Abstract
The non-mevalonate pathway of isoprenoid (terpenoid) biosynthesis is essential in many eubacteria including the major human pathogen, Mycobacterium tuberculosis, in apicomplexan protozoa including the Plasmodium spp. causing malaria, and in the plastids of plants. The metabolic route is absent in humans and is therefore qualified as a promising target for new anti-infective drugs and herbicides. Biochemical and structural knowledge about all enzymes involved in the pathway established the basis for discovery and development of inhibitors by high-throughput screening of compound libraries and/or structure-based rational design.
Keywords: Isoprenoid biosynthesis, Terpenes, Isp proteins, Isopentenyl diphosphate, Dimethylallyl diphosphate, Mevalonate, Deoxyxylulose, Methyerythritol
Introduction
The twentieth century was an era of unprecedented medical progress. The developments in surgery, pharmacology and diagnostic methodology contributed substantially to the doubling of human life expectancy that took place in economically advanced countries over the past 150 years. Cardiovascular medicine, endocrinology and anaesthesiology, to name only a few important areas, experienced tremendous progress. Mortality and morbidity due to infectious disease were dramatically reduced, predominantly but not exclusively in economically advanced countries, by the impact of a plethora of anti-infective drugs [1] and vaccinations. More specifically, the development of sulfonamides in the 1930s and of penicillin in the 1940s was followed by the discovery of a treasure trove of additional antibiotics. In conjunction with vaccines directed against a variety of bacterial and viral infections, these developments caused a major shift in morbidity. Most notably, tuberculosis, which had been a major killer, was dramatically reduced, although not eliminated, in economically advanced areas. Whereas infectious disease had been the dominant factor of mortality in Europe and USA by the end of the nineteenth century, death from infectious disease became a relatively rare event in areas with high level medical services [1]. However, this should not cloud the issue that infectious disease remains a leading factor of mortality on a global scale.
Interestingly, among the numerous pharmacological achievements of the past century, anti-infective agents are unique in so far as they are permanently exposed to a process of attrition. Whereas aspirin is forever, the medical application of antimicrobial drugs triggers off a process of Darwinian selection in the cognate pathogens that is conducive to the emergence and spreading of resistant forms. Typically, this process starts within months to years after the introduction of a specific agent [2]. The development of resistance can be accelerated by the use of antibiotics in animal husbandry, and by poor medical practice involving the indiscriminate use of anti-infective agents, but even in the absence of these, the ultimate development of resistance is the natural fate of every successful anti-infective drug. As a specific example, multidrug-resistant strains of Mycobacterium tuberculosis occur worldwide with increasing frequency [3], and Plasmodium spp. causing malaria have become resistant to most classical antimalarial agents in many areas of the world [4]. In light of this, there is an urgent need for the continued development of novel anti-infective agents in order to counteract the blunting of our anti-infective armament. However, whereas scores of novel antibiotics were introduced in the decades following the Second World War, very few novel agents have been introduced in recent years, and even those that have been introduced were typically modifications of existing drugs rather than novel principles [5].
The success story of the antibiotics era was based predominantly on trial and error procedures. Notably, the systematic screening of natural products was the single most important approach for the identification of novel therapeutic principles. By comparison, the development of antiviral agents has been driven to a significant degree by rational methodology based on data from biochemistry and molecular biology. The rapid development and deployment of a wide variety of anti-HIV drugs is probably the single most striking example for the power of information-based design in the domain of infectious disease if not in the entire area of pharmacology [6].
Superficially, the emerging basis for knowledge-driven design of antibacterial drugs (as opposed to trial and error concepts) is unprecedented. The first genome of a cellular organism reported in 1995 was that of an important human pathogen, the Gram-negative bacterium Haemophilus influenzae [7]. The landmark paper introduced the concept of shotgun sequencing that has rapidly become the technical standard of the unfolding field of genomics. This milestone achievement was rapidly followed by the complete sequences of hundreds of bacterial species including all major bacterial pathogens. Meanwhile, sequencing technology has advanced to a level where bacterial genomes can be sequenced, in principle, within days, and are published under the format of notes rather than full-length articles. The price of bacterial whole-genome sequencing has dropped to the 4-digit US $ level. The total number of sequenced genomes in the public domain is rapidly increasing and may represent up to 0.5‰ of all hitherto reported species [8] including numerous lower and higher animals, several higher plants and representatives of virtually all groups of major human pathogens.
However, the bonanza of novel antibacterial agents that had been expected to result from this wealth of information on pathogen genetics remains elusive. At best, we have witnessed a trickle, and even the few novel agents that have been introduced to the market are typically follow-up drugs rather than novel therapeutic principles. As a notable exception, the mycobacterial adenosine triphosphate synthase was identified as a novel drug target on the basis of genomic sequencing [9]. Tuberculostatic ATPase inhibitors have not yet reached the market, but clinical studies look promising [10].
Critical voices have pointed out that, despite the general pessimism about the growing discrepancy between resistance development and the lack of novel antibiotic principles [11], the conceivable catastrophic developments in the domain of infectious disease have so far not materialized. Thus, even multidrug-resistant tuberculosis can still be contained by the prudent application of available drugs. On the other hand, this favorable state of matters will not necessarily persist forever.
The number of molecular targets that have been addressed hitherto by anti-infective agents is relatively small, in the two-digit range. In other words, the majority of bacterial gene products are not addressed by available antibiotic agents. This could either signify that a large number of targets are still available for future research and discovery, or, alternatively, that only a relatively small subset of gene products can be used for anti-infective therapy and that this subset has been essentially discovered and utilized by the empirical and highly successful approach of past decades [12]. At least for the subgroup of Enterobacteriaceae, whole genome virulence studies suggest tentatively that the set of targets may be rather limited [13, 14]. Notably, however, enzymes of the non-mevalonate pathway appear prominently on the hit-list of these studies.
The present review is focused on the interplay between bacterial genomics, structural biology and assay development in the attempt to discover novel anti-infectives aimed at enzymes of the non-mevalonate pathway of isoprenoid biosynthesis.
Two isoprenoid biosynthetic pathways
Ruzicka’s isoprene rule claiming that all natural terpenes arise from simple five-carbon building blocks was an extremely powerful concept that continues to dominate research on this large group of natural products [15]. Until the late 1980s, the two universal isoprenoid precursors, isopentenyl diphosphate (IPP) (9) and dimethylallyl diphosphate (DMAPP) (10) (Fig. 1), were staunchly believed to be biosynthesized exclusively via the mevalonate pathway (for review, see Refs. [16–19]) that had reached the status of one of the most important drug targets by the introduction of statins for the prevention and treatment of cardiovascular disease [20, 21]. The existence of a second pathway for isoprenoid building blocks was then established independently by the research groups of Rohmer and Arigoni working with eubacteria and plants [22–24]. With hindsight, it is apparent that numerous experimental inconsistencies in earlier studies had been explained away in order to keep the faith in the universality of the “revealed” mevalonate dogma. Work from Rohmer’s and Arigoni’s research groups independently provided evidence that the alternative pathway started from the triose pool of intermediary metabolism [22–24]. Arigoni and his coworkers showed that 1-deoxy-d-xylulose can be incorporated efficiently into isoprenoids [24, 25], and later work established that its 5-phosphate (3) serves as the first intermediate of the novel pathway [26, 27]. Seto and his coworkers could then demonstrate the transformation of that carbohydrate into a branched chain polyol, 2C-methyl-d-erythritol 4-phosphate (4), by a skeletal rearrangement followed by a two-electron reduction step [28].
Fig. 1.
Biosynthetic pathways for the generation of the isoprenoid building units, isopentenyl diphosphate (9) and dimethylallyl diphosphate (10). 1, pyruvate; 2, d-glyceraldehyde 3-phosphate; 3, 1-deoxy-d-xylulose 5-phosphate; 4, 2-C-methyl-d-erythritol 4-phosphate; 5, 4-diphosphocytidyl 2-C-methyl-d-erythritol; 6, 4-diphosphocytidyl 2-C-methyl-d-erythritol 2-phosphate; 7, 2-C-methyl-d-erythritol-2,4-cyclodiphosphate; 8, (E)-1-hydroxy-2-methyl-2-butenyl diphosphate; 11, acetyl-CoA; 12, mevalonate; 13, fosmidomycin
The short and turbulent history of the discovery of the non-mevalonate pathway has been reviewed repeatedly [29–31]. Rather than reiterate these reports, this paper will specifically address the role of the emerging domain of genomics for the elucidation of the non-mevalonate pathway genes and enzymes, and how that information can be harnessed for drug development.
Elucidation of the non-mevalonate pathway using genomics
The sequences of complete genomes became available with increasing frequency after 1995. The first of these, that of H. influenzae, was published in 1995 [7]. It was followed in 1997 by that of Helicobacter pylori [32], and also in 1997 by the long-expected genome of E. coli [33]. Before the end of that prolific decade, the first eukaryote genome (Saccharomyces cerevisiae [34]), the first animal genome (Caenorhabditis elegans [35]), and the first plant genome (Arabidopsis thaliana, [36]) followed, together with many additional bacterial genomes. Since prototype sequences of all mevalonate pathway genes from animals and of two non-mevalonate pathway genes (dxs and dxr/ispC, see below) were already known at the end of the last century, each available genome could be searched for potential orthologs using each of the available templates. Thus, a rapidly progressing number of organisms could be at least tentatively assigned to mevalonate and/or non-mevalonate pathway utilization on basis of sequence comparison (Table 1).
Table 1.
Distribution of genes involved in IPP and DMAPP biosynthesis in completely sequenced organisms
| Organism | Non-mevalonate pathway | Mevalonate pathway | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dxs | ispC | ispD | ispE | ispF | ispG | ispH | hmgs | hmgr | mk | pmk | dpmd | idiI | idiII | |
| Bacteria | ||||||||||||||
| Aquifales (Aquifex aeolicus) | + | + | + | + | + | + | + | − | − | − | − | − | − | − |
| Chlamydia group (Chlamydophila pneumoniae) | + | + | + | + | + | + | + | − | − | − | − | − | − | − |
| Cyanobacteria (Synechocystis sp.) | + | + | + | + | + | + | + | − | − | − | − | − | − | + |
| Deinococcus group (Deinococcus radiodurans) | + | + | + | + | + | + | + | − | − | − | − | − | − | + |
| Firmicutes | ||||||||||||||
| (Bacillus subtilis) | + | + | + | + | + | + | + | − | − | − | − | − | − | + |
| (Mycoplasma genitalium) | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| (Staphyloccus aureus) | − | − | − | − | − | − | − | + | + | + | + | + | − | + |
| (Streptomyces coelicolor) | + | + | + | + | + | + | + | − | − | − | − | − | + | − |
| Proteobacteria | ||||||||||||||
| (Escherichia coli) | + | + | + | + | + | + | + | − | − | − | − | − | + | − |
| (Rickettsia prowazeckii) | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| Spirochaetales | ||||||||||||||
| (Treponema pallidum) | + | + | + | + | + | + | + | − | − | − | − | − | − | − |
| (Borrelia burgdorferi) | − | − | − | − | − | − | − | + | + | + | + | + | − | + |
| Thermotogales (Thermotoga maritima) | + | + | + | + | + | + | + | − | − | − | − | − | − | − |
| Archaea | ||||||||||||||
| Crenarchaeota (Aeropyrum pernix) | − | − | − | − | − | − | − | + | + | + | + | + | − | + |
| Euryarchaeota (Archaeoglobus fulgidus) | − | − | − | − | − | − | − | + | + | + | + | + | − | + |
| Eukaryotes | ||||||||||||||
| Animals (Homo sapiens) | − | − | − | − | − | − | − | + | + | + | + | + | + | − |
| Plants (Arabidopsis thaliana) | + | + | + | + | + | + | + | + | + | + | + | + | + | − |
| Protozoa (Plasmodium falciparum) | + | + | + | + | + | + | + | − | − | − | − | − | − | − |
| Yeasts (Saccharomyces cerevisiae) | − | − | − | − | − | − | − | + | + | + | + | + | + | − |
dxs 1-deoxy-d-xylulose 5-phosphate synthase, ispC 2C-methyl-d-erythritol 4-phosphate synthase, ispD 4-diphosphocytidyl-2C-methyl-d-erythritol synthase, ispE 4-diphosphocytidyl-2C-methyl-d-erythritol kinase, ispF 2C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, ispG 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase, ispH 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase, hmgs 3-hydroxy-3-methylglutaryl-CoA synthase, hmgr 3-hydroxy-3-methylglutaryl-CoA reductase, mk mevalonate kinase, pmk phospomevalonate kinase, dpmd diphospomevalonate decarboxylase, idiI/II isopentenyl diphosphate isomerase type I/II
Using this approach, plants, animals and fungi were shown to carry complete sets of mevalonate pathway gene homologs. Homologs of dxs and dxr/ispC were also identified in plants where they could be assigned as plastid proteins on the basis of sequence arguments. On the other hand, animals and archaea were found to be devoid of non-mevalonate gene homologs. Notably, however, archaea carried only homologs to a subset of animal-type mevalonate genes [37–39]; in retrospect, it is now clear that several mevalonate pathway enzymes of archaea are not the result of divergent evolution but have evolved independently.
