Abstract
Transmissible spongiform encephalopathies form a group of fatal neurodegenerative disorders that have the unique property of being infectious, sporadic, or genetic in origin. Although some doubts about the nature of the responsible agent of these diseases remain, it is clear that a protein called PrPSc plays a central role. PrPSc is a conformational variant of PrPC, the normal host protein. Polyene antibiotics such as amphotericin B have been shown to delay the accumulation of PrPSc and to increase the incubation time of the disease after experimental transmission in laboratory animals. Unlike for Congo red and sulfated polyanions, no effect of amphotericin B has been observed in infected cultures. We show here for the first time that amphotericin B can inhibit PrPSc generation in scrapie-infected GT1-7 and N2a cells. Its activity seems to be related to a modification of the properties of detergent-resistant microdomains. These results provide new insights into the mechanism of action of amphotericin B and confirm the usefulness of infected cultures in the therapeutic research of transmissible spongiform encephalopathies.
Transmissible spongiform encephalopathies (TSEs) are a group of neurodegenerative disorders that include bovine spongiform encephalopathy and scrapie in animals and Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker syndrome, and fatal familial insomnia in humans (for reviews, see references 37 and 38). TSEs can have infectious, sporadic, or genetic origins. In all three cases, brain from affected individuals contains the abnormal isoform of the prion protein, PrPSc, and can be used to transmit disease to laboratory animals. PrPSc is distinguishable from the host protein, PrPC, by its biochemical properties, including proteinase K (PK) resistance and insolubility (32). It is thought that the generation of PrPSc from PrPC involves a conformational transition that is accompanied by changes in the secondary structure of the protein (13, 21, 36). How this conversion is related to the infectious process in TSEs is a matter of controversy (16). However, the central role of PrP in the disease is exemplified by the fact that PrP-null mice are resistant to the disease (6), by the strong genetic linkages between mutations in the PrP gene and genetic forms of TSEs (37), and by the fact that efficiency of experimental transmission of the disease from one species to another is dependent on the similarity of the PrP sequences between species (for a review, see reference 40).
In experimental transmission of TSEs in laboratory animals, different classes of drugs administered either at the moment of inoculation or during incubation have been able to delay the appearance of disease. This was the case with the polyene antibiotic amphotericin B (AmB) and its derivative MS-8209 (17, 39, 55), which were shown to have a strain-specific effect on reducing the accumulation of PrPSc in brain (18). These drugs are among the most studied in TSEs, and they have the advantage of being already used in human therapies as antifungal agents (24). The mechanism of the therapeutic action of AmB in TSEs is uncertain, but it may be related to the ability of the drug to form stable complexes with sterols (1), a property responsible for the antifungal activity of these molecules (24).
PrP molecules are attached to the plasma membrane by a glycosyl phosphatidylinositol (GPI) anchor and are concentrated on the plasma membrane in distinct detergent-resistant microdomains (DRM) (3, 22, 26, 50). These DRM are enriched in cholesterol, sphingolipid, and glycolipid (5). It has been proposed that the conversion of PrPC into PrPSc may occur in these microdomains (54). This hypothesis is based on several major observations. Firstly, PrPC and PrPSc are present in DRM in both infected cell cultures and affected brains (34, 54). Secondly, the use of drugs that influence DRM in culture modifies both the presence of PrP in DRM and the generation of PrPSc (49). Thirdly, modifying the targeting of PrP to DRM by removing or replacing its GPI anchor by a real transmembrane domain prevents PrPSc formation (26). Finally, trafficking studies on PrP have indicated the involvement of DRM and caveolae in the endocytosis and recycling of the molecule, two phenomena known to be essential in the conversion process (4, 12, 50, 54).
With this information in mind, we asked whether the mechanism of action of AmB in TSEs could be analyzed in culture and might involve DRM. To answer these questions, infected N2a and GT1-7 cell lines, which were recently developed in the laboratory (35), were used. After several days of treatment, AmB was able to inhibit PrPSc synthesis, but the drug could not completely cure the infected cells. Finally, AmB was shown to modify the properties of DRM, and we believe that it is this effect which is responsible for the action of the drug.
MATERIALS AND METHODS
Reagents and antibodies.
AmB purchased from Sigma (St. Louis, Mo.) (catalog no. A 2942) was dissolved in sterile distilled water. Pefabloc and PK were purchased from Boehringer Mannheim. Opti-MEM, trypsin, G418, and horse serum were from Life Technologies Inc., MEM-alpha was from ICN, and fetal calf serum was from Bio-Whitaker. Secondary antibodies were from Jackson Immunoresearch (West Grove, Pa.). All other reagents were from Sigma.
