Abstract
Ochratoxin A (OTA) is a toxic mycotoxin produced by some mold species from genera Penicillium and Aspergillus. OTA has been detected in cereals, cereal-derived products, dried fruits, wine, grape juice, beer, tea, coffee, cocoa, nuts, spices, licorice, processed meat, cheese, and other foods. OTA can induce a wide range of health effects attributable to its toxicological properties, including teratogenicity, immunotoxicity, carcinogenicity, genotoxicity, neurotoxicity, and hepatotoxicity. OTA is not only toxic to humans but also harmful to livestock like cows, goats, and poultry. This is why the European Union and various countries regulate the maximum permitted levels of OTA in foods. This review intends to summarize all the main aspects concerning OTA, starting from the chemical structure and fungi that produce it, its presence in food, its toxicity, and methods of analysis, as well as control strategies, including both fungal development and methods of inactivation of the molecule. Finally, the review provides some ideas for future approaches aimed at reducing the OTA levels in foods.
Keywords: ochratoxin A, toxin, contamination, health complication, control
1. Introduction
Mycotoxins, which are naturally synthesized by fungi, can induce toxic responses if contaminated food is consumed by animals or humans. These compounds have gained global attention due to their remarkable economic implications, which are linked to their impact on human health, animal productivity, and food international trade [1,2].
Notably, the presence and proliferation of mycotoxigenic fungi species can exert a considerable influence on the quality and security of food resources [3].
Mycotoxins have been found in a wide range of products, spanning from raw ingredients to processed foods, including staples like bread, flour, pasta, and fruit juice [4]. While numerous mycotoxins have been identified, about 20 families cause issues in human and animal food. Among these, six families frequently occur in food commodities, including aflatoxins (AFs), ochratoxins (OTs), fumonisins (FBs), deoxynivalenol (DON), zearalenone (ZEA), and patulin [5,6]. Furthermore, their thermal stability often exceeds that of the fungi that produce them, making the elimination of mycotoxins difficult or impossible [7,8]. Exposure to mycotoxins can lead to both acute and chronic toxicities, ranging from death to detrimental impacts on various systems, including the central nervous system, cardiovascular system, respiratory system, digestive system, urinary system, and intestinal fibrosis [9]. Of note is their ability to modulate the immune system, thereby reducing resistance to infections, an effect that is now widely recognized as highly significant [10]. Supplementary Table S1 presents a summary of mycotoxins, the producing species, the contaminated foods, and the main effects on humans [11,12,13,14].
AFs, which are produced by Aspergillus species, are notorious for their potent carcinogenicity, and commonly contaminated crops are peanuts, corn, and tree nuts [15,16]. FBs, which are produced by Fusarium species, are common in maize and maize-based products [17]. DON and ZEA, which are also produced by Fusarium species, are found in cereals and grains [18,19]. Patulin, which is produced by Penicillium and Aspergillus species, have been detected in apple products and fruit juices [20,21]. Ochratoxins, mainly ochratoxin A (OTA), which is the subject of this review, are produced by Aspergillus and Penicillium species and can be found in foodstuffs, including cereals, coffee, cheese, and wine [22,23]. A single food product can be susceptible to multiple contaminations. Toxigenic fungi have the capacity to produce several mycotoxins, and the fungi of diverse species can synthesize identical mycotoxins. Additionally, the presence of a mycotoxin-producing fungus does not consistently correspond to the presence of mycotoxins in food products. Thus, humans and animals are often exposed not to a singular mycotoxin but frequently to a mixture of mycotoxins [24,25].
This review aims to cover all primary issues related to OTA, the best-known toxin among ochratoxins because of its widespread occurrence and good stability of the molecule. It contaminates a wide range of agricultural products, including cereal grains (wheat and corn), fruit juices, wine, tea, beer, and coffee [23,26,27,28,29,30]. Because these products are the basis of the human diet, it is virtually impossible to avoid consuming OTA, and in turn it originates toxic effects on humans such as carcinogenicity, immunotoxicity, neurotoxicity, hepatotoxicity, teratogenicity, and genotoxicity [31]. The same report indicates that cereals are the main source of OTA contamination, followed by wine, grape juice, coffee (roasted), and pork. As a consequence, mainly to prevent damage to human health, many countries have established permitted levels of OTA in foodstuffs, e.g., in the European Union, the maximum allowable value in cereals is 3 µg/kg [32]. This review describes the OTA chemical structure and fungi responsible for its production, occurrence in food, analytical methods, and strategies for control (involving both the management of fungal growth and approaches for the deactivation of the toxin). Furthermore, it concludes by presenting suggestions for future approaches aimed at mitigating the OTA issue.
2. Ochratoxin A (OTA): Chemical Structure, Fungal Producers, and Conditions for Formation in Commodities
OTA is produced by different fungi, primarily belonging to the genera Aspergillus, Penicillium, and Fusarium, including species such as A. ochraceus, A. carbonarius, A. niger, and P. verrucosum [33]. The principal fungi capable of toxin production are indicated in Table 1. Contamination generally arises from suboptimal agricultural methods, incomplete or too slow dehydration processes, and inadequate storage practices [6], while the coexistence of mycotoxigenic fungi and mycotoxins in seafood has recently become a source of serious global concern [34].
Table 1.
Aspergillus producing Ochratoxin A.
| Organism | Section | References |
|---|---|---|
| Aspergillus westerdijkiae | Circumdati | [35] |
| A. melleus | Circumdati | |
| A. ochraceus | Circumdati | |
| A. steynii | Circumdati | |
| A. subramanianii | Circumdati | |
| A. sesamicola | Circumdati | |
| A. affinis | Circumdati | [36] |
| A. muricatus | Circumdati | |
| A. occultus | Circumdati | |
| A. ochraceopetaliformis | Circumdati | |
| A. flocculosus | Circumdati | |
| A. pseudoelegans | Circumdati | [37] |
| A. roseoglobulosus | Circumdati | |
| A. sclerotiorum | Circumdati | [35] |
| A. persii | Circumdati | |
| A. salwaensis | Circumdati | |
| A. carbonarius | Nigri | [38,39] |
| A. niger | Nigri | |
| A. lacticoffeatus | Nigri | |
| A. sclerotioniger | Nigri | |
| A. alliaceus | Flavi | [40] |
| A. avenaceus | Flavi | |
| A. bertholletius | Flavi | |
| A. coremiiformis | Flavi | |
| A. leporis | Flavi | [41,42,43,44] |
| A. nomius | Flavi | |
| A. tamarii | Flavi | |
| A. pseudotamarii | Flavi | |
| A. vandermerwei | Flavi | |
| A. neoalliaceus | Flavi |
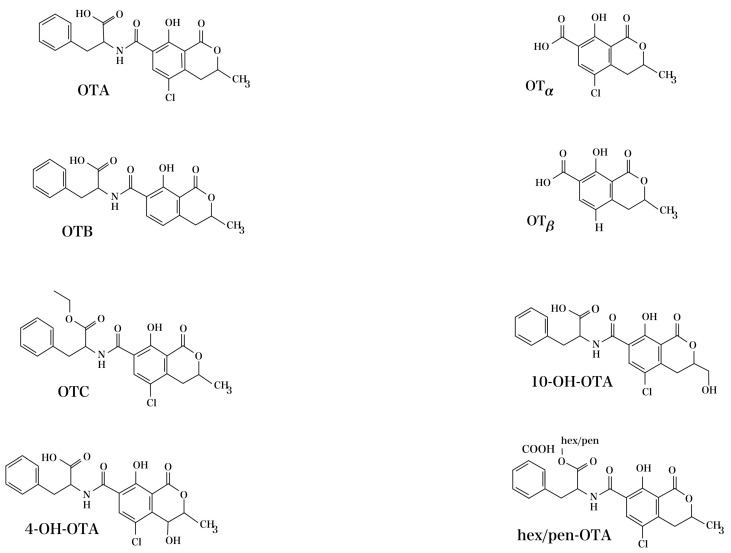
Originally discovered by South African scientists in 1965 [45], the chemical identity of OTA, which is formally known as L-phenylalanine, N-[[(3R)-5-chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl]carbonyl]-, molecular formula C20H18ClNO6, reveals an intricate structure. OTA is a member of the dihydrocoumarins. It is made up of an isocoumarin group that has been substituted (7-carboxy-5-chloro-8-hydroxy-3,4-dihydro-3R methyl isocoumarin) and is joined to L-ß-phenylalanine via an amide bond [46,47]. Figure 1 shows the chemical structure of OTA and its intermediary compounds, including ochratoxin B (OTB), ochratoxin alpha (OTα), and ochratoxin beta (OTβ).
Figure 1.
Chemical structure of OTA and its metabolites.
Pure OTA possesses a molar mass of 403.8 g/mol, and it appears as a white solid, with a melting point of 90 °C when crystallized from benzene; however the melting point climbs to 169 °C if OTA is crystallized from xylene [46]. OTA has a pKa of 7.1, making it a weak acid. OTA exhibits solubility in polar organic solvents under neutral and acidic conditions and has low solubility in aqueous solutions. However, when the pH is basic, it dissolves in alkaline/sodium bicarbonate-containing aqueous solutions. When exposed to ultraviolet (UV) radiation, OTA exhibits extraordinary fluorescence, generating a green fluorescence in an acidic environment and a blue fluorescence in an alkaline environment [46]. The OTA detection and analysis techniques are built on top of this peculiar fluorescence [47]. Generally stable, OTA can be kept in a refrigerator when dissolved in ethanol or methanol, and it is resistant to radiation and temperature [48].
The OTA-producing fungi are influenced by various factors, such as temperature, pH, moisture content, and water activity (aw). The aw is a critical factor for mold germination and growth on nutrient-rich substrates; for example, A. ochraceus produces very little OTA at aw 0.80 on green coffee but a considerable amount of OTA at aw value of 0.95: 7.2 mg/kg [49]. Temperature is another essential factor, with optimal ranges for OTA production being 25–30 °C for A. ochraceus, 10–20 °C for A. carbonarius, and 20–25 °C for A. niger [50,51,52]. In addition, the production of OTA by different fungal species is influenced by specific climate conditions. In South America, South Asia, and Africa, which are characterized by hot and relatively dry climates, Aspergillus species are the primary producers of OTA on maize [53]. Conversely, in temperate countries like the United States, Canada, and Europe, the Penicillium genus is the dominant OTA producer on cereals [54]. Additionally, the development of P. verrucosum and OTA formation varies in different grains, such as wheat, depending on moisture content and temperature, starting at 16% humidity [55] and rising with increasing humidity in the range of 10 to 28 °C [56]. Gas composition also influences fungal growth and OTA production in various food items, including dried fruit, alcoholic beverages, and coffee. On wheat grains, P. verrucosum growth and OTA production are inhibited by 50% CO2 [57]. OTA is mainly produced on moderately acidic foodstuffs, and pH does not have specific effects for A. ochraceus and A. carbonarius [58], even though a pH between 5.5 and 6.5 was optimal for the growth and OTA production of P. verrucosum and P. nordicum [59], and a pH of 5.35 was optimal for A. carbonarius and A. niger OTA production [60].
3. OTA Biosynthetic Pathway and Regulatory Mechanisms
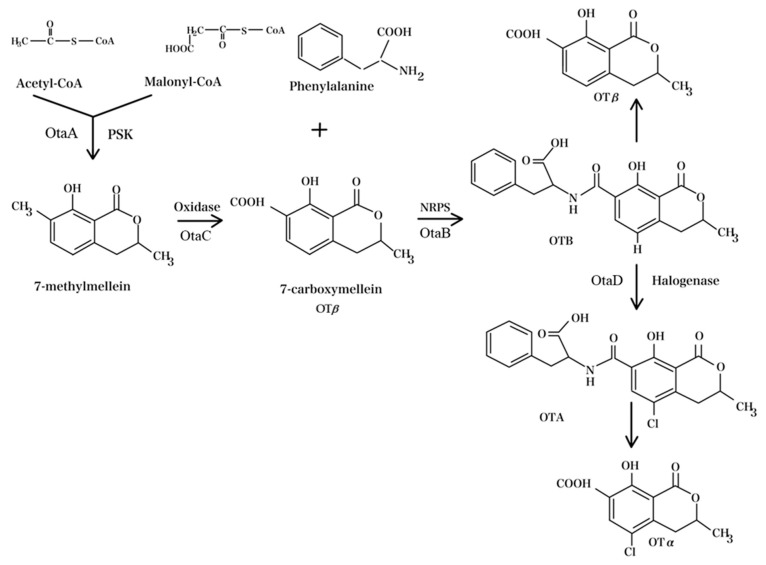
A key point of the OTA biosynthetic pathway was the identification and sequencing of the gene responsible for encoding a halogenase/chloroperoxidase [61]. A mutation in this gene resulted in an increased OTB level and an undetectable amount of OTA in A. carbonarius, indicating that the addition of a chlorine atom by halogenase represents the final biochemical step [62]. Genes encoding OTA biosynthetic enzymes were previously identified in A. carbonarius as multimodule enzymes like polyketide synthases (PKS) and non-ribosomal peptide synthases (NRPS) [63]. Chloroperoxidase’s role in introducing chlorine into OTA was identified in ochratoxigenic strains. The polyketide enzyme family includes domains like KS (β-ketosynthase), AT (acyltransferase), DH (dehydratase), and C-Met (C-methyltransferase), which are responsible for adding the methyl group to the OTA molecule, with ER (enoyl reductase), KR (ketoreductase), and ACP (acyl-CoA precursor carrier) domains located in the C-terminus. NRPS in fungi extends amino acid chains via modules reflecting the final product’s amino acid count and sequence [64,65]. The involvement of chloroperoxidase in the OTA pathway is supported by its chemical structure, with the chloroperoxidase genes, encoding the enzymes that add a chlorine atom at the C5 position, identified in A. carbonarius (AcOTAhal) and P. nordicum (otachlPN) [62,66,67]. In a study by Wang et al. [68], the OTA biosynthesis process (as depicted in Figure 2) was proposed to initiate with the enzyme OtaA, a PKS, which synthesizes 7-methylmellein using acetyl coenzyme A (1 acetyl-CoA) and malonyl-CoA. Subsequently, OtaC oxidizes 7-methylmellein into OTβ (7-carboxymellein), while OtaB, an NRPS, catalyzes the formation of an amide bond between OTβ and L-β-phenylalanine, resulting in OTB. Finally, OtaD, a halogenase, chlorinates OTB, resulting in the formation of OTA.
Figure 2.
Proposed OTA biosynthetic pathway, including intermediate metabolites and catalytic enzymes (redraw after [68]).
Hormonal signaling pathways and transcription factors have been identified as regulators of OTA biosynthesis. One significant regulatory complex is the “VelB/VeA/LaeA” velvet complex, which controls fungal development and secondary metabolite production in fungi also interacting with light receptors [69,70], particularly Aspergillus species [71,72]. In addition, Wang et al. [68] discovered two regulators of OTA biosynthesis in A. ochraceus, OtaR1, and OtaR2. Specifically, a mutation in OtaR1 determines a low-level expression of the OtaABCD genes, while the second regulator, OtaR2, modulates the expression of the OtaA, OtaB, and OtaD genes. The authors asserted that their comprehensive research has clarified uncertainties surrounding the OTA biosynthetic mechanism. Cell redox balance has also been implicated, as demonstrated by the disruption of the Aoyap1 gene in A. ochraceus, which, downregulating the antioxidant response of the cell, triggers OTA biosynthesis [73]. Fungi prefer easily metabolizable carbon sources, reducing the synthesis of enzymes related to other carbon sources, to increase OTA production growing in the presence of sugars, particularly fructose [36,74]. Osmotic stress, particularly in the case of high NaCl levels, influences OTA production differently among fungi. Penicillium species adapt to NaCl-rich environments by producing OTA as a mechanism to maintain cellular homeostasis. However, A. carbonarius lacks this adaptation and has limited OTA biosynthesis under osmotic stress caused by NaCl. The high osmolarity glycerol (HOG) signaling cascade, which is activated by osmotic stress, drives OTA production in NaCl-rich environments, but A. carbonarius appears to decouple OTA biosynthesis from this mechanism [75,76]. Hence, there is a need for further investigation to clarify the mechanisms by which OTA biosynthesis regulation responds to environmental signals [68].
4. Ochratoxin A Occurrence in Food
OTA has been identified in various agricultural products, including cereals and their byproducts, coffee, cocoa beans and chocolate, dried fruits, nuts, chili sauce, spices, ham, pork meat and salami, meat and meat products, poultry, milk, cheese, wine, and beer, all of which are dietary staples globally. These foods, mainly cereals, serve as primary sources of OTA contamination. Table 2 summarizes references concerning OTA occurrence from 2010.
Table 2.
OTA occurrence in food products.
| Matrix | Country | Number of Sample | Occurrence (%) | Maximum µg/kg | Mean µg/kg | Ref. |
|---|---|---|---|---|---|---|
| Corn, wheat | Pakistan | 40 | 27.5 | 360 | / | [77] |
| Rice | Iran | 65 | 4.6 | 11.5 | 5.02 | [78] |
| Barley and wheat | USA | 262 | 12.2 | 185.2 | / | [79] |
| Corn, rice, wheat, and oat-based foods | USA | 489 | 41 | 9.3 | / | [80] |
| Wheat | Canada | 232 | 2.2 | / | 14.7 | [81] |
| Wheat grain clumps around or under manhole openings of storage bins | Canada | 5 | 100 | 370 | [81] | |
| Wheat and derived samples | Algeria | 81 | 76.54 | 34.75 | / | [82] |
| Cereal-based foods | Algeria | 16.9 | 50 | / | 0.15 | [83] |
| Rye | Poland | 60 | 3 | 2.75 | / | [84] |
| Fermented coffees | Brazil | 14 | 21.4 | 0.87 | 0.18 | [85] |
| Roasted and instant coffee | Czech | 103 | 80.6 | 12.8 | / | [86] |
| Coffee bean roasted coffee and soluble coffee | Argentina | 51 | 69 | 20.3 | / | [87] |
| Roasted and instant coffee | Chile | 63 | 33 | 7.25 | 1.3 | [88] |
| Roasted coffee | Spain | 72 | 48.6 | 4.21 | 2.17 | [89] |
| Cocoa bean | Brazil | 123 | 22.8 | 7.2 | 1.2 | [90] |
| Cocoa and chocolate | Canada | 60 | 100 | 7.8 | 0.95 | [91] |
| Dried figs | Turkey | 100 | 8 | 1.72 | 0.64 | [92] |
| Dried grape | Iran | 66 | 40.9 | 8.4 | 2.98 | [93] |
| Palm dates | Tunisia and Algeria | 27 | 11 | 6.07 | 58.7 | [94] |
| Raisin | USA | 40 | 93 | 11.4 | 0.7 | [95] |
| Dried fruit and nuts | China | 253 | 1.6 | 9.39 | 6.23 | [96] |
| Chili sauce | Pakistan | 252 | 71 | 114 | / | [97] |
| Allspice, pepper, chili, cinnamon, ginger, and mixture | Italy | 94 | 30 | 34 | 7.1 | [98] |
| Grounded sweet pepper | Italy | 8 | 100 | / | 23.6 | [99] |
| Dried sweet pepper | Italy | 23 | 39 | / | 53.9 | [99] |
| Salami | Italy | 50 | 10 | 103.69 | / | [100] |
| Dry-cured ham | Italy | 110 | 76.4 | 5.64 | / | [101] |
| Dry-cured ham | Italy | 18 | 22.2 | 69.3 | / | [102] |
| Cheese | Italy | 30 | 13.3 | 4.7 | / | [102] |
| Cheese | Italy | 84 | 8.3 | 22.4 | / | [20] |
| Beef burger | Egypt | 25 | 100 | / | 4.55 | [103] |
| Chicken meat | India | 115 | 41 | / | 1.41 | [104] |
| Eggs | India | 80 | 35 | / | 1.17 | [104] |
| Milk | China | 120 | 25.8 | 18.8 | / | [105] |
| Red wine | Croatia | 110 | 98.2 | 0.163 | 0.040 | [106] |
| Red, rose, and white wine | Serbia | 113 | 52.2 | 0.134 | 0.026 | [107] |
| Wine | Spain | 40 | 47 | 2.28 | 1.13 | [108] |
| Beer | Mainly Portugal | 85 | 10.6 | 11.25 | / | [85] |
Included in Table 2 are matrices, such as cereals and dehydrated products (e.g., raisins, dried figs, spices), that are directly subject to primary contamination in the field. By entering the food chain, farm animals convey OTA into meat, milk, and processed products (e.g., wine and beer), resulting in the intake of OTA by humans. Obviously, storage in the presence of moisture and permissive temperatures for the growth of OTA-producing microorganisms increases the initial contamination. In fact, environmental factors, such as humidity, temperature, and water activity, play critical roles in OTA formation [109]. Therefore, OTA presence has been documented across continents, including Asia, America, Africa, and Europe. In any case, beyond the well-founded perception of the occurrence of OTA in tropical and subtropical regions with warm humid climates, accurate statistical surveys of identical products demonstrating greater contamination in specific countries are not available, especially because warm, humid climate areas are present in many countries in temperate zones, and also because, for example, the OTA spoilage of cereals also occurs in Canada during winter wheat storage, with OTA levels up to 360 µg/kg in grain clumps close to manhole openings [81]. In addition, transport from major grain-producing countries to importing ones is usually by sea, where the high humidity or rewetting may contribute to the occurrence of OTA.
When considering the overall dietary exposure to OTA in Europe, wine ranks as the second most significant source after cereals [31]. Grapes damaged by ochratoxigenic fungal infection, facilitated by the high sugar content, create an optimal environment for OTA synthesis during winemaking. Geographic and climatic variables influence OTA levels in wine, with contamination often occurring in the vineyard. Europe, being a prominent wine producer and consumer, has established a maximum OTA limit of 2 µg/L in wine through the European Commission Regulation 1881/2006 and following Regulations [32]. The maximum level (ML) for OTA is 3 µg/kg in roasted coffee and 5 µg/kg in soluble coffee [32], with coffee being the fifth largest source of OTA exposure in Europe after cereals, wine, beer, and grape juice [31]. In sixth position is cocoa, a key ingredient of chocolate and other food products. The EC has set an ML for OTA in cocoa at 3 µg/kg, 5 µg/kg for unprocessed cereal grains, and between 2 and 4 µg/kg for products derived from cereals, depending on the ingredients. In comparison, in Brazil, the maximum limit for OTA in coffee, cocoa beans, cereals, and cereal products is 10 µg/kg [110]. Although a significant portion of cocoa production occurs in Western Africa, reports of OTA contamination in cocoa are relatively limited [111]. Despite their status as nutrient-dense and widely consumed foods, fruits, dried fruits (e.g., figs, raisins), and nuts (e.g., almonds, pine nuts, hazelnuts, chestnuts, walnuts) are susceptible to OTA contamination [93]. Spices, commonly used in culinary preparations, are also prone to OTA contamination due to their production and storage conditions, even though their consumption is low and not comparable with the previously mentioned food items. In Europe, the OTA ML for dried spices is set at 15 µg/kg, while the limit for dried capsicum spp. is 20 µg/kg [32]. Furthermore, cereals constitute a significant component of animal feed, potentially leading to OTA accumulation in livestock and later in humans through the food chain. In fact, OTA was detected in milk; therefore, in the EU, the ML for OTA in infant formulates is 0.50 µg/kg [32]. Table 3 shows a comparison of the OTA maximum limits set in different countries, which indicates that the European Union [32], along with Brazil [110], Turkey, and Vietnam, have set stringent OTA limits for various plant products or processed products [112]. In contrast, the FAO WHO Codex Alimentarius merely suggests a limit of 5.0 µg/kg for unprocessed cereal grains and 20 µg/kg for Capsicum dried fruits [113]. Other countries, such as China, have set limits for only a few products (dehydrated fruits, cereals, and coffee), while Singapore has established an OTA ML of 2.5 µg/kg any food, in the same way Japan and Switzerland (excepting for baby food, 0.50 µg/kg) determined a unique ML of 5.0 µg/kg, while India fixed an OTA ML only for wheat, barley, and rye (20.0 µg/kg), and Canada stated limits only for swine and poultry food (0.2–2.0 µg/kg). Instead, the US set MLs for aflatoxins, deoxynivalenol, patulin, and fumonisins, but not for OTA [112]. Values set by law are, of course, indicative of the level of contamination and possible harm to health in different countries.
