Abstract
Background and Aims:
Alagille syndrome (ALGS) is characterized by chronic cholestasis with associated pruritus and extrahepatic anomalies. Maralixibat, an ileal bile acid transporter inhibitor, is an approved pharmacologic therapy for cholestatic pruritus in ALGS. Since long-term placebo-controlled studies are not feasible or ethical in children with rare diseases, a novel approach was taken comparing 6-year outcomes from maralixibat trials with an aligned and harmonized natural history cohort from the G lobal AL agille A lliance (GALA) study.
Approach and Results:
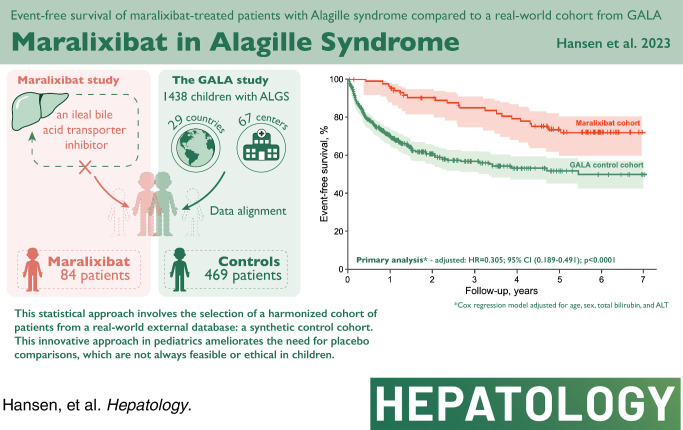
Maralixibat trials comprise 84 patients with ALGS with up to 6 years of treatment. GALA contains retrospective data from 1438 participants. GALA was filtered to align with key maralixibat eligibility criteria, yielding 469 participants. Serum bile acids could not be included in the GALA filtering criteria as these are not routinely performed in clinical practice. Index time was determined through maximum likelihood estimation in an effort to align the disease severity between the two cohorts with the initiation of maralixibat. Event-free survival, defined as the time to first event of manifestations of portal hypertension (variceal bleeding, ascites requiring therapy), surgical biliary diversion, liver transplant, or death, was analyzed by Cox proportional hazards methods. Sensitivity analyses and adjustments for covariates were applied. Age, total bilirubin, gamma-glutamyl transferase, and alanine aminotransferase were balanced between groups with no statistical differences. Event-free survival in the maralixibat cohort was significantly better than the GALA cohort (HR, 0.305; 95% CI, 0.189–0.491; p<0.0001). Multiple sensitivity and subgroup analyses (including serum bile acid availability) showed similar findings.
Conclusions:
This study demonstrates a novel application of a robust statistical method to evaluate outcomes in long-term intervention studies where placebo comparisons are not feasible, providing wide application for rare diseases. This comparison with real-world natural history data suggests that maralixibat improves event-free survival in patients with ALGS.
INTRODUCTION
Alagille syndrome (ALGS) is the most common form of familial intrahepatic cholestasis and is characterized by bile duct paucity, congenital cardiac disease, ocular and skeletal abnormalities, vascular and renal anomalies, and characteristic facial features. Although ALGS-related liver disease can be highly variable, approximately three-quarters of patients have pruritus that is debilitating and frequently refractory to medical therapy and has a negative impact on the quality of life.1,2 Other sequelae of bile duct paucity and intrahepatic retention of toxic bile acids are growth impairment, bone fractures, and biliary cirrhosis. Bile acids are thought to be a major contributor to pruritus, the most debilitating symptom of ALGS and a primary driver of liver transplantation, although the exact mechanism of cholestatic itch remains unclear.3,4 Treatment options for ALGS-related liver disease have generally been limited to supportive care that is directed toward optimizing fat-soluble vitamin levels and nutrition, as well as treating the pruritus with off-label antipruritic agents. Surgical biliary diversion (SBD) has also been used with varying efficacy to promote the disposal of accumulated bile constituents, including bile acids, from the body and thereby to mitigate the pruritus.5–7 Despite these efforts, 50% to 75% of patients with ALGS undergo a liver transplant by adulthood to manage the consequences of cholestasis, or complications associated with cirrhosis.8,9 In a recent analysis of >1000 patients with ALGS, most (72%) were transplanted for ≥1 complication of persistent cholestasis, including pruritus (69%), growth failure (54%), and xanthomas (49%).9
Maralixibat is an ileal bile acid transporter inhibitor that was recently approved by the US Food and Drug Administration and the European Medicines Agency as a pharmacologic therapy in ALGS.10,11 The current indications are limited to cholestatic pruritus.10,11 Maralixibat disrupts the enterohepatic circulation of bile acids by inhibiting their reuptake in the ileum.12 In the pivotal clinical trial (ICONIC; NCT02160782) of maralixibat for children with ALGS, participants demonstrated significant improvement in pruritus with 84% of patients experiencing a pruritus response within the first 48 weeks of treatment, as well as a significant decrease in serum bile acid (sBA). These effects were maintained over time in patients who remained on maralixibat with treatment up to 204 weeks.13 Moreover, improvements in growth, xanthomas, and quality of life were observed versus baseline. Findings of improved pruritus and reduced sBA levels were also observed in two additional randomized clinical trials of maralixibat in children with ALGS.14,15 While all three prior studies affirm the benefit of maralixibat to improve cholestatic pruritus and decrease sBA, its impact on long-term outcomes such as transplantation or SBD has not been previously studied, given that ALGS is a rare condition and that these outcomes are infrequently seen in clinical trials with shorter follow-up. All three studies included optional, open-label, long-term extensions with data now available for up to 6 years of follow-up. These long-term extension studies did not have a corresponding control group because longer-term placebo-controlled studies are not feasible or ethical for children in whom pruritus is debilitating. The G lobal AL agille A lliance (GALA) study, with >1400 patients with ALGS from 29 countries, was developed with the aim of advancing our understanding of the natural history and outcomes of ALGS. As such, the GALA cohort encompasses real-world data on natural history and provides a unique opportunity to compare the long-term outcomes of patients with ALGS treated with maralixibat with untreated controls.
