Abstract
This review delves into the groundbreaking impact of induced pluripotent stem cells (iPSCs) and three-dimensional organoid models in propelling forward neuropathology research. With a focus on neurodegenerative diseases, neuromotor disorders, and related conditions, iPSCs provide a platform for personalized disease modeling, holding significant potential for regenerative therapy and drug discovery. The adaptability of iPSCs, along with associated methodologies, enables the generation of various types of neural cell differentiations and their integration into three-dimensional organoid models, effectively replicating complex tissue structures in vitro. Key advancements in organoid and iPSC generation protocols, alongside the careful selection of donor cell types, are emphasized as critical steps in harnessing these technologies to mitigate tumorigenic risks and other hurdles. Encouragingly, iPSCs show promising outcomes in regenerative therapies, as evidenced by their successful application in animal models.
Keywords: iPSCs, organoids, Alzheimer’s disease, epilepsy, Parkinson’s disease, spinal cord injury
1. Introduction
The investigation of neuropathologies frequently encounters challenges in establishing suitable study models. Neurodegenerative disorders like Alzheimer’s disease (AD) pose significant hurdles due to their complex interplay of extracellular and intracellular factors. For instance, understanding the immune response of patients to pathological protein aggregates like Aβ plaque, the distribution of intracellular neurofibrillary tangles (NTF), and the density of neuritic plaques proves particularly intricate [1]. In neurodevelopmental disorders, delving into the early stages of the disease poses a particular challenge when attempting to replicate it in cellular and animal models. This complexity is evident in conditions like temporal lobe epilepsies [2,3]. Conversely, when it comes to tissue injuries such as spinal cord injury (SCI) and strokes, animal models predominantly serve as the primary investigative tool, while treatment emerges as the principal obstacle [4,5]. Across all these scenarios, there exists a shared objective in exploring the potential for developing disease-specific models or therapeutic interventions.
Induced pluripotent stem cells (iPSCs) were first generated from mouse fibroblasts [6]. Subsequent studies confirmed the ability to differentiate these cells into the main neural cell types, including neurons, astrocytes, and oligodendrocytes [7]. The interest in the use of iPSCs in the field of neuropathology is due to the modeling and treatment of rare and genetically altered diseases. So iPSCs have presented the possibility for cell patient-derived disease modeling, presenting somatic mutations of interest and simulating disease phenotypes, such as AD [8]. In addition, iPSCs also present therapeutic potential in the field of regenerative therapy [9]. In this concise review, we employ a deliberate review methodology and emphasize experimental models for neuropathologies utilizing iPSCs, with a particular focus on organoid models and the prospective utilization of these cells in therapy and drug-screening pipelines.
2. Generation and Characterization of iPSCs
Self-renewal and pluripotency are fundamental attributes of embryonic stem cells (ESCs). Self-renewal refers to the remarkable ability to continuously proliferate without committing to a specific cell fate when cultured in vitro. Pluripotency, on the other hand, indicates the remarkable potential to differentiate into various cell types derived from the three primary embryonic germ layers [10,11]. The generation of iPSCs signifies a remarkable achievement in cellular reprogramming, providing a unique avenue to leverage the regenerative capabilities of pluripotent cells.
The formation of iPSCs was initially showcased in the groundbreaking research of Shinya Yamanaka and his team, who pinpointed a set of transcription factors capable of converting adult somatic cells into a pluripotent state. These factors, namely, Oct4, Sox2, Klf4, and c-Myc (OSKM), have since become synonymous with iPSC reprogramming [6,12]. In recent years, iPSCs have been employed across various applications, such as autologous cell therapy [13], experimental disease modeling [14,15], and as platforms for drug discovery and therapeutic screening [16]. In recent years, a wide range of protocols for iPSC generation has emerged. In the sections below, we summarize the key differences in reprogramming techniques employed for iPSC generation.
2.1. Which Cells Can Be Reprogrammed?
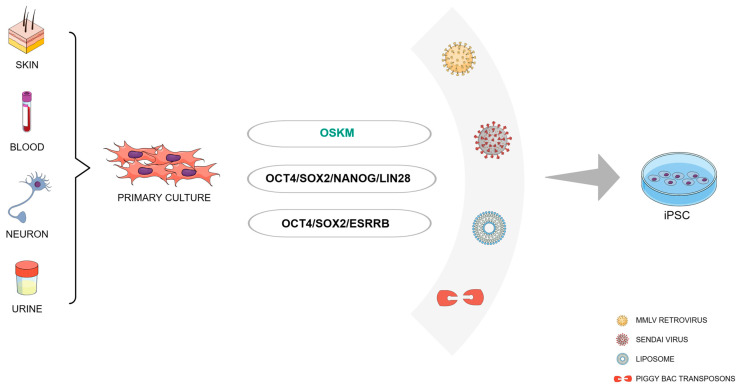
Somatic cells are specialized cells with distinct functions, such as skin cells, blood cells, or nerve cells. The concept of reprogramming posits that virtually every somatic cell in the human body harbors the potential to undergo transformation into an iPSC (Figure 1). Despite this, the efficiency and kinetics of reprogramming can vary depending on the donor cell type. Fibroblasts from both mice and humans continue to be the most prevalent cell types utilized for experimental reprogramming purposes [17]. However, in recent years, there has been a growing interest in other cell types due to their availability, therapeutic significance, and reprogramming simplicity. An example of this is CD133+ cord blood cells, which necessitate only OCT4 and SOX2 for iPSC generation [18]. Human primary keratinocytes exhibit the capability to undergo reprogramming at a rate twice as fast and with 100 times greater efficiency compared to fibroblasts [19]. Additionally, these cells offer the advantage of being obtainable through the cultivation of plucked hair from patients [20].
Figure 1.
Primary cultures can be derived from various tissues including skin, blood, neurons, and urine. Cellular reprogramming for iPSC generation can be conducted using different combinations of transcription factors, with the Yamanaka factors (OSKM) remaining the most commonly employed. Similarly, there are diverse tools available to facilitate cell transfection for reprogramming, such as MMLV, SENDAI virus, liposome, and transposons.
Urine samples also serve as a readily accessible source of cells that can be reprogrammed into iPSCs. Both renal tubular cells and exfoliated renal epithelial cells found in urine have demonstrated successful reprogramming into human iPSCs (hiPSCs) [21,22]. Functional cardiomyocytes derived from urinary cells within the cardiovascular system have displayed the ability to generate action potentials. This phenomenon has been observed both in vitro and in vivo following the differentiation of reprogrammed induced pluripotent stem cells (iPSCs) via lentiviral vector gene transduction [23,24].
Another relevant cell source for reprogramming is blood. Peripheral blood mononuclear cells (PBMCs) can be easily isolated from blood samples and stand as one of the most popular somatic cell sources for iPSC generation [25]. Both terminally differentiated mature B and T lymphocytes were able to generate iPSCs after reprogramming protocol [26,27,28], although reprogrammed T cells have been shown to induce spontaneous T cell lymphomas in mice, limiting the therapeutic applications of these cells [29]. Thus, depending on the goal, it is recommended that protocols be used to eliminate lymphocytes from the PBMCs [30]. An advantage of selecting PBMCs as the donor cells for reprogramming is that these cells can be cryopreserved and reprogrammed at a later time without compromising the kinetics and efficiency of reprogramming [31], thus enabling the utilization of frozen samples stored in blood banks worldwide [32]. It is important to highlight that several ethical, legal and social issues coexist with the availability of biological samples to researchers. Research ethics committees around the world have used policies to protect the interests of research participants, such as confidentiality, ownership, export, storage and secondary use of samples with due consent, obeying specific regulations that differ according to each country [33].
Cell type, degree of differentiation, and maturation level all exert an influence on iPSC generation efficiency. In mice, it has been documented that immature cells undergo reprogramming more readily than terminally differentiated cells [34]. Additionally, a separate study showed that hematopoietic stem and progenitor cells can yield up to 300 times more iPSC colonies compared to terminally differentiated lymphocytes [35]. Hence, selecting the appropriate cell type is a pivotal consideration prior to commencing any experiment. This decision typically hinges on cell accessibility and impacts the need for external factors, as well as the efficiency and kinetics of reprogramming.
2.2. The Reprogramming Recipe
The reprogramming factors, OSKM, remain the most commonly utilized method for generating iPSCs. However, in recent years, numerous alternatives have been described to refine reprogramming protocols. These include exploring other transcription factor combinations and introducing new molecules aimed at enhancing the efficiency of iPSC generation.
Given that the molecules Klf4 and c-Myc are classified as proto-oncogenes, researchers have sought substitute candidates to mitigate potential tumorigenic risks linked with these molecules. One of the initial alternative reprogramming methods was outlined by Yu and colleagues, who utilized Oct4 and Sox2 in conjunction with Nanog and Lin28. This approach resulted in a reprogramming efficiency akin to that achieved with Yamanaka’s OSKM combination [36]. An approach to reprogram mouse embryonic fibroblasts (MEFs) into iPSCs using three factors has also been developed [37]. This technique involved Oct4, Sox2, and the orphan nuclear receptor Esrrb, achieving similar efficiency compared to the OSKM protocol. Subsequent studies have further refined strategies to reprogram cells using only two transcription factors, including various combinations of Oct3/4, Sox2, Klf4, and c-Myc [38,39,40,41,42] (Figure 1).
To enhance efficiency and expedite iPSC generation, additional transcription factors have been explored for their potential to induce cellular reprogramming. Many of these factors are genes typically active during early development, playing a pivotal role in maintaining the pluripotent potential of specific cells that ultimately form the inner cell mass (ICM) in the pre-implantation embryo and contribute to embryonic development. Among the core pluripotency transcription factors are Oct4, Sox2, and Nanog. Co-expression of Nanog with the OSKM factors has been shown to halve the time required for colony appearance compared to OSKM alone [43]. Other pluripotency transcription factors, such as UTF1 and SALL4, have also been found to enhance iPSC generation when co-expressed with the OSKM factors [44,45]. Building on these findings, James Thomson devised a novel combination of transcription factors, incorporating Oct4, Sox2, Nanog, and Lin28 (OSNL), also referred to as the “Thomson factors” [36]. Additionally, Yu and colleagues explored various combinations of transcription factors, including Oct4-Sox2-Nanog-Klf4 and Oct4-Sox2-SV40LT-Klf4, to generate iPSCs [46]. These studies demonstrate that beyond the initially described OSKM factors, multiple pluripotency-related genes can be employed in different combinations to activate the pluripotency genetic program and induce cellular reprogramming into iPSCs.
Other molecules known to regulate cellular processes critical for iPSC generation and maintenance have also been employed to enhance reprogramming efficiency. Proteins that promote proliferation, such as telomerase reverse transcriptase (TERT) and the SV40 large T antigen (SV40LT), have been shown to increase colony formation when combined with OSKM [47]. Chemical compounds that positively regulate cell cycle progression, such as mitogen-activated protein kinase kinase (MAPKK) inhibitors, have also been demonstrated to increase the number of iPSC colonies obtained from reprogrammed neural precursor cells [48]. MicroRNAs (miRNAs) are also known to influence pluripotency and reprogramming [49], and several miRNAs have been tested for their capacity to increase iPSCs generation. Among these, several miRNAs from the miR-290 cluster were able to increase the number of colonies following reprogramming compared to cells using the OSKM factors alone [50]. These miRNAs are believed to be downstream effectors of c-Myc signaling but induce a population of iPSCs that is more homogeneous compared to c-Myc [51]. Numerous cells signaling pathways are regulated by miRNAs, and their potential effects on iPSCs production have been extensively reviewed elsewhere [52,53].
Encouragement for exploring safer reprogramming methods has stemmed from concerns regarding potential genetic alterations and the heightened risk of tumorigenesis associated with integrative reprogramming strategies. It has been proposed that small chemical molecules could regulate gene expression, thereby promoting the pluripotency program without inducing changes in the cell genome. The first chemically induced iPSCs were generated by replacing Oct4 with the small molecule forskolin [54]. A small-molecular combination known as “VC6T”, comprising valproic acid (an HDAC inhibitor), CHIR99021 (a GSK3 inhibitor), E-616452 (a TGF-β inhibitor), and tranylcypromine (an LSD1 inhibitor), facilitated the reprogramming of mouse cells with only Oct4 being genetically induced [55]. Recently, Guan et al. introduced another protocol for generating iPSCs solely through chemical molecules to reprogram human fibroblasts [56]. They highlighted the importance of an initial intermediate state of dedifferentiation for the small molecules to facilitate the complete reprogramming phase [56].
Another promising approach involves employing a variation in CRISPR technology to express the OSKM factors. This method utilizes a modified form of the Cas9 enzyme with inactive nuclease activity (dCas9), fused to either repressive or activating transcriptional domains. This enables CRISPR-mediated transcriptional interference (CRISPRi) or activation (CRISPRa), respectively [57]. Therefore, CRISPRa allows for the activation of gene expression in one or a specific group of genes.
Recently, CRISPRa has been successfully used to reprogram human skin fibroblasts and human leukocytes into iPSCs [58,59]. These studies have illustrated that CRISPRa represents an advanced method for iPSC generation, enabling faster and more efficient reprogramming compared to conventional protocols [58,59]. Moreover, single-cell RNA sequencing (scRNA-seq) analyses have revealed that cells reprogrammed using CRISPRa transition to the pluripotent state with high fidelity, displaying the uniform expression of pluripotency genes and minimal heterogeneity [59].
The versatility of the CRISPRa technique has also been described in direct reprogramming models, where mouse fibroblasts were converted into neurons through the induction of a single transcription factor [60,61]. These studies underscore that with an understanding of the genes essential for converting one cell type into another, the CRISPRa technique emerges as a reliable tool for executing this reprogramming process. This method holds the potential for achieving higher efficiency at a reduced cost and within a shorter timeframe.
Epigenetic modifications, encompassing DNA methylation and histone modifications, govern gene expression without altering the underlying DNA sequence. Throughout iPSC reprogramming, these epigenetic marks are reset to resemble those characteristic of embryonic stem cells (ESCs) [62,63]. This resetting of the epigenetic landscape is crucial for the successful conversion of differentiated cells into pluripotent stem cells [62]. Hence, the application of chemical molecules that regulate DNA methylation or chromatin modifications can enhance reprogramming in numerous cell types [64,65,66]. Treatment with histone deacetylase (HDAC) inhibitors, such as hydroxamic acid (SAHA), trichostatin A (TSA), valproic acid (VPA), and butyrate, has been shown to improve reprogramming in mouse embryonic fibroblasts (MEFs) [64,67,68]. Additionally, VPA has induced pluripotency in dermal fibroblasts and neonatal human foreskin fibroblasts (HFFs) when combined with Oct4 and Sox2 [69].
In summary, the identification of molecules that promote pluripotency and sustain stem cell states is paramount, given the relatively low success rates observed in current iPSC generation protocols. Taking into account a cell’s transcriptome and epigenetic profile is critical for selecting suitable molecules, thereby ensuring that the reprogramming process yields a sufficient number of pluripotent cell colonies.
2.3. Reprogramming Factor Delivery Systems
Originally, the OSKM transcription factors have been delivered into mouse and human fibroblasts using Moloney murine leukemia virus (MMLV)-derived retroviruses [70,71]. Subsequently, reprogramming was also reported using Lentivirus-based vectors [71]. Lentiviral vectors, typically derived from HIV, offer a higher cloning capacity and the ability to infect both dividing and non-dividing cells, often resulting in higher infection efficiency rates compared to MMLV-based models [72]. Additionally, Tet-inducible lentiviruses for reprogramming allow for the controlled expression of reprogramming factors [73]. However, despite achieving acceptable efficiency, their integration into the host genome has raised safety concerns.
Since then, a variety of new delivery systems have emerged, utilizing non-integrative viral vectors such as Sendai virus and adenovirus, as well as non-viral methods including liposomes and vectors based on piggyBac transposon. [74]. (Figure 1).
General delivery systems employed in iPSCs reprogramming have been extensively reviewed elsewhere [72,73,74]. Each delivery method presents both advantages and limitations, making the selection of an appropriate delivery system an important issue to resolve before proceeding to reprogram somatic cells into iPSCs.
3. Organoid Models
What They Are, How They Work, and Applications
An organoid is defined as a cell culture in a 3D structure obtained from stem cells (embryonic or reprogrammed) that after induction differentiation must consist of organ-specific cell types that self-organize. These structures are designed and function in a manner that leads to the expression of characteristics akin to various tissues and organs, including the kidneys, lungs, intestines, brain, and retina, as demonstrated in numerous studies [75,76] (Figure 2). Three-dimensional organoids are formed from human pluripotent stem cells (hPSCs), such as iPSCs and ESCs [77]. In the realm of organoids derived from iPSCs, there exists the potential to harness the combination of self-organization and differentiation capacity through genetic tools. This enables the guidance of these cells and structures toward any specific organ, a capability that could prove fundamental in the treatment of diseases [78]. In a more practical sense, organoids hold immense potential for application in advanced therapies such as organ repair through transplantable structures, as well as in drug studies. Moreover, they serve as invaluable tools for understanding the pathological mechanisms underlying certain diseases [79].
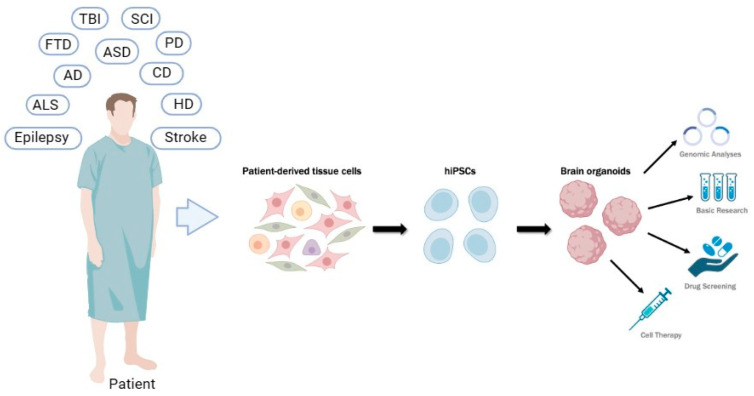
Figure 2.
Organoids can be produced from iPSC cultures using three-dimensional cultivation techniques. Derived from iPSC cultures, these organoids can mimic various organ-specific tissues, serving as invaluable models for studying a wide range of diseases. Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), autism spectrum disorder (ASD), Canavan disease (CD), epilepsy, frontotemporal dementia (FTD), Huntington’s Disease (HD), Parkinson’s disease (PD), spinal cord injury (SCI), stroke, and traumatic brain injury (TBI).
Concerning the utilization of iPSC organoids for transplantation therapies, various protocols have been developed, primarily focusing on neural differentiation to address neurological disorders like Alzheimer’s disease, Parkinson’s disease, and epilepsy (Figure 2). In this context, integrating genome-editing techniques via the CRISPR/Cas9 system can aid in modulating gene expression, thus presenting a promising therapeutic avenue for these diseases [80].
In the field of neuroscience, neural organoid models have become essential for studying various aspects of the brain, particularly neurodegenerative diseases. Their significance lies in their ability to replicate key characteristics of human brain development, which cannot be thoroughly analyzed using animal models alone [77,78]. The application of organoids enabled the formation of “mini-brains” with very specialized zones and structures such as radial glial cells and cerebral cortex, to model human microcephaly [81,82,83].
This methodology has progressed to the point where it is now feasible to generate highly specialized cells such as oligodendrocytes and astrocytes, and subsequently, to develop even more specialized structures in the advanced stages of neural development [84,85]. n addition to 3D organoid models, 2D models are also utilized to study brain structures, as these models facilitate the formation of neural networks [82]. However, 3D models stand as superior candidates for studying and treating neurological diseases [80].
4. Organoid Models in Neurological Diseases
The generation of organoids for studying neurological diseases has been presented as an important strategy for neuropathologies of different orders. Table 1 presents the main examples of neurological diseases studied from the generation of organoids.
4.1. Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disorder marked by gradual memory loss, cognitive impairment, and a decline in the ability to perform daily tasks independently, ultimately becoming the leading cause of dementia [86]. The pathology of AD is characterized by the accumulation of Tau protein and the formation of amyloid beta plaques, which lead to structural changes in the brain, resulting in neuronal destruction and synaptic impairment. As a result, the molecular profile of AD is typified by the presence of amyloid beta and phosphorylated tau protein.
