Highlights
-
•
Bone marrow-sparing (BMS) reduces the rates of concurrent chemotherapy interruptions.
-
•
Patients treated with BMS experience lower rates of high-grade hematologic toxicity.
-
•
Assessing the effect of BMS on survival and disease control is currently not possible.
Keywords: Chemoradiotherapy, Cisplatin, Haematologic toxicity, Bone marrow, Pelvic bones
Abstract
Background
Concurrent chemoradiotherapy (CRT) is the standard treatment for locally advanced cervical cancer. We investigated how additional bone marrow sparing (BMS) affects the clinical outcomes.
Methods
We queried MEDLINE, Embase, Web of Science Core Collection, Google Scholar, Sinomed, CNKI, and Wanfang databases for articles published in English or Chinese between 2010/01/01 and 2023/10/31. Full-text manuscripts of prospective, randomised trials on BMS in cervical cancer patients treated with definitive or postoperative CRT were included. Risk of bias (RoB) was assessed using Cochrane Collaboration’s RoB tool. Random-effects models were used for the meta-analysis.
Results
A total of 17 trials encompassing 1297 patients were included. The majority were single-centre trials (n = 1268) performed in China (n = 1128). Most trials used CT-based anatomical BMS (n = 1076). There was a comparable representation of trials in the definitive (n = 655) and postoperative (n = 582) settings, and the remaining trials included both.
Twelve studies reported data on G ≥ 3 (n = 782) and G ≥ 2 (n = 754) haematologic adverse events. Both G ≥ 3 (OR 0.39; 95 % CI 0.28–0.55; p < 0.001) and G ≥ 2 (OR 0.29; 95 % CI 0.18–0.46; p < 0.001) toxicity were significantly lowered, favouring BMS. Seven studies (n = 635) reported data on chemotherapy interruptions, defined as receiving less than five cycles of cisplatin, which were significantly less frequent in patients treated with BMS (OR 0.44; 95 % CI 0.24–0.81; p = 0.016). There was no evidence of increased gastrointestinal or genitourinary toxicity.
There were no signs of significant heterogeneity. Four studies were assessed as high RoB; sensitivity analyses excluding these provided comparable results for main outcomes. The main limitations include heterogeneity in BMS methodology between studies, low representation of populations most affected by cervical cancer, and insufficient data to assess survival outcomes.
Conclusions
The addition of BMS to definitive CRT in cervical cancer patients decreases hematologic toxicity and the frequency of interruptions in concurrent chemotherapy. However, data are insufficient to verify the impact on survival and disease control.
Introduction
Cervical cancer is the fourth most commonly diagnosed cancer and the fourth leading cause of cancer-related death in women worldwide [1]. Despite the fact that this human papillomavirus-related disease is considered nearly completely preventable, and a collective effort is made to reduce its incidence [2], millions of patients are expected to develop cervical cancer requiring treatment [3]. For more than 20 years, cisplatin-based chemoradiotherapy (CRT) combined with intracavitary brachytherapy has been the standard of care for patients with advanced disease [4]. While cisplatin remains unchallenged, modern-day radiotherapy (RT) is significantly different from the anatomically defined four-field box technique used in the abovementioned trial.
The image-guided RT (IGRT) and intensity-modulated RT (IMRT) were two steps towards better, individually tailored treatments. Trials such as TIME-C and PARCER uncovered significantly reduced gastrointestinal (GI) and genitourinary (GU) toxicity in patients treated with IMRT compared with 3D conformal RT (3D-CRT) [5], [6]. However, they did not investigate the possibility of bone-marrow sparing (BMS) facilitated by the routine use of IGRT and dose painting with IMRT, or more recent volumetric modulated arc therapy (VMAT). Dynamic RT planning techniques lead to decreased volumes of healthy organs exposed to high-dose radiation due to higher dose conformity at the expense of increased volumes of surrounding tissues receiving low-dose radiation [7]. Considering that approximately half of the haematopoietically active bone marrow (BM) in adults is located in the pelvic and lumbar regions [8], and both components of CRT are directly associated with haematologic toxicity (HT) [9], a reduction of the RT contribution to BM damage could improve the quality of care for patients.
In general, BMS can be categorised into anatomical or active BMS. The former relies on widely available computed tomography (CT) scans to identify the external contours of pelvic bones or the low-density areas within. The latter identifies the active BM through functional imaging, often available as an inherent part of RT planning for cervical carcinoma (e.g., positron emission tomography [PET]-CT). Previous reviews have described the rationale, dosimetric benefits, and initial clinical outcomes of pelvic BMS CRT [10], [11]. However, despite the considerable amount of data published in recent years, the early closure of the largest randomised clinical trial (RCT) makes it unlikely that an RCT powered to test for differences in clinically relevant outcomes will be completed [12]. Cervical cancer is mostly prevalent in low-income countries, often dealing with limited resources [13]. In case of no meaningful clinical benefit, BMS could have a relevant adverse impact on the cost-effectiveness and accessibility of treatment. To address this knowledge gap, we performed a systematic review and meta-analysis of RCTs to evaluate the association between the use of BMS in CRT for cervical cancer and relevant clinical outcomes.
