Abstract
Purpose
Chronic diseases often hinder the natural healing process, making wound infections a prevalent clinical concern. In severe cases, complications can arise, potentially leading to fatal outcomes. While allopathic treatments offer numerous options for wound repair and management, the enduring popularity of herbal medications may be attributed to their perceived minimal side effects. Hence, this review aims to investigate the potential of herbal remedies in efficiently treating wounds, presenting a promising alternative for consideration.
Methods
A literature search was done including research, reviews, systematic literature review, meta-analysis, and clinical trials considered. Search engines such as Pubmed, Google Scholar, and Scopus were used while retrieving data. Keywords like Wound healing ‘Wound healing and herbal combinations’, ‘Herbal wound dressing’, Nanotechnology and Wound dressing were used.
Result
This review provides valuable insights into the role of natural products and technology-based formulations in the treatment of wound infections. It evaluates the use of herbal remedies as an effective approach. Various active principles from herbs, categorized as flavonoids, glycosides, saponins, and phenolic compounds, have shown effectiveness in promoting wound closure. A multitude of herbal remedies have demonstrated significant efficacy in wound management, offering an additional avenue for care. The review encompasses a total of 72 studies, involving 127 distinct herbs (excluding any common herbs shared between studies), primarily belonging to the families Asteraceae, Fabaceae, and Apiaceae. In research, rat models were predominantly utilized to assess wound healing activities. Furthermore, advancements in herbal-based formulations using nanotechnology-based wound dressing materials, such as nanofibers, nanoemulsions, nanofiber mats, polymeric fibers, and hydrogel-based microneedles, are underway. These innovations aim to enhance targeted drug delivery and expedite recovery. Several clinical-based experimental studies have already been documented, evaluating the efficacy of various natural products for wound care and management. This signifies a promising direction in the field of wound treatment.
Conclusion
In recent years, scientists have increasingly utilized evidence-based medicine and advanced scientific techniques to validate the efficacy of herbal medicines and delve into the underlying mechanisms of their actions. However, there remains a critical need for further research to thoroughly understand how isolated chemicals extracted from herbs contribute to the healing process of intricate wounds, which may have life-threatening consequences. This ongoing research endeavor holds great promise in not only advancing our understanding but also in the development of innovative formulations that expedite the recovery process.
Graphical abstract
Keywords: Wound healing, Herbs, Wound dressing, Nanotechnology, Clinical trial
Introduction
Wounds encompass a spectrum, ranging from minor cuts to severe injuries like punctures, lacerations, or burns [1]. Factors like diabetes, atherosclerosis, and venous insufficiency in an aging population have led to a surge in chronic wounds [2]. Delayed wound healing escalates risks, potentially culminating in severe complications, including infection, sepsis, and, in extreme cases, necessitating amputation [3]. Pediatric orthopedic surgery for early-onset scoliosis, while often necessary, carries a substantial risk of wound-related complications, averaging an incidence of 15.5% [4]. The rise in chronic wounds has heightened awareness of their associated morbidity and financial burdens over recent decades [5]. Chronic wounds and burns significantly diminish patients’ quality of life, underscoring the need for innovative, cost-effective technologies and treatments to sustain national health systems [6]. Opportunistic bacteria like Staphylococcus aureus and Pseudomonas aeruginosa, due to their inflammatory response-triggering ability, may play a role in sustaining chronic wounds. Research also indicates therapeutic potential in bacterial and host extracellular vesicles, with applications ranging from vaccine candidates to agents modifying bacterial species in chronic wound biofilms [7]. Patients with biofilms, responsible for 60% of burn-related fatalities and contributing to rapid antibiotic resistance spread, require isolation and specialized treatment before full admission to the hospital [8]. Infections in wounds lead to prolonged healing, chronicity, increased hospitalization, potential amputation, and elevated medical costs; biofilm presence exacerbates these issues, underscoring the critical importance of early detection and treatment for improved outcomes [9]. Implementing a structured assessment framework like the Tissue, Inflammation or Infection, Moisture, and Edge of the wound and Epithelial advancement model can enhance wound care, facilitating the detection of deviations from normal healing, including those arising from species-specific factors, and thereby averting potential delays in recovery or further tissue damage, beyond the influence of intrinsic and extrinsic factors [10]. Macrophages play a crucial role in regulating inflammation, fibrosis, and wound healing, owing to their phagocytic capabilities and secretion of cytokines and growth factors [11]. Growth factors serve as primary human regulators in wound healing, while fibroblasts, highly active cells, play a vital role in tissue fibrosis and the healing process [12]. Additionally, platelets, neurons, and glial cells not only aid in tissue repair but also establish the wound microenvironment, influencing the growth of immune cells, fibroblasts, and keratinocytes [13]. The dynamic expression of Programmed Death Ligand-1 on fibroblast-like cells within the granulation tissue during wound healing serves to establish an immunosuppressive microenvironment, facilitating the modulation of macrophage polarization from M1-type to M2-type and initiating the resolution of inflammation, ultimately expediting the wound healing process [14]. Tryptase, a mast cell mediator, triggers bronchial epithelial cells to enhance migration and proliferation, partially regulated by protease-activated receptor-2, ultimately enhancing epithelial wound healing [15]. Mesenchymal stem cells hold promise for cell-based therapy, primarily relying on their paracrine actions for wound healing and tissue repair [16]. Moreover, adipose-derived mesenchymal stem cell exosomes have recently demonstrated involvement in various wound-healing pathways, particularly aiding in the healing of diabetic wounds [17]. Thymosin-4, a naturally occurring protein abundant in various body fluids and cells, especially platelets, plays pivotal roles in wound healing, actively promoting angiogenesis while inhibiting fibrosis, apoptosis, and inflammation [18].
Herbal medicine is increasingly explored for its diverse therapeutic potential, with certain medicinal plants showing significant wound-healing effects in experimental studies [19]. These herbs, supported by evidence-based medicine, offer viable treatment options in various healthcare contexts [20]. For instance, the ancient text, Sushruta Samhita, highlights various medicinal plants with potential for wound cleansing and healing, although no current published data validate these properties [21]. Traditional Persian medicine also provides valuable insights into natural remedies for wound healing [22]. Additionally, research on wound healing agents is a burgeoning field in biomedical sciences, with Chinese medicinal herbs showing promise, particularly bioactive polysaccharides derived from natural resources [23]. Plants like Acacia modesta, Aloe barbadensis, Azadirachta indica, Ficus benghalensis, Nerium oleander, and Olea ferruginea are extensively utilized for wound healing and demonstrate high use values in traditional practices [24]. Certain herbs like Vitis vinifera, Quercus spp., Punica granatum, Polygonum spp., Lilium spp., Gentiana lutea, Arnebia euchroma, Aloe spp., and Caesalpinia spp. possess verified biological and pharmacological mechanisms for wound healing [25]. Similarly, compounds from the convolvulaceae family such as Evolvulus alsinoides, Evolvulus nummularius, Argyreia cuneata, and Ipomoea carnea exhibit notable antidiabetic and wound healing activity, showing potential in diabetic wound care [26].
Antioxidants such as astaxanthin, beta-carotene, epigallocatechin gallate, delphinidin, and curcumin have shown efficacy in promoting cell proliferation, migration, angiogenesis, and inflammation control, presenting a promising approach for developing innovative treatments for cutaneous conditions [27]. Natural dietary antioxidants rich in flavonoids have been shown to influence keratinocyte physiology demonstrating notable skin repair benefits across different stages of the wound-healing process, including cell-cell and cell-matrix interactions, as well as collagen synthesis [28, 29]. In addition, numerous polyherbal formulations have demonstrated the ability to expedite wound healing in experimental models [30]. The effectiveness of combined herbal medications may be attributed to the synergy of diverse plant classes, each contributing different mechanisms that collectively lead to a more comprehensive therapeutic outcome [31]. These formulations show promise in enhancing wound healing by stimulating various physiological functions, warranting clinical trials for further validation and upscaling production, potentially revolutionizing the development of polyherbal wound healing products [32]. For example, polyherbal formulations like Jathyadi Thailam and Jatyadi Ghritam from Indian traditional medicine demonstrate potent antibacterial and anti-inflammatory properties, suggesting their potential as an external adjunct therapy for chronic wound management, particularly in cases of multidrug-resistant bacterial infections [33]. This review aims to investigate the potential of herbal extractions, their combinations (polyherbal formulations), and new developments in wound healing technology to enhance wound recovery abilities.
Methods
Literature search strategy
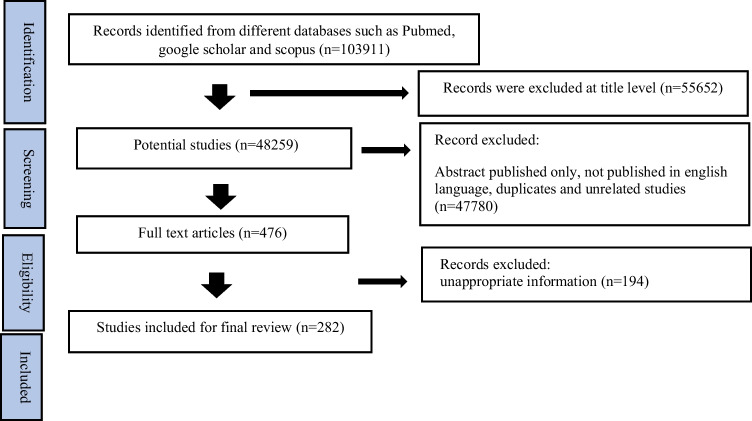
Database searches were conducted in PubMed and Google Scholar to identify articles published between 2014 and 2023, focusing on wound healing, herbal wound treatments, herbal wound dressings, nanotechnology, and wound dressings. Studies concerning the efficacy of herbal drugs in wound treatment were included, while non-English publications were excluded. Initially, 103,911 articles were identified. After removing duplicates, unrelated articles, and those with irrelevant titles, 476 articles remained for eligibility assessment. Ultimately, 282 articles were selected for inclusion in this review. Advanced filters in PubMed and Google Scholar were utilized during the screening process, followed by a manual assessment for eligibility. Data extraction was carried out using spreadsheet software like Microsoft Excel and Google Sheets. The flowchart detailing article inclusion is represented in Fig. 1. This review aimed to evaluate the significant effectiveness of medicinal plants in wound care and explore advancements in wound dressing technology to enhance wound healing capabilities.
Fig. 1.
PRISMA flowchart of included article
Wound healing process
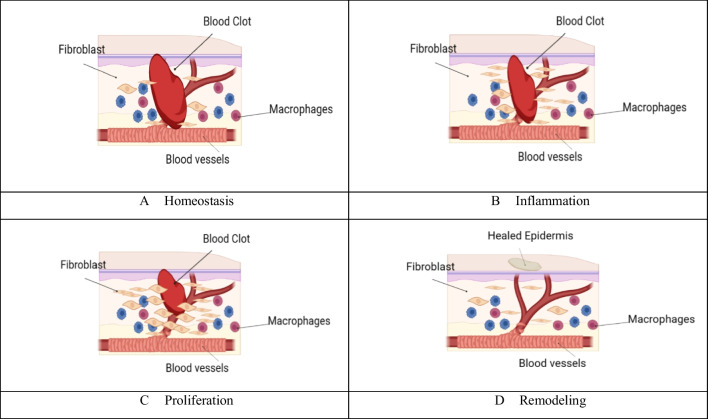
The epidermis, our body’s outermost layer commonly known as the skin, plays a crucial role as a protective barrier, preventing harmful substances, pathogens, and environmental toxins from infiltrating our system [34]. It also plays a pivotal role in temperature regulation, managing heat loss through mechanisms like sweating and blood vessel constriction or dilation [35]. Being the most exposed organ, the skin is susceptible to various forms of injury and damage [36]. Any harm to this protective shield, be it cuts, burns, or wounds, can impair its safeguarding functions [37]. A wound, defined as any injury leading to a break in the skin or mucous membranes, can stem from a range of causes, from abrasions to surgical incisions [38, 39]. Following skin damage, a complex sequence of cellular and molecular events is set into motion, kickstarting the wound-healing process and fortifying the body against infections and further harm [40]. The skin, an intricate organ with diverse cell types, signaling pathways, and functions, makes wound healing a sophisticated undertaking [41]. Effectively enhancing cutaneous wound healing necessitates a diverse array of approaches that acknowledge the intricate nature of the skin and its healing mechanisms [42]. This process hinges on a dynamic interplay of cellular elements, growth factors, cytokines, antioxidants, and essential metal ions [43]. Wound healing, a meticulously organized procedure, seeks to reinstate the skin’s barrier function and mechanical integrity. It unfolds through a well-coordinated series of stages, each playing a pivotal role in repairing tissue damage [44]. Restoring the skin’s integrity and function post-injury is paramount for both wound healing and overall health. This intricate process involves the phased reconstitution of a functional epidermis and other skin layers [45], as depicted in Fig. 2.
Fig. 2.
Wound healing process (A) Homeostasis: After injury, the body initiates homeostasis to stop bleeding and form a blood clot at the wound site. B Inflammation: The inflammatory phase involves the recruitment of immune cells to the wound to combat potential infections and clear debris. C Proliferation: During this stage, new tissue is generated to fill the wound gap. Cells such as fibroblasts produce collagen to build a new extracellular matrix, and new blood vessels form through angiogenesis. D Remodeling: In the final phase, the wound undergoes remodeling as the newly formed tissue matures and gains strength. Collagen fibers realign, and the wound’s overall tensile strength improves
Comprehensive strategies for wound management
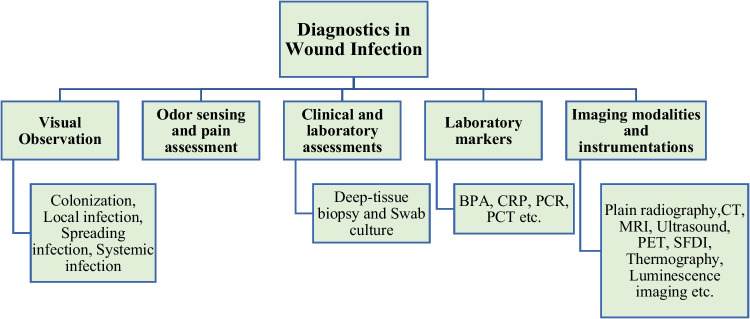
Comprehensive wound management encompasses the intricate multi-stage healing process, distinguishing between acute (pain, redness, warmth, and pus) and chronic infections (slow healing, discolored tissue, clear discharge, pockets, and an unpleasant odor) by their respective symptoms, with Fig. 3 illustrating diagnostic methods to identify wound infections [46]. Optimal wound management prioritizes creating a warm, moist environment to facilitate natural healing, with a strong emphasis on hygiene to reduce infection risks. This comprehensive approach involves crucial steps such as debridement [47–49] and the application of advanced treatments including wound bed preparation, antimicrobial dressings, and silver-based products [50–52]. In burn cases, topical antimicrobial agents like silver sulfadiazine cream play a pivotal role due to the heightened risk of bacterial infections, however, this approach has faced criticism for potential drawbacks including antimicrobial resistance, delayed healing, and cytotoxic effects on host cells [53]. While advanced therapies like negative pressure, growth factors, hyperbaric oxygen, and skin grafts present viable secondary options [54–63], their accessibility is limited by high costs and associated complications, including systemic issues, tissue availability, and donor-site morbidity, particularly in resource-poor settings. This underscores the urgent need for affordable wound treatments [64, 65]. Cost-effective clinical dressings like gauze sterilized absorbent cotton, and bandages offer valuable physical protection in wound healing and infection prevention. However, their adherence to the wound can sometimes lead to secondary damage upon separation [66]. Given the constraints of current wound healing agents, there is a critical imperative for the development of natural products to address non-healing wounds [67, 68].
Fig. 3.
Diagnostic methods for identification of wound infections. Note: BPA: Bacterial protease activity; CRP: C-reactive protein; PCR: Polymerase chain reaction; PCT; Procalcitonin, CT: Computed tomography; MRI: Magnetic resonance imaging; PET: Positron emission tomography; SFDI: Spatial frequency domain imaging
Traditional Herbal Medicines, valued for their cultural significance and minimal side effects, have been esteemed for their proven efficacy, accessibility, growing scientific validation, and commercial viability [69–72]. These traditional therapies include herbal- and animal-derived compounds, living organisms, and traditional dressings [73]. Medicines with bioactive compounds from traditional sources hold promise in treating chronic wounds by reducing inflammation, promoting re-epithelization, and acting as potent antiseptics, even against antibiotic-resistant bacteria [74]. Herbal products and their active constituents like Aloe barbadensis, Adiantum capillus, Commiphora molmol, henna, Nigella sativa, Teucrium polium, Nelumbo nucifera and Boswellia carteri exhibit superior wound healing effects, surpassing the efficacy of standard antimicrobial agents (e.g., silver nitrate, povidone-iodine, silver sulfadiazine, mafenide, mupirocin, bacitracin) and commercially available wound dressings (Comfeel—hydrocolloid dressing, Kaltostat—alginate dressing) [75]. Ayurveda and folk medicine traditions incorporate potent healing agents like Honey, Ghee, and revered medicinal plants including Glycyrrhiza glabra and Nerium indicum, known for their well-established wound-healing properties with minimal adverse effects [76]. Allium sativum, Aloe barbadensis, Centella asiatica, and Hippophae rhamnoides exhibit potent burn wound healing due to their diverse phytochemical composition engaging in antimicrobial, anti-inflammatory, antioxidant, collagen synthesis stimulation, cell proliferation, and angiogenic mechanisms [77]. Incorporating bioactive natural compounds within wound dressings, in forms like nanofiber, hydrogel, film, scaffold, and sponge, coupled with bio- or synthetic polymers, shows remarkable promise in augmenting wound healing by addressing oxidative stress, inflammation, and microbial activity throughout distinct phases of the healing process [78]. Critical to tissue repair, maintaining proper nutrition with adequate protein, vitamin C, zinc, and hydration is essential [79, 80]. Active patient engagement, encompassing vigilant wound care, infection monitoring, timely dressing changes, and knowing when to seek medical help, plays a pivotal role in the recovery process [81, 82].
Herbs used for wound management
Plant-based medications are gaining prominence for their perceived effectiveness, cost-effectiveness, and safety in treating chronic wounds [83]. Research highlights flavonoids, glycosides, saponins, terpenes, and phenolic compounds as key contributors to herbal remedies’ efficacy in wound management, exerting diverse beneficial effects at different stages of the healing process (Table 1).
Table 1.
