Abstract
Ultraviolet B radiation (UV-B, 290–315 nm) can cause damage and induce photomorphogenic responses in plants. The mechanisms that mediate the photomorphogenic effects of UV-B are unclear. In etiolated Arabidopsis seedlings, a daily exposure to 2.5 h of UV-B enhanced the cotyledon opening response induced by a subsequent red light (R) pulse. An R pulse alone, 2.5 h of UV-B terminated with a far-red pulse, or 2.5 h of continuous R caused very little cotyledon opening. The enhancing effect of UV-B increased with fluence rate up to approximately 7.58 μmol m−2 s−1; at higher fluence rates the response to UV-B was greatly reduced. The phyA, phyA cry1, and cry1 cry2 mutants behaved like the wild type when exposed to UV-B followed by an R pulse. In contrast, phyB, phyB cry1, and phyB phyA mutants failed to open the cotyledons. Thus, phytochrome B was required for the cotyledon opening response to UV-B → R treatments, whereas phytochrome A and cryptochromes 1 and 2 were not necessary under the conditions of our experiments. The enhancing effect of low doses of UV-B on cotyledon opening in uvr1 uvr2 and uvr1 uvr3 mutants, deficient in DNA repair, was similar to that found in the wild type, suggesting that this effect of UV-B was not elicited by signals derived from UV-B-induced DNA lesions (cyclobutane pyrimidine dimers and 6-4 photoproducts). We conclude that low doses of UV-B, perceived by a receptor system different from phytochromes, cryptochromes, or DNA, enhance a de-etiolation response that is induced by active phytochrome B.
Plants detect changes in their light environment using specific photoreceptor systems that control plant growth and development. Red light (R) and far red radiation (FR) are perceived by the phytochrome family of photoreceptors. In Arabidopsis there are five phytochromes (known as phyA through phyE), which are encoded by five divergent genes (PHYA through PHYE) (Sharrock and Quail, 1989; Clack et al., 1994). Blue light and UV-A radiation (UV-A, 315–400 nm) are perceived by flavoprotein photoreceptors, which include cryptochrome 1 (cry1), cryptochrome 2 (cry2) (Ahmad and Cashmore, 1993; Guo et al., 1998; Lin et al., 1998), and phototropin (Christie et al., 1998; for review, see Briggs and Huala, 1999). Additional blue light photoreceptors are involved in the control of stomatal opening (Zeiger, 2000).
UV-B radiation induces multiple morphological and physiological responses in plants (for reviews, see Jenkins, 1997; Caldwell et al., 1998; Jansen et al., 1998), but the underlying mechanisms are much less clear than those that control the photomorphogenic effects of blue, R, and FR. The interest for understanding how plants and other organisms respond to UV-B has been stimulated by the demonstration that the increased deterioration of the stratospheric ozone layer at a global scale (Madronich et al., 1998) has lent to augmented UV-B levels at the ground surface over the last decade (McKenzie et al., 1999). Many of the effects of UV-B on plants, such as reduced growth, are likely to be a more or less direct result of cellular damage caused by UV-B photons, which can cause aberrant photoproducts in macromolecules such as DNA (Britt, 1996) and proteins (Gerhardt et al., 1999) and also induce the production of potentially harmful active oxygen species (Foyer et al., 1994; Malanga et al., 1999).
Other effects of UV-B may be the result of signaling cascades engaged by the initial products of damage caused by UV-B. For example, in mammalian cells, DNA damage is clearly involved in the elicitation of certain responses to UV-B, such as the tanning and enhanced-repair responses of skin cells (Eller et al., 1996; Eller et al., 1997; Smith and Fornace, 1997). UV-B can also interact with membrane receptors and subvert signaling cascades normally used by the specific ligands of these receptors (Rosette and Karin, 1996; Rehemtulla et al., 1997; Kulms et al., 1999). Groβ et al. (1999) have suggested that the ligand-independent activation of some membrane receptors is caused by the ability of UV to oxidize (and inactivate) receptor-directed protein-Tyr phosphatases.
In plants some responses to UV-B, such as the synthesis of isoflavonoids in legumes, are thought to be induced by DNA damage because (a) the wavelength dependency of the response is similar to that for DNA absorption, and (b) acceleration of DNA repair by photoreactivating light can lower the magnitude of the response to UV-B (Beggs and Wellmann, 1994). Perturbation of cell membranes and/or activation of lipases has also been proposed to be the initial signal that, through the octadecanoid pathway, induces some responses to UV in plants, such as the accumulation of proteinase inhibitors (Conconi et al., 1996). These perturbations could be elicited by free radicals (Green and Fluhr, 1995; Surplus et al., 1998), which are formed in response to UV-B.
Apart from these damage-related responses, which in general are induced most strongly by the shortest wavelengths of the UV spectrum (UV-C: λ < 280 nm), there are various lines of evidence from physiological experiments that suggest that some responses to UV-B in plants may be triggered by more specific photoreceptor systems. This evidence is based on spectral sensitivity analyses of pigment induction (Yatsuhashi et al., 1982; Beggs and Wellmann, 1994), protein expression (Frohnmeyer et al., 1999), and morphological responses (Ballaré et al., 1995), which frequently show maximum activity in the UV-B region of the spectrum (Ensminger, 1993), inhibitor studies (Ballaré et al., 1995), fluence-response considerations, and comparisons between wild-type (WT) and UV-B-sensitive mutants (Lin et al., 1998). However the nature of this evidence is indirect, and specific UV-B photoreceptors have not yet been identified in plants.
