Abstract
Obesity is an endemic pathophysiological condition and a comorbidity associated with hypercholesterolemia, hypertension, cardiovascular disease, type 2 diabetes mellitus, and cancer. The adipose tissue of obese subjects shows hypertrophic adipocytes, adipocyte hyperplasia, and chronic low-grade inflammation. S100 proteins are Ca2+-binding proteins exclusively expressed in vertebrates in a cell-specific manner. They have been implicated in the regulation of a variety of functions acting as intracellular Ca2+ sensors transducing the Ca2+ signal and extracellular factors affecting cellular activity via ligation of a battery of membrane receptors. Certain S100 proteins, namely S100A4, the S100A8/S100A9 heterodimer and S100B, have been implicated in the pathophysiology of obesity-promoting macrophage-based inflammation via toll-like receptor 4 and/or receptor for advanced glycation end-products ligation. Also, serum levels of S100A4, S100A8/S100A9, S100A12, and S100B correlate with insulin resistance/type 2 diabetes, metabolic risk score, and fat cell size. Yet, secreted S100B appears to exert neurotrophic effects on sympathetic fibers in brown adipose tissue contributing to the larger sympathetic innervation of this latter relative to white adipose tissue. In the present review we first briefly introduce S100 proteins and then critically examine their role(s) in adipose tissue and obesity.
Keywords: Adipocyte, Macrophage, Cytokine, Inflammation, Transdifferentiation, Receptor, White adipose tissue, Brown adipose tissue
Introduction
Obesity is an endemic medical problem, particularly in countries where access to food is virtually unlimited and a sedentary lifestyle is prevailing, with considerable costs to public health [1–3]. It has been estimated that on a global scale, in 2013 ~ 36% of men, ~ 38% of women, and ~ 23% of children were overweight or obese [4, 5]. The relevance of these data stands on the fact that obesity is a comorbidity associated with several pathological conditions such as hypercholesterolemia, hypertension, cardiovascular disease, type 2 diabetes mellitus, and cancer [6–9].
Metabolic obesity defines a condition of increased subcutaneous and visceral adipose tissue (i.e., white adipose tissue, WAT) consequent to excess food intake leading to both hypertrophy of existing adipocytes and adipocyte hyperplasia [6–8]. Histopathologically, obese fat shows infiltration of inflammatory macrophages encircling dying adipocytes to form crown-like structures that sometimes evolve into multinucleated giant cells or foam cells [10, 11]. Since in this condition the local demand for blood exceeds supply, a fraction of adipocytes become stressed or die in consequence of hypoxia, which results in the liberation of damage-associated molecular patterns (DAMPs) into the microenvironment. DAMPs in turn attract innate immune cells such as dendritic cells, macrophages, and granulocytes to trigger type 1 (proinflammatory) immune responses. The local release of proinflammatory cytokines [for example tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), and IL-6] by innate immune cells provokes infiltration of additional immune cells including various granulocytes, group 1 innate lymphoid cells, B cells, and CD8+ T cells, which perpetuate chronic inflammatory responses [6, 12–18]. However, in obesity the population of adipose tissue macrophages (ATMs), though heterogeneous, cannot be distinguished into classically activated (M1, proinflammatory), alternatively activated (M2, anti-inflammatory), and unstimulated (M0) macrophages as found in the context of bacterial infection. Indeed, ATMs from obese humans and mice do not express M1 markers [19]; rather, in the context of metabolic disease (i.e. in high glucose, insulin and palmitate conditions) a distinct population of metabolically activated macrophages could be identified exhibiting a unique (IL1b+, TNFa+, ABCA1+, and CD36 +) proinflammatory macrophage phenotype [19]. Metabolically activated macrophages both fuel inflammation and promote dead adipocyte clearance through lysosomal exocytosis, thus exerting detrimental and beneficial effects via activation of NADPH oxidase 2, toll-like receptor (TLR) 2 and MYD88 [20]. These latter studies also established that targeting NADPH oxidase 2 at early obesity phases might be beneficial reducing the inflammatory response, whereas at later times NADPH oxidase 2 inhibition is detrimental causing inability of macrophages to clear dead adipocytes. Further studies established that in obesity ATMs can be subdivided in at least three phenotypes: a CD9+ phenotype, a CD9‒ phenotype, and an Ly6c phenotype [21]. Proinflammatory CD9+ macrophages are located in crown-like structures, contain high amounts of intracellular lipid, and may influence mammalian metabolism [21]. Indeed, adoptive transfer of CD9+ ATMs to lean mice was sufficient to lead to obesity-associated adipose inflammation [21]. Antiinflammatory Ly6c macrophages are located outside crown-like structures and suggested to release tissue-regulatory factors attempting to repair tissue damage [21]. CD9‒ macrophages are the least represented phenotype in obesity while being the most represented one in lean subjects [21]. Furthermore, adipose tissue CD642‒/CD11c+ dendritic cells appear to be independent contributors to adipose tissue inflammation during obesity [22]. Likewise, important for visceral adipose tissue (VAT) and metabolic homeostasis is the VAT compartment of regulatory T (Treg) lymphocytes [23]. VAT in lean subjects is characterized by a relatively high proportion of Foxp3+/CD4+ Treg cells: obesity is accompanied by a remarkable decrease in the population of Treg cells specific to VAT leading to adipose tissue inflammation [24] and insulin resistance [25]. Treg cells of thymic origin colonize VAT early after birth and expand locally under the action of IL-33 and major histocompatibility complex class-II [23]. In conclusion, excess food intake leads to obesity and a chronic status of low-grade inflammation in adipose tissue in which ATMs play a major role and dysregulated activities of adipose tissue dendritic cells and Treg cells act as independent contributors.
In normal physiological conditions prolonged fasting induces gastric endocrine cells to secrete the hormone, ghrelin, that targets various hypothalamic nuclei to induce feeding [26]. Ingested food transits from the stomach to the small intestine for completion of digestion and absorption of nutrients. Among the various intestinal epithelial cells those known as enteric endocrine cells (EECs) sense nutrients to secrete a plethora of molecules (the gastrointestinal hormones) that act to regulate their own activity and the activity of nearby cells (e.g. other epithelial cells and/or EECs, smooth muscle cells) and/or distant cells—via the bloodstream—located in the gastrointestinal tract and other organs/tissues [27]. Regulatory activities of intestinal hormones are intended to optimize food intake and the products of food digestion not only for energetic and anabolic purposes [27–29] but also for behavior (including eating behavior), mood regulation, cognitive functions, and learning and memory [30–33]. Basically, this gut–brain–gut loop serves the function of inducing eating behavior and halting in an ordered manner so as to maintain energy homeostasis and control body weight.
Excess food intake causes obesity via nutrient-induced hypersecretion of certain intestinal hormones activating specific receptors located on the surface of responsive cells. Among these hormones, glucose-dependent insulinotropic polypeptide (GIP), secreted from K cells in the initial tract of small intestine, is responsible for the majority of the insulinotropic incretin effects in humans and participates in the regulation of energy expenditure to maintain energy balance [27, 29, 34, 35]. GIP induced by absorption of ingested fat or glucose facilitates fat storage in adipocytes and inhibits lipolysis thus promoting obesity and insulin resistance by acting directly on adipocytes [36, 37]. Glucagon-like peptide-1 (GLP-1), the proteolytic product of the prohormone preproglucagon, is another incretin hormone able to stimulate insulin secretion in response to food intake [37–39]. GLP-1 is released postprandial from EECs under the action of diet-derived glucose and following ingestion of prebiotics. Circulating GLP-1 interacts with its receptor, a transmembrane guanine nucleotide-binding protein coupled receptor, located in various tissues including pancreatic β-(insulin secreting) cells, kidney, heart, and hypothalamus. GLP-1 stimulates β-cell insulin biosynthesis and exocytosis while simultaneously promoting β-cell survival, preserving β-cell mass, and inhibiting glucagon secretion. In addition, it slows gastric emptying which contributes to decrease in blood glucose and appetite suppression. This latter effect mainly depends on GLP-1 acting on the hypothalamic paraventricular nucleus, lateral hypothalamus, and the arcuate nucleus leading to attenuation of orexigenic pathways, appetite suppression, and decreased food consumption. Thus, from a functional perspective GIP and GLP-1 exert opposing roles.
Given the association of obesity with several pathological conditions as mentioned above, the cellular and molecular mechanism underpinning chronic low-grade inflammation of the obese adipose tissue is attracting considerable interest among the clinical and preclinical research communities for diagnostic, therapeutic, and preventive purposes. While several excellent reviews have been published on obesity and association of obesity with several diseases [6–9, 14–18], we sought to focus on the role(s) played by certain members of the S100 protein family in the pathophysiology of obesity. Hints for that come from the observation that some S100 proteins have documented roles in inflammation in general and certain S100 proteins have been associated with the pathophysiology of obesity. In the present review we first briefly introduce S100 proteins and then critically examine the role(s) played by some members of the S100 protein family in the pathophysiology of obesity.
The S100 protein family
The S100 family comprises more than 20 proteins exclusively expressed in vertebrates in a cell-specific manner and involved in the regulation of both intracellular and extracellular activities [40]. Of the 24 human S100 genes, 19 (S100 proteins, group A) are located within chromosome 1q21 [41]. Other gene locations include S100A11P, which maps to chromosome 7q22-q3, S100B, which maps to chromosome 21q22, S100G, which maps to chromo-some Xp22, S100P, which maps to chromosome 4p16, and S100Z, which maps to chromosome 5q13 [41]. Within cells the majority of S100 proteins function as Ca2+ sensors becoming activated upon stimuli causing transient increases in cytosolic free Ca2+ concentration. In their Ca2+-bound form these S100 proteins participate in the regulation of cell proliferation, differentiation and motility, apoptosis, enzyme activities, the state of assembly–disassembly of cytoskeleton constituents, the abundance and activity of transcription factors, phagocytosis, redox equilibrium, Ca2+ release from Ca2+ stores, ion channel activities, protein degradation, and the response of immune cells [40, 42]. There is a variable (~ 30 to ~ 50%) degree of sequence identity among the various members of the S100 family with the highest sequence identity being found in their Ca2+-binding sites and the least one found in the linker region between the two Ca2+-binding sites and in the N- and C-terminal regions. This and the cell-specificity of S100 protein expression assure that each cell type expresses a small set of S100 proteins to bring about some of the activities listed above; each S100 protein can bind a defined set of target proteins given that S100 proteins use the least homologous region of their structure to interact with their targets [40, 42]. In the reducing milieu of the cell’s interior S100 proteins exist as homodimers in which two subunits are held together by non-covalent bonds. However, S100A8 and S100A9 associate to form the S100A8/S100A9 heterodimer, also known as calprotectin. Also, at odds with all other S100 proteins S100G exists as a monomer and acts as a Ca2+ modulator protein binding Ca+ with high affinity and buffering cytosolic Ca2+ to extinguish the Ca2+ signal [40, 42].
Some S100 proteins also act as extracellular signals being either constitutively secreted by certain cell types, passively released by damaged or dying cells, or liberated by, e.g. immune cells during the course of inflammatory events [40, 43, 44]. Several extracellular S100 proteins act as DAMPs activating a battery of membrane receptors including the pattern recognition receptors, RAGE (receptor for advanced glycation endproducts) and TLR-4, and G-protein coupled receptors [40, 43–46]. While extracellular S100 proteins affect the activity of a variety of cell types including astrocytes, neurons, microglia, cardiomyocytes, adipocytes, skeletal myoblasts, epithelial cells, and smooth muscle cells in normal and pathological conditions, immune cells (mostly microglia and macrophages) represent a major target of S100 proteins in inflammation [40, 44, 46–52]. Relevant to the present review, some S100 proteins are expressed in and/or act on adipose tissue participating in the pathophysiology of obesity as discussed below.