Herbs used in wound management
| S.No. | Herbs | Family | Traditional uses | Extract | Plant part used | Study model type | Positive control | Analytical method for isolation | Isolated compound | Most active compound for activity | Category of most active compounds for activity | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dioscorea bulbifera | Dioscoreaceae | Wound healing and anti-inflammatory, diuretic, anthelmintic cold, stomach and rectal cancer | Aqueous | Bulbil | In vitro: HDF cell line | Aloe vera gel | VLC | 8-epidiosbulbin E acetate, 15,16-epoxy-6α-O-acetyl-8β-hydroxy-19-nor-clero-13, 14-diene-17,12;18,2-diolide, sitosterol-β-D-glucoside, 3,5-dimethoxyquercetin, catechin, quercetin, kaempferol, allantoin, 2,4,3’,5’-tetrahydroxybibenzyl, 2,4,6,7-tetrahydroxy-9,10 dihydrophenanthrene, myricetin | 15,16-Epoxy-6α-O-acetyl-8β-hydroxy-19-nor-clero-13(16),14-diene-17,12;18,2-diolide, catechin, quercetin and myricetin | Diterpenoid and flavonoid | Cell proliferation and migration | [84, 85] |
| 2 | Boerhavia diffusa | Nyctaginaceae | Anti-inflammatory, diuretic, cancer-preventive, hepatoprotective, antimicrobial, antioxidant and spasmolytic activity | Methanol | Leaf |
In vivo: excision wound model in albino Wistar rat In vitro: HaCaT cell line |
Povidone-iodine ointment | GC-MS | Ethylene glycol, valine, alanine, 2-Pyrrolidinone, proline, isoleucine, threonine, succinic acid, uracil, fumaric acid, serine, citramalic acid, malic acid, threonic acid, asparagine, glutamic acid, phenylalanine, 3,4-Dihydroxy-benzyl alcohol, 4-Methylcatechol, d-Fructofuranose, D-Pinitol, Tyrosine, Glucopyranose, d-Gluconic acid, Oxaloacetic acid, d-Glucuronic acid, Ferulic acid, Caffeic acid, Sucrose, mono palmitin | Caffeic acid, ferulic acid and D-pinitol | Hydrocinnamic acid, phenol, and inositol | Cell migration | [86, 87] |
| 3 | Aegle marmelos | Rutaceae | Anti-diarrheal, gastroprotective, antiviral, antidiabetic, anti-ulcerative colitis, cardioprotective, free-radical scavenging, and hepatoprotective | Hydroalcohol | Flower |
In vivo: full thickness wound model in Sprague-Dawley rat In vitro: HaCaT, Hs68, RAW264.7 cell line |
Betadine | HPLC | Cineol, eugenol, cuminaldehyde, aegelin, HDNC, luvangetin | 1-Hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde (HDNC) | Flavonoid |
Keratinocytes migration by Akt, beta-catenin, and ERK pathway |
[88, 89] |
| 4 | Carthamus caeruleus | Asteraceae | Hair growth and wound healing | Methanol | Root | In vivo: linear incision wound model in Wistar rat | Madecasol | GC-MS | Furfural, 2, 3-dihydro-3,5-dihydroxy-6-methyl-4 H-pyran-4-one, 7-Octenoic caid, 5-HMF, 1-pentadecene, Caryophyllene oxide, 13- tetradece-11-yn-1-ol, 1-octadecene, 8-methylene-3-oxatricyclononane, tetradecanoic acid, 2-ethyl hexyl trans-4-methoxycinnamate, hexadecenoic acid, 1–2 benzene dicarboxylic acid, gamma sitosterol | Palmitic acid, 2-ethylhexyl phthalate, and 5-(hydroxymethyl)-2-furan carboxaldehyde | Fatty acid, Phthalate, and alcohol | Reduced inflammation and oxidation | [90] |
| 5 | Sasa veitchii | Poaceae | Antimicrobial, antidiabetic, and antihypertensive activity | Aqueous | Crude |
In vivo: mice wound model In vitro: HaCaT cell line |
No positive control | - | - | - | - |
Promoted cutaneous aquaporin-3 expression |
[91, 92] |
| 6 | Gynura procumbens | Asteraceae |
Renal protective, antirheumatic, antiarthritic, antidiabetic, and antihypertensive activity |
Ethanol | Crude | In vivo: streptozotocin-induced diabetic model in mice | Solcoseryl jelly | TLC | Stigmasterol, kaempferol and quercetin | Stigmasterol, kaempferol and quercetin | Tetracyclic triterpenes and flavonoids | Cell proliferation and migration | [93, 94] |
| 7 | Reynoutria japonica | Polygonaceae | Used in Inflammation, jaundice and hyperlipemia | Ethanol | Rhizome | In vitro: HGF cell line | Betulinic acid | HPLC-MS | Malic acid, citric acid, procyanidin, catechin, epicatechin, piceatannol glucoside, Piceid, epicatechin-3-O-gallate, Resveratrol derivative, Aloesone hexoside, Emodin-glucoside, Lapathoside D, Torachrysone- hexoside, Torachrysone, Physcionin, Hydropiperoside, Phenylpropanoid-derived disaccharide esters, Vanicoside B, Questin, Physcion | Resveratrol, Procyanidins | Stillbene and flavonoid | Cell proliferation, migration, and increased collagen III synthesis | [95, 96] |
| 8 | Sorocea guilleminina Gaudich | Moraceae | Wound healing, anti-inflammatory, and diuretic activity | Aqueous | Leaf |
In vivo: excision and incision wound model in rat In vitro: fibroblastic N3T3 cell line |
Fitoscar ointment | LC-MS | Salicylic acid, Cinnamic acid, Gallic acid, Siringic acid, Pinocembrin, Chlorogenic acid, Isoquercitrin, Epicatechin | Salicylic acid, gallic acid, pinocembrin and isoquercitrin | Carboxylic acid, Phenol and Flavonoid | Cell proliferation, increased collagen III synthesis, and collagen rearrangement | [97] |
| 9 | Lafoensia pacari | Lythraceae | Wound healing, antiulcer, antifungal, and gastroprotective activity | Hydroalcohol | Leaf |
In vivo: excision model in albino mice In vitro: CHO-K1 and L929 cell line |
Madecassol | ESI-MS | ellagic acid, punicalagin, punicalin, kaempferol, Quercetin-3-O-xylopyranoside, Quercetin-3-O-rhamnopyranoside | ellagic acid, punicalagin, punicalin, kaempferol, Quercetin-3-O-xylopyranoside, Quercetin-3-O-rhamnopyranoside | Ellagic acid derivatives and Flavonoids | Cell proliferation and migration rate of fibroblasts and higher expression of p-ERK 1/2 protein | [98] |
| 10 | Panax ginseng | Araliaceae | Anti-allergic activity | Aqueous (Hot) | Root | In vivo: full-thickness skin wound in Sprague–Dawley rat | Placebo | HPLC | Ginsenoside Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2s, Rg3s, Rg3r | Ginsenoside | Steroid glycoside | Increased expression of TGF-β, VEGF, MMP-1 and MMP-9 | [99] |
| 11 | Coccinia grandis | Cucurbitaceae | Relieving insect bite itching and swelling | Methanol | Leaf | In vitro: HFb and HaCaT cell line | Allantoin | LC-ESI-MS/MS | Rutin, quercetin-hexoside deoxyhexoside, kaempferol-3-O-glucoside, oleuropein and ligstroside | Rutin, quercetin-hexoside deoxyhexoside, kaempferol-3-O-glucoside, oleuropein and ligstroside | Flavonoids and secoiridoids | Reduced hydrogen peroxide-induced oxidative stress by increasing cell survival rate | [100, 101] |
| 12 | Peucedanum ostruthium | Apiaceae | stimulant, stomachic, and diuretic, treats rheumatic, chronic inflammatory, skin problems, and musculoskeletal diseases | Hydroalcohol | Rhizome and Leaf | In vitro: HaCaT, L929 fibroblast cell line | Allantoin | LC-ESI-MS |
Caffeoylquinic acid, p-Coumaroylquinic acid, Feruloylquinic acid, p-Coumaroyl glucose, Quercetin-3-O-rutinoside, Hesperidin, Quercetin-3-O-(6″acetyl-glucoside), 3,7-Dimethylquercetin, Oxypeucedanin-hexoside, Kaempferol 3-O-acetyl-glucoside, Osthenol-7-O-glucoside, Oxypeucedanin, Ostruthol, Isoimperatorin, Imperatorin, Ostruthin |
Caffeoyl and feruloylquinic derivatives, ostruthin, and isoimperatorin, Quercetin | Phenols, coumarins, flavonoids | Inhibit cyclooxygenase and lipoxygenase activity | [102] |
| 13 | Artemisia absinthium | Asteraceae | Used to treat gastrointestinal ailments, helminthiasis, anemia, insomnia, bladder diseases, wounds, and fever | Methanol (Hot) | Leaf | In vivo: wound model in Wistar rat | Povidone Iodine cream | GC-MS |
Epiyangambin, flavone, octadecanoic acid, 2,3-dihydroxypropyl ester, palmitic acid β - monoglyceride, á-D-mannofuranoside, camphor, and terpineol |
Stearic acid and palmitic acid | Fatty acid | Modulated cytokine networks and apoptosis markers levels | [103, 104] |
| 14 | Portulaca oleracea | Portulacaceae | Relieving fever, dysentery, diarrhea, carbuncle, eczema, and hematochezia | Hydroalcohol | Leaf |
In vivo: deep tissue pressure injury model in mice In vitro: HaCaT and HUVEC cell line |
No positive control | - | Hyperoside, kaempferol and quercetin-3-O-α-L-arabino pyranoside | - | - | Increased new blood vessels, collagen deposition, and re-epithelization and decreased inflammatory infiltration | [105, 106] |
| 15 | Premna integrifolia | Lamiaceae | Used in the treatment of bronchitis, diabetes, edema, chyluria, dyspepsia, inflammation, liver problems, constipation, piles, and fever | Standardized extract procured | Crude |
In vivo: wound model In vitro trypsinized cells |
Povidone-iodine ointment | - | - | - | - | Cell proliferation, migration, and keratinization | [107] |
| 16 | Urtica dioica | Urticaceae | Anti-epileptic and treat boils and blisters | Methanol | Crude |
In vivo: full-thickness wound model in rat In vitro: HEK-293 and HaCaT cell line |
Madecassol | 1 H NMR | Saponins, flavonoids, carbohydrates, ketoses, resins, and coumarins | Saponins, flavonoids, carbohydrates, ketoses, resins, and coumarins | Cell proliferation | [108, 109] | |
| 17 | Clinacanthus nutans | Acanthaceae | Anti-venom for snake, scorpion, and insect bites, treat skin rashes, Pruritic rash, burn, inflammation | Sequential extraction with hexane, chloroform, and ethanol | Leaf | In vitro: RAW 264.7 and HGF cell line | TLC, FTIR, HRES-MS | Genistein | Purpurin-18 phytyl ester | Purpurin-18 phytyl ester | Dihydroporphyrin | Inhibit lipopolysaccharide (LPS)-induced NO production | [110, 111] |
| 18 | Cinnamomum verum | Lauraceae | Antidiabetic, antimicrobial skin infections and anticancer activity | Hydroalcohol | Bark | In vivo: Two circular full-thickness excisional wound mouse model | No positive control | HPLC | Caffeic acid, epicatechin, quercetin, coumarin, 2-hydroxyl cinnamaldehyde, cinnamyl alcohol, cinnamic acid, cinnamaldehyde, 2-methoxy cinnamaldehyde, eugenol | Cinnamaldehyde and 2-hydroxyl cinnamaldehyde | Flavonoid |
Fibroblast proliferation, collagen deposition, re-epithelialization, and increased expression of cyclin D1, IGF1, GLUT 1 |
[112] |
| 19 | Zataria multiflora | Lamiaceae | Antibacterial and antioxidant properties | Essential oil | - | In vivo: full-thickness excisional skin mouse model | Mupirocin ointment | GC-MS | α-Thujene, α-Pinene, Camphene, β-Pinene, 3-Octanone, Myrcene, 3-Octanol, α-Phellandrene, α-Terpinene, p-Cymene, Limonene, 1,8-Cineole, γ-Terpinene, cis-Sabinene hydrate, Terpinolene, Linalool, Borneol, Terpinen-4-ol, α-Terpineol, Thymol, Carvacrol, Caryophyllene, Aromadendrene, α-Humulene, Viridiflorene, Spathulenol | Thymol, p-cymene, γ-terpinene, carvacrol | Monoterpene and monoterpene phenol | Increased expression of TGF-β, IL-10 IGF-1, FGF-2, and VEGF | [113] |
| 20 | Glycyrrhiza glabra | Fabaceae | Hepatoprotective, anti-inflammatory, and flavoring agent | Ethanol | Root | In vivo: cutaneous wound model in Wistar rat | No positive group | UPLC-PDA-MS/MS | Licoagroside B, Kaempferol-3-O-rutinoside, HBMA, Isoshaftoside, Isoshaftoside, Liquiritin derivative, Glycyroside, Butein-4-O—glucopyranosyl-apiofuranoside, Licorice glycoside, Isoliquiritin, Licochalcone B, Isoliquiritigenin, Pinocembri, Echinatin, Glycyrrhizin, Formononetin, Prenylated flavonoid, Esculin, Glabrone | Glycyrrhetinic acid, Glycyrrhizic acid, glabridin and licochalcone A | Pentacyclic triterpenoid, Triterpenoid saponin, Hydroxyisoflavan and Chalconoid | Re-epithelialization and collagen synthesis | [114] |
| 21 | Derris scandens | Fabaceae | Analgesic, anti-inflammatory, antimicrobial, antioxidant, and anticancer | Hydroalcohol | Stem | In vitro: HSF cell line | Ascorbic acid | HPLC | Genistein, lupeol | Genistein, lupeol | Flavonoid and Pentacyclic lupane-type triterpenes | Cell migration lowered oxidative stress and proinflammatory markers | [115] |
| 22 | Astragalus floccosus | Leguminosae | Immunomodulatory, antiviral, hepatoprotective, antiperspirant, and antidiabetic activity | Methanol | Root |
In vitro: HDF cell line In vivo: full thickness wound model in rats |
Silver sulfadiazine | LCMS | Calycosin-7-O-beta-D-glucoside, 7,4′-Dihydroxy-3′-methoxyflavone 7-glucoside, 3′-O-Methylorobol-7-O-glucoside, Quercetin derivative, Kaempferol derivative and Formononetin | Calycosin-7-O-beta-D-glucoside and Formononetin | Isoflavonoid | Fibroblast proliferation and epithelization | [116] |
| 23 | Launaea procumbens | Asteraceae | Used in skin problems, tumors, and dysentery, for wound healing activity, painful urination, and reproductive diseases | Methanol | Aerial parts | In vivo: excision wound model in rabbit | MEBO ointment | LC-HRMS | Orientin, Loganic acid, Touruosamine, Esculin, Vulgaxanthin-I, Chlorogeniacidid, Cimigenol, Isobetanidin, Glycerol 1- alkanoates, Bullatacinone, Phytol, Fumaflorine, Catechin-5-o- glucoside | Orientin | Flavonone | Increased expression of TGF-β and decreased levels of TNF-α and IL-1β | [117] |
| 24 | Verbascum sinaiticum | Scrophulariaceae | Curing wounds, abdominal dropsy, anthrax, diarrhea, and fungal infections | Methanol | Leaf | In vivo: excision and Incision wound model in Wistar rats | Nitrofurazone ointment | LC-MS | Quercetin, rutina harpagoside, protocatechuic acid, gentisic acid, p-coumaric acid, ferulic acid, salicylic acid, and rosmarinic acid | - | Saponins, flavonoids, terpenoids | Wound contraction and epithelialization | [118, 119] |
| 25 | Astragalus membranaceus | Fabaceae | Reduce swelling, drain pus, and eradicate toxins | Ethanol | Root |
In vitro: HSF cell line In vivo: full-thickness excision wound in mouse |
Jingwanhong ointment | Ion-exchange chromatography | APS2-1 | APS2-1 | Novel polysaccharide | Increased expression of TGF-β1, bFGF ,and EGF | [120] |
| 26 | Marantodes pumilum | Primulaceae | Used in female reproductive-related problem | Distilled water | Leaf and Root | In vivo: excision wound model in Sprague-Dawley rats | Acriflavine | LC-MS/MS | Cinnamic acid, quinic acid, gallic acid, caffeic acid, ellagic acid, p-hydroxybenzoic acid, catechin, myricetin derivative, protocatechuic acid hexoside | Gallic acid, ellagic acid, and caffeic acid, | Phenolic compound | Fibroblast proliferation, and collagen formation | [121] |
| 27 | Artocarpus communis | Moraceae | Antiinflammatory, antitumor, antimicrobial, and antioxidant activity | Dichloromethane | Heartwood |
In vitro: HaCaT, GM05386, HSF cell line In-vivo excisional wound model in mice |
No positive control | Successive extraction and HPLC | Artocarpin | Artocarpin | Prenylated flavonoid | Fibroblast proliferation and collagen synthesis by activating JNK, Akt, and P38 pathway | [122] |
| 28 | Gliricidia sepium | Fabaceae | Anti-inflammatory, analgesic, and antimicrobial activity | Powder leaves to form an ointment | Leaf | In vivo: wound model Wistar rats | Commercial wound healing agent | - | - | - | - | Lowered the expression of IL-1β and IL-6 | [123] |
| 29 | Olea europaea | Oleaceae | Relieving Skin diseases and wounds | Ethyl alcohol | Leaf | In vivo: full thickness incision wound model in Balb/c mice | No positive group | Solvent extraction and HPLC | Oleuropein | Oleuropein | Glycosylated seco- iridoid | Increased VEGF, collagen deposition and re-epithelisation | [124] |
| 30 | Apium graveolens | Apiaceae | Used in Skin infections, chronic ulcers, antioxidant, and antimicrobial activity | Methanol | Dried celery | In vivo: wound model in Sprague Dawley rat | Positive control | - | - | - | - | Decreased inflammatory cells and increased expression of CK-17 to promote proliferation and epithelization | [125] |
| 31 | Euphorbia hirta | Euphorbiaceae | Analgesic, antiinflammatory, antidiabetic and antimicrobial activity | Methanol | Leaf |
In vivo: excision wound model in Wistar rat In vitro: HDF cell line |
Gentamicin sulfate | - | Euphorbin, A, euphorbin-B, euphorbin-C, euphorbin-E, Quercitrin, myricitrin, rutin, kaempferol, quercetin, gallic acid and protocatechuic acid, β-amyrin, 24-methylenecycloartenol, β-sitosterol, heptacosane, nonacosane, shikimic acid, camphol and quercitol | - | - | Collagen production and fibroblast proliferation | [126] |
| 32 | Blumea balsamifera | Blumea | Anti-rheumatic and used to treat dermatitis, beriberi, lumbago, snake bites, and bruises | Methanol | Leaf | In vivo: excisional wound in Sprague-Dawley rats | Jing Wan Hong ointment | UPLC Q-TOF-MS/DAD | DiCQA, Rutin, 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4 H-chromen-3-yl6-O-(6-deoxy-α-L- mannopyranosyl)-D-glucopyranoside, Hyperoside, Isoquercitrin, Myricitrin, Flavonone derivative, Luteolin and its derivative, Chrysoeriol, Kaempferide, Hydranngetin, Diosmetin, Blumeatin, Ayanin | Rutin, Hyperoside, Isoquercitrin, Myricitrin, Luteolin Chrysoeriol | Flavonoid | Capillary regeneration and re-epithelialization | [127] |
| 33 | Libidibia ferrea | Fabaceae | Antirheumatic, anticancer, antidiabetic, and gastroprotective activity | Ethanol | Leaf, fruit, and flower | In vivo: excision model in dogs | Commercial veterinary ointment (allantoin and zinc oxide) | TLC | Rutin | Rutin | Hydrolysable tannins and flavonoids | Wound retraction by fibroplasia | [128] |
| 34 | Astragalus membranaceus | Leguminosae | Antioxidant, anti-inflammatory, antidiabetic and hepatoprotective activity | Ethanol | Root |
In vitro: HDF and HaCaT cell line In vivo: wound model in mice |
No positive control | Solvent extraction and silica gel chromatography | AS-I, AS-II, AS-III, AS-IV, AS-VI, Iso AS-I, Iso As-II, cycloastragenol-6-O-beta-D-glucoside | Astragaloside VI and cycloastragenol-6-O-beta-D-glucoside | Triterpenoid saponin | Proliferation and migration of skin cells by EGFR/ERK signaling pathway. | [129] |
| 35 | Urtica simensis | Urticaceae | Antirheumatic and antiarthritic activity, treat wounds such as burns and skin rash | Methanol | Leaf | In vivo: excision, incision, and burn wound model in mice | Nitrofurazone ointment | - | - | - | - | Fibroblasts proliferation | [130] |
| 36 | Dipsacus asper | Dipsacaceae | Analgesic and anti-inflammatory activity and treat spermatorrhoea | - | Root |
In vivo: Full-thickness wound model rat In vitro: HUVEC cell line |
bFGF | - | Asperosaponin VI (procured) | Asperosaponin VI | Triterpene saponin |
Enhanced angiogenesis by up-regulating the HIF-1α/VEGF pathway |
[131] |
| 37 | Haplophyllum tuberculatum | Rutaceae | Antiseptic, antiarthritic, antidiabetic, antihypertensive, antiulcer, and analgesic activity | Hydroalcohol | Aerial part |
In vivo: Burn wound model in rat |
Madecassol ointment | LC-MS | p-Coumaric acid, Quercetin-3-O-glucoside, Kaempferol-3-O-glucose, Isorhamnetin-7-O-pentose, Luteolin 7-O-glucoside, Kaempferol-3-O-glucuronic acid, Protocatechuic acid, Salicylic acid, Gentisic acid, Synaptic acid, Ferulic acid, Epigallocatechin, Procyanidins, Rutin, Naringin | p-Coumaric acid, derivative of Quercetin, Kaempferol, Isorhamnetin, Luteolin, Rutin, Procyanidins, Epigallocatechin | Phenolic compound | Regulated growth factors and cytokines | [132] |
| 38 | Oroxylum indicum | Bignoniaceae | Antidiabetic and anticancer activity | Ethanol | Leaf |
In vitro: HaCaT cell line In vivo: excisional wound model in rat |
Sudocrem cream | LC-TOF-MS/MS | Ginkgetin, Orientin, Chrysin, Pinoquercetin, Cupressuflavone, Puerarin xyloside, Forsythiaside, Phlorizin chalcone, Azelaic acid, Luteolin 7-O-glucuronide, Naringenin chalcone, Paederoside, | Orientin, Chrysin, Pinoquercetin, Cupressuflavone, Puerarin xyloside, Forsythiaside and Paederoside | Flavonoid and glycoside | Wound contraction | [133] |
| 39 | Hydnophytum formicarum | Rubiaceae | Antioxidant, anti-inflammatory, and antimicrobial activity | Ethanol | Whole | In vivo: Excision wound model Sprague–Dawley rats | No positive control | - | - | - | - | Promoted angiogenesis and re-epithelialization | [134] |
| 40 | Siegesbeckia orientalis | Asteraceae | Antiarthritic, antimalarial, analgesic and cardioprotective activity | - | - |
In vivo: excision wound model in Wistar rats In vitro: L929 cell line |
No positive control | - | Kirenol (procured) | - | Diterpenoid | Reduced the levels of NF-κB, COX-2, iNOS, MMP-2 and MMP-9 | [135] |
| 41 | Dittrichia viscosa | Asteraceae | Anti-inflammatory, antispasmodic, antiseptic, antirheumatic, treat wound and hemorrhoids | Ethanol | Leaf | In vivo: circular full-thickness wound model Swiss Webster mice | Vehicle is used as a positive control | HPLC-DAD-ESI/MS | Dicaffeoylquinic isomers, quercetin derivatives, isoorientin, apigenin-glucoside, myricetin, and isorhamnetin-O-glucuronopyranoside | Phenolic compounds and Caffeoylquinic Acid | Phenol | Re-epithelialization | [136] |
| 42 | Panax ginseng | Araliaceae | Hepatoprotective and vasoprotective activity | Procured | Root | In vivo: full-thickness wound model Wistar rats | No positive control | - | Ginsenosides | Ginsenosides | Steroid glycosides | Increased expression of VEGF | [137] |
| 43 | Actinidia deliciosa | Actinidiaceae | Anti-inflammatory, anticancer, and cardioprotective activity | Ethanol | Fruit | In vitro: HGF cell line | No positive control | - | - | - | Vitamin C, carotenoids, tannins, and saponin | Fibroblast migration and angiogenesis | [138] |
| 44 | Althaea officinalis | Malvaceae | Analgesic, Antiinflammatory, treat respiratory diseases, skin ailments and digestive diseases | Hydroalcohol | Leaf | In vivo: Excision wound model in rat | Zinc oxide ointment | - | - | - | - | Accelerated wound healing processes | [139] |
| 45 | Aster koraiensis | Asteraceae | Used to treat chronic bronchitis, pneumonia, and pertussis | Ethanol | Aerial parts |
In vivo: Wound model in male Sprague Dawley rat In vitro: HaCaT cell line |
No positive control | HPLC | chlorogenic acid and 3,5-di-O-caffeoylquinic acid | chlorogenic acid and 3,5-di-O-caffeoylquinic acid | Phenol | Inhibited expression of MMP-2/9 | [140] |
| 46 | Amphimas pterocarpoides | Leguminosae | Antimalarial, antiarthritic, anti-inflammatory, analgesic, and used to treat respiratory tract infections | Methanol | leaf and stem bark |
In vivo: excision model in Sprague-Dawley rats |
Silver sulphadiazine | HPLC | - | - | Tannin, triterpenoid, phytosterol, flavonoid, saponin and coumarin | Wound contraction | [141] |
| 47 | Curcuma longa | Zingiberaceae | Anti-inflammatory, antioxidant, and antibacterial activity | - | Rhizome | In vitro: HGF cell line | - | - | Curcumin | Curcumin | Diarylheptanoid | Upregulated expression of KGF-1 and EGFR | [142] |
| 48 | Poincianella pluviosa | Fabaceae | Antimalarial and wound healing activity | Ethanol | Bark | In vivo: wound model in Wistar rat | No positive control | - | - | - | Collagen formation and re-epithelialization | [143] |
Polyherbal a synergistic combination for wound management
Polyherbal compositions, also known as polyherbal therapy, have gained global recognition for their enhanced therapeutic potential compared to individual plant-based treatments, as they harness synergistic effects to amplify medicinal activity while reducing toxicity within specific proportions [144, 145]. This approach offers distinct advantages over single herbal formulations, demonstrating a more potent therapeutic outcome and necessitating lower quantities for desired pharmacological effects, thereby minimizing potential side effects [146, 147]. These collective benefits have substantially bolstered the market appeal of polyherbal remedies. In the context of wound infections, diverse herbal blends tailored for specific effects are available, as detailed in Table 2.
