Abstract
Glial cells have been identified more than 100 years ago, and are known to play a key role in the central nervous system (CNS) function. A recent piece of evidence is emerging showing that in addition to the capacity of CNS modulation and homeostasis, glial cells are also being looked like as a promising cell source not only to study CNS pathologies initiation and progression but also to the establishment and development of new therapeutic strategies. Thus, in the present review, we will discuss the current evidence regarding glial cells’ contribution to neurodegenerative diseases as Parkinson’s disease, providing cellular, molecular, functional, and behavioral data supporting its active role in disease initiation, progression, and treatment. As so, considering their functional relevance, glial cells may be important to the understanding of the underlying mechanisms regarding neuronal-glial networks in neurodegeneration/regeneration processes, which may open new research opportunities for their future use as a target or treatment in human clinical trials.
Keywords: Glial cells, Parkinson's Disease, Dopaminergic neurons, Cell based therapy, PD-related genes
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease affecting around 10 million people worldwide [1]. Clinically, PD is diagnosed based on the identification of cardinal features that affects the motor system, namely, bradykinesia (slowness in the execution of voluntary movements), postural instability (a tendency to fall even in the absence of weakness or cerebellar balance disturbance), muscular rigidity (stiffness), and tremor at rest, with an asymmetric onset, which becomes bilateral along time [2–4]. These motor deficits are the result of progressive degeneration of dopaminergic neurons (DA neurons) in the nigrostriatal pathway at the level of the substantia nigra pars compacta (SNpc) and striatum [3, 5, 6]. Although less explored than motor symptomatology, several studies have been suggesting a critical role for PD non-motor symptoms (NMS) in preceding the appearance of clinical PD motor symptoms [7–9]. Concerning the treatment pipeline for PD, there was not a significant change over the last years, and the use of levodopa stills the gold standard treatment, since its introduction in the early 1960s [10]. However, it is important to highlight that levodopa is just efficient during the first years of the disease, as its chronic administration is associated with the appearance of undesirable side effects, such as dyskinesia, addictive and compulsive behaviors [11–13]. As a therapeutic alternative, dopamine (DA) agonists (e.g., ropinirole, pramipexole, and piribedil) and enzyme inhibitors (e.g., entacapone and selegiline) have been used, although without success in late stages of the disease, in the modulating or delaying PD progression [12, 14]. Safinamide, a recent monoamine oxidase B (MAO-B) inhibitor compound, has been claimed as a promising option for PD treatment [15, 16], due to its multimodal dopaminergic (able to increase dopamine levels and prolong levodopa action) and non-dopaminergic (neuroprotective—able to modulate glutamate release through calcium/sodium channels inhibition, and microglia activation) effects [17]. In addition to these pharmacological treatments, surgical interventions, as deep brain stimulation (DBS) in the globus pallidus internus (GPi), subthalamic nucleus (STN) and pedunculopontine nucleus (PPN) have been applied as an alternative in patients with significant motor complications [18]. Although promising results have been obtained, like in pharmacological approaches, DBS also presents undesirable effects. It was related that STN DBS caused cognitive and psychiatric side effects (e.g., depression, apathy, and impulsivity) [19–21]. In addition to this, it is important to assume that not all PD patients are eligible for DBS, existing specific parameters, and conditions to be considered for this surgical procedure [22–24]. Being so, although improvements in patient’s quality of life were achieved with the above-referred treatments, they do not stop or delay disease progression, which in consequence leads to the increase of drug dosages or constant stimulation frequency adjustments. Therefore, strategies that may overcome the limitations of drug and surgical procedures should be considered and developed. Currently, the use of stem cell-based strategies has been pointed out as a new approach to treat PD patients. The use of embryonic stem cells (ESCs) [25], neural stem cells (NSCs) [26], mesenchymal stem cells (MSCs) [27] and induced pluripotent stem cells (iPSCs) [28] have been investigated and used as a potential therapeutic option to tackle PD [29–31].
More recently, pieces of evidence have been suggesting that glial cells can also be a promising cell source for the establishment of therapeutical strategies for neurodegenerative disorders, including PD [32–35]. However, it remains unclear if glial cells are key players in the development of PD or if they are a potential solution for treatment. Indeed, a dual role has been pointed out, assuming that glial cells can shift from neuroprotective- to neurodegenerative-like profile during PD development and progression, although such assumption remains to be proved [36–38]. Thus, on the scope of the present review, we intend to address the current understanding of glial cells either as a promoter of PD development/progression, either as a therapeutic target/agent for its treatment.
Glial cells
Apart from neurons, CNS is also composed of glial cells, and, for a long time, the function of these cells was not well understood. Actually, for several years, glial cells were only viewed as a “glue” for neurons, being important players in the maintenance of its viability and (trophic) support. Characteristically, glial cells are divided into three major cell groups namely, microglia, oligodendrocytes, and astrocytes, whose function and role in CNS will be further explored. There has been an increasing interest in understanding the role of these cells in the CNS both in normal and pathological conditions, since glial cells have been suggested critical in neuronal development [39–41]. Indeed, studies have shown that astrocytes and microglia are important promoters of axonal outgrowth, dendritic extension as well as modulators of the morphological plasticity of neuronal receptive endings [42–44]. Additionally, these cells have also a unique way to communicate with each other, namely through intracellular waves of calcium (Ca2+) and through the intercellular diffusion of specific gliotransmitters, such as glutamate and ATP [45–47]. Nevertheless, it is important to highlight that gliotransmitters release (e.g., gliotransmission) is still a matter of debate. Some studies showed that this phenomenon requires very specific, temporal and spatial conditions for transmission, thereby suggesting that gliotransmission may not occur under (all) physiological conditions (as remarkably reviewed in [48, 49]).
In contrast to neurons, glial cells are non-excitable cells, but they are also able to respond to various stimuli, like Ca2+ oscillations [50–53]. Under the context of CNS neurodegenerative disorders, particularly in PD, glial cells have been claimed as key players in the disease development, being associated with the occurrence of neuroinflammation and (neuronal) degenerative processes and environments [54–56]. Yet, glial activation has been considered as a secondary phenomenon caused by the neuronal degeneration itself, rather than as a direct contributor to the pathophysiological mechanisms underlying the disease.
Regarding microglial cells, they are considered the resident innate immune cells or “gate-keepers” of the healthy brain. As immune cells, microglia is capable of robust chemotaxis, phagocytosis, and cytokine production [57–59]. These cells are extremely adaptable and undergo a variety of structural changes based on the location and surrounding environment. Under physiological conditions, they exhibit a small soma with long and thin ramified processes, while in pathological conditions, the shape of these cells changes adopting a less ramified morphology with fewer and thicker processes that allow them to easily adapt and react to a novel condition [60, 61]. Actually, over the last years efforts have been done around microglial activation and characterization, as studies have indicated that its presence in the CNS is heterogeneous [62–65]. In fact, under this concept of heterogeneity, microglia has been under intense debate, as studies suggest that they can be phenotypically categorized, namely into M1 and M2 microglial cells [66, 67]. While M1 (pro-inflammatory) microglia appears to respond to injury and infection, acting as the first line of defense of the tissue, M2 (anti-inflammatory) microglia are being described as the major effector with the potential to decrease the pro-inflammatory response and to promote inflammatory attenuation and repair through the expression of anti-inflammatory molecules [66, 68]. However, microglia M1/M2 conformation has been debated [69]. Transcriptomic studies showed that microglia have increased diversity and do not follow specifically an M1 or M2 phenotype, either during homeostatic or activated conditions [69]. Actually, M1 and M2 markers are present during microglia neurodevelopment and through adulthood, thereby indicating that M1 or M2 is not specific to resting or activated phenotypes [69–71].
One of the most well-described roles of microglia is their phagocytic capability. In fact, upon an adverse stimulus in the CNS, microglia are well poised to induce programmed cell death and clean-up accumulating cellular debris [72–74]. The phagocytic activity of microglia relies on a specific receptor expressed on the cell surface and in its downstream signaling pathways. However, in the last years, new insights on microglia roles have been emerging, showing that microglia goes beyond as simple “brain immune cells”. Indeed, neuronal survival and synaptic network development are also other examples in which microglial cells play a role, contributing to a homeostatic function of the CNS. In accordance, studies indicated that during CNS development microglial cells disclose a paracrine activity able to support neuronal differentiation and maturation. For instance, Nayak et al. [72] and Ueno et al. [75] described that insulin-like growth factor-1 (IGF-1) released by surrounding microglia was crucial for the survival of layer V cortical neurons during postnatal development [72, 75]. Such observations were further confirmed by showing that inhibiting or depleting microglial activity led to cell death of layer V cortical neurons, thereby indicating that microglia have a supportive role in neuronal survival and differentiation [75, 76]. Other (neuro)trophic factors, such as basic fibroblast growth factor (FGF), nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) were also found to be released by microglia and are involved in neuronal development and maintenance. Through conditional gene inactivation, Parkhurst et al. [77] showed that microglial-secreted BDNF is involved in the regulation of learning-induced synapse formation. This interplay is mainly due to the microglial-secreted BDNF action on neuronal tropomyosin-related kinase receptor B (TrkB) and in the glutamatergic synaptic transmission modulation and plasticity [77]. In line with this, microglia were also found as being involved in synaptic pruning and maturation, required for the proper maturation of excitatory synapses transmission. In the prenatal mouse brain, microglia regulate the wiring of forebrain circuits, controlling the outgrowth of dopaminergic axons into the forebrain [78, 79]. It is well established that microglia migrate to the site of damage and participate in the phagocytic removal of cellular debris [40]. Moreover, it was recently indicated that microglial cells are also highly motile in an injured brain, continuously extending and retracting functional processes through the extracellular space. Being so, it is possible to hypothesize that microglial cells might be involved in the monitoring of synaptic maturation and functioning [40, 80, 81]. For instance, mice lacking Cx3cr1, a chemokine receptor expressed by microglia in the brain, have lower microglia cell numbers in the developing brain, a fact that was correlated with a synaptic pruning delaying.
