Abstract
Intravaginal delivery offers an effective option for localized, targeted, and potent microbicide delivery. However, an understanding of the physiological factors that impact intravaginal delivery must be considered to develop the next generation of microbicides. In this review, a comprehensive discussion of the opportunities and challenges of intravaginal delivery are highlighted, in the context of the intravaginal environment and currently utilized dosage forms. After a subsequent discussion of the stages of microbicide development, the intravaginal delivery of proteins and oligonucleotides is addressed, with specific application to HSV and HIV. Future directions may include the integration of more targeted delivery modalities to virus and host cells, in addition to the use of biological agents to affect specific genes and proteins involved in infection. More versatile and multipurpose solutions are envisioned that integrate new biologicals and materials into potentially synergistic combinations to achieve these goals.
Keywords: Intravaginal, Microbicides, Drug delivery, Gene delivery, Protein delivery, Sexually transmitted infection (STI), HIV, HSV, Intravaginal ring (IVR), Nanoparticles
The pandemic of sexually transmitted infection
Sexually transmitted diseases (STDs) affect 340 million new people each year [1]. Approximately, 36 million people are living with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), while 536 million people are living with herpes simplex virus 2 (HSV-2) [2–4]. Infection with HIV remains incurable [5, 6], and two million deaths have occurred since 2008 [7]. Furthermore, sexually transmitted infections (STIs), such as HSV-2, predispose women to HIV/AIDS while also facilitating chlamydia and syphilis coinfections, and acting as a potential cofactor in human papillomavirus (HPV) infection. Overall, the presence of untreated STIs has been shown to enhance both the acquisition and transmission of HIV by as much as two- to sixfold [8–14]. This enhanced infection is attributed to infection-associated inflammation that interferes with the integrity of the vaginal epithelium, while recruiting infectible immune cells to the affected region [15]. Therefore, the effective prevention, treatment, and management of all STIs are important HIV prevention strategies [4].
While antiviral drugs are being utilized and progress continues in vaccine development, the incidence of both HIV and HSV has increased [16]. Increased incidence is impacted by a variety of factors affecting both developed and developing countries. In fact, one of the challenges to eradicating STIs includes the global and geographical variations in disease prevalence and incidence [17]. Sub-Saharan Africa, for example, has the highest prevalence and incidence rates, accounting for 20 % of global STIs and 70 % of people living with HIV/AIDS. South and South-East Asia closely follow with rapidly increasing HIV incidence rates approaching 10 % of the global prevalence [18]. Relative to North American levels of HIV infection (~3 % of global infections), these numbers are staggering; however, the epidemic is not limited to underdeveloped countries. The main difference between the United States and Western Europe relative to developing countries is that the number of individuals living with HIV/AIDS has risen, while the rates of HIV infection have plateaued or only slightly increased over the period 2001–2012 [19, 20]. The plateau in infection rates can be broadly attributed to increased preventative measures and educational outreach. Furthermore, the increase in survival rates in countries such as the US and Western Europe is due to the administration of new therapies such as highly active antiretroviral treatment (HAART) that enable better therapeutic outcomes post-infection [20].
The increase in STIs in many non-industrialized countries can be partially attributed to the lack of access to effective and affordable healthcare. Policy makers, health staff, and communities recognize that health services in lower- and middle-income countries need to improve people’s access to and retention in HIV treatment programs [21]. In some cases, major political and economic transitions have contributed to the increase in STIs, such as countries undergoing economic and healthcare reform. While industrialized countries can offer new treatments, such as HAART, that have helped to manage HIV, underdeveloped countries often cannot afford them [18]. The slow implementation of a cost-effective preventative or therapeutic measure has contributed to the lack of progress in decreasing infection rates: such a program requires global investment [22]. However, implementation of effective STI prevention programs is challenging because economical and medical problems, in addition to behavioral and social factors, contribute to increased STI incidence, particularly in women [17].
Current estimates indicate that women acquire 23 % of the new HIV infections domestically, and 50 % of all new global infections [23]. While there are available options to decrease heterosexual transmission of HIV in women, they often require male partner cooperation to be successful. Male condoms provide an excellent example of a widely available prophylactic, in which efficacy depends on the male partner’s level of acceptance [24]. In many situations, it may be difficult for women to negotiate the use of available prophylactics, due to gender inequality, reliance on men for economic security, and unequal relationship dynamics. All of these factors contribute to the challenge in finding better ways to integrate prevention methods [25, 26]. Additionally, cultural conditions can make it difficult for women to insist on safe sex practices, which necessitates methods that women can initiate and control, and apply independently of intercourse (coitally-independent).
Even if safer sex practices and preventative measures are embraced, approved, and implemented, user adherence is critical. As demonstrated in the CAPRISA 004 microbicide trial, suboptimal adherence to a preventative regimen results in lower efficacy [27]. Although implementations of antiretroviral (ARV) therapy for AIDS treatment have demonstrated that high levels of adherence are achievable in real-world settings, obtaining adherence is especially challenging in developing countries. A major difficulty is that clinical testing of products is undertaken in settings where HIV incidence is high, but research infrastructure is limited.
All of these factors contribute to the lack of a successful female-controlled, coitally-independent, preventative and/or treatment strategy—which poses a significant global burden by impacting reproductive health, fertility, child health, and continuing to facilitate transmission of STIs [19]. The financial and societal burden of health care combined with: the increased likelihood of co-infection with other STIs [8, 28–30], and the disproportional effect on women, newborns, and immune-compromised individuals is monumental [31]. Therefore, development of better active agents and drug delivery vehicles is urgently needed. For any solution to be realized, multiple disciplines must collaborate to overcome social, cultural, and technical hurdles.
The focus of this review is to discuss the challenges of female-controlled, intravaginally applied microbicide strategies for preventative and/or therapeutic delivery of proteins and oligonucleotides against STIs. We initially discuss previous generations of microbicide development applied to different formulations and active ingredients (specific and non-specific), to provide the reader with a history of the field. We then discuss current and future protein and oligonucleotide intravaginal delivery strategies to prevent, treat, and mitigate STIs.
Current prevention and treatment strategies
Considerable effort has been spent developing vaccines for the prevention of STIs; however, experts predict that it is unlikely a vaccine for either HIV or HSV will be available in the next decade [32]. While vaccine development has been slow due to several barriers—including inadequate resources, regulatory concerns, and intellectual property issues—the major difficulty lies in the scientific challenges involved in understanding how the human immune response correlates with protection [33, 34]. Thus far, HIV vaccines have had only moderate success in protecting against viral lytic infection and have failed to impact latency [35]. Similarly, vaccines for HSV-2 have proven only moderately successful, despite the identification of targets [9]. Previously, two HSV-2 vaccines successfully completed phase III clinical trials: one showed no protection against HSV-2 acquisition and occurrence of genital ulcers, despite eliciting high titers of neutralizing antibodies (Abs) [36], and the other only protected HSV-2 seronegative women [37]. Further studies are needed to clarify the complex relationship between STIs and the immune system to generate more effective vaccines. A summary of some of the ongoing clinical trials for HSV and HIV vaccines is provided in these references [38–41].
From a treatment perspective, there are a number of antivirals currently used to treat HSV-2 and HIV. Specifically for HSV-2, a deoxyguanosine analogue, Acyclovir (ACV), is the most commonly used antiviral. ACV is preferentially taken up by infected cells, and converted first by virus thymidine kinase and subsequent host enzymes to ACV triphosphate, to inhibit viral DNA synthesis via viral DNA polymerase. While less potent, other antivirals including famciclovir and penciclovir are available that inhibit viral DNA polymerase. Similar in efficacy to ACV is Valaciclovir, a prodrug of ACV that provides similar efficacy, but offers pharmacokinetic advantages including greater bioavailability matching that of intravenous delivery. While these are the preferred treatment choices, other second-tier antivirals exist that offer improved oral solubility, treatment against other HSV variants, and potential treatment of ACV-resistant virus. As a detailed description is outside the scope of this review, please see [42] for more information.
Antivirals used to treat HIV are categorized base on their mechanism of action, and some of these candidates are discussed later in “Stages of intravaginal microbicide development”. Generally, HIV ARVs are classified as nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors, entry inhibitors, HIV integrase strand transfer inhibitors, and multi-class combination products. A more comprehensive listing of antivirals shown to be efficacious in HIV treatment can be found in [43]. A sampling of ARVs from each class of inhibitors includes: NRTIs Truvada (tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC)) and Viread (TDF); NNRTI Edurant (rilpivirine); entry inhibitor Selzentry (maraviroc); fusion inhibitor, Fuzeon [enfuvirtide (ENF)]; and Atripla (a combination of efavirenz, FTC, and TDF). Notably, Truvada has demonstrated marked success in the Partner’s PrEP trial, reducing HIV risk of infection in women by ~73 %, relative to oral TDF alone (62 %) [44–46]. Currently, Truvada is approved as an oral formulation (and is in trials as a vaginal tablet) for those at high risk of getting HIV-1. While a summary of currently promising antivirals and delivery vehicles that have been formulated into intravaginal microbicides for clinical trials are provided in Table 1, more detailed information can be found in [43] and the above references.
Table 1.
Some of the promising intravaginal microbicides in development or holding promise for clinical trials [158, 315–322]
| Delivery vehicle | API delivered | Purpose | Status | Description and outcome |
|---|---|---|---|---|
| Gel | TFV 1 % | Efficacy | Completed | Administer gel 12 h before and after sex; reduced women’s risk of HIV infection by sex by 39 % and also demonstrated decreased risk of acquiring HSV-2 (CAPRISA 004) |
| Efficacy | Completed | Compare effectiveness of women’s daily oral TFV, daily oral TFV/FTC (emtricitabine), and daily TFV 1 % gel for HTV prevention. None of these formulations were shown to prevent HIV, however, participants did not adhere to administration regimen | ||
| Efficacy | Ongoing | Test effectiveness of TFV 1 % gel in preventing HIV and HSV-2 infections in women. Used same dosing regimen as CAPRISA 2004 | ||
| PK/PD, safety | Completed | Evaluated TFV concentrations—low in blood, high in vaginal fluid | ||
| PC-1005 | Safety, efficacy, acceptability | Ongoing | Phase I trial to evaluate MTV-150 (NNRTI), co-formulated with zinc acetate carrageenan gel | |
| Dapivirine | Safety, PK, acceptability | Completed | Evaluate the safety, PK and acceptability of dapivirine gel against HTV-1 | |
| MIV-150, zinc acetate, carrageenan (MZC) | Safety, PK | Ongoing | Phase 1 against HTV, HSV-2, and HPV | |
| GRFT (non-ARV) | Safety, PK, efficacy | Ongoing | Preclinical and clinical studies. Will inform future IVR and fiber designs | |
| IVR | TFV | PK/PD and safety | Pending | Evaluate 90-day ring to assess safety, PK/PD, HIV-1 infectivity, and acceptability |
| Dapivirine | Safety, PK, acceptability, long-term safety | Ongoing | Determine if ring is safe and effective to reduce risk of HIV infection | |
| Dapivirine + maravinoc combination | Safety, absorption | Completed | First clinical study vaginal microbicide with maraviroc and to combine two ARVs. Maraviroc-only did not protect, still looking at continuing optimizing combination. Dapavirine and combination ring prevented HIV challenge | |
| Polyurethane TDF | Safety and PK | Recruiting | Examine safety and PK of polyurethane TDF ring used for 14 days | |
| Maraviroc | Safety and efficacy | Recruiting | Two studies from MTN and IPM evaluating the safety and efficacy of monthly dapivirine vaginal ring | |
| MIV-150, zinc acetate, carrageenan (MZC) | Initial efficacy | Preclinical | Evaluate 90-day sustained-release IVR to prevent HSV-2, HIV, HPV, Trichomonas vaginalis | |
| Film | TFV film (vs. gel) | Safety, efficacy ex vivo | Recruiting | Phase I trial to assess the safety of TFV gels and films |
| Dapivirine film (vs. gel) | Safety, absorption, PK | Completed | Compared safety and PK of dapivirine film to dapivirine gel, both gel and film protected againt HIV in challenge model | |
| Tablets | Vaginal TFV and TFV/FTC (Truvada) | PK/PD and safety | Ongoing | Evaluate fast-dissolve tablets to assess safety, PK/PD, acceptability, anti-HIV activity, and disintegration time |
| Oral TFV/FTC (Truvada) vs. TFV reduced glycerin gel | Safety, acceptability, PK, absorption, adherence | Recruiting | Examine effects of oral Truvada and reduced glycerin l % TFV gel |
While antiviral drugs have increased both the duration and quality of life for people infected with HIV, and have lessened the symptoms and outbreak severity associated with HSV-2 infection and recurrence, effective prevention methods remain elusive [23, 47]. HIV and HSV infections still remain unpreventable and incurable; moreover, not all countries offer routine access to ARV medication [4]. Most recently, ARVs have been incorporated into gels and tablets in clinical trials for HIV/AIDS to enhance efficacy [48], but none have been proven to be fully efficacious.
Considering these challenges, the development of topical, intravaginal microbicides presents a tremendous opportunity for prevention and/or treatment of STIs. Vaginal microbicides are chemical compounds that when applied vaginally or rectally, have the potential to prevent and/or reduce the acquisition of STIs [49]. An inexpensive and safe intervention against STIs, such as locally applied microbicides could delay the need for more expensive drugs such as HAART [16]. Furthermore, it is estimated that a partially effective microbicide, when used in half of coital acts by 20 % of women, could prevent 2.5 million HIV infections over 3 years [50]. Therefore, even a partially effective microbicide may significantly reduce STIs.
Traditionally, microbicides have been classified as non-specific or specific, depending on their mechanism of action, and mediate cell infection or virus integration by attacking viral or cellular targets, usually by affecting virus binding, integrity, and replication [19]. Microbicides work through various mechanisms including blockade of receptors in target host cells (macrophages, T-cells, dendritic cells, epithelial cells) or prevention of viral attachment to host cells. First generation microbicides were typically highly sulfated molecules or detergents, designed to act non-specifically to inhibit virus entry, by disrupting the virus envelope through a charge- or pH-based mechanism [19]. These first generation, non-specific microbicides included: nonoxynol 9 (N9), Savvy (C31G), cellulose sulfate, Carraguard (PC-515), PRO 2000, and BufferGel [51]. Unfortunately, none of these agents produced a significant clinical effect against HIV, with N9 and cellulose sulfate showing increased infection rates associated with use [51, 52].
Because the non-specific first generation microbicides were unsuccessful, second generation microbicides were designed to prevent STI and transmission through more specific mechanisms. These second generation microbicides typically target distinct elements of the viral life cycle, such as viral entry/fusion or critical enzymes (for HIV, usually reverse transcriptase (RT), integrase, or protease) [1]. More recently, researchers have begun to explore products that are multifunctional—to incorporate technologies to prevent unintended pregnancy as well as reproductive infections [47, 53–56]. Whereas the first and early second generation microbicide products were drugs, new technologies seek to incorporate biological and genetic agents to target events in the virus cycle and host response [31, 57–69]. For any of these new, specific products to be successful, it is important to select the right biological target. However, understanding the physiology of intravaginal environment and the dynamics of drug delivery is equally as important to the intelligent design of an effective microbicide.
Opportunities and challenges for intravaginal delivery of microbicides
The local intravaginal environment and its role in infection
Intravaginal delivery has been a site for delivery of agents both locally and systemically [34, 70, 71]. Some of the common intravaginally (locally) delivered agents include: antibacterials, antifungals, antiprotozoals, antivirals, labor-inducing agents, spermicides, prostaglandins, and steroids [72, 73]. The vagina has a number of characteristics, including its: large surface area; vascular, tissue, and mucosal permeability; and abundant vasculature; that promotes the delivery of small molecules, proteins, peptides, oligonucleotides, and plasmid DNA (pDNA) [74–76]. By offering an alternative to non-localized systemic or parenteral administration [1], intravaginal delivery avoids first-pass hepatic clearance associated with most other delivery routes [77, 78]. For prevention of STIs, local vaginal delivery of microbicides also enables interception of the virus at the point of entry and deposition of agents in close proximity to infected or infectible cells. Due to these advantages over other drug delivery methods, intravaginal delivery of microbicides is an area of substantial research interest. However, to optimally design agents for effective local delivery, it is important to understand the anatomy and physiology of the reproductive microenvironment, and how this environment impacts virus infection.
The vagina is a fibromuscular tubular tract that leads from the opening of the vulva to the cervix. The vaginal tract is covered by mucus, a glycoprotein gel, comprised of 95 % water, 1–2 % mucin fibers, and trace constituents including lactic acid, salts, DNA, proteins, and enzymes [79–85]. To establish infection, the virus must travel through the mucous barrier to reach epithelial cells (in the case of HSV) and sub-epithelial cells (in the case of HIV). Furthermore, epithelial morphology varies with location in the vagina; the vagina and ectocervix are comprised of multilayered stratified squamous epithelium, whereas the endocervix epithelium is comprised of a single columnar layer [86]. These structural differences in the epithelium impact the likelihood of virus penetration. While, the columnar layer of the endocervical junction is considered more susceptible to HIV infection because junctions between cells are ordered in a monolayer, the greater relative surface area of the vagina and ectocervix, relative to the endocervix, can allow for greater potential of infection, particularly when the epithelial surface is disrupted [87, 88]. Inflammation in the female reproductive tract caused by trauma or co-infection with other viruses can increase susceptibility to HIV infection [89]. Even small abrasions induced by coitus can enable more efficient entry of HIV to target cells in the basal epithelium and stroma [90]. Furthermore, depending on the virus type, different entry points can facilitate infection.
For HSV infection to occur, virus entry usually occurs via cellular receptors or extracellular glycosaminoglycans. Three prominent receptors are expressed on the vaginal luminal epithelial surface, that enable HSV-2 binding and internalization: nectin-1, herpes virus entry mediator (HVEM), and 3-O-sulfated heparan sulfate [91–95]. One of the first steps in HSV entry is virus binding to heparan sulfate (HS) polysaccharide chains of cell surface heparan sulfate proteoglycans (HSPGs) [96]. HSV-2 binds to HSPGs and “surfs” down cell filipodia, whether entry is via endocytosis or cell fusion [97, 98]. In fact, HS has been found to not only provide a site for virus attachment and subsequent “surfing”, but has also demonstrated that alterations in HS can provide a receptor to virus gD [99]. These receptors and extracellular milieu on epithelial cells, contribute to the unique feature that HSV transmission occurs from skin-to-skin contact (via epithelial cells), instead of bodily fluids. As a result barrier prophylactics such as condoms, while considered the best way to protect against HSV, only protect against the risk of HSV by approximately 30 % with consistent use [100].
Relative to epithelial receptors that enable HSV entry and infection, multiple cellular HIV targets reside in the endocervix and sub-epithelium—including intraepithelial Langerhan’s cells, T-cells, dendritic cells, and macrophages—that provide a proximal source of potentially infectible cells [15, 87]. The primary receptors and co-receptors identified in HIV infection include cluster of differentiation 4 (CD4), chemokine receptor 5 (CCR5), and chemokine (C-X-C motif) receptor 4 (CXCR4). CCR5 is a major co-receptor for macrophage tropic HIV-1 and CXCR4 is a co-receptor for infection by T cell tropic HIV-1 [101]. While chemokine receptors are recognized as essential co-receptors for HIV infection and are expressed on several types of cells including epithelial cells, generally speaking, HIV must surmount epithelial barriers to infect underlying CD4+ cells [87].
As genital epithelial cells do not express the conventional HIV-1 CD4 receptor, this makes them a more challenging target for HIV entry. Alternate receptors, such as gp340, have demonstrated attachment and uptake of HIV-1 in vaginal epithelial cells [102]. Overall however, HIV-1 has been stated to “utilize unconventional mechanisms to cross primary genital epithelial cells” [103]. These mechanisms are not yet fully understood, and the trajectory of HIV virions that are free or released from cells interact with epithelial cells and traverse cell boundaries via transcytosis, endocytosis, infection, inflammation, or penetration through epithelial cell gaps to underlying cells in the epithelium, sub-epithelium, and lamina propria [88, 90, 103]. Transcytosis or alternative mechanisms of HIV through the epithelial cells has been observed to underlying CD4+ cells, yet, virus passage is usually very low [87]. However, the ectocervix, endocervix, and vagina are all portals for HIV infection [103]. In fact, the ecto-to-endocervix transition region can be populated with CD4+ T-cells, making this region particularly susceptible to HIV entry [88]. Additionally, cells such as Langerhans may project cell extensions close to the epithelial surface, and contact virus in this way. However, as mentioned above, HIV also needs to traverse the multilayered epithelial barrier or epithelial gaps between cells, to infect underlying CD4+ cells—a process that is not yet fully understood [87, 104].
