Summary
Adipose tissue, colloquially known as “fat”, is an extraordinarily flexible and heterogeneous organ. While historically viewed as a passive site for energy storage, we now appreciate that adipose tissue regulates many aspects of whole-body physiology, including food intake, maintenance of energy levels, insulin sensitivity, body temperature, and immune responses. A crucial property of adipose tissue is its high degree of plasticity. Physiologic stimuli induce dramatic alterations in adipose tissue metabolism, structure, and phenotype to meet the needs of the organism. Limitations to this plasticity cause diminished or aberrant responses to physiologic cues and drive the progression of cardiometabolic disease along with other pathological consequences of obesity.
Keywords: Adipose tissue, adipocyte, adipocyte progenitor, brown fat, beige fat, thermogenesis, obesity, diabetes
Villanueva eTOC blurb
Fat is an exceptionally heterogeneous and dynamic organ with many functions beyond energy storage. Cellular, molecular, and systems level studies shed light on what fat is and does; how it is regulated; and how it dysfunctions in obesity, metabolic syndrome and aging.
Introduction
Adipose tissue is defined by the presence of specialized lipid handling cells called adipocytes, which function as the body’s primary energy reservoir. Throughout much of our evolution, access to food was sporadic and stores of adipose tissue were advantageous for surviving extended periods of food insecurity. However, in current times, chronic over nutrition is driving an epidemic of obesity and cardiometabolic disease (e.g., type 2 diabetes, coronary artery disease and stroke) in large parts of the world. Furthermore, obesity increases the risk of developing numerous cancers and predisposes to adverse outcomes in other diseases (Donohoe et al., 2017). The increased mortality among obese patients in the COVID-19 pandemic is a notable example. This expanding health crisis is reversing recent gains in life expectancy and imposes an enormous strain on healthcare systems (Mehta et al., 2020).
The association between excess adiposity and disease has been recognized since antiquity, with notable thinkers like Hippocrates writing over 2,000 years ago “sudden death is more common in those who are naturally fat than in the lean” (Haslam and Rigby, 2010). Indeed, obesity, especially central (abdominal) obesity, is associated with several metabolic pathologies, including hyperglycemia, low HDL cholesterol, hypertriglyceridemia, and hypertension, which together are often called “the metabolic syndrome” (Lanktree and Hegele, 2017). Recent discoveries have revealed a complex and nuanced relationship between adipose tissue and health. Epidemiologic studies indicate that excess fat mass strongly correlate with a higher incidence of metabolic disease (Di Angelantonio et al., 2016; Padwal et al., 2016). However, there is substantial inter-individual variation, with some obese people remaining metabolically healthy and some thin people exhibiting metabolic disease. Furthermore, patients with lipodystrophy have low amounts of adipose tissue yet suffer many of the same ailments as those with severe obesity.
The distribution of adipose tissue into multiple heterogeneous depots and their myriad functions add to the challenges in deciphering the roles of adipose tissue in disease. Beyond its critical role in energy storage, adipose tissue produces hormones that regulate many physiological processes, serves as a hub for inflammatory responses, provides mechanical cushioning and insulation, and participates in heat production for the regulation of body temperature (Rosen and Spiegelman, 2014; Zwick et al., 2018). All these processes may change in adaptive or maladaptive ways during weight loss or gain.
How then should we consider the relationship between adipose tissue and metabolic health? Adipose tissue plays a central role in maintaining whole body insulin sensitivity and energy levels. Adipose tissue regulates insulin action via the secretion of insulin-sensitizing factors like Adiponectin and by sequestering lipids, which would otherwise accumulate in other tissues and have deleterious effects. Indeed, adipose tissue insufficiency (as in lipodystrophy) or dysfunction (as in obesity) leads to the excessive deposition of lipids in other organs like liver and muscle, which is a hallmark of and major contributor to insulin resistance (Petersen and Shulman, 2018). Insulin resistance and high insulin secretion define the pre-diabetic state, which often progresses to type 2 diabetes and contributes to the pathogenesis of other disease processes.
This review discusses the function and regulation of adipose tissue, emphasizing its ability to undergo profound metabolic, structural, and phenotypic remodeling in response to physiologic cues (Figure 1). We further consider how the maintenance of adipose tissue plasticity helps to preserve metabolic health.
Figure 1: Adipose Tissue Plasticity.
Adipose tissue engages in multiple adaptive processes to maintain homeostasis which can be classified into distinct categories of plasticity. A) Adipose tissue changes dynamically in response to cold or warm environment. Phenotypic: In response to cold, individual adipocytes remodel their internal architecture to facilitate thermogenesis in a process called beiging or browning. Beiging involves alterations to the structure of lipid droplets, robust mitochondrial biogenesis, and upregulation of a transcriptional program which supports high levels of local fuel oxidation. These changes are reversible and regress with the removal of stimulus through the reverse process called whitening. Metabolic: Cold exposure promotes a metabolic switch from energy storage to fuel utilization and uncoupled respiration. Thermogenesis is classically achieved by a futile cycle involving UCP1, although recent work has demonstrated that adipocytes employ several mechanisms of futile cycling that promote thermogenesis, including calcium and creatine cycling. Adipocytes respond to several cues for thermogenesis, including neuronal, immune, and metabolite derived signals, allowing tight and context specific control of heat production. Structural: During the response to cold, the structure of adipose tissue remodels to facilitate thermogenesis. Cold incudes the production of new adipocytes from adipogenic progenitor cells via de novo differentiation. Additionally, cold induces angiogenesis and sympathetic nerve fiber branching, which regress upon removal of thermogenic stress. B) White adipose tissue plasticity. Phenotypic: In specific contexts, white adipocytes are capable of reversible dedifferentiation in vivo and in vitro, most notably during lactation (dedifferentiation) and involution (redifferentiation), hair follicle cycling, and in “ceiling culture, a specific technique for primary cell culture of isolated adipocytes. Metabolic: White adipocytes switch between two opposing metabolic programs, nutrient storage and nutrient release. Nutrient storage involves uptake of glucose, amino acids, and fatty acids (TAG: triacylglycerol. FFA: Free fatty acid). By the process of de novo lipogenesis (DNL) excess nutrients are converted into fatty acids allowing for efficient storage in lipid droplets. During periods of fasting or high energy demand (ex. exercise), adipocytes release nutrients into the systemic circulation by breaking down stored TAGs and releasing FFAs through lipolysis. Structural: Adipose tissue has a remarkable ability to expand and contract in response to over- and under- nutrition respectively. Expansion is mediated by a combination of one of two mechanisms: hypertrophy (increases in individual adipocyte size) and hyperplasia (increases fat cell number mediated by de novo differentiation of adipocyte progenitor cells). The distribution of adipose tissue is variable and can be modified toward a more metabolically favorable peripheral distribution (green) or a more metabolically maladaptive central distribution (red) by numerous factors including sex hormones, growth hormones, cortisol, and pharmaceuticals. The structure of adipose tissue is in constant flux due to persistent low-level turnover and replacement of adipocytes at a rate of ~10% per year in humans.
Overview of Adipose Tissue
Placental mammals have three main types of adipocytes – white, beige, and brown, organized into discrete depots throughout the body (Figure 2). White adipocytes are specialized for lipid storage and release, while beige and brown adipocytes are specialized thermogenic cells able to expend nutritional energy in the form of heat.
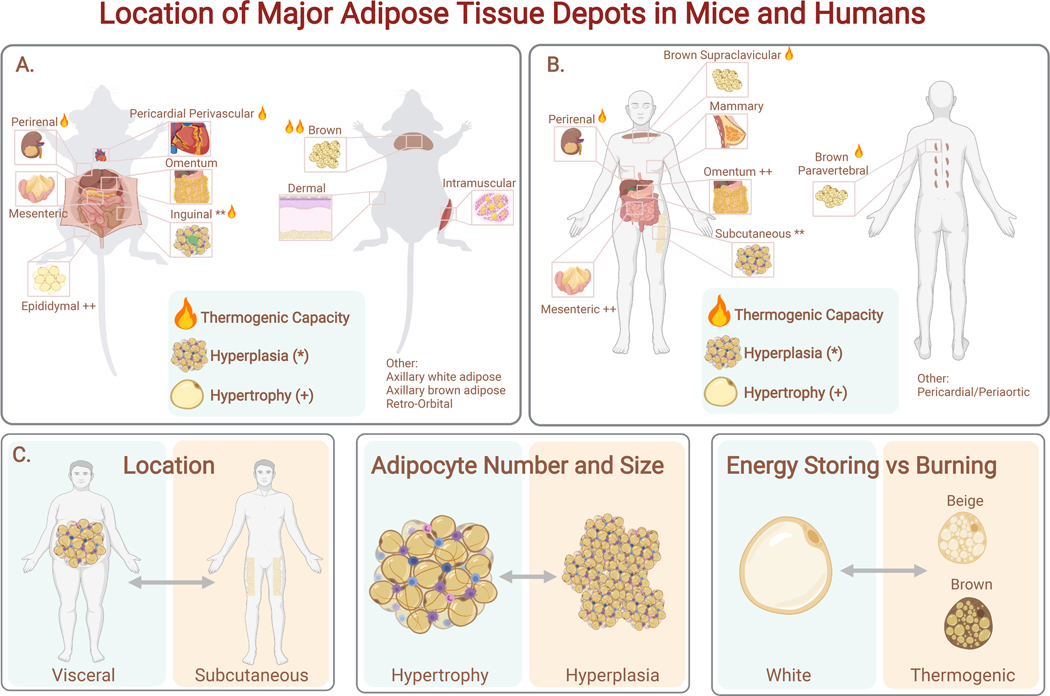
Figure 2. Location of Major Adipose Tissue Depots in Mice and Humans.
Both mice (A) and humans (B) have thermogenic brown adipose tissue (interscapular, cervical, paravertebral). Epididymal adipose tissue (eWAT) is comparable to visceral human adipose tissue (omental, mesenteric adipose tissue (MAT)), while murine inguinal adipose tissue (iWAT) is comparable to human subcutaneous adipose tissue. Fat depots differ in their propensity for thermogenesis. (C) The three axes of adipose tissue variance relevant to metabolic health: location (visceral vs subcutaneous); expansion mechanism (hypertrophy vs hyperplasia), and metabolic phenotype (energy storing vs burning).
White Adipose Tissue (WAT)
WAT is the most abundant form of adipose tissue, found in almost every area of the body (Zwick et al., 2018) (Figure 2). The major WAT depots are classified according to their anatomic location as either subcutaneous or visceral. In humans, visceral fat is located in the peritoneal cavity, corresponding to the omental and mesenteric depots (Chusyd et al., 2016). Subcutaneous fat is located beneath the skin and typically represents 80% or more of total fat mass in humans, concentrated in the abdominal and gluteofemoral depots (Karastergiou and Fried, 2017). Mice and rats have somewhat analogous visceral (mesenteric, perirenal, and gonadal) and subcutaneous (inguinal and axillary) depots (Figure 2). A notable difference is that murine gonadal fat drains into the systemic circulation while human visceral fat drains into the portal circulation (Rytka et al., 2011). In addition to the major fat depots discussed above, smaller deposits of adipocytes serve important mechanical and signaling roles in diverse locations, such as the muscle, breast, bone marrow, orbits, face, joints, feet and dermis (Zwick et al., 2018).
White adipocytes generally possess a single large lipid droplet occupying most of the cell and relatively few mitochondria. A major function of these cells is to store and release energy in response to changes in systemic energy levels. These processes occur on multiple time scales, with lipolysis (fatty acid release) versus lipogenesis (fatty acid uptake/synthesis) acting in the acute setting, the balance of which drives tissue expansion and contraction over longer periods.
WAT is an essential endocrine organ, secreting numerous hormones and other factors, collectively termed adipokines. Adipokines play major roles in regulating whole body metabolism, including promoting insulin sensitivity (e.g. Adiponectin), insulin resistance (e.g. Resistin, RBP4, Lipocalin), and inflammation (e.g. TNF, IL6, IL-1b, IL-8, IL-18, sFRP5) (Funcke and Scherer, 2019). Leptin is particularly well studied as it plays a major role in controlling energy homeostasis. High levels of Leptin signal high levels of energy storage in adipose tissue. Leptin acts in the hypothalamus and other brain regions to promote satiety and augment energy expenditure (Pan and Myers, 2018). Rare loss-of-function mutations in Leptin or the Leptin receptor cause severe forms of monogenic obesity. In common forms of obesity, the brain becomes resistant to higher levels of Leptin. An intriguing recent study shows that reducing leptin levels in obese mice alleviates Leptin resistance, decreases obesity and improves metabolic parameters (Zhao et al., 2019).
Brown and beige adipose tissue
Brown and beige adipocytes, while representing a small proportion of total adipose tissue, can exert a sizable metabolic impact due to their capacity to engage in thermogenesis. When fully active, BAT can increase whole body energy expenditure by over 100% in mice and by 40–80% in humans (Angueira et al., 2020; Ouellet et al., 2012). Both cell types are characterized by multilocular lipid droplets, high mitochondrial density, and expression of Uncoupling Protein 1 (UCP1) (Figure 2). Upon activation, UCP1 separates nutrient catabolism from ATP synthesis by dissipating the proton gradient in the inner mitochondrial membrane, releasing potential energy in the form of heat (Cannon and Nedergaard, 2004).
Brown adipocytes develop in dedicated deposits of brown adipose tissue (BAT) that are specified prior to birth whereas beige adipocytes develop in WAT depots, predominantly in response to cold exposure. The major murine BAT depot is located in the interscapular region, with additional depots found in cervical, axillary, perivascular, and perirenal regions (Zhang et al., 2018) (Figure 2). Human infants also possess an interscapular BAT depot, which later regresses and is absent in adults (Lidell et al., 2013). Adult humans possess substantial, though variable, amounts of BAT and beige fat tissue in the paravertebral junctions, cervical/axillary region, along the trachea and blood vessels, and in perirenal/adrenal locations (Ouellet et al., 2011). Several groups have isolated populations of thermogenic adipocytes from adult humans: some report more transcriptional similarity to mouse beige adipocytes, while others report more similarity to mouse brown adipocytes (Jespersen et al., 2013; Lidell et al., 2013). The results from these studies are probably influenced by the biopsy site and history of cold exposure, so it is likely that humans have both brown and beige adipocytes.
Thermogenic fat is critical for adaptation to environmental cold in mice and humans, but current interest in these tissues focuses on their ability to act as a metabolic sink for excess nutrients. Many studies have shown that mice with increased thermogenic fat activity are protected against weight gain and metabolic dysfunction (Harms and Seale, 2013). Moreover, transplantation of brown or beige fat into obese mice enhances insulin sensitivity and decreases fat mass (Liu et al., 2015; Min et al., 2016). Similarly, in humans, augmenting brown fat activity is associated with beneficial metabolic effects (Chondronikola et al., 2016). In addition to suppressing weight gain by elevating energy expenditure, thermogenic adipocytes improve systemic metabolism and insulin-action via clearing triglyceride-rich lipoproteins, acylcarnitines, glucose and other potentially toxic metabolites such as branched chain amino acids (BCAAs) that have been closely linked to metabolic dysfunction (Bartelt et al., 2011; Yoneshiro et al., 2019).
Metabolic Plasticity of White Adipocytes
WAT metabolism rapidly shifts to meet the energetic needs of the organism, which vary greatly during times of fasting, feeding, cold, and exercise. WAT switches between two opposing metabolic programs, one driving nutrient uptake and the other nutrient release, to ensure that other organs always have an adequate, but not excessive, level of energy (Figure 3). The metabolic plasticity of white adipocytes is controlled by hormonal and neuronal signals acting through a cadre of effector proteins and transcriptional regulators.
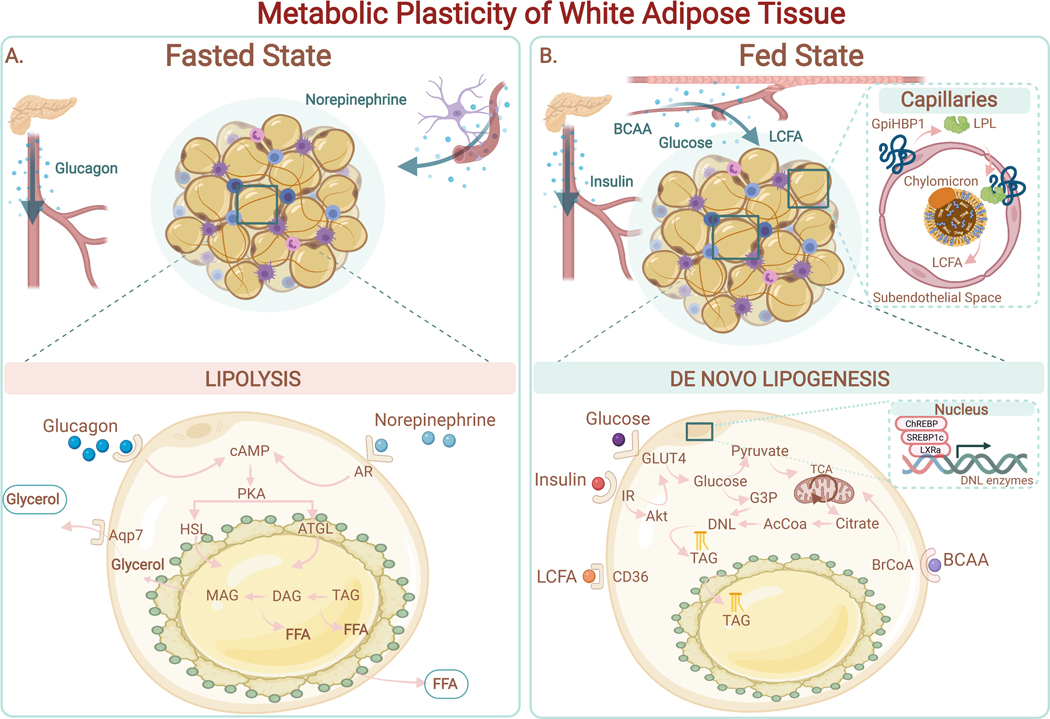
Figure 3. Metabolic Plasticity of White Adipose Tissue.