Table 3.
OTA maximum levels permitted in different countries for main food matrices.
| OTA Maximum Level in Different Countries (μg/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Matrix | European Union | Brazil | China | Turkey | Egypt | Vietnam | Codex Alimentarius |
| Dried vine fruits (currants, raisins and sultanas) and dried figs | 8.0 | 10.0 | 10.0 | 10.0 | 3.0 | ||
| Other dried fruits | 2.0 | 10.0 | |||||
| Dried herbs | 10.0 | ||||||
| Sunflower seeds, pumpkin seeds, (water) melon seeds, hempseeds, soybeans | 5.0 | ||||||
| Unprocessed cereal grains | 5.0 | 10.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5 |
| Bakery wares, cereal snacks and breakfast cereals | 2.0 | 3.0 | 3.0 | 3.0 | |||
| Roasted coffee beans and ground roasted coffee | 3.0 | 10.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| Soluble coffee (instant coffee) | 5.0 | 10.0 | 10.0 | 10.0 | 10.0 | ||
| Cocoa powder | 3.0 | ||||||
| Cocoa beans | 10.0 | ||||||
| Dried spices | 15.0 | 15.0 | |||||
| Capsicum spp. (dried fruits including chillies, chilli powder, cayenne or paprika) | 20.0 | 30.0 | 30.0 | 20.0 | |||
| Wine, fruit wine, aromatized wine, grape juice, grape must | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||
| Baby food and processed cereal-based food, for infants and young children | 0.50 | 2.0 | 0.50 | 0.5 | |||
However, the USA Food and Drug Administration (FDA) covers several essential elements of food safety, such as routine monitoring and sampling of a wide range of food items for the presence of mycotoxins, including OTA. In fact, Mitchell et al. [114] report sampling for the presence of OTA throughout the United States so that exposure can be calculated based on OTA concentrations in food products and consumption for different age groups of the population. Because OTA was not detectable in most of the samples analyzed, except for nuts, breakfast cereals, infant cereals, and cocoa, the (lifetime) margin of safety (MOS) for the USA population, within 95% of consumers of all possible products, was >1, indicating negligible risk. The authors concluded that, even in the absence of OTA regulations, the exposure to OTA in United States would not be associated with a significant risk of adverse effects [114]. Concerning Europe, the European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain estimated the margins of exposure (MOEs), and MOEs calculated for OTA neoplastic adverse effects, particularly those for high consumers and breastfed infants, were less than 10,000, indicating a possible health concern [31]. Thus, in observance of the European precautionary principle, the OTA MLs appear justified.
5. OTA Analytical Methods
Because of the intricate nature of the food composition and the usual minuscule presence of OTA in food, it is necessary to undergo some form of sample processing before testing food samples. Sample preparation is often a pivotal and demanding phase in the entire analytical procedure, and the approach can vary depending on the specific food matrix.
Sampling is crucial when analyzing OTA and other mycotoxins in food, as it significantly impacts the reliability of results and determines whether a food batch complies with safety standards. To ensure representativeness and accuracy, carefully designed sampling plans are essential [115,116].
Sample preparation for OTA analysis in food involves separating OTA from the food matrix, thereby enhancing detection sensitivity and specificity [117,118]. This process is influenced by the OTA chemical properties, food matrix nature, and chosen detection method [115]. OTA is typically extracted using organic solvents like methanol, acetonitrile, or acetone, sometimes with water or an acidic buffer to improve efficiency [115,119,120]. Other solvents tested were ethyl acetate and isopropyl alcohol [121]. An appropriate solvent must possess high OTA extraction capacity and not interfere (be neutral) with the subsequent steps of separation, identification, and quantification. Specifically, Prakasham et al. [121] showed in their work that acetonitrile is the solvent with the highest extraction capacity (greater than 90%), compared with the other four solvents mentioned above, on coffee, tea, and soil samples, while methanol was the least efficient solvent. Moreover, applying Fe3O4 nanoparticles for the analysis of the presence of OTA in sultana raisin samples, chloroform was selected over carbon tetrachloride, dichloromethane, and ethyl acetate [122]. The extraction of OTA from black pepper was better with an 80:20 methanol/water mixture [123], while methanol alone was more efficient than 70:30 methanol/water for OTA extraction from spiked rice samples [124]. Therefore, the solvent or mixture of choice will depend on the matrix type and subsequent cleaning of the extract and the final detection method. Cleaning up the extract is essential to enhance specificity and sensitivity, thereby improving accuracy and precision [119]. Methods for cleanup include liquid–liquid partitioning and, more recently, solid phase extraction (SPE) and immune-affinity columns (IAC). SPE involves specific partitioning of the analyte between a solid adsorbent and an organic solvent [121], while IAC uses antibodies to selectively bind mycotoxins [125]. A method called Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) simplifies extraction and cleanup using acetonitrile, salts, and dispersive SPE, making it a fast and cost-effective option with minimal solvent usage [126,127]. Four absorbents (activated carbon, amorphous graphite, graphitized carbon black, and octadecylsilane) suitable for SPE columns have been evaluated by Prakasham et al. [121], who concluded that the best matrix for cleaning samples is activated carbon. However, immunoaffinity columns represent an effective and robust tool for mycotoxin cleanup, but the prediction is that soon, molecularly imprinted polymers will be competitive with immunoaffinity-based materials [128,129].
Various food products, such cereals, coffee, chocolate, wine, beer, and dried fruits, can be evaluated for the presence of OTA using different methods/techniques.
Thin-layer chromatography (TLC) is a rapid screening method for OTA, in which a thin layer of a stationary phase is used to separate OTA based on their properties. Teixeira et al. [130] efficiently used TLC with a charge-coupled detector (CCD) for Brazilian red wine samples.
High-performance thin-layer chromatography (HPTLC) was introduced as an advanced analytical technology derived from TLC, known for its accuracy and efficiency [131,132].
High-performance liquid chromatography (HPLC) is a technique that is widely employed with a C-18 chromatography column as a stationary phase and a solvent as a mobile phase. This process separates molecules, and detectors record their retention times. Prior to HPLC, immunoaffinity column purification is often employed to remove impurities, ensuring accurate mycotoxin analysis [133,134,135].
Liquid chromatography coupled mass spectroscopy (LC/MS/MS) is an extremely sensitive and accurate technology for mycotoxin analysis, including OTA. Several researchers discussed the use of LC/MS/MS in OTA detection in animal-derived foods and its ability to detect OTA in various food and feed samples [136,137].
Polymerase chain reaction (PCR) and quantitative real-time PCR (qPCR), which are techniques used to quantitatively measure DNA or RNA in a sample, permit us to indirectly detect the presence of OTA by monitoring the gene expression associated with mycotoxin production, particularly in fungal species like Aspergillus and Penicillium [138,139,140].
Enzyme-linked immunosorbent assay (ELISA) is a widely used method for OTA detection, and a new, highly sensitive OTA ELISA has been developed using monoclonal antibodies. Proficiency tests and reference samples validate its accuracy. A direct ELISA format showed promise for OTA quantification in medicinal herbs, exhibiting a low IC50 value and strong correlation with HPLC, as well as a toxin-free ELISA, employing an anti-idiotypic nanobody as a surrogate standard, offer high sensitivity and accuracy, which is confirmed by HPLC correlation [141,142].
Table 4 shows a list of techniques for OTA determination in different matrices and their detection limits.
Table 4.
Analytical methods for OTA determination.
| Methods | Matrix | LOD * | LOQ * | Ref. |
|---|---|---|---|---|
| LC-MS | Cheese | 0.05 µg/kg | 0.15 µg/kg | [143] |
| LC-MS/MS | Bovine meat | 0.059–291.36 μg/kg | 0.081–328.13 μg/kg | [144] |
| 2D-HPLC | Beer, wine, corn, coffee | 21.2 pg/mL | 64.3 pg/mL | [145] |
| HPLC-MS/MS | Maize | 0.26 μg/kg | 0.87 μg/kg | [146] |
| UHPLC-MS/MS | Coffee | 3 pg/g | 1 pg/g | [121] |
| HPLC-fluorescence detection (FLD) | Spices | 0.03 ng/g | 1.0 ng/g | [147] |
| LC- LC-MS/MS | Cereals | 1 pg/kg | _ | [148] |
| Nanofluid extraction coupled with HPLC with fluorescence detector HPLC–FLD | Food | 0.2 μg/kg | 0.5 μg/kg | [122] |
| SPE–HPLC | Wine | 0.03 µg/L | 0.10 µg/L | [149] |
| qPCR | Coffee | 3.85 × 103 copies of the PKS gene | _ | [150] |
| magnetic beads (MBS) ELISA | Cereal | 0.07 ng/mL | 0.249–5.28 ng/mL | [151] |
| magnetic solid-phase extraction (MSPE) GC-MS/MS | Beer, Chinese Baijiu and vinegar | 0.03 µg/L | 0.1–800 μg/L | [152] |
| ELISA | Cereals | 0.001 ng/mL | 0.003–0.673 ng/mL | [142] |
| Electrochemical aptamer sensors | Food | 6.5 µM | _ | [153] |
| Photoluminescence immunosensor | Food commodities | 0.01 ng/mL | _ | [154] |
| Nanobody (Nb) Förster Resonance Energy Transfer (FRET) immunosensor | Cereal | 5 pg/mL | _ | [155] |
| Electrochemical (EC) sensors | Food | 0.38 ng/mL | 1–20 ng/mL | [156] |
| Aptamer based sensors | Food | 0.88 pg/mL | 1 pg to 300 ng/mL | [157] |
| Fluonanobodies (FN) nanosensor | Cereals | 5 pg/mL | 5–5000 pg/mL | [10] |
| Quartz crystal microbalance with dissipation monitoring (QCM-D) | Red wine | 0.16 ng/mL | 0.55 ng/mL | [158] |
| FRET-LFI | Coffee | 0.88 ng/mL | _ | [159] |
* LOD: Limit of detection; LOQ: Limit of quantification.
Chemical and electrochemical biosensors, including immuno, electrochemical, and optical types, based on nanoparticles or not, are effective tools for OTA detection. In fact, antibodies, aptamers, imprinted polymers, peptides, and DNAzymes have been employed as selective recognition receptors for the electrochemical biosensing of mycotoxins, including OTA [160]. Nanoparticles are utilized in colorimetric, fluorometric, enzymatic, and electrochemical assays, contributing to the precision and sensitivity of mycotoxin detection [160,161]. Biosensors are easy to use, require a minimal volume of sample, and are characterized by fast response, a limit of detection (LOD) up to 0.31 fg/mL, and a linear range of 1–50 ng/mL [162], or an LOD of 0.18 ng/mL and a linear range of 0.78–200 ng/mL for OTA [163]. The biosensors, being conceptually designed to be portable, allow for the on-site detection of OTA, making them attractive for the industrial market. Their extensive use is expected in the future, but, at present, biosensors are not yet commercialized.
Infrared spectroscopy uses infrared light to detect OTA without the need for sample preparation. It is fast and nondestructive but faces challenges with heterogeneous food matrices [164].
Fluorescence polarization (FP) measures the rate of rotation of a fluorophore and is used for detecting low-molecular weight materials like mycotoxins in a solution. It has limited sensitivity compared to HPLC [165].
Electronic nose (EN) mimics the human olfactory system and detects volatile compounds. It is still in development for OTA analysis and faces challenges with nonvolatile mycotoxins [166].
6. Strategies for OTA Control
Preventing the formation of OTA has emerged as a significant focal point to safeguard consumers against its detrimental impact on their well-being. The economic detriments stemming from OTA, in addition to its adverse consequences on public health, have spurred scientific endeavors to discover methods for averting the genesis of this toxin in agricultural settings. Equally, researchers are exploring techniques to detoxify OTA from food products. The effectiveness of these techniques varies depending on the chosen methodology, the timing of implementation, and notably, the specific fungal species [167,168].
The implementation of advanced agricultural technologies and good agricultural, manufacturing, and storage practices can mitigate OTA contamination [19]. To prevent fungal growth in plants, employing good agricultural practices like proper soil cultivation, crop rotation, efficient irrigation in drought conditions, accurate use of fungicides and fertilizers, timely harvesting, and adopting fungus-resistant crop varieties is vital [169]. Maintaining product quality during harvest necessitates using dry containers to manage agricultural produce and prevent fungal growth [170]. Inadequate preharvest methods can lead to fungal contamination and the buildup of OTA mycotoxin in stored agricultural products, particularly due to fungi like P. verrucosum and A. flavus infiltrating plants preharvest [171]. Postharvest techniques are essential for addressing OTA mycotoxin post-formation [172].
The control of postharvest OTA contamination revolves around two key strategies, as follows: firstly, the eradication of fungus-infected items, and secondly, the application of a spectrum of treatment methods to the products. These essential practices are incorporated into the storage and distribution phases of the food and feed supply chain. Additionally, this approach extends to the implementation of antagonistic microorganisms such as yeast, fungi, and bacteria, which serve as a shield against OTA production within consumables. Notably, the mere presence of fungus-infected items does not necessarily indicate the concurrent presence of OTA. Instead, the emergence of OTA hinges on a specific set of conditions encompassing nutrient availability, temperature, O2 levels, moisture, and time, all of which must coexist [173].
The methods used to decontaminate mycotoxins in food products should not only inactivate, destroy, or eliminate the toxin but also maintain the nutritional quality of the food, which should be acceptable for both human and animal consumption and should not significantly alter the technological properties of the product. It is important to note that there is no one-size-fits-all decontamination method that can be applied to all mycotoxins, as highlighted by Kamle et al. [19]. To address this challenge, decontamination strategies have been developed to specifically reduce OTA levels in food items. These strategies primarily rely on biological approaches that have no adverse effects on human health or the environment. This approach involves harnessing natural compounds like phenolic compounds and essential oils (Eos), which are derived from plants, as outlined in the studies by Dammak et al. [174] and El Khoury et al. [175]. Moreover, the use of Bacillus amyloliquefaciens, as demonstrated by Chang et al. [176], and lactic acid bacteria (Lactobacillus plantarum, L. graminis and Pediococcus pentosaceus), as shown in the research by Belkacem-Hanfi et al. [177] and Taheur et al. (Lactobacillus kefiri, Kazachstania servazzii and Acetobacter syzygii) [178], offers a safe and effective means of decontaminating food. Additionally, the application of bifunctional enzymes has proven to be a remarkable method for targeting specific mycotoxins, as discussed in the study by Azam et al. [179].
6.1. Chemical Methods
6.1.1. Synthetic Fungicides
The initial phase in controlling fungal contamination involves the strategic application of fungicides in cultivated fields, a critical step that must ensure postharvest product quality, as stressed by Amiri et al. [180]. Throughout the years, fungicides containing chemical compounds like benzimidazoles, aromatic hydrocarbons, and sterol biosynthesis inhibitors have played a pivotal role in agricultural practices, effectively combating various plant diseases. Certain fungicides such as pyrimethanil and the cyprodinil/fludioxonil combination have demonstrated remarkable efficacy in hindering fungal growth and reducing the production of OTA. The latter combination, which is particularly highlighted in studies by Belli et al. [181] and Lagogianni and Tsitsigiannis [182], has shown promising results in Mediterranean countries. However, the indiscriminate and excessive application of fungicides has resulted in the emergence of pathogenic strains that are resistant to these treatments [183]. Consequently, repeated fungicide treatments have become necessary, leading to an increase in toxic residues in food, especially in the case of nonbiodegradable or poorly biodegradable fungicides. Considering these complex issues, the imperative to urgently develop novel, safe, and biodegradable alternatives that remain effective and are economically sustainable over time is evident. This need arises from the necessity to address the limitations and adverse consequences associated with conventional chemical treatments, thereby fostering the exploration of sustainable and safer approaches in the ongoing battle against fungal contamination in agriculture.
6.1.2. Chemical Degradation of OTA
The strategy for detoxifying food commodities contaminated with OTA involves chemical methods to transform the toxin into other harmless substances. Common techniques include ammonization, alkaline hydrolysis, ozonation, and the use of compounds like bisulfites, acids, and oxidizing agents (Table 5).
Table 5.
Some chemical methods employed for OTA degradation.
| Method | Agents | Condition | Food Matrix | Effect on OTA | Ref. |
|---|---|---|---|---|---|
| Alkalinization | Potassium carbonate | 40 °C for pH 10 | Grape | Reduced by up to 50% | [184] |
| Ammonia | High pressure (60 psi) and normal temperature | Wheat | Reduced by 79% | [185] | |
| Potassium carbonate | 2% solution at 90 °C and 6894.76 kPa for 10 min. | Cocoa shells | Reduced by 93% | [186] | |
| Acidification | Acetic acid, citric acid, lactic acid, or hydrochloric acid | 50 °C for 24 h at pH 2 | Grape pomaces | Reduced by up to 67.23% (lactic acid) | [187] |
| Ozonation | ozone | 100 mg/L for 180 min | Corn | Reduced by 70.7% | [188] |
| ozone | gaseous ozone at 12.8 mg/L for 240 min | Sultanas | Reduced by 82.5% | [189] |
Ammonization has historically been a widely utilized method for degrading OTA in various grains, such as corn, wheat, and barley. Despite its effectiveness in reducing OTA levels, this process significantly compromises the taste and quality of the treated foods. Browning of cereals and the loss of essential amino acids are among the noticeable drawbacks, as highlighted by Bhatnagar et al. [190]. Consequently, due to these adverse effects on both sensory perceptions and nutritional value, ammonization is no longer recommended as a suitable method for OTA detoxification. Alkaline hydrolysis, utilizing hydrogen peroxide (H2O2) and sodium hydroxide (NaOH), has shown potential in degrading OTA. However, its practical application is limited by the negative impact it exerts on the sensory and nutritional characteristics of the treated materials. Ozone treatment (O3), when applied briefly, has been an effective method in eliminating OTA in hazelnuts and various plants, as reviewed by Afsah-Hejri et al. [191]. Ozone, which is recognized for its potent oxidizing properties, effectively eliminates fungi and bacteria and aids in food preservation. However, ozone treatment can cause physicochemical changes in the treated food so that it is necessary, prior to industrial use, to test the effect on different matrices [191]. This is because it is necessary to control the oxidation (and peroxidation) of lipids because ozone is able to trigger a rapid accumulation of reactive oxygen species (ROS) in plant tissues [192], causing in turn, negative effects ranging from color changes and the loss of vitamin C in fruits and vegetables to changes in some quality parameters and the formation of an unpleasant smell when O3 is applied to flour [193]. Conversely, it is reported that ozone treatment limits lipid oxidation, inhibits the formation of volatile compounds [194], and enhances the production of antioxidant- and stress-related secondary metabolites (e.g., polyphenols) [195]. Finally, ozone usage is also restricted because it is a toxic gas that demands a controlled and enclosed environment for its application, with precise dosing to prevent potential harm to human health, as indicated by Akbar et al. [196].
6.2. Physical Methods
Research has revealed novel insights into physical detoxification methods, specifically addressing OTA contamination. Initially, sophisticated cleaning methods like microfluidization have undergone scrutiny to improve the extraction of contaminants from harvested grains. This refinement enhances the efficiency of critical processes, such as husking, peeling, and dust particle elimination. The investigation into microfluidization and analogous advanced cleaning techniques seeks to enhance the efficacy of the decontamination process. By employing high-pressure fluid dynamics, these methods ensure a more comprehensive removal of contaminants, thereby mitigating the risk of the presence of OTA in food products. This not only bolsters the safety of the food supply but also contributes to the overall quality and purity of the end products [197]. The optimization of existing processes through advanced cleaning techniques underscores the dedication of recent research to advancing food safety standards. In the face of evolving technology, the ongoing exploration of innovative methods becomes pivotal to guaranteeing the effectiveness of detoxification processes and, consequently, upholding public health (Table 6).
Table 6.
Degradation of OTA by physical methods in food.
| Method | Condition | Food Matrix | Effect | Ref. |
|---|---|---|---|---|
| Heat treatment | 120 °C and 180 °C for 30 min and 60 min | Oats | Reduction of 2–18% | [198] |
| 150 °C for 50 min | Pistachios | Reduction of over 60% | [199] | |
| Ultraviolet radiation | 180 min of UV radiation |
Poultry feed | Completely decontaminated | [200] |
| Gamma radiation | 20 kGy, | Corn | Reduction of 61.1% | [201] |
| 30.5 kGy | Wheat flour, Grape juice, and wine | Reduction of 24%, 12%, and 23%, respectively | [202] |
Regarding heat treatment, OTA displays resistance to heat and can withstand various food processing methods, such as roasting, blending, and cooking, up to a certain limit [203,204]. Based on earlier research findings, it has been observed that while OTA exhibits thermal stability at temperatures of 180 °C and higher, its activity tends to diminish [31]. Aguilar-Alvarez et al. [205] indicated that only 20% of OTA can be degraded through exposure to 100 °C for 160 min or 150 °C for 32 min. Consequently, this approach unavoidably exerts a substantial adverse effect on food quality and is exclusively applicable in the preparation of specific food items, such as coffee roasting. The reduction of OTA at high temperatures and the formation of its degradation products were investigated by Cramer et al. [206]. They demonstrated that when OTA was exposed to temperatures of 175 °C and above, its levels decreased primarily due to the formation of a diastereomer called 2′R-OTA. Sueck et al. [207] observed similar changes, with OTA isomerizing at 120 °C. Up until now, 2′R-OTA has been detected in food items that underwent high manufacturing temperatures. Although the levels of this diastereomer were low, it was confirmed to be present in products like coffee, cocoa, bread, and others [208,209,210]. The efficacy of eliminating OTA from food at elevated temperatures is suboptimal. This is primarily because high-temperature treatments lead to the pyrolysis of other active constituents, especially for conventional food processing temperatures in the range of 80 to 121 °C [200].
Several studies have investigated the use of cold plasma treatment for OTA decontamination. Ouf et al. [211] conducted an experiment in which they intentionally contaminated date palm fruits with A. niger and then subjected them to a 7.5-min treatment using a cold argon plasma under double atmospheric pressure conditions. This treatment led to the complete elimination of OTA. However, because argon (Ar) is a costly noble gas and the 7.5-min treatment duration is relatively lengthy, Durek et al. [212] observed that subjecting barley to CO2 plasma generated through a diffuse coplanar surface barrier discharge (DCSBD) resulted in an increase in OTA production from 49 ± 13.8 ng/g (in the control group) to 72.9 ± 45.8 ng/g after a 3 min treatment. Hoppanova et al. [213] found that OTA production showed a significant increase during the initial 4 days of incubation after treatment with plasma for 60 and 90 s. However, after seven days of incubation, the OTA production was lower than that in the control group. The final yield of OTA in plasma-treated samples was observed to be lower compared to untreated samples. It is important to consider various process conditions like gas composition and storage time in practical applications. Nonetheless, there are also recent studies indicating that plasma treatment can lead to a reduction in OTA content in food; Casas-Junco et al. [214] have employed helium plasma to treat OTA in coffee and noted that a 30 min plasma treatment resulted in a 50% reduction in OTA content and a shift in toxicity from toxic to slightly toxic; a similar OTA reduction (53%) was obtained by Guo et al. [215] for rice grains. Therefore, cold plasma remains a promising method for OTA decontamination because it is very effective against microbial activities [215].
