Abstract
Recovery from infection with the Friend murine leukemia retrovirus complex (FV) requires T-helper cells and cytotoxic T cells as well as neutralizing antibodies. Several host genes, including genes of the major histocompatibility complex (H-2) and an H-2-unlinked gene, Rfv-3, influence these FV-specific immune responses. (B10.A × A/Wy)F1 mice, which have the H-2a/a Rfv-3r/s genotype, fail to mount a detectable FV-specific T-cell proliferative response but nevertheless produce FV-specific neutralizing immunoglobulin M (IgM) antibodies and can eliminate FV viremia. Thus, this IgM response, primarily influenced by the Rfv-3 gene, may be T-cell independent. To test this idea, mice were depleted of either CD4+ or CD8+ T-cell populations in vivo and were monitored for the effect on the neutralizing antibody response following FV infection. Surprisingly, mice in which CD4+ cells were depleted showed undetectable FV-neutralizing antibody responses and high viremia levels compared to nondepleted or CD8-depleted animals. In addition to knocking out the FV antibody response, CD4+ T-cell depletion reduced survival time significantly, further indicating the importance of CD4+ T cells. These studies revealed the first evidence for a functional T-cell response following FV infection in these low-recovery mice and showed that CD4+ T-helper cells are required for the Rfv-3-controlled FV antibody response.
Most mammalian antiviral immune responses involve T lymphocytes, utilizing one or more of their specialized activities. Early induction of CD8+ cytotoxic T lymphocytes (CTL) often results in rapid elimination of virus-infected cells (22, 26). CD4+ T-helper (Th) cells can have a variety of roles in antiviral immunity, including providing help for CTL amplification and memory (2, 18, 25, 28), activation of B-cells (29), and direct antiviral activity (10, 17). In the Friend murine leukemia retrovirus (FV) system, both CD8+ and CD4+ T lymphocytes are critical for an effective immune response leading to spontaneous recovery, although the exact roles of CD4+ cells remain unclear (6, 12, 13, 26). The FV-specific T-cell responses are influenced by several genes of the mouse major histocompatibility complex, H-2 (4, 8, 15, 19). For example, depending on the FV infection dose, H-2a/b and H-2b/b mouse strains exhibit FV-specific CD8+ CTL and CD4+ T-cell proliferation in vitro and recover spontaneously from FV-induced leukemic splenomegaly. In contrast, naive H-2a/a mice mount no detectable FV-specific T-cell proliferative responses and produce few CTL, and these mice succumb to FV-induced splenomegaly and erythroleukemia, even when infected with low doses of virus (8, 15) (Table 1).
TABLE 1.
Immune response parameters of H-2 congenic mouse strainsa
| F1 mouse strain | H-2 genotype | In vitro FV-specific CTLb | In vitro FV-specific T-cell proliferation | FV recoveryc | Rfv-3 genotype | IgG class of FV-neutralizing antibodyd | Viremia (30 dpi)e |
|---|---|---|---|---|---|---|---|
| C57BL/10 × A.BYe | b/b | + | + | High | r/s | IgM + IgG | − |
| B10.A × A.BY | a/b | + | + | Intermediate | r/s | IgM + IgG | − |
| B10.A × A/Wyf | a/a | −g | − | Low | r/s | IgM | − |
Among (B10.A × A.BY)F1 mice, only recovering mice which show regression of splenomegaly (6) exhibit CTL responses.
Refers to long-term regression of FV-induced splenomegaly with failure to progress to fatal erythroleukemia. High, recovery from infection with a high dose of FV; intermediate, recovery from infection with a low dose but not a high dose of FV; low, no recovery even at a low dose of FV.
A.BY mice have H-2b/b Rfv-3s/s genotype and do not make FV-neutralizing antibodies after infection.
A/Wy mice have H-2a/a Rfv-3s/s genotype and do not make FV-neutralizing antibodies after infection.
Rare individuals of this strain have shown evidence of FV-specific CTL.
