INTRODUCTION & PURPOSE
Female stress urinary incontinence (FSUI) is a common condition that affects approximately 10–20% of the population.1 It is associated with negative impacts on quality of life (QoL), socialization, and exercise; it has a significant financial impact, and is associated with depression and anxiety.2
In 2012, the last Canadian Urological Association (CUA) guideline addressing adult urinary incontinence was published; it covered male and female non-neurogenic stress and urgency incontinence.3 Given the considerable changes in the management of FSUI, and the very distinct considerations and surgical procedures for this group, an individual guideline providing a more detailed overview of this topic was requested by the CUA Guidelines Steering Committee.
The specific population this guideline is intended to cover is women over 18 years of age with stress urinary incontinence. This CUA guideline is intended to be used by healthcare providers treating patients with FSUI. The guideline objective is to cover topics important to the evaluation, counselling, non-surgical and surgical treatment of FSUI, and the management of complications of FSUI surgery.
Given the significant variation in patients with FSUI, in some sections we focus on the evaluation and management of the “index patient” (similar to other guidelines).4,5 Other sections are dedicated to specific non-index patients with FSUI. We recognize that guidelines cannot address the management of all FSUI scenarios.
METHODOLOGY
We decided to use a question-and-answer format to provide brief, accessible, and practical answers to common questions addressing the evaluation and management of FSUI. The guideline panel was created to provide representation from a mix of community and academic urologists and allied health professionals from across Canada, in keeping with the CUA guideline rules. Disagreements during the guideline process were resolved by consensus-building. Conflicts of interests for the authors are included at the end of the guideline. The views or interests of the CUA did not influence the final set of recommendations.
The guideline panel was led by Dr. Kevin Carlson and Dr. Blayne Welk. The group first met virtually in December 2021. Objectives to guide the development of this document were agreed upon: 1) to be comprehensive without replicating existing evidence reviews; 2) to provide evidence-based and expert-based opinions on relevant topics within FSUI; and 3) to address the unique needs of Canadian urologists where appropriate.
All guideline members were asked to submit relevant topics that could be addressed in a question-and-answer format, and the final list of questions was agreed upon by the panel. Further feedback was received from CUA members at the 2022 CUA annual meeting. Individual sections were assigned to members, with another panel member acting as primary reviewer. Members reviewed publications relevant to their question using a combination of PubMed, Medline, and/or EMBASE database searches, with an emphasis on identifying existing systematic reviews, and then evaluating any new evidence that was published after the existing review’s search dates. No set limits based on date or study design were used, and English- or French-language studies were used.
Where appropriate, a consensus was sought among all panel members for guideline statements and recommendations. Recommendations were assigned a quality of evidence based on the GRADE working group framework (high, moderate, low, very low quality of evidence).6 The strength of the recommendation was also graded as either “strong” or “weak” based on the summation of the evidence. Where there was unanimous support for a statement that was felt to be standard of care but without a traditional evidence base, it was graded as a “clinical principle.” When making recommendations, we considered the balance of the magnitude of benefits, the magnitude of harms, and patient values and preferences when possible. We did not systematically consider cost or cost-effectiveness, equity, acceptability, or feasibility.
Where appropriate, we have included strong or weak recommendations. We use the term “should” for strong recommendations, which implies that a large majority of patients would benefit from the recommended action. We use the term “may” for weak recommendations, which implies that most patients would benefit from the recommended action, but a large minority may not. Therefore, weak recommendations are more sensitive to individual patient values and preferences and should involve a significant component of shared decision-making. A weak recommendation is typically made because there is important uncertainty in the quality of the evidence, or because there is good evidence but important uncertainty in the tradeoff between benefits and harms (net benefit), or in patient values and preferences. Guideline recommendations were proposed by section authors, and then reviewed and agreed upon by all authors.
The final guideline document was reviewed by the entire panel, and then by external reviewers, as well as the CUA Guidelines Steering Committee. We have abbreviated the guidelines, with more details for some sections placed in an online Appendix (available at cuaj. ca). This guideline is intended to be used for a period of five years, and then should be updated, in keeping with CUA guideline policy.
WHAT IS THE DEFINITION OF THE SUI “INDEX CASE”
Similar to other published guidelines, this CUA guideline also defines the “index case” as a healthy female patient who presents with bothersome stress urinary incontinence (SUI).4,5 While not necessarily representative of most cases in surgical practice, it does provide a good benchmark for patient management. This “index” patient struggles with pure stress incontinence or stress predominant mixed urinary incontinence. She has not undergone prior SUI surgery, has a body mass index (BMI) <40, has not had pelvic radiotherapy, does not have significant pelvic organ prolapse (POP), nor is she in the frail elderly category, where surgical options may not be appropriate. We agree with the recommendation of the European Association of Urology (EAU) guidelines that patients with these characteristics are “complicated” and would potentially be referred to a tertiary center. An example of the “index case” is a healthy, 54-year-old female, who has bothersome SUI while attempting to exercise, at which time she requires the use of incontinence pads.
WHAT ARE THE REQUIREMENTS FOR APPROPRIATE EVALUATION OF AN INDEX CASE?
Evaluation of the “index” patient proceeds in the standard fashion with a history and physical exam, as summarized in Table 1. Additional information can be gathered from a urinalysis (UA), postvoid residual (PVR), questionnaires, and patient-reported outcome measures (PROM s) and/or a three-day bladder diary.
Table 1.
Key historical and physical examination information to gather in evaluating FSUI
| Item | Notes |
|---|---|
| History | |
| Type of incontinence | SUI vs other (Ask: “I leak most when I…”) |
| Severity and impact on QoL | Pad type and number 3-day diary PROMs |
| Other LUTS/conditions | Storage and/or voiding LUTS, pain, hematuria, UTIs |
| Other pelvic floor symptoms | POP, pain, dyspareunia, fecal incontinence |
| Obstetrical and gynecologic history | Menopausal status, sexual function |
| Prior incontinence treatment | Conservative, medical, and surgical treatments |
| Review of systems | Particularly GI, neurologic, cardiac, endocrine |
| Medication and allergy reconciliation | |
| Physical examination | |
| General and neurologic | BMI, cognitive status, mobility, gait |
| Abdominal | Habitus, scars, palpable structures |
| Pelvic | Skin changes, estrogenization, scarring, urethral meatus, urethral mobility, presence of SUI, prolapse (POP-Q), cysts or masses, pelvic floor strength, focal tenderness, reflexes (bulbocavernosus, anal wink), rectal exam (when indicated) |
BMI: body mass index; FSUI: female stress urinary incontinence; GI: gastrointestinal; LUTS: lower urinary tract symptoms; POP-Q: pelvic organ prolapse quantifications system; PROM: patient-reported outcome measures; QoL: quality of life; UTI: urinary tract infection.
Questionnaires and PROMs can be used in both research and clinical practice to guide diagnosis and management, and to measure outcomes. There are many to choose from and they have been summarized well previously.4 One should employ a validated questionnaire that helps differentiate SUI from other types of incontinence, quantifies its severity, and/or quantifies the impact of incontinence on the patient’s QoL.
During the pelvic exam, the clinician should attempt to demonstrate SUI via supine and/or standing stress test. Because the stress test is best done with a comfortably full bladder, timing this part of the exam with assessment of the PVR should be considered. The PVR can be assessed at the time of a uroflow study with a bladder scanner or an in-and-out catheter, later with an outpatient ultrasound, or at the time of cystoscopy if required. The value of a single PVR should be taken in the context of the patient’s symptoms and any clinical findings of a palpable bladder or significant POP. UA is recommended to screen for hematuria, inflammation, infection, glucosuria, and medical renal disease.
WHEN ARE CYSTOSCOPY AND/OR URODYNAMICS INDICATED IN THE EVALUATION OF PATIENTS WITH SUI?
Cystoscopy and/or urodynamic studies (UDS) are often used prior to surgical treatment of FSUI to validate/confirm the preoperative diagnosis, obtain potentially prognostic information with regards to therapeutic outcomes, and/or compliment preoperative therapeutic discussions.
Cystoscopy
There is a paucity of data specifically addressing the influence of cystoscopy on the management of SUI in women, therefore, recommendations in this regard are based on expert opinion. Consistent with guidance from other organizations,5,7 this panel recommends:
■ RECOMMENDATION 1
Cystoscopy should not be performed routinely in the index patient with SUI (Strong recommendation, Moderate quality of evidence). The benefit of cystoscopy in the index patient was considered negligible, and while harms were small (such as urinary infection and patient discomfort), we judged that most patients would prefer not to undergo cystoscopy.
■ RECOMMENDATION 2
Cystoscopy should be performed in patients that have findings on history, physical exam, or other investigations that are concerning for bladder or urethral pathology (pain with incontinence, gross or microscopic hematuria, history of pelvic radiation, suspected fistula, suspected periurethral mass, recurrent urinary tract infections [UTI], elevated PVR, and/or obstructive voiding symptoms) (Clinical principle).
■ RECOMMENDATION 3
Cystoscopy should be performed in patients in whom there is a concern for a structural abnormality of the lower urinary tract or in whom prior pelvic floor/anti-incontinence surgery has been performed (Clinical principle).
Urodynamic studies (UDS)
UDS correlation with clinical diagnosis and test reproducibility
The limited evidence provided by several small studies demonstrates poor correlation between UDS findings and clinical diagnosis.8 Studies comparing the prognostic value of UDS findings to clinical diagnosis are lacking.
Complicating matters is the observation that asymptomatic individuals can have abnormal UDS findings, and conversely, patients with clinical symptoms may have a normal UDS assessment.9–11 Further, studies addressing the reproducibility of UDS findings within patients yield contradictory results, which questions the accuracy of an individual UDS.12–14
Influence of UDS findings on surgical outcomes
The Validation of Urodynamic Evaluation (VALUE) trial randomized 630 index SUI patients to either standard office evaluation alone or standard office evaluation with UDS assessment prior to surgical treatment for SUI.15 Despite observing a change in preoperative diagnosis in 56% of women in the office evaluation + UDS group, there was no difference in treatment outcomes between the two groups. Additional studies have also failed to demonstrate an effect of UDS findings on outcomes post-SUI surgery.16–19
Based on current evidence and in alignment with other consensus guidelines,4,5 the panel recommends:
■ RECOMMENDATION 4
UDS should not be performed in the index patient with SUI (Strong recommendation, High-quality of evidence).
■ RECOMMENDATION 5
UDS may be considered in the workup of non-index patients with SUI (Clinical principle) in keeping with International Continence Society (ICS) best practices,7 such as:
history of prior pelvic floor/pelvic prolapse/anti-incontinence surgery;
pelvic radiation;
elevated PVR;
suspected voiding dysfunction;
neurologic conditions affecting the lower urinary tract;
mixed urinary incontinence – urgency predominant; and/or
discrepancy between history and physical exam (for example, failure to elicit urinary incontinence on physical exam).
WHAT NON-SURGICAL MANAGEMENT OPTIONS ARE AVAILABLE AND/OR SHOULD BE RECOMMENDED BEFORE CONSIDERING SURGERY?
We summarized the various non-surgical options for FSUI in Table 2 and included the strength of our recommendation for pursuing these interventions, as well as the quality of the evidence supporting their use.
Table 2.
| Method | Strength of recommendation | Quality of evidence | |
|---|---|---|---|
|
| |||
| Lifestyle changes | |||
|
| |||
| Weight loss | Diet Exercise Behavioral modification |
Strong | Moderate to high |
|
| |||
| Adjustment of fluid intake | Reduction of the volume of liquid consumed | Weak | Moderate |
|
| |||
| Smoking cessation | Behavioural intervention | Weak | Low |
|
| |||
| Exercise modification | Avoidance of high-impact exercises (jumping, straining) | Weak | Moderate |
|
| |||
| Regular bowel movements | Interventions to prevent constipation and straining during bowel movements | Weak | Low |
|
| |||
| Physiotherapy | |||
| Supervised pelvic floor muscle training | PFMT (only) | Strong There is no clear benefit of adding other modalities to PFMT; however, ES and/or BF may be considered for subgroups of women presenting with significant pelvic floor muscle weakness or atrophy, or reduced proprioception. |
High |
| + ES | |||
| + BF | |||
| + VC | Weak VC with supervised training sessions by a trained professional can be offered to women with SUI who are able and willing to use them. |
Moderate | |
|
| |||
| Self-directed PFMT | Via mobile applications or web platforms | Weak | Weak |
|
| |||
| Bladder training * | Progressive voiding schedule to delay micturition ± distraction techniques | Strong | High |
|
| |||
| Alternative medicine | |||
|
| |||
| Acupuncture | Weak | Low | |
|
| |||
| Yoga, Pilates | Weak | Low | |
Bladder training is mostly used for mixed or urgency incontinence.