In addition, gamma ray irradiation is effective in reducing OTA levels in contaminated corn by disrupting OTA aromatic ring double bonds through the generation of highly reactive radicals. Electron beam irradiation (EBI) possesses the ability to degrade OTA, and the degradation efficiency increases in an irradiation dose-dependent manner, as demonstrated by Peng et al. [216] in aqueous solution. Their findings indicated a degradation process that conforms to a first-order kinetic model. However, the drawbacks of this technology are that the degradation of OTA is influenced by several factors, including irradiation dose, composition of the food matrix, and toxin initial concentration. Additionally, this process may affect the nutritional content/value of food, reducing the content of vitamins, proteins, unsaturated fatty acids, and probiotics [217,218]. Regulatory considerations have evolved alongside these advancements so that a more nuanced approach to dosage is recommended. The permissible dose limit of 10 kGy is being reevaluated based on a comprehensive risk assessment framework, considering both OTA reduction and potential byproducts [219].
Pulsed light (PL) stands as an innovative nonthermal technique for sterilization. This method employs brief bursts of high-intensity, wide-spectrum light energy (λ ranging from 200 to 1100 nm) lasting from 0.1 to 1 s. Its purpose is to inactivate the microbial load in food and, more recently, for the degradation (removal) of toxic molecules. This approach offers benefits, such as sterilization, safe operation, and reduced environmental impact. The mechanism behind the efficacy of PL encompasses photochemical, photothermal, and photophysical effects [220,221,222]. Wang et al. [223] explored the degradation effect of OTA in grape juice by PL, and the highest degradation rate of 95.29% was achieved after optimizing the process using response surface methodology (RSM); the treatment transformed OTA into less toxic compounds like OTα and phenylalanine.
Ultraviolet radiation (UV) has also been used both for inhibiting the growth of ochratoxigenic fungi and detoxification of OTA. Sumbal et al. [200] showed that UV irradiation for 1 h on poultry feed reduced the OTA concentration from 500 μg/kg to 100 μg/kg and achieved a level close to zero after 8 h. More recently, Zhang et al. [224] showed that A. carbonarius and A. ochraceus fungal growth and OTA production were significantly reduced using short-wavelength blue and UV-B light, which both promote OTA degradation and reduce OTA biosynthesis.
Simple mechanical techniques like sorting, cleaning, and milling play a pivotal role in significantly reducing OTA levels, thereby augmenting the safety of the final food product. One crucial advantage lies in the ability of such physical methods to preserve the nutritional quality of the food, as they typically do not involve chemical alterations in the nutrient components. This ensures the overall quality of the food. Another commendable aspect is the environmentally friendly nature of these physical methods, which do not involve chemicals or additives. This aligns with sustainable practices, addressing both consumer concerns regarding food safety and environmental impact. Moreover, the versatility of physical methods allows their application to various food types, rendering them adaptable and effective across different commodities susceptible to OTA contamination.
Despite these advantages, it is imperative to acknowledge certain limitations associated with physical methods for OTA contamination. A significant constraint is an incomplete elimination of mycotoxins, leaving a residual contamination. Additionally, physical methods show a low ability to treat mycotoxins present inside the food matrix, primarily addressing surface contamination. The impact on product quality is another general issue, especially for methods like milling or grinding, which may alter the texture, appearance, or flavor of the final product. Striking a balance between mycotoxin reduction and maintaining product quality can be a complex challenge full of nuances. Furthermore, the high initial investment required for advanced physical methods, such as sorting technologies or specialized equipment, may pose a limiting factor, particularly for smaller producers. Lastly, the applicability of physical methods to certain foods may be reduced, especially when dealing with delicate fruits or vegetables whose quality can be easily compromised. Therefore, for the reduction of OTA through physical methods, a very careful choice is needed considering the specific characteristics of the food product and production scale.
6.3. Adsorption by Physical–Chemical Agents
A different approach to removing OTA from agricultural products involves using adsorbent materials. These materials can bind OTAs, rendering them immobile. This process aims to eliminate OTA through a subsequent simple filtration. Adsorbent materials can be grouped based on their source (Table 7), including (i) natural materials such as jujube stones, oyster mushroom powder, dried fruit shells, olive pomace, and other fruit wastes [225,226,227]; (ii) inorganic minerals mainly represented by activated carbon, aluminum silicate, hydrated sodium calcium aluminosilicate, bentonite, zeolite, diatomaceous earth, and sea foam [228,229,230]; and (iii) organic synthetic materials such as modified silica gel, cellulose polymers, potassium caseinate, and cross-linked chitosan [231,232].
Table 7.
Elimination of OTA through adsorption.
| Material | Adsorbents | Medium/Matrix | Removal | Ref. |
|---|---|---|---|---|
| Ground nuts, coconut fiber, waste coffee grounds, and citrus peel | Liquid (ethanol/water mixture = 14/86, v/v) | Up to 100% | [233] | |
| Natural | Powdered Pleurotus ostreatus | in vitro gastrointestinal digestion | 85% at the end of the intestinal phase | [227] |
| Egg albumin, gelatin, chitin, and chitosan | Red Wine | Between 13% and 34% | [234] | |
| Inorganic Mineral |
Activated carbon 5 mg/mL | In vitro | More than 89% | [226] |
| Activated carbon | PBS and wine | 87–100% | [228] | |
| Activated carbon | Red and white wine | Up to 100% | [230] | |
| Bentonite-orange peel extract | Gastrointestinal fluids | Up to 2.13 mg/g | [235] | |
| Organic Synthetic |
Cyclodextrin-polyurethane polymer | Wine Samples | Up to 10 μg/L | [236] |
| Clay polymer nanocomposite | grape juice and wine | Up to 92% (15 μg/L) | [237] |
Among the various types of adsorbents studied, activated carbon and potassium caseinate have demonstrated the highest efficacy. When activated carbon is employed, around 90% of OTA is removed from red wine, while using 150 g/L of potassium caseinate results in an 82% reduction [238]. However, activated carbon should be used with caution because it also absorbs anthocyanins and other polyphenols from the wine. Similarly, the effectiveness of using oak wood fragments for treatment varies in relation to the quantity of wood chips or powder utilized [239]. Research by Olivares-Marín et al. [240] suggested that activated carbon derived from cherry stones could potentially eliminate up to 50% of OTA in wine without negatively impacting the overall polyphenolic index and color intensity. Conversely, other adsorbents like bentonite, cellulose acetate esters, polyvinylpyrrolidone, cholestyramine, and polygel have proven to be poorly effective in removing OTA [228,234,241,242,243], but Lactobacillus plantarum encapsulated in a polymeric matrix composed of polyvinyl alcohol and alginate, at a concentration of 0.5 g/mL, was able to remove over 50% of the OTA without substantially affecting the presence of total phenols in red wine [244]. Adsorption using physical–chemical agents has emerged as a promising strategy for the control of OTA contamination, demonstrating notable advantages in mycotoxin management. A key strength lies in the versatility of physical–chemical agents, allowing their application across a broad spectrum of food products and feed materials. This methodology preserves the nutritional quality of treated products; nevertheless, it could cause alterations in taste, color, or texture. However, selectivity in adsorption poses a potential limitation, restricting efficacy against a broad spectrum of mycotoxins and necessitating a nuanced approach for comprehensive mycotoxin control. The regeneration and reuse of adsorption agents presents drawbacks, as the saturation of adsorption sites over time may diminish the efficacy. Economic factors, particularly the cost associated with some agents, influence the feasibility of adoption, especially for small-scale producers, while environmental considerations, such as the origin and disposal of adsorbent substances, require careful evaluation to ensure sustainable practices in mycotoxin mitigation strategies.
6.4. Biocontrol Strategies for OTA Control
The negative drawbacks of using physical and chemical methods on the overall quality of food products has raised concerns within the field of toxicology. In response, the use of biological techniques that pose no threat to human health or the environment has been suggested [245]. They involve the utilization of the capabilities of microorganisms like bacteria, yeasts, and fungi, as well as natural compounds derived from plants, such as phenolic compounds and essential oils, to degrade, alter, or absorb OTA or limit the biosynthesis process. This represents a significant shift away from conventional methods, signifying a move towards safer, more environmentally conscious practices aimed at preserving food safety and quality.
6.4.1. Microorganisms and Enzymes
The exploitation of the ability of microorganisms to naturally resist OTA contamination has gained more attention in recent years. Table 8 shows the main results obtained in the attempt to solve OTA contamination.
Table 8.
OTA control by microorganisms and enzymes.
| Microorganism/Enzymes | Source | Reaction Condition | OTA (μg/mL) |
Degradation Rate (%) | Ref. |
|---|---|---|---|---|---|
| Bacteria/Actinobacter | |||||
| Rhodococcus erythropolis GD2A, BRB 1AB | Natural soil | 72 h/liquid medium | 2 | 27–34 | [246] |
| Cupriavidus basilensis ŐR16 | Soil | 5 d/liquid medium | 100 | >90 | [247] |
| Brevundimonas vesicularis | Vineyard soil | 28 °C/water | 1 | 100 | [248] |
| Acinetobacter calcoaceticus strain 396.1 | Vineyard soil | 6 d/liquid medium | 1 | 82 | [249] |
| Acinetobacter sp. Neg1 | Vineyard soil | 6 d/liquid medium | 1 | >70 | [250] |
| Bacillus amyloliquefaciens ASAG1 a | Grain, maize | 31 °C, 10 h/nutrient culture media | 1 | 98.5 | [176] |
| Luteimonas sp. CW574 | Soil and moldy food | 48 h/feed | 0.02 | 48.3 | [251] |
| Alcaligenes faecalis ASAGF 0D-1 | Soil | 48 h/liquid medium | 1 | 92 | [252] |
| Streptomyces AT10, AT8, SN7, MS1, ML5, G10,PT1 | Soil | 5 d/liquid medium | 0.095 | 23–53 | [253] |
| Bifidobacterium bifidum CECT 870T, B. breve CECT 4839T; Lactobacillus casei CECT 475T, Lactobacillus casei CECT 4040, L. casei CECT 4045, L. delbrueckii bulgaricus CECT 4005, L. johnsonii CECT 289, L. paracasei CECT 4022, L. plantarum CECT 220, L. plantarum CECT 221, L. plantarum CECT 222, L. plantarum CECT 223, L. plantarum CECT 748, L. plantarum CECT 749, L. rhamnosus CECT 278T, L. rhamnosus CECT 288, L. salivarius CECT 4062 | Spanish Type Culture Collection | 24 h/liquid medium | 0.6 | 30–97 | [254] |
| Lactobacillus rhamnosu | Winegrapes | TSB medium | 1 | 55 | [255] |
| Bacillus subtilis CW14 | fresh elk | 30 °C, 24 h/liquid medium | 1 | 71.3 | [256] |
| Leuconostoc paracasei ssp. Paracasei (3T3R1), L. mesenteroides ssp. Dextranicum (T2MM3) | Food | 20, 25, 30 °C, 10 days/MRS-CYA20S medium | NR | 7.3–100 | [257] |
| Brevibacillus sp.(ALJ01), Brevibacillus schisleri (ALJ02) | 24 h/MSM medium | 5 | 89.8, 96.5 | [258] | |
| Fungi | |||||
| A. carbonarius SA332 | French grapes | 5 d/liquid medium | 2 | 83 | [259] |
| Botrytis cinerea UdLTA 3·95, UdLTA 3·102, UdLTA 3·115 | Grapes | 7 d/solid grape synthetic medium | 1 | 24.2–26.7 | [260] |
| 124.niger M00120 | Soil | 2 d/liquid medium | 0.2 | 99 | [261] |
| Aureobasidium pullulans AU14-3-1, AU18-3B, AU34-2, LS30 | Apple leaves, Plum fruits, Grapevine leaves, Apple | 6 d/liquid medium | 0.8 | 75–90.5 | [262] |
| A. carbonarius 10614, A. niger 10,443 | Wine grapes | 25 °C, 7 d/Wine | 6 | 83.44 | [263] |
| Yeast | |||||
| Yarrowia lipolytica Y-2 | Vineyard soil | 2 d/liquid medium | 1 | 84 | [249] |
| Yarrowia lipolytica | vineyard | 2 d/liquid medium | 1 | 88 | [264] |
| Yarrowia lipolytica Y-2 | The surface of grapes | 28 °C/liquid medium | 1 | 97.2 | [264] |
| Metschnikowia pulcherrima MACH1, M320; Kloeckera lindneri GAL5; Pichia guilliermondii M8, M29; Rhodococcus erythropolis AR14 | Agroinnova culture collection centre (Italy) | 15 d/liquid medium | 7.5 | 25.8–84 | [265] |
| Enzyme | |||||
| Carboxypeptidase | Bacillus amyloliquefaciens | Overnight/Tris-HCL | 10 | 72 | [177] |
| Hydrolase | Aspergillus niger MUM 03 | 4 h/phosphate buffer | 1000 | Up to 154 ng/min g | [266] |
| Amidase 2 | Aspergillus niger | 30 min/Mops-NaOH | 0.85 | 83 | [267] |
| Carboxypeptidase PJ_1540 | Acinetobacter sp. Neg1 | Overnight/Tris buffer | 1 | 33 | [268] |
| Carboxypeptidase cp4 | Lysobacter sp. CW239 | 24 h | 250 | 86.2 | [269] |
| Peroxidase | Armoracia rusticana | 72 h/PBS | 0.01 | 27 | [270] |
| Laccase | Pleurotus eryngii | 72 h/sodium acetate buffer | 0.05 | 27 | [271] |
| Carboxypeptidase | Bacillus subtilis | 48 h/PBS | 1 | 71.3 | [256] |
| Metalloendopeptidase | Bacillus subtilis | 1 h, 41 °C/Phosphate buffer pH 4.6, 5 and 7 | 0.1 | between 8.2 and 45.3% | [272] |
| Amidohydrolase ADH3 | Stenotrophomonas acidaminiphila CW117 | 24 h, 60 °C/PBS, pH 7.2 | 0.05 | 100 | [273] |
The basic mechanism of OTA degradation involves the enzymatic breakage of its amide bond catalyzed by carboxypeptidase, thereby generating significantly fewer toxic intermediary products. Carboxypeptidase acts specifically on the amide bond within the OTA molecule, resulting in byproducts like Otα and L-β-phenylalanine, which are considered less hazardous than the original compound. In fact, by altering the molecular structure of OTA, carboxypeptidase degrades it into smaller, less harmful components, playing a crucial role in this degradation process. As highlighted in the study by Wei et al. [269], this enzymatic action is a pivotal step in OTA’s natural degradation process, reducing its toxicity. This knowledge is crucial for developing effective strategies to combat OTA contamination across different settings. Actinobacteria strains are characterized by a remarkable capacity to address OTA contamination, making them able to completely degrade OTA in liquid media and reduce OTA levels in solid media. However, it is important to recognize that degradation methods can vary, leading to differences in the observed breakdown products. Detecting common byproducts of degradation might not always be possible, indicating a few mechanisms of action. An example of one such mechanism involves the facilitation of OTA degradation through the hydrolysis of the OTA lactone ring, conducted by microbial enzymes like lactonohydrolases or esterases, as discussed in Campos-Avelar et al. [274]. This process is pivotal in dismantling OTA structure. The varied nature of microbial OTA degradation pathways indicates a microbial adaptability that is crucial in addressing OTA contamination in diverse environments. Understanding these multifaceted mechanisms is critical in crafting comprehensive and effective strategies to combat OTA contamination in various scenarios, covering agricultural fields to food processing and storage facilities, thereby ensuring safer and healthier conditions for consumers. Lactic acid bacteria (LAB) are renowned for their probiotic properties and various beneficial effects. Muhialdin et al. [275] suggested that specific strains of these bacteria have the potential to effectively bind or adsorb OTA. When these bacteria adsorb OTA, they essentially trap or bind the toxin, preventing its absorption by the body upon ingestion. This action could potentially mitigate the adverse effects of OTA on human health. The efficacy of this adsorption process holds significant promise for enhancing food safety. If certain strains of lactic acid bacteria can be harnessed to minimize OTA contamination in food products, it might provide a natural and potentially safer method to control the risks associated with this mycotoxin. In a study conducted by Fuchs et al. [276], Lactobacillus acidophilus VM20, a specific strain of Lactobacillus bacteria, demonstrated an exceptional ability to convert a substantial portion of OTA within a remarkably brief timeframe. The research reported an impressive conversion rate of 96% of OTA, even at a high concentration of 1 μg/mL, achieved in just 4 h, which is a very short time for toxin detoxification. Similar data were obtained by Luz et al. [254] who registered OTA reduction values of up to 97% and 95% with two different strains, L. rhamnosus CECT 278T and L. plantarum CECT 749; OTA reduction was both enzymatic and via adsorption on the cell wall. Similar results were obtained by Domínguez-Gutiérrez [277] when testing the effect of Lactobacillus plantarum cultures on the inhibition of the A. carbonarius growth and OTA production. However, the effectiveness could vary based on the specific bacterial strain, OTA concentration, and the type of food product. Further research and studies are required to better comprehend the mechanisms of OTA adsorption by these bacteria and to verify the safety and efficacy of this approach before it can be implemented as a solution. The study conducted by Shi et al. [278] presented fascinating findings regarding the ability of Bacillus subtilis CW14 in degrading OTA. They reported that the cell-free supernatant of this bacterium was remarkably effective, degrading an impressive 97.6% of OTA at a concentration of 6 μg/mL within a 24 h period. Notably, no byproducts of the degradation process were detected. Additionally, the research highlighted that both live and autoclaved B. subtilis CW14 cells bound more than 60% of OTA. This research presents intriguing possibilities for mycotoxin control in food safety. The effectiveness of the B. subtilis CW 14 cell-free supernatant in degrading OTA without generating byproducts is a significant finding. The understanding of the mechanisms involved in degradation and adsorption is vital for considering the application of such bacterial strains, which could potentially serve as a natural and safer strategy to minimize the risks associated with mycotoxin contamination. The investigation led by Rodriguez et al. [279] unveiled the capacity of Brevibacterium casei RM101 to thoroughly decompose OTA. Specifically, this bacterium accomplished an outstanding 100% degradation of OTA at a concentration of 40 μg/mL within 10 days. This discovery holds significance as it highlights the robust capability of B. casei RM101 to completely degrade OTA, suggesting its potential as an effective solution for managing and eradicating this mycotoxin from diverse food products. Upadhaya et al. [280] highlighted how Eubacterium biforme MM11 also effectively eliminates OTA. Within a 12 h incubation period, E. biforme MM11 successfully removed 77.1% of OTA at 0.1 μg/mL. This outcome underscores the potential of this bacterial strain as a means of bioremediation to minimize OTA contamination. Wei et al. [281] investigated how N-acetyl-L-cysteine (NAC) affects OTA and the combined impact with Cryptococcus podzolicus Y3 in degrading OTA. N-acetyl-L-cysteine, which is known for its antioxidant properties, has been under investigation for its potential to mitigate the toxic effects of OTA. The study findings unveiled that exposing C. podzolicus Y3 to NAC 10 mM resulted in significant improvements of the rate of degradation into Otα. Specifically, the rates of OTA degradation improved by 100% after 24 h and by 92.6% after 48 h. This highlights the substantial enhancement in C. podzolicus Y3′s capacity to break down OTA in the presence of NAC. Zeidan et al. [282] emphasized Burkholderia cepacia’s ability to combat various mycotoxigenic fungi. This bacterium has shown effectiveness against a broad spectrum of fungal genera and species known for producing mycotoxins. Additionally, the research noted that the liquid part/supernatant of B. cepacia culture had the capacity to hinder the production of OTA in A. carbonarius [282]. The antagonistic properties of this bacterium against mycotoxigenic fungi and its capability to limit mycotoxin creation represent another significant discovery in the context of food safety. Some of the strains known for their OTA-degrading abilities also displayed antagonistic activity. Specifically, B. subtilis CW14 [283] and B. megaterium [284] not only exhibited OTA degradation but also effectively inhibited fungal growth.
In addition to bacteria, fungi serve as crucial resources for degrading OTA. In a study conducted by Varga et al. [285], it was found that the atoxigenic A. niger CBS 120.49 strain could efficiently convert OTA (2.5 μg/mL) into Otα within just 5 days when cultivated on solid medium. This process was quicker compared to the 7 days needed when using a liquid medium. During a 7-day growth period on solid substrates, OTα was further converted into an unidentified substance. Among 55 strains of Rhizopus and Mucor, several strains of Rhizopus were able to break down OTA (7.5 μg/mL) to levels that could not be detected within 10 days when grown in a liquid medium. The A. niger (CBS 120.49) strain was especially effective, breaking down more than 90% of OTA within 6 days. In contrast, the R. stolonifer var. stolonifer strain TJM 8A8 required 12 days to achieve similar degradation in a liquid medium. In a separate trial, only R. stolonifer var. stolonifer TJM 8A8 managed to break down 96.5% of OTA (7.5 μg/g) within 10 days when applied to dampened wheat [286]. In addition, several strains of A. niger, A. carbonarius, A. japonicas, A. wentii, A. clavatus, A. ochraceus, A. pullulans, A. versicolor, Alternaria, Botrytis, Cladosporium, and Penicillium have demonstrated varying capacities for OTA degradation, leading to the formation of OTα and phenylalanine byproducts [287,288]. Various yeast strains and yeast-derived products are under scrutiny to determine their efficacy in actively countering or reducing OTA levels across diverse food products. Understanding how yeast can effectively inhibit or diminish OTA in food items represents a critical advancement in enhancing food safety measures.
Ponsone et al. [289] reported the substantial capability of Kluyveromyces thermotolerans-RCKT4 and K. thermotolerans-RCKT5 in reducing the growth of A. niger and A. carbonarius and OTA accumulation in grapes, from as low as 3% up to a remarkable 100% reduction. These results encourage further examination and potential consideration of yeast strains for use in food processing and agricultural practices against mycotoxins. Thereafter, Ponsone et al. [290] evaluated two Lanchancea thermotolerans strains’ efficacy in degrading OTA accumulation by ochratoxigenic fungi over three years in both greenhouse and field settings. Their research demonstrated that these yeast strains effectively controlled OTA accumulation in wine grapes at harvest by 27% to 100%. Saccharomyces cerevisiae have proven effective in diminishing OTA levels in musts and wines by a range of 20.3% to 76.4%. Other yeast species, such as Cyberlindnera jadinii, Candida friedrichii, Candida intermedia, and Lachancea thermotolerans, also contribute to lowering OTA levels, achieving reductions of 20% to 67.5%. Additionally, Trichosporon mycotoxinivorans exhibits complete degradation of OTA during the fermentation process [23,291]. Kloeckera apiculata strains 3187, 3188, 3189, 3197, 3198, and 3200 can eliminate between 25 and 40% of OTA (0.006 μg/mL) over 20 days [292]. In addition, Zou et al. [151] demonstrated that an employed UV–mutated Aspergillus niger (FS-UV-21) exhibited an impressive OTA degradation efficiency up to 89.4% under specific conditions, resulting in a substantial reduction of thein OTA cytotoxic effects of treated food. Finally, Fiori et al. [293], tested the capacity of viable and nonviable yeast strains to adsorb OTA, demonstrating that strains of Candida friedrichii, C. intermedia, Lachancea thermotolerans, and Cyberlindnera jadinii within an 8-day period exhibited OTA adsorption percentages of 70, 73, 75%, and no significant adsorption, respectively, whether vital, and 72, 74, 84, and 82% after autoclaving, respectively. Notably, a significant disparity in OTA adsorption capacity between the viable and nonviable yeast strains Cyberlindnera jadinii was observed in this research.
