Abstract
An association between diabetes and infection has been recognised for many years, with infection being an important cause of death and morbidity in people with diabetes. The COVID-19 pandemic has re-kindled an interest in the complex relationship between diabetes and infection. Some infections occur almost exclusively in people with diabetes, often with high mortality rates without early diagnosis and treatment. However, more commonly, diabetes is a complicating factor in many infections. A reciprocal relationship occurs whereby certain infections and their treatments may also increase the risk of diabetes. People with diabetes have a 1.5- to 4-fold increased risk of infection. The risks are the most pronounced for kidney infection, osteomyelitis and foot infection, but are also increased for pneumonia, influenza, tuberculosis, skin infection and general sepsis. Outcomes from infection are worse in people with diabetes, with the most notable example being a twofold higher rate of death from COVID-19. Hyperglycaemia has deleterious effects on the immune response. Vascular insufficiency and neuropathy, together with altered skin, mucosal and gut microbial colonisation, contribute to the increased risk of infection. Vaccination is important in people with diabetes although the efficacy of certain immunisations may be compromised, particularly in the presence of hyperglycaemia. The principles of treatment largely follow those of the general population with certain notable exceptions.
Graphical Abstract
Supplementary Information
The online version contains a slideset of the figures for download available at 10.1007/s00125-024-06102-x.
Keywords: Antimicrobials, Bacteria, Diabetes, Epidemiology, Infection, Pathogenesis, Review, Virus
Introduction
Historically, infections have been an important cause of death and morbidity in people with diabetes [1, 2]. Although this remains the case, particularly in low- and middle-income countries where infections are commonly presenting features of previously undiagnosed diabetes [3], infection has been an under-studied complication of diabetes. People with diabetes develop infections more often than the general population and the course of infection is more complicated [4–8]. The COVID-19 pandemic has re-kindled interest in the complex relationship between diabetes and infection, following observations that people with diabetes are more likely to progress to severe COVID-19 disease and die than those without diabetes [9].
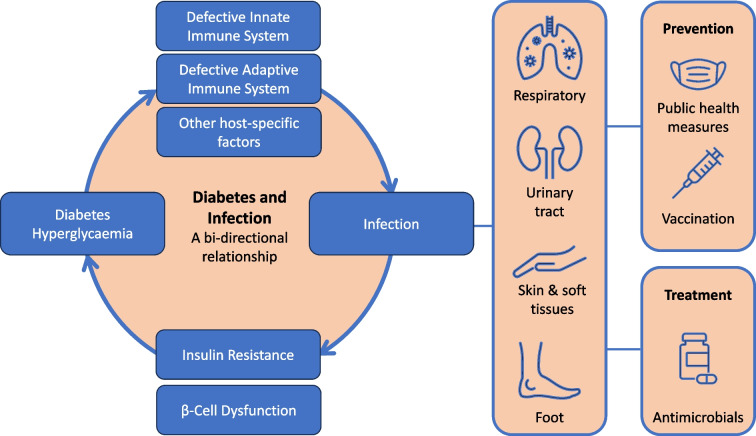
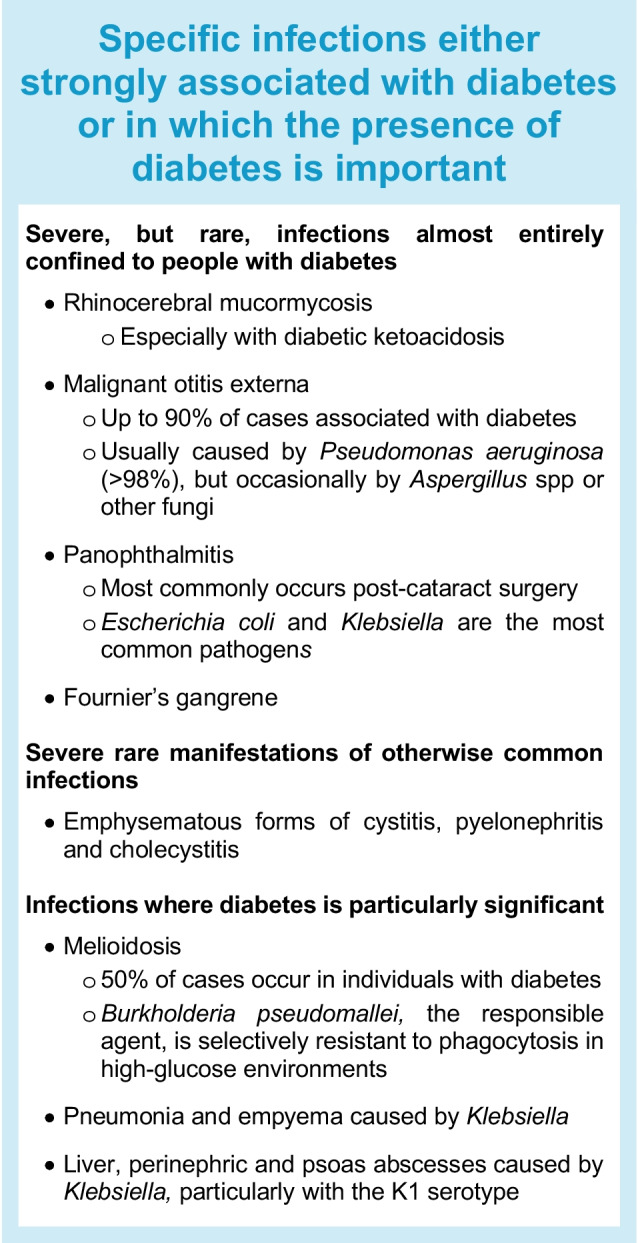
Some severe infections occur predominantly in people with diabetes [10–13]. These tend to be uncommon but convey high mortality rates without early diagnosis and treatment (see Text box). Conversely, diabetes is more often a complicating factor in common infections, where the clinical course may be heterogeneous and affected by factors including glycaemic levels (both recent and longer term), diabetes-related complications and obesity [4–8].
Healthcare professionals should be aware of the relationships between diabetes and infection to watch out for serious manifestations of common infections. This review aims to describe the bi-directional relationship between diabetes and infection, before examining the mechanisms that increase the risk and severity of infection in people with diabetes. It is impossible to describe all infections, but specific mention is made of COVID-19, influenza, tuberculosis, skin and urinary tract infections and diabetes-related foot infections. Finally, we describe the principles of treatment as well as prevention through vaccination.
