Abstract
Metformin is a synthetic biguanide used as an antidiabetic drug in type 2 diabetes mellitus, achieved by studying the bioactive metabolites of Galega officinalis L. It is also used off-label for various other diseases, such as subclinical diabetes, obesity, polycystic ovary syndrome, etc. In addition, metformin is proposed as an add-on therapy for several conditions, including autoimmune diseases, neurodegenerative diseases, and cancer. Although metformin has been used for many decades, it is still the subject of many pharmacodynamic and pharmacokinetic studies in light of its extensive use. Metformin acts at the mitochondrial level by inhibiting the respiratory chain, thus increasing the AMP/ATP ratio and, subsequently, activating the AMP-activated protein kinase. However, several other mechanisms have been proposed, including binding to presenilin enhancer 2, increasing GLP1 release, and modification of microRNA expression. Regarding its pharmacokinetics, after oral administration, metformin is absorbed, distributed, and eliminated, mainly through the renal route, using transporters for cationic solutes, since it exists as an ionic molecule at physiological pH. In this review, particular consideration has been paid to literature data from the last 10 years, deepening the study of clinical trials inherent to new uses of metformin, the differences in effectiveness and safety observed between the sexes, and the unwanted side effects. For this last objective, metformin safety was also evaluated using both VigiBase and EudraVigilance, respectively, the WHO and European databases of the reported adverse drug reactions, to assess the extent of metformin side effects in real-life use.
Keywords: AMPK, miRNA, antidiabetic drugs, ADRs, sex differences, ADMET, pharmacovigilance, T2DM
1. Introduction
Metformin (1,1-dimethylbiguanide) is a low-molecular-weight compound derived from guanidine, which exists in the human body as a cationic molecule (Figure 1). It was synthesized based on the chemical structure of guanidine derivatives extracted from the European medicinal plant Galega officinalis L. (Fabaceae), which has been used for curative purposes since the Middle Ages [1,2]. Various documents attest that as early as the XVIII century, herbal preparations containing the aerial flowering parts of galega were recommended for people affected by persistent thirst and frequent urine emissions, characteristic symptoms of diabetes mellitus (DM) [2]. Subsequently, guanidine and its natural derivative, galegine, were investigated as antidiabetic drugs, demonstrating not only hypoglycemic effects but also some toxicity [2]. In 1922, metformin was first synthesized by Werner and Bell, but it was only later recognized as a hypoglycemic drug [3]. It appears now curious that in the 1950s metformin was used to treat influenza and malaria, considering the antihyperglycemic action a side effect [4,5]. Indeed, only many years later, metformin was approved as an antidiabetic drug in European countries and later in many other states [6]. In 2011, the World Health Organization included metformin among the essential drugs for humanity, and, as such, it is still considered [2,7,8].
Figure 1.
The natural compounds guanidine and galegine recognized in Galega officinalis and the synthetic derivative metformin that is mainly used in the treatment of type 2 diabetes mellitus. Chemical structures were drawn using ACD/ChemSketch 2023.2.1 software.
Its effectiveness as an antidiabetic drug was recognized worldwide in 1998 thanks to the “United Kingdom Prospective Diabetes Study” (UKPDS 34), which showed that intensive glycemic control with metformin reduced cardiovascular mortality and increased survival of 342 overweight and obese type 2 diabetic subjects to a greater extent compared to sulfonylureas (542 subjects) or insulin (409 subjects) [9]. Afterward, other clinical studies confirmed the antidiabetic efficacy of metformin and its usefulness in reducing cardiovascular complications related to uncontrolled hyperglycemia. Among these, a retrospective study published in 2005 by Johnson et al. demonstrated that treatment with metformin alone, or in combination with sulfonylureas, can reduce the risk of cardiovascular-related non-fatal and fatal events compared to patients treated with a sulfonylurea alone [10]. In 2008, Holman et al. published data from monitoring subjects who had been enrolled in the UKPDS for over 10 years. This study showed that metformin treatment of obese diabetic subjects led to a 21% reduction in diabetes-related conditions, a 33% reduction in myocardial infarction, and a 27% reduction in all-cause mortality [11]. Likewise, the efficacy of the drug in non-obese subjects was confirmed by a retrospective observational study by Ong and colleagues published in 2006, involving normal-weight or overweight subjects [12]. Lastly, in a meta-analysis based on 35 clinical trials, metformin monotherapy (15 trials that included 2424 subjects) showed a reduction in glycated hemoglobin (HbA1c) levels of 1.12% (95% CI 0.92–1.32, p < 0.00001) compared to placebo, no treatment, or only diet [13].
The effectiveness of metformin in glycemic control and preventing cardiovascular diseases is widely evidenced, however, new antidiabetic drugs have been developed in recent times, offering themselves as possible therapeutic alternatives. Among these, the main contemporary classes in use include the glucagon-like peptide 1 (GLP1) agonists, the dipeptidyl peptidase-4 (DPP4) inhibitors (known as gliptins), and the sodium–glucose cotransporter 2 (SGLT2) inhibitors (known as gliflozins) [14,15]. Even so, metformin remains one of the most prescribed drugs for the treatment of type 2 diabetes mellitus (T2DM) either alone or in association with other antidiabetic drugs [16,17]. This primacy is due to its therapeutic efficacy, reduced serious side effects, such as hypoglycemic crises, and low cost. However, the new guidelines of the European Society of Cardiology indicate that metformin is the first choice drug in patients without overt cardiovascular events, without heart failure (HF), and with preserved kidney function (eGFR ≥ 60 mL/min) [18]. Similarly, in the American Diabetes Association (ADA) Guidelines 2024, metformin no longer appears as the first-choice drug in all antidiabetic treatments, since it has been indicated as a second-line therapy in patients with a high risk for cardiovascular diseases or overt heart and kidney diseases [19,20]. In detail, in diabetic patients with previous cardiovascular events without HF, metformin is considered the first-choice drug, as well as the analogues of GLP1 and SGLT2 inhibitors, while in patients with HF, the first-line drugs are now the inhibitors of SGLT2, while metformin and the GLP1 receptor agonists are the second-line drugs [18]. Therefore, the predominance of metformin is partially weakened by the newly introduced drugs on the pharmaceutical market. However, metformin retains its status for its wide use in T2DM and also for several off-label uses, such as impaired glucose tolerance, obesity, and polycystic ovary syndrome [21,22].
The main purpose of this review was to point out the data published in the last 10 years concerning the effectiveness and undesirable effects of metformin in its real-world use, also by using VigiBase and EudraVigilance, respectively, the WHO and European databases of the reported adverse drug reactions (ADRs) [23,24]. Its pharmacokinetics and several off-label clinical uses have also been considered. Attention was paid to the potential differences in efficacy and side effects reported in women compared to men in the clinical use of metformin.
2. Results
2.1. Pharmacodynamics: Pharmacological Targets
Metformin is an insulin sensitizer as it improves the tissue response to insulin, acting mainly in the liver, muscles, and adipose tissue [25,26]. It decreases glycemia in T2DM subjects by reducing insulin levels, mainly by decreasing liver gluconeogenesis and glycogenolysis [26,27]. Blood glucose levels are reduced both after meal and in fasting conditions, resulting in a decrease in HbA1c, as reported in several clinical trials [11,13]. Its antihyperglycemic effect does not emerge if no insulin secretion occurs [28]. Of interest, metformin treatment is accompanied by a moderate reduction in body weight (3–4 kg) of the subjects and, in the long-term use, by a decrease in cardiovascular risk with favorable effects on vascular endothelium and antioxidant and anti-inflammatory actions [29,30]. A recent study of sex differences of metformin antihyperglycemic effect was evaluated in Chinese subjects treated for 24 and 48 weeks with this drug, showing a similar reduction in HbA1c between females and males [31].
The pharmacological mechanisms by which metformin reduces blood glucose concentration and exerts tissue-protective effects are still under investigation, increasing the emphasis on its role as an interesting multitarget agent [32,33]. Furthermore, several authors have attributed additional therapeutic properties, including antiviral, anti-inflammatory, immunomodulatory, antitumor, and protective effects on lung and pancreatic tissues [34]. In fact, patients with a recent diagnosis of T2DM have a chronic inflammatory state, evidenced by increased levels of IL-6, TNF-α, IL-1β, IL-2 cytokines, and ferritin, which are curtailed after one year of metformin treatment [35].
Evidence obtained from various experimental models suggests that metformin may have different mechanisms of action, particularly when administered at low doses rather than high doses [36,37]. It is generally accepted that metformin acts at the intracellular level by inhibiting mitochondrial respiratory chain complex-1 and regulating cellular energy metabolism, even at supra-pharmacological doses (~1 mM) [27,38,39]. This inhibition can result in an increase in the AMP/ATP ratio and, consequently, the stimulation of AMP-activated protein kinase (AMPK), which plays an important role as a cell regulator of lipid and glucose metabolisms [39,40]. The ability to control cellular metabolism, leading to a situation of relative “energy poverty”, can also explain many of the pleiotropic effects of metformin related to a reduction in energy availability. Its action could be at least in part similar to that induced by intermittent fasting, which can prevent age-related mitochondrial decline and also increase fatty acid oxidation [41,42,43].
Numerous studies in experimental models of diabetic animals and diabetic or obese human subjects have shown that in insulin resistance there is a significant decrease in glucose uptake by glucose transporter 4 (GLUT4) [44,45,46,47,48,49,50]. Effectively, metformin increases insulin-dependent glucose uptake in skeletal muscle and adipose tissue by GLUT4 [51,52].
Of interest, recent research has shown that a molecular target of metformin is presenilin enhancer 2 (PEN2), which interacts with the lysosomal glucose-sensing pathway to activate AMPK, resulting in benefits similar to those generated by fasting [37]. Furthermore, these authors showed by isothermal calorimetry and surface plasmon resonance measurements that the KD for the metformin–PEN2 interaction is 1.7 and 0.15 µmol/L, which are values consistent with the intracellular concentration of the drug reached in vivo in therapeutic use [37]. However, other molecular mechanisms of metformin that are AMPK-independent have been reported to be related to the oxygen consumption rate, tumor suppression, and blocking important pathways such as mTOR [36]. It has also been suggested that the hypoglycemic effect is in part due to a gut-mediated mechanism mediated by increased secretion of GLP1 and peptide YY (PYY) through the intestinal AMPK-dependent pathway [53]. Furthermore, it has also been reported that the increase in GLP1 is due to an inhibition of the enzyme DPP4, which can also regulate the peptide YY level, causing a reduction in food intake and an increase in satiety [54,55,56]. Recently, Wang and colleagues have highlighted the existence of a DPP4 enzyme produced by the intestinal microbiota that metabolizes human GLP1, which is potentially active in cases of impaired intestinal permeability due to inflammation, as occurs in DM [57]. Moreover, several authors have described that metformin changes the intestinal microbiota while also decreasing glucose absorption [57,58,59,60,61,62]. Therefore, the hypoglycemic action of metformin may also be mediated by intestinal-dependent mechanisms, which could vary depending on microbiota phenotype [63]. Inhibition of IL-6 mediated signaling by decreasing the expression of its receptor at the transcription level via AMPK, mTOR, and miR-34a is one of the newer mechanisms that suggest metformin as a potential treatment for multiple myeloma [64]. MicroRNAs (miRNAs or miR) are small, single-stranded, non-protein-coding RNAs that regulate gene expression post-transcriptionally by degrading target mRNA when there is a perfect match or by inhibiting translation when there is imperfect complementarity [65]. Different miRNAs can promote or inhibit DM complications [66]. Several authors have explored circulating miRNAs in T2DM compared to healthy subjects, suggesting that specific miRNAs might serve as biomarkers for early diagnosis of T2DM [66,67,68]. Various studies have also shown changes in miRNA expression in subjects treated with metformin [69,70,71]. A study with 47 patients (20 women and 27 men) with T2DM showed that a total of 13 miRNAs, such as miR-let-7e-5p, let-7f-5p, miR-21-5p, miR-24-3p, miR-26b-5p, miR-126-5p, miR-129-5p, miR-130b-3p, miR-146a-5p, miR-148a-3p, miR-152-3p, miR-194-5p, and miR-99a-5p, were significantly negatively regulated after a three-month treatment with metformin, while the other 73 miRNAs were not altered [69]. Specifically, metformin significantly altered the expression of miR-148a-3p and miR-194-5p (p < 0.007), potentially affecting the NF-kB and Wnt signaling pathways [69]. Furthermore, metformin can up-regulate miRNA-185-5p expression to suppress glucose-6-phosphatase (G6Pase) and inhibit liver gluconeogenesis, reducing fasting blood glucose levels [72]. Although there are various studies aimed at identifying the effects of metformin on miRNA expression, the currently available data do not allow for definite conclusions, thus, further investigations are required involving a larger number of subjects treated with fixed doses of the drug.
2.2. Pharmacokinetics: Absorption, Distribution, Metabolism, and Excretion (ADME)
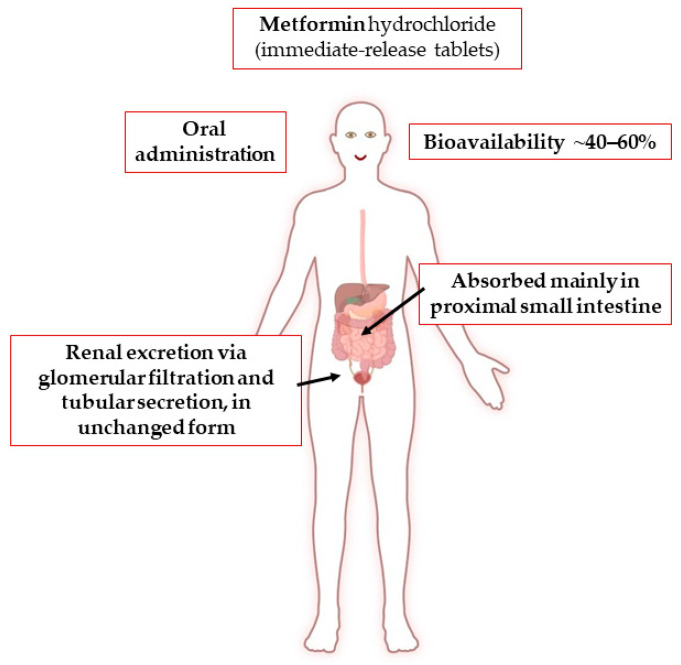
Metformin hydrochloride is administered orally along with meals in doses ranging from 500 to 3000 mg per day, subdivided into two or three times (Figure 2). It has a dose-dependent antihyperglycemic effect [73,74,75]. It is also prescribed for children aged at least 10 years. The pharmacokinetics of metformin have been reported to be similar in women and men, considering the weight differences between the sexes [76].
Figure 2.
Absorption, distribution, metabolism, and excretion of metformin. Created with BioRender software.
