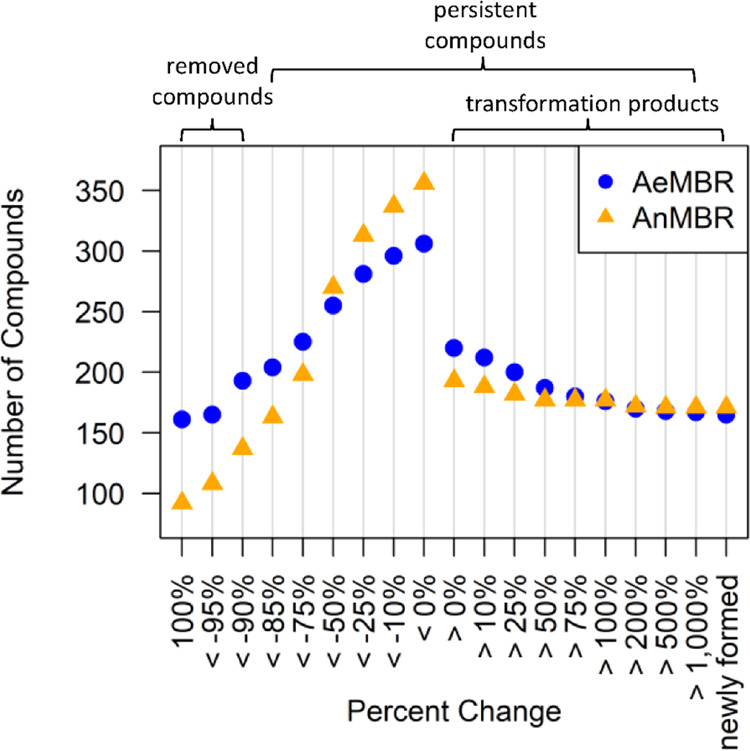
Comparison of AeMBR and AnMBR for treating compounds at various efficiencies. Note: transformation products that were not detected in the influent but were detected in the permeates are referred to as “newly formed”.
Out of the 493 influent compounds, we found that a greater number of compounds could be considered removed (−90% change) by AeMBR (193 compounds) compared to that of AnMBR (137 compounds) (Figure 3). The number of completely removed compounds was higher in AeMBR, but 17 more compounds were partially removed (specifically, between −50 and 0% change) by AnMBR. Biological degradation, sorption to sludge, and volatilization are all potential TOrC removal processes that occur in both MBR treatments. In the anaerobic baffled reactor (ABR), sorption occurs as water passes through the sludge blanket in each chamber, and some volatilization occurs in the membrane tank where nitrogen is applied for scouring.
Out of all of the removed compounds, 119 compounds were removed in both MBR treatment systems, suggesting that these 119 compounds will degrade, transform, or adsorb under either aerobic or anaerobic conditions. Of those 119 compounds, 34 were tentatively identifiable and are listed in Table S14. These included previously reported compounds, such as resorcinol, which is found in acne creams and other personal care products and was observed in rivers receiving wastewater effluent.42
Across all three treatment cycles, there were 44 compounds that were removed by the AeMBR but persisted in the AnMBR, suggesting that these compounds need aerobic conditions or the presence of aerobes to adsorb to biosolids or membrane biofilm, degrade to CO2, or volatize. Twenty-one of those 44 compounds were tentatively identifiable and are listed in Table S15. These included previously reported compounds, such as o-hydroxybiphenyl, which was found to be nonbiodegradable in landfill leachate43 and present in municipal wastewater.44 Meanwhile, only 4 compounds were removed by the AnMBR and persisted through the AeMBR, suggesting that these compounds need anaerobic conditions to adsorb or degrade. It was possible to tentatively identify only one of the 4 compounds, 2-methoxy-4-vinylphenol, which is a naturally occurring volatile organic compound and is also used as a flavoring agent.45
Persistent Compounds
Out of the 493 compounds commonly detected in the influents, 58% of those compounds (n = 288 compounds) persisted through the AnMBR and 44% (n = 217 compounds) persisted through the AeMBR across all treatment cycles. Within the set of persistent compounds, 201 compounds (41% of the 493 influent TOrCs) persisted through both MBR treatments; 113 of those 201 compounds were tentatively identifiable, and their percent changes are reported in Table S16.
Moreover, some persistent compounds had substantial peak area increases after treatment, which may reflect the fact that these persistent compounds were transformation products already present in the San Elijo WRF influent that continued to form during our parallel treatment processes. Of the common compounds detected in the three influent composites, 55 compounds increased in the AeMP, while 22 compounds increased in the AnMP, and 5 compounds commonly increased in both MBRs.