Table 2.
Polyherbal formulation for wound management
| S.No. | Herbal extract | Composition | Family | Part of the plant | Experimental model | Control | Chemical constituents | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chinese herb microneedle patch | Premna microphylla | Lamiaceae | Leaf |
In vivo: excision wound model in rat In vitro: NIH-3T3 cell line |
Microneedle patch without asiatic acid | Pectin and amino acid | Relieved heat, detoxicated, caused detumescence, and treated hemostatis. | [148] |
| Centella asiatica | Apiaceae | Leaf | Asiatic acid | Encouraged the expression of important growth factor genes in fibroblasts | |||||
| 2 | Polyherbal combination | Punica granatum | Punicaceae | Flower | In vivo: excision wound model in rat | No Control group (different concentrations of single and combinational herbs were used to compare with each other) | Tannins, punicalagin, and ellagic acid, gallic acid, maslinic, ursolic acid, and asiatic acid | Strong antioxidant activity and anti-inflammatory activity of the combination | [149] |
| Matricaria chamomilla | Asteraceae | Flower | Quercetin, apigenin, coumarins, and terpenoids, α-bisabolol and chamazulene | ||||||
| 3 | San Huang Powder | Rheum officinale | Polygonaceae | Stem and root |
In vivo: burn wound model in female Lee-Sung pigs In vitro: LPS-induced HMEC-1 and RAW264.7 cell line |
Different combination of herbs was used as comparator for each other | Chrysophanol | Reduced the production of inflammatory mediators such as cytokines and interleukins. | [150] |
| Scutellaria pekinensis | Lamiaceae | Root | Chrysin | ||||||
| Phellodendron amurense | Rutaceae | Bark | Berberine hydrochloride | ||||||
| Coptis chinensis | Ranunculaceae | Rhizome | Berberine hydrochloride | ||||||
| 4 | Chinese medicine ANBP | Agrimonia eupatoria | Rosaceae | Formulation was procured from the market | In vivo: full-layer skin defect model in rat | Control group without treatment | - | Facilitated the wound healing process and reduced the wound healing time. | [151] |
| Nelumbon nucifera | Nelumbonaceae | ||||||||
| Boswellia carteri | Burseraceae | ||||||||
| Typha orientalis | Typhaceae | ||||||||
| 5 | Abnormal Savda Munziq | Lavandula angustifolia | Lamiaceae | Formulation was procured from market | In vivo: comb burn model in Sprague-Dawley rat | Control group without treatment | - | Reduced oxidative stress and apoptosis | [152] |
| Foeniculum vulgare | Apiaceae | ||||||||
| Anchusa italica | Boraginaceae | ||||||||
| Euphorbia humifusae | Euphorbiaceae | ||||||||
| Melissa officinalis | Lamiaceae | ||||||||
| Adiantum capillusveneris | Pteridaceae | ||||||||
| Glycyrrhiza uralensis | Fabaceae | ||||||||
| Cordia dichotoma | Boraginaceae | ||||||||
| Ziziphus jujuba | Rhamnaceae | ||||||||
| Alhagi pseudoalhagi | Fabaceae | ||||||||
| 6 | AnoacH/PiloTab | Mimosa pudica | Fabaceae | Formulation was procured from market | In vivo: human hemorrhoid and fistula specimen | Vehicle control | - | Decreased the migration of immunological cells and mesenchymal cells | [153] |
| Euphorbia hirta | Euphorbiaceae | ||||||||
| Messua ferrea | Calophyllaceae | ||||||||
| Berberis aristata | Berberidaceae | ||||||||
| 7 | Japanese herbal medicine hangeshashinto | Pinellia ternata | Araceae | Tuber | In vitro: HOK Cell line | Vehicle control | - | Improved the migration of human oral keratinocytes. | [154] |
| Scutellaria pekinensis | Lamiaceae | Root | Baicalin, baicalein, wogonin | ||||||
| Zingiber officinale | Zingiberaceae | Rhizome | Shogaol, gingerol | ||||||
| Glycyrrhiza glabra | Fabaceae | Root | Glycyrrhizin, glycyrrhetinic acid, liquiritin, isoliquiritin, liquiritin apioside, liquiritigenin, isoliquiritigenin | ||||||
| Ziziphus jujuba | Rhamnaceae | Fruit | - | ||||||
| Panax ginseng | Araliaceae | Root | - | ||||||
| Coptis occidentalis | Ranunculaceae | Rhizome | - | ||||||
| 8 | Novel Distillate from Fermented Mixture | Angelica gigas | Apiaceae | Root | In vivo: ultraviolet B-induced skin damage in mice | Vehicle control | 2, 6, 10-trimethyldodecane, 2, 6, 11, 15-tetramethylhexadecane, n-heptadecane, n-docosane, Siloxane derivatives | Reduced expressions of TNF-alpha and IL-1 | [155] |
| Lonicera japonica | Caprifoliaceae | Bloom | |||||||
| Dictamnus dasycarpus | Rutaceae | Root | |||||||
| Dioscorea oppositifolia | Dioscoreaceae | Root | |||||||
| Ulmus davidiana | Ulmaceae | Bark | |||||||
| Hordeum vulgare | Gramineae | Seed | |||||||
| Xanthium strumarium | Asteraceae | Seed | |||||||
| Cnidium officinale | Apiaceae | Root | |||||||
| Houttuynia cordata | Saururaceae | Leaf | |||||||
| 9 | Traditional Chinese medicine ARCC | Angelica Sinensis | Apiaceae | Root |
In vivo: full-thickness wound model in mice and also assessed in diabetic patients with gangrene |
Control without treatment | - |
Re-epithelization, vascularization and Increased levels of TGF-β1 and CD31 cells |
[156] |
| Radix Rehmanniae | Scrophulariaceae | Root | |||||||
| 10 | Herbal ointment blend | Punica granatum | Punicaceae | Fruit | In vivo: excision wound model in Wistar rat | Gentamycin | Ellagic tannins, ellagic acid and gallic acid | Increased rate of wound contraction and decreased rate of epithelisation period | [157] |
| Commiphora myrrha | Burseraceae | Stem resinous exudate | Furanosesquiterpenoid, water-soluble and alcohol-soluble resins | ||||||
| Laurus nobilis | Lauraceae | Leaf | Linalool, p-cymene, α-pinene, limonene and β-pinene | ||||||
| 11 | Herbal cream | Pelargonium graveolens | Geraniaceae | Flower | In vivo: diabetic foot ulcers rat animal model | Placebo without treatment | β-citronellol, geraniol, and phenyl ethyl alcohol | Anti-ulcerogenic effect and tissue regeneration | [158] |
| Oliveria decombens | Apiaceae | Flower | Thymol, γ-terpinene, croweacin, and sabinene | ||||||
| 12 | Polyherbal formulation | Elephantopus scaber | Asteraceae | Leaf | In vivo: excision, incision and burn wound model in Swiss albino mice | Povidone iodine | Deoxyelephantopin | Increased antioxidant activity that surges the rate of wound contraction | [159] |
| Clinacanthus nutans | Acanthaceae | Leaf | lupeol, β-sitosterol, stigmasterol, botulin, and myricyl alcohol, vitexin, isovitexin, shaftoside, isomollupentin 7-O-β-glucopyranoside, orientin, and isoorientin | ||||||
| 13 | Jinchuang Ointment | Calamus draco | Arecaceae | Resin | In vitro: HaCaT and HUVEC cell line, also assessed on nonhealing diabetic wounds in patients | VEGF | Dracorhodin perchlorate, Catechin, Epicatechin, Acetyl-11-keto-β-boswellic acid, (E)-Guggulsterone | Stimulated angiogenesis, cell proliferation, and cell migration | [160] |
| Cinnamomum camphora | Lauraceae | Wood | |||||||
| Uncaria gamber | Rubiaceae | Aqueous extract from leaf and shoot | |||||||
| Boswellia serrata | Burseraceae | Resin | |||||||
| Commiphora myrrha | Burseraceae | Resin | |||||||
| 14 | Polyherbal formulation | Vitex negundo | Verbenaceae | Leaf | In vitro: L929 and HaCaT cell line | Cipladine | Flavonoids, phenols, and tannin | Skin regeneration and collagen synthesis and increased levels of antioxidants (catalase and GSH) | [161] |
| Emblica officinalis | Euphorbiaceae | Fruit | |||||||
| Tridax procumbens | Asteraceae | Leaf | |||||||
| 15 | Herbal Mixture | Adiantum capillus-veneris | Adiantaceae | Leaf | In vivo: streptozotocin-induced diabetic rats wound model in Wistar rat | Vaseline control | Tannin, gallic acid, resin, flavonoid, coumarin and anthraquinone | Modulated the expression of TGF-b1, MMP-3/6, IL-6 and TNF-α | [162] |
| Commiphora molmol | Burseraceae | Resin | |||||||
| Aloe barbadensis | Liliaceae | Leaf | |||||||
| Lawsonia inermis | Lythraceae | Leaf | |||||||
| 16 | Aloe vera-based extract of Nerium oleander | Nerium oleander | Apocynaceae | Flower | In vivo: Partial-thickness second-degree burn injury in Wistar albino rat | Silverdin | - | Modulated the levels of MDA, GSH, MPO, TNF-α, IL-1β and DNAT | [163] |
| Aloe barbadensis | Liliaceae | Leaf | |||||||
| 17 | Thai herbal formulation | Centella asiatica | Apiaceae | Leaf |
In vitro: HaCaT cell line |
Untreated cells | - | Upregulate the expression of TIMP-1, VEGF, and TGF-β and downregulated the expression of TNF-α, IL-6, and MMP-9 | [164] |
| Curcuma longa | Zingiberaceae | Rhizome | |||||||
| Zingiber cassumunar | Zingiberaceae | Rhizome | |||||||
| Garcinia mangostana | Guttiferae | Peel | |||||||
| Zingiber officinale | Zingiberaceae | Rhizome | |||||||
| Eleutherine americana | Iridaceae | Rhizome | |||||||
| Piper nigrum | Piperaceae | Seed | |||||||
| Senna alata | Leguminosae | Leaf | |||||||
| Areca catechu | Arecaceae | Fruit | |||||||
| 18 | Iranian traditional medicine | Malva sylvestris | Malvaceae | Leaf | In vivo: Second-degree burn wound in rat | Silver sulfadiazine | Phenol and tannins | Re-epithelialization with remarkable neovascularization | [165] |
| Solanum nigrum | Solanaceae | Leaf | |||||||
| Rosa damascena | Rosaceae | Petal | |||||||
| 19 | Herbal formulation | Adiantum capillus-veneris | Adiantaceae | Leaf | In vitro: mouse skin fibroblasts cell line | Untreated cell | Tannin, gallic acid, resin, flavonoid, coumarin and anthraquinone | Improved the gene expression of TGF-β1 and VEGF-A | [166] |
| Commiphora molmol | Burseraceae | Resin | |||||||
| Aloe barbadensis | Liliaceae | Leaf | |||||||
| Lawsonia inermis | Lythraceae | Leaf | |||||||
| 20 | Tuo-Li-Xiao-Du-San | Radix Angelica sinensis | Apiaceae | Root | In vivo: full-thickness excision wound in Sprague-Dawley rat | Untreated group | - | Reduced infiltration of neutrophils and macrophages and enhanced angiogenesis, and collagen formation | [167] |
| Radix Astragali | Fabaceae | Root | |||||||
| Angelica dahurica | Apiaceae | Root | |||||||
| Gleditsia sinensis | Fabaceae | Thorn | |||||||
| 21 | Traditional Chinese Medicine Herbal Mixture Sophora flavescens | Sophora flavescens | Fabaceae | Root | In vivo: rat model of perianal ulceration | Potassium permanganate solution | - | Inhibited pro-inflammatory cytokines PGE2 and IL-8 | [168] |
| Phellodendron amurense | Rutaceae | Bark | |||||||
| Radix sanguisorbae | Rosaceae | Leaf | |||||||
| Scutellaria baicalensis | Lamiaceae | Root | |||||||
| Paeonia suffruticosa | Paeoniaceae | Root | |||||||
| Gardenia florida | Rubiaceae | Flower | |||||||
| Areca catechu | Arecaceae | Seed | |||||||
| Rheum officinale | Polygonaceae | Rhizome | |||||||
| Glycyrrhiza glabra | Fabaceae | Root | |||||||
| 22 | Iranian Traditional Medicine | Aloe barbadensis | Liliaceae | Leaf | In vivo: excision wound model in rat | Tetracycline ointment | Boswellic acid, sesqui- and triterpenoids, glucomannan, arabinorhamnogalactan, pectic substances, e, and glucuronic acid-containing polysaccharide | Reduced inflammatory cells | [169] |
| Commiphora myrrha | Burseraceae | Resin | |||||||
| Boswellia carteri | Burseraceae | Resin | |||||||
| 23 | Kampo Medicine Rokumigan | Rehmannia glutinosa | Orobanchaceae | Root | In vitro: human gingival epithelial cell line | Aloe vera extract | - | Inhibited IL-6 secretion, fibroblast proliferation and migration | [170] |
| Dioscorea batatas | Dioscoreaceae | Rhizome | |||||||
| Cornus officinalis | Cornaceae | Fruit | |||||||
| Poria cocos | Polyporaceae | Sclerotium | |||||||
| Paeonia suffruticosa | Paeoniaceae | Root cortex | |||||||
| Alisma orientale | Alismataceae | Root | |||||||
| 24 | Topical herbal patch (Perio Patch) | Centella asiatica | Apiaceae | Formulation was procured from the market | In vivo: Full-thickness flaps in rat wound model | Placebo | - | Increased number of proliferating cells, collagen, and blood vessel formation | [171] |
| Echinacea purpurea | Asteraceae | ||||||||
| Sambucus nigra | Adoxaceae |
HDF Human dermal fibroblast, HaCaT: Human keratinocyte cell line, Hs68: human foreskin fibroblast cell line, RAW: macrophage cell line of mouse, VLC Vaccum Liquid Chromatography, GC-MS Gas chromatography- Mass spectroscopy, High-performance liquid chromatography, HGF human gingival fibroblast cell line, CHO-K1: Chinese hamster ovary epithelial cells, ESI-MS Electrospray ionization and mass spectrometry, TGF- β1 transforming growth factor-β1, VEGF vascular endothelial growth factor, MMP matrix metalloproteinase, HFb: Human fibroblast cell line, Human embryonic kidney 293 (HEK-293) cells, HSFs Human skin fibroblast cell, HUVECs Human umbilical vein endothelial cells, bFGF: Human basic fibroblast growth factor, HGF Human gingival fibroblasts cell, HMC-1: Human mast cell, HOKs Human oral keratinocytes, OBA-9: Human gingival epithelial cell line
Recent advances in wound dressing technology for enhanced wound healing capacity
A wound dressing applied directly to a wound plays a crucial role in expediting healing and preventing complications associated with untreated wounds [172]. Wound healing involves four primary stages: hematoma creation, inflammation, neotissue formation, and tissue remodeling [173], with the involvement of macrophages being instrumental [174]. There’s been significant development in wound dressings and technologies aimed at enhancing the body’s natural healing and tissue regeneration processes [175]. Nanotechnologies have emerged, offering unique properties to address issues in wound repair mechanisms [176]. In fields like biomedicine, pharmaceuticals, and medicine, there’s a growing emphasis on nano-formulations for wound care, particularly in cases of diabetes-induced wounds [177]. Herbal preparations have gained attention due to their diverse phytoconstituents and broad pharmacological activity compared to synthetic drugs. They are considered safe for extended use, leading to increased focus on designing herbal-loaded wound dressings [178]. Accelerated wound healing has also been associated with various substances including probiotics, food supplements, metal nanoparticles, polymers, and others [179].
Nanoparticle-based materials excel in wound healing due to their antibacterial properties, compatibility with the body, and ability to provide mechanical strength [180]. Soft nanoparticles, derived from organic sources, encompass liposomes, micelles, nanoemulsions, and polymeric nanoparticles [181]. When incorporated into hydrogels, they show potential for enhancing wound healing, offering improved texture, adherence, skin penetration, controlled drug release, and enhanced user comfort compared to traditional forms [182]. While epidermal growth factor (EGF) is highly effective in wound healing, challenges such as vulnerability to enzymatic degradation and maintaining therapeutic levels at the wound site have been significant hurdles. Encapsulating EGF within chitosan nanoparticles has shown promise, significantly increased wound closure rates, and promoted re-epithelialization and collagen deposition, ultimately contributing to a more efficient wound healing process [183]. The use of silver-modified chitosan and alginate-integrated nanoparticles in wound care provides supplementary benefits, including inhibiting bacterial growth, accelerating re-epithelialization, reducing inflammation, and enhancing collagen fiber deposition [184].