Concerning oligodendrocytes, these are highly specialized cells whose main functions are to myelinate CNS axons, providing axonal metabolic support, and contributing to neuroplasticity [32, 82, 83]. Myelination is critical for the normal functioning of neurons, since it is the process that allows fast and efficient transduction of electrical signals in the nervous system [84]. However, due to the complex architecture and high metabolic demands of these cells, the functionality of them can be easily disturbed. As a consequence of their high metabolic demanding, oligodendrocytes are extremely vulnerable to oxidative stress [85, 86], being for that usually referred to as “the most vulnerable cells of CNS”. Additionally, over the years it was thought that under inflammatory conditions oligodendrocytes do not react; however, recent pieces of evidence have shown the opposite [85, 87], demonstrating that oligodendrocytes can produce immune mediators, such as interleukin (IL)-8 [88], an important cytokine involved in the microglia recruitment [89]. Oligodendrocytes exhibit BDNF mRNA expression and some studies addressing the BDNF expression change in disease/injury conditions. In a model of spinal cord injury, it was found that BDNF expression was upregulated in oligodendrocytes [90].
By last, astrocytes, are the most present class of glial cells in the mammalian CNS, being very heterogeneous at the functional level. Besides structural support provided to neurons [91, 92], astrocytes are also responsible for metabolic support and energy regulation through their capacity to secrete neurotrophic factors [93, 94], maintain blood–brain barrier (BBB) integrity [95] and modulate synaptic transmission and neuronal excitability [96, 97]. In addition to all these functions (in adulthood state), it is also well described that astrocytes play an important role in synapse formation and maturation during the embryonic development. For instance, studies have described that residents and fully differentiated astrocytes in the hippocampus also participate in processes of synaptogenesis [98]. Indeed, like for microglia, it is already known that astrocytes secrete molecules that are important for synaptogenesis, such as d-serine, ATP, BDNF, and glypicans [99–102]. Still, Krzisch et al. [103] found that afferent and efferent synapses of newborn neurons are ensheathed by astrocytic processes, independently of the neuronal age or the size of their synapses. Moreover, astrocytes are also important key players in neurogenesis in the hippocampus [104, 105] and an active role has been pointing out in the formation and integration of adult-born granule neurons through the release of specific growth and neurotrophic factors [104, 106, 107]. Still, astrocytes were also found to be involved in neuronal differentiation (namely into DA neurons) and maturation, due to their trophic ability of synthesis and release of growth factors, such as the basic fibroblast growth factor (bFGF), a relevant neurotrophic factor in embryonic development and neuronal lineage specifications, for instance, on ESCs.
Unlike neurons, astrocytes do not propagate action potentials, but they can sense neuronal inputs through ion channels, neurotransmitter receptors, and transporters [93, 94], thereby modulating a response through a Ca2+ signaling mechanism [108, 109], which regulates the metabolic and trophic support of neurons. Still, the expression of several functional neurotransmitter receptors, such as glutamate or gamma-Aminobutyric acid (GABA) has been correlated with the generation of a Ca2+ flux that propagates within astrocytic populations [93, 94]. Consequently, the generated Ca2+ flux is responsible for the regulation of specific gliotransmitters secretion [110–112]. Astrocytes release different gliotransmitters, such as glutamate, GABA, ATP, and D-serine that lead to the activation of axonal receptors [113]. However, yet remain the challenge to demonstrate if a single astrocyte can release different gliotransmitters or if different astrocytic subpopulations release distinct gliotransmitters [114], although a recent study showed that hippocampal astrocytes release both glutamate and ATP [114].
Glial cells in Parkinson’s disease
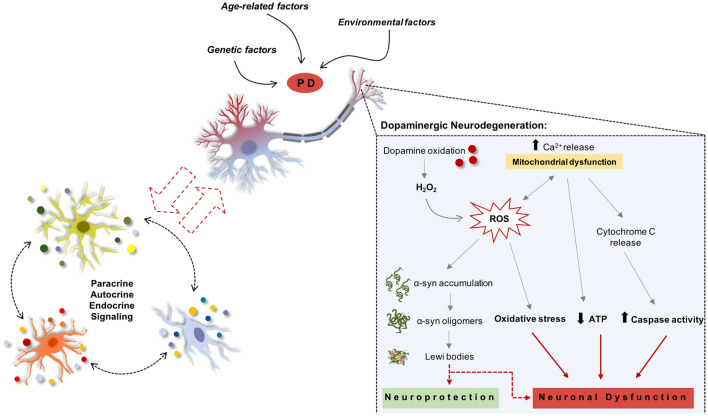
As previously mentioned, PD is the result of progressive degeneration of DA neurons in the nigrostriatal pathway [3, 5], which in consequence leads to striatal malfunctioning due to a drastic reduction of DA production and release within the striatum [3]. Nevertheless, although it has been suggested that norepinephrine and serotonin are also low in PD patients, DA is the most drastically reduced [115], being this loss considered the (main) responsible mechanism for the appearance of the majority of PD motor signs. Still, it has also been documented that PD motor symptoms are caused by increased inhibitory output from the basal nuclei to the thalamus and the prefrontal motor cortex. The motor system involves a wide range of neuronal structures in the midbrain and forebrain, being DA the pivotal neurotransmitter in the modulation of basal ganglia neurons. Another PD hallmark is the formation of Lewy bodies (LBs), used as a post-mortem disease state confirmation. From a broad range of proteins within its constitution, LBs are mainly composed by α-synuclein (α-syn) [116, 117], and although well-defined and characterized, the mechanisms engaged in the formation of LBs and why they play a role in PD pathogenesis remains still unclear [118]. According to the literature, it is known that DA neurons degeneration begins in the axonal and synaptic terminals that retrogradely progresses to the cells’ bodies of the SNpc. Notwithstanding, the starting point of DA neuronal degeneration is still poorly understood. A major question that remains to be answered is, at the cellular level, how PD is triggered? The development of intracellular LBs inclusions is one of the most well-characterized features of PD [4]. Moreover, mutations or overexpression of such proteins has also been associated with the formation of toxic oligomers/insoluble aggregates, which in turn were correlated with alterations in cellular trafficking, disruption of cell morphology, and impairments in mitochondrial function (Fig. 1) [119, 120]. Nevertheless, accumulating pieces of evidence have also been indicating that in addition to abnormal protein accumulation, mitochondrial dysfunction, disruption of protein clearance pathways, excitotoxicity, neuroinflammation, and oxidative stress is also a key player in PD initiation and progression (Fig. 1) [121–124]. In addition to this, nowadays increasing evidence is suggesting the contribution of glial cells in the demise and/or protection of DA neurons (Fig. 1). For instance, activated astrocytes and microglia were found to be either neuroprotector of DA neurons by the secretion of neurotrophic factors (such as GDNF and BDNF), either promoter of neurodegeneration by the release of pro-inflammatory molecules, such as IL-1β and TNF-α [35, 125]. Therefore, understanding this ‘dual’ role of glial cells could be of great importance in the establishment of new concepts and insights for the treatment of PD.
Fig. 1.
Mechanisms involved in the degeneration of Parkinson’s disease. DA cell death may be caused by oxidative stress, mitochondrial dysfunction, and α-synuclein aggregates. However, there is some controversy about how these mechanisms can be activated. Risk factors like genetics, age-related, and surrounding environment are well accepted. Still, the influence of glial cells in these processes is poorly understood and it is still unclear whether these cells are key players in the disease protection or progression. ROS production occurs through the auto-oxidation process of DA, resulting in significant amounts of H2O2 that can further interact with metal ions like iron, leading to DAn generation
Glial cells: are they beneficial for PD?
Trophic support and release of bioactive molecules are some of the most well-known mechanisms of glial cells in normal and pathological conditions. Factors as GDNF, bFGF, BDNF, and NGF have been found in the glial secretory profile, which are important modulators in the development and survival of DA neurons [126, 127]. Datta et al. [128] have recently described that by stimulating astrocytes with nitric oxide (NO) leads to remarkable segregation of BDNF, which was found to have a protective effect against a 6-OHDA insult, thereby promoting DA neurons survival [128]. With engineered astrocytes, Safi et al. demonstrated that GDNF-enriched CM was able to promote DA neuronal survival in vitro [129]. Similarly, Renko et al. [130] by injecting unilaterally GDNF in the striatum of a PD rat model, found that, although GDNF did not affect the extracellular level of DA, it significantly elevated tyrosine hydroxylase (TH) and catechol-O-methyltransferase (COMT) activity [130]. In contrast, with mesencephalic astrocyte-derived neurotrophic factor (MANF) they have observed a positive correlation with DA levels, which were found to be increased within the striatum [130]. Using an adeno-associated virus serotype 9 (AAV9) containing the vector-mediated gene of human MANF (hMANF), Hao et al. [131] demonstrated long term neuroprotection effect on DA neurons, a fact that was correlated with an improvement on rotational asymmetry on parkinsonian rats. Still, the same authors also showed that intracellular MANF protects DA cells via inhibiting 6-OHDA-induced endoplasmic reticulum (ER) stress, while extracellularly was found to modulate PI3K/Akt/mTOR pathway activation [131].
Similarly, Zhang et al. showed that after 6-OHDA exposure, MANF factor protected SH-SY5Y cells through the modulation of autophagy [132]. Concerning mitochondrial damage, MANF was also found as a key player through the attenuation of reactive oxidative species (ROS)-induced damage, thereby increasing mitochondrial functionality [132]. Moreover, Miyazaki et al. [133] have shown that using Levetiracetam (an anti-epileptic drug that increases xCT (a cystine-glutamate antiporter) expression, and also increases glutathione (GSH) production in/from astrocytes) that there was a protection of DA neurons against 6-OHDA-induced neurotoxicity, correlating such observation with an increase of astrocytic-derived GSH, thereby suggesting that xCT astrocytes could be a potential target to prevent DA neuronal degeneration [133]. In addition, and taking advantage of an α-synuclein Caenorhabditis elegans (C. elegans) model, Zhang et al. demonstrated that MANF rescues DA neural degeneration and locomotion defects during PD progression, through its capacity to decrease the aggregation of α-synuclein, and restore DA levels and functionality [134].
