Abstract
Sebaceous glands (SG) are exocrine glands that release their product by holocrine secretion, meaning that the whole cell becomes a secretion following disruption of the membrane. SG may be found in association with a hair follicle, forming the pilosebaceous unit, or as modified SG at different body sites such as the eyelids (Meibomian glands) or the preputial glands. Depending on their location, SG fulfill a number of functions, including protection of the skin and fur, thermoregulation, formation of the tear lipid film, and pheromone-based communication. Accordingly, SG abnormalities are associated with several diseases such as acne, cicatricial alopecia, and dry eye disease. An increasing number of genetically modified laboratory mouse lines develop SG abnormalities, and their study may provide important clues regarding the molecular pathways regulating SG development, physiology, and pathology. Here, we summarize in tabulated form the available mouse lines with SG abnormalities and, focusing on selected examples, discuss the insights they provide into SG biology and pathology. We hope this survey will become a helpful information source for researchers with a primary interest in SG but also as for researchers from unrelated fields that are unexpectedly confronted with a SG phenotype in newly generated mouse lines.
Keywords: Sebaceous gland, Skin, Mouse models
Introduction
As in many other fields of biomedical research, genetically modified laboratory mice became the mainstay of experimental dermatological research [1]. Such mouse lines are employed to better understand skin development, structure and function, to identify the molecular basis of a disease, to study its pathophysiology, and in some cases even to assess a potential therapeutic approach. Many of the engineered (=transgenic, knockin, knockout, and their several derivatives), but also a number of spontaneous, radiation-induced and chemical mutagenesis-induced mutants exhibit various types and degrees of abnormal cutaneous phenotypes. Such mutant lines have been once carefully compiled in a highly influencing but nowadays not fully up-to-date textbook [2]. More recently, a number of review articles focused on mouse lines with abnormalities in hair follicle morphogenesis, cycling, and/or structure [3, 4] or pigmentation [5]. Because mutant mice provide important clues about the function of gene products, such surveys have proved highly useful to researches with different interests, ranging from skin genetics aficionados to investigators from unrelated fields that are confronted with an unexpected skin phenotype in a newly generated or identified mouse line.
The last years witnessed an increased interest in the sebaceous glands (SG) [6–9]. These tiny exocrine glands, most commonly found in the dermis in association with a hair follicle (Fig. 1a), secrete an oily substance with manifold established or putative functions (see below). Recent advances in SG research include the identification of different stem cell pools regulating SG development and homeostasis [10, 11] novel insights into pathways regulating sebaceous lipogenesis [12–15] and a broadening of sebum’s functional repertoire [16]. Here, after a brief introduction to SG physiology and pathology, we summarize in tabulated form the available mouse lines with SG abnormalities and, by concentrating on selected examples, discuss the insights they provide into SG biology. Importantly, in addition to providing insights into the role of the targeted gene/protein in sebocyte development or sebaceous lipogenesis, these mouse lines may be suitable for further applications, including pre-clinical studies assessing the effect of novel compounds in decreasing or increasing SG activity.
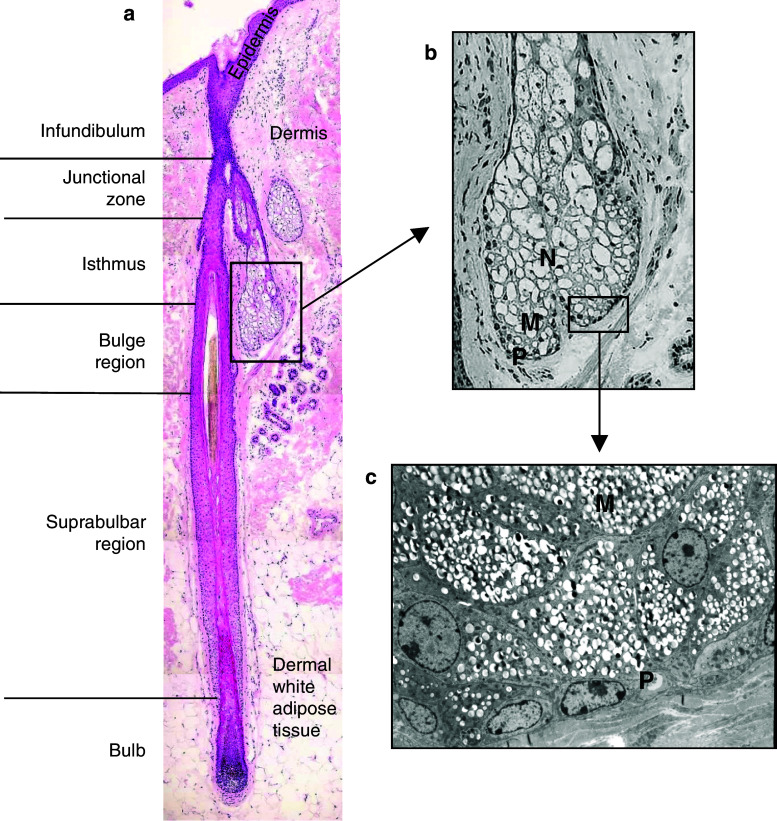
Fig. 1.
Morphology of the pilosebaceous unit and fine structure of the sebaceous gland. a H&E-stained human scalp hair follicle in sagittal section showing the different follicular compartments. b High magnification image of the sebaceous gland. The peripheral (P), maturation (M), and necrosis (N) zones are indicated. c Transmission electron micrograph showing flat, undifferentiated sebocytes in the proliferative (P) zone and cells undergoing sebaceous differentiation and bearing numerous lipid droplets (white spots) in the cytoplasm in the maturation (M) zone
Reproduced with permission from [18]
Morphological and functional diversity of sebaceous glands
SGs are exocrine glands displaying holocrine secretion, meaning that the whole cell forms a secretory product upon disruption of the membrane [8, 17]. Sebocytes, the foremost cell type within SGs, can be distinguished at different stages of differentiation within the same acinus. Sebocytes in the peripheral zone are flattened and mitotically active (Fig. 1b, c). As sebaceous differentiation takes place, these cells accumulate large numbers of cytoplasmic lipid droplets at the expense of other cell structures [18] and are gradually dislodged towards the center of the gland, forming the maturation zone (Fig. 1b, c). Cell disruption and release of lipids and cellular debris eventually take place at the center of the gland, in the necrosis zone (Fig. 1b). Before reaching the skin surface via the infundibulum [19] the SG product passes a glandular excretory duct composed of stratified squamous epithelium. Sebum’s classical function is the formation of a protective film that waterproofs and lubricates the skin and the hair shafts. However, several other functions have been proposed for sebum, including antimicrobial and antioxidative properties [8, 9]. Native (=freshly released) human sebum contains squalene, cholesterol, wax esters, and triglycerides [20]. Triglycerides are partially hydrolyzed as sebum passes the hair canal, making superficial sebum to contain free fatty acids as well as lower amounts of mono- and diglycerides. Notably, sebum composition is remarkably species and age-specific.
Sebocytes derive from leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1)-positive cells during the morphogenesis of the pilosebaceous unit [21]. The transmembrane protein LRIG1, an inhibitor of the EGFR/ERBB receptor family, also marks putative SG stem cells at the isthmus of the HF, which renew the SG and the infundibular epithelium [10, 11]. Numerous additional transcription factors and signaling proteins control SG development, growth, and homeostasis, including MYC, BLIMP1, and Indian hedgehog [8, 9]. In adults, sebum production is strongly influenced by steroid and peptide hormones, growth factors, and neuroendocrine regulators [8, 9].
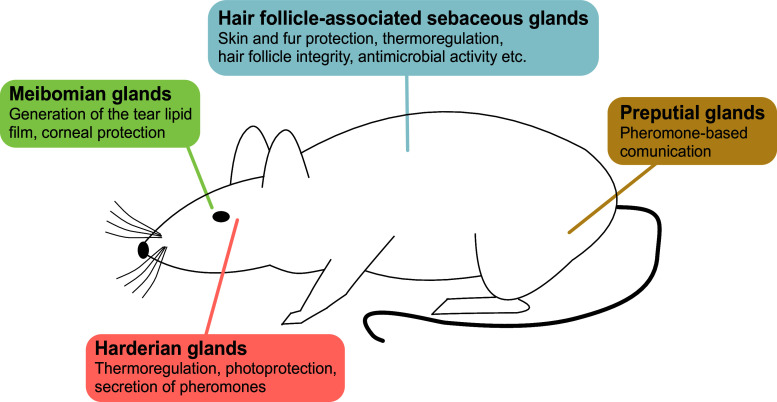
In addition to the hair follicle-associated SG, modified and enlarged SGs (often termed “free” or “ectopic” glands) are found at distinct non-hairy sites such as the nipples, around the genitals, in the oral epithelium, or in the eyelids (Fig. 2). SGs in the latter location are termed Meibomian glands; they secrete a complex mixture of lipids (meibum) that upon delivery to the eye surface form the tear film lipid layer [22]. Another ectopic SG is the preputial gland. This paired gland is located between the skin and the abdominal muscles of male rodents, close to the genital bulb [23–25]. The preputial gland produces a mixture of lipids containing pheromones that have a role in territorial marking and in attracting females [23, 26]. The Harderian gland is located behind the eyeball [27] and is found in all groups of terrestrial vertebrates [28]. The pigment and the lipids with porphyrins produced by this gland reach the surface of the nictitating membrane by a duct and protect the cornea [28]. They are important for the grooming of the fur [27] and seem to facilitate the movement of the third eyelid [29].
Fig. 2.
Major types of sebaceous glands and their localization in mice. See the text for details and references
The SG is also involved in the pathogenesis of diverse diseases. Meibomian gland dysfunction, for instance, frequently characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion, may result in alteration of the tear film and eye irritation or inflammation [30]. More commonly known is the key role of excessive sebum production in the pathogenesis of acne vulgaris, the most frequent cutaneous disorder during adolescence [31, 32]. Finally, SG degeneration is an early event in many types of cicatricial alopecia in humans and in some animal models for the disease [33, 34]. The asebia mouse, for instance, a well-characterized model for primary cicatricial alopecia and one if the earliest mouse mutant lines showing SG abnormalities (Table 1), develops SG atrophy due to a spontaneous mutation in the gene encoding the enzyme stearoyl coenzyme A desaturase 1 [35]. Consequently, normal desquamation of the hair follicle inner root sheath and hair shaft regression are prevented, resulting in inflammatory destruction of the hair follicle [36].
Table 1.