In this analysis, we compare long-term outcomes among individuals with ALGS treated with maralixibat in three placebo-controlled studies and open-label extensions for up to 6 years with a harmonized, aligned, natural history external control cohort from the GALA research database. This statistical approach is novel in pediatrics and allows for the comparison of single-arm clinical trial data with balanced real-world controls. The primary outcomes included event-free survival [EFS; the absence of manifestations of portal hypertension (PHT), biliary diversion, liver transplant, or death] and transplant-free survival (TFS; the absence of liver transplant or death). While the prior published studies of maralixibat focused on short-term outcomes of pruritus and biochemistry, these long-term primary outcomes have not yet been examined in patients treated with maralixibat and are of critical clinical importance.
EXPERIMENTAL PROCEDURES
Patient selection
The control cohort was selected from GALA and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Figure 1). The organization of GALA has been described.9,16 The GALA control cohort for the current analysis consisted of individuals who received standard-of-care treatment for ALGS but were not treated with maralixibat or another ileal bile acid transporter inhibitor. Standard-of-care in GALA represents the standard management of cholestasis, including treatments for pruritus such as antihistamines, rifampicin, cholestyramine, and nutritional support, including fat-soluble vitamins. Individuals from the GALA control cohort were selected based on key inclusion and exclusion criteria from the maralixibat clinical studies, using parameters that were available within the GALA clinical research database (Supplemental Methods, http://links.lww.com/HEP/I186). During the entire selection process of this aligned cohort, the statistician was blinded to the outcomes. The cohort used for comparison consisted of 84 participants with ALGS treated with maralixibat, who participated in long-term studies that have been described elsewhere.13,14 The maralixibat trials followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.17 Of note, cardiac disease was not considered as an exclusion criterion for any of these trials. All aspects of this study were approved by Institutional Review/Ethics Boards at the centers providing data, and informed consent was obtained when appropriate.
FIGURE 1.
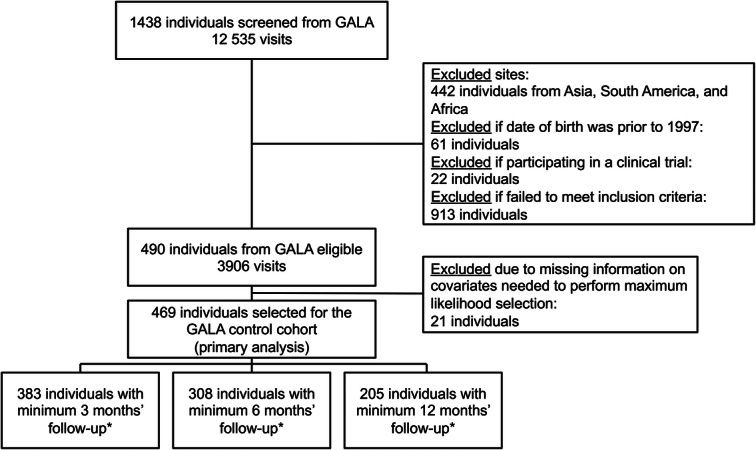
Selection of the GALA control group. *A minimum amount of follow-up time was considered to avoid immortal time bias (ie, an early observed effect due to survivor treatment selection bias). Abbreviation: GALA, Global ALagille Alliance.
Index time
The index time represents the start of follow-up. For the maralixibat cohort, the index time was defined as the date of the first dose of the drug (Supplemental Figure S1, http://links.lww.com/HEP/I186). For the GALA control cohort, patients may have fulfilled the inclusion and exclusion criteria at multiple time points during their disease trajectory and multiple time points were considered (Supplemental Figure S1, http://links.lww.com/HEP/I186). The primary analyses defined the patient-specific index time as the date where the maximum prediction within a patient of all eligible visits compared with the maralixibat cohort (yes/no) was achieved, referred to as “best fit,” applying a logistic regression analysis including age, sex, total bilirubin, and alanine aminotransferase (ALT) as covariates (Supplemental Methods, http://links.lww.com/HEP/I186). Sensitivity analyses were also performed to evaluate the impact of different index times (Supplemental Methods, http://links.lww.com/HEP/I186).
Balance assessment and model selection
The maralixibat and GALA control cohorts at baseline were assessed to confirm balance with respect to the prespecified covariates: age, sex, total bilirubin, and ALT; in addition, the balance of the distribution of gamma-glutamyl transferase (GGT) was investigated. Balance was achieved when the standard mean difference of all covariates was within the borders ± 0.25 (Supplemental Figure S2, http://links.lww.com/HEP/I186). In instances when balance was not achieved using standardized mean differences, two approaches were applied in the time-to-event analysis: (1) Cox regression adjusting for these covariates; and (2) assigning weights using either stabilized inverse probability of treatment weights (stabilized IPTWs) estimated by the propensity scores of the logistic regression: maralixibat yes/no or average treatment effect in the treated (ATT) weights.
Outcomes
An outcome was defined as the time to the first of the following events: manifestations of PHT (ie, varices requiring intervention at endoscopy or ascites requiring therapy), SBD, liver transplant, or death, and EFS was defined as the absence of these events. TFS was defined as the absence of a transplant or death. Hepatocellular carcinoma was prespecified to be excluded as an event. Duration of follow-up was the number of months from the index time until the end of follow-up (Supplemental Methods, http://links.lww.com/HEP/I186).
Sensitivity and subgroup analyses
A series of prespecified sensitivity analyses was performed to determine the robustness of findings: (1) different index times; (2) TFS as a secondary endpoint; (3) the impact of landmarking from follow-up for the first 3, 6, and 12 months to avoid selection biases that may occur in individuals who are potentially too sick to be included in a trial setting (immortal time bias)18; and (4) different approaches to propensity score weighting (ie, IPTW and ATT). Subgroup analyses explored potential variations in outcomes for (1) different geographic regions, (2) overlapping sites that were part of both GALA and maralixibat clinical trials, and (3) individuals for whom sBA data were available.
Statistical analysis
Final analysis datasets and follow-up data for maralixibat-treated participants with ALGS from maralixibat studies were provided to the GALA lead for an independent, blinded, statistical analysis in a staggered manner: first, demographics to permit selection of the GALA control cohort; second, initial outcomes; and lastly, final outcomes. Summaries of patient demographics, disease history, and baseline characteristics were tabulated for the different analysis populations. Kaplan-Meier analyses were performed, and corresponding figures were generated to compare the time to event between the cohorts. Cox regression models were applied to describe and test the differences between the maralixibat-treated cohort and the control cohort adjusting for potential confounders in different models or applying standardized IPTW/ATT weights depending on the balance assessment (Supplemental Methods, http://links.lww.com/HEP/I186).
