Abstract
Camptothecin and its analogues show important antitumor activity and have been used in clinical studies. However, hydrolysis of lactone in the E ring seriously attenuates the antitumor activity. To change this situation, aromathecin alkaloids are investigated in order to replace camptothecins. Potential antitumor activity has obtained more and more attention from organic and pharmaceutical chemists. As a member of the aromathecin alkaloids, rosettacin has been synthesized via different methods. This review summarizes recent advances in the synthesis of rosettacin.
Keywords: rosettacin, camptothecin, aromathecin, heterocycle, antitumor
1. Introduction
Compared with all-carbon cycles, heterocycles often own different physical and chemical properties due to the presence of heteroatoms [1,2,3,4,5,6,7]. Thus far, heterocycles are the largest branch of organic compounds. In addition, they are not only important in biology and industry but also have great significance in the operation of human society. Their involvement in multiple fields should be given more attention [1,2,3,4]. The main portion of commercial drugs based on mimicking biologically active natural products is heterocycles. The heterocyclic scaffold is widely present in natural products, drugs, bioactive molecules and functional materials [8,9,10]. In the Comprehensive Medicinal Chemistry database, more than 60% of the compounds possess heterocycles [11]. Therefore, researchers continuously design and produce drugs, insecticides, rodenticides and herbicides with better effects based on natural product models [1,2,3,4]. Heterocycles play an important role in biochemical processes and are also the most typical and important organic compounds in living cells [1,2,3,4]. Meanwhile, heterocycles play important roles in the metabolism of living cells [12,13,14]. Heterocycles have many applications in other fields, such as being used as additives in various industries involving photocopying, cosmetics, solvents, plastics, information storage and antioxidants [1,2,3,4]. Therefore, heterocyclic chemistry occupies a large proportion of organic chemistry. Moreover, heterocyclic chemistry could be ceaselessly employed to synthesize a wide variety of heterocycles, which is a never-ending resource [1,2,3,4]. The numerous combinations of heteroatoms, carbon and hydrogen could give diverse heterocycles bearing various physical, chemical and biological properties [1,2,3,4]. Thus, developing concise and efficient strategies for the construction of diverse heterocycles is highly desired. The synthesis of novel heterocyclic scaffolds has always been needed, providing the platform and opportunity for the discovery of new drugs.
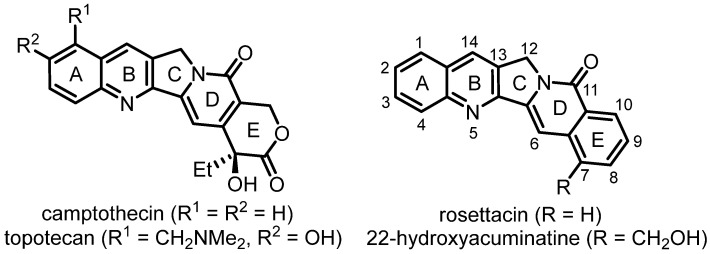
As an important member of heterocyclic scaffolds, indolizino [1,2-b]quinolin-9(11H)-one constitutes the central moiety of camptothecin (CPT) [15] and aromathecin alkaloids [16] (Scheme 1). CPT was first isolated by Wani and Wall in 1966 from the Chinese tree Camptotheca accuminata [17]. In their discovery, the extract exhibited significant anti-tumor activity in in vitro experiments and in mouse leukemia models [18,19]. This result was consistent with the utilization in traditional Chinese medicine as a natural medicine for treating cancer. Meanwhile, some adverse issues with CPT were also observed, such as poor stability and solubility [18,19]. Although the mechanism of action was not clear, CPT was soon approved by the US Food and Drug Administration (FDA) for preliminary clinical trials of colon carcinoma and evaluated as a drug for treating human cancer [18,19]. Although CPT exhibited strong anti-tumor activity in patients with gastric cancer, it also led to serious and unpredictable side-effects such as vomiting, bone marrow suppression, severe hemorrhagic cystic disease and diarrhea [18,19]. These results led to the suspension of the second phase trials in 1972. When further explorations showed that the cellular target of CPT is DNA topoisomerase 1, CPT attracted people’s attention again in the late 1980s [18,19]. Thus far, three compounds in this class (topotecan, belotecan and irinotecan) have been used for clinical treatment of cancer [20]. However, the susceptibility to hydrolysis of the lactone (E ring) generates a hydroxycarboxylate which is inactive and has high affinity for human serum albumin [21]. Thus, more attention has been paid to aromathecin alkaloids, in which the E ring is replaced by a benzene ring [22]. As a member of the aromathecin alkaloids, 22-hydroxyacuminatine is a novel quinoline alkaloid which was isolated along with CPT from the Chinese tree Camptotheca accuminata at an extremely low yield of 0.000006% in 1989 [23]. Further biological activity studies indicated that 22-hydroxyacuminatine showed significant cytotoxic activity against the murine leukemia P-388 cells (ED50 1.32 μg/mL) and KB (ED50 0.61 μg/mL) in vitro [23]. Based on these results, more attention has been paid to the efficient synthesis and biological activity investigations of 22-hydroxyacuminatine and its derivatives in order to obtain better anti-tumor activity. Moreover, rosettacin, belonging to the aromathecin alkaloids, has been employed as a CPT/luotonin A hybrid for inhibiting tumor growth by binding to topoisomerase I [24,25,26]. Initial trials showed that the degree of topoisomerase I-dependent DNA cleavage caused by rosettacin was about 50% of that in luotonin A, suggesting that rosettacin was a weak poison [24,25,26]. Rosettacin was also cytotoxic to yeast strains that did not have yeast topoisomerase I but occupied a plasmid containing the human topoisomerase I gene under the control of a galactose promoter [24,25,26]. However, the expression of human topoisomerase I in the yeast strain indeed reduced the cytotoxicity of rosettacin. Further studies showed that 14 substituted rosettacin derivatives owned better antiproliferative activity and anti-topoisomerase I activity than rosettacin, as well as better topoisomerase I inhibitory activity than 22-hydroxyacuminatine [24,25,26]. These derivatives were proposed to undergo the same mechanism with CPT via an intercalation and poisoning process. Aside from the increased solubility and localization to the DNA-enzyme complex, nitrogenous substituents on the 14 position of rosettacin were proposed to be involved in the major groove of the topoisomerase I-DNA complex and possess hydrogen bonds with the amino acids in the major groove, thus stabilizing the ternary complex [24,25,26]. Considering the significant bioactive activity and unique structure bearing a benzo[6,7]indolizino [1,2-b]quinolin-11(13H)-one core, different strategies have been designed to synthesize rosettacin. These methods not only provide concise and efficient ways to synthesize rosettacin but also give some thoughts on the synthesis of CPT and its analogues as well as other aromathecin alkaloids. This review is focused on the synthesis of rosettacin (Table 1), which is opportune and essential for the rapid growth of this area. This review is organized as a timeline, giving a clear insight for readers to understand the synthesis history of rosettacin.
Scheme 1.
Representative camptothecin and aromathecin alkaloids.
Table 1.
A summary on the synthesis of rosettacin.
| Year | Author | Key Step | Formed Ring | Reference |
|---|---|---|---|---|
| 1972 | Warneke and Winterfeldt | Oxidative rearrangement | B and C | [27] |
| 1980 | Walraven and Pandit | Aminolysis and aldol condensation | D | [28] |
| 2003 | Cushman | Aminolysis and aldol condensation | D | [29] |
| 2008 | Daïch | N-Amidoacylation/aldol condensation | D | [30] |
| 2015 | Daïch | Aryl radical cyclization | C | [31] |
| 2012 | Park | Rh(III)-catalyzed C-H activation | D | [32] |
| 2017 | Glorius | Co(III)-catalyzed C-H activation | D | [33] |
| 2016 | Gao | exo Hydroamination and lactamization | C and D | [34] |
| 2017 | Van der Eycken | Rh(III)-catalyzed C-H activation | C and D | [35] |
| 2018 | Reddy | Rh(III)-catalyzed C-H activation | C and D | [36] |
| 2018 | Van der Eycken | Rh(III)-catalyzed C-H activation | C and D | [37] |
| 2019 | Van der Eycken | Rh(III)-catalyzed C-H activation | C and D | [38] |
| 2018 | Evano | Cu-catalyzed photoinduced radical domino cyclization | C and D | [39] |
| 2018 | Fu and Huang | Carbene-catalyzed aerobic oxidation and Pd-catalyzed cyclization | C | [40] |
| 2023 | Choshi | Thermal cyclization and Reissert–Henze-type reaction | D | [41] |
2. Synthesis of Rosettacin
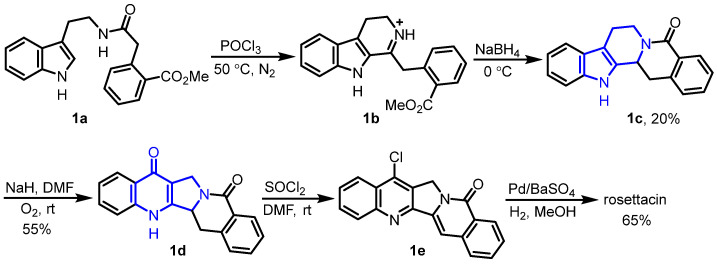
In 1972, Warneke and Winterfeldt reported an oxidative rearrangement from indole to quinolone, which was used as the key step for the synthesis of rosettacin [27] (Scheme 2). Firstly, in the presence of POCl3, amide 1a underwent cyclization to form iminium 1b, followed by sequential NaBH4 reduction and intramolecular amidation to give lactam 1c. By using NaH under O2, lactam 1c underwent oxidative rearrangement, resulting in quinolone 1d. Finally, quinolone 1d underwent sequential chlorination using SOCl2, followed by aromatization and dechlorination using Pd/BaSO4 and H2, leading to rosettacin. This approach provided interesting structural skeleton editing for the formation of B and C rings, while the total yield of rosettacin was quite low.