Methods (evidence acquisition)
Search strategy
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, including the PRISMA 2020 checklist [14] (Supplementary File 1) and the PRISMA 2020 for Abstracts checklist (Supplementary File 2). The PICOS framework was used to formulate the research question (Supplementary File 3). The protocol was registered in the International Prospective Register of Systematic Reviews database (CRD42023437990) prior to the initiation of the review. We conducted searches in MEDLINE (via PubMed), Embase (via Ovid), Web of Science Core Collection (via Web of Science), and the Google Scholar platform (top 200 hits). The search was limited to records published between 2010-01-01 and 2023-06-30, as a previously published systematic review yielded no relevant literature published prior to that date [11]. No language restrictions were imposed. Due to the identification of relevant evidence published in Chinese through backward citation searching, three Chinese-speaking researchers were invited to join the project. Consequently, the search was expanded to include articles from the Sinomed, CNKI, and Wanfang databases. The Chinese-language search included records published between 2010-01-01 and 2023-10-31, and the MEDLINE, Embase, and Web of Science search strategies were subsequently updated to include the period between 2023-07-01 and 2023-10-31 to ensure consistency between search strategies prior to initiating data synthesis.
The main search strategy was developed for PubMed (MEDLINE), including Medical Subject Headings (MESH) “Uterine Cervical Neoplasms”, ‘‘Bone Marrow”, and ‘‘Radiotherapy”, and free-text terms searched in title and abstract, combined with a filter for intervention studies [15], and then adapted for Embase, Web of Science, and Google Scholar. The search strategies for Sinomed, CNKI, and Wanfang were performed using keywords. The strategies were peer-reviewed by the co-authors prior to execution (Supplementary File 4).
Study selection
In brief, we included studies describing patients receiving definitive or postoperative CRT for cervical cancer, with BMS RT performed either through anatomical or active BM protection, compared to standard-of-care non-BMS CRT, reporting pre-defined clinical outcomes based on results of prospective, randomised trials (PICOS; Supplementary File 3). References were imported, combined, and de-duplicated using Rayyan software [16]. Two authors performed independent abstract screening, followed by an independent full-text search by two authors. Additional secondary searches of cited references were performed manually. Any conflicts were resolved through mediation with a third author.
Data extraction
Two authors independently extracted the data and, in cases where it was necessary, contacted the corresponding authors for additional information. Clinical characteristics of the patients and outcome data were systematically extracted, including proportions of patients experiencing specific toxicity or chemotherapy interruption. Hazard ratios (HRs) with corresponding standard errors (SEs) were retrieved for time-to-event outcomes. In cases where the data were presented only on figures, WebPlotDigitizer v. 4.6 software was used to digitise the Kaplan-Meier survival curves [17]. In applicable cases, IPDfromKM software was used to reconstruct individual patient data and subsequently calculate the corresponding HRs and SEs [18].
Risk-of-bias assessment
The risk of bias (RoB) was assessed independently by two authors using the RoB 2.0 tool [19] for English-language studies, and the RoB 1.0 tool for Chinese-language studies [20]. Conflicts were resolved through mediation with a third author.
Statistical analysis
Meta-analysis was performed using the inverse variance method and a random-effect model with Hartung-Knapp adjustment. A continuity correction of 0.5 was added in studies with zero cell frequencies. Meta-analytic averages for toxicity and chemotherapy interruption outcomes were presented as odds ratios (ORs), and for each synthesis, included all studies which provided data on the outcome of interest. Forest plots were used to present ORs with 95 % confidence intervals (95 % CI) for individual studies and meta-analytic averages. Heterogeneity among studies was assessed using τ2 (with Mantel-Haenszel estimator), Higgins&Thompson’s I2 (values > 50 % considered as significant), and Cochran’s Q test. Publication bias was assessed using funnel plots, and in cases where 10 or more studies were included in an analysis, Peters' linear regression test was used to assess plot asymmetry. Sensitivity analyses were performed to assess the impact of the toxicity grading system used to assess the G ≥ 3 haematologic toxicity (excluding non-RTOG studies), and high RoB studies on the G ≥ 3 haematologic toxicity and chemotherapy interruption (excluding studies with high RoB). Meta-regression was performed to assess the association between study results and sample size, and bubble plots were generated to visualise the results. Statistical analyses were performed using R software v4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and the General Package for Meta-Analysis (meta) version 6.5.0. P-values < 0.05 were considered significant. All tests were two-sided.
