Abstract
Human T-cell leukemia virus type 1 (HTLV-1) Tax is a nuclear protein with striking pleiotropic functionality. We recently demonstrated that Tax localizes to a multicomponent nuclear speckled structure (Tax speckled structure [TSS]). Here, we examine these structures further and identify a partial overlap of TSS with transcription hot spots. We used a strategy of directed expression via fusion proteins to determine if these transcription sites are the subtargets within TSS required for Tax function. When fused to human immunodeficiency virus type 1 (HIV-1) Tat, the resulting Tat-Tax fusion protein displayed neither a Tat-like nor a Tax-like pattern but rather was targeted specifically to the transcription subsites. The Tat-Tax fusion was able to activate both the HIV-1 long terminal repeat (LTR) and the HTVL-1 LTR at the same level as the individual component; thus, targeting proteins to transcription hot spots was compatible with both Tax and Tat transcription function. In contrast, the fusion with HIV-1 Rev, Rev-Tax, resulted in a pattern of expression that was largely Rev-like (nucleolar and cytoplasmic). The reduced localization of Rev-Tax to transcription sites was reflected in a 10-fold drop in activation of the HTLV-1 LTR. However, there was no loss in the ability of Tax to activate via NF-κB. Thus, NF-κB-dependent Tax function does not require targeting of Tax to these transcription sites and suggests that activation via NF-κB is a cytoplasmic function. Selective mutation of the nuclear localization signal site in the Rev portion resulted in retargeting of Rev-Tax to TSS and subsequent restoration of transcription function, demonstrating that inappropriate localization preceded loss of function. Mutation of the nuclear export signal site in the Rev portion had no effect on transcription, although the relative amount of Rev-Tax in the cytoplasm was reduced. Finally, in explaining how Tax can occupy multiple subcellular sites, we show that Tax shuttles from the nucleus to the cytoplasm in a heterokaryon fusion assay. Thus, pleiotropic functionality by Tax is regulatable via shuttling between discrete subcellular compartments.
In addition to activation of viral transcription, human T-cell leukemia virus type 1 (HTLV-1) Tax activates various cellular genes involved in cell growth and/or activation (14, 46, 47, 60). Furthermore, Tax has been shown to repress the activity of basic helix-loop-helix proteins, such as c-Myc, that utilize E-box enhancer elements (7, 40, 53, 54). In addition to these specific repressive effects, Tax also binds to the transcription factor CREB-binding protein (CBP) and may squelch the activity of other CBP-dependent transactivators (11). The concerted activity of these functions is believed to play a key role in Tax-mediated immortalization-transformation of cells. Recently, it has been suggested that some of the activities of Tax may contribute to a loss in genetic stability, a characteristic that may be closely linked to progression through oncogenesis (26, 27, 43). In light of these diverse Tax functions, it is becoming clear that uncovering how these various activities are regulated is key to understanding acquisition of adult T-cell leukemia.
It is well established that Tax activates its cognate long terminal repeat (LTR) via indirect targeting of itself to some combination of the three Tax-responsive-elements present in the U3 region (6, 9, 13, 48). This activation also appears to involve the ubiquitous activator of transcription CBP (5, 23, 29, 56, 58). Tax has also been reported to alter the dimerization rates of CREB-like proteins, the biological significance of which is uncertain (1, 3, 21, 29, 38, 59), and Tax activates the c-fos promoter by direct interaction with serum response factor (15–17, 50). In addition to these activating functions, Tax also represses genes regulated by E-box enhancer elements (7, 40, 53, 54). All of these functions are consistent with Tax being a nuclear protein (41). In contrast to the above properties, the ability of Tax to activate NF-κB-containing promoters has been linked to an initial event of Tax interacting with IκB (4, 28, 30, 36). This would imply a cytoplasmic function of Tax. Thus, by inference gained from this example, compartmentalization may be an important regulatory mechanism for Tax pleiotropic function.
We previously showed that Tax targets itself to Tax speckled structure (TSS), which consists of multiple subpopulations of Tax, and suggested that targeting itself to the appropriate region might be one way in which Tax regulates function (41). Here we show that targeting of Tax to transcription hot spots within TSS is a prerequisite to CREB-dependent function. We also show that exclusion of Tax from these transcription sites had no effect on NF-κB-dependent function and conclude that these two activities exist in separate cellular regions. Finally, toward understanding how Tax can participate in both nuclear and cytoplasmic functions, we have demonstrated that Tax shuttles between the nucleus and the cytoplasm.
MATERIALS AND METHODS
Plasmids.
To express the fusion protein, Tat-Tax, the complete Tat cDNA minus the termination codon was inserted in the NcoI site at the ATG of the Tax cDNA in IEX (42). This plasmid is referred to as pTat-Tax. Likewise, pRev-Tax (Rev-Tax) was constructed by inserting the complete Rev cDNA, minus the termination codon, into the NcoI site at the ATG of the Tax cDNA in IEX. The plasmids pU3RCAT (HTLV-1 LTR) (48) and pBennCAT (human immunodeficiency virus type 1 [HIV-1] LTR) (20) have been described previously.
Antiserum.
We employed two anti-Tax sera, a rabbit polyclonal antibody developed to a Tax-specific peptide (40) and the mouse monoclonal antibody 168A (National Institutes of Health AIDS Reference Reagent Program). The anti-Tat rat polyclonal antibody was a gift from K.-T. Jeang (Bethesda, Md.). The anti-SC35 was a mouse monoclonal antibody and a gift from Tom Maniatis (Cambridge, Mass.). The anti-transcription factor IIE (anti-TFIIE) (C-17) was obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Fluorescein isothiocyanate-conjugated antibromodeoxyuridine was a mouse monoclonal preparation from Boehringer Mannheim (Mannheim, Germany).
Preparation of cells for microscopy.
Cells were seeded onto glass coverslips in 100-mm-diameter tissue culture dishes with complete medium (Dulbecco's modified Eagle medium containing 10% fetal calf serum, 2 mM glutamine, and 100 U of penicillin-streptomycin solution per ml) and allowed to adhere overnight. Initial attachment of cells resulted in 40 to 60% confluence. For standard calcium phosphate transfections, precipitates were removed after 16 h of incubation over cell cultures and were replaced with fresh complete medium for 24 h. At the end of this 24-h period, cells were washed twice in phosphate-buffered saline (PBS) followed by incubation with PBS containing 4% paraformaldehyde (pH 8.0) for 12 min at room temperature. The fixative was removed by washing three times with PBS. The cells were permeabilized by exposure to 100% methanol for 2 min at room temperature and then rehydrated with multiple rinses in PBS. Primary antibodies were diluted in PBS containing 4% bovine serum albumin. The antibody was beaded in a 100-μl volume on Parafilm, and the prepared coverslips were inverted onto the antibody and placed in a moisturized chamber. The primary antibody was reacted overnight at 4°C. To remove excess primary antibody, the coverslips were washed five times in PBS-Tween. Fluorochrome-conjugated secondary antibody was reacted in the same manner except that the incubation was for 1 h at room temperature. Excess secondary antibody was removed by washing five times in PBS-Tween. The processed coverslips were then mounted onto glass slides with VectaShield (Vector Laboratories, Burlingame, Calif.). The prepared slides were examined by confocal optics using a Zeiss Axiophot inverted microscope.
In situ transcription runoff.
