Abstract
Background
Exposure to environmental pollutants early in life has been associated with increased prevalence and severity of depression in adolescents; however, the neurobiological mechanisms underlying this association are not well understood. In the current longitudinal study, we investigated whether pollution burden in early adolescence (9–13 years) was associated with altered brain activation and connectivity during implicit emotion regulation and changes in depressive symptoms across adolescence.
Methods
One hundred forty-five participants (n = 87 female; 9–13 years) provided residential addresses, from which we determined their relative pollution burden at the census tract level, and performed an implicit affective regulation task in the scanner. Participants also completed questionnaires assessing depressive symptoms at 3 time points, each approximately 2 years apart, from which we calculated within-person slopes of depressive symptoms. We conducted whole-brain activation and connectivity analyses to examine whether pollution burden was associated with alterations in brain function during implicit emotion regulation of positively and negatively valenced stimuli and how these effects were related to slopes of depressive symptoms across adolescence.
Results
Greater pollution burden was associated with greater bilateral medial prefrontal cortex activation and stronger bilateral medial prefrontal cortex connectivity with regions within the default mode network (e.g., temporoparietal junction, posterior cingulate cortex, precuneus) during implicit regulation of negative emotions, which was associated with greater increases in depressive symptoms across adolescence in those exposed to higher pollution burden.
Conclusions
Adolescents living in communities characterized by greater pollution burden showed altered default mode network functioning during implicit regulation of negative emotions that was associated with increases in depressive symptoms across adolescence.
Keywords: Adolescence, Connectivity, Depression, Emotion regulation, fMRI, Pollution
Plain Language Summary
Exposure to environmental pollution is related to increased risk for depression in youth; however, the neurobiological mechanisms underlying this association are unknown. We found that adolescents living in neighborhoods with greater census tract–level pollution burden had stronger functional connectivity between the medial prefrontal cortex and regions within the default mode network during implicit regulation of negative emotions, which in turn was associated with greater increases in depressive symptoms across adolescence in these pollution-exposed youths.
Plain Language Summary
Exposure to environmental pollution is related to increased risk for depression in youth; however, the neurobiological mechanisms underlying this association are unknown. We found that adolescents living in neighborhoods with greater census tract–level pollution burden had stronger functional connectivity between the medial prefrontal cortex and regions within the default mode network during implicit regulation of negative emotions, which in turn was associated with greater increases in depressive symptoms across adolescence in these pollution-exposed youths.
Adolescence is a period of heightened neuroplasticity and, consequently, heightened sensitivity to environmental exposure, which increases individuals’ opportunities for both adaptive growth and maladaptive development, thereby leading to higher risk for the development of psychopathology. The onset of symptoms of depression and other related disorders increases sharply during adolescence (1) and negatively affects not only individuals and their families (2) but also society more broadly (3, 4, 5). Environmental pollution, which increases risk for adverse physical and mental health outcomes in more than a billion youths worldwide, is also a growing global concern (6). Emerging research suggests that exposure in early life to environmental pollutants, including air pollution, water contaminants, and living in proximity to sources of pollutants (e.g., traffic, power plants), is associated with increased prevalence and severity of depression in adolescents (7, 8, 9, 10) and alterations in neurobiological development (7,11, 12, 13); however, the neurobiological pathways by which environmental pollution exposure is associated with depressive symptoms are not well understood. Given increasing concerns about the adverse consequences of pollution on children’s brain development and neurocognitive functioning (14), it is important that we gain a better understanding of the effects of pollution burden on brain development and mental health in youth.
In this context, exposure to pollution has been found to be associated with alterations in structural and functional brain development in youth, particularly in regions and networks undergoing rapid development and exhibiting heightened plasticity, including subcortical (15, 16, 17), prefrontal, and temporal regions [see (12) for a review]. Most studies that have examined links between environmental pollution and brain function have assessed intrinsic (i.e., resting-state) functional connectivity and have found that exposure to air pollution during childhood is associated with altered development of intrinsic connectivity within and between the default mode network (DMN) (including the medial prefrontal cortex [mPFC] and posterior cingulate cortex [PCC]) and frontoparietal network (including the lateral PFC and parietal regions) and between cortico-subcortical regions (18, 19, 20).
To date, few studies have examined the effects of pollution exposure on task-evoked patterns of brain activation, and most of those have focused on activation during sensory processing. For example, Pujol et al. (18) found that children with greater exposure to vehicle exhaust pollution had less deactivation (i.e., rest > stimulus) in somatosensory and premotor regions during visual and auditory processing. Similarly, Iannilli et al. (21) found that exposure to manganese-contaminated air, soil, and water was associated with lower activation in frontal and parietal regions and in the bilateral insula, precuneus, and PCC during an olfactory task in adolescents. However, we know little about the effects of environmental pollution on the neural circuitry involved in emotion regulation and their implications for adolescent mental health. Some researchers have posited that alterations in the neural circuitry underlying emotion regulation contribute to the onset and maintenance of depression (22). In this context, researchers have found that youth with diagnoses or elevated symptoms of depression were characterized by greater activation in the mPFC and PCC and stronger positive amygdala-PFC and mPFC-DMN connectivity during the regulation of negative emotions (23, 24, 25, 26, 27).
We also know little about the effects of exposure to multiple pollutants. Most investigations have focused on only one type of toxicant class or medium of exposure (e.g., ozone from ambient air pollution); in actuality, however, individuals, especially those living in disadvantaged communities, are exposed to multiple contaminants from multiple sources simultaneously (28, 29, 30). Despite the fact that different pollutants (e.g., ozone, particulate matter, copper, manganese) have distinct physicochemical characteristics, epidemiologic and toxicologic studies indicate that they have common effects on brain structure and function [see (31,32) for reviews], underscoring the importance of examining the cumulative impact of pollutants on brain development.
In the current longitudinal study, we investigated whether pollution burden in early adolescence (ages 9–13 years) was associated with altered brain activation and connectivity during an implicit emotion regulation task and with changes in depressive symptoms across adolescence. We used participants’ residential addresses and the California Environmental Screening Tool (CalEnviroScreen) as a proxy for adolescents’ pollution burden. Participants completed an affect labeling task in the scanner, which assessed implicit emotion regulation in the context of positively and negatively valenced stimuli. We hypothesized that adolescents exposed to greater pollution burden would have greater activation and connectivity in brain regions implicated in implicit emotion regulation (i.e., prefrontal, subcortical, parietal, and temporal regions) (33). We also examined whether pollution burden–related changes in patterns of brain connectivity during implicit emotion regulation were associated with increases in depressive symptoms across adolescence and whether these effects were moderated by pollution burden.