Achieving a complete understanding of the sequence of HIV infection events, has been challenged by inconsistent research results. This inconsistency is primarily attributed to experiments that use epithelial cell types derived from different locations in the body, and primary and immortalized cell lines, resulting in inconsistent experimental practices [88]. For instance, while it has been demonstrated that HIV-1 binds and enters epithelial cells in the lower reproductive tract, transcytosis, while occurring in immortal and primary cells, has not been exhibited in intact tissue [88]. Furthermore, in explants, expression of HIV-1 chemokine co-receptor expression has been inconsistent, ranging from no detection of CCR5 or CXCR4 expression by cervical epithelial cells, to CXCR4 expression by these cells, to expression of CCR5 only [88]. Furthermore, once virions infect leukocytes, there appears a marked difference between cell-associated virions secreted from infected leukocytes and the less efficient transcytosis observed in cell-free virions. While efficient production may occur in cervical epithelial cells as well, it has also been suggested that HIV-1 can be transported by lymphocytes and macrophages, through the epithelium to the draining lymph nodes [88].
In addition to the method through which HIV navigates the epithelium, several receptors have been reported to facilitate entry into CD4− cells. Similar to HSV-2, extracellular constituents have been shown to contribute to infection. Many epithelial cell surface proteins interact with HIV-1 envelope proteins to increase virus attachment to host cells [88]. Specifically, interaction of HIV-1 envelope gp120 and several host cell surface molecules including glycolipids, sulfated lactosylceramide (on vaginal epithelial cells), galactosylceramide adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and lymphocyte function-associated antigen (LFA-1 on ectocervical epithelial cells [103]), transmembrane chondroitin and heparin sulfate proteoglycans (HSPGs), and gp340 (expressed by genital epithelial cells) can also contribute to HIV-1 attachment and entry [87, 88]. Additionally, syndecans, a specific type of HSPG that primarily binds to gp120, can increase HIV-1 attachment to cells when co-expressed with CD4 and chemokine receptors [103]. Syndecan expression does not negate the necessity of CD4 and chemokine receptors for entry, but may increase infection by encouraging virus adsorption to receptive cell surfaces. Due to their adhesive nature, syndecans may play a significant role in obtaining virus-cell adhesion prior to virus entry and infection.
Additionally, C-type lectins such as dendritic cell-specific-ICAM-grabbing non-integrin (receptor) (DC-SIGN, DC-SIGNR), langerin, and the mannose receptor, have been shown to encourage HIV-1 attachment and/or entry into CD4− cells [87, 103]. Fibronectin, a constituent of semen, has been shown to bind to viral glycoprotein 120 (gp120) and also to the β1 integrin, thereby aiding virus-epithelium attachment [89]. In some cervical epithelial explant samples, the β1 integrin subunit bound virions that were likely coated with fibronectin [88]. In addition, envelope specific IgG, which can bind to incoming virions, can bind to the Fc neonatal receptor (FcRN) in the genital mucosa, and may enable transport of HIV through epithelial cells [89]. To date, there has been no demonstration that these receptors are capable of mediating fusion between viral and cellular membranes; however, these receptors represent prime candidates for cell-free HIV-1 passage through genital epithelial cells. For a more comprehensive review of cell receptors and potential virus entry portals, please see [103].
While these physical characteristics of the female reproductive tract provide potential entry points and pathways to virus infection, the intravaginal environment can conversely act as a barrier to virus infection and the delivery of active agents. The intravaginal environment has been studied to understand how this environment innately protects against infection, and this information can be used to suggest ways that local delivery of agents can protect infectible cells from incoming pathogens.
Overview of transport and drug delivery barriers—mucus and epithelium
Localized delivery of microbicides against STIs averts issues inherent to oral or systemic delivery, and provides a promising route to deliver agents in close proximity to both the site of infection and infectible cells. However, the mucus gel and epithelium can act as barriers to intravaginal drug delivery [86, 105]. Mucus, the glycoprotein gel that accumulates on the surface of the vaginal epithelium, is the first barrier encountered in both virus infection and intravaginal drug delivery. Mucus in the vagina—which is produced by cells in the cervix and so appropriately called cervical mucus—forms a gel layer with variable thickness and consistency that changes during the menstrual cycle. Previous studies that investigated the ability of differently sized viruses to diffuse through mucus, originally estimated mucus pore size to be on the order of ~100 nm, with assumptions that mucus impeded larger virus penetration by steric exclusion. More recently, studies have confirmed that mucus has an average pore size of ~340 ± 70 nm, and thickness of a few hundred microns [106–108], yet still hinders pathogen entry into deeper tissue. This indicates that mucoadhesion, rather than steric hindrance garners protection against virus infection [109]. These same properties however, that make mucus a physical barrier to virus infection [110–112], can also impede the diffusion of locally delivered agents that protect and treat against infection [83].
In addition to providing a mucoadhesive path for incoming molecules and virions, cervical mucus has a dynamic role in facilitating sperm movement by altering its viscoelasticity during the menstrual cycle. This change in viscoelasticity can aid or impede drug efficacy by altering transport properties, reducing distribution, and/or inducing leakage, resulting in decreased residence time of a microbicide at the target site. Microbicidal agents can be further compromised by dilution or dissolution in the mucus, thereby weakening the concentration present to induce efficacy. In addition, the mucus covering the epithelium has inherent enzymatic activity that can act as a barrier to drug delivery and absorption. While these antimicrobial peptides and enzymes aid in prevention of infections, they also accelerate degradation of delivered preventative/therapeutic agents. Last, many of the factors that affect mucus fluidity, quantity, consistency, and accumulation, change with age and menstrual cycle, making mucus a dynamic and challenging barrier to drug delivery [1].
The second layer of protection against pathogen infection and drug delivery, which is beyond the mucus gel, is the epithelium [1]. Once the delivered agent traverses the mucus, the epithelium restricts paracellular permeability through the presence of tight junctions. These tight junctions are permeable to water, ions, and even immune cells; therefore, modifications to molecules to alter cellular permeability and improve drug delivery have been investigated [113]. In addition, the apical and basolateral membranes contain lipid bilayers. By partitioning into or associating with these bilayers, small molecules can be transported through these membranes by hydrophobic interactions. Cell receptors located on the apical surface enable the interaction and specific transport of hydrophilic materials across the membrane [86].
Agent distribution, retention, and stability in mucus and epithelium
The effectiveness of vaginally-delivered microbicides is determined by agent distribution, residence time, stability, and permeability to vaginal tissue. As mentioned, mucus can hinder the diffusional transport of molecules, drug-loaded carriers, and pathogens by binding interactions. While these mucosal interactions can facilitate bioadhesion of agents or carriers, helping to increase drug residence time at the mucosal surface, they can also be a barrier to diffusion [83, 106, 107, 109, 114–117]. Any interaction between the agent or carrier and the surrounding negatively-charged mucin glycoprotein chains impedes delivery: binding may occur by physical entanglement, hydrophobic interactions, hydrogen bonding, or electrostatic interactions [117]. Delivery of high molecular weight and hydrophobic drugs is particularly challenging, due to the variety of mechanisms available for interaction with delivered agents [7, 53, 118]. Carriers or vehicles can be used to overcome the physical barriers to mucus penetration: in this case, the vehicle should be small enough to negate physical hindrance, and have net neutral surface charge to minimize interaction with the negatively charged mucin fibers. Drug delivery vehicles with neutral charge have demonstrated enhanced distribution and penetration through intravaginal mucus, despite vehicle sizes that are larger than mucosal pore sizes [83, 106, 107, 109, 115–117, 119].
In addition to the physicochemical transport barrier presented by mucus interactions, mucus has a significant role in agent distribution and retention due to mucosal physical properties and shedding. Macroscopically, mucus is a cervicovaginal surface lubricator that contributes to normal functions including regulation of sperm motility and protection against pathogen entry. At the micro and nanoscales, mucus is heterogeneous: high viscosity mucin glycoproteins containing regions of low viscosity fluid [85, 86]. In addition, secreted lipids, calcium ions, pH, trefoil factor, and non-mucin glycoproteins contribute to the overall viscoelasticity [86, 120, 121]. As an example of the effect of mucus viscosity on transport, consider the effect on sperm motility. Ovulatory mucus, which is thinner and less viscous than non-ovulatory mucus, promotes sperm motility more readily than non-ovulatory mucus [122, 123]. With respect to drug delivery, some agents may be more or less permeable to mucus during different parts of the menstrual cycle, depending on mucus thickness and viscoelasticity. Furthermore, while mucus is secreted and shed continuously, the overall rate of mucus clearance is dependent on an individual’s activity, age, and menstrual cycle stage. Often, secretion and shedding can be stimulated by the presence of irritating substances [86, 120, 121]. Therefore, mucoadhesive carriers trapped in luminal mucus may be shed rapidly, with very few carriers reaching mucus layers that are directly adherent to the epithelial surface [106, 109, 120]. As mucosal shedding can decrease the delivery and efficacy of different dosage forms, mucus penetrating formulations are being designed to more deeply penetrate mucus and to avoid the high turnover of mucus shedding [56, 107, 115].
Even when intravaginally administered agents are designed to traverse mucus and to avoid leakage and mucosal shedding, delivered cargo must maintain its activity through the delivery process. Local enzymatic activity in the vaginal epithelium can affect the stability of locally delivered agents, and contribute to premature degradation. Proteases such as aminopeptidase and proteinase K may induce low vaginal absorption of biological agents, while high enzymatic activity in the outer cell and basal layers of the vaginal epithelium may decrease agent stability [124, 125]. Furthermore, hydrolytic enzyme and dehydrogenase activity can contribute to the destabilization and degradation of intravaginally delivered molecules [126]. Also, being well-vascularized, the vascular and lymph pathways in the vaginal tract can re-route delivery to unintended regions [124, 127].
Cyclic changes in the intravaginal environment
The thickness of the vaginal epithelium can vary with age, stage of the menstrual cycle, and pregnancy. When the vaginal epithelium is thin, absorption of drugs is often easier; however, invasion by pathogens may be easier as well [114]. In addition, it is important to maintain the normal vaginal flora during drug delivery, as the flora provides an innate defense against pathogens, in part by maintaining the acidic pH of the vaginal tract. The microorganisms comprising the vaginal flora are also dynamic, and depend on age, menstrual cycle, pregnancy, and infection [1, 124]. Lactobacillus, a major bacterial component of microflora pre-menopause, helps to maintain the low intravaginal pH that provides pathogen protection. While these “normal” vaginal bacteria maintain a vaginal pH spanning 3.5–5, this pH can be altered in the presence of semen and infections such as bacterial vaginosis and trichomoniasis. Alterations in flora often result in elevated pH levels closer to neutral, and correlate with decreased response to infection [86].
Other: user compliance, acceptability, cost effectiveness, and stability during transport—challenges to developing countries
User compliance, acceptability, cost, and transport/storage stability also impact microbicide development and use, but these subjects are outside the scope of this review. A brief summary of some of the challenges is listed below; however, more resources can be found in these references [18, 22, 23, 27, 56, 124, 128–130].
Compliance
During the last 20 years there have been 12 microbicidal effectiveness trials of seven candidate products; only one product has demonstrated significant protection against HIV infection [22]. The CAPRISA 004 phase IIb safety and effectiveness trial showed that a 1 % tenofovir vaginally administered gel (TFV) reduced HIV acquisition in 18–40 years old women at high risk for HIV infection. While there was a 39 % reduction in infection overall, women who most consistently used the gel in accordance with dosing before and after sexual intercourse (gel adherence greater than 80 %) had a 54 % lower HIV incidence compared with women using the placebo [27, 128, 131]. Data from this trial emphasized that adherence to the prescribed prophylactic/treatment regimen is required for efficacy against infection. Furthermore, this challenge of consistent microbicide use was reinforced by a phase II Carraguard safety trial. While participants reported 94 % usage of Carraguard, a conformation technique that used a mucus-reactive dye revealed that only 61 % of the returned applicators had actually been used [132, 133].
While moderate levels of adherence have been achieved in ARV prophylactic/treatment regimens in both developed and developing countries, it is evident that obtaining user compliance still remains challenging, especially for microbicide regimens that must be adhered to daily [22]. Only more recently have more long-term delivery options (delivery for more than 1-month), such as drug-eluting intravaginal rings (IVRs), been assessed for user adherence [134]. In contrast to the above-mentioned prevention methods, IVRs, which will be discussed more in-depth later, generally depend less on user compliance, due to the decreased frequency with which they must be administered. For instance, in initial studies in Brazil that examined IVR adherence for 28 days, 89 % of women had perfect adherence. In two NuvaRing® trials in the US and Europe, participants were instructed to remove the IVR during coitus and re-insert within three hours. More than 60 % of the women maintained the IVR during 10 months, and 86 % maintained continuous monthly insertion cycles. Furthermore, 81 % of women preferred the IVR to oral contraceptives due to the convenience associated with remembering timing and dosing. Similar results were obtained in a US study where students administered the IVR for 3 months, with 57 % qualifying as perfect users. In the first study to investigate IVR adherence in African women as an HIV prevention method, African women reported high levels of IVR use, with 82 % keeping the IVR in for the entire 12-week study period, and 95 % of women wearing the IVR for at least 12 h per day. These adherence results indicate that sustained-release IVRs are relatively easy and convenient to use, require little effort to achieve adherence, and can be comfortably worn during daily activities [134]. By offering long-term (>30 day) delivery products that promote coital-independence by maintaining microbicide concentrations over weeks to months, hurdles associated with conventional user compliance and acceptability can be overcome [7].
User acceptability
The user acceptability of a product often determines long-term commitment to microbicide use. Despite the lack of efficacy demonstrated by the Carraguard and TFV gel trials following low-to-moderate user compliance, other safety trials have been conducted to assess and understand factors contributing to user acceptability [132]. One of the factors that contribute to compliance is a higher perceived need for protection among HIV-infected women, relative to uninfected women [132]. For example, the acceptability of the gels was particularly high in HIV-infected women, as women were motivated by the potential of the gel to prevent against another STI or strain of HIV, or to protect their partner. Particularly, asymptomatic healthy people may be less likely to adhere to prophylactic/treatment regimens due to a smaller perceived risk, thereby decreasing the incentive to use microbicides consistently. In addition to perceived risk, factors such as trial design, duration, participant characteristics, relationship status, and the physical characteristics of the microbicide have impacted user compliance [132].
Social deterrence, in addition to perceived risk assessment must also be considered to encourage sexual product usage and integration. In particular, the stigma associated with ARV/pre-exposure prophylaxis (PrEP) use contributed to the difficulty in adhering to HIV preventative treatment when healthy. The technology novelty combined with the negative connotations of HIV contributed to women’s and partners’ fear in being identified as HIV-positive (in both gel and tablet users). However, combining a discussion on other aspects of a product, such as its physical characteristics, can stimulate discussions and communication between partners to help overcome social deterrents. A suggestion from a variety of PrEP studies was to encourage couples and male partner’s education and participation as in the Partners PrEP trial [135]. One study focused on assessing the acceptability of barrier and lubricant products among HIV-seropositive Zambian men after group intervention and long-term use [136]. In this study, while adding lubricants to sexual activity was not preferred, men found these products acceptable and used this as a way to better communicate with their partners. The issue of leakage was associated with lower levels of use, making all product types that displayed these issues, preferred similarly. This study highlighted that men have preferences for delivery systems, and prefer a variety of options. In another study, men’s decisions were heavily impacted by whether the product would interfere with sexual pleasure. While women and men from different cultures seemed to differ in opinion on the amount of lubrication that was ideal during coitus, as long as there was moisture, they were willing to compromise for protection against STIs [137]. Therefore, the physical characteristics of a microbicide, namely the ability to lubricate and maintain vaginal moisture (in addition to decreasing vaginal leakage), were important to users, but were beneficial to facilitate discussion about product usage.
In many South African nations, men want to be involved in the decision-making. Similar to other studies in Uganda and Zimbabwe, men desire to have a say in women’s selection of a microbicide [138]. Therefore, increasing male involvement in clinical trials to test microbicides, not only for efficacy and safety, but to also ascertain the short- and long-term user acceptability of a product is recommended. While men recognized the need for women to prevent against STIs, non-contraceptive microbicides may have a greater need than contraceptive vaginal products. By increasing sexual barrier and lubricant product acceptability, in combination with encouraging couples and male partners to communicate about microbicide products, user acceptability may be enhanced [136].
Beyond individual and couple user preferences, geographical location has been shown to impact user acceptability and preferred dosage form [22, 139]. Studies indicate that one microbicide formulation may not be universally acceptable to all populations, and gels may be more accepted in developing countries [22, 139]. On a smaller scale, however, it is also important to note that a woman’s product preference can be impacted by a number of personal factors. These factors may include her partner’s product preference, the perceived risk of acquiring other STIs, her desire to conceive, and/or the convenience of the dosage form. These factors underscore the importance of designing new prevention approaches that allow women to independently control their exposure and risk to STIs. While at-risk women may prefer a variety of options to meet their needs, microbicides that are coitally-independent, and provide the option for sustained release or infrequent administration are highly desirable to increase user adherence and to provide female-controlled methods of protection. Therefore, the development of products that foster compliance, through education in combination with the implementation of convenient, infrequent, and coitally-independent solutions, is expected to enhance adherence and efficacy.
Cost and effectiveness
As technology advances to include biopharmaceuticals such as proteins, nucleic acids, and antibodies, microbicide design must take a new approach to offer cost-effective solutions, especially for developing countries. In recent years, the cost of manufacturing biopharmaceuticals has been reduced; however, the use of some global health products, including microbicides, has been limited due to their high cost and low production capacity. In fact, the FDA identified manufacturing as the rate limiting step for new technology development due to specific challenges including physical design, characterization, scale-up, packaging, and quality control [124].
Overall it is agreed that the cost of microbicides and the program required to deliver them must be affordable, with sufficient financing available to support the development of, and access to future microbicides. In a report by the Alliance for Microbicide Development, R&D groups estimated the cost required by Europe, the US, and G8 countries for phases 1 through 3 clinical trials. Detailed summaries can be found in [129]; however, for phase 1 and 2 trials, cost estimates spanned 1–5 million and 50–62 million US dollars, respectively. These large development costs further support the initiative to more intelligently design microbicides to provide lower-cost alternatives.
Recently, it has been highlighted that a microbicide with relatively low effectiveness could have a substantial impact against the global HIV epidemic, if used by a significant number of women [18]. Some factors that might creatively enable more low-cost alternatives through better design include: developing products that offer non-coital sustained delivery, increasing delivered concentrations by using biopharmaceuticals that have specificity, and developing multipurpose options that are versatile across infections and contraception. In addition, manufacturing advances in bacteria and yeast production (RANTES), bioengineered commensals (cyanovirin, RANTES, CD4), and plants (Abs, griffithsin (GRFT), cyanovirin), may solve cost and capacity constraints for some biopharmaceuticals, and are further discussed below [56]. Furthermore, the increased availability of biologically active pharmaceutical ingredients (APIs) is stimulating the development of biopharmaceuticals formulated for sustained delivery.
Stability during transport
In addition to being easily affordable, maintaining agent stability during transport and storage is a requirement for microbicide development [130]. One of the biggest challenges to microbicides or vaccines—anything that contains biologicals or degradable materials—is temperature regulation. Overexposure to extreme heat or cold can impact the stability of the active ingredients used in microbicides [130]. Furthermore, these conditions can be particularly challenging for faster dissolving formulations and biologicals, e.g. proteins, genes, and peptides [124]. The key differences between industrialized and developing countries for microbicide (and vaccine) distribution are the latter’s lesser resources to pay for the products and the storage, distribution, personnel, and training to properly handle and administer them [140, 141]. Furthermore, in the developing world, microbicides and vaccines travel great distances often from the US or Europe to Africa, where travel continues by vehicle often in rural areas, to finally arrive at a remote clinic with unreliable electricity and minimal refrigeration. Therefore, efforts including the enhancement of the global distribution network of equipment, and the development of procedures for maintaining agent quality during transport and storage will contribute to stability improvements [130, 142]. In addition to these efforts, development of new technologies that ensure efficacy under difficult temperature conditions is critical. Methods are currently being explored to improve the thermostability of new and existing products by drying agents, and changing formulation methods to protect biologicals from heat damage. More information on new methods to create and preserve thermostable formulations can be found in [130, 140, 141].
Overview of intravaginal delivery technologies and dosage forms
Intravaginal delivery offers a promising option to deliver agents locally, specifically, conveniently, and at high concentration to the vaginal tract. Intravaginal administration was first used to deliver drugs against vaginal infections, and for prolonged and local delivery of contraceptive agents [4]. Vaginal formulations, which often employ designs that were successful in rectal suppositories, include tablets, creams, and suppositories for short-term (<1 day) delivery applicable to individual acts of intercourse [4]. In 1970, the first intravaginal controlled drug delivery system was developed: a vaginal ring for the delivery of medroxyprogesterone acetate for contraception [4, 34]. While vaginal rings are more commonly used for long-term delivery (i.e. slow release of an active compound over prolonged periods of days to months [125]), other delivery systems—including gels, films, and micro/nanoparticles—also show promise as sustained-release intravaginal delivery systems [31, 57, 123, 143, 144]. Many first and second generation microbicides consisted of polymers and gels designed to impede the HIV life-cycle by either preventing virus attachment to and fusion with the cell, or by targeting genes and proteins that disrupt the virus replication cycle including reverse transcriptase, integrase, and proteases [86]. Specific targeting of elements of the viral life cycle seeks to increase efficacy, limit cross-resistance, and minimize microbicide-induced non-specific toxicity [145–147].