Similarly to astrocytes, it was already shown that upon activation, microglia enhances neuronal survival by the release of trophic and anti-inflammatory factors such as GDNF [135–138]. Furthermore, resembling astrocytes, microglial cell is also involved in the upregulation of tissue repair and regeneration genes [136, 139]. In contrast, under reactive and inflammatory environments, microglia have been described as promoters of PD neurodegeneration in the nigrostriatal pathway. The secretion of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-1β and IL-6, and multiple chemokines, as well as, ROS-like superoxide and NO, is the major booster of DA neuronal degeneration [136, 140, 141]. Therefore, and due to its importance in mediating neuroinflammation during disease progression, several studies have been suggesting that targeting/modulating microglia activation state may be a new and opportune strategy to target PD. Such modulation can be done through the suppression of deleterious pro-inflammatory neurotoxicity and/or by simultaneously enhance their beneficial anti-inflammatory protective functions [142, 143]. Notably, studies have shown that targeting pro-inflammatory cytokines, such as TNF-α, IL-1β, and interferon-gamma (IFN-γ), by decreasing its ability to interact with microglia receptors may be one of the ways to achieve neuronal protection [143, 144]. For instance, significant positive gains were observed only with a single injection of a lentivirus-expressing dominant-negative TNF (DN-TNF) into the SNpc of a striatal 6-OHDA lesion. Lesioned rats have shown not only amelioration on their behavioral anomalies but also attenuation on the DA loss. These observations were correlated with TNF suppression of microglial M1-associated toxicity, indicating that TNF plays a role in microglial activation and DA degeneration [143, 144]. Parallel et al. showed that Pioglitazone (a PPARγ agonist) can also be an interesting compound to modulate microglial phenotype, demonstrating that microglia can be converted from a pro-inflammatory M1 state to an anti-inflammatory M2 state after exposure, although this concept remains still under discussion [145]. Thus, while promising, the role of microglia in PD remains elusive. Therefore, future studies should be performed to address it as a potential therapeutic target to modify/delay PD progression, which may open new insights for translational clinical studies.
Glial Cells and PD: do they have detrimental effects?
Neuroinflammation
PD is characterized by its neuroinflammatory profile, where both astrocytes and microglia are pivotal players. However, it is important to highlight that remains unclear if this inflammatory response could be or not the cause or one of the leading causes of DA degeneration and consequently of PD initiation and progression.
Under inflammatory cues, microglia become activated (Fig. 2) and display conspicuous functional plasticity, ultimately transforming into a macrophage-like phenotype. These alterations include morphological changes, proliferation, increased expression of cell surface receptors, and the production of neurotoxic factors such as ROS and proinflammatory mediators such as TNF-α (Fig. 2) [33, 146]. In parallel, the immune-competent astrocytes are also able to detect danger signals in their surrounding environment, and, therefore, trigger an immune response through the secretion of important cytokines and chemokines. Moreover, these cells are also important mediators in immune cell trafficking control and activation [55, 147, 148]. Nevertheless, it is believed that an imbalance in the secretion of pro-inflammatory/anti-inflammatory substances contributes to chronic neuroinflammation and neurodegeneration (Fig. 2) [66, 149, 150]. Being so, and considering that in PD there is a progressive loss of DA neuromelanin (NM), Zhang et al. conducted a study to evaluate the impact of extracellular NM on microglial activation. From such a study they found that extracellular NM in the absence of microglia is not toxic to neurons, whereas in the presence of them, NM released by neurons can induce neurotoxicity [151].
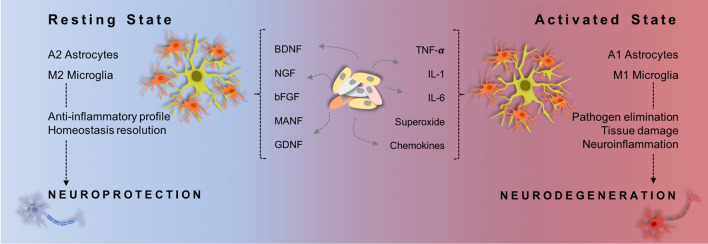
Fig. 2.
Glial cells in healthy and brain disease. Under the different environmental stimuli, astrocytes and microglia can change its activity/function. In the resting state, it is thought that microglia and astrocytes exhibit a characteristic phenotype, M2 and A2 (respectively) which is related to phenomes linked with neuroprotection. Though, under pro-inflammatory environmental this state alters shifting to an activated status: M1 and A1. Being this status associated with neurodegeneration
Although inflammation is an indispensable mechanism of defence, it often damages the surrounding tissues. So, tight control of the inflammation extent, maximizing the antipathogenic effect while minimizing tissue damage is of extreme importance. Astrocytes might play an important role in this regulation by modulating microglial activity, the major responsibility for the PD inflammatory response. For instance, astrocyte-derived plasminogen activator inhibitor type-1 (PAI-1) is being described as a regulator of microglial migration and phagocytic activity, thereby preventing apoptosis [152, 153]. On the other hand, evidence indicates that microglia release pro-inflammatory molecules that consequently activate astrocytes [55, 152]. In most cases, these, in turn, lose their normal function and gain a new toxic function that rapidly kills neurons and mature oligodendrocytes [55]. Therefore, in an attempt to modulate and block these deleterious effect different studies have been conducted. For instance, Sun et al. modulated the protein expression of aquaporin-4 (AQP4), a water membrane transport protein upregulated in PD astrocytes [154, 155]. Using an MPTP PD mice model, these authors demonstrated that knocking down (KO) this protein leads to an increase in NF-kB activity. This increase was followed by an augment in the release of IL-1β and TNF-α by astrocytes and activation of microglia, thereby demonstrating the role of AQP4 in neuroinflammation [155]. Another interesting issue that remains under scientific discussion, is the impact of astrogliosis on brain molecular, cellular, and functional alterations upon CNS injury and disease [156–159], as studies have been suggesting the existence of two main astrocytic phenotypes, namely A1 (described as the “harmful” ones) and the A2 (the protective ones) (Fig. 2). Having this in mind, it seems that the A1 phenotype only appears through microglia activation, gaining a neurotoxic function, and rapidly inducing neuronal death [55]. In contrast, other authors have shown that blocking the microglial mediated conversion of astrocytes to an A1 neurotoxic phenotype through glucagon-like peptide-1 receptor agonists, leads to protection against the loss of DA and behavioral deficits in an α-syn PD mouse model [160]. Even though, to better dissect the role of glial cells in PD, Kuter et al. [161] induced astrocytic dysfunction by chronic infusion of fluorocitrate (FC) into the SNpc of a 6-OHDA PD model. From such a study, they described that prolonged astrocyte dysfunction and death, as well as microglia activation, stressed DA neurons but did not massively degenerate them. Probably these effects were found due to the low concentration of FC used (2 nmol/24 h for 7 days) [161]. However, when combined with 6-OHDA toxin, a reversal effect was observed, with an accelerated DA neuronal degeneration being disclosed. Nevertheless, this study does not provide mechanistic insights on how astrocytes become dysfunctional, whereby further studies are needed to fulfill the role of astrocytes function on PD and in DA system and functionality, as well as how they interplay with microglial cells.
Oxidative stress
Oxidative stress is thought to play an important role in DA neurotoxicity, resulting from an imbalance of ROS production and cellular antioxidant activity [162, 163]. Nevertheless, a well-known endogenous cellular mechanism of defence against oxidative stress is the binding of the transcription factor NF-E2-related factor (Nrf2) to the antioxidant response element (ARE), which leads to antioxidant effects, phase II detoxification enzymes and neuroprotective effects [164]. Indeed, it was already shown that overexpression of Nrf2 in astrocytes protects mice from mutant α-syn [165], and Nrf2-overexpressing astrocytes transplantation into the mouse striatum protects it against 6-OHDA toxicity [166, 167]. Yet, in addition to the segregation of important trophic and neurotrophic molecules, astrocytes are also able to express enzymes with DA roles, as it is the case of MAO-B [168]. MAO-B is a monoamine metabolic enzyme that oxidizes the neurotransmitter dopamine and other amines [169, 170], and hydrogen peroxide (H2O2) is one of the products released during such oxidation. Astrocytes are protected against these oxidative species due to their high content in GSH and glutathione peroxidase, which can detoxify H2O2 within the cells [171]. However, H2O2 has a high membrane permeability, and therefore, it can induce toxic effects not only within the cell of origin but also in neighboring cells [168]. In fact, in the post-mortem brains of PD patients, it was found high levels of MAO-B in astrocytes surrounding the SNpc [172]. In light of this, studies have been performed and proved that the inhibition of MAO-B prolongs the half-life of DA neurons, extending their neurotransmission effect and consequently relieving associated motor symptoms [173].
Like astrocytes, microglia also plays a pivotal role in the pathology of PD by oxidative stress. Microglia activation through LPS leads to activation of the ERK signaling pathway and, consequently, to NADPH oxidase activation [174]. NADPH oxidase is expressed on microglia and is the main ROS producing enzyme during inflammation [175]. Additionally, using an MPTP model, mutant mice defective in NADPH-oxidase have less SNpc DA loss when comparing to their littermates [175].
PD-related genes expressed in Glial cells
Several studies have determined that genes known to have a causative role in the development of PD are expressed in glial cells and have important roles in glial function. Below, we will address some of these genes and their impact on glial cell function.
Park2
This gene encodes ubiquitin ligase (E3)—the Parkin protein—that mediates the link of ubiquitin to its substrate [176], and is also involved in a genetic recessive form of PD [177, 178]. Besides DA neurons, Parkin also plays an important role in astrocyte proliferation. Of note, using cultures of mice Park2-KO, astrocytes seem to have a decreased proliferation rate [179, 180]. Moreover, the absence of Park2 was also found to affect astrocytes neurotrophic and antioxidant capabilities (Fig. 3), leading, for instance, to the reduction of glutathione levels [179–181]. In a different study, it was shown that in a Park2 KO astrocytic model, the neurons had slower growth and a lower oxygen consumption rate. Nevertheless, when WT astrocytes were added to the culture system the phenotype was rescued [182]. Additionally, using toxins to induce the activation of microglia, studies have tried to undisclose the effects of Parkin mRNA in microglial function. For instance, LPS exposure in microglial cultures leads to a decrease of parkin by over-activating c-Jun N-terminal Kinase (JNK) and NF-kB pathways (Fig. 3) [183, 184], thereby suggesting that reductions in parkin levels may contribute to the development of PD by increasing the vulnerability of the nigrostriatal pathway.