Cells were seeded onto glass coverslips and allowed to grow to 60% confluence overnight. Some of the cells were transfected with a Tax-expressing plasmid. Both transfected and nontransfected cells were washed twice in Tris-buffered saline (10 mM Tris-HCl [pH 7.4], 150 mM NaCl2, 5 mM MgCl2). Tris-buffered saline was removed and replaced with glycerol buffer (20 mM Tris-HCl [pH 7.4], 5 mM MgCl2, 0.5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 25% glycerol) for 5 min. Cells were permeabilized with glycerol buffer containing 0.05% Triton X-100 (3 min at room temperature) and then washed once with transcription buffer (50 mM Tris-HCl [pH 7.4], 100 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 25% glycerol, 5 U of RNasin per ml). Transcription was initiated by addition of transcription buffer containing 0.5 mM ATP, CTP, GTP, and BrUTP. Transcription was allowed to proceed for 15 min at room temperature. The reaction was halted by washing in ice-cold PBS and immediate fixation with ice-cold 100% methanol. Fixed cells were processed for microscopy as described above.
Chloramphenicol acetyltransferase assays.
Transcription activity was measured by cotransfection of reporter plasmid (pU3RCAT or pBennCAT) and with the indicated transactivator expression plasmid. The subsequent cell extracts were assayed for the chloramphenicol acetyltransferase activity as previously described (42).
Heterokaryon fusion assay.
The heterokaryon fusion assays were performed essentially as described elsewhere (22, 32). We used HeLa cells transfected with Tax as the donor cell. A COS cell line, which expresses transdominant Rev as a marker, was a gift from M.-L. Hammarskjold (Charlottesville, Va.) and was used as the recipient cell. The TdRev cell line expresses a Rev mutated in the nuclear export signal (NES) that is no longer able to be exported from the nucleus. The nucleolar retention of the mutant Rev serves to identify the recipient cells and acts as a control for leakiness. HeLa cells were seeded onto coverslips at 40% confluence and transfected with Tax-expressing plasmids. At 36 h posttransfection, the TdRev cells were seeded onto the same dishes at concentrations predicted to yield 50% confluence. The donor and recipient cells were treated with 100 μg of cycloheximide per ml at 48 h posttransfection and incubated for 1 h. The cell mix was treated with 50% polyethylene glycol for 90 s to disrupt and/or fuse the cytoplasmic membranes and then washed several times with complete medium. The cells were fixed and subjected to immunofluorescence.
RESULTS
A subpopulation of Tax in TSS colocalizes with sites of transcription.
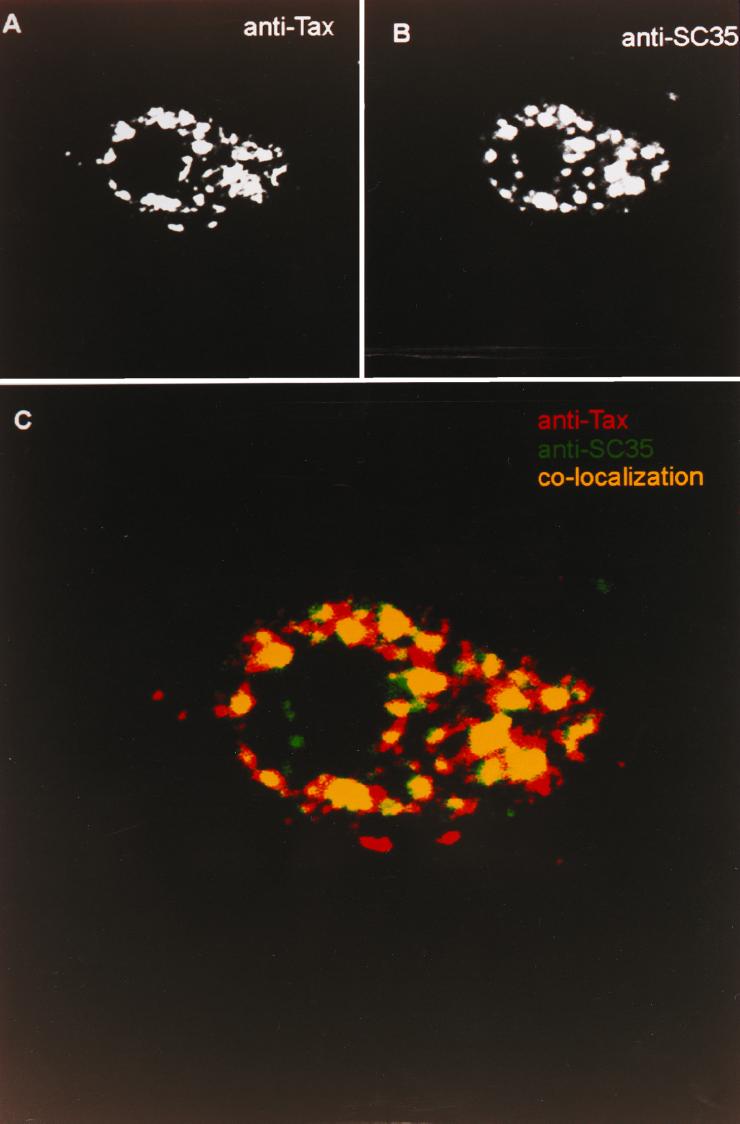
Tax predominately localizes to the nucleus of cells, displays a speckled pattern, and is excluded from the nucleolus (41, 42). We previously noted that the TSS may consist of subpopulations of Tax (41). In the earlier study, we used high-resolution confocal imaging to demonstrate that although TSS colocalizes with SC35-containing nuclear speckles, there is significant Tax-associated fluorescence in the periphery of the SC35 domains. This is significant since nuclear speckles are thought to be subnuclear storage compartments for proteins involved in transcription and splicing (35). The active sites of transcription appear to reside at the periphery of the SC35-containing nuclear speckles (33, 34). In order to demonstrate the existence of subdomains within TSS, we used confocal microscopy to fine map the localization of TSS with respect to known nuclear domains. HeLa cells transiently expressing Tax were analyzed for TSS and SC35 (Fig. 1). The resulting TSS (Fig. 1A) was shown to colocalize with SC35-containing nuclear speckles (Fig. 1B). This agrees with our earlier published accounts. However, upon closer inspection of the overlay of the TSS and SC35 images (Fig. 1C), a significant amount of Tax-associated fluorescence (red) is seen which does not overlap with the SC35 pattern (green). Specifically, most of the SC35-associated fluorescence is contained within the TSS, whereas a significant fraction of the TSS exists outside of the domain prescribed by SC35 staining. Thus, one population of Tax within the TSS resides within nuclear domains identified by SC35 staining, and another population exists peripheral to this region.
FIG. 1.
A subpopulation of Tax within TSS resides outside SC35-containing nuclear speckles. HeLa cells were transiently transfected with the Tax expression plasmid IEX (38). The resulting Tax-expressing cells were fixed onto coverslips and processed for immunofluorescence confocal microscopy. (A) Immunofluorescence image resulting from immunolabeling with anti-Tax rabbit polyclonal antiserum. (B) The same cell immunostained for SC35 using a mouse monoclonal antibody preparation (a gift from T. Maniatis). (C) Overlay of the two images. Tax-specific fluorescence is represented in red, SC35-specific fluorescence is represented in green, and colocalization of TSS and nuclear speckles is shown as yellow.