(A) Fasted state. Adipocytes release free fatty acids (FFAs) and glycerol via lipolysis in response to external stimulation (i.e., norepinephrine, glucagon). Binding of norepinephrine to the adrenergic receptor (AR) on adipocytes drives the elevation of cAMP and PKA activation. PKA stimulates the hydrolysis of triglycerides (TAG), diacylglycerol (DAG), and subsequently monoacylglycerol (MAG) through activation of the endogenous lipases ATGL and HSL. FFAs and glycerol are secreted into the systemic circulation to supply fuel to other tissues.(B) Fed State. Adipocytes have access to multiple sources of circulating nutrients, including: 1) Long Chain Fatty Acids (LCFA) from Very Low-Density Lipoprotein (VLDL) (LPL-mediated hydrolysis of triacylglycerols from VLDL in capillaries to generate FFAs); 2) glucose; 3) branched-chain amino acids (BCAA). De novo lipogenesis (DNL) uses Acetyl-CoA (AcCoA) as the primary building block for fatty acid synthesis. Synthesized fatty acids are esterified into triglycerides (TAG) and stored in lipid droplets. Expression of enzymes involved in DNL (i.e., fatty acid synthase (FAS); acetyl-CoA carboxylase (ACC)) are positively regulated by hormones (i.e., insulin) and by transcription factors such as Carbohydrate response element binding protein (ChREBP), Liver X receptor alpha (LXRa) and Sterol response element binding protein 1c (SREBP1c). TAGs stored in the lipid droplet are released by lipolysis during periods of energy demand.
Nutrient Uptake and Lipogenesis
During periods of positive-energy balance and after feeding, WAT takes up nutrients from the bloodstream and stores them as lipids. This process is mediated by both fatty acid uptake and through the conversion of other nutrients (e.g., glucose) into lipids via de novo lipogenesis. The major signal for nutrient uptake into adipocytes is the hormone insulin, secreted by pancreatic β-cells in response to increased circulating levels of glucose and fatty acids (Petersen and Shulman, 2018). Insulin drives lipid storage in adipocytes by: (1) stimulating glucose uptake, (2) promoting de novo lipogenesis (DNL), and (3) suppressing lipolysis (Carpentier, 2021). Insulin signaling is also critical for the differentiation and maintenance of adipocytes; genetic deletion of the insulin receptor or downstream effectors in adipocytes causes varying degrees of lipodystrophy along with insulin resistance (Sakaguchi et al., 2017; Shearin et al., 2016; Vazirani et al., 2016).
Adipocytes contain specialized machinery to take up FFAs from circulating chylomicrons and very low-density lipoprotein (VLDL) (Figure 3). A major constituent of this machinery is lipoprotein lipase (LPL), an enzyme responsible for the hydrolysis of triacylglycerols (TAG) into FFAs and monoacylglycerols. LPL, produced from adipocytes is transported to the apical membrane of capillaries in adipose tissue via the action of the GPi anchored protein GpiHBP1 (Davies et al., 2010). After LPL releases FFAs, specialized FA binding and transport proteins (FATPs), such as FATP1 and CD36, facilitate the uptake of fatty acids into adipocytes. Insulin stimulates the translocation of FATP1 to the plasma membrane to promote FA uptake. Once taken up by adipocytes, FAs are activated by acyl-CoA synthetase to generate acyl-CoAs, which are the substrate for successive acylation reactions with glycerol through the Kennedy pathway. The last step in triglyceride synthesis joins an acyl-CoA and diacylglycerol (DAG) through diacylglycerol acyltransferase enzymes (DGAT1 and DGAT2) (Carpentier, 2021).
Adipocytes also synthesize acyl chains through DNL. Adipose tissue and liver are the two major sites for DNL, with adipose tissue accounting for more whole-body lipogenesis in humans and the liver accounting for more in rodents (Song et al., 2018). DNL is essential for maintaining energy balance, since it converts excess energy from carbohydrates and protein into fatty acids and ultimately triglycerides, for storage in lipid droplets. DNL initially involves the breakdown of nutrients through the TCA cycle, followed by export of citrate to the cytoplasm which is converted through a series of steps into Acetyl-CoA, Malonyl-CoA, and finally into FAs. DNL is regulated at multiple levels, including: (1) the buildup of malonyl coA, which signals to suppress FA oxidation, and (2) transcriptional activation of key enzymes in the DNL pathway. In particular, carbohydrate response element binding protein (ChREBP), LXRa, and sterol response element binding protein 1c (SREBP1c) stimulate the expression of key DNL, enzymes fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) (Herman et al., 2012). ChREBP is a major transcriptional regulator of DNL in adipocytes, and its expression is controlled by mammalian rapamycin complex 2 (mTORC2), linking the regulation of de novo lipogenesis to growth factor responses (Figure 3) (Tang et al., 2016).
Adipocyte DNL maintains insulin sensitivity by converting excess nutrients into lipids for sequestration in adipocytes. Additionally, DNL in adipocytes results in the production of several lipid species with anti-inflammatory and insulin sensitizing effects (Yilmaz et al., 2016; Yore et al., 2014). These lipids largely correspond to branched fatty acid esters of hydroxy fatty acids (FAHFA), of which there are many variants, based on the position of the branched ester (Zhou et al., 2019). Among these, palmitic acid esters of hydroxy stearic acid (PAHSA) have been singled out for their insulin sensitizing properties. PAHSAs signal through GPR120 to enhance insulin stimulated glucose uptake into adipocytes and also have direct and indirect insulin sensitizing effects in the liver (Yang et al., 2018; Zhou et al., 2019). Finally, branched chain amino acids (BCAAs) are also used as substrate for DNL, thereby limiting their buildup in circulation, which has been linked to insulin resistance (Yoon, 2016).
Energy Mobilization through Adipose Tissue Lipolysis
Lipolysis is the process of hydrolyzing triacylglycerols into glycerol and free fatty acids (FFAs) (Figure 3). Sympathetic nerve-derived catecholamines stimulate lipolysis, and this process is repressed by insulin (Fruhbeck et al., 2014). In particular, epinephrine and norepinephrine release are induced by fasting or exercise and signal through the adrenergic receptor-PKA pathway in adipocytes to increase lipolysis. Lipolysis depends on the inhibitory phosphorylation of the lipid droplet membrane protein PLIN1 (Sztalryd and Brasaemle, 2017). In a basal or anabolic state, PLIN1 is bound to comparative gene identification 58 (CGI-58) (Chouchani and Kajimura, 2019). Upon stimulation of lipolysis, PLIN1 is phosphorylated, triggering the release of CG1–58 and subsequent activation of adipose triglyceride lipase (ATGL). ATGL moves to the lipid droplet surface to hydrolyze triglycerides. PKA also phosphorylates HSL, which binds to PLIN1 to favor the hydrolysis of diacylglycerol, and subsequently monoacylglycerol. The final products, glycerol and FFAs are exported into the bloodstream (Figure 3). While lipolysis is viewed as the main pathway for lipid release, a recent study demonstrates that lipids are also exported from adipocytes in exosomes, providing an important local signal for macrophage differentiation (Flaherty et al., 2019). Lipolysis is further regulated by several endocrine factors. Leptin promotes lipolysis via stimulation of neuro-adipose junctions (Zeng et al., 2015). Growth hormone (GH), adrenocorticotropic hormone, cortisol, thyroid hormones, PTH, and glucagon also provide regulatory roles in lipolysis (Fruhbeck et al., 2014). By contrast, insulin signaling functions as the major anti-lipolytic factor by blocking production of intracellular cAMP, leading to suppression of PKA-activity and lipolysis.
Thermogenic Adaptation of White Adipose Tissue
A striking example of adipose tissue plasticity is observed during environmental cold exposure. Initially, animals shiver and activate pre-existing BAT to help defend their body temperature. Longer exposure recruits additional thermogenic capacity, mediated by increases in BAT mass and elevated expression of thermogenic genes (Cannon and Nedergaard, 2004). In WAT, especially in rodents, cold exposure induces the development of mitochondria-rich, thermogenic beige adipocytes. The rapid induction of beige adipocytes is accompanied by remarkable changes in tissue structure, including increased nerve fiber arborization and angiogenesis. Importantly, these cold-induced changes in BAT and WAT are reversible and regress in the absence of cold, highlighting the flexibility of the tissue.
Beige adipocytes can be generated via three mechanisms: (1) the differentiation of progenitor cells into new beige adipocytes (i.e. de novo beige adipogenesis), (2) the activation (or re-activation) of the thermogenic program in mature adipocytes and (3) the proliferation of mature beige adipocytes (Park et al., 2021; Shao et al., 2019; Wang et al., 2013). Activation of the beige program in adipocytes involves upregulation of thermogenic genes such as Ucp1, mitochondrial biogenesis and lipid droplet remodeling from a unilocular to multilocular morphology (Kim et al., 2019).
BAT undergoes an analogous thermogenic recruitment process during cold exposure. Histological studies show that expression of UCP1 in brown adipocytes is not homogeneous, suggesting a level of cellular heterogeneity in BAT (Cinti et al., 2002). A recent study identified two distinct populations of thermogenic cells in mouse BAT, classical brown adipocytes and ‘low-thermogenic’ brown adipocytes exhibiting fewer mitochondria, lower levels of UCP1, and larger lipid droplets (Song et al., 2020). Interestingly, cold exposure activated the ‘low-thermogenic’ cells to become highly thermogenic. Another recent study identified a new subset of ‘thermogenesis-inhibitory’ adipocytes in mouse and human BAT that restrain the thermogenic capacity of brown adipocytes via local production of acetate (Sun et al., 2020b). These inhibitory adipocytes are enriched in BAT under thermoneutral (non-stimulated) conditions, suggesting that BAT function is regulated by the coordinated activity of distinct adipocyte subpopulations.
Adrenergic signaling is the major physiologic signal controlling both the formation and thermogenic activity of brown and beige adipocytes. Adipose tissue, especially BAT, is densely innervated by sympathetic neurons (Morrison, 2016). Upon cold exposure, sympathetic neurons release the neurotransmitter norepinephrine (NE), which activates the β-adrenergic receptor-cAMP-PKA pathway in adipocytes. This signaling cascade induces lipolysis, thermogenesis and stimulates brown fat-selective gene transcription in brown and beige adipocytes. UCP1 function and thus thermogenic respiration is acutely activated by long-chain FAs and inhibited by purine nucleotides (Bertholet and Kirichok, 2017; Fedorenko et al., 2012).
A key hub of the thermogenic transcriptional response is the coactivator protein PPARg coactivator-1a (PGC1-α), which is upregulated by cold exposure (Puigserver et al., 1998). PGC1-α is phosphorylated and activated by p38 mitogen-activated protein kinase (MAPK) in response to β-adrenergic signaling (Cao et al., 2004). PGC1-α co- activates several transcription factors, including PPAR and ESRR family members. Thyroid Receptor and IRF4 to increase the transcription of Ucp1 and other mitochondrial genes involved in thermogenesis (Shapira and Seale, 2019).
Adrenergic stimulation of adipocytes also activates the nutrient-sensing mTOR pathway, a central integrator of cell and tissue metabolism that functions in two distinct complexes, mTORC1 and mTORC2 (Ye et al., 2019). PKA phosphorylates Raptor and activates the mTORC1 complex in β-adrenergic agonist-stimulated adipocytes (Liu et al., 2016). Mice with genetic loss or inhibition of Raptor display reduced WAT beiging and impaired brown fat activity (Labbe et al., 2016; Liu et al., 2016; Tran et al., 2016). The mTORC2 complex, containing the Rictor subunit, is also required for glucose uptake and glycolysis in brown fat tissue during cold exposure (Albert et al., 2016). Interestingly, inhibition of mTORC2 in brown adipocytes reduces glucose uptake and lipid storage while also leading to enhanced lipid catabolism, associated with protection against cold and obesity (Jung et al., 2019). By contrast, loss of mTORC2 in all adipocytes leads to systemic insulin resistance, which can indirectly decrease BAT function (Tang et al., 2016).
A new study shows that cold and β-adrenergic signaling also activate expression of the ligand-independent G-protein coupled receptor, GPR3 in brown adipocytes (Sveidahl Johansen et al., 2021). GPR3 amplifies the β-adrenergic response to enable high levels of thermogenesis. Forced expression of GPR3 in adipose tissues dramatically augments energy expenditure and can reduce obesity in mice. Finally, numerous other extracellular signals, hormones, and metabolites (e.g. FGF21, natriuretic peptides, acetylcholine, and Irisin) promote WAT beiging and add an additional layer of regulation to the control of thermogenesis (Cohen and Kajimura, 2021).
Immune cells and beiging
Immune cells, including M2 macrophages, mast cells, eosinophils and ILC2s regulate adipose tissue remodeling and thermogenesis during cold exposure. Type 2 cytokines, especially IL-4, promote beige fat biogenesis and ameliorate obesity, although the involved mechanisms remain uncertain (Fischer et al., 2017; Henriques et al., 2020; Qiu et al., 2014). Innate lymphoid ILC2 cells, activated by IL-33, promote beiging through two proposed pathways: (1) the production of methionine-enkephalin peptides, that act on adipocytes to stimulate UCP1 expression (Brestoff et al., 2015); and (2) the induction of IL-4 and IL-13, which act on adipocyte progenitor cells to promote beige adipocyte differentiation (Lee et al., 2015). Recent work has identified stromal cells as a critical source of IL-33 in adipose tissue, illustrating the crosstalk between mesenchymal cells and immune cells in regulating adipose tissue phenotypes (Mahlakoiv et al., 2019; Shan et al., 2021; Spallanzani et al., 2019). The anti-inflammatory cytokine IL-10 suppresses thermogenic genes in adipocytes. Deletion of the IL-10 receptor in adipocytes augments thermogenesis and reduces obesity (Rajbhandari et al., 2019). Additionally, recent studies demonstrate an important role for γδ T cells in regulating innervation, especially in BAT. Specifically, IL-17 secreted from γδ T cells acts on brown adipocytes, leading to TGFβ production and increased sympathetic innervation. Deletion of the γδ T cells or IL-17R on brown adipocytes reduces energy expenditure in mice and exacerbates obesity (Hu et al., 2020).
Adipose tissue whitening
The thermogenic phenotype of fat cells, especially beige fat cells, is unstable, requiring persistent stimulation. Elegant cell tracking studies revealed that UCP1+ beige fat cells become unilocular white-appearing adipocytes following re-warming (Roh et al., 2018; Rosenwald et al., 2013). During this “whitening process”, fat cells lose UCP1 expression and mitochondrial density, and remodel their lipid droplets from a multilocular to a unilocular architecture over the course of ~4 weeks (Roh et al., 2018). This process involves direct conversion of beige adipocytes rather than proceeding through a progenitor cell state and depends on mitochondrial clearance (Altshuler-Keylin et al., 2016). Decreased adrenergic signaling in beige fat cells induces the recruitment of the E3 ubiquitin ligase complex, Parkin, to mitochondria, triggering mitophagy. Impairing this process by deletion of autophagy components, Atg5, Atg12, or Parkin prevents the “beige-to-white” phenotype transition (Lu et al., 2018). Mitophagy in adipocytes is also driven by the kinases STK3 and STK4. STK3 and STK4 are highly expressed in ‘white- appearing’ (unstimulated) adipocytes and downregulated during cold exposure. Genetic loss or inhibition of STK3/4 activity increases mitochondrial content and uncoupled respiratory activity in beige and brown adipocytes via reducing mitophagy (Cho et al., 2021). Remarkably, inhibiting mitochondrial clearance in the above mouse models ameliorates obesity and improves systemic metabolism, though this may be expected to cause aberrant mitochondrial function over the long term. A similar whitening process occurs in BAT with exposure to warmer temperatures and during aging.
The gene expression profile of ‘previously beige adipocytes’ is nearly indistinguishable from ‘white’ adipocytes (never beige) after rewarming (Roh et al., 2018). However, ‘previously beige’ cells rapidly reactivate the thermogenic program upon a second exposure to cold (Rosenwald et al., 2013). Compared to white adipocytes, ‘previously beige’ adipocytes display increased levels of H3K4me1, a chromatin mark associated with active or primed enhancers, at certain thermogenic genes, indicating an epigenetic memory of cold exposure (Roh et al., 2018). Because of the plasticity of mature adipocytes, the balance between new beige adipocyte differentiation and re-activation of ‘previously beige’ adipocytes during beiging depends on the environmental exposure history of the animal. For example, in mice that have recently undergone cold exposure, reactivation of dormant beige cells predominates, whereas in cold naïve animals, de novo beige adipocyte differentiation from progenitor cells is favored (Shao et al., 2019). Interestingly, UCP1+ cells, specifically in the central region of iWAT (near the lymph nodes) exhibit proliferative capacity and generate new beige adipocytes in response to β-adrenergic stimulation (Park et al., 2021).
Metabolic Programming for Thermogenesis
The thermogenic capacity of brown and beige adipocytes relies on burning FAs via oxidative metabolism (Gonzalez-Hurtado et al., 2018). Classically, adipose tissue thermogenesis is driven by sympathetic nerve-mediated adrenergic signaling, which stimulates lipolysis (Figure 4). Free fatty acids (FFAs) serve as both a fuel for thermogenesis and as an allosteric activator of UCP1 function. Surprisingly, lipolysis in UCP1+ adipocytes is dispensable for thermogenesis. However, disrupting lipolysis in all adipocytes compromises thermogenesis in the absence of food, demonstrating that white fat cells can supply the FFAs necessary to support adipose tissue thermogenesis (Schreiber et al., 2017; Shin et al., 2017). Furthermore, even lipid droplets in BAT are dispensable for thermogenesis. Deletion of core lipogenesis components DGAT1 and DGAT2 in UCP1+ adipocytes produces lipid droplet-less adipocytes, that remain competent for thermogenesis (Chitraju et al., 2020).