How infection affects the incidence of diabetes and glucose regulation
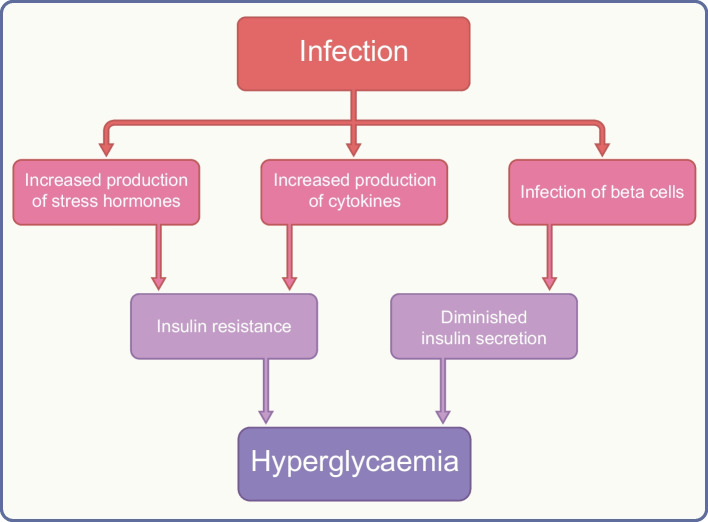
Infection, and in some cases its treatment, may affect glucose homeostasis through effects on insulin secretion and resistance and increase the risk of diabetes (Fig. 1). Infections are an important predisposing factor for both diabetic ketoacidosis and hyperosmolar hyperglycaemia syndrome, and a search for underlying infection is an important part of the clinical management of acute hyperglycaemic complications.
Fig. 1.
Mechanisms by which infection may worsen glycaemia. Stress hormones include glucagon, growth hormone, catecholamines and glucocorticoid. Cytokines include tumour necrosis factor-α and interleukin-1. This figure is available as part of a downloadable slideset
Effects on insulin secretion
Multiple viruses have been associated with type 1 diabetes, in particular enteroviruses (especially Coxsackie B1, B4), mumps, rubella and cytomegalovirus (CMV) [14]. Although the literature spans in vitro studies, animal models, human pancreatic tissue and epidemiological studies, the link has not been unequivocally proven in humans [15]. Interest in this area increased following observations that acute and long COVID-19 may be associated with a higher incidence of diabetes. Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 can infect and replicate in human beta cells by gaining entry via ACE-2 receptors [16], but whether this leads to irreversible harm is uncertain. Although early studies suggested an increased incidence of type 1 diabetes [15, 17–21], not all longer term studies have confirmed a significant effect of SARS-CoV-2 [20, 22–28]. Some of the discrepancy between studies may be explained by detection bias as people may be screened for diabetes when presenting with COVID-19 in the community. By contrast, no differences in risk were found in a hospital study, where there were similar opportunities for detection of previously undiagnosed diabetes [28]. Other social, economic and environmental changes that occurred during the pandemic may provide alternative explanations [19, 25, 27]. The CoviDiab registry (https://COVIDiab.e-dendrite.com) has been established to investigate the extent and characteristics of new-onset, COVID-19-related diabetes and will provide further information on the aetiological role of SARS-CoV-2.
Effect on insulin resistance
Infection induces a stress reaction, increasing the production of counter-regulatory hormones (glucagon, growth hormone, catecholamine and glucocorticoid) and cytokines such as tumour necrosis factor-α and interleukin-1. This combination antagonises insulin action, leading to a failure to suppress hepatic gluconeogenesis and impaired glucose uptake into skeletal muscle [29].
Several viral infections, including infection with hepatitis C virus (HCV) [30, 31] and HIV [32, 33], have been associated with type 2 diabetes. In the case of HCV infection, the inflammation caused by liver damage and subsequent insulin resistance may contribute to the development of hyperglycaemia, but HCV may directly increase insulin resistance by downregulating insulin receptor substrate-1. Successful eradication of HCV improves insulin sensitivity, decreases insulin requirement and lowers blood glucose levels [34]. In addition to the effect of HIV, its treatment with protease inhibitors predisposes to diabetes. These drugs are associated with impaired lipid homeostasis, lipodystrophy, insulin resistance and, to a lesser extent, impaired insulin secretion and mitochondrial dysfunction [35].
Gingivitis and periodontitis are examples of bacterial infection that predispose to diabetes [36]. These infections are associated with local and systemic inflammatory responses that may adversely affect glycaemic levels [37] and increase the risk of diabetes [38, 39]. Treating gingivitis and periodontitis by mechanical removal of dental biofilm and calculus reduces inflammation; meta-analyses of short-duration studies indicate that HbA1c and fasting plasma glucose improve by approximately 3–6 mmol/mol (0.3–0.7%) and 0.5–0.8 mmol/l, respectively, after 3 to 6 months [40–42].
How diabetes affects the incidence and outcomes of infection
The effect of diabetes on infection has been examined in large population-based cohorts [4–8]. Although studies varied in methods used to capture infection, with some studies reporting hospitalisation for infection and others relying on outpatient clinic codes or prescription of antimicrobial therapy, most studies have reported significant risk associations of diabetes with infection independent of comorbid conditions and other confounding factors. Compared with the general population, people with diabetes have a two- to fourfold increased risk of infection-related hospitalisation and a 1.5-fold increased risk of infection presenting in an outpatient setting [7, 8, 43]. By infection type, the risks are the most pronounced for kidney infection (3.0- to 4.9-fold), osteomyelitis (4.4- to 15.7-fold) and foot infection (6.0- to 14.7-fold), but also increased for pneumonia, influenza, tuberculosis, skin infection, surgical site infection and general sepsis [4, 5, 7, 8, 44].
Diabetes confers a worse outcome from infection, with the most notable example being a twofold higher rate of death from COVID-19 [45]. The risk differential for infection is greater in younger vs older people [4], but seems unaffected by ethnicity [43]. Impaired glucose tolerance is also associated with a higher incidence of infection although the magnitude of the risk is smaller than for diabetes [43, 46].
Where studies considered infection rates in type 1 diabetes and type 2 diabetes separately, the risk ratios were generally higher for type 1 diabetes than type 2 diabetes [6, 47]. In a retrospective UK primary care cohort study including 102,493 people, the incidence rate ratio for hospitalisation related to any infection was 3.7 for type 1 diabetes and 1.9 for type 2 diabetes, and death from infection was increased 7.7-fold in type 1 diabetes and 1.9-fold for type 2 diabetes, relative to adults without diabetes. An Australian study reported a 5.8-fold increase for death from pneumonia, 29.6-fold for osteomyelitis and 9.9-fold for sepsis in people with type 1 diabetes compared with those without diabetes [47].
In contemporary studies examining trends of infection, the rates of infection-related hospitalisation have either remained unchanged or fluctuated in the last two decades. In both the USA and Hong Kong, annualised rates of hospitalisation for influenza showed a rising trend in people with and without diabetes, which may be partly explained by more frequent diagnostic testing leading to better case ascertainment (Fig. 2) [4, 7]. In the USA, hospitalisation for pneumonia decreased in the latter half of the observation period in the general population, while remaining static in people with diabetes, similar to what was observed in Hong Kong [4, 7]. The reasons for these divergent trends of pneumonia between people with and without diabetes are unclear. The sex disparity in the rates of pneumonia in people with diabetes has also been seen in the general population and is likely due to biological differences, such as hormonal cycles and cellular immune-mediated responses, and psychosocial reasons, leading to delayed presentation by men [48]. These observations are worrying when contrasted against decreasing trends of other major clinical events such as cardiovascular disease and lower extremity amputation. Hyperglycaemia and obesity are both risk factors for infection, but there is a lack of consistency between reports about the relationship between HbA1c and infection. Nevertheless, there is a tendency towards more severe infections, especially tuberculosis and kidney infections, with higher HbA1c.
Fig. 2.