Methods and Materials
Participants
Starting in 2013 and ending in 2017, 224 participants (n = 132 female, ages 9–13 years, mean = 11.42 years, SD = 1.08) were recruited from the San Francisco Bay Area for a longitudinal study of the effects of early-life adversity on psychobiological functioning across adolescence. Inclusion and exclusion criteria are described in the Supplement. Of the 224 participants, 145 had at least one high-quality functional magnetic resonance imaging (fMRI) run/scan and complete data. Except for differences in the distribution of race/ethnicity and higher pollution burden percentiles, participants with missing/low-quality fMRI data (n = 79) did not differ demographically from those with complete data (n = 145) (Table S1).
Procedure
Study procedures were approved by the Stanford University Institutional Review Board. Eligible families were invited to attend a laboratory session during which research staff obtained consent and assent from parents or legal guardians and adolescents, respectively. During this first session of the time 1 (T1) assessment, parents and adolescents completed interview and questionnaire measures. Approximately 2 weeks later, adolescents completed the MRI scan, and approximately every 2 years over 4 years (i.e., T2, T3), they completed the questionnaires again.
Pollution Burden
The California Office of Environmental Health Hazard Assessment created the CalEnviroScreen, which maps various environmental indicators across California neighborhoods at the census tract level. We used CalEnviroScreen 3.0 (https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-30; updated June 2018), which includes measurements of various environmental indicators (e.g., ozone and PM2.5 concentrations, drinking water contaminants, traffic density, proximity to hazardous waste facilities) between 2012 and 2016, corresponding approximately to the first time point of our data (2013–2017). See the Supplement for detailed descriptions of each indicator and its distribution (Figure S1). Comparison of the indicator values in CalEnviroScreen 3.0 with the most recent values in CalEnviroScreen 4.0 (https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-40, updated October 2021, spanning 2015–2021) indicated that there was generally either stability or increases in exposures over time (Table S2). We accounted for the variability in the time difference between when pollution burden was measured and when participants completed their brain scans by calculating the time difference (in days) between the earliest indicator measurement (May 1, 2012) and the date of each participant’s scan (mean = 1023.85 days, SD = 293.51). CalEnviroScreen combines percentile values of indicators related to exposures and environmental effects to form an index of pollution burden, represented in percentiles across the entire state, that we used in the current study. We used percentile scores to put the environmental indicators on a common scale; using percentile scores also frees us from making assumptions about the distributions of the indicators, which can vary across indicators.
Neighborhood Socioeconomic Disadvantage
We used data from the socioeconomic indicators on the CalEnviroScreen to create a composite measure of neighborhood socioeconomic disadvantage at the census tract level by averaging standardized percentiles of poverty, educational attainment, unemployment, and housing burden indicators. Higher scores on this composite indicate greater neighborhood socioeconomic disadvantage. Detailed descriptions of these indicators and their distributions (Figure S2) are presented in the Supplement.
Household Socioeconomic Disadvantage
We created a household socioeconomic disadvantage composite by averaging standardized scores of household income-to-needs ratio and parental education and then multiplying the composite by −1 so that higher values represent greater household socioeconomic disadvantage. Parents reported total household income over the past 12 months, the number of people in their household, and the highest level of completed education (1 = no General Educational Development/high school diploma; 8 = doctorate). Parents reported income on a 10-point scale as follows: 1 = ≤$5000; 2 = $5001–$10,000; 3 = $10,001–$15,000; 4 = $15,001–$25,000; 5 = $25,001–$35,000; 6 = $35,001–$50,000; 7 = $50,001–$75,000; 8 = $75,001–$100,000; 9 = $100,001–$150,000; 10 = ≥$150,000. Income-to-needs ratios were calculated by dividing the midpoint of the endorsed income bin by the low-income limit for Santa Clara County (80% of the median income) as determined by the Department of Housing and Urban Development based on household size.
Depressive Symptoms
Participants completed the 10-item short form of the Child Depression Inventory (34), a self-report measure of depressive symptoms for youth ages 8 to 17 years. Participants indicated the severity of symptoms of depression that they were experiencing over the past 2 weeks on a 3-point scale from 0 (no symptoms) to 2 (definite symptoms). A summary score was calculated to represent the cumulative frequency and severity of depressive symptoms, with higher scores indicating greater symptoms. Reliabilities for the depressive symptoms scale at T1, T2, and T3 are 0.75 (95% CI, 0.70–0.80), 0.80 (95% CI, 0.76–0.84), and 0.85 (95% CI, 0.82–0.88), respectively.
Implicit Emotion Regulation (Affect Labeling) Task
Participants completed an affect labeling task in the scanner to assess the neural correlates of implicit emotion regulation (35,36). Participants saw a target facial expression at the top of the screen and were instructed to press a button to select the correct option in the bottom left or right of the screen. For label conditions, the 2 options were words describing the emotion; for match conditions, the 2 options were images of facial expressions. Emotions within a block were all either positively valenced (happy, calm, excited) or negatively valenced (fearful, angry, sad). A sensorimotor control condition was included in which participants matched shapes (shape match), resulting in 5 separate conditions/blocks per run: positive match, positive label, negative match, negative label, and shape match. Each block contained 10 trials, each presented on the screen for 5 seconds, with a 15-second fixation rest period between blocks. Conditions and stimuli were presented randomly across all participants, who completed 2 runs of the task. Consistent with other studies (35, 36, 37), we examined the contrast between positive label > positive match and negative label > negative match to isolate the processes specific to implicit emotion regulation.
MRI Acquisition, Preprocessing, and Individual-Level Modeling of fMRI Data
Neuroimaging data were acquired at the Stanford Center for Cognitive and Neurobiological Imaging using a 3T General Electric Discovery MR750 scanner with a 32-channel head coil (Nova Medical). See the Supplement for details on acquisition parameters. Anatomical T1-weighted images were first processed with FreeSurfer (version 6.0.1) (38) for surface-based registration, followed by functional processing using fMRIPrep (version 20.2.1) (39,40) and functional analyses using FSL’s (version 6.0.1) FEAT (41,42). See the Supplement for a detailed description of the fMRIPrep protocol and individual-level modeling.
