Key Points
Question
Does the administration of β-lactam antibiotics by prolonged infusion reduce 90-day mortality compared with intermittent infusion in adult patients with sepsis or septic shock?
Findings
This systematic review and bayesian meta-analysis of 18 randomized trials that included 9108 critically ill adults with sepsis or septic shock reported a 99.1% posterior probability that prolonged infusions were associated with lower 90-day mortality compared with intermittent infusions (risk ratio, 0.86).
Meaning
Prolonged infusions of β-lactam antibiotics are associated with a reduced risk of death in critically ill adult patients with sepsis or septic shock compared with intermittent infusions.
This systematic review and bayesian meta-analysis investigated whether administration of β-lactam antibiotics by prolonged infusion reduces 90-day mortality compared with intermittent infusion in adult patients with sepsis or septic shock.
Abstract
Importance
There is uncertainty about whether prolonged infusions of β-lactam antibiotics improve clinically important outcomes in critically ill adults with sepsis or septic shock.
Objective
To determine whether prolonged β-lactam antibiotic infusions are associated with a reduced risk of death in critically ill adults with sepsis or septic shock compared with intermittent infusions.
Data Sources
The primary search was conducted with MEDLINE (via PubMed), CINAHL, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov from inception to May 2, 2024.
Study Selection
Randomized clinical trials comparing prolonged (continuous or extended) and intermittent infusions of β-lactam antibiotics in critically ill adults with sepsis or septic shock.
Data Extraction and Synthesis
Data extraction and risk of bias were assessed independently by 2 reviewers. Certainty of evidence was evaluated with the Grading of Recommendations Assessment, Development and Evaluation approach. A bayesian framework was used as the primary analysis approach and a frequentist framework as the secondary approach.
Main Outcomes and Measures
The primary outcome was all-cause 90-day mortality. Secondary outcomes included intensive care unit (ICU) mortality and clinical cure.
Results
From 18 eligible randomized clinical trials that included 9108 critically ill adults with sepsis or septic shock (median age, 54 years; IQR, 48-57; 5961 men [65%]), 17 trials (9014 participants) contributed data to the primary outcome. The pooled estimated risk ratio for all-cause 90-day mortality for prolonged infusions of β-lactam antibiotics compared with intermittent infusions was 0.86 (95% credible interval, 0.72-0.98; I2 = 21.5%; high certainty), with a 99.1% posterior probability that prolonged infusions were associated with lower 90-day mortality. Prolonged infusion of β-lactam antibiotics was associated with a reduced risk of intensive care unit mortality (risk ratio, 0.84; 95% credible interval, 0.70-0.97; high certainty) and an increase in clinical cure (risk ratio, 1.16; 95% credible interval, 1.07-1.31; moderate certainty).
Conclusions and Relevance
Among adults in the intensive care unit who had sepsis or septic shock, the use of prolonged β-lactam antibiotic infusions was associated with a reduced risk of 90-day mortality compared with intermittent infusions. The current evidence presents a high degree of certainty for clinicians to consider prolonged infusions as a standard of care in the management of sepsis and septic shock.
Trial Registration
PROSPERO Identifier: CRD42023399434
Introduction
Critically ill adults who develop sepsis and septic shock face high morbidity and mortality. Early and appropriate antibiotic administration is central to the treatment of such patients. There is uncertainty about effective antibiotic dosing, specifically the duration of infusion in this patient population, due to physiologic perturbations and supportive treatments that may alter antibiotic pharmacokinetics.1,2,3 Pathogens causing an infection during an intensive care unit (ICU) admission may have reduced antibiotic susceptibility.
β-Lactam antibiotics are widely used as first-line antibiotics for the treatment of sepsis and septic shock. These agents display time-dependent bactericidal activity that is optimal when the free drug concentration remains above the minimum inhibitory concentration of the infecting pathogen for at least 40% to 70% of the dosing interval.4 There is a biological rationale that prolonged infusions of β-lactam antibiotics may be more effective compared with conventional intermittent dosing.5,6 This rationale is supported by pharmacokinetic-pharmacodynamic studies, which demonstrate that prolonged infusions achieve β-lactam antibiotic exposures associated with maximal bacterial-killing more consistently than intermittent infusions.7 Whether the effects of prolonged β-lactam antibiotic infusion compared with intermittent infusion result in improved patient-centered outcomes remains uncertain.8,9,10,11,12,13
Two recently published multinational randomized clinical trials, the Continuous Infusion vs Intermittent Administration of Meropenem in Critically Ill Patients (MERCY)14 and the Beta-Lactam Infusion Group (BLING) III15 trials, have added substantially to the body of evidence. To provide an updated summary of current evidence, this systematic review and bayesian meta-analysis was conducted to assess whether administration of β-lactam antibiotics by prolonged infusion was associated with reduced 90-day all-cause mortality and other relevant outcomes compared with intermittent infusion.
Methods
A systematic review of randomized clinical trials was performed according to a prespecified published protocol (eAppendix 1 in Supplement 1).16 The review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement17 and was registered at the International Prospective Register of Systematic Reviews (CRD42023399434).
Eligibility Criteria
Randomized clinical trials that recruited critically ill adult participants with sepsis or septic shock and compared the administration of prolonged infusions with intermittent infusions of 1 or more β-lactam antibiotics were included. Conventional and current definitions of sepsis and septic shock at participant recruitment were accepted.18,19,20,21 Prolonged infusion was defined as either an extended infusion (intravenous β-lactam antibiotic administration for 2 hours or longer during a dosing interval) or a continuous infusion (constant intravenous β-lactam antibiotic administration that could be administered as a sequential 6-, 8-, 12-, or 24-hour infusion). Intermittent infusion was defined as intravenous β-lactam antibiotic administration for fewer than 2 hours during a dosing interval.
Search Strategy
A systematic search of MEDLINE (via PubMed), CINAHL, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov from inception to May 2, 2024, was conducted. The search was performed with no restrictions on language, publication date, or publication status. The search terms were created by a research librarian (L.E.) in collaboration with content area experts in antibiotic pharmacokinetics-pharmacodynamics, critical care, and infectious diseases. The search strategy included a combination of key words and Medical Subject Headings terms to identify randomized clinical trials that included “critically ill patients” or “intensive care unit,” “sepsis” or “septic shock,” “beta-lactam” or “carbapenem” or “cephalosporin” or “monobactam” or “penicillin,” and “continuous infusion” or “extended infusion” or “prolonged infusion” or “intermittent infusion.”
Manual searches of reference lists of included studies and other systematic reviews were undertaken to identify additional studies. eAppendix 2 in Supplement 1 provides additional details of the electronic search strategy.
Study Selection
Using the Covidence systematic review software (Veritas Health Innovation), a minimum of 2 reviewers (H.E., E.N., or I.Z.) independently screened all identified references for inclusion based on the study title and abstract. A minimum of 2 reviewers (H.E., E.N., or I.Z.) independently assessed the full text for inclusion of potentially eligible studies, with disagreements resolved by consensus or, if necessary, consultation with a third reviewer (M.H.A.-A., N.E.H., A.D., or J.A.R.).
