Abstract
Background
Currently available behavioral and dietary weight‐loss programs lack magnitude and sustainability compared with bariatric surgery. A novel dietary weight‐loss program was developed to assist participants in achieving sustainable diet changes by building knowledge and skills in food self‐selection. Although the approach worked, a large variation was observed in outcome among participants.
Objective
Determine factors affecting weight‐loss outcomes among participants to further improve the efficacy of the program.
Methods
Participants attended 19 dietary educational sessions during a 1‐year intervention which included prescribed homework. Changes in weight, diet, and body composition were assessed.
Results
Participants (n = 22) achieved mean body weight loss of −6.49(8.37%, p < 0.001) from baseline at 12 months. Nine participants (41%) achieved weight loss >5% of initial bodyweight; two reached a Body Mass Index 25 kg/m2. A large divergence in weight loss among participants was observed; successful (n = 9) achieved −12.9(9.6)% while unsuccessful achieved −2.03(2.78)%. Dietary protein and fiber density by 24‐h records showed a significant and inverse correlation with weight loss (%) throughout the program. Weight loss at 3 months and 12 months showed a strong correlation (r = 0.84). Participants with self‐reported depression lost significantly less weight than those without depression at 12 months (p < 0.03).
Conclusions
Divergence in weight‐loss outcomes among the participants is likely due to a difference in successful dietary implementation. Intra‐cohort analysis indicates early weight‐loss success and early dietary implementation was predictive of long‐term success.
Keywords: fiber, obesity treatment, protein, weight management
1. INTRODUCTION
Weight loss can reduce the risk of obesity‐related comorbidities. 1 , 2 , 3 , 4 , 5 However, available dietary weight‐loss programs, including commercialized diets, very‐low‐calorie diets, and academia‐developed programs, are insufficient in the magnitude of weight loss and sustainability of lost weight. 6 , 7 , 8 , 9 To the best knowledge of the Individualized Diet Improvement Program (iDip) group, only one study demonstrated the major reversal of type 2 diabetes lasting for 2 years using a ketogenic diet. 10 , 11 Outcomes of behavioral weight loss are similar to dietary weight loss in magnitude and sustainability. Only modest weight loss and health benefits are expected from behavioral intervention, and the benefits decline in subsequent years as weight regain commonly occurs. 1 , 3 , 7 , 12 , 13 In pharmacotherapy, stable GLP‐1 analogs showed a higher magnitude of weight loss than dietary, behavioral, and other weight loss medications. 7 , 14 Although the magnitude of weight loss of the GLP‐1 analogs is promising, rapid weight regains occurred after the termination of medication. 14 This underscores that sustainable behavioral and dietary changes are required to maintain lost weight. Moreover, without proper dietary changes, a prolonged GLP‐1 analog medication that reduces food intake would result in deficiency in both macro‐ and micronutrients. As such, bariatric surgery remains the most effective treatment of obesity and its comorbidities. 7 A reduced stomach size by bariatric surgery forces reduced calorie intake and dietary changes, resulting in sustainable weight loss.
A long‐term goal of the iDip group is to develop a dietary weight‐loss program that can reduce an efficiency gap between dietary/behavioral weight loss and bariatric surgery. As the first step toward this goal, the iDip group created a prototype of a novel weight‐management program and tested its feasibility. 15 The premise of iDip's approach is that a sustainable dietary change, which varies among individuals, must be achieved to maintain a healthy weight for a lifetime. The main innovative approaches employed in iDip were twofold. First, most part of the program was dedicated to building the ability to select food based on quantitative nutrient information of individual foods. Second, Protein‐Fiber (PF) plot, a one‐of‐a‐kind quantitative data visualization tool, was utilized to develop the ability of informed decision making in food selection. The program assists participants in developing knowledge of key nutrients to create a personalized, safe, and effective weight‐loss diet. This approach uses intensive dietary education sessions allowing participants to build knowledge and skills from the ground up to create their weight‐loss diet with maximum flexibility. This approach is in contrast with commonly used dietary and behavioral approaches such as strict dietary regimens, dietary weight loss products, weight loss medications, exclusion of certain food groups, and reduction in portion size of foods, all of which are intended for easy implementation. 2 , 3 , 6 , 8 , 9 , 12 However, these approaches bypass the development of lasting cognitive and behavioral flexibility and self‐efficacy in selecting healthy foods. Consequently, weight loss using these approaches often plateaus by 6 months, and participants suffer from weight regain after weight loss. 2 , 3 , 6 , 8 , 9 , 12 The iDip program does not employ these shortcuts but focuses on helping individuals discover a safe and effective weight loss diet by self‐experimenting with various dietary iterations. The knowledge and skills developed during the weight‐loss period will serve as the foundation for subsequent, sustainable weight maintenance.
A well‐designed, 2‐dimensional data visualization is a very effective way to interpret and utilize a quantitative dataset. 16 The iDip utilizes the PF plot as a key tool for participants to develop knowledge and skills for making informed food selections (Figure S1). The PF plot was developed and tested for its efficacy as a data‐visualization tool to easily compare quantitative nutrient values of menu items. 17 When a menu was displayed in the PF plot at a restaurant, customers chose healthier items compared to when there was no provision of nutrition information. In contrast, there was no improvement in food choice when identical nutrient information was presented in the Nutrition Facts Panel, demonstrating easy comparison among menu items was critical to make an informed decision. 17 Using the PF plot, iDip participants created an individualized weight‐loss diet starting from foods they habitually eat by increasing protein and fiber intake while reducing energy intake simultaneously. 15 Strong evidence supports that increasing protein intake per healthy body weight and decreasing energy intake simultaneously is required to optimize the safety and effectiveness of weight loss diet. 18 , 19 This optimization cannot be achieved by simple portion size reduction or calorie counting because when calorie intake is decreased, protein intake also decreases in these approaches. The PF plot enables creating a meal with increased protein and decreased energy by setting a target range of protein/calorie values. Moreover, the focus of the PF plot on protein and fiber prepares individuals for an effective weight maintenance diet because clinical and epidemiological studies have shown that sufficient protein and fiber intake reduces energy overconsumption during weight maintenance. 20 , 21 , 22 The iDip also replaced daily calorie counting with the provision of a weekly weight chart based on daily weighing for participants to visualize their progress and monitor energy balance (Figure S2). 15
The participants of the feasibility study (iDip1) achieved clinically significant weight loss. However, large variations in weight‐loss outcomes were observed among participants. Also, limitations included a short follow‐up period (six months) and the lack of body composition measurements. 15 As such, the objective of this study is to identify factors that affect the weight‐loss outcome of participants using intra‐cohort variation analysis. Here, the first 12‐month outcomes for the revised iDip2 program are reported.