S100A1, a marker of adipocyte differentiation
S100A1 is expressed in skeletal muscle fibers, cardiomyocytes, and certain neuronal populations [47]. S100A1 improves myocardial contractility by interacting with the sarcoplasmic reticulum Ca2+-ATPase [53, 54] and ryanodine receptor 2 (RyR2) [55] in the heart, resulting in improved Ca2+ handling and contractile performance, and targets the cardiac sarcomere and mitochondria, thereby reducing pre-contractile passive tension and enhancing oxidative energy generation. S100A1 deficiency results in abnormal sarcoplasmic reticulum Ca2+ content and fluxes, accelerated deterioration of cardiac performance, and transition to heart failure and S100A1 gene delivery rescues failing myocardium [56, 57]. In skeletal myofibers S100A1 binds to RyR1 [58, 59] and potentiates its open probability and plays role in skeletal muscle excitation–contraction coupling [60]. S100A1 also interacts with the giant sarcomeric kinase, titin, with potential improvement of sarcomeric compliance [61–63]. It also stimulates membrane-bound guanylate cyclase in photoreceptors likely involved in dark adaptation [64] and regulates energy metabolism by stimulating fructose-1,6-biphosphate aldolase and inhibiting phosphoglucomutase and glycogen phosphorylase [40].
S100A1 has been detected in white preadipocytes and considered a marker of adipocyte differentiation [65, 66], with declining S100A1 levels with adipocyte aging in vitro [67] (Table 1). However, the functional meaning of these observations remains elusive. No information is available about extracellular effects of S100A1 in adipose tissue in normal conditions and obesity.
Table 1.
S100 proteins in obesity
| S100 protein | Adipose tissue | Obesity |
|---|---|---|
| S100A1 | Marker of adipocyte differentiation (decreasing levels with increasing differentiation) [65–67]. No functional implications reported | Not investigated |
| S100A4 | Expressed in adipose tissue stromal cells (fibroblasts and macrophages) but not in adipocytes [74]. Decreased S100A4 levels in adipose tissue following HFD [74]. Inhibition of adipogenesis and decreased mRNA levels of inflammatory factors following forced S100A4 expression in adipocytes or administration of S100A4 [74]. S100A4+ fibroblasts adjacent to preadipocytes required for maintenance of the preadipocyte pool [77] | Marker of metabolic complications of excess and/or dysfunctional WAT [71]. Correlation of serum levels of S100A4 with insulin resistance/type 2 diabetes, metabolic risk score, and fat cell size in a BMI-independent manner [71]. Aggravated symptoms of obesity in S100a4 null mice [74] |
| S100A8/S100A9 | Elevated S100A8/S100A9 levels in visceral adipose tissue in obesity and obesity-associated type 2 diabetes [80]. TNF-α-dependent upregulation of S100A8/S100A9 in isolated visceral adipocytes [80]. Elevated S100A8/A9 in adipocytes and SVFCs in animal models of obesity and obese subjects [80, 81]. LC n-3 PUFA-mediated upregulation of S100A8/S100A9 levels in adipocytes along with reduced S100A8 and S100A9 levels in monocytes, in LPS-treated subjects [86] | Marker of obesity [79]. Elevated S100A8/S100A9 levels in visceral adipose tissue in obesity and obesity-associated type 2 diabetes [80]. Elevated S100A8/A9 in adipocytes and SVFCs in animal models of obesity and obese subjects [80, 81]. Association of peripheral blood S100A8 and S100A9 mRNA levels with insulin resistance and inflammation [82]. S100A8/S100A9-dependent recruitment of macrophages to adipose tissue from bone marrow myeloid progenitors during inflammation and in hyperglycemia and obesity [83, 84]. GIP-dependent limitation of S100A8/S100A9 expression in ATMs with consequent restraint of adipose tissue inflammation and preservation of cold-induced adaptive thermogenesis in WAT [89] |
| S100A16 | Adipogenic activity of S100A16 via inhibition of the anti-adipogenic p53 [95, 96, 98] | Not investigated |
| S100A7/S100A15 | Not investigated | Correlation of elevated serum levels of S100A7/S100A15 in psoriatic patients with obesity [101] |
| S100A12 | LC n-3 PUFA-mediated upregulation of S100A12 levels in adipose tissue endothelial, macrophages and stromal cells of LPS-treated subjects [86] | Association of peripheral blood S100A12 mRNA levels with insulin resistance and inflammation [82] |
| S100B | Expressed in white [116] and brown [141, 142] adipocytes. S100B-dependent differentiation of murine embryonic mesenchymal cells into adipocytes via activation of JNK [121]. Oxidative stress/S100B-dependent transition of proliferating myoblasts into brown adipocytes [145]. Correlation of elevated levels of S100B with expression of UCP1 in geriatric myofibers [145] | Correlation of serum levels of S100B with BMI [133] and abdominal obesity and serum levels of triglycerides [134]. S100B/RAGE-induced adipocyte hypertrophy [135]. Elevated plasma and adipose tissue (hypertrophic adipocytes and ATMs) levels of S100B in an animal model of obesity [137]. Implication of adipocyte- and/or macrophage-derived S100B in adipose tissue inflammation via RAGE signaling [136]. Role of secreted S100B as a neurotrophic factor participating in the larger sympathetic innervation of BAT relative to WAT [142]. Role of S100B in WAT acclimation to cold [142] |
S100A4 in obesity: friend or foe?
S100A4, also known as Mts1 or Fsp1, has long been implicated in tumor development and progression and migration of tumor cells by acting from both inside and outside the cell [40, 49]; deletion of S100a4 resulted in suppression of tumor development and metastasis formation in mice [68]. However, S100A4 also was implicated in neuroprotection in an animal model of brain injury via ligation of IL-10R [69] and shown to promote neuronal survival in vitro by activating the ErbB4 receptor and its ligand Neuregulin 1 [70] with no role for the canonical S100 protein receptor, RAGE, in either cases.
S100A4 was proposed as a marker of metabolic complications of excess and/or dysfunctional WAT in humans [71] (Table 1). In this latter study S100A4 levels were higher in adipocyte progenitors than in immune cells and adipocytes; only the expression in adipocytes correlated with body mass index (BMI); and S100A4 levels decreased during adipocyte differentiation (with no quantification of S100A4 protein, yet). Interestingly, S100A4 was released in a time-dependent manner from subcutaneous WAT behaving like a true adipokine, and serum levels of S100A4 correlated with insulin resistance/type 2 diabetes, metabolic risk score, and fat cell size in a BMI-independent manner. However, this latter notion was inferred from the fact that high serum S100A4 levels were only detected in obese, diabetic subjects. Also, this study did not provide clues about the physiological role of S100A4 in adipose tissue and/or the pathophysiological role of S100A4 in obesity/diabetes; it was speculated that S100A4 may be involved in the link of obesity/insulin resistance to cancer given the known association of S100A4 with cancer [49, 68, 72, 73], but no experimental evidence is available that this is the case.
In stark contrast with the study discussed above, in another study S100A4 was found expressed in adipose tissue stromal cells (that is, fibroblasts and macrophages) with essentially no expression in adipocytes, and the S100A4 level in adipose tissue decreased contingent on high-fat diet (HFD) [74] (Table 1). Also, deletion of S100a4 aggravated symptoms of obesity in mice, suppressed insulin signaling after 12 weeks of HFD, and positively correlated with higher liver inflammation. In a specular way, treatment of 3T3-L1 adipocytes with extracellular S100A4 or overexpression of S100A4 in adipocytes inhibited adipogenesis and decreased mRNA levels of inflammatory factors, pointing to a protective effect of the protein via activation of Akt signaling [74]. How extracellular S100A4 exerts its effect on adipocytes has not been elucidated; the participation of RAGE, a known S100A4 receptor [40], was excluded because the RAGE inhibitor, FPS-ZM1, could not prevent S100A4-induced Akt activation, and the participation of heparan sulfate proteoglycans and Gαq-coupled receptor [75], the EGF receptor (EGFR) ligand/EGFR axis [76], IL-10R [69], and/or Neuregulin 1/ErbB4 receptor [70] was not investigated. Likewise, the mechanism by which overexpression of S100A4 in 3T3-L1 adipocytes inhibits adipogenesis was not investigated.
Another study found that WAT contains S100A4+ fibroblasts residing adjacent to preadipocytes and having no adipogenic potential themselves, yet being required for maintenance of the preadipocyte pool via platelet-derived growth factor signaling [77] (Table 1). Indeed, ablation of S100A4+ fibroblasts disturbed adipose homeostasis and resulted in loss of adiposity, similar to activation of canonical Wnt signaling in S100A4+ fibroblasts, and alteration of S100A4+ fibroblasts also made mice resistant to HFD-induced obesity. Further, S100A4+ fibroblasts were shown to regulate adipogenesis through extracellular matrix remodeling and activation of Yes-associated protein signaling. Notably, numbers of S100A4+ fibroblasts were found to increase during obesity suggesting a role for S100A4+ fibroblasts in the pathophysiology of obesity. However, the exact role of S100A4 in adipose tissue fibroblasts is not known. Combined together, the results so far available on the S100A4 role in adipose tissue call for further investigation.
S100A8/S100A9 at the crossroad between immune cells and energy metabolism
S100A8 and S100A9 form a heterodimer, known as calprotectin. They are abundantly expressed in neutrophils and monocytes, and once released, participate in the inflammatory response in chronic inflammatory disorders by activating the pattern recognition receptors TLR-4 and/or RAGE in responsive cells, and promote atherogenesis [40, 44, 46, 48, 52, 78]. Serum S100A8/S100A9 has been proposed as a marker of obesity [79] (Table 1). S100A8/S100A9 levels in VAT were found increased in obesity and obesity-associated type 2 diabetes: circulating and adipose S100A8 and S100A9 mRNA levels correlated with chronic low-grade obesity-associated inflammation and TNF-α upregulated S100A8 but not S100A9 in isolated visceral adipocytes [80]. S100A8/S100A9 was found at elevated levels in obese subjects and found upregulated in adipocytes and even more so in SVFCs in animal models of obesity [80, 81]. Also, peripheral blood mRNA levels of S100A8 and S100A9 correlated with insulin resistance and inflammation [82]. S100A8 and S100A9 were shown to recruit macrophages to adipose tissue from bone marrow myeloid progenitors during inflammation and in the setting of hyperglycemia and obesity [83, 84] and to play a crucial role in the induction of myeloid pro-inflammatory immune responses in WAT with detrimental metabolic implications [85] (Table 1). In particular, S100A8/S100A9 released locally by adipocytes and/or SVFCs in the setting of obesity induced the expression of Il-1β, Nlrp3, and Il-1r as well as the downstream TLR-4 genes, Tnfa and Mcp1, in ATMs with ensuing inflammasome-dependent IL-1β production and release; released IL-1β interacted with the IL-1 receptor on bone marrow myeloid progenitors to stimulate the production of monocytes and neutrophils (Fig. 1). Notably, among a large number of factors, levels of S100A8, but not S100A9, showed the earliest increase in HFD-treated mice compared to controls; S100A8-driven recruitment of myeloid cells in HFD-fed mice was shown to precede the development of metabolic symptoms; and antibody-mediated neutralization of extracellular S100A8 reduced macrophage mobilization in adipose tissue and improved insulin sensitivity [83]. Both TLR-4 and RAGE appeared to have a role, with RAGE playing a major role in the setting of diabetes-associated obesity. Collectively, these findings suggest that obesity promotes enhanced myelopoiesis via release of adipocyte/SVFC S100A8/S100A9 and that S100A8/S100A9 might have a pathogenic role in obesity.
