Abstract
Background:
Although social rejection is among the strongest proximal precipitants of major depressive disorder (MDD), little is known about the underlying neurobiological mechanisms and whether neural sensitivity to social rejection may help explain differences in MDD risk. To address this issue, we tested whether neural responses to social threat differed in female adolescents at high vs. low maternal risk for MDD.
Method:
Female adolescents with (high-risk; n = 22, Mage = 14.68) and without (low-risk; n = 30, Mage = 15.07) a maternal history of depression were experimentally exposed to negative and neutral social evaluation while undergoing an fMRI scan. Neural responses were assessed by event-related activity and functional connectivity, as well as multivoxel pattern analysis. Activity and functional connectivity analyses focused on a priori-selected regions of interest implicated in self-referential processing and emotion regulation.
Results:
Compared to low-risk female adolescents, high-risk female adolescents exhibited greater increases in self-reported depression and social disconnection following social evaluation. Moreover, compared to low-risk female adolescents, high-risk female adolescents exhibited greater amygdala responses to negative social evaluation and a differential pattern of functional connectivity in brain regions related to emotion regulation, self-referential processing, and negative affect. Additionally, these markers of neural threat reactivity were related to depressive symptoms.
Limitations:
A cross-sectional study design and relatively small, Western sample.
Conclusions:
These results suggest that exaggerated neural reactivity to social threat—and an atypical pattern of related functional connectivity—is evident in individuals with a preclinical risk factor for depression. Targeting such responding may thus be a fruitful strategy for preventing depression in at-risk youth.
Keywords: Neural, Connectivity, Social stress, Mechanisms, Risk, Depression
1. Introduction
Depression is a leading cause of disease burden worldwide (Gore et al., 2011; Ferrari et al., 2013), but this burden is not shared equally between males and females. Indeed, while rates of major depressive disorder (MDD) are equally low for males and females before the pubertal transition, MDD rates rise substantially for females following puberty (Salk et al., 2017; Mojtabai et al., 2016). This disease burden is compounded by the fact that MDD is recurrent for some individuals (Slavich and Irwin, 2014) and increases risk for suicide (Smith et al., 2010; Stewart et al., 2019) as well as several somatic conditions including heart disease, chronic pain, and autoimmune and neurode-generative disorders (Slavich and Irwin, 2014). Understanding factors that contribute to the initial onset of depression, especially in female adolescents, is therefore of paramount public importance, especially if such research can help identify preclinical risk processes that could be modified to reduce risk for developing a first onset of MDD.
Nearly all major theories of depression posit that major life stressors increase risk for depression, especially for persons who are already vulnerable (Slavich and Irwin, 2014; Beck and Bredemeier, 2016; Slavich, 2020; Slavich et al., 2010a; Luby et al., 2020; Slavich, 2022). One particularly robust social-psychological antecedent of MDD is social rejection (Slavich et al., 2009). Despite this knowledge, however, the neurobiological mechanisms linking social rejection with risk for depression remain unclear. We addressed this issue using a high-risk family design, which examined how neural responses to social rejection differed for female adolescents at high vs. low risk for developing depression. We also investigated how these neural responses related to both female adolescents’ and their mothers’ depressive symptoms.
1.1. Social rejection and depression
As alluded to above, the lifelong societal and health impacts of adolescent depression have fostered substantial research aimed at better understanding the social-environmental antecedents of MDD. Life stressors, and social rejection in particular, appear to be one of the strongest proximal social-psychological precipitants of depression (Stewart et al., 2019; Slavich et al., 2010a; Slavich et al., 2009). In a retrospective study of individuals diagnosed with MDD, for example, those who experienced targeted rejection (e.g., being broken up with by a romantic partner) developed MDD approximately three times faster than their counterparts who experienced severe life stressors that did not involve targeted rejection (Slavich et al., 2009). Relatedly, recent experiences of social rejection have been found to distinguish individuals who have attempted suicide from those who thought about (but did not attempt) suicide, as well as those with psychiatric diagnoses who had not attempted or thought about suicide (Stewart et al., 2019). Some research has shown that social rejection upregulates inflammatory activity (Slavich et al., 2010b), and that inflammation can induce some depressive symptoms (Slavich and Irwin, 2014; Kappelmann et al., 2018; Shields et al., 2017; Shields and Slavich, 2017; Slavich, 2015). However, what neurobiological processes underlie these links in adolescence remains unclear.
The Social Signal Transduction Theory of Depression (Slavich and Irwin, 2014) posits that experiences of social rejection are converted into neural signals of threat, including greater amygdala and anterior insula activity, as well as altered functional connectivity between these regions and other regions involved in negative affect, self-referential processing, and emotion regulation (e.g., the anterior cingulate cortex [ACC], ventromedial prefrontal cortex [VMPFC]) [see also (Ho et al., 2014; Kaiser et al., 2015)]. These neural representations of threat are then posited to initiate a downstream biological cascade that ultimately increases risk for MDD for vulnerable individuals (Slavich and Irwin, 2014). A key prediction of this theory is that neural responses to social rejection should be stronger for individuals at heightened risk of developing depression, such as those with a maternal lifetime history of MDD (Hammen et al., 1987; Lieb et al., 2002; Lin et al., 2019). In support of this prediction, prior research has found that greater ACC responses to social rejection paradigms, such as “cyberball,” predict increases in depressive symptoms over time (Masten et al., 2011; Silk et al., 2022; Silk et al., 2014), as well as greater inflammatory responses to social stress (Slavich et al., 2010b). To date, however, no study has tested this prediction in youth who are at known high risk for depression but who have not yet experienced depression or any other Axis I disorder.
1.2. Present study
We addressed this issue by examining neural responses to social rejection in female adolescents, 12–16 years old, who were at high vs. low risk of developing MDD. We focused specifically on this age group because it is a critical period when risk for MDD increases significantly but is before most female adolescents experience their first major depressive episode (MDE) (Angold et al., 1998). Risk of depression was determined by maternal lifetime history of MDD, as having a mother with a lifetime history of depression is a strong risk factor for MDD in female adolescents (Hammen et al., 1987; Lieb et al., 2002; Lin et al., 2019). Participants were introduced to another female similar in age (actually a confederate) who was ostensibly going to socially evaluate them while the participant was in the fMRI scanner. We assessed youths’ self-reported changes in social disconnection and depression, event-related neural activity and functional connectivity that was time-locked to adjective selection, and whole-brain functional connectivity that distinguished groups and types of socially evaluative adjectives (i.e., negative or neutral) using multivoxel pattern analysis (MVPA). MVPA is more sensitive to differences than univariate approaches to fMRI, as it detects information contained within patterns of activity, rather than in the level of activity alone (Coutanche and Thompson-Schill, 2012; Norman et al., 2006). MVPA is also more sensitive in some ways than an ROI-based approach to functional connectivity, as it examines patterns of connectivity at the level of voxels—corrected for multiple testing—rather than between ROIs (Norman et al., 2006). MVPA can thus supplement inferences by elucidating clusters of voxels that show strong and complex patterns associated with social threat.
Drawing from Social Signal Transduction Theory of Depression (Slavich and Irwin, 2014) and prior research showing that greater ACC activity (consistent with threat and negative affect reactivity, among other interpretations) during social rejection predicts increases in depressive symptoms (Masten et al., 2011; Silk et al., 2022; Silk et al., 2014), we had three primary hypotheses. In particular, compared to participants in the low MDD risk group, we expected participants in the high MDD risk group to exhibit (a) greater increases in self-reported depression and social disconnectedness, (b) greater functional activity and connectivity to social threat in the amygdala and ACC, and (c) reduced functional activity and connectivity to social threat in and with the VMPFC in response to social evaluation.
