Abstract
Post-transplant cyclophosphamide (PTCy) is increasingly used to reduce graft-versus-host disease after hematopoietic cell transplantation (HCT); however, it might be associated with more infections. All patients who were ≥2 years old, receiving haploidentical or matched sibling donor (Sib) HCT for acute leukemias or myelodysplastic syndrome, and either calcineurin inhibitor (CNI)- or PTCy-based GVHD prophylaxis [Haploidentical HCT with PTCy (HaploCy), 757; Sibling with PTCy (SibCy), 403; Sibling with CNI-based (SibCNI), 1605] were included. Most bacterial infections occurred within the first 100 days; 953 patients (34.5%) had at least 1 infection and 352 patients (13%) had ≥2 infections. Patients receiving PTCy had a greater incidence of bacterial infections by day 180 [HaploCy 46%; SibCy 48%; SibCNI 35%; p<0.001). Compared with the SibCNI without infection cohort, 1.99-fold, 3.33-fold, 2.78-fold, and 2.53-fold increased TRM was seen for the HaploCy cohort without infection and HaploCy, SibCy, and SibCNI cohorts with infection, respectively. Bacterial infections increased mortality [HaploCy (HR1.84, 99% CI: 1.45–2.33, p<0.0001], SibCy cohort (HR,1.68, 99% CI: 1.30–2.19, p<0.0001), and SibCNI cohort (HR,1.76, 99% CI: 1.43–2.16, p<0.0001)]. PTCy was associated with increased bacterial infections regardless of donor, and bacterial infections were associated with increased mortality irrespective of GVHD prophylaxis. Patients receiving PTCy should be monitored carefully for bacterial infections following PTCy.
Keywords: Post-Transplantation cyclophosphamide, allogeneic, hematopoietic cell transplantation, bacterial infections, survival, Graft-versus-host disease
Introduction
Bacterial infections in patients undergoing allogeneic stem cell transplant (alloHCT) are associated with significant morbidity and mortality.1–5 Bacterial infections are highest during the pre-engraftment phase,6 and are influenced by diagnosis,7 graft source,8 and graft-versus-host disease (GVHD) prophylaxis.4, 9, 10
In recent years, the use of post-transplantation cyclophosphamide (PTCy) has significantly increased in alloHCT due to its association with decreased rates of GVHD.11–13 PTCy was first used in haploidentical donor HCT,14 and its use has been extended to other donor type HCTs.15 Despite the lowered incidence of GVHD in patients undergoing alloHCT with PTCy, increased viral infections have been reported in these patients.16–18 Additionally, recent analyses from the Center for International Blood and Marrow Transplant Research (CIBMTR) demonstrated an increased risk of Cytomegalovirus (CMV), community respiratory viral infections, and non-CMV herpes viral infections following PTCy in the haploidentical and matched sibling donor setting.19–21 Furthermore, several single-center analyses suggest increased rates of bacterial infections in patients receiving PTCy.22–24 However, these reports are limited by small sample size and do not include pediatric alloHCT recipients.
The purpose of this study was to evaluate bacterial infections in patients receiving PTCy. To do this, we evaluated three cohorts of patients in the CIBMTR database: patients undergoing alloHCT haploidentical HCT with PTCy (HaploCy), those receiving matched sibling donor grafts with PTCy GVHD prophylaxis (SibCy), and patients who received matched sibling donor grafts and calcineurin inhibitor based GVHD prophylaxis (SibCNI).
Material and Methods
Patient Population:
The study population was previously described.19 Briefly, we included all patients reported to the CIBMTR from 2012 to 2017 who were ≥2 years of age and undergoing first alloHCT for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS). Cohorts were defined by GVHD prophylaxis and included the following:
HaploCy: Individuals undergoing alloHCT and receiving a haploidentical graft from a mismatched related donor. Haploidentical HCT was defined as ≥2 antigen/allele mismatched within the loci HLA-A, -B, -C, -DRB1 and -DQB1 between donor and recipient.
SibCy: Those receiving matched sibling donor grafts with PTCy GVHD prophylaxis (SibCy)
SibCNI: Patients who received matched sibling donor grafts with calcineurin inhibitor based GVHD prophylaxis. This cohort included patients receiving CNI (i.e., tacrolimus or cyclosporine) with either methotrexate (MTX) ± other or mycophenolate mofetil (MMF) ± other.
Patients receiving anti-thymocyte globulin and/or alemtuzumab were excluded. Other exclusion criteria included single mismatch related donors and umbilical cord blood donors. Matched unrelated donor graft were excluded due to smaller numbers of unrelated donors with PTCy and inadequate infection data. To minimize ascertainment bias due to potentially different practices for infection screening and prophylaxis between centers, patients from centers without a patient in both the SibCNI and the HaploCy cohorts were excluded.
Data Source
The CIBMTR is a research consortium consisting of over 500 transplant centers internationally. Through a collaboration between the Medical College of Wisconsin and the National Marrow Donor Program, patient and outcomes data from these centers are collected and analyzed. Central auditing of the data is performed to ensure consistency and quality. The CIBMTR collects the Transplant Essential Data (TED) form and Comprehensive Report Form (CRF) prior to transplantation, at 100 days (D100), 6 months (D180), and 1 year after transplantation and annually thereafter. All patients included in this study gave written consent to participate in the CIBMTR Research Database and to have their data included in observational research. This study was approved by the institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program. Infection data are reported only on the CRF. Centers report infections in accordance with instructions in the forms manual.25 Data collected include an organism, site of infection, and date of onset. There are no data on diagnostic methodology, or treatment of infection. Infection prophylaxis information is limited prior to 2017.
Outcomes and Study Definitions
The primary outcomes of this study were the cumulative incidence of any bacterial infection, excluding Clostridioides difficile, and bacterial infection density by day 180 for the three cohorts defined by donor and GVHD prophylaxis [HaploCy, SibCy, SibCNI]. Infection density is the number of bacterial infections per patient per days at risk during the first 180 days.26 In addition to general bacterial infections, these outcomes were also examined for mucosal barrier injury–laboratory confirmed bloodstream infection (MBI-LCBI) and bacterial blood stream infections (BSI). MBI-LCBI used a modified definition as previously published.7, 27, 28 Other major outcomes were acute and chronic GVHD, overall survival (OS), disease-free survival (DFS), transplant-related mortality (TRM), and infection-related mortality (IRM). Patients were considered an event for IRM if the primary cause of death was infection either concurrent (± 30 days) with or prior to relapse.
Statistical Analysis
Patient-, disease- and transplant-related factors were compared between cohorts using the Chi-square test for categorical variables and the Wilcoxon two sample test for continuous variables. The univariate probability of OS was calculated using the Kaplan Meier estimator, with the variance estimated by Greenwood’s formula. For values for other endpoints, cumulative incidence estimates to account for competing risks were calculated.
Outcomes were examined in six groups defined by cohort and the presence/absence of infection. Because neutropenia and infection as well as acute GVHD (aGVHD) and infection are intertwined time-dependent events, it was necessary to examine the interaction using a dynamic landmark analysis at three landmark times for each univariate analysis, defined as the median and interquartile range for the event [MBI-LCBI, BSI, any bacterial infection, acute GVHD (aGVHD), or neutrophil recovery].29 Death was the competing risk for cumulative incidence of infection, acute and chronic GVHD. Relapse was a competing risk for IRM and TRM.
Cox proportional hazards regression was used for outcomes of OS, TRM, IRM, chronic GVHD (cGVHD), and relapse. The variables considered in the multivariable regression models are listed (Supplemental materials) and all results were examined for center effect.30 The assumption of proportional hazards for each factor in the Cox model was tested. When the proportional hazards assumption was violated, time-dependent variable was added in the model. The stepwise variable selection method was used to identify significant risk factors which associated with the outcomes. Factors significantly associated with the outcome variable at a 1% level were kept in the final model. As infections were expected to have the greatest impact around the time of infection, all outcomes were examined between day 100 to 2 years from transplant.
Data Sharing Statement
The final analysis dataset will be posted to the CIBMTR website at: https://cibmtr.org/CIBMTR/Resources/Publicly-Available-Datasets1#.
Results
Patient Characteristics
Detailed characteristics of 2765 HCTs (HaploCy, 757; SibCy, 403; and SibCNI, 1605) previously published in another CIBMTR study evaluating CMV are provided in Table 1.19 The median age of patients in the SibCNI cohort was significantly lower and had less patients with performance scores <80% than the other two groups. However, the HCT-CI was similar between the three cohorts. The SibCNI cohort received more myeloablative conditioning (MAC) and peripheral blood stem cells (PBSC) but less TBI-based conditioning and lower reported use of growth factor after HCT [HaploCy 82% vs SibCy 79% vs SibCNI 24%, p <0.001]. The SibCNI cohort also had a shorter time from diagnosis to HCT. Finally, in addition to PTCy, 99% of patients in the HaploCy cohort, and 95% in the SibCy cohort, received a calcineurin inhibitor (CNI).