Rabbit polyclonal antibody P45-66, raised against a synthetic peptide encompassing mouse PrP (MoPrP) residues 45 to 66, has been described before (28). Monoclonal antibody (MAb) Pri 308 was generated by J. Grassi (CEA-Saclay, Gif-sur-Yvette, France) against the peptide K-T-N-M-K-H-M-A-G-A-A-A-A-G-A-V-V-G-G-L-G-(C), corresponding to the human PrP sequence from residues 106 to 126, with an additional cysteine residue at the C terminus. Mice (Biozzi strains or PrPo/o) were immunized and hybridoma cells were prepared as previously described (23). By Western blotting, MAb Pri 308 showed a specificity equivalent to that of MAb 3F4 (unpublished data). SAF 60, 69, and 70 are three other MAbs produced by the J. Grassi group. They were obtained by using as an immunogen scrapie-associated fibrils (SAF) that were prepared from infected hamster brains. In enzyme immunometric assays, they were characterized as recognizing peptide epitope 142-160 of hamster PrP (J. Grassi, personal communication). A mixture consisting of equal volumes of ascites containing these three antibodies was used to improve PrPSc detection.
Infected cell cultures.
The GT1-7 cells (subclone 7) employed were graciously provided by David Holtzmann (Washington University, St. Louis, Mo.) and were grown in Dulbecco modified Eagle's medium containing 5% fetal calf serum, 5% horse serum, and penicillin-streptomycin in an atmosphere of 5% CO2 and 95% air. The mouse N2a neuroblastoma cell line (ATCC CCL131), stably transfected with wild-type MoPrP cDNA, was described previously (28, 44). Generation of the infected cultures used here is described elsewhere (35). Briefly, subconfluent cultures were incubated for 4 h with a 2% brain homogenate from mice inoculated with the Chandler strain, and then fresh medium was added volume to volume overnight. Cells were split and infection was assessed by the presence of PrPSc after multiple passages. In this study, a clone of the infected MoPrP-transfected cell line N2a (35), S12, was used, but similar observations were obtained with two other clones. As described by others (46), we tested the conversion of 3F4-tagged wild-type MoPrP after transfection to estimate the effect of the drug on the generation of new PrPSc molecules. In fact, as the 3F4 tag is not present in MoPrP, the use of 3F4-specific antibodies allows the detection of newly synthesized PrPSc molecules. To transiently transfect S12 cells with 3F4-tagged wild-type MoPrP cDNA (27), FuGENE 6 transfection reagent from Boehringer Mannheim was used as described in the manufacturer's instructions.
Detection of PrPSc in infected cells.
Cells from a 35-mm dish were collected in phosphate-buffered saline (PBS) and lysed for 20 min at 4°C in 40 μl of PBS containing 0.5% NP-40 and 0.5% sodium deoxycholate. After 1 min of centrifugation at 10,000 × g, the supernatant was collected. The total protein concentration was then measured using a bicinchoninic acid (BCA) protein assay kit (Pierce), and it was adjusted between samples to an equal value with the buffer used to lyse the cells. Thirty microliters of each of these lysates was treated with PK (16 μg/mg of total protein) for 30 min at 37°C, and digestion was stopped by the addition of Pefabloc (5 mM) for 5 min on ice. An equal volume of 2× sodium dodecyl sulfate (SDS) sample buffer was then added, and the samples were boiled for 5 min. Proteins were electroblotted onto Immobilon membranes and MoPrP was detected by using a mixture of MAbs SAF 60, 69, and 70 (see above) in conjunction with a peroxidase-conjugated goat anti-mouse secondary antibody. The blots were developed by using enhanced chemiluminescence. Films were analyzed using Image Analysis software.
Flotation of MoPrP in sucrose gradients.
The detergent extraction and flotation protocols were adapted from previously described methods (5). Confluent cultures from a 25-cm2 flask were scraped in PBS and pelleted by centrifugation for 5 min at 750 × g, washed in hypotonic buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES [pH 7.4]), 250 mM sucrose, 0.5 mM EDTA], and then resuspended in 0.3 ml of the same buffer containing 0.5 mM EDTA, 1% Triton X-100, and 10 mM PIPES, pH 7.4. Cells were lysed at 4°C for 20 min and then broken by 10 consecutive passages through a 27-gauge needle. Nuclei and debris were removed by centrifugation at 2,500 × g for 10 min at 4°C. The sample was adjusted to 40% sucrose, overlaid with 1 ml of 25% sucrose and 0.5 ml of 5% sucrose (in 10 mM PIPES [pH 7.4] and 0.5 mM EDTA), and then centrifuged for 18 h at 37,000 rpm at 4°C in the TLS-55 rotor of a Beckman Optima TL ultracentrifuge. Ten fractions of 0.2 ml were collected from the top to the bottom. For PrPC detection, the fractions were methanol precipitated and then the protein was pelleted and resuspended directly in SDS loading buffer and analyzed by SDS-polyacrylamide gel electrophoresis using P45-66 antibody. In the case of PrPSc, the fractions were made to 150 mM NaCl–0.5% Triton X-100–0.5% sodium deoxycholate–50 mM Tris-HCl (pH 7.5) and incubated with PK (20 μg/ml) for 30 min at 37°C. Digestion was stopped by the addition of Pefabloc for 5 min on ice. The fractions were then spun at 70,000 rpm for 45 min at 4°C in the TLA 100.4 rotor of a Beckman Optima TL ultracentrifuge, and the pellet was resuspended in SDS loading buffer. PrPSc was analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblotting using a mixture of MAbs SAF 60, 69, and 70.