Laboratory mouse lines with abnormalities in the hair follicle-associated sebaceous glands
| Gene symbol | Mutant name (if applicable) | Type | Characteristics/abnormalities | References | ||
|---|---|---|---|---|---|---|
| Soluble factors | ||||||
| Amphiregulin (AREG) | AREG | tsTg | Enlarged SG producing large amounts of sebum | [51] | ||
| Ectodysplasin A1 | Eda | tsTg | Enlarged SG | [61] | ||
| iTg | Enlarged SG, increased number of sebocytes, excessive sebum production | [62] | ||||
| Epigen (EPGN) | EPGN | itsTg | Enlarged SG, increase in the number of cells per gland, increased sebum production; SG hyperplasia is dependent on continuous epigen supply |
[53] [52] |
||
| Fatty acid-binding protein, epidermal/fatty acid-binding protein 5 (FABP5) | Fabp5 | fKO | Reduced size of SG, reduction in the number of sebocytes, altered lipid composition | [63] | ||
| Neuregulin-3 (NRG-3) | Nrg3 | tsTg | Increased number and size of sebocytes, SG are mis-positioned and hyperplastic | [64] | ||
| Noggin | Nog | tsTg | Hypertrophic SG; pilosebaceous units at the expense of sweat glands in the footpads | [65] | ||
| Nog | tsTg | Ectopic and increased sebocyte differentiation | [66] | |||
| Transforming growth factor alpha (TGFA) | TGFA | Tg | SG hyperplasia | [50] | ||
| Receptors | ||||||
| Activin receptor type-1B (ACTR-IB) | Acvr1b | tsKO | Enlarged SG, increased numbers of SG in the skin | [67] | ||
| Fibroblast growth factor receptor 1 and 2 (FGFR 1 + 2) | Fgfr1 + Fgfr2 | tsKO | Loss of SG | [68] | ||
| Fibroblast growth factor receptor 2b (FGFR 2b) | Fgfr2 | tsKO | SG atrophy; from postnatal day 6 evident differences in the rate of SG growth between the knockout and control mice, by 3 months: virtual absence of SG | [69] | ||
| Glucocorticoid receptor (GR) | Nr3c1 | tsTg | Hypertrophic SG | [70] | ||
| Integrin alpha-V or integrin beta-1 | ITGAV or ITGB1 | tsTg | SG enlargement | [71] | ||
| Integrin beta-1 | Itgb1 | tsKO | At 7 weeks of age: no identifiable remnants of SG | [72] | ||
| Leucine-rich repeat-containing G protein-coupled receptor 5 | LGR5 | itsTg | LGR5 overexpression during embryogenesis: enlarged SG, increased degradation and accelerated maturation of sebocytes | [73] | ||
| Mutated Smoothened | Smo | itsTg | Ectopic sebocytes, increase in size and number of SG upon increased hedgehog signaling | [74] | ||
| Neurogenic locus notch homolog protein 1 (notch 1) | Notch1 | itsTg | Enlarged SG | [75] | ||
| Peroxisome proliferator-activated receptor gamma | Pparg | tsKO | Atrophy of SG, dystrophy of SG | [76] | ||
| Tumor necrosis factor receptor superfamily member EDAR/ectodysplasin-A receptor | Edar | tsTg | Enlarged SG | [77] | ||
| Vitamin D3 receptor (VDR) | Vdr | fKO | Enlarged SG | [78] | ||
| Transcription factors | ||||||
| Catenin beta-1/beta-catenin | Ctnnb1 | itsTg | Initial SG duplication, then inhibition of sebocyte differentiation and loss of SG | [79] | ||
| Delta N87betacat (beta- catenin) | Ctnnb1 | tsTg | Development of ectopic SG | [80] | ||
| CCAAT/enhancer-binding protein alpha and beta (c/EBP alpha and c/EBP beta) | Cebpa/Cebpb | itsKO | Blocked sebocyte differentiation, lack of sebum production, unusual looking sebocytes | [81] | ||
| Homeobox protein BarH-like 2 | Barx2 | fKO | Enlarged SG | [82] | ||
| Homeobox protein DLX-3 | Dlx3 | tsKO | Enlarged SG | [83] | ||
| Krüppel-like factor 4 | KLF4 | itsTg | Atrophy of SG at 9 days after Doxycyclin treatment | [46] | ||
| Delta lymphoid enhancer-binding factor 1 (LEF-1) | Lef1 | itsTg | Enlarged and ectopic SG | [84] | ||
| Delta N lymphoid enhancer-binding factor 1 (LEF-1) | Lef1 | tsTg | Development of skin tumors with sebaceous differentiation | [85] | ||
| Lymphoid enhancer-binding factor 1 (LEF-1) | Lef1 | tsTg | Sebaceous tumors, tumors with differentiated sebocytes | [86] | ||
| Myc proto-oncogene protein | MYC | itsTg | Enlarged SG, increase in cell number | [48] | ||
| itsTg | Enlarged SG, increased number of sebocytes, stimulation of sebaceous differentiation at the expense of hair differentiation | [45] | ||||
| itsTg | Enlarged SG, increase in the number of differentiated sebocytes at the expense of hair differentiation | [87] | ||||
| tsTg | Enlarged SG | [88] | ||||
| itsTg | Enlarged and disorganized SG | [47] | ||||
| Myc | tsKO | Impaired SG secretion | [49] | |||
| PR domain zinc finger protein 1/B lymphocyte-induced maturation protein 1 (Blimp-1) | Prdm1 | itsKO | Increased SG size (in some animals) | [89] | ||
| itsKO | SG enlargement | [90] | ||||
| tsKO | Enlarged SG, sebum lipids: increase in cholesterol esters, triglycerides and cholesterol, increased sebum production | [91] | ||||
| Protein C-ets-1/p54 | Ets1 | iTg | In some cases: enlarged SG | [92] | ||
| Recombining binding protein suppressor of hairless/RBP-J kappa | Rbpj | tsKO | Impaired SG differentiation | [93] | ||
| Trans-acting T-cell-specific transcription factor GATA-3/GATA-binding factor 3 | Gata3 | tsKO | Enlarged SG from P7 onwards | [94] | ||
| Transcription factor A, mitochondrial (mtTFa) | Tfam | tsKO | Lack of SG | [95] | ||
| Transcription factor AP-2-alpha and -gamma (AP2-alpha and AP2-gamma) | Tfap2a and Tfap2c | tsKO | Defects in SG differentiation | [96] | ||
| Transcription factor E2-alpha/transcription factor 3 (TCF-3) | Tcf3 | itsTg | Impairment of SG development | [97] | ||
| Transcription factor SOX-21 | Sox21 | fKO | At P12: enlargement of SG | [98] | ||
| Transcription factor SOX-9 | Sox9 | tsKO | SG morphogenesis is blocked, absence of SG progenitor cells | [99] | ||
| Transcription factor Sp6/krueppel-like factor 14 | Sp6 | fKO | Increase in SG size | [100] | ||
| Tumor protein 63 (p63) | Trp63 | fKO | Absent SG | [101] | ||
| Delta NP63 | Trp63 | itsTg | Absence of SG (no morphogenesis) | [102] | ||
| Zinc finger protein GLI1 | GLI1 | tsTg | Differentiation into cells similar to sebaceous glands and epidermal cysts | [103] | ||
| Zinc finger protein GLI2 | Gli2 | tsTg | Prominent SG duct, additional pairs of highly branched SGs, elongated and enlarged ducts started at p25, at p45 a second pair of SG appears above the existing one; later: additional SG develop at infundibulum- epidermal junctions, ectopic SG | [104] | ||
| Gli2 | tsTg | Deficient/rare SG upon suppression of hedgehog signaling | [74] | |||
| Enzymes | ||||||
| 1-Phosphatidylinositol 4,5-bisphosphate phosphodiesterase delta-1/phospholipase C-delta-1 | Plcd1 | fKO | Hyperplasia of SG, increased number of sebocytes, skin tumors with characteristics of interfollicular epidermis and SG | [105] | ||
| Acyl-CoA desaturase 1/stearoyl -CoA desaturase 1 | Scd1 | Asebia | Spont | Absence of SG | [37] | |
| fKO | Degenerated SG | [106] | ||||
| tsKO | SG hypoplasia, depletion of sebaceous lipids, paucity of lipid- enriched sebocytes/lack of mature sebocytes; large reduction in sebaceous lipids: reduced wax diester and triglyceride content | [107] | ||||
| fKO | SG atrophy | [108] | ||||
| Asebia-2J | Spont | Hypoplasia of SG, skin lipids: reduction in sterol esters and cholesterol, loss of diol esters | [36] | |||
| Flake | ENU | Reduced production of sebum, impaired clearance of skin infections | [109] | |||
| Bis (5′-adenosyl)-triphosphatase | Fhit | fKO | Sebaceous tumors (Fhit±) | [110] | ||
| Cathepsin L1 | Ctsl | nackt | Spont | SG Hyperplasia | [111, 112] | |
| Ceramide synthase 4 (CerS4) | Cers4 | fKO | Enlarged SG with multiple lobules | [113] | ||
| fKO | Altered lipid composition of SG, enlarged SG | [114] | ||||
| Cystathionine beta-synthase | Cbs | fKO | Hyperplastic SG | [115] | ||
| Diacylglycerol O-acyltransferase 1 | Dgat1 | fKO | Atrophy of SG (differences in fur lipid content in older mice) | [116] | ||
| DNA (cytosine-5)-methyltransferase 1 (Dnmt1) | Dnmt1 | tsKO | Hyperplastic SG | [117] | ||
| Elongation of very long-chain fatty acids protein 3 | Elovl3 | fKO | Hyperplasia of SG, imbalance in the sebum lipid content (increase in the hydrophobic components) | [118] | ||
| Exostosin-1 | Ext1 | itsKO | Hyperplasia of SG, increased sebum production from p55, 4- fold increase in SG number (induced from p20 to p55), hyperplastic SG with altered morphology presenting irregular shapes and thickening of the SG canal | [119] | ||
| Fatty acid 2-hydroxylase | Fa2h | fKO | Hyperproliferation of sebocytes, enlarged SG, dilated hair canals are filled with sebum, altered sebum composition (reduced amount of wax diesters, increased amount of wax monoesters, free fatty acids and cholesterol) | [120] | ||
| Focal adhesion kinase (FADK) | Ptk2 | tsKO | SG hypoplasia | [121] | ||
| Gamma secretase | Psen1/Psen2 | tsKO | Failure to form SG | [122] | ||
| Group 2 secretory phospholipase A2 | PLA2G2A | tsTg | SG hyperplasia | [123] | ||
| Group 3 secretory phospholipase A2 | PLA2G3 | Tg | SG hyperplasia in mice older than 9 months of age | [124] | ||
| GTPase HRas (H-RasG12V) | Hras | KI | Lip skin: more SG than in control mice | [125] | ||
| GTPase KRas (kRas G12d) | Kras | KI | Hyperplasia of SG | [126] | ||
| GTPase KRas (kRas G12D) | Kras | KI | Enlargement of SG, sebaceous cysts, dysplasia of SG | [127] | ||
| Histone deacetylases 1 and 2 (HD1 and 2) | Hdac1 and Hdac2 | tsKO | SG hyperplasia | [128] | ||
| Histone deacetylases 1 (HD1) | Hdac1 | tsKO | SG hyperplasia | [128] | ||
| Lysine-specific demethylase hairless | Hr | tsTg | Delayed SG differentiation | [129] | ||
| N-lysine methyltransferase KMT5A (SETD8) | Kmt5a | itsKO | Loss of SG in adult skin | [130] | ||
| Ornithine decarboxylase (ODC) | Odc1 | tsTg | Moderate SG hyperplasia | [131] | ||
| Odc1 | tsTg | At p 12: Moderate sebaceous cell hyperplasia | [132] | |||
| Palmitoyltransferase ZDHHC13 | Zdhhc13 | Spont | SG hyperplasia | [133] | ||
| Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN | Pten | tsKO | Enlarged SG, sebaceous carcinomas | [134] | ||
| Phospholipase A2, membrane associated/enhancing factor (EF) | Pla2g2a | tsTg | Enlarged SG (F2, homozygous) | [135] | ||
| Probable palmitoyltransferase ZDHHC21 | Zdhhc21 | Depilated | Spont | SG hyperplasia with an excess of sebum | [136] | |
| Prostaglandin G/H synthase 2/cyclooxygenase 2 (COX-2) | Ptgs2 | tsTg | Enlargement of SG | [42] | ||
| Ptgs2 | tsTg | SG hyperplasia, increased epicutaneous sebum concentration, enlarged gland duct | [40] | |||
| Ptgs2 | tsTg | SG hyperplasia | [41] | |||
| Protein kinase C lambda | Prkci | tsKO | Increased number of differentiated SG cells, enhanced SG differentiation, enlarged SG | [137] | ||
| Ras-related C3 botulinum toxin substrate 1 | Rac1 | tsKO | Lack of SG | [138] | ||
| tsKO | Enlarged SG | [139] | ||||
| itsKO | 7 to 9 days after treatment: enlarged and disorganized SG; early increase in terminally differentiated sebocytes, followed by progressive sebocyte loss | [140] | ||||
| Receptor tyrosine-protein kinase erbB-2 | Erbb2 | tsTg | Enlargement of SG | [141] | ||
| Serine palmitoyltransferase 2 | Sptlc2 | iKO | SG atrophy | [142] | ||
| Serine/threonine-protein kinase ATR | Atr | iKO | SG cell hypertrophy | [143] | ||
| Serine/threonine-protein kinase B-raf (B-RafV600E) | Braf | KI | Reduced numbers and size of SG | [144] | ||
| Tripeptidyl-peptidase 1 (TPP-1) | Tpp1 | tsKO | Lack of SG | [145] | ||
| Tumor necrosis factor alpha-induced protein 3 (TNF alpha-induced protein 3) | Tnfaip3 | tsKO | Hyperplasia of SG and sebocytes | [146] | ||
| Tyrosine-protein kinase Fyn +Focal adhesion kinase (Fyn−/− + FAK+/−) | Fyn + Ptk2 | fKO | Increased number and size of SG | [147] | ||
| Others | ||||||
| 14-3-3 protein sigma/stratifin | Sfn | Repeated epilation (Er) | Spont | Sfn+/ER (heterozygous dominant- negativ): hyperproliferative SG/enlarged SG | [148] | |
| Acyl-CoA-binding protein (ACBP) | Dbi | Nm1054 | Spont | Sebocyte hyperplasia, increased number of sebocytes, sebaceous lipids with reduced levels of triacylglycerols | [149] | |
| Apolipoprotein C-I (Apo-CI) | APOC1 | Tg | Atrophy of SG, lack of sebum | [150] | ||
| CD109 antigen | Cd109 | fKO | Hyperplasia of SG, accumulation of sebum | [151] | ||
| Cell death activator CIDE-A | Cidea | fKO | Sebocytes accumulate smaller lipid droplets, reduced sebum lipid production | [152] | ||
| Corneodesmosin | Cdsn | itsKO | Hypertrophic SG | [153] | ||
| Disintegrin and metalloproteinase domain-containing protein 10 (ADAM 10) | Adam10 | tsKO | Absence of SG, reduced lipid production | [154] | ||
| itsKO | Deletion from P21 on: no significant loss of sebocytes, decreased lipid production | [154] | ||||
| Gap junction beta-2 protein (Cx26-G45E) | GJB2 | itsTg | Atrophy of SG in animals maintained on doxycycline for 10 weeks | [155] | ||
| Gasdermin-A3 | Gsdma3 | Rim3 | Spont | Abnormal SG differentiation | [156, 157] | |
| Defolliculated (Dfl) | Spont | Sebocytes produce little or no sebum, abnormal differentiation of SG | [158] | |||
| Defolliculated (Dfl) | Spont | Abnormal differentiation of SG, reduced sebum production | [159] | |||
| Finnegan (Fgn) | ENU | Abnormal SG differentiation | [160] | |||
| Reduced coat 2 (RCo2) | ENU | Absent SG | [161] | |||
| Bare skin (Bsk) | ENU | Absent SG | [161] | |||
| Rex denuded (Re den) | ENU | Absent SG | [161] | |||
| ENU | Absence of SG | [162] | ||||
| Golgi pH regulator | Gpr89 | tsKO | Enlargement of SG at 1 month after birth | [163] | ||
| Insulin-induced gene 1 and 2 protein (INSIG-1 and 2) | Insig1 and Insig2 | tsKO | Enlarged SG | [164] | ||
| Keratin, type I cytoskeletal 10/keratin-10 (K10) | Krt10 | fKO | SG started to enlarge at the age of four weeks due to a stronger turnover of sebocytes, increased sebum production | [165] | ||
| Keratin, type I cytoskeletal 25 | Krt25 | Rex | Spont | Enlargement of SG | [166] | |
| ENU | M100573, enlargement of SG | [166] | ||||
| Keratin, type II cytoskeletal 71 | Krt71 | Caracul Rinshoken | Spont | Enlarged SG | [167] | |
| Long-chain fatty acid transport protein 4/Fatty acid transport protein 4 (FATP4) | Slc27a4 | Wrinkle-free | Spont | Dystrophic SG, sebum: reduced level of type II diester wax | [168] | |
| Mothers against decapentaplegic homolog 4/SMAD family member 4 (SMAD 4) | Smad4 | tsKO | Enlarged SG, increased sebocyte differentiation | [169] | ||
| tsKO | Enlarged SG | [170] | ||||
| tsKO | Enlarged SG, sebaceous adenoma | [171] | ||||
| tsKO | One squamous papilloma accompanied by sebaceous hyperplasia | [172] | ||||
| Mothers against decapentaplegic homolog 7 | Smad7 | itsTg | Accelerated SG morphogenesis, rapid growth of SG, hypertrophic enlarged SG, premature SG development | [173] | ||
| Mothers against decapentaplegic homolog 7 and E3 ubiquitin-protein ligase SMURF2 | Smad7 and SMURF2 | itsTg | Hypertrophic SG (more than in Smad7 alone) | [173] | ||
| Myelin protein zero-like protein 3 (predicted) | Mpzl3 | Rough Coat (rc) | spont | SG hypertrophy, sebocyte hyperplasia | [174] | |
| Nuclear receptor coactivator 1 | Ncoa1 | fKO | Heterozygous: enlarged SG | [175] | ||
| Perilipin-2 | Plin2 | fKO | Reduced size of SG, glands contain fewer cells, reduced proliferation | [12] | ||
| Prelamin-A/C (LMNA C1824T) | LMNA | itsTg | Disorganized SG, alterations of SG | [176] | ||
| LMNA | itsTg | Initial hyperplasia is followed by hypoplasia of SG | [177] | |||
| Lmna | Disheveled hair and ear (Dhe) | Spont | Hypoplastic SG | [178] | ||
| KI | Reduced numbers of SG | [179] | ||||
| LMNA | itsTg | Displaced and hyperplastic SG, enlarged and abnormal differentiation | [180] | |||
| Protein Mpv17 (Mpv-17) | Mpv17 | fKO | 2-year-old mice: reduction in number and size of SG | [181] | ||
| Retinoblastoma-like protein 1 (p107) and retinoblastoma- like protein 2 (p130) | Rbl1 and Rbl2 | fKO | Hyperplastic SG | [182] | ||
| RING finger LIM domain-binding protein | Rlim | tsTg | Enlarged SG | [183] | ||
| Sonic hedgehog protein (SHH) | Shh | itsTg | Enlarged SG in the Tabby backround | [184] | ||
| fKO | Failure to produce SG | [185] | ||||
| SV40 large T antigen (SV40T) | SV40 Tag | tsTg | Enlarged SG | [186] | ||
| Telomeric repeat-binding factor 1 | Terf1 | tsKO | Absence of SG | [187] | ||
| TNF receptor-associated factor 6 | Traf6 | fKO | Impairment of SG | [188] | ||
| Unknown | ||||||
| Alopecia-1 | ENU | Lack of SG, at two weeks of age SG are rarely found | [189] | |||
| Alopecia-2 | ENU | Lack of SG, at two weeks of age SG are rarely found | [189] | |||
| Bareskin (Bsk) | ENU | SG consisted of rudimentary buds, cells at site of SG were undergoing abnormal cornification rather than sebaceous differentiation | [2] | |||
| Curly bare (cub) | Spont | Enlarged SG | [190] | |||
| Hairless | Spont | SG hypertrophy (2 months after birth), atrophy (after 1 year of age) | [191] | |||
| Hairless-Rhino | Spont | SG hypertrophy (2 months after birth), atrophy (after 1 year of age) | [191] | |||
| Harlequin Ichthyosis (ichq) | Spont | Small, immature SG | [192] | |||
| Rhino | Spont | SG hypertrophy (2 months after birth), atrophy after 1 year of age | [191] | |||
| Rough-fur (ruf) | ENU | Enlarged SG, lipid droplets are denser, irregular shape of SG | [193] | |||
| Soft coat (soc) | Spont | SG Hyperplasia | [2] | |||
| Uncovered (Uncv) | Spont | SG hyperplasia | [194] | |||
Spont spontaneous, Tg transgen, i induced, ts tissue specific, fKO full knockout, KI knockin, SG sebaceous gland, PG preputial gland, MG Meibomian gland, HG Harderian gland
Mouse lines with sebaceous gland abnormalities: the tables
The mouse lines included in the present tables were gathered with the help of a query at PubMed (http://www.ncbi.nlm.nih.gov/pubmed) with the search terms: “mouse” and “sebaceous/sebocyte/Meibomian/preputial/Harderian” and by searching the Mammalian Phenotype browser (http://www.informatics.jax.org/searches/MP_form.shtml) with the search terms abnormal SG morphology (including “absent sebaceous glands”, “abnormal skin sebaceous gland morphology”, “enlarged sebaceous glands”; “small sebaceous gland”, “sebaceous gland atrophy”, “sebaceous gland hypoplasia”, “abnormal SG number”, “absent SG”, “abnormal sebocyte morphology”), abnormal preputial gland morphology (including “abnormal male preputial morphology”, “squamous metaplasia of the preputial gland”), abnormal Harderian gland morphology (including “abnormal Harderian gland development”, “abnormal Harderian gland pigmentation”, “abnormal Harderian gland size”, “absent Harderian gland”), and abnormal Meibomian gland morphology (including “abnormal Meibomian gland acinus morphology”, “abnormal Meibomian gland development”, “absent Meibomian gland”, “enlarged Meibomian gland”, “Meibomian gland atrophy”, “Meibomian gland cyst”, “small Meibomian gland”). For reasons of clarity and comprehensibility, we present the mouse lines in four tables, depending on whether they show abnormalities in skin SG (Table 1), Meibomian glands (Table 2), preputial glands (Table 3), or Harderian glands (Table 4). In each table, the genes and gene products responsible for the SG abnormalities are grouped in categories (“soluble factors”, “receptors”, “transcription factors”, “enzymes”, “adhesion molecules”, “others” and “unknown”). After indicating whether there is a classical, mostly spontaneous mouse mutation for the gene in question, we list the type of genetic modification, provide a summary of the SG phenotype, and indicate the relevant publication. Although we made every effort to include all known mouse lines with a SG phenotype, we cannot exclude having missed important lines. We apologize for any unintended omission and would be grateful for input in this regard from our readers.
Table 2.