Table 1.
Characteristics of patients receiving first Allogeneic HCT with or without PTCy conditioning regimen, reported to the CIBMTR, from 2012 to 2017
| Variable | Haplo-PTCy N(%) | SibCy N(%) | SibCNI N(%) | P value |
|---|---|---|---|---|
| Number of patients | 757 | 403 | 1605 | |
| Male | 459 (61) | 243 (60) | 933 (58) | |
| Age, median(range), years | 58 (3 – 78) | 46 (3 – 75) | 57 (2 – 78) | <0.001 |
| KPS/LPS <80 | 119 (16) | 65 (16) | 200 (12) | <0.001 |
| HCT-CI =>3 | 348 (46) | 175 (44) | 761 (48) | 0.817 |
| Prior Fungal Infection, Yes | 63 ( 8) | 44 (11) | 125 ( 8) | 0.138 |
| Race/Ethnicity | <0.001 | |||
| Caucasian, non-Hispanic | 444 (59) | 239 (59) | 1109 (69) | |
| African-American, non-Hispanic | 131 (17) | 56 (14) | 107 ( 7) | |
| Asian, non-Hispanic | 52 ( 7) | 29 ( 7) | 97 ( 6) | |
| Hispanic, Caucasian | 72 (10) | 45 (11) | 134 ( 8) | |
| Other | 10(1) | 6 (1) | 31 (2) | |
| Missing | 48 ( 6) | 28 ( 7) | 127 ( 8) | |
| Donor age, median(range), years | 36 (9 – 76) | 45 (4 – 72) | 54 (2 – 82) | <0.001 |
| Donor/Recipient CMV status | 0.04 | |||
| +/+ or −/+ | 543 (72) | 273 (68) | 1067 (67) | |
| +/− or −/− | 185 ( 24) | 115 ( 29) | 490 (30) | |
| Disease Status | <0.001 | |||
| AML/ALL, early | 308 (41) | 189 (47) | 719 (45) | |
| AML/ALL, intermediate | 143 (19) | 77 (19) | 210 (13) | |
| AML/ALL, advanced | 97 (13) | 61 (15) | 144 ( 9) | |
| AML/ALL, unknown | 6 (<1) | 2 (<1) | 15 (<1) | |
| MDS, early | 76 (10) | 24 ( 6) | 179 (11) | |
| MDS, advanced | 127 (17) | 50 (12) | 338 (21) | |
| Peripheral blood stem cells | 449 (59) | 272 (67) | 1405 (88) | <0.001 |
| MAC | 314 (41) | 222 (55) | 935 (58) | <0.001 |
| TBI (cGy) | <0.001 | |||
| No | 226 (30) | 169 (42) | 1169 (73) | |
| Yes and >800 | 115 (15) | 80 (20) | 264 (16) | |
| Growth Factor, Yes | 620 (82) | 319 (79) | 379 (24) | <0.001 |
| Time from diagnosis to transplant, median(range), months | 7 (1 – 165) | 7 (<1 – 396) | 5 (1 – 556) | <0.001 |
| Year of transplant | <0.001 | |||
| 2012–2014 | 170 ( 22) | 87 (22) | 806 ( 50) | |
| 2015–2017 | 587 (78) | 316 (78) | 799 (50) | |
| Median follow-up of survivors, months | 25 (3 – 74) | 25 (3 – 69) | 37 (2 – 75) |
Incidence of Bacterial Infections
A total of 1108 patients (40%) had at least one infection by day 180, with most infections occurring within the first 100 days. The median time to any bacterial infection was 12 – 13 days, which was similar between the three cohorts (p=0.595). Additionally, 5 to 6% of patients in each cohort had a new bacterial infection between days 100 and 180. (Figure 1A).
Figure 1A. Cumulative Incidence of Bacterial Infections by Donor Type/GVHD Prophylaxis in Day 180.
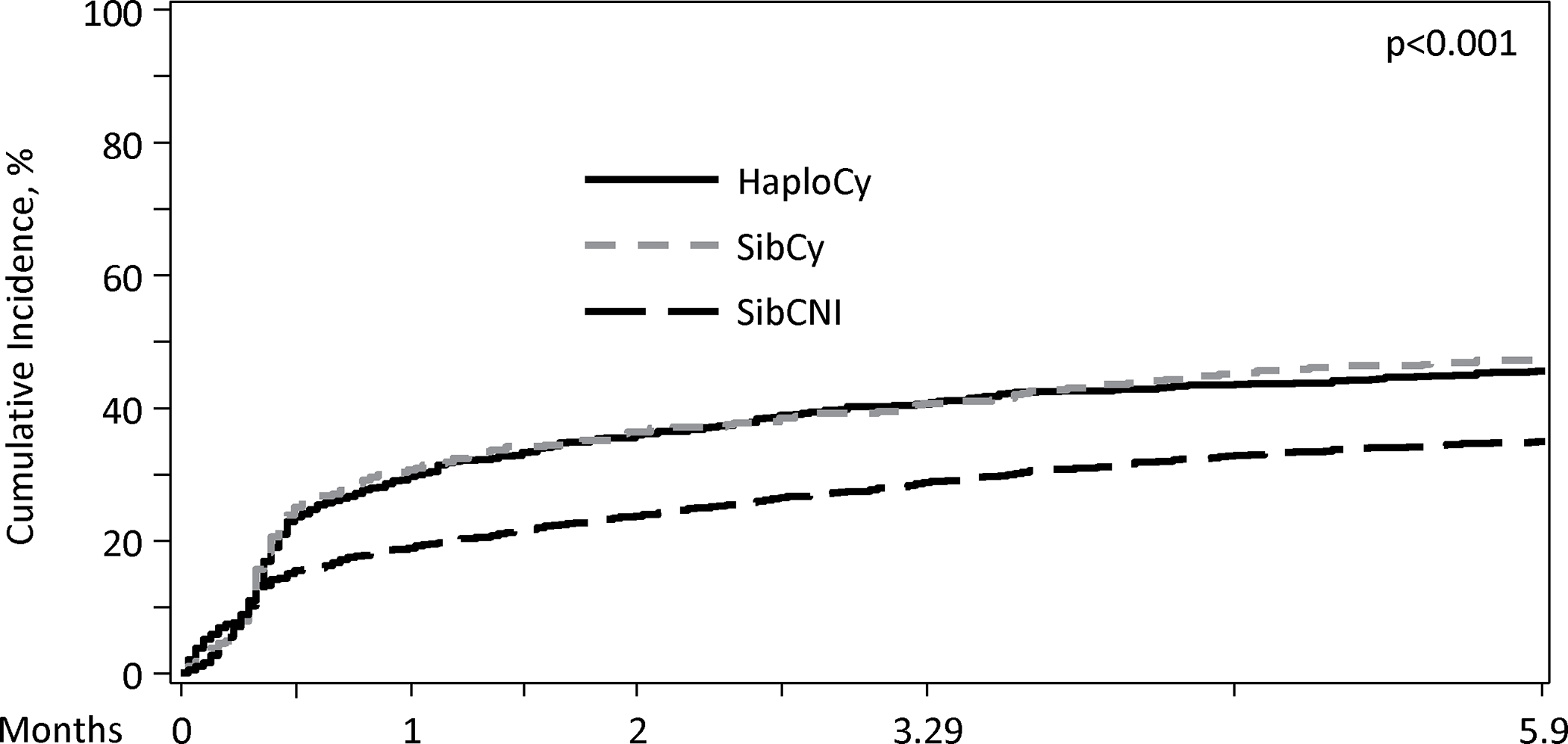
Patients receiving PTCy had a higher cumulative incidence of bacterial infections within first 180 days (HaploCy, 46.1% vs. SibCy, 48% vs. SibCNI, 35.3%; p<0.001) (Figure 1A and 1B). MBI-LCBI occurred more commonly in patients receiving PTCy (MBI-LCBI: HaploCy, 15.5% vs. SibCy,15.4% vs. SibCNI, 7.7%; p<0.001) (Figure 2A and B). Similarly, any bacterial BSI were more common in the PTCy cohorts (HaploCy, 24.7% vs. SibCy, 26.1% vs. SibCNI, 17.7%; p<0.001) within 180 days after alloHCT (Figure 2A and C).
Figure 1B. Frequency ofOne or More Bacterial Infection by Donor Type/GVHD Prophylaxis inDay 180.