Hydrogels, known for their high-water content and excellent flexibility, stand out as highly promising materials for wound dressings. They regulate inflammation by scavenging free radicals, sequestering chemokines, and promoting macrophage transition, thereby promoting effective wound healing [185, 186]. Bioactive polypeptide hydrogel, composed of silk fibroin and angiogenic peptide, demonstrates impressive wound healing capabilities. It effectively reduces inflammation, stimulates angiogenesis, and leads to notable improvements in vessel formation and wound area reduction in a mouse skin wound model [187]. Peptide-based hydrogels, known for their biocompatibility and biodegradability, offer unique benefits in ligand-receptor recognition and stimulus-responsive self-assembly. This makes them highly promising for wound treatment [188]. Stimuli-responsive hydrogels, known as “smart hydrogels,” have gained traction for diabetic wound healing as they possess the unique ability to alter mechanical properties, swelling behavior, hydrophilicity, and permeability to bioactive molecules in response to stimuli like temperature, pH levels, protease activity, and other biological factors [189].
Growth factors hold promise for tissue regeneration, but their instability and rapid clearance from tissues pose significant challenges [190]. Utilizing liposomal drug delivery systems to encapsulate and deliver growth factors has emerged as a potential solution to address these limitations [191]. Liposomes, composed of bilayered lipids, are versatile carriers capable of encapsulating both lipophilic and hydrophilic drugs. This makes them an excellent choice for delivering substances like curcumin effectively [192]. Citicoline-loaded chitosan-coated liposomes have demonstrated remarkable efficacy in enhancing skin wound healing in diabetic rats through a multi-faceted approach, including inflammation reduction, accelerated re-epithelization, enhanced angiogenesis, increased fibroblast proliferation, and improved connective tissue remodeling [193].
Polysaccharide nanofibers, created through electrospinning for wound dressings, hold significant promise for wound healing. They facilitate cell adhesion and proliferation in the wound bed and provide a permeable network structure that mimics the natural extracellular matrix [194]. Polymeric nanofibers are highly prospective as scaffolds for wound healing due to their ability to replicate the extracellular matrix [195]. Another promising avenue lies in hierarchical structure dressings. The top layer, made of hydrophobic polycaprolactone, prevents foreign microbe adherence. The middle layer comprises hydrophilic Janus nanofibers, produced through electrospinning. The bottom layer, consisting of hydrophilic gelatin, creates a moist nurturing environment for the wound [196]. A new class of nanomaterial, electrospun nanofibers, shows great promise in various biological processes. This includes tissue redesigning, using bandages and scaffolds for wound repair, and enabling multimodal drug delivery [197]. Utilizing hyaluronic acid-based nanofibers, which release nitric oxide due to their biodegradable nature, can help control inflammation and eliminate bacterial infections, making it valuable for wound healing [198].
Nanocomposite hydrogels incorporating polymeric micelles offer a dual advantage. They enhance the mechanical, self-healing, and chemical properties of hydrogels while also improving the in vivo stability of the micelles themselves [199]. Polymeric micelles have emerged as a highly auspicious drug delivery platform, particularly for poorly soluble, potent, and potentially toxic compounds. They efficiently encapsulate such molecules [200]. Their strong core-shell structure, exceptional kinetic stability, and innate ability to solubilize hydrophobic drugs make them stand out in this field [201]. Polymeric micelles form through the self-assembly of amphiphilic polymers with both hydrophilic and hydrophobic segments, occurring when polymer concentrations exceed critical micelle concentrations [202]. An innovative approach involves a novel hybrid hydrogel sheet, composed of polyethylene glycol-grafted chitosan and a reactive polymeric micelle. This combination enhances the material’s functionality and improves therapeutic outcomes [203].
A newly developed composite biological dressing, composed of polyvinyl alcohol, carbon nanotubes, and epidermal growth factor, demonstrates a uniformly distributed structure. It effectively releases the epidermal growth factor at a steady rate, creating an environment conducive to expedited wound healing [204]. Even at low concentrations, nanocomposites like carbon nanotube-loaded hydrogels can substantially enhance cell migration within the hydrogel, leading to accelerated tissue regeneration and wound healing [205]. Both single-wall and multi-wall carbon nanotubes, when complexed with chitosan, enhance the re-epithelization of wounds and contribute to increased fibrosis, indicating a positive effect on wound healing and tissue regeneration [206]. The incorporation of zinc oxide nanoparticles and multiwall carbon nanotubes as nanofillers in gellan gum alters the film microstructure, creating a sponge-like texture. This transformation enhances fluid uptake capacity, making it particularly beneficial for wound healing applications [207]. A gold-halloysite nanotubes-chitin composite hydrogel demonstrates dual benefits, exhibiting strong hemostatic activity while also promoting wound healing. This combination maintains low cytotoxicity, making it highly promising for biomedical applications [208]. Advancing the field of biomaterial scaffolds for effective wound healing involves microfabricating biomaterials into various forms, such as 3D-bioprinted structures, microneedles, and electrospun scaffolds [209].
Silicon-based wound dressings, developed into different kinds of scaffolds, are of interest due to their high biocompatibility and mechanical strength [210]. Non-crosslinked collagen-based bi-layered composite dressings have shown promise in promoting wound healing and expediting re-epithelialization [211]. Hydrogels, meticulously designed and prepared to possess specialized qualities, have demonstrated significant promise for skin wound healing [212]. Researchers are increasingly exploring the use of biopolymers in fiber production and their potential applications in wound treatment [213]. Biopolymers like alginate, chitosan, collagen, and hyaluronic acid are frequently employed in wound therapy due to their biocompatibility, biodegradability, and similarity to biomolecules recognized by the human system [214]. The rapidly evolving field of adjustable bioelectronics, with benefits including daily wear, affordability, and easy application, also presents a significant possibility for customized wound therapy [215]. Figure 4 and Table 3 provide examples of advancements in wound dressing technology that have contributed to enhanced wound healing capacity.
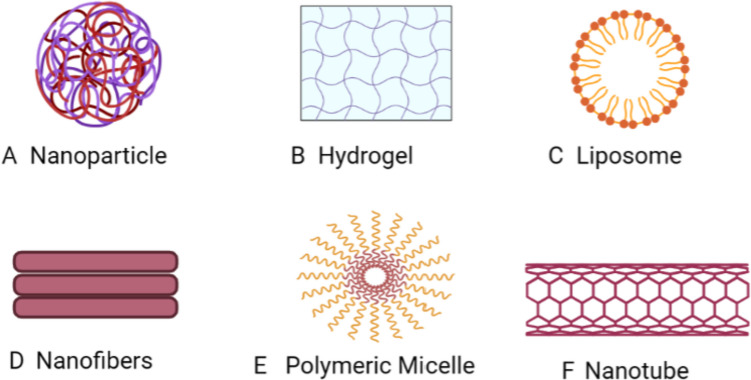
Fig. 4.
Nanotechnology used as drug delivery systems (Created by Biorender.com)
Table 3.
Wound dressing technology for enhanced healing capacity
| S.No. | Herbs | Family | Plant part | Wound dressing technology | Model | Positive control used | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Scutellaria barbata | Lamiaceae | Whole | Nanoparticle: Plant aqueous extract silver nanoparticles coated with cotton fabrics | In vitro: L929 fibroblast cell | Untreated cells | Increased cell proliferation and migration | [216] |
| 2 | Pluchea indica | Asteraceae | Leaf | Nanoparticle: Plant leaf extract nanoparticles oral spray formulation | In vitro: HO-1-N-1 cells | Untreated cells | Increased cell proliferation and migration | [217] |
| 3 | Bletilla striata | Orchidaceae | - | Hydrogel: Plant polysaccharide mixed with methylcellulose and methylparaben |
In vitro: L929 fibroblast cell In vivo: Full-thickness wound model in rat |
wound area treated with only sterile cotton as a control | High efficacy in wound healing | [218] |
| 4 | Aloe barbadensis | Asphodelaceae | Leaf | Electrospun polymer fiber: keratin, chitosan, and polycaprolactone-based- based matrix | In vitro: L929 fibroblast cell | Untreated cells | Increased cellular growth and adhesion | [219] |
| 5 | Centella asiatica (Asiatic acid) | Apiaceae | Active compound purchased from the market | Hydrogel: chitosan-polyvinyl alcohol-based microneedles of asiatic acid (herb-isolated compound) |
In vivo: excision wound model in rat |
Tegaderm | Increased wound closure rate | [220] |
| 6 | Rosmarinus officinalis | Lamiaceae | - | Nanostructured lipid carrier: Plant extract dissolved in Miglyol, Poloxamer |
In vivo: full-thickness wound model in rat |
Mupirocin ointment | Increasing the vascularization, fibroblast infiltration, re-epithelialization, collagen production | [221] |
| 7 | Mentha piperita | Lamiaceae | Leaf | Nanocomposites: γ-AlOOH (bohemite)-based nanocomposite of Au/γ-AlOOH-NC using Chitosan |
In vivo: full-thickness wound model in a mouse |
Mupirocin ointment | Decreased the expression of TNF-α, and increased the expression of Caspase 3, Bcl-2, Cyclin-D1, and FGF-2 | [222] |
| 8 | Moringa oleifera | Moringaceae | Seed | n-hexane Hydrogel |
In vivo: Excision and incision wound model in mouse |
Povidone-iodine | Decreased the no. in inflammatory cells and accelerated tissue regeneration | [223] |
| 9 | Cissus quadrangularis | Vitaceae | - | Electrospun Nanofiber: CQ extract-loaded chitosan nanofibers were coated on chitosan/POSS nanocomposite sponge |
In vitro: NIH/3T3 fibroblast cell line |
Untreated cell | Induced cell proliferation and collagen deposition | [224] |
| 10 | Satureja khuzistanica | Lamiaceae | Leaf and Stem | Hydrogel alginate |
In vivo: Excision wound model in rat |
No positive control | Accelerated wound healing without scar formation | [225] |
| 11 | Narcissus tazetta | Amaryllidaceae | bulb | Non-ionic surfactant vesicles / niosomes by film hydration method |
In vitro: HDF cell line |
Fetal bovine serum | Decreased the gap width on human dermal fibroblasts | [226] |
| 12 | Centella asiatica | Apiaceae | - |
Nanoparticle: Polyurethane foam dressing consists of natural polyols, silver nanoparticles, and asiaticoside |
In vivo: Excision wound model in farm pigs |
No positive control | Increased the absorption property and compressive strength and enhanced the wound closure rate | [227] |
| 13 | Opuntia ficus-indica | Cactaceae | Seed |
Self-nano emulsifying formulation: OFI seed oil poured in 2% HPMC solution |
In vivo: Full-thickness excision wound model in rat |
Mebo ointment | Enhanced expression of transforming factor-beta and VEGF, | [228] |
Discussion
Wounds, encompassing damage to skin integrity from incisions, burns, scalds, or specific lesions (e.g., diabetic foot ulcers, venous ulcers, pressure sores) [229], require proper treatment to prevent complications like bleeding, infection, inflammation, and scarring. These complications can impede angiogenesis and tissue regeneration [230]. Effective wound management plays a crucial role in healthcare, as prolonged healing periods can lead to increased burdens on institutions, healthcare professionals, patients, and their families, both economically and socially [231, 232]. Maintaining proper hygiene is foundational in wound care to minimize infection risks. Wound healing therapies, categorized into traditional and modern (skin grafts, modern dressings, bioengineered skin substitutes, and cell or growth factor therapies), vary in efficacy, clinical acceptance, and side effects notably. The wound management process begins with debridement, involving the removal of necrotic tissue, followed by the application of topical treatments like antimicrobial dressings and products containing silver sulfadiazine, which actively promote optimal wound healing [233]. In cases where wound healing stalls, advanced techniques become crucial. These include negative pressure therapy, growth factors, hyperbaric oxygen, and skin grafts [234]. However, it’s important to note that these treatments may come with a higher cost and limited accessibility, particularly in low-resource settings. Additionally, they carry potential risks such as bleeding, infection, barotrauma, and even the potential development of cancer [235, 236]. Silver dressings are highly effective due to their antimicrobial properties, ease of use, and cost-effectiveness in wound healing, however, their application requires careful consideration, as improper usage may lead to potential cytotoxic effects [237]. Biomaterial-based dressings, including grafts and engineered skin substitutes, play a crucial role in restoring tissue function, especially in cases of severe burns or chronic wounds with significant skin loss but these solutions face challenges such as limited vascularity, weaker mechanical strength, and potential risk of immune rejection [238]. Also, cell and growth factor therapy hold promise for regenerating chronic wounds, but the presence of chronic wound fluid can lead to the rapid degradation of growth factors, hindering stem cell proliferation [239, 240]. Similarly, artificial dressings made from polymers can mimic tissue properties, but they may also lack bioactive components critical for optimal wound healing [241]. Considering the limitations of current wound healing treatments, there is a crucial need for the development of natural products to effectively address wound healing [242–244]. Traditional therapies hold significant value due to their safety, accessibility, established effectiveness, and natural origins, effectively addressing the drawbacks associated with modern approaches, which often entail high costs, lengthy production processes, and the rising challenge of bacterial resistance [245–249]. Recognizing this potential, the World Health Organization advocates for the integration of traditional methods into formal health systems and underscores the power of phytochemicals in not only combating infections but also in supporting the intricate process of wound healing [250]. Ayurveda attributes unique medicinal properties to individual herbs, yet it believes that combining these herbs, termed polyherbal formulations, in specific ratios and proportions can amplify their therapeutic benefits while reducing potential toxicity [251, 252].
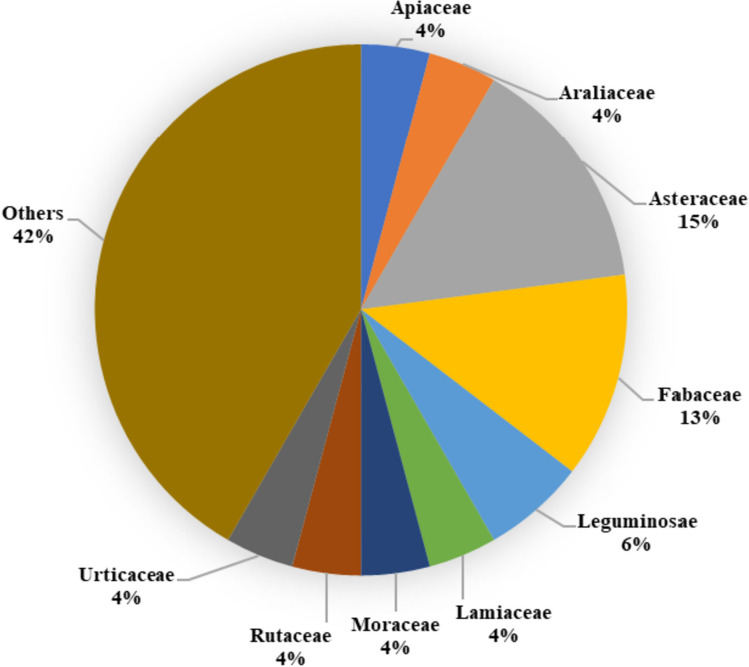
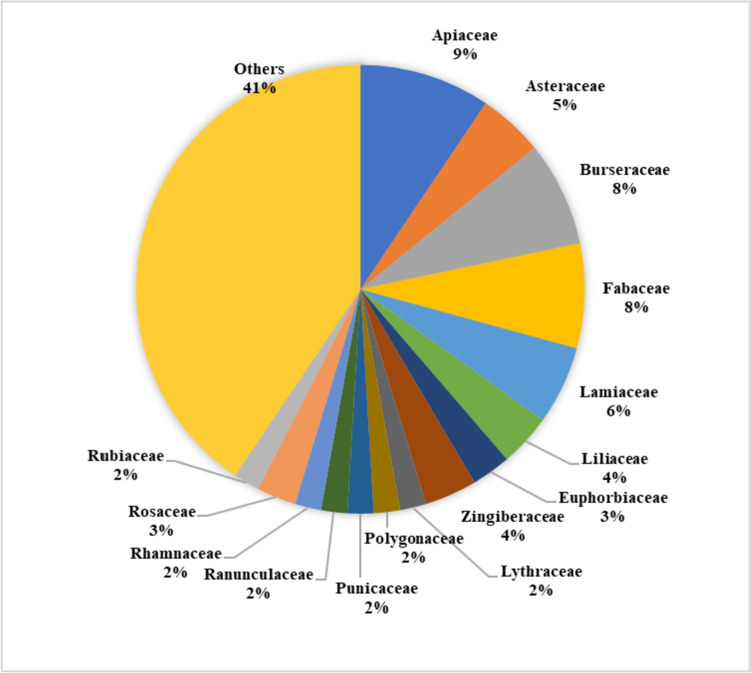
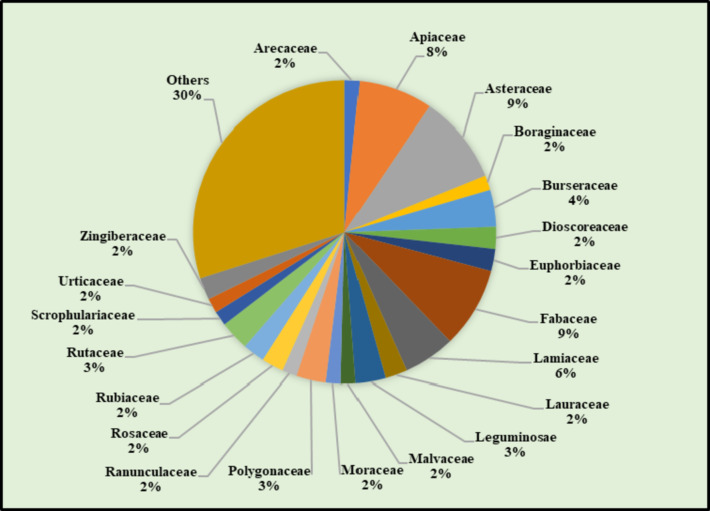
This review offers valuable insights into a diverse range of natural remedies explored for wound healing. When focusing on studies of individual herbs (Fig. 5), a significant number of them were found to belong to the Asteraceae family, followed by the Fabaceae and Leguminosae families in terms of frequency. In the dataset (Fig. 6), the Apiaceae family emerges as predominant, with 24 studies utilizing polyherbal formulations for wound care. It is followed by the Burseraceae and Fabaceae families in terms of representation. When considering both single herbs and those utilized in polyherbal formulations, a total of 72 studies encompassing 127 different herbs (excluding any overlapping herbs) were examined. These herbs were predominantly from the Asteraceae family, followed by the Fabaceae and Apiaceae families (Fig. 7). Noteworthy, herbs with wound healing efficacy belonging to the Asteraceae family include Areca catechu, Calamus draco, Artemisia absinthium, Carthamus caeruleus, Dittrichia viscosa, Echinacea purpurea, Elephantopus scaber, Gynura procumbens, Launaea procumbens, Matricaria chamomilla, Siegesbeckia orientalis, Tridax procumbens, Xanthium strumarium, and Aster koraiensis. Likewise, in another study, the effectiveness of Ageratina pichinchensis and Calendula officinalis in wound healing underscores the potential of Asteraceae plants for the development of impactful wound-healing drugs [253]. Also, Achillea asiatica, commonly known as Asian yarrow, from the Asteraceae family, demonstrates the potential to stimulate wound healing and support the growth of keratinocytes, the predominant cells in the epidermis [254]. Additionally, a study conducted in 2020 focused on the plant’s wound-healing potential reported that the Fabaceae or Leguminosae family exhibited an abundance of herbs beneficial for wound healing [255]. Traditional remedies from the Fabaceae and Rosaceae families have a significant presence among plants used in traditional medicine for various health conditions, including wound care [256].
Fig. 5.
%age of individual herbs from the same botanical family exhibit wound healing properties
Fig. 6.
%age of herbs included in polyherbal formulations, which belong to the same botanical family, possess wound healing properties
Fig. 7.