Data Collection
Two reviewers (H.E. and I.Z.) independently extracted data from each included study by using a standardized data collection form. Discrepancies were resolved by consensus or, if necessary, by consultation with a third reviewer (M.H.A.-A.). Available data were extracted as outlined in the protocol (eAppendix 1 in Supplement 1),16 including characteristics of the included studies, study design, demographic and clinical details of the study population, details of the intervention and comparison group (study antibiotic, study antibiotic dosing regimen, and concomitant antibiotics), and study outcomes. Attempts were made to contact corresponding authors of included studies to obtain essential aggregate-level data. There was no imputation for missing data. Access to aggregate-level data of 2 trials15,22 before their publication was obtained from the respective corresponding authors.
Risk of Bias Assessment
Using the Cochrane Risk of Bias Tool for randomized trials version 2, 2 reviewers (H.E. and I.Z.) with no affiliation with the included trials independently assessed the risk of bias for each trial. The risk of bias was assessed for all outcomes of interest. Any discrepancies were resolved by consensus or, if necessary, consultation with a third reviewer (M.H.A.-A., N.E.H., or A.D.).
Outcomes
The primary outcome was all-cause 90-day mortality. For studies in which 90-day mortality was not reported, the closest time to day 90 (before or after) was used.
Data were also collected for the following secondary outcomes: ICU mortality, ICU length of stay (as reported in the original study), clinical cure (as defined in the original study), microbiologic cure (as defined in the original study), and adverse events (as defined in the original study).
Subgroup Analyses
There were 7 prespecified subgroups for the primary outcome: (1) administration of meropenem vs piperacillin-tazobactam; (2) culture-positive infection vs culture-negative infection; (3) gram-negative infection vs gram-positive infection; (4) receipt of kidney replacement therapy vs no kidney replacement therapy; (5) lung infection vs other infections; (6) sepsis vs septic shock; and (7) male vs female participants. The prespecified hypotheses for these comparisons are detailed in the protocol (eAppendix 1 in Supplement 1).16 When results suggested possible subgroup effects, the Instrument to Assess the Credibility of Effect Modification Analyses (ICEMAN)23 guidelines were used to assess their credibility.
Data Synthesis
A bayesian framework was used as the primary statistical approach, and a frequentist framework was used as the secondary approach. A random-effects model was used in the analyses and pooled estimates of effect sizes as risk ratios (RRs) for binary outcomes, and mean differences for continuous outcomes were presented. Continuous variables presented in formats not readily amenable to pooling were converted to mean and SD with the method described by Wan et al.24 Along with the pooled estimates of effect sizes, 95% credible intervals (CrIs) for the bayesian meta-analysis and 95% CIs for the frequentist model were presented.
For the bayesian approach, primary analysis using vague priors (log of the RR assumed to have a normal distribution with a mean of 0 and an SD of 2) and sensitivity analyses examining treatment effects using weakly informative priors of effect and heterogeneity parameters were conducted.25 The full description of priors is presented in the protocol (eAppendix 1 and eAppendix 3 in Supplement 1).16 For the frequentist approach, a random-effects model using Hartung-Knapp-Sidik-Jonkman26 and DerSimonian-Laird estimates of the between-study variance was used. A random-effects model was chosen a priori for all analyses because of anticipated between-study variation in trial design and implementation of the interventions.
Quantitative heterogeneity was assessed with the posterior estimates of the heterogeneity parameter (τ) with its 95% CrI. The proportion of variation across studies owing to heterogeneity rather than chance was assessed with the I2 statistic. Subgroup heterogeneity was assessed by including an interaction term in the bayesian analysis to obtain an estimate and 95% CrI for the ratio of RRs (RRRs) from the posterior distribution of the interaction estimate. The presence of small-study effects was assessed by visual assessment of the contour-enhanced funnel plots and formal Egger regression test.27,28
All statistical analyses were performed with R version 4.3.1 (R Foundation for Statistical Computing). The bayesian analysis was performed with the bayesmeta package for bayesian analysis,29 and the metafor package was used for the frequentist analysis.30
Confidence in the Cumulative Evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to evaluate the overall certainty of evidence that prolonged infusions of β-lactam antibiotics compared with intermittent infusions improve each outcome measure to any degree.31,32
Results
The results of the search and reasons for study exclusion are detailed in eFigure 1 and eTable 1 in Supplement 1. From 2494 records, 18 eligible randomized clinical trials including 9108 critically ill adult participants with sepsis or septic shock were included (5961 men [65%] and 3147 women [35%]).14,15,22,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 Table 1 presents the characteristics of included trials. Details on microbiologic characteristics, β-lactam antibiotic dosing regimens, and outcome definitions of included trials are summarized in eTables 2 and 3 in Supplement 1. Apart from 1 trial that is not yet published,22 all other trials were published in peer-reviewed journals. Aggregate-level data from the unpublished trial,22 as well as additional unpublished aggregate-level data from 10 trials,14,15,35,36,37,39,40,41,42,43 were obtained directly from study authors (eTable 4 in Supplement 1).
Table 1. Characteristics of Included Randomized Clinical Trialsa.
