Abstract
In addition to its central roles in protein quality control, regulation of cell cycle, intracellular signaling, DNA damage response and transcription regulation, the ubiquitin–proteasome system (UPS) plays specific roles in the nervous system, where it contributes to precise connectivity through development, and later assures functionality by regulating a wide spectrum of neuron-specific cellular processes. Aberrations in this system have been implicated in the etiology of neurodevelopmental and neurodegenerative diseases. In this review, we provide an updated view on the UPS and highlight recent findings concerning its role in normal and diseased nervous systems. We discuss the advantages of the model organism Caenorhabditis elegans as a tool to unravel the major unsolved questions concerning this biochemical pathway and its involvement in nervous system function and dysfunction, and expose the new possibilities, using state-of-the-art techniques, to assess UPS function using this model system.
Keywords: Ubiquitylation, E1, E2, E3, De-ubiquitylase, Nematode, Neurodevelopment, Neurodegeneration, Synaptic plasticity, Neurological disease
Introduction
The UPS is an evolutionarily conserved and tightly regulated biochemical pathway that modulates the levels of critical proteins in specific cellular contexts and is responsible for the degradation of irreversibly damaged proteins. Ubiquitylation can also be uncoupled from proteasomal degradation, regulating protein location and activity. Although the relevance of this system in neuronal function and in several disorders of the nervous system is becoming increasingly evident, in most cases the identity of the molecular players and the detailed mechanisms of UPS regulation remain to be clarified. The aim of this review is to provide an updated overview of the involvement of the UPS in nervous system function and dysfunction, focusing on C. elegans contributions to the field, and to stimulate further development of this organism as a tool to study the UPS.
The ubiquitin–proteasome system
Degradation of proteins via UPS involves two steps: (1) covalent attachment of ubiquitin to the target protein and (2) degradation of the ubiquitin-tagged protein by the 26S proteasome, with release of re-usable ubiquitin, by the action of deubiquitylating enzymes. Two main structures are part of the 26S proteasome: the core or 20S particle, responsible for protein hydrolysis, and the 19S regulatory particle, which is involved in the recognition of the substrate [1]. The C. elegans 26S has 14 conserved subunits in the 20S core and 18 conserved subunits in the 19S core. The interactions between subunits within the 26S complex have been identified recurring to two-hybrid-based protein interaction mapping [2].
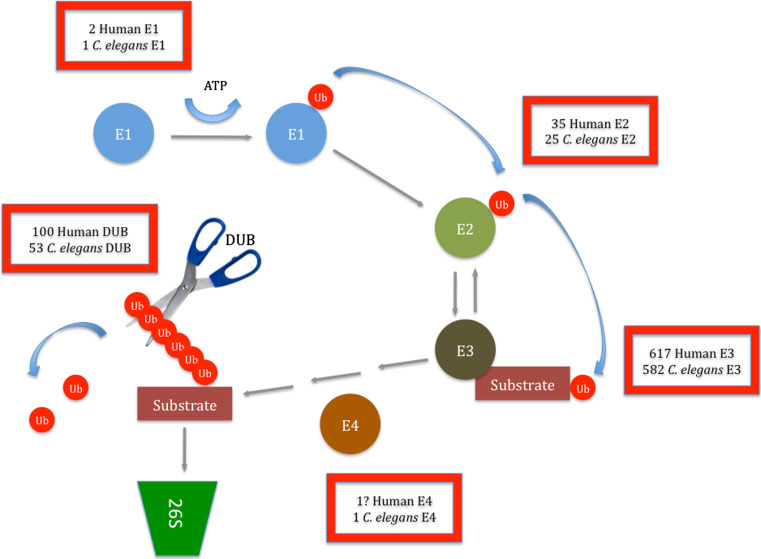
The tagging of a protein for degradation is regulated by consecutive action of three classes of enzymes: E1, E2, and E3. First, ubiquitin is adenylated by an ubiquitin-activating enzyme (E1) and transferred to a cysteine residue in the E1, in an ATP-dependent reaction. Two mammalian E1 enzymes have been described up to now [3, 4], in contrast with C. elegans, where the uba-1 gene encodes the sole E1 enzyme [5, 6]. Activated ubiquitin is then transferred to a cysteine residue on the ubiquitin-conjugating enzyme (E2) through formation of a thioester bond with the glycine 76 of ubiquitin. Members of the E2 family have a highly conserved ubiquitin-conjugating catalytic fold (UBC), which is responsible for the binding to activated ubiquitin, to E1s and ubiquitin ligases (E3s) [7]. A total of 35 E2s have been described up to now in humans [8], whereas in C. elegans there are 22 proteins with homology to ubiquitin-conjugating enzymes, based on the criteria of having a UBC motif and the catalytic cysteine residue that accepts activated ubiquitin. Furthermore, three variant E2s exist in C. elegans that lack the cysteine residue but share the UBC fold [5, 9]. After conjugation, ubiquitin-loaded E2s selectively interact with E3s that recruit and bind specific substrates. Ubiquitin is transferred from the E2 to a cysteine residue located in the active site of the E3 prior to transfer to a lysine residue on the target protein. There are about 617 putative E3s in humans, as described in a genome-wide screen based on the presence of specific catalytic domains [10]. Although the number of E3s present in C. elegans is not yet very clear, until now a few hundreds have been suggested [11, 12]. Ubiquitin ligases are classified into three classes according to the characteristics of their catalytic domain: RING finger, U-Box, or HECT. RING domain are the most abundant E3s and may exist as monomeric RING fingers or as complexes. In the latter E3s, the RING domain binds E2 but the other subunit is responsible for the interaction with the substrate [13, 14]. Through the use of ubiquitin fusion degradation substrate—Ubi-GST—it was found that, in the presence of solely E1, E2, and E3 enzymes, the majority of ubiquitin–protein conjugates contained only one to three ubiquitins. For efficient further elongation, an extra enzyme was needed—UFD2—that was termed an E4 [15]. E4s constitute an extra class of enzymes in the UPS that are necessary in specific contexts for efficient transition from mono- to polyubiquitylation [16]. A comparison of the UPS of humans and C. elegans is presented in Fig. 1.
Fig. 1.
Comparison of the UPS between humans and in C. elegans. E1s for both species have already been identified and characterized. C. elegans E2s have been identified based on the criteria of having a UBC motif and the catalytic cysteine residue that accepts activated ubiquitin, and their function has been determined recurring to RNAi studies; human E2s have been identified through sequence homology using E2 protein sequences from C. elegans and Arabidopsis thaliana as an initial set. Predictions for E3s number in humans and C. elegans have been made based in a genome-wide screen based on the presence of specific catalytic domains. One E4 has been described and characterized up to now in each species, although it is possible that many more exist
Ubiquitin is covalently attached to the target protein via an isopeptide bond between its C-terminal glycine and a lysine in the target protein. After the attachment of the first ubiquitin, others can bind to the target protein, since a substrate can be multi-ubiquitylated in several lysine residues, or polyubiquitylated at a single residue. In the latter case, E3s can elongate the ubiquitin chain by creating ubiquitin–ubiquitin isopeptide bonds. Ubiquitin has seven lysine residues (Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63), and the assembly of a chain with at least four ubiquitins linked through their Lys48 targets the protein for proteasome degradation [17, 18]. Proteins that are monoubiquitylated or contain Lys63-linked Ub chains are known to be directed to the regulation of intracellular traffic or transcription, and to DNA repair [19, 20]. In some instances, monoubiquitylation also enhances protein activity [19, 21]. Although the UPS can negatively regulate transcription by downregulating the levels of transcriptional activators [22], monoubiquitylation leads to increased potency of these activators [23, 24]. This occurs because proteasomal ATPase destabilizes activator–promotor complexes [25] and activator monoubiquitylation protects it from this destabilization [26].
Recent evidence shows that unconventional ubiquitin chains, linked through different lysines—Lys6, Lys11, Lys27, Lys29, Lys33—can also direct proteins to proteasomal degradation, both in S. cerevesiae [27] and in 26S knockout mice [28]. Whether Lys63-linked chains may also target proteins to proteasomal degradation is still controversial [29, 30]. The conflicting results that have been published may arise from the fact that proteins targeted with Lys63 chains are degraded by the proteasome less efficiently, due to reduced accessibility and increased deubiquitylation rates [31]. There are also polyubiquitin chains formed by the ligation of more than one lysine residue, referred as mixed-linkage chains [30]; these are relevant for protein endocytosis and to promote or avoid protein degradation [32–34]. Although ubiquitylation is emerging as a very important mode of regulation of protein activity in cells, akin to phosphorylation and involving an equivalent amount of molecular players (as described below), the “ubiquitin code”, i.e. the correspondence between the type of ubiquitin chain bound to a given protein and its effect, remains to be fully clarified.
Polyubiquitin chains can be totally removed or their length reduced, disassembly occurring through the action of deubiquitylating enzymes (DUBs). These play an important role in cell homeostasis, on the one hand regenerating free ubiquitin for re-use by the UPS, and on the other, facilitating substrate entrance into the proteasome, since the removal of poly-ubiquitin chains by proteasome-associated DUBs precedes the access of unfolded proteins to the proteolytic chamber of the 20S proteasome. DUBs can also protect Lys48-linked polyubiquitin-conjugated proteins from degradation at the proteasome [35, 36]. A chain-editing activity for DUBs has also been described, as highlighted by the in vitro studies of human ataxin-3 by Winborn and co-workers [37]. Another recent study further supports this notion by demonstrating that C. elegans ataxin-3 is also involved in ubiquitin chain editing, producing chains of three to four ubiquitins (that might promote protein degradation), revealing an evolutionarily conserved mechanism of action for this DUB [38].
DUBs can be sub-divided into five classes: four are cysteine proteases, while the other class comprises metalloproteases [jab1/MPN domain-associated metalloisopeptidase class (JAMM)]. Based on their ubiquitin protease domain, cystein proteases can be further divided into ubiquitin C-terminal hydrolases (UCHs), ubiquitin-specific proteases (USPs), otubain proteases (OTUs), and Machado–Joseph disease proteases (MJDs). Mammalian genomes encode approximately 100 DUBs [39], while in C. elegans there are predictions of 53 putative DUBs [11].
The UPS is responsible for the vast majority of regulated protein degradation. It is involved in the degradation of regulatory, short-lived and aberrant proteins, playing a critical role in the maintenance of cellular homeostasis. Regulation of cell cycle, intracellular signaling, DNA damage response and transcription are major known roles of the UPS [40–44]. In addition to assuring that basic cell function is preserved, the UPS also contributes to the response to insults such as ischemia, oxidative stress, DNA damage, inflammation, and others [45–48].
The UPS assumes a particularly important role in the context of the nervous system. The fact that neurons in the adult nervous system do not have the capacity to divide makes the machinery responsible for protein quality control particularly relevant. In addition, UPS degradation of key components seems to be necessary for axonal pathfinding, synaptogenesis, synaptic plasticity, and other neuron-specific activities. In the next sections, we will give examples of the contribution of the UPS for nervous system physiology.
The UPS in the developing nervous system
The function of the nervous system relies on its precise connectivity. The formation of the neuronal networks present in the adult nervous system is a highly dynamic and tightly regulated process, involving the development of neurons with polarised morphology, axon pathfinding, and the establishment of an intricate network of synaptic connections. The UPS has emerged as a crucial component in the regulation of the developing nervous system [49] by controlling the proliferation of neuronal precursors and cell specification [50–54], neuronal migration [55, 56], neuritogenesis [57–63], and synaptic pruning [64–66]. E3 ligases are particularly relevant during the development of the nervous system, selecting key regulators for posterior proteasomal degradation or directing these proteins for specific signaling cascades. The E3 ligases involved in the processes of neural development are listed in Table 1.
Table 1.
List of E3 ligases involved in the processes of neural development
| UPS component | Mechanism of action | References |
|---|---|---|
| Neural stem and progenitor cells | ||
| SCF-bTRCP | REST/NSFR is a master repressor of neural gene expression; SCF-bTRCP is responsible for UPS-mediated REST degradation, allowing derepression of pro-neural REST targets | [50] |
| HUWE1 | HUWE1 supression of a N-Myc-DLL3 cascade, setting cell-cycle withdrawal and neuronal differentiation, restrains proliferation and enables neuronal differentiation | [53] |
| BTBD6 | BTBD6 acts as an adaptor protein in the SCF E3 ligase complex and targets the transcriptional repressor and neurogenesis inhibitor Plzf for degradation | [54] |
| MIB1 | RING-type E3 MIB1 expressing cells generate Notch signaling in neighboring radial glial cells to maintain their stemness and correct differentiation | [52] |
| TRIM11 | TRIM11 mediates the degradation of the development regulator transcription factor Pax6 | [51] |
| Neural cell migration | ||
| E3 ligase complex containing cullin 5 | Cul5 auxiliates Dab1 dgradation in target neurons after a signaling cascade that involves VLDR and ApoE receptors, directing the speed and correct migration of neuronal populations | [55, 56] |
| Axonal growth | ||
| RPM-1 | RPM-1 E3 ligase negatively regulates axon outgrowth by the guidance receptors SAX-3/robo and UNC-5/UNC-5 | [78] |
| CDH1-APC complex | Ubiquitin ligase CDH1-APC operates in the nucleus of neurons to inhibit axon growth through the promotion of transcription factors SnoN and Id2 degradation | [58, 59] |
| NEDD4 | NEDD4 acts as a positive regulator of dendrite extension and arborization through the ubiquitination of RAP2A and PTEN downregulation | [61, 62] |
| SMURF1 | SMURF1 enhances neurite outgrowth ubiquitinating RhoA | [57] |
| SMURF2 | SMURF2 ubiquitinates RAP1B directing it to proteasomal degradation, assuring that only one neurite will become an axon | [60] |
| Synaptic pruning | ||
| DIAP1 | E3 ligase DIAP1 degradation, mediated by UBCD1 conjugating enzyme, leads to a caspase-dependent efficient pruning of the C4da neuron in Drosophila | [65] |
| RPM-1 | RPM-1 E3 ligase negatively regulates a p38 MAPK pathway contibuting to the correct formation of mature synapses in C. elegans | [64] |
| SKR1 | SKR-1, a core component of SCF E3, contributes to synapse elimination in a proteasome-dependent manner in HSNL | [66] |
Axons must then find their target in an environment of negative and positive cues. The axons that fail to hit a target must be dismantled, whereas those that reach their target form functional synaptic contacts [67–69]. Given the key role of the UPS in the regulation of the protein content in the cytoplasm and in the nucleus, it is not surprising that it also contributes to this and other aspects of neuronal development. Thus, during the establishment of neuronal polarity in cultured rat hippocampal neurons, the degradation of Rap1b-GTPase by the UPS, mediated by E3 Smad ubiquitylation regulatory factors 1 and 2 (Smurf1 and Smurf2), defines which neurites become the axon and which are dismantled; only neurites with an accumulation of active Rap1B-GTP are viable [60].
The axon guidance cues netrin-1 and semaphorin 3A are involved in the regulation of growth cone guidance both in Xenopus [70, 71] as well as in mammals [72]. The growth cone collapse inducer L-α-lysophosphatidic acid (LPA) is also involved in this regulation [73], and both netrin-1 and LPA upregulate the amount of ubiquitin conjugates in growth cones. Inhibition of the proteasome has been shown to abolish the attraction of retinal growth cones towards a netrin-1 gradient [74], showing a role for the UPS in the tightly regulated control of protein synthesis/degradation necessary for correct axon guidance.
