Abstract
PTH and Vitamin D are two major regulators of mineral metabolism. They play critical roles in the maintenance of calcium and phosphate homeostasis as well as the development and maintenance of bone health. PTH and Vitamin D form a tightly controlled feedback cycle, PTH being a major stimulator of vitamin D synthesis in the kidney while vitamin D exerts negative feedback on PTH secretion. The major function of PTH and major physiologic regulator is circulating ionized calcium. The effects of PTH on gut, kidney, and bone serve to maintain serum calcium within a tight range. PTH has a reciprocal effect on phosphate metabolism. In contrast, vitamin D has a stimulatory effect on both calcium and phosphate homeostasis, playing a key role in providing adequate mineral for normal bone formation. Both hormones act in concert with the more recently discovered FGF23 and klotho, hormones involved predominantly in phosphate metabolism, which also participate in this closely knit feedback circuit. Of great interest are recent studies demonstrating effects of both PTH and vitamin D on the cardiovascular system. Hyperparathyroidism and vitamin D deficiency have been implicated in a variety of cardiovascular disorders including hypertension, atherosclerosis, vascular calcification, and kidney failure. Both hormones have direct effects on the endothelium, heart, and other vascular structures. How these effects of PTH and vitamin D interface with the regulation of bone formation are the subject of intense investigation.
Parathyroid Hormone
Introduction
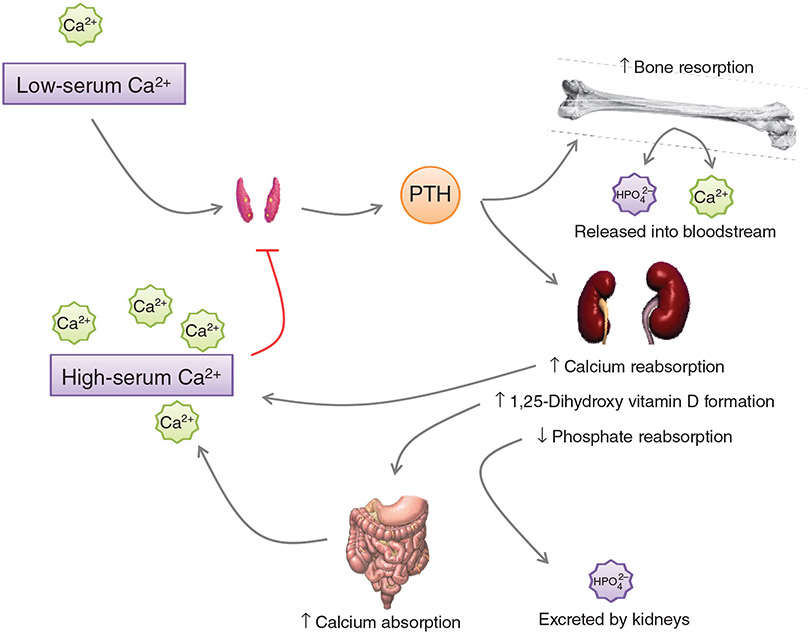
Parathyroid hormone (PTH), a product of the parathyroid glands, embedded in the thyroid (in rodents) or located behind the thyroid (in humans), is a key regulator of calcium and phosphorus homeostasis through its effects on bone, kidney and intestine, and by regulating 1α, 25-dihydroxyvitamin D (372). The serum concentration of PTH is derived both from the release of PTH stored in secretory granules and from de novo synthesis of PTH in response to alterations in the serum levels of calcium, phosphorus, and vitamin D (277). Acute regulation of PTH is accomplished by the release of stored PTH in response to ambient calcium level through the calcium sensing receptor expressed on the chief cells of the parathyroid glands while long-term synthesis and release is dependent upon de novo synthesis through transcription and translation of mRNA encoding pre-pro-PTH (520, 521). PTH restores serum calcium by three different mechanisms: (i) release of calcium and phosphorus from the bones through stimulation of osteoclastic activity; (ii) decrease in calcium excretion and a concomitant decrease in phosphate reabsorption in the kidney; and (iii) increase dietary absorption of calcium and phosphorus in the gut (271) (Fig. 1).
Figure 1.
Regulation of serum Ca2+ by PTH. Low serum Ca2+ stimulates the release of PTH from the parathyroid gland. PTH acts on the bone to increase bone resorption, releasing Ca2+ and HPO42− into the bloodstream. At the kidney, PTH increases Ca2+ reabsorption and decreases HPO42− reabsorption, maintaining the elevated serum Ca2+ from the resorption of bone. Vitamin D becomes activated in the kidney by 1α-hydroxylase, leading to increased Ca2+ absorption from the gut. The restored serum Ca2+ provides a negative feedback signal to the parathyroid glands, discontinuing the release of PTH.
Substantial advances made in the late 1970s and early 1980s to understand the biochemical and cellular regulation of PTH metabolism and mechanisms of action (278-282) led to the development of assays for the detection of PTH in the blood of humans and animals. These efforts uncovered the presence of multiple forms of immunoreactive PTH molecules in circulation adding a previously unappreciated complexity to PTH metabolism. Evidence suggests that the hormone is subjected to proteolysis both in the parathyroid gland and in end organs including liver and kidney. This review will focus on the structure, synthesis, secretion, and functions of the hormone and consider the pathophysiological, pharmacological, and treatment of abnormalities of biosynthesis and secretion of PTH.
History
Although evidence of pathologies now associated with the parathyroid glands can be documented as far back as ancient Egypt (164, 332), the existence of the parathyroid glands was not discovered until the second half of the nineteenth century, and their function not definitively explored until well into the twentieth century. The parathyroid glands went through numerous cycles of naming and discovery, and took several decades to gain traction in the scientific field. It was during the necropsy of a Great Indian Rhinoceros in 1852 that Sir Richard Owen of the Royal College of Surgeons first described the parathyroid glands (219, 497). Remak (544) and Wirchow (690) found the parathyroid glands in humans. Although it had been well established by the beginning of the twentieth century that removal of the glands from humans and animals caused death from tetany, intense debate still existed as to the function of the glands. It was even suggested that the parathyroid glands serve a detoxification purpose, much akin to the liver (409-411). At the turn of the twentieth century, the German pathologist Erdheim determined that patients undergoing thyroid surgery who developed tetany had undergone simultaneous accidental removal of the parathyroid glands (201, 655). In 1915, Schlagenhaufer, a Viennese physician, was the first to draw the conclusion that the osteitis fibrosa cystica observed in his patient was in fact due to an enlarged parathyroid, and not the other way around (332). While previous researchers had implicated a role of the parathyroid in calcium homeostasis, injection of parathyroid extract into animals with tetany failed to relieve the symptoms; thus, the precise relationship between parathyroid glands and calcium homeostasis remained unclear. It was not until Collip developed a method for extracting biologically active parathyroid hormone that the role of the parathyroid glands in calcium homeostasis began to emerge. Collip used a hot acid extraction method to purify potent parathyroid extracts that when injected back into parathyroidectomized animals restored muscle excitability to normal levels (160-162, 522, 524). Although effective, the hot acid extraction was also harsh, and yielded fragmented portions of parathyroid hormone, which frustrated efforts to sequence the polypeptide in the 1950s. In 1954, Handler et al. (284) summarized the frustrations of several scientists in a report by stating that “(i) the active material in the gland may be large protein which in the course of isolation is degraded into fractions of varying size, each of which still has activity; (ii) the active molecule may not be a large molecule at all, but instead a small molecule which adheres to each one of these fractions.” In 1959, Aurbach (35) and Rasmussen and Craig (534) independently isolated the polypeptide and individual fragments without degradation using organic solvent extraction methods. Using standard protein sequencing techniques, they were able to determine the structure of bovine and human PTH. In the 1970s with the development of molecular techniques, it became possible to determine the mechanisms for hormone synthesis, processing, and metabolism.
PTH gene regulation
Several proteins play a critical role in parathyroid gland development. These include glial cells missing (GCM), eyes absent (Eya1), and Hoxa3/Pax1 compound genes. However, there is very limited information available identifying activating and repressing factors that control the transcription of the PTH gene. Early studies suggested that extracellular calcium inhibited PTH gene transcription through a conserved negative calcium-response element (371, 487-489). More recent studies have elucidated an additional role for calcium in regulation of PTH through posttranscriptional repression. Rats that were fed low calcium diets were found to have increased levels of PTH mRNA, whereas rats that were fed low phosphate diets had decreased PTH mRNA expression (457). Low serum calcium and high serum phosphorus are signals that both increase PTH secretion by increasing PTH gene expression posttranscriptionally (60, 61). Additionally, 1,25-dihydroxy-vitamin D decreases PTH expression by decreasing PTH mRNA (471, 472). Vitamin D deficiency increases the PTH mRNA expression through two processes, (i) impaired calcium absorption leading to decreased extracellular calcium and (ii) removal of a known repressor of PTH gene transcription (369). Initial experiments determined that specific sequences in the 3′ UTR of PTH mRNA determine its rate of degradation (646). Calcium and phosphate were identified as regulating PTH posttranscriptionally, through alteration in the interaction of RNA binding protein with the 3′-UTR of the PTH transcript. Two of these proteins are AU-rich element binding factor 1 (AUF1) and KSRP (372). AUF1, an RNA-binding protein, enhances the PTH transcript stability by binding to the 3′UTR region in response to phosphate and calcium concentrations in the serum (61). KSRP destabilizes the PTH transcript through KSRP’s interactions with the 3’-UTR of PTH mRNA (239). Other proteins involved in PTH gene expression include hepatocyte nuclear factor 1β (HNF1β), which binds to the PTH promoter and acts as a translational repressor, as patients with a mutated HNF1β display hyperparathyroidism (223).
Several consensus sequences have been identified in PTH promoter region that regulate its gene expression. A cyclic AMP response element was identified at the transcription start site of the human, bovine, and murine PTH genes. A unique DNA repressor element that binds to the vitamin D receptor has also been identified in the human PTH gene promoter. Alimov et al. (19, 20) identified a highly conserved Sp1 element and a Sp3 element in the human and bovine PTH promoter. Sp1 strongly stimulates the transcription of wild-type bovine and human PTH promoters. The role of Sp3 promoter is not known.
Structure and biochemical properties of PTH
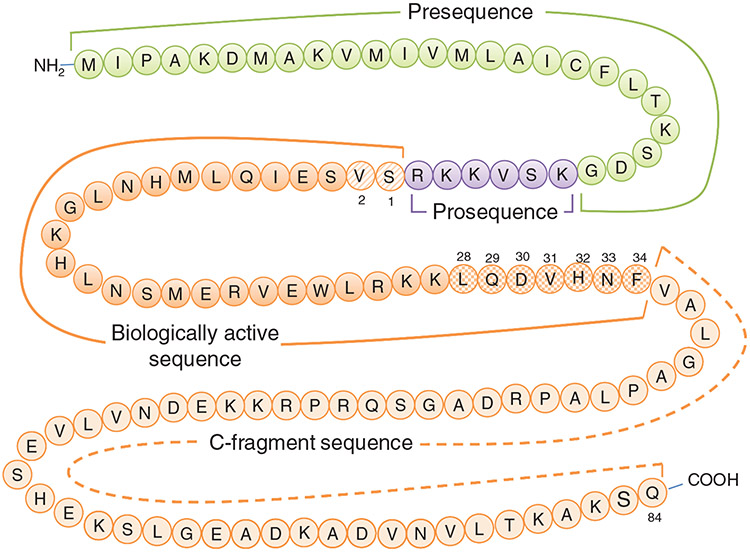
The biosynthetic pathways involved in the synthesis, cellular transport, and metabolism of PTH has been extensively studied. These studies demonstrated that the mRNA encoding the PTH is translated as a 115 amino acid pre-pro-PTH on the rough endoplasmic reticulum (277, 343-346) (Fig. 2). The first two methionines are cleaved during translation by a methionyl amino peptidase releasing the signal peptide such that the protein is directed to a membrane vesicle. During the transit to the Golgi, the N-terminal signal sequence of 23 amino acids is cleaved at the glycyl-lysyl bond to form an intermediate protein of 90 amino acids called the pro-PTH, which is an inactive precursor. The N-terminal six amino acids of the pro-PTH are proteolytically cleaved in the Golgi yielding the mature 84 amino acid PTH which is stored in granules to be released into the circulation by exocytosis after appropriate stimulus (282, 371, 598). Analysis of the region-specific radioimmunoassays demonstrated that pro-PTH constitutes only 7% of the total PTH in normal parathyroid glands and the rest is mostly mature PTH (283). The protein is further cleaved into smaller fragments by cathepsin-B in the parathyroid glands (227, 412, 413). The hormone and its fragments are removed from circulation by receptors predominantly in kidney but also in the bone (282).
Figure 2.
Primary sequence of human parathyroid hormone (sequence from Ensembl.org). The pre- and prosequences are cleaved prior to secretion into the bloodstream. Residues 1 and 2 (diagonally striped) are required for PTH receptor activation. Residues 28-34 (checkered pattern) interact with the extracellular amino domain of the PTH receptor. The C-fragment may be cleaved either prior to or after release from the parathyroid gland.
The naturally occurring PTH 1-37 and 1-34 fragments of PTH maintain full activity of the intact PTH 1-84. Osteoporosis patients treated with PTH1-34 have increased bone density suggesting that this fragment of PTH has all the anabolic activities of the intact 1-84 PTH. Mutation analyses of the PTH molecule have identified amino acids critical for receptor signaling. Truncation of the first two amino acids (PTH 3-34) results in a partially active PTH while removal of the first six amino acids (PTH 7-34) results in a low-affinity antagonist. Further studies have demonstrated that the 17 to 34 amino acid residues are critical for high affinity receptor binding (468).
The structure of PTH has been partially defined through X-ray crystallography and NMR spectroscopy techniques (323). The secondary structure of PTH 1-34 differs depending upon whether it is in aqueous solutions, lipid solutions, or in the presence of secondary structure-inducing solvents such as trifluorethanol (47, 91, 357, 478, 505, 614). X-ray crystallography studies have identified critical amino acids associated with specific structural qualities of PTH. PTH 1-34 has a multihelical structure with a bend between amino acid residues 12 and 21 (323). Mutation analysis demonstrated that the helical structure around Gly12 is important for biological activity and binding of the peptide to its receptor (149, 150). NMR studies have shown three helices between Ser3-Asn10, Ser17 to Lys27, and Asp30 to Leu37 in the N-terminal. An additional poorly defined helix between Asn57 and Ser62 was observed in the C-terminal with evidence of interaction between helix 1 and helix 2. The NMR studies also demonstrated a “U” or “V” shaped tertiary structure formed by the interaction between the N- and C-terminal helices which form a hydrophobic core (47, 141, 270, 699).
Regulation of PTH synthesis, secretion, and metabolism
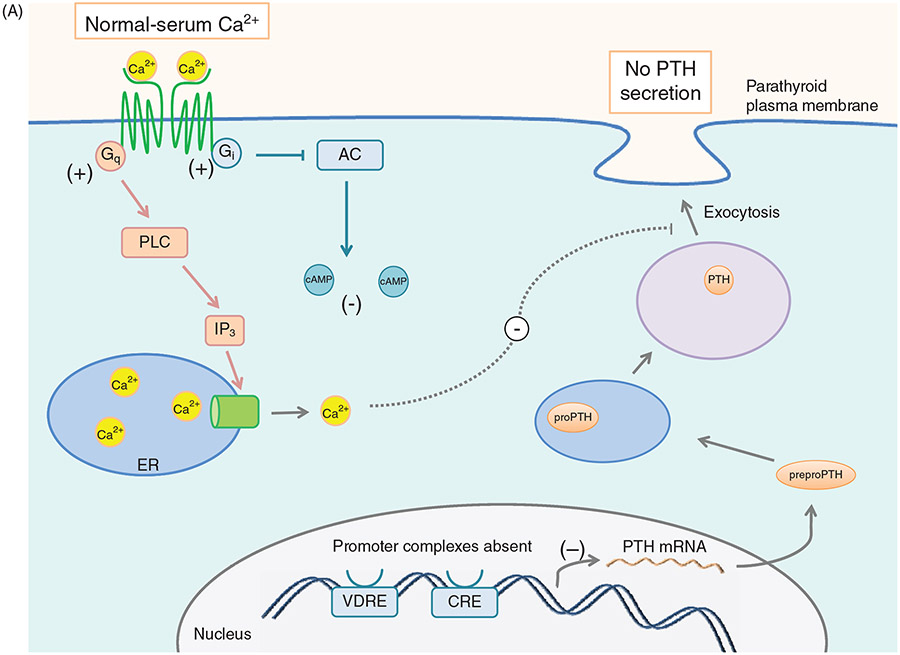
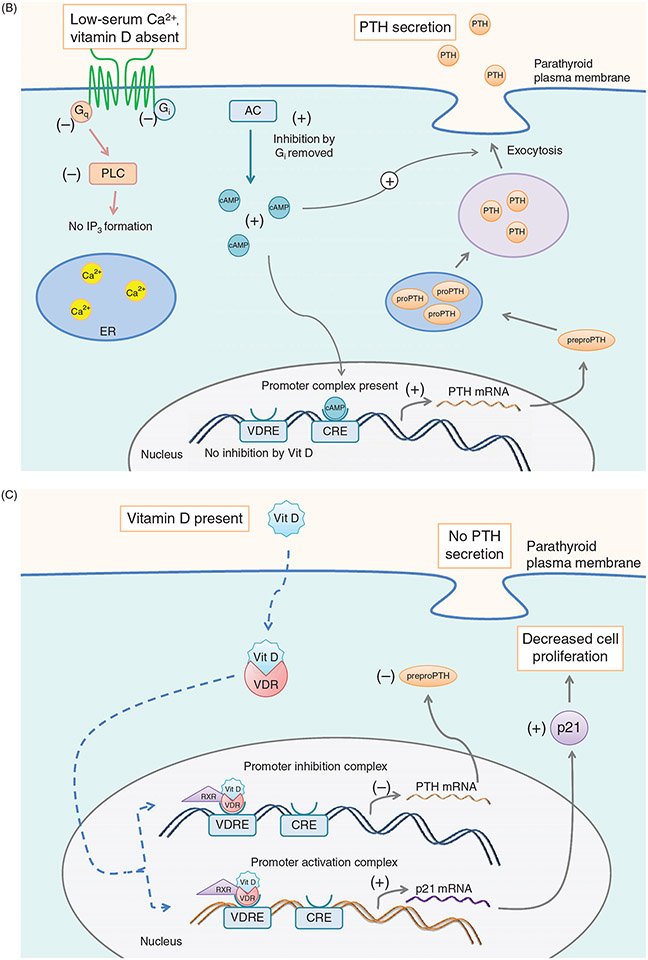
Mature PTH is stored in granules close to the plasma membrane. Electron microscopy of the parathyroid gland demonstrated that the granules containing mature PTH are limited while there are abundant immature vesicles present near the Golgi. These vesicles are transported to the cell surface without incorporation into mature granules. Extracellular ionized calcium concentration is the major physiologic regulator of the synthesis and secretion of PTH (Fig. 3A and B). Decreasing ionized calcium concentration by infusion of the calcium chelator, EGTA, in cows increases the synthesis and secretion of PTH within 20 s of infusion. The initial response to a 0.1 mg/dL decrease in calcium concentration is to release the preformed vesicles from the parathyroid gland (100). Chronic decreases in serum calcium will increase the rate of synthesis and release of PTH (101). Apart from serum calcium, epinephrine, calcitonin, vitamin D, magnesium, and phosphate regulate the synthesis and release of PTH in humans (105, 226). (Fig. 3C) An extracellular calcium receptor (CaSR) acting as a sensor for ionized calcium levels provides the critical link between circulating ionized calcium concentration and PTH secretion, maintaining calcium within a narrow range. High extracellular calcium levels sensed by the CaSR results in decreased PTH secretion and increased Ca++ excretion by the kidney (148, 474). Conversely, lower levels of plasma calcium stimulate PTH secretion and Ca++ reabsorption by the kidney (474-477, 671).
Figure 3.
(A) Effect of serum [Ca2+] on PTH synthesis and secretion. Ca2+ binds to the CaSR and activates Gq and Gi. Gq activation leads to increased [Ca2+]i via PLC signaling and IP3 formation. Increased [Ca2+]i inhibits exocytosis of PTH secretory vesicles. Gi activation inhibits adenylyl cyclase, leading to less cAMP, and therefore less transcription of the PTH gene as well as decreased stimulus for secretory vesicle exocytosis. (B) Effect of low serum [Ca2+] on PTH synthesis and secretion. In the absence of serum Ca2+, CaSR signaling terminates, allowing [cAMP]i to increase, which drives transcription of PTH and secretory vesicle exocytosis, leading to increased serum PTH levels. (C) Effect of Vitamin D on PTH secretion. Vitamin D enters the cell through diffusion across the plasma membrane. Once inside, Vitamin D is bound by the vitamin D receptor (VDR). In the nucleus, the Vit D-VDR complex binds to RXR and the vitamin D response element (VDRE) within the PTH promoter. This promoter complex inhibits transcription of the PTH gene, leading to less preproPTH production. The Vit D-VDR complex also binds to the VDRE within the promoter of p21, activating the transcription of the p21 gene. Increased p21 expression inhibits parathyroid cell proliferation.
Plasma PTH levels exhibit significant fluctuations during the course of the day and about 20% to 30% of its secretion is pulsatile (565). The circadian rhythm of PTH reaches a maximum at late morning, followed by two prominent peaks one in the afternoon and another in early morning (569). However, the maximum PTH secretion occurs at nighttime when the bone resorption activity is highest (202). Both nocturnal increases in PTH secretion and bone resorption can be prevented by administration of calcium in the evening (426). PTH concentration time profiles have revealed a rhythm of secretion consisting of seven secretory pulses per hour, accounting for approximately 30% of spontaneous PTH secretion and regulated by extracellular calcium (572). The mechanisms for the pulsatile secretion of PTH are not very well understood, nor are the functional consequences. It is proposed that the dense autonomic innervation of the parathyroid gland acts as neuronal pacemaker for PTH secretion (123, 208, 613). Acute disruption of sympathetic input by β-adrenergic receptor blockers increases the PTH pulse two fold and increases plasma PTH levels but does not eliminate the pulsatile oscillations (570, 571). It has been suggested that this cyclic release of PTH may be critical for the anabolic effects of bone mass by activating the cyclic AMP pathway while chronically elevated levels of PTH lead to bone destruction through PKC and RGS2-dependent pathways (312, 342). This is based, at least in part, on the observation that daily recombinant PTH injections, which results in exaggerated peaks of serum PTH levels, are an effective treatment for osteoporosis and bone repair, stimulating new bone production in postmenopausal women (75, 209). In contrast, sustained high levels of PTH seen in primary and secondary hyperparathyroidism tend to result in bone destruction. PTH secretion also is dependent upon seasonal fluctuations. It decreases by about 20% in summer and increases by about 20% in winter season (693).
Berson and Yalow demonstrated immunochemical heterogeneity of plasma PTH for the first time and suggested that this may be due to postsecretory modifications of the hormone (70-72). Later studies confirmed the observations of Berson and Yalow and by using RIA demonstrated that the majority of fractions are C-terminal fragments of the hormone (468). Habener et al. demonstrated that direct intravenous injection of intact bovine PTH into calves led to the accumulation of C-terminal fragments generated by peripheral metabolism of the administered hormone (278). In 1973 Canterbury et al. identified N-terminal fragments of PTH that were biologically active (117). This observation was later confirmed by several studies. The N-terminal fragments were found to be more short-lived than the C-terminal fragments. The discovery of biologically active N-terminal fragments led to the hypothesis that peripheral metabolism of secreted PTH is required for biological activity (503). However, this was disproved by the observations of Glotzman et al. who demonstrated that intact PTH could activate adenylate cyclase (260, 507). Fang and Tashjian (215) were the first to demonstrate the contribution of liver in clearance of intact PTH from circulation. This was later confirmed by several studies [reviewed in (468)]. Recent studies demonstrated that the Kupffer cells take up intact PTH, a process that is dependent on amino acids 28-48, and generate C-terminal fragments by proteolysis of the intact hormone (173, 174). Daugaard et al. (183, 184) demonstrated that only C-terminal biologically inactive fragments were generated during liver perfusion. Bringhurst et al. (110-112) demonstrated that the N-terminal fragments are degraded in the Kupffer cells. The demonstration that both intact and fragmented circulating forms of PTH are increased in patients with renal disease (71, 179, 443) and nephrectomized animals (118, 313, 431, 432) suggests that kidneys play a major role in PTH clearance. A portion of PTH is cleared independent of glomerular filtration, through peritubular uptake by binding to PTH1R and involve receptor-mediated endocytosis at the basolateral membrane of the tubule cells (185, 432). Recent studies suggest that megalin/cubulin-dependent endocytosis plays an important role in PTH clearance from urine independent of PTH1R (273, 298).
PTH receptors
Three distinct receptors for PTH viz., PTH1R, PTH2R, and PTH3R have been described in literature. The most common and the classical receptor, the PTH1R, a type II G-protein coupled receptor, is expressed widely, both in the classic PTH target tissues, bone and kidney, as well as many others. PTH1R is activated by both PTH and the PTH-related peptide, a protein that shares the name with PTH but is not derived from the same gene. PTH2R is expressed in very low levels in most tissues, except for the limbic system and the hypothalamus (148). PTH2R is activated by PTH and an unrelated protein, the tuberoinfundibular peptide of 39 amino acids (TIP39). The PTH3R was cloned from zebrafish and is activated 20 times more potently by PTHrP than PTH. The mammalian homologues of PTH3R have not yet been discovered.
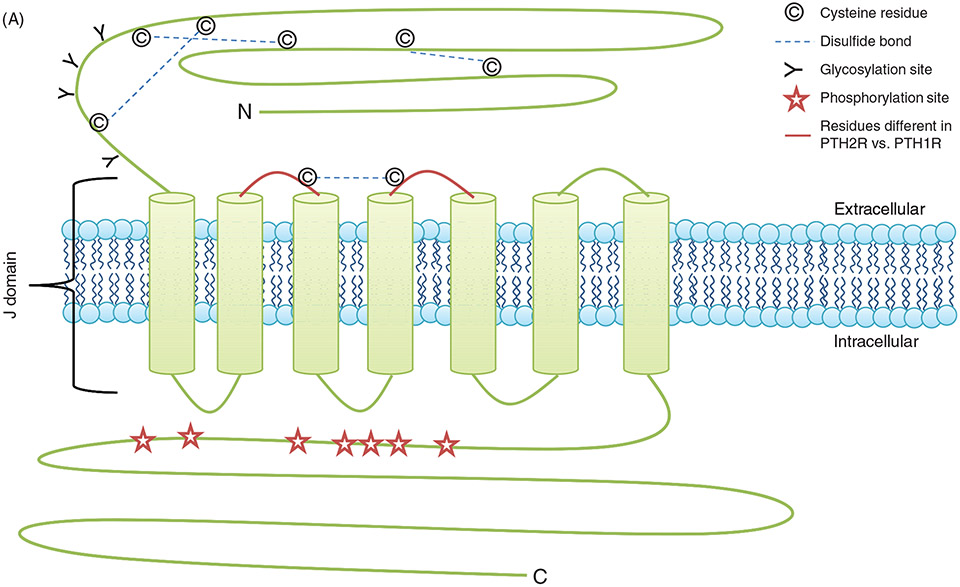
The PTH1R is the best studied PTH receptor. Jüppner et al. in 1991 identified a 585 amino acids protein from COS7 and opossum kidney (OK) cells using 125I-[Tyr36]hPTHrP (1-35)NH2 binding (329). This protein was characterized as type II G-protein-coupled receptor, characterized by the presence of an ~ 150 amino acid N-terminal extracellular domain with four N-glycosylation sites, eight conserved extracellular cysteine residues forming four disulfide bridges, seven transmembrane regions, and a large (150-190 amino acid) intracellular C-terminal tail (148, 362, 578) (Fig. 4A). The PTH1R gene resides on the short (p) arm of chromosome 3 between positions 22 and 21.1 (3p22-p21.1). The PTH1R is encoded by a rather large 22 kb gene that contains 15 exons and 14 introns. The mature transcripts exhibit an extensive poly A tail at the 3′ end (366). Examination of the genomic map of the PTH1R gene has led to the identification of potential splice donor and acceptor sequences (148, 327, 366), and multiple promoters in the 5′ regulatory regions of the mouse, rat, human, and porcine PTH1R resulting in the production of multiple transcripts which show regulation at the developmental and tissue level (327, 441, 442, 595). The mouse, rat, and porcine transcripts have two promoter regions (P1 and P2) while the human transcript has three promoter regions (P1, P2, and P3) (75, 148, 327, 442, 604). They all lack the conventional TATA box homology. However, they contain the GC rich Sp1 motifs and are regulated by ubiquitously expressed transcription factors Sp1, myc-associated zinc-finger protein, and embryonic TEA domain-containing factor (34, 442, 450). The P1 and P3 promoter regions are methylated in a tissue specific manner and the differential methylation patterns are important during development (74). Transcription of the PTH1R is regulated by vitamin D, retinoic acid, and glucocorticoids (23, 335, 643, 701). Vitamin D downregulates the transcription of PTH1R in osteoblasts by inhibiting the activity of P2 promoter while retinoic acid and dexamethasone increases the transcription in mouse embryonal carcinoma P19 cells and ROS 17/2.8 cells respectively but not in OK cells. Glycosylation is an important posttranslational modification of GPCR that regulates the intracellular folding, stabilization, intracellular trafficking, and function (23, 335, 643, 644, 701). The four N-glycosylation sites (N-151, 160, 165, and 175) are located in the putative ligand-binding extracellular amino terminal domain of the PTH1R. Initial studies using inhibition of glycosylation by tunicamycin or by treatment with endoglycosidase F in OK and HEK 293 cells suggested that glycosylation is not required for proper folding, expression, and ligand binding (91, 93, 382, 722). However, more recent data using site-directed mutagenesis of the Asn to Gln showed decreased membrane expression and ligand binding in transiently transfected COS-7 cells suggesting that glycosylation plays an important role in intracellular trafficking, membrane expression, and function of PTH1R (721). Mutations in the six extracellular amino terminal cysteine residues also showed decreased membrane expression and ligand binding of the PTH1R. Two amino acid residues, R233 in the second and Q451 in the seventh transmembrane domain, are highly conserved in type II GPCR, and critical for effective PTH interaction and signaling as mutations in these two sites reduce ligand binding and transmembrane signaling by PTH1R (148, 381) (Fig. 4B).
Figure 4.
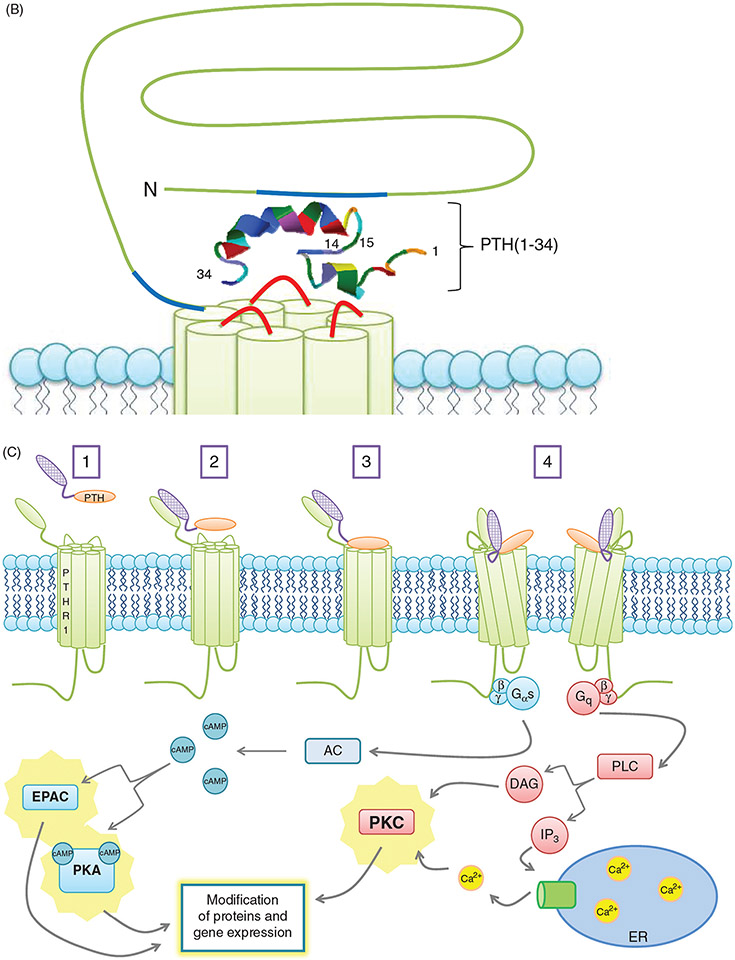
(A) PTH receptor topology and ligand binding. (A)The PTH1R consists of an extracellular amino terminus, a J domain consisting of transmembrane domains as well as intra- and extracellular loops, and an intracellular carboxy terminus. The extracellular amino terminus is 150 residues long, with 4 N-glycosylation sites and 4 disulfide bridges. Less is known regarding PTH2R topology as compared to PTH1R topology. Importantly, residue variations in two of the extracellular loops (highlighted in red) decrease the affinity of the receptor for PTHrP while maintaining specificity for PTH. (B) PTH receptor topology and ligand binding. (B) PTH(1-34) (rainbow structure) interacts with both the extracellular amino terminus of the PTH1R as well as the J domain. Docking of PTH to the PTH1R is thought to occur through initial binding of the C-terminus of PTH (residues 15-34) to the N-terminus (blue regions) of the PTH1R. This interaction is closely followed by the binding of the N-terminus of PTH (residues 1-14) to the J domain (red regions) of the PTH1R, initiating G protein recruitment and intracellular signaling cascade activation. (C) PTH-receptor binding and intracellular signaling. (1) Two-site model of PTH-receptor docking. (2) The N-domain of the PTH receptor (PTHR1) binds the C-domain of PTH. (3) The J-domain binds the amino-terminal region of PTH. (4) Binding of the ligand to the receptor increases the association with the J-domain, while also increasing the affinity of the intracellular beta-gamma binding region of the C-terminal region of the PTH receptor for G proteins, resulting in their subsequent activation and initiation of downstream signaling cascades. Gαs activates adenylate cyclase (AC), which increases intracellular [cAMP], resulting in activation of Epac and PKA. Gq activates phospholipase C (PLC), forming diacylglycerol (DAG) and inositol triphosphate (IP3). DAG directly activates PKC, whereas IP3 indirectly activates PKC by releasing Ca2+ from the ER.
