Abstract
Major depressive disorder (MDD) is associated with T cell activation, but no studies have examined the combined effects of T cell activation and deficits in T regulatory (Treg) cells on the severity of acute phase MDD. Using flow cytometry, we determined the percentage and median fluorescence intensity of CD69, CD71, CD40L, and HLADR-bearing CD3+, CD4+, and CD8+ cells, and cannabinoid type 1 receptor (CB1), CD152 and GARP (glycoprotein A repetitions predominant)-bearing CD25+ FoxP3 T regulatory (Treg) cells in 30 MDD patients and 20 healthy controls in unstimulated and stimulated (anti-CD3/CD28) conditions. Based on cytokine levels, we assessed M1 macrophage, T helper (Th)-1 cell, immune-inflammatory response system (IRS), T cell growth, and neurotoxicity immune profiles. We found that the immune profiles (including IRS and neurotoxicity) were significantly predicted by decreased numbers of CD152 or GARP-bearing CD25+ FoxP3 cells or CD152 and GARP expression in combination with increases in activated T cells (especially CD8+ CD40L+ percentage and expression). MDD patients showed significantly increased numbers of CD3+ CD71+, CD3+ CD40L+, CD4+ CD71+, CD4+ CD40L+, CD4+ HLADR+, and CD8+ HLADR+ T cells, increased CD3+ CD71+, CD4+ CD71+ and CD4+ HLADR+ expression, and lowered CD25+ FoxP3 expression and CD25+ FoxP+ CB1+ numbers as compared with controls. The Hamilton Depression Rating Scale score was strongly predicted (between 30 and 40% of its variance) by a lower number of CB1 or GARP-bearing Treg cells and one or more activated T cell subtypes (especially CD8+ CD40L+). In conclusion, increased T helper and cytotoxic cell activation along with decreased Treg homeostatic defenses are important parts of MDD that lead to enhanced immune responses and, as a result, neuroimmunotoxicity.
Subject terms: Immunology, Neuroscience, Psychology
Introduction
Depression is a major mental health concern that is becoming a more widespread global issue. In 2015, the World Health Organization estimated that approximately 4.4% of the world's population, or approximately 322 million people, were affected by depression1. Additionally, this report found that a similar percentage of the Thai population, or approximately 2.8 million people, suffers from depression1.
There is now evidence that major depressive disorder (MDD) and bipolar disorder (BD) are associated with immune-inflammatory pathway activation. Studies have demonstrated that inflammatory markers, such as cytokines, chemokines, complement factors, and acute phase reactants, are elevated in depressed individuals2–5. Both MDD and BD are characterized by activation of the immune-inflammatory response system (IRS) as indicated by elevated levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8 (CXCL8), IL-12, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α6, and the compensatory immune-regulatory system (CIRS), which prevents hyperinflammation by downregulating the IRS and producing anti-inflammatory cytokines, such as IL-4, IL-10 and transforming growth factor (TGF) -β6. Under physiological conditions, IRS and CIRS interact in a balanced manner6. During acute mood episodes, such as depressive episodes and mania, this balance is disrupted, resulting in an increase in the production of IRS and CIRS cytokines and a new IRS/CIRS setpoint6–8. This is significant because certain IRS and CIRS cytokines have neurotoxic effects that may cause functional injury to neuronal brain circuits, particularly neuronal and astroglial projections9.
As is the case with IRS/CIRS cytokines, there is evidence that imbalances in the number of T helper (CD4+) and T cytotoxic (CD8+) T cells and T regulatory (Treg) cells may contribute to the development of severe mood disorders10–12. Flow cytometry-based immunophenotyping is a method for identifying activated T cells that contribute to IRS and CIRS profiles by producing pro- and anti-inflammatory cytokines. This technique enables us to distinguish between various categories of activated T effector (Teff) and Treg cells. Activated CD4+ and CD8+ cells that express activation markers such as CD69+, CD71+, CD40L+ (CD154+), and HLA-DR+ contribute to the IRS10,13,14 by producing pro-inflammatory cytokines, including IL-2, IL-17 and IFN-γ6,7,10,13,14. Activated Treg cells, including CD4+ CD25+ FoxP3+ cells that can express the cannabinoid receptor CB1, CD152 (CTLA-4) or glycoprotein A repetitions predominant (GARP)7,10,13–15, promote CIRS by regulating the immune response and/or generating anti-inflammatory cytokines such as IL-10 and TGF-β16. By modulating the production and secretion of pro-inflammatory cytokines17, Treg cells play a crucial role in preserving immune balance and promoting immune tolerance. In animal studies, Treg depletion may amplify immune-inflammatory pathways and result in autoimmunity18.
Using flow cytometry results, early precision medicine studies demonstrated that major depression, particularly melancholia, is qualitatively distinct from controls and minor depressed patients19. The most significant CD markers were elevated CD25+ and HLA-DR+ expression on CD4+ T cells, indicating T cell activation in depression and particularly melancholia19. In a separate study, Maes et al.20 determined that 64% of patients with MDD had increased expression of CD7+ CD25+ and CD2+ HLADR+ cells with a specificity of 91%. According to these findings, numerous patients with MDD exhibit T cell activation. The soluble transferrin receptor (sTfR or sCD71) and sIL-2R (CD25+) are elevated in the serum of patients with MDD and BD21–23, corroborating these findings.
Through their involvement in modulating cytokine production, Treg cells have been found to potentially impact mood disorders. Individuals with MDD have lower Treg levels than healthy individuals17, and antidepressant-treated MDD patients have a greater number of CD4+ CD25+ and CD4+ CD25+ FOXP3+ Treg cells24. The remitted phase of BD is characterized by a suppression of Teff cells and an activation of Treg cells, while increasing severity of BD, as measured by duration or number of manic episodes, is associated with Teff cell and Treg cell aberrations10. Mice with depleted CD4+ CD25+ Treg cells exhibit elevated levels of despair behaviors and decreased 5-HT in the hippocampus25.
Nevertheless, no studies have examined whether activation of T cells and depletion of Treg cells are associated with increased severity of depression in an acute phase of MDD and whether MDD is characterized by very early (CD69+), early (CD71+), late (HLADR+) activation markers, and/or increases in CD40L+, a key player in T-cell dependent effector functions and humoral immunity26–29. Consequently, the purpose of this study is to quantify the levels of CD69, CD71, CD40L an HLADR- bearing CD3 (pan T), CD4 and CD8 cells, and CB1, CD152 and GARP-bearing CD25+ FoxP3+ Treg cells in the acute phase of MDD, and to determine if activation of T cells and depletion of Treg are associated with the severity of the acute phase. In addition, the relationships between these T cell subsets and immune cell profiles, based on cytokine production, are assessed.
Methodology
Participants
The investigation included participants from Bangkok's Chulalongkorn University. The Department of Psychiatry's senior psychiatrists recruited outpatients with MDD. Posters and word-of-mouth were used to recruit healthy controls. Before taking part in the study, all participants were required to sign a written consent form. Once participants were recruited, they were required to complete standard questionnaires and provide blood samples. Standard questionnaires and a semi-structured interview were utilized to collect demographic data, including age, gender, and body mass index. Patients between the ages of 18 and 65 who comprehended Thai, had been diagnosed with MDD by a psychiatrist using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria, and had a Hamilton Depression Rating Scale (HAMD) score greater than 17, indicating moderate to severe depression, met the inclusion criteria. Patients with other DSM-5 axis 1 disorders, including schizophrenia, schizoaffective disorders, obsessive compulsive disorder, post-traumatic stress disorder, psycho-organic disorders, or substance misuse disorders, were excluded from the study. Healthy volunteers were recruited from the same catchment area as family or friends of staff members or friends of patients. We excluded individuals with a diagnosis of any axis 1 DSM-5 disorder or a positive family history of MDD, bipolar disorder, psychosis or suicide. Patients and controls were ineligible if they had experienced any allergic or inflammatory responses in the previous three months, if they had neuroinflammatory, neurodegenerative, or neurological disorders such as epilepsy, Alzheimer's disease, multiple sclerosis, or Parkinson's disease, if they had (auto)immune diseases such as COVID-19 infection (lifetime), chronic obstructive pulmonary disease, inflammatory bowel disease, psoriasis, diabetes type 1, asthma, or rheumatoid arthritis, had a history of receiving immunomodulatory drugs, had taken therapeutic doses of antioxidants or omega-3 polyunsaturated fatty acid supplements within three months before the study, had used anti-inflammatory drugs (NSAID or steroids) within one month of the study. We did not include lactating or expectant women. Some patients were taking psychotropic medications, such as sertraline (18 patients), other antidepressants (8 patients, such as fluoxetine, venlafaxine, escitalopram, bupropion, and mirtazapine), benzodiazepines (22 patients), atypical antipsychotics (14 patients), and mood stabilizers (4 patients). In the statistical analysis, the potential effects of these drug variables were taken into consideration.