2. METHODS
2.1. Recruitment
Participants were recruited via University of Illinois Urbana‐Champaign (UIUC) electronic staff newsletters, posters in campus buildings, word‐of‐mouth, and flyers in Carle Clinic (Champaign, IL USA). The primary objective of this study was to determine correlations between weight‐loss outcomes and dietary changes. The previous feasibility study 15 showed that the correlation R 2 between weight loss and protein/calorie by food frequency questionnaire (FFQ) and 24‐h record were 0.32 and 0.17, respectively. Based on the R 2, the sample size for 95% confidence was 24.5. Assuming an attrition rate of 20%, 30 participants were recruited.
Prospective participants contacted investigators and received a detailed description of the study. Interested participants completed a consent form and self‐reported medical history form prior to meeting with investigators. Participants attended individual meetings with investigators where baseline anthropometrics were measured. Inclusion criteria were: aged 18–70 years old; Body Mass Index (BMI) ≥25 kg/m2 (BMI ≥23 kg/m2 if Asian); not pregnant or lactating; WiFi and smartphone access; fluent in English speaking and writing; no self‐reported severe metabolic, cardiovascular, or musculoskeletal problems; not using insulin injection; willing and able to attend 22 dietary improvement sessions; have a goal of losing ≥9.1 kg (20 lb) and maintaining the loss; and willingness to self‐weigh daily for 24 months. Exclusion criteria included failure to submit baseline documents and set up WiFi‐enabled scale. Other than a WiFi scale, no other compensation was offered.
2.2. Study design
iDip2 study follows a similar design to iDip1, a non‐randomized, single‐arm, before‐and‐after study design, which is suitable for analyzing the intra‐cohort correlations between dietary and other factors and weight‐loss outcomes. 15 iDip2 is a 12‐month intervention with an additional 12 months of follow‐up. iDip2 consisted of 19 in‐person group educational sessions and three individual advising sessions. Three identical educational sessions were delivered by registered dietitian nutritionists (RDNs) each week at UIUC. Participants were assigned to one of the three identical sessions but could attend any of the sessions as their schedules allowed. The curriculum is described in Table S1. Education sessions were 50‐min long and consisted of a 30‐min lecture and 20 min of skill‐building activities and discussion. After each session, participants submitted a homework assignment via the secure Box (https://www.box.com/home, Redwood City, CA). Using Box, RDNs communicated about food choices and difficulties in weight loss that participants were facing. Using the individual's PF plot and weight chart, the RDNs reviewed weight and dietary progress with participants and advised them accordingly during in‐person individual advising sessions.
2.3. Dietary intervention
The approach to dietary intervention was described previously 15 with slight modifications. The PF plot and the weekly weight chart were provided as primary tools to assist participants in developing their own weight loss diet. The PF plot (Figure S1) was used throughout the session materials as well as for all dietary analysis and for advising participants. The PF plot displays two target boxes to guide participants to reach protein and fiber density goals derived from the Acceptable Macronutrient Distribution Ranges and Adequate Intakes. 15 , 17 One box is a weight‐loss box with a protein range of 7–11 g/100 kcal and a fiber range of 1.8–3.2 g/100 kcal and the other is a weight‐maintenance box with a protein range of 4–8 g/100 kcal and a fiber range of 1.4–2.8 g/100 kcal (Figure S1). The weekly weight chart allowed participants to self‐monitor energy balance without relying on calorie counting (Figure S2).
iDip 2 further incorporated weight‐management strategies to solve shortcomings identified in iDip1. After each session, participants completed homework assignments and received feedback from RDNs to implement further dietary changes. The follow‐up period was extended up to 12 months after completing all sessions to track the sustainability of weight loss. Monitoring body composition with a bio‐impedance device was incorporated to monitor the loss of fat mass and skeletal muscle mass.
3. OUTCOME MEASURES
3.1. Anthropometrics
Participants self‐weighed daily on a WiFi‐enabled scale (Withings, Issy‐les‐Moulineaux, France). These weights were transmitted to researchers who compiled a weekly weight trend chart. At baseline, 6, and 15 months, height, weight, and hip and waist circumference were collected. Height was measured to the nearest 0.64 cm (0.25″) with shoes removed using a stadiometer (Seca 700, Hanover, MD) and weight was measured to the nearest 0.05 kg (0.1 lb) on the same instrument. Waist and hip circumference were measured to the nearest 0.1 cm using a standard, retractable measuring tape (Gulik II, Gay Mills, WI) by trained investigators.
3.2. Body composition
Body composition was measured at baseline, 6, and 15 months by an electrical impendence method using InBody 270 (InBody USA, Cerritos, CA, US). Measurements initially scheduled at 12 months were delayed to 15 months due to the SARS‐CoV‐2 pandemic in 2020. To accommodate those with virus‐related concerns, the body composition and anthropometric measurements at 15 months were optional and not fully representative of the cohort.
3.3. Dietary intake
At baseline and 12 months, a FFQ was administered to assess changes in habitual diet. At baseline, it was administered via pen‐and‐paper; however, due to the pandemic, it was administered electronically at 12 months. The FFQ was modified from the EPIC‐Norfolk questionnaire and was previously reported. 15 , 23 Written self‐reported 24‐h dietary records were collected six times (1, 3, 4, 6, 7, and 12 months). Dietary records were analyzed, and individualized feedback was provided to participants in a PF plot for dietary guidance. The US Department of Agriculture National Nutrient Database for Standard Reference (https://fdc.nal.usda.gov/) and manufacturer information were used to calculate protein and fiber density.
3.4. Exit survey
At 12 months, a 30‐question survey was administered online via WebTools software (Web Services, UIUC) to evaluate and identify the strengths and weaknesses of the iDip program from the participants' perspectives. Questions were open answers, Likert scales, or checkboxes. Open‐answer questions requested qualitative feedback and improvement suggestions; Likert scale questions addressed personal gain, program and content, homework and feedback, and overall thoughts; check box questions identified the most and least useful parts of the program. The seven‐point Likert scale used had the following options: 1: Strongly disagree; 2: Disagree; 3: Somewhat disagree; 4: Neutral ‐ Neither agree nor disagree; 5: Somewhat agree; 6: Agree; 7: Strongly agree.