The PTH2R was identified by homology screening based on conserved sequences from calcitonin, secretin, and PTH1R from a cerebral cortex cDNA library. The gene for PTH2R, an 88kb gene with 13 exons and several large introns, has been located on chromosome 2q33. Like the PTH1R, the PTH2R is a class II GPCR and 51% identical to PTH1R. Similar to PTH1R, PTH2R has a large hydrophilic amino-terminal domain containing 120 amino acid residues containing four glycosylation sites and six extracellular cysteine residues, seven transmembrane domains, and a large intracellular C-terminal domain. The PTH2R is highly expressed in brain particularly in the limbic system and parts of hypothalamus. Peripherally, the PTH2R is also expressed in pancreatic islet D cells, parafollicular C cells of the thyroid, cells that produce gastrointestinal peptide, cartilage, and heart muscle cells (148). In contrast to the PTH1R, PTH2R selectively binds to PTH but not to PTHrP. The natural ligand of PTH2R in the CNS is the 39 amino acid tubero-infundibular peptide (TIP-39) in the bovine hypothalamus, described for human, rat, and zebrafish PTH2R. PTH also activates human PTH2R but not the rat and zebrafish PTH2R. TIP-39 and PTH do not share sequence homology. Only five residues of bovine PTH and TIP-39 are similar and align with each other. However, NMR studies have demonstrated that the two peptides are structurally very similar with regard to the orientation of polar and nonpolar amino acid residues in the amino-terminal region (57, 245, 300, 645).
The third and the most recently identified PTH receptor, the PTH3R was cloned using genomic PCR from zebrafish DNA. PTH3R is 69% similar and 61% identical to the PTH1R and shares only 48% similarity with PTH2R. PTH3R is almost exclusively activated by PTHrP and is the least studied of the three PTH receptors. The mammalian ortholog of PTH3R has not yet been identified. Similar to PTH1R and PTH2R, PTH3R has been classified as a class II GPCR but little else about its structure-function relationships is known (557, 702, 703).
Recent evidence suggests the presence of PTH receptors specific for the C-terminal of PTH which may play a role in gluconeogenesis, leukocyte migration, and pancreatic secretions [reviewed in (468)].
PTH-PTH1R interactions
Photo-affinity cross-linking studies of the interactions between a modified PTH 1-34 and its cognate receptor (PTH1R) suggest that the position 13 of the PTH (1-34) docks at the positions 169-198 in the N-terminal region of the PTH1R (3, 91, 722) and position 27 of PTH1-34 docks at position 241-285 of the receptor (267, 512). The 1, 3, 23, and 27 positions of the PTH interact with the receptor residues M425, R186, T33/Q87, and L261 (91, 148). Molecular modeling demonstrated that the N-terminal region of hPTH1-34 binds to an invagination of the PTH receptor formed between TM3, TM4, and TM6. (Fig. 4B) Mutation analysis revealed that the residues Trp23, Leu24, and Leu28 of PTH1-34 interact with Phe173 and Leu174 of the PTH receptor through hydrophobic interactions. Hydrophilic interactions are formed between Arg20 of PTH1-34 and residues Glu177, Glu180 of the receptor, and between Lys27 of PTH1-34 and Glu169 of the receptor (250). Mutation of Leu24 and Leu28 results in 4000- and 1600-fold decrease in binding affinity, respectively, while mutation of Asp30 to Lys has no effect on receptor binding (246). The hydrophilic interactions are less important for binding as compared to hydrophobic interactions (246). The human PTH1-37 and 1-34 fragments retain full activity of the intact PTH1-84 and activate both adenylyl cyclase and phospholipase C activity. However, PTH1-31 is nearly equipotent but predominantly stimulates adenylyl cyclase activity, while removal of 2 N-terminal amino acids (PTH3-34) results in loss of adenylyl cyclase activity but activation of phospholipase C activity is retained (45, 46, 231, 685).
Based on molecular modeling, Hoare et al. (299) described a “two-site” dynamic model of interaction between PTH and PTH1R. According to their model, the extracellular N-terminal domain of PTH1R is the docking site for the C-terminal portion of PTH 1-34. The N-terminal of PTH 1-34 interacts with the J domain of the PTH1R which includes extra and intracellular loops and the transmembrane domains. The PTH1R N-domain provides the binding interactions between PTH and PTH1R while the J domain provides a stabilization function involved in receptor activation, G-protein coupling, and signal transduction (65, 330). Residues 15-34 of PTH function primarily as the binding domain (131) while residues 1-14 domain are responsible for the initiation of intracellular signaling through adenylate cyclase and PKC (65, 242, 246, 521). The binding of the N- and C-termini of PTH to the N- and J-domains of the receptor leads to increased affinity of the ligand-receptor complex, G protein complex formation, and activation of the downstream signaling pathways (65, 243, 244, 330). The current data suggest that the PTH receptor and PTH complex is present in an intermediate preactive R0 confirmation where G proteins are not coupled to the receptor ligand complex. The PTH-PTHR1 interaction stimulates the binding of heteromeric G proteins and the complex switches to an active confirmation (RG). Competition binding assays suggest that PTH1-34 binds with greater affinity to the R0 state than the RG state (299).
Signaling mechanisms of PTH1R
When PTH binds to the PTHR, the PTHR undergoes conformational change that promotes binding of G proteins (Gαβγ) to the receptor, followed by exchange of GDP for GTP on the α-subunit, and dissociation of the Gα from Gβγ subunits. (Fig. 4C) Gαs activates adenylyl cyclases to synthesize cyclic-AMP resulting in activation of protein kinase A (PKA). Gαq activates phospholipase C (PLC) which converts PIP2 to diacyl glycerol (DAG) and inositol (1,4,5)-triphosphate (IP3). IP3 stimulates calcium release from the endoplasmic reticulum to the cytosol. Increased Ca++ allows translocation of protein kinase C to the plasma membrane where it is activated by DAG (658, 660). Identification of signal selective peptides has led to better understanding of the PTH-PTHR interactions and downstream signaling mechanisms. For example, PTH1-28 is a cAMP-selective agonist that stimulates PKA activation but does not activate PLC-dependent and -independent PKC activation. This agonist also does not cause receptor internalization or recruitment of β-arrestin. Removal of the first two amino acids of PTH results in loss of cAMP signaling (92). Mutation analysis has demonstrated that the conserved valine at position 2 is critical for signaling through both arms (589). PTH 7-34 acts as a weak antagonist of PTHR, does not stimulate either Gs or Gq-mediated signaling pathways but does cause receptor internalization. The N-terminal truncated PTH fragments like PTH7-34 and PTH39-84 may have inhibitory effects (479). The C-terminal fragments of PTH blunt bone resorption and vitamin-D dependent osteoclastogenesis (196). These actions of PTH are thought to be independent of PTHR but are dependent upon yet an elusive carboxy-terminal PTH fragment receptor and stimulate alkaline phosphatase activity and induce expression of mRNA for both alkaline phosphatase and osteocalcin (195, 617). It is important to note that the ability of PTH to activate cAMP and/or PLC/PKC pathways is cell specific. For example, PTH stimulates cAMP-PKA pathway but not the PLC/PKC pathway in vascular smooth muscle cells (415) while in keratinocytes (495, 684), cardiac myocytes (531, 568), and lymphocytes (359), PTH activates PLC/PKC pathway but not the cAMP/PKA pathway. The development of FRET based assays allowed kinetic studies of binding of PTH to its receptor and signaling in live cells. These studies demonstrated that binding of PTH to its receptor involves two steps. The first step, rapid binding of PTH to the N-terminus of the receptor, requires 150 ms at saturating concentrations of PTH. The second step where the C-terminal of PTH binds to the J-domain of the receptor is considerably slower, requiring about 1 s. The subsequent interaction between PTHR and Gs depends upon the expression levels of Gs and can be completed in about 0.96 s. In about 10 s following receptor coupling with Gs, cAMP production is initiated (130, 222, 656).
The actions of PTH on bone are very well documented and have been the subject of recent excellent reviews (62, 135, 165, 209, 459, 528, 543, 591, 691, 719). PTH interacts directly with osteoblasts and osteocytes through PTH1R to stimulate a number of different pathways including cAMP/PKA, PLC/PKC, β arrestin translocation, and ERK1/2. In bone, the downstream signaling from these pathways is heavily regulated by RGS2 (regulator of G protein signaling 2), one of a family of proteins that modulates G protein activity stimulated by G protein-coupled receptors. Depending on whether the hormone presence is continuous or pulsatile, the overall effect of PTH signaling on bone metabolism will be catabolic or anabolic, respectively. Key to determining which effect predominates is the differential control of the osteoprotegerin-receptor activator of NFκB ligand-receptor activator of NFκB (OPG-RANKL-RANK) pathway. OPG, which is a bone-derived cytokine, and RANK, which is a receptor located on the preosteoclast, compete for binding with RANKL, another bone derived cytokine. Interaction between RANK and RANKL stimulates osteoclastogenesis while OPG prevents that interaction by binding itself to RANKL. It is this pathway that controls the PTH-stimulated interaction with the osteoclast precursor cell, which can mature into a functional osteoclast and mediate bone resorption. Continuous presence of PTH leads to an increase in the mRNA for RANKL and a decrease in the mRNA for OPG through PKA dependent pathways, the result of which is enhanced binding of RANKL to RANK and enhanced osteoclast maturation. PTH stimulates osteoblast differentiation, decreases osteoblast apoptosis, and activates lining cells. The effects of PTH to stimulate osteoblastogenesis can be seen in bone progenitor cells, mediated through changes in the transcriptional program that result in the expression of characteristic bone proteins such as alkaline phosphatase, type I collagen, RUNX2, and others. Recently, several downstream mediators of PTH action on bone have been identified including monocyte chemoattractant protein-1, sclerostin, dickkopf1, and EphrinB2/EphB4 (591) Investigation into the mechanisms of PTH regulation of bone is a dynamic area of inquiry as witnessed by the recent explosion of findings highlighting the intricacies of the process.
The other major target for PTH is the kidney. In human kidneys, the PTH receptors are expressed in proximal tubules, cortical ascending limbs of the Loop of Henle, and distal tubules. The receptors are expressed on both the apical and basolateral membranes of proximal tubules (291, 340, 353, 546, 633). Activation of the receptors on the basolateral membranes activates the PLC/PKC pathway while activation of the receptors on the apical membranes activates cAMP-PKA pathway (546, 633). Both pathways contribute to endocytosis of the type IIa sodium-phosphate cotransporter, leading to inhibition of phosphate reabsorption (350, 351, 380, 467). The differences in the activation of PLC/PKC pathway or cAMP-PKA pathway has been attributed to the binding of PTHR to sodium-hydrogen exchanger regulatory factor-1 (NHERF1), a scaffolding protein that exhibits two internal PDZ domains and an ezrin binding domain at the C terminal (677). Mahon and Segre recognized that the intracellular C-terminal domain of PTHR expresses a PDZ recognition motif D/E-S/T-X-ϕ that preferentially binds to PDZ1 domain of NHERF1 and PDZ2 domain of NHERF2 (418), and confirmed that PTHR binds to both proteins, NHERF1 and NHERF2. NHERF1 and NHERF2, through binding with ezrin, link membrane proteins with the actin cytoskeleton and recruit several proteins including receptors, ion transporters, and signaling proteins to the plasma membrane (97, 167, 676, 677). Using heterologous expression of NHERF2 and PTHR in PS120 fibroblast cells, Mahon and Segre identified that PTH activates PLC/PKC pathway. In the absence of NHERF2, PTH activated cAMP-PKA without affecting PLC/PKC pathway (418, 419). These studies suggest that NHERF provides signaling switch that could explain cell specific functions of PTHR independent of PTHR expression levels and expression of splice variants of PTHR (237). The expression patterns of NHERF1 and PTHR have important physiological consequences for renal phosphate and calcium handling. NHERF1 null mice exhibit phosphaturia and hypophosphatemia in part due to decreased apical membrane expression of type IIa sodium phosphate cotransporter without any changes in serum calcium (121, 167-170, 583, 584, 663, 675, 678-681). Phosphate wasting without changes in serum calcium has also been seen in patients who express NHERF1 polymorphisms or mutations (64, 166, 334, 545). The absence of NHERF1 expression in distal nephrons may explain the differences in the renal regulation of phosphate and calcium by PTH (237).
PTH1R trafficking and desensitization
Following binding and activation, the PTHR undergoes internalization and desensitization similar to most GPCRs (251, 252, 386). PTHR is phosphorylated in the C-terminus by G-protein-coupled receptor kinases (GRKs), resulting in increased association with β-arrestin and internalization through clathrin-coated pit dependent pathways (129, 606, 618, 624, 625, 659). Some of the receptors undergo rapid recycling while the rest are degraded in the lysosomes. The PTHR desensitization is more complex due to its associations with cytoplasmic adapter proteins NHERF1, NHERF2, and disheveled (Dvl2) (552, 607). Binding of these adapter proteins to the C-terminal domain of PTHR alters the selectivity and specificity of PTHR signaling through cell and ligand-dependent effects (273, 416, 417, 420). The laboratory of Peter Friedman extensively studied the internalization of PTHR in kidney cells. Using EGFP tagged PTH1R they demonstrated that GRK2-dependent phosphorylation of PTH1R is required for endocytosis in mouse proximal and distal tubule cells (92, 657). Their studies revealed that the PTH fragments that do not activate the receptor like PTH7-34 cause endocytosis in distal convoluted tubules but not in proximal tubules (237, 660). When they stimulated NHERF1- transfected distal tubule cells with PTH 7-34, they demonstrated blunting of internalization of PTH1R. Similarly, proximal tubule cells expressing dominant negative NHERF1 showed increased internalization of the PTHR in response to PTH7-34. These data suggest that interaction of PTHR with NHERF1 plays an important role in receptor internalization (237, 660). Mutations in the PDZ binding motif of PTHR prevented binding of NHERF1 to the PTHR but did not interfere with signaling or internalization of the receptor. These results suggest that the intact PDZ motif is required for association with NHERF1, but additional studies will be needed to define the nature of association and the impact on receptor internalization. Mutations in the NHERF1 ezrin binding domain prevented NHERF1-PTHR-actin interaction but failed to prevent internalization in response to PTH7-34. Similar results were shown in the presence of actin destabilizing agent, cytochalasin-D. Together these studies suggest that degradation of any component of the interaction between NHERF1-PTHR-actin cytoskeleton allows internalization of the PTHR in response to PTH7-34 (237).
Recent studies from Friedman laboratory suggest that PTHR also associates with the PDZ adaptor protein disheveled 2 (Dvl2). Unlike, the interaction with NHERF1, association with Dvl2 occurs between residues 470 and 480 of the PTHR. Using immunoprecipitation, they demonstrated that PTH1-34 increases interaction between PTHR and Dvl2 transiently. Binding of PTHR with Dvl2 increases its association with AP2 and β-arrestins resulting in internalization of the receptor (140, 552, 710).
Recent studies suggest that internalization of the PTHR in response to PTH1-34 does not completely blunt cAMP-PKA pathway. These observations led to the hypothesis that persistent calcemic responses in animals, prolonged increases in serum 1,25-dihydroxyvitamin D, bone resorption and prolonged cAMP-PKA activation results from signaling through intracellular PTH bound to PTHR in the early endocytic compartment. These provocative studies suggest that GPCR stimulated signaling is not confined to the plasma membrane (116, 192, 222, 360). Further studies are required to determine the physiological consequences of intracellular signaling through endocytosed GPCRs.
Functions of PTH
Several very careful cellular, molecular, and in vivo studies helped to understand the physiological roles of PTH. PTH plays diverse roles in the body from maintaining whole body calcium homeostasis to maintaining bone density. In the kidney, PTH regulates phosphate homeostasis by decreasing expression of sodium-phosphate cotransporters NpT2a (38, 39, 96, 121, 188, 203, 294, 348, 350, 351, 467) and NpT2c (109, 274, 377, 379, 454, 455, 576, 577), thus inhibiting reabsorption of filtered phosphate by the proximal tubules. PTH may also decrease phosphate absorption in the intestine by decreasing membrane expression of NpT2b (115, 255, 562). These two actions that tend to decrease total body phosphate content contrast with the pro-absorptive effects of PTH on calcium and ensure that maintenance of adequate calcium stores is not accompanied by accumulation of excessive phosphate. PTH increases ammoniagenesis (146) and gluconeogenesis (347, 573, 664) in the kidney. In the proximal tubules PTH induces inhibition of bicarbonate partly through inhibition of Na+/H+ exchanger activity (235). The actions of PTH on Na+/H+ exchanger are complex and different from the actions of Na-Pi cotransporters. PTH inhibits the activity of Na+/H+ exchanger but does not decrease the number of transporters whereas it causes endocytosis and degradation of Na-Pi cotransporters (390, 707, 720). The activity of Na+/H+ exchanger returns quickly to normal levels once PTH is removed unlike the activity of Na-Pi cotransporters which requires de novo synthesis (292, 462). PTH activates 25 vitamin D 1α-hydroxylase, thus stimulating conversion of 25-hydroxy vitamin D to its active form, 1, 25-dihydroxy vitamin D (373, 586). The active vitamin D increases calcium reabsorption in the intestine. In the thick ascending limb of the Loop of Henle PTH increases transepithelial transport of sodium, calcium, and magnesium. PTH stimulates distal renal tubular calcium reabsorption by regulating the expression of proteins involved in calcium reabsorption, viz; sodium calcium exchanger, Ca-ATPase, calbindin, TRPV5, and TRPV6 (187, 235, 321, 651). PTH promotes apical calcium entry through dihydropyridine-sensitive calcium channels. Apical calcium entry in this segment of the nephron is favored due to basolateral increase in chloride conductance resulting in increased chloride efflux and fall of intracellular chloride concentrations. Calbindin also plays a crucial role in calcium homeostasis by binding to reabsorbed calcium inside the cells and therefore prevents increases in free intracellular calcium. Expression of calbindin is regulated by PTH-dependent calcitriol synthesis (321). However, the most important function of PTH is to regulate bone mineralization. PTH affects all bone cells; stimulation of osteoblasts enhances bone formation while stimulation of osteoclast maturation increases bone resorption. The stimulatory effects of PTH on bone turnover have been successfully and effectively used pharmacologically to treat osteoporosis (520).
Interaction of PTH with other hormones
The most well studied interaction of PTH with other hormones is the interaction with fibroblast growth factor 23 (FGF23)/klotho complex (328). FGF23 is produced by osteocytes and osteoblasts (68, 429, 525, 527, 588, 597, 704). Both PTH and FGF23 are phosphaturic hormones and regulate phosphate homeostasis by inhibiting phosphate absorption in the intestines and reabsorption in the kidneys through decreasing membrane expression of sodium-phosphate cotransporters NpT2a (26, 247, 248) and NpT2c (66, 247, 248). PTH increases 1α-hydroxylase activity, active vitamin D, and calcium absorption in the intestines and reabsorption in the kidneys whereas FGF23 decreases 1α hydroxylase activity, active vitamin D thereby decreasing the absorption of calcium (289). PTH and FGF23 regulate the synthesis of each other. Higher serum phosphate increases the synthesis of FGF23 and lowers serum calcium, which will trigger synthesis of PTH. While PTH increases calcium reabsorption and indirectly increases FGF23 synthesis. Both of these mechanisms will eventually result in bone disease (328).
In recent years, an association between PTH and aldosterone has been described. New data demonstrate the expression of PTH in the aldosterone secreting zona glomerulosa cells of the adrenal glands and the expression of the mineralocorticoid receptor in parathyroid cells (632). Studies suggest that PTH directly regulate the secretion of aldosterone from the zona glomerulosa cells of adrenal glands and aldosterone in turn regulates PTH secretion from the parathyroid cells (631, 632). Isales et al. (316) and Olgaard et al (490) demonstrated that PTH stimulated aldosterone secretion in a dose dependent manner and potentiated angiotensin 2 stimulated aldosterone secretion. Rosenberg et al confirmed that adrenal glands are a novel target of PTH (554, 555). The effects of PTH on aldosterone secretion were mediated through PTH1R activation of cAMP/PKA and PLC/PKC pathways (213, 214). In patients with primary hyperparathyroidism (pHPT), elevated levels of aldosterone have been demonstrated (249), and parathyroidectomy has resulted in decreased aldosterone levels, decreased blood pressure, decreased risk of metabolic syndrome, and improvement in several parameters of vascular function (21, 216, 217, 407, 496). A prospective study in 226 patients with essential hypertension demonstrated a positive correlation between aldosterone and PTH levels (631, 632). Primary aldosteronism (PA) has been associated with higher PTH and lower calcium. Treatment with spironolactone decreased PTH levels in PA patients (556). Taken together these data suggest aldosterone and PTH cooperatively would cause vascular damage to multiple organs and support the hypothesis that regulation of PTH and aldosterone are associated, though whether these are linked or separate pathways remains unclear. These primarily epidemiologic and associative studies require more investigation to confirm a true cause and effect relationship and to identify the prevalence of these combined disorders.
Pathophysiology
Once it was recognized that parathyroid glands secrete a hormone, PTH, in response to changes in ionized calcium levels, studies were conducted to understand the consequences of hyper- or hyposecretion of PTH. Even in the absence of reliable imaging techniques or assays for the measurement of mineral ions in the early twentieth century, pioneering work of several investigators identified clinical sequelae of excessive (hyperparathyroidism) or insufficient (hypoparathyroidism) PTH.
Hyperparathyroidism
The insights gained from pathological and mechanism-based studies identified and defined the clinical features of hyperparathyroidism (16, 17). Hyperparathyroidism may be primary or secondary (448). pHPT can result from an adenoma, multigland hyperplasia, or carcinoma. In most (about 90%) adults a single adenoma causes pHPT while others (about 5%) may have double or multiple adenomas of the parathyroid glands. About 5% of patients present with glandular hyperplasia and 1% with carcinoma (558). Mechanisms for the development and growth of parathyroid adenomas in the setting of primary and secondary hyperparathyroidism have been investigated extensively. The size of the adenomas is inversely proportional to the nutritional status of vitamin D (532), suggesting a role for vitamin D in regulating the growth of these adenomas, presumably through the VDR. On the other hand, studies of the expression of vitamin D receptor in the adenomas have not demonstrated a consistent pattern (368, 616, 654). Similarly, expression of the CaSR in adenomas has been described as increased, decreased, or unchanged; however, recently Koh and colleagues identified RGS5 as highly expressed in parathyroid adenomas and have suggested that the increased expression of this protein could alter CaSR signaling to decrease its effect on PTH secretion (361). Proteomic analysis comparing normal and adenomatous tissue suggests that proteins involved in apoptosis are decreased in parathyroid adenomas compared to normal parathyroid glands (653). The genetic basis for the development of sporadic hyperparathyroidism has not been fully established. In some circumstances, mutations in the MEN1 gene, the RET gene, or the CaSR gene which are associated with familial forms of hyperparathyroidism have been identified, but not universally (630). Clearly, multiple different mechanisms appear to result in the clinical syndrome of hyperparathyroidism, suggesting that identification of these mechanisms in the individual patient could lead to more directed and effective therapy, perhaps even nonsurgical therapy (585).
Clinically, most patients present with asymptomatic hypercalcemia on routine lab work and a high circulating PTH level. Older literature classically refers to the manifestations of hyperparathyroidism as “stones, bones, abdominal groans, and moans” (58). Stones are due to nephrocalcinosis, hypercalcuria, and renal tubular reabsorption disturbances. Bone pain can be experienced due to fractures resulting from enhanced bone resorption leading to osteoporosis and osteitis fibrosa cystica. Abdominal groans are due to nausea, constipation, anorexia, and pain. Moans refer to both neuromuscular and neuropsychiatric symptoms including depression, anxiety, cognitive dysfunction, and fatigue. Other symptoms include headache, emesis, polydipsia, diarrhea, and joint pains (58). Symptoms are frequently more severe in children than in adults but this may be due in part to delay in diagnosis as serum calcium is not monitored regularly in children (364). Recent data suggest that cyclin D1 mutations also cause pHPT (32, 33, 422, 423).
pHPT can be normocalcemic, asymptomatic, or hereditary (533). Normocalcemic hyperparathyroidism is a fairly newly recognized condition wherein patients present with normal calcium (including ionized calcium) levels and high serum PTH levels with no secondary causes like renal disease, medications, gastrointestinal illness, idiopathic hypercalciuria, or vitamin D deficiency (592). A percentage of these individuals later develop hypercalcemia. It is often diagnosed in patients found to have low bone mass or nephrolithiasis. This two-phase process for the development of full-blown hyperparathyroidism is incompletely understood. One theory holds that initial target organ resistance to the actions of PTH, as would be seen perhaps in premenopausal women with higher circulating estrogen levels, mask the hypercalcemic response to PTH, which then becomes apparent after menopause when estrogen levels decline precipitously. However, most patients with normocalcemic hyperparathyroidism actually are post-menopausal, somewhat negating this theory (18). The epidemiology and natural history of this disorder are not well understood as yet [reviewed in (172)].
Asymptomatic hyperparathyroidism is characterized by mild hypercalcemia, low to deficient vitamin D, and normal serum phosphate levels. It is more prevalent in women than in men and manifests within the first decade after menopause. In asymptomatic patients, densitometric and histomorphometric analyses demonstrate reduced bone mineral density in the distal one-third radius while lumbar region is often preserved and the hip region is intermediate between distal and lumbar regions. PTH levels are high with low 25-hydroxyvitamin D levels [reviewed in (593)].
Familial hyperparathyroidism is a group of inherited autosomal dominant parathyroid disorders (256). These include multiple endocrine neoplasia (MEN) type I MEN1, type 2 MEN2a, type 4 MEN4, familial hypocalciuric hypercalcemia (FHH), neonatal severe hyperparathyroidism (NSHPT), autosomal dominant moderate hyperparathyroidism (ADMH), hyperparathyroidism-jaw tumor syndrome (HPT-JT), and familial isolated hyperparathyroidism (FIHPT). MEN1 is caused by mutations in the MEN1 gene on chromosome 11q13 (388, 389). Patients develop parathyroid adenomas but carcinomas are rare. Patients show multiglandular parathyroid disease with asynchronously and asymmetrically enlarged parathyroid glands (211, 212, 236, 424). MEN2a syndrome is an autosomal dominant condition with high risk of developing medullary thyroid carcinoma. The average onset of PHPT is 38 years of age (256). MEN2 is caused by mutations in RET gene localized to chromosome 10 that encodes a tyrosine kinase (286, 458, 463-465). MEN2 is characterized by parathyroid adenoma or hyperplasia (256). MEN4 is a rare disorder, the result of mutations in CDKN1B gene leading to dysfunctional cell cycle inhibitor p27 (383, 384, 425, 504). Little is known about this illness.
FHH and NSHPT are associated with inactivating mutations in CaSR gene. FHH patients express a mutation in one allele and have hypercalcemia, mild hypermagnesemia, and hypophosphatemia. Generally, these individuals are completely asymptomatic; however, parathyroid glands may be moderately enlarged. This condition is differentiated from the usual sporadic pHPT by very low urine calcium, generally less than 100 mg/day, and requires no treatment. NSHPT is a homozygous form of FHH, resulting in the very rapid development of PHPT at birth or shortly thereafter. Patients have severe hypercalcemia, bone demineralization, and neurodevelopmental disorders. In this disorder, parathyroid glands should be surgically removed within first few days of life to prevent fatal outcome (434-437).
ADMH is caused by mutations in cytoplasmic C-terminal tail of CaSR and is characterized by parathyroid hyperplasia or adenoma. The treatment of choice is surgical removal of the parathyroid gland (126, 127).
HPT-JT is an autosomal dominant disorder caused by mutations in the HRPT2 gene encoding parafibromin, a critical protein for cell growth (128). HPT-JT is associated with a variety of manifestations including fibrous-osseous tumors of the jaw, Wilm’s tumor, papillary renal carcinoma, polycystic kidney disease, renal cysts, and pHPT (132, 133, 136, 582). Sporadic parathyroid carcinomas are very common in the HPT-JT patients (256).
FIHPT is another rare autosomal dominant disorder associated with mutations in CaSR, MEN1, and HRPT2 genes. The disease is characterized by uni- or multiglandular lesions of parathyroid glands, treated by simple surgical removal of adenomas (285, 449, 626, 672).
Secondary hyperparathyroidism is quite common and is caused by decreased levels of vitamin D, hypocalcemia, or in chronic renal disease (233). Patients may present with low bone density, osteoporosis, or fragility fractures. Hypocalcemia of any cause can result in increased PTH secretion. Common clinical situations include intestinal malabsorption or poor diet, which limit calcium intake; pancreatitis or rhabdomyolysis, which sequester calcium; or vitamin D deficiency due to poor diet, lack of sun exposure, nephrotic syndrome, or liver failure. In contrast to pHPT, correction of the underlying disorder will normalize PTH levels. Individuals with secondary hyperparathyroidism more commonly have diffusely hyperplastic glands than those with sporadic pHPT, who more likely will have an adenoma. Secondary hyperparathyroidism complicates the clinical course of nearly all patients with chronic kidney disease, although it is generally not manifest until late in the course. The etiology of secondary hyperparathyroidism associated with chronic kidney disease is complex (210, 275). Early in the development of chronic kidney disease, levels of FGF23 begin to rise, a phenomenon attributed at least in part to the loss of renal expression of klotho and to a diminished ability of the kidney to excrete phosphorus. The rise in FGF23 is mirrored by a decrease in 1,25-dihydroxyvitamin D, resulting in decreased intestinal calcium absorption and hypocalcemia. Clinically, this hypocalcemia may be subtle, asymptomatic, and not recognized. As kidney failure progresses, frank hyperphosphatemia becomes more prominent. The combination of hypocalcemia, decreased active vitamin D, and hyperphosphatemia results in progressive secondary hyperparathyroidism, which is not easily reversible. These individuals may exhibit parathyroid gland hyperplasia or multiple adenomas composed of monoclonal or polyclonal clusters of parathyroid cells. The secondary hyperparathyroidism of chronic kidney disease is implicated in a variety of complications of kidney disease including accelerated vascular disease, vascular calcification, and fractures.
Hypoparathyroidism
Patients with hypoparathyroidism present with severe hypocalcemia, hyperphosphatemia, tetany, hypomagnesemia, and lower levels of vitamin D. Basal ganglia calcifications are another very common feature of this syndrome. The most common cause of hypoparathyroidism is damage or removal of parathyroid glands during neck surgery, especially complicated thyroid surgery. However, hypoparathyroidism may occur as a congenital disorder or as an autoimmune condition, in isolation or in conjunction with other organ failure. The reader is referred to several recent excellent clinical reviews of this rare condition (73, 89, 590). A number of parathyroid-specific autoantibodies have been identified and implicated in the development of hypoparathyroidism in the autoimmune polyendocrinopathy syndrome type I, including antibodies directed against the calcium sensing receptor, tryptophan hydroxylase, interferon omega, and the NACHT leucine rich repeat protein 5 (NALP5) to name a few. However, the autoantibodies responsible for isolated autoimmune hypoparathyroidism and for other forms of autoimmune polyendocrinopathy syndromes are still in question. Standard treatment for symptomatic patients has been high-dose active vitamin D and calcium, but recently, clinical trials of recombinant parathyroid hormone have been initiated (171). Another rare syndrome is pseudohypoparathyroidism where there is resistance to PTH hormone action, either globally or confined to the proximal tubule of the kidney. This condition is caused by mutations in the gene for the Gsα subunit, resulting in abnormal Gsα function or in abnormal Gsα transcription (448, 629).
Human chondrodysplasias
Two devastating forms of chondrodysplasia, Blomstrand’s lethal chondrodysplasia (BLC) and Jansen’s metaphyseal chondrodysplasia (JMC), resulting from mutations in PTH1R gene have been described (567).
BLC was first described by Blomstrand et al in 1985 (99) and is prenatally lethal due to premature bone mineralization, ossification, shortened limbs, and abnormal tooth and mammary gland development (567, 700). Three different inactivating PTH1R mutations have been identified in patients with BLC, which result in failure of PTH to bind to the receptor, diminished PTH1R expression, or impaired signal transduction [reviewed in (567)].
JMC is a rare autosomal dominant disorder associated with severe abnormalities of the growth plate. Clinically, the patients have short stature, disproportionate limbs, and micrognathia. Patients have severe asymptomatic hypercalcemia, hypophosphatemia, increased phosphate and cAMP excretion in urine, and elevated levels of vitamin D with normal or undetectable PTH. JMC is caused by gain of activity single point mutations in PTH1R including H223R, T410P, and I458R that result in PTH-independent activation of cAMP/PKA pathway [reviewed in (567)].
Enchondromatosis is caused due to common solitary or multiple benign tumors of bone. Recently, missense mutation (R150C) in PTH1R has been identified in two patients with enchondromatosis. The mutation results in a constitutively active receptor leading to increased cAMP levels. This mutation is less severe than the mutations observed in JMC [reviewed in (567)].
Conclusions
PTH is a hormone critical for many cell processes, primarily focused on mineral metabolism. This tightly regulated hormone is critical for regulation of calcium and phosphate homeostasis as well as bone metabolism. Dysfunction in the regulation results in dramatic clinical pictures characterized by poor bone mineralization and increased soft tissue mineralization. These abnormalities in turn lead to cardiovascular disease and kidney failure. Key gaps in our understanding of PTH include the role of intracellular signaling, the interaction of PTH with other hormones involved in mineral metabolism, and the mechanisms by which PTH can influence cardiovascular health.
Vitamin D
Introduction
Vitamin D is a steroid hormone, synthesized through conversion of metabolites supplied by skin or intestinal absorption and involved in multiple critical processes for living organisms (Fig. 5). Functioning as a circulating hormone produced by the kidney, active vitamin D’s most prominent role is as a critical regulator of bone mineralization. Vitamin D deficiency results in severe metabolic bone disease both in children and adults. Osteomalacia, a defect in bone mineralization detected by bone biopsy, occurs in both children and adults whereas rickets occurs only in children. Increasingly, vitamin D is recognized as a mediator of multiple other processes in the body including immune function, the renin-angiotensin axis, insulin metabolism, and cell proliferation, to name a few.
Figure 5.