2. Method
2.1. Participants
Participants at high and low risk for developing a first lifetime MDE (N = 52; 22 high-risk, 30 low-risk; Mage = 14.90, SD = 1.35) were recruited using flyers posted in community locations, online advertisements, social media posts, word of mouth, and announcements made at schools located throughout the greater Los Angeles area. This sample size provides 99.1 % power to detect a similar effect size obtained in a study on familial-risk-related differences in fMRI activity to social stimuli (Morgan et al., 2019), though we note that power analyses based on pilot studies with limited sample sizes can minimize the number of participants needed to obtain adequate power (Kraemer et al., 2006). To be eligible, daughters had to be between 12 and 16 years old at time of recruitment, English-speaking, right-handed, not claustrophobic, free of bodily metal (except dental fillings) and other contraindications for MRI, living with their biological mother, and have no current or past history of any Diagnostic and Statistical Manual-IV (DSM-IV) Axis I affective disorder. In addition, daughters must not have had any recent alcohol or substance use or dependence, not have been pregnant as verified with a pregnancy test, and not have had any history of head trauma or a learning disability. Finally, daughters had to be free of past or current inflammatory illness, major sleep disturbance, tobacco use, prescription drug use, excessive caffeine use (i.e., >8 cups/day), or a body mass index of ≥30 due to the measurement of peripheral inflammation (data not analyzed in current manuscript) (O’Connor et al., 2009). Seven adolescents (five low-risk, two high-risk) were excluded from fMRI analyses due to motion (see Materials and Procedure).
The high-risk group was comprised of female adolescents whose mothers met Structured Clinical Interview for DSM-IV [SCID-IV; (First et al., 1995)] diagnostic criteria for major depressive disorder at some point in their lifetime (i.e., positive lifetime history of MDD). Conversely, the low-risk group was comprised of female adolescents whose mothers had no lifetime history of any Axis I disorder. As noted above, none of the female adolescents could have had a current or past history of any Diagnostic and Statistical Manual-IV (DSM-IV) Axis I affective disorder.
2.2. Materials and procedure
All procedures were approved by the UCLA Institutional Review Board. Mothers and daughters interested in the study first completed a phone screening session to introduce the study and preliminarily assess their likelihood of meeting the inclusion and exclusion criteria. If the mothers and daughters both appeared eligible, an intake session was scheduled for the first available date.
At the intake session, informed consent and assent were obtained, and mothers and daughters were separately screened by trained diagnostic interviewers to determine their risk group. Using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version [K-SADS-PL; (Kaufman et al., 1997)], we assessed each daughter’s diagnostic status and, in addition, created interviewer-derived indices of youths’ depressive symptoms and symptom severity. In turn, each mother’s diagnostic status was assessed using the SCID-IV (First et al., 1995). Eligible mother-daughter pairs separately completed questionnaires. Mothers provided reports of their current depressive symptoms using the Beck Depression Inventory-II [BDI-II; (Beck et al., 1996)].
Next, daughters completed a 10-minute video-recorded interview in which an interviewer asked daughters 33 questions about themselves in the absence of their mothers (Sichko et al., 2021). The interview had a conversational feel and focused on the daughters’ opinions, feelings, and memories. Daughters were then scheduled for a second 3.5-hour session, which took place within approximately 1 month of their initial session (median = 26.5 days).
Upon arriving for this second session, daughters were taken to a private testing room and informed of the session’s procedures. Participants completed questionnaires assessing self-reported depression [i.e., the depression subscale of the Profile of Mood States – Short Form; POMS-SF; (Curran et al., 1995)] and social disconnection (Eisenberger et al., 2010). The depression subscale in the POMS-SF consists of eight items. Participants were asked to indicate how well eight words (discouraged, hopeless, sad, blue, helpless, unhappy, worthless, and miserable) described how they felt right then, at that moment, using a scale from 1 (Not at all) to 5 (Extremely). Self-reported feelings of depression could thus range from 8 to 40, with higher values indicating more depression. Similarly, the social disconnection questionnaire asked participants to indicate, using a scale from 1 (Not at all) to 5 (Very much so), the extent to which they felt certain ways right then. Each of the 12 items on this questionnaire was related to social situations or social interaction more broadly (e.g., “I feel alone,” and, “I feel like being around other people”). Scores could thus range from 0 to 60, and the measure was coded such that higher values indicated greater social disconnection. During the time in which the participant completed these questionnaires, they were introduced to “another participant” (actually a confederate) who they were told was completing a related study. The confederate was always a female, college-aged research assistant who dressed and acted like a slightly older adolescent.
Approximately 1 h after the start of this session, both the participant and the confederate were taken to the MRI scanner control room. Participants and the confederate were given instructions for the social evaluation task (Eisenberger et al., 2011). First, participants were reminded of their recorded interview and the first five seconds of their interview was shown. The evaluative nature of the task was introduced by explaining that the confederate would be watching and judging the participant’s video by clicking on 1 of 24 potential adjectives every ten seconds to indicate her impression of the participant. The participant was informed she would see the adjective selections in real time while in the MRI scanner. An example of the display screen was shown to both the participant and the confederate. The confederate always asked a clarifying question (i.e., “How often am I supposed to give a rating?”) to enhance the task’s believability. In reality, all participants watched the same recorded 10-minute video in which the adjectives (one-third positive, one-third neutral, one-third negative) were “selected” in pseudo-random order, with a jittered inter-adjective interval and no more than two similarly valenced words clicked consecutively. This task engages the amygdala (Muscatell et al., 2015) and increases self-reported feelings of social evaluation and rejection (Dedovic et al., 2016), which are common features of depression in youth (Platt et al., 2013).
After the MRI scan, participants returned to the testing room, where they completed the self-reported depression and social disconnection questionnaires again (post-evaluation). Finally, participants were fully debriefed and thanked.
2.3. fMRI image acquisition
Imaging data were acquired using a Prisma 3.0 Tesla whole-body scanner (Siemens Medical Systems, Iselin, New Jersey) at the Staglin One Mind Center for Cognitive Neuroscience at UCLA. High resolution T1-weighted structural images were acquired using a magnetized prepared rapid acquisition gradient echo (MPRAGE) sequence containing 1.1 mm isotropic voxels, TR/TE/flip angle = 2300 ms/2.95 ms/9◦, FOV = 270 mm2, 176 slices. Blood oxygenation level-dependent (BOLD) functional images were acquired containing 3 mm isotropic voxels, TR/ TE/flip angle = 2000 ms/34 ms/76◦, FOV = 208 mm2, 48 slices.
2.4. fMRI preprocessing & analyses
2.4.1. Activity
Functional and structural MRI data used in univariate (i.e., BOLD activation) analyses were preprocessed using SPM12. Preprocessing included realignment (and unwarp) of functional files, functional centering to (0,0,0) coordinates (translation), functional slice-timing correction, motion correction, functional segmentation and normalization, structural translation, and structural segmentation and normalization. Participants with >3 mm and/or 3◦ of movement between slices and/or who were removed from functional connectivity analyses due to excessive motion were excluded from neuroimaging analyses. Contrast images for activity were estimated using SPM12. The mask for the amygdala ROI was taken from the Harvard-Oxford subcortical atlas, the masks for the anterior insula and ACC were taken from the salience network set of ROIs from CONN Toolbox, and the mask of the VMPFC was taken from Bhanji et al. (2019) (Fig. 1). For consistency with our functional connectivity analyses and to make the most of the probabilistic amygdala ROI data, we used REX (https://www.nitrc.org/projects/rex) to extract weighted-sum values of signal intensity from the amygdala for the negative adjective minus neutral adjective contrast. We also used REX to quantify mean signal intensity in nonprobabilistic ROIs. Signal intensities were then standardized prior to analyses.
