Summary
Next-generation sequencing has revolutionized the speed of rare disease (RD) diagnoses. While clinical exome and genome sequencing represent an effective tool for many RD diagnoses, there is room to further improve the diagnostic odyssey of many RD patients. One recognizable intervention lies in increasing equitable access to genomic testing. Rural communities represent a significant portion of underserved and underrepresented individuals facing additional barriers to diagnosis and treatment. Primary care providers (PCPs) at local clinics, though sometimes suspicious of a potential benefit of genetic testing for their patients, have significant constraints in pursuing it themselves and rely on referrals to specialists. Yet, these referrals are typically followed by long waitlists and significant delays in clinical assessment, insurance clearance, testing, and initiation of diagnosis-informed care management. Not only is this process time intensive, but it also often requires multiple visits to urban medical centers for which distance may be a significant barrier to rural families. Therefore, providing early, “direct-to-provider” (DTP) local access to unrestrictive genomic testing is likely to help speed up diagnostic times and access to care for RD patients in rural communities. In a pilot study with a PCP clinic in rural Kansas, we observed a minimum 5.5 months shortening of time to diagnosis through the DTP exome sequencing program as compared to rural patients receiving genetic testing through the “traditional” PCP-referral-to-specialist scheme. We share our experience to encourage future partnerships beyond our center. Our efforts represent just one step in fostering greater diversity and equity in genomic studies.
Keywords: rare diseases, genomics, next-generation sequencing, diagnostics, direct-to-provider, rural populations, genomic equity
Individuals with rare genetic disorders face a long road to obtain a diagnosis and resulting medical care. Those who live in rural areas face even more challenges due to reduced access to testing and resources. Partnerships with rural PCPs can shorten time to diagnosis by delivering specialist-supported genomic testing and counseling.
Introduction
There are more than 7,000 genetic diseases with a known molecular basis, most of which are rare, with roughly half of these affecting children.1,2 For families with rare diseases (RDs), the journey to a genetic diagnosis, often referred to as “diagnostic odyssey,” may be long. Conservative estimates of an average time to diagnosis are at five years (US Department of Health and Human Services).2 Yet, establishing a molecular diagnosis is crucial for determining the best medical care, guiding management, treatment, and referral to additional specialists for evaluation of potential health complications.3 Technology advancements have exponentially increased our ability to make molecular diagnoses, with current rates ranging 25%–35%.2 However, the benefits of these diagnoses only apply to RD patients who have access to testing. There is a shortage and maldistribution of clinical genetic services in the United States, with the limited clinical geneticists and genetic counselors (GCs) available being concentrated in large urban academic medical centers, leaving rural communities severely underserved. Telemedicine has been proposed as an avenue to improved access to clinical genetics services; however, state regulations and insurance requirements can pose barriers to or limit implementation of this model, which still requires significant time from geneticists and GCs and thus does not address workforce shortages.4,5 For many rural RD patients seeking care, their main point of contact for healthcare (if any) is their local primary care provider (PCP) who may be the first to identify the patient’s need for a genetics evaluation.6 PCPs represent a major, underutilized, point of access to RD care in underserved populations. To address this, we explored the benefits and limitations of a direct-to-PCP approach through a pilot study in rural Kansas (KS) that relied on the infrastructure of our well-established genomics program.
GA4K program overview
In 2019, the Children’s Mercy Research Institute in Kansas City, Missouri (MO) established a large-scale genomic RD program named “Genomic Answers for Kids” (GA4K). GA4K aims to collect genomic data and health information from 30,000 children and their families, ultimately creating a sharable database of nearly 100,000 genomes while also investing in expanding diagnostic capabilities. Primary recruitment of RD patients has taken place at Children’s Mercy Kansas City (CMKC), covering a population base of 5.2 million. The clinical catchment is primarily in KS and MO but extends to four neighboring Midwest states and referrals from several external institutions. Recruitment has intentionally been broad, limited only to a suspected underlying genetic diagnosis spanning across all subspecialties without exclusions based on prior genetic testing, availability of parental samples, or insurance coverage.7 The main sequencing methods employed have been short-read exome sequencing (srES), short-read genome sequencing (srGS), and long-read GS (lrGS/HiFi-GS, PacBio). Analysis of the first 1,083 RD patients yielded diagnostic rates ranging from 11% in patients with prior negative genetic testing to 34.5% in untested patients. Incorporating srGS/lrGS analyses added up to 13% of new diagnoses in cases previously unsolved by srES, with lrGS (HiFi-GS) yielding an increased discovery rate of novel complex variants, suggesting that more comprehensive sequencing technologies can simplify testing algorithms.8 Research results of clinical significance, as determined by the guidelines set forth by the American College of Medical Genetics,9,10 are confirmed by orthogonal methods at CMKC’s CLIA/CAP-compliant clinical laboratory and immediately returned to the referring providers and the patient’s family to guide medical care. Sequencing of additional patients across platforms is ongoing, having surpassed 4,700 probands sequenced and over 1,370 diagnosed by the end of 2023.