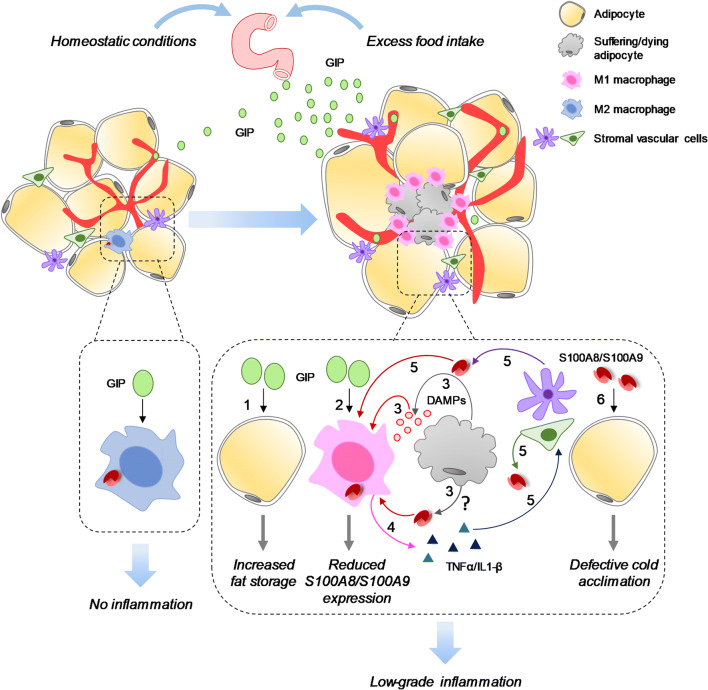
Fig. 1.
Proposed function(s) of S100A8/S100A9 in WAT. In normal physiological conditions ATMs are low in number and express low S100A8/S100A9 levels likely in part due to circulating GIP that selectively restrains S100A8/S100A9 expression in ATMs [89]; the combination of low ATM numbers and low S100A8/S100A9 levels concurs to create a condition of essentially no inflammation. Excess food intake enhances GIP secretion from EECs leading to increased fat storage (1) but reduced S100A8/S100A9 expression in ATMs (2) [89]. However, high fat diet leading to obesity results in the suffering and/or death of adipocytes (3) consequent to insufficient blood supply and liberation of DAMPs (including S100A8/S100A9?) that recruit and activate ATMs to release TNF-α and IL-1β (4) [6, 10, 14, 17, 18]. TNF-α and IL-1β stimulate S100A8/S100A9 release from stromal vascular cells (5) and likely hypertrophic adipocytes with ensuing S100A8/S100A9-dependent recruitment of macrophages and fueling of ATM-dependent inflammation [80, 83]. However, inflammation of the obese adipose tissue is low-grade because of GIPdependent restraint of S100A8/S100A9 expression in ATM [89]. Unrestrained S100A8/S100A9 expression in and release from ATMs (and likely stromal vascular cells) lead to defective cold acclimation in WAT (6) [89]
Interestingly, whereas high circulating S100A8 and S100A9 are found associated with increased chronic inflammation as mentioned above, high adipose S100A8 and S100A9 levels were reported to be associated with reduced inflammation in obese subjects supplemented with long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) [86], compounds proposed to protect against chronic inflammatory cardiometabolic diseases [87, 88]. In an acute inflammatory setting such as that induced by administration of lipopolysaccharide (LPS), supplementation with LC n-3 PUFA was shown to induce rapid upregulation of S100A8 and S100A9 levels in adipose tissue (Table 1). Likewise, LC n-3 PUFA supplementation induced adipose S100A8 and S100A9 upregulation in the setting of obese metabolic syndrome with no significant effects on other known inflammatory genes, e.g. IL6, IL10, CX3CL1, CCL2. In sorted adipocytes, while S100A8 and S100A9 were not significantly upregulated by LPS alone, pre-treatment with LC n-3 PUFA resulted in a significant LPS-induced increase in expression of both genes, as opposed to the reduced S100A8 and S100A9 levels in monocytes. While S100A8 and S100A9 have been proposed to mediate cell-specific LC n-3 PUFA-dependent modulation of systemic inflammation [86], the underpinning mechanism remains to be elucidated. The known decrease in S100A8 and S100A9 levels during maturation of monocytes to either proinflammatory or antiinflammatory macrophages [40, 44, 46] might have a role: the reduced baseline S100A8/S100A9 levels in ATMs compared to monocytes and even more so upon LC n-3 PUFA supplementation might lead to a blunted inflammatory response despite the enhanced S100A8/S100A9 levels in adipocytes. The LC n-3 PUFA-dependent downregulation of S100A8 and S100A9 in monocytes (and likely ATMs) [86] appears to agree with the reported macrophage-derived S100A8/S100A9-mediated inflammation in HFD-treated mice [89]. The mechanism of accumulation of S100A8 and S100A9 in adipocytes following LC n-3 PUFA supplementation remains to be elucidated [86]. One possible explanation is that only PUFA can bind S100A8/S100A9 [90] which might lead to S100A8/S100A9 sequestration within adipocytes. The functional role(s) of S100A8/S100A9 in adipocytes is(are) not known.
A myeloid-GIPR (GIP receptor)-S100A8/S100A9 signaling axis has been suggested to couple nutrient signals to the modulation of inflammation and adaptive thermogenesis [89] (Table 1). GIPR is found on pancreatic β-cells as well as on adipocytes [91] and in many areas of the central nervous system [92]. As mentioned in Introduction, GIP induced by absorption of ingested fat or glucose promotes obesity and insulin resistance by acting directly on adipocytes [26, 27]. However, augmentation of GIPR signaling in animal models of diet-induced obesity reduces adipose tissue infiltration of proinflammatory innate and adaptive immune cells [93, 94]. These apparently contrasting results might be explained by GIP acting on different cell populations in adipose tissue, albeit via the same receptor; GIP acting on adipocytes would promote fat storage while GIP acting on other cell populations in adipose tissue might exert immunomodulatory effects in the context of obesity. Supporting this possibility, GIP was shown to specifically restrain S100A8/S100A9 expression levels in myeloid cells [89]. Indeed, HFD concomitantly induced GIPR and S100A8 expression in WAT and GIP directly and selectively reduced S100a8 expression in ATMs in a GIPR-mediated manner (Fig. 1). Importantly, GIP treatment had no effect on ATM gene expression of the proinflammatory IL-6, TNF-α, and IL-1β which points to a unique inhibitory effect of GIP on S100A8/S100A9 expression in these cells. In HFD-fed mice, deletion of Gipr in bone marrow resulted in increased body weight and enhanced liver steatosis, and hepatic triglyceride content as well as impaired cold-induced adaptive thermogenesis in WAT manifesting as reduced oxygen consumption, heat production, and gene expression of the transcription factor peroxisome proliferator-activated receptor-γ co-activator 1-α, which is known to increase both mitochondrial biogenesis and WAT beiging. However, no such changes were seen in Gipr−/−/S100a9−/− bone marrow mice which points to a pivotal immunoregulatory role for GIP in myeloid cell S100A8/S100A9-driven metabolic dysfunction and myelopoiesis [89]. Of note, the sole deletion of S100a8/S100a9 in immune cells could not induce significant changes in most metabolic parameters, as expected if the GIP/GIPR axis keeps S100A8/S100A9 levels low in ATMs. Remarkably, expression levels of the brown adipocyte marker, uncoupling protein 1 (UCP1), and other thermogenesis-related genes were the same in brown adipose tissue (BAT) from Gipr−/− and Gipr−/−/S100a9−/− bone marrow mice suggesting that S100A8/S100A9 specifically disrupts cold-induced adaptive thermogenesis in WAT [89]. Thus, attenuation of S100A8/S100A9 expression and S100A8/S100A9-driven inflammation appears to be the molecular mechanism by which GIP plays an important immunoregulatory role in adipose tissue immune cells and supports cold-induced adaptive thermogenesis in WAT.
Interestingly, Gipr−/− mice exhibited resistance to weight gain and greater insulin sensitivity under conditions of prolonged nutrient excess in accordance with the reported role of GIP in facilitating fat storage in adipocytes and insulin resistance in the context of a HFD [26, 27]. In contrast, selective GIPR deletion in myeloid cells led to greater weight gain and insulin resistance compared with Gipr−/− mice. Mantelmacher et al. [89] suggested that in the (lean) Gipr−/− mice (that is in the absence of obesity-induced inflammation) resident ATMs are reduced in number and do not become proinflammatory because the reduced release of proinflammatory cytokines in these conditions results in reduced or no recruitment of other myeloid immune cells, whereas deletion of Gipr in bone marrow leads to unrestrained S100A8/S100A9 expression in ATMs and S100A8/S100A9-induced recruitment of immune cells with resultant inflammation of adipose tissue in the context of HFD-induced obesity and insulin resistance (Fig. 1). One issue that deserves attention is regarding the mechanism of increased S100A8/S100A9 in adipose tissue in obesity. Given the quite similar phenotype of most metabolic and immunologic parameters in wild-type and S100a9−/− bone marrow mice [89], SVFCs likely are the main source for pathological S100A8/S100A9 [80, 81] (Fig. 1) although the contribution of adipocyte-derived S100A8 [83] cannot be completely excluded. Thus, in the setting of HFD-induced obesity, adipocyte/SVFC-derived S100A8/S100A9 would support chronic inflammation in adipose tissue in spite of GIP-dependent restraint of S100A8/S100A9 expression in ATMs, and S100A8/S100A9 might disrupt cold-induced adaptive thermogenesis in WAT with a mechanism that awaits elucidation, yet. In addition, several other factors might independently impact the extent of local inflammation and whole-body energy homeostasis. Among these is S100B which is abundantly expressed in white adipocytes and might activate macrophages in adipose tissue in obesity (see below). Whether the GIP-GIPR axis affects S100A8/S100A9 expression levels in adipocytes also remains to be examined.
S100A16 is adipogenic
S100A16 has been proposed to exert adipogenic effects [95] (Table 1). The expression of S100A16 was found to increase during 3T3-L1 preadipocyte differentiation and forced expression of S100A16 in preadipocytes increased their proliferation and markedly enhanced adipogenesis resulting in significant reduction of insulin-stimulated glucose uptake and phosphorylation of Akt. In contrast, downregulation of S100A16 expression significantly inhibited adipogenesis and preadipocyte proliferation. S100A16 was shown to physically interact with and likely inhibits tumor suppressor protein p53, a known inhibitor of adipogenesis [96]. Estrogen treatment blunted S100A16 adipogenic effects by inhibiting S100A16 expression in adipocytes [97]. Also, bone marrow-derived mesenchymal stem cells were found to express S100A16 and increasing its level led to their differentiation into adipocytes [98]. In contrast, decreasing S100A16 level led to bone marrow-derived mesenchymal stem cell differentiation into osteoblasts. Thus, S100A16 appears to have a role in cell fate decision in bone marrow-derived mesenchymal stem cells. S100A16 levels in adipose tissue and body weight were reported to be lower in rats subjected to HFD plus high calcium intake than in rats subjected to HFD plus normal calcium diet and in controls [99]. However, no analysis was conducted of the mechanism by which high calcium intake might lead to reduced adipose S100A16 levels and adipose tissue abundance. S100A16’s extracellular effects, if any, are not known. The role of S100A16 in obesity deserves further investigation.
S100A7/A15: a link between obesity and psoriasis?
S100A7 (also known as psoriasin) is overexpressed in inflammatory skin diseases and induced in keratinocytes by IL-17 and IL-22 and by the TLR-5-ligand, flagellin [40, 100]. It has roles in antimicrobial responses and innate immunity. S100A7 adheres to and reduces E. coli survival. S100A7/RAGE and Zn2+ binding are required for chemotactic activity for lymphocytes, monocytes, and granulocytes, and S100A7 acts synergistically with S100A15, an S100A7 homolog.