Laboratory mouse lines with abnormalities in Meibomian glands
| Gene symbol | Mutant name (if applicable) | Type | Characteristics/abnormalities | References | ||
|---|---|---|---|---|---|---|
| Soluble factors | ||||||
| Noggin | Nog | tsTg | Formation of pilosebaceous units at the expense of MG/suppression of the induction of MG | [65] | ||
| Transforming growth factor alpha (TGFA) | TGFA | itsTg | Abnormal MG morphogenesis, atrophy, and anomalies with a variation of severity | [54] | ||
| Tgfa | fKO | Absence/hypoplasia of MG | [55] | |||
| Receptors | ||||||
| Epidermal growth factor receptor | Egfr | tsKO | Hypoplastic MG | [195] | ||
| Glucocorticoid receptor (GR) | Nr3c1 | tsTg | Lack of MG | [70] | ||
| Neurogenic locus notch homolog protein 1 (Notch1) | Notch1 | tsKO | MG dysfunction, abnormal morphology of MG, lack of lipids | [196] | ||
| Tumor necrosis factor receptor superfamily member EDAR/ectodysplasin-A receptor | Edar | tsTg | Enlarged MG | [77] | ||
| Transcription factors | ||||||
| CCAAT/enhancer-binding protein alpha and beta (c/EBP alpha and c/EBP beta) | Cebpa and Cebpb | itsKO | Reduced lobule size and diminished numbers of differentiated meibocytes with clear vacuolated cytoplasm | [81] | ||
| Homeobox protein BarH-like 2 | Barx2 | fKO | Defects in MG development and structure | [197] | ||
| Krueppel-like factor 5 | Klf5 | tsKO | Malformed MG with disorganized acini, lipid accumulation in the meibomian ducts | [198] | ||
| Myc proto-oncogene protein | MYC | itsTg | Enlarged MG | [45] | ||
| NF-kappaB super-repressor | IkBaDN | KI | Lack of MG | [199] | ||
| PR domain zinc finger protein 1/B lymphocyte-induced maturation protein 1 (Blimp-1) | Prdm1 | tsKO | Enlarged MG | [91] | ||
| Transcription factor AP-1/proto-oncogene c-jun | Jun | tsKO | Hypoplastic MG | [195] | ||
| Transcription factor SOX-9 | Sox9 | tsKO | Reduced number of MG, 40 % fewer glands in the upper and the lower eyelids, most MG had fewer acini | [200] | ||
| Twist-related protein 2 | Twist2 | fKO | Absent/hypoplastic MG | [201] | ||
| Enzymes | ||||||
| Acetyl-CoA acetyltransferase, mitochondrial | Acat1 | fKO | Atrophy of Meibomian gland | [202] | ||
| Acyl-CoA desaturase 1/Stearoyl-CoA desaturase 1 | Scd1 | Asebia-2J | Spont | Small MG, rudimentary duct and glandular structures | [36] | |
| fKO | Atrophy of MG, lack of foamy appearance due to depletion of meibum lipids | [108] | ||||
| Histone deacetylases 1 and 2 (HD1 and 2) | Hdac1 and Hdac2 | tsKO | MG hyperplasia | [128] | ||
| Mitogen-activated protein kinase kinase kinase 1 | Map3k1 | fKO | Hypoplastic MG | [195] | ||
| Superoxide dismutase (Cu–Zn) | Sod1 | fKO | MG alterations including increase in periglandular inflammatory infiltrates, decrease in MG glandular acinar density, increase in periglandular fibrosis | [203] | ||
| Others | ||||||
| 14-3-3 protein sigma/stratifin | Sfn | Repeated epilation (Er) | Spont | MG atrophy and reduced lipid content in aged heterozygotes | [204] | |
| Apolipoprotein C-I (Apo-CI) | APOC1 | Tg | MG atrophy | [150] | ||
| Basigin (CD147) | Bsg | fKO | MG malformation, impaired meibocyte function, secretory acini of MG were poorly developed, small MG, cells in secretory acini failed to produce lipids | [205] | ||
| Cell death activator CIDE-A | Cidea | fKO | Meibocytes accumulate a larger number of smaller-size lipid droplets | [152] | ||
| Gasdermin-A3 | Gsdma3 | Defolliculated (Dfl) | Spont | Decreased lipid production | [158] | |
| Insulin-induced gene 1 and 2 protein (INSIG-1 and 2) | Insig1 and Insig2 | tsKO | Abnormalities in MG | [164] | ||
| Long-chain fatty acid transport protein 4/Fatty acid transport protein 4 (FATP4) | Slc27a4 | Wrinkle-free | Spont | Abnormal development of MG (dystrophic MG), defective meibocyte differentiation | [168] | |
| TNF receptor-associated factor 6 | Traf6 | fKO | Impairment of MG development | [188] | ||
| Unknown | ||||||
| ARMGD | n.r. | MG atrophy | [206] | |||
| crinkled | Chem. | Absence of MG | [207] | |||
| Rhino (hrrhhrrh) | Spont | Progressive loss or atrophy of MG | [208] | |||
| Rhino hrrh | Loss of acini and atrophy of MG | [2] | ||||
| Tabby | Spont | Lack of MG | [209] | |||
| Tabby | Spont | Lack of MG | [2] | |||
| Waved with open eyelids 2 (woe2) | Spont | Absence of MG | [210] | |||
| Waved with open eyes (woe) | Spont | Absence of MG | [211] | |||
Spont spontaneous, Tg transgen, i induced, ts tissue specific, fKO full knockout, KI knockin, SG sebaceous gland, PG preputial gland, MG meibomian gland, HG Harderian Gland, n.r. not reported, chem. chemically
Table 3.
Laboratory mouse lines with abnormalities in the preputial glands
| Gene symbol | Mutant name (if applicable) | Type | Characteristics/abnormalities | References | ||
|---|---|---|---|---|---|---|
| Receptors | ||||||
| Glucocorticoid receptor (GR) | Nr3c1 | tsTg | Underdeveloped PG | [70] | ||
| Gonadotropin-releasing hormone receptor (GnRH-R) | Gnrhr | fKO | Reduced size of PG | [212] | ||
| KiSS-1 receptor (KiSS-1R)/G protein-coupled receptor 54 | Kiss1r | fKO | Lacked preputial separation, small PG | [213] | ||
| fKO | Reduced development of PG | [214] | ||||
| fKO | PG were frequently not identifiable | [215] | ||||
| GKirKO mouse | tsKO | Failure to exhibit PG separation | [216] | |||
| Transcription factors | ||||||
| Catenin beta-1/beta-catenin | Ctnnb1 | KI | Keratinized squamos metaplasia of PG | [217] | ||
| Ctnnb1 | KI | Hyperplasia and squamous metaplasia of PG | [218] | |||
| CCAAT/enhancer-binding protein alpha and beta (c/EBP alpha and c/EBP beta) | Cebpa/Cebpb | itsKO | Atrophy of PG lobules and decreased numbers of finely vacuolated sebocytes | [81] | ||
| Helix-loop-helix protein 2 (HEN-2) | Nhlh2 | fKO | Absent or reduced PG | [219] | ||
| Homeobox protein Hox-D13 | Hoxd13 | Synpolydactyly homolog (spdh) | Spont | Lack of PG | [24] | |
| Digit in Y and carpe (“Dyc”) | Spont | Absent PG | [220] | |||
| PR domain zinc finger protein 1/B lymphocyte-induced maturation protein 1 (Blimp-1) | Prdm1 | tsKO | Enlarged PG | [91] | ||
| Transcription factor GATA-5/GATA-binding factor 5 | Gata5 | fKO | Hypoplastic clitoral glands | [221] | ||
| Enzymes | ||||||
| Cathepsin L1 | Ctsl | Nackt | Spont | Furunculosis and abscesses of PG (mouse maintained non-SPF) | [112] | |
| Ornithine decarboxylase (ODC) | ODC1 | tsTg | Abnormal PG, increased amount of glandular tissue, thicker ducts, metaplastic change | [222] | ||
| Others | ||||||
| Adenomatous polyposis coli protein (APC1638T) | Apc | KI | Absence of PG | [23] | ||
| Autophagy protein 5 | Atg5 | tsKO | Aberrant differentiation of PG | [223] | ||
| DNA cross-link repair 1A protein/SNM1 homolog A | Dclre1a | fKO | Frequent infection of PG | [224] | ||
| DNA repair protein RAD51 homolog 3 | Rad51c | tsKO | Increased keratinization of preputial sebocytes | [225] | ||
| DNA repair protein RAD51 homolog 3 + cellular tumor antigen p53 | Rad51c + Trp53 | tsKO | Increased incidence of PG tumors | [225] | ||
| Gasdermin-A3 | Gsdma3 | Defolliculated (Dfl) | Spont | Decreased PG lipid production | [158] | |
| GTPase KRas and catenin beta-1 | Kras and Ctnnb1 | KI | Keratinized squamos metaplasia of PG | [217] | ||
| Metastasis-suppressor KiSS-1 | Kiss1 | fKO | Lacked preputial separation, small PG | [213] | ||
| fKO | Poor PG development | [226] | ||||
| SV40 large T antigen (SV40T) | SV40 Tag | tsTg | Small PG | [186] | ||
| Protein mab-21-like 1 | Mab21l1 | fKO | Reduction in overall size of PG | [227] | ||
| TNF receptor-associated factor 6 | Traf6 | fKO | Impairment of PG | [188] | ||
| Uveal autoantigen with coiled-coil domains and ankyrin repeats/nuclear membrane-binding protein (nucling) | Uaca | fKO | PG swelling and pathological alterations including keratinization, inflammation and granulomatous lesions | [228] | ||
| fKO | High prevalence of PG abscess, frequent inflammatory lesions of PG in some males younger than 1 year | [229] | ||||
| Unknown | ||||||
| Diabetes | Spont | Small PG | [230] | |||
| Downless | Spont | Absent PG | [2] | |||
| Mesenchymal dysplasia (mes) | Spont | Small PG | [231] | |||
Spont spontaneous, Tg transgen, i induced, ts tissue specific, fKO full knockout, KI knockin, SG sebaceous gland, PG preputial gland, MG Meibomian gland, HG Harderian Gland
Table 4.
Laboratory mouse lines with abnormalities in the Harderian glands
| Gene symbol | Mutant name (if applicable) | Type | Characteristics/abnormalities | References | |
|---|---|---|---|---|---|
| Soluble factors | |||||
| Fibroblast growth factor 10 (FGF-10) | Fgf10 (rat) | tsTg | Ectopic HG in cornea | [232] | |
| Fgf10 | fKO | Lack of HG | [232] | ||
| Fgf10 | fKO | Absent HG epithelium | [233] | ||
| Fgf10 | Aey17 | ENU | HG atrophy (gland replaced by fibrotic pigmented mass) | [234] | |
| Receptors | |||||
| Proto-oncogene tyrosine-protein kinase receptor Ret | Ret | tsTg | HG tumors with hyperplastic and dysplastic lesions | [235] | |
| Receptor tyrosine-protein kinase erbB-2/proto-oncogene Neu | Erbb2/Neu (rat) | tsTg | HG tumors | [236] | |
| Receptor tyrosine-protein kinase erbB-2/tyrosine kinase-type cell surface receptor HER2 | ERBB2/HER2 | tsTg | HG enlargement | [29] | |
| Retinoid acid receptor alpha (RAR alpha) | Rara | fKO | HG agenesis | [237] | |
| Retinoid acid receptor (RAR gamma) | Rarg | fKO | Monolateral or bilateral absence of the HG epithelium | [238] | |
| Retinoid acid receptor (RAR alpha/gamma) | Rara/Rarg | fKO | Agenesis of the HG | [239] | |
| Retinoid acid receptor (RAR beta/gamma) | Rarb/Rarg | fKO | Agenesis of the HG | [239] | |
| Rarb/Rarg | fKO | Unilateral or bilateral absence of HG | [240] | ||
| Transcription factors | |||||
| Catenin beta-1/beta-catenin | Ctnnb1 | KI | Squamos metaplasia with keratinization of the glandular epithelium of HG | [241] | |
| Homeobox protein BarH-like 2 | Barx2 | fKO | Absence of the HG | [197] | |
| Microphthalmia-associated transcription factor | Mitf | Spont | No melanocytes in HG | [242] | |
| NF-kappaB super-repressor | IkBaDN | KI | Lack of HG | [199] | |
| Transcription factor SOX-10 | Sox10 | tsKO | No evidence of secretory acini in HG | [200] | |
| Transcription factor SOX-9 | Sox9 | tsKO | Epithelial component of HG is absent | [200] | |
| Enzymes | |||||
| Acyl-CoA desaturase 1/stearoyl-CoA desaturase 1 | Scd1 | tsKO | HG atrophy | [107] | |
| Aldehyde dehydrogenase family 1 member A3/retinaldehyde dehydrogenase 3 (RALDH-3) | Aldh1a3 | fKO | HG agenesis | [243] | |
| GTPase HRas/c-Ha-ras | HRAS | rasH2 | Tg | HG adenoma | [244] |
| Tg | Some mice developed HG adenocarcinomas | [245] | |||
| GTPase KRas | Kras | KI | Hyperplastic HG | [246] | |
| GTPase NRas | Nras | tsTg | Hyperplasia, degeneration and destruction of HG | [247] | |
| Nras | tsTg | HG tumors and HG hypertrophy | [248] | ||
| Proto-oncogene serine/threonine-protein kinase mos | Mos | Tg | HG hyperplasia in one line | [249] | |
| Retinal dehydrogenase 1 (RALDH 1) and Aldehyde dehydrogenase family 1 member A3/retinaldehyde dehydrogenase 3 (RALDH-3) | Aldh1a3 and Aldh1a1 | fKO | Agenesis of HG | [250] | |
| Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform (PP2A-alpha) | PPP2CA | tsTg | SG hypoplasia | [251] | |
| Others | |||||
| Acyl-CoA-binding protein (ACBP) | Dbi | fKO | Enlarged HG, hypertrophy of acinar cells, vesicles and lumen contain more lipid | [27] | |
| Dickkopf-related protein 2 (Dkk-2) | Dkk2 | fKO | HG hypoplasia | [195] | |
| Human F8B | F8 | Tg | HG tumors | [252] | |
| Neurogenic locus notch homolog protein 4 (Notch 4)/one of three chains: Transforming protein Int-3 | Notch4/Int3 | tsTg | HG hyperplasia | [253] | |
| Transforming growth factor beta regulator 1/nuclear interactor of ARF and Mdm2 | Tbrg1 | fKO | HG adenoma | [254] | |
| v-Ha-ras | Hras | tsTg | Benign hyperplasia of HG | [255] | |
| Hras | tsTg | Hyperplasia of individual HG | [256] | ||
| Hras | tsTg | Bilateral hyperplasia of HG | [257] | ||
| v-Ha-ras and c-myc | Hras and Myc | tsTg | Benign hyperplasia of HG | [255] | |
| v-Ha-ras/cyclin-dependent kinase inhibitor 1A (P21) | Hras/Cdkn1a | tsTg | HG hyperplasia | [258] | |
| Unknown | |||||
| Ichthyosis (ic) | Spont | Absent HG | [259] | ||
| Ocular retardation (or) | Spont | Hypertrophy of HG | [260] | ||
| White-footed mice (two inbred lines: GS109A, GS16A1) | Spont | Harderian adenocarcinomas | [261] | ||
Spont spontaneous, Tg transgen, i induced, ts tissue specific, fKO full knockout, KI knockin, SG sebaceous gland, PG preputial gland, MG Meibomian gland, HG Harderian gland
While it would go beyond the scope of the present review to analyze in detail the phenotype and the significance of each mouse line, glancing through the table immediately reveals some gene products that seem to be of special importance for the SG. A classic model for studying the SG is a mouse line named asebia. Gates and Karasek described in 1965 a spontaneous mouse mutation that is characterized by impaired sebum production due to the absence of SG [37]. Several groups investigated this line in detail [35]. Another enzyme whose expression influences the SG is cyclooxygenase 2 (COX2), also known as prostaglandin endoperoxide H synthase 2. This enzyme uses arachidonic acid to produce prostaglandin H2 [38, 39]. Transgenic mice with overexpression of COX2 in the skin show enlarged SG [40–42], with increased sebum accumulation and SG duct enlargement. These changes support the observation that COX2 inhibits apoptosis [43] and leads to the enlargement of the SG. Another protein whose overexpression increases the size of the SG is the transcription factor myc [44], whose overexpression enhances proliferation and differentiation of the sebocytes at the expense of the hair differentiation [45]. Several groups developed mice with overexpression of myc and observed enlargement of the SG as a consequence [45–49]. Finally, several ligands of the epidermal growth factor receptor (EGFR) influence SG size and sebaceous lipogenesis: Overexpression of transforming growth factor alpha [50], amphiregulin [51], or epigen [52, 53] resulted in enlarged SGs. Mice with inducible expression of transforming growth factor alpha in the eyelid resulted in atrophic MG due to malformation of the eyelid [54]. Conversely, transforming growth factor alpha-deficient mice have hypoplastic MG [55].