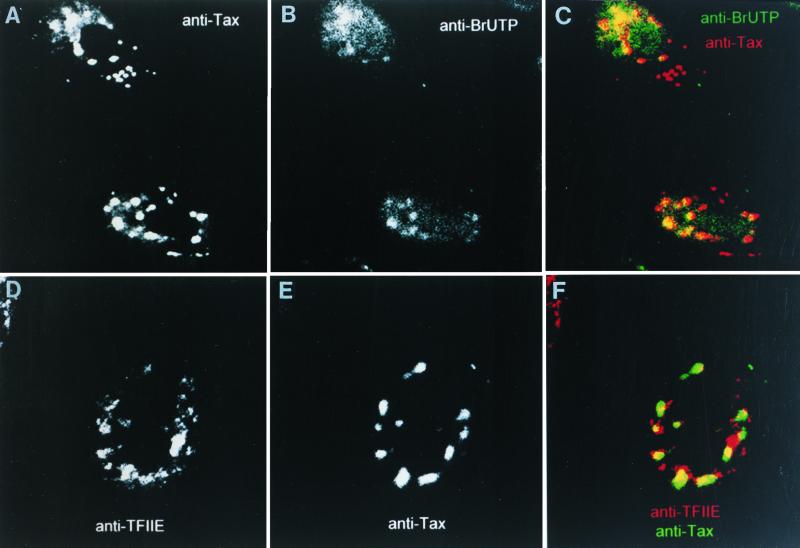
Since transcription has been reported to occur adjacent to the SC35-containing nuclear speckles, we examined whether the population of Tax not residing in these speckles was associated with transcription. We exposed Tax-expressing cells to BrUTP under conditions that specifically label de novo transcription. Using this technique, we identified transcription hot spots within the nucleus of Tax-expressing HeLa cells (Fig. 2B). These hot spots were overlaid with TSS images from the same cell (Fig. 2A). Examination of the overlap patterns (yellow) revealed that a portion of TSS (red) overlapped with anti-BrUTP (green). We also note that the region of overlap is peripheral with respect to the TSS as a whole. The same results were obtained when we examined coimmunostaining of Tax with the transcription factor TFIIE. In this case, the TFIIE-specific staining (Fig. 2D) was at the periphery of the TSS-specific staining (Fig. 2E). This is more clearly seen when the images of TSS (green) are overlaid with the TFIIE images (red) to reveal partial overlap (yellow). Therefore, a subpopulation of Tax within the TSS is associated with sites of active transcription.
FIG. 2.
A subpopulation of TSS overlaps with transcription hot spots. (A to C) HeLa cells expressing Tax were subjected to BrUTP labeling of nascent RNA to identify transcription hot spots and subsequently prepared for immunofluorescence confocal microscopy. Visualization of sites of de novo transcription is aided by immunostaining with antibromodeoxyuridine mouse monoclonal antiserum. Colocalization of TSS (A) with the transcription hot spots (B) is shown in panel C. (D to F) HeLa cells expressing Tax were subjected to immunofluorescence confocal microscopy. Colocalization of TSS (D) with the basal transcription factor TFIIE (E) is shown in panel F.
Tax mutants deficient for CREB-mediated transcription show altered subcellular localization.
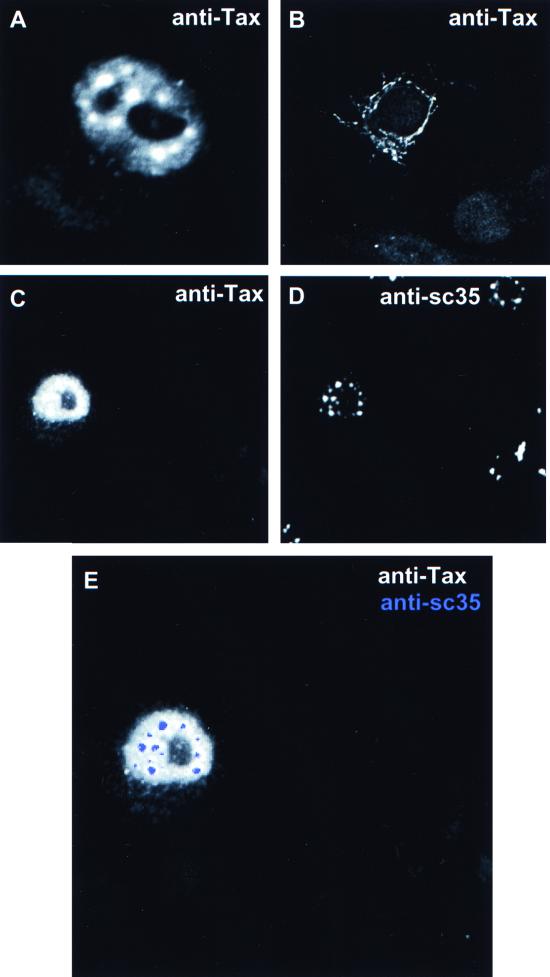
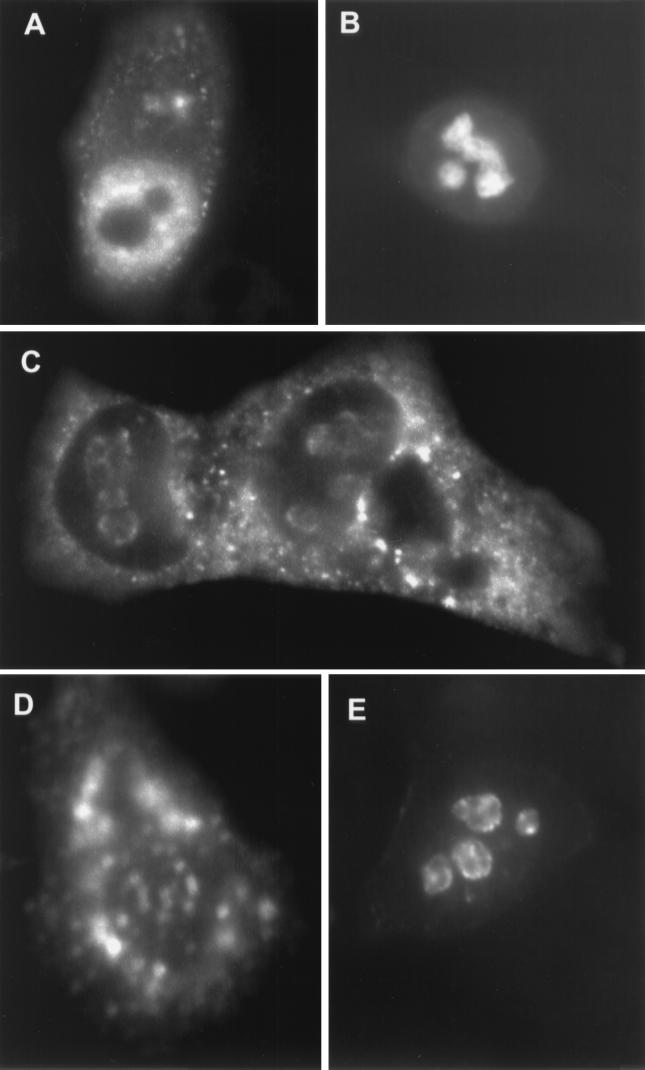
We analyzed the subcellular localization of Tax mutants in order to demonstrate that targeting of Tax to TSS, and thus transcription hot spots, was correlated with CREB-mediated transcription function. We compared wild-type Tax to TaxH43 (able to transactivate the HTLV-1 LTR but unable to activate via NF-κB), TaxG320 (unable to transactivate the HTLV-1 LTR but able to activate via NF-κB), and TaxΔ2-58 (deletion of the nuclear localization signal [NLS] region and inactive for either function). In the case of TaxH43, the subcellular localization was indistinguishable from that seen with wild-type Tax (Fig. 3A). Note the prominence of the TSS, some diffuse nuclear staining, and nucleolar exclusion, a profile identical to that of wild-type Tax (Fig. 1). The NLS mutant, TaxΔ2-58, failed to target the nucleus and accumulated in the cytoplasm (Fig. 3B). The CREB-deficient Tax mutant, TaxG320, showed nucleolar exclusion and nuclear targeting but did not display a TSS pattern (Fig. 3; compare panels C and D; combined image is shown in panel E). These results support the premise that targeting TSS is required for Tax transcription function.
