Abstract
Fibroblasts are typically described as cells that produce extracellular matrix, contribute to the formation of connective tissue, and maintain the structural framework of tissues. Fibroblasts are the first cell type to be transdifferentiated into inducible pluripotent stem cells (iPSCs), demonstrating their versatility and reprogrammability. Currently, there is relatively extensive characterization of the anatomical, molecular, and functional diversity of fibroblasts in different peripheral organs and tissues. With recent advances in single cell RNA sequencing, heterogeneity and diversity of fibroblasts in the central nervous system (CNS) have also begun to emerge. Based on their distinct anatomical locations in the meninges, perivascular space, and choroid plexus, as well as their molecular diversity, important roles for fibroblasts in the CNS have been proposed. Here, we draw inspirations from what is known about fibroblasts in peripheral tissues, in combination with their currently identified CNS locations and molecular characterizations, to propose potential functions of CNS fibroblasts in health and disease. Future studies, using a combination of technologies, will be needed to determine the bona fide in vivo functions of fibroblasts in the CNS.
Keywords: Fibroblasts, Central nervous system, Infection, Aging, Neurodegenerative diseases, Injury
1. Introduction
Fibroblasts were first described as a distinct cell type in 1858 by the German pathologist Rudolf Virchow. The term “fibroblast” was first proposed by Ernst Ziegler and echoed by Santiago Ramon y Cajal, to describe cells that produced new connective tissue upon healing. Fibroblasts were first defined by their spindle-shaped morphology and ability to adhere to plastic. They provide structural frameworks to different tissues by producing diverse extracellular matrix (ECM) components, and provide positional information for neighboring cells by secreting chemokines, cytokines, small molecules, and growth factors [1,2]. They can also serve as progenitors for many cell types [1].
As one of the most widely distributed cell types, fibroblasts from different tissues/organs in the periphery have been extensively studied in the past 160 years. They showed distinct transcriptional profiles, chromatin accessibility, and epigenome profiles [3,4]. The heterogeneity of fibroblasts within and across organs has been characterized using single cell RNA sequencing (scRNA-seq) [2,[5], [6], [7]. Despite extensive studies, a pan fibroblast marker is still lacking [1,2]. In contrast to extensive research in peripheral tissues and organs, characterization of the heterogeneity/diversity of fibroblasts in the central nervous system (CNS), as well as their potential functions, only began recently [8,9].
2. Characteristics of CNS fibroblasts
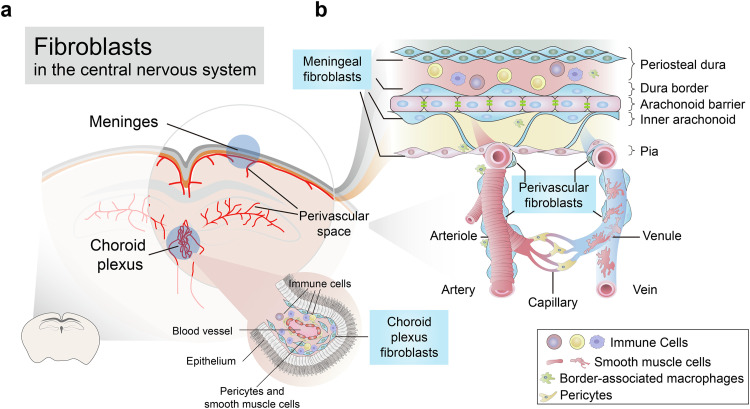
The CNS is highly protected by barrier systems. In the CNS, fibroblasts are mostly distributed in the meninges, perivascular Virchow-Robin space, and choroid plexus [8] (Fig. 1a). Due to the lack of specific molecular markers, fibroblasts are sometimes confounded with pericytes, smooth muscle cells (SMCs), or other mesenchymal cells, especially in the perivascular space. Fibroblasts, pericytes, and SMCs all express PDGFRβ; fibroblasts are in addition PDGFRα positive, a marker that they share with oligodendrocyte precursor cells. Fibroblasts produce collagen, and transgenetic mice (Col1a2CreERT and Col1a1GFP) have been used to label fibroblasts. Pericytes and SMCs express NG2, while SMCs express Acta2 (αSMA). Myofibroblasts differentiating from activated fibroblasts or other mesenchymal cells during pathological conditions share the marker Acta2 with SMCs. In zebrafish, a subset of perivascular fibroblasts has been shown to differentiate into pericytes [10]. However, another study in mice found that Col1a1+ perivascular fibroblasts appeared postnatally, in contrast to pericytes, which appeared during prenatal neurovascular development, suggesting that they have different lineages [11]. In the perivascular Virchow-Robin space, combining positional and morphological information can help to distinguish fibroblasts from pericytes and SMCs. Pericytes wrap around capillaries; along penetrating arterioles, SMCs display ring shapes surrounding blood vessels, while perivascular fibroblasts, which localize to cortical penetrating arterioles and the larger ascending venules, have flattened cell body and thin ruffled processes [8,12] (Fig. 1b). Strong similarities between fibroblasts and mesenchymal stem/stromal cells (MSCs) have also been suggested, including marker expression and differentiation capacities [13]. However, the definition of MSCs is variable between laboratories. Furthermore, most experiments demonstrating their differentiation capabilities have been carried out in vitro, under different culturing conditions. The presence of MSCs in vivo has yet to be demonstrated conclusively [2].