Depending on light conditions, plant photoreceptors can act independently of each other or interact in various ways (Casal, 2000). During de-etiolation of Arabidopsis seedlings, FR and blue light perceived by phyA and cry1, respectively, interact in a synergistic manner with R perceived by phyB (Casal, 1995; Casal and Boccalandro, 1995; Casal and Mazzella, 1998; Hennig et al., 1999). Positive interactions between UV-B and R were reported for flavone glycoside synthesis in cell suspension cultures of Petroselinum hortense (Wellmann, 1971) and also for anthocyanin formation and accumulation in Zea mays coleoptyles (Beggs and Wellmann, 1985), apple skin (Arawaka, 1988), etiolated wheat seedlings (Mohr and Drumm-Herrel, 1983), and in broom sorghum (Yatsuhashi et al., 1982). The photoreceptors involved in these interactions have not been identified. Kim et al. (1998) have shown that in etiolated Arabidopsis seedlings low fluence rates of broad-band UV inhibit hypocotyl elongation to the same extent in WT and uvr2 (photolyase-deficient mutant) seedlings, which suggested that hypocotyl growth inhibition by low doses of UV did not require the accumulation of DNA damage in the form of cyclobutane pyrimidine dimers (CPD). Kim et al. (1998) also reported that either phyA or phyB was required for UV-B-induced elongation inhibition. However, from their experiments it is impossible to discern whether the phytochromes (A or B) directly absorbed UV-B (e.g. Pratt and Butler, 1970), triggering hypocotyl inhibition, or if this response was the result of an interaction between phytochrome/s and UV-B, perceived by a separate UV-B photoreceptor.
In the present work we studied two developmental responses induced by UV-B in de-etiolating Arabidopsis seedlings: cotyledon opening and hypocotyl growth inhibition. We determined the specificity of the UV-B effect and its fluence rate dependence, and we investigated the roles of known plant photoreceptor systems in these responses by using mutants deficient in phytochromes, cryptochromes, and DNA repair.
RESULTS
UV-B Has a Dual Effect on Cotyledon Opening
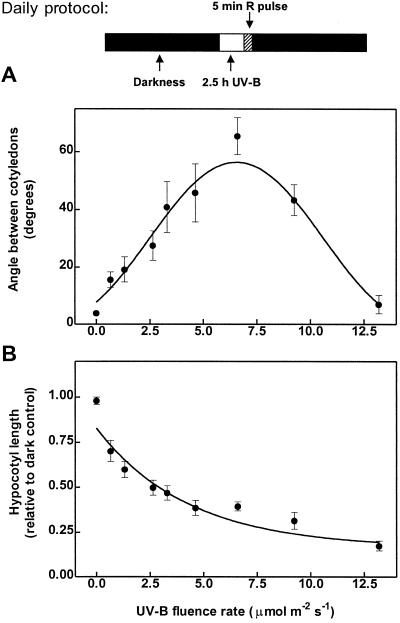
One-day-old etiolated seedlings of Arabidopsis were exposed for 3 d to a daily period of 2.5 h of UV-B followed by an R pulse. Cotyledon opening was enhanced by increasing fluence rates of UV-B between 0.8 and 6.6 μmol m−2 s−1 (Fig. 1A). Higher fluence rates gradually decreased cotyledon unfolding, thereby producing a bell-shaped fluence-rate response curve. Hypocotyl-growth inhibition showed a monophasic response, increasing gradually with fluence rate (Fig. 1B).
Figure 1.
Fluence-rate response curves for UV-B-induced cotyledon opening (A) and hypocotyl growth inhibition (B). One-day-old WT seedlings (24 h after the R pulse used to induce germination) were exposed for 3 d to a daily period of 2.5 h of UV-B (of the indicated fluence rate), followed by a saturating R pulse (see daily protocol at the top of the figure). Each point represents the mean ± se of at least six replicate boxes.
UV-B-Induced Cotyledon Unfolding Requires Pfr
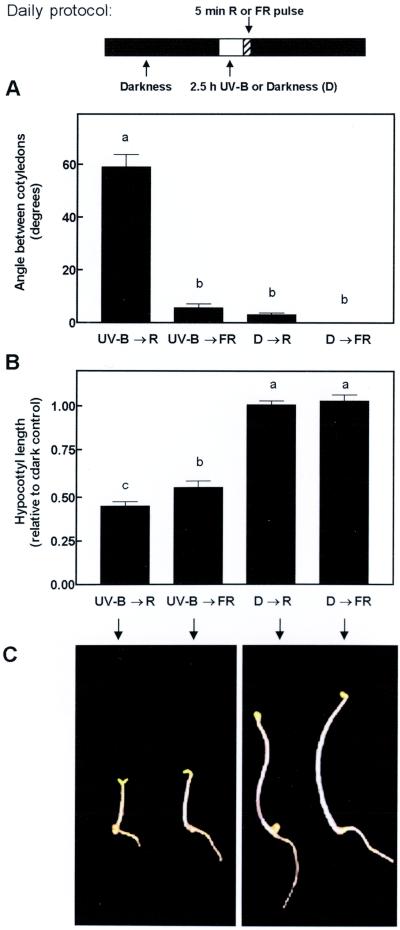
To investigate the interaction between UV-B and phytochrome, etiolated seedlings were exposed daily to 2.5-h UV-B treatments (6.6 μmol m−2 s−1), terminated with a pulse of R or FR (5 min). Neither an R pulse alone nor 2.5 h of UV-B terminated with an FR pulse (low Pfr levels) caused significant cotyledon opening (Fig. 2A). This indicates that the UV-B treatment interacts synergistically with the terminal R pulse (high Pfr level) to produce maximum cotyledon opening. In the case of hypocotyl elongation, UV-B had a strong inhibitory effect per se (i.e. even when the UV-B treatment was followed by an FR pulse, which lowered the level of Pfr) (Fig. 2B), the synergistic interaction between UV-B and R light was marginal but statistically significant.