Major contributions to the understanding of nervous system formation have come from studies using C. elegans as a model organism. This is illustrated by the studies of Shen and co-workers to discover how precise synapse connectivity is established during development. This group first identified the SYG-1 protein (homologous to vertebrates’ NEPH1) as a relevant player in synaptic specificity [75], and additional studies showed SYG-1 as a partner of SKR-1, the core component of Skip1-cullin-F-Box (SCF) E3, during the establishment of hermaphrodite-specific motor neuron (HSNL) synapses. This interaction interferes with the assembly of the SCF complex and, as a consequence, there is a decrease in synapse elimination in the area where synapses are formed in the adult animal, the primary synapse region (PSR). In these studies, it was suggested that the SCF is rate-limiting for synapse elimination in a proteasome-dependent manner. In the area surrounding the PSR, the secondary synapse region, synapses are more likely to be eliminated due to the absence of SYG-1 and consequent presence of more functional E3 [66].
Another E3 that has been implicated in neuronal development, the regulator of pre-synaptic morphology-1 (RPM-1), is localized to pre-synaptic terminals in C. elegans neurons [76]. In rpm-1 C. elegans mutants, the extension and stabilization of synaptic branches are disrupted and an alteration in synaptic vesicle localization is also observed. Furthermore, in this model, the neurons do not form correct mature synapses at the right time and place [77]. More recent evidence identified the specific partners of this E3 in the regulation of axon outgrowth: RPM-1 seems to act by negatively regulating UNC-5/UNC-5 and SAX-3/robo-mediated axon outgrowth-promoting activity [78]. Another target of RPM-1 regulation is GLO-4, a Rab guanine nucleotide exchange factor. The interaction between these proteins is thought to activate the GLO pathway, with RPM-1 acting as a positive regulator, promoting vesicular trafficking essential for correct axon termination and synaptogenesis in mechanosensory neurons [79]. RPM-1 forms a SCF-like complex E3 ligase with the F-box protein FSN-1, the SKP1 ortholog SKR-1, and the Cullin CUL-1 [80], and besides its role in the regulation of axon outgrowth, RPM-1 also acts as a negative modulator of a MAP kinase cascade that regulates pre-synaptic architecture, including the Dual-Leucine zipper Kinase MAPKKK (DLK-1), MKK-4, and the p38 MAPK ortholog, PMK-3 [64].
In the context of nervous system development, DUBs also play an important role, with relevant examples in most DUB classes. Repressor element 1-silencing transcription factor (REST) plays a key role as a transcriptional repressor of neuronal differentiation-associated genes [81]. REST is targeted for proteasomal degradation by the E3 SCF-β-TrCP. The ubiquitin-specific protease herpesvirus-associated ubiquitin-specific protease (HAUSP)—also termed ubiquitin-specific protease 7 (USP7)—negatively regulates REST ubiquitylation, promoting REST stabilization and thus preventing neural precursor cell differentiation [82].
Ubiquitin C-terminal hydrolase 37 (Uch37) was identified as essential for early nervous system development in mouse. The complete deletion of Uch37 resulted in prenatal lethality resultant from deficient formation of the telencephalon, mesencephalon, and metencephalon [83]. In the group of metalloprotease DUBs, the associated molecule with the SH3 domain of STAM (AMSH) [84] was identified as essential for postnatal development in mice. AMSH-deficient mice generated by gene targeting are normal at birth but have postnatal growth retardation and die prematurely. These mice show significant loss of CA1 hippocampal neurons at day 6. Hippocampal neurons cultured in vitro were unable to survive without the presence of AMSH, while other cell types were able to survive, further suggesting a role for this DUB in the normal development of these neurons. Also, neuron numbers in the cerebral cortex were significantly reduced in 16-day-old AMSH−/− mice, revealing an important role for this DUB in the neuronal cell survival in the hippocampus and cerebral cortex [85].
The UPS in the mature nervous system
In addition to its function in neurodevelopment, the UPS also plays a critical role in the mature nervous system, namely in pre- and post-synaptic regulation, and in synaptic plasticity [86].
The first evidence of the involvement of the UPS in synaptic function came from studies in Aplysia demonstrating that the degradation of protein kinase A (PKA) subunits, an event necessary for long-term facilitation, was mediated through the UPS [87]. Not surprisingly, additional studies showed that the UPS is also essential for long-term synaptic depression in this organism [88].
Studies of the Drosophila neuromuscular junction demonstrated unequivocally for the first time that UPS components were part of the presynaptic terminal [89]. Chen and co-workers [90], using rat brain synaptosomes, showed that the influx of calcium following membrane depolarization is rapidly coupled to a general downregulation of ubiquitin-conjugated proteins, and that this effect was reversible.
At the presynaptic terminal, the influx of calcium is coupled to the release of neurotransmitters by exocytosis of synaptic vesicles. The synapse-localized ubiquitin ligase SCRAPPER was shown to be an E3 for Rab3-interacting molecule 1 (RIM1), a Rab-3 effector involved in exocytosis [91] and known to be part of a calcium sensor complex [92]. Studies using scrapper-knockout and scrapper-transgenic mice overexpressing the protein in the hippocampus showed that this E3 regulates the levels of RIM1, thus affecting presynaptic vesicle release [93]. Loss of this E3 increased the frequency of miniature excitatory postsynaptic currents (mEPSCs) and reduced paired pulse facilitation in hippocampal neurons, as a consequence of the increased release probability. This led to anxiety and altered contextual fear-conditioning in heterozygous SCR KO mice [94].
In Drosophila, the E3 anaphase-promoting complex (APC), besides its role in cell cycle regulation [95], is also involved in the regulation of synaptic growth in neuromuscular junctions, with Liprin-α as effector and possible substrate. APC mutants showed increased levels of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAr) subunit GluA2 at the post-synaptic level, which increased the sensitivity to the neurotransmitter glutamate [96].
The activation of post-synaptic receptors by neurotransmitters released from nerve terminals induces several downstream signaling cascades. These events alter the composition of the post-synaptic compartment, and the UPS is required for these changes. The use of C. elegans as a model organism provided significant contributions in the elucidation of some of these mechanisms, including the first reports of ubiquitylation of AMPAr, of great relevance for the current understanding of synaptic plasticity mechanisms. Burbea and co-workers demonstrated in vivo the ubiquitylation of GLR-1, the C. elegans non-NMDA receptor subunit most similar to mammalian AMPAr [97], resulting in removal of the receptors from the synapse [98]. The mechanism of GluA1 AMPA receptor endocytosis was recently described in vertebrates, and it was demonstrated that ubiquitylation is also required for internalization of surface AMPA receptors in response to AMPAr agonist [99]. Studies conducted in C. elegans also showed that the internalization of GLR-1 is mediated by KEL-8 and that this protein forms a complex with the CUL-3 subunit of the CDL3 ubiquitin ligase [100]. Other E3s specifically involved in this regulation were also identified in C. elegans. Temperature-sensitive mutants in APC E3 ligase subunits showed an increase in GLR-1::GFP puncta in the ventral nerve cord at restrictive temperatures. This effect was not observed when blocking endocytosis, suggesting that APC regulates the local recycling of GLR-1 [101].
Besides its role in the organization of the pre-synaptic bouton in C. elegans motor neurons [76], RPM-1 is also involved in the regulation of post-synaptic abundance of GLR-1. This mechanism involves the p38 MAPK pathway and is most likely related to the E3 ubiquitin ligase activity of RPM-1, since loss of FSN-1 (the RPM-1 partner in the E3 complex) also results in the same phenotype [102]. LIN-23 is the substrate-binding subunit of a SCF E3–SCFLIN-23. lin-23 mutants have increased GLR-1 expression in the ventral nerve cord, and in these, an increase in the cytosolic pool of β-catenin homolog BAR-1 was observed. In bar-1;lin-23 double mutants, a smaller increase in GLR-1 puncta was observed compared to bar-1 single mutants, suggesting that the effect of LIN-23 on GLR-1 levels is partially mediated through BAR-1. Another Wnt signaling target, the TCF/Lef transcription factor homolog POP-1, was found to mediate the effects of BAR-1 in GLR-1 levels, since pop-1 mutants significantly decreased the increase of GLR-1::GFP puncta intensity in animals expressing dominant-negative LIN-23. These results suggest an involvement of the Wnt signaling in GLR-1 regulation [103]. A list of the major E3s involved in the regulation of post-synaptic function is presented in Table 2, while the major contributions of C. elegans to our understanding of the role of the UPS in the synapse are shown in Fig. 2.
Table 2.
List of E3 ligases involved in the regulation of post-synaptic function
| E3 ligases | Mechanism of action | References |
|---|---|---|
| UBE3A | Neuronal activity promotes UBE3A expression and consequent downregulation of the substrate ARC, known to be involved in AMPAr endocytosis | [116, 118] |
| APC | APC E3 recruits CDC20 co-factor and regulates the abundance of GLR-1 receptors on the post-synaptic compartment in C. elegans; in Drosophila, APC/C is involved in the regulation of synaptic size in NMJ and also in the regulation of post-synaptic GluA2 levels | [96, 101, 131] |
| PDZRN3A | PDZRN3A synapse-associated RING finger E3 binds to MuSK and downregulates its surface levels, thus regulating the post-synaptic dvelopment of the NMJ | [273] |
| SCF/LIN23 | SCF E3 containing F-box protein LIN-23 regulates GLR-1 abundance in C. elegans ventral cord through the ubiquitination of BAR-1 | [103] |
| KEL-8/CUL3 | KEL-8 binds CUL3 forming an E3 ubiquitin ligase complex that is required for synaptic removal of GLR-1 in C. elegans | [100] |
| RPM-1 | RPM-1 forms an E3 SCF complex with FSN-1, SKR-1, and CUL-1; RPM-1 inhibits p30 MAPK signaling inducing GLR-1 endocytosis in C. elegans synapses | [102] |
| MDM2 | MDM2 E3 ligase ubiquitinates the scaffolding protein PSD95 upon NMDA activation, leading to its proteasomal degradation, thus regulating AMPA receptors synaptic levels | [274] |
| FBX2/CHIP | FBX2, a component of SCF E3 ligase complex, binds CHIP E3, and the interaction facilitates GluN1/2 NMDA receptor subunit degradation | [275] |
| MIB2 | MIB2 ubiquitinates GluN2A and NMDA receptor activity is downregulated by MIB2 in a ubiquitin proteasome-dependent manner | [276] |
| TRIM3 | Upon neuronal activity, the RING finger-containing E3 TRIM3 promotes ubiquitination and proteasome-dependent degradation of the PSD protein GKAP acting as an inhibitor of dendritic spine growth in hippocampal neurons | [277] |
| SCRAPPER | SCRAPPER is the F-box component of a SCF E3; SCRAPPER ubiquitinates RIM-1 regulating its levels and controlling synaptic vesicle release | [93] |
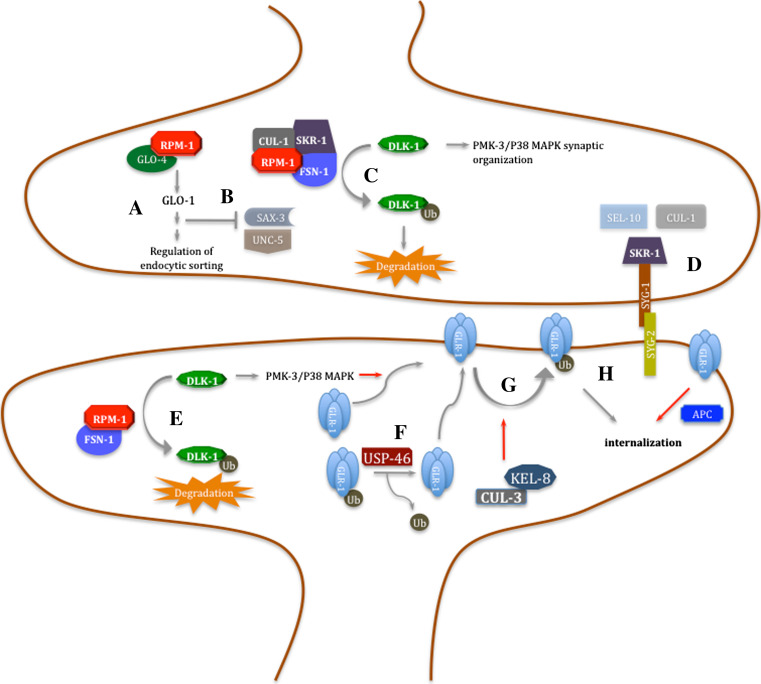
Fig. 2.
Major contributions of C. elegans to the current understanding of the role of the UPS in the synapse. a RPM-1, a RING finger domain protein, regulates pre-synaptic differerentiation by interacting with GLO-4 (Rab GEF), that promotes vesicule-specific membrane proteins trafficking through GLO-1 and AP-3; this pathway is involved in the regulation of axon termination and synaptogenesis. b RPM-1 co-ordinates axon outgrowth by negatively regulating SAX-3 and UNC-5 guidance receptors; this is achieved by controlling vesicular trafficking via the GLO-4 pathway. c RPM-1 contributes to regulate pre-synaptic architecture by forming, with FSN-1 and SKR-1, an SCF complex ubiquitin ligase that leads to DLK-1 ubiquitination and consequent downregulation, thus inhibiting the MAP kinase cascade that includes MKK-4 and PMK-3, known to be involved in synaptic organization. d The transmembrane adhesive molecule SYG-1 determines synaptic sites by preventing SCFSEL−10 ubiquitin ligase complex assembly and synapse elimination, through its interaction with SKR-1. e RPM-1 and FSN-1, working as an E3, negatively regulate DLK-1, and consequently the p38MAPK pathway thus leading to GLR-1 accumulation in neurites. f The deubiquitylase USP-46 cleaves ubiquitin from GLR-1 receptors, thus promoting GLR-1 surface membrane expression and preventing its degradation in the lysosome. g Post-synaptic ubiquitylation of GLR-1, in a mechanism that requires KEL-8, negatively regulates its levels in the membrane by promoting internalization and posterior degradation by the lysosome. h APC E3 ligase promotes loss of GLR-1 containing synapses possibly through the ubiquitination of a scaffold protein associated with GLR-1. Red arrows represent mechanisms/pathways still not fully elucidated
More recently, a DUB was identified that might be regulating AMPAr trafficking in the synapse. The ubiquitin-specific protease 46 (USP-46), homologue to mammalian USP-46, is expressed in C. elegans nervous system and regulates the abundance of GLR-1 in ventral nerve cord neurons by deubiquitylating the receptor and thus preventing its internalization [104]. Recent studies showed that the ubiquitin-conjugating enzyme variant UEV1 is also essential for AMPAr endocytosis in C. elegans [105]. The ubiquitin C-terminal hydrolase 1 (UCH-L1) is another example of a DUB with a relevant role in the maintenance of nervous system function. Besides its DUB activity [39, 106], the enzyme also functions as an E3 ligase in the dimeric form [107] and as a stabilizer of mono-ubiquitin, the latter not requiring enzymatic activity [108]. The expression of AP-Uch (UCH-L1 ortholog) in Aplysia increases in sensory neurons after long-term facilitation (LTF) in vivo and LTF is inhibited upon AP-Uch blockade [109, 110]. In mice, Gong and co-workers [111] discovered that UCH-L1 inhibition reduced both basal synaptic transmission and long-term potentiation (LTP). Moreover, both in APP/PS1 Alzheimer’s mouse model hippocampal slices or in amyloid-β treated hippocampal slices, UCH-L1 perfusion led to improvement in synaptic function [111]. The fact that treatment with amyloid-β led to a decrease of 70% of mono-ubiquitin levels, and that infusion of hippocampal slices with UCH-L1 prior to amyloid-β treatment blocks this decrease [111], points to UCH-L1 as a critical regulator of mono-ubiquitin availability in the synapse.