| Source | Country | Populationb | Participants | Age, y | Male sex | APACHE II scorec | SOFA scored | Mortality time closest to 90 d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolonged infusion | Intermittent infusion | Prolonged infusion | Intermittent infusion | Prolonged infusion | Intermittent infusion | Prolonged infusion | Intermittent infusion | Prolonged infusion | Intermittent infusion | ||||
| Georges et al,33 2005 | France | Sepsise | 26 | 24 | 50 (17) | 46 (24) | 21 (80.8) | 20 (83.3) | 45 (26-72)f | 44 (22-72)f | NR | NR | Not defined |
| Rafati et al,34 2006 | Iran | Sepsisg | 20 | 20 | 50.1 (22.2) | 48 (20.7) | 12 (60) | 15 (75) | 16.4 (6.3) | 14.2 (6.1) | NR | NR | ICU mortality |
| Roberts et al,35 2007 | Australia | Sepsise | 29 | 28 | 43 (19) | 52 (16) | 16 (55) | 17 (61) | 18.8 (5.9) | 16.4 (4.4) | 4 (1.9) | 3.9 (2.0) | ICU mortality |
| Roberts et al,36 2009 | Australia | Sepsise | 5 | 5 | 57 (54-63) | 55 (48-61) | 4 (80) | 3 (60) | NR | NR | 5 (2-8) | 3 (3-4) | ICU mortality |
| Roberts et al,37 2010 | Australia | Sepsise | 8 | 8 | 30 (23-40) | 41 (22-65) | 6 (75) | 5 (62.5) | 20 (16-22) | 24 (18-26) | 4 (3-6) | 3 (3-3) | ICU mortality |
| Chytra et al,38 2012 | Czech Republic | Sepsisg | 120 | 120 | 44.9 (17.8) | 47.2 (16.3) | 78 (65.0) | 83 (69.2) | 21.4 (7.9) | 22.1 (8.79) | 10.4 (2.9) | 10.6 (3.5) | In-hospital mortality |
| Dulhunty et al,39 2013 | Australia, Hong Kong | Severe sepsise | 30 | 30 | 54 (19) | 60 (19) | 23 (76.7) | 19 (63.3) | 21 (8.6) | 23 (7.6) | NR | NR | In-hospital mortality (28 d) |
| Dulhunty et al,40 2015 | Australia, Hong Kong, New Zealand | Severe sepsisg,h | 212 | 220 | 64 (54-72) | 65 (53-72) | 130 (61.3) | 135 (61.4) | 21 (17-26) | 20 (16-25) | NR | NR | 90-d Mortality |
| Jamal et al,41 2015 | Malaysia | Sepsisg,h | 8 | 8 | 44 (33.8-70) | 62.5 (46-70.5) | 8 (100) | 4 (50) | 33 (29.8-34.8) | 33.5 (28.3-40.5) | 15.5 (14-17.5) | 14 (13-17.8) | ICU mortality |
| Jamal et al,42 2015 | Malaysia | Sepsisg,h | 8 | 8 | 47.5 (32-63.3) | 44.5 (29-60.8) | 7 (87.5) | 4 (50) | 30 (26.5-32.5) | 32.5 (29.8-37.8) | 15.5 (13.3-18.5) | 14.5 (14-17.8) | ICU mortality |
| Abdul-Aziz et al,43 2016 | Malaysia | Severe sepsisi | 70 | 70 | 54 (42-63) | 56 (41-68) | 46 (66) | 50 (71) | 21 (17-26) | 21 (15-26) | 8 (6-10) | 7 (5-9) | In-hospital mortality (30 d) |
| Zhao et al,44 2017 | China | Severe sepsis or septic shocki | 25 | 25 | 68 (15.4) | 67 (12.2) | 10 (40) | 11 (44) | 19.4 (5.0) | 19.7 (5.9) | 8.0 (2.8) | 8.5 (2.4) | ICU mortality |
| Khan and Omar,22 2023 | South Africa | Sepsisj | 64 | 58 | 31 (26-39) | 36 (25-50) | 41 (64) | 34 (59) | 8 (5-13) | 10 (6-13) | NR | NR | 90-d Mortality |
| Mirjalili et al,45 2023 | Iran | Sepsis or septic shockj | 68 | 68 | 53.8 (15.8) | 53.1 (16.2) | 37 (54.5) | 38 (55.9) | 19.14 (6.37) | 19.19 (5.82) | NR | NR | In-hospital mortality |
| Monti et al,14 2023 | Croatia, Italy, Kazakhstan, Russia | Sepsis or septic shocke,g,h,j | 303 | 304 | 65.5 (14) | 63.4 (15) | 195 (64) | 209 (69) | 44 (35-55)f | 43 (34-53)f | 9 (6-11) | 9 (6-11) | 90-d Mortality |
| Saad et al,46 2024 | Egypt | Sepsise | 30 | 30 | 54.4 (10.6) | 53.8 (10.7) | NR | NR | 21.49 (6.05) | 22.75 (5.62) | 10.82 (2.73) | 10.84 (3.43) | ICU mortality |
| Álvarez-Moreno et al,47 2024 | Colombia | Sepsis, severe sepsis or septic shockg | 12 | 13 | 60.2 (16.9) | 54.2 (1.4) | 8 (67) | 4 (31) | 12.7 (6.3) | 15.2 (8.01) | 8 (3.5) | 6 (3.3) | Mortality at dischargek |
| Dulhunty et al,15 2024 | Australia, Belgium, France, Malaysia, New Zealand, Sweden, UK | Sepsisg | 3498 | 3533 | 59.3 (16.4) | 59.6 (16.1) | 2308 (66.0) | 2300 (65.1) | 19.6 (7.6) | 19.5 (7.4) | NR | NR | 90-d Mortality |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; NR, not reported; SOFA, Sequential [Sepsis-related] Organ Failure Assessment.
Age, APACHE II score, and SOFA score are presented as mean (SD) or median (IQR). Male sex is presented as counts (percentage).
All trials recruited participants with sepsis or septic shock according to conventional and contemporary definitions in place at recruitment. The population presented in the table refers to the patient population as reported in the trial protocol, original article, or both.
APACHE II is a disease severity classification system that uses 12 routine physiologic measurements, age, and previous health status to provide a general measure of disease severity. A higher score (range, 0-71) indicates higher severity of disease and higher risk of mortality for ICU patients.
The SOFA score is assessment of organ dysfunction for 6 organs/systems (respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems). The score ranges between 0 and 24, and a higher score indicates higher severity of disease and higher risk of mortality for ICU patients.
As defined by ACCP/SCCM Consensus Conference Committee 1992 definitions for sepsis and organ failure.
Simplified Acute Physiology Score II.
As defined by the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.
Included patients receiving kidney replacement therapy before randomization.
As defined by Surviving Sepsis Campaign 2008.
As defined by the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).
Whether mortality was mortality at ICU discharge or hospital discharge was not specified.
The 18 included trials had a median of 59 trial participants (IQR, 28-139 participants). The median age of participants in the included trials was 54 years (IQR, 48-57 years). The median Acute Physiology and Chronic Health Evaluation II score was 20 (IQR, 18-22), and the median Sequential [Sepsis-related] Organ Failure Assessment score was 8 (IQR, 6-11). Of the included randomized clinical trials, 17 trials compared continuous infusions of β-lactam antibiotics with intermittent infusions,14,15,22,33,34,35,36,37,38,39,40,41,42,43,44,46,47 and 1 trial compared extended infusion with intermittent infusion.45 Meropenem was studied in 11 trials,14,15,22,36,38,39,40,41,43,44,46 piperacillin-tazobactam in 8 trials,15,22,34,37,39,40,42,43 cefepime in 3 trials,33,43,47 ticarcillin-clavulanate in 2 trials,39,40 and amoxicillin-clavulanate,22 ampicillin-sulbactam,45 ceftriaxone,35 and imipenem-cilastatin22 in 1 trial each. In 13 trials, an equivalent total daily dose of β-lactam antibiotics was used in both the prolonged and intermittent infusion groups.14,15,33,35,36,39,40,41,42,44,45,46,47 The median duration of randomly assigned β-lactam antibiotic treatment was 7 days (IQR, 6-10 days) and 9 days (IQR, 6-11 days) in the prolonged and intermittent infusion groups, respectively.
Risk of Bias
Risks of bias assessments are presented in eFigure 2 in Supplement 1. Four trials were adjudicated as having low risk of bias in all domains for all outcomes of interest.14,15,39,40 The overall risk of bias was adjudicated as low for 10 of 17 trials contributing all-cause 90-day mortality data.14,15,35,36,38,39,40,41,42,43
Primary Outcome
There were 17 randomized clinical trials (9014 participants) that contributed data to the primary outcome. The times of follow-up differed across the included trials: 7 trials (n = 249) reported mortality at ICU discharge,34,35,36,41,42,44,46 3 trials (n = 436) reported mortality at hospital discharge,38,39,45 4 trials (n = 8117) reported mortality at day 90,14,15,22,40 1 trial (n = 140) reported mortality at day 30,43 and 2 trials (n = 72) did not provide the definition of the mortality end point,33,47 as shown in eTable 3 in Supplement 1.