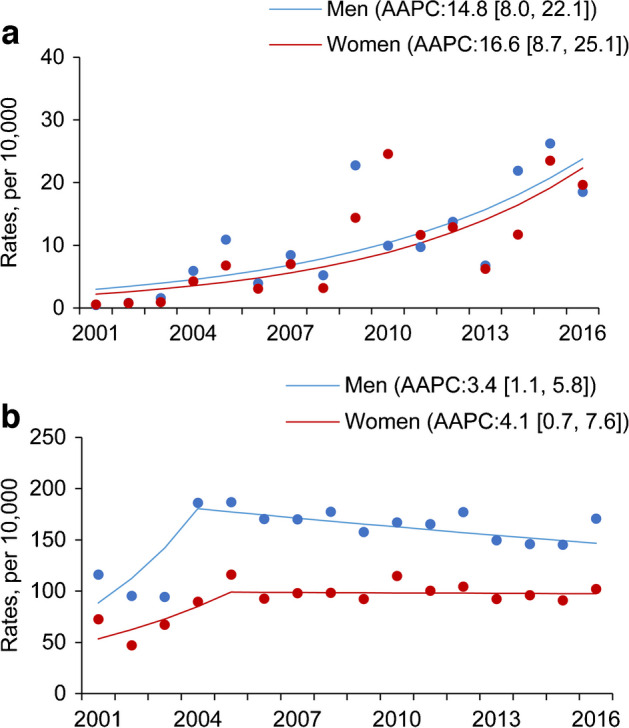
Age-standardised rates of hospitalisation (per 10,000 people) for influenza (a) and community-acquired pneumonia (b) in men (blue) and women (red) with diabetes in Hong Kong between 2001 and 2016 [4]. Similar trends have also been observed in the USA [7]. Data for average annual percentage change (AAPC) with 95% CI are also shown. This figure has been reproduced from [4] with permission from Springer Nature. This figure is available as part of a downloadable slideset
Mechanisms to explain the association of diabetes and infection
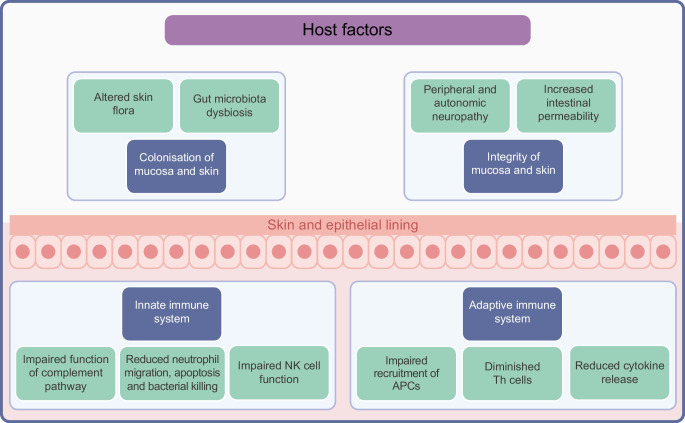
The mechanisms by which diabetes increases the risk of infection can be broadly divided into host factors and organism-specific factors (Fig. 3). Some of the organism-specific factors will be covered in later sections, but in this section we consider the following host factors: the impact of hyperglycaemia on the immune response; vascular insufficiency; sensory peripheral and autonomic neuropathy; skin and mucosal colonisation with pathogens; and increased intestinal permeability and gut-microbiota dysbiosis.
Fig. 3.
Mechanisms that increase the risk of infection in people with diabetes. Diabetes and its complications impair both innate and adaptive immune systems. The green boxes illustrate how host factors associated with diabetes can affect aspects of the immune system (shown in blue boxes). Some of these host factors affect the likelihood of infection (see processes shown above the skin and epithelial lining) and some affect the response to an infection (see processes shown below the skin and epithelial lining). APC, antigen-presenting cells; NK, natural killer. This figure is available as part of a downloadable slideset
Impact of the immune response due to hyperglycaemia
Hyperglycaemia has deleterious effects on the innate immune response and adaptive immunity, both of which contribute towards the increased risk of different infections in individuals affected by diabetes.
Impairment and modulation of the innate immune system
The innate immune system is often considered the first line of defence of any organism against potential pathogens and infections, even in the absence of prior encounter with the pathogens, for example, in neonates. However, it is also required for the subsequent development of the adaptive response to pathogens encountered, mediated by the expansion of specific clones of B and T lymphocytes. The innate immune response works by having a system and group of proteins and phagocytic cells that can recognise certain conserved features of pathogens, thereby acting against pathogens as soon as they come into contact with the body.
The skin and epithelial lining represent an important part of the innate immune system, and a key barrier against infection. Diabetes increases the risk of different skin lesions and ulcers, for example, diabetes-related foot ulcers, which breach this basic defence and increase the risk of infections. Furthermore, recent studies suggest hyperglycaemia disrupts intestinal barrier function, as well as reprogrammes intestinal epithelial cells, thereby increasing the risk of enteric infections [49].
Diabetes and hyperglycaemia represent states of chronic inflammation that are associated with activation of several components of the innate immune system, including the complement pathway, as well as increased production of several cytokines [50]. The complement pathway is a key component of the innate immune system, with C3 being the central component, and activation to C3b is essential for bacterial opsonisation and subsequent destruction by the membrane attack complex (MAC). While circulating C3 and C4 levels are elevated in people with diabetes, hyperglycaemia leads to an altered structure of C3 and inhibits C3-mediated complement effectors, leading to inhibited immune control of bacterial infections [51]. Diabetes is associated with increased production of reactive oxygen species (ROS), increased release of proinflammatory cytokines and elevated formation of neutrophil extracellular trap (NET). However, it is also linked to reduced neutrophil migration, lower levels of apoptosis, impaired intracellular ROS production and reduced bacterial killing [52]. Natural killer (NK) cells, another key part of the innate immune system, are also impaired in individuals with diabetes, in particular those with long duration of type 1 diabetes [53].
Impact of diabetes on the adaptive immune response
Diabetes and hyperglycaemia worsen the adaptive immune response by impairing recruitment and function of antigen-presenting cells (APCs), which results in reduced frequency of T helper (Th)1, Th2 and Th17 cells and the release of cytokines. These, in turn, impact the inflammatory response to encountered pathogens, and contribute to increased risk of infections such as tuberculosis [54]. Interestingly, some of these biological changes are also observed in states of intermediate hyperglycaemia, which highlights the significant role of hyperglycaemia in mediating the risk of infection [55].
Other host-specific factors
Several other host-specific factors, notably peripheral vascular disease, peripheral neuropathy increasing the risk of trauma, autonomic neuropathy, skin colonisation with pathogens and the impact of hyperglycaemia itself, combine to contribute to increased risk and severity of foot ulcers in diabetes. There is also increasing interest in the relationship between diabetes, hyperglycaemia and gut-microbiota dysbiosis. In addition to this being considered an important contributor to the metabolic dysfunction and insulin resistance [56], the microbiome contributes to the increased risk of enteric and other systemic infections in diabetes [49, 57].