The UPS in neurological disease
Given the role of the UPS as a major intracellular pathway for degradation of proteins and a critical regulator of neuronal function, as described above, it is not surprising that its dysfunction is associated with several neurological disorders [112, 113].
The UPS in neurodevelopmental disorders
Dysfunction of the UPS is associated with several neurodevelopmental disorders resulting from genetic mutations, epigenetic modifications, and environmental factors that disturb the normal development, with onset during periods of ongoing maturation. Defects that occur during the establishment of the neuronal networks, either related to migration of neurons and/or to errors in neuronal connections, are key factors in the etiology of these diseases [114].
Probably the best-known example of a neurodevelopmental disorder related to the UPS is Angelman syndrome which, in most cases, is associated with the lack of a functional (and transcriptionally active) copy of the UBE3A gene [115]. Ube3A is an E3 found at the synapse and the loss of the maternal Ube3a allele leads to synaptic dysfunction in mice [116]. Activity-regulated cytoskeleton-associated protein (Arc), a substrate of Ube3A, is involved in AMPAr endocytosis and acts on the consolidation of LTP through regulation of actin dynamics [117]. Transfection of neurons with a Ube3A shRNA was found to decrease AMPAr expression at the membrane, and this effect was mediated by Arc [118]. The cognitive deficits observed in Angelman syndrome patients [119] correlate well with the compromised synaptic function and with the cognitive and behavioral deficits in transgenic mouse models of the disease [120–123].
Point mutations and deletions in the UBE2A/HR6A/UBC2 gene, which encodes an E2, were described to be the cause of an X-linked syndrome characterized by intellectual disability, seizures, obesity, marked hirsutism, and a characteristic facial appearance [124–127]. Although the function of UBE2A is not fully understood, it has been suggested to play a role in ubiquitin-dependent N-end rule targeting, i.e. the cleavage of proteins with destabilizing N-terminal residues (bulky hydrophobic or basic amino acids), through interaction with UBR1, the E3 binding N-end rule target proteins [128]. Interestingly, UBE2A activity is induced in PC12 cells by treatment with nerve growth factor (NGF) and necessary for the induction of neurite outgrowth by this neurotrophin [129]. The fact that proteasome inhibition also leads to a diminished neurite outgrowth led to the concept that this E2 plays an active role in the promotion of neurite extension in response to NGF, through enhanced rates of synthesis of ubiquitin–protein conjugates, but by mechanisms other than targeting them for proteasome degradation. The knockdown of UBE2B (a UBE2A paralogue gene with 80% homology but less abundant in cells) using siRNA also reduced nerve growth factor-induced neurite lengthening in PC12 cells [130]. UBE2B is known to be involved in chromatin structure modification and transcription regulation [131, 132], namely through the ubiquitylation of histone H2B, an activity that is shared by UBE2A. Recently, UBE2A (together with the proteasome subunit Psmd6 and the ubiquitin hydrolase Usp33) was also proposed to be at the core of a gene network playing a key role in fear-conditioning in mice, suggesting a role for this protein in learning and memory [133].
Intriguingly, mutations in UBR1, the RING finger E3 that cooperates with UBE2A/HR6A in N-end rule targeting [128, 134], have been associated with the Johanson–Blizzard syndrome which includes intellectual disability as a key feature [135–138]. Patients with this genetic syndrome suffer from congenital exocrine pancreatic insufficiency, growth retardation, hypothyroidism, hearing loss, and multiple malformations, such as nasal wing aplasia, oligodontia, cardiac anomalies, scalp defects, and imperforate anus, and frequently developmental delay/intellectual disability. UBR1 is a member of the N-recognin class of E3s, proteins that label N-end rule substrates via covalent linkage to ubiquitin, allowing the subsequent substrate delivery to the 26S proteasome. The yeast homologue of Ubr1 is involved in the degradation of misfolded cytosolic proteins and in the regulation of the leucine-mTOR signaling pathway [139–142]. Mice lacking Ubr1 are viable, but have defects that include pancreatic insufficiency, whereas mice lacking both Ubr1 and its paralogue Ubr2 die in mid-gestation, with severe disruption of nervous system and cardiovascular development. In the nervous system, cell proliferation, migration, and differentiation appeared to be affected [143].
A link between UPS dysfunction and neurodevelopment disease was also found in other subtypes of intellectual disability. An alteration in the CUL4B gene was first identified in 8 of the 250 families studied in a large project to identify genes causing X-linked intellectual disability [144]. CUL4B forms a complex that functions as an E3 ubiquitin ligase, known to be involved in cell proliferation and regulation of numerous nuclear processes, such as DNA damage response, DNA replication, and chromatin remodeling, through its action as a regulator of proteins involved in these processes, namely chromatin licensing and DNA replication factor 1 (CDT-1) in cell cycle regulation [145, 146]. CUL4A and CUL4B have also been shown to be components of a conserved Wnt-induced proteasome targeting complex that regulates p27 (KIP1) levels and cell cycle progression in mammalian cells [147]. Cells from patients with CUL4B loss of function mutations exhibit sensitivity to camptothecin (CPT), increased Topo I-induced DNA breaks, and impaired CPT-induced topoisomerase I (Topo I) degradation and ubiquitylation, suggesting Topo I to be a novel Cul4-dependent substrate [148]. Cells from these patients also displayed overexpression of other known CUL4-dependent substrates, such as Cdt1 and p21, but how these biochemical findings are linked to the neurophsychological findings in the patients remains unknown.
The Williams–Beuren syndrome is characterized by a recognizable pattern of malformations, that include growth delay, cognitive disabilities and altered social behaviour (hypersociability), among other deficits [149]. The disease results from hemizygosity of more than 20 genes, one of which, Trim50, encodes an E3 ubiquitin ligase, opening the possibility of UPS involvement also in this disease [150].
Froyen and colleagues identified mutations in the HUWE1 gene in three unrelated families with X-linked syndromic mental retardation [151]. HUWE1 encodes a HECT domain E3 ligase known to interact with the proteasome [152], namely in the brain [153]. Interestingly, prolonged neuronal activity induced 26S disassembly and dissociation of HUWE1, among other proteins, from the proteasome [153], and this change in UPS composition might be relevant for synaptic plasticity. HUWE1 is known to ubiquitylate histones H1, H2A, H2B, H3 and H4 [154], and to modulate transcription of the preprodynorphin gene [155]. Furthermore, it targets CDC6 (involved in the regulation of DNA replication at the initiation step) [156], MCL-1 (regulating apoptosis) [157, 158], and c-MYC [53, 159]. In particular, c-MYC targeting appears essential in neurodevelopment, allowing neural precursors to arrest proliferation and enter neurogenesis [53, 159]. HUWE1 is also relevant for neuron–radial glia interactions that are key to the proper migration and spatial arrangement of the cerebellar cortex [160]. Accordingly, conditional KO mice lacking HUWE1 in cerebellar granule neuron precursors and radial glia show a high rate of postnatal lethality and profound cerebellar abnormalities, resulting from aberrant proliferation and impaired differentiation of the progenitor cell population, as well as from layering aberrations, with persistence of ectopic clusters of granule neurons, due to severe granule neuron migration defects. Although this has been demonstrated for the cerebellum, a parallel role in other parts of the brain may exist.
Using whole genome copy number variant assessment, in a sample of more than 800 cases of individuals with autism spectrum disorders (ASDs), mutations were found in four UPS-related genes previously unrelated with the disease: UBE3A—the ubiquitin ligase also related to Angelman syndrome, PARK2—a ubiquitin ligase also related to Parkinson’s disease (PD), and RFWD2 and FBXO40—also ubiquitin ligases. These genes have been reported as ASDs candidate genes with altered copy number variants in the patients [161].
A region on chromosome 15q24 vulnerable to both deletions and duplications has been previously implicated in a range of phenotypes including autism, Asperger’s syndrome, delayed development, and mild to severe mental retardation [162]. Recent studies allowed narrowing the critical region and identification of ubiquitin-like 7 as a key gene [163, 164]. This protein, also designated BMSC-UbP, contains a UBQ domain at its N-terminus and a ubiquitin-associated domain at its C-terminus. Its role in the nervous system remains unknown.
Overactivation of the UPS in the brain cortex has also been proposed to play a role in Down syndrome (DS), the most common genetic form of intellectual disability, caused by complete or partial trisomy of chromosome 21 [165]. Chymotrypsin and peptidyl-glutamyl peptide-hydrolyzing (PGPH)-like activities of the proteasome are upregulated in the frontal cortex of hAPP-YAC tg mice, which model the overexpression of one of the genes triplicated in DS patients. These proteasome activities were augmented by cholinergic stimulation in control mice, but not in hAPP-YAC tg mice, and a similar upregulation of the UPS activities was detected in the frontal cortex of DS and Alzheimer’s disease (AD) patients. Another link between the UPS and DS comes from the evidence that the tetratricopeptide repeat domain 3 gene, TTC3, located in the DS critical region of chromosome 21, encodes an E3 ligase that targets phosphorylated Akt, facilitating its polyubiquitylation and degradation, and inhibits neuronal differentiation through modulation of the Rho A small GTPase pathway. TTC3 physically interacts with Citron kinase (CIT-K) and Citron N (CIT-N), two Rho A effectors known to be involved in neuronal proliferation and differentiation. In PC12 cells, TTC3 overexpression leads to strong inhibition of neurite extension, which can be prevented by CIT-K RNAi [166]. Therefore, the upregulation of TTC3 may contribute to some of the pathological findings, namely the cognitive deficits, present in DS patients. Ubiquitin-specific protease 25 (USP-25) has also been related to DS, with the protein being overexpressed in human DS fetal brains [167].
The role of the UPS in the development of the nervous system, and its clear involvement in neurodevelopmental diseases, opens a whole new field of research concerning the outcomes of a perturbed UPS. As the system becomes better characterized and its involvement in disease increasingly known, its components may become important targets for pharmacological treatment [168–171], as is happening already in the cancer field [172, 173].
The UPS in neurodegenerative disorders
The accumulation of misfolded protein aggregates is observed in a range of neurological diseases, which includes PD, AD, amyotrophic lateral sclerosis, frontotemporal dementia, and polyglutamine (polyQ) expansion disorders, among others [174–179]. These aggregates are often immunoreactive for components of the UPS, namely ubiquitin [180–182]. The exact significance of this phenomenon is still a matter of debate.
The concept that UPS dysfunction leads to accumulation of misfolded proteins in neurodegenerative diseases is reasonable and may be supported by the available evidence, as described below. However, it is not possible to exclude that protein aggregation is an earlier step in the etiology of the disease that leads itself to UPS dysfunction, even before inclusion body formation [183]. Alternatively, the observed accumulation of UPS components in aggregates may constitute an adaptation, a cellular response to the aggregation phenomenon. In this process, UPS components may become trapped into the protein aggregates, which act simultaneously as the cause of UPS impairment and its consequence, in a positive-feedback mechanism [184]. In normal aging brains, there are reports of protein aggregation [185], decreased activity of the UPS [186, 187], and activation of the “immunoproteasome” [188]. These studies suggest a direct contribution of the UPS dysfunction for age-related neurological disorders. This is certainly the case for PD, where the most common familial disease form, autosomal recessive-juvenile Parkinsonism, results from mutations in parkin, a RING E3 ligase [189]. A mutation in the gene coding for UCH-L1 was also associated with familial forms of the disease, thought to be due to a decrease in the activity of this enzyme [190]. Six of the nine PD genes known to date, α-synuclein being the exception, have an identified C. elegans orthologue, including the UPS components, UCH-L1 and parkin [191]. Springer and co-workers identified the C. elegans parkin homolog, PDR-1, and contributed to elucidate the mechanisms by which parkin is involved in the disease. The nematode strain expressing an in-frame deletion allele of pdr-1 was more prone to protein aggregation and more sensitive to ER stress, suggesting that expressing the mutant form of the enzyme may be more useful to understand the neurotoxicity in the disease than completely abolishing its expression [192], a concept that may be relevant for the study of other diseases.
In polyQ disorders, including Huntington’s disease (HD), spinocerebellar ataxias, Kennedy’s disease, and dentatorubropallidoluysian atrophy, a CAG triplet repeat expansion encodes an expanded polyQ stretch, and the resulting mutant proteins misfold, often forming protein inclusions [193]. An important role for the UPS is suggested in these disorders, namely through the observation that the presence of ubiquitin-positive protein intranuclear inclusions is a common feature [194, 195].
In HD, UPS dysfunction has been demonstrated in several models [183, 196], and ubiquitin-positive polyQ inclusions have been found in the brain of HD as in other neurodegenerative diseases, such as MJD, spinocerebellar ataxia type 7 (SCA7) and dentatorubral-pallidoluysian atrophy patients [194, 197, 198]. However, the question of whether the UPS dysfunction is the cause or a consequence in neurodegenerative diseases is still under debate. In a transversal study using cell lines, HD mice and post-mortem brains of HD patients, it was suggested that accumulation of N-terminal fragments of mutant huntingtin (htt), which may be toxic and drive the formation of aggregates through “seeding” effects, were a consequence of age-related UPS dysfunction rather than its cause [199]. A recent study also suggested that toxic htt forms do not impair the proteasome and that, instead, the impairment of the UPS arises from the accumulation of N-terminal fragments [200]. Supporting this model, an HD mouse study showed no global impairment of UPS activities, and the forms of polyubiquitylated proteins accumulated in this model were of a different nature than those found upon proteasome inhibition [201]. In contrast, impairment of the UPS was demonstrated in transfected HEK293 cells upon polyQ aggregation, even before inclusion of body formation [183]. A recent report reconciles apparently different models, demonstrating a poly-Q-induced global UPS impairment in vivo in a mouse model of HD [196]. These authors attributed the discrepancy between the results obtained in different models to the early onset of the disease in transgenic animals, which are therefore exposed to mutant htt for longer periods. This may lead to adaptation and formation of inclusion bodies, restoring the UPS function, and this was suggested in the earlier studies to be beneficial in the context of the disease [202]. Recent reports trying to further unravel the mechanisms through which the UPS might be related to the disease showed that mutant htt interferes with the degradation of β-catenin, leading to its toxic accumulation [203].
Machado–Joseph disease or spinocerebellar ataxia type 3 is another example of a polyQ disorder [204]. In this case, the polyQ expansion affects ataxin-3, a protein described to have DUB activity in vitro [205]. Although polyQ diseases are thought to result predominantly from a gain of toxic function, due to the presence of the expansion of the polyQ tract, it is nevertheless intriguing that the protein involved in this particular disease has been reported to possess DUB and de-NEDDylase activity [205–207], and interacts with valosin-containing protein (VCP) [208, 209], HHR23A and B [210], parkin [211], and C terminus of Hsc70-interacting protein (CHIP) [212], as well as with components of the proteasome [213]. In the search for physiological roles of ataxin-3 and for the mechanisms of pathogenicity, Pittman and colleagues suggested that ataxin-3 with an expanded polyQ tract displays increased affinity to VCP, preventing the assembly of the complex—VCP/Ufd1-Npl4—responsible for the retranslocation of proteins from the endoplasmatic reticulum to the cytosol, where ubiquitylated proteins are degraded by the proteasome—the endoplasmatic reticulum-associated degradation (ERAD) [214].