Of the 55 compounds that increased in the AeMP, 1H-benzotriazole, a common chemical in wastewater,46 had the greatest percent increase in the AeMP of 2,146 ± 656%, and it increased in the AnMP by 148 ± 5%. Benzoic acid, 3,5-bis(1,1-dimethylethyl)-2-hydroxy-, had the second largest percent increase in the AeMP of 1152 ± 262%, while in the AnMP, it decreased by 35 ± 5%. Benzoic acid, 3,5-bis(1,1-dimethylethyl)-2-hydroxy-, has been identified as a persistent and mobile chemical in surface water47 and as a potential BPA alternative in thermal paper.48
Of the 22 compounds that increased in the AnMP, (1S,4S,9aS)-4-allyl-1-ethyloctahydro-1H-quinolizine had the greatest percent increase in the AnMP of 639 ± 491%, and it increased in the AeMP by 172 ± 147%. This compound has not previously been identified in the wastewater. Ethosuximide had the second largest percent increase in the AnMP of 426 ± 224%, while in the AeMP, it increased by only 18 ± 97%. Ethosuximide is an antiseizure medication and was previously identified in on-site and large-scale sewage treatment plants by a GC×GC/TOF-MS-based nontarget screening.49
Newly Formed Transformation Products
Both AeMBR and AnMBR treatments produced a similar number of newly formed transformation products (165 compounds in AeMBR and 171 in AnMBR), with AnMBR treatment producing slightly more. Of the 165 newly formed compounds present in the AeMP, 136 compounds were absent from the AnMP, suggesting that these 136 compounds unique to the AeMP newly formed only under aerobic conditions. Thirty of those 136 compounds unique to the AeMP were tentatively identifiable and are listed in Table S17. Benzene, 1-azido-3-methoxy-, which has been reported in the synthesis chemistry literature,50 was the most abundant newly formed compound among the 30 compounds unique to the AeMP. The second-most abundant newly formed compound in the AeMP was 5-acetyl-4-methylthiazole, which has been identified as a metabolite of the pharmaceutical, chlormethiazole.51 Neither of these two compounds has been reported in wastewater.
Of the 171 newly formed compounds present in the AnMP, 121 compounds were absent from the AeMP, suggesting that these 121 compounds unique to the AnMP newly formed only under anaerobic conditions. Eighteen of those 121 compounds unique to the AnMP were tentatively identifiable and are listed in Table S18. Pyridine, 3-ethyl, which has previously been reported in sewage sludge that had undergone gasification,52 was the most abundant newly formed compound among the 18 compounds unique to the AnMP. Citronellic acid was the second-most abundant newly formed compound in the AnMP. Citronellic acid has been studied for use as a pest repellant53 and was reported as a transformation product of methoprene, an insect growth regulator.54
The large number of newly formed compounds unique to either AeMP or AnMP suggests that each treatment results in different transformation products. Only 24 transformation products were common to both the AeMPs and AnMPs, suggesting that these 24 compounds newly formed under either aerobic or anaerobic conditions. However, none of the 24 compounds were identifiable and their spectra are presented in the Supporting Information III.
Although our NTA approach identifies a much greater number of compounds than in previous targeted studies, the existing database allowed for the identification of a fraction of the persistent compounds (113 out of 201), aerobic transformation products (30 out of 136), anaerobic transformation products (18 out of 121), and shared transformation products (0 out of 24). This result reveals that there are still many compounds that are not available in the NIST mass spectral library, which remains a challenge for monitoring and regulating future chemicals of emerging concern. Nürenberg et al. evaluated TOrC removal and transformation by biological treatment processes in 11 different WWTFs using an NTA approach.20 They found that transformation products constituted more than half of the number of detected features in biological treatment effluents.20 Additionally, their cumulative area accounted for 36–54% of the total area of all features in the effluents.20 Thus, transformation products are essential to consider, especially given that some can be as harmful as their parent compounds and contribute significantly to the dissolved organic matter in WWTF secondary effluent.2,20
Comparison of Removal and Persistence of TOrCs Identified in Other Studies
Although the removal of TOrCs in systems treating real wastewater may be influenced by different operating conditions, including SRT, HRT, redox conditions, pH, and temperature,55 it is worth evaluating the TOrC removal and persistence among similar studies. Zaouri et al.23 also used NTA to identify compounds in AeMBR and AnMBR permeates, with the goal of evaluating the effects of treated wastewater on seed germination and feed production.23 Their LC-MS/MS-based NTA approach resulted in a total of 36 permeate compounds, including 8 hormones,23 that were tentatively identified in the real treated wastewater. None of those compounds were common with the 351 compounds tentatively identified in our study. While this may be due to a different source of real wastewater, it is also likely that the LC-MS/MS-based and our GC×GC/TOF-MS NTA identified different types of compounds due to different polarities or other properties, further supporting that these are complementary approaches.
Our previous study (Mladenov et al.)18 also employed the GC×GC/TOF-MS NTA and utilized wastewater from the same San Elijo WRF facility as was used in the current study. Therefore, we compared the removal and persistence of 15 compounds in the AeMBR and AnMBR influents of the current study and in the influent of three WWTFs in the study by Mladenov et al.18 These 15 compounds (Table S19) were verified with analytical standards in the current study. The three WWTFs of Mladenov et al.18 included two centralized WWTFs, one in the United States (United States-centralized) and one in South Africa (South Africa-centralized), both of which employ conventional activated sludge biological treatment with post-treatment steps, and one decentralized treatment plant in South Africa (South Africa-decentralized) using an ABR with anaerobic filter, followed by a constructed wetland for polishing. The ABR in the South Africa-decentralized system had an HRT of 33.6 h, although shorter HRTs were also reported for ABRs. The activated sludge treatment processes of the previous study had HRTs between 3 and 15 h. Our HRTs of 24 h for the aeration tank and 48 h for the ABR are longer than those reported in the previous study by about 1.6 and 1.4 times, respectively, and these likely resulted in improved COD removals.