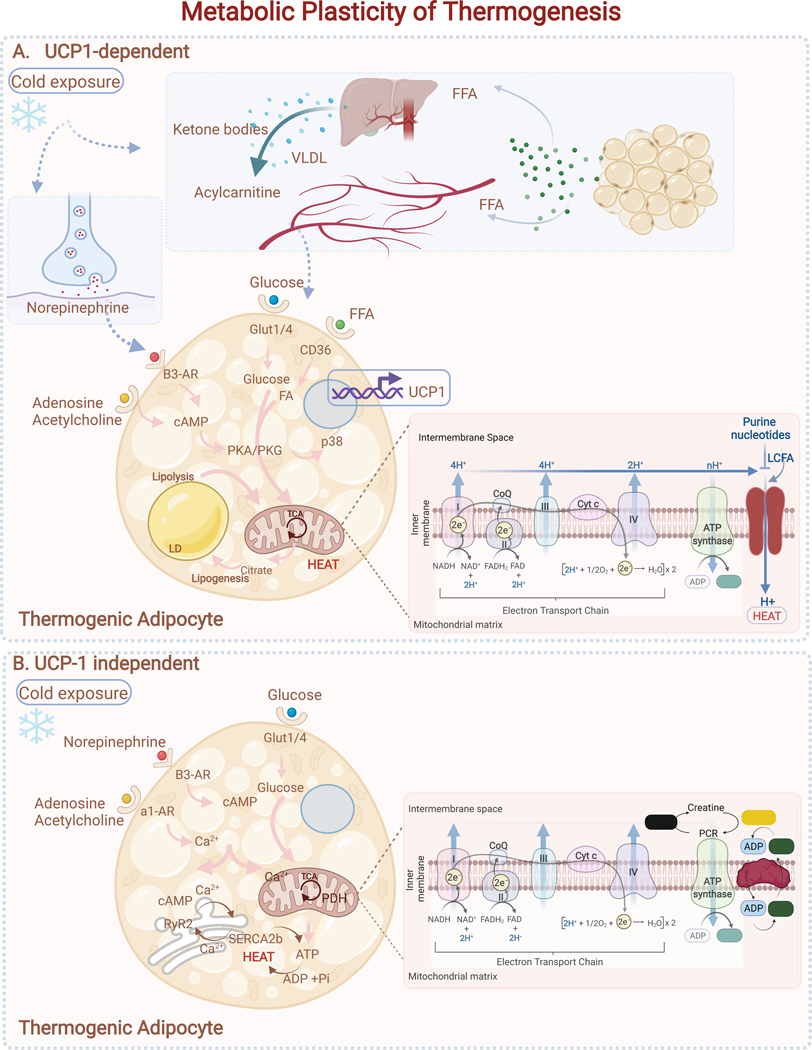
Figure 4. Metabolic Plasticity of Thermogenesis.
(A) UCP-1 dependent: Cold exposure or adrenergic stimulation increases cAMP levels, driving activation of PKA/PKG signaling and lipolysis. P38MAPK (p38) in turn promotes activation of transcriptional regulators that drive mitochondrial biogenesis and expression of thermogenic genes (Ucp1, Cidea, Dio2). When activated, UCP1 drives proton leak in the mitochondria, leading to uncoupling of mitochondrial respiration from ATP synthesis and driving greater consumption of fuels (e.g., glucose and free fatty acids (FFA)). FFA secreted by WAT also drives hepatic production of acylcarnitines and ketones to help fuel thermogenesis.(B) UCP1-independent: beige thermogenic cells use Ca2+ futile cycling through the SERCA2b-RyR2 pathway in the endoplasmic reticulum (ER) to produce heat during cold exposure. Creatine cycling in the mitochondria is an additional mechanism to produce heat independent of UCP1. UCP-1 independent pathways require glycolysis and mitochondrial ATP-synthesis to provide fuel for futile cycling.
Brown fat in mice and humans also oxidize branched-chain amino acids (BCAAs) during cold exposure (Yoneshiro et al., 2019). This pathway likely contributes to the metabolic benefits of BAT given the well-established link between elevated circulating BCAAs and insulin resistance. Interestingly, recent studies show that brown fat cells take up and concentrate large amounts of the TCA intermediate succinate, which promotes thermogenic respiration (Mills et al., 2018). Mechanistically, the oxidation of succinate generates reactive oxygen species that promote UCP1-activity (Chouchani et al., 2016).
Adipocytes can also carry out thermogenesis through an expanding array of UCP1-independent mechanisms. The existence of such mechanisms were initially invoked when it was observed that UCP1-null mice can adapt to the cold if ambient temperature is gradually decreased (Ukropec et al., 2006). These alternative mechanisms have been extensively reviewed elsewhere and include Ca2+ futile cycling, creatine-dependent substrate cycling, and triacylglycerol futile cycling (Chouchani and Kajimura, 2019; Ikeda et al., 2017; Kazak et al., 2015). Of note, the futile creatine cycle is required for high fat diet-induced energy expenditure in adipocytes. Ablation of this pathway in mice sensitizes them to obesity and metabolic complications (Kazak et al., 2017) (Figure 4).
Structural Remodeling to Optimize Thermogenesis
Sympathetic innervation is critical for brown and beige fat thermogenesis. BAT is more densely innervated than WAT depots; however, a recent study shows that >90% of adipocytes in inguinal WAT are closely opposed to sympathetic fibers, which likely relates to the high beiging potential of this depot (Jiang et al., 2017; Murano et al., 2009). Sympathetic arborization increases (by over 3 fold) during cold exposure and is likely essential for sustaining high levels of thermogenic activation (Cao et al., 2018b). Indeed, ablation of nerve fiber arborizations blunts the development of beige adipocytes in response to cold exposure (Cao et al., 2019; Jiang et al., 2017). The expansion of neurites is reversible and neurite density normalizes to baseline within approximately 4 weeks after removal of cold stimulus (Cao et al., 2018b). The growth and branching of sympathetic neurites during cold exposure is regulated by adipocytes. For example, adipocyte-specific deletion of the brown fat transcription factor PRDM16 impairs nerve fiber growth and branching in WAT following cold exposure (Chi et al., 2018). Adipose cells have been shown to produce a variety of neurotrophic factors, including Nerve Growth Factor (NGF), Neuregulin-4 (NRG4), TGFβ and S100b (Hu et al., 2020; Rosell et al., 2014; Zeng et al., 2019).
Vascular density also increases in adipose tissue during cold exposure to support the increase in metabolic activity (Xue et al., 2009). Vascular density doubles within just 5 days and is reversible upon warming (Cao et al., 2018a). As with angiogenesis in other organs, vascular endothelial growth factor (VEGF) is a critical regulator of this process in adipose tissue. Knockout of VEGF in adipose cells leads to whitening of both brown and beige fat (During et al., 2015; Shimizu et al., 2014). Interestingly, overexpression of VEGF stimulates browning of WAT and BAT, suggesting that VEGF and/or angiogenesis plays an instructive role in beiging, in addition to supporting the increase in tissue metabolism (During et al., 2015; Sun et al., 2014).
Phenotypic Plasticity of Adipose Tissue
Intriguing work over the past few years shows that adipocytes are not necessarily terminally differentiated. Under certain conditions adipocytes reversibly de-differentiate and re-differentiate, cycling between a progenitor cell and adipocyte state.
The capacity for adipocytes to dedifferentiate ex vivo was noted in the 1980’s with the development of the ceiling culture method to isolate adipogenic primary cell lines. In this method, mature adipocytes are induced to adhere to the top surface of a flask, where they de-differentiate into a fibroblastic pre-adipocyte state (Cote et al., 2019). However, whether de-differentiation of adipocytes occurs in vivo had been unclear until recently. Bi et al. demonstrated that activation of Notch signaling induces the de-differentiation of adipocytes, leading to the development of liposarcomas (Bi et al., 2016). In lactation, the posterior mammary fat pads (inguinal fat) in mice remodel, with proliferation of mammary alveolar structures and a relative loss of adipocytes in the areas of high ductal density. During this process, mature adipocytes de-differentiate into proliferative fibroblasts that retain their adipocyte differentiation capacity in vitro and in vivo (Wang et al., 2018). Similarly, adipocytes within the dermis undergo reversible de-differentiation during hair follicle cycling and wound healing (Shook et al., 2020; Zhang et al., 2019). In the dermis, mature adipocytes de-differentiate and give rise to myofibroblasts, specialized contractile fibroblasts which secrete extracellular matrix for wound repair. Adipocytes undergoing de- differentiation stimulate lipolysis and release of FFA, which also plays a critical role in regulating the wound inflammatory response (Shook et al., 2020). It remains unknown if adipocyte de-differentiation occurs in the major WAT and BAT depots under other physiological conditions, such as during fasting, weight loss, or wound healing outside of the skin.
Adipose Tissue Expandability
Adipose tissue has an unparalleled ability to expand, and contract compared to other organs. In humans, the proportion of body fat varies widely, ranging from normal levels of 10–20% in men and 15–30% in women, to below 5% in bodybuilders and anorexic patients, and above 70% in severe obesity. These differences in fat mass are driven by long term calorie surplus or deficit, and the structural changes are enabled by the coordinated action of several cell types, including adipocytes, adipocyte progenitor cells, and immune cells.
The Structure of Adipose Tissue
Human subcutaneous WAT is organized by fibrous septa that define progressively smaller tissue compartments at each scale. The highest-level division is formed by a collagen and elastin rich sheet called the fascia superficialis, often referred to as Scarpa’s fascia in the abdominal wall and a “membranous layer” in other body regions (Markman and Barton, 1987). The fascia superficialis runs parallel to the plane of the skin and separates subcutaneous fat into a superficial (sSAT) and a deep compartment (dSAT). At the next level, thinner fibrous septa, sometimes referred to as retinacula cutis superficialis (in sSAT) and profundus (in dSAT), define centimeter scale compartments, and anchor the fascia superficialis to the dermis above and to the deep fascia below. Together, the fascia superficialis, retinacula cutis, and compartments of sSAT and dSAT, are referred to as the superficial fascial system, and are identifiable in nearly all areas of the body (Lockwood, 1991). Finally, within the compartments of the superficial fascial system, 500 μm-1000 μm lobules of adipocyte rich stroma are encapsulated by fibrous septa, representing the smallest structural unit of subcutaneous adipose tissue (Esteve et al., 2019).
The distinctions between sSAT and dSAT are not purely anatomic. During obesity, both the abdominal sSAT and dSAT expand, with males exhibiting a tendency to expand the abdominal dSAT preferentially (Kim et al., 2016). Compared to abdominal sSAT, the dSAT is more prone to inflammation, contains more saturated lipids, and adipocyte progenitor cells from this layer are more resistant to differentiation (Cancello et al., 2013). Accordingly, expansion of the dSAT, especially in men, is associated with adverse metabolic outcomes (Kelley et al., 2000).
Mice have two main subcutaneous WAT depots, the posterior inguinal WAT (iWAT) and anterior axillary WAT (axWAT). The inguinal WAT is heavily studied, due to its larger size, high propensity for beiging, and ease of dissection. Both the iWAT and axWAT lie directly beneath the panniculus carnosus, a layer of striated muscle which separates the subcutaneous structures from the overlying dermis, that some have speculated is an evolutionarily analogous structure to the fascia superficialis (Fodor, 1993). Both depots are encased on all sides by a thin fibrous membrane containing mostly DPP4+ fibroblasts that can also serve as adipocyte progenitors (Merrick et al., 2019; Stefkovich et al., 2021). At the next scale, the tissue can be subdivided into lobular areas (the central areas within the tissue) and non-lobular areas (surrounding, at the periphery) (Barreau et al., 2016; Peurichard et al., 2017). The lobular areas are delineated by fibrous septations, analogous to those found in humans that create discrete compartments of adipocytes on the order of 300 μm (Chi et al., 2018; Dichamp et al., 2019). Several studies have noted clear anatomic regionality within the iWAT, with the more ventral regions and central lobular areas being more prone to cold-induced beiging, as compared to the peripheral and posterior regions (Barreau et al., 2016; Chi et al., 2018).
The structure of visceral adipose tissue has been less well studied. A defining feature of visceral fat is that, like other intraperitoneal organs, it is surrounded by a layer of mesothelium (Chau et al., 2014). Thus, both visceral and subcutaneous fat are encased by a lining of specialized cells (mesothelial cells for visceral fat, DPP4+ fibroblasts for subcutaneous fat), although in contrast to subcutaneous fat, this lining does not appear to contribute to adipocyte generation in visceral adipose tissue (Westcott et al., 2021). Furthermore, a recent report demonstrates the presence of lobules in human visceral adipose tissue, analogous to those present at the smallest scale in subcutaneous fat (Esteve et al., 2019). By contrast, mouse visceral fat depots do not have a readily apparent lobular structure.
Adipose Tissue Expansion
Adipose tissue expansion is intricately linked to metabolic health. While high fat mass generally correlates with poor metabolic health, a high capacity for expansion protects against metabolic disease. The apparent contradiction in this relationship can be understood by considering the fate of excess nutrients. Once ingested and absorbed, excess nutrients must be either burned or stored. WAT is uniquely capable of safely storing large quantities of excess nutrients as lipids. In contrast, accumulation of excess lipids in other tissues drives insulin resistance (Petersen and Shulman, 2018). Therefore, the proper partitioning of excess nutrients into WAT for storage or into BAT for heat generation promote metabolic health. Notably, the site of adipose tissue expansion (into visceral or subcutaneous depots) and the mechanism of expansion, via increases in adipocyte number (hyperplasia) or size (hypertrophy), have profound impacts on metabolic health.
Adipose Tissue Distribution: Metabolic Consequences
Fat tissue distribution is highly variable, driven by differences between sexes, genetics, development, aging, and in response to hormones or drugs. The most common distinction between types of adipose tissue distribution is whether fat is stored viscerally or subcutaneously, and countless studies have examined the relative effects of visceral versus subcutaneous adiposity on overall health. Almost universally, since the first descriptions of “android” (central) vs “gynoid” (subcutaneous/peripheral) obesity by the French physician Jean Vague in the 1950’s, studies have shown that increased visceral/central adiposity correlates with worse insulin resistance and an increased risk of cardiometabolic disease, even in normal weight subjects (Chait and den Hartigh, 2020). By contrast, the preferential expansion of subcutaneous adipose tissue, especially in the superficial region, is associated with a more favorable metabolic profile (Kelley et al., 2000). It should be noted that, despite its metabolic importance, visceral fat represents only a small portion (~6–20%) of total fat mass, with this proportion generally higher in males (Karastergiou and Fried, 2017).
Differences in body fat distribution may also explain the existence of “metabolically healthy obese” and “metabolically unhealthy normal-weight” individuals (Smith et al., 2019). Estimates from the United States suggest that 23.5% of normal weight adults are metabolically unhealthy while 31.7% of obese are metabolically healthy (Wildman et al., 2008). Metabolically healthy obese people have unexpectedly low levels of visceral adiposity for their body weight while the situation is exactly reversed in those who are metabolically unhealthy but normal weight.
What makes visceral fat unhealthy and why do we have it? Visceral adipocytes are more metabolically and lipolytically active, exhibiting higher levels of both basal and catecholamine-induced lipolysis. Mechanistically, these differences may be due to increased expression of the stimulatory β-AR, lower expression of the inhibitory α-AR and reduced insulin-mediated lipolysis suppression in visceral adipocytes (Item and Konrad, 2012). Consistent with these observations, fasting and weight loss in mice induce the preferential mobilization of visceral fat stores, with visceral depots losing mass earlier and losing a greater proportion of their mass overall (Ding et al., 2016; Tang et al., 2017). Similarly, studies of weight loss in humans consistently show that a greater proportion (but not total amount) of the visceral fat is lost compared to subcutaneous fat (Merlotti et al., 2017). It is reasonable to speculate that a rapidly mobilized source of energy for internal organs may be advantageous under certain conditions.
The high lipolytic activity of visceral fat also underlies the basis for the “portal hypothesis”, which posits that visceral depots, since they drain into the portal circulation, expose the liver to high levels of FFAs, which impair hepatic insulin action. However, this version of the portal hypothesis has fallen out of favor because studies in humans show that, while the proportion of portal vein and circulating FFAs from visceral fat increase in obesity (from 5 to 20% and from 6% to 14% respectively), the visceral fat-derived FFAs still only represent a small proportion of the total circulating pool (Nielsen et al., 2004).
Alternative versions of the portal hypothesis highlighting a central role for inflammation are more compelling. Visceral adipose tissue is more prone to immune cell infiltration and inflammatory cytokine production than subcutaneous adipose tissue, especially in obesity (Item and Konrad, 2012). Several factors that are preferentially produced by visceral fat and secreted into the portal circulation have been linked to the development of insulin resistance, including IL-6, IL-1b, and Retinol Binding Protein-4. For example, IL-6 levels are 50% higher and leptin levels are 20% lower in the portal compared to systemic circulation of severely obese subjects (Fontana et al., 2007). Transplantation studies further support the idea that increased intraperitoneal adipose tissue, whether from a visceral or subcutaneous source, is not harmful per se and may even be protective. Instead, the portal delivery of inflammatory cytokines appears to drive the detrimental effects of visceral fat. (Item and Konrad, 2012). In transplant experiments, portal-draining visceral fat transplants impair insulin sensitivity whereas systemic-draining visceral fat transplants improve insulin sensitivity. Furthermore, portal-draining transplants from IL-6-deficient mice did not reduce host insulin sensitivity (Rytka et al., 2011).
The inflammatory properties of visceral adipose tissue may have been selected for during evolution, by providing a defense against intra-peritoneal pathogens and helping to heal abdominal injuries (West-Eberhard, 2019). Consistent with this notion, the omentum has important immunological functions and contains lymphoid cells organized into structures called milky spots, which are key mediators of peritoneal immunity (Meza- Perez and Randall, 2017). Moreover, the omentum and mesenteric fat commonly adhere to sites of injury, including ruptured bowels, ovaries, or surgical trauma (West-Eberhard, 2019). These fat depots can even wall off foreign bodies within the abdomen. A dramatic example of these properties is the phenomenon of creeping fat in Crohn’s disease, in which mesenteric adipose tissue adheres to sites of gut barrier dysfunction, walling of the diseased areas and preventing dissemination of bacteria (Ha et al., 2020). Overall, the metabolic and immunological properties of visceral fat, which serve important protective roles, also trigger metabolic dysfunction in the setting of obesity.
Distributional Plasticity
Body fat distribution is not fixed and can be modified by hormones. Redistribution of adipose tissue is accomplished by varying the rates of nutrient uptake and lipolysis until a new steady state distribution is achieved. A famous example occurs during Cushing’s syndrome, which results from excess secretion or administration of glucocorticoids. In addition to promoting weight gain via effects on the CNS, glucocorticoids induce a redistribution of lipids to visceral adipose tissue, while causing wasting of adipose tissue from the extremities (Lee et al., 2014a).
Androgens and estrogens also produce characteristic effects on adipose tissue leading to the “android” and “gynoid” adipose tissue distributions in men and women, respectively (Karastergiou and Fried, 2017). The plasticity of this distribution is most apparent in studies of gender transition, in which estrogen or androgen treatment produce characteristic shifts toward a gynoid distribution in transwomen and an android distribution in transmen, respectively (Klaver et al., 2018). Prior to puberty there are discernable but small differences in the fat distribution of male and female children which become much more pronounced as sex hormone levels rise (Shen et al., 2009). Likewise, during the transition to menopause, as estrogen levels fall, women begin to accumulate adipose tissue in a more android pattern, with an increase in the amount of centrally stored adipose tissue; these effects are reversed by estrogen replacement therapy (Lovejoy et al., 2008; Reubinoff et al., 1995). Reciprocally, androgens tend to promote preferential visceral fat accumulation in women, as observed in polycystic ovarian syndrome (Dumesic et al., 2016).