Conclusions and outlook
During the compilation of these annotated tables, it became evident that the description and analysis of SG abnormalities differ substantially depending on the laboratory involved. As many reports come from groups whose primary interest is not the SG, the phenotype description is often vague or superficial. For instance, SG enlargement is frequently reported without distinguishing whether it arises from hyperplasia, hypertrophy, or a combination of both events. In addition, dissimilarities in genetic background (different inbreed strains, mixed backgrounds) and environmental differences (nutrition, pathogen status) may result in substantial variations in histological and clinical aspects of the SG abnormality. Finally, the fact that no SG abnormality was reported for a specific mouse line should not lead to the assumption that that such abnormality is not present, as mild changes in SG structure and function may not result in a readily detectable phenotype. These limitations should be kept in mind when consulting the tables provided here.
Genetically modified mouse lines, in association with sebocyte cell culture models [56] significantly contributed to our understanding of SG development, physiology, and pathology. Until now, regulatory sequences of genes encoding keratins or other structural proteins have been used for targeting genes in the epithelial compartment of the skin, including the sebocytes [1]. This approach has the disadvantage that various cell types in the epidermis and in the pilosebaceous unit are targeted concomitantly, potentially causing unspecific phenotypes and side effects. In this regard, the recent report of a mouse line allowing sebocyte-specific gene targeting [57] will allow more precise studies on several aspects of SG biology. We also anticipate that the availability of the CRISPR/Cas9 technology, a novel tool allowing efficient and reliable targeted changes in the genome [58], will further increase the number of genetically modified mouse lines, including those with a SG phenotype. As a detailed guide for SG analysis is now available [59] we also expect future studies to provide a more professional description of the SG alterations.
Although considerable progress has been made in understanding SG biology and pathology, several pathways and processes remain poorly characterized. For instance, while a role for specific enzymes in sebaceous lipogenesis has been demonstrated, our knowledge in this area (particularly in comparison to adipocytes) remains unsatisfactory. Thus, future studies should focus on the systematic characterization of the role played by enzymes as elongases and desaturases [60] in sebum synthesis as well as their regulation. Another worthwhile field for future research is defining the SG stem cells and studying how sebaceous differentiation takes place. Finally, a better understanding of the molecular processes underlying holocrine secretion, in particular the role played by apoptotic pathways, may reveal novel targets for treating SG-associated diseases.
Acknowledgments
Sebaceous gland-related research has been supported by grants from the DFG to MRS.
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest to declare.
References
- 1.Schneider MR. Genetic mouse models for skin research: strategies and resources. Genesis. 2012;50(9):652–664. doi: 10.1002/dvg.22029. [DOI] [PubMed] [Google Scholar]
- 2.Sundberg JP. Handbook of mouse mutations with skin and hair abnormalities. Boca Raton: CRC Press; 1994. [Google Scholar]
- 3.Nakamura M, Sundberg JP, Paus R. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: annotated tables. Exp Dermatol. 2001;10(6):369–390. doi: 10.1034/j.1600-0625.2001.100601.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, et al. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: an update. J Dermatol Sci. 2013;69(1):6–29. doi: 10.1016/j.jdermsci.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, et al. Mutant laboratory mice with abnormalities in pigmentation: annotated tables. J Dermatol Sci. 2002;28(1):1–33. doi: 10.1016/S0923-1811(01)00158-X. [DOI] [PubMed] [Google Scholar]
- 6.Zouboulis CC, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17(6):542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 7.Kurokawa I, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 8.Schneider MR, Paus R. Sebocytes, multifaceted epithelial cells: lipid production and holocrine secretion. Int J Biochem Cell Biol. 2010;42(2):181–185. doi: 10.1016/j.biocel.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Toth BI, et al. “Sebocytes’ makeup”: novel mechanisms and concepts in the physiology of the human sebaceous glands. Pflugers Arch. 2011;461(6):593–606. doi: 10.1007/s00424-011-0941-6. [DOI] [PubMed] [Google Scholar]
- 10.Page ME, et al. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13(4):471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veniaminova NA, et al. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development. 2013;140(24):4870–4880. doi: 10.1242/dev.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlhoff M, et al. PLIN2, the major perilipin regulated during sebocyte differentiation, controls sebaceous lipid accumulation in vitro and sebaceous gland size in vivo. Biochim Biophys Acta. 2013;1830(10):4642–4649. doi: 10.1016/j.bbagen.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camera E, et al. Perilipin 3 modulates specific lipogenic pathways in SZ95 sebocytes. Exp Dermatol. 2014;23(10):759–761. doi: 10.1111/exd.12507. [DOI] [PubMed] [Google Scholar]
- 14.Dahlhoff M, et al. Angiopoietin-like 4, a protein strongly induced during sebocyte differentiation, regulates sebaceous lipogenesis but is dispensable for sebaceous gland function in vivo. J Dermatol Sci. 2014;75(2):148–150. doi: 10.1016/j.jdermsci.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Dahlhoff M, et al. EGFR/ERBB receptors differentially modulate sebaceous lipogenesis. FEBS Lett. 2015;589(12):1376–1382. doi: 10.1016/j.febslet.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Dahlhoff M, Zouboulis CC, Schneider MR. Expression of dermcidin in sebocytes supports a role for sebum in the constitutive innate defense of human skin. J Dermatol Sci. 2016;81(2):124–126. doi: 10.1016/j.jdermsci.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69(2):383–416. doi: 10.1152/physrev.1989.69.2.383. [DOI] [PubMed] [Google Scholar]
- 18.Schneider MR. Lipid droplets and associated proteins in sebocytes. Exp Cell Res. 2016;340(2):205–208. doi: 10.1016/j.yexcr.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358(3):697–704. doi: 10.1007/s00441-014-1999-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49(2):271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Frances D, Niemann C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol. 2012;363(1):138–146. doi: 10.1016/j.ydbio.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Knop E, et al. The international workshop on Meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the Meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits R, et al. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 1999;13(10):1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KR, et al. A new spontaneous mouse mutation of Hoxd13 with a polyalanine expansion and phenotype similar to human synpolydactyly. Hum Mol Genet. 1998;7(6):1033–1038. doi: 10.1093/hmg/7.6.1033. [DOI] [PubMed] [Google Scholar]
- 25.Rudali G, Roudier R, Vives C. The preputial gland of the male mouse. Pathol Biol (Paris) 1974;22(10):895–899. [PubMed] [Google Scholar]
- 26.Bronson FH, Caroom D. Preputial gland of the male mouse; attractant function. J Reprod Fertil. 1971;25(2):279–282. doi: 10.1530/jrf.0.0250279. [DOI] [PubMed] [Google Scholar]
- 27.Bek S, et al. Compromised epidermal barrier stimulates Harderian gland activity and hypertrophy in ACBP−/− mice. J Lipid Res. 2015;56(9):1738–1746. doi: 10.1194/jlr.M060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne AP. The Harderian gland: a tercentennial review. J Anat. 1994;185(Pt 1):1–49. [PMC free article] [PubMed] [Google Scholar]
- 29.Finkle D, et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res. 2004;10(7):2499–2511. doi: 10.1158/1078-0432.CCR-03-0448. [DOI] [PubMed] [Google Scholar]
- 30.Nelson JD, et al. The international workshop on Meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1937. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- 32.Well D. Acne vulgaris: a review of causes and treatment options. Nurse Pract. 2013;38(10):22–31. doi: 10.1097/01.NPR.0000434089.88606.70. [DOI] [PubMed] [Google Scholar]
- 33.McElwee KJ. Etiology of cicatricial alopecias: a basic science point of view. Dermatol Ther. 2008;21(4):212–220. doi: 10.1111/j.1529-8019.2008.00202.x. [DOI] [PubMed] [Google Scholar]
- 34.Ohyama M. Primary cicatricial alopecia: recent advances in understanding and management. J Dermatol. 2012;39(1):18–26. doi: 10.1111/j.1346-8138.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- 35.Schneider MR. Fifty years of the asebia mouse: origins, insights and contemporary developments. Exp Dermatol. 2015;24(5):340–341. doi: 10.1111/exd.12664. [DOI] [PubMed] [Google Scholar]
- 36.Sundberg JP, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156(6):2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gates AH, Karasek M. Hereditary absence of sebaceous glands in the mouse. Science. 1965;148(3676):1471–1473. doi: 10.1126/science.148.3676.1471. [DOI] [PubMed] [Google Scholar]
- 38.Marnett LJ, et al. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem. 1999;274(33):22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 39.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 40.Neufang G, et al. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci USA. 2001;98(13):7629–7634. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller-Decker K, et al. Expression of cyclooxygenase isozymes during morphogenesis and cycling of pelage hair follicles in mouse skin: precocious onset of the first catagen phase and alopecia upon cyclooxygenase-2 overexpression. J Invest Dermatol. 2003;121(4):661–668. doi: 10.1046/j.1523-1747.2003.12473.x. [DOI] [PubMed] [Google Scholar]
- 42.Bol DK, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62(9):2516–2521. [PubMed] [Google Scholar]
- 43.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83(3):493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 44.Watt FM, Frye M, Benitah SA. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nat Rev Cancer. 2008;8(3):234–242. doi: 10.1038/nrc2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11(8):558–568. doi: 10.1016/S0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 46.Foster KW, et al. Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24(9):1491–1500. doi: 10.1038/sj.onc.1208307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun KM, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130(21):5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 48.Bull JJ, et al. Ectopic expression of c-Myc in the skin affects the hair growth cycle and causes an enlargement of the sebaceous gland. Br J Dermatol. 2005;152(6):1125–1133. doi: 10.1111/j.1365-2133.2005.06458.x. [DOI] [PubMed] [Google Scholar]
- 49.Zanet J, et al. Endogenous Myc controls mammalian epidermal cell size, hyperproliferation, endoreplication and stem cell amplification. J Cell Sci. 2005;118(Pt 8):1693–1704. doi: 10.1242/jcs.02298. [DOI] [PubMed] [Google Scholar]
- 50.Halter SA, et al. Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-alpha. Characterization of mammary gland and skin proliferations. Am J Pathol. 1992;140(5):1131–1146. [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y et al (2015) Transgenic expression of human amphiregulin in mouse skin: Inflammatory epidermal hyperplasia and enlarged sebaceous glands. Exp Dermatol [DOI] [PMC free article] [PubMed]
- 52.Dahlhoff M, et al. Epigen transgenic mice develop enlarged sebaceous glands. J. Invest Dermatol. 2010;130(2):623–626. doi: 10.1038/jid.2009.251. [DOI] [PubMed] [Google Scholar]
- 53.Dahlhoff M, et al. Overexpression of epigen during embryonic development induces reversible, epidermal growth factor receptor-dependent sebaceous gland hyperplasia. Mol Cell Biol. 2014;34(16):3086–3095. doi: 10.1128/MCB.00302-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong F, et al. Perturbed Meibomian gland and tarsal plate morphogenesis by excess TGFalpha in eyelid stroma. Dev Biol. 2015;406(2):147–157. doi: 10.1016/j.ydbio.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luetteke NC, et al. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73(2):263–278. doi: 10.1016/0092-8674(93)90228-I. [DOI] [PubMed] [Google Scholar]
- 56.Zouboulis CC, Schagen S, Alestas T. The sebocyte culture: a model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch Dermatol Res. 2008;300(8):397–413. doi: 10.1007/s00403-008-0879-5. [DOI] [PubMed] [Google Scholar]