Results
Evidence synthesis
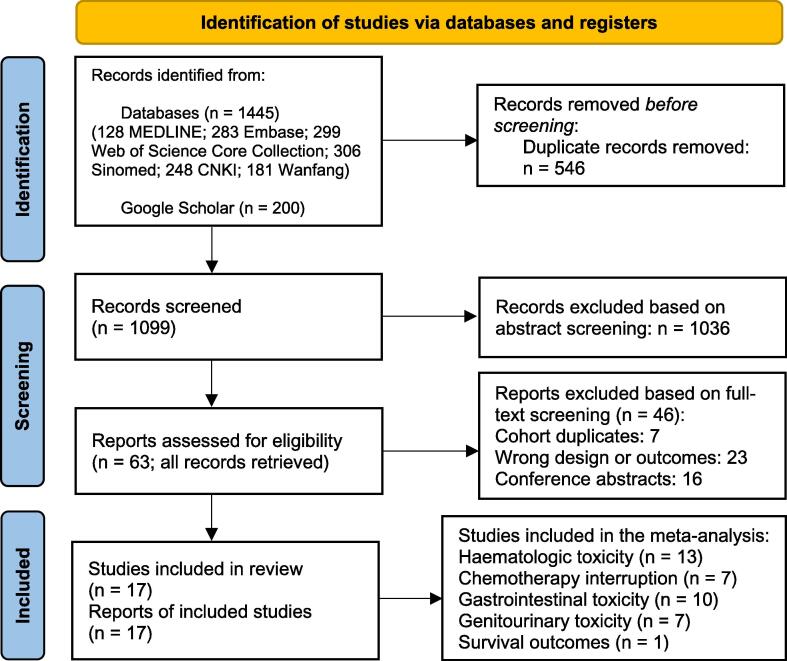
The PRISMA flow diagram is presented in Fig. 1. We identified 17 records published between 2013 and 2023, describing the results of 1297 cervical cancer patients treated in 17 RCTs between 2011 and 2020. The treatment consisted of BMS RT up to 45–50.4 Gy in 1.8–2 Gy fractions (one trial allowed hypofractionation [21]), in most cases combined with cisplatin-based chemotherapy (96 %; n = 1245). The majority of the records (n = 12) were published in Chinese (n = 772) [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [21], and the rest (n = 5) were published in English (n = 525) [12], [33], [34], [35], [36]. All except one international trial, which was prematurely ceased due to futility (n = 29), were single-centre [12]. Fourteen trials were performed in China (n = 1128) [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [36], one in India (n = 80) [34], and the last one in Egypt (n = 60) [35]. There were seven trials on BMS in definitive setting (n = 655) [12], [23], [27], [32], [33], [34], [36], nine in postoperative setting (n = 582) [21], [22], [24], [25], [26], [28], [29], [30], [31], and one study included both (n = 60) [35]. Fifteen trials used CT-based anatomical BMS (n = 1076) [21], [22], [23], [24], [25], [26], [27], [28], [30], [31], [32], [33], [34], [35], [37], and two trials applied PET-CT (n = 29) [12] or SPECT-CT (n = 192) [36] to identify BM. Out of all trials conducted, all but two compared BMS against non-BMS IMRT or VMAT. One trial compared IMRT-BMS with 3D-CRT (n = 80) [34], and one trial lacked a detailed RT description (n = 50) [21]. Table 1 provides a concise summary of individual study details, and Supplementary File 5 provides comprehensive information on BMS CRT.
Fig. 1.
PRISMA flow diagram.
Table 1.
Basic characteristics of 17 prospective randomised trials reporting data bone marrow sparing chemoradiotherapy in the treatment of cervical cancer patients.
| 1st author | Year | Language | Country | # cases | Treatment years | Median age [years] | Median FU [months] | Setting | Brief inclusion criteria |
|---|---|---|---|---|---|---|---|---|---|
| Du C. [1] | 2013 | Chinese | China | 40 | 2011–2012 | 50 | n/a | postoperative | FIGO n/s, age n/s, ECOG 0–2 |
| Zhang D. [5] | 2017 | Chinese | China | 41 | 2016–2017 | 58 | n/a | postoperative | FIGO n/s, age n/s, ECOG 0–2 |
| Luo C. [7] | 2018 | Chinese | China | 102 | 2013–2015 | mean: 50 | n/a | postoperative | FIGO n/s, age n/s, ECOG n/s |
| Sun S. [8] | 2018 | Chinese | China | 40 | 2016–2017 | 50 | 3 | postoperative | FIGO n/s, age n/s, ECOG n/s |
| Fang M. [9] | 2019 | Chinese | China | 78 | 2012–2015 | mean: 50* | n/a | postoperative | FIGO n/s, age n/s, ECOG n/s |
| Xie Y. [12] | 2021 | Chinese | China | 50 | 2018–2020 | mean: 53* | n/a | postoperative | FIGO n/s, age n/s, ECOG n/s |
*estimated based on available data; n/s – not specified; FIGO – disease stage according to The International Federation of Gynecology and Obstetrics; ECOG − Eastern Cooperative Oncology Group performance status; KPS − Karnofsky Performance Scale.
A summary of the RoB analysis is available in Supplementary File 6, including the authors’ judgment for each domain. The majority of studies were assessed as moderate RoB. Two studies raised concerns due to selective reporting and were marked as high risk [28], [31]. In the study by El-Tawab et al., moderate concerns were identified in several domains, ultimately resulting in an overall high RoB [35]. In the study by Sun et al., individual details on haematologic toxicity published in 2018 were inconsistent with a more recent peer-reviewed publication, with all other data being consistent between articles (including statistical test results) [29], [37]. The Mann-Whitney U test result could not be reproduced for the data provided on haematologic toxicity in the 2023 article [37], but was consistent with the outcomes reported in the 2018 dissertation [29]. Therefore, we decided to use the data from the dissertation for meta-analysis [29], and rated the study as having high RoB.