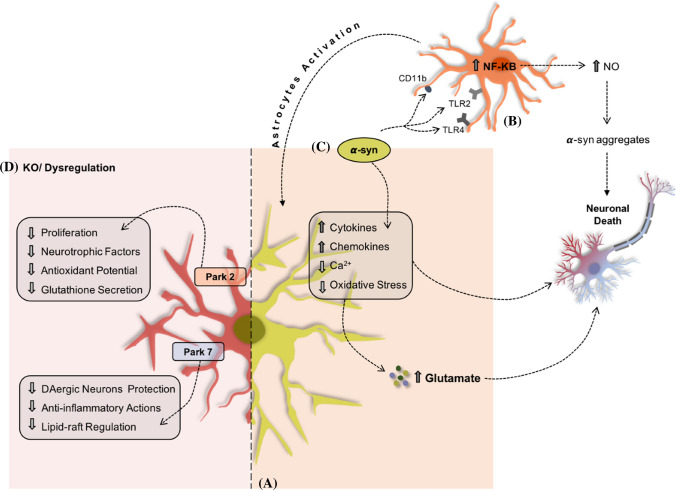
Fig. 3.
PD-related genes expressed in glial cells. Astrocytes (a) can uptake α-syn (c) and, initially, this process is viewed as a protective mechanism. However, reaching a certain threshold, this capability becomes toxic to these cells leading to its malfunction. Also, α-syn can bind to microglial cells (b) through surface receptors and lead to the activation of inflammatory pathways. The dysregulation/depletion of genes, such as Park 2 and Park 7 in astrocytes (d) is also connected to astrocytic malfunction and DA degeneration
Park7
Several studies have determined that genes known to have a causative role in the development of PD are expressed in glial cells and have important roles in glial function. For e.g., DJ-1 (encoded by Park7), which is a chaperone that suppresses α-syn fibrillation [185], mediates neuroprotection through an astrocyte-dependent mechanism involving extracellular-secreted soluble factors (anti-oxidant molecules, bioenergetic molecules, cytokines, and peptide neurotrophic factors) [186–188]. To prove the importance of this protein in neuroprotection, Lev et al. did a KO of Park7 in astrocytes, which resulted in a decrease in astrocytes’ capacity to protect DA neurons both in rotenone and 6-OHDA neurotoxin PD models (Fig. 3) [189, 190]. In a similar study, demonstrating that DJ-1 exerts anti-inflammatory actions in astrocytes and microglia, Kim et al. [191] demonstrated that DJ-1 facilitates the interactions between Src-homology 2-domain containing protein tyrosine phosphate-1 (SHP-1) and specific signal-transducers and activators of transcription (STAT), such as STAT1—important signaling molecules in the modulation of brain inflammation. This protein is also involved in the regulation of lipid raft-dependent endocytosis in astrocytes (Fig. 3) [192, 193], supporting the organization and interaction of proteins involved in several functions, like receptor trafficking and signal transduction [192, 194]. In PD models, it is already known that this composition is altered [195]. Previous reports have shown that lipid rafts associated with several PD-associated proteins, including DJ-1 [193], parkin [196], PINK1 (PTEN-induced kinase 1) [197] and α-syn [198], can protect neurons against oxidative stress by overexpressing human DJ-1 protein in astrocytes. It was already demonstrated that after rotenone exposure, there was a clear reduction in neuronal degeneration and microglial activation in PD animals’ models overexpressing DJ-1 in astrocytes. Also, in an α-syn accumulation and phosphorylation PD model, a remarkable decreased within DA neuronal degeneration was found in DJ-1 transduced animals, thereby indicating DJ-1 as a multiplayer in different PD conditions. Finally, using a transgenic zebrafish model, Frøyset et al. showed that DJ-1 overexpression suppressed mitochondrial complex I inhibition, preventing oxidative stress [199]. Still, DJ-1 was also found to increase the protein levels related to redox status, diluting NO production as well as protein nitrosylation, thereby indicating that DJ-1 may contribute to astrocytic functionality [199].
Park8
Autosomal-dominant missense mutations with the LRRK2 (leucine-rich repeat protein kinase 2, or park8) protein are also involved in PD development [200]. Epidemiologically, about 1% is found in sporadic cases, while 13% are related to familial PD cases [201]. At cellular level LRRK2 is expressed both in neurons and glial cells [202]. Nevertheless, studies have shown that LRRK2 basal levels are higher in cultured microglial cells when compared to neuronal cells [203]. Such evidence could, in that way indicate a potential key role of LRRK2 in microglial functions, like inflammation or phagocytosis [202, 204]. Still, and through RNA-sequencing procedures to characterize the transcriptomic profiles of LRRK2 WT and KO microglial cells treated with α-synuclein pre-formed fibrils (PFFs), it was shown that LRRK2 KO microglia cells reported an attenuated mitochondrial impairment in response to α-synuclein PFFs [205].
Regarding its role in astrocytes, a primary culture system has shown that LRRK2 regulates lysosome size, number, and function by diminishing the lysosomal capacity [206]. Moreover, a recent study characterizing LRRK2 effects on astrocytes (using midbrain-patterned astrocytes from human induced pluripotent stem cells (iPSCs) derived from PD LRRK2 patients) has shown that transforming growth factor-beta 1 (TGFB1, responsible to reduce microglial inflammatory response [207]) and matrix metallopeptidase 2 (MMP2, which is known to degrade α-synuclein aggregates [208]) were down-regulated in LRRK2 astrocytes. Thus, although LRRK2 appears to impact astrocytic (glial) function [209], important studies fully addressing the impact of LRRK-2 on it and in PD development and progression are missing.
SNCA
One evident hallmark of PD is the formation of cellular inclusions in the brain, commonly called Lewy bodies and Lewy neuritis [210, 211]. These are mainly composed of α-syn (SNCA gene) [211], a protein that aggregates into insoluble fibrils via the formation of soluble intermediates. Depositions of α-syn are mainly found in PD DA neurons, but also astrocytes [212]. Studies have been postulating that α-syn can spread from neurons to glial cells via the extracellular space or direct cell-to-cell transfer (Fig. 3) [213, 214]. Astrocytes can rapidly and extensively uptake α-syn oligomers from the extracellular space [213]. Although the neuropathophysiology of α-syn in PD initiation and progression is still not well understood, studies have indicated that in initial phases, the α-syn uptake may be neuroprotective, thereby preventing disease progression. Simplistically, there may exist a threshold that further affects mitochondrial integrity in astrocytes and then leads to neurotoxicity [215]. It was revealed that α-syn accumulation in astrocytes leads to increased levels of expression of cytokines and chemokines, Ca2+ flux, and oxidative stress (Fig. 3) [216, 217]. This ultimately culminates in compromised astrocytic function, such as glutamate uptake and blood–brain barrier integrity [56]. Consequently, microglial cells are activated in the midbrain, where a significant loss of DA neurons is observed [56, 218].
Conversely, soluble α-syn binds with microglia cell surface receptor(TLR2, TLR4, and CD11b) increases oxidative stress leading to the activation of inflammatory pathways, nuclear factor kappa-B (Fig. 3) (NF-kB) [198, 199]. This will lead to the activation of astrocytes and consequently to the upregulation of inflammatory molecules, such as nitric oxide (NO) responsible for creating α-syn aggregates [221, 222]. Recently, Olsen and Feany demonstrated that using Drosophila expressing human α-synuclein in glia culminates in α-synuclein aggregation, death of dopaminergic neurons, impaired locomotor function, and autonomic dysfunction [223], thereby indicating that glial cells may be key players in PD.
GBA
Glucosylceramidase beta is encoded by the GBA gene. Mutations in this gene increase the risk of developing PD, since it leads to increased accumulation of pathological LBs and remarkable cognitive changes than those without GBA mutations [224]. Physiologically, GBA is expressed both in neurons [225] and glial cells [226]. Curiously, astrocytes were found to have higher GBA activity rather than microglial cells [226]. Indeed, GBA expression is relatively enriched in astrocytes and based on the study that was conducted by Sanyal et al. knocking-down GBA in astrocytes led to broad deficits in lysosomal morphology and function, causing inflammatory responses and increasing neurologic damage [227]. Also, studies using GBA-KO astrocytes showed that these astrocytes present reduced LC3-positive puncta, thereby indicating that GBA plays an important role in autophagy [228].
New PD therapies targeting glial cells
Current treatments for PD only ameliorate motor symptoms and do not delay or treat the disease, whereby the main challenge remains: the development of a neuroprotective or disease-modifying strategy for PD. Therefore, targeting both neurons and/or glial cells may offer opportune windows for the establishment of new PD treatments.
Currently, different therapies targeting astrocytes are being developed based on astrocyte transplantation and/or in the pharmacological correction of astrocytic dysfunction. Regarding the first view, disease-relevant human astrocytes can be acquired from primary sources or the differentiation of ESCs or iPSCs [229, 230]. In fact, over the last years, different protocols have been developed to generated feasible ESC- and iPSC-derived astrocytes [231, 232]. After the differentiation of stem cells into astrocytes, they can be transplanted to replace malfunctioning cells or to promote the survival of the existing neurons. Although, there is a general lacking of studies using these approaches, recent pieces of evidence demonstrated that astrocytes can be derived from stem cells in the context of PD [209, 233]. Of note, in the works of di Domenico et al. and Booth et al. it was indicated that astrocytes could be contributors during PD pathogenesis, which open new paths to explore not only new mechanisms to understand PD pathophysiology but also to explore novel therapeutic strategies and targets to tackle PD [179, 233]
In addition to this, stem cell-derived astrocytes can also be widely used both for disease modeling and drug discovery, for instance, to uncover novel compounds that can be protective to insults within the CNS, such as ROS [234, 235]. An example is the use of astrocytes derived from hESCs to find compounds that protect against oxidative stress through a phenotypic assay [235]. Since oxidative stress is well established in the pathology of many neurological diseases, the identification of these stress-protective compounds could be a major help to halt or slow disease progression.