Scheme 2.
Oxidative rearrangement from indole to quinolone as the key step for the synthesis of rosettacin. Created by us and based on the original work.
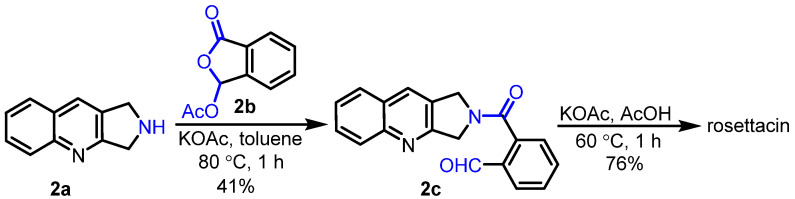
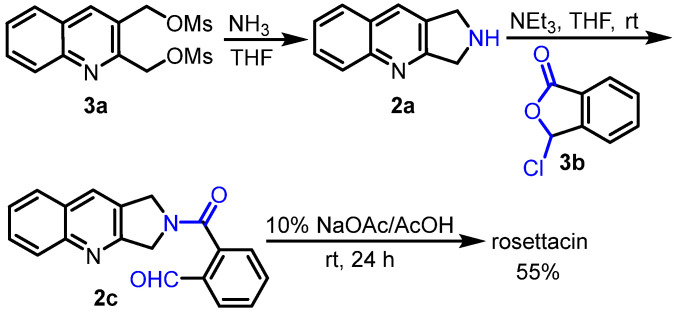
In 1980, Walraven and Pandit reported the synthesis of rosettacin [28] (Scheme 3) by learning from Corey’s strategy for the total synthesis of (S)-CPT [42]. Dihydropyrroloquinoline 2a reacted with pseudo-anhydride 2b under KOAc to give amide 2c, followed by aldol-type condensation in the presence of KOAc and AcOH to deliver rosettacin. Later on, Cushman et al. employed a similar strategy to synthesize rosettacin [29] (Scheme 4). Dimesylate 3a reacted with liquid ammonia to afford dihydropyrroloquinoline 2a, followed by aminolysis of pseudo-anhydride 3b to form amide 2c. Under 10% NaOAc/AcOH, intramolecular cyclization of 2c occurred to produce rosettacin.
Scheme 3.
Synthesis of rosettacin from Walraven and Pandit. Created by us and based on the original work.
Scheme 4.
Synthesis of rosettacin from Cushman. Created by us and based on the original work.
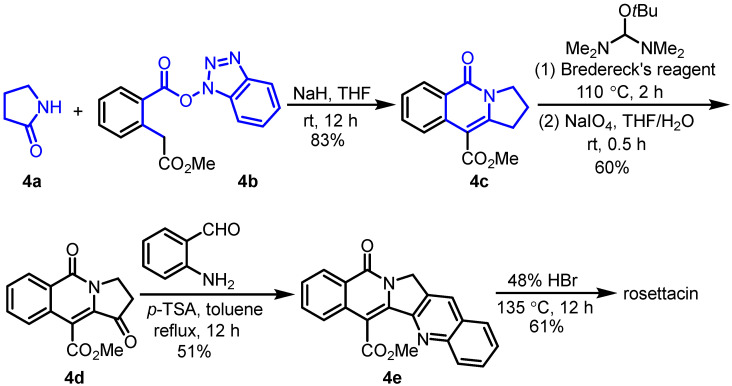
In 2008, Daïch et al. disclosed a form of domino N-amidoacylation/aldol-type condensation to generate poly-N-heterocycles, which was employed as the key step for the synthesis of rosettacin [30] (Scheme 5). Firstly, lactam 4a reacted with HOBt ester 4b in the presence of NaH, resulting in tricyclic product 4c. When subjected to Bredereck’s reagent [43] at 110 ℃ followed by oxidation using NaIO4, keto derivative 4d was formed. Sequential condensation with 2-aminobenzaldehyde in the presence of p-TSA yielded pentacyclic product 4e according to the Friedländer reaction [44]. Final removal of the ester group upon treatment with 48% HBr at 135 ℃ delivered rosettacin. Although this route was not long, the removal of the ester group required concentrated HBr.
Scheme 5.
Domino N-amidoacylation/aldol-type condensation as the key step for the synthesis of rosettacin. Created by us and based on the original work.
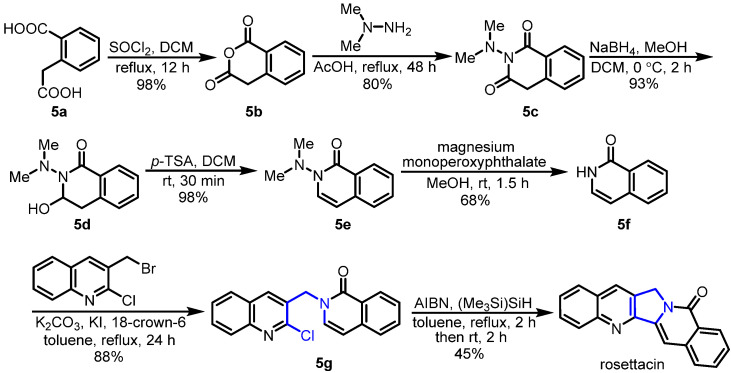
Subsequently, the same group reported another route for the synthesis of rosettacin by using an aryl radical cyclization of enamide as the key ring-closing step [31] (Scheme 6). Firstly, homophthalic acid 5a reacted with SOCl2 under reflux to generate homophthalic anhydride 5b. Subsequent treatment with N,N-dimethylhydrazine in AcOH under reflux resulted in product 5c. This was followed by NaBH4 reduction to afford α-hydroxylactam 5d. In the presence of p-TSA, 5d underwent dehydration to produce enamide 5e. Treatment with magnesium monoperoxyphthalate [45] delivered isoquinolin-1(2H)-one 5f. Under phase transfer conditions using K2CO3, KI and 18-crown-6, N-alkylation of 5f through a reaction with 3-(bromomethyl)-2-chloroquinoline was achieved, affording product 5g. Sequential treatment with AIBN and (Me3Si)3SiH via radical cyclization delivered rosettacin. This route required a multistep sequence to isoquinolinone.
Scheme 6.
Aryl radical cyclization of enamide as the key ring-closing step for the synthesis of rosettacin. Created by us and based on the original work.