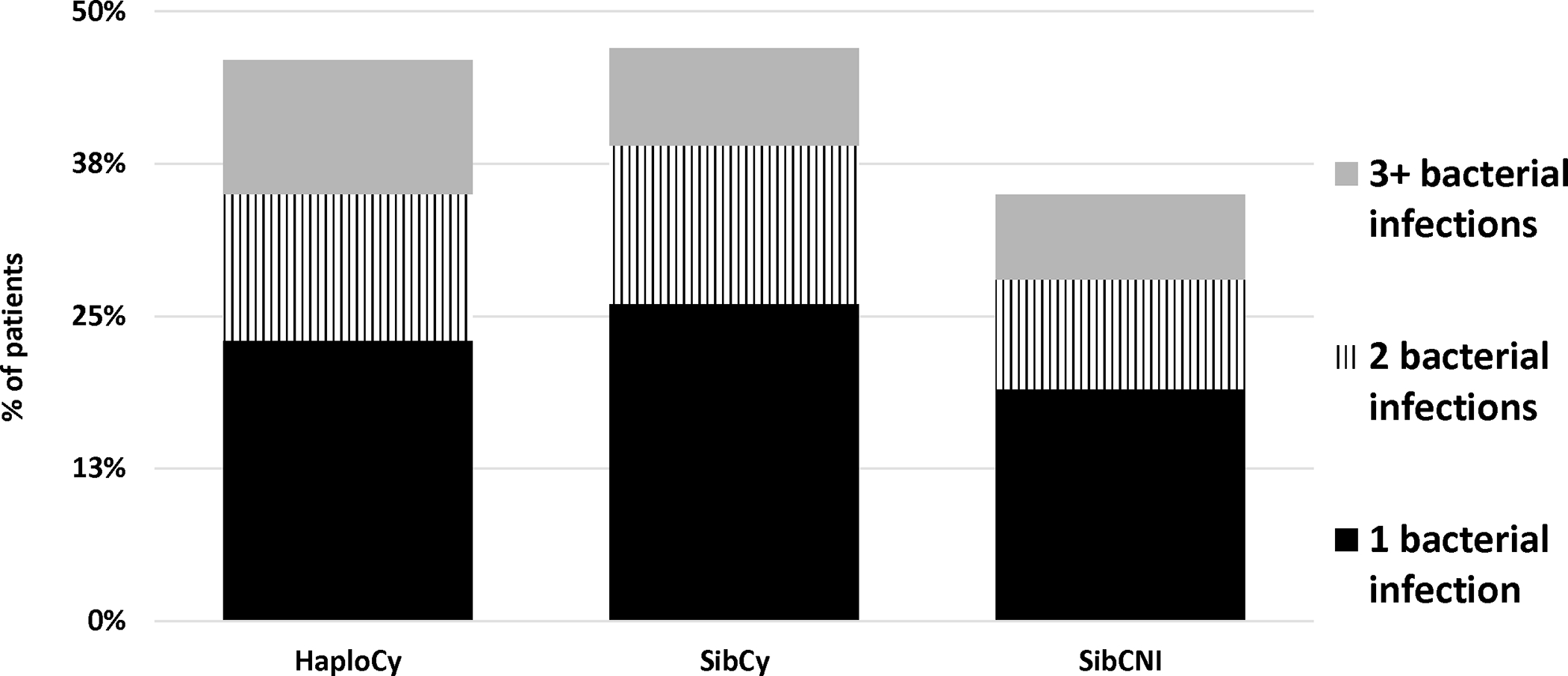
Figure 2A. Rate of MBI, BSI and All Bacterial Infections by Donor Type/GVHD Prophylaxis in Day 180.
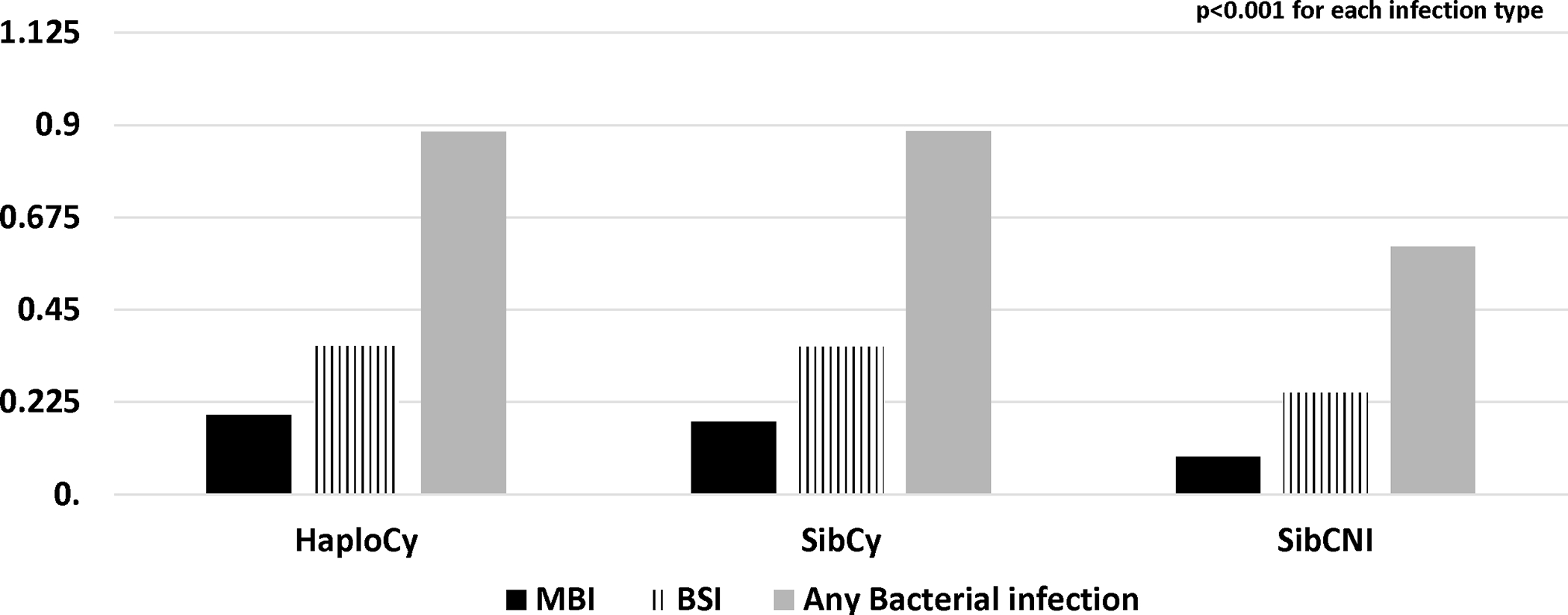
Figure 2B. Cumulative Incidence of MBI by Donor Type/GVHD Prophylaxis in Day 180.
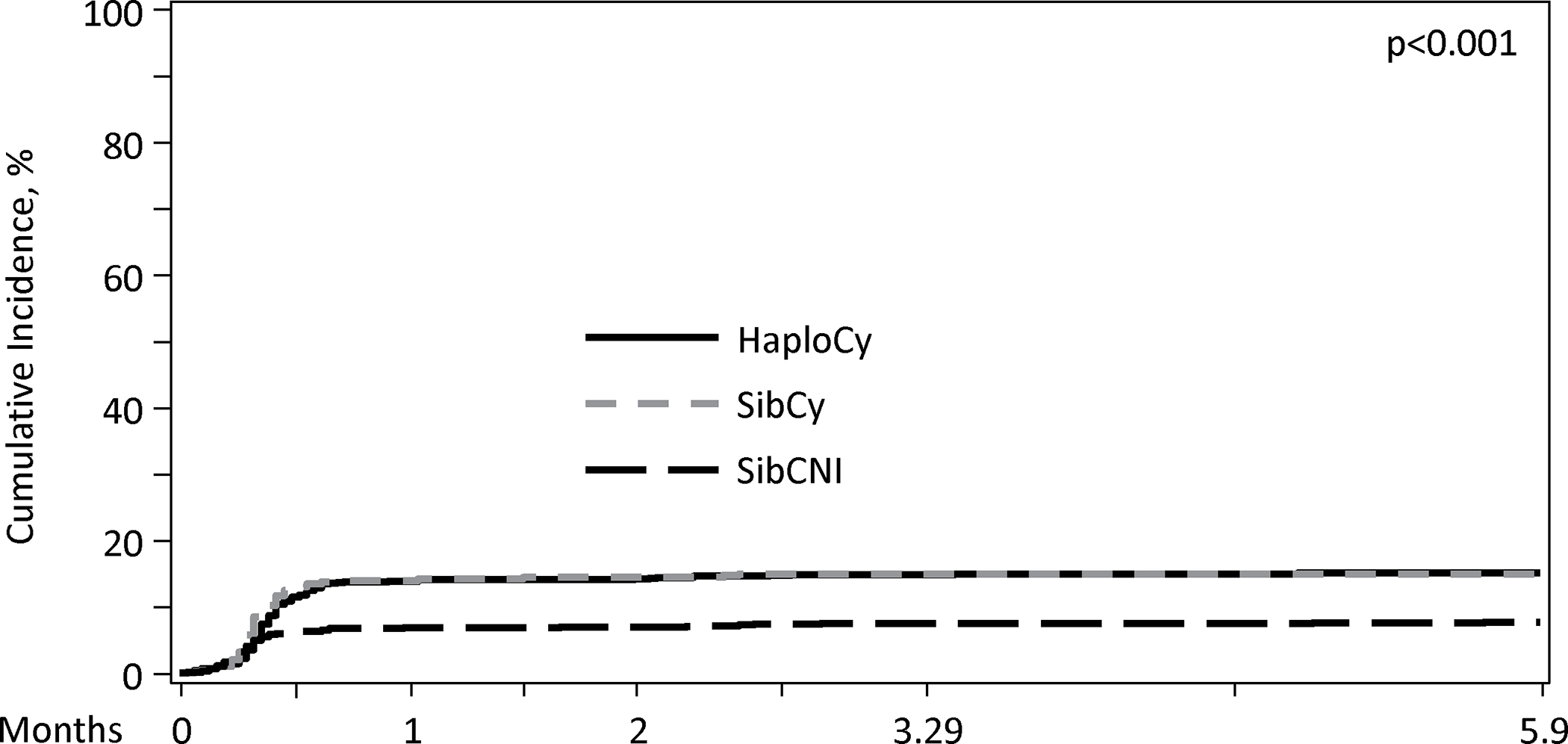
Figure 2C. Cumulative Incidence of any BSI by Donor Type/GVHD Prophylaxis in Day 180.