Fig. 1.
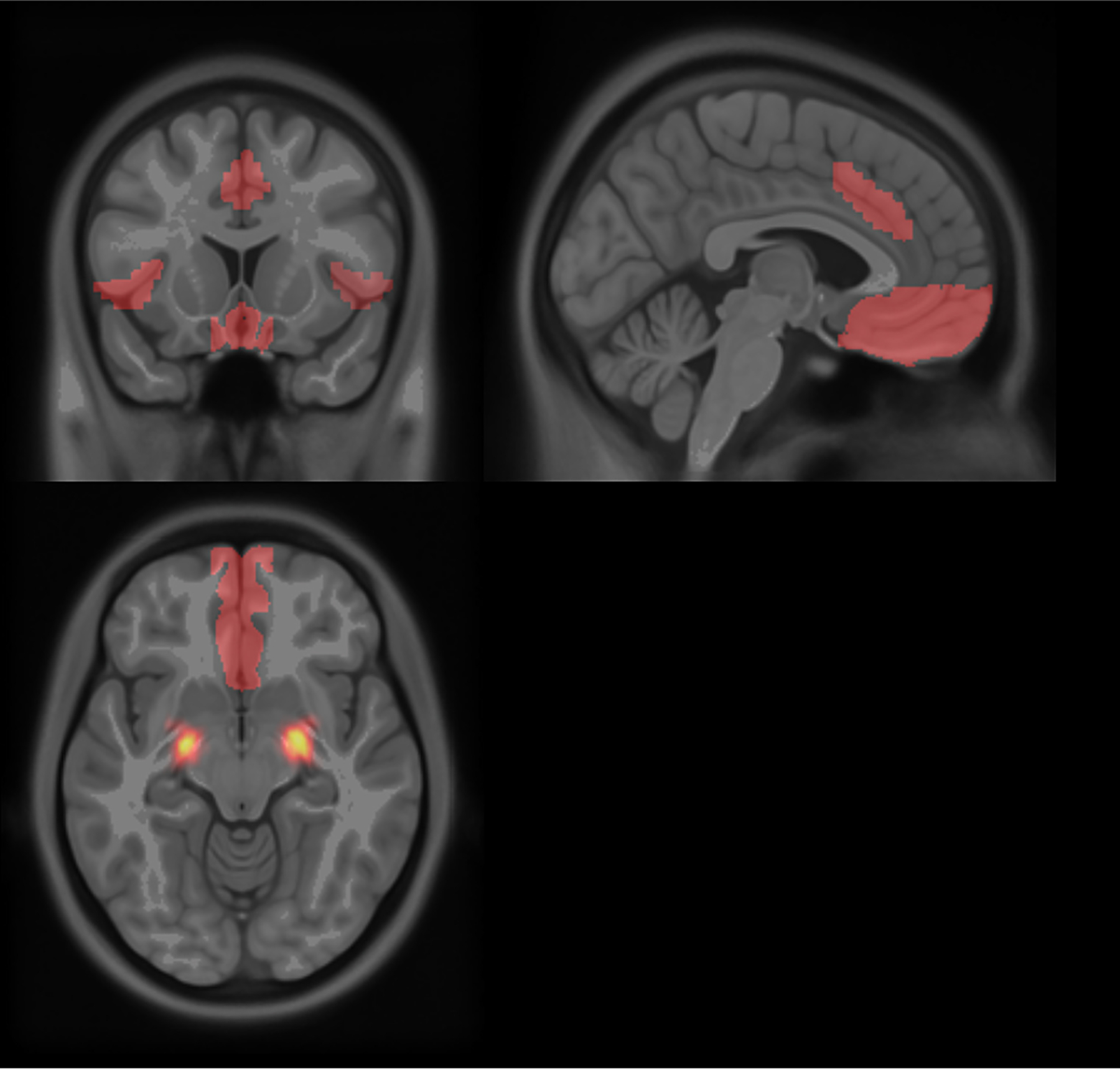
The a priori ROIs examined. Amygdala values were probability-weighted sums in keeping with the probabilistic ROI, whereas values from the remainder of the ROIs were not.
2.4.2. Functional connectivity
Because best practices in preprocessing differ slightly between univariate activation and functional connectivity analyses, for functional connectivity analyses, raw functional and structural MRI data were preprocessed using the default preprocessing pipeline in CONN toolbox v19.b (Whitfield-Gabrieli and Nieto-Castanon, 2012). This pipeline consists of functional realignment (and unwarp) of functional files, functional centering to (0,0,0) coordinates (translation), functional slice-timing correction, functional outlier detection using the artifact detection toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) for scrubbing, functional segmentation and normalization, structural translation, and structural segmentation and normalization. ART parameters were set to flag acquisitions with framewise displacement above 0.9 mm or global BOLD signal changes above five standard deviations as potential outliers. Participants (n = 7) with >20 % of functional images identified as outliers by ART were removed from subsequent analyses. Outlier matrices, three translational and three rotational movement parameters and their first-order temporal derivatives, ten noise component parameters from the anatomical component-based noise correction procedure (aCompCor; five parameters from white matter, five from cerebrospinal areas), and all effects of conditions (done by default in CONN Toolbox to remove illusory correlations) were entered as covariates during first-level analysis. BOLD timeseries were first preprocessed and denoised, then spatially normalized to build the time series for each voxel.
2.4.2.1. ROI-to-ROI analyses.
Event-related functional connectivity (Rissman et al., 2004) for each assessed valence condition was assessed using generalized psychophysiological interaction (gPPI) in CONN Toolbox with contrasts that examined differences in functional connectivity between negative and neutral adjective viewing between groups [(High-Risk Negative – High-Risk Neutral) – (Low-Risk Negative – Low-Risk Neutral)]. The ROIs used were the same as those used in the univariate analyses described above.
2.4.2.2. Multi-voxel pattern analysis (MVPA).
We computed pairwise connectivity between each voxel and the rest of the brain using whole-brain MVPA implemented in CONN Toolbox. We reduced the dimensionality of these data using principal components analysis with nine components to maintain an approximate 5:1 ratio between participants and components, as is recommended (Whitfield-Gabrieli and Nieto-Castanon, 2012; Whitfield-Gabrieli et al., 2016). We then conducted multivariate analyses to identify clusters of functionally connected voxels between any of the nine component scores that differentiated the interaction between Group and Valence [i.e., (High-Risk Negative – High-Risk Neutral) – (Low-Risk Negative – Low-Risk Neutral)] in a data-driven approach. These analyses were thresholded at a cluster level of an FDR-corrected p < .05 and a height level of p < .001.
2.5. Data analysis
Activity values (beta weights) were extracted from REX, and functional connectivity values were extracted from CONN. Connectivity values are Fisher Z-transformed partial correlations. Because we did not have a priori expectations of differences between hemispheres, we averaged across hemisphere for the activity and connectivity analyses. ROI-to-ROI analyses were all specified a priori and thus were not corrected for multiple comparisons. Using false discovery rate corrections for multiple comparisons in ROI-to-ROI analyses reduced the group difference in VMPFC to amygdala connectivity to nonsignificance but did not alter other inferences.