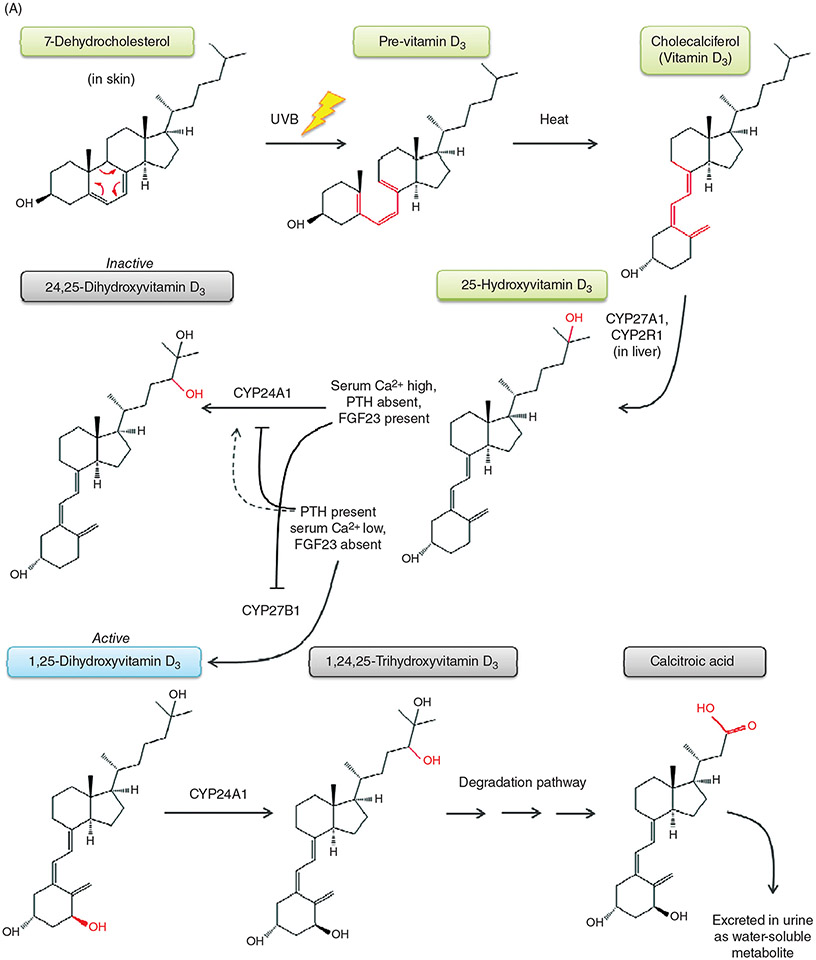
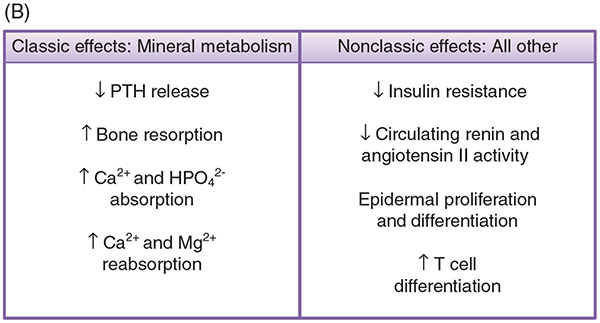
(A) Metabolic pathway of vitamin D. (A) The production of 1,25-dihydroxyvitamin D begins in the skin with the precursor 7-dehydrocholesterol. UVB light catalyzes the conversion of 7-DHC to pre-Vitamin D (structural changes denoted in red), which is further converted to Vitamin D by heat through the process of thermal isomerization. Vitamin D enters the bloodstream, and in the liver undergoes hydroxylation by either CYP27A1 or CYP2R1 to form 25-hydroxyvitamin D. Depending on serum Ca2+ levels and hormones present, in the kidney, 25-hydroxyvitamin D will either be converted to its active form, 1,25-dihydroxyvitamin D, by the enzyme CYP27B1, or be converted to its inactive form, 24,25-dihydroxyvitamin D, by the enzyme CYP24A1. FGF23 upregulates CYP24A1 expression and downregulates CYP27B1. PTH stimulates CYP27B1 expression, and depending on the circumstances, either downregulates CYP24A1 or slightly upregulates its expression. 1,25-dihydroxyvitamin D3 is degraded first by hydroxylation at the 24th position to form 1,24,25-trihydroxyvitamin D, then through a series of steps form the end product calcitroic acid, which is water-soluble and is excreted in the urine. (B) 1,25-Dihydroxyvitamin D3 promotes both classic effects on mineral metabolism as well as nonclassic effects on immune function, cardiovascular protection, and others as listed. (B) Metabolic pathway of vitamin D. (A) The production of 1,25-dihydroxyvitamin D begins in the skin with the precursor 7-dehydrocholesterol. UVB light catalyzes the conversion of 7-DHC to previtamin D, which is further converted to vitamin D by heat. Vitamin D enters the bloodstream, and in the liver undergoes hydroxylation by either CYP27A1 or CYP2R1 to form 25-hydroxyvitamin D. Depending on serum Ca2+ levels and hormones present, in the kidney, 25-hydroxyvitamin D will either be converted to its active form, 1,25-dihydroxyvitamin D, by the enzyme CYP27B1, or be converted to its inactive form, 24,25-dihydroxyvitamin D, by the enzyme CYP24A1. 1,25-dihydroxy-D3 is degraded through a series of steps to the water-soluble product calcitroic acid, which is excreted in the urine. (B) 1,25-Dihydroxy-D3 promotes both classic effects on mineral metabolism as well as nonclassic effects on immune function, cardiovascular protection, and others.
History of discovery
Vitamin D was discovered early in the twentieth century but the fact that there was an active substance in milk apart from the carbohydrate, fat, and protein content that was critical for life was reported first in 1880 by N. Lunin, a Russian scientist who noted that newborn mice fed a diet composed of casein, carbohydrate, fat, and salts died while those fed milk lived (440). In 1922, McCollum reported that cod liver oil, which had been used for a century to treat rickets, contained a fat soluble substance that prevented rickets in rats and named it vitamin D (440). Ensuing work by a number of investigators established the existence of a compound similar but not identical to cholesterol which could be activated by irradiation of skin, liver, and multiple food substances which similarly had antirachitic properties. The compound in food was identified as ergosterol by Windaus and Hess in 1931 and the compound in animal tissue was identified as 7-dehydrocholesterol by Windaus and Bocke in 1937 (697). The full biosynthetic pathway was finally delineated by Holick et al. (189, 309) in 1980.
Structure: Biochemical properties
The vitamin D family compounds are secosteroids, exhibiting a tetracyclic structure with a cleaved ring (153, 307, 309, 311). They differ from conventional steroids in that the B ring is open, lacking a sixth carbon atom. Vitamin D2 or ergocalciferol is synthesized from ergosterol (311, 325), a naturally occurring substance in yeast and plants while vitamin D3 or cholecalciferol is synthesized from 7-dehydrocholesterol, a cholesterol precursor (153, 232, 258, 309). Throughout the metabolic pathway of vitamin D, multiple compounds can be generated, most of which are thought to be inert or which have not been studied (102, 153, 232, 265, 307, 308, 310, 311). Ergocalciferol, cholecalciferol, 25-, and 1,25 vitamin D are fat soluble. The terminal step in vitamin D degradation is calcitroic acid (1α-hydroxy-23-carboxy-24,25,26,27-tetranorvitamin D), which is water soluble and excreted primarily in the urine (537).
Vitamin D synthesis, metabolism, and regulation
Vitamin D metabolism
Vitamin D is a family of steroids originating from ultraviolet light conversion of precursor compounds in the skin (5, 309) or through ingestion of precursor substances in food. In skin, ultraviolet light effects a conformational change in the steroid 7-dehydrocholesterol to produce previtamin D which then is converted to cholecalciferol (vitamin D), a process that requires up to three days (309). The origin of 7-dehydrocholesterol in the skin has been debated, with some arguing for an intestine derived origin; however, more recent studies suggest that 7-dehydrocholesterol is formed de novo by numerous skin cell types (258, 599). Factors regulating this initial step include age, skin pigmentation, degree and duration of skin exposure, and intensity of sunrays (104, 158, 306, 309). Aging and darker skin pigment will limit this process as does, predictably, lesser surface area of skin exposure, lesser time of skin exposure, higher latitude, and the angle of the sun during winter season. A recent study suggested, however, that the degree of conversion correlated more with baseline 25-hydroxy vitamin D25-hydroxy vitamin D levels and total cholesterol levels than with degree of pigmentation (104, 266). The production of cholecalciferol by sun exposure is self-limited as excessive sun results in degradation of previtamin D and vitamin D (574). In addition, sunlight also converts 7 dehydrocholesterol to some inactive metabolites such as tachysterol and lumisterol (190, 266, 306). Cholecalciferol diffuses into the skin capillaries, and circulates as either the free compound or bound to vitamin D binding protein (DBP) (153, 156, 190, 311, 314). Cholecalciferol has no documented direct activity and a relatively short half-life (12-24 h). The free compound enters cells relatively easily and the degree of binding to DBP largely determines the rate of uptake by adipose tissue, muscle, or liver. Cholecalciferol can also be obtained through ingestion of foods containing naturally occurring vitamin D such as egg yolks, fatty fish, and liver, or through foods fortified with vitamin D such as milk, breads, infant formula, and orange juice (95, 686). Absorption of cholecalciferol is dependent on bile acid-mediated formation of micelles. While some of the absorbed vitamin D is transported through the portal system to the liver, the majority of absorbed vitamin D is taken up through chylomicrons into the lymphatics (686). A significant amount of the absorbed vitamin D is taken up into fat tissue and muscle (314). How or whether vitamin D sequestered into these tissues is regulated is unknown. The propensity for adipose tissue absorption of vitamin D may explain the higher vitamin D requirement and/or the lower circulating vitamin D levels in obese individuals. Of note, high bolus ingestions of vitamin D are rapidly cleared by fat and muscle and not released subsequently into the circulation (311). Thus smaller daily doses of cholecalciferol (1000-2000 IU) are preferred to infrequent large doses for maintenance of stable daily serum concentrations of cholecalciferol (306, 311).
Cholecalciferol undergoes 25-hydroxylation to form 25-hydroxyvitamin D, a metabolite that also is considered inactive (27, 293, 724). Several tissues express 25-hydroxylase activity including kidney, intestine, and liver, but the majority of cholecalciferol hydroxylation appears to occur in the liver (77-81, 326, 491, 513). Many 25-hydroxylases capable of performing this function have been identified but most interest has focused on the mitochondrial enzyme CYP27A1 and the microsomal enzyme CYP2R1 (326). Liver exhibits a high expression of the mRNA for 25-hydroxylase, CYP27A1, but, interestingly, mutations in the gene encoding CYP27A1 do not result in significant abnormalities in vitamin D metabolism (315, 398). In contrast, mutations in cytochrome p450 2R1 or vitamin D 25-hydroxylase (CYP2R1) have been reported in individuals with very low vitamin D levels, suggesting that this enzyme is essential for 25-hydroxylation (142). Moreover, CYP2R1 but not CYP27A1 25-hydroxylates both vitamin D2 and vitamin D, while CYP27A1 hydroxylates only D (325, 326). CYP2R1 has a high affinity for vitamin D and is quite specific for the 25 position on vitamin D but not other steroid compounds (615). Thus, CYP2R1 is currently the leading contender for the physiologically relevant CYP governing this aspect of vitamin D metabolism. A very recent study demonstrated that neither Cyp27a1 nor Cyp2r1 is essential for 25-hydroxylation as mice lacking both enzymes, showed only a 50% reduction in 25-hydroxy-vitamin D and exhibited normal 1,25 vitamin D levels (724), suggesting considerable redundancy in this metabolic step. CYP27A1 is regulated by multiple hormones, particularly insulin, glucocorticoids, sex hormones and by 1,25 dihydroxyvitamin D itself (27). Hormonal regulation of CYP2R1 has not been studied. The activity of the 25-hydroxylase enzymes is largely driven by substrate availability; thus this step in vitamin D metabolism is not considered a site of significant regulation. Despite the fact that 25-hydroxy vitamin D25-hydroxy vitamin D is not the active form of vitamin D, it is preferentially measured in clinical medicine to establish vitamin D status because of its long half-life of about 2 to 3 weeks (156, 159, 311). However, concerns about the reproducibility and reliability of the assays raise questions about using 25-hydroxy vitamin D25-hydroxy vitamin D measurement to define vitamin D deficiency (207). 25-Hydroxyvitamin D circulates in three forms: 85% with DBP, 15% albumin-bound, and 0.03% free. The current assays measure total, not free 25-hydroxy vitamin D25-hydroxy vitamin D, and vitamin D-binding protein levels can vary considerably on an individual basis. Nonetheless, at this time, the standard of care is to measure 25-hydroxy vitamin D25-hydroxy vitamin D to evaluate vitamin D status. The presence of 25-hydroxylases capable of cholecalciferol hydroxylation in other tissues has only recently been recognized and potential roles for cholecalciferol in these tissues are currently under investigation (24, 87, 98, 102, 103, 326).
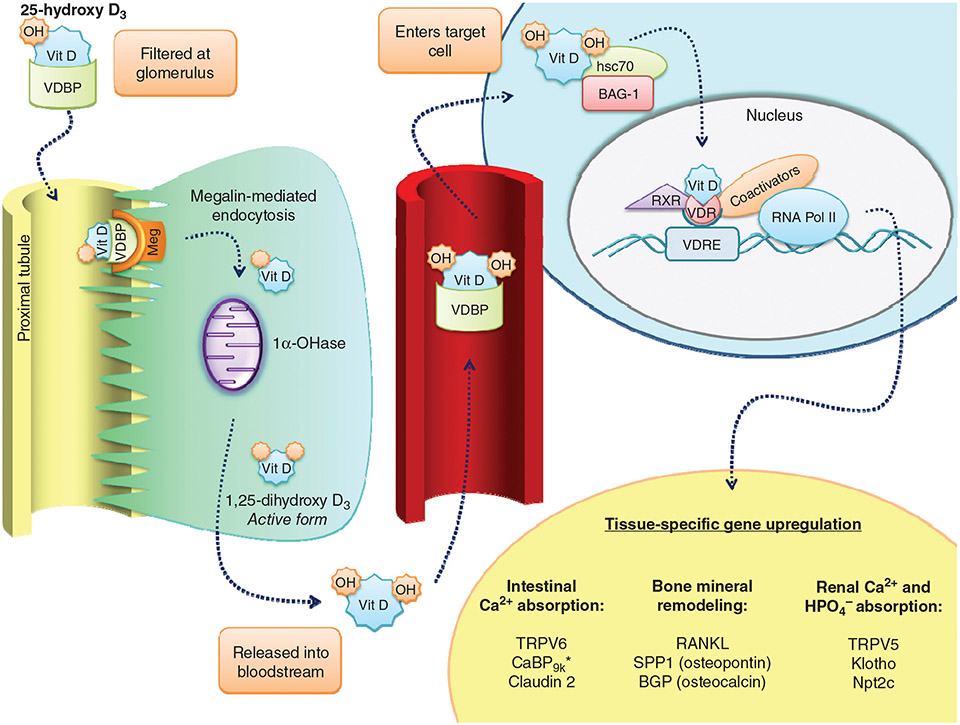
The 25-hydroxy vitamin D25-hydroxy vitamin D-vitamin D-binding protein complex binds to the megalin receptor complex of proximal renal tubule cells and undergoes endocytosis. Once internalized, 25-hydroxy vitamin D25-hydroxy vitamin D is metabolized through one of two pathways: 1α hydroxylation by CYP27B1 to form the active metabolite 1,25-dihydroxyvitamin D or 24-hydroxylation by CYP24A1 to form an inactive metabolite 24,25-dihydroxyvitamin D (6, 11, 29, 98, 153, 337, 367, 492). The balance of the activities of these two enzymes is what determines the ultimate level of active vitamin D. Much of the regulation of these two enzymes is accomplished at the gene level. PTH, a major stimulator of active vitamin D production, increases the level of CYP27B1 activity (12, 29, 42, 228, 241). The effect on the level of CYP24A1 has been variably reported as slightly increased, insufficient to blunt the effect on Cyp27b1, or as decreased with the result being an increase in 1,25 vitamin D synthesis (725, 726). 1,25-dihydroxyvitamin D itself activates CYP24A1, limiting active vitamin D formation. FGF23, a major inhibitor of vitamin D formation, increases CYP24A1 activity, shunting 25-hydroxy vitamin D25-hydroxy vitamin D into the inactive metabolite pathway (290, 293, 427, 523, 526). In addition, FGF23 inhibits CYP27B1. Other factors influencing vitamin D conversion include aging, metabolic acidosis, chronic kidney disease, and a variety of other hormones (28, 30, 31, 153, 275, 385, 514-519, 635-637). Because of its short half-life of hours, 1,25 dihydroxyvitamin D is not considered a useful indicator of vitamin D status (88, 153, 718).
Inactivation of active vitamin D begins with 24-hydroxylation of 1,25 dihydroxyvitamin D, followed by oxidation at carbon 24 and sequential modification of the steroid culminating in the production of calcitroic acid (153, 326, 537). A carbon 23 oxidation pathway has also been described but its significance is unclear. Urinary excretion of the water-soluble calcitroic acid is the major mechanism for vitamin D disposal though a small amount is excreted through the gastrointestinal tract.
The other major source of vitamin D is the plant sterol, ergocalciferol or vitamin D2, which is present in the diet. Intestinal absorption and disposition are very similar to what is seen with vitamin D, although as discussed below, its interaction with DBP is weaker, a property that some investigators believe plays a significant role in their differential clinical efficacies (257).
Vitamin D-binding protein
DBP is central to the metabolism of vitamin D. Originally identified as a member of the albumin family and named GC globulin (group-specific component of serum) in 1959, this abundant serum protein was relabeled DBP after the discovery of this specific property in 1976 by Daiger and colleagues (154, 156, 178, 311, 461). Over 100 isoforms of the protein have been isolated by immunoelectrophoresis, divided into three major isoforms—GC1F, GC1S, and GC2, classified by amino acids at positions 416 and 420—and multiple minor isoforms, grouped around the major isoforms (9, 69, 154, 156, 575). These changes in amino acid sequence result in measurable differences in the glycosylation patterns, especially between the GC1 and GC2 isoforms. Genetic analysis has uncovered an even greater degree of variability with over 1000 variants identified in both major and minor isoforms. Specific patterns of isoform expression are associated with specific ethnicities, allowing the study of population migrations and genetics (69, 134, 175, 176, 575). Many of the genetic variations translate into differences in DBP binding to both 25 and 1,25 vitamin D. Interestingly, GC2 isoforms are very uncommon in the equatorial African populations, while GC1F is the most common. GC1S is seen most frequently in individuals of European ancestry while Asians exhibit both GC1S and GC1F. Binding efficiencies to vitamin D are GC1F>GC1S>GC2, correlating with skin pigmentation and leading to the hypothesis that the different isoforms arose in concert with changes in skin color to facilitate sun-stimulated vitamin D formation and metabolism (461, 470).
DBP is composed of three domains, one which expresses 10 α-helices forming a vitamin D binding cleft, a second which expresses nine α helices and one coil, while the third expresses four α helices (154, 156, 262). Circulating DBP vastly exceeds its vitamin D binding capacity and the protein also binds free actin, which limits actin polymerization and endothelial damage. DBP can also be modified to form DBP-MAF, macrophage-activating factor, which stimulates macrophage and osteoclast activation, thus serving roles in immune function and bone metabolism independent of vitamin D.
The major site of DBP production is the liver, lesser production measured in kidney, testis, and adipose tissue. The human gene exhibits 13 exons and 12 introns and its promoter expresses three binding sites for hepatocyte nuclear factor.
As indicated previously, DBP binds both 25 and 1,25 vitamin D as well as the parent compound, cholecalciferol, in the circulation, transporting the substrates to their intended targets. However, whether DBP is required for vitamin D activity remains an unanswered question. The mouse lacking DBP does not exhibit any discernible defects in bone and mineral metabolism; however, on a vitamin D deficient diet develops metabolic bone defects at an earlier time point than the normal controls (63, 563, 718). DBP appears to play a role in limiting active vitamin D formation, as injection of radiolabeled cholecalciferol into the DBP null mice resulted in a more rapid and enhanced liver uptake. Also, the addition of DBP to cell cultures of monocytes or culture of monocytes in serum derived from DBP-replete mice (when compared to monocytes cultured in serum derived from DBP-deficient mice) inhibited vitamin D-stimulated cathelicidin induction, the high affinity isoforms showing a greater effect than the lower affinity isoforms. These findings suggested that DBP was blunting the uptake of vitamin D into those cells, resulting in diminished responses (155). DBP may also serve as a storage mechanism for vitamin D. Vitamin D bound to DBP is endocytosed into renal proximal tubule cells through a megalin-cubulin-dependent process, allowing the body to recapture filtered 25-hydroxy vitamin D (336, 403, 473, 564). Interestingly, however, DBP null mice exhibit a less extreme phenotype than lrp2 (megalin)-deficient animals (336, 387, 403, 473, 484, 564), suggesting that vitamin D can gain entry into these cells through megalin-independent mechanisms. Likewise, DBP can enter cells that do not express megalin, highlighting the possibility of alternative mechanisms for vitamin D entry into cells.
Gene and its regulation
The gene encoding 25-hydroxy vitamin D 1α hydroxylase (CYP27B1) in humans is located on chromosome 12, 12q14.1, flanked by the genes for METTL21B, methyltransferase 21b; METTL1 methyltransferase like 1; MARCH9, a member of the E3 ubiquitin ligase family; CKD4, cyclin D kinase isoform 4; and mir6759, a microRNA. The gene contains nine exons, including a very large exon 9. The promoter region expresses sequences consistent with three cAMP response elements but no sequence corresponding to a vitamin D response element (108). Consistent with this structure, PTH and direct activators of cAMP such as forskolin increase transcription of the gene but vitamin D has no effect. These findings suggest that PTH stimulates vitamin D production through increased transcription of the CYP27B1 but the well described self-inhibition by 1,25 vitamin D is not mediated through an effect on gene transcription. In contrast, Murayama and colleagues (466) were able to identify a sequence that could serve as a negative regulatory site for 1,25 vitamin D. A recent study found that 1,25 vitamin D could bind to VDR in the proximal tubule brush border and translocate to the nucleus, accompanied by a decrease in CYP27B1 activity and an increase in CYP24A1 activity and suggesting that VDR functions as a sensor for active vitamin D status. Using transfected promoter constructs, Armbrecht et al. (29) reproduced the stimulatory effect of PTH and forskolin on CYP27B1 promoter activity, concluding that these effects were sufficient to explain the stimulation of 25-hydroxy vitamin D25-hydroxy vitamin D activation by PTH. They also showed that PTH and 1,25 vitamin D had a stimulatory effect on CYP24 (25-hydroxy vitamin D 24 hydroxylase) but had an interactive effect. The magnitude of the increase in CYP24 activity was not felt to be sufficient to explain the effect of these hormones on CYP24 mRNA levels. Although several cell types are capable of converting 25-hydroxy to 1,25-dihydroxyvitamin D, only kidney produces sufficient quantity to contribute to circulating levels under typical physiologic conditions. Yoshida et al. (708) have identified an enhancer in the promoter region of the gene specific to proximal tubule which may explain this observation.
Several other factors have been identified that regulate cyp24 and cyp27b1 expression. In mice, absence of the Npt2a (Slc34a1) results in increased cyp27b1 mRNA expression and decreased cyp24 mRNA expression, predictably leading to marked increases in active circulating 1,25 vitamin D (56). These animals have hypercalcemia, hypercalciuria, and nephrocalcinosis as well as hypophosphatemia. Whether this is a direct effect, a result of suppression of FGF23 by hypophosphatemia, or both, is not known. Absence of the other major type II sodium phosphate cotransport Npt2c (Slc34a3) has minimal effect on the mRNA expression of cyp27b1 but does result in significant suppression of cyp24 mRNA (331, 352, 577); thus, 1,25 vitamin D levels are increased as well. However, these animals present a phenotype that differs little from wild-type mice. Interestingly, kidney-specific inducible Npt2c deletion has no effect on phosphate, calcium, or vitamin D homeostasis, suggesting that the phenotype seen in the whole organism constitutive deletion model results from nonrenal phosphate transport mechanisms (469). The differential effects of the sodium phosphate cotransporters on vitamin D metabolism in humans have not been investigated at a molecular level. Unlike mice, humans lacking functional Npt2c exhibit a dramatic clinical phenotype characterized by rickets, hypophosphatemia, hypercalcemia, hypercalciuria, and high vitamin D levels while mutations in Npt2a present a rather mild phenotype of a tendency toward osteopenia and kidney stones (67, 166, 181, 317, 324, 379, 402, 530, 650, 713). The basis for these differences and their implications for regulation of vitamin D metabolism have not been determined. Dietary phosphate deficiency stimulates renal cyp27b1 activity through a growth hormone-dependent mechanism; the sensor for this response remains unknown but may be one of the proximal tubular phosphate transporters (708).
Multiple environmental factors determine the level of vitamin D, including season of the year, vitamin D intake, adiposity, and hormonal status, especially for women (159, 306, 399, 444, 559, 673, 688). Stimulation of renal cyp27B1 by estrogens (433, 510, 511, 536, 621) and the increase in circulating 1,25 vitamin D in pregnancy (536) were demonstrated over 30 years ago. Subsequent studies suggest that the increase in circulating vitamin D levels during pregnancy are of maternal kidney origin as an anephric pregnant woman failed to show the predicted increase (640). However, as this report predated the discovery of FGF23 which is capable of inhibiting extra-renal as well as renal conversion of 25 to 1,25 vitamin D, an exclusive role of the kidney in providing the increase in vitamin D during pregnancy cannot be claimed (37). Exogenous estrogens increase both 25 and 24,25 vitamin D in post-menopausal women, associated with an increase in serum calcium and a decrease in serum phosphorus (44). Cyp27b1 activity is increased by estrogens in extra-renal tissues as well including vascular smooth muscle cells (608); however, it is unlikely that the amount of 1,25 vitamin D produced would alter serum levels.
The level of vitamin D is also subject to significant genetic regulation (51, 482). Twin studies suggest a 43% to 70% heritability for 25-hydroxy vitamin D and a 65% heritability for the active vitamin D (205, 206, 692, 709). These studies have been supported by family studies as well. The few studies of African Americans and Hispanics suggest that heritability may also be influenced by ethnicity, as the estimates of heritability for both forms of vitamin D in these populations are significantly lower. These investigations, however, involve at most approximately 1000 individuals and frequently have been derived from studies of human illnesses such as asthma or multiple sclerosis. Thus, the applicability to the general population could be questioned. Linkage analysis, genome wide association studies, and candidate gene approaches to identify genes important in determining vitamin D levels have uncovered a number of potential candidates including CYP27B1; CYP2R1; CYP24A1; VDR; and DHCR7, the gene encoding the reductase that catalyzes the 7-dehydrocholesterol to cholesterol reaction in the skin; and GC, the gene encoding the DBP (9, 69, 114, 182, 665, 692). CYP27B1 is a member of the cytochrome P450 family most strongly associated with vitamin D levels, but multiple studies support potential contributions from each of the other candidate genes. The reasons for the discrepancies are not known but likely result from differences in study population, small sample sizes, and differences in analytic methods. The reader is referred to a recent review of this topic by Dastani et al. for a more in depth discussion (182).
Receptors
The vitamin D receptor, a member of the steroid hormone receptor superfamily, is expressed in a broad variety of tissues and exhibits significant fluidity in expression dependent on age, underlying pathophysiologic conditions, and even vitamin D status (83, 84, 98, 106, 151, 191). One interesting aspect of VDR is the radical change in expression pattern during development (259, 670). In the mature animal, the receptor is most abundant in the small and large intestine, distal tubule of the kidney nephron, and the parathyroid gland. Other tissues with significant expression include the pancreas, renal proximal tubule, bronchial cells, osteoblasts, thymus, pituitary gland, prostate, and mammary gland. Specifically, the receptor is minimal or not identified in liver, muscle, thyroid, adrenal gland, or nervous tissue, including the central nervous system. It is notable that tissue expression of 1αhydroxylase and the vitamin D receptor show significant overlap, setting the stage for autocrine function and feedback.
Upon interaction with 1,25 vitamin D, the VDR heterodimerizes with the retinoid X receptor (RXR), the complex migrating to the nucleus where it interacts with the nuclear chromatin through VDRE, vitamin D response elements (83, 331) (Figs. 3C and 6). This step also involves two additional layers of regulation (4, 83). First, the transfer of the vitamin D complex to the nucleus is performed by proteins of the Hsc70 chaperone family. Second, the VDRE may be occupied by RNA-binding proteins that compete with the vitamin D complex for occupancy (428). Investigation into the regulation of these two steps is in its infancy. Interaction of the vitamin D receptor complex with the VDRE in some tissues requires the presence of the Williams Syndrome Transcription Factor (WTSF) (49, 238), functioning as one of the subunits of the ATP-dependent chromatin remodeling complex known as WINAC, the WTSF including nucleosome assembly complex. The essential requirement for this interaction has been demonstrated in vitro in knockdown experiments as well as in the skin fibroblasts of Williams Syndrome patients (49, 177, 338, 339, 354, 355). WINAC downregulates VDR-stimulated activation of 1αhydroxylase and activates 24-hydroxylase, thus serving to limit active vitamin D formation (49, 338, 339).
Figure 6.
Vitamin D effector mechanisms. 25-Hydroxy-vitamin D bound to DBP is filtered, binds to the megalin complex and is endocytosed into the renal proximal tubule cell where it undergoes 1 α hydroxylation. Activated vitamin D bound to cofactors and chaperones (BAG-1 and hsc70) enter the nucleus and interacts with the VDR in concert with the RXR. This complex interacts with specific genomic regions including the VDRE to influence gene transcription.
Classically, the VDR-RXR complex has been thought to interact with DNA at specific motifs designated VDRE (vitamin D response elements), thus influencing transcription of its target genes (163, 414, 486, 498). The classic motif is a triple repeat of the PuGG/TTCA sequence separated by three base pairs. In fact, however, the number of gene sites occupied by VDR-RXR has been estimated to be between 2000 and 8000, and many of these genes do not express classic VDRE (124, 125, 561, 581, 639, 641, 667, 687). VDR-RXR bound to active 1,25 vitamin D binds DNA sites that differ from the unbound VDR. Furthermore, the VDR is capable of interacting with other transcription factors to influence their effects. Thus, failure to identify a VDRE motif in the gene of a protein of interest does not exclude the possibility that the gene may be regulated by vitamin D.
There is also evidence that vitamin D may have signaling mechanisms that are nongenomic, specifically activation of protein kinase C and MAP kinases. These pathways have not been studied as intensively as the classic pathway described earlier, but likely contribute substantively to vitamin D actions [reviewed in (456)].
Functions
Classical actions: Mineral metabolism intestine
The major recognized functions of vitamin D revolve around the regulation of mineral metabolism (Fig. 5). The most straightforward and accepted effect of vitamin D is enhanced intestinal absorption of both calcium and phosphate through the stimulation of increased expression of the transporters TRPV6 and Npt2b, respectively (43, 48, 107, 138, 139). Activated vitamin D increases the expression of the calcium channel, TRPV6, in the apical membrane of small intestine, enhancing intestinal cell calcium entry. Predictably, VDREs have been identified on the promoter region for the gene for TRPV6 that play a role in vitamin D stimulation of TRPV6 expression (43, 331, 446, 447). Vitamin D is also important for calbindin D expression but whether this occurs through a classic VDRE has not been established. Vitamin D regulation of the plasma membrane calcium ATPase, the pump responsible for the egress of calcium from the intestinal cell into the blood compartment, has been demonstrated in rats. Lack of significant homology in the VDREs of different species has cast doubt on the applicability of findings in animal models to humans. A recent study using culture explants from endoscopic samples of human duodenum confirmed enhanced expression of TRPV6 mRNA after 6h treatment with either 25 or 1,25 vitamin D (43). Interestingly, treatment with 25-hydroxyvitamin D resulted in increased protein expression of CYP27B1, identified predominantly in the crypt cells, and the increase in TRPV6 correlated strongly with the increase in CYP27B1 expression. These remarkable findings suggest that vitamin D regulation of intestinal calcium absorption may be a function of both autocrine and endocrine actions of vitamin D. How the two systems interact and why and/or when one system or the other will predominate are important questions to be addressed. 1,25-Dihydroxycholecalciferol also significantly increased the mRNA expression of PMCA1 and CYP24 but not calbindin-D9k.
Vitamin D also enhances intestinal phosphate absorption, though the mechanisms and proteins involved are unknown. Studies have shown that 1,25-dihydroxycholecalciferol does not increase the expression of Npt2b, the type IIb sodium dependent phosphate cotransporter, and that the increase in phosphate absorption is sodium-independent (352). A recent study did identify an active vitamin D analog, ED-71, that strongly increased Npt2b expression at the mRNA and protein level through a VDR-dependent mechanism, but had no effect on either Pit-1 or Pit-2, two other sodium-dependent phosphate transporters identified in intestinal epithelium (113). Interestingly, ED-71 also exhibited a profound stimulatory effect on CYP24, increasing levels ten thousand fold over active vitamin D. The authors also noted that serum concentrations of ED-71 level remained constant over greater than 24 h, whereas serum concentrations of active vitamin D levels promptly decreased to baseline within 6 h and suggested that the dramatic differences in pharmacokinetics accounted for the differences in phosphate stimulating potency. This study underscores the role of the rapid counterregulatory processes stimulated by active vitamin D in limiting its actions.
Kidney
1,25-Dihydroxyvitamin D enhances renal calcium reabsorption through stimulation of the expression of virtually all of the proteins involved in calcium transport in the distal nephron (42, 98). Mice lacking expression of CYP27B1 show decreased renal epithelial mRNA and protein expression of TRPV5, calbindin-D28k, calbindin-D9k, and NCX1, the basolateral Na-Ca exchanger responsible for transfer of reabsorbed calcium from the cell to the blood space (194, 301-305, 481). Treatment with calcitriol corrects all of these deficiencies while a high dietary calcium corrects all except for calbindin-D9k. Interestingly, while PTH has a similar effect on the same proteins, it may not be through a vitamin D-dependent mechanism. The coordinated increase in the expression of the calcium transport proteins is dependent on the increase in TRPV5 expression, TRPV5 deficient animals fail to respond to vitamin D with an increase in the additional calcium transport proteins. TRPV5 deficiency results in hypercalciuria, hypocalcemia, and compensatory elevations in 1,25 vitamin D. Thus, stimulation of TRPV5 expression is the key effect of vitamin D on renal calcium absorption (698).
The effect of vitamin D on renal phosphate transport remains very poorly understood (352). Mice-deficient in the VDR fail to express Npt2a and Npt2c but expression can be stimulated by feeding a high calcium diet suggesting that the expression of these two transporters is not dependent on vitamin D (333). The genes for both Npt2a and Npt2c exhibit potential VDRE but an effect of vitamin D on expression of the two proteins has not been specifically demonstrated.