Fig. 1.
The distribution of fibroblasts in the CNS. (a) Fibroblasts are localized in the meninges, choroid plexus, and perivascular spaces. (b) Schematic of cellular composition, zonation, and distribution of cell types in the meninges and perivascular space.
3. CNS fibroblast diversity
Recent scRNA-seq studies have analyzed the transcriptome of CNS fibroblasts in mice and humans [14], [15], [16], [17], [18], [19]. Fibroblasts from three different layers of meninges (dura, arachnoid, and pia) showed distinct gene expression profiles [20]. Differences between perivascular and meningeal fibroblasts have also been described, based on transcriptome analysis. ECM genes are enriched in perivascular fibroblasts, while solute transporter genes are enriched in meningeal fibroblasts, suggesting a potential role of these cells in regulating solute exchange in and out of the brain [17]. The lineage relationship between meningeal and perivascular fibroblasts is currently unknown. Choroid plexus fibroblasts from different ventricles display different gene expression profiles; whether these different expression patterns reflect different functions is currently unknown [21].
4. The function of CNS fibroblasts in health
4.1. Meningeal fibroblasts
The importance of meningeal fibroblasts to CNS development was first uncovered by the Foxc1 mutant. The lack of functional meningeal cells in this mutant led to severe CNS developmental deficits [22]. Contact of meningeal cells and astrocytes have been shown to promote the formation of glia limitans between the meninges and the brain parenchyma [23]. Meningeal fibroblasts are the major source of basement membrane. Radial glia sends out processes and attach to the pial basement membrane; these processes play a structural role in neuronal migration. Meningeal fibroblasts are also the main producers of retinoic acid, which can regulate cortical neuronal migration and blood vessel development [20]. Group 2 innate lymphoid cells (ILC2s) have been found to colocalize with meningeal fibroblasts which express high-level IL33, and likely work together to regulate type 2 immunity, similar to in the lung [24]. Recent studies have also shown that the meninges can serve as a niche for B cell maturation, and that meningeal fibroblasts provide key trophic factors for B cell development [25], [26], [27].
4.2. Perivascular and choroid plexus fibroblasts
The function of perivascular and choroid plexus fibroblasts in CNS development is largely unknown. In the spleen and other niches, stromal cells provide structural anchor and survival factors to macrophages. Could CNS fibroblasts have similar roles? Dural and perivascular macrophages have been proposed to directly interact with fibroblasts in the dural and perivascular space [28]. Notably, perivascular fibroblasts express IL34 and CSF1, ligands of CSF1R, which are essential for the growth and differentiation of macrophages. Both fibroblasts and astrocytes express Connexin 30 (Cx30/Gjb6). Whether functional gap junctions form between these two cell types is unknown. If these connections exit, they may provide a highway for signal transduction from the vasculature to the brain parenchyma. Single cell transcriptome of the mouse brain choroid plexus suggests that fibroblasts in the fourth ventricle may be involved in hindbrain development, by expressing high levels of Hhip, Ptch1, Rbp4, and Wisp1 [21].
5. The function of CNS fibroblasts in diseases
5.1. Acute systemic infection
The location of CNS fibroblasts at the interface between the external milieu and brain parenchyma suggests that they may function as gatekeepers to protect the CNS from external challenges [19,29]. In previous work, we found that within 2 h of systemic infection, CNS fibroblasts (Col1a1 expressing cells) are rapidly activated and express many cytokines at high levels. Some of these cytokines have been shown to affect synaptic transmission and microglia activation, while the function of others is unknown.