FIG. 3.
Tax mutants defective for CREB-dependent function showed altered subcellular localization. HeLa cells expressing selected Tax mutant proteins were subjected to immunofluorescence confocal microscopy. Tax-specific staining was performed using a rabbit polyclonal anti-Tax antiserum. (A to C) Resulting expression patterns of TaxH43 (A), TaxΔ2-58 (B), and TaxG320 (C). (D) Coimmunostaining of cells shown in panel C with anti-SC35. (E) Overlay of images.
Redirecting Tax expression to transcription hot spots retains transactivation function.
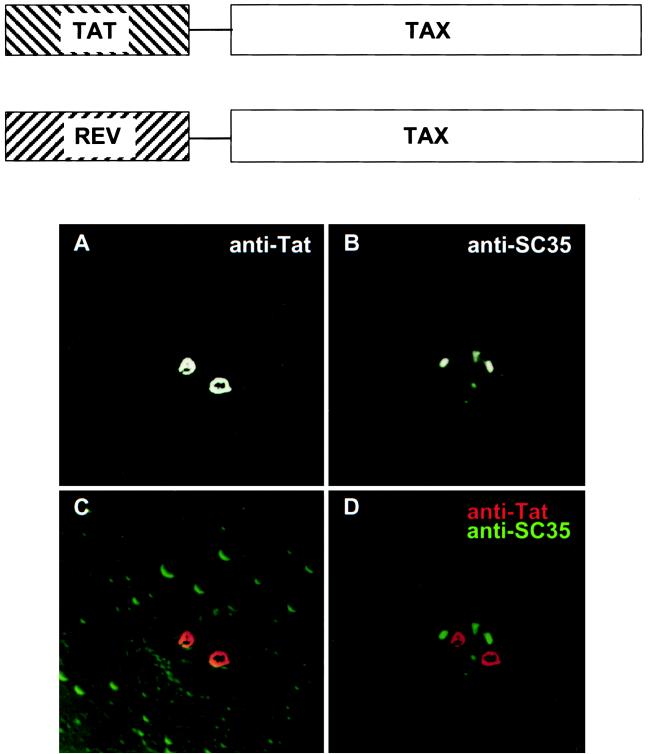
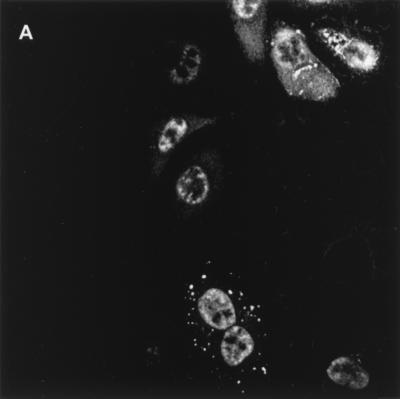
We wanted to know if Tax localization to the transcription domains was a prerequisite for function. However, using Tax mutants to define the role of sub-TSS domains as well as addressing the question of subcellular targeting as prerequisite to function is at best problematic. In the first case, analysis of TSS subdomains lies at the limits of the resolution of available microscopic techniques. In the second case, Tax mutants with impaired function cannot adequately address whether targeting is a prerequisite to function. Therefore, we devised a strategy to alter the localization of wild-type Tax by fusion-directed expression. We reasoned that the functional homologues HIV Tat and Tax would possess a requirement for targeting the same subcellular compartment. Therefore, even though these two proteins have drastically different reported subcellular locations, their function would depend on targeting the same subcellular address. Indeed, Tat has been shown to colocalize with the transcription factor SP1 and to reside in a punctate nuclear pattern when not overexpressed (10). We constructed Tax fusion proteins in which Tax was fused to HIV Tat protein for a functionally homologous fusion (Fig. 4, top). Thus, in this system, we were also able to verify that the reported nucleolar targeting of Tat (Fig. 4, bottom) is not a requirement for transactivation function.
FIG. 4.
(Top) Tax fusion proteins. (Bottom) Subcellular localization of Tat. HeLa cells expressing HIV-1 Tat were subjected to immunofluorescence confocal microscopy. Anti-Tat rat polyclonal antiserum was used to visualize the subcellular localization of Tat. Tat localizes predominately to the nucleolus (A). (C and D) Overlay of Tat-specific fluorescence with SC35-specific fluorescence (B) did not result in any noticeable overlap.
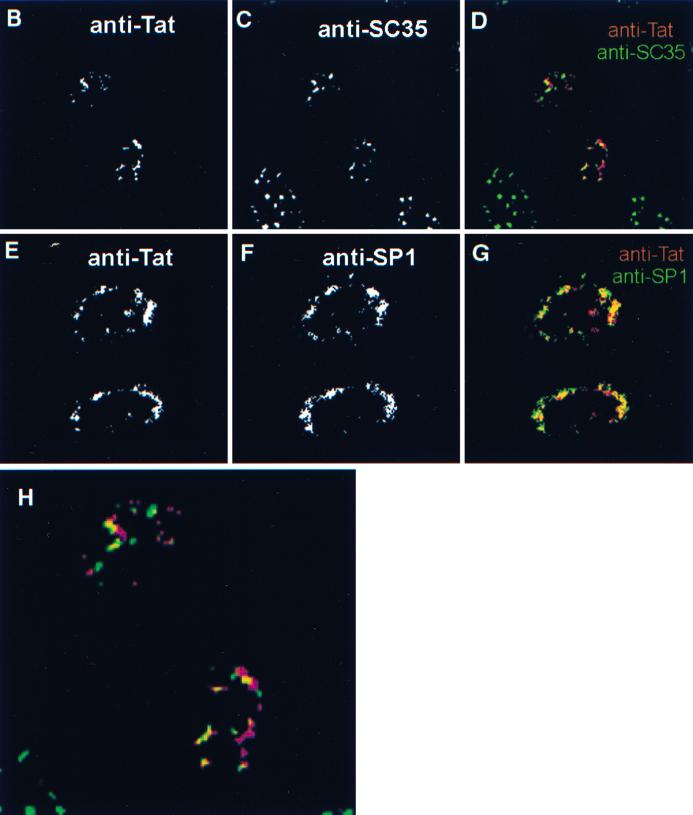
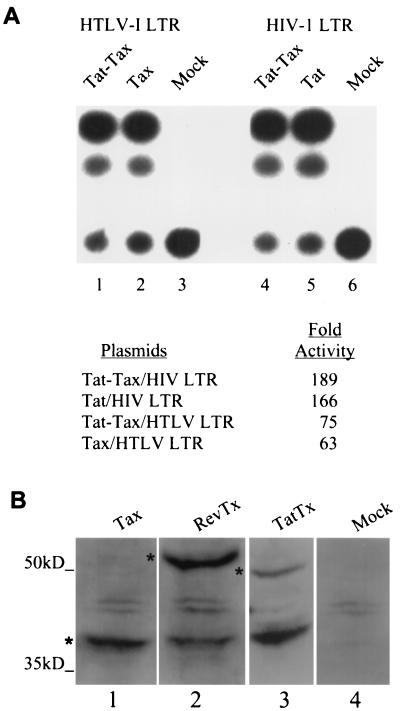
We transiently expressed the Tat-Tax fusion protein in HeLa cells and used indirect immunofluorescence combined with confocal microscopy to observe the pattern of localization (Fig. 5). In contrast to wild-type Tat, the Tat-Tax fusion was excluded from the nucleolus and concentrated in discrete nuclear regions (Fig. 5A). Coimmunostaining of the cells for Tat-Tax and SC35 showed that the expression pattern also differs from that of TSS in that there is a greater amount of the Tat-Tax protein outside the region identified by anti-SC35 staining (Fig. 5; compare panels B, C, and D). This pattern may represent an enlargement of the population of Tax targeting active sites of transcription. Indeed, the Tat-Tax protein overlaps nicely with the transcription factor SP1 (Fig. 5E, F, and G). In this case, we used SP1 because of its role in Tat transcription, but note that SP1 and the basal transcription factors TFIIE, TFIIA, and TATA-binding protein all colocalize within the transcription hot spots (data not shown). Thus, in the context of the Tat-Tax fusion, the Tat-specific nucleolar localization is drastically altered and the Tax-specific pattern has been shifted toward subnuclear transcription sites (compare Fig. 1, 2, and 5). We next tested the Tat-Tax protein for the ability to activate either the HTLV-1 LTR or the HIV-1 LTR. These experiments were conducted in HeLa cells, and under these conditions Tax is unable to activate the HIV-1 LTR; therefore, activation of the HIV-1 LTR is dependent on the Tat portion of the fusion. We show that Tax and Tat alone are each capable of activating their cognate promoters 63- and 166-fold, respectively (Fig. 6A, lanes 2 and 5). Likewise, the Tat-Tax fusion was able to activate either promoter at least as well as the corresponding wild-type protein (Fig. 6A, lanes 1 and 4). Taken together, these data show that directing Tat and Tax to the transcription subnuclear compartment is compatible with each protein's function and demonstrate that these two proteins target the same subcellular site.