Specific infections associated with diabetes
Respiratory tract infections
Community-acquired pneumonia
People with type 2 diabetes have a 1.3- to 2.6-fold higher risk of community-acquired pneumonia and the risks correlate with glycaemic levels [4, 7, 58, 59]. Mortality rates from pneumonia are also increased with diabetes and, in some populations, pneumonia has overtaken cardiovascular disease and cancer as the most common cause of death. Aside from Streptococcus pneumoniae, Staphylococcus aureus and Gram-negative organisms such as Klebsiella pneumoniae are common pathogens leading to lower respiratory tract infections in people with diabetes. In the case of S. aureus pneumonia, this may be attributed to higher rates of nasal carriage in people with diabetes (up to 30%) compared with individuals without diabetes (11%) [60]. Diabetes is the commonest underlying predisposing factor for thoracic empyema, with Klebsiella spp. being notable common pathogens, followed by streptococci, S. aureus and anaerobes [61].
Influenza
Influenza is responsible for half a million deaths globally every year. Diabetes increases the risk of severe disease, defined as the requirement for mechanical ventilation, admission to the intensive care unit (ICU) or in-hospital death in young adults (aged 15–50 years) [62], and overall mortality rates [63] by up to fourfold. Influenza and bacterial pneumonia also increase the risk of acute coronary events, which is sustained for weeks to months after the initial exposure [64]. The reasons are multifactorial, possibly related to systemic inflammation causing activation of inflammatory cells in atherosclerotic plaques, a prothrombotic state triggering coronary thrombosis and increased metabolic demand causing cardiac decompensation. Healthcare professionals should ensure that therapy used in primary or secondary cardiovascular protection, such as aspirin and statins, is not interrupted during the course of the infection. Several studies conducted using real-world databases indicate that the use of renin–angiotensin system inhibitors was associated with reduced hospitalisation and/or death from influenza and pneumonia in people with or without diabetes [65–67]. Other glucose-lowering drugs, for example, metformin, may also lower the risk of pneumonia and related mortality rates but these findings are yet to be verified in large RCTs [68, 69].
Tuberculosis
People with diabetes are approximately two to three times more likely to develop active tuberculosis than the general population [70, 71]. The public health impact may be particularly high in low- or middle-income countries or areas at the forefront of the diabetes epidemic and where tuberculosis remains endemic, namely in Africa and South-East Asia [71, 72]. In Hong Kong, the risk differential for tuberculosis between people with and without diabetes has remained unchanged over a 10 year period at two- to threefold for middle-aged people with diabetes, but reaching sevenfold in younger age groups [4]. In India, diabetes accounts for 15% of pulmonary tuberculosis and 20% of smear-positive tuberculosis, with an excess risk of the latter in urban areas [73].
Comorbid tuberculosis and diabetes worsens the outcome of both conditions. Extrapulmonary or unusual manifestations of tuberculosis including cavitating disease and involvement of the lower lobes are commoner in people with diabetes, especially those with persistent hyperglycaemia [74]. People with diabetes have a higher risk of treatment failure, relapse and death during treatment [75]. Likewise, tuberculosis can exacerbate hyperglycaemia through stress mechanisms and people with pre-existing diabetes may require intensification of glucose-lowering therapy until active infection is resolved. In a large US longitudinal study, latent tuberculosis infection was associated with a 20% increased risk of developing type 2 diabetes and the risk did not attenuate with treatment of the tuberculosis infection [76]. The World Health Organization recommends that all people with newly diagnosed active tuberculosis should be screened for diabetes if local resources allow, as prompt initiation of glucose-lowering therapy can potentially improve treatment outcomes [77]. Conversely, screening for tuberculosis in people with diabetes is not routinely recommended except in areas with high prevalence of tuberculosis (prevalence over 100 per 100,000 population), in people with specific symptoms or signs and in those with unexplained deterioration in glycaemic levels.
Coronavirus
The interaction between diabetes and COVID-19 has been extensively reviewed in the last 3 years [9]. In common with SARS-CoV and Middle East respiratory syndrome-related coronavirus (MERS-CoV), SARS-CoV-2 is an RNA virus that originated from animals. Similar to previous reports that diabetes was an important predictor of severe disease and death in people infected with SARS-CoV and MERS-CoV [78, 79], studies during the pandemic have indicated that diabetes was associated with adverse outcomes and higher mortality rates from COVID-19, although there is no conclusive evidence that diabetes increases the risk of acquiring the viral infection [9, 80]. Between 13% and 58% of people with COVID-19 requiring ICU admission, and between 17% and 35% of people who died, had pre-existing diabetes. Globally, diabetes contributed to 10% of severe forms of COVID-19 and 17% of COVID-19-related deaths [81]. Glycaemic levels appear to affect the outcome of COVID-19. Some, but not all, studies have reported a positive association between HbA1c before hospitalisation and mortality rates from COVID-19 [9]. However, high blood glucose on admission was most consistently predictive of severe disease, irrespective of diabetes status.
Urinary tract infection
People with diabetes have an increased risk of urinary tract infection, spanning all levels of severity from asymptomatic bacteriuria to renal and perinephric abscess and severe life-threatening emphysematous forms of cystitis and pyelonephritis. Fungal infections, for example, candiduria, and infection with organisms other than Escherichia coli are also more frequent in diabetes [82]. The significance of the increased prevalence of asymptomatic bacteriuria in people with diabetes is uncertain because antibiotic treatment does not influence the progression to symptomatic urinary tract infection or risk of pyelonephritis [82]. Autonomic neuropathy is an important predisposing factor; apart from decreased reflex detrusor activity, people with autonomic neuropathy may have impaired bladder sensation resulting in bladder distension, increased residual urine volume and vesicoureteric reflux [83]. The use of sodium–glucose cotransporter 2 (SGLT-2) inhibitors is associated with increased risk of genital tract infections but not urinary tract infections [84].
Skin and soft tissue infections
Skin infections including dermatophyte infection, candidal intertrigo, bacterial cellulitis and skin abscess are common in people with diabetes. Cellulitis or skin abscesses may be a manifestation of systemic bacteraemia, and diabetes confers a higher risk of mortality due to septicaemia especially among older people. Predisposing factors include peripheral sensory neuropathy, in particular sudomotor dysfunction, causing dryness of skin, dermatophytosis and microvasculopathy. Group A Streptococcus and S. aureus are the main culprits for cellulitis, attributable to skin or mucosal colonisation. People with diabetes are more likely to harbour methicillin-resistant S. aureus than methicillin-sensitive S. aureus, partly due to more frequent attendance of healthcare facilities which increases their exposure to more virulent strains.
A less common but serious soft tissue infection which occurs predominantly in people with diabetes is necrotising fasciitis characterised by inflammation and destruction of fascia, fat and muscle with mixed bacterial involvement. Up to 60% of cases of Fournier gangrene, a form of necrotising fasciitis affecting the perineum, had comorbid diabetes [85]. Given the high mortality of this condition, clinicians need a high index of suspicion to ensure prompt diagnosis and treatment, including broad-spectrum antibiotics and surgical debridement of the affected tissues.