All analyses were conducted using R, version 4.3.1. Changes in self-reported feelings of depression and social disconnection were analyzed as difference scores; tests were conducted to determine whether each group differed in change in each of these variables from zero, as well as whether the groups differed in change from each other, using one-sample and two-sample independent groups t-tests, respectively, with change scores as the dependent variables. Extracted fMRI data were also analyzed using two-sample, independent groups t-tests. MVPA clusters were analyzed for group differences in CONN using default corrections before being extracted for association analyses. Associations between extracted activity or functional connectivity data and pre- to post-evaluation change scores were quantified via Pearson correlations. Depressive symptom totals are count data that often violate standard Poisson model assumptions; therefore, associations with depressive symptoms were examined in robust Poisson regressions conducted using the robustbase package, version 0.99–0, using the default Mlqe method. Zero-inflated and hurdle Poisson models produced equivalent results to those presented below; in these models, the associations were significant within the count—not zero—coefficients, as would be expected from our Poisson results. Mediation analyses were conducted using the mediation package, version 4.5.0, using the appropriate outcome distributions (e. g., Gaussian, Poisson) for each model. Mediation analyses were conducted when risk group, depressive symptoms, and fMRI data were all related in order to explore relations among these data; they should be interpreted as exploratory and thus with caution. Excluding outliers, defined as standardized residuals > ±3, did not alter any inferences from analyses of risk group differences or associations reported below.
Results include presentation of Bayes factors (BF10), calculated using the BayesFactor package, version 0.9.12–4.4, in R. A Jeffreys-beta prior was used for the prior in all Bayesian analyses. A Bayes factor quantifies the evidence in favor of the data being observed in the model of interest, such that a Bayes factor >1 (e.g., 2.5) indicates that the data were more likely to be observed in the model of interest than an alternative model (e.g., a Bayes factor of 2.5 indicates that the data are 2.5 times as likely to have occurred given the model of interest than the model it is being tested against, such as a null model), whereas a Bayes factor BF10 <1 indicates evidence against the data occurring under that model. By convention, a Bayes factor BF10 of 3.16 or greater indicates substantial evidence in favor of the data being observed in the model of interest.
3. Results
Participant demographic characteristics by MDD risk group are reported in Table 1.
Table 1.
Sample characteristics by MDD risk group.
| High-risk female adolescents n = 22 mean (SD) |
Low-risk female adolescents n = 30 mean (SD) |
Group difference P |
|
|---|---|---|---|
| Age | 14.68 (1.39) | 15.07 (1.31) | .31 |
| Body mass index | 24.87 (6.70) | 22.24 (4.51) | .10 |
| Adolescents’ depressive symptom severity (K-SADS) | 26.63 (6.79) | 25.08 (3.67) | .34 |
| Mother’s depressive symptoms (BDI-II) | 9.91 (10.41) | 4.95 (4.94) | .03 |
| Race | .45 | ||
| Black or African American | 9 % | 7 % | |
| Asian or Asian American | 0 % | 7 % | |
| White/Caucasian | 23 % | 40 % | |
| Hispanic/Latina | 32 % | 27 % | |
| Other | 5 % | 7 % | |
| Mixed/multiple | 32 % | 13 % |
Note. K-SADS, Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version; BDI-II, Beck Depression Inventory-II. BDI-II cutoffs are 0–13 (minimal depression), 14–19 (mild depression), 20–28 (moderate depression), and 29–63 (severe depression).
3.1. Self-reported depression and social disconnection following social evaluation
As expected, self-reported depressed mood (POMS-SF) increased from pre- to post-social evaluation Mdiff = 0.69, p = .05; moreover, this change significantly differed between groups, with high-risk female adolescents increasing in self-reported depressed mood (Mdiff = 1.77, SE = 0.62, p = .009) to a much greater degree than low-risk female adolescents (Mdiff = −0.10, SE = 0.34, p = .769), t(50) = 2.84, p = .007, 95% CIdiff: [0.55, 3.20] d = 0.80, BF10 = 6.71 (Fig. 2a). Similarly, self-reported social disconnection increased significantly from pre- to post-social evaluation across both groups, Mdiff = 2.74, p = .003. Unlike depressed mood, however, changes in social disconnection were only marginally greater for the high-risk group, with high-risk female adolescents increasing in self-reported social disconnection (Mdiff = 4.48, SE = 1.83, p = .02) to a marginally greater degree than low-risk female adolescents (Mdiff = 1.48, SE = 0.67, p = .04), t(48) = 1.72, p = .09 (p = .045, one-tailed), 95% CIdiff: [0.51, 6.50] d = 0.49, BF10 = 0.94 (Fig. 2b). When analyses were restricted to participants with usable fMRI data, relative to low-risk female adolescents, high-risk female adolescents showed significantly greater increases from pre- to post-evaluation in both self-reported feelings of depression, p = .008, and social disconnection, p = .037.
Fig. 2.
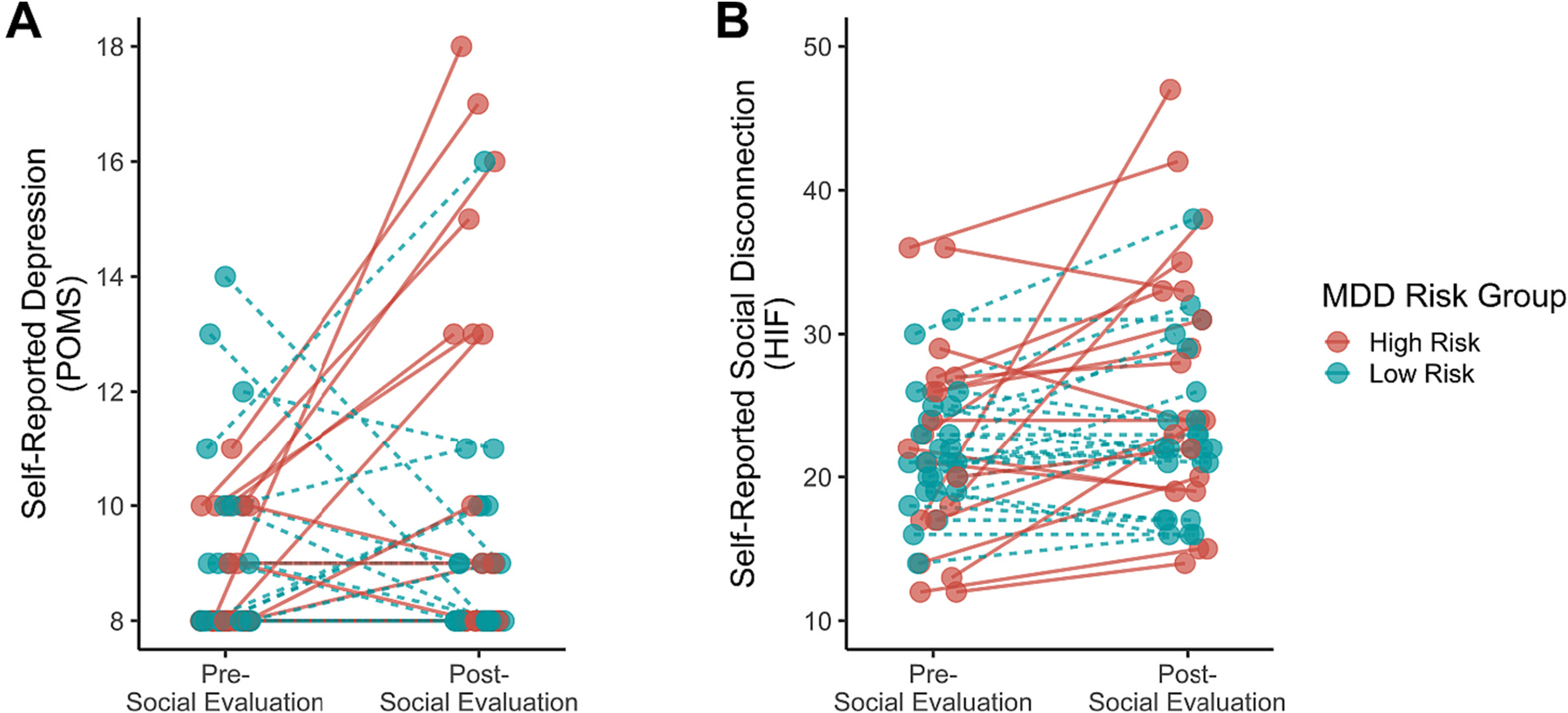
Changes in (A) self-reported depression and (B) self-reported feelings of social disconnection by depression risk group in response to being socially evaluated. Both self-reported depression and feelings of social disconnection increased from pre- to post-social evaluation. As expected, high-risk female adolescents exhibited significantly greater increases in self-reported depression and marginally greater increases in self-reported feelings of social disconnection than low-risk female adolescents.