The sucrose and protein concentrations of the fractions were measured by reflectometry and by using the BCA kit (Pierce), respectively. Ganglioside GM1, a marker of DRM, was detected by dot blotting of fractions onto a nitrocellulose membrane (45). After blocking with 3% bovine serum albumin in PBS, the membrane was incubated with 2 ng of cholera toxin B conjugated to horseradish peroxidase (Sigma) per ml in blocking buffer. Blots were washed four times in Tris-buffered saline (TBS) (100 mM NaCl, 10 mM Tris-HCl [pH 7.8]) containing 0.1% Tween 20 and were developed using enhanced chemiluminescence.
RESULTS AND DISCUSSION
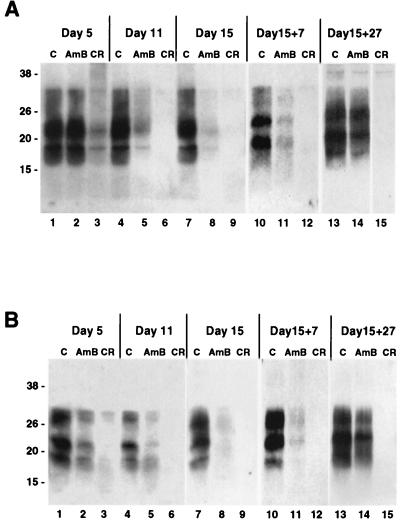
To investigate whether AmB can inhibit PrPSc generation in scrapie-infected GT1-7 and S12 cells, 4.5 μg of the drug per ml was added to the culture medium at each time of cell passage (every 3 to 5 days), and the presence of PrPSc was tested by Western blotting after 5, 11, and 15 days (Fig. 1). Congo red (CR) was used as a control, at a concentration of 1 μg/ml, since it is known to inhibit PrPSc generation in infected cultures (10). At the different time points, no significant difference in the protein concentrations of samples from cells incubated with or without the drugs was noticed. The presence of either drug did not affect significantly the level of MoPrP detected (data not shown). As expected, in both cell lines the PrPSc signal decreased and then disappeared completely after a few days of CR treatment (Fig. 1, lanes 3, 6, and 9). In some cases, a smear related to the formation of SDS-resistant complexes between PrPSc and CR was detected (Fig. 1A, lane 3) (7). Interestingly, the presence of AmB also resulted in a significant decrease in the PrPSc signal, but at a lower rate than with CR (Fig. 1, lanes 2, 5, and 8). The effect of AmB on PrPSc in cultured cells was not reported previously by Caughey and Raymond (11). We believe that in their work, the time of incubation with AmB was insufficient to detect a significant effect of the drug. However, AmB was not as effective as CR, since even after 27 days of treatment with the drug, a low level of PrPSc still remained detectable (data not shown).
FIG. 1.
AmB inhibits PrPSc production in infected GT1-7 and S12 cell lines. Infected GT1-7 (A) and S12 (B) cells were passaged for the indicated periods of time in the absence (control [C]) or the presence of AmB (4.5 μg/ml) or CR (1 μg/ml) for up to 15 days (lanes 1 to 9). The drugs were then removed and cells were passaged in their absence for 7 and 27 additional days (lanes 10 to 15). PrPSc at each time point was detected by Western blotting after limited PK digestion as indicated in Materials and Methods. PrPSc signal disappeared rapidly upon CR treatment in both cell lines. AmB also reduced the amount of PrPSc detected, but at a lower rate; its signal did not disappear completely and was restored after removal of the drug. Molecular masses, on the left, are in kilodaltons.