Statistical inference was assessed at the α = 0.05 significance level and 95% CIs were produced. All analyses were conducted with SAS 9.3.
RESULTS
Study population
The analysis included 84 individuals treated with maralixibat and 469 individuals from the GALA control cohort. The cohorts were well-balanced for the set of prespecified demographic and laboratory characteristics: age, sex, total bilirubin, and ALT (Table 1). The standardized differences between the maralixibat and GALA control cohorts were within the prespecified border of ±0.25 (Supplemental Figure S2, http://links.lww.com/HEP/I186). In addition, there were no significant differences between the groups with respect to year of birth, region, variant type, or GGT. Although baseline sBA was higher in the maralixibat cohort [200 µmol/L (median, Q1: 81; Q3: 371)] versus the GALA control cohort [125 µmol/L (median, Q1: 39; Q3: 260); p = 0.003], these data were only available for 73 of 469 (16%) individuals from the GALA control cohort as sBA levels are not routinely monitored as part of standard-of-care. The mean follow-up/time to censoring in the maralixibat group was 4.4/5.0 years and 1.7/2.4 years in the GALA group. The mean follow-up period appears shorter in the GALA group; however, this represents truncation at the time of a clinical event.
TABLE 1.
Baseline demographics and characteristics
| Characteristic | Maralixibat cohort (n=84) | GALA control cohort (n=469) | P value |
|---|---|---|---|
| Sex | |||
| Male | 49 (58.3) | 274 (58.4) | 0.99 |
| Female | 35 (41.7) | 195 (41.6) | |
| Age at baseline, y | |||
| Median (Q1–Q3) | 5.6 (2.7–9.9) | 4.3 (2.2–9.6) | 0.078 |
| Year of birth | |||
| Median (Q1–Q3) | 2009 (2005–2012) | 2009 (2004–2013) | 0.25 |
| Region | |||
| Europe | 41 (48.8) | 229 (48.8) | 0.95 |
| North America | 34 (40.5) | 195 (41.6) | |
| Australia | 9 (10.7) | 45 (9.6) | |
| Variant | |||
| JAGGED1 | 81 (97.6) | 330 (95.1) | 0.55a |
| NOTCH2 | 2 (2.4) | 17 (4.9) | |
| Other/unknown | 1 (0.2) | 37 (9.6) | |
| Total bilirubin, mg/dL | |||
| Median (Q1–Q3) | 3.15 (1.00–8.15) | 1.99 (0.60–11.52) | 0.39 |
| <2 | 37 (44.0) | 235 (50.1) | 0.31 |
| ≥2 | 47 (56.0) | 234 (49.9) | |
| GGT, log10×ULN | |||
| Median (Q1–Q3) | 1.25 (0.93–1.44) | 1.24 (0.93–1.52) | 0.58 |
| GGT, ×ULN | |||
| <3 | 3 (3.6) | 6 (1.3) | 0.14a |
| ≥3 | 81 (96.4) | 463 (98.7) | |
| ALT, U/L | |||
| Median (Q1–Q3) | 145 (94–207) | 130 (75–203) | 0.12 |
| sBA,b µmol/L | |||
| Median (Q1–Q3) | 200 (81–371) | 125 (39–260) | 0.0033 |
All data are n (%) unless otherwise stated.
Due to >20% of the cells having expected counts <5, chi-square results may be invalid, and the Fisher exact test was used instead.
Baseline sBA data were available for 73 patients in the GALA control cohort. Approximately 85% of the sBA values were not available in the GALA clinical research database as frequent sBA measurement is not part of clinical practice.
Abbreviations: ALT, alanine aminotransferase; GALA, Global ALagille Alliance; GGT, gamma-glutamyl transferase; Q1, first quartile; Q3, third quartile; sBA, serum bile acid; ULN, upper limit of normal.
Outcomes
Over time (0 through year 6), within the maralixibat cohort, 21 patients had an event. These included 10 liver transplants, 4 SBDs, 3 patients who developed manifestations of PHT, and 4 deaths. Among the GALA control cohort, 163 patients had an event, including 110 liver transplants, 33 SBDs, 5 patients who developed manifestations of PHT, and 15 deaths. There were only two deaths due to cardiac disease (both within the GALA control cohort), and they were included in the analysis.
Comparison of EFS
EFS was increased for the maralixibat cohort compared with the GALA control cohort at 6 years of follow-up (71.4% vs. 50.0%; p<0.0001), with an unadjusted HR of 0.380; 95% CI, 0.238–0.604; p<0.0001 (Figure 2). After adjustment for age, sex, bilirubin, and ALT, EFS was significantly higher in the maralixibat cohort [adjusted hazard ratio (aHR), 0.305; 95% CI, 0.189–0.491; p<0.0001] indicating a 70% improvement in EFS with maralixibat treatment. Alternative models were considered, which included GGT, geographic region, and date of birth, and all models yielded similar findings of significantly improved EFS in the maralixibat cohort (Figure 3).
FIGURE 2.
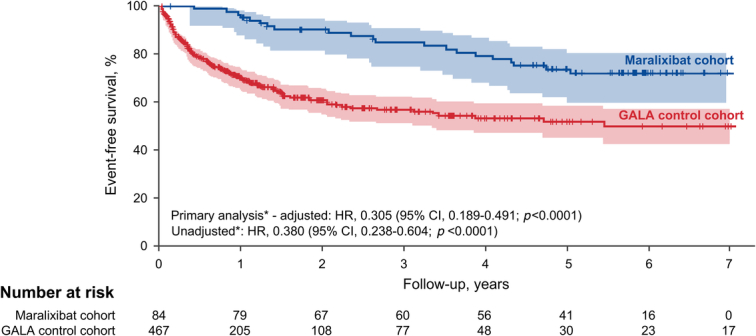
Kaplan-Meier estimates of EFS in the maralixibat and GALA control cohorts. The number at risk is the original number of participants (at time 0) minus those who had an event or were censored (eg, lost to follow-up) before the start of the given period. Shaded areas represent 95% CIs. *Cox regression models for the primary analysis: primary prespecified (adjusted), where the effect of maralixibat versus GALA log-likelihood test was adjusted for age, sex, bilirubin, and alanine aminotransferase (according to the statistical analysis plan), and primary unadjusted. Abbreviations: EFS, event-free survival; GALA, Global ALagille Alliance.