Haematologic toxicity
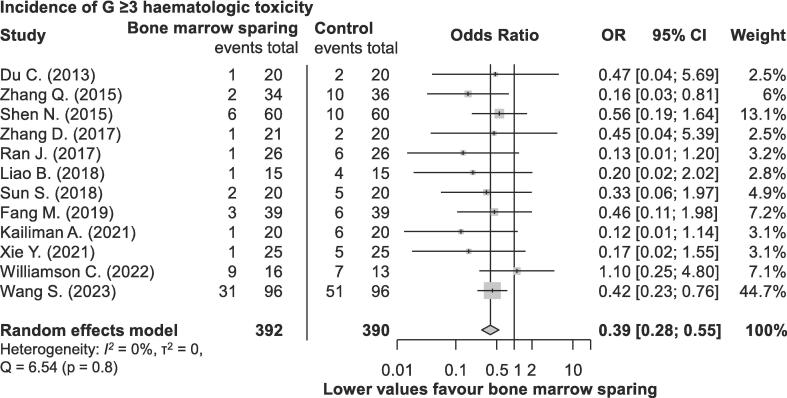
Twelve studies reported data on G ≥ 3 (n = 782) [12], [21], [22], [23], [24], [25], [26], [27], [29], [30], [32], [36], and twelve studies on G ≥ 2 (n = 754) [12], [21], [22], [23], [24], [25], [26], [27], [29], [30], [32], [33] adverse events (AEs) related to haematologic toxicity. Most studies used the Radiation Therapy Oncology Group (RTOG) toxicity scale (n = 749) [22], [23], [24], [25], [26], [27], [29], [33], [36], described in detail in an article by Cox et al. [38]. Additionally, one study used Common Toxicity Criteria for Adverse Events (CTCAE) v. 4.0 (n = 29) [12], while three authors did not specify which toxicity scale was used (n = 168) [21], [30], [32]. As presented in Fig. 2, the incidence of G ≥ 3 haematologic toxicity was significantly decreased in patients receiving BMS RT (OR 0.39; 95 % CI 0.28–0.55; p < 0.001). Similarly, the incidence of G ≥ 2 toxicity was significantly lower in the BMS group (OR 0.29; 95 % CI 0.18–0.46; p < 0.001; Supplementary File 7). There was no evidence of significant heterogeneity for both analyses. Additionally, several articles provided specific information on components of hematologic toxicity, such as neutropenia, leukopenia, anaemia, thrombocytopenia, and lymphopenia. The corresponding data and subset analyses are available in Supplementary File 8.
Fig. 2.
Incidence of grade ≥ 3 hematologic toxicity following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Chemotherapy interruptions
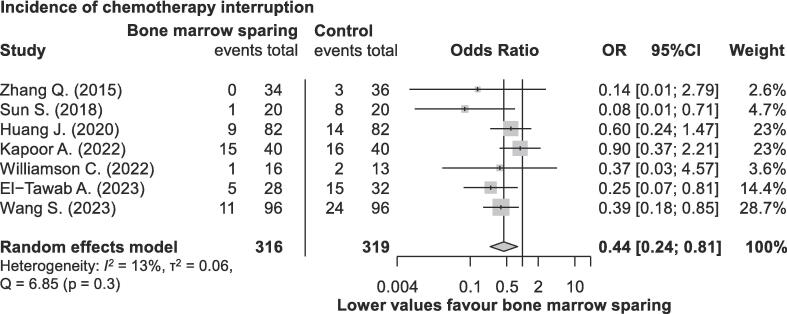
Seven studies, encompassing a total of 635 patients [12], [24], [29], [33], [34], [35], [36], reported on the percentage of patients who experienced systemic treatment interruption, defined as receiving fewer than five cycles of concomitant cisplatin chemotherapy. In total, 19.5 % of patients (124 out of 635) experienced chemotherapy interruption, including 13.3 % in the BMS group (42 out of 316) and 25.7 % in the non-BMS group (82 out of 319). Patients treated with BMS RT exhibited a significantly reduced incidence of completing fewer than five cycles of chemotherapy (OR 0.44; 95 % CI 0.24–0.81; p = 0.016), as presented in Fig. 3. No evidence of significant heterogeneity was found.
Fig. 3.
Incidence of concomitant chemotherapy interruptions during chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Gastrointestinal and genitourinary toxicity
The data on GI toxicity were reported in eight studies for G ≥ 2 AEs (n = 583) [12], [23], [24], [25], [27], [29], [30], [33], and in ten studies for G ≥ 3 AEs (n = 884) [12], [23], [24], [25], [27], [29], [30], [31], [33], [36]. The data on GU toxicity were reported in six studies for G ≥ 2 AEs (n = 390) [23], [24], [25], [27], [29], [30], and in seven studies for G ≥ 3 AEs (n = 499) [23], [24], [25], [27], [29], [30], [31]. As presented in Supplementary File 9, there were no statistically significant differences between BMS and non-BMS groups, except for the incidence of G ≥ 3 GU toxicity (OR 0.55; 95 % CI 0.35–0.87; p = 0.019). That said, the total incidence of G ≥ 3 GU toxicity was relatively low for both BMS (13/270; 4.8 %) and non-BMS patients (24/269; 8.9 %). There was no evidence of significant heterogeneity for any of these analyses.