- 57.Dahlhoff M et al (2016) Sebaceous lipids are essential for water repulsion, protection against UVB-induced apoptosis, and ocular integrity in mice. Development [DOI] [PubMed]
- 58.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinde E, et al. A practical guide for the study of human and murine sebaceous glands in situ. Exp Dermatol. 2013;22(10):631–637. doi: 10.1111/exd.12207. [DOI] [PubMed] [Google Scholar]
- 60.Guillou H, et al. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog Lipid Res. 2010;49(2):186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Mustonen T, et al. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol. 2003;259(1):123–136. doi: 10.1016/S0012-1606(03)00157-X. [DOI] [PubMed] [Google Scholar]
- 62.Cui CY, et al. Inducible mEDA-A1 transgene mediates sebaceous gland hyperplasia and differential formation of two types of mouse hair follicles. Hum Mol Genet. 2003;12(22):2931–2940. doi: 10.1093/hmg/ddg325. [DOI] [PubMed] [Google Scholar]
- 63.Sugawara T, et al. Reduced size of sebaceous gland and altered sebum lipid composition in mice lacking fatty acid binding protein 5 gene. Exp Dermatol. 2012;21(7):543–546. doi: 10.1111/j.1600-0625.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- 64.Panchal H, et al. Neuregulin3 alters cell fate in the epidermis and mammary gland. BMC Dev Biol. 2007;7:105. doi: 10.1186/1471-213X-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plikus M, et al. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164(3):1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guha U, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165(3):729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu W, et al. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J. Invest Dermatol. 2011;131(5):1067–1076. doi: 10.1038/jid.2010.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, et al. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol. 2010;188(6):935–952. doi: 10.1083/jcb.200910126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grose R, et al. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J. 2007;26(5):1268–1278. doi: 10.1038/sj.emboj.7601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cascallana JL, et al. Ectoderm-targeted overexpression of the glucocorticoid receptor induces hypohidrotic ectodermal dysplasia. Endocrinology. 2005;146(6):2629–2638. doi: 10.1210/en.2004-1246. [DOI] [PubMed] [Google Scholar]
- 71.Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83(6):957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 72.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19(15):3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norum JH, et al. A conditional transgenic mouse line for targeted expression of the stem cell marker LGR5 . Dev Biol. 2015;404(2):35–48. doi: 10.1016/j.ydbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Allen M, et al. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163(6):2173–2178. doi: 10.1016/S0002-9440(10)63574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Estrach S, et al. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133(22):4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- 76.Karnik P, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J. Invest Dermatol. 2009;129(5):1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang SH, et al. Enhanced Edar signalling has pleiotropic effects on craniofacial and cutaneous glands. PLoS One. 2009;4(10):e7591. doi: 10.1371/journal.pone.0007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keisala T, et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009;115(3–5):91–97. doi: 10.1016/j.jsbmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131(8):1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 80.Gat U, et al. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95(5):605–614. doi: 10.1016/S0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 81.House JS, et al. C/EBPalpha and C/EBPbeta are required for sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS One. 2010;5(3):e9837. doi: 10.1371/journal.pone.0009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olson LE, et al. Barx2 functions through distinct corepressor classes to regulate hair follicle remodeling. Proc Natl Acad Sci USA. 2005;102(10):3708–3713. doi: 10.1073/pnas.0500519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hwang J, et al. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135(18):3149–3159. doi: 10.1242/dev.022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petersson M, et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 2011;30(15):3004–3018. doi: 10.1038/emboj.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niemann C, et al. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129(1):95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 86.Niemann C, et al. Dual role of inactivating Lef1 mutations in epidermis: tumor promotion and specification of tumor type. Cancer Res. 2007;67(7):2916–2921. doi: 10.1158/0008-5472.CAN-06-3427. [DOI] [PubMed] [Google Scholar]
- 87.Frye M, et al. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 2003;130(12):2793–2808. doi: 10.1242/dev.00462. [DOI] [PubMed] [Google Scholar]
- 88.Waikel RL, et al. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28(2):165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 89.Chiang MF, et al. Inducible deletion of the Blimp-1 gene in adult epidermis causes granulocyte-dominated chronic skin inflammation in mice. Proc Natl Acad Sci USA. 2013;110(16):6476–6481. doi: 10.1073/pnas.1219462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kretzschmar K, et al. BLIMP1 is required for postnatal epidermal homeostasis but does not define a sebaceous gland progenitor under steady-state conditions. Stem Cell Rep. 2014;3(4):620–633. doi: 10.1016/j.stemcr.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126(3):597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagarajan P, et al. Ets1 induces dysplastic changes when expressed in terminally-differentiating squamous epidermal cells. PLoS One. 2009;4(1):e4179. doi: 10.1371/journal.pone.0004179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blanpain C, et al. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20(21):3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurek D, et al. Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development. 2007;134(2):261–272. doi: 10.1242/dev.02721. [DOI] [PubMed] [Google Scholar]
- 95.Hamanaka RB et al (2013) Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal 6(261):ra8 [DOI] [PMC free article] [PubMed]
- 96.Wang X, et al. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J Cell Biol. 2008;183(1):37–48. doi: 10.1083/jcb.200804030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127(1):171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 98.Kiso M, et al. The disruption of Sox21-mediated hair shaft cuticle differentiation causes cyclic alopecia in mice. Proc Natl Acad Sci USA. 2009;106(23):9292–9297. doi: 10.1073/pnas.0808324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nowak JA, et al. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3(1):33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hertveldt V, et al. The development of several organs and appendages is impaired in mice lacking Sp6 . Dev Dyn. 2008;237(4):883–892. doi: 10.1002/dvdy.21355. [DOI] [PubMed] [Google Scholar]
- 101.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 102.Romano RA, et al. DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139(4):772–782. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255(2):238–248. doi: 10.1016/S0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 104.Gu LH, Coulombe PA. Hedgehog signaling, keratin 6 induction, and sebaceous gland morphogenesis: implications for pachyonychia congenita and related conditions. Am J Pathol. 2008;173(3):752–761. doi: 10.2353/ajpath.2008.071089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakamura Y, et al. Phospholipase Cdelta1 is required for skin stem cell lineage commitment. EMBO J. 2003;22(12):2981–2991. doi: 10.1093/emboj/cdg302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Binczek E, et al. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388(4):405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 107.Sampath H, et al. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284(30):19961–19973. doi: 10.1074/jbc.M109.014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and Meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131(9):2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 109.Georgel P, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun. 2005;73(8):4512–4521. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fong LY, et al. Muir-Torre-like syndrome in Fhit-deficient mice. Proc Natl Acad Sci USA. 2000;97(9):4742–4747. doi: 10.1073/pnas.080063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benavides F, et al. Nackt (nkt), a new hair loss mutation of the mouse with associated CD4 deficiency. Immunogenetics. 1999;49(5):413–419. doi: 10.1007/s002510050514. [DOI] [PubMed] [Google Scholar]
- 112.Benavides F, et al. Impaired hair follicle morphogenesis and cycling with abnormal epidermal differentiation in nackt mice, a cathepsin L-deficient mutation. Am J Pathol. 2002;161(2):693–703. doi: 10.1016/S0002-9440(10)64225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peters F, et al. Ceramide synthase 4 regulates stem cell homeostasis and hair follicle cycling. J Invest Dermatol. 2015;135(6):1501–1509. doi: 10.1038/jid.2015.60. [DOI] [PubMed] [Google Scholar]
- 114.Ebel P, et al. Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. Biochem J. 2014;461(1):147–158. doi: 10.1042/BJ20131242. [DOI] [PubMed] [Google Scholar]
- 115.Robert K, et al. Hyperkeratosis in cystathionine beta synthase-deficient mice: an animal model of hyperhomocysteinemia. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(2):1072–1076. doi: 10.1002/ar.a.20082. [DOI] [PubMed] [Google Scholar]
- 116.Chen HC, et al. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Invest. 2002;109(2):175–181. doi: 10.1172/JCI0213880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, et al. Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1. J. Invest Dermatol. 2012;132(12):2681–2690. doi: 10.1038/jid.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Westerberg R, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279(7):5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- 119.Coulson-Thomas VJ, et al. Heparan sulfate regulates hair follicle and sebaceous gland morphogenesis and homeostasis. J Biol Chem. 2014;289(36):25211–25226. doi: 10.1074/jbc.M114.572511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maier H, et al. Normal fur development and sebum production depends on fatty acid 2-hydroxylase expression in sebaceous glands. J Biol Chem. 2011;286(29):25922–25934. doi: 10.1074/jbc.M111.231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Essayem S, et al. Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK . Oncogene. 2006;25(7):1081–1089. doi: 10.1038/sj.onc.1209130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan Y, et al. Gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7(5):731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 123.Grass DS, et al. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J Clin Invest. 1996;97(10):2233–2241. doi: 10.1172/JCI118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sato H, et al. Group III secreted phospholipase A2 transgenic mice spontaneously develop inflammation. Biochem J. 2009;421(1):17–27. doi: 10.1042/BJ20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schuhmacher AJ, et al. A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J Clin Invest. 2008;118(6):2169–2179. doi: 10.1172/JCI34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White AC, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108(18):7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lapouge G, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci USA. 2011;108(18):7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hughes MW, et al. Disrupted ectodermal organ morphogenesis in mice with a conditional histone deacetylase 1, 2 deletion in the epidermis. J Invest Dermatol. 2014;134(1):24–32. doi: 10.1038/jid.2013.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beaudoin GM, 3rd, et al. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci USA. 2005;102(41):14653–14658. doi: 10.1073/pnas.0507609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Driskell I, et al. The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin. EMBO J. 2012;31(3):616–629. doi: 10.1038/emboj.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Megosh L, et al. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res. 1995;55(19):4205–4209. [PubMed] [Google Scholar]