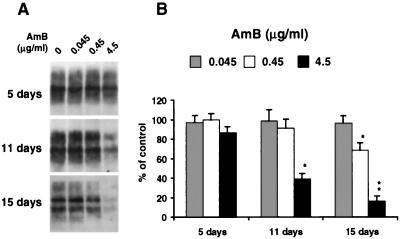
To determine how long the effects of AmB and CR would last after their removal, both drugs were stopped after 15 days of treatment and the cells were cultured in normal medium for a further 27 days (Fig. 1, lanes 10 to 15). This protocol protected against any interference of the drugs with PrPSc detection. As reported earlier (11), no reappearance of PrPSc was observed in CR-treated cells. After the AmB treatment was stopped, the PrPSc signal seemed to increase to its original level (Fig. 1, lane 14). Thus, at the concentration tested, AmB was not able to induce a complete cure of the infected cells, and this may be related to its mechanism of action (see below). We were also able to demonstrate that preincubation of the GT1-7 cells with AmB for 15 days (at 4.5 μg/ml) did not prevent them from being infected after incubation with infectious brain homogenates, as reported elsewhere (35, 42) (data not shown). These results are consistent with in vivo studies where AmB acted mainly on the accumulation of PrPSc and where the drug was not able to completely stop the disease (19). However, the fact that AmB was able to reduce the conversion of 3F4-tagged MoPrP transfected into infected cells (Fig. 2) argues for an effect of the drug on the generation of PrPSc molecules. Similarly, the reappearance of PrPSc after removal of the drug would suggest that AmB does not stop but reduces the efficiency of the conversion. It will now be important to compare the reduction in PrPSc signal in treated cells with the infectivity in an animal assay (study in progress). Finally, to confirm the specificity of action of AmB, various concentrations of the drug were tested on scrapie-infected S12 cells (Fig. 3). A decrease in PrPSc signal was observed at concentrations of 0.45 μg/ml and higher. The concentration range of AmB tested here did not have any obvious effect on the morphology or growth rate of the cells, and a significant toxic effect was observed only with AmB concentrations of 45 μg/ml or higher (data not shown).
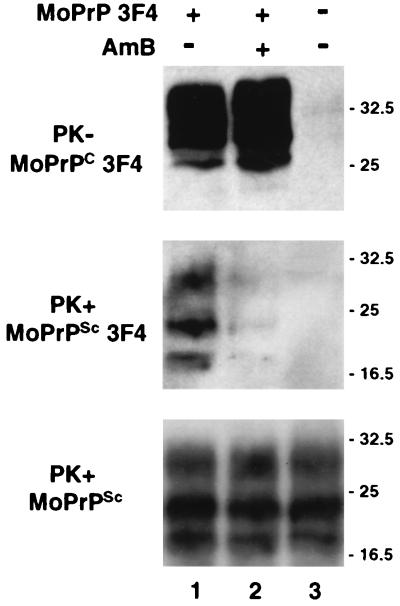
FIG. 2.
AmB inhibits the generation of PrPSc molecules after transfection of infected cells. Infected S12 cells were left untransfected (lane 3) or were transfected with 3F4-tagged MoPrP (lanes 1 and 2). When indicated, AmB (4.5 μg/ml) was added to the culture medium from the time of transfection (lane 2). Seventy-two hours after transfection, cells were lysed, and transfected 3F4-tagged MoPrPC was detected by Western blotting using Pri308 (upper panel). Lysates were subjected to limited PK digestion, and 3F4-tagged and total MoPrPSc was detected using Pri308 (middle panel) or a mixture of MAbs SAF 60, 69, and 70 (lower panel), respectively. Molecular masses, on the right, are in kilodaltons.
FIG. 3.
Dose response and time course of action of AmB. (A) Infected S12 cells were cultured for 5, 11, and 15 days in the presence of various concentrations of AmB. At each time point, PrPSc was detected by immunoblotting after protease digestion as described in Materials and Methods. A decrease in PrPSc signal was observed at concentrations of 0.45 μg/ml and higher. (B) MoPrP-specific bands from panel A and from two other experiments were quantitated by densitometry. The amount of MoPrP remaining after digestion at each time point was plotted as a percentage of the control without AmB. Each bar represents the mean + the standard deviation. Values that are significantly different from those of untreated samples by paired t test are indicated by single (P < 0.05) and double (P < 0.005) asterisks.