To limit the occurrence of gastric and intestinal disorders, namely, nausea and diarrhea, a low initial dose (500 mg, once or twice per day) is administered, and, after 10–15 days, the dose is increased to obtain the target glycemic control (maintenance dose) [15,77]. Furthermore, it has been observed that taking it during or immediately after meals can help reduce the typical gastrointestinal side effects [77]. More recently, various types of delayed-release formulations have been marketed that allow a single evening administration and, potentially, even fewer gastrointestinal disorders [78,79,80]. However, comparative clinical studies between immediate-release and controlled-release formulations are rather limited and difficult to interpret for the different dosages used, the treatment period, and the different number of subjects enrolled [78,81].
Due to its basic hydrophilic nature, the absorption of metformin by simple diffusion through the intestinal mucosa would be extremely low and unsuitable for therapeutic use. In fact, absorption is dose-dependent and occurs through an active and saturable process by means of membrane proteins (transporters) [82]. Thus, after oral administration, about 40–60% of metformin is absorbed primarily in the proximal small intestine (duodenum) through the plasma membrane monoamine transporter (PMAT or SLC29A4) and the organic cation transporters 1 and 3 (OCT1 and 3 or SLC22A1 and 3) [82,83,84]. The drug is eliminated through urine via glomerular filtration and tubular secretion, without enzymatic biotransformation, in its unchanged form [85,86]. A fecal excretion of an unabsorbed fraction (20–30%) is reported [85].
Since metformin exists mostly as a cation in the human organism at the physiological pH, the distribution and renal excretion are also mediated by endogenous transporters, such as OCT1, 2, and 3; PMAT; multidrug and toxin extrusion transporters 1 and 2 (MATE 1 and 2 or SLC47A1 and 2); carnitine/organic cation transporter (OCTN1or SLC22A4); serotonin reuptake transporter (SERT); and thiamine transporter 2 (THTR2 or SLC19A3) [83,87]. Mainly, the OCT1 transporter is expressed at the intestinal level, controlling oral absorption, while OCT1 and 2 regulate renal clearance, respectively, at the luminal and basolateral sides of the proximal and distal tubules [88]. A more detailed description of the transporters involved in metformin life in the human organism is provided in previous reviews [83,88,89]. As is known, the protein transporters involved are expressed differently in human tissues and have high genetic polymorphism, generating wide inter-individual variability in metformin pharmacokinetics, explaining the range of dosage and different antidiabetic effectiveness [87,88,90]. The detected Cmax is around 1 µg/mL, and, in general, the optimal plasma concentration should be less than 2 µg/mL, whereas concentrations greater than 5 µg/mL are considered unsafe due to the increased risk of severe adverse effects, such as lactic acidosis [87,91,92]. However, several authors reported blood concentrations of up to 80 µg/mL in subjects treated with metformin, showing high variability in the pharmacokinetics of this drug [93]. Aging is known to lead to a progressive decline in renal function, which can result in an increase in metformin blood concentration that may require a dose reduction [94]. A recent meta-analysis of 14 clinical trials (408 subjects) shows that food intake reduces Cmax by 40% and delays it by ~29%, decreasing the area under the curve by ~28% [95]. In particular, a diet rich in fat and high in calories can significantly decrease the amount and rate of absorption [95]. Metformin binds marginally to plasma proteins, while it is found within erythrocytes, which form a secondary distribution compartment [82,85,93,96]. The apparent volume of distribution is between 63 and 276 L, while the terminal half-life (t1/2) is 4.0–8.7 h [97,98]. Metformin can cross the human placenta and is detectable in small amounts in breast milk with a milk-to-plasma ratio between 0.35 and 0.71 [99]. Its renal clearance is ~510 ± 120 mL/min, and its half-life is approximately 5 h [82]. When renal function is impaired, renal clearance decreases proportionally with creatinine clearance, thus prolonging the half-life and increasing the plasma concentration. For this, metformin use is contraindicated when the estimated glomerular filtration rate (eGFR) is less than 30 mL/min/1.73 m2 [100].
2.3. Metformin and Vascular Diseases
The macro- and microvascular complications caused by hyperglycemia are widely described in the literature and are also identified as panvascular diabetic disease (PVDD) [101,102,103]. In fact, macro- and microvascular complications can coexist, appearing in specific organ damage according to the characteristics of diabetic patients and resulting in several different diseases, such as heart failure, stroke, peripheral vascular disease, chronic kidney disease, diabetic retinopathy, and autonomic neuropathy [104,105,106]. Metformin, in addition to its proven hypoglycemic action, has the potential to reduce hyperinsulinemia, which is a factor that can worsen metabolic disorders such as hyperlipidemia and endothelial dysfunction [107]. This may provide greater cardiovascular protection than insulin secretagogues such as sulfonylureas and glinides, which raise insulin levels [108,109]. The “2022 Chinese Expert Consensus on Risk Assessment and Management of Panvascular Disease in Patients with T2DM” recommends metformin alone, or in combination with GLP1 receptor agonists or SGLT2 inhibitors, as a first-line hypoglycemic agent in the absence of explicit contraindications [110]. Moreover, the ADA Guidelines 2024 indicate the role of GLP1 agonists and SGLT2 inhibitors in the treatment of atherosclerotic cardiovascular diseases, including peripheral artery disease (PAD) [20].
Several in vitro and in vivo studies have shown that metformin plays a protective role against endothelial dysfunction, protecting vasodilation and decreasing inflammation and oxidative damage [111,112,113,114]. Numerous studies have found that women are more susceptible to PAD than men, and women affected by diabetes have an increased risk of developing symptomatic PAD [115,116,117]. Furthermore, patients with DM and PAD are more likely to develop ischemic ulcers or gangrene, also leading to “diabetic foot”, than non-diabetic subjects [118,119]. Notably, poor glycemic control is an independent risk factor for PAD [120]. It has recently been observed that the prevalence of PAD is the same in both sexes in high-income countries, while it is more recurrent in women in low- and middle-income countries, especially in younger subjects [121]. Furthermore, DM causes higher all-cause mortality in women than in men due to cardiovascular diseases with more manifest endothelial dysfunction than diabetic males [122,123]. A few clinical studies have been planned to evaluate the efficacy of metformin in PAD, and enrolled subjects are generally treated with other drugs, such as antiplatelets, statins, and antihypertensives. For this reason, the effective role of this drug in the progression of PAD is still unclear. In patients undergoing revascularization for chronic ischemia of the limbs, Khan and colleagues showed that subjects treated with metformin (n = 147) had a significant improvement in survival at 60 months compared to those treated with insulin (n = 216) or other oral antihyperglycemic drugs (n = 196). However, unfortunately, there was no significant improvement in patency rates or limb salvage after endovascular reperfusion interventions [124]. A prospective study that enrolled 100 T2DM subjects with PAD after a 12-month follow-up showed the effectiveness of metformin in reducing adverse limb events, suggesting the usefulness of the drug in these patients [125]. Therefore, to determine whether metformin can play a role in symptomatic PAD, regardless of antidiabetic action, a prospective study, compared to placebo, is ongoing in non-diabetic subjects with intermittent claudication, called “Metformin BenefIts Lower Extremities with Intermittent Claudication” (MOBILE IC trial) [126]. The expected date for a first analysis of the results has been set for 2026 [108].
2.4. Metformin and Brain
Metformin can cross the blood–brain barrier and reach a significant concentration in the central nervous system (CNS), causing pleiotropic effects [127]. Although the data obtained are not always consistent, several studies have suggested the use of metformin in various diseases that affect SNC [128,129]. In male and female mice with spinal cord injuries, 200 mg/kg metformin administered subcutaneously for 14 days increased oligodendrogenesis in both sexes [130]. Additionally, it increased the activation of neural stem and progenitor cells in females and reduced the activation of microglia in males [130]. The neurogenesis and oligodendrogenesis induced by metformin were caused by its action on atypical protein kinase C (aPKC)-mediated phosphorylation of the CREB-binding protein (CBP) [131,132,133]. Other authors also suggested that metformin has anti-inflammatory effects in injured brains, reducing microglia activation via the NF-κB-MAPK signaling pathway [134]. An additional molecular pathway has been reported suggesting activation of Ser/Thr kinase partitioning defective 1 (Par1)/MARK, which is a downstream target of tumor suppressor kinase B1 (LKB1) [135].
Cerebral amyloid angiopathy (CAA) often leads to senile dementia and cerebral lobar hemorrhages and is commonly identified in subjects with Alzheimer’s disease (AD) [136]. Numerous epidemiological studies have demonstrated that individuals with T2DM have a significantly higher probability of developing AD [137,138]. In APP23-ob/ob mice, a model of CAA and T2DM, metformin (350 mg/kg daily) administered for about 14 months reduced the number of cerebral blood vessels with amyloid β deposits, increasing the insulin-degrading enzyme (insulysin) [136]. However, there are conflicting data on the development of the effect of metformin on the amyloidosis typical of AD. A protective effect due to a decrease in amyloid secretion, generally described as an AMPK-independent pathway, has been reported [139]. However, an increase in amyloid-β formation with higher doses via AMPK activation has also been described, which may exacerbate the pathogenesis of AD [140,141,142].
The population-based “Singapore Longitudinal Aging Study”, which evaluated 365 subjects with T2DM, aged 55 years and older, who were followed for at least 4 years and treated with metformin compared to placebo, showed a significant inverse association with cognitive impairment in cross-sectional and longitudinal analyses, reporting significant protection after 6 years of metformin therapy [143]. A crossover double-blind RCT was carried out on 24 survivors of pediatric brain tumors who received cranial radiation [144]. With the idea that metformin can increase NPCs and repair brain damage, the authors administered metformin (1000 mg/m2 orally, twice daily for 12 weeks), showing enhanced performance in declarative and working memory tests [144]. Given the small number of enrolled subjects, even if the results obtained are promising, they certainly need to be validated in a larger number of subjects through further RCTs. It should be noted that a recent meta-analysis of 19 RCTs that explored the risk of cognitive dysfunction, dementia, AD, or Parkinson’s disease (PD), considering a total of 285,966 subjects, revealed that exposure to metformin used for antidiabetic treatment did not reduce the incidence of degenerative CNS diseases (OR 1.04, 95% Cl 0.92 to 1.17) and, regrettably, significantly increased the frequency of Parkinson’s disease (OR 1.66, 95% CI 1.14 to 2.42) compared to non-metformin users [145].
Overall, clinical data on the effects of metformin on preventing and treating cognitive and, in general, neurodegenerative CNS diseases are still fragmentary and inconsistent. Consequently, no definitive conclusions can be drawn.
2.5. Metformin and Cancer
It is generally recognized that DM increases the risk of various types of cancer [146,147]. A recent prospective longitudinal cohort study with 428,568 newly diagnosed T2DM subjects, showed that the risk for all-cause cancer was approximately 10% higher in diabetic patients, and the relative risk was higher when T2DM was diagnosed at a younger age [148]. Recently, retrospective studies have shown that T2DM subjects taking metformin display a lower risk of cancer than a healthy population or diabetic patients never treated with metformin [149,150,151]. Several RCTs assessed the impact of metformin on mortality in diabetic patients with colorectal cancer (CRC), showing that it can reduce all-cause mortality and cancer-specific mortality [152,153,154]. However, not all investigations are in agreement; in fact, some have not found a significant protective association between metformin and survival in patients with CRC [155,156].
A recent meta-analysis that considered 8 cohort studies from 2012–2019 showed that CRC subjects with T2DM treated with metformin had a lower overall mortality, even not specific-cause mortality, than CRC patients with T2DM who did not receive metformin [157]. Of interest, this study evidenced a lower mortality in women among CRC patients with T2DM using metformin than in those who did not receive it [157]. Previously, a significant reduction in sex-related incidence was revealed in women treated with low doses of metformin in a prospective cohort study from Taiwan [158]. A meta-analysis based on 37 studies for a total of more than 1.5 million subjects showed that metformin reduces the incidence of cancer in the liver (−78%), pancreas (−46%), colon (−23%), and breast (−6%), as well as mortality from liver and breast cancer [159]. The marked reduction in the incidence of certain types of cancer, such as those of the liver and pancreas, may be explained by the known fact that insulin resistance plays a pivotal role in their growth [160]. Of interest, a large meta-analysis of 121 cohorts, including more than 19 million individuals with about one million all-site cancer events, showed that T2DM is associated with an additional risk of all-site cancer of 6% higher in women than in men [161]. In more detail, DM caused a significantly higher relative risk in women than men for oral, stomach, and kidney cancers and leukemia, whereas the opposite was observed for liver cancer [161].
In general, several systematic reviews and meta-analyses gave comparable results, but with differences in the type of cancer treated, the time and dose of exposure to metformin, the presence of concomitant treatments, etc.; thus, there remains the need to study the real usefulness of metformin in cancer patients regardless of its use as an antidiabetic drug.
2.6. Metformin and COVID-19
Recent studies reported that patients with DM have a higher risk of death and a higher probability of being hospitalized with Severe Acute Respiratory Syndrome CoronaVirus-2 (SARS-CoV-2) than those without diabetes [162,163,164]. A recent systematic review revealed that diabetic patients experienced worse clinical outcomes during the COVID-19 pandemic, with an increase in all-cause mortality and diabetes-related mortality; in particular, clinical outcomes worsened in women, children, and individuals of ethnic minorities [165].
Metformin has given promising results in diabetic subjects with Coronavirus Disease 2019 (COVID-19) because, in addition to its hypoglycemic action, it also has antiviral, anti-inflammatory, and immunomodulatory properties [166,167]. Epidemiological data collected from T2DM subjects treated with metformin for glycemic control during the course of COVID-19 revealed a significant reduction in the mortality rate related to the viral disease by a factor of ~3 times [168]. A meta-analysis of 10,233 subjects confirmed the association between metformin use and mortality reduction after adjustment for various comorbidities such as obesity, hypertension, cardiovascular disease, and kidney disease [169]. Similarly, an observational cohort study with data from T2DM individuals reported a significantly lower risk of COVID-19-related mortality in patients treated with metformin [170]. Another study suggested the existence of differences between female and male subjects with respect to the outcomes of severe SARS-CoV-2 syndrome in T2DM subjects treated with metformin [171]. Indeed, retrospectively analyzing the cohort of diabetic or obese women (BMI ≥ 30 kg/m2) infected with the virus showed a reduction in the severity of the disease and in the mortality rate. The advantage was not proven in the male cohort [171]. Furthermore, a second retrospective cohort study described that metformin use decreased the onset of acute respiratory distress syndrome in women with DMT2 and COVID-19 [172].
One aspect of metformin therapy that has raised concern, especially in the case of severe SARS-CoV-2 infection, is the risk of metabolic acidosis, since there is a general consensus that this drug has a higher risk of lactic acidosis compared to other antidiabetic agents [173]. However, despite this alarm, no increase in mortality from COVID-19 has been observed among metformin-treated individuals [174]. Otherwise, the authors discovered a significant association between metformin use and decreased heart failure and inflammation damage. In particular, metabolic acidosis was observed in patients taking high doses of the drug, with an impairment of renal function and a worsening of the clinical progression of COVID-19 [174]. Although it is one of the most commonly prescribed antidiabetic drugs due to its effectiveness in DM, even for those infected with the SARS-CoV-2 virus, ongoing monitoring is recommended for patients with impaired renal function to accurately determine appropriate therapeutic dosages [175,176]. The discontinuation of metformin is recommended if the eGFR drops below 30 mL/min/1.73 m2, as indicated in patients not affected by COVID-19. Otherwise, the patient would be exposed to excessively high blood concentrations that can cause metabolic acidosis. For this, more attention should be paid to individuals affected by severe COVID-19, among whom a reduced renal function is noted in the various acute sequelae [177,178].