%age of herbs, found in both individual herb and polyherbal formulation studies, and belonging to the same botanical family, demonstrate wound healing properties across a total of 72 studies, which encompass 127 unique herbs (excluding duplicates)
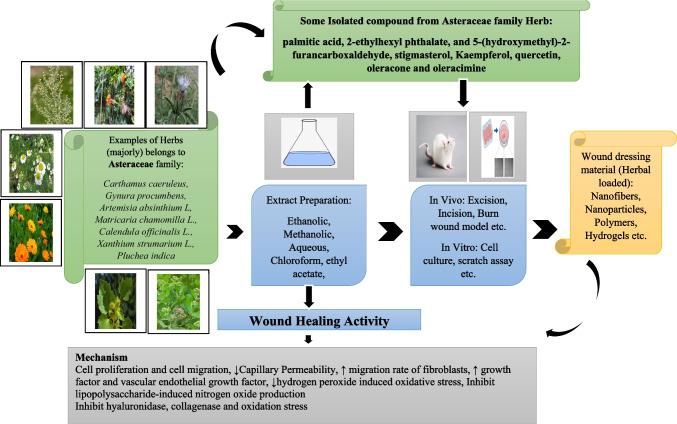
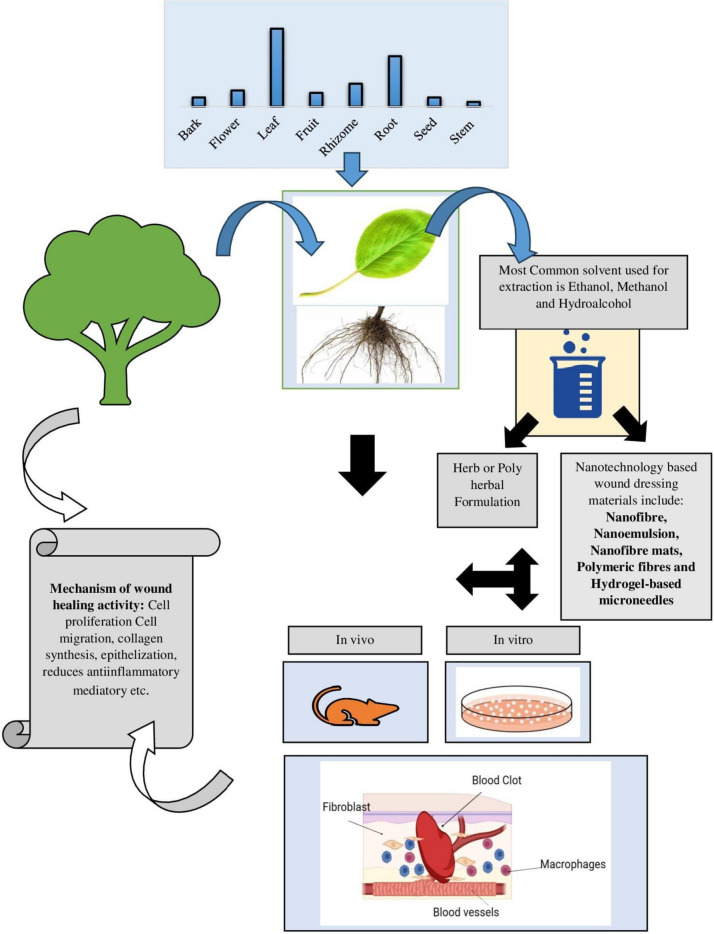
Figure 8 illustrates the sequential steps involved in evaluating the wound healing efficacy of bioactive compounds extracted from herbs. Among the various plant parts studied for wound healing efficacy, leaves were the most utilized, followed by roots, rhizomes, and fruits. These plant parts were initially extracted using solvents such as ethanol, methanol, and hydroalcoholic solvents. This choice of solvents can be attributed to their wide compatibility, high solubility, moderate toxicity, scalability, cost-effectiveness, and stability. Once bioactive compounds were extracted from different plant parts, they were further assessed using in-vivo rat wound models, including incisions, excisions, deep tissue pressure injuries, burns, and medically induced wounds. Additionally, in-vitro models were employed to measure enzyme levels and conduct various cell assays, representing the diverse mechanisms involved in promoting wound healing efficacy. The review highlighted the significant wound-healing efficacy of phenolic acids, flavonoids, glycosides, and other phytochemicals, contributing to wound-healing activity. They operate through diverse mechanisms, enhancing various stages of wound healing, including upregulating vital factors like VEGF and TGF-β, crucial for re-epithelialization, angiogenesis, granulation tissue formation, and collagen deposition. Other studies reported the presence of diverse polyphenols, alkaloids, saponins, terpenes, essential oils, and polyphenols in various plants positively impacts different stages of the wound healing process [257]. These compounds modulate steps in wound healing, including cell proliferation, fibroblast migration, reduction of oxidative stress, improvement of collagen synthesis, and modulation of the expression of various factors. Flavonoids also positively regulate pathways involved in wound healing [258]. Phytochemicals also act as inhibitors of inflammatory factors, conferring antioxidant and anti-inflammatory effects throughout the healing process [259]. Specific compounds like saponins, flavonoids, and quercetin signify the potential wound-healing properties of certain herbs [260]. Molecular approaches are now gaining importance in understanding the underlying mechanisms of action and assessing potential herbal or synthetic compounds for wound management [261]. Phytochemicals, with their potent antimicrobial, antioxidant, and wound-healing properties, play a vital role in encouraging blood clotting, combating infections, and expediting wound recovery. Medicinal plants rich in polyphenols demonstrate notable efficacy in this regard [262–264].
Fig. 8.
Herb-derived bioactive compounds for wound healing activity (from extraction to evaluation). The diagram illustrates the comprehensive process of harnessing bioactive compounds from selected herbs for wound healing. Primarily utilizing leaves, followed by roots, rhizomes, and flowers, the herbs undergo extraction with specific solvents. The extracted compounds are then utilized to prepare either individual herb formulations or polyherbal blends. These formulations are subsequently evaluated for wound healing efficacy through both in vivo and/or in vitro models, showcasing their diverse mechanisms in promoting wound recovery
Conventional treatments for chronic wounds, like skin grafting or negative pressure wound therapy, may lead to tissue damage or functional restrictions, prompting the exploration of nanobiotechnology, an interdisciplinary field integrating engineering, chemistry, and biology, for innovative biomedical applications [265]. By incorporating nanoparticles, both biopolymers and synthetic polymers have been tailored for use as wound dressings, addressing contemporary wound care challenges including tissue repair, scarless healing, and tissue integrity [266]. This review emphasizes advancements in utilizing nanotechnology-based wound dressings in herbal-based formulations to enhance targeted drug delivery and accelerate recovery. These innovative drug delivery systems, benefiting from high stability, extensive surface area, and customizable compositions, have shown promise in both in vitro and in vivo models. Materials for wound dressing include electrospun nanofiber guar gum and polyvinyl alcohol-based matrices, sodium alginate nanofiber mats, cellulosic textile nanoemulsions, polycaprolactone nanofibers with silver nanoparticles, and chitosan-polyvinyl alcohol hydrogel microneedles. In other studies, advanced techniques utilizing nanoparticles and hydrogels loaded with bioactive molecules and non-bioactive substances, particularly smart hydrogels, hold promise for enhancing diabetic wound healing. These approaches can be further enhanced with technologies like photothermal therapy, layer-by-layer self-assembly, and 3D printing [267]. Nanotechnology provides molecularly designed nanostructures for both therapeutic and diagnostic use in burns, categorized as organic and non-organic (e.g., polymeric and silver nanoparticles), with many exhibiting multifunctional properties [268]. Self-assembled nanomaterials, serving as wound dressings and growth factor carriers, characterized by superb biocompatibility and versatile functionalities like mimicking extracellular matrix, drug delivery, and adjustable mechanics, offer promising therapeutic prospects for chronic wound healing [269]. With rising clinical demands, botanical applications are increasingly integrating with nanotechnologies. This fusion, particularly through electrospinning, creates nanofibrous membranes ideal for skin wound healing [270]. Noteworthy breakthroughs in tissue regeneration and skin wound therapy, including 3D-printing, cell-imprinted substrates, nano-architectured surfaces, and gene-editing tools, hold substantial promise for advancing burn wound therapies [271].
Perspectives and future direction
Numerous preclinical research on herbs with shown wound healing properties have been undertaken. Several clinical studies employing single herbs or effective herb combinations have demonstrated efficacy in the therapy of wounds. Here, in “Table 4,” are some of the clinical experiments that were done to create safe medications (herbal or polyherbal formulations) and ensure the quickest recovery period. To achieve this and lessen the significant challenges associated with conducting clinical studies, an appropriate research design that mimics the wound conditions is needed. Future research is required to understand how these herbs’ isolated compound helps wound linked with disease heal and to develop new formulations containing herbal extracts and phytochemicals that will lessen the risk of medication resistance and drug allergies or many other associated factors that interfere with wound healing.
Table 4.
Clinical trials conducted to determine herbs wound healing efficacy
| S.No. | Sample | Treatment | Control | Indication | Efficiency | Reference |
|---|---|---|---|---|---|---|
| 1 | 90 (primiparous women) | Commiphora myrrha and Boswellia carteri | Betadine sitz bath | Episiotomy (wound) | Myrrh demonstrated significantly superior wound healing in episiotomy patients compared to frankincense or betadine | [272] |
| 2 | 210 women diagnosed with vaginitis | St. John’s wort, yarrow, shepherd’s purse chamomile, calendula, and tea tree oil | Probiotic | Vaginitis (wound) | Tea tree oil-based vaginal suppositories exhibited superior effectiveness compared to alternatives | [273] |
| 3 | 12 patients with 24 donor sites | Aloe vera | Placebo | Burns and split-thickness skin graft donor sites | Aloe vera gel topically showed marked improvement in the healing of split-thickness skin graft donor sites | [274] |
| 4 | 60 | Nanocurcumin | Placebo | Diabetic foot ulcer | Incorporating nano curcumin into the treatment of diabetic foot ulcers led to notable enhancement in glycemic control | [275] |
| 5 | 30 | Centella asiatica | Placebo | Facial acne scars | Centella asiatica demonstrated a significantly greater reduction in skin erythema | [276] |
| 6 | 87 (primiparous women) | Silybum marianum | Placebo |
Episiotomy (wound) |
Silybum marianum showed a reduction in episiotomy pain severity and expedited wound healing | [277] |
| 7 | 17 | 30% garlic ointment | Vaseline | Surgical wound | Surgical wounds treated with 30% garlic ointment resulted in more cosmetically appealing scars compared to those treated with Vaseline | [278] |
| 8 | 90 (primiparous women) | Verbascum Thapsus | Placebo |
Episiotomy (wound) |
Verbascum Thapsus is effective in repairing episiotomy wounds | [279] |
| 9 | 50 | Vasconcellea cundinamarcensis (0.1% proteolytic fraction) | Hydrogel | Chronic foot ulcers (neuropathic patients of diabetes type-2) | Vasconcellea cundinamarcensis notably accelerates foot ulcer healing compared to hydrogel treatment | [280] |
| 10 | 129 (women) | 2.5% and 5% grape seed extract | Petrolatum | Cesarean wound | Utilizing 5% grape seed extract may offer therapeutic benefits in enhancing cesarean section wound healing | [281] |
| 11 | 20 patients | Grape seed extract 2% herbal cream | Placebo | Surgical patients | Wounds treated with 2% grape seed extract achieved full repair in fewer days compared to the placebo group | [282] |
Conclusion
This critical overview highlights the multifaceted roles of medicinal plants in advancing wound healing technology. The rich repository of bioactive compounds found in these plants offers a promising avenue for promoting tissue regeneration, combating infections, and reducing inflammation. By leveraging the therapeutic potential of medicinal plants, we can address the complex challenges associated with wound care. There is a need for in-depth studies to unravel the specific mechanisms of action underlying the wound-healing properties of individual bioactive compounds in medicinal plants. This understanding will pave the way for the development of targeted interventions tailored to different types of wounds. Collaborative efforts between traditional healers and scientific researchers can lead to the identification of novel wound-healing agents and innovative treatment approaches.
Funding
None.
Declarations
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chhabra S, Chhabra N, Kaur A, Gupta N. Wound Healing concepts in Clinical Practice of OMFS. J Maxillofac Oral Surg. 2017;16(4):403–423. doi: 10.1007/s12663-016-0880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK. Human wound and its Burden: updated 2020 Compendium of estimates. Adv Wound Care (New Rochelle) 2021;10(5):281–292. doi: 10.1089/wound.2021.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mieczkowski M, Mrozikiewicz-Rakowska B, Kowara M, Kleibert M, Czupryniak L. The problem of wound healing in diabetes—from molecular pathways to the design of an animal model. Int J Mol Sci. 2022;23:7930. doi: 10.3390/ijms23147930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latalski M, Starobrat G, Fatyga M, Sowa I, Wojciak M, Wessely-Szponder J, et al. Wound-related complication in growth-friendly spinal surgeries for early-onset scoliosis—literature review. J Clin Med. 2022;11:2669. doi: 10.3390/jcm11092669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falanga V, Isseroff RR, Soulika AM, Romanelli M, Margolis D, Kapp S, et al. Chronic wounds. Nat Rev Dis Primers. 2022;8:50. doi: 10.1038/s41572-022-00377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira A, Simoes S, Ascenso A, Reis CP. Therapeutic advances in wound healing. J Dermatolog Treat. 2022;33:2–22. doi: 10.1080/09546634.2020.1730296. [DOI] [PubMed] [Google Scholar]
- 7.Brown HL, Clayton A, Stephens P. The role of bacterial extracellular vesicles in chronic wound Infections: current knowledge and future challenges. Wound Repair Regen. 2021;29:864–880. doi: 10.1111/wrr.12949. [DOI] [PubMed] [Google Scholar]
- 8.Thomas RE, Thomas BC. Reducing biofilm Infections in burn patients’ wounds and biofilms on surfaces in hospitals, Medical Facilities and Medical Equipment to improve burn care: a systematic review. Int J Environ Res Public Health. 2021;18:13195. doi: 10.3390/ijerph182413195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoz H. An overview of the prevention and management of wound Infection. Nurs Standard. 2022;37:75–82. doi: 10.7748/ns.2022.e11889. [DOI] [PubMed] [Google Scholar]
- 10.Lux CN. Wound healing in animals: a review of physiology and clinical evaluation. Vet Dermatol. 2022;33:91. doi: 10.1111/vde.13032. [DOI] [PubMed] [Google Scholar]
- 11.Hassanshahi A, Moradzad M, Ghalamkari S, Fadaei M, Cowin AJ, Hassanshahi M. Macrophage-mediated inflammation in skin Wound Healing. Cells. 2022;11:2953. doi: 10.3390/cells11192953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–1180. doi: 10.1016/j.stem.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beura SK, Panigrahi AR, Yadav P, Agrawal S, Singh SK. Role of neurons and Glia Cells in Wound Healing as a Novel Perspective considering platelet as a conventional player. Mol Neurobiol. 2022;59:137–160. doi: 10.1007/s12035-021-02587-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang XH, Guo W, Qiu W, Ao LQ, Yao MW, Xing W, et al. Fibroblast-like cells promote Wound Healing via PD-L1-mediated inflammation resolution. Int J Biol Sci. 2022;18:4388–4399. doi: 10.7150/ijbs.69890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogren S, Berlin F, Ramu S, Sverrild A, Porsbjerg C, Uller L, et al. Mast cell tryptase enhances wound healing by promoting migration in human bronchial epithelial cells. Cell Adh Migr. 2021;15:202–214. doi: 10.1080/19336918.2021.1950594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandoi LPK, Misra S, V RS, Verma KR. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang YX, Chen JJ, Cen Y, Li ZY, Zhang ZY. [Research advances on exosomes derived from adipose-derived mesenchymal stem cells in promoting diabetic wound healing] Zhonghua Shao Shang Za Zhi. 2022;38:491–495. doi: 10.3760/cma.j.cn501120-20210218-00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao YX, Wang LF, Ba SJ, Cao JL, Li F, Li B, et al. Research advances on thymosin β4 in promoting wound healing. Zhonghua Shao Shang Za Zhi. 2022;38:378–384. doi: 10.3760/cma.j.cn501120-20210221-00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derakhshanfar A, Moayedi J, Derakhshanfar G, Poostforoosh Fard A. The role of Iranian medicinal plants in experimental surgical skin wound healing: an integrative review. Iran J Basic Med Sci. 2019;22(6):590–600. doi: 10.22038/ijbms.2019.32963.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornfeldt CR. Therapeutic herbs confirmed by evidence-based medicine. Clin Dermatol. 2018;36(3):289–298. doi: 10.1016/j.clindermatol.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Nesari TM, Ghildiyal S, Sherkhane R. Pharmacodynamic appraisal of wound-healing herbs of Sushruta Samhita. Ayu. 2021;42(1):1–18. doi: 10.4103/ayu.AYU_34_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseinkhani A, Falahatzadeh M, Raoofi E, Zarshenas MM. An evidence-based review on Wound Healing Herbal remedies from reports of traditional Persian Medicine. J Evid Based Complementary Altern Med. 2017;22(2):334–343. doi: 10.1177/2156587216654773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Niu Y, Xing P, Wang C. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin Med. 2018;13:7. doi: 10.1186/s13020-018-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddique Z, Shah GM, Ahmed HM, Nisa S, Khan A, Idrees M, et al. Ethnophytotherapy Practices for Wound Healing among populations of District Haripur, KPK, Pakistan. Evid Based Complement Alternat Med. 2019;2019:4591675. doi: 10.1155/2019/4591675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosein Farzaei M, Abbasabadi Z, Reza Shams-Ardekani M, Abdollahi M, Rahimi R. A comprehensive review of plants and their active constituents with wound healing activity in traditional Iranian medicine. Wounds. 2014;26(7):197–206. [PubMed] [Google Scholar]
- 26.Ambika AP, Nair SN. Wound Healing activity of plants from the Convolvulaceae Family. Adv Wound Care (New Rochelle) 2019;8(1):28–37. doi: 10.1089/wound.2017.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viaña-Mendieta P, Sánchez ML, Benavides J. Rational selection of bioactive principles for wound healing applications: growth factors and antioxidants. Int Wound J. 2022;19:100–113. doi: 10.1111/iwj.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassino E, Gasparri F, Munaron L. Natural dietary antioxidants containing flavonoids modulate keratinocytes physiology: in vitro tri-culture models. J Ethnopharmacol. 2019;238:111844. doi: 10.1016/j.jep.2019.111844. [DOI] [PubMed] [Google Scholar]
- 29.Shirbeigi L, Mohebbi M, Karami S, Nejatbakhsh F. The Role of Nutrition and Edible Medicinal Plants in the treatment of chronic wounds based on the principles of Iranian traditional medicine. Iran J Med Sci. 2016;41(3 Suppl):72. [PMC free article] [PubMed] [Google Scholar]
- 30.Nagoba B, Davane M. Studies on wound healing potential of topical herbal formulations- do we need to strengthen study protocol? J Ayurveda Integr Med. 2019;10(4):316–318. doi: 10.1016/j.jaim.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheikh M, Khan HM, Zafar Khan MU, Sharif A. Formulation, evaluation and optimization of Antimicrobial potential of herbal cream containing Allium sativum, Moringa oleifera extracts and Thymus vulgaris oil. Curr Pharm Biotechnol. 2023 doi: 10.2174/1389201024666230504124838. [DOI] [PubMed] [Google Scholar]
- 32.Dubey S, Dixit AK. Preclinical evidence of polyherbal formulations on wound healing: a systematic review on research trends and perspectives. J Ayurveda Integr Med. 2023;14(2):100688. doi: 10.1016/j.jaim.2023.100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandrika I, Kumar S, Zandersone B, Eranezhath SS, Petrovska R, Liduma I, et al. Antibacterial and anti-inflammatory potential of Polyherbal Formulation used in Chronic Wound Healing. Evid Based Complement Alternat Med. 2021;2021:9991454. doi: 10.1155/2021/9991454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim KM. Skin epidermis and barrier function. Int J Mol Sci. 2021;22(6):3035. doi: 10.3390/ijms22063035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temp (Austin) 2016;4(1):41–59. doi: 10.1080/23328940.2016.1200203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin Wound Healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58:81–94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- 38.Albahri G, Badran A, Hijazi A, Daou A, Baydoun E, Nasser M, et al. The therapeutic Wound Healing bioactivities of various Medicinal plants. Life (Basel) 2023;13(2):317. doi: 10.3390/life13020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Koppen CJ, Hartmann RW. Advances in the treatment of chronic wounds: a patent review. Expert Opin Ther Pat. 2015;25:931–937. doi: 10.1517/13543776.2015.1045879. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37. doi: 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng RJ, Lin CQ, Lin ZH, Chen H, Lu WY, Lin CM, et al. Approaches to cutaneous wound healing: basics and future directions. Cell Tissue Res. 2018;374:217–232. doi: 10.1007/s00441-018-2830-1. [DOI] [PubMed] [Google Scholar]
- 43.Dehkordi AN, Babaheydari FM, Chehelgerdi M, Dehkordi SR. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:111. doi: 10.1186/s13287-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale S, Colanero S, Placidi M, Di Emidio G, Tatone C, Amicarelli F, et al. Phytochemistry and biological activity of medicinal plants in wound healing: an overview of current research. Molecules. 2022;27(11):3566. doi: 10.3390/molecules27113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94–101. doi: 10.1016/j.jcma.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Renick P, Senkowsky J, Nair A, Tang L. Diagnostics for wound infections. Adv Wound Care (New Rochelle) 2021;10(6):317–327. doi: 10.1089/wound.2019.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smet S, Probst S, Holloway S, Fourie A, Beele H, Beeckman D. The measurement properties of assessment tools for chronic wounds: a systematic review. Int J Nurs Stud. 2021;121:103998. doi: 10.1016/j.ijnurstu.2021.103998. [DOI] [PubMed] [Google Scholar]