FIGURE 3.
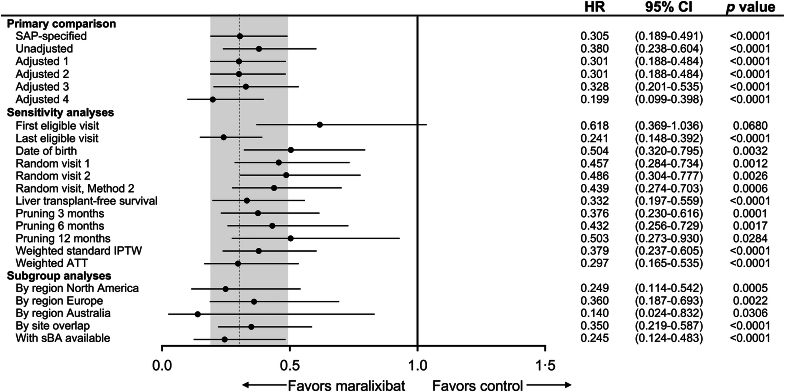
Primary, sensitivity, and subgroup analyses of EFS. Sensitivity and subgroup analyses for the primary comparison included SAP-specified analyses (Cox regression model adjusted for age, sex, total bilirubin, and ALT); Unadjusted [univariate Cox proportional hazards model that only contains treatment as a covariate (EFS)]; Adjusted 1 (Cox regression model adjusted for age, total bilirubin, and GGT); Adjusted 2 (Cox regression model adjusted for age, total bilirubin, GGT, ALT, and region); Adjusted 3 (Cox regression model adjusted for age, total bilirubin, GGT, ALT, sex, and year of birth); and Adjusted 4 (Cox regression model adjusted for age, total bilirubin, GGT, and sBA). Random visit 1 and random visit 2 represent a visit randomly selected uniformly among all eligible visits. Random visit, Method 2 first selected a year at random among all eligible visits and then randomly selected a visit within that year. This was done to account for participants who would have “clusters” of visits due to hospitalization that could skew the selection of a random visit toward that cluster. Landmark time points [x] removed events occurring within either group within the first [x] months of the selected index time. Abbreviations: ALT, alanine aminotransferase; ATT, average treatment effect in the treated; EFS, event-free survival; GGT, gamma-glutamyl transferase; IPTW, inverse probability of treatment weights; SAP, statistical analysis plan; sBA, serum bile acid.
Sensitivity analyses
Additional index times for individuals from the GALA control cohort were selected for sensitivity analyses to assess the robustness of the primary analysis. Using the first eligible visit as the index time so that events occur with maximum possible follow-up, a trend toward improved EFS was evident in the maralixibat-treated cohort compared with the GALA control cohort (aHR, 0.618; 95% CI, 0.369–1.036; p=0.068). In contrast, when using the last eligible visit as the index time, EFS was significantly improved in the maralixibat cohort compared with the GALA control cohort (aHR, 0.241; 95% CI, 0.148–0.392; p<0.0001). With the use of date of birth (for both the maralixibat and GALA control cohorts) as the index time, EFS was significantly higher in the maralixibat cohort compared with the GALA control cohort (aHR, 0.504; 95% CI, 0.320–0.795; p = 0.0032). Three index visits for the start of follow-up were selected at random to further determine the robustness of primary findings and produced similar inferences from the primary analysis for random visit 1 (aHR, 0.457; 95% CI, 0.284–0.734; p = 0.0012), random visit 2 (aHR, 0.486; 95% CI, 0.304–0.777; p = 0.0026), and random visit 3 (aHR, 0.439; 95% CI, 0.274–0.703; p = 0.0006).
In an additional sensitivity analysis that defined an event as liver transplantation or death (ie, TFS), maralixibat-treated individuals had improved TFS compared with the GALA control cohort, after adjusting for age, sex, total bilirubin, and ALT (aHR, 0.332; 95% CI, 0.197–0.559; p<0.0001).
A third set of sensitivity analyses explored the impact of immortal time bias (selection bias for healthier patients to choose a clinical trial) by landmarking time points shortly after inclusion for comparison with the maralixibat cohort. EFS continued to be improved in the maralixibat-treated cohort compared with the GALA control cohort when events were pruned at 3 months (aHR, 0.376; 95% CI, 0.230–0.616; p = 0.0001; Figure 4A), 6 months (aHR, 0.432; 95% CI, 0.256–0.729; p = 0.0017; Figure 4B), and 12 months (aHR, 0.503; 95% CI, 0.273–0.930; p = 0.0284; Figure 4C). A final set of sensitivity analyses explored alternative approaches to balancing the maralixibat and GALA control cohorts. Using weighted standardized IPTW yielded similar results to those of the primary analysis with higher EFS in the maralixibat cohort than in the GALA control cohort (aHR, 0.379; 95% CI, 0.237–0.605; p<0.0001), and similar findings were seen when using weighted ATT (aHR, 0.297; 95% CI, 0.165–0.535; p<0.0001).
FIGURE 4.