Concerning microglial cells, it has been hypothesized that targeting its activation state by suppressing their deleterious pro-inflammatory neurotoxicity and simultaneously enhancing their anti-inflammatory protective functions can be a potential approach for PD treatment [143]. For instance, suppression of the microglial M1 phenotype would decrease the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ [143]. Having this in mind, TNF was already targeted in PD animal models to overwhelm the toxicity associated with the M1 phenotype. Of note, a single injection of lentivirus-expressing dominant TNF into the rat SNpc alongside with 6-OHDA lesion in the striatum was found to attenuate DA loss and correct behavioral deficits in rats [144]. Overall, TNF may be a promising therapeutic target in PD [236]. On the other hand, targeting molecules involved in the activation of the anti-inflammatory M2 microglial phenotype or able to promote the transition from the pro-inflammatory M1 to the M2 could also constitute a promising target, as shown with IL-10 and beta interferons applications [143]. Indeed, cerebral infusion of AAV-expressing human IL-10 in an MPTP PD mouse model was found to promote the decrease of pro-inflammatory iNOS expression, enhancing the levels of anti-inflammatory mediators, such as IFN-γ and transforming TGF-β while preventing the loss of striatal DA neurons [143, 237, 238].
Histone deacetylase (HDAC) has also been looked like a promising therapeutic target for PD, given its role in the modulation of glial cells and α-syn aggregation effects [239, 240]. Indeed, the use of HDAC inhibitors is being presented as a potential treatment of PD, as studies previously shown that using HDAC inhibitors reduce inflammation by preventing the release of pro-inflammatory cytokines from microglia [240, 241]. In line with this, cannabinoid type-2 receptors (CB2) are also being considered a PD therapeutical target, since they are commonly found on activated microglia on PD [242]. Pharmacological activation of microglia CB2 receptors led to a reduction in microglial activation, enhancing the functional motor deficits of an MPTP mouse model of PD [242, 243].
Finally, and as beforementioned, glial cell transplantation has recently emerged as a promising tool for CNS regenerative approaches [244]. However, cell transplantation procedures remain under discussion, and other alternative approaches have been suggested and explored, as the use of the secretome of glial cells [189]. Glial cells secretome was already profiled and it has been suggested as a novel approach for the treatment of CNS disorders, including PD [34, 244, 245]. Actually, and if we think from the clinical point of view, glial secretome could be a strong tool not only to be used as a therapeutical strategy but also as a reliable source for diagnosis and prognosis biomarkers and to the identification of therapeutical targets [246–248]. Nevertheless, the potential application of glial cells secretome as a potential tool for CNS regeneration was already demonstrated by Jeon et al. [249], which found glial cells secretome as a modulator of the phagocytic function of microglia due to the presence of the acute phase protein pentraxin (PTX3) in its composition. Thus, although the intrinsic potential of glial cells secretome appears to be promising, studies regarding its functional impact and its interplay under normal and pathological conditions remain unexplored [245], whereby studies should be performed in the future to explore their role in the pathophysiology of PD, as important gains can be obtained with potential implication to the clinics. In addition to this, although several models are being used to study PD, it becomes important to develop and have new models that could resemble as much as possible PD physiological conditions as it occurs in the human condition, like the preformed fibril model [250–252].
Conclusion
Although studies investigating the contribution of glial cells to the pathogenesis of PD are still sparse compared to those focusing on neurons, its involvement in the disease is now becoming a hot topic. However, the exact role of these cells on the PD pathophysiology is still controversial. Even though, are glial cells key players in the neurodegeneration of DA neurons? Or is the malfunction of glial cells a consequence of DA neurons degeneration? Although such questions remain still to be answered, probably both possibilities might be plausible and coexist in an orchestrated way, which could open new avenues and insights for PD pathophysiology understanding and future therapeutical opportunities.
Acknowledgements
The authors want to acknowledge the financial support from Prémios Santa Casa Neurociências Prize Mantero Belard for Neurodegenerative Diseases Research (MB-28-2019). This work was supported by the European Regional Development Fund (FEDER), through the Competitiveness Internationalization Operational Programme (POCI), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of the projects POCI-01-0145-FEDER-029751, POCI-01-0145-FEDER-007038, UIDB/50026/2020 and UIDP/50026/2020; POCI-01-0145-FEDER-016428 (MEDPERSYST) and PTDC/MED-NEU/29071/2017 (REWSTRESS); and by the projects NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023, supported by Norte Portugal Regional Operational Programme (NORTE 2020). AVD has an FCT grant (SFRH/BD/147066/2019).
Footnotes
Ana. J. Rodrigues and Fábio G. Teixeira share senior authorship
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ana J. Rodrigues, Email: ajrodrigues@med.uminho.pt
Fábio G. Teixeira, Email: fabioteixeira@med.uminho.pt
References
- 1.Pringsheim T, Jette N, Frolkis A, Steeves TDL. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord Off J Mov Disord Soc. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Kim H-J, Lee J-Y, et al. Phenotype analysis in patients with early onset Parkinson’s disease with and without parkin mutations. J Neurol. 2011;258:2260–2267. doi: 10.1007/s00415-011-6110-1. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson’s disease Lancet. Lond Engl. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primer. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 5.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez MC, Guridi OJ, Alvarez L, et al. The subthalamic nucleus and tremor in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 1998;13(Suppl 3):111–118. doi: 10.1002/mds.870131320. [DOI] [PubMed] [Google Scholar]
- 7.Dujardin K, Degreef JF, Rogelet P, et al. Impairment of the supervisory attentional system in early untreated patients with Parkinson’s disease. J Neurol. 1999;246:783–788. doi: 10.1007/s004150050455. [DOI] [PubMed] [Google Scholar]
- 8.Owens-Walton C, Jakabek D, Li X, et al. Striatal changes in Parkinson disease: an investigation of morphology, functional connectivity and their relationship to clinical symptoms. Psychiatry Res. 2018 doi: 10.1016/j.pscychresns.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Daniel W, Burn DJ. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov Disord. 2011;26:1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeWitt PA, Fahn S. Levodopa therapy for Parkinson disease: a look backward and forward. Neurology. 2016;86:S3–12. doi: 10.1212/WNL.0000000000002509. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4:743–757. doi: 10.2147/ndt.s2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rascol O, Payoux P, Ory F, et al. Limitations of current Parkinson’s disease therapy. Ann Neurol. 2003;53(Suppl 3):S3–12. doi: 10.1002/ana.10513. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Shahed J, Telkes I, Viswanathan A, Ince NF. GPi oscillatory activity differentiates tics from the resting state, voluntary movements, and the unmedicated Parkinsonian state. Front Neurosci. 2016;10:436. doi: 10.3389/fnins.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013;62:132–144. doi: 10.1016/j.freeradbiomed.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo C, Jost WH, Bonizzoni E. Long-term efficacy of safinamide on symptoms severity and quality of life in fluctuating Parkinson’s disease patients. J Park Dis. 2020;10:89–97. doi: 10.3233/JPD-191765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29:229–237. doi: 10.1002/mds.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira FG, Gago MF, Marques P, et al. Safinamide: a new hope for Parkinson’s disease? Drug Discov Today. 2018;23:736–744. doi: 10.1016/j.drudis.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367:1529–1538. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- 19.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25:578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 20.Strutt AM, Simpson R, Jankovic J, York MK. Changes in cognitive-emotional and physiological symptoms of depression following STN-DBS for the treatment of Parkinson’s disease. Eur J Neurol. 2012;19:121–127. doi: 10.1111/j.1468-1331.2011.03447.x. [DOI] [PubMed] [Google Scholar]
- 21.Taba HA, Wu SS, Foote KD, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113:1224–1229. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RR, Kotagal V, Patil PG, Chou KL. Validity and efficacy of screening algorithms for assessing deep brain stimulation candidacy in Parkinson disease. Mov Disord Clin Pract. 2014;1:342–347. doi: 10.1002/mdc3.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamberg K, Hariz G-M. The decision-making process leading to deep brain stimulation in men and women with Parkinson’s disease—an interview study. BMC Neurol. 2014;14:89. doi: 10.1186/1471-2377-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishita T, Rahman M, Foote KD, et al. DBS candidates that fall short on a levodopa challenge test: alternative and important indications. The Neurologist. 2011;17:263–268. doi: 10.1097/NRL.0b013e31822d1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brederlau A, Correia AS, Anisimov SV, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells Dayt Ohio. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 26.Daadi MM, Grueter BA, Malenka RC, et al. Dopaminergic neurons from midbrain-specified human embryonic stem cell-derived neural stem cells engrafted in a monkey model of Parkinson’s disease. PLoS ONE. 2012;7:e41120. doi: 10.1371/journal.pone.0041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Wang X, Wang S. Isolation and characterization of mesenchymal stem cells derived from bone marrow of patients with Parkinson’s disease. Vitro Cell Dev Biol Anim. 2008;44:169–177. doi: 10.1007/s11626-008-9093-1. [DOI] [PubMed] [Google Scholar]
- 28.Savchenko E, Marote A, Russ K, et al. Generation of a human induced pluripotent stem cell line (CSC-42) from a patient with sporadic form of Parkinson’s disease. Stem Cell Res. 2018;27:78–81. doi: 10.1016/j.scr.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Goodarzi P, Aghayan HR, Larijani B, et al. Stem cell-based approach for the treatment of Parkinson’s disease. Med J Islam Repub Iran. 2015;29:168. [PMC free article] [PubMed] [Google Scholar]
- 30.Pires AO, Teixeira FG, Mendes-Pinheiro B, et al. Old and new challenges in Parkinson’s disease therapeutics. Prog Neurobiol. 2017;156:69–89. doi: 10.1016/j.pneurobio.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Chen W, Tan S, Lin T. Stem cells for modeling and therapy of Parkinson’s disease. Hum Gene Ther. 2016;28:85–98. doi: 10.1089/hum.2016.116. [DOI] [PubMed] [Google Scholar]
- 32.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol (Berl) 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Hong J-S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 34.Mena MA, García de Yébenes J. Glial cells as players in parkinsonism: the “good”, the “bad”, and the “mysterious” glia. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2008;14:544–560. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Song N, Jiang H, et al. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta. 2013;1832:618–625. doi: 10.1016/j.bbadis.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 36.De Miranda BR, Rocha EM, Bai Q, et al. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol Dis. 2018;115:101–114. doi: 10.1016/j.nbd.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 38.Yue P, Gao L, Wang X, et al. Pretreatment of glial cell-derived neurotrophic factor and geranylgeranylacetone ameliorates brain injury in Parkinson’s disease by its anti-apoptotic and anti-oxidative property. J Cell Biochem. 2018;119:5491–5502. doi: 10.1002/jcb.26712. [DOI] [PubMed] [Google Scholar]