Finally, several drugs produce stereotyped effects on adipose tissue distribution. For example, certain HIV medications promote peripheral subcutaneous fat wasting (lipoatrophy) and central fat accumulation (Koethe et al., 2020). Conversely, TZDs, which promote insulin sensitivity, induce the preferential expansion of subcutaneous adipose tissue (Miyazaki et al., 2002).
Adipocyte hypertrophy and hyperplasia
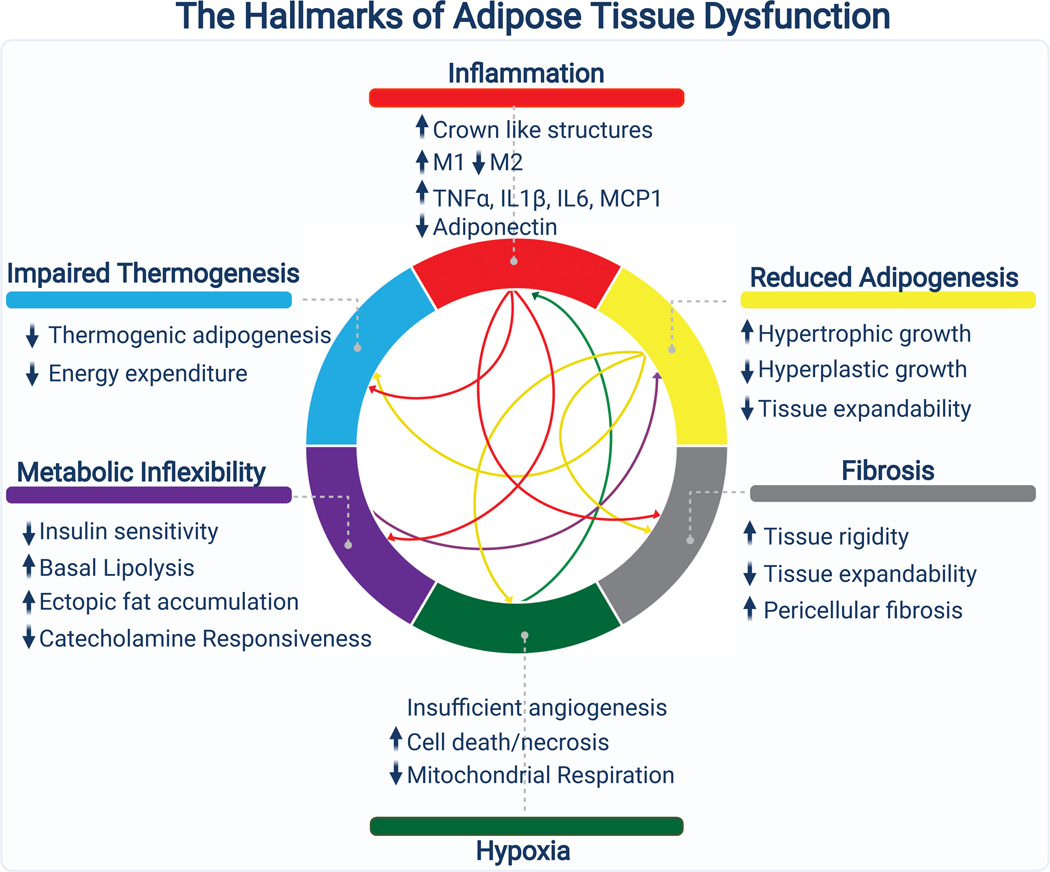
Adipose tissue expands through adipocyte hypertrophy (increases in fat cell size) and/or hyperplasia (increases in fat cell number). Hypertrophic growth is linked with higher levels of adipose tissue inflammation, fibrosis, and hypoxia, along with poor metabolic health (Vishvanath and Gupta, 2019). In contrast, hyperplastic growth does not provoke these pathologic changes and is generally more metabolically favorable.
Association studies in humans provide evidence for the divergent consequences of hypertrophic versus hyperplastic expansion. First, obese subjects, have both more adipocytes and larger, more hypertrophic, adipocytes than normal weight controls (Salans et al., 1973). Adipocyte size increases up to the point of moderate obesity, after which subsequent increases in fat mass are characterized by increases in adipocyte number (Hirsch and Batchelor, 1976). Notably, there is substantial inter-individual variation; at any given fat mass, people can exhibit a more hypertrophic or more hyperplastic adipose tissue phenotype. Second, these studies showed that hypertrophic adipose tissue is associated with poor metabolic health, including increased fasting insulin, decreased insulin sensitivity, and elevated blood glucose levels (Bjorntorp, 1971). Importantly, a body of recent work continues to support these conclusions (McLaughlin et al., 2016). Third, longitudinal and cross sectional studies suggest that the total number of adipocytes increases throughout childhood before stabilizing in adulthood (Spalding et al., 2008). Normal weight children experience two developmental periods (from age 0–2 and from age 13–18 years) characterized by rapid increases in adipocyte number; in contrast, obese children produce significantly more adipocytes than lean children and show ever increasing adipocyte numbers from age 0–18 (Knittle et al., 1979). By the time they reach adulthood, those who were obese as children have about twice as many fat cells as their normal weight counterparts. The apparent stabilization of adipocyte numbers in adulthood has led to considerable confusion, with many erroneously believing that people have a “fixed” number of adipocytes.
While many obese children become obese adults, most obese adults were not obese as children. When do obese adults make their extra adipocytes? Adults produce new adipocytes during the normal process of adipose tissue turnover (Spalding et al., 2008). Therefore, it seems likely that independent of the age of onset, adipocyte numbers increase during the development of obesity. To prove this, a longitudinal study quantifying adipocyte numbers in the transition from leanness to obesity during adulthood would be needed. The converse experiment, tracking adipocyte numbers during weight loss, has been performed. Weight loss induced by dietary changes or bariatric surgery leads to a reduction in subcutaneous adipocyte size but a maintenance of adipocyte number (Andersson et al., 2014; Bjorntorp et al., 1975). These results suggest that adipocyte number might function as a one-way ratchet, expanding in obesity, but not declining after weight loss. This may have evolved to allow the quick expansion of adipose tissue to accommodate calories during cycles of feast and famine.
Hypertrophic adipose tissue is dysfunctional
Hypertrophic expansion of adipose tissue is a risk factor, independent of body mass index, for the development of the metabolic syndrome (Weyer et al., 2000). Interestingly, the WAT of non-obese patients with insulin resistance or diabetes is characterized by large hypertrophic adipocytes further indicating a link between adipocyte hypertrophy (rather than total fat mass) and metabolic dysfunction (Acosta et al., 2016). Molecular and functional analyses of large versus small adipocytes from the same individual provide some insights for why this is the case. In particular, large adipocytes undergo higher rates of lipolysis, and produce higher levels of inflammatory cytokines (Laurencikiene et al., 2011; Xiao et al., 2016). Additionally, small adipocytes may secrete higher levels of the insulin sensitizing hormone adiponectin (Meyer et al., 2013). Consistent with this, WAT from insulin resistant patients features larger adipocytes, more fibrosis, hypoxia, and inflammation (Hepler and Gupta, 2017). At a tissue level, this dysfunctional fat produces lower levels of insulin sensitizing adipokines such as adiponectin (Henninger et al., 2014; Kloting and Bluher, 2014).
Pharmacologic and Genetic Manipulation of Tissue Expandability
Genetic and pharmacological studies suggest that it is not hypertrophic adipocytes per se that drive systemic metabolic dysfunction, but rather a failure of adipose tissue “expandability”. In this model, hypertrophic adipocytes are a symptom more than a cause of dysfunctional adipose tissue. Once adipose tissue becomes “full” and can no longer take up excess nutrients, ectopic lipid begins to accumulate in peripheral organs leading to metabolic decline.
The first line of evidence for this concept comes from two genetic models of healthy obesity, one characterized by extreme adipose tissue hyperplasia and the other by extreme hypertrophy. Leptin deficient (ob/ob) mice, a model of severe obesity, exhibit glucose intolerance, hyperphagia, and adipose tissue replete with large hypertrophic adipocytes and inflammatory macrophages. Strikingly, the metabolic dysfunction of ob/ob mice is ameliorated by concomitant overexpression of the insulin sensitizing hormone adiponectin (AdiponectinTG) or by knockout of collagen 6 (Col6 KO) (Khan et al., 2009; Kim et al., 2007). Both models are characterized by massively increased adipose tissue mass which normalizes insulin sensitivity, presumably by preventing ectopic lipid deposition in other tissues. The adipose tissue of ob/ob AdiponectinTG mice exhibits extreme hyperplasia and contains many small adipocytes. Interestingly, the adipose of ob/ob Col6 KO mice contains enormous, highly hypertrophic adipocytes.
If hypertrophic adipocytes are truly harmful, why do ob/ob Col6 KO mice have less severe metabolic disease than control ob/ob mice? Collagen 6 is selectively produced by adipocytes compared to other cell types (Divoux et al., 2010). It surrounds fat cells and is responsible for the pericellular fibrosis that restrains adipocytes from expanding past a certain size. Mice lacking collagen 6 therefore have a more permissive extracellular matrix, allowing for unrestricted expansion. Importantly, other genetic models which increase adipose ECM flexibility (ex. MMP14 overexpression) produce similar results to Col6 KO (Li et al., 2020). Thus, it appears that hypertrophic adipocytes are not deleterious because they are large, but rather because they are prevented by the ECM from getting even larger.
Further evidence comes from experiments with thiazolidinediones (TZDs) which demonstrate that augmenting the expansion capacity of adipose tissue is beneficial in metabolic disease. TZDs are pharmacological ligands for PPARγ, the master regulator of adipogenesis (Tontonoz, 1994). Activation of PPARγ leads to enhanced adipocyte differentiation (hyperplasia) and, in some depots, to enhanced expansion capacity (hypertrophy) (Tang et al., 2011). Although PPARγ is expressed in other cell types, notably macrophages, endothelium, muscle, and liver, the utility of TZDs as anti-diabetic drugs is believed to come, in large part, from their ability to promote healthy adipose tissue expansion (Yki-Jarvinen, 2004).
Elegant mouse genetic studies further show that enhancing de novo adipocyte differentiation by overexpression of PPARγ in a subset of progenitor cells in visceral adipose tissue improves insulin sensitivity in mice fed a high fat diet (HFD), without affecting body weight. Reciprocally, deletion of PPARγ in these cells provokes adipose tissue fibrosis and inflammation, along with worsened insulin resistance (Shao et al., 2018). Similarly, loss of function mutations in humans and adipocyte specific deletion in mice of phosphate and tensin homologue (PTEN), a negative regulator of adipogenesis, increase nutrient partitioning into adipose tissue and enhance insulin sensitivity despite obesity (Morley et al., 2015; Pal et al., 2012). Other studies have described consistent results, with genetic models characterized by enhanced lipid sequestration into adipocytes and insulin sensitive obesity (Kusminski et al., 2012). Genome wide association studies in humans have also begun to link genetic variants associated with reduced subcutaneous adipocyte storage capacity to increased risk for insulin resistance (Chu et al., 2017; Gulati et al., 2017; Majithia et al., 2014).
Adipose Tissue Turnover
Adipose tissue is in a constant state of low-level turnover, with mature adipocytes dying and being replaced by new adipocytes. Several studies have attempted to estimate the rate of this turnover in mice and humans. The most widely cited study employed 14C measurements of adipocytes, taking advantage of the spike in atmospheric 14C that occurred due to nuclear tests in the 1960’s (Spalding et al., 2008). This group found that about 10% of adipocytes turn over per year in both lean and obese subjects. They note that obese people have similar rates of turnover when normalizing to the number of adipocytes, but higher absolute levels of turnover due to their increased number of adipocytes. Follow up work using 14C measurements to track long term lipid flux in adipose tissue further indicated that there is no long-term lipid pool in fat; i.e. all lipid in the tissue (and thus presumably every adipocyte) is subject to turnover (Arner et al., 2019). Other studies tracking the proliferation of cells and turnover of substrates in slow turnover tissues using 2H2O long term labeling suggested more rapid turnover, of 0.16–0.29% of adipocytes and 4.5% of stromal-vascular cells per day (Neese et al., 2002).
In mice, the rates of adipocyte turnover are higher than in humans. Several studies employing distinct methods largely agree that ~5% of cells in the stromal vascular fraction are replicating at any time and that 1–5% of adipocytes are replaced each day (Neese et al., 2002; Rigamonti et al., 2011). As in humans, obese mice exhibit higher rates of proliferation and adipocyte turnover (Rigamonti et al., 2011). Notably, 15N thymidine labeling studies in mice indicate that the renewal and differentiation of adipocyte progenitors are uncoupled (Kim et al., 2014). The biological basis of this phenomenon is likely accounted for by specialization of adipocyte progenitor cells into discrete cell types, some of which are more proliferative and others which are more primed for differentiation (see below) (Merrick et al., 2019). Therefore, assessments of turnover which rely on assessing proliferation may understate the true rate of de novo adipogenesis, as committed preadipocytes may differentiate without first dividing.
The turnover of adipose tissue requires the coordinated action of multiple cell types. Dying adipocytes must be cleared away in an orderly manner to avoid the harmful effects of releasing lipids into the tissue. This clearing process is dependent upon adipose tissue macrophages, which engulf dying adipocytes and are detected within the tissue as crown-like structures. Interestingly, there is evidence that macrophages recruit adipocyte progenitor cells to sites of dying adipocytes via a CD44-Osteopontin axis, thereby linking the process of adipocyte death to adipogenesis (Lee et al., 2013).
Adipocyte progenitor cells (APCs)
The activity of APCs is a key mechanism by which adipose tissue achieves its plasticity. The major adaptive processes in adipose tissue, including expansion, beiging, and maintenance of adipocyte number, all involve de novo adipogenesis and therefore rely on the proper functioning of APCs. Could imbalances between the rate of adipocyte loss vs. replacement lead to metabolically maladaptive adipose tissue remodeling during aging? Likewise, since APCs must differentiate regularly, could we modulate their cell fate decisions, to encourage the formation of thermogenic adipose tissue instead of white adipose? Finally, do APCs make maladaptive cell fate decisions, for example by differentiating into pro-fibrogenic cell types, and can these decisions be intervened upon?
It has been known for decades that the stromal-vascular fraction (SVF) of adipose tissue contains cells capable of differentiating into adipocytes. The SVF is a heterogeneous mixture containing all the non-adipocyte cells which pellet after tissue digestion, and therefore the identity of the adipogenic cells was unclear. A groundbreaking study in 2008 utilized candidate cell surface markers to prospectively isolate and characterize APCs in WAT (Rodeheffer et al., 2008). In this study, APCs were defined based on their lack of expression of hematopoietic and endothelial cell markers (CD45 and CD31, hereafter celled Lin-) and their selective expression of CD29, CD34, LY6A/Sca1, and CD24. This refined cell population produced adipocytes in vitro and in vivo following cell transplantation (Rodeheffer et al., 2008).
Another landmark study from Graff and colleagues identified a population of Pparg- expressing APCs residing alongside blood vessels in WAT. In addition to Pparg, these cells express the mural (vessel wall cell) marker Pdgfrb (Tang et al., 2008). Genetic lineage tracing studies in mice show that Pdgfrb-expressing cells develop into Pparg+ mural cells and white adipocytes. Further lineage tracing experiments show that Pdgfrb- expressing cells generate new adipocytes in the epididymal WAT upon high fat diet feeding, contributing to 10–30% of the total adipocytes in this depot after several weeks (Gao et al., 2018; Vishvanath et al., 2016). Together, these findings led to the conclusion that (at least a subset of) APCs occupy a peri-vascular niche and are identifiable as a population of PDGFRβ+ cells, often termed mural cells, which are distinct from smooth muscle cells. Many papers in the field have taken this view. However, a parallel body of work suggests that use of the marker PDGFRβ to identify APCs results in the inclusion of numerous, non-perivascular cell types. Indeed, PDGFRβ is also expressed by adventitial fibroblasts that co-express PDGFRα and potentially represent a major source of APCs (Cattaneo et al., 2020; Hong et al., 2015; Vishvanath et al., 2016).
PDGFRα was first identified as a marker of adipogenic cells in regenerating muscle (Joe et al., 2010; Uezumi et al., 2010). Lineage tracing studies indicate that PDGFRα is a common marker of APCs in WAT and BAT depots (Berry and Rodeheffer, 2013; Lee and Granneman, 2012; Lee et al., 2012). These PDGFRα+ cells are characterized as adventitial fibroblasts with multiple elongated processes touching components of the ECM and vasculature. Numerous confirmatory studies have been done, by separate groups employing different Pdgfra-Cre lineage reporters, which consistently demonstrate tracing of Pdgfra+ cells to adipocytes in both visceral and subcutaneous fat (Berry et al., 2014; Cattaneo et al., 2020; Han et al., 2021; Sun et al., 2020a).
An elegant recent study utilized intersectional lineage tracing with Cre/Lox and Dre/Rox reporters, showing that Pdgfra+/Pdgfrb+ and Pdgfra+/Pdgfrb- progenitors, but not Pdgfra-/Pdgfrb+ cells, generated adipocytes during basal turnover and cold-induced adipogenesis in subcutaneous WAT and during wound healing-induced adipogenesis in dermal WAT (Han et al., 2021). Consistent with this, another recent lineage tracing study revealed that Pdgfra+ cells but not Tbx18+ pericytes contribute to adipocyte formation (Cattaneo et al., 2020). Taken together, these results suggest that Pdgfra+ (±Pdgfrb) adventitial fibroblasts rather than mural cells are the primary source of new adipocytes in WAT (Figure 5).