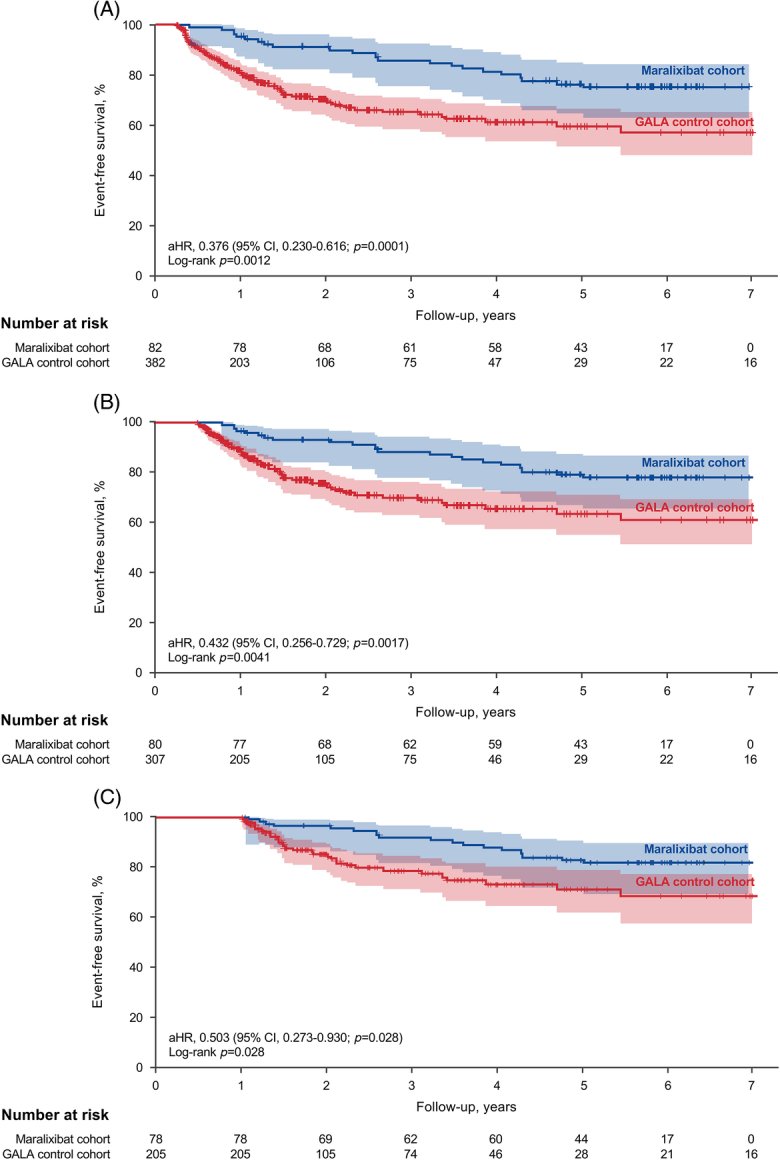
Kaplan-Meier estimates of EFS in the maralixibat and GALA control cohorts with landmark time points for events occurring in the first (A) 3 months, (B) 6 months, and (C) 12 months. The number at risk is the original number of participants (at time 0) minus those who had an event or were censored (eg, lost to follow-up) before the start of the given period. Participants who had events within the first 3, 6, or 12 months were excluded from the analysis (thus, there are no events within these respective periods). This was done to avoid immortal time bias. Shaded areas represent 95% CIs. Abbreviations: aHR, adjusted hazard ratio; EFS, event-free survival; GALA, Global ALagille Alliance.
Subgroup analyses
Analyses of the impact of maralixibat on EFS were stratified by region for individuals from North America, Europe, and Australia. After adjustment for age, sex, total bilirubin, and ALT, improved EFS was observed in maralixibat-treated individuals from North America (aHR, 0.249; 95% CI, 0.114–0.542; p = 0.0005; Figure 3). Similar improvement in EFS was observed in maralixibat-treated individuals from Europe (aHR, 0.360; 95% CI, 0.187–0.693; p = 0.0022) and Australia (aHR, 0.140; 95% CI, 0.024–0.832; p = 0.0306). EFS at overlapping sites (ie, sites that offered both maralixibat clinical trials and participated in GALA; Supplemental Table S1, http://links.lww.com/HEP/I186) was improved for maralixibat-treated individuals (aHR, 0.359; 95% CI, 0.219–0.587; p<0.0001).
Impact of sBA measurement
The inclusion of sBA in the model when available (maralixibat cohort, n = 84; GALA untreated cohort, n = 73) indicated improved EFS following treatment with maralixibat (aHR, 0.245; 95% CI, 0.124–0.483; p<0.0001). To determine whether the limited sBA results in GALA introduce a selection bias, demographic and laboratory values for individuals within GALA who had sBA results available were compared with values of individuals for whom these data were not available (Supplemental Table S2, http://links.lww.com/HEP/I186). Individuals within GALA with and without sBA results had similar baseline biochemical parameters. In addition, there was no difference in the survival curve for those in GALA with and without sBA results, suggesting that the lack of availability of these data in most individuals would not introduce a bias (log-rank p = 0.3359; Supplemental Figure S3, http://links.lww.com/HEP/I186).
DISCUSSION
This analysis incorporates data from three clinical trials of maralixibat in children with ALGS and compares long-term clinically important events in this treated population with the largest global, real-world, natural history cohort of children with ALGS. In a prespecified analysis, maralixibat treatment was associated with a 70% improvement in EFS and a 67% improvement in TFS. This finding of improved EFS was robust and was not dependent on varying approaches to (1) aligning the initial time of follow-up, (2) counting events after the landmark time points, or (3) propensity score matching each cohort. Only the model that was selected for a first eligible visit as the index time failed to show an improvement in EFS for the maralixibat cohort, which may be expected, given that by selecting the first eligible time point, individuals in GALA will necessarily have longer follow-up time until events. Apart from baseline consideration of age and bilirubin, additional adjustments of the model yielded nearly identical results, including a comparison of people from the same center to account for potential variations in standard-of-care practices. The primary finding is not attributable to variations in cardiac disease since there were only two cardiac-related deaths in the GALA cohort.
Historical control comparison is useful when there are ethical concerns regarding the recruitment of patients for long-term control arms requiring several years of study in life-threatening or debilitating diseases.19 In addition, historical controls make the generation of controlled data in rare disease trials feasible.19 ALGS is rare, and the consequences of cholestasis, including pruritus, xanthomas, and growth failure are severe, greatly affecting the quality of life, such that these are leading indications for liver transplantation.9 This setting is therefore the ideal condition for which to invoke a real-world comparison cohort rather than a several-years-long control group.20 The feasibility of selecting a natural history cohort to provide real-world data in ALGS was recently demonstrated by the Childhood Liver Disease Research Network (ChiLDReN), although actual comparisons to trial patients were not performed in this analysis.21 Further, the methodology used in the current analysis has recently been successfully implemented in primary biliary cholangitis to demonstrate improved TFS in patients following treatment with obeticholic acid compared with control patients from two external registries.22 However, this statistical approach has not previously been applied to a pediatric cohort, and this study clearly demonstrates its utility in children, especially those with rare diseases.