A positive correlation has been described between elevated serum levels of S100A7/S100A15 in psoriatic patients and obesity, suggesting that the risk of developing psoriasis is directly related to higher BMI [101] (Table 1). However, this issue has not been investigated further, and the question remains whether S100A7 and/or S100AA15 have a pathogenic role or not in psoriasis in obese people. Recently, breast adipose stromal cells have been shown to promote breast carcinoma cell growth via upregulation of S100A7 expression in breast cancer cell lines [102]. While these results point to an oncogenic role for S100A7 in mammary epithelial cells, no information is available on S100A7/S100A15 expression levels in or effects on adipocytes, and/or stromal vascular fraction cells (SVFCs) in normal tissue or obesity.
S100A12: any role in obesity?
S100A12, also known as calgranulin C and ENRAGE, is constitutively expressed in neutrophils and inducible in macrophages and smooth muscle cells [103]. Overexpression causes several vascular smooth muscle cell dysfunctions such as increased pro-matrix metalloproteinase 2 generation, increased phosphorylation and nuclear translocation of Smad2, and modulation of mitochondrial function leading to vascular remodeling and aneurismal formation [104]. However, S100A12 attenuated chemokine secretion in activated human airway smooth muscle cells. TNF-α and IFN-γ exposure enhanced CCL9 and CXCL10 mRNA and protein levels, and these were attenuated in S100A12 overexpressing cells, with the net effect of dampening allergic inflammation in the airway [105]. Once released, S100A12 exerts proinflammatory effects being chemotactic towards monocytes and mast cells in a G-protein-coupled receptor-mediated manner [106], stimulating RAGE-dependent TNF-α and IL-1β production in microglial cells, IL-2 in lymphocytes, and intercellular adhesion molecule-1 and vascular adhesion molecule expression in endothelial cells [107] and RAGE-independent mast cell activation [108]. Peripheral blood mRNA levels of S100A12 were found to be closely associated with insulin resistance and inflammation [82].
Supplementation with LC n-3 PUFA resulted in S100A12 upregulation in adipose tissue of subjects acutely treated with LPS, similar to S100A8/S100A9 [86] (Table 1). However, S100A12 could not be detected in sorted adipocytes, indicating that expression of this gene in adipose tissue may derive from other adipose cell sources (e.g. endothelial, macrophages, stromal cells). Whether S100A12 regulates adipocyte physiology or not or has a pathogenic role in obesity remains to be elucidated.
S100A2, S100A3, S100A5, S100A6, S100A10, S100A11, S100A13, S100A14, S100P, S100Z, and S100G
No information is available about the expression of S100A2, S100A3, S100A5, S100A6, S100A10, S100A11, S100A13, S100A14, S100P, S100Z, and S100G in and/or effects of these proteins on adipose tissue in normal conditions and obesity.
S100B is adipogenic and participates in the pathophysiology of obesity
S100B is expressed in astrocytes, certain neuronal populations, Schwann cells, melanocytes, chondrocytes, adipocytes, skeletal myofibers and their associated satellite cells, certain dendritic cell and lymphocyte populations, and a few other cell types [109]. S100B can be transiently induced in a restricted number of cell types (e.g. cardiomyocytes, endocrine-resistant breast cancer cells, bronchial epithelial cells, and microglia/macrophages) during the course of inflammatory events, tissue injury, and certain cancers [110–114]. As an intracellular regulator S100B acts as a stimulator of cell proliferation and migration and an inhibitor of apoptosis and differentiation with important implications during tissue development and regeneration and tumorigenesis [40]. Downregulation of S100B expression in precursor cells in a defined temporal window appears to be permissive for cell differentiation; however, cells that downregulate S100B expression at the onset of their differentiation resume S100B expression at completion of development, and in mature cells the protein regulates a large variety of key activities by interacting with a wide array of target proteins [40]. As an extracellular signal S100B activates RAGE in a variety of cell types to regulate cell proliferation, differentiation, migration, and apoptosis with effects depending on the local concentration of S100B [40]. S100B can also activate the bFGF/FGFR1 axis in myoblasts to regulate their proliferation and differentiation during the course of muscle tissue regeneration [114, 115].
S100B was found expressed in adipocytes [116] and shown to be secreted by norepinephrine- and adrenocorticotropin (ACTH)-treated adipocytes [117] (Table 1). Insulin reduced isoproterenol- and ACTH-induced S100B release from isolated adipocytes and the S100B release was 50% decreased in the fat pad obtained from insulin-treated rats [118]. The S100B content in adipose tissue from diabetic or long-term starved rats were significantly lower than those of control rats [118] suggesting that insulin might promote the accumulation of S100B in adipocytes through a mechanism that remains to be elucidated, though. However, the serum of fasting rats contained twice as much S100B as did control rats [119] probably consequent to secretion under the action of lipolytic hormones. Yet, long-term fasting in girls with anorexia nervosa was shown to lead to decreased serum S100B levels [120] by a mechanism that remains to be elucidated. Interestingly, S100B was found expressed in murine embryonic mesenchymal cells and when overexpressed, it stimulated their differentiation into adipocytes via activation of JNK while inhibiting their differentiation into osteoblasts [121] (Table 1). Thus, intracellular S100B might have a role in WAT formation. Interestingly, S100B was shown to inhibit p53 in the context of malignant melanoma, acute colitis, glioma, and ovarian cancer [122–126], suggesting that it might promote adipogenesis by inhibiting the anti-adipogenic p53, as does S100A16.
S100B might participate in the pathophysiology of obesity by acting as an extracellular signal. As mentioned earlier, S100B is one major RAGE ligand [109, 127], and RAGE is involved in the pathophysiology of several diseases including obesity [128–132]. Serum levels of S100B correlate with BMI [133], and a link has been shown between serum S100B levels and abdominal obesity and serum levels of triglycerides [134] (Table 1). Forced expression and downregulation of RAGE in 3T3-L1 preadipocytes led to enhanced and reduced adipocyte hypertrophy, respectively, and double knockdown of high-mobility group box-1 and S100B or knockdown of Tlr2 suppressed RAGE-induced adipocyte hypertrophy [135]. S100B was proposed to function as an adipokine in the interaction between adipocytes and macrophages based on the observation that (1) S100B upregulated TNF-α and proinflammatory markers in RAW264.7 macrophages, and TNF-α augmented S100B secretion from 3T3-L1 preadipocytes; and (2) silencing of S100B in preadipocytes or RAGE neutralization significantly reduced TNF-α secretion from macrophages [136] (Fig. 2). Also, plasma and adipose tissue levels of S100B were found elevated in an animal model of obesity, and S100B was detected in both hypertrophic adipocytes and ATMs [137] (Table 1). Collectively, these results suggest that adipose S100B might promote WAT formation via RAGE-mediated adipocyte hypertrophy and sustain WAT inflammation via RAGE-mediated ATM activation. Given that S100B can be induced in and released from activated macrophages [86], it is possible that adipocyte- and macrophage-derived S100B might participate in RAGE-dependent obesity-associated inflammation.
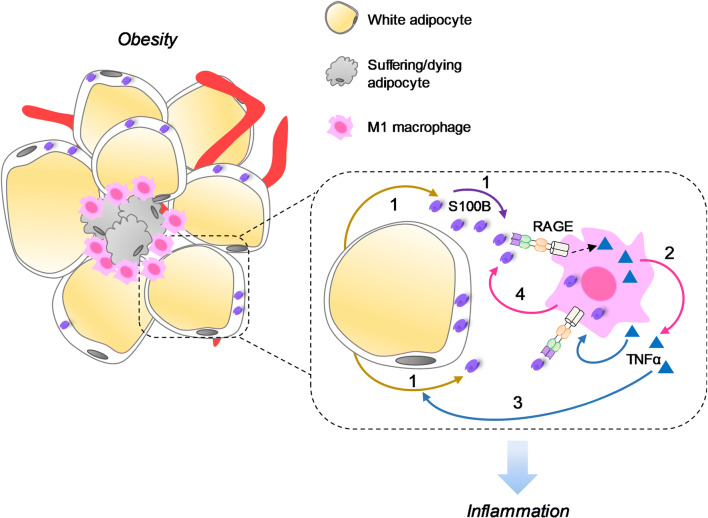
Fig. 2.
S100B might function as an adipokine in the interaction between adipocytes and macrophages. In the contest of obesity hypertrophic adipocytes might release S100B (1) [135] that upregulates TNF-α in ATMs [136] in a RAGE-mediated manner (2). TNF-α can stimulate S100B release from adipocytes (3) [136] thereby fueling inflammation via stimulation of ATMs. S100B is found in both hypertrophic adipocytes and ATMs [137] which makes it possible that ATM-derived S100B might participate in adipose tissue inflammation as well (4)
Protein profiling of VAT of rats exhibiting high or low levels of intrinsic exercise capacity revealed that S100B levels were 4-times greater in low-capacity runners compared to high-capacity runners [138]. Also, S100B and RAGE levels were higher in visceral than in subcutaneous adipose tissue [139]. These results implicate S100B and RAGE in low running capacity associated with a pathological profile in VAT and the pathogenesis of inflammation-induced diseases in the elderly. However, whether S100B/RAGE interactions in VAT are causative of the pathological changes associated with a sedentary lifestyle and aging or not awaits formal demonstration. Administration of S100B to myoblast cultures was shown to impair glycolysis in an insulin-independent manner via inhibition of GAPDH activity through enhanced poly(ADP-ribosyl)ation of the enzyme [140]. S100B decreased glucose consumption, glucose analog uptake, and lactate production increasing the concentration of glycolytic intermediates upstream of GAPDH, with no effects on glycogen accumulation and insulin signaling. The receptor mediating these effects was not investigated. Whether S100B also impairs glycolysis in adipocytes and/or other cell types in adipose tissue in normal physiological conditions and/or in the context of obesity remains to be investigated.
S100B was also found expressed in rat BAT [141] (Table 1). However, its expression was found to be restricted to pauci- or unilocular and inactive (warm acclimatized) adipocytes, very similar to white adipocytes. The functional implications of these observations have remained obscure until recently when (1) S100B was detected in mouse BAT; (2) S100B was localized to the endoplasmic reticulum of brown adipocytes where the previously unknown mammal-specific membrane protein, calsyntenin 3β (CLSTN3β), also localizes; (3) CLSTN3β was shown at act as a chaperon for S100B secretion from brown adipocytes; and (4) secreted S100B was shown to mediate sympathetic nerve–adipose communication enhancing BAT sympathetic innervation [142] (Fig. 3a). Specifically, S100B was the most strongly downregulated protein in the BAT of Clstn3b-knockout mice and S100b-knockout mice showed reduced BAT sympathetic innervation and WAT acclimation to cold compared to wild-type mice [142] (Fig. 3b). Notably, forced expression of S100B in the brown adipocytes of Clstn3b-knockout mice was sufficient to correct the deficits in sympathetic innervation and thermogenesis [142]. Collectively, these observations point to an important role for S100B in the function of BAT and WAT in normal physiological conditions. Whether released brown adipocyte S100B exerts neurotrophic effects on BAT sympathetic fibers via RAGE ligation [143, 144] remains to be determined. Also unknown is the extracellular/intracellular signal leading to CLSTN3β association with S100B for subsequent S100B exportation; norepinephrine might stimulate S100B release from brown adipocytes as in the case of white adipocytes [116]. Furthermore, the fact that S100B protein, but not S100b, is robustly downregulated in Clstn3b-knockout mice [142] suggests that CLSTN3β might not exclusively function as a chaperon for S100B secretion intervening in post-transcriptional regulation of S100B.