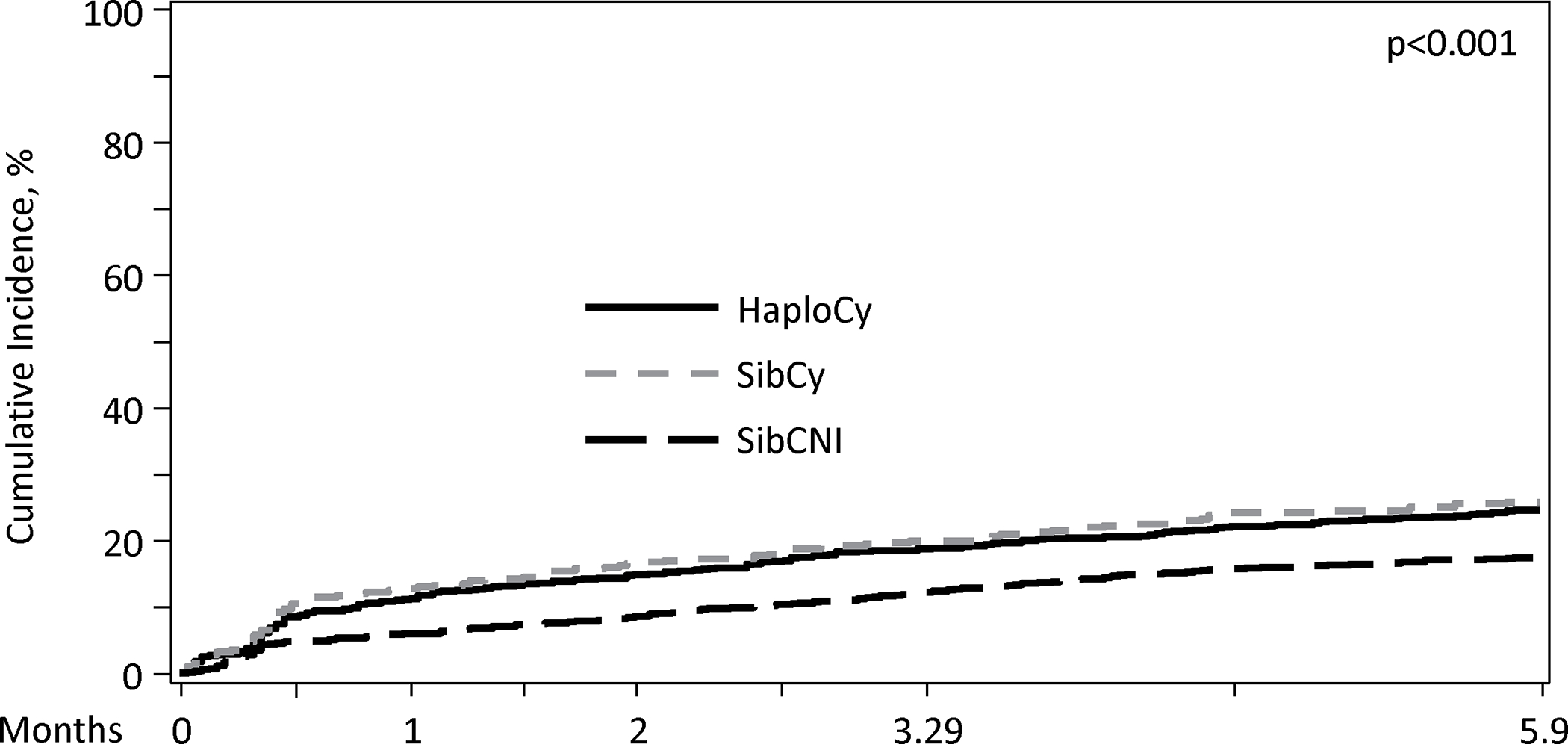
The median day of neutrophil engraftment was 16 days (IQR: 14 – 19) in the entire population [HaploCy: 17 days (range, 1 – 125 days); SibCy: 16 days (<1 – 61 days); SibCNI:15 days (1 – 73 days)]. Using 14 days (lower bound IQR) as the landmark, the cumulative incidence of any bacterial infection by 100 days was higher for patients receiving PTCy, irrespective of donor compared to patients in the SibCNI cohort [HaploCy 19.1% (99% CI, 14 – 24.9%); SibCy 19.4% (12.7–27.2%); SibCNI 12.2% (9.5 – 15.1%); p = 0.002]. Notably, this impact waned by day 180. Furthermore, examining the landmark of the median (16 days) and the upper bound of the IQR (19 days), significance was lost (16 days, p=0.013; 19 days p=0.04). When examining specifically for MBI-LCBI or any BSI, there was no difference between the cohorts at day 100 or day 180 for the median or the upper IQR; however, BSI at the day 14 landmark was higher in the PTCy cohorts [HaploCy 10% (6.5 – 14.1); SibCy 8.2% (4.2 – 13.4); SibCNI 5.1% (3.4 – 7); p = 0.006] (Supplemental Table 1).
Acute GVHD and infection are overlapping time-dependent events. Therefore, dynamic landmark analysis (DLA) was again used to examine the cumulative incidence of infection with the left truncation landmarks of the onset of acute GVHD [median: 38 days, IQR: 26 – 63 days]. There was no difference in the cumulative incidence of any bacterial infection, MBI-LCBI, or BSI between the cohorts at day 100 or day 180 at any of the landmarks assessed (Supplemental Table 2). Of note, in a separate landmark analysis, the impact of infections on acute or chronic GVHD was evaluated and found no impact on acute GVHD or chronic GVHD (Supplemental Table 3 and 4).
As noted previously, 517 patients (19%) had more than one bacterial infection in the first 100 days. To account for multiple infections and varied time evaluable due to early deaths, infection density was examined by cohort to establish the rate of infection in the first 180 days. For any bacterial infection, MBI-LCBI, and any BSI, infections were more likely in the PTCy cohorts irrespective of donor source. The rate of any bacterial infection was 0.884, 0.855, 0.604 (p<0.001) in HaploCy, SibCy, and SibCNI, respectively (Table 2). Overall MBI-LCBSI rates were 0.194, 0.177, and 0.092 (p<0.001) and any bacterial BSI rates were 0.36, 0.36, and 0.24 (p<0.001) in HaploCy, SibCy, and SIBCNI, respectively.
Table 2.
Infection density* by 180 days by cohort.
| Variable (by day 180) | HaploCy N(%) | SibCy N(%) | SibCNI N(%) | P value |
|---|---|---|---|---|
| Infection Density Scores | ||||
| Any bacterial infection | 0.884 | 0.855 | 0.604 | <0.001 |
| MBI-LCBI overall density score | 0.194 | 0.177 | 0.092 | <0.001 |
| BSI overall density score | 0.361 | 0.36 | 0.248 | <0.001 |
Infection density accounts for multiple infections and normalizes for survival to 180 days
Pathogens
In terms of bacteria type, vancomycin-resistant Enterococcus (VRE) and gram-negative rods (GNR) were more common in the PTCy cohorts by day 180. The frequency of VRE was 6%, 5%, and 3% in HaploCy, SibCy, and SibCNI, p<0.001, respectively. The frequency of GNR was 21%, 25%, and 15% in HaploCy, SibCy, and SibCNI, p=<.001, respectively. This was true for non-Enterobacteriaceae as well as Enterobacteriaceae GNR. (Supplemental Table 5 and Supplemental Figure 1).