Inequitable access to testing
Bias in recruitment
Despite GA4K’s effort to make genetic testing accessible to more RD patients, we acknowledge significant inequities in access to our study. Evaluation of the distribution of GA4K study participants recruited until December 2020 by demographics (race, ethnicity, payor type) and location (residential address) confirmed that study enrollment underrepresented non-White individuals and socioeconomically disadvantaged pediatric patients both within and beyond CMKC’s service area.11 This is in addition to prior inequities in access to specialty care at CMKC, our most common referral source. Geolocalization utilized to map enrollment patterns across regions also demonstrated clear inequity in enrollment and participation among children from historically segregated and socially disadvantaged communities, with less than 25% of study participants having a home address in a rural zip code and only 5.9% located in frontier and remote area codes. However, 27.7% and 30.5% of the population in KS and MO, respectively, were reported to live in rural areas (US Census 2020), with higher percentages in the other surrounding Midwest states that CMKC serves, suggesting that physical distance is likely one underexplored obstacle in access to specialist care and therefore to genomic testing and research. Our observed inequities mirror known biases in large sequencing projects, which have historically focused on socio-economically advantaged urban and suburban populations, primarily of White European descent,12,13 warranting a conscious call by experts in the field to increase diversity in genomic studies.14 We believe that supporting more diverse recruitment and inclusion beyond just ethnic and racial minorities, such as rural communities, is key to improving accuracy of population frequencies in genomic datasets as well as our understanding of genomic variation.
From local to specialist care: Bottleneck and delays
RD patients from economically disadvantaged, rural communities are disproportionately impacted by geographical barriers. One major reason is that there is a shortage of genetics providers, and most of them work in large urban academic medical centers,6,15 resulting in reduced access to specialist-initiated genetic testing. As such, many RD patients rely on their local PCPs to recognize their need for a genetics workup.3,6 PCPs have expressed concern that they lack sufficient knowledge about most genetic disorders, would not know how to provide pre- and post-test counseling to initiate genetic testing for their patients, and do not have the time required to provide these services within their fast-paced practices.6,16 So understandably, PCPs typically refer their patients to specialists and rely on them to initiate genetic testing, despite knowing the downstream delays and barriers that their patients will likely face.17 This lack of resources and support to community health institutions leads to unspecific and/or unnecessary referrals, which further overwhelm the limited genetics services available and extend delays in access to care for all. Beyond placing patients on long waitlists, these referrals also burden the families to find time, transportation, and additional accommodations to physically reach the initial specialist consult, at which point genetic testing may or may not be initiated depending on basic stratification and/or insurance restrictions.7 All these steps contribute to extending the diagnostic odyssey.
To illustrate this, we assessed molecular diagnostic timelines based on CMKC’s diagnostic service testing. A total of 1,519 patients that had referrals from external institutions (KS/MO) were seen by Clinical Genetics at CMKC between April 2019 and March 2021. Of these, 527 (34.7%) received testing at CMKC’s clinical diagnostic laboratory, with 183 receiving a molecular diagnosis at first-line testing (12% of total externally referred patients; 34.7% of those tested). Of note, 31 were ascertained due to conditions detected by newborn screen (already presumed positive), and an additional 17 received their molecular diagnosis prior to April 2019 and thus were being referred to Genetics for follow-up care. Therefore, the diagnostic yield of new genetic conditions among externally referred patients assessed in this 2-year period was 9.2% (135/1,471). Given a mean turnaround time for testing of 2.7 months (data not shown) and average wait list of 6–12 months, minimal time from referral to diagnosis can be estimated at 8.7 months. This estimate does not account for additional time delays between pre-authorization of test orders and initiation of testing. The number of patients who received a molecular diagnosis upon subsequent testing is not captured, as such data would illustrate even longer delays in diagnosis. We note that the diagnostic rate among externally referred patients was significantly lower (9%–12%) compared to specialist-referred patients (20%–25%) given in part to lower recognition of clinical features enriched in genetics disorders and less extensive prior workup to exclude other causes of disease. Given the additional barriers that rural RD patients face just to initiate genetic testing, remote assistance could help bridge the gap between small rural clinics and large specialist centers for community health dissemination of genetics knowledge and earlier access to genomic testing. While GA4K had invested in educational, counseling, and other supportive resources to referring physicians within CMKC, efforts to reach external PCPs had previously been limited.