2.7. Metformin and Weight Control
Overweight and obesity in diabetic subjects increase the risk of many cardiovascular diseases and even cancer [179,180]. Obese individuals are believed to have a compromised intestinal barrier, leading to impaired intestinal permeability [181]. Reduced body weight has been found to improve intestinal conditions by restoring the selectivity of the intestinal barrier [182,183]. A moderate low-calorie diet together with increased physical activity after 2 and 12 months improved enteral permeability by decreasing chemerin and lipopolysaccharide-binding protein (LBP) levels in adolescents with abdominal obesity [184]. Assuming that body weight reduction can produce similar results regardless of the method used, metformin has been studied in a group of overweight or obese subjects in the “Survivorship Promotion In Reducing IGF-1 Trial” (SPIRIT) [185]. Contrary to expectations and unlike the diet intervention, 6 and 12 months after the drug treatment, metformin did not decrease the level of blood LBP despite achieving a similar degree of weight loss (3%) [185].
Both metformin and gliflozins have been shown to reduce hyperglycemia and HbA1c and are being approved for the treatment of T2DM [186,187]. Gliflozins act as SGLT2 inhibitors by causing glucose urinary excretion in the proximal tubules, decreasing hyperglycemia and body weight [188,189,190,191]. A sub-analysis of a prospective, multicenter, open-label RCT has considered 29 elderly T2DM subjects, receiving sitagliptin as the base antidiabetic treatment, who were treated with either ipragliflozin (50 mg daily) or metformin (500 mg daily), showing that after 24 weeks, the visceral adiposity index decreased with each one [192]. Furthermore, the recent post-hoc analysis compared the metabolomic changes associated with 50 mg ipragliflozin versus 1000 mg metformin daily for 24 weeks in 30 diabetic subjects receiving sitagliptin as basal treatment, showing that the reduction in the visceral fat area was higher with ipragliflozin (−19.8%) than with metformin (−2.5%, p = 0.002), as well as for waist circumference and body weight [193]. In general, the metabolomic profile of several biomarkers was different between the metformin and ipragliflozin groups. Briefly, with metformin, a moderate reduction in LDL was highlighted, and the levels of citrulline, octanoic acid, indole-3-acetaldehyde, and hexanoic acid were also reduced [193]. Whereas, hypotaurine, methionine, methyl-2-oxovaleric acid, 3-nitrotyrosine, and cyclohexylamine levels increased [193]. Furthermore, ipragliflozin treatment showed a significant increase in hematocrit and a greater increase in severe liver steatosis compared to metformin treatment [193].
A new possibility of metformin use is for weight control in patients treated with antipsychotics, which generally cause an unwanted increase in weight with higher cardiovascular risk. A recent review that considered 5 clinical trials in 227 participants suggests that metformin may be effective in preventing weight gain caused by antipsychotic drugs [194]. However, there were no adequate clinical trials, and the number of patients enrolled was relatively low. Therefore, additional trials are necessary to validate this use.
2.8. Metformin and Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is among the most common reproductive endocrine disorders, affecting approximately 4 to 20% of women in their reproductive years [195,196,197,198]. The “International Evidence-based Guidelines for the Assessment and Management” of PCOS recommended that adult women should be treated with combined oral contraceptive pills (COCP) using low-dose preparations, which is an off-label pill use, in the presence of hyperandrogenism and/or irregular menstrual cycles [199]. Metformin is recommended to treat metabolic disorders that are common in PCSO, either as a standalone treatment or as an additional therapy [199,200]. This endorsement is based on the fact that nearly half of women with PCOS are overweight or obese and may have impaired glucose tolerance, hyperinsulinemia, or overt T2DM [201,202]. It is important to note that metformin aids in regulating menstrual cycles and improving fertility [196,199].
Recently, an open-label prospective RCT compared a combination of metformin (1000 mg twice daily) and canagliflozin (100 mg daily), an SGLT2 inhibitor, with metformin alone for a 3-month treatment in 51 overweight or obese non-diabetic PCOS women aged 18–40 years [203]. Metformin alone showed similar effects to combination therapy on menstrual frequency, weight control, hyperandrogenemia, and insulin resistance, although a greater reduction in testosterone levels and total area under curves of glucose and insulin was observed with combination therapy [203]. Another RCT involving 52 overweight participants compared the efficacy of metformin alone (1000 mg twice daily) with a combination of metformin and liraglutide (metformin taken twice daily, orally, along with liraglutide 1.2 mg once daily, subcutaneously). The results indicated comparable improvements in menstrual cycles, anthropometric measures, and glucose metabolism after the 12-week treatment period [204]. However, the combination of metformin and liraglutide exhibited superior efficacy in improving reproductive abnormalities and hyperandrogenemia, possibly modulating the hypothalamic–pituitary–ovarian axis [204].
Recently, Greff et al. conducted a systematic review of selected 26 RCTs, including 1691 women with PCOS, comparing the efficacy and safety of inositol compared to metformin or placebo treatments [205]. The authors demonstrated that inositol exhibited efficacy comparable to metformin in terms of weight loss, cycle normalization, and testosterone levels. In addition, improvements in hyperinsulinemia and carbohydrate metabolism were observed compared to the placebo group, suggesting that inositol may not be inferior to metformin treatment regarding glucose control [205]. A meta-analysis of 9 RCTs with a total of 612 subjects showed that the administration of myo-inositol (2000 to 4000 mg daily) compared to metformin (1500 to 2550 mg daily) could be more effective in lowering triglycerides and cause fewer side effects of metformin [206].
A very recent meta-analysis of 46 RCTs has been performed as a 2023 update of the “International Evidence-based Guidelines for the Assessment and Management of PCOS”, highlighting that metformin is superior to hormonal treatment in controlling the blood sugar, overweight, and lipid profile of PCSO subjects [207,208]. Therefore, metformin is considered a second-line treatment in PCOS that offers benefits for the management of menstrual and ovulatory disorders, hirsutism, and metabolic and cardiovascular disorders [209,210].
An RCT of 65 women with PCOS treated with COCP (150 mg desogestrel + 30 μg ethinylestradiol) alone, metformin (2000 mg daily) plus COCP, or metformin alone for 12 months showed significant differences in miRNA levels between the metformin-treated groups and those receiving COCP alone [211]. Among 22 selected miRNAs known to be related to PCOS, lipid, and glucose disorders, miR-122, mi-R29a, and miR-223 decreased significantly, suggesting their role in metformin protection in PCOS subjects [211].
However, not all the data reported in the literature are consistent. A systematic review that included 44 RCTs (2253 women), with 39 studies in adult women (2047 subjects) and 5 in adolescent women (206 subjects), found no significant evidence that metformin treatment alone is more effective than COCP, or vice versa, or than combined COCP and metformin administration [212]. New clinical studies stratified by age and symptoms must be performed to validate the therapeutic use of metformin in women with PCOS.
2.9. Metformin and Thyroid Disorders
Endocrine autoimmune diseases account for most thyroid diseases (TDs), which have a high prevalence in the global population (5%), occurring at least 5–8 times more frequently in females than males [213,214,215,216]. TDs are distinguished by either an increase in thyroid activity, that is, Graves’ disease, or a decrease in activity, that is, Hashimoto’s thyroiditis. Autoimmune thyroid diseases can lead to altered glucose metabolism, thereby heightening the risk of developing DM [217,218].
To investigate the influence of metformin on thyroid function, a clinical study has recruited 19 healthy male volunteers, showing that metformin does not alter iodine uptake in the thyroid gland [219]. However, various clinical studies performed in hypothyroid subjects have shown that metformin reduces thyrotropin (TSH) levels even if the mechanism involved is not fully understood [220,221]. A prospective study considered 100 euthyroid subjects (68 females and 32 males) with insulin resistance treated with metformin (1700 mg daily) for six months, resulting in a significant decrease in body weight, insulin resistance, and TSH level and, in addition, a reduction in thyroid volume and the size of thyroid nodules [222]. The authors suggested that hyperinsulinemia per se may promote an increase in thyroid size and the presence of nodules that may improve through the use of metformin, which decreases hyperinsulinemia. In fact, insulin, through IGF-1 signaling, is known to modulate thyroid gene expression, as an additional factor in thyrocyte proliferation and the differentiation of thyrocytes. In addition, metformin can cause antiproliferative effects by suppressing the activity of the mammalian target of rapamycin (mTOR) [223,224].
A systematic review and meta-analysis that considered about 150 subjects showed that metformin moderately reduces the size of thyroid nodules and decreases the level of TSH and insulin resistance, even if it does not change the size of the thyroid gland [225]. These results are in agreement with those obtained in a previous meta-analysis that enrolled 240 patients with benign thyroid nodules and insulin resistance [226].
2.10. Adverse Drug Reactions: The Benefit/Risk Ratio
Subjects taking metformin, especially in the first one–two weeks of intake, frequently experience nausea, vomiting, and abdominal discomfort, with a prevalence of 8 to 21% [206]. Several investigations suggested that the gastrointestinal (GI) side effects are mitigated by starting therapy with low doses and taking the drug during meals [227]. Moreover, the administration of controlled and gradual release formulations may be useful in reducing GI discomforts [228,229]. To explain these gut effects, metformin has been suggested to cause 5-hydroxytryptamine release at the intestinal level, then act by a mechanism independent of 5-HT3 receptors [230]. In fact, several authors, through in vitro and in vivo investigations, showed that GI disorders may depend on the genotype of SERT and OCT1 transporters [231,232]. Furthermore, metformin can also increase intestinal release of GLP1, which is known to slow gastrointestinal transit and reduce appetite (anorectic effect) [54].
Indeed, various clinical studies have shown that about 34% of subjects who begin metformin treatment undergo at least one ADR during a 1-year follow-up period [107,233]. It was also observed that the proportion of undesirable effects was higher among women than among men [233,234]. In particular, the longitudinal study “Lareb Intensive Monitoring (LIM) program” of the Dutch National Pharmacovigilance Center Lareb has included 1712 diabetic subjects with an average age of 58 years, of which 40.9% were women, who were followed for 12 months by an online questionnaire [233]. The study showed that women reported ADRs more frequently in the first period of metformin treatment (at 2 and 6 weeks of starting therapy) than men, while in the following period the recurrence of the reported adverse effects was the same between the two sexes [233]. These studies highlighted the usefulness of carefully evaluating the beginning dose of metformin, as the dose required for antidiabetic treatment in women is generally lower than in men. Women who receive a lower initial dose than men could reduce the appearance of ADRs by improving their adherence to T2DM treatment.
Other side effects commonly reported are headache, dizziness, fatigue, pruritus, and dysgeusia [233,234]. One of the most feared adverse reactions to metformin treatment is lactic acidosis, which is a rare event with an incidence of 1 in 30,000 patients but can lead to fatal outcomes [91,235]. Generally, the observed cases occurred in patients who received high doses and/or had severe hepatic and renal impairment, old age, and alcoholism [236,237]. In particular, the risk of ADRs increases in patients receiving polytherapy because of drug–drug interactions. Various reviews are available in the literature regarding this topic [238,239,240,241,242].
Prolonged use of metformin is linked to the reduced vitamin B12 and elevated levels of homocysteine and methylmalonic acid, which can lead to anemia and diabetic peripheral neuropathy [243,244,245]. Patients with DM2 were studied in an RCT to examine the effects of prolonged use of metformin, observing that vitamin B12 levels were significantly lower in the metformin group (231 vs. 486 pmol/L; p < 0.001), with a frank deficiency in 18 patients (31%) compared to 2 subjects (3%) of the untreated group [246]. It should be noted that the metformin dose is inversely correlated with the amount of serum vitamin and clinically more severe peripheral neuropathy [246]. These data highlight the importance of monitoring the level of vitamin B12 in patients undergoing prolonged metformin therapy and also in subjects who, due to dietary choices, such as vegans, have an additional risk of vitamin B12 deficiency [247]. Recently, a dose-dependent correlation between long-term metformin use and peripheral neuropathy has been confirmed in a clinical trial in Chinese subjects with T2DM [248]. In addition, vitamin B12 deficiency has also been associated with the worsening of various other CNS diseases and, in particular, of PD, explaining the potential correlation between metformin treatment and the worsening of Parkinson’s disease [145,249].
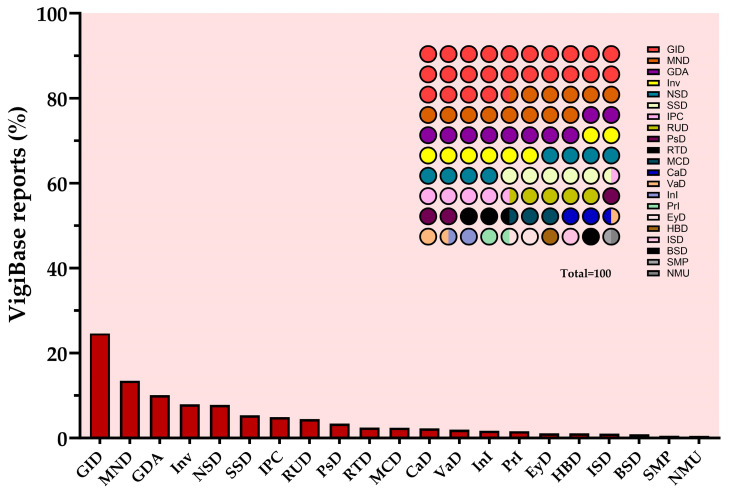
It can be expected that the widespread use of metformin in therapy will be accompanied by increased reports of side effects worldwide. To verify this point, the current search using the VigiBase resource shows a total of 120,083 reports of ADRs since the 1990s until 10 January 2024 [250]. The majority of spontaneous side effects occurred in the Americas (41%), Asia (31%), and Europe (24%) and were mainly distributed in patients aged 45–64 years (35%) of female sex (56%) [250]. Figure 3 shows the side effects found in the database in decreasing order of frequency; among the most recurrent ADRs, those in the GI (24%), in agreement with the data from clinical trials. Some cases of lactic acidosis and hypoglycemia (metabolism and nutrition disorders), drug ineffectiveness, and fatigue (general disorders) were also attested (Figure 3). The ADR ratio (ADRs in women/ADRs in men) obtained from records in VigiBase is 1.43, suggesting a higher risk of ADRs in women than in men. In agreement, several studies have shown that women are more likely to experience adverse reactions caused by hypoglycemic drugs, including metformin [233,234,251]. At least in part, the failure to adjust the dose of metformin administered to women may explain the increased appearance of side effects compared to men.
Figure 3.