The C. elegans orthologue of ataxin-3 (ATX-3) also has DUB activity and interacts with the worm homologue of VCP and with UBXN-5, a VCP adapter thought to be involved in the transport of specific substrates to the proteasome [215]. Although common interactors of VCP and ubxn-5 have been identified, the physiologic substrates of ataxin-3 activity remain mostly unknown. A recent work not only reveals a new functional role for ataxin-3 but also sheds light on possible mechanisms of MJD etiology. The ubiquitin ligase CHIP, that interacts with chaperones to promote the degradation of misfolded proteins [216], has been previously described to interact with ataxin-3 [217], but the functional meaning of this interaction was unknown. In a recent report, Scaglione and co-workers identified the ubiquitin-conjugating enzyme Ube2w as responsible for CHIP mono-ubiquitylation, and showed that this ubiquitylation stabilizes the interaction of CHIP with ataxin-3. In this complex, ataxin-3 is responsible for the editing of the ubiquitin chains of substrates associated to CHIP, promoting their turnover. Ataxin-3 is also able to deubiquitylate CHIP upon the accumulation of polyubiquitylated substrates. This dynamic process is disturbed in the context of expanded-polyQ ataxin-3, since the altered form of the enzyme shows an approximately 6-fold increase in the affinity for CHIP. The functional meaning of this increased affinity is still not known, but in this study it was observed that SCA3 mice (model of MJD) showed a significant decrease in CHIP levels compared with wild-type animals [212], which suggests that the increased affinity of ataxin-3 for CHIP results in CHIP degradation with the consequent deregulation of protein quality control mechanisms. Another contribution to the understanding of the disease came from a recent study that demonstrates that ataxin-3 and parkin—an E3 ligase responsible for a familial form of PD [189]—interact both in vitro and in cells, and that ataxin-3 has DUB activity towards parkin. The expanded-polyQ version of ataxin-3 deubiquitylates parkin more efficiently and is also responsible for increased parkin degradation through the autophagy degradation pathway [211], and this correlates with decreased parkin levels in a transgenic mouse model of the disease [218]. Taking into account the neuroprotective role of parkin [219–221], the fact that expanded-polyQ ataxin-3 induces parkin clearance might help to explain the neurodegeneration in MJD.
Intriguingly, and perhaps counter-intuitively, ATX-3 knock-out C. elegans strains show an improved response to heat stress, with higher expression of chaperones [222], suggesting a negative regulatory role for this protein in the stress response or perhaps an adaptation of the knockout strains to a permanent proteotoxic stress. Another insight to the ATX-3- mediated mechanisms of lifespan regulation was given by the finding that CDC48 and ATX-3 synergistically cooperate in ubiquitin-mediated proteolysis and in ageing regulation. Worms deficient in both ATX-3 and CDC48 have extended lifespan, and it was suggested that this was mediated by the regulation of ubiquitylation of substrates involved in the insulin-IGF-1 signaling [38]. How the normal function of ataxin-3 can be related to disease in this context remains to be further clarified.
The loss of the de-ubiquitylating enzyme Usp14 due to the axJ mutation in ataxia mice was suggested to lead to a decrease in the availability of monomeric ubiquitin [223] and to synaptic transmission defects [224], which may be due to a dysfunction in the Usp14-regulated gamma-aminobutiric acid type A (GABAA) receptor turnover [225]. Neuronal expression of Usp-14 was able to rescue the motor system defects observed in mice carrying the axJ mutation [226], showing that the function of this DUB is highly relevant in neurons. Intriguingly, recent work has described inhibition of ubiquitin–protein conjugate degradation, in vitro and in vivo, by USP-14. This occurs through the trimming of substrate-bound ubiquitin chains on the proteasome. A specific inhibitor of USP-14 was described, 1-[1-(4-fluorophenyl)-2,5-dimethylpyrrol-3-yl]-2-pyrrolidin-1-ylethanone, that can potentially be used to enhance proteasome function, and to improve clearance of misfolded proteins in neurodegenerative diseases [227]. Although, according to this study, performed in cell lines, inhibition of USP-14 might be relevant for the clearance of misfolded proteins, but caution must be taken about the implications of this approach, since it cannot be ignored that decreased levels of USP-14 in USP14 ax J mice also lead to redistributed membrane expression of GABAA receptors and consequent altered synaptic transmission [225].
Alzheimer’s disease, the most common neurodegenerative disease in humans, is characterized by the accumulation of extracellular amyloid-β plaques and intracellular neurofibrillary tangles [228]. As in other neurodegenerative diseases, the identification of ubiquitin immunoreactivity in histological lesions suggested the link between UPS and disease [181]. A more direct link was established with the finding of a mutant form of ubiquitin in AD patients, ubiquitin-B+1 (UBB+1). This aberrant ubiquitin has a 19 amino acid C-terminal extension that lacks the C-terminal glycine essential for substrate ubiquitylation, and has been detected in the cortex in brain sections of AD patients, as well as in elderly controls whenever plaques and tangles were also present [229]. Accumulation of UBB+1 leads to neuritic beading, mitochondrial stress, and neuronal degeneration in primary cultures of cortical neurons. UBB+1 is a UPS-substrate itself, being degraded at low levels; however, at high expression levels, it inhibits the proteasome [230]. Table 3 shows examples of components of the UPS involved in nervous system diseases and their homologues in C. elegans.
Table 3.
List of neurodevelopmental and neurodegenerative diseases for which a component of the UPS is known to be involved in the etiology of the disease and their C. elegans homologues
| Disease | Components of the UPS | C. elegans homologue | C. elegans neuronal expression | References | |
|---|---|---|---|---|---|
| Neurodevelopmental | Angelman syndrome | UBE3A | Y48G8AL.1 | Expression still not defined | [115, 118] |
| Williams–Beuren syndrome | TRIM50 | Not described | Expression still not defined | [150] | |
| Intellectual disability not otherwise specified | CUL4B | cul-4 | No evidence | [144, 267, 268] | |
| HUWE1 | eel-1 | Yes | |||
| Autism spectrum disorders | UBE3A | Y48G8AL.1 | Expression still not defined | [161, 192] | |
| PARK2 | pdr-1 | Yes | |||
| RFWD2 | Not described | Expression still not defined | |||
| FBXO40 | C10E2.2 | Expression still not defined | |||
| Johanson–Blizzard syndrome | UBR1 | C32E8.11 | Expression still not defined | [138] | |
| Down syndrome | TTC3 | Not described | Yes | [166] | |
| USP-25 | Not described | Expression still not defined | |||
| Neurodegenerative | Parkinson’s disease | PARK | pdr-1 | Yes | [189–192, 269–271] |
| UCH-L1 | ubh-1 | Expression still not defined | |||
| DJ-1 | djr-1.1, djr-1.2 | Expression still not defined | |||
| ATP13A2 | catp-6 | Expression still not defined | |||
| Machado–Joseph disease | ATXN3 | atx-3 | Yes | [204, 272] | |
| Ataxia | USP-14 | usp-14 | Expression still not defined | [225] |
Combating neurodegenerative disorders by targeting the UPS is an attractive therapeutic approach [168–171], taking into consideration the significant involvement of UPS components in these disorders, as described above. Perhaps the most challenging aspect of this strategy is to define which step of UPS activity should be interfered with. As substrate selection in the UPS is driven by the ubiquitin ligases, targeting E3s in the conjugation step would confer more specificity to the therapeutics. Anyway, successful strategies interfering with E3s are not easy to devise due to enzyme complexity and lack of knowledge about the mechanisms of catalysis [231], but with the increasing number of neurodegenerative disease-related E3s, this will be a pathway to explore further [232, 233]. The inhibition of neurodegenerative disease-related DUBs has been endorsed with the example cited above, of USP-14 inhibition [227]. Another approach is to facilitate substrate degradation by the UPS; this can be achieved, amongst other possibilities, by induction of heat shock protein expression. This has been attempted with Geranylgeranylacetone [234, 235], 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG), and 17-(Dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) [236]. Taking into account the deleterious effects that disturbing such a widespread system evoke, the combinatorial use of UPS modifiers might have the greatest promise for research in this field.
C. elegans as a model system for the study of the UPS in nervous system
As described previously, the UPS plays crucial roles in the developing and adult nervous system. Furthermore, the dysfunction of the UPS is a hallmark of many human neurological diseases.
Contributions of simple model organisms to unravel human biochemistry and physiology in health and disease are far from being extensively explored. Notwithstanding the phylogenetic distances, those models present many advantages in functional genomics approaches in the initial stages of the search for possible mechanisms of disease or when trying to validate findings of human genetic screens, as in the case of the identification of genes related with diseases. The ability to perform large-scale genetic analysis, the number of mutant strains that are presently available to the community and the fact that they share many molecular mechanisms with mammals are other strong points of these models [237] that can be exploited.
C. elegans has been used as a model to study neuroscience due to its great potentialities as a study platform that can integrate genetics, neuronal circuits, and behavior in the same organism, generating a knowledge that can hardly be obtained in higher organisms, and making it possible to fill the gaps between those apparently distant fields of research. The C. elegans nervous system has been well characterized: it is composed of 302 neurons in hermaphrodites, uses as small molecule neurotransmitters acetylcholine, dopamine, serotonin, GABA, glutamate, and the invertebrate catecholamine–octopamine. In addition, this nematode also uses neuropeptides as neurotransmitters. The genes expressed in the nervous system are quite well preserved across species, with most of the components that exist in mammals also being expressed in C. elegans [238]. Synaptic connectivity was unravelled by serial electron microscopy by Brenner and co-workers [239]. Behavioral assays in C. elegans allow directly relating behavior and genetics, individual neurons or neural circuits, with rare problems relating to phenotypic variations as a consequence of different genetic backgrounds, since the N2 (Bristol) strain is almost invariably used [240]. C. elegans has specific features that allow the conducting of studies that are not possible (or possible but involving much more labor) in mammalian models. Table 4 shows a list of techniques used for genetic modification in C. elegans, all of which allow the generation of modified strains within a 1-month timeframe.
Table 4.
List of methods used in C. elegans for genetic modification, with focus on techniques that are important for nervous system studies
| Methods for genetic modification used in C. elegans | References | |
|---|---|---|
| Random mutagenesis | Recurring to the use of chemical mutagens gene inactivation can be achieved; PCR is used to screen for target gene deletions; backcrossing is essential to remove additional mutations from genetic background | [278] |
| RNAi-increased efficiency in the nervous system | RNAi can be induced in C. elegans through microinjection of dsRNA, soaking of the animal in a solution of dsRNA or feeding with recombinant bacteria expressing dsRNA. Effects remain through generations. Neuronal absence or decreased amount of a transmembrane domain protein—SID-1—is related to the lack of RNA sensitivity in the nervous system; strains expressing SID-1 in the nervous system were generated in which an increased effectiveness of RNAi in the nervous system was observed | [279, 280] |
| Classic transgenesis | Transgenics can be created by microinjection of foreign DNA into the germline of C. elegans; transgenes can remain episomal or be integrated upon exposure to radiation, which then require backcrossing to exclude additional mutations. A wide range of promoters are available | [281] |
| Insertion of single copy transgenes into targeted sites | mos-1 mobilization to a specific site in the DNA chain induces a double-strand break and this can be repaired by copying DNA from an inserted transgene; insertion of the transgene marker unc-119 allows positive selection of the KOs, since it rescues the uncoordinated phenotype observed in unc-119 (-)—that has homology with only one of the DNA broken ends; this method avoids random insertion of transgenes which is frequent using other transgenesis methods | [282, 283] |
| Conditional regulation of gene expression | mec-8 codes an RNA processing factor involved in the splicing of mec-2 (a gene involved in touch sensitivity); adding mec-2 intron 9 to a gene allows its regulation through the expression of MEC-8; the gene silencing system can be made temperature-dependent using a temperature-sensitive allele of mec-8–u218ts; at the non-permissive temperature (15°C), MEC-8 does not splice mec-2 and the expression of the target gene occurs, while at permissive temperature (20°C), the silencing becomes effective; this method allows a temperature-sensitive control of gene and/or RNAi expression for virtually any C. elegans gene | [284–286] |
| Creation of reporter gene fusions | The promoter under study is amplified from worm genomic DNA or from a preparation of cosmid DNA in the first PCR, with the 3′ primer having a 24-nucleotide overhang to the GFP vector pPD95.75; in the second PCR, the GFP coding sequence plus the generically used 3′ untranslated region (UTR) from the unc-54 gene are amplified; the two PCR products are used for the fusion PCR with nested primers and the product is then injected to C. elegans gonad; with this method, besides having the full coding sequence of the gene being expressed allowing subcellular localization information, there is no need for subcloning procedures | [287] |
Once the genetically modified strain has been developed for any gene, nervous system studies are easily amenable in C. elegans. The fact that C. elegans embryonic and post-embryonic cell lineages are known [241, 242] allows detailed neurodevelopmental studies. Many strains bearing neuronal type-specific or synaptic markers are also available, which allow mapping of neuronal networks. Additionally, a battery of tests for assessment of neurological function can be applied, including several motor assessment paradigms, approaches to measure the sensitivity to different stimuli, and even some very basic memory tests. Besides the phylogenetic distance between this nematode and higher organisms, the main current limitations in the use of C. elegans in neurobiological studies are related to the increased difficulty in performing techniques like immunohistochemistry, tissue microdissection, receptor autoradiography, electrophysiology, or microdialysis, which are standard in other organisms.
The use of simple organisms such as C. elegans may be important to tackle the basic mechanisms underlying neurological diseases that at a later point must be further explored in higher organisms. As mentioned above, one of the most obvious constraints to the use of C. elegans as a model in neurological disease stems from its lack of complexity compared with superior animals (simultaneously one of its great advantages). Nevertheless, bioinformatic studies point to 60–80% human genes with a corresponding C. elegans homologue [243–246], and a study comparing human disease gene sequences and potential orthologues in C. elegans identified 42% of human disease genes with orthologues in C. elegans [247], a result that is in accordance with previous predictions [248, 249]. The utility of C. elegans as a model in the study of neurological disease has been attested, namely in studies concerning polyQ disorders, Amyotrophic Lateral Sclerosis, AD, and PD [250–253] (see also Table 3). In these studies, two main strategies have been used: (1) the characterization of the worm orthologues of human disease-relevant genes, or (2) expression of mutant human proteins. The latter studies have demonstrated that proteins associated with neurological diseases in humans, when expressed in C. elegans, are still able to induce toxicity and cause phenotypic changes related to those observed in more complex animal models. Good examples of this are the expression of human α-synuclein at manipulated rates in a C. elegans model of PD [254] or our recent work on the expression of human ataxin-3 in a C. elegans model of MJD [255]. These studies, besides contributing to the understanding of neuron-specific effects of disease-related proteins expression, are also good examples of how C. elegans can be useful to bridge genetics, cell specific phenotypes and behavior in a living organism.
An important application of C. elegans models of neurological disease is the initial screening of therapeutic compounds, that can be later scrutinized in higher organisms [256]. The advantages of this model for large-scale pre-clinical drug screening are supported by highly conserved biochemical pathways, which holds true in the case of the UPS [12], and range from the morphology and lifecycle to the genetic amenability described in this review [257]. This has been attempted with promising results in the study of AD and HD [258, 259].
A recent study to screen for therapeutic suppressors of polyglutamine aggregation constitutes a good example on how useful theses studies can be [260].
Concerning its biological validity as a model for the study of the UPS, the C. elegans UPS has all the main features of mammalian UPS, including all the main components of the system: ubiquitin, E1, E2s, E3s, and DUBs with reports of homology between many of these genes [12]. Until now, this system has been characterized in the nematode mostly using loss of function phenotypes for its components, and in the context of the whole organism [5, 12, 261], and a more local characterization of the system is still missing, namely in the nervous system. A few studies have been reported along these lines and the proof of concept that studying UPS in C. elegans nervous system is viable is established, namely by successful examples such as in the case of the RPM-1 E3, the homologue of murine PHR1 and human protein associated with myc (PAM). The first insights for the physiological function of PHR proteins came from studies in C. elegans [76, 77], contributing to the now well-established role of these proteins in pre-synaptic regulation. These proteins are a good example of how studies in different organisms, from the more simple to the more complex, can elucidate basic molecular mechanisms that are conserved across species, in this case the mechanisms of transition from axon growth to synaptogenesis [262].