Regardless of the treatment type, five compounds (4-(4-hydroxy-3-methoxyphenyl)-2-butanone, 4-ethyl-2-methoxyphenol, benzyl cyanide, piperine, and scopoletin) were removed (<94% change) by both MBR treatments in this study and all three WWTFs (Table S19). In addition, terpineol, a nonsorptive and biodegradable fragrance material, was also removed (<94% change) by all of the treatment systems, except the South Africa-centralized WWTF.
There was also a general agreement for compound removal among the aerobic treatment systems (the two centralized treatment plants and the AeMBR). Six compounds (1,7-dimethylxanthine, 2,4-dihydroxybenzophenone, 5-(methylsulfanyl)pentanenitrile, benzenemethanethiol, nicotine, benzoic acid, 3-(1-methylethyl)-) were removed (<−93% change) by all WWTFs and the AeMBR treatment but were less efficiently removed (−89 to −67% change) by the AnMBR treatment. Although the South Africa-decentralized facility is similar to the AnMBR in that it primarily uses anaerobic biological treatment, the South Africa-decentralized facility also employs a constructed wetland for polishing; therefore, these five compounds’ more efficient removal (<−90% change) in that system could have been attributed to its wetland polishing step. On the other hand, anaerobic processes seemed to be important for the removal of 2-methoxy-4-vinylphenol, which was removed (<−95% change) by the South Africa-decentralized and the AnMBR systems, while persisting through the centralized WWTFs and AeMBR treatments (−81 to −60% change), which rely on aerobic processes.
Bisphenol A (BPA), which is used in the manufacturing of various plastics, was removed (<−91% change) by AeMBR and South Africa-centralized treatments. Its removal was less efficient (−57 and −87% change, respectively) in the United States-centralized and the South Africa-decentralized and even less efficiently removed by the AnMBR treatment, partially decreasing with a −13 ± 41% change. BPA’s removal has been shown to be variable, with other studies demonstrating removals of a −87 to −100% change for an AeMBR treatment13,56 and −25 to −99.9% change for an AnMBR treatment.11,13
Triclosan was removed with a −93 to −89% change by the three WWTFs; however, it slightly increased in the AeMBR (7 ± 81%) and was only partially removed by the AnMBR (−43 ± 4%) (Table S19).18 Incidentally, one of triclosan’s metabolites (methyl triclosan), which was not one of the 15 targeted compounds but was tentatively identified in this study, increased in the AeMBR with a 90 ± 119% change but not in the AnMBR system, where it was partially removed (with a −18% change). The increase of methyl triclosan in the AeMBR is consistent with the previous finding that methylation of triclosan occurred in activated sludge under aerobic conditions but not under anaerobic conditions.57
Persistence, Removal, and Physicochemical and Molecular Properties
PCA was conducted as a QSPR approach to determine if physical–chemical properties or molecular descriptors predicted the percent change of the tentatively identified compounds. Two groups of potentially mechanistically significant descriptors were separately assessed: (1) 16 physical–chemical properties (listed in Table S20) and (2) 12 molecular descriptors (listed in Table S21). Due to the low proportions of explained variance, PCA was unable to predict compound removal based on the physical–chemical properties. For the molecular descriptors, correlations between the percent removal and principal component 1 were further examined but were found to be nonsignificant across the treatment cycles (Figure S3). A more detailed description of the PCA results and other descriptors analyzed, which turned out to be nonsignificant, is discussed in the Principal Component Analysis section in Supporting Information I.
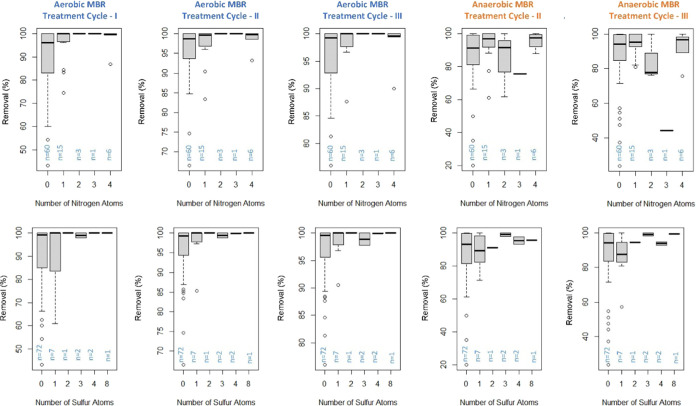
To evaluate the previous findings of Wijekoon et al.12 and Liu et al.13 that nitrogen- or sulfur-containing molecules had a greater removal under anaerobic conditions, we identified the number of N and S atoms in each compound (Tables S22 and S23). For this analysis, to focus on compounds that behaved in a reproducible manner, only the compounds that met the following criteria were considered: (1) the compound was common among all influents, (2) had an average (n = 3 AeMBR, n = 2 AnMBR) peak area reduction between 0 and 100%, and (3) had a coefficient of variation less than 30%. These criteria resulted in a total of 85 tentatively identified compounds commonly detected among influents. The percent peak area reduction versus the number of nitrogen and sulfur atoms is plotted in Figure 4. The boxplots illustrate that the AeMBR had greater removal on average for at least 25 compounds containing nitrogen and 13 compounds containing sulfur compared to the AnMBR. Therefore, our findings differ from those of Wijekoon et al.12 and Liu et al.,13 possibly because the prior experiments evaluated a smaller subset of nitrogen-containing compounds (16 nitrogen-containing compounds and one sulfur- and nitrogen-containing compound).12 By contrast, in this study, we were able to examine 25 N- and 13 S-containing compounds and found that the presence of those elements did not increase removal under anaerobic conditions.