A recent study conducted by Sokol et al identified predictors of EFS in patients with ALGS treated with maralixibat and revealed that improvement in pruritus over 48 weeks, and lower bilirubin and sBA levels at week 48 were associated with fewer clinically important events. The study population in that analysis was well-defined from the outset, per the maralixibat clinical program, and the question answered was which variables (with cut points) differentiated maralixibat responders versus nonresponders. This analysis of improvements in adverse hepatic events was made only in patients enrolled in the clinical trials, all of whom received maralixibat. Most importantly, Sokol et al’s paper lacked an external comparator cohort and therefore no definitive conclusions could be drawn regarding the efficacy of maralixibat in improving EFS in ALGS.23 The current analysis identifies a subset of patients from a real-world database, GALA, aligned to be as similar as possible to patients who were enrolled in the clinical trials. This permitted the creation of a placebo/control cohort to enable, for the first time, an analysis of EFS in patients with ALGS treated with maralixibat versus real-world data.
In this prospectively defined analysis, the finding of improved EFS in maralixibat-treated patients has been shown to be robust, and it is intriguing to explore the potential explanations. The indications for liver transplantation in ALGS are composite, typically encompassing multiple manifestations of cholestasis, such as pruritus, growth failure, and xanthomas. Of these, pruritus is the leading indication in 69% of patients with ALGS.9 Thus, with an efficacious reduction in pruritus, the main indication for liver transplantation may be mitigated. However, since pruritus is rarely the sole indication for liver transplantation in ALGS, and other features, such as growth failure, typically coexist, it is worth considering a broader mechanistic explanation for the improvement in EFS in patients treated with maralixibat. In the pivotal ICONIC study, sBA levels were significantly reduced,13 and it is plausible that this leads to reductions in intrahepatic accumulation of bile acids with reduced hepatic toxicity. This has been demonstrated in mouse models of cholestasis.24 Indeed, the ICONIC study noted positive clinical impacts beyond pruritus, such as reductions in xanthomas and improvement in growth. Thus, although it might be assumed that an improvement in pruritus is the only reason for the observed improvements in EFS seen in the current analysis, broad-based clinical improvements observed in the ICONIC study make this unlikely.13 Interruption of enterohepatic circulation of bile acids and theoretically depleting retained hepatic bile acids may result in improved disease biology to account for the improvement in EFS with maralixibat treatment.
Although our findings were robust across several subgroup and sensitivity analyses, one important limitation when matching to an external control is that there may be important baseline differences that could not be assessed and incorporated to optimally balance both groups. Specifically, we were unable to evaluate baseline differences in pruritus and sBA. Pruritus severity cannot be assessed on a retrospective basis because there is no validated measurement tool that is consistently used by physicians outside of a clinical study setting. Given the importance of pruritus as a manifestation of cholestasis in ALGS, it is included in the GALA data collection as a binary variable, but without an assessment of severity. sBA levels are also largely unavailable in the GALA database because although it is understood that patients with ALGS often have markedly elevated sBA levels, these are not routinely measured in clinical practice. Instead, clinicians rely on standard biochemical markers to assess the severity of cholestasis. To mitigate this limitation, a sensitivity analysis was performed utilizing the subset of GALA patients in whom sBA levels were available, and the results were consistent with the primary result.
This analysis incorporates data from both the largest natural history clinical research database in ALGS with robust and high-quality data and the largest and longest interventional dataset of patients with ALGS enrolled in clinical trials of maralixibat. The novelty of the current study is that it specifically assessed the effect of maralixibat treatment on clinically important liver outcomes (manifestations of PHT, liver transplantation, and death), differentiating it from prior published studies, which focused on pruritus and biochemical endpoints only. Prespecified methodologies were applied to overcome potential biases, which are inherent to any natural history comparison. It is accepted that not every potential confounder can be controlled for in the use of real-world data; however, the statistical rigor applied in this analysis, including the patient selection process and the multiple sensitivity and subgroup analyses, demonstrates the application of such an analysis and reinforces the robustness of the primary result. In addition to demonstrating the power of real-world evidence to generate a control cohort, these findings hold promise for children with cholestatic liver disease related to ALGS. To date, the indications for maralixibat have rested on amelioration of pruritus, which has been clearly demonstrated13,14; however, the reduction of bile acids observed with maralixibat affects additional manifestations of cholestasis (xanthomas, growth failure), all of which contribute to decision-making in the listing for liver transplantation in ALGS. These data are the first to demonstrate a 6-year survival benefit in patients with ALGS using a pharmacologic interventional therapy, suggesting that maralixibat may delay or obviate the need for liver transplantation. This may lead to a paradigm shift in the potential treatment indications for maralixibat beyond pruritus control to a plausible treatment for cholestatic liver disease.
Supplementary Material
DATA AVAILABILITY STATEMENT
Beginning 6 months and ending 5 years after publication, deidentified participant data from the maralixibat clinical trials for data meta-analysis might be made available to investigators whose proposed use of the data has been approved by a review committee, including the primary authors. The study protocol will also be available by means of weblink. Proposals should be directed to Binita M. Kamath (binita.kamath@sickkids.ca). Before being granted access, data requesters will be required to sign a data access agreement.
AUTHOR CONTRIBUTIONS
All authors contributed to data collection, analysis and interpretation, and writing and review of the manuscript. Bettina E. Hansen and Binita M. Kamath also contributed to the design of the study. Bettina E. Hansen, Binita M. Kamath, and Shannon M. Vandriel had access to and verified the data and were responsible for the decision to submit the manuscript. All authors confirm the fidelity of the study to the protocol, accuracy, and completeness of data, and approved manuscript publication. The corresponding author had full access to study data and had final responsibility for manuscript submission.
ACKNOWLEDGMENTS
Editorial assistance was provided by Jeni Fagan, PhD, and Islay Steele, PhD, of Health Interactions, and Charlene Counsellor, ELS, and Eliza Prangley, PhD, of PRECISIONscientia, funded by Mirum Pharmaceuticals, Inc.
FUNDING INFORMATION
Funding for GALA was provided by the Alagille Syndrome Alliance, Mirum Pharmaceuticals, Inc., and Albireo Pharma, Inc., through unrestricted educational grants to the Hospital for Sick Children (SickKids Foundation).