BF: biofeedback; ES: electrical stimulation; PFMT: pelvic floor muscle training; SUI: stress urinary incontinence; VC: vaginal cones.
IN OBESE WOMEN, DOES WEIGHT LOSS/BARIATRIC SURGERY IMPROVE FSUI?
See online Appendix for the supporting text for the recommendations.
■ RECOMMENDATION 6
Overweight or obese women with bothersome stress incontinence should be counselled that weight loss may improve their degree of incontinence (Strong recommendation, High quality of evidence).
■ RECOMMENDATION 7
Surgical interventions for stress incontinence should be delayed in women considering bariatric surgery (Strong recommendation, Moderate quality of evidence).
DOES SMOKING CESSATION IMPROVE FSUI?
See online Appendix for the supporting text for the recommendation.
■ RECOMMENDATION 8
Smoking cessation should be recommended for all patients as a general public health measure and may reduce chronic cough and stress incontinence (Clinical principle).
SHOULD VAGINAL ESTROGEN REPLACEMENT BE OFFERED FOR FSUI?
Estrogen plays an important role in the health and function of the genital and lower urinary tract. Estrogen receptors are present in the vagina, urethra, bladder, and pelvic floor musculature. In patients with genitourinary syndrome of menopause (GSM), topical vaginal estrogen improves several bothersome symptoms, including vaginal dryness, itching, burning, and dyspareunia. Urinary symptoms, such as dysuria, urgency, incontinence, and nocturia, are also improved.32 The use of vaginal estrogen for isolated FSUI has low-quality evidence and results are inconsistent, while for overactive bladder (OAB) and mixed urinary incontinence (MUI), the quality of evidence is moderate.33–38
■ RECOMMENDATION 9
Topical vaginal estrogen should not be used for the treatment of FSUI in isolation (Strong recommendation, Moderate quality of evidence); however, in post-menopausal patients with a constellation of symptoms associated with GSM, the use of topical vaginal estrogen is of demonstrable benefit and may improve incontinence-related symptoms.
DOES EVIDENCE SUPPORT USE OF SELF-DIRECTED PELVIC FLOOR MUSCLE TRAINING TO TREAT FSUI?
Compared to watchful waiting or usual care, self-directed pelvic floor muscle training (PFMT) via app- or web-based programs may remove barriers to treatment access, reduce a patient’s embarrassment, and improve adherence to training. These programs consist of PFM exercises that may or may not include educational and motivational components for managing urinary incontinence.39 More than 100 mobile applications and web platforms with diverse designs and content for urinary incontinence are already available, and most are for SUI.39,40 Despite this widespread use, only two randomized controlled trials (RCTs) have been published, both evaluating the same application and with conflicting evidence regarding its effectiveness.41–44 Women should undergo a pelvic floor muscle (PFM) assessment prior to self-directed PFMT to mitigate the potential adverse sequelae of learning and performing the exercises incorrectly.45
■ RECOMMENDATION 10
Self-directed PFMT may be offered to women who want to manage their symptoms on their own after initial assessment (Weak recommendation, Weak quality of evidence).
WHAT IS THE EVIDENCE FOR USE OF PFMT TO TREAT URINARY INCONTINENCE? SHOULD PARTICULAR METHODS BE RECOMMENDED?
Most of the literature regarding conservative management for SUI relates to PFMT.22 PFMT aims to improve PFM strength, endurance, power, relaxation, or a combination of these parameters.46
PFMT improves FSUI by:
– Using a conscious PFM pre-contraction, prior to and during effort or exertion, preventing urine leakage (the “Knack”); and,
– Supporting the bladder neck with strong, toned PFMs, which are resistant to stretching and which limit downward movement of the bladder neck during effort and exertion.21,22
The most current update of the Cochrane Collaboration’s review and recommendations from the International Consultation on Incontinence support PFMT as the first-line conservative management strategy for women with SUI.21,22
PFMT is effective as a stand-alone therapy, and as part of multicomponent therapies embedding PFMT with concomitant behavioral strategies and lifestyle changes. Benefits of PFMT are shown across age cohorts and urinary incontinence type, in various cultural contexts, using several different training regimens, and assessed by multiple outcome measures.
See online Appendix for additional discussion on this question.
■ RECOMMENDATION 11
For the index patient, providers should recommend a three-month individualized or group-based supervised progressive and intensive PFMT program, taught by a health professional, as first-line treatment for FSUI (Strong recommendation, High quality of evidence).
WHO IS A GOOD CANDIDATE FOR A FEMALE INTERNAL VAGINAL DEVICE FOR FSUI AND HOW EFFECTIVE ARE THEY?
Potential SUI candidates for intravaginal devices (such as a pessary, incontinence tampon, or intravaginal bladder support device) include pregnant patients, elderly women for whom surgery would be a risk, women with primary/recurrent SUI who wish to avoid surgery/repeat surgery, and women who only have SUI in predictable circumstances, such as strenuous exercise.47 To use the device, the patient needs good hand control, memory, and mental function. Patient values and preferences play a strong role in their acceptance of a pessary for treatment of FSUI.
Pessary fittings for SUI are successful in 89–92% of cases.48,49 In prospective, single-center studies, the pessary is associated with 45–58% improvement in SUI symptoms.50,51 Severe complication rates are low and common issues, such as vaginal discharge, odor, and erosion, can usually be successfully treated.47
■ RECOMMENDATION 12
Physicians should offer pessaries and other vaginal devices as a safe and effective treatment for FSUI to women who may be candidates (Weak recommendation, Moderate quality of evidence).
WHAT IS THE EVIDENCE FOR VAGINAL LASER THERAPY FOR FSUI?
Adopted from dermatology, two laser types — erbium-doped yttrium-aluminium-garnet (Er:YAG) and carbon dioxide (CO2) — have been introduced as novel, noninvasive treatments for GSM, and more recently for POP, vaginal laxity, OAB, and SUI.52
Current data evaluating vaginal laser therapy (VLT) is limited by heterogeneity of treatment, short-term followup, and small numbers of subjects. Most studies are observational in nature and rely on subjective measures, and early studies are reported in the laser and esthetics literature. Positive results with the fractional CO2 laser have been reported in small trials and systematic reviews.53–55 Recent RCTs, however, observed no benefit vs. sham treatment in SUI outcomes.52,56,57 While the body of evidence is much smaller, the Er:YAG laser has shown promise in some studies, including one RCT.58 While laser therapy appears to be a low-risk treatment, the short-term nature of most trials limits definitive conclusions, and efficacy is not clear. Early vaginal bleeding and late vaginal scarring have been reported.52
■ RECOMMENDATION 13
Physicians should not recommend the use of VLT for the treatment of SUI (Weak recommendation, Moderate quality of evidence).
WHAT ARE THE KEY ELEMENTS TO INCLUDE IN OBTAINING INFORMED CONSENT FOR FSUI SURGERY?
FSUI is not a life-threatening condition, and treatment is aimed at improving a patient’s QoL. As such, physicians must take increased care in consenting patients for surgery, and the courts may hold a physician at a higher standard of disclosure in such cases. The online version of this guideline document and the Canadian Medical Protective Association website have more detailed information on this subject.59
The essential requirements of a valid consent process are that 1) it is voluntary; 2) the patient has adequate capacity to participate in the process; and 3) the patient is properly informed through the process. Key elements to include in the discussion are summarized in Tables 3 and 4.
Table 3.
Key elements to disclose in consent discussion for FSUI
| Element | Notes |
|---|---|
| Alternatives to surgery (inform patient and offer prior to surgery) | Such as no treatment, absorbent products, lifestyle modifications, PFMT, pessaries |
| Surgical options | Bulking, midurethral sling (MUS), retropubic colposuspension, fascial pubovaginal sling |
| Expected outcomes | Short- and long-term; satisfaction vs. cure |
| Usual postoperative course | Include what to expect and any restrictions/limitations |
| Potential risks | “Material” risks (those that might influence the choice to proceed by a reasonable person) must be disclosed (Table 4) Review signs/symptoms that could require early intervention (“informed discharge”) |
FSUI: female stress urinary incontinence; PFMT: pelvic floor muscle training.
Table 4.
Key material risks associated with FSUI surgery
| Generic | Procedure-specific (as applicable) |
|---|---|
|
|
FSUI: female stress urinary incontinence.
Other considerations include the FDA classification of mesh (for mesh-based procedures), surgeon’s experience, potential off-label use of products if appropriate, and any conflicts of interest.60 Patients should understand that some complications could require further intervention to resolve, and that some can have significant and permanent impact on urinary, bowel and/or sexual function and QoL. Implantation of synthetic materials for FSUI are generally intended to be permanent; surgery to remove them can result in additional morbidity. If mesh-based procedures are being considered, the current advisories and position statements regarding mesh should be discussed and made available.61
See online Appendix for additional discussion on this question.
WHAT ARE THE PRIMARY SURGICAL OPTIONS FOR FSUI?
Numerous surgical procedures have been devised and performed over the last 150 years for the treatment of FSUI; however, there are only a few surgical interventions that are commonly used and relevant to current practice. These include bulking agents, retropubic suspensions, and autologous and synthetic suburethral slings. Shared decision-making should be employed to help women select the correct surgical procedure, based on treatment goals, procedure-specific risks, and special circumstances (Table 5).
Table 5.
Characteristics of FSUI procedures
| Likelihood of cure of SUI | Recovery time | Risk of short-term complications | Long-term complications | Special circumstances | |
|---|---|---|---|---|---|
| Bulking agent | Low-moderate | Immediate | Low | Rare risk of material breakdown | Consider for women pre-childbearing, very mild stress incontinence |
| Colposuspension | Moderate-high | 4–6 weeks | Moderate | Enterocele, overactive bladder | Consider when doing a concurrent abdominal operation, or no vaginal access due to stenosis or limb contractures |
| Retropubic midurethral sling | High | 2–4 weeks | Low | Mesh exposure or extrusion, chronic pain, overactive bladder | |
| Obturator midurethral sling | High | 2–4 weeks | Low | Mesh exposure or extrusion, chronic pain, overactive bladder | Consider when retropubic access is contraindicated (i.e., renal transplant, complex abdominal wall, known bowel adhesions in pelvis, neobladder) |
| Autologous bladder neck fascial sling | High | 4–6 weeks | Moderate | Voiding dysfunction, overactive bladder | Consider for severe sphincteric deficiency, prior mesh complications, or when required during current repair of urethrovaginal fistula or diverticulum |
FSUI: female stress urinary incontinence.
Bulking agents are a minimally invasive treatment option for SUI. Their mechanism of action is hypothesized to result from increasing the power of the urethral sphincter by increasing the sarcomere length of the sphincteric muscle fibers.62 The quality of evidence supporting the use of bulking agents is limited; in general, bulking agents are less effective than other surgical procedures (relative risk [RR ] 0.70, 95% confidence interval [CI] 0.53–0.92), and the CI around the difference in complications compared to surgical procedures is wide (RR 1.30, 95% CI 0.30–5.66).63 Clinical experience, however, would suggest that these procedures have a low risk of complications, and are associated with a rapid recovery.64 Long-term durability of bulking agent procedures may be limited, and repeat procedures are commonly needed.65
Retropubic suspensions (or colposuspensions) have undergone several modifications but essentially, they support the bladder neck through fixation of the periurethral vaginal tissue to the pelvic bone or iliopectineal ligament through an abdominal approach. The technique can be performed laparoscopically to help reduce the morbidity associated with an abdominal incision.