Figure 4.
Removal (%) vs number of nitrogen or sulfur atoms. Each boxplot represents the 85 tentatively identifiable compounds that were common among all influents and both treatments with an average percent removal between 0 and 100%, and a coefficient of variation less than 30% across all treatment cycles. Each treatment cycle (I, II, and III) corresponds to an individual experiment.
Summary of Tentatively Identified TOrCs in This Study
Two hundred eighty-three of the 351 tentatively identifiable compounds were present in at least one chemical list in the EPA CompTox Dashboard, and product use and application information were available for 245 of those tentatively identifiable compounds (access date: 07/12/2023). This enabled us to classify them into use and source groups, as shown in Table 1. Table S24 reports an index defining each category based on the EPA CompTox lists. It makes sense that metabolites and biotransformation products are the largest category of compounds (187 compounds) identified across the wastewater samples, given the fact that these compounds are likely undergoing biodegradation and physicochemical transformations from primarily residential wastewater sources during the MBR treatment processes. The identification of many TOrCs from cosmetics, consumer products, and pharmaceutical drug compounds is also expected due to the source. It is interesting to observe a large number of plastic-related compounds (103 compounds). These chemicals are a growing category of TOrCs with toxicological implications and are potentially detrimental to MBR performance that merits further research.58 In addition, a considerable number of pesticides were identified (47 compounds).
Table 1. Count of Compounds per Product Use Category or Regulatory List of the 245 Tentatively Identifiable Compounds with Product Use Information in the EPA Comptox Dashboard (Access Date: 07/12/2023)a.
| product use and source category | count |
|---|---|
| metabolite/biotransformation product | 187 |
| odorant/aroma compound | 128 |
| plastic-related | 103 |
| consumer product | 92 |
| food contact substance | 86 |
| cosmetic ingredient | 79 |
| pharmaceutical/drug | 68 |
| inert ingredient | 65 |
| food additive | 64 |
| pheromones/semiochemicals | 62 |
| toxin | 59 |
| solvent | 55 |
| pesticide/biocide/insect repellant/antifungal | 47 |
| oil-related (hydraulic fracturing, oil field additive, motor fuel ingredient) | 42 |
| tobacco/cigarette/vaping-related | 38 |
| extractable or leachable from the pharmaceutical product | 35 |
| other | 34 |
| disinfection byproduct | 28 |
| detected in the recycled tire crumb | 22 |
| water distribution leachable substance | 16 |
| flame retardant | 9 |
| antimicrobial | 7 |
| antioxidant | 6 |
| surfactant | 6 |
| industrial chemical or manufacturing waste | 5 |
| regulatory list | count |
|---|---|
| U.S. EPA high production volume list | 65 |
Many of the compounds are identified in multiple product use categories. An index of the EPA Comptox. Lists that define each product use category are found in the Supporting Information (Table S24).
Our findings were somewhat different from a study by Laura-Martin et al., who conducted an LC-high-resolution MS-based NTA of sewage-derived contaminants in ocean water.2 They identified surfactants as the second-most relevant group of contaminants in terms of the number of identified sewage-derived components and first in signal intensities,2 whereas our study only identified 6 surfactants. On another note, we identified 11 fragrances and 6 antioxidants, which are two classes of compounds that are discussed as under-represented in the environmental PPCP literature.25 The 11 fragrance compounds included triethyl citrate, octinoxate, butylated hydroxytoluene, 2-hydroxy-4-methoxybenzophenone, propylparaben, 2-(phenylmethylene)octanal, diethyl phthalate, linalool, benzyl benzoate, α-amyl cinnamaldehyde, and 2-phenoxyethanol. The 6 antioxidants included tert-butylhydroquinone, 2,5-cyclohexadiene-1,4-dione, 2-methyl-5-(1-methylethyl)-, bisphenol A, 4-hydroxybenzeneethanol, N-isopropyl-N′-phenyl-p-phenylenediamine, and 1H-indole-3-propanoic acid. Sixty-five compounds (Table S25) were identified under the U.S. EPA High Production Volume List, which are classified as chemicals that are produced or imported in the United States in quantities of one million pounds or more per year.
Conclusions
Overall, despite the persistence of some TOrCs through both AeMBR and AnMBR systems, the AeMBR and the AnMBR were able to completely remove 34–37% and 19–20%, respectively, of the 493 commonly detected TOrCs across the tested influents. The higher removal of TOrCs under aerobic conditions is important from the perspective of reducing the discharge of chemicals of emerging concern to surface waters. However, the potential for TOrCs to sorb to biosolids and the high sludge production under aerobic conditions should also be weighed when choosing AeMBR or AnMBR treatment, as biosolid disposal in landfills or agricultural land applications represent additional TOrC discharges to the environment.59 Future work should apply NTA to further probe compound removal via sorption and transfer to the solid phase. Additionally, AnMBR technology has garnered increasing interest for its potential for net-zero energy production or biogas (CH4) recovery.16 The finding that the AnMBR had greater partial compound removal (<50% removal) than the AeMBR further suggests that a combination of aerobic and anaerobic treatments may achieve the greatest total removal of TOrCs.