FIG. 5.
Tat-Tax fusion protein was excluded from the nucleolus and targeted to transcription sites. Tax-expressing HeLa cells were subjected to immunofluorescence confocal microscopy. The overall pattern of the Tax-Tat fusion resembled that of TSS (A). However, closer inspection of the Tat-specific fluorescence (B and E) showed that although some of the Tat-Tax overlaps with SC35 (C), unlike TSS, most of the Tat-Tax targeted regions peripheral to SC35 speckles (D). As shown in panel G, most of the Tat-Tax (E) colocalized with transcription factors such as SP1 (F). Panel D is enlarged in panel H for ease of comparison.
FIG. 6.
The Tat-Tax protein retained the transcription function of both Tat and Tax. (A) The transcription assays were performed as described for HeLa cells. Under these conditions, Tax is unable to activate the HIV-1 LTR (38). Tat-Tax was compared to Tax in ability to activate the HTLV-1 LTR (lanes 1 to 3) and to Tat in ability to activate the HIV-1 LTR (lanes 4 to 6). In both assays, the transactivation function of the Tat-Tax fusion was comparable to that of the individual component protein. (B) Western blot analysis of Tax, Rev-Tax, and Tat-Tax. The asterisks in each lane mark the full-length protein. “Mock” was extract from cells transiently transfected with an empty expression vector identical to the one used for expression of the other cDNA.
Targeting of Tax to transcription sites is a prerequisite for CREB-dependent activation but not for NF-κB-dependent activity.
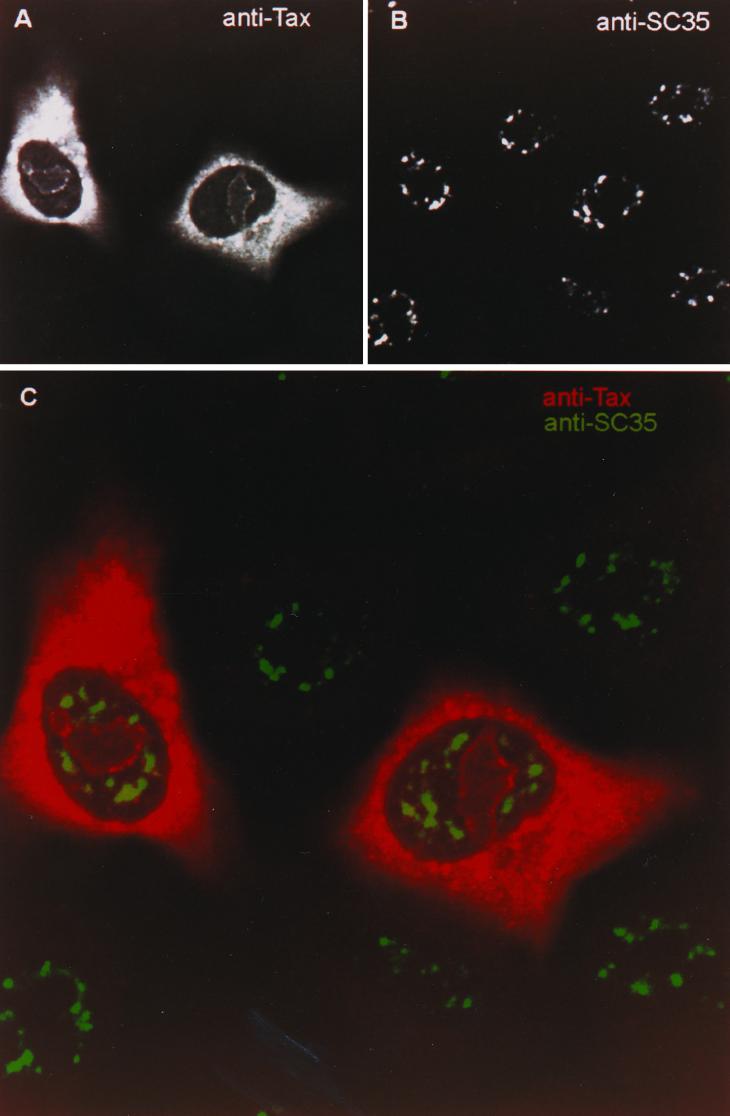
Following the same logic used in the approach described above, we further reasoned that fusion of two proteins with different functions would result in competition between the distinct fusion protein domains for targeting of the fusion protein to separate subcellular compartments. Thus, in the ideal situation, one component targeting profile would be dominant, thereby ablating location-dependent function of the other fusion partner. Specifically, we fused HIV-1 Rev to Tax to form a functionally heterologous fusion protein (Fig. 4, top). The resulting protein was transiently expressed in HeLa cells, and the cells were subsequently examined by indirect immunofluorescence. The pattern of expression was striking in three respects: the presence of Tax-specific staining in the nucleolus, reduced staining in the TSS, and an increased staining in the cytoplasm (Fig. 7A). We clearly show that there is little observable overlap of the Rev-Tax pattern with that of SC35-containing speckles (Fig. 7C). With the exception of the increased cytoplasmic staining, the Rev-Tax expression pattern is largely Rev-like.
FIG. 7.
The Rev-Tax fusion protein failed to target transcription sites or TSS. Tax-expressing HeLa cells were subjected to immunofluorescence confocal microscopy. Tax-specific fluorescence revealed that the Rev-Tax protein targeted the nucleolus and cytoplasm (A). Rev-Tax was noticeably absent from SC35 nuclear speckles (B). Also shown (C) is the overlay of the Tax-specific (red) and SC35-specific (green) signals of the same cell view.