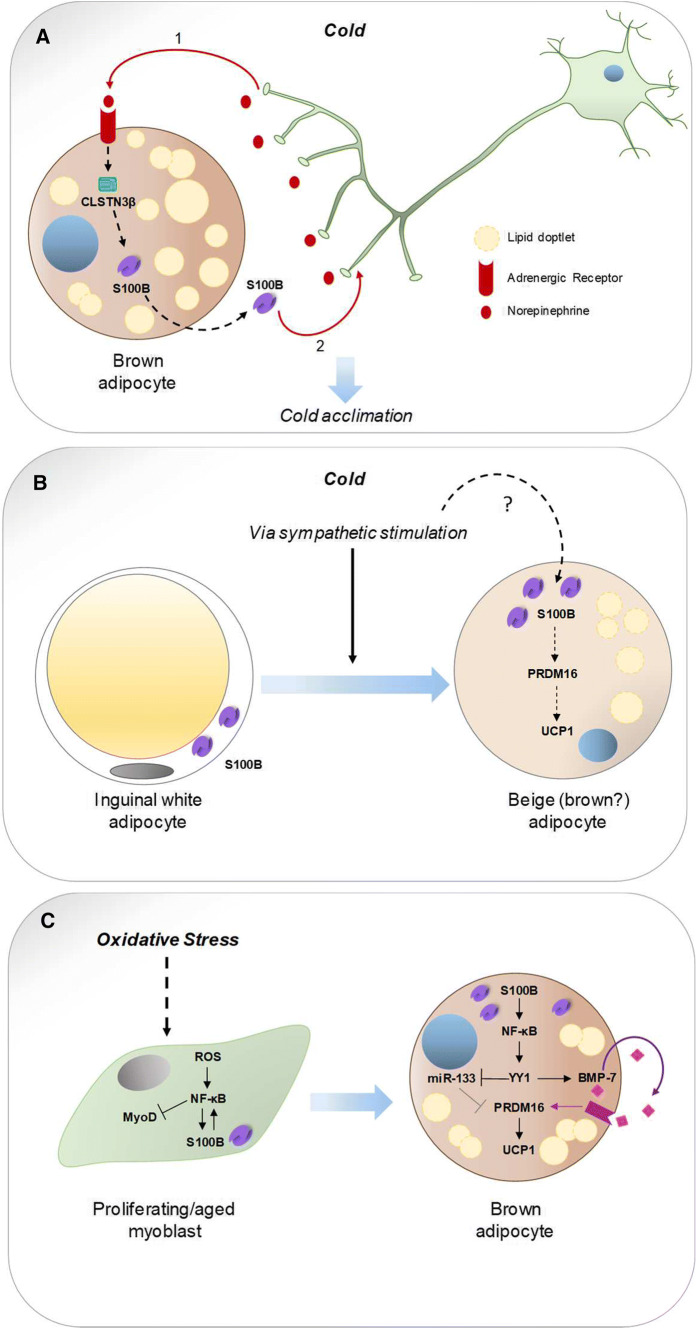
Fig. 3.
Complex role of S100B in brown and white adipocytes, and proliferating myoblasts. a S100B secreted from brown adipocyte acts as a neurotrophic factor participating in the larger sympathetic innervation of BAT relative to WAT and WAT acclimation to cold. Exposure to cold leads to sympathetic stimulation of brown adipocytes that release S100B (1) exerting neurotrophic effects on BAT sympathetic innervation (2) [142]. b Cold exposure induces upregulation of S100B in inguinal adipocytes likely via sympathetic stimulation (?) [142]. Upregulated S100B might induce beiging (browning?) of white adipocytes [142] probably via upregulation of PRDM16 leading to UCP1 expression [117]. Whether beige (brown?) adipocytes release S100B to support sympathetic innervation of WAT and/or perform other still unknown activities remains to be established. c Oxidative stress in proliferating (MyoD+) myoblasts activates NF-κB to upregulate S100B [145] which inhibits the differentiation of myoblasts to fusion-competent myocytes via NF-κB-dependent repression of MyoD expression leading to inhibition of formation of myotubes [170], the precursors of skeletal myofibers. Upregulated S100B also activates an NF-κB/YY1 axis leading to reduced expression levels of miR-133, a promyogenic factor that represses PRDM16, and to upregulation and secretion of BMP-7 [145], that in turn upregulates S100b and Prdm16 leading to induction of UCP1 (that is to transdifferentiation of myoblasts into brown adipocytes) [145]
A hint to a potential functional role of S100B in brown adipogenesis came from the observation that oxidative stress upregulated S100B levels in proliferating myoblasts in an NF-κB-dependent manner and promoted their transdifferentiation into UCP1+ (brown) adipocytes [145] (Table 1). Mechanistically, oxidative stress-induced myoblast-brown adipocyte transition occurred via an S100B/NF-κB/YY1 axis leading to reduced expression levels of miR-133, a promyogenic and anti-adipogenic factor, and to upregulation and secretion of the brown adipogenic factor, bone morphogenetic protein 7 (BMP-7) [146], that in turn upregulated S100b and Prdm16 [145], a zinc-finger transcriptional regulator necessary and sufficient to establish the identity of the brown adipose tissue lineage [147, 148] (Fig. 3c). Notably, PRDM16 was shown to positively regulate S100B in BAT and in inguinal WAT of mice acclimatized to 4 °C [142], suggesting that oxidative stress-induced upregulation of S100B in myoblasts might be regulated by NF-κB [145] and PRDM16. Interestingly, geriatric, but not young mice, exhibited elevated levels of S100B and UCP1 that co-localized in a subset of myofibers and skeletal muscle interstitial cells [145]. It is known that high reactive oxygen species levels and defective activity or abundance of NF-E2-related factor 2, an antioxidant factor playing a fundamental role in the maintenance of intracellular redox homeostasis, characterize the aging muscle tissue [149, 150]. Thus, the higher than normal reactive oxygen species level found in aged myoblasts and myofibers can explain both, the higher levels of S100B in aged myoblasts and myofibers compared to their young counterparts [145, 151] and S100B-induced myoblast-brown adipocyte conversion. Interestingly, myoblast-derived brown adipocytes could be reverted to authentic (i.e. fusion-competent) myoblasts by S100B knockdown [145] suggesting that S100B is an important molecular determinant of myoblast–brown adipocyte interconversion, its levels driving cell fate decision. Relevant to this latter issue is the fact that (1) muscle satellite cells, the adult muscle stem cells of skeletal muscle tissue and precursors of proliferating myoblasts, are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation [152]; and (2) brown adipocytes and myoblasts share a common mesenchymal Myf5+ progenitor [153] (although white and brown adipocytes may arise from transdifferentiation of endothelial, pericytic and mesothelial cells as well [154, 155]). The putative role of S100B as a molecular switch of skeletal muscle fiber progenitors to fusion competent myocytes or brown adipocytes might be important during development as well as in aging muscles. The fact that S100B is expressed at remarkably high levels and co-localizes with the mitochondrial protein, UCP1, in geriatric myofibers—as opposed to the lower S100B levels and absence of UCP1 expression in young myofibers [145]—, might represent an adaptive event to regulate thermogenesis in aged subjects [51].
White and brown adipocytes serve different functions in homeostatic conditions. White adipocytes store fat and provide lipids for energy purposes and adipokines for regulating appetite, inflammation, insulin sensitivity, and skeletal muscle trophism, whereas brown adipocytes regulate adaptive thermogenesis and energy balance [156–158]. Importantly, white-brown conversion can occur during cold exposure, prolonged sympathetic stimulation, exercise, transient activation of inflammatory networks, and cachexia [159–165]. As mentioned earlier, white and brown adipocytes and myoblasts share a common mesenchymal progenitor that differentiates into adipoblasts which give rise to white adipocytes and cells of the myogenic lineage (i.e. Myf5+ cells) which in turn originate brown adipocytes and myoblasts [153]. S100B was reported to be expressed in murine embryonic mesenchymal cells [98] and tonsil-derived mesenchymal stem cells [166]. In contrast, S100B was not detected in human umbilical cord blood mesenchymal stem cells [167], suggesting the possibility that its expression in mesenchymal stem cells might be niche-dependent. Thus, S100B appears to be common to precursors of both, white adipocytes and brown adipocytes/myoblasts, and is expressed in mature white and brown adipocytes and in myoblasts/myofibers. While there is information about the functional role(s) of S100B in myoblasts, such as sensitization of microtubules and desmin filaments to Ca2+, inhibition of premature differentiation into fusion-competent myocytes, protection against oxidative stress-induced apoptosis, and regulation of myoblast–brown adipocyte conversion [145, 151, 168–171], no information is available about S100B’s functional activities in mature myofibers and white adipocytes nor is there information about the role, if any, of S100B in browning of white adipocytes and/or whitening of brown adipocytes [157, 158, 172]. Given the proposed role of the S100B-RAGE axis in obesity-induced inflammation, the role of intracellular S100B in myoblast–brown adipocyte transdifferentiation, and the high S100B level in brown adipocytes and in white adipocytes (see above), S100B might play an important role as a molecular determinant of brown adipocyte identity and a brown adipocyte-derived DAMP fueling adipose tissue inflammation [173]. Yet, in normal physiological conditions released S100B might act as a neurotrophic factor towards sympathetic fibers in BAT [142]. Again, the tissue concentration of extracellular S100B might determine its beneficial or detrimental activity [86, 143, 144]. Future studies should dissect the role(s) of S100B in adipogenesis (including white–brown adipocyte interconversion) and obesity-associated inflammation.
Conclusions
The involvement of a restricted number of S100 proteins in adipogenesis and obesity appears to be in line with the non-redundant functions of these proteins, although the lack of information about the roles played by other members of the S100 protein family might stand on the fact that no targeted studies have been conducted. Therefore, currently S100A4, S100A8/S100A9, S100A16, and S100B seem to be the most relevant S100 proteins with the potential to participate in the physiology of adipose tissue and the pathophysiology of obesity. In these latter contexts, however, evidence for a role in obesity has been presented for S100A4, S100A8/S100A9, and S100B only.
Several questions are open, however. Two relevant questions concern S100A4, that is how its overexpression in 3T3-L1 adipocytes leads to inhibition of adipocyte differentiation, and how the expression of S100A4 in a population of adipose tissue fibroblasts leads to the maintenance of the preadipocyte pool. Information is lacking about the intracellular function(s) of S100A8/S100A9 and S100B in white adipocytes in normal physiological conditions and in the setting of (HFD-induced) obesity. Conditional deletion of S100A8 and/or S100A9 or S100B in white adipocytes could provide a cue for the respective functional role(s) in WAT. Also, whereas there is information about factors able to enhance (i.e., norepinephrine, ACTH, TNF-α) or reduce (i.e., insulin) S100B secretion form white adipocytes (although with a limited insight into the functional relevance of these observations), as mentioned earlier, no information is available on the release of S100A8 and/or S100A9 from adipocytes under normal physiological conditions and the functional consequence of this release. Also unknown is the mechanism by which LC n-3 PUFA increases S100A8 and S100A9 levels in adipocytes while reducing their levels in monocytes and, possibly, ATMs: unraveling this mechanism might offer information about the therapeutic advantage of LC n-3 PUFA supplementation in obese subjects as long as obesity persists. Likewise relevant is unraveling the mechanism by which (excess) S100A8/S100A9 interferes with heat production in the context of cold challenge: while there is enough information about the fact that it is extracellular S100A8/S100A9 that brings about this effect, the receptor transducing this activity and the ensuing cascade of events are not known. Yet another question is whether S100A8, S100A9 and/or S100B are expressed in adipocyte progenitor cells in mature adipose tissue or not and participate in the process of adipocyte progenitor cell differentiation into mature adipocytes in normal physiological conditions and in obesity. Connected with this, the issue of autocrine/paracrine effects of these proteins on white/brown adipocytes themselves in normal WAT/BAT and obesity deserves investigation. Given the importance of beiging/browning of white adipocytes and of whitening of brown adipocytes in physiological and pathophysiological contexts, the potential role of intracellular/extracellular S100B in these processes also deserves investigation. S100B conditional deletion studies as well as usage of S100B pharmacological inhibitors might shed light on this issue. Further, one unresolved question is whether S100B is constitutively released from brown adipocytes thereby assuring a constant neurotrophic support to sympathetic fibers in BAT or whether it is released on demand, e.g. in conditions of tissue suffering and/or during acclimation to cold. Yet another question is whether secreted S100B can enhance sympathetic innervation in WAT or not. Answering these questions could advance our understanding of the functional role(s) of certain S100 proteins in WAT and BAT in normal physiological conditions and in the pathophysiology of obesity.