- 48.Ousey K, Roberts D, Gefen A. Early identification of wound Infection: understanding wound odour. J Wound Care. 2017;26(10):577–582. doi: 10.12968/jowc.2017.26.10.577. [DOI] [PubMed] [Google Scholar]
- 49.Stallard Y. When and how to perform cultures on chronic wounds? J Wound Ostomy Continence Nurs. 2018;45(2):179–186. doi: 10.1097/WON.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 50.Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle) 2021;10(12):685–698. doi: 10.1089/wound.2020.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davini G, Dini V, Janowska A, Macchia M, Gualtieri B, Granieri G, et al. Wound dehiscence after Achilles tendon trauma and repair: treatment with ultraportable negative pressure wound therapy and compression therapy. Wounds. 2021;33(12):E93–98. doi: 10.25270/wnds/2021.e93e98. [DOI] [PubMed] [Google Scholar]
- 52.Simoes D, Miguel SP, Ribeiro MP, Coutinho P, Mendonça AG, Correia IJ. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Skowronska W, Bazylko A. The potential of medicinal plants and natural products in the treatment of burns and sunburn-a review. Pharmaceutics. 2023;15(2):633. doi: 10.3390/pharmaceutics15020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Yu Q, Wang S, Wang G, Li T, Tang PF, et al. Impact of negative-pressure wound therapy on bacterial behaviour and bioburden in a contaminated full-thickness wound. Int Wound J. 2019;16(5):1214–1221. doi: 10.1111/iwj.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tettelbach W, Arnold J, Aviles A, Barrett C, Bhatia A, Desvigne M, et al. Use of mechanically powered disposable negative pressure wound therapy: recommendations and reimbursement update. Wounds. 2019;31(2 Supp):1–S17. [PubMed] [Google Scholar]
- 56.Wang Z, Bai M, Zeng A, Liu Z, Zhao R, Wang X. The role of negative pressure wound therapy in managing Chinese patients with wound-derived acute severe Illness. Wounds. 2018;30(8):235–241. [PubMed] [Google Scholar]
- 57.Xu Q, Shen L, Yang X, Peng H, Liu M. Comparison of changes in wound healing parameters following treatment with three topical wound care products using a laser wound model. Am J Transl Res. 2021;13(4):2644–2652. [PMC free article] [PubMed] [Google Scholar]
- 58.Westby MJ, Dumville JC, Soares MO, Stubbs N, Norman G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst Rev. 2017;6(6):CD011947. doi: 10.1002/14651858.CD011947.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev. 2018;6(6):CD012583. doi: 10.1002/14651858.CD012583.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarl G, Rusaw DF, Terrill AJ, Barnett CT, Woodruff MA, Lazzarini PA. Personalized offloading treatments for Healing Plantar Diabetic Foot Ulcers. J Diabetes Sci Technol. 2023;17(1):99–106. doi: 10.1177/19322968221101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernando ME, Horsley M, Jones S, Martin B, Nube VL, Charles J, et al. Australian diabetes-related Foot Disease Guidelines & Pathways Project. Australian guideline on offloading treatment for foot ulcers: part of the 2021 Australian evidence-based guidelines for diabetes-related foot Disease. J Foot Ankle Res. 2022;15(1):31. doi: 10.1186/s13047-022-00538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daeschlein G. Antimicrobial and antiseptic strategies in wound management. Int Wound J. 2013;10:9–14. doi: 10.1111/iwj.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gacto-Sanchez P. Surgical treatment and management of the severely burn patient: review and update. Med Intensiva. 2017;41(6):356–364. doi: 10.1016/j.medin.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Tyavambiza C, Dube P, Goboza M, Meyer S, Madiehe AM, Meyer M. Wound Healing activities and potential of selected African Medicinal plants and their synthesized biogenic nanoparticles. Plants (Basel) 2021;10(12):2635. doi: 10.3390/plants10122635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang C, Ibrahim A, Bulstrode NW, Ferretti P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int Wound J. 2017;14(3):450–459. doi: 10.1111/iwj.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi C, Wang C, Liu H, Li Q, Li R, Zhang Y, et al. Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol. 2020;8:182. doi: 10.3389/fbioe.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khanam S. A systematic review on wound healing and its promising medicinal plants. IP Int J Compr Adv Pharmacol. 2020;5(4):170–176. doi: 10.18231/j.ijcaap.2020.036. [DOI] [Google Scholar]
- 68.Liu E, Gao H, Zhao Y, Pang Y, Yao Y, Yang Z, et al. Front Pharmacol. 2022;13:900439. doi: 10.3389/fphar.2022.900439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quave CL. Wound healing with botanicals: a review and future perspectives. Curr Dermatol Rep. 2018;7(4):287–295. doi: 10.1007/s13671-018-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ning S, Zang J, Zhang B, Feng X, Qiu F. Botanical drugs in traditional chinese medicine with wound healing properties. Front Pharmacol. 2022;13:885484. doi: 10.3389/fphar.2022.885484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Z, Dong M, Yin S, Dong J, Zhang M, Tian R, et al. Why traditional herbal medicine promotes wound healing: research from immune response, wound microbiome to controlled delivery. Adv Drug Deliv Rev. 2023;195:114764. doi: 10.1016/j.addr.2023.114764. [DOI] [PubMed] [Google Scholar]
- 72.Marume A, Matope G, Katsande S, Khoza S, Mutingwende I, Mduluza T, et al. Wound Healing properties of selected plants used in Ethnoveterinary Medicine. Front Pharmacol. 2017;8:544. doi: 10.3389/fphar.2017.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herman A, Herman AP. Herbal products in postsurgical wound healing - incision, excision and dead space wound models. Planta Med. 2020;86(11):732–748. doi: 10.1055/a-1162-9988. [DOI] [PubMed] [Google Scholar]
- 74.Moses RL, Prescott TAK, Mas-Claret E, Steadman R, Moseley R, et al. Evidence for natural products as alternative wound-healing therapies. Biomolecules. 2023;13(3):444. doi: 10.3390/biom13030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herman A, Herman AP. Herbal products and their active constituents for diabetic wound healing-preclinical and clinical studies: a systematic review. Pharmaceutics. 2023;15(1):281. doi: 10.3390/pharmaceutics15010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotian S, Bhat K, Pai S, Nayak J, Souza A, Gourisheti K, et al. The role of natural medicines on wound healing: a biomechanical, histological, biochemical and molecular study. Ethiop J Health Sci. 2018;28(6):759–770. doi: 10.4314/ejhs.v28i6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future Drugs for the treatment of burn wounds: an integrative review. Arch Dermatol Res. 2014;306(7):601–617. doi: 10.1007/s00403-014-1474-6. [DOI] [PubMed] [Google Scholar]
- 78.El-Sherbeni SA, Negm WA. The wound healing effect of botanicals and pure natural substances used in in vivo models. Inflammopharmacology. 2023;31(2):755–772. doi: 10.1007/s10787-023-01157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nomoto T, Iizaka S. Effect of an oral nutrition supplement containing collagen peptides on stratum corneum hydration and skin elasticity in hospitalized older adults: a multicenter open-label randomized controlled study. Adv Skin Wound Care. 2020;33(4):186–191. doi: 10.1097/01.ASW.0000655492.40898.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Birkbeck R, Donaldson R, Chan DL. Nutritional management of a kitten with thermal Burns and septicaemia. JFMS Open Rep. 2020;6(1):2055116920930486. doi: 10.1177/2055116920930486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2014;2014(12):CD001488. doi: 10.1002/14651858.CD001488.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tobiano G, Walker RM, Chaboyer W, Carlini J, Webber L, Latimer S, et al. Patient experiences of, and preferences for, surgical wound care education. Int Wound J. 2023;20(5):1687–1699. doi: 10.1111/iwj.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramalingam S, Chandrasekar MJN, Nanjan MJ. Plant-based natural products for wound healing: a critical review. Curr Drug Res Rev. 2022;14:37–60. doi: 10.2174/2589977513666211005095613. [DOI] [PubMed] [Google Scholar]
- 84.Chaniad P, Tewtrakul S, Sudsai T, Langyanai S, Kaewdana K. Anti-inflammatory, wound healing and antioxidant potential of compounds from Dioscorea bulbifera L. bulbils. PLoS ONE. 2020;15:e0243632. doi: 10.1371/journal.pone.0243632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaniad P, Wattanapiromsakul C, Pianwanit S, Tewtrakul S. Anti-HIV-1 integrase compounds from Dioscorea bulbifera and molecular docking study. Pharm Biol. 2016;54(6):1077–1085. doi: 10.3109/13880209.2015.1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Juneja K, Mishra R, Chauhan S, Gupta S, Roy P, Sircar D, et al. Metabolite profiling and wound-healing activity of Boerhavia diffusa leaf extracts using in vitro and in vivo models. J Tradit Complement Med. 2019;10:52–59. doi: 10.1016/j.jtcme.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinan KI, Akpulat U, Aldahish AA, Celik Altunoglu Y, Baloglu MC, Zheleva-Dimitrova D, et al. LC-MS/HRMS Analysis, Anti-Cancer, Anti-enzymatic and Anti-oxidant effects of Boerhavia diffusa extracts: a potential raw material for functional applications. Antioxid (Basel) 2021;10:2003. doi: 10.3390/antiox10122003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venthodika A, Chhikara N, Mann S, Garg MK, Sofi SA, Panghal A. Bioactive compounds of Aegle marmelos L., medicinal values and its food applications: a critical review. Phytother Res. 2021;35:1887–1907. doi: 10.1002/ptr.6934. [DOI] [PubMed] [Google Scholar]
- 89.Azmi L, Shukla I, Goutam A, Allauddin, Rao CV, Jawaid T, et al. In vitro wound healing activity of 1-hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde (HDNC) and other isolates of Aegle marmelos L.: enhances keratinocytes motility via Wnt/β-catenin and RAS-ERK pathways. Saudi Pharm J. 2019;27:532–9. 10.1016/j.jsps.2019.01.017 [DOI] [PMC free article] [PubMed]
- 90.Dahmani M, Laoufi R, Selama O, Arab K. Gas chromatography coupled to mass spectrometry characterization, anti-inflammatory effect, wound-healing potential, and hair growth-promoting activity of Algerian Carthamus caeruleus L (Asteraceae) Indian J Pharmacol. 2018;50:123. doi: 10.4103/ijp.IJP_65_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ichimaru Y, Kanaeda N, Tominaga S, Suzui M, Maeda T, Fujii H, et al. Sasa veitchii extract induces anticancer effects via inhibition of cyclin D1 expression in MCF-7 cells. Nagoya J Med Sci. 2020;82:509–518. doi: 10.18999/nagjms.82.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan HL, Chan KG, Pusparajah P, Lee LH, Goh BH. Gynura procumbens: an overview of the Biological activities. Front Pharmacol. 2016;7:52. doi: 10.3389/fphar.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sutthammikorn N, Supajatura V, Yue H, Takahashi M, Chansakaow S, Nakano N, et al. Topical Gynura procumbens as a Novel Therapeutic improves Wound Healing in Diabetic mice. Plants (Basel) 2021;10:1122. doi: 10.3390/plants10061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng W, Qin R, Li X, Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. J Ethnopharmacol. 2013;148:729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 95.Nawrot-Hadzik I, Matkowski A, Pitulaj A, Sterczala B, Olchowy C, Szewczyk A, et al. In Vitro Gingival Wound Healing activity of extracts from Reynoutria japonica Houtt Rhizomes. Pharmaceutics. 2021;13:1764. doi: 10.3390/pharmaceutics13111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Figueiredo FF, Cechinel Filho V, Damazo AS, Arunachalam K, Colodel EM, Ribeiro M, et al. Sorocea Guilleminiana Gaudich.: Wound healing activity, action mechanisms, and chemical characterization of the leaf infusion. J Ethnopharmacol. 2020;248:112307. doi: 10.1016/j.jep.2019.112307. [DOI] [PubMed] [Google Scholar]
- 97.Pereira LOM, Vilegas W, Tangerina MMP, Arunachalam K, Balogun SO, Orlandi-Mattos PE, et al. Lafoensia Pacari A. St.-Hil.: Wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J Ethnopharmacol. 2018;219:337–350. doi: 10.1016/j.jep.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 98.Park KS, Park DH. The effect of Korean Red Ginseng on full-thickness skin wound healing in rats. J Ginseng Res. 2019;43:226–235. doi: 10.1016/j.jgr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Namchaiw P, Jaisin Y, Niwaspragrit C, Malaniyom K, Auvuchanon A, Ratanachamnong P. The leaf extract of coccinia grandis (L.) Voigt Accelerated in Vitro Wound Healing by reducing oxidative stress Injury. Oxid Med Cell Longev. 2021;3963510. 10.1155/2021/3963510 [DOI] [PMC free article] [PubMed]
- 100.Al-Madhagy SA, Mostafa NM, Youssef FS, Awad GEA, Eldahshan OA, Singab ANB. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019;10(10):6267–6275. doi: 10.1039/c9fo01532a. [DOI] [PubMed] [Google Scholar]
- 101.Danna C, Bazzicalupo M, Ingegneri M, Smeriglio A, Trombetta D, Burlando B, et al. Anti-inflammatory and Wound Healing properties of Leaf and Rhizome extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules. 2022;27:4271. doi: 10.3390/molecules27134271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szopa A, Pajor J, Klin P, Rzepiela A, Elansary HO, Al-Mana FA, et al. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants. 2020;9:1063. doi: 10.3390/plants9091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sultan MH, Zuwaiel AA, Moni SS, Alshahrani S, Alqahtani SS, Madkhali O, et al. Bioactive principles and Potentiality of Hot Methanolic Extract of the leaves from Artemisia absinthium L in vitro cytotoxicity against human MCF-7 Breast Cancer cells, antibacterial study and wound healing activity. Curr Pharm Biotechnol. 2020;21:1711–1721. doi: 10.2174/1389201021666200928150519. [DOI] [PubMed] [Google Scholar]
- 104.Zhou YX, Xin HL, Rahman K, Wang SJ, Peng C, Zhang H. Portulaca oleracea L.: a review of Phytochemistry and Pharmacological effects. Biomed Res Int. 2015;2015:925631. doi: 10.1155/2015/925631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo J, Peng J, Han J, Wang K, Si R, Shan H, et al. Extracts of Portulaca oleracea promote wound healing by enhancing angiology regeneration and inhibiting iron accumulation in mice. Chin Herb Med. 2022;14:263–272. doi: 10.1016/j.chmed.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alsareii SA, Alzerwi NAN, AlAsmari MY, Alamri AM, Mahnashi MH, Shaikh IA. Topical application of Premna Integrifolia Linn on skin Wound Injury in rats accelerates the Wound Healing process: evidence from in Vitro and in vivo experimental models. Evid Based Complement Alternat Med. 2022;132022:1–14. doi: 10.1155/2022/6449550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devkota HP, Paudel KR, Khanal S, Baral A, Panth N, Adhikari-Devkota A, et al. Stinging nettle (Urtica dioica L.): nutritional composition, bioactive compounds, and food functional properties. Molecules. 2022;27:5219. doi: 10.3390/molecules27165219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kasouni AI, Chatzimitakos TG, Stalikas CD, Trangas T, Papoudou-Bai A, Troganis AN. The unexplored Wound Healing activity of Urtica dioica L. Extract: an in Vitro and in vivo study. Molecules. 2021;26:6248. doi: 10.3390/molecules26206248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zulkipli IN, Rajabalaya R, Idris A, Sulaiman NA, David SR. Clinacanthus nutans: a review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm Biol. 2017;55:1093–1113. doi: 10.1080/13880209.2017.1288749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roeslan MO, Ayudhya TDN, Yingyongnarongkul B, Koontongkaew S. Anti-biofilm, nitric oxide inhibition and wound healing potential of purpurin-18 phytyl ester isolated from Clinacanthus nutans leaves. Biomed Pharmacother. 2019;113:108724. doi: 10.1016/j.biopha.2019.108724. [DOI] [PubMed] [Google Scholar]
- 111.Daemi A, Lotfi M, Farahpour MR, Oryan A, Ghayour SJ, Sonboli A. Topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol. 2019;57(1):799–806. doi: 10.1080/13880209.2019.1687525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farahpour MR, Sheikh S, Kafshdooz E, Sonboli A. Accelerative effect of topical Zataria multiflora essential oil against infected wound model by modulating inflammation, angiogenesis, and collagen biosynthesis. Pharm Biol. 2021;59(1):1–10. doi: 10.1080/13880209.2020.1861029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Assar DH, Elhabashi N, Mokhbatly AA, Ragab AE, Elbialy ZI, Rizk SA, et al. Wound healing potential of licorice extract in rat model: antioxidants, histopathological, immunohistochemical and gene expression evidences. Biomed Pharmacother. 2021;143:112151. doi: 10.1016/j.biopha.2021.112151. [DOI] [PubMed] [Google Scholar]
- 114.Somwong P, Kamkaen N. Wound-healing activity and quantification of bioactive compounds from Derris scandens extract. J Adv Pharm Technol Res. 2022;13(1):38–43. doi: 10.4103/japtr.japtr_208_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akbari F, Azadbakht M, Bagheri A, Vahedi L. Vitro and in vivo Wound Healing activity of Astragalus Floccosus Boiss. (Fabaceae) Adv Pharmacol Pharm Sci. 2022;2022:7865015. doi: 10.1155/2022/7865015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmed SR, Mostafa EM, Musa A, Rateb EE, Al-Sanea MM, Abu-Baih DH, et al. Wound healing and antioxidant properties of Launaea procumbens supported by metabolomic profiling and molecular docking. Antioxid (Basel) 2022;11(11):2258. doi: 10.3390/antiox11112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lulseged K, Akele MZ, Abiye AA, Abebe B, Assefa Huluka S. Wound healing and antioxidant properties of 80% methanol leaf extract of Verbascum sinaiticum (Scrophulariaceae): an Ethiopian Medicinal Plant. Evid Based Complement Alternat Med. 2022;2022:9836773. doi: 10.1155/2022/9836773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Selseleh M, Nejad ES, Aliahmadi A, Sonboli A, Mirjalili MH. Metabolic profiling, antioxidant, and antibacterial activity of some Iranian Verbascum L. species. Ind Crops Prod. 2020;153. 10.1016/j.indcrop.2020.112609.112609