The C. elegans genome encodes two AMPAr subunits, GLR-1 and GLR-2 [97], that share many functional similarities with their vertebrate homologues [238], having, for example, obligatory auxiliary proteins—TARPs—that perform the same functions in both cases [263]. Together with the fact that the UPS in C. elegans has most of the components that occur in higher species and works in a very similar way, the study of AMPAr trafficking and related synaptic plasticity events is a good example of how useful the detailed study of the UPS in C. elegans nervous system can be.
Recently developed reporter strains are a very promising tool for future studies on the UPS using this nematode as a model system (see Fig. 3). In one of these models, a ubiquitin-conjugated fluorescent reporter protein—Ub-dsRed—was expressed in GABAergic neurons together with wild-type or expanded polyQ-contanining proteins, as a means to assess the effect of these proteins on global UPS activity. It was observed that worms expressing a 127 polyQ stretch had accumulation of aggregates and bright fluorescence, corresponding to an accumulation of the UPS reporter, which was not observed in animals with 19 glutamines [264]. This type of strategy can be used to assess any factor/gene thought to impact on UPS function in the nervous system.
Fig. 3.
Examples of in vivo degradation assays to characterize UPS activity in C. elegans. a In an effort to identify the role of polyQ-containing aggregates in the pathogenesis of neurodegenerative diseases, a construct containing ubiquitin fused with the fluorescent reporter Discosoma Red fluorescent protein (ds-Red) was expressed in C. elegans GABAergic neurons (unc-47 promoter). L1 larvae animals with GABAergic neurons expressing ataxin-3 with 19 glutamine repeats showed no accumulation of aggregates or red fluorescence. Animals expressing ataxin-3 with 127 glutamine repeats and that form aggregates showed red staining corresponding to accumulation of the ubiquitin fusion reporter, indicating UPS impairment. b A ubiquitin fusion degradation substrate was generated by the ligation of GFP to a ubiquitin hidrolase-insensitive (non-cleavable) ubiquitin form (UbV). This construct was stably integrated in the genome of unc-119 (ed4) worms and expressed under the control of the sur-5 promoter in most C. elegans tissues. Mild protein-folding stress using DTT 3 mM or 1% ethanol resulted in UbV-GFP stabilization indicating UPS impairment, whereas acute heat stress—worms grown to day 1 of adulthood and subjected to 37°C for 2 h—enhanced UbV-GFP turnover. RNAi for subunits of the 19S regulatory particles showed different UPS response in different tissues. As an example, rpn-8 subunit depletion resulted in the stabilization of the substrate in the pharynx, vulva, tail, and intestine, whereas rpn-10 depletion resulted in the UPS impairment specifically in the intestine and vulva and not in pharynx or tail. These results show that different types of stress affect the UPS differently and also indicate the tissue-specific requirement of proteasome subunits, besides showing the usefulness of this technique for high-throughput screening approaches. c To understand how the UPS degradation machinery works in different cell types and in a specific time window, a system was developed in which a non-cleavable form of ubiquitin—UbG76V—was fused to the green fluorescent protein Dendra2. Dendra2 can be irreversibly photoconverted from green to red if 405 nm light is used. Making use of specific promoters, this reporter system can be expressed in virtually any cell type and after photoconvertion allows the assessment of UPS activity at the chosen time window
Another biological tool worthy of note is that generated by Segref et al. [265]. To assess the impact of stress in the UPS and to understand the relevance of different proteasome subunits in different tissues in C. elegans, an in vivo degradation assay was generated. A ubiquitin fusion degradation substrate consisting of GFP and a ubiquitin hidrolase-insensitive ubiquitin form (UbV) was created. These studies demonstrated that the UPS is affected differentially in response to different stresses and showed the tissue-specific requirement of proteasome subunits.
Another very promising tool for UPS studies has recently emerged: C. elegans strains were generated carrying the photoconvertible fluorescent protein Dendra2 fused to a modified ubiquitin that is not cleaved by DUBs–UbG76V. This system allows live imaging quantification of UPS activity by measuring the fluorescence after cell-specific photoconversion of Dendra2. Interestingly, this system allows the performing of photoconversion in specific neuronal sub-types and defining differences in degradation rates among them by measuring the persistence of the fluorescent reporter protein [266]. This technique will allow the assessment of the effects of compounds or stimuli in the UPS activity of specific neuronal populations in the C. elegans nervous system in vivo, which could be extremely important in unraveling specific molecular mechanisms in the context of neurological diseases thought to be UPS-related.
Conclusions
Ubiquitylation is emerging as a type of signaling akin to phosphorylation in terms of significance and complexity of the machinery involved. Its effects on protein localization, interactions, activation state, and/or degradation seem to be relevant for many aspects of nervous system function, and its dysfunction is associated with many human neurological diseases. Given its biological features, the nematode C. elegans is an excellent tool for the dissection of ubiquitin-related signaling in the nervous system and for pharmaceutical target development in this context.
Acknowledgments
The work in the authors’ laboratory is funded by Fundação para a Ciência e a Tecnologia, Portugal (PTDC/SAU-GMG/101572/2008) and (PTDC/SAU-NMC/120144/2010), and M.S.B. received a scholarship from Fundação para a Ciência e Tecnologia (SFRH/BD/47963/2008). We thank Andreia Teixeira-Castro for critical review of the manuscript.
Abbreviations
- 17-AAG
17-(Allylamino)-17-demethoxygeldanamycin
- 17-DMAG
17-(Dimethylaminoethylamino)-17-demethoxygeldanamycin
- AD
Alzheimer’s disease
- AMPAr
Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AMSH
Associated molecule with the SH3 domain of STAM
- APC
Anaphase-promoting complex
- Arc
Activity-regulated cytoskeleton-associated protein
- ASDs
Autism spectrum disorders
- ATX-3
Ataxin-3
- CDT-1
Chromatin licensing and DNA replication factor 1
- CHIP
C terminus of Hsc70-interacting protein
- CIT-K
Citron kinase
- CIT-N
Citron N
- CPT
Camptothecin
- DLK-1
Dual-leucine zipper Kinase MAPKKK
- DS
Down syndrome
- DUB
Deubiquitylating enzyme
- DUBs
Deubiquitylating enzymes
- E1
Ubiquitin-activating enzyme
- E2
Ubiquitin-conjugating enzyme
- E3s
Ubiquitin ligases
- ERAD
Endoplasmatic reticulum-associated degradation
- GABA
Gamma-aminobutyric acid
- HAUSP
Herpesvirus-associated ubiquitin-specific protease
- HD
Huntington’s disease
- HSNL
Hermaphrodite-specific motor neuron
- Htt
Huntingtin
- JAMM
Jab1/MPN domain-associated metalloisopeptidase class
- LPA
L-α-lysophosphatidic acid
- LTF
Long-term facilitation
- mEPSCs
Miniature excitatory postsynaptic currents
- MJD
Machado–Joseph disease
- NGF
Nerve growth factor
- OTU
Otubain protease
- PAM
Protein associated with myc
- PD
Parkinson’s disease
- PGPH
Peptidyl-glutamyl peptide-hydrolyzing
- PKA
Protein kinase A
- PolyQ
Polyglutamine
- PSR
Primary synapse region
- RPM-1
Regulator of pre-synaptic morphology-1
- REST
Repressor element 1-silencing transcription factor
- RIM1
Rab3-interacting molecule 1
- SCA7
Spinocerebellar ataxia type 7
- SCF
Skip1-cullin-F-Box
- Smurf1 and Smurf2
Smad ubiquitylation regulatory factors 1 and 2
- UBB+1
Ubiquitin-B+1
- UBC
Ubiquitin-conjugating catalytic fold
- UCH
Ubiquitin C-terminal hydrolase
- Uch37
Ubiquitin C-terminal hydrolase 37
- UCH-L1
Ubiquitin C-terminal hydrolase 1
- UPS
Ubiquitin–proteasome system
- USP
Ubiquitin-specific protease
- USP7
Ubiquitin-specific protease 7
- USP-25
Ubiquitin-specific protease 25
- USP-46
Ubiquitin-specific protease 46
- VCP
Valosin-containing protein
References
- 1.Gallastegui N, Groll M. The 26S proteasome: assembly and function of a destructive machine. Trends Biochem Sci. 2010;35:634–642. doi: 10.1016/j.tibs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Davy A, Bello P, Thierry-Mieg N, Vaglio P, Hitti J, Doucette-Stamm L, Thierry-Mieg D, Reboul J, Boulton S, Walhout AJ, Coux O, Vidal M. A protein–protein interaction map of the Caenorhabditis elegans 26S proteasome. EMBO Rep. 2001;2:821–828. doi: 10.1093/embo-reports/kve184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin J, Li X, Gygi SP, Harper JW. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447:1135–1138. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- 4.Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones D, Crowe E, Stevens TA, Candido EPM. Functional and phylogenetic analysis of the ubiquitylation system in Caenorhabditis elegans: ubiquitin-conjugating enzymes, ubiquitin-activating enzymes, and ubiquitin-like proteins. Genome Biol. 2002;3:RESEARCH0002. doi: 10.1186/gb-2001-3-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni M, Smith HE. E1 ubiquitin-activating enzyme UBA-1 plays multiple roles throughout C. elegans development. PLoS Genet. 2008;4:e1000131. doi: 10.1371/journal.pgen.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burroughs AM, Jaffee M, Iyer LM, Aravind L. Anatomy of the E2 ligase fold: implications for enzymology and evolution of ubiquitin/Ub-like protein conjugation. J Struct Biol. 2008;162:205–218. doi: 10.1016/j.jsb.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michelle C, Vourc’h P, Mignon L, Andres CR. What was the set of ubiquitin and ubiquitin-like conjugating enzymes in the eukaryote common ancestor? J Mol Evol. 2009;68:616–628. doi: 10.1007/s00239-009-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze E, Altmann ME, Adham IM, Schulze B, Fröde S, Engel W. The maintenance of neuromuscular function requires UBC-25 in Caenorhabditis elegans . Biochem Biophys Res Commun. 2003;305:691–699. doi: 10.1016/s0006-291x(03)00824-6. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CAP. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponts N, Yang J, Chung D-WD, Prudhomme J, Girke T, Horrocks P, Le Roch KG. Deciphering the ubiquitin-mediated pathway in apicomplexan parasites: a potential strategy to interfere with parasite virulence. PLoS ONE. 2008;3:e2386. doi: 10.1371/journal.pone.0002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipreos ET (2005) Ubiquitin-mediated pathways in C. elegans. WormBook 2005:1–24 [DOI] [PMC free article] [PubMed]
- 13.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Deshaies RJ, Joazeiro CAP. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 15.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe T. Multiubiquitylation by E4 enzymes: “one size” doesn’t fit all. Trends Biochem Sci. 2005;30:183–187. doi: 10.1016/j.tibs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlbrodt K, Mouysset J, Hoppe T. Orchestra for assembly and fate of polyubiquitin chains. Essays Biochem. 2005;41:1–14. doi: 10.1042/EB0410001. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol. 2004;16:119–126. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Kodadek T. No Splicing, no dicing: non-proteolytic roles of the ubiquitin-proteasome system in transcription. J Biol Chem. 2010;285:2221–2226. doi: 10.1074/jbc.R109.077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todi SV, Scaglione KM, Blount JR, Basrur V, Conlon KP, Pastore A, Elenitoba-Johnson K, Paulson HL. Activity and cellular functions of the deubiquitinating enzyme and polyglutamine disease protein ataxin-3 are regulated by ubiquitination at lysine 117. J Biol Chem. 2010;285:39303–39313. doi: 10.1074/jbc.M110.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treier M, Staszewski LM, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 23.Greer SF, Zika E, Conti B, Zhu X-S, Ting JP-Y. Enhancement of CIITA transcriptional function by ubiquitin. Nat Immunol. 2003;4:1074–1082. doi: 10.1038/ni985. [DOI] [PubMed] [Google Scholar]
- 24.Brès V, Kiernan RE, Linares LK, Chable-Bessia C, Plechakova O, Tréand C, Emiliani S, Peloponese J-M, Jeang K-T, Coux O, Scheffner M, Benkirane M. A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat Cell Biol. 2003;5:754–761. doi: 10.1038/ncb1023. [DOI] [PubMed] [Google Scholar]
- 25.Ferdous A, Sikder D, Gillette T, Nalley K, Kodadek T, Johnston SA. The role of the proteasomal ATPases and activator monoubiquitylation in regulating Gal4 binding to promoters. Genes Dev. 2007;21:112–123. doi: 10.1101/gad.1493207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archer CT, Kodadek T. The hydrophobic patch of ubiquitin is required to protect transactivator-promoter complexes from destabilization by the proteasomal ATPases. Nucleic Acids Res. 2010;38:789–796. doi: 10.1093/nar/gkp1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedford L, Layfield R, Mayer RJ, Peng J, Xu P. Diverse polyubiquitin chains accumulate following 26S proteasomal dysfunction in mammalian neurones. Neurosci Lett. 2011;491:44–47. doi: 10.1016/j.neulet.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 29.Saeki Y, Kudo T, Sone T, Kikuchi Y, Yokosawa H, Toh-e A, Tanaka K. Lysine 63-linked polyubiquitin chain may serve as a targeting signal for the 26S proteasome. EMBO J. 2009;28:359–371. doi: 10.1038/emboj.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem. 2007;282:17375–17386. doi: 10.1074/jbc.M609659200. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson AD, Zhang N-Y, Xu P, Han K-J, Noone S, Peng J, Liu C-W. The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26 s proteasome. J Biol Chem. 2009;284:35485–35494. doi: 10.1074/jbc.M109.052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto E, Yamanaka Y, Ishikawa A, Aoki-Kawasumi M, Mito-Yoshida M, Ohmura-Hoshino M, Matsuki Y, Kajikawa M, Hirano H, Ishido S. Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization. J Biol Chem. 2010;285:35311–35319. doi: 10.1074/jbc.M110.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Hakim AK, Zagorska A, Chapman L, Deak M, Peggie M, Alessi DR. Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem J. 2008;411:249–260. doi: 10.1042/BJ20080067. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. “protein modifications: beyond the usual suspects” review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 36.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winborn BJ, Travis SM, Todi SV, Scaglione KM, Xu P, Williams AJ, Cohen RE, Peng J, Paulson HL. The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J Biol Chem. 2008;283:26436–26443. doi: 10.1074/jbc.M803692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhlbrodt K, Janiesch PC, Kevei É, Segref A, Barikbin R, Hoppe T. The Machado-Joseph disease deubiquitylase ATX-3 couples longevity and proteostasis. Nat Cell Biol. 2011;13:273–281. doi: 10.1038/ncb2200. [DOI] [PubMed] [Google Scholar]
- 39.Nijman SMB, Luna-Vargas MPA, Velds A, Brummelkamp TR, Dirac AMG, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 41.Bhat KP, Greer SF. Proteolytic and non-proteolytic roles of ubiquitin and the ubiquitin proteasome system in transcriptional regulation. Biochim Biophys Acta. 2011;1809:150–155. doi: 10.1016/j.bbagrm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Al-Hakim A, Escribano-Diaz C, Landry M-C, O’Donnell L, Panier S, Szilard RK, Durocher D. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair (Amst) 2010;9:1229–1240. doi: 10.1016/j.dnarep.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grillari J, Grillari-Voglauer R, Jansen-Dürr P. Post-translational modification of cellular proteins by ubiquitin and ubiquitin-like molecules: role in cellular senescence and aging. Adv Exp Med Biol. 2010;694:172–196. doi: 10.1007/978-1-4419-7002-2_13. [DOI] [PubMed] [Google Scholar]