We assumed from the above results that the Rev portion of the fusion protein was dominant over the Tax portion for determining subcellular targeting. This assumption presumes that the Tax portion of the fusion is active and functional in other respects except for proper localization. To demonstrate this, we introduced mutations in the Rev portion of the fusion protein which specifically impair either nuclear localization or nuclear export of Rev. The patterns of localization of the mutant fusion proteins were compared to those of both wild-type Tax (Fig. 8A)and wild-type Rev (Fig. 8B). When mutations were made in the NLS domain of Rev, the Rev-Tax protein retargeted the TSS (Fig. 8, compare panels C and D). Conversely, mutations in the NES region of Rev did not relocate to TSS, although reduced cytoplasmic expression was noted (Fig. 8, compare panels C and E).
FIG. 8.
Incorporating Rev mutants into the fusion protein altered the subcellular location of Rev-Tax. HeLa cells were subjected to immunofluorescence standard microscopy. Tax-expressing cells display nucleolar exclusion and concentration into TSS (A). There is also some cytoplasmic staining of Tax observed. In Rev-expressing cells (B), Rev is localized to the nucleolus predominately, with some diffuse nuclear staining. Rev-Tax-expressing cells display predominately nucleolar and cytoplasmic staining and drastically reduced expression in the extranucleolar nucleus (C). When Rev containing a mutation in the NLS is incorporated into the fusion protein (Rev9-Tax), the targeting pattern of Rev9-Tax is altered to include nuclear speckles (D). In contrast, incorporation of an NES-mutated Rev into the fusion protein (RevM10-Tax) failed to restore localization to nuclear speckles (E). The RevM10-Tax also displayed reduced cytoplasmic staining compared to Rev-Tax (compare panel C to panel E). These images have been computer enhanced, or overexposed, to allow for detection of cellular outline.
We next tested the ability of Rev-Tax and the Rev-Tax mutants to activate either the HTLV-1 LTR or the HIV-1 LTR (Table 1). For these experiments, we used CV-1 cells to examine the activation of the HIV-1 LTR, since under these conditions Tax is capable of activating the HIV-1 LTR in an NF-κB-dependent manner (42). As expected, the Rev-Tax fusion was impaired for activation of the HTLV-1 LTR, consistent with the reduced presence of Tax in the transcription compartment. However, the ability of Tax, in the context of the Rev-Tax fusion, to function via induction of NF-κB was not adversely affected. Thus, redirection of Tax away from the transcription sites impaired CREB-dependent function while having no effect on NF-κB-dependent function.
TABLE 1.
Effect of fusion partner mutations on Tax function
| Protein | Activity (% of control)a
|
|
|---|---|---|
| HTLV-1 LTR | HIV-1 LTR | |
| Tax | 100 | 100 |
| Rev | 0 | 0 |
| Rev-Tax | 8 | 90 |
| Rev9-Tax | 85 | 93 |
| RevM10-Tax | 5 | 62 |
The presented values are averages of three experiments.
When examined for transcription function, the NLS mutant Rev9-Tax, which localized to the transcription compartment, showed restored transcription function, whereas the NES mutant RevM10-Tax was similar to Rev-Tax. None of the mutant proteins differed significantly from Rev-Tax with respect to activation of the NF-κB-dependent pathway. However, CREB-mediated transactivation function strictly correlated with the ability to target the TSS. Thus, reconciliation between Rev and Tax for separate subcellular compartments is the primary factor dictating both localization and function of the fusion protein.
Tax shuttles between the nucleus and cytoplasm.
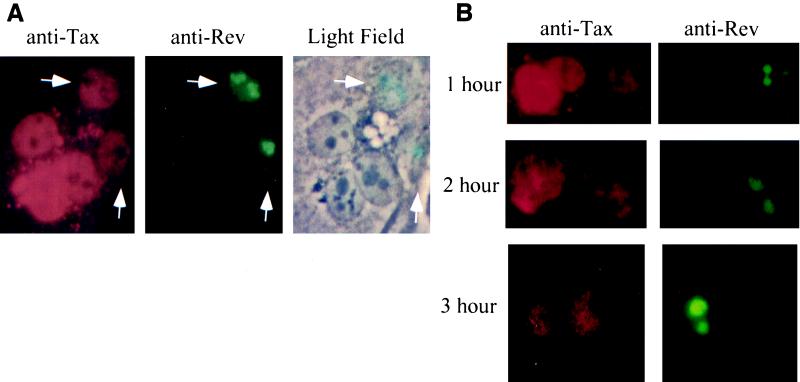
The observation that altering nuclear expression had no effect on NF-κB-dependent function and that the Rev-Tax fusion showed increased cytoplasmic expression prompted us to examine whether Tax can be exported from the nucleus into the cytoplasm. Such a scenario would help explain how Tax can perform both nuclear and cytoplasmic functions. We formed heterokaryons between Tax-expressing donor cells and TdRev-expressing recipient cells and asked whether Tax could shuttle from the nucleus of the donor cell into the cytoplasm and subsequently the recipient cell nucleus. The TdRev cell line expresses a mutant Rev protein that is defective for nuclear export and therefore acts as a marker for the recipient cell and provides an internal control for nuclear shuttling. Figure 9A shows the result of one such fusion. A light-field image of the resulting fused cells shows multiple nuclei contained within a single cytoplasmic entity (rightmost panel). Staining for Tax reveals that all nuclei are positive for the presence of Tax (leftmost panel). The middle panel shows that two of these nuclei were derived from recipient cells and stain with anti-Rev. Therefore, these two nuclei contain a population of Tax that has traveled from the nucleus of the donor cells. We also observed by judging the fluorescent intensities that the recipient cells displayed less Tax than did the donor cells. Therefore, we conducted the heterokaryon fusion assays at increasing times post-fusion formation. Under these conditions, equilibrium was eventually reached between the donor and recipient Tax levels. At 3 h postfusion, the donor and recipient cells showed approximately equal amounts of nuclear Tax (Fig. 9B, 3 h). The intensity of Tax-specific fluorescence is lower at 3 h since the half-life of Tax is about 45 min (data not shown).
FIG. 9.
Tax shuttles between nuclear and cytoplasmic subcellular domains. (A) Tax-expressing HeLa cells were fused with TdRev cells. The TdRev cells constitutively express a transdominant form of the HIV-1 Rev protein that is unable to shuttle out of the nucleus, and thus Rev expression serves as a marker for the heterokaryon recipient cell. Shown is a single heterokaryon consisting of five nuclei. All five nuclei contain Tax (anti-Tax). Two of the nuclei are identified as recipient cells and contain both Tax and Rev (see arrows). (B) Equilibrium of Tax protein between donor and recipient nuclei occurred by 3 h. One hour post-heterokaryon formation allowed for approximately one-fifth of the total Tax to shuttle. The relative concentrations of Tax in donor and recipient cells were roughly equivalent at 3 h post-heterokaryon formation.