A 2019 Cochrane review found that laparoscopic vs. open colposuspension had an equivalent success rate (RR 1.04, 95% CI 0.99–1.08) but a lower risk of perioperative complications (RR 0.67, 95% CI 0.47–0.94); outcomes and complication rates were similar in frequency (but qualitatively different) between laparoscopic colposuspension and synthetic midurethral slings (MUS).66 Smaller case series of robotic colposuspension have also been published, with similar results to laparoscopic procedures.67
Common suburethral slings include midurethral mesh slings (the retropubic tension-free vaginal tape [TVT] and the transobturator transvaginal tension-free vaginal tape-obturator [TVT-O]), and autologous fascial slings (usually placed at the bladder neck with sutures passed through the retropubic space and tied over the abdominal fascia). Midurethral mesh slings are based on the theory that FSUI results from laxity of the pubourethral ligaments, resulting in hypermobility; the mesh sling placed at the midurethra recreates this anatomic support.68
Midurethral mesh slings reduced the morbidity and complexity of FSUI surgery, and in numerous RCTs, the retropubic and obturator approaches have similar subjective efficacy both at one year (RR 0.97, 95% CI 0.87–1.09) and five years (RR 0.95, 95% CI 0.87–1.04),69 although some analyses have suggested that there is a slight advantage to the retropubic approach.70 Objective efficacy at one year for the retropubic or obturator midurethral mesh sling is 81% vs. 78% based on a high-quality RCT.71 Pain can be a complication of both approaches, occurring more commonly in the groin in obturator sling patients (6.4%) vs. retropubic patients (1.3%), and more commonly in the suprapubic region in retropubic sling patients (2.9%) vs. obturator patients (0.8%).69 Autologous slings use rectus fascia or fascia lata to create a sling that is placed at the bladder neck. These procedures can also be performed using segments of mesh. A 2020 Cochrane review found that this surgical approach may be less successful than midurethral mesh slings (odds ratio [OR ] 0.67, 95% CI 0.44–1.02), with medium-term followup of 1–5 years, and has a higher risk of complications (RR 1.74, 95% CI 1.16–2.60).
WHAT IS THE ROLE OF PERIURETHRAL BULKING THERAPY IN SUI MANAGEMENT
Clinical data remains heterogeneous, often involves small trials of low-to-moderate quality, and frequently lack a common objective outcome measure to evaluate effectiveness. Unfortunately, the overall evidence base is insufficient to guide clinical practice, as no clear conclusions can be drawn from multiple separate trials comparing different agents.72
Within the contemporary treatment options for SUI, bulking agents are more effective than PFMT but less effective than surgical management.72,73 Although objective and subjective cure rates are inferior to MUS, patient satisfaction is still high, and the risk of morbidity is low.63,74 Patients should be counselled that efficacy is variable, repeat injections are typically required, and long-term durability has mixed supportive evidence.75,76
Appropriate possible index patients for periurethral bulking therapy (PBT) include those with mild-to-moderate-volume SUI (<3 pads per day), those who may desire future pregnancy, those who are not candidates for more invasive surgery, and women who accept a lower likelihood of success. PBT is also a good option in non-index patients who are not good candidates for surgery, have failed other surgeries, are of advanced age, or who empty their bladder poorly.65,77
Contraindications to urethral bulking agents include a history of hypersensitivity to the bulking agent and active UTI. Potential adverse events include UTI, injection site pain, transient urinary retention, temporary dysuria, hematuria, de novo urgency incontinence, bulking agent extrusion, immune reaction, rare granuloma formation, and possible abscess and pseudocyst formation.65,76,78
■ RECOMMENDATION 14
Physicians may offer periurethral bulking agents as a less invasive treatment option for select patients with mild-to-moderate-volume SUI (Weak recommendation, weak quality of evidence).
WHAT IS THE ROLE OF MUS IN MANAGing THE INDEX PATIENT WITH FSUI? WHAT ARE THE KEY FACTORS THAT DETERMINE SUCCESS?
MUS operations consist of the passage of a small strip of synthetic mesh through either the retropubic (top-down or bottom-up) or obturator space (inside-out or outside-in), with entry or exit points at the lower abdomen or groin, respectively. Variations include the single-incision sling and adjustable slings. The largest systematic review included 55 trials with a total of 8652 patients with SUI or stress-predominant MUI.69 Subjective and objective cure rates were assessed at short (≤1 year), medium (1–5 years), and long-term (>5 years) for all retropubic and transobturator approaches.
The rate of subjective cure of transobturator and retropubic MUS in the short-term were similar, ranging from 62–98% in the transobturator group, and from 71–97% in the retropubic group. Short-term objective cure was similar in the transobturator and retropubic groups. In the long-term, subjective cure rates ranged from 43–92% in the transobturator group, and from 51–88% in the retropubic group.69,79–81
Overall morbidity was low in both groups, with complications including vascular/visceral injury, bladder/urethral perforation, postoperative voiding dysfunction, urgency and urgency urinary incontinence, vaginal mesh exposure, suprapubic or groin pain, and need for further treatment. The risks of prosthesis complications specific to the use of mesh, such as exposure (vaginal, urethral, bladder) and mesh-related pain, should be explicitly discussed with the patient prior to its surgical consideration. The difficulty in removing mesh material, particularly for the transobturator sling, should weigh in the decision-making process.
Predictors of failure
In a meta-analysis, objective success rates of MUS were higher in normal-weight patients vs. overweight or obese patients but the subjective outcomes were not different.82 The presence of intrinsic sphincter deficiency (ISD) or a minimally mobile urethra as a predictor of failure is conflicting across studies due to the inconsistency in methodology and definition of ISD ; however, a few studies suggest a higher rate of failure in patients with ISD with the transobturator sling.83,84 In cases of a non-mobile urethra, it may be more judicious to consider other alternatives to MUS, such as a pubovaginal sling, transurethral bulking agent, or artificial urinary sphincter (AUS).
Contraindications
MUS synthetic mesh should not be used in settings that increase the likelihood of exposure or perforation due to surgical factors or patient factors. For this reason, a mesh MUS should not be placed concomitant to a surgery where the urethra’s integrity may be affected, such as urethral diverticulectomy, repair of urethrovaginal fistula, or urethral mesh excision. As impaired wound healing conditions may increase the likelihood of mesh exposure, physicians should exercise caution when considering mesh MUS in patients with previous radiation, immunosuppression or autoimmune conditions, chronic steroid use or significant periurethral fibrosis. Finally, a woman may choose not to have a mesh-based procedure for SUI, in which case, alternative non-mesh-based procedures should be reviewed.
■ RECOMMENDATION 15
A MUS (retropubic or transobturator) should be offered to index patients with SUI following a careful informed consent process (Strong recommendation, High quality of evidence).
■ RECOMMENDATION 16
A transobturator MUS should not be offered in patients with minimal urethral mobility (Weak recommendation, Weak quality of evidence).
■ RECOMMENDATION 17
A MUS (retropubic or transobturator) should not be placed in cases where the urethral integrity is impaired (Clinical principle).
IS THERE A SUPERIOR MUS ROUTE OR PRODUCT?
Comparing MUS options is challenging, given the heterogeneity in sling materials and implantation methods, as well as in study design and outcomes measured.
Retropubic vs. transobturator sling placement
Results from studies comparing surgical techniques for the placement of retropubic and transobturator slings do not conclusively demonstrate a benefit of one technique over the other.69
Studies with a shorter followup period (<3 years) generally do not find a significant difference in treatment outcomes between retropubic and transobturator slings. Slings placed in a retropubic position may confer better long-term outcomes, although data supporting these findings are limited. Complication rates vary between the sling techniques, with retropubic slings generally conferring a slightly higher risk of voiding/bladder storage issues post-procedure compared to increased leg/groin pain with transobturator slings, although in general, complications rates are low with both.85–87
Single-incision slings vs. retropubic and transobturator slings
A recent pragmatic, non-inferiority RCT comparing single-incision slings to transobturator/retropubic slings found that single-incision slings were non-inferior with respect to patient-reported outcomes at three years;88 however, long-term data demonstrating the efficacy and safety of single-incision slings are lacking. As such, Health Canada considers single-incision slings as a novel treatment for SUI and patients should be counselled appropriately regarding this treatment modality.89
■ RECOMMENDATION 18
In some situations, either the retropubic MUS (for example, a prior failed MUS) or the transobturator MUS (for example, with a renal transplant or complex retropubic anatomy, such as after a neobladder) may be preferrable (Clinical principle).
■ RECOMMENDATION 19
Physicians may offer single-incision slings for the index patient; however, patients should be informed that long-term data on this procedure is lacking (Weak recommendation, Moderate quality of evidence).
WHAT IS THE ROLE OF AUTOLOGOUS FASCIAL SLINGS IN TREATING FSUI?
The autologous fascial sling (AFS) has been used to treat FSUI for almost 100 years. The most used technique was described by be offered by Dr. McGuire, wherein a segment of rectus fascia or fascia lata (approximately 10×2 cm) is placed under the urethrovesical junction. Sutures through the ends of the sling are passed through the space of Retzius and tied over the suprapubic abdominal fascia.89 This results in dynamic support and static elevation of the bladder neck.
The unique risks related to this procedure are due primarily to the fascial harvest (wound infection, seroma, hernia) and the mechanism of action, where a degree of obstruction increases the risk of urinary retention or voiding dysfunction.90,91 Sling implantation requires a degree of experience to place and tension the sling properly. This is critical to success and avoiding urinary retention/voiding dysfunction. Methods of tensioning, such as using a Q-tip in the urethra and tying the sling so that it is elevated to 0–10 degrees, tying the sutures with a rigid cystoscope in place and tilted down 20–30 degrees, or tying the knot over two-three fingers placed on the abdominal wall have been described.
Outcomes are generally similar to MUS and slightly superior to Burch colposuspension procedures.92 Authors have reported using a smaller piece of fascia (“sling-on-a-string”) with equivalent results to the traditional fascial harvest.93
■ RECOMMENDATION 20
AFS may be offered to index patients with SUI following a careful informed consent process (Weak recommendation, Weak quality of evidence).
■ RECOMMENDATION 21
Patients who desire (or have a medical reason) to avoid mesh-based procedures and non-index patients, such as those without evidence of hypermobility (for example, those who have failed previous SUI procedures), those with previous mesh complications, patients undergoing complex repairs (such as concomitant fistula/urethral diverticulum repair), and those with neurologic diseases who use clean intermittent catheterization and require a compressive sling may be offered an AFS over a mesh MUS (Clinical principle).
WHAT IS THE ROLE OF RETROPUBIC COLPOSUSPENSION IN TREATING FSUI?
A retropubic colposuspension (most commonly Burch colposuspension) is performed by placing sutures in the paravaginal fascial lateral to the urethra to stabilize the bladder neck and proximal urethra in a fixed retropubic position; it can be performed either open or laparoscopically.94 These surgeries have reported cure rates of 69–88% in women with SUI over 1–5 years.95
In the index patient, retropubic colposuspension has inferior outcomes compared to pubovaginal slings in several studies (RR of failure of 1.35, 95% CI 1.11–1.64 with medium-term followup); the same Cochrane review suggested that colposuspension outcomes were similar to MUS. There may be a higher risk for future prolapse surgery after retropubic colposuspension compared to pubovaginal or MUS procedures (3.3% vs. 1.1%, p=0.01).96 This is balanced with a lower risk of voiding dysfunction (2% vs. 14% p<0.001), UTI (32% vs. 48%), and retention (OR 0.13, 95% CI 0.05–0.30) compared to the pubovaginal sling.97,98
Contraindications to retropubic colposuspension include inadequate vaginal length or mobility of vaginal tissues (which may be due to prior vaginal surgery, radiotherapy, or prior vaginal incontinence procedures) or inability to safely access the retropubic space. The efficacy of retropubic colposuspension relies on urethral mobility; thus, it is not recommended for patients with ISD.99
■ RECOMMENDATION 22
Retropubic colposuspension may be offered to index patients with SUI following a careful informed consent (Weak recommendation, High quality of evidence).
■ RECOMMENDATION 23
Retropubic colposuspension should be offered to appropriate patients: 1) who are undergoing laparotomy/laparoscopy for concomitant abdominal surgery; 2) who have limited vaginal access or ability to position in lithotomy; or 3) who are unwilling or unable to have a MUS or AFS procedure (Clinical principle).