- 44.Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meller R. The role of the ubiquitin proteasome system in ischemia and ischemic tolerance. Neuroscientist. 2009;15:243–260. doi: 10.1177/1073858408327809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia L, Bickel JS, Wu J, Morgan MA, Li H, Yang J, Yu X, Chan RC, Sun Y. RBX1 (RING box protein 1) E3 ubiquitin ligase is required for genomic integrity by modulating DNA replication licensing proteins. J Biol Chem. 2011;286:3379–3386. doi: 10.1074/jbc.M110.188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wertz IE, Dixit VM. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawabe H, Brose N. The role of ubiquitylation in nerve cell development. Nat Rev Neurosci. 2011;12:251–268. doi: 10.1038/nrn3009. [DOI] [PubMed] [Google Scholar]
- 50.Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, Shi Y, Harper JW, Elledge SJ. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuoc TC, Stoykova A. Trim11 modulates the function of neurogenic transcription factor Pax6 through ubiquitin-proteosome system. Genes Dev. 2008;22:1972–1986. doi: 10.1101/gad.471708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon K-J, Koo B-K, Im S-K, Jeong H-W, Ghim J, Kwon M-C, Moon J-S, Miyata T, Kong Y–Y. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X, D’Arca D, Lim WK, Brahmachary M, Carro MS, Ludwig T, Cardo CC, Guillemot F, Aldape K, Califano A, Iavarone A, Lasorella A. The N-Myc-DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain. Dev Cell. 2009;17:210–221. doi: 10.1016/j.devcel.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobieszczuk DF, Poliakov A, Xu Q, Wilkinson DG. A feedback loop mediated by degradation of an inhibitor is required to initiate neuronal differentiation. Genes Dev. 2010;24:206–218. doi: 10.1101/gad.554510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng L, Allen NS, Simo S, Cooper JA. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007;21:2717–2730. doi: 10.1101/gad.1604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simó S, Jossin Y, Cooper JA. Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J Neurosci. 2010;30:5668–5676. doi: 10.1523/JNEUROSCI.0035-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryan B, Cai Y, Wrighton K, Wu G, Feng X-H, Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005;579:1015–1019. doi: 10.1016/j.febslet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 58.Lasorella A, Stegmüller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- 59.Stegmüller J, Konishi Y, Huynh MA, Yuan Z, Dibacco S, Bonni A. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron. 2006;50:389–400. doi: 10.1016/j.neuron.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Schwamborn JC, Müller M, Becker AH, Püschel AW. Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 2007;26:1410–1422. doi: 10.1038/sj.emboj.7601580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Kawabe H, Neeb A, Dimova K, Young SM, Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang D-E, Umikawa M, Kariya K, Goebbels S, Nave K-A, Rosenmund C, Jahn O, Rhee J, Brose N. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron. 2010;65:358–372. doi: 10.1016/j.neuron.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. 2010;65:341–357. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stegmüller J, Bonni A (2010) Destroy to create: E3 ubiquitin ligases in neurogenesis. F1000 Biol Rep. doi:10.3410/B2-38 [DOI] [PMC free article] [PubMed]
- 64.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Ding M, Chao D, Wang G, Shen K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 2007;317:947–951. doi: 10.1126/science.1145727. [DOI] [PubMed] [Google Scholar]
- 67.Chilton JK. Molecular mechanisms of axon guidance. Dev Biol. 2006;292:13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 68.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 69.Garrity PA, Zipursky SL. Neuronal target recognition. Cell. 1995;83:177–185. doi: 10.1016/0092-8674(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 70.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi H, Koppel AM, Luo Y, Raper JA. A role for collapsin-1 in olfactory and cranial sensory axon guidance. J Neurosci. 1997;17:8339–8352. doi: 10.1523/JNEUROSCI.17-21-08339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pascual M, Pozas E, Barallobre MJ, Tessier-Lavigne M, Soriano E. Coordinated functions of Netrin-1 and Class 3 secreted semaphorins in the guidance of reciprocal septohippocampal connections. Mol Cell Neurosci. 2004;26:24–33. doi: 10.1016/j.mcn.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 73.Saito S. Effects of lysophosphatidic acid on primary cultured chick neurons. Neurosci Lett. 1997;229:73–76. doi: 10.1016/s0304-3940(97)00397-2. [DOI] [PubMed] [Google Scholar]
- 74.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 75.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans . Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 76.Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron. 2000;26:331–343. doi: 10.1016/s0896-6273(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 77.Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans . Neuron. 2000;26:345–356. doi: 10.1016/s0896-6273(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 78.Li H, Kulkarni G, Wadsworth WG. RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J Neurosci. 2008;28:3595–3603. doi: 10.1523/JNEUROSCI.5536-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grill B, Bienvenut WV, Brown HM, Ackley BD, Quadroni M, Jin Y. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron. 2007;55:587–601. doi: 10.1016/j.neuron.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Liao EH, Hung W, Abrams B, Zhen M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 2004;430:345–350. doi: 10.1038/nature02647. [DOI] [PubMed] [Google Scholar]
- 81.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Huang Z, Wu Q, Guryanova OA, Cheng L, Shou W, Rich JN, Bao S. Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells. Nat Cell Biol. 2011;13:142–152. doi: 10.1038/ncb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Shami A, Jhaver KG, Vogel P, Wilkins C, Humphries J, Davis JJ, Xu N, Potter DG, Gerhardt B, Mullinax R, Shirley CR, Anderson SJ, Oravecz T. Regulators of the proteasome pathway, Uch37 and Rpn13, play distinct roles in mouse development. PLoS ONE. 2010;5:e13654. doi: 10.1371/journal.pone.0013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCullough J, Clague MJ, Urbé S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166:487–492. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishii N, Owada Y, Yamada M, Miura S, Murata K, Asao H, Kondo H, Sugamura K. Loss of neurons in the hippocampus and cerebral cortex of AMSH-deficient mice. Mol Cell Biol. 2001;21:8626–8637. doi: 10.1128/MCB.21.24.8626-8637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hegde AN, Goldberg AL, Schwartz JH. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc Natl Acad Sci USA. 1993;90:7436–7440. doi: 10.1073/pnas.90.16.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fioravante D, Liu R-Y, Byrne JH. The ubiquitin-proteasome system is necessary for long-term synaptic depression in Aplysia. J Neurosci. 2008;28:10245–10256. doi: 10.1523/JNEUROSCI.2139-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Speese SD, Trotta N, Rodesch CK, Aravamudan B, Broadie K. The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr Biol. 2003;13:899–910. doi: 10.1016/s0960-9822(03)00338-5. [DOI] [PubMed] [Google Scholar]
- 90.Chen H, Polo S, Di Fiore PP, De Camilli PV. Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proc Natl Acad Sci USA. 2003;100:14908–14913. doi: 10.1073/pnas.2136625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 92.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 93.Yao I, Takagi H, Ageta H, Kahyo T, Sato S, Hatanaka K, Fukuda Y, Chiba T, Morone N, Yuasa S, Inokuchi K, Ohtsuka T, Macgregor GR, Tanaka K, Setou M. SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell. 2007;130:943–957. doi: 10.1016/j.cell.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao I, Takao K, Miyakawa T, Ito S, Setou M. Synaptic E3 ligase SCRAPPER in contextual fear conditioning: extensive behavioral phenotyping of Scrapper heterozygote and overexpressing mutant mice. PLoS ONE. 2011;6:e17317. doi: 10.1371/journal.pone.0017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peters J-M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 96.van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–718. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 97.Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- 98.Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans . Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 99.Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30:16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schaefer H, Rongo C. KEL-8 is a substrate receptor for CUL3-dependent ubiquitin ligase that regulates synaptic glutamate receptor turnover. Mol Biol Cell. 2006;17:1250–1260. doi: 10.1091/mbc.E05-08-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans . Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Park EC, Glodowski DR, Rongo C. The ubiquitin ligase RPM-1 and the p38 MAPK PMK-3 regulate AMPA receptor trafficking. PLoS ONE. 2009;4:e4284. doi: 10.1371/journal.pone.0004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dreier L, Burbea M, Kaplan JM. LIN-23-mediated degradation of beta-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans . Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 104.Kowalski JR, Dahlberg CL, Juo P. The deubiquitinating enzyme USP-46 negatively regulates the degradation of glutamate receptors to control their abundance in the ventral nerve cord of Caenorhabditis elegans . J Neurosci. 2011;31:1341–1354. doi: 10.1523/JNEUROSCI.4765-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kramer LB, Shim J, Previtera ML, Isack NR, Lee M-C, Firestein BL, Rongo C. UEV-1 is an ubiquitin-conjugating enzyme variant that regulates glutamate receptor trafficking in C. elegans neurons. PLoS ONE. 2010;5:e14291. doi: 10.1371/journal.pone.0014291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larsen CN, Krantz BA, Wilkinson KD. Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry. 1998;37:3358–3368. doi: 10.1021/bi972274d. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 108.Osaka H, Wang Y-L, Takada K, Takizawa S, Setsuie R, Li H, Sato Y, Nishikawa K, Sun Y-J, Sakurai M, Harada T, Hara Y, Kimura I, Chiba S, Namikawa K, Kiyama H, Noda M, Aoki S, Wada K. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet. 2003;12:1945–1958. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- 109.Chain DG, Hegde AN, Yamamoto N, Liu-Marsh B, Schwartz JH. Persistent activation of cAMP-dependent protein kinase by regulated proteolysis suggests a neuron-specific function of the ubiquitin system in Aplysia. J Neurosci. 1995;15:7592–7603. doi: 10.1523/JNEUROSCI.15-11-07592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 111.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 112.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 113.Hegde AN, Upadhya SC. Role of ubiquitin-proteasome-mediated proteolysis in nervous system disease. Biochim Biophys Acta. 2011;1809:128–140. doi: 10.1016/j.bbagrm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 116.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 117.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim T-K, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mardirossian S, Rampon C, Salvert D, Fort P, Sarda N. Impaired hippocampal plasticity and altered neurogenesis in adult Ube3a maternal deficient mouse model for Angelman syndrome. Exp Neurol. 2009;220:341–348. doi: 10.1016/j.expneurol.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 121.Sato M, Stryker MP. Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a. Proc Natl Acad Sci USA. 2010;107:5611–5616. doi: 10.1073/pnas.1001281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mulherkar SA, Jana NR. Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome. Neurobiol Dis. 2010;40:586–592. doi: 10.1016/j.nbd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Jiang Y-H, Pan Y, Zhu L, Landa L, Yoo J, Spencer C, Lorenzo I, Brilliant M, Noebels J, Beaudet AL. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS ONE. 2010;5:e12278. doi: 10.1371/journal.pone.0012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nascimento RMP, Otto PA, de Brouwer APM, Vianna-Morgante AM. UBE2A, which encodes a ubiquitin-conjugating enzyme, is mutated in a novel X-linked mental retardation syndrome. Am J Hum Genet. 2006;79:549–555. doi: 10.1086/507047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Honda S, Orii KO, Kobayashi J, Hayashi S, Imamura A, Imoto I, Nakagawa E, Goto Y, Inazawa J. Novel deletion at Xq24 including the UBE2A gene in a patient with X-linked mental retardation. J Hum Genet. 2010;55:244–247. doi: 10.1038/jhg.2010.14. [DOI] [PubMed] [Google Scholar]
- 126.Budny B, Badura-Stronka M, Materna-Kiryluk A, Tzschach A, Raynaud M, Latos-Bielenska A, Ropers HH. Novel missense mutations in the ubiquitination-related gene UBE2A cause a recognizable X-linked mental retardation syndrome. Clin Genet. 2010;77:541–551. doi: 10.1111/j.1399-0004.2010.01429.x. [DOI] [PubMed] [Google Scholar]
- 127.de Leeuw N, Bulk S, Green A, Jaeckle-Santos L, Baker LA, Zinn AR, Kleefstra T, van der Smagt JJ, Vianne Morgante AM, de Vries BBA, van Bokhoven H, de Brouwer APM. UBE2A deficiency syndrome: mild to severe intellectual disability accompanied by seizures, absent speech, urogenital, and skin anomalies in male patients. Am J Med Genet A. 2010;152A:3084–3090. doi: 10.1002/ajmg.a.33743. [DOI] [PubMed] [Google Scholar]
- 128.Kumar B, Lecompte KG, Klein JM, Haas AL. Ser(120) of Ubc2/Rad6 regulates ubiquitin-dependent N-end rule targeting by E3{alpha}/Ubr1. J Biol Chem. 2010;285:41300–41309. doi: 10.1074/jbc.M110.169136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Obin M, Mesco E, Gong X, Haas AL, Joseph J, Taylor A. Neurite outgrowth in PC12 cells. Distinguishing the roles of ubiquitylation and ubiquitin-dependent proteolysis. J Biol Chem. 1999;274:11789–11795. doi: 10.1074/jbc.274.17.11789. [DOI] [PubMed] [Google Scholar]
- 130.Kavakebi P, Hausott B, Tomasino A, Ingorokva S, Klimaschewski L. The N-end rule ubiquitin-conjugating enzyme, HR6B, is up-regulated by nerve growth factor and required for neurite outgrowth. Mol Cell Neurosci. 2005;29:559–568. doi: 10.1016/j.mcn.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 131.Kim J, Guermah M, McGinty RK, Lee J-S, Tang Z, Milne TA, Shilatifard A, Muir TW, Roeder RG. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459–471. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mulugeta Achame E, Wassenaar E, Hoogerbrugge JW, Sleddens-Linkels E, Ooms M, Sun Z-W, IJcken WFJ, Grootegoed JA, Baarends WM. The ubiquitin-conjugating enzyme HR6B is required for maintenance of X chromosome silencing in mouse spermatocytes and spermatids. BMC Genomics. 2010;11:367. doi: 10.1186/1471-2164-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park CC, Gale GD, de Jong S, Ghazalpour A, Bennett BJ, Farber CR, Langfelder P, Lin A, Khan AH, Eskin E, Horvath S, Lusis AJ, Ophoff RA, Smith DJ. Gene networks associated with conditional fear in mice identified using a systems genetics approach. BMC Syst Biol. 2011;5:43. doi: 10.1186/1752-0509-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hwang C-S, Shemorry A, Auerbach D, Varshavsky A. The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nat Cell Biol. 2010;12:1177–1185. doi: 10.1038/ncb2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elting M, Kariminejad A, de Sonnaville M-L, Ottenkamp J, Bauhuber S, Bozorgmehr B, Zenker M, Cobben JM. Johanson-Blizzard syndrome caused by identical UBR1 mutations in two unrelated girls, one with a cardiomyopathy. Am J Med Genet A. 2008;146A:3058–3061. doi: 10.1002/ajmg.a.32566. [DOI] [PubMed] [Google Scholar]