DISCUSSION
Subcellular compartmentalization of function is a common means by which the cell can regulate multiple processes that by necessity occur simultaneously (8, 25, 31, 49, 55). There are many examples of movement from one subcellular compartment to another as a means of regulating the activity of the shuttling protein. From this perspective, one assumes that proteins with the same function would target identical subcellular sites. Therefore, it is somewhat surprising that HTLV-1 Tax and HIV-1 Tat transactivator proteins have strikingly different reported patterns of localization (24, 41, 45). Indeed, it was recently reported that Tat associates with the transcription factor SP1 (10). In this study, we found that HTLV-1 Tax and HIV-1 Tat, when targeted to the same nuclear sites of transcription, retain individual function. These findings support the concept of targeting of Tat to transcription hot spots as a requisite for function. We also show that when we redirect Tax out of these sites, CREB-dependent transcription function is impaired but NF-κB-dependent function is unaffected. Furthermore, we also show that Tax is one of an expanding number of proteins that regulate function by shuttling between cellular compartments.
Mutational analysis is often the approach chosen toward defining the subcellular location of a protein. However, since a functionally inactive protein may have multiple defects, the mutational approach is unable to address whether the specific subcellular targeting is a prerequisite for function. Here, we chose an approach in which we direct the localization of Tax via fusion with functionally defined proteins, in this case Tat and Rev. We reasoned that fusion of Tax with a functional homologue such as Tat would result in a pattern of localization consistent with each protein's function. Conversely, a fusion with the functional heterologue Rev would result in competitive localization inconsistent with the function of at least one of the proteins. As we showed by confocal microscopy, the Tat-Tax protein targeted transcription hot spots, whereas the Rev-Tax protein targeted primarily the nucleolus and cytoplasm, a predominately Rev-like pattern. Thus, our initial premise for directing subcellular expression and/or location was justified.
A close examination of the Tat-Tax expression pattern revealed a pattern unlike the predominately nucleolar pattern of Tat and an apparent subset of the Tax pattern (TSS). The microstructure of the Tat-Tax pattern more closely resembled that of transcription sites and colocalized with the transcription factor SP1. This comparison is most easily made by observing the shift in the color pattern displayed for Tax colocalization with SC35, yellow (colocalization) and red (Tax), to that seen with colocalization of Tat-Tax with SC35, yellow (colocalization), red (Tax), and green (SC35). Thus, Tat-Tax has shifted the Tax-specific pattern toward the periphery of the SC35-containing nuclear speckles as demonstrated by the appearance of SC35-specific (green) fluorescence separable from the Tax-specific signal. However, even though the Tat-Tax pattern was dramatically different from the wild-type Tat pattern and apparently a subset of the TSS pattern, the fusion protein was as active as either component protein. Specifically, Tat-Tax activated the HTLV-1 LTR and HIV-1 LTR as efficiently as did the cognate transactivator. The ability of Tat-Tax to activate the HIV-1 LTR is not a result of NF-κB induction by Tax, since in HeLa cells Tax is unable to activate the HIV-1 LTR. Thus, targeting transcription hot spots is compatible with both Tat and Tax function. In contrast, when Tax was directed out of the hot spots and into predominately the nucleolus and cytoplasm, the ability to activate the HTLV-1 LTR was substantially reduced. We saw a 10-fold decrease in CREB-dependent function of Rev-Tax compared to that of Tax. Since we observed greater steady-state amounts of Rev-Tax, this would suggest that the actual reduction in activity on a molar basis is even higher. Thus, these results strongly suggest that targeting these transcription sites is an absolute prerequisite for CREB-dependent Tax function.
The ability of Tax to activate NF-κB-containing promoters has potentially important biological consequences (15, 46, 59, 60). Activation of interleukin-2–interleukin-2 receptor may play an important role in altering host cell proliferation, and more recently, the link between NF-κB activation and apoptosis provides an important route by which Tax would potentially alter cell survival. NF-κB-dependent Tax function is initiated via induced degradation of IκB and subsequent nuclear translocation of NF-κB. The mechanisms proposed by which Tax mediates IκB degradation are numerous. One of the more consistent findings is a direct binding of Tax to the IκB-like protein p100 followed by subsequent degradation (4, 28, 30, 36). Recently, a very compelling hypothesis has emerged in which Tax mediates degradation of IκB via activation of IKK-specific phosphorylation (19, 57). Either of these models would presumably prescribe cytoplasmic activities to Tax. In fact, cytoplasmic forms of Tax (lacking an NLS) have been shown to retain NF-κB-dependent function (37). Our demonstration that redirecting Tax out of transcription sites had no effect on NF-κB-dependent function is consistent with this function being cytoplasmic.
One mechanism by which a protein can regulate both nuclear and cytoplasmic functions is via shuttling between these two compartments. This ability has been ascribed to numerous proteins to date, including HIV Rev, Epstein-Barr virus Mta, hdm2, and c-abl (2, 12, 18, 32, 39, 44, 51, 52). Our demonstration that Tax can shuttle between heterokaryon nuclei provides evidence for one possible mechanism by which this functionally pleiotropic protein regulates diverse activities. Numerous potential posttranslational modification events such as phosphorylation-dephosphorylation and acetylation can be envisioned as potential pathways for regulating Tax location-function in response to cell state. A scanning of the Tax amino acid sequence revealed a Rev-like NES motif at amino acids 190 to 203. We are currently examining the functional significance of this region of Tax.
In summary, we have verified our earlier observations that suggested that Tax occupies multiple sites within the TSS (41). Specifically, we have extended these findings to show that a Tax subpopulation within the TSS co-occupies sites of de novo transcription. Furthermore, we demonstrate that these sites are key to CREB-dependent function and that targeting these sites is a prerequisite for transcription function. We further show that targeting transcription sites is not required for Tax-mediated NF-κB-dependent function and suggest that this function is cytoplasmic. We provide evidence that nucleocytoplasmic shuttling is a mechanism by which Tax can be involved in both CREB-dependent and NF-κB-dependent functions via shuttling between the nucleus and cytoplasm.
ACKNOWLEDGMENTS
We thank Anne Beyer, Dan Engel, and Maja Zecevic for critical reading of the manuscript.
This work was supported by the American Cancer Society grant RPG99-091-01-MBC, the University of Virginia Research and Development program, the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust, and the Charles H. Ross Fund.