3.5. Statistical analysis
Descriptive analysis was used to summarize demographic characteristics. Weight‐loss of ≥5% from the baseline was considered successful as it has been shown to reduce health risks associated with obesity. 24 Paired t‐test analysis was used to determine time‐course differences in outcome measures. An unpaired t‐test was used to analyze the effects of self‐reported comorbidities, gender, and age groups (18–49 and 50–64 + years old) on the weight‐loss outcome. Correlation analyses were performed to determine associations among bodyweight, protein density, and fiber density. The associations between bodyweight and survey scores were evaluated using correlation analyses. Differences in daily weighing were evaluated using analysis of variance (ANOVA) with weight‐loss outcomes as the grouping variable. The values for weighted fat mass loss (kg)/weighted body weight loss (kg) at 15 months were obtained by multiplying the weight factor with the ratio of fat loss (kg) to weight loss (kg). The weight factor was derived by dividing individual weight loss (kg) by the total weight loss of the sample size of n = 14. Outcomes were analyzed using intention‐to‐treat analysis, with missing data replaced by the latest available data carried forward, and complete case analysis. Both analyses showed similar outcomes in magnitude and statistical significance, and thus, the results of the participants with complete data from measurement timepoints were presented. All statistical and food records analyses were performed with R Computing (Version 3.6.1 © 2019) using the “car” package for ANOVA, “corrr” package for correlation, and “Hmisc” for correlation matrix, or with Microsoft Office Excel 2016, and p < 0.05 was considered statistically significant. Data are reported as mean (SD).
3.6. Institutional approval
This study was approved by the UIUC Institutional Review Board (#18069) and was registered at the US National Institutes of Health (ClinicalTrial.gov) #NCT04605653. Participant recruitment started on 14 January 2019, and the follow‐up period ended on 26 March 2021.
4. RESULTS
4.1. Participants
At baseline, 30 participants (19 females) were enrolled (Figure 1) with demographics listed in Table 1. The mean age was 49.3(SD = 11.5) years, and mean BMI was 36.9(6.3) kg/m2. Enrolled participants had a mean of 3.3(2.1) comorbidities. The most prevalent self‐reported comorbidities included hypercholesterolemia (53.3%) and skeletal problems (46.7%), followed by hypertension (33.3%) (full list in Table S2). Participants reported having prior dieting experiences, including Weight Watchers (43.3%), low‐carbohydrate or ketogenic diet (30%), and research study weight programs (13.3%) (full list in Table S2).
FIGURE 1.
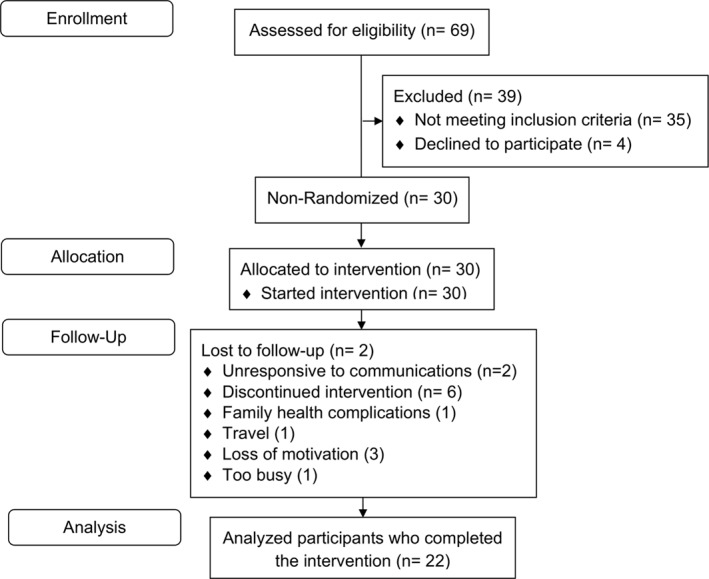
Flow diagram of study participants.
TABLE 1.
Baseline demographics for the iDip 2 study.
| Enrolled participants (n = 30) | Completers (n = 22) | |
|---|---|---|
| Sex, number (%) | ||
| Male | 11 (36.7) | 9 (40.9) |
| Female | 19 (63.3) | 13 (59.1) |
| Age, number (%) | ||
| 18–29 | 1 (3.3) | 1 (4.6) |
| 30–49 | 16 (53.3) | 11 (50.0) |
| 50–64 | 10 (33.3) | 8 (36.4) |
| 64+ | 3 (10.0) | 2 (9.1) |
| Mean age (SD) | 49.3 (11.5) | 49.6 (12.2) |
| Education, number (%) | ||
| High school | 8 (26.7) | 6 (27.8) |
| Bachelor's degree | 8 (26.7) | 5 (22.7) |
| Graduate degree | 13 (43.3) | 11 (50.0) |
| Unknown | 1 (3.3) | 0 (0.0) |
| Ethnicity, number (%) | ||
| White | 25 (83.3) | 18 (81.8) |
| African American | 4 (13.3) | 3 (13.6) |
| Asian | 1 (3.3) | 1 (4.6) |
| BMI (kg/m2) | ||
| Mean BMI (SD) | 36.9 (6.3) | 37.3 (6.1) |
| Previous weight loss attempts | ||
| Mean attempts (SD) | 2.33 (1.18) | 2.36 (1.18) |
| Comorbidities a | ||
| Mean comorbidities (SD) | 3.23 (2.06) | 3.32 (2.15) |
Abbreviation: BMI, Body Mass Index.
Comorbidities include hypercholesterolemia, skeletal problems, hypertension, sleep apnea, depression, irregular periods (female participants only), type 2 diabetes mellitus, asthma, thyroid problems, kidney problems, high blood lipids, previous cancer, muscle pain, nonalcoholic fatty liver disease, and polycystic ovary syndrome.
4.2. Retention and attendance
At 12 months, 22 participants (73.3%) remained in the study (Figure 1, Table 1). Participants were considered enrolled if they continued to self‐weigh and completed the exit survey. Eight participants dropped out due to the following reasons: familial health complications, 1 travel, 1 loss of motivation, 3 too busy, 1 and unresponsive to communications. 2 Of the 22 sessions, the mean attendance was 19.3(2.8) (87.6%), and of the 19 homework assignments, the mean completion was 13.6(2.8) (71.3%). Participants not able to attend a regularly scheduled session were provided a one‐on‐one or small group makeup session and an average of 2.5(1.8) makeup sessions were given per participant (n = 22).
4.3. Weight change
For all completing participants (n = 22 for weight), the mean percentage of body weight loss at 12 months was −6.49(8.36)% (Figure 2A). From baseline to 12 months, mean weight significantly decreased (106.4(21.6) kg versus 99.7(23.2) kg; p < 0.001). Mean BMI change was −2.33(2.98) kg/m2 (37.3 kg/m2 vs. 35.0 kg/m2; p < 0.001) at 12 months.