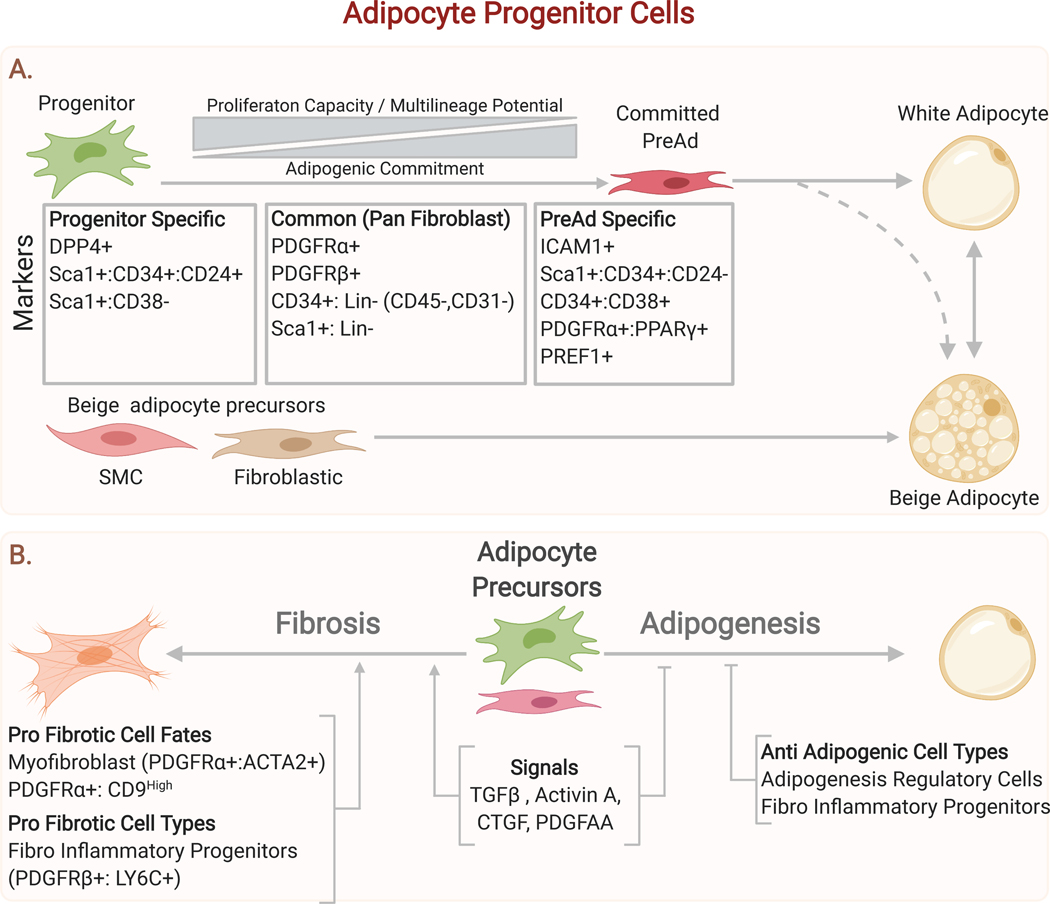
Figure 5. Adipocyte Progenitors and their Contribution to Adipose Tissue Homeostasis.
(A) Adipocyte progenitors are specialized according to their degree of commitment to the adipocyte lineage. A consensus has emerged that the major contributors to the adipocyte lineage are adventitial fibroblasts sharing a common set of fibroblastic markers including Pdgfra and Cd34. These fibroblasts can produce both white and beige adipocytes (which can interconvert in response to environmental temperature).(B) Adipocyte progenitor cells make critical cell fate decisions including whether to differentiate or adopt a more pro-fibrogenic state. Several lines of evidence suggest that, at a tissue level, there is competition between fibrosis and adipogenesis, with key mediators acting on adipocyte progenitors to alter their cell fate decisions.
An APC hierarchy?
Recent single cell transcriptomic-based studies have enabled an unbiased analysis and further refinement of APC populations, suggestion specialization of APCs for different functions (Ferrero et al., 2020). One source of APC specialization relates to their degree of adipocyte lineage commitment. This concept was originally introduced by Rodeheffer et al., who demonstrated that Lin-/CD29+/CD34+ cells could be subdivided into more and less committed cell populations based on CD24 expression. Compared CD24+ cells, CD24- cells are less proliferative and show higher expression of adipocyte identity genes such as Pparg, Lpl, AdipoQ, and Fabp4 (Berry and Rodeheffer, 2013). Moreover, transplantation studies indicate that CD24+ cells produce CD24- cells during adipogenesis (Jeffery et al., 2015; Jeffery et al., 2016).
Recent single cell transcriptomic studies have further refined this concept, identifying distinct cell types on the basis of unique gene expression signatures. Studies in mouse adipose tissue consistently identify a continuum of adipogenic cells which subdivides into two broad categories, namely progenitor cells (also called adipocyte stem cells (ASC) or interstitial progenitors) and preadipocytes (Burl et al., 2018; Cho et al., 2019; Ferrero et al., 2020; Han et al., 2021; Hepler et al., 2018; Merrick et al., 2019; Nguyen et al., 2021; Sarvari et al., 2021; Schwalie et al., 2018; Shao et al., 2021a). Likewise, studies of human subcutaneous adipose tissue have identified a similar continuum of APCs (Hildreth et al., 2021; Raajendiran et al., 2019; Vijay et al., 2020).
Progenitor cells are the most “stem-like” cells found in the tissue and are characterized by expression of Pdgfra, as well as Dpp4, Pi16, Cd55, Ly6a, and numerous Wnt pathway genes. Interestingly, fibroblasts with this gene expression signature are present in nearly every tissue of the body (Buechler et al., 2021). Preadipocytes are characterized by the expression of adipocyte-related genes, including Pparg, Fabp4, Lpl, and Cd36, suggesting commitment to the adipocyte lineage. Interestingly, preadipocytes express similar levels of Pdgfra but higher levels of Pdgfrb than progenitors, suggesting that earlier studies using these markers were in fact isolating distinct APC subtypes (Han et al., 2021; Sarvari et al., 2021).
Computational lineage prediction suggest that the APCs are arranged into a lineage hierarchy, with progenitors producing committed preadipocytes, before finally generating adipocytes (Burl et al., 2018; Merrick et al., 2019). This work implies that APCs likely exist in a continuum, from least to most committed to the adipocyte lineage, rather than occupying discrete states. Consistent with this, transplantation and lineage tracing studies show that DPP4+ progenitors, mainly localized in the layer of fibrous tissue which envelopes adipose tissue depots and subdivides it into lobes, produce preadipocytes and adipocytes in vivo (Figure 5) (Merrick et al., 2019; Stefkovich et al., 2021).
Adipogenesis-inhibitory cells
Several recent studies have identified fibroblast populations that are capable of inhibiting adipogenesis, including: fibro-Inflammatory-Progenitors (FIPs) in visceral fat, CD142High AREGs (adipogenesis-regulatory cells) in visceral and subcutaneous fat and aging-dependent regulatory cells (ARCs) in aged subcutaneous WAT (Hepler et al., 2018; Nguyen et al., 2021; Schwalie et al., 2018). Their anti-adipogenic effects are presumed to come from secretion of inflammatory mediators (ARCs and FIPs) or other secreted factors (AREGs). Of note, the anti-adipogenic properties of AREGs have been called into question, since other groups report robust adipogenesis from this population (Merrick et al., 2019; Nguyen et al., 2021). Overall, the concept that stromal cells modulate the adipogenic commitment and differentiation of APCs is compelling and suggests an added layer of regulation to adipogenesis.
Depot and Development Specific Progenitors
Several groups have investigated the embryonic origin and development of adipocytes and this work has been reviewed in recent articles (Sanchez-Gurmaches et al., 2016; Sebo and Rodeheffer, 2019). To summarize, selective marker genes have been identified for adipocyte lineage cells that give rise to the broad categories of adipose tissue depots. For example, Paired related homeobox 1 (Prx1) is a selective marker of the subcutaneous adipocyte lineage (Sanchez-Gurmaches et al., 2015). Wilms Tumor 1 (WT1), a transcription factor with key roles in heart and kidney development, is a selective marker gene for visceral (versus subcutaneous) APCs (Chau et al., 2014). Notably, since mesothelial cells express WT1, lineage tracing from WT1+ cells into visceral adipocytes initially suggested that mesothelial cells contribute to adipogenesis. However, recent work demonstrates that bona fide mesothelial cells are not adipogenic; instead a population of fibroblastic PDGFRa+/WT1+ accounts for this result (Westcott et al., 2021). Lineage tracing studies show that fat depots in the dorsal anterior aspect of the mouse, including interscapular BAT and WAT, develop from somitic mesodermal cells expressing Myf5, Engrailed1 and Pax7 which also give rise to dermal fibroblasts and skeletal muscle cells (Sebo and Rodeheffer, 2019).
Elegant studies using a Pparg lineage tracing system showed that distinct populations of APCs are responsible for adipose tissue development and maintenance (Jiang et al., 2014). Pparg+ cells are detectable in the region which develops into inguinal WAT as early as E10.5. Interestingly, deletion of Pparg in these embryonic Pparg+ cells at E10.5 does not affect adipose tissue formation but causes progressive lipodystrophy during ageing. These results indicate that the adult progenitor cells responsible for adipocyte renewal are specified early in development and do not mediate the initial development of adipose tissue (organogenesis). The specification of adipocyte progenitors in embryogenesis suggests that in utero exposures may modulate the future differentiation potential or fate of these cells.
White vs. Beige adipogenesis
Beige and white fat-specific progenitor cell populations can be isolated and cloned from subcutaneous WAT, suggesting that beige and white fat cells represent distinct cell types/lineages (Wu et al., 2012). In this regard, PDGFRα+ cells expressing Cd81 have been reported to possess enhanced beige adipogenic potential, though this marker gene appears to be quite broadly expressed in many/most fibroblasts (Oguri et al., 2020). Additionally, smooth muscle-related cells expressing certain SMC marker genes (i.e. Myh11, Acta2, Trpv1) contribute to beige adipocyte development (Berry et al., 2016; Long et al., 2014; Shamsi et al., 2021). Remarkably, in the absence of β-adrenergic receptor signaling, a completely different progenitor cell population expressing skeletal muscle genes, including Myod, are recruited to generate a distinct type of beige fat exhibiting high levels of glycolysis (Chen et al., 2019). These results show that there are multiple paths for beige adipocyte development, though the inter-relationships between these different cell types and their differentiation trajectories are uncertain.
APC Regulation and Adipogenesis
APCs differentiate into adipocytes via the process of adipogenesis. The molecular regulation of this process has been extensively studied using in vitro cell model systems. Adipogenesis is governed by two main waves of transcription factor activation (Lefterova et al., 2014). At the onset of differentiation, C/EBPβ and C/EBPδ bind to “semi-closed” chromatin at adipogenic target genes. At later time points, these regions become transcription factor ‘hotspots’ that are bound and regulated by multiple transcription factors, including Glucocorticoid receptor (GR), retinoid X receptor (RXR), and STAT5a. Amongst the second wave of factors, the master adipogenic factor PPARγ plays a dominant role in activating the expression of adipocyte-selective genes to confer the mature fat cell phenotype (Siersbaek et al., 2012; Steger et al., 2010).
An additional layer of transcriptional regulation in adipocytes is provided by numerous factors that determine the energy-storing white vs. energy-burning brown phenotype. The transcriptional co-activator PGC-1α along with IRF4, ERR factors, c/EBPb, CREB and ZFP516 are key regulators of the β-adrenergic-stimulated thermogenic gene program in adipocytes (Shapira and Seale, 2019). Several other transcriptional factors play pivotal roles in specifying thermogenic adipocyte identity, including EBF2, NFIA, Zc3h10 and PRDM16 (Shapira and Seale, 2019). Interestingly, sustained use of synthetic PPARγ agonists, like thiazolidinediones, can promote thermogenic gene expression, in a PRDM16 dependent manner (Ohno et al., 2012). Conversely, ZFP423 enforces white fat cell identity, acting through suppression of EBF2 activity. Adipocyte-specific deletion of ZFP423 or activation of EBF2 in mice enhances beige fat formation and improves metabolic health (Shao et al., 2016; Shao et al., 2021b; Stine et al., 2016). TLE3 also represses the thermogenic program of adipocytes both by impeding the function of pro-thermogenic factors PRDM16 and EBF2 and by increasing the expression of white-selective genes. Activation of TLE3 in BAT impairs lipid oxidation and thermogenesis, while deletion of TLE3 in WAT promotes thermogenesis and energy expenditure (Pearson et al., 2019; Villanueva et al., 2013).
A full understanding of adipocyte lineage commitment (i.e., progenitor-to-preadipocyte transition) in adult tissues has been hampered by the lack of molecular markers that define these cell types. However, as discussed above, recent studies have identified distinct cell types/states which appear to be positioned at different stages along the adipogenic trajectory. The adipogenic commitment process likely involves the integration of several pro- and anti- adipogenic growth factor signals. Anti-adipogenic signals include: canonical and noncanonical WNT pathways (especially WNT5, WNT6, WNT10a, and WNT10b), TGFβ, platelet derived growth factor (PDGF), and hedgehog signaling (Ghaben and Scherer, 2019). Pro-adipogenic signals include: Insulin, Bone Morphogenic Protein (BMP) signaling (especially BMP2 and BMP4), and extracellular matrix composition(Ghaben and Scherer, 2019). For example, BMP2 and BMP4 induce activation of SMAD4 and its heterodimeric partners, which subsequently stimulates the transcription of PPARy, driving adipogenic commitment (Huang et al., 2009). Of note, ZFP423, a regulator of adipogenic commitment and PPARγ expression, sensitizes cells to the pro-adipogenic effects of BMP signaling (Gupta et al., 2010).
While many pathways and factors have been shown to regulate adipocyte differentiation in cell culture models, the physiologic mechanisms that control adipocyte differentiation in vivo remain poorly defined. There is substantial literature implicating a role for fatty acids in promoting adipocyte differentiation, suggesting that lipolysis or lipid accumulation in fat tissue provides a signal for adipogenesis. In this regard, certain fatty acids can serve as activating ligands for PPAR proteins, providing an attractive mechanistic link between diet, lipid levels and adipocyte differentiation. However, a high affinity natural ligand for PPARγ has yet to be identified. A notable recent study showed that omega-3 fatty acids stimulate preadipocytes to undergo differentiation via the FFAR4 G-protein coupled receptor, located specifically in cilia (Hilgendorf et al., 2019).
A number of pathways have been proposed to inhibit adipocyte differentiation, though in many cases in vivo evidence is lacking. The presence of committed preadipocyte cells expressing detectable levels of PPARγ suggest that the adipocyte differentiation program is actively inhibited in these cells under basal conditions. A widespread problem in the field relates to the misinterpretation of mouse models exhibiting changes in adipose tissue mass. Such effects are often attributed to primary changes in APC activity and adipogenesis. However, adipose tissue size is highly sensitive and responsive to changes in systemic energy levels. Many papers presenting an obesity-resistant mouse model with a metabolically healthy phenotype will attribute the phenotype to a defect in adipogenesis. However, impaired adipogenesis is expected to cause lipodystrophy, resulting in ectopic lipid accumulation in muscle and liver along with insulin resistance.
Limitations to Plasticity
The functional decline of adipose tissue during obesity and aging is associated with a loss of plasticity. A prevailing model posits that maladaptive adipose tissue remodeling, characterized by fibrosis and inflammation, is triggered by a failure of angiogenesis which leads to tissue hypoxia as well as the accumulation of senescent cells (Crewe et al., 2017; Hepler and Gupta, 2017). In this model, adipose tissue expansion outstrips vascular supply, causing local hypoxia, which inhibits adipogenesis and induces hypertrophic adipocytes to secrete inflammatory cytokines, die via necrosis and spill lipid in an uncontrolled manner. Consequently, adipose tissue becomes insulin resistant, inflamed, and fibrotic, further compromising its function. All of these processes are continuous and mutually reinforcing, making it difficult to disentangle cause and effect (Figure 6).
Figure 6. The Hallmarks of Adipose Tissue Dysfunction.
Several interconnected and mutually reinforcing processes contribute to adipose tissue dysfunction during the pathogenesis of metabolic disease. Excessive expansion of adipose tissue and insufficient angiogenesis drives hypoxia, which triggers an inflammatory response and promotes fibrosis. The inflammatory milieu drives the secretion of cytokines that maintain adipose inflammation, promote fibrosis, and impair metabolic flexibility by interfering with both nutrient uptake and nutrient release. Diminished progenitor cell differentiation capacity, due to progenitor autonomous defects, inflammation, or fibrotic ECM, limits expansion via hyperplasia, favoring adipocyte hypertrophy. Thermogenesis, which is highly dependent on the metabolic flexibility of adipose tissue is blunted, diminishing energy expenditure. These processes are continuous and synergistic, leading to a vicious cycle that culminates in adipose tissue dysfunction.
Reduced APC function in aging?
The capacity for hyperplastic adipose tissue expansion declines during aging (Caso et al., 2013; Kim et al., 2014). Aging-induced defects in APCs include decreased expression of sirtuins, reduced expression of pro-adipogenic transcription factors, and impaired proliferative capacity (Caso et al., 2013; Khanh et al., 2018). Moreover, the adipose tissue of aged mice and humans accumulate senescent APCs (Baker et al., 2016; Tabula Muris, 2020). Clearance of senescent cells from the adipose tissue of old mice improves adipogenesis and systemic metabolism (Xu et al., 2015). Similarly, suppression of the SASP in human preadipocytes enhances adipogenic differentiation (Gustafson et al., 2019). Interestingly, aging in mice also leads to the accumulation of a distinct population of anti-adipogenic cells, specifically in subcutaneous fat, called “aging- dependent regulatory cells” (ARCs). ARCs, which express inflammatory markers, inhibit both the proliferation and adipogenic capacity of APCs (Nguyen et al., 2021).
Adipose tissue fibrosis
In healthy adipose tissue, adipocytes are embedded in a loose mesh of ECM, composed of multiple collagens (especially I, III, and VI), fibrillins, and proteoglycans, that provides structural support and modulates the activity of growth factors and signaling molecules (Marcelin et al., 2019). In contrast, fibrosis is a hallmark of dysfunctional fat, characterized by the excessive accumulation of extracellular matrix (ECM) and tissue stiffening. As with fibrosis in other organs, adipose tissue fibrosis is both a symptom of and contributor to the functional decline of the tissue.
Obesity in mice and humans is generally associated with increased adipose tissue fibrosis, especially in visceral depots, with higher levels of fibrosis correlating with more metabolic complications (Sun et al., 2013b). Fibrosis appears to cause tissue dysfunction through several mechanisms. First, adipocytes themselves are mechanosensitive and thus dysregulated ECM can alter mechanical cues and impair adipocyte function. Indeed, mechanical compression of adipocytes impairs lipolysis, decreases the expression of adipokines like leptin and adiponectin, and increases the expression of ECM genes and proinflammatory cytokines (Pellegrinelli et al., 2014). Second, the ECM serves as a reservoir of growth factors and fibrotic ECM can alter tissue function by disrupting the signaling milieu (Marcelin et al., 2019). Third, fibrotic ECM increases the rigidity of the tissue, physically impeding adipose tissue expansion by adipocyte hypertrophy (Khan et al., 2009). Fourth, dysregulated ECM impairs the function of APCs, which must remodel the local ECM to undergo adipocyte differentiation (Chun et al., 2006). Finally, APCs are mechanosensitive and exhibit decreased adipogenic capacity on stiffer substrates (Young et al., 2013).