Figure 2.
Angle between cotyledons (A) and hypocotyl length (B) of WT seedlings after 3 d of exposure to daily periods of 2.5 h of UV-B (6.6 μmol m−2 s−1) or darkness (D), followed by a saturating R or FR pulse (see daily protocol at the top of the figure). C, Phenotype of WT seedlings grown under the different irradiation treatments. Bar data are means and se of at least 16 replicate boxes. Different letters indicate significant differences between treatment means.
The Cotyledon Opening Response to UV-B Requires Active phyB But Not phyA, cry1, or cry2
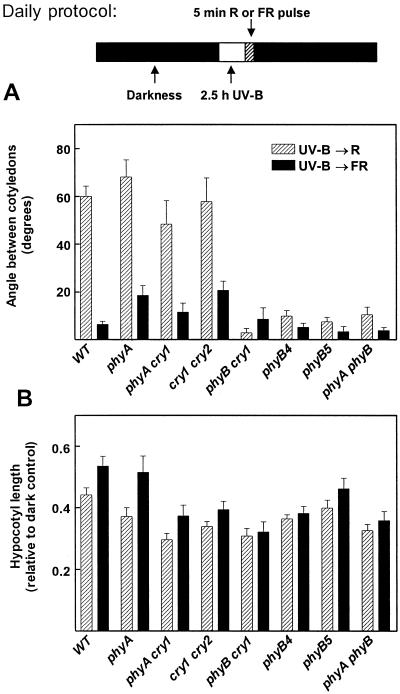
To investigate whether the responses to UV-B require phyA, phyB, cry1, and/or cry2, single and double photoreceptor mutants were exposed daily to 2.5 h of UV-B terminated with either an R or an FR pulse. The phyA, phyA cry1, and cry1 cry2 mutants behaved like the WT, whereas null (phyB-5, phyB-5 cry1, and phyA phyB-5) and weak (phyB-4) mutants of phyB failed to open the cotyledons in response to the UV-B → R treatment (Fig. 3A).
Figure 3.
Angle between cotyledons (A) and hypocotyl length (B) of photoreceptor-deficient single and double mutants. The seedlings were exposed for 3 d to a daily period of 2.5 h of UV-B (6.6 μmol m−2 s−1) or darkness, followed by a saturating R (striped bars) or FR (solid bars) pulse. Bar data are means and se of at least six replicate boxes.
UV-B inhibited hypocotyl elongation in all the genotypes, even when the UV-B treatment was terminated with an FR pulse. A terminal R pulse caused further inhibition only in those genotypes that did not carry a phyB mutation (Fig. 3B).
The Photoreceptor That Mediates the UV-B Effect on Cotyledon Opening Is Not phyB
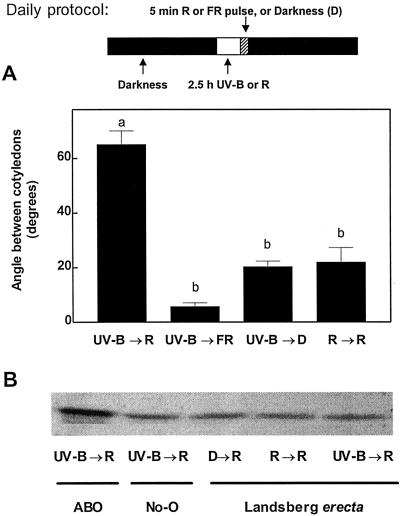
UV-B radiation can be absorbed by the phytochrome apoprotein and drive photoconversion (Pratt and Butler, 1970). Exposure to UV-B is predicted to establish a fractional Pfr level between 0.62 and 0.65 in etiolated tissue (Mancinelli, 1988). Since UV-B failed to elicit cotyledon unfolding in phyB (Fig. 3A), we investigated the possibility that phyB was responsible for the perception of the UV-B treatment. We gave the seedlings 2.5 h of R each day and compared the effect of this treatment with that of the daily UV-B exposures. Although the R treatment is predicted to be more effective than UV-B in generating phyB Pfr (calculated Pfr level, approximately 0.88), UV-B was much more effective than R in terms of enhancing the effect of a terminal R pulse on cotyledon opening (Fig. 4A). PHYB levels were similar in seedlings exposed to UV-B or R treatments (Fig. 4B). Therefore, the enhancing effect of UV-B on cotyledon opening was neither mediated by phyB perception of UV-B (Fig. 4A) nor by UV-B-induced increases in PHYB levels (Fig. 4B).
Figure 4.
A, Angle between cotyledons of WT seedlings after 3 d of exposure to daily periods of 2.5 h of UV-B (6.6 μmol m−2 s−1) followed by darkness or saturating R or FR pulses (UV-B → D, UV-B → R, or UV-B → FR, respectively), or to 2.5 h of R (30 μmol m−2 s−1) followed by a saturating R pulse (R → R). Bar data are means and se of at least six replicate boxes; different letters denote significant differences between treatment means. B, PHYB level of Ler seedlings exposed for 3 d to daily periods of the indicated irradiation treatments (UV-B → R, 2.5 h UV-B followed by an R pulse; R → R, 2.5 h R followed by an R pulse; D → R, one R pulse a day). A phyB overexpressor line (ABO) was included as a positive control along with its corresponding WT (No-O).