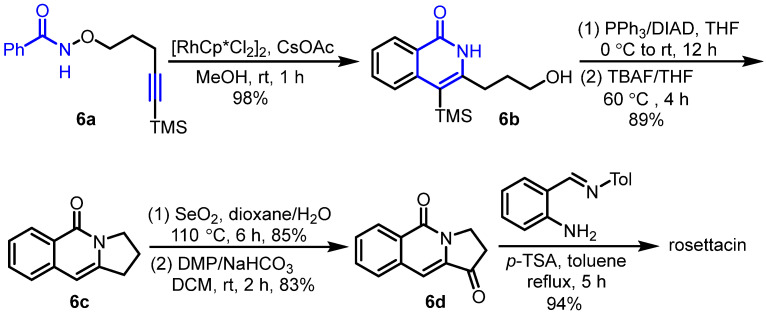
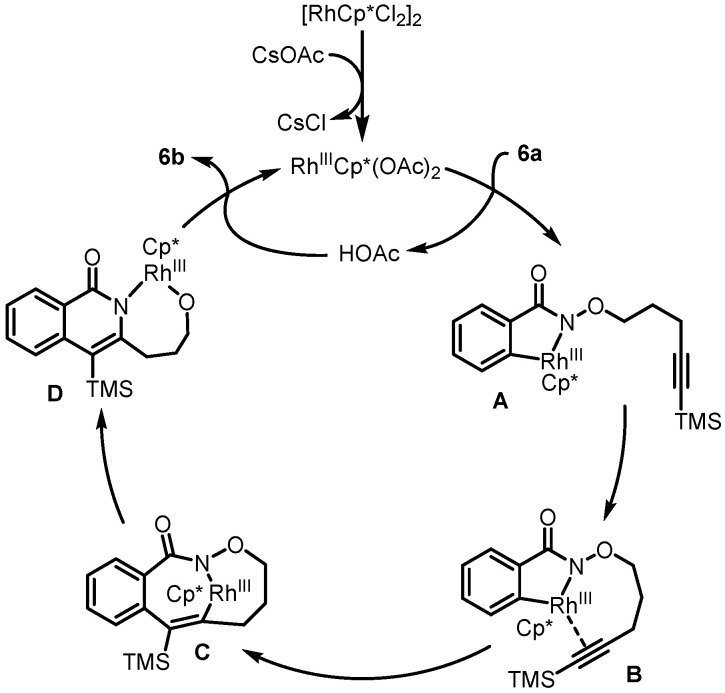
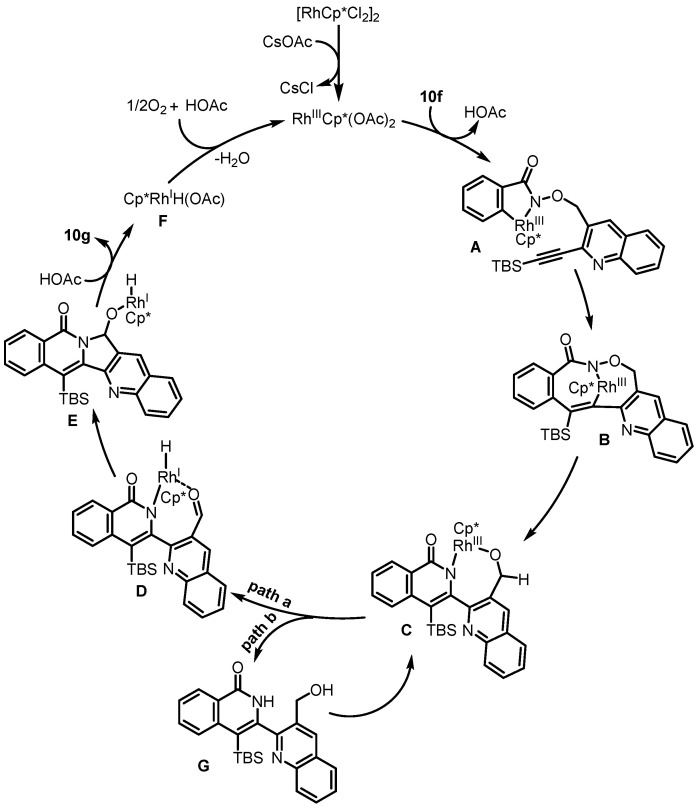
In 2012, Park et al. presented Rh(III)-catalyzed intramolecular C-H activation and annulation of alkyne-tethered hydroxamic esters for the construction of 3-hydroxyalkyl isoquinolones, which served as the key step for the synthesis of rosettacin [32] (Scheme 7). First, hydroxamic ester 6a bearing a TMS-protected alkyne underwent Rh(III)-catalyzed intramolecular C-H activation and annulation to produce isoquinolone 6b. This was followed by a Mitsunobu reaction using a PPh3/DIAD system and subsequent removal of the TMS group using TBAF to give benzoindolizidine 6c. Sequential oxidation using SeO2 and DMP formed ketone 6d, which further reacted with N-(2-aminobenzylydene)-p-toluidine in the presence of p-TSA to deliver rosettacin. This method did not tolerate substrates bearing a terminal alkyne, requiring an additional step to remove the TMS group. The mechanism of the generation of product 6b from substrate 6a was proposed by the authors (Scheme 8). The dimer [RhCp*Cl2]2 undergoes the cleavage of the Rh-Cl bond by using CsOAc to afford an active catalyst RhIIICp*(OAc)2. An irreversible C-H bond activation of substrate 6a catalyzed by the Cp*RhIII complex occurs to give a five-member rhodacycle species A with the concomitant formation of HOAc. Subsequent coordination of alkyne with the RhIII center produces intermediate B. Intermediate B undergoes alkyne insertion into the Rh-C bond to afford a seven-member rhodacycle C. Sequential reductive elimination and oxidative addition into the N-O bond forms intermediate D. Final protonation by HOAc delivers product 6b and regenerates the active catalyst RhIIICp*(OAc)2 for the next cycle.
Scheme 7.
Rh(III)-catalyzed intramolecular C-H activation and annulation of alkyne-tethered hydroxamic ester as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 8.
Proposed mechanism for the construction of 3-hydroxyalkyl isoquinolone 6b from alkyne-tethered hydroxamic ester 6a via Rh(III)-catalyzed intramolecular C-H activation and annulation. Created by us and based on the original work.
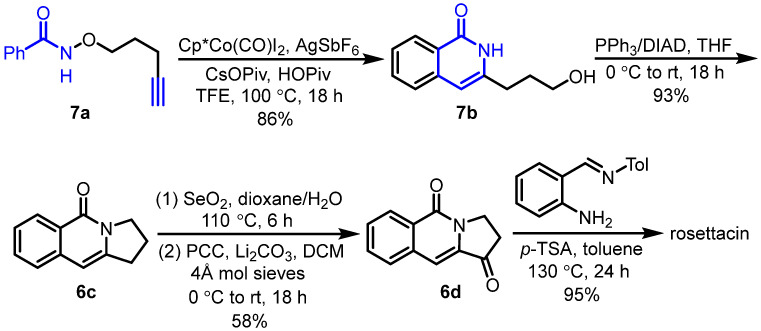
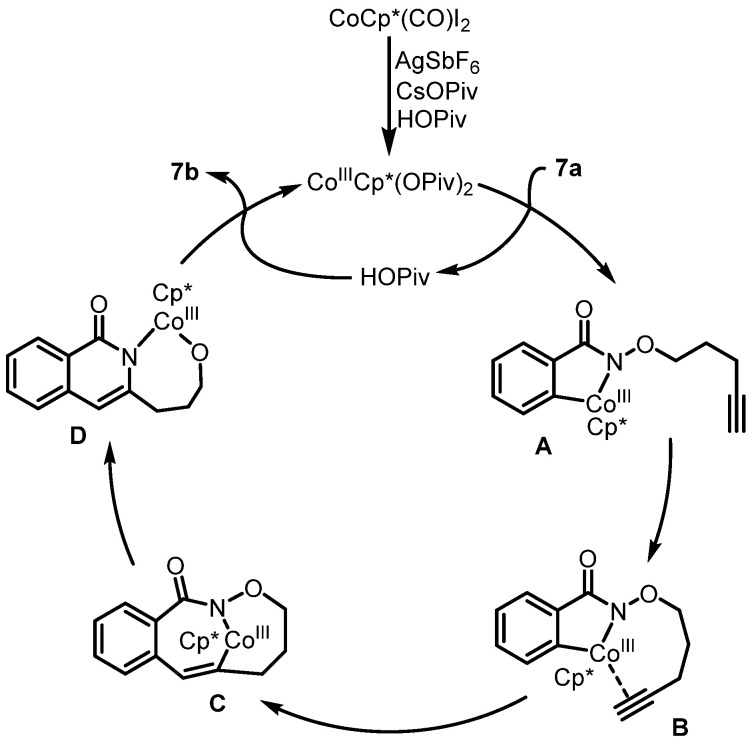
Along the same line, Glorius et al. developed Cp*Co(III)-catalyzed intramolecular C-H activation and annulation of alkyne-tethered hydroxamic esters for the construction of 3-hydroxyalkyl isoquinolones, which was used as the key step for the synthesis of rosettacin [33] (Scheme 9). First, hydroxamic ester 7a bearing a terminal alkyne underwent Cp*Co(III)-catalyzed intramolecular C-H activation and annulation to give isoquinolone 7b. This was followed by a Mitsunobu reaction using a PPh3/DIAD system and sequential oxidation, using SeO2 and PCC to generate ketone 6d. Subsequent treatment with N-(2-aminobenzylydene)-p-toluidine in the presence of p-TSA produced rosettacin. Compared with Rh(III) catalysis, this method not only provided cheap and earth-abundant Co(III) catalysis but also tolerated substrates bearing a terminal alkyne. The mechanism of the generation of product 7b from substrate 7a was proposed by the authors (Scheme 10). CoCp*(CO)I2 undergoes dehalogenation with silver salt to give the active catalyst CoIIICp*(OPiv)2. Subsequent C-H bond cleavage of substrate 7a followed by metalation catalyzed by the Cp*CoIII complex generates a five-member cobaltacycle A with the concomitant formation of HOPiv. The coordination between alkyne and the CoIII center occurs to form intermediate B, followed by alkyne insertion into the Co-C bond to produce a seven-member cobaltacycle C. Subsequent reductive elimination forms a C-N bond followed by oxidative addition into the N-O bond, which affords intermediate D. After protodemetalation of intermediate D, product 7b is released, and the active catalyst CoIIICp*(OPiv)2 is regenerated for the next cycle.