Statistical Analyses
Group-Level Whole-Brain Analyses
We first conducted whole-brain general linear model (GLM) analyses using FLAME1 on the condition × valence contrast (i.e., [negative label > negative match] > [positive label > positive match]) to examine whether pollution burden was associated with neural activation during implicit emotion regulation and whether the effects differed by affective valence (see the Supplement for details about the analytic procedure). Results of these analyses were used to inform the functional connectivity analyses.
Psychophysiological Interaction Analyses
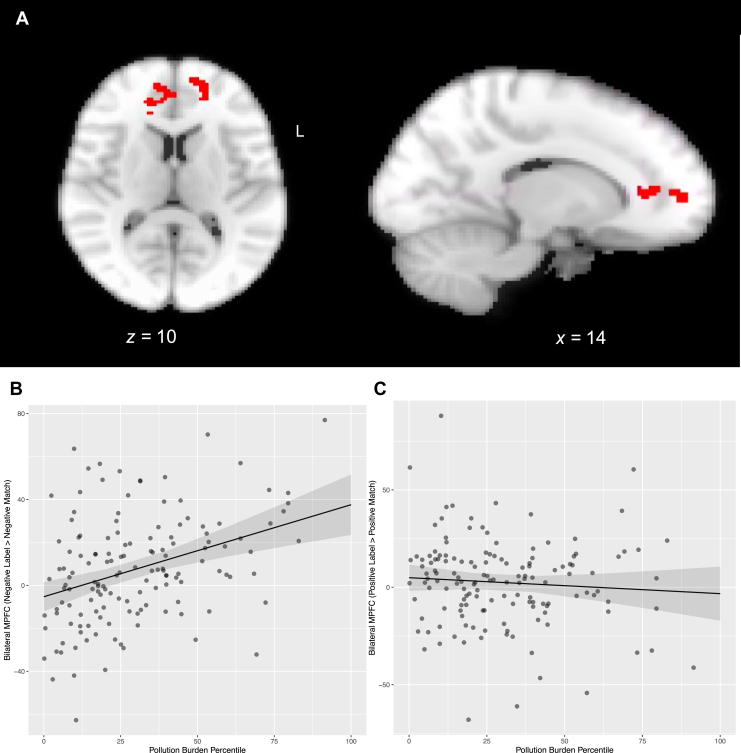
To examine how pollution burden was related to functional connectivity during implicit emotion regulation, we conducted psychophysiological interaction (PPI) analyses (43). Because results from the whole-brain analyses testing the interaction of condition, valence, and pollution burden indicated that pollution burden was associated with bilateral mPFC (left mPFC: −14, 56, 10, Z max = 4.11, 126 voxels; right mPFC: 14, 36, 6, Z max = 3.77, 146 voxels) (Figure 1A) activation during implicit emotion regulation of negatively but not positively valenced stimuli (Figure 1B, C), PPI analyses focused on connectivity during regulation of negatively valenced stimuli (i.e., negative label > negative match). Furthermore, because the associations of pollution burden with the left and right mPFC did not differ in slope (b = 0.07, p = .474) (Table S3 and Figure S5), we combined the left and right mPFC clusters to form a binarized mask that we used as the seed region. For each participant and run, we conducted a PPI GLM analysis in FSL that included the deconvolved time series from the bilateral mPFC seed (physiological regressor), the regressor representing the negative label > negative match contrast (task regressor), the product of the physiological and task regressors (psychophysiological interaction term), and other task regressors (i.e., positive label, positive match, shape match). We convolved task regressors with the canonical double-gamma hemodynamic response function and combined individual-level PPI results from the 2 runs in a fixed-effects model, generating whole-brain maps of functional connectivity with the mPFC seed region, which we then used in group-level whole-brain GLM analysis using FLAME1 with pollution burden percentile scores (mean-centered) entered as a regressor in the GLM. Participant sex (−1 = male, 1 = female), age (mean-centered), household socioeconomic disadvantage (mean-centered), neighborhood socioeconomic disadvantage (mean-centered), time difference between pollution measurement and scan date (mean-centered), and mean framewise displacement (mean-centered) were included as covariates. Z (Gaussianized T) statistic images were thresholded at Z > 3.1, with a corrected cluster significance threshold of p < .025 (Bonferroni-corrected alpha = .05/2 tests [whole-brain activation and PPI]). Anatomical localization of each cluster was defined using the FSL Harvard-Oxford probabilistic atlas.
Figure 1.
Results from whole-brain cluster analyses depicting a significant condition × valence interaction effect ([negative label > negative match] > [positive label > positive match]) in left (L) (−14, 56, 10, Z max = 4.11, 126 voxels) and right (14, 36, 6, Z max = 3.77, 146 voxels) medial prefrontal cortex (mPFC) activation as a function of pollution burden (A). Visual inspection of the association of pollution burden with bilateral mPFC by valence suggests that greater pollution burden was associated with greater bilateral mPFC activation during implicit regulation (label > match) of negative emotions (B), but not of positive emotions (C).
Brain-Behavior Associations
Consistent with previous studies (44,45), using the lme4 package in R, we conducted linear mixed-effects models with random intercepts and age slopes to estimate the intercepts and slopes of depressive symptoms across the 3 time points for each participant. We modeled self-reported depressive symptoms as a function of age (centered at 11 years, the mean age at T1) and extracted the intercept and slope parameter estimates for each participant. To explore the effects of pollution burden on brain-behavior relationships, we first tested whether pollution burden–related alterations in connectivity during regulation of negatively valenced stimuli were related to depressive symptoms at baseline (i.e., intercept) and to increases in depressive symptoms over a 4-year period (i.e., slope) over and above baseline levels of depressive symptoms and covariates. In addition, given findings of differential brain-behavior associations as a function of socioeconomic status (46) and pollution exposure (47), we also tested whether pollution burden moderated associations between functional connectivity during regulation of negatively valenced stimuli and both initial levels and slopes of depressive symptoms. We followed significant interactions with simple slopes analysis using the Johnson-Neyman interval, which identifies the interval of values of pollution burden for which the association of functional connectivity with the depressive symptoms outcome is statistically significant.
For significant brain-behavior associations, we conducted exploratory analyses using regularized (i.e., LASSO) regression to determine which environmental indicator(s) might contribute to observed effects (see the Supplement for details).