Another significant aspect of AD is its multifactorial nature, with a notable genetic component, where the apolipoprotein E (APOE) gene and its alleles (APOE2, APOE3, and APOE4) serve as risk factors for Alzheimer’s disease [87]. Specifically, the APOE4 allele has been strongly associated with the presence, heritability, and progression of AD [88]. Various experimental approaches, including in vivo, in silico, and in vitro methods, have been employed to comprehend and potentially treat AD. However, none have been able to fully replicate the pathological features observed in the human AD brain, despite the significant progress and promising outcomes achieved [89]. Currently, there are three commonly used methods to model AD in cerebral organoids:
Aftin-5 (Aβ42 agonist): In this model, there is an induction of APP amyloid precursor protein (Aβ) using Aftin-5 (an Aβ42 inducer that increases the production and secretion of soluble extracellular amyloid peptides). Aftin-5 treatment leads to a reproducible disruption of the physiological balance between Aβ42 and Aβ40, generating an AD-like condition in human cerebral organoids [90].
Organoids derived from familial AD (FAD): This model was established by generating iPSC-derived brain organoids from patients with familial Alzheimer’s disease (FAD) carrying APP duplications or mutations in the presenilin 1 (PSEN1) gene. Such a model can effectively recapitulate the crucial aspects of the pathology, including the presence of amyloid beta plaques and tau protein accumulation. Moreover, it provides insight into the temporal dynamics of P-tau level increases, contributing to a more comprehensive understanding of disease progression [91,92]. Another variation of this same model is the use of stem cells from patients carrying a missense mutation in the PSEN1 gene linked to early-onset AD. In this case, the cerebral organoids exhibit the same Aβ and P-tau protein aggregates as the previous model [93,94].
Model with APOE3 allele: Due to the significant association between the APOE gene and its impact on AD, organoid models with induced mutations in this gene have been developed. This model involves employing gene-editing techniques such as CRISPR/Cas9 to convert APOE3 to APOE4 in iPSCs derived from patients with sporadic Alzheimer’s disease. This is because the APOE4 variant exerts a stronger genetic influence on AD compared to other variants. In this study, it was observed that APOE4 neurons exhibited an increased number of synapses and an increased secretion of Aβ42 compared to APOE3 cells [95].
4.2. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by the gradual degeneration of motor neurons in the brain and the spinal cord. This degeneration leads to progressive muscle weakness, ultimately culminating in paralysis and, in many cases, death. ALS can manifest in individuals as early as their first or second decade of life, although it can also onset at later ages [96]. ALS typically starts with a localized impact and gradually spreads, resulting in symptoms that initially manifest as mild cramps or weakness in the limbs or bulbar muscles. Over time, these symptoms progress to the paralysis of nearly all skeletal muscles [97]. In terms of prevalence, the majority of ALS cases are sporadic, accounting for approximately 90% of cases, while only about 10% are hereditary, known as familial ALS (FALS). Family genes implicated in ALS are involved in various mechanisms, including proteostasis, RNA binding, and axonal transport. Specifically, genes such as SOD1, TDP-43, and PFN1 are associated with these processes, respectively [98]. One study utilized iPSC lines derived from both healthy controls and individuals affected by ALS. These iPSC lines were used to generate organoids comprising sensory neurons, motor neurons, astrocytes, and other mesodermal derivatives, including vasculature, microglia, and skeletal muscle. Interestingly, motor neurons derived from organoids of ALS-affected patients exhibited compromised Neuromotor Junctions (NMJs). Additionally, iPSC lines carrying mutations in the TARDBP, SOD1, and PFN1 genes were generated to validate the model further, confirming once more the impairment in NMJs [99].
4.3. Attention Deficit Hyperactivity Disorder
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder distinguished by symptoms of inattention, impulsivity, and hyperactivity. These symptoms typically manifest in early childhood and may persist even in adulthood [100,101]. ADHD compromises cognitive, behavioral, and affective domains of attention processing and executive function, response inhibition and impulsive behavior, and hyperactivity [101]. The integration of clinical, neurodevelopmental, and cognitive aspects is crucial in understanding the symptoms of ADHD. Research indicates that structural alterations in the cerebral cortex among individuals with ADHD impact cognitive processes including emotion regulation, response inhibition, and attention [102,103]. Organoid models offer a valuable approach to elucidate various aspects of neurodevelopmental disorders such as ADHD [104]. In a particular study, iPSC-derived telencephalon organoids from individuals with ADHD were utilized. Notable differences were observed between organoids derived from individuals with ADHD and those from the control group. Specifically, the ADHD group exhibited reduced cell proliferation and differentiation, along with disparities in the proportion of symmetric and asymmetric cell division. These findings suggest that the observed alterations may play crucial roles in the pathogenesis of ADHD [105].
4.4. Autism Spectrum Disorder
Autism spectrum disorder (ASD) represents a set of neurodevelopmental disorders with defined behavioral implications. ASD is characterized by a series of conditions, such as persistent deficits in social communication, interaction and repetitive patterns of behaviors, interests, and activities [106]. The etiopathogenesis is multifactorial with complex interactions between genetic and environmental factors [107]. ASD typically manifests in infancy or early childhood, arising from the interplay of genetic and non-genetic factors, which may act independently or in concert to contribute to its development. Over the past two decades, there has been a progressive increase in the prevalence of autism spectrum disorders (ASDs) [108]. ASD is notably more prevalent in men than in women, with data from the 2016 ADDM network indicating that ASD occurs approximately four times more frequently in men [109]. According to the DSM-5 criteria, the diagnosis of ASD requires the presence of at least two typical behavior patterns. Examples of these behaviors include repetitive motor movements, insistence on sameness or adherence to routines, echoing others’ words, hand flapping, finger snapping, fixation on specific interests, and intense attachment to particular objects [110,111]. Various factors contribute to the prevalence of ASD, including maternal factors such as advanced maternal age, obesity, diabetes mellitus, and maternal infection during pregnancy. Perinatal and neonatal factors such as umbilical cord complications, fetal distress, low birth weight or being small for gestational age, congenital malformations, and hyperbilirubinemia are also implicated. Extensive research aimed at unraveling the genetic mechanisms of ASD has identified over 800 genes associated with the condition. Additionally, hundreds of chromosomal aberrations and dozens of syndromes have been recognized. The etiology of ASD involves a complex interplay between genetic inheritance and environmental factors, which are further influenced by epigenetic processes [112]. The utilization of iPSC models and iPSC-derived organoids has been instrumental in advancing our understanding of the genetic and neurochemical mechanisms underlying ASD, as well as in exploring potential treatments for the condition. One of the pioneering studies in this field involved the generation of iPSC-derived brain organoids from individuals affected by idiopathic ASD [113]. The study revealed abnormalities in the proliferation and maturation of neurons, along with an upregulation in the expression of the transcription factor FOXG1, which correlated with the heightened production of inhibitory neurons. These findings underscore the significance of the FOXG1 gene and propose it to be a promising therapeutic target for the management of ASD [107,113]. The application of organoid iPSC models from patients with ASD made it possible to understand the mechanics and influence of genes in the etiopathogenesis of ASD. Several genes have been related to chromatin remodeling and gene transcription (MECP2, MEF2C, HDAC4, CHD8, and CTNNB1) and synaptic functions (GRIN2B, CACNA1, CACNA2D3, SCN2A, GABRA3, and GABRB3) [114]. Moreover, the utilization of cerebral organoids in ASD research has unveiled various phenotypes linked to genetic variations, impacting transcriptional pathways, morphological features, and the development of neuronal networks [115,116]. The utilization of iPSCs provides unique opportunities to investigate brain development and the ramifications of its dysregulation in neurodevelopmental disorders such as ASD. By incorporating the patient’s genetic makeup, organoid models enable the modeling of ASD while preserving the individual’s genetic background and mimicking the 3D structural complexities of the brain. Furthermore, an additional study delved into the impact of a single mutation in autism spectrum disorders (ASDs) using brain organoids. Transcriptomic analysis of these organoids, harboring a mutation in the CHD8 gene, unveiled the differential expression of key genes such as DLX6-AS1 and DLX1, which are associated with interneuronal differentiation in the CHD8 knockout organoids [117].
4.5. Canavan Disease
Canavan disease (CD) is a rare leukodystrophy stemming from the loss of function in the aspartoacylase (ASPA) gene within modified ASPA-expressing mature oligodendrocytes of the central nervous system (CNS) and characterized by macrocephaly, neurodevelopmental delays, and tone abnormalities [118]. The phenotypic spectrum of Canavan disease (CD) ranges from the more severe typical CD, which accounts for 90% of cases worldwide, to the less severe atypical CD, affecting the remaining 10%. Symptoms typically manifest between three and five months of age for neurodevelopmental issues in typical CD, while atypical CD symptoms become evident within the first year of life [119]. The utilization of iPSCs in the study of Canavan disease (CD) remains relatively limited, with only four studies exploring this subject for various objectives. Among these studies, two focused on engrafting mice with ASPA gene-edited iPSCs [120,121] and utilized iPSCs derived from CD subjects as a study model. Another study involved engrafting mice with wild-type ASPA gene iPSCs [122] but did not specifically target CD as a study model. Notably, only one study developed a 3D culture protocol specifically tailored for CD, a rare leukodystrophy resulting from the loss of function in the aspartoacylase (ASPA) gene, which affects altered ASPA-expressing mature oligodendrocytes of the CNS [123]. Despite the limited number of articles, all of them have demonstrated promising results in treatment, modeling, and potentially translational studies.
4.6. Epilepsy
Epilepsy comprises a diverse array of central nervous system (CNS) disorders characterized by an elevated susceptibility to seizures [124]. Seizures arise when neural networks are irregularly formed or disrupted by abnormal structural, infectious, or metabolic issues, resulting in anomalous firing patterns within a specific area of the brain (focal epilepsy) or across the entire brain (generalized epilepsy) [125]. Around 50 million people of all ages worldwide have their lives negatively affected by epilepsy [126].
Utilizing iPSC-derived neurons from individuals with epilepsy can provide valuable insights into the molecular and pathological mechanisms underlying certain epilepsy phenotypes [83]. Therefore, it becomes feasible to investigate neuronal behavior without relying on resected brain tissue. Incorporating editing techniques can further enhance the findings in studies utilizing iPSCs for epilepsy models [80].
For diseases associated with known mutations, cellular models derived from iPSCs become particularly appealing. Conversely, for epileptogenic cortical malformations or developmental epileptic encephalopathies, experimental models utilizing organoids gain prominence, as they enable the creation of intricate multi-tissue cultures.
4.7. Frontotemporal Dementia
Frontotemporal dementia (FTD) manifests as a neurodegenerative clinical syndrome marked by progressive deficits and alterations in behavior, executive function, motor skills, and language [127]. The World Health Organization (WHO) estimates that over 55 million individuals worldwide currently suffer from dementia, with low- and middle-income nations contributing 60% of these cases. Moreover, approximately 10 million new cases are reported annually [128]. Presently, the incidence of FTD is estimated at 1.61 to 4.1 cases per hundred thousand individuals per year [129]. These values allow us to state that frontotemporal dementia is the second most prevalent subtype of dementia, with rates ranging from 3% to 26% [130]. FTD predominantly affects individuals aged between 45 and 65, although cases have been observed in individuals under 30 years of age. While over half of FTD cases are sporadic, up to 40% of cases have a familial history associated with dementia, psychiatric disorders, or motor symptoms. Notably, at least 10% of FTD cases exhibit an autosomal dominant inheritance pattern [131]. Several genes are implicated in the manifestation of FTD symptoms. The GRN gene is accountable for approximately 60% of all cases of inherited frontotemporal lobar degeneration. Another significant gene associated with frontotemporal lobar degeneration is C9orf72, mutations in which contribute to about 25% of familial cases and represent the most prevalent genetic cause of both frontotemporal dementia and amyotrophic lateral sclerosis [132]. Several biological mechanisms that lead to frontotemporal lobar degeneration have been identified and investigated. However, the cause and many other questions remain unanswered [127]. Various studies utilizing iPSCs have contributed significantly to the understanding of disease mechanisms and potential treatments. In the context of FTD, a study employing human iPSC brain organoids expressing tau-V337M elucidated distinct mechanisms linked to MAPT mutation. This research delineated a sequence of events preceding neurodegeneration, uncovering molecular pathways associated with glutamate signaling as potential therapeutic targets for intervention in FTD [133]. Another study employing a derived brain organoid slice model successfully recapitulated the pathological conditions of C9ORF72 ALS/FTD. Within these organoids, distinct disruptions of transcription, proteostasis, and DNA repair in astroglia and neurons were observed. Consequently, patient-specific iPSC-derived cortical organoid slice cultures represent a reproducible translational platform for investigating preclinical mechanisms of ALS/FTD, as well as novel therapeutic strategies [134].
4.8. Huntington’s Disease
Huntington’s disease (HD) is a neurodegenerative disease caused by a CAG trinucleotide repeat increase in the huntingtin gene (HTT) [135]. The HTT mutation can lead to protein aggregation, disrupting cellular processes, particularly the basal ganglia and cortical regions of the brain [136,137]. The neuronal dysfunction ultimately leads to choreiform movements, psychiatric symptoms, dystonia, bradykinesia, and dementia [137,138].
Since HD is caused by a genetic mutation, current treatments primarily focus on managing symptoms through pharmacological interventions targeting mainly dopamine modulation [139,140]. Indeed, a more comprehensive understanding of the pathophysiology and progression of the disease is imperative to develop enhanced treatment options. Various in vitro and in vivo models have been employed to scrutinize the disease mechanisms, encompassing the introduction of the HTT mutation or the insertion of CAG repeats into the cells of both invertebrate and vertebrate animal models [141,142]. While these models have proven instrumental in unraveling the fundamental mechanisms of neuronal dysfunction and neurotransmitter dysregulation, they fall short in recapitulating the more intricate and clinical manifestations of the disease. One promising alternative has been studying the disease using cells from patients carrying the mutated gene. The scarcity of biological material can be overcome by integrating the iPSC technology into these models. In 2008, the Daley laboratory pioneered the creation of human iPSC-based models for Huntington’s disease (HD). Notably, they successfully developed the initial iPSC line from an HD patient with 72 CAG repeats. The cells were subsequently transformed into GABAergic, DARPP32-positive neurons, underscoring the potential of iPSCs to be directed into striatal neurons, a critical cell type vulnerable to degeneration in HD [143,144,145]. Subsequently, other groups developed additional iPSC cell lines from different patients [146,147,148].
Using iPSCs from patients to model HD has revealed many cellular alterations caused by the mutation. Genes related to DNA damage control pathways were downregulated in neurons derived from iPSCs (iNeurons) from patients with high CAG repeat mutations [149]. The malfunction of DNA damage repair systems can be connected to the somatic instability and mosaicism observed in HD [150]. HD-derived iNeurons showed multiple abnormalities in neuronal patterning [151,152] and an observed persistent mitotic population [153]. These changes in neuronal differentiation patterns can be linked to alterations in neurodevelopmental gene expression profiles linked to HD.
Changes in gene expression are believed to be one of the mechanisms that lead to neurodegeneration in HD. Comparisons of gene expression between iNeurons derived from HD iPSCs and gene-corrected control lines revealed the upregulation of the transforming growth factor beta (TGF-β) pathway in HD [154]. Studies conducted on iNeurons from additional HD patients further corroborate this finding [155,156,157]. Recently, the National Institutes of Health (NIH) established a consortium known as the “HD iPSC Consortium” to explore gene expression and functional changes linked to HD. RNA sequencing (RNA-seq) analysis conducted by this consortium revealed transcriptomic alterations in numerous pathways related to the development and master regulators of neurogenesis [157].
Overall, the utilization of iPSCs to comprehend the pathophysiology of HD has unveiled numerous pathways that could be targeted therapeutically to potentially mitigate the disease. Given that iPSCs can be differentiated into various cell subtypes found in brain tissue, including microglia and astrocytes, it would be intriguing to investigate whether the observed alterations in iNeurons persist in cell–cell interaction models.
4.9. Multiple Sclerosis
Multiple sclerosis (MS) is a chronic, inflammatory, and autoimmune disease characterized by demyelination and axonal lesions in focal and generalized regions of the brain and spinal cord. This pathological hallmark results in axonal damage to neurons in both white and gray matter areas. Clinically, MS manifests a range of characteristic symptoms that can lead to irreversible neurological disability and cognitive decline. The etiology of MS is multifactorial, influenced by complex gene–environment interactions, including genetic inheritance and lifestyle factors such as night work, excessive alcohol or caffeine consumption, and a history of infectious mononucleosis [158,159,160]. In 2020, it was estimated that there were 2.8 million people with MS worldwide. The disease is predominantly found in individuals of European descent, but it is relatively rare among individuals of Asian, African, and Native American ethnicities [161]. In terms of gender, MS is more prevalent in women, occurring twice as often as in men. By age group, MS generally affects young adults, ages 20 to 40, although some patients experience a demyelinating event during childhood or adolescence, typically with a form of multiple sclerosis called relapsing-remitting or relapsing-remitting MS (RRMS) [162,163]. The heritability of MS is polygenic, involving polymorphisms in several genes, each associated with a slight increase in the risk of developing the disease. Notably, the HLA class I and HLA class II genes confer the highest risk of MS transmission [158,164]. The use of iPSC-derived organoids is essential both to understand the mechanisms of MS and its clinical subtypes and to try to apply therapies to improve the affected areas and inhibit the effects of MS. The utilization of iPSC-derived organoids is crucial for comprehending the mechanisms underlying MS and its clinical subtypes, as well as for exploring potential therapeutic interventions to ameliorate affected areas and mitigate the effects of MS. A study involving iPSC organoids derived from both MS patients and healthy individuals revealed several key insights into MS pathology. Organoids derived from MS patients exhibited a diminished proliferation capacity compared to those from healthy controls, likely due to a reduction in the pool of stem cells. Furthermore, there was a notable decrease in SOX2+ cells and Olig2+ oligodendrocytes in MS organoids compared to controls, indicating a compromised capacity for differentiation and remyelination in MS patients and their subtypes [165]. hiPSCs can be used in the formation of oligodendrocyte progenitors generated and applied in biomedical studies. The process begins with the formation of the embryoid body, and the standardization of cells using a combination of factors T3, OLIG2, SOX10, A2B5, NG2, PDGFRα, and O4. After 3 weeks of differentiation, OPCs will form oligodendrocyte units with small subpopulations of GFAP+ astrocytes (~1%) and MAP2+ neurons (~5%) [166]. García et al. developed a human oligodendrocyte model specific to primary progressive MS. They achieved this by transducing NPCs derived from two hiPSC lines from primary progressive MS patients with SOX10 lentivirus. Their findings revealed that all lines, whether from healthy or diseased individuals, generated O4+ cells and MBP+ cells within 22 days post-transduction, exhibiting morphology and markers characteristic of intermediate and late oligodendrocytes. This organoid model offers a valuable opportunity to model MS and explore its mechanisms [167].
4.10. Parkinson’s Disease
Parkinson’s disease (PD) is a devastating neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra region of the brain, leading to a reduction in dopamine levels and impaired fine motor coordination. Clinically, PD presents with symptoms such as bradykinesia, muscle rigidity, resting tremors, and postural instability. Pathologically, the disease is characterized by the presence of Lewy body aggregates composed of α-synuclein protein. Genetic factors play a significant role in PD, with several genes implicated in both dominant and recessive familial forms of the disease. These genes include SCNA, LRRK2, PINK1, PARK2 (parkin), and GBA1, along with others such as DJ-1, PARK9 (ATP13A2), SJ-1, and VPS35. Given the complex nature of PD, there is ongoing debate regarding potential in vitro techniques for therapeutic intervention and understanding various aspects of the disease [168].
Indeed, recent advancements in using induced pluripotent stem cells (iPSCs) and 3D brain organoid models offer promising avenues for understanding the pathogenesis of Parkinson’s disease (PD) and identifying potential therapeutic targets. By deriving neurons from patients with PD and forming organoids, researchers can analyze pathogenic mechanisms in detail and conduct drug-screening experiments. While therapeutic measures to modify the course of PD are still lacking, the use of iPSCs and organoid models holds great promise for advancing our understanding of the disease and developing novel therapeutic interventions [169]. Some organoid models are presented below:
α-synuclein (SNCA) model: α-synuclein is a protein that assumes different conformations dictated by cellular stress and is involved in neurodegenerative diseases such as PD [170]. Mutations of A53T mutant α-synuclein or α-synuclein accumulation in neurons lead to increased nitrosative stress, mitochondrial dysfunction, disrupted synaptic connectivity, transcriptional changes in synaptic signaling genes, and reduced ratio of α-synuclein tetramer to monomer, important factors in the pathogenesis of PD [171]. The iPSC-derived neuron model has triplicated levels of α-synuclein and could be a good model for understanding the morphophysiological divergences between healthy neurons and mutant neurons from PD patients.