Scheme 9.
Cp*Co(III)-catalyzed intramolecular C-H activation and annulation of alkyne-tethered hydroxamic ester as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 10.
Proposed mechanism for the construction of 3-hydroxyalkyl isoquinolone 7b from alkyne-tethered hydroxamic ester 7a via Co(III)-catalyzed intramolecular C-H activation and annulation. Created by us and based on the original work.
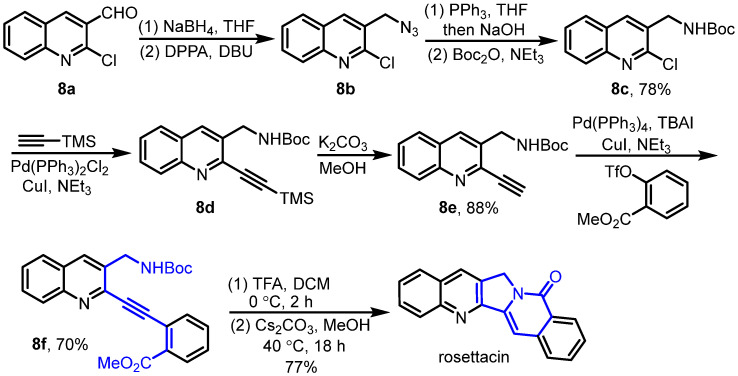
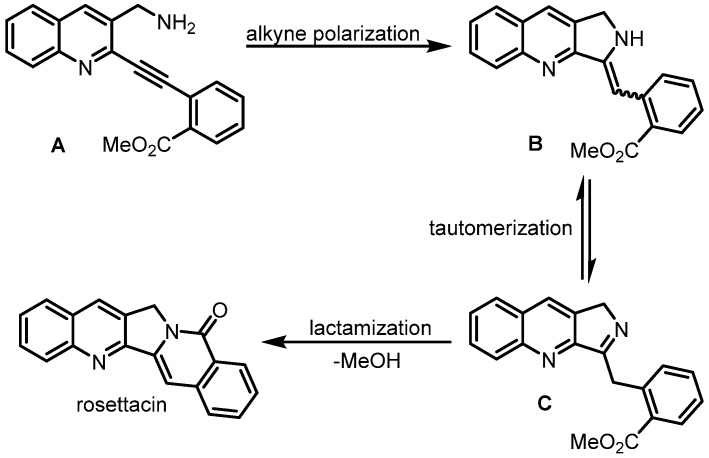
In 2016, Gao et al. developed cascade exo hydroamination followed by spontaneous lactamization, which was used as the key step for the synthesis of rosettacin [34] (Scheme 11). Here, 2-Chloroquinoline-3-carbaldehyde 8a underwent NaBH4 reduction, and subsequent azidation using DPPA and DBU yielded azide 8b. Sequential treatment with PPh3 and NaOH formed the primary amine, which reacted with Boc2O to afford Boc-protected product 8c. Subsequent palladium-catalyzed Sonogashira coupling with TMS-protected alkyne and the removal of the TMS group using K2CO3 generated terminal alkyne 8e, which underwent a second palladium-catalyzed Sonogashira coupling with ortho ester-substituted trifluoromethanesulfonate to give alkyne 8f. Treatment with TFA formed the primary amine, followed by adding Cs2CO3 to deliver rosettacin via exo hydroamination and sequential spontaneous lactamization. Although C and D rings could be formed in one step in this route, the substrate required multistep synthesis. The mechanism of the generation of rosettacin from compound 8f was proposed by the authors (Scheme 12). Compound 8f firstly undergoes treatment with TFA to give the primary amine A. The alkyne in the primary amine A is effectively polarized and activated by the ortho ester-substituted phenyl group, enabling the 5-exo cyclization to form a C-N bond via the primary amine’s addition to a triple bond under basic conditions. This process forms the enamine adduct B with a Z or E geometry, in which the enamine bearing the Z geometry favors intramolecular lactamization to deliver rosettacin. For the enamine bearing the E geometry, E/Z isomerization may occur via the formation of imine intermediate C to complete the intramolecular lactamization, resulting in the generation of rosettacin.
Scheme 11.
Cascade exo hydroamination followed by spontaneous lactamization as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 12.
Proposed mechanism for cascade exo hydroamination followed by spontaneous lactamization. Created by us and based on the original work.
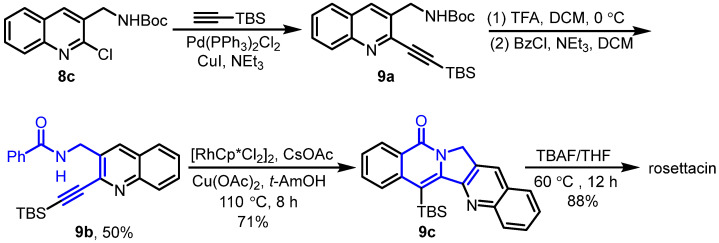
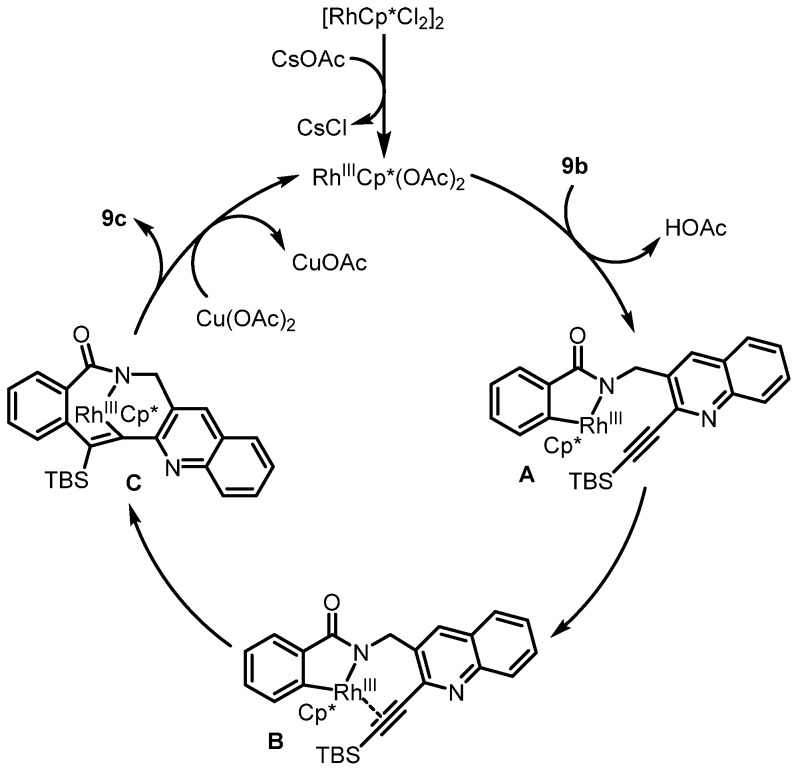
In 2017, Van der Eycken et al. reported the Rh(III)-catalyzed intramolecular C-H activation and annulation of alkyne-tethered benzamides for the construction of poly-N-heterocycles, which served as the key step for the synthesis of rosettacin [35] (Scheme 13). Quinoline 8c underwent palladium-catalyzed Sonogashira coupling with TBS-protected alkyne. This was followed by removal of the Boc group with TFA treatment. Sequential amidation with benzoyl chloride gave amide 9b. Rh(III)-catalyzed intramolecular C-H activation and annulation of the amide 9b afforded pentacyclic product 9c, followed by removal of the TBS group under TBAF to deliver rosettacin. This method did not tolerate substrates bearing a terminal alkyne, requiring an additional step to remove the TBS group. The mechanism of the generation of compound 9c from compound 9b was proposed by the authors (Scheme 14). The dimer [RhCp*Cl2]2 undergoes cleavage of the Rh-Cl bond by using CsOAc to afford the active catalyst RhIIICp*(OAc)2. Irreversible C-H bond cleavage of compound 9b catalyzed by the Cp*RhIII complex occurs to give five-member rhodacycle species A, with the concomitant formation of HOAc. Subsequent coordination of alkyne with the RhIII center produces intermediate B. Intermediate B undergoes alkyne insertion into the Rh-C bond to afford seven-member rhodacycle C. Sequential reductive elimination delivers product 9c, followed by regeneration of the active catalyst RhIIICp*(OAc)2 from the oxidation of Cp*RhI by Cu(OAc)2.