The comparison of results from this study to results from a study of full-scale wastewater treatment systems confirmed the presence of many of the same TOrCs, lending further support to the GC×GC/TOF-MS-based NTA. To achieve a more comprehensive nontargeted screening of wastewater compounds, future AeMBR and AnMBR comparison analyses could use an LC-MS-based NTA as a complementary approach. Nevertheless, the GC×GC/TOF-MS NTA exposed the presence of a much greater number of compounds that have been identified in previous studies using real wastewater, including 11 fragrance compounds that are more amenable to GC analyses. It is worth pointing out that both AeMBR and AnMBR treatments resulted in the formation of a large number of transformation products, many of which are unknown and merit further study. The tentatively identified persistent compounds and transformation products also highlighted a distressing number of plastic-related compounds present in treated effluent and are valuable for informing future monitoring of TOrC contamination in aquatic systems, QSPR analyses, and toxicological studies of MBR-treated wastewater.
Acknowledgments
This research was supported by the National Science Foundation, Award No. 1705901, with traineeships for Jade Johnson and Lauren Steinberg. This work was also supported by the Robert and Patricia Switzer Foundation via a fellowship, the Southern California Society of Environmental Toxicology and Chemistry Graduate Student Research Grant, and the San Diego State University Master’s Research Scholarship awarded to Jade Johnson. The authors thank S. Arredondo and the staff of San Elijo Joint Powers Authority Water Reclamation Facility for assisting with influent sampling, Stephanie Chao, Cheyenne Graves, Jason Simmons, and David Aponte for assisting with the operation and maintenance of the parallel membrane bioreactor systems, and Alma Rocha for assisting with data input. Suez Technologies graciously provided Zeeblok membranes for the study and guidance on the setup of AnMBR and AeMBR systems. The William E. Leonhard Endowment to N.M. supported laboratory remodeling for the experimental setup. The authors also thank Michael Lester for assistance with lab-scale reactor fabrication.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsestwater.3c00542.
Supporting Information I: additional experimental details, materials, and methods, including photographs of the aerobic and anaerobic membranes (PDF)
Supporting Information II: mass spectra of all tentatively identified compounds (PDF)
Supporting Information III: mass spectra of the nontentatively identifiable new transformation products common to both aerobic and anaerobic membrane permeates (PDF)
Author Present Address
∥ Larry Walker Associates, 2525 Ocean Park Blvd, Suite 216, Santa Monica, California 90405, United States
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. CRediT: Jade Lauren Johnson data curation, formal analysis, investigation, methodology, software, validation, visualization, writing-original draft, writing-review & editing; Nathan Gray Dodder conceptualization, formal analysis, funding acquisition, methodology, software, supervision, validation, visualization, writing-review & editing; Natalie Mladenov conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, validation, visualization, writing-original draft, writing-review & editing; Lauren Steinberg formal analysis, investigation, validation, writing-review & editing; William Richardot data curation, investigation, methodology, writing-review & editing; Eunha Hoh conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, validation, writing-review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Wang Z.; Walker G. W.; Muir D. C.; Nagatani-Yoshida K. Toward a Global Understanding of Chemical Pollution: a first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 2020, 54 (5), 2575–2584. 10.1021/acs.est.9b06379. [DOI] [PubMed] [Google Scholar]
- Lara-Martín P. A.; Chiaia-Hernández A. C.; Biel-Maeso M.; Baena-Nogueras R. M.; Hollender J. Tracing urban wastewater contaminants into the Atlantic Ocean by nontarget screening. Environ. Sci. Technol. 2020, 54 (7), 3996–4005. 10.1021/acs.est.9b06114. [DOI] [PubMed] [Google Scholar]
- Ashraf M. A. Persistent organic pollutants (POPs): a global issue, a global challenge. Environ. Sci. Pollut. Res. 2017, 24 (5), 4223–4227. 10.1007/s11356-015-5225-9. [DOI] [PubMed] [Google Scholar]
- Buerge I. J.; Buser H. R.; Kahle M.; Muller M. D.; Poiger T. Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environ. Sci. Technol. 2009, 43 (12), 4381–4385. 10.1021/es900126x. [DOI] [PubMed] [Google Scholar]