CONFLICTS OF INTEREST
Bettina E. Hansen consults and received grants from Calliditas, CymaBay, and Intercept. She consults for Albireo, Enyo, Eiger, Ipsen, and Mirum. Shannon M. Vandriel consults and received grants from Mirum. She consults for Albireo. Pamela Vig is employed by and owns stock in Mirum. Will Garner is employed by and owns stock in Mirum. Douglas B. Mogul is employed by and owns stock in Mirum. Kathleen M. Loomes consults and received grants from Albireo and Mirum. She consults for Travere. Dorota Gliwicz-Miedzińska received grants from ExCEEd Orphan. Emmanuel M. Gonzales consults, advises, is on the speakers’ bureau, and received grants from Albireo and Mirum. He consults and is on the speakers’ bureau for CTRS. He consults for Vivet. Emmanuel Jacquemin consults for CTRS and Vivet. Lorenzo D’Antiga consults and advises Albireo and Mirum. He consults for Advanz, Spark, and Tome. He advises Alexion, AstraZeneca, Genespire, Selecta, and Vivet. Emanuele Nicastro consults for Albireo and Mirum. Henrik Arnell advises Mirum. He is employed by Albireo and Baxter. Étienne Sokal consults, advises, owns stock, holds intellectual property rights, and has other interests with Cellaion. He consults for Ipsen and Mirum. Saul J. Karpen consults for Albireo, HemoShear, Intercept, and Mirum. Rene Romero consults for Albireo and Mirum. He received grants from Gilead. Noelle H. Ebel consults and is on the speakers’ bureau for Ipsen and Mirum. Shikha S. Sundaram advises Albireo and Mirum. Ryan T. Fischer advises and is on the speakers’ bureau for Albireo, Ipsen, and Mirum. Henry C. Lin advises Albireo and Mirum. M. Kyle Jensen consults, advises, and received grants from Albireo. He consults for GLG and Guidepoint Global. Palaniswamy Karthikeyan consults for Cepton Strategies. He advises Albireo. Giuseppe Indolfi advises Albireo and Mirum. Henkjan J. Verkade consults and advises Albireo and Mirum. Pamela L. Valentino is on the speakers’ bureau for Mirum. Antal Dezsőfi advises Mirum. Kathleen Schwarz consults and advises Sarepta. She consults for Mirum and Up to Date. She advises and received grants from Albireo and Gilead. She is employed by NASPGHAN. Pier Luigi Calvo advises Albireo, Ipsen, Mirum, and Nestlé. Andréanne N. Zizzo advises Mirum. Gabriella Nebbia consults and advises Albireo. Rima Fawaz consults for Albireo, the Hepatic Adjudication Committee, and Mirum. Wikrom Karnsakul received grants from Albireo, Gilead, Mirum, and Travere. Cristina Molera Busoms advises Alexion, Mirum, and Orphalan. Deirdre A. Kelly consults, advises, received grants, and is employed by Albireo and Mirum. She consults, advises, and received grants from Intercept. She consults and advises AstraZeneca. She consults for Alnylam, Freeline, GlaxoSmithKline, Orphalan, and Takeda. She received grants from AbbVie and Gilead. Jesus M. Banales consults, advises, and received grants from Albireo and CymaBay. He consults and advises Ikan Biotech and OWL-Rubio Metabolomics. He is on the speakers’ bureau and received grants from Incyte. He is on the speakers’ bureau for AstraZeneca and Intercept. He received grants from Roche. Quais Mujawar advises Medison and Mirum. He owns stock in Bausch Health and Sun Pharma. Orith Waisbourd-Zinman consults for Neopharm Israel. Sabina Wiecek is employed by the Department of Pediatrics, Medical University of Silesia. Raquel Borges Pinto is employed by Hospital Criança Conceição. Nanda Kerkar advises Albireo, Alexion, Ipsen, and Mirum. She received royalties from Elsevier. Jernej Brecelj is on the speakers’ bureau for Swixx Biopharma. Eberhard Lurz consults, advises, and is on the speakers’ bureau for Ipsen, Mirum, Nutricia, and Takeda. Richard J. Thompson consults and owns stock in Generation Bio and Rectify Pharma. He consults for Albireo, Alnylam, and Mirum. Binita M. Kamath consults and received grants from Albireo and Mirum. She consults for Astellas. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: aHR, adjusted hazard ratio; ALGS, Alagille syndrome; ALT, alanine aminotransferase; ATT, average treatment effect in the treated; ChiLDReN, Childhood Liver Disease Research Network; CONSORT, Consolidated Standards of Reporting Trials; EFS, event-free survival; GALA, Global ALagille Alliance; GGT, gamma-glutamyl transferase; IPTW, inverse probability of treatment weights; PHT, portal hypertension; sBA, serum bile acid; SBD, surgical biliary diversion; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; TFS, transplant-free survival.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Antal Dezsőfi, Email: dezsofi.antal@med.semmelweis-univ.hu.
James E. Squires, Email: James.Squires2@chp.edu.
Kathleen Schwarz, Email: kschwarz@rchsd.org.
Pier Luigi Calvo, Email: pcalvo@cittadellasalute.to.it.
Jesus Quintero Bernabeu, Email: jquintero@vhebron.net.
Andréanne N. Zizzo, Email: andreanne.zizzo@lhsc.on.ca.
Gabriella Nebbia, Email: gabriella.nebbia@policlinico.mi.it.
Pinar Bulut, Email: obulut@phoenixchildrens.com.
Ermelinda Santos-Silva, Email: ermelinda.dca@chporto.min-saude.pt.
Rima Fawaz, Email: Rima.Fawaz@yale.edu.
Silvia Nastasio, Email: silvia.nastasio@childrens.harvard.edu.
Wikrom Karnsakul, Email: wkarnsa1@jhmi.edu.
María Legarda Tamara, Email: maria.legardatamara@osakidetza.eus.
Cristina Molera Busoms, Email: cristinamolera@gmail.com.
Deirdre A. Kelly, Email: deirdre@kellyda.co.uk.
Thomas Damgaard Sandahl, Email: thomsand@rm.dk.
Carolina Jimenez-Rivera, Email: cajimenez@cheo.on.ca.
Jesus M. Banales, Email: jesus_banales@yahoo.es.
Quais Mujawar, Email: Qmujawar@hsc.mb.ca.