Completely sequenced eubacterial genomes comprise either mevalonate genes or non-mevalonate genes except for some Listeriae and Mycobacteriae, as well as Nocardia farcinica which contain complete sets of genes of both pathways, and Rickettsiae and Orientiae lacking both pathways. Notably, most Gram-negatives use the non-mevalonate pathway, whereas certain Gram-positive cocci including Staphylococcus and Enterococcus spp. use the mevalonate pathway [40].
An obvious genome mining strategy could be based on a systematic search for un-annotated genes sharing the distribution pattern of the known dxs and dxr/ispC genes. This computer-based approach successively yielded five candidate genes (ispDEFGH) with the required properties (occurrence in eubacteria, in the company of dxr/ispC and in the absence of mevalonate pathway genes) [41]. These hits are summarized in Table 1.
A branched polyol, 2-C-methylerythritol 4-phosphate, is assembled by Dxs and IspC
In a formal sense, Dxs catalyzes the transfer of an acetaldehyde moiety derived from pyruvate (1) to glyceraldehyde 3-phosphate (2) that serves as acceptor. The product is 1-deoxy-d-xylulose 5-phosphate (3, Fig. 1); the carboxylic group of pyruvate is lost in the form of CO2. Dxs is a member of the large transketolase family as shown by sequence comparison and X-ray structure analysis. In close parallel with transketolases, Dxs uses thiamine pyrophosphate as cofactor for the group transfer reaction. The reaction mechanism is well in line with that of transketolases.
X-Ray structures of Dxs from E. coli and Deinococcus radiodurans have been reported [42] (Fig. 2). The monomeric proteins bind thiamine pyrophosphate and a magnesium ion. The folding patterns of the three domains are similar to those from transketolase, but the topologic relationship between the domains differs significantly from that in transketolase.
Fig. 2.
Crystal structure of the Dxs protein of D. radiodurans in stereo view [42]. a Ribbon plot, b surface representation. The subunits of the homodimer are shown in green and blue. Residues 200–242 are not observed in the structure. Thiamin diphosphate and magnesium are shown in ochre and gray, respectively
IspC (also designated Dxr) catalyzes a rearrangement reaction that transforms the substrate 3 into a branched aldose (16) that is immediately reduced to 2-C-methyl-d-erythritol 4-phosphate (4) using NADPH as cofactor (Fig. 3). The same reaction is catalyzed by a paralogous enzyme detected in Brucella abortus and some other bacteria [43]. The branched aldose intermediate 16 has been synthesized and has been confirmed to be accepted as substrate by IspC [44]. A retroaldol/aldol reaction sequence and a sigmatropic rearrangement have been proposed as alternative reaction mechanisms for the skeletal rearrangement (Fig. 3). The proposed retroaldol cleavage of 3 should afford glycolaldehyde phosphate (14) and hydroxyacetone (15) as intermediates, but a quest to document their involvement in the reaction has turned out negative; hence, a strictly intramolecular, sigmatropic rearrangement would be the logical conclusion [45]. On the other hand, however, kinetic isotope effect arguments have been used to support the retroaldol/aldol hypothesis [46, 47]. IspC catalyzes the transfer of a hydride ion from the pro-S position at C-4 of NADPH to the RE position at C-1 of the aldehyde-type reaction product. The stereochemical features are well in line with the results from X-ray structure analysis (Table 2).
Fig. 3.
Hypothetical reaction mechanisms for IspC protein [44–47]. a Sigmatropic rearrangement; b retroaldol/aldol sequence. Since the hypothetical retroaldol fragments 12 and 13 fail to exchange with the bulk solvent, the retroaldol mechanism would require extremely strict containment of the cleavage product
Table 2.
Overview of non-mevalonate isoprenoid pathway proteins that were functionally and/or structurally analysed
| Protein | Organism | Enzymatic properties | Crystal structure |
|---|---|---|---|
| Dxs | B. subtilis | [129] | |
| D. radiodurans | [42] | ||
| E. coli | [26, 27] | [42] | |
| IspC | E. coli | [28] | [55, 133–136] |
| M. tuberculosis | [131] | [137, 138] | |
| P. falciparum | [49] | [139] | |
| T. maritima | [140] | ||
| Synechocytis sp. PCC6803 | [132] | ||
| Z. mobilis | [141] | ||
| IspD | A. thaliana | [144] | |
| E. coli | [72] | [145, 146] | |
| M. tuberculosis | [142, 143] | ||
| IspE | A. aeolicus | [147] | [84, 85, 147] |
| A. tumefaciens | [148] | ||
| E. coli | [73] | [149] | |
| T. thermophilus | [150] | ||
| IspF | A. thaliana | [152] | |
| E. coli | [74] | [81, 153, 154] | |
| H. influenzae | [155] | ||
| P. falciparum | [151] | ||
| T. thermophilus | [156] | ||
| M. smegmatis | [157] | ||
| IspDF | C. jejuni | [158] | [158] |
| A. tumefaciens | [148] | ||
| M. loti | [159] | ||
| IspG | A. aeolicus | [116] | |
| E. coli | [100] | ||
| T. thermophilus | [105] | [160] | |
| IspH | A. aeolicus | [106] | [120] |
| E. coli | [103] | [118, 119] |
The structures of IspC proteins from several microorganisms, including E. coli, M. tuberculosis, and P. falciparum, have been determined by X-ray crystallography (Table 2; Fig. 4). They are all c2-symmetric homodimers which bind a bivalent metal ion (magnesium or manganese) at their active sites, which is essential for catalysis. The structure comprises a flexible loop that can move over the active site cavity.
Fig. 4.
Crystal structure of the IspC protein of E. coli in stereo view [55, 133–136]. a Ribbon plot, b surface representation. The subunits of the c2-symmetric homodimer are shown in green and blue. The substrate 1-deoxyxylulose 5-phosphate (3) and the coenzyme, NADPH, are shown in ochre and magenta, respectively
IspC protein was shown in the late 1990s to be the target of fosmidomycin (13, Fig. 1) [48, 49], an antibiotic produced by Streptomyces lavendulae that had been under development for clinical use in the 1980s but was later abandoned due to unsatisfactory pharmacodynamics [50]. Ki values for orthologs from various microorganisms are in the nanomolar range [51–54]. Extensive X-ray structure work on various IspC orthologs in complex with fosmidomycin has shown that the mode of action is based on the interaction of the fosmidomycin’s hydroxamate group with the divalent metal ion [55].
Fosmidomycin has been used successfully in small clinical studies for the treatment of malaria [56–58]. However, the high recurrence rate necessitated the combination with established second antimalarial [59–61]. More recently, a variety of aryl and alkyl derivatives of fosmidomycin (13) have been synthesized [51, 53, 62–69]. Some derivatives showed improved IC50 values, in the single digit nanomolar range, for IspC from P. falciparum. However, it remains to be established whether these advances translate into improved therapeutic efficiency in animal models and in human malaria. Whereas fosmidomycin is a potent inhibitor for IspC of M. tuberculosis in vitro, it does not affect bacterial cells. This has been attributed to failure of uptake.
Library screening with IspC protein is relatively straightforward since the reaction is directly chromogenic, and no auxiliary enzymes are required [70]. It will be interesting to see whether the protein can be inhibited by compounds without the structural features of fosmidomycin (i.e. phosphonate and hydroxamate motifs, respectively).
2-C-Methylerythritol 4-phosphate is converted into a cyclic diphosphate by IspD, IspE and IspF
Following their identification by the bioinformatics approach described above, proteins specified by the ispD, ispE and ispF genes could be expressed in a homologous as well as a heterologous manner. Orthologs of these proteins from several pathogens have been described in some detail [71]. The reactions that they catalyze were initially identified by assays using putative, 13C-labeled substrates whose consumption and conversion into products could be monitored in real time by high resolution NMR spectroscopy [72–74]. This work has been reviewed elsewhere, and the reader is directed to these respective articles for [75–78]. The somewhat unusual technology might be applicable for the study of other proteins that present particular difficulties to functional analysis.
IspD protein catalyzes the transfer of a cytidyl phosphate moiety to methylerythritol 4-phosphate (4) [72]. IspE protein catalyzes the transfer of a phosphate residue from ATP to the hydroxy group in position 2 of diphosphocytidyl methylerythritol (5) [73], the product of IspD protein. IspF protein catalyzes an intramolecular transphosphorylation which generates a structurally unusual cyclic diphosphate 7 under release of CMP [74]. Obviously, the nucleoside moiety is specifically introduced to enable the formation of the unusual 8-membered ring in 7. The transition states of all three enzymes are characterized by inline attacks on the respective phosphoanhydride motif that serves as the phosphate donor in each respective reaction (Fig. 1). Reaction mechanisms proposed on the basis of X-ray structure analysis were checked and confirmed by site-directed mutagenesis [79–81].