The Cotyledon Opening Response Was Not an Effect of Residual UV-A
As the UV-B lamps used in our experiments emit some residual UV-A (Q-Panel 313 bulbs; approximately 30% of the total energy output), we tested whether the cotyledon opening response was an effect of UV-B or if this residual UV-A, potentially perceived by cryptochromes, was also involved in promoting cotyledon opening. When further UV-A was added to the UV-B emitted by the Q-Panel 313 bulbs, the effect on cotyledon opening was similar to the effect of the UV-B treatment alone (Table I). When the UV-B emitted by the Q-Panel 313 bulbs was cut-off, using a Mylar filter, the opening response was greatly reduced, both in WT and in the cry1 cry2 double mutants (Table I, residual UV-A treatment). Thus, the UV-A component emitted by the UV-B lamps had only a modest effect in eliciting the UV response under our experimental conditions.
Table I.
Angle between cotyledons in WT and cry1 cry2 double mutant seedlings after daily pretreatments of 2.5 h UV-B + UV-A, UV-A, UV-B, or darkness, followed by a saturating R pulse
| Treatment | Angle between Cotyledons (Degrees)
|
|
|---|---|---|
| Genotype
| ||
| WT | cry1 cry2 | |
| UV-B + UV-A → R | 64 ± 4 | 64 ± 7 |
| Residual UV-A → R | 16 ± 2 | 13 ± 2 |
| UV-B → R | 61 ± 4 | 71 ± 7 |
| Darkness → R | 6 ± 2 | 17 ± 5 |
One-day-old seedlings (24 h after the R pulse used to induce germination) were exposed for 3 d to these treatments. Data are means ± se of six replicate boxes.
The Cotyledon Opening Response to UV-B Is Similar in WT and DNA-Repair Mutants
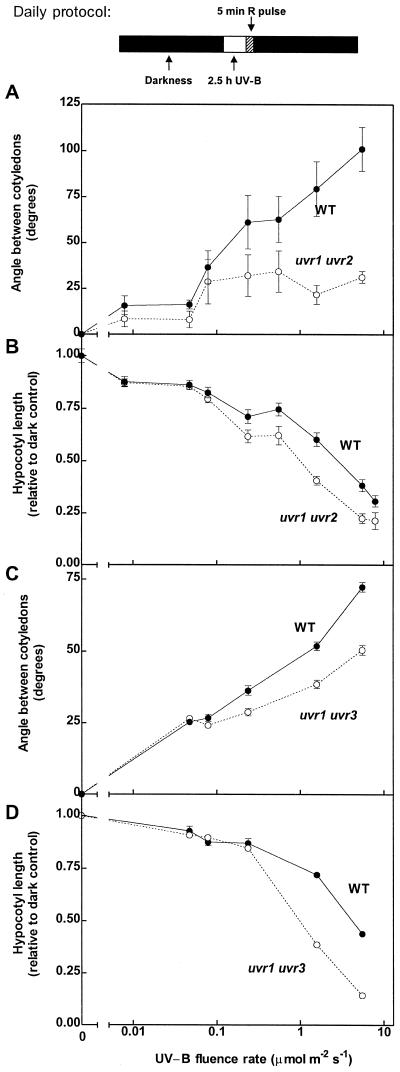
To investigate whether the effects of UV-B were elicited by DNA damage, we compared the de-etiolation responses to UV-B of WT seedlings with those of two DNA photorepair mutant (uvr1 uvr2 and uvr1 uvr3) seedlings. Both mutants are deficient in DNA excision repair; in addition, uvr1 uvr2 is deficient in the photorepair of CPDs, and uvr1 uvr3 is deficient in the photorepair of 6-4 photoproducts (Jiang et al., 1997). CPDs and 6-4 photoproducts are both induced by UV-B, and together they make up almost all of the DNA damage caused by UV radiation (Mitchell and Nairn, 1989; Britt, 1999). If the effect of UV-B on cotyledon unfolding were elicited by CPDs or 6-4 photoproducts, uvr1 uvr2 and/or uvr1 uvr3 should be more sensitive for this response (i.e. display greater opening angles) than the WT at least at low fluence rates. To test this we extended the range of UV-B fluence rates, including additional treatments with UV-B levels less than 0.1 μmol m−2 s−1. Compared with the WT, cotyledon unfolding in uvr1 uvr2 or uvr1 uvr3 seedlings was nearly normal for fluence rates less than 0.1 μmol m−2 s−1 (maximum opening between 25 and 40 degrees), and particularly for uvr1 uvr2, it was reduced rather than increased at higher fluence rates (Fig. 5, A and C). Hypocotyl elongation was not affected by the uvr mutations at fluence rates less than 0.1 μmol m−2 s−1, but both mutants were clearly more inhibited than the WT at greater fluence rates (Fig. 5, B and D).
Figure 5.
Fluence-rate response curves for UV-B-induced cotyledon opening and hypocotyl growth inhibition in WT and DNA repair mutants. Two-day-old seedlings (48 h after the R pulse used to induce germination; compare with Fig. 1) were exposed for 3 d to a daily period of 2.5 h of UV-B (of the indicated fluence rate), followed by a saturating R pulse. Each datum point represents the mean ± se of at least six replicate boxes. The uvr1 uvr2 (A and B) and uvr1 uvr3 mutants (C and D) were tested in two different series of experiments; therefore, separate WT controls are shown for each mutant.