Diabetes-related foot infection
Infection complicates 50% of diabetes-related foot ulcers and the risk of infection is increased with recurrent or chronic ulcers. Diagnosis of diabetes-related foot infection is based on the presence of cardinal signs of inflammation, although in people with peripheral sensory neuropathy and peripheral artery disease, these signs may not be as apparent. Although the incidence rates of diabetes-related foot ulcers and amputation have declined in many countries, the rates remain high in certain subgroups such as young people, those of minority or indigenous ethnicity, those with social deprivation and people with mental health conditions [86, 87].
Principles of treatment
Prevention
Some infection transmission can be reduced through public health measures, such as better personal hygiene, avoidance of crowded conditions and mask wearing, but awareness of and adherence to these preventive measures is low in the general public and in healthcare workers [88–91]. There is no evidence that intensive blood-glucose lowering and weight reduction decreases infection rates in people with diabetes [92].
Some common infections are preventable through vaccination and international guidelines recommend routine vaccination for adults with diabetes (Table 1), although vaccine uptake varies significantly between countries [93]. The evidence on whether diabetes affects immune response to vaccines is unclear and varied depending on the type of vaccine and the age of the population [62]. Vaccination against seasonal influenza is effective both in reducing the likelihood of acquiring the infection and in averting downstream complications. In a large observational cohort of adults with diabetes in Denmark, influenza vaccination was associated with a 15% reduction in risk of all-cause mortality and 16% reduction in risk of cardiovascular mortality [94]. In a population-based study from Canada, influenza vaccination lowered stroke incidence by 31% in people with diabetes vs 17% in those without diabetes, suggesting that vaccination confers similar, if not larger protection against stroke in people with diabetes [95].
Table 1.
Recommended vaccination schedule for people with diabetes
| Vaccine | Age group | Frequency |
|---|---|---|
| COVID-19 | All | Based on local guidelines |
| Hepatitis B | <60 years of age, ≥60 years of age after discussion with healthcare professionals | Two- or three-dose series |
| Human papilloma virus | ≤26 years of age, 27–45 years of age after discussion with healthcare professionals | Three doses over 6 months |
| Influenza | All | Yearly, live attenuated influenza vaccine not recommended |
| Pneumococcal | All |
All children should receive the pneumococcal conjugate vaccine as part of childhood immunisation programme All children and adults with diabetes should receive one dose of pneumococcal conjugate vaccine and one dose of 23-valent pneumococcal polysaccharide vaccine, at least 1 year apart The type of pneumococcal conjugate vaccine and the schedule should be based on local guidelines |
| Tetanus, diphtheria and acellular pertussis | All | One dose followed by booster every 10 years |
| Zoster | ≥50 years | Two doses, 2–6 months apart |
In a study in Israel assessing early antibody response to the BNT162b2 COVID-19 vaccine, adults with diabetes had lower IgG concentration than counterparts without diabetes at 5 weeks after receiving the first vaccine dose [96]. In another study comparing humoral and cell-mediated immunogenicity to a COVID-19 vaccine according to attained HbA1c, those with HbA1c levels of 53 mmol/mol (7.0%) or higher at the time of vaccination had a weaker immune response than those with lower HbA1c levels [97]. Furthermore, improvement in glycaemic levels was associated with an increase in both neutralising antibody titres and CD4+ T cell cytokines. These observations indicate that optimising glycaemic management could be important in maximising the protective effect of COVID-19 vaccine in people with diabetes. In both RCTs and real-world observational studies, COVID-19 vaccine efficacy against SARS-CoV-2 infection and related complications appeared similar between individuals with and without diabetes [98]. In a study in Hong Kong, three doses of COVID-19 vaccine reduced mortality rates by 95% and hospitalisation and ICU admission by 85% to 95% in people with diabetes, comparable to rates in the general population [99].
Treatment
Treatment of common infections largely follows the same principles as for the general population. Clinical practice guidelines do not generally recommend using a different antimicrobial regimen or setting a lower threshold for initiation of antimicrobial therapy in people with diabetes, although there are some exceptions. For instance, antiviral therapy with nirmatrelvir with ritonavir (Paxlovid) for COVID-19 should be considered in those with diabetes, on top of supportive care. Viral shedding may be more prolonged following influenza infections in people with diabetes, which may influence decisions regarding use of antiviral therapy [100].
Treatment regimens for both drug-susceptible and drug-resistant tuberculosis are the same for people with and without diabetes. However, as diabetes is associated with higher risk of treatment failure, recurrence and emergence of drug-resistant species, extending the treatment period from 6 months to 9 months is recommended, especially when directly observed therapy is not implemented [101, 102]. Close medication supervision and a lower threshold for drug susceptibility testing should be considered for people with diabetes.
For diabetes-related foot infection, successful treatment requires the concerted effort of an interdisciplinary team with expertise in diabetic foot management [103]. Careful assessment of the severity of infection and the presence of complications, such as osteomyelitis, is important to guide clinicians with regard to the antimicrobial regimen and length of treatment, as well as the need for hospitalisation and orthopaedic and vascular interventions. There have been new advances in wound management such as hyperbaric oxygen therapy, topical oxygen therapy and negative pressure wound therapy, but evidence supporting their role in treatment of foot infection is currently weak.
People with diabetes should be monitored closely for clinical deterioration and other complications as they are more likely to harbour multidrug-resistant pathogens and have worse outcomes from infections. To minimise antimicrobial resistance, the principles of antibiotic stewardship should be judiciously observed. For instance, antimicrobial therapy for asymptomatic bacteriuria does not reduce the rate of symptomatic urinary tract infection or pyelonephritis in people with diabetes and is generally not recommended [104]. Likewise, antimicrobial therapy should not be used in people with uninfected foot ulcers as prophylactic antimicrobial therapy does not improve outcome [103].
Comorbidities and polypharmacy are common in people with diabetes and due caution should be exercised to avoid clinically relevant drug–drug interactions and serious drug toxicities. For instance, the antimicrobial dose may need to be down-titrated in kidney impairment, while administration of nephrotoxic drugs may worsen kidney function in those with underlying chronic kidney disease. Certain antimicrobials (e.g., rifampicin) affect the hepatic metabolism of glucose-lowering drugs (such as sulfonylureas), thus altering glucose-lowering efficacy. Exposure to drugs that affect vision (e.g., ethambutol) or peripheral nerves (e.g., isoniazid) or drugs that are ototoxic (such as aminoglycosides) may be particularly problematic in people who are partially sighted due to diabetic retinopathy or in those who have other disabilities affecting daily living.
Hyperglycaemia during hospital admission for sepsis and other infections predicts a longer hospital stay and increased mortality rates. In a landmark study examining the effects of glycaemic management in people admitted to the ICU, among whom one-fifth had sepsis, lowering of blood glucose to 4.5–6.0 mmol/l did not lead to improved outcomes compared with moderate blood glucose targets of ≤10 mmol/l [105]. Indeed, intensive blood-glucose lowering was associated with more hypoglycaemic episodes and slightly increased mortality rates. It is recommended that SGLT-2 inhibitors should be temporarily withheld in critically ill people, including those with serious infection, because of the potential risk of diabetic ketoacidosis. However, an RCT of individuals with at least one cardiometabolic comorbidity admitted with COVID-19 showed that in-hospital initiation of dapagliflozin had no effect on organ dysfunction or death vs placebo, and did not increase the rates of diabetic ketoacidosis or acute kidney injury [106].