VigiBase reports of ADRs potentially caused by metformin in real-word therapy. GID = gastrointestinal disorders; MND = metabolism and nutrition disorders; GDA = general disorders and administration side conditions; Inv = investigations; NSD = nervous system disorder; SSD = skin and subcutaneous tissue disorders; IPC = injury, poisoning, and procedural complications; RUD = renal and urinary disorders; PsD = psychiatric disorders; RTD = respiratory, thoracic, and mediastinal disorders; MCD = musculoskeletal and connective tissue disorders; CaD = cardiac disorders; VaD = vascular disorders; InI = infections and infestations; PrI = product issues; EyD = eye disorders; HBD = hepatobiliary disorders; ISD = immune system disorders; BSD = blood and lymphatic system disorders; SMP = surgical and medical procedures; NMU = neoplasms benign, malignant, and unspecified. Created with GraphPad Prism 10.1.2 software.
ADR research has also been implemented in EudraVigilance, the European database of suspected ADR reports, to more closely identify reports in Europe and then make a comparison between the two resources. In EudraVigilance, by a search up to 27 January 2024, a total of 42,557 cases were found, of which 16,436 were in subjects aged 18–64 years, and 16,248 in older subjects aged 65–85 years. Among the reports, those related to women were the majority, equal to 21,635 (50.8%). Most cases were reported in France (25%), followed by Italy (16%), and then Germany (14%). Unlike VigiBase data, the most frequently reported ADR group was “Metabolic and nutritional disorders” (Figure S2). Considering the smaller size of the European database, the information from both databases is generally comparable.
Very few clinical studies have reported sex-related differences in efficacy and safety in the therapeutic use of metformin. The main differences identified in this review are summarized in Table 1.
Table 1.
Sex-related differences in diabetes mellitus complications and therapeutic use of metformin.
| Female versus Male | References | |
|---|---|---|
| Diabetes mellitus | Higher all-cause mortality | [122,123] |
| Higher risk PAD | [115,116,117] | |
| Higher RR for oral, stomach, and kidney cancers, and leukemia | [160] | |
| Met: antihyperglycemic effect | No difference | [31] |
| Met: pharmacokinetics | No difference, considering weight difference and monitoring eGFR. | [76] |
| Met: ADRs | Higher | [23,24] |
| Met: CRC-specific mortality * | Lower | [156] |
| Met: CRC incidence rate * | Lower | [157] |
| Met: COVID-19 * | Lower severity and mortality | [170,171] |
* = Diabetic and/or obese subjects; ADRs = adverse drug reactions; CRC = colorectal cancer; eGFR = estimated glomerular filtration rate; Met = metformin treatment; PAD = peripheral arterial disease; RR = relative risk.
3. Materials and Methods
In this review, publications identified using the PubMed database were considered by searching up to the end of February 2024. The search was restricted to items published in English in the last 10 years by using the keywords “metformin” and “gender differences” (Figure S1). Additionally, five inclusion criteria were added: (1) full text, (2) clinical trial, (3) meta-analysis, (4) randomized controlled trial, and (4) systematic review, selecting in this way 30 items. Studies related to the use of metformin in combination with other antidiabetic agents have generally been excluded. Instead, several other manuscripts were reviewed according to their relevance to the selected topic through a search in PubMed, Google Scholar, and ResearchGate to find additional relevant items. The benefit–risk profile was studied by analyzing the side effects reported in VigiBase, the WHO database, and EudraVigilance, the European database of ADRs [23,24]. The ratio of cases in women versus men was calculated; a deviated value of more than 20% from 1.0 in both directions (0.8–1.2) indicates the potential impact of sex on the frequency of ADRs [252,253].
4. Conclusions
RCT and post-marketing records indicate that metformin is effective and safe in treating hyperglycemia, particularly in type 2 diabetes mellitus. In addition, several clinical studies suggest its use in many other conditions, including polycystic ovary syndrome, weight control in obesity, and as an add-on therapy for various types of cancer. However, there is less evidence for its use in thyroid disorders and neurodegenerative diseases. Despite metformin being on the market for many decades, the interest in validating new clinical uses has not waned, which is surprising. Currently, there are numerous RCTs being developed to verify new applications of metformin. Therefore, new progress can be expected in its clinical use.
The administration of low doses in conjunction with meals can reduce the extent of side effects at the gastrointestinal level, which are generally mitigated gradually in the first two weeks of intake. In order to improve patient compliance and reduce metformin ADRs, it is advised to start with lower doses in all subjects, particularly women, and then gradually increase them to achieve glycemic control. Moreover, monitoring kidney function, particularly in older patients, is recommended.
Existing data on metformin use in real life have not shown significant differences in the antihyperglycemic response between women and men, while adverse drug reactions in women are reported more frequently. A greater care in the choice of posology might decrease the occurrence of side effects in both sexes. It is surprising that women have shown advantages in using metformin to reduce the incidence and mortality of colorectal cancer and improve recovery in COVID-19 compared to men. Additionally, metformin is a drug that has an explicit use, although off-label, in the treatment of polycystic ovary syndrome in women, even at a young age.
In summary, metformin continues to be a drug with satisfactory risk-benefit and cost-benefit ratios, which, by reducing blood glucose, decreases the damage caused by hyperglycemia and is useful in many other diseases related to metabolic disorders.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17040478/s1, Figure S1: Flowchart of articles obtained by the PubMed search for metformin up to February 2024; Figure S2: Reports of spontaneous ADRs on metformin obtained by consulting EudraVigilance.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are reported in the text and in the Supplementary Materials.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bailey C.J., Day C. Metformin: Its Botanical Background. Pract. Diabetes Int. 2004;21:115–117. doi: 10.1002/pdi.606. [DOI] [Google Scholar]
- 2.Bailey C.J. Metformin: Historical Overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 3.Werne E.A., Bell J. The Preparation of Methylguanidine, and of Ββ-Dimethylguanidine by the Interaction of Dicyanodiamide, and Methylammonium and Dimethylammonium Chlorides Respectively. J. Chem. Soc. Trans. 1922;121:1790–1794. doi: 10.1039/CT9222101790. [DOI] [Google Scholar]
- 4.Curd F.H.S., Davey D.G., Rose F.L. Studies on Synthetic Antimalarial Drugs; Some Biguanide Derivatives as New Types of Antimalarial Substances with Both Therapeutic and Causal Prophylactic Activity. Ann. Trop. Med. Parasitol. 1945;39:208–216. doi: 10.1080/00034983.1945.11685237. [DOI] [PubMed] [Google Scholar]
- 5.Garcia E.Y. Flumamine, a New Synthetic Analgesic and Anti-Flu Drug. J. Philipp. Med. Assoc. 1950;26:287–293. [PubMed] [Google Scholar]
- 6.Bailey C.J. Biguanides and NIDDM. Diabetes Care. 1992;15:755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 7.Khan F.N., Hirsch I.B. Diabetes and the WHO Model List of Essential Medicines. Lancet Diabetes Endocrinol. 2022;10:16–17. doi: 10.1016/S2213-8587(21)00318-1. [DOI] [PubMed] [Google Scholar]
- 8.WHO . WHO—Model List of Essential Medicines. World Health Organization; Geneva, Switzerland: 2023. [(accessed on 18 February 2024)]. Essential Medicines, Health Product Policy and Standards, Medicines Selection, IP and Affordability; p. 47. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02. [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J.A., Simpson S.H., Toth E.L., Majumdar S.R. Reduced Cardiovascular Morbidity and Mortality Associated with Metformin Use in Subjects with Type 2 Diabetes. Diabet. Med. 2005;22:497–502. doi: 10.1111/j.1464-5491.2005.01448.x. [DOI] [PubMed] [Google Scholar]
- 11.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 12.Ong C.R., Molyneaux L.M., Constantino M.I., Twigg S.M., Yue D.K. Long-Term Efficacy of Metformin Therapy in Nonobese Individuals with Type 2 Diabetes. Diabetes Care. 2006;29:2361–2364. doi: 10.2337/dc06-0827. [DOI] [PubMed] [Google Scholar]
- 13.Hirst J.A., Farmer A.J., Ali R., Roberts N.W., Stevens R.J. Quantifying the Effect of Metformin Treatment and Dose on Glycemic Control. Diabetes Care. 2012;35:446–454. doi: 10.2337/dc11-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox T., De Block C., Schwartzbard A.Z., Newman J.D. Diabetic Agents, From Metformin to SGLT2 Inhibitors and GLP1 Receptor Agonists. J. Am. Coll. Cardiol. 2020;75:1956–1974. doi: 10.1016/j.jacc.2020.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker C., Retzik-Stahr C., Singh V., Plomondon R., Anderson V., Rasouli N. Should Metformin Remain the First-Line Therapy for Treatment of Type 2 Diabetes? Ther. Adv. Endocrinol. 2021;12:204201882098022. doi: 10.1177/2042018820980225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., Collins B.S., Hilliard M.E., Isaacs D., Johnson E.L., et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46:S140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hur K.Y., Moon M.K., Park J.S., Kim S.-K., Lee S.-H., Yun J.-S., Baek J.H., Noh J., Lee B.-W., Oh T.J., et al. 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes Metab. J. 2021;45:461–481. doi: 10.4093/dmj.2021.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marx N., Federici M., Schütt K., Müller-Wieland D., Ajjan R.A., Antunes M.J., Christodorescu R.M., Crawford C., Di Angelantonio E., Eliasson B., et al. 2023 ESC Guidelines for the Management of Cardiovascular Disease in Patients with Diabetes. Eur. Heart J. 2023;44:4043–4140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Professional Practice Committee. ElSayed N.A., Aleppo G., Bannuru R.R., Bruemmer D., Collins B.S., Ekhlaspour L., Gaglia J.L., Hilliard M.E., Johnson E.L., et al. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47:S158–S178. doi: 10.2337/dc24-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Professional Practice Committee. ElSayed N.A., Aleppo G., Bannuru R.R., Bruemmer D., Collins B.S., Das S.R., Ekhlaspour L., Hilliard M.E., Johnson E.L., et al. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47:S179–S218. doi: 10.2337/dc24-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sam S., Ehrmann D.A. Metformin Therapy for the Reproductive and Metabolic Consequences of Polycystic Ovary Syndrome. Diabetologia. 2017;60:1656–1661. doi: 10.1007/s00125-017-4306-3. [DOI] [PubMed] [Google Scholar]
- 22.Heidari B., Lerman A., Lalia A.Z., Lerman L.O., Chang A.Y. Effect of Metformin on Microvascular Endothelial Function in Polycystic Ovary Syndrome. Mayo Clin. Proc. 2019;94:2455–2466. doi: 10.1016/j.mayocp.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . VigiBase: WHO’s Global Database Signalling Harm and Pointing to Safer Use. WHO; Geneva, Switzerland: 2024. [Google Scholar]
- 24.EudraVigilance . European Database of Suspected Adverse Drug Reaction Reports. EudraVigilance; Amsterdam, The Netherlands: 2024. [Google Scholar]
- 25.Rena G., Hardie D.G., Pearson E.R. The Mechanisms of Action of Metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hundal R.S., Inzucchi S.E. Metformin: New Understandings, New Uses. Drugs. 2003;63:1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 27.LaMoia T.E., Shulman G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiernsperger N.F., Bailey C.J. The Antihyperglycaemic Effect of Metformin: Therapeutic and Cellular Mechanisms. Drugs. 1999;58:31–39. doi: 10.2165/00003495-199958001-00009. [DOI] [PubMed] [Google Scholar]
- 29.Han Y., Xie H., Liu Y., Gao P., Yang X., Shen Z. Effect of Metformin on All-Cause and Cardiovascular Mortality in Patients with Coronary Artery Diseases: A Systematic Review and an Updated Meta-Analysis. Cardiovasc. Diabetol. 2019;18:96. doi: 10.1186/s12933-019-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q., Wang G., Fang D., Gao X., Liang Y., Wang L., Wu J., Zeng M., Luo M. The Role of MicroRNA Networks in Tissue-Specific Direct and Indirect Effects of Metformin and Its Application. Biomed. Pharmacother. 2022;151:113130. doi: 10.1016/j.biopha.2022.113130. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Li J., Shan Z., Yang W., Liu J., Tian H., Zhou Z., Ji Q., Weng J., Jia W., et al. Gender-Differential Effects on Blood Glucose Levels between Acarbose and Metformin in Chinese Patients with Newly Diagnosed Type 2 Diabetes: A Sub-Analysis of the MARCH Trial. Endocr. J. 2021;68:69–79. doi: 10.1507/endocrj.EJ20-0006. [DOI] [PubMed] [Google Scholar]
- 32.Harada N. Effects of Metformin on Blood Glucose Levels and Bodyweight Mediated through Intestinal Effects. J. Diabetes Investig. 2020;11:1420–1421. doi: 10.1111/jdi.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorrenti V., Benedetti F., Buriani A., Fortinguerra S., Caudullo G., Davinelli S., Zella D., Scapagnini G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals. 2022;15:912. doi: 10.3390/ph15080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foretz M., Guigas B., Viollet B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023;19:460–476. doi: 10.1038/s41574-023-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo D., Liu S., Ma H., Tian H., Yu H., Zhang X., Tong N., Liao J., Ren Y. Effects of Acarbose and Metformin on the Inflammatory State in Newly Diagnosed Type 2 Diabetes Patients: A One-Year Randomized Clinical Study. Drug Des. Devel. Ther. 2019;13:2769–2776. doi: 10.2147/DDDT.S208327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L., Patnana P.K., Nimmagadda S.C. Low-Dose Metformin and PEN2-Dependent Lysosomal AMPK Activation: Benefits Outnumber Side Effects. Signal Transduct. Target. Ther. 2022;7:178. doi: 10.1038/s41392-022-01040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma T., Tian X., Zhang B., Li M., Wang Y., Yang C., Wu J., Wei X., Qu Q., Yu Y., et al. Low-Dose Metformin Targets the Lysosomal AMPK Pathway through PEN2. Nature. 2022;603:159–165. doi: 10.1038/s41586-022-04431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng J., Wang X., Ye X., Ares I., Lopez-Torres B., Martínez M., Martínez-Larrañaga M.-R., Wang X., Anadón A., Martínez M.-A. Mitochondria as an Important Target of Metformin: The Mechanism of Action, Toxic and Side Effects, and New Therapeutic Applications. Pharmacol. Res. 2022;177:106114. doi: 10.1016/j.phrs.2022.106114. [DOI] [PubMed] [Google Scholar]