- 136.Alkhouri N, Kaplan B, Kay M, Shealy A, Crowe C, Bauhuber S, Zenker M. Johanson-Blizzard syndrome with mild phenotypic features confirmed by UBR1 gene testing. World J Gastroenterol. 2008;14:6863–6866. doi: 10.3748/wjg.14.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zenker M, Mayerle J, Lerch MM, et al. Deficiency of UBR1, a ubiquitin ligase of the N-end rule pathway, causes pancreatic dysfunction, malformations and mental retardation (Johanson-Blizzard syndrome) Nat Genet. 2005;37:1345–1350. doi: 10.1038/ng1681. [DOI] [PubMed] [Google Scholar]
- 138.Fallahi GH, Sabbaghian M, Khalili M, Parvaneh N, Zenker M, Rezaei N. Novel UBR1 gene mutation in a patient with typical phenotype of Johanson-Blizzard syndrome. Eur J Pediatr. 2011;170:233–235. doi: 10.1007/s00431-010-1239-y. [DOI] [PubMed] [Google Scholar]
- 139.Eisele F, Wolf DH. Degradation of misfolded protein in the cytoplasm is mediated by the ubiquitin ligase Ubr1. FEBS Lett. 2008;582:4143–4146. doi: 10.1016/j.febslet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 140.Nillegoda NB, Theodoraki MA, Mandal AK, Mayo KJ, Ren HY, Sultana R, Wu K, Johnson J, Cyr DM, Caplan AJ. Ubr1 and Ubr2 function in a quality control pathway for degradation of unfolded cytosolic proteins. Mol Biol Cell. 2010;21:2102–2116. doi: 10.1091/mbc.E10-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heck JW, Cheung SK, Hampton RY. Cytoplasmic protein quality control degradation mediated by parallel actions of the E3 ubiquitin ligases Ubr1 and San1. Proc Natl Acad Sci USA. 2010;107:1106–1111. doi: 10.1073/pnas.0910591107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kume K, Iizumi Y, Shimada M, Ito Y, Kishi T, Yamaguchi Y, Handa H. Role of N-end rule ubiquitin ligases UBR1 and UBR2 in regulating the leucine-mTOR signaling pathway. Genes Cells. 2010;15:339–349. doi: 10.1111/j.1365-2443.2010.01385.x. [DOI] [PubMed] [Google Scholar]
- 143.An JY, Seo JW, Tasaki T, Lee MJ, Varshavsky A, Kwon YT. Impaired neurogenesis and cardiovascular development in mice lacking the E3 ubiquitin ligases UBR1 and UBR2 of the N-end rule pathway. Proc Natl Acad Sci USA. 2006;103:6212–6217. doi: 10.1073/pnas.0601700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tarpey PS, Raymond FL, O’Meara S, et al. Mutations in CUL4B, which encodes a ubiquitin E3 ligase subunit, cause an X-linked mental retardation syndrome associated with aggressive outbursts, seizures, relative macrocephaly, central obesity, hypogonadism, pes cavus, and tremor. Am J Hum Genet. 2007;80:345–352. doi: 10.1086/511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 146.Higa LA, Zhang H. Stealing the spotlight: CUL4-DDB1 ubiquitin ligase docks WD40-repeat proteins to destroy. Cell Div. 2007;2:5. doi: 10.1186/1747-1028-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miranda-Carboni GA, Krum SA, Yee K, Nava M, Deng QE, Pervin S, Collado-Hidalgo A, Galic Z, Zack JA, Nakayama K, Nakayama KI, Lane TF. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121–3134. doi: 10.1101/gad.1692808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kerzendorfer C, Whibley A, Carpenter G, Outwin E, Chiang S-C, Turner G, Schwartz C, El-Khamisy S, Raymond FL, O’Driscoll M. Mutations in Cullin 4B result in a human syndrome associated with increased camptothecin-induced topoisomerase I-dependent DNA breaks. Hum Mol Genet. 2010;19:1324–1334. doi: 10.1093/hmg/ddq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Francke U. Williams–Beuren syndrome: genes and mechanisms. Hum Mol Genet. 1999;8:1947–1954. doi: 10.1093/hmg/8.10.1947. [DOI] [PubMed] [Google Scholar]
- 150.Micale L, Fusco C, Augello B, Napolitano LMR, Dermitzakis ET, Meroni G, Merla G, Reymond A. Williams–Beuren syndrome TRIM50 encodes an E3 ubiquitin ligase. Eur J Hum Genet. 2008;16:1038–1049. doi: 10.1038/ejhg.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Froyen G, Corbett M, Vandewalle J, et al. Submicroscopic duplications of the hydroxysteroid dehydrogenase HSD17B10 and the E3 ubiquitin ligase HUWE1 are associated with mental retardation. Am J Hum Genet. 2008;82:432–443. doi: 10.1016/j.ajhg.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Besche HC, Haas W, Gygi SP, Goldberg AL. Isolation of mammalian 26S proteasomes and p97/VCP complexes using the ubiquitin-like domain from HHR23B reveals novel proteasome-associated proteins. Biochemistry. 2009;48:2538–2549. doi: 10.1021/bi802198q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tai H-C, Besche H, Goldberg AL, Schuman EM. Characterization of the Brain 26S Proteasome and its Interacting Proteins. Front Mol Neurosci. 2010 doi: 10.3389/fnmol.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Z, Oughtred R, Wing SS. Characterization of E3Histone, a novel testis ubiquitin protein ligase which ubiquitinates histones. Mol Cell Biol. 2005;25:2819–2831. doi: 10.1128/MCB.25.7.2819-2831.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gu J, Ren K, Dubner R, Iadarola MJ. Cloning of a DNA binding protein that is a tyrosine kinase substrate and recognizes an upstream initiator-like sequence in the promoter of the preprodynorphin gene. Brain Res Mol Brain Res. 1994;24:77–88. doi: 10.1016/0169-328x(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 156.Hall JR, Kow E, Nevis KR, Lu CK, Luce KS, Zhong Q, Cook JG. Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell. 2007;18:3340–3350. doi: 10.1091/mbc.E07-02-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 158.Stankiewicz AR, Livingstone AM, Mohseni N, Mosser DD. Regulation of heat-induced apoptosis by Mcl-1 degradation and its inhibition by Hsp70. Cell Death Differ. 2009;16:638–647. doi: 10.1038/cdd.2008.189. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X, Heng JI-T, Guardavaccaro D, Jiang R, Pagano M, Guillemot F, Iavarone A, Lasorella A. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat Cell Biol. 2008;10:643–653. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.D’Arca D, Zhao X, Xu W, Ramirez-Martinez NC, Iavarone A, Lasorella A. Huwe1 ubiquitin ligase is essential to synchronize neuronal and glial differentiation in the developing cerebellum. Proc Natl Acad Sci USA. 2010;107:5875–5880. doi: 10.1073/pnas.0912874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Glessner JT, Wang K, Cai G, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McInnes LA, Nakamine A, Pilorge M, Brandt T, Jiménez González P, Fallas M, Manghi ER, Edelmann L, Glessner J, Hakonarson H, Betancur C, Buxbaum JD. A large-scale survey of the novel 15q24 microdeletion syndrome in autism spectrum disorders identifies an atypical deletion that narrows the critical region. Mol Autism. 2010;1:5. doi: 10.1186/2040-2392-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cukier HN, Salyakina D, Blankstein SF, Robinson JL, Sacharow S, Ma D, Wright HH, Abramson RK, Menon R, Williams SM, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Microduplications in an autism multiplex family narrow the region of susceptibility for developmental disorders on 15q24 and implicate 7p21. Am J Med Genet B Neuropsychiatr Genet. 2011;156:493–501. doi: 10.1002/ajmg.b.31188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seo H, Isacson O. The hAPP-YAC transgenic model has elevated UPS activity in the frontal cortex similar to Alzheimer’s disease and Down’s syndrome. J Neurochem. 2010;114:1819–1826. doi: 10.1111/j.1471-4159.2010.06902.x. [DOI] [PubMed] [Google Scholar]
- 166.Berto G, Camera P, Fusco C, Imarisio S, Ambrogio C, Chiarle R, Silengo L, Di Cunto F. The Down syndrome critical region protein TTC3 inhibits neuronal differentiation via RhoA and Citron kinase. J Cell Sci. 2007;120:1859–1867. doi: 10.1242/jcs.000703. [DOI] [PubMed] [Google Scholar]
- 167.Valero R, Bayés M, Francisca Sánchez-Font M, González-Angulo O, Gonzàlez-Duarte R, Marfany G (2001) Characterization of alternatively spliced products and tissue-specific isoforms of USP28 and USP25. Genome Biol 2:RESEARCH0043 [DOI] [PMC free article] [PubMed]
- 168.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 169.Hol EM, Fischer DF, Ovaa H, Scheper W. Ubiquitin proteasome system as a pharmacological target in neurodegeneration. Expert Rev Neurother. 2006;6:1337–1347. doi: 10.1586/14737175.6.9.1337. [DOI] [PubMed] [Google Scholar]
- 170.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Edelmann MJ, Nicholson B, Kessler BM. Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases. Expert Rev Mol Med. 2011;13:e35. doi: 10.1017/S1462399411002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Driscoll JJ, Dechowdhury R. Therapeutically targeting the SUMOylation, Ubiquitination and Proteasome pathways as a novel anticancer strategy. Target Oncol. 2010;5:281–289. doi: 10.1007/s11523-010-0165-2. [DOI] [PubMed] [Google Scholar]
- 173.Jia L, Sun Y. SCF E3 ubiquitin ligases as anticancer targets. Curr Cancer Drug Targets. 2011;11:347–356. doi: 10.2174/156800911794519734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dohm CP, Kermer P, Bähr M. Aggregopathy in neurodegenerative diseases: mechanisms and therapeutic implication. Neurodegener Dis. 2008;5:321–338. doi: 10.1159/000119459. [DOI] [PubMed] [Google Scholar]
- 175.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 176.Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- 177.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 178.Chen S, Ferrone FA, Wetzel R. Huntington’s disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci USA. 2002;99:11884–11889. doi: 10.1073/pnas.182276099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thorpe JR, Tang H, Atherton J, Cairns NJ. Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J Neural Transm. 2008;115:1661–1671. doi: 10.1007/s00702-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mayer RJ, Lowe J, Lennox G, Doherty F, Landon M. Intermediate filaments and ubiquitin: a new thread in the understanding of chronic neurodegenerative diseases. Prog Clin Biol Res. 1989;317:809–818. [PubMed] [Google Scholar]
- 181.Lowe J, Blanchard A, Morrell K, Lennox G, Reynolds L, Billett M, Landon M, Mayer RJ. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson’s disease, Pick’s disease, and Alzheimer’s disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988;155:9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- 182.Layfield R, Cavey JR, Lowe J. Role of ubiquitin-mediated proteolysis in the pathogenesis of neurodegenerative disorders. Ageing Res Rev. 2003;2:343–356. doi: 10.1016/s1568-1637(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 183.Bennett EJ, Bence NF, Jayakumar R, Kopito RR. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell. 2005;17:351–365. doi: 10.1016/j.molcel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 184.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 185.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans . PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36:2376–2391. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 187.Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- 188.Gavilán MP, Castaño A, Torres M, Portavella M, Caballero C, Jiménez S, García-Martínez A, Parrado J, Vitorica J, Ruano D. Age-related increase in the immunoproteasome content in rat hippocampus: molecular and functional aspects. J Neurochem. 2009;108:260–272. doi: 10.1111/j.1471-4159.2008.05762.x. [DOI] [PubMed] [Google Scholar]
- 189.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 190.Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 191.Harrington AJ, Hamamichi S, Caldwell GA, Caldwell KA. C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s disease. Dev Dyn. 2010;239:1282–1295. doi: 10.1002/dvdy.22231. [DOI] [PubMed] [Google Scholar]
- 192.Springer W, Hoppe T, Schmidt E, Baumeister R. A Caenorhabditis elegans Parkin mutant with altered solubility couples alpha-synuclein aggregation to proteotoxic stress. Hum Mol Genet. 2005;14:3407–3423. doi: 10.1093/hmg/ddi371. [DOI] [PubMed] [Google Scholar]
- 193.Williams AJ, Paulson HL. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jana NR, Nukina N. Recent advances in understanding the pathogenesis of polyglutamine diseases: involvement of molecular chaperones and ubiquitin-proteasome pathway. J Chem Neuroanat. 2003;26:95–101. doi: 10.1016/s0891-0618(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 195.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6:743–755. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- 196.Ortega Z, Díaz-Hernández M, Maynard CJ, Hernández F, Dantuma NP, Lucas JJ. Acute polyglutamine expression in inducible mouse model unravels ubiquitin/proteasome system impairment and permanent recovery attributable to aggregate formation. J Neurosci. 2010;30:3675–3688. doi: 10.1523/JNEUROSCI.5673-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 198.Paulson HL. Protein fate in neurodegenerative proteinopathies: polyglutamine diseases join the (mis)fold. Am J Hum Genet. 1999;64:339–345. doi: 10.1086/302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou H, Cao F, Wang Z, Yu Z-X, Nguyen H-P, Evans J, Li S-H, Li X-J. Huntingtin forms toxic NH2-terminal fragment complexes that are promoted by the age-dependent decrease in proteasome activity. J Cell Biol. 2003;163:109–118. doi: 10.1083/jcb.200306038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li X, Wang C-E, Huang S, Xu X, Li X-J, Li H, Li S. Inhibiting the ubiquitin-proteasome system leads to preferential accumulation of toxic N-terminal mutant huntingtin fragments. Hum Mol Genet. 2010;19:2445–2455. doi: 10.1093/hmg/ddq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maynard CJ, Böttcher C, Ortega Z, Smith R, Florea BI, Díaz-Hernández M, Brundin P, Overkleeft HS, Li J-Y, Lucas JJ, Dantuma NP. Accumulation of ubiquitin conjugates in a polyglutamine disease model occurs without global ubiquitin/proteasome system impairment. Proc Natl Acad Sci USA. 2009;106:13986–13991. doi: 10.1073/pnas.0906463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mitra S, Tsvetkov AS, Finkbeiner S. Single neuron ubiquitin-proteasome dynamics accompanying inclusion body formation in huntington disease. J Biol Chem. 2009;284:4398–4403. doi: 10.1074/jbc.M806269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S. Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J. 2010;29:2433–2445. doi: 10.1038/emboj.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- 205.Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet. 2003;12:3195–3205. doi: 10.1093/hmg/ddg344. [DOI] [PubMed] [Google Scholar]
- 206.Chow MKM, Mackay JP, Whisstock JC, Scanlon MJ, Bottomley SP. Structural and functional analysis of the Josephin domain of the polyglutamine protein ataxin-3. Biochem Biophys Res Commun. 2004;322:387–394. doi: 10.1016/j.bbrc.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 207.Ferro A, Carvalho AL, Teixeira-Castro A, Almeida C, Tomé RJ, Cortes L, Rodrigues A-J, Logarinho E, Sequeiros J, Macedo-Ribeiro S, Maciel P. NEDD8: a new ataxin-3 interactor. Biochim Biophys Acta. 2007;1773:1619–1627. doi: 10.1016/j.bbamcr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 208.Hirabayashi M, Inoue K, Tanaka K, Nakadate K, Ohsawa Y, Kamei Y, Popiel AH, Sinohara A, Iwamatsu A, Kimura Y, Uchiyama Y, Hori S, Kakizuka A. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001;8:977–984. doi: 10.1038/sj.cdd.4400907. [DOI] [PubMed] [Google Scholar]
- 209.Doss-Pepe EW, Stenroos ES, Johnson WG, Madura K. Ataxin-3 interactions with rad23 and valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis. Mol Cell Biol. 2003;23:6469–6483. doi: 10.1128/MCB.23.18.6469-6483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang G, Sawai N, Kotliarova S, Kanazawa I, Nukina N. Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B. Hum Mol Genet. 2000;9:1795–1803. doi: 10.1093/hmg/9.12.1795. [DOI] [PubMed] [Google Scholar]