A direct-to-provider model to reach rural families
In line with our commitment to seek sustainable interventions to shorten the path to diagnosis for all patients with rare genetic disorders, we designed a model to establish direct partnerships with rural communities, capitalizing on our own genetics expertise while empowering local health providers to deliver the benefits of comprehensive early testing to their patients. We called this a “direct-to(-primary-care)-provider” (DTP) approach, in which we support local PCPs by providing tools to help them recognize patients that would benefit from genomic testing as well as the necessary patient counseling, bypassing the known barriers in traditional access to clinical genetics for assessment and ordering of genetic testing, thereby reaching patients directly in their medical homes. Long-term implementation of a DTP approach should (1) maximize access to genomic medicine in underserved populations, (2) focus limited specialist genetic services on caring for patients with established diagnoses or needing increased support for their undiagnosed condition (beyond first-line testing), and (3) enable earlier interventions, whenever these are available, without the need to visit a large urban academic medical center to qualify. The key to testing this strategy was in doing this within the supported framework of GA4K and with minimal added time and resource pressures for PCPs.
Pilot study: Patients, providers, and project team
To test the DTP model, we partnered with Salina Pediatric Care (SPC) in Salina, KS, for a one-year pilot project (July 2022–June 2023). Salina is located 175 miles from CMKC and has a population of approximately 46,000 individuals, largely rural, with 12% identifying as Hispanic or Latino (US Census 2020). There are approximately 10,600 children in Salina, an estimated 4,000 of whom are served by SPC. This clinic is the main option for pediatric care in the region, with most other children serviced through a family practice office. Participating PCPs were recruited informally by our physician champion (B.Z.) and included physicians, nurse practitioners, and physician assistants; none received participation incentives. We provided simple criteria for referral to the study based on historical data, emphasizing the importance of missed developmental milestones since global developmental delay is the most common and most significantly enriched feature among diagnosed cases at CMKC (data not shown). To better capture the onset of such developmental delays and other early manifestations of pediatric neurologic diseases, we envisioned enrollment would be targeted (though not exclusive) to children ages 6 months to 3 years. Further suspicious presentations, such as unique constellations of congenital anomalies involving multiple organ systems, were also prioritized. Importantly, an in-person visit to our center was not required for enrollment, testing, or return of results. Our GC team was available to SPC providers to help identify additional patients that may be eligible for the study and liaise with CMKC’s clinical team as needed, though this resource was underutilized.
GA4K-supported genomics, from enrollment to return of results
When deemed appropriate, PCPs introduced GA4K to their patients’ families during their clinic visit. If a family expressed interest in enrolling, the provider filled in an electronic nomination form that included basic patient demographics, clinical presentation, and contact information for the patient’s legal guardian(s) and referring provider. Next, the GA4K enrollment team, which included a research coordinator, GC assistants, and a certified GC supervisor, consented the family via phone, with signatures collected electronically. The GA4K enrollment team then coordinated sample collection and shipping at no cost to the family. In most cases, buccal swabs were collected from patients (and family members, if applicable) immediately following their PCP appointment at SPC and mailed to us by SPC staff; otherwise, buccal kits were mailed to patients’ homes with pre-paid materials to mail these back to our center. Upon receipt of the samples, DNA was extracted by standard methods by CMKC’s clinical genetics laboratory to allow for clinical confirmation of research findings downstream. Patient information was added by the enrollment team to PhenoTips, a web-based platform already in use by GA4K that provides a secure way to annotate clinical features and family history, which are crucial in guiding downstream data interpretation.18 PhenoTips also allows for de-identified data sharing with other RD groups for collaborative efforts—a priority of GA4K. Samples were then routed through the already established GA4K research sequencing and analysis pipelines,8 with all samples in this pilot obtaining research srES with sequencing-based copy number variant (CNV) analysis. The analysis team, which included clinically certified laboratory professionals, provided progress updates to the providers, and the GC liaised with SPC providers and patient caregivers for return of results (via phone or telehealth). A clinical report was generated only for positive findings related to the reason of referral (following clinical confirmation by Sanger or qPCR in CMKC’s CLIA-certified laboratory). Variants of uncertain significance (VUSs) deemed compelling candidates were noted internally for follow up if samples from additional family members were to be received for segregation studies or new data were to be obtained (such as functional studies, additional patients identified through publications or GeneMatcher, etc.). Though not encountered in this pilot project, secondary findings with clinical actionability would be reported if the family had specified this in the consent form.