Before participating in the study, all controls and patients gave their written informed consent. The study was carried out following both international and Thai ethics and privacy regulations. The Institutional Review Board of the Faculty of Medicine at Chulalongkorn University in Bangkok, Thailand approved the study (#528/63), which was in accordance with the international guidelines for human research protection, such as the Declaration of Helsinki, the Belmont Report, CIOMS Guideline, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Measurements
A research assistant conducted a semi-structured interview and an experienced psychiatrist administered the 17-item version of the HAMD to assess the intensity of depressive symptoms30. The Mini-International Neuropsychiatric Interview (M.I.N.I.) was used to make the diagnosis of psychiatric disorders31.
After an overnight fast, between 8:00 and 9:00 a.m., participants' blood (20 mL) was collected. The blood was collected in BD Vacutainer® EDTA (10 mL) and BD Vacutainer® SST™ (5 mL) tubes provided by BD Biosciences (Franklin Lakes, NJ, USA). The serum-separating tubes were left to clot at room temperature for 30 min to obtain serum. The tubes were spun at 1100×g for 10 min at 4 °C. Peripheral blood mononuclear cells (PBMCs) were separated from the blood sample through density gradient centrifugation (30 min at 900×g) using Ficoll® Paque Plus (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Utilizing a hemocytometer and trypan blue, 0.4% solution, pH 7.2–7.3 (Sigma-Aldrich Corporation, St. Louis, Missouri, United States), the cell count and viability were determined. Counting total and blue-stained cells ensured that greater than 95% of cells were viable under all conditions. To activate PBMCs, the 96-well plates were coated with 5 µg/mL of the anti-human CD3 antibody (OKT3, from eBioscience), overnight. 3 × 105 PBMCs were added to each well along with 5 μg/mL of the anti-human CD28 antibody (CD28.2, eBioscience). The cells were cultured in RPMI 1640 medium with L-glutamine supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (Gibco Life Technologies, Carlsbad, CA, USA) for 3 days at 37 °C in an incubator with 5% CO2. The unstimulated PBMCs cultured for the same period were used as a negative control. After 3 days, the lymphocyte immunophenotypes were determined through flow cytometry.
To study lymphocyte immunophenotypes, 3 × 105 PBMCs were labeled to surface markers with monoclonal antibodies for 30 min, including CD3-PEcy7, CD4-APCcy7, CD8-APC, CD40L-FITC, CD69-AF700, CD71-PerCPcy5.5, HLA-DR-PE594, CD25-APC, CD152 PE Dazzle594, GARP-PE, CB1-AF700 and 7-AAD (Biolegend, BD Biosciences and R&D Systems). For Treg cells, we firstly stained cells with surface markers (CD3, CD4, CD25) followed by intracellular FoxP3 staining, which was performed using the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) for fixation and permeabilization before staining with antibody to Foxp3-FITC (Biolegend). Electronic Supplementary File (ESF1), Figs. 1 and 2 show our gating strategies. The MFI of the activation markers expression was defined as the MFI of the activation markers expression (e.g. CD71+, HLA-DR+) gated within the CD4+ /CD8+ subpopulations. The flow cytometry was performed using LSRII flow cytometer (BD Biosciences) to evaluate lymphocyte immunophenotypes. All data were analyzed using FlowJo X software (Tree Star Inc., Ashland, OR, USA). Our study focused on characterizing different types of T cells. We did not utilize a Fc receptor blocking agent in our study. Table 1 of the ESF2 shows the different CD and cell surface markers, as well as their key functions, measured in the present study. We assessed both the percentages of T cells and the median fluorescence intensity (MFI) of the markers.
As previously described15, we measured cytokines and chemokines in stimulated diluted whole blood culture supernatant using the same blood samples as those employed for the flow cytometry. We used RPMI-1640 medium supplemented with L-glutamine, phenol red, and 1% penicillin (Gibco Life Technologies, USA), with or without 5 µg/mL PHA (Merck, Germany)+ 25 µg/mL lipopolysaccharide (LPS; Merck, Germany). On sterile 24-well plates, 1.8 mL of each medium was combined with 0.2 mL of 1/10-diluted whole blood. Using the Bio-Plex Pro human cytokine 27-plex assay kit (BioRad, Carlsbad, California, United States of America) and the LUMINEX 200 instrument (BioRad, Carlsbad, California, United States of America), the unstimulated and stimulated levels of cytokines and chemokines were measured. The intra-assay CV values were less than 11%. ESF2, Table 2 shows the cytokines and chemokines that were measured to compute the immune profiles15. ESF2, Table 3 displays how the immune profiles were computed as z unit composite scores using the cytokine and chemokine assessments15. In the current study, we examined whether the immune cell percentages or MFI values could predict the stimulated M1, Th-1, IRS, T cell growth, and neurotoxicity profiles.
Statistics
Among study groups, we compared nominal variables via analysis of contingency tables (χ2-test) and continuous variables via analysis of covariance (ANCOVA). We employed Pearson's product moment correlation coefficients for the purpose of examining the relationships between scale variables. A pre-specified generalized estimating equation (GEE), repeated measures, included fixed categorical effects of diagnosis (differences between patients and controls), cell types (T cell populations, namely CD69, CD71, CD40L, and HLADR-bearing CD3+, CD4+ and CD8+ cells), and the responsivity of the cells to in vitro administration of anti-CD3/CD28 antibodies (unstimulated versus stimulated condition), while allowing for the effects of age, sex, the drug state, BMI and smoking. Using pairwise comparisons, predefined comparisons were examined. Prespecified GEE, repeated measures, were used to examine the fixed categorical effects of diagnosis, treatment (unstimulated versus stimulated), cell type, and their interactions on Treg cells (CD25+ FoxP3+ CB1+, CD25+ FoxP3+ CD152+, and CD25+ FoxP3+ GARP+), while allowing for the effects of the aforementioned background variables. The primary statistical analyses examined the effects of Teff and Treg subsets on the HAMD score using multiple regression analyses. These analyses are the most important because a) a quantitative outcome score such as the HAMD is much more informative or correct than the binary diagnosis MDD32,33, and b) this method allows to examine the combined effects of both activated T and Treg cells on severity of illness. Furthermore, the effects of immune cell numbers or MFI levels on the stimulated production of cytokine profiles (M1, Th-1, IRS, T cell growth and neurotoxicity) were examined using multiple regression analysis with the stimulated production as dependent variables, and GEE analysis, repeated measures, with both unstimulated and stimulated immune profiles as dependent variables. Using manual multiple regression analysis, the effects of cell populations on the HAMD score were evaluated. We utilized an automatic forward stepwise regression strategy with a p-to-enter of 0.05 and a p-to-remove of 0.06 to determine which variables would be included and which would be excluded from the final regression model. We estimated the standardized β coefficients with t-statistics and exact p-values for each explanatory variable, F-statistics (and p-values), and partial eta squared (effect size) of the model. Using the modified Breusch-Pagan test and the White test, heteroskedasticity was investigated. We examined the probability of multicollinearity and collinearity utilizing the tolerance (cut-off value: 0.25) and variance inflation factor (cut-off value: > 4), as well as the condition index and variance proportions from the collinearity diagnostics table. Residuals, residual plots, and data quality were always evaluated in the final model. We also computed partial regression analyses, including partial regression plots, based on the results of the linear modeling analyses. When required, we utilized transformations such as Log10, square-root, rank-based inversed normal, and Winsorization to normalize the data distribution of the indicators. At p = 0.05, all analyses were two-tailed. To conduct the aforementioned statistical analyses, we utilized IBM's Windows version of SPSS 28. Given an effect size of 0.25 (equivalent to 20% explained variance), alpha = 0.05, power = 0.8, and 3 covariates, an a priori power analysis (G*Power 3.1.9.4) for a linear multiple regression analysis (the primary analysis) suggests that the minimum sample size should be 48.
Ethical statement
All subjects gave their written informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki of 1975, revised in 2013, and the protocol was approved by the Ethics Committee of the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (#528/63).
Results
Demographic data
Table 1 displays the sociodemographic and clinical characteristics of the study's patients and controls. There were no significant differences between the study groups in terms of age, gender distribution, education, or smoking. Patients had a higher BMI than controls. In any case, we have controlled all results for possible effects of age, BMI, sex, smoking, and the drug state of the patients but could not find any significant effects. The average HAMD scores of patients were substantially higher than those of controls, indicating that the majority of patients suffered from moderate to severe clinical depression. The outcomes of GEE analyses with immune profiles as dependent variables and stimulation status (baseline vs LPS+ PHA treatment), diagnosis (MDD versus controls), and stimulation by diagnosis interaction as predefined fixed effects are presented in the same table. The Th-1, IRS, T cell growth, and neurotoxicity (but not M1) profiles were all higher in MDD individuals compared to controls.