In the development of a stable, safe, and effective microbicide, the physicochemical properties of the API—a biologically active substance—and the intended carrier or dosage form of these APIs must be considered [19]. In the following sections we address some of the more common [148] microbicide dosage forms; however, reviews on tablets and pessaries used in microbicide applications are also available [4, 47, 51, 114], in addition to more details of the dosage forms referenced in the following sections.
Vaginal gels and creams (semi-solids)
There is a need to establish a coitally-independent microbicide that eliminates application prior to every act of intercourse. However, to maintain an efficacious microbicide while avoiding frequent applications, the residence time in the vagina must be prolonged [149]. Tunable delivery systems that provide long-term prophylactic or therapeutic concentrations of active ingredients following a single dose are a current focus in microbicide development.
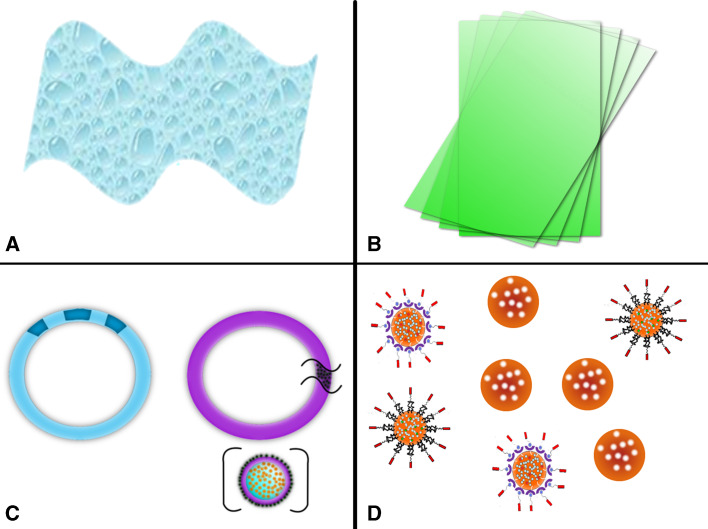
Gels or creams, more broadly referred to as semi-solids, have been the most commonly used delivery vehicles for microbicides [4, 19] (Fig. 1a). As the name implies, gels are primarily liquid by mass, but have some characteristics attributed to solids, due to their three-dimensional cross-linked structure. In fact, cross-linked networks in a liquid environment impart the structural component to gels, and contribute to their adhesive properties [150]. Gels are further sub-classified, depending on the solvent in which they swell. For instance, hydrogels are three-dimensional, hydrophilic polymeric networks capable of absorbing and retaining large amounts of water [151]. Hydrogels can be classified as neutral or ionic, based on the nature of the chemical side groups. The gel networks are insoluble due to the presence of chemical or physical crosslinks, which provide structure and physical integrity [151, 152]. In comparison, creams are defined as a semisolid emulsion that contains suspended or dissolved medication [148].
Fig. 1.
Schematic of the variety of microbicide dosage forms currently available or in development: a vaginal gels (semi-solids); b vaginal films; c vaginal rings; and d nanoparticles
Bioadhesive microbicides have been developed in gel, cream, and solid dosage forms. Bioadhesion defines the interaction of two materials (at least one biological) that are held together for a prolonged duration by interfacial forces [149]. In comparison, mucoadhesion refers to the adhesive interaction that occurs between a mucosal surface, and a synthetic or natural polymer [149, 153]. In the design of drugs and agents targeted for intravaginal applications, bio- and mucoadhesive gels aid in prolonging contact between the rapidly shed mucosa and the active ingredients. By extending the contact time and adhesivity to the mucosa, leakage of the product may be delayed, thereby improving and sustaining the protective and/or therapeutic effects, in addition to increasing user adherence [149]. Furthermore, vaginal gels can enhance spreading and lubrication, relative to other dosage forms, due to their high water content, making this a more comfortable option [149]. In fact, vaginal gel popularity is likely attributed to ease of manufacturing, user comfort level, and uniform mucosal adhesion imparted by the gel.
The inherent physicochemical properties of gels and creams affect the stability, retention, and distribution of these agents. A number of gel formulations have been employed, especially in first generation microbicides, that include BufferGel, a Carbopol®-based gel (a high molecular weight polymer of acrylic acid cross-linked with polyalkenyl ether [154, 155]) that has anti-HIV and HSV-2 activity in vitro, in addition to spermicidal properties, and PRO2000, a naphthalene sulfonate polymer that has been shown to be effective against HIV-1 in macaques. However, both BufferGel and PRO2000 were ineffective at preventing HIV-1 infection in phase II and phase III clinical trials, respectively [19, 138, 156]. More information on each is provided in the section “Stages of intravaginal microbicide development” below.
Similar to gels, creams are often used to provide relief from bacterial and fungal infections, and encounter many of the same user challenges, including leakage and non-uniform vaginal distribution. In one particular study, the efficacy of 3-day clindamycin vaginal cream administration was evaluated against bacterial vaginosis. The trial, conducted in pregnant women, found that the clindamycin cream was accepted and effective [4]. Similarly, a mucoadhesive cyclodextrin-based cream of itraconazole showed effective action against vaginal candidiasis [157]. Many different active agents, including N9, antibacterials, and antifungals have been incorporated into creams for common prevention of these maladies [71].
While gels and creams originally served as vehicles for the delivery of active agents, some active agents (such as antivirals) can be formulated in their active form as these formulations. For example, the NRTIs and non-NRTIs, UC-781, dapivirine, and TFV have shown promise when incorporated into a safe and effective gel [19, 158]. Although these formulations are encouraging, gel-based products are often leaky, causing the microbicide to leak from its site of action, resulting in lower user acceptability. Therefore, microbicides should be optimally designed to spread uniformly over the intravaginal tract, while reducing leakage and enhancing intravaginal adhesion.
Vaginal films
Relative to intravaginal gel formulations, vaginal films are solid, thin strips of polymeric water-soluble material that dissolve and release active ingredients, when placed on the vaginal mucosal surface [159] (Fig. 1b). Vaginal films are convenient dosage forms, as they address user acceptability issues experienced with gel-based microbicides. They offer convenient portability, easy storage, no leakage, discreet use, and low user cost [159]. Vaginal film formulations consist of the active ingredient, water soluble polymers, plasticizers and fillers. Similar to other dosage forms, polymer choice and molecular weight impact properties including strength and sustained release properties (achieved by film disintegration) [159]. Dissolvable polymeric films are being investigated to increase agent retention, and to enhance mucoadhesion, reduce leakage, improve timing of delivery, and accelerate drug release.
Vaginal contraceptive films (VCFs) have been used to deliver N9 and antivirals, and have been shown to be user-friendly and convenient [160, 161]. Polystyrene sulfonate (PSS), was developed as a dual contraceptive and microbicide that was initially found to be safe for intravaginal administration in phase I clinical trials [19, 159]. Similarly cellulose acetate phthalate (CAP) was embedded in a vaginal film and demonstrated activity against HIV-1, HSV-1 and HSV-2 [162]. Delivery of specific anti-HIV agents including entry and replication inhibitors has been studied using vaginal films, however films are challenged with providing longer-lasting formulations, relative to more “quick dissolve” capabilities [160].
Tablets
Tablets and suppositories are also used to deliver agents intravaginally. These generally conical, rod-shaped, or wedge-shaped formulations provide sustained-release on the order of hours to days, by melting or dissolving in the vaginal tract [163]. Similarly to films, tablets provide ease of portability, storage, and administration, precise dosing, large-scale fabrication and low cost [163]. In addition, relative to other dosage forms, tablets are stable at increased temperature and humidity. Similar to other intravaginal dosage forms, tablets can be fabricated to have mucoadhesive properties for sustained residency, sustained-release, and if desired, rapid dissolution [4]. Polymers including chitosan and alginate, carbopol, and sodium carboxymethyl cellulose have been used to achieve bioadhesive material properties [157]. In addition, materials used in IVRs have been used, including silicone-matrices that provide controlled-release profiles with respect to time.
The active agents in vaginal tablets and suppositories include anti-infective agents, hormones, and Lactobacillus spores, as they are one of the most common dosage forms used locally for the treatment of vaginal infections, for drug delivery prior to pregnancy, and for hormone therapy [163]. Some of these specific active agents include antibacterials and antifungals such as neomycin, clotrimazole, miconazole. Within the scope of this review, some microbicide candidates that have been formulated and administered as tablets include cellulose sulfate, ACIDFORM, PSS, dapivirine, TFV, and UC781 [157, 163]. In fact, PSS has been formulated to have antimicrobial activity against both HIV and HSV, and to be safe to vaginal flora and sperm [164]. However, tablets often contain added excipients that work as a diluent, binder, disintegrant, lubricant, anti-adherent, and glidant [4, 159]. Generally, tablets, in addition to gels, creams and suppositories are commonly used in vaginal drug delivery, with intravaginal rings providing options for longer-term delivery [4].
Intravaginal rings (IVRs)
Intravaginal rings (IVRs) are flexible, typically torus-shaped, drug delivery devices that avoid leakage, are coitally-independent, and enable sustained release of substances to the vagina for local or systemic effect [7] (Fig. 1c). IVRs have been designed to provide sustained delivery of an active agent at the mucosal surface, after self-administration and provide long-term (greater than 1 month protection) against disease and unwanted pregnancy [165]. In fact, IVRs have been used for delivery of steroid hormones for up to 3 months and have also demonstrated release of Abs for up to several years [47, 166–169]. Due to their controlled release properties and the ability of women to use them in a coitally-independent manner, IVRs have been the leading choice for long-term intravaginal delivery [7, 19, 118].
In terms of material choice, non-degradable polymers have been widely applied in the fabrication of IVRs, as they provide a material for long-term use. Non-degradable polymers have been fabricated into “matrix-” and “reservoir-” type vaginal rings to tailor release properties [6]. In ring fabrication, active agents may be molecularly dissolved in the solid matrix solution as molecules, or dispersed as crystalline or amorphous particles in a solid dispersion [170]. Often, solid dispersion is an alternative to enhance the solubility of poorly water soluble agents. While a number of vaginal ring designs have been created, the simplest vaginal ring design consists of solid drug particles dispersed throughout the polymer matrix (Fig. 1c, zoom) [7, 118]. These rings are referred to as homogeneous or matrix rings, in which molecule release is dependent on the solubility of the drug in the polymer, drug loading, drug diffusion through the polymer, and the surface area of the ring [118]. Here, solubilized drug is dissolved in the polymer, followed by diffusion of the solubilized molecules through the polymer matrix. In this design, release rates are proportional to drug loading and device surface area, as solute transport from non-degradable polymeric systems is primarily diffusion driven [118]. A drug-deficient layer results as agents near the ring surface diffuse, and molecules within the ring must diffuse through this widened depletion region to be released [118, 171, 172]. Therefore, by tailoring the thickness of the outer membrane, release rates can be tuned for a variety of agents.
Vaginal rings may also be designed in a sandwich (shell) or core (reservoir) formation that provide constant, linear release profiles [7, 118, 165]. As the name implies, the sandwich design contains three concentric layers: a narrow drug-loaded polymer layer located between a non-drug containing (inert) core and a non-drug external polymer membrane. In this design, the proximity of the drug-containing layer with respect to the external surface, is significant to the overall release of active agent, particularly the release of molecules with low diffusivity. In comparison, reservoir or core type designs consist of compartments or dual concentric layers that contain the drug in single or multiple central cores, encapsulated by an inert outer polymer membrane (Fig. 1c, left). Core type designs offer versatility, in that one or more individual drug containing cores can be fabricated within the same ring, providing delivery of multiple agents with differently tailored release rates [118]. In core type designs, the release rate is more dependent on the thickness and permeability of the polymer membrane, than the concentration gradient. Sandwich (or matrix) type devices, in contrast, are dependent on concentration gradient, diffusion distance, and swelling, with release being classified as Fickian diffusion [173]. More information on IVR design and the mathematical details of release can be found in [165].
Specific non-degradable polymer material choice is based on the solubility of the drug in the polymer, biocompatibility, drug permeability, and device flexibility for intravaginal insertion. Generally, vaginal rings are divided into either silicone elastomer or thermoplastic devices, and agent release is dependent on the physicochemical properties of the active agent and its interaction with the elastomer or thermoplastic ring. Thus far, commercially available IVRs are made from silicone elastomer, or thermoplastic materials including poly(ethylene-co-vinyl acetate) (EVAc) or polyurethane (PU). While the specific details of each IVR material can be found in [7, 118, 124], some of the challenges in obtaining release from IVRs include the compatibility between the drug and the polymer to generate sustained and tunable release and the processing conditions for bioactive ingredients.
Silicone elastomers have demonstrated the release of relatively hydrophobic, low molecular weight compounds (such as steroids), indicating they would be useful for controlled release of antiretroviral compounds with similar properties. However, some silicone elastomers have induced burst release dependent on the fabrication method, prompting designs to mitigate burst release in favor of diffusion-mediated processes [118]. In contrast, release from thermoplastics, can be readily tailored by adjusting the components of the reaction mixture; however, poor flexibility has been a challenge. Thermoplastic elastomers such as EVAc have been investigated for use as both the matrix and inert polymer sheath to provide slow-release formulation in the commercial product, Nuvaring®. However the implementation of EVAc IVRs, has been more difficult for microbicide applications, due to the challenges in obtaining clinical grade materials for human testing [118]. A final challenge in fabricating protein or peptide-loaded IVRs using conventional methods, such as hot-melt extrusion, is that processes requiring high heat will result in degradation of the bioactive proteins/peptides. Lyophilization and insertion of biological ingredients after the IVR fabrication process have helped avoid denaturing and aggregation during the fabrication process [174].
Relative to other delivery methods, IVRs provide a long-term method of administration—on the order of weeks to months—with high user acceptability. Overall the current silicone and thermoplastic materials are promising, but still have the challenges of releasing hydrophilic and macromolecular agents. New IVR designs seek to overcome these permeability hurdles, and toward these efforts, new ring designs have been conceived to overcome the transport challenges associated with high molecular weight drugs and hydrophilic microbicide candidates [7, 53, 118, 175]. Currently, three IVR devices are FDA approved, including Estring and Femring (silicone-based) that are used as hormone replacement devices, and Nuvaring®, a thermoplastic elastomer ring [174].
Nanoparticles
To enhance the delivery of APIs, significant research efforts have been focused on developing nanoparticles (NPs) to encapsulate or conjugate a variety of molecules for specific and local delivery [19] (Fig. 1d). NPs are versatile carriers, as they may encapsulate a variety of bioactive agents, and provide a coitally-independent alternative with sustained release and long-term efficacy [176, 177]. A variety of NP platforms exist, including liposomes, dendrimers, and polymeric, metallic, silicon, zinc oxide, carbon, and magnetic NPs; however, only NPs relevant to intravaginal delivery will be discussed here [86, 178–181]. Generally, NPs have a diameter less than 1,000 nm, typically ranging from 10 to 100 nm. This size range enables NPs to penetrate tissues, and be readily available for cell uptake [19].
Polymer NPs are made from synthetic [e.g. polycaprolactone, poly(l-lactide), poly(glycolide)] or natural polymers including chitosan, gelatin, albumin, or DNA [179]. Often, biodegradable NPs offer the advantages of being reabsorbed and broken down by the body, making them a lower toxicity option, relative to non-degradable polymers. One biodegradable polymer, poly (lactic-co-glycolic acid) (PLGA) is one of the most widely accepted and FDA-approved biodegradable polymers [19, 182, 183]. PLGA NPs encapsulate agents for delivery and provide a platform to deliver a variety of agents: hydrophobic/hydrophilic drugs, genetic agents and peptides/proteins, while providing sustained release of the encapsulated agent [183]. Further, in comparison to most genetic delivery reagents—the most common vectors being cationic lipids that are cytotoxic, unstable, or elicit immune activation—by encapsulating active agents, PLGA NPs decrease immune response, shield agents from degradation, enable a greater biological half-life of the delivered agent, increase blood circulation time, and are non-toxic—even at NP concentrations as high as 10 mg/mL [57]. In addition, NP surfaces may be modified to include targeting moieties for cell and virus or stealth properties to penetrate the vaginal mucosa and enhance adhesion to epithelial tissue or targeted cell types [107, 115, 116]. More details on the use of NPs in microbicide applications are discussed below.
Stages of intravaginal microbicide development
Regardless of the physical delivery vehicle or dosage form used to deliver the API, microbicide mechanism of action is typically classified as non-specific or specific. Recently microbicides have been designed to offer more specific delivery and effect to targets of interest. Figure 2 depicts some of the mechanisms used by microbicides to interfere with virus infection, including both non-specific and specific means. While the focus of this review is on the delivery of proteins and oligonucleotides, a brief overview of the evolution of microbicides is provided below.
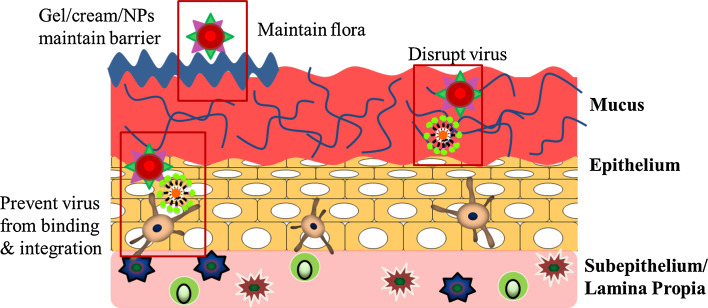
Fig. 2.
Schematic of intravaginal cross-section, denoting microbicide mechanism of action. Adapted in part, and redrawn from [48]. Microbicides have traditionally acted as a physical barrier, in the form of a cream or gel. They can act at the first step of virus contact with the mucus barrier by non-specifically or specifically disrupting the virus membrane prior to attachment by blocking/binding via polyanionic interactions. However, this has often resulted in inflammation, and has failed to provide complete protection. Microbicides must also maintain the normal flora and pH of the vaginal tract, which acts as an innate defense against pathogen and sperm. More recently, second generation microbicides have been designed to more specifically impair virus binding and entry to cells through the incorporation of antivirals, with the most recent generation focusing on the incorporation of proteins and oligonucleotides to protect and treat against infection
First generation
As mentioned above, the earliest microbicide candidates were highly sulfated molecules and detergents that prevented virus attachment or inactivated the virus by disrupting the virus membrane [48]. First generation microbicide products consisted of semi-solid gels including: BufferGel, ACIDFORM, PRO 2000, Carraguard, cellulose sulfate (Ushercell), and Saavy (C31) [47]. These microbicides rely on non-specific charge based interactions and/or buffering of the vaginal flora as their mechanism of action against virus infection. For example, BufferGel and ACIDFORM are based on polyacidic buffers that maintain the vaginal environment at a pH of 4–5, thereby acting as defense agents against virus and sperm. PRO 2000, Carraguard, and cellulose sulfate (Ushercell) are polyanionic polymers designed to inhibit virus entry and fusion by interfering with the virus membrane. It is believed they mimic the charged interaction with the cell, thereby binding to the viral envelope and masking glycoproteins or other structures necessary for cell surface attachment and entry [48]. While PRO 2000 is a synthetic naphthalene sulfonic acid polymer viral entry inhibitor; Carraguard is derived from carrageenan, a natural sulfated polysaccharide from seaweed. In comparison, Saavy combines two different surfactants that act as detergents to disrupt the viral membrane [124]. However, of these first generation microbicides, only PRO 2000 continued to a phase 3 trial evaluating its ability to mitigate HIV infection in women. None have proven to be clinically successful in preventing exposure to viral infection relative to placebo controls.
Second generation: NRTIs and NNRTIs
Second generation microbicides are primarily based on NRTIs and NNRTIs that seek to inhibit viral reverse transcription. Three primary second generation candidates are being or have been evaluated as drugs in solid dose forms, fast dissolve tablets, and IVRs [47]. Included in these is a 1 % gel of TFV,9-[(R)-9-(2-phophonylmethoxyprophyl)propyl] adenine monohydrate or PMPA, a derivative of the NRTI TFV, that is most advanced in development [124]. It has undergone the CAPRISA 004 trial, demonstrating moderately reduced risk of HIV infection when applied before and after intercourse [158]. Similarly, while it reduced HSV-2 acquisition by 51 %, results comparing dosage forms from the VOICE trial indicated that no method was completely effective in HIV prevention [158]. Optimistically, and as mentioned earlier, in the recent Partner’s PrEP trial, oral Truvada significantly reduced the risk of infection in women by ~73 %, relative to oral TDF alone (62 %). The efficacy of TDF alone protected 68 % of women versus 58 % of men, whereas Truvada provided protection for 62 % women and 83 % men, with correspondingly excellent adherence rates of 97 % [44–46]. As a result, Truvada is currently undergoing trials as a vaginal tablet for those at high risk of acquiring HIV-1.