Using a bayesian random-effects model with vague priors, the pooled estimated RR for all-cause 90-day mortality for prolonged infusions of β-lactam antibiotics compared with intermittent infusions was 0.86 (95% CrI, 0.72-0.98; τ = 0.11; I2 = 21.5%), with a 99.1% posterior probability that prolonged infusions were associated with lower 90-day mortality (Figure 1, Figure 2, and Figure 3; eTable 5 in Supplement 1). The certainty in the evidence was adjudicated as high, as presented in Table 2. The primary outcome results were similar in the sensitivity analyses using semi-informative priors and the specified frequentist methods (Figures 1 and 2; eTable 5 in Supplement 1). There was no evidence of small-study effects by visual assessment of the contour-enhanced funnel plots or by Egger regression test (eFigure 3A in Supplement 1).
Figure 1. All-Cause 90-Day Mortality for the Comparison Between Prolonged Infusions of β-Lactam Antibiotics vs Intermittent Infusions.
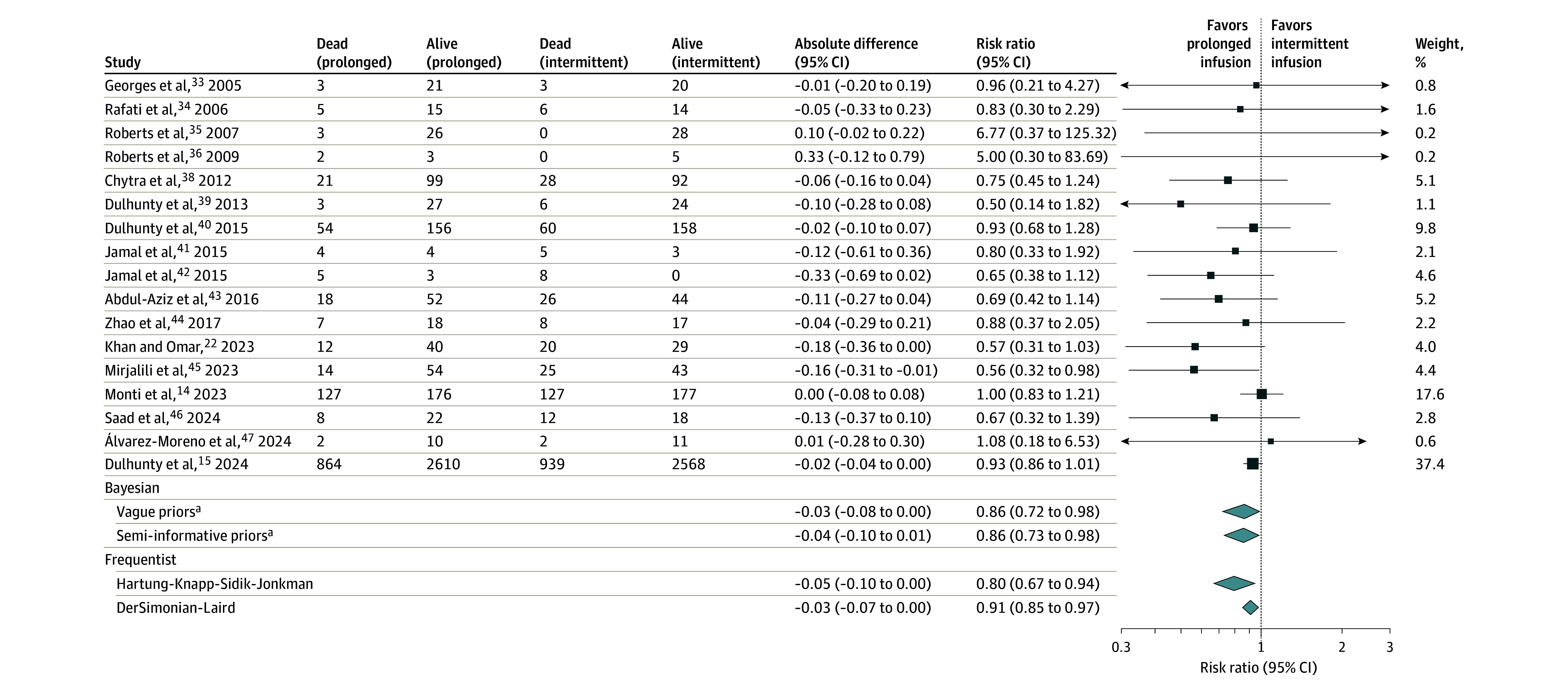
The black boxes represent point estimates, and the areas of the boxes are proportional to the weight of the studies. The weights displayed are based on bayesian analysis with vague priors. The whiskers represent CIs. Width of the diamonds represents the trials’ pooled estimate CI, and the middle point represents the point estimates.
ªCredible intervals are presented for bayesian analysis.
Figure 2. Primary Outcome, Secondary Outcomes, and Subgroup Analyses for the Comparison Between Prolonged Infusions of β-Lactam Antibiotics vs Intermittent Infusions.