HOW SHOULD THE PATIENT WITH STRESS-PREDOMINANT MIXED INCONTINENCE BE COUNSELLED PRIOR TO OPERATIVE INTERVENTION?
MUS offer high cure rates for the SUI component of MUI; however, overall subjective cure rates are low in women with MUI (56%); cure of the urge urinary incontinence (UUI) occurs in 30–85% initially, however, the UUI often recurs over time.100 Preoperative counselling is of utmost importance to discuss the patient’s treatment goals and set realistic expectations, since the persistence of UUI may lead to dissatisfaction with procedural results.101
De novo OAB arises following approximately 9% of MUS surgeries, and pre-existing urgency persists in 15–70%.100,102 Old age, obesity, parity, nocturia, and OAB-related preoperative UDS findings, such as detrusor overactivity and low bladder capacity, have been suggested as risk factors.103,104 Therefore, when invasive therapy is being considered, UDS may help pre-intervention counselling and decision-making.
A pragmatic, individualized approach is necessary for women with MUI that will likely involve a combination of OAB and SUI treatment modalities.105 As medical therapy for OAB can be easily discontinued if women experience side effects or lack of efficacy, it is a logical starting point for women with MUI, although efficacy is quite low among those with a strong stress-predominant incontinence history.106,107 Surgical correction of SUI is recommended for the management of stress-predominant MUI.108 The patient needs to clearly understand that the surgical procedure is designed to treat the stress component, that the overall cure/satisfaction rate is lower, and that they may need further treatment for UUI postoperatively.107
HOW SHOULD A YOUNG WOMAN WHO DESIRES TO HAVE CHILDREN IN THE FUTURE BE COUNSELLED ABOUT HER TREATMENT OPTIONS FOR SUI?
The prevalence of lower urinary tract symptoms in nulliparous women ranges from 8–32%, with 7% documenting significant bother.109 Younger women are often interested in managing their SUI in their reproductive years. Treatment for these women should begin with non-surgical modalities, as outlined in Table 2. PFMT can reduce urinary incontinence during pregnancy and after delivery; for example, at 36 weeks, 32% of patients doing PFMT reported urinary incontinence compared to 48% of those who were not.110–112
Despite historical teaching, there is minimal evidence to support withholding surgical correction of SUI in patients contemplating future pregnancies. Studies have documented good continence outcomes in women who deliver following MUS, and many of these women also experienced satisfactory continence throughout pregnancy.113–116 Similarly, one retrospective study on patients with a previous pubovaginal sling demonstrated unchanged incontinence in eight out of nine women post-delivery.117 Rare complications, such as urinary retention, may occur, with one case study documenting the need for mesh transection during pregnancy due to refractory urinary retention.116
■ RECOMMENDATION 24
Physicians should employ shared decision-making and offer appropriate conservative and surgical options to women who are not done their childbearing (Clinical principle).
HOW IS FEMALE SEXUAL FUNCTION IMPACTED BY FSUI AND ITS TREATMENTS?
Sexual function is an important aspect of well-being, and some women undergoing treatment for SUI do so in the hope that it will improve their sexual function. Improvements are believed to be due to the cessation of coital incontinence, reduction in anxiety, and improved self-image.118
In a secondary analysis from the data obtained from the original SISTEr and TOMUS trials, the relationship to postoperative sexual function of 924 women who had undergone one of the four standard surgical interventions for SUI (Burch colposuspension, AFS, retropubic and transobturator slings) was evaluated. The authors concluded that there is an overall clinically meaningful improvement in sexual function two years after anti-incontinence surgery, independent of the type of procedure performed;119 however, a systematic review and meta-analysis, which included 18 studies and analyzed 1578 women, reported unchanged sexual function after surgery for a majority of patients (55.5%), and it did not report a difference between the retropubic and transobturator sling approaches.120
Worsening of sexual function after MUS is less common but possible. The surgery can lead to adverse effects, such as altered clitoral blood flow, modified vaginal anatomy (fibrosis, cord effect, narrowing), de novo dyspareunia, or genital discomfort for the male partner (hispareunia).121 Some anecdotal cases of anorgasmia have even been described after retropubic sling, hypothetically explained by trauma or irritation of the dorsal nerve of the clitoris during passage of the trocar.122 Data on the effect of bulking agents on overall sexual health is limited.
■ RECOMMENDATION 25
Physicians should counsel patients that sexual function after FSUI surgery may improve or remain the same, and there is a risk of uncommon complications that can negatively affect sexual function (Clinical principle).
HOW SHOULD A WOMAN WHO DEMONSTRATES AN ABDOMINAL VOIDING PATTERN BE COUNSELLED ABOUT HER TREATMENT OPTIONS FOR FSUI?
See online Appendix for the supporting text for the recommendations.
■ RECOMMENDATION 26
Patient with Valsalva voiding considering surgical interventions for FSUI should be counselled on a possible increased risk of urinary retention/worsening voiding dysfunction after surgery, and a possible delayed return to baseline voiding (Clinical principle).
■ RECOMMENDATION 27
Physicians should not rely on preoperative urodynamic parameters to predict postoperative voiding dysfunction (Weak recommendation, Low quality of evidence).
HOW SHOULD A WOMAN WITH URETHRAL DIVERTICULUM AND HISTORY OF SUI BE COUNSELLED ABOUT TREATMENT OPTIONS FOR SUI?
See online Appendix for the supporting text for the recommendations.
■ RECOMMENDATION 28
If a physician identifies a urethral diverticulum (UD) during the workup of FSUI, it should be excised transvaginally before surgically addressing the FSUI component123 (Clinical principle).
■ RECOMMENDATION 29
If a woman has significant preoperative SUI (not postvoid dribbling), the physician may offer simultaneous urethral diverticulectomy and a non-mesh incontinence procedure; however, there is a limited role for an anti-incontinence surgery to prevent de novo SUI, as approximately 60% of patients will have resolution of de novo SUI over time124 (Clinical principle).
HOW SHOULD RECURRENT FSUI AFTER MUS BE INVESTIGATED AND MANAGED?
Approximately 5% of patients by 5–10 years will go on to repeat surgical intervention for SUI.125–131 Proposed risk factors for failure include prior SUI surgery, more severe incontinence, older age, high BMI, ISD, mixed incontinence, type of sling (single-incision and transobturator MUS vs. retropubic slings), and surgical centre volume.125,127,128,132–137
In patients presenting with incontinence and a history of prior MUS surgery, one must first determine the nature of the incontinence (stress or urge, mixed, or overflow incontinence). The patient is re-evaluated with a careful history and examination. It is important to confirm that SUI was present prior to the original surgery and to review the operative records; the degree and duration of success following the initial MUS should be determined. One should next rule out complications of previous surgery, such as obstruction or mesh exposure. UDS and cystoscopy are usually necessary.
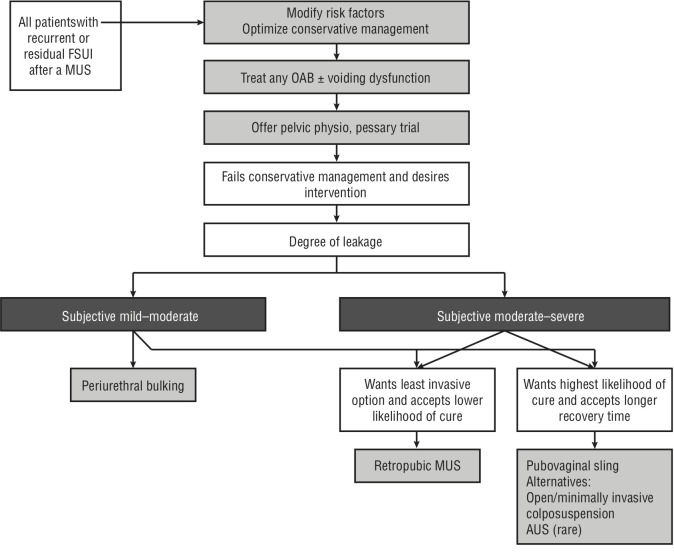
Management in all cases begins with establishing the patient’s goals of treatment, reinforcing conservative measures, and addressing any modifiable risk factors and/or coexisting OAB. Patients should be offered PFMT and/or a pessary trial.138 For patients interested in surgical management, a shared decision-making approach should be undertaken according to the algorithm proposed in Figure 1. Details of individual treatments are available in the full version online. No high-quality studies allow comparison of different treatments as secondary treatments, so guidance is based on the expert opinion of the panel and other authors and organizations.132,139–144 Salvage surgery may be associated with lower success rates, especially in those with more bothersome symptoms, and higher surgical risk than primary surgery.145–148 Referral to a tertiary center should be considered on an individual basis.144
Figure 1.
Suggested management approach for isolated residual or recurrent female stress urinary incontinence (FSUI) following a single prior midurethral sling (MUS). AUS: artificial urinary sphincter; OAB: overactive bladder.
WHAT IS THE APPROPRIATE MANAGEMENT OF URINARY RETENTION AFTER SURGERY FOR FSUI?
Postoperative urinary retention following surgery for FSUI occurs in 2.5–43% of cases.149 Predictive factors may include older age, preoperative UDS findings of detrusor underactivity and Valsalva voiding (which may be available, particularly for the non-index FSUI patient that undergoes UDS), intraoperative fluid administration ≥750 mL and bladder volume ≥270 mL on entry to the post-anesthesia care unit, but not anesthetic type.150–152
Initial management involves immediate catheterization followed by a trial of void. The incidence of postoperative urinary retention beyond four weeks is low (2–4%). Options for managing continued postoperative urinary retention include indwelling or intermittent catheterization, reopening the vaginal incision and pulling down on the sling, loosening the sling sutures, sling incision, or partial sling removal with urethrolysis.
Some authors advocate for early incision of the sling, as this leads to decreased retention rates and reversal of associated urgency symptoms; this is also associated with minimal morbidity.153 Other experts advocate for loosening the sling within one week of insertion.153 Five-year outcomes of transection of MUS for postoperative urinary retention led to 73.9% continence rate, better flow, higher voided volumes, and lower residual volumes in 23 patients.154 In a study comparing sling incision to intermittent catheterization for urinary retention, those who underwent transection of the sling were less likely to have a stress incontinence and had better QoL outcomes.155
■ RECOMMENDATION 30
Physicians should manage postoperative urinary retention after FSUI surgery with 1–2 days of catheterization and a repeat trial of void. In patients who have continued postoperative urinary retention, early sling loosening (within one week) or sling incision (at 4–6 weeks) is recommended when possible (Clinical principle).
HOW SHOULD WORSENING/NEW OAB AFTER SLING SURGERY BE MANAGED?
OAB symptoms are common after sling surgery and can have a negative impact on patient’s QoL. Patients with MUI can experience persistence or aggravation of OAB symptoms, whereas patients with pure SUI can develop de novo urgency with or without UUI. The pathogenesis of de novo OAB remains unclear, the time frame of symptom onset can be quite variable, and, in some patients, symptoms can arise even several years after surgery.
When facing new/worsening OAB after surgery, initial evaluation should attempt to rule out an underlying reversible etiology (Table 6).103 Surgical treatment of any remediable conditions was reported in 28–64% of patients.156 If no remediable cause is found, the initial management of worsening/new OAB is essentially the same as idiopathic OAB.106
Table 6.
Suggested investigations and potential etiologies to new/worsening OAB following SUI surgery
| Investigation | Possible etiology causing OAB |
|---|---|
| History and physical | Constipation Local hematoma |
| Urinalysis ± urine culture | Urinary tract infection |
| Cystoscopy | Surgical complication Foreign body Mesh exposure/stone formation in urethra or bladder |
| Uroflow/Postvoid residual | Urinary retention |
| Urodynamics | Bladder outlet obstruction Dysfunctional voiding Impaired detrusor contractility |
OAB: overactive bladder; SUI: stress urinary incontinence.