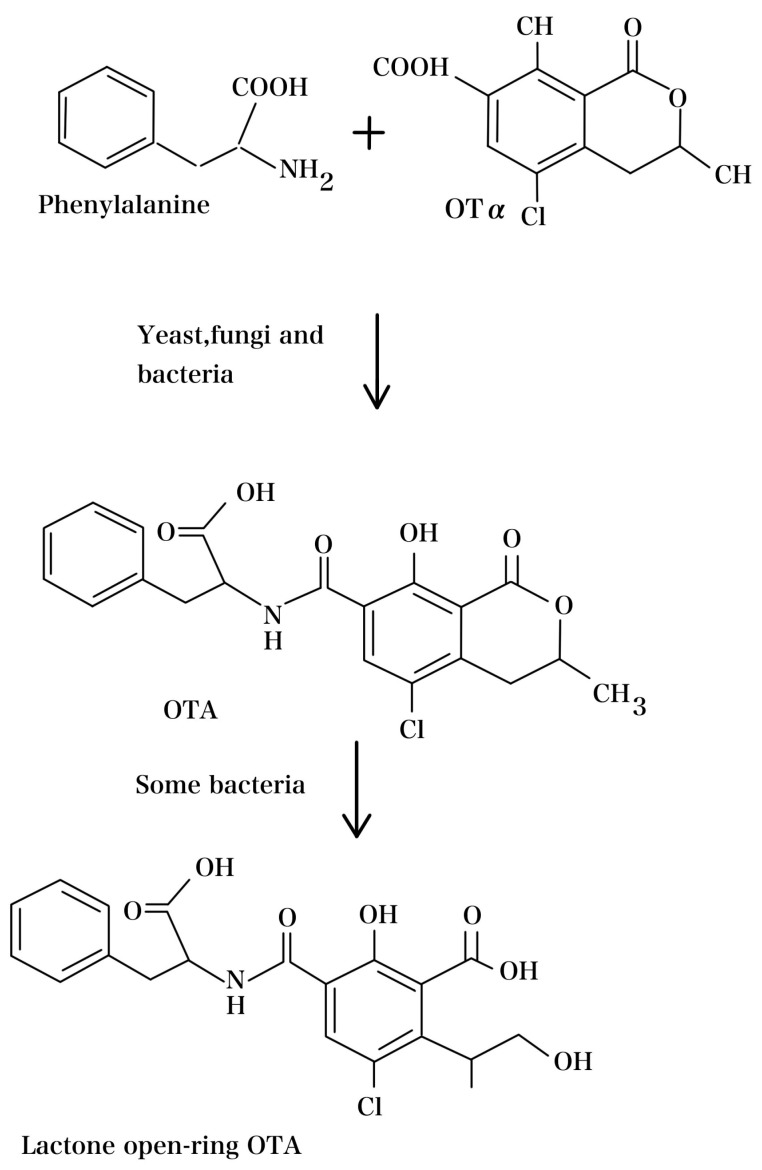
The primary mechanism for OTA degradation relies on enzymatic activity. It is worth noting that these enzymes can exist in either extracellular or intracellular forms [294]. The degradation process involves a range of enzymes, including lipases, laccase, amidases, dioxygenases, ochratoxinases, and proteases, all of which can catalyze the breakdown of OTA. Additionally, researchers have developed a recombinant enzyme with zearalenone hydrolase and carboxypeptidase capabilities for OTA degradation [271,295]. These enzymes have the capacity to degrade the OTA–amide bond or open the lactone ring, leading to the production of less toxic compounds, such as OTα (Figure 3).
Figure 3.
The biodegradation mechanism of OTA (redrawn after [296]).
Dobritzsch et al. [297] showed that a purified recombinant ochratoxinase was more efficient in hydrolyzing OTA than carboxypeptidase A (CPA) and Y (CPY), the two previously known enzymes capable of degrading OTA; the recombinant enzyme is thermostable and has optimal activity at pH 6 and 66 °C. Commercial enzymes protease A and pancreatin achieved 87.3% and 43.4% degradation, respectively, on OTA 1 µg/mL, while commercial prolyve PAC managed only 3% degradation at 37 °C and pH 3 [298]. Moreover, the purified metalloenzyme from A. niger possesses an OTA hydrolytic activity 12.8 times higher than CPA at 37 °C and pH 7.5, showing 36 U/mg activity [299]. In another study conducted by Abrunhosa et al. [300], commercial carboxypeptidase Y from S. cerevisiae exhibited its maximum hydrolytic activity on OTA (1.1 µg/mL) at pH 7.5 and 37 °C; however, the enzyme displayed relatively low specific activity at pH 5.6, as only 52% of OTA was transformed into OTα following a five-day incubation period. Cho et al. [288] found that crude enzymes from A. tubingensis strain M036 removed, within 24 h at 25 °C, 97.5% and 80.3% of the OTA present (0.04 μg/mL) at pH 5 and 7, respectively. Stander et al. [301] investigated the hydrolytic activity of a lipase preparation from A. niger on OTA, observing that the enzyme was able to convert OTA into OTα and phenylalanine; the purified enzyme achieved complete hydrolysis of 80 µg/mL of OTA in a 120 min incubation period at 37 °C pH 7.5. Dalsgaard et al. [302] patented an amidase and a related feed/food additive capable of degrading OTA. This enzyme, termed amidase 2, encoded by A. niger, effectively reduced the OTA concentration by 83% in a 300 µL reaction mixture containing 160 ng/mL amidase 2 and 50 µg/mL OTA. Furthermore, when tested in contaminated milk, the patented enzyme reduced the OTA content from 47 ppb to <2 ppb in just 2.5 h. Similarly, in corn flour, it lowered the OTA concentration from 38 ppm to <2 ppb after 20 h of incubation. Peng et al. [303] isolated from Brevundimonas naejangsanensis (strain ML17) four novel OTA and OTB degradases, denoted as BnOTase1, BnOTase2, BnOTase3, and BnOTase4. These enzymes facilitate the degradation of OTA and OTB into OTα and OTβ by enzymatically hydrolyzing the lactone molecular structure, achieving degradation rate of up to 100%; the Km of the recombinant enzyme that was more active on OTA was 19.38. Ma et al. [304] adopted immobilization technology to enhance the stability and degradability of CPA. By embedding CPA within zeolitic imidazolate framework materials, they achieved significant improvements. The immobilized enzyme demonstrated the ability to be reused over 10 times, and it exhibited a 30.7% higher degradation rate of OTA compared to free CPA. Finally, Luo et al. [273] reported that a recombinant ADH3 from Stenotrophomonas acidaminiphila (at 1.2 µ/mL) completely degrades 50 µ/L OTA within 90 s; the catalytic efficiency of the enzymes was estimated as 35,000 times higher than those of commercial carboxypeptidase A (CPA).
However, the direct employment of microorganisms capable of carrying out enzymatic reactions for OTA degradation on agricultural products and processed foods presents numerous advantages. These organisms offer a biologically sustainable approach to combatting OTA and are capable of either degrading the toxin or inhibiting the growth of its producing fungi, thus representing a natural alternative to chemical fungicides. Additionally, this method proves cost-effective and targets both OTA and OTA-producing fungi specifically, thereby minimizing harm to nontarget organisms and reducing ecological damage. Moreover, employing microorganisms for OTA control also has limitations. Effectiveness can fluctuate based on environmental factors like temperature, pH, and moisture. Regulatory barriers to approval and complex application methods are challenging, as is the search for a single microorganism that is effective against all OTA-producing fungi. Moreover, introducing new microorganisms into ecosystems could upset the existing microbial balance. Instead, enzymes represent a valuable tool in controlling OTA contamination due to their specificity in targeting OTA molecular structure and breaking it down into less harmful metabolites. Enzymes are safe and environmentally friendly because they are unable to reproduce and show versatility in degrading OTA; however, their activity also depends on temperature, pH, and moisture, and regulatory hurdles and cost concerns persist. Overall, the advantages of these biological control methods for managing OTA contamination in food are evident, but their limitations must be carefully taken into account for effective and safe implementation. Balancing these factors is crucial for advancing research, potential industrial integration, and securing regulatory approval in the domain of mycotoxin management.
In fact, a new emerging technique for the biological control of mycotoxins is the utilization of bacterial biofilms. In a recent study by Nahle et al. [305], the effectiveness of bacterial biofilms derived from L. rhamnosus was investigated for the removal of OTA from red grape juice. The assessment of OTA reduction in this beverage was conducted using ELISA, accounting for various influencing factors. The research unveiled the promising potential of this method for mycotoxin elimination. The application of bacterial biofilms, particularly those obtained from L. rhamnosus, offers an innovative and potentially impactful strategy to combat mycotoxin contamination, specifically in beverages like red grape juice. The study cited above contributes to the expanding realm of research exploring inventive methods for mycotoxin mitigation [305,306]. Not only does it demonstrate the efficacy of bacterial biofilms in reducing OTA and other mycotoxin levels but it also underlines the potential for these biofilms to be utilized in the food and beverage industry to uphold product safety and quality. Employing bacterial biofilms for mycotoxin removal signifies a new frontier in food safety strategies encouraging further investigations to refine this technique and potentially apply it to a broader array of food and beverage products.
6.4.2. Plant-Derived Extracts for OTA Biocontrol
Significant scientific research has been devoted to exploring the potential therapeutic applications of plants and medicinal herbs, which are strategically utilized to address minor ailments. The overarching goal of these studies is to enhance our understanding of the intricate mechanisms through which the diverse array of active compounds, including essential oils and extracts, found in these botanical sources effectively counteract the presence of mycotoxins.
The mode of action of plant-derived extracts involves three main aspects that contribute to their inhibitory function. Firstly, the presence of hydroxyl (OH) groups capable of forming hydrogen bonds affects enzymes and intracellular functions. Secondly, these extracts interact with membrane enzymes, altering the rigidity and integrity of hyphal cell walls, thus impacting mold morphology. Thirdly, changes in cell membrane permeability, cytoplasmic granulation, and the rupture of cytoplasmic membranes play a role. These aspects are interconnected, with hydrophobic compounds crossing cell membranes to interact with cellular components, affecting both membrane and intracellular enzymes. Hydrophobic compounds can change membrane permeability for ions, leading to proton flow alteration, cell pH modification, and subsequent cellular disruption. The solubility of these compounds in the lipid phase of cytoplasmic membranes is crucial for their activity, thereby impacting microbial cell permeability.
Within the spectrum of these active constituents, phenolic compounds demonstrate a potent capability not only to slow down fungal growth but also to neutralize and inhibit the toxic effects associated with mycotoxin exposure [35]. Phenolic compounds can affect membrane proteins, causing structural deformations and functional changes [307]. Other effects include the disruption of proton motive force, interference with energy generation, enzyme inhibition, and substrate utilization prevention. These actions collectively lead to the inhibition of spore germination, mycelial growth suppression, and germ tube elongation. Plant compounds may influence the switch from vegetative to reproductive development through signal perception/transduction [308,309].
Passone et al. [310] analyzed the impact of plant essential oils (EOs) on OTA production by fungi belonging to the Aspergillus section Nigri. The primary aim was to discern the influence of different EOs on OTA accumulation and how environmental factors, particularly water activity (aw), contributed to these interactions. Notably, boldo (Pëumus boldus) EO exhibited inhibitory effects on OTA accumulation at a concentration of 2000 μL/L, suggesting its potential to effectively impede OTA production by ochratoxigenic fungi. However, the study uncovered intriguing complexities in the effects of poleo (Lippia turbinata) EO at different aw levels; at the high aw of 0.98, 1000 μL/L of poleo EO unexpectedly stimulated OTA production of the majority of A. niger and A. carbonarius strains. Moreover, clove (Syzygium aromaticum) EO demonstrated a pronounced inhibitory effect at a lower aw of 0.93, and at a concentration of 5000 μL/L, it achieved complete suppression of OTA production, suggesting its potential efficacy in inhibiting OTA synthesis, especially under conditions of lower aw. These results underscore the intricate relationship between EOs, environmental factors like aw, and OTA production by ochratoxigenic fungi. These findings hold promising implications for devising strategies aimed at controlling mycotoxin contamination in food products. In a 2019 study by Dammak et al. [174], the efficacy of EOs obtained from Salvia officinalis, Lavandula dentata, and Laurus nobilis (primary component 1,8-cineole), was tested for their potential to inhibit fungal growth and the production of OTA. L. nobilis EO demonstrated the highest inhibition of fungal growth, surpassing L. dentata and S. officinalis, with inhibition percentages of 47.82%, 37.92%, and 31.71%, respectively. Notably, L. nobilis EO exhibited a remarkable 88.87% reduction in OTA production. Moreover, in contact assays, both L. nobilis and L. dentata EOs displayed heightened antifungal and anti-ochratoxigenic activities compared to S. officinalis. Their minimum inhibitory concentrations (MICs) were approximately 0.3%, while S. officinalis EO exhibited a slightly higher MIC at 0.5%. Importantly, the MIC of 1,8-cineole, a key component in these EOs, was about twice that of L. nobilis and L. dentata EOs. These results underlined the varying effectiveness of different EOs and their components in inhibiting both fungal growth and the production of OTA. Particularly, L. nobilis EO emerged as notably effective in suppressing fungal growth and reducing OTA production. The data also highlight the potential utility of L. dentata EO against ochratoxigenic fungi.
Among EOs, starfruit seed EO has shown promise in significantly reducing OTA production. Researchers have noted a gradual reduction in OTA production with increasing doses of starfruit seed EO. Notably, this reduction did not solely hinge on the inhibition of A. ochraceus fungus growth, a fact supported by studies like the one conducted by Murthy et al. [311].
Scientific investigations have additionally spotlighted EOs as potential regulators of the genes involved in the synthesis of OTA. In the concentration range of 75–150 mg/mL, lemon EO has demonstrated a distinct capacity to impede OTA production in A. ochraceus; its disruption in OTA synthesis appeared to stem from the suppression of both OTA biosynthesis and its associated regulatory genes Hua et al. [312]. What is particularly intriguing is the mechanism of action, which does not merely hinder fungal growth but appears to directly interfere with the molecular processes responsible for OTA biosynthesis, highlighting the potential to target specific step of mycotoxin biosynthesis. Similarly, an independent study conducted by Ferrara et al. [313] revealed a noteworthy reduction in the expression of the polyketide synthase AcOTApks gene in A. carbonarius when exposed to a concentration of 0.065 mg/mL of two hydrocinnamic acids, ferulic acid and p-coumaric acid. These findings served as compelling evidence of the influence of specific compounds present in essential oils on the genetic expression of pivotal genes involved in mycotoxin biosynthesis. The observed marked reduction in the AcOTApks expression emphasizes the potential of EO compounds to disrupt the genetic pathways responsible for mycotoxin production. El Khoury et al. [314] performed a comprehensive investigation into the inhibitory impact of various EOs like fennel, cardamom, anise, chamomile, celery, cinnamon, thyme, taramira, oregano, and rosemary on the growth of A. carbonarius strain S402. Their findings unveiled a direct link between the reduction in OTA production and the suppression of two known biosynthetic genes involved in the OTA biosynthesis (acOTApks and acOTAnrps), as well as the acpks gene and the two regulatory genes (veA and laeA). In the presence of fennel EO at a concentration of 5 μL/mL, acOTApks experienced an impressive reduction of 99%. Moreover, the effects of these EOs on the transcriptional factors veA and LaeA were notable, showing significant decreases at the same 5 μL/mL concentration. Notably, the most substantial reductions in the expression of the veA and laeA genes were observed after the addition of EOs from fennel (30% and 40%), cardamom (71% and 70%), chamomile (94% and 96%), rosemary (95% and 91%), anise (87% and 80%), and celery (88% and 80%), respectively. Lately, El Khoury et al. [175] repeated the analysis of the effects of EOs and phenolic compounds on A. carbonarius S402. They observed that while specific compounds had a limited impact on the dry mass of the fungus, they significantly influenced OTA production. EOs derived from bay leaves, mint, and melissa notably reduced OTA production by 72%, 70%, and 80%, respectively. In contrast, phenolic extracts exhibited effectiveness in inhibiting OTA production but generally to a lesser extent compared to EOs, with fenugreek being a notable exception. In the case of fenugreek, its EOs at a concentration of 1 μL/mL increased OTA production by 45.1%, while its phenolic compounds inhibited it by 32.0%, but the toxin production was reduced by 60% with EO 5 μL/mL. In El-Desouky research [315], aqueous extracts obtained from Sonchus oleraceus, Cichorium pumilum, and Portulaca oleracea hindered the production of OTA by A. ochraceus. The inhibition determined by plant extracts (5 mg/mL) were notably high, 77.5%, 72.3%, and 85.2%, respectively. These results indicated the potential of the aqueous extracts, as well. Recently, Naz et al. [316] assessed the antifungal properties of eight eOs against A. parasiticus using cereal grains as substrate. The researchers emphasized the exceptional efficacy of Cinnamomum verum EO, which displayed the lowest MIC (1.04 μg/mL) among the EOs tested. EOs from Syzygium aromaticum, C. verum, and Eucalyptus globulus exhibited substantial antifungal effects, eradicating A. parasiticus spores within 30, 60, and 90 min, respectively, even in contaminated grains. Additionally, maintaining a 10% moisture content in broken rice resulted in minimal OTA presence (8.16 ng/g) in stored grains. In addition, the study noted an increase in OTA production with moisture levels up to 40%, but this trend reversed at higher moisture levels of 50% to 70%, but no increase in OTA was observed in grains treated with C. verum EO.
Nanoencapsulation formulations stand as a dynamic and evolving research field, showcasing a promising synergy between encapsulated substances and phytochemicals, thereby minimizing potential side effects. Opting for natural formulation-based antifungal agents presents a safer, more efficient, and eco-friendly approach in combating fungi and mycotoxins in agricultural settings, compared to conventional methods. This line of thought finds support from the research by El-Desouky and Ammar [317], Pushpalatha et al. [318], Dhakar et al. [319], Redondo-Blanco et al. [320], Sajid et al. [321], and Thipe et al. [322].
A significant area of interest in this domain involves the development of nanosponges derived from β-cyclodextrin, housing natural bioactive substances. These meticulously engineered nanosponges are crafted to impede the growth of harmful fungi and eradicate mycotoxins present in both food and feed. They are poised to serve as potential biofungicides, aiming to supplant synthetic pesticides. Studies conducted by Pawar et al. [323], Sherje et al. [324], Bhowmik et al. [325], Haimhoffer et al. [326], and Ananya et al. [327] further underscore the potential of these nanosponges in addressing mycotoxin-related threats. The combinatory potential of nanosponges with botanical or other compounds presents a promising avenue in tackling phytotoxic issues. For instance, encapsulated herbicides have exhibited success in combating parasitic weeds while minimizing adverse environmental impacts, as illustrated in the work of Asghari et al. [328]. The study by Appell and Jackson [236] investigated the efficacy of a polymer (nanosponge) composed of β-cyclodextrin and polyurethane in reducing levels of OTA in water and red wine. The lowering of OTA levels in red wine from 10 μg/L to the recommended limit of 2 μg/L is particularly noteworthy, as this is crucial for compliance with regulatory standards and ensuring the safety of the product for consumption. The study revealed the nanosponge maximum binding capacity of 220 μg OTA per gram of polymer. The higher binding capacity suggests that this nanosponge has a considerable affinity for OTA molecules. The use of β-cyclodextrin in the nanosponge is strategic due to its ability to form inclusion complexes with various molecules, which aids in the adsorption and removal of specific compounds like OTA. The combination with polyurethane might provide structural stability and enhance the overall effectiveness of the nanosponge. Appell et al. [329] presented an innovative methodology by creating a solid phase extraction sorbent utilizing a nanosponge formed from a mix of β-cyclodextrin and methylene bis-diphenyl diisocyanate in a 1:5 ratio. The primary aim was to evaluate the efficiency of this polymer in extracting OTA from wine and grape juice samples. Recovery rates within the range of 69.1% to 89.4% for OTA concentrations ranging from 0.5 to 20 ng/mL demonstrated the polymer effectiveness in extracting the toxin, spanning concentrations commonly encountered in these beverages. Additionally, the inclusion of methylene bis-diphenyl diisocyanate in the polymer likely contributes to the nanosponge structural stability and binding properties.
The application of plant extracts and EOs for controlling OTA contamination encounters several significant challenges as variability in their efficacy depends on the wide range of plant types, extraction methods, and the multitude of fungal strains involved in OTA production. Moreover, precise regulations regarding acceptable limits and the food use of essential oils are absent. Also, cost, availability, shelf life, and environmental impact are significant barriers to their practical application. The same is true for nanoencapsulation-coated plant extracts; the complexity of the encapsulation process, associated costs, and the need for continued research and development present significant obstacles for their implementation within food safety strategies.
7. Prospects and Application
A wide range of approaches, both scientific and technological, as well as regulatory and educational, need to be applied in the future to counter OTA contamination. This will take interdisciplinary cooperation, innovation, and a dedication to food safety to pursue the objective. Therefore, it will be necessary to take the following actions:
-
(a)
Continuous improvements in analytical techniques, such as mass spectrometry and molecular approaches, will make it possible to identify low levels of OTA in food items more quickly.
-
(b)
The development of tests based on nanotechnology and biosensors for OTA detection can offer extremely sensitive and selective detection techniques, obviating the requirement for sophisticated laboratory apparatus and knowledgeable staff.
-
(c)
Predictive modelling, risk assessment, and early warning systems can be improved by incorporating machine learning and artificial intelligence algorithms into OTA analysis. These technologies can assist in locating high-risk areas and products, enabling focused monitoring and control efforts.
-
(d)
Researchers should explore diverse avenues to combat OTA contamination. Bioengineered microorganisms or molecular modified enzymes, with optimized OTA-degrading capabilities and stability, offer promise. In fact, it is desirable to obtain new, genetically engineered enzymes with higher pH stability, active over a wider temperature range, stable over time, and characterized by high expression/high yield in microbial culture; this could be a relevant step forward. Incorporating such microorganisms, especially enzymes, into food and beverage fermentation processes could reduce contamination, especially in items like wine, beer, and dairy products.
-
(e)
Crop susceptibility to OTA-producing molds can be decreased by developing best practices for crop management, including breeding programs for OTA-resistant crop varieties. Additionally, crop cultivation may be optimized and fungal contamination minimized with the aid of precision agriculture and weather forecasts.
-
(f)
OTA contamination during food production and distribution can be reduced due to advancements in food processing and storage technologies. Products shelf lives can be increased while OTA levels are decreased using better packing materials, altered atmospheres, and irradiation procedures.
-
(g)
Increased international collaboration and unified rules are also necessary. International standards, stricter laws, and the introduction of monitoring systems to verify compliance across borders are all possibilities for the future.
-
(h)
Demand for safer products may rise with increasing customer knowledge of the dangers of OTA-contaminated food. Future applications might make use of QR codes and mobile apps to inform customers about the OTA (low) content of the goods they buy.
-
(i)
Enhancing food safety measures in emerging markets might contribute significantly to both OTA contamination and control efforts as the world food trade expands.
-
(j)
Individuals may have the opportunity to assess their susceptibility to mycotoxin exposure through advancements in personalized nutrition and healthcare. This information could inform dietary choices and lead to customized recommendations for minimizing OTA intake.
8. Conclusions
Given OTA classification as a food contaminant and its potential to impact human and animal health adversely, the imperative for developing precise and sensitive detection methods has taken precedence. Prior to quantitatively assessing OTA, samples must undergo meticulous cleansing and extraction procedures. OTA holds a prominent position among mycotoxins and is notorious for its association with food security concerns. Its global significance stems from both its toxicity and the resulting implications for human and animal well-being.