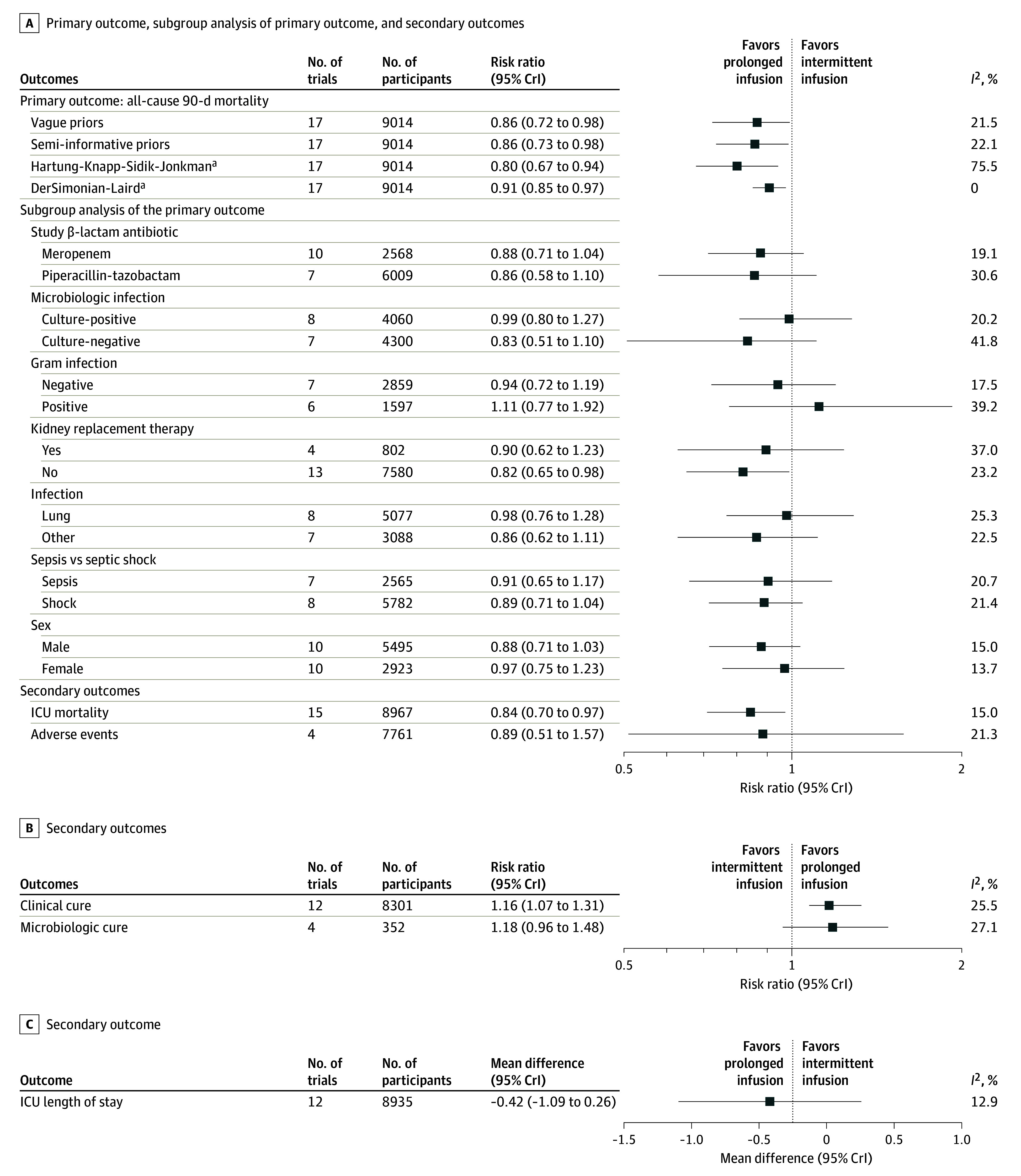
The black boxes represent point estimates, and the whiskers represent the pooled estimate CrIs from bayesian analysis. CrI indicates credible interval; ICU, intensive care unit.
ªCIs are presented for frequentist analysis.
Figure 3. Posterior Probability of the Risk Ratio (RR) for All-Cause 90-Day Mortality for Prolonged Infusions of β-Lactam Antibiotics Compared With Intermittent Infusions.
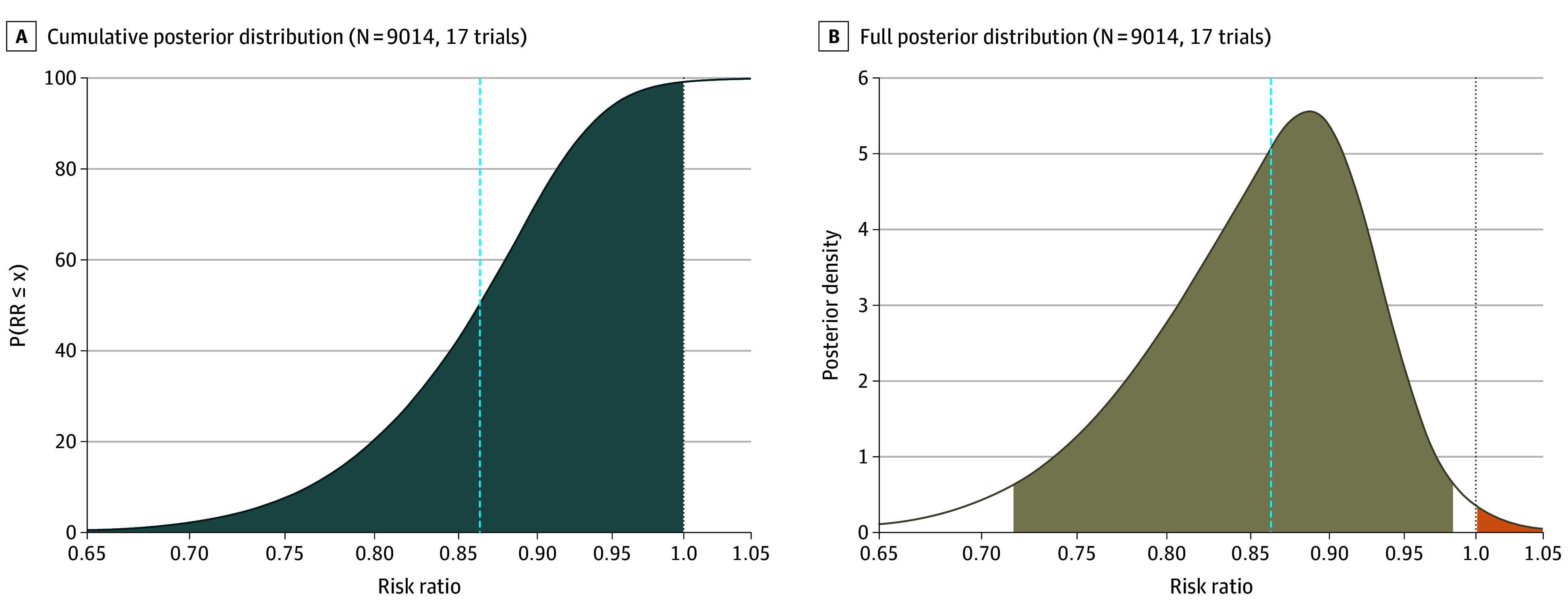
A, The cumulative posterior distribution of the estimated RR, with the y-axis corresponding to the probability the RR is less than or equal to the value on the x-axis. The blue-gray area indicates a beneficial intervention (ie, RR lower than 1). The dashed vertical line indicates the median. B, The full posterior distribution of the estimated RR, with the dashed vertical line indicating the median value and the area highlighted in tan indicating the percentile-based 95% credible interval. The orange area is related to an RR greater than 1 (ie, the intervention is associated with higher mortality vs standard care). The dotted line at an RR of 1 indicates no treatment effect. The figure demonstrates that the probability that prolonged infusions of β-lactam antibiotics is associated with a reduced risk of all-cause 90-day mortality (to any extent) compared with intermittent infusions is more than 99%.