In relation to type of MUS, the incidence of de novo OAB was 9.7% for mini-slings, 8.7–11.2 % for transobturator, and 9.8% for retropubic slings, with no significant differences between groups.102 The incidence of de novo urgency was 0–29% for retropubic MUS, 0.7–25% for transobturator MUS, and 4.3–12.2% for single-incision MUS.157
Risk factors for the development of de novo OAB include older age, early bladder sensation, lower bladder capacity, elevated detrusor pressure, and preoperative urgency.103,158,159 Although not required preoperatively for most patients, UDS may aid the surgeon to identify risk factors for worsening/new OAB and improve patient counselling prior to surgery.
In cases where refractory OAB symptoms develop following SUI surgery and cannot be controlled with conservative management, a sling incision may be considered in select patients. The exact timing of such intervention is highly subjective and not well-guided by present literature. Of patients receiving sling incision primarily for refractory OAB, a 60% symptomatic cure rate was reported in retrospective analyses;160 however, appropriate preoperative patient counselling is paramount, as 60–70% of patients experienced recurrent SUI.
■ RECOMMENDATION 31
Physicians may offer a sling incision in select patients with OAB following sling surgery, including those with bladder outlet obstruction or refractory OAB symptoms (Weak recommendation, Low quality of evidence).
HOW SHOULD PAIN AFTER TRANSVAGINAL MUS BE INVESTIGATED AND MANAGED?
Evaluation of women experiencing pain after MUS begins with a detailed history, including the type of procedure performed (ideally by obtaining the operating room report) and documenting if incontinence has resolved/reoccurred.161 Questions regarding the musculoskel etal system (hips, back, and pelvis), bowel function, and menstrual cycles, if relevant, are necessary.161 Pain should be described as accurately as possible and its timing in relation to insertion of the MUS needs to be determined.156
Patients require a focused abdominal, pelvic, and perineal examination, including palpation of the midpubis, groin/thigh crease, and vaginal course of the MUS. A cystoscopy is required to rule out any bladder/urethral mesh exposure. Imaging with magnetic resonance imaging or a transperineal or transvaginal ultrasound may help locate the mesh tract but are optional and may not change management.162
Initial treatment should be conservative. PFMT (if there is a myofascial component), heat, non-steroidal anti-inflammatory drugs, muscle-relaxants, amitriptyline, neuromodulatory medications (such as gabapentin or pregabalin), and a trial of local block using a combined steroid and local anaesthetic agent can be considered.163,164
Partial or complete surgical removal of mesh may be considered if there is no or minimal improvement after trial of conservative measures; however, this should be performed in experienced centers. Preoperative counselling should highlight the fact that despite improved symptoms for approximately 75% of well-selected patients, some degree of pain may remain and recurrence of SUI is common.163 A recent systematic review found no difference between partial or total mesh removal regarding improvement in pain outcomes, with a postoperative risk of recurrent SUI lower with partial vs. total mesh removal.165,166
WHAT IS THE RISK OF VAGINAL MESH EXPOSURE OR EXTRUSION AFTER MUS? HOW SHOULD IT BE MANAGED?
According to the International Urogynecological Association (IUGA)/ICS Joint Terminology and Classification Report, vaginal complications can range from an epithelial separation to larger exposure or extrusion of mesh. When there is no epithelial separation, the terms prominence (e.g., due to wrinkling or folding), epithelial penetration (without epithelial separation), or contraction (shrinkage) can be used. An exposure is a condition of displaying, revealing, exhibiting, or making accessible (e.g., vaginal mesh visualized through separated vaginal epithelium). An extrusion is passage gradually out of a body structure or tissue.167
In a Cochrane review of 31 trials, the rates of MUS vaginal erosion/exposure/extrusion was low, with 24/1000 instances with the transobturator route compared to 21/1000 with the retropubic route (RR 1.13, 95% CI 0.78–1.65; 31 trials, 4743 women; moderate quality evidence).69 These complications continue to present over time, with studies showing they can present even years after the initial surgery. Possible risk factors are trocar injury, longer vaginal incision, bleeding, diabetes, older age, and smoking.168,169
Other factors that may prevent poor wound healing can predispose to vaginal exposure, such as previous radiation, scar tissue, severe atrophy, immunosuppression, and collagen or autoimmune disorders. Preoperative vaginal estrogen does not impact the incidence of mesh exposure.170,171 Minimizing the risk of mesh exposure perioperatively involves careful tissue handling, hemostasis, minimal tension, and careful inspection for vaginal mucosa perforation.
Patients presenting with this problem should be evaluated with a careful pelvic exam and cystourethroscopy. When possible, old operative reports should be requested. Observation and non-surgical management with local estrogen therapy (6–12 weeks) can be offered to small, minimally symptomatic mesh exposures (although rates of cure with this approach are low).
Local excision in the office can be attempted for small, easily visible exposures. Larger or more symptomatic extrusion should be addressed surgically.172 A retrospective cohort study found that partial sling excision of the suburethral portion and vaginal repair have better outcomes, with less recurrence of sling exposure compared with simple closure.173 Complete removal for mesh exposure resulted in higher rates of postoperative SUI compared to partial removal.166
WHAT IS THE RISK OF MESH EXPOSURE IN THE URINARY TRACT AFTER MUS? HOW SHOULD IT BE MANAGED?
This complication can present years after sling placement, and in some cases, may be asymptomatic and discovered incidentally (suggesting that this complication is likely under-detected). Symptoms such as frequent UTI s, hematuria, urethral pain, and lower urinary tract symptoms are common presentations.174 A Cochrane review of MUS outcomes found little evidence of this complication in the identified randomized trials, and estimated that urinary mesh exposure occurs in <1% of women.69 The risk factors specifically related to urinary exposure of mesh are not well-defined; however, technical mistakes during the procedure, such as placing the MUS in the setting of a urethral injury, improper urethral dissection, lack of intraoperative cystoscopy, or excessive sling tension may be significant.175
The optimal management of these complications is not well-defined. Endoscopic intervention is suitable when there is no significant tissue infection, fistula, or other associated mesh complications (such as diffuse vaginal/pelvic pain or vaginal exposure); this approach has low morbidity and excellent success rates of 92% in well-selected patients.176,177
Transvaginal mesh removal and urethral repair is an alternative treatment approach that is likely associated with more potential morbidity and a higher risk of post-procedure stress incontinence.165,178 To lessen morbidity, a staged primary endoscopic excision/release may be considered prior to open transvaginal excision. In most cases, management of this complication should be referred to an appropriate specialist with experience managing transvaginal mesh complications.
SHOULD YOU REMOVE/REVISE A SLING IN A WOMAN WITH REPEATED UTIS AFTER MUS OR AFS?
New-onset, repeated UTIs occur in 2–7% of women after a MUS, and older age and post-MUS urinary retention are significant risk factors.79 If a patient had recurrent UTIs prior to SUI surgery and the same pattern continues, then logically revising the sling is unlikely to help. A few things must be addressed if the recurrent UTIs are new:
Is the patient obstructed?
Is there any evidence of mesh exposure into the urinary tract?
Once obstruction and mesh exposure have been ruled out, guidelines-based repeated UTI treatment and prevention strategies should be employed.179
■ RECOMMENDATION 32
Due to the potential operative risks of sling removal and the high likelihood of FSUI recurrence, physicians should not recommend sling removal for repeated UTIs in the absence of confirmed related complications (such as obstruction or mesh exposure) (Strong recommendation, Low quality of evidence).
DOES THE USE OF MESH-BASED MUS CAUSE AUTOIMMUNE DISEASES OR CANCER?
See online Appendix for the supporting text for the recommendation.
■ RECOMMENDATION 33
Physicians should counsel patients who ask that there is no evidence mesh MUS are associated with an increased risk of cancer or autoimmune disease (Clinical principle).
Supplementary Information
ACKNOWLEDGEMENT
The authors wish to thank Dr. Phil Violette for his helpful review of the guidelines and assistance with the implementation of the GRADE framework within these guidelines.
Footnotes
Please note, this is the abbreviated version. Additional material is available as an Appendix at cuaj.ca
COMPETING INTERESTS: Dr. Carlson has been an advisory board member for AbbVie and received honoraria for CME development. Dr. Andrews has been an advisory board member for AbbVie. Dr. Baverstock has been a speaker for Astellas and Boston Scientific; and an advisory board member/speaker for AbbVie. Dr. Nadeau has been an advisory board member for AbbVie and Astellas; a consultant for AbbVie, Boston Scientific, and Searchlight Pharma; and a speaker for AbbVie and Laborie. Dr. Welk has been a consultant for BD Company. The remaining authors do not report any competing personal or financial interests related to this work.
Contributor Information
Ashley Cox, Department of Urology, Dalhousie University, Halifax, NS.
Laura Nguyen, Department of Surgery, McMaster University, Hamilton, ON, Canada.