- 39.Jäkel S, Dimou L. Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci. 2017;11:24. doi: 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinhard L, di Bartolomei G, Bolasco G, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto A, Wake H, Ishikawa AW, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:1–12. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitzer-Quintero OK, González-Burgos I (2012) Immune system in the brain: a modulatory role on dendritic spine morphophysiology? In: Neural Plast. https://www.hindawi.com/journals/np/2012/348642/. Accessed 7 Mar 2018 [DOI] [PMC free article] [PubMed]
- 43.Jha MK, Kim J-H, Song GJ, et al. Functional dissection of astrocyte-secreted proteins: implications in brain health and diseases. Prog Neurobiol. 2017 doi: 10.1016/j.pneurobio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Vinet J, van Weering HRJ, Heinrich A, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation. 2012;9:27. doi: 10.1186/1742-2094-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- 47.Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci Off J Soc Neurosci. 2018;38:14–25. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiacco TA, McCarthy KD. Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J Neurosci. 2018;38:3–13. doi: 10.1523/JNEUROSCI.0016-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 51.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato D, Eto K, Nabekura J, Wake H. Activity-dependent functions of non-electrical glial cells. J Biochem (Tokyo) 2018;163:457–464. doi: 10.1093/jb/mvy023. [DOI] [PubMed] [Google Scholar]
- 53.Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:e96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu X-L, Long C-X, Sun L, et al. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michell-Robinson MA, Touil H, Healy LM, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain J Neurol. 2015;138:1138–1159. doi: 10.1093/brain/awv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, et al. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosser C-A, Baptista S, Arnoux I, Audinat E. Microglia in CNS development: shaping the brain for the future. Prog Neurobiol. 2017;149–150:1–20. doi: 10.1016/j.pneurobio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Sierra A, Encinas JM, Deudero JJP, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilbo S, Stevens B. Microglia: the brain’s first responders. Cerebrum Dana Forum Brain Sci. 2017;2017:14–17. [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Nicola D, Perry VH. Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2015;21:169–184. doi: 10.1177/1073858414530512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang CC, Wu CH, Shieh JY, et al. Immunohistochemical study of amoeboid microglial cells in fetal rat brain. J Anat. 1996;189:567–574. [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Arjona del MM, Grondona JM, Granados-Durán P, et al. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front Cell Neurosci. 2017;11:235. doi: 10.3389/fncel.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 67.Ajmone-Cat MA, Mancini M, De Simone R, et al. Microglial polarization and plasticity: evidence from organotypic hippocampal slice cultures. Glia. 2013;61:1698–1711. doi: 10.1002/glia.22550. [DOI] [PubMed] [Google Scholar]
- 68.Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci Off J Soc Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 70.Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammond TR, Dufort C, Dissing-Olesen L, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271.e6. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 74.He H, Zhou Y, Zhou Y, et al. Dexmedetomidine mitigates microglia-mediated neuroinflammation through upregulation of programmed cell death protein 1 in a rat spinal cord injury model. J Neurotrauma. 2018;35:2591–2603. doi: 10.1089/neu.2017.5625. [DOI] [PubMed] [Google Scholar]
- 75.Ueno M, Fujita Y, Tanaka T, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 76.Schafer DP, Stevens B. Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol. 2015;7:a020545. doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tay TL, Savage JC, Hui CW, et al. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2017;595:1929–1945. doi: 10.1113/JP272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Squarzoni P, Oller G, Hoeffel G, et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 80.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 81.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 82.McKenzie IA, Ohayon D, Li H, et al. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nave K-A, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 84.Simons M, Nave K-A. Oligodendrocytes: myelination and axonal support. Cold Spring Harb Perspect Biol. 2016;8:a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peferoen L, Kipp M, Valk P, et al. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014;141:302–313. doi: 10.1111/imm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giacci MK, Bartlett CA, Smith NM, et al. Oligodendroglia are particularly vulnerable to oxidative damage after neurotrauma in vivo. J Neurosci. 2018;38:6491–6504. doi: 10.1523/JNEUROSCI.1898-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeis T, Enz L, Schaeren-Wiemers N. The immunomodulatory oligodendrocyte. Brain Res. 2016;1641:139–148. doi: 10.1016/j.brainres.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 88.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balabanov R, Strand K, Goswami R, et al. Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci Off J Soc Neurosci. 2007;27:2013–2024. doi: 10.1523/JNEUROSCI.4689-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith CM, Cooksey E, Duncan ID. Myelin loss does not lead to axonal degeneration in a long-lived model of chronic demyelination. J Neurosci. 2013;33:2718–2727. doi: 10.1523/JNEUROSCI.4627-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- 92.Schreiner B, Romanelli E, Liberski P, et al. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Rep. 2015;12:1377–1384. doi: 10.1016/j.celrep.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 93.Bosson A, Boisseau S, Buisson A, et al. Disruption of dopaminergic transmission remodels tripartite synapse morphology and astrocytic calcium activity within substantia nigra pars reticulata. Glia. 2015;63:673–683. doi: 10.1002/glia.22777. [DOI] [PubMed] [Google Scholar]
- 94.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Yamamizu K, Iwasaki M, Takakubo H, et al. In vitro modeling of blood-brain barrier with human iPSC-derived endothelial cells, pericytes, neurons, and astrocytes via notch signaling. Stem Cell Rep. 2017;8:634–647. doi: 10.1016/j.stemcr.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.van Deijk A-LF, Camargo N, Timmerman J, et al. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia. 2017;65:670–682. doi: 10.1002/glia.23120. [DOI] [PubMed] [Google Scholar]
- 97.Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Catalani A, Sabbatini M, Consoli C, et al. Glial fibrillary acidic protein immunoreactive astrocytes in developing rat hippocampus. Mech Ageing Dev. 2002;123:481–490. doi: 10.1016/S0047-6374(01)00356-6. [DOI] [PubMed] [Google Scholar]
- 99.Cao X, Li L-P, Wang Q, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19:773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- 100.Martin-Fernandez M, Jamison S, Robin LM, et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci. 2017;20:1540–1548. doi: 10.1038/nn.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panatier A, Theodosis DT, Mothet J-P, et al. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 102.Tan Z, Liu Y, Xi W, et al. Glia-derived ATP inversely regulates excitability of pyramidal and CCK-positive neurons. Nat Commun. 2017;8:13772. doi: 10.1038/ncomms13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krzisch M, Temprana SG, Mongiat LA, et al. Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Struct Funct. 2015;220:2027–2042. doi: 10.1007/s00429-014-0768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sultan S, Li L, Moss J, et al. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron. 2015;88:957–972. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 105.Terrillion CE, Abazyan B, Yang Z, et al. DISC1 in astrocytes influences adult neurogenesis and hippocampus-dependent behaviors in mice. Neuropsychopharmacology. 2017;42:2242–2251. doi: 10.1038/npp.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moss J, Gebara E, Bushong EA, et al. Fine processes of nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci USA. 2016;113:E2536–2545. doi: 10.1073/pnas.1514652113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci Off J Soc Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Srinivasan R, Huang BS, Venugopal S, et al. Ca2+ signaling in astrocytes from IP3R2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Di Castro MA, Chuquet J, Liaudet N, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 111.Osipova ED, Semyachkina-Glushkovskaya OV, Morgun AV, et al. Gliotransmitters and cytokines in the control of blood–brain barrier permeability. Rev Neurosci. 2018 doi: 10.1515/revneuro-2017-0092. [DOI] [PubMed] [Google Scholar]
- 112.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci. 2014;15:327–335. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 113.Perea G, Gómez R, Mederos S, et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife. 2016;5:e20362. doi: 10.7554/eLife.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Covelo A, Araque A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife. 2018;7:e32237. doi: 10.7554/eLife.32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shannak K, Rajput A, Rozdilsky B, et al. Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson’s disease and normal subjects. Brain Res. 1994;639:33–41. doi: 10.1016/0006-8993(94)91761-2. [DOI] [PubMed] [Google Scholar]
- 116.Benskey MJ, Perez RG, Manfredsson FP. The contribution of alpha synuclein to neuronal survival and function—implications for Parkinson’s disease. J Neurochem. 2016;137:331–359. doi: 10.1111/jnc.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mahul-Mellier A-L, Burtscher J, Maharjan N, et al. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci. 2020;117:4971–4982. doi: 10.1073/pnas.1913904117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 119.DeMaagd G, Philip A. Parkinson’s disease and its management. Pharm Ther. 2015;40:504–532. [PMC free article] [PubMed] [Google Scholar]
- 120.Subramaniam SR, Vergnes L, Franich NR, et al. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis. 2014;70:204–213. doi: 10.1016/j.nbd.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ammal Kaidery N, Thomas B. Current perspective of mitochondrial biology in Parkinson’s disease. Neurochem Int. 2018 doi: 10.1016/j.neuint.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Monti DA, Zabrecky G, Kremens D, et al. N-Acetyl cysteine may support dopamine neurons in Parkinson’s disease: preliminary clinical and cell line data. PLoS ONE. 2016;11:e0157602. doi: 10.1371/journal.pone.0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith KM, Eyal E, Weintraub D, Investigators ADAGIO. Combined rasagiline and antidepressant use in Parkinson disease in the ADAGIO study: effects on nonmotor symptoms and tolerability. JAMA Neurol. 2015;72:88–95. doi: 10.1001/jamaneurol.2014.2472. [DOI] [PubMed] [Google Scholar]