DISCUSSION
UV-B induces a wide variety of responses in plants, including alterations in stem elongation and leaf morphology, but the mechanisms involved in these responses are unclear (Jansen et al., 1998). A relatively small fraction of the work on UV-B-induced photomorphogenesis has been carried out in Arabidopsis, and the genetic approaches that were so successful in unraveling the mechanisms of phytochrome and cryptochrome action have not been widely applied in the case of UV-B responses. In the present experiments we have characterized the effects of low levels of UV-B on de-etiolating Arabidopsis seedlings using a combination of physiological and genetic tools. We found that exposure to UV-B enhances the action of a subsequent R pulse, perceived by phyB, promoting cotyledon opening in de-etiolating Arabidopsis seedlings (Figs. 1–3). We also detected a synergistic interaction between UV-B and R for the inhibition of hypocotyl elongation, although this interaction was much weaker than the one for cotyledon opening (Fig. 2). In fact, UV-B caused a significant inhibition of hypocotyl elongation even when the treatment was terminated with an FR pulse (Fig. 2B) and in phyB and phyA phyB mutants (Fig. 3B). This result suggests that the effects of UV-B on cotyledon opening and hypocotyl growth inhibition are mediated through different photosensory mechanisms within the same developmental stage of the Arabidopsis seedling.
FR, perceived by phyA (Casal, 1995; Hennig et al., 1999), and blue light, perceived by cry1 (Casal and Boccalandro, 1995), were reported to have similar enhancing effects on the action of phyB Pfr on cotyledon opening and hypocotyl growth inhibition. The fact that UV-B in low doses can mimic the effects of FR and blue light on cotyledon opening may indicate that (a) UV-B activates the same photoreceptors as FR and blue light (namely phyA and cry1) or (b) using a separate receptor system, it engages transduction cascades that converge with those used by phyA and cry1. With regard to the first possibility, aromatic residues in the protein moiety of protein-pigment photoreceptor systems absorb in the UV-B region, and the energy may be transferred to the chromophores, thereby potentially eliciting biological responses. In the case of phytochrome, for example, UV-B can drive photoconversion and potentially trigger phytochrome-mediated responses (Pratt and Butler, 1970). Kim et al. (1998) reported that UV-B only had a weak effect on hypocotyl growth inhibition in a phyA phyB double mutant, and their results were consistent with the idea that the effect of UV-B was mediated by either phyA or phyB under their experimental conditions.
Obviously phyA was not the UV-B receptor that mediated the effect of UV-B on cotyledon opening in our experiments, because phyA mutants had a normal response to UV-B. The fact that phyB mutants were impaired in their cotyledon response to UV-B might be interpreted as indicating that phyB is involved in the perception of the UV-B treatments. However, the enhancing effect of UV-B could neither be replaced by pretreatments of continuous R nor explained by increased PHYB levels (Fig. 4), suggesting that a photoreceptor system different from phytochrome was responsible for sensing the UV-B treatment.
Regarding activation of blue light receptors by UV-B, recent work by Eisinger et al. (2000) suggests that the UV-B peak (280 nm) in the action spectrum for UV-induced stomatal opening in Vicia faba is mediated by the stomatal blue light receptor; this UV-B sensitivity presumably represents absorbance by the protein portion of the photoreceptor. Protein-chromophore energy transfer could also operate with the cryptochromes. Since cryptochromes show similarity to bacterial (class I) photolyases (the enzymes responsible for undoing DNA damage induced by UV-B), and Trp residues involved in UV-B photon capture and energy transfer have been identified in Escherichia coli photolyase (Kim et al., 1992), it might be reasonable to suspect that the cryptochromes are somehow capable of UV-B perception. From our experiments we can rule out the involvement of both cry1 and cry2 in UV-B signaling leading to cotyledon opening, because we found a WT response to UV-B in the double cry mutants (Table I). The evidence so far available in Arabidopsis agrees with our results, at least in the case of cry1. Fuglevand et al. (1996) studied the UV-B-induced expression of chalcone synthase in seedlings. They found that the UV-B effect is synergistically enhanced by blue and UV-A radiation, but neither the perception of the UV-B signal nor the enhancing actions of blue and UV-A were affected in cry1. Kim et al. (1998) similarly concluded that cry1 had no significant role in UV-B signaling for hypocotyl growth inhibition in Arabidopsis; the role of cry2 was not tested.
If UV-B is not perceived by phytochromes or cryptochromes it is necessary to consider other possibilities. UV-B causes specific photoproducts in DNA, and DNA damage is considered to play a key role in UV signaling in mammalian cells. The evidence in favor of this idea is strong, and it is based on experiments that involve manipulation of repair rates (Kripke et al., 1992; Kulms et al., 1999) and treatment of cells with oligonucleotides that mimic the initial products of DNA damage processing (such as thymine nucleotides, pTpT; Eller et al., 1996, 1997). The lesions caused by UV-B in DNA are CPDs, which may represent between 75% and 90% of the aberrant photoproducts generated, and 6-4 photoproducts, which make up most of the remaining fraction (Mitchell and Nairn, 1989; Britt, 1999). If these lesions were important in UV-B signaling for morphological responses in plants, one should expect a stronger response to UV-B in DNA repair mutants than in the WT, unless the repair mutants are so badly damaged by UV-B that they cannot display normal morphological responses.
Kim et al. (1998) showed that uvr2 seedlings had normal elongation responses to UV-B and interpreted this result as indicating that DNA damage was not involved in UV-B signaling. However, other DNA-repair mechanisms, such as excision repair and photorepair of 6-4 photoproducts are unaltered in uvr2 (Jiang et al., 1997). Moreover, the expression of UVR2 is very low in etiolated seedlings (see also Langer and Wellmann, 1990; Chen et al., 1994), so it is possible that the DNA repair rates of uvr2 were not very different from those of WT seedlings in the experiments of Kim et al. (1998).