Bone
The effect of vitamin D on bone has been extensively studied but remains somewhat enigmatic. Mice lacking VDR develop rickets; however, a diet providing high calcium and phosphate can rescue the bone phenotype, suggesting that the major contribution of vitamin D to bone health is provision of adequate mineral through stimulation of intestinal absorption (563). However, vitamin D clearly has direct effects on bone cells. Osteoblasts express VDR, which upon stimulation by vitamin D leads to the production of receptor activator of NFκB ligand (RANKL) which interacts with RANK on osteoclast precursors, resulting in osteoclast maturation (52, 612, 652). Vitamin D also inhibits the expression of the competing decoy receptor for RANKL, osteoprotegerin, contributing to osteoclast maturation. Another Vitamin D-responsive gene in osteoblasts is LRP5, the Wnt coreceptor that stimulates bone cell proliferation (52, 234, 331, 509). Vitamin D stimulation of osteoblasts and osteoblast precursors enhances the expression of osteoblast specific transcription factors such as RUNX2 and subsequently proteins such as osteocalcin, osteopontin, and alkaline phosphatase. The simultaneous activation of both osteoblasts and osteoclasts would result in an increase in bone turnover but whether the result is predominantly bone formation, bone resorption, or a neutral effect is somewhat unclear. The anti-rachitic effect, although thought to be primarily mediated by the effect of vitamin D on mineral homeostasis, may also result from direct effects on bone. Transgenic mice overexpressing VDR in osteoblasts show an increase in bone formation (376, 452). CYP27B1/VDR double knockout mice fed a rescue diet exhibit fewer osteoblasts, a lower mineralization rate, and decreased bone volume, when compared to control animals (500, 501). These studies are consistent with the hypothesis that the major effect of vitamin D on bone is formative and are consistent with much older studies showing that neither vitamin D deficiency nor vitamin D excess altered bone resorption rate as determined by increases in inner marrow diameter in growing chicks prelabeled with injections of radiolabeled calcium, tetracycline, and proline (358). In this study, chicks given excess vitamin D showed a decrease in bone radiolabeled calcium while the vitamin D-deficient animals showed no change in bone label. Under both conditions, bone mass decreased. The decrease in the bone mineral mass seen in vitamin D deficiency was attributed to lack of intestinal absorption of mineral whereas the decrease seen in vitamin D excess was attributed to failure of calcium reutilization. While these observations suggest a role for vitamin D on the formative arm of bone metabolism, the fact that vitamin D stimulates RANKL, leading to activation of osteoclasts would argue for a physiologic role in bone resorption as well. However, a recent study suggests vitamin D deficiency causes PTH elevation, which leads to cortical thinning and in some cases cause marrow fibrosis (13). An important unanswered question regarding vitamin D effect on bone is whether its effects change with organism age. In human studies, vitamin D administration prevents rickets in children and treats osteomalacia in adults (269, 306); however, the effect of vitamin D in the treatment of osteoporosis—whether vitamin D can prevent or reverse the bone loss associated with aging—remains a controversial topic (36, 542, 560).
Nonclassical actions
Kidney
A variety of other effects of vitamin D on kidney function apart from regulation of mineral metabolism has been described. VDR polymorphisms have been associated with differences in proximal tubule citrate handling, specifically, the presence of the bb genotype of BsmI and the TT genotype of TaqI are associated with hypocitraturia in stone formers when compared to nonstone formers or normocitraturic stone formers(460, 642, 723). Great interest has emerged from studies showing that vitamin D can have an antiproteinuric effect (186, 404, 609, 706), even in patients already on inhibitors of the renin angiotensin aldosterone system. Glomerular epithelial cells, podocytes, expresses VDR and activation of VDR increases podocyte synthesis and expression of nephrin, a key slit-membrane protein important for the integrity of the glomerular filtration apparatus (396, 609, 668). In diabetic animals or in podocytes exposed to high glucose, stimulation or overexpression of the VDR blocked podocyte apoptosis through inhibition of ERK1/2 and caspase 3 activation and blocked activation of the renin angiotensin axis (669).
Vasculature
Several studies support a role for activated vitamin D in maintenance of vascular health (14, 25, 253). VDR deficient mice fed a rescue diet to maintain mineral homeostasis show increased pulse pressure, stiffness of their blood vessels, and a higher heart weight/body weight ratio. Interestingly, blood pressure was not affected. The investigators demonstrated deficient nitric oxide synthesis in endothelial cells (480). Vitamin D blocks vascular plaque formation by inhibiting the ability of macrophages to take up cholesterol (204, 682). Interestingly, both vitamin D deficiency and excess reportedly accelerate atherosclerotic plaque formation (727). VDR deficiency enhances macrophage production of renin and angiotensin, important mediators of atherosclerosis (25, 144, 221, 263, 395, 594). Vitamin D deficiency has also been implicated in the development of preeclampsia (401, 408, 547).
Skin
In addition to being a major contributor to circulating vitamin D, the skin is another site of significant autocrine vitamin D production, active 1,25 vitamin D and a variety of unique vitamin D metabolites, some of which have demonstrable activities (599-602, 638). Epidermal CYP27B1 is required during differentiation of keratinocytes and is associated with the expression of important differentiation markers such as involucrin (84-86). However, the expression of CYP27B1, the VDR, and other components of vitamin D metabolism decrease as differentiation is completed. Interestingly, a variety of skin cancers show increased expression of the vitamin D metabolic pathway and in vitro studies suggest that active vitamin D may be a useful adjunctive therapy by virtue of its prodifferentiation, antiproliferative effects (320, 391-393, 453, 538-541, 600, 622). Vitamin D signaling may also play a role in the development and maintenance of the barrier function of the epithelium, through maintenance of the production of lamellar bodies and antimicrobial peptides (15, 502, 550, 566).
VDR null mice and humans with VDR mutations suffer from alopecia (191, 218, 421). The mechanism is thought to be the loss of dermal stem cells responsible for hair follicle recycling. However, vitamin D deficiency is not accompanied by alopecia. This apparent contradiction suggests alternative roles for the VDR and in fact the mechanism for this phenomenon appears to be a vitamin D independent interaction of VDR with the cWnt and hedgehog target genes (157, 400, 405, 406, 627).
Pancreas
Vitamin D has multiple effects on the pancreatic beta cells that express VDR including protection against cytokine damage (254, 276, 548, 694-696), suppression of the islet renin-angiotensin system (143), and regulation of genes encoding proteins that regulate including neuropeptide production, membrane trafficking, tight junction formation, and ion channels (147, 695). Vitamin D increases beta cell insulin production (717). Vitamin D may also indirectly protect islet cell function through provision of adequate calcium, as calcium deficiency has been demonstrated in some animal models to accelerate the development of diabetes (197, 529, 535, 610).
Immune system
Vitamin D has well-established immunomodulatory capabilities (40, 119, 120, 287, 296, 297). Macrophages are capable of producing significant quantities of active vitamin D from 25-hydroxy vitamin D, described by Adams et al in 1983 in studies utilizing alveolar macrophages from patients with sarcoidosis (7, 8). This phenomenon explains the occurrence of hypercalcemia in patients with sarcoidosis; however, the more physiologically relevant consequence of vitamin D production by macrophages is the significant contribution to the antibacterial activity against certain organisms as first reported by Rook in 1986 (553). Vitamin D enhances the production of cathelicidin, which in turn is responsible for the elaboration of antimicrobial peptides (261, 502, 566, 666, 674). VDR is present on activated but not quiescent lymphocytes and vitamin D exerts an antiproliferative effect on these cells. In addition, vitamin D blunts immunoglobulin and cytokine production by T cells. It is these immunomodulatory properties of vitamin D that have been invoked to explain the effects of vitamin D and/or vitamin D status on autoimmune disorders (2, 120, 224, 225, 397, 493, 494, 551, 605, 619, 705), progression of renal disease (263, 404), response to infections (268, 349, 711), obesity (661), and some cancers (55, 84, 240, 634).
Interaction with other hormones
Effect on other hormones involved in mineral metabolism
Parathyroid gland cells express VDR (52, 151, 180, 318). Stimulation of VDR in this tissue decreases PTH synthesis and secretion, inhibits PTH cell proliferation, and an increases the expression of the calcium sensing receptor. These diverse functions all contribute to a decrease in circulating PTH, thus preventing overcorrection of low calcium levels, the major stimulus to PTH secretion. The PTH gene expresses a VDRE which, when occupied by the VDR-RXR complex, exerts an inhibitory effect on PTH gene transcription, at least in part by displacing another regulatory factor, nuclear factor-Y. Notably, parathyroid gland cells express CYP27B1, thus are capable of generating active vitamin D from circulating vitamin D (82, 151, 241). How circulating and locally produced vitamin D interact to regulate PTH synthesis is not entirely clear; however, the secondary hyperparathyroidism seen with mild vitamin D deficiency may be explained by a deficiency in the autocrine production of active vitamin D (341). PTH, on the other hand, leads to enhanced renal production of 1,25 vitamin D through increasing Cyp27B1 expression and decreasing Cyp24 expression.
Vitamin D stimulates production of FGF23 from bone (52, 331, 363), while it represses the production of PHEX (phosphate-regulating endopeptidase homology, X-linked), an enzyme linked to the degradation and inactivation of FGF23. As would be predicted from both of these actions, treatment with 1,25 vitamin D increases serum FGF23, leading to phosphaturia. Bone cells themselves express CYP27B1; thus, vitamin D regulation of FGF23 production likely results from both circulating and autocrine vitamin D(622, 623). Animals lacking expression of the VDR have suppressed FGF23 levels, even when serum phosphate is corrected by diet, suggesting that vitamin D-stimulated FGF23 is, in fact, mediated through VDR (712). Interestingly, chondrocyte expression of VDR appears to play a key role as chondrocyte-specific VDR deletion results in reduced osteoblast FGF23 expression and enhanced renal 1,25 vitamin D production (438). FGF23 decreases 1,25 vitamin D expression through increasing the renal expression of cyp24, leading to enhanced vitamin D inactivation, and decreased renal expression of cyp27b1, leading to decreased vitamin D production (41). Animals lacking expression of FGF23 have remarkably elevated vitamin D levels associated with elevated cyp27b1 activity (295, 587, 596, 714). FGF23 inhibits vitamin D activation in nonrenal tissues including parathyroid gland (37, 153, 297, 370, 485, 536, 612), monocytes, and placenta.
Effect on hormones not specifically involved in regulation of mineral metabolism
Vitamin D interacts with the renin-angiotensin-aldosterone system at many levels (10, 76, 122, 144). Animals lacking expression of VDR or CYP27B1 show increased renin activity associated with the development of cardiac hypertrophy and hypertension, which can be ameliorated by administration of angiotensin converting enzyme inhibitors. Some, but not all, human studies have demonstrated an inverse relationship between vitamin D levels and renin levels, suggesting a role for vitamin D deficiency in the development of at least some forms of hypertension through activation of the renin angiotensin system (229, 230, 647). 1,25 Vitamin D suppresses ren-1c gene transcription in in vitro studies through interaction at a cAMP response element in the promoter region (365, 715).
Pathophysiology
Vitamin D deficiency
Vitamin D deficiency’s major clinical feature is the result of abnormal mineralization of osteoid in bone. In children, vitamin D deficiency presents as the clinical syndrome of rickets, characterized by decreased bone mineralization and faulty bone development as manifested by significant skeletal deformities including bowing of the legs, knock-knee deformity, flaring of the ends of the ribs (rachitic rosary), pigeon chest malformation, bossing of the skull, and kyphoscoliosis. Adults who develop vitamin D deficiency present a less dramatic clinical picture characterized predominantly by bone pain, low bone mineral density, and fractures. Frank hypocalcemia is infrequently seen, as these individuals will develop secondary hyperparathyroidism in response to the decrease in intestinal calcium absorption; however, hypophosphatemia due to the hyperparathyroidism can be seen. Lesser degrees of vitamin D deficiency, also sometimes termed vitamin D insufficiency, may be associated with no symptoms whatsoever, though decreased bone mass and secondary hyperparathyroidism of a mild degree are often seen.
Vitamin D deficiency has been implicated in a host of disorders including autoimmune diseases such as asthma and multiple sclerosis; cancers such as breast, colon, skin, and prostate cancer; hypertension; diabetes mellitus; and infections such as tuberculosis (see aforementioned Immune system section). These associations have been demonstrated through epidemiologic studies and have been supported experimentally to some extent. For example, breast and colon cancer cells in some individuals and in some experimental conditions express high levels of VDR (55, 157, 193, 240, 375, 392, 506, 580). Treatment with vitamin D suppresses proliferation. The mechanisms for suppression of proliferation that have been described are variable and may be tissue specific. Keratinocytes derived from animals lacking VDR show a higher expression of several oncogenes and a lower expression of several tumor suppressor genes. Jiang and Bikle (322) have shown parallel changes in the expression of long noncoding RNAs for these genes, suggesting that vitamin D may play a critical role in regulating tumor formation through this novel genetic mechanism. Other studies have demonstrated interaction of vitamin D signaling pathways with the Wnt signaling pathway, MAPKinase signaling pathways, and hedgehog signaling (199, 200, 234, 264, 451, 483, 492, 506, 509, 627, 628, 634). Vitamin D deficiency has been associated with the development of hypertension (272, 374, 378). Vitamin D-deficient animals show higher circulating angiotensin II levels and hypertension (394, 648). Vitamin D deficiency has also been associated with endothelial dysfunction, progression of kidney disease, and hyperparathyroidism, all of which may contribute toward the development of hypertension and cardiovascular disease (25, 76, 137, 263, 620). Despite a wealth of studies using either calcitriol or other vitamin D analogs, consistent definitive studies demonstrating that vitamin D therapy can either prevent or treat any of these chronic diseases are lacking (54, 94, 95, 152, 186, 198, 288, 356, 549, 579). The role of vitamin D in extra-skeletal chronic disease pathogenesis is an area of intense interest and research.
Vitamin D deficiency may occur due to poor dietary vitamin D intake, malabsorption syndromes, or suboptimal skin conversion due to lack of sun exposure. Specific ethnic groups exhibit lower vitamin D levels, particularly those of African origin (499, 508, 575, 611). Patients with nephrotic syndrome may become vitamin D deficient due to renal wasting of DBP (50). Certain medications such as antiepileptics cause enhanced vitamin D degradation, and therefore may result in clinical vitamin D deficiency (445, 689). Relative vitamin D deficiency can be seen in syndromes of elevated FGF23 such as X-linked or autosomal dominant hypophosphatemic rickets (220, 683). Vitamin D insufficiency can be seen in up to 70% of certain populations of individuals, such as the elderly, in particular those residing in nursing home facilities (518, 603, 636). True vitamin D deficiency occurs far less frequently.
Vitamin D excess
Excessive vitamin D levels and activity are manifested predominantly as hypercalcemia and hypercalciuria, with symptoms referable to these complications such as altered mental status, polyuria due to nephrogenic diabetes insipidus, nausea and constipation, kidney stones, and kidney failure (145, 662).
Vitamin D intoxication can occur with excessive ingestion of vitamin D supplements, uncommonly with over the counter cholecalciferol but relatively commonly with prescription active vitamin D supplements such as calcitriol. Granulomatous disorders may also be accompanied by high vitamin D levels, which will present as hypercalcemia. The most common is sarcoidosis, a poorly understood autoimmune disorder with a myriad of clinical manifestations including lymphadenopathy, pulmonary nodules and fibrosis, skin lesions, arthritis, neuropathy, and interstitial kidney disease (649). The characteristic lesion of the disease is non-caseating granulomas. The source of the high vitamin D levels is the epithelioid cells of macrophage origin lining the granulomas. Treatment with glucocorticosteroids is highly effective in suppressing the vitamin D production and ameliorating the hypercalcemia. High vitamin D levels are seen considerably less frequently in other granulomatous diseases such as tuberculosis (6, 59, 297, 649, 716). Primary phosphate wasting syndromes such as hereditary hypophosphatemic rickets with hypercalciuria, caused by mutations in one of the sodium phosphate cotransporters responsible for renal phosphate reabsorption, is associated with high vitamin D levels likely due to suppressed FGF23 levels (1, 56, 67, 181, 324). Mutations in Cyp24 have also been identified in individuals with hypercalcemia and high 1,25 vitamin D (319).
Pharmacology
Vitamin D adequacy is assessed by the measurement of serum 25-hydroxyvitamin D. The optimal level remains a source of controversy. Recent Institute of Medicine recommendations, based on an extensive review of multiple studies, suggest that 20 ng/mL is adequate for healthy adults and would not constitute an indication for supplement (22). Ingestion of 600 IU/day of cholecalciferol has been suggested as the minimum daily dietary requirement for maintenance of adequate vitamin D stores for those aged 71 or less, and 800 IU for those above 71 years of age. The upper limit of ingestion is 4000 IU daily. The major sources of dietary vitamin D, either fish oils such as cod liver oil, sardines or vitamin D fortified foods generally are not predictable sources of the daily requirements, as they are not eaten by many people. Twenty minutes of sunlight exposure three times per week has also been shown to result in production of adequate vitamin D in Caucasians. However, darker skinned peoples, older individuals, and those living at high latitudes may not be able to achieve adequate conversion through sunlight exposure. To ensure adequate vitamin D homeostasis, the more recent recommendations for adults suggest 1000 to 2000 IU/day of cholecalciferol (90). Alternative regimens using high dose intermittent therapy are also commonly used, for example, ergocalciferol 50,000 IU weekly or monthly. While some investigators feel that both forms of vitamin D, cholecalciferol or ergocalciferol, are equally efficacious, others suggest that cholecalciferol may be superior (257). Additionally, as indicated previously, there is evidence that high dose, bolus therapy may be less effective due to rapid tissue uptake and sequestration. Taken as prescribed, these compounds rarely produce hypercalcemia.
For individuals with kidney failure who are unable to activate 25-hydroxy vitamin D, an active form of vitamin D can be prescribed. Calcitriol, paracalcitol, and doxercalciferol are the three currently available formulations. Calcitriol is identical to native 1,25 vitamin D. Paracalcitol and doxercalciferol are modified analogs of ergocalciferol(430). Calcitriol and paracalcitol are themselves active compounds while doxercalciferol requires 25-hydroxylation in the liver to become activated (53, 439). The risk of hypercalcemia is much higher with the use of any of these compounds; therefore, patients on these medications should be monitored closely.
Conclusions
Vitamin D, while referred to in the singular, is in fact a family of secosteroid compounds. While 1,25-dihydroxycholecalciferol is recognized as the active circulating and autocrine form of the hormone, it is likely that at least some of the other metabolites may exert unrecognized effects. Vitamin D has widespread effects in a variety of organ systems where for the most part the VDR coexists with the cell machinery to manufacture and metabolize active vitamin D from inactive precursors. These intrinsic mechanisms are carefully regulated such that vitamin D itself, vitamin D precursors, and other hormones may activate synthesis of vitamin D, but simultaneously, mechanisms for limiting active hormone production are activated. In addition to its well-known function as a regulator of mineral metabolism, vitamin D also plays a critical role in skin function, immune regulation, and vascular health. Thus, abnormalities in vitamin D metabolism have been implicated in most if not all chronic disorders including diabetes, hypertension, cancer, and chronic kidney disease as well as a multitude of autoimmune disorders such as multiple sclerosis. Major gaps in our understanding of vitamin D metabolism and effects include the interacting and/or exclusive roles of circulating versus autocrine vitamin D, determinants of circulating levels of vitamin D precursors and metabolites, the genomic versus the nongenomic actions of vitamin D, the roles of the multiple vitamin D metabolites, and, of course, the role of vitamin D dysregulation in the genesis and maintenance of the wide variety of pathologies where it has been implicated.
Acknowledgements
This work was supported by the VA Merit Review Program (E. D. Lederer), the NIA (S. J. Khundmiri), and the University of Louisville (E. D. Lederer).
Dr. Lederer is an employee of the Department of Veterans Affairs. This work was performed in this capacity but represents the opinions of the authors alone, not the Department of Veterans Affairs.
References
- 1.Abe Y, Nagasaki K, Watanabe T, Abe T, Fukami M. Association between compound heterozygous mutations of SLC34A3 and hypercalciuria. Horm Res Paediatr 82: 65–71, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Adamczak DM, Nowak JK, Frydrychowicz M, Kaczmarek M, Sikora J. The role of Toll-like receptors and vitamin D in diabetes mellitus type 1–a review. Scand J Immunol 80: 75–84, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Adams AE, Bisello A, Chorev M, Rosenblatt M, Suva LJ. Arginine 186 in the extracellular N-terminal region of the human parathyroid hormone 1 receptor is essential for contact with position 13 of the hormone. Mol Endocrinol (Baltimore, Md) 12: 1673–1683, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Adams JS, Chen H, Chun R, Gacad MA, Encinas C, Ren S, Nguyen L, Wu S, Hewison M, Barsony J. Response element binding proteins and intracellular vitamin D binding proteins: NMovel regulators of vitamin D trafficking, action and metabolism. J Steroid Biochem Mol Biol 89-90: 461–465, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med 306: 722–725, 1982. [DOI] [PubMed] [Google Scholar]
- 6.Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT. Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol 144pa: 22–27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 72: 1856–1860, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JS, Singer FR, Gacad MA, Sharma OP, Hayes MJ, Vouros P, Holick MF. Isolation and structural identification of 1,25-dihydroxyvitamin D produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab 60: 960–966, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 19: 2739–2745, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajabshir S, Asif A, Nayer A. The effects of vitamin D on the renin-angiotensin system. J Nephropathol 3: 41–43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: Molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D. Endocrinology 138: 2233–2240, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Akerstrom G, Hellman P, Hessman O, Segersten U, Westin G. Parathyroid glands in calcium regulation and human disease. Ann N Y Acad Sci 1040: 53–58, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shoha A, Qiu S, Palnitkar S, Rao DS. Osteomalacia with bone marrow fibrosis due to severe vitamin D deficiency after a gastrointestinal bypass operation for severe obesity. Endocr Pract 15: 528–533, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: Is the evidence solid? Eur Heart J 34: 3691–3698, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ala-Houhala MJ, Karppinen T, Vahavihu K, Kautiainen H, Dombrowski Y, Snellman E, Schauber J, Reunala T. Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral vitamin D supplementation. Acta Derm Venereol 94: 146–151, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Albright F. A page out of the history of hyperparathyroidism. J Clin Endocrinol Metab 8: 637–657, 1948. [DOI] [PubMed] [Google Scholar]
- 17.Albright F, Bauer W, Claflin D, Cockrill JR. Studies in parathyroid physiology: III. The effect of phosphate ingestion in clinical hyperparathyroidism. J Clin Invest 11: 411–435, 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albright F, Reifenstein EC. The Parathyroid Glands and Metabolic Bone Diseases: Selected Studies. Baltimore: The Williams and Wilkins Company, 1948. [Google Scholar]
- 19.AP Alimov, Langub MC, Malluche HH, Koszewski NJ. Sp3/Sp1 in the parathyroid gland: Identification of an Sp1 deoxyribonucleic acid element in the parathyroid hormone promoter. Endocrinology 144: 3138–3147, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Alimov AP, Langub MC, Malluche HH, Park-Sarge OK, Koszewski NJ. Contrasting mammalian parathyroid hormone (PTH) promoters: nuclear factor-Y binds to a deoxyribonucleic acid element unique to the human PTH promoter and acts as a transcriptional enhancer. Endocrinology 145: 2713–2720, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Almqvist EG, Bondeson AG, Bondeson L, Svensson J. Increased markers of inflammation and endothelial dysfunction in patients with mild primary hyperparathyroidism. Scand J Clin Lab Invest 71: 139–144, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Aloia JF. Clinical Review: The 2011 report on dietary reference intake for vitamin D: Where do we go from here? J Clin Endocrinol Metab 96: 2987–2996, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Amizuka N, Lee HS, Kwan MY, Arazani A, Warshawsky H, Hendy GN, Ozawa H, White JH, Goltzman D. Cell-specific expression of the parathyroid hormone (PTH)/PTH-related peptide receptor gene in kidney from kidney-specific and ubiquitous promoters. Endocrinology 138: 469–481, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Anderson PH, Iida S, Tyson JH, Turner AG, Morris HA. Bone CYP27B1 gene expression is increased with high dietary calcium and in mineralising osteoblasts. J Steroid Biochem Mol Biol 121: 71–75, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Andrukhova O, Slavic S, Zeitz U, Riesen SC, Heppelmann MS, Ambrisko TD, Markovic M, Kuebler WM, Erben RG. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol (Baltimore, Md) 28: 53–64, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51: 621–628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araya Z, Tang W, Wikvall K. Hormonal regulation of the human sterol 27-hydroxylase gene CYP27A1. Biochem J 372: 529–534, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armbrecht HJ, Forte LR, Halloran BP. Effect of age and dietary calcium on renal 25(OH)D metabolism, serum 1,25(OH)2D, and pTh. Am J Physiol 246: E266–270, 1984. [DOI] [PubMed] [Google Scholar]
- 29.Armbrecht HJ, Hodam TL, Boltz MA. Hormonal regulation of 25-hydroxyvitamin D-1alpha-hydroxylase and 24-hydroxylase gene transcription in opossum kidney cells. Arch Biochem Biophys 409: 298–304, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Armbrecht HJ, Wongsurawat N, Paschal RE. Effect of age on renal responsiveness to parathyroid hormone and calcitonin in rats. J Endocrinol 114: 173–178, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D by kidney of rat. J Clin Invest 66: 1118–1123, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold A, Kim HG, Gaz RD, Eddy RL, Fukushima Y, Byers MG, Shows TB, Kronenberg HM. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest 83: 2034–2040, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, Gallagher J, Wild Y, Saucier K. Molecular pathogenesis of primary hyperparathyroidism. J Bone Miner Res 17(Suppl 2): N30–36, 2002. [PubMed] [Google Scholar]
- 34.Assil IQ, Qi LJ, Arai M, Shomali M, Abou-Samra AB. Juxtamembrane region of the amino terminus of the corticotropin releasing factor receptor type 1 is important for ligand interaction. Biochemistry 40: 1187–1195, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Aurbach GD. Isolation of parathyroid hormone after extraction with phenol. J Biol Chem 234: 3179–3181, 1959. [PubMed] [Google Scholar]
- 36.Avenell A, Gillespie WJ, Gillespie LD, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev 4: Cd000227, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 28: 46–55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 69: 495–503, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Bacic D, Wagner CA, Hernando N, Kaissling B, Biber J, Murer H. Novel aspects in regulated expression of the renal type IIa Na/Pi-cotransporter. Kidney Int Suppl S5–s12, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: Modulator of the immune system. Curr Opin Pharmacol 10: 482–496, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145: 5269–5279, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Bajwa A, Forster MN, Maiti A, Woolbright BL, Beckman MJ. Specific regulation of CYP27B1 and VDR in proximal versus distal renal cells. Arch Biochem Biophys 477: 33–42, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Balesaria S, Sangha S, Walters JR. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol 297: G1193–1197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal N, Katz R, de Boer IH, Kestenbaum B, Siscovick DS, Hoofnagle AN, Tracy R, Laughlin GA, Criqui MH, Budoff MJ, Li D, Ix JH. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab 98: 4890–4898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbier JR, Gardella TJ, Dean T, MacLean S, Potetinova Z, Whitfield JF, Willick GE. Backbone-methylated analogues of the principle receptor binding region of human parathyroid hormone. Evidence for binding to both the N-terminal extracellular domain and extracellular loop region. J Biol Chem 280: 23771–23777, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Barbier JR, MacLean S, Morley P, Whitfield JF, Willick GE. Structure and activities of constrained analogues of human parathyroid hormone and parathyroid hormone-related peptide: Implications for receptor-activating conformations of the hormones. Biochemistry 39: 14522–14530, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Barden JA, Kemp BE. NMR solution structure of human parathyroid hormone(1-34). Biochemistry 32: 7126–7132, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Barley NF, Prathalingam SR, Zhi P, Legon S, Howard A, Walters JR. Factors involved in the duodenal expression of the human calbindin-D9k gene. Biochem J 341(Pt 3): 491–500, 1999. [PMC free article] [PubMed] [Google Scholar]
- 49.Barnett C, Krebs JE. WSTF does it all: A multifunctional protein in transcription, repair, and replication. Biochem Cell Biol 89:12–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barragry JM, France MW, Carter ND, Auton JA, Beer M, Boucher BJ, Cohen RD. Vitamin-D metabolism in nephrotic syndrome. Lancet 2: 629–632, 1977. [DOI] [PubMed] [Google Scholar]
- 51.Barry EL, Rees JR, Peacock JL, Mott LA, Amos CI, Bostick RM, Figueiredo JC, Ahnen DJ, Bresalier RS, Burke CA, Baron JA. Genetic variants in CYP2R1, CYP24A1, and vDr modify the efficacy of vitamin D supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab 99: E2133–E2137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HAt, Hsieh JC, Slater SA, Hsieh G, Kaczmarska M, Jurutka PW, Kolek OI, Ghishan FK, Haussler MR. 1,25-Dihydroxyvitamin D/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol 103:381–388, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Beaubrun AC, Brookhart MA, Sleath B, Wang L, Kshirsagar AV. Trends and variations in intravenous vitamin D use among hemodialysis patients in the United States. Ren Fail 35: 1–8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyere O. The effects of vitamin D on skeletal muscle strength, muscle mass and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 99: 4336–4345, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Beaudin SG, Robilotto S, Welsh J. Comparative regulation of gene expression by 1,25-dihydroxyvitamin D in cells derived from normal mammary tissue and breast cancer. J Steroid Biochem Mol Biol 148: 96–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A 95: 5372–5377, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behar V, Pines M, Nakamoto C, Greenberg Z, Bisello A, Stueckle SM, Bessalle R, Usdin TB, Chorev M, Rosenblatt M, Suva LJ. The human PTH2 receptor: binding and signal transduction properties of the stably expressed recombinant receptor. Endocrinology 137:2748–2757, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Belcher R, Metrailer AM, Bodenner DL, Stack BC Jr. Characterization of hyperparathyroidism in youth and adolescents: A literature review. Int J Pediatr Otorhinolaryngol 77: 318–322, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Bell NH, Shary J, Shaw S, Turner RT. Hypercalcemia associated with increased circulating 1,25 dihydroxyvitamin D in a patient with pulmonary tuberculosis. Calcif Tissue Int 37: 588–591, 1985. [DOI] [PubMed] [Google Scholar]