Table 2. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Summary of Findings.
| Outcome | No. of trials/No. of participants | Certainty of evidence (quality of the evidence)a | Infusion, No./No. (%) | (95% CrI) | ||
|---|---|---|---|---|---|---|
| Prolonged | Intermittent | Absolute difference | Risk ratio | |||
| All-cause 90-d mortality | 17/9014 | High, ++++ | 1152/4488 (25.7) | 1275/4526 (28.2) | −0.03 (−0.08 to 0.00) | 0.86 (0.72 to 0.98) |
| ICU mortality | 15/8967 | High, ++++ | 806/4466 (18.0) | 911/4501 (20.2) | −0.03 (−0.08 to 0.0) | 0.84 (0.70 to 0.97) |
| Clinical cure | 12/8301 | Moderate,b +++– | 2367/4137 (57.2) | 2106/4164 (50.6) | 0.11 (0.05 to 0.18) | 1.16 (1.07 to 1.31) |
| Microbiologic cure | 4/352 | Very low,c +−−− | 145/174 (83.3) | 126/178 (70.8) | 0.13 (−0.02 to 0.28) | 1.18 (0.96 to 1.48) |
| Adverse events | 4/7761 | Very low,d +−−− | 42/3868 (1.1) | 49/3893 (1.3) | −0.00 (−0.06 to 0.04) | 0.89 (0.51 to 1.57) |
| ICU length of stay, d | 12/8935 | Low,e ++− | 12.6 | 13.1 | −0.42 (−1.09 to 0.26) | NA |
Abbreviations: CrI, credible interval; ICU, intensive care unit; NA, not applicable.
The GRADE approach specifies 4 levels of certainty, as follows: high certainty (++++), very confident that the true effect lies close to that of the estimate of effect; moderate certainty (+++−), moderately confident in the effect estimate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different); low certainty (++−−), limited confidence in the effect estimate (the true effect may be substantially different from the estimate of effect); and very low certainty (+−−−), very little confidence in the effect estimate (the true effect is likely to be substantially different from the estimate of effect).
Downgraded due to inconsistency because most studies used subjective and variable definitions of clinical cure.
Downgraded due to risk of bias of this outcome in the included trials (eFigure 2E in Supplement 1), inconsistency because studies used variable definitions of microbiologic cure, indirectness because microbiologic cure is not directly an important patient outcome, and imprecision due to small sample size with wide CrI.
Downgraded due to inconsistency because most studies used variable definitions of adverse events, indirectness because adverse events may not directly be an important patient outcome, and imprecision because the CrIs for the effect on adverse events (0.51-1.58) are consistent with both an appreciable benefit and appreciable harm.
Downgraded due to risk of bias of this outcome in the included trials (eFigure 2C in Supplement 1) and indirectness because duration of ICU stay is not directly an important patient outcome.
Subgroup Analyses
The primary outcome of all-cause 90-day mortality was evaluated in 7 prespecified subgroups (Figure 2). As presented in eTable 5 and eFigures 4 through 10 in Supplement 1, there was no evidence that the pooled estimate for prolonged infusions of β-lactam antibiotics compared with intermittent infusions for all-cause 90-day mortality was different by meropenem vs piperacillin-tazobactam (RRR, 1.00; 95% CrI, 0.75-1.29); culture-positive vs culture-negative infection (RRR, 1.13; 95% CrI, 0.91-1.72); gram-negative vs gram-positive infection (RRR, 1.13; 95% CrI, 0.85-1.79); kidney replacement therapy vs no kidney replacement therapy (RRR, 1.08; 95% CrI, 0.82-1.53); lung infection vs other infections (RRR, 0.90; 95% CrI, 0.64-1.15); sepsis vs septic shock (RRR, 0.97; 95% CrI, 0.75-1.23); and male vs female participants (RRR, 0.91; 95% CrI, 0.71-1.12).
Secondary Outcomes
The secondary outcomes are presented in Table 2 and Figure 2 (eTable 5 and eFigures 11-15 in Supplement 1). Assessment of small-study effects is presented in eFigure 3B-F in Supplement 1. Compared with the use of intermittent infusions, use of prolonged infusions of β-lactam antibiotics was associated with a reduced risk of ICU mortality (RR, 0.84; 95% CrI, 0.70-0.97; high certainty) (eFigure 11 in Supplement 1) and an increase in clinical cure (RR, 1.16; 95% CrI, 1.07-1.31; moderate certainty) (eFigure 12 in Supplement 1). There were no detectable differences in microbiologic cure (RR, 1.18; 95% CrI, 0.96-1.48; very low certainty) (eFigure 13 in Supplement 1), adverse events (RR, 0.89; 95% CrI, 0.51-1.57; very low certainty) (eFigure 14 in Supplement 1), and duration of ICU length of stay (mean difference, −0.42; 95% CrI, −1.09 to 0.26; low certainty) (eFigure 15 in Supplement 1).
Discussion
In this systematic review and meta-analysis, β-lactam antibiotic administration by prolonged infusion was associated with a reduced risk of mortality at 90 days for critically ill adult participants with sepsis or septic shock compared with intermittent infusion. The bayesian analysis found a 14-percentage-point relative reduction in the risk of mortality at 90 days with prolonged β-lactam antibiotic infusions compared with intermittent infusions. The number needed to treat for prolonged β-lactam antibiotic infusions to prevent 1 death was 26 patients. The use of prolonged infusions of β-lactam antibiotics was associated with a reduced risk of mortality at ICU discharge. In addition, the use of prolonged infusions was associated with an increased probability of clinical cure. Compared with that of intermittent infusions, the effect of prolonged infusions on microbiologic cure, adverse events, and the duration of ICU length of stay was uncertain.
The observation of reduced risk of mortality in the present analysis is consistent with findings from previous meta-analyses.9,10,11,12,13 In combination, the current evidence presents a higher degree of certainty for clinicians to consider prolonged β-lactam antibiotic infusions as a standard of care in the management of sepsis and septic shock.
Strengths and Limitations
The present review has several strengths. Authors of previous meta-analyses9,10,11,12,13 have acknowledged limitations in the quality of the included trials. By incorporating data from 6 recently published trials,14,15,22,45,46,47 this review provides the most up-to-date evidence, to our knowledge, on the treatment effect of prolonged infusions of β-lactam antibiotics compared with intermittent infusions for critically ill adult patients with sepsis or septic shock. This review included trials that recruited critically ill adult participants with sepsis or septic shock to mitigate population heterogeneity reported in previous meta-analyses.48 The addition of these trials has increased the sample size of the present analysis, providing greater confidence and precision in estimating the effects of prolonged infusions of β-lactam antibiotics on clinically important outcomes. Ten trials assessed as having a low risk of bias contributed 95% of the data to this analysis. The inclusion of trials that have recruited patients from geographically diverse regions (18 countries across 5 continents) enhances the generalizability of findings to a broader range of treatment settings. The use of both bayesian and frequentist analyses ensures a comprehensive assessment and robust interpretation of the treatment effect under study.
Potential challenges associated with prolonged infusion administration, including drug instability and incompatibility with other intravenous medications, the need for a dedicated intravenous portal, and the potential effect on clinical workload, require some considerations before broad implementation. Future studies should determine the optimal duration of infusion when β-lactam antibiotics are administered as prolonged infusions. Because no credible subgroup was identified in this analysis, studies to further identify specific subsets of patients with sepsis or septic shock who are most likely to benefit from prolonged β-lactam antibiotic infusions are warranted. Including specific health economic analyses in future trials may provide additional insights for routine use of prolonged β-lactam antibiotic infusions, and this recommendation can then be considered for inclusion in future sepsis treatment guidelines and treatment bundles.