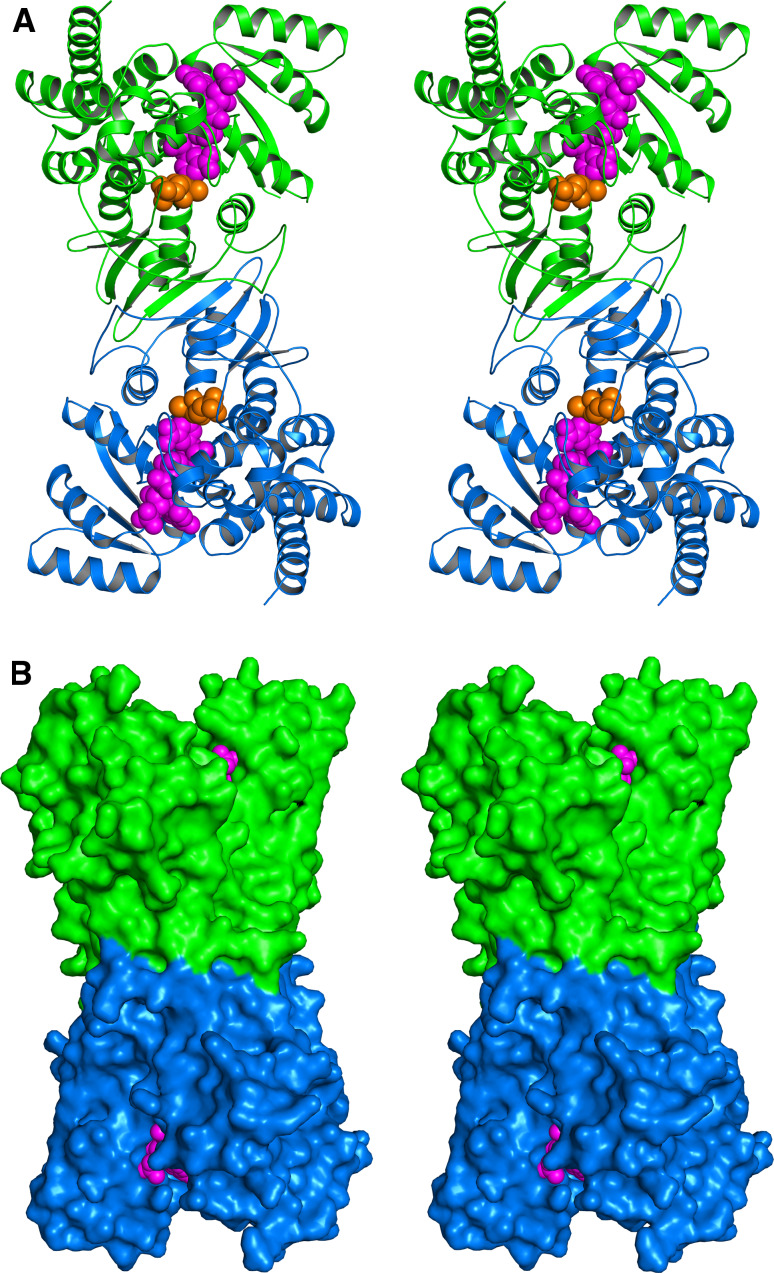
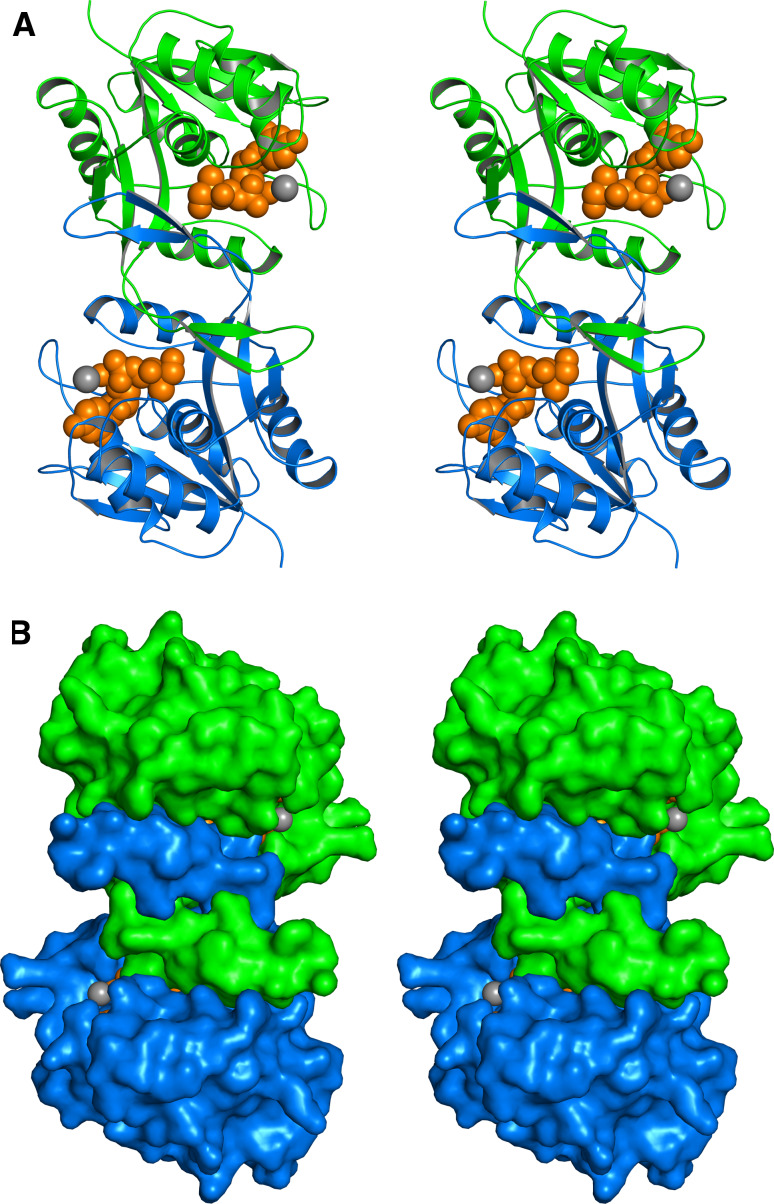
To date, more than a dozen X-ray structures have been reported for IspD, including structures with near-atomic resolution for the E. coli protein in complex with the substrate or product. The protein is a c2-symmetric homodimer, and its subunit consists of a single αβ domain (Fig. 5). The structure of IspD from M. tuberculosis has been released recently (Proton Data Base entry code 3OKR). IspE protein of E. coli is a c2-symmetric homodimer that is characterized by an extended central channel (Fig. 6). The contact areas between the monomers are small. The catalytic site is located close to the subunit interface, but each active site is entirely located within one respective subunit. A recent study on a different crystal form (Protein Data Base entry code 2WW4) suggests that the apparent homodimer structure may be a crystallization artifact [82].
Fig. 5.
Crystal structure of the IspD protein of E. coli in stereo view [145, 146]. a Ribbon plot, b surface representation. The subunits of the c2-symmetric homodimer are shown in green and blue. The product 5 and magnesium are shown in ochre and gray, respectively
Fig. 6.
Crystal structure of IspE protein of E. coli in stereo view [149]. a Ribbon plot, b surface representation. The subunits of the c2-symmetric homodimer are shown in green and blue. The substrate 5 and phosphoaminophosphonic acid-adenylate ester are shown in ochre and magenta, respectively
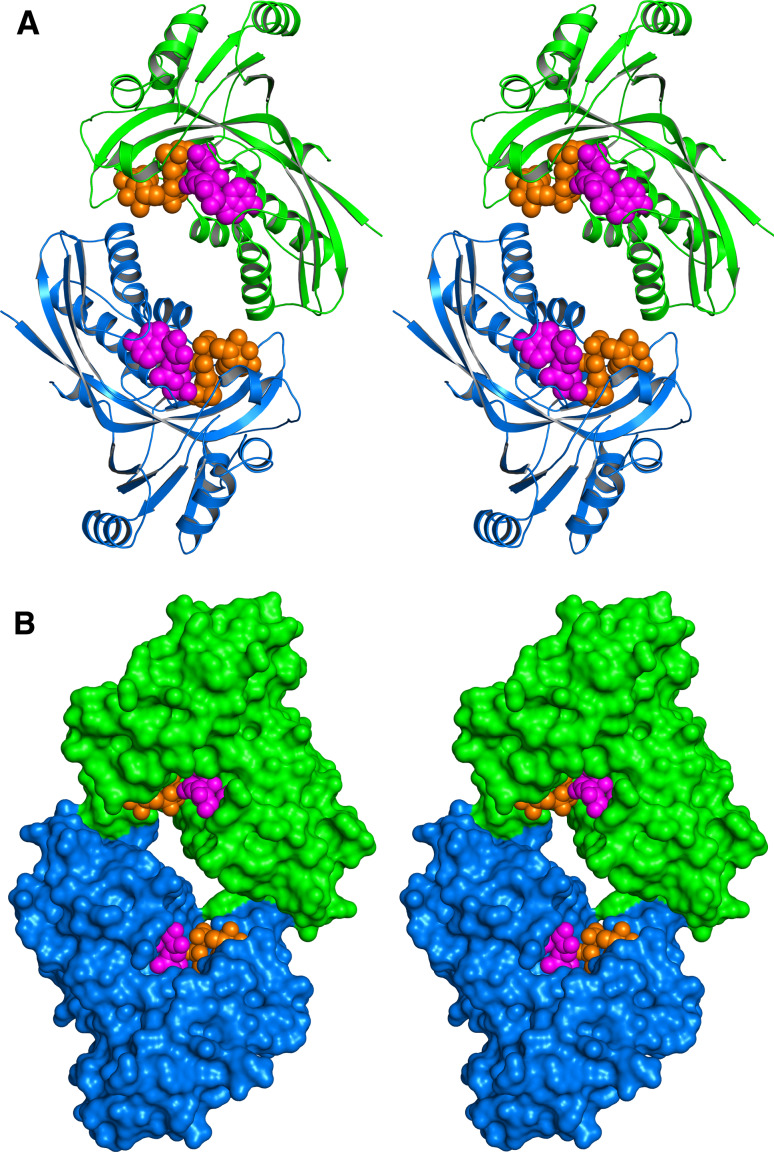
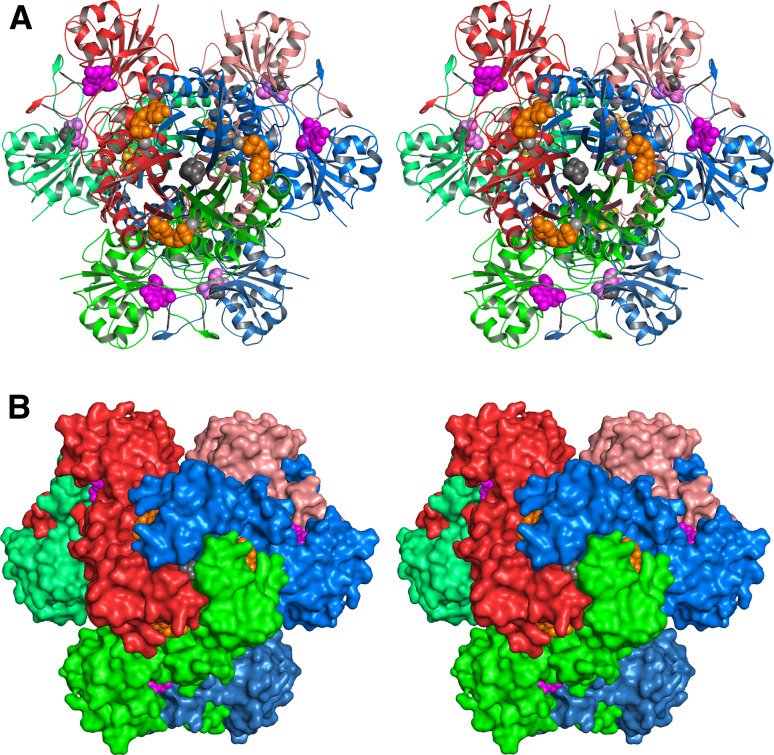
More than 40 X-ray structures have been reported for IspF from various organisms including several important pathogens including Burkholderia pseudomallei, Yersinia pestis, E. coli, and Haemophilus influenzae. Most of these proteins are c3-symmetric homotrimers (Fig. 7). The active sites are located at each respective subunit interface. The enzymes use Zn2+ and Mg2+ ions for catalysis (both cations are required for catalysis). The genomes of the pathogenic Campylobacter jejuni and certain other bacteria specify bifunctional IspDF fusion proteins. IspDF of C. jejuni is a d3-symmetric homohexamer (Fig. 8).
Fig. 7.
Crystal structure of IspF protein of E. coli in stereo view [81, 153, 154]. a Ribbon plot, b surface representation. The subunits of the c3-symmetric homotrimer are shown in red, green and blue. Cytidine-5′-monophosphate, the product 7, and the zink ion are shown in ochre, magenta, and gray, respectively
Fig. 8.
Crystal structure of the IspDF protein of C. jejuni in stereo view [158]. a Ribbon plot, b surface representation. The subunits of the d3-symmetric homohexamer are all shown in different colors. Cytidine-5′-monophosphate is shown in ochre or magenta. The magnesium ion, 1,2-ethanediol, and geranyl diphosphate are shown in light gray, gray, and dark gray, respectively
The structural and mechanistic information summarized above served as the basis for the rational design of inhibitors for IspE and IspF protein [83–90]. In both cases, the cytosine motif present in the substrate and/or product served as the basic template. Substituents that are not structurally related to either substrate or product were designed on basis of computer modeling. Target compounds were then synthesized and were subjected to kinetic analysis. The best IspE inhibitors had IC50 values in the nanomolar range. Some compounds were studied crystallographically in complex with the target enzyme. Interestingly, it turned out that compounds that had been designed with the purpose to inhibit IspF enzyme showed significant inhibitory activity for IspE protein. Based on this, it appears possible to develop drugs designed to inhibit two consecutive reaction steps in the non-mevalonate pathway. This concept appears attractive since dual inhibition could delay resistance development in the microbial target since genetic adaptation of two genetically unrelated proteins would be necessary in order to achieve high-level resistance.