Table 1.
Demographic and clinical data of the depressed patients and healthy controls (HC) included in the present study.
| Variables | HC (n = 20) | MDD (n = 30) | F/W/X2 | df | p |
|---|---|---|---|---|---|
| Sex (male/female) | 6/14 | 11/19 | 0.24 | 1 | 0.626 |
| Age (years) | 33.6 (8.0) | 28.7 (8.6) | F = 8.05 | 1/48 | 0.040 |
| Education (years) | 16.1 (2.2) | 15.6 (1.4) | F = 1.07 | 1/48 | 0.303 |
| Smoking | 18/2 | 23/7 | FEPT | – | 0.285 |
| BMI (kg/m2) | 21.3 (2.5) | 25.5 (5.9) | F = 8.82 | 1/48 | 0.005 |
| HAMD | 1.0 (1.6) | 23.5 (5.8) | F = 281.87 | 1/48 | < 0.001 |
| M1 macrophage (z scores) | −0.131 (0.116) | 0.087 (0.205) | W = 0.86 | 1/48 | 0.354 |
| T helper-1 (z scores) | −0.337 (0.119) | 0.225 (0.198) | W = 5.59 | 1/48 | 0.018 |
| IRS (z scores) | −0.366 (0.114) | 0.244 (0.184) | W = 7.91 | 1/48 | 0.005 |
| T cell growth (z scores) | −0.305 (0.110) | 0.203 (0.169) | W = 6.64 | 1/48 | 0.011 |
| Neurotoxicity (z scores) | −0.286 (0.125) | 0.190 (0.197) | W = 4.09 | 1/48 | 0.043 |
| CD3% | 63.8 (16.7) | 66.1 (10.5) | F = 0.34 | 1/47 | 0.056 |
| CD4% | 53.3 (7.8) | 53.3 (9.2) | F = 0.00 | 1/47 | 0.976 |
| CD8% | 29.8 (8.2) | 32.1 (7.8) | F = 0.91 | 1/47 | 0.344 |
Results are shown as mean (± SD).
FEPT Fisher’s exact probability test, X2 analysis of contingency tables, F results of analysis of variance, W results of general estimating equation (GEE), the p values are results of pairwise comparisons between MDD and controls, BMI body mass index, HAMD Hamilton Depression Rating Scale score, IRS immune-inflammatory responses system.
Baseline and stimulated Teff frequencies and MFI in MDD
Table 1 shows that there were no significant differences in CD3+, CD4+, and CD8+ percentages between MDD patients and controls. GEE analysis performed on the frequency of activated T cells showed significant effects of diagnosis (W = 4.78, df = 1, p = 0.029), stimulation (Wald = 596.28, df = 1, p < 0.001)), cell type (W = 1121.92, df = 1, p < 0.001)), diagnosis X cell type (W = 30.59, df = 11, p = 0.001), stimulation X cell type (W = 542.25, df = 1, p < 0.001), and the three-way interaction cell type X diagnosis X stimulation (W = 25.72, df = 11. P = 0.007). There were no significant effects of age, sex, BMI, smoking or drug state of the patients. Table 2 shows the frequencies of the T cell subtypes according to diagnosis and stimulation status. Anti-CD3/CD28 stimulation significantly increased the frequencies of all subsets. Activation markers were significantly higher in MDD (29.34 ± 0.98%) than in controls (27.6 ± 0.93%). ESF2, Table 4 shows the measurements of the 4 activation markers in CD3+, CD4+ and CD8+ T cells in baseline and stimulated conditions in MDD patients and controls. Pairwise comparisons showed that the number of activated T cells in the baseline condition was greater in patients than controls (p = 0.041). Table 2 shows that in the baseline condition, MDD patients showed higher frequencies of baseline CD3+ CD71+, CD3+ CD40L+, CD4+ CD71+, CD4+ CD40L+, CD4+ HLA-DR+ and CD8+ CD40L+ than normal controls. After stimulation with anti CD3/CD28 antibodies, there were no significant differences in any of the cell types between MDD and controls, except CD8+ CD40L+ which was elevated in MDD.
Table 2.
Differences in unstimulated (UNST) and stimulated (STIM) changes in the percentage of T lymphocyte populations in healthy controls (HC) and major depressed patients (MDD).
| Variables (z scores) | Baseline vs anti-CD3/CD28 | HC (n = 20) | MDD (n = 30) | Pairwise comparisons |
|---|---|---|---|---|
| CD3+ CD69+ % | UNST | −0.487 (0.090) | −0.479 (0.095) | 0.947 |
| STIM | 0.904 (0.024) | 0.921 (0.027) | 0.639 | |
| CD3+ CD71+ % | UNST | −1.673 (0.188) | −0.930 (0.142) | 0.002 |
| STIM | 1.054(0.016) | 1.069 (0.017) | 0.520 | |
| CD3+ CD40L+ % | UNST | −0.955 (0.103) | −0.644 (0.059) | 0.009 |
| STIM | 0.555 (0.026) | 0.553 (0.031) | 0.954 | |
| CD3+ HLADR+ % | UNST | −0.229 (0.081) | −0.132 (0.065) | 0.350 |
| STIM | 0.730 (0.028) | 0.725 (0.021) | 0.510 | |
| CD4+ CD69+ % | UNST | −0.571 (0.125) | −0.445 (0.107) | 0.444 |
| STIM | 0.907 (0.026) | 0.960 (0.024) | 0.130 | |
| CD4+ CD71+ % | UNST | −1.586 (0.187) | −0.802 (0.139) | 0.001 |
| STIM | 1.099 (0.014) | 1.108 (0.014) | 0.636 | |
| CD4+ CD40L+ % | UNST | −0.818 (0.105) | −0.520 (0.059) | 0.013 |
| STIM | 0.718 (0.024) | 0.694 (0.028) | 0.505 | |
| CD4+ HLA-DR+ % | UNST | −0.325 (0.071) | −0.113 (0.061) | 0.024 |
| STIM | 0.676 (0.031) | 0.707 (0.022) | 0.411 | |
| CD8+ CD69+ % | UNST | −0.748 (0.153) | −0.869 (0.126) | 0.541 |
| STIM | 0.831 (0.035) | 0.776 (0.044) | 0.319 | |
| CD8+ CD71+ % | UNST | −1.982 (0.225) | −1.575 (0.174) | 0.152 |
| STIM | 1.060 (0.022) | 1.099 (0.018) | 0.139 | |
| CD8+ CD40L+ % | UNST | −2.026 (0.146) | −1.643 (0.096) | 0.028 |
| STIM | 0.062 (0.055) | 0.218 (0.056) | 0.046 | |
| CD8+ HLADR+ % | UNST | −0.369 (0.113) | −0.346 (0.0.83) | 0.869 |
| STIM | 0.793 (0.031) | 0.791 (0.0226) | 0.953 |
Results are shown as estimated marginal mean values (SE).
CD3%, CD4% and CD8%: results of analysis of variance; shown as mean (standard deviation).
ESF2, Table 5 shows the outcome of a similar GEE analysis performed on the MFI values of the activated T cells. This GEE analysis showed significant effects of anti-CD3/CD28 stimulation (Wald = 825.31, df = 1, p < 0.001), cell type (W = 1875.74, df = 1, p < 0.001), diagnosis X cell type (W = 23.00, df = 11, p = 0.018), stimulation X cell type (W = 1133.54, df = 1, p < 0.001), and the three-way interaction cell type X diagnosis X stimulation (W = 24.12, df = 11. P = 0.012). ESF2, Table 5 displays that MDD patients exhibit increased baseline MFI values of CD3+ CD71+, CD4+ CD71+, and CD4+ HLADR+ and a trend toward increased CD3+ HLADR+ MFI values as compared with controls. In addition, pairwise comparisons showed significant increases in stimulated CD3+ CD71+ and CD4+ HLADR+ MFI values between MDD patients and controls (p < 0.05). There were no significant effects of age, sex, BMI, smoking or drug state of the patients on any of the above measurements (these putative confounders were entered as covariates in the analyses).