This study has several limitations. First, the trials included used various definitions for sepsis and septic shock. To allow for this variation, we accepted all conventional and contemporary definitions for sepsis and septic shock used at the original trials. Second, although the present analysis combined both extended and continuous infusions as prolonged infusions, only 1 trial compared extended infusions with intermittent infusions. Third, variable definitions of clinical cure were used across studies and the determination of cure can be subjective. Fourth, the association between prolonged infusions of β-lactam antibiotics and microbiologic cure, adverse events, and the duration of ICU length of stay remains very uncertain because the quality of evidence concerning these outcomes was very low.
Conclusions
Among adults in the ICU with sepsis or septic shock, the use of prolonged β-lactam antibiotic infusions was associated with a reduced risk of 90-day mortality compared with intermittent infusions. The current evidence presents a high degree of certainty for clinicians to consider prolonged infusions as a standard of care in the management of sepsis and septic shock.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
eAppendix 1. Study Protocol
eAppendix 2. Electronic Search Strategy
eAppendix 3. Semi-Informative Priors for Heterogeneity Parameter Details
eFigure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart of Search Strategy and Included Studies
eTable 1. Excluded Reports and Reasons for Exclusion
eTable 2. Microbiological Characteristics and Beta-Lactam Antibiotic Dosing Regimen Details of Included Randomized Controlled Trials
eTable 3. Definition of Primary and Secondary Outcomes in Studies
eTable 4. Unpublished Outcome Data Obtained From Study Authors
eFigure 2. Risk of Bias Assessments
eTable 5. Additional Outcome Statistics for the Primary Bayesian Model, Sensitivity Analyses, and Secondary Outcomes
eFigure 3. Funnel Plots
eFigure 4. All-Cause 90-Day Mortality by Study Beta-Lactam Antibiotic i.e. Piperacillin/Tazobactam Versus Meropenem
eFigure 5. All-Cause 90-Day Mortality by Culture-Positive Infection Versus Culture-Negative Infection
eFigure 6. All-Cause 90-Day Mortality by Gram-Negative Infection Versus Gram-Positive Infection
eFigure 7. All-Cause 90-Day Mortality by Receipt of Kidney Replacement Therapy Versus No Kidney Replacement Therapy
eFigure 8. All-Cause 90-Day Mortality by Lung Infection Versus Other Infections
eFigure 9. All-Cause 90-Day Mortality by Sepsis Versus Septic Shock
eFigure 10. All-Cause 90-Day Mortality by Sex i.e. Male Versus Female Participants
eFigure 11. Forest Plot for ICU Mortality for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 12. Forest Plot for Clinical Cure for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 13. Forest Plot for Microbiological Cure for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 14. Forest Plot for Adverse Events for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 15. Forest Plot for ICU Length of Stay for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
Data Sharing Statement
References
- 1.Roberts JA, Paul SK, Akova M, et al. ; DALI Study . DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072-1083. doi: 10.1093/cid/ciu027 [DOI] [PubMed] [Google Scholar]
- 2.Roberts JA, Joynt GM, Lee A, et al. ; SMARRT Study Collaborators and the ANZICS Clinical Trials Group . The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: data from the Multinational Sampling Antibiotics in Renal Replacement Therapy study. Clin Infect Dis. 2021;72(8):1369-1378. doi: 10.1093/cid/ciaa224 [DOI] [PubMed] [Google Scholar]
- 3.Shekar K, Abdul-Aziz MH, Cheng V, et al. Antimicrobial exposures in critically ill patients receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2023;207(6):704-720. doi: 10.1164/rccm.202207-1393OC [DOI] [PubMed] [Google Scholar]
- 4.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1-10. doi: 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 5.Mouton JW, Vinks AA. Is continuous infusion of beta-lactam antibiotics worthwhile? efficacy and pharmacokinetic considerations. J Antimicrob Chemother. 1996;38(1):5-15. doi: 10.1093/jac/38.1.5 [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Aziz MH, Portunato F, Roberts JA. Prolonged infusion of beta-lactam antibiotics for gram-negative infections: rationale and evidence base. Curr Opin Infect Dis. 2020;33(6):501-510. doi: 10.1097/QCO.0000000000000681 [DOI] [PubMed] [Google Scholar]
- 7.Dhaese S, Heffernan A, Liu D, et al. Prolonged versus intermittent infusion of β-lactam antibiotics: a systematic review and meta-regression of bacterial killing in preclinical infection models. Clin Pharmacokinet. 2020;59(10):1237-1250. doi: 10.1007/s40262-020-00919-6 [DOI] [PubMed] [Google Scholar]
- 8.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37(6):2071-2078. doi: 10.1097/CCM.0b013e3181a0054d [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Abdul-Aziz MH, Davis JS, et al. Continuous versus intermittent β-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194(6):681-691. doi: 10.1164/rccm.201601-0024OC [DOI] [PubMed] [Google Scholar]
- 10.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108-120. doi: 10.1016/S1473-3099(17)30615-1 [DOI] [PubMed] [Google Scholar]
- 11.Kondo Y, Ota K, Imura H, Hara N, Shime N. Prolonged versus intermittent β-lactam antibiotics intravenous infusion strategy in sepsis or septic shock patients: a systematic review with meta-analysis and trial sequential analysis of randomized trials. J Intensive Care. 2020;8:77. doi: 10.1186/s40560-020-00490-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Long Y, Wu G, et al. Prolonged vs intermittent intravenous infusion of β-lactam antibiotics for patients with sepsis: a systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Ann Intensive Care. 2023;13(1):121. doi: 10.1186/s13613-023-01222-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lokhandwala A, Patel P, Isaak AK, et al. Comparison of the effectiveness of prolonged infusion and intermittent infusion of meropenem in patients with sepsis: a meta-analysis. Cureus. 2023;15(10):e46990. doi: 10.7759/cureus.46990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monti G, Bradic N, Marzaroli M, et al. ; MERCY Investigators . Continuous vs intermittent meropenem administration in critically ill patients with sepsis: the MERCY randomized clinical trial. JAMA. 2023;330(2):141-151. doi: 10.1001/jama.2023.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulhunty JM, Brett SJ, De Waele J, et al. Continuous vs intermittent β-lactam antibiotic infusions in critically ill patients with sepsis: the BLING III randomized clinical trial. JAMA. Published online June 12, 2024. doi: 10.1001/jama.2024.9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdul-Aziz MH, Hammond NE, Brett SJ, et al. Prolonged infusion versus intermittent infusion dosing of beta-lactam antibiotics in critically ill patients with sepsis: a protocol for a systematic review and meta-analysis of randomised controlled trials. Preprint posted online May 16, 2023. medRxiv 2023.05.15.23289889. doi: 10.1101/2023.05.15.23289889 [DOI]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17-60. doi: 10.1007/s00134-007-0934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. ; International Sepsis Definitions Conference . 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530-538. doi: 10.1007/s00134-003-1662-x [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. ; The ACCP/SCCM Consensus Conference Committee; American College of Chest Physicians/Society of Critical Care Medicine . Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655. doi: 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 22.Khan AB, Omar S. Continuous vs intermittent beta-lactam dosing in critically ill patients with sepsis: a randomized controlled trial. World Health Organization. Accessed May 2, 2024. https://trialsearch.who.int/Trial2.aspx?TrialID=PACTR202009811610400