Sensitivity Analyses
We conducted sensitivity analyses to test for the presence of residual confounding and whether we needed to cluster participants by census tract (see the Supplement for details). Furthermore, given that altered functioning of brain regions involved in emotion regulation (36) and depressive/internalizing symptoms (48) have also been associated with exposure to early-life adversity, we reran analyses additionally controlling for early-life adversity (see the Supplement for details). We also tested whether our effects were specific to depressive symptoms or to general psychopathology/behavioral problems by rerunning brain-behavior analyses using intercepts and slopes of externalizing symptoms measured with the Youth Self-Report (49) as outcomes (see the Supplement for details).
Results
Participant Characteristics
Descriptive statistics for participant characteristics are presented in Table 1. Bivariate correlations among variables are presented in Table 2. Pollution burden did not differ as a function of sex, age, motion, time difference, or household socioeconomic disadvantage but was positively associated with neighborhood socioeconomic disadvantage. Main effects of task are reported in the Supplement (Tables S4 and S5; Figures S3 and S4).
Table 1.
Descriptive Statistics for Participant Characteristics (n = 151)
| Variables | n (%) or Mean (SD) [Range] |
|---|---|
| Sex, Female/Male | 90 (59.6%)/61 (40.4%) |
| Age, T1, Years | 11.42 (1.08) [9–13] |
| Race/Ethnicity | |
| African American | 10 (6.6%) |
| Asian | 19 (12.6%) |
| Biracial | 29 (19.2%) |
| Hispanic | 10 (6.6%) |
| Other | 6 (4.0%) |
| White | 77 (51.0%) |
| Pollution Burden Percentile | 30.82 (21.46) [0.16–91.45] |
| Parent Education | 4.96 (1.24) [2–8] |
| Income-to-Needs Ratio | 1.33 (0.52) [0.05–1.97] |
| Depressive Symptoms, T1 | 2.23 (2.57) [0–11] |
| Depressive Symptoms, T2 | 2.26 (2.66) [0–13] |
| Depressive Symptoms, T3 | 3.36 (3.36) [0–13] |
| Mean Framewise Displacement, mm | 0.16 (0.10) [0.05–0.56] |
Parent education was coded as follows: 1 = no General Education Development/no high school diploma; 2 = General Education Development/high school diploma; 3 = some college; 4 = 2-year college degree; 5 = 4-year college degree; 6 = master’s degree; 7 = professional degree (M.D., J.D., D.D.S., etc.); 8 = doctorate.
T, time.
Table 2.
Bivariate Correlations Among Variables
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | −0.34a | −0.15 | −0.13 | −0.07 | 0.02 | 0.16 | −0.15 | −0.10 | −0.14 |
| 2. Sex | – | 0.13 | −0.14 | 0.03 | −0.05 | 0.06 | 0.27b | −0.02 | −0.1 |
| 3. Household Socioeconomic Disadvantage | – | – | 0.37a | 0.16 | −0.01 | 0.06 | 0.13 | 0.01 | −0.05 |
| 4. Neighborhood Socioeconomic Disadvantage | – | – | – | 0.29a | 0.02 | −0.06 | −0.02 | 0.13 | −0.01 |
| 5. Pollution Burden Percentile | – | – | – | – | −0.04 | −0.17 | 0.02 | −0.02 | 0.04 |
| 6. Depressive Symptoms (T1) | – | – | – | – | – | 0.29b | 0.08 | −0.03 | 0.18b |
| 7. Depressive Symptoms (T2) | – | – | – | – | – | – | 0.59a | 0 | −0.02 |
| 8. Depressive Symptoms (T3) | – | – | – | – | – | – | – | 0.02 | 0.04 |
| 9. Mean Framewise Displacement | – | – | – | – | – | – | – | – | 0.12 |
| 10. Time Difference | – | – | – | – | – | – | – | – | – |
Participant sex is coded −1 = male, 1 = female.
T, time.
p < .001.
p < .01.
Pollution Burden and Task-Evoked Functional Connectivity
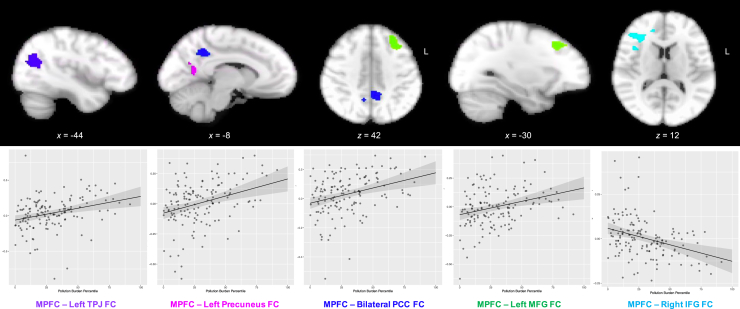
Greater pollution burden was positively associated with bilateral mPFC connectivity with regions that are typically part of the DMN, including the left temporoparietal junction, bilateral PCC, left precuneus, and left middle frontal gyrus, and negatively associated with bilateral mPFC connectivity with the right inferior frontal gyrus (IFG) during implicit emotion regulation of negatively valenced stimuli (Table 3 and Figure 2).
Table 3.
Results From Whole-Brain Psychophysiological Interaction Analyses
| Region | Hemisphere | Voxels | x | y | z | Z Max | p |
|---|---|---|---|---|---|---|---|
| Pollution Burden, Positive | |||||||
| TPJ | Left | 357 | −44 | −62 | 20 | 4.46 | <.001 |
| PCC | Left | 336 | −8 | −48 | 40 | 4.54 | <.001 |
| Right | 6 | −52 | 48 | 3.64 | |||
| Precuneus | Left | 178 | −16 | −64 | 16 | 4.23 | .006 |
| MFG | Left | 157 | −30 | 26 | 42 | 4.29 | .012 |
| Pollution Burden, Negative | |||||||
| IFG | Right | 228 | 32 | 30 | 12 | 4.43 | .002 |
Results indicating regions/clusters that were functionally correlated with bilateral medial prefrontal cortex (the seed region) during implicit regulation of negatively valenced stimuli (negative label > negative match) and which differed as a function of pollution burden, cluster-corrected at Z > 3.1, p < .025.
IFG, inferior frontal gyrus; MFG, middle frontal gyrus; PCC, posterior cingulate cortex; TPJ, temporoparietal junction.
Figure 2.