GEE analysis performed on the frequencies of the three CD25+ FoxP3+ subtypes showed a significant effect of diagnosis (W = 3.96, df = 1, p = 0.047), stimulation (W = 211.51, df = 1, p < 0.001), cell type (W = 529.81, df = 1, p < 0.001) and cell type X simulation (W = 243.14, df = 1, p < 0.001). The CD25+ FoxP3+ cells were significantly lower in MDD (z score ± SE: -0.073 ± 0.055) than in controls (0.111 ± 0.074). As can be seen in Table 3, in vitro administration of anti-CD3/CD28 antibodies increased the number of all three FoxP3 cell types significantly. There was a significant group (diagnosis) by time (unstimulated versus stimulated condition) interaction for CD25+ FoxP3+ CB1, indicating that patients with MDD showed significantly lower CD25+ FoxP3+ CB1+ levels than controls in the unstimulated condition. The results of GEE, repeated measures, performed on the MFI of the CD25+ FoxP+ cells showed a significant effect of stimulation (W = 75.58, df = 1, p < 0.001) and cell types (W = 39.52, df = 1, p < 0.001). ESF2, Table 6 shows the interaction between diagnosis and stimulation with anti-CD3/CD28. Overall, stimulation significantly increased the MFI of FoxP3 cells. Pairwise comparisons show that the unstimulated expression of FoxP3 was significantly lower in MDD as compared with controls (p = 0.027), whereas there were no significant intergroup differences in the stimulated condition (p = 0.772).
Table 3.
Differences in unstimulated (UNST) and stimulated (STIM) changes in the percentage of T regulatory (Treg) lymphocyte populations in healthy controls (HC) and major depressed patients (MDD).
| Condition | Diagnosis | Tests of model effects | ||||
|---|---|---|---|---|---|---|
| Variables (z scores) |
HC (n = 20) | MDD (n = 30) | Effects | Wald df = 1 | p | |
| CD25+ FoxP3+ CB1+ % | UNST | −0.238 (0.290) | −0.879 (0.123) | G | 2.66 | 0.103 |
| STIM | 0.599 (0.124) | 0.643 (0.070) | G X T | 4.468 | 0.035 | |
| CD25+ FoxP3+ CD152+ % | UNST | −0.390 (0.222) | −0.873 (0.159) | G | 3.565 | 0.059 |
| STIM | 0.780 (0.095) | 0.619 (0.077) | G X T | 1.659 | 0.198 | |
| CD25+ FoxP3+ GARP+ % | UNST | −0.523 (0.216) | −0.907 (0.138) | G | 1.134 | 0.287 |
| STIM | 0.719 (0.079) | 0.778 (0.064) | G X T | 3.359 | 0.067 | |
Results are shown as estimated marginal mean values (SE).
Regression analyses of severity of illness on T cell subtype percentages and MFI values
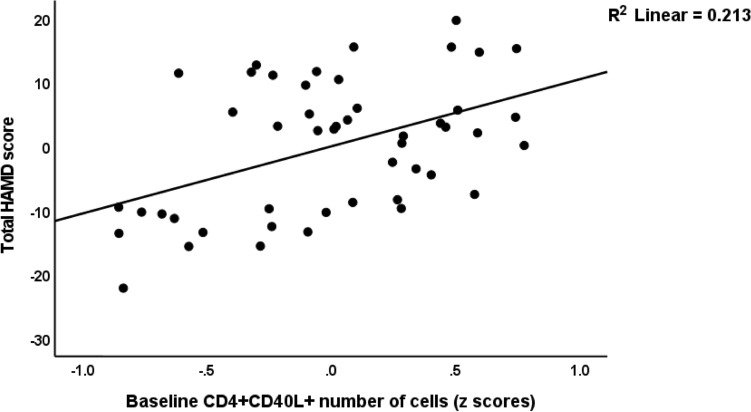
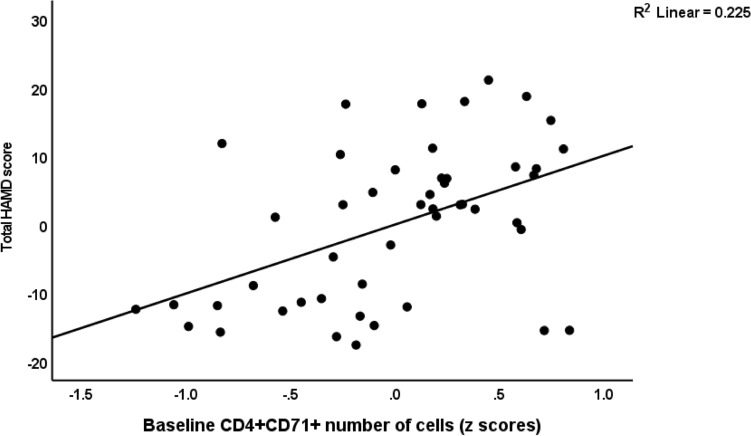
Consequently, we performed the primary outcome statistical analyses, namely multiple regression analyses with the HAMD as outcome variable and the different cell populations (either frequencies or MFI) as explanatory variables. Table 4, regressions #1 and #2 show the outcome of these regressions using the percentages of the cell types, whereas regressions #4, #5 and #6 show the outcome of regressions on the MFI values. We examined the effects of the unstimulated and stimulated cell types. Regression #1 shows that 37.9% of the variance in the HAMD could be explained by unstimulated CD25+ FoxP3+ CB1% (inversely), unstimulated CD4+ CD40L+ % and stimulated CD4+ CD69+ % (positively). Figure 1 shows the partial regression of the total HAMD score on the baseline number of CD4+ CD40L+ cells. Nevertheless, also CD4+ CD71+ % was significantly associated with the HAMD score as shown in Fig. 2. Regression #2 (we omitted the CD4+ populations) shows part of the variance in the HAMD score could be explained by the regression on CD8+ CD40+ % (positively) and age (inversely).
Table 4.
Results of multiple regression analyses with the Hamilton Depression rating Scale (HAMD) score as a dependent variable.
| Dependent variables | Explanatory variables | Parameter estimates | Model | |||||
|---|---|---|---|---|---|---|---|---|
| β | T | p | F | df | P | R2 | ||
| #1. HAMD | Model | 8.94 | 3/44 | < 0.001 | 0.379 | |||
| U_CD25+ FOXP3+ CB1+ % | −0.460 | −3.77 | < 0.001 | |||||
| S_CD4+ CD69+ % | 0.400 | 3.28 | 0.002 | |||||
| U_CD4+ CD40L+ % | 0.284 | 2.38 | 0.022 | |||||
| #2. HAMD | Model | 5.85 | 2/45 | 0.006 | 0.206 | |||
| Age | −0.344 | −2.59 | 0.013 | |||||
| S_CD8+ CD40L+ % | 0.311 | 2.34 | 0.024 | |||||
| #3. HAMD | Model | 5.91 | 4/43 | < 0.001 | 0.355 | |||
| Age | −0.401 | −3.13 | 0.003 | |||||
| S_CD8+ CD40L+ MFI | 0.341 | 2.53 | 0.015 | |||||
| S_CD3+ HLADR+ MFI | 0.362 | 2.65 | 0.011 | |||||
| S_CD4+ CD69+ MFI | 0.270 | 2.04 | 0.048 | |||||
| #4. HAMD | Model | 6.21 | 4/43 | < 0.001 | 0.366 | |||
| Age | −0.417 | −3.29 | 0.002 | |||||
| S_CD8+ CD40L+ MFI | 0.426 | 3.31 | 0.002 | |||||
| S_CD3+ HLADR+ MFI | 0.373 | 2.75 | 0.009 | |||||
| U_CD25+ FOXP3+ CB1+ MFI | −0.278 | −2.24 | 0.030 | |||||
| #5. HAMD | Model | 4.32 | 1/44 | 0.044 | 0.089 | |||
| U_CD25+ FOXP3+ GARP+ MFI | −0.299 | −2.99 | 0.044 | |||||
% prevalence on immune cell populations, MFI median fluorescence intensity, S stimulated, U unstimulated.
Figure 1.
Partial regression of the total Hamilton Depression Rating Scale (HAMD) score on baseline number of CD4+ CD40L+ cells.
Figure 2.
Partial regression of the total Hamilton Depression Rating Scale (HAMD) score on baseline number of CD4+ CD71+ cells.
Regarding the MFI data, we found that (regression #3) 35.5% of the variance in the HAMD could be explained by 3 stimulated MFI values (CD8+ CD40L+, CD3+ HLADR+, CD4+ CD69+, all positively) and age (inversely). Regression #4 shows that this prediction could be slightly improved by adding the unstimulated CD25+ FoxP3+ CB1 MFI data. Regression #5 displays that unstimulated CD25+ FoxP3+ GARP+ MFI was inversely associated with the HAMD.