IspG and IspH proteins convert 2C-methylerythritol 2,4-cyclo-diphoshate into IPP and DMAPP
The final steps of the non-mevalonate pathway are catalyzed by two highly oxygen-sensitive iron–sulfur proteins specified by the ispG (gcpE) and ispH (lytB) genes. Again, bioinformatics provided important contributions for the discovery of the genes [41, 91–94]. However, initial attempts to characterize their catalytic activity failed, and an in vivo approach was therefore used for their biochemical characterization [95, 96]. More specifically, an E. coli strain was engineered in order to enable the generation of large amounts of the cyclic pyrophosphate precursor 7 from proffered, 13C-labeled 1-deoxy-d-xylulose. For that purpose, the strain was endowed with a plasmid specifying the IspCDEF proteins and d-xylulokinase, which had been shown to catalyze the phosphorylation of 1-deoxy-d-xylulose [97], albeit at a reduced rate as compared to the physiological substrate. When that strain was proffered with exogenous 13C-labeled 1-deoxy-d-xylulose, the compound was converted into 7 that reached high intracellular concentrations sufficient for detection by 13C NMR in crude cell extract without any preliminary purification [95].
The additional implementation of a recombinant ispG gene into the recombinant E. coli strain enabled the conversion of the intracellular, 13C-labeled 7 into an unknown compound that was identified as 1-hydroxy-2-methyl-2-(E)-butenyl diphosphate (8) by NMR analysis of the crude cell extract. The additional implementation of yet another recombinant gene, ispH (affording a strain that expressed the D-xylulokinase and the complete set of IspCDEFGH proteins), enabled the conversion of exogenous, 13C-labeled 8 into IPP and DMAPP that were both detected in the crude cell extract by 13C NMR spectroscopy [96]. It should be noted that 1-hydroxy-2-methyl-2-(E)-butenyl diphosphate (8) was also isolated by other authors from cell extract of E. coli lacking IspH [98].
The in vitro transformation of 7 into 8 by IspG was initially achieved with photoactivated deazaflavin as cofactor [99, 100]. Later, reduced methylviologen was used as an artificial cofactor for IspG and IspH. The physiological cofactor of IspG and/or IspH in eubacteria is flavodoxin [101]. That protein has been studied extensively as a model flavoprotein [102], but only recently it was shown that it is an essential protein serving as an obligatory electron transponder for iron–sulfur proteins of the non-mevalonate pathway in Gram-negative bacteria. Plants and apicomplexan parasites are believed to use ferredoxin as electron transponders for IspG and IspH.
In early studies, both IspG and IspH protein had poor apparent catalytic activity that could be improved by in vitro reconstitution of the iron–sulfur cluster [100, 103–108]. However, both proteins can be expressed in fully active form, provided that the isc operon catalyzing the synthesis of iron–sulfur clusters is co-expressed [100, 103].
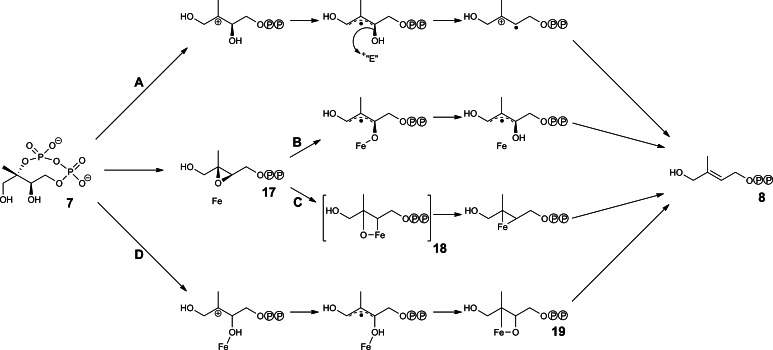
Numerous reaction mechanisms have been proposed for IspG protein (Fig. 9). It is generally assumed that the reaction involves a free radical intermediate that is obtained by electron transfer from the iron–sulfur cluster [100, 104, 105, 109–111]. An oxiran intermediate (17) has been proposed to serve as a reaction intermediate [109, 112–115]. The sequence of electron transfer and bond-breaking steps is still in dispute.
Fig. 9.
Hypothetical reaction mechanisms for IspG protein. a According to [104]; b according to [109]; c according to [115]; d according to [111]
X-ray structure analysis has revealed an unusual homodimer structure for IspG protein from A. aeolicus [116] (Table 2; Fig. 10). Each domain folds into an 8-stranded N-terminal β barrel and a C-terminal domain that binds a [4Fe–4S] cluster. The N-terminal domain is a member of the very large TIM barrel superfamily [116, 117]; in line with that family association, a large patch of strictly conserved, polar amino acid residues at the apical barrel pole qualifies as the binding site for the negatively charged substrate. The C-terminal domain comprises a β-sheet that is flanked on both sides by helices and is similar to ferredoxin domains from various proteins. The iron–sulfur cluster is coordinated by three strictly conserved cystein residues and a strictly conserved glutamate residue. The enzyme has been crystallized in an open conformation where the iron–sulfur cluster is remote from the putative substrate binding site. A substantial conformational change is believed to follow the binding of the substrate and to result in a closed conformation where the iron–sulfur cluster can interact directly with the substrate; however, that hypothetical closed conformation still awaits experimental confirmation.
Fig. 10.
Crystal structure of the IspG protein of A. aeolicus in stereo view [116]. a Ribbon plot, b surface representation. The subunits of the c2-symmetric homodimer are shown in green and blue. The [4Fe–4S] cluster is shown in red (iron) and yellow (sulfur)
Knowledge on IspH protein has also been substantially advanced by recent X-ray structure analysis (Table 2, Fig. 11) [118–121]. The monomeric protein folds into three closely similar domains, although the domains have no detectable sequence similarity. The active site is located at the approximate center of mass and comprises an iron–sulfur cluster which is coordinated by three strictly conserved cysteine residues. Whereas it is now generally agreed that IspH utilizes a [4Fe–4S] cluster as cofactor for catalysis [118, 122], the fourth iron ion, which is not coordinated by an amino acid residue, is easily lost. The substrate is believed to bind to the enzyme in the open conformation [118, 120, 121]. A conformational transition involving a rotation of one of the three folding domains of the monomeric protein is then conducive to a closed conformation where the substrate is completely shielded from the bulk solvent [118, 119, 121]. Only a single water molecule is left inside the loaded active site cavity. This means that hydration water must be almost entirely stripped from the hydrophilic substrate and the active site surface during the formation of a Michaelis complex.
Fig. 11.
Crystal structure of the IspH protein of E. coli in stereo view [118, 119]. a Ribbon plot, b close-up of the active site, c surface representation. The subunits of the c2-symmetric homodimer are shown in green and blue. The [4Fe–4S] cluster is shown in red (iron) and yellow (sulfur). The substrate 8 is shown in ochre
Catalysis is believed to involve the stepwise transfer of two single electrons from the iron–sulfur cluster of IspH. The hydroxy group in position 1 of the substrate is believed to be removed from a free radical intermediate by heterolytic cleavage that requires the prior transfer of a proton to the reactant (Fig. 12) [121, 123]. The subsequent transfer of a second electron affords an allyl carbanion intermediate which can be reprotonated alternatively in two different positions affording either DMAPP or IPP. The formation of these products is thermodynamically controlled. The reprotonation at C-3 affording IPP occurs in the Si position. Since the reactants in the IspH catalyzed trajectory assume a hairpin conformation, the stereochemistry of the reprotonation reaction suggests that the pyrophosphate moiety of the reaction intermediate serves as an intramolecular proton donor for IPP formation [121]. Recent EPR and Endor studies have provided evidence for potential reactive intermediates probably involving metallacycles [124–126]. This has prompted the synthesis of acetylene type substrate analogs that can also coordinate to the iron center. IC50 values in the submicromolar range have been reported for these compounds [126].
Fig. 12.
Hypothetical reaction mechanism of IspH protein. Partitioning in the final reaction step (affording IPP and DMAPP) is kinetically controlled
High-throughput assays for library screening of IspD, IspE and IspF
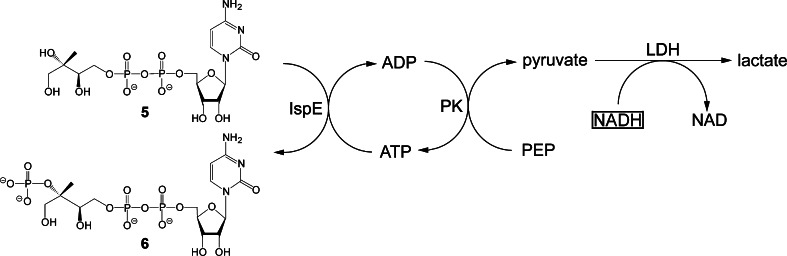
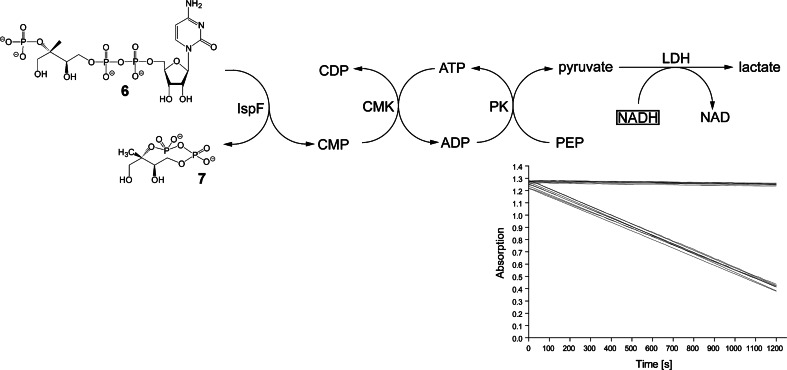
Beside the structure-based, rational design of inhibitors, the screening of compound libraries has become a potent tool for the discovery of lead compounds for drug development. In order to enable the rapid and reliable screening of tens or hundreds of thousands of compounds, the assay readout should be rapid and reliable. Optical methods in general and absorption spectroscopy in particular are best suited to fulfil these requirements. None of the enzyme reactions catalyzed by IspD, IspE or IspF protein is accompanied by a significant absorbance or fluorescence change, but each of the reactions can be coupled to auxiliary chromogenic reactions. Reaction topologies for each of the three enzymes are summarized in Figs. 13, 14 and 15. In each case, the chromogenic reaction consists in the enzyme-catalyzed reduction of pyruvate that is generated by a reaction cascade involving a second enzyme from the non-mevalonate pathway as part of the enzyme auxiliary [70]. The reduction of pyruvate can be monitored optically via the consumption of NADH. For the assays to be specific, it is crucial to use each of the auxiliary enzymes in a large excess in order to establish bottle-neck characteristics for the target enzyme. Notably, since the enzymes of the non-mevalonate pathway are all relatively slow catalysts, screening of large libraries requires multigram amounts of proteins and substrates despite assay miniaturization.
Fig. 13.
High throughput screening assay for IspD protein. PK pyruvate kinase, LDH lactate dehydrogenase. Inset Progression curves of assays and controls monitored photometrically at 340 nm (12 signal assays with substrate (3) and 12 control assays without substrate; assays were run in 96-well microtiter plates)
Fig. 14.
High throughput screening assay for IspE protein. PK pyruvate kinase, LDH lactate dehydrogenase
Fig. 15.
High throughput screening assay for IspF protein. PK pyruvate kinase, LDH lactate dehydrogenase, CMK cytidylate kinase. Inset Progression curves of assays and controls monitored photometrically at 340 nm (8 signal assays with substrate (5) and 8 control assays without substrate; assays were run in 96-well microtiter plates
It should also be noted that at least some of the required enzyme substrates are not easily accessible. Whereas they can all be generated by established enzyme-mediated procedures [70, 127–130], relatively large amounts of protein are required for the purpose. Luckily, genetic engineering technology provides access to efficiently expressed recombinant proteins with optimized properties. It is extremely useful in that sense that genes can be picked from large collections of orthologs representing a wide variety of organisms and can be tailored for optimal expression and other properties. Notably, synthetic genes have been used routinely for that purpose in case of the studies on non-mevalonate pathway enzymes.
Despite the high degree of assay reliability, it is still necessary to cross-check the results of library screens with multi-component multi-reaction screens by an independent method. As mentioned above, the three enzymes discussed in the present section can all be monitored with very high selectivity and acceptable sensitivity by NMR spectroscopy [70], provided that substrates are available in 13C-labeled form. As mentioned above, 13C-labeled substrates for this purpose are accessible via enzyme-assisted synthesis (for review, see [76]). Proof of principle for this approach has been recently achieved by the discovery of heterocyclic IspF inhibitors. The screening of a library of mostly heterocyclic compounds afforded thiazolopyrimidine type inhibitors with IC50 values in the low micromolar range for IspF of P. falciparum and M. tuberculosis [83]. The compounds showed some inhibition of P. falciparum in human erythrocytes.