Acknowledgements
The authors were supported by Association Française contre les Myopathies (Projects 12992 and 16812), Associazione Italiana per la Ricerca sul Cancro (Project 17581), Ministero dell’Istruzione, dell’Università e della Ricerca, Italy (PRIN 2009WBFZYM_002, PRIN 2010R8JK2X_004 and PRIN 2012N8YJC3) and Fondazione Cassa di Risparmio di Perugia (Projects 2012.0241.021, 2015.0325.021 and 2016-0136.021). The authors wish to thank the reviewers for criticism and suggestions.
Abbreviations
- ACTH
Adrenocorticotropin
- ATM
Adipose tissue macrophage
- BAT
Brown adipose tissue
- BMI
Body mass index
- BMP-7
Bone morphogenetic protein 7
- CLSTN3β
Calsyntenin 3β
- DAMP
Damage-associated molecular pattern
- EEC
Enteric endocrine cell
- GIP
Glucose-dependent insulinotropic polypeptide
- GIPR
GIP receptor
- GLP-1
Glucagon-like peptide-1
- HFD
High-fat diet
- IL
Interleukin
- IFN-γ
Interferon-γ
- LC n-3 PUFA
Long-chain omega-3 polyunsaturated fatty acids
- LPS
Lipopolysaccharide
- RAGE
Receptor for advanced glycation endproducts
- RyR
Ryanodine receptor
- SVFC
Stromal vascular fraction cell
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-α
- VAT
Visceral adipose tissue
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francesca Riuzzi and Sara Chiappalupi: Co-first authors.
Guglielmo Sorci and Rosario Donato: Co-senior authors.
References
- 1.James WPT, McPherson K. The costs of overweight. Lancet Public Health. 2017;2:e203–e204. doi: 10.1016/S2468-2667(17)30068-3. [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 3.Kassotis CD, Stapleton HM. Endocrine-mediated mechanisms of metabolic disruption and new approaches to examine the public health threat. Front Endocrinol (Lausanne) 2019;10:39. doi: 10.3389/fendo.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population- based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139–154. doi: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab. 2019;1:189–200. doi: 10.1038/s42255-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaben AL, Schere PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 9.Haider N, Larose L. Harnessing adipogenesis to prevent obesity. Adipocyte. 2019;8:1–7. doi: 10.1080/21623945.2019.1583037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, Maxfield FR. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res. 2016;57:980–992. doi: 10.1194/jlr.M064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev. 2012;13(Suppl 2):83–96. doi: 10.1111/j.1467-789X.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- 15.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, Wollam J, Olefsky JM. An integrated view of immunometabolism. Cell. 2018;172:22–40. doi: 10.1016/j.cell.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue W, Fan Z, Li L, Lu J, Zhai Y, Zhao J. The chemokine system and its role in obesity. J Cell Physiol. 2019;234:3336–3346. doi: 10.1002/jcp.27293. [DOI] [PubMed] [Google Scholar]
- 18.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. doi: 10.1016/j.metabol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, Zhou G, Fernandez S, Zhai L, Hall BA, Haka AS, Shah AM, Reardon CA, Brady MJ, Rhodes CJ, Maxfield FR, Becker L. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20:3149–3161. doi: 10.1016/j.celrep.2017.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, Vallabhajosyula P, Kambayashi T, Won KJ, Lazar MA. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci USA. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O’Rourke RW, Lumeng CN. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197:3650–3661. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2001;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 27.Gögebakan Ö, Andres J, Biedasek K, Mai K, Kühnen P, Krude H, Isken F, Rudovich N, Osterhoff MA, Kintscher U, Nauck M, Pfeiffer AF, Spranger J. Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11β -hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes. 2012;61:292–300. doi: 10.2337/db10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Massadi O, López M, Tschöp M, Diéguez C, Nogueiras R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends Neurosci. 2017;40:167–180. doi: 10.1016/j.tins.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 30.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab Suppl. 2018;1:5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 31.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin JT, McKie S. Human brain responses to gastrointestinal nutrients and gut hormones. Curr Opin Pharmacol. 2016;31:8–12. doi: 10.1016/j.coph.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro MP, Batterham RL. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology. 2017;152:1707–1717. doi: 10.1053/j.gastro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 34.Al-Najim W, Docherty NG, le Roux CW. Food intake and eating behavior after bariatric surgery. Physiol Rev. 2018;98:1113–1141. doi: 10.1152/physrev.00021.2017. [DOI] [PubMed] [Google Scholar]
- 35.Papathanasiou A, Nolen-Doerr E, Farr O, Geoffrey Mantzoros CS, Prize Harris. Novel pathways regulating neuroendocrine function, energy homeostasis and metabolism in humans. Eur J Endocrinol. 2018;180:R59–R71. doi: 10.1530/EJE-18-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeiffer AFH, Keyhani-Nejad F. High glycemic index metabolic damage—a pivotal role of GIP and GLP-1. Trends Endocrinol Metab. 2018;29:289–299. doi: 10.1016/j.tem.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Holst JJ. From the incretin concept and the discovery of GLP-1 to today’s diabetes therapy. Front Endocrinol (Lausanne) 2019;10:260. doi: 10.3389/fendo.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolen-Doerr E, Stockman MC, Rizo I. Mechanism of glucagon-like peptide 1 improvements in type 2 diabetes mellitus and obesity. Curr Obes Rep. 2019 doi: 10.1007/s13679-019-00350-4. [DOI] [PubMed] [Google Scholar]
- 40.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med. 2013;13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 41.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 42.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–842. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 44.Pruenster M, Vogl T, Roth J, Sperandio M. S100A8/A9: from basic science to clinical application. Pharmacol Ther. 2016;167:120–131. doi: 10.1016/j.pharmthera.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Lim SY, Raftery MJ, Geczy CL. Oxidative modifications of DAMPs suppress inflammation: the case for S100A8 and S100A9. Antioxid Redox Signal. 2011;15:2235–2248. doi: 10.1089/ars.2010.3641. [DOI] [PubMed] [Google Scholar]
- 46.Austermann J, Spiekermann C, Roth J. S100 proteins in rheumatic diseases. Nat Rev Rheumatol. 2018;14:528–541. doi: 10.1038/s41584-018-0058-9. [DOI] [PubMed] [Google Scholar]
- 47.Donato R. S100: a multigenic family of calcium modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–638. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 48.Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol. 2012;32:223–229. doi: 10.1161/ATVBAHA.111.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gross SR, Sin CG, Barraclough R, Rudland PS. Joining S100 proteins and migration: for better or for worse, in sickness and in health. Cell Mol Life Sci. 2014;71:1551–1579. doi: 10.1007/s00018-013-1400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donato R, Sorci G, Giambanco I. S100A6 protein: functional roles. Cell Mol Life Sci. 2017;74:2749–2760. doi: 10.1007/s00018-017-2526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riuzzi F, Sorci G, Arcuri C, Giambanco I, Bellezza I, Minelli A, Donato R. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J Cachexia Sarcopenia Muscle. 2018;9:1255–1268. doi: 10.1002/jcsm.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Most P, Bernotat J, Ehlermann P, Pleger ST, Reppel M, Börries M, Niroomand F, Pieske B, Janssen PM, Eschenhagen T, Karczewski P, Smith GL, Koch WJ, Katus HA, Remppis A. S100A1: a regulator of myocardial contractility. Proc Natl Acad Sci USA. 2001;98:13889–13894. doi: 10.1073/pnas.241393598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiewitz R, Acklin C, Schäfer BW, Maco B, Uhrík B, Wuytack F, Erne P, Heizmann CW. Ca2+-dependent interaction of S100A1 with the sarcoplasmic reticulum Ca2+-ATPase2a and phospholamban in the human heart. Biochem Biophys Res Commun. 2003;306:550–557. doi: 10.1016/s0006-291x(03)00987-2. [DOI] [PubMed] [Google Scholar]
- 55.Kettlewell S, Most P, Currie S, Koch WJ, Smith GL. S100A1 increases the gain of excitation-contraction coupling in isolated rabbit ventricular cardiomyocytes. J Mol Cell Cardiol. 2005;39:900–910. doi: 10.1016/j.yjmcc.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Most P, Pleger ST, Völkers M, Heidt B, Boerries M, Weichenhan D, Löffler E, Janssen PM, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Most P, Seifert H, Gao E, Funakoshi H, Völkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR, Jr, Eckhart AD, Feldman AM, Koch WJ. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 58.Treves S, Scutari E, Robert M, Groh S, Ottolia M, Prestipino G, Ronjat M, Zorzato F. Interaction of S100A1 with the Ca2+ release channel (ryanodine receptor) of skeletal muscle. Biochemistry. 1997;36:11496–11503. doi: 10.1021/bi970160w. [DOI] [PubMed] [Google Scholar]
- 59.Prosser BL, Wright NT, Hernãndez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem. 2008;283:5046–5057. doi: 10.1074/jbc.M709231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prosser BL, Hernández-Ochoa EO, Zimmer DB, Schneider MF. The Qγ component of intra-membrane charge movement is present in mammalian muscle fibres, but suppressed in the absence of S100A1. J Physiol. 2009;587:4523–4541. doi: 10.1113/jphysiol.2009.177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heierhorst J, Kobe B, Feil SC, Parker MW, Benian GM, Weiss KR, Kemp BE. Ca2+/S100 regulation of giant protein kinases. Nature. 1996;380:636–639. doi: 10.1038/380636a0. [DOI] [PubMed] [Google Scholar]
- 62.Yamasaki R, Berri M, Wu Y, Trombitás K, McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J. 2001;81:2297–2313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Völkers M, Rohde D, Goodman C, Most P. S100A1: a regulator of striated muscle sarcoplasmic reticulum Ca2+ handling, sarcomeric, and mitochondrial function. J Biomed Biotechnol. 2010;2010:178614. doi: 10.1155/2010/178614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rambotti MG, Giambanco I, Spreca A, Donato R. S100B and S100A1 proteins in bovine retina: their calcium-dependent stimulation of a membrane-bound guanylate cyclase activity as investigated by ultracytochemistry. Neuroscience. 1999;92:1089–1101. doi: 10.1016/s0306-4522(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 65.Kato K, Suzuki F, Ogasawara N. Induction of S100 protein in 3T3-L1 cells during differentiation to adipocytes and its liberating by lipolytic hormones. Eur J Biochem. 1988;177:461–466. doi: 10.1111/j.1432-1033.1988.tb14395.x. [DOI] [PubMed] [Google Scholar]
- 66.Cinti S, Cigolini M, Morroni M, Zingaretti MC. S-100 protein in white preadipocytes: an immunoelectronmicroscopic study. Anat Rec. 1989;224:466–472. doi: 10.1002/ar.1092240403. [DOI] [PubMed] [Google Scholar]