Accelerated diagnoses for rural RD patients
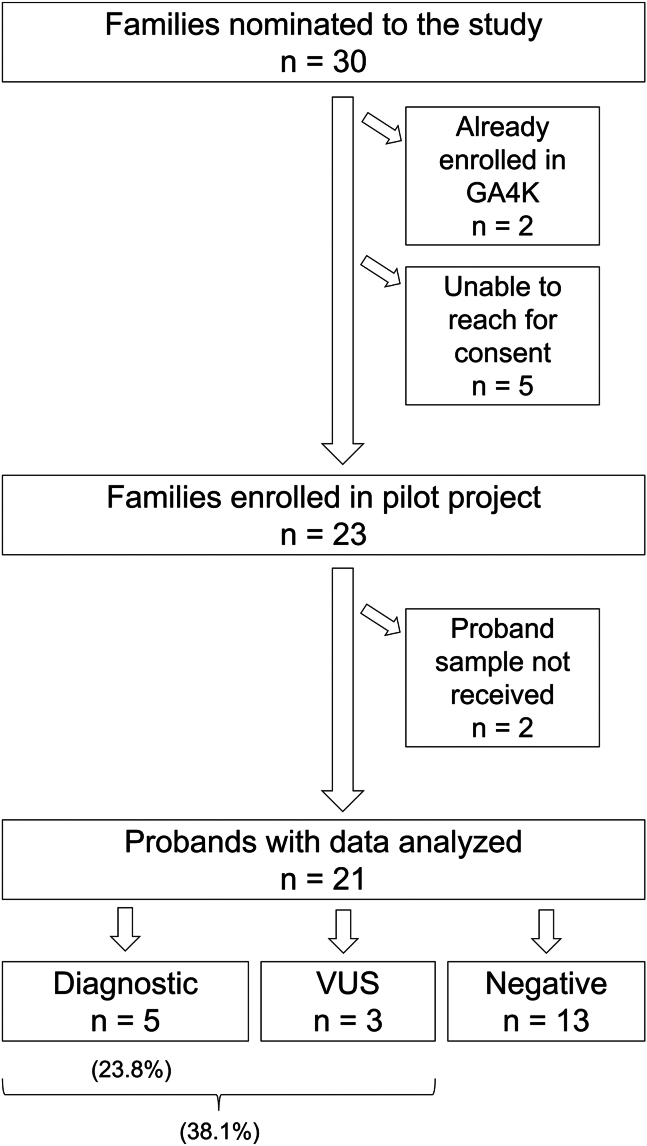
Within the pilot timeline, 30 families were nominated to the study (Figure 1). The enrollment team was unable to reach five of the families, and two had been enrolled previously. Of the 23 families remaining, samples were successfully obtained for 21 probands (Table S1) with variable success in receiving samples from other family members consented. Of these, 15 were male and six were female (∼3:1 ratio). Seventeen were described as White, three as Hispanic (14.3%), and one not specified; these descriptors matched our computational analysis of genetic ancestry using the Somalier tool19 (18 European (EUR)/3 Admixed American (AMR); data not shown). English was listed as the primary language for all. Four of the probands had previously undergone panel testing, which is still a common first line of testing approved by insurance providers despite low yield for most indications.7 We have no knowledge of any prior genetic testing done for the other 17 probands. The most common phenotypic features were global developmental delay (10/21), delayed speech and language development (7/21), and behavioral concern/autism (7/21), as expected based on the recruitment strategy (Table S2). The diagnostic rate was 23.8% (5/21); these findings are summarized in Table 1. Importantly, three of the five diagnostic findings were CNVs, supporting a need for such analysis among previously untested RD patients. One of these CNVs was identified in patient P17 who had previously undergone panel testing that included CNV analysis but not the gene of interest (Table S1). Of note, providers were asked to proceed with standard of care in parallel to study enrollment, and P11 received their diagnosis sooner through commercial testing; it is unknown how many other probands had clinical testing initiated after enrollment in this pilot study. Interestingly, the mean age of probands enrolled was 5.5 years, with three diagnoses found for probands ages 7–16 years, which is much later than our predicted target patient population (0.5–3 years). This finding suggests a potential lack of ascertainment of RDs among underserved populations, particularly past early-intervention years. Time from consent to diagnosis ranged from 9 to 16.5 weeks (mean 13.7 weeks or 3.2 months). Using nomination date as proxy for consult date, mean time from consult to diagnosis only increased by 1.2 weeks (to 14.9 weeks or 3.4 months). The diagnostic rate was higher than historic external referrals (23.8% vs. 9.2%), likely due to absence of prior testing in most patients with predicted enriched features related to neurodevelopmental disorders, and faster than through the traditional referral path, occurring within the first round of data analysis (3–4 months vs. 8–14 months). Three additional probands had a strong candidate VUS, including two that would be upgraded to clinical significance without functional studies if proven de novo, for a potential diagnostic rate at first pass analysis of up to 38.1%. Undiagnosed patients remain in the GA4K research pipeline for further genomic investigations (such as long-read genomes) or may withdraw at any time.