The signaling pathways, gene regulatory networks, and cellular mediators responsible for adipose tissue fibrosis have been extensively reviewed elsewhere (Crewe et al., 2017). Transforming growth factor beta (TGFβ) especially, as well as many other factors, including Activin A, Connective Tissue Growth Factor (CTGF), Platelet Derived Growth Factor (PDGFα), and inflammatory cytokines have been implicated in the development of adipose tissue fibrosis (Iwayama et al., 2015; Yoshino et al., 2019; Zaragosi et al., 2010). Additionally, during obesity, hypoxia-induced signaling through HIF1α exerts potent pro-fibrotic rather than angiogenic effects in adipose tissue, further driving adipose tissue dysfunction (Halberg et al., 2009; Sun et al., 2013a).
The role of APCs in adipose tissue fibrosis has received significant attention. While other cell types in adipose tissue, such as macrophages and adipocytes, produce collagens and secrete pro-fibrotic factors, fibroblasts express the highest levels of collagens and fibrosis genes (Marcelin et al., 2017). Several studies suggest that APCs may have the capacity to adopt either an adipogenic or pro-fibrogenic fate, depending on the signaling context. In this regard, fibrosis would be pathogenic not only because of direct effects on the ECM but also because of aberrant cell fate choices by APCs, compromising their capacity for adipogenesis (Figure 6). Genetic mouse models provide additional evidence that the pro-fibrotic and adipogenic activities of APCs are opposed. As an example, expression of constitutively active PDGFRa in APCs results in lipodystrophy and profoundly fibrotic tissue, while deletion of PDGFRa has opposite effects (Marcelin et al., 2017; Sun et al., 2017; Sun et al., 2020a). Additionally, HIF1α inhibits APC differentiation through inhibitory phosphorylation of PPARγ and targeting this pathway augments adipogenesis and ameliorates metabolic dysfunction (Shao et al., 2021a).
Several studies have defined subsets of APCs in WAT that exhibit high fibrotic and low adipogenic potential. For example, CD9High/PDGFRα+ cells, which increase during obesity in visceral fat, exhibit a pro-fibrotic phenotype and are less adipogenic (Marcelin et al., 2017). Hepler et al. identified a related population of PDGFRβ+/LY6a+/CD9+ cells in visceral WAT, which they termed fibro inflammatory progenitors (FIPs) (Hepler et al., 2018). FIPs are transcriptionally similar to (DPP4+) progenitor cells in subcutaneous WAT, suggesting that the division between pro-fibrotic and pro-adipogenic adipocyte progenitor cell subtypes is conserved across depots (Burl et al., 2018; Ferrero et al., 2020; Merrick et al., 2019).
There is evidence that beige fat biogenesis and the activity of the thermogenic transcription factor PRDM16 is protective against adipose tissue fibrosis. For example, the cold inducible transcription factor GTF2IRD1 recruits the transcription factors PRDM16 and EHMT1 to the promoters and enahncers of TGFβ responsive pro-fibrosis genes, repressing their expression and suppressing fibrosis. Importantly, this mechanism occurs independent of UCP1 (Hasegawa et al., 2018). Additionally, PRDM16 epxression in mature beige adipocytes promotes secretion of beta-hydroxybutyrate, which promotes APC beige adipogenesis and blocks fibrosis (Wang et al., 2019).
Finally, a fibrosis vs. adipogenesis (or lipid storage) fibroblast fate axis exists in other tissues. A well-studied example occurs in the skin, in which conversion of myofibroblasts into adipocytes and vice-versa occurs during wound healing (Marangoni et al., 2015; Plikus et al., 2017; Shook et al., 2020). In skeletal muscle, PDGFRα+ fibroblastic cells also give rise to both adipocytes and pro-fibrogenic cells (Uezumi et al., 2011). Furthermore, in models of idiopathic pulmonary fibrosis (IPF), treatment with PPARγ agonists alleviates fibrosis by promoting differentiation of lung fibroblasts into lipid storing and less fibrogenic lipofibroblasts (El Agha et al., 2017).
Adipose tissue inflammation
Immune cells play many critical roles in regulating adipose tissue phenotypes in response to physiological and pathological stimuli (Lu et al., 2019). Evidence that obesity results in chronic inflammation emerged in the 1990s, through the study of Hotamisligil et al., showing increased concentrations of the inflammatory cytokine TNFα in the adipose tissue of obese rats (Hotamisligil et al., 1993). Neutralization of TNFα signaling improves insulin sensitivity, establishing a link between immune responses and metabolism. Following these early studies, an extensive amount of research has demonstrated that chronic inflammation is a hallmark of adipose tissue dysfunction and systemic metabolic dysregulation.
Obesity in mice and humans dramatically increases the number of adipose tissue macrophages, linked to the activation of several inflammatory pathways (Amano et al., 2014; Patsouris et al., 2008; Weisberg et al., 2003). Seminal work showed that obesity induces a phenotypic switch in adipose tissue macrophages from an anti-inflammatory “type 2” profile to a pro-inflammatory “type 1” state (Lumeng et al., 2007a; Lumeng et al., 2008; Nguyen et al., 2007). These “type 1” macrophages represent a major source of pro- inflammatory cytokines and can be found surrounding dead or dying adipocytes in adipose tissue, forming characteristic crown-like structures. Ablation of pro-inflammatory macrophages in obese mice decreases adipose tissue inflammation and enhances insulin sensitivity (Patsouris et al., 2008). Similarly, reducing macrophage recruitment into adipose tissue ameliorates metabolic complications in high fat fed mice (Kanda et al., 2006; Weisberg et al., 2006).
T cells also increase during obesity and play prominent roles in adipose tissue inflammation (Nishimura et al., 2009; Wu et al., 2007). CD8+ effector T cells infiltrate adipose tissue at early stages of obesity development, stimulating macrophage recruitment and inflammation (Nishimura et al., 2009; Rausch et al., 2008). Of note, high fat feeding in mice led to an accumulation of a particular subset of T cells exhibiting a senescent phenotype and expressing high levels of the pro-inflammatory factor Osteopontin (Spp1) in visceral adipose tissue (Shirakawa et al., 2016). Conversely, regulatory T (Treg) cells play a critical role in suppressing adipose tissue inflammation in the visceral depot (Feuerer et al., 2009; Ilan et al., 2010). Adipose tissue Treg cells are abundant in the lean state and decrease in obesity. Ablation of these cells in fat tissue increases inflammation and insulin resistance, whereas adoptive transfer of Treg cells blunts inflammatory response and improves metabolic parameters.
Another important immune cell type in adipose tissue is innate lymphoid type 2 cells (ILC2). ILC2 cells express IL-5 and IL-13, which regulate the maintenance of alternatively activated macrophages and eosinophils to limit inflammation and promote the development of thermogenic adipocytes (Hams et al., 2013; Molofsky et al., 2013). Like Treg cells, adipose tissue ILC2 decrease in the setting of obesity. ILC2 cells also decrease in abundance and lose their identity in the visceral adipose tissue of mice during aging (Goldberg et al., 2021).
The mechanisms responsible for triggering and sustaining adipose tissue inflammation have been intensively studied over the past decade. Obesity-induced alterations in the gut microbiome, along with increased gut permeability promote the translocation of endotoxins like LPS, driving inflammation in many tissues including adipose tissue. Within adipose tissue, fatty acids released from fat cells (or insufficiently sequestered by fat cells) have been proposed to elicit inflammatory responses, though these effects have not been observed consistently across studies (Tilg et al., 2020). Additionally, the chronic uptake and increased storage of fatty acids as lipid droplets in macrophages may cause lipotoxicity, leading to pro-inflammatory changes within macrophages. In support of this idea, lipid-storing macrophages, resembling foam cells, accumulate in obese adipose tissue (Lumeng et al., 2007b). More recently, single cell transcriptomic studies defined a population of lipid-laden macrophages in adipose tissue of obese animals marked by the expression of CD9. These CD9+ macrophages are sufficient to induce pathologic programing of adipose tissue when transferred into lean mice (Hill et al., 2018). Interestingly, the capacity for macrophages to take up and store lipid exerts beneficial, metabolically protective effects, suggesting that lipid-storage per se is adaptive and that other signals are necessary to provoke inflammatory changes (Aouadi et al., 2014). Further studies show that the lipid receptor TREM2 is a key functional regulator and marker of lipid-storing macrophages in rodent and human fat tissue (Jaitin et al., 2019). Notably, a recent study shows that adipocytes, in addition to releasing fatty acids via lipolysis, transfer lipids to macrophages via exosomes (Flaherty et al., 2019). This exosomal lipid transfer pathway is increased in obesity and promotes macrophage differentiation.
Adipocytes modulate inflammation through the production of adipokines. In particular, Leptin, which increases during obesity, exerts pro-inflammatory effects through direct actions on many types of immune cells (Francisco et al., 2018). By contrast, adiponectin promotes an anti-inflammatory profile in macrophages (Ohashi et al., 2010). An emerging concept in the field demonstrates important functions for various types of fibroblasts in modulating adipose tissue immune responses. For example, the Gupta lab has defined a subset of fibroblasts, called fibro-inflammatory progenitors that stimulate macrophage accrual in adipose tissue during obesity development (Shan et al., 2020). Additionally, certain subpopulations of mesenchymal cells in adipose tissue are a major source of IL-33, which regulates the activity of Treg and ILC2 cells (Spallanzani et al., 2019). Unfortunately, despite a huge body of literature implicating inflammation as a driver of obesity-related metabolic disease, anti-inflammatory therapies have, thus far, not been successful. Given the pleiotropic effects of immune cells in adipose tissue, it will likely be necessary to identify approaches that selectively block the maladaptive effects of inflammation, without compromising the critical functions of immune cells that support adipose tissue health and plasticity.
Limitations to Metabolic Plasticity
Healthy WAT exhibits extensive metabolic flexibility, responding to anabolic and catabolic signals (via lipogenesis and lipolysis respectively) to preserve whole organism energy homeostasis. However, in the setting of chronic positive energy balance, WAT develops metabolic inflexibility, characterized by a decreased amplitude of response to signals regulating both the storage and mobilization of nutrients.
In the fed-state, adipose tissue metabolic inflexibility manifests as insulin resistance, resulting in decreased post-prandial glucose and lipid sequestration, unrestrained lipolysis, and elevated circulating FFA levels (Gastaldelli et al., 2017; Petersen and Shulman, 2018). The molecular pathogenesis of adipose tissue insulin resistance is complex and incompletely understood, but inflammation, hypoxia, fibrosis, and impaired expandability appear to be key contributors; comprehensive reviews offering integrated perspectives on whole body and adipose tissue specific insulin resistance have been recently published elsewhere (Czech, 2020).
In the fasted-state, adipose tissue metabolic inflexibility manifests as diminished catecholamine-stimulated lipolysis in subcutaneous WAT, despite elevations in basal lipolysis in all fat depots (Arner, 2005). This lipolysis impairment is due to alterations in the catecholamine-stimulated signaling cascade, including reduced β2-adrenergic receptor expression, increased anti-lipolytic α2-receptor activity, and decreased HSL stimulation by cAMP; these phenomena have been extensively reviewed elsewhere (Morigny et al., 2016). These changes are likely exacerbated by obesity-induced decreases in sympathetic nerve fiber density (Cao et al., 2018a; Jiang et al., 2017).
Chronic inflammation is believed to be a major contributor to the impairment in the metabolic plasticity of fat (Zatterale et al., 2019). As an example, secretion of the proinflammatory cytokines TNFα and MCP1 by adipocytes and infiltrating macrophages activates JNK signaling, which phosphorylates the insulin receptor and reduces its activity (Hirosumi et al., 2002). Similarly, chronic inflammation diminishes catecholamine responsiveness. For example, TNFα-induced expression of the kinases IKKe and TBK1 activates the phosphodiesterase PDE3B, which directly reduces the levels of cAMP. This reduction in cAMP signaling reduces HSL phosphorylation and UCP1 expression thereby diminishing both lipolysis and thermogenesis (Li et al., 2019; Mowers et al., 2013).
Hypoxia is another key driver of adipose tissue dysfunction and metabolic inflexibility during aging and obesity. Hypoxia is believed to develop due to (1) the presence of hypertrophic adipocytes (reaching 200+ μm in diameter), which exceed the diffusion limit of O2 (typically 100–200 μm in tissues) and (2) defects in post-prandial blood flow and vascular density (Trayhurn, 2013). In obesity, rather than stimulating angiogenesis, HIF1a, the master regulator of the hypoxia response, promotes the expression of pro-inflammatory and pro-fibrotic genes (Halberg et al., 2009; Lee et al., 2014b). Interestingly, expression levels of an anti-angiogenic form of VEGF (VEGFA165b) are elevated in obesity and likely contribute to impaired angiogenesis in adipose tissue (Ngo et al., 2014). Obese humans exhibit diminished adipose tissue blood flow and a notable failure to augment blood flow in response to feeding, prolonged fasting, and exercise (Frayn and Karpe, 2014). Consistent with this finding, obese mice show precipitous declines in adipose tissue capillary density (Cao et al., 2018a). Together these results suggest that targeting adipose tissue angiogenesis to promote healthy vascular growth and avoid hypoxia may be a promoting future therapeutic avenue.
Thermogenesis
Obesity and aging are associated with reductions in the abundance and activity of thermogenic adipose tissue in both mice and humans (Becher et al., 2021; Wang et al., 2019). Interestingly, distinct mechanisms may underlie age- and obesity-linked declines in thermogenic fat activity. For example, Song et al. observed that the conversion of low-thermogenic cells to high-thermogenic cells in BAT is impaired in aging but not in diet-induced obesity (Song et al., 2020). Likewise, Nguyen et al. showed that aging but not obesity causes the emergence of pro-inflammatory precursor (ARC) cells in subcutaneous adipose tissue, which impede adipocyte differentiation (Nguyen et al., 2021). Targeting BAT to increase longevity has been the aim of several studies and is reviewed elsewhere (Darcy and Tseng, 2019).
Conclusions
There is an urgent need to develop new therapies to combat the expanding dual epidemics of obesity and cardiometabolic disease. Adipose tissue lies at the center of these health problems, representing a major contributor to disease pathogenesis and a promising target for therapies. As highlighted in this review, adipose tissue possesses extraordinary plasticity, including its: (1) rapid titration of metabolic programs to maintain systemic energy levels in the face of fluctuating changes in nutrient supply and demand; (2) unparalleled capacity to expand and contract to accommodate long term trends in energy balance; (3) remarkable structural and metabolic transformation during cold exposure to engage in heat production; (4) capacity for de-differentiation to regulate lactation and wound healing.
Obesity often leads to a decline in adipose tissue plasticity, which is associated with fibrosis, inflammation, progenitor cell senescence, and catecholamine resistance. Ultimately, these pathological changes impair the critical nutrient-buffering function of adipose tissue, leading to insulin resistance and metabolic disease. The central role of adipose tissue dysfunction in disease and the incredible plasticity of fat tissue supports the promise of modulating fat tissue phenotypes for therapeutic purposes. The viability of this approach has already been demonstrated with the success of thiazolidinediones, which promote healthy adipose tissue expansion and enhance insulin sensitivity. Unfortunately, unfavorable side effects of some thiazolidinediones have caused this class of drugs to fall out of favor.
New insights into the identity and regulation of APCs, adipocytes, immune cells, and other diverse cell types in adipose tissue promise to reveal novel drug targets to promote metabolically beneficial tissue remodeling. For example, it may be possible to promote favorable APC-fate decisions, encouraging adipogenesis at the expense of adipocyte hypertrophy, fibrosis, and inflammation. Additionally, increasing the abundance and activity of thermogenic adipose tissue is a promising strategy to enhance energy expenditure to combat both metabolic disease and obesity. Many questions and opportunities for future discovery remain, which will yield new insights into adipose tissue biology and hopefully lead to improved therapies for human disease.
Acknowledgements
Research reported in this publication was supported by NIDDK at the National Institutes of Health under R01DK103930 (to C.J.V.), DK123356 and DK120982 (to P.S.), UCLA Life Sciences Fund, UCLA Graduate Council Diversity Fellowship for M.K.S.