- Benotti M. J.; Trenholm R. A.; Vanderford B. J.; Holady J. C.; Stanford B. D.; Snyder S. A. Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ. Sci. Technol. 2009, 43 (3), 597–603. 10.1021/es801845a. [DOI] [PubMed] [Google Scholar]
- Li X.; Ying G. G.; Su H. C.; Yang X. B.; Wang L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010, 36 (6), 557–562. 10.1016/j.envint.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Chen M.; Shan G.; Chen P.; Cui S.; Yi S.; Zhu L. Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Sci. Total Environ. 2017, 598, 814–820. 10.1016/j.scitotenv.2017.04.167. [DOI] [PubMed] [Google Scholar]
- Cossaboon J. M.; Hoh E.; Chivers S. J.; Weller D. W.; Danil K.; Maruya K. A.; Dodder N. G. Apex marine predators and ocean health: Proactive screening of halogenated 23 organic contaminants reveals ecosystem indicator species. Chemosphere 2019, 221, 656–664. 10.1016/j.chemosphere.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack M. E.; Cossaboon J. M.; Tubbs C. W.; Vilchis L. I.; Felton R. G.; Johnson J. L.; Danil K.; Heckel G.; Hoh E.; Dodder N. G. Assessing marine endocrine disrupting chemicals in the critically endangered California condor: implications for reintroduction to coastal environments. Environ. Sci. Technol. 2022, 56 (12), 7800–7809. 10.1021/acs.est.1c07302. [DOI] [PubMed] [Google Scholar]
- Tadkaew N.; Hai F. I.; McDonald J. A.; Khan S. J.; Nghiem L. D. Removal of trace organics by MBR treatment: the role of molecular properties. Water Res. 2011, 45 (8), 2439–2451. 10.1016/j.watres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Monsalvo V. M.; McDonald J. A.; Khan S. J.; Le-Clech P. Removal of trace organics by anaerobic membrane bioreactors. Water Res. 2014, 49, 103–112. 10.1016/j.watres.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Wijekoon K. C.; McDonald J. A.; Khan S. J.; Hai F. I.; Price W. E.; Nghiem L. D. Development of a predictive framework to assess the removal of trace organic chemicals by anaerobic membrane bioreactor. Bioresour. Technol. 2015, 189, 391–398. 10.1016/j.biortech.2015.04.034. [DOI] [PubMed] [Google Scholar]
- Liu W.; Song X.; Huda N.; Xie M.; Li G.; Luo W. Comparison between aerobic and anaerobic membrane bioreactors for trace organic contaminant removal in wastewater treatment. Environ. Technol. Innov. 2020, 17, 100564 10.1016/j.eti.2019.100564. [DOI] [Google Scholar]
- National Academy of Sciences . Water Reuse: Potential for Expanding the Nation’s Water Supply through Reuse of Municipal Wastewater; The National Academies Press: Washington, D.C., 2012; p 49. [Google Scholar]
- Wu B.; Kim J. Anaerobic membrane bioreactors for nonpotable water reuse and energy recovery. J. Environ. Eng. 2020, 146 (2), 03119002 10.1061/(ASCE)EE.1943-7870.0001637. [DOI] [Google Scholar]
- Zhang S.; Lei Z.; Dzakpasu M.; Li Q.; Li Y. Y.; Chen R. Removal of trace organic contaminants in municipal wastewater by anaerobic membrane bioreactor: efficiencies, fates and impact factors. J. Water Process. Eng. 2021, 40, 101953 10.1016/j.jwpe.2021.101953. [DOI] [Google Scholar]
- Pérez-Lemus N.; López-Serna R.; Pérez-Elvira S. I.; Barrado E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: a critical review. Anal. Chim. Acta 2019, 1083, 19–40. 10.1016/j.aca.2019.06.044. [DOI] [PubMed] [Google Scholar]
- Mladenov N.; Dodder N. G.; Steinberg L.; Richardot W.; Johnson J.; Martincigh B. S.; Buckley C.; Lawrence T.; Hoh E. Persistence and removal of trace organic compounds in centralized and decentralized wastewater treatment systems. Chemosphere 2022, 286, 131621 10.1016/j.chemosphere.2021.131621. [DOI] [PubMed] [Google Scholar]
- Blum K. M.; Gallampois C.; Andersson P. L.; Renman G.; Renman A.; Haglund P. Comprehensive assessment of organic contaminant removal from on-site sewage treatment facility effluent by char-fortified filter beds. J. Hazard. Mater. 2019, 361, 111–122. 10.1016/j.jhazmat.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Nürenberg G.; Kunkel U.; Wick A.; Falås P.; Joss A.; Ternes T. A. Nontarget analysis: a new tool for the evaluation of wastewater processes. Water Res. 2019, 163, 114842 10.1016/j.watres.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Choi Y.; Lee J. H.; Kim K.; Mun H.; Park N.; Jeon J. Identification, quantification, and prioritization of new emerging pollutants in domestic and industrial effluents, 24 Korea: Application of LC-HRMS based suspect and non-target screening. J. Hazard. Mater. 2021, 402, 123706 10.1016/j.jhazmat.2020.123706. [DOI] [PubMed] [Google Scholar]