TRM and Infection related mortality (IRM)
Patients diagnosed with a bacterial infection had a higher TRM by 2 years after HCT [HaploCy 27% (19–36); SibCy 20% (11–32%); SibCNI 19% (13 – 26%)] compared with those patients without a bacterial infection by day 16 [HaploCy 20% (15 – 24); SibCy 16% (11 – 23%); SibCNI 12% (10 – 15%); p=0.002] (Supplemental Table 6). The 2-year TRM was higher in the cohorts with any bacterial infection, irrespective of the landmark of median, lower (9 days), or upper (63 days) quartile examined. These findings were consistent when examining by BSI (median onset 48 days) and MBI-LCBI (median onset 10 days). Similar to 2-year TRM, 2-year IRM was lowest in the SibCNI without infection cohort [2.9% (1.7 – 4.5%)] and highest for the HaploCy with infection cohort [10.5% (4.7 – 18.1%)] and intermediate for the other 4 cohorts [with bacterial infection: SibCy 3.4% (0.2 – 10.4); SibCNI 7.1% (3 – 12.7%); without bacterial infection HaploCy 6.9% (4.1 – 10.5); SibCy 5.5% (2.2 – 10); p = 0.002] (Supplemental Table 7).
In multivariable analysis, development of bacterial infection increased TRM 3.33-fold, 2.78-fold, and 2.53-fold higher risk of TRM for the HaploCy, SibCy, and SibCNI cohorts (Table 3). The HaploCy cohort, even without bacterial infection, had a 1.99-fold higher risk of TRM. Additional risk factors associated with increased TRM were female-to-male-donor HCT, transplant for intermediate or high/very high risk MDS, development of acute GVHD, and lack of neutrophil engraftment (Table 3). Bacterial infections were the primary cause of death in 5%, 4%, and 4% of patients in HaploCy, SibCy, and SibCNI, respectively.
Table 3.
Multivariate analysis for OS and TRM
| OS | TRM | |||||||
|---|---|---|---|---|---|---|---|---|
| N | HR (99% CI) | P-value | Overall P-value | N | HR (99% CI) | P-value | Overall P-value | |
| Bacterial Infection | ||||||||
| BI no, SibCNI | 1027 | 1.00 | <.0001 | 1016 | 1.00 | <.0001 | ||
| BI yes, Haplo-PTCy | 345 | 1.84 (1.45 – 2.33) | <.0001 | 342 | 3.33 (2.09– 5.29) | <.0001 | ||
| BI yes, SibCy | 191 | 1.68 (1.30 – 2.19) | <.0001 | 191 | 2.78 (1.79 –4.33) | <.0001 | ||
| BI yes, SibCNI | 558 | 1.76 (1.43 – 2.16) | <.0001 | 551 | 2.53 (1.57 – 4.08) | <.0001 | ||
| BI no, Haplo-PTCy | 396 | 1.32 (1.02 – 1.71) | 0.0058 | 389 | 1.99 (1.24 – 3.18) | 0.0002 | ||
| BI no, SibCy | 205 | 1.21 (0.84 – 1.75) | 0.1727 | 201 | 1.69 (0.88 – 3.25) | 0.0400 | ||
| Donor-Recipient Sex Match | ||||||||
| Male-Male | 924 | 1.00 | 0.0001 | |||||
| Male-Female | 618 | 0.94 (0.68 – 1.30) | 0.6041 | |||||
| Female-Male | 655 | 1.33 (1.01 –1.77) | 0.0082 | |||||
| Female-Female | 493 | 0.76 (0.53 – 1.10) | 0.0601 | |||||
| Age at transplant, Years | ||||||||
| 0–20 | 236 | 1.00 | <.0001 | |||||
| 21–40 | 487 | 0.90 (0.61 – 1.32) | 0.4660 | |||||
| 41–60 | 955 | 1.34 (0.91 – 1.97) | 0.0482 | |||||
| >60 | 1044 | 1.70(1.09 – 2.66) | 0.0021 | |||||
| Disease Risk by disease status and cytogenetic risks | ||||||||
| AL favorable cytogenetic early/intermediate stage | 69 | 1.00 | <.0001 | 68 | 1.00 | 0.0001 | ||
| AL intermediate/nl cytogenetic early stage | 675 | 1.02 (0.58 – 1.81) | 0.9267 | 667 | 1.32 (0.58 – 3.00) | 0.3822 | ||
| AL poor cytogenetic early stage | 439 | 0.99 (0.58 – 1.68) | 0.9598 | 435 | 0.97 (0.44 – 2.12) | 0.9219 | ||
| AL int/nl cytogenetic intermediate stage | 215 | 1.19 (0.68 – 2.09) | 0.4269 | 210 | 1.28 (0.57 – 2.89) | 0.4275 | ||
| AL poor cytogenetic intermediate stage | 126 | 1.32 (0.72 – 2.42) | 0.2306 | 126 | 1.16 (0.45 – 3.00) | 0.6963 | ||
| AL advanced (all cytogenetic categories) | 296 | 1.93 (1.07 – 3.50) | 0.0044 | 294 | 1.26 (0.56 – 2.82) | 0.4691 | ||
| MDS very low/low | 312 | 0.94 (0.54 – 1.66) | 0.7911 | 310 | 1.67 (0.68 – 4.09) | 0.1437 | ||
| MDS intermediate | 227 | 1.69 (1.00 – 2.88) | 0.0105 | 225 | 2.24 (0.92 – 5.45) | 0.0198 | ||
| MDS high/very high | 164 | 2.11 (1.15 – 3.87) | 0.0016 | 160 | 2.49 (0.99 – 6.25) | 0.0108 | ||
| Other/not test/missing | 199 | 1.28 (0.67 – 2.46) | 0.3287 | 195 | 1.44 (0.55 – 3.78) | 0.3353 | ||
| Acute GVHD, grade II - IV | ||||||||
| No | 1744 | 1.00 | <.0001 | 1724 | 1.00 | <.0001 | ||
| Yes | 978 | 1.56 (1.28 – 1.91) | 966 | 2.60 (1.71 – 3.96) | ||||
| Neutrophil engraftment | ||||||||
| No | 69 | 1.00 | <.0001 | 69 | 1.00 | <.0001 | ||
| Yes | 2653 | 0.20 (0.11 – 0.35) | 2621 | 0.12 (0.07 – 0.22) | ||||
| Contrasts | ||||||||
| BI yes, Haplo-PTCy vs. BI yes, SibCy | 1.09 (0.81 – 1.47) | 0.4475 | 1.19 (0.77 – 1.86) | 0.3027 | ||||
| BI yes, Haplo-PTCy vs. BI yes, SibCNI | 1.05 (0.83 – 1.33) | 0.6173 | 1.32 (0.90 – 1.93) | 0.0635 | ||||
| BI yes, Haplo-PTCy vs. BI no, Haplo-PTCy | 1.39 (1.01 – 1.91) | 0.0072 | 1.67 (0.97 – 2.90) | 0.0159 | ||||
| BI yes, Haplo-PTCy vs BI no, SibCy | 1.51 (1.06 – 2.17) | 0.0029 | 1.97 (1.11 – 3.50) | 0.0023 | ||||
| BI yes, SibCy vs . BI yes, SibCNI | 0.96 (0.75 – 1.23) | 0.6616 | 1.10 (0.74 – 1.63) | 0.5243 | ||||
| BI yes, SibCy vs BI no, HaploCy | 1.27 (0.92 – 1.77) | 0.0552 | 1.40 (0.84 – 2.35) | 0.0933 | ||||
| BI yes, SibCy vs. BI no, SibCNI | 1.39 (0.93 – 2.07) | 0.0342 | 1.65 (0.91 – 3.01) | 0.0313 | ||||
| BI yes, SibCNI vs BI no, Haplo-PTCy | 1.33 (1.00 – 1.76) | 0.0098 | 1.27 (0.75 –2.16) | 0.2445 | ||||
| BI yes, SibCNI vs. BI no, SibCy | 1.45 (1.03 – 2.02) | 0.0046 | 1.50 (0.82 – 2.74) | 0.0854 | ||||
| BI no, HaploCy vs BI no, SibCy | 1.09 (0.75 – 1.58) | 0.5560 | 1.18 (0.62 – 2.25) | 0.5106 | ||||
Overall Survival
Relapse was the main cause of all mortalities in each cohort (>60%) (Supplemental Table 8). By the landmark of 16 days, patients with any bacterial infection had a lower OS by 1 year after HCT [HaploCy 61% (51 – 70); SibCy 68% (55–79%); SibCNI 61% (53 – 69%)] compared with those patients without a bacterial infection in the same period [HaploCy 66% (61 – 71); SibCy 68% (60 – 75%); SibCNI 71% (68 – 74%); p<0.001] (Supplemental Table 9). Although the difference became less prominent, inferior survival persisted at 2 years. These findings were consistent when examining by BSI (median onset 48 days). Notably, the 1- and 2-year OS was not impacted by the development of MBI-LCBI by 10 days post-HCT