Conclusion
The scientific investigation of the genes and proteins of the non-mevalonate pathway has progressed rapidly as a result of close interaction between genomic and bioinformatic tools with enzymology and physical biochemistry. This development provides numerous opportunities for translational research directed at drug development. Many pathogenic bacteria including M. tuberculosis and an important group of protozoan parasites causing malaria and toxoplasmosis, respectively, are absolutely dependent on endogenous isoprenoid biosynthesis via the non-mevalonate pathway. The absence of these enzymes in the human host, where isoprenoids are biosynthesized via the mevalonate pathway, appears highly favorable under toxicological aspects, since anti-infective drugs acting against non-mevalonate pathway enzymes should be exempt from target-related toxicity. The combined efforts in enzymology, crystallography and biophysics of the non-mevalonate pathway enzymes can now serve as a solid platform for inhibitor design and development using all the tools of the trade including rational, structure-based and mechanism-based drug design, screening of large compound libraries and virtual screening.
Acknowledgments
Financial support by the Deutsche Forschungsgemeinschaft, and the Hans-Fischer-Gesellschaft is gratefully acknowledged.
References
- 1.Cohen ML. Changing patterns of infectious disease. Nature. 2000;406:762–767. doi: 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- 2.Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- 3.Wade MM, Zhang Y. Mechanisms of drug resistance in Mycobacterium tuberculosis . Front Biosci. 2004;9:975–994. doi: 10.2741/1289. [DOI] [PubMed] [Google Scholar]
- 4.Morlais I, Mori A, Schneider JR, Severson DW. A targeted approach to the identification of candidate genes determining susceptibility to Plasmodium gallinaceum in Aedes aegypti . Mol Genet Genomics. 2003;269:753–764. doi: 10.1007/s00438-003-0882-7. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RP. The antibiotic pipeline–challenges, costs, and values. N Engl J Med. 2004;351:523–526. doi: 10.1056/NEJMp048093. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq E. Emerging anti-HIV drugs. Expert Opin Emerg Drugs. 2005;10:241–273. doi: 10.1517/14728214.10.2.241. [DOI] [PubMed] [Google Scholar]
- 7.Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 8.Nisimov F (2003) The physics factbook. Number of species. http://hypertextbook.com/facts/2003/FelixNisimov.shtml
- 9.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis . Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 10.Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RPG, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, Neeley DFM. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 11.Leeb M. Antibiotics: a shot in the arm. Nature. 2004;431:892–893. doi: 10.1038/431892a. [DOI] [PubMed] [Google Scholar]
- 12.Schmid MB. Do targets limit antibiotic discovery? Nat Biotechnol. 2006;24:419–420. doi: 10.1038/nbt0406-419. [DOI] [PubMed] [Google Scholar]
- 13.Becker D, Selbach M, Rollenhagen C, Ballmaier M, Meyer TF, Mann M, Bumann D. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature. 2006;440:303–307. doi: 10.1038/nature04616. [DOI] [PubMed] [Google Scholar]
- 14.Mdluli K, Spigelman M. Novel targets for tuberculosis drug discovery. Curr Opin Pharmacol. 2006;6:459–467. doi: 10.1016/j.coph.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Ruzicka L. The isoprene rule and the biogenesis of terpenic compounds. Experientia. 1953;9:357–367. doi: 10.1007/BF02167631. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi N, Porter JW. Conversion of acetyl-coenzyme A to isopentenyl pyrophosphate. In: Porter JW, Spurgeon SL, editors. Biosynthesis of isoprenoid compounds. New York: Wiley; 1981. pp. 47–94. [Google Scholar]
- 17.Bloch K. Sterol molecule: structure, biosynthesis, and function. Steroids. 1992;57:378–383. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- 18.Bach TJ. Some aspects of isoprenoid biosynthesis in plants. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- 19.Bochar DA, Friesen JA, Stauffacher CV, Rodwell VW. Biosynthesis of mevalonic acid from acetyl-CoA. In: Cane DE, editor. Comprehensive natural product chemistry. Oxford: Pergamon; 1999. pp. 15–44. [Google Scholar]
- 20.Slater EE, MacDonald JS. Mechanism of action and biological profile of HMG CoA reductase inhibitors. A new therapeutic alternative. Drugs. 1988;36(Suppl 3):72–82. doi: 10.2165/00003495-198800363-00016. [DOI] [PubMed] [Google Scholar]
- 21.Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz MK (1994) Terpen-Biosynthese in Ginkgo biloba: eine überraschende Geschichte. PhD Thesis, ETH Zürich, Zürich
- 24.Broers STJ (1994) Über die frühen Vorstufen der Biosynthese von Isoprenoiden in Escherichia coli. PhD Thesis, ETH Zürich, Zürich
- 25.Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH. Terpenoid biosynthesis from 1-deoxy-d-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprenger GA, Schorken U, Wiegert T, Grolle S, de Graaf AA, Taylor SV, Begley TP, Bringer-Meyer S, Sahm H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange BM, Wildung MR, McCaskill D, Croteau R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi S, Kuzuyama T, Watanabe H, Seto H. A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz MK, Arigoni D. Ginkgolide biosynthesis. In: Cane DE, editor. Comprehensive natural product chemistry, vol 2. Oxford: Pergamon; 1999. pp. 367–399. [Google Scholar]
- 30.Rohmer M. A mevalonate-independent route to isopentenyl diphosphate. In: Cane DE, editor. Comprehensive natural product chemistry. Oxford: Pergamon; 1999. pp. 45–68. [Google Scholar]
- 31.Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 32.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC. The complete genome sequence of the gastric pathogen Helicobacter pylori . Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 33.Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 34.Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG. Life with 6000 genes. Science. 1996;274(546):563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 35.C. elegans Sequencing Consortium Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 36.Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature. (2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 37.Boucher Y, Doolittle WF. The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways. Mol Microbiol. 2000;37:703–716. doi: 10.1046/j.1365-2958.2000.02004.x. [DOI] [PubMed] [Google Scholar]
- 38.Smit A, Mushegian A. Biosynthesis of isoprenoids via mevalonate in Archaea: the lost pathway. Genome Res. 2000;10:1468–1484. doi: 10.1101/gr.145600. [DOI] [PubMed] [Google Scholar]
- 39.Grochowski LL, Xu H, White RH. Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J Bacteriol. 2006;188:3192–3198. doi: 10.1128/JB.188.9.3192-3198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laupitz R, Hecht S, Amslinger S, Zepeck F, Kaiser J, Richter G, Schramek N, Steinbacher S, Huber R, Arigoni D, Bacher A, Eisenreich W, Rohdich F. Biochemical characterization of Bacillus subtilis type II isopentenyl diphosphate isomerase, and phylogenetic distribution of isoprenoid biosynthesis pathways. Eur J Biochem. 2004;271:2658–2669. doi: 10.1111/j.1432-1033.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 41.Adam P, Bacher A, Eisenreich W, Fellermeier M, Hecht S, Rohdich F, Schuhr CA, Wungsintaweekul J, Zenk MH (2001) The non-mevalonate isoprenoid pathway. International Patent WO0194561
- 42.Xiang S, Usunow G, Lange G, Busch M, Tong L. Crystal structure of 1-deoxy-d-xylulose 5-phosphate synthase, a crucial enzyme for isoprenoids biosynthesis. J Biol Chem. 2007;282:2676–2682. doi: 10.1074/jbc.M610235200. [DOI] [PubMed] [Google Scholar]
- 43.Sangari FJ, Perez-Gil J, Carretero-Paulet L, Garcia-Lobo JM, Rodriguez-Concepcion M. A new family of enzymes catalyzing the first committed step of the methylerythritol 4-phosphate (MEP) pathway for isoprenoid biosynthesis in bacteria. Proc Natl Acad Sci USA. 2010;107:14081–14086. doi: 10.1073/pnas.1001962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoeffler JF, Tritsch D, Grosdemange-Billiard C, Rohmer M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway. Mechanistic investigations of the 1-deoxy-d-xylulose 5-phosphate reductoisomerase. Eur J Biochem. 2002;269:4446–4457. doi: 10.1046/j.1432-1033.2002.03150.x. [DOI] [PubMed] [Google Scholar]
- 45.Lauw S, Illarionova V, Bacher A, Eisenreich W, Rohdich F. Biosynthesis of isoprenoids: studies on the mechanism of 2C-methyl-d-erythritol 4-phosphate synthase. FEBS J. 2007;275:4060–4073. doi: 10.1111/j.1742-4658.2008.06547.x. [DOI] [PubMed] [Google Scholar]
- 46.Wong U, Cox RJ. The chemical mechanism of d-1-deoxyxylulose-5-phosphate reductoisomerase from Escherichia coli . Angew Chem Int Ed Engl. 2007;46:4926–4929. doi: 10.1002/anie.200700647. [DOI] [PubMed] [Google Scholar]
- 47.Munos JW, Pu X, Mansoorabadi SO, Kim HJ, Liu H-W. A secondary kinetic isotope effect study of the 1-deoxy-d-xylulose-5-phosphate reductoisomerase-catalyzed reaction: evidence for a retroaldol-aldol rearrangement. J Am Chem Soc. 2009;131:2048–2049. doi: 10.1021/ja807987h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuzuyama T, Shimizu T, Takahashi S, Seto H. Fosmidomycin, a specific inhibitor of 1-deoxy-d-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett. 1998;39:7913–7916. [Google Scholar]
- 49.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 50.Kuemmerle HP, Murakawa T, Sakamoto H, Sato N, Konishi T, De Santis F. Fosmidomycin, a new phosphonic acid antibiotic. Part II: 1. Human pharmacokinetics. 2. Preliminary early phase IIa clinical studies. Int J Clin Pharmacol Ther Toxicol. 1985;23:521–528. [PubMed] [Google Scholar]
- 51.Behrendt CT, Kunfermann A, Illarionova V, Matheeussen A, Gräwert T, Groll M, Rohdich F, Bacher A, Eisenreich W, Fischer M, Maes L, Kurz T. Synthesis and antiplasmodial activity of highly active reverse analogues of the antimalarial drug candidate fosmidomycin. ChemMedChem. 2010;5:1673–1676. doi: 10.1002/cmdc.201000276. [DOI] [PubMed] [Google Scholar]
- 52.Giessmann D, Heidler P, Haemers T, Van Calenbergh S, Reichenberg A, Jomaa H, Weidemeyer C, Sanderbrand S, Wiesner J, Link A. Towards new antimalarial drugs: synthesis of non-hydrolyzable phosphate mimics as feed for a predictive QSAR study on 1-deoxy-d-xylulose-5-phosphate reductoisomerase inhibitors. Chem Biodivers. 2008;5:643–656. doi: 10.1002/cbdv.200890060. [DOI] [PubMed] [Google Scholar]
- 53.Zingle C, Kuntz L, Tritsch D, Grosdemange-Billiard C, Rohmer M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway: structural variations around phosphonate anchor and spacer of fosmidomycin, a potent inhibitor of deoxyxylulose phosphate reductoisomerase. J Org Chem. 2010;75:3203–3207. doi: 10.1021/jo9024732. [DOI] [PubMed] [Google Scholar]