Regression analyses of immune profiles on T cell subtype percentages and MFI values
ESF2, Table 7 shows the measurements of the unstimulated and stimulated M1, Th-1, IRS, T cell growth and immune-associated neurotoxicity profiles in MDD patients and controls. The stimulated M1, IRS, T cell growth and neurotoxicity profiles were significantly increased in MDD than in controls, whereas there was a trend towards an increased Th-1 profile. Table 5 shows the results of multiple regression analyses with stimulated immune profiles (M1, Th-1, IRS, T cell growth and neurotoxicity) as dependent variables and T cells, either percentage (regressions 1–5) or MFI (regressions 6–10) as explanatory variables, while allowing for the effects of extraneous variables. Regression #1 shows that 22.4% of the variance in M1 could be explained by CD8+ CD69+ % (positively) and CD25+ FoxP3+ CD152+ % (inversely). Th-1 was best predicted by CD25+ FoxP3+ CD152+ % (inversely). Regression #3 shows that a larger part (28.8%) of the variance in IRS was explained by baseline CD25+ FoxP3+ CD152+ % (inversely) and stimulated CD4+ CD69+ % and CD4+ CD71+ % (positively). Regression #4 shows that the T cell growth profile was predicted by three different cell types, namely CD3+ CD71+ %, CD4+ CD69+ % (positively) and CD25+ FoxP3+ CD152+ (inversely). Neurotoxicity was best predicted by CD25+ FoxP3+ GARP+ % (inversely).
Table 5.
Results of multiple regression analyses with immune profiles as dependent variables and T cell subsets or expression markers as explanatory variables.
| Dependent variables | Explanatory variables | Estimates | Model | |||||
|---|---|---|---|---|---|---|---|---|
| β | T | p | F | df | p | R2 | ||
| v#1. M1 | Model | 6.48 | 2/45 | 0.003 | 0.224 | |||
| S_CD25+ FoxP3+ CD152+ % | −0.391 | −2.98 | 0.005 | |||||
| S_CD8+ CD69+ % | 0.271 | 2.06 | 0.045 | |||||
| #2. Th-1 | Model | 5.13 | 1/46 | 0.030 | 0.099 | |||
| S_CD25+ FoxP3+ CD152+ % | −0.314 | −2.24 | 0.030 | |||||
| #3. IRS | Model | 5.92 | 3/44 | 0.002 | 0.288 | |||
| S_CD4+ CD69+ % | 0.338 | 2.62 | 0.012 | |||||
| U_CD25+ FoxP3+ CD152+ % | −0.336 | −2.57 | 0.014 | |||||
| S_CD4+ CD71+ % | 0.287 | 2.16 | 0.036 | |||||
| #4. T cell growth | Model | 8.03 | 3/44 | < 0.001 | 0.354 | |||
| S_CD4+ CD69+ % | 0.378 | 3.07 | 0.004 | |||||
| U_CD25+ FoxP3+ CD152+ % | −0.366 | −2.96 | 0.005 | |||||
| U_CD3+ CD71+ % | 0.301 | 2.40 | 0.021 | |||||
| #5. Neurotoxicity | Model | 4.99 | 1/46 | 0.030 | 0.098 | |||
| S_CD25+ FoxP3+ GARP+ % | −0.313 | −2.23 | 0.030 | |||||
| #6. M1 | Model | 7.12 | 2/45 | 0.002 | 0.240 | |||
| S_CD25+ FoxP3+ CD152+ MFI | −0.398 | −3.04 | 0.004 | |||||
| S_CD8+ CD69+ MFI | 0.346 | 2.64 | 0.011 | |||||
| #7. Th-1 | Model | 6.81 | 1/46 | 0.012 | 0.129 | |||
| S_CD8+ CD71+ MFI | 0.359 | 2.61 | 0.012 | |||||
| #8. IRS | Model | 10.10 | 1/46 | 0.003 | 0.180 | |||
| S_CD8+ CD71+ MFI | 0.424 | 3.18 | 0.003 | |||||
| #9. T cell growth | Model | 7.47 | 3/44 | < 0.001 | 0.338 | |||
| S_CD3+ CD71+ MFI | 0.354 | 3.54 | < 0.001 | |||||
| S_CD4+ CD69+ MFI | 0.325 | 2.62 | 0.012 | |||||
| S_CD25+ FoxP3+ CD152+ MFI | −0.299 | −2.43 | 0.019 | |||||
| #10. Neurotoxicity | Model | 6.95 | 2/43 | 0.002 | 0.244 | |||
| U_CD25+ FoxP3+ CD152+ MFI | −0.489 | −3.48 | 0.001 | |||||
| U_CD8+ CD40L+ MFI | 0.339 | −2.41 | 0.020 | |||||
% prevalence on immune cell populations, MFI median fluorescence intensity, S stimulated, U unstimulated.
With regard to the MFI values, we detected that M1 (regression #6) was best predicted by CD25+ FoxP3+ CD152+ MFI (inversely) and CD8+ CD69+ MFI (positively). Regression #7 shows that 12.9% of the variance in Th-1 was predicted by CD4+ CD71+ MFI (positively). IRS was strongly associated with CD8+ CD71+ MFI (regression #8). T cell growth was predicted (33.8% of the variance) by CD3+ CD71+ MFI, CD4+ CD69+ MFI (positively), and CD25+ FoxP3+ CD152+ MFI (inversely). Regression #10 shows that 24.4% of the variance in neurotoxicity was explained by the unstimulated CD25+ FoxP3+ CD152+ MFI (inversely) and CD8+ CD40L+ MFI values.
Table 6 shows that the changes from baseline to the LPS+ PHA-stimulated IRS and neurotoxicity scores were predicted by lowered numbers or expression of CD25+ FoxP3+ CD152+ T reg cells combined with baseline or stimulated CD8+ and CD4+ T activation subsets (either prevalence or MFI values).
Table 6.
Results of general equating estimation, repeated measurement.
| Dependent variables | Explanatory variables | B | SE | Lower | Upper | Wald | df | p |
|---|---|---|---|---|---|---|---|---|
| IRS | U_CD25+ FoxP3+ CD152+ % | −0.233 | 0.0588 | −0.348 | −0.117 | 15.65 | 1 | < 0.001 |
| S_CD4+ CD71+ % | 1.190 | 0.0775 | 1.638 | 1.342 | 235.98 | 1 | < 0.001 | |
| IRS | U_CD25+ FoxP3+ CD152+ % | −0.185 | 0.0467 | −0.277 | −0.094 | 15.80 | 1 | < 0.001 |
| U_CD8+ CD40L+ % | 0.520 | 0.1948 | 0.138 | 0.902 | 7.12 | 1 | 0.008 | |
| IRS | S_CD8+ CD40L+ MFI | 3.199 | 0.1649 | 2.876 | 3.522 | 376.43 | 1 | < 0.001 |
| U_CD25+ FoxP3+ CD152+ MFI | −0.197 | 0.0537 | −0.302 | −0.092 | 13.46 | 1 | < 0.001 | |
| Neurotoxicity | U_CD25+ FoxP3+ CD152+ % | −0.158 | 0.0541 | −0.258 | −0.058 | 9.17 | 1 | 0.002 |
| CD8+ CD40L+ % | 1.958 | 0.0906 | 1.780 | 2.135 | 466.52 | 1 | < 0.001 | |
| Neurotoxicity | CD8+ CD40L+ MFI | 3.420 | 0.1488 | 3.129 | 3.712 | 588.62 | 1 | < 0.001 |
| U_CD25+ FoxP3+ CD152+ MFI | −0.174 | 0.0479 | −0.268 | −0.085 | 13.13 | 1 | < 0.001 |
IRS immune-inflammatory response system, U unstimulated, S stimulated, MFI median fluorescence intensity.
Discussion
Associations between T cells and immune profiles
The first major finding of this study is that there is a strong correlation between the flow cytometric assessments of T cell subtypes and cytokine-based immune-inflammatory profiles, including M1, Th-1, IRS, T cell growth, and neuroimmunotoxic profiles. Thus, these immune profiles were a) positively associated with the number or expression of diverse activated T cells, primarily CD4+ CD71+ and CD8+ CD69+ T cells, but also CD4+ CD69+ T, CD8+ CD71+ and CD8+ CD40L+ cells, and b) negatively associated with the number or expression of diverse Treg cells, including CD152- and GARP-bearing CD25+ FoxP3+ cells.
During T cell activation, distinct phases can be distinguished by the presence of distinct markers. CD69 is a cell surface marker that is expressed immediately after T cell activation and rapidly disappears after the initial stimulation14. CD69 is a costimulatory molecule for T cell differentiation, activation, and proliferation34 as well as lymphocyte retention in tissues14. CD69 activation stimulates the production of IL2 and TGF-β1 and controls the production of pro-inflammatory cytokines such as IL-17, IFN-γ, and IL-22, as well as Treg cells14. CD40L is present in the early phases of T cell activation35 and binds to CD40 on macrophages, CD8+ T cells, B cells, and dendritic cells, thereby promoting the differentiation and proliferation of immune cells, immune-inflammatory responses, and activation and maturation of B cells, which ultimately results in the production of autoantibodies35. After antigen presentation, preformed CD40L molecules are mobilized from the lysosomes of Th-1 cells36, while colligation of CD3 and CD40L increases the production of IFN-γ, TNF-α, and IL-1026. During a later phase of T cell activation, CD71 (TfR) is upregulated, resulting in an increase in iron uptake into the cells37, which stimulates T cell activation and proliferation38. Within twenty-four to forty-eight hours of T cell activation, the late phase activation marker HLA-DR is expressed. This latter molecule is a class II MHC, and its upregulation is associated with an increase in IFN-γ production39.