3.2. Neural activation
We first tested for potential group differences in neural activity in ROIs that we hypothesized would distinguish high- and low-risk female adolescents—namely, the amygdala, anterior insula, ACC, and VMPFC (see Fig. 3)—while participants received negative (vs. neutral) social evaluation. As hypothesized, high-risk female adolescents (M = 0.43, SE = 0.23) exhibited greater activity in the amygdala than low-risk female adolescents (M = −0.34, SE = 0.14) when receiving negative (vs. neutral) social evaluation (Fig. 3a), t(43) = 3.03, p = .004, d = 0.91, BF10 = 9.87. Although high-risk female adolescents showed numerically greater activity in the anterior insula, ACC, or VMPFC in response to negative (vs. neutral) social evaluation as compared to low-risk female adolescents, these differences were not statistically significant, ps > .140 (see Fig. 3b–d).1
Fig. 3.
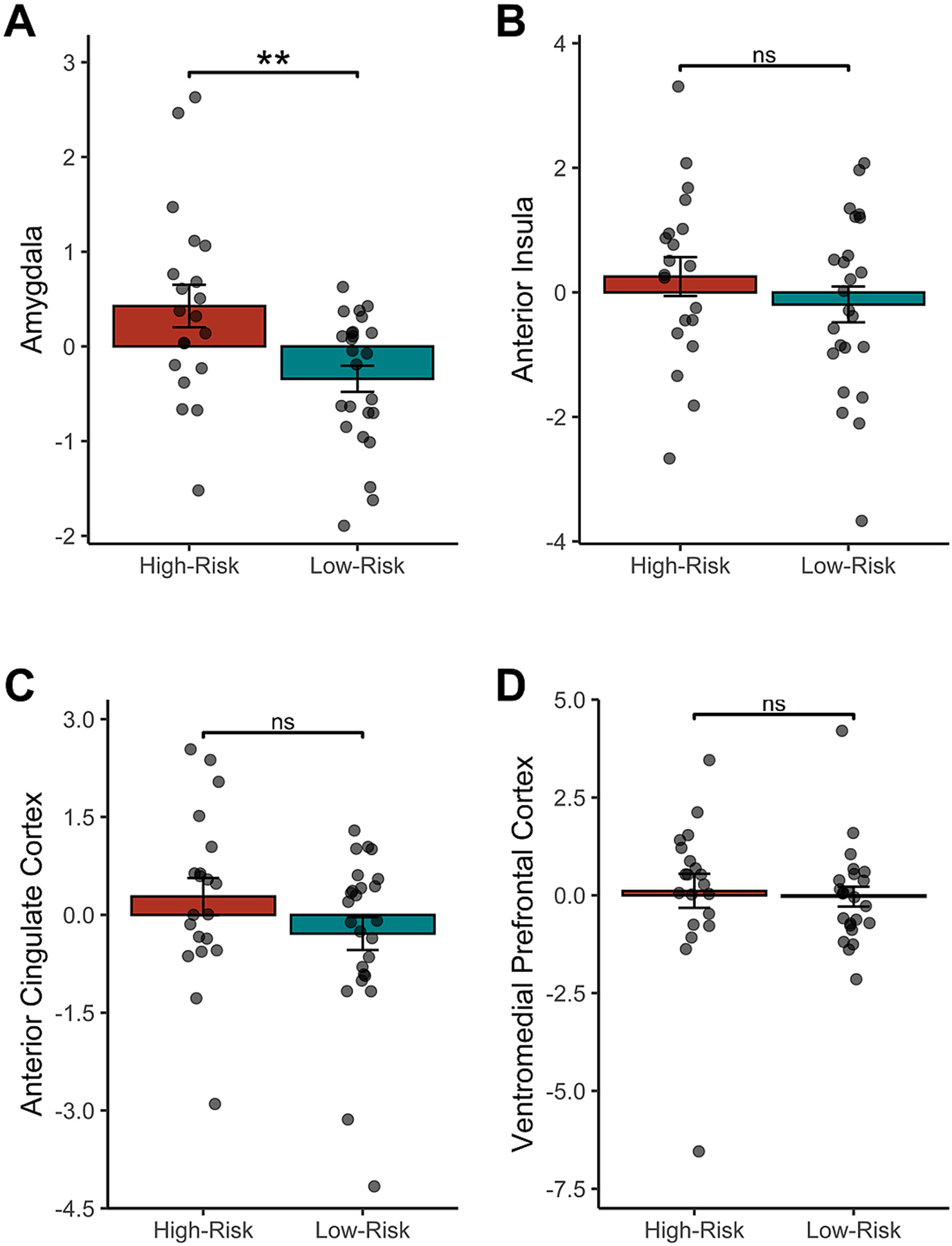
Neural responses to negative (vs. neutral) social evaluation in the a priori regions of interest. High-risk female adolescents exhibited numerically greater activity in all regions of interest (A-D) in response to receiving negative (vs. neutral) social evaluation, but these differences were statistically significant only for the (A) amygdala. Values were standardized to facilitate scaling comparison between the probabilistic weighted-sum regions of interest and the nonprobabilistic regions of interest. The lack of difference between groups in response to negative social evaluation in the ventromedial prefrontal cortex activity persisted after removal of the high-risk outlier.
3.3. Functional connectivity
3.3.1. ROI-to-ROI analyses
We next tested for potential group differences in neural connectivity between the ROIs described above (i.e., amygdala, anterior insula, ACC, and VMPFC). Although only the amygdala differed in activity between these two groups, we examined all four ROIs given the potential for them to exhibit differential coupling in activity over time between the two groups. We again focused on differences between the two diagnostic groups as a function of receiving negative (vs. neutral) social evaluation.
In these analyses, three ROI-to-ROI group differences in functional connectivity emerged. In particular, relative to the low-risk group, high-risk female adolescents exhibited: (a) greater connectivity between the anterior insula and ACC (M = 0.03, SE = 0.01; low-risk group M = 0.00, SE = 0.01), t(43) = 2.30, p = .03, 95 % CIdiff: [0.001, 0.07], d = 0.69, BF10 = 2.35; (b) greater connectivity between the anterior insula and VMPFC (M = 0.02, SE = 0.01; low-risk group M = −0.03, SE = 0.01), t (43) = 2.74, p = .009, 95 % CIdiff: [0.01, 0.08], d = 0.82, BF10 = 5.35; and (c) lesser connectivity between the VMPFC and amygdala (M = −0.04, SE = 0.01; low-risk group M = 0.01, SE = 0.02), t(43) = 2.07, p = .04, 95 % CIdiff: [0.001, 0.09], d = −0.62, BF10 = 1.60, while receiving negative (vs. neutral) social evaluation (Fig. 4).