Addressing the challenges posed by OTA involves incorporating preventive measures before, during, and after harvest, alongside implementing detoxification strategies with natural, synthetic products and standardized microorganisms as the techniques to be applied. By doing so, the potential for highly toxic food products can be mitigated, thereby averting substantial challenges in the realms of food marketing, distribution, and consumption.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13081184/s1, Table S1. Main mycotoxins, fungal producing species, major contaminated foodstuffs, and their effects on humans and animals [11,12,13,14].
Author Contributions
Conceptualization, Investigation, Writing-original draft, review and editing Y.B.M.; Writing-original draft I.C., D.D. and A.B.; Review and editing, supervision A.L. and L.D.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.González-Curbelo M.Á., Kabak B. Occurrence of Mycotoxins in Dried Fruits Worldwide, with a Focus on Aflatoxins and Ochratoxin A: A Review. Toxins. 2023;15:576. doi: 10.3390/toxins15090576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukhtar K., Nabi B.G., Ansar S., Bhat Z.F., Aadil R.M., Mousavi Khaneghah A. Mycotoxins and consumers’ awareness: Recent progress and future challenges. Toxicon. 2023;232:107227. doi: 10.1016/j.toxicon.2023.107227. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen P.A., Strub C., Fontana A., Schorr-Galindo S. Crop molds and mycotoxins: Alternative management using biocontrol. Biol. Control. 2017;104:10–27. doi: 10.1016/j.biocontrol.2016.10.004. [DOI] [Google Scholar]
- 4.Ukwuru M.U., Ohaegbu C.G., Muritala A. An overview of mycotoxin contamination of foods and feeds. J. Biochem. Microb. Toxicol. 2017;1:101. doi: 10.3329/bjlr.v0i0.45441. [DOI] [Google Scholar]
- 5.Ben Miri Y., Ariño A., Djenane D. Study of antifungal, anti-aflatoxigenic, antioxidant activity and phytotoxicity of Algerian Citrus limon var. Eureka and Citrus sinensis var. Valencia essential oils. J. Essent. Oil Bear. Plants. 2018;21:345–361. doi: 10.1080/0972060X.2018.1456363. [DOI] [Google Scholar]
- 6.Li X., Ma W., Ma Z., Zhang Q., Li H. The occurrence and contamination level of ochratoxin A in plant and animal-derived food commodities. Molecules. 2021;26:6928. doi: 10.3390/molecules26226928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla R., Singh P., Prakash B., Dubey N.K. Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm.) Sweet essential oil and its major component 1, 8-cineole against fungal isolates from chickpea seeds. Food Control. 2012;25:27–33. doi: 10.1016/j.foodcont.2011.10.010. [DOI] [Google Scholar]
- 8.Pallarés N., Berrada H., Tolosa J., Ferrer E. Effect of high hydrostatic pressure (HPP) and pulsed electric field (PEF) technologies on reduction of aflatoxins in fruit juices. LWT Food Sci. Technol. 2021;142:3769. doi: 10.1016/j.lwt.2021.111000. [DOI] [Google Scholar]
- 9.Rhee K.H., Yang S.A., Pyo M.C., Lim J.-M., Lee K.-W. MiR-155-5p Elevated by Ochratoxin A Induces Intestinal Fibrosis and Epithelial-to-Mesenchymal Transition through TGF-β Regulated Signaling Pathway In Vitro and In Vivo. Toxins. 2023;15:473. doi: 10.3390/toxins15070473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su B., Zhang Z., Sun Z., Tang Z., Xie X., Chen Q., Hammock B.D. Fluonanobody-based nanosensor via fluorescence resonance energy transfer for ultrasensitive detection of ochratoxin A. J. Hazard. Mater. 2022;422:126838. doi: 10.1016/j.jhazmat.2021.126838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaniou-Grigoriadou I., Eleftheriadou A., Mouratidou T., Katikou P. Determination of aflatoxin M1 in ew’’s milk samples and the produced curd and Feta cheese. Food Control. 2005;16:257–261. doi: 10.1016/j.foodcont.2004.03.003. [DOI] [Google Scholar]
- 12.Hymery N., Sibiril Y., Parent-Massin D. In vitro effects of trichothecenes on human dendritic cells. Toxicol. Vitr. 2006;20:899–909. doi: 10.1016/j.tiv.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Oguz H. Mikotoksinler ve Önemi (Mycotoxins and their importance) Turk. Klin. J. Vet. Sci. Pharmacol. Toxicol. Spec. Top. 2017;3:113–119. [Google Scholar]
- 14.El-Sayed R.A., Jebur A.B., Kang W., El-Demerdash F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Fut. Foods. 2022;2:91–102. doi: 10.1016/j.jfutfo.2022.03.002. [DOI] [Google Scholar]
- 15.Nazhand A., Durazzo A., Lucarini M., Souto E.B., Santini A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods. 2020;9:644. doi: 10.3390/foods9050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickova D., Ostry V., Malir F. A recent overview of producers and important dietary sources of aflatoxins. Toxins. 2021;13:186. doi: 10.3390/toxins13030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamle M., Mahato D.K., Devi S., Lee E.K., Kang S.G., Pradeep K. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins. 2019;11:23. doi: 10.3390/toxins11060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benthem de Grave X., Saltzmann J., Laurain J., Rodriguez M.A., Molist F., Danicke S., Santos R.R. Transmission of zearalenone, deoxynivalenol, and their derivatives from sows to piglets during lactation. Toxins. 2021;13:37. doi: 10.3390/toxins13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Yang C., Mao Y., Hong X., Du D. Zearalenone contamination in corn, corn products, and swine feed in China in 2016–2018 as assessed by magnetic bead immunoassay. Toxins. 2019;11:451. doi: 10.3390/toxins11080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajid M., Mehmood S., Yuan Y., Yue T. Mycotoxin patulin in food matrices: Occurrence and its biological degradation strategies. Drug Metab. Rev. 2019;51:105–120. doi: 10.1080/03602532.2019.1589493. [DOI] [PubMed] [Google Scholar]
- 21.Wei C., Yu L., Qiao N., Zhao J., Zhang H., Zhai Q., Tian F., Chen W. Progress in the distribution, toxicity, control, and detoxification of patulin: A review. Toxicon. 2020;184:83–93. doi: 10.1016/j.toxicon.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Altafini A., Roncada P., Guerrini A., Sonfack G.M., Fedrizzi G., Caprai E. Occurrence of ochratoxin A in different types of cheese offered for sale in Italy. Toxins. 2021;13:540. doi: 10.3390/toxins13080540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Villeda B., Lobos O., Aguilar-Zuniga K., Carrasco-Sánchez V. Ochratoxins in wines: A review of their occurrence in the last decade, toxicity, and exposure risk in humans. Toxins. 2021;13:478. doi: 10.3390/toxins13070478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben Miri Y., Benabdallah A., Taoudiat A., Mahdid M., Djenane D., Tacer-Caba Z., Topkaya C., Simal-Gandara J. Potential of essential oils for protection of Couscous against Aspergillus flavus and aflatoxin B1 contamination. Food Control. 2023;145:109474. doi: 10.1016/j.foodcont.2022.109474. [DOI] [Google Scholar]
- 25.Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 2020;9:13748. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topi D., Babič J., Jakovac-Strajn B., Tavčar-Kalcher G. Incidence of aflatoxins and ochratoxin A in wheat and corn from Albania. Toxins. 2023;15:567. doi: 10.3390/toxins15090567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavioğlu Kaya M., Deveci H.A., Kaya İ., Atar N., Yola M.L. The Electrochemical Detection of Ochratoxin A in Apple Juice via MnCO3 Nanostructures Incorporated into Carbon Fibers Containing a Molecularly Imprinting Polymer. Biosensors. 2023;13:760. doi: 10.3390/bios13080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan H.F., Tashani H., Ballouk F., Daou R., El Khoury A., Abiad M.G., AlKhatib A., Hassan M., El Khatib S., Dimassi H. Aflatoxins and Ochratoxin A in Tea Sold in Lebanon: Effects of Type, Packaging, and Origin. Int. J. Environ. Res. Public Health. 2023;20:6556. doi: 10.3390/ijerph20166556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu A., Zhou H., Yu S., Li Y., Wang L., Wu A., Liang J., Peng S., Liu N. Fusarium Mycotoxins and OTA in Beer from Shanghai, the Largest Megacity in China: Occurrence and Dietary Risk Assessment. Foods. 2023;12:3071. doi: 10.3390/foods12163071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee B.-H., Huang C.H., Liu T.Y., Liou J.S., Hou C.Y., Hsu W.H. Microbial Diversity of Anaerobic-Fermented Coffee and Potential for Inhibiting Ochratoxin-Produced Aspergillus niger. Foods. 2023;12:2967. doi: 10.3390/foods12152967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) Schrenk D., Bodin L., Chipman J.K., del Mazo J., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.-C., Nebbia C.S., et al. Scientific Opinion on the risk assessment of ochratoxin A in food. EFSA J. 2020;18:6113. doi: 10.2903/j.efsa.2020.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006, Consolidated Text. [(accessed on 25 March 2024)]. Available online: http://data.europa.eu/eli/reg/2023/915/2023-08-10.
- 33.Alshannaq A., Yu J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health. 2017;14:632. doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smaoui S., D’Amore T., Agriopoulou S., Mousavi Khaneghah A. Mycotoxins in Seafood: Occurrence, Recent Development of Analytical Techniques and Future Challenges. Separations. 2023;10:217. doi: 10.3390/separations10030217. [DOI] [Google Scholar]
- 35.Han X., Chakrabortti A., Zhu J., Liang Z.X., Li J. Sequencing and functional annotation of the whole genome of the filamentous fungus Aspergillus westerdijkiae. BMC Genom. 2016;17:633. doi: 10.1186/s12864-016-2974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Wang L., Liu F., Wang Q., Selvaraj J.N., Xing F., Liu Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins. 2016;8:83. doi: 10.3390/toxins8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, Drugs and Other Extrolites Produced by Species in Penicillium Subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- 38.Abarca M.L., Accensi F., Cano J., Cabañes F.J. Taxonomy and significance of black Aspergilli. Antonie Leeuwenhoek. 2004;86:33–49. doi: 10.1023/B:ANTO.0000024907.85688.05. [DOI] [PubMed] [Google Scholar]
- 39.Samson R.A., Noonim P., Meijer M., Houbraken J.A.M.P., Frisvad J.C., Varga J. Diagnostic tools to identify black Aspergilli. Stud. Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayman P., Baker J.L., Doster M.A., Michailides T.J., Mahoney N.E. Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl. Environ. Microbiol. 2002;68:2326–2329. doi: 10.1128/AEM.68.5.2326-2329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baranyi N., Jaksić D.D., Palágyi A., Kiss N., Kocsubé S., Szekeres A., Kecskeméti A., Bencsik O., Vágvölgyi C., Segvić K.M., et al. Identification of Aspergillus Species in Central Europe Able to Produce G-Type Aflatoxins. Acta Biol. Hung. 2015;66:339–347. doi: 10.1556/018.66.2015.3.9. [DOI] [PubMed] [Google Scholar]
- 42.Camiletti B.X., Torrico A.K., Maurino M.F., Cristos D., Magnoli C., Lucini E.I., Pecci M.D.L.P.G. Fungal screening and aflatoxin production by Aspergillus section Flavi isolated from pre-harvest maize ears grown in two Argentine regions. Crop. Prot. 2017;92:41–48. doi: 10.1016/j.cropro.2016.10.012. [DOI] [Google Scholar]
- 43.Saldan N.C., Almeida R.T., Avíncola A., Porto C., Galuch M.B., Magon T.F., Oliveira C.C. Development of an analytical method for identification of Aspergillus flavus based on chemical markers using HPLC-MS. Food Chem. 2018;241:113–121. doi: 10.1016/j.foodchem.2017.08.065. [DOI] [PubMed] [Google Scholar]
- 44.Frisvad J.C., Hubka V., Ezekiel C.N., Hong S.B., Nováková A., Chen A.J., Houbraken J. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019;93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Merwe K.J., Steyn P.S., Fourie L., Scott D.B., Theron J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 46.El Khoury A., Atoui A. Ochratoxin A: General overview and actual molecular status. Toxins. 2010;2:461–493. doi: 10.3390/toxins2040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frenette C., Paugh R.J., Tozlovanu M., Juzio M., Pfohl-Leszkowicz A., Manderville R.A. Structure-activity relationships for the fluorescence of ochratoxin A: Insight for detection of ochratoxin A metabolites. Anal. Chim. Acta. 2008;617:153–161. doi: 10.1016/j.aca.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Kiseleva M., Chalyy Z., Sedova I., Aksenov I. Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions. Toxins. 2020;12:94. doi: 10.3390/toxins12020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taniwaki M.H., Urbano G.R., Cabrera Palacios H.A., LeitaÄo M.F.F., Menezes H.C., Vicentini M.C., Iamanaka B.T., Taniwaki N.N. Infuence of water activity on mould growth and ochratoxin A production in coffee; Proceedings of the 19th ASIC Coffee Conference; Trieste, Italy. 14–18 May 2001. [Google Scholar]
- 50.Ramos A., Labernia N., Marín S., Sanchis V., Magan N. Effect of water activity and temperature on growth and ochratoxin production by three strains of Aspergillus ochraceus on a barley extract medium and on barley grains. Int. J. Food Microbiol. 1998;44:133–140. doi: 10.1016/S0168-1605(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 51.Bellí N., Mitchell D., Marín S., Alegre I. Ochratoxin A-producing fungi in Spanish wine grapes and their relationship with meteorological conditions. J. Plant Pathol. 2005;113:233–239. doi: 10.1007/s10658-005-5547-4. [DOI] [Google Scholar]
- 52.Esteban A., Abarca M., Bragulat R., Cabañes F. Effect of water activity on ochratoxin A production by Aspergillus niger aggregate species. Int. J. Food Microbiol. 2006;108:188–195. doi: 10.1016/j.ijfoodmicro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Lee H., Magan N. Impact of environment and interspecific interactions between spoilage fungi and Aspergillus ochraceus on growth and Ochratoxin production in maize grain. Int. J. Food Microbiol. 2000;61:11–16. doi: 10.1016/S0168-1605(00)00385-8. [DOI] [PubMed] [Google Scholar]
- 54.Kuruc J., Hegstad H., Lee K., Wolf-Hall C. Infestation and Quantification of Ochratoxigenic Fungi in Barley and Wheat Naturally Contaminated with Ochratoxin, A. J. Food Prot. 2015;78:1350–1356. doi: 10.4315/0362-028X.JFP-14-578. [DOI] [PubMed] [Google Scholar]
- 55.Magan N., Aldred D. Conditions of formation of Ochratoxin A in drying, transport and in different commodities. Food Addit. Contam. 2005;1:10–16. doi: 10.1080/02652030500412154. [DOI] [PubMed] [Google Scholar]
- 56.Czaban J., Wróblewska B., Stochmal A., Janda B. Growth of Penicillium verrucosum and production of Ochratoxin A on nonsterilized wheat grain incubated at different temperatures and water content. Pol. J. Microbiol. 2006;55:321–331. [PubMed] [Google Scholar]
- 57.Cairns V., Aldred D., Magan N. Water temperature and gas composition interactions affect growth and Ochratoxin A production by isolates of Penicillium verrucosum on wheat grain. J. Appl. Microbiol. 2005;99:1215–1221. doi: 10.1111/j.1365-2672.2005.02695.x. [DOI] [PubMed] [Google Scholar]
- 58.Kapetanakou A.E., Panagou E.Z., Gialitaki M., Drosinos E.H., Skandamis P.N. Evaluating the combined effect of water activity, pH and temperature on ochratoxin A production by Aspergillus ochraceus and Aspergillus carbonarius on culture medium and Corinth raisins. Food Control. 2009;20:725–732. doi: 10.1016/j.foodcont.2008.09.008. [DOI] [Google Scholar]
- 59.Koteswara Rao V., Ramana M.V., Girisham S., Reddy S.M. Culture Media and Factors Influencing Ochratoxin A Production by Two Species of Penicillium Isolated from Poultry Feeds. Natl. Acad. Sci. Lett. 2013;36:101–110. doi: 10.1007/s40009-012-0096-9. [DOI] [Google Scholar]
- 60.Passamani F.R., Hernandes T., Lopes N.A., Bastos S.C., Santiago W.D., Cardoso M.D., Batista L.R. Effect of temperature, water activity, and pH on growth and production of ochratoxin A by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J. Food Prot. 2014;77:1947–1952. doi: 10.4315/0362-028X.JFP-13-495. [DOI] [PubMed] [Google Scholar]
- 61.Huffman J., Gerber R., Du L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers. 2010;93:764–776. doi: 10.1002/bip.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrara M., Perrone G., Gambacorta L., Epifani F., Solfrizzo M., Gallo A. Identification of a Halogenase Involved in the Biosynthesis of Ochratoxin A in Aspergillus carbonarius. Appl. Environ. Microbiol. 2016;82:5631–5641. doi: 10.1128/AEM.01209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallo A., Ferrara M., Perrone G. Phylogenetic study of polyketide synthases and nonribosomal peptide synthetases involved in the biosynthesis of mycotoxins. Toxins. 2013;5:717–742. doi: 10.3390/toxins5040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarzer D., Marahiel M.A. Multimodular Biocatalysts for Natural Product Assembly. Naturwissenschaften. 2001;88:93–101. doi: 10.1007/s001140100211. [DOI] [PubMed] [Google Scholar]
- 65.Amoutzias G.D., Van de Peer Y., Mossialos D. Evolution and taxonomic distribution of nonribosomal peptide and polyketide synthases. Future Microbiol. 2008;3:361–370. doi: 10.2217/17460913.3.3.361. [DOI] [PubMed] [Google Scholar]
- 66.Färber P., Geisen R. Analysis of differentially-expressed ochratoxin A biosynthesis genes of Penicillium nordicum. Eur. J. Plant Pathol. 2004;110:661–669. doi: 10.1023/B:EJPP.0000032405.21833.89. [DOI] [Google Scholar]
- 67.Karolewiez A., Geisen R. Cloning a part of the ochratoxin A biosynthetic gene cluster of Penicillium nordicum and characterization of the ochratoxin polyketide synthase gene. Syst. Appl. Microbiol. 2005;28:588–595. doi: 10.1016/j.syapm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Wang L., Wu F., Liu F., Wang Q., Zhang X., Selvaraj J.N., Zhao Y., Xing F., Yin W.B., et al. A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microbiol. 2018;84:e01009-18. doi: 10.1128/AEM.01009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayram Ö., Braus G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- 70.Sarikaya-Bayram Ö., Palmer J.M., Keller N., Braus G.H., Bayram Ö. One Juliet and four Romeos: VeA and its methyltransferases. Front. Microbiol. 2015;6:1. doi: 10.3389/fmicb.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bok J.W., Keller N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bayram Ö., Feussner K., Dumkow M., Herrfurth C., Feussner I., Braus G.H. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 2016;87:30–53. doi: 10.1016/j.fgb.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 73.Reverberi M., Gazzetti K., Punelli F., Scarpari M., Zjalic S., Ricelli A., Fanelli C. Ao yap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus. Appl. Microbiol. Biotechnol. 2012;95:1293–1304. doi: 10.1007/s00253-012-3985-4. [DOI] [PubMed] [Google Scholar]
- 74.Hashem A., Abd-Allah E.F., Al-Obeed R.S., Alqarawi A.A., Alwathnani H.A. Effect of carbon, nitrogen sources and water activity on growth and ochratoxin production of Aspergillus carbonarius (Bainier) Thom. Jundishapur J. Microbiol. 2015;8:e17569. doi: 10.5812/jjm.17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rispail N., Soanes D.M., Ant C., Czajkowski R., Grünler A., Huguet R., Di Pietro A. Comparative genomics of MAP kinase and calcium–calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 2009;46:287–298. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Stoll D., Schmidt-Heydt M., Geisen R. Differences in the regulation of ochratoxin A by the HOG pathway in Penicillium and Aspergillus in response to high osmolar environments. Toxins. 2013;5:1282–1298. doi: 10.3390/toxins5071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majeed M., Khaneghah A.M., Kadmi Y., Khan M.U., Shariati M.A. Assessment of ochratoxin A in commercial corn and wheat products. Curr. Nutr. Food Sci. 2018;14:116–120. doi: 10.2174/1573401313666170330155823. [DOI] [Google Scholar]
- 78.Nazari F., Sulyok M., Yazdanpanah H., Kobarfard F., Krska R. A survey of mycotoxins in domestic rice in Iran by liquid chromatography tandem mass spectrometry. Toxicol. Mech. Methods. 2014;24:37–41. doi: 10.3109/15376516.2013.844752. [DOI] [PubMed] [Google Scholar]
- 79.Kuruc J.A., Schwarz P., Wolf-Hall C. Ochratoxin A in stored US barley and wheat. J. Food Prot. 2015;78:597–601. doi: 10.4315/0362-028X.JFP-14-418. [DOI] [PubMed] [Google Scholar]
- 80.Lee H.J., Ryu D. Significance of ochratoxin A in breakfast cereals from the United States. J. Agric. Food Chem. 2015;63:9404–9409. doi: 10.1021/jf505674v. [DOI] [PubMed] [Google Scholar]
- 81.Limay-Rios V., Miller J.D., Schaafsma A.W. Occurrence of Penicillium verrucosum, ochratoxin A, ochratoxin B and citrinin in on-farm stored winter wheat from the Canadian Great Lakes Region. PLoS ONE. 2017;12:e0181239. doi: 10.1371/journal.pone.0181239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zebiri S., Mokrane S., Verheecke-Vaessen C., Choque E., Reghioui H., Sabaou N., Riba A. Occurrence of ochratoxin A in Algerian wheat and its milling derivatives. Toxin Rev. 2019;38:206–211. doi: 10.1080/15569543.2018.1438472. [DOI] [Google Scholar]
- 83.Belasli A., Herrera M., Ariño A., Djenane D. Occurrence and Exposure Assessment of Major Mycotoxins in Foodstuffs from Algeria. Toxins. 2023;15:449. doi: 10.3390/toxins15070449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kosicki R., Twarużek M., Dopierała P., Rudzki B., Grajewski J. Occurrence of mycotoxins in winter rye varieties cultivated in Poland (2017–2019) Toxins. 2020;12:423. doi: 10.3390/toxins12060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silva L.J.G., Teixeira A.C., Pereira A.M.P.T., Pena A., Lino C.M. Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment. Toxins. 2020;12:249. doi: 10.3390/toxins12040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jonatova P., Dzuman Z., Prusova N., Hajslova J., Stranska-Zachariasova M. Occurrence of ochratoxin A and its stereoisomeric degradation product in various types of coffee available in the Czech market. World Mycotoxin J. 2020;13:97–107. doi: 10.3920/WMJ2019.2507. [DOI] [Google Scholar]
- 87.Vanesa D., Ana P. Occurrence of Ochratoxin A in coffee beans, ground roasted coffee and soluble coffee and method validation. Food Control. 2013;30:675–678. doi: 10.1016/j.foodcont.2012.09.004. [DOI] [Google Scholar]
- 88.Galarce-Bustos O., Alvarado M., Vega M., Aranda M. Occurrence of ochratoxin A in roasted and instant coffees in Chilean market. Food Control. 2014;46:102–107. doi: 10.1016/j.foodcont.2014.05.014. [DOI] [Google Scholar]
- 89.Coronel M.B., Marin S., Cano G., Ramos A.J., Sanchis V. Ochratoxin A in Spanish retail ground roasted coffee: Occurrence and assessment of the exposure in Catalonia. Food Control. 2011;22:414–419. doi: 10.1016/j.foodcont.2010.09.012. [DOI] [Google Scholar]
- 90.Pires P.N., Vargas E.A., Gomes M.B., Vieira C.B.M., Santos E.A.D., Bicalho A.A.C., Trovatti Uetanabaro A.P. Aflatoxins and ochratoxin A: Occurrence and contamination levels in cocoa beans from Brazil. Food Addit. Contam. Part A. 2019;36:815–824. doi: 10.1080/19440049.2019.1600749. [DOI] [PubMed] [Google Scholar]
- 91.Turcotte A.M., Scott P.M. Ochratoxin A in cocoa and chocolate sampled in Canada. Food Addit. Contam. Part A. 2011;28:762–766. doi: 10.1080/19440049.2010.508796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Celik D., Kabak B. Assessment to propose a maximum permitted level for ochratoxin A in dried figs. J. Food Compos. Anal. 2022;112:104705. doi: 10.1016/j.jfca.2022.104705. [DOI] [Google Scholar]
- 93.Covarelli L., Beccari G., Marini A., Tosi L. A review on the occurrence and control of ochratoxigenic fungal species and ochratoxin A in dehydrated grapes, non-fortified dessert wines and dried vine fruit in the Mediterranean area. Food Control. 2012;26:347–356. doi: 10.1016/j.foodcont.2012.01.044. [DOI] [Google Scholar]
- 94.Abdallah M.F., Krska R., Sulyok M. Occurrence of ochratoxins, fumonisin B2, aflatoxins (B1 and B2), and other secondary fungal metabolites in dried date palm fruits from Egypt: A mini-survey. J. Food Sci. 2018;83:559–564. doi: 10.1111/1750-3841.14046. [DOI] [PubMed] [Google Scholar]
- 95.Nikolchina I., Rodrigues P. A preliminary study on microbiota and ochratoxin a contamination in commercial palm dates (Phoenix dactylifera) Mycotoxin Res. 2021;37:215–220. doi: 10.1007/s12550-021-00432-0. [DOI] [PubMed] [Google Scholar]
- 96.Palumbo J.D., O’Keeffe T.L., Vasquez S.J., Mahoney N.E. Isolation and identification of ochratoxin A producing Aspergillus section Nigri strains from California raisins. Lett. Appl. Microbiol. 2011;52:330–336. doi: 10.1111/j.1472-765X.2011.03004.x. [DOI] [PubMed] [Google Scholar]
- 97.Iqbal S.Z., Mumtaz A., Mahmood Z., Waqas M., Ghaffar A., Ismail A., Pervaiz W. Assessment of aflatoxins and ochratoxin A in Chili sauce samples and estimation of dietary intake. Food Control. 2021;121:107621. doi: 10.1016/j.foodcont.2020.107621. [DOI] [Google Scholar]
- 98.Prelle A., Spadaro D., Garibaldi A., Gullino M.L. Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control. 2014;39:192–197. doi: 10.1016/j.foodcont.2013.11.013. [DOI] [Google Scholar]
- 99.Gambacorta L., Magistà D., Perrone G., Murgolo S., Logrieco A.F., Solfrizzo M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018;11:159–174. doi: 10.3920/WMJ2017.2271. [DOI] [Google Scholar]
- 100.Armorini S., Altafini A., Zaghini A., Roncada P. Ochratoxin A in artisan salami produced in Veneto (Italy) Food Addit. Contam. Part B. 2016;9:9–14. doi: 10.1080/19393210.2015.1098735. [DOI] [PubMed] [Google Scholar]
- 101.Dall’Asta C., Galaverna G., Bertuzzi T., Moseriti A., Pietri A., Dossena A., Marchelli R. Occurrence of ochratoxin A in raw ham muscle, salami and dry-cured ham from pigs fed with contaminated diet. Food Chem. 2010;120:978–983. doi: 10.1016/j.foodchem.2009.11.036. [DOI] [Google Scholar]
- 102.Delfino D., Lucchetti D., Mauti T., Mancuso M., Di Giustino P., Triolone D., Vaccari S., Bonanni R.C., Neri B., Russo K. Investigation of ochratoxin A in commercial cheeses and pork meat products by liquid chromatography-tandem mass spectrometry. J. Food Sci. 2022;87:4465–4475. doi: 10.1111/1750-3841.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abd-Elghany S.M., Sallam K.I. Rapid determination of total aflatoxins and ochratoxins A in meat products by immuno-affinity fluorimetry. Food Chem. 2015;179:253–256. doi: 10.1016/j.foodchem.2015.01.140. [DOI] [PubMed] [Google Scholar]
- 104.Iqbal S.Z., Nisar S., Rafique Asi M., Jinap S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control. 2014;43:98–103. doi: 10.1016/j.foodcont.2014.02.046. [DOI] [Google Scholar]
- 105.Zhang Z., Song Y., Ma L., Huang K., Liang Z. Co-Occurrence of Staphylococcus aureus and Ochratoxin A in Pasteurized Milk. Toxins. 2022;14:718. doi: 10.3390/toxins14100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Žurga P., Vahčić N., Pasković I., Banović M., Malenica Staver M. Occurrence of ochratoxin A and biogenic amines in Croatian commercial red wines. Foods. 2019;8:348. doi: 10.3390/foods8080348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Torović L., Lakatoš I., Majkić T., Beara I. Risk to public health related to the presence of ochratoxin A in wines from Fruška Gora. LWT. 2020;129:109537. doi: 10.1016/j.lwt.2020.109537. [DOI] [Google Scholar]
- 108.Carballo D., Fernández-Franzón M., Ferrer E., Pallarés N., Berrada H. Dietary exposure to mycotoxins through alcoholic and non-alcoholic beverages in Valencia, Spain. Toxins. 2021;13:438. doi: 10.3390/toxins13070438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gruber-Dorninger C., Jenkins T., Schatzmayr G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins. 2019;11:375. doi: 10.3390/toxins11070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Corrêa Ribeiro A.N., Ferreira C.D. Mycotoxins in Grains and Cereals Intended for Human Consumption: Brazilian Legislation, Occurrence Above Maximum Levels and Co-Occurrence. Food Rev. Int. 2023;39:5920–5933. doi: 10.1080/87559129.2022.2098318. [DOI] [Google Scholar]
- 111.Dongo L., Bandyopadhyay R., Kumar M., Ojiambo P.S. Occurrence of Ochratoxin A in Nigeria ready for sale cocoa beans. Agric. J. 2008;3:4–9. [Google Scholar]
- 112.Prognosis Biotech Mycotoxins Legislation Database. [(accessed on 28 March 2024)]. Available online: https://www.prognosis-biotech.com/mycotoxins-legislation/
- 113.FAO WHO Codex Alimentarius—General Standard for Contaminants and Toxins in Food and Feed, CXS 193-1995. [(accessed on 25 March 2024)]. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf.