LRRK2 model: Leucine-rich repeat kinase 2 (LRRK2) is a multikinase involved in roles in neurite outgrowth, phosphorylation of multiple proteins, and endocytic sorting via interactions with Rab-GTPases. Mutations in the gene encoding LRRK2 imply a significant risk for PD as well as other factors [172]. LRRK2 organoid models showed increased levels of oxidized dopamine and lysosomal receptor for chaperone-mediated autophagy. Also, neurons in this model have greater apoptotic activity, reducing neurite growth. Interestingly, LRRK2 also demonstrated irregularities in synaptic vesicle recycling, leading to disrupted synaptic vesicle endocytosis and decreased vesicle density in neurons.
PINK1 model: PINK1 (PTEN-induced kinase 1) is a phosphatase and tensin (PTEN) homologous protein/kinase. PINK1 localizes to the mitochondrial membrane after its depolarization, where it phosphorylates Parkin. Together, PINK1 and Parkin regulate mitochondrial health, and mutations in either related genes are associated with autosomal recessive diseases and early-onset forms of PD. A model with iPSC-derived neurons from patients expressing nonsense (Q456X) or missense (V170G) PINK1 exhibits mitochondrial defects, including impaired recruitment of Parkin to mitochondria [173].
GBA model: The GBA1 gene is responsible for encoding glucocerebrosidase (GCase) or β-glucosidase, a lysosomal enzyme that catalyzes the hydrolysis of glucosylceramide (GlcCer) into glucose and ceramide, but also the hydrolysis of D-glucosyl-N-acylsphingosine into D-glucose and N-acylsphingosine. Studies have confirmed that there is a relationship between PD and GBA mutations, including insertion, deletion, frameshift, and point mutations in GBA. Approximately 5–10% of PD patients carry GBA1 mutations. The result of the GBA mutation is the accumulation of lipids in neurons. In this PD model, alterations of GBA and GCase substrates, glycolipids glucosylceramide (GlcCer), and glucosylsphingosine (GlcSph) are found at increased levels, resulting in defective action of cellular organelles of neurons, making neurons more vulnerable to apoptosis [169].
Idiopathic Parkinson’s: The neuron-based organoid model of idiopathic Parkinson’s seeks to understand aspects of the disease that do not necessarily involve genetic aspects while also seeking to understand PD. Interestingly, it has been shown that neurons derived from patients with idiopathic PD have decreased mitochondrial respiration, increased levels of oxidized dopamine and oxidized DJ-1, and decreased GCase enzyme activity [174].
4.11. Spinal Cord Injury
Spinal cord injury (SCI) refers to damage inflicted upon the spinal cord, leading to a debilitating neurological and pathological condition. It profoundly impacts motor, sensory, and autonomic functions, and can also have far-reaching effects on psychological, social, and vocational aspects, significantly diminishing the patient’s overall well-being and reducing life expectancy [175].
Over the past three decades, the global prevalence of spinal cord injury (SCI) has seen a notable increase, rising from 236 to 1298 cases per million inhabitants. Additionally, it is estimated that between 250,000 and 500,000 new cases of SCI occur worldwide each year. This increase in prevalence underscores the significance of SCI as a global health concern and highlights the need for continued research and advancements in treatment and prevention strategies [176].
Initially, SCI arises from mechanical trauma (primary injury) to the spinal cord. This trauma may result from a fracture, dislocation, compression, shear, laceration, and other forms of injuries to the spine or intervertebral disc that compromise the structure of the spinal cord. Consequently, a secondary injury arises, which involves a cascade of events that are characterized by ischemia and physiological changes such as pro-apoptotic signaling and peripheral inflammation, cellular infiltration, release of pro-inflammatory cytokines and cytotoxic debris (DNA, ATP, and reactive species of oxygen) that cyclically increase the harsh post-injury situation that can result in an expansion of the lesion zone of the affected neural tissue [177].
In any case, evaluating the status of each injury resulting from SCI is essential for making therapeutic decisions. Due to the different aspects involved in the pathology of SCI, different therapeutic strategies have been proposed to treat neurodegenerative events and reduce neuronal damage resulting from SCI [178].
These efforts consist of developing neuroprotective and neuroregenerative therapies that promote neuronal recovery and improve outcomes. These therapies include spinal precautions (logrolling and cervical collar) and protection against other injuries. Additionally, there are treatment options, both surgical and pharmacological, although the efficacy of some of these treatments is still under debate. The pharmacological treatments employed often target alpha-adrenergic and beta-adrenergic functions with the aim of reducing the effects of SCI by controlling pressure and interfering with the mechanisms of action of inflammatory cytokines [176,177].
Although significant progress has been made in the understanding and treatment of LM, a definitive cure has yet to be made a reality due to the complexity of the pathology. However, new therapies based on induced pluripotent stem cells (iPSCs) and organoid models are currently, being studied to promote the treatment of patients affected by SCI and/or other neuromotor diseases. Cell-based treatments include transplants of autologous Schwann cells, olfactory ensheathing cells, mesenchymal stem cells, neural precursor cells, oligodendrocyte progenitor cells, and macrophages to the affected area [175].
Furthermore, iPSC can be used to construct 3D organoid models customized according to the aspects of each disease studied. In the case of SCI, some components studied are part of the structures affected by SCI [179].
Among the organoid models studied are early neuroectodermal cells generated from astrocytes and programmed through the overexpression of OCT4 and p53, along with the supply of molecules such as CHIR99021, SB431542, RepSox, and Y27632. Direct reprogramming of astrocytes into neurons may pave the way for in vivo neural organogenesis from endogenous astrocytes for the repair of central nervous system injuries [180].
Another study generated a three-dimensional culture system and protocol for the production of human spinal cord-like organoids (hSCOs), recapitulating the neurulation-like morphogenesis of the early spinal cord. hSCOs exhibited neurulation-like tube-forming morphogenesis, cellular differentiation into the major types of spinal cord neurons as well as glial cells, and mature synaptic functional activities, among other features of spinal cord development. In this study, the hSCOs generated were used to screen for antiepileptic drugs that can cause neural tube defects. hSCOs may also facilitate the study of human spinal cord development and the modeling of diseases associated with neural tube defects [181].
Organoids modeling the ventral spinal cord have been successfully generated, exhibiting the capacity to differentiate into various constituent cell types, including motor neurons, excitatory V2a interneurons, inhibitory Renshaw interneurons, and astrocytes. The presence of this heterogeneous cellular composition within the organoid framework provides a platform for elucidating the complex pathological mechanisms underlying spinal cord injuries (SCIs). Emerging evidence underscores the significance of cell-to-cell interactions within the three-dimensional microenvironment, emphasizing their pivotal role in deciphering the cellular intricacies governing SCI pathology [182].
4.12. Stroke
Stroke, a cerebrovascular disease resulting from a disruption of blood flow to the brain, stands as a primary cause of neurological impairment and mortality among the elderly population globally. Despite advancements in risk factor management and therapeutic interventions, there persists a concerning rise in the incidence of stroke cases [183]. Since the inception of iPSCs for stroke research, the primary focus has been on cell engraftment, driven by the promising history of stem cell engraftment in ameliorating stroke outcomes. Animal models typically employed include the rodent middle cerebral artery occlusion model for ischemic stroke and intracranial hemorrhagic models for hemorrhagic stroke. While iPSC engraftment in stroke rodent models is hampered by tumorigenicity concerns, both models have exhibited favorable outcomes, including neurological protection and reduced inflammatory responses [184]. Stroke is a tough disease to model in vitro due to the involvement of its tissue heterogeneity. The most common in vitro models for stroke entail oxygen and glucose deprivation. The use of iPSCs has already been reported in this 2D model [185]. Another in vitro model for studying stroke that uses iPSCs involves modeling cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), which leads to vessel weakening due to the accumulation of the NOTCH3 protein. This condition is associated with mutations in the NOTCH3 gene, resulting in the gain or loss of cysteine residues in specific exons. iPSCs have been successfully utilized to model this disease, offering insights into its pathogenesis and potential therapeutic interventions [186,187,188]. Given this context, the utilization of organoids derived from iPSCs has emerged as an attractive model for simulating vessel weakening and neurological damage characteristic of stroke conditions in a 3D in vitro setting [189,190].
4.13. Traumatic Brain Injury
Traumatic brain injury (TBI) is defined as a severe injury to the head resulting from a forceful impact. Its clinical manifestations are diverse and multifaceted, often encompassing edema, traumatic axonal injury, contusion, and hematoma. TBI is a leading cause of mortality and disability, affecting an estimated 69 million individuals worldwide [191]. TBI poses significant challenges for both in vitro and in vivo modeling. In vivo models often struggle to accurately replicate the human response to injury due to differences between human and mouse brains. On the other hand, in vitro models may fall short in capturing the complex interplay of processes that occur throughout the entire body [192]. TBI is divided into two major injuries: The primary injury (PI) occurs in the form of the impact and structural damage simultaneously, causing axonal shear, cell death, and inflammation, enabling the most common clinical signs and even death [193]. Multiple processes that occur during an extended period and include a cascade of metabolic, neuroinflammatory response, and degenerative changes [194], which may lead to several neurodegenerative diseases, including AD, chronic traumatic encephalopathy (CTE), PD, and other forms of dementia or movement disorders characterize the secondary injury (SI) [195,196,197]. The utilization of iPSCs in TBI research primarily centers around potential regenerative therapies and in vitro modeling studies. Various research groups have successfully developed TBI-related assays employing the iPSC technology. The majority of these studies concentrate on specific modeling targets, such as the primary impact (PI) effects [198,199], SI neuroinflammatory response [200], and PI traumatic axonal injury [201].
A protocol developed by Santiago-Ramirez et al. in 2021 introduced a potential approach using 3D organoids to evaluate the effects of TBI. These organoids, derived from iPSCs reprogrammed from fibroblasts, exhibited a variety of cells and structures found in the brain and displayed behavior akin to the cerebral cortex. However, despite the promising results, this model still lacks certain cell types and structural components needed to fully replicate the spectrum of the TBI pathology [202].
Another strategy to study TBI is based on iPSCs (NS/PS), which, through genome editing, expressed the yeast cytosine deaminase/uracil phosphoribosyl transferase enzyme-prodrug gene (yCD-UPRT)These NS/PS exhibited high expression of yCD-UPRT. which was observed in in vivo bioluminescent imaging and histopathological analyses in mice models of TBI. The result obtained was that the group treated with NS/PS showed improvement when compared to the control group. Furthermore, significant functional improvements in motor skills and prevention of brain atrophy were observed in mice treated with NS/PCs. Prevention of brain atrophy was observed in mice transplanted with NS/PCs [192].
In a particular study, iPSCs were derived from reactive glial cells extracted from the adult neocortex affected by TBI. The iPSC generation process involved the use of retroviruses and four transcription factors, i.e., Oct4, Sox2, Klf4, and c-Myc, in an in situ approach, where iPSCs were induced within the tissue affected by TBI. These iPSCs subsequently differentiated into abundant neural stem cells, which further matured into various cell types, including neurons and glia. Remarkably, effective brain repair was observed, characterized by the presence of numerous neurons exhibiting typical neuronal morphology, complete with axons, dendrites, and the ability to generate action potentials [203].
Table 1.
Organoid models generated for studying several neuropathological diseases.
| Organoid Type | Disease | Cell Type | Result | Reference |
|---|---|---|---|---|
| Cerebral Organoid | AD | iPSC | Modeling sporadic Alzheimer’s disease in human brain organoids under serum exposure | [204] |
| Cerebral Organoid | AD | hiPSC | Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs. isogenic controls | [205] |
| Cerebral Organoid | AD | iPSC | Modeling amyloid beta and tau pathology in human cerebral organoids | [206] |
| Disease Stem Cell | AD | iPSC | Familial Alzheimer’s disease mutations in PSEN1 lead to premature human stem cell neurogenesis | [207] |
| Disease Stem Cell | AD | iPSC and hiPSC | iPSC-derived human microglia-like cells to study neurological diseases | [208] |
| Cerebral Organoid | AD | iPSC | APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patients’ iPSC-derived cerebral organoids | [209] |
| Cerebral Organoid | AD | iPSC | A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids | [210] |
| Cerebral Organoid | AD | iPSC | Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids | [211] |
| Cerebral Organoid | AD | iPSC | Tau pathology epigenetically remodels the neuron-glial cross-talk in Alzheimer’s disease | [212] |
| Disease Stem Cell | AD | iPSC | APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types | [95] |
| Disease Stem Cell | AD | iPSC | Type I interferon signaling drives microglial dysfunction and senescence in human iPSC models of Down syndrome and Alzheimer’s disease | [213] |
| Cerebral Organoid | AD | iPSC | Acetylation changes tau interactome to degrade tau in Alzheimer’s disease animal and organoid models | [214] |
| Cerebral Organoid | PD | hiPSC | Modeling G2019S-LRRK2 sporadic Parkinson’s disease in 3D midbrain organoids | [215] |
| Cerebral Organoid | PD | hiPSC | Lewy body-like pathology and loss of dopaminergic neurons in midbrain organoids derived from familial Parkinson’s disease patient | [216] |
| Midbrain Organoid | PD | hiPSC | Human iPSC-derived midbrain organoids functionally integrate into striatum circuits and restore motor function in a mouse model of Parkinson’s disease | [217] |
| Neurospheres | PD | hiPSC and iPSC | Patient-derived three-dimensional cortical neurospheres to model Parkinson’s disease | [218] |
| Midbrain Organoid | PD | hiPSC and iPSC | Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease linked DNAJC6 mutations | [219] |
| Midbrain Organoid | PD | iPSC | Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality | [220] |
| Cerebral Organoid | PD | iPSC | Use of 3D organoids as a model to study idiopathic form of Parkinson’s disease | [221] |
| Cerebral Organoid | PD | iPSC | The Parkinson’s disease-associated mutation LRRK2-G2019S alters dopaminergic differentiation dynamics via NR2F1 | [222] |
| Cerebral Organoid | Rett syndrome | hiPSC | Identification of neural oscillations and epileptiform changes in human brain organoids | [223] |
| Cerebral Organoid | TLE | iPSC | Modeling genetic epileptic encephalopathies using brain organoids | [224] |
| Cerebral Organoid | TSC | hiPSC | Amplification of human interneuron progenitors promotes brain tumors and neurological defects | [225] |
| Motor neurons study | ALS | iPSC | Aberrant axon branching via Fos-B dysregulation in FUS-ALS motor neurons | [226] |
| Sensorimotor organoids | ALS | iPSC | Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions | [99] |
| Cerebral Organoid | ALS | iPSC | Spinal cord extracts of amyotrophic lateral sclerosis spread TDP-43 pathology in cerebral organoids | [227] |
| Motor neurons and brain organoids | ALS and FTD | iPSC | CRISPR/Cas9-mediated excision of ALS/FTD-causing hexanucleotide repeat expansion in C9ORF72 rescues major disease mechanisms in vivo and in vitro | [228] |
| Cerebral organoid slice model | ALS and FTD | iPSC | Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology | [134] |
| Brain organoids | ALS and FTD | iPSC | Granulin loss of function in human mature brain organoids implicates astrocytes in TDP-43 pathology | [229] |
| Motor neurons | ALS | hiPSC | Exploring motor neuron diseases using iPSC platforms | [230] |
| Cerebral organoids | FTD | iPSC | ELAVL4, splicing, and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids | [133] |
| Molecular study | FTD | iPSC | Pathological progression induced by the frontotemporal dementia-associated R406W tau mutation in patient-derived iPSCs | [231] |
| iPSC-derived astrocytes | MS | iPSC | iPSC-derived reactive astrocytes from patients with multiple sclerosis protect cocultured neurons in inflammatory conditions | [232] |
| Model study | MS | iPSC | Selective PDE4 subtype inhibition provides new opportunities to intervene in neuroinflammatory versus myelin-damaging hallmarks of multiple sclerosis | [233] |
| RRMS and PPMS iPSC cellular models | MS | iPSC | Generation of RRMS- and PPMS-specific iPSCs as a platform for modeling multiple sclerosis | [234] |
| Cerebral organoids | MS | iPSC | Cerebral organoids in primary progressive multiple sclerosis reveal stem cell and oligodendrocyte differentiation defect | [165] |
| Model study | MS | iPSC | Generation and characterization of four multiple sclerosis iPSC lines from a single family | [235] |
| Cerebral organoids | ASD | iPSC | Single-cell brain organoid screening identifies developmental defects in autism | [236] |
| Forebrain organoids/Molecular study | ASD | iPSC | Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated autism spectrum disorder | [237] |
| Organoids/Molecular study | ASD | iPSC | FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders | [113] |
| Brain organoids | ASD | iPSC | Superoxide dismutase isozymes in cerebral organoids from autism spectrum disorder patients | [238] |
| Organoids/Molecular study | ASD | iPSC | CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPSC cells | [239] |
| Cell Therapy | TBI | Rat | Combining enriched environment and induced pluripotent stem cell therapy results in improved cognitive and motor function following traumatic brain injury | [240] |
| Cell Therapy | TBI | Mice | Controlled cortical impact model of mouse brain injury with therapeutic transplantation of human induced pluripotent stem cell-derived neural cells | [241] |
| Cerebral Organoid | TBI | hiPSC | Modeling traumatic brain injury in human cerebral organoids | [198] |
| Cell Therapy | CD | Mice | Cell-based therapy for Canavan disease using human iPSC-derived NPCs and OPCs | [122] |
| Cerebral Organoid | Stroke | hiPSC | Gene expression profiles of human cerebral organoids identify PPAR pathway and PKM2 as key markers for oxygen glucose deprivation and reoxygenation | [242] |
| iPSC derived telencephalon organoids | ADHD | iPSC | Telencephalon organoids derived from an individual with ADHD show altered neurodevelopment of early cortical layer structure | [105] |
| Model study | ASD and ADHD | iPSC | Modeling human cerebellar development in vitro in 2D structure | [243] |
| Molecular study | ADHD | iPSC | Generation of a human induced pluripotent stem cell (iPSC) line from a 51-year-old female with attention-deficit/hyperactivity disorder (ADHD) carrying a duplication of SLC2A3 | [244] |
| Model study | ADHD | iPSC | Generation of four iPSC lines from peripheral blood mononuclear cells (PBMCs) of an attention-deficit/hyperactivity disorder (ADHD) individual and a healthy sibling in a Caucasian family in Australia | [245] |
| Model study | ADHD | iPSCs and NSCs | Growth rates of human induced pluripotent stem cells and neural stem cells from attention-deficit/hyperactivity disorder patients: a preliminary study | [246] |
| Molecular study | HD | iPSC | An alternative splicing modulator decreases mutant HTT and improves the molecular fingerprint in Huntington’s disease patient neurons | [247] |
| Molecular study | HD | iPSC-derived neurons (Mice) | CryoET reveals organelle phenotypes in Huntington’s disease patient iPSC-derived and mouse primary neurons | [248] |
| Model study | HD | iPSC-derived neural cells | Bioenergetic deficits in Huntington’s disease iPSC-derived neural cells and rescue with glycolytic metabolites | [249] |
| Model study | HD | iPSC-derived neural cells | Extracellular vesicles improve GABAergic transmission in Huntington’s disease iPSC-derived neurons | [250] |
Legend: AD—Alzheimer’s disease; ADHD—attention-deficit/hyperactivity disorder; ALS—amyotrophic lateral sclerosis; ASD—autism spectrum disorder; CD—Canavan disease; HD—Huntington’s disease; FTD—frontotemporal dementia; MS—multiple sclerosis; PD—Parkinson’s disease; TLE—temporal lobe epilepsy; TSC—tuberous sclerosis complex.