Our experiments extended the range of DNA repair mechanisms that were genetically ablated. The results (Fig. 5) provide convincing evidence against the involvement of CPDs and 6-4 photoproducts in UV-B signaling for cotyledon opening, because the opening response of DNA repair mutants was either similar to (fluence rate < 0.1 μmol m−2 s−1) or lower than that of the WT controls. In contrast, in the case of hypocotyl growth inhibition, we did find higher sensitivity in the uvr double mutants than in the WT. This result indicates that, in the case of hypocotyl growth inhibition, CPD and 6-4 photoproducts are involved, presumably by causing non-specific growth retardation. It is noteworthy, however, that at fluence rates less than 0.1 μmol m−2 s−1, UV-B had only slight effects on hypocotyl elongation, and these effects were similar in uvr mutants and WT seedlings. This observation suggests that, at least within the time frame of our experiments, the toxic growth inhibitory effects of low doses of UV-B were relatively mild in the mutants. Therefore, aberrant DNA photoproducts, which were clearly toxic at high fluence rates, are unlikely to have limited cotyledon opening in the uvr mutants at fluence rates less than 0.1 μmol m−2 s−1. In summary, the lack of an increased opening response in uvr mutants strongly suggests that CPDs and 6-4 photoproducts are not involved in triggering this photomorphogenic effect of UV-B on the cotyledons. A caveat of this interpretation may be that the recognition of DNA photoproducts by the downstream element of the (hypothetical) photomorphogenic transduction cascade might require a functional DNA repair mechanism. If this were the case, the evidence gathered from experiments with DNA repair mutants could not be used to test the role of DNA lesions as informational signals.
Our results are not incompatible with a model in which UV-B activates elicitor receptors at the cell membrane (e.g. by triggering receptor phosphorylation or inhibiting receptor-directed phosphatases) (Rosette and Karin, 1996; Groβ et al., 1999; Kulms et al., 1999), and they neither support nor dismiss the possibility that particular redox states of flavins are involved in UV-B perception (Ensminger and Schäfer, 1992; Khare and Guruprasad, 1993; Ballaré et al., 1995). Isolation and characterization of mutants with altered photomorphogenic responses to UV-B clearly will be necessary to positively identify UV-B receptor systems. Up until now this path has been blocked by the lack of a useful phenotypic marker of UV-B-induced photomorphogenesis, which could be conveniently used in mutant screening programs. Our identification and physiological characterization of a simple photomorphogenic effect induced by UV-B, through a photoreceptor different from the phytochromes, cryptochromes, and DNA, should open the way to isolate mutants with defects in UV-B perception or signaling.
MATERIALS AND METHODS
Plant Material
The ecotype Landsberg erecta (Ler) of Arabidopsis was used as WT in this study. Several photoreceptor mutants (all in Ler background) were included: phyA-201 (formerly fre-1 [Nagatani et al., 1993]), phyB-5 (formerly hy3), and phyB-4 (Koornneef et al., 1980; Reed et al., 1993), the double mutants phyA-201 phyB-5 (Mazzella et al., 1997), phyA-201 cry1, and phyB-1 cry1 (Casal and Mazzella, 1998) and cry1 cry2 (Yanovsky et al., 2000). The PHYB overexpressing line (ABO) and the ecotype Nossen (No-O) (Wagner et al., 1991) were used as additional controls in the western blotting analysis. The uvr1 uvr2 and the uvr1 uvr3 double mutants (Ler background) were kindly provided by Anne B. Britt (University of California, Davis, CA). Both mutants are deficient in DNA excision repair; uvr1 uvr2 is also deficient in the photorepair of CPDs, and uvr1 uvr3 is deficient in the photorepair of 6-4 photoproducts (Jiang et al., 1997).
Ten seeds of each genotype were sown in clear plastic boxes (40 × 33 mm × 15 mm height), containing 3 mL of agar 0.8% (w/v). Boxes were lidded with a UV transparent film (Rolopac, Buenos Aires; 0.025-mm thick), stored 3 d in darkness at 6°C, exposed to a saturating R pulse, and incubated in darkness for 24 h at 25°C before being transferred to the different light treatments. In the western-blotting experiments and in the experiments where the DNA repair mutants were included, the seedlings were incubated 48 h at 25°C in darkness (instead of 24 h) before being transferred to the UV-B treatment to minimize potential deleterious effects of UV-B at early stages of seedling growth.
Light Treatments
UV-B was provided by two UV-B 313 bulbs (Q-Panel 313, Cleveland). UV-A was produced by two UV-A TL 40W/05 bulbs (Philips, Eindhoven, The Netherlands). In all cases a 0.1-mm-thick cellulose di-acetate film (La Casa del Celuloide, Buenos Aires) was placed between the tubes and the seedlings to filter out the UV-C radiation (λ < 290 nm) emitted by the fluorescent tubes. The cellulose di-acetate sheets were replaced after 7.5 h of exposure. Different UV-B irradiances were obtained by varying the distance between the seedlings and the light sources. UV levels were measured with a using an IL-1,700 double-monochromator spectroradiometer (International Light, Newburyport, MA), integrating the spectral irradiance between 290 and 315 nm. The radiometer was calibrated against a standard lamp (OL-40, Optronic, Orlando, FL) in the short-wavelength range and a model 1,800 calibrator (LI-COR, Lincoln, NE) for λ ≥ 320 nm. Wavelength accuracy was checked using a germicidal UV-C lamp. To obtain a UV-A control (Residual UV-A in Table I), we removed the UV-B portion of the spectrum emitted by the Q-Panel 313 bulbs using clear polyester films (Mylar-D, DuPont, Wilmington, DE; 0.1-mm thick). R (30 μmol m−2 s−1) was provided by red fluorescent tubes (40/15, Philips). FR (40 μmol m−2 s−1) was provided by incandescent lamps in combination with a water filter and an RG9 filter (Schott, Mainz, Germany).