Scheme 13.
Rh(III)-catalyzed intramolecular C-H activation and annulation of alkyne-tethered benzamide as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 14.
Proposed mechanism for the construction of poly-N-heterocycle 9c from alkyne-tethered benzamide 9b via Rh(III)-catalyzed intramolecular C-H activation and annulation. Created by us and based on the original work.
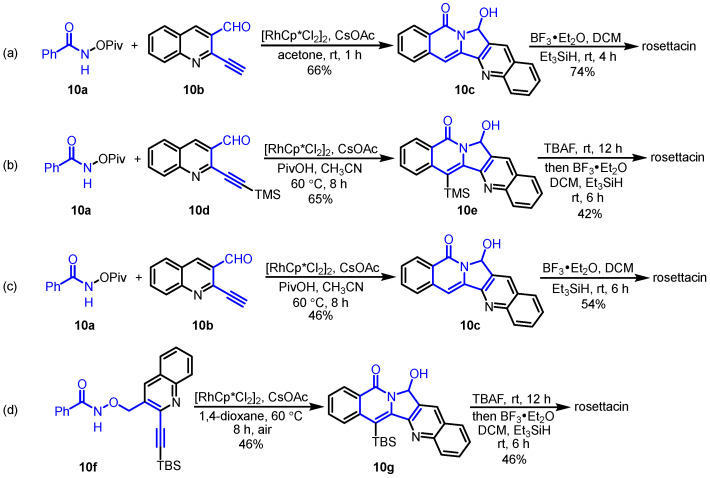
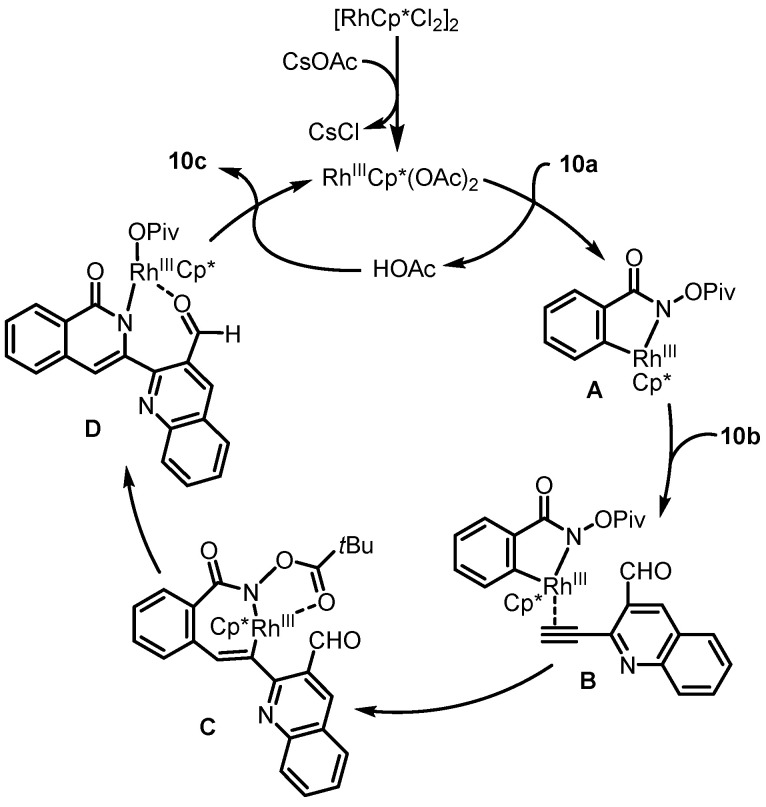
In 2018, Reddy and Mallesh disclosed the Rh(III)-catalyzed intermolecular C-H activation and annulation of N-(pivaloyloxy)benzamides and 2-alkynyl aldehydes for the construction of isoindolo [2,1-b]isoquinolin-5(7H)-one, which served as the key step for the synthesis of rosettacin [36] (Scheme 15a). Rh(III)-catalyzed intermolecular C-H activation and annulation of N-(pivaloyloxy)benzamide 10a and 2-alkynyl quinoline-3-carbaldehyde 10b was performed to yield pentacyclic product 10c. This was followed by the reduction of aminal using BF3•Et2O/Et3SiH to deliver rosettacin. In the same year, Van der Eycken et al. reported a similar form of Rh(III)-catalyzed intermolecular C-H activation and annulation for the synthesis of rosettacin [37] (Scheme 15b,c). Compared with intramolecular versions, the intermolecular methods avoided multistep sequences to synthesize the substrates, providing a concise and efficient approach to rosettacin. The mechanism of the generation of compound 10c from substrates 10a and 10b was proposed by the authors (Scheme 16). The dimer [RhCp*Cl2]2 undergoes dehalogenation with CsOAc to yield the active catalyst RhIIICp*(OAc)2. Subsequent C-H bond cleavage of substrate 10a followed by metalation catalyzed by the Cp*RhIII complex generates five-member rhodacycle species A with the concomitant formation of HOAc. Coordination between the alkyne in substrate 10b and the RhIII center in intermediate A occurs to form intermediate B, followed by alkyne insertion into the Rh-C bond to produce seven-member rhodacycle C. Subsequent reductive elimination forming a C-N bond is followed by oxidative addition into the N-O bond, which affords intermediate D. After adol-type addition and protonation with HOAc, product 10c is released, and the active catalyst RhIIICp*(OAc)2 is regenerated for the next cycle.
Scheme 15.
Rh(III)-catalyzed intermolecular C-H activation and annulation of N-(pivaloyloxy)benzamide and 2-alkynyl aldehyde as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 16.
Proposed mechanism for the construction of isoindolo [2,1-b]isoquinolin-5(7H)-one 10c from N-(pivaloyloxy)benzamide 10a and 2-alkynyl aldehyde 10b via Rh(III)-catalyzed intermolecular C-H activation and annulation. Created by us and based on the original work.
Based on the above work, Van der Eycken et al. presented Rh(III)-catalyzed sequential C(sp2)-H activation and C(sp3)-H amination of alkyne-tethered hydroxamic esters, which were employed as the key steps for the synthesis of rosettacin [38] (Scheme 15d). First, alkyne-tethered hydroxamic ester 10f underwent intramolecular C-H activation and annulation via a rhodium hydride intermediate, resulting in pentacyclic product 10g, followed by treatment with BF3•Et2O/Et3SiH, at which point rosettacin was formed. This method did not tolerate substrates bearing a terminal alkyne, requiring an additional step to remove the TBS group. Additionally, the alkyne-tethered hydroxamic esters required multistep sequences to prepare. The mechanism of the generation of compound 10g from substrate 10f was proposed by the authors (Scheme 17). The dimer [RhCp*Cl2]2 undergoes cleavage of the Rh-Cl bond by using CsOAc to afford the active catalyst RhIIICp*(OAc)2. Irreversible C-H bond cleavage of substrate 10f catalyzed by the Cp*RhIII complex occurs to give five-member rhodacycle species A, with concomitant formation of HOAc. Subsequent coordination of alkyne with the RhIII center and alkyne insertion into the Rh-C bond produces seven-member rhodacycle B. Sequential reductive elimination and oxidative addition into the N-O bond forms intermediate C. Then, two possible pathways are involved. For path a, intermediate C undergoes β-H elimination to yield Cp*RhI species D, followed by adol-type addition of the amide to the aldehyde, forming intermediate E. Sequential protonation with HOAc delivers product 10g and produces Rh-H intermediate F. Through an AcOH-O2-assisted H2O formation process, the active catalyst RhIIICp*(OAc)2 can be regenerated from intermediate F for the next cycle. For path b, intermediate C can undergo protonation to yield intermediate G, which would undergo deprotonation to generate intermediate C again, and then follow path a to deliver product 10g.
Scheme 17.
Proposed mechanism for the construction of isoindolo [2,1-b]isoquinolin-5(7H)-one 10g from alkyne-tethered hydroxamic ester 10f via Rh(III)-catalyzed sequential C(sp2)-H activation and C(sp3)-H amination. Created by us based on the original work.