Fig. 4.
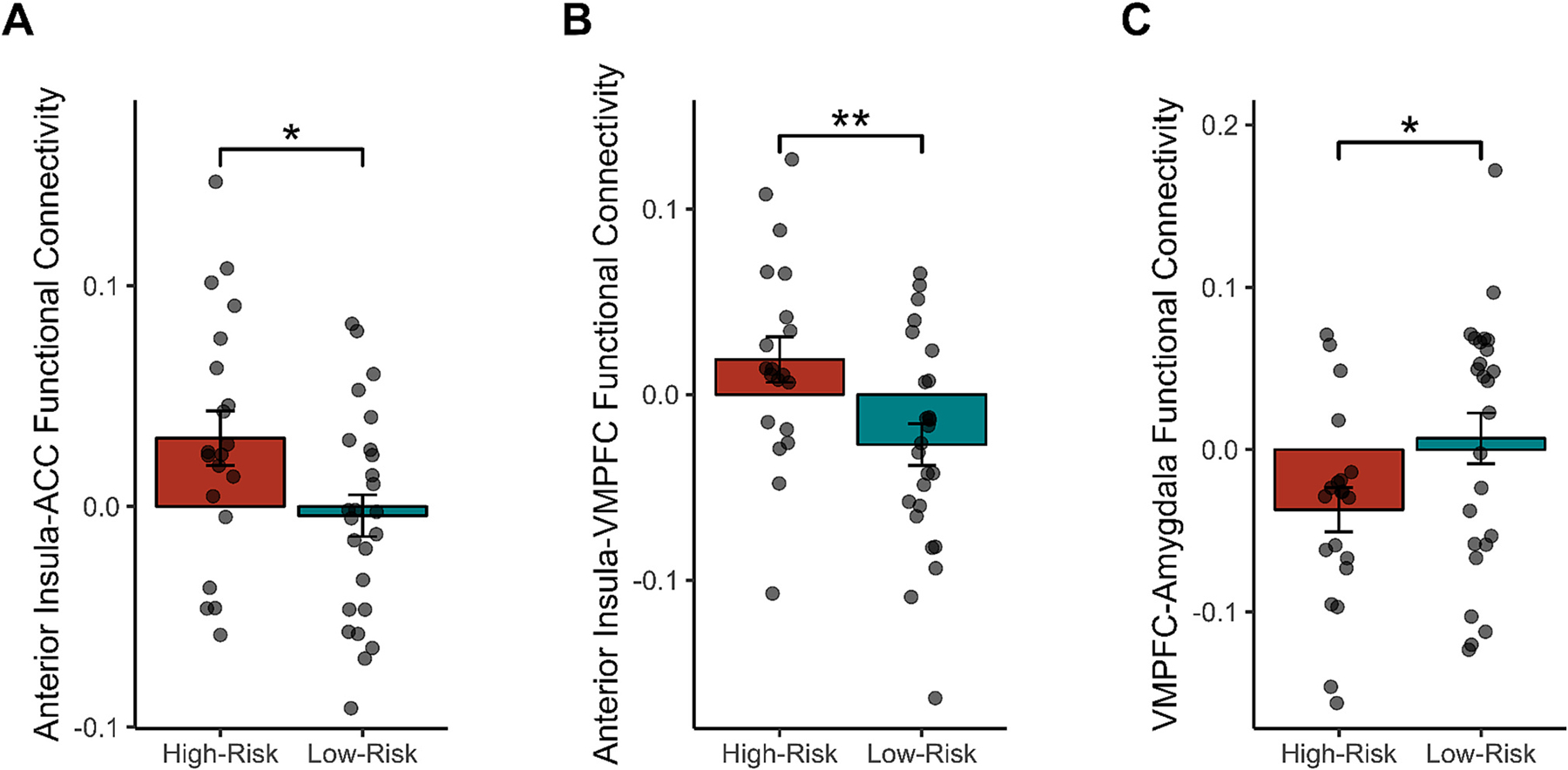
Functional connectivity in the a priori regions of interest that were significant during negative (vs. neutral) social evaluation. Specifically, as compared to low-risk female adolescents, high-risk female adolescents exhibited significantly greater functional connectivity between (A) the anterior cingulate cortex (ACC) and anterior insula, as well as (B) between the anterior insula and ventromedial prefrontal cortex (VMPFC). Additionally, high-risk female adolescents exhibited significantly less coupling between (C) the VMPFC and amygdala as compared to low-risk female adolescents.
3.3.2. Multivoxel pattern analysis
We next used MVPA to determine connectivity patterns in clusters of voxels across the whole brain that distinguished high- vs. low-risk female adolescents when contrasting neural responses to negative vs. neutral social evaluation. Six clusters of voxels survived corrections for multiple comparisons. In particular, clusters in the left precentral sulcus (peak coordinates: −14, −18, 64; pcluster < .001, ppeak < .001), left frontal pole (peak coordinates: −36, 44, 0; pcluster = .007, ppeak < .001), left superior frontal gyrus (SFG; peak coordinates: −18, 16, 68; pcluster = .016, ppeak < .001), left cerebellum (peak coordinates: −16, −64, −50; pcluster = .02, ppeak < .001), left ACC (peak coordinates: −12, 22, 28; pcluster = .020, ppeak < .001), and right frontal orbital cortex (peak coordinates: 14, 34, −22; pcluster = .023, ppeak < .001) distinguished high- and low-risk female adolescents in a negative vs. neutral social evaluation contrast. Low-risk female adolescents had higher values in each of these clusters than high-risk female adolescents, ds > 1.10.
3.4. Associations of neural activity and functional connectivity with depressive symptoms
3.4.1. Neural activity
We then examined how activity in each of the ROIs examined related to depressive symptoms using robust Poisson regressions. Less activity in the VMPFC while receiving negative (vs. neutral) social evaluation was related to more baseline depressive symptoms across all female adolescents, as assessed with the K-SADS, B = −0.32, Z = −4.73, p < .001. There was no association between activity in other regions and depressive symptoms, ps > .21, nor was there an association between activity in these regions and changes in feelings of social disconnection or depression from pre- to post-evaluation, ps > .440. Interestingly, female adolescents’ VMPFC activity while receiving negative (vs. neutral) social evaluation was also inversely related to their mothers’ baseline depressive symptoms as assessed with the BDI-II, B = −0.31, Z = −6.06, p < .001.
3.4.2. Functional connectivity
3.4.2.1. ROI-to-ROI values.
Because anterior insula-to-ACC, anterior insula-to-VMPFC, and VMPFC-to-amygdala functional connectivity differed between the high- and low-risk groups, we next investigated how these connectivity profiles (contrasting negative vs. neutral social evaluation) related to participants’ baseline depressive symptoms, as assessed by the K-SADS for female adolescents and BDI-II for their mothers. Two main associations emerged. First, anterior insula-VMPFC functional connectivity was positively associated with female adolescents’ baseline depressive symptoms as assessed by the K-SADS, B = 0.32, Z = 3.17, p = .002. Statistical mediation analysis showed that MDD risk group (high- vs. low-risk) was indirectly related to depressive symptoms via anterior insula-VMPFC functional connectivity, p = .02. Second, interestingly, female adolescents’ anterior insula-VMPFC functional connectivity values were positively related to their mothers’ baseline depressive symptoms, B = 0.21, Z = 3.16, p = .002. Mothers’ depressive symptoms did not mediate the association between daughters’ MDD risk group and daughters’ insula-VMPFC functional connectivity, p = .17.