FIGURE 2.
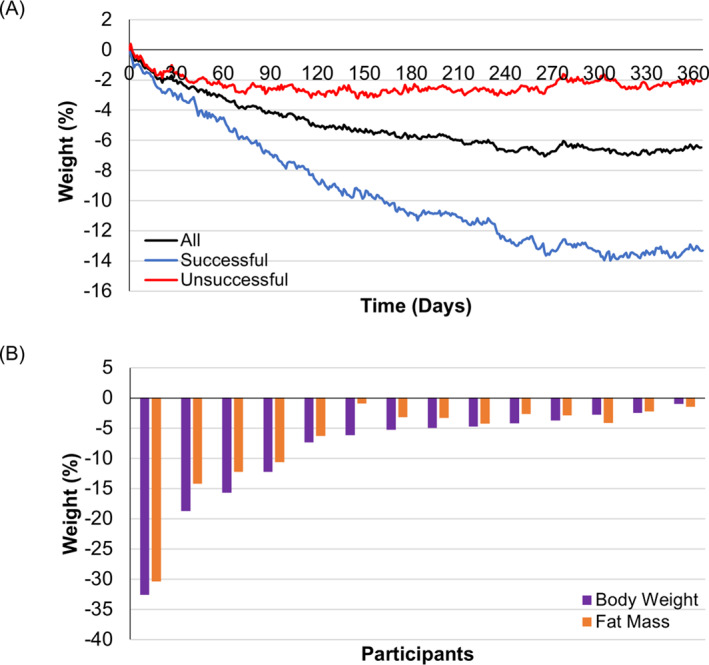
(A) Percentage weight lost over the course of 1 year. Participants are grouped by the weight loss of >5% (n = 9) and <5% (n = 13) at 12 months. Mean of all participants, the successful group achieved significant weight loss from baseline (p = 0.002) while the unsuccessful failed to achieve 5% weight loss (p = 0.02). (B) % body weight and fat mass lost by individuals at 15 months.
Large differences in weight‐loss outcomes at 12 months were observed between the successful (weight loss of >5%) and unsuccessful (weight loss of <5%) groups (Figure 2A). Successful (n = 9) achieved a significant weight loss from the baseline reaching −12.9(9.6%, p = 0.002) while unsuccessful (n = 13) lost −2.03(2.78%, p = 0.02).
4.4. Body composition and anthropometric changes
Of 22 participants, 14 (64%) completed body composition and anthropometric measurements at all three timepoints: baseline, six months, and 15 months (Table 2). Eight participants lost >5% initial bodyweight, achieving clinically significant weight loss (Figure 2B). Among them, four participants lost >10% initial bodyweight (Figure 2B), and two participants reached a BMI <25 kg/m2. Of 8 successful participants, 7 achieved weight loss largely from losing fat mass (Figure 2B). Fat mass significantly decreased at six months (p = 0.0003) and 15 months (p = 0.004). Mean loss of skeletal muscle mass was −1.26(1.32) kg or −1.21(1.23)% of bodyweight at 15 months (p = 0.003), whereas the overall bodyweight reduction at 15 months was −8.39(7.81) kg or −8.41(8.30)% (n = 14). Waist and hip circumference decreased significantly from baseline at 15 months (Table 2). Lean body mass in both successful (weight loss of >5%) and unsuccessful (weight loss of <5% groups were well‐preserved (Table 2).
TABLE 2.
Changes in BMI, weight, anthropometric measures, and body composition over 15 months.
| Baseline | 6 months | 15 months b | |
|---|---|---|---|
| Waist circumference | 114.6 (12.2) | 107.9 (15.1) a | 105.2 (12.9) a |
| Hip circumference | 123.5 (13.06) | 117.7 (14.5) a | 116.7 (15.2) a |
| Waist: Hip ratio | 0.93 (0.07) | 0.92 (0.09) | 0.91 (0.07) a |
| Female (n = 7) | 0.88 (0.06) | 0.86 (0.06) | 0.86 (0.02) |
| Male (n = 7) | 0.99 (0.04) | 0.92 (0.09) | 0.95 (0.06) a |
| Skeletal muscle Mass (kg) | 33.8 (9.8) | 32.9 (9.6) a | 32.5 (9.1) a |
| Weight loss due to skeletal muscle Mass (kg) | N/A | −0.86 (0.87) | −1.26 (1.32) |
| Fat Mass (kg) | 42.6 (14.3) | 35.6 (14.7) a | 35.7 (14.7) a |
| Weight loss due to fat Mass (kg) | N/A | −7.05 (5.36) | −6.92 (7.45) |
| n = 14 | >5% (n = 8) | <5% (n = 6) | ||
|---|---|---|---|---|
| Body weight loss (kg) | −8.7 (7.8) | −13.2 (7.7) | −2.8 (1.15) | |
| Fat mass loss (kg) | −6.9 (7.2) | −10.2 (8.0) | −2.5 (0.56) | |
| Fat mass loss (kg)/body weight loss (kg) | 0.84 (0.33) | 0.70 (0.25) | 1.03 (0.37) | |
| Weighted fat mass loss (kg)/weighted body weight loss (kg) | 0.79 | 0.78 | 0.91 | |
Note: Unless otherwise stated, n = 14. Values are presented as mean (SD).
Abbreviation: BMI, Body Mass Index.
Significantly different from baseline by paired and unpaired t‐tests (p < 0.05).
Delayed from 12 to 15 months due to US 2020 SARS‐CoV‐2 outbreak.
4.5. Dietary changes
Increasing dietary protein and fiber intake while reducing caloric intake are the pillars of the iDip program. Protein and fiber density significantly increased from the 24‐h record at 1 month (5.14 g/100 kcal and 1.33 g/100 kcal, respectively) to 3 (6.36 g/100 kcal (p = 0.007) and 1.73 g/100 kcal (p = 0.03), respectively) and 4 months (5.95 g/100 kcal (p = 0.03) and 1.82 g/100 kcal (p = 0.01), respectively). No further significant protein density increases were seen following 3 months, however fiber density significantly increased from 1 month at 6 months (1.83 g/100 kcal) and 7 months (1.92 g/100 kcal) (Figure 3). Calorie intake was reduced to about 1500 kcal/day at 3 months and later. Protein density assessed by the FFQ significantly increased from baseline to 12 months (protein: 4.7 g/100 kcal to 5.9 g/100 kcal). Fiber density also significantly increased from baseline to 12 months (1.3 g/100 kcal to 1.6 g/100 kcal) (Figure 3). A complete dietary dataset of protein, fiber, and calorie intake at different time points is presented in Table S3.