4.14. Limitations in the Use of Organoid Models
While organoid models offer numerous advantages, it is important to acknowledge their limitations, particularly in the context of disease treatment approaches. One critical consideration is their efficacy, as they may not fully replicate the complex microenvironments found in native tissues and organs. This can pose a significant limitation when translating findings from organoid studies into effective treatments [79].
iPSC-derived organoids present several overarching deficiencies. These encompass issues such as inadequate reproducibility due to variations in culture conditions yielding inconsistent experimental outcomes. Additionally, achieving a precise cell type composition akin to that of native tissues or organs poses challenges, resulting in size and shape heterogeneity. Moreover, organoids often lack crucial components including vasculature, immune cells, neural innervation, and specific morphological attributes, limiting their ability to effectively recapitulate physiological tissue environments. Furthermore, functional capabilities are frequently absent or limited in organoids, posing obstacles for studying complex biological processes and disease mechanisms. Based on these points, it has become a current objective to improve organoid protocols to both reduce these in vitro and organ effects and to promote better adaptation and expression of organ characteristics [78].
5. iPSC-Based Therapies for Neurological Diseases
It is likely possible to model iPSCs into all somatic cell types [251]. Indeed, investigations have pivoted towards leveraging this potential to model specific diseases at the cellular level, particularly those driven by genetic mutations. Patient-derived iPSCs have emerged as a focal point for conducting high-throughput screens aimed at elucidating the developmental mechanisms underlying various pathologies [252]. Therefore, iPSCs have been widely used in research strategies for pharmacological screening and regenerative therapy [253].
The objective of employing stem cell treatment strategies for regenerative therapy is to achieve the remission of pathological changes present in affected tissue, along with the potential to restore lost function resulting from localized damage [254]. The proliferative and differentiating capabilities of this cell type offer the potential for tissue regeneration in conditions requiring surgical resection or tissue loss, such as spinal cord injury (SCI) [255]. iPSCs in the context of regenerative therapy become valuable due to the patient-derived cells, which prevent tissue rejection, in addition to promoting recovery of function [256].
Currently, numerous studies are investigating the regenerative potential of iPSCs for neuropathologies. Animal model studies focused on spinal cord injury (SCI) have shown promising results, with high success rates in motor function recovery [257,258,259]. There have been groups that have attempted to prevent loss of function in animal models of stroke and ischemia [260,261]. In temporal lobe epilepsy, it was carried out to graft modified iPSCs to non-epileptogenic GABAergic neurons [262,263,264]. Even the use of iPSCs has become the target of study as a potential treatment of diseases by replacing tissue in which surgical intervention would not occur, such as the case of PD, metachromatic leukodystrophy (MLD), and HD [265,266,267]. Within all these pathologies, iPSCs have great regenerative and replacement potential for damaged tissue due to the possibility of editing cells to overcome the alterations resulting from the pathology [254].
Pharmacological tests conducted in vitro using various human cell models often face challenges in accurately mimicking the pathology. However, iPSCs offer a valuable opportunity to generate patient-specific cellular models, enabling the investigation of cellular mechanisms underlying diseases [268]. Although iPSCs are less commonly utilized in drug screening, they possess valuable properties for research purposes. Patient-derived cells obtained through iPSCs are highly specific, and the process of acquiring them is less invasive, making them excellent candidates for in vitro study models [269].
The modeling of diseases using iPSCs has seen a significant increase in interest, driven by the need for more precise investigations into pathologies. In diseases such as Alzheimer’s disease (AD), where the pathology is well understood, established drugs have been tested using iPSC-based models to evaluate their efficacy [270]. Currently, the direct use of new drugs in iPSC-based models is uncommon. However, strategies involving the use of iPSCs as a means to validate drug efficacy are increasingly being utilized in cellular models (Table 2) [271].
iPSCs are still under investigation to address their major challenges. While their pluripotent nature is advantageous for forming teratomas, which helps confirm their identity in early-stage studies, their tumorigenic potential is a significant concern for cell therapy. Studies have reported the formation of gliomas in the brain in graft therapy investigations [271]. Since the inception of iPSC research, numerous groups have identified significant risks associated with these cells, particularly concerning their tumorigenic potential linked to transgenic C-MYC expression and viral integration. Furthermore, the Single Cell Transcriptomics methodology has been bolstering disease modeling efforts, enabling a deeper understanding of genetic mechanisms such as gene expression. It is also instrumental in characterizing cell subtypes and identifying novel drug candidates. For instance, Fernandes et al. employed single-cell transcriptome analysis to identify and characterize cellular heterogeneity in human iPSC-derived dopamine neurons. Their study revealed distinct dopaminergic neuron subtypes within iPSC cultures, particularly after exposure to cytotoxic effects and genetic stressors induced by the drug felodipine [272]. Indeed, another study harnessed the Single Cell Transcriptomics methodology to cultivate organoids with precise developmental features and cell type characteristics of their target organ. This technique enabled the differentiation and selection of cell subgroups representing the desired organ characteristics while excluding cells that did not align with those features [273,274].
Hence, ongoing studies aimed at utilizing iPSCs for engraftment purposes are delving deep into examining alterations within the generated cellular model to preempt any undesired modifications [275,276]. Despite the challenges identified in the application of the technique, well-established groups have expressed keen interest in utilizing human pluripotent stem cells (hPSCs) for therapeutic purposes in humans [277].
Table 2.
iPSC usage in cell therapy and drug screening for several neuropathologies.
| Trial Type | Disease | Target | Result | Reference |
|---|---|---|---|---|
| Cell Therapy | AD | Rat | The transplanted rats rescued Alzheimer’s cognition. | [278] |
| Cell Therapy | AD | Mouse | Grafted mice showed improved memory, synaptic plasticity, and reduced AD brain pathology, including a reduction in amyloid and tangle deposits. | [279] |
| Drug Screening | AD | hiPSC | β-secretase inhibitor IV (BSI) and γ-secretase inhibitor XXI/compound E (GSI) showed similar effects as screening in other models. | [280] |
| Drug Screening | AD | hiPSC | Docosahexaenoic acid (DHA) treatment alleviated the stress responses in the AD neural cells. | [270] |
| Drug Screening | AD | hiPSC | The anthelminthic avermectins increase the relative production of short forms of Aβ and reduce the relative production of longer Aβ fragments in human cortical neurons. | [281] |
| Cell Therapy | HD | Mice | iPSCs survived and differentiated into region-specific neurons in both mice groups without tumor formation. | [282] |
| Cell Therapy | HD | Mice | Grafted mice showed a significant increase in lifespan. In iPSC groups, animals showed significant improvement in motor functions and grip strength. | [283] |
| Cell Therapy | HD | Rat | Grafted rats showed significant behavioral improvements for up to 12 weeks. iPSCs enhanced endogenous neurogenesis and reconstituted the damaged neuronal connections. | [166] |
| Cell Therapy | HD | Mice | Improved neuronal dysfunction by SUPT4H1-edited iPSC grafts. | [284] |
| Cell Therapy | MLD | Mice | Transplantation of ARSA-overexpressing precursors into ARSA-deficient mice significantly reduced sulfatide storage up to 300 µm from grafted cells. | [285] |
| Cell Therapy | MLD | Mice | Grafts of iPSCs into neonatal and adult immunodeficient MLD mice stably restored arylsulfatase A (ARSA) activity in the whole CNS and a significant decrease in sulfatide storage when ARSA-overexpressing cells were used. | [165] |
| Cell Therapy | PD | Rat | iPSC graft differentiated into mature mDA neurons that survive over long term and restored motor function. | [286] |
| Cell Therapy | PD | Mice | hiPSCs differentiated into mDA neurons and long-term motor functional recovery was achieved after transplantation. | [287] |
| Cell Therapy | PD | Rat | Grafted iPSCs could survive in Parkinsonian rat brains for at least 150 days, and many of them differentiated into tyrosine hydroxylase (TH)-positive cells. | [288] |
| Cell Therapy | PD | Rat | Intranigral engraftment to the ventral midbrain demonstrated that mDA progenitors cryopreserved on day 17, and cells had a greater capacity than immature mDA neuron cells to innervate over long distances to forebrain structures. | [289] |
| Cell Therapy | PD | Rat | hiPSC-derived dopaminergic progenitor cells integrate better into the striatum of neonates than older rats. | [290] |
| Cell Therapy | PD | Mice | More than 90% of the engrafted cells differentiated into the lineage of mDA neurons, and approximately 15% developed into mature mDA neurons without tumor formation. | [291] |
| Cell Therapy | PD | Rat | There was a neural remodel of basal ganglia circuitry and no tumorigenicity. | [292] |
| Cell Therapy | PD | Mice | iPSCs matured into mDA neurons, reverse motor function, and established bidirectional connections with natural brain target regions without tumor formation. | [217] |
| Cell Therapy | SCI | Rat | Transplanted cells displayed robust integration properties, including synapse formation and myelination by host. | [293] |
| Cell Therapy | SCI | Mice | Due to DREADD expression, it was shown a significant decrease in locomotor dysfunction in SCI-grafted mice, which was exclusively observed following the neurons’ maturation. | [294] |
| Cell Therapy | SCI | Mice | The combination of iPSC graft and rehabilitative training therapy significantly improved motor functions. | [295] |
| Cell Therapy | SCI | Rat | Neuro-pluripotent cells derived from iPSC were able to survive and differentiate into both neurons and astrocytes, which improved forelimb locomotor function. | [296] |
| Cell Therapy | Stroke | Mice | Combination of electroacupuncture and iPSC-derived extracellular vesicle treatment ameliorated neurological impairments and reduced the infarct volume and neuronal apoptosis in MCAO mice. | [297] |
| Cell Therapy | Stroke | Pig | Tanshinone IIA nanoparticles increased iPSC engraftment, enhanced cellular and tissue recovery, and improved neurological function in a translational pig stroke model. | [298] |
| Cell Therapy | Stroke | Rat | Increased glucose metabolism and neurofunctional in iPSC-transplanted rats. | [299] |
| Cell Therapy | Stroke | Rat | Graft of iPSCs inhibited microglial activation and expression of proinflammatory cytokines and suppressed oxidative stress and neuronal death in the cerebral cortex at the ischemic border zone. | [300] |
| Cell Therapy | Stroke | Mice | Graft survived well and primarily differentiated into GABAergic interneurons and significantly restored the sensorimotor deficits of stroke mice for a long time. | [301] |
| Cell Therapy | Stroke | Rat | Generated oligodendrocytes survived and formed myelin-ensheathing human axons in the host tissue after grafting onto adult human cortical organotypic cultures. | [302] |
| Cell Therapy | TLE | Mice | A much-reduced frequency of spontaneous recurrent seizures in grafted animals. | [262] |
Legend: AD—Alzheimer’s disease; HD—Huntington’s disease; MLD—metachromatic leukodystrophy; PD—Parkinson’s disease; SCI—spinal cord injury; TLE—temporal lobe epilepsy; mDA—midbrain dopaminergic. SCI and PD papers presented are only from 2023 and 2022 due to the large number of publications.
6. Conclusions and Future Perspectives
The utilization of induced pluripotent stem cells (iPSCs) in the investigation of neuropathologies offers a promising avenue for comprehending complex diseases and exploring potential therapeutic interventions. The advent of iPSCs represents a significant advancement in cellular reprogramming, enabling the creation of patient-specific models that effectively recapitulate the characteristics of diverse neurological disorders [303].
The versatility of induced pluripotent stem cells (iPSCs) in differentiating into key neural cell types, including neurons, astrocytes, and oligodendrocytes, has greatly facilitated the development of patient-derived disease models for various conditions such as Alzheimer’s disease (AD), Parkinson’s disease (PD), epilepsy, spinal cord injury (SCI), stroke, traumatic brain injury (TBI), Canavan disease (CD), autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), and multiple sclerosis (MS). Moreover, the ability to manipulate iPSCs using gene-editing techniques has further expanded their utility, allowing researchers to study specific disease-related mutations [168,304].
The importance of selecting appropriate donor cell types and optimizing reprogramming protocols cannot be overstated. Continuous efforts are essential to improve efficiency and safety in induced pluripotent stem cell (iPSC) generation. While concerns about their applicability persist, particularly regarding tumorigenesis, several studies using animal models have reported positive results without tumor formation. Despite the controversy surrounding the use of iPSCs as therapies, ongoing research is shedding light on their potential benefits (Table 2).
The integration of induced pluripotent stem cells (iPSCs) into organoid models marks a significant advancement, enabling the in vitro recreation of 3D tissues. Derived from iPSCs, organoids offer a unique platform for studying complex structures like the brain and have yielded insights into the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Through the detailed exploration of organoid models tailored to each neurological condition, including specific genetic mutations and treatment approaches, this technology demonstrates immense potential in disease-modeling and drug-screening endeavors.
While induced pluripotent stem cells (iPSCs) offer tremendous potential for regenerative therapies, there are inherent challenges and limitations that must be addressed. Enhancements in organoid protocols are necessary to improve reproducibility, specificity, and functional characteristics. Moreover, the careful consideration of the tumorigenic potential of iPSCs underscores the importance of rigorous investigations and implementation of safety measures in their application for cell therapy [305,306]. Issues related to iPSC study models include their specificity, which is advantageous for mutation-specific diseases but challenging for sporadic, multifactorial, and epigenetic-dependent diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). In rodent models of iPSCs used for drug screening, the translational efficacy of developed drugs has been limited, highlighting the value of using human iPSCs (hiPSCs). However, using hiPSCs generated from patient-specific cells for drug screening across diseases with diverse origins may compromise the effectiveness of therapy. Therefore, there is a need to discover new drug targets and standardize phenotypes for diseases like AD. Additionally, understanding the lack of translational potential for a particular treatment across multiple patients is crucial for effectively treating individual patients modeled with iPSCs [307]. Therefore, having a data bank for each modeled iPSC is of major importance.
In summary, the extensive investigation into iPSCs and their role in modeling neurological diseases, developing organoids, and exploring potential therapies offers a comprehensive view of the current research landscape in this field. The ongoing endeavors to improve techniques and overcome limitations highlight a dedication to advancing iPSC-based methodologies for gaining deeper insights into neuropathologies and fostering the development of innovative treatments.
7. Future Perspectives
Regenerative medicine seeks to repair and rejuvenate impaired organs or tissues, aiming to bring back their original form and function. Currently, clinical trials are being conducted to explore cell therapies aimed at replenishing vital cell types lost due to various diseases. For instance, in Parkinson’s disease, efforts are focused on replacing neurons [308]. In vivo reprogramming is increasingly seen as an appealing alternative to address the technical challenges associated with the iPSC technology, such as ex vivo reprogramming and large-scale expansion.
The process of in vivo reprogramming takes place amidst a distinctive cellular and extracellular milieu rich in tissue-specific biochemical and mechanical cues. Under these circumstances, cells are generated displaying a greater level of maturation compared to those reprogrammed in vitro [309]. For it to be successful, the identification of appropriate cell sources is crucial. Ideally, these initial cells should be abundant and permissive to reprogramming. Two types of macroglial cells, namely, astrocytes and oligodendrocyte progenitor cells (also referred to as NG2 glia), have been extensively investigated as prime candidates for conversion into induced neurons [310]. NG2 glia possess the ability to self-renew and exhibit high proliferation rates, characteristics that may mitigate the risk of depleting the native population crucial for maintaining tissue homeostasis [311]. For the insights gained from these animal studies to be applicable in clinical settings, it is imperative to attain robust reprogramming within diseased organs safely. Additionally, comprehensive regulatory protocols are required to unify and regulate the endeavors of both academic and industry sectors effectively.
Acknowledgments
I We would like to thank the Brain Institute at Rio Grande do Sul and the Pontifical Catholic University of Rio Grande do Sul for their constant support of research.