None of these three functional connectivity variables were related to changes in feelings of social disconnection or depression from pre- to post-evaluation (ps > .095).
3.4.2.2. MVPA values.
Finally, two main associations emerged between MVPA cluster connectivity values and baseline depressive symptoms. Specifically, connectivity values in the left ACC cluster, B = −0.43, Z = −7.08, p < .001, and in the left superior frontal gyrus cluster, B = −0.50, Z = −8.94, p < .001, were significantly inversely associated with mothers’ depressive symptoms. Statistical mediation analysis showed that MDD risk group was indirectly related to connectivity values in both of these clusters through their mothers’ current depressive symptoms, ACC cluster p = .04, SFG cluster p = .005.
With respect to changes from pre- to post-evaluation, connectivity values in the left precentral sulcus cluster were associated with both changes in feelings of social disconnection (r = −.415, p = .005) and depression (r = −.477, p < .001), such that individuals with lower connectivity values showed greater increases in feelings of social disconnection and depression from pre- to post-evaluation. Statistical mediation analysis showed that MDD risk group was indirectly related to pre- to post-evaluation changes in feelings of depression (p = .04), but not social disconnection (p = .06), through precentral sulcus cluster connectivity. No other MVPA cluster that differentiated between groups was associated with changes in feelings of social disconnection or depression (ps > .148).
4. Discussion
A key prediction derived from Social Signal Transduction Theory of Depression is that individuals who are at heightened risk of depression should exhibit exaggerated neural responses to social threat, which may underlie the strong association observed between social rejection and depression in this group. In testing this hypothesis, we found that female adolescents at high risk of developing MDD exhibited greater increases in self-reported depressed mood and feelings of social disconnection in response to being socially evaluated than those at low risk of developing MDD. Additionally, we found that high-risk female adolescents exhibited greater amygdala activity while being socially evaluated than low-risk female adolescents. Moreover, high-risk female adolescents demonstrated relatively greater functional connectivity between the insula and both the ACC and VMPFC. In turn, MVPA revealed several functional connectivity clusters that distinguished between the high- vs. low-risk groups during viewing of negative (vs. neutral) adjectives, including a cluster with a peak in the ACC. Finally, many of these neural markers were associated with individual differences in both daughters’ and mothers’ depressive symptoms, the latter of which should be considered cautiously, as there are multiple plausible explanations for it (e.g., shared environment, genetics), but which is nonetheless consistent with intergenerational transmission of depression, among other interpretations. Considered together, these results provide converging evidence that never-depressed female adolescents at high risk of developing a first lifetime episode of MDD have potentiated neural responses to social rejection and, moreover, that these neural responses to social evaluation relate to depression severity in both the adolescents and their mothers.
The patterns of activity and functional connectivity that differed between high- and low-risk female adolescents may highlight neurobiological mechanisms that underlie the relatively greater social evaluation-induced increases in self-reported depression and social isolation observed for high- vs. low-risk female adolescents. For example, participants in the high-risk group exhibited greater signaling between the insula, which is involved in self-referential processing and a variety of other functions (Slavich et al., 2010a), and the ACC and VMPFC—regions implicated in multiple processes, including executive control (Sharp et al., 2010; Inzlicht et al., 2015)—when receiving negative (vs. neutral) social evaluation. Although we can only speculate about the psychological processes that coincide with this pattern of functional connectivity, this pattern is consistent with a greater perceived rejection of self and subsequent need to exert executive control over emotions in the high-risk group. Similarly, high-risk female adolescents exhibited less signaling between the VMPFC, which is critical for executive control over emotions (Lamm and Lewis, 2010; Wagner and Heatherton, 2013; Kerestes et al., 2014), and the amygdala, which—when coupled with the heightened amygdala activity evident in the high-risk female adolescents—is consistent with a possible decreased ability to regulate heightened neural threat signaling induced by social stress. However, we note that this interpretation is merely consistent with our data, not required by them. To infer psychological processes from these fMRI data would be reverse inference, and these theoretical interpretations are thus only plausible speculations.
Several limitations of this study should be noted. First, the sample size was limited, decreasing our ability to detect small effects. Second, although this was a diverse community sample, it was still a Western, Educated, Industrialized, Rich, and Democratic (WEIRD) sample, and replicating these results across cultures is needed (Henrich et al., 2010). Third, although all of the mothers of high-risk individuals had a verified history of MDD and none of the mothers of low-risk individuals had ever been depressed, all verified by SCID-IV interview, we cannot rule out the possibility that possible subclinical or subthreshold symptoms in the low-risk individuals’ mothers could have weakened the strength of the group difference. Fourth, although our sample size was large enough to reliably detect a similar effect observed in another small study, small studies—including ours—are likely to overestimate effect sizes (Silk et al., 2014). Future research with larger samples should thus attempt to determine if there exist any smaller differences between groups that we were unable to detect, as well as the extent to which the effect sizes that we observed might differ in magnitude. Finally, even though no daughters had a lifetime episode of MDD, without longitudinal brain and depression data, establishing the temporal precedence of these neural markers vis-à-vis increases in depressive symptoms was not possible.
Notwithstanding these limitations, the present data provide support for the Social Signal Transduction Theory of Depression by showing that adolescents at high risk of developing MDD, but who have not yet experienced depression, exhibit greater increases in social disconnection, increases in depressed mood, and amygdala reactivity to social evaluation than those at low risk for developing the disorder. Moreover, consistent with this theory, high-risk female adolescents exhibited a pattern of functional connectivity that, among other interpretations, is consistent with a greater perceived need to engage in emotion regulation but a lesser ability to successfully exert that emotion regulation during socially evaluative threat. Furthermore, these patterns of activity and functional connectivity related to both mothers’ and daughters’ depressive symptoms.
Although our data are correlational and thus cannot determine whether these markers play a mechanistic role in structuring risk for depression, these neural markers of social threat may be important. For example, if this neural reactivity does contribute to the development of depression as the Social Signal Transduction Theory of Depression predicts, then targeting this pattern of neural reactivity may represent a potentially promising target for interventions aimed at attenuating the link between social rejection and depression to help prevent first onsets of MDD in high-risk youth. Additional research is needed to investigate the generalizability of these findings and to assess how the neural differences documented here relate to the development of MDD over time.
Acknowledgments
We thank the mothers and daughters who participated in this study. We also thank the many students, trainees, and fellows who helped with various aspects of this work, including: Jacob Allely, Sammy Benavidez, Kaitlyn Breiner, Ashley Chipoletti, Kelly Costa, Desiree Delavary, Micah Dombroe, Kishan Ghadiya, Kirsten Gimse, Connie Ha, Marzia Hazara, Kean Hsu, Ashley Huynh, Mark Libowitz, Roman Liccini, Kristy Lin, Abigail Looi, Oria Mimi Lu, Kaivalya Molugu, Riya Mukhopadhyay, Rachel Ogata, Kelly Sun, Evelyn Valencia, Ruben Valentin, Kevin Walsh, and Hilary Wilson. Finally, we thank Keely Muscatell for providing the Social Evaluation Task and several centers at UCLA for supporting the study, including the Cousins Center for Psychoneuroimmunology, Staglin One Mind Center for Cognitive Neuroscience, UCLA Clinical and Translational Research Center (CTRC), UCLA Neuroscience Genomics Core, and Center for Pathology Research Services.