- 54.Kuntz L, Tritsch D, Grosdemange-Billiard C, Hemmerlin A, Willem A, Bach TJ, Rohmer M. Isoprenoid biosynthesis as a target for antibacterial and antiparasitic drugs: phosphonohydroxamic acids as inhibitors of deoxyxylulose phosphate reducto-isomerase. Biochem J. 2005;386:127–135. doi: 10.1042/BJ20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinbacher S, Kaiser J, Eisenreich W, Huber R, Bacher A, Rohdich F. Structural basis of fosmidomycin action revealed by the complex with 2-C-methyl-d-erythritol 4-phosphate synthase (IspC). Implications for the catalytic mechanism and anti-malaria drug development. J Biol Chem. 2003;278:18401–18407. doi: 10.1074/jbc.M300993200. [DOI] [PubMed] [Google Scholar]
- 56.Wiesner J, Borrmann S, Jomaa H. Fosmidomycin for the treatment of malaria. Parasitol Res. 2003;90(Suppl 2):S71–S76. doi: 10.1007/s00436-002-0770-9. [DOI] [PubMed] [Google Scholar]
- 57.Lell B, Ruangweerayut R, Wiesner J, Missinou MA, Schindler A, Baranek T, Hintz M, Hutchinson D, Jomaa H, Kremsner PG. Fosmidomycin, a novel chemotherapeutic agent for malaria. Antimicrob Agents Chemother. 2003;47:735–738. doi: 10.1128/AAC.47.2.735-738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Missinou MA, Borrmann S, Schindler A, Issifou S, Adegnika AA, Matsiegui PB, Binder R, Lell B, Wiesner J, Baranek T, Jomaa H, Kremsner PG. Fosmidomycin for malaria. Lancet. 2002;360:1941–1942. doi: 10.1016/S0140-6736(02)11860-5. [DOI] [PubMed] [Google Scholar]
- 59.Borrmann S, Issifou S, Esser G, Adegnika AA, Ramharter M, Matsiegui PB, Oyakhirome S, Mawili-Mboumba DP, Missinou MA, Kun JF, Jomaa H, Kremsner PG. Fosmidomycin-clindamycin for the treatment of Plasmodium falciparum malaria. J Infect Dis. 2004;190:1534–1540. doi: 10.1086/424603. [DOI] [PubMed] [Google Scholar]
- 60.Borrmann S, Adegnika AA, Matsiegui PB, Issifou S, Schindler A, Mawili-Mboumba DP, Baranek T, Wiesner J, Jomaa H, Kremsner PG. Fosmidomycin-clindamycin for Plasmodium falciparum Infections in African children. J Infect Dis. 2004;189:901–908. doi: 10.1086/381785. [DOI] [PubMed] [Google Scholar]
- 61.Borrmann S, Lundgren I, Oyakhirome S, Impouma B, Matsiegui PB, Adegnika AA, Issifou S, Kun JF, Hutchinson D, Wiesner J, Jomaa H, Kremsner PG. Fosmidomycin plus clindamycin for treatment of pediatric patients aged 1 to 14 years with Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2006;50:2713–2718. doi: 10.1128/AAC.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurz T, Behrendt C, Pein M, Kaula U, Bergmann B, Walter RD. γ-Substituted bis(pivaloyloxymethyl)ester analogues of fosmidomycin and FR900098. Arch Pharm. 2007;340:661–666. doi: 10.1002/ardp.200700107. [DOI] [PubMed] [Google Scholar]
- 63.Kurz T, Schlüter K, Pein M, Behrendt C, Bergmann B, Walter RD. Conformationally restrained aromatic analogues of fosmidomycin and FR900098. Arch Pharm. 2007;340:339–344. doi: 10.1002/ardp.200700013. [DOI] [PubMed] [Google Scholar]
- 64.Devreux V, Wiesner J, Jomaa H, Van der Eycken J, Van Calenbergh S. Synthesis and evaluation of α,β-unsaturated α-aryl-substituted fosmidomycin analogues as DXR inhibitors. Bioorg Med Chem Lett. 2007;17:4920–4923. doi: 10.1016/j.bmcl.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Devreux V, Wiesner J, Jomaa H, Rozenski J, Van der Eycken J, Van Calenbergh S. Divergent strategy for the synthesis of α-Aryl-substituted fosmidomycin analogues. J Org Chem. 2007;72:3783–3789. doi: 10.1021/jo0700981. [DOI] [PubMed] [Google Scholar]
- 66.Ortmann R, Wiesner J, Silber K, Klebe G, Jomaa H, Schlitzer M. Novel deoxyxylulosephosphate-reductoisomerase inhibitors: fosmidomycin derivatives with spacious acyl residues. Arch Pharm. 2007;340:483–490. doi: 10.1002/ardp.200700149. [DOI] [PubMed] [Google Scholar]
- 67.Devreux V, Wiesner J, Goeman JL, Van der Eycken J, Jomaa H, Van Calenbergh S. Synthesis and biological evaluation of cyclopropyl analogues of fosmidomycin as potent Plasmodium falciparum growth inhibitors. J Med Chem. 2006;49:2656–2660. doi: 10.1021/jm051177c. [DOI] [PubMed] [Google Scholar]
- 68.Haemers T, Wiesner J, Van Poecke S, Goeman J, Henschker D, Beck E, Jomaa H, Van Calenbergh S. Synthesis of alpha-substituted fosmidomycin analogues as highly potent Plasmodium falciparum growth inhibitors. Bioorg Med Chem Lett. 2006;16:1888–1891. doi: 10.1016/j.bmcl.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 69.Ortmann R, Wiesner J, Reichenberg A, Henschker D, Beck E, Jomaa H, Schlitzer M. Alkoxycarbonyloxyethyl ester prodrugs of FR900098 with improved in vivo antimalarial activity. Arch Pharm. 2005;338:305–314. doi: 10.1002/ardp.200500976. [DOI] [PubMed] [Google Scholar]
- 70.Illarionova V, Kaiser J, Ostrojenkova E, Bacher A, Eisenreich W, Rohdich F. Non-mevalonate terpene biosynthesis enzymes as antiinfective drug targets. Substrate synthesis and high throughput screening methods. J Org Chem. 2006;71:8824–8834. doi: 10.1021/jo061466o. [DOI] [PubMed] [Google Scholar]
- 71.Rohdich F, Bacher A, Eisenreich W. Isoprenoid biosynthetic pathways as anti-infective drug targets. Biochem Soc Trans. 2005;33:785–791. doi: 10.1042/BST0330785. [DOI] [PubMed] [Google Scholar]
- 72.Rohdich F, Wungsintaweekul J, Fellermeier M, Sagner S, Herz S, Kis K, Eisenreich W, Bacher A, Zenk MH. Cytidine 5’-triphosphate-dependent biosynthesis of isoprenoids: YgbP protein of Escherichia coli catalyzes the formation of 4-diphosphocytidyl-2-C-methylerythritol. Proc Natl Acad Sci USA. 1999;96:11758–11763. doi: 10.1073/pnas.96.21.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lüttgen H, Rohdich F, Herz S, Wungsintaweekul J, Hecht S, Schuhr CA, Fellermeier M, Sagner S, Zenk MH, Bacher A, Eisenreich W. Biosynthesis of terpenoids: YchB protein of Escherichia coli phosphorylates the 2-hydroxy group of 4-diphosphocytidyl-2C-methyl-d-erythritol. Proc Natl Acad Sci USA. 2000;97:1062–1067. doi: 10.1073/pnas.97.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herz S, Wungsintaweekul J, Schuhr CA, Hecht S, Lüttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk MH, Bacher A, Rohdich F. Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate to 2C-methyl-d-erythritol 2,4-cyclodiphosphate. Proc Natl Acad Sci USA. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001;6:78–84. doi: 10.1016/s1360-1385(00)01812-4. [DOI] [PubMed] [Google Scholar]
- 76.Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci. 2004;61:1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rohdich F, Kis K, Bacher A, Eisenreich W. The non-mevalonate pathway of isoprenoids: genes, enzymes and intermediates. Curr Opin Chem Biol. 2001;5:535–540. doi: 10.1016/s1367-5931(00)00240-4. [DOI] [PubMed] [Google Scholar]
- 78.Rohdich F, Hecht S, Bacher A, Eisenreich W. Deoxyxylulose phosphate pathway of isoprenoid biosynthesis. Discovery and function of ispDEFGH genes and their cognate enzymes. Pure Appl Chem. 2003;75:393–405. [Google Scholar]
- 79.Richard SB, Lillo AM, Tetzlaff CN, Bowman ME, Noel JP, Cane DE. Kinetic analysis of Escherichia coli 2-C-methyl-d-erythritol-4-phosphate cytidyltransferase, wild type and mutants, reveals roles of active site amino acids. Biochemistry. 2004;43:12189–12197. doi: 10.1021/bi0487241. [DOI] [PubMed] [Google Scholar]
- 80.Sgraja T, Kemp LE, Ramsden N, Hunter WN. A double mutation of Escherichia coli 2C-methyl-d-erythritol-2,4-cyclodiphosphate synthase disrupts six hydrogen bonds with, yet fails to prevent binding of, an isoprenoid diphosphate. Acta Crystallogr Sect F. 2005;61:625–629. doi: 10.1107/S1744309105018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinbacher S, Kaiser J, Wungsintaweekul J, Hecht S, Eisenreich W, Gerhardt S, Bacher A, Rohdich F. Structure of 2C-methyl-d-erythritol-2,4-cyclodiphosphate synthase involved in mevalonate-independent biosynthesis of isoprenoids. J Mol Biol. 2002;316:79–88. doi: 10.1006/jmbi.2001.5341. [DOI] [PubMed] [Google Scholar]
- 82.Kalinowska-Tluscik J, Miallau L, Gabrielsen M, Leonard GA, McSweeney SM, Hunter WN. A triclinic crystal form of Escherichia coli 4-diphosphocytidyl-2C-methyl-d-erythritol kinase and reassessment of the quaternary structure. Acta Crystallogr Sect F. 2010;66:237–241. doi: 10.1107/S1744309109054591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geist JG, Lauw S, Illarionova V, Illarionov B, Fischer M, Gräwert T, Rohdich F, Eisenreich W, Kaiser J, Groll M, Scheurer C, Wittlin S, Alonso-Gomez JL, Schweizer WB, Bacher A, Diederich F. Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from Mycobacterium tuberculosis and Plasmodium falciparum . ChemMedChem. 2010;5:1092–1101. doi: 10.1002/cmdc.201000083. [DOI] [PubMed] [Google Scholar]
- 84.Hirsch AK, Alphey MS, Lauw S, Seet M, Barandun L, Eisenreich W, Rohdich F, Hunter WN, Bacher A, Diederich F. Inhibitors of the kinase IspE: structure-activity relationships and co-crystal structure analysis. Org Biomol Chem. 2008;6:2719–2730. doi: 10.1039/b804375b. [DOI] [PubMed] [Google Scholar]
- 85.Crane CM, Hirsch AK, Alphey MS, Sgraja T, Lauw S, Illarionova V, Rohdich F, Eisenreich W, Hunter WN, Bacher A, Diederich F. Synthesis and characterization of cytidine derivatives that inhibit the kinase IspE of the non-mevalonate pathway for isoprenoid biosynthesis. ChemMedChem. 2008;3:91–101. doi: 10.1002/cmdc.200700208. [DOI] [PubMed] [Google Scholar]
- 86.Zürcher M, Diederich F. Structure-based drug design: exploring the proper filling of apolar pockets at enzyme active sites. J Org Chem. 2008;73:4345–4361. doi: 10.1021/jo800527n. [DOI] [PubMed] [Google Scholar]
- 87.Crane CM, Kaiser J, Ramsden NL, Lauw S, Rohdich F, Eisenreich W, Hunter WN, Bacher A, Diederich F. Fluorescent inhibitors for IspF, an enzyme in the non-mevalonate pathway for isoprenoid biosynthesis and a potential target for antimalarial therapy. Angew Chem Int Ed Engl. 2006;45:1069–1074. doi: 10.1002/anie.200503003. [DOI] [PubMed] [Google Scholar]
- 88.Hirsch AK, Lauw S, Gersbach P, Schweizer WB, Rohdich F, Eisenreich W, Bacher A, Diederich F. Nonphosphate inhibitors of IspE protein, a kinase in the non-mevalonate pathway for isoprenoid biosynthesis and a potential target for antimalarial therapy. ChemMedChem. 2007;2:806–810. doi: 10.1002/cmdc.200700014. [DOI] [PubMed] [Google Scholar]
- 89.Baumgartner C, Eberle C, Lauw S, Rohdich F, Eisenreich W, Bacher A, Diederich F. Structure-based design and synthesis of the first week non-phosphate inhibitors for IspF, an enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. Helv Chim Acta. 2007;90:1043–1068. [Google Scholar]