Figure 1.
Pilot study overview from recruitment to diagnosis
Table 1.
Summary of diagnoses identified during the DTP pilot study
| ID# | Proband information | Individuals tested | Diagnostic finding |
|---|---|---|---|
| P8 | 2-year-old male with global developmental delay, motor delay, speech delay | proband + parents (trio) | de novo likely pathogenic variant in TET3 (Beck-Fahrner syndrome) |
| P11 | 10-month-old male with hypotonia and gross motor delay | proband only | pathogenic ∼3.62 Mb duplication at 19p13.3p13.2 |
| P13 | 16-year-old male with global developmental delay and autism | proband + mother | likely pathogenic variant in ZNF462 (Weiss-Kruszka syndrome) |
| P16 | 13-year-old female with global developmental delay, autism, abnormal movements, behavior problems | proband + parents (trio) | pathogenic ∼5.05 Mb terminal deletion at 18p11.32p11.31 and adjacent ∼12.38 Mb duplication at 18p11.31p11.1, suggestive of recombinant chromosome |
| P17 | 7-year-old male with macrocephaly, hypotonia, proximal muscle weakness, gross motor delay, ADHD; prior negative comprehensive muscular dystrophy panel (53 genes) | proband + mother | likely pathogenic ∼83.8 kb deletion at 12q13.2q13.3, which includes SMARCC2 (maternal) |
Project challenges and lessons learned
We encountered some logistic setbacks with implementation of the pilot. Given the geographical distance, only virtual meetings had been planned with providers, and this led to a slower uptake than expected and thus lower recruitment within the available time frame. An in-person visit by the pilot study leader and GA4K GC occurred in early December 2022 and resulted in renewed commitment by providers who subsequently nominated more patients to the study. As such, a one-time site visit at the start of similar projects is recommended for successful engagement of providers. Subsequent communication can then take place by secure email, phone, or video communication, though preferred method must be determined for each provider to avoid delays. PCPs agreed that making nominations to the study required minimal added time and effort, and providers did not identify any additional obvious barriers to study participation. Importantly, most indicated that the GA4K GC or other CMKC clinical genetics professional was needed to provide post-result remote counseling. Additional benefits of a site visit included a more engaged educational session and agreement on placement of study brochures in waiting rooms to empower families to inquire about the study during their appointment (as suggested by a parent consultant on this pilot). Though every clinic will have its specific needs, we expect these lessons will lead to more streamlined communication with future collaborating clinics. On the laboratory side, we created a separate, secure system internally to support prioritization of workflow and tracking of the multiple additional process steps needed for incoming samples from rural clinics.