- 23.Schandelmaier S, Briel M, Varadhan R, et al. Development of the Instrument to Assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901-E906. doi: 10.1503/cmaj.200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in bayesian meta-analysis. Stat Med. 2015;34(6):984-998. doi: 10.1002/sim.6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doleman B, Freeman SC, Lund JN, Williams JP, Sutton AJ. Funnel plots may show asymmetry in the absence of publication bias with continuous outcomes dependent on baseline risk: presentation of a new publication bias test. Res Synth Methods. 2020;11(4):522-534. doi: 10.1002/jrsm.1414 [DOI] [PubMed] [Google Scholar]
- 28.Doleman B, Mathiesen O, Jakobsen JC, et al. Methodologies for systematic reviews with meta-analysis of randomised clinical trials in pain, anaesthesia, and perioperative medicine. Br J Anaesth. 2021;126(4):903-911. doi: 10.1016/j.bja.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 29.Röver C. Bayesian random-effects meta-analysis using the bayesmeta R package. J Stat Softw. 2020;93(6):1-51. doi: 10.18637/jss.v093.i06 [DOI] [Google Scholar]
- 30.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines, 1: introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 33.Georges B, Conil JM, Cougot P, et al. Cefepime in critically ill patients: continuous infusion vs an intermittent dosing regimen. Int J Clin Pharmacol Ther. 2005;43(8):360-369. doi: 10.5414/CPP43360 [DOI] [PubMed] [Google Scholar]
- 34.Rafati MR, Rouini MR, Mojtahedzadeh M, et al. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents. 2006;28(2):122-127. doi: 10.1016/j.ijantimicag.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 35.Roberts JA, Boots R, Rickard CM, et al. Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care? a randomized controlled pilot study. J Antimicrob Chemother. 2007;59(2):285-291. doi: 10.1093/jac/dkl478 [DOI] [PubMed] [Google Scholar]
- 36.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64(1):142-150. doi: 10.1093/jac/dkp139 [DOI] [PubMed] [Google Scholar]
- 37.Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. 2010;35(2):156-163. doi: 10.1016/j.ijantimicag.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 38.Chytra I, Stepan M, Benes J, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care. 2012;16(3):R113. doi: 10.1186/cc11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56(2):236-244. doi: 10.1093/cid/cis856 [DOI] [PubMed] [Google Scholar]
- 40.Dulhunty JM, Roberts JA, Davis JS, et al. ; BLING II Investigators for the ANZICS Clinical Trials Group . A multicenter randomized trial of continuous versus intermittent β-lactam infusion in severe sepsis. Am J Respir Crit Care Med. 2015;192(11):1298-1305. doi: 10.1164/rccm.201505-0857OC [DOI] [PubMed] [Google Scholar]
- 41.Jamal JA, Mat-Nor MB, Mohamad-Nor FS, et al. Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;45(1):41-45. doi: 10.1016/j.ijantimicag.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 42.Jamal JA, Roberts DM, Udy AA, et al. Pharmacokinetics of piperacillin in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;46(1):39-44. doi: 10.1016/j.ijantimicag.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 43.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42(10):1535-1545. doi: 10.1007/s00134-015-4188-0 [DOI] [PubMed] [Google Scholar]
- 44.Zhao HY, Gu J, Lyu J, et al. Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: a prospective randomized pilot study. Chin Med J (Engl). 2017;130(10):1139-1145. doi: 10.4103/0366-6999.205859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirjalili M, Zand F, Karimzadeh I, et al. The clinical and paraclinical effectiveness of four-hour infusion vs half-hour infusion of high-dose ampicillin-sulbactam in treatment of critically ill patients with sepsis or septic shock: an assessor-blinded randomized clinical trial. J Crit Care. 2023;73:154170. doi: 10.1016/j.jcrc.2022.154170 [DOI] [PubMed] [Google Scholar]
- 46.Saad SI, Aglan BM, Shaboob EA, Abdelghany HH. Continuous versus intermittent use of meropenem in septic critically ill patients: a randomized controlled trail. Benha Med J. 2024;41(2):38-48. doi: 10.21608/bmfj.2023.247556.1949 [DOI] [Google Scholar]
- 47.Álvarez-Moreno CA, Nocua-Báez LC, Ortiz G, et al. Efficacy of continuous vs intermittent administration of cefepime in adult ICU patients with gram-negative bacilli bacteremia: a randomized double-blind clinical study. Antibiotics (Basel). 2024;13(3):229. doi: 10.3390/antibiotics13030229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdul-Aziz MH, Dulhunty JM, Bellomo R, Lipman J, Roberts JA. Continuous beta-lactam infusion in critically ill patients: the clinical evidence. Ann Intensive Care. 2012;2(1):37. doi: 10.1186/2110-5820-2-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Protocol
eAppendix 2. Electronic Search Strategy
eAppendix 3. Semi-Informative Priors for Heterogeneity Parameter Details
eFigure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart of Search Strategy and Included Studies
eTable 1. Excluded Reports and Reasons for Exclusion
eTable 2. Microbiological Characteristics and Beta-Lactam Antibiotic Dosing Regimen Details of Included Randomized Controlled Trials
eTable 3. Definition of Primary and Secondary Outcomes in Studies
eTable 4. Unpublished Outcome Data Obtained From Study Authors
eFigure 2. Risk of Bias Assessments
eTable 5. Additional Outcome Statistics for the Primary Bayesian Model, Sensitivity Analyses, and Secondary Outcomes
eFigure 3. Funnel Plots
eFigure 4. All-Cause 90-Day Mortality by Study Beta-Lactam Antibiotic i.e. Piperacillin/Tazobactam Versus Meropenem
eFigure 5. All-Cause 90-Day Mortality by Culture-Positive Infection Versus Culture-Negative Infection
eFigure 6. All-Cause 90-Day Mortality by Gram-Negative Infection Versus Gram-Positive Infection
eFigure 7. All-Cause 90-Day Mortality by Receipt of Kidney Replacement Therapy Versus No Kidney Replacement Therapy
eFigure 8. All-Cause 90-Day Mortality by Lung Infection Versus Other Infections
eFigure 9. All-Cause 90-Day Mortality by Sepsis Versus Septic Shock
eFigure 10. All-Cause 90-Day Mortality by Sex i.e. Male Versus Female Participants
eFigure 11. Forest Plot for ICU Mortality for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 12. Forest Plot for Clinical Cure for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 13. Forest Plot for Microbiological Cure for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 14. Forest Plot for Adverse Events for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
eFigure 15. Forest Plot for ICU Length of Stay for the Comparison Between Prolonged Infusions of Beta-Lactam Antibiotics Versus Standard Intermittent Infusions
Data Sharing Statement