Conversely, the inverse associations of the immune profiles with CD152 (CTLA-4) and GARP-bearing Treg cells indicate immunoregulatory Treg effects on different aspects of the immune response. Depending on the surface expression of the proteins, Tregs regulate the immune system through a variety of mechanisms. CTLA-4 also known as CD152 is expressed on Treg cells (and activated T cells) and functions as a negative immune checkpoint that regulates immune responses40. By competing with CD28 for binding to the same ligands on antigen-presenting cells, CD152 inhibits the activation and proliferation of T cells41. GARP is a Treg cell surface molecule that indicates Treg activation and the release of TGF-β1, an anti-inflammatory cytokine. As a result, Treg cells promote immune tolerance41,42. Treg cells, may express CB1, which inhibits the production of pro-inflammatory cytokines and exerts negative immunoregulatory effects43. Previously, we discovered that the number of CD25+ FoxP3+ CB1+ cells was inversely associated with IRS responses15. However, after introducing the effects of GARP- and CD152-bearing Tregs, the effects of CB1 were no longer significant. Intriguingly, the current study detected that decreased numbers of baseline Treg cells predict increased neurotoxic potential, indicating that diminished fitness of Treg cells is associated not only with exaggerated immune responses, but also with increased neuroimmunotoxicity.
T cell activation in MDD
The second major finding of this study is that the numbers of baseline CD3+ CD71+, CD3+ CD40L+, CD4+ CD71+, CD4+ CD40L+, CD4+ HLADR+, and CD8+ CD40L+ and the expression of CD3+ CD71+, CD4+ CD71+, and CD4+ HLADR+ are significantly greater in MDD than in controls. Furthermore, the study demonstrates a strong correlation between the severity of depression and elevated numbers of CD4+ CD40L+ and CD4+ CD69+ cells, as well as increased expression of CD40L on CD8+ cells and HLADR on CD3+ cells.
These results suggest that patients with MDD have an abnormal distribution of CD4+ and CD8+ T cell subsets with elevated T cell activation markers. Maes et al. demonstrated in previous investigations that depressed patients had a higher expression of T cell activation markers, specifically CD25+ and HLADR+ 20. A recent meta-analysis also reported an increase in the mean absolute number of activated T cells expressing CD25+ and CD3+ HLADR+ 44. Moreover, Maes et al.10 found that in the symptomatic remission phase of BD, the frequency of unstimulated CD3+ CD8+ CD71+ cells is lower than in healthy controls. This suggests that the acute phase of major mood disorders is characterized by T cell activation and that this T cell activation is suppressed when the symptoms ameliorate. Therefore, future research on T cell activation (and the immune system in general) should always differentiate between the acute, partially remitted, and remitted phases of mood disorders.
Our findings indicate that the pathogenesis of the acute phase of severe MDD is associated with CD4+ and CD8+ T cell activation. Interaction with the antigen-MHC complex activates CD4+ T cells, which consequently differentiate into distinct subtypes under the influence of cytokines39. Almulla et al.45 reported that increased IL-16, Th-1 activation and Th-1 polarization are hallmarks of the acute phase of severe MDD. IL-16 signaling occurs via the CD4 molecule on Th cells, activating CD4+ cells and upregulating activation markers and the IRS46–48. Ligation of CD40 via CD40L stimulates the secretion of the Th-1-polarizing cytokine IL-1249,50. Moreover, CD40L expression by CD4+ cells is a crucial step in the activation of CD8 T cells29, which may express CDL40, perform T helper functions, and promote their own growth27,29. In addition, CD40L-CD40 interactions mediate the effect of CD4+ cells on CD8+ cytotoxic T lymphocytes51.
Consequently, our findings suggest that CD4+ and CD8+ T cell activation, along with increased Th-1 polarization and IL-16 production, are key phenomena that drive increased T effector, T helper, and T cytotoxic functions, as well as enhanced M1, Th-17, IRS, and neurotoxic immune profiles in the acute phase of severe MDD. These activation markers may contribute to the immune, autoimmune, and neurotoxic processes seen in MDD. First, CD69 is involved in systemic lupus erythematosus (SLE), rheumatoid arthritis, atopic dermatitis, and systemic sclerosis, as well as animal models of arthritis, myocarditis, and inflammatory bowel disease14. Second, increased expression of CD71 on T cells is associated with SLE disease activity and an enhanced Th-17 profile, whereas CD71 may cause autoimmunity in an SLE model52. Targeting CD71 reduces autoimmunity and pathology in a mouse model of SLE and increases the secretion of IL-1052. Third, CD40L-CD40 interactions play a significant role in neurodegenerative and neurological disorders, such as Parkinson's disease, Alzheimer's disease, stroke, multiple sclerosis, and epilepsy53. Increased permeability of the blood–brain barrier (BBB), injury to neuronal and glial cells, neuroinflammation, and the formation of microthrombi are mediated by CD40L-CD40 signaling53. In an animal model of autoimmune disease, CD4+ immune cells, attracted and activated by IL-16, are associated with neuroinflammation54. CD40L+ T cells may cross the BBB and activate microglial CD40, resulting in the production of pro-inflammatory cytokines and reactive nitrogen species, which may cause demyelination of axons53. In addition, activated CD8+ cells may induce IRS responses and induce neurotoxicity55.
Breakdown of immune tolerance in MDD
The third major finding of this study is that lowered numbers/expression of CD3+ CD4+ CD25+ FoxP3+ GARP+ and CD3+ CD4+ CD25+ FOXP3+ CB1+ coupled with T cell activation, is inversely associated with the severity of illness. Jahangard et al. showed that the frequency of FOXP3+ Tregs was decreased in untreated MDD patients compared to healthy controls, while the proliferation of circulating CD4+ T cells was increased in the patients56. Ellul et al.57 found an association between increased risk of MDD and decreased Treg numbers in association with increased inflammation57. Importantly, during the remission phase of mood disorders, Treg functions may be upregulated10.
Treg cells and CIRS activities ensure immune homeostasis by regulating innate and adaptive immune effector cells and restraining the detrimental activities of these effector cells. The transcription factor FoxP3 is a key regulatory factor in Treg activities by producing CIRS cytokines, such as IL-10 and TGF-β1, depleting growth factors, and expressing the co-inhibitory CTLA-4 molecule58. Furthermore, CD25 signaling and the expression levels of FoxP3 are important for the function and stability of these cells59–62. Moreover, CD25+ (or IL-2R) Treg cells may absorb IL-2 precluding IL-2 for the growth of CD8+ T effector and Th-1 cells63. Depletion of Treg cells may be the consequence of reduced fitness, altered apoptosis, and increased inhibitory activities of CD7152.
Overall, the findings indicate that the acute phase of severe MDD is characterized by a breakdown of peripheral immune tolerance. Loss of Treg-associated immune tolerance can lead to immunopathologies and autoimmune diseases64. In addition, Treg cells have neuroprotective properties by suppressing neurotoxic T effector responses and production of neurotoxic cytokines, and favoring anti-inflammatory microglia polarization6,63. When the frequency of Tregs is decreased or their immunosuppressive effects are diminished, the Teff/Treg balance is shifted towards the production of pro-inflammatory Th-1 and Th-7 phenotypes63. As such, diminished Treg functions predispose to neuroinflammatory disorders including multiple sclerosis, stroke, and Alzheimer’s disease. These findings further support the IRS/CIRS theory of mood disorders and previous studies that have highlighted the imbalance of IRS and CIRS activity in patients with MDD57,65–67.
Limitations
This study would have been more interesting if we had measured other immune-related biomarkers of depression including oxidative and nitrosative stress markers. We stimulated PBMCs with anti-CD3/anti-CD28 antibodies and detected Treg cells by gating CD3+ CD4+ CD25+ FoxP3+ cells. Nevertheless, activated conventional T cells can be induced to transiently express Foxp368. However, GARP is only expressed on Treg cells and upregulated in activated Treg cells. Moreover, GARP+ Treg cells clearly show suppressive functions directed at target cells42,69. In our study, we detected surface GARP on Treg cells (CD3+ CD4+ CD25+ FoxP3+ cells) that is likely separated from FoxP3-expression by conventional T cells upon stimulation. In addition, we gated CD25++ which marks Treg cells and excluded activated T cells as shown in our gating strategy figures (in ESF1). Our gating strategy as shown in the ESF1 indicates that no spillover effects were present. Moreover, our unstimulated samples were used to separate negative from positive effects and to determine the best gate placement for the stimulated activation markers70. In addition, the current study also used the fluorescence minus one (FMO) method to identify positive populations.