Funding
This study was supported by NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation and National Institutes of Health (NIH) grant K08 MH103443 to GMS. The UCLA CTRC is supported by NIH grant UL1 TR001881. These organizations had no role in planning, writing, editing, or reviewing this article, or in deciding to submit this article for publication.
Footnotes
CRediT authorship contribution statement
GSS, MV, TB, SS, MRI, and GMS all contributed to the design and conduct of this study, and helped prepare this article for submission. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare no conflicts of interest with regard to this work.
Controlling for either pubic hair development or breast development did not alter the main findings. In particular, the group difference in anterior cingulate activity increased in magnitude to marginal significance, β = −0.294, p = .058. The group difference in amygdala activity remained significant, β = −0.484, p = .001, and the group tests in insula activity, β = −0.224, p = .168, and in VMPFC activity, β = −0.034, p = .813, remained nonsignificant. The same was true of functional connectivity, in that no nonsignificant result became significant and no significant result became nonsignificant, although VMPFC to amygdala functional connectivity fell from being significant (p = .045) to marginal significance (p = .089).
Data availability
The data used for this study are available from the corresponding author on request.
References
- Angold A, Costello EJ, Worthman CM, 1998. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol. Med 28, 51–61. [DOI] [PubMed] [Google Scholar]
- Beck AT, Bredemeier K, 2016. A unified model of depression: integrating clinical, cognitive, biological, and evolutionary perspectives. Clin. Psychol. Sci 4, 596–619. [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory (BDI-II). The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bhanji J, Smith DV, Delgado M, 2019. A brief anatomical sketch of human ventromedial prefrontal cortex. In: PsyArxiv. 10.31234/osf.io/zdt7f. [DOI] [Google Scholar]
- Coutanche MN, Thompson-Schill SL, 2012. The advantage of brief fMRI acquisition runs for multi-voxel pattern detection across runs. Neuroimage 61, 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL, 1995. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol. Assess. 10.1037/1040-3590.7.1.80. [DOI] [Google Scholar]
- Dedovic K, Slavich GM, Muscatell KA, Irwin MR, Eisenberger NI, 2016. Dorsal anterior cingulate cortex responses to repeated social evaluative feedback in young women with and without a history of depression. Front. Behav. Neurosci 10 10.3389/fnbeh.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR, 2010. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav. Immun 24, 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Haltom KEB, Leary MR, 2011. The neural sociometer: brain mechanisms underlying state self-esteem. J. Cogn. Neurosci 23, 3448–3455. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, et al. , 2013. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med. 10 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First Spitzer, Gibbon Williams, Davies Borus J., et al. , 1995. The Structured Clinical Interview for DSM-IV Axis I Disorders-patient Edition. Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Gore FM, Bloem PJN, Patton GC, Ferguson J, Joseph V, Coffey C, et al. , 2011. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet 377, 2093–2102. [DOI] [PubMed] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C, Hiroto D, 1987. Children of depressed mothers: maternal strain and symptom predictors. J. Abnorm. Psychol 96, 190–198. [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A, 2010. Most people are not WEIRD. Nature 466, 29. [DOI] [PubMed] [Google Scholar]
- Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, et al. , 2014. Functional connectivity of negative emotional processing in adolescent depression. J. Affect. Disord 155, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB, 2015. Emotional foundations of cognitive control. Trends Cogn. Sci 19, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM, 2018. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 23, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. , 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ, 2014. Functional brain imaging studies of youth depression: a systematic review. NeuroImage Clin. 4, 209–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA, 2006. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch. Gen. Psychiatry 63, 484–489. [DOI] [PubMed] [Google Scholar]
- Lamm C, Lewis MD, 2010. Developmental change in the neurophysiological correlates of self-regulation in high- and low-emotion conditions. Dev. Neuropsychol 35, 156–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R, Isensee B, Höfler M, Pfister H, Wittchen HU, 2002. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch. Gen. Psychiatry 59, 365–374. [DOI] [PubMed] [Google Scholar]
- Lin B, Kaliush PR, Conradt E, Terrell S, Neff D, Allen AK, et al. , 2019. Intergenerational transmission of emotion dysregulation: part I. Psychopathology, self-injury, and parasympathetic responsivity among pregnant women. Dev. Psychopathol 31, 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Baram TZ, Rogers CE, Barch DM, 2020. Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci. 43, 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Mcnealy K, Pfeifer JH, Dapretto M, 2011. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev. Psychopathol 23, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Han B, 2016. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 138, e20161878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Silk JS, Woods BK, Forbes EE, 2019. Differential neural responding to affective stimuli in 6- to 8-year old children at high familial risk for depression: associations with behavioral reward seeking. J. Affect. Disord 257, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. , 2015. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun 43, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV, 2006. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci 10, 424–430. [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. , 2009. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun 23, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Kadosh KC, Lau JYF, 2013. The role of peer rejection in adolescent depression. Depress. Anxiety 30, 809–821. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M, 2004. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23, 752–763. [DOI] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY, 2017. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA, 2010. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. U. S. A 107, 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Slavich GM, 2017. Lifetime stress exposure and health: a review of contemporary assessment methods and biological mechanisms. Soc. Personal. Psychol. Compass 11. 10.1111/spc3.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Moons WG, Slavich GM, 2017. Inflammation, self-regulation, and health: an immunologic model of self-regulatory failure. Perspect. Psychol. Sci 12, 588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichko S, Bui T, Vinograd M, Shields GS, Saha K, Devkota S, et al. , 2021. Psychobiology of Stress and Adolescent Depression (PSY SAD) study: protocol overview for an fMRI-based multi-method investigation. Brain Behav. Immun. Health 100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE, 2014. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc. Cogn. Affect. Neurosci 9, 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Sequeira SS, Jones NP, Lee KH, Dahl RE, Forbes EE, et al. , 2022. Subgenual anterior cingulate cortex reactivity to rejection vs. acceptance predicts depressive symptoms among adolescents with an anxiety history. J. Clin. Child Adolesc. Psychol 10.1080/15374416.2021.2019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, 2015. Understanding inflammation, its regulation, and relevance for health: a top scientific and public priority. Brain Behav. Immun 45, 13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, 2020. Social safety theory: a biologically based evolutionary perspective on life stress, health, and behavior. Annu. Rev. Clin. Psychol 16, 265–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, 2022. Social safety theory: understanding social stress, disease risk, resilience, and behavior during the COVID-19 pandemic and beyond. Curr. Opin. Psychol 45 10.1016/j.copsyc.2022.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull 140, 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH, 2009. Targeted rejection predicts hastened onset of major depression. J. Soc. Clin. Psychol 28, 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME, 2010a. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev 35, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE, 2010b. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. U. S. A 107, 14817–14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PN, Cukrowicz KC, Poindexter EK, Hobson V, Cohen LM, 2010. The acquired capability for suicide: a comparison of suicide attempters, suicide ideators, and non-suicidal controls. Depress. Anxiety 27, 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JG, Shields GS, Esposito EC, Cosby EA, Allen NB, Slavich GM, Auerbach RP, 2019. Life stress and suicide in adolescents. J. Abnorm. Child Psychol 10.1007/s10802-019-00534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF, 2013. Self-regulatory depletion increases emotional reactivity in the amygdala. Soc. Cogn. Affect. Neurosci 8, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, et al. , 2016. Brain connectomics predict response to treatment in social anxiety disorder. Mol. Psychiatry 21, 680–685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study are available from the corresponding author on request.