Conclusion
This review discusses how infection remains an important cause of morbidity and mortality for people with diabetes. The risks of infection and worse outcomes are higher in people with diabetes. In part, this relates to the effect of hyperglycaemia on the body’s defence mechanisms against infection, but other host-specific and pathogen-specific factors play a role. Infections may worsen glycaemic levels or increase the risk of diabetes. Significant gaps in our knowledge about the relationship between diabetes and infection remain. While trends in incidence of other diabetes complications have been well reported in many regions, the epidemiology of infection is less well described. Most studies do not differentiate between type 1 diabetes and type 2 diabetes; consequently, it is uncertain how infection differs between types of diabetes and whether this has any clinical implications for prevention of infection and responses to antimicrobial therapy. The variety in pathogens and sites of infection further complicate research in this area. Lack of consistency in methods used to define infective episodes in epidemiological studies is a challenge. It is unclear whether people with diabetes have reduced immune responses to vaccine and, if so, what factors may modify vaccine effectiveness (e.g., BMI or glycaemia). Major advances in diabetes treatments and technologies have occurred, but it is unknown whether the improved diabetes care translates to reduction in risk of serious infection. The effects of new anti-diabetes drugs on infection risk should be included as study endpoints in future RCTs of glucose-lowering therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- HCV
Hepatitis C virus
- ICU
Intensive care unit
- MERS-CoV
Middle East respiratory syndrome-related coronavirus
- ROS
Reactive oxygen species
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SGLT-2
Sodium–glucose cotransporter 2
- Th
T helper
Authors’ relationships and activities
RIGH has received honoraria for speaker engagement and conference attendance from Eli Lilly, Novo Nordisk and Rovi. RCWM has received research grants from AstraZeneca, Bayer, Novo Nordisk, Pfizer, Roche Diagnostics (HK) Ltd and Tricida Inc., and consultancy/speaker honoraria from AstraZeneca, Boehringer Ingelheim, Bayer and Merck. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. RCWM is a co-founder of GemVCare, a technology start-up initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU). RCWM is a member of the Editorial Board of Diabetologia. AOYL has served as a member of advisory panels for Amgen, AstraZeneca, Boehringer Ingelheim and Sanofi, and has received research support from Amgen, the Asia Diabetes Foundation, Bayer, Biogen, Boehringer Ingelheim, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Sugardown Ltd. and Takeda. None of these relationships or activities are related to the content of the current article. CSC declares that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors were responsible for drafting the article and reviewing it critically for important intellectual content. All authors approved the final version to be published.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McLaughlin CW, Jr, Wiedman JG. Infection and gangrene in the diabetic extremity. A critical review of surgical management over a seventeen-year period. Nebr State Med J. 1950;35(10):316–319. [PubMed] [Google Scholar]
- 2.Nichols GP. Diabetes among young tuberculous patients; a review of the association of the two diseases. Am Rev Tuberc. 1957;76(6):1016–1030. doi: 10.1164/artpd.1957.76.6.1016. [DOI] [PubMed] [Google Scholar]
- 3.Dunachie S, Chamnan P. The double burden of diabetes and global infection in low and middle-income countries. Trans R Soc Trop Med Hyg. 2019;113(2):56–64. doi: 10.1093/trstmh/try124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luk AOY, Wu H, Lau ESH, et al. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: a retrospective study. Diabetologia. 2021;64(1):109–118. doi: 10.1007/s00125-020-05286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50(3):549–554. doi: 10.1007/s00125-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 6.Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- 7.Harding JL, Benoit SR, Gregg EW, Pavkov ME, Perreault L. Trends in rates of infections requiring hospitalization among adults with versus without diabetes in the U.S., 2000–2015. Diabetes Care. 2020;43(1):106–116. doi: 10.2337/dc19-0653. [DOI] [PubMed] [Google Scholar]
- 8.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 9.Khunti K, Valabhji J, Misra S. Diabetes and the COVID-19 pandemic. Diabetologia. 2023;66(2):255–266. doi: 10.1007/s00125-022-05833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin Grandis J, Branstetter BF, 4th, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis. 2004;4(1):34–39. doi: 10.1016/s1473-3099(03)00858-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159(12):1301–1309. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 12.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 13.Karacal H, Kymes SM, Apte RS. Retrospective analysis of etiopathogenesis of all cases of endophthalmitis at a large tertiary referral center. Int Ophthalmol. 2007;27(4):251–259. doi: 10.1007/s10792-007-9068-3. [DOI] [PubMed] [Google Scholar]
- 14.Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus–why the beta cells? Nat Rev Endocrinol. 2016;12(5):263–273. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14(6):2211–2217. doi: 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller JA, Gross R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 17.Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes care. 2020;43(11):e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 18.Giorda CB, Gnavi R, Tartaglino B, et al. Increased incidence of type 1 diabetes in 2 years of COVID-19 pandemic. Acta Diabetol. 2023;60(4):587–589. doi: 10.1007/s00592-022-01986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knip M, Parviainen A, Turtinen M, et al. SARS-CoV-2 and type 1 diabetes in children in Finland: an observational study. Lancet Diabetes Endocrinol. 2023;11(4):251–260. doi: 10.1016/S2213-8587(23)00041-4. [DOI] [PubMed] [Google Scholar]
- 20.Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years - United States, March 1, 2020–June 28, 2021. MMWR. 2022;71(2):59–65. doi: 10.15585/mmwr.mm7102e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlad A, Serban V, Timar R, et al. Increased incidence of type 1 diabetes during the COVID-19 pandemic in Romanian children. Medicina. 2021;57(9):973. doi: 10.3390/medicina57090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farakla I, Lagousi T, Miligkos M, et al. Stress hyperglycemia, diabetes mellitus and COVID-19 infection: the impact on newly diagnosed type 1 diabetes. Front Clin Diabetes Healthc. 2022;3:818945. doi: 10.3389/fcdhc.2022.818945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noorzae R, Junker TG, Hviid AP, Wohlfahrt J, Olsen SF. Risk of type 1 diabetes in children is not increased after SARS-CoV-2 infection: a nationwide prospective study in Denmark. Diabetes Care. 2023;46(6):1261–1264. doi: 10.2337/dc22-2351. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda F, Itonaga T, Maeda M, Ihara K. Long-term trends of pediatric type 1 diabetes incidence in Japan before and after the COVID-19 pandemic. Sci Rep. 2023;13(1):5803. doi: 10.1038/s41598-023-33037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeigue PM, McGurnaghan S, Blackbourn L, et al. Relation of incident type 1 diabetes to recent COVID-19 infection: cohort study using e-Health record linkage in Scotland. Diabetes Care. 2022;46(5):921–928. doi: 10.2337/dc22-0385. [DOI] [PubMed] [Google Scholar]