Another NNRTI under investigation is the vaginal gel dapivirine (TMC120), which has shown inhibitory effects against a variety of HIV-1 isolates [124]. Gel formulations of dapivirine have been shown to prevent vaginal transmission in hu-SCID mice, be nonirritating in rabbits, and reduce in vitro HIV transmission, in contrast to first generation microbicide gels. After in vivo administration, this gel remained in the vagina, suggesting its use for daily administration. However, due to its inherent solubility issues, it has also been formulated into IVRs, and is most currently being evaluated in clinical trials in silicone-based IVRs [19, 124]. Another NNRTI, thiocarboxanilide UC781, which shares some physical properties as dapivirine is being investigated in different formulations as a potential microbicide. In vitro it has been less potent, although still demonstrates favorable inhibition of HIV in culture. Most recently, the carbopol and methylcellulose based suspensions were under investigation. A phase I safety trial demonstrated that UC781 was well-tolerated after once a day administration for 6 days; however, mild and transient urogenital irritation was observed [124].
In addition to second generation microbicides that act through RT inhibition, an antiviral dendrimer microbicide, VivaGel (SPL7013), which is active against CCR5 and CSCR4-tropic HIV-1 infections in vitro, is under development. It is based on an l-lysine dendrimer that has a polyanionic outer surface. Thus, far it has been shown to be safe in humans in phase I trials, and has undergone phase III trials for bacterial vaginosis [184–188]. In addition, other trials including safety, tolerability and pharmacokinetics have been conducted intravaginally [188]. Similarly, a microbicidal agent, glycerol monolaurate (GML) was formulated in a gel, and has been shown to prevent SIV transmission in infected relative to control macaques [189–191].
Despite these developments, non-specific and antiviral-only based microbicides have been unsuccessful at inhibiting virus infection in clinical trials. Instead, they have often increased vaginal irritation, resulting in the heightened potential for virus infection. These observations have prompted other approaches to microbicide design that target specific cell/virus moieties or induce a targeted cellular response after their administration.
Second generation: antibodies, antigens, and nucleic acids
To achieve more targeted prevention and therapy against viral infection, biopharmaceuticals such as proteins and nucleic acids are being developed as microbicidal candidates. In addition to their specificity for diverse targets, they may be delivered alone or incorporated into delivery systems to provide sustained, non-coital delivery options. As mentioned before, mucus is the first physical barrier of defense against bacterial and viral infections, protecting underlying tissue layers comprised of macrophages, dendritic cells and lymph. As such, delivery to mucosal tissue can elicit an immune response by administering Abs against a given pathogen or by delivering an antigen to stimulate Ab production [168]. The host immune system responds to incoming pathogens by producing high antigen-specific concentrations of Ab to neutralize the incoming pathogen, while recruiting immune cells to eliminate the pathogen and infected cells [168]. The ability to develop microbicides that mimic innate response, by incorporating antibodies, antigens, or oligonucleotides may enable us to specifically target a variety of host or viral factors involved in STIs. While we elaborate on specific biological options later, here we broadly introduce the microbicide advancements to more specifically deliver biological agents against STIs.
Antibodies
Passive immunization is defined as the administration of Abs to a host to neutralize a pathogen [192]. Monoclonal antibodies (mAbs) are molecules synthesized by the immune system to intercept and bind to foreign substances, thereby helping to protect the body against foreign pathogens [4, 193]. With previous success in other prophylactic and therapeutic applications, mAbs are promising options for multipurpose microbicides, due to their high specificity, potency and safety [56]. The development of a topical microbicidal gel, containing human mAbs to protect the intravaginal tract (epithelium and mucosa) in addition to genital skin, by passive immunization is a long-term goal. Furthermore, the ability to deliver locally to mucosal tissue can help to immediately protect against multiple acts of intercourse.
The ability to locally administer Abs intravaginally to mimic innate mucus secretions, would provide high concentrations of specific and potent Abs to prevent infection. Monoclonal Abs against sperm antigens, HIV, and other STI pathogens have been topically applied to the vagina to prevent fertilization, inhibit virus transmission, and induce an immune response, thereby mimicking the normal function of the Abs in the mucosal immune system [166]. Current studies suggest that intravaginally delivered mAbs may provide a multipurpose prevention modality against both pregnancy as well as STIs, including HSV and HIV [194–196].
Monoclonal Abs can be specific to a given target and also offer diversity in terms of mechanism of action by promoting virus neutralization, aggregation, inhibition of binding, mucus trapping, and host receptivity [56]. Yet while mAbs are extremely potent agents for disease prevention in the reproductive system, the challenge is delivering them to the appropriate site for a sustained period [167]. Although mAbs can be absorbed into the vaginal epithelium, the dose required for successful protection or treatment may be frequent and large due to their limited half-life in the vagina (~5 h in mice) [197]. Therefore, for vaginal delivery of proteins to become useful, more convenient and durable delivery methods must be considered. Options for Ab delivery are discussed in greater depth in the next section.
siRNA
Short interfering RNA (siRNA) therapeutics are based on the RNA interference (RNAi) pathway that interferes with specific gene expression by binding complementary mRNA. siRNA is a natural endogenous process for gene inhibition and interference, which has demonstrated promise to prevent and treat genetic diseases [198]. siRNA is comprised of RNA duplexes, typically 19–25 nucleotides in length, that induce sequence-specific cleavage of messenger RNA (mRNA) prior to protein translation [199]. Once double stranded siRNA is denatured by helicase protein, both siRNA sense and antisense strands recruit and bind a group of cellular proteins, known as RNA-induced silencing complexes (RISCs). These strands are loaded into the RISC structure and separated, and one strand guides selective cleavage of target mRNA, by complementary base pairing to the antisense sequence, thus interfering with protein expression or viral replication [4, 200].
Both in vitro vaginal cell culture systems and in vivo models have been used to determine siRNA dose and delivery method to achieve virus inhibition and therapeutic efficacy in the context of microbicide applications [31, 57, 65, 67, 69]. Toward translation of these efforts, in vitro and in vivo studies utilizing fluorescently tagged siRNA and siRNA targeted against virus and host targets indicate that siRNAs are absorbed and distributed throughout the vaginal tissue [31, 57, 65, 67, 68]. Although siRNA offers many advantages in terms of specificity and potency against virus infection, a challenge remains to effectively deliver siRNAs in vivo and translate this into clinical application and efficacy. Similar to proteins, siRNA lifetime is transient—on the order of hours to days—and in addition, their negative charge makes them difficult for cells to internalize. Adaptations to make siRNA amenable to intravaginal delivery are expanded upon in the following section.
Intravaginal delivery of protein, oligonucleotide, and peptide-based microbicides
Advantages and disadvantages of biologically-based microbicides
Intravaginal delivery of proteins and oligonucleotides offers a number of advantages relative to delivery of other active agents. Proteins and oligonucleotides have high specificity for their given cell or virus target, resulting in low toxicity and side effects, and low systemic absorption. Additionally, as proteins and genes have the ability to target virus or to protect the host prior to virus entry, this offers a potent strategy to block infection before the virus has adhered to or bound to target cells. In fact, the multi-stage processes of virus binding, glycoprotein conformation rearrangement, virus fusion, and/or endocytosis all provide opportunities for interception during infection. By utilizing specifically tailored agents against the virus or host cell, a variety of specific interactions between virus glycoproteins and cellular receptors or surface molecules can be inhibited.
Furthermore, to provide less transient delivery of protein-based strategies, protein production by bacteria inherent to the vaginal mucosa or viral vectors may also allow for prolonged expression and delivery. Presentation and production of a live or vectored microbicide, administered as either live bacteria or microbiota, offers the potential of prolonged production and release, while averting the limitations of manufacturing and maintaining agent stability [201–205]. Alternatively, in vivo expression with viral vectors topically or intramuscularly administered may produce protein sustainably for months [205–208]. Similarly, to attain more prolonged effects, delivery vehicles that offer sustained-release properties are attractive options for potent, specific, and long-term administration of biologically-based agents.
While the attributes of high specificity, low toxicity, and prolonged effects have potential, there are some challenges that must be considered with protein and oligonucleotide-based microbicides. Immunogenicity of foreign products and/or additives may promote an immune response, especially upon continued exposure [205]. As some proteins are being investigated as potential vaccine strategies, the wrong type of immune response may promote inflammation and other detrimental effects. However, if human or humanized proteins are used, repeated exposure may induce tolerance. In addition, as mentioned earlier, stability is a challenge for biologics. For a microbicide to maintain efficacy in a temporal and coitally-independent manner, activity loss by degradation, denaturation or proteolysis must be considered. For these purposes, delivery vehicles offer viable options to protect these agents during delivery. Additionally, relative to other microbicide options, proteins are more expensive to produce, store, and transport. However, with the advent of plant production, endogenous intramucosal synthesis, and genetically modified bacteria and vectors, more cost-effective options are likely [209–211]. With these attributes and challenges in mind, here we discuss some specific proteins, oligonucleotides, and peptides, used to combat STIs.
Types of biologically based agents
Antibody delivery
Antibodies that exert activity at the mucosal surface are most effective when delivered locally, and provide female controlled, coitally-independent protection [166]. While mAbs enable specific targeting, their duration of protection is relatively transient—on the order of days to weeks [167, 196, 197, 212]. However, the lifetime of a single Ab dose can be increased by incorporating the Ab into a polymeric formulation. Polymeric IVRs, for instance, have been used to deliver steroids to humans [213, 214] and polymer matrices have been shown to release Abs at a controlled rate for up to several years [166, 182]. In addition, polymer micro/NPs can encapsulate proteins and provide sustained release, thereby extending Ab lifetime and the potential for passive immunity [192].
Early work explored the feasibility of intravaginal Ab delivery to provide immunoprotection against STIs and pregnancy [215]. Relative to systemic immunization, localized and sustained Ab delivery generated similar levels of systemic immunization, while providing a user-friendly method of protection. In parallel, IVR devices were shown to enhance the delivery of contraceptive hormones in humans. By combining the delivery of Abs with a polymeric delivery approach, Radomsky et al. [169] demonstrated prolonged delivery of Abs in relevantly and geometrically scaled EVAc vaginal rings for delivery in mice.
Radomsky et al. [169] designed EVAc devices containing bovine serum albumin (BSA) and anti-hCG (human chorionic gonadotropin) Ab and demonstrated protein release in vitro extending 30 days. Further, following in vivo insertion of the Ab-loaded polymer ring, biologically active Abs were detected in mouse vaginas after 30 days, relative to topically applied Ab-only solutions that lasted only 24 h [169, 197]. In addition to providing a continuous supply of Abs to the vaginal mucus, a high concentration of Abs was achieved within the vaginal lumen, but not significantly in other areas of the reproductive tract. These pioneering studies demonstrated proof-of-concept that an Ab-releasing device provides sustained, well-distributed release of Abs throughout the vaginal mucosa in a mouse model [169].
Despite the success of immunizing oral and nasal mucus epithelia to block bacterial and viral infections, topical intravaginal immunization in humans and animals had been largely neglected [216–218]. Whaley et al. demonstrated the first topical intravaginal passive immunization for protection against HSV-2 in mice, while Saltzman et al. evaluated fluorescently labeled Ab diffusion from EVAc polymers in human cervical mucus [108, 195, 214]. From these studies, it was determined that passive immunization could be achieved [214], and diffusion of Abs from EVAc matrices was similar in mucus as in water [108]. This provided proof-of-concept that sustained and distributed Ab delivery, in addition to topical passive immunization could be achieved from a single EVAc polymer device.
While long-term Ab delivery was achieved using EVAc devices and HSV-2 mAbs protected against vaginal transmission [169, 214] the pharmacokinetics of intravaginal Ab administration were incompletely understood. As passive immunoprotection is only effective as long as the Ab concentration is above a threshold level, it was important to assess the kinetics of Ab distribution. To address this concern, Sherwood et al. [197] used a variety of techniques to assess the residence half-life of Abs delivered topically to the mouse vagina. Using radioactive 125I-IgG, anti-HSV-2 IgG2a mAb, or biotinylated IgG, it was discovered that intravaginally administrated IgG solution was eliminated by two processes with different elimination rates. A significant fraction of vaginally applied IgG was eliminated by leakage from the vagina, whereas IgG that penetrated the mucus layer was dependent on mucosal shedding. Of the Ab absorbed to the mouse vaginal lumen, the half-life was determined to be 5 h, similar to that normally observed with IgG in vaginal mucus. However, due to the 20-fold surface area to volume difference between mice and humans, the expected half-life in humans was estimated to be greater than 5 h [197].
Based on these studies that established the residence time and localization of Abs, small EVAc polymer disks containing two Ab preparations were inserted into the mouse vagina [167]. Radiolabeled Anti-CEA (carcinoembryonic antigen) IgG and lactate dehydrogenase C4 (LDH-C4) were used for autoradiographic and biological activity studies, respectively [167]. Blood and vaginal IgG concentrations were high (8–12 μg/mL) for the first 48 h, with a subsequent decrease over the next 5 days, resulting from Ab accumulation in the vaginal secretions. As a result of this high initial concentration, Abs penetrated the epithelium and diffused through interstitial tissue space, reaching systemic circulation with systemic concentration ~1 % of the intravaginal levels. The results of this study indicated that localized, intravaginal delivery with polymer devices could provide immune protection in two different ways. First, the high local Ab concentration at the mucosal surface enables the neutralization of pathogens at entry, while the systemically available Abs (from penetration) can provide protection by neutralizing pathogens in the blood [167].
To determine that vaginal delivery of Abs from EVAc disks was a viable alternative to systemic delivery, systemic Ab distribution and rates of elimination were measured from the lower female reproductive tract during prolonged vaginal delivery [166]. The release rate of Abs from EVAc matrices was characterized by determining the diffusion coefficient of Ab transport through the polymer matrix. For both Abs, the diffusion coefficient was lower relative to their diffusion in water. However, high concentrations of Ab were found in vaginal lavages, blood, and other tissues for as long as 30 days after administration of the Ab-containing disks in mouse vaginas. The half-life of Ab elimination from the vagina remained similar independent of class IgG or IgM, maintaining that mucus shedding is a primary factor in Ab removal from the vaginal tract. Also Ab transport from the vagina to the blood was similar to the rate observed for permeation across the endothelial barriers, with 1–2 % of IgG found in the blood after 24 h [166]. These studies suggested that while the epithelium may be a barrier to Ab diffusion, it is permeable; vaginal delivery enables long-term systemic administration [166].
Antigen delivery
Development of microbicides that induce protective mucosal immunity holds great promise for the prevention of STIs. As part of the common mucosal immune system, the female reproductive tract can generate Abs in intravaginal mucosal secretions to intercept pathogens and spermatozoa [219]. One pathway toward the development of vaccines and microbicides for intravaginal application is to induce the mucosal immune response, or local production of neutralizing Abs, by administering an antigen close or localized to the reproductive tract. In this way, antigen producing cells (APCs), B-cells and T-cells residing in the cervicovaginal tract can aid in generating mucosal immunity after local application of antigens or immune stimulants.
However, as mentioned above, the delivery of proteins and other molecules to the female reproductive tract is plagued with challenges including low residence time resulting from degradative enzymes and mucosal shedding, high immunotolerance, and alterations in the intravaginal environment (mucus and epithelium) and microbiota due to the fluctuating hormonal cycle [168]. Therefore, effective delivery to the reproductive tract requires a vehicle that can protect the antigenic protein or nucleic acid against degradation, increase its local residence time, and enable controlled release ideally from weeks to months.
Just as IVRs have stimulated protection by sustaining the release of Abs against infection, they can also be formulated to release antigen to stimulate Ab-mediated responses. Early studies conducted by Wyatt et al. [220] demonstrated that IVRs that provided a sustained dose of antigen were able to generate a specific vaginal immune response. Local immune response was compared after vaginal delivery of ferritin from EVAc IVRs and antigen soaked tampons, with antigen delivery by IVRs producing higher and more uniform IgG titers than tampon delivery [220]. Furthermore, sustained antigen delivery promoted higher and more consistent ferritin-specific Ab concentrations in the mucus, with IVRs being more convenient and efficient than other methods to provide prolonged delivery with a single application.
The development of vaccines that can elicit both mucosal and systemic immunity offers great promise for initiating a robust immune response against STIs. Furthermore, delivery of DNA vaccines, as an alternative to protein antigen delivery, offers an additional way of generating mucosal immunoprotection. Initial studies by Wang et al. [221] investigated the feasibility of inducing mucosal immunity following mucosal administration of a DNA vaccine. They demonstrated success of a prototype DNA vaccine by locally delivering pDNA coding the HIV-1 envelope (pcMN160) to stimulate mucosal immunity against HIV-1 in mice. Not only was the mucosally delivered pDNA detected in various tissues both locally (fallopian tubes) and systemically (liver), but it also elicited production of vaginal IgGs specific to the HIV-1 envelope, and neutralized HIV-1 infectivity in vitro. These results suggested that intravaginal delivery of nucleic acid vaccines could provide a novel strategy for stimulating mucosal and systemic immunity [221].
Studies comparing vaginal immunization with intranasal and intrarectal pDNA delivery demonstrated that vaginal immunization provides better mucosal immunity, likely due to the presence of APC and T/B-cells [222–224]. While other studies investigated intravaginal DNA vaccination via invasive and transient means [221, 222, 225], to confront the aforementioned challenges of intravaginal delivery, Shen et al. investigated the potential of EVAc matrices delivering pDNA to protect DNA from the harsh intravaginal environment and to induce local mucosal immunity. Plasmid DNA encoding pcDNA3/LDH-C4, a sperm-specific isozyme of lactate dehydrogenase [219], demonstrated similar release properties from the EVAc matrix as Abs, which was surprising as DNA is larger and negatively charged. Gene expression was detected 28 days after administration and correspondingly, immune response was also sustained. IgA was detected in vaginal secretions by 2 weeks and remained present for 56 days, but was not detected in serum [219].
More recently, Kuo-Haller et al. administered an antigen, ovalbumin (OVA), to demonstrate proof-of-concept intravaginal delivery via EVAc polymer disks. OVA EVac disks were delivered to mice and the efficacy of disks, relative to oral or intranasal delivery was assessed [168]. Two different EVAc disk formulations were compared and demonstrated different rates of OVA release, based on the ratio of OVA to Ficoll. While EVAc disks elicited high antibody titers in mucus and serum, higher titers were present in the vaginal washes. This work indicates that a locally controlled immune response can initiate in the mucosa, and that local, intravaginal vaccine delivery using EVAc disks can achieve high Ab response both locally and systemically. And relative to immunization by other mucosal routes including oral or nasal delivery, intravaginal administration produced higher and more sustained Ab levels [168].
Delivery of Abs or antigens via polymeric materials such as EVAc enables both a sustainable source and controlled release of proteins and DNA. Initial burst release profiles may release a high enough local concentration to generate effective protection against infection, while the long-term release of lesser amounts may provide boosts to the immune system for prolonged efficacy. Release kinetics of these matrices can also be tailored as encapsulation of DNA and protein is convenient and relatively easy to incorporate. Moreover, polymer matrices provide protection to proteins and nucleic acids from degradative enzymes and the acidic pH of the vaginal tract. These studies indicate that increased and long-lasting Ab concentrations and Ab titers can be effectively delivered and induced in animals when administered by intravaginal routes.
siRNA delivery
Gene therapy is challenged with how to optimally deliver oligonucleotides, such as DNA and RNA to cells. Since siRNA is versatile and can theoretically target any gene in specific cell populations, an area of intense research is focused on developing more efficacious delivery strategies [199]. Thus far, siRNAs have been noncovalently complexed with a number of molecules and carriers, some of which include: cationic agents, carriers modified with folate or transferrin, peptides, aptamers, cholesterol, and Ab-mediated formulations [69, 199]. In parallel, siRNA has been covalently attached to targeting ligands, lipophilic molecules, short peptides, Abs, agonist molecules, and nucleic acid based aptamers [199].
In the context of STI inhibition and intravaginal delivery, RNAi is considered to have promising therapeutic potential as a microbicide, as siRNA therapy has been shown to decrease expression of both viral and cellular mRNA involved in infection [31, 60, 65, 68]. The following sections discuss advances in research to date using siRNA microbicides to target STIs.
HSV and HIV applications
RNA interference (RNAi)—using exogenous siRNA molecules delivered as lipoplexes with transfection reagent or conjugated to cholesterol—has been shown to down-regulate gene expression of specific viral and host proteins. For HSV-2 specifically, two genes, UL29.2 and nectin-1 play roles in HSV-2 replication and virus binding and spread. After administration of siRNA lipoplexes against UL29.2 and nectin-1, mRNA were down-regulated 7–14 days after intravaginal administration; however, full viral inhibition was not achieved [65, 68]. By conjugating the siRNA to cholesterol to silence gene expression without causing inflammation, the efficacy of siRNA was improved, enabling better uptake and stabilizing the siRNA from degradation [68]. Due to limited methods thus far available to deliver siRNA or oligonucleotides, notable challenges exist with siRNA delivery systems such as lipid transfection reagents or cholesterol conjugated siRNA. Lipid transfection reagents typically exhibit toxicity at high concentrations, provide transient knockdown, and so far have not incorporated targeting moieties to increase intravaginal tissue adhesion, uptake, or mucosal penetration [57, 68]. In comparison, cholesterol conjugation reduces inflammation and toxicity, while demonstrating transfection efficacy in epithelial cells. However, cholesterol-conjugated molecules may have difficulty decreasing gene expression particularly in immune cells [66, 68]. Therefore, the efficacy of cholesterol conjugation needs to be determined against targets of immune cell infecting viruses like HIV.