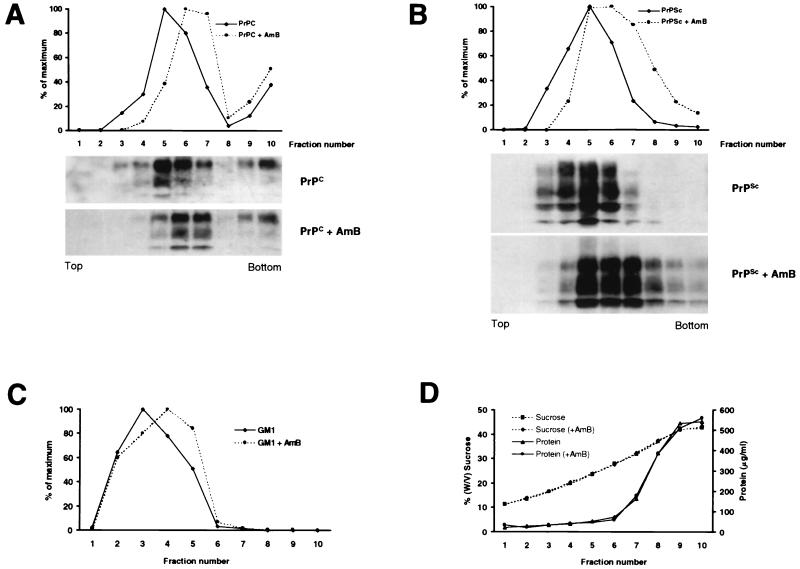
Like other GPI-anchored proteins, PrP is associated with DRM as early as the Golgi apparatus (5, 22, 49). On the cell surface, PrP is clustered in these DRM, which appear to play an important role in both the trafficking of PrP and the generation of PrPSc (26, 49, 54). DRM are endocytosed and recycled in caveola-like structures and are enriched in cholesterol and sphingolipids (5). As AmB antifungal activity appears to be related to its binding to sterols (24), and as this drug is able to disorganize endosomal trafficking in cultured cells (53), DRM would appear to provide a target for AmB. To analyze the impact of AmB on DRM, flotation experiments with GT1-7 cells in a sucrose step gradient system were performed (Fig. 4). In this paradigm, PrPC was recovered mainly in fractions 3 to 7, corresponding to sucrose concentrations between 16 and 32% (Fig. 4A and D). This is consistent with previously published works on N2a cells (22, 49). These fractions contained less than 10% of the total cellular protein, which remained mostly in fractions 8 to 10 (Fig. 4D). PrPSc detected after PK digestion of the fractions also floated and concentrated in fractions similar to those of PrPC (Fig. 4B), consistent with previous observations (49, 54). After AmB treatment, both PrPC and PrPSc molecules shifted toward fractions of higher densities (Fig. 4A, B, and C). This shift was small but reproducible in five independent experiments. Moreover, in CHO cells overexpressing MoPrP (28), a similar effect of AmB on MoPrP flotation was observed (29).
FIG. 4.
AmB affects flotation of PrPC and PrPSc. GT1-7 cells (A) or scrapie-infected GT1-7 cells (B) were incubated for 3 days with or without AmB (4.5 μg/ml). Cells were then collected and flotation gradients were performed as indicated in Materials and Methods. (A and B) Gradient fractions were methanol precipitated (A) or digested with PK (20 μg/ml, 30 min at 37°C) and ultracentrifuged (B). Pellets were resuspended in SDS loading buffer, and MoPrP-specific bands were detected by Western blotting using antibody P45-66 (A) or a mixture of MAbs SAF 60, 69, and 70 (B). In panel A, only 1/10 of fraction 7 and 1/30 of fractions 8 to 10 were loaded. Quantitation of total MoPrP signal in each fraction (from the top, fraction 1, to the bottom, fraction 10) was related to the highest value, which was taken as 100%. Similar results were obtained in three independent experiments, and panel A results were also obtained with infected GT1-7 cells. (C) An aliquot of the fractions of panel A was used in a GM1 dot blot assay (see Materials and Methods). GM1 was concentrated in fractions 2 to 4 prior to AmB treatment and in fractions 3 to 5 after treatment. (D) Samples from panel A were used for this panel, but similar results were obtained with the other gradients used in this work. For each fraction, the sucrose and protein concentrations were determined, respectively, by reflectometry and BCA protein assay. No significant modification of either profile was noted after AmB treatment.
To determine if the action of AmB was restricted to MoPrP or also affected DRM, we used labeled cholera toxin B, which binds to the ganglioside GM1 and can be considered a marker of DRM (45). In the sucrose gradient experiments described above, the GM1 signal was also affected by AmB and shifted toward fractions of higher densities after incubation with the drug (Fig. 4C). This indicates that AmB modified not only the flotation of MoPrP but also that of DRM and suggests that the formation of stable complexes between AmB and cholesterol altered the structure of DRM. Our results are consistent with previous observations showing that cholesterol synthesis inhibitors, such as mevalonate or lovastatin, have an inhibitory effect on PrPSc synthesis in cultured cells (49).