Li-Ting Li, Email: liliting3764@163.com.
Huiyu She, Email: everyshylala@163.com.
Jian-She Wang, Email: jshwang@shmu.edu.cn.
Kyung Mo Kim, Email: kimkyungmo1@gmail.com.
Seak Hee Oh, Email: seakhee.oh@amc.seoul.kr.
Maria Camila Sanchez, Email: mcamilasanchez@gmail.com.
Maria Lorena Cavalieri, Email: maria.cavalieri@hospitalitaliano.org.ar.
Way Seah Lee, Email: leews@um.edu.my.
Christina Hajinicolaou, Email: Christina.Hajinicolaou@wits.ac.za.
Chatmanee Lertudomphonwanit, Email: chatmanee.puk@gmail.com.
Orith Waisbourd-Zinman, Email: oritwz@gmail.com.
Cigdem Arikan, Email: cigdemarikanmd@yahoo.com.
Seema Alam, Email: seema_alam@hotmail.com.
Elisa Carvalho, Email: elisacarvalho@terra.com.br.
Melina Melere, Email: mel_melere@hotmail.com.
John Eshun, Email: jeshun@uthsc.edu.
Zerrin Önal, Email: onalzerrin@gmail.com.
Dev M. Desai, Email: dev.desai@utsouthwestern.edu.
Sabina Wiecek, Email: sabinawk@wp.pl.
Raquel Borges Pinto, Email: raquelborgespinto@gmail.com.
Victorien M. Wolters, Email: V.M.Wolters@umcutrecht.nl.
Jennifer Garcia, Email: JGarcia2@med.miami.edu.
Marisa Beretta, Email: rees.beretta@gmail.com.
Nanda Kerkar, Email: nkerkar@mgh.harvard.edu.
Jernej Brecelj, Email: jernej.brecelj@kclj.si.
Nathalie Rock, Email: Nathalie.Rock@hcuge.ch.
Eberhard Lurz, Email: Eberhard.Lurz@med.uni-muenchen.de.
Niviann Blondet, Email: niviann.blondet@seattlechildrens.org.
Uzma Shah, Email: ushah1@hfhs.org.
Richard J. Thompson, Email: richard.j.thompson@kcl.ac.uk.
Binita M. Kamath, Email: binita.kamath@sickkids.ca.
REFERENCES
- 1.Kamath BM, Baker A, Houwen R, Todorova L, Kerkar N. Systematic review: The epidemiology, natural history, and burden of Alagille syndrome. J Pediatr Gastroenterol Nutr. 2018;67:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamath BM, Spino C, McLain R, Magee JC, Fredericks EM, Setchell KD, et al. Unraveling the relationship between itching, scratch scales, and biomarkers in children with Alagille syndrome. Hepatol Commun. 2020;4:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath BM, Stein P, Houwen RHJ, Verkade HJ. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. 2020;40:1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergasa NV. Pruritus of cholestasis In: Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014:61–88. [Google Scholar]
- 5.Emerick KM, Elias MS, Melin-Aldana H, Strautnieks S, Thompson RJ, Bull LN, et al. Bile composition in Alagille syndrome and PFIC patients having partial external biliary diversion. BMC Gastroenterol. 2008;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang KS, Tiao G, Bass LM, Hertel PM, Mogul D, Kerkar N, et al. Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology. 2017;65:1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemoine C, Superina R. Surgical diversion of enterohepatic circulation in pediatric cholestasis. Semin Pediatr Surg. 2020;29:150946. [DOI] [PubMed] [Google Scholar]
- 8.Kamath BM, Ye W, Goodrich NP, Loomes KM, Romero R, Heubi JE, et al. Outcomes of childhood cholestasis in Alagille syndrome: Results of a multicenter observational study. Hepatol Commun. 2020;4:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandriel SM, Li LT, She H, Wang JS, Gilbert MA, Jankowska I, et al. Natural history of liver disease in a large international cohort of children with Alagille syndrome: Results from the GALA study. Hepatology. 2023;77:512–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livmarli . Prescribing Information. Mirum Pharmaceuticals Inc; 2023. [Google Scholar]
- 11.Livmarli . Summary of Product Characteristics. Mirum Pharmaceuticals International B.V. Accessed July 6, 2023. https://www.ema.europa.eu/documents/product-information/livmarli-epar-product-information_en.pdf. [Google Scholar]
- 12.Shirley M. Maralixibat: First approval. Drugs. 2022;82:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzales E, Hardikar W, Stormon M, Baker A, Hierro L, Gliwicz D, et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): A randomised phase 2 study. Lancet. 2021;398:1581–1592. [DOI] [PubMed] [Google Scholar]
- 14.Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo-controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in Alagille syndrome. Hepatol Commun. 2018;2:1184–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shneider BL, Spino CA, Kamath BM, Magee JC, Ignacio RV, Huang S, et al. Impact of long-term administration of maralixibat on children with cholestasis secondary to Alagille syndrome. Hepatol Commun. 2022;6:1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Houwelingen HC. Dynamic prediction by landmarking in event history analysis. Scand J Statist. 2007;34:70–85. [Google Scholar]
- 19.Ghadessi M, Tang R, Zhou J, Liu R, Wang C, Toyoizumi K, et al. A roadmap to using historical controls in clinical trials—By Drug Information Association Adaptive Design Scientific Working Group (DIA-ADSWG. Orphanet J Rare Dis. 2020;15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millum J, Grady C. The ethics of placebo-controlled trials: Methodological justifications. Contemp Clin Trials. 2013;36:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shneider BL, Kamath BM, Magee JC, Goodrich NP, Loomes KM, Ye W, et al. Use of funded multicenter prospective longitudinal databases to inform clinical trials in rare diseases--Examination of cholestatic liver disease in Alagille syndrome. Hepatol Commun. 2022;6:1910–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murillo Perez CF, Fisher H, Hiu S, Kareithi D, Adekunle F, Mayne T, et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology. 2022;163:1630–1642.e3. [DOI] [PubMed] [Google Scholar]
- 23.Sokol R, Gonzales EM, Kamath BM, Baker A, Vig P, Mogul DB, et al. Predictors of 6-year event-free survival in alagille syndrome patients treated with maralixibat, an ileal bile acid transporter inhibitor. Hepatology. 2023;78:1698–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miethke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]