- 124.Ahuja M, Ammal Kaidery N, Yang L, et al. Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson’s-like disease. J Neurosci Off J Soc Neurosci. 2016;36:6332–6351. doi: 10.1523/JNEUROSCI.0426-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu H, Wang Y, Song N, et al. New progress on the role of glia in iron metabolism and iron-induced degeneration of dopamine neurons in Parkinson’s disease. Front Mol Neurosci. 2018;10:455. doi: 10.3389/fnmol.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rappold PM, Tieu K. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics. 2010;7:413–423. doi: 10.1016/j.nurt.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rocha SM, Cristovão AC, Campos FL, et al. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol Dis. 2012;47:407–415. doi: 10.1016/j.nbd.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 128.Datta I, Ganapathy K, Razdan R, Bhonde R. Location and number of astrocytes determine dopaminergic neuron survival and function under 6-OHDA stress mediated through differential BDNF release. Mol Neurobiol. 2017;1–21:5505. doi: 10.1007/s12035-017-0767-0. [DOI] [PubMed] [Google Scholar]
- 129.Safi R, Gardaneh M, Panahi Y, et al. Optimized quantities of GDNF overexpressed by engineered astrocytes are critical for protection of neuroblastoma cells against 6-OHDA toxicity. J Mol Neurosci MN. 2012;46:654–665. doi: 10.1007/s12031-011-9654-8. [DOI] [PubMed] [Google Scholar]
- 130.Renko J-M, Bäck S, Voutilainen MH, et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) elevates stimulus-evoked release of dopamine in freely-moving rats. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-0872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hao F, Yang C, Chen S-S, et al. Long-term protective effects of AAV9-mesencephalic astrocyte-derived neurotrophic factor gene transfer in Parkinsonian rats. Exp Neurol. 2017;291:120–133. doi: 10.1016/j.expneurol.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 132.Zhang J, Cai Q, Jiang M, et al. Mesencephalic astrocyte-derived neurotrophic factor alleviated 6-OHDA-induced cell damage via ROS-AMPK/mTOR mediated autophagic inhibition. Exp Gerontol. 2017;89:45–56. doi: 10.1016/j.exger.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 133.Miyazaki I, Murakami S, Torigoe N, et al. Neuroprotective effects of levetiracetam target xCT in astrocytes in Parkinsonian mice. J Neurochem. 2016;136:194–204. doi: 10.1111/jnc.13405. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z, Shen Y, Luo H, et al. MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol. 2018;308:59–71. doi: 10.1016/j.expneurol.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 135.Ding YM, Jaumotte JD, Signore AP, Zigmond MJ. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J Neurochem. 2004;89:776–787. doi: 10.1111/j.1471-4159.2004.02415.x. [DOI] [PubMed] [Google Scholar]
- 136.Le W, Wu J, Tang Y. Protective microglia and their regulation in Parkinson’s disease. Front Mol Neurosci. 2016;9:89. doi: 10.3389/fnmol.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nam JH, Leem E, Jeon M-T, et al. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson’s disease. Mol Neurobiol. 2015;51:487–499. doi: 10.1007/s12035-014-8729-2. [DOI] [PubMed] [Google Scholar]
- 138.Schwartz M, Kipnis J. A common vaccine for fighting neurodegenerative disorders: recharging immunity for homeostasis. Trends Pharmacol Sci. 2004;25:407–412. doi: 10.1016/j.tips.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 139.Schwartz M, Ziv Y. Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends Immunol. 2008;29:211–219. doi: 10.1016/j.it.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 141.Dufek M, Rektorova I, Thon V, et al. Interleukin-6 may contribute to mortality in Parkinson’s disease patients: a 4-year prospective study. Park Dis. 2015;2015:898192. doi: 10.1155/2015/898192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carta AR, Frau L, Pisanu A, et al. Rosiglitazone decreases peroxisome proliferator receptor-γ levels in microglia and inhibits TNF-α production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience. 2011;194:250–261. doi: 10.1016/j.neuroscience.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 143.Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci. 2017;9:176. doi: 10.3389/fnagi.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McCoy MK, Ruhn KA, Martinez TN, et al. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther J Am Soc Gene Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dehmer T, Heneka MT, Sastre M, et al. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 146.Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J Off Publ Fed Am Soc Exp Biol. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 147.Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 148.Mohsenzadegan M, Fayazi MR, Abdolmaleki M, et al. Direct immunomodulatory influence of IFN-β on human astrocytoma cells. Immunopharmacol Immunotoxicol. 2015;37:214–219. doi: 10.3109/08923973.2015.1014559. [DOI] [PubMed] [Google Scholar]
- 149.Lecca D, Janda E, Mulas G, et al. Boosting phagocytosis and anti-inflammatory phenotype in microglia mediates neuroprotection by PPARγ agonist MDG548 in Parkinson’s disease models. Br J Pharmacol. 2018;175:3298–3314. doi: 10.1111/bph.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang W, Phillips K, Wielgus AR, et al. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotox Res. 2011;19:63–72. doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jeon H, Kim J-H, Kim J-H, et al. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J Neuroinflammation. 2012;9:149. doi: 10.1186/1742-2094-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jo M, Kim J-H, Song GJ, et al. Astrocytic orosomucoid-2 modulates microglial activation and neuroinflammation. J Neurosci Off J Soc Neurosci. 2017;37:2878–2894. doi: 10.1523/JNEUROSCI.2534-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hoshi A, Tsunoda A, Tada M, et al. Expression of aquaporin 1 and aquaporin 4 in the temporal neocortex of patients with Parkinson’s disease. Brain Pathol. 2017;27:160–168. doi: 10.1111/bpa.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun H, Liang R, Yang B, et al. Aquaporin-4 mediates communication between astrocyte and microglia: implications of neuroinflammation in experimental Parkinson’s disease. Neuroscience. 2016;317:65–75. doi: 10.1016/j.neuroscience.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 156.Mohn TC, Koob AO. Adult Astrogenesis and the etiology of cortical neurodegeneration. J Exp Neurosci. 2015;9:25–34. doi: 10.4137/JEN.S25520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2015;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol (Berl) 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yun SP, Kam T-I, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kuter K, Olech Ł, Głowacka U. Prolonged dysfunction of astrocytes and activation of microglia accelerate degeneration of dopaminergic neurons in the rat substantia nigra and block compensation of early motor dysfunction induced by 6-OHDA. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:551. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bhattacharjee N, Borah A. Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson’s disease. Neurochem Int. 2016;101:48–55. doi: 10.1016/j.neuint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 164.Chen P-C, Vargas MR, Pani AK, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci Off J Soc Neurosci. 2012;32:17775–17787. doi: 10.1523/JNEUROSCI.3049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liddell JR. Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antioxid Basel Switz. 2017;6:65. doi: 10.3390/antiox6030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mallajosyula JK, Kaur D, Chinta SJ, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS ONE. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Finberg JPM, Rabey JM. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front Pharmacol. 2016;7:340. doi: 10.3389/fphar.2016.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Graves SM, Xie Z, Stout KA, et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat Neurosci. 2020;23:15–20. doi: 10.1038/s41593-019-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guo X, Jiang Q, Tuccitto A, et al. The AMPK-PGC-1α signaling axis regulates the astrocyte glutathione system to protect against oxidative and metabolic injury. Neurobiol Dis. 2018;113:59–69. doi: 10.1016/j.nbd.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 172.Tong J, Rathitharan G, Meyer JH, et al. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain. 2017;140:2460–2474. doi: 10.1093/brain/awx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chan HH, Tse MK, Kumar S, Zhuo L. A novel selective MAO-B inhibitor with neuroprotective and anti-Parkinsonian properties. Eur J Pharmacol. 2018;818:254–262. doi: 10.1016/j.ejphar.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 174.Qian L, Tan KS, Wei S-J, et al. (2007) Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol Baltim Md. 1950;179:1198–1209. doi: 10.4049/jimmunol.179.2.1198. [DOI] [PubMed] [Google Scholar]
- 175.Gao H-M, Liu B, Zhang W, Hong J-S. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J Off Publ Fed Am Soc Exp Biol. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 176.Koyano F, Matsuda N. Molecular mechanisms underlying PINK1 and Parkin catalyzed ubiquitylation of substrates on damaged mitochondria. Biochim Biophys Acta BBA Mol Cell Res. 2015;1853:2791–2796. doi: 10.1016/j.bbamcr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 177.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 178.Lücking CB, Dürr A, Bonifati V, et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 179.Booth HDE, Hirst WD, Wade-Martins R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017;40:358–370. doi: 10.1016/j.tins.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Solano RM, Casarejos MJ, Menéndez-Cuervo J, et al. Glial dysfunction in parkin null mice: effects of aging. J Neurosci Off J Soc Neurosci. 2008;28:598–611. doi: 10.1523/JNEUROSCI.4609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Solano RM, Menéndez J, Casarejos MJ, et al. Midbrain neuronal cultures from parkin mutant mice are resistant to nitric oxide-induced toxicity. Neuropharmacology. 2006;51:327–340. doi: 10.1016/j.neuropharm.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 182.Giguere N, Pacelli C, Saumure C, et al. Comparative analysis of Parkinson’s disease-associated genes reveals altered survival and bioenergetics of parkin-deficient dopamine neurons in mice. J Biol Chem. 2018;293(25):9580–9593. doi: 10.1074/jbc.RA117.000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dionísio PEA, Oliveira SR, Amaral JSJD, Rodrigues CMP. Loss of microglial parkin inhibits necroptosis and contributes to neuroinflammation. Mol Neurobiol. 2019;56:2990–3004. doi: 10.1007/s12035-018-1264-9. [DOI] [PubMed] [Google Scholar]
- 184.Tran TA, Nguyen AD, Chang J, et al. Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B. PLoS ONE. 2011;6:e23660. doi: 10.1371/journal.pone.0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shendelman S, Jonason A, Martinat C, et al. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gorshkov K, Aguisanda F, Thorne N, Zheng W. Astrocytes as targets for drug discovery. Drug Discov Today. 2018 doi: 10.1016/j.drudis.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mullett SJ, Hinkle DA. DJ-1 deficiency in astrocytes selectively enhances mitochondrial complex I inhibitor-induced neurotoxicity. J Neurochem. 2011;117:375–387. doi: 10.1111/j.1471-4159.2011.07175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mullett SJ, Di Maio R, Greenamyre JT, Hinkle DA. DJ-1 expression modulates astrocyte-mediated protection against neuronal oxidative stress. J Mol Neurosci MN. 2013;49:507–511. doi: 10.1007/s12031-012-9904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lev N, Barhum Y, Ben-Zur T, et al. Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci MN. 2013;50:542–550. doi: 10.1007/s12031-013-9984-9. [DOI] [PubMed] [Google Scholar]