Several possibilities exist as to the mechanism of action of AmB in TSEs. The direct interaction of the drug with PrP isoforms, as proposed for other agents, such as CR (7, 8, 33), seems unlikely based on the inefficiency of the drug in in vitro conversion assays and on the delayed response in our model. This last point would suggest that AmB acts by changing slowly some metabolic equilibrium inside the cell. Because AmB binds to sterols (24), a property responsible for its antifungal activity, it is possible that it is this binding which is responsible for its action in TSEs (1) through an alteration in the lipid and protein composition of DRM (20, 52). Consequently, as PrP is localized in such domains (22, 34, 54), it is possible that AmB may lead to a modification in the association of PrPC molecules with themselves, with a putative protein X (51), with a PrP receptor (30, 41), or with PrPSc (9). In addition, alteration of DRM on the cell surface and in intracellular organelles by AmB treatment (53) could also have an impact on the pathological events by changing the trafficking of PrP. Importantly, previous studies have shown that depletion of membrane cholesterol with cholesterol-binding drugs or by inhibiting its synthesis disrupted the trafficking of GPI-anchored proteins (14, 43) and in particular, in some cases, accelerated the recycling and diminished the degradation of such proteins (31, 49). These observations fit with the idea that endosomal trafficking and lysosomal trafficking of PrPC are important for the conversion into PrPSc (12, 47). A more complete analysis of PrP trafficking upon AmB treatment (endocytosis, recycling, and cleavage) is needed to identify the exact site of action of the drug. Finally, the fact that a cholesterol gradient appears to exist from the internal organelles to the cell membrane (2, 25) could explain why the generation of PrPSc is affected only partially and temporarily by AmB. In fact, at the concentration used in this work, the external membranes would have been the principal target of the drug (53) and any alternate intracellular conversion pathway of PrPC, as has been suggested previously to occur (48), would have gone unaffected. This hypothesis may be substantiated by our previous work on mutated MoPrPs, where it was found that AmB, at the same concentration range, affected the abnormal biochemical properties of molecules that were transported to the cell surface but did not reduce those of mutant molecules that were retained within the cells (29). It is possible, therefore, that prion strains resistant to AmB treatment (18) rely more on an intracellular conversion pathway and/or are sustained by cells having different membrane compositions (15) and, therefore, different sensitivities to AmB.
In conclusion, our results show for the first time that the effect of AmB can be evaluated in scrapie-infected cell cultures; they also provide new insights into the mechanism of action of AmB in TSEs, suggesting that it acts through modification of the PrP environment and/or trafficking. In the future, similar models can be used to examine other members of the polyene family and to investigate the response of different prion strains to these drugs.
ACKNOWLEDGMENTS
We are grateful to David Harris (Washington University) for antibody P45-66, Jacques Grassi and Yveline Frobert (CEA-Saclay) for Pri308 and the SAF 60, 69, and 70 antibodies, and David Holtzmann for the GT1-7 cell clone.
This work was supported by grants from the FRM (Fondation de la Recherche Médicale), the CCI Prion (Cellule de Coordination Interorganismes sur les Prions), the CNRS (Centre National de la Recherche Scientifique), and the European Community Biotech (BIO4CT98-6055 and BIO4CT98-6064).
REFERENCES
- 1.Adjou K T, Deslys J P, Lasmézas C, Demaimay R, Dormont D. Hypothèse sur les mécanismes d'action de l'amphotéricine B et de ses dérivés dans les encéphalopathies subaigües spongiformes transmissibles. Medecine/Science. 1997;13:892–896. [Google Scholar]
- 2.Boesze-Battaglia K, Schimmel R. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J Exp Biol. 1997;200:2927–2936. doi: 10.1242/jeb.200.23.2927. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt D R, Rogers M, Stahl N, Telling G, Prusiner S B. Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology. 1993;3:319–329. doi: 10.1093/glycob/3.4.319. [DOI] [PubMed] [Google Scholar]
- 4.Borchelt D R, Taraboulos A, Prusiner S B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992;267:16188–16199. [PubMed] [Google Scholar]
- 5.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 6.Bueler H, Aguzzi A, Sailer A, Greiner R A, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 7.Caspi S, Halimi M, Yanai A, Sasson S B, Taraboulos A, Gabizon R. The anti-prion activity of Congo red. Putative mechanism. J Biol Chem. 1998;273:3484–3489. doi: 10.1074/jbc.273.6.3484. [DOI] [PubMed] [Google Scholar]