- Schollée J. E.; Schymanski E. L.; Avak S. E.; Loos M.; Hollender J. Prioritizing unknown transformation products from biologically-treated wastewater using high-resolution mass spectrometry, multivariate statistics, and metabolic logic. Anal. Chem. 2015, 87 (24), 12121–12129. 10.1021/acs.analchem.5b02905. [DOI] [PubMed] [Google Scholar]
- Zaouri N.; Cheng H.; Khairunnisa F.; Alahmed A.; Blilou I.; Hong P. Y. A type dependent effect of treated wastewater matrix on seed germination and food production. Sci. Total Environ. 2021, 769, 144573 10.1016/j.scitotenv.2020.144573. [DOI] [PubMed] [Google Scholar]
- Dallüge J.; Van Stee L. L. P.; Xu X. B.; Williams J.; Beens J.; Vreuls R. J. J.; Brinkman U. A. Th. Unraveling the composition of very complex samples by comprehensive gas chromatography coupled to time-of-flight mass spectrometry: Cigarette smoke. J. Chromatogr. A 2002, 974, 169–184. 10.1016/S0021-9673(02)01384-5. [DOI] [PubMed] [Google Scholar]
- Meyer M. F.; Powers S. M.; Hampton S. E. An evidence synthesis of pharmaceuticals and personal care products (PPCPs) in the environment: imbalances among compounds, sewage treatment techniques, and ecosystem types. Environ. Sci. Technol. 2019, 53 (22), 12961–12973. 10.1021/acs.est.9b02966. [DOI] [PubMed] [Google Scholar]
- Baird R.; Eaton A. D.; Rice E. W.; Bridgewater L., Eds. Standard Methods for the Examination of Water and Wastewater, 23rd, ed., American Public Health Association; American Water Works Association; Water Environment Federation: Washington, D.C., 2017. [Google Scholar]
- Chang D.; Richardot W. H.; Miller E. L.; Dodder N. G.; Sedlak M. D.; Hoh E.; Sutton R. Framework for nontargeted investigation of contaminants released by wildfires into stormwater runoff: case study in the northern San Francisco Bay area. Integr. Environ. Assess. Manag. 2021, 17 (6), 1179–1193. 10.1002/ieam.4461. [DOI] [PubMed] [Google Scholar]
- Migowska N.; Caban M.; Stepnowski P.; Kumirska J. Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography–mass spectrometry and gas chromatography with electron capture detection. Sci. Total Environ. 2012, 441, 77–88. 10.1016/j.scitotenv.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Miège C.; Bados P.; Brosse C.; Coquery M. Method validation for the analysis of estrogens (including conjugated compounds) in aqueous matrices. TrAC Trends Anal. Chem. 2009, 28 (2), 237–244. 10.1016/j.trac.2008.11.005. [DOI] [Google Scholar]
- Krauss M.; Hollender J. Analysis of nitrosamines in wastewater: exploring the trace level quantification capabilities of a hybrid linear ion trap/orbitrap mass spectrometer. Anal. Chem. 2008, 80 (3), 834–842. 10.1021/ac701804y. [DOI] [PubMed] [Google Scholar]
- Miossec C.; Lanceleur L.; Monperrus M. Multi-residue analysis of 44 pharmaceutical compounds in environmental water samples by solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2019, 42 (10), 1853–1866. 10.1002/jssc.201801214. [DOI] [PubMed] [Google Scholar]
- Shaul N. J.; Dodder N. G.; Aluwihare L. I.; Mackintosh S. A.; Maruya K. A.; Chivers S. J.; Hoh E.; et al. Nontargeted biomonitoring of halogenated organic compounds in two ecotypes of bottlenose dolphins (Tursiops truncatus) from the Southern California bight. Environ. Sci. Technol. 2015, 49, 1328–1338. 10.1021/es505156q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. R.; McMahen R. L.; Sobus J. R.; Mansouri K.; Williams A. J.; McEachran A. D.; Strynar M. J. Suspect screening and non-targeted analysis of drinking water using point-of-use filters. Environ. Pollut. 2018, 234, 297–306. 10.1016/j.envpol.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K. P.; Luna R. F.; Richardot W. H.; Lopez-Galvez N.; Plumlee M. H.; Dodder N. G.; Hoh E. Nontargeted analysis of trace organic constituents in reverse osmosis and UV-AOP product waters of a potable reuse facility. ACS ES&T Water. 2022, 2 (1), 96–105. 10.1021/acsestwater.1c00265. [DOI] [Google Scholar]
- Tran C. D.; Dodder N. G.; Quintana P. J.; Watanabe K.; Kim J. H.; Hovell M. F.; Chambers C. D.; Hoh E. Organic contaminants in human breast milk identified by non-targeted analysis. Chemosphere 2020, 238, 124677 10.1016/j.chemosphere.2019.124677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J.; Grulke C. M.; Edwards J.; McEachran A. D.; Mansouri K.; Baker N. C.; Patlewicz G.; Shah I.; Wambaugh J. F.; Judson R. S.; Richard A. M. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Cheminformatics 2017, 9, 61 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman T. W. H.; Cao Y.; Girke T. ChemMine tools: an online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011, 39 (suppl_2), W486–W491. 10.1093/nar/gkr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PubChem Sketcher V2.4. https://pubchem.ncbi.nlm.nih.gov//edit3/index.html.