- 114.Mitchell N.J., Chen C., Palumbo J.D., Bianchini A., Cappozzo J., Stratton J., Wu F. Risk Assessment of Dietary Ochratoxin A in the United States. Food Chem. Toxicol. 2017;100:265–273. doi: 10.1016/j.fct.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ridgway K., Scientific R. Sample preparation for food contaminant analysis. LC GC Eur. 2012;25:60–71. [Google Scholar]
- 116.Shephard G.S. Current status of mycotoxin analysis: A critical review. J. AOAC Int. 2016;99:842–848. doi: 10.5740/jaoacint.16-0111. [DOI] [PubMed] [Google Scholar]
- 117.Krska R., Schubert-Ullrich P., Molinelli A., Sulyok M., Mac Donald S., Crews C. Mycotoxin analysis: An update. Food Addit. Contam. Part A. 2008;25:152–163. doi: 10.1080/02652030701765723. [DOI] [PubMed] [Google Scholar]
- 118.Shephard G.S. Determination of mycotoxins in human foods. Chem. Soc. Rev. 2008;37:2468–2477. doi: 10.1039/b713084h. [DOI] [PubMed] [Google Scholar]
- 119.Rahmani A., Jinap S., Soleimany F. Qualitative and quantitative analysis of mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009;8:202–251. doi: 10.1111/j.1541-4337.2009.00079.x. [DOI] [PubMed] [Google Scholar]
- 120.Turner N.W., Subrahmanyam S., Piletsky S. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta. 2009;632:168–180. doi: 10.1016/j.aca.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 121.Prakasham K., Gurrani S., Shiea J., Wu M.T., Wu C.F., Lin Y.C., Ponnusamy V.K. Ultra-sensitive determination of Ochratoxin A in coffee and tea samples using a novel semi-automated in-syringe based coagulant-assisted fast mycotoxin extraction (FaMEx) technique coupled with UHPLC-MS/MS. Food Chem. 2023;417:135951. doi: 10.1016/j.foodchem.2023.135951. [DOI] [PubMed] [Google Scholar]
- 122.Karami-Osboo R. Nanofluid extraction of Ochratoxin A in food. J. Food Compos. Anal. 2020;87:103425. doi: 10.1016/j.jfca.2020.103425. [DOI] [Google Scholar]
- 123.Jalili M., Jinap S. Optimization and validation of a method for extraction and quantification of ochratoxin A in black pepper. [(accessed on 25 March 2024)];Int. Food Res. J. 2015 22:502–509. Available online: http://www.ifrj.upm.edu.my/22%20(02)%202015/(9).pdf. [Google Scholar]
- 124.Juan C., González L., Soriano J.M., Moltó J.C., Mañes J. Accelerated Solvent Extraction of Ochratoxin A from Rice Samples. J. Agric. Food Chem. 2005;53:9348–9351. doi: 10.1021/jf051560q. [DOI] [PubMed] [Google Scholar]
- 125.Hu X., Hu R., Zhang Z., Li P., Zhang Q., Wang M. Development of a multiple immunoaffinity column for simultaneous determination of multiple mycotoxins in feeds using UPLC-MS/MS. Anal. Bioanal. Chem. 2016;408:6027–6036. doi: 10.1007/s00216-016-9626-5. [DOI] [PubMed] [Google Scholar]
- 126.Koesukwiwat U., Sanguankaew K., Leepipatpiboon N. Evaluation of a modified QuEChERS method for analysis of mycotoxins in rice. Food Chem. 2014;153:44–51. doi: 10.1016/j.foodchem.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 127.National Toxicology Program Toxicology and Carcinogenesis Studies of Ochratoxin A (CAS No. 303-47-9) in F344/N Rats (Gavage Studies) [(accessed on 25 March 2024)];Natl. Toxicol Program Tech. Rep. Ser. 1989 358:1–142. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/lt_rpts/tr358.pdf. [PubMed] [Google Scholar]
- 128.Delaunay N., Combès A., Pichon V. Immunoaffinity Extraction and Alternative Approaches for the Analysis of Toxins in Environmental, Food or Biological Matrices. Toxins. 2020;12:795. doi: 10.3390/toxins12120795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cavalera S., Anfossi L., Di Nardo F., Baggiani C. Mycotoxins Imprinted Polymers: A State-of-the-Art Review. Toxins. 2024;16:47. doi: 10.3390/toxins16010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teixeira T.R., Hoeltz M., Einloft T.C., Dottori H.A., Manfroi V., Noll I.B. Determination of ochratoxin A in wine from the southern region of Brazil by thin layer chromatography with a charge-coupled detector. Food Addit. Contam. Part B. 2011;4:289–293. doi: 10.1080/19393210.2011.638088. [DOI] [PubMed] [Google Scholar]
- 131.Antep H.M., Merdivan M. Determination of ochratoxin A in grape wines after dispersive liquid–liquid microextraction using high-performance thin layer and liquid chromatography–fluorescence detection. Hacet. J. Biol. Chem. 2012;40:155–163. [Google Scholar]
- 132.Kupski L., de Carvalho Silvello M.A., Fontes M.R.V., Lima T.S., Treichel H., Furlong E.B. R. oryzae Cellulases: A New Approach to Degrading Lignocellulosic Material. J. Food Biochem. 2015;39:129–138. doi: 10.1111/jfbc.12097. [DOI] [Google Scholar]
- 133.Mohebbi A., Nemati M., Farajzadeh M.A., Mogaddam M.R.A., Lotfipour F. High-performance liquid chromatography-tandem mass spectrometry determination of patulin and ochratoxin A in commercial fruit juices after their extraction with a green synthesized metal organic framework-based dispersive micro solid-phase extraction procedure. Microchem. J. 2022;179:107558. doi: 10.1016/j.microc.2022.107558. [DOI] [Google Scholar]
- 134.Badji T., Durand N., Bendali F., Piro-Metayer I., Zinedine A., Salah-Abbès J.B., Brabet C. In vitro detoxification of aflatoxin B1 and ochratoxin A by lactic acid bacteria isolated from Algerian fermented foods. Biol. Control. 2023;179:105181. doi: 10.1016/j.biocontrol.2023.105181. [DOI] [Google Scholar]
- 135.Heperkan Z.D., Gunalan-Inci E., Ceyhan T. Unexpectedly high patulin contamination and co-occurrence of ochratoxin A in homemade vinegar. Food Control. 2023;148:109685. doi: 10.1016/j.foodcont.2023.109685. [DOI] [Google Scholar]
- 136.Chen D., Cao X., Tao Y., Wu Q., Pan Y., Huang L., Yuan Z. Development of a sensitive and robust liquid chromatography coupled with tandem mass spectrometry and a pressurized liquid extraction for the determination of aflatoxins and ochratoxin A in animal-derived foods. J. Chromatogr. A. 2012;1253:110–119. doi: 10.1016/j.chroma.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 137.Meerpoel C., Vidal A., di Mavungu J.D., Huybrechts B., Tangni E.K., Devreese M., De Saeger S. Development and validation of an LC–MS/MS method for the simultaneous determination of citrinin and ochratoxin A in a variety of feed and foodstuffs. J. Chromatogr. A. 2018;1580:100–109. doi: 10.1016/j.chroma.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 138.Modh H., Scheper T., Walter J.G. Detection of ochratoxin A by aptamer-assisted real-time PCR-based assay (Apta-qPCR) Eng. Life Sci. 2017;17:923–930. doi: 10.1002/elsc.201700048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rahman H.U., Yue X., Ren X., Zhang W., Zhang Q., Li P. Multiplex PCR assay to detect Aspergillus, Penicillium and Fusarium species simultaneously. Food Addit. Contam. Part A. 2020;37:1939–1950. doi: 10.1080/19440049.2020.1810860. [DOI] [PubMed] [Google Scholar]
- 140.Susca A., Anelli P., Haidukowski M., Probyn C.E., Epifani F., Logrieco A.F., Proctor R.H. A PCR method to identify ochratoxin A-producing Aspergillus westerdijkiae strains on dried and aged foods. Int. J. Food Microbiol. 2021;344:109113. doi: 10.1016/j.ijfoodmicro.2021.109113. [DOI] [PubMed] [Google Scholar]
- 141.Ekhtelat M. Enzyme-linked Immunosorbent Assay and High-performance Liquid Chromatography analysis of Ochratoxin A in Zataria multiflora and Foeniculum vulgare in Ahvaz (Iran) Asian J. Pharm. 2018;12:S523 . doi: 10.22377/ajp.v12i02.2386. [DOI] [Google Scholar]
- 142.Zhang C., Zhang Q., Tang X., Zhang W., Li P. Development of an anti-idiotypic VHH antibody and toxin-free enzyme immunoassay for ochratoxin A in cereals. Toxins. 2019;11:280. doi: 10.3390/toxins11050280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pietri A., Leni G., Mulazzi A., Bertuzzi T. Ochratoxin A and sterigmatocystin in long-ripened grana cheese: Occurrence, wheel rind contamination and effectiveness of cleaning techniques on grated products. Toxins. 2022;14:306. doi: 10.3390/toxins14050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hajrulai-Musliu Z., Uzunov R., Jovanov S., Jankuloski D., Stojkovski V., Pendovski L., Sasanya J.J. A new LC–MS/MS method for multiple residues/contaminants in bovine meat. BMC Chem. 2021;15:62. doi: 10.1186/s13065-021-00788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Armutcu C., Uzun L., Denizli A. Determination of Ochratoxin A traces in foodstuffs: Comparison of an automated on-line two-dimensional high-performance liquid chromatography and off-line immunoaffinity-high-performance liquid chromatography system. J. Chromatogr. A. 2018;1569:139–148. doi: 10.1016/j.chroma.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 146.Wei D., Pan A., Zhang C., Guo M., Lou C., Zhang J., Wang X. Fast extraction of aflatoxins, ochratoxins and enniatins from maize with magnetic covalent organic framework prior to HPLC-MS/MS detection. Food Chem. 2023;404:134464. doi: 10.1016/j.foodchem.2022.134464. [DOI] [PubMed] [Google Scholar]
- 147.Palma P., Calderón R., Godoy M., Vidal M., Rivera A. Human exposure to ochratoxin A and its natural occurrence in spices marketed in Chile (2016–2020): A case study of merkén. J. Food Compos. Anal. 2023;115:104885. doi: 10.1016/j.jfca.2022.104885. [DOI] [Google Scholar]
- 148.Kresse M., Drinda H., Romanotto A., Speer K. Simultaneous determination of pesticides, mycotoxins, and metabolites as well as other contaminants in cereals by LC-LC-MS/MS. J. Chromatogr. B. 2019;1117:86–102. doi: 10.1016/j.jchromb.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 149.Kholová A., Lhotská I., Uhrová A., Špánik I., Machyňáková A., Solich P., Švec F., Šatínský D. Determination of Ochratoxin A and Ochratoxin B in Archived Tokaj Wines (Vintage 1959–2017) Using On-Line Solid Phase Extraction Coupled to Liquid Chromatography. Toxins. 2020;12:739. doi: 10.3390/toxins12120739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Potipun J., Ruaylarb W., Nilthong R., Owatworakit A. Quantification of Ochratoxin A–Producing Fungi in Coffee Products Using Quantitative PCR. Chiang Mai J. Sci. 2018;17:39–46. doi: 10.12982/CMUJNS.2018.0004. [DOI] [Google Scholar]
- 151.Zuo H., Wang X., Liu W., Chen Z., Liu R., Yang H., Ning B. Nanobody-based magnetic chemiluminescence immunoassay for one-pot detection of ochratoxin A. Talanta. 2023;258:124388. doi: 10.1016/j.talanta.2023.124388. [DOI] [PubMed] [Google Scholar]
- 152.Yang X.S., Zhao J., Ma T.T., Li Z.Y., Wang L.L., Ji S.L., Gong H.S. Magnetic covalent organic framework for effective solid-phase extraction and HPLC determination of ochratoxin A in food. LWT. 2023;179:114639. doi: 10.1016/j.lwt.2023.114639. [DOI] [Google Scholar]
- 153.Somerson J., Plaxco K.W. Electrochemical aptamer-based sensors for rapid point-of-use monitoring of the mycotoxin ochratoxin a directly in a food stream. Molecules. 2018;23:912. doi: 10.3390/molecules23040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Viter R., Savchuk M., Iatsunskyi I., Pietralik Z., Starodub N., Shpyrka N., Ramanavicius A. Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosens. Bioelectron. 2018;99:237–243. doi: 10.1016/j.bios.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 155.Tang Z., Liu X., Su B., Chen Q., Cao H., Yun Y., Hammock B.D. Ultrasensitive and rapid detection of ochratoxin A in agro-products by a nanobody-mediated FRET-based immunosensor. J. Hazard. Mater. 2020;387:121678. doi: 10.1016/j.jhazmat.2019.121678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen X., Wu H., Tang X., Zhang Z., Li P. Recent advances in electrochemical sensors for mycotoxin detection in food. Electroanalysis. 2023;35:e202100223. doi: 10.1002/elan.202100223. [DOI] [Google Scholar]
- 157.Zhang S., Luan Y., Xiong M., Zhang J., Lake R., Lu Y. DNAzyme amplified aptasensing platform for ochratoxin A detection using a personal glucose meter. ACS Appl. Mater. Interfaces. 2021;13:9472–9481. doi: 10.1021/acsami.0c20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karczmarczyk A., Haupt K., Feller K.H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta. 2017;166:193–197. doi: 10.1016/j.talanta.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 159.Oh H.K., Joung H.A., Jung M., Lee H., Kim M.G. Rapid and simple detection of ochratoxin a using fluorescence resonance energy transfer on lateral flow immunoassay (FRET-LFI) Toxins. 2019;11:292. doi: 10.3390/toxins11050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kaur M., Gaba J., Singh K., Bhatia Y., Singh A., Singh N. Recent Advances in Recognition Receptors for Electrochemical Biosensing of Mycotoxins- A Review. Biosensors. 2023;13:391. doi: 10.3390/bios13030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clemons T.D., Kerr R.H., Joos A. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. In: Andrews D.L., Lipson R.H., Nann T., Goreham R.V., editors. Comprehensive Nanoscience and Nanotechnology. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2019. pp. 193–210. [DOI] [Google Scholar]
- 162.Guan Y., Si P.B., Yang T., Wu Y., Yang Y.H., Hu R. A novel method for detection of ochratoxin A in foods—Co-MOFs based dual signal radiometric electrochemical aptamer sensor coupled with DNA walker. Food Chem. 2023;403:134316. doi: 10.1016/j.foodchem.2022.134316. [DOI] [PubMed] [Google Scholar]
- 163.Zhang J., Xu D., Zhang Y., Luo Z., Zhao Y., Zheng X., Yang H., Yu Zhou Y. Gold nanoparticle-mediated fluorescence immunoassay for rapid and sensitive detection of Ochratoxin A. Spectrochim. Acta Part A. 2024;304:1386–1425. doi: 10.1016/j.saa.2023.123312. [DOI] [PubMed] [Google Scholar]
- 164.Pettersson H., Aberg L. Near-infrared spectroscopy for determination of mycotoxins in cereals. Food Control. 2003;14:229–232. doi: 10.1016/S0956-7135(03)00011-2. [DOI] [Google Scholar]
- 165.Wendy A., Anton S. Fluorescence Polarization Assays in Small Molecule Screening. Expert Opin. Drug Discov. 2011;6:17–32. doi: 10.1517/17460441.2011.537322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Keshri G., Magan N. Detection and differentiation between mycotoxigenic and non-mycotoxigenic strains of two Fusarium spp. using volatile production profiles and hydrolytic enzymes. J. Appl. Microbiol. 2000;89:825–833. doi: 10.1046/j.1365-2672.2000.01185.x. [DOI] [PubMed] [Google Scholar]
- 167.Kabak B. Aflatoxins and ochratoxin A in chocolate products in Turkey. Food Addit. Contam. Part B. 2019;12:225–230. doi: 10.1080/19393210.2019.1601641. [DOI] [PubMed] [Google Scholar]
- 168.Adebiyi J.A., Kayitesi E., Adebo O.A., Changwa R., Njobeh P.B. Food fermentation and mycotoxin detoxification: An African perspective. Food Control. 2019;106:106731. doi: 10.1016/j.foodcont.2019.106731. [DOI] [Google Scholar]
- 169.Wafula E.N., Muhonja C.N., Kuja J.O., Owaga E.E., Makonde H.M., Mathara J.M., Kimani V.W. Lactic Acid Bacteria from African Fermented Cereal-Based Products: Potential Biological Control Agents for Mycotoxins in Kenya. J. Toxicol. 2022;2022:2397767. doi: 10.1155/2022/2397767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Loi M., Fanelli F., Liuzzi V.C., Logrieco A.F., Mulè G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins. 2017;9:111. doi: 10.3390/toxins9040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tola M., Kebede B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016;2:1191103. doi: 10.1080/23311932.2016.1191103. [DOI] [Google Scholar]
- 172.Mwabulili F., Xie Y., Li Q., Sun S., Yang Y., Ma W. Research progress of ochratoxin a bio-detoxification. Toxicon. 2022;222:107005. doi: 10.1016/j.toxicon.2022.107005. [DOI] [PubMed] [Google Scholar]
- 173.Amézqueta S., González-Peñas E., Murillo-Arbizu M., de Cerain A.L. Ochratoxin A decontamination: A review. Food Control. 2009;20:326–333. doi: 10.1016/j.foodcont.2008.05.017. [DOI] [Google Scholar]
- 174.Dammak I., Hamdi Z., Kammoun El Euch S., Zemni H., Mliki A., Hassouna M., Lasram S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata, and Laurus nobilis essential oils and a major monoterpene constituent 1,8-cineole against Aspergillus carbonarius. Ind. Crop. Prod. 2019;128:85–93. doi: 10.1016/j.indcrop.2018.11.006. [DOI] [Google Scholar]
- 175.El Khoury R., Atoui A., Mathieu F., Kawtharani H., El Khoury A., Maroun R.G., El Khoury A. Antifungal and Antiochratoxigenic Activities of Essential Oils and Total Phenolic Extracts: A Comparative Study. Antioxidants. 2017;6:44. doi: 10.3390/antiox6030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang X., Wu Z., Wu S., Dai Y., Sun C. Degradation of ochratoxin A by Bacillus amyloliquefaciens ASAG1. Food Addit. Contam. Part A. 2015;32:564–571. doi: 10.1080/19440049.2014.991948. [DOI] [PubMed] [Google Scholar]
- 177.Belkacem-Hanfi N., Fhoula I., Semmar N., Guesmi A., Perraud-Gaime I., Ouzari H.-I., Boudabous A., Roussos S. Lactic acid bacteria against post-harvest moulds and ochratoxin A isolated from stored wheat. Biol. Control. 2014;76:52–59. doi: 10.1016/j.biocontrol.2014.05.001. [DOI] [Google Scholar]
- 178.Taheur F.B., Fedhila K., Chaieb K., Kouidhi B., Bakhrouf A., Abrunhosa L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017;251:1–7. doi: 10.1016/j.ijfoodmicro.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 179.Azam M.S., Yu D., Wu A. Enzymes for Degradation of Fusarium Mycotoxins. In: Wu A., editor. Food Safety & Mycotoxins. Springer; Singapore: 2019. pp. 113–135. [DOI] [Google Scholar]
- 180.Amiri A., Dugas R., Pichot A.L., Bompeix G. In vitro and in vitro activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 2008;126:13–19. doi: 10.1016/j.ijfoodmicro.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 181.Belli N., Marin S., Argiles E., Ramos A.J., Sanchis V. Effect of chemical treatments on ochratoxigenic fungi and common mycobiota of grapes (Vitis vinifera) J. Food Prot. 2007;70:157–163. doi: 10.4315/0362-028X-70.1.157. [DOI] [PubMed] [Google Scholar]
- 182.Lagogianni C.S., Tsitsigiannis D.I. Effective chemical management for prevention of aflatoxins in maize. Phytopathol. Mediterr. 2018;57:186–197. doi: 10.14601/Phytopathol_Mediterr-22492. [DOI] [Google Scholar]
- 183.Oiki S., Yaguchi T., Urayama S.I., Hagiwara D. Wide distribution of resistance to the fungicides fludioxonil and iprodione in Penicillium species. PLoS ONE. 2022;17:e0262521. doi: 10.1371/journal.pone.0262521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Özcan S., Gökmen V.U.R.A.L. Alkali-based pre-treatment may prevent ochratoxin A in grapes. World Mycotoxin J. 2016;9:517–523. doi: 10.3920/WMJ2015.2017. [DOI] [Google Scholar]
- 185.Samuel M.S., Jeyaram K., Datta S., Chandrasekar N., Balaji R., Selvarajan E. Detection, contamination, toxicity, and prevention methods of ochratoxins: An update review. J. Agric. Food Chem. 2021;69:13974–13989. doi: 10.1021/acs.jafc.1c05994. [DOI] [PubMed] [Google Scholar]
- 186.Amézqueta S., González-Peñas E., Lizarraga T., Murillo-Arbizu M., López Demmunen A. A simple method reduces Ochratoxin A in contaminated cocoa shells. J. Food Prot. 2008;71:1422–1426. doi: 10.4315/0362-028X-71.7.1422. [DOI] [PubMed] [Google Scholar]