In addition to T cells, spontaneous recovery from FV requires the induction of virus-neutralizing antibodies (7). This antibody response is dependent upon an autosomal dominant, non-H-2 gene, Rfv-3 (Table 1) (7, 8). Interestingly H-2a/a Rfv-3r/s mice can produce an anti-FV immunoglobulin M (IgM) antibody response but fail to switch to IgG following FV infection (16, 20). Furthermore, these mice clear viremia in the presence of ongoing leukemia (7). However, this mouse strain lacks detectable FV-specific in vitro T-cell proliferation (4), suggesting that the IgM response might be T-cell independent. This has been seen in some other antiviral IgM responses (1). Alternatively, the anti-FV antibody response might require specific Th cells that for unknown reasons are not detectable in H-2a/a mice by standard T-cell proliferation assays. In the present study, we tested the role of T cells in the FV-neutralizing antibody response by depleting specific T-cell subsets in vivo. The results showed that CD4+ T cells were required for the FV-neutralizing antibody response in H-2a/a mice and that these cells played a role in prolonging survival time after FV infection.
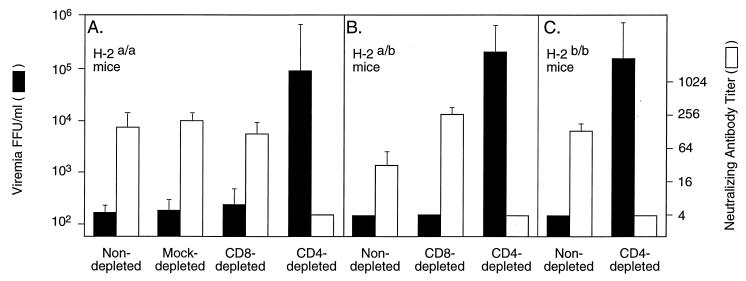
The effect of T-cell depletion was first tested in H-2b/b and H-2a/b Rfv-3r/s F1 congenic mouse strains, both of which normally show detectable FV-specific T-cell proliferation and antibody responses. In addition, because of their H-2 genotype, these strains can switch from IgM to IgG neutralizing antibodies following FV infection (Table 1). Mice were depleted in vivo of CD4+ or CD8+ T cells with monoclonal antibodies (9) and were infected with 1,000 spleen focus-forming units of the B-tropic polycythemia strain of FV as described previously (12). Control groups of nondepleted mice were similarly infected. Neutralizing antibodies and viremia levels were measured in plasma 30 days postinfection (dpi) as previously described (11). CD4 depletion had a dramatic effect on the anti-FV antibody response in both these mouse strains. All CD4-depleted mice lacked detectable FV-neutralizing antibodies (titers of ≤ 1:4) at 30 dpi, whereas all nondepleted mice and CD8-depleted H-2a/b mice showed a detectable range of plasma FV-neutralizing antibodies (Fig. 1B and C). Interestingly, in these strains, neither anti-FV IgM nor IgG was detected after CD4 depletion.
FIG. 1.
Neutralizing antibody and viremia levels in T-cell-depleted or nondepleted H-2 congenic mouse strains. A group of H-2a/a mice was mock depleted with an isotype-matched IgG2b antibody (24). T-cell depletion was considered complete when FACS analysis showed that residual CD4+ or CD8+ T cells were ≤2% of total nucleated peripheral blood cells. Mice were bled at 30 dpi, and plasma was tested as described elsewhere (11). Filled bars (right) display geometric means ± standard errors of the means of plasma virus levels (in focus-forming units per milliliter), and open bars (left) show serum dilutions resulting in 75% neutralization of Friend virus. The neutralizing titer represents total Ig. Control nondepleted H-2a/b and H-2b/b mice had both IgG and IgM neutralizing antibodies. CD4 depletion abolished neutralizing antibodies of both Ig classes. H-2a/a mice are known to make only IgM neutralizing antibodies (16, 20, 23), and therefore these Ig classes were not analyzed separately in this strain. CD4-depleted groups were significantly different (P < 0.0001 by the Student t test) from both CD8-depleted and control groups in terms of neutralizing antibody response and viremia. Data from control groups were not significantly different. (A) H-2a/a mice, 10 nondepleted, 10 CD8 depleted, 5 mock depleted (24), and 9 CD4 depleted. (B) H-2a/b mice, 6 nondepleted, 5 CD8 depleted, and 5 CD4 depleted. (C) H-2b/b mice, 4 nondepleted and 5 CD4 depleted.