- Wegner J. K.; Fröhlich H.; Zell A. Feature selection for descriptor based classification models. 2. Human intestinal absorption (HIA). J. Chem. Inf. Model 2004, 44 (3), 931–939. 10.1021/ci034233w. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Charisi A.; Cheng L. C.; Jiang T.; Girke T. ChemmineR: a compound mining framework for R. Bioinformatics 2008, 24 (15), 1733–1734. 10.1093/bioinformatics/btn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X.; Peldszus S. Selection of representative emerging micropollutants for drinking water treatment studies: a systematic approach. Sci. Total Environ. 2012, 414, 653–663. 10.1016/j.scitotenv.2011.11.035. [DOI] [PubMed] [Google Scholar]
- Kimura K.; Kameda Y.; Yamamoto H.; Nakada N.; Tamura I.; Miyazaki M.; Masunaga S. Occurrence of preservatives and antimicrobials in Japanese rivers. Chemosphere 2014, 107, 393–399. 10.1016/j.chemosphere.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Trzcinski A. P.; Stuckey D. C. Continuous treatment of the organic fraction of municipal solid waste in an anaerobic two-stage membrane process with liquid recycle. Water Res. 2009, 43 (9), 2449–2462. 10.1016/j.watres.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Hrubik J.; Glisic B.; Tubic A.; Ivancev-Tumbas I.; Kovacevic R.; Samardzija D.; Andric N.; Kaisarevic S. Toxicological and chemical investigation of untreated municipal wastewater: fraction-and species-specific toxicity. Ecotoxicol. Environ. Saf. 2016, 127, 153–162. 10.1016/j.ecoenv.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Galarza G.; Figueroa J. G. Volatile compound characterization of coffee (coffea arabica) processed at different fermentation times using SPME–GC–MS. Molecules 2022, 27 (6), 2004. 10.3390/molecules27062004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struk-Sokołowska J.; Kotowska U.; Piekutin J.; Laskowski P.; Mielcarek A. Analysis of 1H-benzotriazole removal efficiency from wastewater in individual process phases of a sequencing batch reactor SBR. Water Resour. Ind. 2022, 28, 100182 10.1016/j.wri.2022.100182. [DOI] [Google Scholar]
- Neuwald I.; Muschket M.; Zahn D.; Berger U.; Seiwert B.; Meier T.; Kuckelkorn J.; Strobel C.; Knepper T. P.; Reemtsma T. Filling the knowledge gap: a suspect 25 screening study for 1310 potentially persistent and mobile chemicals with SFC-and HILIC-HRMS in two German river systems. Water Res. 2021, 204, 117645 10.1016/j.watres.2021.117645. [DOI] [PubMed] [Google Scholar]
- Björnsdotter M. K.; Boer J.; Ballesteros-Gómez A. Bisphenol A and replacements in thermal paper: a review. Chemosphere 2017, 182, 691–706. 10.1016/j.chemosphere.2017.05.070. [DOI] [PubMed] [Google Scholar]
- Blum K. M.; Andersson P. L.; Renman G.; Ahrens L.; Gros M.; Wiberg K.; Haglund P. Non-target screening and prioritization of potentially persistent, bioaccumulating and toxic domestic wastewater contaminants and their removal in on-site and large-scale sewage treatment plants. Sci. Total Environ. 2017, 575, 265–275. 10.1016/j.scitotenv.2016.09.135. [DOI] [PubMed] [Google Scholar]
- Limatibul S.; Watson J. Mechanism of acid hydrolysis of imidazolines. J. Org. Chem. 1971, 36 (24), 3803–3805. 10.1021/jo00823a600. [DOI] [Google Scholar]
- Moore R. G.; Robertson A. V.; Smyth M. P.; Thomas J.; Vine J. Metabolism and urinary excretion of chlormethiazole in humans. Xenobiotica 1975, 5 (11), 687–696. 10.3109/00498257509056138. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Guo L.; Jin H.; Yin J.; Lu Y.; Zhang X. An experimental investigation of sewage sludge gasification in near and super-critical water using a batch reactor. Int. J. Hydrogen Energy 2013, 38 (29), 12912–12920. 10.1016/j.ijhydene.2013.05.076. [DOI] [Google Scholar]
- Phillips A. K.; Appel A. G. Fumigant toxicity of essential oils to the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2010, 103 (3), 781–790. 10.1603/EC09358. [DOI] [PubMed] [Google Scholar]
- Kuo J. N.; McPherson B.; Soon A.; Pasternak J.; Garrett C. Environmental concentrations of methoprene and its transformation products after the treatment of Altosid XR Briquets in the city of Richmond, British Columbia, Canada. Environ. Toxicol. Chem. 2010, 29 (10), 2200–2205. 10.1002/etc.286. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Guo W.; Ngo H. H.; Nghiem L. D.; Hai F. I.; Zhang J.; Liang S.; Xiaochang C. W. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Chen J.; Huang X.; Lee D. Bisphenol A removal by a membrane bioreactor. Process Biochem. 2008, 43 (4), 451–456. 10.1016/j.procbio.2008.01.001. [DOI] [Google Scholar]
- Chen X.; Nielsen J. L.; Furgal K.; Liu Y.; Lolas I. B.; Bester K. Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere 2011, 84 (4), 452–456. 10.1016/j.chemosphere.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Wu X.; Zhao X.; Chen R.; Liu P.; Liang W.; Wang J.; Teng M.; Wang X.; Gao S. Wastewater treatment plants act as essential sources of microplastic formation in aquatic environments: A critical review. Water Res. 2022, 221, 118825 10.1016/j.watres.2022.118825. [DOI] [PubMed] [Google Scholar]
- Harb M.; Lou E.; Smith A. L.; Stadler L. B. Perspectives on the fate of micropollutants in mainstream anaerobic wastewater treatment. Curr. Opin. Biotechnol. 2019, 57, 94–100. 10.1016/j.copbio.2019.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.