Author Contributions
Conceptualization, J.I.B.G., G.Z. and D.R.M.; investigation, D.B.P., T.T.R.P., J.I.B.G. and F.A.C.X.; resources, J.C.d.C.; writing—original draft preparation, D.B.P., T.T.R.P., J.I.B.G. and F.A.C.X.; writing—review and editing, D.B.P., T.T.R.P. and J.I.B.G.; supervision, D.R.M.; funding acquisition, general review and final revision of the manuscript, J.C.d.C. and D.R.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brazil, Finance Code 001.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Trejo-Lopez J.A., Yachnis A.T., Prokop S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics. 2022;19:173–185. doi: 10.1007/s13311-021-01146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curatolo P., Specchio N., Aronica E. Advances in the Genetics and Neuropathology of Tuberous Sclerosis Complex: Edging Closer to Targeted Therapy. Lancet Neurol. 2022;21:843–856. doi: 10.1016/S1474-4422(22)00213-7. [DOI] [PubMed] [Google Scholar]
- 3.Chang B.-L., Chang K.-H. Stem Cell Therapy in Treating Epilepsy. Front. Neurosci. 2022;16:934507. doi: 10.3389/fnins.2022.934507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toman N.G., Grande A.W., Low W.C. Neural Repair in Stroke. Cell Transplant. 2019;28:1123–1126. doi: 10.1177/0963689719863784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro B.F., da Cruz B.C., de Sousa B.M., Correia P.D., David N., Rocha C., Almeida R.D., Ribeiro da Cunha M., Marques Baptista A.A., Vieira S.I. Cell Therapies for Spinal Cord Injury: A Review of the Clinical Trials and Cell-Type Therapeutic Potential. Brain J. Neurol. 2023;146:2672–2693. doi: 10.1093/brain/awad047. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y., Tan S. Direct Lineage Conversion of Astrocytes to Induced Neural Stem Cells or Neurons. Neurosci. Bull. 2015;31:357–367. doi: 10.1007/s12264-014-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinney C.E. Using Induced Pluripotent Stem Cells Derived Neurons to Model Brain Diseases. Neural Regen. Res. 2017;12:1062–1067. doi: 10.4103/1673-5374.211180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paredes-Espinosa M.B., Paluh J.L. Human Stem Cell-Derived Neurons and Neural Circuitry Therapeutics: Next Frontier in Spinal Cord Injury Repair. Exp. Biol. Med. 2022;247:2142–2151. doi: 10.1177/15353702221114099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans M.J., Kaufman M.H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 11.Martello G., Smith A. The Nature of Embryonic Stem Cells. Annu. Rev. Cell Dev. Biol. 2014;30:647–675. doi: 10.1146/annurev-cellbio-100913-013116. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Madrid M., Sumen C., Aivio S., Saklayen N. Autologous Induced Pluripotent Stem Cell-Based Cell Therapies: Promise, Progress, and Challenges. Curr. Protoc. 2021;1:e88. doi: 10.1002/cpz1.88. [DOI] [PubMed] [Google Scholar]
- 14.Ho B.X., Pek N.M.Q., Soh B.-S. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018;19:936. doi: 10.3390/ijms19040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong L., Zhang M., Ly O.T., Chen H., Sridhar A., Lambers E., Chalazan B., Youn S.-W., Maienschein-Cline M., Feferman L., et al. Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes Carrying an SCN5A Mutation Identify Nitric Oxide Signaling as a Mediator of Atrial Fibrillation. Stem Cell Rep. 2021;16:1542–1554. doi: 10.1016/j.stemcr.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasteuning-Vuhman S., de Jongh R., Timmers A., Pasterkamp R.J. Towards Advanced iPSC-Based Drug Development for Neurodegenerative Disease. Trends Mol. Med. 2021;27:263–279. doi: 10.1016/j.molmed.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu G., David B.T., Trawczynski M., Fessler R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020;16:3–32. doi: 10.1007/s12015-019-09935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgetti A., Montserrat N., Aasen T., Gonzalez F., Rodríguez-Pizà I., Vassena R., Raya A., Boué S., Barrero M.J., Corbella B.A., et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood Using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aasen T., Raya A., Barrero M.J., Garreta E., Consiglio A., Gonzalez F., Vassena R., Bilić J., Pekarik V., Tiscornia G., et al. Efficient and Rapid Generation of Induced Pluripotent Stem Cells from Human Keratinocytes. Nat. Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 20.Aasen T., Belmonte J.C.I. Isolation and Cultivation of Human Keratinocytes from Skin or Plucked Hair for the Generation of Induced Pluripotent Stem Cells. Nat. Protoc. 2010;5:371–382. doi: 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- 21.Zhou T., Benda C., Dunzinger S., Huang Y., Ho J.C., Yang J., Wang Y., Zhang Y., Zhuang Q., Li Y., et al. Generation of Human Induced Pluripotent Stem Cells from Urine Samples. Nat. Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 22.Xue Y., Cai X., Wang L., Liao B., Zhang H., Shan Y., Chen Q., Zhou T., Li X., Hou J., et al. Generating a Non-Integrating Human Induced Pluripotent Stem Cell Bank from Urine-Derived Cells. PLoS ONE. 2013;8:e70573. doi: 10.1371/journal.pone.0070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y.-F., Chen M., Zhang N.-N., Yang H.-J., Rui Q., Zhou Y.-F. In Vitro and in Vivo Differentiation of Induced Pluripotent Stem Cells Generated from Urine-Derived Cells into Cardiomyocytes. Biol. Open. 2018;7:bio029157. doi: 10.1242/bio.029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalise M., Marino F., Salerno L., Cianflone E., Molinaro C., Salerno N., De Angelis A., Viglietto G., Urbanek K., Torella D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021;22:13180. doi: 10.3390/ijms222413180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su R.-J., Baylink D.J., Neises A., Kiroyan J.B., Meng X., Payne K.J., Tschudy-Seney B., Duan Y., Appleby N., Kearns-Jonker M., et al. Efficient Generation of Integration-Free Ips Cells from Human Adult Peripheral Blood Using BCL-XL Together with Yamanaka Factors. PLoS ONE. 2013;8:e64496. doi: 10.1371/journal.pone.0064496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanna J., Markoulaki S., Schorderet P., Carey B.W., Beard C., Wernig M., Creyghton M.P., Steine E.J., Cassady J.P., Foreman R., et al. Direct Reprogramming of Terminally Differentiated Mature B Lymphocytes to Pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagano S., Maeda T., Ichise H., Kashima S., Ohtaka M., Nakanishi M., Kitawaki T., Kadowaki N., Takaori-Kondo A., Masuda K., et al. High Frequency Production of T Cell-Derived iPSC Clones Capable of Generating Potent Cytotoxic T Cells. Mol. Ther. Methods Clin. Dev. 2020;16:126–135. doi: 10.1016/j.omtm.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suwanpitak S., Promnakhon N., Netsrithong R., Wattanapanitch M. Efficient Generation of iPSC-Derived Hematoendothelial Progenitors and Specification Toward T Cell Lineage. Methods Mol. Biol. 2022;2454:423–442. doi: 10.1007/7651_2021_355. [DOI] [PubMed] [Google Scholar]
- 29.Serwold T., Hochedlinger K., Swindle J., Hedgpeth J., Jaenisch R., Weissman I.L. T-Cell Receptor-Driven Lymphomagenesis in Mice Derived from a Reprogrammed T Cell. Proc. Natl. Acad. Sci. USA. 2010;107:18939–18943. doi: 10.1073/pnas.1013230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowey S.N., Huang X., Chou B.-K., Ye Z., Cheng L. Generation of Integration-Free Human Induced Pluripotent Stem Cells from Postnatal Blood Mononuclear Cells by Plasmid Vector Expression. Nat. Protoc. 2012;7:2013–2021. doi: 10.1038/nprot.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou H., Martinez H., Sun B., Li A., Zimmer M., Katsanis N., Davis E.E., Kurtzberg J., Lipnick S., Noggle S., et al. Rapid and Efficient Generation of Transgene-Free iPSC from a Small Volume of Cryopreserved Blood. Stem Cell Rev. Rep. 2015;11:652–665. doi: 10.1007/s12015-015-9586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staerk J., Dawlaty M.M., Gao Q., Maetzel D., Hanna J., Sommer C.A., Mostoslavsky G., Jaenisch R. Reprogramming of Human Peripheral Blood Cells to Induced Pluripotent Stem Cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moodley K., Sibanda N., February K., Rossouw T. “It’s My Blood”: Ethical Complexities in the Use, Storage and Export of Biological Samples: Perspectives from South African Research Participants. BMC Med. Ethics. 2014;15:4. doi: 10.1186/1472-6939-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González F., Boué S., Izpisúa Belmonte J.C. Methods for Making Induced Pluripotent Stem Cells: Reprogramming à La Carte. Nat. Rev. Genet. 2011;12:231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 35.Eminli S., Foudi A., Stadtfeld M., Maherali N., Ahfeldt T., Mostoslavsky G., Hock H., Hochedlinger K. Differentiation Stage Determines Potential of Hematopoietic Cells for Reprogramming into Induced Pluripotent Stem Cells. Nat. Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 37.Feng B., Ng J.-H., Heng J.-C.D., Ng H.-H. Molecules That Promote or Enhance Reprogramming of Somatic Cells to Induced Pluripotent Stem Cells. Cell Stem Cell. 2009;4:301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.B., Zaehres H., Wu G., Gentile L., Ko K., Sebastiano V., Araúzo-Bravo M.J., Ruau D., Han D.W., Zenke M., et al. Pluripotent Stem Cells Induced from Adult Neural Stem Cells by Reprogramming with Two Factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 39.Huangfu D., Osafune K., Maehr R., Guo W., Eijkelenboom A., Chen S., Muhlestein W., Melton D.A. Induction of Pluripotent Stem Cells from Primary Human Fibroblasts with Only Oct4 and Sox2. Nat. Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 40.Kim J.B., Greber B., Araúzo-Bravo M.J., Meyer J., Park K.I., Zaehres H., Schöler H.R. Direct Reprogramming of Human Neural Stem Cells by OCT4. Nature. 2009;461:649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.B., Sebastiano V., Wu G., Araúzo-Bravo M.J., Sasse P., Gentile L., Ko K., Ruau D., Ehrich M., van den Boom D., et al. Oct4-Induced Pluripotency in Adult Neural Stem Cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Tsai S.Y., Bouwman B.A., Ang Y.S., Kim S.J., Lee D.F., Lemischka I.R., Rendl M. Single Transcription Factor Reprogramming of Hair Follicle Dermal Papilla Cells to Induced Pluripotent Stem Cells. Stem Cells. 2011;29:964–971. doi: 10.1002/stem.649. [DOI] [PubMed] [Google Scholar]
- 43.Hanna J., Saha K., Pando B., van Zon J., Lengner C.J., Creyghton M.P., van Oudenaarden A., Jaenisch R. Direct Cell Reprogramming Is a Stochastic Process Amenable to Acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y., Yin X., Qin H., Zhu F., Liu H., Yang W., Zhang Q., Xiang C., Hou P., Song Z., et al. Two Supporting Factors Greatly Improve the Efficiency of Human iPSC Generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Tsubooka N., Ichisaka T., Okita K., Takahashi K., Nakagawa M., Yamanaka S. Roles of Sall4 in the Generation of Pluripotent Stem Cells from Blastocysts and Fibroblasts. Genes Cells. 2009;14:683–694. doi: 10.1111/j.1365-2443.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- 46.Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I., Thomson J.A. Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park I.-H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of Human Somatic Cells to Pluripotency with Defined Factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 48.Lee J., Park Y.-J., Jung H. Protein Kinases and Their Inhibitors in Pluripotent Stem Cell Fate Regulation. Stem Cells Int. 2019;2019:e1569740. doi: 10.1155/2019/1569740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N., Long B., Han W., Yuan S., Wang K. microRNAs: Important Regulators of Stem Cells. Stem Cell Res. Ther. 2017;8:110. doi: 10.1186/s13287-017-0551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanyam D., Lamouille S., Judson R.L., Liu J.Y., Bucay N., Derynck R., Blelloch R. Multiple Targets of miR-302 and miR-372 Promote Reprogramming of Human Fibroblasts to Induced Pluripotent Stem Cells. Nat. Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Çağlayan E.S., Güran Ş. Importance of Myc-Related microRNAs in Induced Pluripotency. Cell Biol. Int. 2015;39:987–994. doi: 10.1002/cbin.10467. [DOI] [PubMed] [Google Scholar]
- 52.Lakshmipathy U., Davila J., Hart R.P. miRNA in Pluripotent Stem Cells. Regen. Med. 2010;5:545–555. doi: 10.2217/rme.10.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomes K.M.S., Costa I.C., Santos J.F.D., Dourado P.M.M., Forni M.F., Ferreira J.C.B. Induced Pluripotent Stem Cells Reprogramming: Epigenetics and Applications in the Regenerative Medicine. Rev. Assoc. Med. Bras. 2017;63:180–189. doi: 10.1590/1806-9282.63.02.180. [DOI] [PubMed] [Google Scholar]
- 54.Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K., et al. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Zhang Q., Yin X., Yang W., Du Y., Hou P., Ge J., Liu C., Zhang W., Zhang X., et al. Generation of iPSCs from Mouse Fibroblasts with a Single Gene, Oct4, and Small Molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan J., Wang G., Wang J., Zhang Z., Fu Y., Cheng L., Meng G., Lyu Y., Zhu J., Li Y., et al. Chemical Reprogramming of Human Somatic Cells to Pluripotent Stem Cells. Nature. 2022;605:325–331. doi: 10.1038/s41586-022-04593-5. [DOI] [PubMed] [Google Scholar]
- 57.Dominguez A.A., Lim W.A., Qi L.S. Beyond Editing: Repurposing CRISPR-Cas9 for Precision Genome Regulation and Interrogation. Nat. Rev. Mol. Cell Biol. 2016;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weltner J., Balboa D., Katayama S., Bespalov M., Krjutškov K., Jouhilahti E.-M., Trokovic R., Kere J., Otonkoski T. Human Pluripotent Reprogramming with CRISPR Activators. Nat. Commun. 2018;9:2643. doi: 10.1038/s41467-018-05067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokka J., Yoshihara M., Kvist J., Laiho L., Warren A., Stadelmann C., Jouhilahti E.-M., Kilpinen H., Balboa D., Katayama S., et al. CRISPR Activation Enables High-Fidelity Reprogramming into Human Pluripotent Stem Cells. Stem Cell Rep. 2022;17:413–426. doi: 10.1016/j.stemcr.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter J.L., Halmai J.A.N.M., Fink K.D. The iNs and Outs of Direct Reprogramming to Induced Neurons. Front. Genome Ed. 2020;2:7. doi: 10.3389/fgeed.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chavez A., Scheiman J., Vora S., Pruitt B.W., Tuttle M., P R Iyer E., Lin S., Kiani S., Guzman C.D., Wiegand D.J., et al. Highly Efficient Cas9-Mediated Transcriptional Programming. Nat. Methods. 2015;12:326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brix J., Zhou Y., Luo Y. The Epigenetic Reprogramming Roadmap in Generation of iPSCs from Somatic Cells. J. Genet. Genomics Yi Chuan Xue Bao. 2015;42:661–670. doi: 10.1016/j.jgg.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Van den Hurk M., Kenis G., Bardy C., van den Hove D.L., Gage F.H., Steinbusch H.W., Rutten B.P. Transcriptional and Epigenetic Mechanisms of Cellular Reprogramming to Induced Pluripotency. Epigenomics. 2016;8:1131–1149. doi: 10.2217/epi-2016-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papp B., Plath K. Epigenetics of Reprogramming to Induced Pluripotency. Cell. 2013;152:1324–1343. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huangfu D., Maehr R., Guo W., Eijkelenboom A., Snitow M., Chen A.E., Melton D.A. Induction of Pluripotent Stem Cells by Defined Factors Is Greatly Improved by Small-Molecule Compounds. Nat. Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mali P., Chou B.-K., Yen J., Ye Z., Zou J., Dowey S., Brodsky R.A., Ohm J.E., Yu W., Baylin S.B., et al. Butyrate Greatly Enhances Derivation of Human Induced Pluripotent Stem Cells by Promoting Epigenetic Remodeling and the Expression of Pluripotency-Associated Genes. Stem Cells. 2010;28:713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang S.-J., Park Y.-I., So B., Kang H.-G. Sodium Butyrate Efficiently Converts Fully Reprogrammed Induced Pluripotent Stem Cells from Mouse Partially Reprogrammed Cells. Cell. Reprogram. 2014;16:345–354. doi: 10.1089/cell.2013.0087. [DOI] [PubMed] [Google Scholar]
- 68.Shi Y., Desponts C., Do J.T., Hahm H.S., Schöler H.R., Ding S. Induction of Pluripotent Stem Cells from Mouse Embryonic Fibroblasts by Oct4 and Klf4 with Small-Molecule Compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. Retrovirus-Mediated Gene Transfer and Expression Cloning: Powerful Tools in Functional Genomics. Exp. Hematol. 2003;31:1007–1014. doi: 10.1016/S0301-472X(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi K., Okita K., Nakagawa M., Yamanaka S. Induction of Pluripotent Stem Cells from Fibroblast Cultures. Nat. Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 71.Varas F., Stadtfeld M., de Andres-Aguayo L., Maherali N., di Tullio A., Pantano L., Notredame C., Hochedlinger K., Graf T. Fibroblast-Derived Induced Pluripotent Stem Cells Show No Common Retroviral Vector Insertions. Stem Cells. 2009;27:300–306. doi: 10.1634/stemcells.2008-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao S., Sukonnik T., Kean T., Bharadwaj R.R., Pasceri P., Ellis J. Retrovirus Silencing, Variegation, Extinction, and Memory Are Controlled by a Dynamic Interplay of Multiple Epigenetic Modifications. Mol. Ther. J. Am. Soc. Gene Ther. 2004;10:27–36. doi: 10.1016/j.ymthe.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Sommer C.A., Stadtfeld M., Murphy G.J., Hochedlinger K., Kotton D.N., Mostoslavsky G. Induced Pluripotent Stem Cell Generation Using a Single Lentiviral Stem Cell Cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hämäläinen R., Cowling R., Wang W., Liu P., Gertsenstein M., et al. piggyBac Transposition Reprograms Fibroblasts to Induced Pluripotent Stem Cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clevers H. Modeling Development and Disease with Organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 76.Lee C.-T., Bendriem R.M., Wu W.W., Shen R.-F. 3D Brain Organoids Derived from Pluripotent Stem Cells: Promising Experimental Models for Brain Development and Neurodegenerative Disorders. J. Biomed. Sci. 2017;24:59. doi: 10.1186/s12929-017-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrews M.G., Kriegstein A.R. Challenges of Organoid Research. Annu. Rev. Neurosci. 2022;45:23–39. doi: 10.1146/annurev-neuro-111020-090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garreta E., Kamm R.D., Chuva De Sousa Lopes S.M., Lancaster M.A., Weiss R., Trepat X., Hyun I., Montserrat N. Rethinking Organoid Technology through Bioengineering. Nat. Mater. 2021;20:145–155. doi: 10.1038/s41563-020-00804-4. [DOI] [PubMed] [Google Scholar]
- 79.Turhan A.G., Hwang J.W., Chaker D., Tasteyre A., Latsis T., Griscelli F., Desterke C., Bennaceur-Griscelli A. iPSC-Derived Organoids as Therapeutic Models in Regenerative Medicine and Oncology. Front. Med. 2021;8:728543. doi: 10.3389/fmed.2021.728543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niu W., Parent J.M. Modeling Genetic Epilepsies in a Dish. Dev. Dyn. 2020;249:56–75. doi: 10.1002/dvdy.79. [DOI] [PubMed] [Google Scholar]
- 81.Camp J.G., Badsha F., Florio M., Kanton S., Gerber T., Wilsch-Bräuninger M., Lewitus E., Sykes A., Hevers W., Lancaster M., et al. Human Cerebral Organoids Recapitulate Gene Expression Programs of Fetal Neocortex Development. Proc. Natl. Acad. Sci. USA. 2015;112:15672–15677. doi: 10.1073/pnas.1520760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wray S. Modelling Neurodegenerative Disease Using Brain Organoids. Semin. Cell Dev. Biol. 2021;111:60–66. doi: 10.1016/j.semcdb.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Hirose S., Tanaka Y., Shibata M., Kimura Y., Ishikawa M., Higurashi N., Yamamoto T., Ichise E., Chiyonobu T., Ishii A. Application of Induced Pluripotent Stem Cells in Epilepsy. Mol. Cell. Neurosci. 2020;108:103535. doi: 10.1016/j.mcn.2020.103535. [DOI] [PubMed] [Google Scholar]
- 84.Lancaster M.A., Renner M., Martin C.-A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Renner M., Lancaster M.A., Bian S., Choi H., Ku T., Peer A., Chung K., Knoblich J.A. Self-organized Developmental Patterning and Differentiation in Cerebral Organoids. EMBO J. 2017;36:1316–1329. doi: 10.15252/embj.201694700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaugler J., James B., Johnson T., Reimer J., Solis M., Weuve J., Buckley R.F., Hohman T.J. 2022 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2022;18:700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 87.Gatz M., Reynolds C.A., Fratiglioni L., Johansson B., Mortimer J.A., Berg S., Fiske A., Pedersen N.L. Role of Genes and Environments for Explaining Alzheimer Disease. Arch. Gen. Psychiatry. 2006;63:168. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 88.Van der Lee S.J., Wolters F.J., Ikram M.K., Hofman A., Ikram M.A., Amin N., van Duijn C.M. The Effect of APOE and Other Common Genetic Variants on the Onset of Alzheimer’s Disease and Dementia: A Community-Based Cohort Study. Lancet Neurol. 2018;17:434–444. doi: 10.1016/S1474-4422(18)30053-X. [DOI] [PubMed] [Google Scholar]
- 89.Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., Cummings J., Van Der Flier W.M. Alzheimer’s Disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ranjan V.D., Qiu L., Tan E.K., Zeng L., Zhang Y. Modelling Alzheimer’s Disease: Insights from in Vivo to in Vitro Three-Dimensional Culture Platforms. J. Tissue Eng. Regen. Med. 2018;12:1944–1958. doi: 10.1002/term.2728. [DOI] [PubMed] [Google Scholar]
- 91.Bi F.-C., Yang X.-H., Cheng X.-Y., Deng W.-B., Guo X.-L., Yang H., Wang Y., Li J., Yao Y. Optimization of Cerebral Organoids: A More Qualified Model for Alzheimer’s Disease Research. Transl. Neurodegener. 2021;10:27. doi: 10.1186/s40035-021-00252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavoni S., Jarray R., Nassor F., Guyot A.-C., Cottin S., Rontard J., Mikol J., Mabondzo A., Deslys J.-P., Yates F. Small-Molecule Induction of Aβ-42 Peptide Production in Human Cerebral Organoids to Model Alzheimer’s Disease Associated Phenotypes. PLoS ONE. 2018;13:e0209150. doi: 10.1371/journal.pone.0209150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raja W.K., Mungenast A.E., Lin Y.-T., Ko T., Abdurrob F., Seo J., Tsai L.-H. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS ONE. 2016;11:e0161969. doi: 10.1371/journal.pone.0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan Y., Song L., Bejoy J., Zhao J., Kanekiyo T., Bu G., Zhou Y., Li Y. Modeling Neurodegenerative Microenvironment Using Cortical Organoids Derived from Human Stem Cells. Tissue Eng. Part A. 2018;24:1125–1137. doi: 10.1089/ten.tea.2017.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Y.-T., Seo J., Gao F., Feldman H.M., Wen H.-L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018;98:1141–1154. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischer L.R., Culver D.G., Tennant P., Davis A.A., Wang M., Castellano-Sanchez A., Khan J., Polak M.A., Glass J.D. Amyotrophic Lateral Sclerosis Is a Distal Axonopathy: Evidence in Mice and Man. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Taylor J.P., Brown R.H., Cleveland D.W. Decoding ALS: From Genes to Mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pasinelli P., Brown R.H. Molecular Biology of Amyotrophic Lateral Sclerosis: Insights from Genetics. Nat. Rev. Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 99.Pereira J.D., DuBreuil D.M., Devlin A.-C., Held A., Sapir Y., Berezovski E., Hawrot J., Dorfman K., Chander V., Wainger B.J. Human Sensorimotor Organoids Derived from Healthy and Amyotrophic Lateral Sclerosis Stem Cells Form Neuromuscular Junctions. Nat. Commun. 2021;12:4744. doi: 10.1038/s41467-021-24776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agnew-Blais J.C., Polanczyk G.V., Danese A., Wertz J., Moffitt T.E., Arseneault L. Evaluation of the Persistence, Remission, and Emergence of Attention-Deficit/Hyperactivity Disorder in Young Adulthood. JAMA Psychiatry. 2016;73:713–720. doi: 10.1001/jamapsychiatry.2016.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinshaw S.P. Attention Deficit Hyperactivity Disorder (ADHD): Controversy, Developmental Mechanisms, and Multiple Levels of Analysis. Annu. Rev. Clin. Psychol. 2018;14:291–316. doi: 10.1146/annurev-clinpsy-050817-084917. [DOI] [PubMed] [Google Scholar]
- 102.Arnsten A.F.T., Rubia K. Neurobiological Circuits Regulating Attention, Cognitive Control, Motivation, and Emotion: Disruptions in Neurodevelopmental Psychiatric Disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 103.Ball G., Malpas C.B., Genc S., Efron D., Sciberras E., Anderson V., Nicholson J.M., Silk T.J. Multimodal Structural Neuroimaging Markers of Brain Development and ADHD Symptoms. Am. J. Psychiatry. 2019;176:57–66. doi: 10.1176/appi.ajp.2018.18010034. [DOI] [PubMed] [Google Scholar]
- 104.Hartlaub A.M., McElroy C.A., Maitre N.L., Hester M.E. Modeling Human Brain Circuitry Using Pluripotent Stem Cell Platforms. Front. Pediatr. 2019;7:57. doi: 10.3389/fped.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang D., Eguchi N., Okazaki S., Sora I., Hishimoto A. Telencephalon Organoids Derived from an Individual with ADHD Show Altered Neurodevelopment of Early Cortical Layer Structure. Stem Cell Rev. Rep. 2023;19:1482–1491. doi: 10.1007/s12015-023-10519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanner L. Autistic disturbances of aff ective contact. Nerv. Child. 1943;2:217–250. [Google Scholar]
- 107.Villa C., Combi R., Conconi D., Lavitrano M. Patient-Derived Induced Pluripotent Stem Cells (iPSCs) and Cerebral Organoids for Drug Screening and Development in Autism Spectrum Disorder: Opportunities and Challenges. Pharmaceutics. 2021;13:280. doi: 10.3390/pharmaceutics13020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L., Wang B., Wu C., Wang J., Sun M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023;24:1819. doi: 10.3390/ijms24031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baio J. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC, USA: 2013. Neurodevelopmental disorders. [Google Scholar]
- 111.Iles A. Autism Spectrum Disorders. Prim. Care Clin. Off. Pract. 2021;48:461–473. doi: 10.1016/j.pop.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Genovese A., Butler M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes. 2023;14:677. doi: 10.3390/genes14030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mariani J., Coppola G., Zhang P., Abyzov A., Provini L., Tomasini L., Amenduni M., Szekely A., Palejev D., Wilson M., et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162:375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sassone-Corsi P., Christen Y., editors. A Time for Metabolism and Hormones. Springer; Cham, Switzerland: 2016. [PubMed] [Google Scholar]
- 115.Forsberg S.L., Ilieva M., Maria Michel T. Epigenetics and Cerebral Organoids: Promising Directions in Autism Spectrum Disorders. Transl. Psychiatry. 2018;8:14. doi: 10.1038/s41398-017-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santos J.L.d.S., Araújo C.d.A., Rocha C.A.G., Costa-Ferro Z.S.M., Souza B.S.d.F. Modeling Autism Spectrum Disorders with Induced Pluripotent Stem Cell-Derived Brain Organoids. Biomolecules. 2023;13:260. doi: 10.3390/biom13020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russo F.B., Brito A., de Freitas A.M., Castanha A., de Freitas B.C., Beltrão-Braga P.C.B. The Use of iPSC Technology for Modeling Autism Spectrum Disorders. Neurobiol. Dis. 2019;130:104483. doi: 10.1016/j.nbd.2019.104483. [DOI] [PubMed] [Google Scholar]
- 118.Nagy A., Bley A.E., Eichler F. Canavan Disease. In: Adam M.P., Feldman J., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J., Gripp K.W., Amemiya A., editors. GeneReviews®. University of Washington; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 119.Lotun A., Gessler D.J., Gao G. Canavan Disease as a Model for Gene Therapy-Mediated Myelin Repair. Front. Cell. Neurosci. 2021;15:661928. doi: 10.3389/fncel.2021.661928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng L., Chao J., Ye P., Luong Q., Sun G., Liu W., Cui Q., Flores S., Jackson N., Shayento A.N.H., et al. Developing Hypoimmunogenic Human iPSC-Derived Oligodendrocyte Progenitor Cells as an Off-The-Shelf Cell Therapy for Myelin Disorders. Adv. Sci. 2023;10:e2206910. doi: 10.1002/advs.202206910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chao J., Feng L., Ye P., Chen X., Cui Q., Sun G., Zhou T., Tian E., Li W., Hu W., et al. Therapeutic Development for Canavan Disease Using Patient iPSCs Introduced with the Wild-Type ASPA Gene. iScience. 2022;25:104391. doi: 10.1016/j.isci.2022.104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feng L., Chao J., Tian E., Li L., Ye P., Zhang M., Chen X., Cui Q., Sun G., Zhou T., et al. Cell-Based Therapy for Canavan Disease Using Human iPSC-Derived NPCs and OPCs. Adv. Sci. 2020;7:2002155. doi: 10.1002/advs.202002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng L., Chao J., Zhang M., Pacquing E., Hu W., Shi Y. Developing a Human iPSC-Derived Three-Dimensional Myelin Spheroid Platform for Modeling Myelin Diseases. iScience. 2023;26:108037. doi: 10.1016/j.isci.2023.108037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fisher R.S., Boas W.V.E., Blume W., Elger C., Genton P., Lee P., Engel J., Jr. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 125.Falco-Walter J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020;40:617–623. doi: 10.1055/s-0040-1718719. [DOI] [PubMed] [Google Scholar]
- 126.Epilepsy: A Public Health Imperative: Summary. WHO; Geneva, Switzerland: 2019. [Google Scholar]
- 127.Bang J., Spina S., Miller B.L. Frontotemporal Dementia. Lancet. 2015;386:1672–1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.WHO Dementia Fact Sheet. March 2023. [(accessed on 1 February 2024)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dementia.