In the FV system, control of viremia has been shown to depend on the presence of neutralizing antibodies (5). As predicted, viremia levels in the CD4-depleted H-2b/b and H-2a/b mice correlated inversely with the low or absent neutralizing antibody levels. CD4-depleted mice had high plasma virus levels, whereas nondepleted control mice had low or undetectable (<200 FFU/ml) levels of viremia. The CD8-depleted H-2a/b mice had low or undetectable levels of virus in plasma, similar to those of the nondepleted animals (Fig. 1B and C).
The effect of T-cell depletion was next tested in the low-recovery H-2a/a Rfv-3r/s strain (Table 1). CD4 depletion greatly affected the antibody response, as in the H-2b/b and H-2a/b animals. CD4-depleted mice had no detectable FV-specific antibody response and showed high viremia levels, whereas nondepleted, mock-depleted (24), and CD8-depleted mice had high neutralizing antibody titers and undetectable levels of virus in plasma (Fig. 1A). In summary, these depletion experiments showed an unexpected requirement for CD4+ Th cells in the FV-neutralizing antibody response, which was indistinguishable in the mouse strains with and without T-cell responses.
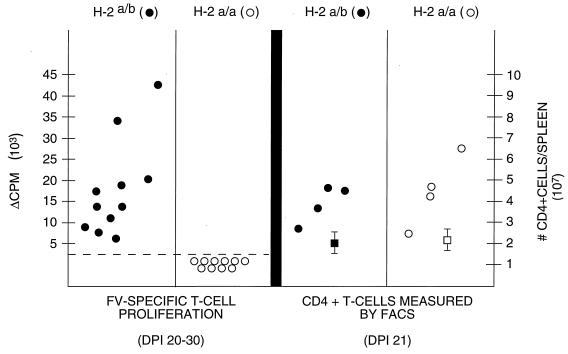
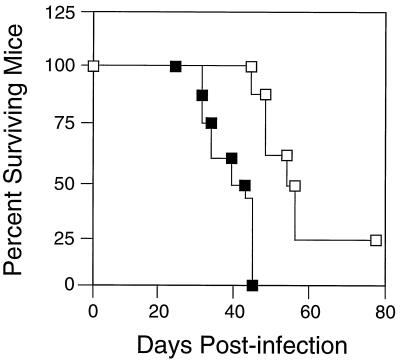
Since our depletion analysis revealed the presence of functional CD4+ cells in the H-2a/a mouse strain, we next used fluorescence-activated cell sorting (FACS) analysis to determine whether overall CD4+ T-cell numbers might increase in vivo following FV infection. We observed an increase in splenic CD4+ T cells in H-2a/a mice with time postinfection, and this CD4+ cell increase was comparable to that seen in H-2a/b mice (Fig. 2). Although the specificity of these cells was unknown, the requirement for CD4+ T cells in the FV-neutralizing antibody response strongly suggested that CD4+ T cells were proliferating in response to FV infection. We further tested the function of these T cells in H-2a/a mice in the FV-specific immune response by comparing survival in CD4-depleted and nondepleted mice. FV-infected, CD4-depleted H-2a/a mice showed significantly decreased survival time compared to nondepleted controls. Depleted mice died or had to be euthanized due to severe splenomegaly between 28 and 45 days, whereas all nondepleted mice of this strain survived beyond 45 days and more than 50% survived 65 days or longer (Fig. 3). Our present studies suggest that one function of these cells in long-term survival following FV infection may be maintenance of the neutralizing antibody response.
FIG. 2.