Patient considerations
At the start of the pilot project, we anticipated that having positive genetic test results in hand would empower the patients’ families to find appropriate follow-up care in an accelerated manner, meaning a reduction in “lost to follow-up” numbers and likely prevention of secondary complications related to delays in establishing appropriate care management. However, we were reminded that patients may receive a diagnosis but remain without access to appropriate care given geographical isolation or lack of means or eligibility. Indeed, though insurance and economic status did not impact eligibility for the study (“upstream” of testing), the GC noted that conversations around economic, travel, and insurance barriers were prominent in the return-of-results sessions with participants. Disappointingly, though we had envisioned that the DTP model would allow routing of RD patients to “downstream” follow-up care and services without an obligatory visit to our urban center, such care was often not readily available and/or not necessarily accessible with a confirmed genetic diagnosis.13,20 All these factors may impact families’ ability to pursue recommended follow-up consultations and/or testing, regardless of how much scheduling and transportation support is provided. This reflects larger systemic problems and can create moral distress for study personnel and healthcare providers.13,21 Although these are beyond the scope of GA4K and would require further qualitative studies for formal assessment, we have taken steps to work more closely with referring providers to support follow-up care while also seeking new partnerships with community groups that may assist families locally by connecting them to additional support services they may need (such as food banks, mental health services, childcare during appointments, advocacy groups, etc.). Despite these post-result challenges, a diagnosis still provides families an answer and the opportunity to find support groups and to receive appropriate counseling for family planning when applicable.2 Most likely, the psychosocial implications of receiving such a diagnosis will vary on a case-by-case basis. As we consider making access to genomic testing more equitable, we must also encourage discussions and research on the implications of this approach on underserved families, as well as the responsibility of researchers toward the patients.
What’s next?
The pilot study allowed us to troubleshoot initial recruitment of underserved rural communities. The same strategy can be applied to additional clinics with the goal of reducing disparities in access to genomic testing at least regionally and shortening diagnostic odysseys overall. This is expected to require translation of recruitment materials into other languages, more extensive telehealth capabilities, and partnering with community advocates to assist locally. To truly seek equity, we must consider likely disparate needs between families and regions, including lack of access to technology and/or internet and lack of physical privacy for remote consent and follow ups (including caretaking responsibilities).22 Altogether, these efforts deploy extensive research resources, and significant modifications to current care delivery systems would be required to implement clinically. Long term, we envision a framework in which “referral rules” could be embedded into patients’ electronic medical record with direct prompts for testing, as currently done for some pharmacogenomic testing prior to initiation of drugs associated with adverse reactions.23 Greater partnership and education between genetic specialists at tertiary care centers and community PCPs would likely be needed to support mutual understanding of resource limitations that more heavily impact rural communities. Contracts to community clinics for genetic counselors supported by tertiary care centers could potentially support telehealth consent, counseling, and consultation with PCPs, though these may be impacted by licensure and billing policies. While such a structure would require genetic counseling resources that are already limited in the workforce, this could be balanced by more targeted and limited referrals for genetics evaluations following a testing-first approach. There may also be a role for continued research-based testing that supports clinical care, as seen more commonly in the oncology field where research participation can both increase access to testing and inform on novel exploratory therapies for a more synergistic approach that can keep up with novel discoveries and bypass possible insurance restrictions.21
Ultimately, data collection from parents and referring DTP PCPs will be needed to formally assess perceived utility of results from a genomics-first approach, challenges in the return-of-results process, barriers to follow-up care, and additional resources needed to support patients and caregivers. Rural stakeholder engagement will be essential to support effective strategies. With expanded studies, we may also be able to gain insight on whether insurance coverage for genetic testing (or lack thereof) represents a more significant barrier to equitable medical care for rural RD patients when compared to our core GA4K population from greater Kansas City. Importantly, we are not capturing RD families that do not have access to PCP care.
With regards to diversity, through our efforts to expand access to genomic testing and research, we expect to continue capturing a subset of the population that is routinely underrepresented in databases. Furthermore, given our commitment to data sharing, we believe the genomic data generated in this program will contribute toward increased representation in RD variation datasets, which in turn should lead to improved data interpretation and reduced VUSs for all. Finally, we call for greater data sharing in RD testing, which remains limited given the large amount of diagnostic testing pursued through commercial laboratories in the United States, including sponsored programs that may impact similarly underrepresented populations.24
Conclusion
In summary, advancing early diagnosis of RDs in rural primary care clinics is promising yet challenging. Our study explored rapid access to testing, coupled with a clinical consulting system to support remote DTP return of genomic results. Expanded recruitment and associated data will facilitate the assessment of real-life impact of this program on testing times and access to medical care of non-urban populations. From the patients’ perspective, the proposed strategy is expected to shorten time to diagnosis but may or may not facilitate access to care. By providing local support to patients at their regular clinics, we can bypass the bottlenecks and disparities of genetics referrals, integrate genomic diagnoses into pediatric primary care with clinical genetics support, and ultimately improve equity in access to genetic diagnoses, care, and research.