- 60.Bell O, Gaberman E, Kilav R, Levi R, Cox KB, Molkentin JD, Silver J, Naveh-Many T. The protein phosphatase calcineurin determines basal parathyroid hormone gene expression. Mol Endocrinol (Baltimore, Md) 19: 516–526, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Bell O, Silver J, Naveh-Many T. Identification and characterization of cis-acting elements in the human and bovine PTH mRNA 3′-untranslated region. J Bone Miner Res 20: 858–866, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone 54:250–257, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berg JP. Vitamin D-binding protein prevents vitamin D deficiency and presents vitamin D for its renal activation. Eur J Endocrinol 141: 321–322, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Bergwitz C, Bastepe M. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359: 2615–2616; author reply 2616-2617, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Bergwitz C, Gardella TJ, Flannery MR, Potts JT Jr, Kronenberg HM, Goldring SR, Juppner H. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin. Evidence for a common pattern of ligand-receptor interaction. J Biol Chem 271: 26469–26472, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 61: 91–104, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berndt TJ, Craig TA, McCormick DJ, Lanske B, Sitara D, Razzaque MS, Pragnell M, Bowe AE, O’Brien sP, Schiavi SC, Kumar R. Biological activity of FGF-23 fragments. Pflugers Arch 454: 615–623, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berry D, Hypponen E. Determinants of vitamin D status: Focus on genetic variations. Curr Opin Nephrol Hypertens 20: 331–336, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Berson SA, Yalow RS. Clinical applications of radioimmunoassay of plasma parathyroid hormone. Am J Med 50: 623–629, 1971. [DOI] [PubMed] [Google Scholar]
- 71.Berson SA, Yalow RS. Immunochemical heterogeneity of parathyroid hormone in plasma. J Clin Endocrinol Metab 28: 1037–1047, 1968. [DOI] [PubMed] [Google Scholar]
- 72.Berson SA, Yalow RS. Radioimmunoassays of peptide hormones in plasma. N Engl J Med 277: 640–647, 1967. [DOI] [PubMed] [Google Scholar]
- 73.Betterle C, Garelli S, Presotto F. Diagnosis and classification of autoimmune parathyroid disease. Autoimmun Rev 13: 417–422, 2014. [DOI] [PubMed] [Google Scholar]
- 74.Bettoun JD, Kwan MY, Minagawa M, Alpert LC, Goodyer CG, Hendy GN, Goltzman D, White JH. Methylation patterns of human parathyroid hormone (PTH)/PTH-related peptide receptor gene promoters are established several weeks prior to onset of their function. Biochem Biophys Res Commun 267: 482–487, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Bettoun JD, Minagawa M, Hendy GN, Alpert LC, Goodyer CG, Goltzman D, White JH. Developmental upregulation of human parathyroid hormone (PTH)/PTH-related peptide receptor gene expression from conserved and human-specific promoters. J Clin Invest 102: 958–967, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int 24: 2167–2180, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Bhattacharyya MH, DeLuca HF. Comparative studies on the 25-hydroxylation of vitamin D 3 and dihydrotachysterol 3. J Biol Chem 248: 2974–2977, 1973. [PubMed] [Google Scholar]
- 78.Bhattacharyya MH, DeLuca HF. The regulation of calciferol-25-hydroxylase in the chick. Biochem Biophys Res Commun 59: 734–741, 1974. [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharyya MH, DeLuca HF. The regulation of rat liver calciferol-25-hydroxylase. J Biol Chem 248: 2969–2973, 1973. [PubMed] [Google Scholar]
- 80.Bhattacharyya MH, DeLuca HF. Subcellular location of rat liver calciferol-25-hydroxylase. Arch Biochem Biophys 160: 58–62, 1974. [DOI] [PubMed] [Google Scholar]
- 81.Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: State of the field and future directions. Trends Endocrinol Metab 23:610–618, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94: 26–34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21: 319–329, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bikle DD. The vitamin D receptor: A tumor suppressor in skin. Adv Exp Med Biol 810: 282–302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122: 984–992, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function. J Steroid Biochem Mol Biol 89-90: 347–353, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Bikle DD, Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology 124: 649–654, 1989. [DOI] [PubMed] [Google Scholar]
- 88.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: A reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab 61: 969–975, 1985. [DOI] [PubMed] [Google Scholar]
- 89.Bilezikian JP, Khan A, Potts JT Jr, Brandi ML, Clarke BL, Shoback D, Juppner H, D’Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J. Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 26: 2317–2337, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol 810: 500–525, 2014. [DOI] [PubMed] [Google Scholar]
- 91.Bisello A, Adams AE, Mierke DF, Pellegrini M, Rosenblatt M, Suva LJ, Chorev M. Parathyroid hormone-receptor interactions identified directly by photocross-linking and molecular modeling studies. J Biol Chem 273: 22498–22505, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL. Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J Biol Chem 277: 38524–38530, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Bisello A, Greenberg Z, Behar V, Rosenblatt M, Suva LJ, Chorev M. Role of glycosylation in expression and function of the human parathyroid hormone/parathyroid hormone-related protein receptor. Biochemistry 35: 15890–15895, 1996. [DOI] [PubMed] [Google Scholar]
- 94.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev 6: Cd007469, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr 142: 1102–1108, 2012. [DOI] [PubMed] [Google Scholar]
- 96.Blaine J, Okamura K, Giral H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M. PTH-induced internalization of apical membrane NaPi2a: role of actin and myosin VI. Am J Physiol Cell Physiol 297: C1339–C1346, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blaine J, Weinman EJ, Cunningham R. The regulation of renal phosphate transport. Adv Chronic Kidney Dis 18: 77–84, 2011. [DOI] [PubMed] [Google Scholar]
- 98.Blomberg Jensen M, Andersen CB, Nielsen JE, Bagi P, Jorgensen A, Juul A, Leffers H. Expression of the vitamin D receptor, 25-hydroxylases, 1alpha-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J Steroid Biochem Mol Biol 121: 376–382, 2010. [DOI] [PubMed] [Google Scholar]
- 99.Blomstrand S, Claesson I, Save-Soderbergh J. A case of lethal congenital dwarfism with accelerated skeletal maturation. Pediatr Radiol 15: 141–143, 1985. [DOI] [PubMed] [Google Scholar]
- 100.Blum JW, Fischer JA, Schwoerer D, Hunziker W, Binswanger U. Acute parathyroid hormone response: sensitivity, relationship to hypocalcemia, and rapidity. Endocrinology 95: 753–759, 1974. [DOI] [PubMed] [Google Scholar]
- 101.Blum JW, Kunz P, Rodriguez SM, Fischer JA. Parathyroid hormone response to hypocalcaemia following hypercalcaemia. Acta Endocrinol (Copenh) 96: 75–80, 1981. [DOI] [PubMed] [Google Scholar]
- 102.Blunt JW, DeLuca HF, Schnoes HK. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D. Biochemistry 7:3317–3322, 1968. [DOI] [PubMed] [Google Scholar]
- 103.Blunt JW, Tanaka Y, DeLuca HF. The biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D. Proc Natl Acad Sci U S A 61: 717–718, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol 130: 546–553, 2010. [DOI] [PubMed] [Google Scholar]
- 105.Born W, Loveridge N, Petermann JB, Kronenberg HM, Potts JT Jr, Fischer JA. Inhibition of parathyroid hormone bioactivity by human parathyroid hormone (PTH)-(3-84) and PTH-(8-84) synthesized in Escherichia coli. Endocrinology 123: 1848–1853, 1988. [DOI] [PubMed] [Google Scholar]
- 106.Bouillon R, Lieben L, Mathieu C, Verstuyf A, Carmeliet G. Vitamin D action: Lessons from VDR and Cyp27b1 null mice. Pediatr Endocrinol Rev 10(Suppl 2): 354–366, 2013. [PubMed] [Google Scholar]
- 107.Boyle IT, Miravet L, Gray RW, Holick MF, Deluca HF. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology 90: 605–608, 1972. [DOI] [PubMed] [Google Scholar]
- 108.Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, DeLuca HF. Parathyroid hormone activation of the 25-hydroxyvitamin D-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci U S A 95: 1387–1391, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Breusegem SY, Takahashi H, Giral-Arnal H, Wang X, Jiang T, Verlander JW, Wilson P, Miyazaki-Anzai S, Sutherland E, Caldas Y, Blaine JT, Segawa H, Miyamoto K, Barry NP, Levi M. Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol 297: F350–361, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bringhurst FR, Segre GV, Lampman GW, Potts JT Jr. Metabolism of parathyroid hormone by Kupffer cells: Analysis by reverse-phase high-performance liquid chromatography. Biochemistry 21:4252–4258, 1982. [DOI] [PubMed] [Google Scholar]
- 111.Bringhurst FR, Stern AM, Yotts M, Mizrahi N, Segre GV, Potts JT Jr. Peripheral metabolism of [35S]parathyroid hormone in vivo: Influence of alterations in calcium availability and parathyroid status. J Endocrinol 122: 237–245, 1989. [DOI] [PubMed] [Google Scholar]
- 112.Bringhurst FR, Stern AM, Yotts M, Mizrahi N, Segre GV, Potts JT Jr. Peripheral metabolism of PTH: Fate of biologically active amino terminus in vivo. Am J Physiol 255: E886–893, 1988. [DOI] [PubMed] [Google Scholar]
- 113.Brown AJ, Zhang F, Ritter CS. Thevitamin D analog ED-71 is a potent regulator of intestinal phosphate absorption and NaPi-IIb. Endocrinology 153:5150–5156, 2012. [DOI] [PubMed] [Google Scholar]
- 114.Bu FX, Armas L, Lappe J, Zhou Y, Gao G, Wang HW, Recker R, Zhao LJ. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum Genet 128: 549–556, 2010. [DOI] [PubMed] [Google Scholar]
- 115.Caldas YA, Giral H, Cortazar MA, Sutherland E, Okamura K, Blaine J, Sorribas V, Koepsell H, Levi M. Liver X receptor-activating ligands modulate renal and intestinal sodium-phosphate transporters. Kidney Int 80: 535–544, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS biology 7: e1000172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Canterbury JM, Levey GS, Reiss E. Activation of renal cortical adenylate cyclase by circulating immunoreactive parathyroid hormone fragments. J Clin Invest 52: 524–527, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Canterbury JM, Reiss E. Multiple immunoreactive molecular forms of parathyroid hormone in human serum. 1. Proc Soc Exp Biol Med (New York, NY) 140: 1393–1398, 1972. [DOI] [PubMed] [Google Scholar]
- 119.Cantorna MT. Why do T cells express the vitamin D receptor? Ann N Y Acad Sci 1217: 77–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci 1317: 70–75, 2014. [DOI] [PubMed] [Google Scholar]
- 121.Capuano P, Bacic D, Roos M, Gisler SM, Stange G, Biber J, Kaissling B, Weinman EJ, Shenolikar S, Wagner CA, Murer H. Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol 292: C927–C934, 2007. [DOI] [PubMed] [Google Scholar]
- 122.Carbone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol 6: 260–276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cardinali DP, Ladizesky MG. Changes in parathyroid hormone and calcium levels after superior cervical ganglionectomy of rats. Neuroendocrinology 40: 291–296, 1985. [DOI] [PubMed] [Google Scholar]
- 124.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol 5: 167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carlberg C. The physiology of vitamin D-far more than calcium and bone. Front Physiol 5: 335, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carling T, Szabo E, Bai M, Ridefelt P, Westin G, Gustavsson P, Trivedi S, Hellman P, Brown EM, Dahl N, Rastad J. Familial hypercalcemia and hypercalciuria caused by a novel mutation in the cytoplasmic tail of the calcium receptor. J Clin Endocrinol Metab 85: 2042–2047, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Carling T, Udelsman R. Parathyroid surgery in familial hyperparathyroid disorders. J Intern Med 257: 27–37, 2005. [DOI] [PubMed] [Google Scholar]
- 128.Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, Gillanders EM, Kennedy AM, Chen JD, Agarwal SK, Sood R, Jones MP, Moses TY, Haven C, Petillo D, Leotlela PD, Harding B, Cameron D, Pannett AA, Hoog A, Heath H III, James-Newton LA, Robinson B, Zarbo RJ, Cavaco BM, Wassif W, Perrier ND, Rosen IB, Kristoffersson U, Turnpenny PD, Farnebo LO, Besser GM, Jackson CE, Morreau H, Trent JM, Thakker RV, Marx SJ, Teh BT, Larsson C, Hobbs MR. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 32: 676–680, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Castro M, Dicker F, Vilardaga JP, Krasel C, Bernhardt M, Lohse MJ. Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins. Endocrinology 143: 3854–3865, 2002. [DOI] [PubMed] [Google Scholar]
- 130.Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci U S A 102:16084–16089, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caulfield MP, McKee RL, Goldman ME, Duong LT, Fisher JE, Gay CT, DeHaven PA, Levy JJ, Roubini E, Nutt RF, Chorev M, Rosenblatt M. The bovine renal parathyroid hormone (PTH) receptor has equal affinity for two different amino acid sequences: the receptor binding domains of PTH and PTH-related protein are located within the 14-34 region. Endocrinology 127: 83–87, 1990. [DOI] [PubMed] [Google Scholar]
- 132.Cavaco BM, Guerra L, Bradley KJ, Carvalho D, Harding B, Oliveira A, Santos MA, Sobrinho LG, Thakker RV, Leite V. Hyperparathyroidism-jaw tumor syndrome in Roma families from Portugal is due to a founder mutation of the HRPT2 gene. J Clin Endocrinol Metab 89: 1747–1752, 2004. [DOI] [PubMed] [Google Scholar]
- 133.Cavaco BM, Santos R, Felix A, Carvalho D, Lopes JM, Domingues R, Sirgado M, Rei N, Fonseca F, Santos JR, Sobrinho L, Leite V. Identification of de novo germline mutations in the HRPT2 gene in two apparently sporadic cases with challenging parathyroid tumor diagnoses. Endocr Pathol 22: 44–52, 2011. [DOI] [PubMed] [Google Scholar]
- 134.Cavalli-Sforza LL, Daiger SP, Rummel DP. Detection of genetic variation with radioactive ligands. I. Electrophoretic screening of plasma proteins with a selected panel of compounds. Am J Hum Genet 29: 581–592, 1977. [PMC free article] [PubMed] [Google Scholar]
- 135.Chan HL, McCauley LK. Parathyroid hormone applications in the craniofacial skeleton. J Dent Res 92: 18–25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen JD, Morrison C, Zhang C, Kahnoski K, Carpten JD, Teh BT. Hyperparathyroidism-jaw tumour syndrome. J Intern Med 253: 634–642, 2003. [DOI] [PubMed] [Google Scholar]
- 137.Chen S, Gardner DG. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. J Steroid Biochem Mol Biol 136: 150–155, 2013. [DOI] [PubMed] [Google Scholar]
- 138.Chen TC, Castillo L, Korycka-Dahl M, DeLuca HF. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr 104: 1056–1060, 1974. [DOI] [PubMed] [Google Scholar]
- 139.Chen TC, DeLuca HF. Receptors of 1,25-dikydroxycholecalciferol in rat intestine. J Biol Chem 248: 4890–4895, 1973. [PubMed] [Google Scholar]
- 140.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science (New York, NY) 301: 1391–1394, 2003. [DOI] [PubMed] [Google Scholar]
- 141.Chen Z, Xu P, Barbier JR, Willick G, Ni F. Solution structure of the osteogenic 1-31 fragment of the human parathyroid hormone. Biochemistry 39: 12766–12777, 2000. [DOI] [PubMed] [Google Scholar]
- 142.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 101: 7711–7715, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng Q, Boucher BJ, Leung PS. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia 56: 553–562, 2013. [DOI] [PubMed] [Google Scholar]
- 144.Cheng Q, Li YC, Boucher BJ, Leung PS. A novel role for vitamin D: Modulation of expression and function of the local renin-angiotensin system in mouse pancreatic islets. Diabetologia 54: 2077–2081, 2011. [DOI] [PubMed] [Google Scholar]
- 145.Chesney RW. Vitamin D: Can an upper limit be defined? J Nutr 119: 1825–1828, 1989. [DOI] [PubMed] [Google Scholar]
- 146.Chobanian MC, Hammerman MR. Parathyroid hormone stimulates ammoniagenesis in canine renal proximal tubular segments. Am J Physiol 255: F847–852, 1988. [DOI] [PubMed] [Google Scholar]
- 147.Choi M, Ozeki J, Hashizume M, Kato S, Ishihara H, Makishima M. Vitamin D receptor activation induces peptide YY transcription in pancreatic islets. Endocrinology 153: 5188–5199, 2012. [DOI] [PubMed] [Google Scholar]
- 148.Chorev M. Parathyroid hormone 1 receptor: Insights into structure and function. Receptors Channels 8: 219–242, 2002. [PubMed] [Google Scholar]
- 149.Chorev M, Behar V, Yang Q, Rosenblatt M, Mammi S, Maretto S, Pellegrini M, Peggion E. Conformation of parathyroid hormone antagonists by CD, NMR, and molecular dynamics simulations. Biopolymers 36: 485–495, 1995. [DOI] [PubMed] [Google Scholar]
- 150.Chorev M, Goldman ME, McKee RL, Roubini E, Levy JJ, Gay CT, Reagan JE, Fisher JE, Caporale LH, Golub EE, Caulfield MP, Nutt RF, Rosenblatt M. Modifications of position 12 in parathyroid hormone and parathyroid hormone related protein: toward the design of highly potent antagonists. Biochemistry 29: 1580–1586, 1990. [DOI] [PubMed] [Google Scholar]
- 151.Chow EC, Quach HP, Vieth R, Pang KS. Temporal changes in tissue 1alpha,25-dihydroxyvitamin D, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D treatment in mice. Am J Physiol Endocrinol Metab 304: E977–989, 2013. [DOI] [PubMed] [Google Scholar]
- 152.Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ (Clinical research ed) 348: g1903, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: Metabolism. Endocrinol Metab Clin North Am 39: 243–253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct 30: 445–456, 2012. [DOI] [PubMed] [Google Scholar]
- 155.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab 95: 3368–3376, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol 144pa: 132–137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A 104: 9428–9433, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D. Lancet 1: 74–76, 1982. [DOI] [PubMed] [Google Scholar]
- 159.Clemens TL, Adams JS, Nolan JM, Holick MF. Measurement of circulating vitamin D in man. Clin Chim Acta 121: 301–308, 1982. [DOI] [PubMed] [Google Scholar]
- 160.Collip JB. Clinical use of the parathyroid hormone. Can Med Assoc J 15: 1158, 1925. [PMC free article] [PubMed] [Google Scholar]
- 161.Collip JB. The internal secretion of the parathyroid glands. Proc Natl Acad Sci U S A 11: 484–485, 1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Collip JB. The physiology of the parathyroid glands. Can Med Assoc J 24: 646–654, 1931. [PMC free article] [PubMed] [Google Scholar]
- 163.Colnot S, Lambert M, Blin C, Thomasset M, Perret C. Identification of DNA sequences that bind retinoid X receptor-1,25(OH)2D-receptor heterodimers with high affinity. Mol Cell Endocrinol 113: 89–98, 1995. [DOI] [PubMed] [Google Scholar]
- 164.Cook M, Molto E, Anderson C. Possible case of hyperparathyroidism in a Roman period skeleton from the Dakhleh Oasis, Egypt, diagnosed using bone histomorphometry. Am J Phys Anthropol 75: 23–30, 1988. [DOI] [PubMed] [Google Scholar]
- 165.Costa AG, Bilezikian JP. Bone turnover markers in primary hyperparathyroidism. J Clin Densitom 16: 22–27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Courbebaisse M, Leroy C, Bakouh N, Salaun C, Beck L, Grandchamp B, Planelles G, Hall RA, Friedlander G, Prie D. A new human NHERF1 mutation decreases renal phosphate transporter NPT2a expression by a PTH-independent mechanism. PloS One 7: e34764, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cunningham R, Biswas R, Steplock D, Shenolikar S, Weinman E. Role of NHERF and scaffolding proteins in proximal tubule transport. Urol Res 38: 257–262, 2010. [DOI] [PubMed] [Google Scholar]
- 168.Cunningham R, E X, Steplock D, Shenolikar S, Weinman EJ. Defective PTH regulation of sodium-dependent phosphate transport in NHERF-1−/− renal proximal tubule cells and wild-type cells adapted to low-phosphate media. Am J Physiol Renal Physiol 289: F933–F938, 2005. [DOI] [PubMed] [Google Scholar]
- 169.Cunningham R, Esmaili A, Brown E, Biswas RS, Murtazina R, Donowitz M, Dijkman HB, van der Vlag J, Hogema BM, De Jonge HR, Shenolikar S, Wade JB, Weinman EJ. Urine electrolyte, mineral, and protein excretion in NHERF-2 and NHERF-1 null mice. Am J Physiol Renal Physiol 294: F1001–F1007, 2008. [DOI] [PubMed] [Google Scholar]
- 170.Cunningham R, Steplock D, Wang F, Huang H, E X, Shenolikar S, Weinman EJ. Defective parathyroid hormone regulation of NHE3 activity and phosphate adaptation in cultured NHERF-1−/− renal proximal tubule cells. J Biol Chem 279: 37815–37821, 2004. [DOI] [PubMed] [Google Scholar]
- 171.Cusano NE, Rubin MR, Bilezikian JP. PTH(1-84) replacement therapy for the treatment of hypoparathyroidism. Expert Rev Endocrinol Metab 10:5–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cusano NE, Silverberg SJ, Bilezikian JP. Normocalcemic primary hyperparathyroidism. J Clin Densitom 16: 33–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.D’Amour P, Huet PM. Evidence of two forms of hepatic extraction of parathyroid hormone in dogs in vivo. Am J Physiol 246: E249–E255, 1984. [DOI] [PubMed] [Google Scholar]
- 174.D’Amour P, Huet PM, Segre GV, Rosenblatt M. Characteristics of bovine parathyroid hormone extraction by dog liver in vivo. Am J Physiol 241: E208–214, 1981. [DOI] [PubMed] [Google Scholar]
- 175.Daiger SP, Cavalli-Sforza LL. Detection of genetic variation with radioactive ligands. II. Genetic variants of vitamin D-labeled group-specific component (Gc) proteins. Am J Hum Genet 29: 593–604, 1977. [PMC free article] [PubMed] [Google Scholar]
- 176.Daiger SP, Miller M, Chakraborty R. Heritability of quantitative variation at the group-specific component (Gc) locus. Am J Hum Genet 36: 663–676, 1984. [PMC free article] [PubMed] [Google Scholar]
- 177.Daiger SP, Miller M, Romeo G, Parsons M, Cavalli-Sforza LL. Vitamin-D-binding protein in the Williams syndrome and idiopathic hypercalcemia. N Engl J Med 298: 687–688, 1978. [PubMed] [Google Scholar]
- 178.Daiger SP, Schanfield MS, Cavalli-Sforza LL. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci U S A 72: 2076–2080, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dambacher MA, Fischer JA, Hunziker WH, Born W, Moran J, Roth HR, Delvin EE, Glorieux FH. Distribution of circulating immunoreactive components of parathyroid hormone in normal subjects and in patients with primary and secondary hyperparathyroidism: The role of the kidney and of the serum calcium concentration. Clin Sci (London, England: 1979) 57: 435–443, 1979. [DOI] [PubMed] [Google Scholar]
- 180.Darwish HM, DeLuca HF. Identification of a transcription factor that binds to the promoter region of the human parathyroid hormone gene. Arch Biochem Biophys 365: 123–130, 1999. [DOI] [PubMed] [Google Scholar]
- 181.Dasgupta D, Wee MJ, Reyes M, Li Y, Simm PJ, Sharma A, Schlingmann KP, Janner M, Biggin A, Lazier J, Gessner M, Chrysis D, Tuchman S, Baluarte HJ, Levine MA, Tiosano D, Insogna K, Hanley DA, Carpenter TO, Ichikawa S, Hoppe B, Konrad M, Savendahl L, Munns CF, Lee H, Juppner H, Bergwitz C. Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J Am Soc Nephrol 25: 2366–2375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dastani Z, Li R, Richards B. Genetic regulation of vitamin D levels. Calcif Tissue Int 92: 106–117, 2013. [DOI] [PubMed] [Google Scholar]
- 183.Daugaard H, Egfjord M, Lewin E, Olgaard K. Metabolism of intact PTH by isolated perfused kidney and liver from uremic rats. Exp Nephrol 2: 240–248, 1994. [PubMed] [Google Scholar]
- 184.Daugaard H, Egfjord M, Olgaard K. Metabolism of intact parathyroid hormone in isolated perfused rat liver and kidney. Am J Physiol 254: E740–748, 1988. [DOI] [PubMed] [Google Scholar]
- 185.Daugaard H, Egfjord M, Olgaard K. Metabolism of parathyroid hormone in isolated perfused rat kidney and liver combined. Kidney Int 38: 55–62, 1990. [DOI] [PubMed] [Google Scholar]
- 186.de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol 24: 1863–1871, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.de Groot T, Kovalevskaya NV, Verkaart S, Schilderink N, Felici M, van der Hagen EA, Bindels RJ, Vuister GW, Hoenderop JG. Molecular mechanisms of calmodulin action on TRPV5 and modulation by parathyroid hormone. Mol Cell Biol 31: 2845–2853, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deliot N, Hernando N, Horst-Liu Z, Gisler SM, Capuano P, Wagner CA, Bacic D, O’Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-P(i) cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol 289: C159–C167, 2005. [DOI] [PubMed] [Google Scholar]
- 189.Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep 3: 479, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.DeLuca HF. The vitamin D story: A collaborative effort of basic science and clinical medicine. FASEB J 2: 224–236, 1988. [PubMed] [Google Scholar]
- 191.Demay MB. Physiological insights from the vitamin D receptor knockout mouse. Calcif Tissue Int 92: 99–105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deupi X, Kobilka BK. Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology (Bethesda, Md) 25: 293–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Di Rosa M, Malaguarnera M, Zanghi A, Passaniti A, Malaguarnera L. Vitamin D insufficiency and colorectal cancer. Crit Rev Oncol Hematol 88: 594–612, 2013. [DOI] [PubMed] [Google Scholar]
- 194.Diepens RJ, den Dekker E, Bens M, Weidema AF, Vandewalle A, Bindels RJ, Hoenderop JG. Characterization of a murine renal distal convoluted tubule cell line for the study of transcellular calcium transport. Am J Physiol Renal Physiol 286: F483–489, 2004. [DOI] [PubMed] [Google Scholar]
- 195.Divieti P, Inomata N, Chapin K, Singh R, Juppner H, Bringhurst FR. Receptors for the carboxyl-terminal region of pth(1-84) are highly expressed in osteocytic cells. Endocrinology 142: 916–925, 2001. [DOI] [PubMed] [Google Scholar]
- 196.Divieti P, John MR, Juppner H, Bringhurst FR. Human PTH-(7-84 inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology 143: 171–176, 2002. [DOI] [PubMed] [Google Scholar]
- 197.Driver JP, Lamont DJ, Gysemans C, Mathieu C, Serreze DV. Calcium insufficiency accelerates type 1 diabetes in vitamin D receptor-deficient nonobese diabetic (NOD) mice. Endocrinology 152: 4620–4629, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Duan S, Lv Z, Fan X, Wang L, Han F, Wang H, Bi S. Vitamin D status and the risk of multiple sclerosis: A systematic review and meta-analysis. Neurosci Lett 570: 108–113, 2014. [DOI] [PubMed] [Google Scholar]
- 199.Dwivedi PP, Hii CS, Ferrante A, Tan J, Der CJ, Omdahl JL, Morris HA, May BK. Role of MAP kinases in the 1,25-dihydroxyvitamin D-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J Biol Chem 277: 29643–29653, 2002. [DOI] [PubMed] [Google Scholar]
- 200.Dwivedi PP, Muscat GE, Bailey PJ, Omdahl JL, May BK. Repression of basal transcription by vitamin D receptor: Evidence for interaction of unliganded vitamin D receptor with two receptor interaction domains in RIP13delta1. J Mol Endocrinol 20: 327–335, 1998. [DOI] [PubMed] [Google Scholar]
- 201.Eknoyan G. A history of the parathyroid glands. Am J Kidney Dis 26: 801–807, 1995. [DOI] [PubMed] [Google Scholar]
- 202.el-Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA. The parathyroid hormone circadian rhythm is truly endogenous–a general clinical research center study. J Clin Endocrinol Metab 82: 281–286, 1997. [DOI] [PubMed] [Google Scholar]
- 203.Elhalel MD, Wald H, Rubinger D, Gal-Moscovici A, Inoue M, Levi M, Popovtzer MM. Regulation of NaPi-IIa mRNA and transporter protein in chronic renal failure: Role of parathyroid hormone (PTH) and dietary phosphate (Pi). Pflugers Arch 449: 265–270, 2004. [DOI] [PubMed] [Google Scholar]
- 204.Ellam T, Hameed A, ul Haque R, Muthana M, Wilkie M, Francis SE, Chico TJ. Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PloS One 9: e88767, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab 93: 3381–3388, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Engelman CD, Meyers KJ, Ziegler JT, Taylor KD, Palmer ND, Haffner SM, Fingerlin TE, Wagenknecht LE, Rotter JI, Bowden DW, Langefeld CD, Norris JM. Genome-wide association study of vitamin D concentrations in Hispanic Americans: the IRAS family study. J Steroid Biochem Mol Biol 122: 186–192, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, Wendler I, Fabian D, Hauptlorenz S, Halwachs-Baumann G. 25-hydroxy-Vitamin D status: Limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab 60: 1541–1550, 2014. [DOI] [PubMed] [Google Scholar]
- 208.Epstein S, Heath H III, Bell NH. Lack of influence of isoproterenol, propranolol, and dopamine on immunoreactive parathyroid hormone and calcitonin in normal man. Calcif Tissue Int 35: 32–36, 1983. [DOI] [PubMed] [Google Scholar]
- 209.Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol 85: 1417–1423, 2013. [DOI] [PubMed] [Google Scholar]
- 210.Evenepoel P, Rodriguez M, Ketteler M. Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Semin Nephrol 34: 151–163, 2014. [DOI] [PubMed] [Google Scholar]
- 211.Falchetti A. MEN syndromes. Tumori 96: 823–826, 2010. [DOI] [PubMed] [Google Scholar]
- 212.Falchetti A, Morelli A, Amorosi A, Tonelli F, Fabiani S, Martineti V, Castello R, Furlani L, Brandi ML. Allelic loss in parathyroid tumors from individuals homozygous for multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 82: 2278–2282, 1997. [DOI] [PubMed] [Google Scholar]
- 213.Fallo F, Cella G, Casonato A, Ermani M, Vettor R, Zanella S, Lumachi F. Biochemical markers of endothelial activation in primary hyperparathyroidism. Horm Metab Res 38: 125–129, 2006. [DOI] [PubMed] [Google Scholar]
- 214.Fallo F, Rocco S, Pagotto U, Zangari M, Luisetto G, Mantero F. Aldosterone and pressor responses to angiotensin II in primary hyperparathyroidism. J Hypertens Suppl 7: S192–193, 1989. [DOI] [PubMed] [Google Scholar]
- 215.Fang VS, Tashjian AH Jr. Studies on the role of the liver in the metabolism of parathyroid hormone. I. Effects of partial hepatectomy and incubation of the hormone with tissue homogenates. Endocrinology 90: 1177–1184, 1972. [DOI] [PubMed] [Google Scholar]
- 216.Farahnak P, Larfars G, Sten-Linder M, Nilsson IL. Mild primary hyperparathyroidism: Vitamin D deficiency and cardiovascular risk markers. J Clin Endocrinol Metab 96:2112–2118, 2011. [DOI] [PubMed] [Google Scholar]
- 217.Farahnak P, Ring M, Caidahl K, Farnebo LO, Eriksson MJ, Nilsson IL. Cardiac function in mild primary hyperparathyroidism and the outcome after parathyroidectomy. Eur J Endocrinol 163: 461–467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Malloy PJ, Zhou Y, Wang J, Hiort O, Feldman D. Mutations in the vitamin D receptor and hereditary vitamin D-resistant rickets. Bonekey Rep 3:510, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Felger EA, Zeiger MA. The death of an Indian Rhinoceros. World J Surg 34: 1805–1810, 2010. [DOI] [PubMed] [Google Scholar]
- 220.Feng JQ, Clinkenbeard EL, Yuan B, White KE, Drezner MK. Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone 54: 213–221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol 304: C1027–C1039, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5: 734–742, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ferre S, Bongers EM, Sonneveld R, Cornelissen EA, van der Vlag J, van Boekel GA, Wetzels JF, Hoenderop JG, Bindels RJ, Nijenhuis T. Early development of hyperparathyroidism due to loss of PTH transcriptional repression in patients with HNF1beta mutations? J Clin Endocrinol Metab 98: 4089–4096, 2013. [DOI] [PubMed] [Google Scholar]
- 224.Ferreira GB, Baeke F, Verstuyf A, De Clercq P, Waelkens E, Mathieu C, Overbergh L. A proteomic approach on the effects of TX527, a 1alpha,25-dihydroxyvitamin D analog, in human T lymphocytes. J Steroid Biochem Mol Biol 144(Pt A): 96–101, 2014. [DOI] [PubMed] [Google Scholar]
- 225.Ferreira GB, Overbergh L, Verstuyf A, Mathieu C. 1alpha,25-Dihydroxyvitamin D and its analogs as modulators of human dendritic cells: A comparison dose-titration study. J Steroid Biochem Mol Biol 136: 160–165, 2013. [DOI] [PubMed] [Google Scholar]
- 226.Fischer JA, Blum JW, Hunziker W, Binswanger U. Regulation of circulating parathyroid hormone levels: Normal physiology and consequences in disorders of mineral metabolism. Klin Wochenschr 53: 939–954, 1975. [DOI] [PubMed] [Google Scholar]
- 227.Fischer JA, Oldham SB, Sizemore GW, Arnaud CD. Calcium-regulated parathyroid hormone peptidase. Proc Natl Acad Sci U S A 69: 2341–2345, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Flanagan JN, Wang L, Tangpricha V, Reichrath J, Chen TC, Holick MF. Regulation of the 25-hydroxyvitamin D-1alpha-hydroxylase gene and its splice variant. Recent Results Cancer Res 164: 157–167, 2003. [DOI] [PubMed] [Google Scholar]