- 129.Coyle-Gilchrist I.T.S., Dick K.M., Patterson K., Vázquez Rodríquez P., Wehmann E., Wilcox A., Lansdall C.J., Dawson K.E., Wiggins J., Mead S., et al. Prevalence, Characteristics, and Survival of Frontotemporal Lobar Degeneration Syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vieira R.T., Caixeta L., Machado S., Silva A.C., Nardi A.E., Arias-Carrión O., Carta M.G. Epidemiology of Early-Onset Dementia: A Review of the Literature. Clin. Pract. Epidemiol. Ment. Health CP EMH. 2013;9:88–95. doi: 10.2174/1745017901309010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Olney N.T., Spina S., Miller B.L. Frontotemporal Dementia. Neurol. Clin. 2017;35:339–374. doi: 10.1016/j.ncl.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bowles K.R., Silva M.C., Whitney K., Bertucci T., Berlind J.E., Lai J.D., Garza J.C., Boles N.C., Mahali S., Strang K.H., et al. ELAVL4, Splicing, and Glutamatergic Dysfunction Precede Neuron Loss in MAPT Mutation Cerebral Organoids. Cell. 2021;184:4547–4563.e17. doi: 10.1016/j.cell.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szebényi K., Wenger L.M.D., Sun Y., Dunn A.W.E., Limegrover C.A., Gibbons G.M., Conci E., Paulsen O., Mierau S.B., Balmus G., et al. Human ALS/FTD Brain Organoid Slice Cultures Display Distinct Early Astrocyte and Targetable Neuronal Pathology. Nat. Neurosci. 2021;24:1542–1554. doi: 10.1038/s41593-021-00923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.MacDonald M.E., Ambrose C.M., Duyao M.P., Myers R.H., Lin C., Srinidhi L., Barnes G., Taylor S.A., James M., Groot N., et al. A Novel Gene Containing a Trinucleotide Repeat That Is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 136.Arrasate M., Finkbeiner S. Protein Aggregates in Huntington’s Disease. Exp. Neurol. 2012;238:1–11. doi: 10.1016/j.expneurol.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Andhale R., Shrivastava D. Huntington’s Disease: A Clinical Review. Cureus. 2022;14:e28484. doi: 10.7759/cureus.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tabrizi S.J., Flower M.D., Ross C.A., Wild E.J. Huntington Disease: New Insights into Molecular Pathogenesis and Therapeutic Opportunities. Nat. Rev. Neurol. 2020;16:529–546. doi: 10.1038/s41582-020-0389-4. [DOI] [PubMed] [Google Scholar]
- 139.Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R., et al. Huntington Disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 140.Ross C.A., Aylward E.H., Wild E.J., Langbehn D.R., Long J.D., Warner J.H., Scahill R.I., Leavitt B.R., Stout J.C., Paulsen J.S., et al. Huntington Disease: Natural History, Biomarkers and Prospects for Therapeutics. Nat. Rev. Neurol. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 141.Pouladi M.A., Morton A.J., Hayden M.R. Choosing an Animal Model for the Study of Huntington’s Disease. Nat. Rev. Neurosci. 2013;14:708–721. doi: 10.1038/nrn3570. [DOI] [PubMed] [Google Scholar]
- 142.Ramaswamy S., McBride J.L., Kordower J.H. Animal Models of Huntington’s Disease. ILAR J. 2007;48:356–373. doi: 10.1093/ilar.48.4.356. [DOI] [PubMed] [Google Scholar]
- 143.Park I.-H., Lerou P.H., Zhao R., Huo H., Daley G.Q. Generation of Human-Induced Pluripotent Stem Cells. Nat. Protoc. 2008;3:1180–1186. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- 144.Park I.-H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M.W., Cowan C., Hochedlinger K., Daley G.Q. Disease-Specific Induced Pluripotent Stem Cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang N., An M.C., Montoro D., Ellerby L.M. Characterization of Human Huntington’s Disease Cell Model from Induced Pluripotent Stem Cells. PLoS Curr. 2010;2:RRN1193. doi: 10.1371/currents.RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chae J.-I., Kim D.-W., Lee N., Jeon Y.-J., Jeon I., Kwon J., Kim J., Soh Y., Lee D.-S., Seo K.S., et al. Quantitative Proteomic Analysis of Induced Pluripotent Stem Cells Derived from a Human Huntington’s Disease Patient. Biochem. J. 2012;446:359–371. doi: 10.1042/BJ20111495. [DOI] [PubMed] [Google Scholar]
- 147.HD iPSC Consortium Induced Pluripotent Stem Cells from Patients with Huntington’s Disease Show CAG-Repeat-Expansion-Associated Phenotypes. Cell Stem Cell. 2012;11:264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mehta S.R., Tom C.M., Wang Y., Bresee C., Rushton D., Mathkar P.P., Tang J., Mattis V.B. Human Huntington’s Disease iPSC-Derived Cortical Neurons Display Altered Transcriptomics, Morphology, and Maturation. Cell Rep. 2018;25:1081–1096. doi: 10.1016/j.celrep.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 149.Moss D.J.H., Pardiñas A.F., Langbehn D., Lo K., Leavitt B.R., Roos R., Durr A., Mead S., TRACK-HD Investigators. REGISTRY Investigators et al. Identification of Genetic Variants Associated with Huntington’s Disease Progression: A Genome-Wide Association Study. Lancet Neurol. 2017;16:701–711. doi: 10.1016/S1474-4422(17)30161-8. [DOI] [PubMed] [Google Scholar]
- 150.Telenius H., Kremer B., Goldberg Y.P., Theilmann J., Andrew S.E., Zeisler J., Adam S., Greenberg C., Ives E.J., Clarke L.A. Somatic and Gonadal Mosaicism of the Huntington Disease Gene CAG Repeat in Brain and Sperm. Nat. Genet. 1994;6:409–414. doi: 10.1038/ng0494-409. [DOI] [PubMed] [Google Scholar]
- 151.Świtońska K., Szlachcic W.J., Handschuh L., Wojciechowski P., Marczak Ł., Stelmaszczuk M., Figlerowicz M., Figiel M. Identification of Altered Developmental Pathways in Human Juvenile HD iPSC With 71Q and 109Q Using Transcriptome Profiling. Front. Cell. Neurosci. 2018;12:528. doi: 10.3389/fncel.2018.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mattis V.B., Tom C., Akimov S., Saeedian J., Østergaard M.E., Southwell A.L., Doty C.N., Ornelas L., Sahabian A., Lenaeus L., et al. HD iPSC-Derived Neural Progenitors Accumulate in Culture and Are Susceptible to BDNF Withdrawal Due to Glutamate Toxicity. Hum. Mol. Genet. 2015;24:3257–3271. doi: 10.1093/hmg/ddv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith-Geater C., Hernandez S.J., Lim R.G., Adam M., Wu J., Stocksdale J.T., Wassie B.T., Gold M.P., Wang K.Q., Miramontes R., et al. Aberrant Development Corrected in Adult-Onset Huntington’s Disease iPSC-Derived Neuronal Cultures via WNT Signaling Modulation. Stem Cell Rep. 2020;14:406–419. doi: 10.1016/j.stemcr.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.An M.C., Zhang N., Scott G., Montoro D., Wittkop T., Mooney S., Melov S., Ellerby L.M. Genetic Correction of Huntington’s Disease Phenotypes in Induced Pluripotent Stem Cells. Cell Stem Cell. 2012;11:253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ring K.L., An M.C., Zhang N., O’Brien R.N., Ramos E.M., Gao F., Atwood R., Bailus B.J., Melov S., Mooney S.D., et al. Genomic Analysis Reveals Disruption of Striatal Neuronal Development and Therapeutic Targets in Human Huntington’s Disease Neural Stem Cells. Stem Cell Rep. 2015;5:1023–1038. doi: 10.1016/j.stemcr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu X., Tay Y., Sim B., Yoon S.-I., Huang Y., Ooi J., Utami K.H., Ziaei A., Ng B., Radulescu C., et al. Reversal of Phenotypic Abnormalities by CRISPR/Cas9-Mediated Gene Correction in Huntington Disease Patient-Derived Induced Pluripotent Stem Cells. Stem Cell Rep. 2017;8:619–633. doi: 10.1016/j.stemcr.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.HD iPSC Consortium Developmental Alterations in Huntington’s Disease Neural Cells and Pharmacological Rescue in Cells and Mice. Nat. Neurosci. 2017;20:648–660. doi: 10.1038/nn.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Olsson T., Barcellos L.F., Alfredsson L. Interactions between Genetic, Lifestyle and Environmental Risk Factors for Multiple Sclerosis. Nat. Rev. Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 159.Filippi M., Bar-Or A., Piehl F., Preziosa P., Solari A., Vukusic S., Rocca M.A. Multiple Sclerosis. Nat. Rev. Dis. Primers. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 160.McCaughey-Chapman A., Connor B. Cell Reprogramming for Oligodendrocytes: A Review of Protocols and Their Applications to Disease Modeling and Cell-Based Remyelination Therapies. J. Neurosci. Res. 2023;101:1000–1028. doi: 10.1002/jnr.25173. [DOI] [PubMed] [Google Scholar]
- 161.Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. Houndmills Basingstoke Engl. 2020;26:1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lublin F.D., Reingold S.C. Defining the Clinical Course of Multiple Sclerosis: Results of an International Survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/WNL.46.4.907. [DOI] [PubMed] [Google Scholar]
- 163.Greer J.M., McCombe P.A. Role of Gender in Multiple Sclerosis: Clinical Effects and Potential Molecular Mechanisms. J. Neuroimmunol. 2011;234:7–18. doi: 10.1016/j.jneuroim.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 164.Compston A., Coles A. Multiple Sclerosis. Lancet Lond. Engl. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 165.Daviaud N., Chen E., Edwards T., Sadiq S.A. Cerebral Organoids in Primary Progressive Multiple Sclerosis Reveal Stem Cell and Oligodendrocyte Differentiation Defect. Biol. Open. 2023;12:bio059845. doi: 10.1242/bio.059845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pouya A., Satarian L., Kiani S., Javan M., Baharvand H. Human Induced Pluripotent Stem Cells Differentiation into Oligodendrocyte Progenitors and Transplantation in a Rat Model of Optic Chiasm Demyelination. PLoS ONE. 2011;6:e27925. doi: 10.1371/journal.pone.0027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.García-León J.A., Kumar M., Boon R., Chau D., One J., Wolfs E., Eggermont K., Berckmans P., Gunhanlar N., de Vrij F., et al. SOX10 Single Transcription Factor-Based Fast and Efficient Generation of Oligodendrocytes from Human Pluripotent Stem Cells. Stem Cell Rep. 2018;10:655–672. doi: 10.1016/j.stemcr.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bose A., Petsko G.A., Studer L. Induced Pluripotent Stem Cells: A Tool for Modeling Parkinson’s Disease. Trends Neurosci. 2022;45:608–620. doi: 10.1016/j.tins.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marotta N., Kim S., Krainc D. Organoid and Pluripotent Stem Cells in Parkinson’s Disease Modeling: An Expert View on Their Value to Drug Discovery. Expert Opin. Drug Discov. 2020;15:427–441. doi: 10.1080/17460441.2020.1703671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Burré J., Sharma M., Südhof T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018;8:a024091. doi: 10.1101/cshperspect.a024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dettmer U., Newman A.J., Soldner F., Luth E.S., Kim N.C., Von Saucken V.E., Sanderson J.B., Jaenisch R., Bartels T., Selkoe D. Parkinson-Causing α-Synuclein Missense Mutations Shift Native Tetramers to Monomers as a Mechanism for Disease Initiation. Nat. Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jeong G.R., Lee B.D. Pathological Functions of LRRK2 in Parkinson’s Disease. Cells. 2020;9:2565. doi: 10.3390/cells9122565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seibler P., Graziotto J., Jeong H., Simunovic F., Klein C., Krainc D. Mitochondrial Parkin Recruitment Is Impaired in Neurons Derived from Mutant PINK1 Induced Pluripotent Stem Cells. J. Neurosci. 2011;31:5970–5976. doi: 10.1523/JNEUROSCI.4441-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eli I., Lerner D.P., Ghogawala Z. Acute Traumatic Spinal Cord Injury. Neurol. Clin. 2021;39:471–488. doi: 10.1016/j.ncl.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 176.Anjum A., Yazid M.D., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., Ismail O.H.R., Athi Kumar R.K., Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ahuja C.S., Nori S., Tetreault L., Wilson J., Kwon B., Harrop J., Choi D., Fehlings M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 178.Eckert M.J., Martin M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017;97:1031–1045. doi: 10.1016/j.suc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 179.Andersen J., Revah O., Miura Y., Thom N., Amin N.D., Kelley K.W., Singh M., Chen X., Thete M.V., Walczak E.M., et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell. 2020;183:1913–1929.e26. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu J., Fang S., Deng S., Li H., Lin X., Huang Y., Chung S., Shu Y., Shao Z. Generation of Neural Organoids for Spinal-Cord Regeneration via the Direct Reprogramming of Human Astrocytes. Nat. Biomed. Eng. 2023;7:253–269. doi: 10.1038/s41551-022-00963-6. [DOI] [PubMed] [Google Scholar]
- 181.Lee J.-H., Shin H., Shaker M.R., Kim H.J., Park S.-H., Kim J.H., Lee N., Kang M., Cho S., Kwak T.H., et al. Production of Human Spinal-Cord Organoids Recapitulating Neural-Tube Morphogenesis. Nat. Biomed. Eng. 2022;6:435–448. doi: 10.1038/s41551-022-00868-4. [DOI] [PubMed] [Google Scholar]
- 182.Winanto, Khong Z.-J., Hor J.-H., Ng S.-Y. Spinal Cord Organoids Add an Extra Dimension to Traditional Motor Neuron Cultures. Neural Regen. Res. 2019;14:1515. doi: 10.4103/1673-5374.255966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 184.Spellicy S.E., Hess D.C. The Immunomodulatory Capacity of Induced Pluripotent Stem Cells in the Post-Stroke Environment. Front. Cell Dev. Biol. 2021;9:647415. doi: 10.3389/fcell.2021.647415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Juntunen M., Hagman S., Moisan A., Narkilahti S., Miettinen S. In Vitro Oxygen-Glucose Deprivation-Induced Stroke Models with Human Neuroblastoma Cell- and Induced Pluripotent Stem Cell-Derived Neurons. Stem Cells Int. 2020;2020:e8841026. doi: 10.1155/2020/8841026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fernández-Susavila H., Bugallo-Casal A., Castillo J., Campos F. Adult Stem Cells and Induced Pluripotent Stem Cells for Stroke Treatment. Front. Neurol. 2019;10:908. doi: 10.3389/fneur.2019.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fernández-Susavila H., Mora C., Aramburu-Núñez M., Quintas-Rey R., Arias S., Collado M., López-Arias E., Sobrino T., Castillo J., Dell’Era P., et al. Generation and Characterization of the Human iPSC Line IDISi001-A Isolated from Blood Cells of a CADASIL Patient Carrying a NOTCH3 Mutation. Stem Cell Res. 2018;28:16–20. doi: 10.1016/j.scr.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 188.Ling C., Liu Z., Song M., Zhang W., Wang S., Liu X., Ma S., Sun S., Fu L., Chu Q., et al. Modeling CADASIL Vascular Pathologies with Patient-Derived Induced Pluripotent Stem Cells. Protein Cell. 2019;10:249–271. doi: 10.1007/s13238-019-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yan Y.-W., Qian E.S., Woodard L.E., Bejoy J. Neural Lineage Differentiation of Human Pluripotent Stem Cells: Advances in Disease Modeling. World J. Stem Cells. 2023;15:530–547. doi: 10.4252/wjsc.v15.i6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wevers N.R., Nair A.L., Fowke T.M., Pontier M., Kasi D.G., Spijkers X.M., Hallard C., Rabussier G., van Vught R., Vulto P., et al. Modeling Ischemic Stroke in a Triculture Neurovascular Unit On-a-Chip. Fluids Barriers CNS. 2021;18:59. doi: 10.1186/s12987-021-00294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lacalle-Aurioles M., Cassel de Camps C., Zorca C.E., Beitel L.K., Durcan T.M. Applying hiPSCs and Biomaterials Towards an Understanding and Treatment of Traumatic Brain Injury. Front. Cell. Neurosci. 2020;14:594304. doi: 10.3389/fncel.2020.594304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Imai R., Tamura R., Yo M., Sato M., Fukumura M., Takahara K., Kase Y., Okano H., Toda M. Neuroprotective Effects of Genome-Edited Human iPS Cell-Derived Neural Stem/Progenitor Cells on Traumatic Brain Injury. Stem Cells. 2023;41:603–616. doi: 10.1093/stmcls/sxad028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Werner C., Engelhard K. Pathophysiology of Traumatic Brain Injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 194.Krishnamurthy K., Laskowitz D.T. Cellular and Molecular Mechanisms of Secondary Neuronal Injury Following Traumatic Brain Injury. In: Laskowitz D., Grant G., editors. Translational Research in Traumatic Brain Injury. Frontiers in Neuroscience; CRC Press/Taylor and Francis Group; Boca Raton, FL, USA: 2016. [PubMed] [Google Scholar]
- 195.Ramos-Cejudo J., Wisniewski T., Marmar C., Zetterberg H., Blennow K., de Leon M.J., Fossati S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. eBioMedicine. 2018;28:21–30. doi: 10.1016/j.ebiom.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang K.K., Yang Z., Zhu T., Shi Y., Rubenstein R., Tyndall J.A., Manley G.T. An Update on Diagnostic and Prognostic Biomarkers for Traumatic Brain Injury. Expert Rev. Mol. Diagn. 2018;18:165–180. doi: 10.1080/14737159.2018.1428089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brett B.L., Gardner R.C., Godbout J., Dams-O’Connor K., Keene C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry. 2022;91:498–507. doi: 10.1016/j.biopsych.2021.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ramirez S., Mukherjee A., Sepulveda S., Becerra-Calixto A., Bravo-Vasquez N., Gherardelli C., Chavez M., Soto C. Modeling Traumatic Brain Injury in Human Cerebral Organoids. Cells. 2021;10:2683. doi: 10.3390/cells10102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Snapper D.M., Reginauld B., Liaudanskaya V., Fitzpatrick V., Kim Y., Georgakoudi I., Kaplan D.L., Symes A.J. Development of a Novel Bioengineered 3D Brain-like Tissue for Studying Primary Blast-Induced Traumatic Brain Injury. J. Neurosci. Res. 2023;101:3–19. doi: 10.1002/jnr.25123. [DOI] [PubMed] [Google Scholar]