- 8.Caughey B, Brown K, Raymond G J, Katzenstein G E, Thresher W. Binding of the protease-sensitive form of prion protein PrP to sulfated glycosaminoglycan and Congo red. J Virol. 1994;68:2135–2141. doi: 10.1128/jvi.68.4.2135-2141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caughey B, Kocisko D A, Raymond G J, Lansbury P T., Jr Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem Biol. 1995;2:807–817. doi: 10.1016/1074-5521(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 10.Caughey B, Race R E. Potent inhibition of scrapie-associated PrP accumulation by congo red. J Neurochem. 1992;59:768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 11.Caughey B, Raymond G J. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol. 1993;67:643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey B, Raymond G J, Ernst D, Race R E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991;65:6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caughey B W, Dong A, Bhat K S, Ernst D, Hayes S F, Caughey W S. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 14.Chang W J, Rothberg K G, Kamen B A, Anderson R G. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charalampous F C. Levels and distributions of phospholipids and cholesterol in the plasma membrane of neuroblastoma cells. Biochim Biophys Acta. 1979;556:38–51. doi: 10.1016/0005-2736(79)90417-6. [DOI] [PubMed] [Google Scholar]
- 16.Chesebro B. BSE and prions: uncertainties about the agent. Science. 1998;279:42–43. doi: 10.1126/science.279.5347.42. [DOI] [PubMed] [Google Scholar]
- 17.Demaimay R, Adjou K, Lasmezas C, Lazarini F, Cherifi K, Seman M, Deslys J P, Dormont D. Pharmacological studies of a new derivative of amphotericin B, MS-8209, in mouse and hamster scrapie. J Gen Virol. 1994;75:2499–2503. doi: 10.1099/0022-1317-75-9-2499. [DOI] [PubMed] [Google Scholar]
- 18.Demaimay R, Adjou K T, Beringue V, Demart S, Lasmézas C I, Deslys J-P, Seman M, Dormont D. Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J Virol. 1997;71:9685–9689. doi: 10.1128/jvi.71.12.9685-9689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demaimay R, Race R, Chesebro B. Effectiveness of polyene antibiotics in treatment of transmissible spongiform encephalopathy in transgenic mice expressing Syrian hamster PrP only in neurons. J Virol. 1999;73:3511–3513. doi: 10.1128/jvi.73.4.3511-3513.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrichson T, Kurzchalia T V. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 21.Gasset M, Baldwin M A, Lloyd D H, Gabriel J M, Holtzman D M, Cohen F, Fletterick R, Prusiner S B. Predicted alpha-helical regions of the prion protein when synthesized as peptides form amyloid. Proc Natl Acad Sci USA. 1992;89:10940–10944. doi: 10.1073/pnas.89.22.10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorodinsky A, Harris D A. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassi J, Frobert Y, Lamourette P, Lagoutte B. Screening of monoclonal antibodies using antigens labeled with acetylcholinesterase: application to the peripheral proteins of photosystem 1. Anal Biochem. 1988;168:436–450. doi: 10.1016/0003-2697(88)90341-7. [DOI] [PubMed] [Google Scholar]
- 24.Hartsel S, Bolard J. Amphotericin B: new life for an old drug. Trends Pharmacol Sci. 1996;17:445–449. doi: 10.1016/s0165-6147(96)01012-7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson W J, Phillips M C, Rothblat G H. Lipoproteins and cellular cholesterol homeostasis. Subcell Biochem. 1997;28:235–276. doi: 10.1007/978-1-4615-5901-6_9. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko K, Vey M, Scott M, Pilkuhn S, Cohen F E, Prusiner S B. COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc Natl Acad Sci USA. 1997;94:2333–2338. doi: 10.1073/pnas.94.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehmann S, Harris D A. Mutant and infectious prion proteins display common biochemical properties in cultured cells. J Biol Chem. 1996;271:1633–1637. doi: 10.1074/jbc.271.3.1633. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann S, Harris D A. A mutant prion protein displays an aberrant membrane association when expressed in cultured cells. J Biol Chem. 1995;270:24589–24597. doi: 10.1074/jbc.270.41.24589. [DOI] [PubMed] [Google Scholar]
- 29.Mangé A, Milhavet O, McMahon H E M, Casanova D, Lehmann S. Effect of amphotericin B on wild-type and mutated prion proteins in cultured cells. J Neurochem. 2000;74:754–762. doi: 10.1046/j.1471-4159.2000.740754.x. [DOI] [PubMed] [Google Scholar]
- 30.Martins V R, Graner E, Garciaabreu J, Desouza S J, Mercadante A F, Veiga S S, Zanata S M, Neto V M, Brentani R R. Complementary hydropathy identifies a cellular prion protein receptor. Nat Med. 1997;3:1376–1382. doi: 10.1038/nm1297-1376. [DOI] [PubMed] [Google Scholar]