With the success of Palliser et al. demonstrating protection against HSV-2 in mice using siRNA against UL29.2 and nectin-1, Zhang et al. [226] demonstrated the durability and specificity of mucosal RNAi in a murine model. They used siRNA lipoplexes to evaluate and improve distribution through the vaginal epithelial layer and submucosa. After establishing that siRNA lipoplexes localized in the epithelial layer and submucosal cells, they determined the durability and extent of lamin A/C knockdown using siRNA lipoplexes. Maximum gene knockdown was achieved on day 4, but reduction in lamin A/C mRNA was observed until day 7, with expression returning to pretreatment levels by day 10. While confocal microscopy determined siRNA penetration into the vaginal submucosa, they evaluated lipoplex penetration of CD4 cells such as lymphocytes and macrophages with an siRNA designed against the chemokine receptor CCR5. After administering CCR5-targeted lipoplexes intravaginally, a decrease in mRNA expression was observed over 10 days, with up to 74 % knockdown occurring on day 10 [226].
In addition to providing a nontoxic alternative to other transfection reagents, encapsulation of siRNA in PLGA NPs provides sustained release, encapsulation of many molecules, and the ability to easily modify the drug delivery vehicle for enhanced delivery. In work by Woodrow et al. [57], siRNA was encapsulated into biodegradable PLGA NPs and administered intravaginally. In vitro studies demonstrated dose and cell-dependent gene silencing after administration. Subsequent in vivo studies tested the efficacy in promoting gene silencing after vaginal instillation targeted against enhanced green fluorescent protein (EGFP) in transgenic mice. After one intravaginal siRNA NP application, potent and prolonged gene silencing resulted throughout the reproductive tract for 14 days [57]. In addition, the PLGA siRNA NPs were less inflammatory relative to siRNA lipoplexes, while producing significant levels of gene knockdown [57].
Using a lipoplex system incorporated in a biodegradable alginate scaffold, Wu et al. [67] complexed siRNA with PEGylated lipoplexes to demonstrate better mucosal penetration and prolonged delivery. Due to the concern of liquid PEGylated lipoplexes leaking from the vaginal cavity after administration, lipoplexes were entrapped in a solid, negatively charged alginate scaffold. After intravaginal administration of the alginate–lipoplex scaffold, nucleic acid delivery into vaginal tissues was significantly enhanced compared to delivery of conventional lipoplexes. Furthermore, this alginate–lipoplex system exhibited 85 % siRNA-mediated knockdown of Lamin A/C, in vaginal tissues after two applications [67]. This hybrid system presents a novel approach to attain prolonged and efficacious delivery of nucleic acids from a solid scaffold in the vaginal cavity.
More recently, we showed that intravaginally applied siRNA PLGA NPs, that encapsulate siRNA against nectin-1, a host cell target necessary to HSV-2 entry, decreases gene expression and improves survival after a lethal HSV-2 infection in mice [31]. PLGA NPs encapsulating siRNA molecules targeted against either UL29.2 or nectin-1 were intravaginally delivered to mice infected with HSV-2. Prolonged in vivo survival was observed in HSV-2 infected mice, with survival out to 28 days after intravaginal administration of si-Nectin PLGA NPs. This study highlights the potential of PLGA siRNA NPs as a potential microbicide for HSV-2 prevention and/or treatment [31].
In addition to providing protection and/or treatment against HSV-2 and uptake in epithelial and immune cells involved in STI, previous work has demonstrated that RNAi can inhibit HIV infection in vitro, and that siRNA targeting conserved sequences in HIV or against cellular receptors and co-receptors can inhibit viruses of different clades [66]. HIV-specific microbicide targets include virus envelope glycoproteins, gp120 and gp41, necessary for infection. These glycoproteins interact with CD4 receptors and subsequently with co-receptors CCR5 and CXCR4 on host cells [48, 227]. Therefore, HIV inhibition approaches have involved interfering with these virus glycoproteins or receptor targets involved in virus-cell fusion, or targeting reverse transcriptase, integrase, or protease after virus fusion and genome release into the cell, to disrupt the virus cycle [48, 227]. Since current anti-HIV products induce variations in virus progeny that allow virus evolution, targeting host genes or proteins might provide better targeting options—assuming they do not adversely affect host function.
Delivery of siRNA to treat HIV is in the early stages of development, but has had promising successes both in vitro and in vivo. Some of the initial siRNA sequences shown to inhibit viral gene expression were designed against Rev, Nef, and Tat—genes involved in HIV protein synthesis, replication, and reverse transcription [228–230]. siRNA against cellular genes considered necessary for infection have also been investigated, including siRNA against CXCR4 and CCR5 to reduce cell surface protein expression. Gene silencing in cell culture was observed 48 h after transfection and receptor expression was reduced by over 50 %, correlating to inhibition of HIV-1 infection in receptor-positive cells [231, 232]. In addition, shRNA lentivirus vectors successfully inhibited the attachment of HIV-1 gp120 to DC-SIGN, a dendritic cell (DC) surface protein, while inhibiting virus transfer to target cells [233].
The ability to target free siRNA against CCR5 to T-cells in an animal model was shown by Kumar et al. To translate these studies to clinical treatment conditions, mice were infused with peripheral blood cells from an HIV-infected patient on antiviral therapy with an undetectable viral load. CCR5-silencing siRNA targeted with anti-CD7 Abs maintained viral load at an undetectable level and preserved CD4 blood cell levels relative to mice treated with a non-specific sequence [234].
Since there are many advantages to encapsulation of molecules for gene delivery, lipid and polymeric NPs have been explored to protect siRNA from enzymatic degradation and the acidic pH of the vagina. Furthermore, surface modifications to the delivery vehicle can improve uptake in and targeting to cells. Specifically for immune cell uptake, encapsulating molecules in NPs was shown to more readily induce phagocytic uptake. In a humanized mouse study, siRNA targeting CCR5 was encapsulated in liposome particles presenting integrin targeting sequences [62]. NPs were surface modified with a lymphocyte function-associated antigen-1 (LFA-1) integrin Ab and infused with an anti-CCR5 siRNA, enabling competition for and decreased virus binding to CCR5. Mice receiving inoculations with these NPs were resistant to HIV-1, as demonstrated by a decrease in viral load and stabilization of CD4 cell counts [62].
In another study, human female reproductive tract explants from the endometrium, endocervix and ectocervix were obtained from HIV-1 seronegative women. NPs with CD4 and CCR5-specific siRNA were administered, the tissue was subsequently challenged with HIV-1, and HIV-1 infection was inhibited. To additionally demonstrate successful mucosal penetration, NPs containing murine CD4 siRNA were instilled into the reproductive tract. Expression of CD4 decreased in uterine tissues 3 days after a single application [59].
While these delivery and targeting measures proved successful, production of Abs and Ab fusion proteins for conjugation to siRNA are still deemed expensive, may be immunogenic, and likely necessitate refrigeration in resource poor environments. Furthermore, conjugation of siRNA to molecules such as cholesterol proved ineffective for transfection of immune cell populations typically infected by HIV [66, 68]. Chimeric siRNA in comparison, consisting of an siRNA fused to an aptamer, may provide a feasible alternative to achieve in vivo knockdown. Aptamers or aptamer–siRNA chimeras (AsiCs) have provided gene transfection in cells with receptors specific to that aptamer. Previous work using AsiCs specific to gp120, inhibited HIV replication in already infected cells both in vitro and in vivo [66]. AsiCs targeting the host CD4 receptor (CD4-AsiCs) with siRNAs targeting viral genes, gag or vif, or host CCR5 were internalized by CD4 cells and decreased expression of their target genes. Moreover, they inhibited HIV infection in primary CD4 T-cells and macrophages in vitro, and in cervicovaginal explants and immunodeficient (BLT) mice [66]. While the CD4 aptamer alone moderately inhibited virus infection, more significant prevention was achieved in explants and BLT mice with chimeric RNAs [66].
Other protein and peptide-based solutions
Proteins
In addition to protein-based solutions such as Abs and antigens mentioned previously that stimulate an immune response, other protein alternatives are being investigated as microbicides to circumvent STIs. Recently, a NP platform delivering PSC-RANTES, a CCR5 chemokine inhibitor, was investigated for protection against HIV [235]. PSC-RANTES is an amino terminus-modified analog of the chemokine RANTES, a ligand for HIV co-receptor CCR5. Relative to unencapsulated PSC-RANTES the NP formulation exhibited comparable anti-HIV activity. However, in an ex vivo cervical tissue model, encapsulated PSC-RANTES improved tissue penetration, while increasing internalization by fivefold, and exhibited greater distribution in the epithelium relative to unencapsulated PSC-RANTES [235]. Thus, far the release of PSC-RANTES from NPs enables bioactivity against HIV comparable to unencapsulated formulations, in addition to maintaining protein stability in, and increased permeability to tissues. PSC-RANTES has also provided protection against vaginal challenge in rhesus macaques by inhibiting the CCR5 co-receptor [86, 235, 236].
As mentioned above, mAbs offer options to inhibit the various stages of viral attachment and fusion to host cells. Some additional antibody studies against HIV-1 and HSV-2 are summarized in the following references [237–239]. A few examples include TMB-355, a mAb specific for CD4 that has inhibited interactions with the HIV envelope, but maintained the ability of CD4 to interact with MHC class II molecules. Currently, in development for systemic administration, this may offer an option for topical delivery. However, clinical resistance in the form of increased viral loads has been observed after missed doses during clinical trials [240]. Recently, a microbicide incorporating three viral envelope binding Abs (MAB-GEL) against HIV entered early phase clinical trials [238, 241]. Initial results indicated that the formulation was safe and that Ab concentrations were sufficient to block viral transmission for hours [241]. With regard to protein-based solutions for HSV-2, single mAbs have also been used to intercept viruses via attachment to viral glycoproteins (such as gD) or host receptors such as nectin-1 [239]. However, the manufacturing of biological therapeutics is still expensive relative to synthesis of chemical agents [240]. Still, mAbs may satisfy several important niches in STI treatment and prevention, in that they may be used for pre-exposure prophylaxis in addition to post-exposure treatment of chronic infection. Especially for these applications where synergistic activity would be beneficial, other treatment formulations may be supplemented with Abs to create efficacious and infrequent dosing regimens. For a more comprehensive review on Abs as microbicides please see McCoy et al. [237] and Brinckmann et al. [241].
While many prevention strategies rely on Ab production or delivery to induce an immune response, vaccines that rely on T-cells have been difficult to develop [242]. In fact, a better understanding of mucosal immune response is required to develop an effective prevention strategy. One recently demonstrated approach stimulated the production of local chemokines that signal immune cells to respond to infection [243]. Shin et al. [242] have recently achieved success delivering chemokines CXCL9 and CXCL10 intravaginally using a “prime and pull” strategy. After conventional vaccination priming the T-cell population, a single topical intravaginal treatment with chemokines (pull) provided protection against HSV-2, with a survival rate of 100 % in mice 10–12 weeks post-pull. The “prime and pull” method decreased HSV-2 spread from the mucosa epithelia to the neurons, revealing a promising strategy for local vaccination against HSV-2 and other STIs [242].
Similar to receptor proteins expressed on immune and epithelial cells, DCs express carbohydrate-binding protein residues such as lectins that bind mannose or other sugars on the virus envelope, thereby enhancing virus transfer efficacy. Mannan, a mannose-rich oligosaccharide, and Abs specific for DC-SIGN inhibited HIV uptake in vitro, but were unable to protect macaques after SHIV challenge by topical application [48, 244]. However, integrating this idea with a multivalent approach, glycodendrimers were synthesized with multiple Lewis-type antigen glycans on the surface. These glycodendrimers prevented HIV transmission by DC-SIGN on DCs, thereby providing a potential alternative for topical application [245].
Another promising candidate, Griffithsin (GRFT), is a lectin that binds clusters of oligomannose N-linked high-mannose oligosaccharide glycans on HIV-1 envelope glycoprotein gp120 to inhibit viral entry [246]. The oligomannose glycans it targets are more prevalent on viral envelope glycoproteins than host cell glycomes, and therefore lend versatility to GRFT as a broad spectrum antiviral. In early trials GRFT has showed synergy with TFV and other antivirals against both HIV and HSV, while provoking negligible immune response. In fact, combinations of GRFT with TFV, maraviroc, and ENF increased antiviral potency against HIV-1 [247]. Additionally, GRFT prevented HSV infection post-entry by inhibiting cell-to-cell spread and successfully protecting mice from genital herpes [246]. Furthermore, when combined with carbohydrate binding agents, GRFT showed synergistic activity against strains of HIV-1 and HIV-2 [248], and also mitigated HCV infection [249]. Most recently, after subcutaneous administration of GRFT in two rodent models, GRFT distributed systemically, achieved antiviral activity against HIV-1 pseudovirus, and accumulated to relevant therapeutic concentrations resulting in minimal toxicity [250].
Peptides
Generally, peptide microbicides have been classified into one of two inhibitory classes: peptides that seek to inhibit STIs via the viral envelope or via host cell receptors.
Virus-targeted peptides against HIV: One class of peptides against HIV infection has focused on targeting the HIV-1 gp41 envelope subunit. Two peptides in this class, T-20 (ENF) and T1249, have been used to combat HIV infection. ENF, the first HIV-1 entry inhibitor to be approved, is a 36 amino acid peptide that targets and binds to the first helical region of gp41. ENF inhibits activation prior to fusion-enabling conformation changes in this envelope glycoprotein [251, 252]. Similarly, a second candidate peptide targeting viral gp41, T-1249, is a 39 amino acid peptide that contains a pocket binding domain of gp41 and inhibits viruses resistant to ENF [253, 254]. While poor bioavailability, relative to ENF impeded further progress, a next-generation C-peptide-based fusion inhibitor, sifuvirtide, has demonstrated improved efficacy over ENF [255, 256].
In similar light, other peptides can be used to bind to HIV-1 binding pockets that are exposed during entry. In this class, d-amino acid peptides have been synthesized, that bind to the N-trimer pocket of HIV-1 gp41, exposed during entry [257]. These peptides’ resistance to proteases, increased serum half-life, systemic absorption after oral administration, and blocking ability against R5 isolates, suggest their potency as a candidate microbicide [258–260]. Additionally, other peptides have been identified to target a variety of regions on the virus. In the design of a recombinant molecule, 5-helix, a promising entry inhibitor, the C-terminal region of gp41 has been identified as a target for HIV-1 entry inhibition. The peptide is comprised of the residues that make contact with the 5-helix protein, but eliminates the fusion core of the sequence. Additionally, another entry receptor for HIV-1, CCR5, is responsible for binding to gp120, and CCR5-mimic peptides targeting this interaction have resulted in relatively low antiviral activity [261, 262].
Receptor-targeted peptides against HIV: The CD4 receptor and co-receptor CCR5 are primary targets for both prophylactic and therapeutic microbicides. Generally co-receptors, CCR5 and CXCR4 are considered the primary clinically relevant candidates, due to their initial binding interaction with gp120 [256, 263]. However, a caution exists in blocking these receptors, due to their contribution in immune functions, including the immunosuppressive effect or receptor-agonist effect that could result in genital inflammation. Candidates in this category must be tested to determine if they elicit such effects in the vaginal mucosa [256].
Similarly, chemokine-derived peptides have been synthesized to include structural motifs of antiviral activity, namely the N-loop and B1-strand [264]. These peptides have been designed to bind to CCR5, much like the RANTES chemokines do. Using these peptides, receptor blocking has been attained without ill CCR5 effects, demonstrating that in fact, structural refinement in design may provide specific and potent candidate microbicides.
An additional receptor, integrin α4β7, has been identified, that may facilitate HIV transmission by interacting with gp120. This integrin has been a candidate in aiding viral passage to activated α4β7 CD4+ T-cells, that highly express CCR5 [265]. Blocking this integrin in macaque studies during acute SIV infection decreased plasma and gastrointestinal tissue viral loads, in addition to binding gp120 [266, 267]. Therefore, α4β7 inhibitory peptides or mAbs, might be viable microbicide candidates for these less commonly highlighted receptors involved in HIV infection.
Peptides against HSV: As mentioned above, one of the first steps in HSV entry is virus binding to HS chains of cell surface HSPGs [96]. In fact, studies have demonstrated that alterations in HS can provide a receptor to gD [99], and that both HS, and its modified 3-O-sulfated HS isoform (3-OS HS), have been shown to regulate infection in vitro [268]. Therefore, peptides that target cell surface HS, can provide innovative strategies to inhibit virus attachment. Some peptides including: human apolipoprotein E-derived peptide, rabbit neutrophil peptide-1, lactoferrin, indolicidin (a tryptophan-rich peptide from bovine neutrophils), and brevinin-1 (a peptide found in frog skin) are peptide candidates to inhibit HSV infection [269–274].
Recently, Tiwari et al. [268] used phage display to screen dodecapeptides that bind specifically to HS and 3-OS HS to prevent HSV entry. Two different groups of anti-HS peptides were identified in the screening process that had high cationic charge densities. The groups were distinguished by either having alternating or repetitive charges, referred to as group 1 (G1, LRSRTKIIRIRH) and group 2 (G2, MPRRRRIRRRQK) peptides, respectively. Both G1 (against HS) and G2 peptides potently inhibited HSV-1 entry into gD-expressing cells in vitro, with G2 (against 3-OS HS), showing more diverse ability to inhibit a variety of herpes virus strains. Furthermore, both peptides demonstrated HSV-1 inhibition in a mouse model of infection. In addition to elucidating the role of positively charged amino acids like lysine and arginine on HSV-1 activity, these discoveries indicate that peptides in these classes can be as potent anti-HSV agents [268].
Antimicrobial peptides: Another category of peptides possess broad antimicrobial properties and are part of the first line of innate immune defense against infection [205, 275]. In fact, many groups of natural host defense peptides are included in this group [276]. These peptides have been proven to have antimicrobial effects on a broad range of bacteria, viruses, fungi, and parasites [277]. Typically, host defense peptides are produced by epithelial and immune cells, and are found in mucosal fluids, where their antiviral properties, as relevant to this review, have been shown to inhibit enveloped viruses. Antimicrobial peptides such as cathelicidins and defensins, or small cysteine-rich host defense peptides, have been shown to inhibit HIV-1 infection by inactivating the virus directly, blocking cell receptor-virus glycoprotein interactions, and decreasing the expression of co-receptors necessary for virus entry [278–283].
As a result, synthetic peptides have been designed and developed to mimic natural host response molecules, and to enhance antiviral response. These antimicrobial peptides are designed based on structurally-derived microbe and host-derived analogues [284–287]. In particular, antimicrobial peptides against HSV have shown that many synthetic peptides, including beta-defensins and others have inhibited HSV in vitro [275, 288–291]. Similarly, four synthetic mimics of bovine bactenecin dodecapeptide reduced HSV-2 infection in vitro by inhibiting virus attachment and entry, and binding to HS, while demonstrating in vivo inhibition of HSV-2 in mice [275].
Similar to the lectins mentioned above, many of the inhibitory properties against HIV-1 are attributed to a lectin-like function which enables ubiquitous binding to a range of glycosylated virus envelopes and cellular surfaces. To target the HIV envelope, other retrocyclin peptides have been made to reduce infection in immune cells [292]. To target the virus glycan shield, recombinant, biologically active retrocyclin peptides were synthesized and shown to bind to HIV-1 gp120 and to significantly reduce HIV-1 infection in PBMC and PM1 cells [293, 294]. Similarly, using antimicrobial peptides led to the discovery of a GLRC peptides (named due to their amino acid content of G, L, R, and C residues), that possess stability and anti-HIV activity [277].
Future directions and challenges
The overall goal of microbicide development is to create an efficacious, convenient, and safe platform to protect against multiple STIs. However, previous generations of microbicides have encountered challenges in obtaining efficacious, and in some cases safe delivery to the vaginal tract. While early generations have provided some promising launching points for new generations of microbicides and intravaginal drug delivery vehicles (e.g. IVRs) [7, 118, 295], other options such as surfactants have highlighted the crucial need to establish a safe and biocompatible modality [51, 138, 184]. Some emerging considerations to improve microbicide design include: more specifically targeted biological carriers, carriers that prolong administration and effect of active agents, multipurpose microbicides for both contraception and infection, combinations of traditional antivirals with new biologicals, and the utilization of nanotechnology to achieve better delivery.