Footnotes
Decleration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Acosta JR, Douagi I, Andersson DP, Backdahl J, Ryden M, Arner P, and Laurencikiene J (2016). Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59, 560–570. [DOI] [PubMed] [Google Scholar]
- Albert V, Svensson K, Shimobayashi M, Colombi M, Munoz S, Jimenez V, Handschin C, Bosch F, and Hall MN (2016). mTORC2 sustains thermogenesis via Akt-induced glucose uptake and glycolysis in brown adipose tissue. EMBO Mol Med 8, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, and Kajimura S. (2016). Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell Metab 24, 402–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, and Aouadi M. (2014). Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab 19, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DP, Eriksson Hogling D, Thorell A, Toft E, Qvisth V, Naslund E, Thorne A, Wiren M, Lofgren P, Hoffstedt J, et al. (2014). Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care 37, 1831–1836. [DOI] [PubMed] [Google Scholar]
- Angueira AR., Shapira SN., Ishibashi J., Sampat S., Sostre-Colon J., Emmett MJ., Titchenell PM., Lazar MA., Lim HW., and Seale P. (2020). Early B Cell Factor Activity Controls Developmental and Adaptive Thermogenic Gene Programming in Adipocytes. Cell Rep 30, 2869-2878 e2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouadi M, Vangala P, Yawe JC, Tencerova M, Nicoloro SM, Cohen JL, Shen Y, and Czech MP (2014). Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab 307, E374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P. (2005). Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19, 471–482. [DOI] [PubMed] [Google Scholar]
- Arner P, Bernard S, Appelsved L, Fu KY, Andersson DP, Salehpour M, Thorell A, Ryden M, and Spalding KL (2019). Adipose lipid turnover and long-term changes in body weight. Nat Med 25, 1385–1389. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. (2016). Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau C, Labit E, Guissard C, Rouquette J, Boizeau ML, Gani Koumassi S, Carriere A, Jeanson Y, Berger-Muller S, Dromard C, et al. (2016). Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity (Silver Spring) 24, 1081–1089. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. (2011). Brown adipose tissue activity controls triglyceride clearance. Nat Med 17, 200–205. [DOI] [PubMed] [Google Scholar]
- Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, Butler SD, Jiang CS, Vaughan R, Schoder H, et al. (2021). Brown adipose tissue is associated with cardiometabolic health. Nat Med 27, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Jiang Y, and Graff JM (2016). Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun 7, 10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Jeffery E, and Rodeheffer MS (2014). Weighing in on adipocyte precursors. Cell Metab 19, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, and Rodeheffer MS (2013). Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet AM, and Kirichok Y. (2017). UCP1: A transporter for H(+) and fatty acid anions. Biochimie 134, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi P, Yue F, Karki A, Castro B, Wirbisky SE, Wang C, Durkes A, Elzey BD, Andrisani OM, Bidwell CA, et al. (2016). Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice. J Exp Med 213, 2019–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P. (1971). Sjostrom L,+SJOSTROM L: Number and size of adipose tissue fat cells in relation to metabolism in human obesity. Metabolism 20, 703–713. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Carlgren G, Isaksson B, Krotkiewski M, Larsson B, and Sjostrom L. (1975). Effect of an energy-reduced dietary regimen in relation to adipose tissue cellularity in obese women. Am J Clin Nutr 28, 445–452. [DOI] [PubMed] [Google Scholar]
- Brestoff JR., Kim BS., Saenz SA., Stine RR., Monticelli LA., Sonnenberg GF., Thome JJ., Farber DL., Lutfy K., Seale P., et al. (2015). Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien R, Roose-Girma M, et al. (2021). Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579. [DOI] [PubMed] [Google Scholar]
- Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, and Granneman JG (2018). Deconstructing Adipogenesis Induced by beta3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metab 28, 300-309 e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R, Zulian A, Gentilini D, Maestrini S, Della Barba A, Invitti C, Cora D, Caselle M, Liuzzi A, and Di Blasio AM (2013). Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity (Silver Spring) 21, 2562–2570. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Cao Q, Jing J, Cui X, Shi H, and Xue B. (2019). Sympathetic nerve innervation is required for beigeing in white fat. Physiol Rep 7, e14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, and Collins S. (2004). p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24, 3057–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Wang H, Wang Q, Han X, and Zeng W. (2018a). Three-dimensional volume fluorescence-imaging of vascular plasticity in adipose tissues. Mol Metab 14, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Wang H, and Zeng W. (2018b). Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein Cell 9, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier AC (2021). 100(th) anniversary of the discovery of insulin perspective: insulin and adipose tissue fatty acid metabolism. Am J Physiol Endocrinol Metab 320, E653–E670. [DOI] [PubMed] [Google Scholar]
- Caso G, McNurlan MA, Mileva I, Zemlyak A, Mynarcik DC, and Gelato MC (2013). Peripheral fat loss and decline in adipogenesis in older humans. Metabolism 62, 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo P, Mukherjee D, Spinozzi S, Zhang L, Larcher V, Stallcup WB, Kataoka H, Chen J, Dimmeler S, Evans SM, et al. (2020). Parallel Lineage-Tracing Studies Establish Fibroblasts as the Prevailing In Vivo Adipocyte Progenitor. Cell Rep 30, 571-582 e572. [DOI] [PubMed] [Google Scholar]
- Chait A, and den Hartigh LJ (2020). Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. (2014). Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ikeda K, Yoneshiro T, Scaramozza A, Tajima K, Wang Q, Kim K, Shinoda K, Sponton CH, Brown Z, et al. (2019). Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J., Wu Z., Choi CHJ., Nguyen L., Tegegne S., Ackerman SE., Crane A., Marchildon F., Tessier-Lavigne M., and Cohe P. (2018). Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab 27, 226-236 e223. [DOI] [PubMed] [Google Scholar]
- Chitraju C, Fischer AW, Farese RV Jr., and Walther TC (2020). Lipid Droplets in Brown Adipose Tissue Are Dispensable for Cold-Induced Thermogenesis. Cell Rep 33, 108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DS, Lee B, and Doles JD (2019). Refining the adipose progenitor cell landscape in healthy and obese visceral adipose tissue using single-cell gene expression profiling. Life Sci Alliance 2, e201900561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Son Y, Saha A, Kim D, Choi C, Kim M, Park JH, Im H, Han J, Kim K, et al. (2021). STK3/STK4 signalling in adipocytes regulates mitophagy and energy expenditure. Nat Metab 3, 428–441. [DOI] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K, et al. (2016). Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab 23, 1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, and Kajimura S. (2019). Metabolic adaptation and maladaptation in adipose tissue. Nat Metab 1, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, et al. (2016). Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Deng X, Fisher VA, Drong A, Zhang Y, Feitosa MF, Liu CT, Weeks O, Choh AC, Duan Q, et al. (2017). Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat Genet 49, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, and Weiss SJ (2006). A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125, 577–591. [DOI] [PubMed] [Google Scholar]
- Chusyd DE, Wang D, Huffman DM, and Nagy TR (2016). Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front Nutr 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Cancello R, Zingaretti MC, Ceresi E, De Matteis R, Giordano A, Himms-Hagen J, and Ricquier D. (2002). CL316,243 and cold stress induce heterogeneous expression of UCP1 mRNA and protein in rodent brown adipocytes. J Histochem Cytochem 50, 21–31. [DOI] [PubMed] [Google Scholar]
- Cohen P, and Kajimura S. (2021). The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol 22, 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote JA, Ostinelli G, Gauthier MF, Lacasse A, and Tchernof A. (2019). Focus on dedifferentiated adipocytes: characteristics, mechanisms, and possible applications. Cell Tissue Res 378, 385–398. [DOI] [PubMed] [Google Scholar]
- Crewe C, An YA, and Scherer PE (2017). The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 127, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP (2020). Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol Metab 34, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy J., and Tseng YH. (2019). ComBATing aging-does increased brown adipose tissue activity confer longevity? Geroscience 41, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BS, Beigneux AP, Barnes RH, 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyren R, Goldberg I, Olivecrona G, et al. (2010). GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab 12, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, et al. (2016). Body- mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet 388, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichamp J, Barreau C, Guissard C, Carriere A, Martinez Y, Descombes X, Penicaud L, Rouquette J, Casteilla L, Plouraboue F, et al. (2019). 3D analysis of the whole subcutaneous adipose tissue reveals a complex spatial network of interconnected lobules with heterogeneous browning ability. Sci Rep 9, 6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Zheng S, Garcia-Ruiz D, Hou D, Wei Z, Liao Z, Li L, Zhang Y, Han X, Zen K, et al. (2016). Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149–3p and suppression of PRDM16. Nat Commun 7, 11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, et al. (2010). Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss.Diabetes 59, 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe CL, Lysaght J, O’Sullivan J, and Reynolds JV (2017). Emerging Concepts Linking Obesity with the Hallmarks of Cancer. Trends Endocrinol Metab 28, 46–62. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, et al. (2016). Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab 101, 4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, and Cao L. (2015). Adipose VEGF Links the White-to-Brown Fat Switch With Environmental, Genetic, and Pharmacological Stimuli in Male Mice. Endocrinology 156, 2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, Szibor M, Kosanovic D, Schwind F, Schermuly RT, et al. (2017). Two-Way Conversion between Lipogenic and Myogenic Fibroblastic Phenotypes Marks the Progression and Resolution of Lung Fibrosis. Cell Stem Cell 20, 261-273 e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve D, Boulet N, Belles C, Zakaroff-Girard A, Decaunes P, Briot A, Veeranagouda Y, Didier M, Remaury A, Guillemot JC, et al. (2019). Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Nat Commun 10, 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, and Kirichok Y. (2012). Mechanism of fatty-acid- dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero R, Rainer P, and Deplancke B. (2020). Toward a Consensus View of Mammalian Adipocyte Stem and Progenitor Cell Heterogeneity. Trends Cell Biol 30, 937–950. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine AB., Benoist C., Shoelson S., et al. (2009). Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15, 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, et al. (2017). Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med 23, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty SE 3rd, Grijalva A, Xu X, Ables E, Nomani A, and Ferrante AW Jr. (2019). A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 363, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor PB (1993). From the panniculus carnosum (PC) to the superficial fascia system (SFS). Aesthetic Plast Surg 17, 179–181. [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, and Klein S. (2007). Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56, 1010–1013. [DOI] [PubMed] [Google Scholar]
- Francisco V, Pino J, Campos-Cabaleiro V, Ruiz-Fernandez C, Mera A, Gonzalez-Gay MA, Gomez R, and Gualillo O. (2018). Obesity, Fat Mass and Immune System: Role for Leptin. Front Physiol 9, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN, and Karpe F. (2014). Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond) 38, 1019–1026. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, and Rodriguez A. (2014). Regulation of adipocyte lipolysis. Nutr Res Rev 27, 63–93. [DOI] [PubMed] [Google Scholar]
- Funcke JB, and Scherer PE (2019). Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res 60, 1648–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Daquinag AC, Su F, Snyder B, and Kolonin MG (2018). PDGFRalpha/PDGFRbeta signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development 145, dev155861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A, Gaggini M, and DeFronzo RA (2017). Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes 66, 815–822. [DOI] [PubMed] [Google Scholar]
- Ghaben AL, and Scherer PE (2019). Adipogenesis and metabolic health. Nat Rev Mol Cell Biol 20, 242–258. [DOI] [PubMed] [Google Scholar]
- Goldberg EL, Shchukina I, Youm YH, Ryu S, Tsusaka T, Young KC, Camell CD, Dlugos T, Artyomov MN, and Dixit VD (2021). IL-33 causes thermogenic failure in aging by expanding dysfunctional adipose ILC2. Cell Metab 33, 2277–2287 e2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hurtado E, Lee J, Choi J, and Wolfgang MJ (2018). Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing. Mol Metab 7, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati P, Day FR, Payne F, Ongen H, Lotta LA, Day FR, van de Bunt M, Gaulton KJ, Eicher JD, Sharp SJ, et al. (2017). Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance (New York, NY :: Nature Pub Co), pp. 17–26. [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, and Spiegelman BM (2010). Transcriptional control of preadipocyte determination by Zfp423. Nature 464, 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B., Nerstedt A., and Smith U. (2019). Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun 10, 2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, et al. (2020). Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 183, 666-683 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, et al. (2009). Hypoxia- inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29, 4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E, Locksley RM, McKenzie AN, and Fallon PG (2013). Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol 191, 5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Zhang Z, He L, Zhu H, Li Y, Pu W, Han M, Zhao H, Liu K, Li Y, et al. (2021). A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell 28, 1160-1176 e1167. [DOI] [PubMed] [Google Scholar]
- Harms M, and Seale P. (2013). Brown and beige fat: development, function and therapeutic potential. Nat Med 19, 1252–1263. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, Hosono T, Maretich P, Yang Y, Ishigaki Y, et al. (2018). Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metabolism 27, 180-194.e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam D, and Rigby N. (2010). A long look at obesity. The Lancet 376, 85–86. [DOI] [PubMed] [Google Scholar]
- Henninger AM, Eliasson B, Jenndahl LE, and Hammarstedt A. (2014). Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS One 9, e105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques F, Bedard AH, Guilherme A, Kelly M, Chi J, Zhang P, Lifshitz LM, Bellve K, Rowland LA, Yenilmez B, et al. (2020). Single-Cell RNA Profiling Reveals Adipocyte to Macrophage Signaling Sufficient to Enhance Thermogenesis. Cell Rep 32, 107998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler C, and Gupta RK (2017). The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol Cell Endocrinol 445, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC, et al. (2018). Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife 7, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, Klein S, and Kahn BB (2012). A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth AD, Ma F, Wong YY, Sun R, Pellegrini M, and O’Sullivan TE (2021). Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol 22, 639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf KI., Johnson CT., Mezger A., Rice SL., Norris AM., Demeter J., Greenleaf WJ., Reiter JF., Kopinke D., and Jackson PK. (2019). Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis. Cell 179, 1289–1305 e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, et al. (2018). Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A 115, E5096–E5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, and Hotamisligil GS (2002). A central role for JNK in obesity and insulin resistance. Nature 420, 333–336. [DOI] [PubMed] [Google Scholar]
- Hirsch J, and Batchelor B. (1976). Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 5, 299–311. [DOI] [PubMed] [Google Scholar]
- Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, et al. (2015). Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 142, 2623–2632. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, and Spiegelman BM (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91. [DOI] [PubMed] [Google Scholar]
- Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, Desai BN, Banks AS, Lowell BB, Mathis D, et al. (2020). gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, and Tang QQ (2009). BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 106, 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, et al. (2017). UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23, 1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, and Weiner HL (2010). Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A 107, 9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Item F, and Konrad D. (2012). Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev 13 Suppl 2, 30–39. [DOI] [PubMed] [Google Scholar]
- Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, and Olson LE (2015). PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29, 1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, et al. (2019). Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 178, 686-698 e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L, and Rodeheffer MS (2015). Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 17, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Pena R, Church CD, Colman L, Berry R, and Rodeheffer MS (2016). The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab 24, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ., Larsen TJ., Peijs L., Daugaard S., Homoe P., Loft A., de Jong J., Mathur N., Cannon B., Nedergaard J., et al. (2013). A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17, 798–805. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ding X, Cao Y, Wang H, and Zeng W. (2017). Dense Intra-adipose Sympathetic Arborizations Are Essential for Cold-Induced Beiging of Mouse White Adipose Tissue. Cell Metab 26, 686-692 e683. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, and Graff JM (2014). Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 9, 1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, and Rossi FMV (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature Cell Biology 12, 153–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SM, Hung CM, Hildebrand SR, Sanchez-Gurmaches J, Martinez-Pastor B, Gengatharan JM, Wallace M, Mukhopadhyay D, Martinez Calejman C, Luciano AK, et al. (2019). Non-canonical mTORC2 Signaling Regulates Brown Adipocyte Lipid Catabolism through SIRT6-FoxO1. Mol Cell 75, 807-822 e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, et al. (2006). MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116, 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, and Fried SK (2017). Cellular Mechanisms Driving Sex Differences in Adipose Tissue Biology and Body Shape in Humans and Mouse Models. In (Springer International Publishing), pp. 29–51. [DOI] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. (2015). A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI, Kumari M, Zhang S, Vuckovic I, Laznik-Bogoslavski D, et al. (2017). Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab 26, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Thaete FL, Troost F, Huwe T, and Goodpaster BH (2000). Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278, E941–948. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, and Scherer PE (2009). Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29, 1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanh VC, Zulkifli AF, Tokunaga C, Yamashita T, Hiramatsu Y, and Ohneda O. (2018). Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochem Biophys Res Commun 500, 682–690. [DOI] [PubMed] [Google Scholar]
- Kim JI, Park J, Ji Y, Jo K, Han SM, Sohn JH, Shin KC, Han JS, Jeon YG, Nahmgoong H, et al. (2019). During Adipocyte Remodeling, Lipid Droplet Configurations Regulate Insulin Sensitivity through F-Actin and G-Actin Reorganization. Mol Cell Biol 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY., van de Wall E., Laplante M., Azzara A., Trujillo ME., Hofmann SM., Schraw T., Durand JL., Li H., Li G., et al. (2007). Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117, 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JH, Song SW, Jung WS, Lee YA, and Kim HN (2016).Relationship between deep subcutaneous abdominal adipose tissue and metabolic syndrome: a case control study. Diabetol Metab Syndr 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, and Steinhauser ML (2014). Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab 20, 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver M, de Blok CJM, Wiepjes CM, Nota NM, Dekker M, de Mutsert R, Schreiner T, Fisher AD, T’Sjoen G, and den Heijer M. (2018). Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol 178, 163–171. [DOI] [PubMed] [Google Scholar]
- Kloting N, and Bluher M. (2014). Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 15, 277–287. [DOI] [PubMed] [Google Scholar]
- Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, and Katz DP (1979). The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest 63, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe JR, Lagathu C, Lake JE, Domingo P, Calmy A, Falutz J, Brown TT, and Capeau J. (2020). HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers 6, 48. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, et al. (2012). MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 18, 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe SM, Mouchiroud M, Caron A, Secco B, Freinkman E, Lamoureux G, Gelinas Y, Lecomte R, Bosse Y, Chimin P, et al. (2016). mTORC1 is Required for Brown Adipose Tissue Recruitment and Metabolic Adaptation to Cold. Sci Rep 6, 37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanktree MB, and Hegele RA (2017). Metabolic Syndrome. Genomic and Precision Medicine: Primary Care: Third Edition 43, 283–299. [Google Scholar]
- Laurencikiene J, Skurk T, Kulyte A, Heden P, Astrom G, Sjolin E, Ryden M, Hauner H, and Arner P. (2011). Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab 96, E2045–2049. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K, and Fried SK (2014a). Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta 1842, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, and Chawla A. (2015). Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, and Granneman JG (2012). Seeking the source of adipocytes in adult white adipose tissues. Adipocyte 1, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, and Granneman JG (2013). Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 18, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, and Granneman JG (2012). In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 15, 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS., Kim JW., Osborne O., Oh DY., Sasik R., Schenk S., Chen A., Chung H., Murphy A., Watkins SM., et al. (2014b). Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova MI, Haakonsson AK, Lazar MA, and Mandrup S. (2014). PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang M, Liu Y, Huang S, Yi X, Yin C, Wang S, Zhang M, Yu Q, Li P, et al. (2019). TNF-alpha Upregulates IKKepsilon Expression via the Lin28B/let-7a Pathway to Induce Catecholamine Resistance in Adipocytes. Obesity (Silver Spring) 27, 767–776. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Y, Chen C, Yang L, Lee HH, Wang Z, Zhang N, Kolonin MG, An Z, Ge X, et al. (2020). Critical Role of Matrix Metalloproteinase 14 in Adipose Tissue Remodeling during Obesity. Mol Cell Biol 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. (2013). Evidence for two types of brown adipose tissue in humans. Nat Med 19, 631–634. [DOI] [PubMed] [Google Scholar]
- Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li JL, Guilherme A, Guntur K, Czech MP, and Collins S. (2016). Activation of mTORC1 is essential for beta-adrenergic stimulation of adipose browning. J Clin Invest 126, 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, Lin J, Zhao Q, Zhang C, Yuan X, et al. (2015). Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 156, 2461–2469. [DOI] [PubMed] [Google Scholar]