- 132.Soler AP, et al. Modulation of murine hair follicle function by alterations in ornithine decarboxylase activity. J Invest Dermatol. 1996;106(5):1108–1113. doi: 10.1111/1523-1747.ep12340155. [DOI] [PubMed] [Google Scholar]
- 133.Perez CJ, et al. Increased susceptibility to skin carcinogenesis associated with a spontaneous mouse mutation in the palmitoyl transferase Zdhhc13 gene. J Invest Dermatol. 2015;135(12):3133–3143. doi: 10.1038/jid.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suzuki A, et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res. 2003;63(3):674–681. [PubMed] [Google Scholar]
- 135.Mulherkar R, et al. Expression of enhancing factor/phospholipase A2 in skin results in abnormal epidermis and increased sensitivity to chemical carcinogenesis. Oncogene. 2003;22(13):1936–1944. doi: 10.1038/sj.onc.1206229. [DOI] [PubMed] [Google Scholar]
- 136.Mill P, et al. Palmitoylation regulates epidermal homeostasis and hair follicle differentiation. PLoS Genet. 2009;5(11):e1000748. doi: 10.1371/journal.pgen.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Niessen MT et al (2013) aPKClambda controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol [DOI] [PMC free article] [PubMed]
- 138.Castilho RM, et al. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene. 2007;26(35):5078–5085. doi: 10.1038/sj.onc.1210322. [DOI] [PubMed] [Google Scholar]
- 139.Chrostek A, et al. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol Cell Biol. 2006;26(18):6957–6970. doi: 10.1128/MCB.00075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Benitah SA, et al. Stem cell depletion through epidermal deletion of Rac1 . Science. 2005;309(5736):933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 141.Kiguchi K, et al. Constitutive expression of erbB2 in epidermis of transgenic mice results in epidermal hyperproliferation and spontaneous skin tumor development. Oncogene. 2000;19(37):4243–4254. doi: 10.1038/sj.onc.1203778. [DOI] [PubMed] [Google Scholar]
- 142.Ohta E, et al. Analysis of development of lesions in mice with serine palmitoyltransferase (SPT) deficiency-Sptlc2 conditional knockout mice. Exp Anim. 2009;58(5):515–524. doi: 10.1538/expanim.58.515. [DOI] [PubMed] [Google Scholar]
- 143.Ruzankina Y, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Urosevic J, et al. Constitutive activation of B-Raf in the mouse germ line provides a model for human cardio-facio-cutaneous syndrome. Proc Natl Acad Sci USA. 2011;108(12):5015–5020. doi: 10.1073/pnas.1016933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tejera AM, et al. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell. 2010;18(5):775–789. doi: 10.1016/j.devcel.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lippens S, et al. Keratinocyte-specific ablation of the NF-kappaB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis. Cell Death Differ. 2011;18(12):1845–1853. doi: 10.1038/cdd.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ilic D, et al. Skin abnormality in aged fyn−/− fak+/− mice. Carcinogenesis. 1997;18(8):1473–1476. doi: 10.1093/carcin/18.8.1473. [DOI] [PubMed] [Google Scholar]
- 148.Hammond NL, Headon DJ, Dixon MJ. The cell cycle regulator protein 14-3-3sigma is essential for hair follicle integrity and epidermal homeostasis. J Invest Dermatol. 2012;132(6):1543–1553. doi: 10.1038/jid.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee L, et al. Loss of the acyl-CoA binding protein (Acbp) results in fatty acid metabolism abnormalities in mouse hair and skin. J. Invest Dermatol. 2007;127(1):16–23. doi: 10.1038/sj.jid.5700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jong MC, et al. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J Clin Invest. 1998;101(1):145–152. doi: 10.1172/JCI791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mii S, et al. Epidermal hyperplasia and appendage abnormalities in mice lacking CD109. Am J Pathol. 2012;181(4):1180–1189. doi: 10.1016/j.ajpath.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 152.Zhang S, et al. Cidea control of lipid storage and secretion in mouse and human sebaceous glands. Mol Cell Biol. 2014;34(10):1827–1838. doi: 10.1128/MCB.01723-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leclerc EA, et al. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J Cell Sci. 2009;122(Pt 15):2699–2709. doi: 10.1242/jcs.050302. [DOI] [PubMed] [Google Scholar]
- 154.Weber S, et al. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development. 2011;138(3):495–505. doi: 10.1242/dev.055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mese G, et al. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol Biol Cell. 2011;22(24):4776–4786. doi: 10.1091/mbc.E11-09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tanaka S, et al. A new Gsdma3 mutation affecting anagen phase of first hair cycle. Biochem Biophys Res Commun. 2007;359(4):902–907. doi: 10.1016/j.bbrc.2007.05.209. [DOI] [PubMed] [Google Scholar]
- 157.Sato H, et al. A new mutation Rim3 resembling Re(den) is mapped close to retinoic acid receptor alpha (Rara) gene on mouse chromosome 11. Mamm Genome. 1998;9(1):20–25. doi: 10.1007/s003359900673. [DOI] [PubMed] [Google Scholar]
- 158.Porter RM, et al. Defolliculated (dfl): a dominant mouse mutation leading to poor sebaceous gland differentiation and total elimination of pelage follicles. J Invest Dermatol. 2002;119(1):32–37. doi: 10.1046/j.1523-1747.2002.01806.x. [DOI] [PubMed] [Google Scholar]
- 159.Ruge F, et al. Delineating immune-mediated mechanisms underlying hair follicle destruction in the mouse mutant defolliculated. J. Invest Dermatol. 2011;131(3):572–579. doi: 10.1038/jid.2010.379. [DOI] [PubMed] [Google Scholar]
- 160.Lunny DP, et al. Mutations in gasdermin 3 cause aberrant differentiation of the hair follicle and sebaceous gland. J. Invest Dermatol. 2005;124(3):615–621. doi: 10.1111/j.0022-202X.2005.23623.x. [DOI] [PubMed] [Google Scholar]
- 161.Runkel F, et al. The dominant alopecia phenotypes Bareskin, Rex-denuded, and Reduced Coat 2 are caused by mutations in gasdermin 3 . Genomics. 2004;84(5):824–835. doi: 10.1016/j.ygeno.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 162.Kumar S, et al. Gsdma 3(I359N) is a novel ENU-induced mutant mouse line for studying the function of Gasdermin A3 in the hair follicle and epidermis. J Dermatol Sci. 2012;67(3):190–192. doi: 10.1016/j.jdermsci.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 163.Tarutani M, et al. GPHR-dependent functions of the Golgi apparatus are essential for the formation of lamellar granules and the skin barrier. J Invest Dermatol. 2012;132(8):2019–2025. doi: 10.1038/jid.2012.100. [DOI] [PubMed] [Google Scholar]
- 164.Evers BM, et al. Hair growth defects in Insig-deficient mice caused by cholesterol precursor accumulation and reversed by simvastatin. J Invest Dermatol. 2010;130(5):1237–1248. doi: 10.1038/jid.2009.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reichelt J, et al. Loss of keratin 10 is accompanied by increased sebocyte proliferation and differentiation. Eur J Cell Biol. 2004;83(11–12):747–759. doi: 10.1078/0171-9335-00429. [DOI] [PubMed] [Google Scholar]
- 166.Tanaka S, et al. Mutations in the helix termination motif of mouse type I IRS keratin genes impair the assembly of keratin intermediate filament. Genomics. 2007;90(6):703–711. doi: 10.1016/j.ygeno.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 167.Kikkawa Y, et al. A small deletion hotspot in the type II keratin gene mK6irs1/Krt2-6g on mouse chromosome 15, a candidate for causing the wavy hair of the caracul (Ca) mutation. Genetics. 2003;165(2):721–733. doi: 10.1093/genetics/165.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin MH, Hsu FF, Miner JH. Requirement of fatty acid transport protein 4 for development, maturation, and function of sebaceous glands in a mouse model of ichthyosis prematurity syndrome. J Biol Chem. 2013;288(6):3964–3976. doi: 10.1074/jbc.M112.416990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Owens P, et al. Smad4-dependent desmoglein-4 expression contributes to hair follicle integrity. Dev Biol. 2008;322(1):156–166. doi: 10.1016/j.ydbio.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang L, Wang L, Yang X. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Mol Biol Cell. 2009;20(3):882–890. doi: 10.1091/mbc.E08-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qiao W, et al. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25(2):207–217. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- 172.Yang L, et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65(19):8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- 173.Han G, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11(3):301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 174.Cao T, et al. Mutation in Mpzl3, a novel [corrected] gene encoding a predicted [corrected] adhesion protein, in the rough coat (rc) mice with severe skin and hair abnormalities. J Invest Dermatol. 2007;127(6):1375–1386. doi: 10.1038/sj.jid.5700706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mahajan MA, et al. The nuclear hormone receptor coactivator NRC is a pleiotropic modulator affecting growth, development, apoptosis, reproduction, and wound repair. Mol Cell Biol. 2004;24(11):4994–5004. doi: 10.1128/MCB.24.11.4994-5004.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McKenna T, et al. Embryonic expression of the common progeroid lamin A splice mutation arrests postnatal skin development. Aging Cell. 2014;13(2):292–302. doi: 10.1111/acel.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sagelius H, et al. Targeted transgenic expression of the mutation causing Hutchinson–Gilford progeria syndrome leads to proliferative and degenerative epidermal disease. J Cell Sci. 2008;121(Pt 7):969–978. doi: 10.1242/jcs.022913. [DOI] [PubMed] [Google Scholar]
- 178.Odgren PR, et al. Disheveled hair and ear (Dhe), a spontaneous mouse Lmna mutation modeling human laminopathies. PLoS One. 2010;5(4):e9959. doi: 10.1371/journal.pone.0009959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mounkes LC, et al. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature. 2003;423(6937):298–301. doi: 10.1038/nature01631. [DOI] [PubMed] [Google Scholar]
- 180.Sagelius H, et al. Reversible phenotype in a mouse model of Hutchinson–Gilford progeria syndrome. J Med Genet. 2008;45(12):794–801. doi: 10.1136/jmg.2008.060772. [DOI] [PubMed] [Google Scholar]
- 181.Viscomi C, et al. Early-onset liver mtDNA depletion and late-onset proteinuric nephropathy in Mpv17 knockout mice. Hum Mol Genet. 2009;18(1):12–26. doi: 10.1093/hmg/ddn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ruiz S, et al. Abnormal epidermal differentiation and impaired epithelial-mesenchymal tissue interactions in mice lacking the retinoblastoma relatives p107 and p130 . Development. 2003;130(11):2341–2353. doi: 10.1242/dev.00453. [DOI] [PubMed] [Google Scholar]
- 183.Xu X, et al. Co-factors of LIM domains (Clims/Ldb/Nli) regulate corneal homeostasis and maintenance of hair follicle stem cells. Dev Biol. 2007;312(2):484–500. doi: 10.1016/j.ydbio.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cui CY, et al. Shh is required for Tabby hair follicle development. Cell Cycle. 2011;10(19):3379–3386. doi: 10.4161/cc.10.19.17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chiang C, et al. Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205(1):1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 186.Held WA, et al. T antigen expression and tumorigenesis in transgenic mice containing a mouse major urinary protein/SV40 T antigen hybrid gene. EMBO J. 1989;8(1):183–191. doi: 10.1002/j.1460-2075.1989.tb03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martinez P, et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23(17):2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Naito A, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci USA. 2002;99(13):8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wood GA, et al. Two mouse mutations mapped to chromosome 11 with differing morphologies but similar progressive inflammatory alopecia. Exp Dermatol. 2005;14(5):373–379. doi: 10.1111/j.1600-0625.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 190.Johnson KR, et al. Curly bare (cub), a new mouse mutation on chromosome 11 causing skin and hair abnormalities, and a modifier gene (mcub) on chromosome 5. Genomics. 2003;81(1):6–14. doi: 10.1016/S0888-7543(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 191.Mann SJ. Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec. 1971;170(4):485–499. doi: 10.1002/ar.1091700409. [DOI] [PubMed] [Google Scholar]
- 192.Sundberg JP, et al. Harlequin ichthyosis (ichq): a juvenile lethal mouse mutation with ichthyosiform dermatitis. Am J Pathol. 1997;151(1):293–310. [PMC free article] [PubMed] [Google Scholar]