Results from whole-brain psychophysiological interaction analyses indicated that greater pollution burden was associated with greater bilateral medial prefrontal cortex (mPFC) connectivity with the left temporoparietal junction (TPJ), left precuneus, bilateral posterior cingulate cortex (PCC), and left middle frontal gyrus (MFG) and lower bilateral mPFC connectivity with the right inferior frontal gyrus (IFG) during implicit regulation of negatively valenced stimuli (negative label > negative match). FC, functional connectivity.
Pollution Burden, Functional Connectivity, and Depressive Symptoms
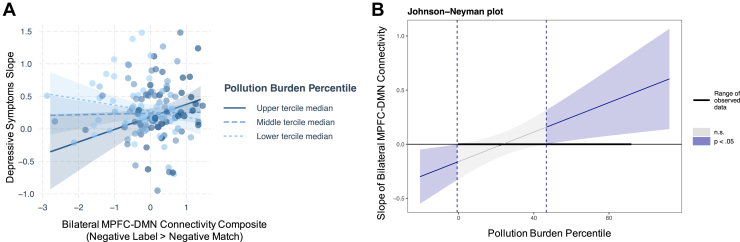
We examined whether mPFC connectivity during implicit regulation of negatively valenced stimuli (negative label > negative match) was associated with depressive symptoms at baseline and/or with changes in depressive symptoms across adolescence and whether the associations were moderated by pollution burden exposure. Clusters that showed greater mPFC connectivity as a function of pollution burden (i.e., the DMN regions) were standardized and averaged across participants to create an mPFC-DMN connectivity composite.
Neither mPFC-DMN nor mPFC-IFG connectivity was associated with baseline depressive symptoms over and above the effects of pollution burden, and there were no significant interactions between pollution burden and mPFC-DMN (Table 4) or mPFC-IFG (Table S6) connectivity in relation to baseline depressive symptoms. In contrast, however, there was a significant interaction between MPFC-DMN connectivity and pollution burden predicting slopes of depressive symptoms, controlling for baseline depressive symptoms and covariates (b = 0.007, SE = 0.026, t132 = 2.63, p = .0095) (Table 4). Simple slopes analysis using the Johnson-Neyman interval indicated that greater mPFC-DMN connectivity was associated with greater increases in depressive symptoms for youth residing in neighborhoods that rank in the 46.61st percentile (0.63 SD above the mean) and above in pollution burden (Figure 3). mPFC-IFG connectivity was not related to changes in depressive symptoms, and pollution burden did not interact with mPFC-IFG connectivity to predict changes in depressive symptoms (Table S6).
Table 4.
Results From Regression Analyses
| Depressive Symptoms |
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (Intercept) |
Longitudinal Changes (Slope) |
|||||||
| b | SE | t | p | b | SE | t | p | |
| Intercept | 1.18 | 0.47 | 2.51 | .013 | 0.05 | 0.21 | 0.24 | .81 |
| Sex | 0.14 | 0.19 | 0.76 | .45 | 0.12 | 0.08 | 1.54 | .12 |
| Age | −0.02 | 0.09 | −0.19 | .85 | −0.02 | 0.04 | −0.63 | .53 |
| Household Socioeconomic Disadvantage | 0.13 | 0.11 | 1.11 | .27 | 0.03 | 0.05 | 0.65 | .52 |
| Neighborhood Socioeconomic Disadvantage | −0.07 | 0.13 | −0.06 | .95 | −0.01 | 0.05 | −0.10 | .92 |
| Mean Framewise Displacement | −0.23 | 0.82 | −0.28 | .78 | 0.09 | 0.35 | 0.25 | .80 |
| Time Difference | 0.0007 | 0.0003 | 2.28 | .024 | −0.00002 | 0.0001 | −0.24 | .81 |
| Pollution Burden Percentile | −0.006 | 0.005 | −1.21 | .23 | −0.002 | 0.002 | −1.00 | .32 |
| mPFC-DMN Connectivity | 0.15 | 0.14 | 1.06 | .29 | 0.06 | 0.06 | 1.05 | .29 |
| mPFC-DMN Connectivity × Pollution Burden Percentile | −0.003 | 0.006 | −0.45 | .65 | 0.007 | 0.026 | 2.63 | .009 |
| Baseline Depressive Symptoms | – | – | – | – | 0.10 | 0.04 | 2.70 | .008 |
Results from regression analyses testing whether mPFC-DMN connectivity during negative label > negative match was related to baseline (left) and/or longitudinal changes (right) in depressive symptoms and whether the association differed by pollution burden. Analysis examining longitudinal changes in depressive symptoms also controlled for baseline depressive symptoms. Age, depressive symptoms intercept, and pollution burden percentile are mean centered.
DMN, default mode network; mPFC, medial prefrontal cortex.
Figure 3.
(A) Functional connectivity between bilateral medial prefrontal cortex (mPFC) and default mode network (DMN) regions (i.e., left temporoparietal junction, left precuneus, bilateral posterior cingulate cortex, and left middle frontal gyrus) during implicit regulation of negative emotions was positively associated with longitudinal increases in depressive symptoms among more (upper tercile median = 51.76st percentile: b = 0.19, SE = 0.09, t132 = 2.14, p = .03) pollution-burdened adolescents (solid line). Bilateral mPFC-DMN connectivity during implicit regulation of negative emotions was not associated with changes in depressive symptoms among less (lower tercile median = 9.86th percentile: b = −0.09, SE = 0.06, t132 = −1.43, p = .16) pollution-burdened adolescents (dashed line). (B) Johnson-Neyman plot depicting the interval of values of pollution burden for which the association of mPFC-DMN connectivity with slopes of depressive symptoms is statistically significant. n.s., not significant.
Exploratory supplemental analyses indicated that the following 7 environmental indicators moderated the association between mPFC-DMN connectivity and slopes of depressive symptoms: ambient ozone, diesel particulate matter, toxic releases from facilities, and living near cleanup sites, groundwater threats, hazardous waste facilities, and impaired water bodies (Table S7).
Including early-life adversity as a covariate did not change the findings (Table S10). The interaction between pollution burden and mPFC-DMN connectivity during negative label > negative match predicting the intercept and slope of externalizing symptoms was not significant (Table S11).