Survival endpoints
Only one of the 17 included trials provided outcome data on survival and oncologic endpoints suitable for meta-analysis (n = 29) [12]. Considering that the trial jointly reported survival outcomes of a phase III RCT, and corresponding non-randomised phase II/III study, we contacted the corresponding author and received updated data on results of interest. There was no significant difference between BMS and non-BMS patients in terms of overall survival (p > 0.9), progression-free survival (p = 0.8), and local–regional control (p = 0.7).
Reporting bias and sensitivity analyses
Funnel plots for all applicable analyses can be found in Supplementary File 11. There were no signs of significant reporting bias. Sensitivity analyses were performed to account for possible sources of heterogeneity associated with study and patient characteristics, and the results are summarised in Supplementary File 10. Both reduction in chemotherapy interruptions (OR 0.54; 95 % CI 0.31–0.96; p = 0.04) and G ≥ 3 haematologic toxicity (OR 0.39; 95 % CI 0.27–0.57; p < 0.001) in patients treated with BMS remained statistically significant after removing studies with high RoB. Similarly, the reduction in G ≥ 3 haematologic toxicity remained statistically significant after excluding studies using undefined or non-RTOG toxicity scales (OR 0.38; 95 % CI 0.27–0.53; p < 0.001). Finally, during the review process it was pointed out that the highest weight study in the G ≥ 3 haematologic toxicity also has a noticeably higher event rate. After excluding it from the analysis, the meta-analytic average remained comparable (OR 0.37; 95 % CI 0.23–0.59; p < 0.001). The meta-regression analysis showed no significant association between the effect size of BMS and sample size in the analysed trials for both G ≥ 3 haematologic toxicity (p = 0.5) and for the rate of chemotherapy interruptions (p = 0.6) (Supplementary File 12).
Discussion
Concurrent CRT has been the standard treatment for locally advanced cervical cancer patients for decades, and BMS presents an appealing opportunity to improve treatment. There are several important findings in our study. First, we found that BMS RT is associated with significantly reduced incidence of concurrent chemotherapy interruptions and, at the same time, significantly reduced overall incidence of haematologic toxicity. Second, there is no evidence that alteration in RT dose distribution leads to increased GI or GU toxicity; on the contrary, we observed a mild reduction in G ≥ 3 GU AEs in patients treated with BMS RT. Third, we identified a concerning lack of evidence assessing BMS effect on survival outcomes.
In this article, we present the first evidence from meta-analysis to confirm that consistent dosimetric benefit and laboratory-based reduction of haematologic toxicity associated with BMS may translate into clinical benefit due to a significantly reduced incidence of chemotherapy interruptions. First, it must be considered that the introduction of BMS to standard-of-care RT should not tangibly increase overall treatment costs. Theoretically, a small clinical benefit would suffice to justify its routine use, but large comparative trials are unlikely due to a lack of commercial interest and the expected reduction in patient population over time. While a minor increase in necessary human resources could be a burden in developing countries, where cervical cancer is most prevalent [13], it could also be compensated by a lower incidence of haematologic toxicity requiring treatment. In particular, subgroups of patients with initially suboptimal blood tests, or those otherwise at risk of developing severe hematologic toxicity, could benefit from BMS. Similarly, it should be considered in trials investigating novel myelotoxic systemic treatment agents due to documented reduction of chemotherapy interruptions. Conceptually, it could be translated to several other pelvic malignancies utilising RT paired with myelotoxic systemic agents, including treatment escalation in locally advanced prostate cancer [39], bladder-sparing trimodal therapy [40], preoperative treatment in rectal cancer [41], or definitive CRT for anal cancer [42], [43], [44]. Such applications, however, require dedicated investigation.
Despite the lack of data to assess the direct impact on OS, the association between BMS and reduced haematologic toxicity is of particular interest, considering that reports indicate inferior survival in patients experiencing radiation-induced lymphopenia [45], [46]. Moreover, studies indicated that a reduction in concomitant chemotherapy dose might increase the risk of distant or nodal failure [47], [48]. While significant confounding must be considered, these findings suggest hypotheses on how BMS could improve OS. Finally, BMS could reduce the risk of pelvic insufficiency fractures, especially common in the sacral region in advanced cervical cancer patients treated with CRT, through reducing the exposure of bones to radiation [49].
The rapidly developing field of artificial intelligence could lead to nearly resourceless integration of BMS into practice. Several authors have proposed solutions automating the bone-marrow contouring process [50], [51], and automatic RT planning has increased in popularity [52]. If implemented, the remaining concern would be to rule out any unlikely negative impact of BMS on survival or clinical outcomes. The majority of the included trials utilised anatomical BMS; however, treatment-planning PET-CT is now routinely performed in many centres, which facilitates active BMS. As presented in Supplementary File 5, there was also significant heterogeneity in terms of assumed dose constraints. For example, all but one trial utilised V20, but the coverage limit varied as much as between 50 % and 80 %. Until better quality data is available, based on the findings of previous meta-analysis, we believe that V10 < 90 %, V20 < 75 %, and V40 < 17.5 % could be recommended as reasonable dose constraints for active BM [11].