- 190.Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim J, Choi D, Jeong H, et al. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: a novel anti-inflammatory function of DJ-1. Neurobiol Dis. 2013;60:1–10. doi: 10.1016/j.nbd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 192.Kim J-M, Cha S-H, Choi YR, et al. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci Rep. 2016;6:28823. doi: 10.1038/srep28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim KS, Kim JS, Park J-Y, et al. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet. 2013;22:4805–4817. doi: 10.1093/hmg/ddt332. [DOI] [PubMed] [Google Scholar]
- 194.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 195.Fabelo N, Martín V, Santpere G, et al. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med. 2011;17:1107–1118. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fallon L, Moreau F, Croft BG, et al. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem. 2002;277:486–491. doi: 10.1074/jbc.M109806200. [DOI] [PubMed] [Google Scholar]
- 197.Silvestri L, Caputo V, Bellacchio E, et al. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 198.Fortin DL, Troyer MD, Nakamura K, et al. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci Off J Soc Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Frøyset AK, Edson AJ, Gharbi N, et al. Astroglial DJ-1 over-expression up-regulates proteins involved in redox regulation and is neuroprotective in vivo. Redox Biol. 2018;16:237–247. doi: 10.1016/j.redox.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 201.Simón-Sánchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Moehle MS, Webber PJ, Tse T, et al. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schapansky J, Nardozzi JD, Felizia F, LaVoie MJ. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum Mol Genet. 2014;23:4201–4214. doi: 10.1093/hmg/ddu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Games D, Seubert P, Rockenstein E, et al. Axonopathy in an α-synuclein transgenic model of Lewy body disease is associated with extensive accumulation of C-terminal—truncated α-synuclein. Am J Pathol. 2013;182:940–953. doi: 10.1016/j.ajpath.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Russo I, Kaganovich A, Ding J, et al. Transcriptome analysis of LRRK2 knock-out microglia cells reveals alterations of inflammatory- and oxidative stress-related pathways upon treatment with α-synuclein fibrils. Neurobiol Dis. 2019;129:67–78. doi: 10.1016/j.nbd.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Henry AG, Aghamohammadzadeh S, Samaroo H, et al. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum Mol Genet. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- 207.Chen X, Liu Z, Cao B-B, et al. TGF-β1 Neuroprotection via inhibition of microglial activation in a rat model of Parkinson’s disease. J Neuroimmune Pharmacol. 2017;12:433–446. doi: 10.1007/s11481-017-9732-y. [DOI] [PubMed] [Google Scholar]
- 208.Oh SH, Kim HN, Park HJ, et al. The cleavage effect of mesenchymal stem cell and its derived matrix metalloproteinase-2 on extracellular α-synuclein aggregates in Parkinsonian models. Stem Cells Transl Med. 2017;6:949–961. doi: 10.5966/sctm.2016-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Booth HDE, Wessely F, Connor-Robson N, et al. RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson’s iPSC-derived astrocytes. Neurobiol Dis. 2019;129:56–66. doi: 10.1016/j.nbd.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 210.Spillantini MG, Crowther RA, Jakes R, et al. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Spillantini MG, Schmidt ML, Lee VM, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 212.Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol (Berl) 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 213.Cavaliere F, Cerf L, Dehay B, et al. In vitro α-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol Dis. 2017;103:101–112. doi: 10.1016/j.nbd.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 214.Reyes JF, Olsson TT, Lamberts JT, et al. A cell culture model for monitoring α-synuclein cell-to-cell transfer. Neurobiol Dis. 2015;77:266–275. doi: 10.1016/j.nbd.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 215.Lindström V, Gustafsson G, Sanders LH, et al. Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol Cell Neurosci. 2017;82:143–156. doi: 10.1016/j.mcn.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 216.Chavarría C, Rodríguez-Bottero S, Quijano C, et al. Impact of monomeric, oligomeric and fibrillar alpha-synuclein on astrocyte reactivity and toxicity to neurons. Biochem J. 2018;475:3153–3169. doi: 10.1042/BCJ20180297. [DOI] [PubMed] [Google Scholar]
- 217.Lee H-J, Suk J-E, Patrick C, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7:a020628. doi: 10.1101/cshperspect.a020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim C, Lee H-J, Masliah E, Lee S-J. Non-cell-autonomous neurotoxicity of α-synuclein through microglial toll-like receptor 2. Exp Neurobiol. 2016;25:113–119. doi: 10.5607/en.2016.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Q-S, Heng Y, Yuan Y-H, Chen N-H. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol Lett. 2017;265:30–37. doi: 10.1016/j.toxlet.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 221.Paxinou E, Chen Q, Weisse M, et al. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci Off J Soc Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tapias V, Hu X, Luk KC, et al. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via inos-mediated nitric oxide production. Cell Mol Life Sci CMLS. 2017;74:2851–2874. doi: 10.1007/s00018-017-2541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Olsen AL, Feany MB. Glial α-synuclein promotes neurodegeneration characterized by a distinct transcriptional program in vivo. Glia. 2019;67:1933–1957. doi: 10.1002/glia.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain J Neurol. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lang C, Campbell KR, Ryan BJ, et al. Single-cell sequencing of iPSC-dopamine neurons reconstructs disease progression and identifies HDAC4 as a regulator of Parkinson cell phenotypes. Cell Stem Cell. 2019;24:93–106.e6. doi: 10.1016/j.stem.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chao DHM, Kallemeijn WW, Marques ARA, et al. Visualization of active glucocerebrosidase in rodent brain with high spatial resolution following in situ labeling with fluorescent activity based probes. PLoS ONE. 2015;10:e0138107. doi: 10.1371/journal.pone.0138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sanyal A, DeAndrade MP, Novis HS, et al. Lysosome and inflammatory defects in GBA1-mutant astrocytes are normalized by LRRK2 inhibition. Mov Disord Off J Mov Disord Soc. 2020 doi: 10.1002/mds.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Osellame LD, Duchen MR. Defective quality control mechanisms and accumulation of damaged mitochondria link Gaucher and Parkinson diseases. Autophagy. 2013;9:1633–1635. doi: 10.4161/auto.25878. [DOI] [PubMed] [Google Scholar]
- 229.Li X, Tao Y, Bradley R, et al. Fast generation of functional subtype astrocytes from human pluripotent stem cells. Stem Cell Rep. 2018;11:998–1008. doi: 10.1016/j.stemcr.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Santos R, Vadodaria KC, Jaeger BN, et al. Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Rep. 2017;8:1757–1769. doi: 10.1016/j.stemcr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L. Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis. 2017;8:e2696. doi: 10.1038/cddis.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Krencik R, Zhang S-C. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.di Domenico A, Carola G, Calatayud C, et al. Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson’s disease. Stem Cell Rep. 2019;12:213–229. doi: 10.1016/j.stemcr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gupta K, Patani R, Baxter P, et al. Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell Death Differ. 2012;19:779–787. doi: 10.1038/cdd.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Thorne N, Malik N, Shah S, et al. High-throughput phenotypic screening of human astrocytes to identify compounds that protect against oxidative stress. Stem Cells Transl Med. 2016;5:613–627. doi: 10.5966/sctm.2015-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Harms AS, Barnum CJ, Ruhn KA, et al. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson’s disease. Mol Ther J Am Soc Gene Ther. 2011;19:46–52. doi: 10.1038/mt.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Joniec-Maciejak I, Ciesielska A, Wawer A, et al. The influence of AAV2-mediated gene transfer of human IL-10 on neurodegeneration and immune response in a murine model of Parkinson’s disease. Pharmacol Rep PR. 2014;66:660–669. doi: 10.1016/j.pharep.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 238.Schwenkgrub J, Joniec-Maciejak I, Sznejder-Pachołek A, et al. Effect of human interleukin-10 on the expression of nitric oxide synthases in the MPTP-based model of Parkinson’s disease. Pharmacol Rep PR. 2013;65:44–49. doi: 10.1016/S1734-1140(13)70962-9. [DOI] [PubMed] [Google Scholar]
- 239.Garbes L, Riessland M, Wirth B. Histone acetylation as a potential therapeutic target in motor neuron degenerative diseases. Curr Pharm Des. 2013;19:5093–5104. doi: 10.2174/13816128113199990356. [DOI] [PubMed] [Google Scholar]
- 240.Tan Y, Delvaux E, Nolz J, et al. Upregulation of histone deacetylase 2 in laser capture nigral microglia in Parkinson’s disease. Neurobiol Aging. 2018;68:134–141. doi: 10.1016/j.neurobiolaging.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 241.Faraco G, Pittelli M, Cavone L, et al. Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis. 2009;36:269–279. doi: 10.1016/j.nbd.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 242.Cassano T, Calcagnini S, Pace L, et al. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Price DA, Martinez AA, Seillier A, et al. WIN55,212–2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the MPTP mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:2177–2186. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.White RE, Barry DS. The emerging roles of transplanted radial glial cells in regenerating the central nervous system. Neural Regen Res. 2015;10:1548–1551. doi: 10.4103/1673-5374.165317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jha MK, Seo M, Kim J-H, et al. The secretome signature of reactive glial cells and its pathological implications. Biochim Biophys Acta. 2013;1834:2418–2428. doi: 10.1016/j.bbapap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 246.Chang M-Y, Son H, Lee Y-S, Lee S-H. Neurons and astrocytes secrete factors that cause stem cells to differentiate into neurons and astrocytes, respectively. Mol Cell Neurosci. 2003;23:414–426. doi: 10.1016/S1044-7431(03)00068-X. [DOI] [PubMed] [Google Scholar]
- 247.Choi SS, Lee HJ, Lim I, et al. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS ONE. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Suk K. Combined analysis of the glia secretome and the CSF proteome: neuroinflammation and novel biomarkers. Expert Rev Proteomics. 2010;7:263–274. doi: 10.1586/epr.10.6. [DOI] [PubMed] [Google Scholar]
- 249.Jeon H, Lee S, Lee W-H, Suk K. Analysis of glial secretome: the long pentraxin PTX3 modulates phagocytic activity of microglia. J Neuroimmunol. 2010;229:63–72. doi: 10.1016/j.jneuroim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 250.Karpinar DP, Balija MBG, Kügler S, et al. Pre-fibrillar α-synuclein variants with impaired β-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dehay B, Bezard E. Intrastriatal injection of alpha-synuclein fibrils induces Parkinson-like pathology in macaques. Brain. 2019;142:3321–3322. doi: 10.1093/brain/awz329. [DOI] [PubMed] [Google Scholar]
- 252.O’Donovan SM, Crowley EK, Brown JR-M, et al. Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2020;32:e13726. doi: 10.1111/nmo.13726. [DOI] [PubMed] [Google Scholar]