Discussion
The current study extends previous investigations to examine the effects of pollution burden on task-evoked brain functioning during implicit emotion regulation through adolescence and whether these effects underlie the link between pollution burden and depressive symptoms in youth. Using a data-driven approach, we localized regions/circuits activated during implicit emotion regulation that differed as a function of census tract–level pollution burden during early adolescence (9–13 years). Then we assessed whether these pollution-related differences in brain functioning were associated with within-person changes in depressive symptoms across 4 years. Consistent with extant structural and intrinsic fMRI findings [e.g., (18,20)], we found that adolescents exposed to relatively high levels of environmental pollution showed greater activation in the mPFC as well as stronger connectivity between the mPFC and regions that are part of the DMN (e.g., temporoparietal junction, PCC, precuneus) during implicit regulation of negatively valenced stimuli, which in turn were associated with greater longitudinal increases in depressive symptoms across adolescence in pollution-burdened adolescents. To our knowledge, the current study is the first to link pollution burden to brain functioning during emotion regulation across the transition to adolescence and to longitudinal changes in depressive symptoms.
Consistent with meta-analytic studies examining the neural correlates of emotion processing and emotion regulation (33,50), we found that matching (vs. labeling) emotional stimuli was associated with frontolimbic activation and that labeling (vs. matching) emotional stimuli was associated with activation in regions implicated in semantic processing. Although activation in these frontolimbic and semantic regions during emotion reactivity and regulation did not differ by pollution burden, pollution burden was associated with the engagement of additional regions within the DMN (e.g., mPFC, PCC, precuneus, temporoparietal junction) during implicit regulation of negatively but not positively valenced stimuli. Activation in these regions has been associated with the conceptualization of emotions (50,51)—that is, making meaning out of external sensory stimuli by drawing from prior experiences (semantic knowledge) and integrating external sensory perceptions with input from the body to create discrete emotions (51,52). Thus, aberrant DMN functioning may lead to dysregulated self-referential and affective processing. In addition to alterations while at rest (53, 54, 55), researchers have found that adults (56,57) and adolescents (27) at risk for depression show heightened activation in DMN regions when processing negative emotional stimuli, which is correlated with greater rumination in adults at risk for depression (57) and greater depression severity and earlier age of depression onset in adolescents (27). Our finding that greater connectivity within DMN regions during implicit regulation of negative emotions was associated with greater increases in depressive symptoms in adolescents exposed to greater pollution burden is consistent with this formulation and suggests that altered DMN functioning during emotion processing and regulation is one mechanism through which pollution exposure is associated with heightened risk for depression. Furthermore, mPFC-DMN connectivity was not related concurrently to depressive symptoms at baseline, suggesting that DMN alterations during emotion regulation in early adolescence is a risk factor for developing depressive symptoms across adolescence and pointing to early adolescence as a period during which emotional health can be improved.
While the precise mechanisms by which pollution and its burden are related to altered DMN connectivity are not well understood, experimental studies suggest that pollutants can have direct effects on brain cells through translocation from systemic circulation (e.g., through lungs) or olfactory transport (58,59). Alternatively, and perhaps more commonly, pollutants can also affect the brain through indirect processes, such as through activation of the hypothalamic-pituitary-adrenal axis, peripheral oxidative stress, and inflammation, the dysregulation of which have been consistently documented to influence brain development and health (14,31,60). For example, studies in adults have found that systemic inflammation is associated with altered connectivity of the DMN at rest (61), in response to affective stimuli (62), and with weaker suppression of the DMN during social cognitive processing in individuals exposed to early adversity (63,64). In adolescents at high risk for depression, systemic inflammation has been associated with stronger connectivity between the ventral striatum and DMN during reward processing (65). These studies suggest that the association between pollution burden and DMN functioning during emotion regulation is mediated by increases in inflammation (and by alterations in stress biology more generally). Future studies that integrate biological indices of stress with measures of environmental pollutants and multimodal brain imaging are needed to test these formulations more explicitly and systematically.
We should note 3 limitations of this study. First, we did not assess participants’ direct exposure to pollutants; instead, we estimated their exposure to pollutants using information from nearby monitoring sites and their geographical proximity to pollution sources. Furthermore, because the time of measurement varied between indicators (i.e., from 2012 to 2016), our estimate of pollution burden is spatially and temporally coarse. Future studies using personal exposure monitors are needed to measure concentrations and durations of individuals’ exposure to pollutants more precisely. Second, we do not have information about participants’ exposure to pollution during the prenatal period or about the length of time that participants resided at the address that they provided at baseline. Consequently, we cannot distinguish chronic from acute effects of pollution on brain development or identify sensitive periods during which participants’ brains are most vulnerable to the effects of pollutants. Longitudinal studies assessing multiple pollutants across development are needed to determine when and how different combinations of exposures affect neurobiology at different stages of development and to identify the most vulnerable individuals. Finally, our participants are from communities within the California Bay Area, which limits the generalizability of our findings. In this context, although the participants in our sample experienced a wide range of pollution burden (ranging from <1st percentile to the 91.5th percentile), the average exposure in our sample was relatively low (mean = 31 percentile). Nevertheless, even at these relatively low levels, pollution burden in our study was associated with brain functioning and increases in depressive symptoms; certainly, it is possible that these effects are stronger in communities with greater pollution burden. Furthermore, we should note that in our sample, participants with higher pollution burden were more likely to be missing fMRI data (either they did not participate in the brain scan portion of the study or they had low-quality fMRI scans), reflecting the longstanding challenges of recruiting and retaining individuals from more disadvantaged backgrounds [e.g., (66, 67, 68, 69)].
Conclusions
Despite these limitations, this study is important in demonstrating that adolescents living in communities characterized by greater pollution burden show stronger activation in the mPFC and stronger connectivity between the mPFC and regions within the DMN during implicit regulation of negatively valenced stimuli, which in turn is associated with greater increases in depressive symptoms across adolescence. Given the ubiquity of pollutants and increasing concerns about climate change and its effects, these findings have important public health implications.
Acknowledgments and Disclosures
This work was supported by the National Institute of Mental Health (Grant No. F32MH135657 [to JPU] and Grant No. R37MH101495 [to IHG]).
We thank Dr. Jessica L. Buthmann for sharing her scripts for some of the supplemental analyses.
Data are available on request.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2024.100322.
Supplementary Material
References
- 1.Breslau J., Gilman S.E., Stein B.D., Ruder T., Gmelin T., Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copeland W.E., Alaie I., Jonsson U., Shanahan L. Associations of childhood and adolescent depression with adult psychiatric and functional outcomes. J Am Acad Child Adolesc Psychiatry. 2021;60:604–611. doi: 10.1016/j.jaac.2020.07.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Lancet Global Health Mental health matters. Lancet Glob Heal. 2020;8 doi: 10.1016/S2214-109X(20)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization “Depression: let’s talk” says WHO, as depression tops list of causes of ill health. 2017. https://www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health Available at: Accessed October 24, 2023.