REFERENCES
- 1.Aoki Y, Brown HW, Brubaker L, et al. Urinary incontinence in women. Nat Rev Dis Primers. 2017;3:17042. doi: 10.1038/nrdp.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milsom I, Altman D, Cartwright R, et al. Epidemiology of urinary incontinence (UI) and other lower urinary tract symptoms (LUTS), pelvic organ prolapse (POP) and anal (AI) incontinence. In: Abrams P, Wagg A, Wein A, editors. International Consultation on Incontinence, C.L. International Continence Society; Bristol: 2017. pp. 1–142. [Google Scholar]
- 3.Bettez M, Tu le M, Carlson K, et al. 2012 update: Guidelines for adult urinary incontinence collaborative consensus document for the Canadian Urological Association. Can Urol Assoc J. 2012;6:354–63. doi: 10.5489/cuaj.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nambiar AK, Arlandis S, Bo K, et al. European Association of Urology guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur Urol. 2022;82:49–59. doi: 10.1016/j.eururo.2022.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline. J Urol. 2017;198:875–83. doi: 10.1016/j.juro.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Schünemann H, Brożek J, Guyatt G, et al. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2013. Feb 23, 2021. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
- 7.Khullar V, Amarenco G, Doumouchtsis SK, et al. Imaging, Neurophysiological Testing and Other Tests. In: Abrams P, et al., editors. Incontinence. 6th Edition. International Continence Society; Bristol, UK: 2017. pp. 671–804. [Google Scholar]
- 8.van Leijsen SA, Hoogstad-van Evert JS, Mol BW, et al. The correlation between clinical and urodynamic diagnosis in classifying the type of urinary incontinence in women. A systematic review of the literature. Neurourol Urodyn. 2011;30:495–502. doi: 10.1002/nau.21047. [DOI] [PubMed] [Google Scholar]
- 9.Brucker BM, Fong E, Kaefer D, et al. Urodynamic findings in women with insensible incontinence. Int J Urol. 2013;20:429–33. doi: 10.1111/j.1442-2042.2012.03146.x. [DOI] [PubMed] [Google Scholar]
- 10.Løwenstein EP, Andersen LL, Møller LA, et al. Urodynamic and questionnaire findings in urinary incontinent women with and without diabetes. Data from a health study. Int Urogynecol J. 2021;32:2847–56. doi: 10.1007/s00192-021-04950-4. [DOI] [PubMed] [Google Scholar]
- 11.Swavely NR, Speich JE, Klausner AP. Artifacts and abnormal findings may limit the use of asymptomatic volunteers as controls for studies of multichannel urodynamics. Minerva Urol Nephrol. 2021;73:655–61. doi: 10.23736/S2724-6051.20.03838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broekhuis SR, Kluivers KB, Hendriks JC, et al. Reproducibility of same session repeated cystometry and pressure-flow studies in women with symptoms of urinary incontinence. Neurourol Urodyn. 2010;29:428–31. doi: 10.1002/nau.20783. [DOI] [PubMed] [Google Scholar]
- 13.Digesu GA, Hutchings A, Salvatore S, et al. Reproducibility and reliability of pressure flow parameters in women. BJOG. 2003;110:774–6. doi: 10.1111/j.1471-0528.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- 14.Rahmanou P, Chaliha C, Kulinskaya E, et al. Reliability testing of urodynamics, pressure flow studies and cough leak point pressure in women with urodynamic stress incontinence with and without detrusor overactivity. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:933–8. doi: 10.1007/s00192-008-0567-1. [DOI] [PubMed] [Google Scholar]
- 15.Nager CW, Brubaker L, Litman HJ, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987–97. doi: 10.1056/NEJMoa1113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rachaneni S, Latthe P. Does preoperative urodynamics improve outcomes for women undergoing surgery for stress urinary incontinence? A systematic review and meta-analysis. BJOG. 2015;122:8–16. doi: 10.1111/1471-0528.12954. [DOI] [PubMed] [Google Scholar]
- 17.Serati M, Braga A, Torella M, et al. The role of urodynamics in the management of female stress urinary incontinence. Neurourol Urodyn. 2019;38:S42–50. doi: 10.1002/nau.23865. [DOI] [PubMed] [Google Scholar]
- 18.Sirls LT, Richter HE, Litman HJ, et al. The effect of urodynamic testing on clinical diagnosis, treatment plan and outcomes in women undergoing stress urinary incontinence surgery. J Urol. 2013;189:204–9. doi: 10.1016/j.juro.2012.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leijsen SA, Kluivers KB, Mol BW, et al. Can preoperative urodynamic investigation be omitted in women with stress urinary incontinence? A non-inferiority randomized controlled trial. Neurourol Urodyn. 2012;31:1118–23. doi: 10.1002/nau.22230. [DOI] [PubMed] [Google Scholar]
- 20.Ayeleke RO, Hay-Smith EJ, Omar MI. Pelvic floor muscle training added to another active treatment versus the same active treatment alone for urinary incontinence in women. Cochrane Database Syst Rev. 2013:CD010551. doi: 10.1002/14651858.CD010551.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi: 10.1002/14651858.CD005654.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumoulin CAT, Booth J, Bradley C, Burgio K, Hagen S, et al. Adult conservative management. In: Abrams P, Wagg A, Wein A, editors. Incontinence 6th International Consultation on Incontinence, C.L. International Continence Society; Bristol: 2017. pp. 1443–628. [Google Scholar]
- 23.Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2000;2000:CD002113. doi: 10.1002/14651858.CD002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay-Smith EJ, Herderschee R, Dumoulin C, et al. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2011;12:CD009508. doi: 10.1002/14651858.CD009508. [DOI] [PubMed] [Google Scholar]
- 25.Herbison GP, Dean N. Weighted vaginal cones for urinary incontinence. Cochrane Database Syst Rev. 2013;2013:CD002114. doi: 10.1002/14651858.CD002114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herderschee R, Hay-Smith EJ, Herbison GP, et al. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2011;7:CD009252. doi: 10.1002/14651858.CD009252. [DOI] [PubMed] [Google Scholar]
- 27.Imamura M, Abrams P, Bain C, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess. 2010;14:1–188. iii–iv.. doi: 10.3310/hta14400. [DOI] [PubMed] [Google Scholar]
- 28.Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database Syst Rev. 2014;12:CD001756. doi: 10.1002/14651858.CD001756.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostaszkiewicz J, Johnston L, Roe B. Habit retraining for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2004;2004:CD002801. doi: 10.1002/14651858.CD002801.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2004;2004:CD002802. doi: 10.1002/14651858.CD002802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todhunter-Brown A, Hazelton C, Campbell P, et al. Conservative interventions for treating urinary incontinence in women: An overview of cochrane systematic reviews. Cochrane Database Syst Rev. 2022;9:CD012337. doi: 10.1002/14651858.CD012337.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: A systematic review. Obstet Gynecol. 2014;124:1147–56. doi: 10.1097/AOG.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber MA, Lim V, Oryszczyn J, et al. The effect of vaginal oestriol cream on subjective and objective symptoms of stress urinary incontinence and vaginal atrophy: An international multi-centre pilot study. Gynecol Obstet Invest. 2017;82:15–21. doi: 10.1159/000445074. [DOI] [PubMed] [Google Scholar]
- 34.Waetjen LE, Dwyer PL. Estrogen therapy and urinary incontinence: What is the evidence and what do we tell our patients? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:541–5. doi: 10.1007/s00192-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 35.Rahn DD, Ward RM, Sanses TV, et al. Vaginal estrogen use in postmenopausal women with pelvic floor disorders: Systematic review and practice guidelines. Int Urogynecol J. 2015;26:3–13. doi: 10.1007/s00192-014-2554-z. [DOI] [PubMed] [Google Scholar]
- 36.Jackson S, Shepherd A, Brookes S, et al. The effect of oestrogen supplementation on post-menopausal urinary stress incontinence: a double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:711–8. doi: 10.1111/j.1471-0528.1999.tb08372.x. [DOI] [PubMed] [Google Scholar]
- 37.Dessole S, Rubattu G, Ambrosini G, et al. Efficacy of low-dose intravaginal estriol on urogenital aging in postmenopausal women. Menopause. 2004;11:49–56. doi: 10.1097/01.GME.0000077620.13164.62. [DOI] [PubMed] [Google Scholar]
- 38.Capobianco G, Donolo E, Borghero G, et al. Effects of intravaginal estriol and pelvic floor rehabilitation on urogenital aging in postmenopausal women. Arch Gynecol Obstet. 2012;285:397–403. doi: 10.1007/s00404-011-1955-1. [DOI] [PubMed] [Google Scholar]
- 39.Bernard S, Boucher S, McLean L, et al. Mobile technologies for the conservative self-management of urinary incontinence: A systematic scoping review. Int Urogynecol J. 2020;31:1163–74. doi: 10.1007/s00192-019-04012-w. [DOI] [PubMed] [Google Scholar]
- 40.Barnes L, Heithoff DM, Mahan SP, et al. Smartphone-based pathogen diagnosis in urinary sepsis patients. EBioMedicine. 2018;36:73–82. doi: 10.1016/j.ebiom.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjostrom M, Lindholm L, Samuelsson E. Mobile app for treatment of stress urinary incontinence: A cost-effectiveness analysis. J Med Internet Res. 2017;19:e154. doi: 10.2196/jmir.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rygh P, Asklund I, Samuelsson E. Real-world effectiveness of app-based treatment for urinary incontinence: A cohort study. BMJ Open. 2021;11:e040819. doi: 10.1136/bmjopen-2020-040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman V, Soderstrom L, Samuelsson E. Self-management of stress urinary incontinence via a mobile app: Two-year followup of a randomized controlled trial. Acta Obstet Gynecol Scand. 2017;96:1180–7. doi: 10.1111/aogs.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asklund I, Nystrom E, Sjostrom M, et al. Mobile app for treatment of stress urinary incontinence: A randomized controlled trial. Neurourol Urodyn. 2017;36:1369–76. doi: 10.1002/nau.23116. [DOI] [PubMed] [Google Scholar]
- 45.Loohuis AMM, Wessels NJ, Dekker JH, et al. App-based treatment in primary care for urinary incontinence: A pragmatic, randomized controlled trial. Ann Fam Med. 2021;19:102–9. doi: 10.1370/afm.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J. 2017;28:191–213. doi: 10.1007/s00192-016-3123-4. [DOI] [PubMed] [Google Scholar]
- 47.Viera AJ, Larkins-Pettigrew M. Practical use of the pessary. Am Fam Physician. 2000;61:2719–26. 29. [PubMed] [Google Scholar]
- 48.Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302–7. doi: 10.1007/s00192-004-1163-7. [DOI] [PubMed] [Google Scholar]
- 49.Nager CW, Richter HE, Nygaard I, et al. Incontinence pessaries: Size, POPQ measures, and successful fitting. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1023–8. doi: 10.1007/s00192-009-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025–9. doi: 10.1016/j.ajog.2003.10.711. [DOI] [PubMed] [Google Scholar]
- 51.Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: A prospective study. Urology. 2016;87:70–5. doi: 10.1016/j.urology.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Alexander JW, Karjalainen P, Ow LL, et al. CO2 surgical laser for treatment of stress urinary incontinence in women: A randomized controlled trial. Am J Obstet Gynecol. 2022;227:473e1–12. doi: 10.1016/j.ajog.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Wang C, Song F, et al. Safety and efficacy of vaginal laser therapy for stress urinary incontinence: A meta-analysis. Ann Palliat Med. 2021;10:2736–46. doi: 10.21037/apm-20-1440. [DOI] [PubMed] [Google Scholar]
- 54.Seki AS, Bianchi-Ferraro A, Fonseca ESM, et al. CO2 Laser and radiofrequency compared to a sham control group in treatment of stress urinary incontinence (LARF study arm 3). A randomized controlled trial. Int Urogynecol J. 2022;33:3535–42. doi: 10.1007/s00192-022-05091-y. [DOI] [PubMed] [Google Scholar]
- 55.Mackova K, Van Daele L, Page AS, et al. Laser therapy for urinary incontinence and pelvic organ prolapse: A systematic review. BJOG. 2020;127:1338–46. doi: 10.1111/1471-0528.16273. [DOI] [PubMed] [Google Scholar]
- 56.Roberts LH, Vollstedt A, Eisner H, et al. PD06-05 Fractional carbon dioxide vaginal laser for the treatment of urinary symptoms: 12 months results. J Urol. 2022;207:e94. doi: 10.1097/JU.0000000000002525.05. [DOI] [Google Scholar]
- 57.Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: A randomized clinical trial. JAMA. 2021;326:1381–9. doi: 10.1001/jama.2021.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153–8. doi: 10.1016/j.ejogrb.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 59.(CMPA) CMPA. Consent: A guide for Canadian physicians. 2021. Apr, Available from: https://www.cmpa-acpm.ca/en/advice-publications/handbooks/consent-a-guide-forcanadian-physicians.