- 193.Park YG, et al. Histological characteristics of the pelage skin of rough fur mice (C3H/HeJ-ruf/ruf) Exp Anim. 2001;50(2):179–182. doi: 10.1538/expanim.50.179. [DOI] [PubMed] [Google Scholar]
- 194.Li SR, et al. Uncv (uncovered): a new mutation causing hairloss on mouse chromosome 11. Genet Res. 1999;73(3):233–238. doi: 10.1017/S0016672399003808. [DOI] [PubMed] [Google Scholar]
- 195.Meng Q, et al. Eyelid closure in embryogenesis is required for ocular adnexa development. Invest Ophthalmol Vis Sci. 2014;55(11):7652–7661. doi: 10.1167/iovs.14-15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vauclair S, et al. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13(2):242–253. doi: 10.1016/j.devcel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 197.Tsau C, et al. Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling. Development. 2011;138(15):3307–3317. doi: 10.1242/dev.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kenchegowda D, et al. Conditional disruption of mouse Klf5 results in defective eyelids with malformed Meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011;356(1):5–18. doi: 10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schmidt-Ullrich R, et al. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128(19):3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 200.Chen Z, et al. FGF signaling activates a Sox9–Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands. Development. 2014;141(13):2691–2701. doi: 10.1242/dev.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tukel T, et al. Homozygous nonsense mutations in TWIST2 cause Setleis syndrome. Am J Hum Genet. 2010;87(2):289–296. doi: 10.1016/j.ajhg.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yagyu H, et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J Biol Chem. 2000;275(28):21324–21330. doi: 10.1074/jbc.M002541200. [DOI] [PubMed] [Google Scholar]
- 203.Ibrahim OM, et al. Oxidative stress induced age dependent Meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS One. 2014;9(7):e99328. doi: 10.1371/journal.pone.0099328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu Q, et al. 14-3-3sigma controls corneal epithelium homeostasis and wound healing. Invest Ophthalmol Vis Sci. 2011;52(5):2389–2396. doi: 10.1167/iovs.09-4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mauris J, et al. Loss of CD147 results in impaired epithelial cell differentiation and malformation of the Meibomian gland. Cell Death Dis. 2015;6:e1726. doi: 10.1038/cddis.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Parfitt GJ, et al. Absence of ductal hyper-keratinization in mouse age-related Meibomian gland dysfunction (ARMGD) Aging (Albany NY) 2013;5(11):825–834. doi: 10.18632/aging.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Falconer DS, Fraser AS, King JW. The genetics and development of ‘crinkled’, a new mutant in the house mouse. J Genet. 1951;50(2):324–344. doi: 10.1007/BF02996227. [DOI] [PubMed] [Google Scholar]
- 208.Jester JV, Rajagopalan S, Rodrigues M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest Ophthalmol Vis Sci. 1988;29(7):1190–1194. [PubMed] [Google Scholar]
- 209.Wang YC, et al. Meibomian gland absence related dry eye in ectodysplasin A mutant mice. Am J Pathol. 2016;186(1):32–42. doi: 10.1016/j.ajpath.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 210.Toonen J, Liang L, Sidjanin DJ. Waved with open eyelids 2 (woe2) is a novel spontaneous mouse mutation in the protein phosphatase 1, regulatory (inhibitor) subunit 13 like (Ppp1r13l) gene. BMC Genet. 2012;13:76. doi: 10.1186/1471-2156-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hassemer EL, et al. The waved with open eyelids (woe) locus is a hypomorphic mouse mutation in Adam17 . Genetics. 2010;185(1):245–255. doi: 10.1534/genetics.109.113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wu S, et al. Disruption of the single copy gonadotropin-releasing hormone receptor in mice by gene trap: severe reduction of reproductive organs and functions in developing and adult mice. Endocrinology. 2010;151(3):1142–1152. doi: 10.1210/en.2009-0598. [DOI] [PubMed] [Google Scholar]
- 213.Lapatto R, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 214.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 215.Funes S, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 216.Novaira HJ, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28(2):225–238. doi: 10.1210/me.2013-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pearson HB, Phesse TJ, Clarke AR. K-ras and Wnt signaling synergize to accelerate prostate tumorigenesis in the mouse. Cancer Res. 2009;69(1):94–101. doi: 10.1158/0008-5472.CAN-08-2895. [DOI] [PubMed] [Google Scholar]
- 218.Bierie B, et al. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22(25):3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- 219.Good DJ, et al. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet. 1997;15(4):397–401. doi: 10.1038/ng0497-397. [DOI] [PubMed] [Google Scholar]
- 220.Cocquempot O, et al. Fork stalling and template switching as a mechanism for polyalanine tract expansion affecting the DYC mutant of HOXD13, a new murine model of synpolydactyly. Genetics. 2009;183(1):23–30. doi: 10.1534/genetics.109.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Molkentin JD, et al. Abnormalities of the genitourinary tract in female mice lacking GATA5 . Mol Cell Biol. 2000;20(14):5256–5260. doi: 10.1128/MCB.20.14.5256-5260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Halmekyto M, et al. Transgenic mice aberrantly expressing human ornithine decarboxylase gene. J Biol Chem. 1991;266(29):19746–19751. [PubMed] [Google Scholar]
- 223.Sukseree S, et al. Targeted deletion of Atg5 reveals differential roles of autophagy in keratin K5-expressing epithelia. Biochem Biophys Res Commun. 2013;430(2):689–694. doi: 10.1016/j.bbrc.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 224.Ahkter S, et al. Snm1-deficient mice exhibit accelerated tumorigenesis and susceptibility to infection. Mol Cell Biol. 2005;25(22):10071–10078. doi: 10.1128/MCB.25.22.10071-10078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tumiati M, et al. Loss of Rad51c accelerates tumourigenesis in sebaceous glands of Trp53-mutant mice. J Pathol. 2015;235(1):136–146. doi: 10.1002/path.4455. [DOI] [PubMed] [Google Scholar]
- 226.d’Anglemont de Tassigny X. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yamada R, et al. Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development. 2003;130(9):1759–1770. doi: 10.1242/dev.00399. [DOI] [PubMed] [Google Scholar]
- 228.Liu L, et al. Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem J. 2004;380(Pt 1):31–41. doi: 10.1042/bj20031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sakai T, et al. Inflammatory disease and cancer with a decrease in Kupffer cell numbers in Nucling-knockout mice. Int J Cancer. 2010;126(5):1079–1094. doi: 10.1002/ijc.24789. [DOI] [PubMed] [Google Scholar]
- 230.Johnson LM, Sidman RL. A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod. 1979;20(3):552–559. doi: 10.1095/biolreprod20.3.552. [DOI] [PubMed] [Google Scholar]
- 231.Sweet HO, et al. Mesenchymal dysplasia: a recessive mutation on chromosome 13 of the mouse. J Hered. 1996;87(2):87–95. doi: 10.1093/oxfordjournals.jhered.a022981. [DOI] [PubMed] [Google Scholar]
- 232.Govindarajan V, et al. Endogenous and ectopic gland induction by FGF-10 . Dev Biol. 2000;225(1):188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- 233.Makarenkova HP, et al. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127(12):2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- 234.Puk O, et al. A new Fgf10 mutation in the mouse leads to atrophy of the Harderian gland and slit-eye phenotype in heterozygotes: a novel model for dry-eye disease? Invest Ophthalmol Vis Sci. 2009;50(9):4311–4318. doi: 10.1167/iovs.09-3451. [DOI] [PubMed] [Google Scholar]
- 235.Iwamoto T, et al. Oncogenicity of the ret transforming gene in MMTV/ret transgenic mice. Oncogene. 1990;5(4):535–542. [PubMed] [Google Scholar]
- 236.Lucchini F, et al. Early and multifocal tumors in breast, salivary, Harderian and epididymal tissues developed in MMTY-Neu transgenic mice. Cancer Lett. 1992;64(3):203–209. doi: 10.1016/0304-3835(92)90044-V. [DOI] [PubMed] [Google Scholar]
- 237.Mascrez B, et al. A transcriptionally silent RXRalpha supports early embryonic morphogenesis and heart development. Proc Natl Acad Sci USA. 2009;106(11):4272–4277. doi: 10.1073/pnas.0813143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lohnes D, et al. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73(4):643–658. doi: 10.1016/0092-8674(93)90246-M. [DOI] [PubMed] [Google Scholar]
- 239.Lohnes D, et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120(10):2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 240.Grondona JM, et al. Retinal dysplasia and degeneration in RARbeta2/RARgamma2 compound mutant mice. Development. 1996;122(7):2173–2188. doi: 10.1242/dev.122.7.2173. [DOI] [PubMed] [Google Scholar]
- 241.Gounari F, et al. Stabilization of beta-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene. 2002;21(26):4099–4107. doi: 10.1038/sj.onc.1205562. [DOI] [PubMed] [Google Scholar]
- 242.Steingrimsson E, et al. The semidominant Mi(b) mutation identifies a role for the HLH domain in DNA binding in addition to its role in protein dimerization. EMBO J. 1996;15(22):6280–6289. [PMC free article] [PubMed] [Google Scholar]
- 243.Dupe V, et al. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA. 2003;100(24):14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tamaoki N. The rasH2 transgenic mouse: nature of the model and mechanistic studies on tumorigenesis. Toxicol Pathol. 2001;29(Suppl):81–89. doi: 10.1080/019262301753178492. [DOI] [PubMed] [Google Scholar]
- 245.Saitoh A, et al. Most tumors in transgenic mice with human c-Ha-ras gene contained somatically activated transgenes. Oncogene. 1990;5(8):1195–1200. [PubMed] [Google Scholar]
- 246.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4(2):111–120. doi: 10.1016/S1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 247.Coto-Montes A, et al. Histopathological features of the Harderian glands in transgenic mice carrying MMTV/N-ras protooncogene . Microsc Res Tech. 1997;38(3):311–314. doi: 10.1002/(SICI)1097-0029(19970801)38:3<311::AID-JEMT11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 248.Mangues R, et al. Tumorigenesis and male sterility in transgenic mice expressing a MMTV/N-ras oncogene. Oncogene. 1990;5(10):1491–1497. [PubMed] [Google Scholar]
- 249.Heath LA, et al. Harderian gland hyperplasia in c-mos transgenic mice. Int J Cancer. 1992;51(2):310–314. doi: 10.1002/ijc.2910510222. [DOI] [PubMed] [Google Scholar]
- 250.Matt N, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132(21):4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- 251.Schild A, et al. Impaired development of the Harderian gland in mutant protein phosphatase 2A transgenic mice. Mech Dev. 2006;123(5):362–371. doi: 10.1016/j.mod.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 252.Valleix S, et al. Expression of human F8B, a gene nested within the coagulation factor VIII gene, produces multiple eye defects and developmental alterations in chimeric and transgenic mice. Hum Mol Genet. 1999;8(7):1291–1301. doi: 10.1093/hmg/8.7.1291. [DOI] [PubMed] [Google Scholar]
- 253.Jhappan C, et al. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6(3):345–355. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- 254.Reed SM, et al. NIAM-deficient mice are predisposed to the development of proliferative lesions including B-cell lymphomas. PLoS One. 2014;9(11):e112126. doi: 10.1371/journal.pone.0112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sinn E, et al. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 256.White V, Sinn E, Albert DM. Harderian gland pathology in transgenic mice carrying the MMTV/v-Ha-ras gene. Invest Ophthalmol Vis Sci. 1990;31(3):577–581. [PubMed] [Google Scholar]
- 257.Tremblay PJ, et al. Transgenic mice carrying the mouse mammary tumor virus ras fusion gene: distinct effects in various tissues. Mol Cell Biol. 1989;9(2):854–859. doi: 10.1128/MCB.9.2.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Adnane J, et al. Loss of p21WAF1/CIP1 accelerates Ras oncogenesis in a transgenic/knockout mammary cancer model. Oncogene. 2000;19(47):5338–5347. doi: 10.1038/sj.onc.1203956. [DOI] [PubMed] [Google Scholar]
- 259.Gruneberg H. Exocrine glands and the Chievitz organ of some mouse mutants. J Embryol Exp Morphol. 1971;25(2):247–261. [PubMed] [Google Scholar]
- 260.Truslove GM. A gene causing ocular retardation in the mouse. J Embryol Exp Morphol. 1962;10:652–660. [PubMed] [Google Scholar]
- 261.Parnell PG, et al. Frequent Harderian gland adenocarcinomas in inbred white-footed mice (Peromyscus leucopus) Comput Med. 2005;55(4):382–386. [PubMed] [Google Scholar]