In various immune responses in the periphery, fibroblastic reticular cells (FRC) in secondary lymphoid organs become activated, undergo remodeling, and attract lymphocytes to secondary lymphoid organs, to mount immune responses. Similarly, following acute infection in the brain, meningeal and perivascular fibroblasts become activated and undergo remodeling. These activated fibroblasts express CCL19 and CCL21, which are critical for recruiting CD8+ T cells to the CNS and for subsequent pathogen clearance [29,30]. While CNS fibroblasts are clearly activated following acute systemic infection, many questions remain unanswered, including: 1) How do CNS fibroblasts sense pathogens? 2) To what extent do CNS fibroblasts contribute to glial activation following acute peripheral infection? 3) How do CNS fibroblasts interact with endothelial cells in vivo to regulate CNS entrance of lymphocytes? In answering these questions, it is important to understand cellular communication among endothelial cells, perivascular fibroblasts, perivascular macrophages, astrocytes, and microglia, especially during the acute phase of systemic infection.
5.2. Chronic inflammation
Over the course of chronic inflammation, tertiary lymphoid organs (TLOs) are formed by lymphoid neogenesis. B cells and T cells are recruited to these sites by follicular dendritic cells (FDC)-like CXCL13 producing fibroblasts and T-zone reticular cells (TRC)-like CCL19, CCL21 producing fibroblasts. TLOs are also found in the meninges, but not the perivascular space, of multiple sclerosis patients. In the experimental autoimmune encephalomyelitis (EAE) model, a mouse model of multiple sclerosis, IL-17 and IL-22 secreted by T helper 17 (Th17) cells are necessary for fibroblast remodeling. Lymphotoxin signaling in fibroblasts is important for lymph node organogenesis and is required for TLO maturation. Blocking lymphotoxin signaling ablated meningeal B cell accumulation and attenuated TLO maturation [29]. The location preference of TLOs suggests that perivascular fibroblasts may have different immunostimulatory potentials from meningeal fibroblasts.
5.3. Aging and neurodegenerative diseases
During aging, lymphocytes such as age-associated B cells, plasma cells, and T cells have been found to accumulate in the CNS [18,25,31]. The exact mechanism driving this accumulation is unclear, although fibroblasts likely play an important role. Reducing CXCL12 level in stromal cells using PdgfrβCreERT2 decreased the number of dural CD4 T cells [18]. CXCL12 also contributes to the migration of B cells, plasma cells, and other cells. Our preliminary results suggest that the expression of CXCL12 is elevated in the CNS of aged mice.
It is important to examine whether other chemokines, including CCL19, CCL21, and CXCL13, with demonstrated roles in T cell and B cell migration in the periphery, also contribute to aging-induced lymphocyte inflation in the CNS. These chemokines are mostly expressed by fibroblasts. In the spleen, the expression of CCL19, CCL21, and CXCL13 are age-dependent [32]; whether their expression in the brain is also age-dependent is unknown.
Fibroblasts are also involved in neurodegenerative diseases. In the pre-symptomatic stages of ALS, increased activity of perivascular fibroblasts in the enlarged perivascular spaces is observed. Increased levels of the perivascular fibroblast marker SPP1 in the plasma predicted shorter survival time in ALS patients [33]. These results suggest that perivascular fibroblasts may contribute to disease progression. In the brain of Alzheimer's disease (AD) patients, there is extensive loss of fibroblasts [17]. Retinoid acid synthesis enzymes are strongly down-regulated in the fibroblasts of AD patients. In Lewy body dementia, high levels of CXCL12 in the cerebrospinal fluid, typically released by the fibroblasts and other stromal cells, is associated with entry of CD4+ T cells into the brain parenchyma, and neuronal damage [34].