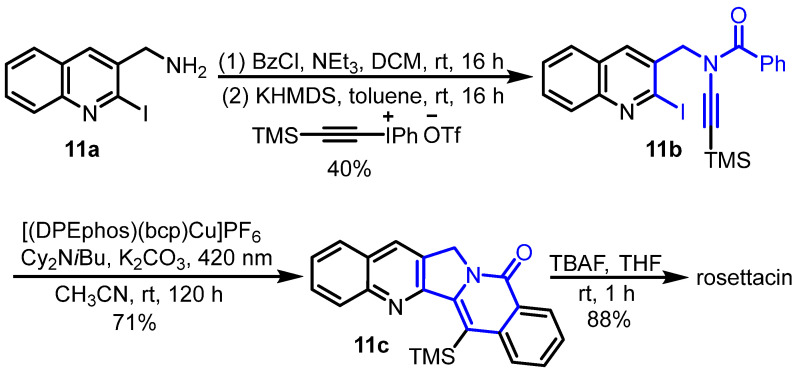
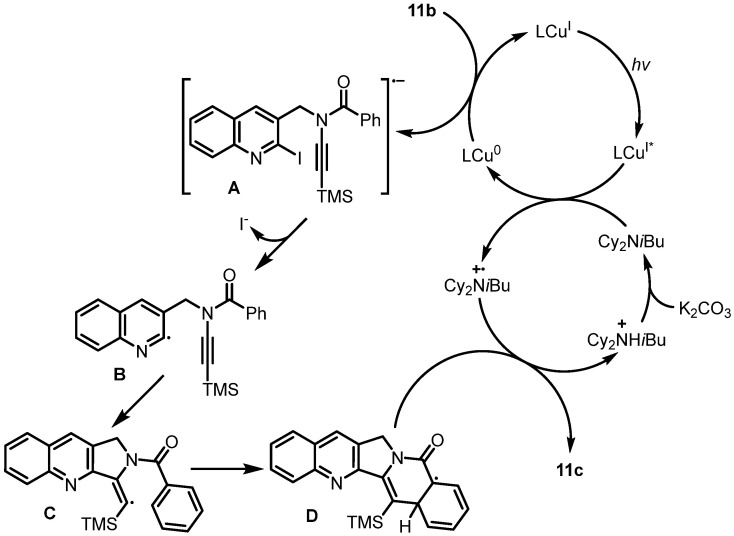
Additionally, Evano et al. reported the copper-catalyzed photoinduced radical domino cyclization of ynamides for the construction of poly-N-heterocycles, which was used as the key step for the synthesis of rosettacin [39] (Scheme 18). Here, 2-Iodo-3-aminomethylquinoline 11a underwent benzoylation with benzoyl chloride. Subsequent alkynylation using Witulski’s method [46] yielded N-benzoylynamine 11b. Subsequent copper-catalyzed photoinduced radical cyclization generated pentacyclic product 11c, followed by the removal of the TMS group under TBAF to deliver rosettacin. In this route, copper-catalyzed photoinduced cyclization provided a mild and green method to construct the C and D rings. The mechanism of the generation of compound 11c from compound 11b was proposed by the authors (Scheme 19). The ground state LCuI photocatalyst is excited by visible light, followed by single-electron transfer (SET) with the tertiary amine to yield LCu0 species and amine radical cation. The subsequent SET between compound 11b and LCu0 affords radical anion A and regenerates the ground state LCuI for the next cycle. After deiodination of intermediate A, radical intermediate B is formed, followed by radical addition to the triple bond via 5-exo-dig cyclization, at which point vinylic radical intermediate C is generated. Intermediate C undergoes radical cyclization to produce intermediate D via a 6-endo-trig process, followed by aromatization via hydrogen atom transfer (HAT) with amine radical cation to deliver product 11c and amine salt. Under K2CO3, the tertiary amine is regenerated from the amine salt for the next cycle.
Scheme 18.
Copper-catalyzed photoinduced radical domino cyclization of ynamide as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 19.
Proposed mechanism for the construction of poly-N-heterocycle 11c from ynamide 11b via copper-catalyzed photoinduced radical domino cyclization. Created by us and based on the original work.
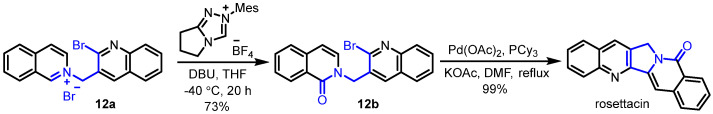
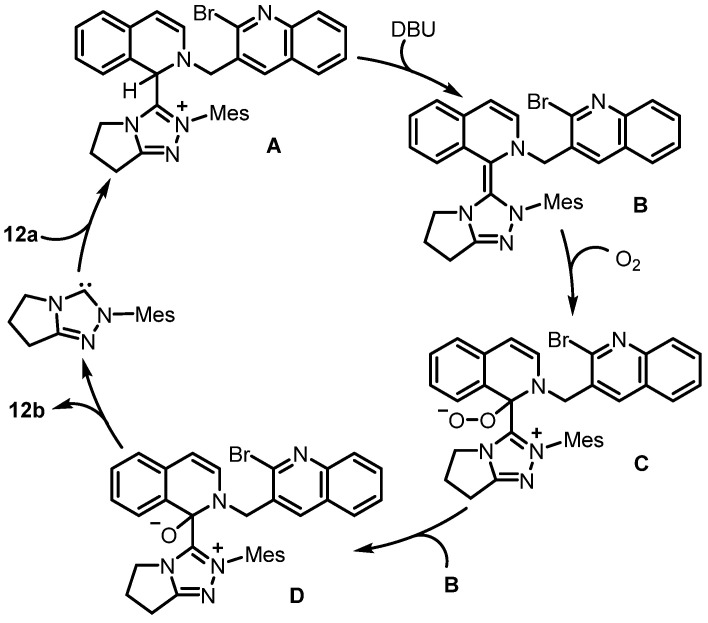
Later on, Fu and Huang developed a form of carbene-catalyzed aerobic oxidation of isoquinolinium salts for the construction of isoquinolinones, which was employed as the key step for the synthesis of rosettacin [40] (Scheme 20). First, the isoquinolinium salt 12a underwent carbene-catalyzed aerobic oxidation to form isoquinolinone 12b. Subsequent palladium-catalyzed intramolecular cyclization of 12b delivered rosettacin. This method provided a metal-free approach to constructing the isoquinolinone scaffold which avoided the employment of transition metals. The mechanism of the generation of compound 12b from substrate 12a was proposed by the authors (Scheme 21). The addition of NHC to substrate 12a yields intermediate A, followed by deprotonation under DBU to generate aza-Breslow intermediate B. Single-electron transfer (SET) between intermediate B and O2 followed by radical recombination affords intermediate C. Intermediate C undergoes interaction with another intermediate B to produce intermediate D, in which intermediate B may serve as the reducing reagent. The final formation of product 12b from intermediate D occurs, regenerating the free NHC for the next cycle.
Scheme 20.
Carbene-catalyzed aerobic oxidation of isoquinolinium salt as the key step for the synthesis of rosettacin. Created by us and based on the original work.
Scheme 21.
Proposed mechanism for the construction of isoquinolinone 12b from isoquinolinium salt 12a via carbene-catalyzed aerobic oxidation. Created by us and based on the original work.
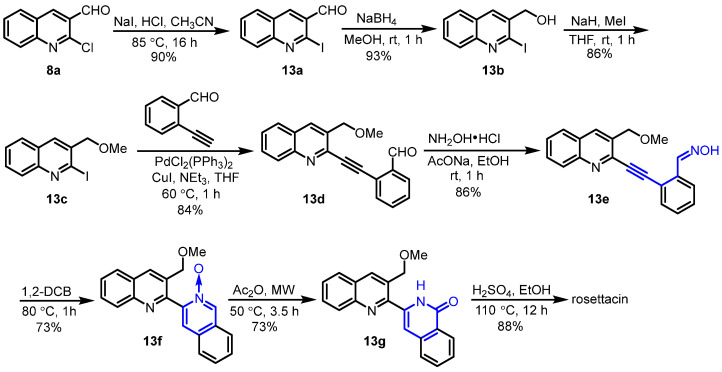
In 2023, Choshi et al. reported a new method for the synthesis of rosettacin by using thermal cyclization and a Reissert–Henze-type reaction as the key step [41] (Scheme 22). Here, 2-Chloroquinoline-3-carbaldehyde 8a reacted with NaI and concentrated HCl to yield 2-iodoquinoline 13a. Sequential NaBH4 reduction and methylation using MeI afforded 3-methoxymethyquinoline 13c. Palladium-catalyzed Sonogashira coupling with 2-ethynylbenzaldehyde followed by treatment with hydroxylamine produced oxime 13e. Oxime 13e underwent thermal cyclization in 1,2-DCB to form N-oxide 13f, followed by a Reissert–Henze-type reaction using Ac2O and microwave conditions to afford isoquinolone 13g. Heating with H2SO4 in EtOH transformed the isoquinolone 13g into rosettacin. This route required a multistep sequence to construct the C/D rings.
Scheme 22.
Thermal cyclization and Reissert-Henze-type reaction as the key step for the synthesis of rosettacin. Created by us and based on the original work.