- Lockwood TE (1991). Superficial fascial system (SFS) of the trunk and extremities: a new concept. Plast Reconstr Surg 87, 1009–1018. [DOI] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. (2014). A smooth muscle-like origin for beige adipocytes. Cell Metab 19, 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, and Smith SR (2008). Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhao J, Meng H, and Zhang X. (2019). Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Front Immunol 10, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Altshuler-Keylin S, Wang Q, Chen Y, Henrique Sponton C, Ikeda K, Maretich P, Yoneshiro T, and Kajimura S. (2018). Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci Signal 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, and Saltiel AR (2007a). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, DelProposto JB, Westcott DJ, and Saltiel AR (2008). Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, and Saltiel AR (2007b). Increased Inflammatory Properties of Adipose Tissue Macrophages Recruited During Diet-Induced Obesity. Diabetes 56, 16–23. [DOI] [PubMed] [Google Scholar]
- Mahlakoiv T., Flamar AL., Johnston LK., Moriyama S., Putzel GG., Bryce PJ., and Artis D. (2019). Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol 4, eaax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majithia AR, Flannick J, Shahinian P, Guo M, Bray MA, Fontanillas P, Gabriel SB, Go TDC, Project NJFAS, Consortium STD, et al. (2014). Rare variants in PPARG with decreased activity in adipocyte differentiation are associated with increased risk of type 2 diabetes. Proc Natl Acad Sci U S A 111, 13127–13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, and Varga J. (2015). Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, Botbol Y, Ambrosini M, Fradet M, Rouault C, et al. (2017). A PDGFRalpha-Mediated Switch toward CD9(high) Adipocyte Progenitors Controls Obesity-Induced Adipose Tissue Fibrosis. Cell Metab 25, 673–685. [DOI] [PubMed] [Google Scholar]
- Marcelin G, Silveira ALM, Martins LB, Ferreira AV, and Clement K. (2019). Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest 129, 4032–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman B, and Barton FE Jr. (1987). Anatomy of the subcutaneous tissue of the trunk and lower extremity. Plast Reconstr Surg 80, 248–254. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Craig C, Liu LF, Perelman D, Allister C, Spielman D, and Cushman SW (2016). Adipose Cell Size and Regional Fat Deposition as Predictors of Metabolic Response to Overfeeding in Insulin-Resistant and Insulin-Sensitive Humans. Diabetes 65, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NK, Abrams LR, and Myrskyla M. (2020). US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc Natl Acad Sci U S A 117, 6998–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlotti C, Ceriani V, Morabito A, and Pontiroli AE (2017). Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis. Int J Obes (Lond) 41, 672–682. [DOI] [PubMed] [Google Scholar]
- Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Percec I, and Seale P. (2019). Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer LK, Ciaraldi TP, Henry RR, Wittgrove AC, and Phillips SA (2013). Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Perez S, and Randall TD (2017). Immunological Functions of the Omentum. Trends Immunol 38, 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, et al. (2018). Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh HL, Kim JK, Cooper MP, Fitzgibbons T, et al. (2016). Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, and DeFronzo RA (2002). Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87, 2784–2791. [DOI] [PubMed] [Google Scholar]
- Molofsky AB., Nussbaum JC., Liang HE., Van Dyken SJ., Cheng LE., Mohapatra A., Chawla A., and Locksley RM. (2013). Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigny P, Houssier M, Mouisel E, and Langin D. (2016). Adipocyte lipolysis and insulin resistance. Biochimie 125, 259–266. [DOI] [PubMed] [Google Scholar]
- Morley TS, Xia JY, and Scherer PE (2015). Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat Commun 6, 7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF (2016). Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci 196, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowers J, Uhm M, Reilly SM, Simon J, Leto D, Chiang SH, Chang L, and Saltiel AR (2013). Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKepsilon and TBK1. Elife 2, e01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano I, Barbatelli G, Giordano A, and Cinti S. (2009). Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat 214, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, Hoh R, Antelo F, Strawford A, McCune JM, et al. (2002). Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A 99, 15345–15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA, et al. (2014). Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 130, 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HP, Lin F, Yi D, Xie Y, Dinh J, Xue P, and Sul HS (2021). Aging- dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev Cell 56, 1437-1451 e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu- Bryan R, Glass CK, Neels JG, and Olefsky JM (2007). A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282, 35279–35292. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, and Jensen MD (2004). Splanchnic lipolysis in human obesity. J Clin Invest 113, 1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. (2009). CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15, 914–920. [DOI] [PubMed] [Google Scholar]
- Oguri Y, Shinoda K, Kim H, Alba DL, Bolus WR, Wang Q, Brown Z, Pradhan RN, Tajima K, Yoneshiro T, et al. (2020). CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 182, 563–577 e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K., Parker JL., Ouchi N., Higuchi A., Vita JA., Gokce N., Pedersen AA., Kalthoff C., Tullin S., Sams A., et al. (2010). Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285, 6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Bruce, and Kajimura S. (2012). PPARγ agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metabolism 15, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, and Carpentier AC (2012). Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, and Richard D. (2011). Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96, 192–199. [DOI] [PubMed] [Google Scholar]
- Padwal R, Leslie WD, Lix LM, and Majumdar SR (2016). Relationship Among Body Fat Percentage, Body Mass Index, and All-Cause Mortality: A Cohort Study. Ann Intern Med 164, 532–541. [DOI] [PubMed] [Google Scholar]
- Pal A, Barber TM, Van de Bunt M, Rudge SA, Zhang Q, Lachlan KL, Cooper NS, Linden H, Levy JC, Wakelam MJ, et al. (2012). PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N Engl J Med 367, 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WW, and Myers MG Jr. (2018). Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 19, 95–105. [DOI] [PubMed] [Google Scholar]
- Park J, Shin S, Liu L, Jahan I, Ong SG, Xu P, Berry DC, and Jiang Y. (2021). Progenitor-like characteristics in a subgroup of UCP1+ cells within white adipose tissue. Dev Cell 56, 985-999 e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, and Neels JG (2008). Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson S, Loft A, Rajbhandari P, Simcox J, Lee S, Tontonoz P, Mandrup S, and Villanueva CJ (2019). Loss of TLE3 promotes the mitochondrial program in beige adipocytes and improves glucose metabolism. Genes Dev 33, 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinelli V, Heuvingh J, du Roure O, Rouault C, Devulder A, Klein C, Lacasa M, Clement E, Lacasa D, and Clement K. (2014). Human adipocyte function is impacted by mechanical cues. J Pathol 233, 183–195. [DOI] [PubMed] [Google Scholar]
- Petersen MC, and Shulman GI (2018). Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev 98, 2133–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurichard D, Delebecque F, Lorsignol A, Barreau C, Rouquette J, Descombes X, Casteilla L, and Degond P. (2017). Simple mechanical cues could explain adipose tissue morphology. J Theor Biol 429, 61–81. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, et al. (2017). Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, and Spiegelman BM (1998). A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Nguyen KD., Odegaard JI., Cui X., Tian X., Locksley RM., Palmiter RD., and Chawla A. (2014). Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raajendiran A, Ooi G, Bayliss J, O’Brien PE, Schittenhelm RB, Clark AK, Taylor RA, Rodeheffer MS, Burton PR, and Watt MJ (2019). Identification of Metabolically Distinct Adipocyte Progenitor Cells in Human Adipose Tissues. Cell Rep 27, 1528-1540 e1527. [DOI] [PubMed] [Google Scholar]
- Rajbhandari P, Arneson D, Hart SK, Ahn IS, Diamante G, Santos LC, Zaghari N, Feng AC, Thomas BJ, Vergnes L, et al. (2019). Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, and Tortoriello DV (2008). Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32, 451–463. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Wurtman J, Rojansky N, Adler D, Stein P, Schenker JG, and Brzezinski A. (1995). Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: a prospective study. Fertil Steril 64, 963–968. [DOI] [PubMed] [Google Scholar]
- Rigamonti A, Brennand K, Lau F, and Cowan CA (2011). Rapid cellular turnover in adipose tissue. PLoS One 6, e17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, and Friedman JM (2008). Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249. [DOI] [PubMed] [Google Scholar]
- Roh HC, Tsai LTY, Shao M, Tenen D, Shen Y, Kumari M, Lyubetskaya A, Jacobs C, Dawes B, Gupta RK, et al. (2018). Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab 27, 1121–1137 e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, et al. (2014). Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 306, E945–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, and Spiegelman BM (2014). What we talk about when we talk about fat. Cell 156, 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, and Wolfrum C. (2013). Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15, 659–667. [DOI] [PubMed] [Google Scholar]
- Rytka JM, Wueest S, Schoenle EJ, and Konrad D. (2011). The portal theory supported by venous drainage-selective fat transplantation. Diabetes 60, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M, Fujisaka S, Cai W, Winnay JN, Konishi M, O’Neill BT, Li M, Garcia-Martin R, Takahashi H, Hu J, et al. (2017). Adipocyte Dynamics and Reversible Metabolic Syndrome in Mice with an Inducible Adipocyte-Specific Deletion of the Insulin Receptor. Cell Metab 25, 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans LB, Cushman SW, and Weismann RE (1973). Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest 52, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hsiao WY, and Guertin DA (2015). Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Reports 4, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Hung CM., and Guertin DA. (2016). Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol 26, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvari AK, Van Hauwaert EL, Markussen LK, Gammelmark E, Marcher AB, Ebbesen MF, Nielsen R, Brewer JR, Madsen JGS, and Mandrup S. (2021). Plasticity of Epididymal Adipose Tissue in Response to Diet-Induced Obesity at Single-Nucleus Resolution. Cell Metab 33, 437-453 e435. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, Kolb D, Hoeks J, Kershaw EE, Sedej S, et al. (2017). Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab 26, 753-763 e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff KU, Soldati G, et al. (2018). A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108. [DOI] [PubMed] [Google Scholar]
- Sebo ZL, and Rodeheffer MS (2019). Assembling the adipose organ: adipocyte lineage segregation and adipogenesis in vivo. Development 146, dev172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi F, Piper M, Ho LL, Huang TL, Gupta A, Streets A, Lynes MD, and Tseng YH (2021). Vascular smooth muscle-derived Trpv1(+) progenitors are a source of cold-induced thermogenic adipocytes. Nat Metab 3, 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B, Shao M, Zhang Q, An YA, Vishvanath L, and Gupta RK (2021). Cold- responsive adipocyte progenitors couple adrenergic signaling to immune cell activation to promote beige adipocyte accrual. Genes Dev 35, 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B, Shao M, Zhang Q, Hepler C, Paschoal VA, Barnes SD, Vishvanath L, An YA, Jia L, Malladi VS, et al. (2020). Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat Metab 2, 1332–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Hepler C, Zhang Q, Shan B, Vishvanath L, Henry GH, Zhao S, An YA, Wu Y, Strand DW, et al. (2021a). Pathologic HIF1alpha signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell 28, 685-701 e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, MacPherson KA, Spurgin SB, Sun K, Holland WL, et al. (2016). Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab 23, 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, Chen S, Yu X, An YA, Zhu Y, et al. (2018). De novo adipocyte differentiation from Pdgfrbeta(+) preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun 9, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE, and Gupta RK (2019). Cellular Origins of Beige Fat Cells Revisited. Diabetes 68, 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Zhang Q, Truong A, Shan B, Vishvanath L, Li L, Seale P, and Gupta RK (2021b). ZFP423 controls EBF2 coactivator recruitment and PPARgamma occupancy to determine the thermogenic plasticity of adipocytes. Genes Dev 35, 1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira SN, and Seale P. (2019). Transcriptional Control of Brown and Beige Fat Development and Function. Obesity (Silver Spring) 27, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearin AL, Monks BR, Seale P, and Birnbaum MJ (2016). Lack of AKT in adipocytes causes severe lipodystrophy. Mol Metab 5, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Punyanitya M., Silva AM., Chen J., Gallagher D., Sardinha LB., Allison DB., and Heymsfield SB. (2009). Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, and Walsh K. (2014). Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest 124, 2099–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, Jackson R, Rumore D, Xue B, Shi H, et al. (2017). Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab 26, 764-777 e765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, et al. (2016). Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest 126, 4626–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BA, Wasko RR, Mano O, Rutenberg-Schoenberg M, Rudolph MC, Zirak B, Rivera-Gonzalez GC, Lopez-Giraldez F, Zarini S, Rezza A, et al. (2020).Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 26, 880-895 e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siersbaek R, Nielsen R, and Mandrup S. (2012). Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab 23, 56–64. [DOI] [PubMed] [Google Scholar]
- Smith GI, Mittendorfer B, and Klein S. (2019). Metabolically healthy obesity: facts and fantasies. J Clin Invest 129, 3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Dai W, Jang MJ, Medrano L, Li Z, Zhao H, Shao M, Tan J, Li A, Ning T, et al. (2020). Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J Clin Invest 130, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Xiaoli AM, and Yang F. (2018). Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 10, 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. (2008). Dynamics of fat cell turnover in humans. Nature 453, 783–787. [DOI] [PubMed] [Google Scholar]
- Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C, and Mathis D. (2019). Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol 4, eaaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefkovich M, Traynor S, Cheng L, Merrick D, and Seale P. (2021). Dpp4+ interstitial progenitor cells contribute to basal and high fat diet-induced adipogenesis. Mol Metab 54, 101357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr., and Lazar MA (2010). Propagation of adipogenic signals through an epigenomic transition state. Genes Dev 24, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine RR, Shapira SN, Lim HW, Ishibashi J, Harms M, Won KJ, and Seale P. (2016). EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol Metab 5, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Berry WL, and Olson LE (2017). PDGFRalpha controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development 144, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Sakashita H., Kim J., Tang Z., Upchurch GM., Yao L., Berry WL., Griffin TM., and Olson LE. (2020a). Mosaic Mutant Analysis Identifies PDGFRalpha/PDGFRbeta as Negative Regulators of Adipogenesis. Cell Stem Cell 26, 707-721 e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Halberg N, Khan M, Magalang UJ, and Scherer PE (2013a). Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol Cell Biol 33, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, and Scherer PE (2014). Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab 3, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Tordjman J, Clement K, and Scherer PE (2013b). Fibrosis and adipose tissue dysfunction. Cell Metab 18, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, Giordano A, Kovanicova Z, Stefanicka P, Balazova L, et al. (2020b). snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102. [DOI] [PubMed] [Google Scholar]
- Sveidahl Johansen O, Ma T, Hansen JB, Markussen LK, Schreiber R, Reverte- Salisa L, Dong H, Christensen DP, Sun W, Gnad T, et al. (2021). Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell 184, 3502-3518 e3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztalryd C, and Brasaemle DL (2017). The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids 1862, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabula Muris C. (2020). A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HN, Tang CY, Man XF, Tan SW, Guo Y, Tang J, Zhou CL, and Zhou HD (2017). Plasticity of adipose tissue in response to fasting and refeeding in male mice. Nutr Metab (Lond) 14, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Seo J, Jo AY, and Graff JM (2011). Thiazolidinediones regulate adipose lineage dynamics. Cell Metab 14, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, and Graff JM (2008). White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wallace M, Sanchez-Gurmaches J, Hsiao WY, Li H, Lee PL, Vernia S, Metallo CM, and Guertin DA (2016). Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat Commun 7, 11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Zmora N, Adolph TE, and Elinav E. (2020). The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 20, 40–54. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, & Spiegelman BM (1994). Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79. [DOI] [PubMed] [Google Scholar]
- Tran CM, Mukherjee S, Ye L, Frederick DW, Kissig M, Davis JG, Lamming DW, Seale P, and Baur JA (2016). Rapamycin Blocks Induction of the Thermogenic Program in White Adipose Tissue. Diabetes 65, 927–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. (2013). Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93, 1–21. [DOI] [PubMed] [Google Scholar]
- Uezumi A., Fukada S., Yamamoto N., Takeda S., and Tsuchida K. (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, et al. (2011). Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124, 3654–3664. [DOI] [PubMed] [Google Scholar]
- Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, and Kozak LP (2006). UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem 281, 31894–31908. [DOI] [PubMed] [Google Scholar]
- Vazirani RP, Verma A, Sadacca LA, Buckman MS, Picatoste B, Beg M, Torsitano C, Bruno JH, Patel RT, Simonyte K, et al. (2016). Disruption of Adipose Rab10-Dependent Insulin Signaling Causes Hepatic Insulin Resistance. Diabetes 65, 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay J, Gauthier MF, Biswell RL, Louiselle DA, Johnston JJ, Cheung WA, Belden B, Pramatarova A, Biertho L, Gibson M, et al. (2020). Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab 2, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, et al. (2013). Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab 17, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, and Gupta RK (2019). Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest 129, 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, and Gupta RK (2016). Pdgfrbeta+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab 23, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L, Jiang L, Ye R, Shao M, Tao C, et al. (2018). Reversible De-differentiation of Mature White Adipocytes into Preadipocyte-like Precursors during Lactation. Cell Metab 28, 282-288 e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, and Scherer PE (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19, 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ishibashi J, Trefely S, Shao M, Cowan AJ, Sakers A, Lim HW, O’Connor S, Doan MT, Cohen P, et al. (2019). A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab 30, 174-189 e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, and Ferrante AW Jr. (2006). CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW Jr. (2003). Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ (2019). Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc Natl Acad Sci U S A 116, 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott GP, Emont MP, Li J, Jacobs C, Tsai L, and Rosen ED (2021).Mesothelial cells are not a source of adipocytes in mice. Cell Rep 36, 109388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Foley JE, Borgadus C, Tataranni PA, and Pratley RE (2000).Enlarged subcutaneous adbominal adipocyte size, but not obesity itself, predicts Type II diabetes independent of insulin resistance. Diabetologia 43, 1498–1506. [DOI] [PubMed] [Google Scholar]
- Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, and Sowers MR (2008). The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168, 1617–1624. [DOI] [PubMed] [Google Scholar]
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, et al. (2007). T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Yang X, Lin Y, Li S, Jiang J, Qian S, Tang Q, He R, and Li X. (2016). Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity. Int J Obes (Lond) 40, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, et al. (2015). Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4, e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, et al. (2009). Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9, 99–109. [DOI] [PubMed] [Google Scholar]
- Yang Q, Vijayakumar A, and Kahn BB (2018). Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 19, 654–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Liu H, Zhang F, and Hu F. (2019). mTOR signaling in Brown and Beige adipocytes: implications for thermogenesis and obesity. Nutr Metab (Lond) 16, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, Claiborn KC, and Hotamisligil GS (2016). De Novo Lipogenesis Products and Endogenous Lipokines. Diabetes 65, 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H. (2004). Thiazolidinediones. N Engl J Med 351, 1106–1118. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, Dai Z, White PJ, McGarrah RW, Ilkayeva OR, et al. (2019). BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MS (2016). The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 8, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, et al. (2014). Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Patterson BW, and Klein S. (2019). Adipose Tissue CTGF Expression is Associated with Adiposity and Insulin Resistance in Humans. Obesity (Silver Spring) 27, 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DA., Choi YS., Engler AJ., and Christman KL. (2013). Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 34, 8581–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, et al. (2010). Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 59, 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, and Beguinot F. (2019). Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol 10, 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, et al. (2015). Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, and Spiegelman BM (2019). Innervation of thermogenic adipose tissue via a calsyntenin 3beta-S100b axis. Nature 569, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Hao G, Shao M, Nham K, An Y, Wang Q, Zhu Y, Kusminski CM, Hassan G, Gupta RK, et al. (2018). An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab 27, 252–262 e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, Zhu Y, Ghaben AL, Wang MY, Li N, et al. (2019). Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 129, 5327–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, Caron A, Zhu Q, Sun K, Xiong W, et al. (2019). Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab 30, 706-719 e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Santoro A, Peroni OD, Nelson AT, Saghatelian A, Siegel D, and Kahn BB (2019). PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J Clin Invest 129, 4138–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick RK., Guerrero-Juarez CF., Horsley V., and Plikus MV. (2018). Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab 27, 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]