FIGURE 3.
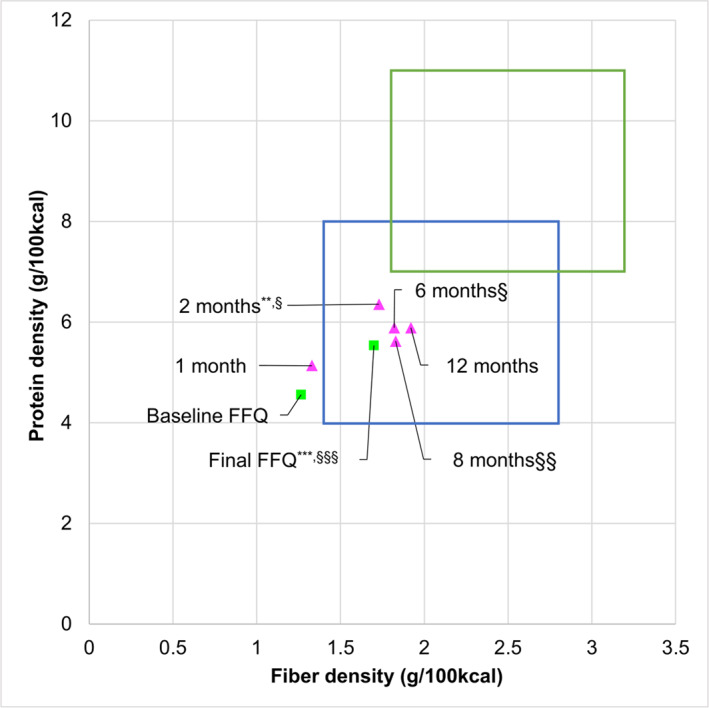
The mean dietary protein and fiber densities (g/100 kcal) of the pre‐and post‐intervention food frequency questionnaires (FFQs) (n = 22) and six 24‐h records (n = 17). The final (at 12 months) FFQ was compared with the baseline FFQ, whereas 24‐h records were compared with the record at one month by a paired t‐test. Significantly different in protein density at *p < 0.05, **p < 0.01, and ***p < 0.001. Significantly different in fiber density at §p < 0.05, § §p < 0.01 and § § §p < 0.001.
4.6. Exit survey
The survey results are summarized in Table S4. Four questions related to satisfaction with the program were inversely correlated with weight loss at 12 months (r = −0.5, p = 0.02). Some participants gave a low score to PF plot understanding, but a correlation between PF plot understanding and weight loss was not significant (r = −0.31, p = 0.15). A mean score on homework assignments showed some participants felt the assignments were not helpful. The most useful sessions were “Starting Weight Loss” (31.8% of responded participants), “Establishing Routine” (31.8%), and “Individual Advising” (27.3%), and the least useful were “Peer Experience Sharing” (36.4%), “Trouble Shooting for Slowing Down” (31.8%), and “Introduction to Weight Maintenance” (27.3%). The session on weight maintenance was held at 6 months, when many participants were not in the weight‐maintenance phase and, thus, did not find the session useful.
5. FACTORS AFFECTING WEIGHT‐LOSS OUTCOME
5.1. Protein and fiber
A complete correlation matrix between diet and weight loss is presented in Figure 4. Protein and fiber densities were calculated using the cumulative average of 24‐h records up to 3, 6, and 12 months. The levels of protein density at 3, 6, and 12 months showed a significant inverse correlation with weights at the respective timepoints, indicating that participants with higher protein density diets experienced greater weight loss (r = −0.62, p = 0.008; r = −0.57, p = 0.018; r = −0.49 p = 0.044, respectively) (Figure 4 and 5B,C and D).
FIGURE 4.
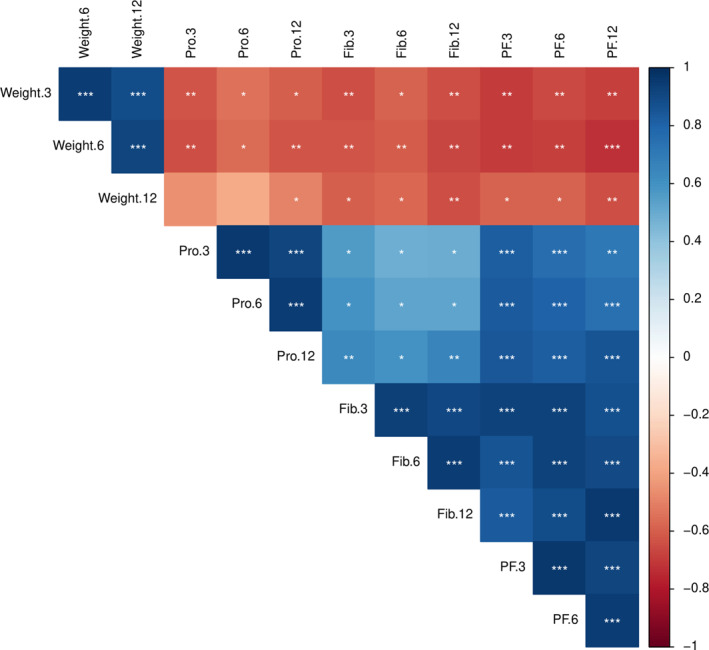
Correlation matrix demonstrating the interrelationship among weight loss, protein and fiber density, and the sum of protein and fiber density at 3 months, 6 months, and 12 months. Cumulative 24‐h records were used at 3, 6, and 12 months. Positive correlations are displayed in blue and negative correlations in red. *p < 0.05, **p < 0.01, ***p < 0.001.
FIGURE 5.