3. Conclusions
Heterocycles are a significant kind of organic compound in synthetic chemistry. Heterocyclic scaffolds widely exist in many natural products, drugs, bioactive molecules and functional materials. Due to their versatile bioactive activities and functions, heterocycles not only play vital roles in biology and industry but also serve as important building blocks for an array of useful transformations. As a representative member of heterocycles, camptothecin (CPT) has been isolated from the Chinese tree Camptotheca accuminata and shows significant anti-tumor activity. Unfortunately, poor solubility and stability as well as unpredictable adverse drug–drug interactions limit its development. A wide range of structural modifications of CPT and the corresponding anti-tumor activity tests produce three compounds (topotecan, belotecan and irinotecan), which have been employed for the clinical treatment of cancer. However, CPT and its analogues face inherent structural problems. The susceptibility to hydrolysis of the lactone in the E ring generates a hydroxycarboxylate, which is inactive and has high affinity for human serum albumin. This seriously reduces antitumor activity. To change this situation, aromathecin alkaloids were investigated in order to replace camptothecins with them.
Rosettacin, belonging to the aromathecin family, has attracted the attention of organic and pharmaceutical chemists. Considering its important bioactive activity and unique structure, an array of strategies have been developed for the synthesis of rosettacin. For example, oxidative rearrangement from indole to quinolone occurs to yield a pentacyclic core. Aminolysis using pseudo-anhydride is performed to afford a tricyclic scaffold. A domino N-amidoacylation/aldol-type condensation process is used to form a tricyclic structure. Aryl radical cyclization of enamide is employed as the key ring-closing step for the construction of rosettacin. Rh(III)-catalyzed or Co(III)-catalyzed intramolecular C-H activation and annulation are used for the synthesis of isoquinolones. Cascade exo hydroamination followed by spontaneous lactamization is utilized as the key ring-closing step for the synthesis of rosettacin. Rh(III)-catalyzed intramolecular or intermolecular C-H activation and annulation are used as the key ring-closing step for the synthesis of a pentacyclic core. Copper-catalyzed photoinduced radical domino cyclization of ynamides serves as the key ring-closing step for constructing a pentacyclic core. Carbene-catalyzed aerobic oxidation of isoquinolinium salts is used for the construction of isoquinolinones. Thermal cyclization and a Reissert–Henze-type reaction are used for the synthesis of isoquinolones. These strategies not only provide a platform for the efficient preparation of rosettacin and its analogues but also bring some directions for the synthesis of CPT and its analogues, as well as other aromathecin alkaloids. This is essential for pharmaceutical chemists studying its antitumor activity. They are also beneficial for the discovery of new anticancer drugs. This review summarizes recent advances in the synthesis of rosettacin, which is timely and desirable for the rapid development of this field. Despite major advances, greener as well as more concise and efficient routes are still in demand. We hope this review will help researchers find hidden opportunities and stimulate the development of novel and concise routes for the synthesis of rosettacin.
Author Contributions
Conceptualization, L.S. and E.V.V.d.E.; methodology, L.S. and E.V.V.d.E.; validation, L.S. and E.V.V.d.E.; writing—original draft preparation, X.T., Y.J., L.S. and E.V.V.d.E.; writing—review and editing, L.S. and E.V.V.d.E.; funding acquisition, L.S. and E.V.V.d.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20220409) (recipient: L.S., supervision and writing) and the Natural Science Research of Jiangsu Higher Education Institutions of China (22KJB150008) (recipient: L.S., supervision and writing). We acknowledge the Fund for Scientific Research-Flanders (Belgium) (FWO) for its financial support (1002720H and 1001920H) (recipient: E.V.V.d.E., supervision and writing) and the research council of KU Leuven (recipient: E.V.V.d.E., supervision and writing). This paper was prepared with the support of the “RUDN University Strategic Academic Leadership Program” (recipient: E.V.V.d.E., supervision and writing).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bur S.K., Padwa A. The Pummerer Reaction: Methodology and Strategy for the Synthesis of Heterocyclic Compounds. Chem. Rev. 2004;104:2401–2432. doi: 10.1021/cr020090l. [DOI] [PubMed] [Google Scholar]
- 2.Martins M.A.P., Frizzo C.P., Moreira D.N., Buriol L., Machado P. Solvent-Free Heterocyclic Synthesis. Chem. Rev. 2009;109:4140–4182. doi: 10.1021/cr9001098. [DOI] [PubMed] [Google Scholar]
- 3.Godoi B., Schumacher R.F., Zeni G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011;111:2937–2980. doi: 10.1021/cr100214d. [DOI] [PubMed] [Google Scholar]
- 4.Tang X., Ding S., Song L., Van der Eycken E.V. Transition Metal-Catalyzed C−H Activation/Annulation Approaches to Isoindolo [2,1-b]isoquinolin-5(7H)-ones. Chem. Rec. 2023;23:e202200255. doi: 10.1002/tcr.202200255. [DOI] [PubMed] [Google Scholar]
- 5.Brandi A., Cicchi S., Cordero F.M., Goti A. Heterocycles from Alkylidenecyclopropanes. Chem. Rev. 2003;103:1213–1270. doi: 10.1021/cr010005u. [DOI] [PubMed] [Google Scholar]
- 6.Patil N.T., Yamamoto Y. Coinage Metal-Assisted Synthesis of Heterocycles. Chem. Rev. 2008;108:3395–3442. doi: 10.1021/cr050041j. [DOI] [PubMed] [Google Scholar]
- 7.Tang X., Song L., Van der Eycken E.V. Post-Ugi Cyclizations towards Polycyclic N-Heterocycles. Chem. Rec. 2023;23:e202300095. doi: 10.1002/tcr.202300095. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Cobo A.A., Franz A.K. Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org. Chem. Front. 2021;8:4315–4348. doi: 10.1039/D1QO00220A. [DOI] [Google Scholar]
- 9.St. Jean D.J., Jr., Fotsch C. Mitigating Heterocycle Metabolism in Drug Discovery. J. Med. Chem. 2012;55:6002–6020. doi: 10.1021/jm300343m. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y. Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014;43:1575–1600. doi: 10.1039/C3CS60369E. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Zhang W.-X., Xi Z. Carbodiimide-based synthesis of N-heterocycles: Moving from two classical reactive sites to chemical bond breaking/forming reaction. Chem. Soc. Rev. 2020;49:5810–5849. doi: 10.1039/C9CS00478E. [DOI] [PubMed] [Google Scholar]
- 12.Xue W., Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Meade J.D., Hellou J., Patel T.R. Aerobic co-metabolism of sulfur, nitrogen and oxygen heterocycles by three marine bacterial consortia. J. Basic Microbiol. 2002;42:19–36. doi: 10.1002/1521-4028(200203)42:1<19::AID-JOBM19>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Turesky R.J., Le Marchand L. Metabolism and Biomarkers of Heterocyclic Aromatic Amines in Molecular Epidemiology Studies: Lessons Learned from Aromatic Amines. Chem. Res. Toxicol. 2011;24:1169–1214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L., Lv Z., Zhang K., Wu Y., Van der Eycken E.V., Cai L. Recent Advances in the Asymmetric Total Synthesis of Camptothecin. Asian J. Org. Chem. 2022;11:e202200515. doi: 10.1002/ajoc.202200515. [DOI] [Google Scholar]
- 16.Yuan J.-M., Wei K., Zhang G.-H., Chen N.-Y., Wei X.-W., Pan C.-X., Mo D.-L., Su G.-F. Cryptolepine and aromathecin based mimics as potent G-quadruplex-binding, DNA-cleavage and anticancer agents: Design, synthesis and DNA targeting-induced apoptosis. Eur. J. Med. Chem. 2019;169:144–158. doi: 10.1016/j.ejmech.2019.02.072. [DOI] [PubMed] [Google Scholar]