- 90.Ramsden NL, Buetow L, Dawson A, Kemp LA, Ulaganathan V, Brenk R, Klebe G, Hunter WN. A structure-based approach to ligand discovery for 2C-methyl-d-erythritol-2,4-cyclodiphosphate synthase: a target for antimicrobial therapy. J Med Chem. 2009;52:2531–2542. doi: 10.1021/jm801475n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campos N, Rodríguez-Concepción M, Seemann M, Rohmer M, Boronat A. Identification of gcpE as a novel gene of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis in Escherichia coli . FEBS Lett. 2001;488:170–173. doi: 10.1016/s0014-5793(00)02420-0. [DOI] [PubMed] [Google Scholar]
- 92.Altincicek B, Kollas AK, Sanderbrand S, Wiesner J, Hintz M, Beck E, Jomaa H. GcpE is involved in the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli . J Bacteriol. 2001;183:2411–2416. doi: 10.1128/JB.183.8.2411-2416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Altincicek B, Kollas A, Eberl M, Wiesner J, Sanderbrand S, Hintz M, Beck E, Jomaa H. LytB, a novel gene of the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli . FEBS Lett. 2001;499:37–40. doi: 10.1016/s0014-5793(01)02516-9. [DOI] [PubMed] [Google Scholar]
- 94.Cunningham FX, Jr, Lafond TP, Gantt E. Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J Bacteriol. 2000;182:5841–5848. doi: 10.1128/jb.182.20.5841-5848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hecht S, Eisenreich W, Adam P, Amslinger S, Kis K, Bacher A, Arigoni D, Rohdich F. Studies on the nonmevalonate pathway to terpenes: the role of the GcpE (IspG) protein. Proc Natl Acad Sci USA. 2001;98:14837–14842. doi: 10.1073/pnas.201399298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rohdich F, Hecht S, Gärtner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W. Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc Natl Acad Sci USA. 2002;99:1158–1163. doi: 10.1073/pnas.032658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wungsintaweekul J, Herz S, Hecht S, Eisenreich W, Feicht R, Rohdich F, Bacher A, Zenk MH. Phosphorylation of 1-deoxy-d-xylulose by d-xylulokinase of Escherichia coli . Eur J Biochem. 2001;268:310–316. doi: 10.1046/j.1432-1033.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 98.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli . FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 99.Wolff M, Seemann M, Grosdemange-Billiard C, Tritsch D, Campos N, Rodriguez-Concepción M, Boronat A, Rohmer M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway. (E)-4-Hydroxy-3-methylbut-2-enyl diphosphate: chemical synthesis and formation from methylertyhritol cyclodiphosphate by a cell-free system from Escherichia coli . Tetrahedron Lett. 2002;43:2555–2559. [Google Scholar]
- 100.Zepeck F, Gräwert T, Kaiser J, Eisenreich W, Rohdich F. Biosynthesis of isoprenoids. Purification and properties of IspG protein from Escherichia coli . J Org Chem. 2005;70:9168–9174. doi: 10.1021/jo0510787. [DOI] [PubMed] [Google Scholar]
- 101.Puan KJ, Wang H, Dairi T, Kuzuyama T, Morita CT. fldA is an essential gene required in the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis. FEBS Lett. 2005;579:3802–3806. doi: 10.1016/j.febslet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 102.Sancho J. Flavodoxins: sequence, folding, binding, function and beyond. Cell Mol Life Sci. 2006;63:855–864. doi: 10.1007/s00018-005-5514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gräwert T, Kaiser J, Zepeck F, Laupitz R, Hecht S, Amslinger S, Schramek N, Schleicher E, Weber S, Haslbeck M, Buchner J, Rieder C, Arigoni D, Bacher A, Eisenreich W, Rohdich F. IspH protein of Escherichia coli: studies on iron–sulfur cluster implementation and catalysis. J Am Chem Soc. 2004;126:12847–12855. doi: 10.1021/ja0471727. [DOI] [PubMed] [Google Scholar]
- 104.Seemann M, Bui BT, Wolff M, Tritsch D, Campos N, Boronat A, Marquet A, Rohmer M. Isoprenoid biosynthesis through the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angew Chem Int Ed Engl. 2002;41:4337–4339. doi: 10.1002/1521-3773(20021115)41:22<4337::AID-ANIE4337>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 105.Kollas AK, Duin EC, Eberl M, Altincicek B, Hintz M, Reichenberg A, Henschker D, Henne A, Steinbrecher I, Ostrovsky DN, Hedderich R, Beck E, Jomaa H, Wiesner J. Functional characterization of GcpE, an essential enzyme of the non-mevalonate pathway of isoprenoid biosynthesis. FEBS Lett. 2002;532:432–436. doi: 10.1016/s0014-5793(02)03725-0. [DOI] [PubMed] [Google Scholar]
- 106.Seemann M, Wegner P, Schünemann V, Bui BT, Wolff M, Marquet A, Trautwein AX, Rohmer M. Isoprenoid biosynthesis in chloroplasts via the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) from Arabidopsis thaliana is a [4Fe–4S] protein. J Biol Inorg Chem. 2005;10:131–137. doi: 10.1007/s00775-004-0619-z. [DOI] [PubMed] [Google Scholar]
- 107.Altincicek B, Duin EC, Reichenberg A, Hedderich R, Kollas AK, Hintz M, Wagner S, Wiesner J, Beck E, Jomaa H. LytB protein catalyzes the terminal step of the 2-C-methyl-d-erythritol-4-phosphate pathway of isoprenoid biosynthesis. FEBS Lett. 2002;532:437–440. doi: 10.1016/s0014-5793(02)03726-2. [DOI] [PubMed] [Google Scholar]
- 108.Wolff M, Seemann M, Bui BT, Frapart Y, Tritsch D, Garcia Estrabot A, Rodríguez-Concepción M, Boronat A, Marquet A, Rohmer M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (LytB/IspH) from Escherichia coli is a [4Fe–4S] protein. FEBS Lett. 2003;541:115–120. doi: 10.1016/s0014-5793(03)00317-x. [DOI] [PubMed] [Google Scholar]
- 109.Rohdich F, Zepeck F, Adam P, Hecht S, Kaiser J, Laupitz R, Gräwert T, Amslinger S, Eisenreich W, Bacher A, Arigoni D. The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: studies on the mechanisms of the reactions catalyzed by IspG and IspH protein. Proc Natl Acad Sci USA. 2003;100:1586–1591. doi: 10.1073/pnas.0337742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brandt W, Dessoy MA, Fulhorst M, Gao W, Zenk MH, Wessjohann LA. A proposed mechanism for the reductive ring opening of the cyclodiphosphate MEcPP, a crucial transformation in the new DXP/MEP pathway to isoprenoids based on modeling studies and feeding experiments. Chem Bio Chem. 2004;5:311–323. doi: 10.1002/cbic.200300743. [DOI] [PubMed] [Google Scholar]
- 111.Xu W, Lees NS, Adedeji D, Wiesner J, Jomaa H, Hoffman BM, Duin EC. Paramagnetic intermediates of (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE/IspG) under steady-state and pre-steady-state conditions. J Am Chem Soc. 2010;132:14509–14520. doi: 10.1021/ja101764w. [DOI] [PubMed] [Google Scholar]
- 112.Nyland RL, 2nd, Xiao Y, Liu P, Freel Meyers CL. IspG converts an epoxide substrate analogue to (E)-4-hydroxy-3-methylbut-2-enyl diphosphate: implications for IspG catalysis in isoprenoid biosynthesis. J Am Chem Soc. 2009;131:17734–17735. doi: 10.1021/ja907470n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao Y, Zahariou G, Sanakis Y, Liu P. IspG enzyme activity in the deoxyxylulose phosphate pathway: roles of the iron–sulfur cluster. Biochemistry. 2009;48:10483–10485. doi: 10.1021/bi901519q. [DOI] [PubMed] [Google Scholar]
- 114.Xiao Y, Nyland RL, 2nd, Meyers CL, Liu P. Methylerythritol cyclodiphosphate (MEcPP) in deoxyxylulose phosphate pathway: synthesis from an epoxide and mechanisms. Chem Commun. 2010;46:7220–7222. doi: 10.1039/c0cc02594a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang W, Li J, Wang K, Huang C, Zhang Y, Oldfield E. Organometallic mechanism of action and inhibition of the 4Fe–4S isoprenoid biosynthesis protein GcpE (IspG) Proc Natl Acad Sci USA. 2010;107:11189–11193. doi: 10.1073/pnas.1000264107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee M, Grawert T, Quitterer F, Rohdich F, Eppinger J, Eisenreich W, Bacher A, Groll M. Biosynthesis of isoprenoids: crystal structure of the [4Fe–4S] cluster protein IspG. J Mol Biol. 2010;404:600–610. doi: 10.1016/j.jmb.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 117.McAteer S, Coulson A, McLennan N, Masters M. The lytB gene of Escherichia coli is essential and specifies a product needed for isoprenoid biosynthesis. J Bacteriol. 2001;183:7403–7407. doi: 10.1128/JB.183.24.7403-7407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gräwert T, Span I, Eisenreich W, Rohdich F, Eppinger J, Bacher A, Groll M. Probing the reaction mechanism of IspH protein by x-ray structure analysis. Proc Natl Acad Sci USA. 2010;107:1077–1081. doi: 10.1073/pnas.0913045107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gräwert T, Rohdich F, Span I, Bacher A, Eisenreich W, Eppinger J, Groll M. Structure of active IspH enzyme from Escherichia coli provides mechanistic insights into substrate reduction. Angew Chem Int Ed Engl. 2009;48:5756–5759. doi: 10.1002/anie.200900548. [DOI] [PubMed] [Google Scholar]
- 120.Rekittke I, Wiesner J, Rohrich R, Demmer U, Warkentin E, Xu W, Troschke K, Hintz M, No JH, Duin EC, Oldfield E, Jomaa H, Ermler U. Structure of (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate reductase, the terminal enzyme of the non-mevalonate pathway. J Am Chem Soc. 2008;130:17206–17207. doi: 10.1021/ja806668q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gräwert T, Span I, Bacher A, Groll M. Reductive dehydroxylation of allyl alcohols by IspH protein. Angew Chem Int Ed Engl. 2010;49:8802–8809. doi: 10.1002/anie.201000833. [DOI] [PubMed] [Google Scholar]
- 122.Seemann M, Janthawornpong K, Schweizer J, Bottger LH, Janoschka A, Ahrens-Botzong A, Tambou EN, Rotthaus O, Trautwein AX, Rohmer M. Isoprenoid biosynthesis via the MEP pathway: in vivo Mößbauer spectroscopy identifies a [4Fe–4S]2+ center with unusual coordination sphere in the LytB protein. J Am Chem Soc. 2009;131:13184–13185. doi: 10.1021/ja9012408. [DOI] [PubMed] [Google Scholar]
- 123.Xiao Y, Zhao ZK, Liu P. Mechanistic studies of IspH in the deoxyxylulose phosphate pathway: heterolytic C–O bond cleavage at C4 position. J Am Chem Soc. 2008;130:2164–2165. doi: 10.1021/ja710245d. [DOI] [PubMed] [Google Scholar]
- 124.Oldfield E. Targeting isoprenoid biosynthesis for drug discovery: bench to bedside. Acc Chem Res. 2010;43:1216–1226. doi: 10.1021/ar100026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang K, Wang W, No JH, Zhang Y, Oldfield E. Inhibition of the Fe(4)S(4)-cluster-containing protein IspH (LytB): electron paramagnetic resonance, metallacycles, and mechanisms. J Am Chem Soc. 2010;132:6719–6727. doi: 10.1021/ja909664j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang W, Wang K, Liu YL, No JH, Li J, Nilges MJ, Oldfield E. Bioorganometallic mechanism of action, and inhibition, of IspH. Proc Natl Acad Sci USA. 2010;107:4522–4527. doi: 10.1073/pnas.0911087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rohdich F, Schuhr CA, Hecht S, Herz S, Wungsintaweekul J, Eisenreich W, Zenk MH, Bacher A. Biosynthesis of Isoprenoids. A rapid method for the preparation of isotope-labeled 4-diphosphocytidyl-2C-methyl-d-erythritol. J Am Chem Soc. 2000;122:9571–9594. [Google Scholar]