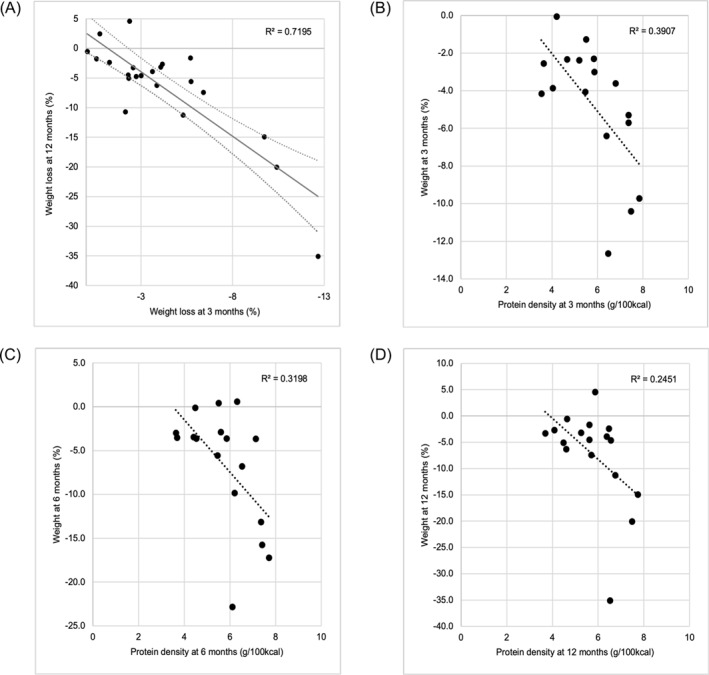
(A) Weight loss at 3 months is predictive of 12‐month weight loss (r = 0.84, p < 0.001, n = 22). Correlation of weight change with dietary protein density at (B) 3 months (r = −0.62, p = 0.008), (C) 6 months (r = −0.57, p = 0.018), and (D) 12 months (r = −0.49 p = 0.044). N = 17 for all figures.
The levels of fiber density at 3, 6, and 12 months also showed significant inverse correlation with weights at the respective timepoints, indicating that participants with higher fiber density diets resulted in greater weight loss (r = −0.64, p = 0.005; r = −0.61, p = 0.01; r = −0.64, p = 0.005, respectively) (Figure 4 and Figure 3A, B, and C). The sum of protein and fiber density at 3, 6, and 12 months correlated with weights at the respective timepoints (r = −0.69, p = 0.002; r = −0.69, p = 0.002; r = −0.64, p = 0.005, respectively) (Figure 4). The sum of protein and fiber density was calculated by dividing each value by 7 g/100 kcal and 1.8 g/100 kcal, respectively, which are the targets of protein and fiber density for weight loss.
5.2. Other factors for weight loss
Three‐month and 12‐month weight loss (%) had a strong correlation (r = 0.84, p < 0.001; Figure 5A), suggesting that weight loss during the first three months was crucial for long‐term success. An inverse correlation was observed between percentage weight loss at 12 months and session attendance, but it did not reach significance (r = −0.34, p = 0.10). There was no trend for weight loss and homework completion rate. Daily weighing frequency was not correlated with weight loss.
The mean number of self‐reported comorbidities was 3.3(2.2) among participants who completed the program (Table 1). Among completers, 54.6% had high blood cholesterol, 50% had skeletal problems, 36.4% had hypertension, 36.4% had sleep apnea, and 31.8% had depression (Table 3). Participants with self‐reported diagnosis of depression lost less weight (n = 7, −2.43(3.59)%) than participants with no depression diagnosis (n = 15, −8.39(9.34)%) (p = 0.04). Weight loss (%) did not significantly differ between participants with other comorbidities and without (high blood cholesterol (p = 0.35), skeletal problems (p = 0.35), hypertension (p = 0.79), and sleep apnea (p = 0.86) (Table 3). Furthermore, no significant results were found between younger and older age groups (p = 0.13) and gender (p = 0.73) in weight‐loss outcomes (Table 3).
TABLE 3.
A comparison table of 12‐month body weight change between the absence and presence of participants' comorbidities, gender and age group.
| Comorbidities | Presence | Absence | p‐value |
|---|---|---|---|
| Sleep apnea | |||
| Participants, n | 14 | 8 | |
| Mean weight change (S.D.) % | −6.09 (7.87) | −6.73 (8.92) | 0.86 |
| Hypertension | |||
| Participants, n | 14 | 8 | |
| Mean weight change (S.D.) % | −5.68 (12.45) | −6.96 (5.38) | 0.79 |
| Skeletal problems | |||
| Participants, n | 11 | 11 | |
| Mean weight change (S.D.) % | −8.22 (11.53) | −4.77 (2.73) | 0.35 |
| Hypercholesterolemia | |||
| Participants, n | 10 | 12 | |
| Mean weight change (S.D.) % | −4.82 (4.82) | −8.51 (11.25) | 0.35 |
| Depression | |||
| Participants, n | 7 | 15 | |
| Mean weight change (S.D.) % | −2.43 (3.59) | −8.39 (9.34) | 0.04* |
| Type 2 diabetes mellitus | |||
| Participants, n | 6 | 16 | |
| Mean weight change (S.D.) % | −4.87 (5.74) | −7.10 (9.25) | 0.51 |
| Asthma | |||
| Participants, n | 4 | 18 | |
| Mean weight change (S.D.) % | −4.25 (7.58) | −6.99 (8.65) | 0.55 |
| Irregular periods, female only | |||
| Participants, n | 4 | 9 | |
| Mean weight change (S.D.) % | −1.48 (3.08) | −8.01 (12.14) | 0.16 |
| Within demographic variables | Participants, n | Mean weight change (SD) % | p‐value |
|---|---|---|---|
| Gender | Male, n = 9 | −7.27 (4.09) | 0.73 |
| Female, n = 13 | −6.00 (10.51) | ||
| Age, years old | 18–49, n = 12 | −4.01 (3.49) | 0.13 |
| 50–70, n = 10 | −9.47 (11.41) |
Note: All participants n = 22.
*p < 0.05 by unpaired t‐test.
6. DISCUSSION
The iDip was built upon two novel quantitative visual aids: the PF plot for select nutrient values of food items in a diet 17 and the weekly weight chart for energy balance and weight‐loss progress. The feasibility study showed that these visual feedback and education sessions enabled participants to lose weight by creating their own weight‐loss diet. 15 In the current study, the mean weight loss was −12.9% for the nine participants who lost more than 5% of their baseline bodyweight after 12 months, whereas the other 13 participants failed to reach 5% of weight loss (mean −2.03%), exhibiting a divergent response to the program among participants. Protein and fiber densities were inversely associated with weight loss throughout the program period. Other factors were identified that may affect weight‐loss outcomes in this participant‐initiated weight‐loss program.