- 60.Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55. doi: 10.1007/s11934-016-0613-3. [DOI] [PubMed] [Google Scholar]
- 61.Hengel B, Welk B, Baverstock RJ. Medicolegal basics and update on transvaginal mesh in Canada. Can Urol Assoc J. 2017;11:S108–11. doi: 10.5489/cuaj.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klarskov N, Lose G. Urethral injection therapy: What is the mechanism of action? Neurourology Urodyn. 2008;27:789–92. doi: 10.1002/nau.20602. [DOI] [PubMed] [Google Scholar]
- 63.Pivazyan L, Kasyan G, Grigoryan B, et al. Effectiveness and safety of bulking agents versus surgical methods in women with stress urinary incontinence: A systematic review and meta-analysis. Int Urogynecol J. 2022;33:777–87. doi: 10.1007/s00192-021-04937-1. [DOI] [PubMed] [Google Scholar]
- 64.Hussain SM, Bray R. Urethral bulking agents for female stress urinary incontinence. Neurourol Urodyn. 2019;38:887–92. doi: 10.1002/nau.23924. [DOI] [PubMed] [Google Scholar]
- 65.Mamut A, Carlson KV. Periurethral bulking agents for female stress urinary incontinence in Canada. Can Urol Assoc J. 2017;11:S152–4. doi: 10.5489/cuaj.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freites J, Stewart F, Omar MI, Mashayekhi A, Agur WI. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2019;2019:CD002239. doi: 10.1002/14651858.CD002239.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sohlberg EM, Elliott CS. Burch colposuspension. Urol Clin North Am. 2019;46:53–9. doi: 10.1016/j.ucl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Petros P, Abendstein B. The mechanics of urethral closure, incontinence and midurethral sling repair. Part 1 original experimental studies. (1990) Neurourol Urodyn. 2019;38:809–13. doi: 10.1002/nau.23888. [DOI] [PubMed] [Google Scholar]
- 69.Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;2017:CD006375. doi: 10.1002/14651858.CD006375.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: Systematic review and network meta-analysis of randomized controlled trials. BMJ. 2019;365:l1842. doi: 10.1136/bmj.l1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richter HE, Albo ME, Zyczynski HM, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066–76. doi: 10.1056/NEJMoa0912658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi: 10.1002/14651858.CD003881.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itkonen Freitas AM, Isaksson C, Rahkola-Soisalo P, et al. Tension-free vaginal tape and polyacrylamide hydrogel injection for primary stress urinary incontinence: 3-year followup from a randomized clinical trial. J Urol. 2022;208:658–67. doi: 10.1097/JU.0000000000002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoe V, Haller B, Yao HH, et al. Urethral bulking agents for the treatment of stress urinary incontinence in women: A systematic review. Neurourol Urodyn. 2021;40:1349–88. doi: 10.1002/nau.24696. [DOI] [PubMed] [Google Scholar]
- 75.Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502–8. doi: 10.1002/nau.24589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang B, Hobbs C, Foley S. Deflux® (NASHATM/Dx) urethral bulking injections: Outcomes over a decade in women with stress urinary incontinence. J Clin Urol. 2022;0:20514158221084823. [Google Scholar]
- 77.Cespedes RD, Serkin FB. Is injection therapy for stress urinary incontinence dead? No. Urology. 2009;73:11–3. doi: 10.1016/j.urology.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 78.Lightner D, Rovner E, Corcos J, et al. Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: Mid-urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically. Urology. 2009;74:771–5. doi: 10.1016/j.urology.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 79.Berger AA, Tan-Kim J, Menefee SA. The impact of midurethral sling surgery on the development of urinary tract infections. Int Urogynecol J. 2022;33:829–34. doi: 10.1007/s00192-021-04779-x. [DOI] [PubMed] [Google Scholar]
- 80.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA Surg. 2017;152:292–8. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 81.Oelke M, De Wachter S, Drake MJ, et al. A practical approach to the management of nocturia. Int J Clin Pract. 2017;71:e13027. doi: 10.1111/ijcp.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia Z, Qian J, Chen Y, et al. Does body mass index influence the outcome of midurethral sling procedures for stress urinary incontinence? Int Urogynecol J. 2017;28:817–22. doi: 10.1007/s00192-016-3181-7. [DOI] [PubMed] [Google Scholar]
- 83.Madjar S. The role of intrinsic sphincteric deficiency diagnosis in the era of midurethral sling. Curr Urol Rep. 2011;12:387–92. doi: 10.1007/s11934-011-0211-3. [DOI] [PubMed] [Google Scholar]
- 84.Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: A randomized controlled trial. Obstet Gynecol. 2008;112:1253–61. doi: 10.1097/AOG.0b013e31818db391. [DOI] [PubMed] [Google Scholar]
- 85.Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203–10. doi: 10.1016/j.juro.2014.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Offiah I, Freeman R MONARC™ study group. Long-term efficacy and complications of a multicentre randomised controlled trial comparing retropubic and transobturator midurethral slings: A prospective observational study. BJOG. 2021;128:2191–9. doi: 10.1111/1471-0528.16899. [DOI] [PubMed] [Google Scholar]
- 87.Zullo MA, Schiavi MC, Luffarelli P, et al. TVT-O vs. TVT-Abbrevo for stress urinary incontinence treatment in women: A randomized trial. Int Urogynecol J. 2020;31:703–10. doi: 10.1007/s00192-019-04077-7. [DOI] [PubMed] [Google Scholar]
- 88.Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230–43. doi: 10.1056/NEJMoa2111815. [DOI] [PubMed] [Google Scholar]
- 89.Canada Go. Summary Safety Review - Single incision mini-sling (SIMS) made from non-absorbable synthetic material (polypropylene) - Health Canada. 2020. Sep 17, 2020. [Accessed Feb 15, 2024]. [cited 2023 February 3]; Available at: https://hpr-rps.hres.ca/reg-content/summary-safety-review-detail.php?lang=en&linkID=SSR00246.
- 90.Plagakis S, Tse V. The autologous pubovaginal fascial sling: An update in 2019. Low Urin Tract Symptoms. 2020;12:2–7. doi: 10.1111/luts.12281. [DOI] [PubMed] [Google Scholar]
- 91.Toledo LG, Korkes F, Romero FR, et al. Bladder outlet obstruction after pubovaginal fascial sling. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:201–5. doi: 10.1007/s00192-008-0759-8. [DOI] [PubMed] [Google Scholar]
- 92.Fusco F, Abdel-Fattah M, Chapple CR, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2017;72:567–91. doi: 10.1016/j.eururo.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 93.Guerrero K, Watkins A, Emery S, et al. A randomised controlled trial comparing two autologous fascial sling techniques for the treatment of stress urinary incontinence in women: Short, medium and long-term followup. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1263–70. doi: 10.1007/s00192-007-0307-y. [DOI] [PubMed] [Google Scholar]
- 94.Veit-Rubin N, Dubuisson J, Ford A, et al. Burch colposuspension. Neurourol Urodyn. 2019;38:553–62. doi: 10.1002/nau.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lapitan MCM, Cody JD, Mashayekhi A. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD002912. doi: 10.1002/14651858.CD002912.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karmakar D, Dwyer PL, Murray C, et al. Long-term effectiveness and safety of open Burch colposuspension vs retropubic midurethral sling for stress urinary incontinence-results from a large comparative study. Am J Obstet Gynecol. 2021;224:593e1–8. doi: 10.1016/j.ajog.2020.11.043. [DOI] [PubMed] [Google Scholar]
- 97.Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143–55. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- 98.Oliveira LM, Dias MM, Martins SB, et al. Surgical treatment for stress urinary incontinence in women: A systematic review and meta-analysis. Rev Bras Ginecol Obstet. 2018;40:477–90. doi: 10.1055/s-0038-1667184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sohlberg EM, Elliott CS. Burch Colposuspension. Urol Clin North Am. 2019;46:53–9. doi: 10.1016/j.ucl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Jain P, Jirschele K, Botros SM, et al. Effectiveness of midurethral slings in mixed urinary incontinence: A systematic review and meta-analysis. Int Urogynecol J. 2011;22:923–32. doi: 10.1007/s00192-011-1406-3. [DOI] [PubMed] [Google Scholar]
- 101.Brucker BM. Expectations of stress urinary incontinence surgery in patients with mixed urinary incontinence. Rev Urol. 2015;17:14–9. [PMC free article] [PubMed] [Google Scholar]
- 102.Pergialiotis V, Mudiaga Z, Perrea DN, et al. De novo overactive bladder following midurethral sling procedures: A systematic review of the literature and meta-analysis. Int Urogynecol J. 2017;28:1631–8. doi: 10.1007/s00192-017-3417-1. [DOI] [PubMed] [Google Scholar]
- 103.Marcelissen T, Van Kerrebroeck P. Overactive bladder symptoms after midurethral sling surgery in women: Risk factors and management. Neurourol Urodyn. 2018;37:83–8. doi: 10.1002/nau.23328. [DOI] [PubMed] [Google Scholar]
- 104.Paick JS, Oh SJ, Kim SW, et al. Tension-free vaginal tape, suprapubic arc sling, and transobturator tape in the treatment of mixed urinary incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:123–9. doi: 10.1007/s00192-007-0401-1. [DOI] [PubMed] [Google Scholar]
- 105.Sung VW, Borello-France D, Newman DK, et al. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence: The ESTEEM randomized clinical trial. JAMA. 2019;322:1066–76. doi: 10.1001/jama.2019.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Corcos J, Przydacz M, Campeau L, et al. CUA guideline on adult overactive bladder. Can Urol Assoc J. 2017;11:E142–73. doi: 10.5489/cuaj.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welk B, Baverstock RJ. The management of mixed urinary incontinence in women. Can Urol Assoc J. 2017;11:S121–4. doi: 10.5489/cuaj.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rovner E, Athanasiou S, Choo MS, et al. Surgery for Urinary Incontinence in Women. In: Abrams P, et al., editors. Incontinence. 6th Edition. International Continence Society; Bristol, UK.: 2017. pp. 1741–854. [Google Scholar]
- 109.Robinson D, Cardozowan L. Urinary incontinence in the young woman: Treatment plans and options available. Womens Health (Lond) 2014;10:201–17. doi: 10.2217/WHE.14.1. [DOI] [PubMed] [Google Scholar]
- 110.Morkved S, Bo K, Schei B, et al. Pelvic floor muscle training during pregnancy to prevent urinary incontinence: A single-blind randomized controlled trial. Obstet Gynecol. 2003;101:313–9. doi: 10.1097/00006250-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 111.Sangsawang B, Sangsawang N. Stress urinary incontinence in pregnant women: A review of prevalence, pathophysiology, and treatment. Int Urogynecol J. 2013;24:901–12. doi: 10.1007/s00192-013-2061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;5:CD007471. doi: 10.1002/14651858.CD007471.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dyrkorn OA, Staff AC, Kulseng-Hanssen S, et al. Childbirth after mid-urethral sling surgery: Effects on long-term success and complications. Int Urogynecol J. 2020;31:485–92. doi: 10.1007/s00192-019-04067-9. [DOI] [PubMed] [Google Scholar]
- 114.Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: A randomized clinical trial. J Urol. 2020;203:372–8. doi: 10.1097/JU.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 115.Tulokas SA, Rahkola-Soisalo P, Gissler M, et al. Pregnancy and delivery after mid-urethral sling operation. Int Urogynecol J. 2021;32:179–86. doi: 10.1007/s00192-020-04497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wieslander CK, Weinstein MM, Handa VL, et al. pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299–305. doi: 10.1097/SPV.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 117.Tan HJ, Siu W, Faerber GJ, et al. Long-term durability of pubovaginal fascial slings in women who then become pregnant and deliver. Int Urogynecol J. 2010;21:631–5. doi: 10.1007/s00192-010-1098-0. [DOI] [PubMed] [Google Scholar]
- 118.Jha S, Radley S, Farkas A, et al. The impact of TVT on sexual function. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:165–9. doi: 10.1007/s00192-008-0743-3. [DOI] [PubMed] [Google Scholar]
- 119.Glass Clark SM, Huang Q, Sima AP, et al. Effect of surgery for stress incontinence on female sexual function. Obstet Gynecol. 2020;135:352–60. doi: 10.1097/AOG.0000000000003648. [DOI] [PubMed] [Google Scholar]
- 120.Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: A systematic review and meta-analysis. J Sex Med. 2012;9:34–43. doi: 10.1111/j.1743-6109.2011.02366.x. [DOI] [PubMed] [Google Scholar]
- 121.Caruso S, Rugolo S, Bandiera S, et al. Clitoral blood flow changes after surgery for stress urinary incontinence: Pilot study on TVT versus TOT procedures. Urology. 2007;70:554–7. doi: 10.1016/j.urology.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 122.Yeni E, Unal D, Verit A, et al. The effect of tension-free vaginal tape (TVT) procedure on sexual function in women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:390–4. doi: 10.1007/s00192-003-1100-1. [DOI] [PubMed] [Google Scholar]
- 123.Lee W, Uberoi P, Coy DL, et al. Erosion of mesh mid-urethral sling into a complex urethral diverticulum: Diagnosis, evaluation, and surgical management. Urology. 2020;141:e1–e2. doi: 10.1016/j.urology.2020.04.073. [DOI] [PubMed] [Google Scholar]