Conclusions
The acute phase of severe MDD is characterized by a breakdown of immune tolerance, and CD40L activation, which are associated with increased neuroimmunotoxic potential. Diminished Treg neuroprotection, T cell activation, Th-1 polarization, increased CD71+ and CD40L expression and IL-16 production are new drug targets to treat the T effector-associated neurotoxicity of the acute phase of severe MDD.
There is now some evidence that anti-inflammatory agents may be useful to treat MDD patients with an elevated immune profile71. A recent meta-analysis shows that different types of anti-inflammatory agents have some clinical efficacy in treating MDD72. Nevertheless, our results show that MDD should not be treated with repurposing anti-inflammatory drugs (e.g., Cox-2 inhibitors) and antioxidant supplements (e.g., curcumin), but rather should boost Treg functions and target CD40L or CD71 and IL-16 to attenuate T cell activation.
Supplementary Information
Author contributions
Design of the study: MM and MR. Recruitment of the participants: MR and KJ. Assays: PS. Statistical analyses: MM. Visualization: MM and PS. Editing: MR, KJ, AS, and PS. All authors agreed to publish the final version of the manuscript.
Funding
This work was supported by AMERI-ASIA MED CO, Ltd, a grant from H.M. the King Bhumibol Adulyadej's 72nd Birthday Anniversary Scholarship support PhD scholarship to MR; and the Thailand Science research and Innovation Fund Chulalongkorn University (HEA663000016) to MM.
Data availability
The dataset generated during and/or analyzed during the current study will be available from the corresponding author (M.M.) upon reasonable request and once the dataset has been fully exploited by the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61865-y.
References
- 1.World Health Organisation. Depression and Other Common Mental Disorders: Global Health Estimates. Contract No.: WHO/MSD/MER/2017.2 (World Health Organization, 2017)
- 2.Felger JC. Role of Inflammation in Depression and Treatment Implications. In: Macaluso M, Preskorn SH, editors. Antidepressants: From Biogenic Amines to New Mechanisms of Action. Springer; 2019. pp. 255–286. [Google Scholar]
- 3.Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(2):445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: A systematic review and meta-analysis. Mol. Psychiatry. 2020;25(2):339–350. doi: 10.1038/s41380-019-0474-5. [DOI] [PubMed] [Google Scholar]
- 5.Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Dębowska W, et al. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;80(Pt C):217–226. doi: 10.1016/j.pnpbp.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Carvalho AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 2018;55(12):8885–8903. doi: 10.1007/s12035-018-1016-x. [DOI] [PubMed] [Google Scholar]
- 7.Ferencova N, Visnovcova Z, Ondrejka I, Funakova D, Hrtanek I, Kelcikova S, et al. Evaluation of inflammatory response system (IRS) and compensatory immune response system (CIRS) in adolescent major depression. J. Inflamm. Res. 2022;15:5959–5976. doi: 10.2147/JIR.S387588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debnath M, Berk M, Maes M. Translational evidence for the inflammatory response system (IRS)/compensatory immune response system (CIRS) and neuroprogression theory of major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;111:110343. doi: 10.1016/j.pnpbp.2021.110343. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hakeim HK, Al-Naqeeb TH, Almulla AF, Maes M. The physio-affective phenome of major depression is strongly associated with biomarkers of astroglial and neuronal projection toxicity which in turn are associated with peripheral inflammation, insulin resistance and lowered calcium. J. Affect. Disord. 2023;331:300–312. doi: 10.1016/j.jad.2023.03.072. [DOI] [PubMed] [Google Scholar]
- 10.Maes M, Nani JV, Noto C, Rizzo L, Hayashi MAF, Brietzke E. Impairments in peripheral blood T effector and T regulatory lymphocytes in bipolar disorder are associated with staging of illness and anti-cytomegalovirus IgG levels. Mol. Neurobiol. 2021;58(1):229–242. doi: 10.1007/s12035-020-02110-1. [DOI] [PubMed] [Google Scholar]
- 11.Bauer ME, Teixeira AL. Neuroinflammation in mood disorders: Role of regulatory immune cells. Neuroimmunomodulation. 2021;28(3):99–107. doi: 10.1159/000515594. [DOI] [PubMed] [Google Scholar]
- 12.Becking K, Haarman BCM, Grosse L, Nolen WA, Claes S, Arolt V, et al. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. J. Neuroimmunol. 2018;319:28–36. doi: 10.1016/j.jneuroim.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Sauls RS, McCausland C, Taylor BN. Histology, T-Cell Lymphocyte. StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 14.Cibrián D, Sánchez-Madrid F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017;47(6):946–953. doi: 10.1002/eji.201646837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maes, M., Rachayon, M., Jirakran, K., Sughondhabirom, A., Almulla, A. & Sodsai, P. Role of T and B Lymphocyte Cannabinoid Type 1 and 2 Receptors in Major Depression and Suicidal Behaviors: Effects of In Vitro Cannabidiol Administration (2023). [DOI] [PubMed]
- 16.Maes M, Moraes JB, Congio A, Bonifacio KL, Barbosa DS, Vargas HO, et al. Development of a novel staging model for affective disorders using partial least squares bootstrapping: Effects of lipid-associated antioxidant defenses and neuro-oxidative stress. Mol. Neurobiol. 2019;56(9):6626–6644. doi: 10.1007/s12035-019-1552-z. [DOI] [PubMed] [Google Scholar]
- 17.Grosse L, Hoogenboezem T, Ambrée O, Bellingrath S, Jörgens S, de Wit HJ, et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav. Immun. 2016;54:38–44. doi: 10.1016/j.bbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl. Acad. Sci. 2015;112(25):E3246–E3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maes M, Stevens W, DeClerck L, Bridts C, Peeters D, Schotte C, et al. Immune disorders in depression: Higher T helper/T suppressor-cytotoxic cell ratio. Acta Psychiatr. Scand. 1992;86(6):423–431. doi: 10.1111/j.1600-0447.1992.tb03292.x. [DOI] [PubMed] [Google Scholar]
- 20.Maes M, Stevens WJ, Declerck LS, Bridts CH, Peters D, Schotte C, et al. Significantly increased expression of T-cell activation markers (interleukin-2 and HLA-DR) in depression: Further evidence for an inflammatory process during that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1993;17(2):241–255. doi: 10.1016/0278-5846(93)90045-T. [DOI] [PubMed] [Google Scholar]
- 21.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J. Affect. Disord. 1995;34(4):301–309. doi: 10.1016/0165-0327(95)00028-L. [DOI] [PubMed] [Google Scholar]
- 22.Chang X, Ma M, Chen L, Song Z, Zhao Z, Shen W, et al. Identification and characterization of elevated expression of transferrin and its receptor TfR1 in mouse models of depression. Brain Sci. 2022;12(10):1267. doi: 10.3390/brainsci12101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motamedi M, Xu L, Elahi S. Correlation of transferrin receptor (CD71) with Ki67 expression on stimulated human and mouse T cells: The kinetics of expression of T cell activation markers. J. Immunol. Methods. 2016;437:43–52. doi: 10.1016/j.jim.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Mohd Ashari NS, Mohamed Sanusi SNF, Mohd Yasin MA, Che Hussin CM, Wong KK, Shafei MN. Major depressive disorder patients on antidepressant treatments display higher number of regulatory T cells. Malays. J. Pathol. 2019;41(2):169–176. [PubMed] [Google Scholar]
- 25.Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, et al. CD4+ CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One. 2012;7(7):e42054. doi: 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blair PJ, Riley JL, Harlan DM, Abe R, Tadaki DK, Hoffmann SC, et al. CD40 ligand (CD154) triggers a short-term CD4(+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J. Exp. Med. 2000;191(4):651–660. doi: 10.1084/jem.191.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay NQ, Lee DCP, Chua YL, Prabhu N, Gascoigne NRJ, Kemeny DM. CD40L expression allows CD8(+) T cells to promote their own expansion and differentiation through dendritic cells. Front. Immunol. 2017;8:1484. doi: 10.3389/fimmu.2017.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henn V, Steinbach S, Büchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98(4):1047–1054. doi: 10.1182/blood.V98.4.1047. [DOI] [PubMed] [Google Scholar]