5.4. CNS injury and regeneration
A major issue following CNS injury is the formation of scar tissue, which interferes with axon regeneration. Scar tissues include inner fibrotic components and outer glial components. Glast+ type A pericytes have been suggested to form fibrotic scar following spinal cord injury (SCI) [35]. Reducing the fibrotic scar through inhibition of Glast+ type A pericyte proliferation facilitated axon regeneration [36]. Another study found that fibrotic scar is generated by Col1a1+NG2− fibroblasts [37]. As fibroblasts and pericytes both express Glast, these studies may refer to the same population by different names. Fibrotic scar formation also occurs in the EAE model, with scars mostly containing Col1a1+ fibroblasts [8], and also following traumatic brain injury (TBI). Meningeal fibroblasts also contribute to astroglial scar formation through direct interaction with astrocytes [38,39]. Remodeling of collagen fibers in the ECM can recruit macrophage through mechanical cue [40]. Macrophages and microglia are the major sources of profibrotic cytokine transforming growth factor-β1 (TGF-β1), which promotes myofibroblasts differentiation. Dissection of the intercellular communication network between fibroblasts and microglia/macrophages may provide new insights into SCI treatment.
Several studies suggested that cell transplantation may be a feasible method for repairing SCI and/or TBI. As fibroblasts are highly plastic, similar to mesenchymal stem cells, would it be possible to reprogram scar-forming fibroblasts into neurons, oligodendrocytes, and/or other cell types, to reduce scaring and repair the injury at the same time? An analogous strategy has been successfully used in skin, liver, and heart repair [41], [42], [43], [44]. Over-expression of Ascl1, Brn2, and Myth1l have transformed fibroblasts into functional neurons [45]. Although previous studies were carried out using cultured cells, it may be possible to transform scar-forming fibroblasts into neurons in vivo following CNS injury, via overexpression of specific sets of transcription factors.
6. Conclusion and future directions
Taking advantage of scRNA-seq, remarkable progress in understanding CNS fibroblasts has been made in the past several years. Different fibroblast subsets and states have been found in different regions of the CNS in health and disease states (Box 1). The unexpected diversity of fibroblasts in the CNS supports the notion that fibroblasts are not only structural cells, but play active roles in CNS development, neuroinflammation, aging, neurodegenerative diseases, and injury.
Box 1.
The functions of CNS fibroblasts in health and disease.
| Health | Disease |
|---|---|
| • Structural support/ECM • Molecular cues for cell (immune cells and neurons) migration and positioning • Regulation of neurogenesis • Providing trophic factors |
• Fibrotic scar/fibrosis in spinal cord injury, traumatic brain injury, experimental autoimmune encephalomyelitis, and stroke • Neuroinflammation • Tertiary lymphoid organ formation |
Many important opportunities/questions are outstanding (Box 2). Could systematic comparison of CNS fibroblasts and those from other tissues help to identify the unique features of CNS fibroblasts? Within the CNS, would single cell level spatial RNA-seq identify region-specific fibroblasts, and could lineage tracing clearly dissect the origin of different subsets of CNS fibroblasts? Can scar-forming fibroblasts be transformed into functional cells to repair CNS injury? Combining GWAS results with the transcriptome of CNS fibroblasts, several human neurological disease-related genes are found to be enriched in CNS fibroblasts. Could dysregulation of gene expression in CNS fibroblasts induce diseases? If so, would targeting fibroblasts provide new strategies for treating neurological diseases?
Box 2.
Open questions and future opportunities in CNS fibroblast research.
|
Development • How are CNS fibroblasts different from fibroblasts in other tissues? • Do CNS fibroblast subtypes have a single common lineage? • What are the key regulators of CNS fibroblast differentiation? • Are there specific markers of CNS fibroblasts? Physiological function • How do fibroblasts transduce information between the external milieu and the brain parenchyma? • Do different CNS fibroblasts subtypes have different functions? • How is the “fibroblast-immune cell niche” organized in the meninges, perivascular space, and choroid plexus? • Do fibroblasts contribute to CSF waste clearance? • Through what signaling mechanisms do fibroblasts communicate with astrocytes, microglia, and neurons following CNS injury or infection? • Can scar-forming fibroblasts be transformed into functional cells to repair CNS injury? • Do fibroblasts play a role in glioblastoma? • How does fibroblast signaling affect the progression of neurodegenerative diseases? |
Interactions between fibroblast and immune cell interactions in health and disease are likely critical for maintaining CNS homeostasis. By raising more questions than providing answers, we hope to draw attention to the emerging role of CNS fibroblasts in health and disease, and encourage investigations into their in vivo physiological functions.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
We thank members of the Duan and Yu laboratories for their comments and suggestions. Work in the Yu Lab is supported by grants from the National Natural Science Foundation of China (32030049) and the Ministry of Science and Technology of China (2021ZD0202500).