- 200.Alam A., Singh T., Kayhanian S., Tjerkaski J., Garcia N.M., Carpenter K.L.H., Patani R., Lindblad C., Thelin E.P., Syed Y.A., et al. Modeling the Inflammatory Response of Traumatic Brain Injury Using Human Induced Pluripotent Stem Cell Derived Microglia. J. Neurotrauma. 2023;40:2164–2173. doi: 10.1089/neu.2022.0508. [DOI] [PubMed] [Google Scholar]
- 201.Sherman S.A., Phillips J.K., Costa J.T., Cho F.S., Oungoulian S.R., Finan J.D. Stretch Injury of Human Induced Pluripotent Stem Cell Derived Neurons in a 96 Well Format. Sci. Rep. 2016;6:34097. doi: 10.1038/srep34097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ramirez S., Mukherjee A., Sepulveda S.E., Gherardelli C., Becerra-Calixto A., Bravo-Vasquez N., Soto C. Protocol for Controlled Cortical Impact in Human Cerebral Organoids to Model Traumatic Brain Injury. STAR Protoc. 2021;2:100987. doi: 10.1016/j.xpro.2021.100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gao X., Wang X., Xiong W., Chen J. In Vivo Reprogramming Reactive Glia into iPSCs to Produce New Neurons in the Cortex Following Traumatic Brain Injury. Sci. Rep. 2016;6:22490. doi: 10.1038/srep22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen X., Sun G., Tian E., Zhang M., Davtyan H., Beach T.G., Reiman E.M., Blurton-Jones M., Holtzman D.M., Shi Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. 2021;8:e2101462. doi: 10.1002/advs.202101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ghatak S., Dolatabadi N., Trudler D., Zhang X., Wu Y., Mohata M., Ambasudhan R., Talantova M., Lipton S.A. Mechanisms of Hyperexcitability in Alzheimer’s Disease hiPSC-Derived Neurons and Cerebral Organoids vs Isogenic Controls. eLife. 2019;8:e50333. doi: 10.7554/eLife.50333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gonzalez C., Armijo E., Bravo-Alegria J., Becerra-Calixto A., Mays C.E., Soto C. Modeling Amyloid Beta and Tau Pathology in Human Cerebral Organoids. Mol. Psychiatry. 2018;23:2363–2374. doi: 10.1038/s41380-018-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arber C., Lovejoy C., Harris L., Willumsen N., Alatza A., Casey J.M., Lines G., Kerins C., Mueller A.K., Zetterberg H., et al. Familial Alzheimer’s Disease Mutations in PSEN1 Lead to Premature Human Stem Cell Neurogenesis. Cell Rep. 2021;34:108615. doi: 10.1016/j.celrep.2020.108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C., et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017;94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhao J., Fu Y., Yamazaki Y., Ren Y., Davis M.D., Liu C.-C., Lu W., Wang X., Chen K., Cherukuri Y., et al. APOE4 Exacerbates Synapse Loss and Neurodegeneration in Alzheimer’s Disease Patient iPSC-Derived Cerebral Organoids. Nat. Commun. 2020;11:5540. doi: 10.1038/s41467-020-19264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park J.-C., Jang S.-Y., Lee D., Lee J., Kang U., Chang H., Kim H.J., Han S.-H., Seo J., Choi M., et al. A Logical Network-Based Drug-Screening Platform for Alzheimer’s Disease Representing Pathological Features of Human Brain Organoids. Nat. Commun. 2021;12:280. doi: 10.1038/s41467-020-20440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pérez M.J., Ivanyuk D., Panagiotakopoulou V., Di Napoli G., Kalb S., Brunetti D., Al-Shaana R., Kaeser S.A., Fraschka S.A.-K., Jucker M., et al. Loss of Function of the Mitochondrial Peptidase PITRM1 Induces Proteotoxic Stress and Alzheimer’s Disease-like Pathology in Human Cerebral Organoids. Mol. Psychiatry. 2021;26:5733–5750. doi: 10.1038/s41380-020-0807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhou L.-T., Liu D., Kang H.-C., Lu L., Huang H.-Z., Ai W.-Q., Zhou Y., Deng M.-F., Li H., Liu Z.-Q., et al. Tau Pathology Epigenetically Remodels the Neuron-Glial Cross-Talk in Alzheimer’s Disease. Sci. Adv. 2023;9:eabq7105. doi: 10.1126/sciadv.abq7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jin M., Xu R., Wang L., Alam M.M., Ma Z., Zhu S., Martini A.C., Jadali A., Bernabucci M., Xie P., et al. Type-I-Interferon Signaling Drives Microglial Dysfunction and Senescence in Human iPSC Models of Down Syndrome and Alzheimer’s Disease. Cell Stem Cell. 2022;29:1135–1153. doi: 10.1016/j.stem.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Choi H., Kim H.J., Yang J., Chae S., Lee W., Chung S., Kim J., Choi H., Song H., Lee C.K., et al. Acetylation Changes Tau Interactome to Degrade Tau in Alzheimer’s Disease Animal and Organoid Models. Aging Cell. 2020;19:e13081. doi: 10.1111/acel.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim H., Park H.J., Choi H., Chang Y., Park H., Shin J., Kim J., Lengner C.J., Lee Y.K., Kim J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019;12:518–531. doi: 10.1016/j.stemcr.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Becerra-Calixto A., Mukherjee A., Ramirez S., Sepulveda S., Sinha T., Al-Lahham R., De Gregorio N., Gherardelli C., Soto C. Lewy Body-like Pathology and Loss of Dopaminergic Neurons in Midbrain Organoids Derived from Familial Parkinson’s Disease Patient. Cells. 2023;12:625. doi: 10.3390/cells12040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zheng X., Han D., Liu W., Wang X., Pan N., Wang Y., Chen Z. Human iPSC-Derived Midbrain Organoids Functionally Integrate into Striatum Circuits and Restore Motor Function in a Mouse Model of Parkinson’s Disease. Theranostics. 2023;13:2673–2692. doi: 10.7150/thno.80271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Raja W.K., Neves E., Burke C., Jiang X., Xu P., Rhodes K.J., Khurana V., Scannevin R.H., Chung C.Y. Patient-Derived Three-Dimensional Cortical Neurospheres to Model Parkinson’s Disease. PLoS ONE. 2022;17:e0277532. doi: 10.1371/journal.pone.0277532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wulansari N., Darsono W.H.W., Woo H.-J., Chang M.-Y., Kim J., Bae E.-J., Sun W., Lee J.-H., Cho I.-J., Shin H., et al. Neurodevelopmental Defects and Neurodegenerative Phenotypes in Human Brain Organoids Carrying Parkinson’s Disease-Linked DNAJC6 Mutations. Sci. Adv. 2021;7:eabb1540. doi: 10.1126/sciadv.abb1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sabate-Soler S., Nickels S.L., Saraiva C., Berger E., Dubonyte U., Barmpa K., Lan Y.J., Kouno T., Jarazo J., Robertson G., et al. Microglia Integration into Human Midbrain Organoids Leads to Increased Neuronal Maturation and Functionality. Glia. 2022;70:1267–1288. doi: 10.1002/glia.24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chlebanowska P., Tejchman A., Sułkowski M., Skrzypek K., Majka M. Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:694. doi: 10.3390/ijms21030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Walter J., Bolognin S., Poovathingal S.K., Magni S., Gérard D., Antony P.M.A., Nickels S.L., Salamanca L., Berger E., Smits L.M., et al. The Parkinson’s-Disease-Associated Mutation LRRK2-G2019S Alters Dopaminergic Differentiation Dynamics via NR2F1. Cell Rep. 2021;37:109864. doi: 10.1016/j.celrep.2021.109864. [DOI] [PubMed] [Google Scholar]
- 223.Samarasinghe R.A., Miranda O.A., Buth J.E., Mitchell S., Ferando I., Watanabe M., Allison T.F., Kurdian A., Fotion N.N., Gandal M.J., et al. Identification of Neural Oscillations and Epileptiform Changes in Human Brain Organoids. Nat. Neurosci. 2021;24:1488–1500. doi: 10.1038/s41593-021-00906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Steinberg D.J., Repudi S., Saleem A., Kustanovich I., Viukov S., Abudiab B., Banne E., Mahajnah M., Hanna J.H., Stern S., et al. Modeling Genetic Epileptic Encephalopathies Using Brain Organoids. EMBO Mol. Med. 2021;13:e13610. doi: 10.15252/emmm.202013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Eichmüller O.L., Corsini N.S., Vértesy Á., Morassut I., Scholl T., Gruber V.-E., Peer A.M., Chu J., Novatchkova M., Hainfellner J.A., et al. Amplification of Human Interneuron Progenitors Promotes Brain Tumors and Neurological Defects. Science. 2022;375:eabf5546. doi: 10.1126/science.abf5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Akiyama T., Suzuki N., Ishikawa M., Fujimori K., Sone T., Kawada J., Funayama R., Fujishima F., Mitsuzawa S., Ikeda K., et al. Aberrant Axon Branching via Fos-B Dysregulation in FUS-ALS Motor Neurons. eBioMedicine. 2019;45:362–378. doi: 10.1016/j.ebiom.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tamaki Y., Ross J.P., Alipour P., Castonguay C.-É., Li B., Catoire H., Rochefort D., Urushitani M., Takahashi R., Sonnen J.A., et al. Spinal Cord Extracts of Amyotrophic Lateral Sclerosis Spread TDP-43 Pathology in Cerebral Organoids. PLoS Genet. 2023;19:e1010606. doi: 10.1371/journal.pgen.1010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Meijboom K.E., Abdallah A., Fordham N.P., Nagase H., Rodriguez T., Kraus C., Gendron T.F., Krishnan G., Esanov R., Andrade N.S., et al. CRISPR/Cas9-Mediated Excision of ALS/FTD-Causing Hexanucleotide Repeat Expansion in C9ORF72 Rescues Major Disease Mechanisms in Vivo and in Vitro. Nat. Commun. 2022;13:6286. doi: 10.1038/s41467-022-33332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.De Majo M., Koontz M., Marsan E., Salinas N., Ramsey A., Kuo Y.-M., Seo K., Li H., Dräger N., Leng K., et al. Granulin Loss of Function in Human Mature Brain Organoids Implicates Astrocytes in TDP-43 Pathology. Stem Cell Rep. 2023;18:706–719. doi: 10.1016/j.stemcr.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Johns A.E., Maragakis N.J. Exploring Motor Neuron Diseases Using iPSC Platforms. Stem Cells. 2022;40:2–13. doi: 10.1093/stmcls/sxab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nakamura M., Shiozawa S., Tsuboi D., Amano M., Watanabe H., Maeda S., Kimura T., Yoshimatsu S., Kisa F., Karch C.M., et al. Pathological Progression Induced by the Frontotemporal Dementia-Associated R406W Tau Mutation in Patient-Derived iPSCs. Stem Cell Rep. 2019;13:684–699. doi: 10.1016/j.stemcr.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kerkering J., Muinjonov B., Rosiewicz K.S., Diecke S., Biese C., Schiweck J., Chien C., Zocholl D., Conrad T., Paul F., et al. iPSC-Derived Reactive Astrocytes from Patients with Multiple Sclerosis Protect Cocultured Neurons in Inflammatory Conditions. J. Clin. Investig. 2023;133:e164637. doi: 10.1172/JCI164637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schepers M., Paes D., Tiane A., Rombaut B., Piccart E., van Veggel L., Gervois P., Wolfs E., Lambrichts I., Brullo C., et al. Selective PDE4 Subtype Inhibition Provides New Opportunities to Intervene in Neuroinflammatory versus Myelin Damaging Hallmarks of Multiple Sclerosis. Brain Behav. Immun. 2023;109:1–22. doi: 10.1016/j.bbi.2022.12.020. [DOI] [PubMed] [Google Scholar]
- 234.Mutukula N., Man Z., Takahashi Y., Iniesta Martinez F., Morales M., Carreon-Guarnizo E., Hernandez Clares R., Garcia-Bernal D., Martinez Martinez L., Lajara J., et al. Generation of RRMS and PPMS Specific iPSCs as a Platform for Modeling Multiple Sclerosis. Stem Cell Res. 2021;53:102319. doi: 10.1016/j.scr.2021.102319. [DOI] [PubMed] [Google Scholar]
- 235.Fortune A.J., Taylor B.V., Charlesworth J.C., Burdon K.P., Blackburn N.B., Fletcher J.L., Mehta A., Young K.M. Generation and Characterisation of Four Multiple Sclerosis iPSC Lines from a Single Family. Stem Cell Res. 2022;62:102828. doi: 10.1016/j.scr.2022.102828. [DOI] [PubMed] [Google Scholar]
- 236.Li C., Fleck J.S., Martins-Costa C., Burkard T.R., Themann J., Stuempflen M., Peer A.M., Vertesy Á., Littleboy J.B., Esk C., et al. Single-Cell Brain Organoid Screening Identifies Developmental Defects in Autism. Nature. 2023;621:373–380. doi: 10.1038/s41586-023-06473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.De Jong J.O., Llapashtica C., Genestine M., Strauss K., Provenzano F., Sun Y., Zhu H., Cortese G.P., Brundu F., Brigatti K.W., et al. Cortical Overgrowth in a Preclinical Forebrain Organoid Model of CNTNAP2-Associated Autism Spectrum Disorder. Nat. Commun. 2021;12:4087. doi: 10.1038/s41467-021-24358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ejlersen M., Ilieva M., Michel T.M. Superoxide Dismutase Isozymes in Cerebral Organoids from Autism Spectrum Disorder Patients. J. Neural Transm. 2022;129:617–626. doi: 10.1007/s00702-022-02472-x. [DOI] [PubMed] [Google Scholar]
- 239.Wang P., Mokhtari R., Pedrosa E., Kirschenbaum M., Bayrak C., Zheng D., Lachman H.M. CRISPR/Cas9-Mediated Heterozygous Knockout of the Autism Gene CHD8 and Characterization of Its Transcriptional Networks in Cerebral Organoids Derived from iPS Cells. Mol. Autism. 2017;8:11. doi: 10.1186/s13229-017-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dunkerson J., Moritz K.E., Young J., Pionk T., Fink K., Rossignol J., Dunbar G., Smith J.S. Combining Enriched Environment and Induced Pluripotent Stem Cell Therapy Results in Improved Cognitive and Motor Function Following Traumatic Brain Injury. Restor. Neurol. Neurosci. 2014;32:675–687. doi: 10.3233/RNN-140408. [DOI] [PubMed] [Google Scholar]
- 241.Furmanski O., Nieves M.D., Doughty M.L. Controlled Cortical Impact Model of Mouse Brain Injury with Therapeutic Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Cells. J. Vis. Exp. 2019;149:e59561. doi: 10.3791/59561. [DOI] [PubMed] [Google Scholar]
- 242.Iwasa N., Matsui T.K., Iguchi N., Kinugawa K., Morikawa N., Sakaguchi Y.M., Shiota T., Kobashigawa S., Nakanishi M., Matsubayashi M., et al. Gene Expression Profiles of Human Cerebral Organoids Identify PPAR Pathway and PKM2 as Key Markers for Oxygen-Glucose Deprivation and Reoxygenation. Front. Cell. Neurosci. 2021;15:605030. doi: 10.3389/fncel.2021.605030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Madencioglu D.A., Kruth K.A., Wassink T.H., Magnotta V.A., Wemmie J.A., Williams A.J. Modeling Human Cerebellar Development In Vitro in 2D Structure. J. Vis. Exp. 2022;187:e64462. doi: 10.3791/64462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jansch C., Günther K., Waider J., Ziegler G.C., Forero A., Kollert S., Svirin E., Pühringer D., Kwok C.K., Ullmann R., et al. Generation of a Human Induced Pluripotent Stem Cell (iPSC) Line from a 51-Year-Old Female with Attention-Deficit/Hyperactivity Disorder (ADHD) Carrying a Duplication of SLC2A3. Stem Cell Res. 2018;28:136–140. doi: 10.1016/j.scr.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 245.Tong J., Lee K.M., Liu X., Nefzger C.M., Vijayakumar P., Hawi Z., Pang K.C., Parish C.L., Polo J.M., Bellgrove M.A. Generation of Four iPSC Lines from Peripheral Blood Mononuclear Cells (PBMCs) of an Attention Deficit Hyperactivity Disorder (ADHD) Individual and a Healthy Sibling in an Australia-Caucasian Family. Stem Cell Res. 2019;34:101353. doi: 10.1016/j.scr.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 246.Yde Ohki C.M., Walter N.M., Bender A., Rickli M., Ruhstaller S., Walitza S., Grünblatt E. Growth Rates of Human Induced Pluripotent Stem Cells and Neural Stem Cells from Attention-Deficit Hyperactivity Disorder Patients: A Preliminary Study. J. Neural Transm. 2023;130:243–252. doi: 10.1007/s00702-023-02600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Krach F., Stemick J., Boerstler T., Weiss A., Lingos I., Reischl S., Meixner H., Ploetz S., Farrell M., Hehr U., et al. An Alternative Splicing Modulator Decreases Mutant HTT and Improves the Molecular Fingerprint in Huntington’s Disease Patient Neurons. Nat. Commun. 2022;13:6797. doi: 10.1038/s41467-022-34419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu G.-H., Smith-Geater C., Galaz-Montoya J.G., Gu Y., Gupte S.R., Aviner R., Mitchell P.G., Hsu J., Miramontes R., Wang K.Q., et al. CryoET Reveals Organelle Phenotypes in Huntington Disease Patient iPSC-Derived and Mouse Primary Neurons. Nat. Commun. 2023;14:692. doi: 10.1038/s41467-023-36096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.The HD iPSC Consortium HD iPSC Consortium Bioenergetic Deficits in Huntington’s Disease iPSC-Derived Neural Cells and Rescue with Glycolytic Metabolites. Hum. Mol. Genet. 2020;29:1757–1771. doi: 10.1093/hmg/ddy430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Beatriz M., Rodrigues R.J., Vilaça R., Egas C., Pinheiro P.S., Daley G.Q., Schlaeger T.M., Raimundo N., Rego A.C., Lopes C. Extracellular Vesicles Improve GABAergic Transmission in Huntington’s Disease iPSC-Derived Neurons. Theranostics. 2023;13:3707–3724. doi: 10.7150/thno.81981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lewitzky M., Yamanaka S. Reprogramming Somatic Cells towards Pluripotency by Defined Factors. Curr. Opin. Biotechnol. 2007;18:467–473. doi: 10.1016/j.copbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 252.Yagi T., Ito D., Okada Y., Akamatsu W., Nihei Y., Yoshizaki T., Yamanaka S., Okano H., Suzuki N. Modeling Familial Alzheimer’s Disease with Induced Pluripotent Stem Cells. Hum. Mol. Genet. 2011;20:4530–4539. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 253.Yamanaka S. A Fresh Look at iPS Cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 254.Okano H., Yamanaka S. iPS Cell Technologies: Significance and Applications to CNS Regeneration and Disease. Mol. Brain. 2014;7:22. doi: 10.1186/1756-6606-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., et al. Grafted Human-Induced Pluripotent Stem-Cell-Derived Neurospheres Promote Motor Functional Recovery after Spinal Cord Injury in Mice. Proc. Natl. Acad. Sci. USA. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 257.Kwokdinata C., Ramanujam V., Chen J., de Oliveira P.N., Nai M.H., Chooi W.H., Lim C.T., Ng S.Y., David L., Chew S.Y. Encapsulation of Human Spinal Cord Progenitor Cells in Hyaluronan-Gelatin Hydrogel for Spinal Cord Injury Treatment. ACS Appl. Mater. Interfaces. 2023;15:50679–50692. doi: 10.1021/acsami.3c07419. [DOI] [PubMed] [Google Scholar]