- 39.Agius L., Ford B.E., Chachra S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020;21:3240. doi: 10.3390/ijms21093240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazmirczak F., Hartweck L.M., Vogel N.T., Mendelson J.B., Park A.K., Raveendran R.M., O-Uchi J., Jhun B.S., Prisco S.Z., Prins K.W. Intermittent Fasting Activates AMP-Kinase to Restructure Right Ventricular Lipid Metabolism and Microtubules. JACC Basic Transl. Sci. 2023;8:239–254. doi: 10.1016/j.jacbts.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weir H.J., Yao P., Huynh F.K., Escoubas C.C., Goncalves R.L., Burkewitz K., Laboy R., Hirschey M.D., Mair W.B. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab. 2017;26:884–896.e5. doi: 10.1016/j.cmet.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontana L., Partridge L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebokova E., Klimes I., Moss R., Mitkova A., Wiersma M., Bohov P. Decreased Glucose Transporter Protein (GLUT4) in Skeletal Muscle of Hypertriglyceridaemic Insulin-Resistant Rat. Physiol. Res. 1995;44:87–92. [PubMed] [Google Scholar]
- 45.Li J., Houseknecht K.L., Stenbit A.E., Katz E.B., Charron M.J. Reduced Glucose Uptake Precedes Insulin Signaling Defects in Adipocytes from Heterozygous GLUT4 Knockout Mice. FASEB J. 2000;14:1117–1125. doi: 10.1096/fasebj.14.9.1117. [DOI] [PubMed] [Google Scholar]
- 46.Abel E.D., Peroni O., Kim J.K., Kim Y.-B., Boss O., Hadro E., Minnemann T., Shulman G.I., Kahn B.B. Adipose-Selective Targeting of the GLUT4 Gene Impairs Insulin Action in Muscle and Liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 47.Leguisamo N.M., Lehnen A.M., Machado U.F., Okamoto M.M., Markoski M.M., Pinto G.H., Schaan B.D. GLUT4 Content Decreases along with Insulin Resistance and High Levels of Inflammatory Markers in Rats with Metabolic Syndrome. Cardiovasc. Diabetol. 2012;11:100. doi: 10.1186/1475-2840-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atkinson B.J., Griesel B.A., King C.D., Josey M.A., Olson A.L. Moderate GLUT4 Overexpression Improves Insulin Sensitivity and Fasting Triglyceridemia in High-Fat Diet–Fed Transgenic Mice. Diabetes. 2013;62:2249–2258. doi: 10.2337/db12-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garvey W.T., Maianu L., Huecksteadt T.P., Birnbaum M.J., Molina J.M., Ciaraldi T.P. Pretranslational Suppression of a Glucose Transporter Protein Causes Insulin Resistance in Adipocytes from Patients with Non-Insulin-Dependent Diabetes Mellitus and Obesity. J. Clin. Investig. 1991;87:1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doehner W., Gathercole D., Cicoira M., Krack A., Coats A.J.S., Camici P.G., Anker S.D. Reduced Glucose Transporter GLUT4 in Skeletal Muscle Predicts Insulin Resistance in Non-Diabetic Chronic Heart Failure Patients Independently of Body Composition. Int. J. Cardiol. 2010;138:19–24. doi: 10.1016/j.ijcard.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Herman R., Kravos N.A., Jensterle M., Janež A., Dolžan V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022;23:1264. doi: 10.3390/ijms23031264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chadt A., Al-Hasani H. Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflug. Arch. Eur. J. Physiol. 2020;472:1273–1298. doi: 10.1007/s00424-020-02417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bahne E., Sun E.W.L., Young R.L., Hansen M., Sonne D.P., Hansen J.S., Rohde U., Liou A.P., Jackson M.L., De Fontgalland D., et al. Metformin-Induced Glucagon-like Peptide-1 Secretion Contributes to the Actions of Metformin in Type 2 Diabetes. JCI Insight. 2018;3:e93936. doi: 10.1172/jci.insight.93936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mannucci E., Ognibene A., Cremasco F., Bardini G., Mencucci A., Pierazzuoli E., Ciani S., Messeri G., Rotella C.M. Effect of Metformin on Glucagon-Like Peptide 1 (GLP-1) and Leptin Levels in Obese Nondiabetic Subjects. Diabetes Care. 2001;24:489–494. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- 55.Melson E., Ashraf U., Papamargaritis D., Davies M.J. What Is the Pipeline for Future Medications for Obesity? Int. J. Obes. 2024 doi: 10.1038/s41366-024-01473-y. [DOI] [PubMed] [Google Scholar]
- 56.Green B.D., Irwin N., Duffy N.A., Gault V.A., O’Harte F.P.M., Flatt P.R. Inhibition of Dipeptidyl Peptidase-IV Activity by Metformin Enhances the Antidiabetic Effects of Glucagon-like Peptide-1. Eur. J. Pharmacol. 2006;547:192–199. doi: 10.1016/j.ejphar.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 57.Wang K., Zhang Z., Hang J., Liu J., Guo F., Ding Y., Li M., Nie Q., Lin J., Zhuo Y., et al. Microbial-Host-Isozyme Analyses Reveal Microbial DPP4 as a Potential Antidiabetic Target. Science. 2023;381:eadd5787. doi: 10.1126/science.add5787. [DOI] [PubMed] [Google Scholar]
- 58.Bryrup T., Thomsen C.W., Kern T., Allin K.H., Brandslund I., Jørgensen N.R., Vestergaard H., Hansen T., Hansen T.H., Pedersen O., et al. Metformin-Induced Changes of the Gut Microbiota in Healthy Young Men: Results of a Non-Blinded, One-Armed Intervention Study. Diabetologia. 2019;62:1024–1035. doi: 10.1007/s00125-019-4848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee Y., Kim A.H., Kim E., Lee S., Yu K.-S., Jang I.-J., Chung J.-Y., Cho J.-Y. Changes in the Gut Microbiome Influence the Hypoglycemic Effect of Metformin through the Altered Metabolism of Branched-Chain and Nonessential Amino Acids. Diabetes Res. Clin. Pract. 2021;178:108985. doi: 10.1016/j.diabres.2021.108985. [DOI] [PubMed] [Google Scholar]
- 60.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., Ståhlman M., Olsson L.M., Serino M., Planas-Fèlix M., et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 61.Napolitano A., Miller S., Nicholls A.W., Baker D., Van Horn S., Thomas E., Rajpal D., Spivak A., Brown J.R., Nunez D.J. Novel Gut-Based Pharmacology of Metformin in Patients with Type 2 Diabetes Mellitus. PLoS ONE. 2014;9:e100778. doi: 10.1371/journal.pone.0100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Krogh Pedersen H., et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Craciun C.-I., Neag M.-A., Catinean A., Mitre A.-O., Rusu A., Bala C., Roman G., Buzoianu A.-D., Muntean D.-M., Craciun A.-E. The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines. 2022;10:308. doi: 10.3390/biomedicines10020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishra A.K., Dingli D. Metformin Inhibits IL-6 Signaling by Decreasing IL-6R Expression on Multiple Myeloma Cells. Leukemia. 2019;33:2695–2709. doi: 10.1038/s41375-019-0470-4. [DOI] [PubMed] [Google Scholar]
- 65.Ismail A., El-Mahdy H.A., Eldeib M.G., Doghish A.S. miRNAs as Cornerstones in Diabetic Microvascular Complications. Mol. Genet. Metab. 2023;138:106978. doi: 10.1016/j.ymgme.2022.106978. [DOI] [PubMed] [Google Scholar]
- 66.Feng J., Xing W., Xie L. Regulatory Roles of MicroRNAs in Diabetes. Int. J. Mol. Sci. 2016;17:1729. doi: 10.3390/ijms17101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chien H.-Y., Lee T.-P., Chen C.-Y., Chiu Y.-H., Lin Y.-C., Lee L.-S., Li W.-C. Circulating microRNA as a Diagnostic Marker in Populations with Type 2 Diabetes Mellitus and Diabetic Complications. J. Chin. Med. Assoc. 2015;78:204–211. doi: 10.1016/j.jcma.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Pordzik J., Jakubik D., Jarosz-Popek J., Wicik Z., Eyileten C., De Rosa S., Indolfi C., Siller-Matula J.M., Czajka P., Postula M. Significance of Circulating microRNAs in Diabetes Mellitus Type 2 and Platelet Reactivity: Bioinformatic Analysis and Review. Cardiovasc. Diabetol. 2019;18:113. doi: 10.1186/s12933-019-0918-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demirsoy İ.H., Ertural D.Y., Balci Ş., Çınkır Ü., Sezer K., Tamer L., Aras N. Profiles of Circulating miRNAs Following Metformin Treatment in Patients with Type 2 Diabetes. J. Med. Biochem. 2018;37:499–506. doi: 10.2478/jomb-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noren Hooten N., Martin-Montalvo A., Dluzen D.F., Zhang Y., Bernier M., Zonderman A.B., Becker K.G., Gorospe M., Cabo R., Evans M.K. Metformin-mediated Increase in DICER1 Regulates microRNA Expression and Cellular Senescence. Aging Cell. 2016;15:572–581. doi: 10.1111/acel.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bridgeman S.C., Ellison G.C., Melton P.E., Newsholme P., Mamotte C.D.S. Epigenetic Effects of Metformin: From Molecular Mechanisms to Clinical Implications. Diabetes Obes. Metab. 2018;20:1553–1562. doi: 10.1111/dom.13262. [DOI] [PubMed] [Google Scholar]
- 72.Zheng H., Wan J., Shan Y., Song X., Jin J., Su Q., Chen S., Lu X., Yang J., Li Q., et al. MicroRNA-185-5p Inhibits Hepatic Gluconeogenesis and Reduces Fasting Blood Glucose Levels by Suppressing G6Pase. Theranostics. 2021;11:7829–7843. doi: 10.7150/thno.46882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grant P.J. The Effects of High- and Medium-Dose Metformin Therapy on Cardiovascular Risk Factors in Patients with Type II Diabetes. Diabetes Care. 1996;19:64–66. doi: 10.2337/diacare.19.1.64. [DOI] [PubMed] [Google Scholar]
- 74.Garber A.J., Duncan T.G., Goodman A.M., Mills D.J., Rohlf J.L. Efficacy of Metformin in Type II Diabetes: Results of a Double-Blind, Placebo-Controlled, Dose-Response Trial. Am. J. Med. 1997;103:491–497. doi: 10.1016/S0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 75.Nagai Y., Kazumori K., Takeshima T., Iwasaki K., Tanaka Y. Effects of Increasing Metformin Dose vs Adding/Switching to Dipeptidyl Peptidase-4 Inhibitors on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Ther. 2021;12:897–911. doi: 10.1007/s13300-021-01017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ilias I., Rizzo M., Zabuliene L. Metformin: Sex/Gender Differences in Its Uses and Effects—Narrative Review. Medicina. 2022;58:430. doi: 10.3390/medicina58030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., Zinman B. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarry-Adkins J.L., Grant I.D., Ozanne S.E., Reynolds R.M., Aiken C.E. Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021;12:1901–1914. doi: 10.1007/s13300-021-01058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali S., Fonseca V. Overview of Metformin: Special Focus on Metformin Extended Release. Expert Opin. Pharmacother. 2012;13:1797–1805. doi: 10.1517/14656566.2012.705829. [DOI] [PubMed] [Google Scholar]
- 80.Blonde L., Dailey G.E., Jabbour S.A., Reasner C.A., Mills D.J. Gastrointestinal Tolerability of Extended-Release Metformin Tablets Compared to Immediate-Release Metformin Tablets: Results of a Retrospective Cohort Study. Curr. Med. Res. Opin. 2004;20:565–572. doi: 10.1185/030079904125003278. [DOI] [PubMed] [Google Scholar]
- 81.Tan J., Wang Y., Liu S., Shi Q., Zhou X., Zhou Y., Yang X., Chen P., Li S. Long-Acting Metformin Vs. Metformin Immediate Release in Patients with Type 2 Diabetes: A Systematic Review. Front. Pharmacol. 2021;12:669814. doi: 10.3389/fphar.2021.669814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graham G.G., Punt J., Arora M., Day R.O., Doogue M.P., Duong J.K., Furlong T.J., Greenfield J.R., Greenup L.C., Kirkpatrick C.M., et al. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 83.Liang X., Giacomini K.M. Transporters Involved in Metformin Pharmacokinetics and Treatment Response. J. Pharm. Sci. 2017;106:2245–2250. doi: 10.1016/j.xphs.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 84.Scheen A.J. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 85.Tucker G., Casey C., Phillips P., Connor H., Ward J., Woods H. Metformin Kinetics in Healthy Subjects and in Patients with Diabetes Mellitus. Brit. J. Clin. Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 87.Christensen M.M.H., Brasch-Andersen C., Green H., Nielsen F., Damkier P., Beck-Nielsen H., Brosen K. The Pharmacogenetics of Metformin and Its Impact on Plasma Metformin Steady-State Levels and Glycosylated Hemoglobin A1c. Pharmacogenetics Genom. 2011;21:837–850. doi: 10.1097/FPC.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 88.Saiz-Rodríguez M., Ochoa D., Zubiaur P., Navares-Gómez M., Román M., Camargo-Mamani P., Luquero-Bueno S., Villapalos-García G., Alcaraz R., Mejía-Abril G., et al. Identification of Transporter Polymorphisms Influencing Metformin Pharmacokinetics in Healthy Volunteers. J. Pers. Med. 2023;13:489. doi: 10.3390/jpm13030489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szymczak-Pajor I., Wenclewska S., Śliwińska A. Metabolic Action of Metformin. Pharmaceuticals. 2022;15:810. doi: 10.3390/ph15070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng A., Gong C., Xu Y., Liang X., Chen X., Hong W., Yan J. Association between Organic Cation Transporter Genetic Polymorphisms and Metformin Response and Intolerance in T2DM Individuals: A Systematic Review and Meta-Analysis. Front. Public Health. 2023;11:1183879. doi: 10.3389/fpubh.2023.1183879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahmood R., Maccourtney D., Vashi M., Mohamed A. A Case of Metformin-Associated Lactic Acidosis. Cureus. 2023;15:e38222. doi: 10.7759/cureus.38222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L., Guan Z., Li R., Zhao W., Hao G., Yan Y., Xu Y., Liao L., Wang H., Gao L., et al. Population Pharmacokinetics and Dosing Optimization of Metformin in Chinese Patients with Type 2 Diabetes Mellitus. Medicine. 2020;99:e23212. doi: 10.1097/MD.0000000000023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lalau J.-D., Lacroix C. Measurement of Metformin Concentration in Erythrocytes:Clinical Implications. Diabetes Obes. Metab. 2003;5:93–98. doi: 10.1046/j.1463-1326.2003.00241.x. [DOI] [PubMed] [Google Scholar]