Preservation of lean body mass during weight loss becomes important to improve overall health, particularly for people who successfully lose >10% of baseline weight. As reviewed in the introduction, increasing protein intake while decreasing calories must be implemented to achieve the preservation of lean body mass. Focusing only on calorie reduction without increasing protein intake during weight loss results in a negative nitrogen balance. 18 An example is the Look AHEAD study, one of the most intensive behavioral weight management intervention studies. 12 Despite a large increase in physical activity in the treatment group, bone density was significantly decreased at year 4. 25 More than half of weight loss from baseline was due to loss of lean body mass, indicating insufficient protein intake among the treatment group. 25 Consistent with the body composition data, incidents of bone fracture over 10 years were significantly higher in the treatment group. 12 The body composition analysis of the current study indicates that lean body mass was well‐preserved in the participants. Even in the group that lost >5% of body weight, 78% of the weight loss came from fat mass. Importantly, the participants were able to create increased protein/calorie meals based on the feedback in the form of the PF plot, not by using weight loss products or following recipes. This indicates that the participants developed self‐efficacy in changing their diet to be healthier.
In addition to preserving lean body mass, increased protein is likely to improve the effectiveness of weight loss by accelerating fat mass loss. A meta‐analysis of 13 randomized controlled trials (RCTs) of 12 weeks or longer demonstrated that a moderately high protein diet (30.5(2.4)% protein, 1576(270) kcal/day) produces more favorable changes in body composition compared to a standard protein diet (17.5(1.5)% protein, 1525(265) kcal/day). 19 Preservation of fat‐free mass during weight loss in the moderately high protein diet groups was significantly better than the standard protein groups, −1.54 kg (ranging from −2.78 to 0.6 kg) and −2.18 kg (−4.06 to −0.3 kg), respectively. 19 Moreover, the moderately high protein groups achieved significantly greater fat mass loss than the standard protein groups, −6.2 kg (−9 to −1.65 kg) and −5.49 kg (−7.6 to −0.64 kg), respectively. 19 As intended, the participants in the current study achieved changes in body composition and diets comparable to the results seen with a moderately high protein diet, as the participants lost −7.1(5.4) kg fat mass, and minimal reduction of skeletal muscle mass, −0.9(0.9) kg at 6 months. The mean protein intake of the participants was 22.9(5.0)% and the mean calories was 1582(383) kcal/day based on the four 24‐h records during six months. In contrast to the previous studies that relied on the provision of prepared meals or strict recipes to follow, 19 this study demonstrated that the participants were able to create their own safe weight‐loss diet by increasing protein while reducing calorie intake.
A difference in the mean weight loss between iDip2 participants (n = 22) and iDip1 participants (n = 12) 15 at 12 months was small, −6.49(8.37)% versus −5.35(5.78)%, and nonsignificant (p = 0.64). The lack of improvement in the magnitude of weight loss can be partly attributable to a large divergence in the weight‐loss outcome as seen in the large SD at 12 months. This large variation in weight‐loss outcomes is consistent with other behavioral and dietary weight loss trials. For example, the mean weight loss % (SD) in the Look AHEAD study at 1 year was 8.6(6.9)%. 26 Using intra‐cohort correlation analysis, the Look AHEAD study identified factors associated with successful weight loss, such as physical activity, treatment attendance, and use of meal replacement products. 26 The present study identified significant associations between protein and fiber densities and weight loss. This finding suggests that the divergence in weight loss can be explained partly by a degree of successful dietary changes.
A strong correlation was observed between weight loss at three and 12 months, indicating the critical importance of the first 3 months of the weight‐loss period. Significant associations were found between weight loss and protein and fiber density as early as 3 months. Thus, the strong correlation between three‐ and 12‐month weight loss suggests that participants who were able to develop sustainable dietary changes within the first three months kept losing weight in the subsequent months, whereas those who had difficulty in implementing sustainable dietary changes in early months rarely succeeded in changing diet in later months. Alternatively, this strong correlation may be due to the increased and sustained motivation of participants who succeeded in their early weight loss stage. Although no data were available in the current study to examine this possibility, studies suggest psychological and behavioral factors, such as motivation, level of satisfaction, and self‐confidence, are associated with early weight‐loss outcomes. 27 , 28
In addition to implementing a sustainable diet, this study identified other possible predictors for successful weight loss that may help improve the program. Among comorbidities, a significantly lower weight‐loss outcome was observed in the participants with a self‐reported diagnosis of depression than those without depression although the sample number was small. Frequent concurrence of depression and obesity has been shown, and the association between them may be bi‐directional. 29 A collaboration with a clinical psychologist to treat depression may improve the weight‐loss outcome of the participants with depression. 7
Even though the program provided identical in‐person group sessions three times a week during the intervention, some participants had difficulties attending one of the three sessions, necessitating the provision of make‐up sessions. Changing in‐person sessions to online may improve accessibility by removing time and transportation restrictions. The exit survey indicated room for improving session materials and homework assignments. Reducing scientific terms in session content could improve comprehension. Homework could be better organized as a step‐by‐step assistance for participants to achieve sustainable dietary changes.
The main limitation of this study was large variations among participants in weight‐loss outcomes to be used as a reliable obesity treatment program. Although the weight‐loss outcome of the successful group was promising, and the study identified factors that affect unsuccessful weight loss, further improvement of the program is warranted. Also, it is yet to be determined by follow‐up whether the self‐discovery approach improves the sustainability of lost weight. Although RCT is standard for clinical studies, we employed a single‐arm, before‐and‐after design for the following reasons. First, the main objective of the study was to identify factors that can explain variable outcomes of the novel participant‐driven program using intra‐cohort variation analysis 26 as discussed above. Second, a large weight‐loss RCT has shown that the weight of the placebo group was unchanged during 1 year, whereas the metformin and lifestyle modification groups lost significant weight. 30 Also, another large‐scale trial that used a standard care group as a control showed much smaller weight change in the control compared with intervention group at 1 year (0.7% vs. 8.6%). 26 Thus, it would be reasonable to assume that in the current study, any significant weight change was due to the intervention. For these two reasons, doubling the participants' size to add a control group is not justifiable.
In conclusion, a dietary weight‐loss program based on facilitating informed decision‐making worked very well for nine participants (41%) who achieved greater than 5% of initial bodyweight, whereas other 13 participants failed to reach 5% weight loss. Several possible causes of the divergence were identified by intra‐cohort analyses. Protein and fiber densities were inversely associated with weight loss, suggesting successful dietary implementation as a key contributor to weight‐loss outcomes. Early weight‐loss success and early dietary implementation were predictive of successful weight loss at 12 months. The presence of depression may be a barrier to weight loss and may need to be addressed together with dietary intervention. Accessibility and delivery of program materials could be improved to expand the reach of the program. At least another year of follow‐up will be necessary to determine if the >5% weight loss group is able to maintain their lost weight.