- 6.World Health Organization More Than 90% of the World’s Children Breathe Toxic Air Every Day. 2018. https://www.who.int/news/item/29-10-2018-more-than-90-of-the-worlds-children-breathe-toxic-air-every-day Available at: Accessed October 24, 2023.
- 7.Xie H., Cao Y., Li J., Lyu Y., Roberts N., Jia Z. Affective disorder and brain alterations in children and adolescents exposed to outdoor air pollution. J Affect Disord. 2023;331:413–424. doi: 10.1016/j.jad.2023.03.082. [DOI] [PubMed] [Google Scholar]
- 8.Zundel C.G., Ryan P., Brokamp C., Heeter A., Huang Y., Strawn J.R., Marusak H.A. Air pollution, depressive and anxiety disorders, and brain effects: A systematic review. Neurotoxicology. 2022;93:272–300. doi: 10.1016/j.neuro.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castagna A., Mascheroni E., Fustinoni S., Montirosso R. Air pollution and neurodevelopmental skills in preschool- and school-aged children: A systematic review. Neurosci Biobehav Rev. 2022;136 doi: 10.1016/j.neubiorev.2022.104623. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C.H., Sears L., Myers J.V., Brock G.N., Sears C.G., Zierold K.M. Proximity to coal-fired power plants and neurobehavioral symptoms in children. J Expo Sci Environ Epidemiol. 2022;32:124–134. doi: 10.1038/s41370-021-00369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wylie A.C., Short S.J. Environmental toxicants and the developing brain. Biol Psychiatry. 2023;93:921–933. doi: 10.1016/j.biopsych.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler C.H., Bagdasarov A., Camacho N.L., Reuben A., Gaffrey M.S. Toxicant exposure and the developing brain: A systematic review of the structural and functional MRI literature. Neurosci Biobehav Rev. 2023;144 doi: 10.1016/j.neubiorev.2022.105006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herting M.M., Younan D., Campbell C.E., Chen J.C. Outdoor air pollution and brain structure and function from across childhood to young adulthood: A methodological review of brain MRI studies. Front Public Health. 2019;7:332. doi: 10.3389/fpubh.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angiulli A. Severe urban outdoor air pollution and children’s structural and functional brain development, from evidence to precautionary strategic action. Front Public Health. 2018;6:95. doi: 10.3389/fpubh.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alemany S., Vilor-Tejedor N., García-Esteban R., Bustamante M., Dadvand P., Esnaola M., et al. Traffic-related air pollution, APOE∊4 status, and neurodevelopmental outcomes among school children enrolled in the BREATHE project (Catalonia, Spain) Environ Health Perspect. 2018;126 doi: 10.1289/EHP2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortamais M., Pujol J., van Drooge B.L., Macià D., Martínez-Vilavella G., Reynes C., et al. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ Int. 2017;105:12–19. doi: 10.1016/j.envint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Cserbik D., Chen J.C., McConnell R., Berhane K., Sowell E.R., Schwartz J., et al. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas I., et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Pujol J., Fenoll R., Macià D., Martínez-Vilavella G., Alvarez-Pedrerol M., Rivas I., et al. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 2016;6 doi: 10.1002/brb3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter D.L., Campbell C.E., Sukumaran K., McConnell R., Berhane K., Schwartz J., et al. Effects of ambient fine particulates, nitrogen dioxide, and ozone on maturation of functional brain networks across early adolescence. Environ Int. 2023;177 doi: 10.1016/j.envint.2023.108001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannilli E., Gasparotti R., Hummel T., Zoni S., Benedetti C., Fedrighi C., et al. Effects of manganese exposure on olfactory functions in teenagers: A pilot study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0144783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Rakesh D., Allen N.B., Whittle S. Balancing act: Neural correlates of affect dysregulation in youth depression and substance use – A systematic review of functional neuroimaging studies. Dev Cogn Neurosci. 2020;42 doi: 10.1016/j.dcn.2020.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler C.H., Miernicki M.E., Rudolph K.D., Telzer E.H. Disrupted amygdala-prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Dev Cogn Neurosci. 2017;27:99–106. doi: 10.1016/j.dcn.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlman G., Simmons A.N., Wu J., Hahn K.S., Tapert S.F., Max J.E., et al. Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon J.A., Thompson J.C., Chaplin T.M. Task-based functional connectivity patterns: Links to adolescent emotion regulation and psychopathology. J Affect Disord. 2022;302:33–40. doi: 10.1016/j.jad.2022.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho T.C., Connolly C.G., Henje Blom E., LeWinn K.Z., Strigo I.A., Paulus M.P., et al. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry. 2015;78:635–646. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaylord A., Osborne G., Ghassabian A., Malits J., Attina T., Trasande L. Trends in neurodevelopmental disability burden due to early life chemical exposure in the USA from 2001 to 2016: A population-based disease burden and cost analysis. Mol Cell Endocrinol. 2020;502 doi: 10.1016/j.mce.2019.110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessum C.W., Apte J.S., Goodkind A.L., Muller N.Z., Mullins K.A., Paolella D.A., et al. Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc Natl Acad Sci USA. 2019;116:6001–6006. doi: 10.1073/pnas.1818859116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T., Wang J., Huang J., Kelly F.J., Li G. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305–313. doi: 10.1001/jamapsychiatry.2022.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson E.M. Air pollution, stress, and allostatic load: Linking systemic and central nervous system impacts. J Alzheimers Dis. 2019;69:597–614. doi: 10.3233/JAD-190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jankowska-Kieltyka M., Roman A., Nalepa I. The air we breathe: Air pollution as a prevalent proinflammatory stimulus contributing to neurodegeneration. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.647643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzi E., Vijayakumar N., Rakesh D., Whittle S. Neural correlates of emotion regulation in adolescents and emerging adults: A meta-analytic study. Biol Psychiatry. 2021;89:194–204. doi: 10.1016/j.biopsych.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs M. In: Cautin R.L., Lilienfeld S.O., editors. John Wiley & Sons; Hoboken, New Jersey: 2015. Children’s Depression Inventory (CDI and CDI 2) pp. 495–499. (The Encyclopedia of Clinical Psychology). [Google Scholar]