- 94.Jang K., Chung H., Yoon J., Moon S., Yoon S.H., Yu K., Kim K., Chung J. Pharmacokinetics, Safety, and Tolerability of Metformin in Healthy Elderly Subjects. J. Clin. Pharma. 2016;56:1104–1110. doi: 10.1002/jcph.699. [DOI] [PubMed] [Google Scholar]
- 95.Sun M.-L., Liu F., Yan P., Chen W., Wang X.-H. Effects of Food on Pharmacokinetics and Safety of Metformin Hydrochloride Tablets: A Meta-Analysis of Pharmacokinetic, Bioavailability, or Bioequivalence Studies. Heliyon. 2023;9:e17906. doi: 10.1016/j.heliyon.2023.e17906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lalau J.-D., Lemaire-Hurtel A.-S., Lacroix C. Establishment of a Database of Metformin Plasma Concentrations and Erythrocyte Levels in Normal and Emergency Situations. Clin. Drug Investig. 2011;31:435–438. doi: 10.2165/11588310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 97.Dutta S., Shah R.B., Singhal S., Dutta S.B., Bansal S., Sinha S., Haque M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug Des. Devel. Ther. 2023;17:1907–1932. doi: 10.2147/DDDT.S409373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sambol N.C., Brookes L.G., Chiang J., Goodman A.M., Lin E.T., Liu C.Y., Benet L.Z. Food Intake and Dosage Level, but Not Tablet vs Solution Dosage Form, Affect the Absorption of Metformin HC1 in Man. Brit. J. Clin. Pharmacol. 1996;42:510–512. doi: 10.1111/j.1365-2125.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 99.Sciacca L., Bianchi C., Burlina S., Formoso G., Manicardi E., Sculli M.A., Resi V. Position Paper of the Italian Association of Medical Diabetologists (AMD), Italian Society of Diabetology (SID), and the Italian Study Group of Diabetes in Pregnancy: Metformin Use in Pregnancy. Acta Diabetol. 2023;60:1421–1437. doi: 10.1007/s00592-023-02137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Orloff J., Min J.Y., Mushlin A., Flory J. Safety and Effectiveness of Metformin in Patients with Reduced Renal Function: A Systematic Review. Diabetes Obes. Metab. 2021;23:2035–2047. doi: 10.1111/dom.14440. [DOI] [PubMed] [Google Scholar]
- 101.Li Y., Liu Y., Liu S., Gao M., Wang W., Chen K., Huang L., Liu Y. Diabetic Vascular Diseases: Molecular Mechanisms and Therapeutic Strategies. Signal Transduct. Target. Ther. 2023;8:152. doi: 10.1038/s41392-023-01400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vadivelu R., Vijayvergiya R. Panvascular Risk Factor—Diabetes. Cor Vasa. 2018;60:e18–e29. doi: 10.1016/j.crvasa.2018.01.004. [DOI] [Google Scholar]
- 103.Cade W.T. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys. Ther. 2008;88:1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sandoval-Garcia E., McLachlan S., Price A.H., MacGillivray T.J., Strachan M.W.J., Wilson J.F., Price J.F. Retinal Arteriolar Tortuosity and Fractal Dimension Are Associated with Long-Term Cardiovascular Outcomes in People with Type 2 Diabetes. Diabetologia. 2021;64:2215–2227. doi: 10.1007/s00125-021-05499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chawla A., Chawla R., Jaggi S. Microvasular and Macrovascular Complications in Diabetes Mellitus: Distinct or Continuum? Indian J. Endocr. Metab. 2016;20:546. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dal Canto E., Ceriello A., Rydén L., Ferrini M., Hansen T.B., Schnell O., Standl E., Beulens J.W. Diabetes as a Cardiovascular Risk Factor: An Overview of Global Trends of Macro and Micro Vascular Complications. Eur. J. Prev. Cardiol. 2019;26:25–32. doi: 10.1177/2047487319878371. [DOI] [PubMed] [Google Scholar]
- 107.Drzewoski J., Hanefeld M. The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals. 2021;14:122. doi: 10.3390/ph14020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seino S., Sugawara K., Yokoi N., Takahashi H. β-Cell Signalling and Insulin Secretagogues: A Path for Improved Diabetes Therapy. Diabetes Obes. Metab. 2017;19:22–29. doi: 10.1111/dom.12995. [DOI] [PubMed] [Google Scholar]
- 109.Suades R., Cosentino F., Badimon L. Glucose-Lowering Treatment in Cardiovascular and Peripheral Artery Disease. Curr. Opin. Pharmacol. 2018;39:86–98. doi: 10.1016/j.coph.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 110.Chu X., Feng B., Ge J., Guo L., Huo Y., Ji L., Jia Q., Jiang S., Li Y., Liu F., et al. Chinese Expert Consensus on the Risk Assessment and Management of Panvascular Disease Inpatients with Type 2 Diabetes Mellitus (2022 Edition) Cardiol. Plus. 2022;7:162–177. doi: 10.1097/CP9.0000000000000029. [DOI] [Google Scholar]
- 111.Salvatore T., Pafundi P.C., Galiero R., Rinaldi L., Caturano A., Vetrano E., Aprea C., Albanese G., Di Martino A., Ricozzi C., et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines. 2020;9:3. doi: 10.3390/biomedicines9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding Y., Zhou Y., Ling P., Feng X., Luo S., Zheng X., Little P.J., Xu S., Weng J. Metformin in Cardiovascular Diabetology: A Focused Review of Its Impact on Endothelial Function. Theranostics. 2021;11:9376–9396. doi: 10.7150/thno.64706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Venu V.K.P., Saifeddine M., Mihara K., Faiza M., Gorobets E., Flewelling A.J., Derksen D.J., Hirota S.A., Marei I., Al-Majid D., et al. Metformin Prevents Hyperglycemia-Associated, Oxidative Stress-Induced Vascular Endothelial Dysfunction: Essential Role for the Orphan Nuclear Receptor Human Nuclear Receptor 4A1 (Nur77) Mol. Pharmacol. 2021;100:428–455. doi: 10.1124/molpharm.120.000148. [DOI] [PubMed] [Google Scholar]
- 114.Triggle C.R., Marei I., Ye K., Ding H., Anderson T.J., Hollenberg M.D., Hill M.A. Repurposing Metformin for Vascular Disease. Curr. Med. Chem. 2023;30:3955–3978. doi: 10.2174/0929867329666220729154615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hiramoto J.S., Katz R., Weisman S., Conte M. Gender-Specific Risk Factors for Peripheral Artery Disease in a Voluntary Screening Population. J. Am. Heart Assoc. 2014;3:e000651. doi: 10.1161/JAHA.113.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kannel W.B., McGee D.L. Update on Some Epidemiologic Features of Intermittent Claudication: The Framingham Study. J. Am. Geriatr. Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 117.Schramm K., Rochon P. Gender Differences in Peripheral Vascular Disease. Semin. Interv. Radiol. 2018;35:009–016. doi: 10.1055/s-0038-1636515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tresierra-Ayala M.Á., García Rojas A. Association between Peripheral Arterial Disease and Diabetic Foot Ulcers in Patients with Diabetes Mellitus Type 2. Med. Univ. 2017;19:123–126. doi: 10.1016/j.rmu.2017.07.002. [DOI] [Google Scholar]
- 119.Thiruvoipati T. Peripheral Artery Disease in Patients with Diabetes: Epidemiology, Mechanisms, and Outcomes. World J. Diabetes. 2015;6:961. doi: 10.4239/wjd.v6.i7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh S., Armstrong E.J., Sherif W., Alvandi B., Westin G.G., Singh G.D., Amsterdam E.A., Laird J.R. Association of Elevated Fasting Glucose with Lower Patency and Increased Major Adverse Limb Events among Patients with Diabetes Undergoing Infrapopliteal Balloon Angioplasty. Vasc. Med. 2014;19:307–314. doi: 10.1177/1358863X14538330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fowkes F.G.R., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., Norman P.E., Sampson U.K., Williams L.J., Mensah G.A., et al. Comparison of Global Estimates of Prevalence and Risk Factors for Peripheral Artery Disease in 2000 and 2010: A Systematic Review and Analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 122.Ballotari P., Ranieri S.C., Luberto F., Caroli S., Greci M., Giorgi Rossi P., Manicardi V. Sex Differences in Cardiovascular Mortality in Diabetics and Nondiabetic Subjects: A Population-Based Study (Italy) Int. J. Endocrinol. 2015;2015:914057. doi: 10.1155/2015/914057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gregg E.W., Gu Q., Cheng Y.J., Venkat Narayan K.M., Cowie C.C. Mortality Trends in Men and Women with Diabetes, 1971 to 2000. Ann. Intern. Med. 2007;147:149. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 124.Khan S.Z., Rivero M., Nader N.D., Cherr G.S., Harris L.M., Dryjski M.L., Dosluoglu H.H. Metformin Is Associated with Improved Survival and Decreased Cardiac Events with No Impact on Patency and Limb Salvage after Revascularization for Peripheral Arterial Disease. Ann. Vasc. Surg. 2019;55:63–77. doi: 10.1016/j.avsg.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 125.Tan S., Goudot G., Arnoux A., Tran Y., Maissoro H., Poenou G., Detriche G., Khider L., Mohamedi N., Mirault T., et al. Occurrence of Major Local Lower Limb Events in Type 2 Diabetic Patients with Lower Extremity Arterial Disease: Impact of Metformin. Ann. Vasc. Surg. 2023;90:153–161. doi: 10.1016/j.avsg.2022.09.064. [DOI] [PubMed] [Google Scholar]
- 126.Reitz K.M., Althouse A.D., Forman D.E., Zuckerbraun B.S., Vodovotz Y., Zamora R., Raffai R.L., Hall D.E., Tzeng E. MetfOrmin BenefIts Lower Extremities with Intermittent Claudication (MOBILE IC): Randomized Clinical Trial Protocol. BMC Cardiovasc. Disord. 2023;23:38. doi: 10.1186/s12872-023-03047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koenig A.M., Mechanic-Hamilton D., Xie S.X., Combs M.F., Cappola A.R., Xie L., Detre J.A., Wolk D.A., Arnold S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data from a Randomized Placebo-Controlled Crossover Study. Alzheimer Dis. Assoc. Disord. 2017;31:107–113. doi: 10.1097/WAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paudel Y.N., Angelopoulou E., Piperi C., Shaikh M.F., Othman I. Emerging Neuroprotective Effect of Metformin in Parkinson’s Disease: A Molecular Crosstalk. Pharmacol. Res. 2020;152:104593. doi: 10.1016/j.phrs.2019.104593. [DOI] [PubMed] [Google Scholar]
- 129.Li Z., Zhang Z., Ren Y., Wang Y., Fang J., Yue H., Ma S., Guan F. Aging and Age-related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology. 2021;22:165–187. doi: 10.1007/s10522-021-09910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gilbert E.A.B., Livingston J., Garcia-Flores E., Kehtari T., Morshead C.M. Metformin Improves Functional Outcomes, Activates Neural Precursor Cells, and Modulates Microglia in a Sex-Dependent Manner After Spinal Cord Injury. Stem Cells Transl. Med. 2023;12:415–428. doi: 10.1093/stcltm/szad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fatt M., Hsu K., He L., Wondisford F., Miller F.D., Kaplan D.R., Wang J. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem Cell Rep. 2015;5:988–995. doi: 10.1016/j.stemcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J., Gallagher D., DeVito L.M., Cancino G.I., Tsui D., He L., Keller G.M., Frankland P.W., Kaplan D.R., Miller F.D. Metformin Activates an Atypical PKC-CBP Pathway to Promote Neurogenesis and Enhance Spatial Memory Formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 133.Dadwal P., Mahmud N., Sinai L., Azimi A., Fatt M., Wondisford F.E., Miller F.D., Morshead C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Rep. 2015;5:166–173. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tao L., Li D., Liu H., Jiang F., Xu Y., Cao Y., Gao R., Chen G. Neuroprotective Effects of Metformin on Traumatic Brain Injury in Rats Associated with NF-κB and MAPK Signaling Pathway. Brain Res. Bull. 2018;140:154–161. doi: 10.1016/j.brainresbull.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 135.DiBona V.L., Shah M.K., Krause K.J., Zhu W., Voglewede M.M., Smith D.M., Crockett D.P., Zhang H. Metformin Reduces Neuroinflammation and Improves Cognitive Functions after Traumatic Brain Injury. Neurosci. Res. 2021;172:99–109. doi: 10.1016/j.neures.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inoue Y., Masuda T., Misumi Y., Ando Y., Ueda M. Metformin Attenuates Vascular Pathology by Increasing Expression of Insulin-Degrading Enzyme in a Mixed Model of Cerebral Amyloid Angiopathy and Type 2 Diabetes Mellitus. Neurosci. Lett. 2021;762:136136. doi: 10.1016/j.neulet.2021.136136. [DOI] [PubMed] [Google Scholar]
- 137.Rojas M., Chávez-Castillo M., Bautista J., Ortega Á., Nava M., Salazar J., Díaz-Camargo E., Medina O., Rojas-Quintero J., Bermúdez V. Alzheimer’s Disease and Type 2 Diabetes Mellitus: Pathophysiologic and Pharmacotherapeutics Links. World J. Diabetes. 2021;12:745–766. doi: 10.4239/wjd.v12.i6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X., Leng S., Song D. Link between Type 2 Diabetes and Alzheimer’s Disease: From Epidemiology to Mechanism and Treatment. Clin. Interv. Aging. 2015;2015:549–560. doi: 10.2147/CIA.S74042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Benito-Cuesta I., Ordóñez-Gutiérrez L., Wandosell F. AMPK Activation Does Not Enhance Autophagy in Neurons in Contrast to MTORC1 Inhibition: Different Impact on β-Amyloid Clearance. Autophagy. 2021;17:656–671. doi: 10.1080/15548627.2020.1728095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Son S.M., Shin H.-J., Byun J., Kook S.Y., Moon M., Chang Y.J., Mook-Jung I. Metformin Facilitates Amyloid-β Generation by β- and γ-Secretases via Autophagy Activation. J. Alzheimer’s Dis. 2016;51:1197–1208. doi: 10.3233/JAD-151200. [DOI] [PubMed] [Google Scholar]
- 141.Chen Y., Zhou K., Wang R., Liu Y., Kwak Y.-D., Ma T., Thompson R.C., Zhao Y., Smith L., Gasparini L., et al. Antidiabetic Drug Metformin (Glucophage R) Increases Biogenesis of Alzheimer’s Amyloid Peptides via up-Regulating BACE1 Transcription. Proc. Natl. Acad. Sci. USA. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.García-Juan M., Ordóñez-Gutiérrez L., Wandosell F. Clearance of Β-amyloid Mediated by Autophagy Is Enhanced by MTORC1 Inhibition but Not AMPK Activation in APP/PSEN1 Astrocytes. Glia. 2024;72:588–606. doi: 10.1002/glia.24492. [DOI] [PubMed] [Google Scholar]