Selection of biologicals for delivery
Prior to the consideration of delivery vehicle is the selection of target. As discussed here, there are many options available for protein and oligonucleotide targets. As part of this selection, consideration will need to be given to those agents that are low-cost, easily manufactured, non-inflammatory, and potent. While Table 1 illustrates intravaginal microbicides that are currently in clinical trials or those that show promise for future clinical trials, most of these are based on antivirals. As discussed in this review, there are many biologics being tested, which is broadening the scope of microbicides to provide not only agents that work against various stages of the infection cycle, but those that do so using different molecular mechanisms. As some of these recently identified protein, peptide, and oligonucleotide candidates are evaluated preclinically, the microbicide trend will likely shift to include a mixture of biologics and antivirals, or just biologics alone.
Targeted delivery
Topically applied microbicides must traverse the mucosa and enhance penetration and delivery to protect epithelial or immune cells. By specifically targeting protein and oligonucleotide uptake to these cell types integral to virus infection, more efficient and localized delivery will be achieved. For nonviral gene delivery in particular, targeting proves beneficial to minimize immunostimulatory effects, reduce off-target effects, and to obtain cytosolic release for more efficacious transfection [296].
There are many new options available to improve the delivery of Abs and nucleic acids, including cell penetrating peptides, endosomal escape peptides, and aptamers that target delivery and increase cell uptake through a variety of mechanisms [199, 297–300]. Enhancement of intravaginal gene and protein delivery has been demonstrated thus far by cholesterol conjugation to improve stability, and by peptides or aptamers to increase gene transfection [66, 68, 301–303]. Moreover, using modifiable drug delivery platforms such as NPs also provides ease of functionalization with ligands to target HIV or HSV-specific cell types, while encapsulating cargo to deliver a higher and more localized dose to the desired site [31, 57, 58, 304]. In addition, specifically delivering a combination of genes or proteins for both host and viral markers will provide more versatile options to combat multiple pathways and stages of infection [65, 68].
Multipurpose microbicides
While the concepts of multivalent vaccines or multiple antiviral treatments such as HAART are widely accepted to target mutating viruses, the microbicide field has traditionally investigated monotherapies to minimize infection [305]. In contrast, combinational therapeutics that have multiple treatment targets—in or on one delivery vehicle—and modulate interactions through multiple biological mechanisms (gene/protein targets, inhibition of virus binding/replication), provide promising routes for exploration [31, 65, 68]. Co-delivery of biologicals with antivirals would also be beneficial against rapidly mutating viruses and their corresponding evolved targets. Recently Boutimah et al. [306] investigated different combinations of antivirals with RNAi-mediated suppression of virus/cell factors, allowing for the possibility of interrupting a variety of stages during virus replication cycle.
Versatile formulations that are effective against multiple STIs are also increasingly desirable. As HSV-2 infections are correlated with an increased risk of HIV-1 acquisition, treatment of HSV-2 or other STIs will likely have an impact on HIV-1 transmission [47, 53]. HSV encoded proteins have been found to promote transcription of HIV-1 in co-infected cells. Furthermore, both symptomatic and asymptomatic HSV-2 reactivation is associated with increased HIV-1 levels in the blood and genital tract. Therefore, alternatives aimed at simultaneously reducing HIV-1 and HSV-2 infection are under development [47, 53].
Perhaps more ideally, dual protection technologies that serve as both a microbicide and contraceptive are appealing prospects, due to their potential of delivering a convenient two-in-one formulation. The option of combining different drug delivery formulations, such as gels, that enable control over frequency of administration, with long-term release devices such as IVRs that reduce dosing, cost, and are coitally-independent is also highly desirable [47, 53]. In addition, intravaginal applications of new material options (in intravaginal applications) such as nanofibers may offer a new approach to deliver a variety of prophylactic, therapeutic, and contraceptive agents [307–309]. Ideally, one platform could simultaneously inhibit STIs while offering contraceptive protection. To attain this goal however, formulation considerations will be important to successfully incorporate and release combinations of different types of agents.
Another important component to microbicide design and intravaginal delivery is the role of immune response in infection. As new discoveries are made that better explain the immune response and its correlation to protection, different modalities may be combined to take advantage of this knowledge. Recently, immune response was tracked in an infected patient, and the question emerged of how to create immunogens or broadly neutralizing antibodies to emulate natural production. As a diverse mixture of agents will likely be required to evoke the desired protective response, understanding and combining multiple immunogens will undoubtedly be beneficial [310].
Utilizing nanotechnology
The overall goals of drug delivery are to enhance efficacy, minimize toxicity, enhance residence time, and provide sustained release with corresponding prolonged preventative or therapeutic effect [171, 311]. In parallel with these aims, major thrusts in microbicide development include increasing user adherence by providing infrequent application doses, convenient protection, and avoiding an adverse immune response after topical application.
With these goals in mind, delivery of Abs, proteins, and nucleotides, can be improved by utilizing nanotechnology. For topical intravaginal delivery this will necessitate navigating mucous barriers and penetrating epithelial cells (tight junctions, etc.), depending on the target. As discussed, lipid, polymer, or macromolecular systems have been explored for many applications to provide these attributes. Thus far, NPs have emerged as versatile carriers that can overcome physical barriers and guide cargo intravaginally [31, 57, 115]. In addition, a carrier that can adapt to the intravaginal environment, and is stimuli sensitive would provide a versatile and “smart” intravaginal delivery platform [312, 313].
Especially in the harsh vaginal environment, delivery of genetic agents and proteins has its challenges. However, drug delivery vehicles enable encapsulation of active agents, uptake of poorly permeable molecules, navigation through the mucus, increased stability and residence time, and sustained release to the mucosa [86]. By increasing the biological half-life of these agents through encapsulation, durability and longevity of protection will be enhanced, while providing the associated clinical benefits of increased user compliance and decreased cost [27]. Due to the low-to-moderate compliance observed in some clinical trials [314], designing delivery systems that offer more effective, robust, and prolonged administration of antibodies, proteins, and oligonucleotides is highly desirable.
Conclusions
Applying the knowledge obtained from microbicide studies thus far, the focus of the next generation of microbicides is to deliver agents more safely and efficaciously. With the identification of new biological targets and delivery platforms, a multitude of options exist that offer specific and unique modalities to interfere with multiple pathways and targets of infection. An emerging research goal has been to establish microbicide candidates that rely on proteins and oligonucleotides to prevent and/or treat STIs, in order to induce a specific and safe preventative or therapeutic effect. By combining proteins and oligonucleotides with carriers that enhance their delivery efficacy intravaginally, this new generation will provide versatile and promising solutions to inhibit a broad spectrum of STIs.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Disease (NIAID) F32 Postdoctoral Fellowship (NIH, NIAID F32-AI093056). The author greatly thanks Dr. W. Mark Saltzman for this opportunity to review the field, for the fortunate experience to be trained in his group, and for his helpful suggestions on the manuscript. The author also thanks Nayeem Moulana for his artistic contribution to Figure 2.
Abbreviations
- Ab
Antibody
- ACV
Acyclovir
- AIDS
Acquired immunodeficiency syndrome
- API
Active pharmaceutical ingredient
- ARV
Antiretroviral
- AsiCs
Aptamer–siRNA chimeras
- BSA
Bovine serum albumin
- CAP
Cellulose acetate phthalate
- CCR
Chemokine receptor
- CD4
Cluster of differentiation four
- CD4+/−
Cluster of differentiation four positive or negative
- CEA
Carcinoembryonic antigen
- CMIS
Common mucosal immune system
- CXCR4
Chemokine (C-X-C motif) receptor four
- DC
Dendritic cell
- DC-SIGN(R)
Dendritic cell-specific-ICAM-grabbing non-integrin (receptor)
- ENF
Enfuvirtide
- EVAc
Ethylene-co-vinyl acetate
- FcRN
Fc neonatal receptor
- FTC
Emtricitabine
- gP
Glycoprotein
- GRFT
Griffithsin
- HAART
Highly active antiretroviral treatment
- hCG
Human chorionic gonadotropin
- HIV
Human immunodeficiency virus
- HSPG
Heparan sulfate proteoglycan
- HSV
Herpes simplex virus
- ICAM
Intercellular adhesion molecule
- IVR
Intravaginal ring
- LDH-C4
Lactate dehydrogenase C4
- LFA
Lymphocyte function-associated antigen
- mAb
Monoclonal antibody
- MZC
MIV-150, zinc acetate, and carrageenan
- N9
Nonoxynol-9
- NP
Nanoparticle
- NRTI
Nucleoside reverse transcriptase inhibitor
- NNRTI
Non-NRTIs
- OVA
Ovalbumin
- pDNA
Plasmid DNA
- PI
Protease inhibitor
- PLGA
Poly(lactic-co-glycolic acid)
- PrEP
Pre-exposure prophylaxis
- PSS
Polystyrene sulfonate
- PU
Polyurethane
- RNAi
RNA interference
- siRNA
Short interfering ribonucleic acid
- STD
Sexually transmitted disease
- STI
Sexually transmitted infection
- TDF
Tenofovir disoproxil fumarate
- TFV
Tenofovir
- VCF
Vaginal contraceptive film
References
- 1.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. Aaps J. 2009;11:78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988–1994. J Infect Dis. 2002;185:1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- 4.Ndesendo VM, Pillay V, Choonara YE, Buchmann E, Bayever DN, Meyer LC. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS Pharm Sci Tech. 2008;9:505–520. doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HIV and its transmission (2003) Center for Disease Control and Prevention. Division of HIV/AIDS Prevention
- 6.(2014) HIV and AIDS Science. Cure for AIDS. AVERT. http://www.avert.org/cure-aids.htm
- 7.Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl 1):S30–S39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines. 2009;8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobngwi-Tambekou J, Taljaard D, Lissouba P, Zarca K, Puren A, Lagarde E, Auvert B. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009;199:958–964. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macmillan L, Ifere GO, He Q, Igietseme JU, Kellar KL, Okenu DM, Eko FO. A recombinant multivalent combination vaccine protects against Chlamydia and genital herpes. FEMS Immunol Med Microbiol. 2007;49:46–55. doi: 10.1111/j.1574-695X.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 12.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, Lutalo T, Kiwanuka N, Kigozi G, Nalugoda F, Meehan MP, Ashley Morrow R, Wawer MJ. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 13.Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Curr Opin HIV AIDS. 2009;4:294–299. doi: 10.1097/COH.0b013e32832c1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 15.Schiffer JT, Corey L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med. 2013;19:280–290. doi: 10.1038/nm.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 17.Mayaud P, McCormick D. Interventions against sexually transmitted infections (STI) to prevent HIV infection. Br Med Bull. 2001;58:129–153. doi: 10.1093/bmb/58.1.129. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (2013) Health Topics HIV/AIDS. World Health Organization website. http://www.who.int/topics/hiv_aids/en/, http://www.who.int/hiv/topics/microbicides/microbicides/en/, http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf
- 19.Buckheit RW, Jr, Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 2010;85:142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(2013) Global Report UNAIDS report onthe global AIDS epidemic 2013, UNAIDS. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf
- 21.Kredo T, Ford N, Adeniyi FB, Garner P. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Database Syst Rev. 2013;6:CD009987. doi: 10.1002/14651858.CD009987.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karim QA, Baxter C. Microbicides for the prevention of sexually transmitted HIV infection. Expert Rev Anti Infect Ther. 2013;11:13–23. doi: 10.1586/eri.12.153. [DOI] [PubMed] [Google Scholar]
- 23.CDC (2012) CDC website: HSV. http://www.cdc.gov/std/herpes/default.htm, http://www.cdc.gov/std/training/std101/presentations-2012/STD-Epidemiology.pdf
- 24.Kulczycki A, Kim DJ, Jamieson DJ, Macaluso M. The acceptability of the female and male condom: a randomized crossover trial. Perspect Sex Reprod Health. 2004;36:114–119. doi: 10.1363/psrh.36.114.04. [DOI] [PubMed] [Google Scholar]
- 25.Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis. 2011;52(Suppl 2):S231–S237. doi: 10.1093/cid/ciq047. [DOI] [PubMed] [Google Scholar]
- 26.Mullick C, Birnkrant D (2012) Vaginal microbicides: development for the prevention of HIV infection. In: Guidance for industry. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm328834.htm
- 27.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corey L. Herpes simplex virus type 2 and HIV-1: the dialogue between the 2 organisms continues. J Infect Dis. 2007;195:1242–1244. doi: 10.1086/513570. [DOI] [PubMed] [Google Scholar]
- 29.Corey L. Synergistic copathogens–HIV-1 and HSV-2. N Engl J Med. 2007;356:854–856. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 30.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 31.Steinbach JM, Weller CE, Booth CJ, Saltzman WM. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. J Control Release. 2012;162:102–110. doi: 10.1016/j.jconrel.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes B, Walker B, Nabel G, Overbaugh J, Burton D. Will there be an HIV vaccine in the next 10 years? Nat Med. 2007;13:518–519. [Google Scholar]
- 33.Brown TJ, Yen-Moore A, Tyring SK. An overview of sexually transmitted diseases. Part II. J Am Acad Dermatol. 1999;41:661–677. doi: 10.1016/s0190-9622(99)70563-3. [DOI] [PubMed] [Google Scholar]
- 34.Weber J, Desai K, Darbyshire J. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med. 2005;2:e142. doi: 10.1371/journal.pmed.0020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padian NS, McCoy SI, Karim SS, Hasen N, Kim J, Bartos M, Katabira E, Bertozzi SM, Schwartlander B, Cohen MS. HIV prevention transformed: the new prevention research agenda. Lancet. 2011;378:269–278. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials, Chiron HSV Vaccine Study Group. JAMA. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 37.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 38.Dropulic LK, Cohen JI. The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev Vaccines. 2012;11:1429–1440. doi: 10.1586/erv.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiffner T, Sattentau QJ, Dorrell L. Development of prophylactic vaccines against HIV-1. Retrovirology. 2013;10:72. doi: 10.1186/1742-4690-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.N.I.O. Health (2013) NIH website. http://www.nih.gov/news/health/nov2013/niaid-08.htm
- 42.Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 43.FDA (2014) Antiretroviral drugs used in the treatment of HIV infection. Drugs used in the treatment of HIV infection. U.S. Food and Drug Administration. http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/hivandaidsactivities/ucm118915.htm
- 44.Opoku-Anane J, Diouf K, Nour NM. New success with microbicides and pre-exposure prophylaxis for human immunodeficiency virus (HIV): is female-controlled prevention the answer to the HIV epidemic? Rev Obstet Gynecol. 2012;5:50–55. [PMC free article] [PubMed] [Google Scholar]
- 45.Coutinho B, Prasad R. Emtricitabine/tenofovir (Truvada) for HIV prophylaxis. Am Fam Physician. 2013;88:535–540. [PubMed] [Google Scholar]
- 46.AIDSMAP (2014) The Partners PrEP Trial. http://www.aidsmap.com/The-Partners-PrEP-trial/page/2213106
- 47.Friend DR, Doncel GF. Combining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: development of dual-protection technologies. Antiviral Res. 2010;88(Suppl 1):S47–S54. doi: 10.1016/j.antiviral.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 49.Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 Gel): a candidate dendrimer—microbicide for the prevention of HIV and HSV infection. Int J Nanomed. 2007;2:561–566. [PMC free article] [PubMed] [Google Scholar]
- 50.du Toit LC, Pillay V, Choonara YE. Nano-microbicides: challenges in drug delivery, patient ethics, and intellectual property in the war against HIV/AIDS. Adv Drug Deliv Rev. 2010;62:532–546. doi: 10.1016/j.addr.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Ramjee G. Microbicide research: current and future directions. Curr Opin HIV AIDS. 2010;5:316–321. doi: 10.1097/COH.0b013e32833a9f66. [DOI] [PubMed] [Google Scholar]
- 52.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, Billitto N, Lynam JD, Pryke K, Graebing P, Hopkins N, Rooney JF, Friend D, Dezzutti CS. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friend DR. Drug delivery in multiple indication (multipurpose) prevention technologies: systems to prevent HIV-1 transmission and unintended pregnancies or HSV-2 transmission. Expert Opin Drug Deliv. 2012;9:417–427. doi: 10.1517/17425247.2012.668183. [DOI] [PubMed] [Google Scholar]
- 54.Aitken RJ, Carey AJ, Beagley KW. Dual purpose contraceptives: targeting fertility and sexually transmitted disease. J Reprod Immunol. 2011;88:228–232. doi: 10.1016/j.jri.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Chandran P, Kabir SN. Dual action microbicides: reappraisal of their roles in contraceptive research. Reprod Biomed Online. 2010;20:103–113. doi: 10.1016/j.rbmo.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Whaley KJ, Hanes J, Shattock R, Cone RA, Friend DR. Novel approaches to vaginal delivery and safety of microbicides: biopharmaceuticals, nanoparticles, and vaccines. Antiviral Res. 2010;88(Suppl 1):S55–S66. doi: 10.1016/j.antiviral.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eszterhas SK, Ilonzo NO, Crozier JE, Celaj S, Howell AL. Nanoparticles containing siRNA to silence CD4 and CCR5 reduce expression of these receptors and inhibit HIV-1 infection in human female reproductive tract tissue explants. Infect Dis Rep. 2011;3:52–61. doi: 10.4081/idr.2011.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katakowski JA, Palliser D. siRNA-based topical microbicides targeting sexually transmitted infections. Curr Opin Mol Ther. 2010;12:192–202. [PMC free article] [PubMed] [Google Scholar]
- 61.Katakowski JA, Palliser D. Optimizing siRNA delivery to the genital mucosa. Discov Med. 2011;11:124–132. [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SS, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, Habiro K, Yang YG, Manjunath N, Shimaoka M, Shankar P. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18:370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lieberman J, Song E, Lee SK, Shankar P. Interfering with disease: opportunities and roadblocks to harnessing RNA interference. Trends Mol Med. 2003;9:397–403. doi: 10.1016/S1471-4914(03)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, Collman RG, Lieberman J, Shankar P, Sharp PA. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- 65.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, Seung E, Deruaz M, Dudek T, Einarsson JI, Yang L, Allen TM, Luster AD, Tager AM, Dykxhoorn DM, Lieberman J. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer–siRNA chimeras. J Clin Investig. 2011;121:2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. J Control Release. 2011;155:418–426. doi: 10.1016/j.jconrel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y, Navarro F, Lal A, Basar E, Pandey RK, Manoharan M, Feng Y, Lee SJ, Lieberman J, Palliser D. Durable protection from Herpes Simplex Virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell Host Microbe. 2009;5:84–94. doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang S, Chen Y, Ahmadie R, Ho EA. Advancements in the field of intravaginal siRNA delivery. J Control Release. 2013;167:29–39. doi: 10.1016/j.jconrel.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 70.Katz DF, Dunmire EN, Henderson MH, Owen DH, Plenys AM (1997) Applications of biomedical engineering in reproductive biomedicine: sensing and drug delivery to the lower female reproductive tract. In: Proceedings of the 19th annual international conference of the IEEE engineering in medicine and biology society, vol 19, Pts 1–6, pp 2656–2658
- 71.Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. J Control Release. 2005;103:301–313. doi: 10.1016/j.jconrel.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 72.Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent KL, Motamedi M, Friend DR, Clark MR, Baum MM. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother. 2012;56:875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gavini E, Sanna V, Juliano C, Bonferoni MC, Giunchedi P. Mucoadhesive vaginal tablets as veterinary delivery system for the controlled release of an antimicrobial drug, acriflavine. AAPS Pharm Sci Tech. 2002;3:E20. doi: 10.1007/BF02830618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bagarazzi ML, Boyer JD, Javadian MA, Chattergoon M, Dang K, Kim G, Shah J, Wang B, Weiner DB. Safety and immunogenicity of intramuscular and intravaginal delivery of HIV-1 DNA constructs to infant chimpanzees. J Med Primatol. 1997;26:27–33. doi: 10.1111/j.1600-0684.1997.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 75.Richardson JL, Illum L, Thomas NW. Vaginal absorption of insulin in the rat: effect of penetration enhancers on insulin uptake and mucosal histology. Pharm Res. 1992;9:878–883. doi: 10.1023/a:1015892630637. [DOI] [PubMed] [Google Scholar]