- 17.Wall M.E., Wani M.C., Cook C.E., Palmer K.H., McPhail A.T., Sim G. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. J. Am. Chem. Soc. 1966;88:3888–3890. doi: 10.1021/ja00968a057. [DOI] [Google Scholar]
- 18.Thomas C.J., Rahier N.J., Hecht S.M. Camptothecin: Current perspectives. Bioorg. Med. Chem. 2004;12:1585–1604. doi: 10.1016/j.bmc.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Martino E., Volpe S.D., Terribile E., Benetti E., Sakaj M., Centamore A., Sala A., Collina S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017;27:701–707. doi: 10.1016/j.bmcl.2016.12.085. [DOI] [PubMed] [Google Scholar]
- 20.Chen L., Chen F.-E. Total Synthesis of Camptothecins: An Update. Synlett. 2017;28:1134–1150. doi: 10.1055/s-0036-1588738. [DOI] [Google Scholar]
- 21.Adams D.J., Dewhirst M.W., Flowers J.L., Gamcsik M.P., Colvin O.M., Manikumar G., Wani M.C., Wall M.E. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother. Pharmacol. 2000;46:263–271. doi: 10.1007/s002800000157. [DOI] [PubMed] [Google Scholar]
- 22.Cinelli M.A., Morrell A.E., Dexheimer T.S., Agama K., Agrawal S., Pommier Y., Cushman M. The structure–activity relationships of A-ring-substituted aromathecin topoisomerase I inhibitors strongly support a camptothecin-like binding mode. Bioorg. Med. Chem. 2010;18:5535–5552. doi: 10.1016/j.bmc.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H., Liu G., Yao Z.-J. Short and Efficient Total Synthesis of Luotonin A and 22-Hydroxyacuminatine Using A Common Cascade Strategy. J. Org. Chem. 2007;72:6270–6272. doi: 10.1021/jo070837d. [DOI] [PubMed] [Google Scholar]
- 24.Cheng K., Rahier N.J., Eisenhauer B.M., Gao R., Thomas S.J., Hecht S.M. 14-Azacamptothecin: A Potent Water-Soluble Topoisomerase I Poison. J. Am. Chem. Soc. 2005;127:838–839. doi: 10.1021/ja0442769. [DOI] [PubMed] [Google Scholar]
- 25.Cinelli M.A., Morrell A., Dexheimer T.S., Scher E.S., Pommier Y., Cushman M. Design, Synthesis, and Biological Evaluation of 14-Substituted Aromathecins as Topoisomerase I Inhibitors. J. Med. Chem. 2008;51:4609–4619. doi: 10.1021/jm800259e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamid A., Souizi A., Lawson A.M., Othman M., Ghinet A., Rigo B., Daïch A. Benzo[7,8]indolizinoquinoline scaffolds based on Mg(ClO4)2-promoted regiospecific imide reduction and π-cyclization of N-acyliminium species. Analogues of the topo-1 poison rosettacin and 22-hydroxyacuminatine alkaloids. Arab. J. Chem. 2019;12:680–693. doi: 10.1016/j.arabjc.2018.03.009. [DOI] [Google Scholar]
- 27.Warneke J., Winterfeldt E. Reaktionen an Indolderivaten, XVI. Die autoxydative Indol-Chinolon-Umwandlung eines Camptothecin-Modells. Chem. Ber. 1972;105:2120–2125. doi: 10.1002/cber.19721050705. [DOI] [PubMed] [Google Scholar]
- 28.Walraven H.G.M., Pandit U.K. A facile two synthon approach to the camptothecin skeleton. Tetrahedron. 1980;36:321–327. doi: 10.1016/0040-4020(80)80022-6. [DOI] [Google Scholar]
- 29.Fox B.M., Xiao X., Antony S., Kohlhagen G., Pommier Y., Staker B.L., Stewart L., Cushman M. Design, Synthesis, and Biological Evaluation of Cytotoxic 11-Alkenylindenoisoquinoline Topoisomerase I Inhibitors and Indenoisoquinoline−Camptothecin Hybrids. J. Med. Chem. 2003;46:3275–3282. doi: 10.1021/jm0300476. [DOI] [PubMed] [Google Scholar]
- 30.Pin F., Comesse S., Sanselme M., Daïch A. A Domino N-Amidoacylation/Aldol-Type Condensation Approach to the Synthesis of the Topo-I Inhibitor Rosettacin and Derivatives. J. Org. Chem. 2008;73:1975–1978. doi: 10.1021/jo702387q. [DOI] [PubMed] [Google Scholar]
- 31.El Blidi L., Namoune A., Bridoux A., Nimbarte V.D., Lawson A.M., Comesse S., Daïch A. Expeditious Synthesis of the Topoisomerase I Inhibitors Isoindolo [2,1-b]isoquinolin-7(5H)-one and the Alkaloid Rosettacin Based on Aryl Radical Cyclization of Enamide Generated by Using N-Acyliminium Chemistry. Synthesis. 2015;47:3583–3592. [Google Scholar]
- 32.Xu X., Liu Y., Park C.-M. Rhodium(III)-Catalyzed Intramolecular Annulation through C-H Activation: Total Synthesis of (±)-Antofine, (±)-Septicine, (±)-Tylophorine, and Rosettacin. Angew. Chem. Int. Ed. 2012;51:9372–9376. doi: 10.1002/anie.201204970. [DOI] [PubMed] [Google Scholar]
- 33.Lerchen A., Knecht T., Koy M., Daniliuc C.G., Glorius F. A General Cp*CoIII-Catalyzed Intramolecular C−H Activation Approach for the Efficient Total Syntheses of Aromathecin, Protoberberine, and Tylophora Alkaloids. Chem. Eur. J. 2017;23:12149–12152. doi: 10.1002/chem.201702648. [DOI] [PubMed] [Google Scholar]
- 34.Li K., Ou J., Gao S. Total Synthesis of Camptothecin and Related Natural Products by a Flexible Strategy. Angew. Chem. Int. Ed. 2016;55:14778–14783. doi: 10.1002/anie.201607832. [DOI] [PubMed] [Google Scholar]
- 35.Song L., Tian G., He Y., Van der Eycken E.V. Rhodium(iii)-catalyzed intramolecular annulation through C–H activation: Concise synthesis of rosettacin and oxypalmatime. Chem. Commun. 2017;53:12394–12397. doi: 10.1039/C7CC06860C. [DOI] [PubMed] [Google Scholar]
- 36.Raji Reddy C., Mallesh K. Rh(III)-Catalyzed Cascade Annulations To Access Isoindolo [2,1-b]isoquinolin-5(7H)-ones via C–H Activation: Synthesis of Rosettacin. Org. Lett. 2018;20:150–153. doi: 10.1021/acs.orglett.7b03509. [DOI] [PubMed] [Google Scholar]
- 37.Song L., Tian G., Van der Eycken E.V. Rhodium(III)-catalyzed intermolecular cascade annulation through C-H activation: Concise synthesis of rosettacin. Mol. Catal. 2018;459:129–134. doi: 10.1016/j.mcat.2018.09.004. [DOI] [Google Scholar]
- 38.Song L., Zhang X., Tian G., Robeyns K., Van Meervelt L., Harvey J.N., Van der Eycken E.V. Intramolecular cascade annulation triggered by CH activation via rhodium hydride intermediate. Mol. Catal. 2019;463:30–36. doi: 10.1016/j.mcat.2018.11.016. [DOI] [Google Scholar]
- 39.Baguia H., Deldaele C., Romero E., Michelet B., Evano G. Copper-Catalyzed Photoinduced Radical Domino Cyclization of Ynamides and Cyanamides: A Unified Entry to Rosettacin, Luotonin A, and Deoxyvasicinone. Synthesis. 2018;50:3022–3030. [Google Scholar]
- 40.Wang G., Hu W., Hu Z., Zhang Y., Yao W., Li L., Fu Z., Huang W. Carbene-catalyzed aerobic oxidation of isoquinolinium salts: Efficient synthesis of isoquinolinones. Green Chem. 2018;20:3302–3307. doi: 10.1039/C8GC01488D. [DOI] [Google Scholar]
- 41.Mizuno S., Nishiyama T., Endo M., Sakoguchi K., Yoshiura T., Bessho H., Motoyashiki T., Hatae N., Choshi T. Novel Approach to the Construction of Fused Indolizine Scaffolds: Synthesis of Rosettacin and the Aromathecin Family of Compounds. Molecules. 2023;28:4059. doi: 10.3390/molecules28104059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corey E., Crouse D.N., Anderson J.E. Total synthesis of natural 20 (S)-camptothecin. J. Org. Chem. 1975;40:2140–2141. doi: 10.1021/jo00902a034. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman H.H., Ives J.L. Reaction of singlet oxygen with enamino carbonyl systems. A general method for the synthesis of. alpha.-keto derivatives of lactones, esters, amides, lactams, and ketones. J. Org. Chem. 1985;50:3573–3580. doi: 10.1021/jo00219a025. [DOI] [Google Scholar]
- 44.Marco-Contelles J., Pérez-Mayoral E., Samadi A., Carreiras M.d.C., Soriano E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009;109:2652–2671. doi: 10.1021/cr800482c. [DOI] [PubMed] [Google Scholar]
- 45.Deniau E., Enders D. Synthesis of 3-alkyl-1-isoindolinones by alkylation of a benzotriazolyl substituted N-dimethylamino-phthalimidine. Tetrahedron. 2001;57:2581–2588. doi: 10.1016/S0040-4020(01)00073-4. [DOI] [Google Scholar]
- 46.Witulski B., Stengel T. N-Functionalized 1-Alkynylamides: New Building Blocks for Transition Metal Mediated Inter- and Intramolecular [2+2+1] Cycloadditions. Angew. Chem. Int. Ed. 1998;37:489–492. doi: 10.1002/(SICI)1521-3773(19980302)37:4<489::AID-ANIE489>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.