Biographies

Lihui Duan(BRID: 01361.00.85109) is a principal investigator at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences. He received his Ph.D. in neuroscience in 2018 from the Institute of Neuroscience, Chinese Academy of Sciences. He pursued post-doctoral research in immunology at the University of California San Francisco during 2018–2022. His research interests focus on the development and function of fibroblasts in the central nervous system.

Xiang Yu(BRID: 09363.00.05797) is a professor in the School of Life Sciences at Peking University, and investigator in the Peking-Tsinghua Center for Life Sciences and Peking University McGovern Institute. After receiving her Bachelor's degree from Trinity College, University of Cambridge, she completed her Ph.D. at the MRC Laboratory of Molecular Biology in Cambridge and her post-doctoral work at Stanford University. During 2005–2019 she was a principle investigator at the Institute of Neuroscience, Chinese Academy of Sciences. Her laboratory is interested in identifying key molecules, cell types and circuits regulating neural circuit development and plasticity, especially in the context of developmental neurological disorders.
Contributor Information
Lihui Duan, Email: lihui.duan@genetics.ac.cn.
Xiang Yu, Email: yuxiang01@pku.edu.cn.
References
- 1.Plikus M.V., Wang X., Sinha S., et al. Fibroblasts: Origins, definitions, and functions in health and disease. Cell. 2021;184(15):3852–3872. doi: 10.1016/j.cell.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lendahl U., Muhl L., Betsholtz C. Identification, discrimination and heterogeneity of fibroblasts. Nat. Commun. 2022;13(1):3409. doi: 10.1038/s41467-022-30633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang H.Y., Chi J.T., Dudoit S., et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 2002;99(20):12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krausgruber T., Fortelny N., Fife-Gernedl V., et al. Structural cells are key regulators of organ-specific immune responses. Nature. 2020;583(7815):296–302. doi: 10.1038/s41586-020-2424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buechler M.B., Pradhan R.N., Krishnamurty A.T., et al. Cross-tissue organization of the fibroblast lineage. Nature. 2021;593(7860):575–579. doi: 10.1038/s41586-021-03549-5. [DOI] [PubMed] [Google Scholar]
- 6.Korsunsky I., Wei K., Pohin M., et al. Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases. Med. 2022;3(7):481–518. doi: 10.1016/j.medj.2022.05.002. e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhl L., Genove G., Leptidis S., et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020;11(1):3953. doi: 10.1038/s41467-020-17740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorrier C.E., Jones H.E., Pintaric L., et al. Emerging roles for CNS fibroblasts in health, injury and disease. Nat. Rev. Neurosci. 2021;23(1):23–24. doi: 10.1038/s41583-021-00525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derk J., Jones H.E., Como C., et al. Living on the edge of the CNS: Meninges cell diversity in health and disease. Front. Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.703944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajan A.M., Ma R.C., Kocha K.M., et al. Dual function of perivascular fibroblasts in vascular stabilization in zebrafish. PLoS Genet. 2020;16(10) doi: 10.1371/journal.pgen.1008800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly K.K., MacPherson A.M., Grewal H., et al. Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neurosci. 2016;17(1):49. doi: 10.1186/s12868-016-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonney S.K., Sullivan L.T., Cherry T.J., et al. Distinct features of brain perivascular fibroblasts and mural cells revealed by in vivo two-photon imaging. J. Cereb. Blood Flow Metab. 2021;42(6):966–978. doi: 10.1177/0271678X211068528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soundararajan M., Kannan S. Fibroblasts and mesenchymal stem cells: Two sides of the same coin? J. Cell. Physiol. 2018;233(12):9099–9109. doi: 10.1002/jcp.26860. [DOI] [PubMed] [Google Scholar]
- 14.Vanlandewijck M., He L., Mae M.A., et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 15.Winkler E.A., Kim C.N., Ross J.M., et al. A single-cell atlas of the normal and malformed human brain vasculature. Science. 2022:eabi7377. doi: 10.1126/science.abi7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia F.J., Sun N., Lee H., et al. Single-cell dissection of the human brain vasculature. Nature. 2022;603(7903):893–899. doi: 10.1038/s41586-022-04521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang A.C., Vest R.T., Kern F., et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature. 2022;603(7903):885–892. doi: 10.1038/s41586-021-04369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rustenhoven J., Drieu A., Mamuladze T., et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–1016. doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan L., Zhang X.D., Miao W.Y., et al. PDGFRbeta cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron. 2018;100(1):183–200. doi: 10.1016/j.neuron.2018.08.030. e188. [DOI] [PubMed] [Google Scholar]