- 128.Schuhr CA, Hecht S, Kis K, Eisenreich W, Wungsintaweekul J, Bacher A, Rohdich F. Studies on the non-mevalonate pathway—preparation and properties of isotope-labeled 2C-methyl-d-erythritol 2,4-cyclodiphosphate. Eur J Org Chem. 2001;17:3221–3226. [Google Scholar]
- 129.Hecht S, Kis K, Eisenreich W, Amslinger S, Wungsintaweekul J, Herz S, Rohdich F, Bacher A. Enzyme-assisted preparation of isotope-labeled 1-deoxy-d-xylulose 5-phosphate. J Org Chem. 2001;66:3948–3952. doi: 10.1021/jo0100300. [DOI] [PubMed] [Google Scholar]
- 130.Hecht S, Wungsintaweekul J, Rohdich F, Kis K, Radykewicz T, Schuhr CA, Eisenreich W, Richter G, Bacher A. Biosynthesis of terpenoids: efficient multistep biotransformation procedures affording isotope-labeled 2C-methyl-d-erythritol 4-phosphate using recombinant 2C-methyl-d-erythritol 4-phosphate synthase. J Org Chem. 2001;66:7770–7775. doi: 10.1021/jo015890v. [DOI] [PubMed] [Google Scholar]
- 131.Argyrou A, Blanchard JS. Kinetic and chemical mechanism of Mycobacterium tuberculosis 1-deoxy-d-xylulose-5-phosphate isomeroreductase. Biochemistry. 2004;43:4375–4384. doi: 10.1021/bi049974k. [DOI] [PubMed] [Google Scholar]
- 132.Yin X, Proteau PJ. Characterization of native and histidine-tagged deoxyxylulose 5-phosphate reductoisomerase from the cyanobacterium Synechocystis sp. PCC6803. Biochim Biophys Acta. 2003;1652:75–81. doi: 10.1016/j.bbapap.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 133.Yajima S, Nonaka T, Kuzuyama T, Seto H, Ohsawa K. Crystal structure of 1-deoxy-d-xylulose 5-phosphate reductoisomerase complexed with cofactors: implications of a flexible loop movement upon substrate binding. J Biochem. 2002;131:313–317. doi: 10.1093/oxfordjournals.jbchem.a003105. [DOI] [PubMed] [Google Scholar]
- 134.Yajima S, Hara K, Iino D, Sasaki Y, Kuzuyama T, Ohsawa K, Seto H. Structure of 1-deoxy-d-xylulose 5-phosphate reductoisomerase in a quaternary complex with a magnesium ion, NADPH and the antimalarial drug fosmidomycin. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:466–470. doi: 10.1107/S1744309107024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mac Sweeney A, Lange R, Fernandes RP, Schulz H, Dale GE, Douangamath A, Proteau PJ, Oefner C. The crystal structure of E. coli 1-deoxy-d-xylulose-5-phosphate reductoisomerase in a ternary complex with the antimalarial compound fosmidomycin and NADPH reveals a tight-binding closed enzyme conformation. J Mol Biol. 2005;345:115–127. doi: 10.1016/j.jmb.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 136.Reuter K, Sanderbrand S, Jomaa H, Wiesner J, Steinbrecher I, Beck E, Hintz M, Klebe G, Stubbs MT. Crystal structure of 1-deoxy-d-xylulose-5-phosphate reductoisomerase, a crucial enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. J Biol Chem. 2002;277:5378–5384. doi: 10.1074/jbc.M109500200. [DOI] [PubMed] [Google Scholar]
- 137.Henriksson LM, Unge T, Carlsson J, Aqvist J, Mowbray SL, Jones TA. Structures of Mycobacterium tuberculosis 1-deoxy-d-xylulose-5-phosphate reductoisomerase provide new insights into catalysis. J Biol Chem. 2007;282:19905–19916. doi: 10.1074/jbc.M701935200. [DOI] [PubMed] [Google Scholar]
- 138.Henriksson LM, Bjorkelid C, Mowbray SL, Unge T. The 1.9 A resolution structure of Mycobacterium tuberculosis 1-deoxy-d-xylulose 5-phosphate reductoisomerase, a potential drug target. Acta Crystallogr D Biol Crystallogr. 2006;62:807–813. doi: 10.1107/S0907444906019196. [DOI] [PubMed] [Google Scholar]
- 139.Umeda T, Tanaka N, Kusakabe Y, Nakanishi M, Kitade Y, Nakamura KT. Crystallization and preliminary X-ray crystallographic study of 1-deoxy-d-xylulose 5-phosphate reductoisomerase from Plasmodium falciparum . Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:330–332. doi: 10.1107/S1744309110001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takenoya M, Ohtaki A, Noguchi K, Endo K, Sasaki Y, Ohsawa K, Yajima S, Yohda M. Crystal structure of 1-deoxy-d-xylulose 5-phosphate reductoisomerase from the hyperthermophile Thermotoga maritima for insights into the coordination of conformational changes and an inhibitor binding. J Struct Biol. 2010;170:532–539. doi: 10.1016/j.jsb.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 141.Ricagno S, Grolle S, Bringer-Meyer S, Sahm H, Lindqvist Y, Schneider G. Crystal structure of 1-deoxy-d-xylulose-5-phosphate reductoisomerase from Zymomonas mobilis at 1.9-A resolution. Biochim Biophys Acta. 2004;1698:37–44. doi: 10.1016/j.bbapap.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 142.Eoh H, Brown AC, Buetow L, Hunter WN, Parish T, Kaur D, Brennan PJ, Crick DC. Characterization of the Mycobacterium tuberculosis 4-diphosphocytidyl-2-C-methyl-d-erythritol synthase: potential for drug development. J Bacteriol. 2007;189:8922–8927. doi: 10.1128/JB.00925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shi W, Feng J, Zhang M, Lai X, Xu S, Zhang X, Wang H. Biosynthesis of isoprenoids: characterization of a functionally active recombinant 2-C-methyl-d-erythritol 4-phosphate cytidyltransferase (IspD) from Mycobacterium tuberculosis H37Rv. J Biochem Mol Biol. 2007;40:911–920. doi: 10.5483/bmbrep.2007.40.6.911. [DOI] [PubMed] [Google Scholar]
- 144.Gabrielsen M, Kaiser J, Rohdich F, Eisenreich W, Laupitz R, Bacher A, Bond CS, Hunter WN. The crystal structure of a plant 2C-methyl-d-erythritol 4-phosphate cytidylyltransferase exhibits a distinct quaternary structure compared to bacterial homologues and a possible role in feedback regulation for cytidine monophosphate. FEBS J. 2006;273:1065–1073. doi: 10.1111/j.1742-4658.2006.05133.x. [DOI] [PubMed] [Google Scholar]
- 145.Richard SB, Bowman ME, Kwiatkowski W, Kang I, Chow C, Lillo AM, Cane DE, Noel JP. Structure of 4-diphosphocytidyl-2-C-methylerythritol synthetase involved in mevalonate-independent isoprenoid biosynthesis. Nat Struct Biol. 2001;8:641–648. doi: 10.1038/89691. [DOI] [PubMed] [Google Scholar]
- 146.Kemp LE, Bond CS, Hunter WN. Crystallization and preliminary X-ray diffraction studies of recombinant Escherichia coli 4-diphosphocytidyl-2-C-methyl-d-erythritol synthetase. Acta Crystallogr D Biol Crystallogr. 2001;57:1189–1191. doi: 10.1107/s0907444901010137. [DOI] [PubMed] [Google Scholar]
- 147.Sgraja T, Alphey MS, Ghilagaber S, Marquez R, Robertson MN, Hemmings JL, Lauw S, Rohdich F, Bacher A, Eisenreich W, Illarionova V, Hunter WN. Characterization of Aquifex aeolicus 4-diphosphocytidyl-2C-methyl-d-erythritol kinase—ligand recognition in a template for antimicrobial drug discovery. FEBS J. 2008;275:2779–2794. doi: 10.1111/j.1742-4658.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lherbet C, Pojer F, Richard SB, Noel JP, Poulter CD. Absence of substrate channeling between active sites in the Agrobacterium tumefaciens IspDF and IspE enzymes of the methyl erythritol phosphate pathway. Biochemistry. 2006;45:3548–3553. doi: 10.1021/bi0520075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miallau L, Alphey MS, Kemp LE, Leonard GA, McSweeney SM, Hecht S, Bacher A, Eisenreich W, Rohdich F, Hunter WN. Biosynthesis of isoprenoids: crystal structure of 4-diphosphocytidyl-2C-methyl-d-erythritol kinase. Proc Natl Acad Sci USA. 2003;100:9173–9178. doi: 10.1073/pnas.1533425100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wada T, Kuzuyama T, Satoh S, Kuramitsu S, Yokoyama S, Unzai S, Tame JR, Park SY. Crystal structure of 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol kinase, an enzyme in the non-mevalonate pathway of isoprenoid synthesis. J Biol Chem. 2003;278:30022–30027. doi: 10.1074/jbc.M304339200. [DOI] [PubMed] [Google Scholar]
- 151.Rohdich F, Eisenreich W, Wungsintaweekul J, Hecht S, Schuhr CA, Bacher A. Biosynthesis of terpenoids. 2C-Methyl-d-erythritol 2,4-cyclodiphosphate synthase (IspF) from Plasmodium falciparum . Eur J Biochem. 2001;268:3190–3197. doi: 10.1046/j.1432-1327.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- 152.Calisto BM, Perez-Gil J, Bergua M, Querol-Audi J, Fita I, Imperial S. Biosynthesis of isoprenoids in plants: structure of the 2C-methyl-d-erithrytol 2,4-cyclodiphosphate synthase from Arabidopsis thaliana. Comparison with the bacterial enzymes. Protein Sci. 2007;16:2082–2088. doi: 10.1110/ps.072972807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Richard SB, Ferrer JL, Bowman ME, Lillo AM, Tetzlaff CN, Cane DE, Noel JP. Structure and mechanism of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase. An enzyme in the mevalonate-independent isoprenoid biosynthetic pathway. J Biol Chem. 2002;277:8667–8672. doi: 10.1074/jbc.C100739200. [DOI] [PubMed] [Google Scholar]
- 154.Kemp LE, Bond CS, Hunter WN. Structure of 2C-methyl-d-erythritol 2,4-cyclodiphosphate synthase: an essential enzyme for isoprenoid biosynthesis and target for antimicrobial drug development. Proc Natl Acad Sci USA. 2002;99:6591–6596. doi: 10.1073/pnas.102679799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lehmann C, Lim K, Toedt J, Krajewski W, Howard A, Eisenstein E, Herzberg O. Structure of 2C-methyl-d-erythrol-2,4-cyclodiphosphate synthase from Haemophilus influenzae: activation by conformational transition. Proteins. 2002;49:135–138. doi: 10.1002/prot.10182. [DOI] [PubMed] [Google Scholar]
- 156.Kishida H, Wada T, Unzai S, Kuzuyama T, Takagi M, Terada T, Shirouzu M, Yokoyama S, Tame JR, Park SY. Structure and catalytic mechanism of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MECDP) synthase, an enzyme in the non-mevalonate pathway of isoprenoid synthesis. Acta Crystallogr D Biol Crystallogr. 2003;59:23–31. doi: 10.1107/s0907444902017705. [DOI] [PubMed] [Google Scholar]
- 157.Buetow L, Brown AC, Parish T, Hunter WN. The structure of Mycobacteria 2C-methyl-d-erythritol-2,4-cyclodiphosphate synthase, an essential enzyme, provides a platform for drug discovery. BMC Struct Biol. 2007;7:68. doi: 10.1186/1472-6807-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gabrielsen M, Rohdich F, Eisenreich W, Gräwert T, Hecht S, Bacher A, Hunter WN. Biosynthesis of isoprenoids: a bifunctional IspDF enzyme from Campylobacter jejuni . Eur J Biochem. 2004;271:3028–3035. doi: 10.1111/j.1432-1033.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 159.Testa CA, Lherbet C, Pojer F, Noel JP, Poulter CD. Cloning and expression of IspDF from Mesorhizobium loti. Characterization of a bifunctional protein that catalyzes non-consecutive steps in the methylerythritol phosphate pathway. Biochim Biophys Acta. 2006;1764:85–96. doi: 10.1016/j.bbapap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 160.Rekittke I, Nonaka T, Wiesner J, Demmer U, Warkentin E, Jomaa H, Ermler U. Structure of the E-1-hydroxy-2-methyl-but-2-enyl-4-diphosphate synthase (GcpE) from Thermus thermophilus . FEBS Lett. 2010;585:447–451. doi: 10.1016/j.febslet.2010.12.012. [DOI] [PubMed] [Google Scholar]