- 211.Durcan TM, Kontogiannea M, Thorarinsdottir T, Fallon L, Williams AJ, Djarmati A, Fantaneanu T, Paulson HL, Fon EA. The Machado-Joseph disease-associated mutant form of ataxin-3 regulates parkin ubiquitination and stability. Hum Mol Genet. 2011;20:141–154. doi: 10.1093/hmg/ddq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Scaglione KM, Zavodszky E, Todi SV, Patury S, Xu P, Rodríguez-Lebrón E, Fischer S, Konen J, Djarmati A, Peng J, Gestwicki JE, Paulson HL. Ube2w and ataxin-3 coordinately regulate the ubiquitin ligase CHIP. Mol Cell. 2011;43:599–612. doi: 10.1016/j.molcel.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Doss-Pepe EW, Stenroos ES, Johnson WG, Madura K. Ataxin-3 interactions with rad23 and valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis. Mol Cell Biol. 2003;23:6469–6483. doi: 10.1128/MCB.23.18.6469-6483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhong X, Pittman RN. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum Mol Genet. 2006;15:2409–2420. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]
- 215.Rodrigues A-J, Neves-Carvalho A, Ferro A, Rokka A, Corthals G, Logarinho E, Maciel P. ATX-3, CDC-48 and UBXN-5: a new trimolecular complex in Caenorhabditis elegans. Biochem Biophys Res Commun. 2009;386:575–581. doi: 10.1016/j.bbrc.2009.06.092. [DOI] [PubMed] [Google Scholar]
- 216.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Höhfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 217.Jana NR, Dikshit P, Goswami A, Kotliarova S, Murata S, Tanaka K, Nukina N. Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J Biol Chem. 2005;280:11635–11640. doi: 10.1074/jbc.M412042200. [DOI] [PubMed] [Google Scholar]
- 218.Cemal CK, Carroll CJ, Lawrence L, Lowrie MB, Ruddle P, Al-Mahdawi S, King RHM, Pook MA, Huxley C, Chamberlain S. YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum Mol Genet. 2002;11:1075–1094. doi: 10.1093/hmg/11.9.1075. [DOI] [PubMed] [Google Scholar]
- 219.Hasegawa T, Treis A, Patenge N, Fiesel FC, Springer W, Kahle PJ. Parkin protects against tyrosinase-mediated dopamine neurotoxicity by suppressing stress-activated protein kinase pathways. J Neurochem. 2008;105:1700–1715. doi: 10.1111/j.1471-4159.2008.05277.x. [DOI] [PubMed] [Google Scholar]
- 220.Ulusoy A, Kirik D. Can overexpression of parkin provide a novel strategy for neuroprotection in Parkinson’s disease? Exp Neurol. 2008;212:258–260. doi: 10.1016/j.expneurol.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 221.Yasuda T, Hayakawa H, Nihira T, Ren Y-R, Nakata Y, Nagai M, Hattori N, Miyake K, Takada M, Shimada T, Mizuno Y, Mochizuki H. Parkin-mediated protection of dopaminergic neurons in a chronic MPTP-minipump mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2011;70:686–697. doi: 10.1097/NEN.0b013e3182269ecd. [DOI] [PubMed] [Google Scholar]
- 222.Rodrigues AJ, Neves-Carvalho A, Teixeira-Castro A, Rokka A, Corthals G, Logarinho E, Maciel P. Absence of ataxin-3 leads to enhanced stress response in C. elegans . PLoS ONE. 2011;6:e18512. doi: 10.1371/journal.pone.0018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Anderson C, Crimmins S, Wilson JA, Korbel GA, Ploegh HL, Wilson SM. Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. J Neurochem. 2005;95:724–731. doi: 10.1111/j.1471-4159.2005.03409.x. [DOI] [PubMed] [Google Scholar]
- 224.Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet. 2002;32:420–425. doi: 10.1038/ng1006. [DOI] [PubMed] [Google Scholar]
- 225.Lappe-Siefke C, Loebrich S, Hevers W, Waidmann OB, Schweizer M, Fehr S, Fritschy J-M, Dikic I, Eilers J, Wilson SM, Kneussel M. The ataxia (axJ) mutation causes abnormal GABAA receptor turnover in mice. PLoS Genet. 2009;5:e1000631. doi: 10.1371/journal.pgen.1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Crimmins S, Jin Y, Wheeler C, Huffman AK, Chapman C, Dobrunz LE, Levey A, Roth KA, Wilson JA, Wilson SM. Transgenic rescue of ataxia mice with neuronal-specific expression of ubiquitin-specific protease 14. J Neurosci. 2006;26:11423–11431. doi: 10.1523/JNEUROSCI.3600-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee B-H, Lee MJ, Park S, Oh D-C, Elsasser S, Chen P-C, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 229.van Leeuwen FW, de Kleijn DP, van den Hurk HH, Neubauer A, Sonnemans MA, Sluijs JA, Köycü S, Ramdjielal RD, Salehi A, Martens GJ, Grosveld FG, Peter J, Burbach H, Hol EM. Frameshift mutants of beta amyloid precursor protein and ubiquitin-B in Alzheimer’s and Down patients. Science. 1998;279:242–247. doi: 10.1126/science.279.5348.242. [DOI] [PubMed] [Google Scholar]
- 230.Tan Z, Sun X, Hou F-S, Oh H-W, Hilgenberg LGW, Hol EM, van Leeuwen FW, Smith MA, O’Dowd DK, Schreiber SS. Mutant ubiquitin found in Alzheimer’s disease causes neuritic beading of mitochondria in association with neuronal degeneration. Cell Death Differ. 2007;14:1721–1732. doi: 10.1038/sj.cdd.4402180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Eldridge AG, O’Brien T. Therapeutic strategies within the ubiquitin proteasome system. Cell Death Differ. 2010;17:4–13. doi: 10.1038/cdd.2009.82. [DOI] [PubMed] [Google Scholar]
- 232.Ardley HC, Robinson PA. The role of ubiquitin-protein ligases in neurodegenerative disease. Neurodegener Dis. 2004;1:71–87. doi: 10.1159/000080048. [DOI] [PubMed] [Google Scholar]
- 233.Dikshit P, Jana NR. Role of ubiquitin protein ligases in the pathogenesis of polyglutamine diseases. Neurochem Res. 2008;33:945–951. doi: 10.1007/s11064-007-9459-x. [DOI] [PubMed] [Google Scholar]
- 234.Katsuno M, Sang C, Adachi H, Minamiyama M, Waza M, Tanaka F, Doyu M, Sobue G. Pharmacological induction of heat-shock proteins alleviates polyglutamine-mediated motor neuron disease. Proc Natl Acad Sci USA. 2005;102:16801–16806. doi: 10.1073/pnas.0506249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yasuda H, Shichinohe H, Kuroda S, Ishikawa T, Iwasaki Y. Neuroprotective effect of a heat shock protein inducer, geranylgeranylacetone in permanent focal cerebral ischemia. Brain Res. 2005;1032:176–182. doi: 10.1016/j.brainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 236.Herbst M, Wanker EE. Small molecule inducers of heat-shock response reduce polyQ-mediated huntingtin aggregation. A possible therapeutic strategy. Neurodegener Dis. 2007;4:254–260. doi: 10.1159/000101849. [DOI] [PubMed] [Google Scholar]
- 237.Teschendorf D, Link CD. What have worm models told us about the mechanisms of neuronal dysfunction in human neurodegenerative diseases? Mol Neurodegener. 2009;4:38. doi: 10.1186/1750-1326-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 239.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans . Philos Trans R Soc Lond B. 1983;2:633–640. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 240.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans . Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 241.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans . Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 242.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans . Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 243.Sonnhammer EL, Durbin R. Analysis of protein domain families in Caenorhabditis elegans . Genomics. 1997;46:200–216. doi: 10.1006/geno.1997.4989. [DOI] [PubMed] [Google Scholar]
- 244.Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000;10:703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kuwabara PE, O’Neil N. The use of functional genomics in C. elegans for studying human development and disease. J Inherit Metab Dis. 2001;24:127–138. doi: 10.1023/a:1010306731764. [DOI] [PubMed] [Google Scholar]
- 246.Harris TW, Chen N, Cunningham F, et al. WormBase: a multi-species resource for nematode biology and genomics. Nucleic Acids Res. 2004;32:D411–417. doi: 10.1093/nar/gkh066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Culetto E, Sattelle DB. A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum Mol Genet. 2000;9:869–877. doi: 10.1093/hmg/9.6.869. [DOI] [PubMed] [Google Scholar]
- 248.Ahringer J. Turn to the worm! Curr Opin Genet Dev. 1997;7:410–415. doi: 10.1016/s0959-437x(97)80157-8. [DOI] [PubMed] [Google Scholar]
- 249.Wheelan SJ, Boguski MS, Duret L, Makałowski W. Human and nematode orthologs–lessons from the analysis of 1800 human genes and the proteome of Caenorhabditis elegans . Gene. 1999;238:163–170. doi: 10.1016/s0378-1119(99)00298-x. [DOI] [PubMed] [Google Scholar]
- 250.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans . Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Faber PW, Alter JR, MacDonald ME, Hart AC. Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc Natl Acad Sci USA. 1999;96:179–184. doi: 10.1073/pnas.96.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kuwahara T, Koyama A, Koyama S, Yoshina S, Ren C-H, Kato T, Mitani S, Iwatsubo T. A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in alpha-synuclein transgenic C. elegans . Hum Mol Genet. 2008;17:2997–3009. doi: 10.1093/hmg/ddn198. [DOI] [PubMed] [Google Scholar]
- 253.Zhang T, Mullane PC, Periz G, Wang J. TDP-43 neurotoxicity and protein aggregation modulated by heat shock factor and insulin/IGF-1 signaling. Hum Mol Genet. 2011;20:1952–1965. doi: 10.1093/hmg/ddr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vartiainen S, Pehkonen P, Lakso M, Nass R, Wong G. Identification of gene expression changes in transgenic C. elegans overexpressing human alpha-synuclein. Neurobiol Dis. 2006;22:477–486. doi: 10.1016/j.nbd.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 255.Teixeira-Castro A, Ailion M, Jalles A, Brignull HR, Vilaça JL, Dias N, Rodrigues P, Oliveira JF, Neves-Carvalho A, Morimoto RI, Maciel P. Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: rescue by the DAF-16 and HSF-1 pathways. Hum Mol Genet. 2011;20:2996–3009. doi: 10.1093/hmg/ddr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 257.Artal-Sanz M, de Jong L, Tavernarakis N. Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol J. 2006;1:1405–1418. doi: 10.1002/biot.200600176. [DOI] [PubMed] [Google Scholar]
- 258.Diomede L, Cassata G, Fiordaliso F, Salio M, Ami D, Natalello A, Doglia SM, De Luigi A, Salmona M. Tetracycline and its analogues protect Caenorhabditis elegans from β amyloid-induced toxicity by targeting oligomers. Neurobiol Dis. 2010;40:424–431. doi: 10.1016/j.nbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 259.Voisine C, Varma H, Walker N, Bates EA, Stockwell BR, Hart AC. Identification of potential therapeutic drugs for huntington’s disease using Caenorhabditis elegans . PLoS ONE. 2007;2:e504. doi: 10.1371/journal.pone.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Silva MC, Fox S, Beam M, Thakkar H, Amaral MD, Morimoto RI. A genetic screening strategy identifies novel regulators of the proteostasis network. PLoS Genet. 2011;7:e1002438. doi: 10.1371/journal.pgen.1002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 262.Po MD, Hwang C, Zhen M. PHRs: bridging axon guidance, outgrowth and synapse development. Curr Opin Neurobiol. 2010;20:100–107. doi: 10.1016/j.conb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 263.Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron. 2008;59:997–1008. doi: 10.1016/j.neuron.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Khan LA, Bauer PO, Miyazaki H, Lindenberg KS, Landwehrmeyer BG, Nukina N. Expanded polyglutamines impair synaptic transmission and ubiquitin-proteasome system in Caenorhabditis elegans . J Neurochem. 2006;98:576–587. doi: 10.1111/j.1471-4159.2006.03895.x. [DOI] [PubMed] [Google Scholar]
- 265.Segref A, Torres S, Hoppe T. A screenable in vivo assay to study proteostasis networks in Caenorhabditis elegans . Genetics. 2011;187:1235–1240. doi: 10.1534/genetics.111.126797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hamer G, Matilainen O, Holmberg CI. A photoconvertible reporter of the ubiquitin-proteasome system in vivo. Nat Methods. 2010;7:473–478. doi: 10.1038/nmeth.1460. [DOI] [PubMed] [Google Scholar]
- 267.Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11:171–176. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 268.Zhong W, Feng H, Santiago FE, Kipreos ET. CUL-4 ubiquitin ligase maintains genome stability by restraining DNA-replication licensing. Nature. 2003;423:885–889. doi: 10.1038/nature01747. [DOI] [PubMed] [Google Scholar]
- 269.Ha MK, Soo Cho J, Baik O-R, Lee KH, Koo H-S, Chung KY. Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics. 2006;6:3339–3351. doi: 10.1002/pmic.200500395. [DOI] [PubMed] [Google Scholar]
- 270.Cornejo Castro EM, Waak J, Weber SS, Fiesel FC, Oberhettinger P, Schütz M, Autenrieth IB, Springer W, Kahle PJ. Parkinson’s disease-associated DJ-1 modulates innate immunity signaling in Caenorhabditis elegans . J Neural Transm. 2010;117:599–604. doi: 10.1007/s00702-010-0397-4. [DOI] [PubMed] [Google Scholar]
- 271.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet J-C, Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rodrigues A-J, Coppola G, Santos C, Costa M do C, Ailion M, Sequeiros J, Geschwind DH, Maciel P (2007) Functional genomics and biochemical characterization of the C. elegans orthologue of the Machado-Joseph disease protein ataxin-3. FASEB J 21:1126–1136 [DOI] [PubMed]
- 273.Lu Z, Je H-S, Young P, Gross J, Lu B, Feng G. Regulation of synaptic growth and maturation by a synapse-associated E3 ubiquitin ligase at the neuromuscular junction. J Cell Biol. 2007;177:1077–1089. doi: 10.1083/jcb.200610060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nelson RF, Glenn KA, Miller VM, Wen H, Paulson HL. A novel route for F-box protein-mediated ubiquitination links CHIP to glycoprotein quality control. J Biol Chem. 2006;281:20242–20251. doi: 10.1074/jbc.M602423200. [DOI] [PubMed] [Google Scholar]
- 276.Jurd R, Thornton C, Wang J, Luong K, Phamluong K, Kharazia V, Gibb SL, Ron D. Mind bomb-2 is an E3 ligase that ubiquitinates the N-methyl-D-aspartate receptor NR2B subunit in a phosphorylation-dependent manner. J Biol Chem. 2008;283:301–310. doi: 10.1074/jbc.M705580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hung AY, Sung CC, Brito IL, Sheng M. Degradation of postsynaptic scaffold GKAP and regulation of dendritic spine morphology by the TRIM3 ubiquitin ligase in rat hippocampal neurons. PLoS ONE. 2010;5:e9842. doi: 10.1371/journal.pone.0009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jansen G, Hazendonk E, Thijssen KL, Plasterk RH. Reverse genetics by chemical mutagenesis in Caenorhabditis elegans . Nat Genet. 1997;17:119–121. doi: 10.1038/ng0997-119. [DOI] [PubMed] [Google Scholar]
- 279.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 280.Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods. 2010;7:554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans . Mol Cell Biol. 1985;5:3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen S-P, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans . Nat Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Frøkjaer-Jensen C, Davis MW, Hollopeter G, Taylor J, Harris TW, Nix P, Lofgren R, Prestgard-Duke M, Bastiani M, Moerman DG, Jorgensen EM. Targeted gene deletions in C. elegans using transposon excision. Nat Methods. 2010;7:451–453. doi: 10.1038/nmeth.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Calixto A, Ma C, Chalfie M. Conditional gene expression and RNAi using MEC-8-dependent splicing in C. elegans . Nat Methods. 2010;7:407–411. doi: 10.1038/nmeth.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans . Nature. 1995;378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- 286.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans . Dev Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 287.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans . Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]