- 229.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, Giovannucci EL, Fuchs CS, Chan AT. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension 61: 779–785, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 55: 1283–1288, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fraher LJ, Avram R, Watson PH, Hendy GN, Henderson JE, Chong KL, Goltzman D, Morley P, Willick GE, Whitfield JF, Hodsman AB. Comparison of the biochemical responses to human parathyroid hormone-(1-31)NH2 and hPTH-(1-34) in healthy humans. J Clin Endocrinol Metab 84: 2739–2743, 1999. [DOI] [PubMed] [Google Scholar]
- 232.Fraser DR, Kodicek E. Conformational similarities of vitamin D and cholesterol as enzyme substrates. Nature 220: 1031–1032, 1968. [DOI] [PubMed] [Google Scholar]
- 233.Fraser WD. Hyperparathyroidism. Lancet 374: 145–158, 2009. [DOI] [PubMed] [Google Scholar]
- 234.Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene’s transcriptional start site. J Steroid Biochem Mol Biol 103: 440–445, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Friedlander G, Amiel C. Cellular mode of action of parathyroid hormone. Adv Nephrol Necker Hosp 23: 265–279, 1994. [PubMed] [Google Scholar]
- 236.Friedman E, Sakaguchi K, Bale AE, Falchetti A, Streeten E, Zimering MB, Weinstein LS, McBride WO, Nakamura Y, Brandi ML, Norton JA, Aurbach GD, Spiegel AM, Marx SJ. Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med 321: 213–218, 1989. [DOI] [PubMed] [Google Scholar]
- 237.Friedman PA. PTH revisited. Kidney Int Suppl S13–S19, 2004. [DOI] [PubMed] [Google Scholar]
- 238.Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. EMBO J 24: 3881–3894, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 239.Galitzer H, Lavi-Moshayoff V, Nechama M, Meir T, Silver J, Naveh-Many T. The calcium-sensing receptor regulates parathyroid hormone gene expression in transfected HEK293 cells. BMC Biol 7: 17, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gandini S, Gnagnarella P, Serrano D, Pasquali E, Raimondi S. vitamin D receptor polymorphisms and cancer. Adv Exp Med Biol 810: 69–105, 2014. [DOI] [PubMed] [Google Scholar]
- 241.Gao XH, Dwivedi PP, Choe S, Alba F, Morris HA, Omdahl JL, May BK. Basal and parathyroid hormone induced expression of the human 25-hydroxyvitamin D 1alpha-hydroxylase gene promoter in kidney AOK-B50 cells: role of Sp1, Ets and CCAAT box protein binding sites. Int J Biochem Cell Biol 34: 921–930, 2002. [DOI] [PubMed] [Google Scholar]
- 242.Gardella TJ, Axelrod D, Rubin D, Keutmann HT, Potts JT Jr, Kronenberg HM, Nussbaum SR. Mutational analysis of the receptor-activating region of human parathyroid hormone. J Biol Chem 266: 13141–13146, 1991. [PubMed] [Google Scholar]
- 243.Gardella TJ, Juppner H, Wilson AK, Keutmann HT, Abou-Samra AB, Segre GV, Bringhurst FR, Potts JT Jr., Nussbaum SR, Kronenberg HM. Determinants of [Arg2]PTH-(1-34) binding and signaling in the transmembrane region of the parathyroid hormone receptor. Endocrinology 135:1186–1194, 1994. [DOI] [PubMed] [Google Scholar]
- 244.Gardella TJ, Luck MD, Jensen GS, Schipani E, Potts JT Jr, Juppner H. Inverse agonism of amino-terminally truncated parathyroid hormone (PTH) and PTH-related peptide (PTHrP) analogs revealed with constitutively active mutant PTH/PTHrP receptors. Endocrinology 137: 3936–3941, 1996. [DOI] [PubMed] [Google Scholar]
- 245.Gardella TJ, Luck MD, Jensen GS, Usdin TB, Juppner H. Converting parathyroid hormone-related peptide (PTHrP) into a potent PTH-2 receptor agonist. J Biol Chem 271: 19888–19893, 1996. [DOI] [PubMed] [Google Scholar]
- 246.Gardella TJ, Wilson AK, Keutmann HT, Oberstein R, Potts JT Jr, Kronenberg M, Nussbaum SR. Analysis of parathyroid hormone’s principal receptor-binding region by site-directed mutagenesis and analog design. Endocrinology 132: 2024–2030, 1993. [DOI] [PubMed] [Google Scholar]
- 247.Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Renal Physiol 306: F351–358, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 297: F282–291, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gennari C, Nami R, Gonnelli S. Hypertension and primary hyperparathyroidism: the role of adrenergic and renin-angiotensinaldosterone systems. Miner Electrolyte Metab 21: 77–81, 1995. [PubMed] [Google Scholar]
- 250.Gensure RC, Gardella TJ, Juppner H. Multiple sites of contact between the carboxyl-terminal binding domain of PTHrP-(1–36) analogs and the amino-terminal extracellular domain of the PTH/PTHrP receptor identified by photoaffinity cross-linking. J Biol Chem 276: 28650–28658, 2001. [DOI] [PubMed] [Google Scholar]
- 251.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281: 10856–10864, 2006. [DOI] [PubMed] [Google Scholar]
- 252.Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci. Transl. Med 1: 1ra1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gezmish O, Black MJ. Vitamin D deficiency in early life and the potential programming of cardiovascular disease in adulthood. J Cardiovasc Transl Res 6: 588–603, 2013. [DOI] [PubMed] [Google Scholar]
- 254.Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol (Baltimore, Md: 1950) 173: 2280–2287, 2004. [DOI] [PubMed] [Google Scholar]
- 255.Giral H, Cranston D, Lanzano L, Caldas Y, Sutherland E, Rachelson J, Dobrinskikh E, Weinman EJ, Doctor RB, Gratton E, Levi M. NHE3 regulatory factor 1 (NHERF1) modulates intestinal sodium-dependent phosphate transporter (NaPi-2b) expression in apical microvilli. J Biol Chem 287: 35047–35056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Giusti F, Cavalli L, Cavalli T, Brandi ML. Hereditary hyperparathyroidism syndromes. J Clin Densitom 16: 69–74, 2013. [DOI] [PubMed] [Google Scholar]
- 257.Glendenning P, Chew GT, Inderjeeth CA, Taranto M, Fraser WD. Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone 56: 271–275, 2013. [DOI] [PubMed] [Google Scholar]
- 258.Glossmann HH. Origin of 7-dehydrocholesterol (provitamin D) in the skin. J Invest Dermatol 130: 2139–2141, 2010. [DOI] [PubMed] [Google Scholar]
- 259.Goltzman D, Hendy GN, White JH. Vitamin D and its receptor during late development. Biochim Biophys Acta 1849: 171–180, 2015. [DOI] [PubMed] [Google Scholar]
- 260.Goltzman D, Peytremann A, Callahan EN, Segre GV, Potts JT Jr. Metabolism and biological activity of parathyroid hormone in renal cortical membranes. J Clin Invest 57: 8–19, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D. FASEB J 19: 1067–1077, 2005. [DOI] [PubMed] [Google Scholar]
- 262.Gomme PT, Bertolini J. Therapeutic potential of vitamin D-binding protein. Trends Biotechnol 22: 340–345, 2004. [DOI] [PubMed] [Google Scholar]
- 263.Goncalves JG, de Braganca AC, Canale D, Shimizu MH, Sanches TR, Moyses RM, Andrade L, Seguro AC, Volpini RA. Vitamin D deficiency aggravates chronic kidney disease progression after ischemic acute kidney injury. PloS One 9: e107228, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gras DE, Persinoti GF, Peres NT, Martinez-Rossi NM, Tahira AC, Reis EM, Prade RA, Rossi A. Transcriptional profiling of Neurospora crassa Deltamak-2 reveals that mitogen-activated protein kinase MAK-2 participates in the phosphate signaling pathway. Fungal Genet Biol 60: 140–149, 2013. [DOI] [PubMed] [Google Scholar]
- 265.Gray R, Boyle I, DeLuca HF. Vitamin D metabolism: The role of kidney tissue. Science (New York, NY) 172: 1232–1234, 1971. [DOI] [PubMed] [Google Scholar]
- 266.Gray RW, Omdahl JL, Ghazarian JG, DeLuca HF. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem 247: 7528–7532, 1972. [PubMed] [Google Scholar]
- 267.Greenberg Z, Bisello A, Mierke DF, Rosenblatt M, Chorev M. Mapping the bimolecular interface of the parathyroid hormone (PTH)-PTH1 receptor complex: Spatial proximity between Lys(27) (of the hormone principal binding domain) and leu(261) (of the first extracellular loop) of the human PTH1 receptor. Biochemistry 39: 8142–8152, 2000. [DOI] [PubMed] [Google Scholar]
- 268.Griffin AT, Arnold FW. Review of metabolic, immunologic, and virologic consequences of suboptimal vitamin D levels in HIV infection. AIDS Patient Care STDS 26: 516–525, 2012. [DOI] [PubMed] [Google Scholar]
- 269.Grober U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol 5: 331–347, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gronwald W, Schomburg D, Harder MP, Mayer H, Paulsen J, Wingender E, Wray V. Structure of recombinant human parathyroid hormone in solution using multidimensional NMR spectroscopy. Biol Chem Hoppe Seyler 377: 175–186, 1996. [DOI] [PubMed] [Google Scholar]
- 271.Gruson D, Buglioni A, Burnett JC Jr. PTH: Potential role in management of heart failure. Clin Chim Acta 433c: 290–296, 2014. [DOI] [PubMed] [Google Scholar]
- 272.Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol 9: 337–347, 2013. [DOI] [PubMed] [Google Scholar]
- 273.Guo J, Song L, Liu M, Mahon MJ. Fluorescent ligand-directed co-localization of the parathyroid hormone 1 receptor with the brush-border scaffold complex of the proximal tubule reveals hormone-dependent changes in ezrin immunoreactivity consistent with inactivation. Biochim Biophys Acta 1823: 2243–2253, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Guo J, Song L, Liu M, Segawa H, Miyamoto K, Bringhurst FR, Kronenberg HM, Juppner H. Activation of a non-cAMP/PKA signaling pathway downstream of the PTH/PTHrP receptor is essential for a sustained hypophosphatemic response to PTH infusion in male mice. Endocrinology 154: 1680–1689, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gutierrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: Updating the “trade-off” hypothesis. Clin J Am Soc Nephrol 5: 1710–1716, 2010. [DOI] [PubMed] [Google Scholar]
- 276.Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, Eizirik DL, Mathieu C. 1,25-Dihydroxyvitamin D modulates expression of chemokines and cytokines in pancreatic islets: Implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 146: 1956–1964, 2005. [DOI] [PubMed] [Google Scholar]
- 277.Habener JF, Kronenberg HM. Parathyroid hormone biosynthesis: Structure and function of biosynthetic precursors. Fed Proc 37: 2561–2566, 1978. [PubMed] [Google Scholar]
- 278.Habener JF, Mayer GP, Dee PC, Potts JT Jr. Metabolism of amino- and carboxyl-sequence immunoreactive parathyroid hormone in the bovine: Evidence for peripheral cleavage of hormone. Metabolism 25: 385–395, 1976. [DOI] [PubMed] [Google Scholar]
- 279.Habener JF, Potts JT Jr. Relative effectiveness of magnesium and calcium on the secretion and biosynthesis of parathyroid hormone in vitro. Endocrinology 98: 197–202, 1976. [DOI] [PubMed] [Google Scholar]
- 280.Habener JF, Potts JT Jr. Subcellular distributions of parathyroid hormone, hormonal precursors, and parathyroid secretory protein. Endocrinology 104: 265–275, 1979. [DOI] [PubMed] [Google Scholar]
- 281.Habener JF, Rosenblatt M, Dee PC, Potts JT Jr. Cellular processing of pre-proparathyroid hormone involves rapid hydrolysis of the leader sequence. J Biol Chem 254: 10596–10599, 1979. [PubMed] [Google Scholar]
- 282.Habener JF, Rosenblatt M, Potts JT Jr. Parathyroid hormone: Biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev 64: 985–1053, 1984. [DOI] [PubMed] [Google Scholar]
- 283.Habener JF, Stevens TD, Tregear GW, Potts JT Jr. Radioimmunoassay of human proparathyroid hormone: analysis of hormone content in tissue extracts and in plasma. J Clin Endocrinol Metab 42: 520–530, 1976. [DOI] [PubMed] [Google Scholar]
- 284.Handler P, Cohn DV, DeMaria WJ. Effect of parathyroid extract on renal function. Am J Physiol 165:434–441, 1951. [DOI] [PubMed] [Google Scholar]
- 285.Hannan FM, Nesbit MA, Christie PT, Fratter C, Dudley NE, Sadler GP, Thakker RV. Familial isolated primary hyperparathyroidism caused by mutations of the MEN1 gene. Nat Clin Pract Endocrinol Metab 4: 53–58, 2008. [DOI] [PubMed] [Google Scholar]
- 286.Hansford JR, Mulligan LM. Multiple endocrine neoplasia type 2 and RET: From neoplasia to neurogenesis. J Med Genet 37: 817–827, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Haroon M, Fitzgerald O. Vitamin D and its emerging role in immunopathology. Clin Rheumatol 31: 199–202, 2012. [DOI] [PubMed] [Google Scholar]
- 288.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, Bishop NJ, Baird J, Cooper C. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess (Winchester, England) 18: 1–190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010. [DOI] [PubMed] [Google Scholar]
- 290.Haussler MR, Whitfield GK, Kaneko I, Forster R, Saini R, Hsieh JC, Haussler CA, Jurutka PW. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev Endocr Metab Disord 13: 57–69, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hayes G, Forgo J, Bringhurst FR, Segre G, Murer H. Expression of parathyroid hormone receptors in MDCK and LLC-PK1 cells. Pflugers Arch 430: 636–644, 1995. [DOI] [PubMed] [Google Scholar]
- 292.Helmle-Kolb C, Montrose MH, Murer H. Parathyroid hormone regulation of Na+/H+ exchange in opossum kidney cells: polarity and mechanisms. Pflugers Arch 416: 615–623, 1990. [DOI] [PubMed] [Google Scholar]
- 293.Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab 25: 531–541, 2011. [DOI] [PubMed] [Google Scholar]
- 294.Hernando N, Gisler SM, Pribanic S, Deliot N, Capuano P, Wagner CA, Moe OW, Biber J, Murer H. NaPi-IIa and interacting partners. J Physiol 567: 21–26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol 26: 75–84, 2007. [DOI] [PubMed] [Google Scholar]
- 296.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 76: 315–325, 2012. [DOI] [PubMed] [Google Scholar]
- 297.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103: 316–321, 2007. [DOI] [PubMed] [Google Scholar]
- 298.Hilpert J, Nykjaer A, Jacobsen C, Wallukat G, Nielsen R, Moestrup SK, Haller H, Luft FC, Christensen EI, Willnow TE. Megalin antagonizes activation of the parathyroid hormone receptor. J Biol Chem 274:5620–5625, 1999. [DOI] [PubMed] [Google Scholar]
- 299.Hoare SR, Gardella TJ, Usdin TB. Evaluating the signal transduction mechanism of the parathyroid hormone 1 receptor. Effect of receptor-G-protein interaction on the ligand binding mechanism and receptor conformation. J Biol Chem 276: 7741–7753, 2001. [DOI] [PubMed] [Google Scholar]
- 300.Hoare SR, Rubin DA, Juppner H, Usdin TB. Evaluating the ligand specificity of zebrafish parathyroid hormone (PTH) receptors: comparison of PTH, PTH-related protein, and tuberoinfundibular peptide of 39 residues. Endocrinology 141: 3080–3086, 2000. [DOI] [PubMed] [Google Scholar]
- 301.Hoenderop JG, Chon H, Gkika D, Bluyssen HA, Holstege FC, St-Arnaud R, Braam B, Bindels RJ. Regulation of gene expression by dietary Ca2+ in kidneys of 25-hydroxyvitamin D-1 alpha-hydroxylase knockout mice. Kidney Int 65: 531–539, 2004. [DOI] [PubMed] [Google Scholar]
- 302.Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, St -Arnaud R, Bindels RJ. Modulation of renal Ca2+ transport protein genes by dietary Ca2 +and 1,25-dihydroxyvitamin D in 25-hydroxyvitamin D-1alpha-hydroxylase knockout mice. FASEB J 16: 1398–1406, 2002. [DOI] [PubMed] [Google Scholar]
- 303.Hoenderop JG, Muller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 12: 1342–1349, 2001. [DOI] [PubMed] [Google Scholar]
- 304.Hoenderop JG, van der Kemp AW, Urben CM, Strugnell SA, Bindels RJ. Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D-1alpha-hydroxylase knockout mice. Kidney Int 66: 1082–1089, 2004. [DOI] [PubMed] [Google Scholar]
- 305.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112: 1906–1914, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Holick MF. McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. Am J Clin Nutr 60: 619–630, 1994. [DOI] [PubMed] [Google Scholar]
- 307.Holick MF, DeLuca HF. A new chromatographic system for vitamin D and its metabolites: Resolution of a new vitamin D metabolite. J Lipid Res 12: 460–465, 1971. [PubMed] [Google Scholar]
- 308.Holick MF, Kleiner-Bossaller A, Schnoes HK, Kasten PM, Boyle IT, DeLuca HF. 1,24,25-Trihydroxyvitamin D. A metabolite of vitamin D effective on intestine. J Biol Chem 248: 6691–6696, 1973. [PubMed] [Google Scholar]
- 309.Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D in human skin and the physiologic consequences. Science (New York, NY) 210:203–205, 1980. [DOI] [PubMed] [Google Scholar]
- 310.Holick SA, Holick MF, MacLaughlin JA. Chemical synthesis of [1 beta-3H] 1 alpha, 25-dihydroxyvitamin D and [1 alpha-3H] 1 beta, 25-dihydroxyvitamin D2: Biological activity of 1 beta, 25-dihydroxyvitamin D. Biochem Biophys Res Commun 97: 1031–1037, 1980. [DOI] [PubMed] [Google Scholar]
- 311.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 98:4619–4628, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Homme M, Schaefer F, Mehls O, Schmitt CP. Differential regulation of RGS-2 by constant and oscillating PTH concentrations. Calcif Tissue Int 84: 305–312, 2009. [DOI] [PubMed] [Google Scholar]
- 313.Hruska KA, Martin K, Mennes P, Greenwalt A, Anderson C, Klahr S, Slatopolsky E. Degradation of parathyroid hormone and fragment production by the isolated perfused dog kidney. The effect of glomerular filtration rate and perfusate CA++ concentrations. J Clin Invest 60: 501–510, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Imawari M, Kida K, Goodman DS. The transport of vitamin D and its 25-hydroxy metabolite in human plasma. Isolation and partial characterization of vitamin D and 25-hydroxyvitamin D binding protein. J Clin Invest 58: 514–523, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Inanloorahatloo K, Zand Parsa AF, Huse K, Rasooli P, Davaran S, Platzer M, Fan JB, Amini S, Steemers F, Elahi E. Mutation in CYP27A1 identified in family with coronary artery disease. Eur J Med Genet 56: 655–660, 2013. [DOI] [PubMed] [Google Scholar]
- 316.Isales CM, Barrett PQ, Brines M, Bollag W, Rasmussen H. Parathyroid hormone modulates angiotensin II-induced aldosterone secretion from the adrenal glomerulosa cell. Endocrinology 129: 489–495, 1991. [DOI] [PubMed] [Google Scholar]
- 317.Iwaki T, Sandoval-Cooper MJ, Tenenhouse HS, Castellino FJ. A missense mutation in the sodium phosphate co-transporter Slc34a1 impairs phosphate homeostasis. J Am Soc Nephrol 19: 1753–1762, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Jaaskelainen T, Huhtakangas J, Maenpaa PH. Negative regulation of human parathyroid hormone gene promoter by vitamin D through nuclear factor Y. Biochem Biophys Res Commun 328: 831–837, 2005. [DOI] [PubMed] [Google Scholar]
- 319.Jacobs TP, Kaufman M, Jones G, Kumar R, Schlingmann KP, Shapses S, Bilezikian JP. A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab 99: 708–712, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr, Slominski AT. 20,23-dihydroxyvitamin D, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol 223: 36–48, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Jeon US. Kidney and calcium homeostasis. Electrolyte Blood Press 6: 68–76, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Jiang YJ, Bikle DD. LncRNA profiling reveals new mechanism for VDR protection against skin cancer formation. J Steroid Biochem Mol Biol 144(Pt A): 87–90, 2014. [DOI] [PubMed] [Google Scholar]
- 323.Jin L, Briggs SL, Chandrasekhar S, Chirgadze NY, Clawson DK, Schevitz RW, Smiley DL, Tashjian AH, Zhang F. Crystal structure of human parathyroid hormone 1-34 at 0.9-A resolution. J Biol Chem 275: 27238–27244, 2000. [DOI] [PubMed] [Google Scholar]
- 324.Jones A, Tzenova J, Frappier D, Crumley M, Roslin N, Kos C, Tieder M, Langman C, Proesmans W, Carpenter T, Rice A, Anderson D, Morgan K, Fujiwara T, Tenenhouse H. Hereditary hypophosphatemic rickets with hypercalciuria is not caused by mutations in the Na/Pi cotransporter NPT2 gene. J Am Soc Nephrol 12: 507–514, 2001. [DOI] [PubMed] [Google Scholar]
- 325.Jones G. Extrarenal vitamin D activation and interactions between vitamin D(2), vitamin D(3), and vitamin D analogs. Annu Rev Nutr 33: 23–44, 2013. [DOI] [PubMed] [Google Scholar]
- 326.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55: 13–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Joun H, Lanske B, Karperien M, Qian F, Defize L, Abou-Samra A. Tissue-specific transcription start sites and alternative splicing of the parathyroid hormone (PTH)/PTH-related peptide (PTHrP) receptor gene: a new PTH/PTHrP receptor splice variant that lacks the signal peptide. Endocrinology 138: 1742–1749, 1997. [DOI] [PubMed] [Google Scholar]
- 328.Juppner H. Phosphate and FGF-23. Kidney Int Suppl 2011: S24–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF Jr, Hock J, Potts JT Jr, Kronenberg HM, Segre GV. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science (New York, NY) 254: 1024–1026, 1991. [DOI] [PubMed] [Google Scholar]
- 330.Juppner H, Schipani E, Bringhurst FR, McClure I, Keutmann HT, Potts JT Jr, Kronenberg HM, Abou-Samra AB, Segre GV, Gardella TJ. The extracellular amino-terminal region of the parathyroid hormone (PTH)/PTH-related peptide receptor determines the binding affinity for carboxyl-terminal fragments of PTH-(1-34). Endocrinology 134: 879–884, 1994. [DOI] [PubMed] [Google Scholar]
- 331.Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, Hsieh JC, Kaczmarska M, Haussler CA, Haussler MR. vitamin D receptor: Key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res 22(Suppl 2): V2–10, 2007. [DOI] [PubMed] [Google Scholar]
- 332.Kafetzis ID, Diamantopoulos A, Christakis I, Leoutsakos B. The history of the parathyroid glands. Hormones (Athens, Greece) 10: 80–84, 2011. [DOI] [PubMed] [Google Scholar]
- 333.Kaneko I, Segawa H, Furutani J, Kuwahara S, Aranami F, Hanabusa E, Tominaga R, Giral H, Caldas Y, Levi M, Kato S, Miyamoto K. Hypophosphatemia in vitamin D receptor null mice: effect of rescue diet on the developmental changes in renal Na+-dependent phosphate cotransporters. Pflugers Arch 461: 77–90, 2011. [DOI] [PubMed] [Google Scholar]
- 334.Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prie D. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359: 1128–1135, 2008. [DOI] [PubMed] [Google Scholar]
- 335.Karperien M, Farih-Sips H, Hendriks JA, Lanske B, Papapoulos SE, Abou-Samra AB, Lowik CW, Defize LH. Identification of a retinoic acid-inducible element in the murine PTH/PTHrP (parathyroid hormone/parathyroid hormone-related peptide) receptor gene. Mol Endocrinol (Baltimore, Md) 13: 1183–1196, 1999. [DOI] [PubMed] [Google Scholar]
- 336.Kaseda R, Hosojima M, Sato H, Saito A. Role of megalin and cubilin in the metabolism of vitamin D(3). Ther Apher Dial 15(Suppl 1): 14–17, 2011. [DOI] [PubMed] [Google Scholar]
- 337.Kato S. Genetic mutation in the human 25-hydroxyvitamin D 1alpha-hydroxylase gene causes vitamin D-dependent rickets type I. Mol Cell Endocrinol 156:7–12, 1999. [DOI] [PubMed] [Google Scholar]
- 338.Kato S, Fujiki R, Kim MS, Kitagawa H. Ligand-induced transrepressive function of VDR requires a chromatin remodeling complex, WINAC. J Steroid Biochem Mol Biol 103: 372–380, 2007. [DOI] [PubMed] [Google Scholar]
- 339.Kato S, Fujiki R, Kitagawa H. Vitamin D receptor (VDR) promoter targeting through a novel chromatin remodeling complex. J Steroid Biochem Mol Biol 89-90: 173–178, 2004. [DOI] [PubMed] [Google Scholar]
- 340.Kaufmann M, Muff R, Stieger B, Biber J, Murer H, Fischer JA. Apical and basolateral parathyroid hormone receptors in rat renal cortical membranes. Endocrinology 134: 1173–1178, 1994. [DOI] [PubMed] [Google Scholar]
- 341.Kawahara M, Iwasaki Y, Sakaguchi K, Taguchi T, Nishiyama M, Nigawara T, Tsugita M, Kambayashi M, Suda T, Hashimoto K. Predominant role of 25OHD in the negative regulation of PTH expression: Clinical relevance for hypovitaminosis D. Life Sci 82: 677–683, 2008. [DOI] [PubMed] [Google Scholar]
- 342.Keinan D, Yang S, Cohen RE, Yuan X, Liu T, Li YP. Role of regulator of G protein signaling proteins in bone. Front Biosci (Landmark edition) 19: 634–648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kemper B, Habener JF, Ernst MD, Potts JT Jr, Rich A. Pre-proparathyroid hormone: Analysis of radioactive tryptic peptides and amino acid sequence. Biochemistry 15: 15–19, 1976. [DOI] [PubMed] [Google Scholar]
- 344.Kemper B, Habener JF, Potts JT Jr, Rich A. Pre-proparathyroid hormone: Fidelity of the translation of parathyroid messenger RNA by extracts of wheat germ. Biochemistry 15: 20–25, 1976. [DOI] [PubMed] [Google Scholar]
- 345.Kemper B, Habener JF, Potts JT Jr, Rich A. Proparathyroid hormone: Identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A 69: 643–647, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kemper B, Habener JF, Rich A, Potts JT Jr. Parathyroid secretion: Discovery of a major calcium-dependent protein. Science (New York, NY) 184: 167–169, 1974. [DOI] [PubMed] [Google Scholar]
- 347.Kempson SA, Kowalski JC, Puschett JB. Inhibition of renal brush border phosphate transport and stimulation of renal gluconeogenesis by cyclic amp and parathyroid hormone. Biochem Pharmacol 32: 1533–1537, 1983. [DOI] [PubMed] [Google Scholar]
- 348.Kempson SA, Lotscher M, Kaissling B, Biber J, Murer H, Levi M. Parathyroid hormone action on phosphate transporter mRNA and protein in rat renal proximal tubules. Am J Physiol 268: F784–791, 1995. [DOI] [PubMed] [Google Scholar]
- 349.Keynan Y, Malik S, Fowke KR. The role of polymorphisms in host immune genes in determining the severity of respiratory illness caused by pandemic H1N1 influenza. Public Health Genomics 16: 9–16, 2013. [DOI] [PubMed] [Google Scholar]
- 350.Khundmiri SJ, Ahmad A, Bennett RE, Weinman EJ, Steplock D, Cole J, Baumann PD, Lewis J, Singh S, Clark BJ, Lederer ED. Novel regulatory function for NHERF-1 in Npt2a transcription. Am J Physiol Renal Physiol 294: F840–849, 2008. [DOI] [PubMed] [Google Scholar]
- 351.Khundmiri SJ, Rane MJ, Lederer ED. Parathyroid hormone regulation of type II sodium-phosphate cotransporters is dependent on an A kinase anchoring protein. J Biol Chem 278: 10134–10141, 2003. [DOI] [PubMed] [Google Scholar]
- 352.Kido S, Kaneko I, Tatsumi S, Segawa H, Miyamoto K. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib Nephrol 180:86–97, 2013. [DOI] [PubMed] [Google Scholar]
- 353.Kilav R, Silver J, Biber J, Murer H, Naveh-Many T. Coordinate regulation of rat renal parathyroid hormone receptor mRNA and Na-Pi cotransporter mRNA and protein. Am J Physiol 268: F1017–F1022, 1995. [DOI] [PubMed] [Google Scholar]
- 354.Kitagawa H, Fujiki R, Yoshimura K, Mezaki Y, Uematsu Y, Matsui D, Ogawa S, Unno K, Okubo M, Tokita A, Nakagawa T, Ito T, Ishimi Y, Nagasawa H, Matsumoto T, Yanagisawa J, Kato S. The chromatin-remodeling complex WINAC targets a nuclear receptor to promoters and is impaired in Williams syndrome. Cell 113: 905–917, 2003. [DOI] [PubMed] [Google Scholar]
- 355.Kitagawa H, Fujiki R, Yoshimura K, Oya H, Kato S. Williams syndrome is an epigenome-regulator disease. Endocr J 58: 77–85, 2011. [DOI] [PubMed] [Google Scholar]
- 356.Kitson MT, Sarrazin C, Toniutto P, Eslick GD, Roberts SK. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: A systematic review and meta-analysis. J Hepatol 61: 1247–1252, 2014. [DOI] [PubMed] [Google Scholar]
- 357.Klaus W, Dieckmann T, Wray V, Schomburg D, Wingender E, Mayer H. Investigation of the solution structure of the human parathyroid hormonefragment(1-34) by 1H NMR spectroscopy, distance geometry, and molecular dynamics calculations. Biochemistry 30: 6936–6942, 1991. [DOI] [PubMed] [Google Scholar]
- 358.Klein L. Direct measurement of bone resorption and calcium conservation during vitamin D deficiency or hypervitaminosis D. Proc Natl Acad Sci U S A 77: 1818–1822, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Klinger M, Alexiewicz JM, Linker-Israeli M, Pitts TO, Gaciong Z, Fadda GZ, Massry SG. Effect of parathyroid hormone on human T cell activation. Kidney Int 37: 1543–1551, 1990. [DOI] [PubMed] [Google Scholar]
- 360.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28: 397–406, 2007. [DOI] [PubMed] [Google Scholar]
- 361.Koh J, Dar M, Untch BR, Dixit D, Shi Y, Yang Z, Adam MA, Dressman H, Wang X, Gesty-Palmer D, Marks JR, Spurney R, Druey KM, Olson JA Jr. Regulator of G protein signaling 5 is highly expressed in parathyroid tumors and inhibits signaling by the calcium-sensing receptor. Mol Endocrinol (Baltimore, Md) 25: 867–876, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kolakowski LF Jr. GCRDb: A G-protein-coupled receptor database. Receptors Channels 2: 1–7, 1994. [PubMed] [Google Scholar]
- 363.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1alpha,25-Dihydroxyvitamin D upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289: G1036–G1042, 2005. [DOI] [PubMed] [Google Scholar]
- 364.Kollars J, Zarroug AE, van Heerden J, Lteif A, Stavlo P, Suarez L, Moir C, Ishitani M, Rodeberg D. Primary hyperparathyroidism in pediatric patients. Pediatrics 115: 974–980, 2005. [DOI] [PubMed] [Google Scholar]
- 365.Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int 74: 1577–1581, 2008. [DOI] [PubMed] [Google Scholar]
- 366.Kong XF, Schipani E, Lanske B, Joun H, Karperien M, Defize LH, Juppner H, Potts JT Jr, Segre GV, Kronenberg HM, Abou-Samra AB. The rat, mouse and human genes encoding the receptor for parathyroid hormone and parathyroid hormone-related peptide are highly homologous. Biochem Biophys Res Commun 200: 1290–1299, 1994. [DOI] [PubMed] [Google Scholar]
- 367.Kong XF, Zhu XH, Pei YL, Jackson DM, Holick MF. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D-1alpha-hydroxylase gene. Proc Natl Acad Sci U S A 96: 6988–6993, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Korkor AB. Reduced binding of [3H]1,25-dihydroxyvitamin D in the parathyroid glands of patients with renal failure. N Engl J Med 316: 1573–1577, 1987. [DOI] [PubMed] [Google Scholar]
- 369.Koszewski NJ, Alimov AP, Langub MC, Park-Sarge OK, Malluche HH. Contrasting mammalian PTH promoters: Identification of transcription factors controlling PTH gene expression. Clin Nephrol 63: 158–162, 2005. [DOI] [PubMed] [Google Scholar]
- 370.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195: 125–131, 2007. [DOI] [PubMed] [Google Scholar]
- 371.Kronenberg HM, Igarashi T, Freeman MW, Okazaki T, Brand SJ, Wiren KM, Potts JT Jr. Structure and expression of the human parathyroid hormone gene. Recent Prog Horm Res 42: 641–663, 1986. [PubMed] [Google Scholar]
- 372.Kumar R, Thompson JR. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol 22: 216–224, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Kuntziger H, Amiel C, Roinel N, Morel F. Effects of parathyroidectomy and cyclic AMP on renal transport of phosphate, calcium, and magnesium. Am J Physiol 227: 905–911, 1974. [DOI] [PubMed] [Google Scholar]
- 374.Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: Causal association or epiphenomenon? Eur J Epidemiol 29: 1–14, 2014. [DOI] [PubMed] [Google Scholar]
- 375.Lagishetty V, Chun RF, Liu NQ, Lisse TS, Adams JS, Hewison M. 1alpha-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol 121: 228–233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Lam NN, Triliana R, Sawyer RK, Atkins GJ, Morris HA, O’Loughlin PD, Anderson PH. Vitamin D receptor overexpression in osteoblasts and osteocytes prevents bone loss during vitamin D-deficiency. J Steroid Biochem Mol Biol 144(Pt A): 128–131, 2014. [DOI] [PubMed] [Google Scholar]
- 377.Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O, Gratton E, Levi M, Blaine J. Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Cell Physiol 301: C850–C861, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lavie CJ, Dinicolantonio JJ, Milani RV, O’Keefe JH. Vitamin D and cardiovascular health. Circulation 128: 2404–2406, 2013. [DOI] [PubMed] [Google Scholar]
- 379.Lederer E, Miyamoto K. Clinical consequences of mutations in sodium phosphate cotransporters. Clin J Am Soc Nephrol 7: 1179–1187, 2012. [DOI] [PubMed] [Google Scholar]
- 380.Lederer ED, Khundmiri SJ, Weinman EJ. Role of NHERF-1 in regulation of the activity of Na-K ATPase and sodium-phosphate co-transport in epithelial cells. J Am Soc Nephrol 14: 1711–1719, 2003. [DOI] [PubMed] [Google Scholar]
- 381.Lee C, Gardella TJ, Abou-Samra AB, Nussbaum SR, Segre GV, Potts JT Jr, Kronenberg HM, Juppner H. Role of the extracellular regions of the parathyroid hormone (PTH)/PTH-related peptide receptor in hormone binding. Endocrinology 135: 1488–1495, 1994. [DOI] [PubMed] [Google Scholar]
- 382.Lee C, Luck MD, Juppner H, Potts JT Jr, Kronenberg HM, Gardella TJ. Homolog-scanning mutagenesis of the parathyroid hormone (PTH) receptor reveals PTH-(1-34) binding determinants in the third extracellular loop. Mol Endocrinol (Baltimore, Md) 9: 1269–1278, 1995. [DOI] [PubMed] [Google Scholar]
- 383.Lee M, Pellegata NS. Multiple endocrine neoplasia syndromes associated with mutation of p27. J Endocrinol Invest 36: 781–787, 2013. [DOI] [PubMed] [Google Scholar]
- 384.Lee M, Pellegata NS. Multiple endocrine neoplasia type 4. Front Horm Res 41: 63–78, 2013. [DOI] [PubMed] [Google Scholar]
- 385.Lee SW, Russell J, Avioli LV. 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol: conversion impaired by systemic metabolic acidosis. Science (New York, NY) 195: 994–996, 1977. [DOI] [PubMed] [Google Scholar]
- 386.Lefkowitz RJ. Arrestins come of age: A personal historical perspective. Prog Mol Biol Transl Sci 118:3–18, 2013. [DOI] [PubMed] [Google Scholar]
- 387.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Lemmens I, Merregaert J, Van de Ven WJ, Kas K, Zhang CX, Giraud S, Wautot V, Buisson N, De Witte K, Salandre J, Lenoir G, Calender A, Parente F, Quincey D, Courseaux A, Carle GF, Gaudray P, De Wit MJ, Lips CJ, Hoppener JW, Khodaei S, Grant AL, Weber G, Kytola S, Teh BT, Farnebo F, Grimmond S, Phelan C, Larsson C, Forbes SA, Bassett JHD, Pannett AAJ, Thakker RV. Construction of a 1.2-Mb sequence-ready contig of chromosome 11q13 encompassing the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Genomics 44: 94–100, 1997. [DOI] [PubMed] [Google Scholar]