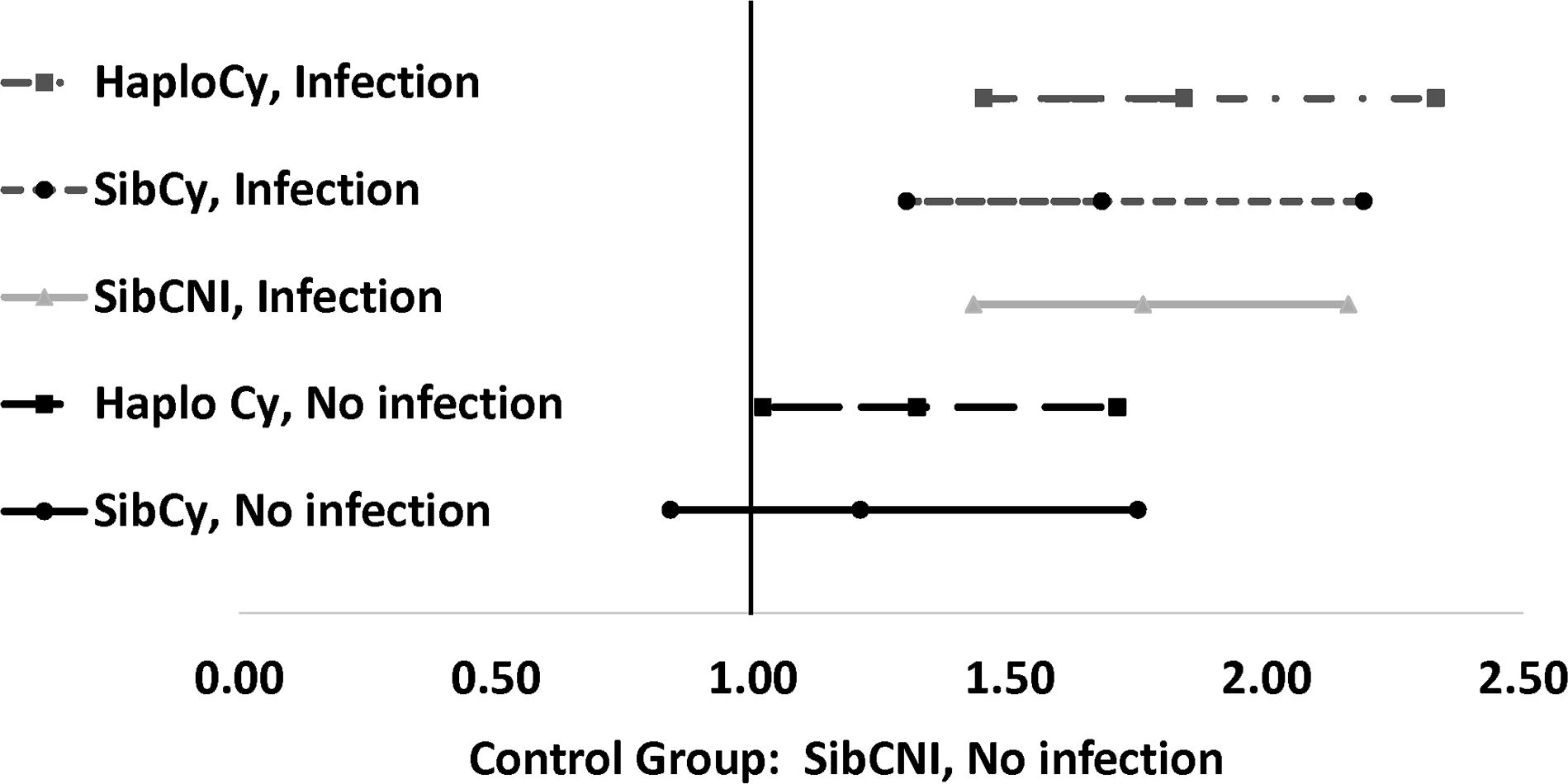
In multivariable analysis, compared with the SibCNI without bacterial infection cohort, development of bacterial infection increased mortality for the HaploCy, SibCy, and SIBCNI cohorts (Table 3 and Figure 3). The HaploCy cohort, even without bacterial infection, had a 1.32-fold higher risk of mortality. Additional factors increasing the risk of death in the MVA included age of recipient >60 years, advanced acute leukemia, high/very high risk MDS, development of acute GVHD prior to infection, and lack of neutrophil engraftment prior to infection.
Figure 3. 2-year Hazard Ratio of Mortality by Bacterial Infection by Day 180.
Discussion
In this large study, our main finding is that bacterial infections are more common with PTCy regardless of donor type (i.e., haploidentical siblings or HLA-matched siblings). In addition, we demonstrated that early bacterial infections are associated with increased TRM as well as overall mortality in all 3 types of transplantation investigated.
Many studies showed a high incidence of bacterial infections (e.g., 40% to 64%) after HaploCy following PTCy prophylaxis within 1 year.31–33 The preponderance of these bacterial infections (approximately 30–40%) occurred in early phase (e.g., 30 days).31–33 This is likely increased risk of infections in the pre-engraftment period given that median time to neutrophil engraftment is approximately 3 weeks after HaploCy.31, 33, 34
It is well-known that bacterial infections after an allogeneic HCT are associated with mortality.5 Most studies also showed bacterial infections after Haploidentical HCT following PTCy was associated with a higher mortality. Slade et al reported that risk of mortality increases by 2.32 (95%CI, 1.23–4.3) times when bacterial infection occurred after haploidentical HCT.33 In a Spanish Group for Hematopoietic Stem Cell Therapy study, IRM at year 1 was found to be 17% after HaploCy, and bacterial infections were the most common cause of IRM (51%). Likewise, the French stem cell transplantation group showed that bacterial infections constituted 46% of TRM.32 In a comparison study, patients with acute lymphoblastic leukemia receiving HaploCy (>90% patients receiving PTCy) had significantly higher IRM compared with those receiving SibCNI (>90% patients receiving non-PTCy) (33.1% vs. 19.7%).35
Following studies that used PTCy not only in Haploidentical HCT but also in other donor types shed some light whether this increased bacterial infections and higher IRM primarily due to donor type, GVHD prophylaxis or both. Khimani et al showed similar results in a relatively smaller single center study from Moffit Cancer Center.36 PTCy was used in 75 haploidentical and in 38 MUD HCTs whereas a CNI-based GVHD prophylaxis was used in 470 MUD HCTs. Overall infection density was significantly more common in PTCy patients (5.0 vs approximately 2 per 1000-person days; p<0.01) within the first year of alloHCT, and this difference resulted from higher bacterial and viral infections. Immune reconstitution analysis showed that PTCy led to slower CD4+ cell but faster CD19+ cell recovery. Salas et al. recent study revealed 2.4 times higher BSI in the first 30 days after PTCy compared with other GVHD prophylaxis regimens in 330 patients receiving an alloHCT from various donors, including in MRD, MUD, and MMUD, and Haploidentical an alloHCT.37
Supporting further the suggestion that GVHD prophylaxis has a more prominent impact than donor per se on infections, the study by Ciurea et al evaluated haploidentical HCT patients’ outcomes in two cohorts; the ones received PTCy and unmanipulated stem cells vs. those received antithymocyte globulin (ATG) and CD34+ selected stem cells.38 IRM (although mainly due to viral and fungal infections) was significantly higher in the latter group (9% vs.24%, p=0.01). This indicates that GVHD prophylaxis affects infections and IRM even in the same donor type HCT (i.e., HaploHCT). Indeed, Goldsmith et al using our same CIBMTR patient population showed that cumulative incidences of CMV infection by day 180 in patients receiving HaploCy, (n = 757), SibCy (n = 403) and SibCNI (n= 1605) were 42%, 37%, and 23%, respectively (p <0.001).19 PTCy also increased other viral infections in the haploidentical and matched sibling donor setting.20, 21 This observation is further supported by other studies using PTCy in different donor types; a single center, recent study used PTCy for 3 different donor type HCTs (i.e., haploidentical, MRD, and 1-allele mismatched unrelated donor, n=117).34 In this study, neither bacterial infections nor IRM was different among the donor types. Another study from Italy investigated PTCy in 235 patients, including 62% Hapto-PTCy, 21% MUD-PTCy, and 17% SibCy.24
Donor type had no effect on pre-engraftment bacterial infections although impacted viral and fungal infections. IRM at day 180 was 8%, patients had pre-engraftment bacterial infection had a higher mortality: GN BSI, 14% vs. GP BSI,7% vs. no BSI,2%, (Gray’s test: p =0.010). IRM was not impacted by donor type.24
In this study, we showed that PTCy increases bacterial infections and TRM regardless of donor type. However, it is also important to note that patients receiving HaploCy, irrespective of bacterial infection, had a higher risk of death compared to the SibCNI cohort that did not develop bacterial infections. Furthermore, looking at the patients who developed any bacterial infection, there was no impact of donor or PTCy as all patients had a higher risk of death ranging for 1.68x – 1.84x that of the SibCNI cohort without infection. This effect of infection did not seem related to increased acute or chronic GVHD in our study.