Comparison of FV-specific T-lymphocyte proliferation and CD4+ T-cell expansion following FV infection in H-2 congenic F1 mouse strains. (Left) Splenic T cells from FV-infected H-2a/b or H-2a/a mice were assayed for FV-specific T-cell proliferation as measured by [3H]thymidine uptake (4). Specific T-cell proliferation was noted only in H-2a/b mice. (Right) Twenty-one days following FV infection, spleen cells were directly labeled with phycoerythrin-conjugated anti-CD4 antibody (Pharmingen) and CD4+ cell numbers were measured by FACS. Statistically significant increases in CD4+ cells were seen in both H-2a/b (P < 0.0023) and H-2a/a (P < 0.0025) mice at 21 dpi. Squares represent mean numbers of CD4+ cells in uninfected H-2a/b (■) and H-2a/a (□) mice. At 21 dpi the percentage of CD4+ T cells in the spleen was lower in H-2a/a than in H-2a/b mice due to more severe splenomegaly (an average of 4 times more total spleen cells) in the H-2a/a strain.
FIG. 3.
Comparison of duration of survival in H-2a/a CD4-depleted (■) and nondepleted (□) mice. CD4-depleted mice (n = 8) began to die at 28 dpi, and at day 46 the remaining 4 mice were moribund and were euthanized. All control nondepleted mice (n = 8) survived until 47 dpi, and 50% remained alive >65 days. Survival data were statistically significant (P < 0.001 by the Fisher exact test) from day 47 on.
Although these experiments did not test antigen specificity directly, these data demonstrated, for the first time, the presence of functional CD4+ Th cells in H-2a/a mice following FV infection. In contrast, FV-specific T-cell proliferation has been readily observed in H-2b/b and H-2a/b mice, which are able to isotype switch from IgM to IgG neutralizing antibodies (16, 20) (Table 1). Since FACS analysis showed similar numbers of splenic CD4+ cells following FV infection in H-2a/b and H-2a/a mice (Fig. 2), the in vitro proliferation assay may preferentially detect the particular subsets of FV-specific Th cells which facilitate the class switch in H-2a/b mice, not the CD4+ cells detected in H-2a/a mice in our in vivo experiments (Fig. 1 through 3). Previous studies, using various FV vaccines, have linked FV-specific T-cell proliferation in vitro with the ability to induce antibody isotype class switching (16).
The FV retrovirus complex and human immunodeficiency virus type 1 (HIV-1) both induce severe immunosuppressive effects in vivo. Nevertheless, infected individuals in both systems can recover from viremia following initial infection (3, 7). In the HIV system, CD8+ T cells appear to play a major role in the early control of viremia, apparently acting before antiviral antibodies become effective (3). However, in the present study with the FV system, CD8 depletion appeared to have little or no effect on FV viremia levels at 30 dpi in either the H-2a/a or the H-2a/b mice tested. While CD8+ CTL are known to be critical for spontaneous recovery from FV disease, the present data provide further evidence that control of viremia in FV infection is primarily dependent upon CD4+ cells and neutralizing antibodies (5). Recent evidence suggests that CD4+ T cells may also play a role in the control of viremia in some HIV-infected humans. Although HIV-specific CD4+ Th responses have rarely been detected in HIV-infected individuals, a subset of individuals who appear to control HIV-1 replication in the absence of drug therapy has been recognized. These long-term AIDS nonprogressors have now been shown to exhibit vigorous anti-HIV-1 CD4+ T-proliferative responses which correlate with the ability to clear HIV-1 viremia (27). Whether this CD4+ T cell response is directly linked to the production of antiviral antibodies, as in the FV system, is as yet unknown.
Although we have shown that the anti-FV antibody response controlled by the Rfv-3 gene is CD4+ T-cell dependent, little is known about the Rfv-3 gene itself. FV-infected Rfv-3s/s mice are defective specifically in their antibody response to FV, while remaining responsive to other antigens (21), indicating that Rfv-3 has a unique immune regulatory mechanism. Rfv-3 maps to chromosome 15 and is linked to a number of interleukin receptor protein genes, including IL-2rb, IL-3rb1, and IL-3rb2 (14). Although it is unknown at this time whether one of these receptor genes is the Rfv-3 gene itself, linkage to one of these genes might indirectly influence the immune response of T-cell populations, ultimately affecting the FV-specific immune response.