There are several limitations of our study. First, although most authors used CT to identify BM, there were noticeable differences in definitions, and imprecise or missing descriptions, including a lack of consensus on optimal dose constraints for BMS, and limited data on the actual achieved dose-volume parameters. Second, BMS through functional imaging, which is likely to increase in popularity along with the routine use of PET-CT, was underrepresented in our meta-analysis. Third, there were differences in definitions of haematologic toxicity, and limited data was provided on the methodology and frequency of blood tests. Fourth, the majority of included studies were performed in China, while cervical cancer has the highest incidence in African countries, possibly reducing the generalizability of results [13]. Fifth, despite no significant evidence of heterogeneity or reporting bias for major analyses, the data was retrieved predominantly from small, single-institutional trials, rather than high-quality phase III trials. Finally, missing data on survival and oncologic outcomes made it impossible to perform three major pre-defined analyses.
Conclusions
BMS represents a valuable addition to standard CRT for cervical cancer patients, effectively reducing hematologic toxicity and minimizing interruptions in concurrent chemotherapy.
Currently available data do not allow for the assessment of the impact of BMS on survival or disease control. Further studies would be necessary to rule out the unlikely adverse effect of BMS-related dose redistribution on these outcomes.
Authors' contributions
The study was designed by MM, with input from PZ and LKM. The formal steps of the systematic review were carried out by MM, TW, MS, ES, ZC, PR, and PZ. Risk of bias analysis was performed by MM, KK, ZN, TW, ZC, and PZ. Statistical analysis and interpretation of results were performed by MM, KK, and ZN. The manuscript was written by MM with input of TW, KK, ZN, JW, MS, RT, PR, SFS, and PZ. All authors approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2024.100801.
Contributor Information
Marcin Miszczyk, Email: marcin.miszczyk@meduniwien.ac.at.
Pixiao Zhou, Email: zpx2019969@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
PRISMA checklist.
PRISMA for Abstracts checklist.
PICOS.
Search strategy.
Details of bone marrow-sparing strategies in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Risk of bias analysis.
Incidence of grade ≥2 hematologic toxicity following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Subset analyses of grade ≥2 and ≥3 toxicities for specific blood test components following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Gastrointestinal and genitourinary toxicity following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Sensitivity analyses.
Funnel plots.
Meta-regression analysis.
Data Availability
Research data are not available at this time.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.PATH. Global HPV Vaccine Introduction Overview 2022. https://www.path.org/resources/global-hpv-vaccine-introduction-overview/ (accessed December 18, 2023).
- 3.Brisson M., Kim J.J., Canfell K., Drolet M., Gingras G., Burger E.A., et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:575–590. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose P.G., Bundy B.N., Watkins E.B., Thigpen J.T., Deppe G., Maiman M.A., et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 5.Klopp A.H., Yeung A.R., Deshmukh S., Gil K.M., Wenzel L., Westin S.N., et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology–RTOG 1203. J Clin Oncol. 2018;36:2538–2544. doi: 10.1200/JCO.2017.77.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra S., Gupta S., Kannan S., Dora T., Engineer R., Mangaj A., et al. Late toxicity after adjuvant conventional radiation versus image-guided intensity-modulated radiotherapy for cervical cancer (PARCER): a randomized controlled trial. J Clin Oncol. 2021;39:3682–3692. doi: 10.1200/JCO.20.02530. [DOI] [PubMed] [Google Scholar]
- 7.Miszczyk M., Majewski W. Hematologic toxicity of conformal radiotherapy and intensity modulated radiotherapy in prostate and bladder cancer patients. Asian Pac J Cancer Prev. 2018;19:2803–2806. doi: 10.22034/APJCP.2018.19.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis R.E. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255–258. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 9.Mauch P., Constine L., Greenberger J., Knospe W., Sullivan J., Liesveld J.L., et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 10.Franco P., Arcadipane F., Ragona R., Mistrangelo M., Cassoni P., Racca P., et al. Hematologic toxicity in anal cancer patients during combined chemo-radiation: a radiation oncologist perspective. Expert Rev Anticancer Ther. 2017;17:335–345. doi: 10.1080/14737140.2017.1288104. [DOI] [PubMed] [Google Scholar]
- 11.Zhou P., Zhang Y., Luo S., Zhang S. Pelvic bone marrow sparing radiotherapy for cervical cancer: A systematic review and meta-analysis. Radiother Oncol. 2021;165:103–118. doi: 10.1016/j.radonc.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Williamson C.W., Sirák I., Xu R., Portelance L., Wei L., Tarnawski R., et al. Positron emission tomography-guided bone marrow-sparing radiation therapy for locoregionally advanced cervix cancer: final results from the INTERTECC Phase II/III Trial. Int J Radiat Oncol Biol Phys. 2022;112:169–178. doi: 10.1016/j.ijrobp.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avau B., Van Remoortel H., De Buck E. Translation and validation of PubMed and Embase search filters for identification of systematic reviews, intervention studies, and observational studies in the field of first aid. J Med Libr Assoc. 2021;109:599–608. doi: 10.5195/jmla.2021.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohatgi A. WebPlotDigitizer2022. https://automeris.io/.