Risk factors for IRM after PTCy were found to be delayed neutrophil engraftment, older age, lymphoid malignancy, and the presence of severe GVHD also increased IRM.24, 34 In our study, we showed that the neutropenia following PTCy increased bacterial infections but that the incidence of bacterial infections was not different between the cohorts following the onset of aGVHD.
Enterobacteriaceae seemed to be more in PTCy patients in our study. Similar to our findings, in another HaploCy study, gram-negative bacilli were the most prevalent bacteria (59.3%) with a majority of Enterobacteriaceae.32 In another study, Enterobacteriaceae species were the most common documented gram-negative (66%) and all bacteria (31.6%) after HaploCy.33 The majority of gram-negative bacterial infection occurred after engraftment32, 34 and associated with more mortality34 whereas gram-positive bacteria were more common in the pre-engraftment period.24, 34 Although it is logical to think that gram-negative infections were associated with acute GVHD, the findings in the literature remain controversial.33, 34 In fact, given that PTCy is generally associated with less GVHD, this also undermines the potential relation between acute GVHD and gram negative bacteremia observed in the later phases of HCTs.
Our study limitations are inherent to the retrospective nature of the study including lacking the the reasons a center chose to use a PTCy for GVHD prophylaxis in a matched sibling transplant. Another limitation is that data on antibiotic prophylaxis, treatment, or evidence of multidrug-resistance were not captured in this transplant registry. Lack of these data might limit our interpretation of the results and outcomes following HCT. Our goal was to create as homogeneous population as possible to discern if PTCy or a haploidentical donor or both increased the risk of infection. The number of patients receiving MUD with PTCy was limited, therefore the effect of PTCy could not be evaluated. Similarly, there were low number of patients who received ATG; thus, to avoid additional confounders, these patients were not included in the study. However, our study has several important strengths including, first, a robust sample size from 102 centers from diverse geographic locations and reflecting current transplant practices. The inclusion of multiple centers provides a diverse population of all ages (our study included pediatric population as well), most of the common stem cell sources and transplant types; however, it also results in a small percentage of missing data. Given that it is less than 5% for nearly all pertinent variables, these data are unlikely to change the overall outcomes in this large dataset. It is also likely to minimize over or underreporting biases inherent in single center studies. Uniform definitions were used for data collection stipulated by CIBMTR and long term follow up is ensured. Second bacterial infections especially in 180 days are significant clinical events that are likely to be reported, even if the patient is no longer at the HCT center.
In conclusion, PTCY is a risk factor for early bacterial infections, TRM and OS regardless of donor type. As PTCy is almost always used with a combination of immunosuppressive drugs (e.g., CNI), increased infections may be more expected compared with only CNI-based regimens. A subset of patients enrolled in the BMT CTN 1703 trial co-enrolled in the BMT CTN 1801 trial which prospectively captured detailed infectious complications including antimicrobial prophylaxis and treatment.39 These forthcoming results will add to the literature and include MUD HCT patients. Regardless, this study shows that PTCy (another great GVHD prophylaxis option e.g., decreased chronic GVHD) has adverse effects on infections. Therefore, appropriate preventions and close monitoring of patients for high-risk infections are needed.
Supplementary Material
Funding Statement:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); 75R60222C00011 from the Health Resources and Services Administration (HRSA); N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Regimmune; Sanofi; Sanofi-Aventis U.S. Inc.; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
Footnotes
Conflict-of-interest disclosure: Dr. Batista reports compensation (honoraria) from from United Medical, Astra Zeneca, and Merck Sharp Dohme; and research support from National Health Institute-National Cancer Institute (NIH-NCI), National Council for Scientific and Technological Development (CNPq) and FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo.
Dr. Battiwalla reports research support (institution) from Novartis.
Dr. Cerny reports consulting fees (Advisory Board Membership) for Jazz Pharmaceuticals, Pfizer, and Amgen; Data Safety Monitoring Board member for Allovir Inc.; and stocks with Actinium Pharmaceuticals, Bluebird Bio/2Seventy, Dynavax Technologies, aTyr Pharma, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart, and Veru.
Dr. Hill reports compensation (consulting) for Karius; research support from Karius; and significant payments (Scientific Advisory Board) for Karius (limit $10,000).
Dr. Liu reports compensation (advisor/consultant/speaker) for Agios, Pfizer, CTI Biopharm, Nkarta, Servier; research support (funding paid to my institution) for clinical trials from Miltenyi Biotec, Alexion, Astellas, Nkarta, ImmuOnco, Cellularity and Marker.
Dr. Munshi reports Speaker’s Bureau participation for Incyte and Kite; and consultancy for Incyte.
Dr. Wingard reports compensation (consultant) for Cidara, Celgene, Merck, Shire, Orca and Ansun.
Dr. Chemaly reports compensation (advisor/consultant/speaker) for Merck, Takeda, Karius, Therapeutics Partners, Ansun, Eurofins-Viracor, Genentech, Qiagen, Oxford Immunotec, Astellas, Roche, Aseptico, AiCuris, and Shinogi; and significant payments (grants and funding paid to my institution for research) from Merck, Ansun, AiCuris, Eurofins-Viracor, Karius, Genentech, Freestyle, and Takeda.
Dr. Perales reports compensation (honoraria) from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma; Data Safety Monitoring Board participation for Cidara Therapeutics, Medigene, and Sellas Life Sciences; scientific advisory board participation for NexImmune; ownership interests in NexImmune and Omeros; significant payments (honoraria in excess of $5,000) from Bristol-Myers Squibb, Incyte, Kite/Gilead, Merck, Nektar Therapeutics, Novartis, Omeros, Cidara Therapeutics (DSMB) and research support (institutional in excess of $5000) for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; and equity interest in Omeros.
Dr. Riches is an employee of IQVIA Biotech, a clinical research organization.
Dr. Papanicolaou reports compensation (consultant/other) for Allovir, Astellas, Cidara, Merck, Takeda, Therapeutics Partners and Octapharma; and research funding (paid to my institution) from Merck and Takeda.
Data Use Statement:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Data Availability Statement:
Materials described in the manuscript, including all relevant raw data, belongs to CIBMTR and will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality per discussion with CIBMTR.