- 35.Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 36.Colich N.L., Williams E.S., Ho T.C., King L.S., Humphreys K.L., Price A.N., et al. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Dev Psychopathol. 2017;29:1851–1864. doi: 10.1017/S0954579417001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan J.P., Ho T.C., Coury S.M., Chahal R., Colich N.L., Gotlib I.H. Early life stress, systemic inflammation, and neural correlates of implicit emotion regulation in adolescents. Brain Behav Immun. 2022;105:169–179. doi: 10.1016/j.bbi.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 39.Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban O., Ciric R., Finc K., Blair R.W., Markiewicz C.J., Moodie C.A., et al. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nat Protoc. 2020;15:2186–2202. doi: 10.1038/s41596-020-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 43.Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 44.Cooper R., Di Biase M.A., Bei B., Allen N.B., Schwartz O., Whittle S., Cropley V. Development of morning–eveningness in adolescence: Implications for brain development and psychopathology. J Child Psychol Psychiatry. 2023;64:449–460. doi: 10.1111/jcpp.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uy J.P., Gotlib I.H. Associations among early life adversity, sleep disturbances, and depressive symptoms in adolescent females and males: A longitudinal investigation. J Child Psychol Psychiatry. 2023 doi: 10.1111/jcpp.13942. [published online Dec 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellwood-Lowe M.E., Whitfield-Gabrieli S., Bunge S.A. Brain network coupling associated with cognitive performance varies as a function of a child’s environment in the ABCD study. Nat Commun. 2021;12:7183. doi: 10.1038/s41467-021-27336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson B.S., Bansal R., Sawardekar S., Nati C., Elgabalawy E.R., Hoepner L.A., et al. Prenatal exposure to air pollution is associated with altered brain structure, function, and metabolism in childhood. J Child Psychol Psychiatry. 2022;63:1316–1331. doi: 10.1111/jcpp.13578. [DOI] [PubMed] [Google Scholar]
- 48.LeMoult J., Humphreys K.L., Tracy A., Hoffmeister J.A., Ip E., Gotlib I.H. Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59:842–855. doi: 10.1016/j.jaac.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- 50.Brooks J.A., Shablack H., Gendron M., Satpute A.B., Parrish M.H., Lindquist K.A. The role of language in the experience and perception of emotion: A neuroimaging meta-analysis. Soc Cogn Affect Neurosci. 2017;12:169–183. doi: 10.1093/scan/nsw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. The brain basis of emotion: A meta-analytic review. Behav Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mancuso L., Cavuoti-Cabanillas S., Liloia D., Manuello J., Buzi G., Cauda F., Costa T. Tasks activating the default mode network map multiple functional systems. Brain Struct Funct. 2022;227:1711–1734. doi: 10.1007/s00429-022-02467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon B. Towards a new model of understanding – The triple network, psychopathology and the structure of the mind. Med Hypotheses. 2019;133 doi: 10.1016/j.mehy.2019.109385. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser R.H., Kang M.S., Lew Y., Van Der Feen J., Aguirre B., Clegg R., et al. Abnormal frontoinsular-default network dynamics in adolescent depression and rumination: A preliminary resting-state co-activation pattern analysis. Neuropsychopharmacology. 2019;44:1604–1612. doi: 10.1038/s41386-019-0399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou T., Deckersbach T., Dougherty D.D., Hooley J.M. The default mode network and rumination in individuals at risk for depression. Soc Cogn Affect Neurosci. 2023;18 doi: 10.1093/scan/nsad032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucchini R.G., Dorman D.C., Elder A., Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2012;33:838–841. doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costa L.G., Cole T.B., Dao K., Chang Y.C., Garrick J.M. Developmental impact of air pollution on brain function. Neurochem Int. 2019;131 doi: 10.1016/j.neuint.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marsland A.L., Kuan D.C.-H., Sheu L.K., Krajina K., Kraynak T.E., Manuck S.B., Gianaros P.J. Systemic inflammation and resting state connectivity of the default mode network. Brain Behav Immun. 2017;62:162–170. doi: 10.1016/j.bbi.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alvarez G.M., Hackman D.A., Miller A.B., Muscatell K.A. Systemic inflammation is associated with differential neural reactivity and connectivity to affective images. Soc Cogn Affect Neurosci. 2020;15:1024–1033. doi: 10.1093/scan/nsaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King S., Mothersill D., Holleran L., Patlola S., McManus R., Kenyon M., et al. Childhood trauma, IL-6 and weaker suppression of the default mode network (DMN) during theory of mind (ToM) performance in schizophrenia. Brain Behav Immun Health. 2022;26 doi: 10.1016/j.bbih.2022.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King S., Mothersill D., Holleran L., Patlola S.R., Burke T., McManus R., et al. Early life stress, low-grade systemic inflammation and weaker suppression of the default mode network (DMN) during face processing in Schizophrenia. Transl Psychiatry. 2023;13:213. doi: 10.1038/s41398-023-02512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rengasamy M., Nance M., Eckstrand K., Forbes E. Splitting the reward: Differences in inflammatory marker associations with neural connectivity between reward anticipation and reward outcome in adolescents at high risk for depression. J Affect Disord. 2023;327:128–136. doi: 10.1016/j.jad.2023.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellard-Gray A., Jeffrey N.K., Choubak M., Crann S.E. Finding the hidden participant: Solutions for recruiting hidden, hard-to-reach, and vulnerable populations. Int J Qual Methods. 2015;14:1–10. [Google Scholar]
- 67.Fisher-Hoch S.P., Below J.E., North K.E., McCormick J.B. Challenges and strategies for recruitment of minorities to clinical research and trials. J Clin Transl Sci. 2023;7:e154. doi: 10.1017/cts.2023.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogel A., Comtesse H., Rosner R. Challenges in recruiting and retaining adolescents with abuse-related posttraumatic stress disorder: Lessons learned from a randomized controlled trial. Child Adolesc Psychiatry Ment Health. 2020;14:14. doi: 10.1186/s13034-020-00320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cosgrove K.T., McDermott T.J., White E.J., Mosconi M.W., Thompson W.K., Paulus M.P., et al. Limits to the generalizability of resting-state functional magnetic resonance imaging studies of youth: An examination of ABCD Study® baseline data. Brain Imaging Behav. 2022;16:1919–1925. doi: 10.1007/s11682-022-00665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.