- 76.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 77.Kimberlin DW, Whitley RJ. Antiviral therapy of HSV-1 and -2. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 78.Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm. 2013;10:459–466. doi: 10.1021/mp3002045. [DOI] [PubMed] [Google Scholar]
- 79.Burruano BT, Schnaare RL, Malamud D. Synthetic cervical mucus formulation. Contraception. 2002;66:137–140. doi: 10.1016/s0010-7824(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 80.Katz DF. Human cervical mucus: research update. Am J Obstet Gynecol. 1991;165:1984–1986. doi: 10.1016/s0002-9378(11)90559-6. [DOI] [PubMed] [Google Scholar]
- 81.Zavos PM, Cohen MR. The pH of cervical mucus and the postcoital test. Fertil Steril. 1980;34:234–238. doi: 10.1016/s0015-0282(16)44953-8. [DOI] [PubMed] [Google Scholar]
- 82.Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, Gioia CJ, Spongberg EJ, Kauffman SM, McRaven MD, Lakougna HY, Hammond C, Kiser PF, Hope TJ. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6:427–434. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cu Y, Saltzman WM. Mathematical modeling of molecular diffusion through mucus. Adv Drug Deliv Rev. 2009;61:101–114. doi: 10.1016/j.addr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.das Neves J, Amiji M, Sarmento B. Mucoadhesive nanosystems for vaginal microbicide development: friend or foe? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:389–399. doi: 10.1002/wnan.144. [DOI] [PubMed] [Google Scholar]
- 85.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mallipeddi R, Rohan LC. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert Opin Drug Deliv. 2010;7:37–48. doi: 10.1517/17425240903338055. [DOI] [PubMed] [Google Scholar]
- 87.Fanibunda SE, Modi DN, Gokral JS, Bandivdekar AH. HIV gp120 binds to mannose receptor on vaginal epithelial cells and induces production of matrix metalloproteinases. PLoS ONE. 2011;6:e28014. doi: 10.1371/journal.pone.0028014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci USA. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hladik F, Doncel GF. Preventing mucosal HIV transmission with topical microbicides: challenges and opportunities. Antiviral Res. 2010;88(Suppl 1):S3–S9. doi: 10.1016/j.antiviral.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 92.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 94.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 95.Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce de Leon M, Peng T, Nicola AV, Montgomery RI, Warner MS, Soulika AM, Spruce LA, Moore WT, Lambris JD, Spear PG, Cohen GH, Eisenberg RJ. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ali MM, Karasneh GA, Jarding MJ, Tiwari V, Shukla D. A 3-O-sulfated heparan sulfate binding peptide preferentially targets herpes simplex virus 2-infected cells. J Virol. 2012;86:6434–6443. doi: 10.1128/JVI.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin ET, Krantz E, Gottlieb SL, Magaret AS, Langenberg A, Stanberry L, Kamb M, Wald A. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med. 2009;169:1233–1240. doi: 10.1001/archinternmed.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O’Connell DM, Asin SN, Wira CR, Fanger MW. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinlock BL, Wang Y, Turner TM, Wang C, Liu B. Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. PLoS ONE. 2014;9:e96760. doi: 10.1371/journal.pone.0096760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, Corti D, Moldt B, Hel Z, Lanzavecchia A, Ruprecht RM, Burton DR, Mestecky J, Anderson DJ, Forthal DN. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brunelli R, Papi M, Arcovito G, Bompiani A, Castagnola M, Parasassi T, Sampaolese B, Vincenzoni F, De Spirito M. Globular structure of human ovulatory cervical mucus. FASEB J. 2007;21:3872–3876. doi: 10.1096/fj.07-8189com. [DOI] [PubMed] [Google Scholar]
- 106.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cu Y, Saltzman WM. Controlled surface modification with poly(ethylene)glycol enhances diffusion of PLGA nanoparticles in human cervical mucus. Mol Pharm. 2009;6:173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci USA. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- 114.Nayak BS, Ellaiah P, Sudhahar D. Novel approaches in vaginal drug delivery systems for local and systemic treatments. J Pharm Res. 2010;3:675–680. [Google Scholar]
- 115.Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. J Control Release. 2011;156:258–264. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cu Y, Saltzman WM. Drug delivery: stealth particles give mucus the slip. Nat Mater. 2009;8:11–13. doi: 10.1038/nmat2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med. 2012;4:138ra179. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malcolm RK, Fetherston SM, McCoy CF, Boyd P, Major I. Vaginal rings for delivery of HIV microbicides. Int J Women’s Health. 2012;4:595–605. doi: 10.2147/IJWH.S36282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 121.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mrsny RJ. Lessons from nature: “Pathogen-Mimetic” systems for mucosal nano-medicines. Adv Drug Deliv Rev. 2009;61:172–192. doi: 10.1016/j.addr.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 123.Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv Drug Deliv Rev. 2005;57:1692–1712. doi: 10.1016/j.addr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 124.Friend DR. Pharmaceutical development of microbicide drug products. Pharm Dev Technol. 2010;15:562–581. doi: 10.3109/10837450903369879. [DOI] [PubMed] [Google Scholar]
- 125.Woolfson AD, Malcolm RK, Gallagher R. Drug delivery by the intravaginal route. Crit Rev Ther Drug Carrier Syst. 2000;17:509–555. [PubMed] [Google Scholar]
- 126.Blackwell RE. Detection of ovulation. Fertil Steril. 1984;41:680–681. doi: 10.1016/s0015-0282(16)47831-3. [DOI] [PubMed] [Google Scholar]
- 127.Sedlis A. Diseases of the vagina. New York: Springer; 1987. [Google Scholar]
- 128.Gengiah TN, Abdool Q. Karim, Implementing microbicides in low-income countries, Best practice & research. Clin Obstet Gynaecol. 2012;26:495–501. doi: 10.1016/j.bpobgyn.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stone A, Harrison PF (2010) Microbicides: way forward. In: Boyce L (ed) Alliance for microbicide development. http://www.avac.org/ht/a/GetDocumentAction/i/27266
- 130.PATH, protecting vaccines from extremes. http://www.path.org/projects/vaccine-stabilization.php
- 131.Gengiah TN, Baxter C, Mansoor LE, Kharsany AB, Abdool Karim SS. A drug evaluation of 1 % tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection. Expert Opin Investig Drugs. 2012;21:695–715. doi: 10.1517/13543784.2012.667072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Whitehead SJ, McLean C, Chaikummao S, Braunstein S, Utaivoravit W, van de Wijgert JH, Mock PA, Siraprapasiri T, Friedland BA, Kilmarx PH, Markowitz LE. Acceptability of Carraguard vaginal microbicide gel among HIV-infected women in Chiang Rai, Thailand. PLoS ONE. 2011;6:e14831. doi: 10.1371/journal.pone.0014831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cairns G (2008) Microbicides 2008: will adherence issues affect all the first-generation trials? http://www.aidsmap.com/Microbicides-2008-Will-adherence-issues-affect-all-the-first-generation-trials/page/1429752
- 134.Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, Bekker L, Ganesh S, Young K, Romano J, Nel A, Woodsong C. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;16:1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- 135.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, Schwartz K, Laborde N, Soto-Torres L. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9:e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jones DL, Weiss SM, Chitalu N, Mumbi M, Shine A, Vamos S, Villar O. Acceptability and use of sexual barrier products and lubricants among HIV-seropositive Zambian men. AIDS Patient Care STDs. 2008;22:1015–1020. doi: 10.1089/apc.2007.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Braunstein S, van de Wijgert J. Preferences and practices related to vaginal lubrication: implications for microbicide acceptability and clinical testing. J Women’s Health. 2005;14:424–433. doi: 10.1089/jwh.2005.14.424. [DOI] [PubMed] [Google Scholar]
- 138.Ramjee G, Kamali A, McCormack S. The last decade of microbicide clinical trials in Africa: from hypothesis to facts. AIDS. 2010;24(Suppl 4):S40–S49. doi: 10.1097/01.aids.0000390706.81383.f3. [DOI] [PubMed] [Google Scholar]
- 139.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, Elliott J, Janocko L, Khanukhova E, Dennis R, Cumberland WG, Ju C, Carballo-Dieguez A, Mauck C, McGowan I. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28:1412–1421. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kristensen D, Zaffran M. Designing vaccines for developing-country populations: ideal attributes, delivery devices, and presentation formats. Proc Vaccinol. 2010;2:119–123. [Google Scholar]
- 141.Kristensen D, Chen D. Stabilization of vaccines: lessons learned. Hum Vaccin. 2010;6:229–231. doi: 10.4161/hv.6.3.11618. [DOI] [PubMed] [Google Scholar]
- 142.CDC (2014) http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/vac-storage.pdf, http://www.cdc.gov/vaccines/pubs/
- 143.Bonferoni MC, Giunchedi P, Scalia S, Rossi S, Sandri G, Caramella C. Chitosan gels for the vaginal delivery of lactic acid: relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS Pharm Sci Tech. 2006;7:104. doi: 10.1208/pt0704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang C, Yin C, Pen Y. New superporous hydrogel composites based on aqueous carbopol solution (SPHScs): synthesis, characterization and in vitro bioadhesive force studies. Eur Polym J. 2005;41:557–562. [Google Scholar]
- 145.Al-Tahami K, Singh J. Smart polymer based delivery systems for peptides and proteins. Recent Pat Drug Deliv Formul. 2007;1:65–71. doi: 10.2174/187221107779814113. [DOI] [PubMed] [Google Scholar]
- 146.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 147.Lee SJ, Kim SW, Chung H, Park YT, Choi YW, Cho YH, Yoon MS. Bioadhesive drug delivery system using glyceryl monooleate for the intravesical administration of paclitaxel. Chemotherapy. 2005;51:311–318. doi: 10.1159/000088953. [DOI] [PubMed] [Google Scholar]
- 148.http://www.reference.md/files/D014/mD014622.html. Accessed 13 Oct 2014
- 149.Acarturk F. Mucoadhesive vaginal drug delivery systems. Recent Pat Drug Deliv Formul. 2009;3:193–205. doi: 10.2174/187221109789105658. [DOI] [PubMed] [Google Scholar]
- 150.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 152.Pal K, Banthia AK, Majumdar DK. Polymeric hydrogels: characterization and biomedical applications. Des Monomers Polym. 2009;12:197–220. [Google Scholar]
- 153.Shaikh R, RajSingh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3:89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Forbes CJ, Lowry D, Geer L, Veazey RS, Shattock RJ, Klasse PJ, Mitchnick M, Goldman L, Doyle LA, Muldoon BC, Woolfson AD, Moore JP, Malcolm RK. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Release. 2011;156:161–169. doi: 10.1016/j.jconrel.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hosmani AH (2006) Carbopol and its pharmaceutical significance. http://www.pharmainfo.net/reviews/carbopol-and-its-pharmaceutical-significance-review
- 156.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choudhury A, Das S, Kar M. A review on novelty and potentiality of vaginal drug delivery. Int J Pharm Tech Res. 2011;3:1033–1044. [Google Scholar]
- 158.CONRAD (2014) CONRAD: clinical trials. antiretrovirals as microbides: tenofovir (TFV). http://www.conrad.org/microbicides-trials.html
- 159.Garg S, Vermani K, Garg A, Anderson RA, Rencher WB, Zaneveld LJ. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm Res. 2005;22:584–595. doi: 10.1007/s11095-005-2490-1. [DOI] [PubMed] [Google Scholar]
- 160.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, Rohan LC. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1:209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 162.Neurath AR, Strick N, Li YY. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infect Dis. 2003;3:27. doi: 10.1186/1471-2334-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garg S, Goldman D, Krumme M, Rohan LC, Smoot S, Friend DR. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antiviral Res. 2010;88(Suppl 1):S19–S29. doi: 10.1016/j.antiviral.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 164.Alam MA, Ahmad FJ, Khan ZI, Khar RK, Ali M. Development and evaluation of acid-buffering bioadhesive vaginal tablet for mixed vaginal infections. AAPS Pharm Sci Tech. 2007;8:E109. doi: 10.1208/pt0801015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saltzman WM. Drug delivery: engineering principles for drug therapy. Oxford: Oxford University Press; 2001. [Google Scholar]
- 166.Saltzman WM, Sherwood JK, Adams DR, Haller P. Long-term vaginal antibody delivery: delivery systems and biodistribution. Biotechnol Bioeng. 2000;67:253–264. [PubMed] [Google Scholar]
- 167.Kuo PY, Sherwood JK, Saltzman WM. Topical antibody delivery systems produce sustained levels in mucosal tissue and blood. Nat Biotechnol. 1998;16:163–167. doi: 10.1038/nbt0298-163. [DOI] [PubMed] [Google Scholar]
- 168.Kuo-Haller P, Cu Y, Blum J, Appleton JA, Saltzman WM. Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity. Mol Pharm. 2010;7:1585–1595. doi: 10.1021/mp100009e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Radomsky ML, Whaley KJ, Cone RA, Saltzman WM. Controlled vaginal delivery of antibodies in the mouse. Biol Reprod. 1992;47:133–140. doi: 10.1095/biolreprod47.1.133. [DOI] [PubMed] [Google Scholar]
- 170.Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersions: a review. Pak J Pharm Sci. 2009;22:234–246. [PubMed] [Google Scholar]
- 171.Saltzman WM. Case Studies in Drug Delivery. In: Gubbins KE, editor. Drug delivery: engineering principles for drug therapy. New York: Oxford University Press; 2001. pp. 281–315. [Google Scholar]
- 172.Wyatt TL, Saltzman WM. Protein delivery from nondegradable polymer matrices. In: Saunders L, Hendren W, editors. Protein delivery—physical systems. New York: Plenum Press; 1997. pp. 119–137. [DOI] [PubMed] [Google Scholar]
- 173.Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7:429–444. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ho EA. Intravaginal rings as a novel platform for mucosal vaccination. Mol Pharm Orgnic Process Res. 2013;1:2. [Google Scholar]
- 175.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, McNicholl JM, Hendry RM, Dinh CT, Martin A, Herold BC, Kiser PF. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci USA. 2013;110:16145–16150. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Avgoustakis K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr Drug Deliv. 2004;1:321–333. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- 178.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 179.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64:1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 180.Antoine TE, Mishra YK, Trigilio J, Tiwari V, Adelung R, Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antiviral Res. 2012;96:363–375. doi: 10.1016/j.antiviral.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mishra YK, Adelung R, Rohl C, Shukla D, Spors F, Tiwari V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Res. 2011;92:305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21:387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 183.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 184.Moscicki AB, Kaul R, Ma Y, Scott ME, Daud II, Bukusi EA, Shiboski S, Rebbapragada A, Huibner S, Cohen CR. Measurement of mucosal biomarkers in a phase 1 trial of intravaginal 3% StarPharma LTD 7013 gel (VivaGel) to assess expanded safety. J Acquir Immune Defic Syndr. 2012;59:134–140. doi: 10.1097/QAI.0b013e31823f2aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cohen CR, Brown J, Moscicki AB, Bukusi EA, Paull JR, Price CF, Shiboski S. A phase I randomized placebo controlled trial of the safety of 3% SPL7013 Gel (VivaGel(R)) in healthy young women administered twice daily for 14 days. PLoS ONE. 2011;6:e16258. doi: 10.1371/journal.pone.0016258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McGowan I, Gomez K, Bruder K, Febo I, Chen BA, Richardson BA, Husnik M, Livant E, Price C, Jacobson C. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004) AIDS. 2011;25:1057–1064. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, Xia S, Spelman T, Hodsman P, Moench TR, Humberstone A, Paull JR, Tachedjian G. SPL7013 Gel (VivaGel(R)) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS ONE. 2011;6:e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Starpharma (2014) VivaGel Clinical Trials. Starpharma
- 189.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 190.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schlievert PM, Strandberg KL, Brosnahan AJ, Peterson ML, Pambuccian SE, Nephew KR, Brunner KG, Schultz-Darken NJ, Haase AT. Glycerol monolaurate does not alter rhesus macaque (Macaca mulatta) vaginal lactobacilli and is safe for chronic use. Antimicrob Agents Chemother. 2008;52:4448–4454. doi: 10.1128/AAC.00989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kuntz RM, Saltzman WM. Polymeric controlled delivery for immunization. Trends Biotechnol. 1997;15:364–369. doi: 10.1016/s0167-7799(97)01087-1. [DOI] [PubMed] [Google Scholar]
- 193.Cheshenko N, Keller MJ, MasCasullo V, Jarvis GA, Cheng H, John M, Li JH, Hogarty K, Anderson RA, Waller DP, Zaneveld LJ, Profy AT, Klotman ME, Herold BC. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother. 2004;48:2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Castle PE, Whaley KJ, Hoen TE, Moench TR, Cone RA. Contraceptive effect of sperm-agglutinating monoclonal antibodies in rabbits. Biol Reprod. 1997;56:153–159. doi: 10.1095/biolreprod56.1.153. [DOI] [PubMed] [Google Scholar]
- 195.Cone RA, Whaley KJ. Monoclonal antibodies for reproductive health: part I. Preventing sexual transmission of disease and pregnancy with topically applied antibodies. Am J Reprod Immunol. 1994;32:114–131. doi: 10.1111/j.1600-0897.1994.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 196.Sherwood JK, Zeitlin L, Whaley KJ, Cone RA, Saltzman M. Controlled release of antibodies for long-term topical passive immunoprotection of female mice against genital herpes. Nat Biotechnol. 1996;14:468–471. doi: 10.1038/nbt0496-468. [DOI] [PubMed] [Google Scholar]
- 197.Sherwood JK, Zeitlin L, Chen X, Whaley KJ, Cone RA, Saltzman WM. Residence half-life of IgG administered topically to the mouse vagina. Biol Reprod. 1996;54:264–269. doi: 10.1095/biolreprod54.1.264. [DOI] [PubMed] [Google Scholar]
- 198.Gavrilov K, Saltzman WM. Therapeutic siRNA: principles, challenges, and strategies. Yale J Biol Med. 2012;85:187–200. [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou J, Rossi JJ. Aptamer-targeted cell-specific RNA interference. Silence. 2010;1:4. doi: 10.1186/1758-907X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Crombie R, Kawasaki K, Hojo K, Laurence J. Peptides derived from salivary thrombospondin-1 replicate its anti-HIV effect: potential role in microbicide development. J Acquir Immune Defic Syndr. 2001;27:91–93. doi: 10.1097/00126334-200105010-00016. [DOI] [PubMed] [Google Scholar]
- 201.Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, Fekete RA, Kar S, Adhya S, Hamer DH. Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci USA. 2005;102:11993–11998. doi: 10.1073/pnas.0504881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu JJ, Reid G, Jiang Y, Turner MS, Tsai CC. Activity of HIV entry and fusion inhibitors expressed by the human vaginal colonizing probiotic Lactobacillus reuteri RC-14. Cell Microbiol. 2007;9:120–130. doi: 10.1111/j.1462-5822.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 203.Pusch O, Boden D, Hannify S, Lee F, Tucker LD, Boyd MR, Wells JM, Ramratnam B. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J Acquir Immune Defic Syndr. 2005;40:512–520. doi: 10.1097/01.qai.0000187446.76579.d3. [DOI] [PubMed] [Google Scholar]
- 204.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, Lewicki JA, Lee PP. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci USA. 2003;100:11672–11677. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dey B, Lagenaur LA, Lusso P. Protein-based HIV-1 microbicides. Curr HIV Res. 2013;11:576–594. doi: 10.2174/1570162x11666140101120709. [DOI] [PubMed] [Google Scholar]
- 206.Abdel-Motal UM, Sarkis PT, Han T, Pudney J, Anderson DJ, Zhu Q, Marasco WA. Anti-gp120 minibody gene transfer to female genital epithelial cells protects against HIV-1 virus challenge in vitro. PLoS ONE. 2011;6:e26473. doi: 10.1371/journal.pone.0026473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sexton A, Harman S, Shattock RJ, Ma JK. Design, expression, and characterization of a multivalent, combination HIV microbicide. FASEB J. 2009;23:3590–3600. doi: 10.1096/fj.09-131995. [DOI] [PubMed] [Google Scholar]
- 210.Sexton A, Drake PM, Mahmood N, Harman SJ, Shattock RJ, Ma JK. Transgenic plant production of cyanovirin-N, an HIV microbicide. FASEB J. 2006;20:356–358. doi: 10.1096/fj.05-4742fje. [DOI] [PubMed] [Google Scholar]
- 211.Hamorsky KT, Grooms-Williams TW, Husk AS, Bennett LJ, Palmer KE, Matoba N. Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides. Antimicrob Agents Chemother. 2013;57:2076–2086. doi: 10.1128/AAC.02588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Saltzman WM. Antibodies for treating and preventing disease: the potential role of polymeric controlled release. Crit Rev Ther Drug Carrier Syst. 1993;10:111–142. [PubMed] [Google Scholar]