- 389.Lemmens I, Van de Ven WJ, Kas K, Zhang CX, Giraud S, Wautot V, Buisson N, De Witte K, Salandre J, Lenoir G, Pugeat M, Calender A, Parente F, Quincey D, Gaudray P, De Wit MJ, Lips CJ, Hoppener JW, Khodaei S, Grant AL, Weber G, Kytola S, Teh BT, Farnebo F, Phelan C, Hayward N, Larsson C, Pannett AAJ, Forbes SA, Bassett JHD, Thakker RV. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet 6:1177–1183, 1997. [DOI] [PubMed] [Google Scholar]
- 390.Leong PK, Yang LE, Lin HW, Holstein-Rathlou NH, McDonough AA. Acute hypotension induced by aortic clamp vs. PTH provokes distinct proximal tubule Na+ transporter redistribution patterns. Am J Physiol Regul Integr Comp Physiol 287: R878–R885, 2004. [DOI] [PubMed] [Google Scholar]
- 391.Li C, Liu Z, Zhang Z, Strom SS, Gershenwald JE, Prieto VG, Lee JE, Ross MI, Mansfield Pf, Cormier JN, Duvic M, Grimm EA, Wei Q. Genetic variants of the vitamin D receptor gene alter risk of cutaneous melanoma. J Invest Dermatol 127: 276–280, 2007. [DOI] [PubMed] [Google Scholar]
- 392.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D, which shows antiproliferative activity. Steroids 75: 926–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Li XY, Boudjelal M, Xiao JH, Peng ZH, Asuru A, Kang S, Fisher GJ, Voorhees JJ. 1,25-Dihydroxyvitamin D increases nuclear vitamin D receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol Endocrinol (Baltimore, Md) 13: 1686–1694, 1999. [DOI] [PubMed] [Google Scholar]
- 394.Li YC. Discovery of vitamin D hormone as a negative regulator of the renin-angiotensin system. Clin Chem 60: 561–562, 2014. [DOI] [PubMed] [Google Scholar]
- 395.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89-90: 387–392, 2004. [DOI] [PubMed] [Google Scholar]
- 396.Li YC. Vitamin D receptor signaling in renal and cardiovascular protection. Semin Nephrol 33: 433–447, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Lin CH, Kadakia S, Frieri M. New insights into an autoimmune mechanism, pharmacological treatment and relationship between multiple sclerosis and inflammatory bowel disease. Autoimmun Rev 13: 114–116, 2014. [DOI] [PubMed] [Google Scholar]
- 398.Lin CJ, Dardis A, Wijesuriya SD, Abdullah MA, Casella SJ, Miller WL. Lack of mutations in CYP2D6 and CYP27 in patients with apparent deficiency of vitamin D 25-hydroxylase. Mol Genet Metab 80:469–472, 2003. [DOI] [PubMed] [Google Scholar]
- 399.Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of vitamin D status. Ann N Y Acad Sci 1317: 92–98, 2014. [DOI] [PubMed] [Google Scholar]
- 400.Lisse TS, Saini V, Zhao H, Luderer HF, Gori F, Demay MB. The Vitamin D Receptor Is Required for Activation of cWnt and Hedgehog Signaling in Keratinocytes. Mol Endocrinol (Baltimore, Md) 28: 1698–1706, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Liu NQ, Ouyang Y, Bulut Y, Lagishetty V, Chan SY, Hollis BW, Wagner C, Equils O, Hewison M. Dietary vitamin D restriction in pregnant female mice is associated with maternal hypertension and altered placental and fetal development. Endocrinology 154: 2270–2280, 2013. [DOI] [PubMed] [Google Scholar]
- 402.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78: 193–201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Lou YR, Molnar F, Perakyla M, Qiao S, Kalueff AV, St-Arnaud R, Carlberg C, Tuohimaa P. 25-Hydroxyvitamin D(3)is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol 118: 162–170, 2010. [DOI] [PubMed] [Google Scholar]
- 404.Lucisano S, Buemi M, Passantino A, Aloisi C, Cernaro V, Santoro D. New insights on the role of vitamin D in the progression of renal damage. Kidney Blood Press Res 37: 667–678, 2013. [DOI] [PubMed] [Google Scholar]
- 405.Luderer HF, Demay MB. The vitamin D receptor, the skin and stem cells. J Steroid Biochem Mol Biol 121: 314–316, 2010. [DOI] [PubMed] [Google Scholar]
- 406.Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-beta signaling during the inflammatory response to cutaneous injury. Endocrinology 154: 16–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Luigi P, Chiara FM, Laura Z, Cristiano M, Giuseppina C, Luciano C, Giuseppe P, Sabrina C, Susanna S, Antonio C, Giuseppe C, Giorgio de T, Claudio L. Arterial hypertension, metabolic syndrome and subclinical cardiovascular organ damage in patients with asymptomatic primary hyperparathyroidism before and after parathyroidectomy: Preliminary results. Int J Endocrinol 2012: 408295, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab 303: E928–E935, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Maccallum WG, Lambert RA, Vogel KM. The removal of calcium from the blood by dialysis in the study of tetany. J Exp Med 20: 149–168, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Maccallum WG, Voegtlin C. On the relation of tetany to the parathyroid glands and to calcium metabolism. J Exp Med 11: 118–151, 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Maccallum WG, Vogel KM. Further experimental studies in tetany. J Exp Med 18: 618–650, 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.MacGregor RR, Hamilton JW, Kent GN, Shofstall RE, Cohn DV. The degradation of proparathormone and parathormone by parathyroid and liver cathepsin B. J Biol Chem 254: 4428–4433, 1979. [PubMed] [Google Scholar]
- 413.MacGregor RR, Hamilton JW, Shofstall RE, Cohn DV. Isolation and characterization of porcine parathyroid cathepsin B. J Biol Chem 254: 4423–4427, 1979. [PubMed] [Google Scholar]
- 414.Mackey SL, Heymont JL, Kronenberg HM, Demay MB. Vitamin D receptor binding to the negative human parathyroid hormone vitamin D response element does not require the retinoid x receptor. Mol Endocrinol (Baltimore, Md) 10: 298–305, 1996. [DOI] [PubMed] [Google Scholar]
- 415.Maeda S, Wu S, Juppner H, Green J, Aragay AM, Fagin JA, Clemens TL. Cell-specific signal transduction of parathyroid hormone (PTH)-related protein through stably expressed recombinant PTH/PTHrP receptors in vascular smooth muscle cells. Endocrinology 137: 3154–3162, 1996. [DOI] [PubMed] [Google Scholar]
- 416.Mahon MJ. The parathyroid hormone 1 receptor directly binds to the FERM domain of ezrin, an interaction that supports apical receptor localization and signaling in LLC-PK1 cells. Mol Endocrinol (Baltimore, Md) 23: 1691–1701, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Mahon MJ, Bonacci TM, Divieti P, Smrcka AV. A docking site for G protein betagamma subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol Endocrinol (Baltimore, Md) 20: 136–146, 2006. [DOI] [PubMed] [Google Scholar]
- 418.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na(+)/H(+) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417: 858–861, 2002. [DOI] [PubMed] [Google Scholar]
- 419.Mahon MJ, Segre GV. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cbeta, and actin increases intracellular calcium in opossum kidney cells. J Biol Chem 279: 23550–23558, 2004. [DOI] [PubMed] [Google Scholar]
- 420.Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett 579: 803–807, 2005. [DOI] [PubMed] [Google Scholar]
- 421.Malloy PJ, Feldman D. The role of vitamin D receptor mutations in the development of alopecia. Mol Cell Endocrinol 347: 90–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Mallya SM, Arnold A. Cyclin D1 in parathyroid disease. Front Biosci 5: D67–371, 2000. [DOI] [PubMed] [Google Scholar]
- 423.Mallya SM, Wu HI, Saria EA, Corrado KR, Arnold A. Tissue-specific regulatory regions of the PTH gene localized by novel chromosome 11 rearrangement breakpoints in a parathyroid adenoma. J Bone Miner Res 25: 2606–2612, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Marini F, Falchetti A, Luzi E, Tonelli F, Maria Luisa B. Multiple endocrine neoplasia type 1 (MEN1) syndrome. In: Riegert-Johnson DL, Boardman LA, Hefferon T, Roberts M, editors. Cancer Syndromes. Bethesda (MD): National Center for Biotechnology Information (US) Douglas L Riegert-Johnson, 2009. [PubMed] [Google Scholar]
- 425.Marinoni I, Lee M, Mountford S, Perren A, Bravi I, Jennen L, Feuchtinger A, Drouin J, Roncaroli F, Pellegata NS. Characterization of MENX-associated pituitary tumours. Neuropathol Appl Neurobiol 39: 256–269, 2013. [DOI] [PubMed] [Google Scholar]
- 426.Markowitz ME, Arnaud S, Rosen JF, Thorpy M, Laximinarayan S. Temporal interrelationships between the circadian rhythms of serum parathyroid hormone and calcium concentrations. J Clin Endocrinol Metab 67: 1068–1073, 1988. [DOI] [PubMed] [Google Scholar]
- 427.Marsell R, Jonsson KB. The phosphate regulating hormone fibroblast growth factor-23. Acta physiologica (Oxford, England) 200: 97–106, 2010. [DOI] [PubMed] [Google Scholar]
- 428.Marshall PA, Hernandez Z, Kaneko I, Widener T, Tabacaru C, Aguayo I, Jurutka PW. Discovery of novel vitamin D receptor interacting proteins that modulate 1,25-dihydroxyvitamin D signaling. J Steroid Biochem Mol Biol 132: 147–159, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 92: 131–155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Martin KJ, Gonzalez EA. Vitamin D analogues for the management of secondary hyperparathyroidism. Am J Kidney Dis 38: S34–40, 2001. [DOI] [PubMed] [Google Scholar]
- 431.Martin KJ, Hruska KA, Freitag JJ, Klahr S, Slatopolsky E. The peripheral metabolism of parathyroid hormone. N Engl J Med 301:1092–1098, 1979. [DOI] [PubMed] [Google Scholar]
- 432.Martin KJ, Hruska KA, Lewis J, Anderson C, Slatopolsky E. The renal handling of parathyroid hormone. Role of peritubular uptake and glomerular filtration. J Clin Invest 60: 808–814, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Martz A, Forte LR, Langeluttig SG. Renal cAMP and 1,25(OH)2D synthesis in estrogen-treated chick embryos and hens. Am J Physiol 249: E626–633, 1985. [DOI] [PubMed] [Google Scholar]
- 434.Marx SJ, Attie MF, Levine MA, Spiegel AM, Downs RW Jr, Lasker RD. The hypocalciuric or benign variant of familial hypercalcemia: Clinical and biochemical features in fifteen kindreds. Medicine 60: 397–412, 1981. [DOI] [PubMed] [Google Scholar]
- 435.Marx SJ, Aurbach GD, Spiegel AM. Incidence of primary hyperparathyroidism. N Engl J Med 302: 1313, 1980. [PubMed] [Google Scholar]
- 436.Marx SJ, Spiegel AM, Brown EM, Aurbach GD. Family studies in patients with primary parathyroid hyperplasia. Am J Med 62: 698–706, 1977. [DOI] [PubMed] [Google Scholar]
- 437.Marx SJ, Spiegel AM, Levine MA, Rizzoli RE, Lasker RD, Santora AC, Downs RW Jr, Aurbach GD. Familial hypocalciuric hypercalcemia: The relation to primary parathyroid hyperplasia. N Engl J Med 307: 416–426, 1982. [DOI] [PubMed] [Google Scholar]
- 438.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116: 3150–3159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Mazzaferro S, Goldsmith D, Larsson TE, Massy ZA, Cozzolino M. Vitamin D metabolites and/or analogs: which D for which patient? Curr Vasc Pharmacol 12: 339–349, 2014. [DOI] [PubMed] [Google Scholar]
- 440.McCollum EV. The paths to the discovery of vitamins A and D. J Nutr 91(Suppl 1): 11–16, 1967. [DOI] [PubMed] [Google Scholar]
- 441.McCuaig KA, Clarke JC, White JH. Molecular cloning of the gene encoding the mouse parathyroid hormone/parathyroid hormone-related peptide receptor. Proc Natl Acad Sci U S A 91: 5051–5055, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.McCuaig KA, Lee HS, Clarke JC, Assar H, Horsford J, White JH. Parathyroid hormone/parathyroid hormone related peptide receptor gene transcripts are expressed from tissue-specific and ubiquitous promoters. Nucleic Acids Res 23: 1948–1955, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Melick RA, Martin TJ. Parathyroid hormone metabolism in man: Effect of nephrectomy. Clin Sci 37: 667–674, 1969. [PubMed] [Google Scholar]
- 444.Mellerup B, Mellerup ET. Seasonal variation in urinary excretion of calcium, magnesium and phosphate in manic-melancholic patients. Chronobiol Int 1: 81–86, 1984. [DOI] [PubMed] [Google Scholar]
- 445.Menon B, Harinarayan CV. The effect of anti epileptic drug therapy on serum 25-hydroxyvitamin D and parameters of calcium and bone metabolism—a longitudinal study. Seizure 19: 153–158, 2010. [DOI] [PubMed] [Google Scholar]
- 446.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D in intestinal cells. Mol Endocrinol 20: 1447–1461, 2006. [DOI] [PubMed] [Google Scholar]
- 447.Meyer MB, Zella LA, Nerenz RD, Pike JW. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D in mouse kidney and intestine in vivo. J Biol Chem 282: 22344–22352, 2007. [DOI] [PubMed] [Google Scholar]
- 448.Michels TC, Kelly KM. Parathyroid disorders. Am Fam Physician 88: 249–257, 2013. [PubMed] [Google Scholar]
- 449.Miedlich S, Lohmann T, Schneyer U, Lamesch P, Paschke R. Familial isolated primary hyperparathyroidism—a multiple endocrine neoplasia type 1 variant? Eur J Endocrinol 145: 155–160, 2001. [DOI] [PubMed] [Google Scholar]
- 450.Minagawa M, Kwan MY, Bettoun JD, Mansour FW, Dassa J, Hendy GN, Goltzman D, White JH. Dissection of differentially regulated (G+C)-rich promoters of the human parathyroid hormone (PTH)/PTH-related peptide receptor gene. Endocrinology 141: 2410–2421, 2000. [DOI] [PubMed] [Google Scholar]
- 451.Miodovnik M, Koren R, Ziv E, Ravid A. The inflammatory response of keratinocytes and its modulation by vitamin D: the role of MAPK signaling pathways. J Cell Physiol 227: 2175–2183, 2012. [DOI] [PubMed] [Google Scholar]
- 452.Misof BM, Roschger P, Tesch W, Baldock PA, Valenta A, Messmer P, Eisman JA, Boskey AL, Gardiner EM, Fratzl P, Klaushofer K. Targeted overexpression of vitamin D receptor in osteoblasts increases calcium concentration without affecting structural properties of bone mineral crystals. Calcif Tissue Int 73: 251–257, 2003. [DOI] [PubMed] [Google Scholar]
- 453.Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gartner BC, Kamradt J, Meese E, Tilgen W, Reichrath J. Analysis of the vitamin D system in basal cell carcinomas (BCCs). Lab Invest 84: 693–702, 2004. [DOI] [PubMed] [Google Scholar]
- 454.Miyamoto K, Haito-Sugino S, Kuwahara S, Ohi A, Nomura K, Ito M, Kuwahata M, Kido S, Tatsumi S, Kaneko I, Segawa H. Sodium-dependent phosphate cotransporters: Lessons from gene knockout and mutation studies. J Pharm Sci 100: 3719–3730, 2011. [DOI] [PubMed] [Google Scholar]
- 455.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol 27: 503–515, 2007. [DOI] [PubMed] [Google Scholar]
- 456.Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal 2: re4, 2009. [DOI] [PubMed] [Google Scholar]
- 457.Moallem E, Kilav R, Silver J, Naveh-Many T. RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem 273: 5253–5259, 1998. [DOI] [PubMed] [Google Scholar]
- 458.Mole SE, Mulligan LM, Healey CS, Ponder BA, Tunnacliffe A. Localisation of the gene for multiple endocrine neoplasia type 2A to a 480 kb region in chromosome band 10q11.2. Hum Mol Genet 2: 247–252, 1993. [DOI] [PubMed] [Google Scholar]
- 459.Mosekilde L, Torring O, Rejnmark L. Emerging anabolic treatments in osteoporosis. Curr Drug Saf 6: 62–74, 2011. [DOI] [PubMed] [Google Scholar]
- 460.Mossetti G, Vuotto P, Rendina D, Numis FG, Viceconti R, Giordano F, Cioffi M, Scopacasa F, Nunziata V. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 253: 194–200, 2003. [DOI] [PubMed] [Google Scholar]
- 461.Mourant AE, Tills D, Domaniewska-Sobczak K. Sunshine and the geographical distribution of the alleles of the Gc system of plasma proteins. Hum Genet 33: 307–314, 1976. [DOI] [PubMed] [Google Scholar]
- 462.Muff R, Fischer JA, Biber J, Murer H. Parathyroid hormone receptors in control of proximal tubule function. Annu Rev Physiol 54: 67–79, 1992. [DOI] [PubMed] [Google Scholar]
- 463.Mulligan LM. From genes to decisions: Evolving views of genotype-based management in MEN 2. Cancer Treat Res 122: 417–428, 2004. [PubMed] [Google Scholar]
- 464.Mulligan LM, Eng C, Healey CS, Clayton D, Kwok JB, Gardner E, Ponder MA, Frilling A, Jackson CE, Lehnert H, Neumann HPH, Thibodeau SN, Ponder BAJ. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet 6: 70–74, 1994. [DOI] [PubMed] [Google Scholar]
- 465.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363: 458–460, 1993. [DOI] [PubMed] [Google Scholar]
- 466.Murayama A, Takeyama K, Kitanaka S, Kodera Y, Hosoya T, Kato S. The promoter of the human 25-hydroxyvitamin D 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 alpha,25(OH)2D. Biochem Biophys Res Commun 249: 11–16, 1998. [DOI] [PubMed] [Google Scholar]
- 467.Murray RD, Holthouser K, Clark BJ, Salyer SA, Barati MT, Khundmiri SJ, Lederer ED. Parathyroid hormone (PTH) decreases sodium-phosphate cotransporter type IIa (NpT2a) mRNA stability. Am J Physiol Renal Physiol 304: F1076–1085, 2013. [DOI] [PubMed] [Google Scholar]
- 468.Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: Evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev 26: 78–113, 2005. [DOI] [PubMed] [Google Scholar]
- 469.Myakala K, Motta S, Murer H, Wagner CA, Koesters R, Biber J, Hernando N. Renal-specific and inducible depletion of NaPi-IIc/Slc34A3, the cotransporter mutated in HHRH, does not affect phosphate or calcium homeostasis in mice. Am J Physiol Renal Physiol 306: F833–F843, 2014. [DOI] [PubMed] [Google Scholar]
- 470.Nagasawa H, Uto Y, Sasaki H, Okamura N, Murakami A, Kubo S, Kirk KL, Hori H. Gc protein (vitamin D-binding protein): Gc genotyping and GcMAF precursor activity. Anticancer Res 25: 3689–3695, 2005. [PubMed] [Google Scholar]
- 471.Naveh-Many T, Marx R, Keshet E, Pike JW, Silver J. Regulation of 1,25-dihydroxyvitamin D receptor gene expression by 1,25-dihydroxyvitamin D in the parathyroid in vivo. J Clin Invest 86: 1968–1975, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Naveh-Many T, Silver J. Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. J Clin Invest 86: 1313–1319, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Negri AL. Proximal tubule endocytic apparatus as the specific renal uptake mechanism for vitamin D-binding protein/25-(OH)D complex. Nephrology (Carlton, Vic) 11: 510–515, 2006. [DOI] [PubMed] [Google Scholar]
- 474.Nemeth EF. Allosteric modulators of the extracellular calcium receptor. Drug Discov Today Technol 10: e277–284, 2013. [DOI] [PubMed] [Google Scholar]
- 475.Nemeth EF, Steffey ME, Fox J. The parathyroid calcium receptor: A novel therapeutic target for treating hyperparathyroidism. Pediatr Nephrol (Berlin, Germany) 10: 275–279, 1996. [DOI] [PubMed] [Google Scholar]
- 476.Nemeth EF. Pharmacological regulation of parathyroid hormone secretion. Curr Pharm Des 8: 2077–2087, 2002. [DOI] [PubMed] [Google Scholar]
- 477.Nemeth EF. The parathyroid polyhormone hypothesis revisited. Kidney Int Suppl S22–28, 2006. [DOI] [PubMed] [Google Scholar]
- 478.Neugebauer W, Surewicz WK, Gordon HL, Somorjai RL, Sung W, Willick GE. Structural elements of human parathyroid hormone and their possible relation to biological activities. Biochemistry 31: 2056–2063, 1992. [DOI] [PubMed] [Google Scholar]
- 479.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D’Amour P. Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology 142: 1386–1392, 2001. [DOI] [PubMed] [Google Scholar]
- 480.Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 64: 1290–1298, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14: 2731–2740, 2003. [DOI] [PubMed] [Google Scholar]
- 482.Nissen J, Vogel U, Ravn-Haren G, Andersen EW, Nexo BA, Andersen R, Mejborn H, Madsen KH, Rasmussen LB. Real-life use of vitamin D-fortified bread and milk during a winter season: the effects of CYP2R1 and GC genes on 25-hydroxyvitamin D concentrations in Danish families, the VitmaD study. Genes Nutr 9: 413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Nutchey BK, Kaplan JS, Dwivedi PP, Omdahl JL, Ferrante A, May BK, Hii CS. Molecular action of 1,25-dihydroxyvitamin D and phorbol ester on the activation of the rat cytochrome P450C24 (CYP24) promoter: Role of MAP kinase activities and identification of an important transcription factor binding site. Biochem J 389: 753–762, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D. Cell 96: 507–515, 1999. [DOI] [PubMed] [Google Scholar]
- 485.Ohata Y, Yamazaki M, Kawai M, Tsugawa N, Tachikawa K, Koinuma T, Miyagawa K, Kimoto A, Nakayama M, Namba N, Yamamoto H, Okano T, Ozono K, Michigami T. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res 29: 1627–1638, 2014. [DOI] [PubMed] [Google Scholar]
- 486.Ohyama Y, Ozono K, Uchida M, Shinki T, Kato S, Suda T, Yamamoto O, Noshiro M, Kato Y. Identification of a vitamin D-responsive element in the 5′-flanking region of the rat 25-hydroxyvitamin D 24-hydroxylase gene. J Biol Chem 269: 10545–10550, 1994. [PubMed] [Google Scholar]
- 487.Okazaki R, Matsumoto T, Furukawa Y, Fujimoto Y, Niimi H, Seino Y, Fujita T, Nagataki S, Ogata E. Serum intact parathyroid hormone concentration measured by a two-site immunoradiometric assay in normal subjects and patients with various parathyroid disorders. Endocrinol Jpn 39: 115–120, 1992. [DOI] [PubMed] [Google Scholar]
- 488.Okazaki T, Ando K, Igarashi T, Ogata E, Fujita T. Conserved mechanism of negative gene regulation by extracellular calcium. Parathyroid hormone gene versus atrial natriuretic polypeptide gene. J Clin Invest 89: 1268–1273, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Okazaki T, Zajac JD, Igarashi T, Ogata E, Kronenberg HM. Negative regulatory elements in the human parathyroid hormone gene. J Biol Chem 266:21903–21910, 1991. [PubMed] [Google Scholar]
- 490.Olgaard K, Lewin E, Bro S, Daugaard H, Egfjord M, Pless V. Enhancement of the stimulatory effect of calcium on aldosterone secretion by parathyroid hormone. Miner Electrolyte Metab 20: 309–314, 1994. [PubMed] [Google Scholar]
- 491.Olson EB Jr, Knutson JC, Bhattacharyya MH, DeLuca HF. The effect of hepatectomy on the synthesis of 25-hydroxyvitamin D. J Clin Invest 57: 1213–1220, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: Expression, function, and regulation. Annu Rev Nutr 22: 139–166, 2002. [DOI] [PubMed] [Google Scholar]
- 493.Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: Why do T cells have vitamin D receptors? Mol Aspects Med 33: 77–82, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Ooi JH, McDaniel KL, Weaver V, Cantorna MT. Murine CD8+ T cells but not macrophages express the vitamin D 1alpha-hydroxylase. J Nutr Biochem 25: 58–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Orloff JJ, Kats Y, Urena P, Schipani E, Vasavada RC, Philbrick WM, Behal A, Abou-Samra AB, Segre GV, Juppner H. Further evidence for a novel receptor for amino-terminal parathyroid hormone-related protein on keratinocytes and squamous carcinoma cell lines. Endocrinology 136:3016–3023, 1995. [DOI] [PubMed] [Google Scholar]
- 496.Osto E, Fallo F, Pelizzo MR, Maddalozzo A, Sorgato N, Corbetti F, Montisci R, Famoso G, Bellu R, Luscher TF, Iliceto S, Tona F. Coronary microvascular dysfunction induced by primary hyperparathyroidism is restored after parathyroidectomy. Circulation 126: 1031–1039, 2012. [DOI] [PubMed] [Google Scholar]
- 497.Owen R. On the anatomy of the Indian rhinoceros. Trans Zool Soc Lond 4:31–58, 1862. [Google Scholar]
- 498.Owen TA, Bortell R, Yocum SA, Smock SL, Zhang M, Abate C, Shalhoub V, Aronin N, Wright KL, van Wijnen AJ, et al. Coordinate occupancy of AP-1 sites in the vitamin D-responsive and CCAAT box elements by Fos-Jun in the osteocalcin gene: Model for phenotype suppression of transcription. Proc Natl Acad Sci U S A 87: 9990–9994, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Paik JM, Farwell WR, Taylor EN. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 23: 1727–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 279: 16754–16766, 2004. [DOI] [PubMed] [Google Scholar]
- 501.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 98: 7498–7503, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, Uchida Y. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem 286: 34121–34130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Parsons JA, Robinson CJ. A rapid indirect hypercalcaemic action of parathyroid hormone deonstrated in isolated blood-perfused bone. Proc R Soc Med 62: 239, 1969. [PMC free article] [PubMed] [Google Scholar]
- 504.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Hofler H, Fend F, Graw J, Atkinson MJ. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A 103: 15558–15563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Pellegrini M, Royo M, Rosenblatt M, Chorev M, Mierke DF. Addressing the tertiary structure of human parathyroid hormone-(1-34). J Biol Chem 273: 10420–10427, 1998. [DOI] [PubMed] [Google Scholar]
- 506.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res 29: 3687–3698, 2009. [PubMed] [Google Scholar]
- 507.Peytremann A, Goltzman D, Callahan EN, Tregear GW, Potts JT Jr. Metabolism and biological activity of proparathyroid hormone and synthetic analogues in renal cortical membranes. Endocrinology 97: 1270–1280, 1975. [DOI] [PubMed] [Google Scholar]
- 508.Pibiri F, Kittles RA, Sandler RS, Keku TO, Kupfer SS, Xicola RM, Llor X, Ellis NA. Genetic variation in vitamin D-related genes and risk of colorectal cancer in African Americans. Cancer Causes Control 25: 561–570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Pike JW, Meyer MB, Watanuki M, Kim S, Zella LA, Fretz JA, Yamazaki M, Shevde NK. Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D and its receptor. J Steroid Biochem Mol Biol 103: 389–395, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Pike JW, Parker JB, Haussler MR, Boass A, Toverud SV. Dynamic changes in circulating 1,25-dihydroxyvitamin D during reproduction in rats. Science (New York, NY) 204: 1427–1429, 1979. [DOI] [PubMed] [Google Scholar]
- 511.Pike JW, Spanos E, Colston KW, MacIntyre I, Haussler MR. Influence of estrogen on renal vitamin D hydroxylases and serum 1alpha,25-(OH)2D in chicks. Am J Physiol 235: E338–343, 1978. [DOI] [PubMed] [Google Scholar]
- 512.Piserchio A, Bisello A, Rosenblatt M, Chorev M, Mierke DF. Characterization of parathyroid hormone/receptor interactions: Structure of the first extracellular loop. Biochemistry 39: 8153–8160, 2000. [DOI] [PubMed] [Google Scholar]
- 513.Ponchon G, DeLuca HF. The role of the liver in the metabolism of vitamin D. J Clin Invest 48: 1273–1279, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Portale AA, Booth BE, Halloran BP, Morris RC Jr. Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest 73: 1580–1589, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Portale AA, Halloran BP, Harris ST, Bikle DD, Morris RC Jr. Metabolic acidosis reverses the increase in serum 1,25(OH)2D in phosphorus-restricted normal men. Am J Physiol 263: E1164–E1170, 1992. [DOI] [PubMed] [Google Scholar]
- 516.Portale AA, Halloran BP, Morris RC Jr. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 80: 1147–1154, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Portale AA, Halloran BP, Morris RC Jr. Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest 83: 1494–1499, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Portale AA, Halloran BP, Morris RC Jr, Lonergan ET. Effect of aging on the metabolism of phosphorus and 1,25-dihydroxyvitamin D in healthy men. Am J Physiol 270:E483–490, 1996. [DOI] [PubMed] [Google Scholar]
- 519.Portale AA, Halloran BP, Murphy MM, Morris RC Jr. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest 77:7–12, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Potts JT. Parathyroid hormone: Past and present. J Endocrinol 187: 311–325, 2005. [DOI] [PubMed] [Google Scholar]
- 521.Potts JT, Gardella TJ. Progress, paradox, and potential: Parathyroid hormone research over five decades. Ann N Y Acad Sci 1117: 196–208, 2007. [DOI] [PubMed] [Google Scholar]
- 522.Potts JT Jr. A short history of parathyroid hormone, its biological role, and pathophysiology of hormone excess. J Clin Densitom 16: 4–7, 2013. [DOI] [PubMed] [Google Scholar]
- 523.Prie D, Friedlander G. Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol 5: 1717–1722, 2010. [DOI] [PubMed] [Google Scholar]
- 524.Pugsley LI, Collip JB. The effect of parathyroid hormone upon the serum calcium and calcium excretion of normal and adrenalectomized rats. Biochem J 30: 1274–1279, 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: Implications in chronic kidney disease. Exp Cell Res 318: 1040–1048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 8: 276–286, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Quarles LD. A systems biology preview of the relationships between mineral and metabolic complications in chronic kidney disease. Semin Nephrol 33: 130–142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Rabinovitch A, Suarez-Pinzon WL, Sooy K, Strynadka K, Christakos S. Expression of calbindin-D(28k) in a pancreatic islet beta-cell line protects against cytokine-induced apoptosis and necrosis. Endocrinology 142: 3649–3655, 2001. [DOI] [PubMed] [Google Scholar]
- 530.Rajagopal A, Debora B, James TL, Soledad K, Florencia C, Hamilton C, David L, Jose Miguel L, Graciela V, Ignacio B, Richard G, Campeau P, Lee B. Exome sequencing identifies a novel homozygous mutation in the phosphate transporter SLC34A1 in hypophosphatemia and nephrocalcinosis. J Clin Endocrinol Metab 99: E2451–E2456, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Rampe D, Lacerda AE, Dage RC, Brown AM. Parathyroid hormone: An endogenous modulator of cardiac calcium channels. Am J Physiol 261: H1945–H1950, 1991. [DOI] [PubMed] [Google Scholar]
- 532.Rao DS, Honasoge M, Divine GW, Phillips ER, Lee MW, Ansari MR, Talpos GB, Parfitt AM. Effect of vitamin D nutrition on parathyroid adenoma weight: Pathogenetic and clinical implications. J Clin Endocrinol Metab 85: 1054–1058, 2000. [DOI] [PubMed] [Google Scholar]
- 533.Rao DS, Wilson RJ, Kleerekoper M, Parfitt AM. Lack of biochemical progression or continuation of accelerated bone loss in mild asymptomatic primary hyperparathyroidism: Evidence for biphasic disease course. J Clin Endocrinol Metab 67: 1294–1298, 1988. [DOI] [PubMed] [Google Scholar]
- 534.Rasmussen H, Craig LC. Purification of parathyroid hormone by use of counter-current distribution. J Am Chem Soc 81: 5003–5003, 1959. [Google Scholar]
- 535.Reddy D, Pollock AS, Clark SA, Sooy K, Vasavada RC, Stewart AF, Honeyman T, Christakos S. Transfection and overexpression of the calcium binding protein calbindin-D28k results in a stimulatory effect on insulin synthesis in a rat beta cell line (RIN 1046-38). Proc Natl Acad Sci U S A 94: 1961–1966, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Reddy GS, Norman AW, Willis DM, Goltzman D, Guyda H, Solomon S, Philips DR, Bishop JE, Mayer E. Regulation of vitamin D metabolism in normal human pregnancy. J Clin Endocrinol Metab 56: 363–370, 1983. [DOI] [PubMed] [Google Scholar]
- 537.Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D through C-24 oxidation pathway. Biochemistry 28:1763–1769, 1989. [DOI] [PubMed] [Google Scholar]
- 538.Reichrath J. Unravelling of hidden secrets: The role of vitamin D in skin aging. Dermatoendocrinol 4: 241–244, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gartner BC, Tilgen W, Holick MF. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol 31: 224–231, 2004. [DOI] [PubMed] [Google Scholar]
- 540.Reichrath J, Rech M, Moeini M, Meese E, Tilgen W, Seifert M. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D-responsive and-resistant melanoma cells. Cancer Biol Ther 6: 48–55, 2007. [DOI] [PubMed] [Google Scholar]
- 541.Reichrath J, Reichrath S, Heyne K, Vogt T, Roemer K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front Physiol 5: 166, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 383: 146–155, 2014. [DOI] [PubMed] [Google Scholar]
- 543.Rejnmark L, Sikjaer T, Underbjerg L, Mosekilde L. PTH replacement therapy of hypoparathyroidism. Osteoporos Int 24: 1529–1536, 2013. [DOI] [PubMed] [Google Scholar]
- 544.Remak R. Untersuchungen über die Entwicklung der Wirbeltiere Reimer G (ed). Berlin: 1855, pp. 39–40. [Google Scholar]
- 545.Rendina D, De Filippo G, Strazzullo P. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359: 2616; author reply 2616-2617,2008. [PubMed] [Google Scholar]
- 546.Reshkin SJ, Forgo J, Murer H. Apical and basolateral effects of PTH in OK cells: Transport inhibition, messenger production, effects of pertussis toxin, and interaction with a PTH analog. J Membr Biol 124: 227–237, 1991. [DOI] [PubMed] [Google Scholar]
- 547.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem 8: 204–226, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, Muharram G, Kerr Conte J, Pattou F. 1,25-Dihydroxyvitamin D protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis 11: 151–159, 2006. [DOI] [PubMed] [Google Scholar]
- 549.Richter B. Vitamin D for preventing cancer: Evidence and health beliefs. Cochrane Database Syst Rev 6: Ed000085, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Roider E, Ruzicka T, Schauber J. Vitamin d, the cutaneous barrier, antimicrobial peptides and allergies: Is there a link? Allergy Asthma Immunol Res 5: 119–128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Rolf L, Muris AH, Hupperts R, Damoiseaux J. Vitamin D effects on B cell function in autoimmunity. Ann N Y Acad Sci 1317:84–91, 2014. [DOI] [PubMed] [Google Scholar]
- 552.Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC, Friedman PA. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem 285: 14756–14763, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J. Vitamin D, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 57: 159–163, 1986. [PMC free article] [PubMed] [Google Scholar]
- 554.Rosenberg J, Pines M, Hurwitz S. Response of adrenal cells to parathyroid hormone stimulation. J Endocrinol 112: 431–437, 1987. [DOI] [PubMed] [Google Scholar]
- 555.Rosenberg J, Pines M, Levy JJ, Nutt RF, Caulfield MP, Russell J, Sherwood LM, Hurwitz S. Renal and adrenal adenosine 3′,5′-monophosphate production and corticosteroid secretion in response to synthetic chicken parathyroid hormone-(1-34). Endocrinology 125: 1082–1089, 1989. [DOI] [PubMed] [Google Scholar]