- 119.Zhao B, Zhang X, Han W, Cheng J, Qin Y. Wound healing effect of an Astragalus membranaceus polysaccharide and its mechanism. Mol Med Rep. 2017;15(6):4077–4083. doi: 10.3892/mmr.2017.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahmad SU, Binti Aladdin NA, Jamal JA, Shuid AN, Mohamed IN. Evaluation of wound-healing and antioxidant effects of Marantodes Pumilum (Blume) Kuntze in an Excision Wound Model. Molecules. 2021;26(1):228. doi: 10.3390/molecules26010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yeh CJ, Chen CC, Leu YL, Lin MW, Chiu MM, Wang SH. The effects of artocarpin on wound healing: in vitro and in vivo studies. Sci Rep. 2017;7(1):15599. doi: 10.1038/s41598-017-15876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aulanniam A, Ora KM, Ariandini NA, Wuragil DK, Permata FS, Riawan W, et al. Wound healing properties of Gliricidia sepium leaves from Indonesia and the Philippines in rats (Rattus norvegicus) Vet World. 2021;14(3):820–824. doi: 10.14202/vetworld.2021.820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mehraein F, Sarbishegi M, Aslani A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014;16(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- 124.Prakoso YA, Rini CS, Rahayu A, Sigit M, Widhowati D. Celery (Apium graveolens) as a potential antibacterial agent and its effect on cytokeratin-17 and other healing promoters in skin wounds infected with methicillin-resistant Staphylococcus aureus. Vet World. 2020;13(5):865–871. doi: 10.14202/vetworld.2020.865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chattopadhyay P, Goyary D, Mazumder PM, Veer V. Euphorbia hirta accelerates fibroblast proliferation and smad-mediated collagen production in rat excision wound. Pharmacogn Mag. 2014;10(Suppl 3):534–542. doi: 10.4103/0973-1296.139801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pang Y, Zhang Y, Huang L, Xu L, Wang K, Wang D, et al. Effects and mechanisms of total flavonoids from Blumea balsamifera (L.) DC. On skin wound in rats. Int J Mol Sci. 2017;18(12):2766. doi: 10.3390/ijms18122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Americo AVLDS, Nunes KM, de Assis FFV, Dias SR, Passos CTS, Morini AC, et al. Efficacy of phytopharmaceuticals from the amazonian plant libidibia ferrea for wound healing in dogs. Front Vet Sci. 2020;12:244. doi: 10.3389/fvets.2020.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee SY, Chang WL, Li ZX, Kirkby NS, Tsai WC, Huang SF, et al. Astragaloside VI and cycloastragenol-6-O-beta-D-glucoside promote wound healing in vitro and in vivo. Phytomedicine. 2018;38:183–191. doi: 10.1016/j.phymed.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Abeje BA, Bekele T, Getahun KA, Asrie AB. Evaluation of Wound Healing activity of 80% hydromethanolic crude extract and solvent fractions of the leaves of Urtica simensis in mice. J Exp Pharmacol. 2022;14:221–241. doi: 10.2147/JEP.S363676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang CG, Lou YT, Tong MJ, Zhang LL, Zhang ZJ, Feng YZ, et al. Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up-regulating HIF-1α/VEGF signaling. Acta Pharmacol Sin. 2018;39(3):393–404. doi: 10.1038/aps.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Agour A, Mssillou I, Es-Safi I, Conte R, Mechchate H, Slighoua M, et al. The antioxidant, Analgesic, anti-inflammatory, and Wound Healing activities of Haplophyllum tuberculatum (Forsskal) A. Juss Aqueous and Ethanolic Extract. Life (Basel) 2022;12(10):1553. doi: 10.3390/life12101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdulhafiz F, Reduan MFH, Hisam AH, Mohammad I, Abdul Wahab IR, Abdul Hamid FF, et al. LC-TOF-MS/MS and GC-MS based phytochemical profiling and evaluation of wound healing activity of Oroxylum Indicum (L.) Kurz (Beka) Front Pharmacol. 2022;13:1050453. doi: 10.3389/fphar.2022.1050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ananda N, Ariawan D, Juniantito V. Effects of the hydnophytum formicarum plant extract on collagen density, angiogenesis, wound length, and re-epithelialization in wound healing: experimental study on rats. Dent Med Probl. 2022;59(1):67–73. doi: 10.17219/dmp/140208. [DOI] [PubMed] [Google Scholar]
- 134.Ren J, Yang M, Chen J, Ma S, Wang N. Anti-inflammatory and wound healing potential of kirenol in diabetic rats through the suppression of inflammatory markers and matrix metalloproteinase expressions. Biomed Pharmacother. 2020;129:110475. doi: 10.1016/j.biopha.2020.110475. [DOI] [PubMed] [Google Scholar]
- 135.Rhimi W, Hlel R, Ben Salem I, Boulila A, Rejeb A, Saidi M. Dittrichia viscosa L. Ethanolic Extract based ointment with Antiradical, antioxidant, and Healing Wound activities. Biomed Res Int. 2019;2019:4081253. doi: 10.1155/2019/4081253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duymus ME, Aydin HA, Bulgurcu A, Bayramoglu Z, Durhan A, Salih S, et al. Effect of topical application and/or systemic use of red ginseng extract on wound healing in rats with experimentally induced diabetes. Wound Manag Prev. 2022;68(6):28–37. doi: 10.25270/wmp.2022.6.2837. [DOI] [PubMed] [Google Scholar]
- 137.Rahman S, Karibasappa SN, Mehta DS. Evaluation of the wound-healing potential of the kiwifruit extract by assessing its effects on human gingival fibroblasts and angiogenesis. Dent Med Probl. 2023;60(1):71–77. doi: 10.17219/dmp/146635. [DOI] [PubMed] [Google Scholar]
- 138.Rezaei M, Dadgar Z, Noori-Zadeh A, Mesbah-Namin SA, Pakzad I, Davodian E. Evaluation of the antibacterial activity of the Althaea officinalis L. leaf extract and its wound healing potency in the rat model of excision wound creation. Avicenna J Phytomed. 2015;5(2):105–112. [PMC free article] [PubMed] [Google Scholar]
- 139.Hyun SW, Kim J, Jo K, Kim JS, Kim CS. Aster Koraiensis extract improves impaired skin wound healing during hyperglycemia. Integr Med Res. 2018;7(4):351–357. doi: 10.1016/j.imr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Asante-Kwatia E, Adjei S, Jibira Y, Gyimah L, Adjei-Hinneh G, Amponsah IK, et al. Amphimas pterocarpoides harms.: an evaluation of flavonoid and phenolic contents, wound healing, anthelmintic and antioxidant activities of the leaves and stem bark. Heliyon. 2021;7(11):e08261. doi: 10.1016/j.heliyon.2021.e08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rujirachotiwat A, Suttamanatwong S. Curcumin promotes collagen Type I, Keratinocyte growth Factor-1, and epidermal growth factor receptor expressions in the In Vitro wound healing model of human gingival fibroblasts. Eur J Dent. 2021;15(1):63–70. doi: 10.1055/s-0040-1715781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bueno FG, Moreira EA, Morais GR, Pacheco IA, Baesso ML, Leite-Mello EV, et al. Enhanced cutaneous wound healing in vivo by standardized crude extract of Poincianella Pluviosa. PLoS ONE. 2016;11(3):e0149223. doi: 10.1371/journal.pone.0149223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev. 2014;8(16):73–80. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nagore SG, Kuber DH, Gupta VV, Patil PK. Design and development of a stable polyherbal formulation based on the results of compatibility studies. Pharmacognosy Res. 2011;3(2):122–129. doi: 10.4103/0974-8490.81960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amin AH, Fatima F, Shafiq B, Rehman A, Haq NU, Gilani IU. Systematic review of polyherbal combinations used in metabolic syndrome. Front Pharmacol. 2021;12:752926. doi: 10.3389/fphar.2021.752926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Das R, Pal P, Bhutia S. Pharmacognostical characterization and formulation of herbal-based low-cost mosquito repellents from Elettaria cardamomum (Linn.) Seed by using natural binder. Futur J Pharm Sci. 2021;7:15. doi: 10.1186/s43094-020-00166-3. [DOI] [Google Scholar]
- 147.Chi J, Sun L, Cai L, Fan L, Shao C, Shang L, et al. Chinese herb microneedle patch for wound healing. Bioact Mater. 2021;6:3507–3514. doi: 10.1016/j.bioactmat.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Niknam S, Tofighi Z, Faramarzi MA, Abdollahifar MA, Sajadi E, Dinarvand R, et al. Polyherbal combination for wound healing: Matricaria chamomilla L. and Punica granatum L. Daru. 2021;29:133–145. doi: 10.1007/s40199-021-00392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu JR, Lu YC, Hung SJ, Lin JH, Chang KC, Chen JK, et al. Antimicrobial and immunomodulatory activity of Herb extracts used in burn Wound Healing: San Huang Powder. Evid Based Complement Alternat Med. 2021;2021:1–13. doi: 10.1155/2021/2900060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou Z, Chen L, Su Y, Li M, Zhong L, Liao L. A New Target of the four-Herb Chinese Medicine for Wound Repair promoted by mitochondrial metabolism using protein acetylation analysis. Med Sci Monit. 2022;28:e934816. doi: 10.12659/MSM.934816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou H, Fang Q, Li N, Yu M, Chen H, Guo S. ASMq protects against early burn wound progression in rats by alleviating oxidative stress and secondary mitochondria associated apoptosis via the Erk/p90RSK/Bad pathway. Mol Med Rep. 2021;23:390. doi: 10.3892/mmr.2021.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Porwal A, Kundu G, Bhagwat G, Butti R. Polyherbal formulation Anoac H suppresses the expression of RANTES and VEGF for the management of bleeding hemorrhoids and fistula. Mol Med Rep. 2021;24:736. doi: 10.3892/mmr.2021.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miyano K, Eto M, Hitomi S, Matsumoto T, Hasegawa S, Hirano A, et al. The Japanese herbal medicine Hangeshashinto enhances oral keratinocyte migration to facilitate healing of chemotherapy-induced oral ulcerative mucositis. Sci Rep. 2020;10:625. doi: 10.1038/s41598-019-57192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Her Y, Lee TK, Ahn JH, Lim SS, Kang BG, Park JS, et al. Chemical composition of a Novel Distillate from Fermented mixture of nine anti-inflammatory herbs and its UVB-Protective efficacy in mouse dorsal skin via Attenuating Collagen Disruption and Inflammation. Molecules. 2020;26:124. doi: 10.3390/molecules26010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhong L, Shi C, Hou Q, Yang R, Li M, Fu X. Promotive effects of four herbal medicine ARCC on wound healing in mice and human. Health Sci Rep. 2022;5(3):e494. doi: 10.1002/hsr2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elzayat EM, Auda SH, Alanazi FK, Al-Agamy MH. Evaluation of wound healing activity of henna, pomegranate and myrrh herbal ointment blend. Saudi Pharm J. 2018;26(5):733–738. doi: 10.1016/j.jsps.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mahboubi M, Taghizadeh M, Khamechian T, Tamtaji OR, Mokhtari R, Talaei SA. The Wound Healing effects of Herbal cream containing Oliveria Decumbens and Pelargonium Graveolens essential oils in Diabetic Foot Ulcer Model. World J Plast Surg. 2018;7(1):45–50. [PMC free article] [PubMed] [Google Scholar]
- 158.Aslam MS, Ahmad MS, Mamat AS, Ahmad MZ, Salam F. Antioxidant and Wound Healing activity of Polyherbal fractions of Clinacanthus nutans and Elephantopus scaber. Evid Based Complement Alternat Med. 2016;2016:4685246. doi: 10.1155/2016/4685246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ho TJ, Jiang SJ, Lin GH, Li TS, Yiin LM, Yang JS, et al. The in vitro and in vivo wound healing properties of the Chinese Herbal Medicine Jinchuang Ointment. Evid Based Complement Alternat Med. 2016;2016:1654056. doi: 10.1155/2016/1654056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Talekar YP, Apte KG, Paygude SV, Tondare PR, Parab PB. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J Ayurveda Integr Med. 2017;8(2):73–81. doi: 10.1016/j.jaim.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Galehdari H, Negahdari S, Kesmati M, Rezaie A, Shariati G. Effect of the herbal mixture composed of Aloe Vera, Henna, Adiantum capillus-veneris, and Myrrha on wound healing in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2016;16(1):386. doi: 10.1186/s12906-016-1359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akgun SG, Aydemir S, Ozkan N, Yuksel M, Sardas S. Evaluation of the wound healing potential of Aloe vera-based extract of Nerium oleander. North Clin Istanb. 2017;4(3):205–12. doi: 10.14744/nci.2017.94914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chumpolphant S, Suwatronnakorn M, Issaravanich S, Tencomnao T, Prasansuklab A. Polyherbal formulation exerts wound healing, anti-inflammatory, angiogenic and antimicrobial properties: potential role in the treatment of diabetic foot ulcers. Saudi J Biol Sci. 2022;29(7):103330. doi: 10.1016/j.sjbs.2022.103330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound Healing activity of a traditionally used Poly Herbal product in a burn Wound Model in rats. Iran Red Crescent Med J. 2015;17(9):e19960. doi: 10.5812/ircmj.19960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Negahdari S, Galehdari H, Kesmati M, Rezaie A, Shariati G. Wound Healing activity of extracts and formulations of Aloe vera, Henna, Adiantum capillus-veneris, and myrrh on mouse dermal fibroblast cells. Int J Prev Med. 2017;8:18. doi: 10.4103/ijpvm.IJPVM_338_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang XN, Ma ZJ, Wang Y, Li YZ, Sun B, Guo X, et al. The four-Herb Chinese Medicine Formula Tuo-Li-Xiao-Du-San accelerates cutaneous wound healing in streptozotocin-induced diabetic rats through reducing inflammation and increasing angiogenesis. J Diabetes Res. 2016;2016:5639129. doi: 10.1155/2016/5639129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu X, Li X, Zhang L, Liu Z, Pan Y, Chen D, et al. Enhancement of wound healing by the traditional Chinese Medicine Herbal Mixture Sophora flavescens in a rat model of Perianal Ulceration. In Vivo. 2017;31(4):543–549. doi: 10.21873/invivo.11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jahandideh M, Hajimehdipoor H, Mortazavi SA, Dehpour A, Hassanzadeh G. Evaluation of the Wound Healing activity of a traditional compound Herbal Product using rat excision wound model. Iran J Pharm Res. 2017;16(Supp):153–163. [PMC free article] [PubMed] [Google Scholar]
- 169.Liao J, Azelmat J, Zhao L, Yoshioka M, Hinode D, Grenier D. The Kampo medicine Rokumigan possesses antibiofilm, anti-inflammatory, and wound healing properties. Biomed Res Int. 2014;2014:436206. doi: 10.1155/2014/436206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chaushu L, Weinreb M, Beitlitum I, Moses O, Nemcovsky CE. Evaluation of a topical herbal patch for soft tissue wound healing: an animal study. J Clin Periodontol. 2015;42(3):288–293. doi: 10.1111/jcpe.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang N, Zheng Y, Cui D, Haick H. Multifunctional dressing for Wound diagnosis and Rehabilitation. Adv Healthc Mater. 2021;10:e2101292. doi: 10.1002/adhm.202101292. [DOI] [PubMed] [Google Scholar]
- 172.Kaur G, Narayanan G, Garg D, Sachdev A, Matai I. Biomaterials-based regenerative strategies for skin tissue Wound Healing. ACS Appl Bio Mater. 2022;5:2069–2106. doi: 10.1021/acsabm.2c00035. [DOI] [PubMed] [Google Scholar]
- 173.Joorabloo A, Liu T. Recent advances in nanomedicines for regulation of macrophages in wound healing. J Nanobiotechnol. 2022;20:407. doi: 10.1186/s12951-022-01616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hawthorne B, Simmons JK, Stuart B, Tung R, Zamierowski DS, Mellott AJ. Enhancing wound healing dressing development through interdisciplinary collaboration. J Biomed Mater Res B Appl Biomater. 2021;109:1967–1985. doi: 10.1002/jbm.b.34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Blanco-Fernandez B, Castano O, Mateos-Timoneda MA, Engel E, Perez-Amodio S. Nanotechnology approaches in Chronic Wound Healing. Adv Wound Care (New Rochelle) 2021;10:234–256. doi: 10.1089/wound.2019.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bhadauria SS, Malviya R. Advancement in Nanoformulations for the management of Diabetic Wound Healing. Endocr Metab Immune Disord Drug Targets. 2022;22:911–926. doi: 10.2174/1871530322666220304214106. [DOI] [PubMed] [Google Scholar]
- 177.Nikam A, Thomas A, Giram P, Nagore D, Chitlange S. Herbal-based dressings in Wound Management. Curr Diabetes Rev. 2022;18. 10.2174/1573399818666220401105256 [DOI] [PubMed]
- 178.Banerjee K, Madhyastha R, Nakajima Y, Maruyama M, Madhyastha H. Nanoceutical adjuvants as Wound Healing Material: precepts and prospects. Int J Mol Sci. 2021;22:4748. doi: 10.3390/ijms22094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dam P, Celik M, Ustun M, Saha S, Saha C, Kacar EA, et al. Wound healing strategies based on nanoparticles incorporated in hydrogel wound patches. RSC Adv. 2023;13(31):21345–21364. doi: 10.1039/d3ra03477a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chu W, Wang P, Ma Z, Peng L, Guo C, Fu Y, et al. Lupeol-loaded chitosan-Ag+ nanoparticle/sericin hydrogel accelerates wound healing and effectively inhibits bacterial Infection. Int J Biol Macromol. 2023;243:125310. doi: 10.1016/j.ijbiomac.2023.125310. [DOI] [PubMed] [Google Scholar]
- 181.Sari MHM, Cobre AF, Pontarolo R, Ferreira LM. Status and future scope of Soft nanoparticles-based hydrogel in Wound Healing. Pharmaceutics. 2023;15(3):874. doi: 10.3390/pharmaceutics15030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Montazeri S, Rastegari A, Mohammadi Z, Nazari M, Yousefi M, Samadi FY, et al. Chitosan nanoparticle loaded by epidermal growth factor as a potential protein carrier for wound healing: in vitro and in vivo studies. IET Nanobiotechnol. 2023;17(3):204–211. doi: 10.1049/nbt2.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Froelich A, Jakubowska E, Wojtylko M, Jadach B, Gackowski M, Gadzinski P, et al. Alginate-based materials loaded with nanoparticles in Wound Healing. Pharmaceutics. 2023;41142. 10.3390/pharmaceutics15041142 [DOI] [PMC free article] [PubMed]
- 184.Huang C, Dong L, Zhao B, Lu Y, Huang S, Yuan Z, et al. Anti-inflammatory hydrogel dressings and skin wound healing. Clin Transl Med. 2022;12(11):e1094. doi: 10.1002/ctm2.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang X, Wei P, Yang Z, Liu Y, Yang K, Cheng Y, et al. Current Progress and Outlook of Nano-based hydrogel dressings for Wound Healing. Pharmaceutics. 2022;15(1):68. doi: 10.3390/pharmaceutics15010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen Z, Wang L, Guo C, Qiu M, Cheng L, Chen K, et al. Vascularized polypeptide hydrogel modulates macrophage polarization for wound healing. Acta Biomater. 2023;155:218–234. doi: 10.1016/j.actbio.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 187.Guan T, Li J, Chen C, Liu Y. Self-assembling peptide-based hydrogels for Wound tissue repair. Adv Sci (Weinh) 2022;9(10):e2104165. doi: 10.1002/advs.202104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang S, Ge G, Qin Y, Li W, Dong J, Mei J, et al. Recent advances in responsive hydrogels for diabetic wound healing. Mater Today Bio. 2022;18:100508. doi: 10.1016/j.mtbio.2022.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Umar AK, Sriwidodo S, Maksum IP, Wathoni N. Film-forming spray of Water-Soluble Chitosan containing liposome-coated human epidermal growth factor for Wound Healing. Molecules. 2021;26(17):5326. doi: 10.3390/molecules26175326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Natsaridis E, Mouzoura P, Gkartziou F, Marazioti A, Antimisiaris SG. Development of growth factor-incorporating liposomes for integration into scaffolds as a method to improve tissue regeneration. Int J Dev Biol. 2022;66(1–2–3):137–154. doi: 10.1387/ijdb.210108sa. [DOI] [PubMed] [Google Scholar]
- 191.Cardoso-Daodu IM, Ilomuanya MO, Amenaghawon AN, Azubuike CP. Artificial neural network for optimizing the formulation of curcumin-loaded liposomes from statistically designed experiments. Prog Biomater. 2022;11(1):55–65. doi: 10.1007/s40204-022-00179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eid HM, Ali AA, Ali AMA, Eissa EM, Hassan RM, Abo El-Ela FI, et al. Potential use of tailored citicoline Chitosan-Coated liposomes for Effective Wound Healing in Diabetic Rat Model. Int J Nanomedicine. 2022;17:555–575. doi: 10.2147/IJN.S342504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tan G, Wang L, Pan W, Chen K. Polysaccharide Electrospun nanofibers for Wound Healing Applications. Int J Nanomedicine. 2022;17:3913–3931. doi: 10.2147/IJN.S371900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patel Z, Gharat SA, Al-Tabakha MM, Ashames A, Boddu SHS, Momin MM. Recent advancements in Electrospun nanofibers for Wound Healing: polymers, Clinical and Regulatory Perspective. Crit Rev Ther Drug Carrier Syst. 2022;39:83–118. doi: 10.1615/CritRevTherDrugCarrierSyst.2022039840. [DOI] [PubMed] [Google Scholar]
- 195.Xu H, Zhang F, Wang M, Lv H, Yu DG, Liu X, et al. Electrospun hierarchical structural films for effective wound healing. Biomater Adv. 2022;136:212795. doi: 10.1016/j.bioadv.2022.212795. [DOI] [PubMed] [Google Scholar]
- 196.Behere I, Ingavle G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: innovative nanofiber designs, stem cell approaches, and future perspectives. J Biomed Mater Res. 2022;110:443–461. doi: 10.1002/jbm.a.37290. [DOI] [PubMed] [Google Scholar]
- 197.Gwon K, Choi WI, Lee S, Lee JS, Shin JH. Biodegradable hyaluronic acid-based, nitric oxide-releasing nanofibers for potential wound healing applications. Biomater Sci. 2021;9:8160–8170. doi: 10.1039/d1bm01019k. [DOI] [PubMed] [Google Scholar]