- 67.Zoico E, Di Francesco V, Olioso D, Fratta Pasini AM, Sepe A, Bosello O, Cinti S, Cominacini L, Zamboni M. In vitro aging of 3T3-L1 mouse adipocytes leads to altered metabolism and response to inflammation. Biogerontology. 2010;11:111–122. doi: 10.1007/s10522-009-9236-0. [DOI] [PubMed] [Google Scholar]
- 68.Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, Ambartsumian N. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. 2005;65:3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 69.Dmytriyeva O, Pankratova S, Owczarek S, Sonn K, Soroka V, Ridley CM, Marsolais A, Lopez-Hoyos M, Ambartsumian N, Lukanidin E, Bock E, Berezin V, Kiryushko D. The metastasis-promoting S100A4 protein confers neuroprotection in brain injury. Nat Commun. 2012;3:1197. doi: 10.1038/ncomms2202. [DOI] [PubMed] [Google Scholar]
- 70.Pankratova S, Klingelhofer J, Dmytriyeva O, Owczarek S, Renziehausen A, Syed N, Porter AE, Dexter DT, Kiryushko D. The S100A4 protein signals through the ErbB4 receptor to promote neuronal survival. Theranostics. 2018;8:3977–3990. doi: 10.7150/thno.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arner P, Petrus P, Esteve D, Boulomié A, Näslund E, Thorell A, Gao H, Dahlman I, Rydén M. Screening of potential adipokines identifies S100A4 as a marker of pernicious adipose tissue and insulin resistance. Int J Obes (Lond) 2018;42:2047–2056. doi: 10.1038/s41366-018-0018-0. [DOI] [PubMed] [Google Scholar]
- 72.EL Naaman C, Grum-Schwensen B, Mansouri A, Grigorian M, Santoni-Rugiu E, Hansen T, Kriajevska M, Schafer BW, Heizmann CW, Lukanidin E, Ambartsumian N. Cancer predisposition in mice deficient for the metastasis-associated Mts1(S100A4) gene. Oncogene. 2004;23:3670–3680. doi: 10.1038/sj.onc.1207420. [DOI] [PubMed] [Google Scholar]
- 73.Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. Expression of the calcium-binding protein S100A4 (p9Ka) in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene. 1996;13:1631–1637. [PubMed] [Google Scholar]
- 74.Hou S, Jiao Y, Yuan Q, Zhai J, Tian T, Sun K, Chen Z, Wu Z, Zhang J. S100A4 protects mice from high-fat diet-induced obesity and inflammation. Lab Invest. 2018;98:1025–1038. doi: 10.1038/s41374-018-0067-y. [DOI] [PubMed] [Google Scholar]
- 75.Kiryushko D, Novitskaya V, Soroka V, Klingelhofer J, Lukanidin E, Berezin V, Bock E. Molecular mechanisms of Ca2+ signaling in neurons induced by the S100A4 protein. Mol Cell Biol. 2006;26:3625–3638. doi: 10.1128/MCB.26.9.3625-3638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klingelhöfer J, Møller HD, Sumer EU, Sumer EU, Berg CH, Poulsen M, Kiryushko D, Soroka V, Ambartsumian N, Grigorian M, Lukanidin EM. Epidermal growth factor receptor ligands as new extracellular targets for the metastasis-promoting S100A4 protein. FEBS J. 2009;276:5936–5948. doi: 10.1111/j.1742-4658.2009.07274.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang R, Gao Y, Zhao X, Gao M, Wu Y, Han Y, Qiao Y, Luo Z, Yang L, Chen J, Ge G. FSP1-positive fibroblasts are adipogenic niche and regulate adipose homeostasis. PLoS Biol. 2018;16:e2001493. doi: 10.1371/journal.pbio.2001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediat Inflamm. 2013;2013:828354. doi: 10.1155/2013/828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mortensen OH, Nielsen AR, Erikstrup C, Plomgaard P, Fischer CP, Krogh-Madsen R, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK. Calprotectin: a novel marker of obesity. PLoS One. 2009;4:e7419. doi: 10.1371/journal.pone.0007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Fernández-Real JM, Salvador J, Frühbeck G. Increased levels of calprotectin in obesity are related to macrophage content: impact on inflammation and effect of weight loss. Mol Med. 2011;17:1157–1167. doi: 10.2119/molmed.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sekimoto R, Kishida K, Nakatsuji H, Nakagawa T, Funahashi T, Shimomura I. High circulating levels of S100A8/A9 complex (calprotectin) in male Japanese with abdominal adiposity and dysregulated expression of S100A8 and S100A9 in adipose tissues of obese mice. Biochem Biophys Res Commun. 2012;419:782–789. doi: 10.1016/j.bbrc.2012.02.102. [DOI] [PubMed] [Google Scholar]
- 82.Yamaoka M, Maeda N, Nakamura S, Mori T, Inoue K, Matsuda K, Sekimoto R, Kashine S, Nakagawa Y, Tsushima Y, Fujishima Y, Komura N, Hirata A, Nishizawa H, Matsuzawa Y, Matsubara K, Funahashi T, Shimomura I. Gene expression levels of S100 protein family in blood cells are associated with insulin resistance and inflammation (Peripheral blood S100 mRNAs and metabolic syndrome) Biochem Biophys Res Commun. 2013;433:450–455. doi: 10.1016/j.bbrc.2013.02.096. [DOI] [PubMed] [Google Scholar]
- 83.Sekimoto R, Fukuda S, Maeda N, Tsushima Y, Matsuda K, Mori T, Nakatsuji H, Nishizawa H, Kishida K, Kikuta J, Maijima Y, Funahashi T, Ishii M, Shimomura I. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc Natl Acad Sci USA. 2015;112:E2058–E2066. doi: 10.1073/pnas.1409480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah RD, Xue C, Zhang H, Tuteja S, Li M, Reilly MP, Ferguson JF. Expression of calgranulin genes S100A8, S100A9 and S100A12 is modulated by n-3 PUFA during inflammation in adipose tissue and mononuclear cells. PLoS One. 2017;12:e0169614. doi: 10.1371/journal.pone.0169614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 88.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 89.Mantelmacher FD, Zvibel I, Cohen K, Epshtein A, Pasmanik-Chor M, Vogl T, Kuperman Y, Weiss S, Drucker DJ, Varol C, Fishman S. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat Metab. 2018;1:58–69. doi: 10.1038/s42255-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 90.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 91.Yip RG, Boylan MO, Kieffer TJ, Wolfe MM. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139:4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 92.Nyberg J, Jacobsson C, Anderson MF, Eriksson PS. Immunohistochemical distribution of glucose dependent insulinotropic polypeptide in the adult rat brain. J Neurosci Res. 2007;85:2099–2119. doi: 10.1002/jnr.21349. [DOI] [PubMed] [Google Scholar]
- 93.Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CH. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7:e40156. doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varol C, Zvibel I, Spektor L, Mantelmacher FD, Vugman M, Thurm T, Khatib M, Elmaliah E, Halpern Z, Fishman S. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J Immunol. 2014;193:4002–4009. doi: 10.4049/jimmunol.1401149. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Zhang R, Xin J, Sun Y, Li J, Wei D, Zhao AZ. Identification of S100A16 as a novel adipogenesis promoting factor in 3T3-L1 cells. Endocrinology. 2011;152:903–911. doi: 10.1210/en.2010-1059. [DOI] [PubMed] [Google Scholar]
- 96.Inoue N, Yahagi N, Yamamoto T, Ishikawa M, Watanabe K, Matsuzaka T, Nakagawa Y, Takeuchi Y, Kobayashi K, Takahashi A, Suzuki H, Hasty AH, Toyoshima H, Yamada N, Shimano H. Cyclin-dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem. 2008;283:21220–21229. doi: 10.1074/jbc.M801824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang R, Su D, Zhu W, Huang Q, Liu M, Xue Y, Zhang Y, Li D, Zhao A, Liu Y. Estrogen suppresses adipogenesis by inhibiting S100A16 expression. J Mol Endocrinol. 2014;52:235–244. doi: 10.1530/JME-13-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li D, Zhang R, Zhu W, Xue Y, Zhang Y, Huang Q, Liu M, Liu Y. S100A16 inhibits osteogenesis but stimulates adipogenesis. Mol Biol Rep. 2013;40:3465–3473. doi: 10.1007/s11033-012-2413-2. [DOI] [PubMed] [Google Scholar]
- 99.Zhang R, Zhu W, Du X, Xin J, Xue Y, Zhang Y, Li D, Liu Y. S100A16 mediation of weight gain attenuation induced by dietary calcium. Metabolism. 2012;61:157–163. doi: 10.1016/j.metabol.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 100.D’Amico F, Skarmoutsou E, Granata M, Trovato C, Rossi GA, Mazzarino MC. S100A7: a rAMPing up AMP molecule in psoriasis. Cytokine Growth Factor Rev. 2016;32:97–104. doi: 10.1016/j.cytogfr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 101.Salama RH, Al-Shobaili HA, Al Robaee AA, Alzolibani AA. Psoriasin: a novel marker linked obesity with psoriasis. Dis Markers. 2013;34:33–39. doi: 10.3233/DMA-2012-120945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakurai M, Miki Y, Takagi K, Suzuki T, Ishida T, Ohuchi N, Sasano H. Interaction with adipocyte stromal cells induces breast cancer malignancy via S100A7 upregulation in breast cancer microenvironment. Breast Cancer Res. 2017;19:70. doi: 10.1186/s13058-017-0863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vogl T, Pröpper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 104.Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res. 2010;106:145–154. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hofmann Bowman MA, Heydemann A, Gawdzik J, Shilling RA, Camoretti-Mercado B. Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation. Clin Exp Allergy. 2011;41:878–889. doi: 10.1111/j.1365-2222.2011.03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan WX, Armishaw C, Goyette J, Yang Z, Cai H, Alewood P, Geczy CL. Mast cell and monocyte recruitment by S100A12 and its hinge domain. J Biol Chem. 2008;283:13035–13043. doi: 10.1074/jbc.M710388200. [DOI] [PubMed] [Google Scholar]
- 107.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 108.Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG, Hunt J, Hart P, Hughes JM, Perry MA, Alewood PF, Geczy CL. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119:106–114. doi: 10.1016/j.jaci.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 109.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta Mol Cell Res. 2009;1793:1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 110.Tsoporis JN, Marks A, Haddad A, Dawood F, Liu PP, Parker TG. S100B expression modulates left ventricular remodeling after myocardial infarction in mice. Circulation. 2005;111:598–606. doi: 10.1161/01.CIR.0000154554.65287.F5. [DOI] [PubMed] [Google Scholar]
- 111.McIlroy M, McCartan D, Early S, Gaora PO, Pennington S, Hill AD, Young LS. Interaction of developmental transcription factor HOXC11 with steroid receptor coactivator SRC-1 mediates resistance to endocrine therapy in breast cancer [corrected] Cancer Res. 2010;70:1585–1594. doi: 10.1158/0008-5472.CAN-09-3713. [DOI] [PubMed] [Google Scholar]
- 112.Sorci G, Giovannini G, Riuzzi F, Bonifazi P, Zelante T, Zagarella S, Bistoni F, Donato R, Romani L. The danger signal S100B integrates pathogen- and danger-sensing pathways to restrain inflammation. PLoS Pathog. 2011;7:e1001315. doi: 10.1371/journal.ppat.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J, Badie SA, Badie B. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486–498. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riuzzi F, Beccafico S, Sagheddu R, Chiappalupi S, Giambanco I, Bereshchenko O, Riccardi C, Sorci G, Donato R. Levels of S100B protein drive the reparative process in acute muscle injury and muscular dystrophy. Sci Rep. 2017;7:12537. doi: 10.1038/s41598-017-12880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Riuzzi F, Sorci G, Donato R. S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGFdependent manner. J Cell Sci. 2011;124:2389–2400. doi: 10.1242/jcs.084491. [DOI] [PubMed] [Google Scholar]