- 26.Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022;65(6):949–954. doi: 10.1007/s00125-022-05670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holman N, Barron E, Young B, et al. Comparative incidence of diabetes following hospital admission for COVID-19 and pneumonia: a cohort study. Diabetes Care. 2023;46(5):938–943. doi: 10.2337/dc22-0670. [DOI] [PubMed] [Google Scholar]
- 29.Vedantam D, Poman DS, Motwani L, Asif N, Patel A, Anne KK. Stress-induced hyperglycemia: consequences and management. Cureus. 2022;14(7):e26714. doi: 10.7759/cureus.26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecube A, Hernandez C, Genesca J, Simo R. Glucose abnormalities in patients with hepatitis C virus infection: epidemiology and pathogenesis. Diabetes Care. 2006;29(5):1140–1149. doi: 10.2337/diacare.2951140. [DOI] [PubMed] [Google Scholar]
- 31.Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: systematic review and meta-analysis of the literature. Rev Endocr Metab Disord. 2018;19(4):405–420. doi: 10.1007/s11154-017-9440-1. [DOI] [PubMed] [Google Scholar]
- 32.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–1229. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology. 2018;29(3):431–441. doi: 10.1097/EDE.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 34.Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40(9):1173–1180. doi: 10.2337/dc17-0485. [DOI] [PubMed] [Google Scholar]
- 35.Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125. doi: 10.1007/s11892-018-1076-3. [DOI] [PubMed] [Google Scholar]
- 36.Lin SY, Lin CL, Liu JH, et al. Association between periodontitis needing surgical treatment and subsequent diabetes risk: a population-based cohort study. J Periodontol. 2014;85(6):779–786. doi: 10.1902/jop.2013.130357. [DOI] [PubMed] [Google Scholar]
- 37.Borgnakke WS. IDF Diabetes Atlas: diabetes and oral health - a two-way relationship of clinical importance. Diabetes Res Clin Pract. 2019;157:107839. doi: 10.1016/j.diabres.2019.107839. [DOI] [PubMed] [Google Scholar]
- 38.Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the Study of Health in Pomerania (SHIP) Diabetes Care. 2010;33(5):1037–1043. doi: 10.2337/dc09-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes: an update of the EFP-AAP review. J Clin Periodontol. 2018;45(2):167–187. doi: 10.1111/jcpe.12837. [DOI] [PubMed] [Google Scholar]
- 40.Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig. 2013;4(5):502–509. doi: 10.1111/jdi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. 2013;84(7):958–973. doi: 10.1902/jop.2012.120377. [DOI] [PubMed] [Google Scholar]
- 42.Artese HPC, Foz AM, de Sousa Rabelo M, et al. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: a meta-analysis. PLoS One. 2015;10(5):e0128344. doi: 10.1371/journal.pone.0128344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carey IM, Critchley JA, Chaudhry UAR, et al. Evaluating ethnic variations in the risk of infections in people with prediabetes and type 2 diabetes: a matched cohort study. Diabetes Care. 2023;46(6):1209–1217. doi: 10.2337/dc22-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang M, Ishigami J, Echouffo-Tcheugui JB, Lutsey PL, Pankow JS, Selvin E. Diabetes and the risk of hospitalisation for infection: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2021;64(11):2458–2465. doi: 10.1007/s00125-021-05522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Wu H, Fan B, et al. The role of age on the risk relationship between prediabetes and major morbidities and mortality: analysis of the Hong Kong diabetes surveillance database of 2 million Chinese adults. Lancet Reg Health West Pac. 2023;30:100599. doi: 10.1016/j.lanwpc.2022.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magliano DJ, Harding JL, Cohen K, Huxley RR, Davis WA, Shaw JE. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care. 2015;38(7):1274–1280. doi: 10.2337/dc14-2820. [DOI] [PubMed] [Google Scholar]
- 48.Barbagelata E, Cilloniz C, Dominedo C, Torres A, Nicolini A, Solidoro P. Gender differences in community-acquired pneumonia. Minerva Med. 2020;111(2):153–165. doi: 10.23736/S0026-4806.20.06448-4. [DOI] [PubMed] [Google Scholar]
- 49.Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 50.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26. doi: 10.1038/nrneph.2015.175. [DOI] [PubMed] [Google Scholar]
- 51.Hair PS, Echague CG, Rohn RD, Krishna NK, Nyalwidhe JO, Cunnion KM. Hyperglycemic conditions inhibit C3-mediated immunologic control of Staphylococcus aureus. J Transl Med. 2012;10:35. doi: 10.1186/1479-5876-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dowey R, Iqbal A, Heller SR, Sabroe I, Prince LR. A bittersweet response to infection in diabetes; targeting neutrophils to modify inflammation and improve host immunity. Front Immunol. 2021;12:678771. doi: 10.3389/fimmu.2021.678771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodacki M, Svoren B, Butty V, et al. Altered natural killer cells in type 1 diabetic patients. Diabetes. 2007;56(1):177–185. doi: 10.2337/db06-0493. [DOI] [PubMed] [Google Scholar]
- 54.Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J Immunol Res. 2019;2019:6196532. doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eckold C, Kumar V, Weiner J, et al. Impact of intermediate hyperglycemia and diabetes on immune dysfunction in tuberculosis. Clin Infect Dis. 2021;72(1):69–78. doi: 10.1093/cid/ciaa751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vangipurapu J, Fernandes Silva L, Kuulasmaa T, Smith U, Laakso M. Microbiota-related metabolites and the risk of type 2 diabetes. Diabetes Care. 2020;43(6):1319–1325. doi: 10.2337/dc19-2533. [DOI] [PubMed] [Google Scholar]
- 57.Anhe FF, Barra NG, Schertzer JD. Glucose alters the symbiotic relationships between gut microbiota and host physiology. Am J Physiol Endocrinol Metab. 2020;318(2):E111–E116. doi: 10.1152/ajpendo.00485.2019. [DOI] [PubMed] [Google Scholar]
- 58.Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–989. doi: 10.1136/thoraxjnl-2015-206780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luk AOY, Lau ESH, Cheung KKT, et al. Glycaemia control and the risk of hospitalisation for infection in patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2017;33(8):e2923. doi: 10.1002/dmrr.2923. [DOI] [PubMed] [Google Scholar]
- 60.Lipsky BA, Pecoraro RE, Chen MS, Koepsell TD. Factors affecting staphylococcal colonization among NIDDM outpatients. Diabetes Care. 1987;10(4):483–486. doi: 10.2337/diacare.10.4.483. [DOI] [PubMed] [Google Scholar]
- 61.Chen KY, Hsueh PR, Liaw YS, Yang PC, Luh KT. A 10-year experience with bacteriology of acute thoracic empyema: emphasis on Klebsiella pneumoniae in patients with diabetes mellitus. Chest. 2000;117(6):1685–1689. doi: 10.1378/chest.117.6.1685. [DOI] [PubMed] [Google Scholar]
- 62.Verstraeten T, Fletcher MA, Suaya JA, et al. Diabetes mellitus as a vaccine-effect modifier: a review. Expert Rev Vaccines. 2020;19(5):445–453. doi: 10.1080/14760584.2020.1760098. [DOI] [PubMed] [Google Scholar]