Data and code availability
All raw data are being made available through standard controlled access mechanisms in NIH/NCBI dbGAP (phs002206.v4.p1 NHGRI GA4K) and are also being staged at NHGRI/AnVIL. Processed data for rare variants, de-identified pedigrees and coded phenotypes are available to registered users through a cloud-hosted PhenoTips web UI (https://phenotips-ga4k.cmh.edu/). Access inquiries for investigators should be directed to GA4k@cmh.edu.
Acknowledgments
We would like to thank the families for participating in our study. We also thank Kelly Baesel-Freund for serving as our parent consultant in the pilot project and the providers at Salina Pediatric Care for their collaboration. This work was made possible by the generous gifts to Children’s Mercy Research Institute and the Genomic Answers for Kids program at Children’s Mercy Kansas City. The pilot project for rural expansion was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002366; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All studies were approved by the Children’s Mercy Institutional Review Board (IRB) (study # 11120514; pilot study # 00002314). Informed written consent was obtained from all participants prior to study inclusion.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2024.03.016.
Supplemental information
References
- 1.Nguengang Wakap S., Lambert D.M., Olry A., Rodwell C., Gueydan C., Lanneau V., Murphy D., Le Cam Y., Rath A. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur. J. Hum. Genet. 2020;28:165–173. doi: 10.1038/s41431-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marwaha S., Knowles J.W., Ashley E.A. A guide for the diagnosis of rare and undiagnosed disease: beyond the exome. Genome Med. 2022;14:23. doi: 10.1186/s13073-022-01026-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraiman Y.S., Wojcik M.H. The influence of social determinants of health on the genetic diagnostic odyssey: who remains undiagnosed, why, and to what effect? Pediatr. Res. 2021;89:295–300. doi: 10.1038/s41390-020-01151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubendran S., Sivamurthy S., Schaefer G.B. A novel approach in pediatric telegenetic services: geneticist, pediatrician and genetic counselor team. Genet. Med. 2017;19:1260–1267. doi: 10.1038/gim.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye C., Bodurtha J., Edick M., Ginsburg S., Keehn A., Lloyd-Puryear M., Doyle D.L., Lyon M., Ostrander R., Taylor M., National Coordinating Center for the Regional GeneticService Collaboratives Regional Support Service Model Workgroup and AdvisoryCommittee Regional models of genetic services in the United States. Genet. Med. 2020;22:381–388. doi: 10.1038/s41436-019-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou A.F., Duncan A.R., Hallford G., Kelley D.M., Dean L.W. Barriers and strategies to integrate medical genetics and primary care in underserved populations: a scoping review. J. Community Genet. 2021;12:291–309. doi: 10.1007/s12687-021-00508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zion T.N., Berrios C.D., Cohen A.S.A., Bartik L., Cross L.A., Engleman K.L., Fleming E.A., Gadea R.N., Hughes S.S., Jenkins J.L., et al. Insurance denials and diagnostic rates in a pediatric genomic research cohort. Genet. Med. 2023;25 doi: 10.1016/j.gim.2023.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen A.S.A., Farrow E.G., Abdelmoity A.T., Alaimo J.T., Amudhavalli S.M., Anderson J.T., Bansal L., Bartik L., Baybayan P., Belden B., et al. Genomic answers for children: Dynamic analyses of >1000 pediatric rare disease genomes. Genet. Med. 2022;24:1336–1348. doi: 10.1016/j.gim.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs E.R., Andersen E.F., Cherry A.M., Kantarci S., Kearney H., Patel A., Raca G., Ritter D.I., South S.T., Thorland E.C., et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen) Genet. Med. 2020;22:245–257. doi: 10.1038/s41436-019-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane N.J., Cohen A.S.A., Berrios C., Jones B., Pastinen T., Hoffman M.A. Committing to genomic answers for all kids: Evaluating inequity in genomic research enrollment. Genet. Med. 2023;25 doi: 10.1016/j.gim.2023.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolan D.D., Cho M.K., Lee S.S.J. Innovating for a Just and Equitable Future in Genomic and Precision Medicine Research. Am. J. Bioeth. 2023;23:1–4. doi: 10.1080/15265161.2023.2215201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galasso I. Precision Medicine for Whom? Public Health Outputs from "Genomics England" and "All of Us" to Make Up for Upstream and Downstream Exclusion. Am. J. Bioeth. 2024;24:71–85. doi: 10.1080/15265161.2023.2180108. [DOI] [PubMed] [Google Scholar]