- 116.Michetti F, Dell’Anna E, Tiberio G, Cocchia D. Immunochemical and immunocytochemical study of S-100 protein in rat adipocytes. Brain Res. 1983;262:352–356. doi: 10.1016/0006-8993(83)91032-6. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki F, Kato K, Nakajima T. Hormonal regulation of adipose S-100 protein release. J Neurochem. 1984;43:1336–1341. doi: 10.1111/j.1471-4159.1984.tb05391.x. [DOI] [PubMed] [Google Scholar]
- 118.Suzuki F, Kato K. Inhibition of adipose S-100 protein release by insulin. Biochim Biophys Acta. 1985;845:311–316. doi: 10.1016/0167-4889(85)90193-4. [DOI] [PubMed] [Google Scholar]
- 119.Netto CB, Conte S, Leite MC, Pires C, Martins TL, Vidal P, Benfato MS, Giugliani R, Gonçalves CA. Serum S100B protein is increased in fasting rats. Arch Med Res. 2006;37:683–686. doi: 10.1016/j.arcmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Holtkamp K, Bühren K, Ponath G, von Eiff C, Herpertz-Dahlmann B, Hebebrand J, Rothermundt M. Serum levels of S100B are decreased in chronic starvation and normalize with weight gain. J Neural Transm (Vienna) 2008;115:937–940. doi: 10.1007/s00702-008-0041-8. [DOI] [PubMed] [Google Scholar]
- 121.Li D, Li K, Chen G, Xia J, Yang T, Cai P, Yao C, Yang Y, Yan S, Zhang R, Chen H. S100B suppresses the differentiation of C3H/10T1/2 murine embryonic mesenchymal cells into osteoblasts. Mol Med Rep. 2016;14:3878–3886. doi: 10.3892/mmr.2016.5697. [DOI] [PubMed] [Google Scholar]
- 122.Lin J, Yang Q, Wilder PT, Carrier F, Weber DJ. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J Biol Chem. 2010;285:27487–27498. doi: 10.1074/jbc.M110.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esposito G, Capoccia E, Sarnelli G, Scuderi C, Cirillo C, Cuomo R, Steardo L. The antiprotozoal drug pentamidine ameliorates experimentally induced acute colitis in mice. J Neuroinflammation. 2012;9:277. doi: 10.1186/1742-2094-9-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Capoccia E, Cirillo C, Marchetto A, Tiberi S, Sawikr Y, Pesce M, D’Alessandro A, Scuderi C, Sarnelli G, Cuomo R, Steardo L, Esposito G. S100B-p53 disengagement by pentamidine promotes apoptosis and inhibits cellular migration via aquaporin-4 and metalloproteinase-2 inhibition in C6 glioma cells. Oncol Lett. 2015;9:2864–2870. doi: 10.3892/ol.2015.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang T, Cheng J, Yang Y, Qi W, Zhao Y, Long H, Xie R, Zhu B, S100B Mediates stemness of ovarian cancer stem-like cells through inhibiting p53. Stem Cells. 2017;35:325–336. doi: 10.1002/stem.2472. [DOI] [PubMed] [Google Scholar]
- 126.Yang T, Cheng J, You J, Yan B, Liu H, Li F. S100B promotes chemoresistance in ovarian cancer stem cells by regulating p53. Oncol Rep. 2018;40:1574–1582. doi: 10.3892/or.2018.6527. [DOI] [PubMed] [Google Scholar]
- 127.Sorci G, Riuzzi F, Giambanco I, Donato R. RAGE in tissue homeostasis, repair and regeneration. Biochim Biophys Acta Mol Cell Res. 2013;1833:101–109. doi: 10.1016/j.bbamcr.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 128.Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol. 2013;24:4–11. doi: 10.1097/MOL.0b013e32835aea13. [DOI] [PubMed] [Google Scholar]
- 129.Boyer F, Vidot JB, Dubourg AG, Rondeau P, Essop MF, Bourdon E. Oxidative stress and adipocyte biology: focus on the role of AGEs. Oxid Med Cell Longev. 2015;2015:534873. doi: 10.1155/2015/534873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE-opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Ther Targ. 2016;20:431–446. doi: 10.1517/14728222.2016.1111873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.López-Díez R, Shekhtman A, Ramasamy R, Schmidt AM. Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim Biophys Acta. 2016;1862:2244–2252. doi: 10.1016/j.bbadis.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, Yang P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol. 2017;454:103–111. doi: 10.1016/j.mce.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 133.Steiner J, Schiltz K, Walter M, Wunderlich MT, Keilhoff G, Brisch R, Bielau H, Bernstein HG, Bogerts B, Schroeter ML, Westphal S. S100B serum levels are closely correlated with body mass index: an important caveat in neuropsychiatric research. Psychoneuroendocrinology. 2010;35:321–324. doi: 10.1016/j.psyneuen.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 134.Kheirouri S, Ebrahimi E, Alizadeh M. Association of S100B serum levels with metabolic syndrome and its components. Acta Med Port. 2018;31:201–206. doi: 10.20344/amp.9073. [DOI] [PubMed] [Google Scholar]
- 135.Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A, Emoto M, Yamamoto Y, Yamamoto H, Nishizawa Y, Kurajoh M, Yamamoto T, Inaba M. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes. 2013;62:478–489. doi: 10.2337/db11-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fujiya A, Nagasaki H, Seino Y, Okawa T, Kato J, Fukami A, Himeno T, Uenishi E, Tsunekawa S, Kamiya H, Nakamura J, Oiso Y, Hamada Y. The role of S100B in the interaction between adipocytes and macrophages. Obesity (Silver Spring) 2014;22:371–379. doi: 10.1002/oby.20532. [DOI] [PubMed] [Google Scholar]
- 137.Buckman LB, Anderson-Baucum EK, Hasty AH, Ellacott KLJ. Regulation of S100B in white adipose tissue by obesity in mice. Adipocyte. 2014;3:215–220. doi: 10.4161/adip.28730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bowden-Davies K, Connolly J, Burghardt P, Koch LG, Britton SL, Burniston JG. Label-free profiling of white adipose tissue of rats exhibiting high or low levels of intrinsic exercise capacity. Proteomics. 2015;15:2342–2349. doi: 10.1002/pmic.201400537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Son KH, Son M, Ahn H, Oh S, Yum Y, Choi CH, Park KY, Byun K. Age-related accumulation of advanced glycation end-products-albumin, S100β, and the expressions of advanced glycation end product receptor differ in visceral and subcutaneous fat. Biochem Biophys Res Commun. 2016;477:271–276. doi: 10.1016/j.bbrc.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 140.Hosokawa K, Hamada Y, Fujiya A, Murase M, Maekawa R, Niwa Y, Izumoto T, Seino Y, Tsunekawa S, Arima H. S100B impairs glycolysis via enhanced poly(ADP-ribosyl)ation of glyceraldehyde-3-phosphate dehydrogenase in rodent muscle cells. Am J Physiol Endocrinol Metab. 2017;312:E471–E481. doi: 10.1152/ajpendo.00328.2016. [DOI] [PubMed] [Google Scholar]
- 141.Barbatelli G, Morroni M, Vinesi P, Cinti S, Michetti F. S-100 protein in rat brown adipose tissue under different functional conditions: a morphological, immunocytochemical, and immunochemical study. Exp Cell Res. 1993;208:226–231. doi: 10.1006/excr.1993.1241. [DOI] [PubMed] [Google Scholar]
- 142.Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, Spiegelman BM. Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis. Nature. 2019 doi: 10.1038/s41586-019-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275(51):40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 144.Businaro R, Leone S, Fabrizi C, Sorci G, Donato R, Lauro GM, Fumagalli L. S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J Neurosci Res. 2006;83:897–906. doi: 10.1002/jnr.20785. [DOI] [PubMed] [Google Scholar]
- 145.Morozzi G, Beccafico S, Bianchi R, Riuzzi F, Bellezza I, Giambanco I, Arcuri C, Minelli A, Donato R. Oxidative stress-induced S100B accumulation converts myoblasts into brown adipocytes via an NF-κB/YY1/miR-133 axis and NF-κB/YY1/BMP-7 axis. Cell Death Differ. 2017;24:2077–2088. doi: 10.1038/cdd.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8:349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 151.Beccafico S, Riuzzi F, Puglielli C, Mancinelli R, Fulle S, Sorci G, Donato R. Human muscle satellite cells show age-related differential expression of S100B protein and RAGE. Age (Dordr) 2011;33:523–541. doi: 10.1007/s11357-010-9197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 153.Frühbeck G, Sesma P, Burrell MA. PRDM16: the interconvertible adipo-myocyte switch. Trends Cell Biol. 2009;19:141–146. doi: 10.1016/j.tcb.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 154.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, Spiegelman BM. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S, Cinti S. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov. 2016;15:405–424. doi: 10.1038/nrd.2016.31. [DOI] [PubMed] [Google Scholar]
- 158.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17:691–702. doi: 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nüsing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 160.Diaz MB, Herzig S, Vegiopoulos A. Thermogenic adipocytes: from cells to physiology and medicine. Metabolism. 2014;63:1238–1249. doi: 10.1016/j.metabol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 161.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sidossis LS, Porter C, Saraf MK, Børsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18:355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Babaei R, Schuster M, Meln I, Lerch S, Ghandour RA, Pisani DF, Bayindir-Buchhalter I, Marx J, Wu S, Schoiswohl G, Billeter AT, Krunic D, Mauer J, Lee YH, Granneman JG, Fischer L, Müller-Stich BP, Amri EZ, Kershaw EE, Heikenwälder M, Herzig S, Vegiopoulos A. Jak-TGFβ cross-talk links transient adipose tissue inflammation to beige adipogenesis. Sci Signal. 2018;11:eaai7838. doi: 10.1126/scisignal.aai7838. [DOI] [PubMed] [Google Scholar]
- 166.Jung N, Park S, Choi Y, Park JW, Hong YB, Park HH, Yu Y, Kwak G, Kim HS, Ryu KH, Kim JK, Jo I, Choi BO, Jung SC. Tonsil-derived mesenchymal stem cells differentiate into a schwann cell phenotype and promote peripheral nerve regeneration. Int J Mol Sci. 2016;17(11):E1867. doi: 10.3390/ijms17111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiao YZ, Wang S. Differentiation of Schwann-like cells from human umbilical cord blood mesenchymal stem cells in vitro. Mol Med Rep. 2015;11:1146–1152. doi: 10.3892/mmr.2014.2840. [DOI] [PubMed] [Google Scholar]
- 168.Garbuglia M, Verzini M, Giambanco I, Spreca A, Donato R. Effects of calcium-binding proteins (S100a0, S100a, S100b) on desmin assembly in vitro. FASEB J. 1996;10:317–324. doi: 10.1096/fasebj.10.2.8641565. [DOI] [PubMed] [Google Scholar]
- 169.Sorci G, Agneletti AL, Donato R. Effects of S100A1 and S100B on microtubule stability. An in vitro study using triton-cytoskeletons from astrocyte and myoblast cell lines. Neuroscience. 2000;99:773–783. doi: 10.1016/s0306-4522(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 170.Tubaro C, Arcuri C, Giambanco I, Donato R. S100B protein in myoblasts modulates myogenic differentiation via NF-κB-dependent inhibition of MyoD expression. J Cell Physiol. 2010;223:270–282. doi: 10.1002/jcp.22035. [DOI] [PubMed] [Google Scholar]
- 171.Tubaro C, Arcuri C, Giambanco I, Donato R. S100B in myoblasts regulates the transition from activation to quiescence and from quiescence to activation, and reduces apoptosis. Biochim Biophys Acta Mol Cell Res. 2011;1813:1092–1104. doi: 10.1016/j.bbamcr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 172.Cinti S. Adipose Organ Development and Remodeling. Compr Physiol. 2018;8:1357–1431. doi: 10.1002/cphy.c170042. [DOI] [PubMed] [Google Scholar]
- 173.Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, Cecchini MP, Kershaw EE, Barbatelli G, Haemmerle G, Zechner R, Cinti S. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res. 2018;59:784–794. doi: 10.1194/jlr.M079665. [DOI] [PMC free article] [PubMed] [Google Scholar]