- 20.DeSisto J., O'Rourke R., Jones H.E., et al. Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Dev. Cell. 2020;54(1):43–59. doi: 10.1016/j.devcel.2020.06.009. e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dani N., Herbst R.H., McCabe C., et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–3074. doi: 10.1016/j.cell.2021.04.003. e3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegenthaler J.A., Ashique A.M., Zarbalis K., et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139(3):597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abnet K., Fawcett J.W., Dunnett S.B. Interactions between meningeal cells and astrocytes in vivo and in vitro. Dev. Brain Res. 1991;59(2):187–196. doi: 10.1016/0165-3806(91)90099-5. [DOI] [PubMed] [Google Scholar]
- 24.Dahlgren M.W., Jones S.W., Cautivo K.M., et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity. 2019;50(3):707–722. doi: 10.1016/j.immuni.2019.02.002. e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brioschi S., Wang W.L., Peng V., et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373(6553):eabf9277. doi: 10.1126/science.abf9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafflick D., Wolbert J., Heming M., et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci. 2021;24(9):1225–1234. doi: 10.1038/s41593-021-00880-y. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Chen D., Xu D., et al. Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity. 2021;54(12):2784–2794. doi: 10.1016/j.immuni.2021.09.016. e2786. [DOI] [PubMed] [Google Scholar]
- 28.Sato T., Konishi H., Tamada H., et al. Morphology, localization, and postnatal development of dural macrophages. Cell Tissue Res. 2021;384(1):49–58. doi: 10.1007/s00441-020-03346-y. [DOI] [PubMed] [Google Scholar]
- 29.Pikor N.B., Cupovic J., Onder L., et al. Stromal cell niches in the inflamed central nervous system. J. Immunol. 2017;198(5):1775–1781. doi: 10.4049/jimmunol.1601566. [DOI] [PubMed] [Google Scholar]
- 30.Ramaglia V., Florescu A., Zuo M., et al. Stromal cell-mediated coordination of immune cell recruitment, retention, and function in brain-adjacent regions. J. Immunol. 2021;206(2):282–291. doi: 10.4049/jimmunol.2000833. [DOI] [PubMed] [Google Scholar]
- 31.Fung I.T.H., Sankar P., Zhang Y., et al. Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline. J. Exp. Med. 2020;217(4):282–291. doi: 10.1084/jem.20190915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefebvre J.S., Maue A.C., Eaton S.M., et al. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell. 2012;11(5):732–740. doi: 10.1111/j.1474-9726.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manberg A., Skene N., Sanders F., et al. Altered perivascular fibroblast activity precedes ALS disease onset. Nat. Med. 2021;27(4):640–646. doi: 10.1038/s41591-021-01295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gate D., Tapp E., Leventhal O., et al. CD4(+) T cells contribute to neurodegeneration in Lewy body dementia. Science. 2021;374(6569):868–874. doi: 10.1126/science.abf7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goritz C., Dias D.O., Tomilin N., et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333(6039):238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 36.Dias D.O., Kim H., Holl D., et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173(1):153–165. doi: 10.1016/j.cell.2018.02.004. e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soderblom C., Luo X., Blumenthal E., et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 2013;33(34):13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanner I.B., Anderson M.A., Song B., et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 2013;33(31):12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Klett F., Priller J. The fibrotic scar in neurological disorders. Brain Pathol. 2014;24(4):404–413. doi: 10.1111/bpa.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pakshir P., Alizadehgiashi M., Wong B., et al. Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat. Commun. 2019;10(1):1850. doi: 10.1038/s41467-019-09709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto K., Akiyama M., Tamura F., et al. Direct in vivo reprogramming with sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell. 2018;22(1):91–103. doi: 10.1016/j.stem.2017.11.010. e105. [DOI] [PubMed] [Google Scholar]
- 42.Rezvani M., Espanol-Suner R., Malato Y., et al. In vivo hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell. 2016;18(6):809–816. doi: 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song G., Pacher M., Balakrishnan A., et al. Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell. 2016;18(6):797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Kurita M., Araoka T., Hishida T., et al. In vivo reprogramming of wound-resident cells generates skin epithelial tissue. Nature. 2018;561(7722):243–247. doi: 10.1038/s41586-018-0477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vierbuchen T., Ostermeier A., Pang Z.P., et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]