- 29.Frentsch M, Stark R, Matzmohr N, Meier S, Durlanik S, Schulz AR, et al. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013;122(3):405–412. doi: 10.1182/blood-2013-02-483586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. J. Affect. Disord. 2013;150(2):384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Pettersson A, Modin S, Wahlström R, Af Winklerfelt Hammarberg S, Krakau I. The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: A mixed-methods study. BMC Fam. Pract. 2018;19(1):19. doi: 10.1186/s12875-017-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maes MH, Stoyanov D. False dogmas in mood disorders research: Towards a nomothetic network approach. World J. Psychiatry. 2022;12(5):651–667. doi: 10.5498/wjp.v12.i5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes M, Almulla AF. Research and diagnostic algorithmic rules (RADAR) and RADAR plots for the first episode of major depressive disorder: Effects of childhood and recent adverse experiences on suicidal behaviors, neurocognition and phenome features. Brain Sci. (Internet) 2023;13(5):714. doi: 10.3390/brainsci13050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12(5):456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 35.Kyttaris VC. Chapter 64—New treatments for systemic lupus erythematosus. In: Tsokos GC, editor. Systemic Lupus Erythematosus. Academic Press; 2016. pp. 551–557. [Google Scholar]
- 36.Koguchi Y, Buenafe AC, Thauland TJ, Gardell JL, Bivins-Smith ER, Jacoby DB, et al. Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8− thymocytes and invariant NKT cells but not in Treg cells. PLoS One. 2012;7(2):e31296. doi: 10.1371/journal.pone.0031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg V, Modak M, Brell J, Puck A, Künig S, Jutz S, et al. Iron Deprivation in human T cells induces nonproliferating accessory helper cells. ImmunoHorizons. 2020;4(4):165–177. doi: 10.4049/immunohorizons.2000003. [DOI] [PubMed] [Google Scholar]
- 38.Brekelmans P, van Soest P, Leenen PJ, van Ewijk W. Inhibition of proliferation and differentiation during early T cell development by anti-transferrin receptor antibody. Eur. J. Immunol. 1994;24(11):2896–2902. doi: 10.1002/eji.1830241147. [DOI] [PubMed] [Google Scholar]
- 39.Saraiva DP, Azeredo-Lopes S, Antunes A, Salvador R, Borralho P, Assis B, et al. Expression of HLA-DR in cytotoxic T lymphocytes: A validated predictive biomarker and a potential therapeutic strategy in breast cancer. Cancers (Internet) 2021;13(15):3841. doi: 10.3390/cancers13153841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 41.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol. Life Sci. 2009;66(16):2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun L, Jin H, Li H. GARP: A surface molecule of regulatory T cells that is involved in the regulatory function and TGF-β releasing. Oncotarget. 2016;7(27):42826–42836. doi: 10.18632/oncotarget.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan BL. The role of CB1 in immune modulation by cannabinoids. Pharmacol. Ther. 2013;137(3):365–374. doi: 10.1016/j.pharmthera.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Foley ÉM, Parkinson JT, Mitchell RE, Turner L, Khandaker GM. Peripheral blood cellular immunophenotype in depression: A systematic review and meta-analysis. Mol. Psychiatry. 2023;28(3):1004–1019. doi: 10.1038/s41380-022-01919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almulla AF, Al-Hakeim HK, Maes M. Chronic fatigue and affective symptoms in acute and long COVID are attributable to immune-inflammatory pathways. Psychiatry Clin. Neurosci. 2023;77(2):125–126. doi: 10.1111/pcn.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathy NL, Bannert N, Norley SG, Kurth R. Cutting edge: CD4 is not required for the functional activity of IL-16. J. Immunol. 2000;164(9):4429–4432. doi: 10.4049/jimmunol.164.9.4429. [DOI] [PubMed] [Google Scholar]
- 47.Hall BM. CD4+CD25+ T regulatory cells in transplantation tolerance: 25 years on. Transplantation. 2016;100(12):2533–2547. doi: 10.1097/TP.0000000000001436. [DOI] [PubMed] [Google Scholar]
- 48.Cullen JG, McQuilten HA, Quinn KM, Olshansky M, Russ BE, Morey A, et al. CD4(+) T help promotes influenza virus-specific CD8(+) T cell memory by limiting metabolic dysfunction. Proc. Natl. Acad. Sci. USA. 2019;116(10):4481–4488. doi: 10.1073/pnas.1808849116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruedl C, Bachmann MF, Kopf M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur. J. Immunol. 2000;30(7):2056–2064. doi: 10.1002/1521-4141(200007)30:7<2056::AID-IMMU2056>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 50.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 2009;21(5):265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 1999;5(7):774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 52.Voss K, Sewell AE, Krystofiak ES, Gibson-Corley KN, Young AC, Basham JH, et al. Elevated transferrin receptor impairs T cell metabolism and function in systemic lupus erythematosus. Sci. Immunol. 2023;8(79):eabq0178. doi: 10.1126/sciimmunol.abq0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ots HD, Tracz JA, Vinokuroff KE, Musto AE. CD40-CD40L in neurological disease. Int. J. Mol. Sci. 2022;23(8):4115. doi: 10.3390/ijms23084115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hridi SU, Barbour M, Wilson C, Franssen AJ, Harte T, Bushell TJ, et al. Increased levels of IL-16 in the central nervous system during neuroinflammation are associated with infiltrating immune cells and resident glial cells. Biology (Basel) 2021;10(6):472. doi: 10.3390/biology10060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu D, Xia W, Weiner HL. CD8(+) T cells in neurodegeneration: Friend or foe? Mol. Neurodegener. 2022;17(1):59. doi: 10.1186/s13024-022-00563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jahangard L, Behzad M. Diminished functional properties of T regulatory cells in major depressive disorder: The influence of selective serotonin reuptake inhibitor. J. Neuroimmunol. 2020;344:577250. doi: 10.1016/j.jneuroim.2020.577250. [DOI] [PubMed] [Google Scholar]
- 57.Ellul P, Mariotti-Ferrandiz E, Leboyer M. Regulatory T cells as supporters of psychoimmune resilience: toward immunotherapy of major depressive disorder. Front. Neurol. 2018;9:167. doi: 10.3389/fneur.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu L, Bai Y, Wang Z. Elevated T cell activation score is associated with improved survival of breast cancer. Breast Cancer Res. Treat. 2017;164(3):689–696. doi: 10.1007/s10549-017-4281-x. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colamatteo A, Carbone F, Bruzzaniti S, Galgani M, Fusco C, Maniscalco GT, et al. Molecular mechanisms controlling Foxp3 expression in health and autoimmunity: From epigenetic to post-translational regulation. Front. Immunol. 2019;10:3136. doi: 10.3389/fimmu.2019.03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K, Gu J, Ni X, Ding Z, Wang Q, Zhou H, et al. CD25 signaling regulates the function and stability of peripheral Foxp3+ regulatory T cells derived from the spleen and lymph nodes of mice. Mol. Immunol. 2016;76:35–40. doi: 10.1016/j.molimm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Permanyer M, Bošnjak B, Glage S, Friedrichsen M, Floess S, Huehn J, et al. Efficient IL-2R signaling differentially affects the stability, function, and composition of the regulatory T-cell pool. Cell. Mol. Immunol. 2021;18(2):398–414. doi: 10.1038/s41423-020-00599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machhi J, Kevadiya BD, Muhammad IK, Herskovitz J, Olson KE, Mosley RL, et al. Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders. Mol. Neurodegener. 2020;15(1):32. doi: 10.1186/s13024-020-00375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitz A, Singer E, Hafler D. Regulatory T cells: From discovery to autoimmunity. Cold Spring Harb. Perspect. Med. 2018;8(12):29041. doi: 10.1101/cshperspect.a029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tubbs JD, Ding J, Baum L, Sham PC. Immune dysregulation in depression: Evidence from genome-wide association. Brain Behav. Immun. Health. 2020;7:100108. doi: 10.1016/j.bbih.2020.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Müller N. Immunological aspects of the treatment of depression and schizophrenia. Dialogues Clin. Neurosci. 2017;19(1):55–63. doi: 10.31887/DCNS.2017.19.1/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: Towards precision medicine. Nat. Rev. Drug Discov. 2022;21(3):224–244. doi: 10.1038/s41573-021-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Ioan-Facsinay A, van der Voort EIH, Huizinga TWJ, Toes REM. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37(1):129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 69.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. 2009;106(32):13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry Part A. 2006;69A(9):1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 71.Kopschina Feltes P, Doorduin J, Klein HC, Juárez-Orozco LE, Dierckx RA, Moriguchi-Jeckel CM, et al. Anti-inflammatory treatment for major depressive disorder: Implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J. Psychopharmacol. 2017;31(9):1149–1165. doi: 10.1177/0269881117711708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bai S, Guo W, Feng Y, Deng H, Li G, Nie H, et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: A systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry. 2020;91(1):21–32. doi: 10.1136/jnnp-2019-320912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during and/or analyzed during the current study will be available from the corresponding author (M.M.) upon reasonable request and once the dataset has been fully exploited by the authors.