- 18.Liu N., Zhou Y., Lee J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21:111. doi: 10.1186/s12874-021-01308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y., Li L. Analysis of the relationship between pelvic bone marrow protection and acute hematologic adverse effects of chemoradiotherapy for cervical cancer. Chin J Clinical Rational Drug Use. 2021;14:173–174. doi: 10.15887/j.cnki.13-1389/r.2021.23.072. [DOI] [Google Scholar]
- 22.Du C., Cai M., Lin F. Clinical observation of hematologic toxicity in intensity modulated radiotherapy for cervical cancer to restrict dosimetry of bone marrow irradiated. Med J West China. 2013;25:1857–1858. doi: 10.3969/j.issn.1672-3511.2013.12.036. [DOI] [Google Scholar]
- 23.Shen N. Medical University; HEBEI: 2015. Analysis of Dosimetry and Clinical Efficacy of Pelvic Bone Marrow-Sparing Intensity-Modulated Radiotherapy in Advanced Cervical Cancer. [Google Scholar]
- 24.Zhang Q., Wang H. Clinical study of bone marrow-sparing intensity-modulated radiation therapy for postoperative cervical cancer. Chin J Radiol Med Prot. 2015;35:441–444. doi: 10.3760/cma.j.issn.0254-5098.2015.06.009. [DOI] [Google Scholar]
- 25.Ran J., Zhang Y., Xue X., Shen J., Cui Y. Clinical study of bone marrow- sparing volumetric- modulated arc-radiation therapy for postoperative cervical cancer. Chin J General Pract. 2017;15:2021–2023. doi: 10.16766/j.cnki.issn.1674-4152.2017.12.005. [DOI] [Google Scholar]
- 26.Zhang D., Guo H., Zhang Q., Liu L. Dosimetric analysis of bone marrow-sparing pelvic intensity-modulated radiotherapy after surgery for cervical cancer. Chin. J Radiat Oncol. 2017;26:1303–1307. doi: 10.3760/cma.j.issn.1004-4221.2017.11.013. [DOI] [Google Scholar]
- 27.Liao B. Guangzhou Medical University; 2018. Analysis of dosimetry and acute toxicity of bone marrow sparing intensity modulated radiotherapy in lymph-node positive cervical cancer. [Google Scholar]
- 28.Luo C., Lai L., Huang J., Xu S., Mo W., Tang H. Application of whole-pelvic intensity modulated radiotherapy to protect bone marrow in postoperative concurrent chemotherapy for cervical cancer. J Chin Res. 2018;35:347–349. doi: 10.3969/j.issn.1671-7171.2018.02.046. [DOI] [Google Scholar]
- 29.Sun S. Wenzhou Medical University; 2018. Clinical Study on Acute Toxicity of Pelvic Bone Marrow-Sparing Intensity-Modulated Radiotherapy in Cervical Cancer after Hysterectomy. [Google Scholar]
- 30.Fang M., Zhou Y., Yang H., Zheng J., Gao Y., Li Z., et al. Comparison of the effects of limited and unlimited pelvic bone marrow doses in postoperative intensity-modulated radiation therapy for cervical cancer. China Med Pharmacy. 2019;9:149–151. doi: 10.3969/j.issn.2095-0616.2019.15.044. [DOI] [Google Scholar]
- 31.Feng J., Lin J., Liao S., Luo H., Fu Z. The relationship between bone marrow suppression and dose volume of bone marrow irradiation for the postoperative cervical cancer patients received intensity modulated radiotherapy. Int J Radiat Med Nucl Med. 2020;44:143–150. doi: 10.3760/cma.j.cn121381-201811039-00002. [DOI] [Google Scholar]
- 32.Kailiman A., Qi X., Zhao H. Dosimetry of pelvic bone marrow sparing intensity modulated radiotherapy simultaneous chemotherapy in patients with lymph node-positive cervical cancer. Oncol Prog. 2021;19(2133–6):2148. doi: 10.11877/j.issn.1672-1535.2021.19.20.22. [DOI] [Google Scholar]
- 33.Huang J., Gu F., Ji T., Zhao J., Li G. Pelvic bone marrow sparing intensity modulated radiotherapy reduces the incidence of the hematologic toxicity of patients with cervical cancer receiving concurrent chemoradiotherapy: a single-center prospective randomized controlled trial. Radiat Oncol. 2020;15:180. doi: 10.1186/s13014-020-01606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapoor A.R., Bhalavat R.L., Chandra M., Pareek V., Moosa Z., Markana S., et al. A randomized study for dosimetric assessment and clinical impact of bone marrow sparing intensity-modulated radiation therapy versus 3-dimensional conformal radiation therapy on hematological and gastrointestinal toxicities in cervical cancer. J Cancer Res Ther. 2022;18:1490. doi: 10.4103/jcrt.JCRT_1242_20. [DOI] [PubMed] [Google Scholar]
- 35.El-Tawab ASMMAEN, Barakat AF, Hussien FZ, Ghanam AAEA, Hakim MMA. Bone marrow sparing intensity modulated radiotherapy concurrent with chemotherapy for treatment of cervical malignancy. Oncology and Radiotherapy 2023;17:108–15.