- 198.Zeng N, Jiang L, Miao Q, Zhi Y, Shan S, Su H. [Preparation and applications of the polymeric micelle/hydrogel nanocomposites as biomaterials] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021;38(3):609–620. doi: 10.7507/1001-5515.202011024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble Drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80–118. doi: 10.1016/j.addr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kotta S, Aldawsari HM, Badr-Eldin SM, Nair AB, Yt K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics. 2022;14(8):1636. doi: 10.3390/pharmaceutics14081636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perumal S, Atchudan R, Lee W. A review of polymeric micelles and their applications. Polym (Basel) 2022;14(12):2510. doi: 10.3390/polym14122510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yoshida C, Uchida Y, Ito T, Takami T, Murakami Y. Chitosan gel sheet containing polymeric micelles: synthesis and Gelation properties of PEG-Grafted Chitosan. Mater (Basel) 2017;10(9):1075. doi: 10.3390/ma10091075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liao JL, Zhong S, Wang SH, Liu JY, Chen J, He G, et al. Preparation and properties of a novel carbon nanotubes/poly(vinyl alcohol)/epidermal growth factor composite biological dressing. Exp Ther Med. 2017;14(3):2341–2348. doi: 10.3892/etm.2017.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ravanbakhsh H, Bao G, Mongeau L. Carbon nanotubes promote cell migration in hydrogels. Sci Rep. 2020;10(1):2543. doi: 10.1038/s41598-020-59463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kittana N, Assali M, Abu-Rass H, Lutz S, Hindawi R, Ghannam L, et al. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. Int J Nanomedicine. 2018;13:7195–7206. doi: 10.2147/IJN.S183342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu J, Ismail NA, Yusoff M, Razali MH. Physicochemical Properties and Antibacterial Activity of Gellan Gum incorporating Zinc Oxide/Carbon nanotubes Bionanocomposite Film for Wound Healing. Bioinorg Chem Appl. 2022;2022:3158404. doi: 10.1155/2022/3158404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao P, Feng Y, Zhou Y, Tan C, Liu M. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact Mater. 2022;20:355–367. doi: 10.1016/j.bioactmat.2022.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ali Zahid A, Chakraborty A, Shamiya Y, Ravi SP, Paul A. Leveraging the advancements in functional biomaterials and scaffold fabrication technologies for chronic wound healing applications. Mater Horiz. 2022;9:1850–1865. doi: 10.1039/d2mh00115b. [DOI] [PubMed] [Google Scholar]
- 209.Zulkiflee I, Masri S, Zawani M, Salleh A, Amirrah IN, Wee MFMR, et al. Silicon-based Scaffold for Wound Healing skin regeneration applications: a concise review. Polym (Basel) 2022;14:4219. doi: 10.3390/polym14194219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sun L, Li L, Wang Y, Li M, Xu S, Zhang C. A collagen-based bi-layered composite dressing for accelerated wound healing. J Tissue Viability. 2022;31:180–189. doi: 10.1016/j.jtv.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 211.Hao R, Cui Z, Zhang X, Tian M, Zhang L, Rao F, et al. Rational design and preparation of functional hydrogels for skin wound healing. Front Chem. 2022;9:9. doi: 10.3389/fchem.2021.839055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sharma A, Puri V, Kumar P, Singh I. Biopolymeric, nanopatterned, fibrous carriers for wound healing applications. Curr Pharm Des. 2020;26:4894–908. doi: 10.2174/1381612826666200701152217. [DOI] [PubMed] [Google Scholar]
- 213.Gardikiotis I, Cojocaru FD, Mihai CT, Balan V, Dodi G. Borrowing the features of biopolymers for emerging Wound Healing dressings: a review. Int J Mol Sci. 2022;23:8778. doi: 10.3390/ijms23158778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang C, Shirzaei Sani E, Gao W. Wearable bioelectronics for chronic wound management. Adv Funct Mater. 2022;32:2111022. doi: 10.3760/cma.j.cn501120-20210218-00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Veeraraghavan VP, Periadurai ND, Karunakaran T, Hussain S, Surapaneni KM, Jiao X. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929) Saudi J Biol Sci. 2021;28:3633–3640. doi: 10.1016/j.sjbs.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Buranasukhon W, Athikomkulchai S, Tadtong S, Chittasupho C. Wound healing activity of Pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm Biol. 2017;55:1767–1774. doi: 10.1080/13880209.2017.1326511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jakfar S, Lin TC, Chen ZY, Yang IH, Gani BA, Ningsih DS, et al. A polysaccharide isolated from the Herb Bletilla striata combined with methylcellulose to Form a Hydrogel via Self-Assembly as a Wound Dressing. Int J Mol Sci. 2022;23:12019. doi: 10.3390/ijms231912019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zahedi E, Esmaeili A, Eslahi N, Shokrgozar MA, Simchi A. Fabrication and characterization of Core-Shell electrospun fibrous mats containing medicinal herbs for wound healing and skin tissue engineering. Mar Drugs. 2019;17:27. doi: 10.3390/md17010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ryall C, Chen S, Duarah S, Wen J. Chitosan-based microneedle arrays for dermal delivery of Centella asiatica. Int J Pharm. 2022;627:122221. doi: 10.1016/j.ijpharm.2022.122221. [DOI] [PubMed] [Google Scholar]
- 220.Khezri K, Farahpour MR, Mounesi Rad S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif Cells Nanomed Biotechnol. 2019;47(1):980–988. doi: 10.1080/21691401.2019.1582539. [DOI] [PubMed] [Google Scholar]
- 221.Parastar H, Farahpour MR, Shokri R, Jafarirad S, Kalantari M. Acceleration in healing of infected full-thickness wound with novel antibacterial γ-AlOOH-based nanocomposites. Prog Biomater. 2023;12(2):123–136. doi: 10.1007/s40204-022-00216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ali A, Garg P, Goyal R, Kaur G, Li X, Negi P, et al. A Novel Herbal Hydrogel Formulation of Moringa oleifera for Wound Healing. Plants (Basel) 2020;10(1):25. doi: 10.3390/plants10010025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 223.Aker SD, Tamburaci S, Tihminlioglu F. Development of Cissus quadrangularis-Loaded POSS-Reinforced Chitosan-based bilayer sponges for Wound Healing Applications: drug release and in Vitro Bioactivity. ACS Omega. 2023;8(22):19674–19691. doi: 10.1021/acsomega.3c00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Beyranvand F, Gharzi A, Abbaszadeh A, Khorramabadi RM, Gholami M, Gharravi AM. Encapsulation of Satureja Khuzistanica extract in alginate hydrogel accelerate wound healing in adult male rats. Inflamm Regen. 2019;39:2. doi: 10.1186/s41232-019-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rameshk M, Sharififar F, Mehrabani M, Pardakhty A, Farsinejad A, Mehrabani M. Proliferation and in Vitro Wound Healing effects of the microniosomes containing Narcissus tazetta L. Bulb extract on primary human fibroblasts (HDFs) Daru. 2018;26(1):31–42. doi: 10.1007/s40199-018-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Namviriyachote N, Lipipun V, Akkhawattanangkul Y, Charoonrut P, Ritthidej GC. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J Pharm Sci. 2019;14(1):63–77. doi: 10.1016/j.ajps.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Koshak AE, Algandaby MM, Mujallid MI, Abdel-Naim AB, Alhakamy NA, Fahmy UA, et al. Wound Healing activity of Opuntia ficus-indica fixed oil formulated in a self-nanoemulsifying formulation. Int J Nanomedicine. 2021;16:3889–3905. doi: 10.2147/IJN.S299696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4(9):560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sharifi S, Hajipour MJ, Gould L, Mahmoudi M. Nanomedicine in Healing Chronic wounds: opportunities and challenges. Mol Pharm. 2021;18(2):550–575. doi: 10.1021/acs.molpharmaceut.0c00346. [DOI] [PubMed] [Google Scholar]
- 230.Oguntibeju OO. Medicinal plants and their effects on diabetic wound healing. Vet World. 2019;12(5):653. doi: 10.14202/vetworld.2019.653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ghomi ER, Shakiba M, Ardahaei AS, Kenari MA, Faraji M, Ataei S, et al. Innovations in drug delivery for chronic wound healing. Curr Pharm Des. 2022;28(5):340–351. doi: 10.2174/1381612827666210714102304. [DOI] [PubMed] [Google Scholar]
- 232.Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle) 2014;3(8):511–529. doi: 10.1089/wound.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Park JW, Hwang SR, Yoon IS. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22(8):1259. doi: 10.3390/molecules22081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li Z, Yu A. Complications of negative pressure wound therapy: a mini review. Wound Repair Regen. 2014;22(4):457–461. doi: 10.1111/wrr.12190. [DOI] [PubMed] [Google Scholar]
- 236.Khansa I, Schoenbrunner AR, Kraft CT, Janis JE. Silver in Wound Care-Friend or Foe? A Comprehensive Review. Plast Reconstr Surg Glob Open. 2019;7(8):e2390. doi: 10.1097/GOX.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346(6212):941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 238.Borena BM, Martens A, Broeckx SY, Meyer E, Chiers K, Duchateau L, et al. Regenerative skin wound healing in mammals: state-of-the-art on growth factor and stem cell based treatments. Cell Physiol Biochem. 2015;36(1):1–23. doi: 10.1159/000374049. [DOI] [PubMed] [Google Scholar]
- 239.Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, et al. Stem cells in wound healing: the future of Regenerative Medicine? A Mini-review. Gerontology. 2016;62(2):216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 240.Parani M, Lokhande G, Singh A, Gaharwar AK. Engineered nanomaterials for Infection control and Healing Acute and Chronic wounds. ACS Appl Mater Interfaces. 2016;8(16):10049–10069. doi: 10.1021/acsami.6b00291. [DOI] [PubMed] [Google Scholar]
- 241.Sharma A, Khanna S, Kaur G, Singh I. Medicinal plants and their components for wound healing applications. Future J Pharm Sci. 2021;7(1):1–3. doi: 10.1186/s43094-021-00202-w. [DOI] [Google Scholar]
- 242.Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid-Based Complementary Altern Med. 2019;2019.10.1155/2019/2684108 [DOI] [PMC free article] [PubMed]
- 243.Ghuman S, Ncube B, Finnie JF, McGaw LJ, Njoya EM, Coopoosamy RM, et al. Antioxidant, anti-inflammatory and wound healing properties of medicinal plant extracts used to treat wounds and dermatological disorders. South Afr J Bot. 2019;126:232–240. doi: 10.1016/j.sajb.2019.07.013. [DOI] [Google Scholar]
- 244.Solati K, Karimi M, Rafieian-Kopaei M, Abbasi N, Abbaszadeh S, Bahmani M. Phytotherapy for wound healing: the most important herbal plants in wound healing based on Iranian ethnobotanical documents. Mini Rev Med Chem. 2021;21(4):500–519. doi: 10.2174/1389557520666201119122608. [DOI] [PubMed] [Google Scholar]
- 245.Malabadi RB, Kolkar KP, Acharya M, Nityasree BR, Chalannavar RK. Wound healing: role of traditional herbal medicine treatment. IJISRR. 2022;4(6):2856–2874. [Google Scholar]
- 246.Pereira RF, Bartolo PJ. Traditional therapies for skin Wound Healing. Adv Wound Care (New Rochelle) 2016;5(5):208–229. doi: 10.1089/wound.2013.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Balap AR, Gaikwad AA. Challenges, advances and opportunities of herbal medicines in wound healing:a review. Int J Pharm Sci Rev Res. 2021;71(1):125–136. doi: 10.47583/ijpsrr.2021.v71i01.015. [DOI] [Google Scholar]
- 248.Monika P, Chandraprabha MN, Rangarajan A, Waiker PV, Chidambara Murthy KN. Challenges in healing wound: role of complementary and alternative medicine. Front Nutr. 2022;8:791899. doi: 10.3389/fnut.2021.791899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ayaz M, Subhan F, Ahmed J, Khan AU, Ullah F, Ullah I, et al. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J Biol Res (Thessalon) 2015;22(1):4. doi: 10.1186/s40709-015-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dev SK, Choudhury PK, Srivastava R, Sharma M. Antimicrobial, anti-inflammatory and wound healing activity of polyherbal formulation. Biomed Pharmacother. 2019;111:555–567. doi: 10.1016/j.biopha.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 251.Lordani TVA, de Lara CE, Ferreira FBP, de Souza Terron Monich M, Mesquita da Silva C, Felicetti Lordani CR, et al. Therapeutic effects of Medicinal plants on Cutaneous Wound Healing in humans: a systematic review. Mediators Inflamm. 2018;2018:7354250. doi: 10.1155/2018/7354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Carvalho AR, Jr, Diniz RM, Suarez MA, Figueiredo CS, Zagmignan A, Grisotto MA, et al. Use of some asteraceae plants for the treatment of wounds: from ethnopharmacological studies to scientific evidences. Front Pharmacol. 2018;9:784. doi: 10.3389/fphar.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dorjsembe B, Lee HJ, Kim M, Dulamjav B, Jigjid T, Nho CW. Achillea Asiatica extract and its active compounds induce cutaneous wound healing. J Ethnopharmacol. 2017;206:306–314. doi: 10.1016/j.jep.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 254.Neto JA, Tarôco BR, Dos Santos HB, Thomé RG, Wolfram E, de Ribeiro A. Using the plants of Brazilian cerrado for wound healing: from traditional use to scientific approach. J Ethnopharmacol. 2020;260:112547. doi: 10.1016/j.jep.2020.112547. [DOI] [PubMed] [Google Scholar]
- 255.Fazil M, Nikhat S. Topical medicines for wound healing: a systematic review of Unani literature with recent advances. J Ethnopharmacol. 2020;257:112878. doi: 10.1016/j.jep.2020.112878. [DOI] [PubMed] [Google Scholar]
- 256.Criollo-Mendoza MS, Contreras-Angulo LA, Leyva-Lopez N, Gutierrez-Grijalva EP, Jimenez-Ortega LA, Heredia JB. Wound healing properties of natural products: mechanisms of action. Molecules. 2023;28(2):598. doi: 10.3390/molecules28020598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Carvalho MT, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JS, Barreto RS. Wound healing properties of flavonoids: a systematic review highlighting the mechanisms of action. Phytomedicine. 2021;90:153636. doi: 10.1016/j.phymed.2021.153636. [DOI] [PubMed] [Google Scholar]
- 258.Yazarlu O, Iranshahi M, Kashani HRK, Reshadat S, Habtemariam S, Iranshahy M, et al. Perspective on the application of medicinal plants and natural products in wound healing: a mechanistic review. Pharmacol Res. 2021;174:105841. doi: 10.1016/j.phrs.2021.105841. [DOI] [PubMed] [Google Scholar]
- 259.Nejjari R, Benabbes M, Amrani M, Meddah B, Bouatia M, Taoufik J. Phytochemical screening and wound healing activity of Telephium Imperati (L.) in rats. South Afr J Bot. 2019;123:147–151. doi: 10.1016/j.sajb.2019.03.023. [DOI] [Google Scholar]
- 260.Shrivastav A, Mishra AK, Ali SS, Ahmad A, Abuzinadah MF, Khan NA. In vivo models for assesment of wound healing potential: a systematic review. Wound Med. 2018;20:43–53. doi: 10.1016/j.wndm.2018.01.003. [DOI] [Google Scholar]
- 261.Mehta P, Shah R, Lohidasan S, Mahadik KR. Pharmacokinetic profile of phytoconstituent(s) isolated from medicinal plants-A comprehensive review. J Tradit Complement Med. 2015;5(4):207–227. doi: 10.1016/j.jtcme.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ovais M, Ahmad I, Khalil AT, Mukherjee S, Javed R, Ayaz M, et al. Wound healing applications of biogenic colloidal silver and gold nanoparticles: recent trends and future prospects. Appl Microbiol Biotechnol. 2018;102(10):4305–4318. doi: 10.1007/s00253-018-8939-z. [DOI] [PubMed] [Google Scholar]
- 263.Zohra T, Ovais M, Khalil AT, Qasim M, Ayaz M, Shinwari ZK. Extraction optimization, total phenolic, flavonoid contents, HPLC-DAD analysis and diverse pharmacological evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat Prod Res. 2019;33(1):136–142. doi: 10.1080/14786419.2018.1437428. [DOI] [PubMed] [Google Scholar]
- 264.Jiang T, Li Q, Qiu J, Chen J, Du S, Xu X, et al. Nanobiotechnology: applications in Chronic Wound Healing. Int J Nanomedicine. 2022;17:3125–3145. doi: 10.2147/IJN.S372211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gobi R, Ravichandiran P, Babu RS, Yoo DJ. Biopolymer and Synthetic polymer-based nanocomposites in Wound dressing applications: a review. Polym (Basel) 2021;13(12):1962. doi: 10.3390/polym13121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bai Q, Han K, Dong K, Zheng C, Zhang Y, Long Q, et al. Potential applications of nanomaterials and Technology for Diabetic Wound Healing. Int J Nanomedicine. 2020;15:9717–9743. doi: 10.2147/IJN.S276001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, Sahandi Zangabad K, Ghamarypour A, Aref AR, Karimi M, Hamblin MR. Nanomedicine and advanced technologies for Burns: preventing Infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64. doi: 10.1016/j.addr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kang HJ, Chen N, Dash BC, Hsia HC, Berthiaume F. Self-assembled nanomaterials for chronic skin Wound Healing. Adv Wound Care (New Rochelle) 2021;10(5):221–233. doi: 10.1089/wound.2019.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Guo S, Wang P, Song P, Li N. Electrospinning of botanicals for skin wound healing. Front Bioeng Biotechnol. 2022;10:1006129. doi: 10.3389/fbioe.2022.1006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnol. 2019;17(1):82. doi: 10.1186/s12951-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Faraji A, Aghdaki M, Hessami K, Hosseinkhani A, Roozmeh S, Asadi N, et al. Episiotomy wound healing by Commiphora myrrha (Nees) Engl. And Boswellia carteri Birdw. In primiparous women: a randomized controlled trial. J Ethnopharmacol. 2021;264:113396. doi: 10.1016/j.jep.2020.113396. [DOI] [PubMed] [Google Scholar]
- 272.Duric K, Kovcic Hadziabdic S, Duric M, Niksic H, Uzunovic A, Dzudzevic Cancar H. Efficacy and safety of three plant extracts based formulations of vagitories in the treatment of vaginitis: a randomized controlled trial. Med Glas (Zenica) 2021;18:47–54. doi: 10.17392/1261-21. [DOI] [PubMed] [Google Scholar]
- 273.Burusapat C, Supawan M, Pruksapong C, Pitiseree A, Suwantemee C. Topical Aloe Vera Gel for Accelerated Wound Healing of Split-Thickness skin graft Donor sites: a Double-Blind, randomized, controlled trial and systematic review. Plast Reconstr Surg. 2018;142(1):217–226. doi: 10.1097/PRS.0000000000004515. [DOI] [PubMed] [Google Scholar]
- 274.Mokhtari M, Razzaghi R, Momen-Heravi M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot Ulcer: a randomized, double‐blind, placebo‐controlled trial. Phytother Res. 2021;35:2099–2107. doi: 10.1002/ptr.6957. [DOI] [PubMed] [Google Scholar]
- 275.Damkerngsuntorn W, Rerknimitr P, Panchaprateep R, Tangkijngamvong N, Kumtornrut C, Kerr SJ, et al. The effects of a standardized extract of Centella asiatica on Postlaser Resurfacing Wound Healing on the Face: a Split-Face, Double-Blind, randomized, placebo-controlled trial. J Altern Complement Med. 2020;26:529–536. doi: 10.1089/acm.2019.0325. [DOI] [PubMed] [Google Scholar]
- 276.Toomari E, Hajian S, Mojab F, Omidkhah T, Nasiri M. Evaluation the effect of Silybum marianum ointment on episiotomy wound healing and pain intensity in primiparous women: a randomized triple-blind clinical trial. BMC Complement Med Ther. 2021;21:253. doi: 10.1186/s12906-021-03413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.lhashim M, Lombardo J. Effect of Topical Garlic on Wound Healing and Scarring: a clinical trial. Dermatol Surg. 2020;46:618–627. doi: 10.1097/DSS.0000000000002123. [DOI] [PubMed] [Google Scholar]
- 278.Taleb S, Saeedi M. The effect of the Verbascum Thapsus on episiotomy wound healing in nulliparous women: a randomized controlled trial. BMC Complement Med Ther. 2021;21:166. doi: 10.1186/s12906-021-03339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tonaco LAB, Gomes FL, Velasquez-Melendez G, Lopes MTP, Salas CE. The proteolytic fraction from latex of Vasconcellea cundinamarcensis (P1G10) enhances wound healing of diabetic foot ulcers: a double-blind Randomized Pilot Study. Adv Ther. 2018;35:494–502. doi: 10.1007/s12325-018-0684-2. [DOI] [PubMed] [Google Scholar]
- 280.Izadpanah A, Soorgi S, Geraminejad N, Hosseini M. Effect of grape seed extract ointment on cesarean section wound healing: a double-blind, randomized, controlled clinical trial. Complement Ther Clin Pract. 2019;35:323–328. doi: 10.1016/j.ctcp.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 281.Hemmati AA, Foroozan M, Houshmand G, Moosavi ZB, Bahadoram M, Maram NS. The topical effect of grape seed extract 2% cream on Surgery wound healing. Glob J Health Sci. 2014;7(3):52–58. doi: 10.5539/gjhs.v7n3p52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ikarashi N, Kaneko M, Fujisawa I, Fukuda N, Yoshida R, Kon R, et al. Wound-Healing and skin-moisturizing effects of Sasa veitchii Extract. Healthcare. 2021;9:761. doi: 10.3390/healthcare9060761. [DOI] [PMC free article] [PubMed] [Google Scholar]