References:
- 1.Vydra J, Shanley RM, George I, Ustun C, Smith AR, Weisdorf DJ et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55(6): 764–770. e-pub ahead of print 20120612; doi: 10.1093/cid/cis550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M et al. Infection Rates among Acute Leukemia Patients Receiving Alternative Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2016; 22(9): 1636–1645. e-pub ahead of print 20160622; doi: 10.1016/j.bbmt.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanicolaou GA, Ustun C, Young JH, Chen M, Kim S, Ahn KW et al. Bloodstream infection (BSI) due to Vancomycin-Resistant Enterococcus (VRE) is associated with increased mortality after hematopoietic cell transplantation for acute leukemia and myelodysplastic syndrome: A multicenter, retrospective cohort study. Clin Infect Dis 2019. e-pub ahead of print 2019/01/17; doi: 10.1093/cid/ciz031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ustun C, Kim S, Chen M, Beitinjaneh AM, Brown VI, Dahi PB et al. Increased overall and bacterial infections following myeloablative allogeneic HCT for patients with AML in CR1. Blood Adv 2019; 3(17): 2525–2536. doi: 10.1182/bloodadvances.2019000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustun C, Young JH, Papanicolaou GA, Kim S, Ahn KW, Chen M et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2019; 54(8): 1254–1265. e-pub ahead of print 20181213; doi: 10.1038/s41409-018-0401-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhmedov M, Klyasova G, Kuzmina L, Fedorova A, Vasilyeva V, Drokov M et al. Incidence, etiology, risk factors, and outcomes of pre-engraftment bloodstream infections after first and second allogeneic hematopoietic cell transplantation. Transpl Infect Dis 2022; 24(3): e13842. e-pub ahead of print 2022/05/03; doi: 10.1111/tid.13842 [DOI] [PubMed] [Google Scholar]
- 7.Dandoy CE, Kim S, Chen M, Ahn KW, Ardura MI, Brown V et al. Incidence, Risk Factors, and Outcomes of Patients Who Develop Mucosal Barrier Injury-Laboratory Confirmed Bloodstream Infections in the First 100 Days After Allogeneic Hematopoietic Stem Cell Transplant. JAMA Netw Open 2020; 3(1): e1918668. e-pub ahead of print 20200103; doi: 10.1001/jamanetworkopen.2019.18668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi S, Ogura S, Araoka H, Uchida N, Mitsuki T, Yuasa M et al. The impact of graft cell source on bloodstream infection in the first 100 days after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2021; 56(7): 1625–1634. e-pub ahead of print 2021/02/21; doi: 10.1038/s41409-021-01229-6 [DOI] [PubMed] [Google Scholar]
- 9.Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transplant 2017; 52(8): 1091–1106. e-pub ahead of print 20170327; doi: 10.1038/bmt.2017.14 [DOI] [PubMed] [Google Scholar]
- 10.Nathan S, Ustun C. Complications of Stem Cell Transplantation that Affect Infections in Stem Cell Transplant Recipients, with Analogies to Patients with Hematologic Malignancies. Infect Dis Clin North Am 2019; 33(2): 331–359. e-pub ahead of print 20190330; doi: 10.1016/j.idc.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 11.Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016; 127(11): 1502–1508. e-pub ahead of print 2016/01/15; doi: 10.1182/blood-2015-10-672071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14(6): 641–650. doi: 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saliba RM, Alousi AM, Pidala J, Arora M, Spellman SR, Hemmer MT et al. Characteristics of Graft-Versus-Host Disease (GvHD) After Post-Transplantation Cyclophosphamide Versus Conventional GvHD Prophylaxis. Transplant Cell Ther 2022; 28(10): 681–693. e-pub ahead of print 20220716; doi: 10.1016/j.jtct.2022.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCurdy SR, Luznik L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Hematology Am Soc Hematol Educ Program 2019; 2019(1): 513–521. doi: 10.1182/hematology.2019001323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster JA, Luznik L, Tsai HL, Imus PH, DeZern AE, Pratz KW et al. Allogeneic transplantation for Ph+ acute lymphoblastic leukemia with posttransplantation cyclophosphamide. Blood Adv 2020; 4(20): 5078–5088. doi: 10.1182/bloodadvances.2020002945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith SR, Slade M, DiPersio JF, Westervelt P, Lawrence SJ, Uy GL et al. Cytomegalovirus viremia, disease, and impact on relapse in T-cell replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Haematologica 2016; 101(11): e465–e468. e-pub ahead of print 20160721; doi: 10.3324/haematol.2016.149880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huntley D, Gimenez E, Pascual MJ, Hernandez-Boluda JC, Gago B, Vazquez L et al. Incidence, features, and outcomes of cytomegalovirus DNAemia in unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Transpl Infect Dis 2020; 22(1): e13206. e-pub ahead of print 20191115; doi: 10.1111/tid.13206 [DOI] [PubMed] [Google Scholar]
- 18.Jamy O, Hebert C, Dunn-Valadez S, Magnusson T, Watts N, McGwin G et al. Risk of Cytomegalovirus Infection with Post-Transplantation Cyclophosphamide in Haploidentical and HLA-Matched Unrelated Donor Transplantation. Transplant Cell Ther 2022. e-pub ahead of print 20220121; doi: 10.1016/j.jtct.2022.01.011 [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood 2021; 137(23): 3291–3305. doi: 10.1182/blood.2020009362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Dandoy CE, Chen M, Kim S, Mulroney CM, Kharfan-Dabaja MA et al. Post-transplantation cyclophosphamide is associated with an increase in non-cytomegalovirus herpesvirus infections in patients with acute leukemia and myelodysplastic syndrome. Transplantation and cellular therapy 2022; 28(1): 48. e41–48. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulroney CM, Abid MB, Bashey A, Chemaly RF, Ciurea SO, Chen M et al. Incidence and impact of community respiratory viral infections in post-transplant cyclophosphamide-based graft-versus-host disease prophylaxis and haploidentical stem cell transplantation. British journal of haematology 2021; 194(1): 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esquirol A, Pascual MJ, Kwon M, Pérez A, Parody R, Ferra C et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant 2021; 56(10): 2432–2444. e-pub ahead of print 2021/06/02; doi: 10.1038/s41409-021-01328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikulska M, Raiola AM, Galaverna F, Balletto E, Borghesi ML, Varaldo R et al. Pre-Engraftment Bloodstream Infections after Allogeneic Hematopoietic Cell Transplantation: Impact of T Cell-Replete Transplantation from a Haploidentical Donor. Biol Blood Marrow Transplant 2018; 24(1): 109–118. e-pub ahead of print 20170830; doi: 10.1016/j.bbmt.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 24.Oltolini C, Greco R, Galli L, Clerici D, Lorentino F, Xue E et al. Infections after Allogenic Transplant with Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biol Blood Marrow Transplant 2020; 26(6): 1179–1188. e-pub ahead of print 20200128; doi: 10.1016/j.bbmt.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 25.manual Cfi. Q428–440: infection. In: Milwaukee, WI: The Medical College of Wisconsin, Inc, 2020. [Google Scholar]
- 26.Tomblyn M, Young JA, Haagenson MD, Klein JP, Trachtenberg EA, Storek J et al. Decreased infections in recipients of unrelated donor hematopoietic cell transplantation from donors with an activating KIR genotype. Biol Blood Marrow Transplant 2010; 16(8): 1155–1161. e-pub ahead of print 20100301; doi: 10.1016/j.bbmt.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See I, Iwamoto M, Allen-Bridson K, Horan T, Magill SS, Thompson ND. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol 2013; 34(8): 769–776. e-pub ahead of print 20130627; doi: 10.1086/671281 [DOI] [PubMed] [Google Scholar]
- 28.Epstein L, See I, Edwards JR, Magill SS, Thompson ND. Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections (MBI-LCBI): Descriptive Analysis of Data Reported to National Healthcare Safety Network (NHSN), 2013. Infect Control Hosp Epidemiol 2016; 37(1): 2–7. e-pub ahead of print 20151012; doi: 10.1017/ice.2015.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Logan B, Riches M, Chen M, Ahn KW. Statistical Methods for Time-Dependent Variables in Hematopoietic Cell Transplantation Studies. Transplant Cell Ther 2021; 27(2): 125–132. e-pub ahead of print 20201002; doi: 10.1016/j.bbmt.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime data analysis 1995; 1(2): 145–156. [DOI] [PubMed] [Google Scholar]
- 31.Crocchiolo R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis 2015; 17(2): 242–249. doi: 10.1111/tid.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fayard A, Daguenet E, Blaise D, Chevallier P, Labussiere H, Berceanu A et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: a study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant 2019; 54(10): 1586–1594. e-pub ahead of print 20190215; doi: 10.1038/s41409-019-0475-7 [DOI] [PubMed] [Google Scholar]
- 33.Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis 2017; 19(1). e-pub ahead of print 20161228; doi: 10.1111/tid.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irene GC, Albert E, Anna BV, Rahinatu A, Silvana N, Silvana S et al. Patterns of infection and infectious-related mortality in patients receiving post-transplant high dose cyclophosphamide as graft-versus-host-disease prophylaxis: impact of HLA donor matching. Bone Marrow Transplant 2021; 56(4): 818–827. e-pub ahead of print 20201026; doi: 10.1038/s41409-020-01092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. J Hematol Oncol 2021; 14(1): 53. e-pub ahead of print 20210401; doi: 10.1186/s13045-021-01065-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khimani F, Ranspach P, Elmariah H, Kim J, Whiting J, Nishihori T et al. Increased Infections and Delayed CD4(+) T Cell but Faster B Cell Immune Reconstitution after Post-Transplantation Cyclophosphamide Compared to Conventional GVHD Prophylaxis in Allogeneic Transplantation. Transplant Cell Ther 2021; 27(11): 940–948. e-pub ahead of print 20210728; doi: 10.1016/j.jtct.2021.07.023 [DOI] [PubMed] [Google Scholar]
- 37.Salas MQ, Charry P, Puerta-Alcalde P, Martinez-Cibrian N, Solano MT, Serrahima A et al. Bacterial Bloodstream Infections in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation With Post-Transplantation Cyclophosphamide. Transplant Cell Ther 2022. e-pub ahead of print 20220909; doi: 10.1016/j.jtct.2022.09.001 [DOI] [PubMed] [Google Scholar]
- 38.Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18(12): 1835–1844. e-pub ahead of print 20120711; doi: 10.1016/j.bbmt.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtan SG, Hamadani M, WU J, AL Malki MM, Runaas L, Elmariah H et al. Post-Transplant Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil As the New Standard for Graft-Versus-Host Disease (GVHD) Prophylaxis in Reduced Intensity Conditioning: Results from Phase III BMT CTN 1703. Blood 2022; 140(Supplement 2): LBA-4–LBA-4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Materials described in the manuscript, including all relevant raw data, belongs to CIBMTR and will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality per discussion with CIBMTR.