- 143.Ng T.P., Feng L., Yap K.B., Lee T.S., Tan C.H., Winblad B. Long-Term Metformin Usage and Cognitive Function among Older Adults with Diabetes. J. Alzheimer’s Dis. 2014;41:61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- 144.Ayoub R., Ruddy R.M., Cox E., Oyefiade A., Derkach D., Laughlin S., Ades-aron B., Shirzadi Z., Fieremans E., MacIntosh B.J., et al. Assessment of Cognitive and Neural Recovery in Survivors of Pediatric Brain Tumors in a Pilot Clinical Trial Using Metformin. Nat. Med. 2020;26:1285–1294. doi: 10.1038/s41591-020-0985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ping F., Jiang N., Li Y. Association between Metformin and Neurodegenerative Diseases of Observational Studies: Systematic Review and Meta-Analysis. BMJ Open Diabetes Res. Care. 2020;8:e001370. doi: 10.1136/bmjdrc-2020-001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tsilidis K.K., Kasimis J.C., Lopez D.S., Ntzani E.E., Ioannidis J.P.A. Type 2 Diabetes and Cancer: Umbrella Review of Meta-Analyses of Observational Studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 147.Noto H., Tsujimoto T., Sasazuki T., Noda M. Significantly Increased Risk of Cancer in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr. Pract. 2011;17:616–628. doi: 10.4158/EP10357.RA. [DOI] [PubMed] [Google Scholar]
- 148.Li Y., Tian J., Hou T., Gu K., Yan Q., Sun S., Zhang J., Sun J., Liu L., Sheng C.-S., et al. Association Between Age at Diabetes Diagnosis and Subsequent Incidence of Cancer: A Longitudinal Population-Based Cohort. Diabetes Care. 2024;47:353–361. doi: 10.2337/dc23-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Evans J.M.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and Reduced Risk of Cancer in Diabetic Patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Libby G., Donnelly L.A., Donnan P.T., Alessi D.R., Morris A.D., Evans J.M.M. New Users of Metformin Are at Low Risk of Incident Cancer. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lega I.C., Shah P.S., Margel D., Beyene J., Rochon P.A., Lipscombe L.L. The Effect of Metformin on Mortality Following Cancer among Patients with Diabetes. Cancer Epidemiol. Biomark. Prev. 2014;23:1974–1984. doi: 10.1158/1055-9965.EPI-14-0327. [DOI] [PubMed] [Google Scholar]
- 152.Ki Y.-J., Kim H.J., Kim M.-S., Park C.M., Ko M.J., Seo Y.S., Moon S.M., Choi J.A. Association between Metformin Use and Survival in Nonmetastatic Rectal Cancer Treated with a Curative Resection: A Nationwide Population Study. Cancer Res. Treat. 2017;49:29–36. doi: 10.4143/crt.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fransgaard T., Thygesen L.C., Gögenur I. Metformin Increases Overall Survival in Patients with Diabetes Undergoing Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016;23:1569–1575. doi: 10.1245/s10434-015-5028-8. [DOI] [PubMed] [Google Scholar]
- 154.Lee J.H., Kim T.I., Jeon S.M., Hong S.P., Cheon J.H., Kim W.H. The Effects of Metformin on the Survival of Colorectal Cancer Patients with Diabetes Mellitus. Int. J. Cancer. 2012;131:752–759. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 155.Mc Menamin Ú.C., Murray L.J., Hughes C.M., Cardwell C.R. Metformin Use and Survival after Colorectal Cancer: A Population-based Cohort Study. Int. J. Cancer. 2016;138:369–379. doi: 10.1002/ijc.29720. [DOI] [PubMed] [Google Scholar]
- 156.Fransgaard T., Thygesen L.C., Gögenur I. Association between Metformin Use after Surgery for Colorectal Cancer and Oncological Outcomes: A Nationwide Register-based Study. Int. J. Cancer. 2018;143:63–72. doi: 10.1002/ijc.31305. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y., Xiao J., Zhao Y., Du S., Du J. Effect of Metformin on the Mortality of Colorectal Cancer Patients with T2DM: Meta-Analysis of Sex Differences. Int. J. Color. Dis. 2020;35:827–835. doi: 10.1007/s00384-020-03539-5. [DOI] [PubMed] [Google Scholar]
- 158.Lee M.-S., Hsu C.-C., Wahlqvist M.L., Tsai H.-N., Chang Y.-H., Huang Y.-C. Type 2 Diabetes Increases and Metformin Reduces Total, Colorectal, Liver and Pancreatic Cancer Incidences in Taiwanese: A Representative Population Prospective Cohort Study of 800,000 Individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang P., Li H., Tan X., Chen L., Wang S. Association of Metformin Use with Cancer Incidence and Mortality: A Meta-Analysis. Cancer Epidemiol. 2013;37:207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 160.Szablewski L. Insulin Resistance: The Increased Risk of Cancers. Curr. Oncol. 2024;31:20075. doi: 10.3390/curroncol31020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ohkuma T., Peters S.A.E., Woodward M. Sex Differences in the Association between Diabetes and Cancer: A Systematic Review and Meta-Analysis of 121 Cohorts Including 20 Million Individuals and One Million Events. Diabetologia. 2018;61:2140–2154. doi: 10.1007/s00125-018-4664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu J., Zhang J., Sun X., Wang L., Xu Y., Zhang Y., Liu X., Dong C. Influence of Diabetes Mellitus on the Severity and Fatality of SARS-CoV-2 (COVID-19) Infection. Diabetes Obes. Metab. 2020;22:1907–1914. doi: 10.1111/dom.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gęca T., Wojtowicz K., Guzik P., Góra T. Increased Risk of COVID-19 in Patients with Diabetes Mellitus—Current Challenges in Pathophysiology, Treatment and Prevention. Int. J. Environ. Res. Public Health. 2022;19:6555. doi: 10.3390/ijerph19116555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Froldi G., Dorigo P. Endothelial Dysfunction in Coronavirus Disease 2019 (COVID-19): Gender and Age Influences. Med. Hypotheses. 2020;144:110015. doi: 10.1016/j.mehy.2020.110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hartmann-Boyce J., Highton P., Rees K., Onakpoya I., Suklan J., Curtis F., O’Mahoney L., Morris E., Kudlek L., Morgan J., et al. The Impact of the COVID-19 Pandemic and Associated Disruptions in Health-Care Provision on Clinical Outcomes in People with Diabetes: A Systematic Review. Lancet Diabetes Endocrinol. 2024;12:132–148. doi: 10.1016/S2213-8587(23)00351-0. [DOI] [PubMed] [Google Scholar]
- 166.Petakh P., Griga V., Mohammed I., Loshak K., Poliak I., Kamyshnyiy A. Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes. Med. Arch. 2022;76:329. doi: 10.5455/medarh.2022.76.329-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Erickson S.M., Fenno S.L., Barzilai N., Kuchel G., Bartley J.M., Justice J.N., Buse J.B., Bramante C.T. Metformin for Treatment of Acute COVID-19: Systematic Review of Clinical Trial Data Against SARS-CoV-2. Diabetes Care. 2023;46:1432–1442. doi: 10.2337/dc22-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Crouse A.B., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin Use Is Associated with Reduced Mortality in a Diverse Population With COVID-19 and Diabetes. Front. Endocrinol. 2021;11:600439. doi: 10.3389/fendo.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Khunti K., Knighton P., Zaccardi F., Bakhai C., Barron E., Holman N., Kar P., Meace C., Sattar N., Sharp S., et al. Prescription of Glucose-Lowering Therapies and Risk of COVID-19 Mortality in People with Type 2 Diabetes: A Nationwide Observational Study in England. Lancet Diabetes Endocrinol. 2021;9:293–303. doi: 10.1016/S2213-8587(21)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bramante C.T., Ingraham N.E., Murray T.A., Marmor S., Hovertsen S., Gronski J., McNeil C., Feng R., Guzman G., Abdelwahab N., et al. Metformin and Risk of Mortality in Patients Hospitalised with COVID-19: A Retrospective Cohort Analysis. Lancet Healthy Longev. 2021;2:e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jiang N., Chen Z., Liu L., Yin X., Yang H., Tan X., Wang J., Li H., Tian M., Lu Z., et al. Association of Metformin with Mortality or ARDS in Patients with COVID-19 and Type 2 Diabetes: A Retrospective Cohort Study. Diabetes Res. Clin. Pract. 2021;173:108619. doi: 10.1016/j.diabres.2020.108619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., et al. Practical Recommendations for the Management of Diabetes in Patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng X., Liu Y.-M., Li H., Zhang X., Lei F., Qin J.-J., Chen Z., Deng K.-Q., Lin L., Chen M.-M., et al. Metformin Is Associated with Higher Incidence of Acidosis, but Not Mortality, in Individuals with COVID-19 and Pre-Existing Type 2 Diabetes. Cell Metab. 2020;32:537–547.e3. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bielka W., Przezak A., Pawlik A. Therapy of Type 2 Diabetes in Patients with SARS-CoV-2 Infection. Int. J. Mol. Sci. 2021;22:7605. doi: 10.3390/ijms22147605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lim S., Bae J.H., Kwon H.-S., Nauck M.A. COVID-19 and Diabetes Mellitus: From Pathophysiology to Clinical Management. Nat. Rev. Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Adapa S., Aeddula N.R., Konala V.M., Chenna A., Naramala S., Madhira B.R., Gayam V., Balla M., Muppidi V., Bose S. COVID-19 and Renal Failure: Challenges in the Delivery of Renal Replacement Therapy. J. Clin. Med. Res. 2020;12:276–285. doi: 10.14740/jocmr4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 179.Piché M.-E., Tchernof A., Després J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 180.Krupa-Kotara K., Dakowska D. Impact of Obesity on Risk of Cancer. Cent. Eur. J. Public Health. 2021;29:38–44. doi: 10.21101/cejph.a5913. [DOI] [PubMed] [Google Scholar]
- 181.Bona M.D., Torres C.H.D.M., Lima S.C.V.C., Morais A.H.D.A., Lima A.Â.M., Maciel B.L.L. Intestinal Barrier Permeability in Obese Individuals with or without Metabolic Syndrome: A Systematic Review. Nutrients. 2022;14:3649. doi: 10.3390/nu14173649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Y., Zhu X., Yu X., Novák P., Gui Q., Yin K. Enhancing Intestinal Barrier Efficiency: A Novel Metabolic Diseases Therapy. Front. Nutr. 2023;10:1120168. doi: 10.3389/fnut.2023.1120168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Portincasa P., Bonfrate L., Khalil M., Angelis M.D., Calabrese F.M., D’Amato M., Wang D.Q.-H., Di Ciaula A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines. 2021;10:83. doi: 10.3390/biomedicines10010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marti A., Martínez I., Ojeda-Rodríguez A., Azcona-Sanjulian M.C. Higher Lipopolysaccharide Binding Protein and Chemerin Concentrations Were Associated with Metabolic Syndrome Features in Pediatric Subjects with Abdominal Obesity during a Lifestyle Intervention. Nutrients. 2021;13:289. doi: 10.3390/nu13020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tilves C., Yeh H.-C., Maruthur N., Juraschek S.P., Miller E.R., Appel L.J., Mueller N.T. A Behavioral Weight-Loss Intervention, but Not Metformin, Decreases a Marker of Gut Barrier Permeability: Results from the SPIRIT Randomized Trial. Int. J. Obes. 2022;46:655–660. doi: 10.1038/s41366-021-01039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tentolouris A., Vlachakis P., Tzeravini E., Eleftheriadou I., Tentolouris N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public Health. 2019;16:2965. doi: 10.3390/ijerph16162965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Keller D.M., Ahmed N., Tariq H., Walgamage M., Walgamage T., Mohammed A., Chou J.T.-T., Kałużna-Oleksy M., Lesiak M., Straburzyńska-Migaj E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. J. Clin. Med. 2022;11:1470. doi: 10.3390/jcm11061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rieg T., Vallon V. Development of SGLT1 and SGLT2 Inhibitors. Diabetologia. 2018;61:2079–2086. doi: 10.1007/s00125-018-4654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dominguez Rieg J.A., Xue J., Rieg T. Tubular Effects of Sodium–Glucose Cotransporter 2 Inhibitors: Intended and Unintended Consequences. Curr. Opin. Nephrol. Hypertens. 2020;29:523–530. doi: 10.1097/MNH.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Feijóo-Bandín S., Aragón-Herrera A., Otero-Santiago M., Anido-Varela L., Moraña-Fernández S., Tarazón E., Roselló-Lletí E., Portolés M., Gualillo O., González-Juanatey J.R., et al. Role of Sodium-Glucose Co-Transporter 2 Inhibitors in the Regulation of Inflammatory Processes in Animal Models. Int. J. Mol. Sci. 2022;23:5634. doi: 10.3390/ijms23105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koshizaka M., Ishikawa K., Ishibashi R., Maezawa Y., Sakamoto K., Uchida D., Nakamura S., Yamaga M., Yokoh H., Kobayashi A., et al. Comparing the Effects of Ipragliflozin versus Metformin on Visceral Fat Reduction and Metabolic Dysfunction in Japanese Patients with Type 2 Diabetes Treated with Sitagliptin: A Prospective, Multicentre, Open-label, Blinded-endpoint, Randomized Controlled Study (PRIME-V Study) Diabetes Obes. Metab. 2019;21:1990–1995. doi: 10.1111/dom.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koshizaka M., Ishikawa K., Ishibashi R., Takahashi S., Sakamoto K., Yokoh H., Baba Y., Ide S., Ide K., Ishikawa T., et al. Comparison of Visceral Fat Reduction by Ipragliflozin and Metformin in Elderly Type 2 Diabetes Patients: Sub-Analysis of a Randomized-Controlled Study. Diabetes Ther. 2021;12:183–196. doi: 10.1007/s13300-020-00949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsukagoshi-Yamaguchi A., Koshizaka M., Ishibashi R., Ishikawa K., Ishikawa T., Shoji M., Ide S., Ide K., Baba Y., Terayama R., et al. Metabolomic Analysis of Serum Samples from a Clinical Study on Ipragliflozin and Metformin Treatment in Japanese Patients with Type 2 Diabetes: Exploring Human Metabolites Associated with Visceral Fat Reduction. Pharmacotherapy. 2023;43:1317–1326. doi: 10.1002/phar.2884. [DOI] [PubMed] [Google Scholar]
- 194.Agarwal S.M., Stogios N., Ahsan Z.A., Lockwood J.T., Duncan M.J., Takeuchi H., Cohn T., Taylor V.H., Remington G., Faulkner G.E.J., et al. Pharmacological Interventions for Prevention of Weight Gain in People with Schizophrenia. Cochrane Database Syst. Rev. 2022:CD013337. doi: 10.1002/14651858.CD013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deswal R., Narwal V., Dang A., Pundir C. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020;13:261. doi: 10.4103/jhrs.JHRS_95_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nasri H., Rafieian-Kopaei M. Metformin: Current Knowledge. J. Res. Med. Sci. 2014;19:658–664. [PMC free article] [PubMed] [Google Scholar]
- 197.Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B.O. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 198.Wolf W., Wattick R., Kinkade O., Olfert M. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health. 2018;15:2589. doi: 10.3390/ijerph15112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., Piltonen T., Norman R.J., Andersen M., Azziz R., et al. Recommendations from the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barber T.M., Franks S. Obesity and Polycystic Ovary Syndrome. Clin. Endocrinol. 2021;95:531–541. doi: 10.1111/cen.14421. [DOI] [PubMed] [Google Scholar]