- 36.Wang S., Liu J., Lei K., Jia Y., Wang C., Zhang X., et al. Single-photon emission computed tomography-defined active bone marrow-sparing volumetric-modulated arc therapy reduces the incidence of acute hematologic toxicity in locally advanced cervical cancer patients who receive chemoradiotherapy: A single-center prospective randomized controlled trial. Cancer. 2023;129:1995–2003. doi: 10.1002/cncr.34771. [DOI] [PubMed] [Google Scholar]
- 37.Sun S., Chen Z., Li P., Wu J., Zhu B., Zhang X., et al. Clinical study of acute toxicity of pelvic bone marrow-sparing intensity-modulated radiotherapy for cervical cancer. Ginekol Pol. 2023;94:101–106. doi: 10.5603/GP.a2021.0234. [DOI] [PubMed] [Google Scholar]
- 38.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiation Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 39.Rajwa P., Pradere B., Gandaglia G., van den Bergh R.C.N., Tsaur I., Shim S.R., et al. Intensification of systemic therapy in addition to definitive local treatment in nonmetastatic unfavourable prostate cancer: a systematic review and meta-analysis. Eur Urol. 2022;82:82–96. doi: 10.1016/j.eururo.2022.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Ploussard G., Daneshmand S., Efstathiou J.A., Herr H.W., James N.D., Rödel C.M., et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66:120–137. doi: 10.1016/j.eururo.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 41.Bahadoer R.R., Dijkstra E.A., van Etten B., Marijnen C.A.M., Putter H., Kranenbarg E.-M.-K., et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 42.Rao S., Guren M.G., Khan K., Brown G., Renehan A.G., Steigen S.E., et al. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32:1087–1100. doi: 10.1016/j.annonc.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Arcadipane F., Silvetti P., Olivero F., Gastino A., De Luca V., Mistrangelo M., et al. Bone Marrow-Sparing IMRT in Anal Cancer Patients Undergoing Concurrent Chemo-Radiation: Results of the First Phase of a Prospective Phase II Trial. Cancers (basel) 2020;12:3306. doi: 10.3390/cancers12113306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arcadipane F., Silvetti P., Olivero F., Gastino A., Carlevato R., Chiovatero I., et al. Concurrent Chemoradiation in Anal Cancer Patients Delivered with Bone Marrow-Sparing IMRT: Final Results of a Prospective Phase II Trial. J Personalized Med. 2021;11:427. doi: 10.3390/jpm11050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damen P.J.J., Kroese T.E., van Hillegersberg R., Schuit E., Peters M., Verhoeff J.J.C., et al. The Influence of Severe Radiation-Induced Lymphopenia on Overall Survival in Solid Tumors: A Systematic Review and Meta-Analysis. Int J Radiation Oncol Biol Phys. 2021;111:936–948. doi: 10.1016/j.ijrobp.2021.07.1695. [DOI] [PubMed] [Google Scholar]
- 46.Taguchi A., Furusawa A., Ito K., Nakajima Y., Shimizuguchi T., Hara K., et al. Postradiotherapy persistent lymphopenia as a poor prognostic factor in patients with cervical cancer receiving radiotherapy: a single-center, retrospective study. Int J Clin Oncol. 2020;25:955–962. doi: 10.1007/s10147-020-01623-y. [DOI] [PubMed] [Google Scholar]
- 47.Schmid M.P., Franckena M., Kirchheiner K., Sturdza A., Georg P., Dörr W., et al. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecol Oncol. 2014;133:256–262. doi: 10.1016/j.ygyno.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Hou X., Hu K., Zhang F., Wang W., Ren K. Risk Factors for Nodal Failure in Patients with FIGO IIIC Cervical Cancer Receiving Definitive Image-Guided Radiotherapy. Curr Oncol. 2023;30:10385–10395. doi: 10.3390/curroncol30120756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramlov A., Pedersen E.M., Røhl L., Worm E., Fokdal L., Lindegaard J.C., et al. Risk Factors for Pelvic Insufficiency Fractures in Locally Advanced Cervical Cancer Following Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;97:1032–1039. doi: 10.1016/j.ijrobp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Fiandra C., Rosati S., Arcadipane F., Dinapoli N., Fato M., Franco P., et al. Active bone marrow segmentation based on computed tomography imaging in anal cancer patients: A machine-learning-based proof of concept. Phys Med. 2023;113 doi: 10.1016/j.ejmp.2023.102657. [DOI] [PubMed] [Google Scholar]
- 51.Andreychenko A., Kroon P.S., Maspero M., Jürgenliemk-Schulz I., De Leeuw A.A.C., Lam M.G.E.H., et al. The feasibility of semi-automatically generated red bone marrow segmentations based on MR-only for patients with gynecologic cancer. Radiother Oncol. 2017;123:164–168. doi: 10.1016/j.radonc.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Wortel G., Eekhout D., Lamers E., van der Bel R., Kiers K., Wiersma T., et al. Characterization of automatic treatment planning approaches in radiotherapy. Phys Imaging Radiation Oncol. 2021;19:60–65. doi: 10.1016/j.phro.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
PRISMA for Abstracts checklist.
PICOS.
Search strategy.
Details of bone marrow-sparing strategies in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Risk of bias analysis.
Incidence of grade ≥2 hematologic toxicity following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Subset analyses of grade ≥2 and ≥3 toxicities for specific blood test components following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Gastrointestinal and genitourinary toxicity following chemo-radiotherapy for cervical cancer in randomised controlled trials investigating bone marrow-sparing chemo-radiotherapy.
Sensitivity analyses.
Funnel plots.
Meta-regression analysis.
Data Availability Statement
Research data are not available at this time.