- 556.Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens 8: 884–893, 1995. [DOI] [PubMed] [Google Scholar]
- 557.Rubin DA, Hellman P, Zon LI, Lobb CJ, Bergwitz C, Juppner H. A G protein-coupled receptor from zebrafish is activated by human parathyroid hormone and not by human or teleost parathyroid hormone-related peptide. Implications for the evolutionary conservation of calcium-regulating peptide hormones. J Biol Chem 274: 23035–23042, 1999. [DOI] [PubMed] [Google Scholar]
- 558.Ruda JM, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 132: 359–372, 2005. [DOI] [PubMed] [Google Scholar]
- 559.Rudnicki M, Thode J, Jorgensen T, Heitmann BL, Sorensen OH. Effects of age, sex, season and diet on serum ionized calcium, parathyroid hormone and vitamin D in a random population. J Intern Med 234: 195–200, 1993. [DOI] [PubMed] [Google Scholar]
- 560.Ryan JW, Anderson PH, Turner AG, Morris HA. Vitamin D activities and metabolic bone disease. Clin Chim Acta 425: 148–152, 2013. [DOI] [PubMed] [Google Scholar]
- 561.Ryynanen J, Neme A, Tuomainen TP, Virtanen JK, Voutilainen S, Nurmi T, de Mello VD, Uusitupa M, Carlberg C. Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Mol Nutr Food Res 58: 2036–2045, 2014. [DOI] [PubMed] [Google Scholar]
- 562.Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal phosphate transport. Adv Chronic Kidney Dis 18: 85–90, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest 103: 239–251, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Saito A, Iino N, Takeda T, Gejyo F. Role of megalin, a proximal tubular endocytic receptor, in calcium and phosphate homeostasis. Ther Apher Dial 11(Suppl 1): S23–S26, 2007. [DOI] [PubMed] [Google Scholar]
- 565.Schaefer F. Pulsatile parathyroid hormone secretion in health and disease. Novartis Found Symp 227: 225–239; discussion 239-243, 2000. [PubMed] [Google Scholar]
- 566.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117: 803–811, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Schipani E, Provot S. PTHrP, PTH, and the PTH/PTHrP receptor in endochondral bone development. Birth Defects Res C Embryo Today 69:352–362, 2003. [DOI] [PubMed] [Google Scholar]
- 568.Schluter KD, Weber M, Piper HM. Parathyroid hormone induces protein kinase C but not adenylate cyclase in adult cardiomyocytes and regulates cyclic AMP levels via protein kinase C-dependent phosphodiesterase activity. Biochem J 310(Pt 2): 439–444, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Schmitt CP, Homme M, Schaefer F. Structural organization and biological relevance of oscillatory parathyroid hormone secretion. Pediatr Nephrol (Berlin, Germany) 20: 346–351, 2005. [DOI] [PubMed] [Google Scholar]
- 570.Schmitt CP, Locken S, Mehls O, Veldhuis JD, Lehnert T, Ritz E, Schaefer F. PTH pulsatility but not calcium sensitivity is restored after total parathyroidectomy with heterotopic autotransplantation. J Am Soc Nephrol 14: 407–414, 2003. [DOI] [PubMed] [Google Scholar]
- 571.Schmitt CP, Obry J, Feneberg R, Veldhuis JD, Mehls O, Ritz E, Schaefer F. Beta1-adrenergic blockade augments pulsatile PTH secretion in humans. J Am Soc Nephrol 14: 3245–3250, 2003. [DOI] [PubMed] [Google Scholar]
- 572.Schmitt CP, Schaefer F, Bruch A, Veldhuis JD, Schmidt-Gayk H, Stein G, Ritz E, Mehls O. Control of pulsatile and tonic parathyroid hormone secretion by ionized calcium. J Clin Endocrinol Metab 81: 4236–4243, 1996. [DOI] [PubMed] [Google Scholar]
- 573.Schoolwerth AC, Smith BC, Culpepper RM. Renal gluconeogenesis. Miner Electrolyte Metab 14: 347–361, 1988. [PubMed] [Google Scholar]
- 574.Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids 66: 399–408, 2001. [DOI] [PubMed] [Google Scholar]
- 575.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab 99: 1631–1637, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Segawa H, Aranami F, Kaneko I, Tomoe Y, Miyamoto K. The roles of Na/Pi-II transporters in phosphate metabolism. Bone 45(Suppl 1): S2–7, 2009. [DOI] [PubMed] [Google Scholar]
- 577.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol 20: 104–113, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Segre GV, Goldring SR. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagonlike peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab 4: 309–314, 1993. [DOI] [PubMed] [Google Scholar]
- 579.Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Effect of vitamin D supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 99: 3551–3560, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Seifert M, Tilgen W, Reichrath J. Expression of 25-hydroxyvitamin D-1alpha-hydroxylase (1alphaOHase, CYP27B1) splice variants in HaCaT keratinocytes and other skin cells: modulation by culture conditions and UV-B treatment in vitro. Anticancer Res 29: 3659–3667, 2009. [PubMed] [Google Scholar]
- 581.Seuter S, Neme A, Carlberg C. Characterization of genomic vitamin D receptor binding sites through chromatin looping and opening. PloS One 9: e96184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Shattuck TM, Valimaki S, Obara T, Gaz RD, Clark OH, Shoback D, Wierman ME, Tojo K, Robbins CM, Carpten JD, Farnebo LO, Larsson C, Arnold A. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med 349: 1722–1729, 2003. [DOI] [PubMed] [Google Scholar]
- 583.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci U S A 99: 11470–11475, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Shenolikar S, Weinman EJ. NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol 280: F389–395, 2001. [DOI] [PubMed] [Google Scholar]
- 585.Shi Y, Hogue J, Dixit D, Koh J, Olson JA Jr. Functional and genetic studies of isolated cells from parathyroid tumors reveal the complex pathogenesis of parathyroid neoplasia. Proc Natl Acad Sci U S A 111: 3092–3097, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Shigematsu T, Horiuchi N, Ogura Y, Miyahara T, Suda T. Human parathyroid hormone inhibits renal 24-hydroxylase activity of 25-hydroxyvitamin D by a mechanism involving adenosine 3′,5′-monophosphate in rats. Endocrinology 118: 1583–1589, 1986. [DOI] [PubMed] [Google Scholar]
- 587.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6505, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Shimizu N, Dean T, Tsang JC, Khatri A, Potts JT Jr, Gardella TJ. Novel parathyroid hormone (PTH) antagonists that bind to the juxtamembrane portion of the PTH/PTH-related protein receptor. J Biol Chem 280: 1797–1807, 2005. [DOI] [PubMed] [Google Scholar]
- 590.Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 359: 391–403, 2008. [DOI] [PubMed] [Google Scholar]
- 591.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol 22: 41–50, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: A “forme fruste” of an old disease. J Clin Endocrinol Metab 88: 5348–5352, 2003. [DOI] [PubMed] [Google Scholar]
- 593.Silverberg SJ, Walker MD, Bilezikian JP. Asymptomatic primary hyperparathyroidism. J Clin Densitom 16: 14–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol 103: 521–524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Singh AT, Radeff JM, Kunnel JG, Stern PH. Phosphatidylcholine-specific phospholipase C inhibitor, tricyclodecan-9-yl xanthogenate (D609), increases phospholipase D-mediated phosphatidylcholine hydrolysis in UMR-106 osteoblastic osteosarcoma cells. Biochim Biophys Acta 1487: 201–208, 2000. [DOI] [PubMed] [Google Scholar]
- 596.Sitara D. Correlation among hyperphosphatemia, type II sodium phosphate transporter activity, and vitamin D metabolism in Fgf-23 null mice. Ann N Y Acad Sci 1116: 485–493, 2007. [DOI] [PubMed] [Google Scholar]
- 597.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23: 421–432, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Slatopolsky E, Martin K, Hruska K. Parathyroid hormone metabolism and its potential as a uremic toxin. Am J Physiol 239: F1–12, 1980. [DOI] [PubMed] [Google Scholar]
- 599.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol 5: 7–19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol 300: C526–541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol 144(Pt A): 28–39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol 383: 181–192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Slovik DM, Adams JS, Neer RM, Holick MF, Potts JT Jr. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med 305: 372–374, 1981. [DOI] [PubMed] [Google Scholar]
- 604.Smith DP, Zhang XY, Frolik CA, Harvey A, Chandrasekhar S, Black EC, Hsiung HM. Structure and functional expression of a complementary DNA for porcine parathyroid hormone/parathyroid hormone-related peptide receptor. Biochim Biophys Acta 1307: 339–347, 1996. [DOI] [PubMed] [Google Scholar]
- 605.Smyk DS, Orfanidou T, Invernizzi P, Bogdanos DP, Lenzi M. vitamin D in autoimmune liver disease. Clin Res Hepatol Gastroenterol 37: 535–545, 2013. [DOI] [PubMed] [Google Scholar]
- 606.Sneddon WB, Magyar CE, Willick GE, Syme CA, Galbiati F, Bisello A, Friedman PA. Ligand-selective dissociation of activation and internalization of the parathyroid hormone (PTH) receptor: Conditional efficacy of PTH peptide fragments. Endocrinology 145: 2815–2823, 2004. [DOI] [PubMed] [Google Scholar]
- 607.Sneddon WB, Syme CA, Bisello A, Magyar CE, Rochdi MD, Parent JL, Weinman EJ, Abou-Samra AB, Friedman PA. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50). J Biol Chem 278: 43787–43796, 2003. [DOI] [PubMed] [Google Scholar]
- 608.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin D-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 111: 1666–1671, 2005. [DOI] [PubMed] [Google Scholar]
- 609.Sonneveld R, Ferre S, Hoenderop JG, Dijkman HB, Berden JH, Bindels RJ, Wetzels JF, van der Vlag J, Nijenhuis T. Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am J Pathol 182: 1196–1204, 2013. [DOI] [PubMed] [Google Scholar]
- 610.Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, Fleischer N, Sharp GW, Christakos S. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and beta cell lines. J Biol Chem 274: 34343–34349, 1999. [DOI] [PubMed] [Google Scholar]
- 611.Specker BL, Lichtenstein P, Mimouni F, Gormley C, Tsang RC. Calcium-regulating hormones and minerals from birth to 18 months of age: a cross-sectional study. II. Effects of sex, race, age, season, and diet on serum minerals, parathyroid hormone, and calcitonin. Pediatrics 77: 891–896, 1986. [PubMed] [Google Scholar]
- 612.St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys 473: 225–230, 2008. [DOI] [PubMed] [Google Scholar]
- 613.Stern JE, Cardinali DP. Influence of the autonomic nervous system on calcium homeostasis in the rat. Biol Signals 3: 15–25, 1994. [DOI] [PubMed] [Google Scholar]
- 614.Strickland LA, Bozzato RP, Kronis KA. Structure of human parathyroid hormone(1-34) in the presence of solvents and micelles. Biochemistry 32: 6050–6057, 1993. [DOI] [PubMed] [Google Scholar]
- 615.Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. Structural analysis of CYP2R1 in complex with vitamin D. J Mol Biol 380: 95–106, 2008. [DOI] [PubMed] [Google Scholar]
- 616.Sudhaker Rao D, Han ZH, Phillips ER, Palnitkar S, Parfitt AM. Reduced vitamin D receptor expression in parathyroid adenomas: Implications for pathogenesis. Clin Endocrinol (Oxf) 53: 373–381, 2000. [DOI] [PubMed] [Google Scholar]
- 617.Sutherland MK, Rao LG, Wylie JN, Gupta A, Ly H, Sodek J, Murray TM. Carboxyl-terminal parathyroid hormone peptide (53-84) elevates alkaline phosphatase and osteocalcin mRNA levels in SaOS-2 cells. J Bone Miner Res 9: 453–458, 1994. [DOI] [PubMed] [Google Scholar]
- 618.Syme CA, Friedman PA, Bisello A. Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J Biol Chem 280: 11281–11288, 2005. [DOI] [PubMed] [Google Scholar]
- 619.Takiishi T, Van Belle T, Gysemans C, Mathieu C. Effects of vitamin D on antigen-specific and non-antigen-specific immune modulation: Relevance for type 1 diabetes. Pediatr Diabetes 14: 81–89, 2013. [DOI] [PubMed] [Google Scholar]
- 620.Tamez H, Kalim S, Thadhani RI. Does vitamin D modulate blood pressure? Curr Opin Nephrol Hypertens 22: 204–209, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Tanaka Y, Castillo L, DeLuca HF. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc Natl Acad Sci U S A 73:2701–2705, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, Tuckey RC. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D, producing 1alpha,20,23-trihydroxyvitamin D, which has altered biological activity. Drug Metab Dispos 38: 1553–1559, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Tang WJ, Wang LF, Xu XY, Zhou Y, Jin WF, Wang HF, Gao J. Autocrine/paracrine action of vitamin D on FGF23 expression in cultured rat osteoblasts. Calcif Tissue Int 86: 404–410, 2010. [DOI] [PubMed] [Google Scholar]
- 624.Tawfeek HA, Che J, Qian F, Abou-Samra AB. Parathyroid hormone receptor internalization is independent of protein kinase A and phospholipase C activation. Am J Physiol Endocrinol Metab 281: E545–557, 2001. [DOI] [PubMed] [Google Scholar]
- 625.Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Mol Endocrinol (Baltimore, Md) 16: 1–13, 2002. [DOI] [PubMed] [Google Scholar]
- 626.Teh BT, Farnebo F, Twigg S, Hoog A, Kytola S, Korpi-Hyovalti E, Wong FK, Nordenstrom J, Grimelius L, Sandelin K, Robinson B, Farnebo LO, Larsson C. Familial isolated hyperparathyroidism maps to the hyperparathyroidism-jaw tumor locus in 1q21-q32 in a subset of families. J Clin Endocrinol Metab 83: 2114–2120, 1998. [DOI] [PubMed] [Google Scholar]
- 627.Teichert A, Elalieh H, Bikle D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J Cell Physiol 225: 482–489, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol 131: 2289–2297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Thakker RV, Juppner H. Genetic Disorders of Calcium Homeostasis Caused by Abnormal Regulation of Parathyroid Hormone Secretion or Responsiveness. Philadelphia: Elsevier, 2005. [Google Scholar]
- 630.Toka HR, Pollak MR. The role of the calcium-sensing receptor in disorders of abnormal calcium handling and cardiovascular disease. Curr Opin Nephrol Hypertens 23: 494–501, 2014. [DOI] [PubMed] [Google Scholar]
- 631.Tomaschitz A, Ritz E, Pieske B, Fahrleitner-Pammer A, Kienreich K, Horina JH, Drechsler C, Marz W, Ofner M, Pieber TR, Pilz S. Aldosterone and parathyroid hormone: A precarious couple for cardiovascular disease. Cardiovasc Res 94: 10–19, 2012. [DOI] [PubMed] [Google Scholar]
- 632.Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, Gaksch M, Grubler M, Fahrleitner-Pammer A, Mrak P, Toplak H, Kraigher-Krainer E, Marz W, Pilz S. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism 63: 20–31, 2014. [DOI] [PubMed] [Google Scholar]
- 633.Traebert M, Volkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1-34 and 3-34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am J Physiol Renal Physiol 278: F792–798, 2000. [DOI] [PubMed] [Google Scholar]
- 634.Trowbridge R, Kizer RT, Mittal SK, Agrawal DK. 1,25-dihydroxyvitamin D in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Expert Rev Clin Immunol 9: 517–533, 2013. [DOI] [PubMed] [Google Scholar]
- 635.Tsai KS, Chen JS, Hwang KM, Chieng PU, Su CT. Age-related changes in vitamin D metabolites, osteocalcin, alkaline phosphatase and parathyrin in normal Chinese women in Taipei. J Formos Med Assoc 90: 1033–1037, 1991. [PubMed] [Google Scholar]
- 636.Tsai KS, Heath H III, Kumar R, Riggs BL. Impaired vitamin D metabolism with aging in women. Possible role in pathogenesis of senile osteoporosis. J Clin Invest 73: 1668–1672, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Tsai KS, Wahner HW, Offord KP, Melton LJ III, Kumar R, Riggs BL. Effect of aging on vitamin D stores and bone density in women. Calcif Tissue Int 40: 241–243, 1987. [DOI] [PubMed] [Google Scholar]
- 638.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy metabolites of vitamin D by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos 39: 1577–1588, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Tuoresmaki P, Vaisanen S, Neme A, Heikkinen S, Carlberg C. Patterns of genome-wide VDR locations. PloS One 9: e96105, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Turner M, Barre PE, Benjamin A, Goltzman D, Gascon-Barre M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy? Miner Electrolyte Metab 14: 246–252, 1988. [PubMed] [Google Scholar]
- 641.Turunen MM, Dunlop TW, Carlberg C, Vaisanen S. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res 35: 2734–2747, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Unwin RJ, Capasso G, Shirley DG. An overview of divalent cation and citrate handling by the kidney. Nephron Physiol 98: 15–20, 2004. [DOI] [PubMed] [Google Scholar]
- 643.Urena P, Iida-Klein A, Kong XF, Juppner H, Kronenberg HM, Abou-Samra AB, Segre GV. Regulation of parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acid by glucocorticoids and PTH in ROS 17/2.8 and OK cells. Endocrinology 134: 451–456, 1994. [DOI] [PubMed] [Google Scholar]
- 644.Urena P, Kong XF, Abou-Samra AB, Juppner H, Kronenberg HM, Potts JT Jr, Segre GV. Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 133: 617–623, 1993. [DOI] [PubMed] [Google Scholar]
- 645.Usdin TB, Bonner TI, Hoare SR. The parathyroid hormone 2 (PTH2) receptor. Receptors Channels 8: 211–218, 2002. [PubMed] [Google Scholar]
- 646.Vadher S, Hawa NS, O’Riordan JL, Farrow SM. Translational regulation of parathyroid hormone gene expression and RNA: Protein interactions. J Bone Miner Res 11: 746–753, 1996. [DOI] [PubMed] [Google Scholar]
- 647.Vaidya A, Forman JP. Vitamin D and hypertension: Current evidence and future directions. Hypertension 56: 774–779, 2010. [DOI] [PubMed] [Google Scholar]
- 648.Valcheva P, Cardus A, Panizo S, Parisi E, Bozic M, Lopez Novoa JM, Dusso A, Fernandez E, Valdivielso JM. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 235: 247–255, 2014. [DOI] [PubMed] [Google Scholar]
- 649.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet 383: 1155–1167, 2014. [DOI] [PubMed] [Google Scholar]
- 650.van den Heuvel L, Op de Koul K, Knots E, Knoers N, Monnens L. Autosomal recessive hypophosphataemic rickets with hypercalciuria is not caused by mutations in the type II renal sodium/phosphate cotransporter gene. Nephrol Dial Transplant 16: 48–51, 2001. [DOI] [PubMed] [Google Scholar]
- 651.van der Hagen Ea, Lavrijsen M, van Zeeland F, Praetorius J, Bonny O, Bindels RJ, Hoenderop JG. Coordinated regulation of TRPV5-mediated Ca transport in primary distal convolution cultures. Pflugers Arch 2014. [DOI] [PubMed] [Google Scholar]
- 652.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1Alpha-hydroxylase expression and activity in human bone cells. FASEB J 20: 2417–2419, 2006. [DOI] [PubMed] [Google Scholar]
- 653.Varshney S, Bhadada SK, Arya AK, Sharma S, Behera A, Bhansali A, Rao SD. Changes in parathyroid proteome in patients with primary hyperparathyroidism due to sporadic parathyroid adenomas. Clinical endocrinology 81: 614–620, 2014. [DOI] [PubMed] [Google Scholar]
- 654.Varshney S, Bhadada SK, Sachdeva N, Arya AK, Saikia UN, Behera A, Rao SD. Methylation status of the CpG islands in vitamin D and calcium-sensing receptor gene promoters does not explain the reduced gene expressions in parathyroid adenomas. J Clin Endocrinol Metab 98: E1631–E1635, 2013. [DOI] [PubMed] [Google Scholar]
- 655.Vermeulen AH. The birth of endocrine pathology: How Erdheim misunderstood parathyroids. Virchows Arch 457: 283–290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol 21: 807–812, 2003. [DOI] [PubMed] [Google Scholar]
- 657.Vilardaga JP, Frank M, Krasel C, Dees C, Nissenson RA, Lohse MJ. Differential conformational requirements for activation of G proteins and the regulatory proteins arrestin and G protein-coupled receptor kinase in the G protein-coupled receptor for parathyroid hormone (PTH)/PTH-related protein. J Biol Chem 276: 33435–33443, 2001. [DOI] [PubMed] [Google Scholar]
- 658.Vilardaga JP, Gardella TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends Pharmacol Sci 33: 423–431, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J Biol Chem 277: 8121–8129, 2002. [DOI] [PubMed] [Google Scholar]
- 660.Vilardaga JP, Romero G, Friedman PA, Gardella TJ. Molecular basis of parathyroid hormone receptor signaling and trafficking: A family B GPCR paradigm. Cell Mol Life Sci 68: 1–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, Wood AR, Michaelsson K, Vandenput L, Zgaga L, Yerges-Armstrong LM, McCarthy MI, Dupuis J, Kaakinen M, Kleber ME, Jameson K, Arden N, Raitakari O, Viikari J, Lohman KK, Ferrucci L, Melhus H, Ingelsson E, Byberg L, Lind L, Lorentzon M, Salomaa V, Campbell H, Dunlop M, Mitchell BD, Herzig KH, Pouta A, Hartikainen AL, Streeten EA, Theodoratou E, Jula A, Wareham NJ, Ohlsson C, Frayling TM, Kritchevsky SB, Spector TD, Richards JB, Lehtimaki T, Ouwehand WH, Kraft P, Cooper C, Marz W, Power C, Loos RJ, Wang TJ, Jarvelin mR, Whittaker jC, Hingorani AD, Hypponen E. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10: e1001383, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. Vitamin D supplementation and risk of toxicity in pediatrics: A review of current literature. J Clin Endocrinol Metab 99: 1132–1141, 2014. [DOI] [PubMed] [Google Scholar]
- 663.Wade JB, Liu J, Coleman RA, Cunningham R, Steplock DA, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285: C1494–C1503, 2003. [DOI] [PubMed] [Google Scholar]
- 664.Wang MS, Kurokawa K. Renal gluconeogenesis: Axial and internephron heterogeneity and the effect of parathyroid hormone. Am J Physiol 246: F59–F66, 1984. [DOI] [PubMed] [Google Scholar]
- 665.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper jD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth dJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 376: 180–188, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-Dihydroxyvitamin D is a direct inducer of antimicrobial peptide gene expression. J Immunol 173: 2909–2912, 2004. [DOI] [PubMed] [Google Scholar]
- 667.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D target genes. Mol Endocrinol 19: 2685–2695, 2005. [DOI] [PubMed] [Google Scholar]
- 668.Wang Y, Borchert ML, Deluca HF. Identification of the vitamin D receptor in various cells of the mouse kidney. Kidney Int 81: 993–1001, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Wang Y, Deb DK, Zhang Z, Sun T, Liu W, Yoon D, Kong J, Chen Y, Chang A, Li YC. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol 23: 1977–1986, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys 523: 123–133, 2012. [DOI] [PubMed] [Google Scholar]
- 671.Ward DT, Riccardi D. New concepts in calcium-sensing receptor pharmacology and signalling. Br J Pharmacol 165: 35–48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Warner J, Epstein M, Sweet A, Singh D, Burgess J, Stranks S, Hill P, Perry-Keene D, Learoyd D, Robinson B, Birdsey P, Mackenzie E, Teh BT, Prins JB, Cardinal J. Genetic testing in familial isolated hyperparathyroidism: unexpected results and their implications. J Med Genet 41:155–160, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr 51: 1075–1081, 1990. [DOI] [PubMed] [Google Scholar]
- 674.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol 124: 1080–1082, 2005. [DOI] [PubMed] [Google Scholar]
- 675.Weinman EJ, Biswas RS, Peng G, Shen L, Turner CL, E X, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412–3420, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 676.Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: A tale of three hormones. Am J Physiol Renal Physiol 303: F321–F327, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Weinman EJ, Lederer ED. PTH-mediated inhibition of the renal transport of phosphate. Exp Cell Res 318: 1027–1032, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, Shenolikar S, Cunningham R. Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am J Physiol Renal Physiol 290: F838–843, 2006. [DOI] [PubMed] [Google Scholar]
- 679.Weinman EJ, Shenolikar S. The Na-H exchanger regulatory factor. Exp Nephrol 5: 449–452, 1997. [PubMed] [Google Scholar]
- 680.Weinman EJ, Steplock D, Tate K, Hall RA, Spurney RF, Shenolikar S. Structure-function of recombinant Na/H exchanger regulatory factor (NHE-RF). J Clin Invest 101: 2199–2206, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Weinman EJ, Steplock D, Zhang X, Akhter S, Shenolikar S. Molecular cloning of the cDNA and promoter sequences for the mouse sodium-hydrogen exchanger regulatory factor. Biochim Biophys Acta 1447: 71–76, 1999. [DOI] [PubMed] [Google Scholar]
- 682.Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PloS One 8: e54625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.White KE, Evans WE, O’Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, Grabowski M, Meitinger T, Strom TM. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000. [DOI] [PubMed] [Google Scholar]
- 684.Whitfield JF, Chakravarthy BR, Durkin JP, Isaacs RJ, Jouishomme H, Sikorska M, Williams RE, Rixon RH. Parathyroid hormone stimulates protein kinase C but not adenylate cyclase in mouse epidermal keratinocytes. J Cell Physiol 150: 299–303, 1992. [DOI] [PubMed] [Google Scholar]
- 685.Whitfield JF, Morley P, Willick G, Langille R, Ross V, MacLean S, Barbier JR. Cyclization by a specific lactam increases the ability of human parathyroid hormone (hPTH)-(1-31)NH2 to stimulate bone growth in ovariectomized rats. J Bone Miner Res 12: 1246–1252, 1997. [DOI] [PubMed] [Google Scholar]
- 686.Wikvall K. Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form (review). Int J Mol Med 7: 201–209, 2001. [DOI] [PubMed] [Google Scholar]
- 687.Wilfinger J, Seuter S, Tuomainen TP, Virtanen JK, Voutilainen S, Nurmi T, de Mello VD, Uusitupa M, Carlberg C. Primary vitamin D receptor target genes as biomarkers for the vitamin D status in the hematopoietic system. J Nutr Biochem 25: 875–884, 2014. [DOI] [PubMed] [Google Scholar]
- 688.Winkelman JW, Cannon DC, Pileggi CJ, Reed AH. Estimation of norms from a controlled sample survey. II. Influence of body habitus, oral contraceptives, and other factors on values for the normal range derived from the SMA 12-60 screening group of tests. Clin Chem 19: 488–491, 1973. [PubMed] [Google Scholar]
- 689.Winnacker JL, Yeager H, Saunders JA, Russell B, Anast CS. Rickets in children receiving anticonvulsant drugs. Biochemical and hormonal markers. Am J Dis Child (I960) 131: 286–290, 1977. [DOI] [PubMed] [Google Scholar]
- 690.Wirchow R. Die krankhaften Geschwulste Hirschwall A (ed). Berlin: 1863. [Google Scholar]
- 691.Witteveen JE, van Thiel S, Romijn JA, Hamdy NA. Hungry bone syndrome: Still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol 168: R45–R53, 2013. [DOI] [PubMed] [Google Scholar]
- 692.Wjst M, Altmuller J, Braig C, Bahnweg M, Andre E. A genome-wide linkage scan for 25-OH-D(3) and 1,25-(OH)2-D serum levels in asthma families. J Steroid Biochem Mol Biol 103: 799–802, 2007. [DOI] [PubMed] [Google Scholar]
- 693.Woitge HW, Knothe A, Witte K, Schmidt-Gayk H, Ziegler R, Lemmer B, Seibel MJ. Circaannual rhythms and interactions of vitamin D metabolites, parathyroid hormone, and biochemical markers of skeletal homeostasis: a prospective study. J Bone Miner Res 15: 2443–2450, 2000. [DOI] [PubMed] [Google Scholar]
- 694.Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: Its importance for beta cell and immune function. Mol Cell Endocrinol 347: 106–120, 2011. [DOI] [PubMed] [Google Scholar]
- 695.Wolden-Kirk H, Overbergh L, Gysemans C, Brusgaard K, Naamane N, Van Lommel L, Schuit F, Eizirik DL, Christesen H, Mathieu C. Unraveling the effects of 1,25OH2D on global gene expression in pancreatic islets. J Steroid Biochem Mol Biol 136: 68–79, 2013. [DOI] [PubMed] [Google Scholar]
- 696.Wolden-Kirk H, Rondas D, Bugliani M, Korf H, Van Lommel L, Brusgaard K, Christesen HT, Schuit F, Proost P, Masini M, Marchetti P, Eizirik DL, Overbergh L, Mathieu C. Discovery of molecular pathways mediating 1,25-dihydroxyvitamin D protection against cytokine-induced inflammation and damage of human and male mouse islets of Langerhans. Endocrinology 155: 736–747, 2014. [DOI] [PubMed] [Google Scholar]
- 697.Wolf G. The discovery of vitamin D: The contribution of Adolf Windaus. J Nutr 134: 1299–1302, 2004. [DOI] [PubMed] [Google Scholar]
- 698.Woudenberg-Vrenken TE, Bindels RJ, Hoenderop JG. The role of transient receptor potential channels in kidney disease. Nat Rev Nephrol 5: 441–449, 2009. [DOI] [PubMed] [Google Scholar]
- 699.Wray V, Federau T, Gronwald W, Mayer H, Schomburg D, Tegge W, Wingender E. The structure of human parathyroid hormone from a study of fragments in solution using 1H NMR spectroscopy and its biological implications. Biochemistry 33: 1684–1693, 1994. [DOI] [PubMed] [Google Scholar]
- 700.Wysolmerski JJ, Cormier S, Philbrick WM, Dann P, Zhang JP, Roume J, Delezoide AL, Silve C. Absence of functional type 1 parathyroid hormone (PTH)/PTH-related protein receptors in humans is associated with abnormal breast development and tooth impaction. J Clin Endocrinol Metab 86: 1788–1794, 2001. [DOI] [PubMed] [Google Scholar]
- 701.Yaghoobian J, Drueke TB. Regulation of the transcription of parathyroid-hormone/parathyroid-hormone-related peptide receptor mRNA by dexamethasone in ROS 17/2.8 osteosarcoma cells. Nephrol Dial Transplant 13: 580–586, 1998. [DOI] [PubMed] [Google Scholar]
- 702.Yamamoto S, Morimoto I, Yanagihara N, Zeki K, Fujihira T, Izumi F, Yamashita H, Eto S. Parathyroid hormone-related peptide-(1-34) [PTHrP-(1-34)] induces vasopressin release from the rat supraoptic nucleus in vitro through a novel receptor distinct from a type I or type II PTH/PTHrP receptor. Endocrinology 138: 2066–2072, 1997. [DOI] [PubMed] [Google Scholar]
- 703.Yamamoto S, Morimoto I, Zeki K, Ueta Y, Yamashita H, Kannan H, Eto S. Centrally administered parathyroid hormone (PTH)-related protein(1-34) but not PTH(1-34) stimulates arginine-vasopressin secretion and its messenger ribonucleic acid expression in supraoptic nucleus of the conscious rats. Endocrinology 139:383–388, 1998. [DOI] [PubMed] [Google Scholar]
- 704.Yamashita T. Structural and biochemical properties of fibroblast growth factor 23. Ther Apher Dial 9: 313–318, 2005. [DOI] [PubMed] [Google Scholar]
- 705.Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: A comprehensive review. Clin Rev Allergy Immunol 45: 217–226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Yang L, Ma J, Zhang X, Fan Y, Wang L. Protective role of the vitamin D receptor. Cell Immunol 279: 160–166, 2012. [DOI] [PubMed] [Google Scholar]
- 707.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896–F906, 2004. [DOI] [PubMed] [Google Scholar]
- 708.Yoshida T, Yoshino J, Hayashi M, Saruta T. Identification of a renal proximal tubular cell-specific enhancer in the mouse 25-hydroxyvitamin d 1alpha-hydroxylase gene. J Am Soc Nephrol 13: 1455–1463, 2002. [DOI] [PubMed] [Google Scholar]
- 709.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab 94: 3306–3313, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell 12: 129–141, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Yu X, Li C, Hong W, Pan W, Xie J. Autophagy during Mycobacterium tuberculosis infection and implications for future tuberculosis medications. Cell Signal 25: 1272–1278, 2013. [DOI] [PubMed] [Google Scholar]
- 712.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36: 971–977, 2005. [DOI] [PubMed] [Google Scholar]
- 713.Yu Y, Sanderson SR, Reyes M, Sharma A, Dunbar N, Srivastava T, Juppner H, Bergwitz C. Novel NaPi-IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: Long-term follow-up in one kindred. Bone 50: 1100–1106, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Yuan Q, Sitara D, Sato T, Densmore M, Saito H, Schuler C, Erben RG, Lanske B. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology 152: 4053–4061, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-dihydroxyvitamin D suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007. [DOI] [PubMed] [Google Scholar]
- 716.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86: 888–894, 2001. [DOI] [PubMed] [Google Scholar]
- 717.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 17: 509–511, 2003. [DOI] [PubMed] [Google Scholar]
- 718.Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology 149: 3656–3667, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Zhang D, Potty A, Vyas P, Lane J. The role of recombinant PTH in human fracture healing: a systematic review. J Orthop Trauma 28: 57–62, 2014. [DOI] [PubMed] [Google Scholar]
- 720.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol 276: F711–F719, 1999. [DOI] [PubMed] [Google Scholar]
- 721.Zhou AT, Assil I, Abou-Samra AB. Role of asparagine-linked oligosaccharides in the function of the rat PTH/PTHrP receptor. Biochemistry 39: 6514–6520, 2000. [DOI] [PubMed] [Google Scholar]
- 722.Zhou AT, Bessalle R, Bisello A, Nakamoto C, Rosenblatt M, Suva LJ, Chorev M. Direct mapping of an agonist-binding domain within the parathyroid hormone/parathyroid hormone-related protein receptor by photoaffinity crosslinking. Proc Natl Acad Sci U S A 94: 3644–3649, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Zhu C, Ye Z, Chen Z, Xia D, Hu J. Association between vitamin D receptor gene polymorphisms and idiopathic hypocitraturia in the Chinese population. Urol Int 85: 100–105, 2010. [DOI] [PubMed] [Google Scholar]
- 724.Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A 110: 15650–15655, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Zierold C, Mings JA, DeLuca HF. Regulation of 25-hydroxyvitamin D-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D and parathyroid hormone. J Cell Biochem 88: 234–237, 2003. [DOI] [PubMed] [Google Scholar]
- 726.Zierold C, Nehring JA, DeLuca HF. Nuclear receptor 4A2 and C/EBPbeta regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D-1alpha-hydroxylase. Arch Biochem Biophys 460: 233–239, 2007. [DOI] [PubMed] [Google Scholar]
- 727.Zittermann A. Vitamin D and cardiovascular disease. Anticancer Res 34: 4641–4648, 2014. [PubMed] [Google Scholar]