- 201.Barber T.M., Hanson P., Weickert M.O., Franks S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health. 2019;13:117955811987404. doi: 10.1177/1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Long C., Feng H., Duan W., Chen X., Zhao Y., Lan Y., Yue R. Prevalence of Polycystic Ovary Syndrome in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022;13:980405. doi: 10.3389/fendo.2022.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang J., Xing C., Cheng X., He B. Canagliflozin Combined with Metformin versus Metformin Monotherapy for Endocrine and Metabolic Profiles in Overweight and Obese Women with Polycystic Ovary Syndrome: A Single-Center, Open-Labeled Prospective Randomized Controlled Trial. Front. Endocrinol. 2022;13:1003238. doi: 10.3389/fendo.2022.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xing C., Zhao H., Zhang J., He B. Effect of Metformin versus Metformin plus Liraglutide on Gonadal and Metabolic Profiles in Overweight Patients with Polycystic Ovary Syndrome. Front. Endocrinol. 2022;13:945609. doi: 10.3389/fendo.2022.945609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Greff D., Juhász A.E., Váncsa S., Váradi A., Sipos Z., Szinte J., Park S., Hegyi P., Nyirády P., Ács N., et al. Inositol Is an Effective and Safe Treatment in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Reprod. Biol. Endocrinol. 2023;21:10. doi: 10.1186/s12958-023-01055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang J.-Q., Xing C., He B. Short Period-Administration of Myo-Inositol and Metformin on Hormonal and Glycolipid Profiles in Patients with Polycystic Ovary Syndrome: A Systematic Review and Updated Meta-Analysis of Randomized Controlled Trials. Eur. Rev. Med. Pharmacol. Sci. 2022;26:1792–1802. doi: 10.26355/eurrev_202203_28322. [DOI] [PubMed] [Google Scholar]
- 207.Melin J., Forslund M., Alesi S., Piltonen T., Romualdi D., Spritzer P.M., Tay C.T., Pena A., Witchel S.F., Mousa A., et al. Metformin and Combined Oral Contraceptive Pills in the Management of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2024;109:e817–e836. doi: 10.1210/clinem/dgad465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Joham A.E., Norman R.J., Stener-Victorin E., Legro R.S., Franks S., Moran L.J., Boyle J., Teede H.J. Polycystic Ovary Syndrome. Lancet Diabetes Endocrinol. 2022;10:668–680. doi: 10.1016/S2213-8587(22)00163-2. [DOI] [PubMed] [Google Scholar]
- 209.Stadtmauer L.A. Should Patients with Polycystic Ovary Syndrome Be Treated with Metformin?: Benefits of Insulin Sensitizing Drugs in Polycystic Ovary Syndrome—Beyond Ovulation Induction. Hum. Reprod. 2002;17:3016–3026. doi: 10.1093/humrep/17.12.3016. [DOI] [PubMed] [Google Scholar]
- 210.Legro R.S., Arslanian S.A., Ehrmann D.A., Hoeger K.M., Murad M.H., Pasquali R., Welt C.K. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Udesen P.B., Glintborg D., Sørensen A.E., Svendsen R., Nielsen N.L.S., Wissing M.L.M., Andersen M.S., Englund A.L.M., Dalgaard L.T. Metformin Decreases miR-122, miR-223 and miR-29a in Women with Polycystic Ovary Syndrome. Endocr. Connect. 2020;9:1075–1084. doi: 10.1530/EC-20-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fraison E., Kostova E., Moran L.J., Bilal S., Ee C.C., Venetis C., Costello M.F. Metformin versus the Combined Oral Contraceptive Pill for Hirsutism, Acne, and Menstrual Pattern in Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 2020;2020:CD005552. doi: 10.1002/14651858.CD005552.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lauretta R., Sansone M., Romanelli F., Appetecchia M. Gender in Endocrinological Diseases: Biological and Clinical Differences. Ital. J. Gend. Specif. Med. 2017;3:109–116. doi: 10.1723/2882.29060. [DOI] [Google Scholar]
- 214.Beeson P.B. Age and Sex Associations of 40 Autoimmune Diseases. Am. J. Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 215.Effraimidis G., Wiersinga W.M. Mechanisms in Endocrinology: Autoimmune Thyroid Disease: Old and New Players. Eur. J. Endocrinol. 2014;170:R241–R252. doi: 10.1530/EJE-14-0047. [DOI] [PubMed] [Google Scholar]
- 216.Adams R., Oh E.S., Yasar S., Lyketsos C.G., Mammen J.S. Endogenous and Exogenous Thyrotoxicosis and Risk of Incident Cognitive Disorders in Older Adults. JAMA Intern. Med. 2023;183:1324. doi: 10.1001/jamainternmed.2023.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Biondi B., Kahaly G.J., Robertson R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019;40:789–824. doi: 10.1210/er.2018-00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Eom Y.S., Wilson J.R., Bernet V.J. Links between Thyroid Disorders and Glucose Homeostasis. Diabetes Metab. J. 2022;46:239–256. doi: 10.4093/dmj.2022.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sloot Y.J.E., Janssen M.J.R., Van Herwaarden A.E., Peeters R.P., Netea-Maier R.T., Smit J.W.A. The Influence of Energy Depletion by Metformin or Hypocaloric Diet on Thyroid Iodine Uptake in Healthy Volunteers: A Randomized Trial. Sci. Rep. 2019;9:5396. doi: 10.1038/s41598-019-41997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pappa T., Alevizaki M. Metformin and Thyroid: An Update. Eur. Thyroid. J. 2013;2:22–28. doi: 10.1159/000346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rotondi M., Cappelli C., Magri F., Botta R., Dionisio R., Iacobello C., De Cata P., Nappi R.E., Castellano M., Chiovato L. Thyroidal Effect of Metformin Treatment in Patients with Polycystic Ovary Syndrome: Metformin Therapy and TSH. Clin. Endocrinol. 2011;75:378–381. doi: 10.1111/j.1365-2265.2011.04042.x. [DOI] [PubMed] [Google Scholar]
- 222.Anil C., Kut A., Atesagaoglu B., Nar A., Bascil Tutuncu N., Gursoy A. Metformin Decreases Thyroid Volume and Nodule Size in Subjects with Insulin Resistance: A Preliminary Study. Med. Princ. Pract. 2016;25:233–236. doi: 10.1159/000442821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dowling R.J.O., Zakikhani M., Fantus I.G., Pollak M., Sonenberg N. Metformin Inhibits Mammalian Target of Rapamycin–Dependent Translation Initiation in Breast Cancer Cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 224.Kheder S., Sisley K., Hadad S., Balasubramanian S.P. Effects of Prolonged Exposure to Low Dose Metformin in Thyroid Cancer Cell Lines. J. Cancer. 2017;8:1053–1061. doi: 10.7150/jca.16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.He X., Wu D., Hu C., Xu T., Liu Y., Liu C., Xu B., Tang W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid. 2019;29:359–367. doi: 10.1089/thy.2017.0707. [DOI] [PubMed] [Google Scholar]
- 226.Sui M., Yu Y., Zhang H., Di H., Liu C., Fan Y. Efficacy of Metformin for Benign Thyroid Nodules in Subjects with Insulin Resistance: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018;9:494. doi: 10.3389/fendo.2018.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bonnet F., Scheen A. Understanding and Overcoming Metformin Gastrointestinal Intolerance. Diabetes Obes. Metab. 2017;19:473–481. doi: 10.1111/dom.12854. [DOI] [PubMed] [Google Scholar]
- 228.Kim C., Han K., Oh H., Tan K.E., Sothiratnam R., Tjokroprawiro A., Klein M. Safety, Tolerability, and Efficacy of Metformin Extended-release Oral Antidiabetic Therapy in Patients with Type 2 Diabetes: An Observational Trial in Asia. J. Diabetes. 2012;4:395–406. doi: 10.1111/j.1753-0407.2012.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McCreight L.J., Bailey C.J., Pearson E.R. Metformin and the Gastrointestinal Tract. Diabetologia. 2016;59:426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cubeddu L.X., Bönisch H., Göthert M., Molderings G., Racké K., Ramadori G., Miller K.J., Schwörer H. Effects of Metformin on Intestinal 5-Hydroxytryptamine (5-HT) Release and on 5-HT3 Receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000;361:85–91. doi: 10.1007/s002109900152. [DOI] [PubMed] [Google Scholar]
- 231.Dujic T., Zhou K., Tavendale R., Palmer C.N.A., Pearson E.R. Effect of Serotonin Transporter 5-HTTLPR Polymorphism on Gastrointestinal Intolerance to Metformin: A GoDARTS Study. Diabetes Care. 2016;39:1896–1901. doi: 10.2337/dc16-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yee S.W., Lin L., Merski M., Keiser M.J., Gupta A., Zhang Y., Chien H.-C., Shoichet B.K., Giacomini K.M. Prediction and Validation of Enzyme and Transporter Off-Targets for Metformin. J. Pharmacokinet. Pharmacodyn. 2015;42:463–475. doi: 10.1007/s10928-015-9436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.de Vries S.T., Denig P., Ekhart C., Mol P.G.M., van Puijenbroek E.P. Sex Differences in Adverse Drug Reactions of Metformin: A Longitudinal Survey Study. Drug Saf. 2020;43:489–495. doi: 10.1007/s40264-020-00913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.de Jong L., Härmark L., van Puijenbroek E. Time Course, Outcome and Management of Adverse Drug Reactions Associated with Metformin from Patient’s Perspective: A Prospective, Observational Cohort Study in the Netherlands. Eur. J. Clin. Pharmacol. 2016;72:615–622. doi: 10.1007/s00228-016-2019-z. [DOI] [PubMed] [Google Scholar]
- 235.The Diabetes Prevention Program Research Group Long-Term Safety, Tolerability, and Weight Loss Associated with Metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Inzucchi S.E., Lipska K.J., Mayo H., Bailey C.J., McGuire D.K. Metformin in Patients with Type 2 Diabetes and Kidney Disease: A Systematic Review. JAMA. 2014;312:2668. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hung S.-C., Chang Y.-K., Liu J.-S., Kuo K.-L., Chen Y.-H., Hsu C.-C., Tarng D.-C. Metformin Use and Mortality in Patients with Advanced Chronic Kidney Disease: National, Retrospective, Observational, Cohort Study. Lancet Diabetes Endocrinol. 2015;3:605–614. doi: 10.1016/S2213-8587(15)00123-0. [DOI] [PubMed] [Google Scholar]
- 238.Stage T.B., Brøsen K., Christensen M.M.H. A Comprehensive Review of Drug–Drug Interactions with Metformin. Clin. Pharmacokinet. 2015;54:811–824. doi: 10.1007/s40262-015-0270-6. [DOI] [PubMed] [Google Scholar]
- 239.Pakkir Maideen N.M., Jumale A., Balasubramaniam R. Drug Interactions of Metformin Involving Drug Transporter Proteins. Adv. Pharm. Bull. 2017;7:501–505. doi: 10.15171/apb.2017.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tasnim J., Hashim N.M., Han H.C. A Comprehensive Review on Potential Drug–Drug Interactions of Proton Pump Inhibitors with Antidiabetic Drugs Metformin and DPP-4 Inhibitors. Cell Biochem. Funct. 2024;42:e3967. doi: 10.1002/cbf.3967. [DOI] [PubMed] [Google Scholar]
- 241.Özyiğit F., Paşalı Kilit T., Onbaşı K. Polypharmacy and drug-drug interactions among patients with Diabetes Mellitus. Hippocrates Med. J. 2023;3:7–15. doi: 10.58961/hmj.1394987. [DOI] [Google Scholar]
- 242.Ansari A.I., Rizvi A.A., Verma S., Abbas M., Siddiqi Z., Mishra D., Verma S., Raza S.T., Mahdi F. Interactions between Diabetic and Hypertensive Drugs: A Pharmacogenetics Approach. Mol. Genet. Genom. 2023;298:803–812. doi: 10.1007/s00438-023-02011-7. [DOI] [PubMed] [Google Scholar]
- 243.Schwartz A.V., Pan Q., Aroda V.R., Crandall J.P., Kriska A., Piromalli C., Wallia A., Temprosa M., Florez H., for the Diabetes Prevention Program Research Group Long-Term Effects of Lifestyle and Metformin Interventions in DPP on Bone Density. Osteoporos Int. 2021;32:2279–2287. doi: 10.1007/s00198-021-05989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Infante M., Leoni M., Caprio M., Fabbri A. Long-Term Metformin Therapy and Vitamin B12 Deficiency: An Association to Bear in Mind. World J. Diabetes. 2021;12:916–931. doi: 10.4239/wjd.v12.i7.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang W., Cai X., Wu H., Ji L. Associations between Metformin Use and Vitamin B 12 Levels, Anemia, and Neuropathy in Patients with Diabetes: A Meta-analysis. J. Diabetes. 2019;11:729–743. doi: 10.1111/1753-0407.12900. [DOI] [PubMed] [Google Scholar]
- 246.Wile D.J., Toth C. Association of Metformin, Elevated Homocysteine, and Methylmalonic Acid Levels and Clinically Worsened Diabetic Peripheral Neuropathy. Diabetes Care. 2010;33:156–161. doi: 10.2337/dc09-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Niklewicz A., Smith A.D., Smith A., Holzer A., Klein A., McCaddon A., Molloy A.M., Wolffenbuttel B.H.R., Nexo E., McNulty H., et al. The Importance of Vitamin B12 for Individuals Choosing Plant-Based Diets. Eur. J. Nutr. 2023;62:1551–1559. doi: 10.1007/s00394-022-03025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yang R., Yu H., Wu J., Chen H., Wang M., Wang S., Qin X., Wu T., Wu Y., Hu Y. Metformin Treatment and Risk of Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus in Beijing, China. Front. Endocrinol. 2023;14:1082720. doi: 10.3389/fendo.2023.1082720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Christine C.W., Auinger P., Saleh N., Tian M., Bottiglieri T., Arning E., Tran N.K., Ueland P.M., Green R., The Parkinson Study Group—DATATOP Investigators Relationship of Cerebrospinal Fluid Vitamin B12 Status Markers with Parkinson’s Disease Progression. Mov. Disord. 2020;35:1466–1471. doi: 10.1002/mds.28073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.WHO . VigiBase—WHO’s Global Database VigiBase. WHO; Geneva, Switzerland: 2024. Metformin. [Google Scholar]
- 251.Oktora M.P., de Vos S., de Vries S.T., Hak E., Denig P. Sex Disparities in Treatment Patterns after Metformin Initiation among Patients with Type 2 Diabetes Mellitus. Pharmacoepidemiol. Drug. 2023;32:1395–1405. doi: 10.1002/pds.5672. [DOI] [PubMed] [Google Scholar]
- 252.Oi Yan Chan J., Moullet M., Williamson B., Arends R.H., Pilla Reddy V. Harnessing Clinical Trial and Real-World Data Towards an Understanding of Sex Effects on Drug Pharmacokinetics, Pharmacodynamics and Efficacy. Front. Pharmacol. 2022;13:874606. doi: 10.3389/fphar.2022.874606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zucker I., Prendergast B.J. Sex Differences in Pharmacokinetics Predict Adverse Drug Reactions in Women. Biol. Sex Differ. 2020;11:32. doi: 10.1186/s13293-020-00308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are reported in the text and in the Supplementary Materials.