- 187.Yu J., Smith I.N., Mikiashvili N. Reducing Ochratoxin A Content in Grape Pomace by Different Methods. Toxins. 2020;12:424. doi: 10.3390/toxins12070424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qi L., Li Y., Luo X., Wang R., Zheng R., Wang L., Chen Z. Detoxification of zearalenone and ochratoxin A by ozone and quality evaluation ofozonised corn. Food Addit. Contam. A. 2016;33:1700–1710. doi: 10.1080/19440049.2016.1232863. [DOI] [PubMed] [Google Scholar]
- 189.Torlak E. Use of gaseous ozone for reduction of ochratoxin A and fungal populations on sultanas. Aust. J. Grape Wine Res. 2019;25:25–29. doi: 10.1111/ajgw.12362. [DOI] [Google Scholar]
- 190.Bhatnagar D., Ehrlich K., Cleveland T. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2003;61:83–93. doi: 10.1007/s00253-002-1199-x. [DOI] [PubMed] [Google Scholar]
- 191.Afsah-Hejri L., Hajeb P., Ehsani R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020;19:1777–1808. doi: 10.1111/1541-4337.12594. [DOI] [PubMed] [Google Scholar]
- 192.Krasensky J., Carmody M., Sierla M., Kangasjärvi J. Ozone and reactive oxygen species. eLS. 2017:1–9. doi: 10.1002/9780470015902.a0001299.pub3. [DOI] [Google Scholar]
- 193.Trombete F.M., Freitas-Silva O., Saldanha T., Venâncio A.A., Fraga M.E. Ozone against mycotoxins and pesticide residues in food: Current applications and perspectives. Int. Food Res. J. 2016;23:2545–2556. [Google Scholar]
- 194.Çelebi Y., Koç G.Ç., Süfer Ö., Tekgül Barut Y., Şahin Ercan S., Yüksel A.N., Sezer S., Ramniwas S., Rastogi S., Chandra Khanashyam A., et al. Impact of Ozone Treatment on Lipid Oxidation in Foods: A Critical Review. Ozone Sci. Eng. 2024:1–25. doi: 10.1080/01919512.2024.2312907. [DOI] [Google Scholar]
- 195.Kaur K., Pandiselvam R., Kothakota A., Ishwarya S.P., Zalpouri R., Mahanti N.K. Impact of ozone treatment on food polyphenols—A comprehensive review. Food Control. 2022;142:109207. doi: 10.1016/j.foodcont.2022.109207. [DOI] [Google Scholar]
- 196.Akbar A., Medina A., Magan N. Potential Control of Mycotoxigenic Fungi and Ochratoxin A in Stored Coffee Using Gaseous Ozone Treatment. Microorganisms. 2020;8:1462. doi: 10.3390/microorganisms8101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Awuchi C.G., Nwozo O.S., Aja P.M., Odongo G.A. High-pressure acidified steaming with varied citric acid dosing can successfully detoxify mycotoxins. Food Sci. Nutr. 2023;11:2677–2685. doi: 10.1002/fsn3.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee H.J. Stability of ochratoxin A in oats during roasting with reducing sugars. Food Control. 2020;118:107382. doi: 10.1016/j.foodcont.2020.107382. [DOI] [Google Scholar]
- 199.Jalili M., Selamat J., Rashidi L. Effect of thermal processing and traditional flavouring mixture on mycotoxin reduction in pistachio. World Mycotoxin J. 2020;13:381–389. doi: 10.3920/WMJ2019.2486. [DOI] [Google Scholar]
- 200.Sumbal G.A., Hussain Shar Z., Hussain Sherazi S.T., Sirajuddin Nizamani S.M., Mahesar S.A. Decontamination of poultry feed from ochratoxin A by UV and sunlight radiations. J. Sci. Food Agric. 2016;96:2668–2673. doi: 10.1002/jsfa.7384. [DOI] [PubMed] [Google Scholar]
- 201.Khalil O.A., Hammad A.A., Sebaei A.S. Aspergillus flavus and Aspergillus ochraceus inhibition and reduction of aflatoxins and ochratoxin A in maize by irradiation. Toxicon. 2021;198:111–120. doi: 10.1016/j.toxicon.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 202.Calado T., Fernández-Cruz M.L., Verde S.C., Venâncio A., Abrunhosa L. Gamma irradiation effects on ochratoxin A: Degradation, cytotoxicity and application in food. Food Chem. 2018;240:463–471. doi: 10.1016/j.foodchem.2017.07.136. [DOI] [PubMed] [Google Scholar]
- 203.Krogh P., Hald B., Gjertsen P., Myken F. Fate of ochratoxin A and citrinin during malting and brewing experiments. Appl. Microbiol. 1974;28:31–34. doi: 10.1128/am.28.1.31-34.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scott P. Effects of processing and detoxification treatments on ochratoxin A: Introduction. Food Addit. Contam. 1996;13:19–21. [PubMed] [Google Scholar]
- 205.Aguilar-Alvarez M.E., Saucedo-Castañeda G., Durand N., Perraud-Gaime I., González-Robles R.O., Rodríguez-Serrano G.M. The variety, roasting, processing, and type of cultivation determine the low OTA levels of commercialized coffee in Chiapas State, Mexico. Food Control. 2021;126:108088. doi: 10.1016/j.foodcont.2021.108088. [DOI] [Google Scholar]
- 206.Cramer B., Konigs M., Humpf H.U. Identification and in vitro cytotoxicity of ochratoxin A degradation products formed during coffee roasting. J. Agric. Food Chem. 2008;56:5673–5681. doi: 10.1021/jf801296z. [DOI] [PubMed] [Google Scholar]
- 207.Sueck F., Hemp V., Specht J., Torres O., Cramer B., Humpf H.U. Occurrence of the ochratoxin A degradation product 2’R-ochratoxin A in coffee and other food: An update. Toxins. 2019;11:329. doi: 10.3390/toxins11060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bittner A., Cramer B., Harrer H., Humpf H.U. Structure elucidation and in vitro cytotoxicity of ochratoxin α amide, a new degradation product of ochratoxin A. Mycotoxin Res. 2015;31:83. doi: 10.1007/s12550-014-0218-y. [DOI] [PubMed] [Google Scholar]
- 209.Bryła M., Ksieniewicz-Woźniak E., Stępniewska S., Modrzewska M., Waśkiewicz A., Szymczyk K., Szafrańska A. Transformation of ochratoxin A during bread-making processes. Food Control. 2021;125:107950. doi: 10.1016/j.foodcont.2021.107950. [DOI] [Google Scholar]
- 210.Zapaśnik A., Bryła M., Waśkiewicz A., Ksieniewicz-Woźniak E., Podolska G. Ochratoxin A and 2′R-Ochratoxin A in selected foodstuffs and dietary risk assessment. Molecules. 2022;27:188. doi: 10.3390/molecules27010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ouf S.A., Basher A.H., Mohamed A.A.H. Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J. Sci. Food Agric. 2015;95:3204–3210. doi: 10.1002/jsfa.7060. [DOI] [PubMed] [Google Scholar]
- 212.Durek J., Schlüter O., Roscher A., Durek P., Fröhling A. Inhibition or stimulation of ochratoxin A synthesis on inoculated barley triggered by diffuse coplanar surface barrier discharge plasma. Front. Microbiol. 2018;9:2782. doi: 10.3389/fmicb.2018.02782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hoppanova L., Dylikova J., Kovacik D., Medvecka V., Durina P., Krystofova S., Kalinakova B. The effect of cold atmospheric pressure plasma on Aspergillus ochraceus and ochratoxin A production. Antonie Leeuwenhoek. 2020;113:1479–1488. doi: 10.1007/s10482-020-01457-8. [DOI] [PubMed] [Google Scholar]
- 214.Casas-Junco P.P., Solís-Pacheco J.R., Ragazzo-Sánchez J.A., Aguilar-Uscanga B.R., Bautista-Rosales P.U., Calderón-Santoyo M. Cold plasma treatment as an alternative for ochratoxin A detoxification and inhibition of mycotoxigenic fungi in roasted coffee. Toxins. 2019;11:337. doi: 10.3390/toxins11060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guo J., He Z., Ma C., Li W., Wang J., Lin F., Liu X., Li L. Evaluation of cold plasma for decontamination of molds and mycotoxins in rice grain. Food Chem. 2023;402:134159. doi: 10.1016/j.foodchem.2022.134159. [DOI] [PubMed] [Google Scholar]
- 216.Peng C., Ding Y., An F., Wang L., Li S., Nie Y., Zhou L., Li Y., Wang C., Li S. Degradation of ochratoxin A in aqueous solutions by electron beam irradiation. J. Radioanal. Nucl. Chem. 2015;306:39–46. doi: 10.1007/s10967-015-4086-5. [DOI] [Google Scholar]
- 217.Luo X., Qi L., Liu Y., Wang R., Yang D., Li K., Wang L., Li Y., Zhang Y., Chen Z. Effects of electron beam irradiation on zearalenone and ochratoxin A in naturally contaminated corn and corn quality parameters. Toxins. 2017;9:84. doi: 10.3390/toxins9030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang K., Li K., Pan L., Luo X., Xing J., Wang J., Wang L., Wang R., Zhai Y., Chen Z. Effect of ozone and electron beam irradiation on degradation of zearalenone and ochratoxin A. Toxins. 2020;12:138. doi: 10.3390/toxins12020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Feliciano C.P. High-dose irradiated food: Current progress, applications, and prospects. Radiat. Phys. Chem. 2018;144:34–36. doi: 10.1016/j.radphyschem.2017.11.010. [DOI] [Google Scholar]
- 220.Söbeli C., Uyarcan M., Kayaardı S. Pulsed UV-C radiation of beef loin steaks: Effects on microbial inactivation, quality attributes, and volatile compounds. Innov. Food Sci. Emerg. Technol. 2021;67:102558. doi: 10.1016/j.ifset.2020.102558. [DOI] [Google Scholar]
- 221.Chen J., Wei Z., Wang Y., Long M., Wu W., Kuca K. Fumonisin B1: Mechanisms of toxicity and biological detoxification progress in animals. Food Chem. Toxicol. 2021;149:111977. doi: 10.1016/j.fct.2021.111977. [DOI] [PubMed] [Google Scholar]
- 222.Kaya Z., Unluturk S., Martin-Belloso O., Soliva-Fortuny R. Effectiveness of pulsed light treatments assisted by mild heat on Saccharomyces cerevisiae inactivation in verjuice and evaluation of its quality during storage. Innov. Food Sci. Emerg. Technol. 2020;66:102517. doi: 10.1016/j.ifset.2020.102517. [DOI] [Google Scholar]
- 223.Wang L., Liu X., Cai R., Ge Q., Zhao Z., Yue T., Yuan Y., Gao Z., Wang Z. Detoxification of ochratoxin A by pulsed light in grape juice and evaluation of its degradation products and safety. Innov. Food Sci. Emerg. Technol. 2022;78:103024. doi: 10.1016/j.ifset.2022.103024. [DOI] [Google Scholar]
- 224.Zhang H., Wang G., Yang Q., Yang X., Zheng Y., Liu Y., Xing F. Effects of Light on the Ochratoxigenic Fungi Aspergillus ochraceus and A. carbonarius. Toxins. 2021;13:251. doi: 10.3390/toxins13040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abdelnaby A., Abdelaleem N.M., Elshewy E., Mansour A.H., Ibrahim S. The efficacy of clay bentonite, date pit, and chitosan nanoparticles in the detoxification of aflatoxin M1 and ochratoxin A from milk. Environ. Sci. Pollut. Res. Int. 2022;29:20305–20317. doi: 10.1007/s11356-021-17254-3. [DOI] [PubMed] [Google Scholar]
- 226.Fernandes J.M., Calado T., Guimaraes A., Rodrigues M.A.M., Abrunhosa L. In vitro adsorption of aflatoxin B1, ochratoxin A, and zearalenone by micronized grape stems and olive pomace in buffer solutions. Mycotoxin Res. 2019;35:243–252. doi: 10.1007/s12550-019-00349-9. [DOI] [PubMed] [Google Scholar]
- 227.Nobre C., Gonzalez A., Losoya C., Teixeira J.A., Belmares R., Abrunhosa L. Detoxification of ochratoxin A and zearalenone by Pleurotus ostreatus during in vitro gastrointestinal digestion. Food Chem. 2022;384:132525. doi: 10.1016/j.foodchem.2022.132525. [DOI] [PubMed] [Google Scholar]
- 228.Var I., Kabak B., Erginkaya Z. Reduction in ochratoxin A levels in white wine, following treatment with activated carbon and sodium bentonite. Food Control. 2008;19:592–598. doi: 10.1016/j.foodcont.2007.06.013. [DOI] [Google Scholar]
- 229.Carraro Di Gregorio M., de Valganon Neeff D., Vincenzi Jager A., Corassin C.H., de Pinho Carão Á., de Albuquerque R., de Azevedo A.C., Fernandes Oliveira C.A. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014;33:125–135. doi: 10.3109/15569543.2014.905604. [DOI] [Google Scholar]
- 230.Cosme F., Inês A., Silva D., Filipe-Ribeiro L., Abrunhosa L., Nunes F.M. Elimination of ochratoxin A from white and red wines: Critical characteristics of activated carbons and impact on wine quality. LWT. 2021;140:110838. doi: 10.1016/j.lwt.2020.110838. [DOI] [Google Scholar]
- 231.Zhao Z., Liu N., Yang L., Wang J., Song S., Nie D., Yang X., Hou J., Wu A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control. 2015;57:362–369. doi: 10.1016/j.foodcont.2015.05.014. [DOI] [Google Scholar]
- 232.Solis-Cruz B., Hernandez-Patlan D., Beyssac E., Latorre J.D., Hernandez-Velasco X., Merino-Guzman R., Tellez G., Lopez-Arellano R. Evaluation of Chitosan and Cellulosic Polymers as Binding Adsorbent Materials to Prevent Aflatoxin B1, Fumonisin B1, Ochratoxin, Trichothecene, Deoxynivalenol, and Zearalenone Mycotoxicoses Through an In Vitro Gastrointestinal Model for Poultry. Polymers. 2017;9:529. doi: 10.3390/polym9100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Loffredo E., Scarcia Y., Parlavecchia M. Removal of ochratoxin A from liquid media using novel low-cost biosorbents. Environ. Sci. Pollut. Res. Int. 2020;27:34484–34494. doi: 10.1007/s11356-020-09544-z. [DOI] [PubMed] [Google Scholar]
- 234.Quintela S., Villarán M.C., De López Armentia I., Elejalde E. Ochratoxin A removal from red wine by several oenological fining agents: Bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan. Food Addit. Contam. Part A. 2012;29:1168–1174. doi: 10.1080/19440049.2012.682166. [DOI] [PubMed] [Google Scholar]
- 235.Rasheed U., Ain Q.U., Yaseen M., Fan X., Yao X., Tong Z., Liu B. Modification of bentonite with orange peels extract and its application as mycotoxins’ binder in buffered solutions and simulated gastrointestinal fluids. J. Clean. Prod. 2020;267:122105. doi: 10.1016/j.jclepro.2020.122105. [DOI] [Google Scholar]
- 236.Appell M., Jackson M.A. Sorption of ochratoxin A from aqueous solutions using beta-cyclodextrin-polyurethane polymer. Toxins. 2012;4:98–109. doi: 10.3390/toxins4020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alford R., Mishael Y.G. Bifunctional clay based sorbent for ‘Ochratoxin’ removal and wine fining. Food Chem. 2023;416:135827. doi: 10.1016/j.foodchem.2023.135827. [DOI] [PubMed] [Google Scholar]
- 238.Dumeau F., Trione D. Influence de Différents Traitements sur la Concentration en Ochratoxine A des Vins Rouges. Rev. Des Anol. 2000;95:37–38. [Google Scholar]
- 239.Savino F., Pelle E., Palumeri E., Oggero R., Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus Simethicone in the Treatment of Infantile Colic: A Prospective Randomized Study. Pediatrics. 2007;119:e124–e130. doi: 10.1542/peds.2006-1222. [DOI] [PubMed] [Google Scholar]
- 240.Olivares-Marín M., Del Prete V., Garcia-Moruno E., Fernández-González C., Macías-García A., Gómez-Serrano V. The Development of an Activated Carbon from Cherry Stones and Its Use in the Removal of Ochratoxin A from Red Wine. Food Control. 2009;20:298–303. doi: 10.1016/j.foodcont.2008.05.008. [DOI] [Google Scholar]
- 241.Gambuti A., Strollo D., Genovese A., Ugliano M., Ritieni A., Moio L. Influence of Enological Practices on Ochratoxin A Concentration in Wine. Am. J. Enol. Vitic. 2005;56:155–162. doi: 10.5344/ajev.2005.56.2.155. [DOI] [Google Scholar]
- 242.Visconti A., Perrone G., Cozzi G., Solfrizzo M. Managing Ochratoxin A Risk in the Grape-Wine Food Chain. Food Addit. Contam. 2008;25:193–202. doi: 10.1080/02652030701744546. [DOI] [PubMed] [Google Scholar]
- 243.Quintela S., Villarán M.C., de López Armentia I., Elejalde E. Ochratoxin A removal in wine: A review. Food Control. 2013;30:439–445. doi: 10.1016/j.foodcont.2012.08.014. [DOI] [Google Scholar]
- 244.Castro R.I., Laurie V.F., Padilla C., Carrasco-Sánchez V. Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. plantarum (APLP) Complexes: A Preliminary Study. Toxins. 2022;14:230. doi: 10.3390/toxins14040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bleve G., Grieco F., Cozzi G., Logrieco A., Visconti A. Isolation of Epiphytic Yeasts with Potential for Biocontrol of Aspergillus carbonarius and A. niger on Grape. Int. J. Food Microbiol. 2006;108:204–209. doi: 10.1016/j.ijfoodmicro.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 246.Cserha´ti M., Kriszt B., Krifaton C., Szoboszlay S., Kukolya J. Mycotoxin-degradation profile of Rhodococcus strains. Int. J. Food Microbiol. 2013;166:176–185. doi: 10.1016/j.ijfoodmicro.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 247.Ferenczi S., Cserhati M., Krifaton C., Szoboszlay S., Kukolya J., Szoke Z., Kriszt B. A new ochratoxin A biodegradation strategy using Cupriavidus basilensis Or16 strain. PLoS ONE. 2014;9:e109817. doi: 10.1371/journal.pone.0109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang J.J., Zhang H.Y., Yang Q.Y. Screening and identification of microorganisms for ochratoxin A degradation. Food Sci. 2014;35:154–158. doi: 10.7506/spkx1002-6630-201421030. [DOI] [Google Scholar]
- 249.De Bellis P., Tristezza M., Haidukowski M., Fanelli F., Sisto A., Mulè G., Grieco F. Biodegradation of Ochratoxin A by Bacterial Strains Isolated from Vineyard Soils. Toxins. 2015;7:5079–5093. doi: 10.3390/toxins7124864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fanelli F., Chiara M., Liuzzi V.C., Haidukowski M., Tristezza M., Caterina M., D’Erchia A.M., Pesole G., Horner D.S., Mule’ G. Draft genome sequence of Acinetobacter sp. neg1 capable of degrading ochratoxin A. FEMS Microbiol. Lett. 2015;362:fnv004. doi: 10.1093/femsle/fnv004. [DOI] [PubMed] [Google Scholar]
- 251.Jiang N., Wang X., Zhou Y., Wei C., Wu Y. New Luteimonas Species Useful in Preparing Biodegradable Agents for Detoxification Treatment of Moldy Food Materials and Animal Feed. State Intellectual Property Office; Beijing, China: 2016. [Google Scholar]
- 252.Zhang H.H., Wang Y., Zhao C., Wang J., Zhang X.L. Biodegradation of ochratoxin A by Alcaligenes faecalis isolated from soil. J. Appl. Microbiol. 2017;123:661–668. doi: 10.1111/jam.13537. [DOI] [PubMed] [Google Scholar]
- 253.El Khoury R., Mathieu F., Atoui A., Kawtharani H., El Khoury A., Afif C., El Khoury A. Ability of soil isolated actinobacterial strains to prevent, bind, and biodegrade ochratoxin A. Toxins. 2017;9:222. doi: 10.3390/toxins9070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Luz C., Ferrer J., Mañes J., Meca G. Toxicity reduction of ochratoxin A by lactic acid bacteria. Food Chem. Toxicol. 2018;112:60–66. doi: 10.1016/j.fct.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 255.Minguez C.L., Garrigues M.A.R., Ocana L.L., Novella R.A., Vinuesa J.M., Meca G. Transformation of ochratoxin A by microorganisms isolated from Tempranillo grapes in wine systems. Am. J. Enol. Viticult. 2020;71:167–174. doi: 10.5344/ajev.2019.19041. [DOI] [Google Scholar]
- 256.Xu X., Pang M., Liu J., Wang Y., Wu X., Huang K., Liang Z. Genome mining reveals the genes of carboxypeptidase for OTA detoxification in Bacillus subtilis CW14. Int. J. Biol. Macromol. 2021;186:800–810. doi: 10.1016/j.ijbiomac.2021.07.085. [DOI] [PubMed] [Google Scholar]
- 257.Mateo E.M., Tarazona A., Jiménez M., Mateo F. Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi. Toxins. 2022;14:807. doi: 10.3390/toxins14110807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu C., Zhao C., Liu H., Du W., Sun J., Zhou W., Sun C. Biodegradation of ochratoxin A by two novel strains of Brevibacillus sp. isolated from wheat (Triticum aestivum L.) Food Biosci. 2023;54:102847. doi: 10.1016/j.fbio.2023.102847. [DOI] [Google Scholar]