REFERENCES
- 1.Bachmann M F, Zinkernagel R M. The influence of virus structure on antibody responses and virus serotype formation. Immunol Today. 1996;17:553–558. doi: 10.1016/s0167-5699(96)10066-9. [DOI] [PubMed] [Google Scholar]
- 2.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Hahn B, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt W J, Chesebro B. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 1983;157:1736–1745. doi: 10.1084/jem.157.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt W J, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130:2363–2367. [PubMed] [Google Scholar]
- 6.Chesebro B, Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. II. Cell-mediated immunity. J Exp Med. 1976;143:85–99. doi: 10.1084/jem.143.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesebro B, Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci USA. 1979;76:425–429. doi: 10.1073/pnas.76.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro B, Miyazawa M, Britt W J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- 9.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 10.Corey L, Spear P G. Infections with herpes simplex viruses. N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 11.Earl P L, Moss B, Morrison R P, Wehrly K, Nishio J, Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986;234:728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- 12.Hasenkrug K J, Brooks D M, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995;92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasenkrug K J, Brooks D M, Nishio J, Chesebro B. Differing T-cell requirements for recombinant retrovirus vaccines. J Virol. 1996;70:368–372. doi: 10.1128/jvi.70.1.368-372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasenkrug K J, Valenzuela A, Letts V A, Nishio J, Chesebro B, Frankel W N. Chromosome mapping of Rfv3, a host resistance gene to Friend murine retrovirus. J Virol. 1995;69:2617–2620. doi: 10.1128/jvi.69.4.2617-2620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara C, Miyazawa M, Nishio J, Chesebro B. Induction of protective immunity to Friend murine leukemia virus in genetic nonresponders to virus envelope protein. J Immunol. 1991;146:3958–3963. [PubMed] [Google Scholar]
- 17.Kurane I, Zeng L, Brinton M A, Ennis F A. Definition of an epitope on NS3 recognized by human CD4+ cytotoxic T lymphocyte clones cross-reactive for dengue virus types 2, 3, and 4. Virology. 1998;240:169–174. doi: 10.1006/viro.1997.8925. [DOI] [PubMed] [Google Scholar]
- 18.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazawa M, Nishio J, Chesebro B. Genetic control of T cell responsiveness to the Friend murine leukemia virus envelope antigen. Identification of class II loci of the H-2 as immune response genes. J Exp Med. 1988;168:1587–1605. doi: 10.1084/jem.168.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison R P, Earl P L, Nishio J, Lodmell D L, Moss B, Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature. 1987;329:729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- 21.Morrison R P, Nishio J, Chesebro B. Influence of the murine MHC (H-2) on Friend leukemia virus-induced immunosuppression. J Exp Med. 1986;163:301–314. doi: 10.1084/jem.163.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldstone M B A. The role of cytotoxic T lymphocytes in infectious disease: history, criteria, and state of the art. Curr Top Microbiol Immunol. 1994;189:1–8. doi: 10.1007/978-3-642-78530-6_1. [DOI] [PubMed] [Google Scholar]
- 23.Perry L L, Miyazawa M, Hasenkrug K, Wehrly K, David C S, Chesebro B. Contrasting effects from a single major histocompatibility complex class II molecule (H-2E) in recovery from Friend virus leukemia. J Virol. 1994;68:4921–4926. doi: 10.1128/jvi.68.8.4921-4926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pincus S H, Carmack C E. Variable regions of antibodies to synthetic polypeptides. III. Antibodies arising in response to administration of anti-idiotope. Mol Immunol. 1992;29:811–819. doi: 10.1016/0161-5890(92)90118-h. [DOI] [PubMed] [Google Scholar]
- 25.Planz O, Ehl S, Furrer E, Horvath E, Brundler M-A, Hengartner H, Zinkernagel R M. A critical role for neutralizing-antibody-producing B cells, CD4+ T cells and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson M N, Spangrude G J, Hasenkrug K, Perry L, Nishio J, Wehrly K, Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992;66:3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1477–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 28.von Herrath M G, Yokoyama M, Dockter J, Oldstone M B A, Whitton J L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yewdell J W, Bennink J R. Immune responses to viruses. In: Richman D D, Whitley R J, Hayden F G, editors. Clinical virology. New York, N.Y: Churchill Livingstone; 1997. pp. 271–305. [Google Scholar]