AUTHOR CONTRIBUTIONS
Contributions (in alphabetical order): designed research: Jennie C. Hsu, Mindy H. Lee, Manabu T. Nakamura. Conducted research: Annabelle Shaffer, Catherine C. Applegate, Jennie C. Hsu, Mindy H. Lee, Manabu T. Nakamura, Nouf W. Alfouzan. Performed statistical analysis and data interpretation: Annabelle Shaffer, Mindy H. Lee, Manabu T. Nakamura, Nouf W. Alfouzan. Wrote paper: Annabelle Shaffer, Mindy H. Lee, Manabu T. Nakamura. Other manuscript revision: Annabelle Shaffer, Catherine C. Applegate, John W. Erdman, Jennie C. Hsu, Mindy H. Lee, Manabu T. Nakamura, Nouf W. Alfouzan.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CLINICAL TRIAL REGISTRATION
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The project was supported by a grant (ILLU‐698‐908) from the United States Department of Agriculture of the National Institute of Food and Agriculture. CA was supported by a grant (T32EB019944) from the National Institute of Biomedical Imaging and Bioengineering of the National Institute of Health.
Lee MH, Shaffer A, Alfouzan NW, et al. Successful dietary changes correlate with weight‐loss outcomes in a new dietary weight‐loss program. Obes Sci Pract. 2024;e764. 10.1002/osp4.764
Mindy H. Lee and Annabelle Shaffer, these authors contributed equally to the work.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Look ARG, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long‐term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post‐hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stevens VJ, Obarzanek E, Cook NR, et al. Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1‐11. 10.7326/0003-4819-134-1-200101020-00007 [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Prevention Program Research G, Knowler WC, Fowler SE, et al. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677‐1686. 10.1016/s0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes ‐ 5‐year outcomes. N Engl J Med. 2017;376(7):641‐651. 10.1056/nejmoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen JB. Hypertension in obesity and the impact of weight loss. Curr Cardiol Rep. 2017;19(10):98. 10.1007/s11886-017-0912-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969‐977. 10.1001/jama.297.9.969 [DOI] [PubMed] [Google Scholar]
- 7. Elmaleh‐Sachs A, Schwartz JL, Bramante CT, Nicklas JM, Gudzune KA, Jay M. Obesity management in adults: a review. JAMA. 2023;330(20):2000‐2015. 10.1001/jama.2023.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas JG, Raynor HA, Bond DS, et al. Weight loss in Weight Watchers Online with and without an activity tracking device compared to control: a randomized trial. Obesity. 2017;25(6):1014‐1021. 10.1002/oby.21846 [DOI] [PubMed] [Google Scholar]
- 9. Ard JD, Lewis KH, Rothberg A, et al. Effectiveness of a total meal replacement program (OPTIFAST program) on weight loss: results from the OPTIWIN study. Obesity. 2019;27(1):22‐29. 10.1002/oby.22303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 Year: an open‐label, non‐randomized, controlled study. Diabetes Ther. 2018;9:583‐612. 10.1007/s13300-018-0373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long‐term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2‐year non‐randomized clinical trial. Front Endocrinol. 2019;10. 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Look Ahead Research G. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145‐154. 10.1056/nejmoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Force USPST, Curry SJ, Krist AH, et al. Behavioral weight loss interventions to prevent obesity‐related morbidity and mortality in adults: US preventive Services task force recommendation statement. JAMA. 2018;320(11):1163‐1171. 10.1001/jama.2018.13022 [DOI] [PubMed] [Google Scholar]
- 14. Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553‐1564. 10.1111/dom.14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee MH, Applegate CC, Shaffer AL, Emamaddin A, Erdman JW, Jr. , Nakamura MT. A feasibility study to test a novel approach to dietary weight loss with a focus on assisting informed decision making in food selection. PLoS One. 2022;17(5):e0267876. 10.1371/journal.pone.0267876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilke CO. Fundamentals of Data Visualization: A Primer on Making Informative and Compelling Figures. O'Reilly Media; 2019. [Google Scholar]
- 17. Pratt NS, Ellison BD, Benjamin AS, Nakamura MT. Improvements in recall and food choices using a graphical method to deliver information of select nutrients. Nutr Res. 2016;36(1):44‐56. 10.1016/j.nutres.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 18. Hoffer LJ, Bistrian BR, Young VR, Blackburn GL, Matthews DE. Metabolic effects of very low calorie weight reduction diets. J Clin Invest. 1984;73(3):750‐758. 10.1172/jci111268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy‐restricted high‐protein, low‐fat compared with standard‐protein, low‐fat diets: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281‐1298. 10.3945/ajcn.112.044321 [DOI] [PubMed] [Google Scholar]
- 20. Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight‐loss maintenance. New Engl J Med. 2010;363(22):2102‐2113. 10.1056/nejmoa1007137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu SM, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle‐aged women. Am J Clin Nutr. 2003;78(5):920‐927. 10.1093/ajcn/78.5.920 [DOI] [PubMed] [Google Scholar]
- 22. Koh‐Banerjee P, Franz MV, Sampson L, et al. Changes in whole‐grain, bran, and cereal fiber consumption in relation to 8‐y weight gain among men. Am J Clin Nutr. 2004;80(5):1237‐1245. 10.1093/ajcn/80.5.1237 [DOI] [PubMed] [Google Scholar]
- 23. Mulligan AA, Luben RN, Bhaniani A, et al. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open. 2014;4(3):e004503. 10.1136/bmjopen-2013-004503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3(suppl 2):211s‐216s. 10.1002/j.1550-8528.1995.tb00466.x [DOI] [PubMed] [Google Scholar]
- 25. Lipkin EW, Schwartz AV, Anderson AM, et al. The Look AHEAD Trial: bone loss at 4‐year follow‐up in type 2 diabetes. Diabetes Care. 2014;37(10):2822‐2829. 10.2337/dc14-0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wadden TA, West DS, Neiberg RH, et al. One‐year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17(4):713‐722. 10.1038/oby.2008.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carels RA, Cacciapaglia HM, Douglass OM, Rydin S, O'Brien WH. The early identification of poor treatment outcome in a women's weight loss program. Eat Behav. 2003;4(3):265‐282. 10.1016/s1471-0153(03)00029-1 [DOI] [PubMed] [Google Scholar]
- 28. James BL, Roe LS, Loken E, Rolls BJ. Early predictors of weight loss in a 1‐year behavioural weight‐loss programme. Obes Sci Pract. 2018;4(1):20‐28. 10.1002/osp4.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta‐analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220‐229. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- 30. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