- 259.Bejaoui H., Mathieu F., Taillandier P., Lebrihi A. Biodegradation of Ochratoxin A by Aspergillus section Nigri species isolated from French grapes: A potential means of Ochratoxin A decontamination in grape juices and musts. FEMS Microbiol. Lett. 2006;255:203–208. doi: 10.1111/j.1574-6968.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 260.Valero A., Marín S., Ramos A.J., Sanchis V. Survey: Ochratoxin A in European Special Wines. Food Chem. 2008;108:593–599. doi: 10.1016/j.foodchem.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 261.Xiong K., Wang X.L., Zhi H.W., Sun B.G., Li X.T. Identification and safety evaluation of a product from the biodegradation of ochratoxin A by an Aspergillus strain. J. Sci. Food Agric. 2017;97:434–443. doi: 10.1002/jsfa.7742. [DOI] [PubMed] [Google Scholar]
- 262.de Felice D.V., Solfrizzo M., De Curtis F., Lima G., Visconti A., Castoria R. Strains of Aureobasidium pullulans can lower ochratoxin A contamination in wine grapes. Phytopathology. 2008;98:1261–1270. doi: 10.1094/PHYTO-98-12-1261. [DOI] [PubMed] [Google Scholar]
- 263.Freire L., Braga P.A.C., Furtado M.M., Delafiori J., Dias-Audibert F.L., Pereira G.E., Reyes F.G., Catharino R.R., Sant’Ana A.S. From grape to wine: Fate of ochratoxin A during red, rose, and white winemaking process and the presence of ochratoxin derivatives in the final products. Food Control. 2020;113:107167. doi: 10.1016/j.foodcont.2020.107167. [DOI] [Google Scholar]
- 264.Yang Q., Wang J., Zhang H., Li C., Zhang X. Ochratoxin A is degraded by Yarrowia lipolytica and generates non-toxic degradation products. World Mycotoxin J. 2016;9:269–278. doi: 10.3920/WMJ2015.1911. [DOI] [Google Scholar]
- 265.Patharajan S., Reddy K., Karthikeyan V., Spadaro D., Lore A., Gullino M.L., Garibaldi A. Potential of yeast antagonists on in vitro biodegradation of ochratoxin A. Food Control. 2011;22:290–296. doi: 10.1016/j.foodcont.2010.07.024. [DOI] [Google Scholar]
- 266.Abrunhosa L., Venâncio A., Teixeira J.A. Optimization of process parameters for the production of an OTA-hydrolyzing enzyme from Aspergillus niger under solid-state fermentation. J. Biosci. Bioeng. 2011;112:351–355. doi: 10.1016/j.jbiosc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 267.Yu S., Poulsen C.H., Dalsgaard S., Wang H., Nikolaev I. Food Additive Comprising an Amidase for Detoxifying Ochratoxin. State Intellectual Property Office; Beijing, China: 2015. [Google Scholar]
- 268.Liuzzi V.C., Fanelli F., Tristezza M., Haidukowski M., Picardi E., Manzari C. Transcriptional analysis of Acinetobacter sp. neg1 capable of degrading Ochratoxin A. Front. Microbiol. 2016;7:2162. doi: 10.3389/fmicb.2016.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wei W., Qian Y., Wu Y., Chen Y., Zhou Y. Detoxification of ochratoxin A by Lysobacter sp. CW239 and characteristics of a novel degrading gene carboxypeptidase cp4. Environ. Pollut. 2020;258:113677. doi: 10.1016/j.envpol.2019.113677. [DOI] [PubMed] [Google Scholar]
- 270.de Oliveira Garcia S., Sibaja K.V.M., Nogueira W.V., Feltrin A.C.P., Pinheiro D.F.A., Cerqueira M.B.R., Furlong E.B., Garda-Buffon J. Peroxidase as a simultaneous degradation agent of ochratoxin A and zearalenone applied to model solution and beer. Food Res. Int. 2020;131:109039. doi: 10.1016/j.foodres.2020.109039. [DOI] [PubMed] [Google Scholar]
- 271.Loi M., Fanelli F., Cimmarusti M.T., Mirabelli V., Haidukowski M., Logrieco A.F., Caliandro R., Mule G. In vitro single and combined mycotoxins degradation by Ery4 laccase from Pleurotus eryngii and redox mediators. Food Control. 2018;90:401–406. doi: 10.1016/j.foodcont.2018.02.032. [DOI] [Google Scholar]
- 272.Orozco-Cortes P.C., Flores-Ortiz C.M., Hernandez-Portilla L.B., Vazquez Medrano J., Rodriguez-Pena O.N. Molecular docking and in vitro studies of Ochratoxin A (OTA) Biodetoxification Testing Three Endopeptidases. Molecules. 2023;28:2019. doi: 10.3390/molecules28052019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Luo H., Wang G., Chen N., Fang Z., Xiao Y., Zhang M., Gerelt K., Qian Y., Lai R., Zhou Y. A Superefficient Ochratoxin A Hydrolase with Promising Potential for Industrial Applications. Appl. Environ. Microbiol. 2022;88:e01964-21. doi: 10.1128/AEM.01964-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Campos-Avelar I., Colas de la Noue A., Durand N., Fay B., Schorr-Galindo S. Minimizing ochratoxin A contamination through the use of actinobacteria and their active molecules. Toxins. 2020;12:296. doi: 10.3390/toxins12050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Muhialdin B.J., Saari N., Meor Hussin A.S. Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of food supply. Molecules. 2020;25:2655. doi: 10.3390/molecules25112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fuchs S., Sontag G., Stidl R., Ehrlich V., Kundi M., Knasmueller S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008;46:1398–1407. doi: 10.1016/j.fct.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 277.Domínguez-Gutiérrez G.A., Perraud-Gaime I., Escalona-Buendía H., Durand N., Champion-Martínez E.I., Fernández-Soto R.R., Saucedo-Castañeda G., Rodríguez-Serrano G. Inhibition of Aspergillus carbonarius growth and Ochratoxin A production using lactic acid bacteria cultivated in an optimized medium. Int. J. Food Microbiol. 2023;404:110320. doi: 10.1016/j.ijfoodmicro.2023.110320. [DOI] [PubMed] [Google Scholar]
- 278.Shi L., Liang Z., Li J., Hao J., Xu Y., Huang K., Tian J., He X., Xu W. Ochratoxin A biocontrol and biodegradation by Bacillus subtilis CW14. J. Sci. Food Agric. 2014;94:1879–1885. doi: 10.1002/jsfa.6507. [DOI] [PubMed] [Google Scholar]
- 279.Rodríguez A., Rodríguez M., Luque M.I., Justesen A.F., Córdoba J.J. Quantification of Ochratoxin A-Producing Molds in Food Products by SYBR Green and TaqMan Real-Time PCR Methods. Int. J. Food Microbiol. 2011;149:226–235. doi: 10.1016/j.ijfoodmicro.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 280.Upadhaya S.D., Song J.Y., Park M.A., Seo J.K., Yang L., Lee C.H., Cho K.J., Ha J.K. Isolation, screening, and identification of swine gut microbiota with Ochratoxin A biodegradation ability. Asian-Australas. J. Anim. Sci. 2012;25:114–121. doi: 10.5713/ajas.2011.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wei M., Dhanasekaran S., Ji Q., Yang Q., Zhang H. Sustainable and efficient method utilizing N-acetyl-L-cysteine for complete and enhanced ochratoxin A clearance by antagonistic yeast. J. Hazard. Mater. 2023;448:130975. doi: 10.1016/j.jhazmat.2023.130975. [DOI] [PubMed] [Google Scholar]
- 282.Zeidan R., Ul-Hassan Z., Al-Thani R., Migheli Q., Jaoua S. In vitro application of a Qatari Burkholderiacepacia strain (QBC03) in the biocontrol of mycotoxigenic fungi and in the reduction of ochratoxin A biosynthesis by Aspergillus carbonarius. Toxins. 2019;11:700. doi: 10.3390/toxins11120700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zou D., Ji J., Ye Y., Yang Y., Yu J., Wang M., Zheng Y., Sun X. Degradation of Ochratoxin A by a UV-Mutated Aspergillus niger Strain. Toxins. 2022;14:343. doi: 10.3390/toxins14050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shang L., Bai X., Chen C., Liu L., Li M., Xia X., Wang Y. Isolation and identification of a Bacillus megaterium strain with ochratoxin A removal ability and antifungal activity. Food Control. 2019;106:106743. doi: 10.1016/j.foodcont.2019.106743. [DOI] [Google Scholar]
- 285.Varga J., Rigó K., Téren J. Degradation of Ochratoxin A by Aspergillus species. Int. J. Food Microbiol. 2000;59:1–7. doi: 10.1016/S0168-1605(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 286.Varga J., Péteri Z., Tábori K., Téren J., Vágvölgyi C. Degradation of Ochratoxin A and other mycotoxins by Rhizopus isolates. Int. J. Food Microbiol. 2005;99:321–328. doi: 10.1016/j.ijfoodmicro.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 287.Abrunhosa L., Serra R., Venâncio A. Biodegradation of Ochratoxin A by fungi isolated from grapes. J. Agric. Food Chem. 2002;50:7493–7496. doi: 10.1021/jf025747i. [DOI] [PubMed] [Google Scholar]
- 288.Cho S.M., Jeong S.E., Lee K.R., Sudhani H.P., Kim M., Hong S.Y., Chung S.H. Biodegradation of Ochratoxin A by Aspergillus tubingensis Isolated from Meju. J. Microbiol. Biotechnol. 2016;26:1687–1695. doi: 10.4014/jmb.1606.06016. [DOI] [PubMed] [Google Scholar]
- 289.Ponsone M.L., Chiotta M.L., Combina M., Dalcero A., Chulze S. Biocontrol as a strategy to reduce the impact of ochratoxin A and Aspergillus section Nigri in grapes. Int. J. Food Microbiol. 2011;151:70–77. doi: 10.1016/j.ijfoodmicro.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 290.Ponsone M.L., Nally M.C., Chiotta M.L., Combina M., Köhl J., Chulze S.N. Evaluation of the effectiveness of potential biocontrol yeasts against black sur rot and ochratoxin A occurring under greenhouse and field grape production conditions. Biol. Control. 2016;103:78–85. doi: 10.1016/j.biocontrol.2016.07.012. [DOI] [Google Scholar]
- 291.Petruzzi L., Corbo M.R., Baiano A., Beneduce L., Sinigaglia M., Bevilacqua A. In vivo stability of the complex ochratoxin A-Saccharomyces cerevisiae starter strains. Food Control. 2015;50:516–520. doi: 10.1016/j.foodcont.2014.09.042. [DOI] [Google Scholar]
- 292.Angioni A., Caboni P., Garau A., Farris A., Orro D., Budroni M., Cabras P. In Vitro Interaction Between Ochratoxin A and Different Strains of Saccharomyces cerevisiae and Kloeckeraapiculata. J. Agric. Food Chem. 2007;55:2043–2048. doi: 10.1021/jf062768u. [DOI] [PubMed] [Google Scholar]
- 293.Fiori S., Urgeghe P.P., Hammami W., Razzu S., Jaoua S., Migheli Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014;189:45–50. doi: 10.1016/j.ijfoodmicro.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 294.Wei M., Dhanasekaran S., Legrand Ngolong Ngea G., Abiso Godana E., Zhang X., Yang Q., Zheng X., Zhang H. Cryptococcus podzolicus Y3 degrades ochratoxin A by intracellular enzymes and simultaneously eliminates citrinin. Biol. Control. 2022;168:104857. doi: 10.1016/j.biocontrol.2022.104857. [DOI] [Google Scholar]
- 295.Taheur F.B., Kouidhi B., Al Qurashi Y.M.A., Salah-Abbès J.B., Chaieb K. Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon. 2019;160:12–22. doi: 10.1016/j.toxicon.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 296.Ding L., Han M., Wang X., Guo Y. Ochratoxin A: Overview of Prevention, Removal, and Detoxification Methods. Toxins. 2023;15:565. doi: 10.3390/toxins15090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Dobritzsch D., Wang H., Schneider G., Yu S. Structural and functional characterization of ochratoxinase, a novel mycotoxin-degrading enzyme. Biochem. J. 2014;462:441–452. doi: 10.1042/BJ20140382. [DOI] [PubMed] [Google Scholar]
- 298.Abrunhosa L., Santos L., Venâncio A. Degradation of Ochratoxin A by Proteases and by a Crude Enzyme of Aspergillus niger. Food Biotechnol. 2006;20:231–242. doi: 10.1080/08905430600904369. [DOI] [Google Scholar]
- 299.Abrunhosa L., Venâncio A. Isolation and purification of an enzyme hydrolyzing ochratoxin A from Aspergillus niger. Biotechnol. Lett. 2007;29:1909–1914. doi: 10.1007/s10529-007-9479-2. [DOI] [PubMed] [Google Scholar]
- 300.Abrunhosa L., Paterson R.R., Venâncio A. Biodegradation of Ochratoxin A for food and feed decontamination. Toxins. 2010;2:1078–1099. doi: 10.3390/toxins2051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Stander M.A., Bornscheuer U.T., Henke E., Steyn P.S. Screening of commercial hydrolases for the degradation of ochratoxin A. J. Agric. Food Chem. 2000;48:5736–5739. doi: 10.1021/jf000413j. [DOI] [PubMed] [Google Scholar]
- 302.Dalsgaard P.W., Petersen B.O., Duus J.Ø., Zidorn C., Frisvad J.C., Christophersen C., Larsen T.O. Atlantinone A, a Meroterpenoid Produced by Penicillium ribeum and Several Cheese Associated Penicillium Species. Metabolites. 2012;2:214–220. doi: 10.3390/metabo2010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Peng M., Zhang Z., Xu X., Zhang H., Zhao Z., Liang Z. Purification and characterization of the enzymes from Brevundimonas naejangsanensis that degrade ochratoxin A and B. Food Chem. 2023;419:135926. doi: 10.1016/j.foodchem.2023.135926. [DOI] [PubMed] [Google Scholar]
- 304.Ma L., Qiu X., Li Y., Tang S., Shen W., Xing C., Kong D., Sheng J. Carboxypeptidase A immobilization with zeolitic imidazolate framework for enhancement of ochratoxin A degradation ability. Food Agric. Immunol. 2020;31:587–599. doi: 10.1080/09540105.2020.1749570. [DOI] [Google Scholar]
- 305.Nahle S., Atoui A., Assaf J.C., El Khoury A., Louka N., Chokr A. Time-Dependent Effect of Surface Material on Lactobacillus rhamnosus GG Biofilm Formation and Gene Expression. Microbiology. 2023;92:55–65. doi: 10.1134/S0026261721102142. [DOI] [Google Scholar]
- 306.Nahle S., El Khoury A., Savvaidis I., Chokr A., Louka N., Atoui A. Detoxification approaches of mycotoxins: By microorganisms, biofilms and enzymes. Int. J. Food Contam. 2022;9:3. doi: 10.1186/s40550-022-00089-2. [DOI] [Google Scholar]
- 307.Da Cruz Cabral L., Pinto F.V., Patriarca A. Application of plant-derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 308.de Coutinho Oliveira T.L., de Araújo Soares R., Mendes Ramos E., das Graças Cardoso M., Alves E., Piccoli R.H. Antimicrobial activity of Satureja montana L. essential oil against Clostridium perfringens type A inoculated in mortadella-type sausages formulated with different levels of sodium nitrite. Int. J. Food Microbiol. 2011;144:546–555. doi: 10.1016/j.ijfoodmicro.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 309.El Mogy M.M., Alsanius B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control. 2012;28:157–162. doi: 10.1016/j.foodcont.2012.04.036. [DOI] [Google Scholar]
- 310.Passone M.A., Girardi N.S., Etcheverry M. Evaluation of the control ability of five essential oils against Aspergillus section Nigri growth and ochratoxin A accumulation in peanut meal extract agar conditioned at different water activities levels. Int. J. Food Microbiol. 2012;159:198–206. doi: 10.1016/j.ijfoodmicro.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 311.Murthy P.S., Borse B.B., Khanum H., Srinivas P. Inhibitory effects of Ajowan (Trachyspermum ammi) ethanolic extract on A. ochraceus growth and ochratoxin production. Turkish J. Biol. 2009;33:211–217. doi: 10.3906/biy-0805-13. [DOI] [Google Scholar]
- 312.Hua H., Xing F., Selvaraj J.N., Wang Y., Zhao Y., Zhou L., Liu X., Liu Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. PLoS ONE. 2014;9:e108285. doi: 10.1371/journal.pone.0108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ferrara M., Gallo A., Scalzo R.L., Haidukowski M., Picchi V., Perrone G. Inhibition of ochratoxin A production in Aspergillus carbonarius by hydroxycinnamic acids from grapes. World Mycotoxin J. 2015;8:283–289. doi: 10.3920/WMJ2014.1753. [DOI] [Google Scholar]
- 314.El Khoury R., Atoui A., Verheecke C., Maroun R., El Khoury A., Mathieu F. Essential Oils Modulate Gene Expression and Ochratoxin A Production in Aspergillus carbonarius. Toxins. 2016;8:242. doi: 10.3390/toxins8080242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.El-Desouky T.A. Evaluation of effectiveness aqueous extract for some leaves of wild edible plants in Egypt as anti-fungal and anti-toxigenic. Heliyon. 2021;7:e06209. doi: 10.1016/j.heliyon.2021.e06209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Naz G., Anjum A.A., Nawaz M., Iqbal S., Azeem S., Ali T., Manzoor R. Evaluation of Cinnamomum verum Essential Oils against Ochratoxin A-producing Aspergillus parasiticus in Stored Wheat, Maize, and Rice. Polish J. Environ. Stud. 2023;32:667–676. doi: 10.15244/pjoes/155084. [DOI] [Google Scholar]
- 317.El-Desouky T., Ammar H. Honey-mediated silver nanoparticles and their inhibitory effect on aflatoxins and ochratoxin A. J. Appl. Pharm. Sci. 2016;6:83–90. doi: 10.7324/JAPS.2016.60615. [DOI] [Google Scholar]
- 318.Pushpalatha R., Selvamuthukumar S., Kilimozhi D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery: Physicochemical characterization, drug release, stability, and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018;45:45–53. doi: 10.1016/j.jddst.2018.03.004. [DOI] [Google Scholar]
- 319.Dhakar N.K., Matencio A., Caldera F., Argenziano M., Cavalli R., Dianzani C., Zanetti M., Lopez-Nicolas J.M., Trotta F. Comparative evaluation of solubility, cytotoxicity, and photostability studies of resveratrol and oxyresveratrol loaded nanosponges. Pharmaceutics. 2019;11:545. doi: 10.3390/pharmaceutics11100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Redondo-Blanco S., Fernandez J., Lopez-Ibanez S., Miguelez E.M., Villar C.J., Lombo F. Plant phytochemicals in food preservation: Antifungal bioactivity: A review. J. Food Prot. 2019;83:163–171. doi: 10.4315/0362-028X.JFP-19-163. [DOI] [PubMed] [Google Scholar]
- 321.Sajid M., Cameotra S.S., Sajjad M., Khan A., Ahmad I. Nanoparticle-based delivery of phytomedicines: Challenges and opportunities. In: Ahmad Khan M.S., Ahmad I., Chattopadhyay D., editors. New Look to Phytomedicine. Elsevier; Amsterdam, The Netherlands: 2019. pp. 597–623. [DOI] [Google Scholar]
- 322.Thipe V.C., Panjtan Amiri K., Bloebaum P., Raphael Karikachery A., Khoobchandani M., Katti K.K., Jurisson S.S., Katti K.V. Development of resveratrol-conjugated gold resveratrol corona on anti-tumor efficacy against nanoparticles: Interrelationship of increased breast, pancreatic, and prostate cancers. Int. J. Nanomed. 2019;14:4413–4428. doi: 10.2147/IJN.S204443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pawar A.Y., Naik A.K., Jadhav K.R. Nanosponges: A novel drug delivery system. Asian J. Pharm. Pharmacol. 2016;10:S456–S463. doi: 10.2174/1872210512666180925102842. [DOI] [Google Scholar]
- 324.Sherje A.P., Dravyakar B.R., Kadam D., Jadhav M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017;173:37–49. doi: 10.1016/j.carbpol.2017.05.086. [DOI] [PubMed] [Google Scholar]
- 325.Bhowmik H., Venkatesh D.N., Kuila A., Kumar K.H. Nanosponges: A review. Int. J. Appl. Pharm. 2018;10:1. doi: 10.22159/ijap.2018v10i4.25026. [DOI] [Google Scholar]
- 326.Haimhoffer Á., Rusznyák Á., Réti-Nagy K., Vasvári G., Váradi J., Vecsernyés M., Bácskay I., Fehér P., Ujhelyi Z., Fenyvesi F. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019;87:33. doi: 10.3390/scipharm87040033. [DOI] [Google Scholar]
- 327.Ananya K.V., Preethi S., Amit B.P., Gowda D.V. Recent review on Nano sponge. Int. J. Res. Pharm. Sci. 2020;11:1085–1096. doi: 10.26452/ijrps.v11i1.1940. [DOI] [Google Scholar]
- 328.Asghari F., Jahanshiri Z., Imani M., Shams-Ghahfarokhi M., Razzaghi-Abyaneh M. Antifungal nanomaterials: Synthesis, properties, and applications. In: Mihai Grumezescu A., editor. Nanobiomaterials in Antimicrobial Therapy. Elsevier; Amsterdam, The Netherlands: 2016. pp. 343–383. [DOI] [Google Scholar]
- 329.Appell M., Evans K.O., Jackson M.A., Compton D.L. Determination of ochratoxin A in grape juice and wine using nanosponge solid phase extraction clean-up and liquid chromatography with fluorescence detection. J. Liq. Chromatogr. Relat. Technol. 2018;41:949–954. doi: 10.1080/10826076.2018.1544148. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.