Western Blotting
Eighty seedlings per irradiation treatment were grown under the appropriate light conditions. Proteins were extracted as described in Martinez-García et al.(1999). Fifty microliters were loaded per lane, separated on 8% (w/v) SDS-PAGE gels, and western blotted. The blots were probed with the monoclonal antibody anti-phyB MAb B6-B3, kindly provided by Peter H. Quail (University of California, Berkeley, and U.S. Department of Agriculture Plant Gene Expression Center, Albany, CA). After washing, the membrane was incubated with 1:500 affinity-purified alkaline phosphatase-conjugated antibody against mouse IgG developed in goat (Sigma, St. Louis). The bands were visualized by incubating the blots in 0.1 m Tris (pH 9.5), 100 nm NaCl, 5 mm MgCl2 containing 0.165 mg mL−1 5-bromo-4-chloro-3-indoyl phosphate, p-toluidine salt, and 0.33 mg mL−1 nitro blue tetrazolium (both from Sigma).
Plant Observations and Statistics
Hypocotyl length was measured to the nearest 0.5 mm with a ruler and the angle between the cotyledons was measured with a protractor. Average values were calculated for each seed box (i.e. one replicate) and used for statistical analysis. Each experiment was conducted on four to eight independent occasions; the data (replicate boxes) were pooled for the analysis.
ACKNOWLEDGMENTS
We thank Pedro Gundel and Laura Luccioni for technical assistance, Dr. Anne B. Britt for the provision of the uvr mutants, Dr. Peter H. Quail for the provision of the monoclonal antibody directed against phyB, and the Arabidopsis Biological Resource Center for the provision of Arabidopsis mutants. H.E.B., C.A.M., and M.A.M. were supported by fellowships from the Universidad de Buenos Aires, the Antorchas Foundation, and Consejo Nacional de Investigaciones Científicas y Técnicas, respectively.
Footnotes
This research was supported by grants from the Secretariat of Science and Technology (Agencia Nacional de Promoción Científica y Tecnológica, BID OC-AR802 PID no. 394 and PICT nos. 00342 and 05292).
LITERATURE CITED
- Ahmad M, Cashmore AR. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Arawaka O. Photoregulation of anthocyanin synthesis in apple fruit under UV-B and red light. Plant Cell Physiol. 1988;29:1385–1389. [Google Scholar]
- Ballaré CL, Barnes PW, Flint SD. Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolating tomato seedlings: I. The photoreceptor. Physiol Plant. 1995;93:584–592. [Google Scholar]
- Beggs CJ, Wellmann E. Analysis of light-controlled anthocyanin formation in coleoptiles of Zea mays L.: the role of UV-B, blue, red and far-red light. Photochem Photobiol. 1985;41:481–486. [Google Scholar]
- Beggs CJ, Wellmann E. Photocontrol of flavonoid biosynthesis. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 733–751. [Google Scholar]
- Briggs WR, Huala E. Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- Britt AB. DNA damage and repair in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:75–100. doi: 10.1146/annurev.arplant.47.1.75. [DOI] [PubMed] [Google Scholar]
- Britt AB. Molecular genetics of DNA repair in higher plants. Trends Plant Sci. 1999;4:20–25. doi: 10.1016/s1360-1385(98)01355-7. [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Teramura AH, Tevini M, Bornman JF, Björn LO, Kulandaivelu G. Effects of increase solar ultraviolet radiation on terrestrial ecosystems. J Photochem Photobiol B Biol. 1998;46:40–52. doi: 10.1039/b211159b. [DOI] [PubMed] [Google Scholar]
- Casal JJ. Coupling of phytochrome B to the control of hypocotyl growth in Arabidopsis. Planta. 1995;196:23–29. doi: 10.1007/BF00193213. [DOI] [PubMed] [Google Scholar]
- Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB and hy4 simple, double and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA. The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature. 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Mitchell DL, Britt AB. A light-dependent pathway for the elimination of UV-induced pyrimidine (6-4) pyrimidinone photoproducts in Arabidopsis. Plant Cell. 1994;6:1311–1317. doi: 10.1105/tpc.6.9.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR. Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- Eisinger W, Swartz TE, Bogomolni RA, Taiz L. The ultraviolet action spectrum for stomatal opening in broad bean. Plant Physiol. 2000;122:99–106. doi: 10.1104/pp.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller M, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci USA. 1996;93:1087–1092. doi: 10.1073/pnas.93.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller MS, Maeda T, Magnoni C, Atwal D, Gilchrest BA. Enhancement of DNA repair in human skin cells by thymidine dinucleotides: evidence for a p53-mediated mammalian SOS response. Proc Natl Acad Sci USA. 1997;94:12627–12632. doi: 10.1073/pnas.94.23.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger PA. Control of development in plants and fungi by far-UV radiation. Physiol Plant. 1993;88:501–508. [Google Scholar]
- Ensminger PA, Schäfer E. Blue and ultraviolet-B light photoreceptors in parsley cells. Photochem Photobiol. 1992;55:437–447. [Google Scholar]
- Foyer CR, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Frohnmeyer H, Loyall L, Blatt MR, Grabov A. Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J. 1999;20:109–117. doi: 10.1046/j.1365-313x.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- Fuglevand G, Jackson JA, Jenkins GI. UV-B, UV-A, blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell. 1996;8:2347–2357. doi: 10.1105/tpc.8.12.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt KE, Wilson MI, Greenberg BM. Tryptophan photolysis leads to a UVB-induced 66 kDa photoproduct of ribulose-1,5-biphosphate carboxylase/oxygenase (Rubisco) in vitro and in vivo. Photochem Photobiol. 1999;70:49–56. [Google Scholar]