- 14.Green E.D., Gunter C., Biesecker L.G., Di Francesco V., Easter C.L., Feingold E.A., Felsenfeld A.L., Kaufman D.J., Ostrander E.A., Pavan W.J., et al. Strategic vision for improving human health at The Forefront of Genomics. Nature. 2020;586:683–692. doi: 10.1038/s41586-020-2817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins B.D., Fischer C.G., Polito C.A., Maiese D.R., Keehn A.S., Lyon M., Edick M.J., Taylor M.R.G., Andersson H.C., Bodurtha J.N., et al. The 2019 US medical genetics workforce: a focus on clinical genetics. Genet. Med. 2021;23:1458–1464. doi: 10.1038/s41436-021-01162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikat-Stevens N.A., Larson I.A., Tarini B.A. Primary-care providers' perceived barriers to integration of genetics services: a systematic review of the literature. Genet. Med. 2015;17:169–176. doi: 10.1038/gim.2014.101. [DOI] [PubMed] [Google Scholar]
- 17.Truong T.K., Kenneson A., Rosen A.R., Singh R.H. Genetic Referral Patterns and Responses to Clinical Scenarios: A Survey of Primary Care Providers and Clinical Geneticists. J. Prim. Care Community Health. 2021;12 doi: 10.1177/21501327211046734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girdea M., Dumitriu S., Fiume M., Bowdin S., Boycott K.M., Chénier S., Chitayat D., Faghfoury H., Meyn M.S., Ray P.N., et al. PhenoTips: patient phenotyping software for clinical and research use. Hum. Mutat. 2013;34:1057–1065. doi: 10.1002/humu.22347. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen B.S., Bhetariya P.J., Brown J., Kravitz S.N., Marth G., Jensen R.L., Bronner M.P., Underhill H.R., Quinlan A.R. Somalier: rapid relatedness estimation for cancer and germline studies using efficient genome sketches. Genome Med. 2020;12:62. doi: 10.1186/s13073-020-00761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman S.L., Brown J.E.H., Zamora A., Outram S. "I Have Fought for so Many Things": Disadvantaged families' Efforts to Obtain Community-Based Services for Their Child after Genomic Sequencing. AJOB Empir. Bioeth. 2023;14:208–217. doi: 10.1080/23294515.2023.2209747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasquez E.E., Foti N., McMahon C.E., Jeske M., Bentz M., Fullerton S., Shim J.K., Lee S.S.J. Rethinking Benefit and Responsibility in in the Context of Diversity: Perspectives from the Frontlines of Precision Medicine Research. Public Health Genomics. 2023;26:103–112. doi: 10.1159/000531656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastin M., Odgis J.A., Suckiel S.A., Bonini K.E., Di Biase M., Brown K., Marathe P., Kelly N.R., Ramos M.A., Rodriguez J.E., et al. The TeleKidSeq pilot study: incorporating telehealth into clinical care of children from diverse backgrounds undergoing whole genome sequencing. Pilot Feasibility Stud. 2023;9:47. doi: 10.1186/s40814-023-01259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker C.I.S., Groeneweg G., Maitland-van der Zee A.H., Rieder M.J., Hawcutt D.B., Hubbard T.J., Swen J.J., Carleton B.C. Pharmacogenomic testing in paediatrics: Clinical implementation strategies. Br. J. Clin. Pharmacol. 2022;88:4297–4310. doi: 10.1111/bcp.15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson S.A., Liu J., Flannery D., Michie M., Ford P.J. The cost of 'free': Advising patients about sponsored genetic testing. Cleve. Clin. J. Med. 2023;90:161–164. doi: 10.3949/ccjm.90a.22010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data are being made available through standard controlled access mechanisms in NIH/NCBI dbGAP (phs002206.v4.p1 NHGRI GA4K) and are also being staged at NHGRI/AnVIL. Processed data for rare variants, de-identified pedigrees and coded phenotypes are available to registered users through a cloud-hosted PhenoTips web UI (https://phenotips-ga4k.cmh.edu/). Access inquiries for investigators should be directed to GA4k@cmh.edu.