- 124.Barratt R, Malde S, Pakzad M, et al. The incidence and outcomes of urodynamic stress urinary incontinence in female patients with urethral diverticulum. Neurourol Urodyn. 2019;38:1889–900. doi: 10.1002/nau.24090. [DOI] [PubMed] [Google Scholar]
- 125.Berger AA, Tan-Kim J, Menefee SA. The impact of surgical center volume on reoperation risk after mid urethral sling. J Urol. 2021;206:1454–60. doi: 10.1097/JU.0000000000002140. [DOI] [PubMed] [Google Scholar]
- 126.Denman MA, Gregory WT, Boyles SH, et al. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2008;198:555e1–5. doi: 10.1016/j.ajog.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 127.Trabuco EC, Carranza D, El Nashar SA, et al. Reoperation for urinary incontinence after retropubic and transobturator sling procedures. Obstet Gynecol. 2019;134:333–42. doi: 10.1097/AOG.0000000000003356. [DOI] [PubMed] [Google Scholar]
- 128.Tulokas S, Rahkola-Soisalo P, Gissler M, et al. Long-term re-procedure rate after midurethral slings for stress urinary incontinence. Int Urogynecol J. 2020;31:727–35. doi: 10.1007/s00192-019-04223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Welk B, Winick-Ng J. Repeat surgical intervention for stress urinary incontinence after a failed mid urethral sling. Urol Pract. 2016;3:475–80. doi: 10.1016/j.urpr.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Wu MP, Long CY, Liang CC, et al. Trends in reoperation for female stress urinary incontinence: A nationwide study. Neurourol Urodyn. 2015;34:693–8. doi: 10.1002/nau.22648. [DOI] [PubMed] [Google Scholar]
- 131.Fialkow M, Symons RG, Flum D. Reoperation for urinary incontinence. Am J Obstet Gynecol. 2008;199:546e1–8. doi: 10.1016/j.ajog.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 132.Berger AA, Tan-Kim J, Menefee SA. Long-term risk of reoperation after synthetic mesh midurethral sling surgery for stress urinary incontinence. Obstet Gynecol. 2019;134:1047–55. doi: 10.1097/AOG.0000000000003526. [DOI] [PubMed] [Google Scholar]
- 133.Kavanagh A, Sanaee M, Carlson KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11:S143–6. doi: 10.5489/cuaj.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lo TS, Pue LB, Tan YL, et al. Risk factors for failure of repeat midurethral sling surgery for recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2016;27:923–31. doi: 10.1007/s00192-015-2912-5. [DOI] [PubMed] [Google Scholar]
- 135.Richter HE, Litman HJ, Lukacz ES, et al. Demographic and clinical predictors of treatment failure one year after midurethral sling surgery. Obstet Gynecol. 2011;117:913–21. doi: 10.1097/AOG.0b013e31820f3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holmgren C, Nilsson S, Lanner L, et al. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38–43. doi: 10.1097/01.AOG.0000167393.95817.dc. [DOI] [PubMed] [Google Scholar]
- 137.Anger JT, Litwin MS, Wang Q, et al. The effect of age on outcomes of sling surgery for urinary incontinence. J Am Geriatr Soc. 2007;55:1927–31. doi: 10.1111/j.1532-5415.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 138.Dumoulin C, Hay-Smith EJ, Mac Habee-Seguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2014:CD005654. doi: 10.1002/14651858.CD005654.pub3. [DOI] [PubMed] [Google Scholar]
- 139.Bakali E, Johnson E, Buckley BS, et al. Interventions for treating recurrent stress urinary incontinence after failed minimally invasive synthetic midurethral tape surgery in women. Cochrane Database Syst Rev. 2019;9:Cd009407. doi: 10.1002/14651858.CD009407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brennand EA, Ugurlucan FG, Brown HW, et al. Female pelvic medicine and reconstructive surgery challenges on behalf of the collaborative research in pelvic surgery consortium: Managing complicated cases : Series 5: Management of recurrent stress urinary incontinence after midurethral sling exposure. Int Urogynecol J. 2020;31:1747–54. doi: 10.1007/s00192-020-04385-3. [DOI] [PubMed] [Google Scholar]
- 141.El-Hamamsy D, Tincello DG. Recurrent stress urinary incontinence surgery in the United Kingdom: An analysis of the British Society of Urogynaecology database (2007–2015) Int Urogynecol J. 2021;32:167–72. doi: 10.1007/s00192-020-04420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giarenis I, Thiagamoorthy G, Zacchè M, et al. Management of recurrent stress urinary incontinence after failed midurethral sling: A survey of members of the International Urogynecological Association (IUGA) Int Urogynecol J. 2015;26:1285–91. doi: 10.1007/s00192-015-2696-7. [DOI] [PubMed] [Google Scholar]
- 143.Tincello DG, Armstrong N, Hilton P, et al. Surgery for recurrent stress urinary incontinence: The views of surgeons and women. Int Urogynecol J. 2018;29:45–54. doi: 10.1007/s00192-017-3376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van der Doelen MJ, Withagen MI, Vierhout ME, et al. Results of primary vs. recurrent surgery to treat stress urinary incontinence in women. Int Urogynecol J. 2015;26:997–1005. doi: 10.1007/s00192-015-2627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim TH, You HW, Ryu DS, et al. Surgical outcome of a repeat midurethral sling procedure after failure of a first procedure. Int Urogynecol J. 2015;26:1759–66. doi: 10.1007/s00192-015-2773-y. [DOI] [PubMed] [Google Scholar]
- 146.Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: A systematic review. Acta Obstet Gynecol Scand. 2015;94:568–76. doi: 10.1111/aogs.12625. [DOI] [PubMed] [Google Scholar]
- 147.Sabadell J, Montero-Armengol A, Rodríguez-Mias N, et al. Long-term outcomes of retropubic tension-free vaginal tape for stress urinary incontinence after a transobturator tape failure: A retrospective study. Int Urogynecol J. 2020;31:755–60. doi: 10.1007/s00192-019-04169-4. [DOI] [PubMed] [Google Scholar]
- 148.Smithling KR, Adams-Piper EE, Tran AM, et al. Efficacy of repeat midurethral sling for persistent or recurrent stress urinary incontinence: A fellows pelvic research network study. Female Pelvic Med Reconstr Surg. 2019;25:430–3. doi: 10.1097/SPV.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 149.Geller EJ. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Womens Health. 2014;6:829–38. doi: 10.2147/IJWH.S55383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alas A, Martin L, Devakumar H, et al. Anesthetics’ role in postoperative urinary retention after pelvic organ prolapse surgery with concomitant midurethral slings: A randomized clinical trial. Int Urogynecol J. 2020;31:205–13. doi: 10.1007/s00192-019-03917-w. [DOI] [PubMed] [Google Scholar]
- 151.Gracely A, Major N, Zheng Y, et al. Do urodynamics predict urinary retention after sling placement in the complex patient: The value of reproducing symptoms on urodynamics. Int Urogynecol J. 2021;32:81–6. doi: 10.1007/s00192-020-04623-8. [DOI] [PubMed] [Google Scholar]
- 152.Keita H, Diouf E, Tubach F, et al. Predictive factors of early postoperative urinary retention in the postanesthesia care unit. Anesth Analg. 2005;101:592–6. doi: 10.1213/01.ANE.0000159165.90094.40. [DOI] [PubMed] [Google Scholar]
- 153.Bazi T, Kerkhof MH, Takahashi SI, et al. Management of post-midurethral sling voiding dysfunction. International Urogynecological Association research and development committee opinion. Int Urogynecol J. 2018;29:23–8. doi: 10.1007/s00192-017-3509-y. [DOI] [PubMed] [Google Scholar]
- 154.Baekelandt F, Van Oyen P, Ghysel C, et al. Long-term functional results after unilateral mid-urethral sling transection for voiding dysfunction. Eur J Obstet Gynecol Reprod Biol. 2016;207:89–93. doi: 10.1016/j.ejogrb.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 155.Moksnes LR, Svenningsen R, Schiotz HA, et al. Sling mobilization in the management of urinary retention after midurethral sling surgery. Neurourol Urodyn. 2017;36:1091–6. doi: 10.1002/nau.23046. [DOI] [PubMed] [Google Scholar]
- 156.Blaivas JG, Purohit RS, Benedon MS, et al. Safety considerations for synthetic sling surgery. Nat Rev Urol. 2015;12:481–509. doi: 10.1038/nrurol.2015.183. [DOI] [PubMed] [Google Scholar]
- 157.YH, Lee CK, Chang SD, et al. Focusing on long-term complications of mid-urethral slings among women with stress urinary incontinence as a patient safety improvement measure: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e26257. doi: 10.1097/MD.0000000000026257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lo TS, Ng KL, Lin YH, et al. De novo detrusor overactivity and urgency after mid-urethral slings for urodynamic stress incontinence. Int Urogynecol J. 2021;32:2737–45. doi: 10.1007/s00192-021-04911-x. [DOI] [PubMed] [Google Scholar]
- 159.Manodoro S, Barba M, Locatelli L, et al. Urodynamic predictors of de novo overactive bladder after single-incision sling. Int J Gynaecol Obstet. 2021;153:412–6. doi: 10.1002/ijgo.13503. [DOI] [PubMed] [Google Scholar]
- 160.Viereck V, Rautenberg O, Kociszewski J, et al. Midurethral sling incision: Indications and outcomes. Int Urogynecol J. 2013;24:645–53. doi: 10.1007/s00192-012-1895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duckett J, Bodner-Adler B, Rachaneni S, et al. Management of complications arising from the use of mesh for stress urinary incontinence-International Urogynecology Association Research and Development Committee opinion. Int Urogynecol J. 2019;30:1413–7. doi: 10.1007/s00192-019-03935-8. [DOI] [PubMed] [Google Scholar]
- 162.Duckett J, Baranowski A. Pain after suburethral sling insertion for urinary stress incontinence. Int Urogynecol J. 2013;24:195–201. doi: 10.1007/s00192-012-1863-3. [DOI] [PubMed] [Google Scholar]
- 163.Goldman HB. Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707–10. doi: 10.1097/SPV.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 164.Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015:CD008242. doi: 10.1002/14651858.CD011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doyle PJ, Grimes CL, Balk EM, et al. Surgical removal of midurethral sling in women undergoing surgery for presumed mesh-related complications: A systematic review. Obstet Gynecol. 2022;139:277–86. doi: 10.1097/AOG.0000000000004646. [DOI] [PubMed] [Google Scholar]
- 166.Jambusaria LH, Heft J, Reynolds WS, et al. Incontinence rates after midurethral sling revision for vaginal exposure or pain. Am J Obstet Gynecol. 2016;215:764e1–5. doi: 10.1016/j.ajog.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 167.Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourol Urodyn. 2011;30:2–12. doi: 10.1002/nau.21036. [DOI] [PubMed] [Google Scholar]
- 168.Kokanali MK, Cavkaytar S, Kokanali D, et al. A comperative study for short-term surgical outcomes of midurethral sling procedures in obese and non-obese women with stress urinary incontinence. J Obstet Gynaecol. 2016;36:1080–5. doi: 10.1080/01443615.2016.1209169. [DOI] [PubMed] [Google Scholar]
- 169.Osborn DJ, Dmochowski RR, Harris CJ, et al. Analysis of patient and technical factors associated with midurethral sling mesh exposure and perforation. Int J Urol. 2014;21:1167–70. doi: 10.1111/iju.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun Z, Zhu L, Xu T, et al. Effects of preoperative vaginal estrogen therapy for the incidence of mesh complication after pelvic organ prolapse surgery in postmenopausal women: Is it helpful or a myth? A 1-year randomized controlled trial. Menopause. 2016;23:740–8. doi: 10.1097/GME.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 171.Developed by the Joint Writing Group of the American Urogynecologic Society and the International Urogynecological Association. Joint position statement on the management of mesh-related complications for the FPMRS specialist. Int Urogynecol J. 2020;31:679–94. doi: 10.1007/s00192-020-04248-x. [DOI] [PubMed] [Google Scholar]
- 172.Giusto LL, Zahner PM, Goldman HB. Management of the exposed or perforated midurethral sling. Urol Clin North Am. 2019;46:31–40. doi: 10.1016/j.ucl.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 173.Karmakar D, Dwyer PL, Nikpoor P. Mid-urethral sling revision for mesh exposure-long-term outcomes of two surgical techniques from a comparative clinical retrospective cohort study. BJOG. 2020;127:1027–33. doi: 10.1111/1471-0528.16149. [DOI] [PubMed] [Google Scholar]
- 174.Lee D, Zimmern PE. Management of complications of mesh surgery. Curr Opin Urol. 2015;25:284–91. doi: 10.1097/MOU.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 175.Zheng Y, Major N, Silverii H, et al. Is it the surgeon? A re-examination of midurethral sling complications. Urology. 2021;157:269–73. doi: 10.1016/j.urology.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 176.Allagany F, Dekalo S, Welk B. Endoscopic management of intraurethral mesh extrusion with the holmium:YAG laser is an acceptable treatment option in selected patients. Neurourol Urodyn. 2022;41:1511–6. doi: 10.1002/nau.24999. [DOI] [PubMed] [Google Scholar]
- 177.Karim SS, Pietropaolo A, Skolarikos A, et al. Role of endoscopic management in synthetic sling/mesh erosion following previous incontinence surgery: A systematic review from European Association of Urologists Young Academic Urologists (YAU) and Uro-technology (ESUT) groups. Int Urogynecol J. 2020;31:45–53. doi: 10.1007/s00192-019-04087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hermieu N, Ouzaid I, Aoun R, et al. Urethral exposure of mid-urethral sling: Diagnosis, management and functional outcomes. Urology. 2022;164:100–5. doi: 10.1016/j.urology.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 179.Anger J, Lee U, Ackerman AL, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol. 2019;202:282–9. doi: 10.1097/JU.0000000000000296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.