- 63.Lina B, Georges A, Burtseva E, et al. Complicated hospitalization due to influenza: results from the Global Hospital Influenza Network for the 2017–2018 season. BMC Infect Dis. 2020;20(1):465. doi: 10.1186/s12879-020-05167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380(2):171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 65.Christiansen CF, Heide-Jorgensen U, Rasmussen TB, et al. Renin-angiotensin system blockers and adverse outcomes of influenza and pneumonia: a Danish cohort study. J Am Heart Assoc. 2020;9(19):e017297. doi: 10.1161/JAHA.120.017297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang A, Shi M, Wu H, et al. Time-varying risk associations of renin angiotensin system inhibitors with pneumonia and related deaths in a cohort of 252,616 patients with diabetes (2002–2019) Diabetes Res Clin Pract. 2022;185:109233. doi: 10.1016/j.diabres.2022.109233. [DOI] [PubMed] [Google Scholar]
- 67.Das A, Stroud S, Mehta A, Rangasamy S. New treatments for diabetic retinopathy. Diabetes ObesMetab. 2015;17(3):219–230. doi: 10.1111/dom.12384. [DOI] [PubMed] [Google Scholar]
- 68.Yang A, Shi M, Wu H, et al. Long-term metformin use and risk of pneumonia and related death in type 2 diabetes: a registry-based cohort study. Diabetologia. 2021;64(8):1760–1765. doi: 10.1007/s00125-021-05452-0. [DOI] [PubMed] [Google Scholar]
- 69.Mohammed T, Bowe M, Plant A, Perez M, Alvarez CA, Mortensen EM. Metformin use is associated with lower mortality in veterans with diabetes hospitalized with pneumonia. Clin Infect Dis. 2023;76(7):1237–1244. doi: 10.1093/cid/ciac900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre MJ. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. 2012;66(6):519–523. doi: 10.1136/jech.2010.114595. [DOI] [PubMed] [Google Scholar]
- 71.Remais JV, Zeng G, Li G, Tian L, Engelgau MM. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int J Epidemiol. 2013;42(1):221–227. doi: 10.1093/ije/dys135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevenson CR, Forouhi NG, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pizzol D, Di Gennaro F, Chhaganlal KD, et al. Tuberculosis and diabetes: current state and future perspectives. Trop Med Int Health. 2016;21(6):694–702. doi: 10.1111/tmi.12704. [DOI] [PubMed] [Google Scholar]
- 76.Magee MJ, Khakharia A, Gandhi NR, et al. Increased risk of incident diabetes among individuals with latent tuberculosis infection. Diabetes Care. 2022;45(4):880–887. doi: 10.2337/dc21-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Y, Harries AD, Kumar AMV, et al. Tackling diabetes mellitus and tuberculosis: a new Union guide on the management of diabetes-tuberculosis. Int J Tuberc Lung Dis. 2019;23(7):771–772. doi: 10.5588/ijtld.19.0119. [DOI] [PubMed] [Google Scholar]
- 78.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 79.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartmann-Boyce J, Rees K, Perring JC, et al. Risks of and from SARS-CoV-2 infection and COVID-19 in people with diabetes: a systematic review of reviews. Diabetes Care. 2021;44(12):2790–2811. doi: 10.2337/dc21-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li R, Shen M, Yang Q, et al. Global diabetes prevalence in COVID-19 patients and contribution to COVID-19–related severity and mortality: a systematic review and meta-analysis. Diabetes Care. 2023;46(4):890–897. doi: 10.2337/dc22-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stapleton A. Urinary tract infections in patients with diabetes. Am J Med. 2002;113(Suppl 1A):80S–84S. doi: 10.1016/s0002-9343(02)01062-8. [DOI] [PubMed] [Google Scholar]
- 83.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 84.Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith GL, Bunker CB, Dinneen MD. Fournier's gangrene. Br J Urol. 1998;81(3):347–355. doi: 10.1046/j.1464-410x.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 86.Chamberlain RC, Fleetwood K, Wild SH, et al. Foot ulcer and risk of lower limb amputation or death in people with diabetes: a national population-based retrospective cohort study. Diabetes Care. 2022;45(1):83–91. doi: 10.2337/dc21-1596. [DOI] [PubMed] [Google Scholar]
- 87.Riley J, Antza C, Kempegowda P, et al. Social deprivation and incident diabetes-related foot disease in patients with type 2 diabetes: a population-based cohort study. Diabetes Care. 2021;44(3):731–739. doi: 10.2337/dc20-1027. [DOI] [PubMed] [Google Scholar]
- 88.Jefferson T, Dooley L, Ferroni E et al (2023) Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev Issue 1, Art. no.: CD006207. 10.1002/14651858.CD006207.pub6 [DOI] [PMC free article] [PubMed]
- 89.Mouajou V, Adams K, DeLisle G, Quach C. Hand hygiene compliance in the prevention of hospital-acquired infections: a systematic review. J Hosp Infect. 2022;119:33–48. doi: 10.1016/j.jhin.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 90.Houghton C, Meskell P, Delaney H et al (2020) Barriers and facilitators to healthcare workers' adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev Issue 4, Art no.: CD013582. 10.1002/14651858.CD013582 [DOI] [PMC free article] [PubMed]
- 91.Alhumaid S, Al Mutair A, Al Alawi Z, et al. Knowledge of infection prevention and control among healthcare workers and factors influencing compliance: a systematic review. Antimicrob Resist Infect Control. 2021;10(1):86. doi: 10.1186/s13756-021-00957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41(10):2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 93.ElSayed NA, Aleppo G, Aroda VR, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes—2023. Diabetes Care. 2023;46(Suppl 1):S49–S67. doi: 10.2337/dc23-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Modin D, Claggett B, Kober L, et al. Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes: a nationwide cohort study. Diabetes Care. 2020;43(9):2226–2233. doi: 10.2337/dc20-0229. [DOI] [PubMed] [Google Scholar]
- 95.Holodinsky JK, Zerna C, Malo S, Svenson LW, Hill MD. Association between influenza vaccination and risk of stroke in Alberta, Canada: a population-based study. Lancet Public Health. 2022;7(11):e914–e922. doi: 10.1016/S2468-2667(22)00222-5. [DOI] [PubMed] [Google Scholar]
- 96.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marfella R, D'Onofrio N, Sardu C, et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: the CAVEAT study. Diabetes Obes Metab. 2022;24(1):160–165. doi: 10.1111/dom.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moreira ED, Jr, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386(20):1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan EYF, Mok AHY, Yan VKC, et al. Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA.2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: a case control study. J Infect. 2022;85(5):e140–e144. doi: 10.1016/j.jinf.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Q, Li W, Xue M, et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: a meta-analysis. Sci Rep. 2017;7(1):1090. doi: 10.1038/s41598-017-01213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang JY, Lee MC, Shu CC, et al. Optimal duration of anti-TB treatment in patients with diabetes: nine or six months? Chest. 2015;147(2):520–528. doi: 10.1378/chest.14-0918. [DOI] [PubMed] [Google Scholar]
- 103.Lipsky BA, Senneville E, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(Suppl 1):e3280. doi: 10.1002/dmrr.3280. [DOI] [PubMed] [Google Scholar]
- 104.Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611–1615. doi: 10.1093/cid/ciz021. [DOI] [PubMed] [Google Scholar]
- 105.The NICE-SUGAR Study Investigatorsl Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 106.Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(9):586–594. doi: 10.1016/S2213-8587(21)00180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.UK Health Security Agency (2023) Immunisation against infectious disease. Available from: www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book. Accessed: 15 January 2024
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.