REFERENCES
- 1.Armstrong A P, Franklin A A, Uittenbogaard M N, Giebler H A, Nyborg J K. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc Natl Acad Sci USA. 1993;90:7303–7307. doi: 10.1073/pnas.90.15.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachelerie F, Rodriguez M S, Dargemont C, Rousset D, Thomas D, Virelizier J L, Arenzana-Seisdedos F. Nuclear export signal of IκBα interferes with the Rev-dependent posttranscriptional regulation of human immunodeficiency virus type I. J Cell Sci. 1997;110:2883–2893. doi: 10.1242/jcs.110.22.2883. [DOI] [PubMed] [Google Scholar]
- 3.Baranger A M, Palmer C R, Hamm M K, Giebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 4.Beraud C, Sun S C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady J, Jeang K-T, Duvall J, Khoury G. Identification of p40X-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 8.Carter K C, Lawrence J B. DNA and RNA within the nucleus: how much sequence-specific spatial organization. J Cell Biochem. 1991;47:124–129. doi: 10.1002/jcb.240470205. [DOI] [PubMed] [Google Scholar]
- 9.Chen I, Slamon D, Rosenblatt J, Shah N, Quan S, Wachsman W. The x gene is essential for HTLV replication. Science. 1985;229:54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- 10.Chun R, Semmes O, Neuveut C, Jeang K. Modulation of SP1 phosphorylation by human immunodeficiency virus type 1 Tat. J Virol. 1998;72:2615–2629. doi: 10.1128/jvi.72.4.2615-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colgin M A, Nyborg J K. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J Virol. 1998;72:9396–9399. doi: 10.1128/jvi.72.11.9396-9399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felber B, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis G. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 14.Feuer G, Chen I. Mechanisms of human T-cell leukemia virus-induced leukemogenesis. Biochim Biophys Acta. 1992;1114:223–233. doi: 10.1016/0304-419x(92)90017-s. [DOI] [PubMed] [Google Scholar]
- 15.Fujii M, Sassone-Corsi P, Verma I. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 17.Fujii M, Tsuchiya H, Chuhjo T, Minamino T, Miyamoto K, Seiki M. Serum response factor has functional roles both in indirect binding to the CArG box and in the transcriptional activation function of human T-cell leukemia virus type I Tax. J Virol. 1994;68:7275–7283. doi: 10.1128/jvi.68.11.7275-7283.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997;272:32642–32648. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- 19.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gendelman H E, Phelps W, Feigenbaum L, Ostrove J M, Adachi A, Howley P M, Khoury G, Ginsberg H S, Martin M A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci USA. 1986;83:9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodrum F D, Shenk T, Ornelles D A. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J Virol. 1996;70:6323–6335. doi: 10.1128/jvi.70.9.6323-6335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauber J, Malim M H, Cullen B R. Mutational analysis of the conserved basic domain of human immunodeficiency virus tat protein. J Virol. 1989;63:1181–1187. doi: 10.1128/jvi.63.3.1181-1187.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hozak P, Hassan A B, Jackson D A, Cook P R. Visualization of replication factories attached to nucleoskeleton. Cell. 1993;73:361–373. doi: 10.1016/0092-8674(93)90235-i. [DOI] [PubMed] [Google Scholar]
- 26.Jeang K-T, Widen S, Semmes O J, Wilson S. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 27.Jin D Y, Spencer F, Jeang K-T. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 28.Kanno T, Franzoso G, Siebenlist U. Human T-cell leukemia virus type I Tax-protein-mediated activation of NF-κB from p100 (NF-κB2)-inhibited cytoplasmic reservoirs. Proc Natl Acad Sci USA. 1994;91:12634–12638. doi: 10.1073/pnas.91.26.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 30.Lanoix J, Lacoste J, Pepin N, Rice N, Hiscott J. Overproduction of NFKB2 (lyt-10) and c-Rel: a mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signalling pathway. Oncogene. 1994;9:841–852. [PubMed] [Google Scholar]
- 31.Lawrence J B, Carter K C, Xing X. Probing functional organization within the nucleus: is genome structure integrated with RNA metabolism. Cold Spring Harbor Symp Quant Biol. 1993;58:807–818. doi: 10.1101/sqb.1993.058.01.088. [DOI] [PubMed] [Google Scholar]
- 32.Meyer B E, Malim M H. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 33.Misteli T, Caceres J, Clement J, Kfainer A, Wilkinson M, Spector D. Serine phosphorylation of SP proteins is required for their recruitment to sites of transcription in vivo. J Cell Biol. 1998;143:297–307. doi: 10.1083/jcb.143.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misteli T, Caceres J, Spector D. The dynamics of a pre-mRNA splicing factor in living cells. Nature. 1997;387:523–527. doi: 10.1038/387523a0. [DOI] [PubMed] [Google Scholar]
- 35.Misteli T, Spector D. The cellular organization of gene expression. Curr Opin Cell Biol. 1998;10:323–331. doi: 10.1016/s0955-0674(98)80007-0. [DOI] [PubMed] [Google Scholar]
- 36.Murakami T, Hirai H, Suzuki T, Fujisawa J, Yoshida M. HTLV-1 Tax enhances NF-κB2 expression and binds to the products p52 and p100, but does not suppress the inhibitory function of p100. Virology. 1995;206:1066–1074. doi: 10.1006/viro.1995.1029. [DOI] [PubMed] [Google Scholar]
- 37.Nicot C, Tie F, Giam C Z. Cytoplasmic forms of human T-cell leukemia virus type 1 Tax induce NF-κB activation. J Virol. 1998;72:6777–6784. doi: 10.1128/jvi.72.8.6777-6784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perini G, Wagner S, Green M R. Recognition of bZIP proteins by the human T-cell leukaemia virus transactivator Tax. nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 39.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. Nucleocytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semmes O, Barret J, Dang C, Jeang K-T. Human T-cell leukemia virus type I tax masks c-Myc function through a cAMP-dependent pathway. J Biol Chem. 1996;271:9730–9738. doi: 10.1074/jbc.271.16.9730. [DOI] [PubMed] [Google Scholar]
- 41.Semmes O, Jeang K-T. Localization of human T-cell leukemia virus type 1 Tax to subnuclear compartments that overlap with interchromatin speckles. J Virol. 1996;70:6347–6357. doi: 10.1128/jvi.70.9.6347-6357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semmes O, Jeang K-T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semmes O, Majone F, Cantemir C, Turchetto L, Hjelle B, Jeang K-T. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology. 1996;217:373–379. doi: 10.1006/viro.1996.0126. [DOI] [PubMed] [Google Scholar]
- 44.Semmes O J, Chen L, Sarisky R T, Gao Z, Zhong L, Hayward D. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mRNA. J Virol. 1998;72:9526–9534. doi: 10.1128/jvi.72.12.9526-9534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siomi H, Shida H, Maki M, Hatanaka M. Effects of a highly basic region of human immunodeficiency virus Tat protein on nucleolar localization. J Virol. 1990;64:1803–1807. doi: 10.1128/jvi.64.4.1803-1807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith M, Greene W. Molecular biology of the type I human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J Clin Investig. 1991;87:761–766. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sodroski J. The human T-cell leukemia virus (HTLV) transactivator (Tax) protein. Biochim Biophys Acta. 1992;1114:19–29. doi: 10.1016/0304-419x(92)90003-h. [DOI] [PubMed] [Google Scholar]
- 48.Sodroski J, Rosen C, Wong-Staal F, Salahuddin S, Popovic M, Arya S, Gallo R, Haseltine W. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985;227:171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- 49.Spector D L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki T, Hirai H, Fujisawa J, Fujita T, Yoshida M. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κB site and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- 51.Taagepera S, McDonald D, Loeb J E, Whitaker L L, McElroy A K, Wang J Y, Hope T J. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uittenbogaard M N, Armstrong A P, Chiaramello A, Nyborg J K. Human T-cell leukemia virus type I Tax protein represses gene expression through the basic helix-loop-helix family of transcription factors. J Biol Chem. 1994;269:22466–22469. [PubMed] [Google Scholar]
- 54.Uittenbogaard M N, Giebler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type I Tax protein. J Biol Chem. 1995;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 55.Wansink D G, van Driel R, de Jong L. Organization of pre-mRNA metabolism in the cell nucleus. Mol Biol Rep. 1994;20:45–55. doi: 10.1007/BF00996353. [DOI] [PubMed] [Google Scholar]
- 56.Yan J P, Garrus J E, Giebler H A, Stargell L A, Nyborg J K. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J Mol Biol. 1998;281:395–400. doi: 10.1006/jmbi.1998.1951. [DOI] [PubMed] [Google Scholar]
- 57.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 58.Yin M J, Gaynor R B. HTLV-1 21 bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida M. HTLV-1 oncoprotein Tax deregulates transcription of cellular genes through multiple mechanisms. J Cancer Res Clin Oncol. 1995;121:521–528. doi: 10.1007/BF01197764. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida M. Molecular biology of HTLV-I: recent progress. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:63–68. doi: 10.1097/00042560-199600001-00012. [DOI] [PubMed] [Google Scholar]