- Green R, Fluhr R. UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell. 1995;7:203–212. doi: 10.1105/tpc.7.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groβ S, Knebel A, Tenev T, Neninger A, Gaestel M, Herrlich P, Böhmer F. Inactivation of protein-tyrosine phosphatases as mechanism of UV-induced signal transduction. J Biol Chem. 1999;274:26378–26386. doi: 10.1074/jbc.274.37.26378. [DOI] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Unger S, Schäfer E. Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta. 1999;208:257–263. doi: 10.1007/s004250050557. [DOI] [PubMed] [Google Scholar]
- Jansen M, Gaba V, Greenberg B. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Jenkins GI. UV and blue light signal transduction in Arabidopsis. Plant Cell Environ. 1997;20:773–778. doi: 10.1046/j.1365-3040.1997.d01-105.x. [DOI] [PubMed] [Google Scholar]
- Jiang CZ, Yee J, Mitchell D, Britt AB. Photorepair mutants of Arabidopsis. Proc Natl Acad Sci USA. 1997;94:7441–7445. doi: 10.1073/pnas.94.14.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare M, Guruprasad KN. UV-B-induced anthocyanin synthesis in maize regulated by FMN and inhibitors of FMN photoreactions. Plant Sci. 1993;91:1–5. [Google Scholar]
- Kim BC, Tennessen DJ, Last RL. UV-B-induced photomorphogenesis in Arabidopsis thaliana. Plant J. 1998;16:667–674. doi: 10.1046/j.1365-313x.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Li YF, Sancar A. The third chromophore of DNA photolyase: Trp-277 of Escherichia coli DNA photolyase repairs thymine dimers by direct electron transfer. Proc Natl Acad Sci USA. 1992;89:900–904. doi: 10.1073/pnas.89.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolf E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;15:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulms D, Pöppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T. Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA. 1999;96:7974–7979. doi: 10.1073/pnas.96.14.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer B, Wellmann E. Phytochrome induction of photoreactivating enzyme in Phaseolus vulgaris L. seedlings. Photochem Photobiol. 1990;52:861–863. [Google Scholar]
- Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR. Enhancement of blue-light sensitivity of Arabidopsis seedlings by blue light receptor cryptochrome 2. Proc Natl Acad Sci USA. 1998;95:2686–2690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madronich S, McKenzie RL, Björn LO, Caldwell MM. Changes in biologically active ultraviolet radiation reaching the Earth's surface. J Photochem Photobiol B Biol. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Malanga R, Kozak R, Puntarulo S. N-Acetylcysteine-dependent protection against UV-B damage in two photosynthetic organisms. Plant Sci. 1999;141:129–137. [Google Scholar]
- Mancinelli AL. Some thought about the use of predicted values of the state of phytochrome in plant photomorphogenesis research. Plant Cell Environ. 1988;11:429–439. [Google Scholar]
- Martinez-García JF, Monte E, Quail PH. A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J. 1999;20:251–257. doi: 10.1046/j.1365-313x.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- McKenzie RL, Connor B, Bodeker G. Increased summertime UV radiation in New Zealand in response to ozone loss. Science. 1999;285:1709–1711. doi: 10.1126/science.285.5434.1709. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Nairn R. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49:805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Mohr H, Drumm-Herrel H. Co-action between phytochrome and blue/UV light in anthocyanin synthesis in seedlings. Physiol Plant. 1983;58:408–414. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L, Butler W. Phytochrome conversion by ultraviolet light. Photochem Photobiol. 1970;11:503–509. doi: 10.1111/j.1751-1097.1970.tb06021.x. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A, Hamilton C, Chinnaiyan A, Dixit V. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Smith ML, Fornace AJJ. p53-mediated protective responses to UV irradiation. Proc Natl Acad Sci USA. 1997;94:12255–12257. doi: 10.1073/pnas.94.23.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surplus S, Jordan B, Murphy A, Carr J, Thomas B, Mackerness SAH. Ultraviolet-B-induced responses in Arabidopsis thaliana: role of salicylic acid and reactive oxygen species in the regulation of transcripts encoding photosynthetic and acidic pathogenesis-related proteins. Plant Cell Environ. 1998;21:685–694. [Google Scholar]
- Wagner D, Tepperman JM, Quail PH. Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell. 1991;3:1275–1288. doi: 10.1105/tpc.3.12.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann E. Phytochrome-mediated flavone glycoside synthesis in cell suspension cultures of Petroselinum hortense after preirradiation with ultraviolet light. Planta. 1971;101:283–286. doi: 10.1007/BF00386835. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Mazzella MA, Casal JJ. A quadruple photoreceptor mutant still keeps track of time. Curr Biol. 2000;10:1013–1015. doi: 10.1016/s0960-9822(00)00651-5. [DOI] [PubMed] [Google Scholar]
- Yatsuhashi H, Hashimoto T, Shimizu S. Ultraviolet action spectrum for anthocyanin formation in broom sorghum first internodes. Plant Physiol. 1982;70:735–741. doi: 10.1104/pp.70.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E. Sensory transduction of blue light in guard cells. Trends Plant Sci. 2000;5:183–185. doi: 10.1016/s1360-1385(00)01602-2. [DOI] [PubMed] [Google Scholar]