- 31.Mayor S, Sabharanjak S, Maxfield F R. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer R K, McKinley M P, Bowman K A, Braunfeld M B, Barry R A, Prusiner S B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci USA. 1986;83:2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milhavet O, Mangé A, Casanova D, Lehmann S. Effect of Congo red on wild-type and mutated prion proteins in cultured cells. J Neurochem. 2000;74:222–230. doi: 10.1046/j.1471-4159.2000.0740222.x. [DOI] [PubMed] [Google Scholar]
- 34.Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem. 1997;272:6324–6331. doi: 10.1074/jbc.272.10.6324. [DOI] [PubMed] [Google Scholar]
- 35.Nishida N, Harris D A, Vilette D, Laude H, Frobert Y, Grassi J, Casanova D, Milhavet O, Lehmann S. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J Virol. 2000;74:320–325. doi: 10.1128/jvi.74.1.320-325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan K M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parchi P, Gambetti P. Human prion diseases. Curr Opin Neurol. 1995;8:286–293. doi: 10.1097/00019052-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Pocchiari M. Prions and related neurological diseases. Mol Asp Med. 1994;15:195–291. doi: 10.1016/0098-2997(94)90042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pocchiari M, Schmittinger S, Masullo C. Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J Gen Virol. 1987;68:219–223. doi: 10.1099/0022-1317-68-1-219. [DOI] [PubMed] [Google Scholar]
- 40.Prusiner S B, Scott M R, Dearmond S J, Cohen F E. Prion protein biology. Cell. 1998;93:337–348. doi: 10.1016/s0092-8674(00)81163-0. [DOI] [PubMed] [Google Scholar]
- 41.Rieger R, Edenhofer F, Lasmezas C I, Weiss S. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med. 1997;3:1383–1388. doi: 10.1038/nm1297-1383. [DOI] [PubMed] [Google Scholar]
- 42.Schätzl H M, Laszlo L, Holtzman D M, Tatzelt J, DeArmond S J, Weiner R I, Mobley W C, Prusiner S B. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol. 1997;71:8821–8831. doi: 10.1128/jvi.71.11.8821-8831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnitzer J E, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shyng S L, Lehmann S, Moulder K L, Harris D A. Sulfated glycans stimulate endocytosis of the cellular isoform of the prion protein, PrPC, in cultured cells. J Biol Chem. 1995;270:30221–30229. doi: 10.1074/jbc.270.50.30221. [DOI] [PubMed] [Google Scholar]
- 45.Stulnig T M, Berger M, Sigmund T, Raederstorff D, Stockinger H, Waldhausl W. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supattapone S, Nguyen H O, Cohen F E, Prusiner S B, Scott M R. Elimination of prions by branched polyamines and implications for therapeutics. Proc Natl Acad Sci USA. 1999;96:14529–14534. doi: 10.1073/pnas.96.25.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taraboulos A, Raeber A J, Borchelt D R, Serban D, Prusiner S B. Synthesis and trafficking of prion proteins in cultured cells. Mol Biol Cell. 1992;3:851–863. doi: 10.1091/mbc.3.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taraboulos A, Rogers M, Borchelt D R, McKinley M P, Scott M, Serban D, Prusiner S B. Acquisition of protease resistance by prion proteins in scrapie-infected cells does not require asparagine-linked glycosylation. Proc Natl Acad Sci USA. 1990;87:8262–8266. doi: 10.1073/pnas.87.21.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taraboulos A, Scott M, Semenov A, Avrahami D, Laszlo L, Prusiner S B. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J Cell Biol. 1995;129:121–132. doi: 10.1083/jcb.129.1.121. . (Erratum, 130:501.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taraboulos A, Scott M, Semenov A, Avrahami D, Prusiner S B. Biosynthesis of the prion proteins in scrapie-infected cells in culture. Braz J Med Biol Res. 1994;27:303–307. [PubMed] [Google Scholar]
- 51.Telling G C, Scott M, Mastriani J, Gabizon R, Torchia M, Cohen F E, DeArmond S J, Prusiner S B. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell. 1995;83:79–90. doi: 10.1016/0092-8674(95)90236-8. [DOI] [PubMed] [Google Scholar]
- 52.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 53.Vertut-Doï A, Ohnishi S-I, Bolard J. The endocytic process in CHO cells, a toxic pathway of the polyene antibiotic amphotericin B. Antimicrob Agents Chemother. 1994;38:2373–2379. doi: 10.1128/aac.38.10.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vey M, Pilkuhn S, Wille H, Nixon R, DeArmond S J, Smart E J, Anderson R G, Taraboulos A, Prusiner S B. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc Natl Acad Sci USA. 1996;93:14945–14949. doi: 10.1073/pnas.93.25.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xi Y G, Ingrosso L, Ladogana A, Masullo C, Pocchiari M. Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature. 1992;356:598–601. doi: 10.1038/356598a0. [DOI] [PubMed] [Google Scholar]