- 213.Apter D, Cacciatore B, Stenman UH, Alapiessa U, Assendorp R. Clinical performance and endocrine profiles of contraceptive vaginal rings releasing 3-keto-desogestrel and ethinylestradiol. Contraception. 1990;42:285–295. doi: 10.1016/0010-7824(90)90016-o. [DOI] [PubMed] [Google Scholar]
- 214.Whaley KJ, Zeitlin L, Barratt RA, Hoen TE, Cone RA. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 215.Castle PE, Whaley KJ, Moench TR, H. J.E., Saltzman WM, Radomsky ML (1991) Monoclonal IgM antibodies against rabbit sperm for vaginal contraception. J Androl Suppl, 27–29
- 216.Bessen D, Fischetti VA. Passive acquired mucosal immunity to group A streptococci by secretory immunoglobulin A. J Exp Med. 1988;167:1945–1950. doi: 10.1084/jem.167.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ma JK, Smith R, Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans . Infect Immun. 1987;55:1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tacket CO, Binion SB, Bostwick E, Losonsky G, Roy MJ, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47:276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 219.Shen H, Goldberg E, Saltzman WM. Gene expression and mucosal immune responses after vaginal DNA immunization in mice using a controlled delivery matrix. J Control Release. 2003;86:339–348. doi: 10.1016/s0168-3659(02)00354-1. [DOI] [PubMed] [Google Scholar]
- 220.Wyatt TL, Whaley KJ, Cone RA, Saltzman WM. Antigen-releasing polymer rings and microspheres stimulate mucosal immunity in the vagina. J Control Release. 1998;50:93–102. doi: 10.1016/s0168-3659(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 221.Wang B, Dang K, Agadjanyan MG, Srikantan V, Li F, Ugen KE, Boyer J, Merva M, Williams WV, Weiner DB. Mucosal immunization with a DNA vaccine induces immune responses against HIV-1 at a mucosal site. Vaccine. 1997;15:821–825. doi: 10.1016/s0264-410x(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 222.Livingston JB, Lu S, Robinson H, Anderson DJ. Immunization of the female genital tract with a DNA-based vaccine. Infect Immun. 1998;66:322–329. doi: 10.1128/iai.66.1.322-329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Edwards JN, Morris HB. Langerhans’ cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynaecol. 1985;92:974–982. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 224.Parr MB, Kepple L, Parr EL. Langerhans cells phagocytose vaginal epithelial cells undergoing apoptosis during the murine estrous cycle. Biol Reprod. 1991;45:252–260. doi: 10.1095/biolreprod45.2.252. [DOI] [PubMed] [Google Scholar]
- 225.Livingston JB, Lu S, Robinson HL, Anderson DJ. The induction of mucosal immunity in the female genital tract using gene-gun technology. Part 1: antigen expression. Ann N Y Acad Sci. 1995;772:265–267. doi: 10.1111/j.1749-6632.1995.tb44755.x. [DOI] [PubMed] [Google Scholar]
- 226.Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T, Hovanesian V, Monfils PR, Resnick M, Moss SF, Ramratnam B. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14:336–342. doi: 10.1016/j.ymthe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 227.Shattock RJ, Rosenberg Z (2012) Microbicides: topical prevention against HIV-1. Cold Spring Harbor Perspect Med 4 [DOI] [PMC free article] [PubMed]
- 228.Boden D, Pusch O, Silbermann R, Lee F, Tucker L, Ramratnam B. Enhanced gene silencing of HIV-1 specific siRNA using microRNA designed hairpins. Nucleic Acids Res. 2004;32:1154–1158. doi: 10.1093/nar/gkh278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Das AT, Brummelkamp TR, Westerhout EM, Vink M, Madiredjo M, Bernards R, Berkhout B. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J Virol. 2004;78:2601–2605. doi: 10.1128/JVI.78.5.2601-2605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee NS, Dohjima T, Bauer G, Li H, Li MJ, Ehsani A, Salvaterra P, Rossi J. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol. 2002;20:500–505. doi: 10.1038/nbt0502-500. [DOI] [PubMed] [Google Scholar]
- 231.Martinez MA, Gutierrez A, Armand-Ugon M, Blanco J, Parera M, Gomez J, Clotet B, Este JA. Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. AIDS. 2002;16:2385–2390. doi: 10.1097/00002030-200212060-00002. [DOI] [PubMed] [Google Scholar]
- 232.Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N, Shankar P. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Arrighi JF, Pion M, Wiznerowicz M, Geijtenbeek TB, Garcia E, Abraham S, Leuba F, Dutoit V, Ducrey-Rundquist O, van Kooyk Y, Trono D, Piguet V. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J Virol. 2004;78:10848–10855. doi: 10.1128/JVI.78.20.10848-10855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26:502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, Piatak M, Jr, Lifson JD, Salkowitz JR, Rodriguez B, Blauvelt A, Hartley O. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 237.McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med. 2013;210:209–223. doi: 10.1084/jem.20121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Morris GC, Lacey CJ. Microbicides and HIV prevention: lessons from the past, looking to the future. Curr Opin Infect Dis. 2010;23:57–63. doi: 10.1097/QCO.0b013e328334de6d. [DOI] [PubMed] [Google Scholar]
- 239.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fessel WJ, Anderson B, Follansbee SE, Winters MA, Lewis ST, Weinheimer SP, Petropoulos CJ, Shafer RW. The efficacy of an anti-CD4 monoclonal antibody for HIV-1 treatment. Antiviral Res. 2011;92:484–487. doi: 10.1016/j.antiviral.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Brinckmann S, da Costa K, van Gils MJ, Hallengard D, Klein K, Madeira L, Mainetti L, Palma P, Raue K, Reinhart D, Reudelsterz M, Ruffin N, Seifried J, Schafer K, Sheik-Khalil E, Skold A, Uchtenhagen H, Vabret N, Ziglio S, Scarlatti G, Shattock R, Wahren B, Gotch F. Rational design of HIV vaccines and microbicides: report of the EUROPRISE network annual conference 2010. J Transl Med. 2011;9:40. doi: 10.1186/1479-5876-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rancez M, Couedel-Courteille A, Cheynier R. Chemokines at mucosal barriers and their impact on HIV infection. Cytokine Growth Factor Rev. 2012;23:233–243. doi: 10.1016/j.cytogfr.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 244.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 245.Garcia-Vallejo JJ, Koning N, Ambrosini M, Kalay H, Vuist I, Sarrami-Forooshani R, Geijtenbeek TB, van Kooyk Y. Glycodendrimers prevent HIV transmission via DC-SIGN on dendritic cells. Int Immunol. 2013;25:221–233. doi: 10.1093/intimm/dxs115. [DOI] [PubMed] [Google Scholar]
- 246.Nixon B, Stefanidou M, Mesquita PM, Fakioglu E, Segarra T, Rohan L, Halford W, Palmer KE, Herold BC. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J Virol. 2013;87:6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ferir G, Palmer KE, Schols D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology. 2011;417:253–258. doi: 10.1016/j.virol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 248.Ferir G, Huskens D, Palmer KE, Boudreaux DM, Swanson MD, Markovitz DM, Balzarini J, Schols D. Combinations of griffithsin with other carbohydrate-binding agents demonstrate superior activity against HIV Type 1, HIV Type 2, and selected carbohydrate-binding agent-resistant HIV Type 1 strains. AIDS Res Hum Retroviruses. 2012;28:1513–1523. doi: 10.1089/aid.2012.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Meuleman P, Albecka A, Belouzard S, Vercauteren K, Verhoye L, Wychowski C, Leroux-Roels G, Palmer KE, Dubuisson J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Barton C, Kouokam JC, Lasnik AB, Foreman O, Cambon A, Brock G, Montefiori DC, Vojdani F, McCormick AA, O’Keefe BR, Palmer KE. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Reeves JD, Simmons G. Chemokine inhibition of HIV infection. Methods Mol Biol. 2000;138:209–222. doi: 10.1385/1-59259-058-6:209. [DOI] [PubMed] [Google Scholar]
- 252.Xu L, Hue S, Taylor S, Ratcliffe D, Workman JA, Jackson S, Cane PA, Pillay D. Minimal variation in T-20 binding domain of different HIV-1 subtypes from antiretroviral-naive and -experienced patients. AIDS. 2002;16:1684–1686. doi: 10.1097/00002030-200208160-00016. [DOI] [PubMed] [Google Scholar]
- 253.Martin-Carbonero L. Discontinuation of the clinical development of fusion inhibitor T-1249. AIDS Rev. 2004;6:61. [PubMed] [Google Scholar]
- 254.Lalezari JP, Bellos NC, Sathasivam K, Richmond GJ, Cohen CJ, Myers RA, II, Henry DH, Raskino C, Melby T, Murchison H, Zhang Y, Spence R, Greenberg ML, Demasi RA, Miralles GD, Group TS. T-1249 retains potent antiretroviral activity in patients who had experienced virological failure while on an enfuvirtide-containing treatment regimen. J Infect Dis. 2005;191:1155–1163. doi: 10.1086/427993. [DOI] [PubMed] [Google Scholar]
- 255.He Y, Xiao Y, Song H, Liang Q, Ju D, Chen X, Lu H, Jing W, Jiang S, Zhang L. Design and evaluation of sifuvirtide, a novel HIV-1 fusion inhibitor. J Biol Chem. 2008;283:11126–11134. doi: 10.1074/jbc.M800200200. [DOI] [PubMed] [Google Scholar]
- 256.Lusso P. HIV and the chemokine system: 10 years later. EMBO J. 2006;25:447–456. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 258.Welch BD, Francis JN, Redman JS, Paul S, Weinstock MT, Reeves JD, Lie YS, Whitby FG, Eckert DM, Hill CP, Root MJ, Kay MS. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J Virol. 2010;84:11235–11244. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pappenheimer JR, Karnovsky ML, Maggio JE. Absorption and excretion of undegradable peptides: role of lipid solubility and net charge. J Pharmacol Exp Ther. 1997;280:292–300. [PubMed] [Google Scholar]
- 260.Eckert DM, Kim PS. Design of potent inhibitors of HIV-1 entry from the gp41N-peptide region. Proc Natl Acad Sci USA. 2001;98:11187–11192. doi: 10.1073/pnas.201392898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dogo-Isonagie C, Lam S, Gustchina E, Acharya P, Yang Y, Shahzad-ul-Hussan S, Clore GM, Kwong PD, Bewley CA. Peptides from second extracellular loop of C-C chemokine receptor type 5 (CCR5) inhibit diverse strains of HIV-1. J Biol Chem. 2012;287:15076–15086. doi: 10.1074/jbc.M111.332361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Agrawal L, VanHorn-Ali Z, Berger EA, Alkhatib G. Specific inhibition of HIV-1 coreceptor activity by synthetic peptides corresponding to the predicted extracellular loops of CCR5. Blood. 2004;103:1211–1217. doi: 10.1182/blood-2003-08-2669. [DOI] [PubMed] [Google Scholar]
- 263.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 264.Nardese V, Longhi R, Polo S, Sironi F, Arcelloni C, Paroni R, DeSantis C, Sarmientos P, Rizzi M, Bolognesi M, Pavone V, Lusso P. Structural determinants of CCR5 recognition and HIV-1 blockade in RANTES. Nat Struct Biol. 2001;8:611–615. doi: 10.1038/89653. [DOI] [PubMed] [Google Scholar]
- 265.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 266.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J. The genotype of early-transmitting HIV gp120 s promotes alpha (4) beta(7)-reactivity, revealing alpha (4) beta(7)+/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7:e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, Wang X, Li J, Price AA, Little DM, Zaidi M, Lyles R, Villinger F. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–1059. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tiwari V, Liu J, Valyi-Nagy T, Shukla D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem. 2011;286:25406–25415. doi: 10.1074/jbc.M110.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tiwari V, O’Donnell CD, Oh MJ, Valyi-Nagy T, Shukla D. A role for 3-O-sulfotransferase isoform-4 in assisting HSV-1 entry and spread. Biochem Biophys Res Commun. 2005;338:930–937. doi: 10.1016/j.bbrc.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 270.Sinha S, Cheshenko N, Lehrer RI, Herold BC. NP-1, a rabbit alpha-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob Agents Chemother. 2003;47:494–500. doi: 10.1128/AAC.47.2.494-500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bhattacharjee PS, Neumann DM, Hill JM. A human apolipoprotein E mimetic peptide effectively inhibits HSV-1 TK-positive and TK-negative acute epithelial keratitis in rabbits. Curr Eye Res. 2009;34:99–102. doi: 10.1080/02713680802647662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Andersen JH, Jenssen H, Sandvik K, Gutteberg TJ. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J Med Virol. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 273.Andersen JH, Jenssen H, Gutteberg TJ. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antiviral Res. 2003;58:209–215. doi: 10.1016/s0166-3542(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 274.AlbiolMatanic VC, Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 275.Shestakov A, Jenssen H, Hancock RE, Nordstrom I, Eriksson K. Synthetic analogues of bovine bactenecin dodecapeptide reduce herpes simplex virus type 2 infectivity in mice. Antiviral Res. 2013;100:455–459. doi: 10.1016/j.antiviral.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 276.Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 277.Wang L, Althoff EA, Bolduc J, Jiang L, Moody J, Lassila JK, Giger L, Hilvert D, Stoddard B, Baker D. Structural analyses of covalent enzyme-substrate analog complexes reveal strengths and limitations of de novo enzyme design. J Mol Biol. 2012;415:615–625. doi: 10.1016/j.jmb.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Furci L, Tolazzi M, Sironi F, Vassena L, Lusso P. Inhibition of HIV-1 infection by human alpha-defensin-5, a natural antimicrobial peptide expressed in the genital and intestinal mucosae. PLoS ONE. 2012;7:e45208. doi: 10.1371/journal.pone.0045208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Furci L, Sironi F, Tolazzi M, Vassena L, Lusso P. Alpha-defensins block the early steps of HIV-1 infection: interference with the binding of gp120 to CD4. Blood. 2007;109:2928–2935. doi: 10.1182/blood-2006-05-024489. [DOI] [PubMed] [Google Scholar]
- 280.Seidel A, Ye Y, de Armas LR, Soto M, Yarosh W, Marcsisin RA, Tran D, Selsted ME, Camerini D. Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS ONE. 2010;5:e9737. doi: 10.1371/journal.pone.0009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 282.Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci USA. 2006;103:1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bai F, Town T, Pradhan D, Cox J, Ashish, Ledizet M, Anderson JF, Flavell RA, Krueger RA, Koski RA, Fikrig E. Antiviral peptides targeting the west Nile virus envelope protein. J Virol. 2007;81:2047–2055. doi: 10.1128/JVI.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jenssen H, Andersen JH, Mantzilas D, Gutteberg TJ. A wide range of medium-sized, highly cationic, alpha-helical peptides show antiviral activity against herpes simplex virus. Antiviral Res. 2004;64:119–126. doi: 10.1016/j.antiviral.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 286.Mohan KV, Rao SS, Atreya CD. Antiviral activity of selected antimicrobial peptides against vaccinia virus. Antiviral Res. 2010;86:306–311. doi: 10.1016/j.antiviral.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cho NJ, Dvory-Sobol H, Xiong A, Cho SJ, Frank CW, Glenn JS. Mechanism of an amphipathic alpha-helical peptide’s antiviral activity involves size-dependent virus particle lysis. ACS Chem Biol. 2009;4:1061–1067. doi: 10.1021/cb900149b. [DOI] [PubMed] [Google Scholar]
- 288.Scudiero O, Galdiero S, Cantisani M, Di Noto R, Vitiello M, Galdiero M, Naclerio G, Cassiman JJ, Pedone C, Castaldo G, Salvatore F. Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob Agents Chemother. 2010;54:2312–2322. doi: 10.1128/AAC.01550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Luganini A, Nicoletto SF, Pizzuto L, Pirri G, Giuliani A, Landolfo S, Gribaudo G. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Antimicrob Agents Chemother. 2011;55:3231–3239. doi: 10.1128/AAC.00149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Krepstakies M, Lucifora J, Nagel CH, Zeisel MB, Holstermann B, Hohenberg H, Kowalski I, Gutsmann T, Baumert TF, Brandenburg K, Hauber J, Protzer U. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J Infect Dis. 2012;205:1654–1664. doi: 10.1093/infdis/jis273. [DOI] [PubMed] [Google Scholar]
- 291.Egal M, Conrad M, MacDonald DL, Maloy WL, Motley M, Genco CA. Antiviral effects of synthetic membrane-active peptides on herpes simplex virus, type 1. Int J Antimicrob Agents. 1999;13:57–60. doi: 10.1016/s0924-8579(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 292.Munk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR, Cole AM. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res Hum Retroviruses. 2003;19:875–881. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- 293.Owen SM, Rudolph DL, Wang W, Cole AM, Waring AJ, Lal RB, Lehrer RI. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2004;20:1157–1165. doi: 10.1089/aid.2004.20.1157. [DOI] [PubMed] [Google Scholar]
- 294.Wu Z, Cocchi F, Gentles D, Ericksen B, Lubkowski J, Devico A, Lehrer RI, Lu W. Human neutrophil alpha-defensin 4 inhibits HIV-1 infection in vitro. FEBS Lett. 2005;579:162–166. doi: 10.1016/j.febslet.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 295.Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012;14:62–77. [PubMed] [Google Scholar]
- 296.Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012;20:1298–1304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chu TC, Twu KY, Ellington AD, Levy M. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006;34:e73. doi: 10.1093/nar/gkl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 299.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 300.Zhou J, Li H, Li S, Zaia J, Rossi JJ. Novel dual inhibitory function aptamer–siRNA delivery system for HIV-1 therapy. Mol Ther. 2008;16:1481–1489. doi: 10.1038/mt.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kanazawa T, Takashima Y, Okada H. Vaginal DNA vaccination against infectious diseases transmitted through the vagina. Front Biosci. 2012;4:2340–2353. doi: 10.2741/e546. [DOI] [PubMed] [Google Scholar]
- 302.Kanazawa T, Takashima Y, Shibata Y, Tsuchiya M, Tamura T, Okada H. Effective vaginal DNA delivery with high transfection efficiency is a good system for induction of higher local vaginal immune responses. J Pharm Pharmacol. 2009;61:1457–1463. doi: 10.1211/jpp/61.11.0004. [DOI] [PubMed] [Google Scholar]
- 303.Kanazawa T, Tamura T, Yamazaki M, Takashima Y, Okada H. Needle-free intravaginal DNA vaccination using a stearoyl oligopeptide carrier promotes local gene expression and immune responses. Int J Pharm. 2013;447:70–74. doi: 10.1016/j.ijpharm.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 304.Zhou J, Rossi JJ. Current progress in the development of RNAi-based therapeutics for HIV-1. Gene Ther. 2011;18:1134–1138. doi: 10.1038/gt.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Grant RM, Hamer D, Hope T, Johnston R, Lange J, Lederman MM, Lieberman J, Miller CJ, Moore JP, Mosier DE, Richman DD, Schooley RT, Springer MS, Veazey RS, Wainberg MA. Whither or wither microbicides? Science. 2008;321:532–534. doi: 10.1126/science.1160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Boutimah F, Eekels JJ, Liu YP, Berkhout B. Antiviral strategies combining antiretroviral drugs with RNAi-mediated attack on HIV-1 and cellular co-factors. Antiviral Res. 2013;98:121–129. doi: 10.1016/j.antiviral.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 307.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS ONE. 2012;7:e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013;100(Suppl):S9–S16. doi: 10.1016/j.antiviral.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 309.Huang C, Soenen SJ, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, Vervaet C, Coenye T, Verstraelen H, Temmerman M, Demeester J, De Smedt SC. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33:962–969. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 310.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Becker J, Benjamin B, Blakesley R, Bouffard G, Brooks S, Coleman H, Dekhtyar M, Gregory M, Guan X, Gupta J, Han J, Hargrove A, Ho SL, Johnson T, Legaspi R, Lovett S, Maduro Q, Masiello C, Maskeri B, McDowell J, Montemayor C, Mullikin J, Park M, Riebow N, Schandler K, Schmidt B, Sison C, Stantripop M, Thomas J, Thomas P, Vemulapalli M, Young A, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gao W, Xiao Z, Radovic-Moreno A, Shi J, Langer R, Farokhzad OC. Progress in siRNA delivery using multifunctional nanoparticles. Methods Mol Biol. 2010;629:53–67. doi: 10.1007/978-1-60761-657-3_4. [DOI] [PubMed] [Google Scholar]
- 312.Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur J Pharm Biopharm. 2011;79:526–536. doi: 10.1016/j.ejpb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang T, Zhang C, Agrahari V, Murowchick JB, Oyler NA, Youan BB. Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antiviral Res. 2013;97:334–346. doi: 10.1016/j.antiviral.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. Adherence support approaches in biomedical hiv prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17:2143–2155. doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.AVAC (2014) AVAC: Trial Summary Table, in, http://www.avac.org/trial-summary-table/microbicides
- 316.AIDS Info (2014) Clinical trial search results. http://aidsinfo.nih.gov/clinical-trials/search/q/1/category/59/prevention-research/63/microbicides
- 317.ClinicalTrials.gov, ClinicalTrials.gov: Safety and Acceptability Study of Oral Emtricitabine/Tenofovir Disoproxil Fumarate Tablet and Rectally-Applied Tenofovir Reduced-Glycerin 1% Gel, in, http://clinicaltrials.gov/show/NCT01687218, 2014.
- 318.IMPT for Reproductive Health (2014) MPT Product Development Database. http://mpts101.org/mpt-database/product-page/35-candidate-single-indication-agents-for-mpts/anti-hiv-topical-rings/87-maraviroc-international-partnership-for-microbicides-ipm-usaid-niaid-daids-vaginal-ring-ivr-hiv
- 319.Population Council (2014) Microbicides and multipurpose prevention technologies. http://www.popcouncil.org/research, 2014.
- 320.MTN (2011) MTN statement on decision to discontinue use of oral tenofovir tablets in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/node/3619
- 321.M.T. Network (2014) MTN: microbicide trials network—studies. http://www.mtnstopshiv.org/studies
- 322.IPM (2014) International partnership for microbicides webpage. http://www.ipmglobal.org/