- 258.Sieber-Blum M. Epidermal Neural Crest Stem Cells and Their Use in Mouse Models of Spinal Cord Injury. Brain Res. Bull. 2010;83:189–193. doi: 10.1016/j.brainresbull.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 259.Obara K., Shirai K., Hamada Y., Arakawa N., Yamane M., Takaoka N., Aki R., Hoffman R.M., Amoh Y. Chronic Spinal Cord Injury Functionally Repaired by Direct Implantation of Encapsulated Hair-Follicle-Associated Pluripotent (HAP) Stem Cells in a Mouse Model: Potential for Clinical Regenerative Medicine. PLoS ONE. 2022;17:e0262755. doi: 10.1371/journal.pone.0262755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Polentes J., Jendelova P., Cailleret M., Braun H., Romanyuk N., Tropel P., Brenot M., Itier V., Seminatore C., Baldauf K., et al. Human Induced Pluripotent Stem Cells Improve Stroke Outcome and Reduce Secondary Degeneration in the Recipient Brain. Cell Transplant. 2012;21:2587–2602. doi: 10.3727/096368912X653228. [DOI] [PubMed] [Google Scholar]
- 261.Kawai H., Yamashita T., Ohta Y., Deguchi K., Nagotani S., Zhang X., Ikeda Y., Matsuura T., Abe K. Tridermal Tumorigenesis of Induced Pluripotent Stem Cells Transplanted in Ischemic Brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010;30:1487–1493. doi: 10.1038/jcbfm.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Upadhya D., Hattiangady B., Castro O.W., Shuai B., Kodali M., Attaluri S., Bates A., Dong Y., Zhang S.-C., Prockop D.J., et al. Human Induced Pluripotent Stem Cell-Derived MGE Cell Grafting after Status Epilepticus Attenuates Chronic Epilepsy and Comorbidities via Synaptic Integration. Proc. Natl. Acad. Sci. USA. 2019;116:287–296. doi: 10.1073/pnas.1814185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cunningham M., Cho J.-H., Leung A., Savvidis G., Ahn S., Moon M., Lee P.K.J., Han J.J., Azimi N., Kim K.-S., et al. hPSC-Derived Maturing GABAergic Interneurons Ameliorate Seizures and Abnormal Behavior in Epileptic Mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Martin P., Wagh V., Reis S.A., Erdin S., Beauchamp R.L., Shaikh G., Talkowski M., Thiele E., Sheridan S.D., Haggarty S.J., et al. TSC Patient-Derived Isogenic Neural Progenitor Cells Reveal Altered Early Neurodevelopmental Phenotypes and Rapamycin-Induced MNK-eIF4E Signaling. Mol. Autism. 2020;11:2. doi: 10.1186/s13229-019-0311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cai J., Yang M., Poremsky E., Kidd S., Schneider J.S., Iacovitti L. Dopaminergic Neurons Derived from Human Induced Pluripotent Stem Cells Survive and Integrate into 6-OHDA-Lesioned Rats. Stem Cells Dev. 2010;19:1017–1023. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Meneghini V., Frati G., Sala D., De Cicco S., Luciani M., Cavazzin C., Paulis M., Mentzen W., Morena F., Giannelli S., et al. Generation of Human Induced Pluripotent Stem Cell-Derived Bona Fide Neural Stem Cells for Ex Vivo Gene Therapy of Metachromatic Leukodystrophy. Stem Cells Transl. Med. 2017;6:352–368. doi: 10.5966/sctm.2015-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yoon Y., Kim H.S., Hong C.P., Li E., Jeon I., Park H.J., Lee N., Pei Z., Song J. Neural Transplants From Human Induced Pluripotent Stem Cells Rescue the Pathology and Behavioral Defects in a Rodent Model of Huntington’s Disease. Front. Neurosci. 2020;14:558204. doi: 10.3389/fnins.2020.558204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chang C.-Y., Ting H.-C., Liu C.-A., Su H.-L., Chiou T.-W., Lin S.-Z., Harn H.-J., Ho T.-J. Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening. Molecules. 2020;25:2000. doi: 10.3390/molecules25082000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Aboul-Soud M.A.M., Alzahrani A.J., Mahmoud A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells. 2021;10:2319. doi: 10.3390/cells10092319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kondo T., Asai M., Tsukita K., Kutoku Y., Ohsawa Y., Sunada Y., Imamura K., Egawa N., Yahata N., Okita K., et al. Modeling Alzheimer’s Disease with iPSCs Reveals Stress Phenotypes Associated with Intracellular Aβ and Differential Drug Responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 271.Miura K., Okada Y., Aoi T., Okada A., Takahashi K., Okita K., Nakagawa M., Koyanagi M., Tanabe K., Ohnuki M., et al. Variation in the Safety of Induced Pluripotent Stem Cell Lines. Nat. Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 272.Fernandes H.J.R., Patikas N., Foskolou S., Field S.F., Park J.-E., Byrne M.L., Bassett A.R., Metzakopian E. Single-Cell Transcriptomics of Parkinson’s Disease Human In Vitro Models Reveals Dopamine Neuron-Specific Stress Responses. Cell Rep. 2020;33:108263. doi: 10.1016/j.celrep.2020.108263. [DOI] [PubMed] [Google Scholar]
- 273.Wu H., Uchimura K., Donnelly E.L., Kirita Y., Morris S.A., Humphreys B.D. Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell. 2018;23:869–881.e8. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cowan C.S., Renner M., Gennaro M.D., Gross-Scherf B., Goldblum D., Hou Y., Munz M., Rodrigues T.M., Krol J., Szikra T., et al. Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell. 2020;182:1623–1640.e34. doi: 10.1016/j.cell.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S. Generation of Induced Pluripotent Stem Cells without Myc from Mouse and Human Fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 276.Okita K., Nakagawa M., Hyenjong H., Ichisaka T., Yamanaka S. Generation of Mouse Induced Pluripotent Stem Cells without Viral Vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 277.Sugai K., Sumida M., Shofuda T., Yamaguchi R., Tamura T., Kohzuki T., Abe T., Shibata R., Kamata Y., Ito S., et al. First-in-Human Clinical Trial of Transplantation of iPSC-Derived NS/PCs in Subacute Complete Spinal Cord Injury: Study Protocol. Regen. Ther. 2021;18:321–333. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hoveizi E., Mohammadi T., Moazedi A.A., Zamani N., Eskandary A. Transplanted Neural-like Cells Improve Memory and Alzheimer-like Pathology in a Rat Model. Cytotherapy. 2018;20:964–973. doi: 10.1016/j.jcyt.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 279.Armijo E., Edwards G., Flores A., Vera J., Shahnawaz M., Moda F., Gonzalez C., Sanhueza M., Soto C. Induced Pluripotent Stem Cell-Derived Neural Precursors Improve Memory, Synaptic and Pathological Abnormalities in a Mouse Model of Alzheimer’s Disease. Cells. 2021;10:1802. doi: 10.3390/cells10071802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yahata N., Asai M., Kitaoka S., Takahashi K., Asaka I., Hioki H., Kaneko T., Maruyama K., Saido T.C., Nakahata T., et al. Anti-Aβ Drug Screening Platform Using Human iPS Cell-Derived Neurons for the Treatment of Alzheimer’s Disease. PLoS ONE. 2011;6:e25788. doi: 10.1371/journal.pone.0025788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Brownjohn P.W., Smith J., Portelius E., Serneels L., Kvartsberg H., De Strooper B., Blennow K., Zetterberg H., Livesey F.J. Phenotypic Screening Identifies Modulators of Amyloid Precursor Protein Processing in Human Stem Cell Models of Alzheimer’s Disease. Stem Cell Rep. 2017;8:870–882. doi: 10.1016/j.stemcr.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Al-Gharaibeh A., Culver R., Stewart A.N., Srinageshwar B., Spelde K., Frollo L., Kolli N., Story D., Paladugu L., Anwar S., et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington’s Disease. Front. Neurosci. 2017;11:628. doi: 10.3389/fnins.2017.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cho I.K., Hunter C.E., Ye S., Pongos A.L., Chan A.W.S. Combination of Stem Cell and Gene Therapy Ameliorates Symptoms in Huntington’s Disease Mice. NPJ Regen. Med. 2019;4:7. doi: 10.1038/s41536-019-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Park H.J., Han A., Kim J.Y., Choi J., Bae H.S., Cho G.-B., Shin H., Shin E.J., Lee K.-I., Kim S., et al. SUPT4H1-Edited Stem Cell Therapy Rescues Neuronal Dysfunction in a Mouse Model for Huntington’s Disease. NPJ Regen. Med. 2022;7:8. doi: 10.1038/s41536-021-00198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Doerr J., Böckenhoff A., Ewald B., Ladewig J., Eckhardt M., Gieselmann V., Matzner U., Brüstle O., Koch P. Arylsulfatase A Overexpressing Human iPSC-Derived Neural Cells Reduce CNS Sulfatide Storage in a Mouse Model of Metachromatic Leukodystrophy. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:1519–1531. doi: 10.1038/mt.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Alekseenko Z., Dias J.M., Adler A.F., Kozhevnikova M., van Lunteren J.A., Nolbrant S., Jeggari A., Vasylovska S., Yoshitake T., Kehr J., et al. Robust Derivation of Transplantable Dopamine Neurons from Human Pluripotent Stem Cells by Timed Retinoic Acid Delivery. Nat. Commun. 2022;13:3046. doi: 10.1038/s41467-022-30777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Brot S., Thamrin N.P., Bonnet M.-L., Francheteau M., Patrigeon M., Belnoue L., Gaillard A. Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model. Cells. 2022;11:1596. doi: 10.3390/cells11101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Guo Y., Guan Y., Zhu H., Sun T., Wang Y., Huang Y., Ma C., Emery R., Guan W., Wang C., et al. Therapeutic Function of iPSCs-Derived Primitive Neuroepithelial Cells in a Rat Model of Parkinson’s Disease. Neurochem. Int. 2022;155:105324. doi: 10.1016/j.neuint.2022.105324. [DOI] [PubMed] [Google Scholar]
- 289.Hiller B.M., Marmion D.J., Thompson C.A., Elliott N.A., Federoff H., Brundin P., Mattis V.B., McMahon C.W., Kordower J.H. Optimizing Maturity and Dose of iPSC-Derived Dopamine Progenitor Cell Therapy for Parkinson’s Disease. NPJ Regen. Med. 2022;7:24. doi: 10.1038/s41536-022-00221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Grinand L., Takahashi J. Automated Measurement of Fluorescence Signals Reveals a Significant Increase of the Graft-Derived Neurite Extension in Neonates Compared to Aged Rats. Regen. Ther. 2022;19:97–106. doi: 10.1016/j.reth.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nakamura R., Nonaka R., Oyama G., Jo T., Kamo H., Nuermaimaiti M., Akamatsu W., Ishikawa K.-I., Hattori N. A Defined Method for Differentiating Human iPSCs into Midbrain Dopaminergic Progenitors That Safely Restore Motor Deficits in Parkinson’s Disease. Front. Neurosci. 2023;17:1202027. doi: 10.3389/fnins.2023.1202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Guo Y., Zhu H., Wang Y., Sun T., Xu J., Wang T., Guan W., Wang C., Liu C., Ma C. Miniature-Swine iPSC-Derived GABA Progenitor Cells Function in a Rat Parkinson’s Disease Model. Cell Tissue Res. 2023;391:425–440. doi: 10.1007/s00441-022-03736-4. [DOI] [PubMed] [Google Scholar]
- 293.Lavoie N.S., Truong V., Malone D., Pengo T., Patil N., Dutton J.R., Parr A.M. Human Induced Pluripotent Stem Cells Integrate, Create Synapses and Extend Long Axons after Spinal Cord Injury. J. Cell. Mol. Med. 2022;26:1932–1942. doi: 10.1111/jcmm.17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kitagawa T., Nagoshi N., Kamata Y., Kawai M., Ago K., Kajikawa K., Shibata R., Sato Y., Imaizumi K., Shindo T., et al. Modulation by DREADD Reveals the Therapeutic Effect of Human iPSC-Derived Neuronal Activity on Functional Recovery after Spinal Cord Injury. Stem Cell Rep. 2022;17:127–142. doi: 10.1016/j.stemcr.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shibata T., Tashiro S., Shibata S., Shinozaki M., Shindo T., Hashimoto S., Kawai M., Kitagawa T., Ago K., Matsumoto M., et al. Rehabilitative Training Enhances Therapeutic Effect of Human iPSC-Derived Neural Stem/Progenitor Cells Transplantation in Chronic Spinal Cord Injury. Stem Cells Transl. Med. 2023;12:83–96. doi: 10.1093/stcltm/szac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zheng Y., Gallegos C.M., Xue H., Li S., Kim D.H., Zhou H., Xia X., Liu Y., Cao Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells. 2022;11:2765. doi: 10.3390/cells11172765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Deng P., Wang L., Zhang Q., Chen S., Zhang Y., Xu H., Chen H., Xu Y., He W., Zhang J., et al. Therapeutic Potential of a Combination of Electroacupuncture and Human iPSC-Derived Small Extracellular Vesicles for Ischemic Stroke. Cells. 2022;11:820. doi: 10.3390/cells11050820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kaiser E.E., Waters E.S., Yang X., Fagan M.M., Scheulin K.M., Sneed S.E., Cheek S.R., Jeon J.H., Shin S.K., Kinder H.A., et al. Tanshinone IIA-Loaded Nanoparticle and Neural Stem Cell Therapy Enhances Recovery in a Pig Ischemic Stroke Model. Stem Cells Transl. Med. 2022;11:1061–1071. doi: 10.1093/stcltm/szac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Chen Y., Song F., Tu M., Wu S., He X., Liu H., Xu C., Zhang K., Zhu Y., Zhou R., et al. Quantitative Proteomics Revealed Extensive Microenvironmental Changes after Stem Cell Transplantation in Ischemic Stroke. Front. Med. 2022;16:429–441. doi: 10.1007/s11684-021-0842-9. [DOI] [PubMed] [Google Scholar]
- 300.Arakawa M., Sakamoto Y., Miyagawa Y., Nito C., Takahashi S., Nitahara-Kasahara Y., Suda S., Yamazaki Y., Sakai M., Kimura K., et al. iPSC-Derived Mesenchymal Stem Cells Attenuate Cerebral Ischemia-Reperfusion Injury by Inhibiting Inflammatory Signaling and Oxidative Stress. Mol. Ther. Methods Clin. Dev. 2023;30:333–349. doi: 10.1016/j.omtm.2023.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cao S.-Y., Tao M.-D., Lou S.-N., Yang D., Lin Y.-H., Wu H.-Y., Chang L., Luo C.-X., Xu Y., Liu Y., et al. Functional Reconstruction of the Impaired Cortex and Motor Function by hMGEOs Transplantation in Stroke. Biochem. Biophys. Res. Commun. 2023;671:87–95. doi: 10.1016/j.bbrc.2023.06.010. [DOI] [PubMed] [Google Scholar]
- 302.Martinez-Curiel R., Jansson L., Tsupykov O., Avaliani N., Aretio-Medina C., Hidalgo I., Monni E., Bengzon J., Skibo G., Lindvall O., et al. Oligodendrocytes in Human Induced Pluripotent Stem Cell-Derived Cortical Grafts Remyelinate Adult Rat and Human Cortical Neurons. Stem Cell Rep. 2023;18:1643–1656. doi: 10.1016/j.stemcr.2023.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hulme A.J., Maksour S., St-Clair Glover M., Miellet S., Dottori M. Making Neurons, Made Easy: The Use of Neurogenin-2 in Neuronal Differentiation. Stem Cell Rep. 2022;17:14–34. doi: 10.1016/j.stemcr.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Barak M., Fedorova V., Pospisilova V., Raska J., Vochyanova S., Sedmik J., Hribkova H., Klimova H., Vanova T., Bohaciakova D. Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: From Neural Stem Cells to Cerebral Organoids. Stem Cell Rev. Rep. 2022;18:792–820. doi: 10.1007/s12015-021-10254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Andrews P.W., Cavagnaro J., Deans R., Feigal E., Horowitz E., Keating A., Rao M., Turner M., Wilmut I., Yamanaka S. Harmonizing standards for producing clinical-grade therapies from pluripotent stem cells. Nat. Biotechnol. 2014;32:724–726. doi: 10.1038/nbt.2973. [DOI] [PubMed] [Google Scholar]
- 306.Okano H., Nakamura M., Yoshida K., Okada Y., Tsuji O., Nori S., Ikeda E., Yamanaka S., Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ. Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 307.Okano H., Morimoto S. iPSC-Based Disease Modeling and Drug Discovery in Cardinal Neurodegenerative Disorders. Cell Stem Cell. 2022;29:189–208. doi: 10.1016/j.stem.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 308.Barker R.A., Parmar M., Studer L., Takahashi J. Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell. 2017;21:569–573. doi: 10.1016/j.stem.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 309.Gascón S., Masserdotti G., Russo G.L., Götz M. Direct Neuronal Reprogramming: Achievements, Hurdles, and New Roads to Success. Cell Stem Cell. 2017;21:18–34. doi: 10.1016/j.stem.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 310.Magnusson J.P., Göritz C., Tatarishvili J., Dias D.O., Smith E.M.K., Lindvall O., Kokaia Z., Frisén J. A Latent Neurogenic Program in Astrocytes Regulated by Notch Signaling in the Mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 311.Kang S.H., Fukaya M., Yang J.K., Rothstein J.D., Bergles D.E. NG2+ CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and Following Neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]